Introduction
Hereditary angioedema (HAE) is a rare, autosomal dominant disorder related to C1 inhibitor (C1-INH). It is characterized by recurrent attacks of localized swelling of different body parts mostly involving the face and limbs. The prevalence of hereditary angioedema is 1 in 50,000 (Santacroce et al., 2023). A large case series of patients with HAE diagnosed based on clinical and laboratory criteria as well as the mutation spectrum of SERPING1 gene have been reported from India (Jindal et al., 2021;Perumalla et al., 2021). Being rare, HAE is mostly misdiagnosed due to symptoms mimicking other disorders involving edema of body parts.
There is a mean delay of more than 10 years between the onset of symptoms and diagnosis due to lack of awareness and diagnostic facilities. Most deaths occur in undiagnosed cases since conventional therapies such as corticosteroids and antihistaminics are ineffective. Involvement of larynx or tongue may be life-threatening. There are three types of HAE depending on the etiology. Therapies have become available for the treatment of episodes and short or long-term prophylaxis. Awareness of this rare disease is important for correct and timely diagnosis and initiation of lifesaving treatments.
Etiolopathology of HAE
Based on quantitative or qualitative defects of the C1-inhibitor, HAE has been categorized as HAE with deficient C1-INH (type I) (MIM #106100), HAE with dysfunctional C1-INH (type II), and HAE with normal C1-INH. More than 150 different pathogenic variants have been identified in patients with type I and II HAE. Type I HAE results from a quantitative deficiency of C1-INH due to variants in the SERPING1 gene, accounting for approximately 85% of cases. In type I HAE, the different SERPING1 mutations result in truncated or misfolded proteins that cannot be secreted; hence a deficiency in C1-INH level. Type II HAE which has dysfunctional C1-INH protein is responsible for approximately 15% of cases. In type II, SERPING1 mutations include residues at or near the active site on the reactive mobile loop that result in a mutant C1-INH protein which is secreted but dysfunctional. As a result, type II HAE has normal or even elevated C1-INH with decreased protein function (Zhang et al., 2018). Variants in six other different genes have been identified in affected individuals with normal C1-INH (HAE III). These genes are in the factor XII (F12), plasminogen (PLG), angiopoietin 1 (ANGPT1), kininogen 1 (KNG1), myoferlin (MYOF), and heparan sulfate (HS)-glucosamine 3-O-sulfotransferase 6 (HS3ST6) and mostly act through plasmin.
Clinical manifestations of HAE
The onset of symptoms of HAE is around adolescence or during childhood in most cases. It manifests with any combination of painless, non-pruritic, nonpitting swelling of submucosal, dermal, or cutaneous tissue with or without severe abdominal pain, or acute airway obstruction due to laryngeal edema. The attacks last for 2 to 5 days, usually slowly increasing and then resolving even without therapy. Triggers for acute attacks of HAE are trauma, infections, stress, or procedures in 40% of cases. Other triggers include ACE inhibitors and estrogens. Prodromal symptoms such as rash (erythema marginatum) occur before majority of attacks. The most frequent sites of swelling include the skin (100%), the abdomen (97%), and the larynx (54%). The life-threatening episodes of laryngeal edema are not infrequent. The disease-associated mortality rates can be up to 33% in hospitalized patients (Agostoni et al., 1992).
Diagnosis of HAE
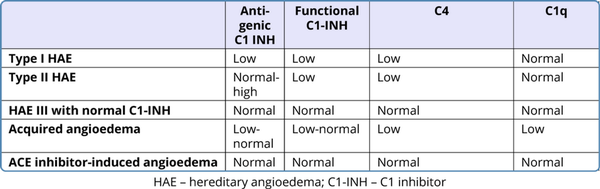
The inappropriately increased levels of bradykinin along with low levels of C4, C1-INH protein and/or C1-INH function is diagnostic. Low serum C1-INH level is characteristic of HAE I. In type II HAE the quantitative level of C1-INH is normal but functional assay shows deficiency of C1-INH function. The diagnosis is based on laboratory assessment as shown in Table 1. C1-INH deficiency or dysfunction increases bradykinin production by inhibiting proteases involved in the complement, contact-system, coagulation, and fibrinolytic pathway (Figure 1). The critical functional threshold for C1-INH control of the plasma contact system is approximately 40%.
The acquired angioedema (AAE) with C1 esterase inhibitor deficiency associated with underlying malignancy or rheumatologic disease, angiotensin-converting enzyme inhibitor-induced angioedema, and hypersensitivity reactions or urticaria/angioedema syndromes needs to be considered as differential diagnosis in appropriate clinical scenarios. The acquired type has histamine as the main mediator while in HAE, increased bradykinin is responsible for recurrent episodes of swelling.
Management of HAE
The unavailability of specific therapy leads to significant morbidity and mortality even in diagnosed cases. In most cases, there is inappropriate and rampant use of antihistaminics, corticosteroids, adrenaline, etc. Sometimes, misdiagnosis leads to unnecessary surgical intervention in cases with HAE presenting with pain abdomen mimicking acute abdomen. Often the recurrent nature of angioedema attacks with no proper treatment modalities leads to self-searching for unconventional treatment options by the patients. The utility of unconventional therapies without proper trials or approval is questionable and can be quite dangerous for the patient acutely or in the long term.
Historical aspects and recent advances
In 1962, Landerman observed a lack of kallikrein inhibitory activity in the plasma of HAE patient plasma. The inherited functional deficiency of C1 INH in affected individuals was first identified by Donaldson and Evans in 1963. Replacement therapy with fresh frozen plasma was tried in 1964-65 as the treatment for acute attacks since the patients did not respond satisfactorily to treatment with epinephrine, antihistaminic agents, or corticosteroids. Subsequently, C1 inhibitors purified from human plasma were found to be effective in both prevention as well as in terminating attacks of angioedema when used as the first specific treatment for HAE in 1973. The use of androgens was the first treatment modality tried for abatement for acute attacks. In 1980, Gadek conducted the first well-controlled study for replacement therapy in 8 HAE patients during an HAE attack by using partly purified C1INH from pooled plasma. In this study, 5 out of 8 patients showed abatement of symptoms in addition to increased serum C4 activity. The first C1 esterase inhibitor therapy (plasma derived C1INH concentrate) to be approved by FDA was Cinryze(r) in 2008. It is pasteurized with an additional nanofiltration step providing additional protection against enveloped and non-enveloped viral particles and prions. In 2009, Berinert(r) (a pasteurized plasma derived C1INH concentrate) licensed in Europe for over 20 years, got approval from FDA after the completion of a phase III study for use in the treatment of acute attacks in adolescent and adult patients. Early investigations prepared from the incubation of plasma from HAE patients identified a vascular permeability-enhancing factor named kallikrein assumed to be mediator of swelling in HAE. Trysolol with activity against trypsin, plasmin, and plasma kallikrein was the first plasma kallikrein inhibitor (other than C1INH) to be used. The major drawback associated was severe anaphylactoid reactions. To avoid such adverse effects, a specific plasma kallikrein inhibitor, Ecallantide, FDA-approved in 2009, was used to treat an angioedema attack with lesser risk of anaphylaxis. Inhibition of the binding of bradykinin to its receptor resolves most attacks of acute attack of HAE. Icatibant, FDA-approved in 2011, acts as a specific and selective competitive antagonist of the bradykinin B2 receptor. It can be self-administered as an on-demand treatment of all types of HAE attacks in adults and children with good safety and tolerability. In 2013, Lanadelumab, a fully human monoclonal antibody inhibitor of plasma kallikrein, got FDA global approval in the USA in 2018 after a succession of trials where it was used as a prophylaxis to prevent HAE attacks in patients aged 12 years or more (Syed et al., 2018).
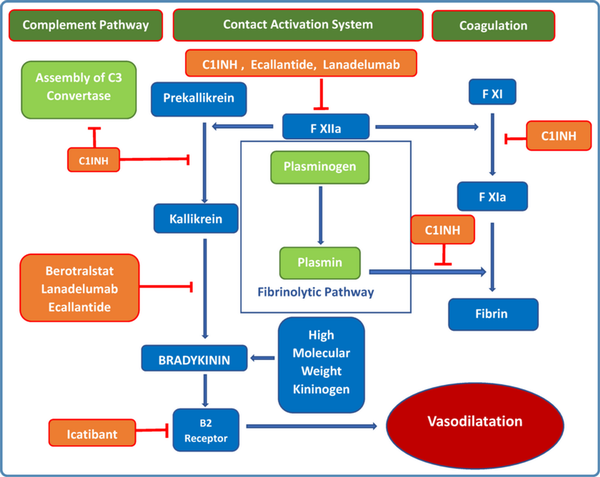
Figure 1
Pathophysiology of hereditary angioedema (HAE) and the site of action of the different drugs used for treatment of HAE. The names of the drugs are mentioned in the orange boxes.
Current therapies in practice
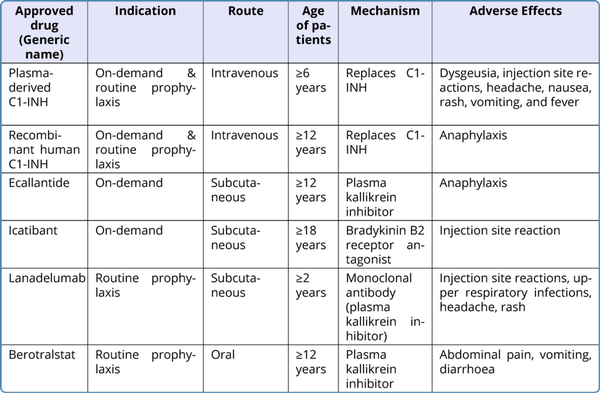
There are three different principles in the management of HAE. Table 2 summarizes currently available drugs. Due to easy availability and preventive action on plasminogen activation, Tranexamic acid is also used for acute attacks and prophylactically to treat hereditary angioedema. But the data about efficacy is not convincing.
A. On-demand therapy
On-demand therapy is for the treatment of acute attacks with an FDA-approved on-demand HAE medication (Ecallantide, Icatibant, plasma derived C1-INH, or recombinant C1-INH). The drug becomes effective in 60 min with relief in 2 hours. Second dose may be required. In situations of unavailability of specific therapy, solvent-detergent-treated plasma or FFP can be used along with supportive care (intravenous fluids, antiemetics, narcotic pain medication, or intubation).
B. Short-term prophylaxis
Recommended for all medical, surgical, and dental procedures associated with any mechanical impact to the upper aerodigestive tract. Intravenous plasma-derived C1-INH is the first-line short-term prophylaxis drug for HAE with fresh frozen plasma (FFP) as the second-line agent.
C. Long-term prophylaxis
In hereditary angioedema, long-term prophylaxis is indicated for frequent and/or severe episodes of angioedema.
Management of HAE in the pediatric age group
The first attack of HAE occurs in children before 12 years and 23 years of age in 50% and 90% of cases respectively. Most of the attacks are in the form of angioedema of the skin, may manifest as erythema marginatum in 42%-58% of cases. The frequency and severity of attacks may increase during puberty and adolescence. The earlier the onset of symptoms, the more severe the subsequent course of HAE type 1 or 2. Cases may get critical with delayed diagnosis since abdominal attacks may often go unrecognized. Also, asphyxia can ensue rapidly in children, probably because of the small airway diameter. C1-INH, icatibant and ecallantide are approved on-demand treatments for children with HAE 1 or 2.
Management of HAE in women
The symptoms of HAE are more severe in women. There should be cautious use of exogenous steroids in women. During pregnancy, C1-INH is recommended as first-line therapy for pregnant or breastfeeding HAE-1/2 patients. Short term prophylaxis is indicated for procedures. Ecallantide, Lanadelumab and Berotralstat are not recommended in pregnancy. Androgens are contraindicated. Short-term prophylaxis is not indicated in vaginal delivery, since attacks are uncommon during vaginal delivery. But there is increased angioedema of the vulva after delivery. A dose of plasma derived C1-INH is recommended during vacuum or forceps delivery. A preprocedural dose of plasma derived C1-INH is indicated for planned cesarean delivery. General anesthesia should be given with endotracheal intubation.
Plasma derived C1-INH or recombinant C1-INH for on-demand or prophylaxis is recommended. During lactation, anabolic androgens and tranexamic acid are contraindicated with no safety data on the use of Ecallantide, Icatibant, or Lanadelumab.
Gene therapy and other emerging therapies
Various therapies to cater to the unmet needs of HAE like long duration of action and oral drugs are in various stages of trial. Table 3 provides an overview of these emerging therapeutic modalities.
Like all other monogenic disorders, cure by gene therapy is the target of research. Ting Qiu et al in 2019, created a heterozygote C1EI deficient mouse model (S63+/-) that shared characteristics associated with HAE in humans including decreased plasma C1EI and C4 levels with increased vascular permeability of skin and internal organs. Single-time intravenous administration of an adeno-associated virus (AAV) gene transfer vector expressing the genetic sequence of the normal human C1 esterase-inhibitor to the gene-deficient mice resulted in sustained human C1EI activity levels above the predicted therapeutic levels and the correction of the vascular leak in the skin and internal organs.
Another promising gene therapy that is being developed for the treatment of HAE with C1-INH deficiency is BMN331. BMN331 is identified as AAV5 hSERPINGI, an adeno-associated virus (AAV5)-based gene therapy vector that expresses wild-type human C1 Esterase Inhibitor (hC1-INH), under the control of a liver-selective promoter. BMN331 is currently under a phase 1 / 2 open-label, dose-escalation study to determine the safety tolerability and efficacy.
Conclusion
Currently available therapies are effective and with improved understanding of pathophysiology, newer modalities are in research mode. C1-INH therapy is now available in India and though the cost is high, it has become accessible for Indian patients through funding support provided by the Government of India under the National Policy for Rare Diseases. Awareness about the disease will help in avoiding misdiagnosis. Gene therapy is an exciting approach with promising results in research studies and this is likely to significantly benefit patients with HAE.
References
- 1. Agostoni A, et al. Hereditary and acquired Cl-inhibitor deficiency: Biological and clinical characteristics in 235 patients. Medicine (Baltimore) 1992;71: 206–15.
- 2. Craig TJ, et al. Efficacy and safety of garadacimab, a factor XIIa inhibitor for hereditary angioedema prevention (VANGUARD): a global, multicentre, randomised, double-blind, placebo-controlled, phase 3 trial. Lancet. 2023; 401(10382):1079–1090.
- 3. Ferrone JD, et al. IONIS-PKKRx a Novel Antisense Inhibitor of Prekallikrein and Bradykinin Production. Nucleic Acid Ther. 2019; 29(2): 82–91.
- 4. Jindal AK, et al. Novel SERPING1 gene mutations and clinical experience of type 1 hereditary angioedema from North India. Pediatr Allergy Immunol. 2021; 32(3): 599–611.
- 5. Lesage A, et al. In vitro pharmacological profile of PHA-022121, a small molecule bradykinin B2 receptor antagonist in clinical development. Int Immunopharmacol 2022;105:108523.
- 6. Liu J, et al. An investigational RNAi therapeutic targeting Factor XII (ALN-F12) for the treatment of hereditary angioedema. RNA. 2019; 25(2): 255–263.
- 7. Perumalla S, et al. Clinical profile of hereditary angioedema from a tertiary care centre in India. Indian J Med Microbiol. 2021; 39(4): 509–512.
- 8. Santacroce R, et al. The Genetics of Hereditary Angioedema: A Review. J Clin Med. 2021; 10(9): 2023.
- 9. Sinnathamby ES, et al. Hereditary Angioedema: Diagnosis, Clinical Implications, and Pathophysiology. Adv Ther. 2023; 40(3): 814–827.
- 10. Spaulding W. Methyl Testesterone therapy for hereditary episodic edema (hereditary angioneurotic edema). Ann Intern Med 1960;1: 739–744.
- 11. Syed YY, et al. Lanadelumab: First Global Approval. Drugs. 2018; 78(15):1633–1637.
- 12. Qiu T, et al. Gene therapy for C1 esterase inhibitor deficiency in a Murine Model of Hereditary angioedema. Allergy. 2019; 74(6):1081–1089.
- 13. Zhang Y, et al. Exposure-Response Model of Subcutaneous C1-Inhibitor Concentrate to Estimate the Risk of Attacks in Patients with Hereditary Angioedema. CPT Pharmacometrics Syst Pharmacol. 2018; 7(3):158–165.