Introduction
Puberty is a dynamic stage in development, characterized by abrupt changes in height, weight, and body composition, all of which are sexually dimorphic. In this period of life, linear growth is at its fastest rate since infancy, and sexual differentiation is the greatest since fetal life []. Inadequate nutrition, hormonal deficiencies, and chronic disease may attenuate the pubertal growth spurt and impair final adult height []. Adolescence can provide a second opportunity to catch up on growth if environmental conditions, especially relating to nutrient intake, are favorable [].
In a previous study in similarly lean and short children of younger age (3–9 years) [, ], we showed that intervention with a multi-nutrient nutritional formula was a feasible, effective, and safe approach for promoting linear growth and weight gain in children without an obesogenic effect. We subsequently adapted the formula to the greater nutritional needs of adolescent boys to meet age- and gender-specific requirements (60% more energy and 45% more protein than in the previous formula, and higher doses of micronutrients). We recently reported the findings of the randomized double-blind placebo-controlled phase of this nutritional supplementation study, which we carried out in short and lean, healthy peripubertal children []. We found that 6 months of nutritional supplementation significantly improved weight, muscle mass, and the body mass index standard deviation score (BMI-SDS) in all the participants and prevented a Δ height-SDS decline in the participants who were older than 11.4 years at inclusion.
We now report the results of the open-labeled extension phase (6–12 months) of our study. In this phase, all the participants, both those who had received placebo and those who had received the formula, were offered to consume the formula for 6 months. The aims of the extension phase were to determine the 1-year effectiveness and safety of consumption of the formula on linear growth, weight gain, and changes in body composition of the original cohort of short and lean peripubertal children and to validate the previously reported findings in those initially treated with placebo.
Materials and Methods
The study design, sample size calculation, inclusion and exclusion criteria, dietary composition of the formula and placebo, study procedures, and methods have been previously reported in full []. Following 6 months of double-blinded intervention with nutritional formula or placebo (1:1), an open-label extension phase was carried out for another 6 months. In this phase, we provided the nutritional formula to all the participants in both the intervention and placebo arms. In the present work, we report the findings of the extension phase of the study and a 12-month analysis (clinicaltrials.gov; NCT02389803). A study checklist according to the CONSORT statement is provided in the supplement.
As reported previously [], eligible participants were healthy, short, and lean prepubertal boys (age ≥10 years) referred to the pediatric endocrinology units at three medical centers (Schneider Children’s Medical Center of Israel, the principal research center, and two other centers, Soroka Medical Center and Shamir Medical Center) for growth assessment. Inclusion criteria included Tanner stage 1 (testicular volume <4 mL), height and weight ≤10th percentile, and weight percentile ≤ height percentile for age and sex according to the 2000 Centers for Disease Control (CDC 2000) growth charts []. Exclusion criteria were the presence of chronic diseases including malabsorption or other gastrointestinal diseases; genetic syndromes or malignancies; growth hormone deficiency or treatment with recombinant growth hormone or any chronic medical treatment; and severe stunting (height-SDS <−2.5 SD) or wasting (BMI-SDS <−3 SD).
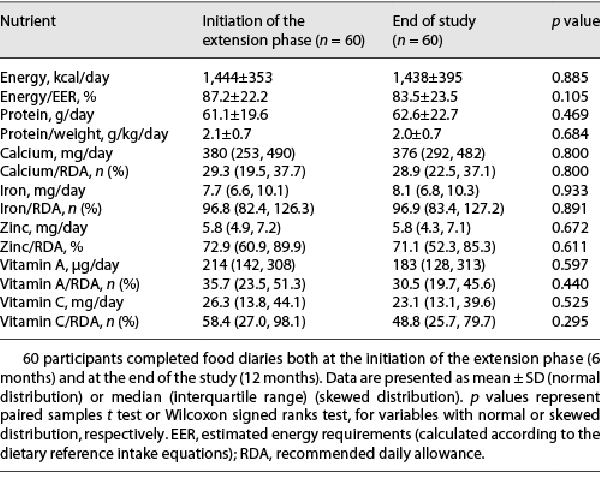
The study population flow diagram is presented in Figure 1. Overall, 126 boys completed the first 6 months of the double-blinded phase (65 – formula, 61 – placebo). Of these, 98 proceeded to the extension phase of the study, while 28 (15 – formula, 13 – placebo) did not. Of the latter, 25 rejected the offer to continue participation in the study, 11 due to poor consumption during the double-blind phase and 14 for other reasons, mainly COVID-19 limitations (lockdowns and parents’ concern about visiting the hospital during the pandemic). Two participants did not continue due to the initiation of growth hormone treatment and one participant due to a diagnosis of an eating disorder.
Fig. 1
Study flow diagram.
The nutritional supplementation formula (powder added to water) in vanilla or chocolate flavor contained about 25% of the recommended dietary reference intake for calories (the total daily amount was based on target nutritional needs for weight and height in the 50th percentile for age and gender and a low activity level of 570 kcal per recommended daily portion size), high protein (whey; 25% of calories; 36 g), 64 g carbohydrates, 19 g fat, and added vitamins and minerals (40–100% of the recommended daily allowance) [].
All the participants were instructed to consume one sachet of formula mixed with 200 mL of water after dinner (a total volume of 250 mL), in addition to their regular diet. Between visits, the participants and their parents were asked to record in a chart, the proportion consumed of the recommended daily dose from the total volume of nutritional supplement. The average consumption rate (% of the recommended dose) was calculated according to the parents’ report in the daily consumption chart. Consumption of ≥50% of the recommended dose was defined as “good,” while consumption of <50% of the formula was defined as “poor.” The consumption categories (good/poor) were defined according to the consumption of the formula and not of the placebo, as dose-response correlations between consumption and outcome parameters in the first phase of the study were found in the formula group only and not in the placebo group []. The participants were categorized into four groups according to initial randomization and formula consumption rate. Those who received formula in both phases of the study (a total of 12 months) were subcategorized as either good consumers (average intake ≥50% of the recommended dose) – Good-Formula-Formula (Good-FF) or poor consumers (average intake <50%) – Poor-Formula-Formula (Poor-FF). Participants who initially received 6 months of placebo were subcategorized as good consumers of the formula – Good-Placebo-Formula (Good-PF) or poor consumers of the formula – Poor-Placebo-Formula (Poor-PF), according to consumption in the extension phase only.
An anthropometric assessment at baseline and thereafter every 3 months up to 12 months included height and weight measurements. BMI was calculated as weight (kg)/height (m2). Height, weight, and BMI were expressed as SDS according to the recommendations of the U.S. Centers for Disease Control and Prevention []. Body composition was evaluated by bio-impedance analysis using a single-frequency 50-kHz leg-to-leg bio-impedance analysis system combined with a digital scale body composition analyzer (SC-330, Tanita Corporation America Inc., Ill., USA). Tanner stage was assessed by a pediatric endocrinologist at baseline and every 6 months up to 12 months. For assessing the safety of the intervention, fasting blood tests (hemoglobin, glucose, lipid profile, total proteins, albumin liver function, and basal levels of LH, FSH, and testosterone) were performed at baseline and every 6 months up to 12 months.
Dietary intake was assessed at baseline, and after 6 and 12 months of intervention, based on a 3-day food diary (2 weekdays and the weekend), and analyzed using Tzameret 3 (2016), a software developed in collaboration with the Israel Center for Disease Control and the Ministry of Health []. Safety assessments included reporting of adverse events and laboratory tests. IGF-1 concentrations were measured using an immunoassay (LIAISON Analyzer; DiaSorin, Saluggi, Italy). IGF-1 levels were converted to IGF-1-SDS.
Statistical Analysis
The data were analyzed using the SPSS software version 27 []. Distributions of continuous variables were examined by visual methods (histogram and QQ plot) and a Kolmogorov–Smirnov test. Continuous data are presented as the mean ± SD (symmetrical distribution) or median (interquartile range, IQR) (skewed distribution), and as number and percentage for categorical variables.
Spearman’s correlation was used to analyze correlations between formula consumption (skewed data) and changes in anthropometric and body composition parameters. Linear regression models were used to evaluate the effect of consumption rate, adjusted to baseline age and puberty at the end of the study (Tanner stage ≥2 vs. Tanner stage 1 as a reference group), on changes in anthropometric and body composition parameters.
As our cohort comprised short and lean children, a population that is often characterized as poor eaters, the consumption of the full dose of the formula was challenging. Therefore, all outcome parameters were analyzed according to consumption category (good/poor), as defined by the study statistical plan (clinicaltrials.gov; NCT02389803). Changes in anthropometrics and body composition after 6 and 12 months of intervention were compared between four groups, defined according to initial randomization and consumption rate, as detailed above. Differences between groups and consumption categories were evaluated by 1-way ANOVA analysis and post hoc Tukey.
One-sample t tests were used to evaluate the null hypothesis that a change in the relevant parameter equals 0. A significant test (p ≤ 0.05) represents a significant change in the parameter. The χ2 test and Fisher’s exact test were used to examine differences in categorical data.
Nutrient intake (without the supplement) was compared between two time points: the initiation of the extension phase (6 months) and the end of the study (12 months). The paired samples t test and Wilcoxon signed ranks test were used for variables with normal and skewed distributions, respectively.
Results
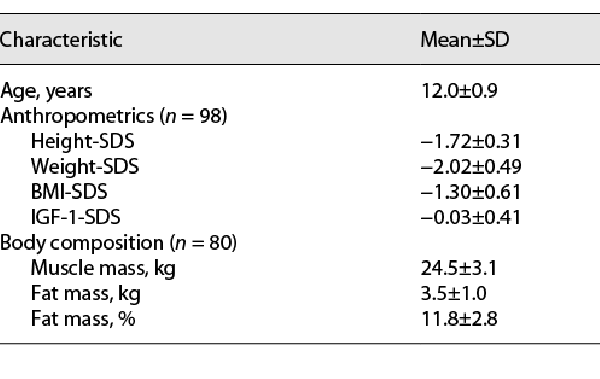
At initiation of the extension phase of the study, the mean age of the boys who proceeded to this phase (n = 98) was 12.0 ± 0.9 years. Anthropometrics and body composition of the participants at the initiation of the extension phase are presented in Table 1. Their mean height-SDS was −1.72 ± 0.31, weight-SDS −2.02 ± 0.49, BMI-SDS −1.30 ± 0.61, and IGF-1-SDS −0.03 ± 0.41. Seventy-nine completed this phase, while 19 dropped out (7 due to poor consumption, 11 due to COVID-19 limitations, and 1 due to initiation of Depalept treatment) (Fig. 1). The dropout rate was similar between participants subcategorized as good and poor consumers (8/57 vs. 11/41, respectively, p = 0.114).
At the beginning of the extension phase, 58 participants (62%) were at Tanner stage 1, 33 (36%) at Tanner stage 2, and 3 (2%) at Tanner stage 3 (four participants refused to have a Tanner stage evaluation by a pediatric endocrinologist). At the end of the study, 26 participants (35%) were at Tanner stage 1, 40 (54%) at Tanner stage 2, and 8 (11%) at Tanner stage 3.
Dose-Response Correlations
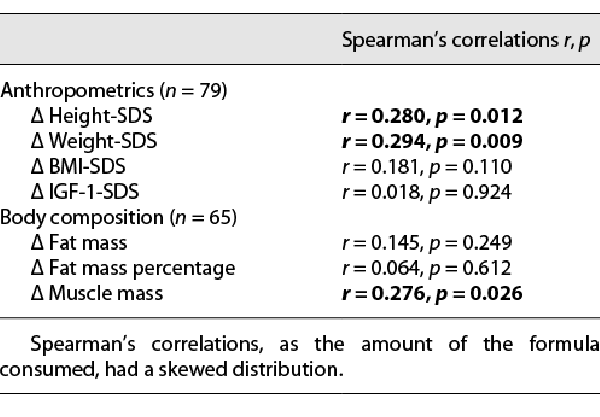
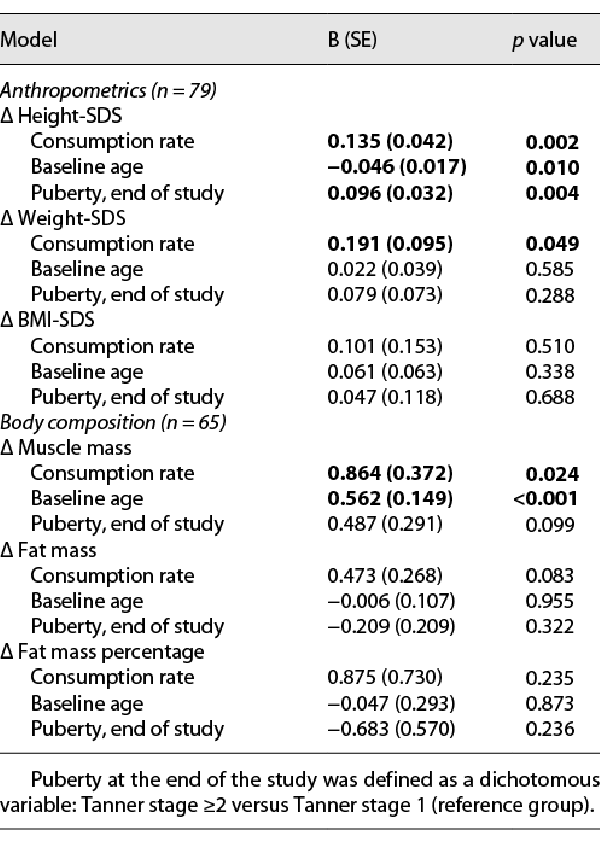
Dose-response correlations of the amount of formula consumed (average daily consumption rate) and changes in anthropometrics and body composition parameters during the extension phase are presented in Table 2 (crude correlations) and Table 3 (linear regression adjusted to baseline age and pubertal status at the end of the study). Significant positive dose-response correlations were found between formula consumption and changes in height-SDS, weight-SDS, and muscle mass (crude correlations and after adjustment for all the above parameters). In the linear regression analysis, a younger baseline age and Tanner stage ≥2 at the end of the study were also positively associated with the change in height-SDS. Older baseline age was positively associated with the change in muscle mass.
No significant dose-response correlations were found between formula consumption and changes in BMI-SDS, fat mass, fat mass percentage, and IGF-1-SDS. However, when analyzing these dose-response correlations separately among participants who consumed formula or placebo in the blinded phase, significant correlations were found only in the placebo-first group (Δ BMI-SDS: r = 0.561, p < 0.001; Δ fat mass: r = 0.502, p = 0.004; Δ fat mass percentage: r = 0.395, p = 0.028), but not among participants who consumed formula in the first 6 months of the study.
Changes in Tanner Stage, Anthropometrics, and Body Composition after 6 and 12 Months
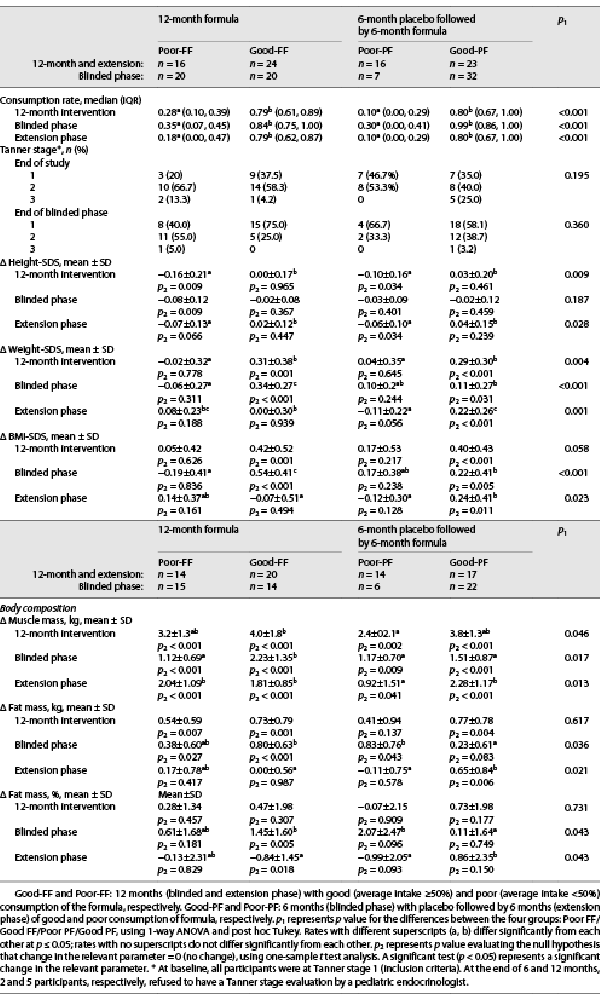
Table 4 presents Tanner stages and changes in outcome measures after 6 and 12 months of intervention for the participants who completed the study, according to the four groups defined above (Good-FF, Poor-FF, Good-PF, and Poor-PF). The results of the blinded phase for the participants who completed 12 months of intervention were similar to the results published for all the participants who completed the first phase. No between-group differences were observed in the Tanner stage at the end of the blinded phase (6 months) and at the end of the open-labeled extension phase (12 months).
Height-SDS
During the extension phase, height-SDS was maintained among good consumers of the formula (Good-FF and Good-PF) but declined among poor consumers (Poor-FF and Poor-PF) (p = 0.028 for the between-group difference). During the 12 months of intervention, Good-FF and Good-PF (those who consumed the formula for either 6 or 12 months) maintained their height-SDS, while Poor-FF and Poor-PF showed a decline in height-SDS (p = 0.009) (Fig. 2a).
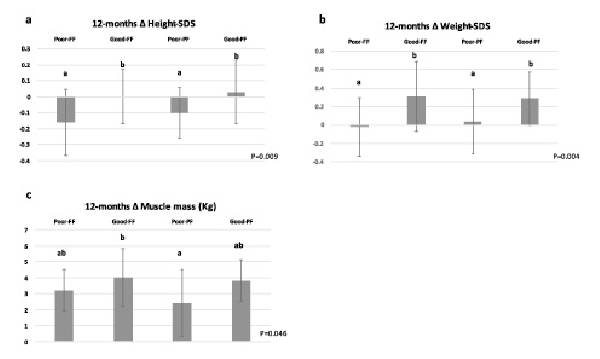
Fig. 2
12-month changes in height-SDS (a), weight-SDS (b), muscle mass (c). Good-FF and Poor-FF: 12 months (blinded and extension phase) with good (average intake ≥50%) and poor (average intake <50%) consumption of the formula, respectively. Good-PF and Poor-PF: 6 months (blinded phase) with placebo followed by 6 months (extension phase) of good and poor consumption of formula, respectively. P represents p value for the differences between the four groups: Poor FF/Good FF/Poor PF/Good PF, using 1-way ANOVA and post hoc Tukey. Rates with different superscripts (a, b) differ significantly from each other at p ≤ 0.05; rates with identical superscripts do not differ significantly from each other.
Weight-SDS and BMI-SDS
During the extension phase, only Good-PF gained significantly in weight-SDS (p2 < 0.001) and BMI-SDS (p2 = 0.011), while the other groups maintained their weight- and BMI-SDS (p2 > 0.05) (between-group differences, p = 0.001 and p = 0.023, respectively). During the 12 months of intervention, Good-FF and Good-PF gained more in weight-SDS compared to Poor-FF and Poor-PF (p = 0.004) (Fig. 2b). However, the between-group difference in BMI-SDS was not significant (p = 0.058).
Muscle Mass
During the extension phase, Poor-PF gained less muscle mass than did all the other subgroups (p = 0.013). During the 12 months of intervention, the mean gain in muscle mass was significantly higher for Good-FF than Poor-PF (4.0 vs. 2.4 kg), while the mean gains for Good-PF and Poor-FF, 3.8 kg and 3.2 kg, respectively, were not significantly different from those of the other groups (between-group difference, p = 0.046) (Fig. 2c).
Fat Mass and Fat Mass Percent
During the extension phase, the mean gain in fat mass was greater for Good-PF than for the other three groups (fat mass p = 0.021, fat mass percentage p = 0.043). However, the changes in fat mass and fat mass percentage throughout the 12 months of the study were relatively small (mean changes <0.8 kg and <0.8%, respectively, in all four groups); between-group differences were not significant (p = 0.671 and p = 0.731, respectively).
Statistically significant differences were not observed in the participants’ nutrient intake (energy, macronutrients, and micronutrients) other than the supplement between the beginning and the end of the extension phase (6–12 months) (Table 5). This indicates that the intervention did not affect regular daily nutritional intake.
Clinical adverse events included mild gastrointestinal symptoms after consumption of the formula, as reported by seven participants: sporadic stomachache (n = 3), nausea (n = 2), constipation (n = 1), and “feeling full” (n = 1). No laboratory adverse events were found in hemoglobin levels, serum chemistry, lipid profile, and urinalysis. No serious adverse events were reported during the study.
Discussion
Catch-up growth during adolescence is possible, according to studies of children with chronic diseases and of malnourished children from low- and middle-income countries, after resolution of the cause of growth failure []. Several longitudinal observational studies from such regions showed a reduction in the rate of stunting along childhood []. On the other hand, in many countries across the globe, children were found to be of optimal height at age 5 years but to fall behind by age 19 years when their height measured shorter than the median of the WHO reference, by ∼ 2 cm or more [].
This 1-year nutritional intervention study was aimed at improving growth in healthy, short, and lean peripubertal boys living in an affluent country. During the open-label extension phase, we found significant dose-response correlations between the consumption of the nutritional formula and Δ height-SDS, Δ weight-SDS, and Δ muscle mass (both crude correlations and after adjustment for baseline age and Tanner stage at the end of the study). These results are in line with the results of the first 6 months of the blinded phase of the study, in which we found similar significant dose-response correlations in the formula group but not in the placebo group []. Our results showed that good consumers of the formula for either 6 or 12 months maintained their height-SDS throughout the year, while poor consumers did not. These results suggest that the beneficial effect of the supplementation is not limited to the first 6 months of its consumption but is maintained for at least 12 months. Our results are presented in SDS to account for the big heterogeneity in the expected changes in cm for children of different ages and different height percentiles. This can be clarified by an example of the change in cm for a 12-year-old boy whose height is in the 10th percentile: A “good FF” consumer, whose change in height-SDS is 0.02 SDS over 6 months, will grow 3.1 cm in this time period. The equivalent boy in the “poor FF” group, whose change in height-SDS is −0.07 SDS in 6 months, will grow 2.5 cm at the same time. The difference in height gain is 0.6 cm/6 months (e.g., 1.2 cm/year). Similar estimations for 10–14-year-old boys in the 10th percentile at initiation show differences of 0.6–0.8 cm/6 months between good and poor consumers. This impact, although modest, may become clinically meaningful over time. No serious adverse events were reported, supporting the safety of the intervention, in line with the results of the first phase of the study.
Positive associations were also found between height-SDS and younger baseline age and Tanner stage ≥2 at the end of study. This may be explained by the fact that the older participants, who still had not begun puberty by the end of the study, were relatively late-maturing boys. For these boys, growth velocity is slower than in their age- and gender-matched peers [] and results in a temporary decline in height-SDS. In the extension phase and the 12-month analysis, participants who were good formula consumers managed to maintain their height-SDS score, while the poor formula consumers showed a significant decline in their height-SDS. This result is in line with the positive effect on height-SDS observed in the first phase of the study among the older participants [].
Muscle mass was positively correlated with baseline age. This is in line with the known muscle mass accrual in male adolescents, with increasing age and the progression of puberty during the second decade of life []. Participants who were good consumers of the formula throughout the year gained an average of 4 kg in muscle mass during the study.
During the first 6 months of the study, we witnessed positive correlations between formula consumption and Δ BMI-SDS, Δ fat mass, and Δ fat mass percent []. However, in the second phase of the study, this did not persist among the participants who continued to consume the formula, but only among those who had previously consumed the placebo. Similarly, during the extension phase, gains in BMI-SDS and fat mass were observed among good consumers of the formula who were initially in the placebo group but not among good consumers who were initially in the formula group. This suggests that the gains in BMI-SDS and fat mass attributed to formula consumption were attenuated after an initial gain. Importantly, the 12-month analysis showed similar gains in both parameters for all consumer groups. Furthermore, throughout the study, the net changes in fat mass and fat mass percentage were minor in all the groups (mean changes of <0.8 kg and <0.8%, respectively). These results support the safety of the intervention in terms of a possible obesogenic effect.
As no difference in Tanner stage was observed between the good and poor consumers of the formula at any stage of the study, our results do not support an effect of consumption of the formula on pubertal status. This result also suggests that the effect of the formula on growth parameters was not mediated by an enhancement of the pubertal tempo but by a direct effect of the nutritional intervention. Similarly to our previous study in 3–9-year-old short and lean boys and girls (5), no dose response was observed between formula consumption and change in IGF-1-SDS.
As the dietary intake (not including the supplement) of energy, protein, and micronutrients at the beginning of the extension phase and at the end of the study was similar, we presume that the growth observed was stimulated by the nutritional supplement and not by changes in the participants’ diets.
Assessment of the impact of nutritional support in older children and adolescents is challenging due to inter-individual differences in pubertal timing and tempo []. Only a few studies have attempted to improve growth by nutritional interventions in late childhood [], and none of them included a placebo-controlled design. Other nutritional interventional studies that aimed to enhance growth were either aimed at younger or otherwise different populations or were of a shorter duration [, -]. None of the aforementioned studies evaluated changes in body composition throughout 1 year of intervention to examine a possible negative metabolic effect. Therefore, the strengths of this study lie in its double-blinded, randomized placebo-controlled design, the relatively large sample size, the long duration, and the evaluation of body composition. The homogenous population of male adolescents in the current study enabled postulating causal relations between the intervention and outcomes.
The main limitation of our study is the partial consumption of the formula. The partial consumption could be attributed to the high protein and energy content of the formula, which may have affected the texture of the drink. However, this enabled the evaluation of dose-response correlations between consumption and the change in outcome measures. The dropout rate was in part due to the COVID-19 outbreak and participants’ reluctance to conduct hospital visits. Like many other studies on children’s growth, one of our primary endpoints was the change in height-SDS after a relatively short intervention and not the ultimate endpoint of final height.
In conclusion, the extension phase and the overall 1-year intervention with a multi-nutrient, protein-rich supplement were efficacious and safe. The induced changes in growth and body composition, although modest, may be clinically significant. Further research is needed to determine the optimal dose of the nutritional formula required to further improve the long-term growth response, to determine the effects of a similar intervention in lean and short healthy girls, and to determine the effect of such interventions throughout puberty. To isolate the effect of an intervention on growth in adolescents and to overcome the differences in pubertal timing and tempo of progression, well-designed, longitudinal interventions are required.
Acknowledgments
The authors thank the participating children and their families who made this study possible. Appreciation is also expressed to our research coordinators and to the study nurses. Data from this study were presented at the International Nutrition and Growth Conference in March 2022 as an oral lecture named “Food supplement for short and lean adolescent boys.”
Statement of Ethics
The study was approved by the Rabin Medical Center Institutional Review Board (067614RMC) (clinicaltrials.gov; NCT02389803). Written informed consent from parents was obtained prior to enrollment in the study.
Conflict of Interest Statement
Moshe Phillip is the chief scientific officer in NG Solutions and owns stock and options in the company. Raanan Shamir is the chief medical officer and board director in NG Solutions and owns stock and options in the company. Michal Yackobovitch-Gavan is the director of clinical studies in NG Solutions and owns stock and options in the company.
Funding Sources
This work is an investigator-initiated study that received grant support from NG Solutions.
Author Contributions
Michal Yackobovitch-Gavan: perceived the study concept and design, acquired data, contributed to the analysis, and drafted the manuscript. Liora Lazar: contributed substantially to the conception and interpretation of data and revised the manuscript critically for important intellectual content. Sharon Demol, Marie Mouler, Marianna Rachmiel, and Eli Hershkovitz: contributed substantially to the acquisition and interpretation of data and revised the manuscript critically for important intellectual content. Raanan Shamir and Moshe Phillip: perceived the study concept and design, contributed to the analysis, and revised the manuscript critically for important intellectual content. Naama Fisch Shvalb: contributed substantially to the conception of the study, acquired data, contributed to the analysis, and drafted the manuscript.
Data Availability Statement
All data generated or analyzed during this study are included in this article. Further inquiries can be directed to the corresponding author (Michal Yackobovitch-Gavan).
References
- 1. Rogol AD, Roemmich JN, Clark PA. Growth at puberty. J Adolesc Health. 2002 Dec;31(6):192–200. https://doi.org/10.1016/s1054-139x(02)00485-8.
- 2. Modan-Moses D, Yaroslavsky A, Pinhas-Hamiel O, Levy-Shraga Y, Kochavi B, Iron-Segev S, et al. Prospective Longitudinal Assessment of linear growth and adult height in female adolescents with anorexia nervosa. J Clin Endocrinol Metab. 2021 Jan;106(1):e1–e10. https://doi.org/10.1210/clinem/dgaa510.
- 3. Jacob JA, Nair MKC. Protein and micronutrient supplementation in complementing pubertal growth. Indian J Pediatr. 2012 Jan;79(S1):S84–91. https://doi.org/10.1007/s12098-011-0430-0.
- 4. Lebenthal Y, Yackobovitch-Gavan M, Lazar L, Shalitin S, Tenenbaum A, Shamir R, et al. Effect of a nutritional supplement on growth in short and lean prepubertal children: a prospective, randomized, double-blind, placebo-controlled study. J Pediatr. 2014 Dec;165(6):1190–3.e1. https://doi.org/10.1016/j.jpeds.2014.08.011.
- 5. Yackobovitch-Gavan M, Lebenthal Y, Lazar L, Shalitin S, Demol S, Tenenbaum A, et al. Effect of nutritional supplementation on growth in short and lean prepubertal children after 1 year of intervention. J Pediatr. 2016 Dec;179:154–9.e1. https://doi.org/10.1016/j.jpeds.2016.08.100.
- 6. Fisch Shvalb N, Lazar L, Demol S, Mouler M, Rachmiel M, Hershkovitz E, et al. Effect of a nutritional supplementation on growth and body composition in short and lean preadolescent boys: a randomised, double-blind, placebo-controlled study. Acta Paediatr. 2022 Jan;111(1):141–50. https://doi.org/10.1111/apa.16054.
- 7. Kuczmarski RJ, Ogden CL, Grummer-Strawn LM, Flegal KM, Guo SS, Wei R, et al. CDC growth charts: United States. Adv Data. 2000 Jun;(314):1–27.
- 8. Tzameret 3 software. Israel Center for Disease Control and the Ministry of Health. 2016.
- 9. IBM Corp. Released 2017. IBM SPSS Statistics for Windows. Armonk, NY: IBM Corp; 2017. Version 25.0.
- 10. Campisi S, Carducci B, Söder O, Bhutta Z. The Intricate relationship between chronic undernutrition, Impaired linear growth and delayed puberty: Is ‘catch-up’ growth possible during adolescence?Innocenti Working Papers no. 2018-12. Florence: UNICEF Office of Research - Innocenti.
- 11. Lassi ZS, Moin A, Das JK, Salam RA, Bhutta ZA. Systematic review on evidence-based adolescent nutrition interventions. Ann N Y Acad Sci. 2017 Apr;1393(1):34–50. https://doi.org/10.1111/nyas.13335.
- 12. NCD Risk Factor Collaboration NCD-RisC. Height and body-mass index trajectories of school-aged children and adolescents from 1985 to 2019 in 200 countries and territories: a pooled analysis of 2181 population-based studies with 65 million participants. Lancet. 2020 Nov;396(10261):1511–24. https://doi.org/10.1016/S0140-6736(20)31859-6.
- 13. Zhang Z, Li F, Hannon BA, Hustead DS, Aw MM, Liu Z, et al. Effect of oral nutritional supplementation on growth in children with undernutrition: a systematic review and meta-analysis. Nutrients. 2021 Aug;13(9):3036. https://doi.org/10.3390/nu13093036.
- 14. Han JC, Damaso L, Welch S, Balagopal P, Hossain J, Mauras N. Effects of growth hormone and nutritional therapy in boys with constitutional growth delay: a randomized controlled trial. J Pediatr. 2011 Mar;158(3):427–32. https://doi.org/10.1016/j.jpeds.2010.09.006.
- 15. Alarcon PA, Lin LH, Noche M Jr, Hernandez VC, Cimafranca L, Lam W, et al. Effect of oral supplementation on catch-up growth in picky eaters. Clin Pediatr. 2003 Apr;42(3):209–17. https://doi.org/10.1177/000992280304200304.
- 16. Khanna D, Yalawar M, Saibaba PV, Bhatnagar S, Ghosh A, Jog P, et al. Oral nutritional supplementation improves growth in children at malnutrition risk and with picky eating behaviors. Nutrients. 2021 Oct;13(10):3590. https://doi.org/10.3390/nu13103590.