Introduction
Pubertal onset is a complex biological process that is affected by genetic, hormonal, nutritional, environmental, and socioeconomic factors. These interactions culminate in an increase in the pulsatile secretion of gonadotropin-releasing hormone (GnRH), with the subsequent release of gonadotropins from the anterior pituitary, which in turn stimulate the gonad, to activate the steroidogenic machinery which gives rise to the appearance of secondary sexual characteristics and reproductive ability. The most plausible hypothesis involved in the activation of the reproductive axis is a decrease in the inhibitory tone in conjunction with a stimulation of the excitatory tone of the GnRH-producing neurons in the preoptic area of the hypothalamus []. Insulin-like growth factor-1 (IGF-1) has emerged as a candidate neuromodulator of pubertal onset []. Studies in animal models have shown IGF-1 receptor (IGF-1R) expression in the median eminence [, ] and the ability of IGF-1 to stimulate glial release of prostaglandin E2 [, ] which in turn stimulates the release of GnRH []. In prepubertal rats and sheep, IGF-1 administration induces LH secretion [, ]. Moreover, in a murine model, the administration of IGF-1 in the third ventricle increases the expression of neurokinin B and kisspeptin (excitatory tone) and decreases dynorphin (inhibitory tone) [, ]. In primates, increases in IGF-1 plasma levels accelerate the first ovulation []. In humans, the GH/IGF-I axis has been implicated in pubertal timing. Delayed puberty is a characteristic feature of disorders with lower activity in this axis []. There is evidence of an inverse association between IGF-1 peripubertal concentrations and pubertal development in girls [, ]. Despite the existence of animal and girls’ data, there is a paucity of this evidence in boys. Our goal was to test the association between prepubertal IGF-1 levels and pubertal developmental milestones in both sexes, considering other covariates known to affect pubertal timing [].
Materials and Methods
Study Population
The Growth and Obesity Chilean Cohort Study (GOCS) is a longitudinal follow-up of 1,190 girls and boys, recruited at ages between 3.0 and 4.9 years, attending Chilean National Nursery School Council Program from the southeast area of Santiago, and with no physical or psychological conditions that could severely affect growth. All included children were singleton birth with a birth weight (BW) between 2,500 and 4,500 g; further details of the cohort have been previously published []. For this study, we included those with available blood samples in year 2009 (mean age ≈6.7 years) with measurement of prepubertal concentrations of IGF-1 and dehydroepiandrosterone sulfate (DHEAS).
Pubertal Assessment
Yearly clinical anthropometric evaluation was performed until year 2009. Thereafter, at age 6.7 years, a single pediatric endocrinologist (VM) assessed breast (B), pubarche (P), and genital development by palpation and classified breast, pubic hair, and testes (G) according to Tanner [, ]. Thereafter, every 6 months, secondary sex characteristics were evaluated by a single dietitian (same sex) trained for this purpose, with permanent supervision of VM. Concordance between the dietitian and pediatric endocrinologist was 0.9 for breast and genitalia evaluation []. Age at menarche (M) was self-reported. Among the 1,190 participants in the cohort, 943 (489 girls and 454 boys) met all inclusion criteria. Seventeen girls had no data at thelarche (B2), but we had their age at M. These girls were not included in B2 analysis.
Anthropometry
Weight and height were collected using standardized protocols by two dietitians (one female and one male) with inter- and intra-observer correlation coefficients over 0.80. Measurements were taken as follows: weight using a portable electronic scale (Seca 770), with precision of 0.1 kg, and height with a portable stadiometer (Harpenden 603) to the nearest 0.1 cm. Body mass index (BMI) was calculated by dividing weight (kilograms) by (height)2 (meters). We estimated height for age and BMI for age using the World Health Organization 2007 growth reference []. BW was obtained from medical records []. Body composition was assessed using bioimpedance analysis measurements using a TANITA segmental body composition analyzer (model BC-418).
Blood Markers’ Measurement
At a mean age of 6.7 years, a fasting venous sample was collected upon arrival to the Institute of Nutrition and Food Technology outpatient clinic. Mothers were contacted the day before sample drawing to confirm the absence of symptoms of acute infection in the children. DHEAS and IGF-1 analyses were conducted at the Institute of Maternal and Child Research, University of Chile. Serum DHEAS was determined by competitive specific binding RIA (Diagnostic System Laboratories [Webster, TX]) with intra- and inter-assay coefficients of variation of 3.5% and 5.1%, respectively. Serum IGF-1 was measured using a standardized, locally developed radioimmunoassay requiring sample extraction as a first step, with intra- and inter-assay coefficients of variation of 8.6% and 10.2%, respectively [].
Ethical Aspects
This study protocol was reviewed and approved by the Ethics Review Board of the Institute of Nutrition and Food Technology of the University of Chile, approval certificate #18. Informed consent and assent were provided accordingly.
Statistical Analysis
Age at B2 was defined as breast bud appearance between visits. Age at gonadarche (G2) in boys was defined as a testicular volume ≥4 mL. Timing of these events was defined as the mid-point of 2 consecutive visits, the last visit without signs of sexual development and the first visit in which one of these signs were detected. The group of girls was divided into 4 groups according to age at B2 and M, using the age of the events in the data of the GOCS cohort []: precocious (<−2 SD), early (−2 and −1 SD), normal (−1 and +1 SD), and late (>+1 SD). The same classification was used for boys []. An ANOVA was performed together with a post hoc test of multiple comparisons with Sidak correction to evaluate differences between the 4 groups and prepubertal concentrations of IGF-1.
The hazard proportional regression models which estimates the hazard ratio (HR), were performed to assess the risk of early onset B2, M, and G2 for an increase of one standard deviation in prepubertal IGF-1 concentrations. Survival time for M onset was defined as the time from birth to age at M, while for B2 and G2, a Weibull survival model was performed with interval-censored data since the exact date was unknown. To assess the risk of presenting a predefined early B2 (<8 years), early M (<10 years), and early G2 (9 years) for an increase of one standard deviation in concentrations of IGF-1, a logistic regression was performed. All models were adjusted for covariates known to be associated with age at pubertal development as follows: BW, height, BMI, fat mass percentage, and DHEAS levels, all at the time of measuring IGF-1 concentrations. In girls, we also adjusted for age at maternal M. This latter adjustment attempts to account for the genetic background which is relevant [].
A p value <0.05 was considered significant. Statistical analysis was performed with STATA program, version 16.
Results
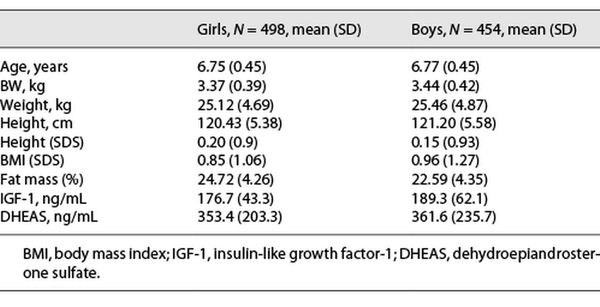
A total of 943 children (489 girls and 454 boys) were evaluated at a mean age of 6.7 years, all in Tanner 1 during year 2009. Participant’s clinical characteristics as well as mean concentrations of IGF-1 and DHEAS are detailed in Table 1.
Girls
Thelarche
We collected data at Tanner 2 in 472 girls, at a mean age 9.15 ± 1.38 years. Of these girls, 22 had a precocious B2 (mean age ± SD in years, 5.9 ± 1.15); 48 had early B2 (7.1 ± 0.40); 311 had normal B2 (9.2 ± 0.79); and 91 had a late B2 (mean age 10.9 ± 0.44). Prepubertal IGF-1 levels showed an inverse trend with earlier B2 ages (189.5 ng/mL [49.6], 190.8 ng/mL [46.1], 175.7 ng/mL [43.3], and 166.1 ng/mL [39.1], p = 0.003; shown in Fig. 1). There was not a clear trend in prepubertal height SDS with age at B2. Only those girls with early age at B2 were taller (0.63 SDS) versus those with normal or late age at B2 (0.13 and −0.03 SDS, respectively) (both p < 0.001; shown in Fig. 1).
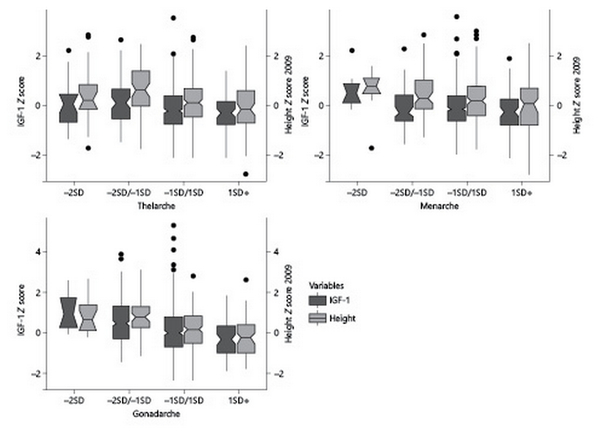
Fig. 1
Prepubertal IGF-1 concentrations and height in girls and boys, stratified by age at B2, M, and G2.
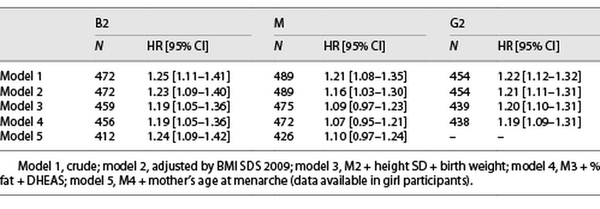
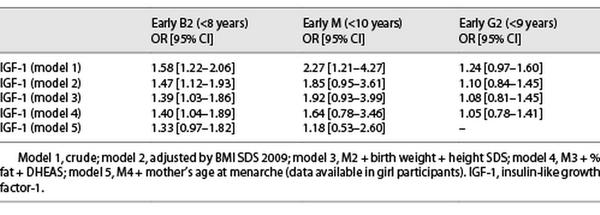
Girls with higher levels of IGF-1 at Tanner 1 were more likely to have a younger age at B2 onset in the crude and all adjusted models for covariates (HR: 1.24, 95% CI: 1.09–1.42) (Table 2), meaning that for an increase in one standard deviation of IGF-1 at Tanner 1, there is a 24% increased risk of having an earlier B2. Furthermore, we analyzed whether higher levels of IGF-1 were associated with a B2 onset before age 8; we found similar results; however, results were not significant after adjusting by maternal age at M (Table 3).
Menarche
We collected age at M in 489 girls, at a mean age 11.90 ± 1.00 years. Of these girls, 7 had a precocious M (mean age ± SD in years, 9.2 ± 0.49); 63 had early M (10.5 ± 0.26); 333 had normal M (11.9 ± 0.56); and 86 had late M (13.5 ± 0.49). IGF-1 levels showed an inverse trend with earlier ages of M (217.4 ng/mL [43.7], 174.8 ng/mL [43.3], 179 ng/mL [42.7], and 167.2 ng/mL [41.6] for precocious, early, normal, and late M, respectively, p = 0.041) (shown in Fig. 1). There was not a clear trend in height SDS with age at M (shown in Fig. 1). Only the girls with precocious M were taller (0.55 SDS) compared to their counterparts with late M (−0.03 SDS) (p = 0.017) (shown in Fig. 1).
Higher levels of IGF-1 were also associated with a younger age at M but only in the models adjusted by BMI SDS (HR 1.16, 95% CI: 1.03–1.30). The association lost significance after further confounders were added into the model (Table 2). Furthermore, we analyzed whether higher levels of IGF-1 were associated with early M (<10 years), and only in the crude analysis, IGF-1 was associated with an early age at M onset (before age of 10 years) (OR 2.27, 95% CI: 1.21–4.27; Table 3).
Boys
Gonadarche
We analyzed 454 boys, at a mean age 10.89 ± 1.49 years. Of these boys, 14 had a precocious G2 (mean age ± SD in years, 7.6 ± 0.36); 58 early G2 (8.9 ± 0.39); 288 normal G2 (10.9 ± s0.94); and 94 late G2 (12.8 ± 0.39). We observed a trend of higher prepubertal IGF-1 levels at earlier ages of G2 (237.1 ng/mL [48.2], 218.2 ng/mL [71.7], 188.5 ng/mL [60.8], and 164.6 ng/mL for precocious, early, normal, and late G2, respectively, p=<0.001; shown in Fig. 1). There was a clear inverse trend in height SDS with age at G2 (p < 0.001).
Boys with higher levels of IGF-1 before puberty onset had a younger age at G2 in the crude and all adjusted models (HR 1.19, 95% CI: 1.09–1.31), meaning that for an increase in one standard deviation of IGF-1 at Tanner 1, there is a 19% increased the risk of earlier G2 (Table 2), but no association was found between higher IGF-1 levels at Tanner 1 and G2 onset before the age of 9 years (Table 3). The results for B2 and M in girls and G2 in boys were similar when removing the premature and early pubertal groups.
Discussion
In the present study, higher IGF-1 concentrations increased the risk of belonging to a younger age group for B2 in girls and G2 in boys, suggesting that these hormones might directly promote an earlier timing of pubertal development in both sexes, even after adjusting for known covariates, but not necessarily a clinically meaningful earlier M (<10 y) or G2 onset (<9 y). These results are in line with the observation that IGF-1 has emerged as a candidate neuromodulator of pubertal onset [] both in animals and in humans [-]. Patients with mutations that impair the GH/IGF-1 axis are short and present with pubertal delay [].
IGF-1 may modulate the reproductive system through widespread effects on the hypothalamus, pituitary, and ovaries by its endocrine, paracrine, and autocrine actions based on the developmental and hormonal state []. IGF-1 plays a role in ovarian follicular formation and enhances FSH-mediated steroidogenesis []. IGF-1 is also a hormone critical for breast development [] and breast density []. Moreover, IGF-1 receptor is also expressed in the human adrenal tissue and might be involved in reticularis maturation [].
Only two studies have investigated the association of prepubertal IGF-1 and age at puberty in girls. In the study of Thankamony et al. [], 329 girls from a prospective UK birth cohort study provided blood samples at a mean age of 8.1 years (range 8–8.5 years). The authors found higher IGF-1 was associated with earlier age at M, and these findings persisted when adjusted for BMI and height at the time of blood sampling. We found a similar association, but in our cohort, the findings only persisted after adjustment of BMI at the time of IGF-1 assessment and were lost when we included BW and prepubertal height. Indeed, we explored this model separating the 2 confounders; adjusting only for BW, the association lost strength but persisted significantly (HR 1.14, 95% CI: 1.01–1.28). We believe that including this confounder is of most importance considering the evidence on the association of lower BW and earlier age at M []. Infancy weight gain predicts childhood body fat and age at M in girls [], and higher IGF-1 is already observed by 1 year of age in children born with lower BW and faster postnatal weight gain []. Furthermore, these authors also assessed age at B2 but did not analyze which hormone predicted this event. The difference in our results compared to those of Thankamony with regards to prepubertal height may be attributed to the reliability of G2 assessment as follows: palpation versus parental questionnaire at age of 8 years and more importantly the ages of evaluation (≈6.7 vs. 8 years). It is possible that higher IGF-1 and adrenal steroids were simply markers of those girls who were already in early puberty at age of 8 years. Later ages might be already influenced by early peri/pubertal estrogens which in turn increase height and IGF-1 concentrations, raising the possibility that height and IGF-1 in the regression models were collinear, decreasing the statistical significance of IGF-1. In the same aforementioned study by Thankamony et al. [], DHEAS, androstenedione, and adrenal androgens were also associated with earlier age of M. The observation by Thankamony et al. [] was also observed in this cohort, where higher DHEAS concentrations were associated to earlier pubertal events in girls []. These findings support a role of DHEAS as another relevant factor in the regulation of pubertal timing in girls, despite the classical theory that adrenarche and the onset of puberty are independent phenomena. The mechanism of this regulation is unclear, but one theory is that adrenal androgens would slow the negative feedback of gonadal steroids on the central nervous system, thus leading to an increase in GnRH pulsatility []. These results support the complex interaction of a myriad of variables that trigger the onset and culmination of female pubertal development and suggest that an increase in IGF-1 concentrations in girls could be mediated by these other variables (i.e., leptin, insulin), although current evidence points to IGF-1 as more plausible factor [].
The second study exploring this association by Biro et al. [] also in a longitudinal fashion included 183 girls (119 Caucasian and 64 African American). The authors observed that the association between age at B2 and age at M and prepubertal (≈6 months prior to B2) IGF-1 concentrations was lost when adjusting for prepubertal BMI and race. The partial disagreement with our results may be due to inherent differences in the study methods (i.e., timing of IGF-1 concentration with regards to the appearance of breast tissue) and number of included girls. In our results, the association of IGF-1 concentrations and earlier B2 was maintained after all the confounders were added in the models. Earlier B2 is usually accompanied by a longer time before age at M [, ].
On the other hand, we observed that the IGF-1 effects on age at M were less consistent. This divergence in the effects at age at B2 and M may be explained by differences in the activation of the infundibular and preoptic kisspeptin-secreting neurons, by IGF-1. The pulsatile regulation of GnRH neurons in both sexes depends on synchronized activity of specialized kisspeptin, neurokinin B, and dynorphin neurons located in the infundibular hypothalamus. Kisspeptin-expressing neurons are also located in the preoptic area, and these neurons do not contribute to pulsatile GnRH release but do contribute to the preovulatory surge of gonadotropins []. Hence, IGF-1 could directly promote breast tissue proliferation and activate a GnRH-FSH-predominant secretion, leading to a longer period between B2 and M [].
To our knowledge, there is no robust information on prepubertal IGF-1 levels and G2 in boys. In our cohort, higher IGF-1 concentrations before puberty onset had earlier G2. Of note, GH treatment with increased IGF-1 concentrations increased odds of testicular growth in a retrospective registry []. Interestingly, higher concentrations of IGF-1 may persist over the course of life [], and adult height is associated with risk of several cancer sites; there is no strong evidence to support IGF-1 moderating this latter association. Interestingly, in a recent GWAS study of pubertal timing determinants, Hollis et al. [] reported an snp near the IGF-1R implicated in both sexes. A better understanding of life course determinants of the IGF system may provide new insights into the modulation of puberty onset disease etiology and primary prevention.
Our study is not without limitations. We did not count with paternal age at pubertal development for adjusting boys’ results for this covariate and mid-parental height. Recently, a study by Upners E. et al. [] suggests that taller children have higher IGF-1, and both traits were associated to earlier age at M and B2. This is a complex observation. In children who are growing above mid-parental height centiles, hence taller for their expected growth, usually higher IGF-1 concentrations are associated to earlier pubertal maturation. In our study, we did not find a clear association of height and pubertal timing measured as B2 in girls and G2 in boys (shown in Fig. 1). Only the extreme ages of at M had a significant prepubertal height difference. However, the complexity of hormonal interactions associated with pubertal development, and their differences with males have been previously reported [].
On the other hand, our study offers great strengths; it is a longitudinal study that includes anthropometric variables of the ethnically homogeneous participants from the newborn period. It is also the first study to analyze prepubertal IGF-1 levels and pubertal tempo in both sexes with similar ethnicity and with pubertal tempo evaluated by a reliable method (breast palpation and testicular volume).
In conclusion, our findings support the hypothesis that IGF-1 could serve as a link between growth and sexual maturation. IGF-1 determination in subjects with early pubertal maturation could add to predict which patients will progress in their pubertal development. These findings support the association of IGF-1 with the regulation of pubertal maturation. These associations may reflect common early-life programming events that determine IGF-1 levels, the timing of adrenarche, and the timing of puberty.
Acknowledgments
We thank GOCS families and children for their participation.
Statement of Ethics
The Ethics Review Board of the Institute of Nutrition and Food Technology (INTA) of the University of Chile approved the study protocol, Chile approval certificate #18. All parents or guardians of the children provided written informed consent, and the children gave their assent.
Conflict of Interest Statement
The authors have no conflicts of interest to declare.
Funding Sources
FONDECYT support (Fondo Nacional de ciencia y tecnologia) through Grant No: #1140447 grant to V.M., 1120326 grant to V.M., 1170670 grant to A.P., and 1190346 grant to V.M. All the funds of each of these grants served to recruit the cohort and maintain the logistic for follow-up of this longitudinal cohort and fund the blood assays and statistical analysis.
Author Contributions
Ingrid Baier and German Iñiguez participated in the methodology, investigation, formal analysis, and writing of the first draft. Pedro Ferrer participated in the methodology, supervision formal analysis, and writing of the draft. Drs. Veronica Mericq and Ana Pereira conceptualized the study and participated in the methodology, supervision, formal analysis, and writing of the draft. All authors approved the final manuscript as submitted and agree to be accountable for all aspects of the work.
Data Availability Statement
All data generated or analyzed during this study are included in this article. Further inquiries can be directed to the corresponding author.
References
- 1. Livadas S, Chrousos GP. Control of the onset of puberty. Curr Opin Pediatr. 2016;28(4):551–8. https://doi.org/10.1097/MOP.0000000000000386.
- 2. Wolfe A, Divall S, Wu S. The regulation of reproductive neuroendocrine function by insulin and insulin-like growth factor-1 (IGF-1). Front Neuroendocrinol. 2014;35(4):558–72. https://doi.org/10.1016/j.yfrne.2014.05.007.
- 3. Aguado F, Rodrigo J, Cacicedo L, Mellstrom B. Distribution of insulin-like growth factor-I receptor mRNA in rat brain. Regulation in the hypothalamo-neurohypophysial system. J Mol Endocrinol. 1993;11(2):231–9. https://doi.org/10.1677/jme.0.0110231.
- 4. Bohannon NJ, Figlewicz DP, Corp ES, Wilcox BJ, Porte D Jr, Baskin DG. Identification of binding sites for an insulin-like growth factor (IGF-I) in the median eminence of the rat brain by quantitative autoradiography. Endocrinology. 1986;119(2):943–5. https://doi.org/10.1210/endo-119-2-943.
- 5. Ojeda SR, Negro-Vilar A, McCann SM. Release of prostaglandin Es by hypothalamic tissue: evidence for their involvement in catecholamine-induced luteinizing hormone-releasing hormone release. Endocrinology. 1979;104(3):617–24. https://doi.org/10.1210/endo-104-3-617.
- 6. Hiney JK, Dees WL. Ethanol inhibits luteinizing hormone-releasing hormone release from the median eminence of prepubertal female rats in vitro: investigation of its actions on norepinephrine and prostaglandin-E2. Endocrinology. 1991;128(3):1404–8. https://doi.org/10.1210/endo-128-3-1404.
- 7. Hiney JK, Srivastava V, Nyberg CL, Ojeda SR, Dees WL. Insulin-like growth factor I of peripheral origin acts centrally to accelerate the initiation of female puberty. Endocrinology. 1996;137(9):3717–28. https://doi.org/10.1210/endo.137.9.8756538.
- 8. Hiney JK, Srivastava V, Lara T, Dees WL. Ethanol blocks the central action of IGF-1 to induce luteinizing hormone secretion in the prepubertal female rat. Life Sci. 1998;62(4):301–8. https://doi.org/10.1016/s0024-3205(97)01111-9.
- 9. Adam CL, Findlay PA, Moore AH. Effects of insulin-like growth factor-1 on luteinizing hormone secretion in sheep. Anim Reprod Sci. 1998;50(1–2):45–56. https://doi.org/10.1016/s0378-4320(97)00089-4.
- 10. Wilson ME. Premature elevation in serum insulin-like growth factor-I advances first ovulation in rhesus monkeys. J Endocrinol. 1998;158(2):247–57. https://doi.org/10.1677/joe.0.1580247.
- 11. Dees WL, Hiney JK, Srivastava VK. Regulation of prepubertal dynorphin secretion in the medial basal hypothalamus of the female rat. J Neuroendocrinol. 2019;31(12):e12810. https://doi.org/10.1111/jne.12810.
- 12. Hiney JK, Srivastava VK, Vaden Anderson DN, Hartzoge NL, Dees WL. Regulation of kisspeptin synthesis and release in the preoptic/anterior hypothalamic region of prepubertal female rats: actions of IGF-1 and alcohol. Alcohol Clin Exp Res. 2018;42(1):61–8. https://doi.org/10.1111/acer.13539.
- 13. Veldhuis JD, Roemmich JN, Richmond EJ, Bowers CY. Somatotropic and gonadotropic axes linkages in infancy, childhood, and the puberty-adult transition. Endocr Rev. 2006;27(2):101–40. https://doi.org/10.1210/er.2005-0006.
- 14. Thankamony A, Ong KK, Ahmed ML, Ness AR, Holly JMP, Dunger DB. Higher levels of IGF-I and adrenal androgens at age 8 years are associated with earlier age at menarche in girls. J Clin Endocrinol Metab. 2012;97(5):E786–90. https://doi.org/10.1210/jc.2011-3261.
- 15. Biro FM, Huang B, Wasserman H, Gordon CM, Pinney SM. Pubertal growth, IGF-1, and windows of susceptibility: puberty and future breast cancer risk. J Adolesc Health. 2021;68(3):517–22. https://doi.org/10.1016/j.jadohealth.2020.07.016.
- 16. Corvalán C, Uauy R, Stein AD, Kain J, Martorell R. Effect of growth on cardiometabolic status at 4 y of age. Am J Clin Nutr. 2009;90(3):547–55. https://doi.org/10.3945/ajcn.2008.27318.
- 17. Marshall WA, Tanner JM. Variations in pattern of pubertal changes in girls. Arch Dis Child. 1969;44(235):291–303. https://doi.org/10.1136/adc.44.235.291.
- 18. Marshall WA, Tanner JM. Variations in the pattern of pubertal changes in boys. Arch Dis Child. 1970;45(239):13–23. https://doi.org/10.1136/adc.45.239.13.
- 19. Pereira A, Garmendia ML, González D, Kain J, Mericq V, Uauy R, et al. Breast bud detection: a validation study in the Chilean growth obesity cohort study. BMC Womens Health. 2014;14:96. https://doi.org/10.1186/1472-6874-14-96.
- 20. de Onis M, Garza C, Victora CG, Onyango AW, Frongillo EA, Martines J. The WHO Multicentre Growth Reference Study: planning, study design, and methodology. Food Nutr Bull. 2004;25(1 Suppl):S15–26. https://doi.org/10.1177/15648265040251S103.
- 21. Kain J, Galván M, Taibo M, Corvalán C, Lera L, Uauy R. Evolution of the nutritional status of Chilean children from preschool to school age: anthropometric results according to the source of the data. Arch Latinoam Nutr. 2010;60(2):155–9.
- 22. Iniguez G, Villavicencio A, Gabler F, Palomino A, Vega M. Effect of nitric oxide on the expression of insulin-like growth factors and the insulin-like growth factor binding proteins throughout the lifespanof the human corpus luteum. Reproduction. 2001;122(6):865–73. https://doi.org/10.1530/rep.0.1220865.
- 23. Pereira A, Corvalan C, Merino PM, Leiva V, Mericq V. Age at pubertal development in a hispanic-latina female population: should the definitions Be revisited?J Pediatr Adolesc Gynecol. 2019;32(6):579–83. https://doi.org/10.1016/j.jpag.2019.08.008.
- 24. Pereira A, Busch AS, Solares F, Baier I, Corvalan C, Mericq V. Total and central adiposity are associated with age at gonadarche and incidence of precocious gonadarche in boys. J Clin Endocrinol Metab. 2021;106(5):1352–61. https://doi.org/10.1210/clinem/dgab064.
- 25. Perry JR, Day F, Elks CE, Sulem P, Thompson DJ, Ferreira T, et al. Parent-of-origin-specific allelic associations among 106 genomic loci for age at menarche. Nature. 2014;514(7520):92–7. https://doi.org/10.1038/nature13545.
- 26. Messina MF, Arrigo T, Valenzise M, Ghizzoni L, Caruso-Nicoletti M, Zucchini S, et al. Long-term auxological and pubertal outcome of patients with hereditary insulin-like growth factor-I deficiency (Laron and growth hormone-gene deletion syndrome) treated with recombinant human insulin-like growth factor-I. J Endocrinol Invest. 2011;34(4):292–5. https://doi.org/10.1007/BF03347088.
- 27. Daftary SS, Gore AC. IGF-1 in the brain as a regulator of reproductive neuroendocrine function. Exp Biol Med. 2005;230(5):292–306. https://doi.org/10.1177/153537020523000503.
- 28. Zhao J, Xiong DH, Guo Y, Yang TL, Recker RR, Deng HW. Polymorphism in the insulin-like growth factor 1 gene is associated with age at menarche in caucasian females. Hum Reprod. 2007;22(6):1789–94. https://doi.org/10.1093/humrep/dem052.
- 29. Neville MC, Daniel CW, editors. The mammary gland: development, regulation and function. New York, NY: Plenum Press; 1987. p. 67–93.
- 30. Diorio C, Pollak M, Byrne C, Mâsse B, Hébert-Croteau N, Yaffe M, et al. Insulin-like growth factor-I, IGF-binding protein-3, and mammographic breast density. Cancer Epidemiol Biomarkers Prev. 2005;14(5):1065–73. https://doi.org/10.1158/1055-9965.EPI-04-0706.
- 31. Belgorosky A, Baquedano MS, Guercio G, Rivarola MA. Expression of the IGF and the aromatase/estrogen receptor systems in human adrenal tissues from early infancy to late puberty: implications for the development of adrenarche. Rev Endocr Metab Disord. 2009;10(1):51–61. https://doi.org/10.1007/s11154-008-9105-1.
- 32. Tam CS, de Zegher F, Garnett SP, Baur LA, Cowell CT. Opposing influences of prenatal and postnatal growth on the timing of menarche. J Clin Endocrinol Metab. 2006;91(11):4369–73. https://doi.org/10.1210/jc.2006-0953.
- 33. Adair LS. Size at birth predicts age at menarche. Pediatrics. 2001;107(4):E59. https://doi.org/10.1542/peds.107.4.e59.
- 34. Iñiguez G, Ong K, Bazaes R, Avila A, Salazar T, Dunger D, et al. Longitudinal changes in insulin-like growth factor-I, insulin sensitivity, and secretion from birth to age three years in small-for-gestational-age children. J Clin Endocrinol Metab. 2006;91(11):4645–9. https://doi.org/10.1210/jc.2006-0844.
- 35. Pereira A, Iñiguez G, Corvalan C, Mericq V. High DHEAS is associated with earlier pubertal events in girls but not in boys. J Endocr Soc. 2017;1(7):800–8. https://doi.org/10.1210/js.2017-00120.
- 36. Nader S. Adrenarche and polycystic ovary syndrome: a tale of two hypotheses. J Pediatr Adolesc Gynecol. 2007;20(6):353–60. Erratum in: J Pediatr Adolesc Gynecol. 2008;21(2):111. https://doi.org/10.1016/j.jpag.2007.05.001.
- 37. Lam BYH, Williamson A, Finer S, Day FR, Tadross JA, et alGenes & Health Research Team. MC3R links nutritional state to childhood growth and the timing of puberty. Nature. 2021;599(7885):436–41. https://doi.org/10.1038/s41586-021-04088-9.
- 38. German A, Shmoish M, Belsky J, Hochberg Z. Outcomes of pubertal development in girls as a function of pubertal onset age. Eur J Endocrinol. 2018;179(5):279–85. https://doi.org/10.1530/EJE-17-1025.
- 39. Hrabovszky E, Ciofi P, Vida B, Horvath MC, Keller E, Caraty A, et al. The kisspeptin system of the human hypothalamus: sexual dimorphism and relationship with gonadotropin-releasing hormone and neurokinin B neurons. Eur J Neurosci. 2010;31(11):1984–98. https://doi.org/10.1111/j.1460-9568.2010.07239.x.
- 40. Pescovitz OH, Hench KD, Barnes KM, Loriaux DL, Cutler GB Jr. Premature thelarche and central precocious puberty: the relationship between clinical presentation and the gonadotropin response to luteinizing hormone-releasing hormone. J Clin Endocrinol Metab. 1988;67(3):474–9. https://doi.org/10.1210/jcem-67-3-474.
- 41. Cannarella R, Caruso M, Crafa A, Timpanaro TA, Lo Bianco M, Presti S, et al. Testicular growth and pubertal onset in GH-deficient children treated with growth hormone: a retrospective study. Front Endocrinol. 2021;12:619895. https://doi.org/10.3389/fendo.2021.619895.
- 42. Sandhu J, Davey Smith G, Holly J, Cole TJ, Ben-Shlomo Y. Timing of puberty determines serum insulin-like growth factor-I in late adulthood. J Clin Endocrinol Metab. 2006;91(8):3150–7. https://doi.org/10.1210/jc.2005-2318.
- 43. Hollis B, Day FR, Busch AS, Thompson DJ, Soares ALG, Timmers PRHJ, et al. Genomic analysis of male puberty timing highlights shared genetic basis with hair colour and lifespan. Nat Commun. 2020;11(1):1536. https://doi.org/10.1038/s41467-020-14451-5.
- 44. Upners EN, Busch AS, Almstrup K, Petersen JH, Assens M, Main KM, et al. Does height and IGF-I determine pubertal timing in girls?Pediatr Res. 2021;90(1):176–83. https://doi.org/10.1038/s41390-020-01215-6.