Established Facts
Some endocrine-disrupting chemicals can interfere with the pubertal process in children, causing them to develop earlier.
Phytosterol is a precursor of steroid hormone synthesis and shows certain hormone activity in the body.
Novel Insights
Long-term and excessive intake of phytosterols in children may lead to precocious puberty.
Introduction
Precocious puberty is defined as the onset of secondary sexual characteristics development before 8 years in girls and 9 years in boys []. Precocious puberty is classified into central precocious puberty and peripheral precocious puberty (PPP). Central precocious puberty is dependent on activation of the hypothalamic-pituitary-gonadal axis, while PPP, which results from peripheral production of sex steroids, is independent of activation of the hypothalamic-pituitary-gonadal axis. The secondary sexual characteristic development, progression of puberty, and sequence of adolescent events in PPP may be significantly different from normal pubertal development []. PPP often appears among boys, and its etiologies are diverse, both congenital, such as congenital adrenocortical hyperplasia, McCune-Albright syndrome, and familial testotoxicosis, and acquired. Some tumors can secrete sex hormones, including adrenocortical tumors, gonadal tumors, and tumors that can secrete human chorionic gonadotropin, and are common acquired factors leading to PPP. In recent years, cases of PPP in children caused by exogenous steroids have been reported. However, PPP caused by substances contained in a daily diet flavoring is relatively rare. In the present study, we describe a case of PPP due to long-term and excessive intake of a phytosterol-containing diet in a 20-month-old boy.
Case Presentation
A 20-month-old boy was referred to the Department of Endocrinology and Metabolism of Shanghai Children’s Medical Center in October 2020, who came to see a doctor because of acne, hairiness, increased penis size, and coarse voice. The case was the first singleton born to a physically healthy and non-consanguineous couple by natural delivery at full-term, with a birth weight of 2,400 g. Recently, he showed language retardation and accelerated growth (86.5 cm tall 16 months old and 93 cm tall 20 months old). Since he was 5 months old, two cans of face cream have been used for repeated eczema. Before coming to our hospital, the patient went to two other hospitals for treatment. The patient’s mother reported that the boy had taken chicken essence seasoning for every meal since he was 6 months old, with a daily intake of about 15 g. There was no fever, vomiting, diarrhea, or other discomforts on the onset of the symptoms.
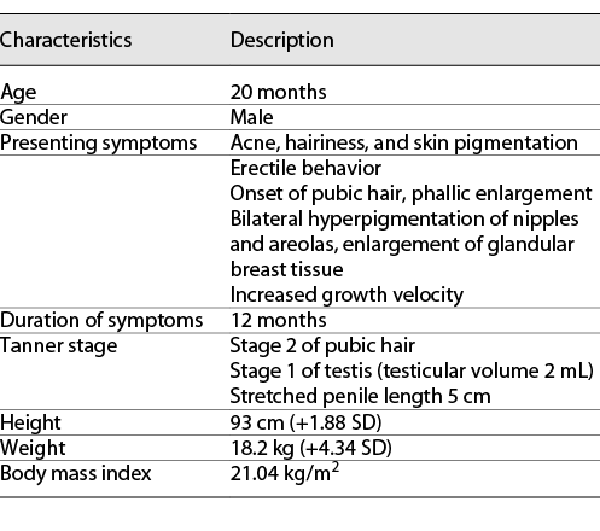
On physical examination, the patient’s length was 93 cm (1.88 SD above the mean for the sex and age []), and the weight was 18.2 kg (4.34 SD above the mean for the sex and age []). His muscles were developed, and the body fat distribution was decreased. Acne-like papules in the size of rice grains could be seen on the face and trunk, mainly on the face, red in color, with abscesses at the tip (Fig. 1a). The skin on the abdomen was darkly pigmented (Fig. 1b). Increased hairs were distributed in the forehead, back of the neck, upper limbs, both sides of the thighs, and lumbosacral region (Fig. 1c). He was found to have pigmentation on both nipples and areola. The breast tissue can be touched bilaterally, and the size is about 0.5 × 1 cm (Tanner stage of 2). Genitourinary examination showed that his two-sided testicles had descended to the scrotum with a volume of about 2 mL. The hyperpigmented penis was 5 cm (mean ± standard deviation for 2 years old: 3.54 ± 0.34 cm []) in length and 1.8 cm in diameter. Pubic hair can be seen at the root of the penis, reaching at the Tanner stage of 2. The clinical characteristics of this patient are shown in Table 1.
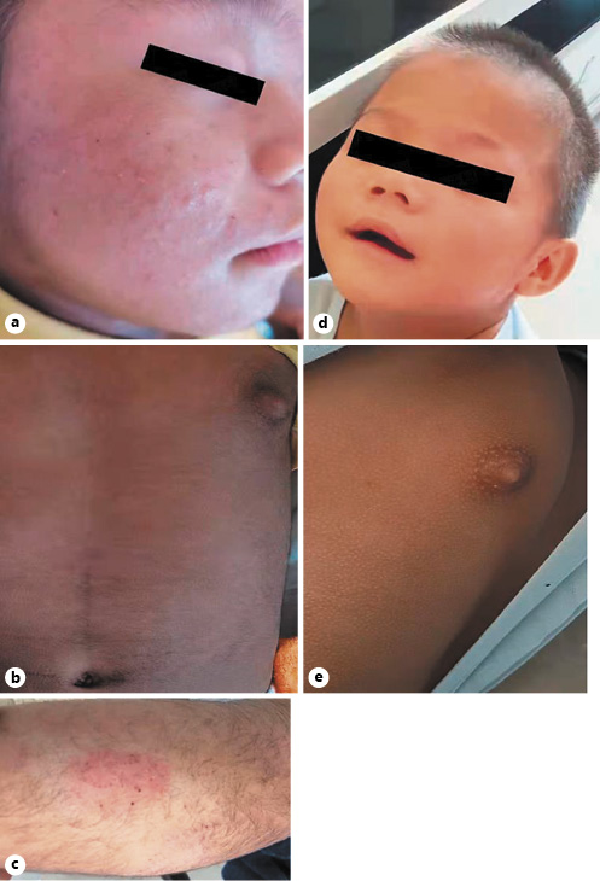
Fig. 1
Clinical symptoms of the patient at admission and 10 months later. The symptoms at admission were shown on the left side; it is shown the patient’s acne-like papules on face (a), pigmentation of abdominal skin (b), and thick and long body hair on his legs (c). It also showed the improvement of those symptoms after 10 months on the right side, and it showed the patient’s improved facial acne (d) and the recovery of skin color (e).
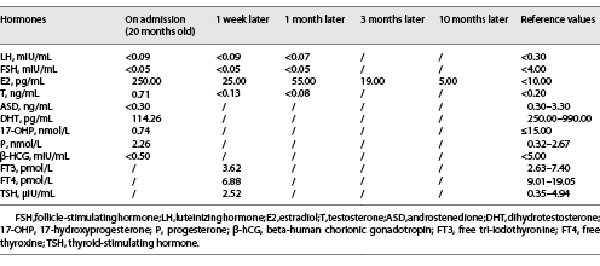
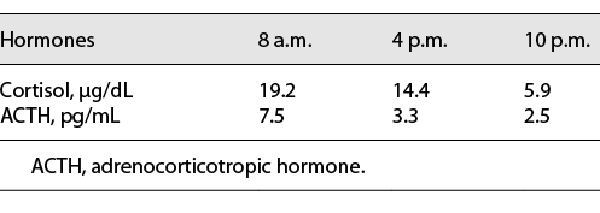
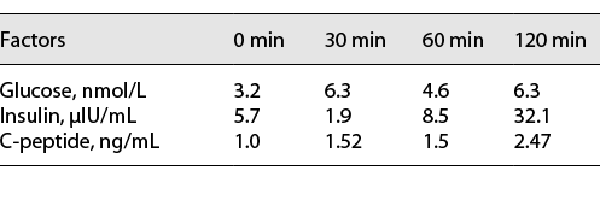
Biochemical examinations showed that the patient had damaged liver function (ALT 121 U/L, reference range: 0–55 U/L, AST 87 U/L, reference range: 5–34 U/L), abnormal blood lipid (increased total cholesterol [10.03 mmol/L, reference range: 0.00–5.20 mmol/L], triglyceride [3.47 mmol/L, reference range: <2.26 mmol/L], and low-density lipoprotein cholesterol [8.06 mmol/L, reference range: <3.37 mmol/L]) and increased the number of white blood cells. Hormone detection (Table 2) revealed that luteinizing hormone (LH), follicle-stimulating hormone (FSH), dihydrotestosterone, and androstenedione were decreased. The levels of estradiol (E2) and testosterone (T) were increased. Cortisol and ACTH rhythms and concentrations were normal (Table 3). In addition, the low-dose dexamethasone suppression test was negative. For the oral glucose tolerance test, after the analysis of the results, there was no insulin resistance (Table 4). The levels of tumor biomarkers (alpha fetoprotein, carcinoembryonic antigen, carbohydrate antigen 199) were also in the normal reference ranges.
The imaging examination showed there were no obvious abnormalities in adrenal ultrasound and adrenal enhanced computed tomography (CT). The thyroid ultrasound test revealed normal as well. An abdominal ultrasound examination revealed that the liver was enlarged. Pituitary magnetic resonance imaging (MRI) showed pituitary hyperplasia, and skull MRI showed pituitary hyperplasia and uneven signal, and the sellar diaphragm was raised. Further sellar diaphragm-enhanced MRI showed no space occupying lesion. No abnormality was found in the positron emission tomography-computed tomography examination. The bone age (based on the X-rays of the left-hand bone) was 5.5 years old, which was much older than the chronological age. The chromosome karyotype of the patient was 46XY, and the results of the whole exon and Sanger sequencing of the CYP21A2 gene were normal and inconsistent with the clinical features. The cream and chicken essence seasoning were analyzed by high-performance liquid chromatography-tandem mass spectrometry (HPLC-MS) to detect whether there were exogenous steroids. A kind of phytosterol (avenasterol/clerosterol/chondrillasterol) with the molecular formula C29H48O was found in chicken essence seasoning; the chromatogram (Fig. 2a) shows that retention time is 1.64 min, peak area (AA) is 946,850, and the signal to noise ratio (S/N) is 1,319, and the mass spectrum (Fig. 2b) shows that the mass-to-charge ratio (m/z) is 413.3761. No hormones and steroids were found in the cream. For the chromatographic conditions, the extraction samples were analyzed using a Q Exactive plus mass spectrometer (ThermoFisher Scientific, Waltham, MA, USA) linked to an Ultimate 3000 UPLC (Dionex, Sunnyvale, CA, USA), and a hydrophilic pHILIC column (150 mm × 2.1 mm, 5 µm, Merck) was used for chromatographic separation. For the MS acquisition, the Q-Exacitve plus spectrometer with electrospray ionization was operated in the data-dependent acquisition mode using Xcalibur 4.0.27 software (ThermoFisher Scientific). The sample was detected in full scan mode with positive and negative switching scan.
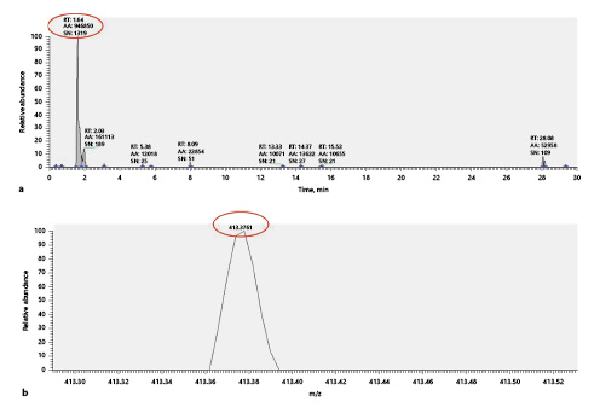
Fig. 2
The steroids in chicken essence seasoning were determined by HPLC-MS, and a kind of phytosterol (avenasterol/clerosterol/chondrillasterol) with molecular formula C29H48O was detected. a Chromatogram of phytosterol, it is showing that retention time is 1.64 min, peak area (AA) is 946,850, and the signal to noise ratio (S/N) is 1,319. b Mass spectrum of phytosterol, it is showing that the mass-to-charge ratio (m/z) is 413.3761.
The patient was treated with compound glycyrrhizin tablets [] (12.5 mg oral administration, three times a day) to protect the liver, ursodeoxycholic acid tablets [] (200 mg oral administration, once a day) to reduce free cholesterol, and cetirizine hydrochloride tablets for anti-allergy treatment. At the same time, the patient was advised to avoid chicken essence seasoning food and cream and to monitor the level of sex hormones dynamically. After stopping the use of cream and avoiding the chicken essence seasoning diet for 1 month, examinations at the clinic follow-up showed that the level of E2 and T declined dramatically. At a follow-up after 10 months, the concentration of E2 decreased to a normal level. The decline trends of LH, FSH, E2, and T levels are shown in Figure 3. Furthermore, all of the clinical symptoms of the patient improved. We can see the improvements in facial acne (Fig. 1d), the recovery of skin color (Fig. 1e), the regression of whole-body hair, and the growth and development returned to normal. The differences between admission and 10 months later at clinical follow-up were compared and shown in Figure 1.
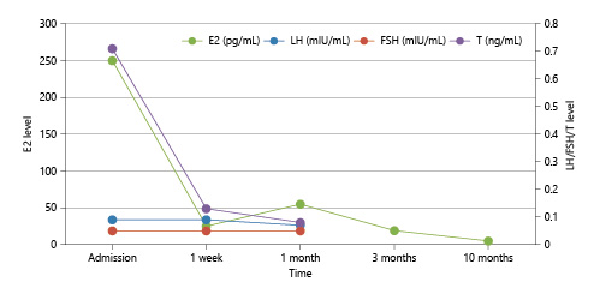
Fig. 3
Changes of LH, FSH, E2, and T levels since decomposition of chicken essence seasoning. At admission, the patient had high levels of E2 and T but relatively low level of LH and FSH. As time went by, the level of E2 and T gradually returned to normal.
Discussion
The developmental procedure of peripheral precocious puberty is not completely consistent with that of normal puberty. The absence of testicular enlargement but the penis and pubic hair developed in this case, in combination with low gonadotrophins (LH, FSH) and high sex hormones (E2, T), confirmed a gonadotrophin-independent process. The normal cortisol rhythm and baseline 17-OH-P and ACTH concentration, along with the normal sequencing results of the CYP21A2 gene in the patient, did not support the diagnosis of congenital adrenal hyperplasia secondary to 21-hydroxylase deficiency. The possibility of adrenal tumors can also be excluded by adrenal image and biochemical examination of tumor biomarkers. The results of the MRI examination of the pituitary and skull were not consistent with the manifestations of intracranial tumors. According to some research, estrogen can stimulate pituitary cell proliferation in the pituitary gland through its receptor-mediated signaling pathway, leading to pituitary hyperplasia []. We speculated that the pituitary hyperplasia of the boy may be associated with the stimulation of increased estrogen. The hyperplasia of the pituitary gland may lead to the decline of FT4 in the patient, and the symptoms of language development delay appear.
The chicken essence seasoning taken in by the patient was analyzed by HPLC-MS, and a kind of phytosterol was detected. Chicken essence seasoning is a compound seasoning commonly used in Chinese cooking to bolster flavors. It is processed based on monosodium glutamate by adding edible salt, chicken, chicken bone, flavored disodium nucleotide, and other accessories, and it has the flavor of chicken. Phytosterol is often used as a chicken feed additive (approved by the Ministry of Agriculture of China in 2008) to improve animal protein synthesis, promote animal growth, and lessen the content of cholesterol in eggs and meat. Therefore, phytosterol residues in chicken essence seasoning may result from the chicken fed with phytosterol.
As a steroid compound, phytosterol has a similar chemical structure to steroids, which is a precursor of steroid hormone synthesis and shows certain hormone activity in the body []. Some animal research findings indicate that phytosterols produce an increase in circulating testosterone concentration in males [] or are related to precursor chemicals of testosterone []. In addition, phytosterols can be converted to testosterone by microorganisms []. These studies show that phytosterols may lead to increased testosterone levels in the body. Furthermore, several research studies have revealed that phytosterols have estrogen-like effects. The β-sitosterol and the soysterol extracts, which are typical phytosterols, had estrogenic effects on MCF-7 cell growth in vitro and induced cell proliferation []. Petteri Nieminen and others [] used 50 mg/kg/day phytosterols to intervene for 2 weeks in the European Polecat; the results showed an increase in the plasma estradiol. In the study by Petteri Nieminen et al. [] on the effects of phytosterols on endocrinology and metabolism in the field voles, the results showed that the plasma estradiol and testosterone concentrations of males were higher due to phytosterol supplement at 5 mg/kg/day. In a human trial [], 11 adult female subjects ingested 8.6 g of phytosterols daily for 28 days, with no significant effect on serum estrogen levels, but there was no comparison of male sex hormone levels. The case we report is a 20-month-old boy, which is quite different from the subjects in the human trial described above. Considering that children are extremely sensitive to any alteration of their hormonal environment [, ], and in combination with the findings of animal research, it is highly reasonable to suspect that the high testosterone and estradiol levels in the patient are related to the long-term intake of phytosterols, which in turn led to the development of penis and pubic hair as well as breast development in this boy.
In recent years, some cases of premature development in children caused by percutaneous exposure to androgen-containing preparations [-], lavender products [, ], and endocrine-disrupting chemicals such as pesticides [] have been reported, but precocious puberty directly caused by a daily diet flavoring is relatively rare. As a flavoring, chicken essence seasoning is only used to improve food flavor and should be added properly. Moreover, the work of the liver and kidneys of children is not yet fully developed, and the additives and sodium salts in the seasoning will cause a burden to the liver and kidneys, so they should have less or not. This case had a long-term and excessive consumption of chicken essence seasoning, which led to the accumulation of phytosterols in the body and in turn triggered a series of early development symptoms and even affected liver function.
For one thing, this report shows the importance of lifestyle for the pubertal development in children. To explore the reasons for children’s precocious puberty (especially for young patients) when pathological causes are excluded, we should pay more attention to the patient’s lifestyle and consider exogenous substances that may cause these symptoms. For another, more attention should be paid to the effect of phytosterols on the growth and development of children. The effects of phytosterols on individual endocrine and metabolism differ depending on the type of phytosterol, dose, duration of exposure, species, and developmental stage of the individual. More research on phytosterols’ sex hormone-like effects and mechanism in humans (particularly in prepubertal children) is needed. This case report provided an essential research direction.
Acknowledgments
We thank the patient and his family members for their support and participation.
Statement of Ethics
Ethical approval of the Local Review Board was not required for this study in accordance with local guidelines. Written informed consent was obtained from the patient’s parents for the publication of any data and images included in this article.
Conflict of Interest Statement
The authors have nothing to disclose.
Funding Sources
This study was supported by the Natural Science Foundation of China (81872637, 81903341, 82173534), Shanghai Professional and Technical Services Platform (18DZ2294100), Key Subject Program for Clinical Nutrition from the Shanghai Municipal Health Commission (2019ZB0103), and Key discipline construction project of the 3-year action Plan of Shanghai Public Health System (GWV-10.1-XK07).
Author Contributions
Shijian Liu and Xiumin Wang performed study design and supervision. Peng Xue, Yirou Wang, and Yao Chen drafted the manuscript. Peng Xue, Yirou Wang, Xiaoyu Wu, Yao Chen, Yijun Tang, and Yu Ding collected and analyzed the data. Peng Xue, Yirou Wang, Yao Chen, Xiumin Wang, and Shijian Liu performed data interpretation. Shijian Liu and Xiumin Wang critically reviewed and revised the manuscript.
Data Availability Statement
All data analyzed during this study are included in this article. Further inquiries can be directed to the corresponding author.
References
- 1. Nathan BM, Palmert MR. Regulation and disorders of pubertal timing. Endocrinol Metab Clin North Am. 2005 Sep;34(3):617–41, ix. https://doi.org/10.1016/j.ecl.2005.04.015.
- 2. Haddad NG, Eugster EA. Peripheral precocious puberty including congenital adrenal hyperplasia: causes, consequences, management and outcomes. Best Pract Res Clin Endocrinol Metab. 2019 Jun;33(3):101273. https://doi.org/10.1016/j.beem.2019.04.007.
- 3. Zhang YQ, Li H, Wu HH, Zong XN, Zhu ZH, Pan Y, et al. The 5th national survey on the physical growth and development of children in the nine cities of China: anthropometric measurements of Chinese children under 7 years in 2015. Am J Phys Anthropol. 2017 Jul;163(3):497–509. https://doi.org/10.1002/ajpa.23224.
- 4. Fu C, Li XL. Normal penile growth amongst Chinese. Chin J Pediatr Surg. 2010;31:432–4. (in Chinese). http://doi.org/10.3760/cma.j.issn.0253-3006.2010.06.008.
- 5. Shi Q, Wang Q, Chen J, Xia F, Qiu C, Li M, et al. Transcriptome and lipid metabolomics-based discovery: glycyrrhizic acid alleviates tripterygium glycoside tablet-induced acute liver injury by regulating the activities of CYP and the metabolism of phosphoglycerides. Front Pharmacol. 2022 Feb;12:822154. https://doi.org/10.3389/fphar.2021.822154.
- 6. Simental-Mendía LE, Simental-Mendía M, Sánchez-García A, Banach M, Serban MC, Cicero AFG, et al. Impact of ursodeoxycholic acid on circulating lipid concentrations: a systematic review and meta-analysis of randomized placebo-controlled trials. Lipids Health Dis. 2019 Apr;18(1):88. https://doi.org/10.1186/s12944-019-1041-4.
- 7. Watson CS, Jeng YJ, Kochukov MY. Nongenomic actions of estradiol compared with estrone and estriol in pituitary tumor cell signaling and proliferation. FASEB J. 2008 Sep;22(9):3328–36. https://doi.org/10.1096/fj.08-107672.
- 8. De Brabander HF, Verheyden K, Mortier V, Le Bizec B, Verbeke W, Courtheyn D, et al. Phytosterols and anabolic agents versus designer drugs. Anal Chim Acta. 2007 Mar;586(1–2):49–56. https://doi.org/10.1016/j.aca.2006.07.031.
- 9. Sudeep HV, Thomas JV, Shyamprasad K. A double blind, placebo-controlled randomized comparative study on the efficacy of phytosterol-enriched and conventional saw palmetto oil in mitigating benign prostate hyperplasia and androgen deficiency. BMC Urol. 2020 Jul 3;20(1):86. https://doi.org/10.1186/s12894-020-00648-9.
- 10. Decloedt AI, Bailly-Chouriberry L, Vanden Bussche J, Garcia P, Popot MA, Bonnaire Y, et al. In vitro simulation of the equine hindgut as a tool to study the influence of phytosterol consumption on the excretion of anabolic-androgenic steroids in horses. J Steroid Biochem Mol Biol. 2015 Aug;152:180–92. https://doi.org/10.1016/j.jsbmb.2015.06.001.
- 11. Lo CK, Pan CP, Liu WH. Production of testosterone from phytosterol using a single-step microbial transformation by a mutant of Mycobacterium sp. J Ind Microbiol Biotechnol. 2002 May;28(5):280–3. https://doi.org/10.1038/sj/jim/7000243.
- 12. Sriraman S, Ramanujam GM, Ramasamy M, Dubey GP. Identification of beta-sitosterol and stigmasterol in Bambusa bambos (L.) Voss leaf extract using HPLC and its estrogenic effect in vitro. J Pharm Biomed Anal. 2015 Nov;115:55–61. https://doi.org/10.1016/j.jpba.2015.06.024.
- 13. Nieminen P, Mustonen AM, Lindström-Seppä P, Asikainen J, Mussalo-Rauhamaa H, Kukkonen JVK. Phytosterols act as endocrine and metabolic disruptors in the European polecat (Mustela putorius). Toxicol Appl Pharmacol. 2002 Jan;178(1):22–8. https://doi.org/10.1006/taap.2001.9315.
- 14. Nieminen P, Mustonen AM, Lindström-Seppä P, Kärkkäinen V, Mussalo-Rauhamaa H, Kukkonen JVK. Phytosterols affect endocrinology and metabolism of the field vole (Microtus agrestis). Exp Biol Med. 2003 Feb;228(2):188–93. https://doi.org/10.1177/153537020322800209.
- 15. Ayesh R, Weststrate JA, Drewitt PN, Hepburn PA. Safety evaluation of phytosterol esters. Part 5. Faecal short-chain fatty acid and microflora content, faecal bacterial enzyme activity and serum female sex hormones in healthy normolipidaemic volunteers consuming a controlled diet either with or without a phytosterol ester-enriched margarine. Food Chem Toxicol. 1999 Dec;37(12):1127–38. https://doi.org/10.1016/s0278-6915(99)00109-x.
- 16. Fudvoye J, Bourguignon JP, Parent AS. Endocrine-disrupting chemicals and human growth and maturation: a focus on early critical windows of exposure. Vitam Horm. 2014;94:1–25. https://doi.org/10.1016/B978-0-12-800095-3.00001-8.
- 17. Aksglaede L, Juul A, Leffers H, Skakkebaek NE, Andersson A-M. The sensitivity of the child to sex steroids: possible impact of exogenous estrogens. Hum Reprod Update. 2006 Jul–Aug;12(4):341–9. https://doi.org/10.1093/humupd/dml018.
- 18. Azova S, Wolfsdorf J. Precocious sexual development in a male toddler caused by unrecognized transdermal exposure to testosterone: case report and review of the literature. J Pediatr Endocrinol Metab. 2021 Mar 3;34(5):675–8. https://doi.org/10.1515/jpem-2020-0616.
- 19. Ramos CdO, Macedo DB, Bachega TASS, Nascimento ML, Madureira G, Latronico AC, et al. Premature pubarche due to exogenous testosterone gel or intense diaper rash prevention cream use: a case series. Horm Res Paediatr. 2019;91(6):411–5. https://doi.org/10.1159/000495664.
- 20. Huynh T, Stewart CI. Virilisation in siblings secondary to transdermal “bioidentical" testosterone exposure. J Paediatr Child Health. 2017 Mar;53(3):301–5. https://doi.org/10.1111/jpc.13466.
- 21. Martinez-Pajares JD, Diaz-Morales O, Ramos-Diaz JC, Gomez-Fernandez E. Peripheral precocious puberty due to inadvertent exposure to testosterone: case report and review of the literature. J Pediatr Endocrinol Metab. 2012;25(9–10):1007–12. https://doi.org/10.1515/jpem-2012-0124.
- 22. Ramsey JT, Li Y, Arao Y, Naidu A, Coons LA, Diaz A, et al. Lavender products associated with premature thelarche and prepubertal gynecomastia: case reports and endocrine-disrupting chemical activities. J Clin Endocrinol Metab. 2019 Nov 1;104(11):5393–405. https://doi.org/10.1210/jc.2018-01880.
- 23. Linklater A, Hewitt JK. Premature thelarche in the setting of high lavender oil exposure. J Paediatr Child Health. 2015 Feb;51(2):235. https://doi.org/10.1111/jpc.12837.
- 24. Gaspari L, Paris F, Jeandel C, Sultan C. Peripheral precocious puberty in a 4-month-old girl: role of pesticides?Gynecol Endocrinol. 2011 Sep;27(9):721–4. https://doi.org/10.3109/09513590.2010.526666.
Peng Xue and Yirou Wang contributed equally to this work.