Background
The incidence of attention deficit hyperactivity disorder (ADHD) in children has increased in the USA over the last two decades []. According to a 2016 national parent survey, 9.4% of children and 12.9% of boys were diagnosed with ADHD []. Optimal management of children with ADHD represents an area of debate among pediatricians, developmental pediatricians, psychologists, and educators with an overarching goal to address their unique behavioral, medical, and educational needs and to ensure functionality and success [, ]. Almost two thirds of children with ADHD were medically treated, and slightly less than half received behavioral therapy []. Children with ADHD are often referred to pediatric endocrinology clinics for evaluation and management of impaired growth. The analysis of the Pfizer International Growth Study (KIGS) database performed in 2014 showed that 7.4% of children treated with recombinant human growth hormone (rhGH) had a diagnosis of ADHD and 5.3% were prescribed a stimulant medication [].
The exact pathophysiology of ADHD is not fully understood, but dysregulation of several neurotransmitter systems including dopaminergic (DA) and noradrenergic (NE) pathways has been proposed as a possible mechanism []. The prefrontal cortex, caudate, and cerebellum have been shown as the areas of deficits in ADHD [, ]. These areas are interconnected by a network of DA and NE neurons []. The DA and NE pathways are also involved in growth hormone regulation [], suggesting a possible mechanistic link between impaired growth and ADHD [].
Growth impairment in a subgroup of children with ADHD has been widely recognized by pediatricians [] and psychiatrists []. Studies investigating growth inhibiting side effects of stimulants (methylphenidate, dextroamphetamine, lisdexamfetamine etc.) and nonstimulant medications (atomexitine, guanfacine, and clonidine) have shown controversial results: some suggest an association between stimulant medications and reduction in growth velocity [, ] and expected heights [], but others do not [-]. Previous studies have demonstrated that stimulant therapy temporarily impairs growth in children with ADHD in preadolescence through mid-adolescence, but does not affect the final target height [, ]. In the KIGS, children with either growth hormone deficiency or idiopathic short stature prescribed stimulant therapy for ADHD demonstrated impaired growth response to rhGH in the first year of treatment []. Conversely, the National Cooperative Growth Study (NCGS) database analysis revealed similar first-year growth responses in growth hormone-treated children receiving ADHD medications compared to those not taking ADHD medications []. Sixty-four percent of children with ADHD have psychiatric comorbidities and in addition to/instead of a stimulant are treated with other psychotropic medications, including clonidine, trazodone, serotonin selective reuptake inhibitors (SSRIs), and antipsychotics []. Effects of these other medications on growth are less known.
Children with ADHD and growth failure frequently undergo growth hormone stimulation testing (GHST) as part of the evaluation for GH deficiency despite the fact that the utility of GHSTs is controversial due to significant pitfalls in validity and reproducibility [-]. The GH secretagogues are known to release various neurohormones, such as somatostatin and GHRH via the alpha adrenergic pathways [] which might be impaired in ADHD and affected by medications used to treat ADHD. There are numerous studies on the growth suppressing effect of stimulant medications used in ADHD, but limited data on the GHST outcomes in children with ADHD treated or untreated with a stimulant or a nonstimulant medication. The NCGS did report similar peak-stimulated growth hormone responses to provocative stimuli in the ADHD-treated and non-ADHD-treated groups [].
In this study, we evaluate whether children with growth failure and ADHD are more likely to fail the GHST than children without ADHD. We also attempt to evaluate the association between the use of stimulant and other psychotropic medication and the outcome of the GHST in children with ADHD.
Clinicians are often faced with the dilemma of whether to prescribe growth hormone to these children with ADHD and impaired linear growth. The questionable validity of the GHST may result in overdiagnosis of GH deficiency leading to GH therapy with its significant cost implications. A better understanding of the outcomes of the GHST in this subset of children will help inform appropriate workup and counseling families.
Methods
The study was performed at an academic tertiary health care center. The data were collected from a single pediatric endocrinology group that provides services as a referral center for primary pediatric practices and community hospitals in western Massachusetts.
All children between the ages of 3–18 years evaluated in pediatric endocrinology for growth failure during a 16-year period (2002–2018) and who had a GHST performed were studied. Patients with pituitary abnormalities, hypopituitarism/septo-optic dysplasia, or genetic syndromes associated with short stature were excluded.
Retrospective chart review was performed using a pediatric endocrinology clinic database PEDRO Electronic Medical Record System (Pedrosoft LLC, Basking Ridge, NJ, USA) and Clinical Information System general hospital medical records (Cerner, Kansas City, MO, USA). Demographic data including age, sex, and ethnicity were collected to assess the differences among the groups. Baseline auxological information, height, weight, and body mass index (BMI), and pubertal staging were also extracted. Mid-parental heights (MPHs) were calculated. Results of the bone age study were collected as read by a pediatric radiologist and classified as normal if bone age was read within 2SD, delayed if under −2 SD, or advanced if over 2 SD. Tables V and VI from the Radiographic Atlas of Skeletal Development of the Hand and Wrist by Gruelich and Pyle were used for reference on the SD intervals for age []. In addition, we extracted medication entries for each subject with a diagnosis of ADHD based on the different classes of medications used in the treatment of ADHD.
The stimulation tests were performed after an overnight fast of 8–10 h. We requested that the family hold any noncritical medications on the morning of the GHST. The designated nurse in the infusion center discussed this with the family over the phone when the test was scheduled and a patient letter with the instructions was mailed to each patient. We did not collect the data on whether psycho-stimulant medications were held or not.
Testing protocol: Our practice used a combination of two agents – oral clonidine and either IV/IM glucagon or IV arginine. Testing was conducted using a one- or two-day test protocol based on the weight of the subject. Arginine followed by clonidine was used between January 2002 and October 2012. We subsequently adopted clonidine and IV glucagon as a second GH provocative agent in November 2012 due to shortage and cost of arginine. We changed our method of delivery of glucagon from IV to IM in November following a QI project that revealed that the mean peak GH levels form IV glucagon were not comparable with clonidine. A review of our protocol, in November 2018, revealed that glucagon was a weaker secretagogue, and as a result, we switched our testing protocol back to using arginine and clonidine.
The duration of the GH stimulation test was 4 h. The initial pharmacological agent was administered at baseline (time 0), and the second agent was administered at time 120 min after the first agent. GH levels were obtained at baseline and at 30-min intervals throughout the duration of the test. No children were primed with sex steroid before the provocative tests.
Statistical Analysis
Univariable comparisons of children with or without ADHD were conducted with t tests for continuous outcomes and Fisher’s exact tests for categorical variables. Variables with an ordinal scale (sexual maturity rating [SMR]) were analyzed using the Wilcoxon rank-sum test. Due to varying opinions and practices and because GH assays have evolved to become more sensitive, we evaluated response using GH peak cut-offs to diagnose GHD of <10 ng/mL, <7 ng/mL, and <5 ng/mL. Multivariable analysis of the proportion of children failing the GHST for these cut-off points was conducted using logistic regression to adjust for potential confounding factors. The outcomes of the GHST depend on the age/sexual maturity []; therefore, we also stratified our outcomes by age. All analyses were conducted in Stata (version 16.1, StataCorp, College Station, TX, USA). The odds ratios (ORs) and adjusted proportion of children failing the GHST are reported with 95% confidence intervals. Statistical significance was evaluated at an alpha level of 5%. To estimate statistical power and sample size, we assumed that about 50% of patients would fail the GHST at a cut-point of 10. Thus, a sample size of about 262 patients, approximately equally divided between those with and without ADHD, would provide about 80% power to detect an OR of at least 2.0 for a two-sided test at a critical test level of 5%.
Results
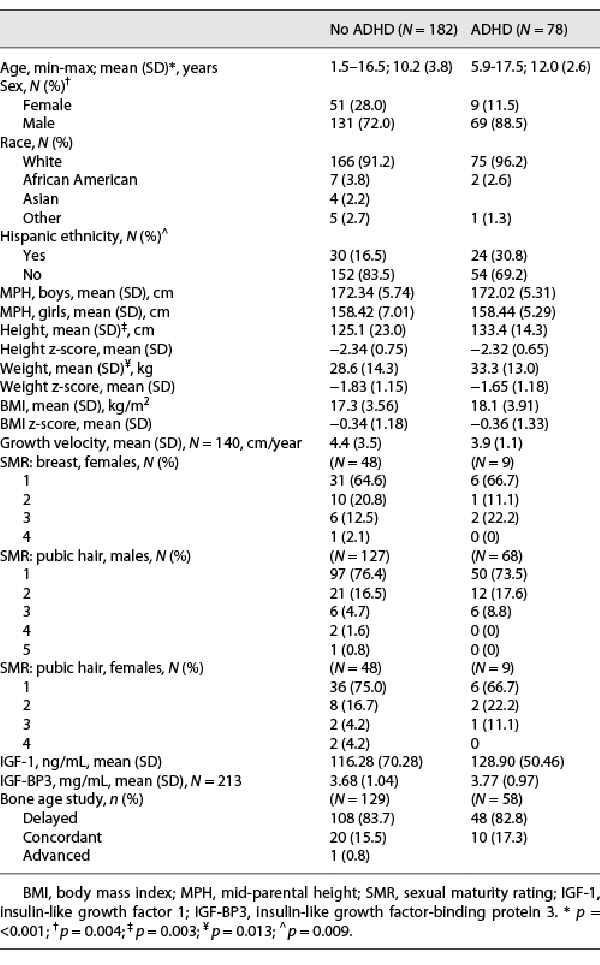
We included 260 children in the analysis who underwent GHST due to concerns for impaired linear growth and had normal pituitary morphology; the results are presented in Table 1. Thirty percent of the children had ADHD. Boys largely outnumbered girls in both the ADHD and non-ADHD groups. Both groups had a striking predominance of Caucasians (96% and 91%, respectively). Children with ADHD were 2 years older and taller and weighed more than those without ADHD, but there was no difference in the height z-score, weight z-score, BMI, and BMI z-score between these two groups. A large proportion of the children in the two groups were pre-pubertal, SMR 1 (82% and 80% for males and 77% and 78% for females). Groups had similar growth velocities and MPHs. Also, there was no difference in prevalence of other chronic conditions known to be associated with growth failure, such as hypothyroidism (2 children in each group), asthma (19 and 32), and inflammatory bowel disease (2 and 1) between the ADHD and non-ADHD groups. The mean insulin growth factors (insulin-like growth factor 1 [IGF-1] and insulin-like growth factor-binding protein 3 [IGF-BP3]) obtained as part of the initial biochemical evaluation were similar in both groups. A majority of children in both groups had a delayed bone age (82.8% in ADHD group and 83.7% in non-ADHD group).
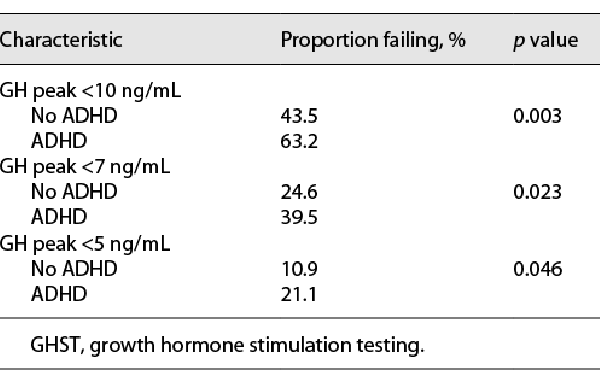
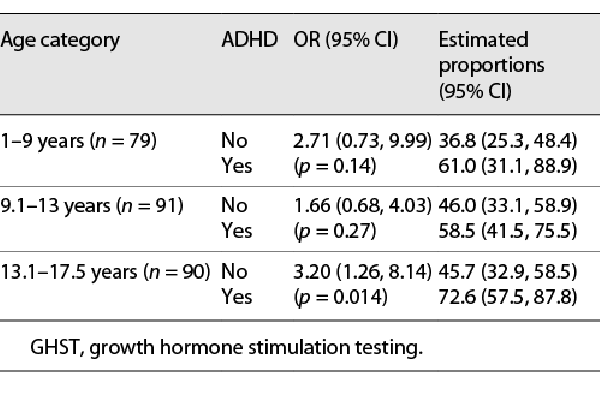
We analyzed the likelihood of failing the panel of two tests as part of GHST at three different cut-offs (<10 ng/mL, <7 ng/mL, and <5 ng/mL) in children with ADHD and without it. Subjects with ADHD had a higher likelihood of failing GHST across all three cut-off points (Table 2). The same was true across three age groups when logistic analysis was performed for the peak GH cut-off <10 ng/mL with adjustment for sex and stratification by age (Table 3). The effect was more robust in the older age group. The time from initial visit to GHST was similar between the two groups. The mean (SD) GH level in children with ADHD was 10.3 (7.4) ng/mL vs. 12.7 (7.5) ng/mL in children without ADHD (p = 0.018).
Our testing protocol changed twice during the duration of our study; about half of the children underwent the GHST using IV glucagon/oral clonidine, and about a quarter of children were tested using arginine/oral clonidine or IM glucagon/oral clonidine each. We assessed the consistency of GHST outcomes in both ADHD and non-ADHD group across the above three testing protocols and found no difference (see Fig. 1). There was however a difference in the overall performance between the types of GHST protocols with the IV glucagon/clonidine having a significantly higher failure rate than the IM glucagon/clonidine in the total sample (estimated probability 0.55 vs. 0.39 accordingly, p = 0.01).
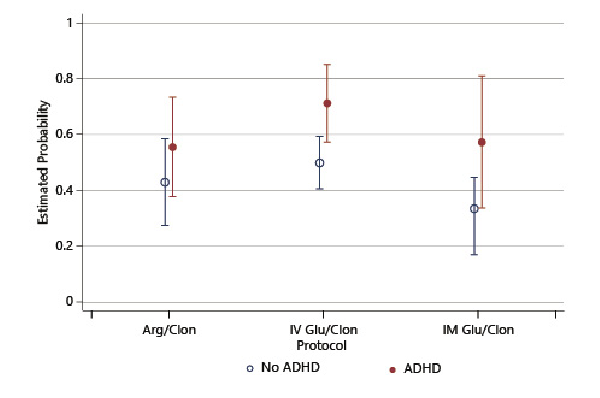
Fig. 1
Probability of failing growth hormone stimulation testing by protocol. Arg/Clon, arginine/clonidine; IV Glu/Clon, intravenous glucagon/clonidine; IM Glu/Clon, intramuscular glucagon/clonidine.
In our study group, nearly all children who failed GHST at the peak of 10 ng/mL were eventually prescribed and treated with rhGH including all children in ADHD group and 75 out of 79 subjects in the non-ADHD group. For children with the diagnosis of ADHD (n = 78), we attempted to assess the association between the use of stimulants and other psychiatric medications and the outcome of the GHST. Only 9 out of 78 children with ADHD did not receive pharmacological treatment. There was no difference in the GHST failure rate between children prescribed medication for ADHD and those 9 who were not treated. There was also no statistical difference in GHST failure rate between children prescribed only one stimulant medication versus other options (nonstimulants, SSRIs, antipsychotics as monotherapy, or in combination between those agents). There was a trend for a higher likelihood of failing a GHST when more than one psychotropic medication was prescribed; e.g., all children prescribed 2 or 3 stimulants (n = 7), a stimulant and atomoxetine (n = 2), a stimulant and an antipsychotic (n = 3) failed GHST (assessed at a peak <10), as well as 7/10 children who received a combination of a stimulant and guanfacine, 6/7 who received a stimulant and clonidine, and 9/11 who received a stimulant and an SSRI.
Discussion
Our study provides an intriguing observation about the physiology of pituitary regulation in children with ADHD – namely, the growth hormone response to a panel of growth hormone provocative agents in GHST. In a sample of 260 children with poor linear growth and normal pituitary morphology, we show that children with ADHD were more likely to fail GHST across 3 cut-offs (10, 7, and 5 ng/mL). At the same time, similar IGF-1 and IGF-BP3 levels in both groups suggest no impact of ADHD and its treatment on chronic GH secretion.
In accordance with the previous studies, we showed disproportionately high prevalence of ADHD in children with growth concerns: ∼30% versus ∼10% in general population nationwide [, ] which could be due to referral biases and not necessarily indicating that children with ADHD are shorter. Anxious parents might be requesting evaluations, and children with ADHD might be getting more medical attention than children without ADHD. The causes of impaired linear growth in children with ADHD have been previously investigated [, ], and the appetite-suppressing role of the stimulant medications leading to decreased weight gain was proposed as a main factor. In contrast, in our cohort we show that children with ADHD were similar in weight and BMI z-scores to children without ADHD. Children with ADHD were about 2 years older at the time of the GHST that might be explained by a more conservative approach in the group. It is possible that pediatricians waited longer to refer. It is also possible that growth decline occurs later in this group, possibly related to timing of medical treatment initiation []. Despite being older, children with ADHD and without were similar in pubertal stage, which means that children with ADHD were actually more delayed in puberty than the comparison group. Pubertal delay significantly affects function and regulation within the GH axis. The higher rate of CDGP in ADHD group could have affected GHST outcomes.
The diagnosis of GH deficiency using the GHST continues to be controversial. A substantial number of children with normal height fail to achieve a GH level above the cut-off during provocative testing []. Pre-pubertal and early pubertal children can exhibit very low GH secretion peaks but show normal increases in GH response with progress through puberty []. In our study, the majority of children were pre-pubertal and the overall GH failure rates were similar to those previously reported []. The children with ADHD in this study were 2 years older and more affected with CDGP. Previous studies have found decreases in GHST failure rate with age and pubertal maturation and proposed priming with sex steroids prior to GHST [, ]. In our study, children with ADHD were more likely to fail GHST despite being older. More than that, the effect was stronger in the older age group (Table 3). We could not statistically assess the effect of the medication due to a low number of individuals not prescribed medications for ADHD. It is possible that older children were more likely to be medically treated or used more psychotropic medications. Our study also supports previous findings of predisposition to CDGP in children with ADHD and poor linear growth. Constitutional delay is considered a major cause of slow growth in children with ADHD as final target heights are less affected or not affected [, ].
Studies have provided consistent evidence of deficits in the prefrontal cortex, caudate, and cerebellum in children with ADHD []. These areas are interconnected by network of neurons using dopamine and norepinephrine that have also been implicated in the regulation of growth hormone. The agents that are used for growth provocative tests facilitate GH secretion via central adrenergic mechanisms []. It is possible that our finding of a higher failure rate of GH to respond to GH provocative agents is due to inherent differences in the DA and NE neurons in children with ADHD.
The growth suppressing effects of psycho-stimulants in ADHD have been studied, but nothing is reported on the association of the medication and the GHST. The NCGS showed no differences in the maximum stimulated growth hormone response between the ADHD-treated and non-ADHD-treated groups []. We attempted to assess the effects of psycho-stimulants on the failure rate of the GHST but did not have sufficient numbers of untreated subjects. The immediate impact of these medications was not possible to assess given that our subjects were given the option to hold ADHD medications on the day of GHST based on severity of their ADHD symptoms. A larger sample size and probably a multicenter study are needed to evaluate the effects of the different psycho-stimulant medications on the GHST.
Strength and Limitations
The study has the major limitation of being retrospective. Our patients were given the option of holding any nonlife-threatening medications on the morning of the GHST, but their choices of taking or not taking the medications were not documented; thus, we cannot assess whether GHST results were impacted by the morning dose of medications.
None of the children included in this retrospective analysis received sex steroid priming before the GHST. Sex hormone priming could have helped discriminate the effect of delayed puberty on GH axis regulation from the impact of ADHD and its treatment, but the ethical side of this approach is still debated and therefore its use is not universal [].
Over the duration of the study, our practice utilized different pharmacological agents in our GH stimulation testing. The nonhomogenous nature of our testing procedure is also a limitation of our study, but we did not observe a difference in the GHST outcomes in relation to ADHD across three different tests used.
The setting of this study may limit generalizability given that the population was largely Caucasian; we did not collect data on the socioeconomic status of our subjects. Despite the above limitations, our study has the advantage of being the first study looking into the outcome of GHST in a large population of children with and without ADHD and short stature or growth failure.
Conclusion
Our study shows an association between ADHD and increased risk of failure of the GHST in children with short stature or impaired linear growth. Whether these findings reflect an impact of the ADHD itself or the effects of the psycho-stimulant medications on the hypothalamic-pituitary regulation of GH needs to be further assessed. It remains unclear whether the higher GHST failure rate in children with ADHD is due to ADHD and/or its treatment or related CDGP.
Acknowledgments
Author Obiageli Obi was not available to confirm co-authorship, but the corresponding author Ksenia N. Tonyushkina affirms that author Obiageli Obi contributed to the paper, had the opportunity to review the final version to be published, and guarantees author Obiageli Obi co-authorship status and the accuracy of the author contribution and conflict of interest statements.
Statement of Ethics
This study protocol was reviewed and approved by the Institutional Review Board of Baystate Medical Center, approval number BH19-099.
Conflict of Interest Statement
Authors state no conflict of interest.
Funding Sources
Authors declared no funding sources related to this study.
Author Contributions
Each author has made substantial contributions to the conception or design of the work, or to the acquisition, analysis, or interpretation of data for the work; and participated in drafting the work or revising it critically for important intellectual content. Ksenia N. Tonyushkina conceived the project, interpreted the data, and substantially edited the initial draft. Obiageli Obi reviewed the background literature, collected the data, and drafted the manuscript. Paul Visintainer performed statistical analysis and interpreted the data; Victoria Cobb collected the data and assisted with data analysis; Holley F. Allen and Edward Reiter contributed to data interpretation and editing the manuscript. All authors have accepted responsibility for the entire content of this manuscript and approved its submission.
Data Availability Statement
All data generated or analyzed during this study are included in this article. Further inquiries can be directed to the corresponding author.
References
- 1. Xu G, Strathearn L, Liu B, Yang B, Bao W. Twenty-year trends in diagnosed attention-deficit/hyperactivity disorder among US children and adolescents, 1997-2016. JAMA Netw Open. 2018;1(4):e181471. https://doi.org/10.1001/jamanetworkopen.2018.1471.
- 2. Danielson ML, Bitsko RH, Ghandour RM, Holbrook JR, Kogan MD, Blumberg SJ. Prevalence of parent-reported ADHD diagnosis and associated treatment among U.S. Children and adolescents, 2016. J Clin Child Adolesc Psychol. 2018 Mar–Apr;47(2):199–212. https://doi.org/10.1080/15374416.2017.1417860.
- 3. Tarver J, Daley D, Sayal K. Attention-deficit hyperactivity disorder (ADHD): an updated review of the essential facts. Child Care Health Dev. 2014 Nov;40(6):762–74. https://doi.org/10.1111/cch.12139.
- 4. Wolraich ML, Hagan JF, Allan C, Chan E, Davison D, Earls M, et al. Clinical practice guideline for the diagnosis, evaluation, and treatment of attention-deficit/hyperactivity disorder in children and adolescents. Pediatrics. 2019;144(4):e20192528. https://doi.org/10.1542/peds.2019-2528.
- 5. Miller BS, Aydin F, Lundgren F, Lindberg A, Geffner ME. Stimulant use and its impact on growth in children receiving growth hormone therapy: an analysis of the KIGS International Growth Database. Horm Res Paediatr. 2014;82(1):31–7. https://doi.org/10.1159/000360005.
- 6. Raskin LA, Shaywitz SE, Shaywitz BA, Anderson GM, Cohen DJ. Neurochemical correlates of attention deficit disorder. Pediatr Clin North Am. 1984 Apr;31(2):387–96. https://doi.org/10.1016/s0031-3955(16)34584-9.
- 7. Shaw P, Eckstrand K, Sharp W, Blumenthal J, Lerch JP, Greenstein D, et al. Attention-deficit/hyperactivity disorder is characterized by a delay in cortical maturation. Proc Natl Acad Sci U S A. 2007 Dec 4;104(49):19649–54. https://doi.org/10.1073/pnas.0707741104.
- 8. Arnsten AF, Pliszka SR. Catecholamine influences on prefrontal cortical function: relevance to treatment of attention deficit/hyperactivity disorder and related disorders. Pharmacol Biochem Behav. 2011 Aug;99(2):211–6. https://doi.org/10.1016/j.pbb.2011.01.020.
- 9. Kesner RP, Churchwell JC. An analysis of rat prefrontal cortex in mediating executive function. Neurobiol Learn Mem. 2011 Oct;96(3):417–31. https://doi.org/10.1016/j.nlm.2011.07.002.
- 10. Martin JB. Neural regulation of growth hormone secretion. N Engl J Med. 1973 Jun;288(26):1384–93. https://doi.org/10.1056/nejm197306282882606.
- 11. Waxmonsky JG, Pelham WE 3rd, Baweja R, Hale D, Pelham WE Jr. Predictors of changes in height, weight, and body mass index after initiation of central nervous system stimulants in children with attention deficit hyperactivity disorder. J Pediatr. 2022 Feb;241:115–25.e2. https://doi.org/10.1016/j.jpeds.2021.09.030.
- 12. Baweja R, Hale DE, Waxmonsky JG. Impact of CNS stimulants for attention-deficit/hyperactivity disorder on growth: epidemiology and approaches to management in children and adolescents. CNS Drugs. 2021 Aug;35(8):839–59. https://doi.org/10.1007/s40263-021-00841-w.
- 13. Schneider G, Banaschewski T, Feldman BL, Gustafsson PA, Murphy B, Reynolds M, et al. Weight and height in children and adolescents with attention-deficit/hyperactivity disorder: a longitudinal database study assessing the impact of guanfacine, stimulants, and no pharmacotherapy. J Child Adolesc Psychopharmacol. 2019 May;29(4):285–304. https://doi.org/10.1089/cap.2018.0132.
- 14. Yackobovitch-Gavan M, Mimouni-Bloch A, Gabbay U, Carmi D, Goldstein B, Keinan-Boker L, et al. Sex-specific long-term height and body mass index trajectories of children diagnosed with attention-deficit/hyperactivity disorder and treated with stimulants. J Pediatr. 2021 Nov;238:296–304.e4. https://doi.org/10.1016/j.jpeds.2021.07.018.
- 15. Faraone SV, Spencer TJ, Kollins SH, Glatt SJ. Effects of lisdexamfetamine dimesylate treatment for ADHD on growth. J Am Acad Child Adolesc Psychiatry. 2010 Jan;49(1):24–32. https://doi.org/10.1097/00004583-201001000-00006.
- 16. Bereket A, Turan S, Karaman MG, Haklar G, Ozbay F, Yazgan MY. Height, weight, IGF-I, IGFBP-3 and thyroid functions in prepubertal children with attention deficit hyperactivity disorder: effect of methylphenidate treatment. Horm Res Paediatr. 2005;63(4):159–64. https://doi.org/10.1159/000084683.
- 17. Biederman J, Spencer TJ, Monuteaux MC, Faraone SV. A naturalistic 10-year prospective study of height and weight in children with attention-deficit hyperactivity disorder grown up: sex and treatment effects. J Pediatr. 2010 Oct;157(4):635–40.e1. https://doi.org/10.1016/j.jpeds.2010.04.025.
- 18. Harstad EB, Weaver AL, Katusic SK, Colligan RC, Kumar S, Chan E, et al. ADHD, stimulant treatment, and growth: a longitudinal study. Pediatrics. 2014 Oct;134(4):e935–44. https://doi.org/10.1542/peds.2014-0428.
- 19. Faraone SV, Biederman J, Morley CP, Spencer TJ. Effect of stimulants on height and weight: a review of the literature. J Am Acad Child Adolesc Psychiatry. 2008 Sep;47(9):994–1009. https://doi.org/10.1097/chi.0b013e31817e0ea7.
- 20. Frindik JP, Morales A, Fowlkes J, Kemp S, Thrailkill K, Lippe B, et al. Stimulant medication use and response to growth hormone therapy: an NCGS database analysis. Horm Res. 2009;72(3):160–6. https://doi.org/10.1159/000232491.
- 21. Stanley T. Diagnosis of growth hormone deficiency in childhood. Curr Opin Endocrinol Diabetes Obes. 2012 Feb;19(1):47–52. https://doi.org/10.1097/med.0b013e32834ec952.
- 22. Grimberg A, DiVall SA, Polychronakos C, Allen DB, Cohen LE, Quintos JB, et al. Guidelines for growth hormone and insulin-like growth factor-I treatment in children and adolescents: growth hormone deficiency, idiopathic short stature, and primary insulin-like growth factor-I deficiency. Horm Res Paediatr. 2016;86(6):361–97. https://doi.org/10.1159/000452150.
- 23. Allen DB. Diagnosis of growth hormone deficiency remains a judgment call: and that is good. Horm Res Paediatr. 2021;94(11–12):406–9. https://doi.org/10.1159/000521628.
- 24. Giustina A, Veldhuis JD. Pathophysiology of the neuroregulation of growth hormone secretion in experimental animals and the human. Endocr Rev. 1998;19(6):717–97. https://doi.org/10.1210/er.19.6.717.
- 25. Greulich WW, Pyle SI. Radiographic atlas of skeletal development of the hand and wrist. Stanford, California: Stanford University Press; 1959.
- 26. Gandrud LM, Wilson DM. Is growth hormone stimulation testing in children still appropriate?Growth Horm IGF Res. 2004 Jun;14(3):185–94. https://doi.org/10.1016/j.ghir.2003.11.003.
- 27. Faraone SV, Sergeant J, Gillberg C, Biederman J. The worldwide prevalence of ADHD: is it an American condition?World Psychiatry. 2003;2(2):104–13.
- 28. Spencer T, Biederman J, Wilens T. Growth deficits in children with attention deficit hyperactivity disorder. Pediatrics. 1998 Aug;102(2 Pt 3):501–6. https://doi.org/10.1542/peds.102.s3.501.
- 29. Davallow Ghajar L, DeBoer MD. Children with attention-deficit/hyperactivity disorder are at increased risk for slowed growth and short stature in early childhood. Clin Pediatr. 2020 May;59(4–5):401–10. https://doi.org/10.1177/0009922820902437.
- 30. Mauras N, Walton P, Nicar M, Welch S, Rogol AD. Growth hormone stimulation testing in both short and normal statured children: use of an immunofunctional assay. Pediatr Res. 2000 Nov;48(5):614–8. https://doi.org/10.1203/00006450-200011000-00010.
- 31. Shalet SM, Toogood A, Rahim A, Brennan BM. The diagnosis of growth hormone deficiency in children and adults. Endocr Rev. 1998 Apr;19(2):203–23. https://doi.org/10.1210/edrv.19.2.0329.
- 32. Martin LG, Clark JW, Connor TB. Growth hormone secretion enhanced by androgen. J Clin Endocrinol Metab. 1968 Mar;28(3):425–8. https://doi.org/10.1210/jcem-28-3-425.
- 33. Martha PM Jr, Rogol AD, Veldhuis JD, Kerrigan JR, Goodman DW, Blizzard RM. Alterations in the pulsatile properties of circulating growth hormone concentrations during puberty in boys. J Clin Endocrinol Metab. 1989 Sep;69(3):563–70. https://doi.org/10.1210/jcem-69-3-563.
- 34. Rubia K, Alegría AA, Brinson H. Brain abnormalities in attention-deficit hyperactivity disorder: a review. Rev Neurol. 2014 Feb 24;58(Suppl 1):S3–16.
- 35. Wetterau LA. The pros and cons of sex steroid priming in growth hormone stimulation testing. J Pediatr Endocrinol Metab. 2012;25(11–12):1049–55. https://doi.org/10.1515/jpem.2011.327.