Introduction
Folliculogenesis is controlled by a complex interaction among hormones in the hypothalamus, anterior pituitary gland and the ovaries. Luteinizing hormone (LH) and follicle-stimulating hormone (FSH) are secreted from the anterior pituitary gland in response to GnRH (; ) and play a complementary role in follicle development and ovulation (). In ovarian theca cells, LH stimulates the secretion of androgens that are transferred to granulosa cells to be converted to oestradiol (E2) by aromatase. In granulosa cells, FSH stimulates the development of ovarian follicles, while LH action is involved in follicle development and maturation. A deficiency in LH and FSH production or action compromises gametogenesis and gonadal steroid production thereby reducing female fertility (; ; ; ). Recently, the International Committee for Monitoring Assisted Reproductive Technologies (ICMART) highlighted the importance of gonadotropin action in determining a deficiency of LH and FSH, and provided a broader definition of hypogonadotropic hypogonadism that encompasses this notion, namely ‘Gonadal failure associated with reduced gametogenesis and reduced gonadal steroid production due to reduced gonadotropin production or action’ (). While the causes of reduced LH and FSH production are well described (), deficiency of LH and FSH action has received much less attention, even though it is now recognised that both production and action are relevant for human fertility and are of clinical interest for medically assisted reproduction (MAR).
The action of LH and FSH is determined by a variety of factors, i.e. the frequency and amplitude of GnRH peaks, the different isoforms of LH and FSH, polymorphisms of FSH and LH and their receptors, and intracellular signalling. Furthermore, in MAR, inter-individual demographic, clinical and treatment factors, such as ageing, comorbidities, and the effect of oral contraceptives and GnRH analogue protocols, can influence gonadotropin action and the response to exogenous gonadotropins (; ; ; ). In this review, we focus on the determinants of reduced LH and FSH action that are associated with a reduced quantitative and qualitative response to ovarian stimulation (OS) in the attempt to gain insights that may help to improve the clinical management of women with LH and FSH deficiency undergoing MAR.
LH and FSH action in physiological and altered conditions
The hypothalamic–pituitary–gonadal axis
LH and FSH production and activity depend on the precise orchestration of numerous elements of the hypothalamic–pituitary–gonadal (HPG) axis (Fig. 1), the functioning of which is regulated by positive and negative feedback loops that occur in parallel with follicular development (; ). Oestradiol is the hormone most involved in the regulation of the HPG axis, together with progesterone and inhibins. In the early follicular phase, FSH levels increase thereby triggering E2 secretion which, in turn, selectively inhibits FSH release and maintains rapid GnRH pulsatility during the late follicular phase (). Negative feedback control of FSH release is also supported by the release of inhibin B from the granulosa cell mass of the follicle. In the late follicular phase, accelerated GnRH pulsatility causes an increase in LH level, which further stimulates E2 secretion. At mid-menstrual cycle, E2 provides a positive feedback to the hypothalamus, thereby causing the LH (and FSH) surge (). After ovulation, luteinization of the ruptured follicle induces progesterone secretion, which has a negative feedback on the hypothalamus, thus reducing the GnRH pulse frequency. Finally, with the demise of the corpus luteum, the levels of E2, progesterone and inhibin A sharply decrease, and the GnRH pulse frequency and FSH secretion increase leading to the next cycle (; ). Moreover, increasing evidence indicates that anti-Müllerian hormone (AMH) plays a role in neuroendocrine control of reproduction and in gonadotropin action (). Anti-Müllerian hormone receptors were found in hypothalamic GnRH neurons and in gonadotrope-derived cell lines (; ). In vivo and in vitro experiments demonstrated that AMH acts on GnRH neurons thereby increasing LH pulsatility and secretion () and that it interacts with both hypothalamic and pituitary cells to facilitate gonadotropin secretion (). In addition, AMH inhibits follicle growth by decreasing the sensitivity of ovarian follicles to FSH () and exogenous AMH has been reported to decrease aromatase activity and the number of luteinizing hormone/choriogonadotropin receptors (LHCGRs) on granulosa cells in vitro (; ).
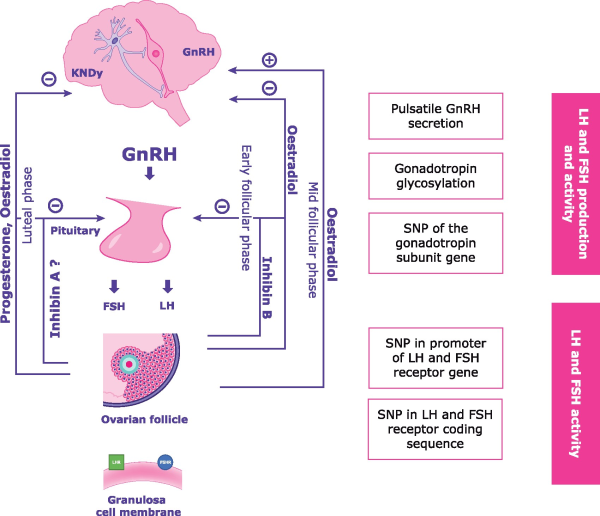
Figure 1
The hypothalamus–pituitary–gonadal axis interaction, feedback system and disrupting factors. KNDy, kisspeptin, neurokinin B, and dynorphin; SNP, single nucleotide polymorphism.
Synergic action of LH and FSH in follicles
The action of both FSH and LH is required for follicular growth. In the mid-follicular phase, LH-dependent and FSH-dependent synergistic action in granulosa cells ensures adequate steroidogenesis to stimulate follicle growth (; ; ). In vitro studies confirmed that the synergistic action of LH and FSH regulates the proliferative and anti-apoptotic effects that occur across the menstrual cycle i.e. folliculogenesis, granulosa cell growth, ovulation triggering, and maintenance of the corpus luteum (, , ). Furthermore, the combination of FSH and LH was found to activate progesterone and E2 production in human granulosa lutein cells (). The synergy between LH and FSH is even more striking at receptor level. FSH receptors (FSHRs) are expressed on the granulosa cells of the developing ovarian follicle, while LHCGRs are found on three distinct cell types: the theca cells of the early antral follicle, the mural granulosa cells of the peri-ovulatory Graafian follicle induced by FSH/FSHR, and the luteal cells of the corpus luteum. Like many G protein-coupled receptors (GPCRs), FSHR and LHCGR can form receptor homodimers/oligomers, and can also form heteromers (; ) (Fig. 2). This complexity may explain why in mid-late follicular phase, when both LHCGRs and FSHRs are expressed on granulosa cells, FSH is sufficient for follicular growth even in the presence of very low LH levels, while as LH levels rise, LHCGR/FSHR heteromers may then promote the LH-mediated pathways required for ovulation and luteinization (; ). Also the precise intracellular location of both LHCGR and FSHR is critical for gonadotropin signalling (; , ). The downstream functions of receptor signalling from intracellular compartments have yet to be determined, but LHCGR may be needed for oocyte maturation () and androgen production, as shown in mouse and bovine ovarian models (). At the receptor level, diverse FSH glycoforms may activate distinct FSH signalling with greater or lesser efficacy, a phenomenon known as ‘biased agonism’ (; ), and this may have a profound effect on the cell proliferation rate and/or steroidogenesis.
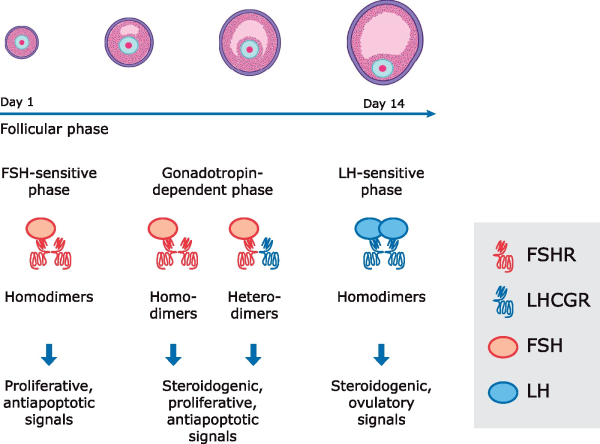
Figure 2
Model of antral follicular development mediated by FSH receptor (FSHR) and LH/choriogonadotropin receptor (LHCGR) dimers. Modified from .
The effect of different LH and FSH glycosylation variants and female age on LH and FSH action
The glycosylation of LH and FSH varies throughout the menstrual cycle and across reproductive life, thus impacting the half-life and activity of gonadotropins (; ; ). Glycosylation variants have differential activity in vivo (). While the proportion of the acidic FSH isoforms is higher during the early to mid-follicular phase than in the pre-ovulatory phase, the more basic FSH isoforms are secreted before ovulation (). Moreover, the half-lives of the LH and FSH isoforms are shorter, whereas their activity is greater in young post-pubertal women than in post-menopausal women (; ).
The decreased fertility observed with ageing (women > 35 years of age) is associated with an increase of fully glycosylated FSH variants that have a lower affinity for the FSHR versus the most common isoforms found in younger women (; ; ). Also LH isoforms change to less bioactive isoforms with ageing, i.e. more sialylated and less sulfonated LH isoforms (). The reduced action of circulating gonadotropins caused by ageing results in reduced steroidogenesis and decreased ovarian function (; ; ). In the perimenopausal transition period, there is a tendency towards increasing levels of serum gonadotropins and decreasing levels of E2, and a statistically significant negative correlation between LHCGRs and serum LH levels (). Moreover, various studies reported that decreased LH activity in ageing women impairs androgen production (; ). In fact, circulating levels of androgens have been reported to be much lower in advanced maternal age (AMA) women than in younger women (; ; ).
The effect of genetic variants of LH, FSH and their receptors on LH and FSH action
LH and FSH deficiency can also be associated with genetic variants in either the β subunit or in LH and FSH receptors (; ). Mutations in FSHB have been reported in women with delayed puberty, absent breast development, and primary amenorrhea (; ). The FSHR gene variant at position −29 was associated with increased serum FSH levels in subjects with primary amenorrhea (). LHB gene mutations seem to be less frequent, but in males, these mutations were related to delayed puberty, low testosterone, and arrested spermatogenesis in the presence of elevated serum LH (). Heterozygous LHB mutation in women may be associated with infertility (). In addition, homozygous LHCGR mutations are associated with oligomenorrhea or amenorrhea despite normal pubertal development (). Also, polymorphisms of FSH, LH, and their receptors have been related to reduced fertility in terms of reduced activity of endogenous gonadotropins or resistance to OS that result in a lower than expected oocyte yield (; ; ). Furthermore, polymorphisms of the FSHB promoter and FSHR were associated with lower FSH levels, a longer menstrual cycles, and age at menopause (; ). The specific implications of polymorphisms in assisted reproduction are discussed below.
Clinical presentation of LH and FSH deficiency
Deficiency of LH and FSH is caused by reduced gonadotropin production or action due to internal and external factors (Fig. 3). These conditions are generally characterised by low E2 and low-normal gonadotropins (; ). Congenital abnormalities are well described rare conditions that usually present with deficient GnRH secretion occurring in isolation or in association with anosmia (Kallmann syndrome) (; ). Among acquired conditions, intensive exercise and eating disorders are widely recognised as life-style factors that could suppress the HPG axis (; ). Furthermore, poorly controlled diabetes and thyroid disorders could significantly affect gonadotropin secretion and action. To restore reproductive function, stress habits should be corrected and the underlying endocrine disorder be treated (; ). Other acquired conditions of reduced LH and FSH action could be linked to pituitary tumours (e.g. prolactinomas) or pituitary infarct (e.g. Sheehan’s syndrome) that are usually characterised by specific symptoms due to pituitary dysfunction or compression of tissues surrounding the pituitary or that can severely affect pituitary function beyond reproductive function (e.g. panhypopituitarism) (). All these conditions are in line with the ICMART definition of hypogonadotropic hypogonadism being associated with reduced gametogenesis and steroidogenesis due to reduced gonadotropin production or action. In MAR, a combination of factors such as AMA and genetic variants of gonadotropins, or their receptors that impair gonadotropin action, may further exacerbate the transient reduced LH and FSH production caused by GnRH analogues, and result in a low or sub-optimal response to OS (; , ).
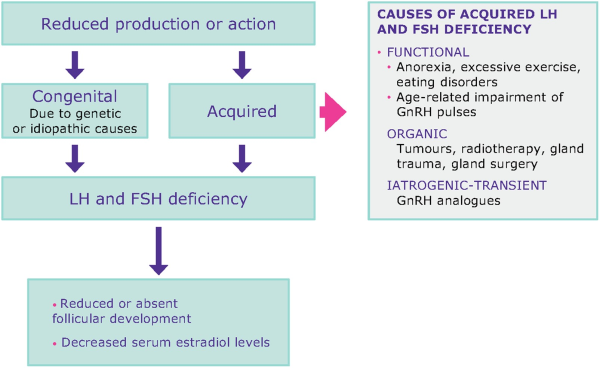
Figure 3
LH and FSH deficiency is a spectrum of conditions with various aetiologies. Women with LH and FSH deficiency have low/normal gonadotropin levels and low oestradiol levels.
LH and FSH deficiency in medically assisted reproduction
LH and FSH deficiency induced by GnRH analogue protocols
GnRH agonists and antagonists cause transient LH and FSH deficiency and are used during OS to prevent premature ovulation, thus enabling retrieval of more oocytes (). GnRH agonists and antagonists have different modes of action. GnRH agonists, after an initial increase in LH and FSH secretion (flare up), induce downregulation of the GnRH receptor (). Conversely, GnRH antagonists competitively block the GnRH receptor thereby preserving pituitary gland responsiveness, so that gonadotropin levels are restored within a few hours of discontinuing suppression (). Usually, the residual circulating levels of LH are sufficient to support steroidogenesis in theca cells, and recombinant human FSH (r-hFSH) is sufficient for OS. However, LH levels much lower than baseline can negatively affect MAR outcomes in some women (; ; ; ; ; ).
In a study of 200 normo-gonadotropic women treated with a standard long GnRH agonist protocol and OS with r-hFSH, found that, in 48% of women, LH levels remained profoundly suppressed (< 0.5 IU/l) on stimulation Day 8, and that the early pregnancy loss was significantly higher in this group than in women whose LH levels were not profoundly suppressed (45% versus 9%, P < 0.005). Similarly, found that a 50% decrease in the LH level from the early to mid-follicular phase after GnRH agonist downregulation was associated with a lower live birth rate than in women with a less sharp decrease in LH levels. It can be speculated that an abrupt LH drop during OS results in a decrease in the conversion of androgen precursors to oestrogens thereby resulting in insufficient E2 production by the growing follicles and a drop in circulating E2 levels (). However, other studies did not find that low serum LH levels after pituitary downregulation with GnRH agonists negatively affected reproductive outcomes (; ). Thus, it is not clear to which extent serum LH levels are predictive of reproductive outcome in GnRH agonist cycles. However, it should be noted that different LH threshold values were used in different studies to define low LH groups (i.e. <1.5–0.5 IU/l LH) (; ; ).
Profound LH suppression has also been reported in some women after the administration of GnRH antagonists. found that if the change in serum LH during GnRH antagonist administration was −2.2 IU/l below baseline values, the chance of achieving a clinical pregnancy under standard OS was almost nil. reported that, after treatment with a GnRH antagonist, LH was over suppressed in 26% of women and these patients had a significantly lower increase in E2 during the first 24 h after antagonist administration compared to women who were not over suppressed. The effect was reversed in these patients after the addition of recombinant human LH (r-hLH). It remains unclear if this strong suppression of LH levels in response to GnRH antagonists is observed only in some subgroups of patients. It is conceivable that it may be associated with the individual pharmacodynamic response to the GnRH antagonist. However, it has been suggested that the severity of the LH deficiency caused by GnRH antagonist treatment is not linked to absolute LH serum levels but rather to the magnitude of suppression over time versus the baseline ().
Several studies have shown that r-hFSH and r-hLH co-treatment can improve OS in women with transient LH and FSH deficiency after GnRH agonist or antagonist treatment (; ), whereas two systematic reviews did not observe such an effect in the general population (; ). To evaluate the benefits of r-hFSH:r-hLH treatment, studies should include subgroups of patients who are more prone to develop LH and FSH deficiency after GnRH analogues, such as women of AMA and hypo-responders (, ).
Reduced LH and FSH action in AMA women undergoing MAR
The impaired functioning of the LH and FSH systems in AMA women, reviewed above, could be exacerbated by the transient gonadotropin deficiency induced by GnRH analogue regimens during OS. Co-treatment with r-hFSH:r-hLH has been proposed in AMA women undergoing MAR (; ). A meta-analysis of studies in women between the ages of 35 and 40 years found that r-hFSH:r-hLH co-treatment resulted in higher clinical pregnancy rates than r-hFSH monotherapy (). demonstrated that r-hLH addition could compensate for the LH deficiency observed in women above 35 years, and three randomised controlled trials, which were included in the Hill meta-analysis (), found that co-treatment with r-hLH and r-hFSH in women between the ages of 35 and 39 years improved both live birth and implantation rates (; ). This effect was not found in studies of women over the age of 40 years (; ; ). The latter finding is not surprising as there is a dramatic reduction in the proportion of normal euploid embryos in women over the age of 40 years (), and currently there are no data to suggest that this effect can be compensated by r-hLH.
The benefit of r-hFSH:r-hLH treatment for AMA women may also be related to the effect of LH on oocyte maturation and embryo implantation. In fact, LH exerts an anti-apoptotic effect on cumulus cells and promotes the paracrine signalling involved in cell expansion and oocyte maturation during folliculogenesis (; ). In addition, LH modulates various signalling molecules involved in endometrial implantation namely, leukaemia inhibiting factor, colony-stimulating factor-1, interleukin-1, integrins, glycodelin and mucin 1, and may improve endometrial receptivity (; ).
Hypo-response to ovarian stimulation due to reduced LH and FSH action
LH and FSH action may be reduced by receptor and post-receptor defects that cause a reduced response to OS. In clinical trials, about 10–15% of normo-ovulatory and normo-gonadotropic women undergoing MAR had a hypo-response to OS with exogenous gonadotropins ().
Ovarian hypo-response can be defined as an unexpected slow response or stagnated follicle growth during OS with standard-dose FSH monotherapy, or that may require a higher than expected dose of gonadotropins depending on age, body mass index and ovarian reserve (). A meta-analysis on hypo-response found that OS with r-hFSH:r-hLH resulted in significantly higher clinical pregnancy and implantation rates versus r-hFSH alone (). Recently, two indices based on individual ovarian reserve have been proposed to measure the ovarian response to OS: the follicle output rate (FORT) () and the follicle-to-oocyte index (FOI) (). FORT measures the ratio of the number of pre-ovulatory follicles on the day of hCG trigger × 100 over the number of small antral follicles at baseline prior to OS, while FOI is the ratio between the number of oocytes collected at ovum pick up and the number of antral follicles at the beginning of OS. These two indices are a direct measure of the individual response to OS and can help to identify a hypo-response to OS better than oocyte number alone.
The pathophysiological mechanisms of hypo-response are not yet fully understood. In some cases, a hypo-response may be associated with LH and FSH single nucleotide polymorphisms (SNPs) and their receptors (; ; ), but the exact prevalence of these SNPs in MAR is unknown because they are not routinely tested.
Reduced LH and FSH action in ovarian stimulation due to genetic variants
Some SNPs related to LH, FSH, and their receptors that reduce the activity of endogenous gonadotropins or induce ovarian resistance to OS due to receptor and post-receptor defects have been associated with reduced fertility (; ; ). A polymorphism of the FSH molecule that affects the FSH beta chain promoter (c.-221 G > T) seems to affect gonadotropin function (; ). Indeed, in a study of Finnish women of reproductive age, the T allele carriers were characterised by increased baseline serum LH and FSH levels, and by idiopathic infertility (). In another study of 63,350 women, the T allele was associated with a longer menstrual cycle, later age at menopause and a reduced incidence of endometriosis ().
Also FSHR SNPs may be associated with a hypo-response to OS (). Some variants of FSHR 680 c.2039G>A (rs6166) are related to higher FSH levels and to higher FSH consumption (; ; ). FSHR -29 G > A (rs1394205) allele A carriers were found to have fewer FSHRs than allele G carriers, which translated in a higher FSH consumption during OS and a lower ovarian sensitivity in A carriers (; ). Also the FSHR c. G919A (rs6165) variant may affect ovarian response but not ovarian reserve (; ; ). Interestingly, different combinations of genetic variants of the FSH beta chain and of FSHR affect menstrual Day 3 serum FSH levels in women of reproductive age ().
Variants of the LH beta chain and of LHCGRs that affect OS have also been identified. A common variant of the LH beta chain (rs1800447) affects the LH system during OS (; , ). Clinically, carriers of this LH variant are characterised by a less active form of LH that does not adequately support FSH activity during follicle stimulation, and consequently results in a reduced response to OS with FSH (, ). The LHCGR c.872A>G (rs12470652) polymorphism could affect ovarian response when associated with other polymorphisms. Indeed, the ratio between the cumulative FSH dose and the total number of oocytes retrieved was higher in carriers of allele C of FSHR -29 rs1394205, FSHR rs6166, and LHCGR 291 rs12470652 than in women who did not have allele C in at least one of these SNPs (). In addition, LHCGR S312N (rs2293275) carriers required a higher FSH dosage during OS versus asparagine carriers (). Thus, polymorphisms of the LH beta chain and of LHCGRs could reduce ovarian sensitivity to FSH, which suggests that the function of both gonadotropins is crucial during OS. In addition, the presence of serine instead of asparagine in Position 312 might lead to reduced sensitivity to LH action and may explain why carriers of this polymorphism required more r-hLH during OS than non-carriers (). Moreover, in the same study, the pregnancy rate was significantly higher in serine carriers who received r-hFSH:r-hLH than in those receiving r-hFSH alone (P = 0.04) ().
Understanding LH and FSH deficiency in MAR: where do we stand? Limitations and implications for future research
LH and FSH deficiency is characterised by reduced LH and FSH production or action in the presence of normal-low LH and FSH and low E2 levels. In specific subgroups of women (AMA women and women with a hypo-response to OS), LH and FSH deficiency may be the underlying cause of an unfavourable prognosis. Indeed, OS with r-hFSH:r-hLH has been reported to improve reproductive outcomes in these subgroups (). In addition, preliminary findings suggest that serine carriers of the LHCGR variant (rs 2293275) might require higher amounts of r-hLH during OS together with r-hFSH than do aspargine homozygous carriers (). Should these data be confirmed in larger studies, they will facilitate the identification of subpopulations of women with potential LH and FSH deficiency and consequently a poor MAR prognosis. Recently, the POSEIDON (Patient-Oriented Strategies Encompassing IndividualizeD Oocyte Number) group proposed a classification of low prognosis based on a confirmed or expected inappropriate ovarian response to gonadotropin stimulation (; ). The POSEIDON concept contributes to identifying more homogeneous populations for clinical studies. Indeed, a large retrospective study of 18 455 cycles found that, while the cumulative live birth rate was similar in younger patients with normal ovarian parameters and a poor prognosis (POSEIDON Groups 1 and 3, respectively), it was significantly lower in women of advanced age with a normal ovarian reserve (POSEIDON Group 2 vs. Group 1) (). Interestingly, according to systematic reviews, pregnancy rate and implantation rate were higher in hypo-responders treated with r-FSH and r-hLH versus those treated with r-hFSH alone (; ). Nevertheless, to evaluate the effect of gonadotropins in women with potential LH and FSH deficiency, studies should include information on endocrinological and clinical outcomes. Relevant endocrine endpoints, including serum testosterone, serum E2 values and the E2/oocyte ratio could elucidate the stimulatory effect exerted by LH during OS on steroidogenesis in both theca and granulosa cells, and may lead to biomarkers for the diagnosis of LH and FSH deficiency before and during OS. Moreover, clinical end points such as FORT and FOI can directly measure the downstream clinical effect of gonadotropins in relation to the baseline antral follicle count. Ultimately, individualised treatment aimed at optimising FORT and FOI will reveal the highest number of oocytes attainable for each cycle and each patient. This in turn could increase the probability of live birth because oocyte number is positively correlated with the number of good quality embryos (). Lastly, given that the probability of live birth is also related to endometrial receptivity, it is important to evaluate if and how different gonadotropin regimens affect endometrial receptivity, how the luteal phase can be effectively supported in women with potential LH and FSH deficiency (), and when a freeze all strategy with subsequent frozen embryo transfer cycles is superior to embryo transfer in a fresh cycle with respect to efficacy and safety.
Conclusions
Reduced gonadotropin production or action may cause clinically significant LH and FSH deficiency associated with reduced gametogenesis and steroidogenesis. This may explain why some women treated with MAR have an ‘unexpected’ hypo-response to standard OS with r-hFSH alone, notwithstanding their normal gonadotropin levels and normal ovarian reserve, and may contribute to a reduced ovarian response in women of AMA. It may also pave the way to precision medicine solutions for fertility patients (), and thus improve reproductive outcomes, particularly in AMA and hypo-responder patients who may benefit from OS with r-hFSH and r-hLH.
Data availability
No new data were generated or analysed in support of this research.
Acknowledgements
The authors thank Daniela Finizio and Jean Ann Gilder, Scientific Communication and Event Planet, Naples, Italy, for writing support.
Authors’ roles
All authors equally contributed to the conception of the work, co-wrote the initial draft, critically reviewed the content and approved the final version of the work.
Funding
Merck KGaA, Darmstadt, Germany funded the preparation of this article, including independent medical writing assistance.
Conflict of interest
D.C., T.D.H., and M.L are employees of Merck KGaA, Darmstadt, Germany; C.A., A.C., and P.C. report personal fees from Merck KGaA, Darmstadt, Germany during the submitted work; E.B. received consulting fees from Abbott, Ferring, Gedeon Richter, Merck KGaA, Darmstadt, Germany, and Roche, speakers bureau and honoraria fees from Ferring, Gedeon Richter, Merck KGaA, Darmstadt, Germany, and Roche and Research Cooperation with Gedeon Richter; A.C.H. reports personal fees from Imperial Consultants during the conduct of the study; P.H. received personal fees from Merck KGaA, Darmstadt, Germany during the conduct of the study; grants and personal fees from IBSA, Gedeon Richter, Merck KGaA, Darmstadt, Germany and MSD outside the submitted work; S.K. received personal fees and grants from Merck KGaA, Darmstadt, Germany during and outside the submitted work; M.S. received grants and personal fees from Ferring, IBSA and Merck KGaA, Darmstadt, Germany during and outside the conduct of the submitted work; N.R.F. has nothing to disclose; V.R. reports personal fees from Merck KGaA, Darmstadt, Germany and Novartis, and grants from Ipsen outside the submitted work.
References
- Achermann JC, Ozisik G, Meeks JJ, Jameson JL. Genetic causes of human reproductive disease. J Clin Endocrinol Metab2002;87:2447–2454.
- Achrekar SK, Modi DN, Desai SK, Mangoli VS, Mangoli RV, Mahale SD. Follicle-stimulating hormone receptor polymorphism (Thr307Ala) is associated with variable ovarian response and ovarian hyperstimulation syndrome in Indian women. Fertil Steril2009;91:432–439.
- Achrekar SK, Modi DN, Meherji PK, Patel ZM, Mahale SD. Follicle stimulating hormone receptor gene variants in women with primary and secondary amenorrhea. J Assist Reprod Genet2010;27:317–326.
- Alviggi C, Andersen CY, Buehler K, Conforti A, De Placido G, Esteves SC, Fischer R, Galliano D, Polyzos NP, Sunkara SK, POSEIDON Group (Patient-Oriented Strategies Encompassing IndividualizeD Oocyte Number) et alA new more detailed stratification of low responders to ovarian stimulation: from a poor ovarian response to a low prognosis concept. Fertil Steril2016a;105:1452–1453.
- Alviggi C, Clarizia R, Pettersson K, Mollo A, Humaidan P, Strina I, Coppola M, Ranieri A, D'Uva M, De Placido G. Suboptimal response to GnRHa long protocol is associated with a common LH polymorphism. Reprod Biomed Online2009a;18:9–14.
- Alviggi C, Conforti A, Cariati F, Alfano S, Strina I, Huhtaniemi I, Santi D, De PG, Humaidan P. Impact of polymorphisms of gonadotropins and their receptors on controlled ovarian stimulation: a prospective observational study. Abstr 32nd Annu Meet ESHRE Hels Finl 3 July – 6 July 20162016b.
- Alviggi C, Conforti A, Esteves SC, Andersen CY, Bosch E, Bühler K, Ferraretti AP, De Placido G, Mollo A, Fischer R. et al Recombinant luteinizing hormone supplementation in assisted reproductive technology: a systematic review. Fertil Steril2018a;109:644–664.
- Alviggi C, Conforti A, Esteves SC, Vallone R, Venturella R, Staiano S, Castaldo E, Andersen CY, De Placido G. Understanding ovarian hypo-response to exogenous gonadotropin in ovarian stimulation and its new proposed Marker-The Follicle-To-Oocyte (FOI) Index. Front Endocrinol (Lausanne)2018b;9:589.
- Alviggi C, Conforti A, Santi D, Esteves SC, Andersen CY, Humaidan P, Chiodini P, De Placido G, Simoni M. Clinical relevance of genetic variants of gonadotrophins and their receptors in controlled ovarian stimulation: a systematic review and meta-analysis. Hum Reprod Update2018c;24:599–614.
- Alviggi C, Humaidan P, Howles CM, Tredway D, Hillier SG. Biological versus chronological ovarian age: implications for assisted reproductive technology. Reprod Biol Endocrinol2009b;7:101.
- Alviggi C, Mollo A, Clarizia R, De Placido G. Exploiting LH in ovarian stimulation. Reprod Biomed Online2006;12:221–233.
- Alviggi C, Pettersson K, Longobardi S, Andersen CY, Conforti A, De Rosa P, Clarizia R, Strina I, Mollo A, De Placido G. et al A common polymorphic allele of the LH beta-subunit gene is associated with higher exogenous FSH consumption during controlled ovarian stimulation for assisted reproductive technology. Reprod Biol Endocrinol2013;11:51.
- Anobile CJ, Talbot JA, McCann SJ, Padmanabhan V, Robertson WR. Glycoform composition of serum gonadotrophins through the normal menstrual cycle and in the post-menopausal state. Mol Hum Reprod1998;4:631–639.
- Ata B, Kaplan B, Danzer H, Glassner M, Opsahl M, Tan SL, Munné S. Array CGH analysis shows that aneuploidy is not related to the number of embryos generated. Reprod Biomed Online2012;24:614–620.
- Balasch J, Vidal E, Peñarrubia J, Casamitjana R, Carmona F, Creus M, Fábregues F, Vanrell JA. Suppression of LH during ovarian stimulation: analysing threshold values and effects on ovarian response and the outcome of assisted reproduction in down-regulated women stimulated with recombinant FSH. Hum Reprod Oxf Engl2001;16:1636–1643.
- Barbotin A-L, Peigné M, Malone SA, Giacobini P. Emerging roles of anti-Müllerian hormone in hypothalamic-pituitary function. Neuroendocrinology2019;109:218–229.
- Barrenetxea G, Agirregoikoa JA, Jiménez MR, de Larruzea AL, Ganzabal T, Carbonero K. Ovarian response and pregnancy outcome in poor-responder women: a randomized controlled trial on the effect of luteinizing hormone supplementation on in vitro fertilization cycles. Fertil Steril2008;89:546–553.
- Benmachiche A, Benbouhedja S, Zoghmar A, Humaidan P. Low LH level on the day of GnRH agonist trigger is associated with reduced ongoing pregnancy and live birth rates and increased early miscarriage rates following IVF/ICSI treatment and fresh embryo transfer. Front Endocrinol2019;10:639.
- Berga S, Naftolin F. Neuroendocrine control of ovulation. Gynecol Endocrinol Off Endocrinol2012;28 Suppl 1:9–13.
- Bianco SDC, Kaiser UB. The genetic and molecular basis of idiopathic hypogonadotropic hypogonadism. Nat Rev Endocrinol2009;5:569–576.
- Boehm U, Bouloux P-M, Dattani MT, N de R, Dodé C, Dunkel L, Dwyer AA, Giacobini P, Hardelin J-P, Juul A. et al Expert consensus document: European Consensus Statement on congenital hypogonadotropic hypogonadism–pathogenesis, diagnosis and treatment. Nat Rev Endocrinol2015;11:547–564.
- Bosch E, Labarta E, Crespo J, Simón C, Remohí J, Pellicer A. Impact of luteinizing hormone administration on gonadotropin-releasing hormone antagonist cycles: an age-adjusted analysis. Fertil Steril2011;95:1031–1036.
- Bousfield GR, May JV, Davis JS, Dias JA, Kumar TR. In vivo and in vitro impact of carbohydrate variation on human follicle-stimulating hormone function. Front Endocrinol (Lausanne)2018;9:216.
- Boyar RM, Katz J, Finkelstein JW, Kapen S, Weiner H, Weitzman ED, Hellman L. Anorexia nervosa. Immaturity of the 24-hour luteinizing hormone secretory pattern. N Engl J Med1974;291:861–865.
- Casarini L, Moriondo V, Marino M, Adversi F, Capodanno F, Grisolia C, La Marca A, La Sala GB, Simoni M. FSHR polymorphism p.N680S mediates different responses to FSH in vitro. Mol Cell Endocrinol2014;393:83–91.
- Casarini L, Pignatti E, Simoni M. Effects of polymorphisms in gonadotropin and gonadotropin receptor genes on reproductive function. Rev Endocr Metab Disord2011;12:303–321.
- Casarini L, Riccetti L, De Pascali F, Nicoli A, Tagliavini S, Trenti T, La Sala GB, Simoni M. Follicle-stimulating hormone potentiates the steroidogenic activity of chorionic gonadotropin and the anti-apoptotic activity of luteinizing hormone in human granulosa-lutein cells in vitro. Mol Cell Endocrinol2016;422:103–114.
- Casarini L, Santi D, Brigante G, Simoni M. Two hormones for one receptor: evolution, biochemistry, actions and pathophysiology of LH and hCG. Endocr Rev2018a;39:549–592.
- Casarini L, Santi D, Simoni M, Potì F. “Spare” luteinizing hormone receptors: facts and fiction. Trends Endocrinol Metab TEM2018b;29:208–217.
- Chang H-M, Klausen C, Leung PCK. Antimüllerian hormone inhibits follicle-stimulating hormone-induced adenylyl cyclase activation, aromatase expression, and estradiol production in human granulosa-lutein cells. Fertil Steril2013;100:585–592.e1.
- Chen C-D, Chiang Y-T, Yang P-K, Chen M-J, Chang C-H, Yang Y-S, Chen S-U. Frequency of low serum LH is associated with increased early pregnancy loss in IVF/ICSI cycles. Reprod Biomed Online2016;33:449–457.
- Choi J, Smitz J. Luteinizing hormone and human chorionic gonadotropin: origins of difference. Mol Cell Endocrinol2014;383:203–213.
- Cimino I, Casoni F, Liu X, Messina A, Parkash J, Jamin SP, Catteau-Jonard S, Collier F, Baroncini M, Dewailly D. et al Novel role for anti-Müllerian hormone in the regulation of GnRH neuron excitability and hormone secretion. Nat Commun2016;7:10055.
- Comim FV, Hardy K, Franks S. Adiponectin and its receptors in the ovary: further evidence for a link between obesity and hyperandrogenism in polycystic ovary syndrome. PLoS One2013;8:e80416.
- Conforti A, Esteves SC, Cimadomo D, Vaiarelli A, Di Rella F, Ubaldi FM, Zullo F, De Placido G, Alviggi C. Management of women with an unexpected low ovarian response to gonadotropin. Front Endocrinol2019a;10:387.
- Conforti A, Esteves SC, Di Rella F, Strina I, De Rosa P, Fiorenza A, Zullo F, De Placido G, Alviggi C. The role of recombinant LH in women with hypo-response to controlled ovarian stimulation: a systematic review and meta-analysis. Reprod Biol Endocrinol2019b;17:18.
- Conforti A, Esteves SC, Picarelli S, Iorio G, Rania E, Zullo F, De Placido G, Alviggi C. Novel approaches for diagnosis and management of low prognosis patients in assisted reproductive technology: the POSEIDON concept. Panminerva Med2019c;61:24–29.
- Conforti A, Vaiarelli A, Cimadomo D, Bagnulo F, Peluso S, Carbone L, Di Rella F, De Placido G, Ubaldi FM, Huhtaniemi I. et al Pharmacogenetics of FSH action in the female. Front Endocrinol (Lausanne)2019d;10:398.
- Conn PM, Crowley WF. Gonadotropin-releasing hormone and its analogs. Annu Rev Med1994;45:391–405.
- Davison SL, Bell R, Donath S, Montalto JG, Davis SR. Androgen levels in adult females: changes with age, menopause, and oophorectomy. J Clin Endocrinol Metab2005;90:3847–3853.
- Desai SS, Roy BS, Mahale SD. Mutations and polymorphisms in FSH receptor: functional implications in human reproduction. Reproduction2013;146:R235–R248.
- Duggavathi R, Murphy BD. Development. Ovulation signals. Science2009;324:890–891.
- Duijkers IJ, Klipping C, Willemsen WN, Krone D, Schneider E, Niebch G, Hermann R. Single and multiple dose pharmacokinetics and pharmacodynamics of the gonadotrophin-releasing hormone antagonist Cetrorelix in healthy female volunteers. Hum Reprod Oxf Reprod1998;13:2392–2398.
- Durlinger AL, Gruijters MJ, Kramer P, Karels B, Kumar TR, Matzuk MM, Rose UM, Jong FH, de Uilenbroek JT, Grootegoed JA. et al Anti-Müllerian hormone attenuates the effects of FSH on follicle development in the mouse ovary. Endocrinology2001;142:4891–4899.
- Feng X, Zhang M, Guan R, Segaloff DL. Heterodimerization between the lutropin and follitropin receptors is associated with an attenuation of hormone-dependent signaling. Endocrinology2013;154:3925–3930.
- Filicori M, Cognigni GE, Pocognoli P, Ciampaglia W, Bernardi S. Current concepts and novel applications of LH activity in ovarian stimulation. Trends Endocrinol Metab TEM2003;14:267–273.
- Garcia-Velasco JA, Coelingh Bennink HJT, Epifanio R, Escudero E, Pellicer A, Simón C. High-dose recombinant LH add-back strategy using high-dose GnRH antagonist is an innovative protocol compared with standard GnRH antagonist. Reprod Biomed Online2007;15:280–287.
- Garrel G, Racine C, L'Hôte D, Denoyelle C, Guigon CJ, di Clemente N, Cohen-Tannoudji J. Anti-Müllerian hormone: a new actor of sexual dimorphism in pituitary gonadotrope activity before puberty. Sci Rep2016;6:23790.
- Genro VK, Grynberg M, Scheffer JB, Roux I, Frydman R, Fanchin R. Serum anti-Müllerian hormone levels are negatively related to Follicular Output RaTe (FORT) in normo-cycling women undergoing controlled ovarian hyperstimulation. Hum Reprod Oxf Engl2011;26:671–677.
- Gordon CM. Clinical practice. Functional hypothalamic amenorrhea. N Engl J Med2010;363:365–371.
- Gordon CM, Ackerman KE, Berga SL, Kaplan JR, Mastorakos G, Misra M, Murad MH, Santoro NF, Warren MP. Functional hypothalamic amenorrhea: An Endocrine Society Clinical Practice Guideline. J Clin Endocrinol Metab2017;102:1413–1439.
- Griesinger G, Shapiro DB. Luteinizing hormone add-back: is it needed in controlled ovarian stimulation, and if so, when? J Reprod Med 2011;56:279–300.
- Grigorova M, Punab M, Ausmees K, Laan M. FSHB promoter polymorphism within evolutionary conserved element is associated with serum FSH level in men. Hum Reprod Oxf Engl2008;23:2160–2166.
- Grossman MP, Nakajima ST, Fallat ME, Siow Y. Müllerian-inhibiting substance inhibits cytochrome P450 aromatase activity in human granulosa lutein cell culture. Fertil Steril2008;89:1364–1370.
- Haavisto AM, Pettersson K, Bergendahl M, Virkamäki A, Huhtaniemi I. Occurrence and biological properties of a common genetic variant of luteinizing hormone. J Clin Endocrinol Metab1995;80:1257–1263.
- Hanyaloglu A, Fanelli F, Jonas K. Class A GPCR: Di/oligomerization of glycoprotein hormone receptors. In Receptors. Totowa, NJ: Humana Press Springer International2017, 207–231. [Internet]. Available from: .
- Herrler A, von RU, Beier HM. Embryo-maternal signalling: how the embryo starts talking to its mother to accomplish implantation. Reprod Biomed Online2003;6:244–256.
- Hill MJ, Levens ED, Levy G, Ryan ME, Csokmay JM, DeCherney AH, Whitcomb BW. The use of recombinant luteinizing hormone in patients undergoing assisted reproductive techniques with advanced reproductive age: a systematic review and meta-analysis. Fertil Steril2012;97:1108–1114.e1.
- Huang Y, Zhao Y, Yu Y, Li R, Lin S, Zhang C, Liu P, Qiao J. Altered amphiregulin expression induced by diverse luteinizing hormone receptor reactivity in granulosa cells affects IVF outcomes. Reprod Biomed Online2015;30:593–601.
- Huhtaniemi IT, Themmen APN. Mutations in human gonadotropin and gonadotropin-receptor genes. Endocrine2005;26:207–217.
- Huirne JAF, Loenen ACD, van Schats R, McDonnell J, Hompes PGA, Schoemaker J, Homburg R, Lambalk CB. Dose-finding study of daily GnRH antagonist for the prevention of premature LH surges in IVF/ICSI patients: optimal changes in LH and progesterone for clinical pregnancy. Hum Reprod Oxf Reprod2005;20:359–367.
- Humaidan P, Alviggi C, Fischer R, Esteves SC. The novel POSEIDON stratification of ‘Low prognosis patients in Assisted Reproductive Technology’ and its proposed marker of successful outcome. F1000Res2016;5:2911.
- Humaidan P, Bungum L, Bungum M, Andersen CY. Ovarian response and pregnancy outcome related to mid-follicular LH levels in women undergoing assisted reproduction with GnRH agonist down-regulation and recombinant FSH stimulation. Hum Reprod Oxf Engl2002;17:2016–2021.
- Humaidan P, Bungum M, Bungum L, Yding Andersen C. Effects of recombinant LH supplementation in women undergoing assisted reproduction with GnRH agonist down-regulation and stimulation with recombinant FSH: an opening study. Reprod Biomed Online2004;8:635–643.
- Jean-Alphonse F, Bowersox S, Chen S, Beard G, Puthenveedu MA, Hanyaloglu AC. Spatially restricted G protein-coupled receptor activity via divergent endocytic compartments. J Biol Chem2014;289:3960–3977.
- Jonas KC, Chen S, Virta M, Mora J, Franks S, Huhtaniemi I, Hanyaloglu AC. Temporal reprogramming of calcium signalling via crosstalk of gonadotrophin receptors that associate as functionally asymmetric heteromers. Sci Rep2018;8:2239.
- Kaiser UB, Jakubowiak A, Steinberger A, Chin WW. Differential effects of gonadotropin-releasing hormone (GnRH) pulse frequency on gonadotropin subunit and GnRH receptor messenger ribonucleic acid levels in vitro. Endocrinology1997;138:1224–1231.
- Kallmann FJ, Schoenfeld WA, Barrera SE. The genetic aspects of primary eunuchoidsm. Am J Ment Def1944;48:203–236.
- Kol S. Individualized treatment from theory to practice: the private case of adding LH during GnRH antagonist-based stimulation protocol. Clin Med Insights Reprod Health2014;8:59–64.
- Kol S, Homburg R. Change, change, change: hormonal actions depend on changes in blood levels. Hum Reprod Oxf Reprod2008;23:1004–1006.
- König TE, van der Houwen LEE, Overbeek A, Hendriks ML, Beutler-Beemsterboer SN, Kuchenbecker WKH, Renckens CNM, Bernardus RE, Schats R, Homburg R. et al Recombinant LH supplementation to a standard GnRH antagonist protocol in women of 35 years or older undergoing IVF/ICSI: a randomized controlled multicentre study. Hum Reprod Oxf Reprod2013;28:2804–2812.
- La Marca A, Sighinolfi G, Argento C, Grisendi V, Casarini L, Volpe A, Simoni M. Polymorphisms in gonadotropin and gonadotropin receptor genes as markers of ovarian reserve and response in in vitro fertilization. Fertil Steril2013;99:970–978.e1.
- Lahoud R, Jefout MA, Tyler J, Ryan J, Driscoll G. A relative reduction in mid-follicular LH concentrations during GnRH agonist IVF/ICSI cycles leads to lower live birth rates. Hum Reprod Oxf Engl2006;21:2645–2649.
- Laisk-Podar T, Kaart T, Peters M, Salumets A. Genetic variants associated with female reproductive ageing–potential markers for assessing ovarian function and ovarian stimulation outcome. Reprod Biomed Online2015;31:199–209.
- Lambalk CB, Banga FR, Huirne JA, Toftager M, Pinborg A, Homburg R, Veen F, van der Wely M. GnRH antagonist versus long agonist protocols in IVF: a systematic review and meta-analysis accounting for patient type. Hum Reprod Update2017;23:560–579.
- Landomiel F, De PF, Raynaud P, Jean-Alphonse F, Yvinec R, Pellissier LP, Bozon V, Bruneau G, Crépieux P, Poupon A. Biased signaling and allosteric modulation at the FSHR. Front Endocrinol2019;10:148.
- Latronico AC, Chai Y, Arnhold IJ, Liu X, Mendonca BB, Segaloff DL. A homozygous microdeletion in helix 7 of the luteinizing hormone receptor associated with familial testicular and ovarian resistance is due to both decreased cell surface expression and impaired effector activation by the cell surface receptor. Mol Endocrinol Baltim Endocrinol1998;12:442–450.
- Layman LC, Lee EJ, Peak DB, Namnoum AB, Vu KV, Lingen BL, van Gray MR, McDonough PG, Reindollar RH, Jameson JL. Delayed puberty and hypogonadism caused by mutations in the follicle-stimulating hormone beta-subunit gene. N Engl J Med1997;337:607–611.
- Li RHW, Ng EHY. Management of anovulatory infertility. Best Pract Res Clin Obstet Gynaecol2012;26:757–768.
- Lindgren I, Bååth M, Uvebrant K, Dejmek A, Kjaer L, Henic E, Bungum M, Bungum L, Cilio C, Leijonhufvud I. et al Combined assessment of polymorphisms in the LHCGR and FSHR genes predict chance of pregnancy after in vitro fertilization. Hum Reprod Oxf Reprod2016;31:672–683.
- Lyga S, Volpe S, Werthmann RC, Götz K, Sungkaworn T, Lohse MJ, Calebiro D. Persistent cAMP signaling by internalized LH receptors in ovarian follicles. Endocrinology2016;157:1613–1621.
- Marshall JC, Dalkin AC, Haisenleder DJ, Paul SJ, Ortolano GA, Kelch RP. Gonadotropin-releasing hormone pulses: regulators of gonadotropin synthesis and ovulatory cycles. Recent Prog Horm Res1991;47:155–187–189. discussion 188–189.
- Marut EL, Williams RF, Cowan BD, Lynch A, Lerner SP, Hodgen GD. Pulsatile pituitary gonadotropin secretion during maturation of the dominant follicle in monkeys: estrogen positive feedback enhances the biological activity of LH. Endocrinology1981;109:2270–2272.
- Matorras R, Prieto B, Exposito A, Mendoza R, Crisol L, Herranz P, Burgués S. Mid-follicular LH supplementation in women aged 35-39 years undergoing ICSI cycles: a randomized controlled study. Reprod Biomed Online2009;19:879–887.
- Matthews CH, Borgato S, Beck-Peccoz P, Adams M, Tone Y, Gambino G, Casagrande S, Tedeschini G, Benedetti A, Chatterjee VK. Primary amenorrhoea and infertility due to a mutation in the beta-subunit of follicle-stimulating hormone. Nat Genet1993;5:83–86.
- Moenter SM, Brand RC, Karsch FJ. Dynamics of gonadotropin-releasing hormone (GnRH) secretion during the GnRH surge: insights into the mechanism of GnRH surge induction. Endocrinology1992;130:2978–2984.
- Mol BW, Bossuyt PM, Sunkara SK, Garcia Velasco JA, Venetis C, Sakkas D, Lundin K, Simon C, Taylor HS, Wan R. et al Personalized ovarian stimulation for assisted reproductive technology: study design considerations to move from hype to added value for patients. Fertil Steril2018;109:968–979.
- Mushayandebvu T, Castracane VD, Gimpel T, Adel T, Santoro N. Evidence for diminished midcycle ovarian androgen production in older reproductive aged women. Fertil Steril1996;65:721–723.
- Overbeek A, Kuijper EAM, Hendriks ML, Blankenstein MA, Ketel IJG, Twisk JWR, Hompes PGA, Homburg R, Lambalk CB. Clomiphene citrate resistance in relation to follicle-stimulating hormone receptor Ser680Ser-polymorphism in polycystic ovary syndrome. Hum Reprod Oxf Reprod2009;24:2007–2013.
- Pau KY, Berria M, Hess DL, Spies HG. Preovulatory gonadotropin-releasing hormone surge in ovarian-intact rhesus macaques. Endocrinology1993;133:1650–1656.
- Perez Mayorga M, Gromoll J, Behre HM, Gassner C, Nieschlag E, Simoni M. Ovarian response to follicle-stimulating hormone (FSH) stimulation depends on the FSH receptor genotype. J Clin Endocrinol Metab2000;85:3365–3369.
- Pezzuto A, Ferrari B, Coppola F, Nardelli GB. LH supplementation in down-regulated women undergoing assisted reproduction with baseline low serum LH levels. Gynecol Endocrinol Off Endocrinol2010;26:118–124.
- Ramaraju GA, Cheemakurthi R, Prathigudupu K, Balabomma KL, Kalagara M, Thota S, Kota M. Role of Lh polymorphisms and r-hLh supplementation in GnRh agonist treated ART cycles: a cross sectional study. Eur J Obstet Gynecol Reprod Biol2018;222:119–125.
- Revel A. Defective endometrial receptivity. Fertil Steril2012;97:1028–1032.
- Riccetti L, De Pascali F, Gilioli L, Santi D, Brigante G, Simoni M, Casarini L. Genetics of gonadotropins and their receptors as markers of ovarian reserve and response in controlled ovarian stimulation. Best Pract Res Clin Obstet Gynaecol2017;44:15–25.
- Rull K, Grigorova M, Ehrenberg A, Vaas P, Sekavin A, Nõmmemees D, Adler M, Hanson E, Juhanson P, Laan M. FSHB -211 G>T is a major genetic modulator of reproductive physiology and health in childbearing age women. Hum Reprod Oxf Reprod2018;33:954–966.
- Ruth KS, Beaumont RN, Tyrrell J, Jones SE, Tuke MA, Yaghootkar H, Wood AR, Freathy RM, Weedon MN, Frayling TM. et al Genetic evidence that lower circulating FSH levels lengthen menstrual cycle, increase age at menopause and impact female reproductive health. Hum Reprod2016;31:473–481.
- Ruvolo G, Bosco L, Pane A, Morici G, Cittadini E, Roccheri MC. Lower apoptosis rate in human cumulus cells after administration of recombinant luteinizing hormone to women undergoing ovarian stimulation for in vitro fertilization procedures. Fertil Steril2007;87:542–546.
- Santi D, Casarini L, Alviggi C, Simoni M. Efficacy of follicle-stimulating hormone (FSH) alone, FSH + luteinizing hormone, human menopausal gonadotropin or FSH + human chorionic gonadotropin on assisted reproductive technology outcomes in the “Personalized” medicine era: a meta-analysis. Front Endocrinol (Lausanne)2017;8:114.
- Santoro N, Filicori M, Crowley WF. Hypogonadotropic disorders in men and women: diagnosis and therapy with pulsatile gonadotropin-releasing hormone. Endocr Rev1986;7:11–23.
- Schüring AN, Busch AS, Bogdanova N, Gromoll J, Tüttelmann F. Effects of the FSH-β-subunit promoter polymorphism -211G->T on the hypothalamic-pituitary-ovarian axis in normally cycling women indicate a gender-specific regulation of gonadotropin secretion. J Clin Endocrinol Metab2013;98:E82–E86.
- Shangold MM. Causes, evaluation, and management of athletic oligo-/amenorrhea. Med Clin North Am1985;69:83–95.
- Shi W, Zhou H, Tian L, Zhao Z, Zhang W, Shi J. Cumulative live birth rates of good and low prognosis patients according to POSEIDON criteria: a single center analysis of 18,455 treatment cycles. Front Endocrinol2019;10:409.
- Silva MSB, Giacobini P. New insights into anti-Müllerian hormone role in the hypothalamic-pituitary-gonadal axis and neuroendocrine development. Cell Mol Life Sci CMLS2020;78(1):1–16.
- Simoni M, Nieschlag E, Gromoll J. Isoforms and single nucleotide polymorphisms of the FSH receptor gene: implications for human reproduction. Hum Reprod Update2002;8:413–421.
- Sposini S, De Pascali F, Richardson R, Sayers NS, Perrais D, Yu H, Palmer S, Nataraja S, Reiter E, Hanyaloglu AC. Pharmacological programming of endosomal signaling activated by small molecule ligands of the follicle stimulating hormone receptor. Front Pharmacol2020;11:593492. [Internet] Frontiers.
- Sposini S, Jean-Alphonse FG, Ayoub MA, Oqua A, West C, Lavery S, Brosens JJ, Reiter E, Hanyaloglu AC. Integration of GPCR signaling and sorting from very early endosomes via opposing APPL1 mechanisms. Cell Rep2017;21:2855–2867.
- Strotmeyer ES, Steenkiste AR, Foley TP, Berga SL, Dorman JS. Menstrual cycle differences between women with type 1 diabetes and women without diabetes. Diabetes Care2003;26:1016–1021.
- Tesarik J, Hazout A, Mendoza C. Luteinizing hormone affects uterine receptivity independently of ovarian function. Reprod Biomed Online2003;7:59–64.
- Trevisan CM, Peluso C, Cordts EB, R de O, Christofolini DM, Barbosa CP, Bianco B. Ala307Thr and Asn680Ser polymorphisms of FSHR gene in human reproduction outcomes. Cell Physiol Biochem Int Biochem2014;34:1527–1535.
- Ulloa-Aguirre A, Espinoza R, Damian-Matsumura P, Chappel SC. Immunological and biological potencies of the different molecular species of gonadotrophins. Hum Reprod Oxf Reprod1988;3:491–501.
- Ulloa-Aguirre A, Timossi C, Méndez JP. Is there any physiological role for gonadotrophin oligosaccharide heterogeneity in humans? I. Gondatrophins are synthesized and released in multiple molecular forms. A matter of fact. Hum Reprod Oxf Reprod2001;16:599–604.
- Vermey BG, Chua SJ, Zafarmand MH, Wang R, Longobardi S, Cottell E, Beckers F, Mol BW, Venetis CA, D'Hooghe T. Is there an association between oocyte number and embryo quality? A systematic review and meta-analysis. Reprod Biomed Online2019;39:751–763.
- Vihko KK. Gonadotropins and ovarian gonadotropin receptors during the perimenopausal transition period. Maturitas1996;23 Suppl:S19–S22.
- Vuong TNL, Phung HT, Ho MT. Recombinant follicle-stimulating hormone and recombinant luteinizing hormone versus recombinant follicle-stimulating hormone alone during GnRH antagonist ovarian stimulation in patients aged ≥35 years: a randomized controlled trial. Hum Reprod Oxf Reprod2015;30:1188–1195.
- Wang P-Y, Protheroe A, Clarkson AN, Imhoff F, Koishi K, McLennan IS. Müllerian inhibiting substance contributes to sex-linked biases in the brain and behavior. Proc Natl Acad Sci U S A2009;106:7203–7208.
- Weiss J, Axelrod L, Whitcomb RW, Harris PE, Crowley WF, Jameson JL. Hypogonadism caused by a single amino acid substitution in the beta subunit of luteinizing hormone. N Engl J Med1992;326:179–183.
- Westergaard LG, Laursen SB, Andersen CY. Increased risk of early pregnancy loss by profound suppression of luteinizing hormone during ovarian stimulation in normogonadotrophic women undergoing assisted reproduction. Hum Reprod Oxf Engl2000;15:1003–1008.
- Wide L, Eriksson K. Dynamic changes in glycosylation and glycan composition of serum FSH and LH during natural ovarian stimulation. Ups J Med Sci2013;118:153–164.
- Wide L, Eriksson K. Low-glycosylated forms of both FSH and LH play major roles in the natural ovarian stimulation. Ups J Med Sci2018;123:100–108.
- Wide L, Eriksson K, Sluss PM, Hall JE. Serum half-life of pituitary gonadotropins is decreased by sulfonation and increased by sialylation in women. J Clin Endocrinol Metab2009;94:958–964.
- Wide L, Naessén T, SundströM-Poromaa I, Eriksson K, Sulfonation and sialylation of gonadotropins in women during the menstrual cycle, after menopause, and with polycystic ovarian syndrome and in men. J Clin Endocrinol Metab2007;92:4410–4417.
- Yasmin E, Davies M, Conway G, Balen AH. British Fertility Society null. British Fertility Society. “Ovulation induction in WHO Type 1 anovulation: guidelines for practice”. Produced on behalf of the BFS Policy and Practice Committee. Hum Fertil Camb Engl2013;16:228–234.
- Younis JS, Izhaki I, Ben-Ami M. The effect of rLH supplementation to the GnRH-antagonist protocol on endocrine dynamics in the advanced reproductive age. J Endocrinol Invest2017;40:831–839.
- Zariñán T, Butnev VY, Gutiérrez-Sagal R, Maravillas-Montero JL, Martínez-Luis I, Mejía-Domínguez NR, Juárez-Vega G, Bousfield GR, Ulloa-Aguirre A. In vitro impact of FSH glycosylation variants on FSH receptor-stimulated signal transduction and functional selectivity. J Endocr Soc [Internet]2020;4:bvaa019.
- Zegers-Hochschild F, Adamson GD, Dyer S, Racowsky C, de Mouzon J, Sokol R, Rienzi L, Sunde A, Schmidt L, Cooke ID. et al The international glossary on infertility and fertility care, 2017. Hum Reprod Oxf Reprod2017;32:1786–1801.
- Zumoff B, Strain GW, Miller LK, Rosner W. Twenty-four-hour mean plasma testosterone concentration declines with age in normal premenopausal women. J Clin Endocrinol Metab1995;80:1429–1430.
