I EXECUTIVE SUMMARY
I.A Introduction
The International Consensus Statement on Allergy and Rhinology: Allergic Rhinitis 2023 (ICAR‐Allergic Rhinitis 2023) was developed as an update to the original ICAR‐Allergic Rhinitis 2018 document. The goal of this document is to summarize and critically review the best evidence related to allergic rhinitis (AR). Through a systematic approach including literature review, semi‐blinded stepwise iterative review process, and consensus and oversight by associate editors, all steps of document development have been rigorous and of high quality.
ICAR‐Allergic Rhinitis 2023 is not intended to be a clinical practice guideline, meta‐analysis, or expert panel report. The ICAR authors have carefully reviewed all relevant literature and determined the strength of the available evidence. Based upon this evidence, where applicable, recommendations are made for various diagnostic and treatment options in the realm of AR. A secondary goal of this document is to identify updates in the field as compared to the previous ICAR‐Allergic Rhinitis 2018 document and highlight advances in our understanding of AR, as well as its diagnosis and treatment. Through this in‐depth investigation, we are also able to identify areas in which further work is needed.
Since the publication of ICAR‐Allergic Rhinitis 2018, there are numerous new high‐level publications in various aspects of AR. There have been updates in levels of evidence and recommendations. These findings, along with a comparison to the ICAR‐Allergic Rhinitis 2018 available publications, and levels of evidence, are shown in the tables in this executive summary. Still, several important areas of future investigation remain.
I.B Methods
In the ICAR‐Allergic Rhinitis 2023 update, there were a total of 144 individual topics assigned to 87 primary authors. A multidisciplinary group of expert authors from around the world, often with a notable publication record in the field, were invited to contribute to both authorship and iterative peer review aspects of the ICAR process. Topics were assigned as literature reviews, evidence‐based reviews without recommendations, or evidence‐based reviews with recommendations, depending on the available literature, strength of evidence, and type of intervention. Topics that had sufficient evidence to substantiate clinical recommendations were assigned as evidence‐based reviews with recommendations, based on the work of Rudmik and Smith.
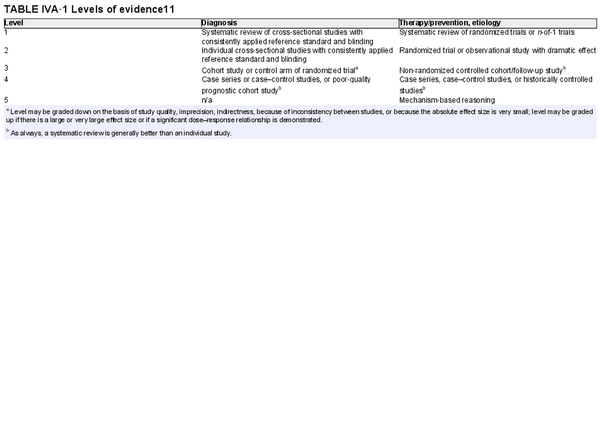
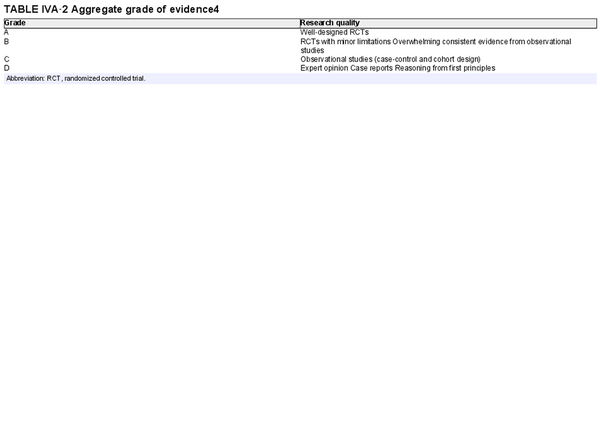
For each section, authors were instructed to perform systematic reviews, which included the Ovid MEDLINE, EMBASE, and Cochrane Review databases, and generally followed PRISMA guidelines (Preferred Reporting for Systematic Reviews and Meta‐Analyses). Included studies were presented in table format, indicating the level of evidence. Systematic reviews, meta‐analyses, and randomized controlled trials were noted as providing the highest levels of evidence. An aggregate grade of evidence was determined for each topic, and an evidence‐based recommendation was made considering benefit, harm, and cost for each topic, where appropriate.
Each section then underwent a stepwise review in a semi‐blinded fashion by two additional experts. Consensus was reached after each stage in the iterative review process. The review process was overseen by an associate editor to ensure adherence to the ICAR methodology and assist in resolution of any concerns. Following completion of all topics, the individual sections were collated into major content areas (e.g., Evaluation and Diagnosis, Management, Associated Conditions) and each major content area was reviewed by three to five associate editors. The final ICAR‐Allergic Rhinitis 2023 document was then compiled and reviewed by all authors for consensus.
The ICAR process aims to be systematic, consistent, and thorough; however, certain limitations exist. The literature search for each topic was performed by the individual invited author for that topic. This has the potential to introduce some variability in search results despite detailed literature search instructions. Also, for some topics, there is extensive high‐quality literature available. This may allow an aggregate grade of evidence to be delineated without listing every published study on that topic. In these cases, an exhaustive list of lower‐level studies may not be provided in the evidence tables.
I.C Results
I.C.1 Definitions, classification, and differential diagnosis
AR is primarily driven by an immunoglobulin E (IgE)‐mediated type 1 hypersensitivity response, due to an allergen exposure. Classically, seasonal AR was thought to be associated with outdoor allergens and perennial AR with indoor year‐round exposure to allergens. However, climate change and polysensitization may make these classifications challenging. Intermittent AR is defined as symptoms for less than 4 days per week or less than four consecutive weeks. Persistent AR is defined as symptoms for more than 4 days per week for at least 1 month. Sensitization to allergens may be identified on skin or in vitro testing which assesses the presence of allergen‐specific IgE (sIgE). However, many people that are sensitized do not exhibit allergy symptoms, so correlation with clinical symptoms upon allergen exposure is critical. Classic AR symptoms include sneezing, rhinorrhea, and nasal congestion/obstruction. These symptoms are non‐specific, and the differential diagnosis of AR is broad. Section V of the ICAR‐Allergic Rhinitis 2023 document explores AR definition, classification, and differential diagnosis (Table I.C.1).
I.C.2 Pathophysiology and mechanisms
Shortly after IgE receptor stimulation, mast cells secrete proteins due to stimulated gene transcription. Multiple cytokines and chemokines are released, which recruit inflammatory cells such as eosinophils, basophils, neutrophils, macrophages, and T cells.
Various inflammatory processes occur at different stages of AR. These processes are driven by the type 2 immune response. Considering the pathophysiology of AR, the ICAR‐Allergic Rhinitis 2023 document explores local and systemic IgE‐mediated inflammation, cellular infiltrates, cytokines and soluble mediators, neural mechanisms, histologic and epithelial changes, epithelial barrier alterations, association with vitamin D, alterations in nitric oxide and the microbiome, as well as the unified airway concept. Section VI of the ICAR‐Allergic Rhinitis 2023 document discusses AR pathophysiology and mechanisms.
I.C.3 Epidemiology
The prevalence of AR has been reported from 5% to 50% worldwide. Prevalence reporting is dependent on the method of diagnosis and age of participants studied, which may explain some of the variability in reported AR prevalence. There have been increased attempts to provide more uniformity in the terminology and diagnostic criteria for AR. The available literature suggests that AR had been previously increasing across the globe. While recent evidence indicates this upward trend may have leveled off, notable geographic differences exist. The rate of AR typically increases with age until young adulthood. The effects of geographic influences on epidemiology of AR and the role of climate change are active areas of research. Section VII of the ICAR‐Allergic Rhinitis 2023 document reviews the epidemiology of AR.
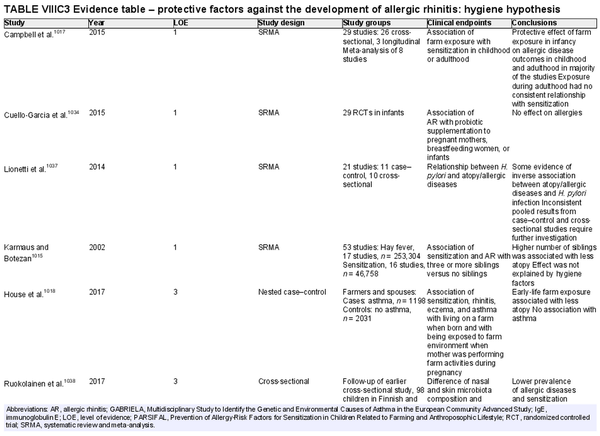
I.C.4 Risk factors and protective factors for the development of allergic rhinitis
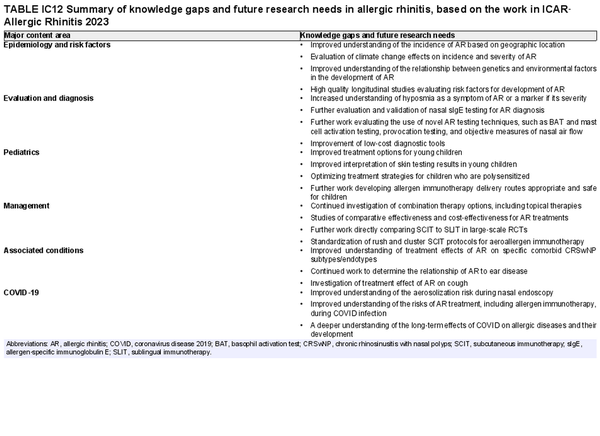
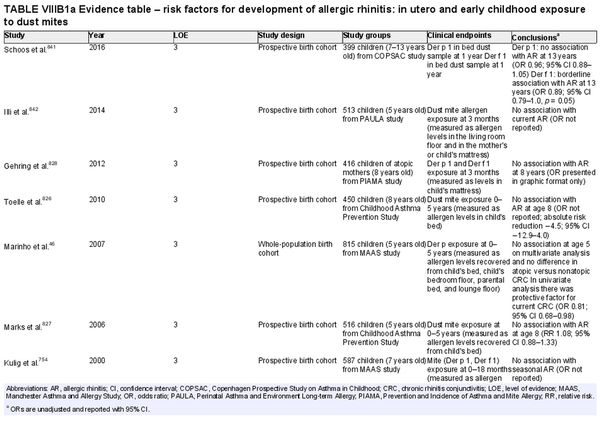
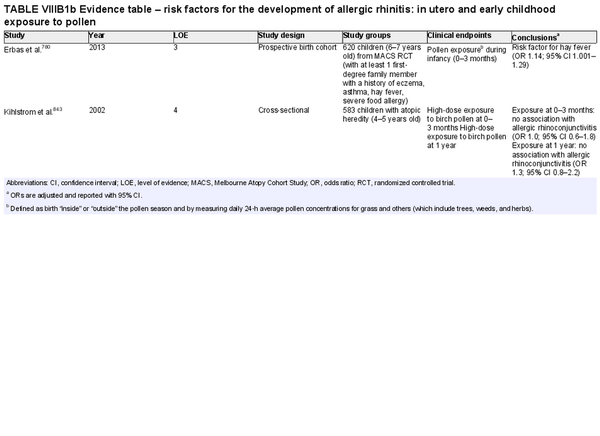
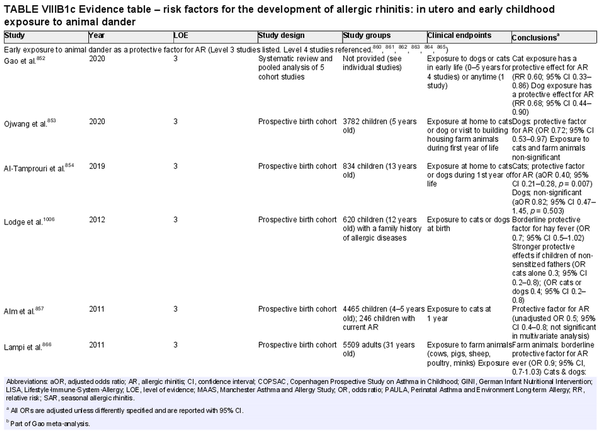
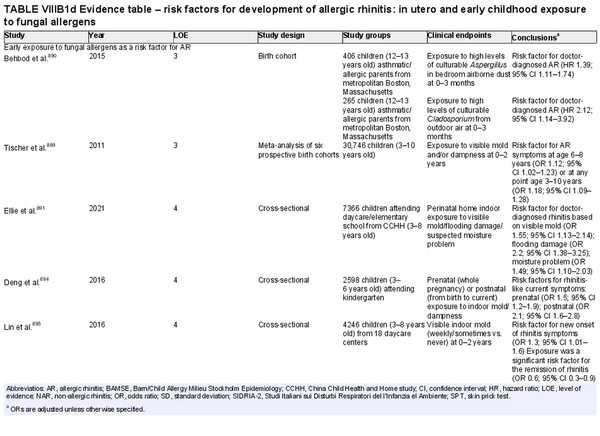
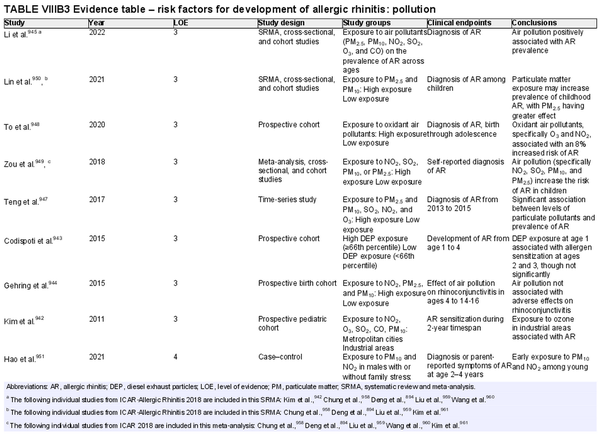
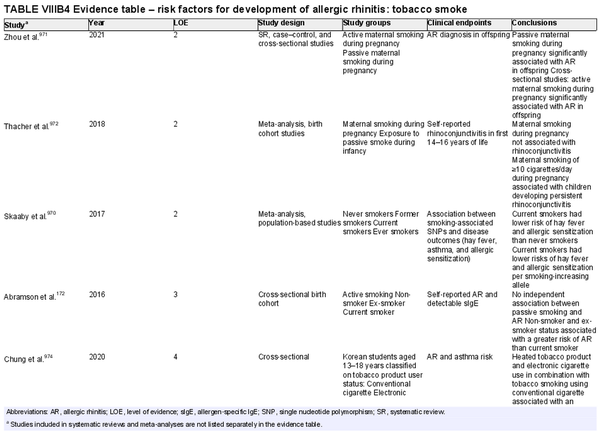
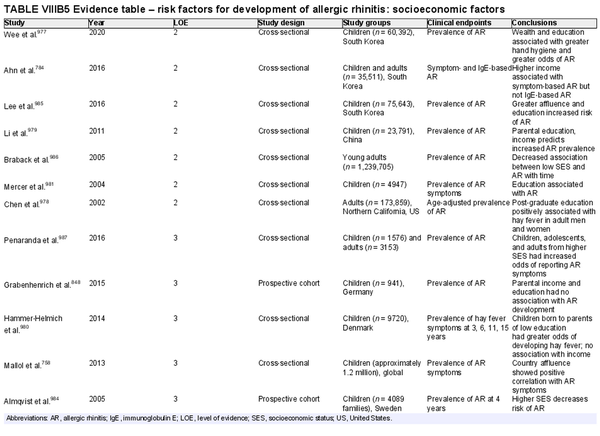
Several risk factors for the development of AR have been investigated. There is conflicting data for many of these potential risk factors, and this area of work remains a topic of active investigation. Section VIII of the ICAR‐Allergic Rhinitis 2023 document explores risk factors and potential protective factors for the development of AR (Tables I.C.4.‐1 and I.C.4.‐2).
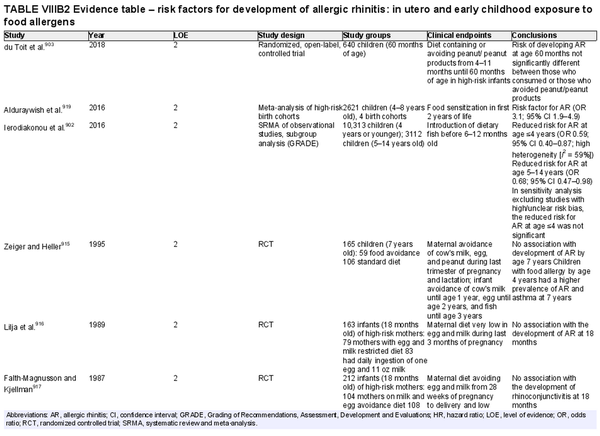
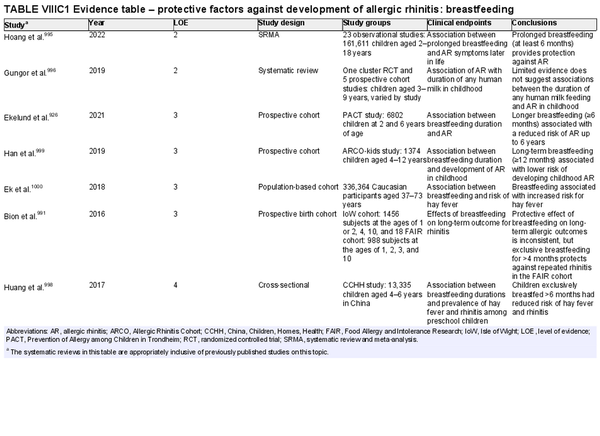
Breastfeeding
Aggregate grade of evidence: C (Level 2: 2 studies, level 3: 4 studies, level 4: 1 study)
Benefit: Benefits on general health of infant and possible protection against AR, especially in young children.
Harm: None.
Cost: Low.
Benefits‐harm assessment: Slight preponderance of benefit over harm for protection against AR. Large preponderance of benefit over harm for breastfeeding for all infants, unless there is a contraindication. The benefit of breastfeeding for all infants inextricably influences this recommendation.
Value judgments: Evidence suggests that breastfeeding may reduce the risk of AR without harm.
Policy level: Recommendation for breastfeeding due to various positive effects on general health and possible protective effects on AR.
Intervention: Breastfeeding for at least 4–6 months should be encouraged unless contraindicated.
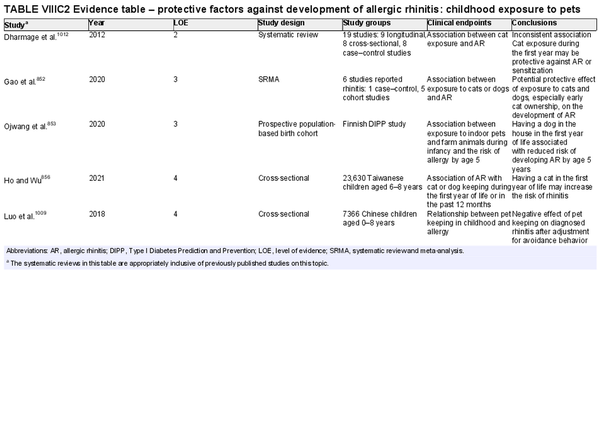
Childhood exposure to pets
Aggregate grade of evidence: C (Level 2: 1 study, level 3: 2 studies, level 4: 2 studies)
Benefit: Exposure to pets at birth and in the first year of life has potential benefits of decreasing risk of AR.
Harm: Pet keeping in childhood could have a negative effect, especially in Asians.
Cost: Various.
Benefits‐harm assessment: Difficulty distinguishing between benefits and harm.
Value judgments: There is conflicting evidence that childhood pet exposure prevents the development of AR.
Policy level: Option.
Intervention: Recommendation to expose or avoid pets for the prevention of AR in children cannot be provided based on current evidence.
I.C.5 Disease burden
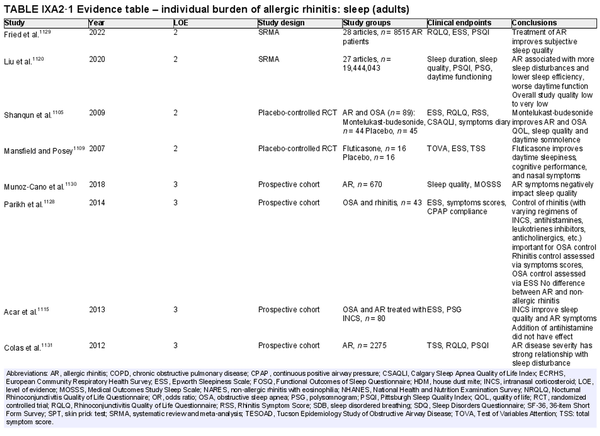
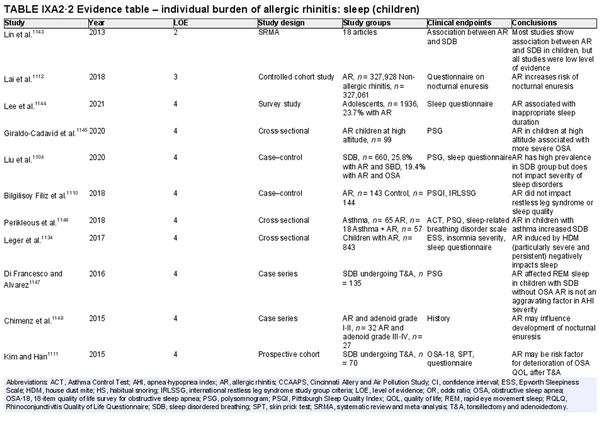
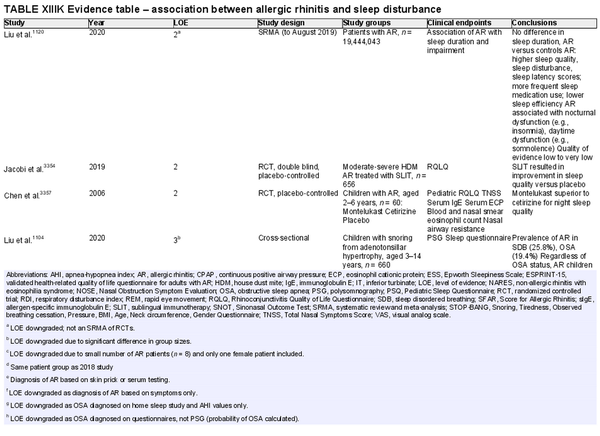
ICAR‐Allergic Rhinitis 2023 reviewed the disease burden of AR as it relates to quality of life (QOL) and sleep disturbance. Several new studies have been added in each of these categories since ICAR‐Allergic Rhinitis 2018. AR also has substantial impact at a societal level, which may be quantified in direct and indirect costs, absenteeism or presenteeism, and other measures. Individual and societal burdens of AR are significant and addressed further in the full ICAR‐Allergic Rhinitis 2023 document (Table I.C.5).
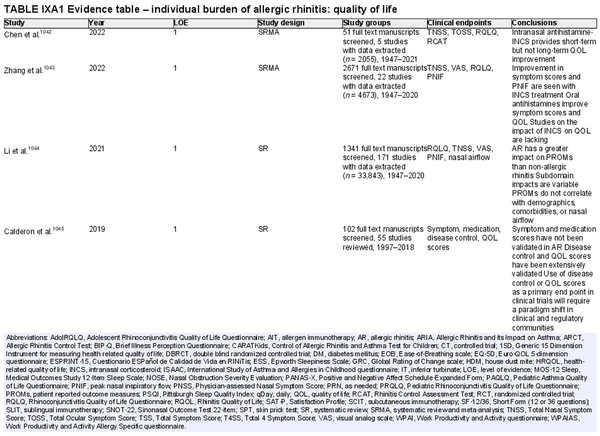
Disease burden – quality of life
Aggregate grade of evidence: B (Level 1: 6 studies, level 2: 35 studies, level 3: 15 studies)
Benefit: Successful treatment of AR leads to improved overall and disease specific QOL.
Harm: Depending on the specific treatments for AR, there are variable levels of harm.
Cost: Treatments for AR have variable costs.
Benefits‐harm assessment: The benefits of treating patients with AR to improve QOL likely outweigh risks of treatment.
Value judgments: Validated measures of QOL should be utilized in future studies of treatments for AR.
Policy level: Recommendation.
Intervention: Validated measures of QOL should be utilized in future studies of treatments for AR.
Disease burden – sleep disturbance
Aggregate grade of evidence: B (Level 2: 5 studies, level 3: 8 studies, level 4: 50 studies)
Benefit: AR negatively impacts sleep quality. Successful management of AR leads to decreased sleep disturbance in adults and children.
Harm: Medical management of AR is generally low risk and medications have low side‐effect profiles. allergen immunotherapy (AIT) is associated with rare serious adverse events.
Cost: Associated costs consist of the direct costs of allergy testing and medical management, and indirect cost of increased time and effort for AIT.
Benefits‐harm assessment: The benefits of treating patients with AR may outweigh any associated risks.
Value judgments: In patients with AR, the successful control of symptoms with medical management or AIT can lead to important improvements in sleep disturbance. The level of available evidence is stronger for the adult population compared with the pediatric population.
Policy level: Treatment of AR to improve sleep disturbance – Recommended in adults. Option in children.
Intervention: Intranasal corticosteroids (INCS), oral antihistamines, montelukast, and AIT are appropriate options, when medically indicated, to improve sleep disturbance in patients with AR.
I.C.6 Evaluation and diagnosis
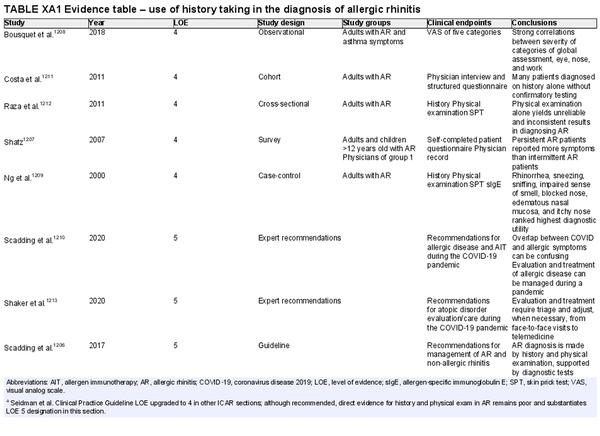
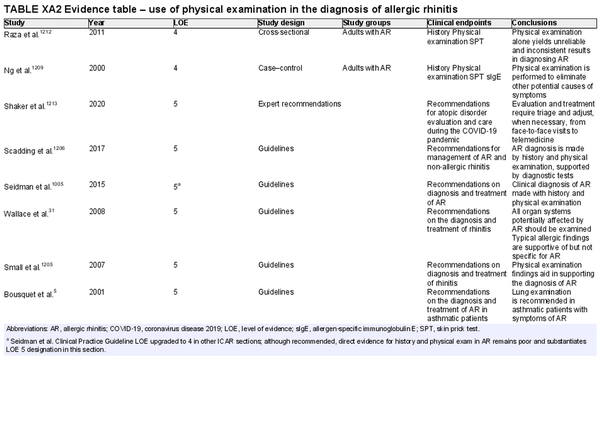
A thorough history is critical to AR diagnosis. This should be complemented by an appropriate physical examination, and nasal endoscopy may also be considered. Various diagnostic testing modalities may also be employed to solidify a diagnosis of AR or when considering an alternate etiology for the patient's symptoms. A summary of various diagnostic modalities for AR is presented in Table I.C.6.
The section that follows includes the recommendation summaries for AR diagnostic modalities considered in the ICAR‐Allergic Rhinitis 2023 document.
Patient history
Aggregate grade of evidence: D (Level 4: 5 studies, level 5: 7 guidelines or expert recommendations)
Benefit: Improves accuracy of diagnosis, avoid unnecessary referrals, testing, or treatment.
Harm: Potential misdiagnosis or inappropriate treatment.
Cost: Minimal.
Benefits‐harm assessment: Preponderance of benefit over harm.
Value judgments: Using history to make a presumptive diagnosis of AR is reasonable and would not delay treatment initiation. History should be combined with physical examination, which may not be possible in some scenarios such as telemedicine. Confirmation with diagnostic testing is required for progression to AIT or targeted avoidance therapy, or desirable with inadequate response to treatment.
Policy level: Recommendation.
Intervention: Despite low level evidence specifically addressing this area, history is essential in the diagnosis of AR.
Physical examination
Aggregate grade of evidence: D (Level 4: 2 studies, level 5: 6 guidelines)
Benefit: Possible improved diagnosis of AR with physical examination findings, along with evaluation and/or exclusion of alternative diagnoses.
Harm: Possible patient discomfort from routine examination, not inclusive of endoscopy.
Cost: Minimal.
Benefits‐harm assessment: Preponderance of benefit over harm, potential misdiagnosis, and inappropriate treatment if used in isolation.
Value judgments: Telemedicine is a safe and useful tool in pandemic conditions but does limit what can be gleaned from physical examination. Without the use of nasal endoscopy, it is possible some physical examination findings may be missed.
Policy level: Recommendation.
Intervention: When possible, physical examination should be performed with appropriate personal protective equipment to aid in the diagnosis of AR and exclusion of other conditions. When combined with patient history, it increases diagnostic accuracy and may exclude alternative causes of symptoms.
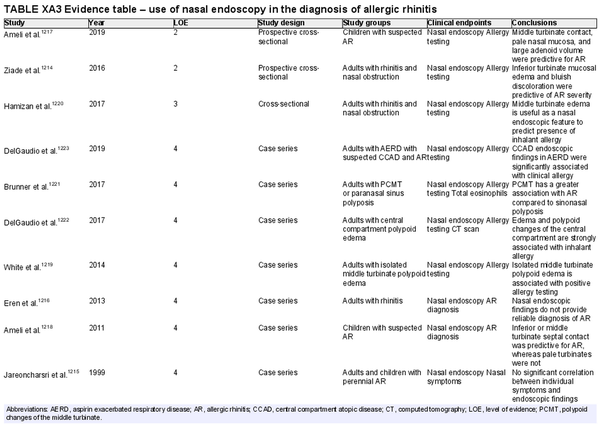
Nasal endoscopy
Aggregate grade of evidence: C (Level 2: 2 studies, level 3: 1 study, level 4: 7 studies)
Benefit: Possible improved diagnosis with visualization of middle or inferior turbinate edema, pale/bluish discoloration, or isolated central compartment polypoid changes and/or edema, which have been associated with AR.
Harm: Possible patient discomfort.
Cost: Moderate equipment and processing costs, as well as procedural charges.
Benefits‐harm assessment: Balance of benefit and harm.
Value judgments: Nasal endoscopy may increase diagnostic sensitivity among children and adults with AR.
Policy level: Option.
Intervention: Nasal endoscopy may be considered as a diagnostic adjunct in the evaluation of patients with suspected AR.
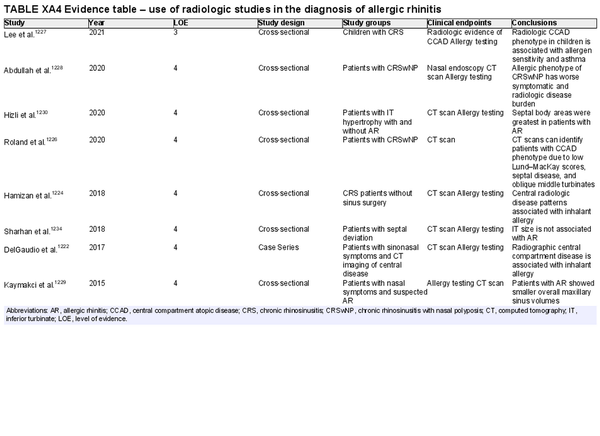
Radiologic studies
Aggregate grade of evidence: D (level 3: 1 study, level 4: 7 studies)
Benefit: Some radiologic findings, particularly those associated with central compartment edema/polyposis, may alert the clinician to the possibility of an associated allergic etiology.
Harm: Unnecessary radiation exposure, unnecessary cost.
Cost: High equipment and processing costs. Additional costs for interpretation of studies by radiologist.
Benefits‐harm assessment: Preponderance of harm over benefit.
Value judgments: Long‐term risks of ionizing radiation outweigh potential benefit.
Policy level: Recommendation against.
Intervention: Routine use of imaging is not recommended for the diagnosis of AR.
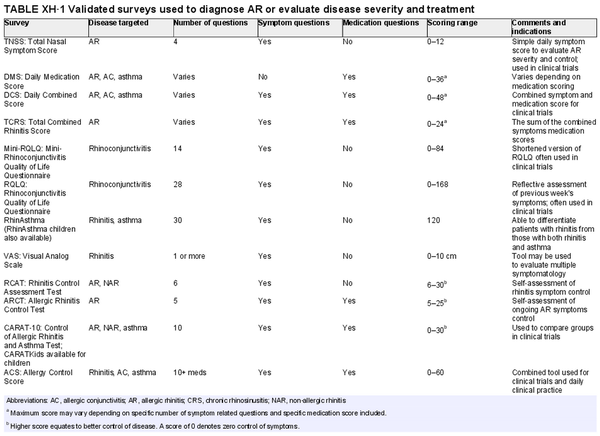
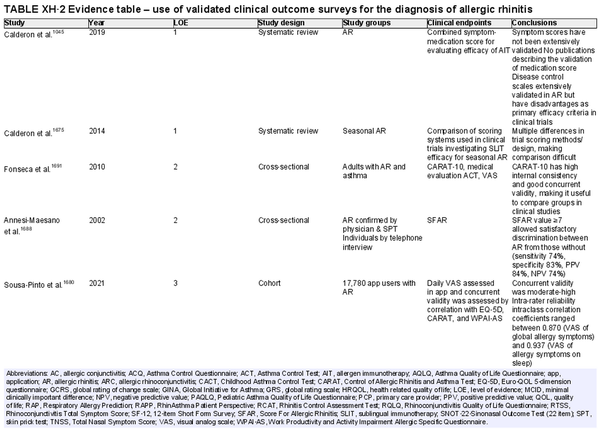
Use of validated subjective instruments and patient‐reported outcome measures
Aggregate grade of evidence: B (Level 1: 2 studies, level 2: 2 studies, level 3: 5 studies, level 4: 13 studies)
Benefit: Validated surveys offer a simple point‐of‐care option for screening and tracking symptoms, QOL, and control of allergic disease.
Harm: Minimal. Time to complete survey. Potential risk of misdiagnosis when based on survey data alone.
Cost: No financial burden to patients. Some fees associated with validated tests used for clinical research.
Benefits‐harm assessment: Preponderance of benefit over harm. Risk of misdiagnosis leading to unnecessary additional testing. Likewise, there is a risk that false negative responses may lead to delay in testing and further management.
Value judgments: Validated surveys may be used as a screening tool and primary or secondary outcome measure.
Policy level: Recommendation.
Intervention: Validated surveys may be used to screen for AR, follow treatment outcomes and as a primary outcome measure for clinical trials. Specific tests are optimized for various clinicopathological scenarios.
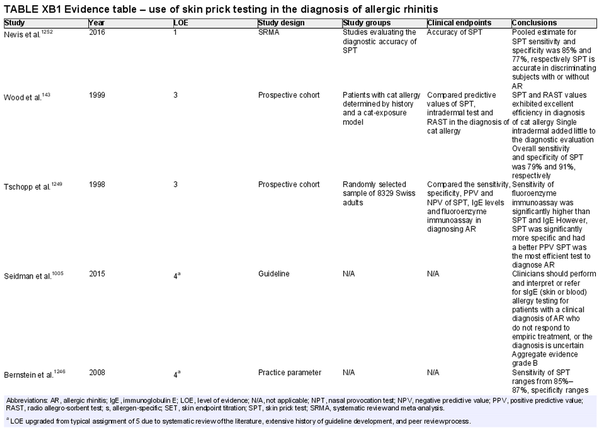
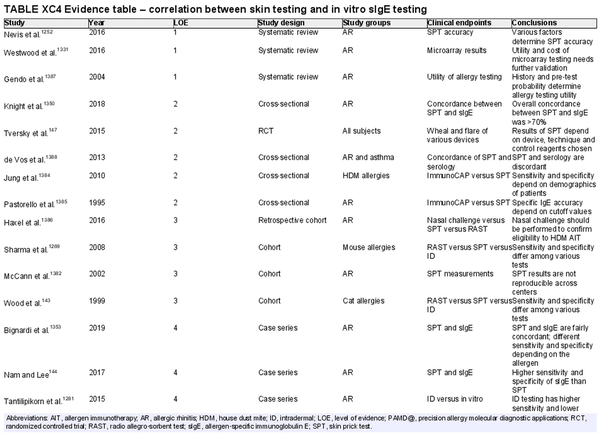
Skin prick testing
Aggregate grade of evidence: B (Level 1: 1 study, level 3: 2 studies, level 4: 7 studies, level 5: 2 studies)
Benefit: Confirm AR diagnosis and direct appropriate pharmacologic therapy, initiation of AIT, as well as avoidance measures.
Harm: Adverse events from testing including discomfort, pruritus, erythema, worsening of asthma symptoms, anaphylaxis, inaccurate test results, and misinterpreted test results. See Table II.C. in full ICAR document.
Cost: Moderate cost of testing procedure.
Benefits‐harm assessment: Preponderance of benefit over harm.
Value judgments: Patients can benefit from identification of their specific sensitivities. Skin prick testing (SPT) is a quick and relatively comfortable way to test several antigens with accuracy similar to other available methods of testing.
Policy level: Recommendation.
Intervention: Regular use of the same SPT device type will allow clinicians to familiarize themselves with it and interpretation of results may therefore be more consistent. The use of standardized allergen extracts can further improve consistency of interpretation.
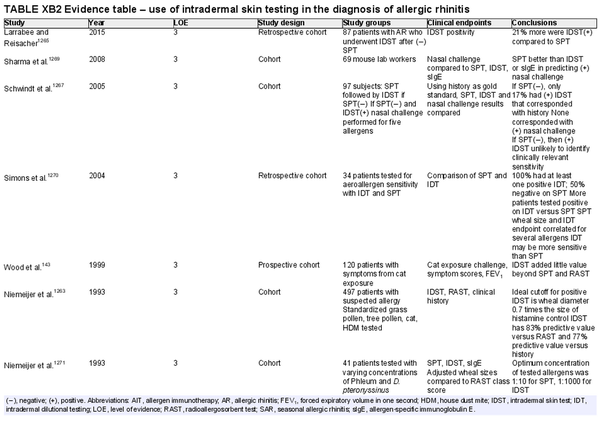
Skin intradermal testing
Aggregate grade of evidence: C (Level 3: 7 studies, level 4: 13 studies)
Benefit: May improve identification of allergic sensitization in patients with low‐level skin sensitivity or with non‐standardized allergens.
Harm: Adverse events from testing including discomfort, pruritus, erythema, worsening of asthma symptoms, anaphylaxis, inaccurate test results, and misinterpreted test results. See Table II.C. in full ICAR document.
Cost: Moderate cost of testing procedure.
Benefits‐harm assessment: Benefit over harm when used as a stand‐alone diagnostic test, when used to confirm the results of SPT, and as a quantitative diagnostic test.
Value judgments: Intradermal skin tests may not perform as well as SPT in most clinical situations.
Policy level: Option for using intradermal testing as a stand‐alone diagnostic test for individuals with suspected AR. Option for using intradermal testing as a confirmatory test following negative SPT for non‐standardized allergens.
Intervention: Intradermal testing may be used to determine aeroallergen sensitization in individuals suspected of having AR.
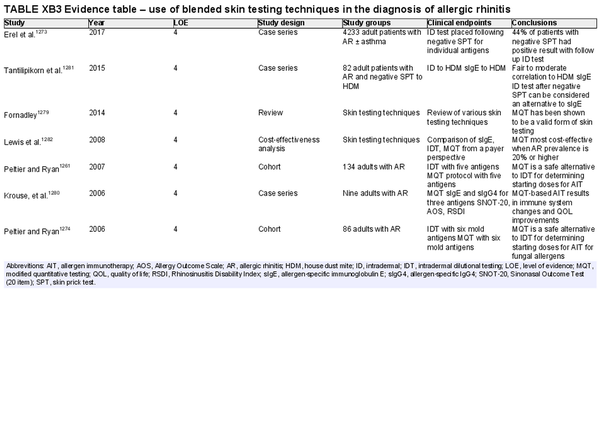
Blended skin testing techniques
Aggregate grade of evidence: D (Level 4: 7 studies)
Benefit: Ability to establish an endpoint in less time than intradermal dilutional testing, potential to determine allergen sensitization after negative SPT.
Harm: Adverse events from testing including discomfort, pruritus, erythema, worsening of asthma symptoms, anaphylaxis, inaccurate test results, and misinterpreted test results. Additional time and discomfort versus SPT alone. See Table II.C. in full ICAR document.
Cost: Moderate cost of testing procedure.
Benefits‐harm assessment: Preponderance of benefit over harm.
Value judgments: While AIT can be based off SPT results alone, endpoint‐based immunotherapy may have possible benefits of decreased time to therapeutic dosage.
Policy level: Option.
Intervention: Blended skin testing techniques, such as modified quantitative testing, are methods that can be used to determine a starting point for AIT or confirm allergic sensitization.
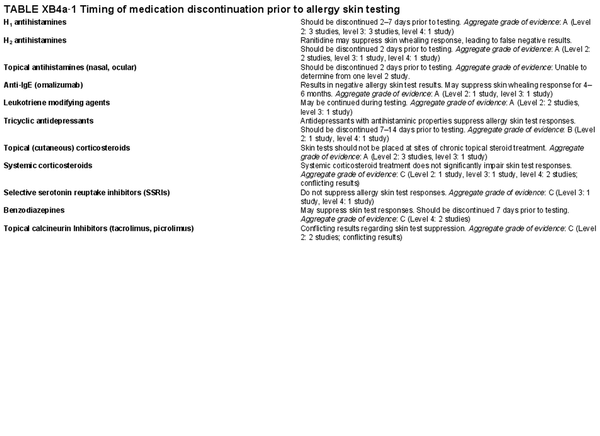
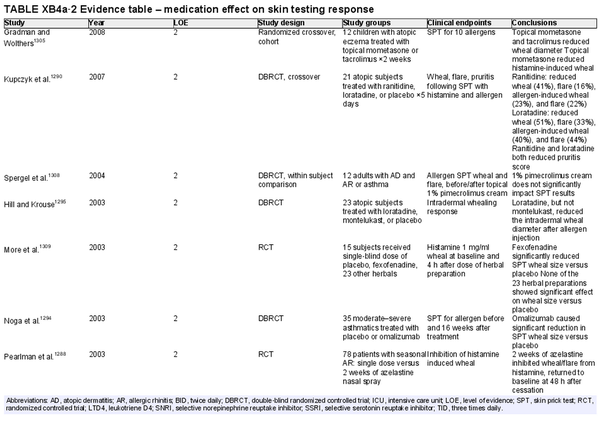
Issues that may affect the performance and interpretation of skin tests – medications:
H1 antihistamines– Aggregate grade of evidence: A (Level 2: 3 studies, level 3: 3 studies, level 4: 1 study). Should be discontinued 2–7 days prior to testing.
H2 antihistamines – Aggregate grade of evidence: A (Level 2: 2 studies, level 3: 1 study, level 4: 1 study). Ranitidine may suppress skin whealing response, leading to false negative results. Should be discontinued 2 days prior to testing.
Topical antihistamines – Aggregate grade of evidence: Unable to determine from one level 2 study. Should be discontinued 2 days prior to testing.
Anti‐IgE (omalizumab) – Aggregate grade of evidence: A (Level 2: 1 study, level 3: 1 study). Results in negative allergy skin test results. May suppress skin whealing response for 4–6 months.
Leukotriene modifying agents – Aggregate grade of evidence: A (Level 2: 2 studies, level 3: 1 study). May be continued during testing.
Tricyclic antidepressants – Aggregate grade of evidence: B (Level 2: 1 study, level 4: 1 study). Antidepressants with antihistaminic properties suppress allergy skin test responses. Should be discontinued 7–14 days prior to testing.
Topical (cutaneous) corticosteroids – Aggregate grade of evidence: A (Level 2: 3 studies, level 3: 1 study). Skin tests should not be placed at sites of chronic topical steroid treatment.
Systemic corticosteroids – Aggregate grade of evidence: C (Level 2: 1 study, level 3: 1 study, level 4: 2 studies; conflicting results). Systemic corticosteroid treatment does not significantly impair skin test responses.
Selective serotonin reuptake inhibitors – Aggregate grade of evidence: C (Level 3: 1 study, level 4: 1 study). Selective serotonin reuptake inhibitors do not suppress allergy skin test responses.
Benzodiazepines – Aggregate grade of evidence: C (Level 4: 2 studies). May suppress skin test responses. Should be discontinued 7 days prior to testing.
Topical calcineurin inhibitors (tacrolimus, picrolimus) – Aggregate grade of evidence: C (Level 2: 2 studies; conflicting results). Conflicting results regarding skin test suppression.
Issues that may affect the performance and interpretation of skin tests – skin conditions: Common sense dictates that allergy skin tests should not be performed at sites of active dermatitis, but clinical studies to investigate this phenomenon are lacking. There are insufficient studies published on this topic, and an Aggregate Grade of Evidence could not be assigned.
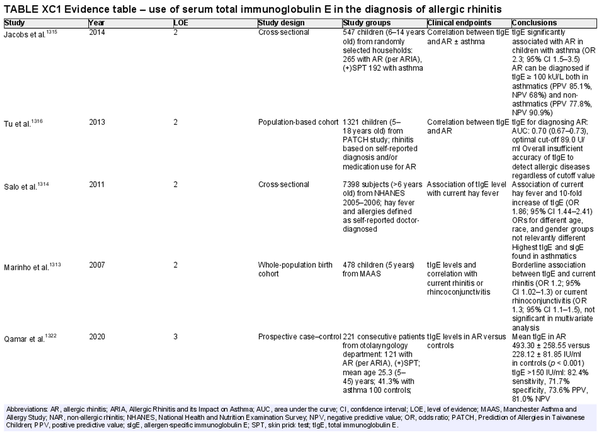
Serum total immunoglobulin E
Aggregate grade of evidence: C (Level 2: 4 studies, level 3: 11 studies)
Benefit: Possibility to suspect allergy or atopy in a wide screening.
Harm: Cost of test, undergoing of venipuncture, low level does not exclude AR.
Cost: Low, dependent on country and local healthcare environment.
Benefits‐harm assessment: Slight preponderance of benefit over harm. In addition, the ratio of total to allergen‐specific IgE (sIgE) may be useful to interpret the real value of sIgE production and predict treatment outcomes with AIT.
Value judgments: The evidence does not support routine use.
Policy level: Option.
Intervention: Assessment of total IgE may be useful to assess overall atopic status; furthermore, in selected cases it might help guide therapy (i.e., predict outcome of AIT).
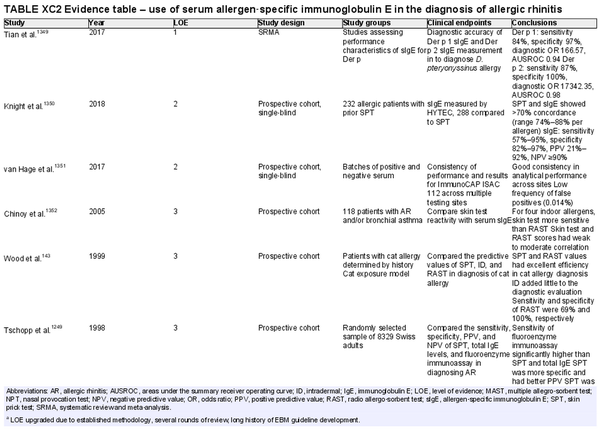
Serum allergen‐specific immunoglobulin E
Aggregate grade of evidence: B (Level 1: 1 study, level 2: 2 studies, level 3: 6 studies, level 4: 6 studies, level 5: 1 study)
Benefit: Confirms diagnosis and directs appropriate pharmacological therapy while possibly avoiding unnecessary/ineffective treatment, guides avoidance, directs AIT.
Harm: Adverse events from testing including discomfort from blood draw, inaccurate test results, false positive test results, misinterpreted test results.
Cost: Moderate cost of testing.
Benefits‐harm assessment: Preponderance of benefit over harm.
Value judgments: Patients can benefit from identification of their specific sensitivities. Further, in some patients who cannot undergo SPT, serum sIgE testing is a safe and effective alternative.
Policy level: Recommendation.
Intervention: Serum sIgE testing may be used in patients who cannot undergo allergy skin testing. The use of highly purified allergen or recombinants can increase the sensitivity, specificity, and diagnostic accuracy of sIgE tests. Rigorous proficiency testing on the part of laboratories may also improve accuracy.
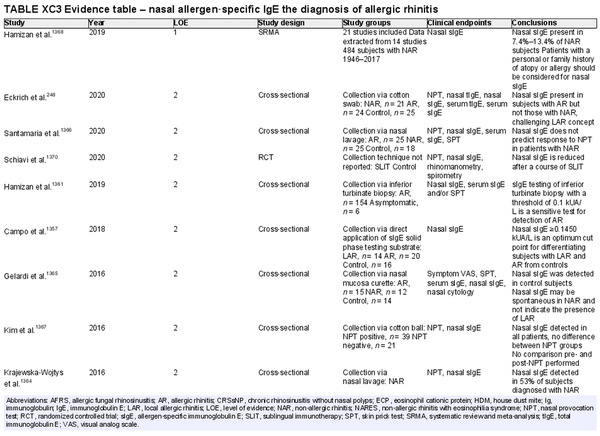
Nasal allergen‐specific immunoglobulin E
Aggregate grade of evidence: C (Level 1: 1 study, level 2: 21 studies, level 3: 3 studies, level 4: 11 studies)
Benefit: Patients with non‐allergic rhinitis found to have nasal sIgE may have local AR and could benefit from avoidance or AIT.
Harm: Measurement of nasal sIgE is minimally invasive. No significant adverse effects have been reported. Possible discomfort from sample collection.
Cost: Associated costs include the direct costs of testing and indirect cost of increased time and effort for performing nasal sIgE diagnostic test.
Benefits‐harm assessment: The benefits of identifying patients with an allergic component to their rhinitis may outweigh associated risks.
Value judgments: In patients with non‐allergic rhinitis who also have risk factors for atopic disease and have inadequate response to pharmacotherapy, testing for nasal sIgE may be helpful in confirming a diagnosis of local AR and allowing for treatment with AIT. There is no consensus for levels of nasal sIgE that indicate sensitivity.
Policy level: Option.
Intervention: Measurement of nasal sIgE is an option in patients with non‐allergic rhinitis suspected of having local AR to support this diagnosis and guide AIT if pharmacologic therapies are inadequate. Consensus for levels of nasal sIgE indicating AR need to be established.
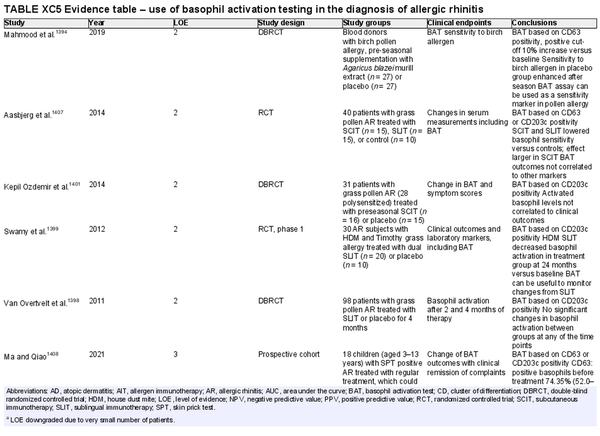
Basophil activation test
Aggregate grade of evidence: C (Level 2: 5 studies, level 3: 13 studies, level 4: 1 study)
Benefit: May help diagnose AR in specific cases where common approaches are not possible or show conflicting results.
Harm: Discomfort of venipuncture.
Cost: Moderate cost of performing the test, plus venipuncture. Depending on the local situation and availability.
Benefits‐harm assessment: Balance of benefit and harm.
Value judgments: The evidence does not support routine use for the diagnosis of AR or for following AIT response.
Policy level: Option.
Intervention: Application of basophil activation test in specific situations where other diagnostic procedures for AR are not possible or conflicting. Potentially useful for monitoring AIT if other methods fail or show conflicting results.
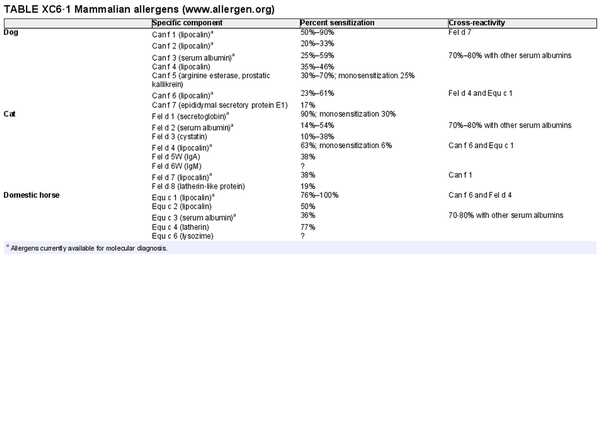
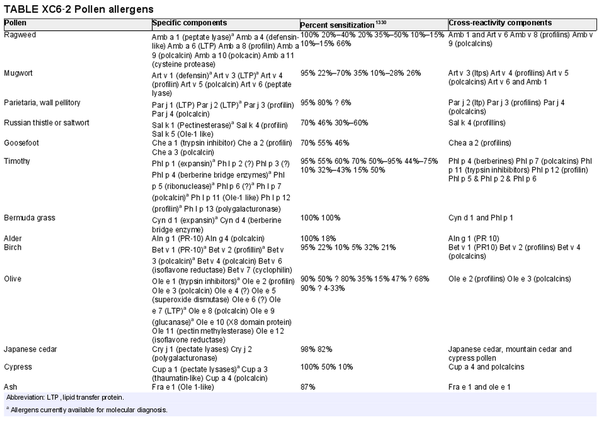
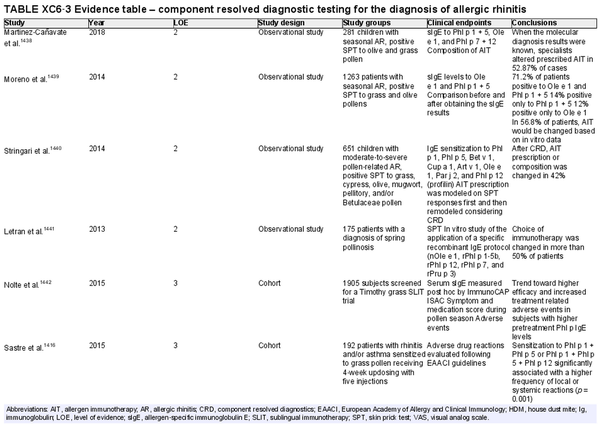
Component resolved diagnostic testing
Aggregate grade of evidence: C (Level 2: 4 studies, level 3: 2 studies, level 4: 11 studies, level 5: 1 study)
Benefit: Reliable. May help in identification and selection of suitable allergens for AIT, as well as possibly improving safety of AIT.
Harm: Discomfort of venipuncture.
Cost: Moderate cost of testing, minimal cost of venipuncture; depends on local availability.
Benefits‐harm assessment: Balance of benefit and harm.
Value judgments: Molecular diagnosis may be a useful tool for assessment of AR in some scenarios, especially in polysensitized patients.
Policy level: Option.
Intervention: Component resolved diagnostic testing is an option for diagnosis of AR by specialists.
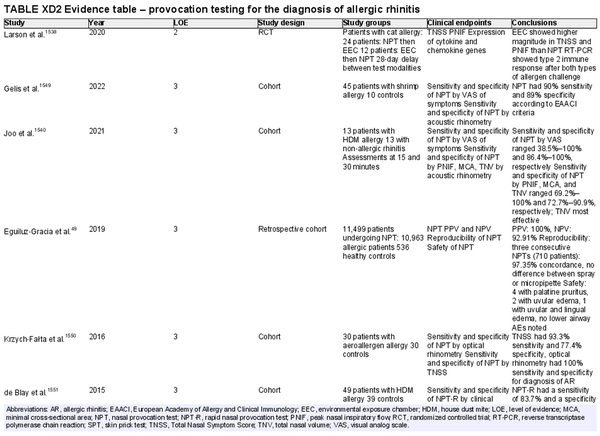
Nasal provocation testing
Aggregate grade of evidence: C (Level 2: 1 study, level 3: 7 studies)
Benefit: May assist in confirming diagnosis of AR in specific cases when immunological tests are unavailable or unreliable. Nasal provocation testing is crucial in diagnosing occupational rhinitis and local AR.
Harm: Not necessary if first‐ and second‐line tests are indicative for AR diagnosis.
Cost: Depending on the local situation and availability of equipment and staff, costs may be high.
Benefits‐harm assessment: Balance of benefit and harm.
Value judgments: The evidence does not support routine use for diagnosis of AR, but provocation testing is useful for diagnosis of occupational rhinitis and local AR.
Policy level: Option for diagnosis of AR when skin or in vitro tests are equivocal or unreliable. Recommendation for diagnosis of local AR and occupational rhinitis.
Intervention: Application of nasal provocation testing is useful in local AR and to confirm occupational rhinitis.
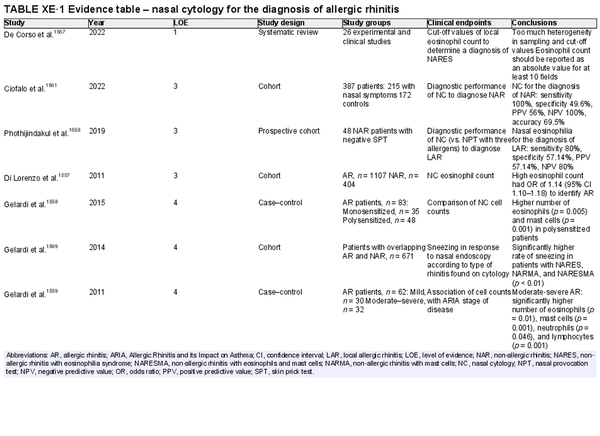
Nasal cytology
Aggregate grade of evidence: C (Level 1: 1 study, level 3: 3 studies, level 4: 3 studies)
Benefit: Low costs and low invasiveness. Could help to detect eosinophils in non‐allergic rhinitis and to diagnose a mixed rhinitis.
Harm: Nasal cytology is minimally invasive and minimal adverse effects have been reported.
Cost: Associated costs include the direct cost of nasal cytology and indirect cost of increased time and effort for performing nasal cytology.
Benefits‐harm assessment: Preponderance of benefit over harm.
Value judgments: The evidence does not support routine clinical use.
Policy level: Option.
Intervention: Nasal cytology could help in cases of non‐allergic rhinitis to suspect local AR or in cases of AR to diagnose a mixed rhinitis. It could be considered an option in cases of negative SPT and/or serum sIgE to evaluate the presence of mucosal eosinophils and consideration of local AR or type 2 inflammation. The cut‐off values for determining non‐allergic rhinitis with eosinophilia syndrome (NARES) are not yet clear.
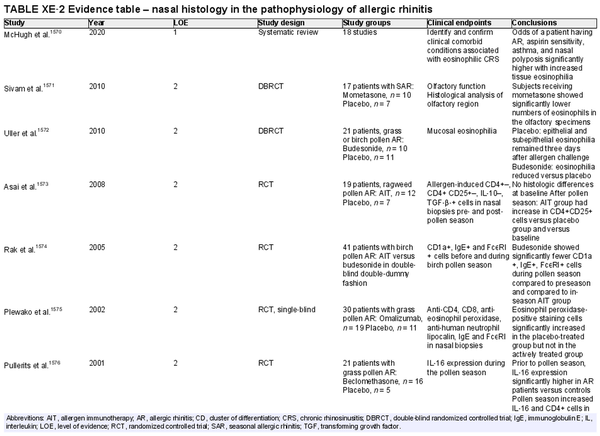
Nasal histology
Aggregate grade of evidence: B (Level 1: 1 study, level 2: 7 studies, level 4: 2 studies)
Benefit: May assist in evaluation of tissue eosinophilia and expression of mediators. May be useful in clinical research.
Harm: Small risk of complications (e.g., bleeding, infection).
Cost: Associated costs consist of the direct cost of nasal histology and indirect cost of increased time and effort for performing nasal histology.
Benefits‐harm assessment: Preponderance of benefit over harm.
Value judgments: The evidence does not support routine clinical use.
Policy level: Recommendation against.
Intervention: Nasal histology may be helpful in clinical research or selected cases (e.g., evaluation of tissue eosinophils during surgery). Recommendation against in routine clinical practice for AR evaluation due to invasive nature of obtaining a specimen.
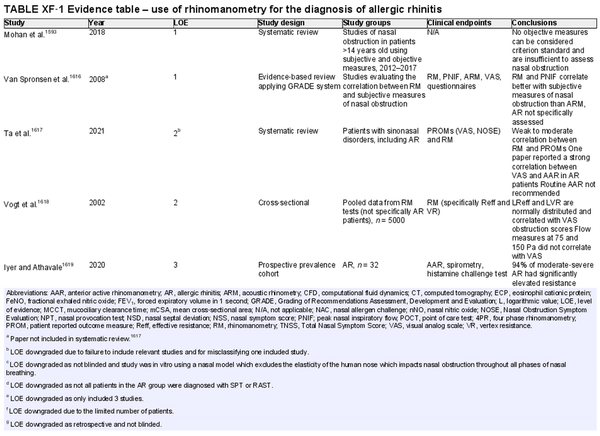
Rhinomanometry
Aggregate grade of evidence: B (Level 1: 2 studies, level 2: 2 studies, level 3: 5 studies, level 4: 4 studies, level 5: 6 studies)
Benefit: Rhinomanometry is useful to improve patient selection for surgery, distinguish between structural and functional causes of nasal obstruction, diagnose nasal valve collapse, clarify conflicting symptoms and exam findings, use as a medicolegal tool and in nasal allergen challenges. Four‐phase rhinomanometry correlates with subjective scores.
Harm: Low. Rhinomanometry has limited effectiveness in patients with complete nasal obstruction or septal perforation. The equipment is not portable and therefore requires a clinic visit and trained staff. The procedure may be considered time consuming.
Cost: High.
Benefits‐harm assessment: Benefits outweigh harm.
Value judgments: For some patients, it may be important to avoid unnecessary costs in the diagnosis of AR; therefore, this procedure is less preferred.
Policy level: Option.
Intervention: Rhinomanometry is useful in distinguishing between structural and soft tissue causes of obstruction, when history and examination findings are not congruent, as well as a research tool. Better with individual nasal cavity assessment and four‐phase rhinomanometry.
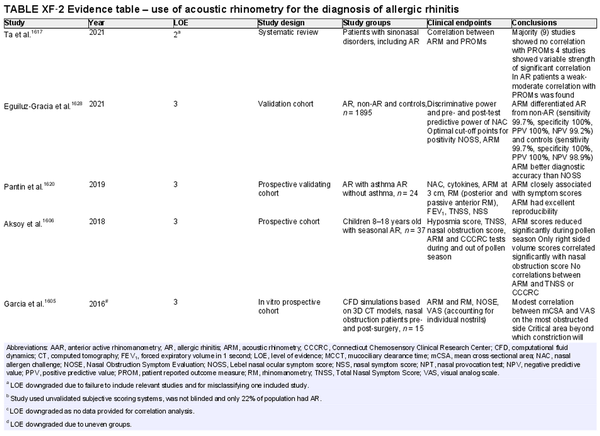
Acoustic rhinometry
Aggregate grade of evidence: C (Level 2: 1 study, level 3: 5 studies, level 4: 3 studies, level 5: 2 studies)
Benefit: Improves patient selection for surgery, helps distinguish between structural and functional causes of nasal obstruction, evaluates a response in nasal allergen challenges, and functions as a medicolegal tool to demonstrate objective evidence of effectiveness of an intervention.
Harm: Low. Equipment is not portable therefore, requires a clinic visit and trained staff. Time‐consuming. Leakage into sinuses may provide inaccurate results and lead to inappropriate treatment.
Cost: High.
Benefits‐harm assessment: Benefits outweigh harm as harm is low.
Value judgments: For some patients, it may be important to avoid unnecessary cost in the diagnosis of AR, and thus acoustic rhinometry is less preferred.
Policy level: Option.
Intervention: Acoustic rhinometry is most useful in research setting as opposed to as a clinical diagnostic tool.
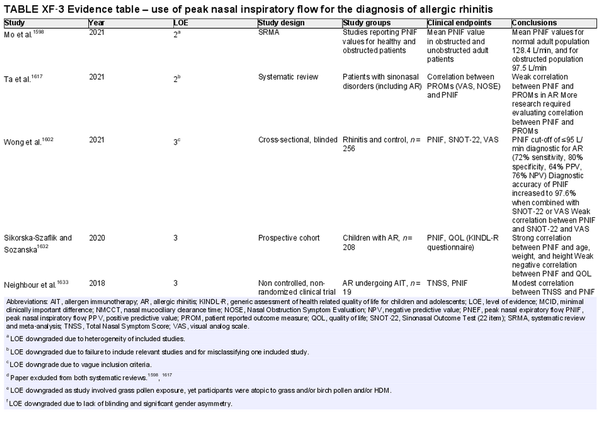
Peak nasal inspiratory flow
Aggregate grade of evidence: B (Level 2: 2 studies, level 3: 4 studies, level 4: 1 study, level 5: 1 study)
Benefit: Can improve patient selection for surgery, can evaluate a response in nasal allergen challenges, and can be used as a medicolegal tool to demonstrate objective evidence of effectiveness of an intervention.
Harm: Low. Risk of missing valve collapse and septal deviation as causes of obstruction.
Cost: Low.
Benefits‐harm assessment: Benefits likely to outweigh harm as harm is low.
Value judgments: Relies on patient effort and does not assess individual nasal cavities. Unable to evaluate nasal valve collapse.
Policy level: Option.
Intervention: Use in conjunction with patient reported outcome measures to improve utility.
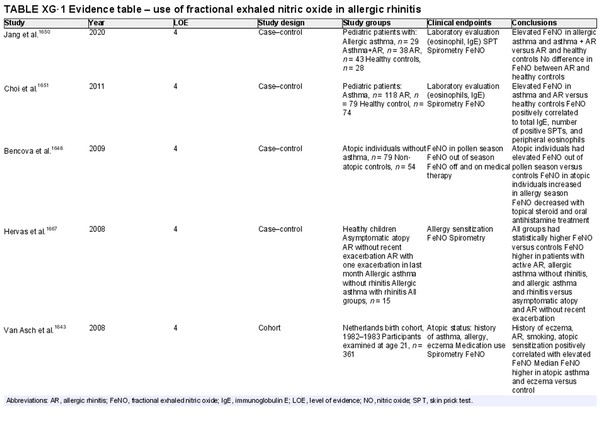
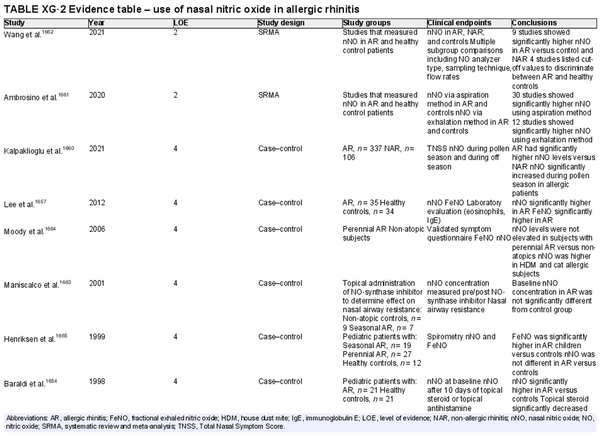
Nitric oxide measurements
Aggregate grade of evidence:
Fractional exhaled nitric oxide (FeNO): D (Level 4: 7 studies)
Nasal nitric oxide (nNO): C (Level 2: 2 studies, level 4: 6 studies)
Benefit: Possible benefit in differentiation of allergic and non‐allergic rhinitis through non‐invasive testing. Possible benefit in monitoring treatment response.
Harm: No studies have shown harm with either exam.
Cost:
FeNO: Relatively high. FeNO analyzers are approximately $7000–10,000 US, but testing is covered by some insurance plans.
nNO: High. Chemiluminescence NO analyzers are approximately $30,000–50,000 US, and clinical testing is not covered by insurance in the US.
Benefits‐harm assessment: Preponderance of benefit over harm.
Value judgments: There is inconsistent evidence in the ability of FeNO or nNO to differentiate adults and children with AR and non‐allergic rhinitis. Most studies were of low evidence or small impact. There is no agreed upon cut‐off value when performing FeNO or nNO for the diagnosis of AR.
Policy level:
FeNO: Recommend against for routine diagnosis of AR.
nNO: Recommend against for routine diagnosis of AR.
Intervention: History and physical, diagnostic skin testing, or sIgE testing should be the first‐line evaluation of AR. FeNO or nasal NO testing may provide additional diagnostic information if necessary but should not be routinely employed for AR diagnosis.
I.C.7 Management
I.C.7.a Avoidance measures and environmental controls
Allergen avoidance is generally low risk and may provide some benefit in controlling AR symptoms. Both physical interventions and chemical applications may reduce allergen load in the environment, although assessment of the effects of these interventions on control of AR symptoms is lacking in some studies. ICAR‐Allergic Rhinitis 2023 evaluated allergen avoidance and environmental control measures for house dust mite, cockroach, pets, rodents, pollen, and occupational allergens. Section XI.A of the ICAR‐Allergic Rhinitis 2023 document summarizes studies of avoidance measures and environmental controls employed for the treatment of AR (Table I.C.7.a).
The section that follows includes recommendation summaries for allergen avoidance and environmental controls that are included in the ICAR‐Allergic Rhinitis 2023 document.
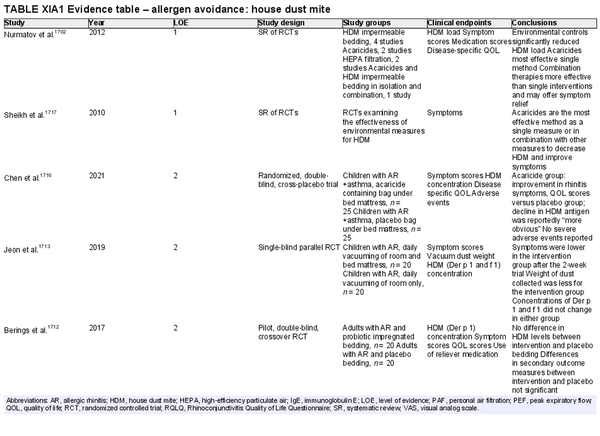
Avoidance – house dust mite (HDM)
Aggregate grade of evidence: B (Level 1: 2 studies, level 2: 12 studies)
Benefit: Potential improvement in AR symptoms and QOL with reduced concentration of environmental HDM antigens.
Harm: None.
Cost: Low to moderate. However, cost‐effectiveness was not evaluated.
Benefits‐harm assessment: Benefit outweighs harm.
Value judgments: There is supporting evidence for the use of acaricides in reducing HDM concentration in children who have AR coexistent with asthma. In adults and children without concomitant asthma, the use of acaricides with/without bedroom‐based control programs for reducing HDM concentration are promising, but further, high‐quality studies are needed to evaluate clinical outcomes.
Policy level: Option.
Intervention: Acaricides used independently or alongside environmental control measures, such as air filtration devices, could be considered as options in the management AR.
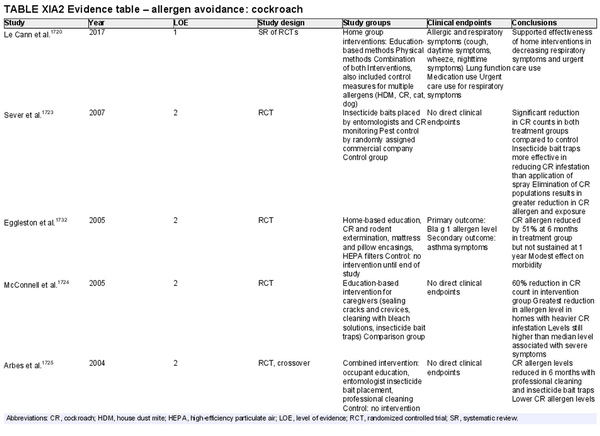
Avoidance – cockroach
Aggregate grade of evidence: B (Level 1: 1 study, level 2: 8 studies, level 3: 2 studies, level 4: 1 study)
Benefit: Reduction in cockroach count but allergen concentrations (Bla g 1 and Bla g 2) often above acceptable levels for clinical benefits. No studies included clinical endpoints related to AR.
Harm: None noted.
Cost: Direct costs include multiple treatment applications or multi‐interventional approaches. Indirect costs include potential time off work for interventions in home and substantial labor of cleaning measures to eradicate allergens.
Benefits‐harm assessment: Balance of benefits and harms since lack of clear clinical benefits.
Value judgments: Control of cockroach populations especially in densely populated multi‐family dwellings is important to control cockroach allergen levels.
Policy level: Option.
Intervention: Combination of physical measures (e.g., insecticide bait traps, house cleaning) and education‐based methods seem to have the greatest efficacy. Additional research on single intervention approaches is needed with cost analysis, as well as investigation of clinical outcomes related to AR.
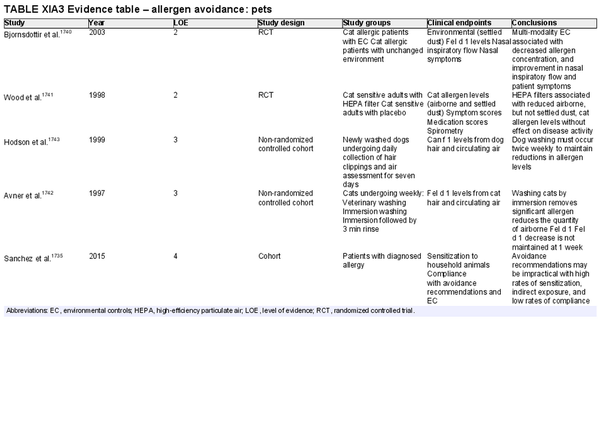
Avoidance – pets
Aggregate grade of evidence: C (Level 2: 2 studies, level 3: 2 studies, level 4: 1 study)
Benefit: Decreased environmental allergen exposure with possible reduction in symptoms and secondary prevention of asthma.
Harm: Emotional distress caused by removal of household pets. Financial and time costs of potentially ineffective intervention.
Cost: Low to moderate.
Benefits‐harm assessment: Equivocal.
Value judgments: While several studies have demonstrated an association between environmental controls and reductions in environmental antigens, only a single, multi‐modality randomized controlled trial has demonstrated clinical improvement in nasal symptoms among patients with Fel d 1 sensitivity. The secondary prevention and treatment of asthma in sensitized individuals must also be considered.
Policy level: Option.
Intervention: Pet avoidance and environmental control strategies, particularly multi‐modality environmental controls among patients with diagnosed Fel d 1 sensitivity, may be presented as an option for the treatment of AR.
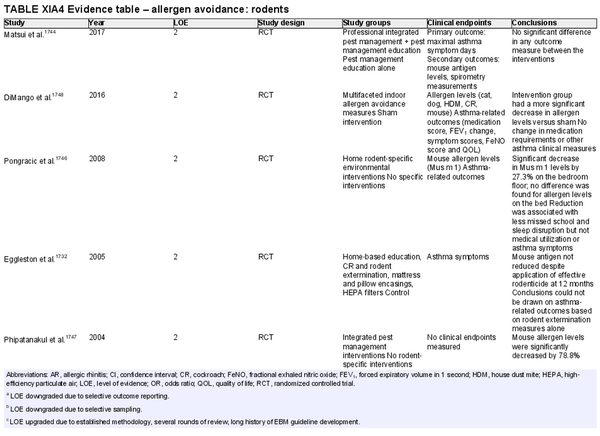
Avoidance – rodents
Aggregate grade of evidence: C (Level 2: 5 studies, level 3: 5 studies, level 4: 4 studies, level 5: 1 study)
Benefit: Reduces rodent allergen levels (specifically mouse allergen) but no information on AR outcomes.
Harm: Reduction in patient QOL due to removal of pet rodent to whom patient is emotionally attached. Change in job position or role if primary rodent exposure is work‐related.
Cost: Direct costs include the cost of interventions such as extermination and mitigating causal factors or loss of income if a job change occurs. Indirect costs include time off work for pest control appointments.
Benefits‐harm assessment: Balance of benefit and harm.
Value judgments: Careful patient selection based on exposure history. Heterogeneity of integrated pest management protocols makes quantification of benefit difficult.
Policy level: Option.
Intervention: Avoidance likely improves rodent‐specific allergen exposure, especially when the interaction can be eliminated such as when it is work‐related or with a pet rodent. Integrated pest management should be considered in select patients, such as pediatric inner‐city patients that suffer from asthma and are mouse sensitized.
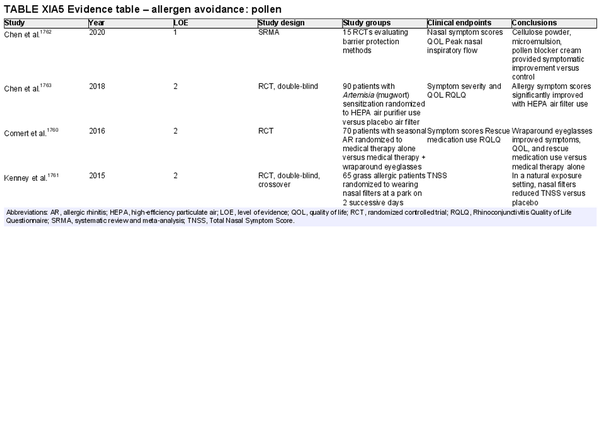
Avoidance – pollen
Aggregate grade of evidence: B (Level 1: 1 study, level 2: 3 studies)
Benefit: Decreased symptoms and medication use with potential for improved QOL.
Harm: Interventions may vary in cost and efficacy of each may be inadequately defined.
Cost: Generally low monetary cost depending on strategy.
Benefits‐harm assessment: Equivocal, most interventions with lower harm but not well‐defined benefits.
Value judgments: Most pollen avoidance measures are based on clinical and expert opinion although trial‐based evidence is available for some interventions.
Policy level: Option.
Intervention: Pollen avoidance strategies are generally well tolerated and lower cost, non‐medication‐based interventions that may have benefit with minimal harm to the patient, but further randomized controlled trials with larger populations would be needed to better characterize efficacy.
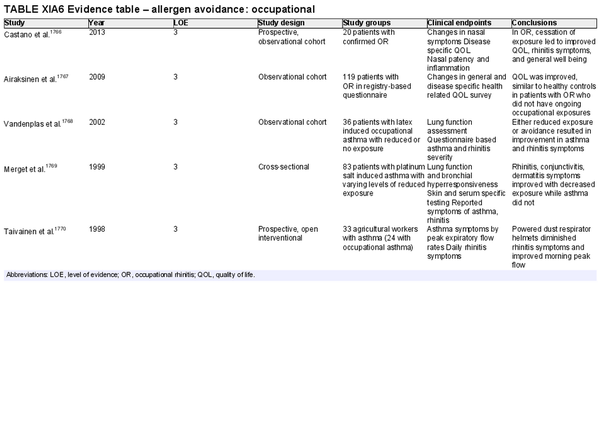
Avoidance – occupational
Aggregate grade of evidence: C (Level 3: 5 studies)
Benefit: Decreased allergen exposure may lead to reduction in symptoms, improvement in QOL, and possible reduced likelihood of developing occupational asthma.
Harm: Potential for socioeconomic harm with loss of wages or requiring changes in occupation.
Cost: Individually may vary if avoidance results in loss of income; for employers, potentially high cost depending on interventions or environmental controls required.
Benefits‐harm assessment: Where possible from a patient‐centered perspective, in occupational rhinitis complete avoidance is likely beneficial in improving health quality compared to ongoing exposures.
Value judgments: Based primarily on observational studies, allergen avoidance or decreasing exposure is recommended for all patients but can be nuanced depending on the resulting socioeconomic impact.
Policy level: Recommendation.
Intervention: Patients should be counseled to avoid or decrease exposure to inciting agents in occupational respiratory disease.
I.C.7.b Pharmacotherapy and procedural options
Pharmacologic treatments are frequently employed to control AR symptoms. Depending on the specific therapy and geographic region, these may be available by prescription or over‐the‐counter. The evidence for pharmacologic options for AR has been reviewed (Table I.C.7.b).
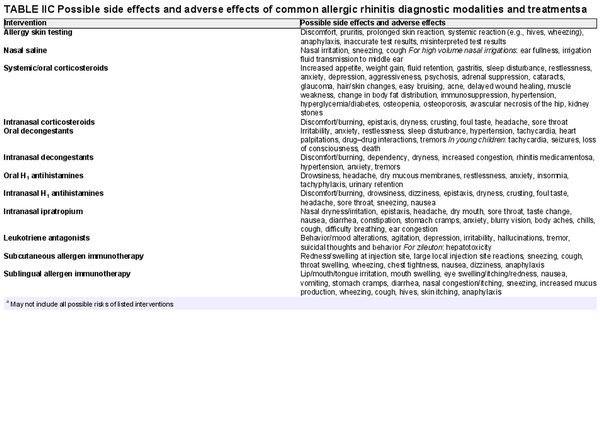
The section that follows includes recommendation summaries for pharmacotherapies and procedural interventions that are included in the ICAR‐Allergic Rhinitis 2023 document. A standard listing of side effect and adverse effects of most AR management options may be found in Table II.C. within the full ICAR‐Allergic Rhinitis 2023 document.
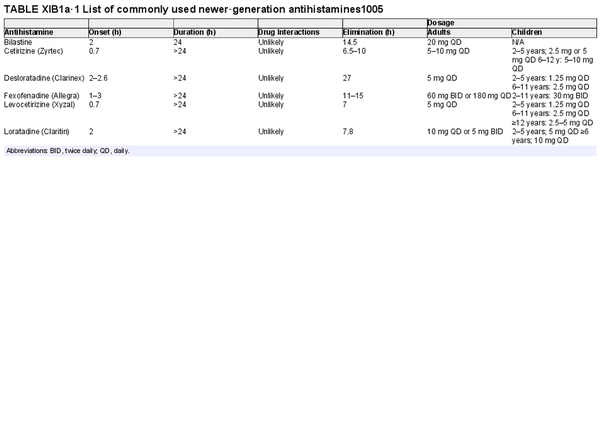
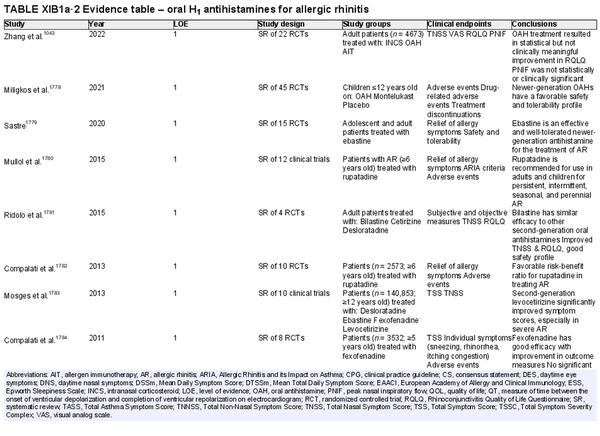
Oral H1 antihistamines
Aggregate grade of evidence: A (Level 1: 19 studies, level 4: 5 studies)
Benefit: Reduction in symptoms of AR.
Harm: Compared to first‐generation oral antihistamines, newer‐generation antihistamines have fewer central nervous system and anticholinergic side effects. The side effects of first‐generation antihistamines can be more pronounced in the elderly. See Table II.C. in full ICAR document.
Cost: Inexpensive. Given their improved side effect profile, newer‐generation oral antihistamines also have lower indirect costs than first generation oral H1 antihistamines.
Benefits‐harm assessment: The benefits outweigh harm for use of newer‐generation H1 oral antihistamines for AR.
Value judgments: First‐generation oral antihistamines are not recommended for the treatment of AR because of their central nervous system and anticholinergic side effects.
Policy level: Strong recommendation for the use of newer‐generation oral antihistamines for AR.
Intervention: Newer‐generation oral antihistamines can be considered in the treatment of AR.
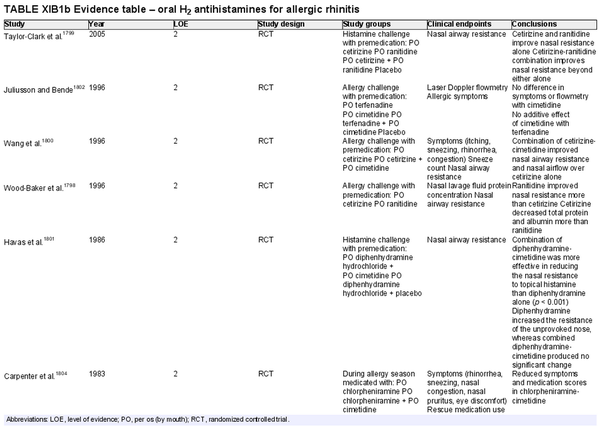
Oral H2 antihistamines
Aggregate grade of evidence: B (Level 2: 7 studies)
Benefit: Decreased objective nasal resistance, and improved symptom control in 4 studies when used in combination with H1 antagonists.
Harm: Drug–drug interaction (p450 inhibition, inhibited gastric secretion, and absorption).
Cost: Increased cost associated with H2 antagonist over H1 antagonist alone.
Benefits‐harm assessment: Unclear benefit and possible harm.
Value judgments: No studies evaluating efficacy of H2 antihistamines in context of INCS. There were 2 studies that showed no benefit for H2 antagonist when used alone or as an additive to H1 antagonist therapy.
Policy level: No recommendation. Available evidence does not adequately address the benefit of H2 antihistamines in AR.
Intervention: Addition of an oral H2 antagonist to an oral H1 antagonist may improve symptom control in AR, but data is limited.
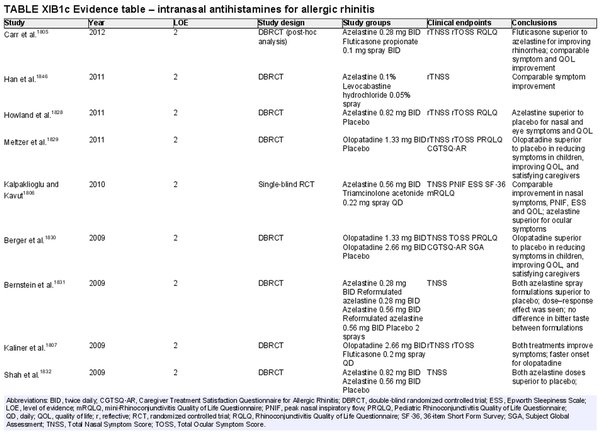
Intranasal antihistamines
Aggregate grade of evidence: A (Level 2: 44 studies)
Benefit: Rapid onset; more effective for nasal congestion than oral antihistamines; more effective for ocular symptoms than INCS; consistent reduction in symptoms and improvement in QOL in randomized controlled trials compared to placebo.
Harm: Patient tolerance, typically related to taste aversion; less effective for congestion than INCS. See Table II.C. in full ICAR document.
Cost: Low to moderate financial burden; available as prescription or nonprescription product.
Benefits‐harm assessment: Preponderance of benefit over harm. Intranasal antihistamine as monotherapy is consistently more effective than placebo. Most studies show intranasal antihistamines superior to INCS for sneezing, itching, rhinorrhea, and ocular symptoms. Adverse effects are minor and infrequent. Generic prescription and over‐the‐counter formulations now available.
Value judgments: Extensive high‐level evidence comparing intranasal antihistamine monotherapy to active and placebo controls demonstrates overall effectiveness and safety.
Policy level: Strong recommendation.
Intervention: Intranasal antihistamines may be used as first‐ or second‐line therapy in the treatment of AR.
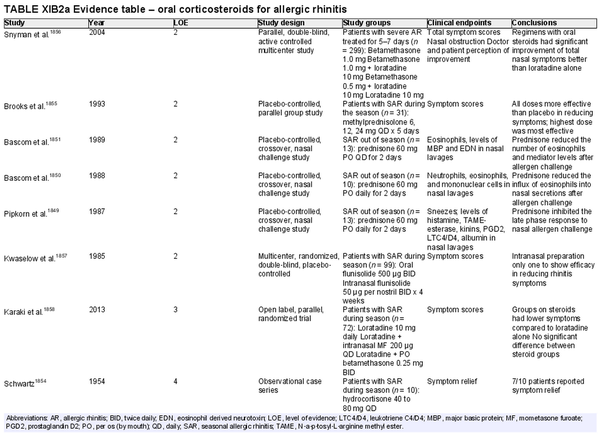
Oral corticosteroids
Aggregate grade of evidence: B (Level 2: 6 studies, level 3: 1 study, level 4: 3 studies)
Benefit: Oral corticosteroids can attenuate symptoms of AR and ongoing allergen induced inflammation.
Harm: Oral corticosteroids have multiple potential adverse effects, including hypothalamic‐pituitary axis suppression. Prolonged use may lead to growth retardation in pediatric populations. See Table II.C. in full ICAR document.
Cost: Low.
Benefits‐harm assessment: The risks of oral corticosteroids outweigh the benefits, given similar symptomatic improvement observed with the use of safer INCS.
Value judgments: In the presence of effective symptom control using INCS, the risk of adverse effects from using oral corticosteroids for AR outweighs potential benefits.
Policy level: Strong recommendation against routine use.
Intervention: Although not recommended for routine use in AR, certain clinical scenarios may warrant the use of short courses of systemic corticosteroids, following a discussion of the risks and benefits with the patient. For example, oral steroids could be considered in select patients with significant nasal obstruction that precludes adequate penetration of intranasal agents (corticosteroids or antihistamines). In these cases, a short course of systemic corticosteroids may improve congestion and facilitate access of topical medications. No evidence supports this suggestion, and thus careful clinical judgment and risk discussion are advocated.
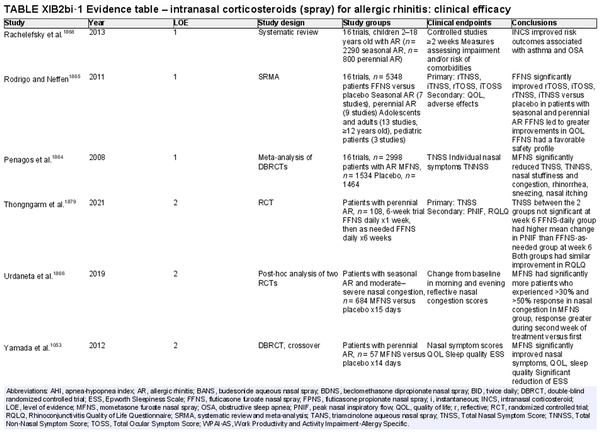
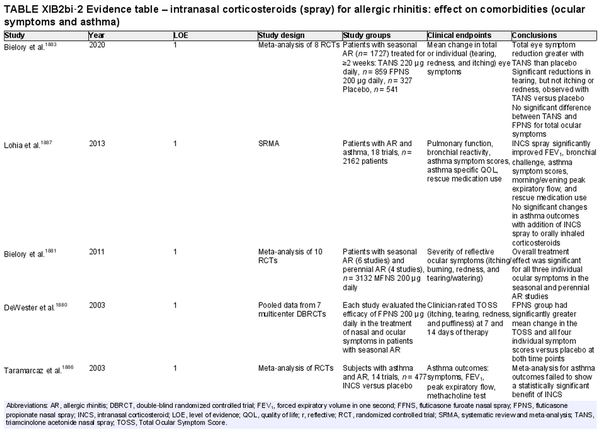
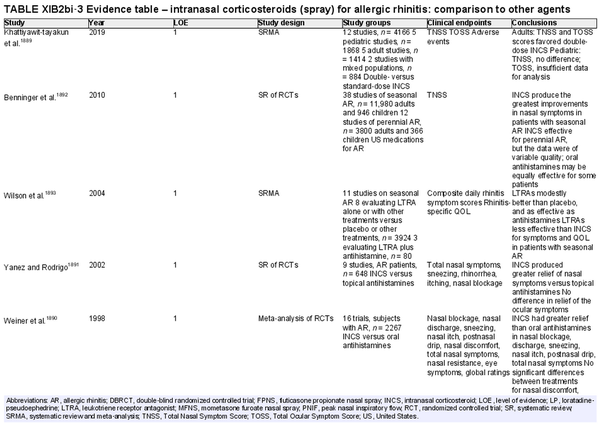
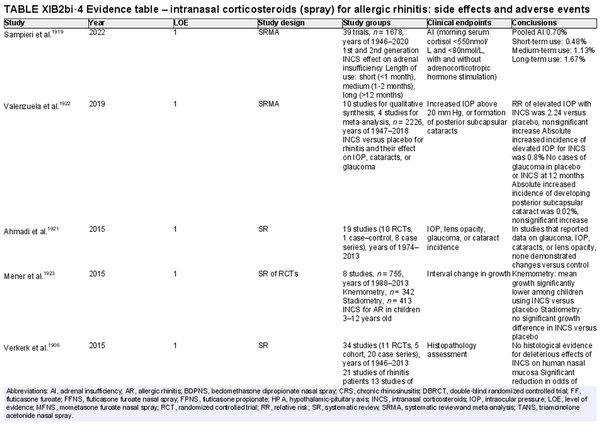
Intranasal corticosteroid sprays
Aggregate grade of evidence: A (Level 1: 18 studies, level 2: 29 studies, level 3: 3 studies)
Benefit: INCS sprays are effective in reducing nasal and ocular symptoms of AR. Studies have demonstrated superior efficacy compared to oral antihistamines and leukotriene receptor antagonists (LTRAs).
Harm: INCS sprays have undesirable local adverse effects, such as epistaxis, with increased frequency compared to placebo in prolonged administration studies. There are no apparent negative effects on the hypothalamic‐pituitary axis. There might be some negative effects on short‐term growth in children, but it is unclear whether these effects translate into long‐term growth suppression. See Table II.C. in full ICAR document.
Cost: Low.
Benefits‐harm assessment: The benefits of using INCS sprays outweigh the risks when used to treat seasonal or perennial AR.
Value judgments: INCS sprays are first line therapy for the treatment of AR by virtue of their superior efficacy in controlling nasal symptoms. Subjects with seasonal AR should start prophylactic treatment with INCS sprays several days before the pollen season with an evaluation of the patient's response a few weeks after initiation, including a nasal exam to evaluate for local irritation or mechanical trauma. Children receiving INCS sprays should be on the lowest effective dose to avoid negative growth effects.
Policy level: Strong recommendation.
Intervention: The demonstrated efficacy of INCS sprays, as well as their superiority over other agents, make them first‐line therapy in the treatment of AR.
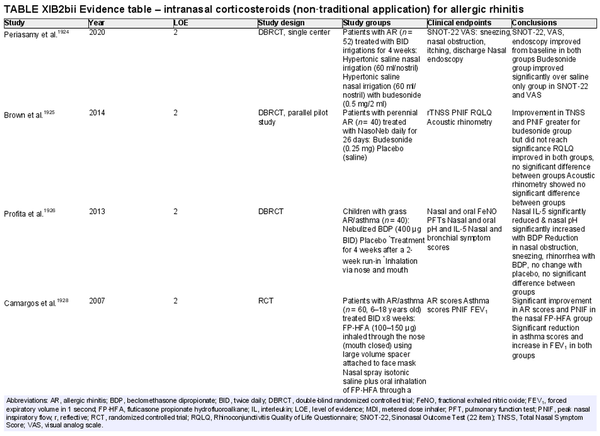
Intranasal corticosteroids: non‐traditional application
Aggregate grade of evidence: B (Level 2: 4 studies, level 3: 1 study)
Benefit: Nebulized steroids or those used via irrigation show some benefit in the treatment of AR in limited studies. Furthermore, steroids inhaled or exhaled through the nose in patients with asthma and rhinitis also show some benefit for rhinitis. Nasal steroid drops are not approved for treatment of rhinitis but are used in certain countries.
Harm: Nasal steroid drops have significant systemic side effects. See Table II.C. in full ICAR document.
Cost: Low.
Benefits‐harm assessment: The risks of using corticosteroid nasal drops for AR outweigh the benefits. Limited evidence suggests that nasal steroid irrigations for rhinitis lead to significant improvement of symptoms. Scarce evidence does not support routine recommendation for this route of therapy.
Value judgments: In the presence of effective symptom control using traditional spray administration for INCS, there is no solid data to support other routes of administration.
Policy level: Recommendation against routine use.
Intervention: There is some evidence that inhaled steroids, when exhaled through the nose might improve AR symptoms. Similar benefit is seen when steroids are inhaled by first passing through the nose. These routes might be useful in patients with both rhinitis and asthma.
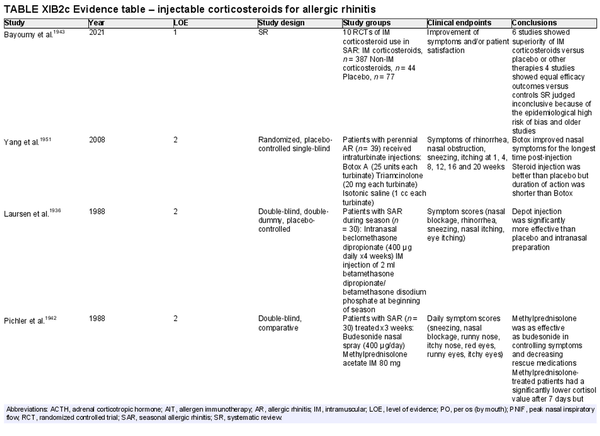
Injectable corticosteroids
Aggregate grade of evidence: B (Level 1: 1 study, level 2: 11 studies, level 4: 2 studies)
Benefit: Injectable corticosteroids improved symptoms of AR in clinical studies.
Harm: Injectable corticosteroids have known undesirable adverse effects on the hypothalamic‐pituitary axis, growth, osteoporosis, glycemic control, and other systemic adverse effects, for varied periods of time after injection. Intraturbinate corticosteroids have a small but potentially serious risk of ocular side effects including decline or loss of vision. See Table II.C. in full ICAR document.
Cost: Low.
Benefits‐harm assessment: In routine management of AR, the risk of serious adverse effects outweighs the demonstrated clinical benefit.
Value judgments: Injectable corticosteroids are effective for the treatment of AR. However, given the risk of significant systemic adverse effects, the risk of serious ocular side effects, and the availability of effective alternatives (e.g., INCS sprays), injectable corticosteroids are not recommended for the routine treatment of AR.
Policy level: Recommendation against.
Intervention: None.
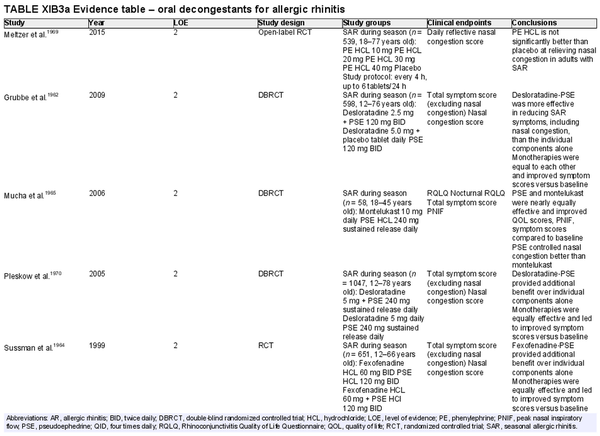
Oral decongestants
Aggregate grade of evidence: A (Level 2: 12 studies)
Benefit: Reduction of nasal congestion with pseudoephedrine. No benefit with phenylephrine.
Harm: Oral decongestants have known undesirable adverse effects. See Table II.C. in full ICAR document.
Cost: Low.
Benefits‐harm assessment: Balance of benefit and harm for pseudoephedrine. Possible harm for phenylephrine.
Value judgments: Little evidence for benefit in controlling symptoms other than nasal congestion.
Policy level: Strong recommendation against for routine use in AR. In certain cases, combination therapy with an oral antihistamine may be beneficial to alleviate severe nasal congestion in short courses.
Intervention: Although not recommended for routine use in AR, pseudoephedrine can be effective in reducing nasal congestion in patients with AR; however, it should only be used as short‐term/rescue therapy after a discussion of the risks and benefits with the patient (comorbidities) and consideration of alternative intranasal therapy options.
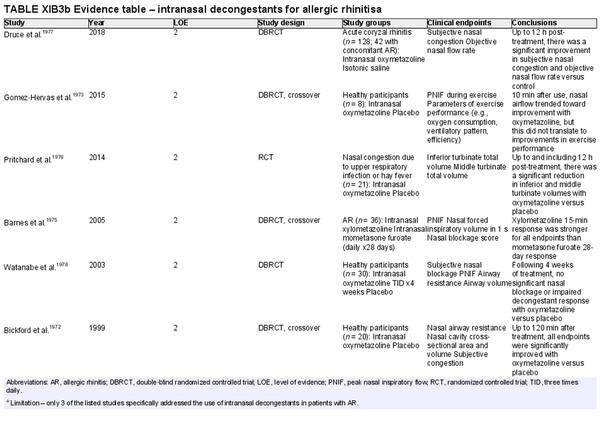
Intranasal decongestants
Aggregate grade of evidence: B (Level 2: 10 studies, level 3: 2 studies) Limitation – only 3 studies included subjects with AR.
Benefit: Reduction in symptoms of nasal congestion/blockage and corresponding objective markers with intranasal decongestants compared to placebo.
Harm: Side effects include nasal discomfort/burning, dependency, dryness, hypertension, anxiety, and tremors. Potential for rebound congestion with long‐term use. See Table II.C. in full ICAR document.
Cost: Low.
Benefits‐harm assessment: Harm likely outweighs benefit if used long‐term, with adverse effects appearing as early as 3 days.
Value judgments: Intranasal decongestants can be helpful for short‐term relief of nasal congestion.
Policy level: Option for short‐term use.
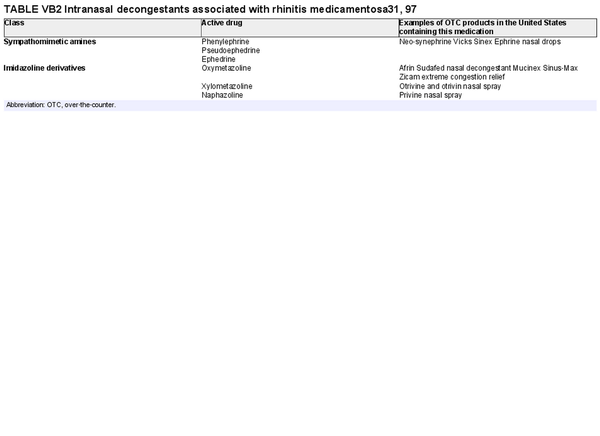
Intervention: Intranasal decongestants can provide effective short‐term relief of nasal congestion in patients with AR during an acute flare but recommend against chronic use due to risk of rhinitis medicamentosa.
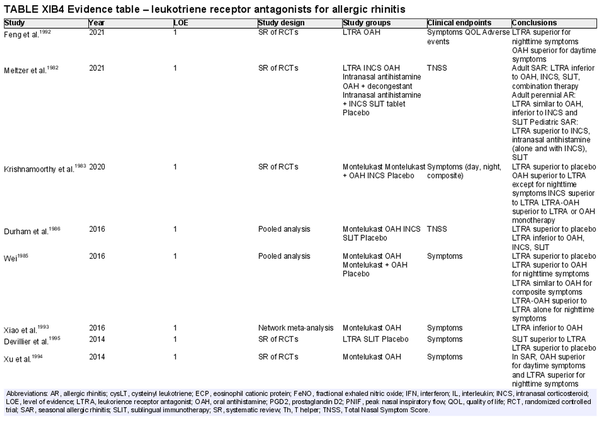
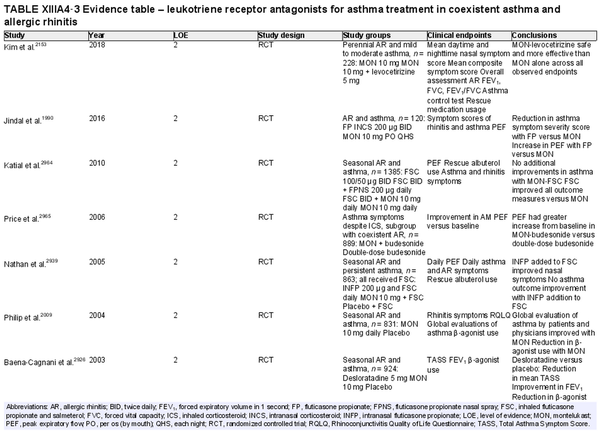
Leukotriene receptor antagonists (LTRA)
Aggregate grade of evidence: A (Level 1: 13 studies; level 2: 21 studies)
Benefit: Consistent reduction in symptoms and improvement in QOL compared to placebo.
Harm: United States Food and Drug Administration (FDA) boxed warning regarding neuropsychiatric side effects, including suicidal ideation. Consistently inferior compared to INCS at symptom reduction and improvement in QOL. Equivalent or inferior effect compared to oral antihistamines in symptom reduction and improvement of QOL. See Table II.C. in full ICAR document.
Cost: Moderate.
Benefits‐harm assessment: LTRAs are effective as monotherapy compared to placebo. However, there is a consistently inferior or equivalent effect to other, less expensive agents used as monotherapy. The FDA boxed warning is associated with LTRAs as well.
Value judgments: LTRAs are more effective than placebo at controlling both asthma and AR symptoms in patients with both conditions. However, in the light of significant concerns over its safety profile and the availability of effective alternatives such as INCS and oral antihistamines, evidence is lacking to recommend LTRAs as monotherapy in the management of AR.
Policy level: Recommendation against LTRAs as first‐line monotherapy for patients with AR. Option for LTRA as monotherapy in patients with contraindications to other preferred treatments.
Intervention: LTRAs should not be used as monotherapy in the treatment of AR but can be considered in select situations where patients have contraindications to alternative treatments.
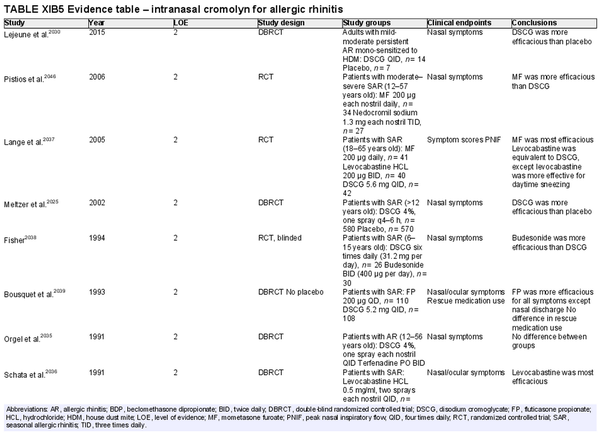
Intranasal cromolyn
Aggregate grade of evidence: A (Level 2: 25 studies)
Benefit: Disodium cromoglycate (DSCG) is effective in reducing sneezing, rhinorrhea, and nasal congestion.
Harm: Rare local side effects.
Cost: Low.
Benefits‐harm assessment: Preponderance of mild to moderate benefit over harm. Less effective than INCS and intranasal antihistamines.
Value judgments: DSCG is useful for preventative short‐term use in adult patients, children (2 years and older), and pregnant patients with known exposure risks.
Policy level: Recommendation as a second‐line treatment in AR.
Intervention: DSCG may be used as a second‐line treatment for AR in patients who fail INCS or intranasal antihistamines, or for short‐term preventative benefit prior to allergen exposures.
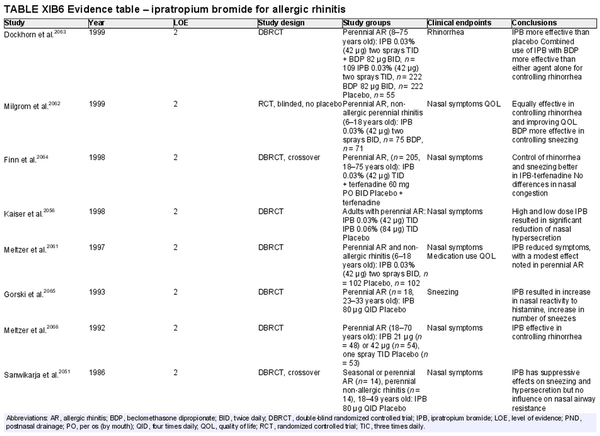
Intranasal anticholinergics (ipratropium bromide (IPB))
Aggregate grade of evidence: A (Level 2: 10 studies, level 3: 2 studies)
Benefit: Reduction of rhinorrhea with topical anticholinergics.
Harm: Care should be taken to avoid overdosage leading to systemic side effects. See Table II.C. in full ICAR document.
Cost: Low.
Benefits‐harm assessment: Preponderance of benefit over harm in AR patients with rhinorrhea.
Value judgments: Benefits limited to controlling rhinorrhea. Can be used as add on treatment for AR patients with persistent rhinorrhea despite first‐line medical management.
Policy level: Option.
Intervention: IPB nasal spray may be used as an adjunct medication to INCS in AR patients with persistent rhinorrhea.
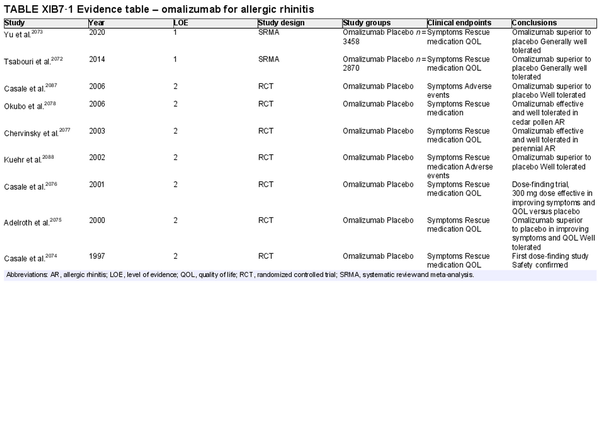
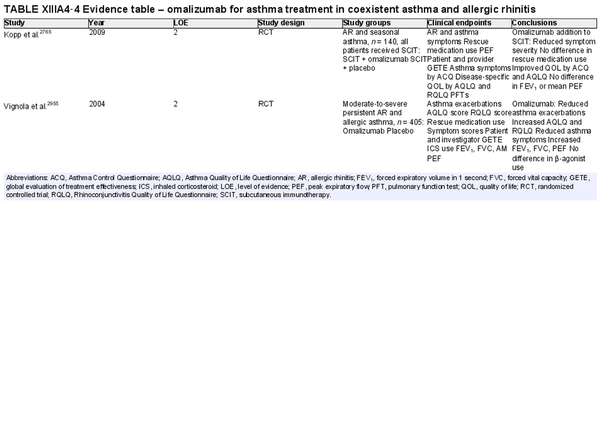
Biologic therapies
Aggregate grade of evidence: A (Level 1: 2 studies, level 2: 8 studies, level 3: 2 studies)
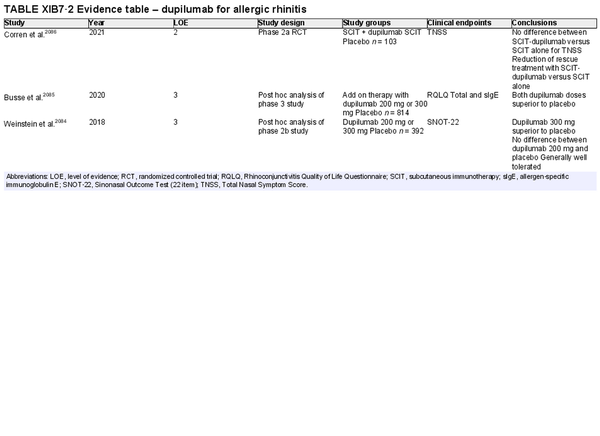
Benefit: Omalizumab treatment resulted in improvement of symptoms, rescue medication, and QOL as a monotherapy. Dupilumab data is less robust and needs further investigation.
Harm: Local reaction at injection site and risk of anaphylaxis.
Cost: High.
Benefits‐harm assessment: Benefit outweighs harm.
Value judgments: Biologic therapies show promise as a treatment option for AR; however, no biologic therapies have been approved by the US FDA for this indication.
Policy level: Option based upon published evidence, although not currently approved for this indication.
Intervention: Monoclonal antibody (biologic) therapies are not currently approved for the treatment of AR.
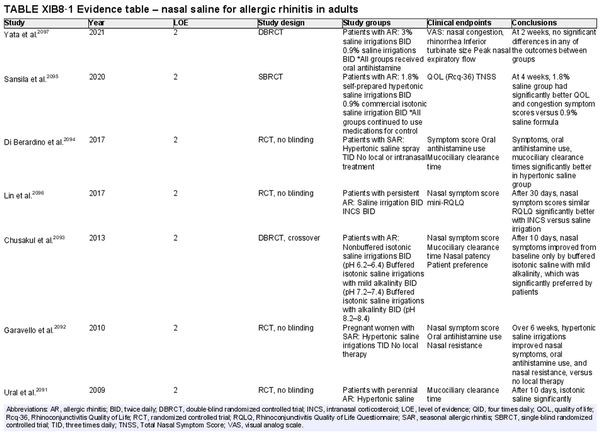
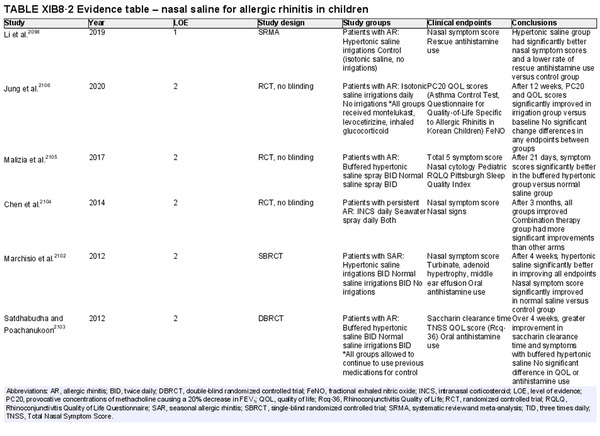
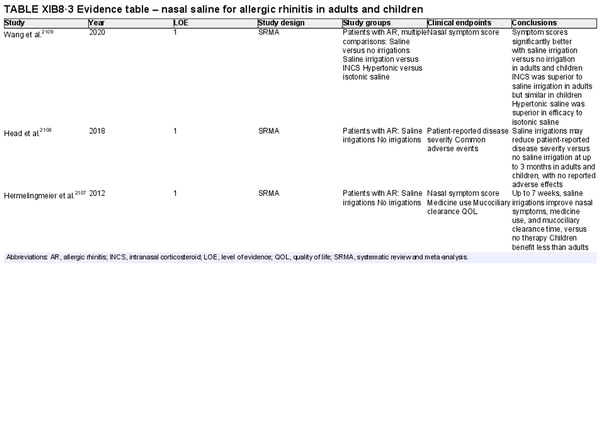
Intranasal saline
Aggregate grade of evidence: A (Level 1: 4 studies, level 2: 17 studies)
Benefit: Improved nasal symptoms and QOL, reduction in oral antihistamine use, and improved mucociliary clearance. Well‐tolerated with excellent safety profile.
Harm: Nasal irritation, sneezing, cough, and ear fullness. See Table II.C. in full ICAR document.
Cost: Minimal.
Benefits‐harm assessment: Preponderance of benefit over harm.
Value judgments: Nasal saline can and should be used as a first line treatment in patients with AR, either alone or combined with other pharmacologic treatments as evidence supports an additive effect. Hypertonic saline may be more effective in children. Data is otherwise inconclusive on optimal salinity, buffering, and frequency and volume of administration.
Policy level: Strong recommendation.
Intervention: Nasal saline is strongly recommended as part of the treatment strategy for AR.
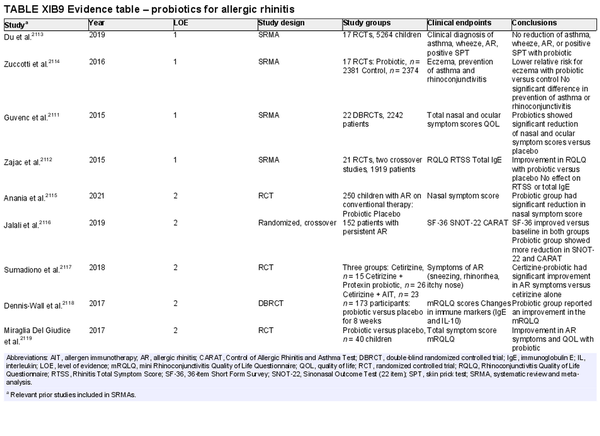
Probiotics
Aggregate grade of evidence: A (Level 1: 4 studies, level 2: 5 studies)
Benefit: Improved nasal/ocular symptoms or QOL in most studies.
Harm: Mild gastrointestinal side effects.
Cost: Low.
Benefits‐harm assessment: Balance of benefit and harm.
Value judgments: Minimal harm associated with probiotics. Heterogeneity across studies makes magnitude of benefit difficult to quantify. Variation in organism and dosing across trials prevents specific recommendations for treatment.
Policy level: Option.
Intervention: Consider adjuvant use of probiotics for patients with symptomatic seasonal or perennial AR.
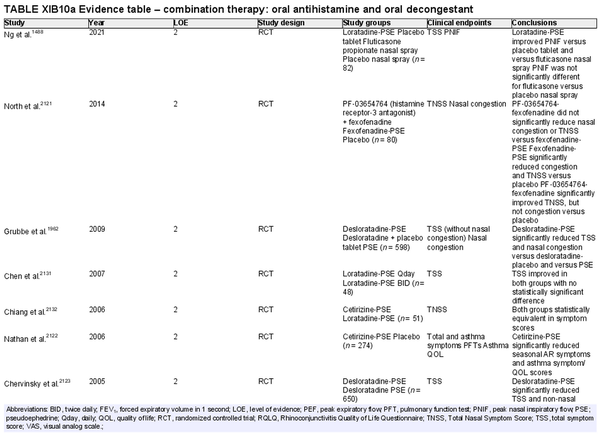
Combination oral antihistamine and oral decongestant
Aggregate grade of evidence: A (Level 2: 30 studies)
Benefit: Improved nasal congestion and total symptom scores with combination oral antihistamine‐oral decongestants.
Harm: Oral decongestants can cause adverse events in patients with cardiac conditions, hypertension, or benign prostatic hypertrophy and are not indicated in patients under age 12 or pregnant patients. Oral antihistamines are not indicated in patients under 2 years of age, and caution should be exercised in patients aged 2–5 years old. See Table II.C. in full ICAR document.
Cost: Low.
Benefits‐harm assessment: Combination oral antihistamine‐oral decongestant medications carry relatively low risks of adverse events when used as needed for episodic AR symptoms in well‐selected patients. Risk may be higher if used daily or in patients with certain comorbidities. There is not a preponderance of benefit or harm when used appropriately as a treatment option.
Value judgments: Oral antihistamine‐oral decongestants may be an effective option for acute AR symptoms such as nasal congestion and sneezing. Caution should be exercised with long‐term use.
Policy level: Option for episodic or acute AR symptoms.
Intervention: Combination oral antihistamine‐oral decongestant medications may provide effective relief of nasal symptoms of AR on an episodic basis. Caution should be exercised in chronic or long‐term use as the adverse effect profile of oral decongestants is greater for chronic use.
Combination oral antihistamine and intranasal corticosteroid
Aggregate grade of evidence: A (Level 1: 1 study, level 2: 12 studies)
Benefit: The addition of oral antihistamine to INCS has not consistently demonstrated a benefit over INCS alone for symptoms of AR.
Harm: Oral antihistamines generally not recommended in patients under 2 years old, and attention to dosing is necessary in patients 2–12 years old. See Table II.C. in full ICAR document.
Cost: Low.
Benefits‐harm assessment: Benefit likely outweighs potential harms in patients with significant nasal congestion symptoms in addition to symptoms such as sneezing and ocular itching. Addition of an INCS may be limited benefit versus potential harm in patients without significant nasal congestion symptoms.
Value judgments: Adding oral antihistamine to INCS spray has not been demonstrated to confer additional benefit over INCS spray alone. INCS improves congestion with or without oral antihistamine.
Policy level: Option.
Intervention: Current evidence is mixed to support antihistamines as an additive therapy to INCS, as several randomized trials have not demonstrated a benefit over INCS alone for symptoms of AR.
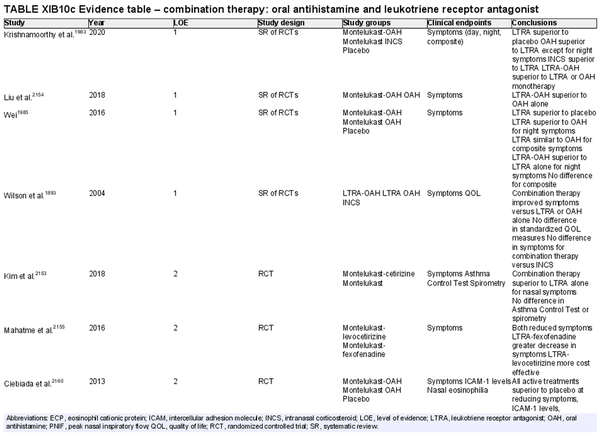
Combination oral antihistamine and leukotriene receptor antagonist
Aggregate grade of evidence: A (Level 1: 4 studies, level 2: 13 studies)
Benefit: Combination oral antihistamine‐LTRA was superior in symptom reduction and QOL improvement versus placebo and versus either agent as monotherapy.
Harm: FDA boxed warning due to risks of mental health side effects limiting use for AR. See Table II.C. in full ICAR document.
Cost: Generic montelukast added to generic loratadine or cetirizine is more expensive per month than generic fluticasone furoate nasal sprays, according to National Average Drug Acquisition Cost data provided by the Centers for Medicare and Medicaid Services.
Benefits‐harm assessment: Combination LTRA and oral antihistamine is superior to placebo, and superior to either agent as monotherapy. However, there is an inferior effect versus INCS, which is also less costly. In addition, there is a boxed warning associated with montelukast.
Value judgments: Combination therapy of LTRA and oral antihistamines is effective, but in light of concerns over the safety profile of montelukast, and the availability of effective alternatives such as INCS, evidence is lacking to recommend combination therapy in the management of AR.
Policy level: Recommendation against as first line therapy.
Intervention: Combination LTRA and oral antihistamines should not be used as first line therapy for AR but can be considered in patients with contraindications to other alternatives. This combination should be used judiciously after carefully weighing potential risks and benefits.
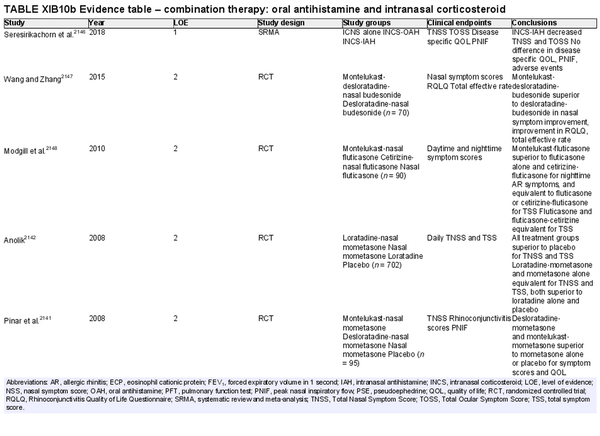
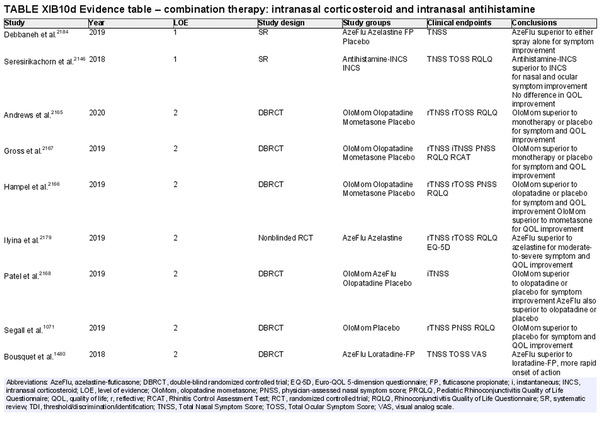
Combination intranasal corticosteroid and intranasal antihistamine
Aggregate grade of evidence: A (Level 1: 2 studies, level 2: 18 studies, level 4: 3 studies)
Benefit: Rapid onset; more effective for relief of multiple symptoms than either INCS or intranasal antihistamine alone.
Harm: Patient tolerance, especially due to taste. See Table II.C. in full ICAR document.
Cost: Moderate financial burden for combined formulation. Concurrent use of individual intranasal antihistamine and corticosteroid sprays is likely a more economical option.
Benefits‐harm assessment: Preponderance of benefit over harm. Combination therapy with intranasal antihistamine and INCS is consistently more effective than placebo or monotherapy. Low risk of non‐serious adverse effects.
Value judgments: High‐level evidence demonstrates that combination spray therapy with INCS plus intranasal antihistamine is more effective than monotherapy or placebo, as well as more effective than combination of INCS plus oral antihistamine. The increased financial cost and need for prescription limit the value of combination therapy as a routine first‐line treatment for AR. When a combined formulation is financially prohibitive, the concurrent use of two separate formulations (antihistamine and corticosteroid) is an alternative option.
Policy level: Strong recommendation for the treatment of AR when monotherapy fails to control symptoms.
Intervention: Combination therapy with INCS and intranasal antihistamine may be used as second‐line therapy in the treatment of AR when initial monotherapy with either INCS or antihistamine does not provide adequate control.
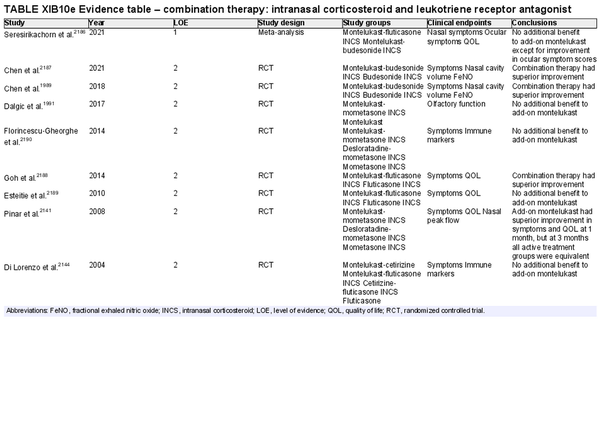
Combination intranasal corticosteroid and leukotriene receptor antagonist
Aggregate grade of evidence: B (Level 1: 1 study, level 2: 8 studies)
Benefit: Some studies demonstrate improvement of symptoms and QOL with combination therapy. One meta‐analysis did not show benefit with the exception of ocular itching.
Harm: Boxed warning due to risks of serious neuropsychiatric events for LTRA limiting use for AR. See Table II.C. in full ICAR document.
Cost: Low.
Benefits‐harm assessment: Boxed warning for AR limits use. If comorbid asthma and AR, treatment is an option with consideration of mental health risks.
Value judgments: Possibly useful for symptom control, especially in patients with comorbid asthma, however, boxed warning limits use in AR without asthma.
Policy level: Option as combination therapy if comorbid asthma present and mental health risks are considered. Not recommended for AR alone.
Intervention: Consider use in patients with AR and asthma, after weighing therapeutic benefits against risks of mental health adverse effects.
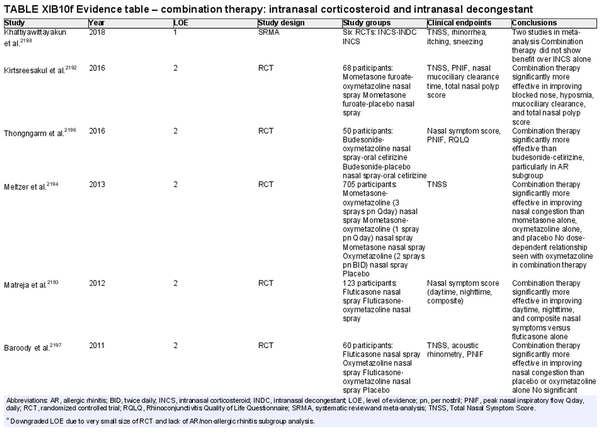
Combination intranasal corticosteroid and intranasal decongestant
Aggregate grade of evidence: B (Level 1: 1 study, level 2: 5 studies, level 3: 1 study)
Benefit: Some evidence in randomized studies of benefit from addition of intranasal decongestant to INCS therapy in refractory AR patients. The evidence regarding the magnitude of effect is unclear, and a meta‐analysis that tried to estimate this effect was significantly limited by study heterogeneity and low sample size (two trials).
Harm: See Table II.C. in full ICAR document.
Cost: Low.
Benefits‐harm assessment: Balance of benefit and harm with current evidence base.
Value judgments: While combination therapy of intranasal decongestant and INCS is superior to INCS therapy alone with low risk of tachyphylaxis in patients with refractory AR, the magnitude of effect is still unclear. There may be a role in patients with AR refractory to INCS and intranasal antihistamine combination therapy prior to consideration of surgery or in patients uninterested in surgery.
Policy level: Option.
Intervention: Short‐term combination therapy with INCS and intranasal decongestant may be considered in patients with AR refractory to combination therapy with INCS and intranasal antihistamine prior to consideration of inferior turbinate reduction or in patients declining surgery.
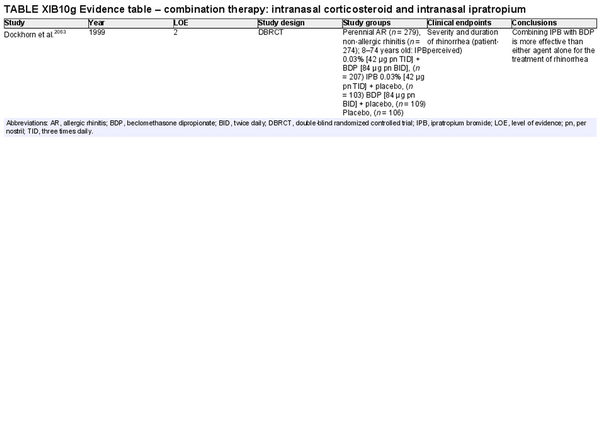
Combination intranasal corticosteroid and intranasal ipratropium bromide
Aggregate grade of evidence: Unable to determine based on one study. (Level 2: 1 study)
Benefit: Reduction of rhinorrhea in INCS‐treatment‐refractory AR.
Harm: Usually no systemic anticholinergic activity if administered intranasally in the recommended doses. See Table II.C. in full ICAR document.
Cost: Low.
Benefits‐harm assessment: Benefit for combined INCS and IPB therapy in patients with treatment refractory AR and the main symptom of rhinorrhea.
Value judgments: No evidence for benefit in controlling symptoms other than rhinorrhea. Evidence is limited, but results are encouraging for patients with persistent rhinorrhea.
Policy level: Option.
Intervention: Combining IPB with beclomethasone dipropionate can be more effective than either agent alone for the treatment of rhinorrhea in refractory AR in children and adults. Although multiple consensus guidelines have recommended, and there is evidence to support this recommendation, it is important to note that there has only been one randomized controlled trial (RCT) to study the efficacy of combined INCS and IPB therapy compared to either agent alone, and this study was performed in a combined population of patients with AR and non‐allergic rhinitis.
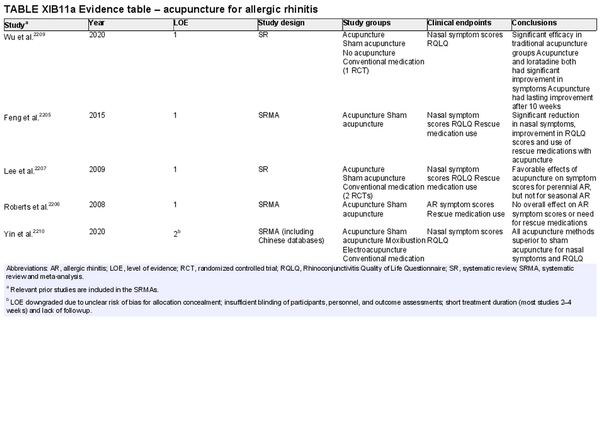
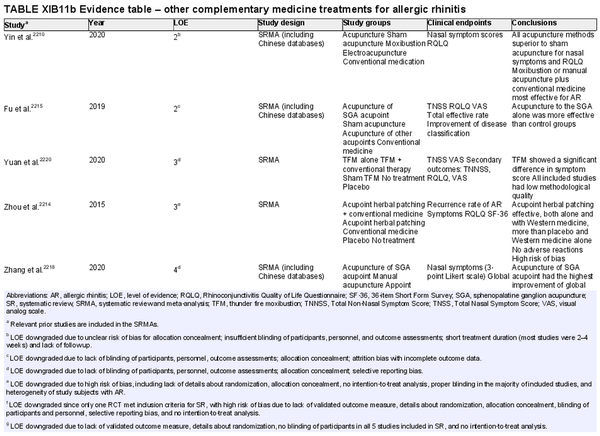
Acupuncture
Aggregate grade of evidence: A (Level 1: 4 studies, level 2: 1 study)
Benefit: Improvement of QOL and symptoms. Fairly well tolerated with no systemic adverse effects.
Harm: Needle sticks associated with minor adverse events including skin irritation, erythema, subcutaneous hemorrhage, pruritus, numbness, fainting, and headache. Electroacupuncture can interfere with pacemakers and other implantable devices. Caution is recommended in pregnant patients as some acupoints can theoretically induce labor. Need for multiple treatments and possible ongoing treatment to maintain any benefit gained. Relatively long treatment period.
Cost: Moderate‐high. Cost and time associated with acupuncture treatment; multiple treatments required.
Benefits‐harm assessment: Balance of benefit and harm.
Value judgments: The evidence is generally supportive of acupuncture. Acupuncture may be appropriate for some patients to consider as an adjunct/alternative therapy.
Policy level: Option.
Intervention: In patients who are interested in avoiding medications, acupuncture can be suggested as a possible therapeutic adjunct.
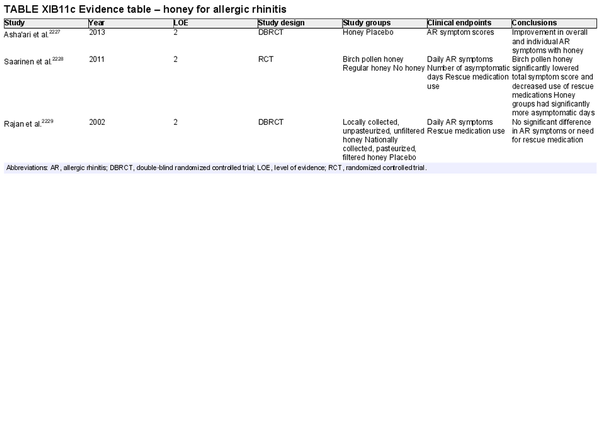
Honey
Aggregate grade of evidence: D (Level 2: 3 studies, conflicting evidence)
Benefit: Unclear as studies have shown differing results and include different preparations of honey in the trials. Local honey may be able to modulate symptoms and decrease need for antihistamines.
Harm: Potential compliance issues with patients not tolerating the level of sweetness. Potential risk of allergic reaction and rarely anaphylaxis. Caution should be exercised in in pre‐diabetics and diabetics for concern of elevated blood glucose levels.
Cost: Cost of honey and associated healthcare costs with increased consumption.
Benefits‐harm assessment: Balance of benefit and harm.
Value judgments: More studies are required before honey intake can be widely recommended.
Policy level: No recommendation.
Intervention: None.
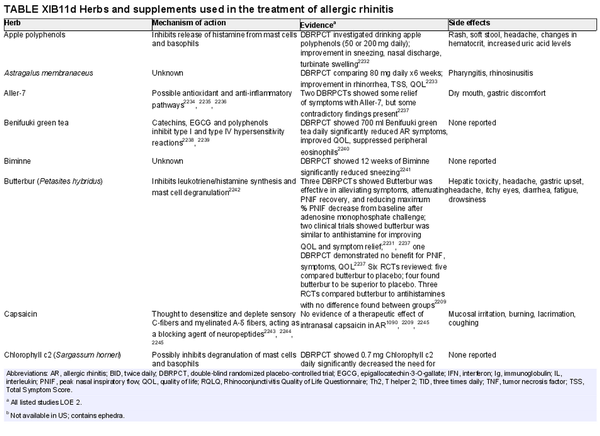
Herbal therapies
Aggregate grade of evidence: Uncertain.
Benefit: Unclear, but some herbs may be able to provide symptomatic relief.
Harm: Some herbs are associated with mild side effects. Also, the safety, quality, and standardization of herbal remedies and supplements are unclear.
Cost: Cost of herbal supplements.
Benefits‐harm assessment: Unknown.
Value judgments: There is a lack of sufficient evidence to recommend the use of herbal supplements in AR.
Policy level: No recommendation.
Intervention: None.
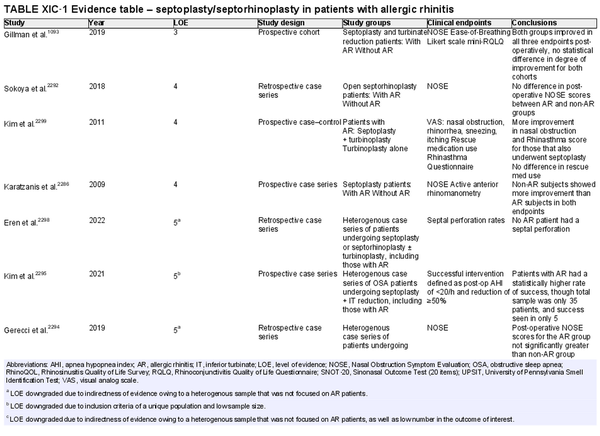
Septoplasty/septorhinoplasty
Aggregate grade of evidence: C (Level 3: 1 study, level 4: 3 studies, level 5: 11 studies)
Benefit: Improved postoperative symptoms and nasal airway.
Harm: Risk of complications (e.g., septal hematoma or perforation, nasal dryness, cerebrospinal fluid leak, epistaxis, unfavorable aesthetic change); persistent obstruction.
Cost: Surgical/procedural costs, time off from work.
Benefits‐harm assessment: Potential benefit must be weighed against low risk of harm and cost of procedure.
Value judgments: Properly selected patients with septal deviation impacting their nasal patency can experience improved nasal obstruction symptoms.
Policy level: Option for those with obstructive septal deviation.
Intervention: Septoplasty/septorhinoplasty may be considered in AR patients that have failed medical management and who have anatomic, obstructive features that may benefit from this intervention.
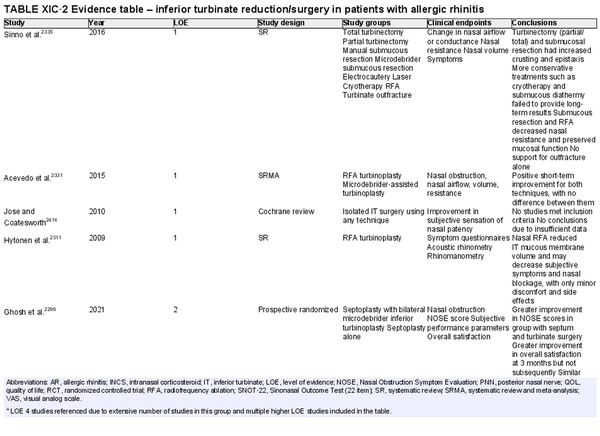
Inferior turbinate (IT) surgery
Aggregate grade of evidence: B (Level 1: 4 studies, level 2: 13 studies, level 3: 18 studies, level 4: 50 studies)
Benefit: Improvement in rhinitis symptoms including nasal breathing, congestion, sneezing, and itching. Improved nasal cavity area via objective measures, as well as increased QOL via subjective measures.
Harm: Risk of complications (e.g., swelling, crusting, empty nose syndrome, epistaxis).
Cost: Surgical/procedural costs, potential time off from work.
Benefits‐harm assessment: Potential benefit outweighs low risk of harm.
Value judgments: Current evidence suggests that patients with AR who suffer from IT hypertrophy will likely experience improvement in symptoms, nasal patency, and QOL.
Policy level: Recommendation in patients with medically refractory nasal obstruction.
Intervention: In AR patients with IT hypertrophy that have failed medical management, IT reduction is a safe and effective treatment to reduce symptoms and improve nasal function. More studies are warranted to directly compare IT surgery methods (e.g., radiofrequency ablation, laser‐assisted, microdebrider‐assisted) for the most efficacious and long‐lasting outcome.
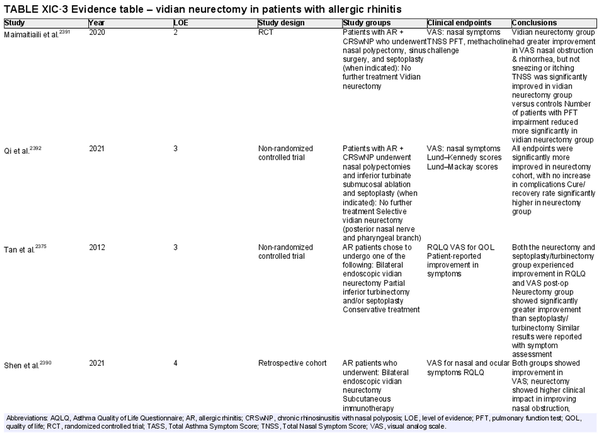
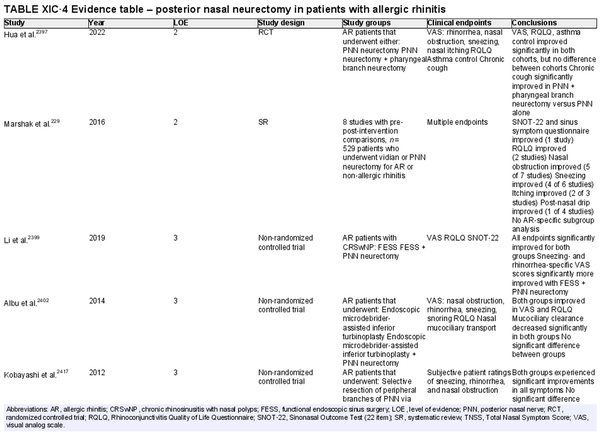
Vidian neurectomy, posterior nasal neurectomy
Aggregate grade of evidence: B (Level 2: 3 studies, level 3: 5 studies, level 4: 7 studies, level 5: 2 studies)
Benefit: Improvement in rhinorrhea.
Harm: Risk of complications (e.g., dry eye and decreased lacrimation, numbness in lip/palate, nasal dryness, damage to other nerves).
Cost: Surgical/procedural costs, potential time off from work.
Benefits‐harm assessment: Potential benefit must be balanced with low risk of harm but consider that long‐term results may be limited.
Value judgments: Patients may experience an improvement in symptoms.
Policy level: Option.
Intervention: Vidian neurectomy or posterior nasal neurectomy may be considered in AR patients that have failed medical management, particularly for rhinorrhea.
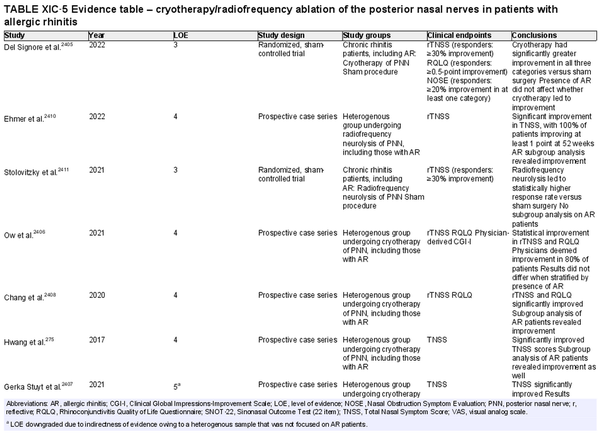
Cryotherapy/radiofrequency ablation of posterior nasal nerve
Aggregate grade of evidence: C (Level 3: 2 studies, level 4: 4 studies, level 5: 5 studies)
Benefit: Improvement in rhinorrhea.
Harm: Risk of complications (e.g., epistaxis, temporary facial pain and swelling, headaches), limited long‐term results.
Cost: Surgical/procedural costs, cost of device, potential time off from work.
Benefits‐harm assessment: Potential benefit must be balanced with low risk of harm, especially considering limited long‐term results.
Value judgments: Patients may experience an improvement in symptoms.
Policy level: Option.
Intervention: Cryoablation and radiofrequency ablation of the posterior nasal nerve may be considered in AR patients that have failed medical management, particularly for rhinorrhea.
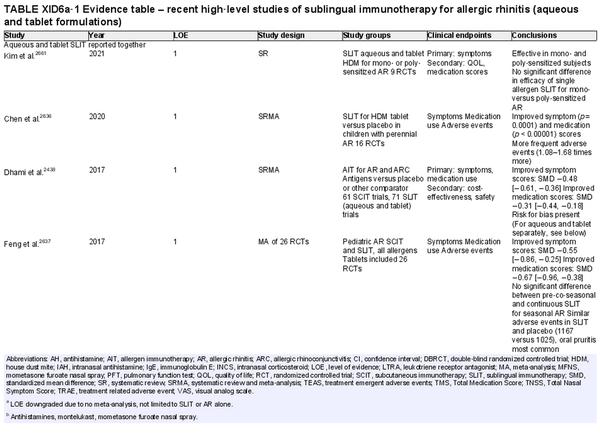
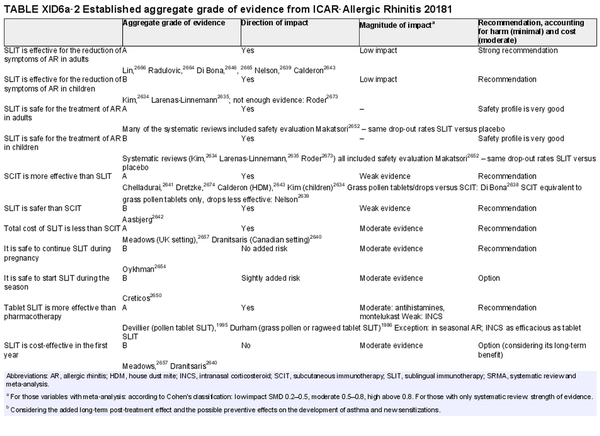
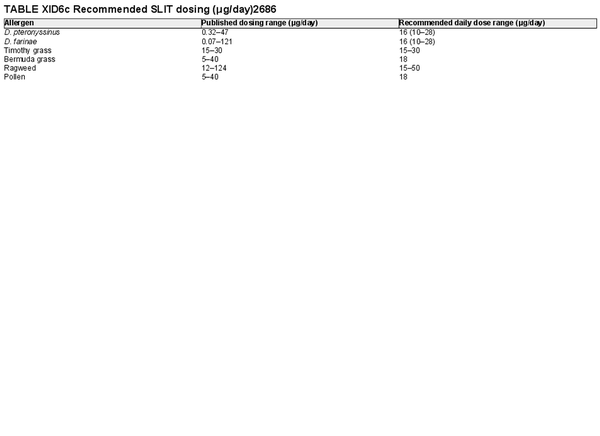
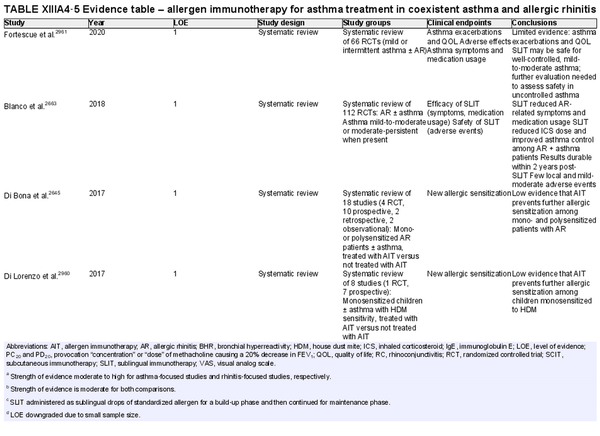
I.C.7.c Allergen immunotherapy
Unlike allergen avoidance, environmental controls, and pharmacotherapy, AIT has the benefit of initiating and sustaining immunologic alterations. Following AIT, which involves scheduled administration of allergen extracts at effective doses for a specified time frame, controlled trials demonstrate reduction in allergy symptoms and medication use.
The AIT portion of ICAR‐Allergic Rhinitis 2023 discusses AIT candidacy, benefits, and contraindications. Allergen units and standardization are addressed, along with allergen extract adjuvants and modified allergen extracts. Overall, there is high level evidence supporting the use of AIT for AR (Table I.C.7.c).
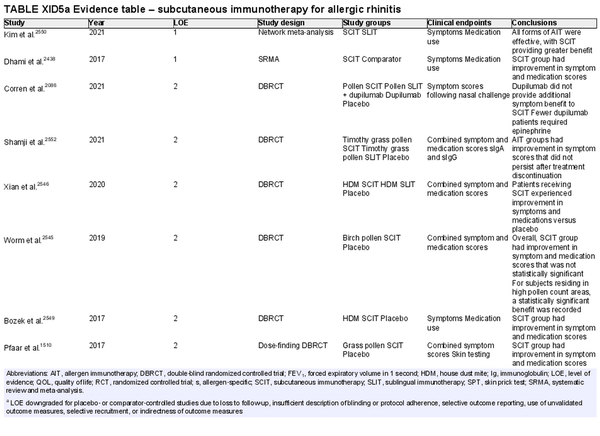
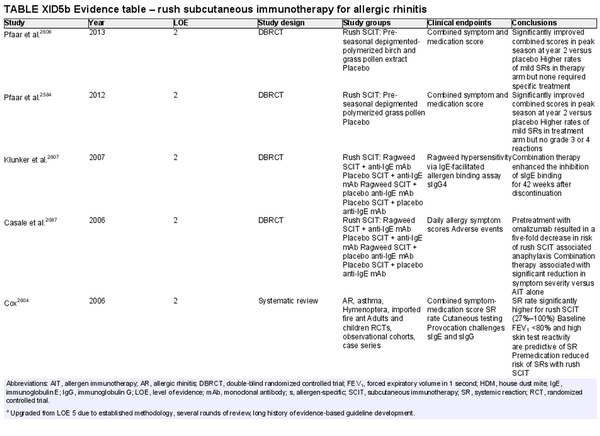
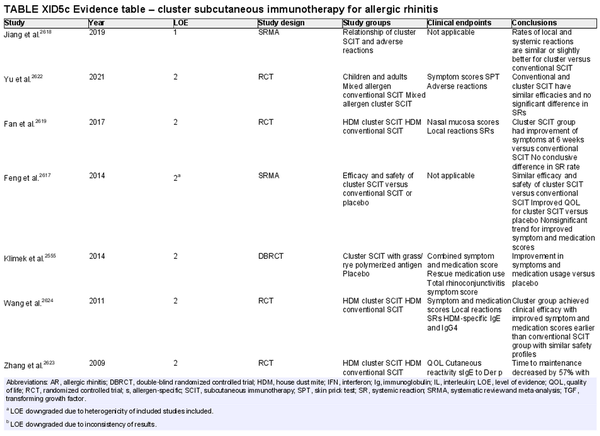
Conventional subcutaneous immunotherapy (SCIT)
Aggregate grade of evidence: A (Level 1: 2 studies, level 2: 46 studies, level 3: 29 studies)
Benefit: SCIT reduces symptom and medication use, as demonstrated in multiple high‐quality studies.
Harm: Risks of SCIT include frequent local reactions and rare systemic reactions, which may be severe and potentially fatal if not managed appropriately. This risk must be discussed with patients prior to initiation of therapy.
Cost: SCIT is cost‐effective, with some studies demonstrating value that dominates the alternative strategy with improved health outcomes at lower cost. Direct and indirect costs of AIT vary based on the third‐party payer, the office/region, co‐payment responsibilities, and travel/opportunity related costs in being able to adhere to the frequency of office visits required.
Benefits‐harm assessment: For patients with symptoms lasting longer than a few weeks per year and for those who cannot obtain adequate relief with symptomatic treatment or who prefer an immunomodulation option, benefits of SCIT outweigh harm. The potential benefit of secondary disease‐modifying effects, especially in children and adolescents, should be considered.
Value judgments: A patient preference‐sensitive approach to therapy is needed. Comparatively, the potential for harm and burden associated with medications are significantly lower, although the potential for benefit is also lower (with no potential for any disease‐modifying effect or long‐term benefit) as medications do not induce immunomodulation. Logistical issues surrounding time commitment involved with AIT may be prohibitive for some patients. The strength of evidence for SCIT efficacy, along with the benefit relative to cost, would support coverage by third party payers.
Policy level: Strong recommendation for SCIT as a patient preference‐sensitive option for the treatment of AR.
Strong recommendation for SCIT over no therapy for the treatment of AR.
Option for SCIT over sublingual immunotherapy (SLIT) for the treatment of AR.
Intervention: SCIT is an appropriate treatment consideration for patients who have not obtained adequate relief with symptomatic therapy or who prefer this therapy as a primary management option, require prolonged weeks of treatment during the year, and/or wish to start treatment for the benefit of the potential secondary disease‐modifying effects of SCIT.
Rush subcutaneous immunotherapy
Aggregate grade of evidence: B (Level 2: 12 studies, level 3: 4 studies, level 4: 4 studies)
Benefit: Accelerates the time to reach therapeutic dosing which may improve compliance, lead to earlier clinical benefit, and be more convenient for the patient. Improvement of symptoms and decreased need for rescue medication.
Harm: Higher rates of local and systemic reactions with rush SCIT protocols compared to conventional and cluster SCIT. Inconvenience of visits to a medical facility to receive injections.
Cost: Direct costs may be similar or slightly less compared to conventional SCIT, which includes cost of extract preparation and injection visits. Indirect costs are improved due to the reduced number of appointment visits, which reduces work and school absenteeism.
Benefits‐harm assessment: Balance of benefit and harm.
Value judgments: Careful patient selection and shared decision making would reduce risks. Heterogeneity of protocols, extract types, and dosing across studies makes quantification of risk difficult.
Policy level: Option.
Intervention: Aeroallergen rush SCIT is an option for AR in appropriately selected patients that do not have adequate control of their symptoms with symptomatic therapies. If available at practice location, the use of depigmented‐polymerized allergen extracts for rush SCIT has a better safety profile compared with standard extracts.
Cluster subcutaneous immunotherapy
Aggregate grade of evidence: B (Level 1: 1 study, level 2: 12 studies, level 4: 2 studies)
Benefit: Accelerates the time to reach therapeutic dosing which may improve compliance, lead to earlier clinical benefit, and be more convenient for the patient. Improvement of symptoms and decreased need for rescue medication. Similar safety profile compared to conventional SCIT.
Harm: Minimal harm with occasional, but mild, local adverse events and rare systemic adverse events when premedication is used. Inconvenience of visits to a medical facility to receive injections.
Cost: Direct costs may be similar, slightly more, or slightly less compared to conventional SCIT, depending on how the practicing provider bills for the services. This includes cost of extract preparation, injection visits, and possibly rapid desensitization codes. Indirect costs are lower due to the reduced number of appointment visits, which reduces work and school absenteeism.
Benefits‐harm assessment: Preponderance of benefit over harm for patients that cannot achieve adequate relief with symptomatic management. Balance of benefit and harm compared to conventional SCIT but in slight favor of cluster SCIT due to convenience.
Value judgments: Careful patient selection and shared decision making would reduce risks. Heterogeneity of protocols, extract types, and dosing across studies makes risk quantification difficult.
Policy level: Option.
Intervention: Cluster SCIT can be safely implemented in clinical practice and offered to those patients eligible for SCIT that may prefer this protocol compared to conventional build‐up protocols due to convenience. Premedication should be strongly considered.
Sublingual immunotherapy (SLIT): general considerations
Aggregate grade of evidence: A (Level 1: 17 studies, level 2: 12 studies, level 4: 1 study)
Due to heterogeneity of SLIT study reporting, it is difficult to separate out overall versus aqueous SLIT versus tablet SLIT.
Benefit: SLIT improves patient symptom scores, even as add‐on treatment with rescue medication. SLIT reduces medication use. The effect of SLIT lasts for at least 2 years after a 3‐year course of therapy. In AR patients, there is some evidence that SLIT reduces the frequency of onset of asthma and the development of new sensitizations up to 2 years after treatment termination. Benefit is generally higher than with single‐drug pharmacotherapy; however, it may be less than with SCIT (low quality evidence).
Harm: Minimal harm with very frequent, but mild local adverse events, and very rare systemic adverse events. SLIT seems to be safer than SCIT.
Cost: Intermediate. SLIT becomes cost‐effective compared to pharmacotherapy after several years of administration. Total costs seem to be lower than with SCIT.
Benefits‐harm assessment: Benefit of treatment over placebo is small but tangible and occurs in addition to improvement with medication. There is a lasting effect at least 2 years off treatment. Minimal harm with SLIT, greater risk for SCIT.
Value judgments: SLIT improved patient symptoms with low risk for adverse events.
Policy level: Strong recommendation for the use of SLIT grass pollen tablet, ragweed tablet, HDM tablet, and tree pollen aqueous solution. Recommendation for SLIT for Alternaria allergy. Option for SLIT for animal allergy. Recommendation for dual‐therapy SLIT in bi‐allergic patients.
Intervention: Recommend tablet or aqueous SLIT in patients (adults and children) with seasonal and/or perennial AR who wish to reduce their symptoms and medication use, as well as possibly reduce the propensity to develop asthma or new allergen sensitizations.
Sublingual immunotherapy tablets
Aggregate grade of evidence: A (Level 1: 11 studies, level 2: 4 studies)
Benefit: Improvement of symptoms, rescue medication, and QOL.
Harm: Local reaction at oral administration site and low risk of anaphylaxis.
Cost: Intermediate. More expensive than standard pharmacotherapy, but persistent benefit may result in cost‐saving in the long‐term.
Benefits‐harm assessment: Benefit outweighs harm.
Value judgments: Useful for patients with severe or refractory symptoms of AR.
Policy level: Strong recommendation.
Intervention: SLIT tablets are recommended for patients with severe or refractory AR. Epinephrine auto‐injector is recommended in the FDA labeling for approved tablets due to the rare but serious risk of anaphylaxis. Tablets for select antigens are available in various countries.
Aqueous sublingual immunotherapy
Aggregate grade of evidence: B (Level 1: 7 studies, level 2: 5 studies, level 4: 1 study)
Benefit: Aqueous SLIT improves patient symptom scores and decreases rescue medication use. There is some indication of less benefit from aqueous versus tablet SLIT, but the lack of standardized dosing across multiple trials does not allow for adequate comparison.
Harm: Common mild to moderate local adverse events. Very rare cases of systemic adverse events. No reported cases of life‐threatening reactions
Cost: Intermediate. More expensive than standard pharmacotherapy, but there are indications of lasting benefit and cost‐saving in the long‐term.
Benefits‐harm assessment: Appreciable benefit in patient symptoms and minimal harm.
Value judgments: Aqueous SLIT improves patient symptoms and rescue medication usage with minimal risk of serious adverse events but common local mild adverse events. Single allergen therapy has been extensively tested. Multiallergen AIT requires future studies to validate its use.
Policy level: Recommendation.
Intervention: High‐dose aqueous SLIT is recommended for those patients who wish to reduce their symptoms and rescue medication use.
Epicutaneous/transcutaneous immunotherapy
Aggregate grade of evidence: B (Level 2: 5 studies)
Benefit: Epicutaneous AIT to grass pollen resulted in limited and variable improvement in symptoms, medication use, and allergen provocation tests in patients with AR or conjunctivitis.
Harm: Epicutaneous AIT resulted in systemic and local reactions, with a relative risk of 4.65 and 2.29, respectively. Systemic reactions occurred in up to 14.6% of patients receiving grass transcutaneous AIT.
Cost: Unknown.
Benefits‐harm assessment: There is limited and inconsistent data on benefit of the treatment, while there is a concerning rate of adverse effects. Three out of 4 studies on this topic were published by the same investigators from 2009 to 2015.
Value judgments: Epicutaneous AIT could offer a potential alternative to SCIT and SLIT, but further research is needed.
Policy level: Recommendation against.
Intervention: While epicutaneous AIT may potentially have a future clinical application in the treatment of AR, at this juncture there are limited studies that show variable and limited effectiveness, and a significant rate of adverse reactions. Given the above and the availability of alternative treatments, epicutaneous AIT is not recommended at this time.
Intralymphatic immunotherapy
Aggregate grade of evidence: A (Level 1: 2 studies, level 2: 11 studies, level 4: 3 studies)
Benefit: Shorter treatment period, decreased number of injections, smaller amount of allergen, lower risk of adverse events versus SCIT.
Harm: Local reaction at injection site and risk of anaphylaxis.
Cost: Cost savings due to shorter treatment duration and fewer injections. Additional cost for training required.
Benefits‐harm assessment: Benefit outweighs harm.
Value judgments: Apparent short‐term favorable effect, but long‐term effect is lacking.
Policy level: Option.
Intervention: More studies are essential to establish the long‐term effects of ILIT.
Combination subcutaneous immunotherapy and biologics
Aggregate grade of evidence: B (Level 2: 5 studies)
Benefit: Improved safety of accelerated cluster and rush SCIT protocols, with decreased symptom and rescue medication scores among a carefully selected population.
Harm: Financial cost and low risk of anaphylactic reactions to omalizumab.
Cost: Moderate to high.
Benefits‐harm assessment: Preponderance of benefit over harm.
Value judgments: Combination therapy increases the safety of SCIT, with decreased systemic reactions following cluster and rush protocols. Associated treatment costs must be considered. While two high‐quality RCTs have demonstrated improved symptom control with combination therapy over SCIT or anti‐IgE alone, not all patients will require this approach. Rather, an individualized approach to patient management must be considered, with evaluation of alternative causes for persistent symptoms, such as unidentified allergen sensitivity. Also, the studies did not compare optimal medical treatment of AR (INCS, antihistamine, allergen avoidance measures) to combination therapy versus SCIT alone. The current evidence does not support the utilization of combination therapy for all patients failing to benefit from SCIT alone.
Policy level: Option.
Intervention: Current evidence supports that anti‐IgE may be beneficial as a premedication prior to induction of cluster or rush SCIT protocols, and combination therapy may be advantageous as an option for carefully selected patients with persistent symptomatic AR following AIT. However, at the time of this writing, biologic therapies are not approved by the US FDA for AR alone. An individualized approach to patient management must be considered.
I.C.8 Pediatric considerations
The pediatric section is a new addition for ICAR‐Allergic Rhinitis 2023 and encompasses several literature reviews. AR takes a few years to develop in children. A family history of AR, atopy, or asthma is important to discuss as children may be at an increased risk of developing AR or other allergic diseases. The “allergic march,” described as a specific sequence of atopic disorders, should be considered in children with clinical suspicion. Diagnosis may be challenging in the pediatric population, and some diagnostic clues include chapped lips from mouth breathing, fatigue, irritability, poor appetite, and attention issues. Physical exam findings include posterior pharyngeal cobblestoning, clear nasal drainage, and enlarged/boggy inferior turbinates, “allergic” or “adenoid” facies, the allergic salute, allergic crease, allergic shiners, or Dennie–Morgan lines. The diagnosis of AR in children should be based on both clinical history and testing. SPT is generally accepted as the preferred method of testing in children. Treatment options for children under age 2 are limited. For older children, treatment options are similar to the adult population. AIT is also an option for children with persistent symptoms. AIT may reduce the risk of asthma development in pediatric patients with AR.
I.C.9 Associated conditions
There is evidence for the association of several comorbid conditions with AR, which are listed in Table I.C.9. Several additional conditions have been added since ICAR‐Allergic Rhinitis 2018.
I.C.10 Special section on COVID‐19
Coronavirus disease 2019 (COVID‐19) case rates have changed practice strategies. AR has not been identified as a risk factor for severe COVID‐19. However, there have been challenges with overlapping symptoms of AR and COVID‐19. Telemedicine visits have been helpful for initial evaluation; however, many diagnostic techniques for AR require face‐to‐face encounters. Recommendations have continued to evolve during the pandemic. Standard therapies for AR were not shown to increase the risk of severe COVID‐19. Additionally, anti‐IgE therapy has not increased susceptibility or severity of COVID‐19 infection.
I.C.11 Summary figure for allergic rhinitis diagnosis and management
See Figure I.C.11 for summary diagnosis and management options for AR, based upon current evidence.
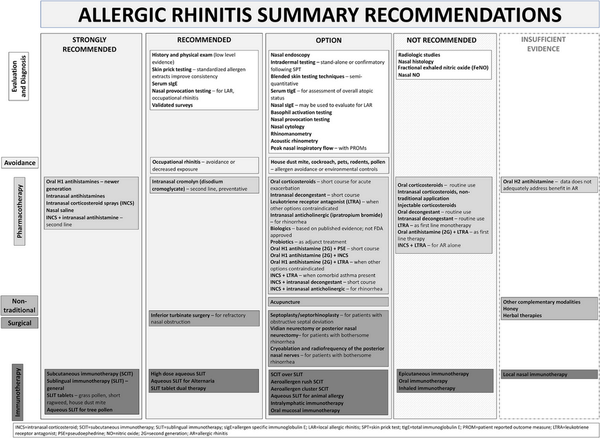
FIGURE I
C.11. Allergic Rhinitis Summary Recommendations
I.C.12 Knowledge gaps
Evidence in the realm of AR continues to grow at a steady pace. We have seen substantial progress in many aspects of the AR literature in recent years. However, several knowledge gaps remain. Table I.C.12. lists knowledge gaps and future research needs that have been identified as a result of the work in ICAR‐Allergic Rhinitis 2023.
I.D Discussion
In the executive summary for ICAR‐Allergic Rhinitis 2023, we highlight the current evidence levels and recommendations (where applicable) for AR diagnosis, management, and associated conditions. Over 40 new topics have been added to this evidence‐based assessment since the initial ICAR‐Allergic Rhinitis 2018 publication. In many individual topic areas, numerous additional studies were identified and evaluated. In certain cases, the recommendation level changed. While these advances in our current literature are exciting, there are several knowledge gaps that remain – and there is still work to be done to further our understanding of various aspects of AR pathophysiology, epidemiology, disease burden, diagnosis, management, and associated conditions.
I.E Lay summary
The International Consensus Statement on Allergy and Rhinology: Allergic Rhinitis 2023
ICAR‐Allergic Rhinitis 2023 contains the most complete and up‐to‐date information on how allergic rhinitis develops, how medical teams can identify it, how it may be treated, and other conditions that can be seen with allergic rhinitis. The document has been written and reviewed by a large group of medical and research experts from around the world. ICAR‐Allergic Rhinitis 2023 may be used by medical providers who treat allergic rhinitis.
What is allergic rhinitis?
Allergic rhinitis is a reaction that occurs from substances that we breathe in from the environment. Patients often have drainage and blockage from their nose, along with sneezing and itching. While there are many possible causes of these symptoms, allergic rhinitis is due to a specific trigger in the environment that the body is sensitive to. Allergic rhinitis may be associated with other diseases, such as asthma, sleep problems, sinus and ear problems, cough, and more.
How common is allergic rhinitis?
Allergic rhinitis is a common problem. Depending on the specific research study and the location where the study is done, allergic rhinitis has been reported in 5%–50% of the population. It is more common in children.
How severe is allergic rhinitis?
Allergic rhinitis can affect quality of life. It may also interrupt sleep. Allergic rhinitis medicines, other treatments, and medical visits cost money directly. There are added costs related to missing work or school – or not functioning as well at work. Research suggests that treating allergic rhinitis helps improve overall quality of life and sleep.
How is allergic rhinitis treated?
People may avoid their allergic triggers if they are aware of the specific things that they react to – and if these things can be easily avoided. Using different types of medications can also help control allergic symptoms. Immunotherapy, such as allergy shots or drops/tablets under the tongue, introduces the known allergen to the body in small amounts at first. Over time, the body will not react to the allergen. There are also some procedures and surgeries that can decrease drainage from the nose or improve breathing through the nose.
What disorders are associated with allergic rhinitis?
Asthma, atopic dermatitis (a condition of the skin), eye symptoms, food allergies, and sleep problems are all associated with allergic rhinitis. Some studies report that certain ear issues and sinus problems may be related to allergic rhinitis, although more studies should be done to understand these better.
II TABLE OF CONTENTS AND NAVIGATING THROUGH THE DOCUMENT
II.A Detailed table of contents
II.B List of abbreviations
AAO‐HNSF: American Academy of Otolaryngology‐Head and Neck Surgery Foundation
AAP: American Academy of Pediatrics
AC: allergic conjunctivitis
ACC: allergen challenge chamber
ACEI: angiotensin converting enzyme inhibitors
AD: atopic dermatitis
AERD: aspirin‐exacerbated respiratory disease
AFRS: allergic fungal rhinosinusitis
AH: adenoid hypertrophy
AHI: apnea‐hypopnea index
AIDS: acquired immunodeficiency syndrome
AIT: allergen‐specific immunotherapy
ANA: antinuclear antibody
ANCA: anti‐neutrophil cytoplasmic antibody
AP: activator protein
AR: allergic rhinitis
ARIA: Allergic Rhinitis and its Impact on Asthma
ARS: acute rhinosinusitis
ASHMI: Anti‐Asthma Simplified Herbal Medicine Intervention
ATH: adenotonsillar hypertrophy
AU: allergy units
BAT: basophil activation test
BAU: biologic allergy units
cAMP: cyclic adenosine monophosphate
CBER: Center for Biologics Evaluation and Research
CC: central compartment
CCAD: central compartment atopic disease
CCL5: C‐C chemokine ligand‐5
CD: cluster of differentiation
CDC: Centers for Disease Control
cGMP: cyclic guanosine monophosphate
CGRP: calcitonin gene‐related protein
CI: confidence interval
CMV: cytomegalovirus
COPD: chronic obstructive pulmonary disease
COVID: coronavirus disease
COX: cyclooxygenase
CPAP: continuous positive airway pressure
CPT: conjunctival provocation test
CRD: component‐resolved diagnostics
CRS: chronic rhinosinusitis
CRSsNP: chronic rhinosinusitis without nasal polyps
CRSwNP: chronic rhinosinusitis with nasal polyps
CS: combined score
CSF: cerebrospinal fluid
CT: computed tomography
DAMP: damage‐associated molecular pattern
DSCG: disodium cromoglycate
dsDNA: double stranded DNA
EAACI: European Academy of Allergy and Clinical Immunology
EBRR: evidence‐based review with recommendations
ECHRS: European Community Respiratory Health Survey
ECP: eosinophil cationic protein
EEC: environmental exposure chamber
EGPA: eosinophilic granulomatosis with polyangiitis
EGR: early growth response
ELISA: enzyme‐linked immunosorbent assay
eNOS: endothelial nitric oxide synthase
ENS: empty nose syndrome
EoE: eosinophilic esophagitis
ET: Eustachian tube
ETD: Eustachian tube dysfunction
FDA: Food and Drug Administration
FeNO: fractional exhaled nitric oxide
FEV1: forced expiratory volume in 1 second
FITC: fluorescein isothiocyanate
FOXP3: forkhead‐box P3
GA2LEN: Global Allergy and Asthma European Network
GATA: GATA binding protein
GINA: Global Initiative for Asthma
GITRL: glucocorticoid‐induced TNF receptor ligand
GM‐CSF: granulocyte‐macrophage colony stimulating factor
GPA: granulomatosis with polyangiitis
GWAS: genome‐wide association studies
HDM: house dust mite
HEPA: high‐efficiency particulate air [filtration]
HIV: human immunodeficiency virus
HMGB‐1: high mobility group box‐1
HMW: high molecular weight
HNS: head and neck surgery
HSP: heat shock protein
ICAM: intercellular adhesion molecule
ICAR: International Consensus Statement on Allergy and Rhinology
ICD: International Classification of Disease
IDT: intradermal dilutional testing
IFN: interferon
Ig: immunoglobulin
IgE: immunoglobulin E
IL: interleukin
ILC: innate lymphoid cell
ILIT: intralymphatic immunotherapy
IMAP: inferior meatus augmentation procedure
INCS: intranasal corticosteroid
INDC: intranasal decongestant
iNOS: inducible nitric oxide synthase
IPB: ipratropium bromide
ISAAC: International Studies of Asthma and Allergies in Childhood
IT: inferior turbinate
ITAM: immunoreceptor tyrosine‐based activation motif
KNHANES: South Korean National Health and Nutrition Examination Survey
LAR: local allergic rhinitis
LMW: low molecular weight
LOE: level of evidence
LPR: laryngopharyngeal reflux
LSR: lipolysis‐stimulated lipoprotein receptor
LTRA: leukotriene receptor antagonist
MBP: major basic protein
MCP: monocyte chemoattractant protein
MD: molecular diagnostics
MEE: middle ear effusion
MMP: matrix metalloproteinase
MQT: modified quantitative testing
mRQLQ: mini‐Rhinoconjunctivitis Quality of Life Questionnaire
MT: middle turbinate
NARES: non‐allergic rhinitis with eosinophilia syndrome
NC: nasal cytology
NF: nuclear factor
NFAT: nuclear factor of activated T cells
NGF: neural growth factor
NH: nasal histology
NHANES: National Health and Nutrition Examination Survey
NK: natural killer
nNO: nasal nitric oxide
nNOS: neuronal nitric oxide synthase
NO: nitric oxide
NOS: nitric oxide synthase
NOSE: Nasal Obstruction Symptom Evaluation
NPT: nasal provocation test
NPV: negative predictive value
NSAID: non‐steroidal anti‐inflammatory drug
OAS: oral allergy syndrome
OME: otitis media with effusion
OMIT: oral mucosal immunotherapy
OR: odds ratio
OSA: obstructive sleep apnea
PAMD@: precision allergy molecular diagnostic applications
PAMP: pathogen‐associated molecular pattern
PDE: phosphodiesterase
PEF: peak expiratory flow
PFAS: pollen food allergy syndrome
PFT: pulmonary function test
PG: prostaglandin
PM: particulate matter
PNEF: peak nasal expiratory flow
PNIF: peak nasal inspiratory flow
PNN: posterior nasal nerve
PO: per os (by mouth)
ppb: parts per billion
ppm: parts per million
PPV: positive predictive value
4PR: four‐phase rhinomanometry
PROM: patient reported outcome measure
PRQLQ: Pediatric Rhinoconjunctivitis Quality of Life Questionnaire
PSG: polysomnogram
QALY: quality adjusted life year
QID: four times daily
QOL: quality of life
RANTES: regulated upon activation, normal T cell expressed and presumably secreted
RAP: Respiratory Allergy Prediction
RAPP: RhinAsthma Patient Perspectives
RARS: recurrent acute rhinosinusitis
RAST: radio allegro‐sorbent test
RCT: randomized controlled trial
RDI: respiratory disturbance index
REM: rapid eye movement
RMS: rescue medication score
RQLQ: Rhinoconjunctivitis Quality of Life Questionnaire
RR: relative risk
RSDI: Rhinosinusitis Disability Index
RTSS: Rhinitis Total Symptom Score
SARS‐CoV‐2: virus that causes COVID‐19
SCIT: subcutaneous immunotherapy
SDB: sleep disordered breathing
SES: socioeconomic status
sIgE: allergen‐specific immunoglobulin E
sIgG: allergen‐specific immunoglobulin G
SLIT: sublingual immunotherapy
SMA: smooth muscle actin
SMD: standardized mean difference
SNHL: sensorineural hearing loss
SNOT: SinoNasal Outcome Test
SNP: single nucleotide polymorphism
SPT: skin prick test
SRMA: systematic review and meta‐analysis
STAT: signal transducer and activator of transcription
TARC: thymus and activation‐regulated chemokine
TCM: Traditional Chinese Medicine
TGF: transforming growth factor
Th: T helper
tIgE: total immunoglobulin E
TJ: tight junction
TL1A: tumor necrosis factor‐like cytokine 1A
TLR: toll‐like receptor
TNF: tumor necrosis factor
TNSS: Total Nasal Symptom Score
TOSS: Total Ocular Symptom Score
TPRV: transient receptor potential vanilloid
Treg: T regulatory cell
TRP: transient receptor potential
TSLP: thymic stromal lymphopoietin
TSS: total symptom score
UK: United Kingdom
US: Unites States
VAS: visual analog scale
VCAM: vascular cell adhesion molecule
VCOS: validated clinical outcome survey
VD3: vitamin D
VDR: vitamin D receptor
VHI: voice handicap index
WAO: World Allergy Organization
WHO: World Health Organization
ZO: zonula occludens
II.C Possible adverse effects of common allergic rhinitis treatments
Various aspects of the International Consensus Statement on Allergy and Rhinology (ICAR): Allergic Rhinitis (ICAR‐Allergic Rhinitis) 2023 document include possible side effects or treatment risks of interventions under consideration. In order to standardize listing of these potential side effects and treatment risks within the document text and recommendation summaries, Table II.C. defines known and typical side effects and adverse effects for commonly utilized treatment modalities that should be considered when determining policy level recommendations. Table II.C. may not include all possible risks of listed interventions.
III INTRODUCTION
The original ICAR‐Allergic Rhinitis 2018 document was developed to summarize and critically review the best available evidence for allergic rhinitis (AR), including major content areas of epidemiology, risk factors, diagnosis, management, conditions associated with AR, and others. Since the publication of ICAR‐Allergic Rhinitis 2018, the AR literature has continued to grow. We previously reported that there were 8212 publications related to AR between 2010 and the final writing of ICAR‐Allergic Rhinitis 2018. Between 2018 and June 2022, 5803 additional AR publications have been logged in PubMed. The methodology, results, evidence levels, and quality of scientific publications vary widely, and it can be challenging to distill important findings from such a large body of work. ICAR‐Allergic Rhinitis 2023 aims to evaluate and summarize the AR evidence for each topic in a succinct format to provide the clinician, researcher, or medical professional with a reference document that contains useful, relevant information. Given the recent expansion of the AR literature, an update of the original ICAR‐Allergic Rhinitis 2018 document was deemed appropriate.
When evaluating a scientific publication, it is important to critically assess the study methods and presentation of results, as these contribute to the evidence levels and ultimate recommendations for patient care. ICAR‐Allergic Rhinitis 2023 aims to incorporate new high‐level evidence into an updated document and utilizes this evidence, along with assessment of benefit, harm, and cost to determine recommendations for AR diagnostic and management strategies, where appropriate. ICAR‐Allergic Rhinitis 2023 follows previously developed methodology that has produced multiple evidence‐based reviews with recommendations (EBRR) in the International Forum of Allergy and Rhinology, as well as several ICAR documents, including those covering topics of AR, rhinosinusitis, endoscopic skull base surgery, and olfaction., , , ,
ICAR‐Allergic Rhinitis 2023 was created by conducting systematic literature searches on 144 individual AR topics, by 87 primary authors and 40 additional consultant authors. Over 40 new topics have been added for this
ICAR‐Allergic Rhinitis update, and the number of cited references has expanded by over 1400. Like previous ICAR documents, structured grading of evidence was performed, recommendations were created where appropriate, and each section underwent stepwise semi‐blinded iterative review (blinded for initial peer review then un‐blinded to reach consensus). Finally, a panel of editors critiqued each major content area, and the collated manuscript was circulated to all authors for review. The EBRR and ICAR methodology appears to be effective and robust and continues to be used regularly in evaluation of the rhinology and allergy literature.
Throughout the ICAR‐Allergic Rhinitis 2023 document, it is evident that many AR topics have grown in literature citations compared to 2018. This may be noted by a simple increase in the number of publications; however, the reader will also recognize that many topic areas contain new systematic reviews and meta‐analyses (SRMA) that have been published since ICAR‐Allergic Rhinitis 2018. This is an exciting development, as SRMAs represent the highest level of evidence and, when performed with robust methodology, collate the available evidence into a single report that should be easily understood by the reader. Still, while some areas of AR have very strong evidence, others are lacking in high‐level evidence.
It is important to recognize the limitations of ICAR‐Allergic Rhinitis 2023. Recommendations in this document are based on the available evidence. Each recommendation is only as strong as the evidence that supports it and the population/sample included in the studies. Practicing evidence‐based medicine takes into account the available evidence, along with clinical expertise and the patient's values and expectations. ICAR‐Allergic Rhinitis 2023 presents evidence‐based recommendations, but it is not a manual, flowchart, or algorithm for care of an individual AR patient. The clinician should continue to evaluate and treat each AR patient individually, using an evidence‐based foundation combined with clinical acumen/expertise and consideration of patient values and principles. Recommendations in ICAR‐Allergic Rhinitis 2023, as in previous ICAR documents, do not define the standard of care or medical necessity, nor do they dictate the care of individual patients.
Through the ICAR‐Allergic Rhinitis 2023 process, several gaps in knowledge have been identified and may encourage further research in AR. Additionally, some evidence grades have changed since 2018, and we anticipate that we will continue to see evidence grow and evolve in the future. Ultimately, improved patient outcomes should result as we continue to evaluate the growing body of AR literature.
IV METHODS
IV.A Topic development
The methods of ICAR‐Allergic Rhinitis 2023 largely follow previous ICAR documents,, , with utmost reliance on published evidence and minimal influence of expert opinion and other biases. The 2011 EBRR method described by Rudmik and Smith is the foundation of ICAR and aims to evaluate existing literature on each AR topic, grade the evidence, and provide literature‐based recommendations where appropriate.
To complete ICAR‐Allergic Rhinitis 2023, the subject of AR was initially divided into 144 individual topics, representing 41 additional topics compared to ICAR‐Allergic Rhinitis 2018. A primary author who is a recognized expert in allergy, rhinology, or the designated topic was assigned to evaluate each topic. Authors were initially selected via online literature searches for each ICAR‐Allergic Rhinitis 2023 topic. Authors of high‐quality publications in each topic area were invited as ICAR contributors. Other invited authors included experts in the EBRR process, experts in education on specific AR topic areas, and those with knowledge of the systematic review process. The invited primary author was able to choose a secondary/consultant author for each section if desired.
Certain topics, such as those providing background or definitions, were assigned as literature reviews without evidence grades or recommendations. Some topics were not appropriate for clinical recommendations and were assigned as evidence‐based reviews without recommendations (EBRs). Topics that had evidence to inform clinical recommendations were assigned as EBRRs. For topics included in ICAR‐Allergic Rhinitis 2018, the author was instructed to perform a new literature search and include updated evidence since the previous ICAR‐Allergic Rhinitis document as well as any other relevant studies previously published. Aggregate grades of evidence and recommendations summaries were updated accordingly.
Creation of the content for each individual AR topic area began with a literature search. Authors received instructions to perform a systematic review of the literature for each topic area based upon Preferred Reporting Items for Systematic Reviews and Meta‐analyses (PRISMA) standardized guidelines. Ovid MEDLINE (1947‐2021), EMBASE (1974‐2021), and Cochrane Review databases were included. The search began by identifying any previously published systematic reviews or guidelines pertaining to the assigned topic. Since clinical recommendations are best supported by high quality evidence, the search focused on identifying randomized controlled trials (RCT) and meta‐analyses of RCTs to provide the highest level of evidence (LOE). Reference lists of all identified studies were examined to ensure all relevant studies were captured. If the authors felt that a non‐English study should be included in the review, it was instructed that the paper be appropriately translated to minimize the risk of missing important data during the development of recommendations.
To optimize transparency of the evidence, all included studies in EBR and EBRR topic sections are presented in a standardized table format and the quality of each study was evaluated to receive a level based on the Oxford LOEs (level 1–5, Table IV.A.‐1). Adjustments were made to the LOE due the quality of each study based on accepted standards, with specific changes often highlighted in the text or evidence tables. At the completion of the systematic review and research quality evaluation for each EBR or EBRR topic, an aggregate grade of evidence (A–D) was produced for the topic based on the guidelines from the American Academy of Pediatrics (AAP) Steering Committee on Quality Improvement and Management (Table IV.A.‐2). For AR topics that addressed a diagnostic or therapeutic intervention and contained evidence to appropriately support formulation of a recommendation, the AAP guidelines for recommendation development were followed, thus completing the EBRR process (Table IV.A.‐3). Each evidence‐based recommendation was formulated with consideration of the aggregate grade of evidence, benefit, harm, and cost. A summary of the EBRR topic development process is provided in Figure IV.A.
It is important to note that assignment of LOE for each publication is not always straightforward. In some instances, individual studies do not fit neatly into one of the Oxford LOE categories. Also, Oxford LOE grading has changed over time, adding complexity to the evidence grading when undertaking updates such as this one. This becomes even more difficult when evaluating certain documents that employ advanced systematic evidence searches to formulate guidelines, practice parameters, position papers, and recommendation documents (e.g., Clinical Practice Guidelines, ICAR statements, European Position Statements on Sinusitis). In these instances, even methodological experts may disagree on evidence levels – some seeing the document as a systematic review with a high evidence level, while others would assign a lower LOE typical of a consensus statement, guideline, or expert opinion. Furthermore, these documents often contain multiple subsections that vary in the amount and quality of available evidence. Therefore, when these types of documents are included in individual topic areas, the assigned LOEs may differ.
Throughout the ICAR‐Allergic Rhinitis process, when a single publication was cited in multiple sections with differing LOEs initially assigned, this was returned to the authors/reviewers of each section for collective discussion. In some circumstances, the discussion resulted in the group deciding to revise the LOE to a consistent assignment across sections. In other cases, the groups supported their initial LOE assignment with appropriate reasoning – and the original LOE assignments remained. Therefore, the reader may notice occasional fluctuation in LOE assignment throughout the ICAR document.
IV.B Iterative review
Following the development of the initial topic text and any associated evidence tables, evidence grades, and recommendations, each section underwent a two‐stage online iterative review process using two independent reviewers that were initially blinded to the author's identity (Figure IV.B.). The purpose of the individual AR topic iterative review process was to evaluate the completeness of the identified literature and ensure any EBRR recommendations were appropriate. The content of the first draft from each topic section was reviewed by the first reviewer in a blinded fashion. The process was then unblinded, and necessary changes were agreed upon and incorporated by the initial author and this first reviewer – arriving at a consensus for the first stage. The revised topic section was subsequently reviewed by a second reviewer in a blinded fashion. Following the second review, the process was again unblinded. Initial topic authors and both assigned reviewers agreed upon necessary changes before each section was considered finalized and appropriate to proceed into the final ICAR statement stage.
IV.C ICAR‐Allergic Rhinitis statement development
After the content of each of topic was reviewed and consensus reached amongst the initial author and two iterative reviewers, the principal editor (S.K.W.) compiled associated topics into major content areas. The first draft of each major content area (i.e., Evaluation and Diagnosis, Pharmacotherapy, Immunotherapy, etc.) then underwent additional reviews for consistency and flow by a group of three to five ICAR associate editors. Finally, the full draft of ICAR‐Allergic Rhinitis 2023 was compiled and circulated to all authors. The final ICAR‐Allergic Rhinitis 2023 manuscript was produced when all authors agreed upon the literature and final recommendations (Figure IV.C).
IV.D Limitations of methods and data presentation
It is important to note that each topic author individually performed the literature search for his/her assigned topic. Therefore, search results may contain some inherent variability despite specific and detailed search instructions. Furthermore, while aiming to be as comprehensive as possible, this document may not present every study published on every topic. For certain topics, the literature is extensive and only high‐quality studies or systematic reviews are listed. If the aggregate evidence on a topic reached a high evidence grade with only high‐level studies, an exhaustive list of lower‐level studies (or all studies ever performed) is not provided.
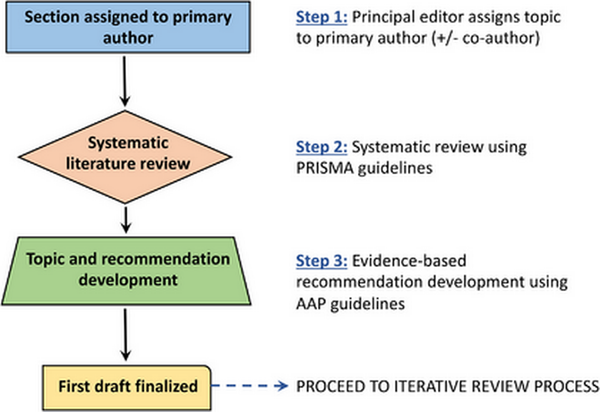
FIGURE IV
A. Topic development (Stage 1). Abbreviations: AAP, American Academy of Pediatrics; PRISMA, Preferred Reporting Items for Systematic Reviews and Meta‐Analyses
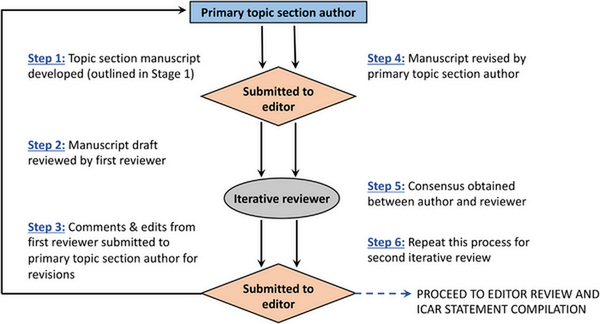
FIGURE IV
B. Topic iterative review process (Stage 2)
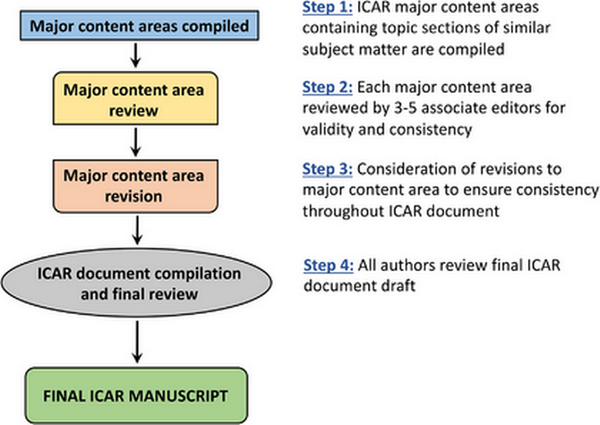
FIGURE IV
C. ICAR‐Allergic Rhinitis 2023 statement development (Stage 3). Abbreviation: ICAR, International Consensus Statement on Allergy and Rhinology
V DEFINITIONS, CLASSIFICATION, AND DIFFERENTIAL DIAGNOSIS OF ALLERGIC RHINITIS
V.A General definition and classification
V.A.1 Definition, classification, and severity of allergic rhinitis
AR is an immunoglobulin E (IgE)‐mediated, type 1 hypersensitivity response of the nasal mucosal membranes, resulting from allergen exposure in a sensitized individual. Symptomatically, it is characterized by anterior or posterior rhinorrhea, nasal congestion/blockage, nasal pruritis, and sneezing. AR is widely prevalent and can result in significant physical sequelae and recurrent or persistent morbidities. Additionally, it is strongly associated with asthma, supporting the unified airway theory which postulates that upper and lower airway inflammation share common pathophysiologic mechanisms. (See Section VI.K. Unified Airway for additional information on this topic.)
The prevalence of AR ranges from approximately 5%–50% worldwide, with the highest incidence in the pediatric population. While this range of AR prevalence is wide, it is important to recognize that published studies may vary in their definition of AR and some may define AR as sensitization to allergens. (See Section VII. Epidemiology of Allergic Rhinitis for additional information on this topic.) AR is essentially absent in infants and typically develops in school age children. Since sensitization takes years to develop, it is unlikely to manifest before 2 years of age. This is likely secondary to the rapidly evolving immune system inherent in a child's early development. AR often results from an overactive response of T helper (Th)‐2 lymphocytes and initiation of a systemic IgE‐driven reaction, which can dominate a child's immune system until completely mature.
In the atopic individual, exposure to allergens may prompt allergen‐specific IgE (sIgE) production. Subsequent exposure triggers both early and late‐stage reactions, leading to the clinical manifestations of AR. The early‐stage reaction typically occurs within minutes after re‐introduction of the sensitized allergen, producing a rapid onset of nasal itching, congestion, and rhinorrhea. The late‐stage reaction often occurs during the 4‐ to 8‐h period after allergen re‐introduction and results in congestion, hyposmia, increased anterior and posterior rhinorrhea, and nasal hyper‐responsiveness. (See Section VI. Pathophysiology and Mechanisms of Allergic Rhinitis for additional information on this topic.)
Allergic Rhinitis and its Impact on Asthma (ARIA) proposals have categorized AR by presumed cause and the timing during which it occurs. Classically, this has been categorized as seasonal AR (i.e., hay fever) and perennial AR. Seasonal AR is typically associated with outdoor allergens, such as pollens, and usually occurs during seasons with high pollen counts.Perennial AR is typically associated with indoor allergens, such as house dust mites (HDM), insects, and animal dander, and has been considered to occur consistently throughout the year. Mold exposure may occur indoors or outdoors depending on the specific environmental situation.
Of note, the classification of seasonal versus perennial AR can potentially be in conflict. For example, seasonal AR may persist for longer periods secondary to the effects of climate change, with resultant prolonged elevations in pollen counts. Seasonal AR may also continue across multiple seasons secondary to polysensitization. Furthermore, manifestations of perennial allergy may not occur throughout the entire year. This is particularly the case for patients allergic to HDM, who may demonstrate mild or moderate/severe intermittent AR., , ,
Because of the priming effect on the nasal mucosa introduced by low levels of pollen exposure,, , , , , and minimal but persistent nasal inflammation in patients with “symptom‐free rhinitis,”, , symptoms may not occur entirely in conjunction with allergen exposure. This may result in non‐specific exacerbations. Additionally, air pollution may also contribute to variations in allergen sensitivity, resulting in fluctuating symptom severity depending on location/air quality. (See Section VIII.B.3. Risk Factors for Allergic Rhinitis – Pollution for additional information on this topic.)
Subsequently, ARIA proposed a new method of classification based on the length and persistence of symptoms.Intermittent AR is characterized by symptoms for less than 4 days per week or less than four consecutive weeks. Persistent AR is characterized by symptoms occurring more than 4 days per week for at least four consecutive weeks. Additionally, it was demonstrated that the previous categories of seasonal and perennial AR cannot be used along with the new classification of intermittent/persistent AR, as they do not represent the same stratification of the disease state. As such, intermittent AR and persistent AR are not synonymous with seasonal and perennial classifications., , ,
The ARIA guidelines have likewise proposed another stratification of severity (mild and moderate‐severe) with respect to these disabilities. AR can result in problematic symptoms, including sleep disturbance; impairment of daily, leisure, or sport activities; impairment of school or work; or troublesome symptoms. AR is considered mild if none of these occur. If one or more of these symptoms exist, AR is classified as moderate–severe.
V.A.2 Sensitization versus clinical allergy
Atopic diseases comprise of a range of linked conditions presenting as multiple heterogeneous clinical phenotypes ranging from single organ to multi‐system disease., Currently used taxonomy is largely organ‐based and does not fully take into account the mechanisms leading to symptoms. For example, the 2016 Melbourne epidemic thunderstorm asthma event saw a dramatic increase in asthma‐related hospitalizations and 10 deaths over a 30‐h period. Interestingly, most patients hospitalized with severe asthma attack did not have a diagnosis of asthma. They did have a diagnosis of AR and allergen‐specific immunotherapy (AIT) appeared to offer protection. It can be postulated that these patients suffered from a single IgE‐driven condition with a clear pathophysiological mechanism, for which there are available biomarkers (e.g., sIgE) and mechanism‐based treatment (e.g., AIT).
Although patients with AR and allergic asthma are by definition sensitized, many individuals with allergic sensitization do not have symptoms of allergic disease, and in a proportion of patients with AR and allergic asthma, sensitization is not related to the presence or severity of symptoms. Furthermore, the reliability of skin testing depends greatly on allergen extracts and methods used. Thus, clinicians face a problem that sensitization on standard allergy tests does not prove that symptoms are caused by allergy. Some subtypes of allergic sensitization are benign and not associated with clinical symptoms, while others are pathologic and lead to a spectrum of disease from single‐organ disease to allergic multi‐morbidity. (See Sections XI.D.11.a.ii. Multi‐allergen Immunotherapy and XI.D.11.b.ii. Polysensitization and for additional information on this topic.)
Better ways of differentiating clinically significant sensitization are needed. Quantification of sensitization through standard diagnostic tests (i.e., sIgE titer, size of skin test wheal) can increase the specificity, both in terms of diagnostic accuracy and the capacity to predict the persistence of symptoms., , , However, the problem of false‐positive test results remains. Currently, nasal allergen challenges is the most accurate way to confirm clinical allergy. Recent studies show that this is highly sensitive and specific, with negative and positive predictive values greater than 90%., It can also be helpful in the diagnosis of local nasal allergy, which may otherwise be missed on skin testing or in vitro testing methods. However, in most healthcare systems, this procedure is restricted to centers with specialist expertise.
We can now assess sensitization in greater detail using component‐resolved diagnostics (CRD), which measures sIgE to multiple allergenic molecules and may be more informative than standard tests., , , , Recent novel analyses of CRD data demonstrated that the pattern of interaction between allergen component‐specific IgEs predicts asthma and that networks of interactions between sIgE to multiple components are predictors of asthma severity across the lifespan. These findings offer clues about mechanisms contributing to presence and severity of allergic airway disease and suggest that it may be possible to develop biomarkers/prediction tools based on CRD to help in diagnosis, severity assessment, prediction of future risk, and ultimately, the prediction of response to treatment.
V.B Differential diagnosis
V.B.1 Drug induced rhinitis
Rhinitis secondary to systemic medications can be classified into local inflammatory, neurogenic, and idiopathic types., , The local inflammatory type occurs when usage of a drug causes a direct change in inflammatory mediators within the nasal mucosa. The neurogenic type occurs after use of a drug that systemically modulates neural stimulation, leading to downstream changes in the nasal mucosa. The idiopathic classification is applied when a well‐defined mechanism has not been elucidated. Rhinitis medicamentosa and hormone‐induced rhinitis are discussed in later sections (Table V.B.1).
Local inflammatory type. Systemic ingestion of nonsteroidal anti‐inflammatory drugs (NSAIDs) in specific patients can cause respiratory symptoms and may be associated with nasal polyposis and asthma due to abnormal arachidonic acid metabolism. NSAIDs inhibit cyclooxygenase (COX)‐1, leading to decreased prostaglandin (PG) E2 and increased leukotriene production due to an imbalance toward the lipoxygenase pathway. Reduction in PGE2, and increased leukotriene C4, D4, and E4 production contributes to eosinophilic and mast cell inflammation within the upper and lower respiratory tracts., , ,
Neurogenic type. Neurogenic‐type non‐allergic rhinitis is caused by drug‐induced modulation of the autonomic nervous system. Antihypertensives and vasodilators are among the many classes of drugs that cause neurogenic drug‐induced non‐allergic rhinitis. Other nonspecific drugs, such as psychotropics and immunosuppressants, have unknown direct mechanisms and are categorized as idiopathic type, but can also cause neuromodulatory effects. Modulation of the autonomic nervous system leads to downstream changes in the nasal mucosa, blood vessels, and secretory glands.
Alpha‐ and beta‐adrenergic modulators. Alpha‐ and β‐adrenergic receptor modulators are indicated for various cardiovascular and respiratory diseases. The nasal mucosa is replete with sympathetic and parasympathetic end‐units that influence nasal physiology during systemic drug use. Alpha‐ and β‐adrenergic antagonists, and presynaptic α‐agonists cause decreased sympathetic tone and unopposed parasympathetic stimulation producing mucosal engorgement, nasal congestion, and rhinorrhea., ,
Phosphodiesterase inhibitors. Phosphodiesterease (PDE) inhibitors prevent enzymatic breakdown of cyclic nucleotides. This inhibition has diverse effects including smooth muscle relaxation, vasodilation, and bronchodilation, making PDE inhibitors useful for numerous disease processes. PDE‐3 and PDE‐5 inhibitors are commonly used to treat intermittent claudication, heart failure, pulmonary hypertension, lower urinary tract symptoms, and erectile dysfunction., PDE‐3 and nonselective PDE inhibitors inhibit cyclic adenosine monophosate (cAMP) hydrolysis, which ultimately prevents platelet aggregation and encourages vasodilation with increased extremity blood flow. PDE‐5‐specific inhibitors encourage smooth muscle relaxation through inhibition of nitric oxide (NO)‐generated cyclic guanosine monophosphate (cGMP), causing vasodilation of the corpus cavernosum and pulmonary vasculature as well as changes in the lower urinary tract. NO/cyclic nucleotide mediated vasodilation occurs in the nasal mucosa causing nasal mucosal engorgement and edema, , , , (Table V.B.1).
Angiotensin converting enzyme inhibitors. Angiotensin converting enzyme inhibitors (ACEI) inhibit the conversion of angiotensin I to angiotensin II in the lungs and are commonly used for cardiac and renal diseases. ACEI upregulate the formation of bradykinin, an inflammatory peptide that causes vasodilation and smooth muscle contraction. Bradykinin B1 and B2 receptors have been demonstrated in nasal mucosa, and bradykinin application to nasal mucosa has resulted in increased sneezing., In addition to cough, rhinorrhea and nasal obstruction have been associated with ACEI.
Illicit drug use. The nose provides a unique portal for illicit drug use due to well vascularized and easily accessible nasal mucosa. Applying a crushed solid, liquid, or aerosolized form of a drug to the nasal cavity avoids invasive intravascular or intramuscular administration. For some drugs, nasal administration increases bioavailability and shortens time to onset when compared to oral ingestion., In contrast to oral agents, intranasal administration bypasses portal filtration.
Cocaine is most commonly associated with nasal illicit drug use and exerts its effect by modulating dopamine transporters to inhibit synaptic reuptake, increasing dopamine for post‐synaptic stimulation. After application to nasal mucosa, cocaine is quickly metabolized by native mucosal esterases into its bioactive metabolite, which then passively diffuses across the nasal mucosa and the olfactory bulb, leading to elevated systemic and brain concentrations resulting in a psychotropic euphoria. Cocaine‐induced rhinitis is a result of vasoconstrictive events, which can be followed by rebound nasal mucosal edema and mucus production, similar to rhinitis medicamentosa., , , In the repeat user, vasoconstriction, direct trauma compounded by anesthetic effects, and/or injury secondary to contaminants may result in tissue necrosis., , , Similarly, prescription narcotics, antidepressants, anticholinergics, and psychostimulants can be abused by intranasal administration., Tissue necrosis has also been associated with intranasal opioid and acetaminophen abuse., , Possible mechanisms of injury include hyperosmotic conditions, vasculitic‐like inflammation, or direct injury secondary to talc.,
Drug‐induced rhinitis is a subtype of non‐allergic rhinitis that can cause mucosal edema, vasodilation, and inflammatory mediator production. Vasoconstriction and mucosal injury often accompany illicit drug use. Drug‐induced rhinitis differs from AR as it is not allergen‐induced nor dependent on IgE mechanisms, although symptomatology may be similar.
V.B.2 Rhinitis medicamentosa
Rhinitis medicamentosa is a drug‐induced rhinitis resulting from prolonged topical intranasal decongestant (INDC) use., Topical INDCs are readily available without a prescription and often lack appropriate warnings of prolonged use, potentially resulting in overuse and dependence. Although no consensus diagnostic criteria exist, rhinitis medicamentosa was originally associated with the triad of prolonged INDC use, persistent nasal obstruction, and rebound swelling of the nasal mucosa. Patients present with nasal congestion, often lack rhinorrhea or sneezing, and may note reduced efficacy, or tachyphylaxis, with further use of INDCs., , Physical examination is variable, but often reveals nasal mucosal edema, erythema, and hyperemia (Table V.B.2).
Nasal anatomy and physiology. Vasculature within the nasal mucosa consists of resistance vessels (arterioles), whose sympathetic innervation is predominated by α‐2 adrenergic receptors, and capacitance vessels (venous sinusoids), that are innervated by α‐1 and α‐2 receptors. Stimulation of these receptors results in vasoconstriction with resultant decongestion due to decreased blood flow and increased sinusoid emptying., The two classes of nasal decongestants are imidazolines and sympathomimetic amines. Imidazolines are α‐2 receptor agonists, while sympathomimetic amines encourage presynaptic norepinephrine release. Norepinephrine stimulates α‐adrenergic receptors and weakly stimulates β‐adrenergic receptors. Both medication classes have a rapid onset, are potent, and are long‐acting.,
The exact pathophysiologic mechanism causing rhinitis medicamentosa is unclear, although several hypotheses exist: (1) chronic vasoconstriction causes recurrent nasal tissue hypoxia and ischemia, which may cause interstitial edema; (2) changes in endothelial permeability may result in increased edema; and (3) continuous INDC use may decrease endogenous norepinephrine and downregulate α‐receptors, through negative neural feedback, causing decreased adrenergic responsiveness., , , , , Inflammatory cells, local inflammatory mediators, uninhibited parasympathetic stimulation, and increased mucin production also contribute to symptomatology.
Histologic changes within the mucosa after prolonged INDC use include ciliary damage and ciliary loss, epithelial cell injury, epithelial metaplasia and hyperplasia, dilated intercellular spaces, goblet cell hyperplasia, and edema., , Benzalkonium chloride, an antimicrobial preservative used in many nasal sprays, has been implicated in the mechanism of rhinitis medicamentosa. Studies have demonstrated that benzalkonium chloride is toxic to nasal epithelium and induces mucosal edema, propagating rhinitis medicamentosa, although the data are inconclusive., , , , Neither duration, nor cumulative dose of INDC needed to initiate rhinitis medicamentosa is known. Rebound congestion has developed after 3 to 10 days of medication use,, but may not occur until after 30 days., Other studies have demonstrated a lack of rebound congestion after 8 weeks of continuous use., , , Furthermore, doubling the dose of intranasal imidazoline did not increase the extent of rebound edema. Although inconclusive, studies suggest that INDC use should be discontinued after 3 days to avoid rebound congestion., ,
Treatment of rhinitis medicamentosa. Despite the lack of formal treatment guidelines for rhinitis medicamentosa, discontinuation of INDCs is paramount. Patients should be educated regarding common over‐the‐counter products containing decongestants as labeling may be inadequate. Various treatments have been trialed including nasal cromolyn, nasal saline spray, oral/intranasal antihistamines, turbinate steroid injections, and oral/intranasal corticosteroids., , , , , , , Intranasal corticosteroids (INCS) are the most common treatment for rhinitis medicamentosa. Many initiate INCSs while weaning INDCs., , , , , Often there is an underlying undiagnosed rhinitis and/or anatomic issue that initiated decongestant use, and this should be addressed to relieve the drive to use INDCs. For refractory cases, oral steroids and inferior turbinate (IT) reduction have been considered.
Rhinitis medicamentosa is typically associated with repeated exposure to INDCs, with increasing symptoms when the medication is withheld. In contrast, AR is classically associated with an allergic trigger with similar symptoms increasing upon allergen exposure and is dependent upon IgE‐mediated inflammation. It is possible that both may coexist, and a careful history should be obtained regarding these triggers to obtain an accurate diagnosis and provide appropriate treatment.
V.B.3 Occupational rhinitis
Occupational rhinitis is an inflammatory disease of the nose, characterized by intermittent or persistent symptoms of nasal congestion, sneezing, rhinorrhea, itching, and/or variable nasal airflow obstruction due to causes and conditions attributable to a particular work environment., While many social activities or hobbies can result in overlapping symptoms, stimuli that are encountered outside the workplace are not considered occupationally related.
The pathophysiological mechanisms of occupational rhinitis are the same as other forms of chronic rhinitis although symptoms may be intimately tied to work exposure., , Occupational rhinitis may be classified as allergic, resulting from an immunological exposure to a sensitizing high molecular weight protein (HMW > 5 kDa) or non‐allergic, mediated by non‐immunological low molecular weight chemical irritant (LMW < 5 kDa)., Non‐allergic occupational rhinitis is sometimes subdivided into annoyance (e.g., perfumes), irritant‐induced (e.g., formaldehyde or smoke), or corrosive rhinitis (e.g., ammonia or acids), the latter of which may include permanent inflammation of the nasal mucosa, ulcerations, and perforation of the nasal septum.,
Cross sectional studies of various workers show a wide range of occupational rhinitis prevalence rates (3%–87%),, , although rates are higher for HMW agents compared to lower for LMW agents. Occupations and commonly implicated agents are reported in Table V.B.3., , , , , Pre‐existing AR or allergic asthma, baseline total IgE >150 kIU/L, or occupations with frequent exposure to animals have been shown to be risk factors for occupational rhinitis.,
Occupational rhinitis tends to be three times more prevalent than occupational asthma, but the two disorders are often associated (up to 92% of cases). In most cases, work‐related nasal symptoms develop 5–6 months before the onset of bronchial symptoms., Consequently, occupational rhinitis may be considered a marker of the likelihood of developing occupational asthma. Previous practice parameters and consensus documents suggest that workers in certain high‐risk occupations be periodically monitored by survey and/or skin prick testing (SPT) so that risk mitigation strategies can reduce sensitization, and potentially limit progression of occupational rhinitis or the development of occupational asthma., ,
The clinical presentation of occupational rhinitis does not differ from those of non‐occupational chronic rhinitis. Diagnostic assessment must include a thorough clinical and occupational history, aimed to investigate the type of symptoms and work‐related temporality, and to collect information on specific occupational exposures. Documentation of noxious compounds in the workplace should include examination of available Material Safety Data Sheets. The presence of a latency period between beginning of occupational exposure and symptom onset (months or even years) suggests an immunologic mechanism. This contrasts to non‐allergic irritant occupational rhinitis which may occur immediately upon first exposure.
Nasal endoscopy, assessing nasal patency, inflammation, and secretions minimize patient misclassification., , Sensitization to a suspected HMW agent by SPT may be preferred over serum sIgE assessment as skin testing has been reported to be more sensitive and specific in various reports., , , However, the reliability of sIgE testing depends on the equipment, materials, and technique employed; therefore, a standardized approach and validated extracts are required, which are often not available especially for LMW agents., , , , A truly definitive diagnosis can only be established by objective demonstration of the causal relationship between rhinitis and the work environment through nasal provocation test (NPT) with the suspected agent(s). However, irritant triggers, LMW agents, and delayed type reactions are often not easily identified by NPT, , , , (Figure V.B.3). Validated clinical assessment tools such as the Total Nasal Symptom Score (TNSS) or and/or sneeze counts administered pre‐and‐post exposure may aid in quantifying the severity of the response. At some institutions, rhinomanometry is also available to obtain additional quantitative data.
If NPT is negative, further evaluation of work‐related changes in nasal parameters at the workplace is recommended, especially in the presence of a highly suggestive clinical history. When possible, a formal site visit may allow the technician to directly observe the workplace environment, symptomatology, and Material Safety Data Sheets, and suggest specific workplace modifications. Due to the strict relationships between upper and lower airways, spirometry and exhaled NO assessment should be performed in patients with occupational rhinitis.,
The primary treatment of allergic occupational rhinitis is avoidance or reduction of culprit exposures. Pharmacologic treatment does not differ from that of non‐occupational rhinitis, although medications alone may be insufficient given the intensity and frequency of many workplace exposures. In allergic occupational rhinitis due to HMW sensitizers, AIT may be considered when validated extracts are available. However, AIT may have limitations in those individuals with continued high workplace exposure; therefore, simultaneous mitigation and avoidance strategies are essential.
Occupational rhinitis has both medical and socioeconomic implications, and may be the cause of leaving work. Since occupational rhinitis is acknowledged as a risk factor for the development of occupational asthma, the prevention and early identification of occupational rhinitis of exposed workers may provide an excellent opportunity to prevent the development of occupational asthma. (See Section XI.A.6. Allergen Avoidance – Occupational for additional information on this topic.)
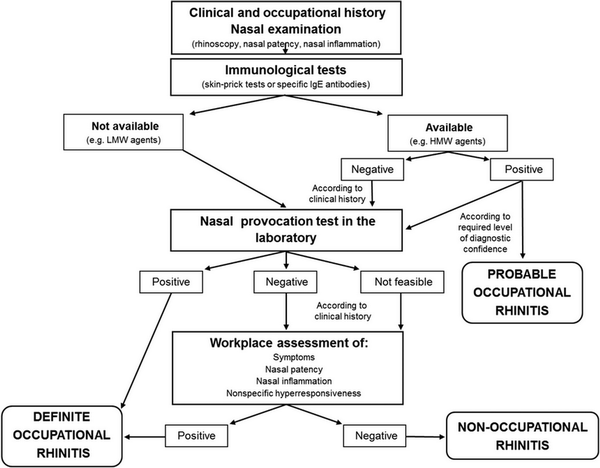
FIGURE V
B.3. Diagnostic algorithm for occupational rhinitis
V.B.4 Chemical rhinitis
As exposure to environmental chemicals and pollutants increases in daily life, patients may present with rhinitis symptoms that do not necessarily fall within a traditional allergic profile. Chemicals may cause sensory irritation which can include congestion, sneezing, rhinorrhea, nasal discomfort, post‐nasal drainage, headache, olfactory function, and epistaxis. This is often associated with lower airway symptoms and conjunctival irritation. The differential diagnosis of chemical rhinitis is broad, including occupational rhinitis, but not all chemical rhinitis is occupational. Typically, the differential should include causes of both AR and non‐allergic rhinitis, as well as mixed rhinitis, recurrent acute rhinosinusitis (RARS), and chronic rhinosinusitis (CRS).
Exposures at home and work are important elements to obtain in the history. There are many chemicals with which specific occupations are closely associated, and household chemicals may play a role as well. Volatile organic compounds such as benzene, toluene, and the secondary production of formaldehyde can be found in cleaning products, furniture, plastics, flooring and can cause barrier dysfunction and inflammation in both the upper and lower airway., , Larger chemical particles greater than 10 μm in diameter are generally deposited in the upper airway and agents such as ammonia, formaldehyde, nitrogen dioxide, or sulfur dioxide among others may readily disrupt the epithelial barrier.
In general, inquiring about exposure to vapors, fumes, smoke, and dust can be helpful to determine if a patient has an element of chemical rhinitis. These responses are typically non‐IgE‐mediated by a reflex response which is often termed neurogenic inflammation. A subset of these individuals involved in single exposure incidents may develop persistent and chronic symptoms. This phenomenon has been described as reactive upper airways dysfunction syndrome when only rhinitis symptoms are present, and reactive airways dysfunction syndrome when asthma‐like symptoms are present.,
Chemicals known to cause respiratory inflammation and in some cases, allergic sensitization include diisocyanates, acid anhydrides, some platinum salts, reactive dyes, and many cleaning products that are used in hospitals and in the pandemic era including glutaraldehyde, quaternary ammonium compounds, and chloramine., , , There is still debate concerning the exact mechanism behind sensitization to these chemicals. However, smaller chemical compounds must associate with larger protein molecules in order to induce an immune response. As a result, evaluation of sensitization through skin testing and/or evaluation of sIgE can be helpful and in the future, immunoassays based on cellular responses may serve as better biomarkers of exposure to chemicals.,
V.B.5 Smoke induced rhinitis
Tobacco smoke exposure is associated with chronic rhinitis and CRS., , Other smoke exposure sources besides conventional cigarettes, cigars, and pipes include electronic cigarettes, vaping, and cannabis. Although there is limited research on these other methods of smoke exposure, initial studies support that there may be an increased risk of rhinitis with some of these products and these exposures should be considered in the differential diagnosis., Symptoms common to both AR and smoke‐induced rhinitis include rhinorrhea and congestion, but smoke‐induced rhinitis is not driven by IgE‐mediated hypersensitivity which tends to also exhibit sneezing on exposure to a specific allergen., , ,
Symptoms of rhinitis are provoked by exposure to the chemicals in smoke and can correlate with serum cotinine levels in patients using tobacco. Furthermore, smoking in combination with occupational irritants are additive risk factors for nasal symptoms and may be independent of allergic sensitization. Although smoke‐induced rhinitis does not require allergen sensitization, there has been at least one report of potential allergenic compounds in smoke. Interestingly, active smokers show elevated total serum IgE, although they exhibit a lower skin test reactivity to specific allergens compared to non‐smokers despite well documented increased rates of lower respiratory disorders such as asthma, cough, sputum production, and wheezing. This may be due in part to the fact that tobacco smoke exposure results in decreased mucociliary clearance.
One of the mechanisms to explain nasal irritation resulting from smoke exposure may be related to capsaicin‐sensitive neurons in the nasal mucosa. This neurogenic type of nasal inflammation is mediated by neuropeptides such as substance P, neurokinin A, and calcitonin gene‐related peptide (CGRP). These mediators are released by sensory nerve fibers in the nose and result in vasodilation, edema, and inflammation.
Patients who are reactive to tobacco exposure are identified by both subjective (congestion, rhinorrhea, sneezing) and an objective response (increased nasal resistance) to controlled challenge with tobacco smoke. In a prospective study, patients were defined as demonstrating reactivity if nasal resistance increased by more than 35% by acoustic rhinometry in response to tobacco smoke; patients with less than 5% increase in nasal resistance were defined as nonreactive. Congestive responses have been demonstrated on challenge with both brief and prolonged exposure to tobacco smoke. In individuals who report a history of smoke induced rhinitis, only brief smoke exposure (45 parts per million [ppm] for 15 min) leads to increased nasal resistance as measured by posterior rhinometry (although there were no significant increases in histamine levels noted). However, prolonged exposure to moderate levels of smoke (15 ppm for 2 h) induced a congestive response lasting for an hour or longer in both individuals with and without a history of smoke‐induced rhinitis. While objective response may be short lived, patients reported symptoms lasting hours to days following exposure. Since significant symptom overlap exists, a thorough history and allergy testing can help further differentiate smoke‐induced rhinitis from other types of rhinitis.
V.B.6 Infectious rhinitis
Infectious rhinitis is a very common diagnosis in general practice. Differences in onset and pathogenic cause lead to various pathophysiologies and forms. Common conditions in general practice are acute viral and bacterial rhinitis. Nasal symptoms include clear or discolored nasal discharge, nasal obstruction, postnasal drip, cough, and facial pressure depending on the etiology. These symptoms may also be present in non‐infectious rhinitis; most commonly AR. This diagnostic distinction is important to avoid inappropriate treatment and diagnostic procedures. Distinctive clinical characteristics suggestive of AR are sneezing, nasal or ocular itching, the presence of an obvious allergic trigger, and the presence of recurrent seasonal‐related symptoms – these symptoms are less frequent in infectious rhinitis.,
Rhinitis symptoms are the result of nasal mucosa and/or sinus inflammation. The mucosa of the nose and sinuses are contiguous. Thus, the clinical presentations of rhinitis and rhinosinusitis are overlapping, and it is difficult to differentiate between them. Infectious rhinitis or rhinosinusitis are classified by duration and pathogenic cause into subtypes including acute viral (common cold), post‐viral, and bacterial. (See Sections V.B.15. Differential Diagnosis ‐ Rhinosinusitis and XIII.B. Associated Conditions ‐ Rhinosinusitis for additional information on this topic.)
Acute viral rhinitis, or the common cold, is responsible for most acute infectious rhinitis, especially in children. The incidence of acute viral rhinosinusitis is expected to be as high as 98%., Common organisms are rhinovirus, adenovirus, influenza virus, and parainfluenza virus. Viral rhinitis is a self‐limited illness and only requires supportive treatment. Most symptoms resolve by day five; nasal discharge and cough may last longer. Prolonged symptoms of more than 2 weeks duration suggest a non‐infectious etiology or post‐viral rhinosinusitis.
The relationship between viral infection and AR has been studied. The upregulation of intercellular adhesion molecule (ICAM)‐1, which is the major human receptor of rhinovirus, was shown in patients with underlying allergic disease., , The increased expression of ICAM‐1 was demonstrated in both upper and lower allergic airway diseases compared with healthy controls., , This enhances the susceptibility of airway epithelial cells to viral infection.
In some cases, viral rhinitis episodes are secondarily infected by bacterial organisms such as Streptococcus pneumonia, Haemophilus influenza, and Moraxella catharralis., This occurs in 0.5%–2.0% of all viral infections., Clinical presentation distinguishing viral from bacterial rhinitis/rhinosinusitis is often impossible., , , Inappropriate prescribing of antibiotics and diagnostic tools is often secondary to misdiagnosis of the symptoms and signs of viral and bacterial origin with up to 60% starting a course of antibiotics at first symptom presentation., ,
The possibility of bacterial infection increases if there is deterioration in symptoms after day 5. Predicting criteria for bacterial infection have been suggested using clinical characteristics, the pattern of symptoms, and laboratory reports., , However, the maximum sensitivity and specificity only reach 69% and 81%, respectively, among various criteria., Additionally, a collection of factors contribute to developing an infection of bacterial origin. These factors include dental infection or procedure, previous sinus surgery/nasogastric tube insertion/nasal packing, underlying immunodeficiency, structural nasal problems, and evidence of underlying nasal mucosa edema such as AR.
V.B.7 Rhinitis of pregnancy and hormonally induced rhinitis
Rhinitis of pregnancy. Pregnancy‐induced rhinitis describes nasal symptoms that occur during pregnancy, are independent of other etiologies for rhinitis, and remit after delivery., , Symptoms include rhinorrhea, sneezing, hyposmia, and nasal itching. In a multicenter study of 599 previously asymptomatic women, prevalence of rhinitis of pregnancy was 22%. A history of AR and smoking increase risk for its development., ,
Quantifying the impact of pregnancy‐induced rhinitis has been done objectively and subjectively. Acoustic rhinometry, rhinomanometry, peak nasal airflow measurements, and saccharin testing confirm that changes to nasal airway patency occur., , Electron microscopy demonstrates glandular hyperactivity, increased phagocytotic activity, and increased amounts of acid mucopolysaccharides in the ground substance. Studies using validated patient reported outcome measures (PROMs) (e.g., Nasal Obstruction Symptom Evaluation [NOSE] scale, Rhinitis Quality of Life Questionnaire [RQLQ]), confirm the subjective component of pregnancy‐induced rhinitis., ,
The precise pathophysiology of pregnancy‐induced rhinitis remains unknown., , Estrogen, progesterone, and placental growth hormonal have all been implicated., , , Increased expression of histamine receptors secondary to β‐estradiol and progesterone in nasal epithelial and endothelial cells has been demonstrated and is proposed as a potential mechanism of nasal hyperreactivity in pregnancy‐induced rhinitis. Additionally, serum levels of placental growth hormone were significantly higher in patients with pregnancy‐induced rhinitis throughout their pregnancy.
Pregnancy‐induced rhinitis has been implicated in potential risks for the mother and fetus., , Mouth breathing from pregnancy‐induced rhinitis bypasses the benefits of nasal breathing, including preparation of inspired air for the lungs and NO release from the maxillary sinuses, which reduces pulmonary vascular resistance and contributes to increased pulmonary oxygenation., Additionally, maternal sleep disruption, when severe, can be associated with snoring and obstructive sleep apnea (OSA) and may contribute to increased risk for pre‐eclampsia and maternal hypertension. Intrauterine growth retardation and decreased Apgar scores are also possible.,
Treatment is conservative and relies on education. Reassurance regarding the temporary nature of pregnancy‐induced rhinitis is beneficial. Regular use of nasal saline lavage is safe and provides symptomatic relief., , Counseling against the routine use of oral and topical decongestants is critical due to the risk for congenital gastroschisis, pyloric stenosis, endocardial cushion defects, renal anomalies, and limb defects. These risks are greater in the first trimester, but caution should be maintained throughout the pregnancy., , INCS are generally considered safe for use during pregnancy; however, triamcinolone is associated with congenital respiratory defects. A treatment option under investigation is topical hyaluronate, which facilitates mucociliary clearance and hydration. In a 2019 pilot study of pregnancy‐induced rhinitis, sodium hyaluronate use decreased snoring, mucosa congestion, and nasal secretions and had no adverse events. More studies are needed before recommending its routine use during pregnancy.
Hormonally‐induced rhinitis. Cytological changes and cell turnover of the nasal epithelium during the phases of the menstrual cycle have been demonstrated. In general, estrogens are thought to cause nasal vascular engorgement, resulting in obstruction and rhinorrhea. As with pregnancy‐induced rhinitis, the mechanism of these changes remains unclear., , , The expression of histamine H1‐receptors within the nasal epithelium and microvascular endothelial cells are increased in response to β‐estradiol and progesterone. These hormones may also induce eosinophil migration and/or degranulation.
Rhinitis can also occur in patients with endocrine pathologies. Hypothyroidism can cause hypertrophy of mucous glands, increased submucosal connective tissue, and resultant nasal obstruction and rhinorrhea., , These patients may also have prolonged mucociliary clearance time. Rhinitis with sinonasal mucosal hypertrophy and polyp formation can also be seen in acromegaly, though it is unclear if elevated serum levels of growth hormone are the cause.
V.B.8 Food and alcohol induced rhinitis
Food‐induced rhinitis. Gustatory rhinitis is characterized by watery, unilateral, and/or bilateral rhinorrhea within a few minutes after the ingestion of food, usually hot and spicy foods such as tabasco sauce, hot chili peppers, horseradish, red cayenne or black pepper, and other foods that contain capsaicin. The rhinorrhea lasts as long as the food is ingested., , , , Gustatory rhinitis can be confused with IgE‐mediated food allergy, but there is no sneezing, pruritus, or facial pain and the time course of the rhinorrhea is self‐limited. There is also no associated disturbance of smell or taste. Gustatory rhinitis occurs more often in patients with AR and patients who have a history of smoking, but not those with asthma or food allergies.
The pathophysiology has been confirmed through pharmacologic observations and immunohistology studies to occur through a neural reflex arc initiated upon the stimulation of afferent sensory nerves. This leads to the stimulation of the parasympathetic efferent nerve supply to the submucosal glands in the nasal mucosa., It is additionally possible that interactions between the sympathetic and parasympathetic nervous system could lead to uninhibited activity of the parasympathetic system with resultant rhinorrhea. For example, the chemical capsaicin is known to cause gustatory rhinitis. The capsaicin receptor is a transient receptor potential vanilloid subtype 1 (TRPV1) receptor and exists in neuronal as well as non‐neuronal cells along the nasal mucosa and oral epithelium. A direct effect on goblet cell secretion may be triggered when capsaicin is ingested. A well‐known culprit of gustatory rhinitis is chili peppers, which contain capsaicin. A variety of other foods are associated with gustatory rhinitis including horseradish, wasabi, black pepper, hot mustard, and vinegar.,
Treatment of gustatory rhinitis is avoidance of the inciting food. Topical anticholinergic medications such as ipratropium bromide (IPB) are used when avoidance is impractical., , The use of topical capsaicin and resection of the posterior nasal nerve (PNN) have been proposed as a last resort for intractable gustatory rhinitis.,
Alcohol‐induced rhinitis. Exacerbation of respiratory symptoms after ingestion of alcohol occurs in approximately 3%–4% of the general population. Among the nasal symptoms that occur, blockage is the most common and may be accompanied by rhinorrhea, sneezing and lower airway symptoms. This is reportedly more common in patients with AR, asthma, chronic obstructive pulmonary disease (COPD), and emphysema. Up to 75% of aspirin‐exacerbated respiratory disease (AERD) patients suffer exacerbations of respiratory symptoms when they consume alcohol., , Symptom exacerbations occur relatively soon after alcohol ingestion, are often associated with the ingestion of small volumes, and seem to correlate with peak blood alcohol levels. Such symptoms can arise regardless of the type of alcohol ingested., These reactions to alcohol consumption are more prevalent in chronic rhinosinusitis with nasal polyp (CRSwNP) patients who suffer with severe and recurrent disease and are related to the severity of upper airway inflammation.
In AERD patients, the severity of aspirin‐induced respiratory symptoms is positively correlated with the severity of alcohol‐induced reactions. Exacerbations of respiratory symptoms in response to alcohol have been shown to be decreased after aspirin‐desensitization in patients with AERD. Patients with AERD have elevated baseline cysteinyl leukotriene levels, which are proposed to mediate the upper and lower airway reactions to aspirin., Cardet et al. propose that cysteinyl leukotrienes mediate the response to alcohol in these patients as well, though the pathway for such a mechanism is unknown.
High alcohol consumption is observationally and genetically associated with high serum IgE levels, though not with allergic disease. Two possible mechanisms have been proposed as the etiology for this observation: (1) alcohol changes the balance of the Th1 and Th2 responses toward a Th2 immune response with a direct effect on B cells, or (2) alcohol induces increased uptake of endotoxins from the gut resulting in elevated IgE levels.
V.B.9 Eosinophilic rhinitis and non‐allergic rhinitis with eosinophilia syndrome (NARES)
Non‐allergic rhinitis with eosinophilia syndrome (NARES) is a clinical disorder comprising symptoms consistent with perennial AR in which there is an absence of atopy but presence of local eosinophilia found on nasal cytology. The pathophysiology of NARES is not well understood, but a key component involves chronic local eosinophilic, self‐perpetuating inflammation, with non‐specific histamine release. It is one of the most common type of inflammatory non‐allergic rhinitis that was first described by Jacobs and colleagues in 1981.
NARES patients report symptoms that are similar to those of perennial AR: nasal congestion, profuse aqueous rhinorrhea, sneezing, and nasal and ocular pruritis. A prominent feature of NARES is olfactory dysfunction. NARES patients demonstrate significantly higher thresholds on olfactory testing than seasonal and perennial AR patients. NARES is diagnosed by obtaining a careful history, findings on physical exam, not unlike those found in perennial AR patients (pale, boggy turbinates), and negative skin or in vitro allergy testing. Cytologic examination in NARES reveals the presence of prominent eosinophilia, usually 10%–20% on nasal smear, with a diagnostic criterion of 25% or more eosinophils., In addition, nasal biopsies from these patients commonly show increased numbers of mast cells with prominent degranulation.,
Research has supported the role of chronic inflammation in the development of NARES. Though there is still a lack of understanding as to the exact pathophysiology, studies have shown an increased transendothelial migration of eosinophils in nasal lavage fluid, which are attracted and activated by chemokines and cytokines., Specifically, NARES is characterized by elevated nasal fluid levels of tryptase (which is also seen in perennial AR) and eosinophilic cationic protein. Elevated levels of interleukin (IL)‐1β, IL‐17, interferon (IFN)‐γ, tumor necrosis factor (TNF)‐α, monocyte chemoattractant protein (MCP)‐1, and RANTES (regulated upon activation, normal T cell expressed and presumably secreted) in nasal fluid were found in NARES compared to controls.,
A correlation between the concentration of RANTES with nasal symptoms and eosinophil counts in perennial AR patients has been shown. However, levels of MCP‐1 and RANTES were significantly higher in the nasal fluid of NARES compared to perennial AR subjects. Elevation of these cytokines correlated with the ratio of nasal symptom scores/percentage of eosinophils in NARES patients, where nasal symptoms of nasal obstruction, rhinorrhea, hyposmia, sneezing, and itching were each measured using a 3‐point scale. Several studies from European cohorts have found a lack of nasal mucosal IgE in NARES patients., More recent studies of Chinese cohorts of NARES patients have found increased expression of Charcot‐Leyden crystals which correlated with severity of symptoms and degree of eosinophilia. Elevated cysteine protease inhibitor cystatin SN was also observed with greater loss of sense of smell. Neuropeptide mediated eosinophil chemotaxis, including substance P, CGRP, and cholecystokinin octapeptide, has also been described as a contributing factor to the symptomatology in NARES patients.
NARES may occur in isolation, but it can be associated with (and may be a precursor for) AERD. NARES has also been identified as a risk factor for the induction or exacerbation of OSA and has been associated with increased tendency for lower airway hyperresponsiveness.
The treatment of non‐allergic rhinitis centers on its underlying cause. NARES is primarily treated with INCS, which decrease neutrophil and eosinophil chemotaxis, reduce mast cell and basophil mediator release, and result in decreased mucosal edema and local inflammation., A combined analysis of three double‐blind, randomized, prospective, placebo‐controlled studies of 983 patients (309 of whom were classified as NARES) demonstrated a positive treatment effect using INCS with improvement in symptoms of nasal obstruction, postnasal drip, and rhinorrhea. Additionally, the intranasal antihistamine azelastine and leukotriene receptor antagonists (LTRA) have been shown to reduce symptoms of rhinitis, including postnasal drainage, sneezing, rhinorrhea, and congestion., , ,
V.B.10 Non‐allergic rhinopathy
Non‐allergic rhinopathy/rhinitis is a chronic rhinitis made by a diagnosis of exclusion of other etiological factors. These include CRSwNP, NARES, AERD, infectious rhinitis, anatomical abnormalities, rhinitis medicamentosa, drug side effects, cerebrospinal fluid (CSF) rhinorrhea, and rhinitis of pregnancy. Clinical characteristics of non‐allergic rhinopathy/rhinitis include primary symptoms of nasal congestion and rhinorrhea, postnasal drip in the absence of acid reflux, throat clearing, cough, Eustachian tube dysfunction (ETD), sneezing, hyposmia, and facial pressure/headache. These symptoms may be perennial, persistent, or seasonal, and are typically elicited by defined triggers, such as cold air, climate changes (e.g., temperature, humidity, barometric pressure), strong smells, tobacco smoke, changes in sexual hormone levels, environmental pollutants, physical exercise, and alcohol. Notably, the lack of a defined trigger does not preclude the diagnosis of non‐allergic rhinopathy.
The prevalence of non‐allergic rhinopathy, the second most common form of rhinitis, is between 7% and 9.6% in the adult population in the United States (US) and Europe., Vasomotor rhinitis is the most common cause of non‐allergic rhinitis, and is found in 71% of cases., , Non‐allergic rhinopathy occurs with a female‐to‐male ratio of 2:1 to 3:1 and is typically seen after the age of 20. It is defined by the absence of an IgE‐mediated immune response. The term “non‐allergic rhinopathy” has been suggested to replace vasomotor rhinitis, as allergic inflammation is absent in the pathogenesis, although vasomotor causes may not account for the entirety of non‐allergic rhinopathy/rhinitis cases.
The nasal mucosa of patients with non‐allergic rhinopathy displays erythema and clear rhinorrhea. Allergy testing can be used to differentiate between non‐allergic rhinopathy and AR. Vasomotor rhinitis, the most common subtype of non‐allergic rhinopathy, has been linked to autonomic dysfunction and has been attributed to an imbalance between the parasympathetic and sympathetic systems.
Local allergic rhinitis (LAR) is a distinct rhinitis that presents with features in between AR and non‐allergic rhinopathy. Patients with LAR demonstrate entopy or local IgE production in the nasal mucosa but lack skin test positivity. Individuals with LAR suffer from typical allergic symptoms upon allergen exposure but display a lack of systemic IgE sensitization. Local provocation is necessary to definitively exclude this diagnosis., The prevalence of LAR among non‐allergic rhinopathy has been reported to be 26.5%. (See Section VI.A.3. Local IgE Production for additional information on this topic.) Additional forms of non‐allergic rhinopathy include food‐induced rhinorrhea and age‐related rhinitis. (See Section V.B.8. Food and Alcohol Induced Rhinitis and Section V.B.11. Age‐related Rhinitis for additional information on this topic.)
Neurosensory abnormalities are thought to play an important role the development of non‐allergic rhinopathy. In previous evaluation of central responses to olfactory stimuli, subjects with non‐allergic rhinopathy underwent functional magnetic resonance imaging following exposure to different odors (vanilla and hickory smoke). Findings included increased blood flow to the olfactory cortex, leading to the hypothesis of an altered neurologic response.,
Medical management of non‐allergic rhinopathy includes topical nasal sprays that have variable responses which have been used alone or in combination: INCS,, topical azelastine, and IPB. In addition adjunctive treatments include nasal saline sprays or lavage, especially with tenacious post nasal drip.
For severely symptomatic patients refractory to medical therapy, surgical approaches targeting the vidian nerve and its branches have been shown to result in symptom control., These include botulinum toxin injections which result in temporary symptom improvement, endoscopic vidian neurectomy, endoscopic posterior nasal neurectomy, and cryoablation of the posterior nasal nerve. Posterior nasal neurectomy is purported to result in lower rates of dry eye complications than vidian neurectomy. Recent studies show that office based cryotherapy can achieve improvement in rhinorrhea and congestion for up to 1 year.,
V.B.11 Age‐related rhinitis
As the percentage of the adult population aged 65 years and older continues to increase, so does the prevalence of diseases associated with aging. Specific to rhinologic disease, the physiological process of aging results in neural, hormonal, mucosal, and histologic alterations that cause morphological and functional changes in the nasal cavity., This, in turn, can result in symptoms of rhinorrhea, nasal congestion, postnasal drip, dry nose, intranasal crusting, and decreased olfaction in the elderly population.,
Rhinorrhea. A questionnaire distributed to a cohort of adults in Pittsburgh demonstrated that 33% of the younger age group respondents (n = 76, mean age 19 years) regularly reported clear anterior nasal drainage as compared to 74% of the older age group respondents (n = 82, mean age 86 years). It is known that autonomic function declines with age as α− and β−receptors become less sensitive. Therefore, an imbalance of this system with decreased sympathetic tone and unopposed parasympathetic stimulation could result in a rise in glandular activity in the nasal cavity, leading to increased nasal drainage., , , This mechanism is similar to the process classically termed “vasomotor rhinitis,” where the autonomic response to certain stimulants causes the nasal mucosal blood vessels to dilate and the mucus glands to become overactive, resulting in hypersecretion and excessive drainage. Vasomotor rhinitis is the most common type of non‐allergic rhinopathy/rhinitis, and the highest prevalence of non‐allergic rhinopathy is seen in the elderly,, , , supporting an autonomic nervous system mechanism as the physiologic reason for increased rhinorrhea in this population.
Nasal obstruction and congestion. Other changes that occur in the aging nose include thicker mucus secondary to a decrease in body water content,, , loss of nasal cartilage elasticity and tip support,, , mucus stasis secondary to a less effective mucociliary clearance system, , , and age‐related central nervous system changes that affect the physiologic nasal cycle,, all of which can result in nasal obstruction/congestion.
Nasal dryness and intranasal crusting. Nasal dryness and intranasal crusting in the elderly often occurs due to decreases in mucosal blood flow and an increase in epithelial degeneration. This, in turn, results in intranasal volume increase due to nasal mucosal atrophy. Schrodter et al. evaluated nasal mucosa samples from the middle turbinate (MT) of 40 healthy subjects 5–75 years old, and found an age‐related increase in atrophic epithelium (only seen in patients over 40 years) with thickened basement membranes. Nasal crusting may also occur due to a decrease in intranasal temperature and humidity in the aging nose.
Allergic rhinitis. The worldwide growth of both the aging population and allergic disease has caused an increase in the prevalence of AR in the elderly, with the prevalence estimated to be around 5%–10%., However, epidemiologic data is overall lacking and AR in the elderly population is likely under‐diagnosed and under‐treated. Although there is symptomatic overlap between age‐related rhinitis and AR in the elderly, AR is a type I hypersensitivity IgE‐mediated reaction,, whereas age‐related rhinitis is more similar to vasomotor or non‐allergic rhinopathy/rhinitis in that allergens do not play a role in the aforementioned physiologic changes of the aging nose. AR in the elderly should be treated similarly to AR in the younger population, with INCS, oral and topical antihistamines,, and AIT. For age‐related/non‐allergic rhinitis rhinorrhea, saline lavage and topical anticholinergics may be therapeutic. However, both conditions can be concomitantly present in the elderly population, presenting as a “mixed rhinitis,” and should be considered in elderly patients who are refractory to typical medical management for a singular disease.
V.B.12 Atrophic rhinitis
Atrophic rhinitis is a chronic disease of the nose presenting with symptoms of nasal dryness and crusting, persistent fetid odor, recurrent epistaxis, and nasal obstruction., It is characterized by progressive atrophy of the nasal mucosa and bone, leading to anatomically wider nasal airways, albeit many patients paradoxically complain about the symptom of nasal obstruction. Upon removing crusts, the nasal cavity appears enlarged, with significant atrophy of the nasal turbinates. Atrophic rhinitis can be classified into primary or if occurring as a sequela of a causative factor, secondary. Both primary and secondary atrophic rhinitis are significantly different in their clinical presentation and underlying pathophysiology compared to AR.
The prevalence of primary atrophic rhinitis varies across regions worldwide, with a higher prevalence in tropical countries such as India or Thailand compared to Europe or the US., , , , It is also more commonly found in young to middle‐aged adults, with a predominance of females. Primary atrophic rhinitis has also been linked to environmental and socioeconomic factors. For example, it has been more commonly found in industrial workers, those with lower socioeconomic status (SES), and those in rural areas. While there are no universally accepted guidelines for diagnosing primary atrophic rhinitis, it usually consists of a structured medical history and physical examination, including nasal endoscopy.,
The differentiation with secondary atrophic rhinitis includes the exclusion of potential causative etiologies related to secondary atrophic rhinitis, such as excessive nasal surgery, chronic granulomatous infections (e.g., tuberculosis, syphilis, leprosy), autoimmune/inflammatory disorders (e.g., granulomatosis with polyangiitis [GPA] or sarcoidosis), and excessive drug use (nasal sprays and cocaine). Studies in the US on atrophic rhinitis patients revealed that secondary atrophic rhinitis accounted for more than 80% of atrophic rhinitis cases and was most commonly found in middle‐aged adults. Compared to the diagnosis of primary atrophic rhinitis, which mainly consists of excluding potential causative etiologies related to secondary atrophic rhinitis, a complete medical history to evaluate for causative factors represents the most correctly step for correctly diagnosing secondary atrophic rhinitis.
To work up atrophic rhinitis, accurate and comprehensive medical history is important. Nasal endoscopy, cultures, and histopathology can also help clarify the diagnosis. Ly et al. identified seven key symptoms that can be used to establish the diagnosis of atrophic rhinitis: purulence, nasal obstruction, history of nasal/sinus surgeries (at least two), crusting, recurrent epistaxis, smell loss, and chronic inflammatory disease of the upper airway. While more symptoms are associated with a higher sensitivity to diagnose atrophic rhinitis, the authors proposed that the presence of at least two symptoms (excluding nasal obstruction) enhances the sensitivity and specificity to 95% and 77%, respectively, to support the diagnosis of atrophic rhinitis. Endoscopic findings usually include nasal crusting and enlarged lateral sidewalls.
The underlying etiology and pathophysiology of primary atrophic rhinitis are still unknown, although persistent bacterial infection is commonly believed to be the causative agent. Microbiological cultures from the middle meatus can aid in the diagnosis. The most common bacteria found in affected individuals is Klebsiella ozaenae,, , , albeit many other bacteria such as Staphylococcus aureus or Pseudomonas aeruginosa have also been isolated from nasal cultures., Histopathological changes in both primary and secondary atrophic rhinitis may include partial or total squamous metaplasia, granulation tissue, atrophy, reduction of the seromucous glands, and vascular changes (e.g., reduced vascularity, dilated blood vessels, and in some cases endarteritis). Interestingly, there have also been case reports which suggest primary atrophic rhinitis may have a genetic inheritance pattern.
V.B.13 Empty nose syndrome
Empty nose syndrome (ENS) is a rare and complex acquired upper airway disease. “ENS” was coined nearly three decades ago to describe the “empty” or “wide open” nasal cavity examination and imaging in patients following turbinoplasty with excess loss of turbinate tissue or contour., , , , , Clinically, it is characterized by a spectrum of debilitating symptoms like nasal burning, dryness, and crusting, accompanied by symptoms quite unique to ENS like severe suffocation, paradoxical sensation of nasal obstruction, or excessive nasal airflow (i.e., “nose feels too open”)., ,
ENS is linked to several IT reduction approaches, such as total turbinectomy, IT trimming, and radiofrequency ablation., Presentation can be immediate or delayed, secondary to over‐aggressive IT reduction or suboptimal post‐surgical healing and scarring, respectively., , While ENS is mostly associated with inferior turbinoplasty (ENS‐IT), ENS from MT tissue loss (ENS‐MT) has been reported.
The physiologic basis for perceiving reduced and/or unpleasant nasal breathing may be related to altered signaling through trigeminal sensory receptors, specifically TRPM8. Resultant aberrant thermosensation and neurosensory deprivation manifest as muted airflow sensation., , , , , Damage to, and/or delayed recovery of, the trigeminal sensory nerve has also been implicated in the development of ENS in a minority of patients. Additionally, objective shifts in nasal airflow support a novel “aberrant airflow” hypothesis., , Computational fluid dynamics modeling of nasal airflow demonstrates abnormally high velocity airflow to the middle meatus and dampened airflow vectors to the inferior meatus in ENS.
There has been welcome progress in the diagnosis and treatment of ENS in the past decade. In addition to a history of nasal surgery and abnormally expansive unilateral/bilateral nasal airway with concomitant IT tissue loss, thickened central nasal septum mucosa has been shown to be present in longstanding ENS. The validated patient reported outcome measure Empty Nose Syndrome 6‐item Questionnaire (ENS6Q) can be used to quantify the severity of six cardinal ENS symptoms on a 5‐point Likert scale. A score ≥11 indicates ENS. Placement of a cotton plug in the inferior meatus to simulate turbinate bulk (the cotton test) has been validated as an office‐based tool to assess/alleviate ENS symptoms. A positive blinded cotton test both confirms the ENS diagnosis and informs candidacy for possible treatment interventions.
ENS has historically been a challenging disease to effectively treat due to debilitating nasal symptoms and, in a minority of patients, concerning psychiatric overtones., , , , Past therapies were confined to reducing the daily burden of ENS symptoms via nasal maintenance strategies including moisturizers and emollients, increasing nasal airflow (supplemental oxygen, CPAP [continuous positive airway pressure] use), and psychiatric interventions like cognitive behavioral therapy.,
Current published interventions focus on restoring tissue volume to the truncated ITs or the adjacent inferior meatus. Submucosal injection of slow‐resorbing gel fillers can be trialed for the effect of “transient turbinate augmentation” lasting 1–3 months. A wide variety of biomaterials – including acellular dermis, implants, and xenografts – have been published as bulking options to sites of inferior meatus and IT tissue loss., , , , , Importantly, a procedure originally reported by Houser, now termed the inferior meatus augmentation procedure (IMAP), where missing turbinate contour is replaced with fashioned rounded rib grafts placed in the anterolateral nasal airway, has accumulated strong evidence for effectively treating ENS. IMAP has yielded statistically significant short and long term reductions in the ENS6Q and the Sinonasal Outcome Test (SNOT)‐22. Mechanistically, comparing computational fluid dynamics airflow modeling pre/post‐surgery, the cotton test and IMAP procedures both normalize disordered vectors of ENS airflow, highlighting a novel function of the turbinates in guiding and/or enhancing nasal airflow. Future ENS research will determine anatomic versus physiologic prognostic factors to identify “at risk” subpopulations for developing ENS, and design more nuanced airflow metrics for upper airway function in health and disease.
V.B.14 Autoimmune, granulomatous, and vasculitic rhinitis
Differential diagnosis. Vasculitic, granulomatous, and autoimmune diseases may cause non‐specific sinonasal symptoms (e.g., nasal obstruction, rhinorrhea, facial pain, and loss of smell) often mimicking AR. Therefore, broadening the differential diagnosis to consider systemic etiologies when evaluating these sinonasal symptoms is crucial. Crusting, recurrent epistaxis, or negative skin and/or blood allergy tests are among the signs that should heighten one's suspicion of alternative systemic diseases.,
Granulomatosis with polyangiitis (GPA). This is an uncommon disease with highest prevalence amongst people of Northern European descent, with men and women equally affected and incidence peaking in the seventh decade of life. It is a chronic, relapsing, and idiopathic disease characterized by necrotizing and granulomatous inflammation affecting predominantly small to medium sized blood vessels. Potential triggers include Staphylococcus aureus as well as other infectious, environmental, chemical, or pharmacologic agents.
Sinonasal manifestations (e.g., nasal obstruction, crusting, epistaxis, anosmia, cacosmia, and paranasal sinus inflammation) are the presenting symptoms of GPA in about 73% of patients. Recurrent serous otitis, mastoiditis causing hearing loss, and lower respiratory tract symptoms (e.g., cough, breathlessness, stridor, wheeze) occur in 80%–90% of patients., Additionally, renal (75% of patients), ocular (50% of patients), and systemic manifestations (e.g., fever, arthritis, weight loss) are also possible.
Diagnosis is often dependent on a multidisciplinary approach and based on a combination of suggestive local and systemic clinical manifestations, positive ANCA (anti‐neutrophil cytoplasmic antibody) serology, and histological evidence of necrotizing vasculitis or glomerulonephritis by a positive organ biopsy (skin, lung, or kidney).,
Before the introduction of effective therapy, GPA was a potentially life‐threatening disease. Treatment includes corticosteroids and immunosuppressive agents to induce remission. Cyclophosphamide and rituximab are often used for induction and maintenance. Patients can be transitioned to other immunosuppressive agents (e.g., azathioprine, mycophenolate, or methotrexate) with fewer potential side effects when disease remission is obtained.
Eosinophilic granulomatosis with polyangiitis (EGPA). EGPA (formerly Churg–Strauss syndrome) is a small‐vessel vasculitis. Defining features include eosinophil‐rich, necrotizing granulomatous inflammation involving the respiratory tract. It is associated with asthma, eosinophilia, and CRSwNP. It is a rare disease with a prevalence of 10–15 people per million in Europe and appears in patients 40–60 years old. EGPA has different triggers and frequently progresses through three stages gradually appearing over years. An initial phase with rhinitis (75%), asthma, and CRSwNP is often followed by peripheral eosinophilia and additional organ involvement, and finally diffuse clinical manifestations secondary to small vessel vasculitis. Diagnosis should be suspected in patients with asthma, increased peripheral‐blood eosinophil count (>10%) and pulmonary infiltrates. CRSwNP is present in approximately 50% of patients. Nasal crusting, purulent, or bloody discharge can be present, but is less common than in GPA. Treatment includes high doses of corticosteroids with rituximab in specific cases. Mepolizumab, an anti‐IL‐5 antibody, has shown efficacy in the eosinophilic inflammation and was approved for the treatment of EGPA in 2017 by the Food and Drug Administration (FDA).,
Sarcoidosis. This is chronic multisystem disorder characterized by bilateral hilar lymphadenopathy and pulmonary infiltrates. Ocular and skin lesions are more common in young and middle‐aged adults. Sinonasal involvement occurs in 1%–4% of cases and symptoms are non‐specific: chronic crusting (70%–90%), nasal obstruction (80%–90%), anosmia (70%), and epistaxis (2%)., , Aggressive non‐caseating granulomas can cause hard or soft palate erosions as well as a saddle‐nose deformity. Intranasal findings include erythematous, edematous, and friable mucosa, as well as submucosal yellow nodules (representative of intramucosal granulomas). Diagnosis is usually made by a lung (transbronchial), skin, minor salivary gland, or lymph node biopsy.
Sinonasal sarcoidosis treatment depends on its location, extension, and severity going from topical to systemic therapy (when nasal obstruction is severe). Endoscopic sinus surgery can be effective when medical treatment has failed, particularly in cases of sinus drainage blockage. Sinus surgery improves quality of life (QOL) but does not eradicate the disease nor prevent recurrence. Biological therapy with anti‐TNF agents has improved the therapeutic options in refractory organ‐threatening sarcoidosis.
Systemic lupus erythematosus. This is an autoimmune disease that predominantly affects women (10:1) with an incidence of 5.6 per 100,000 people. Oral, nasal (nasal skin or vestibule), and pharyngeal mucosal lesions are seen in 9%–18% of cases., Diagnosis requires a detailed medical history, physical examination, and laboratory tests (ANA [antinuclear antibody] or anti‐dsDNA [double stranded DNA]).,
Therapy with corticosteroids, immunomodulators (e.g., prasterone, vitamin D, hydroxychloroquine), or immunosuppressants (e.g., azathioprine, cyclophosphamide, mycophenolate) is used for symptom control. Belimumab, an anti‐BAFF (B cell activating factor) monoclonal antibody, is the only therapy currently utilized for extrarenal disease due to its modest effect on lupus activity. Anifrolumab, an IFN‐type 1 monoclonal antibody, has substantial evidence in effectively and safely treating moderate to severe active lupus.
V.B.15 Rhinosinusitis
The symptoms of AR may overlap with those of rhinosinusitis., Rhinosinusitis is a broad term that includes the diagnosis of acute rhinosinusitis (ARS), RARS, and CRS. Symptomatically, these conditions are characterized by nasal obstruction, nasal congestion, facial pressure or pain, anterior or posterior nasal discharge, and anosmia/hyposmia., AR and rhinosinusitis have several overlapping symptoms, namely rhinorrhea and nasal congestion, which can make it challenging to differentiate these conditions., , It is important to differentiate between AR and rhinosinusitis to ensure the correct diagnosis and subsequent treatment.
ARS is defined as the sudden onset of sinonasal symptoms outlined above with associated sinonasal inflammation that lasts less than 4 weeks – it may be viral or bacterial in nature., , , , In ARS, nasal discharge is often unilateral and purulent., Associated facial pressure and pain is described as moderate to severe. Viral ARS is typically present for less than 10 days, whereas a longer duration of illness suggests bacterial ARS., Progressive worsening over a short period of time (i.e., 5 days) is also suggestive of bacterial ARS., RARS is defined as at least four episodes of ARS per year., , , CRS is an inflammatory condition of the sinonasal cavity, defined as sinonasal inflammation persisting for more than 12 weeks with at least two of the sinonasal symptoms outlined above., , , , In addition, patients must have objective evidence of sinonasal inflammation on either nasal endoscopy (polyps, edema, mucopurulent rhinorrhea) or on computed tomography (CT) scan of the sinuses., , ,
Comparatively, AR is characterized by nasal obstruction, nasal congestion, clear watery rhinorrhea (anterior or posterior), and allergic symptoms such as nasal itching, sneezing, and allergic conjunctivitis., AR is not typically associated with purulent or unilateral nasal discharge. Moderate to severe facial pain is also atypical and may indicate an episode of ARS or an acute exacerbation of CRS., , AR symptoms are variable in duration and tend to have daily and/or local environmental fluctuations., , As a result, AR symptoms have been classified by duration (intermittent vs. persistent) and severity. AR symptoms, in general, present for at least 1 h on most days; however, patients may have symptom‐free intervals., AR symptoms are also exacerbated by exposure to allergens in a time‐dependent fashion. The early reaction occurs immediately after exposure, lasting approximately 30 min (sneezing, nasal/ocular itching, rhinorrhea), while the late reaction occurs up to 6 h after exposure (nasal obstruction and congestion). Superimposed late reactions from multiple exposures may blunt the manifestation of acute phase symptoms and make the diagnosis of AR less obvious.
When attempting to determine whether a patient has AR, ARS, RARS, or CRS, it is important to elicit the onset and duration of symptoms. A history of allergic symptoms or allergen exposure‐related symptoms is more consistent with AR., The development of acute, unilateral, moderate to severe symptoms, and nasal purulence may be consistent with ARS or RARS., , A prolonged duration of symptoms (greater than 12 weeks) as well as presence of smell loss, which is not as common in AR, should raise suspicion for CRS and prompt further investigation., , Of note, these conditions are not mutually exclusive. It is possible to have concurrent AR and rhinosinusitis, and this should be considered when patient symptomatology or response to treatment does not fit a single diagnosis., , (See Section XIII.B. Associated Conditions – Chronic Rhinosinusitis for additional information on this topic.) Careful consideration of these symptoms and environmental triggers may help guide clinicians to the correct diagnoses.
V.B.16 Non‐rhinitis conditions
There are a variety of non‐rhinitis conditions which can be included in the differential diagnosis of AR. In general, non‐rhinitis conditions can be differentiated from AR based on a thorough history and physical exam, with an emphasis on laterality, timing, and associated symptoms (Table V.B.16).
Anatomical conditions, such as septal deviation, turbinate hypertrophy, or nasal valve collapse, overlap symptomatically with AR largely by causing nasal obstruction. Septal deviations often have an asymmetry in airflow, with one side being more obstructed than the other., , Nasal valve collapse is often associated with obstruction on inspiration or during exercise., , Some congenital anatomical abnormalities such as piriform aperture stenosis or choanal atresia also cause nasal obstruction, which typically results in lifelong symptoms, which may or may not be identified in childhood. The majority of these structural conditions should be evident on a physical examination including nasal endoscopy.
Sinonasal neoplasms often present with nasal obstruction. The differential for sinonasal masses is extensive, including papillomas, hemangiomas, encephaloceles, osseous lesions, congenital masses, carcinomas, melanomas, and lymphomas., , , , Sinonasal neoplasms are typically associated with unilateral nasal obstruction, but they can cause bilateral obstruction if they grow larger or if they block the nasopharynx. When sinonasal neoplasms cause unilateral nasal obstruction, they can also be associated with unilateral rhinorrhea, which is more likely to be thick or mucopurulent. Rarely, neoplasms can erode through the skull base and cause CSF rhinorrhea, discussed below., The onset of symptoms in sinonasal neoplasms usually spans weeks to months with a progressive worsening of symptoms. Associated symptoms including epistaxis, hypoesthesia, visual changes, epiphora, trismus, or dental changes should raise the clinical suspicion for a nasal mass versus AR., , These symptoms would be highly atypical for AR and would warrant a careful physical exam, endoscopy, and sinonasal imaging, which can localize the sinonasal lesion if present.
There are a variety of other less common non‐rhinitis conditions to consider in the evaluation of AR. CSF rhinorrhea is associated with episodes of thin, watery rhinorrhea, much like AR. Unlike AR, CSF rhinorrhea is most commonly unilateral and often reproducible with positional maneuvers. While many CSF leaks are spontaneous, a history of significant head trauma or previous sinonasal surgery preceding the onset of symptoms should raise suspicion for a CSF leak over AR., Retained foreign bodies or rhinolithiasis can also cause nasal obstruction and rhinorrhea, though these are usually associated with unilateral symptoms and purulent nasal drainage., , Disorders which affect mucociliary clearance, including primary ciliary dyskinesia or cystic fibrosis, can also lead to nasal obstruction and rhinorrhea., These persistent rhinitis symptoms without allergic variation, with viscous secretions and systemic organ dysfunction are not consistent with AR and should raise suspicion for alternative diagnoses.,
There is increasing evidence suggesting an association between reflux disease and sinonasal symptoms. Reflux disease (gastroesophageal, laryngopharyngeal) has been associated with nasal congestion and postnasal drip., Congestion and inflammation of the nasal mucosa may result from acidic content directly affecting the mucosa or from esophageal‐nasal reflexes triggered by the vagal nerve., Reflux symptoms may warrant treatment but whether this improves sinonasal symptoms or not is unclear.
While many of these non‐rhinitis conditions have symptoms that overlap with AR, a careful assessment of the laterality, timing, and associated symptoms can help differentiate these conditions from AR. Similarly, a careful physical examination and nasal endoscopy will aid in identifying the correct diagnosis. A high degree of clinical suspicion will help clinicians accurately diagnose AR versus alternative diagnoses.
VI PATHOPHYSIOLOGY AND MECHANISMS
VI.A IgE‐mediated allergic rhinitis
VI.A.1 IgE/IgE‐receptor cascade
In the last several years, much has been learned about the immunologic cascade that follows antigen cross‐linking of IgE bound to cellular receptors. Three different IgE receptors have been described. The type I high‐affinity IgE receptor (FcεRI) is found on mast cells and basophils through which it mediates cellular degranulation and cytokine production. It is also found on dendritic cells and macrophages where it mediates the internalization of IgE‐bound antigens for processing and presentation, and facilitates production of cytokines promoting the Th2 immune response. The low affinity cluster of differentiation (CD)23/FcεRII receptor is found on macrophages and epithelial cells and mediates the uptake of IgE‐antigen complexes. FcεRIII is expressed by B cells and regulates IgE production and facilitates antigen processing and presentation. This section will focus on the cascade that follows activation of the high‐affinity receptor FcεRI.
FcεRI consists of an α chain which is a transmembrane protein that binds the IgE FC portion, a β chain which is a receptor‐stabilizing and signal‐amplifying subunit with four transmembrane domains, and disulfide‐linked dimeric γ chains which act as signal‐triggering subunits. Secreted IgE binds to FcεRI on mast cells or basophils. When an antigen binds or cross‐links two IgE/FcεRI complexes, activation of mast cells and basophils is triggered and degranulation occurs causing the release of histamine, tryptase, cysteinyl leukotrienes, and platelet activating factors among others., This process is known as the early allergic response and is associated with vasodilation, edema, and bronchoconstriction.,
Within the β and γ subunits of the FcεRI receptor is the immunoreceptor tyrosine‐based activation motif (ITAM). Following receptor stimulation, ITAM on the β and γ subunits undergo phosphorylation by Src family protein tyrosine kinases and recruitment of another tyrosine kinase Syk. Through conformational changes and tyrosine phosphorylation, Syk is activated. Syk is critical for most activation events within the mast cell which lead to degranulation as well as the de novo synthesis and production of chemokines, cytokines, and lipid mediators.,
Within a few hours of IgE receptor stimulation by IgE cross‐linking, activated mast cells secrete a large amount of newly synthesized proteins, a result of de novo gene transcription prompted by receptor stimulation., Following stimulation of the FcεRI receptor, human mast cells have been demonstrated to upregulate 260 genes and downregulate 84 genes for up to 2 h. The upregulated genes include gene sets encoding cell surface molecules, cytokines/chemokines, signaling molecules, transcription factors, proteases, and other enzymes. The downregulated genes include gene sets involved in signal transduction, apoptosis, cell proliferation, and genes encoding receptors.
Cross‐linking of the FcεRI receptors by antigen‐bound IgE leads to the activation of several transcription factors. These signal dependent transcription factors including signal transducer and activator of transcription (STAT)‐5, nuclear factor of activated T cells (NFAT), activator protein (AP)‐1, nuclear factor (NF)‐κB, and early growth response (EGR)‐2 function in FcεR1 upregulated gene expression. Ultimately, this complex process of de novo gene transcription and upregulation/downregulation of genes results in the production and release of cytokines and chemokines. This includes IL‐3, IL‐4, IL‐5, IL‐13, C‐C chemokine ligand‐5 (CCL5), and granulocyte‐macrophage colony stimulating factor (GM‐CSF)., , The effect of these cytokines and chemokines is the recruitment of inflammatory cells including eosinophils, basophils, neutrophils, macrophages, and T cells., , This is referred to as the late allergic response characterized by airway inflammation, hyperresponsiveness, airway remodeling, and mucus hypersecretion.
VI.A.2 Systemic mechanisms and manifestations of allergic rhinitis
Allergic diseases such as asthma, atopic dermatitis (AD), and AR share a common inflammatory pathway involving the adaptive immune system mediated by sIgE. The adaptive immune system can generally be categorized into Th1, Th2, and Th17 responses, named after the Th cells that orchestrate the corresponding immune responses. The Th1 response provides defense against intracellular pathogens, and has IFN‐γ as its canonical cytokine. The Th17 response also provides defense against pathogens, such as bacteria and fungi, and is characterized by neutrophilic inflammation and its canonical cytokine, IL‐17. The Th2 response provides defense against parasites and is marked by the expression of IL‐4, IL‐5, and IL‐13., These ILs represent integral mediators responsible for driving IgE‐ and eosinophil‐associated inflammation that often characterizes atopic disease. Type 2 innate lymphoid cells (ILC2s) are a newly characterized group of effector cells of the innate immune response that also have the capacity to produce large quantities of the type 2 cytokines, especially IL‐4, IL‐5, and IL‐13, playing a critical early role in the initiation of Th2 responses to aeroallergens during allergic inflammation., ,
In AR, aeroallergens are inhaled onto the nasal mucosa. When mucosal epithelial integrity is disrupted, epithelial cells release alarmins and other damage‐associated molecular patterns (DAMPs)., These mediators possess pro‐inflammatory properties and have been shown to assist in initiating and maintaining a Th2 immune response., For example, thymic stromal lymphopoietin (TSLP) is an important alarmin which can promote the recruitment of inflammatory cells (i.e., eosinophils, basophils, and mast cells) and the maturation of dendritic cells into Th2‐promoting subtypes, further enhancing Th2 polarization., , , It is theorized that in AR, this pathway is similarly activated and there are aeroallergens (e.g., dust mite allergens), that directly compromise the mucosa through protease activity or by activating pattern recognition receptors of which the toll‐like receptor (TLR) family is the most well‐known.
On first exposure to an allergen, dendritic cells in the nasal mucosa process the allergen and then migrate to present it on MHC class II to naive helper T (Th0) cells in secondary lymphoid organs. Once exposed to antigen/allergen in the appropriate costimulatory environment, Th0 cells become activated and differentiate into allergen‐specific Th2 cells. Th2 differentiation requires co‐stimulation via the interaction of CD28 on T cells with CD80 and CD86 on antigen presenting cells and the presence of IL‐4., IL‐4 binds STAT‐6 on Th0 cells which activates the master switch GATA‐3 (GATA‐binding protein 3). As a result, Th2 cells release cytokines such as IL‐4, IL‐5, and IL‐13 which activate B cells and initiate IgE class switching., Class switching occurs via upregulation of ε‐germline gene transcription and clonal expansion, as well as the interaction between surface CD40 ligand on T cells with surface CD40 on B cells. This process allows B cells to differentiate into plasma cells that produce sIgE. The end result is the creation of a pool of memory Th2 and B cells. sIgE is released into circulation and binds to high‐affinity FcεRI IgE receptors on the surface of effector cells such as mast cells and basophils. During IgE‐mediated reactions, prostaglandin D2 (PGD2) which is mainly synthesized by mast cells has recently been shown to exert an important role in recruitment and activation of ILC2s, in addition to leukotrienes, and innate cytokines., Crosslinking of IgE on the surface of these effector cells causes degranulation and the release of inflammatory mediators such as histamine and leukotrienes, resulting in classic symptoms of AR.
AR has traditionally been thought of as resulting from an immune response leading to systemic IgE production., The classic example of systemic reactivity in AR is the cutaneous reaction elicited during traditional skin testing. The concept of LAR is discussed in the section that follows.
VI.A.3 Local IgE production
When systemic allergen sensitization is present, sIgE is detected via serum in vitro testing or allergy skin testing. However, systemic allergy testing methods do not provide direct information regarding the target‐organ immunological response., , , Studies in recent decades support the concept of local IgE production. LAR is characterized by allergic nasal symptoms in patients with negative systemic allergy testing. However, in these patients, positive NPT and/or detection of nasal sIgE and/or positive basophil activation test (BAT) demonstrate a localized allergic response., , , , , ,
Local IgE production has been demonstrated in patients with AR, , , and LAR., , , , , , , , , In LAR, sIgE in nasal secretions has been confirmed after natural exposure,, after controlled exposure to aeroallergens by NPT,, , , , and also during periods of non‐exposure to aeroallergens., It is theorized that in LAR individuals, sIgE produced at the mucosal level can be enough to sensitize nasal effector cells, but not to reach skin mast cells or to be detected in the free state in serum.
The immunopathology of local sIgE production in LAR is not completely understood. Flow cytometry of nasal lavage confirms a nasal IgE‐mediated inflammatory response in LAR patients, with increased eosinophils, basophils, mast cells, CD3+ and CD4+ T cells, and local sIgE, along with characteristic pro‐inflammatory mediators such as tryptase and eosinophil cationic protein (ECP) during natural exposure to aeroallergens., , , , , , , , , , , , ,
NPT studies to assess potential mechanisms of local sIgE production have revealed characteristic immediate/early and late phases of the allergic response in LAR. In these patients, nasal mucosal reaction to administered allergen is immediate and occurs mostly by stimulation of IgE‐coated mast cells and basophils. This results in the secretion of tryptase, histamine, cys‐leukotriene, and PGD2, which then stimulate the local sensory nerve and vascular receptors in nasal mucosa. Mast cells secrete chemotactic agents and platelet activating factor, contributing to the development of inflammation with local production of sIgE and eosinophil activation. As a result, serum IL‐5 levels increase and IL‐5 is transported into the pulmonary circulation, causing increased exhaled NO and bronchial hyperreactivity., Finally, in a study by Campo et al., following NPT with nOle e 1 (the most significant allergen of Olea europea), 83% of LAR O. europaea sensitized subjects responded. Further, ECP levels in nasal lavage significantly increased after NPT in LAR patients indicating that secretion of ECP following NPT could potentially act as a confirmatory biomarker.
Additional studies have shown that sIgE produced in the nasal mucosa of patients with LAR sensitized to HDM and pollens has the capability of binding to the FcεRI high‐affinity receptor on basophils., Furthermore, the sIgE‐related mechanism of basophil activation in LAR has been demonstrated by performing BAT with wortmannin pretreatment, showing reversal of positive results when wortmannin was added to the assay. These findings suggest that after local IgE production, basophils might be the first target cells for sIgE produced in the target organ transported from the site of inflammation (nasal mucosa) to the general circulation.
Studies report LAR prevalence is approximately 26% in Mediterranean countries (Portugal, Spain, Italy and Greece) and 7%‐10% in Asian countries (China and Korea)., , LAR may affect approximately 47% of children previously classified as non‐allergic rhinitis., , , , Exposure to environmental factors such as temperature, humidity and pollution are associated with higher incidence of LAR., There is a low rate of conversion (∼3%) to systemic detection of allergen sensitivity, development of asthma, and worsening clinical progression is rarely seen., , , ,
VI.B Non‐IgE‐mediated inflammation in allergic rhinitis
AR is thought of as mainly an IgE‐driven response. Nonetheless, our awareness and comprehension of the important contributions of the nasal innate immune response to the pathogenesis of AR has grown immensely in recent years.
The pathophysiological mechanisms of inflammatory airway diseases are associated with large biological networks involving the environment and the host. The nasal epithelium first encounters aeroallergens in the host. Disruption of epithelial barrier function by proteolytic mechanisms, lipid‐binding activity, and interactions with polysaccharides and polysaccharide molecular recognition systems of allergens may allow allergen to penetrate into local tissues, perpetuating chronic and ongoing inflammatory processes., This may also occur with irritants like chlorine and air pollution. Epithelial barrier dysfunction has been shown to contribute to the development of inflammatory diseases including AR. However, additional research is needed to determine the extent to which primary (genetic) versus secondary (inflammatory) mechanisms drive barrier dysfunction. (see Section VI.G. Epithelial Barrier Alterations for additional information on this topic.)
Epithelial cells act as a physical barrier toward inhaled allergens and actively contribute to airway inflammation by detecting and responding to environmental factors. Nasal epithelial cells bear TLR pattern recognition receptors., , Exposure of the nasal epithelium to molecules such as allergens and pathogens results in stimulation of TLRs and the production of alarmins: IL‐25, IL‐33, and TSLP, which in turn activate dendritic cells, T cells, and type 2 ILCs. ILCs are key players in the pathogenesis of Th2 type diseases like AR, CRSwNP, and asthma., , Three major subsets have been defined based on their phenotype and functional similarities to Th1 (ILC1), Th2 (ILC2), and Th17 (ILC3) cells. The release of the cytokines IL‐25, IL‐33, and TSLP by epithelial cells directly activate ILC2s, then they produce the prototypical type 2 cytokines IL‐5 and IL‐13.
Allergen challenge in AR subjects induces increased numbers of peripheral blood ILC2s, and results in and influx of ILC2 in the nasal mucosa. Pre‐treatment with INCS attenuates allergen‐induced increases in ILC2s in the nasal mucosa of AR patients. ILC2s also contribute to epithelial barrier leakiness through IL‐13. Treatment with anti‐IL13 has shown significant reduction of AR symptoms, pointing to the important role of the innate immune system in the development of symptoms and signs of disease. AIT reduces ILC2's and increases IL‐10‐producing ILCs in the peripheral blood of AR patients. Moreover, the frequency of IL‐10‐producing ILCs correlated with improvement in clinical parameters. More novel therapies directed toward the innate immune system are in development for treatment of AR.
VI.C Cellular inflammatory infiltrates
Various types of inflammation are involved at different AR stages, including sensitization, exacerbations, remodeling, and remission. Different mediators orchestrate a type 2 immune response. Most commonly a type 2 inflammatory environment is observed with Th2 cells, M2 macrophages, eosinophils, and type 2 ILCs playing important roles. Other patterns with mixed type 2 and type 3, or even type 1 may arise depending on the allergen protease activity and the microbial and inorganic environments., As it is virtually impossible to define one inflammatory pattern, endotyping in AR seems highly important to drive personalized medicine.
Cellular interactions are important, including the role of a defective barrier and the release of epithelial alarmins. IL‐33 acts on Type 2 ILCs and promotes mast cell degranulation through inhibition of autophagy. In the induction of a type 2 response, IL‐25 acts on Th2 cells and ILC2s while TSLP mainly activates dendritic cells.
Allergen‐specific CD4+ T cells regulate multiple facets of allergen‐specific responses: IgE production in B cells, regulation of eosinophilia by IL‐5, and enhancement of type 2 inflammation by IL‐9. Antigen‐presenting cells, such as dendritic cells, are increased in frequency, higher in maturation markers CD40, and loaded with sIgE contributing to atopy, while elimination of dendritic cells suppresses AR. Dendritic cells are crucial in the initiation of a Th2 response, while basophils will merely amplify it. Myeloic dendritic cells may activate ILC2s and plasmacytoid dendritic cells play important roles in AR through IL‐2 and IL‐6 pathway alterations.
Innate and effector mechanisms affect allergic disease. A skew toward Th2 with GATA‐3 overexpression are hallmark findings in AR mucosa., Tissue γ/δ‐T cells and CD4+ memory T cells are increased. Different type 2 cytokines orchestrate the production of sIgE, eosinophilia, mucus, tissue migration of Th2 cells, and regulation of tight junctions (TJ) and barrier integrity., , , ,
Distinct phenotypes of regulatory T cells (Treg) subsets include CD4+CD25+ Forkhead‐box P3 (FOXP3)+ Tregs and type 1 Tregs., , Allergen‐specific Tregs suppress other T cells, IgE, eosinophils, and dendritic cell maturation to control AR development. They increase in the mucosa after AIT correlating with clinical remission., , The ratio between effector and regulatory cell types determines whether an allergic response is triggered. Regulatory B cells and Th17 cells may play important roles in intolerance and AR., Increased levels of CD4+ T cells were identified in AR patients’ blood with reduced CXCR3 expression.
ILCs, introduced and described in prior sections, lack rearranged antigen receptor or lineage markers. In addition to their contribution to type 2 inflammation, ILC1s increase in local sinonasal infections and ILC3s increase in remodeling. ILC2s closely interact with epithelial cells and others leading to a type 2 favoring cytokine environment. They particularly open epithelial barriers and make the tissues prone to environmental insults.
IgE‐producing B cells reside in the lymphoid follicles of the Waldeyer's ring where antibodies are transferred to the mucosa. However, B cells and plasma cells also produce IgE locally which is becoming a hallmark finding of AR. In AR, numbers of circulating memory B cells were found to be increased.
Major basic protein (MBP)‐positive and activated eosinophils can increase locally during the pollen season. This increase is not observed in the T lymphocyte subsets, neutrophils, and macrophages. Yet, mast cells seem to infiltrate the mucosa and the submucosal layer similarly to eosinophils.
Both mast cell and basophil granulocyte degranulation are relevant components of the early and late phases of a type I hypersensitivity reaction after an allergen is encountered and crosslinking of IgE occurs., Basophils accumulate within 1 h after allergen provocation in the lamina propria.
Adhesion molecules are upregulated and chemoattractants facilitate the influx of inflammatory cells during the late phase. This allows for further accumulation of cells promoting remodeling with upregulation of matrix metalloproteinases and angiogenic factors.
VI.D Cytokine network and soluble mediators
The pathophysiology of AR involves IgE‐mediated inflammation which is a type 2 immune response. IgE crosslinking results in mast cell activation and release of inflammatory cytokines such as IL‐4, IL‐5, IL‐6, IL‐13, IL‐25, and IL‐33 as well as preformed bioactive mediators and newly formed mediators including histamine, leukotrienes, prostaglandins, and kinins. These cytokines regulate the allergic inflammatory cascade through induction of IgE synthesis, upregulation of IgE production, and production of other cytokines and chemokines from epithelial cells which results in the mucosal recruitment of inflammatory cells., , Numerous cell types act as sources for type 2 cytokines including T cells, nasal epithelial cells, ILC2s, mast cells, and eosinophils.
Nasal epithelial cells secrete inflammatory cytokines including TSLP, IL‐25, and IL‐33. TSLP is a critical upstream cytokine for ILC2s, mast cells, dendritic cells, T cells, and basophils., , IL‐25, IL‐33, and TSLP secreted by epithelial cells act on surrounding cells resulting in the release of IL‐4, IL‐5, and IL‐13 which recruit additional inflammatory cells leading to a type 2 response. Nasal epithelial cells are also a source for IL‐1, IL‐6, IL‐8, and TNF‐α, and through these signals, play a role in the migration and activation of eosinophils, basophils, and Th2 cells.
ILC2s are tissue resident cells that can be stimulated to secrete IL‐4, IL‐5, and IL‐13 by the alarmins TSLP, IL‐25, and IL‐33 (which are secreted by epithelial cells or myeloid dendritic cells) via the IL‐33/ST2 pathway., , Survival factors or co‐stimulators including IL‐2, IL‐4, IL‐7, IL‐9, TNF‐like cytokine 1A (TL1A), and glucocorticoid‐induced TNF receptor ligand (GITRL) serve to maintain basic functionality of ILC2s. Both TL1A and GITRL are responsible for ILC2 proliferation and the release of type 2 cytokines from these cells. IL‐2, IL‐7, and IL‐9 are regulatory factors necessary for the development, maintenance, and survival of ILC2s. IL‐2 activates ILC2s and induces them to secrete IL‐9, which is also critical for maintaining the activity and survival of ICL2s., ,
Airway mast cells are a source of type 2 cytokines, proinflammatory cytokines, chemokines, and TSLP., , , IL‐13 from mast cells plays a role in mast cell‐induced local IgE synthesis by B cells, which in turn upregulate FcεRI expression on mast cells. Along with IL‐4 and IL‐13, TNF‐α, a proinflammatory cytokine produced by mast cells, enhances the production of thymus and activation‐regulated chemokine (TARC), TSLP, and eotaxin from epithelial cells. This suggests a crucial interplay between mast cells and epithelial cells in promoting and regulating the allergic inflammatory cascade.
Both mast cells and epithelial cells directly produce or upregulate eosinophil chemoattractants including eotaxin, macrophage/monocyte chemotactic protein 4, RANTES, and cysteinyl leukotrienes., , Eosinophils are a key factor in type 2 inflammation and are regulated by IL‐4, IL‐5, and IL‐13. These cells are also a major source of inflammatory cytokines including macrophage migration inhibitory factor, eosinophil peroxidase, and nerve growth factor.,
Finally, Th17 cells may play an important role in AR. The major cytokine of Th17 cells is IL‐17. Six isoforms of IL‐17 exist denoted as IL‐17a to IL‐17f. Currently, it is understood that IL‐17a and IL‐17f play roles in allergic‐type inflammation. Studies have shown that the production of IL‐1, IL‐6, IL‐8, matrix metalloproteinases, and TNF‐α can be induced via IL‐17 receptors on different cell types. A recent systematic review by Hofmann et al. evaluated 10 studies looking at IL‐17 levels in either serum or nasal fluid in patients with AR. In all studies, elevated IL‐17 levels in either serum or nasal fluid were observed in patients with AR compared to controls. These findings could indicate that Th17 cells and associated type 3 inflammation play a role in the pathophysiology of AR, but the exact role remains unclear.
VI.E Neural mechanisms
The pathophysiology of AR is heavily influenced by sensory neurons, axonal reflexes, and neurotransmitters. The trigeminal sensory, sympathetic, and parasympathetic nervous systems work in concert to form a protective barrier in the upper airway mucosa and regulate epithelial, glandular, and vascular processes. Branches of the trigeminal nerve innervate blood vessels and mucous membranes in the nasal cavity. The trigeminal nerve has nociceptive Aδ and C fibers that are stimulated by physical and chemical ligands as well as products of allergic reactions. Inflammatory mediators (e.g., bradykinin, histamine, acetylcholine, and capsaicin) are capable of activating sensory neurons in the trigeminal nerve, largely through TRP ion channels., , , Through repeated depolarization, lasting changes develop in TRP channels as demonstrated for the TRP cation channel subfamily V member 1 (TRPV1) and subfamily A member 1 (TRPA1). This leads to hyperexcitability of neurons in AR patients through changes in stimulation threshold and membrane potentials., Studies investigating treatment with intranasal capsaicin, the prototypic ligand for TRPV1, have demonstrated significant improvement in nasal congestion, sinus pressure, pain, and headache within 5 min after administration in patients with non‐allergic and mixed rhinitis but not clearly in AR. Furthermore, treatment with azelastine nose spray, approved by the FDA for treatment of AR and non‐allergic rhinitis, has been shown to downregulate TRP receptors.,
Depolarization of these nociceptive channels on sensory nerves leads to the release of neuropeptides including substance P, CGRP, and neurokinin‐A. Substance P receptors are located on nasal epithelium, glands, and arterial and venous vessels, and sinusoidal vessels, which leads to glandular secretion, increased vessel permeability, edema, vasodilation, and further activation of inflammatory cells., , Substance P has been recognized as a short acting vasodilator while CGRP is a long‐acting arterial vasodilator found in increased concentrations in AR patients compared to controls., , Substance P and CGRP also activate mast cells to release more inflammatory mediators, such as histamine, that further propagate the hypersensitivity reaction. Neurokinin A, a tachykinin that acts similarly to substance P, causes increased vascular permeability, vasodilation, bronchial smooth muscle contraction, mucus secretion, mast cell degranulation, as well as leukocyte chemotaxis and activation., , Understanding these biologic pathways has led to investigation of novel therapies including bradykinin antagonists and TRP receptor calcium ion channel blockers.
Parasympathetic and sympathetic nerves also play a central role in the neural response to allergens. Acetylcholine and vasoactive intestinal peptide are released during the parasympathetic response leading to mucous cell secretion, vasodilation, and epithelial cell activation via muscarinic receptors found on the nasal epithelium, submucosal glands, and blood vessels., Sympathetic nerves respond to neurokinin Y leading to vasoconstriction and nasal decongestion. A widely accepted mechanism of non‐allergic rhinitis has been an imbalance between the sympathetic and parasympathetic response leading to parasympathetic overactivity and manifests as nasal congestion, rhinorrhea, and postnasal drainage.
The neuropeptides previously discussed are significantly increased in nasal lavage of AR patients compared to controls., Upregulation of these inflammatory mediators and neuropeptides leads to peripheral sensitization of nerve fibers which can subsequently cause central sensitization or a lowered threshold for a given stimulus. Neural growth factor (NGF) is a neurotrophin that leads to survival and growth of neurons that express an NGF receptor. Sources of NGF, such as mast cells and eosinophils, are chronically activated in AR patients and may account in part for the nasal hyper‐responsiveness, increased sensory nerve concentration, and increase in neuropeptides that further propagate this inflammatory response., , , Unfortunately, clinical trials investigating neuropeptide and TRP antagonists in seasonal AR have been unsuccessful this far., ,
VI.F Histologic and epithelial changes
The nasal mucosa warms, conditions, and humidifies air entering the respiratory tract. It is also the first line of defense against pathogens, through both the innate and acquired immunity., , The structure of the nasal mucosa is well adapted to carry out these roles. The normal sinonasal epithelium forms a physical barrier, comprised of pseudostratified columnar ciliated and non‐ciliated cells, goblet cells and basal cells. The epithelial cells are linked by apical junctional complexes. At the superior nasal septum and superior turbinate, olfactory epithelium is also present, which consists of bipolar olfactory receptor neurons, sustentacular (supporting) cells, basal cells, and Bowman glands. Overlying the sinonasal epithelium is a mucus blanket, which consists of water, mucin glycoproteins, and antimicrobial peptides such as lactoferrin, lysozyme, and defensins. The mucus blanket forms a double layer, consisting of an inner serous (sol or periciliary) layer and an outer viscous (gel) layer. The basement membrane separates the epithelium from the submucosa or lamina propria.
In the presence of conditions that impair mucosal integrity, the epithelium releases alarmins and other DAMPs or pathogen‐associated molecular patterns (PAMPs) that initiate repair mechanisms and induce protective inflammation., The epithelial inflammatory response to allergens is a key feature of AR. The histological characteristics of airway inflammation are commonly goblet cell hyperplasia, mucus hypersecretion, basal membrane thickening, and airway smooth muscle hyperplasia. This inflammatory response translates into mucosal edema, increased mucosal secretions and hyper‐responsiveness common in AR. Allergens (e.g., Alternaria and HDM) are shown to enhance the chemical mediator production from nasal epithelial cells, and these allergens may induce not only a type 2 inflammatory response but also other, for example, type 1, inflammatory responses in the nasal mucosa. Nasal epithelial cells of AR patients showed increased expression of pro‐inflammatory and IL‐1 family cytokines at baseline and under stimulation, which could contribute to a micromilieu which is favorable for type 2 of inflammation. Whether robust type 2 inflammation contributes to the development of airway remodeling in AR remains controversial. One study demonstrated that after repeated nasal allergen challenge, no differences were observed in epithelial integrity, reticular basement membrane thickness, glandular area, expression of markers of activation of airway remodeling including α‐smooth muscle actin (SMA), heat shock protein (HSP‐47), extracellular matrix (matrix metalloproteinase [MMP]‐7, MMP‐9, and TIMP [metallopeptidase inhibitor]‐1), angiogenesis, and lymphangiogenesis for AR patients compared with healthy controls.
The nasal lavage samples from patients with ongoing grass pollen AR showed distinct gene expression profiles and functional gene pathways which reflect their anatomical and functional origins. Mucin production, regulated by the mucin genes MUC5AC and MUC5B in particular, is upregulated by allergens. Goblet cell hyperplasia in allergic airway inflammation is partially due to high expression of CD44v3, a surface marker for intermediate progenitor cells from basal cells. AR may be associated with increased epithelial permeability or defective epithelial barriers as a result of decreased expression of the TJ proteins occludin and zonula occludens (ZO)‐1. Impairment of ZO proteins are observed in AR patients and dysfunction of ZOs allows allergens to pass into the subepithelium. This may also be mediated by various factors such as histone deacetylase activity and deficiency of the MUC1 gene. Some allergens, such as Der p 1 in HDM, have protease activity and can directly compromise the epithelial barrier. Dysfunction of the epithelial barrier and allergen entry into the submucosa may trigger the inflammatory cascade observed in AR. (see Section VI.G. Epithelial Barrier Alterations for additional information on this topic.)
VI.G Epithelial barrier alterations
The epithelial barrier consists of different layers that defend against airborne pollutants, allergens, and pathogens, while maintaining homeostasis within the subepithelial compartment. Over 40 years ago, epithelial barrier leakiness was described in AR. A defective epithelial barrier may facilitate allergens and pathogens entering the mucosa, thus perpetuating inflammation.
Within the supra‐epithelial layer different proteins and peptides (including mucins) are found, mainly protecting against pathogens, but also against allergens. Furthermore, a large part of the nasal microbiome is found within this layer. However, improperly cleared bacteria and fungi may lead to colonization and activation of the adaptive immune system, accentuating the cycle of inflammation. Proinflammatory cytokines produced during allergic inflammation, in particular IL‐13, are known to affect mucin expression (i.e., MUC5AC), leading to viscous secretions and impairment of mucocilliary clearance. Microbial derived short chain fatty acids also impact the epithelial barrier. Sodium butyrate leads to blocking of histone deacetylase, restoring defective TJs. Synthetic histone deacetylase inhibitors show strong antiallergic effects in a HDM‐sensitized mouse model.
The epithelium itself creates the main barrier. Intercellular junctions are prerequisites of an intact barrier. TJs, adherens junctions, (hemi‐)desmosomes, and gap junctions with their connecting proteins are the main determinants of an intact epithelial barrier. They also polarize the epithelium into an apical and basolateral compartment. TJs are defective in both AR and rhinosinusitis patients., Disruption of different parts of the TJs in AR has been demonstrated microscopically and in functional analyses comparing diseased mucosa with healthy controls. Type 2 cytokines like IL‐4 and IL‐13 can disrupt the epithelial barrier leading to leakiness as shown by fluorescently labeled small molecule (fluorescein isothiocyanate [FITC])‐dextran assays. Pollen peptidases and Der p 1 were shown to actively disrupt the epithelial barrier specifically at the level of TJs., Interestingly, fluticasone treatment of air–liquid interfaces in IL‐4 exposed primary nasal epithelial cells could restore TJs even in the absence of inflammatory cells. INCS are also effective ex vivo in restoring the barrier in HDM‐sensitive AR patients’ derived mucosa (Table VI.G).
AR derived nasal secretions and histamine are strong disruptors of the epithelial barrier function. Very recently, high mobility group box‐1 (HMGB1), which is increased by transforming growth factor (TGF)‐β1 in AR, was shown to disrupt the epithelial barrier by decreasing angulin‐1/LSR (lipolysis‐stimulated lipoprotein receptor) in vitro in human nasal epithelial cell cultures. Even particulate matter (PM)‐2.5, a very fine particle found in air pollution, affects the epithelial barrier in an AR mouse model by reducing ZO‐1 expression. TSLP seems to play an important role in AR; interestingly it increases TJ proteins thus preserving the epithelial barrier. Finally, epithelial to mesenchymal transition has been shown to occur in type 2 CRS affecting the barrier function of the epithelium. Similar findings are expected to occur in AR.
There are several features of the epithelial barrier that seem impaired in AR and can contribute to the cycle of inflammation at different levels of the epithelium. This may contribute to the recently observed increase in allergies worldwide. The cause and consequence of a defective epithelial barrier in AR remains open for additional research.
VI.H Vitamin D
Vitamin D (VD3) circulates in its inactive form (25‐VD3) and is converted to its active form (1,25‐VD3) by 1‐α hydroxylase. VD3 is obtained from two distinct sources, diet and ultraviolet‐mediated synthesis in the epidermal layer of the skin. In the skin, ultraviolet rays promote biochemical reactions converting 25‐VD3 to 1,25‐VD3. The liver and kidneys also play important roles in 1,25‐VD3 synthesis. The active form of VD3 binds to vitamin D receptors (VDR), ultimately modulating gene transcription and expression. VDRs are present in several organ systems including bone, skin, intestines, kidneys, brain, eyes, heart, pancreas, and immune cells. VD3 is an important immune mediator influencing T cell activation, cytokine production, and B lymphocyte inhibition. VD3's role in AR has been a focus of investigation and the discovery of VDR on immune cells has led to research aiming to elucidate the immunomodulatory action of 1,25‐VD3.
Many immune cells, including macrophages and dendritic cells, are capable of synthesizing 1,25‐VD3 potentially shaping adaptive immune responses. While conflicting data exists, most studies suggest that type 1 inflammatory cytokines (e.g., IFN‐γ, IL‐2, TNF‐α, IL‐12) are suppressed by exposure to 1,25‐VD3 while type 2 cytokines are upregulated. The impact of VD3 on the Th1/Th2 balance has been a focus of research as it may potentially explain, in part, the role of VD3 in allergic diseases. In recent studies Th17 and Treg cells have been implicated in the development of AR as well, and among the various T cells, elevated VDR expression is found on differentiated Th17 cells., ,
Increasing numbers of epidemiological studies have linked VD3 levels with allergic disorders, especially asthma. Recent systematic reviews have demonstrated some support for VD3 in reducing asthma exacerbations, but further well‐designed studies are required., This has led to more recent investigations into the relationship between VD3 and AR.
Clinical studies investigating an association between VD3 and AR are conflicting. A recent clinical study investigating the relationship between VD3 levels and allergen sensitization to 59 aeroallergens in adults demonstrated no significant association after controlling for confounders (sex, age, and winter season). A separate cross‐sectional study looking at a pediatric population (<16 years old) found a high prevalence of vitamin D deficiency in children with asthma and AR. A recent systematic review investigating VD3 levels in AR found that prior VD3 levels were not predictive of developing AR, but lower VD3 levels were associated with higher AR prevalence in children. The precise relationship between VD3 and AR, however, is still a subject of investigation.
Similarly, the data on VD3 supplementation for AR is inconclusive. Multiple RCTs looking specifically at children with AR have demonstrated symptom improvement following VD3 supplementation., However, a recent systematic review concluded that there is insufficient evidence to support VD3 supplementation for AR prevention. Given the widespread prevalence of VD3 deficiency and its impact upon a spectrum of health aspects, physicians should consider evaluating VD3 levels, especially in children.
In summary, VD3 has critical immunomodulatory effects and has been implicated in other allergic disease processes such as asthma. There appears to be a stronger association between VD3 and AR in the pediatric population and assessing VD3 levels is a low‐risk intervention that may provide useful information in the management of AR, as well as other aspects of health. Further research is needed to elucidate the relationship between AR and VD3.
VI.I Nitric oxide
The nose and paranasal sinuses are a major site of intrinsic NO production in human airways, and AR is characterized by increased release of NO., , , , , NO plays several important roles in the maintenance of physiological homeostasis and regulation of airway inflammation, through the expression of three isoforms: neuronal NO synthase (nNOS), endothelial NO synthase (eNOS), and inducible NO synthase (iNOS).
NO is a key molecular player in the primary host defense and its cytotoxic effects are essential to prevent pathogen infection., , , However, the bacteriostatic or bactericidal effects of NO may be species‐specific. Recent studies demonstrate that bactericidal activities could elicit bitter taste receptor‐activated downstream responses, enhancing the production of NO., , NO has also shown antiviral effects against DNA and RNA viruses, including SARS‐CoV‐2, by partially inhibiting virus replication., , Moreover, NO is an important modulator of epithelial ciliary beating – important for the clearance of pathogens – through activation of the sGC‐GMPc‐PKG pathway., , , Based on these findings, NO plays a protective role against a variety of microbial infections, , , , , and has been considered an important mediator in pathophysiological events underlying inflammatory airway responses.,
NO also causes disruption of Treg cell‐mediated tolerance. Accordingly, NO derived from iNOS and eNOS affects the differentiation of helper T cells and the effector functions of T lymphocytes., The function of T cell mediated immunity can be regulated by endogenous NO at various concentrations., , NO secreted by activated dendritic cells plays a complicated role in restricting T cell activity, by inducing dendritic cell stimulatory capacity on T cells., , , , , Therefore, NO might have potential impact in the regulation of inflammatory responses through its interaction with Treg cells.
NO further links innate and adaptive immunity, regulates the adaptive immune response,, , , , and is believed to participate in both type 1 and type 2 immune responses, which may depend on the concentration of NO. Type 1 inflammation is triggered by low NO concentrations and inhibited by high concentrations,, , whereas type 2 cell proliferation can be induced by higher NO concentrations., , , , Moreover, NO is involved in T cell differentiation at the transcriptional level, and high levels of NO may activate Th2 transcription factors, upregulating IL‐4‐mediated Th2 cell differentiation., In this sense, NO is a key molecule in maintaining the Th1/Th2 balance that regulates the evolution of airway inflammation.
NO is also presumably involved in the regulation of various signaling pathways related to transcription factor activation and gene expression, as well as posttranslational regulation. NF‐κB is a key mediator regulated by NO in the airway epithelial inflammatory response, which is either increased or decreased after NO exposure, dependent on the NO concentration and the time of exposure. NO increases IL‐8 expression in airway epithelial cells, which may be important to initiate an inflammatory response in the airway epithelium., In addition, the IL‐33–ST2 axis is believed to control Th2 and Th17 immune responses in allergic airway diseases, and the balance between oxidative stress and antioxidant responses plays a key role in controlling IL‐33 release in airway epithelium.
Therefore, expression of NO and NOS in innate and adaptive immune cells reveals new functions and modes of NO action. These are particularly notable in the control and escape of microbes, T lymphocyte differentiation, interaction with NO reaction partners, and regulation of NOS by micromilieu factors, micro RNAs, and “unexpected” cytokines. However, we only understand the “tip of the iceberg” regarding NO and its role in nasal mucosal physiopathology. (See Section X.G. Evaluation and Diagnosis – Exhaled Nitric Oxide for additional information on this topic.)
VI.J Microbiome
Humans are colonized by an estimated 100 trillion microorganisms. The aggregate of these microorganisms that live on or within human tissue and fluids is termed the human microbiome. The microbiome is extraordinarily diverse – both within an individual at various anatomic sites and between individuals., , , With modern technology we can use culture‐independent high throughput sequencing techniques to gain insight into the composition of the microbiome among organs and individuals to try and understand its role in health and disease.
ICAR‐Allergic Rhinitis 2018 presented a number of studies that linked the gut microbiome to the development of allergic disease, specifically in children., , , , , However, differing methodologies, sample sizes, and culture techniques used in each study made it difficult to interpret results and draw conclusions. In the years since then, the role of the microbiome in the development of AR has been further investigated.
In an analysis of gut microbial composition of adults with AR compared to healthy controls, Watts et al. concluded that the AR cohort had reduced overall microbial diversity, with more abundant Bacteroidetes and decreased Firmicutes phyla. Similar results were reported by Zhou et al. in a smaller patient series and by Hua et al. in an evaluation of the association of the gut microbiome and self‐reported allergy utilizing data from the American Gut Project. The Firmicutes phyla is associated with butyrate production, which is an important regulator of the intestinal barrier via TJ modulation. It is hypothesized that decreased butyrate may lead to increased pro‐inflammatory molecular activity in the submucosa. In a mouse model studying the effect of intranasal sodium butyrate in AR, Wang et al. demonstrate that nasal mucosal epithelial morphology improved and levels of pro‐inflammatory markers corrected, supporting this proposed mechanism.
Although the gut is the most well studied microbiome, the nasal microbiome may also influence pathologic states, including allergic inflammation. In a study comparing the nasal microbiome of patients with AR, CRS, and a control group, Gan et al. did not find a significant difference in microorganism richness or diversity between the groups. Similarly, in a study evaluating the role of AIT on the nasal microbiome of patients with AR, Bender et al. showed no difference in the nasal microbial richness between patients with AR and controls, although they did conclude that AR patients have more similar microbiomes to each other than to controls. Gan et al. identified an association between Spirochaetae and AR, a higher abundance of Pseudomonas and Peptostreptococcaceae in AR, and lower abundance of Lactobacillus in AR. These findings may suggest a possible role of microbial dysbiosis as the pathogenesis of local mucosal inflammation. However, a mechanism for this is not yet elucidated and the validation of these results remains uncertain.
Interestingly, the differentially detected microorganism species in the adult population studied by Watts et al. were not always consistent with those found in reports that included children. The reason for this is unclear. Nonetheless, the microbes present in infancy cannot be extrapolated to adults. However, there is evidence that altered DNA methylation patterns in upper airway mucosal cells during infancy contributes to the development of AR into childhood. Longitudinal studies to understand shifts in the microbiome of AR patients over time will be required.
While it seems apparent that microbiome biodiversity is associated with microbiome fitness and alterations are associated with disease states, including AR, there are studies that contradict this assertion. Specific mechanisms of the microbe–host relationship are not well understood. Future research should provide a more complete understanding of the dynamic human microbiome during all ages and at all anatomic sites and its impact on AR. (See Section VIII.C.3. Hygiene Hypothesis and Section XI.B.9. Management – Probiotics for additional information on this topic.)
VI.K Unified airway
The upper and lower airways are linked anatomically, histologically, and immunologically to form a united airway system. Inflammation in either the upper or lower airway influences the other, giving rise to the concept of united airway disease., As the development of biological treatments options progresses, understanding the unified airway system has been recently underscored.,
The upper and lower airways share several histological features, such as in the mucosa, which is composed of columnar pseudo‐stratified epithelium and ciliated cells on a basement membrane. Likewise, the submucosa of both airway portions consists of mucus glands, fibroblasts, and inflammatory cells. Differences in histology lie in the absence of smooth muscles in the upper airways, while the lower airways lack extensive sub‐epithelial capillaries, arterial systems, and venous cavernous sinusoids, all of which are instrumental in oxygen exchange.
In the allergy realm, the concept of unified airway disease has arisen with the observation that upper and lower airway allergic diseases often coexist. Indeed, evidence has uncovered the association between AR and asthma, as well as between CRS and asthma., , Moreover, both AR and non‐allergic rhinitis have been suggested to be risk factors for asthma onset and asthma persistence, while CRSwNP has been suggested to share a common pathogenic mechanism. Interestingly, both AR and asthma have similar hyperreactivity, further solidifying the concept a unified response between the upper and lower airways., ,
Similarities between the upper and lower airways extend to endotypes, such as in type 2 immune responses. Type 2 inflammation is a prominent endotype in allergic diseases and can involve Th2 cells, type 2 B cells, IL‐4 producing natural killer (NK)/T cells, basophils, eosinophils, mast cells, ILC2, IL‐4, IL‐5, IL‐13, IL‐25, IL‐31, IL‐33., , , , In general, the type 2 profile in AR and asthma is related to a good response to corticosteroids. However, systemic corticosteroids carry serious adverse effects and side effects which generally outweigh the benefits especially in the upper airways., Alternative type 2 inflammation‐targeted treatments include anti‐IgE antibodies, anti‐IL5 (mepolizumab), and anti‐IL4/13 (dupulimab), which have been used to treat asthma – a lower airway disease – with greater efficacy. These drugs have also been shown to be effective in the treatment of upper airway disease such as CRSwNP, due to the similarities in endotype response between upper and lower airway inflammatory diseases.,
Shared characteristics between the upper and lower airways extend from acquired immune response to the role of innate immunity like epithelial barrier function and innate lymphoid cells., , , , (See Section VI.B. Non‐IgE‐mediated Inflammation in Allergic Rhinitis for additional information on this topic.) Mechanisms proposed for the interaction between upper and lower airway dysfunction include altered breathing patterns, nasal‐bronchial reflex, and uptake of inflammatory mediators in the systemic circulation. Most convincingly, AR may result in nasal blockage and the preference for oral breathing, which is associated with asthma. Additionally, small molecules such as molds and cat dander – which may pass through the upper airway into the lower airway – are associated with an increased risk for asthma; larger molecules such as tree and grass pollen are primarily associated with upper airway symptoms. The evidence supporting other hypotheses are weak. Although a clear relationship exists between postnasal drip and cough, the relationship between nasal secretions and its contact with bronchial mucosa remains unclear, since radio‐labeled allergen deposited in the upper airway it is not detected in the lower airway. Instead, stimulation of pharyngolaryngeal receptors has been suggested as the more likely cause of a postnasal drip‐related cough. Likewise, evidence supporting nasal‐bronchial reflex as an important contributor to the unified airways is lacking. Nasal allergen challenge could be blocked with a vasoconstrictor but not with lidocaine, and the lower airway responses after allergen challenge were generally more delayed than would be expected following a nasal‐bronchial reflex.
Allergen provocation studies have provided a greater understanding of the nasal‐bronchial interaction in allergic airway disease. In patients with AR, segmental bronchial provocation, as well as nasal provocation, induced allergic inflammation in both the nasal and bronchial mucosa., , Presumably, absorption of inflammatory mediators (e.g., IL‐5 and eotaxin) from sites of inflammation into the systemic circulation results in the release of eosinophils, basophils, and their progenitor cells from the bone marrow. The systemic allergic response is further characterized by increased expression of adhesion molecules, such as vascular cell adhesion molecule (VCAM)‐1 and E‐selectin, on nasal and bronchial endothelium, which facilitates the migration of inflammatory cells into the tissue. Increases in CD34+ cells capable of eosinophil differentiation, as well as other circulatory mediators (IL‐5, eotaxin, and cysteinyl leukotrienes), are associated with impaired lung function parameters and enhanced mucosal inflammation in asthmatic patients and can be inhibited by local corticosteroids in rhinitis patients. Supporting evidence suggests that treatment with biologics against type 2 inflammation has been shown to be effective in both asthma and eosinophilic upper airway disease., Overall, these studies demonstrate that AR is not a local disease but that the entire respiratory tract is involved, even in the absence of clinical asthma. Systemic factors, such as the number of blood eosinophils and atopy severity, are indicative of a more extensive airway disease.
VII EPIDEMIOLOGY OF ALLERGIC RHINITIS
VII.A Epidemiology of allergic rhinitis in adults
To assist in concretely defining the prevalence of AR in adults, recent literature has attempted to provide more uniformity in the terminology and diagnostic criteria used to identify it. The International Study of Asthma and Allergies in Childhood (ISAAC), ARIA, the European Community Respiratory Health Survey (ECHRS), and International Classification of Diseases (ICD) have recognized and adopted a more standardized definition and methodology for diagnosing AR in a given population., , As such, there has been more consistency in the response data obtained from study subjects and clarity in the criteria used in identifying AR. Nonetheless, the prevalence estimates of AR still differ widely across studies, with an approximate range of 5%–50%.,
As noted in ICAR‐Allergic Rhinitis 2018, differing AR definitions affect prevalence estimates. Incidence of physician‐diagnosed AR, which entails the precondition of being diagnosed or informed of AR affliction, potentially underestimates AR, as reflected in the South Korean National Health and Nutrition Examination Survey (KNHANES) data from 2008 to 2012 (35.02% according to questionnaire responses and ARIA guidelines; 14.89% when “diagnosed with AR by a medical doctor”). Likewise, the inclusion of at least one allergen test reaction (e.g., positive reaction to SPT) resulted in a lower prevalence estimates for AR in a Danish study in 2010 (AR, 39.0%; AR with SPT reaction, 25.9%), a Chinese study in 2018 (AR, 32.4%; AR with SPT reaction, 18.5%), and KNHANES data from 2008 to 2012 (current AR, 35.02%; AR based on allergy tests: 17.56%)., , Identification of AR according to ICD codes from databases generally yielded lower estimates for AR (German AOK Saxony database study, 6.2%). Conversely, estimates for lifetime AR were slightly higher than that of current AR, which was often defined as occurring within 12 months; this was observed in the Tromsø Study Fit Future 2, an expansion of the Tromsø Study (current AR, 26.0%; ever AR, 28.9%)., ,
Additionally, age ranges of given study samples may also capture subjects at different stages of the putative atopic march. KNHANES identified a falling AR prevalence from 21.1% in 20‐ to 29‐year‐olds, to 5.4% in over 60‐year‐olds. Considering all age ranges, AR prevalence in a Swedish study of 18‐ to 65‐year‐olds was 24%, and 27.2% in an Iranian study of 20‐ to 65‐year‐olds., Although time of year and study location may potentially affect the presence of allergens and manifestations of AR, this discrepancy can often be obviated by including the temporal range of any time “in the last 12 months.”
Notably, studies spanning longer periods of time have noted changes in the prevalence of AR. A Finnish study of conscripts’ medical data identified a 100‐fold‐increase in AR prevalence from 1966 to 1993, and reached an approximate plateau around 10.7% in 2017. Similarly, in Italy, prevalence of AR increased from 16.2% in 1985–1988, to 20.2% in 1991–1993, to 37.4% in 2009–2011 another study comprising randomly selected ECRHS subjects estimated that prevalence for AR changed from 19.7% in 1990–1994, to 23.1% in 1999–2001, to 24.7% in 2010–2012, with an overall change of 5.1%. In contrast, in Brazil the prevalence of ever having hay fever in adults decreased from 52.0% in 2011 to 43.3% in 2018.
Overall, the AR prevalence in Asia ranges approximately 5%–35%, depending on the method of diagnosis. In Europe, the most recent estimates put AR prevalence at around 25%. Variations in the prevalence were likely due to differences in participants’ age, and thus the corresponding stage of the atopic march. Regardless, considering the data available, the worldwide prevalence of AR likely ranges between 5% and 50%.
VII.B Epidemiology of allergic rhinitis in children
Several studies have attempted to describe the incidence and prevalence of AR in the pediatric population. AR symptoms have been shown to manifest in children as young as 12 months of age. A separate study of 1850, 18‐month‐olds found AR‐like symptoms and biological evidence of atopy, giving an AR prevalence estimate of 9.1%. Kulig et al., however, performed a multi‐center longitudinal study in 587 children from birth to 7 years of age in Germany and posited that two periods of seasonal allergen exposure are typically required to develop clinically significant AR. In their cohort, no children were diagnosed with seasonal AR by age 1. The remission rate of AR in children is relatively low, cited as occurring at a rate of 12% by one study performed in 2024 children from ages 4 to 8 years old.
Most studies regarding AR prevalence in children are cross‐sectional in design, of which the Phase 1 and Phase 3 ISAAC remain among the largest undertaken to date. Therein, patient‐reported symptom questionnaires were administered to hundreds of thousands of children comprising two age groups (6–7‐year‐olds and 13–14‐year‐olds) in 98 countries., , , The average prevalence of AR across all centers was 8.5% for 6–7‐year‐olds and 14.6% in 13–14‐year‐olds. In the 6–7‐year age group, the lowest current symptom prevalence was observed in the Indian subcontinent (4.2%) and the highest in Latin America (12.7%). In the 13–14‐year age group, the lowest prevalence was in Northern and Eastern Europe (9.2%), and the highest regional prevalence rates were recorded in Africa (18%) and Latin America (17.3%). Several follow‐up studies of similar design have been performed on smaller scales in several countries across the world. For instance, such survey‐based epidemiologic studies have been performed in children from Costa Rica (42.6% prevalence), Japan (18.7% in 6–8‐year‐olds, 26.7% in 13–15‐year‐olds), United Arab Emirates (46.5% in 6–7‐year‐olds, 51.3% in 13–14‐year‐olds), Nigeria (19.4% in 6–17‐year‐olds), Brazil (range of 45.3%–35.4% in children over 10 years of age), and Ecuador (48% in 3–5‐year‐olds)., , , , , These studies also indicate an overall increase in AR prevalence with age into young adulthood. Recent Chinese studies have estimated an AR prevalence averaging 28.6% in 6–12‐year‐olds in Wuhan and 28.9% in 5–18‐year‐olds in Zhongshan.,
The regional variations in reported AR prevalence highlight some limitations in questionnaire‐based, “open” studies of AR prevalence. Many of these studies might be over‐ or underestimating prevalence of AR because of disparities in responder education and researcher definitions of AR. Also, one must consider differences accounted for by measuring point prevalence and lifetime prevalence of AR. Pols et al. investigated AR prevalence by using physician‐diagnosed and treated atopic disease in a primary care database consisting of 478,076 children and found the peak point‐prevalence of AR to be 5.7% at 18 years. The lifetime cumulative incidence in this study was much higher at 16%–22.5%. A separate study conducted by Kurukulaaratchy et al. in the Isle of Wight birth cohort (1456 participants) performed SPT to define AR and observed prevalence from 5.4% at 4 years to 27.3% at 18 years. In a separate longitudinal study comprising 5471 children from birth to 10 years, de Jong et al. estimated a prevalence of allergic sensitization to be 32.2% when using skin testing results and 12.4% when using physician diagnosis.
Taken together, the available evidence indicates that the prevalence of AR in children increases with age into young adulthood. Moreover, the prevalence of AR has previously been reported to be increasing across the globe. It should be noted, however, that recently published data indicate that this trend of increasing AR prevalence may not persist into the future, although substantial geographic differences exist. The underlying factors that determine prevalence are complex, multifactorial, and reviewed in detail in the sections that follow.
VII.C Geographic variation and effect of climate on prevalence of allergic rhinitis
The prevalence of AR varies significantly based on geographic location. However, other factors such as population density (urban vs. rural) can further alter AR rates within the same locale. One important challenge in meaningfully comparing AR rates between locations is the variability created by differences in study subject recruitment and method of diagnosing AR. For example, Bauchau and Durham, who diagnosed patients via serological IgE testing after a positive telephone screen, reported that Belgium had an AR prevalence of 28.5% (the highest of the European countries he evaluated). On the other hand, Bousquet et al., who skin tested randomly sampled subjects, reported a rate in Belgium of 16.4%, one of the lowest of 15 countries examined.
Given the difficulty in standardizing AR prevalence studies across different locations, there have been major international efforts to examine national prevalence rates of AR using standardized methods (i.e., ECRHS and ISAAC). These studies show marked geographic variation with a higher prevalence of AR in “English speaking” countries (i.e., United Kingdom [UK], Australia, New Zealand), a higher rate in Western Europe than in Eastern Europe, and a higher prevalence in countries with higher rates of asthma and sensitization to seasonal allergens., However, these studies have evaluated national rates from only one or a few centers within each country, and substantial intra‐country variation may occur. For example, the prevalence of AR varies from 9.6% to 23.9% in 18 major cities in China.
Geographic variation in AR prevalence may also be impacted by climate change, which has an association with lengthening pollen seasons, increasing pollen counts, and broadening/altering the typical vegetative species for a location. Climate change has been estimated to be associated with increased seasonal pollen exposures, and as a result, sensitizations are anticipated to more than double in the next few decades, particularly in colder climates that previously were spared from higher rates of seasonal AR. Additionally, this increased environmental exposure has been shown to be associated with an increased risk of AR as well as patient symptoms of atopic nasal diseases.,
When assessing geographic variations associated with AR, differentiating between seasonal and perennial AR is also an important consideration not examined in the ECRHS or ISAAC studies. Smaller studies over more limited geographic regions which have examined perennial AR suggest increased sensitivity rates in urban settings and colder climates., , , Li et al. theorized that urban dwellers participate in more indoor activities compared to their rural counterparts, amplifying their exposure to dust mites and possibly leading to increased sensitization to these perennial allergens. Additionally, some reports suggest exposure to urban pollutants may be associated with increased AR in children.
Latitude plays a more questionable role with regards to perennial AR. For example, the prevalence of persistent AR was found to be higher in both Northern Europe and Northern China compared to their southern counterparts., This may occur because those in colder climates spend more time indoors, increasing their exposure to dust mites and other perennial allergens. However, it has also been reported that peak months for AR outpatient visits were the same in most regions of China, regardless of the latitude. Latitude may also an important determinant of seasonal AR. Allergenic plants are often characteristic for certain locations and the pollen concentrations of various species depend on the climate of a specific region.
Overall, improved knowledge of the geographic influences, seasonal variations, and the role of climate change on AR prevalence is important in that it allows patients to anticipate and better self‐manage their symptoms through avoidance techniques and preemptive use of pharmacologic therapies.,
VIII RISK FACTORS AND PROTECTIVE FACTORS FOR ALLERGIC RHINITIS
VIII.A Genetics
Hereditary factors play a role in both AR and non‐allergic rhinitis with presence of disease in family members being the strongest risk factor. Studies on twins have shown that genetic factors account for up to 70%–80% of interindividual variability in susceptibility to development of AR., However, no single gene or polymorphism can account entirely for the hereditary effect. Many genes, along with their respective variants and complex interactions, contribute to disease initiation, persistence, and severity. In this section, the current literature on the genetics of AR is reviewed, with a focus on recent large‐scale genome‐wide association studies (GWASs) and evidence for shared genetics between allergic diseases. In addition, gene–environment interaction effects and epigenetics studies are briefly covered.
VIII.A.1 Single nucleotide polymorphisms (SNPs) associated with allergic rhinitis
Genome‐wide association studies. GWASs, with their unbiased approach that includes hundreds of thousands of common variants, have successfully identified important genes for complex diseases over the past decade (https://www.ebi.ac.uk/gwas/). Thirty‐four GWASs involving AR (or seasonal AR/hay fever) have been published up to November 2021, of which nine (one exome‐sequencing project) reported genome‐wide significant hits (Table VIII.A). SNPs in LRRC32 (leucine‐rich repeat‐containing protein 32) have been strongly associated with AR in five of the GWASs,, , , , as well as with asthma,, eczema,, and other allergy‐related co‐morbidities., , LRRC32 is known to regulate T cell proliferation, cytokine secretion, and TGF‐β activation. These associations support the concept of shared genetic mechanisms for AR and other allergy‐related diseases. This concept is further supported by a GWAS on self‐reported cat, dust mite, and pollen sensitization (as well as AR), which revealed 16 shared susceptibility loci with strong association (p < 5 × 10−8; TLR‐locus top hit). Strong overlap between top loci for sensitization and self‐reported allergies also are found in two of the larger GWASs., In a recent GWAS specifically designed to evaluate pleiotropy between asthma, eczema, and hay fever, a total number of 136 SNPs were identified at the genome‐wide significant level (including 73 novel at the time), of which only six SNPs showed evidence for disease‐specific effects. In a follow‐up study, additional novel loci for comorbid allergic disease were identified by applying a gene‐based test of association. The only larger exome‐sequencing study published to date identified rare variants in IL33, a well‐known gene associated with other types airway inflammation, including asthma.
As expected, larger studies with better power allow for improved ability to accurately detect novel loci and potentially novel AR‐related disease mechanisms. Recently, very large GWASs were able to confirm many of the previously identified susceptibility loci for AR, with top hits HLA‐DQB1/DQA1, IL1RL1, TLR1/10, WDR36, and LRRC32., A recent multi‐institutional study comprising over 50,000 cases of AR identified the novel loci IL7R, which encodes the receptor for IL‐7 (and TSLP) involved in immunoregulation, and CXCR5, a chemokine receptor involved in B cell migration.
Candidate gene studies. The candidate gene approach for selecting disease‐relevant genes is based on known molecular biology or gene function relevant to disease pathophysiology. Such studies in AR have identified several well‐replicated genes, as summarized previously., , Notably, results from many candidate gene studies often overlap with GWASs results. For example, SNPs in genes involved in antigen presentation (e.g., HLA‐DQA1), pathogen recognition (e.g., TLR2,7,8), IL signaling, and pro‐inflammatory signaling (e.g., IL13, IL18, TSLP) have been highlighted., , , , , , However, many of the candidate gene study findings have not been well‐replicated across studies and populations., This could be due to lack of power from small sample sizes, inconsistent phenotype definition, or lack of true disease association.
VIII.A.2 Gene‐environment interactions and epigenetic effects
Epigenetic mechanisms, defined as changes in phenotype or gene expression caused by mechanisms (e.g., methylation) other than changes in the underlying DNA sequence, have been proposed to constitute a link between genetic and environmental factors. Recent studies show that DNA methylation in children is very strongly influenced by well‐known risk factors for allergic diseases, such as tobacco smoking/maternal smoking during pregnancy, air pollution exposure, and length of pregnancy. However, it is not currently known if these methylation changes are part of a causal pathway in the development of AR (and asthma), or if these epigenetic biomarkers are simply markers of exposure. Still, several studies have convincingly linked methylation profiles to AR, , and IgE‐related outcomes., Recently, methylation signatures in nasal epithelial brushes were shown to be strongly associated with AR (and also asthma). Also, epigenetic studies have highlighted shared molecular mechanisms underlying asthma, eczema and AR pathophysiology.
In summary, a family history of AR remains one of the strongest risk factors for disease development, and strong associations with genes involved in antigen presentation (e.g., HLA genes), T cell activation (e.g., LRRC32), and innate immunity (e.g., TLRs) have been identified. Shared genetic mechanisms for AR and other allergy‐related diseases clearly exist. These novel findings lend insight into mechanisms underlying the pathogenesis of AR, as well as comorbid atopic conditions, and may aid drug discovery efforts for novel disease targets. With increasing evidence for the role of epigenetics in AR, future research should also focus on investigating mechanisms, thereby providing a functional explanation for the link between genetics variants, environmental exposures, and disease development.
Risk factors – genetics
Aggregate grade of evidence: C (Level 3: 8 GWASs and 1 exome sequencing study. Candidate gene studies not assessed regarding grade of evidence, Table VIII.A).
VIII.B Risk factors
VIII.B.1 Inhalant allergens – in utero and early childhood exposure
VIII.B.1.a Mites
While there have not been any major new studies published on this topic since 2016, three older prospective birth cohorts (not included in ICAR‐Allergic Rhinitis 2018) concur with the conclusion that there is no established association of early mite exposure and the development of AR., , Studies showing that early life dust mite exposure results in early sensitization (e.g., positive skin tests without symptoms) and AR later in childhood are often limited in that they fail to measure and account for dust mite allergen concentrations in the home. Likewise, other studies implement dust mite reduction interventions without pre and post dust mite allergen measurements and/or combine environmental changes with dietary changes, , (Table VIII.B.1.a).
It has been suggested that the effect of dust mite exposure on sensitization may follow a bell‐shaped dose response curve, with both very low and very high exposure being protective., , , , Exposure levels that are less than 2 mg dust mite allergen/gram of house dust may be a “safe” level for atopic children for primary allergic disease prevention., The risk of allergic disease in childhood may also depend upon mono‐ versus polysensitization at age 1 or 2.
Risk factors – in utero and early childhood exposure to mites
Aggregate grade of evidence: C (Level 3: 7 studies, Table VIII.B.1.a)
VIII.B.1.b Pollen
Since ICAR‐Allergic Rhinitis 2018, no new studies were identified that addressed the impact of early pollen exposure on the development of AR; furthermore, the two previous studies were inconclusive., While very few studies longitudinally track pollen counts and the subsequent development of AR, several studies have demonstrated that the development of pollen sensitization in early life is associated with AR in later childhood., In fact, following initial pollen sensitization in children, there is a progressive increase in both the level and number of pollen sensitizations. While seasonal AR symptoms are rare before age 3, between 3 and 12 years, the percentage of new cases increases at a rate of approximately 2% per year., , With the environmental changes associated with global warming, such as increased length of pollination season, we are starting to see higher rates of pollen sensitization in young children which will likely lead to increased AR in adolescence and adulthood (Table VIII.B.1.b).
Focusing on early life sensitization rather than pollen exposure may be a more productive research pathway. Sensitization to one or more allergenic molecules (e.g., Phl p 1) at age 4 has been shown to be a better predictor of AR at age 16, then a positive test to Timothy extract. Likewise, higher levels of Bet v 1 or finding multiple pathogenesis‐related class 10 allergens at age 4 helped to predict AR to birch in adolescence. With the difficulty of conducting longitudinal pollen studies and the inability to control the year‐to‐year variation in pollen counts or the young child's level of exposure, the use of CRD in early childhood may prove to be the best tool for predicting pollen‐induced AR in adolescence and adulthood.
Risk factors – in utero and early childhood exposure to pollen
Aggregate grade of evidence: C (Level 3: 1 study, level 4: 1 study; Table VIII.B.1.b)
VIII.B.1.c Animal dander
Since the ICAR‐Allergic Rhinitis 2018, high quality studies have found that early life exposure to animal dander may be protective from the development of AR,, , while two lower quality studies concluded that it was a risk factor., A 2020 systematic review and pooled analysis of five cohort studies found a protective effect for early life exposure to cats and dogs. Two additional prospective birth cohorts found a similar protective effect., Animal exposure during the first 2 years of life offers the best possibility for protection., , , However, when reviewing all the major studies published since 2000 one finds that the majority of studies find early life animal dander exposure to be either a risk factor or unassociated with the development of AR. One possibility for this disparity is that lower quality studies were unable to account for all the confounding factors (e.g., atopic family history; community prevalence of pets; pet gender and breed; number of household pets; exposure to other indoor allergens, irritants, microorganisms; and child's microbiome). A combination of factors, such as the addition of probiotics to the child's diet, may enhance the protective effect of early animal dander exposure. At this time, it is not possible to make evidence‐based recommendations regarding early life animal exposure (Table VIII.B.1.c).
Risk factors – in utero and early childhood exposure to pets
Aggregate grade of evidence: C (Level 3: 18 studies, level 4: 28 studies*; Table VIII.B.1.c)
*Level 3 studies are listed in table; level 4 studies are referenced.
VIII.B.1.d Fungal allergens
Further supporting the ICAR‐Allergic Rhinitis 2018 conclusions, all newly reviewed studies, many having a higher evidence level, concluded that early life exposure to fungal allergens or dampness is a risk factor for AR., , Unfortunately, existing studies have not been able to establish a dose–response relationship for mold exposure and the subsequent development of AR nor have they been able to define a threshold below which no effect of mold exposure on the health of the general or high‐risk population would be expected., It may be that the presence of fungal diversity alone or in combination with microbial diversity could play an even greater role than levels of indoor mold. The role of outdoor fungal spores, which can vary widely by geographical location, has rarely been considered. While most studies adjust for demographic characteristics, the co‐exposure levels or symptoms produced by other allergens (e.g., HDM, pollen, pet dander) are rarely studied. Consistent results from well‐designed longitudinal studies are needed before one can determine the causal effect of early life exposure to fungal components on the future development of AR (Table VIII.B.1.d).
Risk factors – in utero and early childhood exposure to fungal allergens or dampness
Aggregate grade of evidence: C (Level 3: 3 studies, level 4: 12 studies; Table VIII.B.1.d)
Summary for the effect of inhalant allergens (in utero and early childhood exposure) as a risk factor for the development of AR. The impact of early inhalant allergen exposure (HDM, pollen, animal dander, fungal allergens) on the development of AR remains ambiguous. Early life allergen exposures identified as significant risk factors for AR at age 6 are often found to be insignificant by age 12 or later. Despite several in‐depth reviews and a growing body of literature,, , , no definitive conclusions may be drawn regarding risk‐benefit of early inhalant allergen exposure, and further research is welcomed to address this unmet need.
VIII.B.2 Food allergens
Historically, there has been concern that highly allergenic foods in the maternal as well as the infant's diet would lead to the development of food allergy and subsequently to other atopic diseases, such as AR. Since ICAR‐Allergic Rhinitis 2018, six publications have looked at the effect of early introduction of specific foods (e.g., fish and peanut) and diverse foods into the infant's diet and the subsequent development of AR., , , , , Older publications (not part of ICAR‐Allergic Rhinitis 2018) have looked at the effect of fish and tree nuts in the maternal diet, , and early introduction of specific or diverse foods into the infant's diet, , , (Table VIII.B.2).
A maternal diet that avoids or strictly limits highly allergenic foods, for example, cow's milk, egg, peanut, and fish has not been shown to reduce the risk of AR., , , However, a maternal diet high in oily fish or tree nuts has been reported to reduce the risk of AR.,
Early sensitization to food has been linked to the development of AR in childhood., , A meta‐analysis of high‐risk infants found that food sensitization at age less than 24 months increased the risk of AR during childhood. In a prospective birth cohort, food allergy at 4–10 years old, however, had no association with AR at age 18 or 26; whereas food sensitization (independent of symptoms) increased the risk of AR at both age 18 and 26. Additional cohort studies have found that food sensitization at age less than 24 months, especially when combined with inhalant sensitization, increases the risk of AR in childhood., , , ,
Multiple studies have evaluated the effect of early introduction of highly allergenic foods into the infant's diet. In a prospective RCT, cow's milk, egg, and peanut were avoided during the last trimester of pregnancy and during lactation and infants avoided milk, egg, peanut, and fish for 1, 2, 3, and 3 years respectively. By age 7, the food avoidance group had no reduced rates of AR. In an open label RCT, there was no association of avoiding or consuming peanuts from 4 to 11 months on the risk of developing AR at age 5 years.
In a subgroup meta‐analysis of observational studies, the introduction of fish into the infant's diet before 6–12 months was associated with a reduced risk for AR at 4 and 14 years. Three additional prospective birth cohort studies support this conclusion., , One prospective birth cohort found that introduction of rye, oat, and barley before 5–5.5 months and egg before 11 months reduced the risk of AR at 5 years old. However, there are conflicting conclusions regarding the timing of introduction of complementary foods and risk for AR.,
While guidelines have recommended that all infants have a diverse diet, the evidence is both limited and conflicting on whether this reduces the risk of AR. Food diversity has been reported to increase, decrease, decrease if there are concurrent skin symptoms, or have no effect on the risk of developing AR in childhood.
Current guidelines as well as a Cochrane systematic review recommend an unrestricted maternal diet during pregnancy as avoidance of highly allergenic foods is unlikely to substantially reduce the risk of atopic disease, including AR, in the offspring., , , Furthermore, it is recommended that complementary foods are introduced into the diet of all infants, regardless of atopic risk, at 4–6 months of age as avoidance or delayed introduction has not been shown to reduce atopic disease. Guidelines have not made recommendation on the early introduction into the infant's diet of any specific foods to prevent the development of AR.
Risk factors – in utero and early childhood exposure to food allergens
Aggregate grade of evidence: A (Level 2: 6 studies, level 3: 12 studies; Table VIII.B.2)
VIII.B.3 Pollution
According to the World Health Organization (WHO), air pollution is defined as “contamination of the indoor or outdoor environment by any chemical, physical, or biological agent that modifies the natural characteristics of the atmosphere.” Pollutants, produced through traffic‐related combustion and industrial activity, generally include NO and nitrogen dioxide (NO2), sulfur dioxide (SO2), carbon monoxide and dioxide (CO and CO2), as well as PM <10 μm (PM10) and PM <2.5 μm (PM2.5). The effect of air pollution on human morbidity is well‐known, though the relationship with AR is complex., , It is thought that through oxidative stress pathways, pollutants may stimulate the expression of antioxidant genes and recruitment of inflammatory cells to the nasal mucosa, though the mechanisms remain unclear.,
At the time of ICAR‐Allergic Rhinitis 2018, the strongest evidence in the literature suggested minimal or no significant associations between air pollutants and AR development., , , , , Kim et al. found that the incidence of AR was not significantly associated with exposure to air pollutants, while Codispoti et al. reported that diesel exhaust particle exposure at age 1 was associated with allergen sensitization at ages 2 and 3, though not to a significant degree. In a pooled prospective cohort, air pollution was reported to not be associated with adverse effects on rhinoconjunctivitis.
In more recent years, the interest in understanding a potential relationship between air pollution and AR has further increased. Li et al. reported a positive association between air pollution and AR while Burte et al. found that individuals with AR living in highly polluted areas were more likely to experience more severe nasal symptoms. Evaluating environmental air pollutants from 2013 to 2015, Teng et al. reported that levels of PM are strongly associated with the prevalence of AR. In another study, ozone and NO2, oxidant air pollutants, were associated with an 8% increased risk of AR. A meta‐analysis by Zou et al. reported increased AR prevalence in children with exposure to high levels of NO2, SO2, PM10, and PM2.5. This was further supported by an SRMA by Lin et al. who reported that PM2.5 exposure may be correlated with childhood AR. Hao et al. studied children aged 2–4 years and found that those with family stress and boys compared to girls were particularly vulnerable to increased risk of AR with early exposure to traffic‐related air pollution (Table VIII.B.3).
Co‐exposure of diesel exhaust and indoor or outdoor inhalant allergens were found to induce changes in lung protein concentrations, alter DNA methylation patterns of bronchial epithelial cells, and result in lung function impairment., , In a controlled allergen challenge facility study by Ellis et al., participants with ragweed‐induced AR aggravated by exposure to diesel exhaust particle were effectively treated with fexofenadine hydrochloride, resulting in reduced AR symptoms, compared to placebo.
The evidence demonstrating the role of air pollution on AR severity has certainly advanced. In 2018, the European Institute of Innovation and Technology launched the “Impact of air POLLution on sleep, Asthma and Rhinitis” (POLLAR) project, in efforts to use machine learning to better evaluate the relationship between sleep disorders, air pollution, and AR across six European countries. The recognition of the impact of pollution on AR is highlighted by the 2020 consensus paper published in the World Allergy Organization Journal which summarizes strategies to manage pollution‐induced AR symptoms.
Much of the current literature demonstrating the detrimental effects of air pollution on AR prevalence and severity has been from Europe and Asia. As air pollution affects all countries, future studies from all continents are needed to explore this global problem.
Risk factors – pollution
Aggregate grade of evidence: C (Level 3: 8 studies, level 4: 7 studies; Table VIII.B.3)
VIII.B.4 Tobacco smoke
Most prospective cohort studies and systematic reviews presented in ICAR‐Allergic Rhinitis 2018 have found no correlation between active or passive tobacco smoke and AR., , , One study suggested that tobacco smoke may have a protective effect against the development of AR. Similarly, pathophysiology studies examining this relationship have contradictory findings. It has been shown that tobacco smoke negatively impacts the barrier function of the bronchial epithelium leading to increased allergen penetration. A recent study in an AR mouse model showed that intranasal exposure to a tobacco smoke solution exacerbated the allergic response and increased eosinophil levels and IL‐5 expression in the respiratory epithelium. Conversely, nicotine has been shown to suppress type 2 responses to allergens, effectively acting as an immunosuppressant.
Since the last ICAR‐Allergic Rhinitis 2018, two large meta‐analyses have investigated the impact of tobacco smoke on AR., Skaaby et al. performed a Mendelian randomization meta‐analysis of data from 22 studies in the Causal Analysis Research in Tobacco and Alcohol (CARTA) consortium and the UK Biobank. The smoking‐increasing allele of rs1051730/rs16969968 was associated with a lower odds ratio of AR in current smokers. They saw similar results in their observational analysis; current smokers had a lower risk of hay fever than never smokers, and, accordingly, they saw an inverse dose–response relationship between smoking heaviness and hay fever. These results suggest that smoking may decrease the risk of AR. Zhou et al. also systematically reviewed 16 studies in a meta‐analysis of maternal tobacco smoke exposure during pregnancy and AR. This study found that maternal passive smoking during pregnancy but not maternal active smoking during pregnancy increases the risk of their offspring developing AR (Table VIII.B.4).
Recent birth cohort and prospective cohort studies have contributed to our understanding of tobacco's effect on AR development. A meta‐analysis was performed on the Mechanisms of the Development of ALLergy consortium, including five European birth cohort studies and 10,080 participants followed from pregnancy to 14–16 years of age. In this cohort, maternal smoking was not associated with a significant increase in rhinoconjunctivitis during childhood and adolescence. However, in children who developed AR, maternal smoking of 10 or more cigarettes per day during pregnancy was associated with persistent, rather than transient, rhinoconjunctivitis. Abramson et al. performed an analysis of questionnaire and sIgE data from the Swiss Cohort Study on Air Pollution and Lung and Heart Diseases in Adults (SAPALDIA) to assess secondhand smoking's impact on AR risk. They found that while those with AR were significantly less likely to be current or former smokers, there were no significant associations between secondhand smoking and AR.
It is known that AR represents a risk factor for asthma onset or worsening. A cross‐sectional study by Ciprandi et al. reported a clustering analysis to identify the subset of patients with AR at a higher risk of asthma development. This subset of patients had characteristics that included longer AR history and smoking, among others that also represent risk factors for evolving asthma. These results suggest that smoking may be a possible risk factor for asthma development in people with AR.
Another area of interest is electronic cigarettes and heated tobacco products and their impact on AR. In 2020, a survey study of Korean youth reported that current smokers of conventional tobacco cigarettes had a higher risk of AR than those using heated tobacco products and electronic cigarettes. However, the use of heated tobacco products and electronic cigarettes among conventional tobacco smokers increases the apparent risk of AR and asthma. Future research should focus on understanding the effects of these new products on a mechanistic level.
In summary, there have been few large prospective cohort studies or systematic reviews examining the effect of tobacco smoke exposure on the development of AR since ICAR‐Allergic Rhinitis 2018. The studies presented herein predominantly found no correlation between active or passive tobacco smoke and AR. However, some studies suggest that tobacco may decrease AR risk, a finding that warrants further investigation.
Risk factors – tobacco smoke
Aggregate grade of evidence: C (Level 2: 3 studies, level 3: 1 study, level 4: 2 studies; Table VIII.B.4)
VIII.B.5 Socioeconomic factors
SES describes the social standing of a group or individual and is determined by a combination of income, occupation, and education. The association of SES with AR was described as early as the 1800s. The concept of SES and its correlation with AR is similar to the hygiene hypothesis, which theorizes that a potential reduction in an individual's microbial colonization can result in an increase in allergic disease (discussed below). (See Section VIII.C.3. Hygiene Hypothesis for additional information on this topic.) As an example, Wee et al. conducted a large cross‐sectional study in over 60,000 school‐aged children and found that higher SES was associated with both improved hand hygiene and increased odds of developing AR. The role of SES in the development of AR has additional, complex underpinnings, and likely accounts for variations in a multitude of factors, including housing conditions, air quality, water supply, education, and access to care, to name a few (Table VIII.B.5).
The ISAAC studies are among the largest multi‐institutional studies evaluating prevalence of AR in children across the globe. Phase 1 and 3 ISAAC studies examined prevalence patterns of AR in ∼1.2 million children in 98 countries., , , Like most studies of AR prevalence, these studies were open, survey‐based cross‐sectional studies. A post‐hoc analysis of the ISAAC Phase 1 and 3 study data found a positive correlation between a country's gross national income per capita and national prevalence of AR. However, while statistically significant, the correlation was weak (r = 0.328 for 6–7 years, 0.206 for 13–14 years).
Chen et al. performed a large survey‐based cross‐sectional study in 173,859 adults participating in a Kaiser Permanente multiphasic health check‐up from 1964 and 1972. Their study used educational level as a marker for SES and found that post‐graduate education was associated with increased odds of hay fever. A subsequent study by Li et al. conducted in 23,971 children aged 6–13 years old in eight metropolitan cities in China found that both parental education and household income per capita predicted a higher prevalence of allergic disease. Hammer‐Helmich et al. performed a cross‐sectional, survey‐based study of SES and its association with hay fever in 9720 participants aged 3, 6, 11, and 15 years in Denmark. They found parental education level was a socioeconomic factor associated with increased risk of hay fever (OR 1.68). Income showed no association.
Studies of SES and its impact on risk of AR highlight the role that study participant education may play on the reporting of AR symptoms, or its diagnosis. This is illustrated by a study performed by Mercer et al., who evaluated 4947 children aged 13–14 in South Africa and found that residents living in low SES, but attending high SES schools, showed significantly higher prevalence of rhinitis symptoms than children in low SES schools. This suggests that education and access to medical care may affect differences in reporting in survey‐based, cross‐sectional studies.
Not all studies have demonstrated a positive relationship of AR with higher SES. A cross‐sectional study performed in Bolu, Turkey including 1403 subjects observed that poor living conditions and income was associated with a greater risk of self‐reported AR. Similarly, Lewis et al. examined allergen sensitization patterns in 458 adult women and found that lower SES was associated with increases in tIgE, number of allergen sensitizations, and sIgE levels. In a separate prospective cohort study performed in 4089 families in Sweden, Almqivst et al. found increased SES (using parent occupation as a measure of SES) to be associated with lower risk of AR at age 4. Similarly, a prospective cohort performed by Grabenhenrich et al. among 941 children up to age 20 in Germany showed no association between SES and AR development. And finally, using IgE‐based sensitivity testing (in addition to symptom‐based testing), Ahn et al. found that only high income (and not education or occupation) was associated with symptom‐based AR, but not IgE‐based AR.
Thus, while most of the available evidence indicates that higher SES is associated with increased risk of AR, the data is not uniform. SES is related to a myriad of factors, many of which play an important role in the development of AR.
Risk factors – socioeconomic factors
Aggregate grade of evidence: C (Level 2: 7 studies, level 3: 9 studies, level 4: 1 study; Table VIII.B.5)
VIII.C Protective factors
VIII.C.1 Breastfeeding
Breastfeeding is considered to have several benefits for mothers and infants. WHO guidelines recommend breastfeeding for 6 months and European Academy of Allergy and Clinical Immunology (EAACI) guidelines advise exclusive breastfeeding for 4–6 months., ICAR‐Allergic Rhinitis 2018 also documented that breastfeeding has been strongly recommended due to its multiple benefits in general; the policy level was “option” for the specific purpose of AR prevention. Several mechanisms have been suggested to explain how breastfeeding might prevent allergic disease. Breast milk contains immunomodulatory factors that stimulate host defense mechanisms and immune response., Although the association of breastfeeding with the development of allergic disease has been investigated in many studies, there is no consensus on whether breastfeeding is effective in preventing AR.
A recent SRMA revealed that exclusive or non‐exclusive breastfeeding for 6 or more months may have protective effects on the development of AR up to 18 years of age. A 2019 systematic review that included one cluster RCT and five prospective cohort studies examined the relationship between shorter versus longer durations of any human milk feeding (whether or not it was fed at the breast) and AR in childhood. The only statistically significant association was found by Codispoti et al., noting that longer duration of breastfeeding was associated with a lower risk of AR in 3‐year‐old African Americans (odds ratio [OR] 0.8; 95% CI 0.6–0.9). The authors stated that published data are insufficient to determine whether the duration of any human milk feeding was associated with AR (Table VIII.C.1).
The results from a questionnaire‐based cross‐sectional study of 4–6‐year‐old Shanghai children suggested that exclusive breastfeeding for greater than 6 months reduced the risk of hay fever (OR 0.93; 95% CI 0.89–0.97) and rhinitis (OR 0.97; 95% CI 0.94–0.99) compared to those who were never breastfed. Food Allergy and Intolerance Research (FAIR) birth cohort in the Isle of Wight, UK, also showed exclusive breastfeeding for greater than 4 months reduced the risk of rhinitis (OR 0.36; 95% CI 0.18–0.71) from birth up to 10 years of age. A recent cohort study of children with AR compared to non‐allergic rhinitis in Korea showed that breastfeeding for 12 or more months had a significantly lower prevalence of AR compared with breastfeeding for less than 6 months, and the association was still valid, accounting for age, sex, mode of delivery, number of siblings, parental atopy history, and living area (OR 0.54; 95% CI 0.34–0.88). However, in one study using a large population‐based cohort (336,364 participants) from the UK, researchers found that breastfeeding increased the risk of hay fever when adjusted for body mass index, birth weight, SES, home area, and year of birth (OR 1.11; 95% CI 1.06–1.16).
These inconsistencies in studies, which are mainly observational surveys, can possibly be influenced by demographic, socioeconomic, educational, ethnic, cultural, psychological status, and study design., , In addition, since it is difficult to distinguish between AR and viral respiratory infection at a young age, the protective effect of breastfeeding against viral infection has possibly been confused as a protective effect on AR. Furthermore, differences in methodological factors such as duration of breastfeeding, any or exclusive breastfeeding, diagnostic criteria of AR, comorbid allergic disease, and the follow‐up period may account for discrepancies in assessing the association between breastfeeding and AR.
Overall, considering the literature review on the association between breastfeeding and AR, breastfeeding should be recommended due to various positive effects on general health and possible protective effects on AR.
1 Protective factors – breastfeeding
Aggregate grade of evidence: C (Level 2: 2 studies, level 3: 4 studies, level 4: 1 study; Table VIII.C.1)
Benefit: Benefits on general health of infant and possible protection against AR, especially in young children.
Harm: None.
Cost: Low.
Benefits‐harm assessment: Slight preponderance of benefit over harm for protection against AR. Large preponderance of benefit over harm for breastfeeding for all infants, unless there is a contraindication. The benefit of breastfeeding for all infants inextricably influences this recommendation.
Value judgments: Evidence suggests that breastfeeding may reduce the risk of AR without harm.
Policy level: Recommendation for breastfeeding due to various positive effects on general health and possible protective effects on AR.
Intervention: Breastfeeding for at least 4–6 months should be encouraged unless contraindicated.
VIII.C.2 Childhood exposure to pets
Pet‐keeping families are concerned about the effects of pets on their children with regard to allergic diseases; however, the recommendations of guidelines for AR in relation to childhood pet exposure remain conflicting., , ICAR‐Allergic Rhinitis 2018 stated that early pet exposure may reduce the development of AR and its protective effect is stronger in non‐allergic families with dog exposure.
A recent SRMA investigating the association between pet exposure and the risk of AR revealed the protective effect of early cat exposure (RR 0.60; 95% CI 0.33–0.86) or dog exposure (RR 0.68; 95% CI 0.44–0.90) on the development of AR. Furthermore, early cat ownership in the first 2 years of life has been associated with a significantly lower risk of AR compared to non‐ownership (OR 0.51; 95% CI 0.28–0.92) (Table VIII.C.2).
A prospective birth cohort study in Finland revealed that having a dog in the house in the first year of life seemed to protect against AR (OR 0.72; 95% CI 0.53–0.97) by the age of 5 years compared to those without. Additional studies support the finding that exposure to pets during childhood reduces the risk of AR., Nevertheless, these studies did not make a firm conclusion about the protective effect of pet exposure on the development of AR. Heterogeneous factors such as the timing of exposure, duration of exposure, animal species, dose of exposure (number of household pets, environmental exposure vs. ownership), and avoidance behavior may be the reason.,
Furthermore, some studies have shown conflicting results. A cross‐sectional survey conducted in first graders (6–8 years old) in Taiwan demonstrated that having a cat in the first year of life was associated with an increased risk of AR. In addition, one study in Chinese children aged 0–8 years old showed a negative effect of pet keeping (aOR 3.60; 95% CI 2.07–6.27) for AR after adjustment for avoidance behavior. However, these results should be interpreted with caution because of ethnic differences, family inheritance, and other environmental risk factors that may confound of the association between pet keeping and AR. Although the exact mechanism of the effects of pet exposure on allergic disease remains unclear, it has been suggested that environmental exposure may increase or decrease the risk of AR according to the stage of immune system development., , ,
Overall, the causal relationship between pet exposure in childhood and the protective effect of AR is inconsistent; thus, no strong advice can be provided regarding childhood exposure to pets. Nevertheless, pet exposure at birth or in the first year of life may reduce the risk of AR.
Protective factors – childhood exposure to pets
Aggregate grade of evidence: C (Level 2: 1 study, level 3: 2 studies, level 4: 2 studies; Table VIII.C.2)
Benefit: Exposure to pets at birth and in the first year of life has potential benefits of decreasing risk of AR.
Harm: Pet keeping in childhood could have a negative effect, especially in Asians.
Cost: Various.
Benefits‐harm assessment: Difficulty distinguishing between benefits and harm.
Value judgment: There is conflicting evidence that childhood pet exposure prevents the development of AR.
Policy level: Option.
Intervention: Recommendation to expose or avoid pets for the prevention of AR in children cannot be provided based on current evidence.
VIII.C.3 Hygiene hypothesis
The hygiene hypothesis originated from the observation that frequent and recurrent infections in early childhood appear to protect against the development of AR later in life. Over time, the hygiene hypothesis evolved to the biodiversity hypothesis, which expands the scope from the protective effect of infection from single microbes to the protective effect of microbial variety during development. The microbiota hypothesis was later proposed to confine the causative microbes specifically to those living in or on the human body and their impact on our immune system.,
An SRMA was conducted to determine the effect of the number of siblings on AR development; this analysis assessed 53 studies with 300,062 participants. They saw a strong inverse association between many siblings (three or more) and the development of AR. Similarly, a large international cohort study based on questionnaire data for children aged 6–7 and 13–14 years also saw an inverse association between the number of siblings and AR but only in affluent countries (Table VIII.C.3).
It has also been observed in several studies that exposure to early‐life farming may protect against childhood allergic diseases particularly, exposure to farm animals and stables., , , , , , , , , , In a recent meta‐analysis by Campbell et al., the risk of sensitization measured by sIgE or SPT in childhood or adulthood was 40% lower among children who had lived on a farm during the first year of life. Further, a 2017 US case–control study showed farm exposure in utero provides even greater protection against sensitization in adulthood. While an isolated exposure to bacterial endotoxin was claimed to have a similar protective effect, the results thus far have been inconclusive.,
Increased diversity in the gut and skin microbiome has been associated with a protective effect on atopy., , , , , , Recently, three large cohort studies have reported that reduced bacterial diversity in the infant's intestinal flora within the first 6 years of life predisposes them to a higher risk of developing AR., , Notwithstanding this, a meta‐analysis of 29 trials did not find supplementation of probiotics to pregnant mothers or infants beneficial in preventing atopy. A publicly available American Gut Project questionnaire and database was used in a study to determine the fecal microbiota richness and composition in adults with AR. They found an imbalance (dysbiosis) of gut flora with higher Bacteriodes and reduced Clostridia taxa in this population. In addition, the role of Helicobacter pylori has been investigated, with inconsistent findings., , Interestingly, in a meta‐analysis of 21 studies assessing the association between H. pylori infection and allergic diseases, a significant inverse association was found between H. pylori infection with atopy from the case–control studies while an association was seen between allergic disease and H. pylori infection from the cross‐sectional studies.
Lower biodiversity on the skin and in the home living environment is associated with an increased risk of atopy. Ruokolainen et al. performed a comparative study of the microbiota of skin and nose in randomly selected school children from urban and rural areas. They saw that rural school children had increased microbial diversity on their skin and in their noses and this was associated with lower allergy prevalence compared urban school children.
In summary, there is some evidence of the protective effect of the hygiene hypothesis on AR from epidemiological studies but more studies that evaluate causality are needed. (See Section VI.J. Microbiome and Section XI.B.9. Probiotics for additional information on this topic.)
Protective factors – hygiene hypothesis
Aggregate grade of evidence: B (Level 1: 4 studies, level 3: 12 studies, level 4: 3 studies, level 5: 2 studies; Table VIII.C.3)
IX ALLERGIC RHINITIS DISEASE BURDEN
IX.A Individual burden
IX.A.1 Quality of life
High quality evidence evaluating the impact of AR on QOL continues to show AR patients suffer from decreased general and disease‐specific QOL due to impacts on physical and mental health., , , , , These studies also show that treatment of AR with INCS, oral antihistamines, and AIT leads to improved QOL. Validation of QOL metrics in AR continues. There has been a trend toward use of disease specific QOL metrics, especially the RQLQ. As this has become more accepted, the use of general health related QOL metrics such as Short Form 12 and 36 (SF‐12/36) has decreased., A measure of QOL used in CRS, the SNOT‐22, has now been studied in AR. This study showed SNOT‐22 was able to assess QOL and response to treatment in AR. Olfaction, an objective measure of QOL also typically used in CRS, has also been studied in AR recently. Olfactory dysfunction was identified in 44% of patients with AR. The use of SNOT‐22 and objective measures of olfaction could simplify implementation of QOL monitoring for both diseases from a clinical standpoint (Table IX.A.1).
Despite the availability of disease specific QOL instruments, many studies continue to rely on unvalidated methods to assess QOL. This leads to difficulty comparing outcomes between some studies. A recent SRMA evaluated the outcomes of medical therapy with INCS, oral antihistamines, or AIT for AR. Treatment with oral antihistamines and AIT had a statistically significant impact on QOL. Despite near universal acceptance of INCS for the treatment of AR, meta‐analysis of the impact of INCS on QOL could not be performed due to a lack of available data. There are numerous individual RCTs evaluating the effect of INCS, oral antihistamines,, , , and AIT., , , The overarching findings in these individual RCTs is that these treatments improve QOL.
While numerous studies exist comparing changes in symptoms with treatment for AR, direct, head‐to‐head comparisons of changes in QOL with different treatments for AR are lacking. There is only one study comparing the impact of monotherapy with INCS (mometasone) to combination therapy with INCS and oral antihistamine (mometasone + levocetirizine) or INCS and leukotriene D4 receptor antagonist (mometasone + montelukast) on QOL as measured with the 14‐question mini‐RQLQ. This study found that polytherapy with mometasone and levocetirizine or montelukast improved QOL more than mometasone alone; no difference was seen between montelukast or levocetirizine when added to mometasone.
New evidence evaluating the impact of AR on QOL in children and in the parents of children with AR is emerging. As expected, these studies show impacts on QOL in this population. More surprisingly, they show impacts on parental QOL as well., , , In one study, parents overestimate their children's QOL. This focus on assessing QOL in children and adolescents with AR was built on prior work measuring general QOL in children with instruments such as KINDL. Disease‐specific instruments (Pediatric Rhinoconjunctivitis Quality of Life Questionnaire [PRQLQ] and RhinAsthma Patient Perspective [RAPP]‐children) have now been developed to measure the impact of AR on QOL in pediatric and adolescent populations., In children and adolescents with persistent AR, those with nasal obstruction secondary to septal deviation or turbinate hypertrophy have the worst QOL. Nasal endoscopy should be considered in patients in this population not responding to therapy to ensure nasal obstruction is not contributing.
Variations in QOL in AR patients have not been prospectively studied over time. Most studies are either cross‐sectional or have short follow‐up periods with few time points at which QOL is assessed. Control groups from RCTs and meta‐analyses of RCTs can provide insight into long‐term variation in QOL in AR, however. Two RCTs have studied the effect of oral antihistamines with a follow‐up period of at least 6 months., These RCTs show that both the placebo and treatment groups experience clinically and statistically significantly improvements in generic and disease specific QOL, but the improvement is greater in the treatment arm. A more recent meta‐analysis of a combination INCS and intranasal antihistamine showed short‐term but not long‐term QOL improvement with this treatment. This latter finding, however, was based on a single study. AIT RCTs have longer follow‐up periods (12 months to 3 years) and show similar results, with placebo patients either remaining at baseline or improving to a lesser degree than the treatment arms., , As expected, patients with seasonal AR have worse QOL during seasons in which they are exposed to allergens and improved QOL outside of these seasons.
Disease burden – quality of life
Aggregate grade of evidence: B (Level 1: 6 studies, level 2: 35 studies, level 3: 15 studies; Table IX.A.1)
Benefit: Successful treatment of AR leads to improved overall and disease specific QOL.
Harm: Depending on the specific treatments for AR, there are variable levels of harm (Table II.C).
Cost: Treatments for AR have variable costs.
Benefits‐harm assessment: The benefits of treating patients with AR to improve QOL likely outweigh risks of treatment.
Value judgments: Validated measures of QOL should be utilized in future studies of treatments for AR.
Policy level: Recommendation.
Intervention: Validated measures of QOL should be utilized in future studies of treatments for AR.
IX.A.2 Sleep disturbance
AR affects 20%–30% of adults and children with OSA and sleep disordered breathing (SDB)., Multiple studies have investigated the relationship between AR and sleep in adults and children. The general conclusion from the aggregate data is that similar to overall and rhinitis specific QOL, AR negatively impacts sleep quality, and the successful treatment of AR reduces sleep disturbance. Overall, the data is of low to moderate strength, with the overall quality of the data being higher for adults than for the pediatric population. For the adult population, there is strong evidence supporting the conclusion that AR negatively impacts sleep., , , , This data deals with subjective reporting of daytime sleepiness, sleep quality, and symptoms usually through validated tools, in the setting of testing the effect of INCS and montelukast (Tables IX.A.2.‐1 and IX.A.2.‐2).
In children, lower quality data suggest that AR is associated with sleep disturbance in the form of increased risk of snoring, SDB, and OSA. However, the findings here are not uniform, with some studies suggesting that while the prevalence of AR is high in the OSA population, AR might not impact disease severity., Furthermore, AR has been suggested to be a risk factor for deterioration of OSA QOL after adenotonsillectomy. Additionally, AR may increase the risk of nocturnal enuresis in children.
Two studies looked at variations in sleep symptoms with changes in nasal inflammation over time. Nasal cytokine level alterations are associated with changes in the polysomnogram (PSG) and AR patients have worse PSG parameters and sleep disturbance when their symptoms are present or during their peak allergen season. The data on PSG parameters in adults is mixed. Most studies that perform PSG found that AR worsens PSG parameters, , , , , , , , , , ; however, two studies found either no difference or a modest change.,
AR patients have improvements of sleep quality, daytime sleepiness, sinonasal symptoms, and QOL after treatment with INCS, , , or a combination of INCS and montelukast. Additionally, AR has been associated with worse sleep fragmentation, and snoring., In addition to reducing sleep disturbance, treatment of AR has been suggested to also improve CPAP compliance. (See Section XIII.K. Associated Conditions – Sleep Disturbance for additional information on this topic.)
Disease burden ‐ sleep disturbance
Aggregate grade of evidence: B (Level 2: 5 studies, level 3: 8 studies, level 4: 50 studies Tables IX.A.2.‐1 and IX.A.2.‐2).
Benefit: AR negatively impacts sleep quality. Successful management of AR leads to decreased sleep disturbance in adults and children.
Harm: Medical management of AR is generally low risk and medications have low side‐effect profiles. AIT is associated with rare serious adverse events (Table II.C).
Cost: Associated costs consist of the direct costs of allergy testing and medical management, and indirect cost of increased time and effort for AIT.
Benefits‐harm assessment: The benefits of treating patients with AR may outweigh any associated risks.
Value judgments: In patients with AR, the successful control of symptoms with medical management or AIT can lead to important improvements in sleep disturbance. The level of available evidence is stronger for the adult population compared with the pediatric population.
Policy level: Treatment of AR to improve sleep disturbance – Recommended in adults. Option in children.
Intervention: INCS, oral antihistamines, montelukast, and AIT are appropriate options, when medically indicated, to improve sleep disturbance in patients with AR.
IX.B Societal burden
AR has a high prevalence globally and imposes negative effects on QOL and therefore a burden to individuals and society. Due to its chronicity and prevalence, AR poses a significant socioeconomic burden., The true burden of AR involves direct, indirect, and societal costs. Direct costs relate to financial expenditures on healthcare related to AR, including the diagnosis, prevention, and management of disease. Indirect costs are due to loss of productivity related to disease including job loss, absenteeism, and presenteeism. Additional costs include costs due to reduced QOL and societal costs related to an individual's symptoms and subsequent reduced QOL., , ,
In the US, AR is the fifth most burdensome chronic condition when considering total cost. Direct costs of AR in the US exceed $4.5 billion per year., , , , Likewise, AR represents a large direct economic burden in several other countries., , Medication expense makes up most of the direct cost, but additional costs include office visits, testing, and procedures. These costs are even higher when considering patients with related illnesses such as asthma, allergic conjunctivitis, and CRS., , Despite many treatments being available over‐the‐counter, US medication costs for only AR are estimated to exceed $1 billion (US), and patients with AR are also more likely to utilize clinic visits, further driving direct costs.,
AR leads to increased direct costs in countries around the world. A 2021 US study demonstrated that AR patients had annual mean costs of $218 (US) for clinic visits and procedures, and additional $111 (US) for medications. In a 2020 Dutch study comparing 350 AR patients to controls, those with AR spent an additional €208 per year in direct costs. In a 2016 study of 8001 Swedish residents, direct costs attributable to AR were €210 per individual per year. A 2017 French study demonstrated median direct costs of €159 for AR without asthma and €375 for AR with asthma. Studies from Turkey showed increased costs of $79 to $139 (US) for AR patients. Studies from South Korea and India also demonstrate significant direct costs., ,
Despite its perception as a nuisance disorder, AR has significant effect on QOL and accounts for substantial indirect costs related to missed work or school and poorer productivity. AR results in 3.5 million missed workdays and 2 million missed school days. However, indirect costs account for a larger proportion of the burden of AR than the direct costs. In the US, AR has been shown to contribute to greater than $5 billion (US) in lost productivity yearly. These costs include absenteeism, but health impairments of AR are often not severe enough to cause absenteeism. AR symptoms can interfere with cognitive functioning, resulting in fatigue and impaired learning, concentration, and critical thinking leading to presenteeism or reduced productivity while at work. As such, presenteeism accounts for the majority of reduced productivity related to AR., ,
In the US, AR is the most prevalent condition among the workforce, and accounted for 52 symptomatic days per year with a mean productivity loss of $518 (US) per employee per year. In the UK, impaired productivity and/or missed work occurred as a result of AR in 52% of patients. In India, 37% percent of surveyed patients with AR endorsed presenteeism and AR was responsible for $460 (US) loss per patient annually. In Sweden, indirect costs were calculated to be €751 per patient annually. In The Netherlands, indirect costs were estimated to be €3681 per patient annually, and presenteeism accounted for the majority of lost productivity. In a Spanish study, presenteeism made up 95% of the loss in productivity and was estimated €1772 per year.
Additionally, there are indirect economic losses that come from caregivers missing work while a child is absent from school. In a Swedish study, the cost of caregiver absenteeism comprised 19% of the mean total costs per year. The cost related to caregiver absenteeism was highest for women aged 30–44 years.
AR is also the most prevalent chronic disorder among children, as such it has a significant impact on education., On any given day in the US, approximately 10,000 children are absent from school because of AR. AR can alter sleep quality resulting in daytime sleepiness, impaired cognition, and poorer memory in children that significantly affects the learning process and impacts school performance., , Even when present during school hours, children with AR exhibit decreased productivity. Conditions associated with AR such as rhinosinusitis, ETD and associated conductive hearing loss may enhance the learning dysfunction.
Additionally, AR has been associated with negative impact on mental health with functional decline as well as major depression, further reducing overall QOL., , This relationship has been shown in studies from Europe, the US, and Asia.
AR represents a significant personal and socioeconomic burden that will likely worsen as the prevalence continues to increase., It can reduce productivity and QOL in affected patients and contribute to comorbid conditions. This results in a significant impact to the overall health system.
X EVALUATION AND DIAGNOSIS
X.A History and physical examination
X.A.1 History
A crucial component in the diagnosis of suspected AR rests on clinical history., , , , This includes symptoms experienced, timing of symptoms, duration, frequency, patient occupation/school/home environmental exposures that elicit symptoms, and any measures or medications that improve or worsen symptoms., , , , , Other comorbid conditions in the past medical history, such as asthma, OSA, family history of atopic disorders, and medications currently taken should be gathered., , , , , Patient response to self‐treatment with over‐the‐counter medications is helpful information, and with advancing technology mobile applications may allow for the potential collection of patient symptomatology to identify symptom patterns that may be very useful for treating providers.
Classic symptoms of AR include nasal congestion or obstruction, nasal pruritis, rhinorrhea, and sneezing. In addition, patients may complain of other symptoms associated with comorbidities including ocular pruritis, erythema, and/or tearing (allergic conjunctivitis), oral cavity or pharyngeal pruritis (allergic pharyngitis), throat clearing, and wheezing or cough (reactive airway disease and/or asthma)., , , , , Snoring or sleep‐disordered breathing, aural congestion or pruritis, and wheezing are other frequent symptoms., , , In the coronavirus disease 2019 (COVID‐19) era, symptoms of hyposmia or anosmia, cough, and/or sore throat, which potentially may also be associated with AR, may cause confusion, and should prompt consideration for other diagnoses, such as active COVID‐19 infection., ,
Patients with suspected AR will commonly present with multiple complaints, frequently with two or more symptoms., , Perennial AR patients have a tendency to report more congestive symptoms (sinus pressure, nasal blockage/congestion, and snoring) than seasonal AR patients. Also, perennial AR patients more frequently complain of sore throat, cough, sneezing, rhinorrhea, and postnasal drip. Prior to the COVID‐19 pandemic, symptoms of rhinorrhea, sneezing, sniffing, hyposmia/anosmia, nasal obstruction, and itchy nose ranked highest in diagnostic utility among symptoms of AR; however, the diagnostic utility of hyposmia/anosmia, nasal obstruction, and congestion may be less given the overlap in COVID‐19 symptomology., ,
Despite the dearth of high‐level evidence, many guidelines suggest that history of two or more symptoms consistent with AR is sufficient for making the diagnose of AR, , , , , (Table X.A.1). Since AR lacks pathognomonic physical examination findings, physical examination alone to diagnose AR has been shown to have poor predictive value. The reliability and predictive value of the patient history for AR exceeds that of the physical exam alone. In clinical practice, the presumptive diagnosis of AR is often made by only history, even more so during the pandemic with increased utilization of telemedicine where a physical examination is limited., ,
Patient history
Aggregate grade of evidence: D (Level 4: 5 studies, level 5: 7 guidelines or expert recommendations; Table X.A.1)
Benefit: Improves accuracy of diagnosis, avoids unnecessary referrals, testing, or treatment.
Harm: Potential misdiagnosis or inappropriate treatment.
Cost: Minimal.
Benefits‐harm assessment: Preponderance of benefit over harm.
Value judgments: Using history to make a presumptive diagnosis of AR is reasonable and would not delay treatment initiation. History should be combined with physical examination, which may not be possible in some scenarios such as telemedicine. Confirmation with diagnostic testing is required for progression to AIT or targeted avoidance therapy, or desirable with inadequate response to treatment.
Policy level: Recommendation.
Intervention: Despite low level evidence specifically addressing this area, history is essential in the diagnosis of AR.
X.A.2 Physical examination
Whenever possible, it is important to include physical examination as part of the evaluation of suspected AR patients., , , , , Telemedicine may complicate this part of the evaluation, but a limited visual examination may be obtained. An assessment of head and neck organ systems should be completed with the use of any necessary personal protective equipment., , , If there are patient complaints of wheezing or coughing with allergic triggers or comorbid conditions of asthma, the physical examination may include auscultation of the lungs.
An unremarkable physical examination is common for AR patients, particularly those with intermittent exposure. Observation alone may reveal possible signs suggestive of AR, which can be useful during telemedicine visits. These signs include mouth‐breathing, nasal itching or a transverse supratip nasal crease, throat clearing, periorbital edema, or “allergic shiners” (dark discoloration of the lower lids and periorbital area)., Ear examination may reveal retraction of the tympanic membrane or transudative fluid, although evidence for association of effusion with AR is low level. Anterior rhinoscopy may reveal IT hypertrophy, congested/edematous nasal mucosa, purplish or bluish nasal mucosa, and clear rhinorrhea., , Eye examination may reveal conjunctival erythema and/or chemosis.,
Physical examination by itself is more variable and poorly predictive of the diagnosis of AR when compared to history‐taking, with the average sensitivity, specificity, positive predictive value (PPV), and negative predictive values (NPV) of the patient history higher than those of the physical examination. Most guidelines recommend a physical examination as part of the diagnosis of AR, despite a lack of high level evidence; however, pandemic conditions and the utilization of telemedicine may limit the completeness or possibility of physical examination (Table X.A.2). Without a physical examination, other potential causes of symptoms such as CRS may not be fully evaluated or eliminated, so if there are limits placed by telemedicine, additional diagnostic measures may need to be considered, such as a CT scan of the sinuses. A patient history combined with a physical examination improves diagnostic accuracy.
Physical examination
Aggregate grade of evidence: D (Level 4: 2 studies, level 5: 6 guidelines; Table X.A.2)
Benefit: Possible improved diagnosis of AR with physical examination findings, along with evaluation and/or exclusion of alternative diagnoses.
Harm: Possible patient discomfort from routine examination, not inclusive of endoscopy.
Cost: Minimal.
Benefits‐harm assessment: Preponderance of benefit over harm, potential misdiagnosis, and inappropriate treatment if used in isolation.
Value judgments: Telemedicine is a safe and useful tool in pandemic conditions but does limit what can be gleaned from physical examination. Without the use of nasal endoscopy, it is possible some physical examination findings may be missed.
Policy level: Recommendation.
Intervention: When possible, physical examination should be performed with appropriate personal protective equipment to aid in the diagnosis of AR and exclusion of other conditions. When combined with patient history, it increases diagnostic accuracy and may exclude alternative causes of symptoms.
X.A.3 Nasal endoscopy
Diagnostic nasal endoscopy may complement the evaluation of patients with suspected AR. Several case series and cross‐sectional studies have evaluated the association of endoscopic findings with the diagnosis and severity of AR (Table X.A.3).
Ziade et al. studied a prospective cohort of adult patients with AR symptoms and skin testing confirmation, showing that mucosal edema and bluish discoloration of the ITs were highly predictive of the severity of AR disease (p < 0.05) when comparing patients with mild versus moderate/severe AR. Conversely, early studies by Jareoncharsri et al. and Eren et al. evaluated a population of adults and children with AR confirmed by allergy testing, concluding that findings of nasal endoscopy do not provide a reliable diagnosis or correlate with specific nasal symptoms of AR.
Additionally, Ameli et al. evaluated a large cohort of children with suspected AR and confirmed with skin testing, reporting that endoscopic findings of IT or MT septal contact as well as pale mucosa and large adenoid volume were highly predictive for AR. Notably, there were conflicting results in a previous study by the same group that reported no predictive role of pale mucosa as an endoscopic sign for AR. The possible explanation could be related to the smaller sample analyzed in the previous study.
Polypoid change of the MT has also been correlated with the diagnosis of AR as shown by White et al., who described 16 patients with polypoid changes/polyps of the MT, all of which had positive allergy testing. Hamizan et al. reported that multifocal, diffuse, and polypoid edema – the highest grades of MT edema – had the strongest association with allergy, with positive predictive values of 85.15%, 91.7%, and 88.9%, respectively. Brunner et al. compared the clinical characteristics of patients with isolated polypoid change of the MT versus paranasal sinonasal polyposis, finding a higher prevalence of AR in patients with polypoid MT changes compared to patients with conventional sinonasal polyposis (83% vs. 34%, p < 0.001).
Central compartment atopic disease (CCAD), first described in the multi‐institutional case series by DelGaudio et al. in 2017, is a phenotype of nasal inflammatory disease which presents with isolated polypoid changes involving the superior nasal septum with or without the MT and/or superior turbinate, and is strongly associated with inhalant allergy. All patients in the series had positive allergy testing. In a subsequent case series, the same authors found that 81.9% of patients with AERD had central involvement of disease, with 100% of patients with endoscopic central compartment disease having clinical AR. (See Section XIII.B.3. Central Compartment Atopic Disease for additional information on this topic.)
Despite early inconsistent reports, the current body of evidence has shown that certain nasal endoscopy findings, particularly central compartment polypoid changes, are predictive factors for the presence and severity of AR and nasal endoscopy may aid in the identification or exclusion of other possible causes of symptoms, such as nasal polyposis or CRS.
Nasal endoscopy
Aggregate grade of evidence: C (Level 2: 2 studies, level 3: 1 study, level 4: 7 studies; Table X.A.3)
Benefit: Possible improved diagnosis with visualization of middle or inferior turbinate edema, pale/bluish discoloration, or isolated central compartment polypoid changes and/or edema, which have been associated with AR.
Harm: Possible patient discomfort.
Cost: Moderate equipment and processing costs, as well as procedural charges.
Benefits‐harm assessment: Balance of benefit and harm.
Value judgments: Nasal endoscopy may increase diagnostic sensitivity among children and adults with allergic rhinitis.
Policy level: Option.
Intervention: Nasal endoscopy may be considered as a diagnostic adjunct in the evaluation of patients with suspected AR.
X.A.4 Radiologic studies
Radiographic workup is not recommended for the routine diagnosis of AR. Although some radiographic findings have been associated with AR, there are no high‐quality studies demonstrating a role for imaging in the diagnosis of AR.
For patients that undergo imaging, certain radiologic patterns described in the literature may indicate an allergic role in their disease process. Several studies have demonstrated association between inflammatory changes to the central compartment mucosa and aeroallergen reactivity, resulting in the CRS phenotype of CCAD., , , , Other studies have described evidence of radiographic changes among patients with known AR, including the association for smaller maxillary sinuses and enlargement of the septal swell region.,
Radiology studies incur additional cost and demonstrate little diagnostic value for AR. There is also concern for ionizing radiation with CT scanning, along with risk for future malignancy., , These factors preclude the routine utilization of radiographic studies for the diagnosis of AR.
Radiologic studies
Aggregate grade of evidence: D (Level 3: 1 study, level 4: 7 studies; Table X.A.4)
Benefit: Some radiologic findings, particularly those associated with central compartment edema/polyposis, may alert the clinician to the possibility of an associated allergic etiology.
Harm: Unnecessary radiation exposure, unnecessary cost.
Cost: High equipment and processing costs. Additional costs for interpretation of studies by radiologist.
Benefits‐harm assessment: Preponderance of harm over benefit.
Value judgments: Long‐term risks of ionizing radiation outweigh potential benefit.
Policy level: Recommendation against.
Intervention: Routine use of imaging is not recommended for the diagnosis of AR.
X.B Skin testing
X.B.1 Skin prick testing
SPT, in conjunction with clinical history and physical examination, can confirm the diagnosis of AR and help to differentiate AR from non‐allergic types of rhinitis. The confirmation of an IgE‐mediated process can guide avoidance measures and direct appropriate pharmacologic therapy. Allergy testing is crucial for the initiation of AIT, and therefore, skin testing should be utilized in eligible patients when AIT is being considered.
SPT is performed with lancets, which come in a variety of forms. Generally, lancets are designed to limit skin penetration depth to 1 mm. However, varying amounts of pressure applied to the delivery device can alter the depth of skin penetration, which ultimately influences the skin reaction to an antigen. Prick testing devices can come as single or multiple lancet devices. Multiple lancet devices have the advantage of being able to rapidly apply multiple antigens to the skin at one time with a more consistent amount of pressure., Wheal size, sensitivity, and reproducibility all differ from one device to another; therefore, any clinician performing SPT must thoroughly familiarize themselves with the testing device they choose to utilize in their practice., , The lancet can be dipped into a well containing an antigen and then applied to the skin, or droplets of antigen can be placed on the skin and then using the lancet, a prick made through the droplet. When an antigen is applied to the skin of a sensitized patient, the antigen cross‐links IgE antibodies on the surface of cutaneous mast cells resulting in degranulation and release of mediators (including histamine) which leads to the formation of a wheal and flare reaction within 15–20 min.,
The volar surfaces of the forearms and the back are the most common testing sites for SPT. Choice of site is directed by the age and size of the patient, the presence of active skin conditions in a testing location, or significant tattooing in the testing area, which could impact interpretation. Reactivity of different body sites can vary, as the back is overall more reactive than the forearm. Within each site, there may be variability as well, as middle and upper parts of the back are more reactive than the lower back. Tests should be applied 2 cm or greater apart as placing them closer to one another can allow spreading of allergen solution between test sites. After approximately 20 min, the results are read by measuring the size of the wheal by its greatest diameter. Wheals that are greater than or equal to 3 mm in diameter, when compared to the negative control, are considered positive.
The number and choice of antigens used in testing vary considerably between clinical practices. A panel of antigens representing an appropriate geographical profile of allergens that a patient would routinely be exposed to is recommended. Positive (histamine) and negative (saline, 50% glycerin or 50% glycerinated human serum albumin with saline) controls should always be included. Regarding allergen extracts, variability in quality and potency between commercially available extracts has been demonstrated., Therefore, whenever possible, standardized allergens should be used. With advancements in molecular biology, new techniques for extraction, characterization, and production of allergens have been developed allowing for production of recombinant or purified allergens which may increase the sensitivity, specificity, and diagnostic accuracy of tests.
Given the limited depth of penetration, SPT is safe with very rare reports of anaphylaxis and no reported fatalities. SPT can be performed in any age group and is of value in pediatric populations given the speed at which multiple antigens can be applied and the limited discomfort experienced during testing. Aside from an excellent safety profile, SPT has reported sensitivity and specificity of around 80%., , It is felt to be more sensitive than serum sIgE testing with the added benefits of lower cost and immediate results., , Despite numerous studies aimed at comparing SPT, single intradermal tests, and serum sIgE testing, evidence marking one form of testing as superior to the others is lacking.
Skin testing is not appropriate in all patients. Absolute contraindications to SPT in the evaluation of AR include uncontrolled or severe asthma, severe or unstable cardiovascular disease, and pregnancy. Skin conditions including dermatographia and AD are relative contraindications to SPT given the possibility of false positives. Concurrent β‐blocker therapy is also a relative contraindication. Certain medications and skin conditions can interfere with skin testing and are covered in detail in other sections. (See Section X.B.4. Issues that may Affect the Performance or Interpretation of Skin Tests for additional information on this topic.)
Several errors may occur during SPT and impact the results and reliability. Since heterogeneity can be introduced when using multiple different test devices, it is recommended that the same device type can be used routinely in one's clinical practice to improve the reliability, comparability, and interpretation of testing. Personnel who apply tests should be appropriately trained and periodically monitored for quality control. Common errors with SPT include placing the test sites too close together (less than 2 cm), pressing too hard or creating deep punctures that cause bleeding, insufficient penetration of the skin by the puncture instrument, and spreading of allergen solutions across the field during the test by wiping away the solution.
There is a large body of evidence detailing the use of SPT in clinical practice. Based upon several prospective studies and systematic reviews, SPT has been demonstrated to be a safe method of allergy testing with sensitivity and specificity of greater than 80% (Table X.B.1). It has not been shown to be inferior to serum sIgE testing or single intradermal testing and is less expensive than serum sIgE testing. SPT does carry a risk of anaphylaxis, but no deaths from SPT have been reported. It is also associated with some discomfort during testing; however, the discomfort is generally less than that experienced during an intradermal test. Reviewing the available literature, a preponderance of benefit over harm exists for SPT. Therefore, the use of SPT is recommended in situations where the diagnosis of AR needs to be confirmed or a patient with presumed AR has failed appropriate empiric medical therapy and AIT is being considered.
Skin prick testing
Aggregate grade of evidence: B (Level 1: 1 study, level 3: 2 studies, level 4: 7 studies, level 5: 2 studies; Table X.B.1)
Benefit: Confirm AR diagnosis and direct appropriate pharmacologic therapy, initiation of AIT, as well as avoidance measures.
Harm: Adverse events from testing including discomfort, pruritus, erythema, worsening of asthma symptoms, anaphylaxis, inaccurate test results, and misinterpreted test results. See Table II.C.
Cost: Moderate cost of testing procedure.
Benefits‐harm assessment: Preponderance of benefit over harm.
Value judgments: Patients can benefit from identification of their specific sensitivities. SPT is a quick and relatively comfortable way to test several antigens with accuracy similar to other available methods of testing.
Policy level: Recommendation.
Intervention: Regular use of the same SPT device type will allow clinicians to familiarize themselves with it and interpretation of results may therefore be more consistent. The use of standardized allergen extracts can further improve consistency of interpretation.
X.B.2 Intradermal skin testing
Intradermal skin testing is one of the oldest forms of allergy testing, originally described in 1911. In this technique, 0.02–0.05 ml of diluted allergen extract is introduced into the dermis with a needle. The dilutions used are 100‐ to 1000‐fold less concentrated than those used for SPT. The response is measured at 10–15 min after injection. A significant wheal and flare reaction suggests the presence of preformed IgE bound to the surface of cutaneous mast cells, and thus a type 1 hypersensitivity to the tested allergen. Intradermal testing is considered to be more sensitive than SPT, but not necessarily more capable of identifying clinically relevant allergy. Intradermal testing may be used as a primary diagnostic modality and its performance for some allergens, such as Alternaria, may be similar to SPT or in vitro testing. A more common approach is to perform intradermal testing after a negative SPT to identify lower level allergic sensitivity. Some allergists also use intradermal testing in a titrated fashion (using multiple allergen dilutions) with the goal of more accurately quantifying allergic sensitization or as a means to select a starting dose for AIT. Intradermal dilutional testing (IDT) is roughly equivalent to SPT in the diagnosis of inhalant allergy, and IDT endpoint correlates with SPT wheal size. However, the role of intradermal testing for aeroallergen sensitivity is controversial due to concerns about the performance characteristics (sensitivity and specificity) of single intradermal tests relative to SPT.
As with any skin test, intradermal skin testing should be performed in conjunction with appropriate positive and negative controls. A negative control should include appropriately diluted test solutions (e.g., glycerin for aqueous glycerinated extracts). A positive control should contain diluted histamine base (e.g., 10 mg/ml). Measurement of the wheal and flare response is used to determine a positive result; however, thresholds for a positive test may vary because studies have not been performed to standardize test grading. A wheal size 2–4 mm larger than the negative control is often used as the threshold for a positive test.,
Assessment of the sensitivity and specificity of intradermal testing is hampered by multiple variables in the published studies. These include the concentration and volume of allergen injected, the definitions of a positive test, variation in allergens tested, and the “gold standard” comparator used for analysis. As a stand‐alone diagnostic test for AR, using studies with nasal provocation as the reference standard, estimates for sensitivity for intradermal testing range between 60% and 79%, while specificity is in the range of 68%–69%., In comparison, a meta‐analysis of SPT trials had pooled estimates of 88.4% sensitivity and 77.1% specificity for SPT, suggesting superiority of SPT as a stand‐alone allergy diagnostic test. Nevertheless, intradermal tests are still used when a highly sensitive skin test is desired. This may be particularly important when testing with non‐standardized allergen extracts (e.g., molds, trees) (Table X.B.2).
Intradermal tests are also employed when SPT is negative but history strongly suggests an allergic sensitivity, and may be particularly useful in patients with lower skin sensitivity. Negative intradermal testing may be helpful in ruling out IgE‐mediated disease. On the other hand, the addition of intradermal testing in the setting of SPT negativity may result in 20% more positive allergy skin testing results, and the clinical significance of these results is an important question that needs to be resolved. Positive intradermal tests may merely be due to non‐specific irritant phenomena.
Because intradermal testing has traditionally been considered more sensitive than SPT, it is often used as an add‐on test in the setting of a negative SPT result when allergy is suspected. Theoretically, an intradermal test will be able to identify a clinically significant sensitivity that is otherwise not detected on SPT. However, many studies have failed to show an added benefit of intradermal testing in this setting. For example, Krouse et al. showed that adding intradermal testing to SPT only increased the sensitivity from 87% to 93% for Timothy grass allergy when nasal provocation was used as the comparator. In a similar study with Alternaria, Krouse et al. determined that adding intradermal testing to SPT increased the sensitivity from 42% to 58%. These studies suggest marginal increase in sensitivity that may vary based upon the allergen being tested.
Nelson et al. studied individuals with a history of seasonal AR and clinical history of grass allergy. One group had negative SPT but positive intradermal tests, while another group had negative SPT and negative intradermal tests. In both groups, 11% of individuals had a positive nasal challenge with timothy grass, demonstrating that the addition of an intradermal test did not improve the diagnostic accuracy of skin testing as judged by the “gold standard” of nasal provocation plus clinical history. Additionally, in a study of patients with clinical cat allergy and negative SPT, a positive intradermal test did not increase the likelihood of a positive cat allergen challenge. There was no difference between those who had positive or negative intradermal testing (24% vs. 31%). Thus, while about 30% of patients with a clear clinical history of cat allergy had a positive cat allergen challenge despite a negative SPT, the addition of an intradermal test did not improve the diagnostic accuracy of skin testing.
Schwindt et al. studied 97 subjects with allergic rhinoconjunctivitis symptoms. SPT was followed by intradermal testing if SPT was negative. If patients were SPT negative and intradermal test positive, a nasal challenge was performed against five different allergens. If SPT with the multi‐test II device was negative, only 17% of subjects had a positive intradermal test that corresponded with clinical history. None of these positive intradermal results corresponded with a positive nasal challenge. Taken together, these studies suggest that intradermal testing may not improve the diagnosis of allergy in subjects with a negative SPT.
Intradermal testing for inhalant allergens is considered safe. However, systemic reactions, such as anaphylaxis, and even death, have been reported after intradermal testing. The risks of intradermal testing may be reduced by testing with more dilute solutions in individuals with suspected high‐level sensitivity or by performing SPT as an initial screening test. The risk of intradermal testing is significantly higher in medication allergy and IgE‐mediated food allergy and therefore not recommended.
In summary, intradermal testing is an option for the diagnosis of AR due to aeroallergens, especially when using non‐standardized allergen extracts. This form of testing demonstrates no clear superiority over SPT when comparing sensitivity and specificity, though results may vary by allergen tested. Single dilution intradermal testing has not been adequately studied in comparison to IDT, though IDT results may approximate SPT results, especially in patients with high level sensitivity. For some allergens such as Alternaria, there appears to be a gain in sensitivity when intradermal testing is used as a confirmatory test following negative SPT.
Intradermal skin testing
Aggregate grade of evidence: C (Level 3: 7 studies, level 4: 13 studies; Table X.B.2)
Benefit: May improve identification of allergic sensitization in patients with low‐level skin sensitivity or with non‐standardized allergens.
Harm: Adverse events from testing including discomfort, pruritus, erythema, worsening of asthma symptoms, anaphylaxis, inaccurate test results, and misinterpreted test results. See Table II.C.
Cost: Moderate cost of testing procedure.
Benefits‐harm assessment: Benefit over harm when used as a stand‐alone diagnostic test, when used to confirm the results of SPT, and as a quantitative diagnostic test.
Value judgments: Intradermal skin tests may not perform as well as SPT in most clinical situations.
Policy level: Option for using intradermal testing as a stand‐alone diagnostic test for individuals with suspected AR. Option for using intradermal testing as a confirmatory test following negative SPT for non‐standardized allergens.
Intervention: Intradermal testing may be used to determine aeroallergen sensitization in individuals suspected of having AR.
X.B.3 Blended skin testing techniques
The combined use of SPT and intradermal testing for a specific allergen is referred to as “blended” allergy testing., , One example, originally described by Krouse and Krouse as a method to establish an “end‐point” for a specified allergen, was described as “modified quantitative testing” (MQT) and serves as an example of a blended technique. MQT involves an algorithm where SPT is used initially to apply an antigen. Depending upon the SPT result, an intradermal test may or may not be applied., , , With these results, the algorithm is used to determine an endpoint for each antigen tested., , , The endpoint is considered to be a safe starting point for AIT. Other protocols may combine the use of SPT and intradermal testing but not for the purposes of establishing an endpoint., Instead, an intradermal test may be used following a negative SPT to determine allergen sensitization.,
AIT based on the results of MQT has shown to be successful and to induce immune system changes in line with other skin testing techniques. However, literature is lacking in protocols involving blended skin testing (Table X.B.3).
Specifically for MQT, advantages attributed to it include the provision of both qualitative data (sensitization to a specific allergen) and quantitative data (testing endpoint upon which AIT starting dose can be based) in less time than IDT., , Disadvantages include the additional risk and time involved in placing intradermal tests. MQT has been shown to be more cost‐effective when the prevalence of AR in a population is 20% or higher when compared to IDT and in vitro testing methods.,
Blended skin testing techniques
Aggregate grade of evidence: D (Level 4: 7 studies; Table X.B.3)
Benefit: Ability to establish an endpoint in less time than intradermal dilutional testing, potential to determine allergen sensitization after negative SPT.
Harm: Adverse events from testing including discomfort, pruritus, erythema, worsening of asthma symptoms, anaphylaxis, inaccurate test results, and misinterpreted test results. Additional time and discomfort versus SPT alone. See Table II.C.
Cost: Moderate cost of testing procedure.
Benefits‐harm assessment: Preponderance of benefit over harm.
Value judgments: While AIT can be based off SPT results alone, endpoint‐based AIT may have possible benefits of decreased time to therapeutic dosage.
Policy level: Option.
Intervention: Blended skin testing techniques, such as MQT, are methods that can be used to determine a starting point for AIT or confirm allergic sensitization.
X.B.4 Issues that may affect the performance or interpretation of skin tests
X.B.4.a Medications
Medications that inhibit mast cell degranulation or block histamine H1 receptors antagonists may suppress appropriate skin test responses. For this reason, it is important to assess the medications patients are taking prior to allergy skin testing.
There is substantial variation in the suppressive effects that H1 antihistamines have on the allergen and histamine induced wheal and flare responses,, with the duration of suppression dependent on the tissue concentration and half‐life of the medication. Orally ingested antihistamines typically suppress skin test responses for 2–7 days after stopping the medication., Topical antihistamines may also suppress skin wheal and flare responses. Furthermore, H2 receptor antagonists like ranitidine can reduce skin whealing responses,, and a combined suppressive effect of H1 and H2 antihistamines on skin whealing has been demonstrated. Antidepressants with antihistaminic properties (such as doxepin) impair the wheal and flare, but newer antidepressant classes such as selective serotonin reuptake inhibitors do not alter allergy skin test reactivity (Tables X.B.4.a.‐1 and X.B.4.a.‐2).
Omalizumab, a monoclonal anti‐IgE antibody, suppresses the allergy the skin test response by interfering with IgE‐mediated mast cell degranulation. A placebo‐controlled RCT noted significant reduction in the allergen‐induced skin wheal response after 4 months of omalizumab; whereas skin test response returned to normal within 8 weeks of discontinuation of omalizumab in another study.
Hill and Krouse and Simons et al. found no effect of montelukast on intradermal skin tests, and Cuhadaroglu et al. noted that allergic patients treated with zafirlukast had no change in SPT results. Therefore, leukotriene modifying agents do not appear to affect skin test results.
Most studies indicate that systemic steroid treatment does not alter skin test results,, but some less rigorous retrospective studies contradict these findings., Topical steroid treatment does suppress the wheal and flare reaction in treated skin areas, according to several studies., , , Allergy skin tests should not be performed in areas that are being treated with topical steroid medications in order to avoid false negative results.
Several classes of medications have not been adequately studied with respect to their effect on allergy skin test responses. Benzodiazepines have been implicated as possibly suppressing skin test responses., Calcineurin inhibitors demonstrate conflicting findings. Tacrolimus has been shown to inhibit SPT whealing, whereas pimecrolimus does not appear to affect skin whealing responses. Herbal preparations are understudied in this area, so it is unclear which of these agents could interfere with allergy skin test responses. More et al. performed a double‐blind placebo‐controlled, single dose crossover study in 15 healthy volunteers, examining the histamine induced skin test response. None of the 23 herbal supplements evaluated suppressed the histamine induced wheal response.
All allergy skin testing should be performed after application of appropriate positive controls (e.g., histamine) to verify that the histamine induced skin test reaction is intact at the time of testing. This practice helps to mitigate against unknown factors – potentially medications – causing inappropriate interpretation of skin test results.
X.B.4.b Skin conditions
Allergy skin tests rely upon the wheal and flare reaction induced by allergen‐specific mast cell degranulation. However, mast cell degranulation can occur via a variety of non‐immunologic mechanisms including minor skin trauma. Individuals with an exaggerated “triple response of Lewis” are considered to have “dermatographia” or “urticaria factitia,” and may comprise 2%–5% of the population. Dermatographism may interfere with interpretation of allergy skin tests. Therefore, a negative control test should also be performed at the time of skin testing. In general, the negative control test consists of a prick with an applicator device (including the diluent), or placement of an intradermal wheal with inert diluent, in the case of intradermal testing. While an allergen induced skin wheal and flare may be compared to that induced by a test with mere diluent, results must always be interpreted with caution in the setting of dermatographia.
The skin of patients with other urticarias, AD, allergic contact dermatitis, etc. also may not respond appropriately to the trauma, histamine, glycerin, or allergen that are inherent in skin testing. Skin reactions could be exaggerated, or the effect of allergen‐induced mast cell degranulation could be obscured. Common sense dictates that allergy skin tests should not be performed at sites of active dermatitis, but clinical studies to investigate this phenomenon are lacking. In some cases, it may be preferable to perform in vitro sIgE testing in patient with skin disease or dermatographism, but this is not based on data or outcomes from controlled studies.
Issues that may affect the performance or interpretation of skin tests – skin conditions
Aggregate grade of evidence: N/A (no identified studies)
Benefit: Correct identification of aeroallergen sensitivity.
Harm: Discomfort of skin test.
Cost: Low‐moderate.
Benefits‐harm assessment: Accurate skin test results justify discomfort and negligible cost of control tests.
Value judgments: In vitro allergy tests may be more appropriate than skin tests, in patients with dermatographia, urticaria, or other generalized dermatitis.
Policy level: Recommendation.
Intervention: Allergy skin tests should be performed in areas without active dermatitis or other lesions. Positive and negative control tests should be used in conjunction with allergy skin testing for AR.
X.C In vitro testing
X.C.1 Serum total IgE
IgE is the hallmark immunoglobulin in atopic disease. Atopy, or reactivity to otherwise innocent allergens can be determined by dermal reactivity (e.g., SPT), or by determining sIgE to a certain allergen in serum. The total IgE (tIgE) level in serum can also be determined. As atopy is not disease‐specific, the question arises whether serum tIgE has any place in the evaluation and diagnosis of AR.
From the literature, roughly two study approaches to determine the role of tIgE are identified: population‐based studies (e.g., birth cohorts, school health surveys, or general population approaches) and hospital‐based studies including patients visiting otorhinolaryngology or allergy clinics. Data from the first approach show conflicting evidence. In some studies, tIgE is related to AR diagnosis;, , , in others it is less clear., Moreover, it seems from these studies that other comorbidities, especially asthma, give rise to elevated tIgE., However, the presence of asthma is not accounted for in most studies, possibly confounding the outcomes. Another weakness of population‐based studies is that the diagnosis of AR depends on questionnaires, symptom scores, or self‐reported diagnosis. This might lead to overdiagnosis of AR in these studies as the distinction with non‐allergic rhinitis, common colds, or other nasal diseases can be challenging (Table X.C.1).
Hospital‐based studies have the advantage of improved diagnostics but have the risk of selection bias. At any rate, these studies also show a mixed picture about the role of tIgE in the diagnosis of AR. Overall, the levels of tIgE are higher in AR versus non‐allergic rhinitis, , or versus controls., Some studies investigated the correlation between serum sIgE and tIgE, showing a good overall fit. In hospital‐based studies, the influence of asthma is seen as well but again not accounted for in most reports.
Taken together, an elevated tIgE is indicative of an atopic condition, though not necessarily AR specifically. As such, tIgE is not required in the diagnostic pathway for AR. Many authors conclude that obtaining a serum tIgE can be helpful but is only a preliminary or supportive criterion for AR. Especially if an SPT is performed, there seems to be little added value of obtaining a serum tIgE, as it requires venipuncture which can be bothersome for children. In population‐based studies, tIgE can be supportive of AR, given that the study methodology allows for differentiation between atopic conditions such as asthma or AD in the study population.
Although in general obtaining a serum tIgE is not advised as a routine diagnostic approach, it can be needed or helpful in specific situations. For example, it has been suggested that monitoring of the efficiency of AIT may be done by evaluating the ratio between sIgE and tIgE; this is discussed in detail in a position paper from EAACI. Allergic broncho‐pulmonary aspergillosis is the only clinical condition described to date, where the presence of high levels of tIgE is strictly related to disease severity. However, these specific cases are exceptions to the rule that serum tIgE is not needed for the diagnosis and evaluation of AR.
Serum total IgE
Aggregate grade of evidence: C (Level 2: 4 studies, level 3: 11 studies; Table X.C.1)
Benefit: Possibility to suspect allergy or atopy in a wide screening.
Harm: Cost of test, undergoing of venipuncture, low level does not exclude AR.
Cost: Low, dependent on country and local healthcare environment.
Benefits‐harm assessment: Slight preponderance of benefit over harm. In addition, the ratio tIgE/sIgE may be useful to interpret the real value of sIgE production and predict treatment outcomes with AIT.
Value judgments: The evidence does not support routine use.
Policy level: Option.
Intervention: Assessment of tIgE may be useful to assess overall atopic status; furthermore, in selected cases it might help guide therapy (i.e., monitor efficacy of AIT).
X.C.2 Serum allergen‐specific IgE
Determining the presence of sIgE that verifies allergen sensitization is the cornerstone of diagnostic testing in suspected allergic conditions. The assessment of sIgE can be done by skin tests, serological immunoassays, and/or cellular immunoassays.
Serological immunoassays detect and measure the level of serum sIgE. Innovations in molecular biology have revolutionized the procurement, characterization, and production of allergens through recombinant and phage methods. The ability to perform serum sIgE immunoassays with recombinant or highly purified allergens has increased the sensitivity, specificity, and diagnostic accuracy of these tests. Additionally, development of miniature computer‐driven autoanalyzers and nanotechnology‐based devices, enhanced signal detection instrumentation, and new solid phase chip and particle materials have improved the diagnostic accuracy and consistency of in vitro tests., Furthermore, increased knowledge of molecular allergen components allow clinicians to predict the risk of severe allergic reactions and to identify the most appropriate AIT extract selections for each patient.
Derived from the original radio allegro‐sorbent test (RAST), new methods of sIgE immunoassay, like enzyme‐linked immunosorbent assay (ELISA), fluorescent enzyme immunoassays, and/or chemiluminescent assays are available. These measurements of serum sIgE can be done using single allergen (singleplex: one assay per sample) or through a predefined panel that includes several allergens (multiplex: multiple assays per sample). Singleplex tests allow the clinician to choose select allergens as dictated by the clinical history. Multiplex tests provide results of a broad array of preselected allergens.
The multiplex test is important in diagnosis of polysensitized patients. Multiplex platforms are slowly being implemented in many allergy care centers outside of research and tertiary care centers, although currently the most widely used systems are singleplex. Some, like Thermo Fisher ImmunoCAP, have an extensive amount of scientific literature demonstrating their efficacy. Each test has certain characteristics based on the detection method used, the dynamic range of reading of the instrument, time and conditions for the incubation, amount of allergen in the tube, and characteristics of the anti‐IgE., There are three different kinds of serum sIgE assays available: qualitative, semi‐quantitative, and quantitative. Qualitative assays are useful to determine if the patient is sensitized to common allergens, providing positive, negative, or borderline sIgE results to a mix of allergens without measuring the IgE concentration. Semi‐quantitative assays grade response by reporting a series of classes (e.g., class I–VI). Quantitative assays report sIgE antibody concentration. Most singleplex platforms are quantitative assays; multiplex is semi‐quantitative.
Multiplex platforms or panels of 10–12 selected allergens (i.e., pollens, cat, and mite) will detect up to 95% of patients who would have been identified on a larger battery., If the test is negative, absence of allergy is probable.
Serum sIgE testing may also be beneficial for selecting allergens for AIT. In polysensitized patients, it can be difficult to determine the most relevant allergen(s) on SPT. In these situations, molecular allergy using components will help to discriminate the most relevant allergens and thus better guide AIT. In addition, serum sIgE seems to correlate with the severity of AR symptoms., , , , Since patients with more severe symptoms appear to respond better to AIT than those with milder symptoms, serum sIgE may help in the selection of candidates for AIT and possibly predicting the response.,
SPT has advantages and disadvantages when compared to sIgE tests. As a general concept, SPT is more sensitive, whereas serum sIgE detection is more quantitative than SPT.
There are several advantages of serum sIgE over skin testing. The safety profile is excellent as the risk for anaphylaxis is non‐existent. It is the preferred testing method in individuals at high risk for anaphylaxis. Undergoing SPT is also limited by the presence of certain medical conditions. When SPT is contraindicated, serum sIgE testing offers a safe and effective option for determining the presence of IgE‐mediated hypersensitivities. Additionally, where certain medications can alter SPT results, serum sIgE testing is not similarly impacted. Finally, in very young patients in which SPT may prove too stressful, serum sIgE can be considered.
There are some important limitations to serum sIgE testing. While patients are accepting of both in vitro and in vivo allergy testing, many prefer SPT because it allows for immediate feedback and visible results. Unless molecular allergy diagnostic approach with allergenic components is used (precision allergy medicine diagnosis or PAMD@), serum sIgE to regular allergens cannot accurately predict the risk of severe allergic reaction. If PAMD@ is not used, cross‐reacting allergens and poly‐sensitizations can confound in vitro testing, leading to false positive results.
While SPT results may vary based on the quality of the extracts, as well as clinicians administering and interpreting the test, serum sIgE testing results can vary from one laboratory to another. One study sent blinded samples of the same sera, diluted and undiluted, to 6 major commercial laboratories and compared the results to the expected curve from an ideal assay. Out of the six laboratories, only two demonstrated precision and accuracy in their results. Further studies have demonstrated poor agreement on results from testing the same sera by different commercially available assay systems., , These factors introduce notable heterogeneity in serum sIgE testing. Clinicians should be familiar with the platform used for serum sIgE testing at their institution and to understand any limitations inherent to that platform.
Studies have shown that serum sIgE testing has a sensitivity range of 67%–96% and specificity range of 80%–100%., , , , Further, serum sIgE correlates well with NPT and SPT for AR diagnosis., , , , While there is good evidence to show that serum sIgE is often equivalent to SPT, it is generally accepted that SPT is more sensitive., , A recent position paper from the World Allergy Organization (WAO) stated that skin tests are still considered first line and that serum sIgE testing should be considered as a complimentary or alternative diagnostic tool. Based on the literature, serum sIgE testing is a reasonable alternative to SPT and is safe to use in patients who are not candidates for SPT. All sIgE tests should be evaluated within the framework of a patient's clinical history (Table X.C.2).
Serum allergen‐specific IgE
Aggregate grade of evidence: B (Level 1: 1 study, level 2: 2 studies, level 3: 6 studies, level 4: 6 studies, level 5: 1 study; Table X.C.2)
Benefit: Confirms diagnosis and directs appropriate pharmacological therapy while possibly avoiding unnecessary/ineffective treatment, guides avoidance, directs AIT.
Harm: Adverse events from testing including discomfort from blood draw, inaccurate test results, false positive test results, misinterpreted test results.
Cost: Moderate cost of testing.
Benefits‐harm assessment: Preponderance of benefit over harm.
Value judgments: Patients can benefit from identification of their specific sensitivities. Further, in some patients who cannot undergo SPT, serum sIgE testing is a safe and effective alternative.
Policy level: Recommendation.
Intervention: Serum sIgE testing may be used in patients who cannot undergo allergy skin testing. Use of highly purified allergen or recombinants can increase the sensitivity, specificity, and diagnostic accuracy of sIgE tests. Rigorous proficiency testing on the part of laboratories may also improve accuracy.
X.C.3 Nasal allergen‐specific IgE
AR is frequently diagnosed by history alone in clinical practice. When objective testing for confirmation of the diagnosis is needed, SPT or in vitro testing for serum sIgE is performed. However, the nasal mucosa of patients with AR has been shown to produce sIgE locally, providing a potential alternative method for objective testing for AR., , , , ,
Collection of nasal secretions is typically done by nasal lavage, through absorption of the secretions with absorbent materials, or directly with solid sIgE testing substrates., , , Collection of mucosal tissue can be achieved with either tissue biopsy or with a cytology brush., There is no consensus on which technique is superior, and most appear to yield similar results in identifying nasal sIgE., Cut‐off values for nasal sIgE levels that indicate a diagnosis of AR are debated and consensus has yet to be established. It is generally accepted that levels of nasal sIgE will be lower than levels of serum sIgE in patients with AR, , (Table X.C.3).
Outside of a few circumstances, the clinical utility of nasal sIgE testing in patients with AR is limited. However, in patients with negative SPT and negative serum sIgE with a history suggestive of AR, nasal sIgE testing may detect sIgE in their nasal secretions and/or mucosa., , , , , , , , This phenomenon is referred to as LAR. LAR is a type of rhinitis characterized by typical allergic symptoms with local sIgE production and positive response to NPT, without positive SPT or serum sIgE testing. (See Section VI.A.3. Local IgE Production and Section X.D.2. Local Allergen Challenge Testing for additional information on these topics.) The strictest diagnostic criteria for LAR require a positive NPT and evidence of sIgE in nasal secretions or nasal mucosa, as some studies have shown sIgE in control patients with negative results on NPT., , ,
Currently, patients with negative SPT and/or negative serum sIgE testing are given the diagnosis of non‐allergic rhinitis. Several studies have investigated the results of nasal sIgE testing in patients with non‐allergic rhinitis to achieve a greater understanding of what portion of patients diagnosed with non‐allergic rhinitis have evidence of LAR. A recent systematic review of studies that measured nasal sIgE in mucus collected from the nasal cavity in patients diagnosed with non‐allergic rhinitis showed sIgE to be present in 7.4%–13.4% of subjects. The results of this study contrast with a 2017 systematic review that analyzed the results of NPT in patients with AR and non‐allergic rhinitis. The 2017 study found 24.7% of patients with non‐allergic rhinitis had positive NPT. This analysis did not include measurements of nasal sIgE limiting direct comparison to the more recent study. The origin of this disagreement between these two reviews is unclear but may be related to low quantities of nasal sIgE in nasal secretions or flaws in the methodology for testing for nasal sIgE.
Differentiating LAR from non‐allergic rhinitis is important in patients with symptoms of rhinitis that are not adequately managed with pharmacologic therapy. While both would typically respond to treatment, identification of offending allergens in LAR may permit allergen avoidance and/or allow for treatment with AIT. Patients who are classified as non‐allergic rhinitis would not typically be candidates for AIT; however, for patients with LAR, treatment with AIT is an option. In this population, early studies suggest that AIT can decrease symptoms and medication usage and improve QOL. Therefore, in patients with symptoms of AR but negative SPT and/or negative in vitro testing for serum sIgE whose symptoms are not fully controlled on appropriate pharmacologic therapy, assessment of nasal sIgE to investigate for possible LAR could be considered.
Nasal allergen‐specific IgE
Aggregate grade of evidence: C (Level 1: 1 study, level 2: 21 studies, level 3: 3 studies, level 4: 11 studies; Table X.C.3)
Benefit: Patients with non‐allergic rhinitis found to have nasal sIgE may have LAR and could benefit from avoidance or AIT.
Harm: Measurement of nasal sIgE is minimally invasive. No significant adverse effects have been reported. Possible discomfort from sample collection.
Cost: Associated costs include the direct costs of testing and indirect cost of increased time and effort for performing nasal sIgE diagnostic test.
Benefits‐harm assessment: The benefits of identifying patients with an allergic component to their rhinitis may outweigh associated risks.
Value judgments: In patients with non‐allergic rhinitis who also have risk factors for atopic disease and have inadequate response to pharmacotherapy, testing for nasal sIgE may be helpful in confirming a diagnosis of LAR and allowing for treatment with AIT. There is no consensus for levels of nasal sIgE that indicate sensitivity.
Policy level: Option.
Intervention: Measurement of nasal sIgE is an option in patients with non‐allergic rhinitis suspected of having LAR to support this diagnosis and guide AIT if pharmacologic therapies are inadequate. Consensus for levels of nasal sIgE indicating AR need to be established.
X.C.4 Correlation between skin testing and in vitro sIgE testing
Factors that influence sensitivity and specificity of SPT include patient demographics, technician expertise, specific methodologies employed, quality of reagents, and what allergen is being tested., , , , , , SPT wheal size and sensitivity depend on the choice of control reagents used for testing, specific device selection, angle of penetration, amount of allergen, and skill of the technician., , A 2016 SRMA indicates that SPT is an accurate test that when utilized along with a detailed clinical history, helps confirm the diagnosis AR.
The performance and reliability of serum sIgE testing depends on choice of reagents, age of equipment, and patient demographics. Sensitivity and specificity are affected by the cutoff value of a positive test. In a Korean population, SPT was found to be superior to ImmunoCAP for measuring HDM sensitivity if the patient was less than 30 years of age; for the group older than age 50, ImmunoCAP was more sensitive.
Several studies have compared serum sIgE to SPT., , , , , , Both techniques yield good sensitivity and are generally well correlated; however, interpretation of the results depends to some extent upon the gold standard reference used to define allergic status, namely environmental chambers, nasal challenge, and validated questionnaires.
Microarray allergy testing systems have been introduced more recently to offer a comprehensive in vitro allergen test panel. There are several commercially available multiplex platforms: Thermo Fisher ImmunoCAP ISAC (Immuno‐solid phase Allergen Chip) which contains 112 allergen molecules; MADx Allergen Explorer 2 (ALEX2) containing 117 purified allergens plus 178 allergenic components and Euroline microstrips. The implementation of molecular allergy diagnostic approach (PAMD@) is increasingly entering into routine care.
Selection and interpretation of allergen testing is not based on sensitivity and specificity alone. The intended physiological mechanism to be evaluated also needs to be considered. SPT measures end‐organ pathological mechanisms associated with sIgE bound to the surface of mast cells. Serum sIgE and microarray approaches measure circulating IgE that may or may not represent downstream allergic inflammatory responses.
The average pooled sensitivity of SPT is 85% which tends to be slightly higher than that of serum sIgE. This can vary depending on the allergen being tested and the characteristics of the patient. SPT is often chosen as the first line diagnostic instrument to detect sensitivity to aeroallergens based on accuracy, convenience, cost, and speed. In cases where dermatographism is present and/or patients are unable to wean off medications that affect skin testing, serum sIgE testing may be a better choice.
The role of small volume blood testing through emerging microarray multiplex (multiple assays per sample) technology is evolving. Multiplex assays are especially suited for use in patients with complex sensitization patterns or symptoms. In polysensitized patients, PAMD@ makes it possible to distinguish between primary and cross‐sensitization. This is very important for appropriate prescription of AIT. Specific molecular sensitization patterns obtained in multiplex platforms may predict the risk for AR and asthma. PAMD@ is beginning to be used worldwide.
Correlation between skin testing and in vitro sIgE testing
Aggregate grade of evidence: B (Level 1: 3 studies, level 2: 5 studies, level 3: 4 studies, level 4: 5 studies, level 5: 2 studies, Table X.C.4)
X.C.5 Basophil activation testing
The BAT is an in vitro test for reactivity to specific allergens. It uses the propensity of activated basophils to express CD63 or CD203c. A BAT may have various ways of reporting results: the number of activated basophils as a full number or dichotomized (negative/positive, often at a cut‐off of 10% or 15%) and dose–response curves to indicate basophil sensitivity to increasing allergen extract concentrations. As such, BAT is a functional measurement. Per allergen, different concentrations and cut‐offs might be needed, making the comparison of studies challenging at times.
BAT is often performed in food, medication, and insect venom allergies, as it avoids bothersome or high‐risk provocations. To diagnose AR, the clinical history, along with measurement of sIgE or skin testing is usually sufficient. As these tests are inexpensive, fast, and safe, one may wonder whether there is a place for BAT in diagnosis of AR.
In HDM sensitive children, BAT has excellent sensitivity (82%–100%) and specificity (96%–100%). Similar findings were reached in 31 grass pollen sensitive adults: sensitivity 87%–100% and specificity 100%. In a combined study in 47 children with HDM and/or grass pollen allergy, sensitivity of BAT for HDM allergy was 90%, with 73% specificity at a cut‐off of 12.5% activated basophils, whereas sensitivity for grass pollen was 96%, with 93% specificity at 11% cut‐off. BAT is also able to distinguish between AR based on HDM allergy and irrelevant HDM‐sensitization. For birch allergy, BAT sensitivity was shown to increase after the pollen season compared to placebo. Results of BAT are valid in both in‐season and pre‐season measurements. A more general approach with a mixed group of 30 allergic children with aeroallergen AR or asthma showed increased levels of activated basophils compared to controls (Table X.C.5).
These studies show that BAT can be used as a diagnostic tool in AR. The usefulness of BAT as evaluation for the effect of treatment (especially AIT) is less clear.
In a very small study with Japanese cedar AR patients, clinical effects were not correlated to BAT outcomes. In a double‐blind RCT with 98 grass pollen sensitive patients receiving sublingual immunotherapy (SLIT) or placebo, there were no differences in BAT outcomes after 2 and 4 months of therapy. In another study, long‐term differences were found between HDM and grass pollen sensitive patients treated with dual SLIT or placebo; basophil activation in the treatment group was significantly decreased after 24 months compared with baseline. SLIT for Parietaria showed reduced basophil activation in 16 patients after 12 months of treatment.
For grass pollen subcutaneous immunotherapy (SCIT), some changes were found in BAT outcomes in 16 patients after 9 months of follow‐up compared to placebo, but these changes were not correlated to clinical outcomes. In another study with 50 grass pollen sensitized patients, SCIT gave a clear reduction in BAT outcomes 3–5 years after treatment. These results were confirmed in a smaller study with 18 patients treated with grass pollen SCIT; here, early changes in BAT outcomes were related to late clinical improvement.
In HDM‐sensitized patients, no apparent changes in BAT outcomes 24 months after SCIT were found, whereas in mugwort‐sensitized patients, basophil reactivity was reduced at this timepoint. Feng et al. were able to find changes in basophil activation after 2 years of SCIT for HDM in 35 patients. Two months of SCIT in HDM sensitive patients with (n = 24) or without (n = 19) other sensitizations showed improved clinical scores but increased BAT outcomes, especially in polysensitized patients. When comparing SCIT and SLIT in grass pollen sensitive patients, both lowered basophil sensitivity compared to controls at 15 months. However, the effect was larger in SCIT.
The evidence summarized above suggests that BAT is possibly of value in long‐term outcomes of AIT and possibly more sensitive in SCIT treated patients. However, the lack of correlation of BAT outcomes to clinical parameters in many studies shows that the application in BAT to evaluate AIT in clinical practice is not obvious.
The studies mentioned above used either CD63 or CD203c positivity as marker for basophil activation. In a small study with 16 SLIT‐treated patients, both markers were compared, showing that both were sensitive to treatment, but only CD203c data were correlated to clinical improvement. Ma and Qiao used a mixed cohort of 18 children treated for AR showing that both CD63 and CD203c‐based BAT correlated to clinical remission of symptoms. This suggests that technical choices in the execution of BAT influence outcomes and usability in practice.
In summary, the role of BAT in the diagnosis and evaluation of AR in clinical practice is limited. In most cases a detailed history with sIgE measurements or skin testing will suffice. In specific cases (e.g., contraindication for skin testing or conflicting results), though, BAT could be considered. The use of BAT to monitor reactivity to treatment is not advised in daily clinical practice.
Basophil activation testing
Aggregate grade of evidence: C (Level 2: 5 studies, level 3: 13 studies, level 4: 1 study; Table X.C.5)
Benefit: May help diagnose AR in specific cases where common approaches are not possible or show conflicting results.
Harm: Discomfort of venipuncture.
Cost: Moderate cost of performing the test, plus venipuncture. Cost depends on the local situation and availability.
Benefits‐harm assessment: Balance of benefit and harm.
Value judgments: The evidence does not support routine use for the diagnosis of AR or for following AIT response.
Policy level: Option.
Intervention: Application of BAT in specific situations where other diagnostic procedures for AR are not possible or conflicting. Potentially useful for monitoring AIT if other methods fail or show conflicting results.
X.C.6 Component resolved diagnostic testing
The implementation of molecular allergy diagnostic approach, or PAMD@, is increasingly entering into routine clinical care. Although PAMD@ may initially appear complex to interpret, with increasing experience, the information gained is relevant and allows improved management of allergic diseases. By measuring sIgE to purified natural or recombinant allergens, PAMD@ allows clinicians to evaluate allergen sensitization at the individual protein level, thus allowing potential identification of disease‐eliciting molecules.
In addition to potentially improving diagnostic accuracy, molecular diagnostics (MD) can also aid in distinguishing cross‐reactivity phenomena from true co‐sensitization and resolving low‐risk markers from high‐risk markers of disease activity. When compared to diagnosis based on sIgE determination and/or SPT with raw commercial extracts, MD may improve the identification of disease‐causing allergen sources and the prescription of AIT., , , , Changes in AIT prescriptions as a result of MD have demonstrated cost‐effectiveness. A real‐life study showed that although SPT was less expensive, MD allowed a more precise prescription of AIT, which substantially reduced treatment costs and the combined costs for diagnosis and treatment. MD may also aid with risk stratification by identifying certain patterns of sensitization to pollen allergens that are at higher risk of adverse reaction during AIT., Clinicians should keep in mind that all in vitro test results should be evaluated in context of the clinical history since allergen sensitization does not necessarily imply clinical symptoms.
Patients with a broader polymolecular IgE sensitization pattern to mites, epithelia, and pollen allergens have a trend toward more severe disease and more comorbidities., The presence of IgE antibodies against allergenic molecules may be determined using a singleplex or multiplex measurement platform (ISAC, Thermofisher‐Scientific, Uppsala, Sweden; Alex MacroArray Diagnostics, Vienna, Austria). It should be noted that the results of singleplex and multiplex platforms are not interchangeable, and, in general, sensitivity is higher for singleplex platforms., Singleplex platforms are quantitative assays and multiplex are semi‐quantitative.
In the case of mite sensitivity, Der p 1 and Der p 2 for D. pteronyssinus sensitize the majority of mite‐allergic patients, with double sensitization to groups 1 and 2 being common. Recently, Der p 23 has been described also as a frequent allergen and associated with increased asthma risk., Other good markers of sensitization are Lep d 2 for Lepidoglyphus destructor (storage mite, with limited cross‐reactivity with other HDMs) and Blo t 5 for Blomia tropicalis (non‐Pyroglyphidae mite). Der p 10 is a tropomyosin, which can cause cross‐reaction with tropomyosin from crustaceans (shrimp, crab, lobster) and mollusks (oyster, mussel, scallop), but it is not a marker of sensitization to mites., A better clinical response to AIT was observed in patients sensitized only to Der p 1 and/or Der p 2, when compared to patients with a broader IgE response.
In dog allergy, patients display a more complex pattern, with several allergens being recognized by around 50% of patients and 25% of patients being monosensitized to Can f 5., , , The pattern of sensitization should be kept in mind since the content of dog allergens in AIT extracts is very heterogeneous. In the case of cat allergic patients, Fel d 1 is clearly the major allergen, but other allergens also seem important such as Fel d 4 and Fel d 7., , A list of dog, cat, and horse aeroallergens is shown in Table X.C.6.‐1.
Allergens related to sensitization to cockroaches are Bla g 1, Bla g 2, Bla g 4, and Bla g 5, although in certain populations, tropomyosins (Bla g 7 and/or Per a 7) can be important.
Alt a 1 is a major allergen that is recognized in approximately 80%–100% of Alternaria‐allergic patients. There are 23 Aspergillus fumigatus allergens, but the main ones are Asp f 1, Asp f 2, Asp f 3, Asp f 4, and Asp f 6, with Asp f 1 being the most important.,
Markers of sensitization to several pollens are summarized in Table X.C.6.‐2. Sensitization to profilin has been associated with more severe respiratory symptoms in grass‐allergic patients, as well as sensitization to the minor olive allergens Ole e 7 and Ole e 9., Specific markers of sensitization to grass pollen include IgE antibodies to Phl p 1 and/or Phl p 5. Phl p 6 is contained only in Pooideae grasses and Phl p 4 can be used as a marker of sensitization to non‐Pooideae grasses. As allergens from groups 1, 2, 5, and 6 are only expressed in grasses and not in other plants, they detect a genuine sensitization to grasses.
In summary, PAMD@ in AR can help to better define the sensitization, better predict disease severity, better select patients and allergens for AIT and may predict the efficacy of AIT. However, it is not recommended for routine use in daily clinical practice at this time.
Component resolved diagnostic testing
Aggregate grade of evidence: C (Level 2: 4 studies, level 3: 2 studies, level 4: 11 studies, level 5: 1 study; Table X.C.6.‐3)
Benefit: Reliable. May help in identification and selection of suitable allergens for AIT, as well as possibly improving safety of AIT.
Harm: Discomfort of venipuncture.
Cost: Moderate cost of testing, minimal cost of venipuncture; depends on local availability.
Benefits‐harm assessment: Balance of benefit and harm.
Value judgments: Molecular diagnosis may be a useful tool for assessment of AR in some scenarios, especially in polysensitized patients.
Policy level: Option.
Intervention: Component resolved diagnostic testing is an option for diagnosis of AR by specialists.
X.D Allergen challenge testing
X.D.1 Environmental exposure chambers (allergen challenge chambers)
Environmental exposure chambers (EEC) have been used for decades to study the impact of exposures to well‐defined atmospheres of a variety of substances such as allergens, particulate and gaseous air pollutants, chemicals, or climate conditions. Valid exposure conditions with high temporal and spatial stability are technically demanding, limiting the number of EECs worldwide. In addition to the opportunity to use EEC for mechanistic studies on the effect of environmental pollutants on human health, it is also an interesting way to do efficacy testing of new drugs by allergen challenge in the chamber setting with induction of symptoms in patients with allergic disease. Presently, there are 15 allergen challenge chamber (ACC) facilities around the globe focusing on allergen exposure.
Our understanding of the pathophysiology of allergic diseases has been enhanced by ACC studies. A prime example of this is knowledge gained that controlled allergen exposure exacerbates AD. Also, the impact of exposure with pollen allergen fragments and the aggravating effect of diesel exhaust particles on AR symptoms have been shown. Furthermore, the importance of the integrity of the epithelial barrier for induction of local and systemic inflammatory responses has been investigated in patients with allergic rhinoconjunctivitis using the ACC setting, as well as severity phenotypes of allergic asthma and rhinoconjunctivitis.,
The use of ACC in clinical trials for efficacy testing of investigational new drugs and their acceptance by regulatory authorities is peremptorily dependent on the technical and clinical validation of ACCs. ACC have been intensively validated regarding specificity and dose‐dependency of symptom induction, as well as technical aspects such as temporal stability and spatial homogeneity of the allergen exposure., , , , , , , , Also, repeatability of outcome measures in the ACC has been systematically investigated and verified for TNSS, peak nasal inspiratory flow (PNIF), conjunctivitis symptoms,, and inflammatory nasal biomarkers. Remarkably, epigenetic changes in peripheral blood mononuclear cells and nasal epithelia after allergen challenge have recently been demonstrated, with baseline epigenetic status predicting symptom severity. Given the level of technical and clinical validation, ACCs have been used in clinical drug development to study pharmacological properties of new drugs during phase 2 trials, such as optimal dose,, , onset of action,, , , , , , and duration of action., , In this respect, numerous clinical trials have been conducted using parallel‐group or cross‐over designs in order to test the efficacy of drugs with prophylactic therapeutic potential, such as INCS,, , , , or with immediate therapeutic activity, such as antihistamines., , , , , , Novel anti‐inflammatory compounds,, , , , drug‐free nasal fluids,, and probiotics, have also been tested by this method. Additionally, the efficacy of AIT, , , , , , , , , , , and air cleaners, has been tested, as well as the influence of allergic nasal symptoms on the absorption of nasally applied drugs. Major advantages in the ACC setting compared to field studies are better signal‐to‐noise ratios, a safeguarded minimum level of symptomatology in the ACC, and reproducibility of symptoms through allergen dose consistency allowing intra‐individual comparisons.
A variety of validation studies of allergen atmospheres in ACCs have been published, including grass,, birch, HDM,, , Japanese cypress, and ragweed. While regulatory authorities accept the use of ACC in phase 2 of drug development, they have been reluctant to approve them in pivotal phase 3 studies because their clinical validation is still imperfect., , Differences between natural exposure and ACC studies exist, for example, with regards to exposure time (continuous versus intermittent), exposure atmosphere complexity (natural mix versus artificial purity), selection of study population (all‐comers vs. allergen challenge responders), and sample size (higher in field studies than in ACC to achieve comparable statistical power). To promote the implementation of ACC in phase 3 clinical trials, an EAACI initiated task force gathers and evaluates data on their clinical validation. Minimal technical requirements have already been identified. Hybrid approaches combining ACC and field study might provide proper robustness to determine drug efficacy.,
In summary, numerous well‐designed RCTs using technically validated ACCs for efficacy testing of investigational new drugs with detailed analysis of dose–response, onset of action, and duration of action underline the value of ACCs in clinical drug development of AR medicines.
X.D.2 Local allergen challenge testing
Challenging target organs with allergens could demonstrate reactivity when SPT and/or serum sIgE tests are unconvincing or inconsistent with patient symptoms and exam. NPT and conjunctival provocation test (CPT) may be used for AR and rhinoconjunctivitis diagnosis, respectively, in these circumstances., ,
NPT aims to reproduce the upper airway response to nasal allergen exposure., The only test fulfilling such requirements directly is the EEC; allergens administered during NPT usually exceed the levels of natural exposure. (See Section X.D.1. Environmental Exposure Chambers for additional information on this topic.) NPT can be administered by several devices: syringes, droppers, sprays, or disks, each with limitations. Positive NPT can be assessed by symptom scales, rhinometry, PNIF, nasal lavage inflammatory markers, and nasal nitric oxide (nNO). NPT contraindications include acute rhinosinusitis, recent AR exacerbation, history of anaphylactic reactions, severe general diseases (cardiopulmonary diseases with reduced lung capacity), and pregnancy. Reported sensitivities and specificities of NPT range between 83.7%–93.3.% and 72.7%–100%, respectively (Table X.D.2). A standardized NPT, suggested by Gosepath et al., has been defined by the EAACI position paper, although NPT utilization for AR diagnosis may decrease due to emerging tools like molecular allergy diagnostics and BAT., , ,
The characteristics and safety of NPT were investigated in 518 children and 5830 adults by Eguiluz‐Gracia et al., with 11,499 challenges and only four local adverse reactions noted. Reproducibility, positive and negative predictive values of three consecutive NPT in 710 subjects were 97.32%, 100%, and 92.91%, respectively, with no false‐positive results. Comparison between NPT and EEC in patients with cat allergy resulted in similar clinical and immunological responses. The authors suggested that selecting a specific allergen challenge method should depend on the study objectives and costs when investigating cat allergy. Regarding HDM, Wanjun et al. studied the relationship between the severity of AR and various diagnostic tests noting that NPT, SPT wheal size, and serum sIgE correlated with each other; only NPT was associated with the nasal symptom severity. Joo et al. evaluated the EAACI NPT protocol, concluding that standardized NPT could help diagnose AR caused by HDM. Finally, Xiao et al. found that, in assessing HDM allergic patients’ candidacy for AIT, NPT is valuable and safe for confirming the diagnosis before treatment, especially in Der p 1‐positive or low sIgE patients.
NPT is crucial in diagnosing occupational rhinitis and LAR. Occupational rhinitis diagnosis requires “objective demonstration of the causal relationship between rhinitis and the work environment through NPT with the suspected agent(s).” Occupational rhinitis diagnosis is challenging and should be suspected in patients with adult‐onset rhinitis; NPT is the gold standard for diagnosis when immunological tests are unavailable or unreliable.
For LAR, the SPT and serum sIgE are negative and diagnosis requires the measurement of local IgE in nasal secretions or a positive NPT. Measuring local sIgE in the clinic is not readily available or practical, making NPT critical. Of note, NPT with HDM, pollens, and Alternaria was positive in 100% of 22 adults with previously diagnosed LAR; however, in 28 children with non‐allergic rhinitis, NPT was positive in only 25% of subjects. In another study involving 62 symptomatic patients with negative SPT, the prevalence of LAR to HDM was 24.2%, with sneezing noted as a more dominant symptom in LAR versus non‐allergic rhinitis.
CPT is generally performed by instilling 20–30 μl of an allergen solution into the inferolateral quadrant of the conjunctiva, using a control diluent in the contralateral eye. A positive CPT response results in a reaction 5–20 min after testing with ocular itching/pruritis, tearing, redness/conjunctival erythema, and possibly edema. A study of 20 children with seasonal rhinoconjunctivitis tested three times with CPT reported good reproducibility. CPT sensitivity and specificity in HDM‐allergic patients were reported as 90% and 100%, respectively. A systematic review contributed to the EAACI guidelines for the practice of CPT with grade B evidence for identifying the allergen trigger. It was concluded that allergists should be more familiar with CPT due to its simplicity. However, symptom scales need to be validated, allergen extract standardization should be improved, and CPT indications in patients with non‐allergic conjunctivitis remain uncertain. Only one recent trial has been published which assessed a group of children monosensitized to Can f 5 from dogs. Interestingly, reference SPT and CPT demonstrated different reactions to male and female dog extracts, suggesting tolerance to female dogs.
Local allergen challenge testing (provocation testing)
Aggregate grade of evidence: C (Level 2: 1 study, level 3: 7 studies; Table X.D.2)
Benefit: May assist in confirming diagnosis of AR in specific cases when immunological tests are unavailable or unreliable. NPT is crucial in diagnosing occupational rhinitis and LAR.
Harm: Not necessary if first‐ and second‐ line tests are indicative for AR diagnosis.
Cost: Depending on the local situation and availability of equipment and staff, costs may be high.
Benefits‐harm assessment: Balance of benefit and harm.
Value judgments: The evidence does not support routine use for diagnosis of AR, but provocation testing is useful for diagnosis of occupational rhinitis and LAR.
Policy level: Option for diagnosis of AR when skin or in vitro tests are equivocal or unreliable. Recommendation for diagnosis of LAR and occupational rhinitis.
Intervention: Application of NPT is useful in LAR and to confirm occupational rhinitis.
X.E Nasal cytology and histology
Nasal cytology (NC) is a diagnostic procedure that evaluates cell types present in the nasal mucosa. NC starts with sampling the surface cells of the nasal mucosa; typically with a Rhino‐probe (Arlington Scientific, Springville, UT, USA). After sampling, staining using the May–Grunwald–Giemsa method allows identification of inflammatory (i.e., eosinophils, neutrophils, mast cells, and lymphocytes) and normal cells (ciliated and mucinous). At least 50 microscopic fields of the slides are then examined through a 1000× optical microscope. NC may directly detect bacteria, viruses, and fungi, as well as biofilms, demonstrating that biofilm is present not only in infectious rhinitis, but also in inflammatory and/or immune‐mediated diseases. Specific cytological patterns can aid in classifying various forms of rhinitis, including AR, non‐allergic rhinitis, and overlapping forms. The predominant cell type assessed by NC in AR is the eosinophil, followed by mast cells and basophils., , , Elevated nasal eosinophil counts had an OR of 1.14 (95% CI 1.10–1.18) of identifying AR. NC in poly‐allergic patients showed a more intense inflammatory infiltrate than in mono‐allergic patients, and demonstrated seasonal changes of inflammatory cells, probably due to changes in allergen exposure.
Studies on NC performance in diagnosing AR or non‐allergic rhinitis are limited (Table X.E.‐1). In 2021, a study on 387 patients assessed the diagnostic performance of NC showing 100% sensitivity (95% CI 97–100), 49.6% specificity (95% CI 43%–56%); PPV of 56% (95% CI 50%–62%), and NPV of 100% (95% CI 96%–100%) with a non‐allergic rhinitis prevalence of 39%. The accuracy of the test was 69.5% (95% CI 64.6%–74.0%). Such performance does not help to identify when it might be valuable to use, particularly with poor PPV. The ability of the NC to identify subjects affected by non‐allergic rhinitis helps the clinician to inform the patient about the possibility or the reason for the low efficacy of the AR therapy in mixed rhinitis. NC has been evolving in the last years, and novel approaches have recently been proposed using nasal scraping to collect samples for measurement of inflammatory mediators and cytokines.,
Nasal histology (NH) was the only technique to study nasal tissues and cells for many decades. Biopsy‐based investigations in the 1990s allowed researchers to define the role of the different inflammatory cells in AR. After a tissue sample is taken from the MT, it is placed in buffered formalin and then stained with reagents (Giemsa, hematoxylin/eosin, periodic acid‐Schiff, Masson trichrome, azure A, and chloroacetate esterase)., The slides are then examined by an optical double‐headed light microscope.
NC made it possible to obtain similar information as NH but without the potential risk for bleeding and allowing sequential sampling. Furthermore, following allergen challenge, NC revealed an increase in inflammatory cells not detected by histology; thus suggesting that the nasal secretions, which the NC collects together with the cells, and the nasal mucosa may represent two distinct cellular compartments with different expression of inflammatory cells. While NH is useful in pathophysiology research, it is hardly feasible for routine clinical use due to the expertise in tissue sampling and biopsy processing required. Table X.E.‐2 shows studies on AR as evaluated by NH.
Nasal cytology
Aggregate grade of evidence: C (Level 1: 1 study, level 3: 3 studies, level 4: 3 studies; Table X.E.‐1)
Benefit: Low costs and low invasiveness. Could help to detect eosinophils in non‐allergic rhinitis and to diagnose a mixed rhinitis.
Harm: NC is minimally invasive and minimal adverse effects have been reported.
Cost: Associated costs include the direct cost of NC and indirect cost of increased time and effort for performing NC.
Benefits‐harm assessment: Preponderance of benefit over harm.
Value judgments: The evidence does not support routine clinical use.
Policy level: Option.
Intervention: NC could help in cases of non‐allergic rhinitis to suspect LAR or in cases of AR to diagnose a mixed rhinitis. It could be considered an option in cases of negative SPT and/or serum sIgE to evaluate the presence of mucosal eosinophils and consideration of LAR or type 2 inflammation. The cut‐off values for determining NARES are not yet clear.
Nasal histology
Aggregate grade of evidence: B (Level 1: 1 study, level 2: 7 studies, level 4: 2 studies; Table X.E.‐2)
Benefit: May assist in evaluation of tissue eosinophilia and expression of mediators. May be useful in clinical research.
Harm: Small risk of complications (e.g., bleeding, infection).
Cost: Associated costs consist of the direct cost of NH and indirect cost of increased time and effort for performing NH.
Benefits‐harm assessment: Preponderance of benefit over harm.
Value judgments: The evidence does not support routine clinical use.
Policy level: Recommendation against.
Intervention: NH may be helpful in clinical research or selected cases (e.g., evaluation of tissue eosinophils during surgery). Recommendation against in routine clinical practice for AR evaluation due to invasive nature of obtaining a specimen.
X.F Rhinometry, acoustic rhinometry, and peak nasal inspiratory flow
Subjective measures of nasal obstruction have proven difficult to quantify as patient perceptions vary widely and often do not correlate with examination findings. Therefore, objective measures of nasal obstruction have been developed which measure physiologic parameters (e.g., peak nasal inspiratory/expiratory flow [PNIF/PNEF], airflow resistance or rhinomanometry) and non‐physiologic parameters (e.g., nasal cavity cross‐sectional area and volume, or acoustic rhinometry). These measures may be utilized pre‐ and post‐decongestion to distinguish between nasal obstruction secondary to dynamic or fixed structural deformities. Objective tests can also be used to assess the effectiveness of interventions or treatments, to provide objective data when clinical examination findings are not consistent with patient symptoms, and to evaluate a response in NPT and as a medicolegal tool.
Rhinomanometry. This involves the objective measure of nasal airflow resistance or the ratio of nasal airway pressure to flow. A clinical classification for five classes of nasal obstruction based on rhinomanometry measures in the reference population has been published by a European group., Rhinomanometry can be used in adults and children, and normative/reference values exist for both., , , , , , , However, reference values vary widely as rhinomanometry results depend on factors such as ethnicity, height, sex, smoking status, adenoid tissue, and age.,
Rhinomanometry has certain disadvantages. It is expensive, time consuming and requires trained personnel. Further, rhinomanometry is ineffective in the presence of complete obstruction of one or both nasal cavities or in the presence of a septal perforation.
Traditionally, nasal resistance has been calculated on one single volume value at one single pressure (i.e., 75 or 150 Pa). This is no longer recommended as this represents a portion of the curve where the pressure/volume flux relationship is non‐linear and a pressure of 150 Pa is often not achieved in normal relaxed breathing cycles., To address these limitations, four‐phase rhinomanometry (4PR) measures airflow resistance throughout the breathing cycle in four phases: the accelerating inspiratory phase, decelerating inspiratory phase, accelerating expiratory phase and decelerating expiratory phase., Logarithmic measures taken during 4PR correlate significantly with subjective scores of nasal obstruction. 4PR overcomes many of the limitations of standard rhinomanometry; however, more studies using and validating 4PR and evaluating nasal cavities individually are required.
Acoustic rhinometry. This is a measure of nasal cavity volume, geometry, and cross‐sectional area. Acoustic rhinometry can also localize the site of obstruction. Results of acoustic rhinometry are impacted by septal perforation and therefore, endoscopic examination is vital prior to acoustic rhinometry use. Acoustic rhinomanometry is limited in that it provides a static measure of a dynamic process. Further, acoustic rhinometry may overestimate the cross‐sectional area of the posterior nasal cavity due to leakage into patent sinuses.
Peak nasal inspiratory and expiratory flow. PNIF/PNEF is a test which carries the advantages of relatively low cost and ease of use. A minimally clinically important difference of 20 L/min has been defined and a lack of improvement of 20 L/min or 20% after decongestion may indicate a structural cause of obstruction., , An SRMA reported mean PNIF values in normal adults of 128.4 and 97.5 L/min for obstructed adults. However, standardized values have yielded inconsistent results due to multiple confounding factors including patient effort, pulmonary status, nasal valve collapse, smoking, height, and recent physical exercise., It would appear that PNEF correlates best with symptoms of nasal obstruction. PNIF/PNEF measures should be supported by subjective measures to improve diagnostic accuracy.
In summary, many papers have reported a lack of correlation between objective measures of nasal patency and subjective perceptions of nasal obstruction. Possible reasons for this discrepancy include the failure to accommodate septal deviations and to evaluate individual nasal cavities separately and measuring values at one single pressure rather than the entire breathing cycle. In fact, correlations between objective and subjective measures have been found when nasal cavities were assessed individually., , , , It has also been shown that patient symptoms do not necessarily correlate with the degree of measured obstruction., , This discordance has been illustrated in studies that applied substances such as menthol or local anaesthetic to the nasal mucosa, resulting in a subjective change in nasal airflow with no corresponding change in resistance., , , , , , Therefore, nasal cavity volume, airflow, and resistance may only be a few of many factors contributing to the sensation of nasal obstruction. Finally, whilst symptoms are paramount, objective measures of the nasal airway are useful beyond correlating with patient symptoms. They are useful in identifying or excluding other causes of nasal obstruction (such as psychiatric or sensory pathology), in nasal allergen challenges, in patient selection for surgery, and in the research setting.
Rhinomanometry
Aggregate grade of evidence: B (Level 1: 2 studies, level 2: 2 studies, level 3: 5 studies, level 4: 4 studies, level 5: 6 studies; Table X.F.‐1)
Benefit: Rhinomanometry is useful to improve patient selection for surgery, distinguish between structural and functional causes of nasal obstruction, diagnose nasal valve collapse, clarify conflicting symptoms and exam findings, use as a medicolegal tool and in nasal allergen challenges. Four‐phase rhinomanometry correlates with subjective scores.
Harm: Low. Rhinomanometry has limited effectiveness in patients with complete nasal obstruction or septal perforation. The equipment is not portable and therefore requires a clinic visit and trained staff. The procedure may be considered time consuming.
Cost: High.
Benefits‐harm assessment: Benefits outweigh harm.
Value judgments: For some patients, it may be important to avoid unnecessary costs in the diagnosis of AR; therefore, this procedure is less preferred.
Policy level: Option.
Intervention: Rhinomanometry is useful in distinguishing between structural and soft tissue causes of obstruction, when history and examination findings are not congruent, as well as a research tool. Better with individual nasal cavity assessment and 4PR.
Acoustic rhinometry
Aggregate grade of evidence: C (Level 2: 1 study, level 3: 5 studies, level 4: 3 studies, level 5: 2 studies; Table X.F.‐2)
Benefit: Improves patient selection for surgery, helps distinguish between structural and functional causes of nasal obstruction, evaluates a response in nasal allergen challenges, and functions as a medicolegal tool to demonstrate objective evidence of effectiveness of an intervention.
Harm: Low. Equipment is not portable therefore, requires a clinic visit and trained staff. Time‐consuming. Leakage into sinuses may provide inaccurate results and lead to inappropriate treatment.
Cost: High.
Benefits‐harm assessment: Benefits outweigh harm as harm is low.
Value judgments: For some patients, it may be important to avoid unnecessary cost in the diagnosis of AR, and thus acoustic rhinometry is less preferred.
Policy level: Option.
Intervention: Acoustic rhinometry is most useful in research setting as opposed to as a clinical diagnostic tool.
Peak nasal inspiratory flow
Aggregate grade of evidence: B (Level 2: 2 studies, level 3: 4 studies, level 4: 1 study, level 5: 1 study; Table X.F.‐3)
Benefit: Can improve patient selection for surgery, can evaluate a response in nasal allergen challenges, and can be used as a medicolegal tool to demonstrate objective evidence of effectiveness of an intervention.
Harm: Low. Risk of missing valve collapse and septal deviation as causes of obstruction.
Cost: Low.
Benefits‐harm assessment: Benefits likely to outweigh harm as harm is low.
Value judgments: Relies on patient effort and does not assess individual nasal cavities. Unable to evaluate nasal valve collapse.
Policy level: Option.
Intervention: Use in conjunction with PROMs to improve utility.
X.G Exhaled nitric oxide
NO is a volatile gas which functions as a vasodilator, bronchodilator, neurotransmitter, and inflammatory mediator in the airway. NO is formed in the upper and lower respiratory tract with high concentrations found in the nasal cavity and paranasal sinuses,, , and NO synthase is upregulated in ciliated respiratory epithelium and inflammatory cells in atopic patients. In adults, sex, menstrual cycle, pregnancy, recent consumption of high nitrate foods, recent exercise, and tobacco exposure may modify NO levels. Height and body surface area may also modify NO in pediatric population., , ,
Fractional exhaled nitric oxide (FeNO). FeNO is a measurement of NO in orally exhaled breath. The American Thoracic Society published recommendations for FeNO measurement. Briefly, the participant inhales through a NO filter to remove ambient NO. Then exhalation through a flow restrictor results in airflow limitation and creates a positive pressure exhalation, closing the velum and preventing contamination of the measurement with nasal NO. The orally exhaled breath is analyzed.
Although FeNO is highly variable in the healthy population, elevated levels are indicative of various types of inflammation in the respiratory tract. Elevated levels are found in AR, asthma, COPD, bronchiectasis, pulmonary sarcoidosis, and acute lung allograft rejection. FeNO is primarily utilized in the diagnosis and monitoring of therapeutic response and compliance in asthma,, , , but recent research has attempted to expand this testing for diagnosis of AR. Small studies have shown increased FeNO in AR patients, especially those with concomitant asthma., , , This finding was also seen in a large population study from The Netherlands which showed independent association of elevated FeNO in patients with positive skin testing, eczema, or AR (Table X.G.‐1).
FeNO is positively correlated with symptoms of AR and allergic sensitization in pediatric patients, with one study showing a sensitivity and specificity of 81.1% and 78.6%, respectively, at a FeNO cut‐off level of 18.4 ppb. Pediatric patients also show decreased FeNO after appropriate medical therapy., ,
There are potential cofounders when using FeNO as a biomarker. First, a wide variety of normal results for FeNO are possible in a given population and are influenced by age, sex, smoking status, and lab sampling. Additionally, there is no agreed upon cut off to indicate an abnormal result for the diagnosis of AR versus asthma.
Nasal nitric oxide (nNO). Due to the non‐invasive nature of NO measurement, there is interest in using this tool to differentiate allergic and non‐allergic rhinitis. nNO is measured by chemiluminescence. A small catheter is placed into one nostril and ambient nasal gas is measured while the patient orally exhales through a flow resistor tube to ensure the velum is closed and only nasal cavity gas is measured. nNO is reduced in several rhinologic diseases, including primary ciliary dyskinesia and cystic fibrosis, but is elevated in AR., , ,
Three small case–control studies have shown significant increase in nNO when comparing non‐atopic healthy adults with atopic adults without asthma., , Additionally, two systematic reviews (total n = 953 and n = 4093, respectively) showed significant increase in nNO in healthy controls versus patients with AR., However, these results conflict with other small case‐control studies showing no difference., , There is a reported nNO increase during pollen season in AR patients, and reduction after appropriate medical treatment of atopy (Table X.G.‐2).
Various factors influence nNO values including medication use, recent allergen exposure, recent viral respiratory infection, and concomitant asthma. Additionally, there is no standardized application of nNO measurement, with groups performing testing on a variety of analyzers with variations in sampling flow rate and carbon dioxide monitoring. Even small differences in testing application dramatically changes captured NO, making comparisons across research groups and establishment of normative values challenging. There is currently no agreed upon cut off point for the diagnosis of AR.
1 Nitric oxide measurements
Aggregate grade of evidence:
Fractional exhaled nitric oxide (FeNO): D (Level 4: 7 studies; Table X.G.‐1)
Nasal nitric oxide (nNO): C (Level 2: 2 studies, level 4: 6 studies; Table X.G.‐2)
Benefit: Possible benefit in differentiation of allergic and non‐allergic rhinitis through non‐invasive testing. Possible benefit in monitoring treatment response.
Harm: No studies have shown harm with either exam.
Cost:
FeNO: Relatively high. FeNO analyzers are approximately $7000–10,000 US, but testing is covered by some insurance plans.
nNO: High. Chemiluminescence NO analyzers are approximately $30,000–50,000 US, and clinical testing is not covered by insurance in the US.
Benefits‐harm assessment: Preponderance of benefit over harm.
Value judgments: There is inconsistent evidence in the ability of FeNO or nNO to differentiate adults and children with AR and non‐allergic rhinitis. Most studies were of low evidence or small impact. There is no agreed upon cut‐off value when performing FeNO or nNO for the diagnosis of AR.
Policy level:
FeNO: Recommend against for routine diagnosis of AR.
nNO: Recommend against for routine diagnosis of AR.
Intervention: History and physical, diagnostic skin testing, or sIgE testing should be the first line evaluation of AR. FeNO or nasal NO testing may provide additional diagnostic information if necessary but should not be routinely employed for AR diagnosis.
X.H Use of validated subjective instruments and patient reported outcome measures
Validated clinical outcome surveys (VCOS) are simple, effective tools that may be used to evaluate and screen patients with suspected or known AR. They can be helpful in establishing a diagnosis of AR, assessing severity, or evaluating treatment response. Typical survey questions inquire about symptoms such as congestion, rhinorrhea, and sneezing; the questions may be referring to that instant, or to a time period of days or weeks. Although objective testing such as allergy skin testing and sIgE serology can help confirm or rule out the diagnosis, clinical history is indispensable in the evaluation of AR. In resource‐poor settings, SPT, serologic testing, or other advanced technologies may not be available to confirm the diagnosis., , , Furthermore, VCOS offer a more structured and standardized means of obtaining the clinical history and assessing treatment response.
These PROMs focus on varying aspects of AR. They may primarily be symptom severity surveys such as the TNSS, or health‐related QOL questionnaires such as the RQLQ. Surveys of medication usage (Daily Medication Score), disease prediction (Respiratory Allergy Prediction [RAP]), and disease control (Rhinitis Control Test) are also available. VCOS can be cross‐validated with more objective tools such as NPT and SPT. These instruments are routinely utilized in clinical trials as objective, standardized measures to assess the efficacy of AR medications and are widely accepted in the academic allergy and rhinology community., , , , , Recently, VCOS have been adapted for use in smartphone applications that track AR symptomatology and medication use., , , , ,
Table X.H.‐1 lists several frequently used VCOS, outlining the targeted disease, number of questions, score range, symptoms, and/or medication questions included, and the context in which each is typically employed., , , , , , , , , , , , , , , , , , The TNSS is typically administered as a daily survey comprised of only four questions focusing on runny nose, nasal itching, sneezing, and congestion. Some studies have used the TNSS as a reflective score calculated as the average of both the 12‐h nighttime and 12‐h daytime average (rTNSS). The TNSS can be combined with questions about rescue medication use to yield the Daily Combined Score and the Total Combined Rhinitis Score. Both have been used in many therapeutic intervention studies. The RQLQ is a more comprehensive survey that asks the patient to reflect upon the past week and includes global QOL questions. It can be administered either in the office or at home so that it may be easier to obtain daily scores. A limitation of this test may be potential recall bias attributable to the 7‐day recall period (Table X.H.‐2).
The Control of Allergic Rhinitis and Asthma Test (CARAT‐10) evaluates rhinoconjunctivitis and asthma symptoms with a recall period of the preceding 4 weeks giving a broader evaluation of seasonal symptom control. The RAP test is a 9‐question survey incorporating upper and lower respiratory queries as well as a question about medication use. It was validated in a study in which primary care physicians used it as a screening tool to determine whether patients needed referral for allergy testing.
If conjunctivitis is to be assessed simultaneously with rhinitis symptoms, then the Rhinoconjunctivitis Total Symptom Score (RTSS) can be combined with Rescue Medication Score (RMS) to yield the combined score (CS). The Rhinosinusitis Disability Index (RSDI) was initially developed for CRS, but was validated for AR, non‐allergic rhinitis, and nasal obstruction. It has the unique property of evaluating sexual function in AR patients., The SNOT‐22 has also been validated for use in AR patients.
In summary, VCOS are simple, effective tools that may be used to assist in making the diagnosis of AR, and in evaluating the efficacy of various therapies.
Use of validated subjective instruments and patient‐reported outcome measures
Aggregate grade of evidence: B (Level 1: 2 studies, level 2: 2 studies, level 3: 5 studies, level 4: 13 studies; Table X.H.‐2)
Benefit: Validated surveys offer a simple point‐of‐care option for screening and tracking symptoms, QOL, and control of allergic disease.
Harm: Minimal. Time to complete survey. Potential risk of misdiagnosis when based on survey data alone.
Cost: No financial burden to patients. Some fees associated with validated tests used for clinical research.
Benefits‐harm assessment: Preponderance of benefit over harm. Risk of misdiagnosis leading to unnecessary additional testing. Likewise, there is a risk that false negative responses may lead to delay in testing and further management.
Value judgments: Validated surveys may be used as a screening tool and primary or secondary outcome measure.
Policy level: Recommendation.
Intervention: Validated surveys may be used to screen for AR, follow treatment outcomes and as a primary outcome measure for clinical trials. Specific tests are optimized for various clinicopathological scenarios.
XI MANAGEMENT
XI.A Allergen avoidance and environmental controls
XI.A.1 House dust mites
HDMs are a common trigger for AR. Therefore, reducing exposure to HDM through physical barriers and chemical treatments are potentially important options in the management of AR, , , , (Table XI.A.1).
Physical techniques for HDM reduction, including heating, ventilation, barrier methods, air filtration, vacuuming, and ionizers, have shown inconsistent results for the treatment of AR., , , , , , While several interventions have reduced the concentration of environmental HDM antigens,, , , , an associated improvement in clinical symptoms has not been reliably demonstrated. Ghazala et al. and Terreehorst et al. demonstrated a reduction in HDM antigen concentration with impermeable bedding as an isolated intervention but found no clinical benefits. Similar findings were reported by Antonicelli et al. following a trial of high‐efficiency particulate air (HEPA) filtration.
Acaricides in household cleaners have been utilized as a chemical technique to reduce HDM concentration. Geller‐Bernstein et al. evaluated an acaricide spray in the bedrooms of patients with HDM sensitization, demonstrating improved mean symptom scores versus control patients without acaricide. Similar findings were reported by Kneist et al. Using a crossover study design, Chen et al. investigated an acaricide containing bag placed beneath bed mattresses in children with AR and asthma, reporting improved AR symptom scores and disease specific QOL (measured using the RQLQ) for those in the intervention group compared to control.
Overall, no serious adverse effects were reported from the evaluated interventions. None of the studies evaluated cost‐effectiveness.
Recent findings, as well as a 2010 Cochrane review suggest acaricides, either as a single measure or in combination with other measures, are the most effective intervention for reducing HDM levels and improving AR symptoms.
Allergen avoidance and environmental controls – house dust mite
Aggregate grade of evidence: B (Level 1: 2 studies, level 2: 12 studies; Table XI.A.1)
Benefit: Potential improvement in AR symptoms and QOL with reduced concentration of environmental HDM antigens.
Harm: None.
Cost: Low to moderate. However, cost‐effectiveness was not evaluated.
Benefits‐harm assessment: Benefit outweighs harm.
Value judgments: There is supporting evidence for the use of acaricides in reducing HDM concentration in children who have AR coexistent with asthma. In adults and children without concomitant asthma, the use of acaricides with/without bedroom‐based control programs for reducing HDM concentration are promising, but further, high‐quality studies are needed to evaluate clinical outcomes.
Policy level: Option.
Intervention: Acaricides used independently or alongside environmental control measures, such as air filtration devices, could be considered as options in the management AR.
XI.A.2 Cockroach
Measures to control cockroach allergen concentrations within the home environment have been targeted at eliminating infestations and abating cockroach allergen. The three main intervention strategies used are: (1) education‐based methods consisting of house cleaning measures and sealing cracks and crevices in highly infested areas; (2) physical methods using insecticides or bait traps; and (3) treatments combining education‐based interventions with physical methods. The greatest challenges in controlling cockroach infestation and reducing allergen concentrations are in densely populated inner‐city areas that contain multi‐occupant housing.,
Most studies contain one or more interventions focused on German cockroach (Blattella germanica antigen 1 and 2 [Bla g 1, Bla g 2]) allergen levels,, , , , , , , , however some studies included treatments targeted at reducing multiple allergens (e.g., HDM, cockroach, rodent, cat, and dog)., The majority of studies were RCTs designed to evaluate the efficacy of specific environmental control measures in reducing environmental allergens. These studies used a variety of interventions that included home‐based education as well as physical methods such as pest control and insecticides., , , , , , , Although Bla g 1 and Bla g 2 allergen levels were reduced below 8 U/g in some homes, clinical benefits in sensitized individuals were not achieved., , , , One study found Bla g 1 concentrations could be decreased below targeted thresholds for most apartments using a building‐wide cockroach control program (Table XI.A.2).
The most effective treatment for eliminating infestation and reducing allergen load was professional pest control. In one study that monitored cockroach populations and allergen concentrations over a 12‐month period, findings revealed that insecticide bait traps placed by professional entomologists were more effective in reducing cockroach populations and cockroach allergen compared to dwellings that received numerous commercial applications of insecticide formulations to baseboards, cracks, and crevices. Bait traps, including labor and monitoring costs, were estimated to be less expensive than commercially applied insecticide sprays. The expense of integrated home management that consists of professional cleaning, education, and pest control was not found to be cost‐effective. Thus, most investigators focused on assessing the efficacy of single interventions, such as extermination alone, in assessing potential cost benefits., Arbes et al. and Sever et al. have noted that these measures were not found to be cost effective. Detailed information may be found in their publications, as this discussion was beyond the scope of this section. Families often had difficulty adhering to home‐based intervention regimens over the course of the study, which reduced the efficacy of these treatments and subsequently resulted in increased cockroach allergen levels.
Although cockroach count could be significantly reduced in single‐family homes using bait traps, reinfestation and high allergen levels remained an ongoing problem in multi‐family buildings. Effectively controlling cockroach infestation and allergen levels within multi‐family buildings and apartments requires implementation of a building‐wide management program. Thus, it is difficult to dramatically reduce cockroach allergen levels in the home unless a significant reduction in cockroach counts is maintained over time. Most studies did not include clinical endpoints. However, those that did evaluate clinical outcomes focused on asthma symptoms, hospitalizations or emergency room visits, and medication usage., No studies included any assessment of symptoms or clinical endpoints associated with AR.
Allergen avoidance and environmental controls – cockroach
Aggregate grade of evidence: B (Level 1: 1 study, level 2: 8 studies, level 3: 2 studies, level 4: 1 study; Table XI.A.2)
Benefit: Reduction in cockroach count but allergen concentrations (Bla g 1 and Bla g 2) often above acceptable levels for clinical benefits. No studies included clinical endpoints related to AR.
Harm: None noted.
Cost: Direct costs include multiple treatment applications or multi‐interventional approaches. Indirect costs include potential time off work for interventions in home and labor intensity of cleaning measures to eradicate allergens.
Benefits‐harm assessment: Balance of benefits and harms since lack of clear clinical benefits.
Value judgments: Control of cockroach populations especially in densely populated multi‐family dwellings is important to control cockroach allergen levels.
Policy level: Option.
Intervention: Combination of physical measures (e.g., insecticide bait traps, house cleaning) and education‐based methods seem to have the greatest efficacy. Additional research on single intervention approaches is needed with cost analysis, as well as investigation of clinical outcomes related to AR.
XI.A.3 Pets
Pet avoidance and environmental control represent treatment options for AR due to animal allergy. Pet removal is a commonly cited strategy without high‐quality outcomes evaluation and is associated with extremely poor compliance., , , One study evaluated compliance of 288 sensitized patients with pet removal recommendations; only 4% of those with direct exposure to home animals adhered to removal recommendations. However, pet avoidance has shown benefit in the secondary prevention of asthma among previously sensitized individuals and current asthma treatment guidelines recommend pet removal from a sensitized individual's home, (Table XI.A.3).
Environmental controls have been evaluated as strategies to decrease antigen exposure and symptoms of AR with mixed results. While most pet allergen environmental control studies focus on cats, less evidence is available for other allergenic pets, such as dogs, birds, and others. The utility of multi‐modality environmental control (cat avoidance, weekly cleaning with removal of carpeting and upholstered furniture, etc.) was studied in 40 patients diagnosed with cat (Fel d 1) sensitization and resulted in significant improvements in nasal airflow and clinical symptoms. However, single‐modality environmental control has not been associated with improved symptoms despite identified reductions in environmental antigens. Wood et al. evaluated HEPA filtration in a high‐quality randomized controlled study of 35 patients with Fel d 1 sensitization, finding unchanged nasal symptom scores, sleep disturbance, rescue medication usage, and spirometry following a 3‐month trial. Likewise, there is not good evidence to support the impact of dog allergen mitigation on improvement in clinical symptoms. Several studies of lower‐quality evidence have evaluated the duration of antigen reduction following pet washing, finding that washing of cats and dogs must be completed at least twice weekly to maintain significant reductions in environmental antigens.,
Allergen avoidance and environmental controls – pets
Aggregate grade of evidence: C (Level 2: 2 studies, level 3: 2 studies, level 4: 1 study; Table XI.A.3)
Benefit: Decreased environmental allergen exposure with possible reduction in symptoms and secondary prevention of asthma.
Harm: Emotional distress caused by removal of household pets. Financial and time costs of potentially ineffective intervention.
Cost: Low to moderate.
Benefits‐harm assessment: Equivocal.
Value judgments: While several studies have demonstrated an association between environmental controls and reductions in environmental antigens, only a single, multi‐modality RCT has demonstrated clinical improvement in nasal symptoms among patients with Fel d 1 sensitivity. The secondary prevention and treatment of asthma in sensitized individuals must also be considered.
Policy level: Option.
Intervention: Pet avoidance and environmental control strategies, particularly multi‐modality environmental controls among patients with diagnosed Fel d 1 sensitivity, may be presented as an option for the treatment of AR.
XI.A.4 Rodents
Only a few high‐quality studies have been published on rodent (i.e., mouse, rat, guinea pig, and hamster) avoidance and interventions to reduce exposure specifically related to AR. Most studies focus on changes in mouse allergen levels and asthma‐related outcomes in inner‐city children, which may not directly correlate with AR symptoms in other populations., , , , , While some RCTs have been conducted for mouse allergen, none have been performed for non‐mouse rodent allergens. Demonstrating efficacy of rodent avoidance or interventions targeted to reduce exposure is difficult as most environmental interventions lead to non‐specific removal of multiple allergens (Table XI.A.4).
Observation studies of early exposure to rodents in childhood have yielded mixed results when evaluating future risk of rodent sensitization and the development of AR or allergic asthma., , , Larger controlled studies are needed.
Avoidance of workplace rodent exposure. Removal of rodent exposure is a management option for AR and asthma in those that are sensitized; however, as exposure can occur in various environments, comprehensively accomplishing this is challenging. When exposure primarily occurs at the workplace (e.g., laboratory worker handling rodents), reduction of allergen exposure can be accomplished by changing jobs or roles, use of personal protective devices, maintaining ventilation systems, and proper staff training.,
Rodents as pets or pests. As various rodents can be kept as pets, many sensitized individuals or their caregivers are reluctant to remove the rodent from the living space, similar to other furry animals., Conversely, individuals are generally willing to comply with recommendations to remove things they consider pests. Rodent predators such as cats can reduce rodent populations but are unlikely to eliminate an infestation. One observational inner‐city study showed that the number of cats and cat allergen levels are inversely correlated with mouse allergen levels. No clinical outcomes were reported in this study. No recommendations can be made at this time, but the risks likely outweigh potential benefit due to the high reported co‐sensitization rate for cat and mouse allergens, which could lead to worsening of allergic symptoms with cat introduction.
Integrated pest management for rodent infestation. Integrated pest management encompasses the initial removal of allergen reservoirs and habit modifications to reduce the risk of infestation recurrence. These interventions include home‐based education, rodent extermination via traps and rodenticide, HEPA filtration, sealing of holes and cracks with copper mesh, and thorough cleaning. Singular interventions, such as placing rodent traps alone, are unlikely to provide meaningful benefit, which is consistent with cockroach allergen mitigation literature. (See Section XI.A.2. Allergen Avoidance – Cockroach for additional information on this topic.)
Several RCTs have been performed to evaluate the efficacy of integrated pest management in reducing indoor allergen levels; however, only six specifically address mouse allergen., , , , , Integrated pest management methods were highly variable between these studies, making direct comparisons difficult. In addition, the outcome measures evaluated were primarily mouse antigen levels and asthma‐related outcomes (no rhinitis outcomes were reported) in low‐income, inner‐city populations, which limits the generalizability of the results. Three out of the six showed a reduction of mouse antigen levels with integrated pest management, one did not report this outcome, and two showed no significant difference. Asthma‐related clinical endpoint results were mixed, but 1 study that utilized extensive integrated pest management interventions showed an increase in FEV1 (forced expiratory volume in 1 second) in inner‐city children when ≥75% reduction of mouse allergen levels was achieved.
In summary, avoidance measures for work‐related exposures and pet rodent exposures may have significant benefit. For rodent infestations, integrated pest management reduces mouse allergen levels in the household, but meaningful clinical improvement remains unclear in mouse‐sensitized patients., , , , , The generalizability of rodent‐specific integrated pest management RCTs is very limited as they all mainly included low‐income, inner‐city populations in the northeastern US. No well‐conducted studies have evaluated allergen reduction interventions for other rodents. Future research should concentrate on the effects of integrated pest management on rodent allergen levels in non‐inner‐city populations, rhinitis outcomes, and determining which interventions are highest yield to maximize cost‐efficiency.
Allergen avoidance and environmental controls – rodents
Aggregate grade of evidence: C (Level 2: 5 studies, level 3: 5 studies, level 4: 4 studies, level 5: 1 study; Table XI.A.4)
Benefit: Reduces rodent allergen levels (specifically mouse allergen) but no information on AR outcomes.
Harm: Reduction in QOL of patient due to removal of pet rodent to whom patient is emotionally attached. Change in job position or role if primary rodent exposure is work‐related.
Cost: Direct costs include the cost of interventions such as extermination and mitigating causal factors or loss of income if a job change occurs. Indirect costs include time off work for pest control appointments.
Benefits‐harm assessment: Balance of benefit and harm.
Value judgments: Careful patient selection based on exposure history. Heterogeneity of integrated pest management protocols makes quantification of benefit difficult.
Policy level: Option.
Intervention: Avoidance likely improves rodent‐specific allergen exposure, especially when the interaction can be eliminated such as when it is work‐related or with a pet rodent. Integrated pest management should be considered in select patients, such as pediatric inner‐city patients that suffer from asthma and are mouse sensitized.
XI.A.5 Pollen
For pollen sensitized patients, avoidance or environmental control measures are often the first recommended intervention to decrease exposure and symptoms. This approach is derived from the experience in which nasal or inhalational allergen challenges induce inflammatory changes and clinical symptoms after exposure. Education and avoidance measures often involve personal behavior changes, particularly when pollen counts are elevated. While complete avoidance of pollen triggers is rarely achievable, it also has undesirable consequences such as avoiding the outdoors. A more realistic goal is a reduction in exposure to pollens rather than complete elimination Further, evidence supporting such recommendations is often limited to expert opinion and clinical experience.
Dominant aeroallergens may vary significantly by geographical location, climate, and season. Understanding an individual's specific sensitization pattern is best characterized by the combination of history and physical examination along with skin testing or serum sIgE testing. This combined with local pollen data can guide when a patient may be most likely exposed to a particular allergen and, therefore, when avoidance measures may be most effective. Local pollen counts can be ascertained by various sources including local media, phone applications, and trusted internet websites.
Practical interventions for pollen avoidance include keeping windows in homes and cars closed, drying clothes indoors, and staying inside when possible. Cabin air filters in cars, pollen screens, eyeglasses, and mouth‐nose covering masks may reduce exposures. Pollen counts tend to be higher on sunny, windy days with lower humidity. HEPA filters in air purifiers can decrease exposure and, when studied in Artemisia pollen sensitized patients, led to decreased allergy symptom scores compared to placebo filters. For individuals able to change immediate landscaping, choosing entomophilous or insect pollinated plants may be helpful in addition to selecting plants less likely to induce allergic symptoms. While allergen avoidance is endorsed by national and international guidelines,, the clinical efficacy of these interventions has not been rigorously evaluated.
The previously mentioned pollen avoidance approaches apply more generally to one's surroundings. There have also been attempts with physical barriers in direct or close contact with mucosal membrane surfaces where pollens may adhere and cascade immune responses. One study enrolled 70 individuals with seasonal AR (primarily to grass) or polysensitized individuals without perennial sensitizations, where patients were randomized to receive wraparound eyeglasses in addition to medical treatment versus medical treatment alone for three successive pollen seasons. Patients provided wraparound glasses had improved ocular and nasal symptoms, in addition to improved RQLQ compared to medical therapy alone. Nasal filters have also been used as an avoidance tool to prevent symptoms of AR. In a randomized, double‐blind placebo‐controlled crossover trial, 65 grass sensitized adults were monitored in a natural exposure setting at a park while either wearing a nasal filter or placebo. Patients wearing nasal filters had significantly reduced TNSS scores compared to placebo. Other barrier protection measures have been assessed, including cellulose powder applied to the nose, pollen blocker cream, and microemulsion. In a systematic review, 15 RCTs involving data of these measures from 1154 patients were assessed with subgroup analysis according to the type of barrier protection studied. Compared to placebo, the barrier protection methods assessed each had improved symptom control by meta‐analysis without increased adverse events (of note, nasal filter was not analyzed by meta‐analysis due to insufficient data). Most of the included studies were small with heterogeneous study designs, but overall barrier methods may offer non‐pharmacologic, symptomatic improvement to motivated patients (Table XI.A.5).
Allergen avoidance and environmental controls – pollen
Aggregate grade of evidence: B (Level 1: 1 study, level 2: 3 studies; Table XI.A.5)
Benefit: Decreased symptoms and medication use with potential for improved QOL.
Harm: Interventions may vary in cost and efficacy of each may be inadequately defined.
Cost: Generally low monetary cost depending on strategy.
Benefits‐harm assessment: Equivocal, most interventions with lower harm but not well‐defined benefits.
Value judgments: Most pollen avoidance measures are based on clinical and expert opinion although trial‐based evidence is available for some interventions.
Policy level: Option.
Intervention: Pollen avoidance strategies are generally well tolerated and lower cost, non‐medication‐based interventions that may have benefit with minimal harm to the patient, but further RCTs with larger populations would be needed to better characterize efficacy.
XI.A.6 Occupational
Occupational rhinitis may be secondary to allergic or irritant responses and has been associated with a variety of agents, including animals, particulate matter from woods, grains, chemicals, and other substances. Early diagnosis is crucial not only for managing rhinitis symptoms but also potentially preventing the development of coexisting occupational asthma., Regarding management, the most common strategy is avoidance or implementation of environmental controls. However, it is critical to prevent sensitization through appropriate occupational hygiene and safety practices with surveillance of symptoms and exposures in high risk environments.
Accurate diagnosis of occupational rhinitis may be suggested by periods of improvement during work avoidance such as planned time away from the workplace, when not exposed regularly to occupational allergens. NPT may be pursued but the validity of this testing is often poorly defined. For patients with high clinical suspicion of occupational rhinitis, complete avoidance is recommended as the safest and most effective therapeutic option. If this is not possible due to socioeconomic consequences or otherwise, environmental control measures to reduce exposure may be an acceptable alternative. This may be accomplished with escalating interventions, starting with avoidance by the use of less problematic materials, improving ventilation of the areas involved, reducing time spent working with implicated materials, or utilizing protective gear for the patient.
Symptom improvement has been reported in clinical settings following effective avoidance. In a prospective study, 20 patients with specific inhalation challenge‐confirmed occupational rhinitis (exposures including flour, animal proteins, tea, isocyanates, resins, and acrylates) were assessed at diagnosis and follow‐up, with a mean time interval of 4.7 ± 1.3 years. At follow‐up assessment, all patients had been removed from exposure and reported significant decreases in nasal symptoms and improvement in QOL. Similarly, a separate Finnish cohort of 119 patients was diagnosed with occupational rhinitis (exposures including flour, animal proteins, storage mites, latex, flowers or indoor plants, dried egg powder, organic acid anhydrides with human serum proteins, abache wood dust, human dandruff, and enzymes) with an average of 10 years since diagnosis. Health‐related QOL for those no longer exposed to occupational allergens was similar to healthy controls, while it was impaired among those with continued exposures. Thus, complete avoidance appears to improve rhinitis symptoms and QOL, and when feasible, may be the best approach (Table XI.A.6).
However, if complete avoidance is not able to be achieved, there can be benefit to treatment approaches including decreased levels of exposure. In a group of 36 patients with latex‐induced occupational asthma and a median follow up time of 56 months, 20 subjects with reduced exposure had improved asthma severity along with reduced rhinitis symptom severity scores. The other 16 patients without ongoing exposure (defined as latex gloves never used in the working environment) also had improvement in asthma and rhinitis symptom severity but had more loss of income and work disability. In a separate cross‐sectional survey of patients with occupational asthma to platinum salts, transfer to low‐exposure areas at work resulted in improved rhinitis symptoms compared to high exposure areas. Where avoidance or decreased exposure by job location is not achievable, personal protective equipment may be sufficient to decrease symptoms of occupational rhinitis. In a group of agricultural workers, predominately with occupational asthma to cow dander or grains, use of a powered dust respirator helmet worn over a period of 10 months resulted in significantly reduced rhinitis symptoms and improved morning peak flow rate.
Overall, while most of the evidence is limited to small observational studies, complete avoidance of an inciting agent in occupational rhinitis likely provides the best improvement in symptoms and QOL and should be pursued when possible. Alternatively, occupation‐specific interventions to decrease exposure may offer benefit to patients when complete avoidance cannot be accomplished. Further characterization of levels of exposure and most effective means of decreasing exposure is needed. (See Section V.B.3 Occupational Rhinitis for additional information on this topic.)
Allergen avoidance and environmental controls – occupational
Aggregate grade of evidence: C (Level 3: 5 studies; Table XI.A.6)
Benefit: Decreased allergen exposure may lead to reduction in symptoms, improvement in QOL, and possible reduced likelihood of developing occupational asthma.
Harm: Potential for socioeconomic harm with loss of wages or requiring changes in occupation.
Cost: Individually may vary if avoidance results in loss of income; for employers, potentially high cost depending on interventions or environmental controls required.
Benefits‐harm assessment: Where possible from a patient‐centered perspective, in occupational rhinitis complete avoidance is likely beneficial in improving health quality compared to ongoing exposures.
Value judgments: Based primarily on observational studies, allergen avoidance or decreasing exposure is recommended for all patients but can be nuanced depending on the resulting socioeconomic impact.
Policy level: Recommendation.
Intervention: Patients should be counseled to avoid or decrease exposure to inciting agents in occupational respiratory disease.
XI.B Pharmacotherapy
XI.B.1 Antihistamines
XI.B.1.a Oral H1 antihistamines
In AR, sIgE binds to mast cells and basophils which triggers the release of histamine. The effects of histamine include vasodilation, smooth muscle bronchoconstriction, increased endothelial permeability, and sensory nerve stimulation, contributing to the classic symptoms of AR. Antihistamines are inverse agonists of histamine and cause histamine receptors to convert to an inactive state. Antihistamines are classified as first, second, and third generation. However, herein we classify the second and third generation as newer‐generation antihistamines (Table XI.B.1.a.‐1). First‐generation antihistamines (e.g., diphenhydramine and chlorpheniramine) have anticholinergic side effects and can cross the blood–brain barrier, resulting in central nervous system effects such as sedation and drowsiness., These side effects can be more pronounced in the elderly, so first‐generation antihistamines should be used with caution. Newer‐generation antihistamines (e.g., bilastine, cetirizine, desloratadine, fexofenadine, levocetirizine, and loratadine) block peripheral H1 receptors without crossing the blood–brain barrier which prevents central nervous system side effects. Several newer‐generation antihistamines are metabolized in the liver by cytochrome p450 enzymes. As a result, prescribers should be conscious of concomitant administration of other drugs that are either processed by cytochrome p450 or drugs that are cytochrome p450 inducers because concurrent administration can either increase or decrease the plasma concentration of the antihistamine.
Given their use since the 1940s, there are numerous RCTs regarding the use of oral antihistamines for the management of AR. With this in mind, a summary of the highest grade of evidence published is provided (Table XI.B.1.a.‐2).
There are several published guidelines regarding the use of oral antihistamines for the management of AR. In 2004 the ARIA group and EAACI released recommendations regarding the pharmacological criteria that commonly used AR medications should meet. Taking into consideration the efficacy, safety, and pharmacology, newer‐generation antihistamines were shown to have a favorable risk‐benefit profile and were recommended over first‐generation oral antihistamines for the treatment of AR. The 2015 American Academy of Otolaryngology‐Head and Neck Surgery Foundation (AAO‐HNSF) Clinical Practice Guidelines and the 2019 Canadian Society of Allergy and Clinical Immunology position statement also recommended newer‐generation antihistamines over first‐generation antihistamines for the management of AR.,
The ARIA guidelines 2010 revision made a strong recommendation for newer‐generation antihistamines that are non‐sedating and do not interact with cytochrome p450. The ARIA guidelines 2016 revision made several recommendations regarding when to consider the use of oral antihistamines, taking into context other drugs available for the management of seasonal and perennial AR. In 2020, the ARIA group published the first GRADE‐based guidelines that integrated real‐world patient‐reported experience and clinical studies to inform the management of AR. It provided a treatment algorithm that, in a nuanced manner, considered a patient's symptom severity with past and current medication use to clarify the role of newer‐generation antihistamines for the management of AR. The standard dosing for newer‐generation antihistamines is listed in Table XI.B.1.a.‐1.
The decision on which newer‐generation antihistamine to prescribe should be individualized to the patient and the dosing, drug interactions, side effects, the onset of action, and cost should be considered. A large study that examined all e‐prescriptions of oral antihistamines (n = 2280) in Poland in 2018 found that approximately one in five prescriptions was not redeemed. This finding suggests the need for further studies regarding patient adherence to oral antihistamines, noting that various factors could influence patient adherence including lack of trust in the prescriber, cost and availability of the medication over the counter.
Excluding oral antihistamines only available by prescription, the cost of most newer‐generation oral antihistamines is similar at ∼$2 per day. As newer‐generation oral antihistamines have fewer central nervous system side effects than first‐generation oral antihistamines, their indirect costs to society are lower than first‐generation oral antihistamines., , The indirect costs amongst newer‐generation oral antihistamines are similar given the similar side effect profiles.
Oral H1 antihistamines
Aggregate grade of evidence: A (Level 1: 19 studies, level 4: 5 studies; Table XI.B.1.a.‐2)
Benefit: Reduction in symptoms of AR.
Harm: Compared to first‐generation oral antihistamines, newer‐generation antihistamines have fewer central nervous system and anticholinergic side effects. The side effects of first‐generation antihistamines can be more pronounced in the elderly. See Table II.C.
Cost: Inexpensive. Given their improved side effect profile, newer‐generation oral antihistamines also have lower indirect costs than first‐generation oral H1 antihistamines.
Benefits‐harm assessment: The benefits outweigh harm for use of newer‐generation H1 oral antihistamines for AR.
Value judgments: First‐generation oral antihistamines are not recommended for the treatment of AR because of their central nervous system and anticholinergic side effects.
Policy level: Strong recommendation for the use of newer‐generation oral antihistamines for AR.
Intervention: Newer‐generation oral antihistamines can be considered in the treatment of AR.
XI.B.1.b Oral H2 antihistamines
Our understanding of the role of the H2 receptor in mediating histamine‐related nasal symptoms in AR is limited. There is no data comparing H2‐receptor antagonism efficacy to common first line therapy such as INCS, and only a few relatively small studies have investigated the impact of H2‐receptor antagonism. Most importantly, the clinical significance of the changes associated with H2 antihistamines has not been clearly defined. Nonetheless, H2 antihistamines possess relatively low risk (drug–drug interactions through decreased gastric acidity and inhibition of cytochrome p450) and low cost and have been supported by some studies for use in patients with recalcitrant nasal airway obstruction in combination with oral H1 antihistamines.
There have been several RCTs that investigated the efficacy of H2 antihistamines in improving objective measures such nasal airway resistance and nasal secretion. Wood‐Baker et al. compared oral cetirizine to oral ranitidine. Objective measures of nasal airway resistance showed greater improvement with ranitidine; however, objective measures of nasal secretion decreased more with cetirizine. Despite very few studies showing efficacy of H2 blockers alone, several studies have emphasized their potential utility in combination with H1 antagonists. Taylor‐Clark et al. found similar improvement in nasal airway resistance between cetirizine and ranitidine, but a significant improvement with the use of combination therapy. Wang et al. also showed improvement in nasal airflow with combination therapy of cimetidine and cetirizine. Havas et al. measured the nasal airflow resistive response to topical histamine and also found that combined histamine antagonism with diphenhydramine hydrochloride and cimetidine was significantly more effective in reducing the nasal resistive response than H1 antagonist alone. However, not all data regarding combination therapy has been conclusive with other studies finding no improvement in nasal airflow with the addition of an H2 antihistamine., Moreover, the clinical significance of these objective measures remain unclear (Table XI.B.1.b).
Alternatively, several studies have investigated the impact of H2 antagonism on symptoms by employing PROMs. Subjects were asked to report some combination of congestion, blockage, itch, drainage, sneeze, eye symptoms, and asthma with a categorical severity measure. Three of the four studies examined symptoms after nasal allergen challenge, and none of these demonstrated efficacy of H2 antihistamines in diminishing allergic symptoms, either alone, or conjunction with an H1 antihistamine., , , The majority of RCTs investigating the efficacy of H2 antihistamines are within the context of pre‐treatment of a patient prior to a nasal histamine or allergen challenge. Only one study investigated the impact of an H2 antagonist, cimetidine, in conjunction with chlorpheniramine in a real‐world setting. Carpenter et al. randomized 23 subjects with known late‐summer AR to receive alternating 2‐week courses of either chlorpheniramine plus placebo during the season, or chlorpheniramine plus cimetidine. Symptom scores were recorded twice daily along with adjuvant medical therapies taken (specifically, oral corticosteroids). A significant reduction in medication use was reported by patients receiving both H1 and H2 antagonists (28 corticosteroid days vs. 44 corticosteroid days, p < 0.02) and decreased symptoms scores during one of the 8 weeks when weed pollen counts were high. A limitation of this study is its utilization of a first‐generation antihistamine which is no longer utilized as first‐line treatment of rhinitis symptoms. No current studies exist comparing INCS with second‐generation antihistamines in combination with H2 blockers.
The data existing on the use of H2 antihistamines in AR is limited in scope and quality, with very little addition to the literature in the past decade. The objective findings of improved nasal airway resistance suggest that the H2 histamine receptor does modulate nasal tissue response to histamine., , , However, the clinical significance of this mechanism is not clear, particularly in the context of modern treatment algorithms., , , , Given the relatively manageable side effect profile and costs of H2 antihistamines, they may offer patients with otherwise recalcitrant AR symptoms an additional treatment option. However, additional investigation on the efficacy of H2 antihistamines in combination with other topical medications may be beneficial in the future.
Oral H2 antihistamines
Aggregate grade of evidence: B (Level 2: 7 studies; Table XI.B.1.b)
Benefit: Decreased objective nasal resistance, and improved symptom control in 4 studies when used in combination with H1 antagonists.
Harm: Drug–drug interaction (p450 inhibition, inhibited gastric secretion and absorption). See Table II.C.
Cost: Increased cost associated with H2 antagonist over H1 antagonist alone.
Benefits‐harm assessment: Unclear benefit and possible harm.
Value judgments: No studies evaluating efficacy of H2 antihistamines in context of INCS. There were two studies that showed no benefit for H2 antagonist when used alone or as an additive to H1 antagonist therapy.
Policy level: No recommendation. Available evidence does not adequately address the benefit of H2 antihistamines in AR.
Intervention: Addition of an oral H2 antagonist to an oral H1 antagonist may improve symptom control in AR, but data is limited.
XI.B.1.c Intranasal antihistamines
Two formulations of intranasal antihistamine are currently available in North America for use as a topical spray, azelastine hydrochloride, and olopatadine hydrochloride. The English‐language literature was systematically reviewed for clinical trials of either of these formulations for the treatment of AR. A total of 44 papers were identified that reported results of RCTs of intranasal antihistamine monotherapy. This included 24 studies with an active treatment comparator arm, , , , , , , , , , , , , , , , , , , , , , , and 29 studies with an inactive placebo arm., , , , , , , , , , , , , , , , , , , , , , , , , , , , Monotherapy with azelastine was reported in 37 studies, , , , , , , , , , , , , , , , , , , , , , , , , , , , , , , , , , , , while monotherapy with olopatadine was reported in 10 studies., , , , , , , , , Some studies utilized multiple active treatment arms of antihistamine and/or corticosteroid (Table XI.B.1.c).
Patient‐reported symptom scores or QOL assessments were the most frequently utilized outcome measures in the included studies. The most common outcome measure was the TNSS (23 studies), which summarizes the severity of the cardinal symptoms of sneezing, itching, congestion, and runny nose. Other outcome measures included the RQLQ (seven studies), the Total Ocular Symptom Score (TOSS, five studies), the Caregiver Treatment Satisfaction Questionnaire (two studies), the Pediatric RQLQ (one study), the SF‐36 (one study), the ESS (one study), the Rhinitis Severity Score (one study), and a Subjective Global Assessment (one study). Multiple studies, particularly those published more than 20 years ago, relied upon arbitrary, non‐validated symptom scores for reporting treatment outcomes (19 studies). A minority of studies included objective measures such as nasal lavage (three studies), response to methacholine challenge (two studies), nasal flow rate (two studies), and rhinomanometry (one study).
The most frequent treatment duration was 14 days in the included studies, with a range from 2 days to 8 weeks. Study enrollment ranged from 20 to 1188 subjects. In the 29 studies using placebo as a comparison group,, , , , , , , , , , , , , , , , , , , , , , , , , , , , intranasal antihistamine showed superiority for the primary outcome of nasal symptom improvement. An active treatment comparator of a different medication was used in 24 studies., , , , , , , , , , , , , , , , , , , , , , , The intranasal antihistamine spray treatment group consistently had a more rapid onset of action than the treatment comparator, occurring as early as 15 min after administration, although this was not reported in all studies. Azelastine and olopatadine were directly compared in three studies, with no significant difference in symptom relief between agents., , Azelastine was compared with an experimental formulation of intranasal levocabastine in two additional studies, with either comparable or superior results for azelastine., Levocabastine is not available as a commercial product.
The active treatment comparators utilized in 24 studies consisted of an INCS or oral antihistamine. Twelve studies compared intranasal antihistamine with INCS, with the primary outcome of nasal symptom improvement favoring antihistamine in two studies,, INCS in three studies,, , and showing equivalency in seven studies., , , , , , Superiority of the antihistamine for treating ocular symptoms was found in two studies, one of which was nearly 30 years old., The three studies showing superiority of INCS were over 20 years old and reported outcomes using heterogeneous non‐validated symptom scores.
Intranasal antihistamine was compared to oral antihistamine monotherapy in eight studies, with superiority of intranasal antihistamine in three studies,, , and equivalency in five studies., , , , One study included a treatment arm with oral chlorpheniramine as a positive control without intent to compare efficacy with azelastine. Azelastine monotherapy was at least as effective as combination therapy in a single study comparing azelastine spray versus oral loratadine plus intranasal beclomethasone. Combination therapy with intranasal azelastine plus oral antihistamine was not found to confer additional benefit in two studies compared to intranasal azelastine monotherapy., An overall dose–response relationship was found in 11 studies that included comparison of multiple dose concentrations of intranasal antihistamine. , , , , , , , , , ,
Most of the included studies set a minimum enrollment age of 12 years or older. Three studies that included children aged between 6 and 12 years old found superiority of intranasal antihistamine to placebo in improving symptoms and QOL., ,
No study reported any serious adverse effects from use of an intranasal antihistamine. These formulations are noted to be generally well tolerated, with taste aversion being the most reported adverse effect. One study that compared a reformulated vehicle against the commercially available form of azelastine found no difference in taste aversion. Olopatadine was reported to have better sensory attributes than azelastine in one study. Other reported adverse effects were uncommon, with somnolence, headache, epistaxis, and nasal discomfort each occurring in less than 10% of patients treated with azelastine or olopatadine (Table II.C).
In 2021, the US FDA approved azelastine hydrochloride as an over‐the‐counter formulation, making intranasal antihistamines available for the first time without a prescription. This change may remove some financial barriers to patient use and improve access to this medication as a treatment option for AR.
Intranasal antihistamines
Aggregate grade of evidence: A (Level 2: 44 studies; Table XI.B.1.c)
Benefit: Rapid onset; more effective for nasal congestion than oral antihistamines; more effective for ocular symptoms than INCS; consistent reduction in symptoms and improvement in QOL in RCTs compared to placebo.
Harm: Patient tolerance, typically related to taste aversion; less effective for congestion than INCS. See Table II.C.
Cost: Low to moderate financial burden; available as prescription or nonprescription product.
Benefits‐harm assessment: Preponderance of benefit over harm. Intranasal antihistamine as monotherapy is consistently more effective than placebo. Most studies show intranasal antihistamines superior to INCS for sneezing, itching, rhinorrhea, and ocular symptoms. Adverse effects are minor and infrequent. Generic prescription and over‐the‐counter formulations now available.
Value judgments: Extensive high‐level evidence comparing intranasal antihistamine monotherapy to active and placebo controls demonstrates overall effectiveness and safety.
Policy level: Strong recommendation.
Intervention: Intranasal antihistamines may be used as first‐ or second‐line therapy in the treatment of AR.
XI.B.2 Corticosteroids
XI.B.2.a Oral corticosteroids
Early work using the nasal challenge model has elucidated the anti‐inflammatory effects of oral corticosteroids in AR. Pipkorn et al. premedicated patients with seasonal AR with either prednisone or placebo for 2 days prior to an allergen challenge. When compared to placebo, patients receiving prednisone demonstrated a significant reduction in sneezing as well as reduced levels of histamine and other mediators of vascular permeability in nasal lavages during the late phase response. Active treatment also reduced the priming response to consecutive allergen challenges. In similar placebo‐controlled studies, Bascom et al., demonstrated a reduction in the influx of eosinophils and levels of eosinophil mediators (MBP and eosinophil derived neurotoxin) in nasal secretions during the late phase response in patients receiving 60 mg oral prednisone for 2 days prior to nasal challenge (Table XI.B.2.a).
The efficacy of oral corticosteroids in seasonal clinical disease has also been demonstrated with less rigorous studies that did not include a placebo control. Schwartz et al. demonstrated that 15 days of cortisone (25 mg QID [four times daily]) during the ragweed season resulted in significant relief of symptoms in 21 of 25 patients. Schiller and Lowell showed that cortisone (100 mg daily) for 4 day courses during the pollen season resulted in rhinitis symptom relief in 42 of 51 patients. Twenty of those patients had a relapse of symptoms within 7 days of cessation of therapy. Oral hydrocortisone (40–80 mg daily) has been shown to reduce symptoms of ragweed allergies. In a placebo‐controlled study performed during the ragweed season, Brooks et al. compared the efficacy of methylprednisolone (6, 12, or 24 mg PO [per os, by mouth] daily for 5 days) to placebo in controlling nasal symptoms. They reported a significant reduction in congestion, postnasal drainage, and ocular symptoms compared to placebo after 6 and 12 mg doses. The higher, 24 mg, dose was more effective and resulted in a significant reduction in all symptoms queried (congestion, runny nose, sneezing, itching, postnasal drainage, and ocular symptoms) compared to placebo. Snyman et al. performed a parallel, double blind study comparing betamethasone 1 mg alone to a combination of betamethasone and loratadine and loratadine alone in patients with severe AR. The group on oral steroids had a significant improvement from baseline in total nasal symptoms and was superior to loratadine alone.
Although effective, oral corticosteroids have well recognized systemic adverse events, and therefore, their use has been largely replaced by intranasal preparations (Table II.C). In a double‐blind, placebo‐controlled trial conducted during the ragweed season, the effect of intranasal flunisolide and its oral dose bioequivalent (an oral dose that would lead to similar systemic levels) were compared. The intranasal preparation reduced rhinitis symptoms compared to placebo whereas the oral dosing did not, suggesting that INCS achieve their benefit primarily through local activity as opposed to systemic bioavailability.
Karaki et al. compared the efficacy of INCS to systemic steroids by performing an open label, parallel, randomized trial during the cedar pollen season in Japan. Patients were randomized to receive loratadine 10 mg daily alone, loratadine with intranasal mometasone furoate (200 μg once daily), or loratadine with oral betamethasone 0.25 mg twice daily for 1 week. Participants receiving any form of steroids demonstrated significantly reduced symptoms of sneezing, rhinorrhea, and nasal obstruction compared to loratadine alone, with no significant difference between the intranasal and oral preparations noted. The oral steroid was more effective than the INCS, however, in controlling allergic eye symptoms.
In summary, oral corticosteroids are effective for the treatment of AR. However, given the significant systemic adverse effects related to using these agents for prolonged periods of time, and the availability of effective and less systemically available intranasal preparations, oral corticosteroids are not recommended for the routine treatment of AR.
Oral corticosteroids
Aggregate grade of evidence: B (Level 2: 6 studies, level 3: 1 study, level 4: 3 studies; Table XI.B.2.a)
Benefit: Oral corticosteroids can attenuate symptoms of AR and ongoing allergen induced inflammation.
Harm: Oral corticosteroids have multiple potential adverse effects, including hypothalamic‐pituitary axis suppression. Prolonged use may lead to growth retardation in pediatric populations. See Table II.C.
Cost: Low.
Benefits‐harm assessment: The risks of oral corticosteroids outweigh the benefits, given similar symptomatic improvement observed with the use of safer INCS.
Value judgments: In the presence of effective symptom control using INCS, the risk of adverse effects from using oral corticosteroids for AR outweighs potential benefits.
Policy level: Strong recommendation against routine use.
Intervention: Although not recommended for routine use in AR, certain clinical scenarios may warrant the use of short courses of systemic corticosteroids, following a discussion of the risks and benefits with the patient. For example, oral steroids could be considered in select patients with significant nasal obstruction that precludes adequate penetration of intranasal agents (corticosteroids or antihistamines). In these cases, a short course of systemic corticosteroids may improve congestion and facilitate access of topical medications. No evidence supports this suggestion, and thus careful clinical judgement and risk discussion are advocated.
XI.B.2.b Intranasal corticosteroids
XI.B.2.b.i Traditional spray application
INCS have potent anti‐inflammatory properties and lead to a significant reduction in mediator and cytokine release along with a significant inhibition in the recruitment of inflammatory cells to nasal secretions and the nasal mucosa., , , , INCS also reduce the antigen‐induced hyperresponsiveness of the nasal mucosa to subsequent challenge., ,
Clinical trials in adults and children have demonstrated the effectiveness of INCS in the reduction of nasal symptoms in AR., , INCS also significantly improve patients’ QOL, , and sleep., , , , Onset of action starts at time points ranging from 3–5 h to 60 h after dosing., , , Although the continuous daily use of INCS is overall superior,, studies have demonstrated the superiority of as needed use of intranasal fluticasone propionate over placebo, and one study showed equivalence of as needed to continuous dosing (Table XI.B.2.b.i.‐1).
INCS have beneficial effects on allergic eye symptoms,, , , secondary to a reduction in the naso‐ocular reflex. This effect is not equal among preparations. Some, but not all, studies have suggested that INCS improve asthma control measures and asthma exacerbations, , (Table XI.B.2.b.i.‐2).
In comparative studies there are no significant differences in efficacy between the available agents, and one study shows an advantage of using double dosing. INCS have shown superior efficacy to H1 antihistamines in controlling nasal symptoms, including nasal congestion, with no significant difference in the relief of ocular symptoms., , However, for fast relief of nasal congestion (1 h after dosing) a combination of loratadine‐pseudoephedrine was superior to intranasal fluticasone propionate. INCS are more effective than LTRAs, , (Table XI.B.2.b.i.‐3).
Different preparations of INCS are comparable in efficacy, making sensory attributes an important factor in patient preference. These include aftertaste, nose runout, throat rundown, and odor; there are minor differences between preparations. Two intranasal nonaqueous preparations with hydrofluoroalkane aerosols, beclomethasone dipropionate, and ciclesonide address some of these concerns., , , , ,
The most common side effects of INCS are a result of local irritation and include dryness, burning, stinging, blood‐tinged secretions, and epistaxis (Table II.C). The incidence of epistaxis with different preparations ranges 4%–8% over short treatment periods (2–12 weeks) with no differences between placebo and active therapy., In studies carried over 1 year, epistaxis is as high as 20%., Septal perforations are rare complications of INCS. In a systematic review of biopsy studies in patients using INCS, none of the studies that evaluated atrophy of the nasal mucosa reported any atrophy with INCS. Studies in adults and children evaluating effects of INCS on the hypothalamic pituitary axis and adrenal insufficiency show no clinically relevant adverse effects., , , , , , , , , , , , , Although there exists a report of association between INCS use and development of posterior subcapsular cataracts, two systematic reviews of controlled trials did not demonstrate a clinically relevant impact of INCS on either ocular pressure, glaucoma, lens opacity, or cataract formation., Therefore, it is reasonable to use these agents with caution in patients with increased intraocular pressure, glaucoma, or cataracts. The effect of INCS on growth in children has been investigated in controlled short‐term (2–4 weeks) and long‐term (12 months) studies. A meta‐analysis of eight RCTs showed that in the short‐term, mean growth was significantly lower among children using INCS compared to placebo in trials using knemometry (n = 4), but that in the long‐term, there was no significant growth difference in studies using stadiometry (n = 4). The data suggest that INCS might have deleterious effects on short‐term growth in children, but the heterogeneity of the results in the stadiometry studies (two studies show growth increase and two show growth decrease) makes the effects on long‐term growth suppression unclear. It is therefore wise to check growth periodically in children on long‐term INCS (Table XI.B.2.b.i.‐4).
Intranasal corticosteroid spray
Aggregate grade of evidence: A (Level 1: 18 studies, level 2: 29 studies, level 3: 3 studies; Tables XI.B.2.b.i.‐1, XI.B.2.b.i.‐2, XI.B.2.b.i.‐3, and XI.B.2.b.i.‐4)
Benefit: INCS are effective in reducing nasal and ocular symptoms of AR. Studies have demonstrated superior efficacy compared to oral antihistamines and LTRAs.
Harm: INCS sprays have undesirable local adverse effects, such as epistaxis, with increased frequency compared to placebo in prolonged administration studies. There are no apparent negative effects on the hypothalamic‐pituitary axis. There might be some negative effects on short‐term growth in children, but it is unclear whether these effects translate into long‐term growth suppression. See Table II.C.
Cost: Low.
Benefits‐harm assessment: The benefits of using INCS outweigh the risks when used to treat seasonal or perennial AR.
Value judgments: INCS are first line therapy for the treatment of AR by virtue of their superior efficacy in controlling nasal symptoms. Subjects with seasonal AR should start prophylactic treatment with INCS several days before the pollen season with an evaluation of the patient's response a few weeks after initiation, including a nasal exam to evaluate for local irritation or mechanical trauma. Children receiving INCS should be on the lowest effective dose to avoid negative growth effects.
Policy level: Strong recommendation.
Intervention: The demonstrated efficacy of INCS, as well as their superiority over other agents, make them first line therapy in the treatment of AR.
XI.B.2.b.ii Non‐traditional application
INCS are typically administered with metered devices for AR. Alternate routes of delivery (irrigation and nebulization) have been studied. Periasamy et al. conducted a prospective, single center double‐blind RCT in 52 patients with AR. Patients received buffered hypertonic saline nasal irrigation (60 ml each nostril twice daily) with either a placebo or a budesonide respule (0.5 mg/2 ml) for 4 weeks. Patients were assessed using the SNOT‐22 questionnaire, visual analog scale (VAS) for sneezing, nasal obstruction, itching, and nasal discharge, and nasal endoscopy findings. SNOT‐22, VAS, and endoscopy score improved from baseline in both groups. The group on budesonide had significantly more improvement than the saline only group in SNOT‐22 and VAS but not endoscopy scores. Study results suggest a beneficial effect of saline irrigations on AR symptoms that is enhanced when steroids are added (Table XI.B.2.b.ii).
Brown et al. investigated the effect of budesonide administered by nebulization in patients with perennial AR. Patients received either budesonide (0.25 mg) or placebo (saline) delivered by nebulization once daily for 4 weeks. The patients on budesonide had significant increases in PNIF, decreases in symptoms and improvement in QOL compared to baseline but the changes were not significantly different from placebo.
Some studies evaluated the effect of corticosteroids in patients with both asthma and AR. Profita et al. randomized children with rhinitis and asthma to either nebulized beclomethasone (administered via face mask breathing through mouth and nose) or placebo twice daily for 4 weeks. Compared to baseline, concentrations of nasal IL‐5 were significantly decreased, and nasal pH levels were significantly increased after beclomethasone treatment. Nasal symptom scores showed a significant reduction in obstruction, sneezing, and rhinorrhea after treatment with beclomethasone dipropionate, but no change after placebo. When the data were compared between beclomethasone and placebo groups, there were significant differences in favor of beclomethasone in nasal IL‐5 and pH but not symptom scores. The significance of nasal pH increase is not clear but could lead to better mucociliary function. Active treatment did improve FEV1 and asthma symptoms. In a similar study, Camargos et al. randomized patients with AR and asthma to either fluticasone propionate hydrofluoroalkane (FP‐HFA) (100–150 μg) inhaled through the nose (mouth closed) using a large volume spacer attached to a face mask or a nasal spray of isotonic saline plus oral inhalation of FP‐HFA through a mouthpiece attached to the same spacer. After 8 weeks of treatment, there was a significant improvement in AR scores and nasal peak flow in the group who received FP‐HFA through the nose compared to the group who received FP by mouth inhalation. There was a significant reduction in asthma scores and increase in FEV1 values in both groups. Shaikh performed an open, parallel crossover trial in patients with asthma and rhinitis and compared budesonide administered inhaled/intranasal to budesonide inhaler alone, exhaled through the nose. When exhaled through the nose, budesonide resulted in an improvement in nasal symptoms and nasal flow to a lesser extent than using intranasal budesonide but allowed for a significant reduction in the dose of intranasal budesonide required to improve nasal symptoms.
INCS are also used in drop form, usually for treatment of nasal polyps. In a few cases where they were used for AR, there was systemic absorption leading to unfavorable side effects such as growth inhibition and adrenal suppression or iatrogenic Cushing syndrome. In a study comparing fluticasone propionate administered as nasal drops or aqueous spray, the drops had eight times more systemic bioavailability than the spray.
Intranasal corticosteroid, non‐traditional application
Aggregate grade of evidence: B (Level 2: 4 studies, level 3: 1 study; Table XI.B.2.b.ii). Some studies noted in the text were not performed in patients with AR or were case reports so are not summarized in the table.
Benefit: Nebulized steroids or those used via irrigation show some benefit in the treatment of AR in limited studies. Furthermore, steroids inhaled or exhaled through the nose in patients with asthma and rhinitis also show some benefit for rhinitis. Nasal steroid drops are not approved for treatment of rhinitis but are used in certain countries.
Harm: Nasal steroid drops have significant systemic side effects.
Cost: Low.
Benefits‐harm assessment: The risks of using corticosteroid nasal drops for AR outweigh the benefits. Limited evidence suggests that nasal steroid irrigations for rhinitis lead to significant improvement of symptoms. Scarce evidence does not support routine recommendation for this route of therapy.
Value judgments: In the presence of effective symptom control using traditional spray administration for INCS, there is no solid data to support other routes of administration.
Policy level: Recommendation against routine use.
Intervention: There is some evidence that inhaled steroids, when exhaled through the nose might improve AR symptoms. Similar benefit is seen when steroids are inhaled by first passing through the nose. These routes might be useful in patients with both rhinitis and asthma.
XI.B.2.c Injectable corticosteroids
Corticosteroids have been injected intramuscularly or into the turbinates for management of AR. Several early studies demonstrated significant improvement in subjective allergy symptoms after intramuscular corticosteroid injections. Four of these studies were single center RCTs with a placebo arm and modest numbers of participants, , , (Table XI.B.2.c).
Studies comparing different intramuscular steroid preparations have showed improvement of symptoms with all variations but some differences in efficacy among them., , , When compared to other agents, intramuscular corticosteroids demonstrated similar or superior efficacy in controlling symptoms of AR. Specifically, pre‐seasonal betamethasone injection was as effective as daily oral prednisolone and more effective than daily intranasal beclomethasone dipropionate in controlling nasal itching, congestion, rhinorrhea and eye symptoms. In another seasonal study, a single injection of methylprednisolone was as effective as intranasal budesonide over a 3 week treatment period. Although these studies show a favorable effect of intramuscular steroids on symptoms of AR, a recent systematic review was inconclusive based on a high risk of bias of the available studies that mostly dated back to more than 30 years ago.
Injectable corticosteroid preparations have significant potential side effects which can include adrenal suppression and growth retardation (Table II.C). Injectable corticosteroids affected adrenal function in two out of four relevant studies, (Table XI.B.2.c). Evidence from a study of Danish National Registries shows that the relative risk and incidence of both osteoporosis and diabetes were higher in allergic individuals receiving at least one depot corticosteroid injection yearly for three consecutive years during the allergy season compared to those receiving AIT. Laursen et al. reported that ACTH testing performed at 3 weeks showed significant suppression of adrenal function in the oral steroid treatment group but no evidence of suppression after a single corticosteroid injection. This discrepancy may relate to the short‐lasting adrenal suppression after a single injection of corticosteroids compared to continuous administration of the oral formulation, although Kronholm also did not show any effect of intramuscular preparations on adrenal function.
Corticosteroid injection into the nasal turbinates has also been studied for the management of AR; however, this route is less widely utilized than previously observed. Several early reports detailed significant improvement in symptoms of AR in a large proportion of patients who received intra‐turbinate injections of various steroid formulations., , , , A placebo‐controlled, single‐blind RCT showed that intra‐turbinate injections of botulinum toxin A or triamcinolone in patients with perennial AR resulted in improved control of nasal symptoms, including nasal congestion, compared to isotonic saline, although botulinum toxin had the longest duration of clinical effect.
Enthusiasm for intra‐turbinate steroid injection has been tempered by reports of orbital complications associated with intra‐turbinate, but not intramuscular, deposition. Complications have included transient visual loss and diplopia; blurred vision and temporary blindness; and temporary distorted vision, decreased visual acuity, and paresis of the medial rectus. Martin reported on the rapid onset of ocular pain, blurred vision, and decreased visual acuity after an intra‐turbinate injection of triamcinolone acetonide. Symptoms were caused by choroidal and retinal arterial embolization and resolved completely within 24 h. A more recent report detailed progression of glaucoma‐related optic neuropathy after intra‐turbinate injection associated with chorioretinal microvascular embolism. The mechanism of embolization is likely related to retrograde flow from the anterior tip of the IT to the ophthalmic artery, followed by anterograde flow with the particles lodging in the end arteries of the choroid and retinal vessels. Larger particle size steroids (e.g., methylprednisolone) are thought to present higher risk than smaller sized particles (e.g., triamcinolone). Moss et al. reported on personal experience with 152 turbinate and 85 intra‐polyp injections of triamcinolone acetonide, noting one transient subjective decrease in vision after intra‐polyp injection. They reviewed the literature for an estimated 117,000 individual intra‐turbinate and polyp injections and reported an estimated visual complication rate of 0.003% (three instances), with a 0.00% (0 instances) rate of permanent visual complications.
Injectable corticosteroids
Aggregate grade of evidence: B (Level 1: 1 study, level 2: 11 studies, level 4: 2 studies; Table XI.B.2.c)
Benefit: Injectable corticosteroids improved symptoms of AR in clinical studies.
Harm: Injectable corticosteroids have known undesirable adverse effects on the hypothalamic‐pituitary axis, growth, osteoporosis, glycemic control, and other systemic adverse effects, for varied periods of time after injection. Intraturbinate corticosteroids have a small but potentially serious risk of ocular side effects including decline or loss of vision. See Table II.C.
Cost: Low.
Benefits‐harm assessment: In routine management of AR, the risk of serious adverse effects outweighs the demonstrated clinical benefit.
Value judgments: Injectable corticosteroids are effective for the treatment of AR. However, given the risk of significant systemic adverse effects, the risk of serious ocular side effects, and the availability of effective alternatives (e.g., INCS), injectable corticosteroids are not recommended for the routine treatment of AR.
Policy level: Recommendation against.
Intervention: None.
XI.B.3 Decongestants
XI.B.3.a Oral decongestants
Oral decongestants are medications that act on adrenergic receptors, which leads to vasoconstriction of small blood vessels (such as those in the nasal mucosa), resulting in relief of nasal congestion symptoms in AR patients. The most commonly used oral decongestants are pseudoephedrine and phenylephrine, which are sympathomimetic vasoconstrictors that differ in their selectivity to adrenoceptors. Due to the oral administration of pseudoephedrine and phenylephrine, both drugs act systemically and can lead to side effects such as insomnia, headache, nervousness, anxiety, tremors, palpitations, urinary retention, increased blood pressure, and other adverse effects, , , (Table II.C).
Our review of the literature found 12 studies that evaluate the use of oral decongestants in AR and are summarized in Table XI.B.3.a. Individual studies evaluating the effect of oral decongestants in AR patients as monotherapy during allergy season have shown that pseudoephedrine monotherapy led to improved symptom scores (total nasal symptom and individual symptom scores) compared to baseline., , , , One study also compared pseudoephedrine monotherapy against placebo and found that pseudoephedrine monotherapy is more effective in reducing total nasal symptom and nasal stuffiness scores than placebo. With regard to the comparison of pseudoephedrine monotherapy against the combination therapy, including an oral antihistamine and pseudoephedrine, studies have shown that pseudoephedrine monotherapy is less effective than combination therapy in treating primary outcomes such as total nasal symptom and individual symptom scores., , , , ,
Studies on the effectiveness of oral decongestants in AR patients as premedication monotherapy before allergy challenge have shown that pseudoephedrine is equally effective compared to montelukast and more effective than placebo, in treating primary outcomes. One study showed that pseudoephedrine monotherapy was less effective than a combination therapy of an oral antihistamine and pseudoephedrine, while another study showed no difference in outcome. The results in head‐to‐head comparisons between antihistamine and pseudoephedrine monotherapy are contradictory. While some studies showed that antihistamine monotherapy was more efficient than pseudoephedrine,, other studies have had different findings., , , , Nonetheless, either monotherapy (i.e., pseudoephedrine or antihistamine) was more effective than placebo., , , Interestingly, an analysis of the effectiveness of phenylephrine compared to placebo has shown that phenylephrine (up to 40 mg six times daily) is not superior to placebo in relieving nasal congestion symptoms in AR patients.
Oral decongestants
Aggregate grade of evidence: A (Level 2: 12 studies; Table XI.B.3.a)
Benefit: Reduction of nasal congestion with pseudoephedrine. No benefit with phenylephrine.
Harm: Oral decongestants have known undesirable adverse effects. See Table II.C.
Cost: Low.
Benefits‐harm assessment: Balance of benefit and harm for pseudoephedrine. Possible harm for phenylephrine.
Value judgments: Little evidence for benefit in controlling symptoms other than nasal congestion.
Policy level: Strong recommendation against for routine use in AR. In certain cases, combination therapy with an oral antihistamine may be beneficial to alleviate severe nasal congestion in short courses.
Intervention: Although not recommended for routine use in AR, pseudoephedrine can be effective in reducing nasal congestion in patients with AR; however, it should only be used as short‐term/rescue therapy after a discussion of the risks and benefits with the patient (comorbidities) and consideration of alternative intranasal therapy options.
XI.B.3.b Intranasal decongestants
INDC – oxymetazoline, xylometazoline, and phenylephrine – are α‐adrenergic agonists acting as topical vasoconstrictors reducing edema/tissue thickness. The highest level of evidence consists of seven RCTs, , , , , , looking at short‐term effects of INDC. There are also three RCTs, , and two cohort studies, evaluating prolonged effects of INDC.
Clinically, short‐term use results in reduction of nasal congestion/blockage, with little to no effect on allergic symptoms such as sneezing, rhinorrhea, or nasal itching., , , Onset of action is within 10 min, and duration of the effect lasts up to 12 h. There are also improvements in objective measures of nasal congestion/blockage, including nasal airway resistance, measures of nasal cavity volume for airflow, and PNIF., , , , Measures of nasal cavity volume for airflow exhibit a clear dose–response relationship across doses ranging from 6.25 to 50 μg, with nasal airway resistance requiring a higher threshold dose of 25 μg before significant changes in nasal patency are seen. Despite oxymetazoline's vasoconstrictive effects, it does not seem to affect histamine‐induced plasma exudation. The majority of studies compared INDC to placebo,, , , , but Barnes et al. found that the decongestant response was stronger for intranasal xylometazoline after 15 min than daily administration of intranasal mometasone furoate after 28 days. It is worth noting that only three studies included patients with AR,, , the remainder consisted of healthy participants., , ,
Rhinitis medicamentosa, which is a condition thought to result from prolonged usage of INDC, is characterized by an increase in symptomatic nasal congestion, thereby precluding a recommendation for long‐term use of these medications. Studies to identify the duration of intranasal decongestant use that leads to rhinitis medicamentosa have shown variable results. Some studies show prolonged use (up to 6 weeks) does not produce any symptoms of rebound nasal congestion or objective markers of impaired decongestant response., , Another study, however, noted development of rhinitis medicamentosa after as little as 3 days of use. This may be due to nasal hyperreactivity and mucosal swelling. Additionally, Graf et al. looked at the impact of the presence of the preservative benzalkonium chloride, which can be found in INDC sprays. Compared to oxymetazoline and placebo nasal sprays, a nasal spray with benzalkonium chloride alone induces mucosal swelling, suggesting the presence of this preservative may aggravate rhinitis medicamentosa. (See Section V.B.2 Rhinitis Medicamentosa for additional information on this topic.)
Known adverse effects of INDC include nasal discomfort/burning, dependency, dryness, increased congestion, rhinitis medicamentosa, hypertension, anxiety, and tremors (Table II.C). One study noted significantly decreased ciliary beat frequencies at 1000 μg/ml, but no significant difference at 500 μg/ml. The 500 μg/ml (0.5 mg/ml,.05%) concentration is typical for available formulations. In sum, while intranasal decongestants are effective at reducing nasal congestion, short‐term use of the medication, approximately 3 days or less, is recommended to avoid the potential for rebound nasal congestion and rhinitis medicamentosa.
Intranasal decongestants
Aggregate grade of evidence: B (Level 2: 10 studies, level 3: 2 studies; Table XI.B.3.b). Limitation – only 3 studies included subjects with AR.
Benefit: Reduction in symptoms of nasal congestion/blockage and corresponding objective markers with INDC compared to placebo.
Harm: Side effects include nasal discomfort/burning, dependency, dryness, hypertension, anxiety, and tremors. See Table II.C. Potential for rebound congestion with long‐term use.
Cost: Low.
Benefits‐harm assessment: Harm likely outweighs benefit if used long‐term, with adverse effects appearing as early as 3 days.
Value judgments: INDC can be helpful for short‐term relief of nasal congestion.
Policy level: Option for short‐term use.
Intervention: INDC can provide effective short‐term relief of nasal congestion in patients with AR during an acute flare but recommend against chronic use due to risk of rhinitis medicamentosa.
XI.B.4 Leukotriene receptor antagonists
LTRAs have been studied in the treatment of AR. Montelukast is approved by the US FDA for the treatment of seasonal AR in adults and children over 2 years of age, and for perennial AR in adults and children over 6 months of age. Other LTRAs include pranlukast (approved for treatment of AR in Japan) and zafirlukast (FDA‐approved for treatment of asthma).
Since the 2018 ICAR‐Allergic Rhinitis consensus statement, the body of evidence surrounding LTRA monotherapy has grown. A systematic search revealed 15 SRMAs of RCTs published since 2014. This gave a total of 34 studies examining the use of LTRA in AR which are considered high‐level evidence (Table XI.B.4).
Most recent studies, , , , demonstrate concordance with previous findings that LTRA monotherapy is superior to placebo in controlling symptoms and improving QOL in both seasonal and perennial AR, except a single RCT which showed no difference between the two. Yoshihara et al. found that LTRA showed promise as a prophylactic agent in children with seasonal AR when administered before the Japanese cedar pollen season.
However, there remains consistent evidence that LTRA is inferior to INCS in terms of symptom reduction and QOL improvement., , In a RCT by Chen et al, LTRA was inferior to INCS in improving acoustic rhinometry readings, concentrations of inflammatory mediators in nasal secretions, and the inflammatory cell composition (Th1, Th2, Treg) from turbinate brush cytology. Dalgic et al. found LTRA to be inferior to INCS in improving olfactory function in patients with seasonal AR. In comparison to oral antihistamines, there remains mixed evidence for relative efficacy,, , with recent studies favoring oral antihistamines. Comparing diurnal symptoms of AR, Feng et al. found LTRA to be superior to oral antihistamines for controlling nighttime symptoms, but inferior for daytime symptoms. LTRA monotherapy was further compared against AIT and found to be inferior for symptom control., Li et al. compared LTRA monotherapy to acupoint‐application of Chinese herbal medication and found no difference in symptom control for children with perennial AR.
In March 2020, the US FDA announced a safety concern regarding montelukast and potential serious neuropsychiatric events, including suicidal thoughts. A boxed warning, the FDA's most prominent warning, was added to prescribing information. The FDA advised further that in AR, montelukast should be reserved for patients who are not treated effectively with or cannot tolerate other allergy medications.
In their 2015 Clinical Practice Guidelines for AR, the AAO‐HNSF recommended against LTRA monotherapy, as it was less effective than other first‐line medications and more costly. In 2020, this guideline was endorsed by the American Academy of Family Physicians. In the same year, the Joint Task Force on Practice Parameters issued an update recommending against the selection of LTRA as initial treatment of AR.
While LTRA monotherapy has been consistently shown to be superior to placebo for the treatment of AR, there is now significant evidence that alternative agents such as INCS are superior and less costly. Given the increased risk profile of LTRA highlighted by the FDA boxed warning, LTRA monotherapy is not recommended as first‐line therapy for patients with AR but may be considered in selected patients who have contraindications to both oral antihistamines and INCS.
Leukotriene receptor antagonists
Aggregate grade of evidence: A (Level 1: 13 studies, level 2: 21 studies; Table XI.B.4)
Benefit: Consistent reduction in symptoms and improvement in QOL compared to placebo.
Harm: FDA boxed warning regarding neuropsychiatric side effects, including suicidal ideation. Consistently inferior compared to INCS at symptom reduction and improvement in QOL. Equivalent or inferior effect compared to oral antihistamines in symptom reduction and improvement of QOL. See Table II.C.
Cost: Moderate.
Benefits‐harm assessment: LTRAs are effective as monotherapy compared to placebo. However, there is a consistently inferior or equivalent effect to other, less expensive agents used as monotherapy. Also, there is an FDA boxed warning associated with LTRAs.
Value judgments: LTRAs are more effective than placebo at controlling both asthma and AR symptoms in patients with both conditions. However, in the light of significant concerns over its safety profile and the availability of effective alternatives such as INCS and oral antihistamines, evidence is lacking to recommend LTRAs as monotherapy in the management of AR.
Policy level: Recommendation against LTRAs as first‐line monotherapy for patients with AR. Option for LTRA as monotherapy in patients with contraindications to other preferred treatments.
Intervention: LTRAs should not be used as monotherapy in the treatment of AR but can be considered in select situations where patients have contraindications to alternative treatments.
XI.B.5 Intranasal cromolyn
Disodium cromoglycate (DSCG) [synonyms: cromolyn sodium, sodium cromoglycate, disodium 4,4’‐dioxo‐5,5’‐(2‐hydroxytrimethylenedioxy)di(4H‐chromene‐2‐carboxylate] is a mast cell stabilizer that inhibits the release of mast cell mediators that promote IgE‐mediated inflammation., DSCG is FDA‐approved for adults and children (2 years and older) for the prevention and relief of nasal symptoms of AR and is available as an over‐the‐counter nasal spray. It has a rapid onset of action with efficacy lasting up to 8 h, taken as one spray 3‐6 times daily, and is primarily used to prevent the onset of symptoms prior to allergen exposure, but it also can be used to treat symptoms once they occur., , ,
DSCG exhibits an excellent safety profile with only minor adverse effects including nasopharyngeal irritation, sneezing, rhinorrhea, and headache. There are very rare reports of immediate IgE‐mediated reaction to the medication., Due to its high safety profile, this medication can be considered for very young children and pregnant patients.,
DSCG has been shown to be more effective than placebo in patients with seasonal AR in controlling nasal symptoms of sneezing, rhinorrhea, and nasal congestion as treatment during their peak allergy season., , , , The largest double‐blind placebo‐controlled trial included 1150 patients with seasonal AR treated for 2 weeks (580 patients on DSCG, 570 treated with placebo). Patients received DSCG as a 4% nasal solution, one spray every 4–6 h, no more than six times per day. DSCG was significantly better than placebo in controlling overall symptoms (p = 0.02), sneezing (p = 0.01), and nasal congestion (p = 0.03). Studies on the superiority of DSCG versus placebo in perennial AR have been controversial and with relatively small sample sizes., , , , In the most recent study that demonstrated a benefit of DSCG in perennial AR (n = 14), DCSG resulted in significant improvement in the symptom scores of runny nose, nasal congestion, sneezing, and nose blowing, when compared to placebo (p < 0.005). Additionally, factors that were found to be associated with a good clinical response to the medication included: (1) patients with higher IgE levels, (2) patients with markedly positive skin test reactions to foods and animal dander compared to pollen allergy, and (3) female gender (Table XI.B.5).
In a small study, DSCG demonstrated similar efficacy for controlling nasal symptoms compared to oral antihistamines and significantly reduced the number of nasal eosinophils, whereas oral antihistamines did not. When compared to intranasal antihistamines, and INCS,, , , , , , , , , , DSCG has been shown to be less effective in controlling nasal symptoms. Ultimately, the role of DSCG as a primary treatment for AR is limited given its lower efficacy when compared to INCS and potential compliance challenges secondary to a frequent dosing regimen. The medication can also be administered as a preventive strategy, prior to allergen exposure to reduce the development of AR symptoms.
Intranasal cromolyn
Aggregate grade of evidence: A (Level 2: 25 studies; Table XI.B.5)
Benefit: DSCG is effective in reducing sneezing, rhinorrhea, and nasal congestion.
Harm: Rare local side effects.
Cost: Low.
Benefits‐harm assessment: Preponderance of mild to moderate benefit over harm. Less effective than INCS and intranasal antihistamines.
Value judgments: DSCG is useful for preventative short‐term use in adult patients, children (2 years and older), and pregnant patients with known exposure risks.
Policy level: Recommendation as a second‐line treatment in AR.
Intervention: DSCG may be used as a second‐line treatment for AR in patients who fail INCS or intranasal antihistamines, or for short‐term preventative benefit prior to allergen exposures.
XI.B.6 Intranasal anticholinergics
IPB is a synthetic quaternary ammonium anticholinergic compound that is related to atropine. Effects of IPB have been explored prior to nasal methacholine challenge in patients with AR. It was found to reduce rhinorrhea and sneezing with no effects on nasal airway resistance., In addition, administration of IPB resulted in the reduction of rhinorrhea following cold air exposure and following the ingestion of hot soup, which suggested that this type of rhinorrhea is mediated through a reflex leading to hypersecretion from nasal glands. IPB is effective in controlling anterior rhinorrhea with no effect on nasal congestion or sneezing., , , , , IPB is available at 0.03% and 0.06% concentration and is effective in adults and children with perennial rhinitis (0.03%) and common cold (0.06%)., It has a quick onset of action and short half‐life and can be administered up to six times per day, with less than 10% absorption over a range of 84–336 μg/day.
Intranasal IPB is poorly absorbed, and systemic side effects have not been observed with therapeutic dosing, as plasma concentrations of greater than 1.8 ng/ml are needed to produce systemic anticholinergic effects. However, care should be taken to avoid overdosage that could lead to high serum concentrations of ipratropium. Side effects of topical IPB are mostly local (Table II.C).
IPB is FDA‐approved for the treatment of seasonal AR in both adults and children (5 years and older). IPB also controls rhinorrhea in children and adults with perennial AR.
The largest study that compared IPB to placebo was conducted on perennial AR and perennial non‐allergic rhinitis in pediatric patients aged 6–18 years. A total of 204 patients were included in this double‐blind RCT, divided equally between IPB and placebo subgroups. There was a significant reduction in the severity and duration of rhinorrhea and improvement in QOL in the IPB group. The effect was more pronounced in the perennial non‐allergic rhinitis group compared to the perennial AR group (Table XI.B.6).
Evidence on the efficacy of IPB in seasonal AR is derived from two studies, a prospective study and a double‐blind RCT. The prospective study included a total of 230 children aged 2–5 years old with seasonal or perennial AR and found that IPB was safe and effective in controlling rhinorrhea. In the double‐blind crossover trial (n = 24), adults aged 18–49 with seasonal AR, perennial AR, and non‐allergic perennial rhinitis the local pretreatment with IPB effect on methacholine challenge was studied. IPB was found to be more effective than placebo in suppressing sneezing and nasal hypersecretion with no effect on nasal airway resistance.
When compared to other medications for treating AR, IPB has been shown to be equally effective compared to INCS with respect to nasal drainage. Despite its beneficial effects on rhinorrhea and sneezing, IPB was shown to be inferior to INCS in controlling sneezing. No head‐to‐head studies have compared IPB to other AR medications.
Intranasal anticholinergics (ipratropium bromide)
Aggregate grade of evidence: A (Level 2: 10 studies; level 3: 2 studies; Table XI.B.6)
Benefit: Reduction of rhinorrhea with topical anticholinergics.
Harm: Care should be taken to avoid overdosage leading to systemic side effects. See Table II.C.
Cost: Low.
Benefits‐harm assessment: Preponderance of benefit over harm in AR patients with rhinorrhea.
Value judgments: Benefits limited to controlling rhinorrhea. Can be used as add on treatment for AR patients with persistent rhinorrhea despite first line medical management.
Policy level: Option.
Intervention: IPB nasal spray may be used as an adjunct medication to INCS in AR patients with persistent rhinorrhea.
XI.B.7 Biologics
The biologics investigated for treating allergic conditions include omalizumab, mepolizumab, dupilumab, benralizumab, and reslizumab. These compounds work by targeting specific components of the pathways involved in type 2 inflammation. Omalizumab acts on IgE; dupilumab on the IL‐4 receptor α subunit (recognized by IL‐4 and IL‐13); and mepolizumab, benralizumab, and reslizumab on IL‐5 or its receptor. Only omalizumab and dupilumab have been studied specifically for AR. Biologics are currently FDA approved for the treatment of moderate to severe persistent asthma, AD, CRSwNP, chronic idiopathic urticaria, and eosinophilic esophagitis (EoE), but not for AR.
Omalizumab interferes with the allergic cascade by binding the serum free IgE molecules and preventing them from attaching to mast cells and basophils. Trials using omalizumab as a monotherapy in treating AR have been favorable (Table XI.B.7.‐1). Two systematic reviews demonstrated decreased use of rescue medication, improvement of overall symptoms and QOL in patients treated with omalizumab., The effectiveness of omalizumab monotherapy was assessed for both seasonal and perennial AR., , , , Omalizumab monotherapy achieved significant improvement of nasal symptom score, ocular symptom score, medication symptom score, and QOL with the corresponding reduction of emergency drug use and serum IgE levels. Together with the marked reduction of free serum IgE level, there was notable inhibition of specific inflammatory mediators tryptase and ECP in the nasal secretions., When compared to suplatast tosilate, a selective Th2 cytokine inhibitor (a drug sometimes used as a prophylaxis for atopic asthma), omalizumab was superior in treating patients with seasonal AR.
Studies showed favorable safety profiles with adverse events such as local injection site reactions and anaphylaxis, with no significant difference observed compared to placebo. The dosing is based on the serum tIgE level (IU/ml) and the body weight (kg) prior to the initiation of treatment where most studies used dosing from 75 to 375 mg of omalizumab administered every 2–4 weeks and mean duration of treatment of 16 weeks. Given the weight‐based dosing regimen, cost of treatment with omalizumab varies between $10,000 and $32,000 per year.
Omalizumab has been evaluated as a combination therapy with AIT. This is addressed in Section XI.D.10. Combination Biologic Therapy and Subcutaneous Immunotherapy.
Another biologic investigated for the treatment of allergic airway diseases is dupilumab, which works through binding of IL‐4Rα to inhibit IL‐4 and IL‐13. Dupilumab was shown to be effective when administered as an adjunct treatment in patients with uncontrolled persistent asthma and comorbid AR. Similar findings were observed in a post hoc analysis of patients having uncontrolled moderate‐to‐severe asthma and comorbid perennial AR receiving add on dupilumab therapy. In another multicenter trial, combination therapy did not significantly improve total symptom score but it resulted in better tolerance to AIT with less withdrawal and fewer requirement of rescue medicine. These results suggest dupilumab may have a role in treating AR, at the time of this writing it is not FDA approved for this indication (Table XI.B.7.‐2).
In treating refractory AR that has failed optimal pharmacological treatment, biologics show promising results. Omalizumab has been the most studied and appears to be efficacious in symptom reduction, medicine use, and improvement in QOL with favorable safety profile. Current limitations in the widespread use of biologics for the treatment of AR are related mostly to the high cost of treatment and lack of FDA approval. In addition, it is foreseeable that the use of biologics will be long‐term and once discontinued the symptoms may recur. Although there is no subgroup analysis to determine the efficacy of biologics in AR with comorbid bronchial asthma, the cost to benefit analysis is expected to improve considerably in such cases.
Biologics
Aggregate grade of evidence: A (Level 1: 2 studies, level 2: 8 studies, level 3: 2 studies; Tables XI.B.7.‐1 and XI.B.7.‐2)
Benefit: Omalizumab treatment resulted in improvement of symptoms, rescue medication and QOL as a monotherapy. Dupilumab data is less robust and needs further investigation.
Harm: Local reaction at injection site and risk of anaphylaxis.
Cost: High.
Benefits‐harm assessment: Benefit outweighs harm.
Value judgments: Biologic therapies show promise as a treatment option for AR; however, no biologic therapies have been approved by the US FDA for this indication.
Policy level: Option based upon published evidence, although not currently approved for this indication.
Intervention: Monoclonal antibody (biologic) therapies are not currently approved for the treatment of AR.
XI.B.8 Intranasal saline
Nasal saline is a frequently utilized therapy in the treatment of AR. The term “nasal saline,” however, encompasses a wide variety of therapeutic regimens. These can include differences in solution characteristics, such as salinity (hypertonic versus isotonic/normal saline) and buffering (buffered versus non‐buffered), and differences in frequency, volume, and mode of administration.
This review included only level 1 and 2 evidence published in the English language evaluating nasal saline in the treatment of AR. Search methodologies identified nine RCTs in adults, , , , , , , , (Table XI.B.8.‐1) and one systematic review and eight RCTs, , , , , , , in children (Table XI.B.8.‐2). Three SRMAs, , have been performed including both adults and children (Table XI.B.8.‐3). Compared to no irrigations, all found symptoms and patient‐reported disease severity were significantly better in the saline irrigation group., , Hermelingmeier et al. also identified a 24%–100% reduction in medication usage, as well as an improvement of 30%–37% in QOL, and suggested that children may benefit less than adults.
Adult population. All studies found improvements in clinical outcomes with the utilization of nasal saline, with formulas varying in salinity, buffering, and frequency, volume, and mode of administration. Studies also varied in the types of AR evaluated., , , , , , , , Compared to no intranasal treatment, hypertonic saline was found to significantly improve outcomes, including nasal symptoms, QOL, and oral antihistamine use., , Ural et al. further compared hypertonic and isotonic saline irrigations, finding improved mucociliary clearance with the isotonic solution only. Looking at subjective outcomes with hypertonic versus isotonic solutions, however, Cordray et al. and Sansila et al. found QOL and symptom score were better with hypertonic solutions. Finally, Yata et al. evaluated both subjective and objective outcomes and found no difference between hypertonic and isotonic saline irrigations. Focusing on isotonic saline with various degrees of buffering, Chusakul et al. found that after 10 days buffered isotonic saline with mild alkalinity had the greatest impact on reducing nasal symptom scores and was preferred by most patients. Both Cordray et al. and Lin et al. found INCS had similar efficacy in improving nasal symptoms but showed statistically significant improvement in QOL outcomes compared to saline spray.
Pediatric population. All studies found an improvement in clinical outcomes with the incorporation of nasal saline., , , , , , , , Compared to no irrigations, hypertonic and isotonic saline were found to improve outcomes, including nasal symptoms, oral antihistamine use, and QOL., , Supporting these findings, a 2019 SRMA found significantly better nasal symptom scores and a lower rate of rescue antihistamine use with hypertonic saline irrigations compared to the control group (isotonic saline and no irrigations). Further, studies have shown that that hypertonic saline irrigations resulted in a greater improvement in nasal symptom scores in children than isotonic saline., , Finally, Li et al. and Chen et al. found an additive effect in the utilization of nasal saline spray as an adjunct to INCS when compared to either therapy independently.
Overall, there is substantial evidence to support the use of nasal saline in the treatment of AR. In adults, the data is conflicting regarding optimal salinity of the solution. In children, there is some data to support a hypertonic solution being more effective. Although nasal saline demonstrates improvement in symptoms and QOL outcomes when used alone, it is often implemented with other therapies, such as INCS, intranasal antihistamines, or oral antihistamines. In both adults and children, nasal saline appears to have an additive effect when used in combination with other standard AR treatments. Further, nasal saline is of relatively low cost and has an excellent safety profile. While adverse effects are rare, they can include nasal irritation, sneezing, cough, and ear fullness (Table II.C).
Intranasal saline
Aggregate grade of evidence: A (Level 1: 4 studies, level 2: 17 studies; Tables XI.B.8.‐1, XI.B.8.‐2, and XI.B.8.‐3)
Benefit: Improved nasal symptoms and QOL, reduction in oral antihistamine use, and improved mucociliary clearance. Well‐tolerated with excellent safety profile.
Harm: Nasal irritation, sneezing, cough, and ear fullness. See Table II.C.
Cost: Minimal.
Benefits‐harm assessment: Preponderance of benefit over harm.
Value judgments: Nasal saline can and should be used as a first line treatment in patients with AR, either alone or combined with other pharmacologic treatments as evidence supports an additive effect. Hypertonic saline may be more effective in children. Data is otherwise inconclusive on optimal salinity, buffering, and frequency and volume of administration.
Policy level: Strong recommendation.
Intervention: Nasal saline is strongly recommended as part of the treatment strategy for AR.
XI.B.9 Probiotics
The relationship between the microbiome and the development of atopy is complex and incompletely understood. The hygiene hypothesis theorizes that modern sanitized living conditions reduce microbial exposure resulting in inadequate immune priming. Low biodiversity in early life affects the immune system and can result in a pro‐inflammatory response, including allergic over‐sensitization. Conversely, appropriate microbial exposure in infancy influences gut biodiversity, thereby increasing regulatory T cell action and immune tolerance. (See Section VI.J. Microbiome and Section VIII.C.3. Hygiene Hypothesis for additional information on this topic.)
Probiotics induce immunomodulatory effects on gut‐associated lymphoid tissue. The gut microbiome and the immune system interact via dendritic cells, regulatory T cells, bacterial metabolites, and cytokines. Probiotic exposure induces a Th1 response via IL‐12, IFN‐γ, with upregulation of Treg cells via IL‐10 and TGF‐β. Furthermore, the allergy‐associated Th2 pathway is suppressed through downregulation of IL‐4, tIgE, IgG1, and IgA.
Numerous RCTs have examined the therapeutic role of probiotic administration for the control of AR symptoms. Several high‐quality meta‐analyses have been performed on aggregate data from RCTs. Results in children and adults have been mixed.
Guvenc et al. performed a meta‐analysis of 22 RCTs comprising 2242 patient aged 2–65 years with seasonal or perennial AR who were treated with daily probiotic or placebo in addition to standard allergy therapies for 4 weeks to 12 months. The primary outcomes of the study were nasal/ocular symptom scores and QOL. Seventeen trials demonstrated clinical benefit of probiotics with improvement in nasal symptoms (standardized mean difference [SMD)] −1.23, p < 0.001), ocular symptoms (SMD −1.84, p < 0.001), total QOL (SMD −1.84, p < 0.001), nasal QOL (SMD −2.30, p = 0.006), and ocular QOL (SMD −3.11, p = 0.005).
Zajac et al. performed a meta‐analysis of 21 RCTs and two randomized crossover studies that included 1919 adult and pediatric patients with seasonal or perennial AR. Patients were treated with 3 weeks to 12 months of probiotic or placebo. The primary outcomes were validated QOL, symptom scores, and immunologic variables. Seventeen studies demonstrated clinical benefit of probiotics for AR. Meta‐analysis demonstrated improvement in RQLQ global score (SMD −2.23, p = 0.02) and RQLQ nasal symptom score (SMD −1.21, p < 0.00001). No effect of probiotic administration was found for Rhinitis Total Symptom Score, tIgE, or sIgE.
Du et al. published a meta‐analysis of 19 RCTs comprising a total of 5264 healthy children treated with at least 6 months of probiotic or placebo. Ten RCTs reported no difference in the risk of developing AR (RR 1.03; p = 0.83) or a positive SPT (RR 0.74; p = 0.13) after administration of oral probiotics.
Zuccotti et al. reported a meta‐analysis of 17 RCTs comparing probiotics versus placebo in 4755 children. The primary endpoint was to determine if supplementation of probiotics in pregnancy or early infancy reduced the relative risk of eczema, asthma, wheezing, and rhinoconjunctivitis. No significant difference in terms of prevention of asthma, wheezing or rhinoconjunctivitis was noted (RR 0.91; p = 0.53), whereas the relative risk of eczema in the treatment group was significantly lower than controls (RR = 0.78; p = 0.0003).
Probiotics are inexpensive and well tolerated in patients with minimal side effects (e.g., flatulence, diarrhea, and abdominal pain). The data from meta‐analyses and RCTs suggests a potential benefit of probiotics in reduction of symptoms of seasonal and perennial AR in both adults and children but interpretation is limited by the heterogeneity of age, diagnosis, interventions, and outcomes included in the studies. The current data indicate that administration of probiotics in infancy does not reduce the diagnosis of most atopic diseases, with exception of eczema.
Probiotics
Aggregate grade of evidence: A (Level 1: 4 studies, level 2: 5 studies; Table XI.B.9)
Benefit: Improved nasal/ocular symptoms or QOL in most studies.
Harm: Mild gastrointestinal side effects.
Cost: Low.
Benefits‐harm assessment: Balance of benefit and harm.
Value judgments: Minimal harm associated with probiotics. Heterogeneity across studies makes magnitude of benefit difficult to quantify. Variation in organism and dosing across trials prevents specific recommendations for treatment.
Policy level: Option.
Intervention: Consider adjuvant use of probiotics for patients with symptomatic seasonal or perennial AR.
XI.B.10 Combination therapy
XI.B.10.a Oral antihistamine and oral decongestant
Oral antihistamines, commonly used for treatment of AR, target the H1 histamine receptor, block histamine receptor binding, and prevent histamine‐mediated symptoms of AR such as pruritus, sneezing, vasodilation, and flushing. The effect of oral antihistamines on nasal obstruction in AR may be less pronounced. Oral decongestants such as phenylephrine or pseudoephedrine, which are typically sympathomimetic drugs that target α‐1 receptors causing blood vessel constriction, cause more pronounced nasal decongestion. Oral antihistamines can thus be combined with oral decongestants to reduce histamine‐mediated symptoms of AR while concomitantly improving nasal airflow., , ,
RCTs have demonstrated that combination antihistamine‐decongestant medications including fexofenadine‐pseudoephedrine, desloratadine‐pseudoephedrine, cetirizine‐pseudoephedrine, loratadine‐pseudoephedrine, and others reduce AR symptoms including rhinorrhea, nasal congestion, nasal itching, and sneezing when compared to placebo., , , , , , , , , , , , , , , , , , Combination oral antihistamine‐oral decongestant medications have also been shown to reduce nasal congestion symptoms versus oral antihistamine alone or versus oral decongestant alone., , , , , , , , , , , , , , , , , , Studies have also demonstrated that once daily dosing of combination oral antihistamine‐oral decongestant medications are statistically equivalent to twice daily dosing with regard to symptom relief, and that different antihistamine‐decongestant combinations are statistically equivalent in improving symptom scores., , , , In some studies, oral antihistamine‐oral decongestant combination medications are reported to be superior to INCS with regard to improving AR symptoms, particularly nasal congestion., , In contrast, cetirizine‐pseudoephedrine was not superior to xylometazoline nasal decongestant spray alone in improving nasal airflow and nasal obstruction symptoms (Table XI.B.10.a).
Oral antihistamines may cause sedation and dry mouth, especially in the case of first‐generation antihistamines such as doxylamine and diphenhydramine; oral antihistamines may also cause urinary retention., Oral decongestants, through their actions on α‐1 receptors may cause palpitations, insomnia, jitteriness, and dry mouth. Oral decongestants or oral antihistamine‐decongestant combinations are typically not recommended by their manufacturers in patients under 12 years old, while oral antihistamines other than cetirizine are typically not recommended in patients under age 2., Over‐the‐counter sales of oral decongestants and oral antihistamine‐oral decongestant combinations are typically monitored or restricted given their potential use in the illicit manufacture of methamphetamines. Oral decongestants should be used with caution in pregnant patients and patients with cardiac arrythmias, hypertension, or benign prostatic hypertrophy. Oral antihistamines should be used with caution in patients with preexisting cardiac conditions, patients taking monoamine oxidase inhibitors, narcotic pain medications or other sedating medications, and some antiseizure medications, (Table II.C).
Combination oral antihistamine and oral decongestant
Aggregate grade of evidence: A (Level 2: 30 studies; Table XI.B.10.a)
Benefit: Improved nasal congestion and total symptom scores (TSS) with combination oral antihistamine‐oral decongestants.
Harm: Oral decongestants can cause adverse events in patients with cardiac conditions, hypertension, or benign prostatic hypertrophy and are not indicated in patients under age 12 or pregnant patients. Oral antihistamines are not indicated in patients under 2 years of age, and caution should be exercised in patients aged 2–5 years old. See Table II.C.
Cost: Low.
Benefits‐harm assessment: Combination oral antihistamine‐oral decongestant medications carry relatively low risks of adverse events when used as needed for episodic AR symptoms in well‐selected patients. Risk may be higher if used daily or in patients with certain comorbidities. There is not a preponderance of benefit or harm when used appropriately as a treatment option.
Value judgments: Oral antihistamine‐oral decongestants may be an effective option for acute AR symptoms such as nasal congestion and sneezing. Caution should be exercised with long‐term use.
Policy level: Option for episodic or acute AR symptoms.
Intervention: Combination oral antihistamine‐oral decongestant medications may provide effective relief of nasal symptoms of AR on an episodic basis. Caution should be exercised in chronic or long‐term use as the adverse effect profile of oral decongestants is greater for chronic use.
XI.B.10.b Oral antihistamine and intranasal corticosteroid
A combination of an oral antihistamine with INCS is a commonly used treatment option for patients with AR. First‐generation antihistamines include diphenhydramine, chlorpheniramine, and hydroxyzine, while newer second‐generation medications include cetirizine, levocetirizine, fexofenadine, loratadine, and desloratadine. Typically, second‐generation antihistamines are preferred given their improved safety profile compared to first‐generation antihistamines. INCS reduce inflammatory mediator and cytokine release; decrease the recruitment of nasal eosinophils, neutrophils, basophils, lymphocytes, monocytes, and macrophages; and can decrease hyperresponsive effects to antigen challenge. INCS have an excellent safety profile and low systemic absorption.
There have been several RCTs examining the use of oral antihistamine‐INCS combinations in the treatments of AR. Pinar et al. used TNSS, rhinoconjunctivitis scores, and PNIF to compare 4 groups: (1) intranasal mometasone‐oral desloratadine, (2) intranasal mometasone‐oral montelukast, (3) intranasal mometasone alone, and (4) placebo. This study found that intranasal mometasone with desloratadine or montelukast was superior to intranasal mometasone alone or placebo for improving TNSS and QOL (Table XI.B.10.b).
Anolik examined TNSS and TSS in patients treated with intranasal mometasone‐oral loratadine, intranasal mometasone alone, oral loratadine alone, or placebo. This study noted that intranasal mometasone plus loratadine and intranasal nasal mometasone alone were statistically equivalent for TNSS and TSS. All treatment groups were superior to placebo in improving TNSS and TSS. The study also reported that intranasal mometasone and mometasone‐loratadine were superior to loratadine alone or placebo for TNSS and TSS, while loratadine alone was superior to placebo for TNSS.
Barnes et al. compared RQLQ scores, PNIF, TNSS, and nNO in patients treated with intranasal fluticasone‐oral cetirizine versus intranasal fluticasone‐oral placebo. Their study found that nasal symptom score was statistically equivalent for cetirizine‐fluticasone patients versus fluticasone‐placebo patients.
Di Lorenzo et al. evaluated five groups: (1) oral cetirizine‐intranasal fluticasone, (2) oral montelukast‐intranasal fluticasone, (3) intranasal fluticasone alone, (4) oral cetirizine‐oral montelukast, or (5) placebo. This study reported that all treatment groups were superior to the placebo group in improving TSS and rhinorrhea, sneezing, and nasal itching scores. They also noted that the fluticasone alone and fluticasone‐cetirizine groups were superior to placebo or cetirizine‐montelukast in improving TSS, nasal congestion on waking, and daily nasal congestion.
Ratner et al. examined intranasal fluticasone‐oral loratadine versus fluticasone alone, loratadine alone, or placebo. They found that fluticasone and fluticasone‐loratadine were superior to loratadine only and placebo groups for clinician and patient total and individual nasal symptom scores, and that loratadine alone was equivalent to placebo for nasal symptom score. QOL improvement was greater for fluticasone and fluticasone‐loratadine compared to loratadine alone or placebo. QOL improvement was statistically equivalent for fluticasone‐loratadine versus fluticasone.
A SRMA in 2018 by Seresirikachorn et al. showed no added benefit for oral antihistamines plus INCS. This is in contrast to intranasal antihistamines plus INCS, which did show additional benefit. Potential side effects of oral antihistamine with INCS combinations are typically low and are included in the combined table of AR treatment side effects (Table II.C).
Combination oral antihistamine and intranasal corticosteroid
Aggregate grade of evidence: A (Level 1: 1 study, level 2: 12 studies; Table XI.B.10.b)
Benefit: The addition of oral antihistamine to INCS has not consistently demonstrated a benefit over INCS alone for symptoms of AR.
Harm: Oral antihistamines generally not recommended in patients under 2 years old, and attention to dosing is necessary in patients 2–12 years old. See Table II.C.
Cost: Low.
Benefits‐harm assessment: Benefit likely outweighs potential harms in patients with significant nasal congestion symptoms in addition to symptoms such as sneezing and ocular itching. Addition of an INCS may be limited benefit versus potential harm in patients without significant nasal congestion symptoms.
Value judgments: Adding oral antihistamine to INCS spray has not been demonstrated to confer additional benefit over INCS spray alone. INCS improves congestion with or without oral antihistamine.
Policy level: Option.
Intervention: Current evidence is mixed to support antihistamines as an additive therapy to INCS, as several randomized trials have not demonstrated a benefit over INCS alone for symptoms of AR.
XI.B.10.c Oral antihistamine and leukotriene receptor antagonist
The combination of oral antihistamine‐LTRA in the treatment of AR was reviewed as a therapeutic option in the previous ICAR‐Allergic Rhinitis 2018 consensus statement. An updated systematic search revealed three additional systematic reviews and two RCTs,, , , , giving a total of 17 studies meeting criteria for level 1 or 2 evidence (Table XI.B.10.c).
Combination oral antihistamine‐LTRA has been shown to be superior to placebo in multiple RCTs. Recent studies have sought to clarify the comparative efficacy of combination therapy against monotherapy with LTRA or oral antihistamines, which was previously unclear. Compared to LTRA alone, Kim et al. found that oral antihistamine‐LTRA therapy was superior in reducing nasal symptoms. However, in asthmatic patients, no difference was reported between the two treatment arms in improving spirometry readings or Asthma Control Test scores.
Krishnamoorthy et al. found that oral antihistamine‐LTRA therapy was superior to monotherapy with either LTRA or oral antihistamines in improving daytime and nighttime symptoms of AR, as well as ocular symptoms. Additional systematic reviews by Liu et al. and Wei are concordant with these findings.
There have been no new studies comparing combination oral antihistamine‐LTRA therapy to monotherapy with INCS. Previous evidence suggests that combination therapy is equivalent to, or less effective than INCS alone for reduction of symptoms and nasal eosinophil counts., , , Comparing different antihistamines with LTRA, Mahatme et al. found that fexofenadine added to LTRA led to a greater decrease in symptoms, although the combination with levocetirizine was more cost‐effective.
Regarding objective measures, there is mixed evidence for the use of combination oral antihistamine‐LTRA. Cingi et al. found that combination oral antihistamine‐LTRA was superior to oral antihistamines alone in reducing nasal resistance on rhinomanometric testing, and Li et al. found that the former was superior to the latter in increasing nasal volume as measured by acoustic rhinometry. However, Moinuddin et al. found that there was no significant difference in PNIF values between the two. Combination oral antihistamine‐LTRA was superior to placebo in reducing peripheral and nasal eosinophil counts, but inferior to INCS and equivalent to oral antihistamines alone.
It is important to note that in the Joint Task Force Practice Parameters, INCS were recommended when symptoms were not controlled with an oral antihistamine alone. Although the combination of LTRA and oral antihistamines was previously found to be well tolerated with minimal concerns for drug interactions, recent concerns regarding the safety of LTRA have been raised, with the US FDA now requiring a boxed warning for serious neuropsychiatric events on montelukast.
Overall, the combination of oral antihistamine‐LTRA is an effective therapy option when compared to placebo. However, in view of the adverse effect profile of montelukast, we recommend the consideration of other efficacious agents such as INCS which have been shown to result in superior symptom control, and that combination oral antihistamine‐LTRA therapy be reserved for rare patients with contraindications to alternative treatments.
Combination oral antihistamine and leukotriene receptor antagonist
Aggregate grade of evidence: A (Level 1: 4 studies, level 2: 13 studies; Table XI.B.10.c)
Benefit: Combination oral antihistamine‐LTRA was superior in symptom reduction and QOL improvement versus placebo and versus either agent as monotherapy.
Harm: Boxed warning due to risks of mental health side effects limiting use for AR. See Table II.C.
Cost: Generic montelukast added to generic loratadine or cetirizine is more expensive per month than generic fluticasone furoate nasal sprays, according to National Average Drug Acquisition Cost data provided by the Centers for Medicare and Medicaid Services.
Benefits‐harm assessment: Combination LTRA and oral antihistamine is superior to placebo, and superior to either agent as monotherapy. However, there is an inferior effect versus INCS, which is also less costly. In addition, there is a boxed warning associated with montelukast.
Value judgments: Combination therapy of LTRA and oral antihistamines is effective, but in light of concerns over the safety profile of montelukast, and the availability of effective alternatives such as INCS, evidence is lacking to recommend combination therapy in the management of AR.
Policy level: Recommendation against as first line therapy.
Intervention: Combination LTRA and oral antihistamines should not be used as first line therapy for AR but can be considered in patients with contraindications to other alternatives. This combination should be used judiciously after carefully weighing potential risks and benefits.
XI.B.10.d Intranasal corticosteroid and intranasal antihistamine
Combination therapy of INCS plus intranasal antihistamine spray is available for the treatment of AR. One combined formulation is currently available in North America for intranasal use as a combination of azelastine hydrochloride and fluticasone propionate (AzeFlu). This agent is alternatively designated in the literature as MP‐AzeFlu or MP29‐02 and is marketed in the US under the trade name Dymista (Viatris, Canonsburg, PA). A second combination of olopatadine and mometasone (OloMom) was FDA approved in January 2022 and is marketed in the US under the trade name Ryaltris (Glenmark Pharmaceuticals, Mahwah, NJ).
A systematic review of the English‐language literature was performed for clinical trials of combination INCS and intranasal antihistamine for the treatment of AR. A total of 18 RCTs (16 double‐blind, two non‐blinded) evaluated the efficacy of combination therapy against either placebo or active control., , , , , , , , , , , , , , , , , An additional three observational studies reported outcomes of AzeFlu as a single treatment arm., , This evidence has been summarized in two previous systematic reviews, (Table XI.B.10.d).
Patient‐reported symptom scores and QOL assessments are the most commonly reported outcome measures. The most common outcome measure was the TNSS (16 studies), which records the severity of runny nose, sneezing, itching, and congestion. Other outcome measures included the TOSS Score (eight studies), VAS (four studies), the RQLQ (seven studies), the PRQLQ (one study), and odor threshold/discrimination/identification score (one study).
The majority of included studies enrolled patients with a minimum age of 12 years or older. Most studies reported outcomes from 14 days of treatment, with the exception of two studies with a 3‐month duration, and one study with a 52‐week duration. The number of subjects in each study ranged from 47 to 3398. AzeFlu as a single formulation was compared to placebo in seven studies, with primary outcomes showing superiority to placebo in all studies., , , , , , Superiority of combination therapy with AzeFlu was also demonstrated over active treatment with fluticasone propionate monotherapy in six studies., , , , , Similarly, superiority of combination therapy with AzeFlu was demonstrated over active treatment with azelastine hydrochloride monotherapy in four studies., , , A single study evaluated combination therapy with non‐proprietary azelastine hydrochloride and fluticasone propionate applied using two separate spray bottles, which found superiority over either azelastine or fluticasone as monotherapy.
OloMom was compared to olopatadine or mometasone monotherapy in four studies, all of which showed superiority of the combination therapy., , , One study comparing AzeFlu with OloMom found comparable symptom reduction. AzeFlu was directly compared to combination therapy with intranasal olopatadine and fluticasone in one study, with no significant difference in symptom relief between treatment groups. An experimental combination of solubilized azelastine and budesonide was found in a single study to be superior to either a suspension‐type formulation of azelastine and budesonide or placebo. A recent meta‐analysis found that intranasal antihistamines plus INCS is superior to oral antihistamines plus INCS in improving nasal symptoms in patients with AR.
Current FDA approval for the AzeFlu combined formulation extends to children ages 6 years and up, although indications for monotherapy are as low as 4 years for fluticasone and 6 months for azelastine. Children aged 6 to 12 years old were evaluated in two studies, with superiority of AzeFlu over placebo in improving symptoms and QOL., Several studies reported time to onset of AzeFlu was more rapid than INCS alone.
No study reported serious adverse effects from the use of combination INCS plus intranasal antihistamine. This combination therapy was generally well tolerated, with the most common adverse effect being taste aversion. Other reported adverse effects occurred in less than 5% of cases in any study, and included somnolence, headache, epistaxis, and nasal discomfort (Table II.C). One study that compared combination therapy of fluticasone propionate with either azelastine or olopatadine reported more treatment‐related events for the azelastine group than the olopatadine group. Ocular changes such as increased intraocular pressure and cataract formation are unlikely; nonetheless, caution may be warranted in patients with a history of glaucoma. Additional specific patient factors may be considered when selecting options for combination therapy.
Combination intranasal corticosteroid and intranasal antihistamine
Aggregate grade of evidence: A (Level 1: 2 studies, level 2: 18 studies, level 4: 3 studies; Table XI.B.10.d)
Benefit: Rapid onset; more effective for relief of multiple symptoms than either INCS or intranasal antihistamine alone.
Harm: Patient tolerance, especially due to taste. See Table II.C.
Cost: Moderate financial burden for combined formulation. Concurrent use of individual intranasal antihistamine and corticosteroid sprays is likely a more economical option.
Benefits‐harm assessment: Preponderance of benefit over harm. Combination therapy with intranasal antihistamine and INCS is consistently more effective than placebo or monotherapy. Low risk of non‐serious adverse effects.
Value judgments: High‐level evidence demonstrates that combination spray therapy with INCS plus intranasal antihistamine is more effective than monotherapy or placebo, as well as more effective than combination of INCS plus oral antihistamine. The increased financial cost and need for prescription limit the value of combination therapy as a routine first‐line treatment for AR. When a combined formulation is financially prohibitive, the concurrent use of two separate formulations (antihistamine and corticosteroid) is an alternative option.
Policy level: Strong recommendation for the treatment of AR when monotherapy fails to control symptoms.
Intervention: Combination therapy with INCS and intranasal antihistamine may be used as second‐line therapy in the treatment of AR when initial monotherapy with either INCS or antihistamine does not provide adequate control.
XI.B.10.e Intranasal corticosteroid and leukotriene receptor antagonist
LTRAs have been studied in conjunction with INCS for the treatment of AR. Montelukast is the only LTRA approved by the FDA for the treatment of seasonal AR in adults and children over 2 years of age, and for perennial AR in adults and children over 6 months of age. However, a boxed warning from the FDA in 2020 advises restricting use of montelukast for AR due to serious neuropsychiatric events, ranging from behavioral changes to suicidal thoughts or behavior. For patients with both asthma and AR, LTRAs may be considered with awareness of the mental health risks.
Montelukast has been studied in combination with INCS to determine if add‐on therapy to INCS provides improved outcomes. Nasal symptoms, olfaction, QOL, nasal airflow measures, and immunologic markers have been used to compare combination therapy with LTRA and INCS to INCS monotherapy for AR – with conflicting results reported in controlled trials. There is one meta‐analysis and eight controlled trials, , , , , , , where montelukast was studied as add‐on therapy to INCS. The meta‐analysis included four studies that used fluticasone propionate and one that used budesonide as the INCS; all used oral montelukast as the LTRA. No difference was demonstrated in nasal symptoms, disease specific QOL, or adverse effects, when comparing combination therapy with LTRA and INCS to INCS as monotherapy. However, significant improvement in ocular symptoms with combination therapy was reported in one RCT included in the meta‐analysis (Table XI.B.10.e).
Four trials demonstrated benefit with LTRA added to INCS., , , Chen et al. studied budesonide alone or in combination with montelukast. Outcome measures of symptoms, nasal cavity volume, and expired NO all demonstrated improvement in with combination therapy. A follow‐up study by Chen et al. showed similar favorable outcomes in all three outcomes categories for combination therapy. Goh et al. reported an RCT with fluticasone propionate compared to montelukast‐fluticasone propionate; combination therapy demonstrated improvement in symptom scores and QOL. Pinar et al. reported a trial with mometasone alone or in combination with desloratadine or montelukast. Add‐on montelukast had superior improvement in symptoms and QOL compared to all other active treatment groups after 1 month of treatment but not at 3 months (when all active treatment groups showed comparable efficacy).
Four other studies did not show additional benefit with add‐on montelukast., , , Di Lorenzo et al. studied symptoms and eosinophil‐specific inflammatory markers in four cohorts: fluticasone propionate alone, cetirizine‐fluticasone propionate, montelukast‐fluticasone propionate, and cetirizine‐montelukast. There was no additional benefit to add‐on montelukast besides a decrease in nasal itching with the combination therapy of montelukast‐fluticasone propionate compared to fluticasone propionate alone. Inflammatory markers were not different when LTRA was added to INCS.
Esteitie et al. studied symptoms and QOL in patients on fluticasone propionate compared to montelukast‐fluticasone propionate. There was no additional benefit to add‐on montelukast for nasal symptom scores and QOL measures.
Dalgic et al. studied objective measures of olfactory function in patients on mometasone furoate, montelukast, or montelukast‐mometasone. They found no difference in olfactory function with combination therapy. Florincescu‐Gheorghe et al. studied eosinophils in nasal secretions and symptoms in patients on mometasone furoate, desloratadine‐mometasone furoate, and montelukast‐mometasone furoate. There was no additional benefit to adding montelukast to mometasone furoate for all outcomes measured.
Overall, there are varying outcomes from trials reporting combination therapy with LTRA and INCS. Differences in the corticosteroid preparation may affect study findings – two studies with budesonide had favorable outcomes, whereas those with fluticasone propionate and mometasone furoate had variable outcomes. There was heterogeneity between the studies with variations in allergy sensitizations and seasonal symptoms, and the studies had modest sample sizes. Given the FDA boxed warning and variable study outcomes, use of LTRA with INCS should primarily be considered for patients with comorbid asthma, rather than AR alone. Proper counselling regarding mental health risks to patients and families, highlighting the importance of monitoring for any neuropsychiatric symptoms regardless of prior history of psychiatric disorders.
Combination intranasal corticosteroid and leukotriene receptor antagonist
Aggregate grade of evidence: B (Level 1: 1 study, level 2: 8 studies; Table XI.B.10.e)
Benefit: Some studies demonstrate improvement of symptoms and QOL with combination therapy. One meta‐analysis did not show benefit with the exception of ocular itching.
Harm: Boxed warning due to risks of serious neuropsychiatric events limiting use for AR. See Table II.C.
Cost: Low.
Benefits‐harm assessment: Boxed warning for AR limits use. If comorbid asthma and AR, treatment is an option with consideration of mental health risks.
Value judgments: Possibly useful for symptom control, especially in patients with comorbid asthma, however, boxed warning limits use in AR without asthma.
Policy level: Option as combination therapy if comorbid asthma present and mental health risks are considered. Not recommended for AR alone.
Intervention: Consider use in patients with AR and asthma, after weighing therapeutic benefits against risks of mental health adverse effects.
XI.B.10.f Intranasal corticosteroid and intranasal decongestant
Combination therapy of INCS and INDC is used less frequently in clinical practice for the treatment of refractory AR. Most INDC (e.g., oxymetazoline, phenylephrine, and xylometazoline) are α‐receptor agonists, and decrease nasal congestion by reducing nasal mucosal volume through sympathomimetic vasoconstriction of mucosal blood vessels. Prolonged use of INDCs alone has been shown to cause rhinitis medicamentosa, or rebound rhinitis symptoms that respond increasingly poorly to INDCs. INCSs, on the other hand, as detailed in the preceding sections, have been widely validated and shown to be safe and effective in the first‐line treatment of AR.
In patients refractory to first‐line therapy, several RCTs have examined combination therapy using INCS and INDC. Five RCTs, varying in size from 23 to 705 participants, showed that combination therapy with INCS and INDC was significantly more effective in improving nasal symptom scores compared to INCS alone., , , , Three of these studies also reported no rhinitis medicamentosa in patients receiving combination therapy., , In contrast, Baroody et al, in a 2011 randomized cohort with refractory AR, showed that TNSS improved with fluticasone‐oxymetazoline compared to placebo or oxymetazoline alone, but not over fluticasone alone. Additionally, while Meltzer et al. showed combination therapy to be superior to mometasone alone in their AR cohort, they did not demonstrate a dose‐dependent relationship of oxymetazoline as part of the combination therapy in reducing nasal congestion (Table XI.B.10.f).
This controversy extends to higher level evidence as well. A 2018 SRMA of two studies by Khattiyawittayakun et al. determined that there was no demonstrable benefit to the addition of an INDC to INCS, and an IT reduction should be recommended in AR patients refractory to first‐line therapy with INCS. Several limitations in the current data exist that make comparing published RCTs challenging, including heterogeneity of methods and medications used, inconsistency between studies in their cohort construction (some including seasonal and perennial AR and others including non‐allergic rhinitis), and differences in antihistamine use in various trials. This is reflected in the measured statements issued in current guidelines. The 2020 Joint Task Force Practice Parameter on Rhinitis suggests that combination therapy of INCS‐INDC can be offered for up to 4 weeks to patients with nasal congestion unresponsive to INCS or INCS‐intranasal antihistamine combination therapy. The 2015 AAO‐HNSF Clinical Practice Guideline for AR cautions that such combination therapy with INDC should be limited to a few days to prevent rebound congestion.
Combination intranasal corticosteroid and intranasal decongestant
Aggregate grade of evidence: B (Level 1: 1 study, level 2: 5 studies, level 3: 1 study; Table XI.B.10.f)
Benefit: Some evidence in randomized studies of benefit from addition of INDC to INCS therapy in refractory AR patients. The evidence regarding the magnitude of effect is unclear, and a meta‐analysis that tried to estimate this effect was significantly limited by study heterogeneity and low sample size (two trials).
Harm: See Table II.C.
Cost: Low.
Benefits‐harm assessment: Balance of benefit and harm with current evidence base.
Value judgments: While combination therapy of INDC and INCS is superior to INCS therapy alone with low risk of tachyphylaxis in patients with refractory AR, the magnitude of effect is still unclear. There may be a role in patients with AR refractory to INCS and intranasal antihistamine combination therapy prior to consideration of surgery or in patients uninterested in surgery.
Policy level: Option.
Intervention: Short‐term combination therapy with INCS and INDC may be considered in patients with AR refractory to combination therapy with INCS and intranasal antihistamine prior to consideration of IT reduction or in patients declining surgery.
XI.B.10.g Intranasal corticosteroid and intranasal ipratropium
Current treatment algorithms for children, and adult patients, with moderate to severe AR with insufficient symptom control or treatment failure with INCS monotherapy uniformly recommend adding nasal IPB to the established INCS therapy if one of the main symptoms is predominant or refractory rhinorrhea. Although most guidelines recommend the combined use of both INCS and IPB in those patients, only one study assessed the effectiveness of this combination therapy in AR patients. Dockhorn et al. conducted a double‐blind RCT in patients with AR and non‐allergic rhinitis and demonstrated that the combination therapy of 14 days of IPB 0.03%, 42 μg per nostril TID and beclomethasone dipropionate, 84 μg per nostril BID was superior to either agent alone, or placebo, in reducing the severity and duration of rhinorrhea. The combination therapy resulted in a clinically relevant reduction in severity and duration of rhinorrhea in 74% and 66% of patients, respectively, compared to 57% and 50% for IPB monotherapy, 64% and 54% for beclomethasone dipropionate monotherapy, and 47% and 38% for placebo. Of note, in evaluation of nasal congestion alone, combination therapy was more effective than IBP monotherapy or placebo, but not statistically better than beclomethasone dipropionate alone. Similarly, better improvements in QOL PROMs, including the SF‐36 Health Survey and the RQLQ, were seen in the combination therapy group relative to monotherapy or placebo. The QOL effects of the combination therapy were most pronounced on the three RQLQ questions that focus on rhinorrhea. A clinically relevant improvement from: “somewhat troubled‐extremely troubled” at baseline to “not troubled‐hardly troubled” after 2 weeks of treatment was found in 48.8% of patients with the combined treatment compared to 38.9%, 25.2%, and 16% in the IPB, beclomethasone dipropionate, and placebo groups. The combination therapy was generally well tolerated. The most reported adverse effects included nasal dryness, epistaxis, blood‐streaked sputum, nasal irritation, and congestion (Table II.C). Interestingly, the percentage of patients reporting these adverse events was comparable to the treatment groups receiving monotherapy. Of note, this study population included patients with both AR and non‐allergic rhinitis and therefore these conclusions may only apply to this combination population. Nonetheless, as there is only evidence that the combination therapy effectively controls rhinorrhea, add‐on IPB should only be prescribed if one of the predominant refractory symptoms is rhinorrhea (Table XI.B.10.g).
Combination intranasal corticosteroid and intranasal ipratropium
Aggregate grade of evidence: Unable to determine based on one study. (Level 2: 1 study; Table XI.B.10.g)
Benefit: Reduction of rhinorrhea in INCS‐treatment‐refractory AR.
Harm: Usually no systemic anticholinergic activity if administered intranasally in the recommended doses. See Table II.C.
Cost: Low.
Benefits‐harm assessment: Benefit for combined INCS and IPB therapy in patients with treatment refractory AR and the main symptom of rhinorrhea.
Value judgments: No evidence for benefit in controlling symptoms other than rhinorrhea. Evidence is limited, but results are encouraging for patients with persistent rhinorrhea.
Policy level: Option.
Intervention: Combining IPB with beclomethasone dipropionate can be more effective than either agent alone for the treatment of rhinorrhea in refractory AR in children and adults. Although multiple consensus guidelines have recommended, and there is evidence to support this recommendation, it is important to note that there has only been one RCT to study the efficacy of combined INCS and IPB therapy compared to either agent alone, and this study was performed in a combined population of patients with AR and non‐allergic rhinitis.
XI.B.11 Non‐traditional and alternative therapies
XI.B.11.a Acupuncture
Since the 5th century BC, acupuncture has been used as a therapeutic modality for otolaryngologic disorders. A central tenet of Traditional Chinese Medicine (TCM) is the concept of qi, which represents the body's vital energy and flows through a network of meridians beneath the skin. Acupuncture involves insertion of thin needles at specific acupoints located along these meridians with the goal of achieving a therapeutic “de qi” effect. Studies have shown that acupuncture may potentially reset the Th2‐Th1 imbalance by modulating IgE and IL‐10 levels in patients with AR significantly more than controls., Acupuncture has an excellent safety profile with only mild reported adverse effects.,
Several SRMAs have been performed on acupuncture for the treatment of AR. In 2008, Roberts et al. reviewed seven RCTs and found a high degree of heterogeneity between studies with most studies being of low quality. No overall effects of acupuncture on AR symptom scores or use of relief medications were identified. In 2009, Lee et al. performed a systematic review with pooled analysis of 152 patients demonstrating that the results of acupuncture for AR are mixed – with acupuncture superior to sham acupuncture in symptom scores for perennial AR, but not for seasonal AR. In 2015, a meta‐analysis by Feng et al., which included 13 studies, showed a significant improvement of nasal symptoms, RQLQ scores, and use of rescue medications in the group receiving acupuncture. This meta‐analysis included data from a large multicenter RCT (n = 422) demonstrating improvement of seasonal AR with true acupuncture. In 2020, a systematic review by Wu et al. analyzed 15 RCTs and found acupuncture as a useful adjunct to allopathic standard of care or as monotherapy for AR. Yin et al. reviewed 39 studies, which included several studies from China and a meta‐analysis showing that acupuncture was superior to sham acupuncture with improvement in nasal symptom and RQLQ scores (Table XI.B.11.a).
Most important to note is the paucity of trials with head‐to‐head comparisons between acupuncture and standard conventional AR medication, with most RCTs using medication primarily as rescue treatment. The uncontrolled use of AR medications can significantly impact outcomes and underscores the critical need for comparative effectiveness research, as prioritized by the National Academy of Medicine.
Acupuncture
Aggregate grade of evidence: A (Level 1: 4 studies, level 2: 1 study; Table XI.B.11.a)
Benefit: Improvement of QOL and symptoms. Fairly well tolerated with no systemic adverse effects.
Harm: Needle sticks associated with minor adverse events including skin irritation, erythema, subcutaneous hemorrhage, pruritus, numbness, fainting, and headache. Electroacupuncture can interfere with pacemakers and other implantable devices. Caution is recommended in pregnant patients as some acupoints can theoretically induce labor. Need for multiple treatments and possible ongoing treatment to maintain any benefit gained. Relatively long treatment period.
Cost: Moderate‐high. Cost and time associated with acupuncture treatment; multiple treatments required.
Benefits‐harm assessment: Balance of benefit and harm.
Value judgments: The evidence is generally supportive of acupuncture. Acupuncture may be appropriate for some patients to consider as an adjunct/alternative therapy.
Policy level: Option.
Intervention: In patients who are interested in avoiding medications, acupuncture can be suggested as a possible therapeutic adjunct.
XI.B.11.b Other complementary modalities
Several SRMAs and RCTs have been performed on complementary interventions other than traditional acupuncture. These include: (1) ear acupressure; (2) acupoint catgut implantation; (3) acupoint herbal patching; (4) sphenopalatine ganglion acupuncture – a modern version of acupuncture developed by a Chinese otolaryngologist in the 1960s and first reported in 1990 for the treatment of AR, , , ; and (5) moxibustion/thunder fire moxibustion – a therapy based upon TCM theory that entails the burning of mugwort leaves as a warming treatment to promote circulation of qi., , SRMA results are mixed, with several of the SRMAs including studies of low methodological quality or high risk of bias (Table XI.B.11.b).
Other complementary modalities
Aggregate grade of evidence: Uncertain. Various complementary modalities assessed. Studies included in several SRMAs had poor methodological quality or high risk of bias.
Benefit: Unclear but some of these complementary therapies may be able to provide symptomatic relief.
Harm: Minimal side effects reported.
Cost: Moderate‐high cost of therapies with multiple treatments required.
Benefits‐harm assessment: Unknown.
Value judgments: There is lack of sufficient evidence to recommend the use of these interventions in AR.
Policy level: No recommendation.
Intervention: None.
XI.B.11.c Honey
A long‐held belief has been that honey is effective in treating symptoms of AR; however, evidence for this is scarce. It is postulated that environmental antigens contained within locally produced honey could, when ingested regularly, lead to the development of tolerance in a manner similar to SLIT. Primary sources of antigens can include pollen and microflora from the digestive tract of honeybees, which typically contains microorganisms present in dust, air, and flowers. It is important to note, however, that heavy insect‐borne pollens do not meet Thomen's postulates, as they are not airborne and hence should not be able to induce allergic sensitivity. Studies in animals have demonstrated the ability of honey to suppress IgE antibody responses against different allergens and to inhibit IgE‐mediated mast cell activation,, , while studies in humans have demonstrated various anti‐inflammatory properties of honey.,
There have been three RCTs looking at honey in the treatment of AR. The studies all differed on geographic location, length of treatment, dose of honey, and timing with respect to specific allergy seasons. One double‐blind RCT and an additional RCT showed a significant decrease in total symptoms scores in the treatment group compared to control. In contrast, another double‐blind RCT found no benefit of honey ingestion for the relief of AR symptoms compared to controls (Table XI.B.11.c).
Of note, it has been reported that higher doses (50–80 g daily intake) of honey are required to achieve health benefits from honey, and only the trial by Asha'ari et al. dosed patients at that level. In addition, the benefit of birch pollen honey in the trial by Saarinen et al. might be explained by a specific immunotolerance developed during oral intake of birch pollen with honey acting as a vehicle.
Honey
Aggregate grade of evidence: D (Level 2: 3 studies, conflicting evidence; Table XI.B.11.c)
Benefit: Unclear as studies have shown differing results and include different preparations of honey in the trials. Local honey may be able to modulate symptoms and decrease need for antihistamines.
Harm: Potential compliance issues with patients not tolerating the level of sweetness. Potential risk of allergic reaction and rarely anaphylaxis. Caution should be exercised in in pre‐diabetics and diabetics for concern of elevated blood glucose levels.
Cost: Cost of honey and associated healthcare costs with increased consumption.
Benefits‐harm assessment: Balance of benefit and harm.
Value judgments: More studies are required before honey intake can be widely recommended.
Policy level: No recommendation.
Intervention: None.
XI.B.11.d Herbal therapies
There are a vast number of studies looking at the effectiveness of various herbs and supplements in the treatment of AR; however, most are small and of poor quality. Herbal remedies that have been subjected to more rigorous study are summarized in Table XI.B.11.d.
Herbs often contain active pharmacologic ingredients, which can be difficult to measure clinically. Given the lack of robust and repeated large double‐blind placebo‐controlled RCTs for any particular herbal remedy, further research is needed before recommendations can be made regarding routine use of any particular herb or supplement.
Herbal therapies
Aggregate grade of evidence: Uncertain.
Benefit: Unclear, but some herbs may be able to provide symptomatic relief.
Harm: Some herbs are associated with mild side effects. Also, the safety, quality and standardization of herbal remedies and supplements are unclear.
Cost: Cost of herbal supplements.
Benefits‐harm assessment: Unknown.
Value judgments: There is a lack of sufficient evidence to recommend the use of herbal supplements in AR.
Policy level: No recommendation.
Intervention: None.
XI.B.11.e Guideline summary recommendations for non‐traditional and alternative therapies
See Table XI.B.11.e. for a summary of current guideline recommendations for non‐traditional and alternative therapies for AR.
XI.C Intranasal procedural interventions
Although medical therapy has largely been considered the cornerstone of treatment for AR, surgical/procedural management may play a role when patients are refractory to medical treatment. In these instances, surgery aims to improve structural problems that may lead to nasal obstruction/congestion, or to directly address physiologic causes of symptoms (e.g., rhinorrhea, mucosal swelling).
The literature surrounding the role of septoplasty/septorhinoplasty as a structural treatment for AR has expanded recently. While early evidence suggested that AR patients may benefit less from septoplasty/septorhinoplasty than non‐AR counterparts,, , most of the recent literature suggests the contrary,, , , , , , , , , with overall low complication rates., Kim et al. found that AR patients with septal deviation that underwent septoplasty with turbinoplasty had greater improvement in nasal obstruction than those that who underwent turbinoplasty alone. Nevertheless, the evidence is low‐quality overall, with a preponderance of retrospective case series and no RCTs. Furthermore, many applicable studies did not directly evaluate the role of septoplasty/septorhinoplasty in AR, but instead include it peripherally in the analysis. Therefore, in the properly selected patient, septoplasty/septorhinoplasty may represent an option at best (Table XI.C.‐1).
IT surgery can improve symptoms by structurally reducing nasal obstruction/congestion caused by enlarged turbinates, reducing volume of mucosal tissue that reacts with allergens, and allow improved accommodation of AR‐induced turbinate swelling. Inferior turbinoplasty is done via various surgical techniques: (1) bony lateral outfracture; (2) energy‐related submucous reduction techniques (e.g., radiofrequency ablation, electrocautery, coblation, laser‐assisted); (3) microdebrider‐assisted submucous reduction, and (4) bony and submucosal resection, including medial flap turbinoplasty. Total turbinectomy or turbinate resection was not covered as part of this review as they are typically not performed for inflammatory disease.
There are numerous studies investigating the efficacy of IT surgery for AR. Bony outfracture, the most atraumatic and conservative IT surgery, can reduce the distance between IT and lateral nasal wall and enlarge the dimensions of the nasal airway when performed alone, or in conjunction with other techniques., IT surgery via energy‐related techniques, , , , , , , , , , , , , , , , , , , , , , , , , , , , , , , , , , , , , , , , , , , , , , , , , , , , , , , , , , , and via direct tissue removal, , , , , , , , , , , , , , , , , , , , , , , , have both been extensively studied, with reported high efficacy in reducing symptoms and increasing nasal volume and airflow with minimal complications. Of note, botulinum toxin injection, , and high‐intensity focused ultrasound may also provide symptomatic relief,, though there remains limited evidence for their utility. As such, the current literature suggests that, in the properly selected AR patient with concomitant IT hypertrophy, IT surgery is an effective and safe treatment to reduce symptoms and improve QOL. More rigorous studies are warranted to directly compare various IT reduction techniques for optimal and durable outcomes (Table XI.C.‐2).
Another structural target is the nasoseptal swell body, with newer interventions directed toward volumetric reduction to improve airflow. Though ablation of the swell body (whether through radiofrequency, laser, or coblation) has shown promise in reducing symptoms,, , , , its effectiveness has yet to be tested with an AR‐specific cohort. However, the advent of devices intended for office use (e.g., Vivaer, Aerin Medical, Sunnyvale, CA) may provide opportunities for further study.
Rhinorrhea, as part of both AR and non‐allergic rhinitis, may arise from overactivity of parasympathetic nerve fibers originating from the vidian nerve. A vidian neurectomy with permanent sectioning of the most proximally accessible nerve segment is a potential surgical approach to reduce rhinorrhea in these patients. Evidence published from 2011 onwards provides support regarding its use in AR patients. Observational studies and a non‐randomized controlled trial found that AR patients experienced improvements in sneezing, nasal discharge, obstruction, itching, and QOL., , , , An RCT and another non‐randomized controlled trial of patients with both AR and CRSwNP found similar results, as well as improvement on pulmonary functions tests., There remains some concern that symptom recurrence may be high based on earlier studies, especially with longer‐term follow up, though this remains in contention and recent series have reported durable outcomes. Additionally, vidian neurectomy also carries the risk of dry eye due to the rami lacrimales that diverge from the nerve. Though recent evidence suggests that the properly selected patient does not experience symptomatic dry eye postoperatively, newer, more directed techniques targeting distal nerve segments have been developed. Specifically, the PNN, a branch of the vidian, appears to be an appropriate target given its specific nasal innervation. Though there is no study that evaluates vidian and PNN neurectomy head‐to‐head in AR patients, PNN neurectomy has been similarly shown to be effective for reducing symptoms,, , , , , , , though one non‐randomized controlled trial did not find a benefit to adding PNN neurectomy to microdebrider‐assisted turbinoplasty. Given the evidence, neurectomy is an option for treating refractory rhinorrhea following failed medical management (Tables XI.C.‐3 and XI.C.‐4).
Alternatively, energy‐based ablation of the PNN (RhinAer, Aerin Medical, Sunnyvale, CA) utilizing radiofrequency or cryotherapy (ClariFix, Stryker, Kalamazoo, MI) are office‐based alternatives to direct nerve section. The earliest report of utilizing cryotherapy for this indication was by Terao et al. in 1983. Studies utilizing cryoablation, including a randomized, sham‐controlled trial, have shown improvement in symptoms and QOL., , , , , , Though no study specifically evaluated an AR‐specific cohort, many performed subgroup analysis (which showed similar improvement) or controlled for the presence of AR (which showed that AR did not modify outcomes). Similar results were seen with radiofrequency ablation, also in the form of a randomized, sham‐controlled trial., In‐office endoscopic laser ablation of the PNN has also been reported with positive improvement. These procedures seem to be well‐tolerated, with minimal complication risk. There is also evidence to suggest that appropriate response to IPB nasal spray seems to correlate with improved cryotherapy treatment response. Ultimately, as the current evidence is largely based on industry‐sponsored studies with limited long‐term data, these interventions remain an option for properly selected patients (Table XI.C.‐5).
Septoplasty/septorhinoplasty
Aggregate grade of evidence: C (Level 3: 1 study, level 4: 3 studies, level 5: 11 studies; Table XI.C.‐1)
Benefit: Improved postoperative symptoms and nasal airway.
Harm: Risk of complications (e.g., septal hematoma or perforation, nasal dryness, cerebrospinal fluid leak, epistaxis, unfavorable aesthetic change); persistent obstruction.
Cost: Surgical/procedural costs, time off from work.
Benefits‐harm assessment: Potential benefit must be weighed against low risk of harm and cost of procedure.
Value judgments: Properly selected patients with septal deviation impacting their nasal patency can experience improved nasal obstruction symptoms.
Policy level: Option for those with obstructive septal deviation.
Intervention: Septoplasty/septorhinoplasty may be considered in AR patients that have failed medical management and who have anatomic, obstructive features that may benefit from this intervention.
Inferior turbinate surgery
Aggregate grade of evidence: B (Level 1: 4 studies, level 2: 13 studies, level 3: 18 studies, level 4: 50 studies*; Table XI.C.‐2)
*Level 1, 2, and 3 studies are listed in the table; level 4 studies are referenced.
Benefit: Improvement in rhinitis symptoms including nasal breathing, congestion, sneezing, and itching. Improved nasal cavity area via objective measures, as well as increased QOL via subjective measures.
Harm: Risk of complications (e.g., swelling, crusting, empty nose syndrome, epistaxis).
Cost: Surgical/procedural costs, potential time off from work.
Benefits‐harm assessment: Potential benefit outweighs low risk of harm.
Value judgments: Current evidence suggests that patients with AR who suffer from IT hypertrophy will likely experience improvement in symptoms, nasal patency, and QOL.
Policy level: Recommendation in patients with medically refractory nasal obstruction.
Intervention: In AR patients with IT hypertrophy that have failed medical management, IT reduction is a safe and effective treatment to reduce symptoms and improve nasal function. More studies are warranted to directly compare IT surgery methods (e.g., radiofrequency ablation, laser‐assisted, microdebrider‐assisted) for the most efficacious and long‐lasting outcome.
Neurectomy (vidian neurectomy, posterior nasal neurectomy)
Aggregate grade of evidence: B (Level 2: 3 studies, level 3: 5 studies, level 4: 7 studies, level 5: 2 studies; Tables XI.C.‐3 and XI.C.‐4)
Benefit: Improvement in rhinorrhea.
Harm: Risk of complications (e.g., dry eye and decreased lacrimation, numbness in lip/palate, nasal dryness, damage to other nerves).
Cost: Surgical/procedural costs, potential time off from work.
Benefits‐harm assessment: Potential benefit must be balanced with low risk of harm but consider that long‐term results may be limited.
Value judgments: Patients may experience an improvement in symptoms.
Policy level: Option.
Intervention: Vidian neurectomy or PNN neurectomy may be considered in AR patients that have failed medical management, particularly for rhinorrhea.
Cryotherapy/radiofrequency ablation of the posterior nasal nerve
Aggregate grade of evidence: C (Level 3: 2 studies, level 4: 4 studies, level 5: 5 studies; Table XI.C.‐5)
Benefit: Improvement in rhinorrhea.
Harm: Risk of complications (e.g., epistaxis, temporary facial pain and swelling, and headaches), limited long‐term results.
Cost: Surgical/procedural costs, cost of device, potential time off from work.
Benefits‐harm assessment: Potential benefit must be balanced with low risk of harm, especially considering limited long‐term results.
Value judgments: Patients may experience an improvement in symptoms
Policy level: Option.
Intervention: Cryoablation and radiofrequency ablation of the PNN may be considered in AR patients that have failed medical management, particularly for rhinorrhea.
XI.D Immunotherapy
XI.D.1 Allergen immunotherapy candidacy
Of the three primary modalities used to manage AR – allergen avoidance, pharmacotherapy, and AIT – immunotherapy is the only treatment that that has a disease‐modifying effect through induction of immunologic tolerance. AIT may be considered when a patient has an IgE‐positive skin or in vitro test to an allergen that can be correlated with a patient's exposures and symptoms. The presence of sIgE antibodies alone indicates sensitivity to the allergen but may not result in clinically significant allergic symptoms.
Most position papers on AIT recommend its use in patients with moderate to severe symptoms that are not controlled with avoidance and/or pharmacotherapy., However, there is evidence that SCIT is at least as potent as pharmacotherapy in controlling symptoms of seasonal AR as early as the first season after initiating treatment. Although there is no direct evidence that AIT is as effective as pharmacotherapy as a primary treatment for AR, most RCTs evaluating the efficacy of SLIT or SCIT showed improvement in symptoms and/or medication requirement compared to placebo. One caveat to these studies is the fact that patients in the placebo groups were allowed to use allergy medications and were essentially a pharmacotherapy treatment group rather than a true placebo group.,
Patients who have adverse reactions to traditional pharmacotherapy or decline long‐term medication use are also excellent candidates for AIT. There is strong evidence of decreased medication use up to 3 years after stopping both SCIT and SLIT., , In a double‐blind, placebo‐controlled RCT, there was no difference in symptom scores in patients who discontinued AIT after 4 years of use and those who continued it.
One perceived benefit, and perhaps indication, for AIT has been the long‐held theory that it may prevent or reduce the development of new allergic disease. However, a recent meta‐analysis of 32 studies found no conclusive evidence that AIT reduced the risk of long‐term new allergic disease and sensitizations both in the pediatric and adult population. This study did find a reduction in short‐term risk of developing asthma in patients with diagnosed AR (RR 0.4; 95% CI 0.30–0.54). There is evidence from other studies indicating that AIT helps reduce the risk of development of asthma., In a double‐blind RCT of 812 children (5–12 years old) with clinically relevant AR and no history of asthma, patients were treated with 3 years of grass SLIT versus placebo with 2 years of follow‐up. The SLIT group had a significantly reduced risk of experiencing asthma symptoms or using asthma medication during the treatment and at the end of the 5‐year period.
Clinicians should be aware that there is a subset of patients for whom AIT is not an option. Absolute and relative contraindications for AIT are addressed in Section XI.D.3 Contraindications to Allergen Immunotherapy.
There is limited evidence for the efficacy of AIT for the treatment of AR in children younger than 5. However, there is data to show the efficacy and safety of both SLIT and SCIT in children 5 years and older., Patient adherence with AIT can be challenging, so consideration of risks and benefits, QOL impairment, financial concerns, and patient preference are important in treatment selection.
XI.D.2 Benefits of allergen immunotherapy for allergic rhinitis
SCIT is the best studied form of AIT and is effective for AR and rhinoconjunctivitis, allergic asthma, and Hymenoptera venom allergy. SCIT has been practiced for over a century using aqueous extracts of the naturally occurring allergens; its effectiveness and safety have improved over time with the advent of extract standardization and research into mechanisms of action. SCIT involves the repeated subcutaneous injection of the allergen extract in question, beginning with very small doses of allergen and gradually increasing to higher doses. This is followed by repeated injections of the highest or maintenance dose for periods of 3–5 years to reduce symptoms upon exposure to that allergen. Clinical and physiological improvement can be demonstrated shortly after the patient reaches a maintenance dose. AIT can also be provided in the sublingual form [SLIT]; dissolvable tablets are FDA approved for a limited number of allergens.
In contrast to other treatment options for allergic disease, AIT helps achieve sustained immunological changes, by altering the immune system's response and inducing long‐lasting immune tolerance to allergens. Despite extensive experience with this therapy and decades of research, the mechanisms underlying clinical improvement have not been fully elucidated. Although less mechanistic research exists for SLIT compared with SCIT, data suggest that both forms of AIT induce similar immunologic changes. These include a reduction in mast cell and basophil degranulation; an initial increase then decrease in sIgE and increase in allergen‐specific IgG (sIgG) blocking antibodies; generation of allergen‐specific regulatory T and B cells and suppression of allergen‐specific effector T cell subsets and ILCs; and reduction in tissue mast cells and eosinophils accompanied by a decrease in type I skin test reactivity., The clinically evident changes occur earlier with SCIT, and more pronounced sIgG4 responses are observed compared with SLIT.
The effectiveness of AIT for the treatment of AR is supported by an extensive body of evidence and is generally measured via improvement in allergy symptoms and reduction in allergy medication use., , Although meta‐analyses conclude that AIT is effective, this positive judgment of efficacy (and safety) should be limited to products tested in the clinical trials. It is incorrect to make a general assumption that all forms of AIT are effective since this may lead to the clinical use of products that have not been properly studied.
The severity and duration of AR symptoms, as well as coexisting medical conditions such as asthma, should be considered in assessing the need for AIT. The decision to initiate AIT depends on a number of factors, including but not limited to patient's preference, adherence, response to avoidance measures, medication requirements, and adverse effects of medications. Patients should be evaluated at least every 12 months while receiving AIT. While many patients experience sustained clinical remission of their allergic disease after discontinuing AIT, others may relapse. A decision about continuation of effective AIT should generally be made after the initial period of 3–5 years of treatment.
As noted in the preceding section, a 2017 meta‐analysis evaluating the preventative effects of AIT (SCIT and SLIT) found evidence of a reduction in the short‐term (<2 years) risk of developing asthma among patients with AR. The analysis also examined the longer term risk of asthma development, as well as the ability of AIT to prevent the occurrence of a first allergic disease in sensitized but asymptomatic individuals or to prevent sensitization to new allergens. There were trends toward benefit but inconclusive findings regarding these measures.
XI.D.3 Contraindications to allergen immunotherapy
Contraindications to AIT are uncommon but must be reviewed in all patients prior to initiating treatment. For both SLIT and SCIT, the adverse event of greatest severity is anaphylaxis. Therefore, many of the absolute and relative contraindications to AIT are directly related to this risk, including uncontrolled asthma, concomitant β‐blocker use, contraindication to injectable epinephrine, and pregnancy.
Uncontrolled asthma may be the single most important risk factor. There were fewer severe injection reactions reported among practices that routinely screened for and withheld injections from patients with asthma that was not controlled. Most fatal reactions were associated with bronchospasm and/or respiratory failure.,
Due to the inability to engage the β‐adrenergic receptor with injectable epinephrine, β‐blocker use is considered a relative contraindication for AIT. Since approximately 0.1% of allergy injections may lead to systemic symptoms, and 0.003% can be considered severe, the ability to emergently treat these reactions with epinephrine when indicated is essential. β‐blocker use does not appear to increase the likelihood of systemic reactions but, although not consistently observed, may be associated with higher anaphylaxis severity., Thus, the lack of effect of typical subcutaneous epinephrine dosing in a β‐blocked patient creates the treatment dilemma.
Although there is some variability, some guidelines consider active systemic autoimmune diseases and active malignancy as contraindications to AIT. This is based on case reports and case series and generally lower quality evidence that the risk of anaphylaxis from AIT is greater in patients with these conditions or that the immunomodulatory effect might negatively affect the underlying disease process. Successful AIT has been reported in several patients with malignancy. Similarly, the theoretical concerns in autoimmune disease are offset by several case series demonstrating relative safety and effectiveness. Furthermore, in a large observational study of 1888 patients, there was no increase in the development of autoimmune disease in AR treated with AIT over a 20 year observation period.
Initiating AIT during pregnancy is contraindicated although most consensus documents state that continuing maintenance immunotherapy during pregnancy is not contraindicated., Avoiding the initiation of AIT is presumably based on the concern that severe anaphylaxis is more likely to occur during buildup immunotherapy and that anaphylaxis, or treatment thereof, could harm the developing fetus. There are limited data to guide decision making, but in a cohort of 102 pregnancies during AIT, there were no increased fetal complications compared with untreated pregnancies. Three patients had systemic reactions requiring epinephrine – none resulting in pregnancy complication. A more recent study demonstrated the relative safety of SLIT initiated during pregnancy.
SLIT is available for several allergens as an FDA approved tablet. Contraindications for this therapy include unstable or uncontrolled asthma. Therapy should not be initiated in a patient with a medical condition impairing recovery from anaphylaxis, or in those for whom epinephrine or β‐agonist therapy might be less effective. SLIT tablets are also contraindicated in patients with EoE., , ,
There are a variety of relative contraindications that merit shared decision making. Cardiovascular disease, systemic autoimmune diseases in remission, severe psychiatric disorders, poor adherence, primary and secondary immunodeficiencies, and a history of serious systemic reactions to AIT have all been considered as relative contraindications. A 2019 EAACI task force summary also reviews some additional considerations. ACEI therapy in venom immunotherapy is a relative contraindication, but not for aeroallergen immunotherapy. Inability to communicate symptoms that might herald the beginning of anaphylaxis are a potential contraindication and might be especially challenging in very young children (less than 5 years old). Human immunodeficiency virus (HIV) is usually not considered a contraindication unless the patient has acquired immunodeficiency syndrome (AIDS). This and other chronic infections should be factored into the overall risk/benefit evaluation.
XI.D.4 Allergen extracts
XI.D.4.a Overview, units, and standardization
Overview. Allergy testing began with pollen grains placed on the conjunctiva., As skin testing and SCIT evolved, injectable allergen extracts were required. Inhaled allergenic particles are composed of a heterogeneous mixture of allergenic and non‐allergenic proteins and macromolecules. Allergen extracts are created by refining raw materials and extracting proteins in a solution.
There are multiple sources of variance in allergen extracts. The composition of allergenic proteins can vary, conferring different degrees of total antigenicity through genetic or epigenetic mechanisms., Impurities in the source materials, such as mold growing on pollen granules or bacteria on cat pelts, may affect immunogenicity. Variation also occurs in the raw material collection and in the extraction process., , , Additionally, there is biologic variation in individual sensitizations to major and minor allergens within a source. Only a very small fraction of the proteins extracted are allergenic. Given that the antigenic composition of allergen extracts is not uniformly assessed, assuring extracts are both safe and effective is challenging.
Units and potency. Allergen extracts are labeled with a variety of units, many of which do not convey information about allergenic content or allergenic potency. Potency can refer to the qualitative allergenicity of a source material's proteins or the quantitative concentration of allergens in an extract. Measures of an allergen extract may refer to quantity of extracted material in the solution (a concentration) or be standardized to the biologic activity in allergic individuals. The different techniques of assessing allergen extracts lead to multiple types of units, which can be grouped into non‐standardized, standardized, and proprietary.
Non‐standardized allergen extracts. The majority of allergen extracts available in the US are non‐standardized. Allergen extracts are regulated by the Center for Biologics Evaluation and Research (CBER) under the US FDA. The FDA requires that allergen extracts list the biologic source, a potency unit, and an expiration date. This labeling allows for significant variation between manufacturers and between lots produced by the same manufacturer.
There are two US non‐standardized units, weight/volume (w/v) and protein nitrogen units (PNU). Weight/volume refers to the ratio of grams of dry raw material to milliliters of extract solvent. An allergen extract labeled 1:20 w/v indicates for every 1 g of raw material (e.g., pollen) 20 ml of extract solvent was used. This does not provide direct information about the amount of allergenic protein in the extract nor its reactivity in allergic individuals. However, it implies a reproducible extraction methodology was employed. PNU is the second most common non‐standardized unit currently used in the US. PNU refers to an assay of the precipitable protein nitrogen by phosphotungstic acid that correlates with the total protein in the extract. While most of the protein is non‐allergenic, the total protein is another method to quantitate an allergen extract's content.
In Europe, many manufacturers use proprietary units and internal quality controls which must utilize a validated assay. This European manufacturer based quality control is known as “In House Reference Preparation” or “IHRP.” However, the European Medical Agency has been developing a standardized framework based on protein homology rather than source species. The European Union is also developing additional allergen standards with the WHO starting with Bet v 1 and Phl p 5a. Extract units in Europe, the US, and other countries vary without agreed upon references available for conversion.
Standardized allergen extracts. Standardized allergen extracts in the US are tested by the manufacturers to be within a reference range (70%–140%) when compared to a standard provided by the FDA's CBER. Standardized inhalant allergens within the US include cat, Dermatophatoides pteronyssinus, Dermatophagoides farinae, short ragweed, and multiple grass species.
The CBER creates the reference standardized extract through skin testing in known “highly allergic” individuals. They use serial intradermal skin testing with three‐fold titrations and measure potency by how many dilutions are needed to produce a flare reaction measured by adding the largest diameter and its 90° (orthogonal) diameter. The orthogonal sums are plotted for each dilution and a best‐fit line drawn. The concentration that corresponds to where the orthogonal sum of the flare totals 50 mm (ID50EAL) determines the units listed in either allergy units (AU) or biologic allergy units (BAU). AU is used for HDM historically. A mean ID50EAL of fourteen three‐fold dilutions is defined as 100,000 BAUs/ml and 12 three‐fold dilutions 10,000 BAUs/ml. Manufacturers then compare their extract lots to the CBER allergen standard through competition ELISA using pooled serum IgE from known allergic subjects.
The process is different for extracts where the major allergen reactivity strongly correlates with overall allergen reactivity (cat and ragweed). A major allergen is defined as a specific protein that elicits an allergic reaction in more than 50% of individuals allergic to that species. If there is a major allergen that correlates strongly with the population's clinical reactivity, the manufacturer compares their extract to the CBER's standard by gel electrophoresis employing monoclonal IgG antibodies to the major allergen protein. When standardized by major allergen, the units are listed in μg/ml (Fel d 1 for cat; Antigen E or Amb a 1 for ragweed). For cat extracts, the presence of Fel d 2 is also required. Also, cat extract with 10–19.9 Fed d 1 U/ml is designated as 10,000 BAU/ml. Short ragweed extract of 350 Amb a 1 U/ml is designated as 100,000 BAU/ml.
Some allergen extracts in Europe use the Nordic method where 10,000 biologically standardized units/ml is comparable to a SPT response elicited by 10 mg/ml of histamine. Most allergen extracts in Europe are proprietary; however, the European effort to develop cross‐product comparability is summarized nicely by Zimmer et al. The WHO has identified allergen standardization as a problem and the European Union funds a project known as CREATE to “develop certified reference materials for allergenic products and validation of methods for their quantification.”,
In summary, there is not an international consensus on allergen units or standardization for allergen extracts. While cross‐manufacturer standardization and biologic potency labeling increase manufacturing costs, it is widely agreed that greater standardization would benefit patient efficacy and safety. Variations in allergen extracts between manufacturers may discourage medical providers from changing vendors, thus reducing competition's effect on price. Non‐standardized and proprietary units also complicate the interpretation of published efficacy and safety studies. As of 2022, multiple opaquely referenced allergen units remain in use worldwide. (See Section XI.D.11.a.i. Allergen Standardization and Heterogeneity for additional information on this topic.)
XI.D.4.b Allergen extract adjuvants
Although AIT is an effective treatment for AR, it is not without limitations including cumbersome‐up‐dosing regimens, systemic reactions, and variable efficacy. Adjuvants are chemicals and proteins that may enhance the safety, convenience, and immunological effects of AIT., , , , , , Effective AIT attenuates pro‐inflammatory Th2 responses in favor of tolerogenic Treg responses. This immunological transformation can be enhanced with adjuvants that are subdivided into several broad categories (Table XI.D.4.b).
Of the potential adjuvants listed, several have reached Phase 1 or Phase 2 clinical trials for treating AR. Some have already received FDA approval for use in modern infectious disease vaccines. Next generation AIT products may very well incorporate adjuvants in combination with peptides and other allergenic molecules. A few adjuvants deserve specific mention.
Mineral salts and crystalline molecules. Alum (aluminum hyroxide salt) was the first adjuvant to be tested in AIT and has recently been considered for COVID‐19 vaccines., Early studies with alum‐precipitated extracts demonstrated an augmented immunologic response but with some undesirable IgE‐mediated response that hindered its therapeutic application., Microcrystalline tyrosine has been tested as an alternative with less IgE production., Alum formulations are currently being considered for certain allergen peptide vaccines.
Toll like receptor constructs. It has been proposed that danger signal molecules synthesized from virus, parasites, and bacteria and used in combination with allergens could help induce tolerance by augmenting TLR mediated innate immune responses., , , Tversky et al., showed that traditional SCIT alone results in a partial restoration in the impaired TLR function demonstrated among AR sufferers and that this effect could potentially be augmented with certain adjuvants.
Among the specific TLR targeted clinical studies, Creticos et al. first reported a study using synthetic bacterial derived DNA (CpG oligodeoxynucleotide) bound to ragweed protein Amb a 1 designed to upregulate the immunostimulatory responses via TLR‐9. This TLR‐9 agonist bound to Amb a 1 (Tolamba) was administered in a double‐blind, placebo‐controlled study of ragweed‐allergic subjects with a single season 6‐injection regimen. Efficacy was observed over two ragweed seasons indicating that the vaccine conferred some clinical tolerance. A follow‐up study did not reach statistical significance. In 2021, Leonard et al. reported on the use of CpG and a Fel d 1 specific mouse immunotherapy model to elucidate important signaling elements that may be capitalized upon moving forward.
CYT003‐QbG10 is another TLR targeted immunotherapeutic product in development for the treatment of AR and asthma. It is based on Cytos Biotechnology's modified Immunodrug platform, which incorporates virus‐like particle Qb, a TLR‐9 immunostimulatory DNA sequence to induce targeted T cell responses. In a Phase 2b double‐blind, placebo‐controlled study of 300 patients with allergic rhinoconjunctivitis, QbG10 was shown to be safe, well‐tolerated and efficacious.
A TLR‐4 adjuvant has also been in clinical development (Pollinex Quattro, Allergy Therapeutics). This construct is comprised of monophosphoryl lipid A and formulated with pollen allergoids. A large grass study showed significant improvement in symptom and medication scores versus placebo. A brief ragweed trial also showed positive clinical effect.
Nanoparticle based constructs. Synthetic nanoparticles have been proffered since 1959 to deliver a host physiologically active substances including vaccines., A successful recent example of this is the use of liposomes to deliver mRNA encoded spike protein instructions in the Pfizer and Moderna COVID‐19 vaccines. This same approach has been proposed to deliver genetic instructions encoding allergenic proteins for immunotherapy. These so‐called allergen “vaccines” have the potential to synergistically activate TLR receptors while simultaneously encoding allergenic proteins.
Naturally occurring adjuvants. Certain naturally occurring immune modulators have been shown to act as potential adjuvants. Nutritional compounds and probiotics may be ingested directly or administered subcutaneously in tandem with allergen., One example is VD3 which has been shown to reduce effector T cell stimulation and cytokine production and promote the effect of AIT in both mice and humans., , One mouse immunotherapy study successfully employed the use of Fel d 1 covalently bound to VD3. (See Section VI.H. Vitamin D for additional information on this topic.)
Components isolated from Ganoderma Lucidum, a Chinese herb contained in Anti‐Asthma Simplified Herbal Medicine Intervention (ASHMI), induce levels of IL‐10, IFN‐γ, and Foxp3 in response to environmental allergens. Like TLR ligands, ASHMI has shown some limited effectiveness in treating certain allergic diseases by itself without the presence of an allergen. However, because of its unique tolerogenic cytokine profile, ASHMI and other naturally occurring herb combinations may also prove to be advantageous when used as an adjuvant for AIT.
In summary, various adjuvants have been proposed and studied in animal models and tested in humans, but there is currently no adjuvant FDA approved for use in AIT. Improving the immunologic profiles of immunotherapies while maintaining safety standards remains challenging. Recent Phase 1 and Phase 2 studies have been reported for select adjuvants, and there is promise for future AIT protocols to incorporate adjuvants which outperform traditional therapies.
XI.D.4.c Modified allergen extracts
Traditionally the disease‐modifying capability and potential for long‐lasting therapeutic effect of AIT has been accomplished via SCIT or SLIT with native, unmodified extracts. However, reliance on native extracts has limitations for widespread use including production costs and availability, as well as consistency and comparability among extracts. Furthermore, while generally safe, AIT with natural extracts has the potential for inducing hypersensitivity reactions that can rarely be life‐threatening. The use of modified allergen extracts has been studied as an alternative to native extracts as a means of providing improved AIT efficacy, safety, and reliability. This section discussed several approaches of modified allergen extracts.
Recombinant allergen extracts. Recombinant‐derived allergens rely on recombinant DNA technology to produce clones of natural allergens in the case of wild type recombinant allergens, or clones of partial allergen sequences in hypoallergenic recombinant allergens. For wild type recombinant allergens, this technique produces consistent structures that preserve allergenic epitopes and potencies. However, the disadvantage is that as a clone, there is potential for inducing hypersensitivity reactions. Hypoallergenic recombinant extracts, on the other hand, maintain certain T cell epitopes but may induce less IgE driven responses. Immunotherapy trials using recombinant birch and Timothy grass allergens have been reported. Timothy grass AIT with recombinant allergen induced immunologic changes, including increased IgG4 and down trending sIgE while decreasing symptoms and medication use compared to placebo., Similarly for birch AIT, recombinant allergen use resulted in reduced rhinoconjunctivitis symptoms and rescue medication use, with symptom improvement similar to treatment with natural extract; immunological changes included increased IgG levels compared to placebo., Together, these studies show potential for comparable performance of recombinant allergen extracts, with the advantage over natural extract of using a more consistent, pure allergen that could be precisely dosed.
Synthetic peptides. These are linear fragments of amino acids derived from T cell epitopes of allergens. Peptides do not induce early phase responses because they lack the conformational structure to bind to IgE receptors. When used for AIT, they do not generate a robust blocking IgG but do have the capability of inducing immunologic T cell changes. AIT with synthetic peptides has been studied for several allergens including cat, grass, HDM, ragweed, and birch with somewhat inconsistent efficacy. Grass allergen peptides were effective in reducing rhinoconjunctivitis symptom scores when injected at 2‐week intervals over a brief trial, and ragweed peptide therapy improved symptom scores compared to natural extract and placebo. Birch pollen pre‐seasonal treatment induced immunologic changes, but clinical symptoms were not significantly improved. Cat peptide AIT in particular had promising initial results reducing symptoms in sensitized individuals, but Phase 3 data of one product did not significantly outperform the placebo group., , , Longer sequences, termed contiguous overlapping peptides, have been alternatively used in an attempt to generate a more robust immunogenic response; birch AIT resulted in improved symptom scores and medication use as well as induction of IgG antibodies., ,
Allergoids. These involve native allergens that have been modified or denatured with the use of additional chemical agents, such as aldehydes and polyethylene glycol. These modified structures have the potential to retain immunogenicity, largely via T cell responses, but also decrease the risk for IgE‐mediated reactions. In addition to improved safety, this may offer ability to decrease the number of injections required during a build‐up period. While immediate hypersensitivity reactions are reduced, late phase adverse reactions can still occur. Allergoid preparations have been evaluated to several different allergens. Initially utilized in ragweed allergic patients, allergoid preparations reduced symptom scores and increased blocking antibodies., Subsequent studies with grass pollen allergoid also showed effectiveness in reducing clinical symptom scores and medication use., , Allergoids in HDM allergic patients also demonstrated improved symptom scores, in both subcutaneous and sublingual routes., More recently, in an open label study a glutaraldehyde‐modified allergoid in birch pollen allergic patients induced initial humoral responses as well as T cell augmentation of IL‐10 production. While allergoids are commercially available in Europe, standardization criteria have been a limiting factor in receiving regulatory approval in the US.
Encapsulated allergens. Encapsulation of allergens involves use of nanoparticles or microparticles to envelop allergens of interest which can then be injected or ingested orally. This process has the potential to decrease the dose required for immunologic responses, protect the allergen from degradation, and improve uptake of allergen while limiting adverse reactions. Encapsulation can be accomplished with biodegradable nanoparticles including synthetic or natural polymers, liposomes, and virus‐like particles, or with nonbiodegradable nanoparticles such as dendrimers or carbon‐based particles. Most of the research involving encapsulated allergens has yet to be evaluated in human trials. In one study, a liposome encapsulated HDM extract was evaluated in patients with asthma, who had improved symptom scores over a 12‐month period compared to placebo. Separately, an oral microencapsulated form of Timothy grass allergen was used to treat patients with AR over a period of 10 weeks; patients in the active treatment group experienced decreased symptom scores compared to placebo. Limited human trial data suggest that encapsulated allergens may induce immune responses but further understanding of their role in AIT is needed.
Overall, a variety of modified allergen extracts hold promising clinical and immunologic findings. Further research is needed involving larger clinical groups to study the efficacy and safety of these agents as compared to the native allergen extracts.
XI.D.5 Subcutaneous immunotherapy for allergic rhinitis
XI.D.5.a Conventional subcutaneous immunotherapy for allergic rhinitis
Efficacy. Over the past 68 years, multiple RCTs have supported the therapeutic efficacy of SCIT for AR. SCIT efficacy is contingent upon an appropriate treatment duration and dose, with an optimal target maintenance dose between 5 and 20 μg of major allergen for each clinically relevant aeroallergen. SCIT has been associated with effective symptom amelioration and potential disease modification that can persist after stopping treatment.
Evidence suggests that a SCIT treatment duration of 3–5 years is appropriate. A clinically significant relapse rate has been observed with SCIT discontinuation prior to 3 years. Currently, there are no validated biomarkers to reliably identify when SCIT can be discontinued and clinical remission sustained. The determination to discontinue SCIT in patients who have responded should balance the potential for benefit with the potential for harm and burden, in an open discussion with patient participation in the medical decision‐making process.
High‐quality data have substantiated the therapeutic utility of SCIT for AR patients with particular aeroallergens and certain formulations. Therefore, SCIT efficacy for AR treatment is contextual, and should not be interpreted as an “umbrella” description based on favorable outcomes observed in RCTs focused on a limited number of products.
SCIT is efficacious for AR sensitive to pollen, mold, HDM, and animal allergens., , , , , , , Such efficacy has been demonstrated based on rigorous RCTs for pollens (e.g., ragweed, grass, and birch), cat, and HDM (Dermatophagoides farinae and Dermatophagoides pteronyssinus), where a standardized extract target concentration is available and was studied. However, these data cannot be interpreted as a “class effect” that necessarily extends to other aeroallergens. Data supporting the SCIT efficacy for dog, cockroach, and mold spores (particularly Alternaria and Cladosporium) are encouraging, but limited, and additional studies are needed to substantiate the therapeutic efficacy of SCIT for AR related to these inhalant allergens., , , , ,
The majority of RCTs supporting SCIT for AR have been studies of single aeroallergens. There have been very few studies of multi‐allergen SCIT, which are heterogeneous and suffer from methodological shortcomings. While multi‐allergen SCIT is a mainstay of clinical practice in the US, and patients report favorable treatment benefits, additional high‐quality studies are needed to provide rigorous support for the efficacy of multi‐allergen SCIT in treating AR.
Safety. SCIT is associated with localized reactions occurring in the majority of patients. Evidence indicates local reactions do not reliably predict occurrence of subsequent systemic reactions; dosage adjustment is not typically required after their occurrence. While there is a low risk for systemic reactions from SCIT, potentially life‐threatening and fatal reactions may occur. Non‐fatal systemic reactions occur at a rate of approximately 2 per 1000 injections in patients receiving SCIT. Severe grade 4 anaphylactic reactions occur in approximately 1 per million injections, and fatal reactions in approximately 1 in 23 million injection visits.,
Risk factors for systemic reactions from SCIT include poorly controlled asthma, exquisite aeroallergen sensitivity, concomitant β‐blocker use, rush SCIT protocols, prior systemic reaction, high dose SCIT, injection from a new SCIT vial (i.e., higher potency), and dosing error., , , A recent decline in fatal systemic reaction rate has been observed, which has been attributed to greater awareness and identification of patients with risk factors.
Cost‐effectiveness. Data support SCIT as a cost‐effective intervention, in large part due to the potential for reductions in long‐term symptom burden, disease complications, disease progression, and medication costs. US studies demonstrate SCIT superiority over alternative approaches – providing clinical benefit while improving health outcomes., However, practice variation may produce cost disparities. As an example, some physicians may require SCIT patients to be provided a self‐injectable epinephrine prescription, which has not been shown to be cost‐effective (incremental cost‐effectiveness ratio $669,327,730 per QALY [quality adjusted life year]).
Evidence. Dhami et al. undertook a systematic review appraising SCIT efficacy for AR, with 61 robustly conducted double‐blind RCTs of SCIT satisfying inclusion criteria (Table XI.D.5.a). Study quality was high, with the majority of RCTs having low risk of bias. Significant improvements were seen in symptom scores (standardized mean difference [SMD] −0.65 [95% CI −0.86, −0.43]), medication use (SMD −0.52 [95% CI −0.75, −0.29]), combined symptom/medication score (SMD −0.51 [95% CI −0.77, −0.26]), and QOL (SMD −0.35 [95% CI −0.74, −0.04]; six trials). Analysis of safety was obfuscated by variation in reporting of adverse effects. In 19 RCTs, the overall relative risk of adverse events was 1.58 (95% CI 1.13, 2.20). Local adverse event relative risk was 2.21 (95% CI 1.43–3.41, nine RCTs). Systemic adverse event relative risk was 1.15 (95% CI 0.67–2.00, 15 RCTs). This systematic review provides evidence for short‐term benefit in symptoms and medication reliance, as well as a limited effect on disease specific QOL.
Several studies imply SCIT for AR is associated with continued benefit after stopping treatment, including a reduced risk for developing asthma, and new allergen sensitivities., However, data meta‐analyzed by Dhami et al. are more limited in terms of persistence of benefit in symptoms scores after treatment discontinuation. Additional studies are required to support this important and desirable outcome of SCIT treatment.
An updated systematic review of RCTs of SCIT for AR was performed from January 1, 2015, through October 1, 2021. All studies did not evaluate clinical endpoints, heterogeneity between studies was significant, and there was variable risk of bias. In general, studies demonstrated significant SCIT treatment benefit across age groups., , Arroabarren et al. evaluated children 5–15 years old in a prospective study comparing a 3‐year versus a 5‐year course of SCIT, demonstrating a 44% reduction in symptom and medication scores from baseline after 3 years of therapy (p = 0.002) and a 50% decrease after 5 years of therapy (p = 0.001). Wang and Shi reported 77% reduction in TNSS in children with a similar decrease in medication scores. In an elderly cohort, Bozek et al. evaluated subjects 65–75 years old with moderate or severe intermittent AR, comparing 3 years of grass SCIT to placebo and finding a 41% decrease in combined symptom and medication scores versus baseline (p = 0.004).
Recent evidence demonstrates SCIT benefit for HDM and grass allergens., , , , , Kim et al. demonstrated through network meta‐analysis that efficacy of SCIT for HDM was greater than SLIT drops or tablets.
Recent studies support the safety of SCIT; however, the rate of SCIT‐associated hypersensitivity reactions has shown a wide range. In the study by Arroabarren et al., systemic adverse effects were noted in 2.5% of patients overall, while Scadding et al. reported hypersensitivity events (mostly mild) in 47.2% of subjects with grade 3 systemic reactions in 5.5%.
Values and preferences. While the recommendation for AIT is strong with high certainty evidence, given the potential for harm associated with potentially life‐threatening anaphylaxis (with very rare SCIT associated fatality), and the burden associated with receiving SCIT, patient preference is important. Comparatively, the potential for harm and burden associated with medications is lower; the potential for benefit is also lower, with no potential for disease‐modifying immunomodulation. Some patients may prefer safety and a reduced risk of therapy‐associated anaphylaxis, despite reduced therapeutic efficacy. Patient motivation and choice are important considerations in AR treatment.
Summary. ICAR‐Allergic Rhinitis 2018 recommended SCIT for AR with an Aggregate Grade of Evidence “A.” Recently, evidence has continued to accrue in support of the therapeutic efficacy of SCIT in properly selected patients with AR, across age ranges and with selected standardized allergens. SCIT carries a strong recommendation and high certainty of evidence. The data concerning safety support a favorable potential for benefit with SCIT in patients with AR compared with the potential for harm or burden, though patients started and continued on SCIT must be counseled on the risk of anaphylaxis and potential fatality and presented treatment alternatives that may be safer though less efficacious. It should be noted that while SCIT remains the predominant method for AIT administration in the US, in the past two decades SLIT became the dominant approach for AIT in several European countries; recommendations for SLIT in Europe include tablet formulations and sublingual drops. Additional studies are required to substantiate the long‐term effectiveness of SCIT for AR, including its potential for reducing risk for future development of asthma and sensitization to novel antigens in monosensitized patients treated with SCIT, and the safety and efficacy of multi‐allergen SCIT.
Conventional subcutaneous immunotherapy
Aggregate grade of evidence: A (Level 1: 2 studies, level 2: 46 studies, level 3: 29 studies; Table XI.D.5.a)
Benefit: SCIT reduces symptom and medication use, as demonstrated in multiple high‐quality studies.
Harm: Risks of SCIT include frequent local reactions and rare systemic reactions, which may be severe and potentially fatal if not managed appropriately. This risk must be discussed with patients prior to initiation of therapy. See Table II.C.
Cost: SCIT is cost‐effective, with some studies demonstrating value that dominates the alternative strategy with improved health outcomes at lower cost. Direct and indirect costs of AIT vary based on the third‐party payer, the office/region, co‐payment responsibilities, and travel/opportunity related costs in being able to adhere to the frequency of office visits required.
Benefits‐harm assessment: For patients with symptoms lasting longer than a few weeks per year and for those who cannot obtain adequate relief with symptomatic treatment or who prefer an immunomodulation option, benefits of SCIT outweigh harm. The potential benefit of secondary disease‐modifying effects, especially in children and adolescents, should be considered.
Value judgments: A patient preference‐sensitive approach to therapy is needed. Comparatively, the potential for harm and burden associated with medications are significantly lower, although the potential for benefit is also lower (with no potential for any disease‐modifying effect or long‐term benefit) as medications do not induce immunomodulation. Logistical issues surrounding time commitment involved with AIT may be prohibitive for some patients. The strength of evidence for SCIT efficacy, along with the benefit relative to cost, would support coverage by third party payers.
Policy level: Strong recommendation for SCIT as a patient preference‐sensitive option for the treatment of AR.
Strong recommendation for SCIT over no therapy for the treatment of AR.
Option for SCIT over SLIT for the treatment of AR.
Intervention: SCIT is an appropriate treatment consideration for patients who have not obtained adequate relief with symptomatic therapy or who prefer this therapy as a primary management option, require prolonged weeks of treatment during the year, and/or wish to start treatment for the benefit of the potential secondary disease‐modifying effects of SCIT.
XI.D.5.b Rush subcutaneous immunotherapy for allergic rhinitis
Rush SCIT rapidly reaches the target therapeutic dose by administering incremental allergen doses over a much shorter period compared to conventional SCIT. Rush SCIT has successfully been implemented for venom immunotherapy. Evaluating rush SCIT for aeroallergen immunotherapy is difficult due to study heterogeneity with escalation protocols, target doses, premedication regimens, and extracts utilized. Furthermore, there remains a lack of standardization of what constitutes rush SCIT versus other immunotherapy protocols.
The main benefit of rush SCIT is the expedited build‐up phase, decreasing the time to reach maintenance dosing and office visits required. Patient convenience is improved, but evidence has not yet determined if the expedited process leads to more rapid clinical improvement. Potential disadvantages include increased risk of systemic reactions, higher staff/resource utilization, and decreased long‐term compliance with one study at a military medical center citing a decrease from 80% (conventional schedule) to 48% (rush schedule).
Efficacy and safety. Aeroallergen rush SCIT has demonstrated effectiveness for AR and asthma. The majority of double‐blind RCTs utilized single‐allergen extracts, primarily grass pollen., , , Other allergens investigated include ragweed, various tree pollens, Alternaria, cat, dog, and HDM., , , , , , , These studies report significant benefit over placebo in clinical outcomes (most commonly reported with combined symptom‐medication scores), SPT, and provocation challenges (Table XI.D.5.b).
Safety remains a limiting factor for aeroallergen rush SCIT due to a greater risk of systemic reactions, which range 15%–100% of patients without premedication for standardized extracts, depot preparations, and allergoids. This improves to 12%–38% when using routine premedication. Depigmented‐polymerized extracts have a significantly better safety profile with systemic reactions occurring in less than 2% of patients., , , Local reactions do not appear to predict systemic reactions and delayed systemic reactions are reported rarely with rush SCIT. Only one double‐blind RCT specifically evaluated safety and efficacy of rush versus conventional SCIT. In this small Der p 1 trial (n = 18), the efficacy was similar, but the rush SCIT group had significantly higher side effect scores without any severe systemic reactions. One retrospective observational study found an increase in systemic reactions on subsequent doses following initial rush SCIT, although additional studies are needed due to the variability in rush SCIT protocols.
Rush, ultra‐rush, and modified rush. Rush SCIT has traditionally been defined as achieving target therapeutic dose within 1–3 days, ; however, lack of universal standardization has led to variations of rush SCIT schedules. Modified rush designates accelerated SCIT protocols that reach a target dose within 3 days, then follow a more conventional build‐up to reach maintenance. Ultra‐rush classifies those that attain maintenance dose within several hours.
Due to the increased risk of systemic reactions with ultra‐rush, traditional extracts have not generally been used. Depigmented‐polymerized extracts, which are approved and commercially available in several regions of Europe, have been utilized via an ultra‐rush protocol with good efficacy in adults and children., , , Local reactions occurred in 21%–70.4% of patients, while systemic reactions ranged 2%–12.7%; all considered non‐severe (no grade 3 or 4 reactions).
Pre‐medication for rush SCIT. Limited studies specifically evaluated the effects of premedication on aeroallergen rush SCIT., Premedication regimens varied, including H1 and H2 histamine antagonists, systemic steroids, theophylline, and anti‐IgE monoclonal antibodies.
In one double‐blind, placebo‐controlled study of 22 children undergoing multiallergen rush SCIT over 1.5 days, a significant reduction in systemic reactions was observed in those receiving pretreatment with astemizole, ranitidine, and prednisone versus placebo (27% vs. 73%, respectively). A larger non‐randomized study involving children and adults undergoing rush SCIT to Dermatophagoides pteronyssinus evaluated the effects of premedication (methylprednisolone, ketotifen, and theophylline) and preventive measures (modifying dosing schedule after local reactions of >10 cm) on systemic reaction rates. The systemic reaction rate declined from 36% of patients with rush SCIT alone to 16% of patients that received premedication. This further declined to 7.3% when preventive measures were added to the premedication regimen.
Omalizumab has also been investigated as part of a 9‐week pretreatment regimen for ragweed rush SCIT., A five‐fold reduction in anaphylaxis was reported for the omalizumab‐premedicated group compared to the placebo‐premedicated group. Combination omalizumab and rush SCIT also led to lower symptom severity scores compared to either intervention alone.
In summary, rush SCIT has increasing availability globally with moderate evidence demonstrating improvement in clinical/immunologic outcomes versus placebo. The lack of SRMAs is notable and a key research need. There is also insufficient data directly comparing rush to conventional SCIT. Systemic reactions are a limiting factor but can be mitigated with premedication, use of depigmented‐polymerized extracts, and careful patient selection. Due to the heterogeneity of rush SCIT protocols, extract types, and premedication regimens, studying rush SCIT remains challenging.
Rush subcutaneous immunotherapy
Aggregate grade of evidence: B (Level 2: 12 studies, level 3: 4 studies, level 4: 4 studies; Table XI.D.5.b)
Benefit: Accelerates the time to reach therapeutic dosing which may improve compliance, lead to earlier clinical benefit, and be more convenient for the patient. Improvement of symptoms and decreased need for rescue medication.
Harm: Higher rates of local and systemic reactions with rush SCIT protocols compared to conventional and cluster SCIT. Inconvenience of visits to a medical facility to receive injections.
Cost: Direct costs may be similar or slightly less compared to conventional SCIT, which includes cost of extract preparation and injection visits. Indirect costs are improved due to the reduced number of appointment visits, which reduces work and school absenteeism.
Benefits‐harm assessment: Balance of benefit and harm.
Value judgments: Careful patient selection and shared decision making would reduce risks. Heterogeneity of protocols, extract types, and dosing across studies makes quantification of risk difficult.
Policy level: Option.
Intervention: Aeroallergen rush SCIT is an option for AR in appropriately selected patients that do not have adequate control of their symptoms with symptomatic therapies. If available at practice location, the use of depigmented‐polymerized allergen extracts for rush SCIT has a better safety profile compared with standard extracts.
XI.D.5.c Cluster subcutaneous immunotherapy for allergic rhinitis
Cluster SCIT is a method to shorten the build‐up phase for SCIT. Cluster schedules entail two or more injections during each visit on non‐consecutive days. Typically, target maintenance dosing can be reached in 4–8 weeks. This improves convenience for patients and may lead to more rapid symptom improvement, without a significant rise in systemic reactions when premedication is used., ,
Efficacy and safety. Like rush SCIT, cluster SCIT is difficult to study due to the heterogenicity of study protocols, extract types, target maintenance dosing, and premedication regimens. One SRMA evaluated the cluster SCIT efficacy for single allergen extracts and included eight RCTs comparing cluster SCIT to conventional SCIT or placebo. While no differences were found between cluster SCIT and placebo for symptom and medication scores, the high level of heterogenicity between the studies creates difficulty with interpretation. Several individual RCTs showed benefit in symptom, medication, and QOL benefit, consistent with other forms of SCIT., Two additional RCTs not included in the meta‐analysis show improvement in symptom/medication scores for cluster SCIT over placebo using depot or polymerized pollen extracts., Compared to conventional SCIT, cluster SCIT demonstrates similar efficacy for multiple extracts including pollens and HDM., , , , Cluster and rush SCIT have not been directly compared in RCTs (Table XI.D.5.c).
Two meta‐analyses of RCTs and observational studies have assessed cluster SCIT safety., When evaluating for local and systemic adverse reactions by number of patients, no difference was found with cluster versus conventional SCIT. The meta‐analysis by Jiang et al. showed a lower rate of grade 1 systemic and local adverse reactions if analysis is done per injection. Additional studies are needed to further explore these findings, as non‐randomized studies may favor inclusion of less vulnerable patient populations in the cluster cohort. High heterogeneity was noted which limits study conclusions.
A more recent RCT from China and large retrospective study of a multiple‐physician practice in the US with over 2.5 million injections given during the study period showed no difference in systemic reactions between cluster and conventional SCIT on a per‐patient basis, but the retrospective trial did show a slightly increased risk on a per‐injection basis., Minimal data is available on delayed reactions with cluster SCIT and no conclusions can be drawn.,
Factors that affect systemic reactions with cluster SCIT. Only one RCT specifically assessed the use of premedication in cluster SCIT with standardized pollen extracts. Use of loratadine prior to cluster dosing showed a decline in systemic reactions from 79% of patients to 33% for the study duration. While no life‐threatening systemic reactions occurred, there was a reduction in severity of systemic reactions with premedication. Other RCTs and observational studies had high variability in premedication regimens (e.g., oral antihistamines, oral systemic steroids, and leukotriene modifying agents) and most do not provide relevant information. Timing of the premedication has not been directly studied.
Other factors may affect the frequency and severity of systemic reactions during cluster SCIT including dosing frequency, extract formulation (standardized, depot, polymerized), number of injections administered during a cluster session, and number of clusters given to reach maintenance. Currently there is insufficient data to draw any conclusions, but this should be an area of emphasis for future research.
In summary, cluster SCIT has a similar safety profile as conventional SCIT and fewer systemic reactions than rush SCIT., , Importantly, the safety of cluster SCIT is comparable to standard regimens overall because the number of injections required for buildup can be less, not because the per injection risk is necessarily lower. Additionally, premedication use appears to be necessary to reach this comparable safety profile for cluster SCIT. Some practices may translate this as the need to observe patients during cluster sessions more closely and for longer periods. Efficacy remains difficult to investigate due to the significant study heterogeneity but does appear to be similar to conventional SCIT, which is strongly recommended to manage refractory AR. Standardization of cluster protocols through additional large‐scale RCTs should be a key area of research as there remain many understudied topics including dosing frequency, number of injections per visit, and the optimal duration of the build‐up phase.
Cluster subcutaneous immunotherapy
Aggregate grade of evidence: B (Level 1: 1 study, level 2: 12 studies, level 4: 2 studies; Table XI.D.5.c)
Benefit: Accelerates the time to reach therapeutic dosing which may improve compliance, lead to earlier clinical benefit, and be more convenient for the patient. Improvement of symptoms and decreased need for rescue medication. Similar safety profile compared to conventional SCIT.
Harm: Minimal harm with occasional, but mild, local adverse events, and rare systemic adverse events when premedication is used. Inconvenience of visits to a medical facility to receive injections.
Cost: Direct costs may be similar, slightly more, or slightly less compared to conventional SCIT, depending on how the practicing provider bills for the services. This includes cost of extract preparation, injection visits, and possibly rapid desensitization codes. Indirect costs are lower due to the reduced number of appointment visits, which reduces work and school absenteeism.
Benefits‐harm assessment: Preponderance of benefit over harm for patients that cannot achieve adequate relief with symptomatic management. Balance of benefit and harm compared to conventional SCIT but in slight favor of cluster SCIT due to convenience.
Value judgments: Careful patient selection and shared decision making would reduce risks. Heterogeneity of protocols, extract types, and dosing across studies makes risk quantification difficult.
Policy level: Option.
Intervention: Cluster SCIT can be safely implemented in clinical practice and offered to those patients eligible for SCIT that may prefer this protocol compared to conventional build‐up protocols due to convenience. Premedication should be strongly considered.
XI.D.6 Sublingual immunotherapy for allergic rhinitis
XI.D.6.a Sublingual immunotherapy for allergic rhinitis – general efficacy
While SCIT was first practiced over a century ago by Noon et al.,, the first double‐blind placebo‐controlled trial of SLIT dates from 1986 by Scadding and Brostoff. Over the next two decades several small trials were conducted. From 2006 onward, the “big trials” finally demonstrated the clinical efficacy and safety of SLIT. , Since then, a wealth of high‐quality SLIT trials have been conducted.
In ICAR‐Allergic Rhinitis 2018, the joint outcomes of the best quality trials gathered in over two dozen SRMAs on SLIT were presented. Since then, further trials have been conducted taking better care to define the exact dosing, focus on specific allergens, and separate the two different sublingual administration routes: aqueous or tablets. In this section, evidence for SLIT efficacy in general is reviewed, and subsections on aqueous and tablet SLIT follow. SRMAs were primarily analyzed. Several RCTs that have been published since ICAR‐Allergic Rhinitis 2018 were added as well. For the interpretation of the SMD of meta‐analyses, an effect size 0.3‐0.5 indicates a mild effect, 0.5‐0.8 indicates a moderate effect, and above 0.8 indicates a large effect the intervention on the disease.
Table XI.D.6.a.‐1 shows the cumulative recent evidence from SRMAs, primarily over the past 5 years. Additional notable studies prior to ICAR‐Allergic Rhinitis 2018 are also listed. Combined evidence previously published in ICAR‐Allergic Rhinitis 2018 is presented in Table XI.D.6.a.‐2 for an Aggregate Grade of Evidence of SLIT efficacy in general.
Efficacy in adults. The majority of the SRMAs show mild‐to‐moderate symptom and medication reduction in patients on SLIT compared to placebo. Symptom score improvements have also been demonstrated to be higher with longer treatment duration (greater than 12 months treatment, SMD = 0.70). All subjects, both those in the SLIT and in the placebo arms, had open access to rescue medication. As such, symptom reduction with SLIT comes on top of the symptom improvement obtained with rescue medication. SLIT efficacy in adults is judged to be grade A, with mild‐to‐moderate impact.
Efficacy in children. Studies on SLIT efficacy in children were previously limited by the heterogeneity of trials and the considerable risk of bias. In addition to the ICAR‐Allergic Rhinitis 2018 evidence demonstrating moderate efficacy for symptom relief in pollen and HDM liquid SLIT and grass pollen tablet SLIT, there is additional evidence for a moderate reduction in symptoms and medication scores in pediatric perennial AR., SLIT efficacy in children is judged to be grade A, with moderate impact.
Efficacy of SLIT over pharmacotherapy. For perennial AR, HDM SLIT tablets are more effective than antihistamines, LTRAs, and INCS. For seasonal AR, grass pollen and ragweed tablet SLIT are almost as effective as INCS and more effective than the other pharmacotherapies. An additional study showed that the 5‐grass tablet had the highest relative clinical impact on symptom score over all other pharmacotherapy treatments. SLIT efficacy over pharmacotherapy is judged to be grade B.
Efficacy of SLIT compared to SCIT. Several investigators have tried to compare the efficacy of SLIT against that of SCIT., , , , , Most meta‐analyses show superiority of SCIT over SLIT, but they are of low grade evidence as they are based on indirect comparisons. There are very few direct head‐to‐head randomized trials comparing both treatments. One recent head‐to‐head study was powered for the comparison against the placebo‐group, but not for SCIT versus SLIT. In children, SCIT seems more effective than SLIT, but the quality of evidence is low. SLIT efficacy compared to SCIT is judged to be grade B, with low grade evidence of SCIT superiority.
Short‐term preventative effects of SLIT. There is moderate grade evidence for a high impact of SLIT in patients with AR to prevent them from developing asthma, during three years of treatment and within the first 2 years off‐treatment. However, there is no evidence for primary prevention with SLIT, nor for long‐term secondary preventive effects. For the development of new sensitizations, there are a few systematic reviews. The most comprehensive meta‐analysis showed only a tendency for SLIT, and the effect did not withstand the sensitivity analysis, while another systematic review found only low‐grade evidence. Evidence for short‐term preventative effects of SLIT is judged to be grade B.
SLIT safety. Rare systemic and serious adverse events have been reported with SLIT. In general, meta‐analyses, including the most recent in 2019, found SLIT to be safer than SCIT. In the complete dataset of systemic reviews, there were seven reports of the use of epinephrine in the SLIT group. There was no administration of epinephrine in trials outside of the US. There were several reports of symptoms suggestive of anaphylaxis with the first grass pollen tablet, and three with the first HDM tablet; this supports the recommendation in the package insert for administration under the supervision of a physician with experience in the diagnosis and treatment of allergic diseases and observation in the office for at least 30 min following the initial dose. Starting SLIT in‐season seemed to be safe. Although there were two serious treatment related adverse events with co‐seasonal SLIT initiation, none needed epinephrine administration.
Grass pollen SLIT tablets were noted to be equally safe in AR patients with and without mild asthma. Dropout rates have been raised as a concern for trial safety, but there is no evidence of differences in drop‐out rates between SLIT and placebo groups. There have been a few case‐reports of EoE after a course of grass pollen SLIT tablets. Continuing SLIT during pregnancy did not increase the incidence of adverse outcomes during delivery nor alter the risk of developing atopic disease in the offspring. However, there is insufficient data to draw conclusions about safety and efficacy in pregnant women.
Evidence that SLIT is generally safe is judged to be grade A. Evidence that SLIT is safer than SCIT is judged to be grade B.
Cost‐effectiveness of SLIT. The meta‐analysis comparing the efficacy and cost‐savings of the 5‐grass SLIT tablet versus the Timothy grass tablet has several flaws, making direct comparison of outcomes not possible., The 5‐grass tablet was associated with cost savings against year‐round SCIT, seasonal SCIT, and the Timothy grass tablet during the first year of therapy, which persisted during the second and third year of treatment. The higher costs for SCIT were due to elevated indirect costs from missing working hours and transportation costs related to in‐office SCIT administration. The higher costs for the Timothy grass tablet are due to the year‐round dosing versus the pre‐ and co‐seasonal 6‐month total dosing of the 5‐grass tablet.
After a previous positive UK meta‐analysis on costs, a more recent one also concluded that the body of evidence suggests that SLIT and SCIT could be considered cost‐effective using the National Institute for Health and Clinical Excellence cost‐effectiveness threshold of £20,000 per QALY.
Additional data not included in systematic reviews. Investigators showed after a 3‐year course of Japanese cedar pollen tablet SLIT, there was a reduction in symptom‐medication score of 45.3% one year post‐treatment and 34.0% two years post‐treatment (p < 0.001). A post‐hoc analysis demonstrated symptom and medication reduction with the birch SLIT tablet during the oak pollen season in adults with allergic rhinoconjunctivitis.
There have been several studies on immunologic changes and biomarkers for AIT. There seems to be a differential induction of allergen‐specific antibody responses after grass pollen AIT, with SCIT primarily inducing sIgG4 and SLIT inducing sIgA.
Sublingual immunotherapy – general
Aggregate grade of evidence for SLIT overall: A (Level 1: 17 studies, level 2: 12 studies, level 4: 1 study; Tables XI.D.6.a.‐1 and XI.D.6.a.‐2)
Due to heterogeneity of SLIT study reporting, it is difficult to separate out overall versus aqueous SLIT versus tablet SLIT.
Benefit: SLIT improves patient symptom scores, even as add‐on treatment with rescue medication. SLIT reduces medication use. The effect of SLIT lasts for at least 2 years after a 3‐year course of therapy. In AR patients, there is some evidence that SLIT reduces the frequency of onset of asthma and the development of new sensitizations up to 2 years after treatment termination. Benefit is generally higher than with single‐drug pharmacotherapy; however, it may be less than with SCIT (low quality evidence).
Harm: Minimal harm with very frequent, but mild local adverse events, and very rare systemic adverse events. SLIT seems to be safer than SCIT. See Table II.C.
Cost: Intermediate. SLIT becomes cost‐effective compared to pharmacotherapy after several years of administration. Total costs seem to be lower than with SCIT.
Benefits‐harm assessment: Benefit of treatment over placebo is small but tangible and occurs in addition to improvement with medication. There is a lasting effect at least 2 years off treatment. Minimal harm with SLIT, greater risk for SCIT.
Value judgments: SLIT improved patient symptoms with low risk for adverse events.
Policy level: Strong recommendation for use of SLIT grass pollen tablet, ragweed tablet, HDM tablet, and tree pollen aqueous solution. Recommendation for SLIT for Alternaria allergy. Option for SLIT for animal allergy. Recommendation for dual‐therapy SLIT in bi‐allergic patients.
Intervention: Recommend tablet or aqueous SLIT in patients (adults and children) with seasonal and/or perennial AR who wish to reduce their symptoms and medication use, as well as possibly reduce the propensity to develop asthma or new allergen sensitizations.
XI.D.6.b Sublingual immunotherapy for allergic rhinitis – tablets
SLIT tablets have been studied for HDM, as well as short ragweed, grass, birch, and Japanese cedar pollens. US FDA‐approved tablets encompass Timothy grass, short ragweed, a 5‐grass combination, and HDM allergens. Administration schedules and age ranges of approved use vary based on the specific tablet prescribed.
Since 2017, numerous SRMAs were identified for SLIT tablets (Table XI.D.6.a.‐1). Eight reported both aqueous and tablet SLIT,, , , , , , , six presented aqueous and tablet SLIT separately,, , , , , and nine reported on tablet SLIT alone., , , , , , , , All studies reported outcomes for HDM, grass pollen, and/or ragweed pollen. There were no SRMAs for birch or Japanese cedar pollen tablets. Studies focusing only on SLIT tablets demonstrated safety and efficacy for HDM, grass pollen, and ragweed pollen. Improvement in symptom scores, medication scores, and QOL metrics are evident with minimal adverse reactions.
Meltzer et al. published a meta‐analysis evaluating the efficacy of pharmacotherapies and SLIT tablets versus placebo on nasal symptoms in seasonal and perennial AR. Active treatments significantly improved nasal symptoms versus placebo. Trial heterogeneity and publication bias limited comparison of treatment classes. Of note, comparison groups were not equally matched. SLIT is generally used for pharmacotherapy‐recalcitrant patients, resulting in a more severe group using SLIT. Additionally, patients often use supplement SLIT with rescue medications, confounding individual comparison of medical treatments.
Analysis of pediatric studies demonstrated that HDM SLIT reduced symptoms and medication scores versus placebo, with a slight increase in adverse reactions. A similar study of HDM SLIT tablets in adults showed improvement in symptom scores and QOL compared to placebo. Nelson et al. published a systematic review of 12 double‐blind RCTs for HDM SLIT tablets and concluded that efficacy was established with all 12 studies, with statistically significant symptom score improvement.
SRMAs including SLIT tablet and aqueous preparations also reported favorable outcomes for symptoms scores, medications, and QOL. Findings for aqueous SLIT are discussed in the next section.
Examples of dose–response studies for grass pollen and HDM tablets include those by Didier et al., Horak et al., Malling et al., and Bergmann et al. Dose‐finding studies aim to identify effective therapeutic doses while minimizing adverse effects.
The efficacy findings from 2017 to 2022 SLIT tablet studies are consistent with the findings reported in the first ICAR‐Allergic Rhinitis 2018. The majority of the SRMAs show mild‐to‐moderate efficacy of SLIT tablets over placebo. There is strong evidence that grass pollen SLIT tablets and HDM tablets reduce symptoms of AR in children.
Rare systemic and serious adverse events have been reported with SLIT, but in general, meta‐analyses found SLIT to be safer than SCIT. One study found seven of 2259 patients on grass pollen SLIT tablets were given epinephrine for treatment related adverse effects. Presence of mild asthma did not affect adverse reactions for grass pollen SLIT tablets. Starting SLIT in‐season is generally deemed to be safe; although there were two serious treatment related adverse events with co‐seasonal SLIT initiation, none needed epinephrine.
SLIT tablet options are limited compared to off‐label aqueous SLIT extracts. Since HDM is the only tablet approved for patients with non‐seasonal AR, data regarding polysensitized patients is important. Kim et al. reported a meta‐analysis of HDM AIT in mono‐ or polysensitized patients. Nine studies, five SLIT and four SCIT, revealed no differences for nasal symptom score, medication use, and QOL scores between mono‐ and polysensitized patients.
The use of multiple concurrent SLIT tablets (Timothy grass and short ragweed) has been studied by Maloney et al. Simultaneous co‐administration within 5 min did not result in severe swelling, systemic allergic reactions, asthma attacks, or reactions requiring epinephrine. Gotoh et al. reported the first study of dual administration of SLIT tablets for perennial and seasonal AR using HDM and Japanese cedar pollen tablets administered alone and as dual therapy. The percentage of subjects with adverse events and reactions was similar between the two groups and between the two periods of monotherapy and dual therapy. There were no serious events and immunologic marker responses were not altered by co‐administration of tablets. These studies provide support for the contention that co‐administration of tablets does not adversely affect the safety or efficacy of tablet SLIT.
Sublingual immunotherapy – tablets
Aggregate grade of evidence: A (Level 1: 11 studies, level 2: 4 studies; Table XI.D.6.a.‐1)
Benefit: Improvement of symptoms, rescue medication, and QOL.
Harm: Local reaction at oral administration site and low risk of anaphylaxis.
Cost: Intermediate. More expensive than standard pharmacotherapy, but persistent benefit may result in cost‐saving in the long‐term.
Benefits‐harm assessment: Benefit outweighs harm.
Value judgments: Useful for patients with severe or refractory symptoms of AR.
Policy level: Strong recommendation.
Intervention: SLIT tablets are recommended for patients with severe or refractory AR. Epinephrine auto‐injector is recommended in the FDA labeling for approved tablets due to the rare but serious risk of anaphylaxis. Tablets for select antigens are available in various countries.
XI.D.6.c Sublingual immunotherapy for allergic rhinitis – aqueous
SLIT can be administered via tablets or aqueous drops. Like sublingual tablets, this offers easy at‐home administration with a similar safety profile. While some aqueous extracts are approved for use in Europe, aqueous SLIT products are not FDA approved in the US; many providers currently use subcutaneous allergen extracts off‐label for sublingual desensitization.
Aqueous SLIT has a mild to moderate effect on improving patient symptoms and reducing medication usage., , , , Although it is difficult to compare studies due to methodologic or extract differences, improvement in symptom/medication outcomes is prevalent across most studies. The FDA has approved SLIT tablets for HDM, grass pollen, and ragweed pollen allergy – these antigens have standardized dosages; however, many allergens cannot be treated with the limited number of available tablets. Additionally, there is currently no head‐to‐head data comparing aqueous SLIT to tablet SLIT. Some meta‐analyses have undertaken subgroup analysis between aqueous SLIT and tablet SLIT and found both to be effective without clear superiority of one over the other.,
Aqueous SLIT seems to be efficacious for adults and children. An earlier meta‐analysis noted no significant improvement in symptom score for children treated with SLIT. However, most of the included studies had a low monthly allergen dose that has been shown to be ineffective in subsequent meta‐analyses., , , Lack of dosing standardization across multiple studies in different countries using extracts from various manufacturers has led to heterogeneity in aqueous SLIT data (Table XI.D.6.a.‐1).
Leatherman et al. provided recommendations for effective doses of aqueous SLIT based on micrograms per day administered in RCTs that demonstrated efficacy. Published and recommended dosing ranges for common allergens are shown in Table XI.D.6.c. However, many allergens such as cat, dog, mold/fungi, and cockroach did not have enough data to provide specific recommendations. There is expert opinion that for allergens without current effective ranges, daily SLIT dose equal to the monthly SCIT dose may be in the effective dose range; further studies should validate this.
While single allergen SLIT has been shown to be effective in both monosensitized and polysenstized patients,, , there is equivocal evidence on added benefit of multi‐allergen immunotherapy in the polyallergic patient. This is pertinent to tablet SLIT as well because of the limited number of antigens available as tablets. Most RCTs demonstrate significant benefit over placebo with multi‐allergen SLIT but have not compared monotherapy to polytherapy. One open‐label, controlled trial in patients with grass and birch sensitization randomized patients to treatment with grass pollen, birch pollen, grass and birch pollen, or placebo. Monotherapy with grass or birch showed clinically significant improvement and nasal eosinophil reduction versus baseline, but polytherapy with grass and birch showed improvement over the monotherapy groups. Alternatively, comparing Timothy extract alone or with nine additional pollen extracts against a placebo group demonstrated secondary outcome efficacy (e.g., SPT reactivity, nasal challenge, sIgE) in favor of the mono‐Timothy group, though neither treatment group showed symptom/medication improvement over placebo, as the grass pollen season was too mild. Another study randomized polysensitized patients to single, pauci, or multi‐allergen SLIT. Symptom scores significantly improved in all groups, yet there was no significant efficacy difference shown for single versus pauci‐ versus multi‐allergen SLIT. Of note, this study had only 16 patients total and follow up was 9 months. Further study is needed to determine the role of monotherapy or polytherapy SLIT on specific seasonal symptoms and QOL measures over several seasons.
Safety of aqueous SLIT is comparable to its SCIT and tablet SLIT counterparts. There is no standardized mechanism of reporting safety outcomes across RCTs but reported adverse outcomes have been modest. Local reactions range 0.2%–97%. Life‐threatening reactions or anaphylaxis were largely absent from most meta‐analyses, except for one meta‐analysis of SCIT and SLIT for grass allergens which found one case of anaphylaxis in the SLIT group. Notably the SCIT group had 12 cases of anaphylaxis and the placebo group had two cases, suggesting that the risk of anaphylaxis in SLIT is significantly lower than in SCIT. There were no cases of anaphylaxis or life‐threatening events in children (Table II.C).
Sublingual immunotherapy – aqueous
Aggregate grade of evidence: B (Level 1: 7 studies, level 2: 5 studies, level 4: 1 study; Table XI.D.6.a.‐1)
Benefit: Aqueous SLIT improves patient symptom scores and decreases rescue medication use. There is some indication of less benefit from aqueous versus tablet SLIT, but the lack of standardized dosing across multiple trials does not allow for adequate comparison.
Harm: Common mild to moderate local adverse events. Very rare cases of systemic adverse events. No reported cases of life‐threatening reactions. See Table II.C.
Cost: Intermediate. More expensive than standard pharmacotherapy, but there are indications of lasting benefit and cost‐saving in the long‐term.
Benefits‐harm assessment: Appreciable benefit in patient symptoms and minimal harm.
Value judgments: Aqueous SLIT improves patient symptoms and rescue medication usage with minimal risk of serious adverse events but common local mild adverse events. Single allergen therapy has been extensively tested. Multiallergen AIT requires future studies to validate its use.
Policy level: Recommendation.
Intervention: High‐dose aqueous SLIT is recommended for those patients who wish to reduce their symptoms and rescue medication use.
XI.D.7 Subcutaneous versus sublingual allergen immunotherapy for allergic rhinitis – comparison table
Table XI.D.6.d.
XI.D.7 Epicutaneous/transcutaneous immunotherapy
Epicutaneous or transcutaneous immunotherapy is a non‐invasive form of AIT that consists of the application of allergens to the skin without involving injections. Allergen is applied through patches kept on the skin for several hours. The epidermal barrier is usually impermeable to molecules larger than 500 Da. In order to increase/improve antigen delivery to the immune cells of the epidermis and dermis, different techniques have been used including adhesive tape stripping, abrasion of the skin, and sweat accumulation through patch application., Newly engineered techniques are being evaluated for the delivery of powder‐based AIT into the epidermis with minimal skin reaction, including microneedle arrays and laser‐mediated microporation; these have primarily been studied in food allergy (peanut). To date, four clinical trials of aeroallergen epicutaneous AIT have been published (three of them by the same group of investigators) reporting the efficacy of grass pollen extract coated patches in varying doses, numbers of weekly patches, and duration in contact with the skin (Table XI.D.7).
The first pilot study of aeroallergen epicutaneous AIT was a monocentric, placebo‐controlled, double‐blind trial of 37 adults with positive SPT and nasal challenge tests to grass pollen randomized to treatment with allergen or placebo patches. Symptom scores after NPT scores showed notable reduction in the grass‐treated patients, but the difference was not statistically significant. Grass‐treated patients had improved subjective symptom scores, both after the pollen seasons of 2006 (p = 0.02) and 2007 (p = 0.005). Eczema at application sites was significantly higher in the treatment arm; there were no serious adverse events.
A second monocentric double‐blind study randomized 15 children to grass epicutaneous AIT versus placebo. There were no significant differences in skin test wheal size between groups before and after treatment. Both groups had an increase in symptoms, but the treatment group had lower rhinorrhea, nasal obstruction, dyspnea, and ocular tearing. The treatment group had a significant reduction in antihistamine use (p = 0.019). There were no systemic or local reactions.
A third monocentric trial randomized 132 adults to placebo, low, medium, or high dose grass extract patches. Significant improvement in rhinoconjunctivitis symptoms was found only in the high dose treated patients one year later (p = 0.017). There were no differences in conjunctival provocation test, SPT, or rescue medication use. Local reactions were more frequent in high dose treated patients and decreased with subsequent applications. Systemic reactions treated with intravenous antihistamines and corticosteroids occurred in 8.3% of patients.
A fourth monocentric double‐blind RCT randomized 98 adults to grass patches or placebo. There was a 48% improvement in seasonal symptom scores in the first year (placebo 10%) but no significant differences in combined treatment and medication scores. CPT scores improved after the first year in the active treatment group. Allergen‐specific IgG4 was significantly increased in the active treatment group only during the first pollen season; sIgE did not show any variation. Local adverse events occurred in 18%; eight systemic reactions led to study exclusion.
A systematic review of the efficacy and safety of epicutaneous AIT for food and pollen allergy; the four clinical trials above on grass allergy were included. Given the lack of original data on means and standard deviation of symptom scores, a meta‐analysis on the efficacy was not possible and the authors concluded that the effectiveness of epicutaneous AIT for grass pollen allergy is unclear. Subgroup analyses concluded that epicutaneous grass pollen AIT significantly increased the risk of local (relative risk [RR] 2.29; 95% 1.05–4.96) and systemic (RR 4.65; 95% CI 1.10–19.64) adverse reactions. It is interesting to note that the cited clinical trials were conducted more than 10 years ago suggesting little progress in this area for AR.
Epicutaneous/transcutaneous immunotherapy
Aggregate grade of evidence: B (Level 2: 5 studies; Table XI.D.7)
Benefit: Epicutaneous AIT to grass pollen resulted in limited and variable improvement in symptoms, medication use, and allergen provocation tests in patients with AR or conjunctivitis.
Harm: Epicutaneous AIT resulted in systemic and local reactions, with a RR of 4.65 and 2.29, respectively. Systemic reactions occurred in up to 14.6% of patients receiving grass transcutaneous AIT.
Cost: Unknown.
Benefits‐harm assessment: There is limited and inconsistent data on benefit of the treatment, while there is a concerning rate of adverse effects. Three out of 4 studies on this topic were published by the same investigators from 2009 to 2015.
Value judgments: Epicutaneous AIT could offer a potential alternative to SCIT and SLIT, but further research is needed.
Policy level: Recommendation against.
Intervention: While epicutaneous AIT may potentially have a future clinical application in the treatment of AR, at this juncture there are limited studies that show variable and limited effectiveness, and a significant rate of adverse reactions. Given the above and the availability of alternative treatments, epicutaneous AIT is not recommended at this time.
XI.D.8 Intralymphatic immunotherapy
Notwithstanding the long‐term benefits to AR patients by AIT, the recommended treatment duration of 3–5 years is time consuming, expensive, and demands strict adherence from patients. SCIT requires monthly maintenance injections, and SLIT requires daily oral intake. Intralymphatic immunotherapy (ILIT) was introduced to address these concerns. ILIT involves the application of low dose allergens via ultrasound‐guided injection into the lymph nodes, mainly the inguinal nodes. The treatment protocol of ILIT has a shorter duration, usually comprising three injections over a period of 8 weeks. The cumulative dose for ILIT is dramatically lower than that used for conventional AIT and there are significantly fewer adverse events.
Thus far, two systematic reviews are available (Table XI.D.8). The first systematic review included 11 trials and two cohorts in a qualitative and quantitative analyses of 483 participants with the average age of 33 years. The second systematic review involved quantitative analysis of 11 trials with 452 participants aged 15 years and above. The outcomes assessed in both reviews include the combined symptom‐medication score, symptom score, VAS, medication score, overall improvement score, medication reduction, QOL, sIgE level, sIgG level, and adverse events. The overall level of evidence of the included trials ranged from very low to moderate.
ILIT was administered by injecting aluminum hydroxide‐adsorbed antigen vaccine into inguinal lymph nodes for all patients under ultrasound guidance., , , , , , , , , , In one pilot study, the cervical lymph nodes were used as the injected site. Single allergen was evaluated in seven trials,, , , , , , two different allergens assessed simultaneously in four trials,, , , and one trial assessed two different allergens individually. Grass pollen extract was injected in eight trials,, , , , , , , cedar pollen extract in two trials,, birch pollen extract in four trials,, , , and cat dander allergen extract (MAT‐Fel d 1) in one trial. Placebo injections were used in all but two trials, which used SCIT as control groups.
All trials performed three injections at 4‐week intervals except for one trial which used a 2‐week interval. Short‐term relief of the combined symptoms and medication score was achieved in the 4‐week but not for the 2‐week interval. Increased sIgG4 levels have been associated with the effectiveness of AIT. While a short‐term increase of sIgG4 level has been documented following ILIT, there has not been any medium‐term or long‐term effects. The reduction of sIgE in the short, medium, and long‐term is frequently reported with SCIT; however, this has been notably absent with ILIT.,
ILIT was shown to confer short‐term relief of AR symptoms in one review. Despite being safe and well tolerated, both meta‐analyses determined that the efficacy of ILIT for long‐term relief of AR symptoms was inconclusive., The safety of ILIT and reported adverse events were investigated in all eleven trials. While more local reactions were noted from ILIT compared to placebo, systemic adverse events were similar in both the ILIT and placebo groups. The major advantage in favor of ILIT compared to SCIT is fewer adverse effects of local and systemic reactions compared to SCIT. At present, there is no trial comparing ILIT versus SLIT with regard to adverse effects. Overall, two anaphylactic events have been reported for ILIT but no deaths. The anaphylaxis following ILIT transpired following the first injection in one patient and following the second injection in another patient, both patients receiving non‐standardized aqueous allergen extract compared to aluminum‐based extract used in most trials.
ILIT trials varied as to the dose of allergen administered and the interval between injections. Increased efficacy was associated with a 4‐week (vs. 2‐week) interval, and future trials should use and establish a standard treatment regimen. Another shortcoming is a lack of standardization of clinical endpoints. The use of standardized assessment such as combined symptoms‐medication score could better reflect the actual potential of ILIT. The high heterogeneity among the trials could be due, in part, to the use of different allergens. The immunogenicity effect may differ between allergens when administered as a single or multiple allergens. One trial used both grass and birch allergen to treat polysensitized patients and found elevated sIgE and sIgG4 levels for grass pollen but not for birch pollen. ILIT could be beneficial as an alternative to other forms of AIT due to its shorter treatment period, reduced number of injections, and fewer adverse events; however, the long‐term efficacy has to be supported by more studies prior to its incorporation into clinical practice.
Intralymphatic immunotherapy
Aggregate grade of evidence: A (Level 1: 2 studies, level 2: 11 studies, level 4: 3 studies; Table XI.D.8)
Benefit: Shorter treatment period, decreased number of injections, smaller amount of allergen, lower risk of adverse events versus SCIT.
Harm: Local reaction at injection site and risk of anaphylaxis.
Cost: Cost savings due to shorter treatment duration and fewer injections. Additional cost for training required.
Benefits‐harm assessment: Benefit outweighs harm.
Value judgments: Apparent short‐term favorable effect, but long‐term effect is lacking.
Policy level: Option.
Intervention: More studies are essential to establish the long‐term effects of ILIT.
XI.D.9 Other forms of immunotherapy – oral, nasal, inhaled
Oral, nasal, and inhaled (intra‐bronchial) routes of AIT administration have been investigated for AR to bypass some challenges of SCIT, including resource utilization and discomfort. Today, SCIT remains commonly used while these alternative techniques have been largely supplanted by SLIT and are relegated to primarily historical significance.
Oral, nasal, and inhaled AIT involve the topical absorption of allergen extracts via the oral cavity/gastrointestinal tract, nasal cavity, or bronchial mucosa, respectively. RCTs have evaluated oral/gastrointestinal AIT for the treatment of birch, cat, and ragweed allergy without a significant decline in nasal symptoms, improvement in provocation testing, or reduction in medication utilization. Moreover, oral/gastrointestinal allergen administration requires extract concentrations approaching 200‐times greater than SCIT, and is associated with adverse gastrointestinal side effects., In contrast to AR, the efficacy of oral/gastrointestinal immunotherapy has been demonstrated for the treatment of food hypersensitivity (Table XI.D.9).
Oral mucosal immunotherapy (OMIT) is an alternative form of AIT distinct from both SLIT and oral/gastrointestinal administration. OMIT utilizes a glycerin‐based toothpaste vehicle to introduce antigen to high‐density antigen processing oral Langerhans cells in the oral vestibular and buccal mucosa. Theoretical benefits include induction of immune tolerance using lower antigen concentrations, decreased local side effects, and higher adherence versus SLIT. Currently, OMIT has been investigated in a single pilot study versus SLIT with findings of clinically significant improvements in disease specific QOL measures and a significant rise in specific IgG4 over the first 6 months of treatment. No adverse events were reported, and there were no significant differences between outcome measures for both treatment arms. Further study is needed to define the role of OMIT in the treatment of AR.
Local nasal AIT has been established as an effective and well‐tolerated approach for the treatment of pollen and HDM hypersensitivity in adults., However, high rates of local adverse reactions have been identified in pediatric patients and may limit patient compliance, with one study finding that 43.9% of children abandoned this treatment option within the first year of therapy. No high quality studies of inhaled/intra‐bronchial AIT exist for the treatment of AR, with current studies limited to the treatment of allergic asthma.
Current evidence suggests limited utility of oral/gastrointestinal, nasal, and inhaled AIT in the treatment of AR due to limited efficacy, increased adverse events, and poor treatment compliance. However, OMIT represents a possible alternative to SCIT/SLIT warranting further study.
Other forms of immunotherapy – oral, nasal, inhaled
Aggregate grade of evidence: B (Level 2: 3 studies, level 3: 3 studies; Table XI.D.9)
Benefit: OMIT and local nasal AIT represent alternative AIT administration methods for individuals who are unable to comply with SCIT or SLIT treatment regimens. Oral AIT has not consistently shown benefit for the treatment of AR. Inhaled AIT has not demonstrated benefit for the treatment of AR.
Harm: OMIT may be associated with increased cost to patients due to non‐standard preparation methods. Oral AIT is associated with increased risk of gastrointestinal side effects and treatment noncompliance and has not consistently demonstrated benefit for AR symptoms. Inhaled AIT has not shown benefit for AR.
Cost: Moderate.
Benefits‐harm assessment: OMIT equivocal to SLIT; possible benefit for local nasal AIT with low risk for harm; balance of harm over benefit for oral AIT and inhaled AIT.
Value judgments: While a single study has demonstrated OMIT to be non‐inferior to SLIT in objective and subjective patient outcomes, further study of OMIT is needed to substantiate these results prior to widespread clinical use. Local nasal AIT may have utility for the treatment of AR not associated with additional atopic symptoms; however, further study is needed to demonstrate clinical efficacy. Oral AIT and inhaled IT do not appear to be beneficial for the treatment of AR.
Policy level: Option for OMIT as an alternative to SCIT or SLIT, pending additional studies. Local nasal AIT has not shown benefit as alternative to SCIT or SLIT at present, further study may find benefit for patients with AR without additional atopic symptoms. Recommend against oral AIT. Recommend against inhaled AIT.
Intervention: OMIT may be presented as an option for the administration of AIT in patients unable to tolerate SCIT or SLIT; further study is encouraged. Local nasal AIT has not yet shown clinical efficacy for the treatment of AR relative to conventional forms of immunotherapy; further study may yet find benefit. Oral AIT and inhaled AIT do not appear to be effective for the treatment of AR.
XI.D.10 Combination therapy – monoclonal antibody (biologic) therapy and subcutaneous immunotherapy
There are currently six biologics/monoclonal antibodies approved by the US FDA for the treatment of asthma and allergic diseases: omalizumab (anti‐IgE), mepolizumab (anti‐IL5), reslizumab (anti‐IL5), benralizumab (anti‐IL5Rα), dupilumab (anti‐IL4Rα), and tezepelumab (anti‐TSLP). Omalizumab, mepolizumab, and dupilumab are also approved for the treatment of CRSwNP, and benralizumab is pending approval for this indication.
None of the six biologics are approved as an adjunctive therapy to AIT. However, there have been several studies examining the concomitant use of AIT with omalizumab. The only other biologic to be studied in this manner is dupilumab, and only in a single study. In a Phase 2a, multicenter, double‐blind, placebo‐controlled, parallel‐group study conducted in 103 adults with grass pollen‐induced seasonal AR, patients were randomized 1:1:1:1 to SCIT, dupilumab (300 mg every 2 weeks), SCIT plus dupilumab, or placebo. SCIT was administered using an 8‐week cluster protocol (escalating doses of 1–3 SCIT injections weekly to approximately 20 μg Phl p 5) followed by 8 weeks of maintenance injections. The investigators found that 16 weeks of SCIT plus dupilumab may improve SCIT tolerability but did not incrementally reduce post‐allergen challenge nasal symptoms compared with SCIT alone (Table XI.D.10).
The remainder of this section will focus on the efficacy and safety of the combination of omalizumab plus AIT. Prior to many of the studies examining the combination, omalizumab as a standalone therapy was shown to be effective for the treatment of seasonal and perennial AR.,
The first clinical trial that investigated the effects of omalizumab plus AIT was conducted by Kuehr et al. In this double‐blind placebo‐controlled multisite RCT, 221 patients aged 6–17 years with moderate to severe AR and sensitization to birch and grass pollen were randomized to one of four different treatments: SCIT (either grass or birch pollen), starting at least 14 weeks before the local birch pollen season and after the 12‐week SCIT titration phase, and either omalizumab or placebo therapy was added. This combination therapy with SCIT and omalizumab or placebo lasted 24 weeks. Combination therapy with omalizumab reduced symptom load over the two pollen seasons (birch and grass) by 48% over SCIT alone (p < 0.001). Combination therapy also reduced the need for rescue medication, days with allergy symptoms, and symptom severity compared with SCIT alone (p < 0.001). A safety analyses of these data indicated that redness and swelling at the SCIT injection sites appeared significantly more often in the placebo group versus the omalizumab group (p < 0.05) suggesting a positive effect of omalizumab on local reactions induced by SCIT. Subgroup analysis of grass allergic patients confirmed the primary study results.
Because omalizumab reduces free IgE resulting in a decrease in the high affinity IgE receptor, FcεR1, pretreatment with omalizumab should allow for safer and more effective AIT., Casale et al. conducted a three‐center, double‐blind placebo‐controlled RCT in patients with ragweed‐induced seasonal AR to examine whether omalizumab given 9 weeks before rush SCIT (1‐day rush, maximal dose 1.2–4.0 μg Amb a 1), followed by 12 weeks of dual omalizumab and SCIT, is safer and more effective than AIT alone. Patients receiving both omalizumab and SCIT showed a significant improvement in severity scores during the ragweed season compared with those receiving SCIT alone (0.69 vs. 0.86; p = 0.044). Omalizumab pretreatment resulted in fewer adverse events during rush SCIT, and a post hoc analysis found a five‐fold decrease in risk of anaphylaxis caused by ragweed SCIT (SCIT alone 25.6% vs. SCIT with omalizumab 5.6%; p = 0.03). The combination also resulted in prolonged inhibition of allergen‐IgE binding compared with either treatment alone, events that might contribute to enhanced efficacy.
Kopp et al. performed a double‐blind, placebo‐controlled, multicenter RCT of omalizumab versus placebo in combination with depigmented SCIT during the grass pollen season in patients with seasonal AR and co‐morbid seasonal allergic asthma. Omalizumab or placebo was started 2 weeks before SCIT, and the entire treatment lasted 18 weeks. Combination therapy reduced daily symptom load by 39% (p < 0.05), improved control of rhinoconjunctivitis and asthma, and improved QOL, but no significant improvements in SCIT safety were observed.,
Massanari et al. conducted a study to evaluate the efficacy of omalizumab in improving the safety and tolerability of SCIT given to a high‐risk population of adults with persistent asthma uncontrolled on inhaled corticosteroids. This multicenter, double‐blind, parallel‐group study randomized patients to treatment with omalizumab or placebo for 8 weeks, after which they received SCIT to at least one of three perennial aeroallergens (cat, dog, HDM) according to a 4‐week, 18‐injection cluster regimen, followed by 7 weeks of maintenance therapy. Use of omalizumab was associated with 50% fewer systemic allergic reactions to AIT and enabled more patients to achieve the target immunotherapy maintenance dose.
Combination biologic therapy and subcutaneous immunotherapy
Aggregate grade of evidence: B (Level 2: 5 studies; Table XI.D.10)
Benefit: Improved safety of accelerated cluster and rush SCIT protocols, with decreased symptom and rescue medication scores among a carefully selected population.
Harm: Financial cost and low risk of anaphylactic reactions to omalizumab.
Cost: Moderate to high.
Benefits‐harm assessment: Preponderance of benefit over harm.
Value judgments: Combination therapy increases the safety of SCIT, with decreased systemic reactions following cluster and rush protocols. Associated treatment costs must be considered. While two high‐quality RCTs have demonstrated improved symptom control with combination therapy over SCIT or anti‐IgE alone, not all patients will require this approach. Rather, an individualized approach to patient management must be considered, with evaluation of alternative causes for persistent symptoms, such as unidentified allergen sensitivity. Also, the studies did not compare optimal medical treatment of AR (INCS, antihistamine, allergen avoidance measures) to combination therapy versus SCIT alone. The current evidence does not support the utilization of combination therapy for all patients failing to benefit from SCIT alone.
Policy level: Option
Intervention: Current evidence supports that anti‐IgE may be beneficial as a premedication prior to induction of cluster or rush SCIT protocols, and combination therapy may be advantageous as an option for carefully selected patients with persistent symptomatic AR following AIT. However, at the time of this writing, biologic therapies are not approved by the US FDA for AR alone. An individualized approach to patient management must be considered.
XI.D.11 Efficacy considerations for immunotherapy
XI.D.11.a Extract factors
XI.D.11.a.i Allergen standardization and heterogeneity
Although the efficacy of AIT is well‐established, one factor that limits its widespread application is the heterogeneity of natural allergen extracts. Maintenance of product‐specific standardization (or batch‐to‐batch consistency) and cross‐product standardization (or consistency among products from different manufacturers) both pose unique challenges. This is due, in large part, to the natural origin of allergen product from biologic sources.,
Traditionally, the active ingredients of AIT extracts have been mixtures of crude proteins and allergens extracted from biological sources, such as pollens, animal dander, or HDM. In fact, prior to the 1970s it was common practice for allergists to manufacture their own extracts using allergen materials provided by regional suppliers. Understandably, this resulted in a high degree of variability among allergen extracts.
Even now with extraction methods subject to regulatory standards, allergen extracts remain heterogeneous. Today, allergens are still manufactured by extracting mixtures of allergen and other proteins from biological sources. Impurities in source materials may exist, and there is biologic variability in the raw material. While there is inherent variance in the product related to the sourcing and collection of allergenic materials, the extraction process has become more standardized across the industry. Extraction typically occurs using Coca solution (physiologic saline, bicarbonate buffer, and phenol) with or without glycerin. All allergen extracts must be sterilized and must contain bacteriostatic and fungistatic preservative. In the US, manufacturers typically use phenol at 0.2%–0.5% with or without 50% glycerin. These extracts may then be used unmodified, as is the case with most US extracts, or they may be treated with aldehydes and then processed with or without an adjuvant, such as aluminum hydroxide, as is the case with a majority of European SCIT extracts.,
In the US, the CBER is responsible for the regulation of allergenic extracts. Two important features of CBER's regulatory program have focused on the establishment of safe, consistent allergen manufacturing processes, as well as allergen standardization. The primary purpose of allergen standardization is to characterize the biologic potency of allergen extracts in a consistent manner. CBER mandates which test defines potency and the units by which potency is assigned. For example, one allergen may have potency determined by ELISA, while another may be determined by IDT (ID50EAL). These standardization practices then result in potency measurements in either BAU or AU. This aids in decreasing variability among lots as well as across manufacturers. In the US, 19 allergen extracts are currently standardized. These include HDM, cat pelt and cat hair, grasses, ragweed, and venoms. A majority of allergens in the US remain non‐standardized and carry labeled units (PNU or weight/volume) that do not correlate with biologic activity or potency. One caveat to CBER's standardization effort is the fact that potency units are typically assigned based on only one or two major allergen proteins, such as Fel d 1 for cat or Amb a 1 for ragweed. Even with strides made toward standardization, limitations persist and CBER continues to investigate novel approaches toward determining extract potency.
Further complicating efforts to minimize antigen heterogeneity and facilitate intercontinental evidence‐based recommendations, US standardization efforts are difficult to compare with European and other global standardization practices. In fact, standardization in Europe is largely based on in‐house references, and different units based on biological activity are utilized. Since no international consensus is established for the standardization of extracts, comparison of different products is difficult, and this variability interferes with intelligent interpretation of published studies across the continents. The CREATE project aimed to support the introduction of major allergen‐based standardization using recombinant or purified natural allergens as reference materials, as well as to validate existing ELISA tests for the measurement of major allergens.
One additional evolving challenge is the practice (more widespread in Europe) of modifying aeroallergen extracts via formulation with adjuvants or allergoids, as well as the use of recombinant allergens. While these novel approaches to allergen preparation may ultimately lead to improved safety and efficacy of AIT, there is currently no sufficient evidence to show clear advantage over the use of crude allergen extract in a majority of cases. These modifications further contribute to questions regarding the impact on efficacy of AIT, as well as allergen standardization and heterogeneity. (See Section XI.D.4. Allergen Extracts for additional information on this topic.)
XI.D.11.a.ii Multi‐allergen immunotherapy
The approach to treatment of polysensitized patients has been the subject of international debate. In the US, it is common practice for allergists to first characterize a sensitization profile, and subsequently provide multi‐allergen immunotherapy, whereby several allergen extracts are administered simultaneously throughout the treatment course. Conversely, a common practice in Europe entails identification of the most clinically problematic allergen followed by single‐allergen administration., If a single allergen cannot be identified as the predominant culprit for allergic symptoms, additional extracts may be given so long as they are administered at separate sites with at least 30‐min intervals., The Allermix survey conducted across 16 countries in 2016 revealed that 98% of providers reported management of polyallergic patients. Approximately 58% of these providers used single‐allergen immunotherapy while the remaining 42% used multi‐allergen immunotherapy.
Given that polysensitized patients are not necessarily polyallergic, the overuse and efficacy of multi‐allergen immunotherapy has been questioned. Skin testing or sIgE blood tests may be positive but may not correlate with clinical symptoms or disease. Furthermore, positive testing may reflect cross‐reactivity with proteins within other allergens that are not associated with symptoms. CRD may play an important role in clarifying the primary sensitizations but is not widely available. The multi‐allergen approach is scientifically supported by four double‐blind placebo‐controlled RCTs from the 1960s to 1980s (two studies with AR). These trials demonstrated significant improvement in patients who received mixtures of multiple, unrelated allergen extracts, but these studies were done prior to better standardization of extracts., , , More recent studies based in Spain have also supported multi‐allergen immunotherapy., A systematic review in 2009 evaluated 13 multi‐allergen immunotherapy studies (11 SCIT, one SLIT, and one both) and corroborated that co‐administration of two extracts is in fact clinically effective. Nevertheless, the results were less clear when more than two extracts were administered contemporaneously, a practice often used by US allergists. In fact, a survey comprising 670 patients across six US and Canadian practices reported a mean of 18 extracts in their mixtures.,
Although few prior studies have directly evaluated multi‐allergen immunotherapy compared to single‐allergen immunotherapy in polysensitized AR patients, there is growing evidence that the efficacy of these two strategies may not differ. Potential limitations in multi‐allergen SLIT were highlighted in a previous double‐blind placebo‐controlled RCT in which efficacy outcomes were suboptimal compared to single‐allergen SLIT. Ortiz et al. recently demonstrated that despite significant improvement in allergic symptoms across all subject groups, there was no significant difference observed in efficacy of single‐allergen SLIT versus pauci‐allergen (three to six allergens) or multi‐allergen SLIT in polysensitized patients. Additionally, Wang and Shi concluded that single‐allergen SLIT response is comparable to multi‐allergen SCIT in children with AR secondary to HDM. On the other hand, several studies, including a meta‐analysis for HDM, have substantiated comparable efficacy of single‐allergen immunotherapy in monosensitized and polysensitized AR patients., , , , , ,
A clear knowledge gap is the need for further evidence to support the use of multi‐allergen immunotherapy in polysensitized patients. Unfortunately, well‐controlled studies in the polysensitized population are difficult to design and conduct. Sensitization profiles can vary drastically among patients, resulting in a heterogeneous population that is difficult to investigate. Moreover, comparison of single‐allergen immunotherapy versus multi‐allergen immunotherapy is challenging as each unique polysensitization profile contains a different single dominant allergen to target which in turn may be difficult to distinguish clinically. At the time of this writing, there were 11 active or recruiting clinical trials investigating efficacy of AIT in AR patients (five SCIT, two SLIT, one both SCIT and SLIT, and three ILIT). None of the studies compare single‐allergen to multi‐allergen IT.
If multi‐allergen SCIT is administered, several considerations must be accounted for prior to the mixing process., First, one must be careful to maintain therapeutic amounts of each allergen in the mixture. Second, the chosen preservative must be compatible with all allergens in the mixture. Moreover, attention must be paid to the proteolytic activity of fungal and some insect body extracts. When extracts with greater proteolytic activity are mixed with certain allergens susceptible to proteolysis such as pollen, mite, and animal dander allergens, the effective concentrations in the extract mixture may be reduced.,
Given the widely varied practice patterns and challenges inherent in the study of polysensitized individuals, the evidence supporting multi‐allergen immunotherapy is not as strong as that supporting single‐antigen immunotherapy strategies. Although it is difficult to directly compare multi‐allergen and single‐allergen treatment strategies, the literature strongly supports the efficacy of single‐antigen immunotherapy even in polysensitized patients, while there remains a need for more careful analysis of the efficacy of multi‐allergen immunotherapy. (See Section XI.D.11.b.ii. Polysensitization for additional information on this topic.)
XI.D.11.b Patient factors
XI.D.11.b.i Patient age
Patient age is not a contraindication for AIT, but unique characteristics of the extremes of age merit discussion. First, older adult patients with multiple or particular comorbidities might be regarded as having a higher risk associated with AIT. Second, immunosenescence is also a concern, as older adults may theoretically have reduced benefit due to a less plastic immune response from the intended immunomodulatory effects of AIT. Yet, multiple studies in older adults have confirmed AIT is effective in treating clinical symptoms with associated positive effects on immunologic biomarkers. In four separate RCTs, Bozek et al. demonstrated the clinical effects of SLIT and SCIT for dust mite and grass pollen mixture in patients ranging 60–75 years of age, showing improvement in TNSS and medication usage, as well as an increase in antigen‐specific IgG4 levels., , , These effects remained durable 3 years after completing a 3‐year course of SCIT.
In children, several studies have demonstrated AIT has short‐term and long‐term effectiveness, including decreasing the dose of inhaled corticosteroids in asthmatic patients., , , , , Literature supports the efficacy of both SCIT and SLIT in the pediatric population. There is no lower age limit delineated in the US for initiating SCIT, but FDA‐approved SLIT products are only approved beginning at age 5.
Pediatric AIT may have additional benefit of prolonged disease modifying effects. In the Preventive Allergy Treatment (PAT) study, 205 children aged 5–13 with rhinoconjunctivitis to birch and/or grass pollen were randomized to AIT versus pharmacotherapy. AIT patients had less asthma symptoms, improved methacholine response, and potential for asthma prevention., SLIT using a grass tablet was shown to have a similar asthma prevention effect in the Grass immunotherapy tablet Asthma Prevention (GAP) trial. Similarly, in a retrospective analysis of 1099 children with AR receiving grass pollen SLIT tablets were compared with 27,475 rhinitis‐control patients only 1.8% of SLIT treated children developed asthma versus 5.3% of control patients. A meta‐analysis concluded that AIT decreases the risk of neo‐sensitization and asthma development in the short‐term (asthma RR 0.40; neo‐sensitization RR 0.72), although the long‐term benefit is unclear.
Safety and tolerability are important considerations in the pediatric population. In a retrospective evaluation of systemic reactions in pediatric and adult patients, the unadjusted systemic reaction rate was higher in children (0.2%) but not when adjusted for asthma, gender and phase of SCIT. In a Chinese population, systemic reactions were more common in younger children (3.28% of injections) compared with adolescents (1.47% of injections) but were treatable without requiring hospitalization. AIT is not customarily initiated in infants and toddlers given fears of the child not being able to communicate symptoms, in particular those of systemic reactions, and concerns that injections may be poorly tolerated in very young children. Every potential pediatric AIT case merits consideration of balancing the potential benefits versus risks and inviting child and parent to participate in shared decision‐making to express their values and preferences regarding the trade‐offs of AIT, which are likely quite individualized. Similar processes and considerations are recommended for older adults.
XI.D.11.b.ii Polysensitization
Polysensitization, or sensitization to more than one allergen, is common in the general population, and a factor which potentially challenges AIT efficacy. In an effort to identify the prevalence of sensitization in the general population, a 2010 study showed that among 11,355 participants in the first ECRHS, 57%–67.8% of the population was not sensitized to any test allergens, 16.2%–19.6% were monosensitized, and 23.8%–25.3% were polysensitized. Similarly, the National Health and Nutrition Examination Survey III (NHANES) studied skin sensitization to common aeroallergens in the US general population. Among the 10,863 participants 45.7% were not sensitized to any test allergens, 15.5% were monosensitized, and 38.8% were polysensitized. Hence, polysensitization appears to be more prevalent than monosensitization in the general population. More recent evidence suggests that polysensitization may be an entirely distinct phenotype compared to monosensitization, possibly predictive of more severe comorbid allergic disease expression., ,
Once polysensitization is established via skin testing or sIgE testing, the conundrum facing allergists is whether this polysensitization represents true polyallergy. To have polyallergy, the individual must have relevant symptoms upon exposure to two or more specific, sensitizing allergens.
In some patients showing positive test responses to multiple allergens, this may be caused by cross‐reactivity to highly conserved proteins, or panallergens. These related proteins, which have highly conserved sequence regions and structures, trigger IgE cross‐recognition. Separating the clinical relevance of positive test responses to pollens known to demonstrate cross‐reactivity can be challenging because the seasonality of symptoms may overlap. New technologies focused on CRD may prove useful in determining whether cross‐reactive allergens are the cause of polysensitization, and may help to direct AIT decisions.
The issue of whether the polyallergic patient is best treated with more than one (or even several) clinically relevant allergens versus a single allergen deemed most responsible for the patient's symptoms, is a subject of debate, and one characterized by trans‐continental practice variations. The predominant approach in the US is to treat the polyallergic patient with multiple allergens simultaneously, while the European approach is to focus AIT on one, or at most two, clinically significant allergens.
While the published literature comparing the efficacy of single‐ or multi‐allergen immunotherapy in the polysensitized patient continues to evolve, there are published guidelines which can help to direct practical decision making. Not unexpectedly, these guidelines reflect regional bias. The 2018 EAACI Guidelines on Allergen Immunotherapy specify that polysensitized patients who are monoallergic receive AIT only for the specific allergen driving their symptoms. The EAACI guidelines further specify that for the polyallergic patient sensitized to two homologous allergens (i.e., two grass pollens), a single allergen preparation or a mixture of two homologous allergens may be used, and for the polyallergic patient sensitized to allergens which are not homologous, AIT should be limited to one or two of the clinically most important allergens administered separately at distinct anatomic locations and separated by 30–60 min. Similarly, the 2010 Global Allergy and Asthma European Network (GA2LEN)/EAACI pocket guide does not recommend the use of allergen mixtures in AIT. The Practice Parameter Third Update guidelines developed by the Joint Task Force acknowledges that there have been few studies investigating the efficacy of multiallergen SCIT, and that these studies have considerable heterogeneity, yielding conflicting results. The Practice Parameter emphasizes the importance of treating patients with only relevant allergens but does not discourage prescribing multi‐allergen immunotherapy in properly selected patients. (See Section XI.D.11.a.ii. Multi‐allergen Immunotherapy for additional information on this topic.)
XI.D.11.b.iii Adherence to therapy
Adherence to AIT is variable and dependent upon route of administration, SLIT versus SCIT, dosing frequency/regimen, patient characteristics, and AIT‐associated adverse events. A review of the literature indicates no reported prospective double‐blind, placebo‐controlled RCT examining and/or comparing the adherence of SLIT versus SCIT as the primary endpoint. However, there are data on the adherence of AIT in prospective double‐blind, placebo‐controlled RCT of clinical efficacy, but these data are somewhat artificial in that adherence is closely monitored and patients are selected based on criteria that would promote better compliance to therapy. Furthermore, since optimal efficacy of either SLIT or SCIT is not appreciated until a minimum of 2 and optimally 3 years of therapy, adherence rates must be determined over a prolonged period. AIT adherence is reported to be much lower in real‐life studies versus clinical trials. For example, in an analysis of sales figures from two SLIT manufacturers in Italy that account for more than 60% of the Italian immunotherapy market, sales decreased from 100% at the start to approximately 44% in the first year, 28% in the second year, and 13% in the third year. This indicates that less than 20% of patients were adherent to the prescribed SLIT regimen.
A non‐interventional, prospective, observational, multicenter, open label study examined the adherence of 399 patients (236 adults and 163 children) with moderate‐to‐severe grass‐induced allergic rhinoconjunctivitis to a 3‐year regimen of grass SLIT tablets. The authors found that only 55% of patients completed the 3‐year treatment period. These data are similar to many retrospective analyses of adherence to SLIT at the end of a 3‐year regimen, ranging 10%–61%, , and illustrate that even though self‐administration of AIT could be advantageous over injections requiring office visits, adherence is a significant problem.
The adherence rate to SCIT regimens have also been studied in retrospective and a few prospective uncontrolled studies. In a real‐world study examining claims data, 103,207 patients were reported to have at least one AIT claim, but only approximately 44% of these patients reached maintenance AIT. There was no follow‐up of these patients to determine how many of the 56% that reached maintenance continued AIT for a full 3 years. A retrospective cohort analysis of a German longitudinal prescription database indicated that at the end of 3 years, adherence to SCIT was 35%–37%, and higher than that reported for SLIT (10%–18%). A data management retrospective study compared adherence to SCIT and SLIT at the end of 3 years and found that SLIT patients had a higher dropout rate (39%) versus SCIT (32.4%). In a retrospective analysis of a community pharmacy database, only 18% of 6486 patients starting AIT reached a minimal duration of 3 years, 23% for SCIT and 7% for SLIT. A retrospective analysis compared attrition rates in patients prescribed SCIT or SLIT found at the end of the prescribed period, attrition rates were similar, 45% and 41%, respectively. Another retrospective analysis comparing SLIT versus SCIT adherence found that only about 30% of patients completed a 3‐year course of either therapy.
Overall, the strength of evidence is low since most studies involved retrospective analyses and none reported efficacy outcomes. However, data strongly suggest that adherence to either regimen of AIT is very low which likely results in poorer efficacy. Reasons for the poor adherence are many and include inconvenience of taking a daily medication (SLIT) or frequent office visits (SCIT), adverse events especially during the first months of therapy, cost, and perceived lack of benefit.
XI.D.11.b.iv Pregnancy
AR and asthma affect 20%–30% of women of childbearing age and are considered two of the most common medical conditions that can affect pregnancy. One‐third of these women will suffer from worsening symptoms during pregnancy and up to 20% will experience exacerbations of asthma resulting in hospitalization or even death. AIT is an effective treatment option for AR, and its role in pregnancy continues to be investigated. The evidence regarding the efficacy and safety of AIT during pregnancy is scarce with a single large‐scale prospective study published to date. In the most recent Practice Parameter update, it is stated that AIT can be continued, but not initiated, in the pregnant patient. Furthermore, if pregnancy occurs during the build‐up phase and the patient has not reached a therapeutic dose, discontinuation of AIT should be considered.
The first study to assess the safety of AIT in pregnancy was published in 1978 by Metzger et al. This retrospective study analyzed the incidence of prematurity, toxemia, abortion, neonatal death, and congenital malformation in 90 atopic women who received SCIT during their pregnancy compared to a group of 147 untreated atopic mothers. No significant difference in these outcomes was found between the two groups suggesting that continuation of AIT during pregnancy was safe.
Over the next 10 years questions regarding the safety of AIT during pregnancy continued. In 1993, Shaikh et al. published a retrospective study that investigated 81 atopic women who underwent SCIT during pregnancy, for a total of 109 pregnancies. Similar variables as the Metzger et al. study were analyzed, and when compared to the control group of 60 patients (82 pregnancies) who declined AIT, the incidence of prematurity, gestational hypertension, and proteinuria were actually lower. Of note, only seven of the 109 pregnancies initiated SCIT for the first‐time during pregnancy. This study supported that SCIT was not only safe during pregnancy, but control of allergies and asthma during pregnancy may decrease adverse perinatal outcomes.
To date, only one RCT has been performed to demonstrate the safety of starting SLIT in the pregnant population. Shaikh et al. separated 280 atopic women (326 total pregnancies) into one of three groups: 155 patients received SLIT during 185 pregnancies (with 24 patients receiving SLIT for the first time during pregnancy). The remaining patients were separated into two control groups, receiving either daily budesonide (group A) or rescue inhaled salbutamol (group B). The study showed no significant differences in perinatal outcomes, suggesting that both initiation and continuation of SLIT was safe during pregnancy. Although this study concludes that initiation of SLIT during pregnancy is safe, it is important to note that only 24 patients, 13% of the treatment group, fell into the initiation arm of the study.
Continuation of AIT during pregnancy has not shown to be harmful to either the mother or the fetus. There is limited data, however, to draw conclusions regarding the safety of first‐time initiation of AIT during pregnancy. Lastly, no conclusion can be made regarding the effects of pregnancy on efficacy of AIT due to lack of literature.
XII PEDIATRIC CONSIDERATIONS IN ALLERGIC RHINITIS
XII.A History and physical exam
As repeated exposure to allergens is required, AR takes a few years to develop in children. Food and indoor allergies are more common in children under the age of 3, with seasonal outdoor allergy risk increasing after the age of 3. A family history of AR, atopy, or asthma is important to assess as children may be at an increased risk of developing AR or other allergic diseases. The future development of AR should be considered in children exhibiting signs of the “allergic march”. Certain risk factors may have a link to the development of AR in children. (See Sections VIII. A‐B. Risk Factors for Allergic Rhinitis for additional information on this topic.)
Common findings consistent with AR in children include nasal congestion, sneezing, postnasal drip, cough, sniffling, throat clearing, palatal click, and mouth breathing., , , , Defining a seasonal timeline or triggers for symptoms can help identify a cause and help determine if rhinitis is allergic or non‐allergic in nature.
Although evidence is conflicting and variable, there are several conditions possibly associated with AR in children, which should be assessed during clinical evaluation. The most common comorbidities associated with childhood AR are asthma, conjunctivitis, and AD. Other comorbidities include rhinosinusitis, SDB, ETD, otitis media, and oral allergy syndrome., , , Oral allergy syndrome may be suspected in patients with mouth itching or swelling after eating raw fruits or vegetables.
There is data to suggest that AR is more common in children with otitis media with effusion (OME) than those without. While the results vary based on the age of the children studied, this highlights the importance of ear evaluation during the physical exam., , (See Section XIII.G.2. Otitis Media for additional information on this topic.) Similarly, the association of adenoid hypertrophy (AH) with AR is debated, but some studies have suggested the importance of the correlation between these two diseases., , , , (See Section XIII.F. Adenoid Hypertrophy for additional information on this topic.) This may help to explain the association between AR and OSA in children.
Diagnosing AR in the pediatric population may be challenging due to difficulty clearly communicating symptoms. There is also overlap of symptoms with frequent illnesses experienced in childhood, for example, upper respiratory infection. Diagnostic clues, which may be reported by a parent or caregiver include chapped lips from mouth breathing, fatigue, irritability, poor appetite, and attention issues.,
After a complete history, there are several elements of the physical exam that may aid in diagnosis. An important aspect of the physical exam is to rule out other etiologies of nasal obstruction and rhinitis such as nasal foreign body or choanal atresia. Some physical exam findings are similar to the adult population including posterior pharyngeal cobblestoning, clear nasal drainage, serous middle ear effusions, and enlarged/boggy ITs., Specifically in the pediatric population, “allergic” or “adenoid facies” may be present, characterized by mouth breathing, high‐arched palate, and dental malocclusion. Additionally, the “allergic salute” is defined as repeated rubbing of the nose, which can lead to a transverse nasal crease or “allergic crease.” “Allergic shiners” are caused by infraorbital venous stasis and Dennie‐Morgan lines are folds below the lower eyelids suggesting allergic conjunctivitis (AC)., , , , Voice changes including hoarseness and hyponasality are common in pediatric AR. Anterior rhinoscopy can reveal IT bogginess, paleness, and/or hypertrophy. Nasal endoscopy has been evaluated as a tool for diagnosis in pediatric AR, with IT and MT contact with other nasal structures as predictive factors for positive SPT results. There are no specific recommendations for the use of nasal endoscopy in children with suspected AR, but this assessment may be important in ruling out other, less common, causes of nasal obstruction or rhinitis.
Of note, one important goal of early diagnosis of AR is to identify young children at risk of developing other allergic disorders. Non‐allergic rhinitis, viral URI, and anatomical causes of nasal obstruction should be on the differential diagnosis in children evaluated for AR.
XII.B Diagnostic techniques
Allergy testing recommendations for the pediatric population are similar to those for adults. Allergy testing should be considered in children with insufficient response to medical treatment. The EAACI Section on Pediatrics recommends that allergy testing be considered in children presenting with AR clinical symptoms and signs in order to initiate treatment and lifestyle changes, such as avoidance of allergens. Clinical practice guidelines exclude children younger than 2 years of age as causes of rhinitis may be different in this population. However, there are no age limits for allergy testing and young children are eligible.
The diagnosis of AR in children should be based on both clinical history and testing. Allergy testing without clinical suspicion has been shown to lead to false‐positive SPT results over 50% of the time. SPT is generally accepted as the preferred method of testing in children; it is faster and less painful than intradermal testing, and it is less expensive than in vitro serum testing. Although intradermal testing or SPT may be considered in the pediatric population, SPT is often considered superior due to ease, minimal discomfort, and timeliness of results. There are indications for in vitro testing in children as there are in adults, including skin disorders (e.g., dermatographism, dermatitis at the proposed testing site) and medication usage (e.g., inability to hold antihistamines for testing). It is also important to note that a positive SPT in a young child will result in a smaller wheal size than in an older child or adult due to relatively lower circulating IgE levels.
There is limited data regarding nasal eosinophil and basophil levels for the purpose of AR diagnosis. Nasal eosinophilia has been associated with AR in children but is not widely used to diagnose AR., , , Additionally, nasal basophilic metachromic cells have shown high sensitivity for AR., While there is limited data on BAT in general, and it is considered an option for AR diagnosis in adults; one small pediatric study has shown that BAT has sensitivity and specificity of 90% and 73%, respectively.
XII.C Pharmacotherapy
Most patients with symptoms of AR will use some form of pharmacotherapy for satisfactory symptom control. The specific management of each patient is influenced by the frequency and intensity of symptoms, response to treatment, the presence of comorbid conditions as well as the patient's age and preference. Current pharmacologic options in the treatment of AR include INCS, intranasal and oral antihistamines, decongestants, mast cell stabilizers, intranasal anticholinergics, and LTRAs., ,
Children less than 2 years of age. In this age group AR is less prevalent, but children may have frequent bouts of allergy‐type symptoms including rhinorrhea, sneezing, itchy eyes, etc. which could be due to other, more common triggers, such as recurrent viral illness, AH, or rhinosinusitis. Before treating a young child for AR, other causes should be investigated and ruled out.
The pharmacologic options for AR in children under 2 years old are limited. Second‐ and third‐generation antihistamines such as cetirizine, levocetirizine, and desloratadine have indications down to 6 months of age and are an option in the treatment of the young patient with AR. First‐generation antihistamines (diphenhydramine, chlorpheniramine) have the disadvantage of being lipophilic and cross the brain–blood barrier. Unwanted side effects of these medications make them difficult and dangerous to use and not indicated in children less than 2 years old (Table II.C).
Children 2 years old and older. For the older child, treatment of AR is very similar to that in the adult patient and depends largely on the frequency and severity of symptoms.
Mild or episodic symptoms may be treated with medications aimed at addressing the specific symptom(s). A second‐ or third‐generation antihistamine may be used on an as needed basis for rhinitis, sneezing, and itchy watery eyes. Intranasal antihistamine preparations are another option in children over the age of 5 (azelastine 0.1%) and 6 years old (olapatadine); benefits include targeted delivery, decreased side effects, and rapid onset of action., , , Intranasal antihistamines have been recommended over oral antihistamines in the appropriate patient population.,
For persistent or moderate‐to‐severe symptoms, INCS are recommended as the best single therapy in the treatment of allergic symptoms affecting QOL., , , The effectiveness of INCS in the reduction of nasal symptoms including sneezing, itching, rhinorrhea, and congestion in children with AR has been demonstrated., , , INCS are usually well tolerated; however, because adverse effects are possible, growth in children using INCS should be monitored and dosages should be tapered to the lowest effective dose in all patients.
INCS preparations approved for children aged 2 years and older include mometasone furoate, triamcinolone acetonide, and fluticasone furoate. Most others are indicated for children aged 6 years and older, except for fluticasone propionate and beclomethasone dipropionate, which are indicated down to age 4 years.
When response to initial INCS is suboptimal, a second agent can be considered. Options include intranasal or oral antihistamines, combination intranasal INCS/antihistamine, or antihistamine/decongestant products. The choice should be made based on the persistent symptoms being addressed, patient preference, possible side effects and coexistent conditions (Table II.C).
LTRAs, such as montelukast, have been used in the management of AR and asthma. LTRA efficacy has been shown to be less effective than INCS, but more effective than placebo., , , , , Due to its potential for neuropsychiatric effects, the US FDA has recommended against the use of montelukast in patients with AR in favor of other treatment options. In the latest Clinical Practice Guideline on AR published by the AAO‐HNSF, montelukast is not recommended as first line therapy.
Cromolyn nasal spray is a mast cell stabilizer that can inhibit the allergic response. It is most effective when used as a preventive measure when allergy exposure is anticipated. It has a low side effect profile (sneezing, bad taste, etc.), but due to its short half‐life must be administered three to six times daily. It has been approved for use in children as young as 2 years old. Though less effective than INCS or second‐generation antihistamines, some parents and clinicians prefer it due to its excellent safety profile., ,
IPB nasal spray has been shown to decrease rhinorrhea. It has a quick onset of action and must be used frequently. It is not recommended as a first‐line drug in AR but has had some success in patients with profuse rhinorrhea not otherwise controlled with INCS. It has been shown to be more effective when combined with a nasal steroid than when either medication is used alone in the treatment of chronic rhinitis. It is indicated down to age 5 years.
Oral decongestants are also a consideration in the treatment of AR, but due to their side effect profile and potential for central nervous system stimulation in the pediatric population, the risk/benefit ratio should be carefully considered when used in children between the ages of 2 and 6 year old., , Oral decongestants are not recommended in younger children (Table II.C).
XII.D Immunotherapy
AIT is a treatment option when other strategies, such as avoidance and pharmacotherapy, have failed. It may also be considered for patients who cannot tolerate standard therapies, those who want to avoid prolonged used of medications, and those wishing to obtain a lasting response by modifying the immunologic process. Consideration for AIT should only be undertaken in patients with documented sIgE response to aeroallergens correlating with the patient's allergic symptoms. As long as these recommendations are followed, AIT is an option for allergic patients regardless of age. However, due to the required environmental exposure for the development of clinically relevant sensitization(s) to aeroallergens, combined with the limited evidence for the efficacy of AIT for AR in children under 5 years of age, the decision to provide AIT should consider the above factors along with a discussion with the family regarding its limitations and safety concerns.
Modalities for AIT administration include SCIT and SLIT (available in the form of a dissolvable tablet or as a liquid extract). Both options are available for adults and children, with specific age indications depending on the individual SLIT tablet. Usually patient demographics, preference, and treatment goals are used to guide the choice of AIT modality. For example, in young children who may be traumatized by or unable to tolerate repeated injections, and who may be unable to report early symptoms of an allergic reaction, SLIT may be considered due to its ease of administration and superior safety profile.
Dosing of SCIT and SLIT liquid extract is the same in the adult and pediatric populations. SLIT tablets currently available in the United States for use in children include a single grass (Timothy) tablet, a multi‐grass (sweet vernal, orchard, perennial rye, Timothy, Kentucky bluegrass) tablet, and a short ragweed tablet, all indicated down to age 5 years. The HDM tablet available for adults has not received approval for pediatric use as of this writing.
Though the literature regarding efficacy of AIT is less robust in the pediatric population, it has been shown to be effective in the treatment of AR,, , and both SCIT and SLIT have resulted in improved control of comorbid conditions such as asthma and AC. Of particular interest is the research that has demonstrated that AIT has the potential added benefit of decreasing the development of asthma in pediatric patients with AR, as well as reducing the onset of new allergen sensitizations although additional studies are warranted., ,
In all populations, potential contraindications to AIT (SCIT and SLIT) include uncontrolled or poorly controlled asthma, active autoimmune disorders, and malignancy. EoE is also a contraindication to SLIT., , , Special consideration should be given when treating patients with cardiovascular disease, those on β‐blocker medications, and those with partially controlled asthma due to their impaired ability to respond to resuscitation efforts should an allergic reaction occur.
Challenges systematically being addressed in the practice of adult AIT extend to the pediatric population. These include the use of one or multiple allergens in the treatment of AR; whether mixtures of multiple allergens can compromise efficacy; the standardization of the allergen extracts for consistency, quality, and potency; and effective dose ranges for the pertinent allergens used.
XIII ASSOCIATED CONDITIONS
XIII.A Asthma
XIII.A.1 Asthma definition
Asthma is a common chronic lung disease comprising a heterogeneous group of phenotypes, including allergic and non‐allergic, and further subtypes based on demographic, clinical, and/or pathophysiological characteristics. The definition of asthma has appreciably changed over time. The latest Global Initiative for Asthma (GINA) Guidelines define asthma as “a heterogenous disease, usually characterized by chronic airway inflammation. It is defined by the history of respiratory symptoms such as wheeze, shortness of breath, chest tightness and cough that vary over time and in intensity, together with variable expiratory airflow limitation.”
In addition to the aforementioned respiratory symptoms, a diagnosis of asthma typically requires evidence of variable obstruction of expiratory airflow, by bronchodilator reversibility testing or bronchial hyperreactivity tests. In clinical practice patients have a variety of clinical presentations, and when patients are well, most tests show no abnormalities. Increasingly, asthma is being recognized as a disease of airway inflammation and disordered immunology, as well as aberrant physiology, with combinations of “treatable traits” in different patients. Most patients have mild or moderate disease. A small proportion (up to 10%) has severe disease that is refractory to standard inhaled medications. These patients have more severe symptoms, frequent exacerbations and need more intensive treatment regimens.
XIII.A.2 Asthma association with allergic and non‐allergic rhinitis
AR and non‐allergic rhinitis have been established as important comorbidities of asthma. Increasingly, there has been a shift toward conceptualizing multimorbid chronic upper airway inflammation and asthma as a single “unified airway” pathology affecting both the upper and lower airway. (See Section VI.K Unified Airway for additional information on this topic).
The prevalence of comorbid AR and asthma varies. Recent population‐based studies have shown rates between 20.3% and 93.5%., , , , , In one study, AR was found to be an independent determinant of current asthma among adults (OR 7.72; 95% CI 6.56–9.09, p < 0.001). Some studies have shown that patients with comorbid AR tend to have poorer asthma control, a greater number of exacerbations per year, and more visits to the emergency department., , , Interestingly, the association of allergy with asthma weakens with more severe asthma (Table XIII.A.2).
Non‐allergic rhinitis is also commonly associated with comorbid asthma., Increasingly, asthma is being considered a multifactorial disease with variable endotype and phenotype presentations, particularly with regards to aberrant type 2 inflammation, which may or may not be allergic., The functional relevance of this upper airway association can be summarized as follows:
In line with the unified airway hypothesis, allergen and irritant challenge to the nose and upper airway elicits lower airway inflammation through shared immunological and neurogenic pathways.
Nasal obstruction results in mouth breathing, which leads to reduced filtration and humidification of inspired air, facilitating reactive lower airways.
Nasal blockage resulting in mouth breathing can be associated with breathing pattern disorders and increased breathlessness in patients with asthma.,
Several recent molecular studies have shed light on the mechanisms underlying the phenomenon of this multimorbidity. GWAS studies have demonstrated independent risk variants, which are common between asthma, AR, and eczema. Moreover, gene expression analyses suggest that type 2 mediated inflammation has a similar molecular basis across disease types. These findings underscore the proposed “one airway” model, which recognizes similar disease mechanisms occurring in both the upper airway and the lower airway.
In summary, upper airway symptoms can impact asthma disease control and patient QOL. Assessment and treatment via a multidisciplinary approach, encompassing pulmonologists, allergists, immunologists, otolaryngologists/rhinologists, should be considered.
Asthma association with allergic and non‐allergic rhinitis
Aggregate grade of evidence: B (Level 1: 3 studies, level 2: 3 studies, level 3: 3 studies, level 4: 8 studies; Table XIII.A.2)
XIII.A.3 Allergic rhinitis and asthma – association of risk factors
Up to 30% of patients with AR develop asthma. Indeed, several large epidemiological studies have demonstrated that AR is an independent risk factor for developing asthma. Specifically, persistent AR appears to portend a significantly greater risk for development of asthma compared to intermittent AR (Table XIII.A.3).
The Children's Respiratory Study showed that there is a doubling of the risk of developing asthma by age 11 when AR is diagnosed by a physician during infancy. Rhinitis is also a significant risk factor for adult‐onset asthma whether patients are atopic or non‐atopic., , , In contrast, in childhood, asthma is frequently associated with allergy., Limited data fail to demonstrate a relationship between a diagnosis of AR and severity of comorbid asthma. Nevertheless, data on whether the severity of AR itself impacts the prevalence of comorbid asthma remains conflicting.,
Asthma and AR have overlapping risk factors. Aeroallergen sensitization may be the most important and has been demonstrated among adults and children across different geographic regions and populations around the world., , Indeed, most inhaled allergens are associated with both nasal and bronchial hyperresponsiveness. Occupational rhinitis is also a risk factor for occupational asthma caused by HMW agents. Genetic polymorphisms common to AR and asthma, such as unique subtypes of deregulated circulating microRNAs, may also provide a mechanistic link between the two disease processes.
There is growing evidence that exposure to traffic related air pollutants, (i.e., black carbon, NO2, NO, SO2, CO, CO2, and PM) may increase the risk of developing both asthma and AR. Nevertheless, additional studies with improved study designs incorporating confounder variables (e.g., allergens), and standardized definitions of traffic related air pollutants are needed., , (See Section VIII.B.3. Pollution for additional information on this topic.)
Similarly, a cross‐sectional study of 325 non‐asthmatic AR patients suggest that cigarette smoking may be an independent risk factor for the development of new asthma among patients with AR, although confirmatory studies are still needed. (see Section VIII.B.4. Tobacco Smoke for additional information on this topic.)
In summary, AR is a significant risk factor for asthma. However, there is currently limited evidence for the role of traffic related air pollutants and smoking as additional risk factors in the development of asthma among patients with AR.
Allergic rhinitis and asthma – association of risk factors
Aggregate grade of evidence: C (Level 2: 3 studies, level 3: 19 studies; Table XIII.A.3)
XIII.A.4 Treatment of allergic rhinitis and its effect on asthma
AR and asthma are linked both epidemiologically and pathophysiologically along one common airway., , , , Indeed, there is a body of evidence to suggest that the following AR therapies may benefit both conditions: INCS,, , , intranasal antihistamine, oral antihistamines,, LTRAs, and AIT., , AIT has shown promising results in altering the course of the allergic inflammation seen in both AR and asthma., , There is extensive literature in this area; therefore, this section focuses primarily on prospective randomized trials and systematic reviews to minimize inherent biases and weaknesses of retrospective studies.
Allergen avoidance
Allergen avoidance is often recommended for allergies, specifically for AR and allergic asthma., , Despite being intuitive and having reasonable biological plausibility, the actual evidence for benefit in AR and asthma is limited. No benefit was identified for chemical or physical methods to reduce HDM methods in a 2008 Cochrane review examining randomized trials of subjects with asthma. Similarly, single allergen avoidance or elimination plans such as removing or washing pets, mattress coverings, removing carpeting, and use of HEPA filters have not shown strong evidence‐based clinical benefit for reducing asthma and/or AR symptoms, although there are some exceptions (e.g., acaricides for HDM allergy)., , Nevertheless, there is theoretical benefit of reducing allergen exposure, a paucity of data on multimodality approaches to reduce allergen load, and minimal downside to attempting these various techniques. (See Section XI.A. Allergen Avoidance for additional information on this topic.) Allergen avoidance is mentioned here for completeness in discussing treatment modalities for AR with an effect on asthma, but given poor evidence of effect, an aggregate grade of evidence and literature summary table are deferred.
Pharmacotherapy
Oral H1 antihistamines. Six RCTs were identified that specifically evaluated H1 antihistamines for the treatment of asthma in the context of coexistent AR., , , , , Cetirizine and loratadine are the two most highly studied second‐generation antihistamines used concomitantly in AR and asthma. Elevated histamine levels after allergen challenge are associated with bronchoconstriction responses in acute asthma episodes. Cetirizine also has bronchodilatory effects which are significant both as monotherapy and in combination with albuterol. Despite biological plausibility of antihistamines as effective treatment and improvement in subjective asthma symptoms, objective measures using PFT and PEF have failed to demonstrate significant improvements., , Antihistamines may also have a preventive effect on the development of asthma in atopic patients. In a subgroup analysis, the Early Treatment of the Atopic Child trial found a near 50% reduced risk of developing asthma among cetirizine‐treated patients with grass pollen and HDM sensitivities. (See Section XI.B.1. Antihistamines for additional information on this topic.) (Table XIII.A.4.‐1).
Oral corticosteroids. Oral corticosteroids are commonly used in asthma patients who are inadequately controlled with bronchodilators and inhaled corticosteroids. They are also effective for symptoms of rhinitis. Due to the side‐effect profile associated with these medications, especially with increasing duration of use, oral steroids are not recommended for the routine treatment of AR. For these reasons, an aggregate grade of evidence and evidence summary table are deferred. (See Section XI.B.2.a. Oral Corticosteroids for additional information on this topic.)
Intranasal corticosteroids. In the 1980s, INCS were reported to improve asthma symptoms in patients with coexistent AR and asthma., Two meta‐analyses and 12 RCTs address the potential “unified airway” effect of INCS on asthma, and a single historical cohort study evaluates the impact of combination INCS and intranasal antihistamine on asthma outcomes in patients with both AR and asthma., , , , , , , , , , , , , , A 2003 Cochrane review evaluated the efficacy of INCS on asthma outcomes in patients with coexistent rhinitis, finding no significant improvement in asthma outcomes with INCS. Heterogeneity in study designs may have limited the findings of this meta‐analysis and explain the discrepancy of the results compared to high‐quality RCTs. Alternatively, a 2013 SRMA demonstrated improvements in asthma outcomes with the use of INCS compared to placebo in patients with asthma and AR, although the addition of INCS to inhaled corticosteroids was not associated with improved asthma outcomes. Patient education was noted to be important as patients with concomitant AR and asthma who received training on the proper use of INCS and education on the relationship of AR and asthma demonstrated significant reductions in asthma symptoms and albuterol use compared to patients receiving INCS without additional education. Finally, intranasal azelastine‐fluticasone propionate spray is a known effective treatment for AR alone. Recently, a pre‐post historical cohort also reported its potential utility in asthmatics with AR, demonstrating a significant reduction in acute respiratory events and rescue inhaler medication usage, as well as an increase in the overall number of well‐controlled asthmatics (See Section XI.B.2.b. Intranasal Corticosteroids for additional information on this topic.) (Table XIII.A.4.‐2).
Leukotriene receptor antagonists. LTRAs (montelukast and zafirlukast), often in combination with topical corticosteroids, have demonstrated benefit for the treatment of both asthma and AR, consistent with efficacy in addressing inflammation in the “unified airway.” ARIA 2008 guidelines supported the effectiveness of montelukast in treating patients with asthma and AR, finding improvement of both nasal and bronchial symptoms as well as reduction of β‐agonist use. The 2010 ARIA update specified that LTRAs are not recommended over other first‐line therapies for the respective conditions, recommending treatment of asthma and AR with a nasal and inhaled corticosteroid as first‐line therapies, rather than an LTRA to treat both conditions. A more recent review in 2015 also identified some utility of LTRAs for patients with concomitant AR and asthma. However, the limited additional benefit must be weighed against added cost and an FDA boxed warning regarding serious neuropsychiatric events when comparing inhaled corticosteroids to LTRAs for single‐modality treatment of asthma in patients with comorbid AR (See Section XI.B.4. Leukotriene Receptor Antagonists for additional information on this topic) (Table XIII.A.4.‐3).
Pharmacotherapy treatment of AR and its effect on asthma
Aggregate grade of evidence: A
Oral H1 antihistamines (Level 2: 4 studies, level 3: 2 studies; Table XIII.A.4.‐1)
Intranasal corticosteroids (Level 1: 2 studies, level 2: 5 studies, level 3: 8 studies; Table XIII.A.4.‐2)
Leukotriene receptor antagonists (Level 2: 7 studies; Table XIII.A.4.‐3)
Biologics
Omalizumab. Omalizumab is a monoclonal anti‐IgE antibody which binds free IgE, preventing interactions with high‐affinity IgE receptors and resulting in receptor downregulation on inflammatory cells. Omalizumab has demonstrated effectiveness separately for asthma as well as AR., , , , There are several published studies evaluating omalizumab in AR or asthma,, with one RCT specifically evaluating the efficacy of omalizumab in patients with concomitant moderate‐to‐severe asthma and persistent AR. Omalizumab as an adjunct to SCIT has also been evaluated. Both studies show a reduction in symptoms as well as an improvement in QOL measures., Additional biologics are currently in varying stages of development/emergence with further evaluation needed to determine their role for the treatment of coexistent AR and asthma. (See Sections XI.B.7. Biologics and XI.D.10. Combination Biologic Therapy and Subcutaneous Immunotherapy for additional information on this topic.) (Table XIII.A.4.‐4).
Biologic treatment of AR and its effect on asthma
Aggregate grade of evidence: B (Level 2: 2 studies; Table XIII.A.4.‐4)
**Note: There is high level evidence with multiple RCTs and reviews for asthma individually, but only one RCT specifically evaluating omalizumab versus placebo in patients with concurrent conditions.
Allergen immunotherapy
Both SCIT and SLIT improve control of AR and comorbid asthma., , , , Several studies indicate that AIT, often in addition to traditional antihistamine pharmacotherapies, may help halt the progression of allergic disease, including prevention of new allergic sensitivities and the development of asthma., , , , , , , , However, several systematic reviews have concluded that the evidence for AIT possible prevention of further allergic sensitization is low, due to limited analyses of asthma exacerbations, mixed population recruitment, and a focus on mild disease only., , Further evaluation is required to assess safety in patients with uncontrolled asthma. Of note, the 2010 ARIA statement recommended both SCIT and SLIT for the treatment of asthma in patients with AR and asthma. The 2019 GINA guidelines recommend adding HDM SLIT for adult patients with AR and FEV1 >70% who are suboptimally controlled on high dose inhaled corticosteroids. Finally, the National Heart Lung and Blood Institute Expert Panel conditionally recommends SCIT as an adjunct treatment to standard pharmacotherapy for those 5 years and older with mild to moderate persistent asthma who show clear evidence of a relationship between symptoms and exposure to an allergen to which the individual is sensitive. (See Section XI.D. Allergen Immunotherapy for additional information on this topic.) (Table XIII.A.4.‐5).
Allergen immunotherapy treatment of AR and its effect on asthma
Aggregate grade of evidence: A (Level 1: 7 studies, level 2: 3 studies, level 3: 3 studies; Table XIII.A.4.‐5)
XIII.B Rhinosinusitis
XIII.B.1 General association of allergic rhinitis with chronic rhinosinusitis
AR may be associated with CRS in several clinical settings. CRS is a condition of the sinonasal cavity characterized by persistent inflammation. While the causes of inflammation vary, CRSwNP is generally associated with type 2 mediated inflammation, while CRSsNP tends to have less predominance of type 2 inflammation., AR is predominantly driven by type 2 mediated inflammation and is thought to potentially be an inciting factor in the development of CRS, though the relationship remains unclear., This section will discuss the overall association between AR and CRSsNP as well as CRSwNP.
Allergic rhinitis and chronic rhinosinusitis without nasal polyposis. Since the previous iteration of ICAR‐AR, there have been no new studies examining CRSsNP and AR., There are no controlled studies examining the role of AR in the development of CRSsNP and no studies showing that the treatment of allergic disease alters the progression of CRSsNP, or vice versa., The Wilson et al. review continues to provide the most robust assessment of the relationship between allergy and CRSsNP, reporting four studies that supported an association between allergy and CRSsNP and five that do not. Because the correlation remains unclear, allergy testing is listed as an option in CRSsNP patients based on the theoretical benefit of identifying and treating comorbid allergic disease, (Table XIII.B.1.‐1).
Associated conditions – chronic rhinosinusitis without nasal polyps
Aggregate grade of evidence: D (Level 2: 1 study, level 3: 1 study, level 4: 8 studies, conflicting evidence; Table XIII.B.1.‐1) Table adapted from Wilson et al.
Allergic rhinitis and chronic rhinosinusitis with nasal polyposis. The pathogenesis of CRSwNP is strongly associated with type 2 inflammation., Additionally, nasal polyps have high levels of tissue eosinophils, as well as mast cells and basophils., AR follows a similar inflammatory pathway and this suggests there may be a pathophysiologic similarities between CRSwNP and AR., , However, the clinical evidence for or against an association between AR and CRSwNP has been mixed., Similar to CRSsNP, there have been no new studies specifically examining CRSwNP and AR since ICAR‐Allergic Rhinitis 2018. There is an expanding area of research on CCAD. (See Section XIII.B.3. Central Compartment Atopic Disease for additional information on this topic.) The evidence for a relationship between AR and CRSwNP remains conflicted. Ten studies support an association while ten do not, or have equivocal findings. Hypersensitivity to HDM, cockroach, and Candida have been associated with CRSwNP. Despite the overlapping pathophysiologic features between allergy and CRSwNP, conflicting evidence exists regarding an association between AR and CRSwNP. Allergy testing remains an option in CRSwNP patients based on the theoretical benefit of identifying and treating comorbid allergic disease, especially since allergy may be seen in these patients, (Table XIII.B.1.‐2).
Associated conditions – chronic rhinosinusitis with nasal polyps
Aggregate grade of evidence: D (Level 3: 5 studies, level 4: 16 studies, conflicting evidence; Table XIII.B.1.‐2) Table adapted from Wilson et al.
In summary, the association between AR and CRSwNP or CRSsNP remains unclear, with conflicting evidence. The available literature is limited by varying definitions of allergy versus AR as well as a failure to separate CRSwNP and CRSsNP. Studies that combined CRSwNP and CRSsNP in their evaluation of a potential CRS‐AR association were excluded from the Wilson et al. review and the ICAR‐Allergic Rhinitis 2018 and are not included here. As our understanding of CRS endotypes and inflammatory patterns evolves, it becomes more pertinent to specify the relationship of AR with specific CRS disease processes (allergic fungal rhinosinusitis [AFRS], CCAD, AERD), which are discussed in the following sections.
Despite the unclear relationship, the diagnosis and treatment of comorbid allergy is an option in rhinosinusitis patients balancing the cost and low evidence with the low risk of allergic rhinosinusitis treatment and the theoretical benefits of reducing allergic sinonasal inflammation.
XIII.B.2 Allergic fungal rhinosinusitis
AFRS is a non‐invasive, chronic, hypertrophic form of rhinosinusitis that affects immunocompetent hosts and is associated with an IgE‐mediated local inflammatory response to extramucosal fungi present in the sinonasal cavities., The Bent and Kuhn criteria are the most commonly cited diagnostic criteria for AFRS and include type I IgE‐mediated hypersensitivity, recognizing that the diagnosis of AFRS requires a positive allergy history and that type I hypersensitivity can be used to distinguish IgE‐mediated forms of rhinosinusitis, such as AFRS and CCAD, from other forms of non‐IgE‐mediated rhinosinusitis.
Various studies have demonstrated the importance of IgE in the pathophysiology of AFRS, with both systemic and local IgE and fungal sIgE production consistently shown to be elevated in this disease process., , Additionally, it has been determined that most AFRS patients have detectable fungal sIgE in their allergic mucin., Wise et al. further established that there is a significant increase in localized IgE staining of the sinus epithelium and subepithelium in AFRS patients compared to controls and CRSsNP patients. The role of type I hypersensitivity in AFRS, even in the absence of positive serum sIgE to fungal allergens, has also been demonstrated, (Table XIII.B.2).
Although generally both CRSsNP and CRSwNP have been found to have an equivocal association with allergy, 100% of AFRS patients in a study by Marcus et al. demonstrated positive allergy testing. Allergy testing and treatment is not recommended in CRS unless there are concurrent AR symptoms and sensitivities, respectively, but some data support a role for AIT in improving AFRS patient outcomes in terms of reliance on systemic or topical corticosteroids, need for revision surgery, sinonasal crusting, QOL scores, and objective endoscopy scores., Still, a systematic review by Gan et al. reported a grade C in quality of evidence for AIT in AFRS, so it is considered an option in refractory AFRS cases.
The exact role of allergy and fungal hypersensitivity in the pathogenesis of AFRS has long been debated, partially due to a vague understanding of eosinophilic mucin CRS subtypes, including those classified as CRS with eosinophilic mucin but without the presence of fungi. Furthermore, eosinophilic mucin and polyps, which must be present to diagnose AFRS, can occur in the absence of allergy., Pant et al. showed that elevated IgG3 levels specific to Alternaria alternata and Aspergillus fumigatus could distinguish eosinophilic mucin CRS from control groups, which suggests a possible fungal‐specific non‐allergic immune response in AFRS, and Clark et al. found significantly higher levels of Staphylococcus aureus in AFRS patients as compared to non‐AFRS patients, again suggesting a different type of immune mechanism in the pathophysiology of AFRS. In addition, with improved fungal culture techniques, some studies report the presence of fungi in nearly 100% of non‐AFRS CRS patients and control subjects, further complicating the true role of fungi in AFRS., , , Despite these debates, there is evidence demonstrating the important role allergy and type 2 inflammation play in the pathophysiology, diagnosis, and treatment of AFRS.
Associated conditions – allergic fungal rhinosinusitis
Aggregate grade of evidence: C (Level 2: 1 study, level 3: 9 studies, level 4: 5 studies; Table XIII.B.2)
XIII.B.3 Central compartment atopic disease
CCAD is a distinct variant of CRS described as polypoid changes of central compartment (CC) structures where airflow is most prominent, including the MT, superior turbinate, and or/posterosuperior nasal septum. There is relative disease sparing of the peripheral sinus cavities, and studies suggest a strong association with allergy. In 2014 White et al. first described the association between allergy and isolated MT polypoid edema, with 16/16 patients having allergen sensitization. Hamizan et al. found that MT edema/polyposis has a high specificity and positive predictive value for the presence of inhalant allergy, with the highest grades of MT edema having the strongest association. In comparing patients with isolated MT polyposis to those with paranasal sinus polyposis, Brunner et al. found clinically distinct features as patients with isolated MT polyposis were more commonly younger, female, had lower Lund–Mackay CT scores, and had a significantly higher association with AR compared to those with diffuse polyposis (p < 0.001) (Table XIII.B.3).
In 2017, DelGaudio et al. introduced the term CCAD to describe this distinct variant of sinonasal disease. Further progression of CCAD results in involvement of the sinuses by lateralization or polypoid changes of the MT causing secondary obstruction of the sinuses in a medial to lateral progression. In a multi‐institutional case series including 15 patients, all patients had symptoms consistent with AR and allergen sensitization was seen in the 14 patients who underwent allergy testing. Based on computational fluid dynamics, the proposed pathophysiology is a local immune response related to antigen deposition in CC structures exposed to inhaled allergens. To further characterize CCAD, Roland et al. described radiologic features that differentiate CCAD from other CRSwNP subtypes, including oblique MT orientation, septal involvement, and lower Lund–Mackay score.
While there is conflicting data regarding the association between allergy and CRS in general, there is evidence to support an association between allergy and CCAD. In a subtype analysis of patients with CRSwNP, Marcus et al. reported significantly higher allergy prevalence in patients with CCAD compared with CRSwNP not otherwise specified (p<0.001). In patients with radiologic features of CCAD, Hamizan et al. noted a significantly higher association with allergen sensitization compared to the non‐CCAD group (p = 0.03). Abdullah et al. reported similar results with 100% of patients with CCAD having sensitization to HDM, compared to only 13.6% of non‐CCAD patients (p = 0.00). Additionally, Lee et al. found higher blood eosinophil and serum IgE levels, and higher prevalence of allergen sensitization in pediatric patients with CCAD compared to non‐CCAD (p = 0.008). While no association between CCAD and allergy sensitization was noted in CRS patients in East Asia, patients with CCAD had significantly higher peripheral eosinophils (p = 0.001), tissue eosinophils (p = 0.005), and IL‐13 (p < 0.05) and IL‐5 levels (p < 0.05) in MT tissue compared to the non‐CCAD group, suggesting an eosinophilic/type 2 inflammatory response. Radiologic features can be predictive of CCAD, but edema/polyposis of the CC on endoscopy remains the current diagnostic standard. In a study by Lin et al., patients with minor CC radiologic findings and essentially normal endoscopy were included in the CC‐CRSsNP group, which may not meet the definition of CCAD according to DelGaudio et al. While CCAD is a distinct variant of sinonasal disease, CC disease can be found in other processes such as AERD and respiratory epithelial adenomatoid hamartoma, with studies reporting a positive association with AR., ,
Associated conditions – central compartment atopic disease
Aggregate grade of evidence: C (Level 3: 2 studies, level 4: 11 studies; Table XIII.B.3)
XIII.B.4 Aspirin exacerbated respiratory disease
AERD is a chronic inflammatory condition that includes the tetrad of asthma, nasal polyposis, eosinophilic rhinosinusitis, and a non‐IgE‐mediated reaction to inhibitors of the COX‐1 enzyme. Although considered an inflammatory disease that results from dysregulation of arachidonic acid metabolism leading to an overproduction of leukotrienes and not a true allergic condition, there are data that suggest an association between AERD and IgE‐mediated allergy.
Historically, Samter and Beers reported the prevalence of atopy in AERD as less than 3% (n = 182) using the criteria of positive SPT, and either a family history of atopy or a correlation between allergen exposure and clinical symptoms. However, recent evidence supports a higher atopic rate in AERD., , , In one cohort, 200 of 300 (66%) AERD subjects had a history of positive SPT, and in a latent class analysis of AERD sub‐phenotypes, 105 of 201 (52.2%) patients had positive aeroallergen SPT responses, with the most common allergen being HDM (29.6%). In another study that evaluated personal atopic history, SPT, and elevated total and specific IgE, AERD subjects had a higher rate of atopy than controls (53.9% vs. 14%, p < 0.001) (Table XIII.B.4).
When compared to other forms of CRS, greater rates of physician diagnosed AR and positive SPT were found in AERD subjects when compared with CRSwNP subjects (80% vs. 66%, p < 0.001). Recently, a retrospective study investigated the prevalence of atopy in patients with various CRS phenotypes (n = 380) and found that a significantly higher percentage of atopic CRS patients had AERD (9.4% atopic vs. 1.1% non‐atopic subjects).
Although the aforementioned studies demonstrate a higher rate of atopy in AERD compared to other forms of CRS, it should be noted that AERD is not driven by sIgE‐mediated reactions. Even though local IgE levels within AERD nasal polyps are significantly elevated when compared with nasal tissue from other CRSwNP patients and healthy controls, this does not reflect atopic status. Similarly, serum tIgE is often elevated in AERD patients but does not discriminate atopic from non‐atopic AERD populations.
The understanding that AERD is not driven by traditional atopic mechanisms has important ramifications regarding treatment. In a survey of 190 patients with AERD, 86 (45%) of respondents had concomitant AR treated with AIT. More than half did not perceive any clinical benefit, and only 8% reported significant efficacy. This contrasts with non‐AERD patients with AR, in whom rates of improvement with AIT are greater than 80%. The high failure rate of AIT in AERD suggests that amelioration of any atopic component of their symptoms is overwhelmed by the non‐allergic AERD mechanisms. Although it is important to note that AIT has not been properly studied as a treatment option for AERD.
In summary, despite the high rate of concomitant atopy in AERD, symptoms related to inhalant sensitization are not responsible for the majority of AERD symptoms. Therefore, allergen‐directed therapies, such as standard AIT, are unlikely to be efficacious for most AERD patients. Nevertheless, clinicians should elicit atopic histories for contributory comorbid AR, as recent expert guidance suggests routine allergy testing in AERD for sensitization to inhalant allergens. However, AIT may only be highest yield for candidates with obvious seasonal variation to their symptoms and identifiable environmental triggers.
Associated conditions – aspirin exacerbated respiratory disease
Aggregate grade of evidence: C (Level 3: 3 studies, level 4: 3 studies; Table XIII.B.4)
XIII.C Conjunctivitis
Although the association between AR and AC is well recognized, accurate insight into ocular allergy prevalence is complicated by multiple factors., Most prevalence studies use variable definitions of AC and may employ several different assessment questionnaires. Additionally, most studies do not distinguish specifically between AR and AC symptoms. Rather, AC is considered a secondary manifestation of AR., There is phenotypic diversity of both AR and AC, with very few studies adequately characterizing the phenotypes of their study samples. Further, many epidemiologic studies are based solely on subjective questionnaires rather than incorporating objective evidence of allergic sensitization (Table XIII.C).
Overall, there is a significant burden of associated AC in patients with AR. In the US, the 1988–1994 NHANES III survey (n = 33,994) found a 30% prevalence of concomitant AR and AC. Isolated ocular symptoms were reported by 6%, more frequently in patients over 50 years old – which may be attributable to dry eye and concomitant ocular conditions contributing to symptom severity. AC was associated with skin test positivity to all allergen classes except mold.
Similar AC prevalence trends are echoed globally,, , , , , with higher rates noted in some studies. In one report, 95% of 187 Australian patients with allergist‐diagnosed AR reported ocular allergy. A Swiss survey of hay fever patients showed 85% prevalence of concomitant nasal and eye symptoms. A cross‐sectional Italian study of 2150 adolescents determined that more than half of the respondents with AR also had AC. Comorbid AC also conferred an increased risk of asthma (OR 5.23) versus AR alone (OR 2.28).
The largest global data source regarding the AR–AC association derives from the ISAAC investigations, a series of worldwide studies established in 1991 with the aim of investigating the epidemiology of allergic diseases. ISAAC used a standardized questionnaire and obtained unified assessments of the time trends of the global prevalence in different regions or countries. Current rhinoconjunctivitis was defined as self‐reported “current rhinitis” along with a positive answer to “In the past 12 months, has this nose problem been accompanied by itchy‐watery eyes?”
ISAAC Phase 1 reported AC prevalence in 257,800 children aged 6–7 years in 91 centers (38 countries) and 463,801 children aged 13–14 years in 155 centers (56 countries). Although the ISAAC survey was not validated for the diagnosis of AC, ISAAC studies support the frequent association of AR with itchy/watery eyes; Phase 1 results revealed that ocular symptoms affect 33%–50% of children with AR. ISAAC Phase 3 analyzed temporal trends in prevalence of allergic rhinoconjunctivitis over 7 years in the two age groups (n = 498,083). There was a global increase in rhinoconjunctivitis prevalence, with considerable heterogeneity between test centers. The average overall prevalence of allergic rhinoconjunctivitis was 14.6% for adolescents.
Recently, the Global Asthma Network used ISAAC methodology to update the prevalence of pediatric atopic diseases. The study surveyed 74,361 adolescents and 45,434 6–7‐year‐olds from 27 centers (14 countries). Overall, the prevalence of current rhinoconjunctivitis had decreased slightly from ISAAC Phase 3 among young children (−0.44%) and adolescents (−1.32%). Additionally, an analysis of 2914 patients from the Alergológica 2015 study revealed AC in one‐third of participants, and AC was associated with AR in 88%. The duration and severity of AC was also associated with that of AR (p < 0.001).
Underreporting of ocular allergy may be attributable to symptom variability and increased attention to non‐ocular allergy symptoms. Although the burden of illness (i.e., QOL impairment) associated with AC is established, AC is often underrecognized and undertreated except when severe. More than half of AR patients endorsed that red/itchy/watery eyes were moderately to extremely bothersome in the Allergies in America Survey. Another survey of allergic rhinoconjunctivitis patients (n = 2765) ranked red/itchy eyes as the second most bothersome symptom after nasal obstruction.
Ocular allergy symptoms also contribute significantly to QOL impairment associated with AR. Ocular symptoms of allergic rhinoconjunctivitis are among the most common symptoms which cause patients to seek allergy treatment. When assessing AR patients, one should evaluate ocular symptoms and consider treatment specific to AC. AIT may have a role in AC management; however, most studies investigating AIT efficacy have studied allergic rhinoconjunctivitis rather than AC alone. In a prospective study of patients with AC receiving SCIT or SLIT, both groups had similar rates of clinical improvement in terms of decreased symptoms, medications, tIgE and skin test wheal diameters after 1 year.
Associated conditions – allergic conjunctivitis
Aggregate grade of evidence: C (Level 2: 4 studies, level 3: 8 studies; Table XIII.C)
XIII.D Atopic dermatitis
AD is a chronic/relapsing, inflammatory skin disorder characterized by recurrent eczematous lesions and pruritis that affects all ages and ethnicities. AD is the leading cause of the global burden from skin disease. AD is associated with increased risk of multiple allergic comorbidities, including food allergy, asthma, and AR., AD that starts in infancy usually precedes the development of other atopic diseases, and therefore, is considered the first step of the “atopic march,” or an early marker of the predisposition toward type I hypersensitivity.,
AD and AR are the most prevalent allergic diseases, but many epidemiological studies focus on asthma; only 15.7% and 24.5% of epidemiological studies provide data on AD and AR, respectively. Studying the epidemiology of AR and its comorbidities, in particular AD, is complicated by different disease definitions and reporting, and different testing to confirm diagnoses. In one study, for example, less than half of all patients reporting AR had a physician‐confirmed diagnosis of AR. Therefore, the link between AR and AD remains poorly defined due to methodologic differences and limitations of the studies that have examined this association, , , , , , , , , , , , (Table XIII.D).
The largest study to assess the association between AR and AD was based on data collected in the ISAAC study, which started in 1991 and aimed to investigate the epidemiology and etiology of asthma, rhinitis, and AD in each country using standard questionnaires, SPT, and flexural dermatitis examination. The study involved 256,410 children age 6–7 years in 90 centers from 37 countries, and 458,623 children age 13–14 years in 153 centers from 56 countries, demonstrating a prevalence of AD between 5% and 20%. Several longitudinal studies show improvement or resolution of AD with age, but children often remain atopic for the rest of their lives with a prevalence of AR among those with AD ranging from 15% to 61%., , ,
Multiple studies performed in different countries and age groups, using a variety of methodologies, conclude that there is a disease association between AR and AD. The available evidence suggests that there is a two‐ to four‐fold increase in AR among people with AD., , , , , , , , , , , , For example, in the cross‐sectional multicenter study titled “Epidemiology of Allergic Diseases in Poland” conducted in children age 6–7 and 13–14 years and adults aged 20–44 years, allergic diseases were common in children and young adults. Single disease AR occurred in 29.3% and AD in 7.2%. A single disease (asthma, AR, or AD) was observed in 27.7% of the subjects and allergic multimorbidity was noted in 9.3%. Allergic multimorbidity was more common in children (10.7%–10.9%) than in adults. There was an increasing risk of multimorbidity depending on the number of positive SPTs.
High prevalences of AR and AD were also shown in an independent Phase 3 follow‐up study of unselected 8th‐grade school children in Denmark participating in the Odense Adolescence Cohort Study. The participating children were reassessed after reaching 28–30 years of age. The lifetime prevalence of atopic diseases increased significantly from adolescence (31%) to adulthood (57%), particularly AR (incidence 17.5/1000 person‐years). The lifetime prevalence of AD was 34.1%. Childhood predictors for adult AR were AR, asthma, asymptomatic sensitization to pollen, and AD (OR 1.7; 95% CI 1.1–2.5, p = 0.021). Seven percent of subjects with AD developed AR.
The Canadian Healthy Infant Longitudinal Development study recruited pregnant women from the general population across four Canadian provinces and followed them until their children were 5 years old. The authors defined five distinct classes of individuals: healthy (81.8%), AD (7.6%), inhalant sensitization (3.5%), transient sensitization (4.1%), and persistent sensitization (3.2%). Children in the AD groups were at increased risk of developing AR (OR 2.36; 95% CI 2.13–2.62).
The increased risk of AR in patients with AD has been seen in multiple studies using different research strategies (i.e., prospective, population‐based, cross‐sectional) in different age groups and in different continents (Asia, Europe). This supports the notion that AR and AD are related diseases., , , , , , , , , , ,
Associated conditions – atopic dermatitis
Aggregate grade of evidence: C (Level 2: 16 studies, level 3: 12 studies, level 4: 3 studies; Table XIII.D)
XIII.E Food allergy
XIII.E.1 Pollen food allergy syndrome
Immune responses to foods may produce a spectrum of symptoms and disorders including pollen food allergy syndrome (PFAS; also known as oral allergy syndrome [OAS])., PFAS is an IgE‐mediated allergy which localizes to the oral mucosa, leading to transient itching, perioral hives, angioedema, and rarely systemic symptoms. Patients with pollen allergies may have allergic reactions confined to the oral cavity after consuming specific fruits, vegetables, nuts, or spices. PFAS symptoms manifest as a result of cross‐reactivity of IgE specific for an offending pollen with highly homologous proteins found in a variety of fruits, vegetables, and nuts. The most common example of this cross‐reactivity in Western populations is birch pollen and apples, which is due to the high degree of sequence homology between Bet v 1 (major allergen of birch pollen) and Mal d 1 (major allergen of apple), leading to IgE‐mediated cross‐reactivity. Table XIII.E.1.‐1 lists common pollen allergens with plant‐derived foods that may demonstrate cross‐reactivity. A 2018 review by Carlson et al. reported PFAS prevalence ranged from 4.7% to over 20% among children and 13%–58% among adults, with prevalence varying widely by geographic region. A study conducted in 1360 Italian children with pollen‐related AR noted that a longer duration of AR symptoms was related to developing PFAS, suggesting that individuals living in areas with more pollen seasons have a higher rate of PFAS, possibly reflecting the higher range of prevalence in adults., Table XIII.E.1.‐2 summarizes the evidence link between PFAS and AR.
The diagnosis of PFAS is typically established by a detailed history and physical exam that explores a given patient's underlying allergy to pollen and raw foods with shared homologous proteins. As per the Joint Task Force Practice Parameters, sIgE testing to pollens is recommended in patients with a suggestive clinical history. The estimated rates of systemic and anaphylactic reactions from a pollen‐food allergy are 10% and 2%–10%,, respectively, and such a history must be thoroughly elicited. The gold standard for establishing a diagnosis of PFAS is a double‐blind food challenge, but this can still be confounded by biases inherent to the appearance, texture, and taste of foods. It is important to note that skin testing using commercially available fruit or vegetable extracts may not be useful as the allergens are heat labile. Oral food challenge, SPT, and food sIgE levels have also been used to diagnose PFAS or food allergy., , , Another technique that has also shown promise in accurate diagnosis of PFAS and food allergy is CRD utilizing pure and potentially cross‐reactive allergenic components in certain foods. This has been demonstrated in refining diagnosis of true peanut allergy, where the component Ara h 2 has been identified as a better predictor of clinical allergy.
The standard recommendation for the treatment of PFAS has been to identify and eliminate offending foods from the diet. There is no consensus on whether patients should be provided auto‐injectable epinephrine. Some pollen‐associated foods may lose their cross‐reactivity potential once the often‐labile proteins are denatured by heat. In one study, food challenges were performed with apple, carrot, or celery in patients with AD and birch pollen allergy, who reported oral allergy symptoms and dermatologic symptoms upon ingestion of the raw foods. Cooked versions of the offending foods did not cause oral allergy symptoms.
Several studies have evaluated the effect of targeted AIT for pollen allergy at reducing PFAS symptoms with mixed results. There has been some published evidence of pollen‐specific AIT resulting in increased tolerance to the PFAS‐associated offending foods., , , However, one RCT failed to demonstrate any improved tolerance to apple in birch allergic patients treated with birch specific AIT compared to placebo. One study evaluating the persistence of tolerance for apple after birch AIT demonstrated that AIT resulted in increased apple tolerance for some patients up to 30 months; however, there was no difference between the AIT and control groups. Currently, AIT is not recommended for the sole purpose of treating PFAS, although patients receiving AIT should be counseled on the potential benefit of improved food tolerance (Table XIII.E.1.‐3).
Associated conditions – pollen food allergy syndrome
Aggregate grade of evidence: C (Level 3: 3 studies, level 4: 5 studies, level 5: 5 studies; Table XIII.E.1.‐2) for link between AR and PFAS, including cross‐reactivity; C (Level 2: 2 studies, level 3: 2 studies; Table XIII.E.1.‐3) for AIT in treatment of PFAS
XIII.E.2 Anaphylactic food allergy
Like AR, food allergy may be driven by an IgE‐mediated response and as a result may sometimes lead to anaphylactic reactions. There is an abundance of consistent evidence, largely in the form of large sample cross‐sectional and retrospective analyses, that the occurrence of food allergy is independently associated with AR, , , , , , , , , , , , , , , (Table XIII.E.2). In an analysis of over 8000 families, Alm et al. found a strong, independent association between the development of food allergy and AR (OR 10.21; 95% CI 4.22–24.73). A separate analysis of more than 300,000 children by Hill et al. found that a diagnosis of food allergy was highly associated with later development of AR (OR 2.72; 95% CI 2.45–3.03).
Peanut allergy is one of the most common and well‐studied food allergies, and its prevalence has been linked to AR in the existing literature., , , Similarly, AR is a relatively more common atopic condition among people with allergies to shellfish,, , , and specifically shrimp., , Identifying infants at high risk of peanut allergy and introducing peanuts to them early can significantly decrease the frequency of developing peanut allergy, ; however, it is currently unclear whether such measures can have a protective effect on developing AR in the future. There is reported low‐ to very low‐certainty evidence that early fish introduction to the diet before age 6–12 months can be associated with reduced AR before age 14.
Long‐term management of food allergies mainly includes identification and avoidance of each food item and provision of counseling regarding food‐related systemic or anaphylactic reactions; in some circumstances, oral immunotherapy may be an option. Epinephrine auto‐injectors with associated instructions for use should be provided to patients who are at risk for anaphylactic reactions., Finally, there are ongoing studies investigating several possible type 2 targeted biologics in treatment of food allergy.
It is suggested that AIT is perhaps the only possible disease‐modifying treatment for allergic diseases by inducing long‐term tolerance against specific allergens. AIT prompts the inhibition of early and late‐phase allergic responses and induction of immunological tolerance of AR and food allergy via diverse mechanisms on T cells (e.g., Th1/2, Treg), regulatory B cells, innate lymphoid cells, dendritic cells, mast cells, eosinophils, and basophils. When studied separately, AIT treatment has been shown to lead to several years of symptomatic remission in AR, or sustained responsiveness for various food allergies.,
Associated conditions – anaphylactic food allergy
Aggregate grade of evidence: C (Level 1: 1 study, level 2: 3 studies, level 3: 6 studies, level 4: 9 studies, level 5: 1 study; Table XIII.E.2)
XIII.F Adenoid hypertrophy
Children with AH and AR may exhibit similar symptoms including nasal obstruction and rhinorrhea. Adenoids commonly enlarge through the preschool years but typically involute with puberty.,
Literature evaluating the relationship between AH and allergic sensitization draws from two populations. The first is allergic children assessed for AH. Several studies assessing allergic children found an association with AH. In one study, the prevalence of AH in 1322 allergic children (12.4%) was higher than in 100 age‐matched non‐allergic controls (3%), p < 0.0001. Similarly, Dogru et al. found a relatively high rate (21.2%) of AH amongst 566 children with AR. Modrynksi and Zawisza reported that seasonal adenoid enlargement in birch pollen allergic children was more frequent than in controls but the increased adenoid size resolved after pollen season. However, this study was small (n = 67) and did not comment on blinding (Table XIII.F).
Three cohort studies have assessed the relationship of mold sensitivity and AH with mixed results. Atan Sahin et al. compared 242 children living in an arid environment to 142 children living on the coast and found no correlation between mold and pollen sensitization with AH. However, HDM‐sensitive children in the coastal group had an increased prevalence of AH (p = 0.01). Huang and Giovanni compared 315 children who had AH with AR to age‐matched controls with AR alone and found a higher prevalence of mold sensitivity in AH with AR versus AR alone (p = 0.013 to p < 0.0001). Dogru et al. also reported an increased sensitization to Alternaria in the AH with AR group compared to AR alone (p = 0.032).
The second population studied is children suspected of AH who are assessed for allergic sensitization; these studies also have mixed results. Cassano et al. reported that inhalant allergen sensitization decreased as AH size increased. Karaca et al. compared allergy sensitization to radiographic adenoid size in 82 children and found no association. Ameli et al. assessed 205 children with nasal endoscopy and SPT and found a negative association between SPT positivity and adenoid volume (p < 0.0001). Conversely, Sadeghi‐Shabestari et al. compared SPT results and tIgE levels amongst 117 children with adenotonsillar hypertrophy (ATH) and 100 controls. Over 70% of the ATH group had a positive SPT versus 10% of the control group (p = 0.04), but this study is limited by the inclusion of SPT for foods (highest positive allergen subgroup) and latex.
In two additional studies, children referred from allergy practices were assessed for both AH with nasal endoscopy and SPT sensitivity. Both studies excluded children on allergy medication and observed a significant negative correlation between AH and SPT positivity (r = −0.208, p = 0.009) and (p = 0.04). The variability in study population recruitment and age range may explain the mixed findings.
Several studies have found immunologic evidence of allergic physiology in adenoid tissue. Ni et al. found a higher Th17/Treg ratio in adenoid tissue from children with AR versus non‐allergic controls. Masieri et al. reported Th1 gene expression in non‐allergic adenoid tissue, Th1 and Th2 gene expression in adenoid tissue of children with AH and AR, and downregulation of Th1 and Th2 gene expression in adenoid tissue during SLIT. Zhu et al. found increased tissue eosinophilia and markers of Th2 inflammation in the adenoid tissue of children with AH with AR, compared to AH alone. Local allergy may also play a role. One cohort of 102 children with ATH showing 53.9% sero‐atopy and 68.6% with sIgE detected in their adenotonsillar tissue. sIgE positive adenoid tissue was found in 36.2% of the sero‐negative children. Independently, Shin et al., detected HDM and Alternaria local sIgE in adenoid tissue. Therefore, studies of allergic markers in adenoid tissue are present more often in atopic children, and there is some evidence of local allergic sensitization in children testing negative for sero‐atopy.
The effect of INCS on reducing nasal obstruction in the setting of AH has been demonstrated in systematic reviews and is independent of allergy., Whether INCS reduce adenoid size is unclear. One retrospective study (n = 47) reported improvement in rhinitis symptoms in similar percentages of AR (86%) and non‐allergic rhinitis (76%) after adenoidectomy. At least one study suggests that AR is a risk factor for refractory nasal symptoms after adenoidectomy.
In summary, AH occurs in allergic children more often than non‐allergic controls., , A recent systematic review concluded that clinical and biomarker evidence favored an association between allergy and AH. However, in children referred to otolaryngology for nasal obstruction, the association between allergic sensitivity and AH is inconsistent., , , , One possible explanation for this discrepancy is that symptomatic AH peaks earlier in childhood than AR. This is supported in the literature by Pagella et al, who reviewed records of children referred to otolaryngology for nasal symptoms (n = 795) and found no association between AR and AH in children aged 1–7 years (p = 0.34), but noted an association for children aged 8–14 years (p = 0.0043).
Associated conditions – adenoid hypertrophy
Aggregate grade of evidence: C (Level 2: 1 study, level 4: 12 studies; Table XIII.F)
XIII.G Otologic conditions
XIII.G.1 Eustachian tube dysfunction
The Eustachian tube (ET) is a bony and cartilaginous canal that connects the middle ear to the nasopharynx and functions to equalize pressure between the middle ear and the environment, protect the middle ear from harmful sounds and nasopharyngeal pathogens, and provide mucociliary clearance of middle ear secretions., Obstructive ETD refers primarily to ventilatory dysfunction and is considered to have multifactorial etiologies including inflammation around the ET orifice (e.g., upper respiratory tract infection, rhinosinusitis, and reflux), pressure dysregulation (e.g., air travel, scuba diving), and obstructive lesions (e.g., nasopharyngeal tumor, AH). Evidence suggests a causal role of AR in the etiology of ETD due to allergic secretions, nasal mucosa edema, and hypersecretion of nasal cavity seromucous glands, all resulting in obstruction of the ET lumen., ,
Data supporting a causal role of AR in the development of ETD comes from experimental studies using intranasal and transtympanic allergen challenges. Multiple studies have demonstrated transient ETD following allergen challenges in adult and pediatric subjects with, , , and without AR, as well as in animal models,, , although ET responses have not been found to correlate with IgE levels (Table XIII.G.1).
In addition to experimental evidence suggesting a link between AR and ETD, observational data also supports this association. For example, ET obstruction is observed during natural exposure to allergens during pollen season, even without subjects being intranasally or transtympanically challenged., Furthermore, in a representative adult cohort from the NHANES data, odds of reporting allergies was 1.71 times higher in subjects with ETD compared to those without ETD. Similarly, a pediatric population study found that significantly more children with AR had abnormal tympanograms compared to those without AR. Histologically, increased levels of allergic cytokines such as IL‐4, IL‐5, and eosinophils have been found at both ends of the ET, suggesting that an allergic response could be activated at the ET in sensitized patients.
However, despite both experimental and observational data supporting an association between allergy and ETD, studies have failed to consistently demonstrate improvement in ETD and its associated symptoms with allergy treatment. Gluth et al. found no significant normalization of abnormal tympanometric signs and no improvement in ETD symptoms between patients treated with INCS and those in placebo groups, and a clinical consensus statement found no role for systemic decongestants, antihistamines, nasal topical decongestants, or INCS in the diagnosis or treatment of patients with ETD. On the other hand, Pollock et al. found that ETD could be prevented in sensitized rats when pre‐treated with IL‐4 receptor decoys, and Derebery et al. reported improvement in the ETD symptom of ear fullness in allergic patients treated with AIT in a retrospective case series (although the presence of reported food allergy in this group may confound the results).
Overall, there is experimental and observational evidence to support a causal role of allergy in the development of ETD. However, the exact pathophysiologic mechanism behind this association is unclear since not all patients with ETD have AR, and traditional allergy treatment has not consistently shown benefit in reducing symptoms of ETD.
Associated conditions – Eustachian tube dysfunction
Aggregate grade of evidence: C (Level 2: 1 study, level 3: 12 studies, level 4: 3 studies; Table XIII.G.1)
XIII.G.2 Otitis media
OME is a common pediatric condition characterized by pressure changes and inflammation in the middle ear resulting in serous or mucoid fluid buildup behind the tympanic membrane. A relationship between middle ear effusion (MEE) and allergy and has long been a subject of epidemiologic study. The reported prevalence of allergy amongst patients with OME has varied widely, from essentially no difference compared to controls,, to varying degrees of difference,, , , , , , , to a near universal association., , , , , However, cross‐sectional studies and one recent SRMA have reported that AR and atopy are independent risk factors for OME., , The inconsistencies of findings in these observational studies likely represent differences between highly selected populations and OME diagnostic criteria, variability of allergy testing methods, and sensitivities and the challenges of accounting for cofounders, such as age or OME phenotype (Table XIII.G.2).
Proposed pathogenic mechanisms of the development of OME center around Eustachian tube dysfunction; and theories regarding causal mechanisms that directly link allergy and otitis media without concurrent Eustachian tube dysfunction are controversial. (See Section XIII.G.1. Eustachian Tube Dysfunction for additional information on this topic.) Some have proposed that the middle ear itself can be a site of targeted allergic reaction. Several cohort studies suggest that the middle ear is capable of developing a local IgE‐mediated inflammatory reaction irrespective of a systemic inflammatory reaction., , , Additionally, type 2 inflammatory patterns, such as eosinophil growth, mucus production, and mast cell presence, have been found in effusions of atopic patients when compared to non‐atopic patients., , Furthermore, the chemoattractant cytokine RANTES, ECP, IL‐4, IL‐5, and MBP were found to be higher in effusions of atopic children than non‐atopic children., , , , Arguably the strongest evidence to date directly establishing the middle ear as an allergic target and linking it with the upper airway is the presence of similar cytokine expression patterns from biopsies of middle ear and nasopharyngeal specimens in atopic patients with OME.
Despite evidence suggesting that the middle ear is a site of allergic inflammation in patients with OME, high quality evidence has failed to demonstrate significant improvement or resolution of effusions after traditional allergy treatments. Placebo‐controlled RCTs have shown that INCS do not improve OME outcomes., Two Cochrane reviews have demonstrated the statistical ineffectiveness of antihistamines, decongestants, antihistamine/decongestant combinations, and INCS in resolution of OME., In two RCTs of children with OME, LTRAs provided no benefit over placebo in resolution of effusions., Finally, though one prospective cohort demonstrated a significant improvement in OME after targeted SCIT compared to a group of controls self‐selected to avoid AIT, some aspects of the study design are flawed, including significant selection bias and inclusion of a generally older population than that most affected by OME.
In summary, observational studies provide low grade evidence of an association between allergy and OME. Nevertheless, moderate grade evidence from histologic studies suggest that the middle ear could be a primary site of allergy. Additionally, a high level of evidence suggests that traditional allergy treatment is not effective in resolving OME.
Associated conditions – otitis media
Aggregate grade of evidence: C (Level 1: 3 studies, level 2: 8 studies, level 3: 1 study, level 4: 24 studies; Table XIII.G.2)
XIII.G.3 Meniere's and inner ear disease
Meniere's disease is a chronic condition that occurs almost exclusively in adults and is characterized by aural fullness, tinnitus, fluctuating sensorineural hearing loss (SNHL), and episodic vertigo. While the underlying pathophysiologic mechanism of Meniere's disease remains uncertain, it is associated with a dysregulation of inner ear fluid volume resulting in endolymphatic hydrops. Theories linking allergy to Meniere's disease have centered on the role of the endolymphatic sac in the development of hydrops and clinical symptoms through its release of allergic mediators or its susceptibility to circulating immune complexes and dormant viral antigens. A causal relationship between allergy and Meniere's disease is supported by limited studies, though there have been a number of observations of association between Meniere's disease and allergic conditions. Patient‐reported and physician‐reported data suggest that Meniere's disease patients have higher rates of concurrent AR than expected in the general population and have increased odds of allergies versus controls. Similar patient‐reported data suggests higher rates of allergy and migraine in Meniere's disease patients. Overall, these studies generally provide low grade evidence (Table XIII.G.3).
Objective evidence of heightened immunopathologic profiles and reactivity in Meniere's disease patients has been mixed. Higher rates of serum IgE levels were observed in Meniere's disease patients versus controls,, as well as in patients with acute low frequency SNHL compared to those with sudden SNHL. However, in another small study, there was no difference in serum tIgE levels between Meniere's disease and controls. In two small studies, electrocochleographic summation potential/action potential [SP/AP] ratios increased in response to allergen challenge in Meniere's disease patients,, suggesting that allergy may worsen endolymphatic hydrops. Likewise, serum IgE levels were found to correlate with elevated SP/AP ratios in patients with low frequency SNHL. Overall, studies on IgE levels and electrocochleography are of low‐grade evidence with significant shortcomings in design.
Lastly, there have been two studies on the treatment of allergies in Meniere's disease patients, both of low‐grade evidence, suggesting that AIT results in improvement of Meniere's disease symptoms in patients with concurrent allergies (although potentially confounded by inclusion of non‐IgE‐mediated food allergy)., However, a double‐blind RCT, expected to conclude in April 2022, is being conducted to investigate the efficacy of a leukotriene inhibitor in reducing vertigo and hearing loss in Meniere's disease patients. In conclusion, though observational studies have found associations between Meniere's disease and allergy, no data to date supports reflexive allergy testing and treatment in Meniere's disease patients without a concurrent history of allergies.
Associated conditions – Meniere's and inner ear disease
Aggregate grade of evidence: C (Level 2: 1 study, level 3: 1 study, level 4: 10 studies; Table XIII.G.3)
XIII.H Cough
Cough clears the lower airways of irritants. Vagal afferent nerves regulate involuntary cough, yet there is cortical control of the overall visceral cough reflex. AR has been associated with cough. Allergens may stimulate the nasal mucosa, resulting in the rhinobronchial reflex and bronchospasm. Inflammation in the upper airways with eosinophil activation and cytokine release may also lead to inflammation of the lower airways and cough. There is a complex interplay between cells and inflammatory cytokines, and the upper and lower airways can be considered a single functional unit. The exact pathways and mechanisms of this unified airway model continue to unfold.
Patients with AR and concomitant cough may have asthma and/or a nonspecific bronchial hyper‐reactivity, and generalized inflammation of the upper and lower airways can be present. Patients with cough and AR may cough due to their underlying asthma. However, many patients with AR and cough do not have the diagnostic airflow obstruction or bronchodilator‐associated FEV1 reversibility that is necessary to meet asthma diagnostic criteria. Krzych‐Falta et al. performed nasal allergen challenges in AR patients and noted extra‐nasal symptoms, including cough and breathlessness, especially in those with perennial AR. Additionally, Chakir et al showed increased lymphocytes, eosinophil recruitment, and IL‐5 expression in the bronchial mucosa after exposure with natural pollen in patients with AR without current or prior asthma. The same group noted deposition of type I and III collagens and fibronectin by bronchial myofibroblasts in patients with AR in a previous study, suggesting structural remodeling of the lower airways in patients with AR which was similar to asthma, albeit less severe. In an animal model, HDM‐sensitized guinea pigs had a significantly enhanced cough response compared to non‐sensitized animals. These studies demonstrate that AR, independent of asthma, may result in bronchial inflammation, lower airway remodeling, and ultimately cough (Table XIII.H).
Several publications in 2016 reported results of relatively large studies evaluating the characteristics of respiratory diseases in the Asia Pacific region. In a 1000‐person cross‐sectional observational study, it was noted that patients with asthma and/or COPD present to physicians with a primary complaint of cough, whereas AR patients typically present with watery rhinorrhea and/or sneezing., In addition, combined respiratory disease may be seen; this occurred in 33.5%, with the most common combination being AR and asthma., A multi‐country observational study of 5250 subjects reported that 47% of patients with AR reported cough; however, only 11% of these patients reported cough as the main reason for seeking medical care. Interestingly, for patients with asthma, 61% reported cough, and for 33% cough was the primary reason for seeing medical care. In a prospective study of 2713 patients with AR, He et al. found the prevalence of comorbidities, including cough, to gradually increase with increasing AR severity and frequency.
Publications from 2020 to 2021 provide additional evidence to support the association between cough and AR. In two RCTs that enrolled patients with either refractory or unexplained cough, concomitant AR was present in 15% and 20% of patients. Kim et al. found that more patients presenting with AR for allergy testing reported cough in the 2010s (27.9%) compared to the 1990s (22%). Increasing evidence associates AR with cough or, more commonly, cough as a comorbidity of AR., , Therefore, diagnostic and treatment modalities for cough in patients with AR have an increasingly important role.
Recent studies have proposed FeNO as a tool to differentiate causes of cough in patients with AR. Elevated FeNO is associated with airway eosinophilia in asthma patients. Elevated FeNO may raise suspicion for AR in patients with cough variant asthma or cough predominant asthma., When AR and chronic cough are both present, FeNO may be able to differentiate between chronic cough due to cough variant asthma or non‐asthmatic eosinophilic bronchitis from other forms of chronic cough.,
It is not clear if treatment of AR with INCS improves the associated cough,, but an RCT by Kim et al. suggests that nasal saline irrigations decrease cough associated with AR. Posterior nasal neurectomy with or without pharyngeal neurectomy in patients with AR may decrease cough.
Associated conditions – cough
Aggregate grade of evidence: C (Level 2: 3 studies, level 3: 3 studies, level 4: 11 studies, level 5: 1 study; Table XIII.H)
XIII.I Laryngeal disease
AR and inhalant allergy have been associated with laryngeal disease; however, understanding of their precise role in laryngeal disease is limited. This section evaluates studies that examine the relationship between inhalant allergy and laryngeal disease, including allergic laryngitis. Allergic laryngitis is characterized by allergen‐induced laryngeal inflammation and can present with dysphonia, coughing, throat clearing, and globus. Some studies have evaluated laryngeal symptoms in individuals with AR while others have evaluated the direct effects of allergen exposure on the larynx (Table XIII.I).
Establishing a causal relationship between AR and laryngeal disease has proven difficult, although associations have been reported. Lee et al. found an association between the diagnosis of chronic laryngitis and AR in a Korean nationwide cohort. Subsequently, Wang et al. identified a strong association between AR and developing laryngeal pathology in a Taiwanese nationwide cohort. Several studies have reported higher Voice Handicap Index (VHI) scores in AR patients versus controls., , , Ohlsson et al. reported that vocal symptoms in those with AR worsen during the allergy season and may be associated with a decrease in speech fundamental frequency. Velickovic et al. found that overall AR is common and occurs in 44.2% of professional voice users presenting with dysphonia. Singers with self‐perceived voice issues were 15% more likely to have AR than those without vocal complaints. The likelihood of AR increased as the number of vocal symptoms increased.
The adverse effects of AR on voice‐related QOL have also been reported,, , and Turley et al. supported this association by showing that patients who reported poor rhinitis‐related QOL also had poor voice‐related QOL and increased severity of chronic laryngeal symptoms. Furthermore, increased allergen load was associated with greater severity of vocal symptoms. Overall, there is a higher than anticipated incidence of AR in patients with vocal dysfunction and vice versa., , ,
Findings of laryngeal inflammation have largely been attributed to laryngopharyngeal reflux (LPR), but recent studies have questioned its role as the primary source of laryngeal dysfunction., Allergic laryngitis associated with AR can be difficult to distinguish from other laryngeal inflammatory disorders, including LPR, due to limitations of current diagnostic methods including poor specificity and inter‐rater reliability. Patients with clinically significant LPR may be more likely to report AR symptoms. However, the opposite may be true in professional voice users presenting with dysphonia. Randhawa et al. studied patients presenting with voice concerns and reported one‐third were diagnosed with LPR, whereas two‐thirds of patients were diagnosed with allergies. Laryngeal findings in LPR and allergic laryngitis and LPR may be similar; laryngeal edema, laryngeal erythema, and excessive thick mucus are often seen., Eren et al. demonstrated no significant difference in laryngeal appearance between allergy‐positive and LPR‐positive subjects. However, thick endolaryngeal mucus may predict allergy.
Several studies have evaluated the direct effect of allergens on the larynx. Belafsky et al. and Mouadeb et al. examined Dermatophagoides farinae exposure to the laryngeal mucosa of guinea pigs and found an increase in eosinophilia compared to saline exposure, providing some support for allergens contributing to laryngeal disease. Two studies from the same voice laboratory evaluated direct laryngeal stimulation by nebulized Dermatophagoides pteronyssinus in allergic patients to assess laryngeal symptoms, appearance, and function., In the first study, Reidy et al. did not identify a significant difference between antigen‐ and placebo‐challenged subjects on any of the evaluated measures, such as VHI, Sinus Symptoms Questionnaire, laryngoscopy, and acoustic/aerodynamic testing. In a follow‐up, Dworkin et al. used increased allergen concentration for the challenge and noted an increase in endolaryngeal mucus, throat clearing, and coughing. Roth et al. performed a similar study but isolated the larynx by utilizing a nose clip to ensure oral inhalation and eliminated patients with reactive airways based on methacholine challenge, thus demonstrating a causal relationship between allergen stimulation and impaired vocal function. Suzuki et al. also utilized a nose clip and found more laryngeal symptoms when patients were exposed to cypress pollen compared to placebo. However, there were no corresponding objective changes in acoustic analysis or flexible laryngoscopy. These studies suggest that in subjects with inhalant allergy there can be laryngeal dysfunction due to direct allergen stimulation of the larynx as well as possible symptoms secondary to the nasal congestion, inflammation, and drainage of AR.
There is increasing evidence suggesting a relationship between AR, inhalant allergy, and laryngeal disease. Although laryngeal findings specific to allergic laryngitis are not consistently demonstrated, thick endolaryngeal mucus should raise suspicion for underlying allergy. AR should be considered in the differential diagnosis of patients with vocal complaints. Additional studies are needed on the effect of AR treatment on associated laryngeal disease.
Associated conditions – laryngeal disease
Aggregate grade of evidence: C (Level 2: 7 studies, level 3: 4 studies, level 4: 10 studies, level 5: 2 studies; Table XIII.I)
XIII.J Eosinophilic esophagitis
EoE is a chronic inflammatory condition of the esophagus defined symptomatically by esophageal dysfunction and histologically by eosinophil‐predominant inflammation. EoE is widely considered a type 2 inflammatory disease, and patients with EoE often have other comorbid atopic conditions such as AD, asthma, food allergies, and AR.
Several studies have examined the prevalence of clinician‐diagnosed AR and aeroallergen sensitization in patients with EoE. Among both pediatric and adult patients with EoE, 50%–75% have consistently been found to have AR., , , , , , , , , , , , , , , , There is also evidence for a higher prevalence of AR among EoE patients compared with the general population., , Although most studies were case series, the consistency of findings strongly suggests that a majority of patients with EoE have comorbid AR and that the presence of AR in EoE patients may be higher compared with the general population (Table XIII.J).
While the above associations have been well documented, the pathophysiology underpinning the specific relationship between IgE sensitization and EoE remains unclear. Hill et al. demonstrated that the presence of AR was associated with subsequent EoE diagnosis, suggesting that sensitization to aeroallergens early in life may predispose to EoE development. Additionally, several case series noted an increase in EoE diagnosis, symptoms, and/or esophageal eosinophilia during pollen season, typically with peaks during spring and summer., , , , , , , AIT has also demonstrated efficacy in the treatment of EoE in one case‐control study and two case reports., , Of note, several case reports described the development of EoE in patients undergoing SLIT and resolution with cessation, raising the possibility that repeated esophageal stimuli with offending allergens might elicit esophageal eosinophilia. However other studies, including a systematic review by Lucendo et al., demonstrated no seasonal variation in EoE diagnosis or exacerbations, suggesting a limited role for aeroallergens as a relevant trigger for initiating or aggravating EoE., , Therefore, there is limited observational data suggesting a potential association between aeroallergens and EoE pathogenesis, with some conflicting data.
Associated conditions – eosinophilic esophagitis
Aggregate grade of evidence: C (Level 3: 6 studies, level 4: 29 studies; Table XIII.J)
XIII.K Sleep disturbance and obstructive sleep apnea
AR negatively impacts sleep and is a risk factor for OSA. Various symptoms of AR may contribute to sleep dysfunction. However, nasal obstruction, which is present in up to 90% of AR patients, seems to have the greatest impact and is a major independent contributor to poor sleep quality and SDB., , , , , , , , , , , This may be due to increased nasal obstruction during the night with a peak in the early morning. The mechanisms underlying the association between AR and sleep disturbance include inflammatory cytokines causing fatigue, direct impact of AR symptoms, combination of recumbency and diurnal variation in turbinate size and pathophysiologic changes, and as sequelae of autonomic dysfunction in AR., , Histamine plays a role in the regulation of the sleep‐wake cycle and arousal, and cysteinyl leukotrienes are involved in sleep disruption., Excessive histamine results in insomnia and inadequate amounts cause hypersomnolence., Cytokines released in AR patients, such as IL‐1β and IL‐4, are thought to reduce sleep onset latency and increase the time to onset of rapid eye movement (REM) sleep., , Patients with OSA also have increased mediators which activate Th2 cells, such as TNF, IL‐1, and IL‐6, further exacerbating symptoms of AR and potentiating the severity of OSA. Further, nasal airflow stimulates respiration and improves upper airway dilatory muscle tone via the nasal‐ventilatory reflex and also stimulates the genioglossus muscle, resulting in tongue protrusion and improved airway patency via the trigemino‐hypoglossal reflex., , , , , Therefore, nasal obstruction may reduce the stimulation of these mechanoreceptors resulting in collapsibility of the downstream pharyngeal segment of the upper airway, thereby leading to OSA (Table XIII.K).
Sleep is critical for mood, cognitive function, immune function, and endocrine functions. OSA is associated with hypertension, coronary artery disease, cerebrovascular disease, arrhythmias, insulin resistance, congestive heart failure, pulmonary hypertension, and behavioral problems in children., , , , , Further, in children, SDB may negatively impact brain development, impair psychomotor, and cognitive performance, and contribute to hyperactivity., , REM sleep is associated with memory, cognition, dreams, and restorative sleep., As the nasal cycle is prolonged, worsening nasal obstruction, people with AR have impaired REM sleep., , , , However, as the diagnosis of SDB typically relies upon the measurement of all‐night AHI and RDI via polysomnography, many patients with AR and SDB have normal indices by this method. By considering respiratory effort‐related arousals, as well as AHI and RDI measured specifically in REM sleep (REM‐AHI, REM‐RDI), sleep disorders in AR patients will be detected more often.
CPAP treatment for OSA may present a non‐allergic trigger to AR patients with OSA and worsen nasal symptoms. Further, persistent nasal symptoms are a common reason for early CPAP non‐compliance., , However, correction of nasal obstruction can improve CPAP compliance/tolerance,, , though there is typically no direct impact on OSA severity.
It is important to assess AR patients for sleep disorders due to their negative impact on health. Numerous instruments are available to assess the impact of AR on sleep. These include the Stanford Sleepiness Score, Jenkins Questionnaire, Epworth Sleepiness Score, Pittsburgh Sleep Quality Index, University of Pennsylvania Functional Outcomes of Sleep, Sleep Scale from the Medical Outcome Study, Sleep Disorders Questionnaire, The Pediatric Sleep Questionnaire, and The Pediatric Daytime Sleepiness Scale.
Treatment of nasal congestion in AR patients improves sleep quality, daytime somnolence, and QOL. Numerous medical therapies have been investigated regarding the link between AR treatment and sleep quality. INCS and isolated nasal surgery have also been shown to improve sleep quality in AR patients, particularly those with moderate‐to‐severe pre‐treatment obstruction., , , , INCS may improve sleep in patients with AR due to improvement in nasal obstruction, but also due to reduction in local inflammatory cytokines., A recent RCT and case series found significant improvements in sleep parameters following AR treatment with HDM SLIT., First generation H1 antihistamines cross the blood–brain barrier and cause sedation which may exacerbate daytime somnolence in patients with AR and SDB. Therefore, newer‐generation H1 antihistamines are favored, such as fexofenadine and loratadine, which are lipophobic and do not cross the blood–brain barrier., , Although leukotriene antagonists have not demonstrated benefit when added to INCS in the treatment of AR, one RCT found that montelukast was more effective than cetirizine in improving sleep quality in children according to patient diaries., Nasal decongestants may result in stimulatory effects causing insomnia. Nasal decongestant sprays do not significantly improve AHI. A crossover RCT comparing xylometazoline to placebo in patients with OSA and nasal congestion found that xylometazoline did not improve sleep quality and resulted in a transient improvement in AHI at the time of peak effectiveness only. As these sprays carry the potential for rhinitis medicamentosa, insomnia, and palpitations, they are not recommended for the treatment of AR in OSA patients.
Sleep disorders should be considered in any patient diagnosed with AR due to their significant association and the negative impact that SDB has on QOL. Changes in sleep parameters should also be considered when evaluating the impact of treatment of AR. (See Section IX.A.2. Allergic Rhinitis Disease Burden – Sleep Disturbance for additional information on this topic)
Associated conditions – sleep disturbance and obstructive sleep apnea
Aggregate grade of evidence: B (Level 2: studies, level 3: 4 studies, level 4: 9 studies; Table XIII.K)
XIV SPECIAL SECTION ON COVID‐19
XIV.A COVID‐19 effect on patient presentation for allergic rhinitis evaluation
The WHO declared COVID‐19 a pandemic on March 11, 2020. With mounting evidence of rapid spread, high morbidity and mortality, and a push to maintain the healthcare system infrastructure, routine ambulatory care for conditions like AR was often reduced. As the pandemic endured, expert group consensus generally applied different recommendation strategies depending on case rates. When case rates were high, it was reasonable to suspend care temporarily, particularly if providers and healthcare facilities were redeployed., However, as case rates fell, it was necessary to find ways to evaluate patients for AR., Telemedicine, using phone or video where available, was rapidly implemented and provided significant access to specialty care while limiting exposure for patients and providers., , , , However, implementation of telemedicine practices may exacerbate gaps in access for populations already at risk for health disparities.
Another evident issue became the similarities in presentation between AR and COVID‐19, and it was important to identify ways to differentiate the diseases., AR was not a risk factor for severe COVID‐19 infection., , , , , , , The consensus from a survey distributed to members of the ARIA/EAACI study group was that AR presented with runny nose, sneezing, stuffy nose, nasal pruritus, ocular pruritis, and redness compared to COVID‐19 which presented with more smell and taste dysfunction, dyspnea, and cough. Patients scored validated questionnaires like the SNOT‐22 and mini‐RQLQ differently., SNOT‐22 scores were higher in patients with COVID‐19 infection (with more frequent cough, dizziness, loss of smell/taste, psychiatric, and sleep dysfunction) compared to patients with AR (with more frequent nose blowing and sneezing). In patients with allergic rhinoconjunctivitis with COVID‐19 infection, mini‐RQLQ scores were lower in COVID‐19 infection compared to their allergies. They specifically reported less sneezing, runny nose, itchy eyes, sore eyes, and watery eyes and generally noted a difference in their symptoms with COVID‐19 infection compared to typical allergies.
Changes in exposure associated with widespread lockdowns affected the clinical presentation of patients with AR. Visits for AR increased during the COVID pandemic, with patients reporting ongoing nasal symptoms as an impetus for seeking care., However, in general, AR symptoms and medication use decreased., , , The decrease in AR symptoms was attributed to reduced outdoor exposures, use of face masks, and decreased pollution as a result of COVID‐19 lockdowns., However, changes in symptom presentation depended on sensitization pattern – patients with cypress pollen allergy reported decreased symptoms but those with dust mite allergy noted increased symptoms., The COVID pandemic also led to increased exposure to indoor respiratory irritants such as tobacco, cooking smoke, and cleaning products. And although use of face masks were reliably associated with fewer nasal symptoms compared to no mask, the effect on ocular symptoms was mixed., Finally, patients who discontinued their therapies for AR due to pandemic concerns expectedly reported loss of symptom control.
Comorbid mental health diagnoses including depression and anxiety are commonly reported in patients with AR and positively correlated with symptom scores. This correlation persisted during the pandemic with atopic patients reporting higher symptoms of post‐traumatic stress disorder, higher depression risk scores, and higher hyperarousable subscale scores than non‐atopic patients.
XIV.B Changes in allergic rhinitis diagnostic techniques related to COVID‐19
Although the initial clinical evaluation of patients often could be done through telemedicine, many diagnostic techniques for AR require a face‐to‐face encounter with potentially aerosol generating procedures (e.g., performing spirometry on an asthmatic patient prior to allergy skin testing). Because SARS‐CoV‐2 viral loads are highest in the upper airway, these procedures are particularly high risk., In many cases, if in‐person encounters were not appropriate, diagnostic testing was deferred. In vitro serum sIgE was an alternative option to evaluate for allergen sensitization, although phlebotomy still required healthcare contact. Additionally, there was often national, regional, and/or institutional guidance for in person visits and procedures., , , , , , , Policies to contain and reduce spread of COVID‐19 are still evolving. At the time of this writing, available publications often stemmed from early pandemic practices and expert opinion. Adjustments to the recommendation with changing COVID‐19 community transmission levels are ongoing but typically involved phased de‐escalation of these recommendations.
For in‐person encounters, general considerations included measures to screen for COVID‐19 infection, enhance social distancing, and reduce transmission. Early in the COVID‐19 pandemic, screening prior to healthcare facility encounters included survey screening of symptoms suggestive of COVID‐19 for patients and staff, , and, in some countries, body temperature screening and epidemiologic tracking via smartphone., Social distancing of at least 6 feet was recommended when possible., , This was important in clinical spaces and the waiting room. Visitor limitations (with one adult allowed for children and none for adult patients when possible) were enacted., Clinical care modifications included asking patients to fill out health information prior to visits, using telemedicine to obtain history to minimize in person time, and adjusting clinic schedule templates to allow for social distancing and room ventilation. Finally, measures to reduce transmission included hand hygiene, appropriate personal protective equipment (generally including a mask), removing reading material to minimize indirect transmission, and enhanced cleaning of facilities., , , ,
For aerosol‐generating procedures, additional action was recommended. There have not been clinical studies of COVID‐19 transmission with any allergy or otolaryngologic procedures. As stated earlier in ICAR‐Allergic Rhinitis 2023, nasal endoscopy is an option when evaluating the AR patient, used primarily to evaluate potential intranasal signs associated with allergy or to rule out alternate causes presenting symptoms. Studies of nasal endoscopy has provided conflicting reports on aerosol generation., Initial studies by two research groups using cadaveric heads did not demonstrate aerosol generation during cold instrumentation, although further studies in live patients undergoing nasal endoscopy detected increased airborne particles., Another study did not detect a significant change in particle concentration from pre‐scope to scope, but there was a trend for increased particle concentrations in patients who required sinonasal debridement. There is also concern that nasal endoscopy can induce behaviors including sneezing, breathing, speaking, and possibly coughing that are aerosol generating., , However, some modifications including nasal endoscopy using modified surgical or N95 masks could prevent aerosol generation,, , as well as repositioning at the back of the patient or using a tower with camera, screen, and light source. Local anesthetics and decongestants could be applied with actuated pump sprays or soaked pledgets rather than atomized forms to avoid aerosol generation., , Immediate decontamination of equipment, especially the endoscope, was also recommended. Expert groups generally recommended against certain procedures including nasal provocation, nasal cytology, anterior rhinomanometry, and PNIF., , If supplies were not constrained, rapid and accurate pre‐procedural screening for SARS‐CoV‐2 was also recommended. For personal protective equipment, the WHO recommended an N95 face mask, full eye protection, and full body protective clothing., , Techniques to improve donning and doffing included one‐step glove and gown removal, double‐gloving, spoken instructions during doffing, and glove disinfection.
Aerosol clearance depends on ventilation and air exchange. The Centers for Disease Control (CDC) recommended at least 12 air changes per hour and controlled direction of airflow although the WHO recommends double this. After the patient leaves the room and 5 air exchanges occur, less than 1% of airborne contaminants will remain. With at least 12 air changes per hour, this would occur in 30 min. The COVID‐19 pandemic led to changes in access to in‐person healthcare and potentially aerosol‐generating procedures. In making the diagnosis of AR, there were strategies employed to help contain and reduce spread of COVID‐19.,
XIV.C Changes in allergic rhinitis management related to COVID‐19
Much of the standard management of AR was recommended by expert groups to be continued during the COVID‐19 pandemic. There was specific motivation to control AR symptoms given concern that sneezing increased viral spreading and poorly controlled upper airway symptoms serve as a trigger for asthma exacerbations., , , , In Beijing, providers made public efforts to develop pollen monitoring networks, television, and online lectures, and suggested over‐the‐counter drug recommendations for all patients with AR. In addition, AR is not a contraindication to receiving the COVID‐19 vaccine. Patients with AR were able to tolerate COVID‐19 vaccination without severe reactions., ,
As always, the first step in management of AR remains allergen avoidance. The pandemic demonstrated that allergen avoidance could significantly improve symptoms. Practices like face masks and handwashing appear to be mutually beneficial for management of AR and COVID‐19. Standard therapies for AR, including INCS, oral and topical antihistamines, montelukast, and AIT, were not identified as increasing susceptibility or severity of COVID‐19 infection., , , , Systemic corticosteroids may be a concern although this is not a standard therapy for AR. Patients on INCS were found to have a lower risk for COVID‐19 related hospitalization, admission to the intensive care unit, and in‐hospital mortality compared to patients who were not on INCS. Montelukast has also been associated with a reduction in COVID‐infection in a small retrospective cohort study of elderly asthmatics.
AIT has been shown to improve symptom control with a decrease in respiratory infections and antibiotic use. Prior studies with viral infections including influenza, cytomegalovirus (CMV), and HIV have not shown changes in the efficacy or safety of AIT. When COVID‐19 cases were high, initiating AIT was generally not recommended. However, consideration for continuing AIT includes lengthening the injection interval which minimizes healthcare visits., , , Consensus from one expert panel recommended lengthening the interval to every 2 weeks during the build‐up phase and every 6 weeks during maintenance. Therapy should be stopped if COVID‐19 infection is suspected or diagnosed, until resolution. There was evidence that patients were more likely to be nonadherent and discontinue AIT during the pandemic leading to higher symptom scores, decreased QOL, and higher medication use than before the pandemic., , , , Consideration for switching patients to or starting patients on SLIT, both tablet and aqueous forms, may be a preferred therapy since maintenance does not require in‐person administration., , In case of COVID‐associated quarantine, an adequate supply of SLIT should be maintained at home., Finally, home SCIT in selected patients was cost effective under pandemic considerations alone., Of note, this is not currently approved and is not the standard of care.
Finally, anti‐IgE therapy has been approved for severe cases of Japanese cedar pollinosis. There is no evidence of altered susceptibility or severity of COVID‐19 infection with anti‐IgE therapy. In fact, clinical studies have shown that pre‐seasonal treatment with anti‐IgE therapy decreases seasonal exacerbations of asthma related to viral infections., , IgE has been found to suppress the ability of dendritic cells to produce type I interferons and theorized to increase the susceptibility for respiratory viral infections., , However, as there is limited evidence, physician judgment is recommended.
XV SUMMARY OF KNOWLEDGE GAPS AND RESEARCH OPPORTUNITIES
Through the ICAR‐Allergic Rhinitis 2023 update process, we have seen an increased number of scientific publications in many areas. We are also encouraged to see additional high‐quality studies, including many SRMAs, addressing numerous individual AR topics. As highlighted in previous ICAR documents, one of the most important aspects of this process is to identify knowledge gaps and key areas where future research may further advance our knowledge in AR. The sections that follow emphasize several important areas where additional research may further expand and solidify our understanding of AR.
Epidemiology and risk factors. Studies have been undertaken to understand the prevalence of AR around the world. These are limited by differing methodology and reporting. Since ICAR‐Allergic Rhinitis 2018, the Aggregate Grades of Evidence remain largely unchanged. However, there has been significant work evaluating the hygiene hypothesis, SES, and in utero influences on AR development. Challenges of these studies are the retrospective nature of most work evaluating risk factors. Randomization is difficult in such studies, and the confounding effects of other risk factors are difficult to assess. Several gaps in knowledge exist and may be helpful to address. The following are areas where we suggest additional study:
Improved understanding of the incidence of AR based on geographic location
Evaluation of climate change effects on incidence and severity of AR
Improved understanding of the relationship between genetics and environmental factors in the development of AR
High quality longitudinal studies evaluating risk factors for development of AR
Evaluation and diagnosis. Diagnosis of AR begins with history and physical exam. Classic symptoms of AR (e.g., nasal/ocular pruritis, rhinorrhea, nasal congestion) are well documented. Since the early months of the COVID‐19 pandemic, awareness of hyposmia and its association with nasal pathology has been heightened, but research on the association between hyposmia and AR remains limited. Studies have suggested that AR can affect smell during pollen season, but the cause of hyposmia in AR is unclear., The effect of AR on olfaction will be important to understand in more detail in the future.
Beyond history and physical exam, skin testing or in vitro sIgE are used for further evaluation. Since ICAR‐Allergic Rhinitis 2018, several new sections have been added, evaluating the use of additional diagnostic techniques for AR. In addition to BAT, mast cell activation testing is a new option for in vitro allergy testing., The use of this test for AR specific evaluation is currently limited, reported techniques are time consuming, and human mast cells are heterogeneous. Additional understanding of mast cell activation testing and its application in AR is needed.
The following are areas in which AR evaluation and diagnosis may be improved in the future:
Increased understanding of hyposmia as a symptom of AR or a marker if its severity
Further evaluation and validation of nasal sIgE testing for AR diagnosis
Further work evaluating the use of novel AR testing techniques, such as BAT and mast cell activation testing, provocation testing, and objective measures of nasal air flow
Improvement of low‐cost diagnostic tools
Pediatrics. The pediatrics section has been added for the ICAR‐Allergic Rhinitis 2023 update. This section summarizes the existing literature on pediatric allergy diagnosis and treatment. We have identified areas in which more work is needed:
Improved treatment options for young children
Improved interpretation of skin testing results in young children
Optimizing treatment strategies for children who are polysensitized
Further work developing AIT delivery routes appropriate and safe for children
Management. There are several well documented strategies for AR management with high levels of evidence and effectiveness. Avoidance strategies are cost‐effective, but high‐level data is lacking. However, many pharmacotherapy and AIT options have been shown to be effective, and several of these treatment strategies are strongly recommended. Since ICAR‐Allergic Rhinitis 2018, additional studies have been completed; however, all avoidance strategies other than reduction of occupational exposures remain as an “option” due to relatively low‐quality evidence in assessment of clinical benefit. Pharmacotherapy and AIT treatment option aggregate grades of evidence remain largely stable since ICAR‐Allergic Rhinitis 2018, although there are a few notable recommendation updates including strong recommendations against oral steroids and oral decongestants for routine use in the treatment of AR. Areas of future work in AR management include:
Continued investigation of combination therapy options, including topical therapies
Studies of comparative effectiveness and cost‐effectiveness for AR treatments
Further work directly comparing SCIT to SLIT in large‐scale RCTs
Standardization of rush and cluster SCIT protocols for aeroallergen immunotherapy
Associated conditions. The evidence supporting the relationship between AR and other conditions is often conflicting. Since ICAR‐Allergic Rhinitis 2018, the relationship of asthma to AR has been extensively studied with an increase in the Aggregate Grades of Evidence. In addition, several new sections in ICAR‐Allergic Rhinitis 2023 highlight the potential relationship of allergy to various subtypes/endotypes of CRS, however the evidence remains conflicting. More research is needed in the following domains:
Improved understanding of treatment effects of AR on specific comorbid CRSwNP subtypes/endotypes
Continued work to determine the relationship of AR to ear disease
Investigation of treatment effect of AR on cough
COVID‐19. One of the notable effects of the identification of the novel coronavirus disease in 2019 was a rapid expansion in research efforts, scientific publications, and dissemination of knowledge related to the transmission, health consequences, and risk to patients and healthcare workers. The work on AR and COVID‐19 continues to evolve. The following are topics of interest regarding COVID‐19 and AR:
Improved understanding of the aerosolization risk during nasal endoscopy
Improved understanding of the risks of AR treatment, including AIT, during COVID infection
A deeper understanding of the long‐term effects of COVID on allergic diseases and their development
XVI CONCLUSION
In this document, we summarized the available literature for AR and created recommendations based on the highest levels of evidence. Through this, we have identified several areas with robust literature and a strong evidence base. There have been many advances in the field since the publication of ICAR‐Allergic Rhinitis 2018, but notable knowledge gaps remain. There are several areas of AR research which will be limited based on inherent conditions of study design. For example, it is not feasible to blind or randomize for some AR treatments, and epidemiological studies to evaluate risk factors may be inherently limited by their retrospective nature and confounding variables. Therefore, for each major content area, we have suggested practical and feasible areas of study that we believe could advance our knowledge of AR in a productive manner.
AUTHOR CONFLICT OF INTEREST DISCLOSURE
See table at the end document.
FUNDING
None.
ICAR‐Allergic Rhinitis 2023 Author Disclosure of Financial Relationships and Potential COI
Authors:
Consultant authors:
REFERENCES
- 1. Wise SK, Lin SY, Toskala E, et al. International Consensus Statement on Allergy and Rhinology: Allergic Rhinitis. Int Forum Allergy Rhinol. 2018;8(2):108–352. https://doi.org/10.1002/alr.22073
- 2. Rudmik L, Smith TL. Development of an evidence‐based review with recommendations using an online iterative process. Int Forum Allergy Rhinol. 2011;1(6):431–437. https://doi.org/10.1002/alr.20095
- 3. Moher D, Liberati A, Tetzlaff J, Altman DG, Group P. Preferred reporting items for systematic reviews and meta‐analyses: the PRISMA statement. Open Med. 2009;3(3):e123–e130.
- 4. American Academy of Pediatrics Steering Committee on Quality I, Management . Classifying recommendations for clinical practice guidelines. Pediatrics. 2004;114(3):874–877. https://doi.org/10.1542/peds.2004‐1260
- 5. Bousquet J, Van Cauwenberge P, Khaltaev N, Aria Workshop Group , World Health Organization . Allergic rhinitis and its impact on asthma. J Allergy Clin Immunol. 2001;108(5 Suppl):S147–S334. https://doi.org/10.1067/mai.2001.118891
- 6. Orlandi RR, Kingdom TT, Hwang PH, et al. International Consensus Statement on Allergy and Rhinology: Rhinosinusitis. Int Forum Allergy Rhinol. 2016;6(suppl 1):S22–S209. https://doi.org/10.1002/alr.21695
- 7. Orlandi RR, Kingdom TT, Smith TL, et al. International consensus statement on allergy and rhinology: rhinosinusitis 2021. Int Forum Allergy Rhinol. 2021;11(3):213–739. https://doi.org/10.1002/alr.22741
- 8. Wang EW, Zanation AM, Gardner PA, et al. ICAR: endoscopic skull‐base surgery. Int Forum Allergy Rhinol. 2019;9(S3):S145–S365. https://doi.org/10.1002/alr.22326
- 9. Patel ZM, Holbrook EH, Turner JH, et al. International consensus statement on allergy and rhinology: Olfaction. Int Forum Allergy Rhinol. 2022;12(4):327–680. https://doi.org/10.1002/alr.22929
- 10. Sackett DL, Rosenberg WM, Gray JA, Haynes RB, Richardson WS. Evidence based medicine: what it is and what it isn't. BMJ. 1996;312(7023):71–72. https://doi.org/10.1136/bmj.312.7023.71
- 11. OCEBM Levels of Evidence Working Group : The Oxford 2011 Levels of Evidence. Accessed April 4, 2019. http://www.cebm.net/index.aspx?o=5653
- 12. Handbook for grading the quality of evidence and the strength of recommendations using the GRADE approach, updated October 2013. Accessed April 2, 2019. https://gdt.gradepro.org/app/handbook/handbook.html
- 13. Bousquet J, Bachert C, Canonica GW, et al. Unmet needs in severe chronic upper airway disease (SCUAD). J Allergy Clin Immunol. 2009;124(3):428–433. https://doi.org/10.1016/j.jaci.2009.06.027
- 14. Bousquet JJ, Schunemann HJ, Togias A, et al. Next‐generation ARIA care pathways for rhinitis and asthma: a model for multimorbid chronic diseases. Clin Transl Allergy. 2019;9:44. https://doi.org/10.1186/s13601‐019‐0279‐2
- 15. Asher MI, Montefort S, Bjorksten B, et al. Worldwide time trends in the prevalence of symptoms of asthma, allergic rhinoconjunctivitis, and eczema in childhood: ISAAC Phases One and Three repeat multicountry cross‐sectional surveys. Lancet. 2006;368(9537):733–743. https://doi.org/10.1016/S0140‐6736(06)69283‐0
- 16. Vinke JG, KleinJan A, Severijnen LW, Hoeve LJ, Fokkens WJ. Differences in nasal cellular infiltrates between allergic children and age‐matched controls. Eur Respir J. 1999;13(4):797–803. https://doi.org/10.1034/j.1399‐3003.1999.13d17.x
- 17. Bauchau V, Durham SR. Prevalence and rate of diagnosis of allergic rhinitis in Europe. Eur Respir J. 2004;24(5):758–764. https://doi.org/10.1183/09031936.04.00013904
- 18. Bauchau V, Durham SR. Epidemiological characterization of the intermittent and persistent types of allergic rhinitis. Allergy. 2005;60(3):350–353. https://doi.org/10.1111/j.1398‐9995.2005.00751.x
- 19. Ciprandi G, Buscaglia S, Pesce G, et al. Minimal persistent inflammation is present at mucosal level in patients with asymptomatic rhinitis and mite allergy. J Allergy Clin Immunol. 1995;96(6 pt 1):971–979. https://doi.org/10.1016/s0091‐6749(95)70235‐0
- 20. Platts‐Mills TA, Hayden ML, Chapman MD, Wilkins SR. Seasonal variation in dust mite and grass‐pollen allergens in dust from the houses of patients with asthma. J Allergy Clin Immunol. 1987;79(5):781–791. https://doi.org/10.1016/0091‐6749(87)90211‐9
- 21. Connell JT. Quantitative intranasal pollen challenges. 3. The priming effect in allergic rhinitis. J Allergy. 1969;43(1):33–44. https://doi.org/10.1016/0021‐8707(69)90018‐5
- 22. Wachs M, Proud D, Lichtenstein LM, Kagey‐Sobotka A, Norman PS, Naclerio RM. Observations on the pathogenesis of nasal priming. J Allergy Clin Immunol. 1989;84(4 pt 1):492–501. https://doi.org/10.1016/0091‐6749(89)90362‐x
- 23. Juliusson S, Bende M. Priming effect of a birch pollen season studied with laser Doppler flowmetry in patients with allergic rhinitis. Clin Allergy. 1988;18(6):615–618. https://doi.org/10.1111/j.1365‐2222.1988.tb02913.x
- 24. Naito K, Ishihara M, Senoh Y, Takeda N, Yokoyama N, Iwata S. Seasonal variations of nasal resistance in allergic rhinitis and environmental pollen counts. II: Efficacy of preseasonal therapy. Auris Nasus Larynx. 1993;20(1):31–38. https://doi.org/10.1016/s0385‐8146(12)80208‐2
- 25. Koh YY, Lim HS, Min KU, Min YG. Airways of allergic rhinitics are ‘primed’ to repeated allergen inhalation challenge. Clin Exp Allergy. 1994;24(4):337–346. https://doi.org/10.1111/j.1365‐2222.1994.tb00244.x
- 26. Assing K, Bodtger U, Poulsen LK, Malling HJ. Grass pollen symptoms interfere with the recollection of birch pollen symptoms – a prospective study of suspected, asymptomatic skin sensitization. Allergy. 2007;62(4):373–377. https://doi.org/10.1111/j.1398‐9995.2006.01280.x
- 27. Knani J, Campbell A, Enander I, Peterson CG, Michel FB, Bousquet J. Indirect evidence of nasal inflammation assessed by titration of inflammatory mediators and enumeration of cells in nasal secretions of patients with chronic rhinitis. J Allergy Clin Immunol. 1992;90(6 pt 1):880–889. https://doi.org/10.1016/0091‐6749(92)90460‐j
- 28. Ricca V, Landi M, Ferrero P, et al. Minimal persistent inflammation is also present in patients with seasonal allergic rhinitis. J Allergy Clin Immunol. 2000;105(1 pt 1):54–57. https://doi.org/10.1016/s0091‐6749(00)90177‐5
- 29. Riediker M, Monn C, Koller T, Stahel WA, Wuthrich B. Air pollutants enhance rhinoconjunctivitis symptoms in pollen‐allergic individuals. Ann Allergy Asthma Immunol. 2001;87(4):311–318. https://doi.org/10.1016/S1081‐1206(10)62246‐6
- 30. Bousquet J, Annesi‐Maesano I, Carat F, et al. Characteristics of intermittent and persistent allergic rhinitis: DREAMS study group. Clin Exp Allergy. 2005;35(6):728–732. https://doi.org/10.1111/j.1365‐2222.2005.02274.x
- 31. Wallace DV, Dykewicz MS, Bernstein DI, et al. The diagnosis and management of rhinitis: an updated practice parameter. J Allergy Clin Immunol. 2008;122(2 suppl):S1–S84. https://doi.org/10.1016/j.jaci.2008.06.003
- 32. Van Hoecke H, Vastesaeger N, Dewulf L, Sys L, van Cauwenberge P. Classification and management of allergic rhinitis patients in general practice during pollen season. Allergy. 2006;61(6):705–711. https://doi.org/10.1111/j.1398‐9995.2006.01057.x
- 33. Demoly P, Allaert FA, Lecasble M, Bousquet J, Pragma. Validation of the classification of ARIA (allergic rhinitis and its impact on asthma). Allergy. 2003;58(7):672–675. https://doi.org/10.1034/j.1398‐9995.2003.t01‐1‐00202.x
- 34. Bachert C, van Cauwenberge P, Olbrecht J, van Schoor J. Prevalence, classification and perception of allergic and nonallergic rhinitis in Belgium. Allergy. 2006;61(6):693–698. https://doi.org/10.1111/j.1398‐9995.2006.01054.x
- 35. Todo‐Bom A, Loureiro C, Almeida MM, et al. Epidemiology of rhinitis in Portugal: evaluation of the intermittent and the persistent types. Allergy. 2007;62(9):1038–1043. https://doi.org/10.1111/j.1398‐9995.2007.01448.x
- 36. Custovic A, Henderson J, Simpson A. Does understanding endotypes translate to better asthma management options for all? J Allergy Clin Immunol. 2019;144(1):25–33. https://doi.org/10.1016/j.jaci.2019.05.016
- 37. Akar‐Ghibril N, Casale T, Custovic A, Phipatanakul W. Allergic Endotypes and Phenotypes of Asthma. J Allergy Clin Immunol Pract. 2020;8(2):429–440. https://doi.org/10.1016/j.jaip.2019.11.008
- 38. Saglani S, Wisnivesky JP, Charokopos A, Pascoe CD, Halayko AJ, Custovic A. Update in Asthma 2019. Am J Respir Crit Care Med. 2020;202(2):184–192. https://doi.org/10.1164/rccm.202003‐0596UP
- 39. Thien F, Beggs PJ, Csutoros D, et al. The Melbourne epidemic thunderstorm asthma event 2016: an investigation of environmental triggers, effect on health services, and patient risk factors. Lancet Planet Health. 2018;2(6):e255–e263. https://doi.org/10.1016/S2542‐5196(18)30120‐7
- 40. Thien F. Melbourne epidemic thunderstorm asthma event 2016: lessons learnt from the perfect storm. Respirology. 2018;23(11):976–977. https://doi.org/10.1111/resp.13410
- 41. O'Hehir RE, Varese NP, Deckert K, et al. Epidemic Thunderstorm asthma protection with five‐grass pollen tablet sublingual immunotherapy: a clinical trial. Am J Respir Crit Care Med. 2018;198(1):126–128. https://doi.org/10.1164/rccm.201711‐2337LE
- 42. Custovic A, Custovic D, Kljaic Bukvic B, Fontanella S, Haider S. Atopic phenotypes and their implication in the atopic march. Expert Rev Clin Immunol. 2020;16(9):873–881. https://doi.org/10.1080/1744666X.2020.1816825
- 43. Oksel C, Custovic A. Development of allergic sensitization and its relevance to paediatric asthma. Curr Opin Allergy Clin Immunol. 2018;18(2):109–116. https://doi.org/10.1097/ACI.0000000000000430
- 44. Shtessel M, Tversky J. Reliability of allergy skin testing. Ann Allergy Asthma Immunol. 2018;120(1):80–83. https://doi.org/10.1016/j.anai.2017.10.015
- 45. Simpson A, Soderstrom L, Ahlstedt S, Murray CS, Woodcock A, Custovic A. IgE antibody quantification and the probability of wheeze in preschool children. J Allergy Clin Immunol. 2005;116(4):744–749. https://doi.org/10.1016/j.jaci.2005.06.032
- 46. Marinho S, Simpson A, Lowe L, Kissen P, Murray C, Custovic A. Rhinoconjunctivitis in 5‐year‐old children: a population‐based birth cohort study. Allergy. 2007;62(4):385–393. https://doi.org/10.1111/j.1398‐9995.2006.01294.x
- 47. Marinho S, Simpson A, Marsden P, Smith JA, Custovic A. Quantification of atopy, lung function and airway hypersensitivity in adults. Clin Transl Allergy. 2011;1(1):16. https://doi.org/10.1186/2045‐7022‐1‐16
- 48. Roberts G, Ollert M, Aalberse R, et al. A new framework for the interpretation of IgE sensitization tests. Allergy. 2016;71(11):1540–1551. https://doi.org/10.1111/all.12939
- 49. Eguiluz‐Gracia I, Testera‐Montes A, Gonzalez M, et al. Safety and reproducibility of nasal allergen challenge. Allergy. 2019;74(6):1125–1134. https://doi.org/10.1111/all.13728
- 50. Ramchandani R, Linton S, Hossenbaccus L, Ellis AK. Comparing the nasal allergen challenge and environmental exposure unit models of allergic rhinitis. Ann Allergy Asthma Immunol. 2021;127(2):163–164. https://doi.org/10.1016/j.anai.2021.04.012
- 51. Custovic A, Sonntag HJ, Buchan IE, Belgrave D, Simpson A, Prosperi MCF. Evolution pathways of IgE responses to grass and mite allergens throughout childhood. J Allergy Clin Immunol. 2015;136(6):1645–1652.e8. https://doi.org/10.1016/j.jaci.2015.03.041
- 52. Howard R, Belgrave D, Papastamoulis P, Simpson A, Rattray M, Custovic A. Evolution of IgE responses to multiple allergen components throughout childhood. J Allergy Clin Immunol. 2018;142(4):1322–1330. https://doi.org/10.1016/j.jaci.2017.11.064
- 53. Simpson A, Lazic N, Belgrave DC, et al. Patterns of IgE responses to multiple allergen components and clinical symptoms at age 11 years. J Allergy Clin Immunol. 2015;136(5):1224–1231. https://doi.org/10.1016/j.jaci.2015.03.027
- 54. Prosperi MC, Marinho S, Simpson A, Custovic A, Buchan IE. Predicting phenotypes of asthma and eczema with machine learning. BMC Med Genomics. 2014;7(suppl 1):S7. https://doi.org/10.1186/1755‐8794‐7‐S1‐S7
- 55. Prosperi MC, Belgrave D, Buchan I, Simpson A, Custovic A. Challenges in interpreting allergen microarrays in relation to clinical symptoms: a machine learning approach. Pediatr Allergy Immunol. 2014;25(1):71–79. https://doi.org/10.1111/pai.12139
- 56. Fontanella S, Frainay C, Murray CS, Simpson A, Custovic A. Machine learning to identify pairwise interactions between specific IgE antibodies and their association with asthma: a cross‐sectional analysis within a population‐based birth cohort. PLoS Med. 2018;15(11):e1002691. https://doi.org/10.1371/journal.pmed.1002691
- 57. Roberts G, Fontanella S, Selby A, et al. Connectivity patterns between multiple allergen specific IgE antibodies and their association with severe asthma. J Allergy Clin Immunol. 2020;146(4):821–830. https://doi.org/10.1016/j.jaci.2020.02.031
- 58. Niespodziana K, Borochova K, Pazderova P, et al. Toward personalization of asthma treatment according to trigger factors. J Allergy Clin Immunol. 2020;145(6):1529–1534. https://doi.org/10.1016/j.jaci.2020.02.001
- 59. Varghese M, Glaum MC, Lockey RF. Drug‐induced rhinitis. Clin Exp Allergy. 2010;40(3):381–384. https://doi.org/10.1111/j.1365‐2222.2009.03450.x
- 60. Settipane RA, Kaliner MA. Chapter 14: nonallergic rhinitis. Am J Rhinol Allergy. 2013;27(suppl 1):S48–S51. https://doi.org/10.2500/ajra.2013.27.3927
- 61. Agnihotri NT, McGrath KG. Allergic and nonallergic rhinitis. Allergy Asthma Proc. 2019;40(6):376–379. https://doi.org/10.2500/aap.2019.40.4251
- 62. Walgama ES, Hwang PH. Aspirin‐exacerbated respiratory disease. Otolaryngol Clin North Am. 2017;50(1):83–94. https://doi.org/10.1016/j.otc.2016.08.007
- 63. Laidlaw TM, Levy JM. NSAID‐ERD syndrome: the new hope from prevention, early diagnosis, and new therapeutic targets. Curr Allergy Asthma Rep. 2020;20(4):10. https://doi.org/10.1007/s11882‐020‐00905‐9
- 64. Kowalski ML, Agache I, Bavbek S, et al. Diagnosis and management of NSAID‐Exacerbated Respiratory Disease (N‐ERD) – a EAACI position paper. Allergy. 2019;74(1):28–39. https://doi.org/10.1111/all.13599
- 65. Sousa AR, Parikh A, Scadding G, Corrigan CJ, Lee TH. Leukotriene‐receptor expression on nasal mucosal inflammatory cells in aspirin‐sensitive rhinosinusitis. N Engl J Med. 2002;347(19):1493–1499. https://doi.org/10.1056/NEJMoa013508
- 66. Barnes PJ. Neurogenic inflammation in the airways. Respir Physiol. 2001;125(1‐2):145–154. https://doi.org/10.1016/s0034‐5687(00)00210‐3
- 67. Kaliner MA, Baraniuk JN, Benninger M, et al. Consensus definition of nonallergic rhinopathy, previously referred to as vasomotor rhinitis, nonallergic rhinitis, and/or idiopathic rhinitis. World Allergy Organ J. 2009;2(6):119–120. https://doi.org/10.1097/WOX.0b013e3181a8e15a
- 68. Settipane RA, Charnock DR. Epidemiology of rhinitis: allergic and nonallergic. Clin Allergy Immunol. 2007;19:23–34.
- 69. Mah GT, Tejani AM, Musini VM. Methyldopa for primary hypertension. Cochrane Database Syst Rev. 2009;(4):CD003893. https://doi.org/10.1002/14651858.CD003893.pub3
- 70. Boswell‐Smith V, Spina D, Page CP. Phosphodiesterase inhibitors. Br J Pharmacol. 2006;147(suppl 1):S252–S257. https://doi.org/10.1038/sj.bjp.0706495
- 71. Andersson KE. PDE5 inhibitors – pharmacology and clinical applications 20 years after sildenafil discovery. Br J Pharmacol. 2018;175(13):2554–2565. https://doi.org/10.1111/bph.14205
- 72. Kiroglu AF, Bayrakli H, Yuca K, Cankaya H, Kiris M. Nasal obstruction as a common side‐effect of sildenafil citrate. Tohoku J Exp Med. 2006;208(3):251–254. https://doi.org/10.1620/tjem.208.251
- 73. Motamed M, Sandhu D, Murty GE. Sildenafil and nasal obstruction. J Otolaryngol. 2003;32(4):259–261. https://doi.org/10.2310/7070.2003.41631
- 74. Cingi C, Ozdoganoglu T, Songu M. Nasal obstruction as a drug side effect. Ther Adv Respir Dis. 2011;5(3):175–182. https://doi.org/10.1177/1753465811403348
- 75. Ahmed WS, Geethakumari AM, Biswas KH. Phosphodiesterase 5 (PDE5): structure‐function regulation and therapeutic applications of inhibitors. Biomed Pharmacother. 2021;134:111128. https://doi.org/10.1016/j.biopha.2020.111128
- 76. Togias A. Unique mechanistic features of allergic rhinitis. J Allergy Clin Immunol. 2000;105(6 pt 2):S599–S604. https://doi.org/10.1067/mai.2000.106885
- 77. Pinargote P, Guillen D, Guarderas JC. ACE inhibitors: upper respiratory symptoms. BMJ Case Rep. 2014;2014. https://doi.org/10.1136/bcr‐2014‐205462
- 78. Riccio MM, Proud D. Evidence that enhanced nasal reactivity to bradykinin in patients with symptomatic allergy is mediated by neural reflexes. J Allergy Clin Immunol. 1996;97(6):1252–1263. https://doi.org/10.1016/s0091‐6749(96)70193‐8
- 79. Shirasaki H, Kanaizumi E, Himi T. Immunohistochemical localization of the bradykinin B1 and B2 receptors in human nasal mucosa. Mediators Inflamm. 2009;2009:102406. https://doi.org/10.1155/2009/102406
- 80. Trimarchi M, Miluzio A, Nicolai P, Morassi ML, Bussi M, Marchisio PC. Massive apoptosis erodes nasal mucosa of cocaine abusers. Am J Rhinol. 2006;20(2):160–164.
- 81. Tan TH, Stevenson B, Yip D. Docetaxel‐induced nasal septal perforation. Intern Med J. 2006;36(7):471–472. https://doi.org/10.1111/j.1445‐5994.2006.01105.x
- 82. Lanier B, Kai G, Marple B, Wall GM. Pathophysiology and progression of nasal septal perforation. Ann Allergy Asthma Immunol. 2007;99(6):473–479; quiz 480‐1, 521. https://doi.org/10.1016/S1081‐1206(10)60373‐0
- 83. Alexander D, Alexander K, Valentino J. Intranasal hydrocodone‐acetaminophen abuse induced necrosis of the nasal cavity and pharynx. Laryngoscope. 2012;122(11):2378–2381. https://doi.org/10.1002/lary.23542
- 84. Wang SH, Wang HW, Wang JY. Effects of cocaine on human nasal mucosa. Eur Arch Otorhinolaryngol. 1993;250(4):245–248. https://doi.org/10.1007/BF00171534
- 85. Snyder RD, Snyder LB. Intranasal cocaine abuse in an allergists office. Ann Allergy. 1985;54(6):489–492.
- 86. Hall LJ, Jackson RT. Effects of alpha and beta adrenergic agonists on nasal blood flow. Ann Otol Rhinol Laryngol. 1968;77(6):1120–1130. https://doi.org/10.1177/000348946807700610
- 87. Walker JS. Rhinitis medicamentosa. J Allergy. 1952;23(2):183–186. https://doi.org/10.1016/0021‐8707(52)90093‐2
- 88. Kim D, Steinhart B. Seizures induced by recreational abuse of bupropion tablets via nasal insufflation. CJEM. 2010;12(2):158–161. https://doi.org/10.1017/s1481803500012203
- 89. Sataloff RT, Gullane PJ, Goldstein DP. Sataloff's Comprehensive Textbook of Otolaryngology, Head and Neck Surgery. Jaypee Brothers Medical Publishing; 2016.
- 90. Daws LC, Callaghan PD, Moron JA, et al. Cocaine increases dopamine uptake and cell surface expression of dopamine transporters. Biochem Biophys Res Commun. 2002;290(5):1545–1550. https://doi.org/10.1006/bbrc.2002.6384
- 91. Middleton LS, Nuzzo PA, Lofwall MR, Moody DE, Walsh SL. The pharmacodynamic and pharmacokinetic profile of intranasal crushed buprenorphine and buprenorphine/naloxone tablets in opioid abusers. Addiction. 2011;106(8):1460–1473. https://doi.org/10.1111/j.1360‐0443.2011.03424.x
- 92. Zhang H, Prisinzano TE, Donovan MD. Permeation and metabolism of cocaine in the nasal mucosa. Eur J Drug Metab Pharmacokinet. 2012;37(4):255–262. https://doi.org/10.1007/s13318‐012‐0085‐x
- 93. Lin RJ, Smith LJ. Laryngeal Manifestation of intranasal acetaminophen abuse and review of literature. Ear Nose Throat J. 2019;98(4):192–194. https://doi.org/10.1177/0145561319836807
- 94. Hardison SA, Marcum KK, Lintzenich CR. Severe necrosis of the palate and nasal septum resulting from intranasal abuse of acetaminophen. Ear Nose Throat J. 2015;94(10‐11):E40–E42.
- 95. Lin Y, Lu JY, Pinheiro‐Neto CD, Jones DM, Gildener‐Leapman N. Intranasal acetaminophen abuse and nasal, pharyngeal, and laryngotracheal damage. Cureus. 2019;11(8):e5432. https://doi.org/10.7759/cureus.5432
- 96. Morrison DA, Wise SK, DelGaudio JM, Chowdhury NI, Levy JM. Intranasal tissue necrosis associated with opioid abuse: case report and systematic review. Laryngoscope. 2018;128(8):1767–1771. https://doi.org/10.1002/lary.27069
- 97. Ramey JT, Bailen E, Lockey RF. Rhinitis medicamentosa. J Investig Allergol Clin Immunol. 2006;16(3):148–155.
- 98. Graf PM. Rhinitis medicamentosa. Clin Allergy Immunol. 2007;19:295–304.
- 99. Min YG, Kim HS, Suh SH, Jeon SY, Son YI, Yoon S. Paranasal sinusitis after long‐term use of topical nasal decongestants. Acta Otolaryngol. 1996;116(3):465–471. https://doi.org/10.3109/00016489609137874
- 100. Mortuaire G, de Gabory L, Francois M, et al. Rebound congestion and rhinitis medicamentosa: nasal decongestants in clinical practice. Critical review of the literature by a medical panel. Eur Ann Otorhinolaryngol Head Neck Dis. 2013;130(3):137–144. https://doi.org/10.1016/j.anorl.2012.09.005
- 101. Zucker SM, Barton BM, McCoul ED. Management of rhinitis medicamentosa: a systematic review. Otolaryngol Head Neck Surg. 2019;160(3):429–438. https://doi.org/10.1177/0194599818807891
- 102. Graf P, Juto JE. Sustained use of xylometazoline nasal spray shortens the decongestive response and induces rebound swelling. Rhinology. 1995;33(1):14–17.
- 103. Vicks Sinex . Thompson PDR; 2004.
- 104. Fleece L, Mizes JS, Jolly PA, Baldwin RL. Rhinitis medicamentosa. Conceptualization, incidence, and treatment. Ala J Med Sci. 1984;21(2):205–208.
- 105. Knipping S, Holzhausen HJ, Goetze G, Riederer A, Bloching MB. Rhinitis medicamentosa: electron microscopic changes of human nasal mucosa. Otolaryngol Head Neck Surg. 2007;136(1):57–61. https://doi.org/10.1016/j.otohns.2006.08.025
- 106. Marple B, Roland P, Benninger M. Safety review of benzalkonium chloride used as a preservative in intranasal solutions: an overview of conflicting data and opinions. Otolaryngol Head Neck Surg. 2004;130(1):131–141. https://doi.org/10.1016/j.otohns.2003.07.005
- 107. Graf P. Adverse effects of benzalkonium chloride on the nasal mucosa: allergic rhinitis and rhinitis medicamentosa. Clin Ther. 1999;21(10):1749–1755. https://doi.org/10.1016/S0149‐2918(99)80053‐8
- 108. Graf P. Rhinitis medicamentosa: a review of causes and treatment. Treat Respir Med. 2005;4(1):21–29. https://doi.org/10.2165/00151829‐200504010‐00003
- 109. Graf P. Benzalkonium chloride as a preservative in nasal solutions: re‐examining the data. Respir Med. 2001;95(9):728–733. https://doi.org/10.1053/rmed.2001.1127
- 110. Kawabata M, Ohori J, Kurono Y. Effects of benzalkonium chloride on histamine H1 receptor mRNA expression in nasal epithelial cells. Auris Nasus Larynx. 2016;43(6):685–688. https://doi.org/10.1016/j.anl.2016.02.003
- 111. Morris S, Eccles R, Martez SJ, Riker DK, Witek TJ. An evaluation of nasal response following different treatment regimes of oxymetazoline with reference to rebound congestion. Am J Rhinol. 1997;11(2):109–115. https://doi.org/10.2500/105065897782537197
- 112. Chodirker WB. Rhinitis medicamentosa. Can Med Assoc J. 1981;124(4):370, 372.
- 113. May M, West JW. The “stuffy” nose. Otolaryngol Clin North Am. 1973;6(3):655–674.
- 114. Graf P, Hallen H, Juto JE. The pathophysiology and treatment of rhinitis medicamentosa. Clin Otolaryngol Allied Sci. 1995;20(3):224–229. https://doi.org/10.1111/j.1365‐2273.1995.tb01853.x
- 115. Elwany S, Abdel‐Salaam S. Treatment of rhinitis medicamentosa with fluticasone propionate – an experimental study. Eur Arch Otorhinolaryngol. 2001;258(3):116–119. https://doi.org/10.1007/s004050000309
- 116. Tas A, Yagiz R, Yalcin O, et al. Use of mometasone furoate aqueous nasal spray in the treatment of rhinitis medicamentosa: an experimental study. Otolaryngol Head Neck Surg. 2005;132(4):608–612. https://doi.org/10.1016/j.otohns.2005.01.010
- 117. Stephens AL Jr., Boggs PB. Intranasal dexamethasone: an adjunct in the treatment of chemical rhinitis. Ann Allergy. 1968;26(11):612–613.
- 118. Elwany SS, Stephanos WM. Rhinitis medicamentosa. An experimental histopathological and histochemical study. ORL J Otorhinolaryngol Relat Spec. 1983;45(4):187–194. https://doi.org/10.1159/000275642
- 119. Settipane RA. Other causes of rhinitis: mixed rhinitis, rhinitis medicamentosa, hormonal rhinitis, rhinitis of the elderly, and gustatory rhinitis. Immunol Allergy Clin North Am. 2011;31(3):457–467. https://doi.org/10.1016/j.iac.2011.05.011
- 120. Dykewicz MS, Fineman S, Skoner DP, et al. Diagnosis and management of rhinitis: complete guidelines of the Joint Task Force on Practice Parameters in Allergy, Asthma and Immunology. American Academy of Allergy, Asthma, and Immunology. Ann Allergy Asthma Immunol. 1998;81(5 pt 2):478–518. https://doi.org/10.1016/s1081‐1206(10)63155‐9
- 121. Akerlund A, Bende M. Sustained use of oxymetazoline nose drops aggrevates vasomotor rhinitis. Amer J Rhinol. 1991;5:157–160.
- 122. Fowler J, Chin CJ, Massoud E. Rhinitis medicamentosa: a nationwide survey of Canadian otolaryngologists. J Otolaryngol Head Neck Surg. 2019;48(1):70. https://doi.org/10.1186/s40463‐019‐0392‐1
- 123. Yoo JK, Seikaly H, Calhoun KH. Extended use of topical nasal decongestants. Laryngoscope. 1997;107(1):40–43. https://doi.org/10.1097/00005537‐199701000‐00010
- 124. Moscato G, Vandenplas O, Van Wijk RG, et al. EAACI position paper on occupational rhinitis. Respir Res. 2009;10:16. https://doi.org/10.1186/1465‐9921‐10‐16
- 125. Kotz S, Pechtold L, Jorres RA, Nowak D, Chaker AM. Occupational rhinitis. Allergol Select. 2021;5:51–56. https://doi.org/10.5414/ALX02165E
- 126. Vandenplas O, Hox V, Bernstein D. Occupational rhinitis. J Allergy Clin Immunol Pract. 2020;8(10):3311–3321. https://doi.org/10.1016/j.jaip.2020.06.047
- 127. Tarlo SM, Lemiere C. Occupational asthma. N Engl J Med. 2014;370(7):640–649. https://doi.org/10.1056/NEJMra1301758
- 128. Ronsmans S, Steelant B, Backaert W, Nemery B, Van Gerven L. Diagnostic approach to occupational rhinitis: the role of nasal provocation tests. Curr Opin Allergy Clin Immunol. 2020;20(2):122–130. https://doi.org/10.1097/ACI.0000000000000608
- 129. Siracusa A, Desrosiers M, Marabini A. Epidemiology of occupational rhinitis: prevalence, aetiology and determinants. Clin Exp Allergy. 2000;30(11):1519–1534. https://doi.org/10.1046/j.1365‐2222.2000.00946.x
- 130. Pala G, Pignatti P, Perfetti L, et al. Occupational rhinitis and asthma due to cabreuva wood dust. Ann Allergy Asthma Immunol. 2010;104(3):268–269. https://doi.org/10.1016/j.anai.2010.01.009
- 131. Lopata AL, Jeebhay MF. Airborne seafood allergens as a cause of occupational allergy and asthma. Curr Allergy Asthma Rep. 2013;13(3):288–297. https://doi.org/10.1007/s11882‐013‐0347‐y
- 132. Siracusa A, De Blay F, Folletti I, et al. Asthma and exposure to cleaning products – a European Academy of Allergy and Clinical Immunology task force consensus statement. Allergy. 2013;68(12):1532–1545. https://doi.org/10.1111/all.12279
- 133. Siracusa A, Folletti I, Moscato G. Non‐IgE‐mediated and irritant‐induced work‐related rhinitis. Curr Opin Allergy Clin Immunol. 2013;13(2):159–166. https://doi.org/10.1097/ACI.0b013e32835e12e7
- 134. Folletti I, Zock JP, Moscato G, Siracusa A. Asthma and rhinitis in cleaning workers: a systematic review of epidemiological studies. J Asthma. 2014;51(1):18–28. https://doi.org/10.3109/02770903.2013.833217
- 135. Szeszenia‐Dabrowska N, Swiatkowska B, Wilczynska U. Occupational diseases among farmers in Poland. Med Pr. 2016;67(2):163–171. Choroby zawodowe rolnikow w Polsce. https://doi.org/10.13075/mp.5893.00303
- 136. Rodier F, Gautrin D, Ghezzo H, Malo JL. Incidence of occupational rhinoconjunctivitis and risk factors in animal‐health apprentices. J Allergy Clin Immunol. 2003;112(6):1105–1111. https://doi.org/10.1016/j.jaci.2003.08.011
- 137. Ruoppi P, Koistinen T, Susitaival P, Honkanen J, Soininen H. Frequency of allergic rhinitis to laboratory animals in university employees as confirmed by chamber challenges. Allergy. 2004;59(3):295–301. https://doi.org/10.1046/j.1398‐9995.2003.00204.x
- 138. Schyllert C, Ronmark E, Andersson M, et al. Occupational exposure to chemicals drives the increased risk of asthma and rhinitis observed for exposure to vapours, gas, dust and fumes: a cross‐sectional population‐based study. Occup Environ Med. 2016;73(10):663–669. https://doi.org/10.1136/oemed‐2016‐103595
- 139. Krop EJ, Heederik DJ, Lutter R, et al. Associations between pre‐employment immunologic and airway mucosal factors and the development of occupational allergy. J Allergy Clin Immunol. 2009;123(3):694–700, 700.e1‐3. https://doi.org/10.1016/j.jaci.2008.12.021
- 140. Phipatanakul W, Matsui E, Portnoy J, et al. Environmental assessment and exposure reduction of rodents: a practice parameter. Ann Allergy Asthma Immunol. 2012;109(6):375–387. https://doi.org/10.1016/j.anai.2012.09.019
- 141. Pignatti P, Pala G, Pisati M, Perfetti L, Banchieri G, Moscato G. Nasal blown secretion evaluation in specific occupational nasal challenges. Int Arch Occup Environ Health. 2010;83(2):217–223. https://doi.org/10.1007/s00420‐009‐0459‐9
- 142. Ottaviano G, Fokkens WJ. Measurements of nasal airflow and patency: a critical review with emphasis on the use of peak nasal inspiratory flow in daily practice. Allergy. 2016;71(2):162–174. https://doi.org/10.1111/all.12778
- 143. Wood RA, Phipatanakul W, Hamilton RG, Eggleston PA. A comparison of skin prick tests, intradermal skin tests, and RASTs in the diagnosis of cat allergy. J Allergy Clin Immunol. 1999;103(5 pt 1):773–779. https://doi.org/10.1016/s0091‐6749(99)70419‐7
- 144. Nam YH, Lee SK. Comparison between skin prick test and serum immunoglobulin E by CAP system to inhalant allergens. Ann Allergy Asthma Immunol. 2017;118(5):608–613. https://doi.org/10.1016/j.anai.2017.03.005
- 145. Raulf M, Quirce S, Vandenplas O. Addressing molecular diagnosis of occupational allergies. Curr Allergy Asthma Rep. 2018;18(1):6. https://doi.org/10.1007/s11882‐018‐0759‐9
- 146. Amirneni A, Tversky J. High histamine control concentration leads to false negative allergy skin testing. Am J Rhinol Allergy. 2021;35(6):854–860. https://doi.org/10.1177/19458924211008685
- 147. Tversky JR, Chelladurai Y, McGready J, Hamilton RG. Performance and pain tolerability of current diagnostic allergy skin prick test devices. J Allergy Clin Immunol Pract. 2015;3(6):888–893. https://doi.org/10.1016/j.jaip.2015.07.022
- 148. Tversky J, MacGlashan D. Short‐wave infrared camera as a novel solution to allergy skin testing. Allergy. 2020;75(4):965–968. https://doi.org/10.1111/all.14089
- 149. Kim YH, Jang TY. Nasal provocation test using allergen extract versus cold dry air provocation test: which and when? Am J Rhinol Allergy. 2013;27(2):113–117. https://doi.org/10.2500/ajra.2013.27.3870
- 150. Jang TY, Kim YH. Nasal provocation test is useful for discriminating allergic, nonallergic, and local allergic rhinitis. Am J Rhinol Allergy. 2015;29(4):e100–e104. https://doi.org/10.2500/ajra.2015.29.4214
- 151. Gomez F, Rondon C, Salas M, Campo P. Local allergic rhinitis: mechanisms, diagnosis and relevance for occupational rhinitis. Curr Opin Allergy Clin Immunol. 2015;15(2):111–116. https://doi.org/10.1097/ACI.0000000000000150
- 152. Bousquet J, Khaltaev N, Cruz AA, et al. Allergic Rhinitis and its Impact on Asthma (ARIA) 2008 update (in collaboration with the World Health Organization, GA(2)LEN and AllerGen). Allergy. 2008;63(suppl 86):8–160. https://doi.org/10.1111/j.1398‐9995.2007.01620.x
- 153. Moscato G, Pala G, Sastre J. Specific immunotherapy and biological treatments for occupational allergy. Curr Opin Allergy Clin Immunol. 2014;14(6):576–581. https://doi.org/10.1097/ACI.0000000000000105
- 154. Moscato G, Rolla G, Siracusa A. Occupational rhinitis: consensus on diagnosis and medicolegal implications. Curr Opin Otolaryngol Head Neck Surg. 2011;19(1):36–42. https://doi.org/10.1097/MOO.0b013e328341e228
- 155. Gerth van Wijk R, Patiwael JA, de Jong NW, de Groot H, Burdorf A. Occupational rhinitis in bell pepper greenhouse workers: determinants of leaving work and the effects of subsequent allergen avoidance on health‐related quality of life. Allergy. 2011;66(7):903–908. https://doi.org/10.1111/j.1398‐9995.2011.02556.x
- 156. Foss‐Skiftesvik MH, Winther L, Johnsen CR, et al. High occurrence of rhinitis symptoms in hairdressing apprentices. Int Forum Allergy Rhinol. 2017;7(1):43–49. https://doi.org/10.1002/alr.21834
- 157. Rouadi PW, Idriss SA, Naclerio RM, et al. Immunopathological features of air pollution and its impact on inflammatory airway diseases (IAD). World Allergy Organ J. 2020;13(10):100467. https://doi.org/10.1016/j.waojou.2020.100467
- 158. Moscato G, Pala G, Folletti I, Siracusa A, Quirce S. Occupational rhinitis affects occupational asthma severity. J Occup Health. 2016;58(3):310–313. https://doi.org/10.1539/joh.15‐0067‐BR
- 159. Tai CF, Baraniuk JN. Upper airway neurogenic mechanisms. Curr Opin Allergy Clin Immunol. 2002;2(1):11–19. https://doi.org/10.1097/00130832‐200202000‐00003
- 160. Meggs WJ. RADS and RUDS–the toxic induction of asthma and rhinitis. J Toxicol Clin Toxicol. 1994;32(5):487–501. https://doi.org/10.3109/15563659409011053
- 161. Brooks SM, Weiss MA, Bernstein IL. Reactive airways dysfunction syndrome (RADS). Persistent asthma syndrome after high level irritant exposures. Chest. 1985;88(3):376–384. https://doi.org/10.1378/chest.88.3.376
- 162. Bello A, Quinn MM, Perry MJ, Milton DK. Characterization of occupational exposures to cleaning products used for common cleaning tasks – a pilot study of hospital cleaners. Environ Health. 2009;8:11. https://doi.org/10.1186/1476‐069X‐8‐11
- 163. Chary A, Hennen J, Klein SG, Serchi T, Gutleb AC, Blomeke B. Respiratory sensitization: toxicological point of view on the available assays. Arch Toxicol. 2018;92(2):803–822. https://doi.org/10.1007/s00204‐017‐2088‐5
- 164. Kimber I, Dearman RJ, Basketter DA. Diisocyanates, occupational asthma and IgE antibody: implications for hazard characterization. J Appl Toxicol. 2014;34(10):1073–1077. https://doi.org/10.1002/jat.3041
- 165. Kimber I, Dearman RJ. Chemical respiratory allergy: role of IgE antibody and relevance of route of exposure. Toxicology. 2002;181‐182:311–315. https://doi.org/10.1016/s0300‐483x(02)00299‐8
- 166. Wisnewski AV. Developments in laboratory diagnostics for isocyanate asthma. Curr Opin Allergy Clin Immunol. 2007;7(2):138–145. https://doi.org/10.1097/ACI.0b013e3280895d22
- 167. Christensen DN, Franks ZG, McCrary HC, Saleh AA, Chang EH. A systematic review of the association between cigarette smoke exposure and chronic rhinosinusitis. Otolaryngol Head Neck Surg. 2018;158(5):801–816. https://doi.org/10.1177/0194599818757697
- 168. Eriksson J, Ekerljung L, Sundblad BM, et al. Cigarette smoking is associated with high prevalence of chronic rhinitis and low prevalence of allergic rhinitis in men. Allergy. 2013;68(3):347–354. https://doi.org/10.1111/all.12095
- 169. Yao TC, Chang SW, Chang WC, et al. Exposure to tobacco smoke and childhood rhinitis: a population‐based study. Sci Rep. 2017;7:42836. https://doi.org/10.1038/srep42836
- 170. Lee A, Lee SY, Lee KS. The use of heated tobacco products is associated with asthma, allergic rhinitis, and atopic dermatitis in Korean adolescents. Sci Rep. 2019;9(1):17699. https://doi.org/10.1038/s41598‐019‐54102‐4
- 171. Phulka JS, Howlett JW, Hu A. Cannabis related side effects in otolaryngology: a scoping review. J Otolaryngol Head Neck Surg. 2021;50(1):56. https://doi.org/10.1186/s40463‐021‐00538‐6
- 172. Abramson MJ, Schindler C, Schikowski T, et al. Rhinitis in Swiss adults is associated with asthma and early life factors, but not second hand tobacco smoke or obesity. Allergol Int. 2016;65(2):192–198. https://doi.org/10.1016/j.alit.2015.11.004
- 173. Pallasaho P, Kainu A, Juusela M, Meren M, Sovijarvi A. High prevalence of rhinitis symptoms without allergic sensitization in Estonia and Finland. Eur Clin Respir J. 2015;2. https://doi.org/10.3402/ecrj.v2.25401
- 174. Shargorodsky J, Garcia‐Esquinas E, Galan I, Navas‐Acien A, Lin SY. Allergic sensitization, rhinitis and tobacco smoke exposure in US adults. PLoS One. 2015;10(7):e0131957. https://doi.org/10.1371/journal.pone.0131957
- 175. Hisinger‐Molkanen H, Piirila P, Haahtela T, Sovijarvi A, Pallasaho P. Smoking, environmental tobacco smoke and occupational irritants increase the risk of chronic rhinitis. World Allergy Organ J. 2018;11(1):6. https://doi.org/10.1186/s40413‐018‐0184‐5
- 176. Gleich GJ, Welsh PW, Yunginger JW, Hyatt RE, Catlett JB. Allergy to tobacco: an occupational hazard. N Engl J Med. 1980;302(11):617–619. https://doi.org/10.1056/NEJM198003133021107
- 177. Burrows B, Halonen M, Lebowitz MD, Knudson RJ, Barbee RA. The relationship of serum immunoglobulin E, allergy skin tests, and smoking to respiratory disorders. J Allergy Clin Immunol. 1982;70(3):199–204. https://doi.org/10.1016/0091‐6749(82)90042‐2
- 178. Bascom R, Kesavanathan J, Fitzgerald TK, Cheng KH, Swift DL. Sidestream tobacco smoke exposure acutely alters human nasal mucociliary clearance. Environ Health Perspect. 1995;103(11):1026–1030. https://doi.org/10.1289/ehp.951031026
- 179. Andre E, Campi B, Materazzi S, et al. Cigarette smoke‐induced neurogenic inflammation is mediated by alpha, beta‐unsaturated aldehydes and the TRPA1 receptor in rodents. J Clin Invest. 2008;118(7):2574–2582. https://doi.org/10.1172/JCI34886
- 180. Meggs WJ. Neurogenic inflammation and sensitivity to environmental chemicals. Environ Health Perspect. 1993;101(3):234–238. https://doi.org/10.1289/ehp.93101234
- 181. Bascom R, Kulle T, Kagey‐Sobotka A, Proud D. Upper respiratory tract environmental tobacco smoke sensitivity. Am Rev Respir Dis. 1991;143(6):1304–1311. https://doi.org/10.1164/ajrccm/143.6.1304
- 182. Dykewicz MS, Wallace DV, Amrol DJ, et al. Rhinitis 2020: a practice parameter update. J Allergy Clin Immunol. 2020;146(4):721–767. https://doi.org/10.1016/j.jaci.2020.07.007
- 183. Fokkens WJ, Lund VJ, Hopkins C, et al. European Position Paper on rhinosinusitis and nasal polyps 2020. Rhinology. 2020;58(suppl S29):1–464. https://doi.org/10.4193/Rhin20.600
- 184. Meltzer EO, Hamilos DL, Hadley JA, et al. Rhinosinusitis: establishing definitions for clinical research and patient care. Otolaryngol Head Neck Surg. 2004;131(6 suppl):S1–S62. https://doi.org/10.1016/j.otohns.2004.09.067
- 185. Fokkens WJ, Lund VJ, Mullol J, et al. European Position Paper on rhinosinusitis and nasal polyps 2012. Rhinol Suppl. 2012;23:3 p preceding table of contents, 1‐298.
- 186. Aring AM, Chan MM. Current concepts in adult acute rhinosinusitis. Am Fam Physician. 2016;94(2):97–105.
- 187. Canonica GW, Ciprandi G, Pesce GP, Buscaglia S, Paolieri F, Bagnasco M. ICAM‐1 on epithelial cells in allergic subjects: a hallmark of allergic inflammation. Int Arch Allergy Immunol. 1995;107(1‐3):99–102. https://doi.org/10.1159/000236943
- 188. Ciebiada M, Gorska‐Ciebiada M, Gorski P. sICAM‐1 and TNF‐alpha in asthma and rhinitis: relationship with the presence of atopy. J Asthma. 2011;48(7):660–666. https://doi.org/10.3109/02770903.2011.604886
- 189. Tantilipikorn P. The relationship between allergic rhinitis and viral infections. Curr Opin Otolaryngol Head Neck Surg. 2014;22(3):249–252. https://doi.org/10.1097/MOO.0000000000000049
- 190. Gorska‐Ciebiada M, Ciebiada M, Gorska MM, Gorski P, Grzelewska‐Rzymowska I. Intercellular adhesion molecule 1 and tumor necrosis factor alpha in asthma and persistent allergic rhinitis: relationship with disease severity. Ann Allergy Asthma Immunol. 2006;97(1):66–72. https://doi.org/10.1016/S1081‐1206(10)61372‐5
- 191. Shiota Y, Wilson JG, Marukawa M, Ono T, Kaji M. Soluble intercellular adhesion molecule 1 (ICAM‐1) antigen in sera of bronchial asthmatics. Chest. 1996;109(1):94–99. https://doi.org/10.1378/chest.109.1.94
- 192. Gosset P, Tillie‐Leblond I, Janin A, et al. Expression of E‐selectin, ICAM‐1 and VCAM‐1 on bronchial biopsies from allergic and non‐allergic asthmatic patients. Int Arch Allergy Immunol. 1995;106(1):69–77. https://doi.org/10.1159/000236892
- 193. Kenealy T, Arroll B. Antibiotics for the common cold and acute purulent rhinitis. Cochrane Database Syst Rev. 2013;(6):CD000247. https://doi.org/10.1002/14651858.CD000247.pub3
- 194. Kaper NM, Breukel L, Venekamp RP, Grolman W, van der Heijden GJ. Absence of evidence for enhanced benefit of antibiotic therapy on recurrent acute rhinosinusitis episodes: a systematic review of the evidence base. Otolaryngol Head Neck Surg. 2013;149(5):664–667. https://doi.org/10.1177/0194599813505841
- 195. Lemiengre MB, van Driel ML, Merenstein D, Young J, De Sutter AI. Antibiotics for clinically diagnosed acute rhinosinusitis in adults. Cochrane Database Syst Rev. 2012;10:CD006089. https://doi.org/10.1002/14651858.CD006089.pub4
- 196. van den Broek MF, Gudden C, Kluijfhout WP, et al. No evidence for distinguishing bacterial from viral acute rhinosinusitis using symptom duration and purulent rhinorrhea: a systematic review of the evidence base. Otolaryngol Head Neck Surg. 2014;150(4):533–537. https://doi.org/10.1177/0194599814522595
- 197. Stjarne P, Odeback P, Stallberg B, Lundberg J, Olsson P. High costs and burden of illness in acute rhinosinusitis: real‐life treatment patterns and outcomes in Swedish primary care. Prim Care Respir J. 2012;21(2):174–179; quiz 10p following 179. https://doi.org/10.4104/pcrj.2012.00011
- 198. Jaume F, Quinto L, Alobid I, Mullol J. Overuse of diagnostic tools and medications in acute rhinosinusitis in Spain: a population‐based study (the PROSINUS study). BMJ Open. 2018;8(1):e018788. https://doi.org/10.1136/bmjopen‐2017‐018788
- 199. Seresirikachorn K, Snidvongs K, Chitsuthipakorn W, et al. EPOS2012 has better specificity compared to IDSA2012 for diagnosing acute bacterial rhinosinusitis. Rhinology. 2018;56(3):241–244. https://doi.org/10.4193/Rhin17.261
- 200. Chow AW, Benninger MS, Brook I, et al. IDSA clinical practice guideline for acute bacterial rhinosinusitis in children and adults. Clin Infect Dis. 2012;54(8):e72–e112. https://doi.org/10.1093/cid/cir1043
- 201. Rosenfeld RM, Piccirillo JF, Chandrasekhar SS, et al. Clinical practice guideline (update): adult sinusitis. Otolaryngol Head Neck Surg. 2015;152(2 suppl):S1–S39. https://doi.org/10.1177/0194599815572097
- 202. Lindbaek M, Hjortdahl P, Johnsen UL. Use of symptoms, signs, and blood tests to diagnose acute sinus infections in primary care: comparison with computed tomography. Fam Med. 1996;28(3):183–188.
- 203. Ellegard EK. Pregnancy rhinitis. Immunol Allergy Clin North Am. 2006;26(1):119–135, vii. https://doi.org/10.1016/j.iac.2005.10.007
- 204. Ellegard EK. Clinical and pathogenetic characteristics of pregnancy rhinitis. Clin Rev Allergy Immunol. 2004;26(3):149–159. https://doi.org/10.1385/CRIAI:26:3:149
- 205. Ellegard E, Karlsson G. Nasal congestion during pregnancy. Clin Otolaryngol Allied Sci. 1999;24(4):307–311. https://doi.org/10.1046/j.1365‐2273.1999.00264.x
- 206. Baudoin T, Simunjak T, Bacan N, Jelavic B, Kuna K, Kosec A. Redefining pregnancy‐induced rhinitis. Am J Rhinol Allergy. 2021;35(3):315–322. https://doi.org/10.1177/1945892420957490
- 207. Ellegard E, Hellgren M, Toren K, Karlsson G. The incidence of pregnancy rhinitis. Gynecol Obstet Invest. 2000;49(2):98–101. https://doi.org/10.1159/000010223
- 208. Philpott CM, Conboy P, Al‐Azzawi F, Murty G. Nasal physiological changes during pregnancy. Clin Otolaryngol Allied Sci. 2004;29(4):343–351. https://doi.org/10.1111/j.1365‐2273.2004.00815.x
- 209. Toppozada H, Michaels L, Toppozada M, El‐Ghazzawi I, Talaat M, Elwany S. The human respiratory nasal mucosa in pregnancy. An electron microscopic and histochemical study. J Laryngol Otol. 1982;96(7):613–626. https://doi.org/10.1017/s0022215100092902
- 210. Juniper EF, Guyatt GH, Andersson B, Ferrie PJ. Comparison of powder and aerosolized budesonide in perennial rhinitis: validation of rhinitis quality of life questionnaire. Ann Allergy. 1993;70(3):225–230.
- 211. Kumar R, Hayhurst KL, Robson AK. Ear, nose, and throat manifestations during pregnancy. Otolaryngol Head Neck Surg. 2011;145(2):188–198. https://doi.org/10.1177/0194599811407572
- 212. Orban N, Maughan E, Bleach N. Pregnancy‐induced rhinitis. Rhinology. 2013;51(2):111–119. https://doi.org/10.4193/Rhino12.045
- 213. Hamano N, Terada N, Maesako K, et al. Expression of histamine receptors in nasal epithelial cells and endothelial cells–the effects of sex hormones. Int Arch Allergy Immunol. 1998;115(3):220–227. https://doi.org/10.1159/000023904
- 214. Ellegard E, Oscarsson J, Bougoussa M, et al. Serum level of placental growth hormone is raised in pregnancy rhinitis. Arch Otolaryngol Head Neck Surg. 1998;124(4):439–443. https://doi.org/10.1001/archotol.124.4.439
- 215. Franklin KA, Holmgren PA, Jonsson F, Poromaa N, Stenlund H, Svanborg E. Snoring, pregnancy‐induced hypertension, and growth retardation of the fetus. Chest. 2000;117(1):137–141. https://doi.org/10.1378/chest.117.1.137
- 216. Favilli A, Laurenti E, Stagni GM, Tassi L, Ricci G, Gerli S. Effects of sodium hyaluronate on symptoms and quality of life in women affected by pregnancy rhinitis: a pilot study. Gynecol Obstet Invest. 2019;84(2):159–165. https://doi.org/10.1159/000493137
- 217. Hellings PW, Klimek L, Cingi C, et al. Non‐allergic rhinitis: position paper of the European Academy of Allergy and Clinical Immunology. Allergy. 2017;72(11):1657–1665. https://doi.org/10.1111/all.13200
- 218. Ellegard EK, Karlsson NG, Ellegard LH. Rhinitis in the menstrual cycle, pregnancy, and some endocrine disorders. Clin Allergy Immunol. 2007;19:305–321.
- 219. Navarrete‐Palacios E, Hudson R, Reyes‐Guerrero G, Guevara‐Guzman R. Correlation between cytological characteristics of the nasal epithelium and the menstrual cycle. Arch Otolaryngol Head Neck Surg. 2003;129(4):460–463. https://doi.org/10.1001/archotol.129.4.460
- 220. Proetz AW. Further observations of the effects of thyroid insufficiency on the nasal mucosa. Laryngoscope. 1950;60(7):627–633. https://doi.org/10.1288/00005537‐195007000‐00004
- 221. Kulekci Ozturk S, Sakci E, Kavvasoglu C. Rhinitis in patients with acquired hypothyroidism. Eur Arch Otorhinolaryngol. 2021;278(1):87–92. https://doi.org/10.1007/s00405‐020‐06254‐7
- 222. Skinner DW, Richards SH. Acromegaly – the mucosal changes within the nose and paranasal sinuses. J Laryngol Otol. 1988;102(12):1107–1110. https://doi.org/10.1017/s0022215100107455
- 223. Sampson HA, Aceves S, Bock SA, et al. Food allergy: a practice parameter update‐2014. J Allergy Clin Immunol. 2014;134(5):1016–1025.e43. https://doi.org/10.1016/j.jaci.2014.05.013
- 224. Jovancevic L, Georgalas C, Savovic S, Janjevic D. Gustatory rhinitis. Rhinology. 2010;48(1):7–10. https://doi.org/10.4193/Rhin07.153
- 225. Waibel KH, Chang C. Prevalence and food avoidance behaviors for gustatory rhinitis. Ann Allergy Asthma Immunol. 2008;100(3):200–205. https://doi.org/10.1016/S1081‐1206(10)60443‐7
- 226. Raphael G, Raphael MH, Kaliner M. Gustatory rhinitis: a syndrome of food‐induced rhinorrhea. J Allergy Clin Immunol. 1989;83(1):110–115. https://doi.org/10.1016/0091‐6749(89)90484‐3
- 227. Georgalas C, Jovancevic L. Gustatory rhinitis. Curr Opin Otolaryngol Head Neck Surg. 2012;20(1):9–14. https://doi.org/10.1097/MOO.0b013e32834dfb52
- 228. Seki N, Shirasaki H, Kikuchi M, Sakamoto T, Watanabe N, Himi T. Expression and localization of TRPV1 in human nasal mucosa. Rhinology. 2006;44(2):128–134.
- 229. Marshak T, Yun WK, Hazout C, Sacks R, Harvey RJ. A systematic review of the evidence base for vidian neurectomy in managing rhinitis. J Laryngol Otol. 2016;130(suppl 4):S7–S28. https://doi.org/10.1017/S0022215116008008
- 230. Nihlen U, Greiff LJ, Nyberg P, Persson CG, Andersson M. Alcohol‐induced upper airway symptoms: prevalence and co‐morbidity. Respir Med. 2005;99(6):762–769. https://doi.org/10.1016/j.rmed.2004.11.010
- 231. Glicksman JT, Parasher AK, Doghramji L, et al. Alcohol‐induced respiratory symptoms improve after aspirin desensitization in patients with aspirin‐exacerbated respiratory disease. Int Forum Allergy Rhinol. 2018;8(10):1093–1097. https://doi.org/10.1002/alr.22168
- 232. Cardet JC, White AA, Barrett NA, et al. Alcohol‐induced respiratory symptoms are common in patients with aspirin exacerbated respiratory disease. J Allergy Clin Immunol Pract. 2014;2(2):208–213. https://doi.org/10.1016/j.jaip.2013.12.003
- 233. De Schryver E, Derycke L, Campo P, et al. Alcohol hyper‐responsiveness in chronic rhinosinusitis with nasal polyps. Clin Exp Allergy. 2017;47(2):245–253. https://doi.org/10.1111/cea.12836
- 234. Lomholt FK, Nielsen SF, Nordestgaard BG. High alcohol consumption causes high IgE levels but not high risk of allergic disease. J Allergy Clin Immunol. 2016;138(5):1404–1413.e13. https://doi.org/10.1016/j.jaci.2016.05.022
- 235. Ellis AK, Keith PK. Nonallergic rhinitis with eosinophilia syndrome. Curr Allergy Asthma Rep. 2006;6(3):215–220. https://doi.org/10.1007/s11882‐006‐0037‐0
- 236. Jacobs RL, Freedman PM, Boswell RN. Nonallergic rhinitis with eosinophilia (NARES syndrome). Clinical and immunologic presentation. J Allergy Clin Immunol. 1981;67(4):253–262. https://doi.org/10.1016/0091‐6749(81)90019‐1
- 237. Simola M, Malmberg H. Sense of smell in allergic and nonallergic rhinitis. Allergy. 1998;53(2):190–194. https://doi.org/10.1111/j.1398‐9995.1998.tb03869.x
- 238. Moneret‐Vautrin DA, Jankowski R, Bene MC, et al. NARES: a model of inflammation caused by activated eosinophils? Rhinology. 1992;30(3):161–168.
- 239. Powe DG, Huskisson RS, Carney AS, Jenkins D, Jones NS. Evidence for an inflammatory pathophysiology in idiopathic rhinitis. Clin Exp Allergy. 2001;31(6):864–872. https://doi.org/10.1046/j.1365‐2222.2001.01106.x
- 240. Berger G, Goldberg A, Ophir D. The inferior turbinate mast cell population of patients with perennial allergic and nonallergic rhinitis. Am J Rhinol. 1997;11(1):63–66. https://doi.org/10.2500/105065897781446775
- 241. De Corso E, Baroni S, Battista M, et al. Nasal fluid release of eotaxin‐3 and eotaxin‐2 in persistent sinonasal eosinophilic inflammation. Int Forum Allergy Rhinol. 2014;4(8):617–624. https://doi.org/10.1002/alr.21348
- 242. De Corso E, Baroni S, Lucidi D, et al. Nasal lavage levels of granulocyte‐macrophage colony‐stimulating factor and chronic nasal hypereosinophilia. Int Forum Allergy Rhinol. 2015;5(6):557–562. https://doi.org/10.1002/alr.21519
- 243. Kramer MF, Burow G, Pfrogner E, Rasp G. In vitro diagnosis of chronic nasal inflammation. Clin Exp Allergy. 2004;34(7):1086–1092. https://doi.org/10.1111/j.1365‐2222.2004.01989.x
- 244. Groger M, Klemens C, Wendt S, et al. Mediators and cytokines in persistent allergic rhinitis and nonallergic rhinitis with eosinophilia syndrome. Int Arch Allergy Immunol. 2012;159(2):171–178. https://doi.org/10.1159/000336169
- 245. Marcella R, Croce A, Moretti A, Barbacane RC, Di Giocchino M, Conti P. Transcription and translation of the chemokines RANTES and MCP‐1 in nasal polyps and mucosa in allergic and non‐allergic rhinopathies. Immunol Lett. 2003;90(2‐3):71–75. https://doi.org/10.1016/s0165‐2478(03)00163‐9
- 246. Peric A, Sotirovic J, Spadijer‐Mirkovic C, Matkovic‐Jozin S, Peric AV, Vojvodic D. Nonselective chemokine levels in nasal secretions of patients with perennial nonallergic and allergic rhinitis. Int Forum Allergy Rhinol. 2016;6(4):392–397. https://doi.org/10.1002/alr.21684
- 247. Becker S, Rasp J, Eder K, Berghaus A, Kramer MF, Groger M. Non‐allergic rhinitis with eosinophilia syndrome is not associated with local production of specific IgE in nasal mucosa. Eur Arch Otorhinolaryngol. 2016;273(6):1469–1475. https://doi.org/10.1007/s00405‐015‐3769‐4
- 248. Eckrich J, Hinkel J, Fischl A, et al. Nasal IgE in subjects with allergic and non‐allergic rhinitis. World Allergy Organ J. 2020;13(6):100129. https://doi.org/10.1016/j.waojou.2020.100129
- 249. Zhang M, Yan B, Wang Y, Wang C, Zhang L. Charcot‐Leyden crystal protein in nasal secretions of patients with nonallergic rhinitis with eosinophilia syndrome. Int Arch Allergy Immunol. 2020;181(11):888–896. https://doi.org/10.1159/000509252
- 250. Meng Y, Yan B, Wang Y, Wu D, Zhang L, Wang C. Diagnosis and management of nonallergic rhinitis with eosinophilia syndrome using cystatin SN together with symptoms. World Allergy Organ J. 2020;13(7):100134. https://doi.org/10.1016/j.waojou.2020.100134
- 251. Numao T, Agrawal DK. Neuropeptides modulate human eosinophil chemotaxis. J Immunol. 1992;149(10):3309–3315.
- 252. Kramer MF, de la Chaux R, Fintelmann R, Rasp G. NARES: a risk factor for obstructive sleep apnea? Am J Otolaryngol. 2004;25(3):173–177. https://doi.org/10.1016/j.amjoto.2003.12.004
- 253. Wang Q, Ji J, Xie Y, et al. Lower airway inflammation and hyperresponsiveness in non‐asthmatic patients with non‐allergic rhinitis. J Thorac Dis. 2015;7(10):1756–1764. https://doi.org/10.3978/j.issn.2072‐1439.2015.10.26
- 254. Settipane RA, Lieberman P. Update on nonallergic rhinitis. Ann Allergy Asthma Immunol. 2001;86(5):494–507; quiz 507‐8. https://doi.org/10.1016/S1081‐1206(10)62896‐7
- 255. Pipkorn U, Proud D, Lichtenstein LM, Kagey‐Sobotka A, Norman PS, Naclerio RM. Inhibition of mediator release in allergic rhinitis by pretreatment with topical glucocorticosteroids. N Engl J Med. 1987;316(24):1506–1510. https://doi.org/10.1056/NEJM198706113162403
- 256. Webb DR, Meltzer EO, Finn AF Jr., et al. Intranasal fluticasone propionate is effective for perennial nonallergic rhinitis with or without eosinophilia. Ann Allergy Asthma Immunol. 2002;88(4):385–390. https://doi.org/10.1016/S1081‐1206(10)62369‐1
- 257. Bachert C, van Cauwenberge P, Khaltaev N, World Health Organization . Allergic rhinitis and its impact on asthma. In collaboration with the World Health Organization. Executive summary of the workshop report. 7‐10 December 1999, Geneva, Switzerland. Allergy. 2002;57(9):841–855. https://doi.org/10.1034/j.1398‐9995.2002.23625.x
- 258. Banov CH, Lieberman P, Vasomotor Rhinitis Study Groups . Efficacy of azelastine nasal spray in the treatment of vasomotor (perennial nonallergic) rhinitis. Ann Allergy Asthma Immunol. 2001;86(1):28–35. https://doi.org/10.1016/S1081‐1206(10)62352‐6
- 259. De Corso E, Anzivino R, Galli J, et al. Antileukotrienes improve naso‐ocular symptoms and biomarkers in patients with NARES and asthma. Laryngoscope. 2019;129(3):551–557. https://doi.org/10.1002/lary.27576
- 260. Settipane RA. Epidemiology of vasomotor rhinitis. World Allergy Organ J. 2009;2(6):115–118. https://doi.org/10.1097/WOX.0b013e3181ac91ae
- 261. Mullarkey MF, Hill JS, Webb DR. Allergic and nonallergic rhinitis: their characterization with attention to the meaning of nasal eosinophilia. J Allergy Clin Immunol. 1980;65(2):122–126. https://doi.org/10.1016/0091‐6749(80)90196‐7
- 262. Enberg RN. Perennial nonallergic rhinitis: a retrospective review. Ann Allergy. 1989;63(6 pt 1):513–516.
- 263. Nozad CH, Michael LM, Betty Lew D, Michael CF. Non‐allergic rhinitis: a case report and review. Clin Mol Allergy. 2010;8:1. https://doi.org/10.1186/1476‐7961‐8‐1
- 264. Pattanaik D, Lieberman P. Vasomotor rhinitis. Curr Allergy Asthma Rep. 2010;10(2):84–91. https://doi.org/10.1007/s11882‐010‐0089‐z
- 265. Campo P, Rondon C, Gould HJ, Barrionuevo E, Gevaert P, Blanca M. Local IgE in non‐allergic rhinitis. Clin Exp Allergy. 2015;45(5):872–881. https://doi.org/10.1111/cea.12476
- 266. James LK, Durham SR. Rhinitis with negative skin tests and absent serum allergen‐specific IgE: more evidence for local IgE? J Allergy Clin Immunol. 2009;124(5):1012–1013. https://doi.org/10.1016/j.jaci.2009.09.029
- 267. Hamizan AW, Rimmer J, Alvarado R, et al. Positive allergen reaction in allergic and nonallergic rhinitis: a systematic review. Int Forum Allergy Rhinol. 2017;7(9):868–877. https://doi.org/10.1002/alr.21988
- 268. Eifan AO, Durham SR. Pathogenesis of rhinitis. Clin Exp Allergy. 2016;46(9):1139–1151. https://doi.org/10.1111/cea.12780
- 269. Bernstein JA, Hastings L, Boespflug EL, Allendorfer JB, Lamy M, Eliassen JC. Alteration of brain activation patterns in nonallergic rhinitis patients using functional magnetic resonance imaging before and after treatment with intranasal azelastine. Ann Allergy Asthma Immunol. 2011;106(6):527–532. https://doi.org/10.1016/j.anai.2011.02.014
- 270. Segboer C, Gevorgyan A, Avdeeva K, et al. Intranasal corticosteroids for non‐allergic rhinitis. Cochrane Database Syst Rev. 2019;2019(11):CD010592. https://doi.org/10.1002/14651858.CD010592.pub2
- 271. Lieberman P, Kaliner MA, Wheeler WJ. Open‐label evaluation of azelastine nasal spray in patients with seasonal allergic rhinitis and nonallergic vasomotor rhinitis. Curr Med Res Opin. 2005;21(4):611–618. https://doi.org/10.1185/030079905X41408
- 272. Grossman J, Banov C, Boggs P, et al. Use of ipratropium bromide nasal spray in chronic treatment of nonallergic perennial rhinitis, alone and in combination with other perennial rhinitis medications. J Allergy Clin Immunol. 1995;95(5 pt 2):1123–1127. https://doi.org/10.1016/s0091‐6749(95)70216‐4
- 273. Yan CH, Hwang PH. Surgical management of nonallergic rhinitis. Otolaryngol Clin North Am. 2018;51(5):945–955. https://doi.org/10.1016/j.otc.2018.05.010
- 274. Ikeda K, Yokoi H, Saito T, Kawano K, Yao T, Furukawa M. Effect of resection of the posterior nasal nerve on functional and morphological changes in the inferior turbinate mucosa. Acta Otolaryngol. 2008;128(12):1337–1341. https://doi.org/10.1080/00016480801935525
- 275. Hwang PH, Lin B, Weiss R, Atkins J, Johnson J. Cryosurgical posterior nasal tissue ablation for the treatment of rhinitis. Int Forum Allergy Rhinol. 2017;7(10):952–956. https://doi.org/10.1002/alr.21991
- 276. Kompelli AR, Janz TA, Rowan NR, Nguyen SA, Soler ZM. Cryotherapy for the treatment of chronic rhinitis: a qualitative systematic review. Am J Rhinol Allergy. 2018;32(6):491–501. https://doi.org/10.1177/1945892418800879
- 277. Sahin‐Yilmaz AA, Corey JP. Rhinitis in the elderly. Clin Allergy Immunol. 2007;19:209–219.
- 278. Edelstein DR. Aging of the normal nose in adults. Laryngoscope. 1996;106(9 pt 2):1–25. https://doi.org/10.1097/00005537‐199609001‐00001
- 279. Lindemann J, Sannwald D, Wiesmiller K. Age‐related changes in intranasal air conditioning in the elderly. Laryngoscope. 2008;118(8):1472–1475. https://doi.org/10.1097/MLG.0b013e3181758174
- 280. Pinto JM, Jeswani S. Rhinitis in the geriatric population. Allergy Asthma Clin Immunol. 2010;6(1):10. https://doi.org/10.1186/1710‐1492‐6‐10
- 281. Rodriguez K, Rubinstein E, Ferguson BJ. Clear anterior rhinorrhea in the population. Int Forum Allergy Rhinol. 2015;5(11):1063–1067. https://doi.org/10.1002/alr.21583
- 282. Parashar R, Amir M, Pakhare A, Rathi P, Chaudhary L. Age related changes in autonomic functions. J Clin Diagn Res. 2016;10(3):CC11–CC15. https://doi.org/10.7860/JCDR/2016/16889.7497
- 283. Hotta H, Uchida S. Aging of the autonomic nervous system and possible improvements in autonomic activity using somatic afferent stimulation. Geriatr Gerontol Int. 2010;10(suppl 1):S127–S136. https://doi.org/10.1111/j.1447‐0594.2010.00592.x
- 284. Lal D, Corey JP. Vasomotor rhinitis update. Curr Opin Otolaryngol Head Neck Surg. 2004;12(3):243–247. https://doi.org/10.1097/01.moo.0000122310.13359.79
- 285. Kimmelman CP, Ali GH. Vasomotor rhinitis. Otolaryngol Clin North Am. 1986;19(1):65–71.
- 286. Georgitis JW. Prevalence and differential diagnosis of chronic rhinitis. Curr Allergy Asthma Rep. 2001;1(3):202–206. https://doi.org/10.1007/s11882‐001‐0006‐6
- 287. Baptist AP, Nyenhuis S. Rhinitis in the elderly. Immunol Allergy Clin North Am. 2016;36(2):343–357. https://doi.org/10.1016/j.iac.2015.12.010
- 288. Janzen VD. Rhinological disorders in the elderly. J Otolaryngol. 1986;15(4):228–230.
- 289. Ciftci Z, Catli T, Hanci D, Cingi C, Erdogan G. Rhinorrhoea in the elderly. Eur Arch Otorhinolaryngol. 2015;272(10):2587–2592. https://doi.org/10.1007/s00405‐014‐3182‐4
- 290. Bozek A. Pharmacological management of allergic rhinitis in the elderly. Drugs Aging. 2017;34(1):21–28. https://doi.org/10.1007/s40266‐016‐0425‐7
- 291. Ho JC, Chan KN, Hu WH, et al. The effect of aging on nasal mucociliary clearance, beat frequency, and ultrastructure of respiratory cilia. Am J Respir Crit Care Med. 2001;163(4):983–988. https://doi.org/10.1164/ajrccm.163.4.9909121
- 292. Mirza N, Kroger H, Doty RL. Influence of age on the ‘nasal cycle’. Laryngoscope. 1997;107(1):62–66. https://doi.org/10.1097/00005537‐199701000‐00014
- 293. Slavin RG. Treating rhinitis in the older population: special considerations. Allergy Asthma Clin Immunol. 2009;5(1):9. https://doi.org/10.1186/1710‐1492‐5‐9
- 294. Schrodter S, Biermann E, Halata Z. Histological evaluation of age‐related changes in human respiratory mucosa of the middle turbinate. Anat Embryol (Berl). 2003;207(1):19–27. https://doi.org/10.1007/s00429‐003‐0326‐5
- 295. Milgrom H, Huang H. Allergic disorders at a venerable age: a mini‐review. Gerontology. 2014;60(2):99–107. https://doi.org/10.1159/000355307
- 296. Wheatley LM, Togias A. Clinical practice. Allergic rhinitis. N Engl J Med. 2015;372(5):456–463. https://doi.org/10.1056/NEJMcp1412282
- 297. Seidman MD, Gurgel RK, Lin SY, et al. Clinical practice guideline: allergic rhinitis executive summary. Otolaryngol Head Neck Surg. 2015;152(2):197–206. https://doi.org/10.1177/0194599814562166
- 298. Slavin RG. Special considerations in treatment of allergic rhinitis in the elderly: role of intranasal corticosteroids. Allergy Asthma Proc. 2010;31(3):179–184. https://doi.org/10.2500/aap.2010.31.3342
- 299. Bozek A, Cudak A, Walter Canonica G. Long‐term efficacy of injected allergen immunotherapy for treatment of grass pollen allergy in elderly patients with allergic rhinitis. Allergy Asthma Proc. 2020;41(4):271–277. https://doi.org/10.2500/aap.2020.41.200035
- 300. Jain T, Sanju Kumar H, Guerrieri M, Ralli M, Di Mauro R. Primary atrophic rhinitis: ozeana and other infective forms. In: Di Girolamo S, ed. Atrophic Rhinitis. Springer; 2020:3–12.
- 301. Wright J. Atrophic rhinitis in its historical, etiological and histological aspects. Laryngoscope. 1913;23:641–666.
- 302. Ruskin S. A differential diagnosis and therapy of atrophic rhinitis and ozena. Arch Otolaryngol. 1932;15:222–257.
- 303. Bunnag C, Jareoncharsri P, Tansuriyawong P, Bhothisuwan W, Chantarakul N. Characteristics of atrophic rhinitis in Thai patients at the Siriraj Hospital. Rhinology. 1999;37(3):125–130.
- 304. Moore EJ, Kern EB. Atrophic rhinitis: a review of 242 cases. Am J Rhinol. 2001;15(6):355–361.
- 305. Bist SS, Bisht M, Purohit JP, Saxena R. Study of histopathological changes in primary atrophic rhinitis. ISRN Otolaryngol. 2011;2011:269479. https://doi.org/10.5402/2011/269479
- 306. Bist SS, Bisht M, Purohit JP. Primary atrophic rhinitis: a clinical profile, microbiological and radiological study. ISRN Otolaryngol. 2012;2012:404075. https://doi.org/10.5402/2012/404075
- 307. Sinha SN, Sardana DS, Rajvanshi VS. A nine years' review of 273 cases of atrophic rhinitis and its management. J Laryngol Otol. 1977;91(7):591–600. https://doi.org/10.1017/s0022215100084097
- 308. Mishra A, Kawatra R, Gola M. Interventions for atrophic rhinitis. Cochrane Database Syst Rev. 2012;(2):CD008280. https://doi.org/10.1002/14651858.CD008280.pub2
- 309. Gigant L, Zoli A, Guiacomini PG, Zoli A. A secondary atrophis rhinitis: autoimmune and granulomatous forms. In: Di Girolamo S, ed. Atrophic Rhinitis. Springer; 2020:13–30.
- 310. Ly TH, deShazo RD, Olivier J, Stringer SP, Daley W, Stodard CM. Diagnostic criteria for atrophic rhinosinusitis. Am J Med. 2009;122(8):747–753. https://doi.org/10.1016/j.amjmed.2008.12.025
- 311. Taylor M, Young A. Histopathological and histochemical studies on atrophic rhinitis. J Laryngol Otol. 1961;75:574–590. https://doi.org/10.1017/s0022215100058138
- 312. Zohar Y, Talmi YP, Strauss M, Finkelstein Y, Shvilli Y. Ozena revisited. J Otolaryngol. 1990;19(5):345–349.
- 313. Chand MS, MacArthur CJ. Primary atrophic rhinitis: a summary of four cases and review of the literature. Otolaryngol Head Neck Surg. 1997;116(4):554–558. https://doi.org/10.1016/s0194‐5998(97)70311‐5
- 314. Sibert JR, Barton RP. Dominant inheritance in a family with primary atrophic rhinitis. J Med Genet. 1980;17(1):39–40. https://doi.org/10.1136/jmg.17.1.39
- 315. Scheithauer MO. Surgery of the turbinates and "empty nose" syndrome. GMS Curr Top Otorhinolaryngol Head Neck Surg. 2010;9:Doc03. https://doi.org/10.3205/cto000067
- 316. Coste A, Dessi P, Serrano E. Empty nose syndrome. Eur Ann Otorhinolaryngol Head Neck Dis. 2012;129(2):93–97. https://doi.org/10.1016/j.anorl.2012.02.001
- 317. Chhabra N, Houser SM. The diagnosis and management of empty nose syndrome. Otolaryngol Clin North Am. 2009;42(2):311–330, ix. https://doi.org/10.1016/j.otc.2009.02.001
- 318. Houser SM. Surgical treatment for empty nose syndrome. Arch Otolaryngol Head Neck Surg. 2007;133(9):858–863. https://doi.org/10.1001/archotol.133.9.858
- 319. Kuan EC, Suh JD, Wang MB. Empty nose syndrome. Curr Allergy Asthma Rep. 2015;15(1):493. https://doi.org/10.1007/s11882‐014‐0493‐x
- 320. Velasquez N, Thamboo A, Habib AR, Huang Z, Nayak JV. The Empty Nose Syndrome 6‐Item Questionnaire (ENS6Q): a validated 6‐item questionnaire as a diagnostic aid for empty nose syndrome patients. Int Forum Allergy Rhinol. 2017;7(1):64–71. https://doi.org/10.1002/alr.21842
- 321. Payne SC. Empty nose syndrome: what are we really talking about? Otolaryngol Clin North Am. 2009;42(2):331–337, ix‐x. https://doi.org/10.1016/j.otc.2009.02.002
- 322. Chang MT, Nayak JV. In Response to Inferior Meatus Augmentation Procedure (IMAP) for treatment of empty nose syndrome. Laryngoscope. 2022;132(6):E22. https://doi.org/10.1002/lary.30119
- 323. Thamboo A, Velasquez N, Ayoub N, Nayak JV. Distinguishing computed tomography findings in patients with empty nose syndrome. Int Forum Allergy Rhinol. 2016;6(10):1075–1082. https://doi.org/10.1002/alr.21774
- 324. Malik J, Li C, Maza G, et al. Computational fluid dynamic analysis of aggressive turbinate reductions: is it a culprit of empty nose syndrome? Int Forum Allergy Rhinol. 2019;9(8):891–899. https://doi.org/10.1002/alr.22350
- 325. Bautista DM, Siemens J, Glazer JM, et al. The menthol receptor TRPM8 is the principal detector of environmental cold. Nature. 2007;448(7150):204–208. https://doi.org/10.1038/nature05910
- 326. Zhao K, Blacker K, Luo Y, Bryant B, Jiang J. Perceiving nasal patency through mucosal cooling rather than air temperature or nasal resistance. PLoS One. 2011;6(10):e24618. https://doi.org/10.1371/journal.pone.0024618
- 327. Zhao K, Jiang J, Blacker K, et al. Regional peak mucosal cooling predicts the perception of nasal patency. Laryngoscope. 2014;124(3):589–595. https://doi.org/10.1002/lary.24265
- 328. Willatt DJ, Jones AS. The role of the temperature of the nasal lining in the sensation of nasal patency. Clin Otolaryngol Allied Sci. 1996;21(6):519–523. https://doi.org/10.1111/j.1365‐2273.1996.tb01102.x
- 329. Kimbell JS, Frank DO, Laud P, Garcia GJ, Rhee JS. Changes in nasal airflow and heat transfer correlate with symptom improvement after surgery for nasal obstruction. J Biomech. 2013;46(15):2634–2643. https://doi.org/10.1016/j.jbiomech.2013.08.007
- 330. Lindemann J, Tsakiropoulou E, Scheithauer MO, Konstantinidis I, Wiesmiller KM. Impact of menthol inhalation on nasal mucosal temperature and nasal patency. Am J Rhinol. 2008;22(4):402–405. https://doi.org/10.2500/ajr.2008.22.3194
- 331. Sozansky J, Houser SM. Pathophysiology of empty nose syndrome. Laryngoscope. 2015;125(1):70–74. https://doi.org/10.1002/lary.24813
- 332. Li C, Farag AA, Leach J, et al. Computational fluid dynamics and trigeminal sensory examinations of empty nose syndrome patients. Laryngoscope. 2017;127(6):E176–E184. https://doi.org/10.1002/lary.26530
- 333. Li C, Farag AA, Maza G, et al. Investigation of the abnormal nasal aerodynamics and trigeminal functions among empty nose syndrome patients. Int Forum Allergy Rhinol. 2018;8(3):444–452. https://doi.org/10.1002/alr.22045
- 334. Malik J, Dholakia S, Spector BM, et al. Inferior meatus augmentation procedure (IMAP) normalizes nasal airflow patterns in empty nose syndrome patients via computational fluid dynamics (CFD) modeling. Int Forum Allergy Rhinol. 2021;11(5):902–909. https://doi.org/10.1002/alr.22720
- 335. Thamboo A, Velasquez N, Habib AR, Zarabanda D, Paknezhad H, Nayak JV. Defining surgical criteria for empty nose syndrome: validation of the office‐based cotton test and clinical interpretability of the validated Empty Nose Syndrome 6‐Item Questionnaire. Laryngoscope. 2017;127(8):1746–1752. https://doi.org/10.1002/lary.26549
- 336. Talmadge J, Nayak JV, Yao W, Citardi MJ. Management of postsurgical empty nose syndrome. Facial Plast Surg Clin North Am. 2019;27(4):465–475. https://doi.org/10.1016/j.fsc.2019.07.005
- 337. Kanjanawasee D, Campbell RG, Rimmer J, et al. Empty nose syndrome pathophysiology: a systematic review. Otolaryngol Head Neck Surg. 2021:1945998211052919. https://doi.org/10.1177/01945998211052919
- 338. Manji J, Nayak JV, Thamboo A. The functional and psychological burden of empty nose syndrome. Int Forum Allergy Rhinol. 2018;8(6):707–712. https://doi.org/10.1002/alr.22097
- 339. Huang CC, Wu PW, Fu CH, Huang CC, Chang PH, Lee TJ. Impact of psychologic burden on surgical outcome in empty nose syndrome. Laryngoscope. 2021;131(3):E694–E701. https://doi.org/10.1002/lary.28845
- 340. Huang CC, Wu PW, Lee CC, Chang PH, Huang CC, Lee TJ. Suicidal thoughts in patients with empty nose syndrome. Laryngoscope Investig Otolaryngol. 2022;7(1):22–28. https://doi.org/10.1002/lio2.730
- 341. Tian P, Hu J, Ma Y, et al. The clinical effect of psychosomatic interventions on empty nose syndrome secondary to turbinate‐sparing techniques: a prospective self‐controlled study. Int Forum Allergy Rhinol. 2021;11(6):984–992. https://doi.org/10.1002/alr.22726
- 342. Lemogne C, Consoli SM, Limosin F, Bonfils P. Treating empty nose syndrome as a somatic symptom disorder. Gen Hosp Psychiatry. 2015;37(3):273.e9–10. https://doi.org/10.1016/j.genhosppsych.2015.02.005
- 343. Borchard NA, Dholakia SS, Yan CH, Zarabanda D, Thamboo A, Nayak JV. Use of intranasal submucosal fillers as a transient implant to alter upper airway aerodynamics: implications for the assessment of empty nose syndrome. Int Forum Allergy Rhinol. 2019;9(6):681–687. https://doi.org/10.1002/alr.22299
- 344. Modrzynski M. Hyaluronic acid gel in the treatment of empty nose syndrome. Am J Rhinol Allergy. 2011;25(2):103–106. https://doi.org/10.2500/ajra.2011.25.3577
- 345. Saafan ME. Acellular dermal (alloderm) grafts versus silastic sheets implants for management of empty nose syndrome. Eur Arch Otorhinolaryngol. 2013;270(2):527–533. https://doi.org/10.1007/s00405‐012‐1955‐1
- 346. Jiang C, Shi R, Sun Y. Study of inferior turbinate reconstruction with Medpor for the treatment of empty nose syndrome. Laryngoscope. 2013;123(5):1106–1111. https://doi.org/10.1002/lary.23908
- 347. Bastier PL, Bennani‐Baiti AA, Stoll D, de Gabory L. beta‐Tricalcium phosphate implant to repair empty nose syndrome: preliminary results. Otolaryngol Head Neck Surg. 2013;148(3):519–522. https://doi.org/10.1177/0194599812472436
- 348. Jung JH, Baguindali MA, Park JT, Jang YJ. Costal cartilage is a superior implant material than conchal cartilage in the treatment of empty nose syndrome. Otolaryngol Head Neck Surg. 2013;149(3):500–505. https://doi.org/10.1177/0194599813491223
- 349. Velasquez N, Huang Z, Humphreys IM, Nayak JV. Inferior turbinate reconstruction using porcine small intestine submucosal xenograft demonstrates improved quality of life outcomes in patients with empty nose syndrome. Int Forum Allergy Rhinol. 2015;5(11):1077–1081. https://doi.org/10.1002/alr.21633
- 350. Chang MT, Bartho M, Kim D, et al. Inferior Meatus Augmentation Procedure (IMAP) for treatment of empty nose syndrome. Laryngoscope. 2022;132(6):1285–1288. https://doi.org/10.1002/lary.30001
- 351. Thamboo A, Dholakia SS, Borchard NA, et al. Inferior Meatus Augmentation Procedure (IMAP) to treat empty nose syndrome: a pilot study. Otolaryngol Head Neck Surg. 2020;162(3):382–385. https://doi.org/10.1177/0194599819900263
- 352. Dholakia SS, Yang A, Kim D, et al. Long‐term outcomes of inferior meatus augmentation procedure to treat empty nose syndrome. Laryngoscope. 2021;131(11):E2736–E2741. https://doi.org/10.1002/lary.29593
- 353. Malik J, Thamboo A, Dholakia S, et al. The cotton test redistributes nasal airflow in patients with empty nose syndrome. Int Forum Allergy Rhinol. 2020;10(4):539–545. https://doi.org/10.1002/alr.22489
- 354. Alobid I, Mullol J, Cid MC. Rhinitis of granulomatous and vasculitic diseases. Clin Allergy Immunol. 2007;19:221–239.
- 355. Afiari A, Gabriel A, Gaiki MR. Concurrent use of mepolizumab and rituximab for eosinophilic granulomatosis with polyangiitis and multisystem involvement. Cureus. 2020;12(7):e9242. https://doi.org/10.7759/cureus.9242
- 356. Falk RJ, Gross WL, Guillevin L, et al. Granulomatosis with polyangiitis (Wegener's): an alternative name for Wegener's granulomatosis. Arthritis Rheum. 2011;63(4):863–864. https://doi.org/10.1002/art.30286
- 357. Sardana K, Goel K. Nasal septal ulceration. Clin Dermatol. 2014;32(6):817–826. https://doi.org/10.1016/j.clindermatol.2014.02.022
- 358. Erickson VR, Hwang PH. Wegener's granulomatosis: current trends in diagnosis and management. Curr Opin Otolaryngol Head Neck Surg. 2007;15(3):170–176. https://doi.org/10.1097/MOO.0b013e3281568b96
- 359. Nasser M, Cottin V. The respiratory system in autoimmune vascular diseases. Respiration. 2018;96(1):12–28. https://doi.org/10.1159/000486899
- 360. Holle JU, Gross WL. Neurological involvement in Wegener's granulomatosis. Curr Opin Rheumatol. 2011;23(1):7–11. https://doi.org/10.1097/BOR.0b013e32834115f9
- 361. Beltran Rodriguez‐Cabo O, Reyes E, Rojas‐Serrano J, Flores‐Suarez LF. Increased histopathological yield for granulomatosis with polyangiitis based on nasal endoscopy of suspected active lesions. Eur Arch Otorhinolaryngol. 2018;275(2):425–429. https://doi.org/10.1007/s00405‐017‐4841‐z
- 362. Al‐Hussain T, Hussein MH, Conca W, Al Mana H, Akhtar M. Pathophysiology of ANCA‐associated vasculitis. Adv Anat Pathol. 2017;24(4):226–234. https://doi.org/10.1097/PAP.0000000000000154
- 363. Lynch 3rd JP, Derhovanessian A, Tazelaar H, Belperio JA. Granulomatosis with polyangiitis (Wegener's granulomatosis): evolving concepts in treatment. Semin Respir Crit Care Med. 2018;39(4):434–458. https://doi.org/10.1055/s‐0038‐1660874
- 364. Noth I, Strek ME, Leff AR. Churg‐Strauss syndrome. Lancet. 2003;361(9357):587–594. https://doi.org/10.1016/S0140‐6736(03)12518‐4
- 365. Groh M, Pagnoux C, Baldini C, et al. Eosinophilic granulomatosis with polyangiitis (Churg‐Strauss) (EGPA) Consensus Task Force recommendations for evaluation and management. Eur J Intern Med. 2015;26(7):545–553. https://doi.org/10.1016/j.ejim.2015.04.022
- 366. Chaigne B, Dion J, Guillevin L, Mouthon L, Terrier B. Physiopathologie de la granulomatose eosinophilique avec polyangeite (Churg‐Strauss. Rev Med Interne. 2016;37:337–342.
- 367. Wechsler ME, Akuthota P, Jayne D, et al. Mepolizumab or placebo for eosinophilic granulomatosis with polyangiitis. N Engl J Med. 2017;376(20):1921–1932. https://doi.org/10.1056/NEJMoa1702079
- 368. Crouser ED, Maier LA, Wilson KC, et al. Diagnosis and detection of sarcoidosis. An official American Thoracic Society Clinical Practice Guideline. Am J Respir Crit Care Med. 2020;201(8):e26–e51. https://doi.org/10.1164/rccm.202002‐0251ST
- 369. Cereceda‐Monteoliva N, Rouhani MJ, Maughan EF, et al. Sarcoidosis of the ear, nose and throat: A review of the literature. Clin Otolaryngol. 2021;46(5):935–940. https://doi.org/10.1111/coa.13814
- 370. Judson MA. The clinical features of sarcoidosis: a comprehensive review. Clin Rev Allergy Immunol. 2015;49(1):63–78. https://doi.org/10.1007/s12016‐014‐8450‐y
- 371. Send T, Tuleta I, Koppen T, et al. Sarcoidosis of the paranasal sinuses. Eur Arch Otorhinolaryngol. 2019;276(7):1969–1974. https://doi.org/10.1007/s00405‐019‐05388‐7
- 372. Lisnevskaia L, Murphy G, Isenberg D. Systemic lupus erythematosus. Lancet. 2014;384(9957):1878–1888. https://doi.org/10.1016/S0140‐6736(14)60128‐8
- 373. Thong B, Olsen NJ. Systemic lupus erythematosus diagnosis and management. Rheumatology (Oxford). 2017;56(suppl_1):i3–i13. https://doi.org/10.1093/rheumatology/kew401
- 374. Samotij D, Reich A. Biologics in the treatment of lupus erythematosus: a critical literature review. Biomed Res Int. 2019;2019:8142368. https://doi.org/10.1155/2019/8142368
- 375. Tanaka Y, Tummala R. Anifrolumab, a monoclonal antibody to the type I interferon receptor subunit 1, for the treatment of systemic lupus erythematosus: an overview from clinical trials. Mod Rheumatol. 2021;31(1):1–12. https://doi.org/10.1080/14397595.2020.1812201
- 376. Helman SN, Barrow E, Edwards T, DelGaudio JM, Levy JM, Wise SK. The role of allergic rhinitis in chronic rhinosinusitis. Immunol Allergy Clin North Am. 2020;40(2):201–214. https://doi.org/10.1016/j.iac.2019.12.010
- 377. Min YG. The pathophysiology, diagnosis and treatment of allergic rhinitis. Allergy Asthma Immunol Res. 2010;2(2):65–76. https://doi.org/10.4168/aair.2010.2.2.65
- 378. Kakli HA, Riley TD. Allergic rhinitis. Prim Care. 2016;43(3):465–475. https://doi.org/10.1016/j.pop.2016.04.009
- 379. Benninger MS, Ferguson BJ, Hadley JA, et al. Adult chronic rhinosinusitis: definitions, diagnosis, epidemiology, and pathophysiology. Otolaryngol Head Neck Surg. 2003;129(3 suppl):S1–S32. https://doi.org/10.1016/s0194‐5998(03)01397‐4
- 380. Shapiro DJ, Gonzales R, Cabana MD, Hersh AL. National trends in visit rates and antibiotic prescribing for children with acute sinusitis. Pediatrics. 2011;127(1):28–34. https://doi.org/10.1542/peds.2010‐1340
- 381. Whyte A, Boeddinghaus R. Imaging of adult nasal obstruction. Clin Radiol. 2020;75(9):688–704. https://doi.org/10.1016/j.crad.2019.07.027
- 382. Hsu DW, Suh JD. Anatomy and Physiology of Nasal Obstruction. Otolaryngol Clin North Am. 2018;51(5):853–865. https://doi.org/10.1016/j.otc.2018.05.001
- 383. Cox DR, Wise SK. Medical Treatment of Nasal Airway Obstruction. Otolaryngol Clin North Am. 2018;51(5):897–908. https://doi.org/10.1016/j.otc.2018.05.004
- 384. Villwock JA, Kuppersmith RB. Diagnostic Algorithm for Evaluating Nasal Airway Obstruction. Otolaryngol Clin North Am. 2018;51(5):867–872. https://doi.org/10.1016/j.otc.2018.05.002
- 385. Samra S, Steitz JT, Hajnas N, Toriumi DM. Surgical Management of Nasal Valve Collapse. Otolaryngol Clin North Am. 2018;51(5):929–944. https://doi.org/10.1016/j.otc.2018.05.009
- 386. Alvo A, Villarroel G, Sedano C. Neonatal nasal obstruction. Eur Arch Otorhinolaryngol. 2021;278(10):3605–3611. https://doi.org/10.1007/s00405‐020‐06546‐y
- 387. Harvey RJ, Sheahan PO, Schlosser RJ. Surgical management of benign sinonasal masses. Otolaryngol Clin North Am. 2009;42(2):353–375, x. https://doi.org/10.1016/j.otc.2009.01.006
- 388. Kuo MJ, Reid AP, Smith JE. Unilateral nasal obstruction: an unusual presentation of a complication of nasotracheal intubation. J Laryngol Otol. 1994;108(11):991–992. https://doi.org/10.1017/s0022215100128701
- 389. Tuluc M, Zhang X, Inniss S. Giant cell tumor of the nasal cavity: case report. Eur Arch Otorhinolaryngol. 2007;264(2):205–208. https://doi.org/10.1007/s00405‐006‐0143‐6
- 390. Hongmei Y, Zhe W, Jing W, Daokui W, Peicheng C, Yongjie L. Delayed cerebrospinal fluid rhinorrhea after gamma knife surgery in a patient with a growth hormone‐secreting adenoma. J Clin Neurosci. 2012;19(6):900–902. https://doi.org/10.1016/j.jocn.2011.09.016
- 391. Marchiano E, Carniol ET, Guzman DE, Raikundalia MD, Baredes S, Eloy JA. An Analysis of Patients Treated for Cerebrospinal Fluid Rhinorrhea in the United States from 2002 to 2010. J Neurol Surg B Skull Base. 2017;78(1):18–23. https://doi.org/10.1055/s‐0036‐1584297
- 392. Tufano RP, Thaler ER, Lanza DC, Goldberg AN, Kennedy DW. Endoscopic management of sinonasal inverted papilloma. Am J Rhinol. 1999;13(6):423–426. https://doi.org/10.2500/105065899781329665
- 393. Taylor MA, Saba NF. Cancer of the Paranasal Sinuses. Hematol Oncol Clin North Am. 2021;35(5):949–962. https://doi.org/10.1016/j.hoc.2021.05.006
- 394. Oakley GM, Alt JA, Schlosser RJ, Harvey RJ, Orlandi RR. Diagnosis of cerebrospinal fluid rhinorrhea: an evidence‐based review with recommendations. Int Forum Allergy Rhinol. 2016;6(1):8–16. https://doi.org/10.1002/alr.21637
- 395. Kinoshita Y, Wasita B, Akatsuka K, Kambe A, Kurosaki M, Watanabe T. Choroid plexus papilloma presenting with cerebrospinal fluid rhinorrhea and otorrhea: case report. Neurol Med Chir (Tokyo). 2010;50(10):930–933. https://doi.org/10.2176/nmc.50.930
- 396. Kose OD, Kose TE, Erdem MA, Cankaya AB. Large rhinolith causing nasal obstruction. BMJ Case Rep. 2015;2015. https://doi.org/10.1136/bcr‐2014‐208260
- 397. Sumbullu MA, Tozoglu U, Yoruk O, Yilmaz AB, Ucuncu H. Rhinolithiasis: the importance of flat panel detector‐based cone beam computed tomography in diagnosis and treatment. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2009;107(6):e65–e67. https://doi.org/10.1016/j.tripleo.2009.02.029
- 398. Okafor S, Kelly KM, Halderman AA. Management of sinusitis in the cystic fibrosis patient. Immunol Allergy Clin North Am. 2020;40(2):371–383. https://doi.org/10.1016/j.iac.2019.12.008
- 399. Shoemark A, Harman K. Primary ciliary dyskinesia. Semin Respir Crit Care Med. 2021;42(4):537–548. https://doi.org/10.1055/s‐0041‐1730919
- 400. Finocchio E, Locatelli F, Sanna F, et al. Gastritis and gastroesophageal reflux disease are strongly associated with non‐allergic nasal disorders. BMC Pulm Med. 2021;21(1):53. https://doi.org/10.1186/s12890‐020‐01364‐8
- 401. Dagli E, Yuksel A, Kaya M, Ugur KS, Turkay FC. Association of oral antireflux medication with laryngopharyngeal reflux and nasal resistance. JAMA Otolaryngol Head Neck Surg. 2017;143(5):478–483. https://doi.org/10.1001/jamaoto.2016.4127
- 402. Lou Z, Lou ZH. Laryngopharyngeal reflux is a potential cause of nasal congestion and obstructive sleep apnea syndrome. Eur Arch Otorhinolaryngol. 2018;275(9):2409–2411. https://doi.org/10.1007/s00405‐017‐4782‐6
- 403. Kraft S, Kinet JP. New developments in FcepsilonRI regulation, function and inhibition. Nat Rev Immunol. 2007;7(5):365–378. https://doi.org/10.1038/nri2072
- 404. Acharya M, Borland G, Edkins AL, et al. CD23/FcepsilonRII: molecular multi‐tasking. Clin Exp Immunol. 2010;162(1):12–23. https://doi.org/10.1111/j.1365‐2249.2010.04210.x
- 405. Wu LC, Zarrin AA. The production and regulation of IgE by the immune system. Nat Rev Immunol. 2014;14(4):247–259. https://doi.org/10.1038/nri3632
- 406. Blank U, Huang H, Kawakami T. The high affinity IgE receptor: a signaling update. Curr Opin Immunol. 2021;72:51–58. https://doi.org/10.1016/j.coi.2021.03.015
- 407. Humbert M, Bousquet J, Bachert C, et al. IgE‐mediated multimorbidities in allergic asthma and the potential for omalizumab therapy. J Allergy Clin Immunol Pract. 2019;7(5):1418–1429. https://doi.org/10.1016/j.jaip.2019.02.030
- 408. Paolini R, Jouvin MH, Kinet JP. Phosphorylation and dephosphorylation of the high‐affinity receptor for immunoglobulin E immediately after receptor engagement and disengagement. Nature. 1991;353(6347):855–858. https://doi.org/10.1038/353855a0
- 409. Siraganian RP, de Castro RO, Barbu EA, Zhang J. Mast cell signaling: the role of protein tyrosine kinase Syk, its activation and screening methods for new pathway participants. FEBS Lett. 2010;584(24):4933–4940. https://doi.org/10.1016/j.febslet.2010.08.006
- 410. Costello PS, Turner M, Walters AE, et al. Critical role for the tyrosine kinase Syk in signalling through the high affinity IgE receptor of mast cells. Oncogene. 1996;13(12):2595–2605.
- 411. Zhang J, Berenstein EH, Evans RL, Siraganian RP. Transfection of Syk protein tyrosine kinase reconstitutes high affinity IgE receptor‐mediated degranulation in a Syk‐negative variant of rat basophilic leukemia RBL‐2H3 cells. J Exp Med. 1996;184(1):71–79. https://doi.org/10.1084/jem.184.1.71
- 412. Mukai K, Tsai M, Saito H, Galli SJ. Mast cells as sources of cytokines, chemokines, and growth factors. Immunol Rev. 2018;282(1):121–150. https://doi.org/10.1111/imr.12634
- 413. Draber P, Halova I, Polakovicova I, Kawakami T. Signal transduction and chemotaxis in mast cells. Eur J Pharmacol. 2016;778:11–23. https://doi.org/10.1016/j.ejphar.2015.02.057
- 414. Motakis E, Guhl S, Ishizu Y, et al. Redefinition of the human mast cell transcriptome by deep‐CAGE sequencing. Blood. 2014;123(17):e58–e67. https://doi.org/10.1182/blood‐2013‐02‐483792
- 415. Li Y, Gao J, Kamran M, et al. GATA2 regulates mast cell identity and responsiveness to antigenic stimulation by promoting chromatin remodeling at super‐enhancers. Nat Commun. 2021;12(1):494. https://doi.org/10.1038/s41467‐020‐20766‐0
- 416. Cildir G, Pant H, Lopez AF, Tergaonkar V. The transcriptional program, functional heterogeneity, and clinical targeting of mast cells. J Exp Med. 2017;214(9):2491–2506. https://doi.org/10.1084/jem.20170910
- 417. Jayapal M, Tay HK, Reghunathan R, et al. Genome‐wide gene expression profiling of human mast cells stimulated by IgE or FcepsilonRI‐aggregation reveals a complex network of genes involved in inflammatory responses. BMC Genomics. 2006;7:210. https://doi.org/10.1186/1471‐2164‐7‐210
- 418. Anto JM, Bousquet J, Akdis M, et al. Mechanisms of the Development of Allergy (MeDALL): introducing novel concepts in allergy phenotypes. J Allergy Clin Immunol. 2017;139(2):388–399. https://doi.org/10.1016/j.jaci.2016.12.940
- 419. Oettgen HC, Geha RS. IgE in asthma and atopy: cellular and molecular connections. J Clin Invest. 1999;104(7):829–835. https://doi.org/10.1172/JCI8205
- 420. Galli SJ, Tsai M. IgE and mast cells in allergic disease. Nat Med. 2012;18(5):693–704. https://doi.org/10.1038/nm.2755
- 421. Berger A. Th1 and Th2 responses: what are they? BMJ. 2000;321(7258):424. https://doi.org/10.1136/bmj.321.7258.424
- 422. Chaplin DD. Overview of the immune response. J Allergy Clin Immunol. 2010;125(2 suppl 2):S3–S23. https://doi.org/10.1016/j.jaci.2009.12.980
- 423. Moro K, Yamada T, Tanabe M, et al. Innate production of T(H)2 cytokines by adipose tissue‐associated c‐Kit(+)Sca‐1(+) lymphoid cells. Nature. 2010;463(7280):540–544. https://doi.org/10.1038/nature08636
- 424. Neill DR, Wong SH, Bellosi A, et al. Nuocytes represent a new innate effector leukocyte that mediates type‐2 immunity. Nature. 2010;464(7293):1367–1370. https://doi.org/10.1038/nature08900
- 425. Halim TY, Steer CA, Matha L, et al. Group 2 innate lymphoid cells are critical for the initiation of adaptive T helper 2 cell‐mediated allergic lung inflammation. Immunity. 2014;40(3):425–435. https://doi.org/10.1016/j.immuni.2014.01.011
- 426. Lambrecht BN, Hammad H. Allergens and the airway epithelium response: gateway to allergic sensitization. J Allergy Clin Immunol. 2014;134(3):499–507. https://doi.org/10.1016/j.jaci.2014.06.036
- 427. Cayrol C, Duval A, Schmitt P, et al. Environmental allergens induce allergic inflammation through proteolytic maturation of IL‐33. Nat Immunol. 2018;19(4):375–385. https://doi.org/10.1038/s41590‐018‐0067‐5
- 428. Roan F, Obata‐Ninomiya K, Ziegler SF. Epithelial cell‐derived cytokines: more than just signaling the alarm. J Clin Invest. 2019;129(4):1441–1451. https://doi.org/10.1172/JCI124606
- 429. Hammad H, Lambrecht BN. Barrier epithelial cells and the control of type 2 immunity. Immunity. 2015;43(1):29–40. https://doi.org/10.1016/j.immuni.2015.07.007
- 430. Osguthorpe JD. Pathophysiology of and potential new therapies for allergic rhinitis. Int Forum Allergy Rhinol. 2013;3(5):384–392. https://doi.org/10.1002/alr.21120
- 431. Sin B, Togias A. Pathophysiology of allergic and nonallergic rhinitis. Proc Am Thorac Soc. 2011;8(1):106–114. https://doi.org/10.1513/pats.201008‐057RN
- 432. Pawankar R, Mori S, Ozu C, Kimura S. Overview on the pathomechanisms of allergic rhinitis. Asia Pac Allergy. 2011;1(3):157–167. https://doi.org/10.5415/apallergy.2011.1.3.157
- 433. Liu YJ. Thymic stromal lymphopoietin: master switch for allergic inflammation. J Exp Med. 2006;203(2):269–273. https://doi.org/10.1084/jem.20051745
- 434. Bousquet J, Anto JM, Bachert C, et al. Allergic rhinitis. Nat Rev Dis Primers. 2020;6(1):95. https://doi.org/10.1038/s41572‐020‐00227‐0
- 435. Geha RS. Regulation of IgE synthesis in humans. J Allergy Clin Immunol. 1992;90(2):143–150. https://doi.org/10.1016/0091‐6749(92)90064‐9
- 436. Nurieva RI, Liu X, Dong C. Yin‐Yang of costimulation: crucial controls of immune tolerance and function. Immunol Rev. 2009;229(1):88–100. https://doi.org/10.1111/j.1600‐065X.2009.00769.x
- 437. Luna‐Gomes T, Magalhaes KG, Mesquita‐Santos FP, et al. Eosinophils as a novel cell source of prostaglandin D2: autocrine role in allergic inflammation. J Immunol. 2011;187(12):6518–6526. https://doi.org/10.4049/jimmunol.1101806
- 438. Xue L, Salimi M, Panse I, et al. Prostaglandin D2 activates group 2 innate lymphoid cells through chemoattractant receptor‐homologous molecule expressed on TH2 cells. J Allergy Clin Immunol. 2014;133(4):1184–1194. https://doi.org/10.1016/j.jaci.2013.10.056
- 439. Togias A. Systemic effects of local allergic disease. J Allergy Clin Immunol. 2004;113(1 suppl):S8–S14. https://doi.org/10.1016/j.jaci.2003.09.051
- 440. Pinart M, Benet M, Annesi‐Maesano I, et al. Comorbidity of eczema, rhinitis, and asthma in IgE‐sensitised and non‐IgE‐sensitised children in MeDALL: a population‐based cohort study. Lancet Respir Med. 2014;2(2):131–140. https://doi.org/10.1016/S2213‐2600(13)70277‐7
- 441. Togias AG. Systemic immunologic and inflammatory aspects of allergic rhinitis. J Allergy Clin Immunol. 2000;106(5 suppl):S247–S250. https://doi.org/10.1067/mai.2000.110157
- 442. Scadding G, Hellings P, Alobid I, et al. Diagnostic tools in Rhinology EAACI position paper. Clin Transl Allergy. 2011;1(1):2. https://doi.org/10.1186/2045‐7022‐1‐2
- 443. Rondon C, Bogas G, Barrionuevo E, Blanca M, Torres MJ, Campo P. Nonallergic rhinitis and lower airway disease. Allergy. 2017;72(1):24–34. https://doi.org/10.1111/all.12988
- 444. Fuiano N, Fusilli S, Passalacqua G, Incorvaia C. Allergen‐specific immunoglobulin E in the skin and nasal mucosa of symptomatic and asymptomatic children sensitized to aeroallergens. J Investig Allergol Clin Immunol. 2010;20(5):425–430.
- 445. Rondon C, Campo P, Togias A, et al. Local allergic rhinitis: concept, pathophysiology, and management. J Allergy Clin Immunol. 2012;129(6):1460–1467. https://doi.org/10.1016/j.jaci.2012.02.032
- 446. Powe DG, Jagger C, Kleinjan A, Carney AS, Jenkins D, Jones NS. ‘Entopy’: localized mucosal allergic disease in the absence of systemic responses for atopy. Clin Exp Allergy. 2003;33(10):1374–1379. https://doi.org/10.1046/j.1365‐2222.2003.01737.x
- 447. Rondon C, Campo P, Zambonino MA, et al. Follow‐up study in local allergic rhinitis shows a consistent entity not evolving to systemic allergic rhinitis. J Allergy Clin Immunol. 2014;133(4):1026–1031. https://doi.org/10.1016/j.jaci.2013.10.034
- 448. Rondon C, Campo P, Eguiluz‐Gracia I, et al. Local allergic rhinitis is an independent rhinitis phenotype: the results of a 10‐year follow‐up study. Allergy. 2018;73(2):470–478. https://doi.org/10.1111/all.13272
- 449. Sennekamp J, Joest I, Filipiak‐Pittroff B, von Berg A, Berdel D. Local allergic nasal reactions convert to classic systemic allergic reactions: a long‐term follow‐up. Int Arch Allergy Immunol. 2015;166(2):154–160. https://doi.org/10.1159/000380852
- 450. Coker HA, Durham SR, Gould HJ. Local somatic hypermutation and class switch recombination in the nasal mucosa of allergic rhinitis patients. J Immunol. 2003;171(10):5602–5610. https://doi.org/10.4049/jimmunol.171.10.5602
- 451. Durham SR, Gould HJ, Thienes CP, et al. Expression of epsilon germ‐line gene transcripts and mRNA for the epsilon heavy chain of IgE in nasal B cells and the effects of topical corticosteroid. Eur J Immunol. 1997;27(11):2899–2906. https://doi.org/10.1002/eji.1830271123
- 452. Platts‐Mills TA. Local production of IgG, IgA and IgE antibodies in grass pollen hay fever. J Immunol. 1979;122(6):2218–2225.
- 453. Takhar P, Smurthwaite L, Coker HA, et al. Allergen drives class switching to IgE in the nasal mucosa in allergic rhinitis. J Immunol. 2005;174(8):5024–5032. https://doi.org/10.4049/jimmunol.174.8.5024
- 454. Powe DG, Huskisson RS, Carney AS, et al. Mucosal T‐cell phenotypes in persistent atopic and nonatopic rhinitis show an association with mast cells. Allergy. 2004;59(2):204–212. https://doi.org/10.1046/j.1398‐9995.2003.00315.x
- 455. Rondon C, Dona I, Lopez S, et al. Seasonal idiopathic rhinitis with local inflammatory response and specific IgE in absence of systemic response. Allergy. 2008;63(10):1352–1358. https://doi.org/10.1111/j.1398‐9995.2008.01695.x
- 456. Rondon C, Romero JJ, Lopez S, et al. Local IgE production and positive nasal provocation test in patients with persistent nonallergic rhinitis. J Allergy Clin Immunol. 2007;119(4):899–905. https://doi.org/10.1016/j.jaci.2007.01.006
- 457. Wedback A, Enbom H, Eriksson NE, Moverare R, Malcus I. Seasonal non‐allergic rhinitis (SNAR) – a new disease entity? A clinical and immunological comparison between SNAR, seasonal allergic rhinitis and persistent non‐allergic rhinitis. Rhinology. 2005;43(2):86–92.
- 458. Huggins KG, Brostoff J. Local production of specific IgE antibodies in allergic‐rhinitis patients with negative skin tests. Lancet. 1975;2(7926):148–150. https://doi.org/10.1016/s0140‐6736(75)90056‐2
- 459. Bozek A, Ignasiak B, Kasperska‐Zajac A, Scierski W, Grzanka A, Jarzab J. Local allergic rhinitis in elderly patients. Ann Allergy Asthma Immunol. 2015;114(3):199–202. https://doi.org/10.1016/j.anai.2014.12.013
- 460. Klimek L, Bardenhewer C, Spielhaupter M, Harai C, Becker K, Pfaar O. Lokale allergische Rhinitis auf Alternaria alternata: Nachweis bei Patienten mit persistierender nasaler Symptomatik [Local allergic rhinitis to Alternaria alternata: Evidence for local IgE production exclusively in the nasal mucosa]. HNO. 2015 May;63(5):364–72. German. https://doi.org/10.1007/s00106‐015‐0005‐x
- 461. Lopez S, Rondon C, Torres MJ, et al. Immediate and dual response to nasal challenge with Dermatophagoides pteronyssinus in local allergic rhinitis. Clin Exp Allergy. 2010;40(7):1007–1014. https://doi.org/10.1111/j.1365‐2222.2010.03492.x
- 462. Samolinski B, Rapiejko P, Krzych‐Falta E. Standards of nasal provocation tests. Postepy Dermatol Alergol. 2010;27:1669.
- 463. Rondon C, Fernandez J, Lopez S, et al. Nasal inflammatory mediators and specific IgE production after nasal challenge with grass pollen in local allergic rhinitis. J Allergy Clin Immunol. 2009;124(5):1005–1011.e1. https://doi.org/10.1016/j.jaci.2009.07.018
- 464. Blanca‐Lopez N, Campo P, Salas M, et al. Seasonal local allergic rhinitis in areas with high concentrations of grass pollen. J Investig Allergol Clin Immunol. 2016;26(2):83–91. https://doi.org/10.18176/jiaci.0018
- 465. Campo P, Eguiluz‐Gracia I, Bogas G, et al. Local allergic rhinitis: implications for management. Clin Exp Allergy. 2019;49(1):6–16. https://doi.org/10.1111/cea.13192
- 466. Rondon C, Campo P, Galindo L, et al. Prevalence and clinical relevance of local allergic rhinitis. Allergy. 2012;67(10):1282–1288. https://doi.org/10.1111/all.12002
- 467. Campo P, Villalba M, Barrionuevo E, et al. Immunologic responses to the major allergen of Olea europaea in local and systemic allergic rhinitis subjects. Clin Exp Allergy. 2015;45(11):1703–1712. https://doi.org/10.1111/cea.12600
- 468. Gomez E, Campo P, Rondon C, et al. Role of the basophil activation test in the diagnosis of local allergic rhinitis. J Allergy Clin Immunol. 2013;132(4):975‐676.e1‐5. https://doi.org/10.1016/j.jaci.2013.07.016
- 469. Campo P, Salas M, Blanca‐Lopez N, Rondon C. Local allergic rhinitis. Immunol Allergy Clin North Am. 2016;36(2):321–332. https://doi.org/10.1016/j.iac.2015.12.008
- 470. Reitsma S, Subramaniam S, Fokkens WWJ, Wang Y. Recent developments and highlights in rhinitis and allergen immunotherapy. Allergy. 2018;73(12):2306–2313. https://doi.org/10.1111/all.13617
- 471. Shin YS, Jung CG, Park HS. Prevalence and clinical characteristics of local allergic rhinitis to house dust mites. Curr Opin Allergy Clin Immunol. 2018;18(1):10–15. https://doi.org/10.1097/ACI.0000000000000413
- 472. Cheng KJ, Xu YY, Liu HY, Wang SQ. Serum eosinophil cationic protein level in Chinese subjects with nonallergic and local allergic rhinitis and its relation to the severity of disease. Am J Rhinol Allergy. 2013;27(1):8–12. https://doi.org/10.2500/ajra.2013.27.3845
- 473. Zicari AM, Occasi F, Di Fraia M, et al. Local allergic rhinitis in children: novel diagnostic features and potential biomarkers. Am J Rhinol Allergy. 2016;30(5):329–334. https://doi.org/10.2500/ajra.2016.30.4352
- 474. Duman H, Bostanci I, Ozmen S, Dogru M. The relevance of nasal provocation testing in children with nonallergic rhinitis. Int Arch Allergy Immunol. 2016;170(2):115–121. https://doi.org/10.1159/000447635
- 475. Altintoprak N, Kar M, Bayar Muluk N, et al. Update on local allergic rhinitis. Int J Pediatr Otorhinolaryngol. 2016;87:105–109. https://doi.org/10.1016/j.ijporl.2016.06.008
- 476. Buntarickpornpan P, Veskitkul J, Pacharn P, et al. The proportion of local allergic rhinitis to Dermatophagoides pteronyssinus in children. Pediatr Allergy Immunol. 2016;27(6):574–579. https://doi.org/10.1111/pai.12606
- 477. Rondon C, Campo P, Blanca‐Lopez N, Torres MJ, Blanca M. More research is needed for local allergic rhinitis. Int Arch Allergy Immunol. 2015;167(2):99–100. https://doi.org/10.1159/000436970
- 478. Papadopoulos NG, Bernstein JA, Demoly P, et al. Phenotypes and endotypes of rhinitis and their impact on management: a PRACTALL report. Allergy. 2015;70(5):474–494. https://doi.org/10.1111/all.12573
- 479. Toppila‐Salmi S, van Drunen CM, Fokkens WJ, et al. Molecular mechanisms of nasal epithelium in rhinitis and rhinosinusitis. Curr Allergy Asthma Rep. 2015;15(2):495. https://doi.org/10.1007/s11882‐014‐0495‐8
- 480. Scadding GK, Scadding GW. Innate and adaptive immunity in allergic airway disease. Curr Opin Allergy Clin Immunol. 2022;22(1):10–15. https://doi.org/10.1097/ACI.0000000000000800
- 481. Jacquet A, Robinson C. Proteolytic, lipidergic and polysaccharide molecular recognition shape innate responses to house dust mite allergens. Allergy. 2020;75(1):33–53. https://doi.org/10.1111/all.13940
- 482. Kortekaas Krohn I, Seys SF, Lund G, et al. Nasal epithelial barrier dysfunction increases sensitization and mast cell degranulation in the absence of allergic inflammation. Allergy. 2020;75(5):1155–1164. https://doi.org/10.1111/all.14132
- 483. Shim JS, Lee HS, Park DE, et al. Aggravation of asthmatic inflammation by chlorine exposure via innate lymphoid cells and CD11c(intermediate) macrophages. Allergy. 2020;75(2):381–391. https://doi.org/10.1111/all.14017
- 484. Kim J, Kim YC, Ham J, et al. The effect of air pollutants on airway innate immune cells in patients with asthma. Allergy. 2020;75(9):2372–2376. https://doi.org/10.1111/all.14323
- 485. Steelant B, Farre R, Wawrzyniak P, et al. Impaired barrier function in patients with house dust mite‐induced allergic rhinitis is accompanied by decreased occludin and zonula occludens‐1 expression. J Allergy Clin Immunol. 2016;137(4):1043–1053.e5. https://doi.org/10.1016/j.jaci.2015.10.050
- 486. Pat Y, Ogulur I. The epithelial barrier hypothesis: a 20‐year journey. Allergy. 2021;76(11):3560–3562. https://doi.org/10.1111/all.14899
- 487. van Tongeren J, Golebski K, Van Egmond D, de Groot EJ, Fokkens WJ, van Drunen CM. Synergy between TLR‐2 and TLR‐3 signaling in primary human nasal epithelial cells. Immunobiology. 2015;220(4):445–451. https://doi.org/10.1016/j.imbio.2014.11.004
- 488. Radman M, Golshiri A, Shamsizadeh A, et al. Toll‐like receptor 4 plays significant roles during allergic rhinitis. Allergol Immunopathol (Madr). 2015;43(4):416–420. https://doi.org/10.1016/j.aller.2014.04.006
- 489. Mjosberg JM, Trifari S, Crellin NK, et al. Human IL‐25‐ and IL‐33‐responsive type 2 innate lymphoid cells are defined by expression of CRTH2 and CD161. Nat Immunol. 2011;12(11):1055–1062. https://doi.org/10.1038/ni.2104
- 490. Matsushita K, Kato Y, Akasaki S, Yoshimoto T. Proallergic cytokines and group 2 innate lymphoid cells in allergic nasal diseases. Allergol Int. 2015;64(3):235–240. https://doi.org/10.1016/j.alit.2014.12.008
- 491. Bartemes KR, Kephart GM, Fox SJ, Kita H. Enhanced innate type 2 immune response in peripheral blood from patients with asthma. J Allergy Clin Immunol. 2014;134(3):671–678.e4. https://doi.org/10.1016/j.jaci.2014.06.024
- 492. Hong H, Liao S, Chen F, Yang Q, Wang DY. Role of IL‐25, IL‐33, and TSLP in triggering united airway diseases toward type 2 inflammation. Allergy. 2020;75(11):2794–2804. https://doi.org/10.1111/all.14526
- 493. Doherty TA, Scott D, Walford HH, et al. Allergen challenge in allergic rhinitis rapidly induces increased peripheral blood type 2 innate lymphoid cells that express CD84. J Allergy Clin Immunol. 2014;133(4):1203–1205. https://doi.org/10.1016/j.jaci.2013.12.1086
- 494. Lao‐Araya M, Steveling E, Scadding GW, Durham SR, Shamji MH. Seasonal increases in peripheral innate lymphoid type 2 cells are inhibited by subcutaneous grass pollen immunotherapy. J Allergy Clin Immunol. 2014;134(5):1193–1195.e4. https://doi.org/10.1016/j.jaci.2014.07.029
- 495. Dhariwal J, Cameron A, Trujillo‐Torralbo MB, et al. Mucosal type 2 innate lymphoid cells are a key component of the allergic response to aeroallergens. Am J Respir Crit Care Med. 2017;195(12):1586–1596. https://doi.org/10.1164/rccm.201609‐1846OC
- 496. Xie Y, Ju X, Beaudin S, et al. Effect of intranasal corticosteroid treatment on allergen‐induced changes in group 2 innate lymphoid cells in allergic rhinitis with mild asthma. Allergy. 2021;76(9):2797–2808. https://doi.org/10.1111/all.14835
- 497. Sugita K, Steer CA, Martinez‐Gonzalez I, et al. Type 2 innate lymphoid cells disrupt bronchial epithelial barrier integrity by targeting tight junctions through IL‐13 in asthmatic patients. J Allergy Clin Immunol. 2018;141(1):300–310.e11. https://doi.org/10.1016/j.jaci.2017.02.038
- 498. Boguniewicz M, Beck LA, Sher L, et al. Dupilumab improves asthma and sinonasal outcomes in adults with moderate to severe atopic dermatitis. J Allergy Clin Immunol Pract. 2021;9(3):1212–1223.e6. https://doi.org/10.1016/j.jaip.2020.12.059
- 499. Orimo K, Tamari M, Saito H, Matsumoto K, Nakae S, Morita H. Characteristics of tissue‐resident ILCs and their potential as therapeutic targets in mucosal and skin inflammatory diseases. Allergy. 2021;76(11):3332–3348. https://doi.org/10.1111/all.14863
- 500. Akdis M, Aab A, Altunbulakli C, et al. Interleukins (from IL‐1 to IL‐38), interferons, transforming growth factor beta, and TNF‐alpha: Receptors, functions, and roles in diseases. J Allergy Clin Immunol. 2016;138(4):984–1010. https://doi.org/10.1016/j.jaci.2016.06.033
- 501. Zheng H, Zhang Y, Pan J, et al. The role of type 2 innate lymphoid cells in allergic diseases. Front Immunol. 2021;12:586078. https://doi.org/10.3389/fimmu.2021.586078
- 502. Schuijs MJ, Willart MA, Vergote K, et al. Farm dust and endotoxin protect against allergy through A20 induction in lung epithelial cells. Science. 2015;349(6252):1106–1110. https://doi.org/10.1126/science.aac6623
- 503. Lambrecht BN, Hammad H. The immunology of asthma. Nat Immunol. 2015;16(1):45–56. https://doi.org/10.1038/ni.3049
- 504. Muraro A, Lemanske Jr. RF, Hellings PW, et al. Precision medicine in patients with allergic diseases: airway diseases and atopic dermatitis‐PRACTALL document of the European Academy of Allergy and Clinical Immunology and the American Academy of Allergy, Asthma & Immunology. J Allergy Clin Immunol. 2016;137(5):1347–1358. https://doi.org/10.1016/j.jaci.2016.03.010
- 505. Nian JB, Zeng M, Zheng J, et al. Epithelial cells expressed IL‐33 to promote degranulation of mast cells through inhibition on ST2/PI3K/mTOR‐mediated autophagy in allergic rhinitis. Cell Cycle. 2020;19(10):1132–1142. https://doi.org/10.1080/15384101.2020.1749402
- 506. Cheng KJ, Zhou ML, Liu YC, Wang C, Xu YY. The role of CD40 in allergic rhinitis and airway remodelling. Mediators Inflamm. 2021;2021:6694109. https://doi.org/10.1155/2021/6694109
- 507. KleinJan A, Willart M, van Rijt LS, et al. An essential role for dendritic cells in human and experimental allergic rhinitis. J Allergy Clin Immunol. 2006;118(5):1117–1125. https://doi.org/10.1016/j.jaci.2006.05.030
- 508. Hammad H, Plantinga M, Deswarte K, et al. Inflammatory dendritic cells–not basophils–are necessary and sufficient for induction of Th2 immunity to inhaled house dust mite allergen. J Exp Med. 2010;207(10):2097–2111. https://doi.org/10.1084/jem.20101563
- 509. Meng Y, Wang C, Zhang L. Advances and novel developments in allergic rhinitis. Allergy. 2020;75(12):3069–3076. https://doi.org/10.1111/all.14586
- 510. Annunziato F, Romagnani C, Romagnani S. The 3 major types of innate and adaptive cell‐mediated effector immunity. J Allergy Clin Immunol. 2015;135(3):626–635. https://doi.org/10.1016/j.jaci.2014.11.001
- 511. Durham SR, Ying S, Varney VA, et al. Cytokine messenger RNA expression for IL‐3, IL‐4, IL‐5, and granulocyte/macrophage‐colony‐stimulating factor in the nasal mucosa after local allergen provocation: relationship to tissue eosinophilia. J Immunol. 1992;148(8):2390–2394.
- 512. Sogut A, Yilmaz O, Kirmaz C, et al. Regulatory‐T, T‐helper 1, and T‐helper 2 cell differentiation in nasal mucosa of allergic rhinitis with olive pollen sensitivity. Int Arch Allergy Immunol. 2012;157(4):349–353. https://doi.org/10.1159/000329159
- 513. Pawankar RU, Okuda M, Okubo K, Ra C. Lymphocyte subsets of the nasal mucosa in perennial allergic rhinitis. Am J Respir Crit Care Med. 1995;152(6 pt 1):2049–2058. https://doi.org/10.1164/ajrccm.152.6.8520775
- 514. Soyka MB, Wawrzyniak P, Eiwegger T, et al. Defective epithelial barrier in chronic rhinosinusitis: the regulation of tight junctions by IFN‐gamma and IL‐4. J Allergy Clin Immunol. 2012;130(5):1087–1096.e10. https://doi.org/10.1016/j.jaci.2012.05.052
- 515. Kubo T, Wawrzyniak P, Morita H, et al. CpG‐DNA enhances the tight junction integrity of the bronchial epithelial cell barrier. J Allergy Clin Immunol. 2015;136(5):1413‐1416.e1‐8. https://doi.org/10.1016/j.jaci.2015.05.006
- 516. Georas SN, Rezaee F. Epithelial barrier function: at the front line of asthma immunology and allergic airway inflammation. J Allergy Clin Immunol. 2014;134(3):509–520. https://doi.org/10.1016/j.jaci.2014.05.049
- 517. Akdis M. Healthy immune response to allergens: T regulatory cells and more. Curr Opin Immunol. 2006;18(6):738–744. https://doi.org/10.1016/j.coi.2006.06.003
- 518. Akdis M, Akdis CA. Therapeutic manipulation of immune tolerance in allergic disease. Nat Rev Drug Discov. 2009;8(8):645–660. https://doi.org/10.1038/nrd2653
- 519. Raedler D, Ballenberger N, Klucker E, et al. Identification of novel immune phenotypes for allergic and nonallergic childhood asthma. J Allergy Clin Immunol. 2015;135(1):81–91. https://doi.org/10.1016/j.jaci.2014.07.046
- 520. Akdis M, Verhagen J, Taylor A, et al. Immune responses in healthy and allergic individuals are characterized by a fine balance between allergen‐specific T regulatory 1 and T helper 2 cells. J Exp Med. 2004;199(11):1567–1575. https://doi.org/10.1084/jem.20032058
- 521. Suarez‐Fueyo A, Ramos T, Galan A, et al. Grass tablet sublingual immunotherapy downregulates the TH2 cytokine response followed by regulatory T‐cell generation. J Allergy Clin Immunol. 2014;133(1):130‐138.e1‐2. https://doi.org/10.1016/j.jaci.2013.09.043
- 522. Fox EM, Torrero MN, Evans H, Mitre E. Immunologic characterization of 3 murine regimens of allergen‐specific immunotherapy. J Allergy Clin Immunol. 2015;135(5):1341‐1351.e1‐7. https://doi.org/10.1016/j.jaci.2014.07.052
- 523. Akdis CA, Akdis M. Advances in allergen immunotherapy: aiming for complete tolerance to allergens. Sci Transl Med. 2015;7(280):280ps6. https://doi.org/10.1126/scitranslmed.aaa7390
- 524. Jansen K, Cevhertas L, Ma S, Satitsuksanoa P, Akdis M, van de Veen W. Regulatory B cells, A to Z. Allergy. 2021;76(9):2699–2715. https://doi.org/10.1111/all.14763
- 525. Hofmann MA, Fluhr JW, Ruwwe‐Glosenkamp C, Stevanovic K, Bergmann KC, Zuberbier T. Role of IL‐17 in atopy – a systematic review. Clin Transl Allergy. 2021;11(6):e12047. https://doi.org/10.1002/clt2.12047
- 526. Yu X, Wang M, Cao Z. Reduced CD4(+)T cell CXCR3 expression in patients with allergic rhinitis. Front Immunol. 2020;11:581180. https://doi.org/10.3389/fimmu.2020.581180
- 527. Morita H, Arae K, Unno H, et al. An interleukin‐33‐mast cell‐interleukin‐2 axis suppresses papain‐induced allergic inflammation by promoting regulatory T cell numbers. Immunity. 2015;43(1):175–186. https://doi.org/10.1016/j.immuni.2015.06.021
- 528. Ganzer U, Bachert C. Localization of IgE synthesis in immediate‐type allergy of the upper respiratory tract. ORL J Otorhinolaryngol Relat Spec. 1988;50(4):257–264. https://doi.org/10.1159/000276000
- 529. KleinJan A, Vinke JG, Severijnen LW, Fokkens WJ. Local production and detection of (specific) IgE in nasal B‐cells and plasma cells of allergic rhinitis patients. Eur Respir J. 2000;15(3):491–497. https://doi.org/10.1034/j.1399‐3003.2000.15.11.x
- 530. Yao Y, Wang N, Chen CL, et al. CD23 expression on switched memory B cells bridges T‐B cell interaction in allergic rhinitis. Allergy. 2020;75(10):2599–2612. https://doi.org/10.1111/all.14288
- 531. Bentley AM, Jacobson MR, Cumberworth V, et al. Immunohistology of the nasal mucosa in seasonal allergic rhinitis: increases in activated eosinophils and epithelial mast cells. J Allergy Clin Immunol. 1992;89(4):877–883. https://doi.org/10.1016/0091‐6749(92)90444‐7
- 532. Gomez E, Corrado OJ, Baldwin DL, Swanston AR, Davies RJ. Direct in vivo evidence for mast cell degranulation during allergen‐induced reactions in man. J Allergy Clin Immunol. 1986;78(4 pt 1):637–645. https://doi.org/10.1016/0091‐6749(86)90082‐5
- 533. Haenuki Y, Matsushita K, Futatsugi‐Yumikura S, et al. A critical role of IL‐33 in experimental allergic rhinitis. J Allergy Clin Immunol. 2012;130(1):184–194.e11. https://doi.org/10.1016/j.jaci.2012.02.013
- 534. KleinJan A, McEuen AR, Dijkstra MD, Buckley MG, Walls AF, Fokkens WJ. Basophil and eosinophil accumulation and mast cell degranulation in the nasal mucosa of patients with hay fever after local allergen provocation. J Allergy Clin Immunol. 2000;106(4):677–686. https://doi.org/10.1067/mai.2000.109621
- 535. Semik‐Orzech A, Barczyk A, Wiaderkiewicz R, Pierzchala W. Eotaxin, but not IL‐8, is increased in upper and lower airways of allergic rhinitis subjects after nasal allergen challenge. Allergy Asthma Proc. 2011;32(3):230–238. https://doi.org/10.2500/aap.2011.32.3435
- 536. Kim TH, Lee JY, Lee HM, et al. Remodelling of nasal mucosa in mild and severe persistent allergic rhinitis with special reference to the distribution of collagen, proteoglycans, and lymphatic vessels. Clin Exp Allergy. 2010;40(12):1742–1754. https://doi.org/10.1111/j.1365‐2222.2010.03612.x
- 537. Pawankar R, Yamagishi S, Yagi T. Revisiting the roles of mast cells in allergic rhinitis and its relation to local IgE synthesis. Am J Rhinol. 2000;14(5):309–317. https://doi.org/10.2500/105065800781329582
- 538. Pawankar R. Mast cells in allergic airway disease and chronic rhinosinusitis. Chem Immunol Allergy. 2005;87:111–129. https://doi.org/10.1159/000087639
- 539. Powe DG, Hiskisson RS, Carney AS, Jenkins D, Jones NS. Idiopathic and allergic rhinitis show a similar inflammatory response. Clin Otolaryngol Allied Sci. 2000;25(6):570–576. https://doi.org/10.1046/j.1365‐2273.2000.00422‐2.x
- 540. Divekar R, Kita H. Recent advances in epithelium‐derived cytokines (IL‐33, IL‐25, and thymic stromal lymphopoietin) and allergic inflammation. Curr Opin Allergy Clin Immunol. 2015;15(1):98–103. https://doi.org/10.1097/ACI.0000000000000133
- 541. Wang W, Li Y, Lv Z, et al. Bronchial allergen challenge of patients with atopic asthma triggers an alarmin (IL‐33, TSLP, and IL‐25) response in the airways epithelium and submucosa. J Immunol. 2018;201(8):2221–2231. https://doi.org/10.4049/jimmunol.1800709
- 542. Hussain M, Borcard L, Walsh KP, et al. Basophil‐derived IL‐4 promotes epicutaneous antigen sensitization concomitant with the development of food allergy. J Allergy Clin Immunol. 2018;141(1):223–234.e5. https://doi.org/10.1016/j.jaci.2017.02.035
- 543. Corren J, Ziegler SF. TSLP: from allergy to cancer. Nat Immunol. 2019;20(12):1603–1609. https://doi.org/10.1038/s41590‐019‐0524‐9
- 544. London Jr. NR, Lane AP. Innate immunity and chronic rhinosinusitis: what we have learned from animal models. Laryngoscope Investig Otolaryngol. 2016;1(3):49–56. https://doi.org/10.1002/lio2.21
- 545. Pawankar R. Epithelial cells as immunoregulators in allergic airway diseases. Curr Opin Allergy Clin Immunol. 2002;2(1):1–5. https://doi.org/10.1097/00130832‐200202000‐00001
- 546. Peng YQ, Qin ZL, Fang SB, et al. Effects of myeloid and plasmacytoid dendritic cells on ILC2s in patients with allergic rhinitis. J Allergy Clin Immunol. 2020;145(3):855–867.e8. https://doi.org/10.1016/j.jaci.2019.11.029
- 547. Kabata H, Moro K, Koyasu S. The group 2 innate lymphoid cell (ILC2) regulatory network and its underlying mechanisms. Immunol Rev. 2018;286(1):37–52. https://doi.org/10.1111/imr.12706
- 548. Wilhelm C, Hirota K, Stieglitz B, et al. An IL‐9 fate reporter demonstrates the induction of an innate IL‐9 response in lung inflammation. Nat Immunol. 2011;12(11):1071–1077. https://doi.org/10.1038/ni.2133
- 549. Turner JE, Morrison PJ, Wilhelm C, et al. IL‐9‐mediated survival of type 2 innate lymphoid cells promotes damage control in helminth‐induced lung inflammation. J Exp Med. 2013;210(13):2951–2965. https://doi.org/10.1084/jem.20130071
- 550. Wilson AM, Duong M, Crawford L, Denburg J. An evaluation of peripheral blood eosinophil/basophil progenitors following nasal allergen challenge in patients with allergic rhinitis. Clin Exp Allergy. 2005;35(1):39–44. https://doi.org/10.1111/j.1365‐2222.2004.02072.x
- 551. Bradding P, Holgate ST. The mast cell as a source of cytokines in asthma. Ann N Y Acad Sci. 1996;796:272–281. https://doi.org/10.1111/j.1749‐6632.1996.tb32589.x
- 552. Pawankar RU, Okuda M, Hasegawa S, et al. Interleukin‐13 expression in the nasal mucosa of perennial allergic rhinitis. Am J Respir Crit Care Med. 1995;152(6 pt 1):2059–2067. https://doi.org/10.1164/ajrccm.152.6.8520776
- 553. Pawankar R, Okuda M, Yssel H, Okumura K, Ra C. Nasal mast cells in perennial allergic rhinitics exhibit increased expression of the Fc epsilonRI, CD40L, IL‐4, and IL‐13, and can induce IgE synthesis in B cells. J Clin Invest. 1997;99(7):1492–1499. https://doi.org/10.1172/JCI119311
- 554. Pawankar R. Inflammatory mechanisms in allergic rhinitis. Curr Opin Allergy Clin Immunol. 2007;7(1):1–4. https://doi.org/10.1097/ACI.0b013e3280145347
- 555. Nonaka M, Pawankar R, Fukumoto A, Ogihara N, Sakanushi A, Yagi T. Induction of eotaxin production by interleukin‐4, interleukin‐13 and lipopolysaccharide by nasal fibroblasts. Clin Exp Allergy. 2004;34(5):804–811. https://doi.org/10.1111/j.1365‐2222.2004.1954.x
- 556. Nair P, Pizzichini MM, Kjarsgaard M, et al. Mepolizumab for prednisone‐dependent asthma with sputum eosinophilia. N Engl J Med. 2009;360(10):985–993. https://doi.org/10.1056/NEJMoa0805435
- 557. Nakamaru Y, Oridate N, Nishihira J, Takagi D, Furuta Y, Fukuda S. Macrophage migration inhibitory factor in allergic rhinitis: its identification in eosinophils at the site of inflammation. Ann Otol Rhinol Laryngol. 2004;113(3 pt 1):205–209. https://doi.org/10.1177/000348940411300306
- 558. Kobayashi H, Gleich GJ, Butterfield JH, Kita H. Human eosinophils produce neurotrophins and secrete nerve growth factor on immunologic stimuli. Blood. 2002;99(6):2214–2220. https://doi.org/10.1182/blood.v99.6.2214
- 559. Pappu R, Ramirez‐Carrozzi V, Sambandam A. The interleukin‐17 cytokine family: critical players in host defence and inflammatory diseases. Immunology. 2011;134(1):8–16. https://doi.org/10.1111/j.1365‐2567.2011.03465.x
- 560. Mandhane SN, Shah JH, Thennati R. Allergic rhinitis: an update on disease, present treatments and future prospects. Int Immunopharmacol. 2011;11(11):1646–1662. https://doi.org/10.1016/j.intimp.2011.07.005
- 561. Kim D, Baraniuk JN. Neural aspects of allergic rhinitis. Curr Opin Otolaryngol Head Neck Surg. 2007;15(4):268–273. https://doi.org/10.1097/MOO.0b013e328259c372
- 562. Pfaar O, Raap U, Holz M, Hormann K, Klimek L. Pathophysiology of itching and sneezing in allergic rhinitis. Swiss Med Wkly. 2009;139(3‐4):35–40. doi:smw‐12468.
- 563. Singh U, Bernstein JA, Haar L, Luther K, Jones WK. Azelastine desensitization of transient receptor potential vanilloid 1: a potential mechanism explaining its therapeutic effect in nonallergic rhinitis. Am J Rhinol Allergy. 2014;28(3):215–224. https://doi.org/10.2500/ajra.2014.28.4059
- 564. Singh U, Bernstein JA, Lorentz H, et al. A pilot study investigating clinical responses and biological pathways of azelastine/fluticasone in nonallergic vasomotor rhinitis before and after cold dry air provocation. Int Arch Allergy Immunol. 2017;173(3):153–164. https://doi.org/10.1159/000478698
- 565. Kuruvilla M, Kalangara J, Lee FEE. Neuropathic pain and itch mechanisms underlying allergic conjunctivitis. J Investig Allergol Clin Immunol. 2019;29(5):349–356. https://doi.org/10.18176/jiaci.0320
- 566. Gawlik R, Jawor B, Rogala B, Parzynski S, DuBuske L. Effect of intranasal azelastine on substance P release in perennial nonallergic rhinitis patients. Am J Rhinol Allergy. 2013;27(6):514–516. https://doi.org/10.2500/ajra.2013.27.3955
- 567. Baraniuk JN, Kaliner MA. Neuropeptides and nasal secretion. J Allergy Clin Immunol. 1990;86(4 pt 2):620–627. https://doi.org/10.1016/s0091‐6749(05)80226‐x
- 568. Bernstein JA, Davis BP, Picard JK, Cooper JP, Zheng S, Levin LS. A randomized, double‐blind, parallel trial comparing capsaicin nasal spray with placebo in subjects with a significant component of nonallergic rhinitis. Ann Allergy Asthma Immunol. 2011;107(2):171–178. https://doi.org/10.1016/j.anai.2011.05.016
- 569. Golpanian RS, Smith P, Yosipovitch G. Itch in organs beyond the skin. Curr Allergy Asthma Rep. 2020;20(9):49. https://doi.org/10.1007/s11882‐020‐00947‐z
- 570. Mosimann BL, White MV, Hohman RJ, Goldrich MS, Kaulbach HC, Kaliner MA. Substance P, calcitonin gene‐related peptide, and vasoactive intestinal peptide increase in nasal secretions after allergen challenge in atopic patients. J Allergy Clin Immunol. 1993;92(1 pt 1):95–104. https://doi.org/10.1016/0091‐6749(93)90043‐f
- 571. Singh U, Bernstein JA. Intranasal capsaicin in management of nonallergic (vasomotor) rhinitis. Prog Drug Res. 2014;68:147–170. https://doi.org/10.1007/978‐3‐0348‐0828‐6_6
- 572. Sanico AM, Koliatsos VE, Stanisz AM, Bienenstock J, Togias A. Neural hyperresponsiveness and nerve growth factor in allergic rhinitis. Int Arch Allergy Immunol. 1999;118(2‐4):154–158. https://doi.org/10.1159/000024054
- 573. Bresciani M, Laliberte F, Laliberte MF, Gramiccioni C, Bonini S. Nerve growth factor localization in the nasal mucosa of patients with persistent allergic rhinitis. Allergy. 2009;64(1):112–117. https://doi.org/10.1111/j.1398‐9995.2008.01831.x
- 574. O'Hanlon S, Facer P, Simpson KD, Sandhu G, Saleh HA, Anand P. Neuronal markers in allergic rhinitis: expression and correlation with sensory testing. Laryngoscope. 2007;117(9):1519–1527. https://doi.org/10.1097/MLG.0b013e3180ca7846
- 575. Abbott‐Banner K, Poll C, Verkuyl JM. Targeting TRP channels in airway disorders. Curr Top Med Chem. 2013;13(3):310–321. https://doi.org/10.2174/1568026611313030008
- 576. Ba G, Tang R, Sun X, Li Z, Lin H, Zhang W. Therapeutic effects of SKF‐96365 on murine allergic rhinitis induced by OVA. Int J Immunopathol Pharmacol. 2021;35:20587384211015054. https://doi.org/10.1177/20587384211015054
- 577. Backaert W, Steelant B, Hellings PW, Talavera K, Van Gerven L. A TRiP through the roles of transient receptor potential cation channels in type 2 upper airway inflammation. Curr Allergy Asthma Rep. 2021;21(3):20. https://doi.org/10.1007/s11882‐020‐00981‐x
- 578. Nam JH, Kim WK. The role of TRP channels in allergic inflammation and its clinical relevance. Curr Med Chem. 2020;27(9):1446–1468. https://doi.org/10.2174/0929867326666181126113015
- 579. Vareille M, Kieninger E, Edwards MR, Regamey N. The airway epithelium: soldier in the fight against respiratory viruses. Clin Microbiol Rev. 2011;24(1):210–229. https://doi.org/10.1128/CMR.00014‐10
- 580. Wang DY, Li Y, Yan Y, Li C, Shi L. Upper airway stem cells: understanding the nose and role for future cell therapy. Curr Allergy Asthma Rep. 2015;15(1):490. https://doi.org/10.1007/s11882‐014‐0490‐0
- 581. Akira S. Pathogen recognition by innate immunity and its signaling. Proc Jpn Acad Ser B Phys Biol Sci. 2009;85(4):143–156. https://doi.org/10.2183/pjab.85.143
- 582. Chen CR, Kachramanoglou C, Li D, Andrews P, Choi D. Anatomy and cellular constituents of the human olfactory mucosa: a review. J Neurol Surg B Skull Base. 2014;75(5):293–300. https://doi.org/10.1055/s‐0033‐1361837
- 583. Bustamante‐Marin XM, Ostrowski LE. Cilia and mucociliary clearance. Cold Spring Harb Perspect Biol. 2017;9(4):a028241. https://doi.org/10.1101/cshperspect.a028241
- 584. Scherzad A, Hagen R, Hackenberg S. Current understanding of nasal epithelial cell mis‐differentiation. J Inflamm Res. 2019;12:309–317. https://doi.org/10.2147/JIR.S180853
- 585. Hamelmann E. Development of allergic airway inflammation in early life – interaction of early viral infections and allergic sensitization. Allergol Select. 2018;2(1):132–137. https://doi.org/10.5414/ALX01635E
- 586. Shin SH, Ye MK, Lee DW, Chae MH, Han BD. Nasal epithelial cells activated with alternaria and house dust mite induce not only Th2 but also Th1 immune responses. Int J Mol Sci. 2020;21(8):2693. https://doi.org/10.3390/ijms21082693
- 587. Bergougnan C, Dittlein DC, Hummer E, et al. Physical and immunological barrier of human primary nasal epithelial cells from non‐allergic and allergic donors. World Allergy Organ J. 2020;13(3):100109. https://doi.org/10.1016/j.waojou.2020.100109
- 588. Orban NT, Jacobson MR, Nouri‐Aria KT, Durham SR, Eifan AO. Repetitive nasal allergen challenge in allergic rhinitis: priming and Th2‐type inflammation but no evidence of remodelling. Clin Exp Allergy. 2021;51(2):329–338. https://doi.org/10.1111/cea.13775
- 589. Watts AM, West NP, Cripps AW, Smith PK, Cox AJ. Distinct gene expression patterns between nasal mucosal cells and blood collected from allergic rhinitis sufferers. Int Arch Allergy Immunol. 2018;177(1):29–34. https://doi.org/10.1159/000489609
- 590. Groneberg DA, Peiser C, Dinh QT, et al. Distribution of respiratory mucin proteins in human nasal mucosa. Laryngoscope. 2003;113(3):520–524. https://doi.org/10.1097/00005537‐200303000‐00023
- 591. Lee SN, Kim SJ, Yoon SA, et al. CD44v3‐positive intermediate progenitor cells contribute to airway goblet cell hyperplasia. Am J Respir Cell Mol Biol. 2021;64(2):247–259. https://doi.org/10.1165/rcmb.2020‐0350OC
- 592. Siti Sarah CO, Md Shukri N, Mohd Ashari NS, Wong KK. Zonula occludens and nasal epithelial barrier integrity in allergic rhinitis. PeerJ. 2020;8:e9834. https://doi.org/10.7717/peerj.9834
- 593. Steelant B, Wawrzyniak P, Martens K, et al. Blocking histone deacetylase activity as a novel target for epithelial barrier defects in patients with allergic rhinitis. J Allergy Clin Immunol. 2019;144(5):1242–1253.e7. https://doi.org/10.1016/j.jaci.2019.04.027
- 594. Zhou LB, Zheng YM, Liao WJ, et al. MUC1 deficiency promotes nasal epithelial barrier dysfunction in subjects with allergic rhinitis. J Allergy Clin Immunol. 2019;144(6):1716–1719.e5. https://doi.org/10.1016/j.jaci.2019.07.042
- 595. Buckle FG, Cohen AB. Nasal mucosal hyperpermeability to macromolecules in atopic rhinitis and extrinsic asthma. J Allergy Clin Immunol. 1975;55(4):213–221. https://doi.org/10.1016/0091‐6749(75)90139‐6
- 596. Zhang Y, Derycke L, Holtappels G, et al. Th2 cytokines orchestrate the secretion of MUC5AC and MUC5B in IL‐5‐positive chronic rhinosinusitis with nasal polyps. Allergy. 2019;74(1):131–140. https://doi.org/10.1111/all.13489
- 597. Wang J, Wen L, Wang Y, Chen F. Therapeutic effect of histone deacetylase inhibitor, sodium butyrate, on allergic rhinitis in vivo. DNA Cell Biol. 2016;35(4):203–208. https://doi.org/10.1089/dna.2015.3037
- 598. Runswick S, Mitchell T, Davies P, Robinson C, Garrod DR. Pollen proteolytic enzymes degrade tight junctions. Respirology. 2007;12(6):834–842. https://doi.org/10.1111/j.1440‐1843.2007.01175.x
- 599. Henriquez OA, Den Beste K, Hoddeson EK, Parkos CA, Nusrat A, Wise SK. House dust mite allergen Der p 1 effects on sinonasal epithelial tight junctions. Int Forum Allergy Rhinol. 2013;3(8):630–635. https://doi.org/10.1002/alr.21168
- 600. Steelant B, Seys SF, Van Gerven L, et al. Histamine and T helper cytokine‐driven epithelial barrier dysfunction in allergic rhinitis. J Allergy Clin Immunol. 2018;141(3):951–963.e8. https://doi.org/10.1016/j.jaci.2017.08.039
- 601. Ohwada K, Konno T, Kohno T, et al. Effects of HMGB1 on tricellular tight junctions via TGF‐beta signaling in human nasal epithelial cells. Int J Mol Sci. 2021;22(16):8390. https://doi.org/10.3390/ijms22168390
- 602. Fukuoka A, Matsushita K, Morikawa T, Takano H, Yoshimoto T. Diesel exhaust particles exacerbate allergic rhinitis in mice by disrupting the nasal epithelial barrier. Clin Exp Allergy. 2016;46(1):142–152. https://doi.org/10.1111/cea.12597
- 603. Kamekura R, Kojima T, Koizumi J, et al. Thymic stromal lymphopoietin enhances tight‐junction barrier function of human nasal epithelial cells. Cell Tissue Res. 2009;338(2):283–293. https://doi.org/10.1007/s00441‐009‐0855‐1
- 604. Hupin C, Gohy S, Bouzin C, Lecocq M, Polette M, Pilette C. Features of mesenchymal transition in the airway epithelium from chronic rhinosinusitis. Allergy. 2014;69(11):1540–1549. https://doi.org/10.1111/all.12503
- 605. Akdis CA. Does the epithelial barrier hypothesis explain the increase in allergy, autoimmunity and other chronic conditions? Nat Rev Immunol. 2021;21(11):739–751. https://doi.org/10.1038/s41577‐021‐00538‐7
- 606. Wawrzyniak P, Wawrzyniak M, Wanke K, et al. Regulation of bronchial epithelial barrier integrity by type 2 cytokines and histone deacetylases in asthmatic patients. J Allergy Clin Immunol. 2017;139(1):93–103. https://doi.org/10.1016/j.jaci.2016.03.050
- 607. Nur Husna SM, Siti Sarah CO, Tan HT, Md Shukri N, Mohd Ashari NS, Wong KK. Reduced occludin and claudin‐7 expression is associated with urban locations and exposure to second‐hand smoke in allergic rhinitis patients. Sci Rep. 2021;11(1):1245. https://doi.org/10.1038/s41598‐020‐79208‐y
- 608. Baeke F, Takiishi T, Korf H, Gysemans C, Mathieu C. Vitamin D: modulator of the immune system. Curr Opin Pharmacol. 2010;10(4):482–496. https://doi.org/10.1016/j.coph.2010.04.001
- 609. Adams JS, Hewison M. Update in vitamin D. J Clin Endocrinol Metab. 2010;95(2):471–478. https://doi.org/10.1210/jc.2009‐1773
- 610. Kongsbak M, Levring TB, Geisler C, von Essen MR. The vitamin d receptor and T cell function. Front Immunol. 2013;4:148. https://doi.org/10.3389/fimmu.2013.00148
- 611. Tian HQ, Cheng L. The role of vitamin D in allergic rhinitis. Asia Pac Allergy. 2017;7(2):65–73. https://doi.org/10.5415/apallergy.2017.7.2.65
- 612. Hamzaoui A, Berraies A, Hamdi B, Kaabachi W, Ammar J, Hamzaoui K. Vitamin D reduces the differentiation and expansion of Th17 cells in young asthmatic children. Immunobiology. 2014;219(11):873–879. https://doi.org/10.1016/j.imbio.2014.07.009
- 613. Zhang H, Shih DQ, Zhang X. Mechanisms underlying effects of 1,25‐dihydroxyvitamin D3 on the Th17 cells. Eur J Microbiol Immunol (Bp). 2013;3(4):237–240. https://doi.org/10.1556/EuJMI.3.2013.4.1
- 614. Urry Z, Chambers ES, Xystrakis E, et al. The role of 1alpha,25‐dihydroxyvitamin D3 and cytokines in the promotion of distinct Foxp3+ and IL‐10+ CD4+ T cells. Eur J Immunol. 2012;42(10):2697–2708. https://doi.org/10.1002/eji.201242370
- 615. Jolliffe DA, Greenberg L, Hooper RL, et al. Vitamin D supplementation to prevent asthma exacerbations: a systematic review and meta‐analysis of individual participant data. Lancet Respir Med. 2017;5(11):881–890. https://doi.org/10.1016/S2213‐2600(17)30306‐5
- 616. Riverin BD, Maguire JL, Li P. Vitamin D supplementation for childhood asthma: a systematic review and meta‐analysis. PLoS One. 2015;10(8):e0136841. https://doi.org/10.1371/journal.pone.0136841
- 617. Wee JH, Cho SW, Kim JW, Rhee CS. Non‐association between low vitamin d levels and aeroallergen‐positivity evaluated using multiple allergen simultaneous test in Korean adults. Allergy Asthma Clin Immunol. 2021;17(1):23. https://doi.org/10.1186/s13223‐021‐00525‐6
- 618. Bener A, Ehlayel MS, Bener HZ, Hamid Q. The impact of Vitamin D deficiency on asthma, allergic rhinitis and wheezing in children: an emerging public health problem. J Family Community Med. 2014;21(3):154–161. https://doi.org/10.4103/2230‐8229.142967
- 619. Kim YH, Kim KW, Kim MJ, et al. Vitamin D levels in allergic rhinitis: a systematic review and meta‐analysis. Pediatr Allergy Immunol. 2016;27(6):580–590. https://doi.org/10.1111/pai.12599
- 620. Bakhshaee M, Sharifian M, Esmatinia F, Rasoulian B, Mohebbi M. Therapeutic effect of vitamin D supplementation on allergic rhinitis. Eur Arch Otorhinolaryngol. 2019;276(10):2797–2801. https://doi.org/10.1007/s00405‐019‐05546‐x
- 621. Jerzynska J, Stelmach W, Rychlik B, et al. Clinical and immunological effects of vitamin D supplementation during the pollen season in children with allergic rhinitis. Arch Med Sci. 2018;14(1):122–131. https://doi.org/10.5114/aoms.2016.61978
- 622. Takeno S, Yoshimura H, Kubota K, Taruya T, Ishino T, Hirakawa K. Comparison of nasal nitric oxide levels between the inferior turbinate surface and the middle meatus in patients with symptomatic allergic rhinitis. Allergol Int. 2014;63(3):475–483. https://doi.org/10.2332/allergolint.14‐OA‐0689
- 623. Takeno S, Osada R, Furukido K, Chen JH, Yajin K. Increased nitric oxide production in nasal epithelial cells from allergic patients–RT‐PCR analysis and direct imaging by a fluorescence indicator: DAF‐2 DA. Clin Exp Allergy. 2001;31(6):881–888. https://doi.org/10.1046/j.1365‐2222.2001.01093.x
- 624. Yuksel H, Kirmaz C, Yilmaz O, et al. Nasal mucosal expression of nitric oxide synthases in patients with allergic rhinitis and its relation to asthma. Ann Allergy Asthma Immunol. 2008;100(1):12–16. https://doi.org/10.1016/S1081‐1206(10)60398‐5
- 625. Hou J, Lou H, Wang Y, et al. Nasal ventilation is an important factor in evaluating the diagnostic value of nasal nitric oxide in allergic rhinitis. Int Forum Allergy Rhinol. 2018;8(6):686–694. https://doi.org/10.1002/alr.22087
- 626. Ren L, Zhang W, Zhang Y, Zhang L. Nasal nitric oxide is correlated with nasal patency and nasal symptoms. Allergy Asthma Immunol Res. 2019;11(3):367–380. https://doi.org/10.4168/aair.2019.11.3.367
- 627. Liu C, Zheng K, Liu X, et al. Use of nasal nitric oxide in the diagnosis of allergic rhinitis and nonallergic rhinitis in patients with and without sinus inflammation. J Allergy Clin Immunol Pract. 2020;8(5):1574–1581.e4. https://doi.org/10.1016/j.jaip.2019.12.017
- 628. Maniscalco M, Sofia M, Pelaia G. Nitric oxide in upper airways inflammatory diseases. Inflamm Res. 2007;56(2):58–69. https://doi.org/10.1007/s00011‐006‐6111‐1
- 629. Barnes PJ, Dweik RA, Gelb AF, et al. Exhaled nitric oxide in pulmonary diseases: a comprehensive review. Chest. 2010;138(3):682–692. https://doi.org/10.1378/chest.09‐2090
- 630. Maniscalco M, Bianco A, Mazzarella G, Motta A. Recent advances on nitric oxide in the upper airways. Curr Med Chem. 2016;23(24):2736–2745. https://doi.org/10.2174/0929867323666160627115335
- 631. Alam MS, Akaike T, Okamoto S, et al. Role of nitric oxide in host defense in murine salmonellosis as a function of its antibacterial and antiapoptotic activities. Infect Immun. 2002;70(6):3130–3142. https://doi.org/10.1128/IAI.70.6.3130‐3142.2002
- 632. Poljakovic M, Persson K. Urinary tract infection in iNOS‐deficient mice with focus on bacterial sensitivity to nitric oxide. Am J Physiol Renal Physiol. 2003;284(1):F22–F31. https://doi.org/10.1152/ajprenal.00101.2002
- 633. Rajaram K, Nelson DE. Chlamydia muridarum infection of macrophages elicits bactericidal nitric oxide production via reactive oxygen species and cathepsin B. Infect Immun. 2015;83(8):3164–3175. https://doi.org/10.1128/IAI.00382‐15
- 634. Yadav R, Samuni Y, Abramson A, et al. Pro‐oxidative synergic bactericidal effect of NO: kinetics and inhibition by nitroxides. Free Radic Biol Med. 2014;67:248–254. https://doi.org/10.1016/j.freeradbiomed.2013.10.012
- 635. Workman AD, Carey RM, Kohanski MA, et al. Relative susceptibility of airway organisms to antimicrobial effects of nitric oxide. Int Forum Allergy Rhinol. 2017;7(8):770–776. https://doi.org/10.1002/alr.21966
- 636. Carey RM, Chen B, Adappa ND, et al. Human upper airway epithelium produces nitric oxide in response to Staphylococcus epidermidis. Int Forum Allergy Rhinol. 2016;6(12):1238–1244. https://doi.org/10.1002/alr.21837
- 637. Freund JR, Mansfield CJ, Doghramji LJ, et al. Activation of airway epithelial bitter taste receptors by Pseudomonas aeruginosa quinolones modulates calcium, cyclic‐AMP, and nitric oxide signaling. J Biol Chem. 2018;293(25):9824–9840. https://doi.org/10.1074/jbc.RA117.001005
- 638. Antosova M, Bencova A, Mokra D, Plevkova J, Pepucha L, Buday T. Exhaled and nasal nitric oxide – impact for allergic rhinitis. Physiol Res. 2020;69(suppl 1):S123–S130. https://doi.org/10.33549/physiolres.934393
- 639. Rolim WR, Pieretti JC, Reno DLS, et al. Antimicrobial activity and cytotoxicity to tumor cells of nitric oxide donor and silver nanoparticles containing PVA/PEG films for topical applications. ACS Appl Mater Interfaces. 2019;11(6):6589–6604. https://doi.org/10.1021/acsami.8b19021
- 640. Akaberi D, Krambrich J, Ling J, et al. Mitigation of the replication of SARS‐CoV‐2 by nitric oxide in vitro. Redox Biol. 2020;37:101734. https://doi.org/10.1016/j.redox.2020.101734
- 641. Pieretti JC, Rubilar O, Weller RB, Tortella GR, Seabra AB. Nitric oxide (NO) and nanoparticles – potential small tools for the war against COVID‐19 and other human coronavirus infections. Virus Res. 2021;291:198202. https://doi.org/10.1016/j.virusres.2020.198202
- 642. Li D, Shirakami G, Zhan X, Johns RA. Regulation of ciliary beat frequency by the nitric oxide‐cyclic guanosine monophosphate signaling pathway in rat airway epithelial cells. Am J Respir Cell Mol Biol. 2000;23(2):175–181. https://doi.org/10.1165/ajrcmb.23.2.4022
- 643. Hariri BM, Payne SJ, Chen B, et al. In vitro effects of anthocyanidins on sinonasal epithelial nitric oxide production and bacterial physiology. Am J Rhinol Allergy. 2016;30(4):261–268. https://doi.org/10.2500/ajra.2016.30.4331
- 644. Fowler CJ, Olivier KN, Leung JM, et al. Abnormal nasal nitric oxide production, ciliary beat frequency, and Toll‐like receptor response in pulmonary nontuberculous mycobacterial disease epithelium. Am J Respir Crit Care Med. 2013;187(12):1374–1381. https://doi.org/10.1164/rccm.201212‐2197OC
- 645. Jiao J, Wang H, Lou W, et al. Regulation of ciliary beat frequency by the nitric oxide signaling pathway in mouse nasal and tracheal epithelial cells. Exp Cell Res. 2011;317(17):2548–2553. https://doi.org/10.1016/j.yexcr.2011.07.007
- 646. Serbina NV, Salazar‐Mather TP, Biron CA, Kuziel WA, Pamer EG. TNF/iNOS‐producing dendritic cells mediate innate immune defense against bacterial infection. Immunity. 2003;19(1):59–70. https://doi.org/10.1016/s1074‐7613(03)00171‐7
- 647. Cobb JP, Hotchkiss RS, Swanson PE, et al. Inducible nitric oxide synthase (iNOS) gene deficiency increases the mortality of sepsis in mice. Surgery. 1999;126(2):438–442.
- 648. MacMicking JD, North RJ, LaCourse R, Mudgett JS, Shah SK, Nathan CF. Identification of nitric oxide synthase as a protective locus against tuberculosis. Proc Natl Acad Sci U S A. 1997;94(10):5243–5248. https://doi.org/10.1073/pnas.94.10.5243
- 649. Mishra BB, Lovewell RR, Olive AJ, et al. Nitric oxide prevents a pathogen‐permissive granulocytic inflammation during tuberculosis. Nat Microbiol. 2017;2:17072. https://doi.org/10.1038/nmicrobiol.2017.72
- 650. Bajwa G, DeBerardinis RJ, Shao B, Hall B, Farrar JD, Gill MA. Cutting Edge: Critical Role of Glycolysis in Human Plasmacytoid Dendritic Cell Antiviral Responses. J Immunol. 2016;196(5):2004–2009. https://doi.org/10.4049/jimmunol.1501557
- 651. Kopincova J, Calkovska A. Meconium‐induced inflammation and surfactant inactivation: specifics of molecular mechanisms. Pediatr Res. 2016;79(4):514–521. https://doi.org/10.1038/pr.2015.265
- 652. Heffler E, Carpagnano GE, Favero E, et al. Fractional Exhaled Nitric Oxide (FENO) in the management of asthma: a position paper of the Italian Respiratory Society (SIP/IRS) and Italian Society of Allergy, Asthma and Clinical Immunology (SIAAIC). Multidiscip Respir Med. 2020;15(1):36. https://doi.org/10.4081/mrm.2020.36
- 653. Soares MP, Teixeira L, Moita LF. Disease tolerance and immunity in host protection against infection. Nat Rev Immunol. 2017;17(2):83–96. https://doi.org/10.1038/nri.2016.136
- 654. Garcia‐Ortiz A, Serrador JM. Nitric oxide signaling in T cell‐mediated immunity. Trends Mol Med. 2018;24(4):412–427. https://doi.org/10.1016/j.molmed.2018.02.002
- 655. Akdis CA, Arkwright PD, Bruggen MC, et al. Type 2 immunity in the skin and lungs. Allergy. 2020;75(7):1582–1605. https://doi.org/10.1111/all.14318
- 656. Monga N, Sethi GS, Kondepudi KK, Naura AS. Lipid mediators and asthma: scope of therapeutics. Biochem Pharmacol. 2020;179:113925. https://doi.org/10.1016/j.bcp.2020.113925
- 657. Huang F, Yin JN, Wang HB, Liu SY, Li YN. Association of imbalance of effector T cells and regulatory cells with the severity of asthma and allergic rhinitis in children. Allergy Asthma Proc. 2017;38(6):70–77. https://doi.org/10.2500/aap.2017.38.4076
- 658. Amiel E, Everts B, Fritz D, et al. Mechanistic target of rapamycin inhibition extends cellular lifespan in dendritic cells by preserving mitochondrial function. J Immunol. 2014;193(6):2821–2830. https://doi.org/10.4049/jimmunol.1302498
- 659. Everts B, Amiel E, van der Windt GJ, et al. Commitment to glycolysis sustains survival of NO‐producing inflammatory dendritic cells. Blood. 2012;120(7):1422–1431. https://doi.org/10.1182/blood‐2012‐03‐419747
- 660. Lawless SJ, Kedia‐Mehta N, Walls JF, et al. Glucose represses dendritic cell‐induced T cell responses. Nat Commun. 2017;8:15620. https://doi.org/10.1038/ncomms15620
- 661. Linke M, Fritsch SD, Sukhbaatar N, Hengstschlager M, Weichhart T. mTORC1 and mTORC2 as regulators of cell metabolism in immunity. FEBS Lett. 2017;591(19):3089–3103. https://doi.org/10.1002/1873‐3468.12711
- 662. Chen C, Pore N, Behrooz A, Ismail‐Beigi F, Maity A. Regulation of glut1 mRNA by hypoxia‐inducible factor‐1. Interaction between H‐ras and hypoxia. J Biol Chem. 2001;276(12):9519–9525. https://doi.org/10.1074/jbc.M010144200
- 663. Xu L, Huang Y, Yang J, et al. Dendritic cell‐derived nitric oxide is involved in IL‐4‐induced suppression of experimental allergic encephalomyelitis (EAE) in Lewis rats. Clin Exp Immunol. 1999;118(1):115–121. https://doi.org/10.1046/j.1365‐2249.1999.01029.x
- 664. Nathan C. Nitric oxide as a secretory product of mammalian cells. FASEB J. 1992;6(12):3051–3064.
- 665. Bogdan C. Nitric oxide and the immune response. Nat Immunol. 2001;2(10):907–916. https://doi.org/10.1038/ni1001‐907
- 666. Nathan C. Specificity of a third kind: reactive oxygen and nitrogen intermediates in cell signaling. J Clin Invest. 2003;111(6):769–778. https://doi.org/10.1172/JCI18174
- 667. Bogdan C. Regulation of lymphocytes by nitric oxide. Methods Mol Biol. 2011;677:375–393. https://doi.org/10.1007/978‐1‐60761‐869‐0_24
- 668. Wink DA, Hines HB, Cheng RY, et al. Nitric oxide and redox mechanisms in the immune response. J Leukoc Biol. 2011;89(6):873–891. https://doi.org/10.1189/jlb.1010550
- 669. Ibiza S, Serrador JM. The role of nitric oxide in the regulation of adaptive immune responses. Immunologia. 2008;27:103–117.
- 670. Bogdan C. Nitric oxide synthase in innate and adaptive immunity: an update. Trends Immunol. 2015;36(3):161–178. https://doi.org/10.1016/j.it.2015.01.003
- 671. Bailey JD, Diotallevi M, Nicol T, et al. Nitric oxide modulates metabolic remodeling in inflammatory macrophages through TCA cycle regulation and itaconate accumulation. Cell Rep. 2019;28(1):218–230.e7. https://doi.org/10.1016/j.celrep.2019.06.018
- 672. Lee M, Rey K, Besler K, Wang C, Choy J. Immunobiology of nitric oxide and regulation of inducible nitric oxide synthase. Results Probl Cell Differ. 2017;62:181–207. https://doi.org/10.1007/978‐3‐319‐54090‐0_8
- 673. Pavord ID, Afzalnia S, Menzies‐Gow A, Heaney LG. The current and future role of biomarkers in type 2 cytokine‐mediated asthma management. Clin Exp Allergy. 2017;47(2):148–160. https://doi.org/10.1111/cea.12881
- 674. Bachert C, Han JK, Desrosiers M, et al. Efficacy and safety of dupilumab in patients with severe chronic rhinosinusitis with nasal polyps (LIBERTY NP SINUS‐24 and LIBERTY NP SINUS‐52): results from two multicentre, randomised, double‐blind, placebo‐controlled, parallel‐group phase 3 trials. Lancet. 2019;394(10209):1638–1650. https://doi.org/10.1016/S0140‐6736(19)31881‐1
- 675. Nesi RT, Barroso MV, Souza Muniz V, et al. Pharmacological modulation of reactive oxygen species (ROS) improves the airway hyperresponsiveness by shifting the Th1 response in allergic inflammation induced by ovalbumin. Free Radic Res. 2017;51(7‐8):708–722. https://doi.org/10.1080/10715762.2017.1364377
- 676. Bove PF, van der Vliet A. Nitric oxide and reactive nitrogen species in airway epithelial signaling and inflammation. Free Radic Biol Med. 2006;41(4):515–527. https://doi.org/10.1016/j.freeradbiomed.2006.05.011
- 677. Sparkman L, Boggaram V. Nitric oxide increases IL‐8 gene transcription and mRNA stability to enhance IL‐8 gene expression in lung epithelial cells. Am J Physiol Lung Cell Mol Physiol. 2004;287(4):L764–L773. https://doi.org/10.1152/ajplung.00165.2004
- 678. Gottipati KR, Bandari SK, Nonnenmann MW, et al. Transcriptional mechanisms and protein kinase signaling mediate organic dust induction of IL‐8 expression in lung epithelial and THP‐1 cells. Am J Physiol Lung Cell Mol Physiol. 2015;308(1):L11–L21. https://doi.org/10.1152/ajplung.00215.2014
- 679. Vocca L, Di Sano C, Uasuf CG, et al. IL‐33/ST2 axis controls Th2/IL‐31 and Th17 immune response in allergic airway diseases. Immunobiology. 2015;220(8):954–963. https://doi.org/10.1016/j.imbio.2015.02.005
- 680. Uchida M, Anderson EL, Squillace DL, et al. Oxidative stress serves as a key checkpoint for IL‐33 release by airway epithelium. Allergy. 2017;72(10):1521–1531. https://doi.org/10.1111/all.13158
- 681. Ursell LK, Metcalf JL, Parfrey LW, Knight R. Defining the human microbiome. Nutr Rev. 2012;70(suppl 1):S38–S44. https://doi.org/10.1111/j.1753‐4887.2012.00493.x
- 682. Qin J, Li R, Raes J, et al. A human gut microbial gene catalogue established by metagenomic sequencing. Nature. 2010;464(7285):59–65. https://doi.org/10.1038/nature08821
- 683. International Human Genome Sequencing Consortium . Finishing the euchromatic sequence of the human genome. Nature. 2004;431(7011):931–945. https://doi.org/10.1038/nature03001
- 684. Fierer N, Hamady M, Lauber CL, Knight R. The influence of sex, handedness, and washing on the diversity of hand surface bacteria. Proc Natl Acad Sci U S A. 2008;105(46):17994–17999. https://doi.org/10.1073/pnas.0807920105
- 685. Turnbaugh PJ, Hamady M, Yatsunenko T, et al. A core gut microbiome in obese and lean twins. Nature. 2009;457(7228):480–484. https://doi.org/10.1038/nature07540
- 686. Abrahamsson TR, Jakobsson HE, Andersson AF, Bjorksten B, Engstrand L, Jenmalm MC. Low gut microbiota diversity in early infancy precedes asthma at school age. Clin Exp Allergy. 2014;44(6):842–850. https://doi.org/10.1111/cea.12253
- 687. Sjogren YM, Jenmalm MC, Bottcher MF, Bjorksten B, Sverremark‐Ekstrom E. Altered early infant gut microbiota in children developing allergy up to 5 years of age. Clin Exp Allergy. 2009;39(4):518–526. https://doi.org/10.1111/j.1365‐2222.2008.03156.x
- 688. Melli LC, do Carmo‐Rodrigues MS, Araujo‐Filho HB, Sole D, de Morais MB. Intestinal microbiota and allergic diseases: a systematic review. Allergol Immunopathol (Madr). 2016;44(2):177–188. https://doi.org/10.1016/j.aller.2015.01.013
- 689. Fujimura KE, Sitarik AR, Havstad S, et al. Neonatal gut microbiota associates with childhood multisensitized atopy and T cell differentiation. Nat Med. 2016;22(10):1187–1191. https://doi.org/10.1038/nm.4176
- 690. Ipci K, Altintoprak N, Muluk NB, Senturk M, Cingi C. The possible mechanisms of the human microbiome in allergic diseases. Eur Arch Otorhinolaryngol. 2017;274(2):617–626. https://doi.org/10.1007/s00405‐016‐4058‐6
- 691. Bisgaard H, Li N, Bonnelykke K, et al. Reduced diversity of the intestinal microbiota during infancy is associated with increased risk of allergic disease at school age. J Allergy Clin Immunol. 2011;128(3):646‐652.e1‐5. https://doi.org/10.1016/j.jaci.2011.04.060
- 692. Watts AM, West NP, Zhang P, Smith PK, Cripps AW, Cox AJ. The gut microbiome of adults with allergic rhinitis is characterised by reduced diversity and an altered abundance of key microbial taxa compared to controls. Int Arch Allergy Immunol. 2021;182(2):94–105. https://doi.org/10.1159/000510536
- 693. Zhou MS, Zhang B, Gao ZL, et al. Altered diversity and composition of gut microbiota in patients with allergic rhinitis. Microb Pathog. 2021;161(pt A):105272. https://doi.org/10.1016/j.micpath.2021.105272
- 694. Hua X, Goedert JJ, Pu A, Yu G, Shi J. Allergy associations with the adult fecal microbiota: analysis of the American Gut Project. EBioMedicine. 2016;3:172–179. https://doi.org/10.1016/j.ebiom.2015.11.038
- 695. Choi CH, Poroyko V, Watanabe S, et al. Seasonal allergic rhinitis affects sinonasal microbiota. Am J Rhinol Allergy. 2014;28(4):281–286. https://doi.org/10.2500/ajra.2014.28.4050
- 696. Gan W, Yang F, Meng J, Liu F, Liu S, Xian J. Comparing the nasal bacterial microbiome diversity of allergic rhinitis, chronic rhinosinusitis and control subjects. Eur Arch Otorhinolaryngol. 2021;278(3):711–718. https://doi.org/10.1007/s00405‐020‐06311‐1
- 697. Bender ME, Read TD, Edwards TS, et al. A Comparison of the Bacterial Nasal Microbiome in Allergic Rhinitis Patients Before and After Immunotherapy. Laryngoscope. 2020;130(12):E882–E888. https://doi.org/10.1002/lary.28599
- 698. Hu B, Kuang Y, Jing Y, Li Y, Zhao H, Ouyang H. Pediatric allergic rhinitis with functional gastrointestinal disease: Associations with the intestinal microbiota and gastrointestinal peptides and therapeutic effects of interventions. Hum Exp Toxicol. 2021;40(11):2012–2021. https://doi.org/10.1177/09603271211017325
- 699. Morin A, McKennan CG, Pedersen CT, et al. Epigenetic landscape links upper airway microbiota in infancy with allergic rhinitis at 6 years of age. J Allergy Clin Immunol. 2020;146(6):1358–1366. https://doi.org/10.1016/j.jaci.2020.07.005
- 700. Zhu L, Xu F, Wan W, et al. Gut microbial characteristics of adult patients with allergy rhinitis. Microb Cell Fact. 2020;19(1):171. https://doi.org/10.1186/s12934‐020‐01430‐0
- 701. Giavina‐Bianchi P, Aun MV, Takejima P, Kalil J, Agondi RC. United airway disease: current perspectives. J Asthma Allergy. 2016;9:93–100. https://doi.org/10.2147/JAA.S81541
- 702. Genuneit J, Seibold AM, Apfelbacher CJ, et al. Overview of systematic reviews in allergy epidemiology. Allergy. 2017;72(6):849–856. https://doi.org/10.1111/all.13123
- 703. Agache I, Beltran J, Akdis C, et al. Efficacy and safety of treatment with biologicals (benralizumab, dupilumab, mepolizumab, omalizumab and reslizumab) for severe eosinophilic asthma. A systematic review for the EAACI Guidelines – recommendations on the use of biologicals in severe asthma. Allergy. 2020;75(5):1023–1042. https://doi.org/10.1111/all.14221
- 704. Shamji MH, Palmer E, Layhadi JA, Moraes TJ, Eiwegger T. Biological treatment in allergic disease. Allergy. 2021;76(9):2934–2937. https://doi.org/10.1111/all.14954
- 705. Kanda A, Kobayashi Y, Asako M, Tomoda K, Kawauchi H, Iwai H. Regulation of interaction between the upper and lower airways in united airway disease. Med Sci (Basel). 2019;7(2):27. https://doi.org/10.3390/medsci7020027
- 706. Akdis CA, Bachert C, Cingi C, et al. Endotypes and phenotypes of chronic rhinosinusitis: a PRACTALL document of the European Academy of Allergy and Clinical Immunology and the American Academy of Allergy, Asthma & Immunology. J Allergy Clin Immunol. 2013;131(6):1479–1490. https://doi.org/10.1016/j.jaci.2013.02.036
- 707. Asano K, Ueki S, Tamari M, Imoto Y, Fujieda S, Taniguchi M. Adult‐onset eosinophilic airway diseases. Allergy. 2020;75(12):3087–3099. https://doi.org/10.1111/all.14620
- 708. Backaert W, Steelant B, Jorissen M, et al. Self‐reported nasal hyperreactivity is common in all chronic upper airway inflammatory phenotypes and not related to general well‐being. Allergy. 2021;76(12):3806–3809. https://doi.org/10.1111/all.15060
- 709. Feijen J, Seys SF, Steelant B, et al. Prevalence and triggers of self‐reported nasal hyperreactivity in adults with asthma. World Allergy Organ J. 2020;13(6):100132. https://doi.org/10.1016/j.waojou.2020.100132
- 710. Doulaptsi M, Steelant B, Prokopakis E, et al. Prevalence and impact of nasal hyperreactivity in chronic rhinosinusitis. Allergy. 2020;75(7):1768–1771. https://doi.org/10.1111/all.14199
- 711. Agache I, Sugita K, Morita H, Akdis M, Akdis CA. The Complex Type 2 Endotype in Allergy and Asthma: From Laboratory to Bedside. Curr Allergy Asthma Rep. 2015;15(6):29. https://doi.org/10.1007/s11882‐015‐0529‐x
- 712. Avdeeva KS, Fokkens WJ, Reitsma S. Towards a new epidemiological definition of chronic rhinitis: prevalence of nasal complaints in the general population. Rhinology. 2021;59(3):258–266. https://doi.org/10.4193/Rhin20.637
- 713. Viiu B, Christer J, Fredrik S, et al. Asthma in combination with rhinitis and eczema is associated with a higher degree of type‐2 inflammation and symptom burden than asthma alone. Allergy. 2021;76(12):3827–3829. https://doi.org/10.1111/all.15082
- 714. Camp J, Cane JL, Bafadhel M. Shall we focus on the eosinophil to guide treatment with systemic corticosteroids during acute exacerbations of COPD?: PRO. Med Sci (Basel). 2018;6(3):74. https://doi.org/10.3390/medsci6030074
- 715. Hox V, Lourijsen E, Jordens A, et al. Benefits and harm of systemic steroids for short‐ and long‐term use in rhinitis and rhinosinusitis: an EAACI position paper. Clin Transl Allergy. 2020;10:1. https://doi.org/10.1186/s13601‐019‐0303‐6
- 716. Hox V, Lourijsen E, Jordens A, et al. Correction to: benefits and harm of systemic steroids for short‐ and long‐term use in rhinitis and rhinosinusitis: an EAACI position paper. Clin Transl Allergy. 2020;10:38. https://doi.org/10.1186/s13601‐020‐00343‐w
- 717. Agache I, Song Y, Alonso‐Coello P, et al. Efficacy and safety of treatment with biologicals for severe chronic rhinosinusitis with nasal polyps: a systematic review for the EAACI guidelines. Allergy. 2021;76(8):2337–2353. https://doi.org/10.1111/all.14809
- 718. Hellings PW, Verhoeven E, Fokkens WJ. State‐of‐the‐art overview on biological treatment for CRSwNP. Rhinology. 2021;59(2):151–163. https://doi.org/10.4193/Rhin20.570
- 719. Tomassen P, Vandeplas G, Van Zele T, et al. Inflammatory endotypes of chronic rhinosinusitis based on cluster analysis of biomarkers. J Allergy Clin Immunol. 2016;137(5):1449–1456.e4. https://doi.org/10.1016/j.jaci.2015.12.1324
- 720. Kato A, Peters AT, Stevens WW, Schleimer RP, Tan BK, Kern RC. Endotypes of chronic rhinosinusitis: relationships to disease phenotypes, pathogenesis, clinical findings, and treatment approaches. Allergy. 2022;77(3):812–826. https://doi.org/10.1111/all.15074
- 721. Steelant B, Seys SF, Boeckxstaens G, Akdis CA, Ceuppens JL, Hellings PW. Restoring airway epithelial barrier dysfunction: a new therapeutic challenge in allergic airway disease. Rhinology. 2016;54(3):195–205. https://doi.org/10.4193/Rhino15.376
- 722. Scadding GK, Scadding GW. Innate and adaptive immunity: ILC2 and Th2 cells in upper and lower airway allergic diseases. J Allergy Clin Immunol Pract. 2021;9(5):1851–1857. https://doi.org/10.1016/j.jaip.2021.02.013
- 723. van der Ploeg EK, Golebski K, van Nimwegen M, et al. Steroid‐resistant human inflammatory ILC2s are marked by CD45RO and elevated in type 2 respiratory diseases. Sci Immunol. 2021;6(55):eabd3489. https://doi.org/10.1126/sciimmunol.abd3489
- 724. Braunstahl GJ, Fokkens W. Nasal involvement in allergic asthma. Allergy. 2003;58(12):1235–1243. https://doi.org/10.1046/j.0105‐4538.2003.00354.x
- 725. Izuhara Y, Matsumoto H, Nagasaki T, et al. Mouth breathing, another risk factor for asthma: the Nagahama study. Allergy. 2016;71(7):1031–1036. https://doi.org/10.1111/all.12885
- 726. Braunstahl GJ. United airways concept: what does it teach us about systemic inflammation in airways disease? Proc Am Thorac Soc. 2009;6(8):652–654. https://doi.org/10.1513/pats.200906‐052DP
- 727. Corren J, Adinoff AD, Irvin CG. Changes in bronchial responsiveness following nasal provocation with allergen. J Allergy Clin Immunol. 1992;89(2):611–618. https://doi.org/10.1016/0091‐6749(92)90329‐z
- 728. Braunstahl GJ, Kleinjan A, Overbeek SE, Prins JB, Hoogsteden HC, Fokkens WJ. Segmental bronchial provocation induces nasal inflammation in allergic rhinitis patients. Am J Respir Crit Care Med. 2000;161(6):2051–2057. https://doi.org/10.1164/ajrccm.161.6.9906121
- 729. Braunstahl GJ, Overbeek SE, Fokkens WJ, et al. Segmental bronchoprovocation in allergic rhinitis patients affects mast cell and basophil numbers in nasal and bronchial mucosa. Am J Respir Crit Care Med. 2001;164(5):858–865. https://doi.org/10.1164/ajrccm.164.5.2006082
- 730. Braunstahl GJ, Overbeek SE, Kleinjan A, Prins JB, Hoogsteden HC, Fokkens WJ. Nasal allergen provocation induces adhesion molecule expression and tissue eosinophilia in upper and lower airways. J Allergy Clin Immunol. 2001;107(3):469–476. https://doi.org/10.1067/mai.2001.113046
- 731. Allakhverdi Z, Comeau MR, Smith DE, et al. CD34+ hemopoietic progenitor cells are potent effectors of allergic inflammation. J Allergy Clin Immunol. 2009;123(2):472–478. https://doi.org/10.1016/j.jaci.2008.10.022
- 732. Sergejeva S, Malmhall C, Lotvall J, Pullerits T. Increased number of CD34+ cells in nasal mucosa of allergic rhinitis patients: inhibition by a local corticosteroid. Clin Exp Allergy. 2005;35(1):34–38. https://doi.org/10.1111/j.1365‐2222.2004.02038.x
- 733. Lans R, Fokkens WJ, Adriaensen G, Hoven DR, Drubbel JJ, Reitsma S. Real‐life observational cohort verifies high efficacy of dupilumab for chronic rhinosinusitis with nasal polyps. Allergy. 2022;77(2):670–674. https://doi.org/10.1111/all.15134
- 734. ISAAC Steering Committee . ISAAC – The International Study of Asthma and Allergies in Childhood. Accessed November 1, 2021. http://isaac.auckland.ac.nz
- 735. World Health Organization . International Statistical Classigfication of Diseases and Related Health Problems (ICD). Accessed November 1, 2021. https://www.who.int/standards/classifications/classification‐of‐diseases
- 736. Alqahtani JM. Atopy and allergic diseases among Saudi young adults: a cross‐sectional study. J Int Med Res. 2020;48(1):300060519899760. https://doi.org/10.1177/0300060519899760
- 737. Oliveira TB, Persigo ALK, Ferrazza CC, Ferreira ENN, Veiga ABG. Prevalence of asthma, allergic rhinitis and pollinosis in a city of Brazil: a monitoring study. Allergol Immunopathol (Madr). 2020;48(6):537–544. https://doi.org/10.1016/j.aller.2020.03.010
- 738. Nam JS, Hwang CS, Hong MP, Kim KS. Prevalence and clinical characteristics of allergic rhinitis in the elderly Korean population. Eur Arch Otorhinolaryngol. 2020;277(12):3367–3373. https://doi.org/10.1007/s00405‐020‐06256‐5
- 739. Mortz CG, Andersen KE, Poulsen LK, Kjaer HF, Broesby‐Olsen S, Bindslev‐Jensen C. Atopic diseases and type I sensitization from adolescence to adulthood in an unselected population (TOACS) with focus on predictors for allergic rhinitis. Allergy. 2019;74(2):308–317. https://doi.org/10.1111/all.13630
- 740. Wang XY, Ma TT, Wang XY, et al. Prevalence of pollen‐induced allergic rhinitis with high pollen exposure in grasslands of northern China. Allergy. 2018;73(6):1232–1243. https://doi.org/10.1111/all.13388
- 741. Schmitt J, Stadler E, Kuster D, Wustenberg EG. Medical care and treatment of allergic rhinitis: a population‐based cohort study based on routine healthcare utilization data. Allergy. 2016;71(6):850–858. https://doi.org/10.1111/all.12838
- 742. Sorensen M, Wickman M, Sollid JU, Furberg AS, Klingenberg C. Allergic disease and Staphylococcus aureus carriage in adolescents in the Arctic region of Norway. Pediatr Allergy Immunol. 2016;27(7):728–735. https://doi.org/10.1111/pai.12595
- 743. Winther A, Dennison E, Ahmed LA, et al. The Tromso Study: Fit Futures: a study of Norwegian adolescents' lifestyle and bone health. Arch Osteoporos. 2014;9:185. https://doi.org/10.1007/s11657‐014‐0185‐0
- 744. Jacobsen BK, Eggen AE, Mathiesen EB, Wilsgaard T, Njolstad I. Cohort profile: the Tromso study. Int J Epidemiol. 2012;41(4):961–967. https://doi.org/10.1093/ije/dyr049
- 745. Yang L, Fu J, Zhou Y. Research progress in Atopic March. Front Immunol. 2020;11:1907. https://doi.org/10.3389/fimmu.2020.01907
- 746. Park S, Jung PK, Choi M, et al. Association between occupational clusters and allergic rhinitis in the Korean population: analysis of the Korean National Health and Nutrition Examination Survey data. J Occup Health. 2018;60(4):312–319. https://doi.org/10.1539/joh.2017‐0234‐OA
- 747. Cardell LO, Olsson P, Andersson M, et al. TOTALL: high cost of allergic rhinitis‐a national Swedish population‐based questionnaire study. NPJ Prim Care Respir Med. 2016;26:15082. https://doi.org/10.1038/npjpcrm.2015.82
- 748. Idani E, Raji H, Madadizadeh F, Cheraghian B, Haddadzadeh Shoshtari M, Dastoorpoor M. Prevalence of asthma and other allergic conditions in adults in Khuzestan, southwest Iran, 2018. BMC Public Health. 2019;19(1):303. https://doi.org/10.1186/s12889‐019‐6491‐0
- 749. Reijula J, Latvala J, Makela M, Siitonen S, Saario M, Haahtela T. Long‐term trends of asthma, allergic rhinitis and atopic eczema in young Finnish men: a retrospective analysis, 1926‐2017. Eur Respir J. 2020;56(6):1902144. https://doi.org/10.1183/13993003.02144‐2019
- 750. Maio S, Baldacci S, Carrozzi L, et al. Respiratory symptoms/diseases prevalence is still increasing: a 25‐yr population study. Respir Med. 2016;110:58–65. https://doi.org/10.1016/j.rmed.2015.11.006
- 751. Janson C, Johannessen A, Franklin K, et al. Change in the prevalence asthma, rhinitis and respiratory symptom over a 20 year period: associations to year of birth, life style and sleep related symptoms. BMC Pulm Med. 2018;18(1):152. https://doi.org/10.1186/s12890‐018‐0690‐9
- 752. Sucharew H, Ryan PH, Bernstein D, et al. Exposure to traffic exhaust and night cough during early childhood: the CCAAPS birth cohort. Pediatr Allergy Immunol. 2010;21(2 pt 1):253–259. https://doi.org/10.1111/j.1399‐3038.2009.00952.x
- 753. Herr M, Clarisse B, Nikasinovic L, et al. Does allergic rhinitis exist in infancy? Findings from the PARIS birth cohort. Allergy. 2011;66(2):214–221. https://doi.org/10.1111/j.1398‐9995.2010.02467.x
- 754. Kulig M, Klettke U, Wahn V, Forster J, Bauer CP, Wahn U. Development of seasonal allergic rhinitis during the first 7 years of life. J Allergy Clin Immunol. 2000;106(5):832–839. https://doi.org/10.1067/mai.2000.110098
- 755. Westman M, Stjarne P, Asarnoj A, et al. Natural course and comorbidities of allergic and nonallergic rhinitis in children. J Allergy Clin Immunol. 2012;129(2):403–408. https://doi.org/10.1016/j.jaci.2011.09.036
- 756. Ait‐Khaled N, Pearce N, Anderson HR, et al. Global map of the prevalence of symptoms of rhinoconjunctivitis in children: The International Study of Asthma and Allergies in Childhood (ISAAC) Phase Three. Allergy. 2009;64(1):123–148. https://doi.org/10.1111/j.1398‐9995.2008.01884.x
- 757. Bjorksten B, Clayton T, Ellwood P, Stewart A, Strachan D, Group IPIS. Worldwide time trends for symptoms of rhinitis and conjunctivitis: Phase III of the International Study of Asthma and Allergies in Childhood. Pediatr Allergy Immunol. 2008;19(2):110–124. https://doi.org/10.1111/j.1399‐3038.2007.00601.x
- 758. Mallol J, Crane J, von Mutius E, et al. The International Study of Asthma and Allergies in Childhood (ISAAC) Phase Three: a global synthesis. Allergol Immunopathol (Madr). 2013;41(2):73–85. https://doi.org/10.1016/j.aller.2012.03.001
- 759. Strachan D, Sibbald B, Weiland S, et al. Worldwide variations in prevalence of symptoms of allergic rhinoconjunctivitis in children: the International Study of Asthma and Allergies in Childhood (ISAAC). Pediatr Allergy Immunol. 1997;8(4):161–176. https://doi.org/10.1111/j.1399‐3038.1997.tb00156.x
- 760. Soto‐Martinez ME, Yock‐Corrales A, Camacho‐Badilla K, et al. The current prevalence of asthma, allergic rhinitis, and eczema related symptoms in school‐aged children in Costa Rica. J Asthma. 2019;56(4):360–368. https://doi.org/10.1080/02770903.2018.1455860
- 761. Morikawa E, Sasaki M, Yoshida K, Adachi Y, Odajima H, Akasawa A. Nationwide survey of the prevalence of wheeze, rhino‐conjunctivitis, and eczema among Japanese children in 2015. Allergol Int. 2020;69(1):98–103. https://doi.org/10.1016/j.alit.2019.08.010
- 762. Ibrahim NM, Almarzouqi FI, Al Melaih FA, Farouk H, Alsayed M, AlJassim FM. Prevalence of asthma and allergies among children in the United Arab Emirates: a cross‐sectional study. World Allergy Organ J. 2021;14(10):100588. https://doi.org/10.1016/j.waojou.2021.100588
- 763. Ozoh OB, Aderibigbe SA, Ayuk AC, et al. The prevalence of asthma and allergic rhinitis in Nigeria: a nationwide survey among children, adolescents and adults. PLoS One. 2019;14(9):e0222281. https://doi.org/10.1371/journal.pone.0222281
- 764. de Oliveira TB, Moscon JG, Ferreira E, da Veiga ABG. Prevalence of symptoms of asthma and allergic rhinitis in children in Southern Brazil: a ten‐year monitoring study. J Asthma. 2020;57(4):373–380. https://doi.org/10.1080/02770903.2019.1573253
- 765. Ochoa‐Aviles C, Morillo D, Rodriguez A, et al. Prevalence and risk factors for asthma, rhinitis, eczema, and atopy among preschool children in an Andean city. PLoS One. 2020;15(7):e0234633. https://doi.org/10.1371/journal.pone.0234633
- 766. Tong H, Gao L, Deng Y, et al. Prevalence of allergic rhinitis and associated risk factors in 6 to 12 years schoolchildren from Wuhan in Central China: a cross‐sectional study. Am J Rhinol Allergy. 2020;34(5):632–641. https://doi.org/10.1177/1945892420920499
- 767. Zhang HL, Wang BY, Luo Y, et al. Association of pet‐keeping in home with self‐reported asthma and asthma‐related symptoms in 11611 school children from China. J Asthma. 2021;58(12):1555–1564. https://doi.org/10.1080/02770903.2020.1818772
- 768. Pols DH, Wartna JB, Moed H, van Alphen EI, Bohnen AM, Bindels PJ. Atopic dermatitis, asthma and allergic rhinitis in general practice and the open population: a systematic review. Scand J Prim Health Care. 2016;34(2):143–150. https://doi.org/10.3109/02813432.2016.1160629
- 769. Van Wonderen KE, Van Der Mark LB, Mohrs J, Bindels PJ, Van Aalderen WM, Ter Riet G. Different definitions in childhood asthma: how dependable is the dependent variable? Eur Respir J. 2010;36(1):48–56. https://doi.org/10.1183/09031936.00154409
- 770. Pols DHJ, Nielen MMJ, Korevaar JC, Bindels PJE, Bohnen AM. Reliably estimating prevalences of atopic children: an epidemiological study in an extensive and representative primary care database. NPJ Prim Care Respir Med. 2017;27(1):23. https://doi.org/10.1038/s41533‐017‐0025‐y
- 771. Kurukulaaratchy RJ, Karmaus W, Raza A, Matthews S, Roberts G, Arshad SH. The influence of gender and atopy on the natural history of rhinitis in the first 18 years of life. Clin Exp Allergy. 2011;41(6):851–859. https://doi.org/10.1111/j.1365‐2222.2011.03765.x
- 772. de Jong NW, Elbert NJ, Mensink‐Bout SM, et al. Parental and child factors associated with inhalant and food allergy in a population‐based prospective cohort study: the Generation R Study. Eur J Pediatr. 2019;178(10):1507–1517. https://doi.org/10.1007/s00431‐019‐03441‐5
- 773. Strachan DP, Rutter CE, Asher MI, et al. Worldwide time trends in prevalence of symptoms of rhinoconjunctivitis in children: Global Asthma Network Phase I. Pediatr Allergy Immunol. 2022;33(1):e13656. https://doi.org/10.1111/pai.13656
- 774. Bousquet PJ, Leynaert B, Neukirch F, et al. Geographical distribution of atopic rhinitis in the European Community Respiratory Health Survey I. Allergy. 2008;63(10):1301–1309. https://doi.org/10.1111/j.1398‐9995.2008.01824.x
- 775. Variations in the prevalence of respiratory symptoms, self‐reported asthma attacks, and use of asthma medication in the European Community Respiratory Health Survey (ECRHS). Eur Respir J. 1996;9(4):687–695. https://doi.org/10.1183/09031936.96.09040687
- 776. Weinmayr G, Forastiere F, Weiland SK, et al. International variation in prevalence of rhinitis and its relationship with sensitisation to perennial and seasonal allergens. Eur Respir J. 2008;32(5):1250–1261. https://doi.org/10.1183/09031936.00157807
- 777. Wang XD, Zheng M, Lou HF, et al. An increased prevalence of self‐reported allergic rhinitis in major Chinese cities from 2005 to 2011. Allergy. 2016;71(8):1170–1180. https://doi.org/10.1111/all.12874
- 778. Anderegg WRL, Abatzoglou JT, Anderegg LDL, Bielory L, Kinney PL, Ziska L. Anthropogenic climate change is worsening North American pollen seasons. Proc Natl Acad Sci U S A. 2021;118(7):e2013284118. https://doi.org/10.1073/pnas.2013284118
- 779. Lake IR, Jones NR, Agnew M, et al. Climate change and future pollen allergy in Europe. Environ Health Perspect. 2017;125(3):385–391. https://doi.org/10.1289/EHP173
- 780. Erbas B, Lowe AJ, Lodge CJ, et al. Persistent pollen exposure during infancy is associated with increased risk of subsequent childhood asthma and hayfever. Clin Exp Allergy. 2013;43(3):337–343. https://doi.org/10.1111/cea.12071
- 781. Toth I, Peternel R, Gajnik D, Vojnikovic B. Micro‐regional hypersensitivity variations to inhalant allergens in the city of Zagreb and Zagreb County. Coll Antropol. 2011;35(suppl 2):31–37.
- 782. Kim J, Han Y, Seo SC, et al. Association of carbon monoxide levels with allergic diseases in children. Allergy Asthma Proc. 2016;37(1):e1–e7. https://doi.org/10.2500/aap.2016.37.3918
- 783. Li CW, Chen DD, Zhong JT, et al. Epidemiological characterization and risk factors of allergic rhinitis in the general population in Guangzhou City in China. PLoS One. 2014;9(12):e114950. https://doi.org/10.1371/journal.pone.0114950
- 784. Ahn JC, Kim JW, Lee CH, Rhee CS. Prevalence and risk factors of chronic rhinosinusitus, allergic rhinitis, and nasal septal deviation: results of the Korean National Health and Nutrition Survey 2008‐2012. JAMA Otolaryngol Head Neck Surg. 2016;142(2):162–167. https://doi.org/10.1001/jamaoto.2015.3142
- 785. Song WJ, Sohn KH, Kang MG, et al. Urban‐rural differences in the prevalence of allergen sensitization and self‐reported rhinitis in the elderly population. Ann Allergy Asthma Immunol. 2015;114(6):455–461. https://doi.org/10.1016/j.anai.2015.03.008
- 786. Zheng M, Wang X, Wang M, et al. Clinical characteristics of allergic rhinitis patients in 13 metropolitan cities of China. Allergy. 2021;76(2):577–581. https://doi.org/10.1111/all.14561
- 787. Beggs PJ, Katelaris CH, Medek D, et al. Differences in grass pollen allergen exposure across Australia. Aust N Z J Public Health. 2015;39(1):51–55. https://doi.org/10.1111/1753‐6405.12325
- 788. Westman M, Kull I, Lind T, et al. The link between parental allergy and offspring allergic and nonallergic rhinitis. Allergy. 2013;68(12):1571–1578. https://doi.org/10.1111/all.12267
- 789. Thomsen SF, Ulrik CS, Kyvik KO, et al. Genetic and environmental contributions to hay fever among young adult twins. Respir Med. 2006;100(12):2177–2182. https://doi.org/10.1016/j.rmed.2006.03.013
- 790. Rasanen M, Laitinen T, Kaprio J, Koskenvuo M, Laitinen LA. Hay fever – a Finnish nationwide study of adolescent twins and their parents. Allergy. 1998;53(9):885–890. https://doi.org/10.1111/j.1398‐9995.1998.tb03996.x
- 791. Ferreira MA, Matheson MC, Tang CS, et al. Genome‐wide association analysis identifies 11 risk variants associated with the asthma with hay fever phenotype. J Allergy Clin Immunol. 2014;133(6):1564–1571. https://doi.org/10.1016/j.jaci.2013.10.030
- 792. Hinds DA, McMahon G, Kiefer AK, et al. A genome‐wide association meta‐analysis of self‐reported allergy identifies shared and allergy‐specific susceptibility loci. Nat Genet. 2013;45(8):907–911. https://doi.org/10.1038/ng.2686
- 793. Ramasamy A, Curjuric I, Coin LJ, et al. A genome‐wide meta‐analysis of genetic variants associated with allergic rhinitis and grass sensitization and their interaction with birth order. J Allergy Clin Immunol. 2011;128(5):996–1005. https://doi.org/10.1016/j.jaci.2011.08.030
- 794. Johansson A, Rask‐Andersen M, Karlsson T, Ek WE. Genome‐wide association analysis of 350 000 Caucasians from the UK Biobank identifies novel loci for asthma, hay fever and eczema. Hum Mol Genet. 2019;28(23):4022–4041. https://doi.org/10.1093/hmg/ddz175
- 795. Waage J, Standl M, Curtin JA, et al. Genome‐wide association and HLA fine‐mapping studies identify risk loci and genetic pathways underlying allergic rhinitis. Nat Genet. 2018;50(8):1072–1080. https://doi.org/10.1038/s41588‐018‐0157‐1
- 796. Ferreira MA, Matheson MC, Duffy DL, et al. Identification of IL6R and chromosome 11q13.5 as risk loci for asthma. Lancet. 2011;378(9795):1006–1014. https://doi.org/10.1016/S0140‐6736(11)60874‐X
- 797. Weidinger S, Willis‐Owen SA, Kamatani Y, et al. A genome‐wide association study of atopic dermatitis identifies loci with overlapping effects on asthma and psoriasis. Hum Mol Genet. 2013;22(23):4841–4856. https://doi.org/10.1093/hmg/ddt317
- 798. Marenholz I, Esparza‐Gordillo J, Ruschendorf F, et al. Meta‐analysis identifies seven susceptibility loci involved in the atopic march. Nat Commun. 2015;6:8804. https://doi.org/10.1038/ncomms9804
- 799. Stockis J, Colau D, Coulie PG, Lucas S. Membrane protein GARP is a receptor for latent TGF‐beta on the surface of activated human Treg. Eur J Immunol. 2009;39(12):3315–3322. https://doi.org/10.1002/eji.200939684
- 800. Bonnelykke K, Matheson MC, Pers TH, et al. Meta‐analysis of genome‐wide association studies identifies ten loci influencing allergic sensitization. Nat Genet. 2013;45(8):902–906. https://doi.org/10.1038/ng.2694
- 801. Ferreira MA, Vonk JM, Baurecht H, et al. Shared genetic origin of asthma, hay fever and eczema elucidates allergic disease biology. Nat Genet. 2017;49(12):1752–1757. https://doi.org/10.1038/ng.3985
- 802. Ferreira MAR, Vonk JM, Baurecht H, et al. Eleven loci with new reproducible genetic associations with allergic disease risk. J Allergy Clin Immunol. 2019;143(2):691–699. https://doi.org/10.1016/j.jaci.2018.03.012
- 803. Backman JD, Li AH, Marcketta A, et al. Exome sequencing and analysis of 454,787 UK Biobank participants. Nature. 2021;599(7886):628–634. https://doi.org/10.1038/s41586‐021‐04103‐z
- 804. Davila I, Mullol J, Ferrer M, et al. Genetic aspects of allergic rhinitis. J Investig Allergol Clin Immunol. 2009;19(suppl 1):25–31.
- 805. Andiappan AK, Nilsson D, Hallden C, et al. Investigating highly replicated asthma genes as candidate genes for allergic rhinitis. BMC Med Genet. 2013;14:51. https://doi.org/10.1186/1471‐2350‐14‐51
- 806. Nilsson D, Andiappan AK, Hallden C, et al. Toll‐like receptor gene polymorphisms are associated with allergic rhinitis: a case control study. BMC Med Genet. 2012;13:66. https://doi.org/10.1186/1471‐2350‐13‐66
- 807. Kang I, Oh YK, Lee SH, Jung HM, Chae SC, Lee JH. Identification of polymorphisms in the Toll‐like receptor gene and the association with allergic rhinitis. Eur Arch Otorhinolaryngol. 2010;267(3):385–389. https://doi.org/10.1007/s00405‐009‐1100‐y
- 808. Kormann MS, Ferstl R, Depner M, et al. Rare TLR2 mutations reduce TLR2 receptor function and can increase atopy risk. Allergy. 2009;64(4):636–642. https://doi.org/10.1111/j.1398‐9995.2008.01891.x
- 809. Moller‐Larsen S, Nyegaard M, Haagerup A, Vestbo J, Kruse TA, Borglum AD. Association analysis identifies TLR7 and TLR8 as novel risk genes in asthma and related disorders. Thorax. 2008;63(12):1064–1069. https://doi.org/10.1136/thx.2007.094128
- 810. Sun Q, Liu Y, Zhang S, et al. Thymic stromal lymphopoietin polymorphisms and allergic rhinitis risk: a systematic review and meta‐analysis with 6351 cases and 11472 controls. Int J Clin Exp Med. 2015;8(9):15752–15758.
- 811. Nilsson D, Andiappan AK, Hallden C, et al. Poor reproducibility of allergic rhinitis SNP associations. PLoS One. 2013;8(1):e53975. https://doi.org/10.1371/journal.pone.0053975
- 812. Vercelli D. Discovering susceptibility genes for asthma and allergy. Nat Rev Immunol. 2008;8(3):169–182. https://doi.org/10.1038/nri2257
- 813. London SJ, Melen E. Genomic interactions with exposure to inhaled pollutants. J Allergy Clin Immunol. 2019;143(6):2011–2013.e1. https://doi.org/10.1016/j.jaci.2019.04.008
- 814. Gruzieva O, Xu CJ, Breton CV, et al. Epigenome‐wide meta‐analysis of methylation in children related to prenatal NO2 air pollution exposure. Environ Health Perspect. 2017;125(1):104–110. https://doi.org/10.1289/EHP36
- 815. Merid SK, Novoloaca A, Sharp GC, et al. Epigenome‐wide meta‐analysis of blood DNA methylation in newborns and children identifies numerous loci related to gestational age. Genome Med. 2020;12(1):25. https://doi.org/10.1186/s13073‐020‐0716‐9
- 816. Li JY, Zhang Y, Lin XP, et al. Association between DNA hypomethylation at IL13 gene and allergic rhinitis in house dust mite‐sensitized subjects. Clin Exp Allergy. 2016;46(2):298–307. https://doi.org/10.1111/cea.12647
- 817. Nestor CE, Barrenas F, Wang H, et al. DNA methylation changes separate allergic patients from healthy controls and may reflect altered CD4+ T‐cell population structure. PLoS Genet. 2014;10(1):e1004059. https://doi.org/10.1371/journal.pgen.1004059
- 818. Sarnowski C, Laprise C, Malerba G, et al. DNA methylation within melatonin receptor 1A (MTNR1A) mediates paternally transmitted genetic variant effect on asthma plus rhinitis. J Allergy Clin Immunol. 2016;138(3):748–753. https://doi.org/10.1016/j.jaci.2015.12.1341
- 819. Liang L, Willis‐Owen SAG, Laprise C, et al. An epigenome‐wide association study of total serum immunoglobulin E concentration. Nature. 2015;520(7549):670–674. https://doi.org/10.1038/nature14125
- 820. Everson TM, Lyons G, Zhang H, et al. DNA methylation loci associated with atopy and high serum IgE: a genome‐wide application of recursive Random Forest feature selection. Genome Med. 2015;7:89. https://doi.org/10.1186/s13073‐015‐0213‐8
- 821. Qi C, Jiang Y, Yang IV, et al. Nasal DNA methylation profiling of asthma and rhinitis. J Allergy Clin Immunol. 2020;145(6):1655–1663. https://doi.org/10.1016/j.jaci.2019.12.911
- 822. Xu CJ, Gruzieva O, Qi C, et al. Shared DNA methylation signatures in childhood allergy: the MeDALL study. J Allergy Clin Immunol. 2021;147(3):1031–1040. https://doi.org/10.1016/j.jaci.2020.11.044
- 823. Andiappan AK, de Wang Y, Anantharaman R, et al. Genome‐wide association study for atopy and allergic rhinitis in a Singapore Chinese population. PLoS One. 2011;6(5):e19719. https://doi.org/10.1371/journal.pone.0019719
- 824. Bunyavanich S, Schadt EE, Himes BE, et al. Integrated genome‐wide association, coexpression network, and expression single nucleotide polymorphism analysis identifies novel pathway in allergic rhinitis. BMC Med Genomics. 2014;7:48. https://doi.org/10.1186/1755‐8794‐7‐48
- 825. Sakaue S, Kanai M, Tanigawa Y, et al. A cross‐population atlas of genetic associations for 220 human phenotypes. Nat Genet. 2021;53(10):1415–1424. https://doi.org/10.1038/s41588‐021‐00931‐x
- 826. Toelle BG, Ng KK, Crisafulli D, et al. Eight‐year outcomes of the Childhood Asthma Prevention Study. J Allergy Clin Immunol. 2010;126(2):388–389. 389.e1‐3. https://doi.org/10.1016/j.jaci.2010.04.031
- 827. Marks GB, Mihrshahi S, Kemp AS, et al. Prevention of asthma during the first 5 years of life: a randomized controlled trial. J Allergy Clin Immunol. 2006;118(1):53–61. https://doi.org/10.1016/j.jaci.2006.04.004
- 828. Gehring U, de Jongste JC, Kerkhof M, et al. The 8‐year follow‐up of the PIAMA intervention study assessing the effect of mite‐impermeable mattress covers. Allergy. 2012;67(2):248–256. https://doi.org/10.1111/j.1398‐9995.2011.02739.x
- 829. Gabet S, Ranciere F, Just J, et al. Asthma and allergic rhinitis risk depends on house dust mite specific IgE levels in PARIS birth cohort children. World Allergy Organ J. 2019;12(9):100057. https://doi.org/10.1016/j.waojou.2019.100057
- 830. Arshad SH, Bateman B, Sadeghnejad A, Gant C, Matthews SM. Prevention of allergic disease during childhood by allergen avoidance: the Isle of Wight prevention study. J Allergy Clin Immunol. 2007;119(2):307–313. https://doi.org/10.1016/j.jaci.2006.12.621
- 831. Chan‐Yeung M, Ferguson A, Watson W, et al. The Canadian Childhood Asthma Primary Prevention Study: outcomes at 7 years of age. J Allergy Clin Immunol. 2005;116(1):49–55. https://doi.org/10.1016/j.jaci.2005.03.029
- 832. Calderon MA, Linneberg A, Kleine‐Tebbe J, et al. Respiratory allergy caused by house dust mites: what do we really know? J Allergy Clin Immunol. 2015;136(1):38–48. https://doi.org/10.1016/j.jaci.2014.10.012
- 833. Wahn U, Lau S, Bergmann R, et al. Indoor allergen exposure is a risk factor for sensitization during the first three years of life. J Allergy Clin Immunol. 1997;99(6 pt 1):763–769. https://doi.org/10.1016/s0091‐6749(97)80009‐7
- 834. Tovey ER, Almqvist C, Li Q, Crisafulli D, Marks GB. Nonlinear relationship of mite allergen exposure to mite sensitization and asthma in a birth cohort. J Allergy Clin Immunol. 2008;122(1):114–118. 118.e1‐5. https://doi.org/10.1016/j.jaci.2008.05.010
- 835. Schram‐Bijkerk D, Doekes G, Boeve M, et al. Nonlinear relations between house dust mite allergen levels and mite sensitization in farm and nonfarm children. Allergy. 2006;61(5):640–647. https://doi.org/10.1111/j.1398‐9995.2006.01079.x
- 836. Torrent M, Sunyer J, Munoz L, et al. Early‐life domestic aeroallergen exposure and IgE sensitization at age 4 years. J Allergy Clin Immunol. 2006;118(3):742–748. https://doi.org/10.1016/j.jaci.2006.04.059
- 837. Cullinan P, MacNeill SJ, Harris JM, et al. Early allergen exposure, skin prick responses, and atopic wheeze at age 5 in English children: a cohort study. Thorax. 2004;59(10):855–861. https://doi.org/10.1136/thx.2003.019877
- 838. Lau S, Falkenhorst G, Weber A, et al. High mite‐allergen exposure increases the risk of sensitization in atopic children and young adults. J Allergy Clin Immunol. 1989;84(5 pt 1):718–725. https://doi.org/10.1016/0091‐6749(89)90300‐x
- 839. Kuehr J, Frischer T, Meinert R, et al. Mite allergen exposure is a risk for the incidence of specific sensitization. J Allergy Clin Immunol. 1994;94(1):44–52. https://doi.org/10.1016/0091‐6749(94)90070‐1
- 840. Lodge CJ, Lowe AJ, Gurrin LC, et al. House dust mite sensitization in toddlers predicts current wheeze at age 12 years. J Allergy Clin Immunol. 2011;128(4):782–788.e9. https://doi.org/10.1016/j.jaci.2011.06.038
- 841. Schoos AM, Chawes BL, Jelding‐Dannemand E, Elfman LB, Bisgaard H. Early indoor aeroallergen exposure is not associated with development of sensitization or allergic rhinitis in high‐risk children. Allergy. 2016;71(5):684–691. https://doi.org/10.1111/all.12853
- 842. Illi S, Weber J, Zutavern A, et al. Perinatal influences on the development of asthma and atopy in childhood. Ann Allergy Asthma Immunol. 2014;112(2):132–139.e1. https://doi.org/10.1016/j.anai.2013.11.019
- 843. Kihlstrom A, Lilja G, Pershagen G, Hedlin G. Exposure to birch pollen in infancy and development of atopic disease in childhood. J Allergy Clin Immunol. 2002;110(1):78–84. https://doi.org/10.1067/mai.2002.125829
- 844. Scadding GK, Smith PK, Blaiss M, et al. Allergic Rhinitis in Childhood and the New EUFOREA Algorithm. Front Allergy. 2021;2:706589. https://doi.org/10.3389/falgy.2021.706589
- 845. Lipiec A, Sybilski A, Komorowski J, et al. Sensitisation to airborne allergens as a risk factor for allergic rhinitis and asthma in the Polish population. Postepy Dermatol Alergol. 2020;37(5):751–759. https://doi.org/10.5114/ada.2019.84231
- 846. Hatzler L, Panetta V, Lau S, et al. Molecular spreading and predictive value of preclinical IgE response to Phleum pratense in children with hay fever. J Allergy Clin Immunol. 2012;130(4):894–901.e5. https://doi.org/10.1016/j.jaci.2012.05.053
- 847. Gough H, Grabenhenrich L, Reich A, et al. Allergic multimorbidity of asthma, rhinitis and eczema over 20 years in the German birth cohort MAS. Pediatr Allergy Immunol. 2015;26(5):431–437. https://doi.org/10.1111/pai.12410
- 848. Grabenhenrich LB, Keil T, Reich A, et al. Prediction and prevention of allergic rhinitis: a birth cohort study of 20 years. J Allergy Clin Immunol. 2015;136(4):932–940.e12. https://doi.org/10.1016/j.jaci.2015.03.040
- 849. Lee KS, Kim K, Choi YJ, et al. Increased sensitization rates to tree pollens in allergic children and adolescents and a change in the pollen season in the metropolitan area of Seoul, Korea. Pediatr Allergy Immunol. 2021;32(5):872–879. https://doi.org/10.1111/pai.13472
- 850. Westman M, Aberg K, Apostolovic D, et al. Sensitization to grass pollen allergen molecules in a birth cohort‐natural Phl p 4 as an early indicator of grass pollen allergy. J Allergy Clin Immunol. 2020;145(4):1174–1181.e6. https://doi.org/10.1016/j.jaci.2020.01.006
- 851. Westman M, Lupinek C, Bousquet J, et al. Early childhood IgE reactivity to pathogenesis‐related class 10 proteins predicts allergic rhinitis in adolescence. J Allergy Clin Immunol. 2015;135(5):1199‐1206.e1‐11. https://doi.org/10.1016/j.jaci.2014.10.042
- 852. Gao X, Yin M, Yang P, et al. Effect of exposure to cats and dogs on the risk of asthma and allergic rhinitis: a systematic review and meta‐analysis. Am J Rhinol Allergy. 2020;34(5):703–714. https://doi.org/10.1177/1945892420932487
- 853. Ojwang V, Nwaru BI, Takkinen HM, et al. Early exposure to cats, dogs and farm animals and the risk of childhood asthma and allergy. Pediatr Allergy Immunol. 2020;31(3):265–272. https://doi.org/10.1111/pai.13186
- 854. Al‐Tamprouri C, Malin B, Bill H, Lennart B, Anna S. Cat and dog ownership during/after the first year of life and risk for sensitization and reported allergy symptoms at age 13. Immun Inflamm Dis. 2019;7(4):250–257. https://doi.org/10.1002/iid3.267
- 855. Sultesz M, Horvath A, Molnar D, et al. Prevalence of allergic rhinitis, related comorbidities and risk factors in schoolchildren. Allergy Asthma Clin Immunol. 2020;16(1):98. https://doi.org/10.1186/s13223‐020‐00495‐1
- 856. Ho CL, Wu WF. Risk factor analysis of allergic rhinitis in 6‐8 year‐old children in Taipei. PLoS One. 2021;16(4):e0249572.
- 857. Alm B, Goksor E, Thengilsdottir H, et al. Early protective and risk factors for allergic rhinitis at age 4(1/2) yr. Pediatr Allergy Immunol. 2011;22(4):398–404. https://doi.org/10.1111/j.1399‐3038.2011.01153.x
- 858. Dunlop J, Matsui E, Sharma HP. Allergic rhinitis: environmental determinants. Immunol Allergy Clin North Am. 2016;36(2):367–377. https://doi.org/10.1016/j.iac.2015.12.012
- 859. Sandini U, Kukkonen AK, Poussa T, Sandini L, Savilahti E, Kuitunen M. Protective and risk factors for allergic diseases in high‐risk children at the ages of two and five years. Int Arch Allergy Immunol. 2011;156(3):339–348. https://doi.org/10.1159/000323907
- 860. Fasce L, Tosca MA, Silvestri M, Olcese R, Pistorio A, Rossi GA. “Early” cat ownership and the risk of sensitization and allergic rhinitis in Ligurian children with respiratory symptoms. Ann Allergy Asthma Immunol. 2005;94(5):561–565. https://doi.org/10.1016/S1081‐1206(10)61134‐9
- 861. Dimich‐Ward H, Chow Y, Chung J, Trask C. Contact with livestock–a protective effect against allergies and asthma? Clin Exp Allergy. 2006;36(9):1122–1129. https://doi.org/10.1111/j.1365‐2222.2006.02556.x
- 862. Majkowska‐Wojciechowska B, Pelka J, Korzon L, et al. Prevalence of allergy, patterns of allergic sensitization and allergy risk factors in rural and urban children. Allergy. 2007;62(9):1044–1050. https://doi.org/10.1111/j.1398‐9995.2007.01457.x
- 863. Matheson MC, Dharmage SC, Abramson MJ, et al. Early‐life risk factors and incidence of rhinitis: results from the European Community Respiratory Health Study – an international population‐based cohort study. J Allergy Clin Immunol. 2011;128(4):816–823.e5. https://doi.org/10.1016/j.jaci.2011.05.039
- 864. Perkin MR, Bader T, Rudnicka AR, Strachan DP, Owen CG. Inter‐relationship between rhinitis and conjunctivitis in allergic rhinoconjunctivitis and associated risk factors in rural UK children. PLoS One. 2015;10(11):e0143651. https://doi.org/10.1371/journal.pone.0143651
- 865. Vargas C, Bustos P, Diaz PV, Amigo H, Rona RJ. Childhood environment and atopic conditions, with emphasis on asthma in a Chilean agricultural area. J Asthma. 2008;45(1):73–78. https://doi.org/10.1080/02770900701752540
- 866. Lampi J, Canoy D, Jarvis D, et al. Farming environment and prevalence of atopy at age 31: prospective birth cohort study in Finland. Clin Exp Allergy. 2011;41(7):987–993. https://doi.org/10.1111/j.1365‐2222.2011.03777.x
- 867. Perzanowski MS, Chew GL, Divjan A, et al. Cat ownership is a risk factor for the development of anti‐cat IgE but not current wheeze at age 5 years in an inner‐city cohort. J Allergy Clin Immunol. 2008;121(4):1047–1052. https://doi.org/10.1016/j.jaci.2008.02.005
- 868. Nafstad P, Magnus P, Gaarder PI, Jaakkola JJ. Exposure to pets and atopy‐related diseases in the first 4 years of life. Allergy. 2001;56(4):307–312. https://doi.org/10.1034/j.1398‐9995.2001.00881.x
- 869. Tamay Z, Akcay A, Ones U, Guler N, Kilic G, Zencir M. Prevalence and risk factors for allergic rhinitis in primary school children. Int J Pediatr Otorhinolaryngol. 2007;71(3):463–471. https://doi.org/10.1016/j.ijporl.2006.11.013
- 870. Batlles‐Garrido J, Torres‐Borrego J, Rubi‐Ruiz T, et al. Prevalence and factors linked to allergic rhinitis in 10 and 11‐year‐old children in Almeria. Isaac Phase II, Spain. Allergol Immunopathol (Madr). 2010;38(3):135–141. https://doi.org/10.1016/j.aller.2009.09.005
- 871. Lombardi E, Simoni M, La Grutta S, et al. Effects of pet exposure in the first year of life on respiratory and allergic symptoms in 7‐yr‐old children. The SIDRIA‐2 study. Pediatr Allergy Immunol. 2010;21(2 pt 1):268–276. https://doi.org/10.1111/j.1399‐3038.2009.00910.x
- 872. Ibargoyen‐Roteta N, Aguinaga‐Ontoso I, Fernandez‐Benitez M, et al. Role of the home environment in rhinoconjunctivitis and eczema in schoolchildren in Pamplona, Spain. J Investig Allergol Clin Immunol. 2007;17(3):137–144.
- 873. Kurosaka F, Terada T, Tanaka A, et al. Risk factors for wheezing, eczema and rhinoconjunctivitis in the previous 12 months among six‐year‐old children in Himeji City, Japan: food allergy, older siblings, day‐care attendance and parental allergy history. Allergol Int. 2011;60(3):317–330. https://doi.org/10.2332/allergolint.10‐OA‐0246
- 874. Brunekreef B, Von Mutius E, Wong G, et al. Exposure to cats and dogs, and symptoms of asthma, rhinoconjunctivitis, and eczema. Epidemiology. 2012;23(5):742–750. https://doi.org/10.1097/EDE.0b013e318261f040
- 875. Tamay Z, Akcay A, Ergin A, Guler N. Prevalence of allergic rhinitis and risk factors in 6‐ to 7‐yearold children in Istanbul, Turkey. Turk J Pediatr. 2014;56(1):31–40.
- 876. Yang SI, Lee E, Jung YH, et al. Effect of antibiotic use and mold exposure in infancy on allergic rhinitis in susceptible adolescents. Ann Allergy Asthma Immunol. 2014;113(2):160–165.e1. https://doi.org/10.1016/j.anai.2014.05.019
- 877. Hesselmar B, Aberg N, Aberg B, Eriksson B, Bjorksten B. Does early exposure to cat or dog protect against later allergy development? Clin Exp Allergy. 1999;29(5):611–617. https://doi.org/10.1046/j.1365‐2222.1999.00534.x
- 878. Leynaert B, Neukirch C, Jarvis D, et al. Does living on a farm during childhood protect against asthma, allergic rhinitis, and atopy in adulthood? Am J Respir Crit Care Med. 2001;164(10 pt 1):1829–1834. https://doi.org/10.1164/ajrccm.164.10.2103137
- 879. Anyo G, Brunekreef B, de Meer G, Aarts F, Janssen NA, van Vliet P. Early, current and past pet ownership: associations with sensitization, bronchial responsiveness and allergic symptoms in school children. Clin Exp Allergy. 2002;32(3):361–366. https://doi.org/10.1046/j.1365‐2222.2002.01254.x
- 880. Waser M, von Mutius E, Riedler J, et al. Exposure to pets, and the association with hay fever, asthma, and atopic sensitization in rural children. Allergy. 2005;60(2):177–184. https://doi.org/10.1111/j.1398‐9995.2004.00645.x
- 881. Sultesz M, Katona G, Hirschberg A, Galffy G. Prevalence and risk factors for allergic rhinitis in primary schoolchildren in Budapest. Int J Pediatr Otorhinolaryngol. 2010;74(5):503–509. https://doi.org/10.1016/j.ijporl.2010.02.008
- 882. Kim WK, Kwon JW, Seo JH, et al. Interaction between IL13 genotype and environmental factors in the risk for allergic rhinitis in Korean children. J Allergy Clin Immunol. 2012;130(2):421–426.e5. https://doi.org/10.1016/j.jaci.2012.04.052
- 883. Lam A, Wong GW, Poon CM, Lee SS. A GIS‐based assessment of environmental influences on allergy development in children. Asia Pac J Public Health. 2014;26(6):575–587. https://doi.org/10.1177/1010539511428488
- 884. Torfi Y, Bitarafan N, Rajabi M. Impact of socioeconomic and environmental factors on atopic eczema and allergic rhinitis: a cross sectional study. EXCLI J. 2015;14:1040–1048. https://doi.org/10.17179/excli2015‐519
- 885. Kellberger J, Dressel H, Vogelberg C, et al. Prediction of the incidence and persistence allergic rhinitis in adolescence: a prospective cohort study. J Allergy Clin Immunol. 2012;129(2):397–402, 402.e1‐3. https://doi.org/10.1016/j.jaci.2011.08.016
- 886. Lodrup Carlsen KC, Roll S, Carlsen KH, et al. Does pet ownership in infancy lead to asthma or allergy at school age? Pooled analysis of individual participant data from 11 European birth cohorts. PLoS One. 2012;7(8):e43214. https://doi.org/10.1371/journal.pone.0043214
- 887. Chen CM, Morgenstern V, Bischof W, et al. Dog ownership and contact during childhood and later allergy development. Eur Respir J. 2008;31(5):963–973. https://doi.org/10.1183/09031936.00092807
- 888. Chen CM, Rzehak P, Zutavern A, et al. Longitudinal study on cat allergen exposure and the development of allergy in young children. J Allergy Clin Immunol. 2007;119(5):1148–1155. https://doi.org/10.1016/j.jaci.2007.02.017
- 889. Tischer CG, Hohmann C, Thiering E, et al. Meta‐analysis of mould and dampness exposure on asthma and allergy in eight European birth cohorts: an ENRIECO initiative. Allergy. 2011;66(12):1570–1579. https://doi.org/10.1111/j.1398‐9995.2011.02712.x
- 890. Behbod B, Sordillo JE, Hoffman EB, et al. Asthma and allergy development: contrasting influences of yeasts and other fungal exposures. Clin Exp Allergy. 2015;45(1):154–163. https://doi.org/10.1111/cea.12401
- 891. Ellie AS, Sun Y, Hou J, Wang P, Zhang Q, Sundell J. Prevalence of childhood asthma and allergies and their associations with perinatal exposure to home environmental factors: a cross‐sectional study in Tianjin, China. Int J Environ Res Public Health. 2021;18(8):4131. https://doi.org/10.3390/ijerph18084131
- 892. Caillaud D, Leynaert B, Keirsbulck M, Nadif R, Anses Working Group – mould . Indoor mould exposure, asthma and rhinitis: findings from systematic reviews and recent longitudinal studies. Eur Respir Rev. 2018;27(148):170137. https://doi.org/10.1183/16000617.0137‐2017
- 893. Nevalainen A, Taubel M, Hyvarinen A. Indoor fungi: companions and contaminants. Indoor Air. 2015;25(2):125–156. https://doi.org/10.1111/ina.12182
- 894. Deng Q, Lu C, Ou C, Chen L, Yuan H. Preconceptional, prenatal and postnatal exposure to outdoor and indoor environmental factors on allergic diseases/symptoms in preschool children. Chemosphere. 2016;152:459–467. https://doi.org/10.1016/j.chemosphere.2016.03.032
- 895. Lin Z, Norback D, Wang T, et al. The first 2‐year home environment in relation to the new onset and remission of asthmatic and allergic symptoms in 4246 preschool children. Sci Total Environ. 2016;553:204–210. https://doi.org/10.1016/j.scitotenv.2016.02.040
- 896. Kuyucu S, Saraclar Y, Tuncer A, et al. Epidemiologic characteristics of rhinitis in Turkish children: the International Study of Asthma and Allergies in Childhood (ISAAC) phase 2. Pediatr Allergy Immunol. 2006;17(4):269–277. https://doi.org/10.1111/j.1399‐3038.2006.00407.x
- 897. Bornehag CG, Sundell J, Hagerhed‐Engman L, et al. 'Dampness' at home and its association with airway, nose, and skin symptoms among 10,851 preschool children in Sweden: a cross‐sectional study. Indoor Air. 2005;15(suppl 10):48–55. https://doi.org/10.1111/j.1600‐0668.2005.00306.x
- 898. Thacher JD, Gruzieva O, Pershagen G, et al. Mold and dampness exposure and allergic outcomes from birth to adolescence: data from the BAMSE cohort. Allergy. 2017;72(6):967–974. https://doi.org/10.1111/all.13102
- 899. Biagini JM, LeMasters GK, Ryan PH, et al. Environmental risk factors of rhinitis in early infancy. Pediatr Allergy Immunol. 2006;17(4):278–84. https://doi.org/10.1111/j.1399‐3038.2006.00386.x
- 900. Testa D, DI Bari M, Nunziata M, et al. Allergic rhinitis and asthma assessment of risk factors in pediatric patients: a systematic review. Int J Pediatr Otorhinolaryngol. 2020;129:109759. https://doi.org/10.1016/j.ijporl.2019.109759
- 901. Hardjojo A, Shek LP, van Bever HP, Lee BW. Rhinitis in children less than 6 years of age: current knowledge and challenges. Asia Pac Allergy. 2011;1(3):115–122. https://doi.org/10.5415/apallergy.2011.1.3.115
- 902. Ierodiakonou D, Garcia‐Larsen V, Logan A, et al. Timing of allergenic food introduction to the infant diet and risk of allergic or autoimmune disease: a systematic review and meta‐analysis. JAMA. 2016;316(11):1181–1192. https://doi.org/10.1001/jama.2016.12623
- 903. du Toit G, Sayre PH, Roberts G, et al. Allergen specificity of early peanut consumption and effect on development of allergic disease in the Learning Early About Peanut Allergy study cohort. J Allergy Clin Immunol. 2018;141(4):1343–1353. https://doi.org/10.1016/j.jaci.2017.09.034
- 904. Fong WCG, Chan A, Zhang H, et al. Childhood food allergy and food allergen sensitisation are associated with adult airways disease: a birth cohort study. Pediatr Allergy Immunol. 2021;32(8):1764–1772. https://doi.org/10.1111/pai.13592
- 905. Erkkola M, Kaila M, Nwaru BI, et al. Maternal vitamin D intake during pregnancy is inversely associated with asthma and allergic rhinitis in 5‐year‐old children. Clin Exp Allergy. 2009;39(6):875–82. https://doi.org/10.1111/j.1365‐2222.2009.03234.x
- 906. Oien T, Schjelvaag A, Storro O, Johnsen R, Simpson MR. Fish Consumption at one year of age reduces the risk of eczema, asthma and wheeze at six years of age. Nutrients. 2019;11(9):1969. https://doi.org/10.3390/nu11091969
- 907. Markevych I, Standl M, Lehmann I, von Berg A, Heinrich J. Food diversity during the first year of life and allergic diseases until 15 years. J Allergy Clin Immunol. 2017;140(6):1751–1754.e4. https://doi.org/10.1016/j.jaci.2017.08.011
- 908. Maslova E, Granstrom C, Hansen S, et al. Peanut and tree nut consumption during pregnancy and allergic disease in children‐should mothers decrease their intake? Longitudinal evidence from the Danish National Birth Cohort. J Allergy Clin Immunol. 2012;130(3):724–732. https://doi.org/10.1016/j.jaci.2012.05.014
- 909. Maslova E, Strom M, Oken E, et al. Fish intake during pregnancy and the risk of child asthma and allergic rhinitis ‐ longitudinal evidence from the Danish National Birth Cohort. Br J Nutr. 2013;110(7):1313–1325. https://doi.org/10.1017/S000711451300038X
- 910. Willers SM, Wijga AH, Brunekreef B, et al. Maternal food consumption during pregnancy and the longitudinal development of childhood asthma. Am J Respir Crit Care Med. 2008;178(2):124–131. https://doi.org/10.1164/rccm.200710‐1544OC
- 911. Nwaru BI, Takkinen HM, Kaila M, et al. Food diversity in infancy and the risk of childhood asthma and allergies. J Allergy Clin Immunol. 2014;133(4):1084–1091. https://doi.org/10.1016/j.jaci.2013.12.1069
- 912. Roduit C, Frei R, Depner M, et al. Increased food diversity in the first year of life is inversely associated with allergic diseases. J Allergy Clin Immunol. 2014;133(4):1056–1064. https://doi.org/10.1016/j.jaci.2013.12.1044
- 913. Nwaru BI, Takkinen HM, Niemela O, et al. Timing of infant feeding in relation to childhood asthma and allergic diseases. J Allergy Clin Immunol. 2013;131(1):78–86. https://doi.org/10.1016/j.jaci.2012.10.028
- 914. Virtanen SM, Kaila M, Pekkanen J, et al. Early introduction of oats associated with decreased risk of persistent asthma and early introduction of fish with decreased risk of allergic rhinitis. Br J Nutr. 2010;103(2):266–273. https://doi.org/10.1017/S0007114509991541
- 915. Zeiger RS, Heller S. The development and prediction of atopy in high‐risk children: follow‐up at age seven years in a prospective randomized study of combined maternal and infant food allergen avoidance. J Allergy Clin Immunol. 1995;95(6):1179–1190. https://doi.org/10.1016/s0091‐6749(95)70074‐9
- 916. Lilja G, Dannaeus A, Foucard T, Graff‐Lonnevig V, Johansson SG, Oman H. Effects of maternal diet during late pregnancy and lactation on the development of atopic diseases in infants up to 18 months of age – in‐vivo results. Clin Exp Allergy. 1989;19(4):473–479. https://doi.org/10.1111/j.1365‐2222.1989.tb02416.x
- 917. Falth‐Magnusson K, Kjellman NI. Development of atopic disease in babies whose mothers were receiving exclusion diet during pregnancy – a randomized study. J Allergy Clin Immunol. 1987;80(6):868–875. https://doi.org/10.1016/s0091‐6749(87)80279‐8
- 918. Willers SM, Devereux G, Craig LC, et al. Maternal food consumption during pregnancy and asthma, respiratory and atopic symptoms in 5‐year‐old children. Thorax. 2007;62(9):773–779. https://doi.org/10.1136/thx.2006.074187
- 919. Alduraywish SA, Lodge CJ, Campbell B, et al. The march from early life food sensitization to allergic disease: a systematic review and meta‐analyses of birth cohort studies. Allergy. 2016;71(1):77–89. https://doi.org/10.1111/all.12784
- 920. Brockow I, Zutavern A, Hoffmann U, et al. Early allergic sensitizations and their relevance to atopic diseases in children aged 6 years: results of the GINI study. J Investig Allergol Clin Immunol. 2009;19(3):180–187.
- 921. Garden FL, Simpson JM, Marks GB, Investigators C. Atopy phenotypes in the Childhood Asthma Prevention Study (CAPS) cohort and the relationship with allergic disease: clinical mechanisms in allergic disease. Clin Exp Allergy. 2013;43(6):633–641. https://doi.org/10.1111/cea.12095
- 922. Kulig M, Bergmann R, Tacke U, Wahn U, Guggenmoos‐Holzmann I. Long‐lasting sensitization to food during the first two years precedes allergic airway disease. The MAS Study Group, Germany. Pediatr Allergy Immunol. 1998;9(2):61–67. https://doi.org/10.1111/j.1399‐3038.1998.tb00305.x
- 923. Chiu CY, Huang YL, Tsai MH, et al. Sensitization to food and inhalant allergens in relation to atopic diseases in early childhood: a birth cohort study. PLoS One. 2014;9(7):e102809. https://doi.org/10.1371/journal.pone.0102809
- 924. Kjaer HF, Eller E, Andersen KE, Host A, Bindslev‐Jensen C. The association between early sensitization patterns and subsequent allergic disease. The DARC birth cohort study. Pediatr Allergy Immunol. 2009;20(8):726–734. https://doi.org/10.1111/j.1399‐3038.2009.00862.x
- 925. Zutavern A, Brockow I, Schaaf B, et al. Timing of solid food introduction in relation to eczema, asthma, allergic rhinitis, and food and inhalant sensitization at the age of 6 years: results from the prospective birth cohort study LISA. Pediatrics. 2008;121(1):e44–e52. https://doi.org/10.1542/peds.2006‐3553
- 926. Ekelund L, Gloppen I, Oien T, Simpson MR. Duration of breastfeeding, age at introduction of complementary foods and allergy‐related diseases: a prospective cohort study. Int Breastfeed J. 2021;16(1):5. https://doi.org/10.1186/s13006‐020‐00352‐2
- 927. Venter C, Agostoni C, Arshad SH, et al. Dietary factors during pregnancy and atopic outcomes in childhood: a systematic review from the European Academy of Allergy and Clinical Immunology. Pediatr Allergy Immunol. 2020;31(8):889–912. https://doi.org/10.1111/pai.13303
- 928. Fleischer DM, Spergel JM, Assa'ad AH, Pongracic JA. Primary prevention of allergic disease through nutritional interventions. J Allergy Clin Immunol Pract. 2013;1(1):29–36. https://doi.org/10.1016/j.jaip.2012.09.003
- 929. Greer FR, Sicherer SH, Burks AW, American Academy of Pediatrics Committee on Nutrition , American Academy of Pediatrics Section on Allergy and Immunology . Effects of early nutritional interventions on the development of atopic disease in infants and children: the role of maternal dietary restriction, breastfeeding, timing of introduction of complementary foods, and hydrolyzed formulas. Pediatrics. 2008;121(1):183–191. https://doi.org/10.1542/peds.2007‐3022
- 930. Donovan S, Dewey K, Novotny R, et al. Maternal Diet during Pregnancy and Lactation and Risk of Child Food Allergies and Atopic Allergic Diseases: A Systematic Review [Internet]. Alexandria (VA): USDA Nutrition Evidence Systematic Review; 2020 Jul. https://doi.org/10.52570/NESR.DGAC2020.SR0207
- 931. Kramer MS, Kakuma R. Maternal dietary antigen avoidance during pregnancy or lactation, or both, for preventing or treating atopic disease in the child. Cochrane Database Syst Rev. 2012;(9):CD000133. https://doi.org/10.1002/14651858.CD000133.pub3
- 932. Air pollution . Accessed November 18, 2021. https://www.who.int/health‐topics/air‐pollution
- 933. Gorr MW, Falvo MJ, Wold LE. Air pollution and other environmental modulators of cardiac function. Compr Physiol. 2017;7(4):1479–1495. https://doi.org/10.1002/cphy.c170017
- 934. Li J, Sun S, Tang R, et al. Major air pollutants and risk of COPD exacerbations: a systematic review and meta‐analysis. Int J Chron Obstruct Pulmon Dis. 2016;11:3079–3091. https://doi.org/10.2147/COPD.S122282
- 935. Carlsten C, Blomberg A, Pui M, et al. Diesel exhaust augments allergen‐induced lower airway inflammation in allergic individuals: a controlled human exposure study. Thorax. 2016;71(1):35–44. https://doi.org/10.1136/thoraxjnl‐2015‐207399
- 936. Hernandez M, Brickey WJ, Alexis NE, et al. Airway cells from atopic asthmatic patients exposed to ozone display an enhanced innate immune gene profile. J Allergy Clin Immunol. 2012;129(1):259‐261.e1‐2. https://doi.org/10.1016/j.jaci.2011.11.007
- 937. Anderson HR, Ruggles R, Pandey KD, et al. Ambient particulate pollution and the world‐wide prevalence of asthma, rhinoconjunctivitis and eczema in children: phase one of the International Study of Asthma and Allergies in Childhood (ISAAC). Occup Environ Med. 2010;67(5):293–300. https://doi.org/10.1136/oem.2009.048785
- 938. Chiang TY, Yuan TH, Shie RH, Chen CF, Chan CC. Increased incidence of allergic rhinitis, bronchitis and asthma, in children living near a petrochemical complex with SO2 pollution. Environ Int. 2016;96:1–7. https://doi.org/10.1016/j.envint.2016.08.009
- 939. Singh S, Sharma BB, Salvi S, et al. Allergic rhinitis, rhinoconjunctivitis, and eczema: prevalence and associated factors in children. Clin Respir J. 2018;12(2):547–556. https://doi.org/10.1111/crj.12561
- 940. Jung DY, Leem JH, Kim HC, et al. Effect of traffic‐related air pollution on allergic disease: results of the children's health and environmental research. Allergy Asthma Immunol Res. 2015;7(4):359–366. https://doi.org/10.4168/aair.2015.7.4.359
- 941. Shirinde J, Wichmann J, Voyi K. Allergic rhinitis, rhinoconjunctivitis and hayfever symptoms among children are associated with frequency of truck traffic near residences: a cross sectional study. Environ Health. 2015;14:84. https://doi.org/10.1186/s12940‐015‐0072‐1
- 942. Kim BJ, Kwon JW, Seo JH, et al. Association of ozone exposure with asthma, allergic rhinitis, and allergic sensitization. Ann Allergy Asthma Immunol. 2011;107(3):214–219.e1. https://doi.org/10.1016/j.anai.2011.05.025
- 943. Codispoti CD, LeMasters GK, Levin L, et al. Traffic pollution is associated with early childhood aeroallergen sensitization. Ann Allergy Asthma Immunol. 2015;114(2):126–133. https://doi.org/10.1016/j.anai.2014.10.020
- 944. Gehring U, Wijga AH, Hoek G, et al. Exposure to air pollution and development of asthma and rhinoconjunctivitis throughout childhood and adolescence: a population‐based birth cohort study. Lancet Respir Med. 2015;3(12):933–942. https://doi.org/10.1016/S2213‐2600(15)00426‐9
- 945. Li S, Wu W, Wang G, et al. Association between exposure to air pollution and risk of allergic rhinitis: a systematic review and meta‐analysis. Environ Res. 2022;205:112472. https://doi.org/10.1016/j.envres.2021.112472
- 946. Burte E, Leynaert B, Marcon A, et al. Long‐term air pollution exposure is associated with increased severity of rhinitis in 2 European cohorts. J Allergy Clin Immunol. 2020;145(3):834–842.e6. https://doi.org/10.1016/j.jaci.2019.11.040
- 947. Teng B, Zhang X, Yi C, et al. The association between ambient air pollution and allergic rhinitis: further epidemiological evidence from Changchun, Northeastern China. Int J Environ Res Public Health. 2017;14(3):226. https://doi.org/10.3390/ijerph14030226
- 948. To T, Zhu J, Stieb D, et al. Early life exposure to air pollution and incidence of childhood asthma, allergic rhinitis and eczema. Eur Respir J. 2020;55(2):1900913. https://doi.org/10.1183/13993003.00913‐2019
- 949. Zou QY, Shen Y, Ke X, Hong SL, Kang HY. Exposure to air pollution and risk of prevalence of childhood allergic rhinitis: a meta‐analysis. Int J Pediatr Otorhinolaryngol. 2018;112:82–90. https://doi.org/10.1016/j.ijporl.2018.06.039
- 950. Lin L, Li T, Sun M, et al. Effect of particulate matter exposure on the prevalence of allergic rhinitis in children: a systematic review and meta‐analysis. Chemosphere. 2021;268:128841. https://doi.org/10.1016/j.chemosphere.2020.128841
- 951. Hao S, Yuan F, Pang P, Yang B, Jiang X, Yan A. Early childhood traffic‐related air pollution and risk of allergic rhinitis at 2‐4 years of age modification by family stress and male gender: a case‐control study in Shenyang, China. Environ Health Prev Med. 2021;26(1):48. https://doi.org/10.1186/s12199‐021‐00969‐7
- 952. Mookherjee N, Piyadasa H, Ryu MH, et al. Inhaled diesel exhaust alters the allergen‐induced bronchial secretome in humans. Eur Respir J. 2018;51(1):1701385. https://doi.org/10.1183/13993003.01385‐2017
- 953. Clifford RL, Jones MJ, MacIsaac JL, et al. Inhalation of diesel exhaust and allergen alters human bronchial epithelium DNA methylation. J Allergy Clin Immunol. 2017;139(1):112–121. https://doi.org/10.1016/j.jaci.2016.03.046
- 954. Wooding DJ, Ryu MH, Huls A, et al. Particle depletion does not remediate acute effects of traffic‐related air pollution and allergen. A randomized, double‐blind crossover study. Am J Respir Crit Care Med. 2019;200(5):565–574. https://doi.org/10.1164/rccm.201809‐1657OC
- 955. Ellis AK, Murrieta‐Aguttes M, Furey S, Picard P, Carlsten C. Effect of fexofenadine hydrochloride on allergic rhinitis aggravated by air pollutants. ERJ Open Res. 2021;7(2):00806–2020. https://doi.org/10.1183/23120541.00806‐2020
- 956. Bousquet J, Anto JM, Annesi‐Maesano I, et al. POLLAR: Impact of air POLLution on Asthma and Rhinitis; a European Institute of Innovation and Technology Health (EIT Health) project. Clin Transl Allergy. 2018;8:36. https://doi.org/10.1186/s13601‐018‐0221‐z
- 957. Naclerio R, Ansotegui IJ, Bousquet J, et al. International expert consensus on the management of allergic rhinitis (AR) aggravated by air pollutants: impact of air pollution on patients with AR: current knowledge and future strategies. World Allergy Organ J. 2020;13(3):100106. https://doi.org/10.1016/j.waojou.2020.100106
- 958. Chung HY, Hsieh CJ, Tseng CC, Yiin LM. Association between the first occurrence of allergic rhinitis in preschool children and air pollution in Taiwan. Int J Environ Res Public Health. 2016;13(3):268. https://doi.org/10.3390/ijerph13030268
- 959. Liu W, Huang C, Hu Y, et al. Associations of gestational and early life exposures to ambient air pollution with childhood respiratory diseases in Shanghai, China: a retrospective cohort study. Environ Int. 2016;92‐93:284–293. https://doi.org/10.1016/j.envint.2016.04.019
- 960. Wang IJ, Tung TH, Tang CS, Zhao ZH. Allergens, air pollutants, and childhood allergic diseases. Int J Hyg Environ Health. 2016;219(1):66–71. https://doi.org/10.1016/j.ijheh.2015.09.001
- 961. Kim HH, Lee CS, Yu SD, et al. Near‐road exposure and impact of air pollution on allergic diseases in elementary school children: a cross‐sectional study. Yonsei Med J. 2016;57(3):698–713. https://doi.org/10.3349/ymj.2016.57.3.698
- 962. Hur K, Liang J, Lin SY. The role of secondhand smoke in sinusitis: a systematic review. Int Forum Allergy Rhinol. 2014;4(1):22–28. https://doi.org/10.1002/alr.21232
- 963. Saulyte J, Regueira C, Montes‐Martinez A, Khudyakov P, Takkouche B. Active or passive exposure to tobacco smoking and allergic rhinitis, allergic dermatitis, and food allergy in adults and children: a systematic review and meta‐analysis. PLoS Med. 2014;11(3):e1001611. https://doi.org/10.1371/journal.pmed.1001611
- 964. Keil T, Lau S, Roll S, et al. Maternal smoking increases risk of allergic sensitization and wheezing only in children with allergic predisposition: longitudinal analysis from birth to 10 years. Allergy. 2009;64(3):445–451. https://doi.org/10.1111/j.1398‐9995.2008.01867.x
- 965. Lin SY, Reh DD, Clipp S, Irani L, Navas‐Acien A. Allergic rhinitis and secondhand tobacco smoke: a population‐based study. Am J Rhinol Allergy. 2011;25(2):e66–e71. https://doi.org/10.2500/ajra.2011.25.3580
- 966. Bendtsen P, Gronbaek M, Kjaer SK, Munk C, Linneberg A, Tolstrup JS. Alcohol consumption and the risk of self‐reported perennial and seasonal allergic rhinitis in young adult women in a population‐based cohort study. Clin Exp Allergy. 2008;38(7):1179–1185. https://doi.org/10.1111/j.1365‐2222.2008.02945.x
- 967. Gangl K, Reininger R, Bernhard D, et al. Cigarette smoke facilitates allergen penetration across respiratory epithelium. Allergy. 2009;64(3):398–405. https://doi.org/10.1111/j.1398‐9995.2008.01861.x
- 968. Ueha R, Ueha S, Kondo K, Nishijima H, Yamasoba T. Effects of cigarette smoke on the nasal respiratory and olfactory mucosa in allergic rhinitis mice. Front Neurosci. 2020;14:126. https://doi.org/10.3389/fnins.2020.00126
- 969. Mishra NC, Rir‐Sima‐Ah J, Langley RJ, et al. Nicotine primarily suppresses lung Th2 but not goblet cell and muscle cell responses to allergens. J Immunol. 2008;180(11):7655–7663. https://doi.org/10.4049/jimmunol.180.11.7655
- 970. Skaaby T, Taylor AE, Jacobsen RK, et al. Investigating the causal effect of smoking on hay fever and asthma: a Mendelian randomization meta‐analysis in the CARTA consortium. Sci Rep. 2017;7(1):2224. https://doi.org/10.1038/s41598‐017‐01977‐w
- 971. Zhou Y, Chen J, Dong Y, et al. Maternal tobacco exposure during pregnancy and allergic rhinitis in offspring: a systematic review and meta‐analysis. Medicine (Baltimore). 2021;100(34):e26986. https://doi.org/10.1097/MD.0000000000026986
- 972. Thacher JD, Gehring U, Gruzieva O, et al. Maternal smoking during pregnancy and early childhood and development of asthma and rhinoconjunctivitis – a MeDALL Project. Environ Health Perspect. 2018;126(4):047005. https://doi.org/10.1289/EHP2738
- 973. Ciprandi G, Silvestri M, Pistorio A, Tosca MA, Cirillo I. Clustering analysis in outpatients with allergic rhinitis in clinical practice. Allergy. 2019;74(3):607–610. https://doi.org/10.1111/all.13645
- 974. Chung SJ, Kim BK, Oh JH, et al. Novel tobacco products including electronic cigarette and heated tobacco products increase risk of allergic rhinitis and asthma in adolescents: analysis of Korean youth survey. Allergy. 2020;75(7):1640–1648. https://doi.org/10.1111/all.14212
- 975. Waite KJ. Blackley and the development of hay fever as a disease of civilization in the nineteenth century. Med Hist. 1995;39(2):186–196. https://doi.org/10.1017/s0025727300059834
- 976. Strachan DP. Family size, infection and atopy: the first decade of the “hygiene hypothesis”. Thorax. 2000;55(suppl 1):S2–S10. https://doi.org/10.1136/thorax.55.suppl_1.s2
- 977. Wee JH, Park MW, Min C, Park IS, Park B, Choi HG. The association between high hygiene scores and allergic rhinitis in Korean adolescents. Int Forum Allergy Rhinol. 2020;10(8):1024–1030. https://doi.org/10.1002/alr.22569
- 978. Chen JT, Krieger N, Van Den Eeden SK, Quesenberry CP. Different slopes for different folks: socioeconomic and racial/ethnic disparities in asthma and hay fever among 173,859 U.S. men and women. Environ Health Perspect. 2002;110(Suppl 2):211–216. https://doi.org/10.1289/ehp.02110s2211
- 979. Li F, Zhou Y, Li S, et al. Prevalence and risk factors of childhood allergic diseases in eight metropolitan cities in China: a multicenter study. BMC Public Health. 2011;11:437. https://doi.org/10.1186/1471‐2458‐11‐437
- 980. Hammer‐Helmich L, Linneberg A, Thomsen SF, Glumer C. Association between parental socioeconomic position and prevalence of asthma, atopic eczema and hay fever in children. Scand J Public Health. 2014;42(2):120–127. https://doi.org/10.1177/1403494813505727
- 981. Mercer MJ, Joubert G, Ehrlich RI, et al. Socioeconomic status and prevalence of allergic rhinitis and atopic eczema symptoms in young adolescents. Pediatr Allergy Immunol. 2004;15(3):234–241. https://doi.org/10.1111/j.1399‐3038.2004.00125.x
- 982. Talay F, Kurt B, Tug T, Kurt OK, Goksugur N, Yasar Z. The prevalence of asthma and allergic diseases among adults 30‐49 years of age in Bolu, Western Black Sea Region of Turkey. Clin Ter. 2014;165(1):e59–e63. https://doi.org/10.7471/CT.2014.1673
- 983. Lewis SA, Weiss ST, Platts‐Mills TA, Syring M, Gold DR. Association of specific allergen sensitization with socioeconomic factors and allergic disease in a population of Boston women. J Allergy Clin Immunol. 2001;107(4):615–622. https://doi.org/10.1067/mai.2001.113523
- 984. Almqvist C, Pershagen G, Wickman M. Low socioeconomic status as a risk factor for asthma, rhinitis and sensitization at 4 years in a birth cohort. Clin Exp Allergy. 2005;35(5):612–618. https://doi.org/10.1111/j.1365‐2222.2005.02243.x
- 985. Lee KS, Rha YH, Oh IH, Choi YS, Choi SH. Socioeconomic and sociodemographic factors related to allergic diseases in Korean adolescents based on the Seventh Korea Youth Risk Behavior Web‐based Survey: a cross‐sectional study. BMC Pediatr. 2016;16:19. https://doi.org/10.1186/s12887‐016‐0549‐2
- 986. Braback L, Hjern A, Rasmussen F. Social class in asthma and allergic rhinitis: a national cohort study over three decades. Eur Respir J. 2005;26(6):1064–1068. https://doi.org/10.1183/09031936.05.00022105
- 987. Penaranda A, Garcia E, Barragan AM, et al. Factors associated with allergic rhinitis in Colombian subpopulations aged 1 to 17 and 18 to 59. Rhinology. 2016;54(1):56–67. https://doi.org/10.4193/Rhino14.234
- 988. Bergmann RL, Edenharter G, Bergmann KE, Lau S, Wahn U. Socioeconomic status is a risk factor for allergy in parents but not in their children. Clin Exp Allergy. 2000;30(12):1740–1745. https://doi.org/10.1046/j.1365‐2222.2000.00927.x
- 989. Lewis SA, Britton JR. Consistent effects of high socioeconomic status and low birth order, and the modifying effect of maternal smoking on the risk of allergic disease during childhood. Respir Med. 1998;92(10):1237–1244. https://doi.org/10.1016/s0954‐6111(98)90427‐9
- 990. Goh DY, Chew FT, Quek SC, Lee BW. Prevalence and severity of asthma, rhinitis, and eczema in Singapore schoolchildren. Arch Dis Child. 1996;74(2):131–135. https://doi.org/10.1136/adc.74.2.131
- 991. Bion V, Lockett GA, Soto‐Ramirez N, et al. Evaluating the efficacy of breastfeeding guidelines on long‐term outcomes for allergic disease. Allergy. 2016;71(5):661–670. https://doi.org/10.1111/all.12833
- 992. Muraro A, Halken S, Arshad SH, et al. EAACI food allergy and anaphylaxis guidelines. Primary prevention of food allergy. Allergy. 2014;69(5):590–601. https://doi.org/10.1111/all.12398
- 993. Hoppu U, Kalliomaki M, Laiho K, Isolauri E. Breast milk – immunomodulatory signals against allergic diseases. Allergy. 2001;56(suppl 67):23–26. https://doi.org/10.1034/j.1398‐9995.2001.00908.x
- 994. Friedman NJ, Zeiger RS. The role of breast‐feeding in the development of allergies and asthma. J Allergy Clin Immunol. 2005;115(6):1238–1248. https://doi.org/10.1016/j.jaci.2005.01.069
- 995. Hoang MP, Samuthpongtorn J, Seresirikachorn K, Snidvongs K. Prolonged breastfeeding and protective effects against the development of allergic rhinitis: a systematic review and meta‐analysis. Rhinology. 2022;60(2):82–91. https://doi.org/10.4193/Rhin21.274
- 996. Gungor D, Nadaud P, LaPergola CC, et al. Infant milk‐feeding practices and food allergies, allergic rhinitis, atopic dermatitis, and asthma throughout the life span: a systematic review. Am J Clin Nutr. 2019;109(suppl_7):772S–799S. https://doi.org/10.1093/ajcn/nqy283
- 997. Codispoti CD, Levin L, LeMasters GK, et al. Breast‐feeding, aeroallergen sensitization, and environmental exposures during infancy are determinants of childhood allergic rhinitis. J Allergy Clin Immunol. 2010;125(5):1054–1060.e1. https://doi.org/10.1016/j.jaci.2010.02.004
- 998. Huang C, Liu W, Cai J, et al. Breastfeeding and timing of first dietary introduction in relation to childhood asthma, allergies, and airway diseases: a cross‐sectional study. J Asthma. 2017;54(5):488–497. https://doi.org/10.1080/02770903.2016.1231203
- 999. Han DH, Shin JM, An S, et al. Long‐term breastfeeding in the prevention of allergic rhinitis: Allergic Rhinitis Cohort Study for Kids (ARCO‐Kids Study). Clin Exp Otorhinolaryngol. 2019;12(3):301–307. https://doi.org/10.21053/ceo.2018.01781
- 1000. Ek WE, Karlsson T, Hernandes CA, Rask‐Andersen M, Johansson A. Breast‐feeding and risk of asthma, hay fever, and eczema. J Allergy Clin Immunol. 2018;141(3):1157–1159.e9. https://doi.org/10.1016/j.jaci.2017.10.022
- 1001. Heinrich J. Modulation of allergy risk by breast feeding. Curr Opin Clin Nutr Metab Care. 2017;20(3):217–221. https://doi.org/10.1097/MCO.0000000000000366
- 1002. Nuzzi G, Di Cicco ME, Peroni DG. Breastfeeding and allergic diseases: what's new? Children (Basel). 2021;8(5):330. https://doi.org/10.3390/children8050330
- 1003. Lodge CJ, Tan DJ, Lau MX, et al. Breastfeeding and asthma and allergies: a systematic review and meta‐analysis. Acta Paediatr. 2015;104(467):38–53. https://doi.org/10.1111/apa.13132
- 1004. Brozek JL, Bousquet J, Baena‐Cagnani CE, et al. Allergic Rhinitis and its Impact on Asthma (ARIA) guidelines: 2010 revision. J Allergy Clin Immunol. 2010;126(3):466–476. https://doi.org/10.1016/j.jaci.2010.06.047
- 1005. Seidman MD, Gurgel RK, Lin SY, et al. Clinical practice guideline: allergic rhinitis. Otolaryngol Head Neck Surg. 2015;152(1 suppl):S1–S43. https://doi.org/10.1177/0194599814561600
- 1006. Lodge CJ, Lowe AJ, Gurrin LC, et al. Pets at birth do not increase allergic disease in at‐risk children. Clin Exp Allergy. 2012;42(9):1377–1385. https://doi.org/10.1111/j.1365‐2222.2012.04032.x
- 1007. Takkouche B, Gonzalez‐Barcala FJ, Etminan M, Fitzgerald M. Exposure to furry pets and the risk of asthma and allergic rhinitis: a meta‐analysis. Allergy. 2008;63(7):857–864. https://doi.org/10.1111/j.1398‐9995.2008.01732.x
- 1008. Lodge CJ, Allen KJ, Lowe AJ, et al. Perinatal cat and dog exposure and the risk of asthma and allergy in the urban environment: a systematic review of longitudinal studies. Clin Dev Immunol. 2012;2012:176484. https://doi.org/10.1155/2012/176484
- 1009. Luo S, Sun Y, Hou J, et al. Pet keeping in childhood and asthma and allergy among children in Tianjin area, China. PLoS One. 2018;13(5):e0197274. https://doi.org/10.1371/journal.pone.0197274
- 1010. Chen CM, Heinrich J. Re: exposure to furry pets and the risk of asthma and allergic rhinitis: a meta‐analysis. Allergy. 2009;64(3):494–495. https://doi.org/10.1111/j.1398‐9995.2008.01930.x
- 1011. Holt PG, Sly PD. Non‐atopic intrinsic asthma and the ‘family tree’ of chronic respiratory disease syndromes. Clin Exp Allergy. 2009;39(6):807–811. https://doi.org/10.1111/j.1365‐2222.2009.03258.x
- 1012. Dharmage SC, Lodge CL, Matheson MC, Campbell B, Lowe AJ. Exposure to cats: update on risks for sensitization and allergic diseases. Curr Allergy Asthma Rep. 2012;12(5):413–423. https://doi.org/10.1007/s11882‐012‐0288‐x
- 1013. Strachan DP. Hay fever, hygiene, and household size. BMJ. 1989;299(6710):1259–1260. https://doi.org/10.1136/bmj.299.6710.1259
- 1014. von Hertzen L, Hanski I, Haahtela T. Natural immunity. Biodiversity loss and inflammatory diseases are two global megatrends that might be related. EMBO Rep. 2011;12(11):1089–1093. https://doi.org/10.1038/embor.2011.195
- 1015. Karmaus W, Botezan C. Does a higher number of siblings protect against the development of allergy and asthma? A review. J Epidemiol Community Health. 2002;56(3):209–217. https://doi.org/10.1136/jech.56.3.209
- 1016. Strachan DP, Ait‐Khaled N, Foliaki S, et al. Siblings, asthma, rhinoconjunctivitis and eczema: a worldwide perspective from the International Study of Asthma and Allergies in Childhood. Clin Exp Allergy. 2015;45(1):126–136. https://doi.org/10.1111/cea.12349
- 1017. Campbell BE, Lodge CJ, Lowe AJ, Burgess JA, Matheson MC, Dharmage SC. Exposure to ‘farming’ and objective markers of atopy: a systematic review and meta‐analysis. Clin Exp Allergy. 2015;45(4):744–757. https://doi.org/10.1111/cea.12429
- 1018. House JS, Wyss AB, Hoppin JA, et al. Early‐life farm exposures and adult asthma and atopy in the Agricultural Lung Health Study. J Allergy Clin Immunol. 2017;140(1):249–256.e14. https://doi.org/10.1016/j.jaci.2016.09.036
- 1019. Riedler J, Eder W, Oberfeld G, Schreuer M. Austrian children living on a farm have less hay fever, asthma and allergic sensitization. Clin Exp Allergy. 2000;30(2):194–200. https://doi.org/10.1046/j.1365‐2222.2000.00799.x
- 1020. Von Ehrenstein OS, Von Mutius E, Illi S, Baumann L, Bohm O, von Kries R. Reduced risk of hay fever and asthma among children of farmers. Clin Exp Allergy. 2000;30(2):187–193. https://doi.org/10.1046/j.1365‐2222.2000.00801.x
- 1021. Riedler J, Braun‐Fahrlander C, Eder W, et al. Exposure to farming in early life and development of asthma and allergy: a cross‐sectional survey. Lancet. 2001;358(9288):1129–1133. https://doi.org/10.1016/S0140‐6736(01)06252‐3
- 1022. Barnes M, Cullinan P, Athanasaki P, et al. Crete: does farming explain urban and rural differences in atopy? Clin Exp Allergy. 2001;31(12):1822–1828. https://doi.org/10.1046/j.1365‐2222.2001.01240.x
- 1023. Downs SH, Marks GB, Mitakakis TZ, Leuppi JD, Car NG, Peat JK. Having lived on a farm and protection against allergic diseases in Australia. Clin Exp Allergy. 2001;31(4):570–575. https://doi.org/10.1046/j.1365‐2222.2001.01070.x
- 1024. Wickens K, Lane JM, Fitzharris P, et al. Farm residence and exposures and the risk of allergic diseases in New Zealand children. Allergy. 2002;57(12):1171–1179. https://doi.org/10.1034/j.1398‐9995.2002.t01‐1‐23644.x
- 1025. Remes ST, Pekkanen J, Soininen L, Kajosaari M, Husman T, Koivikko A. Does heredity modify the association between farming and allergy in children? Acta Paediatr. 2002;91(11):1163–1169. https://doi.org/10.1111/j.1651‐2227.2002.tb00122.x
- 1026. Remes ST, Iivanainen K, Koskela H, Pekkanen J. Which factors explain the lower prevalence of atopy amongst farmers' children? Clin Exp Allergy. 2003;33(4):427–434. https://doi.org/10.1046/j.1365‐2222.2003.01566.x
- 1027. Martinez FD, Holt PG. Role of microbial burden in aetiology of allergy and asthma. Lancet. 1999;354(suppl 2):SII12–SII15. https://doi.org/10.1016/s0140‐6736(99)90437‐3
- 1028. Simpson A, Martinez FD. The role of lipopolysaccharide in the development of atopy in humans. Clin Exp Allergy. 2010;40(2):209–223. https://doi.org/10.1111/j.1365‐2222.2009.03391.x
- 1029. Tischer C, Gehring U, Chen CM, et al. Respiratory health in children, and indoor exposure to (1,3)‐beta‐D‐glucan, EPS mould components and endotoxin. Eur Respir J. 2011;37(5):1050–1059. https://doi.org/10.1183/09031936.00091210
- 1030. Arrieta MC, Stiemsma LT, Dimitriu PA, et al. Early infancy microbial and metabolic alterations affect risk of childhood asthma. Sci Transl Med. 2015;7(307):307ra152. https://doi.org/10.1126/scitranslmed.aab2271
- 1031. Hanski I, von Hertzen L, Fyhrquist N, et al. Environmental biodiversity, human microbiota, and allergy are interrelated. Proc Natl Acad Sci U S A. 2012;109(21):8334–8339. https://doi.org/10.1073/pnas.1205624109
- 1032. Fyhrquist N, Ruokolainen L, Suomalainen A, et al. Acinetobacter species in the skin microbiota protect against allergic sensitization and inflammation. J Allergy Clin Immunol. 2014;134(6):1301–1309. e11. https://doi.org/10.1016/j.jaci.2014.07.059
- 1033. Stsepetova J, Sepp E, Julge K, Vaughan E, Mikelsaar M, de Vos WM. Molecularly assessed shifts of Bifidobacterium ssp. and less diverse microbial communities are characteristic of 5‐year‐old allergic children. FEMS Immunol Med Microbiol. 2007;51(2):260–269. https://doi.org/10.1111/j.1574‐695X.2007.00306.x
- 1034. Cuello‐Garcia CA, Brozek JL, Fiocchi A, et al. Probiotics for the prevention of allergy: a systematic review and meta‐analysis of randomized controlled trials. J Allergy Clin Immunol. 2015;136(4):952–961. https://doi.org/10.1016/j.jaci.2015.04.031
- 1035. Holster IL, Vila AM, Caudri D, et al. The impact of Helicobacter pylori on atopic disorders in childhood. Helicobacter. 2012;17(3):232–237. https://doi.org/10.1111/j.1523‐5378.2012.00934.x
- 1036. Akiner U, Yener HM, Gozen ED, Kuzu SB, Canakcioglu S. Helicobacter pylori in allergic and non‐allergic rhinitis does play a protective or causative role? Eur Arch Otorhinolaryngol. 2020;277(1):141–145. https://doi.org/10.1007/s00405‐019‐05659‐3
- 1037. Lionetti E, Leonardi S, Lanzafame A, et al. Helicobacter pylori infection and atopic diseases: is there a relationship? A systematic review and meta‐analysis. World J Gastroenterol. 2014;20(46):17635–17647. https://doi.org/10.3748/wjg.v20.i46.17635
- 1038. Ruokolainen L, Paalanen L, Karkman A, et al. Significant disparities in allergy prevalence and microbiota between the young people in Finnish and Russian Karelia. Clin Exp Allergy. 2017;47(5):665–674. https://doi.org/10.1111/cea.12895
- 1039. Valkonen M, Wouters IM, Taubel M, et al. Bacterial exposures and associations with atopy and asthma in children. PLoS One. 2015;10(6):e0131594. https://doi.org/10.1371/journal.pone.0131594
- 1040. Ege MJ, Mayer M, Normand AC, et al. Exposure to environmental microorganisms and childhood asthma. N Engl J Med. 2011;364(8):701–709. https://doi.org/10.1056/NEJMoa1007302
- 1041. von Hertzen L, Laatikainen T, Pitkanen T, et al. Microbial content of drinking water in Finnish and Russian Karelia – implications for atopy prevalence. Allergy. 2007;62(3):288–292. https://doi.org/10.1111/j.1398‐9995.2006.01281.x
- 1042. Chen R, Zheng D, Zhang Y, Sima G. Efficacy and safety of twice‐daily olopatadine‐mometasone combination nasal spray (GSP301) in the treatment of allergic rhinitis: a systematic review and meta‐analysis. Eur Arch Otorhinolaryngol. 2022;279(4):1691–1699. https://doi.org/10.1007/s00405‐021‐07085‐w
- 1043. Zhang K, Li AR, Miglani A, Nguyen SA, Schlosser RJ. Effect of medical therapy in allergic rhinitis: a systematic review and meta‐analysis. Am J Rhinol Allergy. 2022;36(2):269–280. https://doi.org/10.1177/19458924211041438
- 1044. Li AR, Zhang K, Reddy PD, et al. Systematic review of measures of disease severity in rhinitis. Int Forum Allergy Rhinol. 2021;11(9):1367–1377. https://doi.org/10.1002/alr.22794
- 1045. Calderon MA, Casale TB, Demoly P. Validation of Patient‐reported outcomes for clinical trials in allergic rhinitis: a systematic review. J Allergy Clin Immunol Pract. 2019;7(5):1450–1461.e6. https://doi.org/10.1016/j.jaip.2019.01.015
- 1046. Linneberg A, Dam Petersen K, Hahn‐Pedersen J, Hammerby E, Serup‐Hansen N, Boxall N. Burden of allergic respiratory disease: a systematic review. Clin Mol Allergy. 2016;14:12. https://doi.org/10.1186/s12948‐016‐0049‐9
- 1047. Hahn‐Pedersen J, Boxall N, Maier W, Linneberg A, Serup‐Hansen N. Systematic literature review assessing data on the burden of allergic rhinitis from a cost and quality of life perspective. Value Health. 2014;17(7):A602. https://doi.org/10.1016/j.jval.2014.08.2087
- 1048. Juniper EF, Guyatt GH. Development and testing of a new measure of health status for clinical trials in rhinoconjunctivitis. Clin Exp Allergy. 1991;21(1):77–83. https://doi.org/10.1111/j.1365‐2222.1991.tb00807.x
- 1049. McHorney CA, Ware JE Jr., Lu JF, Sherbourne CD. The MOS 36‐item Short‐Form Health Survey (SF‐36): III. Tests of data quality, scaling assumptions, and reliability across diverse patient groups. Med Care. 1994;32(1):40–66. https://doi.org/10.1097/00005650‐199401000‐00004
- 1050. Ware J Jr., Kosinski M, Keller SD. A 12‐Item Short‐Form Health Survey: construction of scales and preliminary tests of reliability and validity. Med Care. 1996;34(3):220–233. https://doi.org/10.1097/00005650‐199603000‐00003
- 1051. Husain Q, Hoehle L, Phillips K, Caradonna DS, Gray ST, Sedaghat AR. The 22‐Item Sinonasal Outcome Test as a tool for the assessment of quality of life and symptom control in allergic rhinitis. Am J Rhinol Allergy. 2020;34(2):209–216. https://doi.org/10.1177/1945892419884789
- 1052. Passali FM, Passali GC, Passali D, Ciprandi G. Smell impairment in patients with allergic rhinitis. Int Forum Allergy Rhinol. 2021;11(6):1031–1032. https://doi.org/10.1002/alr.22786
- 1053. Yamada T, Yamamoto H, Kubo S, et al. Efficacy of mometasone furoate nasal spray for nasal symptoms, quality of life, rhinitis‐disturbed sleep, and nasal nitric oxide in patients with perennial allergic rhinitis. Allergy Asthma Proc. 2012;33(2):e9–e16. https://doi.org/10.2500/aap.2012.33.3509
- 1054. Bousquet J, Zuberbier T, Canonica GW, Fokkens WJ, Gopalan G, Shekar T. Randomized controlled trial of desloratadine for persistent allergic rhinitis: correlations between symptom improvement and quality of life. Allergy Asthma Proc. 2013;34(3):274–282. https://doi.org/10.2500/aap.2013.34.3668
- 1055. Holmberg K, Tonnel AB, Dreyfus I, et al. Desloratadine relieves nasal congestion and improves quality‐of‐life in persistent allergic rhinitis. Allergy. 2009;64(11):1663–1670. https://doi.org/10.1111/j.1398‐9995.2009.02096.x
- 1056. Walter Canonica G, Bousquet J, Van Hammee G, et al. Levocetirizine improves health‐related quality of life and health status in persistent allergic rhinitis. Respir Med. 2006;100(10):1706–1715. https://doi.org/10.1016/j.rmed.2006.03.039
- 1057. Bachert C, Bousquet J, Canonica GW, et al. Levocetirizine improves quality of life and reduces costs in long‐term management of persistent allergic rhinitis. J Allergy Clin Immunol. 2004;114(4):838–844. https://doi.org/10.1016/j.jaci.2004.05.070
- 1058. Pedregal‐Mallo D, Pacheco E, Rodrigo JP, Llorente JL, Alvarez‐Marcos C. Impact of immunotherapy on quality of life in patients with house dust mite allergic rhinitis. Allergy. 2020;75(7):1783–1785. https://doi.org/10.1111/all.14215
- 1059. Hoiby AS, Strand V, Robinson DS, Sager A, Rak S. Efficacy, safety, and immunological effects of a 2‐year immunotherapy with Depigoid birch pollen extract: a randomized, double‐blind, placebo‐controlled study. Clin Exp Allergy. 2010;40(7):1062–1070. https://doi.org/10.1111/j.1365‐2222.2010.03521.x
- 1060. Di Rienzo V, Pucci S, D'Alo S, et al. Effects of high‐dose sublingual immunotherapy on quality of life in patients with cypress‐induced rhinitis: a placebo‐controlled study. Clin Exp Allergy Reviews. 2006;6(3):67–70.
- 1061. Colas C, Monzon S, Venturini M, Lezaun A. Double‐blind, placebo‐controlled study with a modified therapeutic vaccine of Salsola kali (Russian thistle) administered through use of a cluster schedule. J Allergy Clin Immunol. 2006;117(4):810–816. https://doi.org/10.1016/j.jaci.2005.11.039
- 1062. Juel‐Berg N, Darling P, Bolvig J, et al. Intranasal corticosteroids compared with oral antihistamines in allergic rhinitis: a systematic review and meta‐analysis. Am J Rhinol Allergy. 2017;31(1):19–28. https://doi.org/10.2500/ajra.2016.30.4397
- 1063. Tatar EC, Surenoglu UA, Ozdek A, Saylam G, Korkmaz H. The effect of combined medical treatment on quality of life in persistent allergic rhinitis. Indian J Otolaryngol Head Neck Surg. 2013;65(suppl 2):333–337. https://doi.org/10.1007/s12070‐012‐0486‐9
- 1064. Fasola S, Montalbano L, Ferrante G, et al. RAPP‐children: a new tool for assessing quality of life in patients with asthma and rhinitis. Clin Exp Allergy. 2020;50(6):662–671. https://doi.org/10.1111/cea.13599
- 1065. Bosnic‐Anticevich S, Smith P, Abramson M, et al. Impact of allergic rhinitis on the day‐to‐day lives of children: insights from an Australian cross‐sectional study. BMJ Open. 2020;10(11):e038870. https://doi.org/10.1136/bmjopen‐2020‐038870
- 1066. Hwang TY, Kim SK, Kim SH, Kim M. A cross sectional survey on health‐related quality of life among parents of children with allergic symptoms using the EQ‐5D‐5L. J Asthma. 2019;56(11):1239–1245. https://doi.org/10.1080/02770903.2019.1571086
- 1067. Valls‐Mateus M, Marino‐Sanchez F, Ruiz‐Echevarria K, et al. Nasal obstructive disorders impair health‐related quality of life in adolescents with persistent allergic rhinitis: a real‐life study. Pediatr Allergy Immunol. 2017;28(5):438–445. https://doi.org/10.1111/pai.12724
- 1068. Sikorska‐Szaflik H, Sozanska B. Quality of life in allergic rhinitis – children's and their parents' perspective in polish urban and rural population. Health Qual Life Outcomes. 2020;18(1):64. https://doi.org/10.1186/s12955‐020‐01315‐1
- 1069. Ravens‐Sieberer U, Bullinger M. Assessing health‐related quality of life in chronically ill children with the German KINDL: first psychometric and content analytical results. Qual Life Res. 1998;7(5):399–407. https://doi.org/10.1023/a:1008853819715
- 1070. Juniper EF, Howland WC, Roberts NB, Thompson AK, King DR. Measuring quality of life in children with rhinoconjunctivitis. J Allergy Clin Immunol. 1998;101(2 pt 1):163–170. https://doi.org/10.1016/s0091‐6749(98)70380‐x
- 1071. Segall N, Prenner B, Lumry W, Caracta CF, Tantry SK. Long‐term safety and efficacy of olopatadine‐mometasone combination nasal spray in patients with perennial allergic rhinitis. Allergy Asthma Proc. 2019;40(5):301–310. https://doi.org/10.2500/aap.2019.40.4233
- 1072. Majani G, Baiardini I, Giardini A, et al. Health‐related quality of life assessment in young adults with seasonal allergic rhinitis. Allergy. 2001;56(4):313–317. https://doi.org/10.1034/j.1398‐9995.2001.00852.x
- 1073. Aruthra R, Kumar M. To study the impact of allergic rhinitis on quality of life in a tertiary care hospital. Intern J Cur Res Rev. 2021;13(2):118–120.
- 1074. Zhu R, Wang J, Wu Y, et al. The Allergic Rhinitis Control Test Questionnaire is valuable in guiding step‐down pharmacotherapy treatment of allergic rhinitis. J Allergy Clin Immunol Pract. 2019;7(1):272–278. https://doi.org/10.1016/j.jaip.2018.05.028
- 1075. Bousquet J, Arnavielhe S, Bedbrook A, et al. The Allergic Rhinitis and its Impact on Asthma (ARIA) score of allergic rhinitis using mobile technology correlates with quality of life: The MASK study. Allergy. 2018;73(2):505–510. https://doi.org/10.1111/all.13307
- 1076. Hoehle LP, Speth MM, Phillips KM, et al. Association between symptoms of allergic rhinitis with decreased general health‐related quality of life. Am J Rhinol Allergy. 2017;31(4):235–239. https://doi.org/10.2500/ajra.2017.31.4444
- 1077. Filanowicz M, Szynkiewicz E, Cegla B, Bartuzi Z. Analysis of the quality of life of patients with asthma and allergic rhinitis after immunotherapy. Postepy Dermatol Alergol. 2016;33(2):134–141. https://doi.org/10.5114/pdia.2015.48061
- 1078. Jaruvongvanich V, Mongkolpathumrat P, Chantaphakul H, Klaewsongkram J. Extranasal symptoms of allergic rhinitis are difficult to treat and affect quality of life. Allergol Int. 2016;65(2):199–203. https://doi.org/10.1016/j.alit.2015.11.006
- 1079. Song Y, Wang M, Xie J, et al. Prevalence of allergic rhinitis among elementary and middle school students in Changsha city and its impact on quality of life. J Laryngol Otol. 2015;129(11):1108–1114. https://doi.org/10.1017/S0022215115002492
- 1080. Bousquet PJ, Demoly P, Devillier P, Mesbah K, Bousquet J. Impact of allergic rhinitis symptoms on quality of life in primary care. Int Arch Allergy Immunol. 2013;160(4):393–400. https://doi.org/10.1159/000342991
- 1081. Katelaris CH, Sacks R, Theron PN. Allergic rhinoconjunctivitis in the Australian population: burden of disease and attitudes to intranasal corticosteroid treatment. Am J Rhinol Allergy. 2013;27(6):506–509. https://doi.org/10.2500/ajra.2013.27.3965
- 1082. de la Hoz Caballer B, Rodriguez M, Fraj J, Cerecedo I, Antolin‐Amerigo D, Colas C. Allergic rhinitis and its impact on work productivity in primary care practice and a comparison with other common diseases: the Cross‐sectional study to evAluate work Productivity in allergic Rhinitis compared with other common dIseases (CAPRI) study. Am J Rhinol Allergy. 2012;26(5):390–394. https://doi.org/10.2500/ajra.2012.26.3799
- 1083. Meltzer EO, Gross GN, Katial R, Storms WW. Allergic rhinitis substantially impacts patient quality of life: findings from the Nasal Allergy Survey Assessing Limitations. J Fam Pract. 2012;61(2 suppl):S5–S10.
- 1084. Stull DE, Schaefer M, Crespi S, Sandor DW. Relative strength of relationships of nasal congestion and ocular symptoms with sleep, mood and productivity. Curr Med Res Opin. 2009;25(7):1785–1792. https://doi.org/10.1185/03007990903021968
- 1085. Witt CM, Reinhold T, Jena S, Brinkhaus B, Willich SN. Cost‐effectiveness of acupuncture in women and men with allergic rhinitis: a randomized controlled study in usual care. Am J Epidemiol. 2009;169(5):562–571. https://doi.org/10.1093/aje/kwn370
- 1086. Brinkhaus B, Witt CM, Jena S, Liecker B, Wegscheider K, Willich SN. Acupuncture in patients with allergic rhinitis: a pragmatic randomized trial. Ann Allergy Asthma Immunol. 2008;101(5):535–543. https://doi.org/10.1016/S1081‐1206(10)60294‐3
- 1087. Petersen KD, Kronborg C, Gyrd‐Hansen D, Dahl R, Larsen JN, Lowenstein H. Quality of life in rhinoconjunctivitis assessed with generic and disease‐specific questionnaires. Allergy. 2008;63(3):284–291. https://doi.org/10.1111/j.1398‐9995.2007.01583.x
- 1088. Ciprandi G, Klersy C, Cirillo I, Marseglia GL. Quality of life in allergic rhinitis: relationship with clinical, immunological, and functional aspects. Clin Exp Allergy. 2007;37(10):1528–1535. https://doi.org/10.1111/j.1365‐2222.2007.02809.x
- 1089. Radcliffe MJ, Lewith GT, Turner RG, Prescott P, Church MK, Holgate ST. Enzyme potentiated desensitisation in treatment of seasonal allergic rhinitis: double blind randomised controlled study. BMJ. 2003;327(7409):251–254. https://doi.org/10.1136/bmj.327.7409.251
- 1090. Gerth Van Wijk R, Terreehorst IT, Mulder PG, Garrelds IM, Blom HM, Popering S. Intranasal capsaicin is lacking therapeutic effect in perennial allergic rhinitis to house dust mite. A placebo‐controlled study. Clin Exp Allergy. 2000;30(12):1792–1798. https://doi.org/10.1046/j.1365‐2222.2000.00920.x
- 1091. Leynaert B, Neukirch C, Liard R, Bousquet J, Neukirch F. Quality of life in allergic rhinitis and asthma. A population‐based study of young adults. Am J Respir Crit Care Med. 2000;162(4 pt 1):1391–1396. https://doi.org/10.1164/ajrccm.162.4.9912033
- 1092. Cuesta‐Herranz J, Laguna JJ, Mielgo R, et al. Quality of life improvement with allergen immunotherapy treatment in patients with rhinoconjunctivitis in real life conditions. Results of an observational prospective study (ICARA). Eur Ann Allergy Clin Immunol. 2019;51(5). https://doi.org/10.23822/EurAnnACI.1764‐1489.104
- 1093. Gillman GS, Staltari GV, Chang YF, Mattos JL. A prospective study of outcomes of septoplasty with turbinate reductions in patients with allergic rhinitis. Otolaryngol Head Neck Surg. 2019;160(6):1118–1123. https://doi.org/10.1177/0194599819838761
- 1094. Baiardini I, Fasola S, Montalbano L, et al. RHINASTHMA‐Children: a new quality of life tool for patients with respiratory allergy. Pediatr Allergy Immunol. 2017;28(1):102–105. https://doi.org/10.1111/pai.12667
- 1095. Novakova SM, Staevska MT, Novakova PI, et al. Quality of life improvement after a three‐year course of sublingual immunotherapy in patients with house dust mite and grass pollen induced allergic rhinitis: results from real‐life. Health Qual Life Outcomes. 2017;15(1):189. https://doi.org/10.1186/s12955‐017‐0764‐z
- 1096. Schwanke T, Carragee E, Bremberg M, Reisacher WR. Quality‐of‐life outcomes in patients who underwent subcutaneous immunotherapy and sublingual immunotherapy in a real‐world clinical setting. Am J Rhinol Allergy. 2017;31(5):310–316. https://doi.org/10.2500/ajra.2017.31.4465
- 1097. Bukstein D, Parikh R, Eid S, Ferro T, Morello JP. Beclomethasone Dipropionate Nasal Aerosol in Patients with Perennial Allergic Rhinitis (BALANCE) study: 6‐month results. Allergy Asthma Proc. 2016;37(2):121–130. https://doi.org/10.2500/aap.2016.37.3939
- 1098. Cingi C, Oghan F, Eskiizmir G, Yaz A, Ural A, Erdogmus N. Desloratadine‐montelukast combination improves quality of life and decreases nasal obstruction in patients with perennial allergic rhinitis. Int Forum Allergy Rhinol. 2013;3(10):801–806. https://doi.org/10.1002/alr.21185
- 1099. Demoly P, Bousquet PJ, Mesbah K, Bousquet J, Devillier P. Visual analogue scale in patients treated for allergic rhinitis: an observational prospective study in primary care: asthma and rhinitis. Clin Exp Allergy. 2013;43(8):881–888. https://doi.org/10.1111/cea.12121
- 1100. Ciprandi G, Cadario G, Valle C, et al. Sublingual immunotherapy in polysensitized patients: effect on quality of life. J Investig Allergol Clin Immunol. 2010;20(4):274–279.
- 1101. Cadario G, Ciprandi G, Di Cara G, et al. Comparison between continuous or intermittent schedules of sublingual immunotherapy for house dust mites: effects on compliance, patients satisfaction, quality of life and safety. Int J Immunopathol Pharmacol. 2008;21(2):471–473. https://doi.org/10.1177/039463200802100229
- 1102. Laforest L, Bousquet J, Neukirch F, et al. Influence of sociodemographic factors on quality of life during pollen season in seasonal allergic rhinitis patients. Ann Allergy Asthma Immunol. 2005;95(1):26–32. https://doi.org/10.1016/S1081‐1206(10)61184‐2
- 1103. Zheng M, Wang X, Ge S, et al. Allergic and non‐allergic rhinitis are common in obstructive sleep apnea but not associated with disease severity. J Clin Sleep Med. 2017;13(8):959–966. https://doi.org/10.5664/jcsm.6694
- 1104. Liu J, Wu Y, Wu P, Xu Z, Ni X. Analysis of the impact of allergic rhinitis on the children with sleep disordered breathing. Int J Pediatr Otorhinolaryngol. 2020;138:110380. https://doi.org/10.1016/j.ijporl.2020.110380
- 1105. Shanqun L, Shenyuan L, Zhou J, Bai C. The role of montelukast and intranasal budesonide on OSAHS and allergic rhinitis. Allergy. 2009;64:591.
- 1106. Gurevich F, Glass C, Davies M, et al. The effect of intranasal steroid budesonide on the congestion‐related sleep disturbance and daytime somnolence in patients with perennial allergic rhinitis. Allergy Asthma Proc. 2005;26(4):268–274.
- 1107. Hughes K, Glass C, Ripchinski M, et al. Efficacy of the topical nasal steroid budesonide on improving sleep and daytime somnolence in patients with perennial allergic rhinitis. Allergy. 2003;58(5):380–385. https://doi.org/10.1034/j.1398‐9995.2003.00093.x
- 1108. Craig TJ, Teets S, Lehman EB, Chinchilli VM, Zwillich C. Nasal congestion secondary to allergic rhinitis as a cause of sleep disturbance and daytime fatigue and the response to topical nasal corticosteroids. J Allergy Clin Immunol. 1998;101(5):633–637. https://doi.org/10.1016/s0091‐6749(98)70171‐x
- 1109. Mansfield LE, Posey CR. Daytime sleepiness and cognitive performance improve in seasonal allergic rhinitis treated with intranasal fluticasone propionate. Allergy Asthma Proc. 2007;28(2):226–229. https://doi.org/10.2500/aap.2007.28.2950
- 1110. Bilgilisoy Filiz M, Filiz S, Baran RT, et al. Restless legs syndrome in children with allergic rhinitis: a comparative study on frequency, severity and sleep quality. Turk J Phys Med Rehabil. 2018;64(3):198–204. https://doi.org/10.5606/tftrd.2018.2265
- 1111. Kim DK, Han DH. Impact of allergic rhinitis on quality of life after adenotonsillectomy for pediatric sleep‐disordered breathing. Int Forum Allergy Rhinol. 2015;5(8):741–746. https://doi.org/10.1002/alr.21529
- 1112. Lai PH, Yang PS, Lai WY, Lin CL, Hsu CY, Wei CC. Allergic rhinitis and the associated risk of nocturnal enuresis in children: a population‐based cohort study. Int Forum Allergy Rhinol. 2018;8(11):1260–1266. https://doi.org/10.1002/alr.22219
- 1113. Krouse HJ, Davis JE, Krouse JH. Immune mediators in allergic rhinitis and sleep. Otolaryngol Head Neck Surg. 2002;126(6):607–613. https://doi.org/10.1067/mhn.2002.125300
- 1114. McNicholas WT, Tarlo S, Cole P, et al. Obstructive apneas during sleep in patients with seasonal allergic rhinitis. Am Rev Respir Dis. 1982;126(4):625–628. https://doi.org/10.1164/arrd.1982.126.4.625
- 1115. Acar M, Cingi C, Sakallioglu O, San T, Fatih Yimenicioglu M, Bal C. The effects of mometasone furoate and desloratadine in obstructive sleep apnea syndrome patients with allergic rhinitis. Am J Rhinol Allergy. 2013;27(4):e113–e116. https://doi.org/10.2500/ajra.2013.27.3921
- 1116. Young T, Finn L, Kim H. Nasal obstruction as a risk factor for sleep‐disordered breathing. The University of Wisconsin Sleep and Respiratory Research Group. J Allergy Clin Immunol. 1997;99(2):S757–S762. https://doi.org/10.1016/s0091‐6749(97)70124‐6
- 1117. Lavigne F, Petrof BJ, Johnson JR, et al. Effect of topical corticosteroids on allergic airway inflammation and disease severity in obstructive sleep apnoea. Clin Exp Allergy. 2013;43(10):1124–1133. https://doi.org/10.1111/cea.12158
- 1118. Lavie P, Gertner R, Zomer J, Podoshin L. Breathing disorders in sleep associated with "microarousals' in patients with allergic rhinitis. Acta Otolaryngol. 1981;92(5‐6):529–533. https://doi.org/10.3109/00016488109133292
- 1119. Berson SR, Klimczak J, Prezio EA, Hu S, Abraham M. Clinical associations between allergies and rapid eye movement sleep disturbances. Int Forum Allergy Rhinol. 2018;8(7):817–824. https://doi.org/10.1002/alr.22099
- 1120. Liu J, Zhang X, Zhao Y, Wang Y. The association between allergic rhinitis and sleep: a systematic review and meta‐analysis of observational studies. PLoS One. 2020;15(2):e0228533. https://doi.org/10.1371/journal.pone.0228533
- 1121. Berson SR, Klimczak JA, Prezio EA, Abraham MT. House dust mite related allergic rhinitis and REM sleep disturbances. Am J Otolaryngol. 2020;41(6):102709. https://doi.org/10.1016/j.amjoto.2020.102709
- 1122. Pace A, Iannella G, Rossetti V, et al. Diagnosis of obstructive sleep apnea in patients with allergic and non‐allergic rhinitis. Medicina (Kaunas). 2020;56(9):454. https://doi.org/10.3390/medicina56090454
- 1123. Meng J, Xuan J, Qiao X, et al. Assessment of sleep impairment in persistent allergic rhinitis patients using polysomnography. Int Arch Allergy Immunol. 2011;155(1):57–62. https://doi.org/10.1159/000317244
- 1124. Bozkurt B, Serife Ugur K, Karamanli H, Kucuker F, Ozol D. Polysomnographic findings in persistent allergic rhinitis. Sleep Breath. 2017;21(2):255–261. https://doi.org/10.1007/s11325‐016‐1390‐4
- 1125. Thompson A, Sardana N, Craig TJ. Sleep impairment and daytime sleepiness in patients with allergic rhinitis: the role of congestion and inflammation. Ann Allergy Asthma Immunol. 2013;111(6):446–451. https://doi.org/10.1016/j.anai.2013.05.020
- 1126. Rimmer J, Downie S, Bartlett DJ, Gralton J, Salome C. Sleep disturbance in persistent allergic rhinitis measured using actigraphy. Ann Allergy Asthma Immunol. 2009;103(3):190–194. https://doi.org/10.1016/S1081‐1206(10)60180‐9
- 1127. Camhi SL, Morgan WJ, Pernisco N, Quan SF. Factors affecting sleep disturbances in children and adolescents. Sleep Med. 2000;1(2):117–123. https://doi.org/10.1016/s1389‐9457(99)00005‐2
- 1128. Parikh NG, Junaid I, Sheinkopf L, Randhawa I, Santiago SM, Klaustermeyer WB. Clinical control in the dual diagnosis of obstructive sleep apnea syndrome and rhinitis: a prospective analysis. Am J Rhinol Allergy. 2014;28(1):e52–e55. https://doi.org/10.2500/ajra.2014.28.3977
- 1129. Fried J, Yuen E, Zhang K, et al. Impact of treatment for nasal cavity disorders on sleep quality: systematic review and meta‐analysis. Otolaryngol Head Neck Surg. 2022;166(4):633–642. https://doi.org/10.1177/01945998211029527
- 1130. Munoz‐Cano R, Ribo P, Araujo G, Giralt E, Sanchez‐Lopez J, Valero A. Severity of allergic rhinitis impacts sleep and anxiety: results from a large Spanish cohort. Clin Transl Allergy. 2018;8:23. https://doi.org/10.1186/s13601‐018‐0212‐0
- 1131. Colas C, Galera H, Anibarro B, et al. Disease severity impairs sleep quality in allergic rhinitis (The SOMNIAAR study). Clin Exp Allergy. 2012;42(7):1080–1087. https://doi.org/10.1111/j.1365‐2222.2011.03935.x
- 1132. Romano M, James S, Farrington E, Perry R, Elliott L. The impact of perennial allergic rhinitis with/without allergic asthma on sleep, work and activity level. Allergy Asthma Clin Immunol. 2019;15:81. https://doi.org/10.1186/s13223‐019‐0391‐9
- 1133. Roxbury CR, Qiu M, Shargorodsky J, Lin SY. Association between allergic rhinitis and poor sleep parameters in U.S. adults. Int Forum Allergy Rhinol. 2018;8(10):1098–1106. https://doi.org/10.1002/alr.22174
- 1134. Leger D, Bonnefoy B, Pigearias B, de La Giclais B, Chartier A. Poor sleep is highly associated with house dust mite allergic rhinitis in adults and children. Allergy Asthma Clin Immunol. 2017;13:36. https://doi.org/10.1186/s13223‐017‐0208‐7
- 1135. Gadi G, Wali S, Koshak E, et al. The prevalence of allergic rhinitis and atopic markers in obstructive sleep apnea. J Epidemiol Glob Health. 2017;7(1):37–44. https://doi.org/10.1016/j.jegh.2016.06.001
- 1136. Park CE, Shin SY, Lee KH, Cho JS, Kim SW. The effect of allergic rhinitis on the degree of stress, fatigue and quality of life in OSA patients. Eur Arch Otorhinolaryngol. 2012;269(9):2061–2064. https://doi.org/10.1007/s00405‐011‐1888‐0
- 1137. Udaka T, Suzuki H, Fujimura T, et al. Chronic nasal obstruction causes daytime sleepiness and decreased quality of life even in the absence of snoring. Am J Rhinol. 2007;21(5):564–569. https://doi.org/10.2500/ajr.2007.21.3087
- 1138. Leger D, Annesi‐Maesano I, Carat F, et al. Allergic rhinitis and its consequences on quality of sleep: an unexplored area. Arch Intern Med. 2006;166(16):1744–1748. https://doi.org/10.1001/archinte.166.16.1744
- 1139. Canova CR, Downs SH, Knoblauch A, Andersson M, Tamm M, Leuppi JD. Increased prevalence of perennial allergic rhinitis in patients with obstructive sleep apnea. Respiration. 2004;71(2):138–143. https://doi.org/10.1159/000076674
- 1140. Mintz M, Garcia J, Diener P, Liao Y, Dupclay L, Georges G. Triamcinolone acetonide aqueous nasal spray improves nocturnal rhinitis‐related quality of life in patients treated in a primary care setting: the Quality of Sleep in Allergic Rhinitis study. Ann Allergy Asthma Immunol. 2004;92(2):255–261. https://doi.org/10.1016/S1081‐1206(10)61557‐8
- 1141. Stuck BA, Czajkowski J, Hagner AE, et al. Changes in daytime sleepiness, quality of life, and objective sleep patterns in seasonal allergic rhinitis: a controlled clinical trial. J Allergy Clin Immunol. 2004;113(4):663–638. https://doi.org/10.1016/j.jaci.2003.12.589
- 1142. Janson C, De Backer W, Gislason T, et al. Increased prevalence of sleep disturbances and daytime sleepiness in subjects with bronchial asthma: a population study of young adults in three European countries. Eur Respir J. 1996;9(10):2132–2138. https://doi.org/10.1183/09031936.96.09102132
- 1143. Lin SY, Melvin TA, Boss EF, Ishman SL. The association between allergic rhinitis and sleep‐disordered breathing in children: a systematic review. Int Forum Allergy Rhinol. 2013;3(6):504–509. https://doi.org/10.1002/alr.21123
- 1144. Lee K, Choi IH, Hong Y, Lee H, Lee SH, Kim TH. Association between allergic rhinitis‐related factors and sleep duration in adolescents: Korea National Health and Nutrition Examination Survey V (2010–2012). Int J Pediatr Otorhinolaryngol. 2021;142:110613. https://doi.org/10.1016/j.ijporl.2021.110613
- 1145. Giraldo‐Cadavid LF, Perdomo‐Sanchez K, Cordoba‐Gravini JL, et al. Allergic rhinitis and OSA in children residing at a high altitude. Chest. 2020;157(2):384–393. https://doi.org/10.1016/j.chest.2019.09.018
- 1146. Perikleous E, Steiropoulos P, Nena E, et al. Association of asthma and allergic rhinitis with sleep‐disordered breathing in childhood. Front Pediatr. 2018;6:250. https://doi.org/10.3389/fped.2018.00250
- 1147. Di Francesco RC, Alvarez J. Allergic rhinitis affects the duration of rapid eye movement sleep in children with sleep‐disordered breathing without sleep apnea. Int Forum Allergy Rhinol. 2016;6(5):465–471. https://doi.org/10.1002/alr.21689
- 1148. Chimenz R, Manti S, Fede C, et al. Primary nocturnal enuresis in children with allergic rhinitis and severe adenotonsillar hypertrophy: a single center pilot study. J Biol Regul Homeost Agents. 2015;29(2 suppl 1):73–79.
- 1149. Koinis‐Mitchell D, Kopel SJ, Boergers J, et al. Asthma, allergic rhinitis, and sleep problems in urban children. J Clin Sleep Med. 2015;11(2):101–110. https://doi.org/10.5664/jcsm.4450
- 1150. Poachanukoon O, Kitcharoensakkul M. Snoring and sleep problems in children with and without allergic rhinitis: a case control study. J Med Assoc Thai. 2015;98(suppl 2):S138–S144.
- 1151. Kwon JA, Lee M, Yoo KB, Park EC. Does the duration and time of sleep increase the risk of allergic rhinitis? Results of the 6‐year nationwide Korea youth risk behavior web‐based survey. PLoS One. 2013;8(8):e72507. https://doi.org/10.1371/journal.pone.0072507
- 1152. Bhattacharjee R, Kheirandish‐Gozal L, Spruyt K, et al. Adenotonsillectomy outcomes in treatment of obstructive sleep apnea in children: a multicenter retrospective study. Am J Respir Crit Care Med. 2010;182(5):676–683. https://doi.org/10.1164/rccm.200912‐1930OC
- 1153. Li AM, Au CT, So HK, Lau J, Ng PC, Wing YK. Prevalence and risk factors of habitual snoring in primary school children. Chest. 2010;138(3):519–527. https://doi.org/10.1378/chest.09‐1926
- 1154. Vichyanond P, Suratannon C, Lertbunnaphong P, Jirapongsananuruk O, Visitsunthorn N. Clinical characteristics of children with non‐allergic rhinitis vs with allergic rhinitis. Asian Pac J Allergy Immunol. 2010;28(4):270–274.
- 1155. Barone JG, Hanson C, DaJusta DG, Gioia K, England SJ, Schneider D. Nocturnal enuresis and overweight are associated with obstructive sleep apnea. Pediatrics. 2009;124(1):e53–e59. https://doi.org/10.1542/peds.2008‐2805
- 1156. Sogut A, Yilmaz O, Dinc G, Yuksel H. Prevalence of habitual snoring and symptoms of sleep‐disordered breathing in adolescents. Int J Pediatr Otorhinolaryngol. 2009;73(12):1769–1773. https://doi.org/10.1016/j.ijporl.2009.09.026
- 1157. Liukkonen K, Virkkula P, Aronen ET, Kirjavainen T, Pitkaranta A. All snoring is not adenoids in young children. Int J Pediatr Otorhinolaryngol. 2008;72(6):879–884. https://doi.org/10.1016/j.ijporl.2008.02.018
- 1158. Kalra M, Lemasters G, Bernstein D, et al. Atopy as a risk factor for habitual snoring at age 1 year. Chest. 2006;129(4):942–946. https://doi.org/10.1378/chest.129.4.942
- 1159. Goldbart AD, Goldman JL, Veling MC, Gozal D. Leukotriene modifier therapy for mild sleep‐disordered breathing in children. Am J Respir Crit Care Med. 2005;172(3):364–370. https://doi.org/10.1164/rccm.200408‐1064OC
- 1160. Ng DK, Kwok KL, Cheung JM, et al. Prevalence of sleep problems in Hong Kong primary school children: a community‐based telephone survey. Chest. 2005;128(3):1315–1323. https://doi.org/10.1378/chest.128.3.1315
- 1161. Sogut A, Altin R, Uzun L, et al. Prevalence of obstructive sleep apnea syndrome and associated symptoms in 3–11‐year‐old Turkish children. Pediatr Pulmonol. 2005;39(3):251–256. https://doi.org/10.1002/ppul.20179
- 1162. Chng SY, Goh DY, Wang XS, Tan TN, Ong NB. Snoring and atopic disease: a strong association. Pediatr Pulmonol. 2004;38(3):210–216. https://doi.org/10.1002/ppul.20075
- 1163. Kidon MI, See Y, Goh A, Chay OM, Balakrishnan A. Aeroallergen sensitization in pediatric allergic rhinitis in Singapore: is air‐conditioning a factor in the tropics? Pediatr Allergy Immunol. 2004;15(4):340–343. https://doi.org/10.1111/j.1399‐3038.2004.00152.x
- 1164. Mansfield LE, Diaz G, Posey CR, Flores‐Neder J. Sleep disordered breathing and daytime quality of life in children with allergic rhinitis during treatment with intranasal budesonide. Ann Allergy Asthma Immunol. 2004;92(2):240–244. https://doi.org/10.1016/S1081‐1206(10)61554‐2
- 1165. Anuntaseree W, Rookkapan K, Kuasirikul S, Thongsuksai P. Snoring and obstructive sleep apnea in Thai school‐age children: prevalence and predisposing factors. Pediatr Pulmonol. 2001;32(3):222–227. https://doi.org/10.1002/ppul.1112
- 1166. McColley SA, Carroll JL, Curtis S, Loughlin GM, Sampson HA. High prevalence of allergic sensitization in children with habitual snoring and obstructive sleep apnea. Chest. 1997;111(1):170–173. https://doi.org/10.1378/chest.111.1.170
- 1167. Brozek JL, Bousquet J, Agache I, et al. Allergic Rhinitis and its Impact on Asthma (ARIA) guidelines–2016 revision. J Allergy Clin Immunol. 2017;140(4):950–958. https://doi.org/10.1016/j.jaci.2017.03.050
- 1168. Katelaris CH, Lee BW, Potter PC, et al. Prevalence and diversity of allergic rhinitis in regions of the world beyond Europe and North America. Clin Exp Allergy. 2012;42(2):186–207. https://doi.org/10.1111/j.1365‐2222.2011.03891.x
- 1169. Dierick BJH, van der Molen T, Flokstra‐de Blok BMJ, et al. Burden and socioeconomics of asthma, allergic rhinitis, atopic dermatitis and food allergy. Expert Rev Pharmacoecon Outcomes Res. 2020;20(5):437–453. https://doi.org/10.1080/14737167.2020.1819793
- 1170. Kritikos V, Price D, Papi A, et al. The burden of self‐reported rhinitis and associated risk for exacerbations with moderate‐severe asthma in primary care patients. J Asthma Allergy. 2020;13:415–428. https://doi.org/10.2147/JAA.S266204
- 1171. Strozek J, Samolinski BK, Klak A, et al. The indirect costs of allergic diseases. Int J Occup Med Environ Health. 2019;32(3):281–290. https://doi.org/10.13075/ijomeh.1896.01275
- 1172. Al‐Digheari A, Mahboub B, Tarraf H, et al. The clinical burden of allergic rhinitis in five Middle Eastern countries: results of the SNAPSHOT program. Allergy Asthma Clin Immunol. 2018;14:63. https://doi.org/10.1186/s13223‐018‐0298‐x
- 1173. Goetzel RZ, Long SR, Ozminkowski RJ, Hawkins K, Wang S, Lynch W. Health, absence, disability, and presenteeism cost estimates of certain physical and mental health conditions affecting U.S. employers. J Occup Environ Med. 2004;46(4):398–412. https://doi.org/10.1097/01.jom.0000121151.40413.bd
- 1174. Workman AD, Dattilo L, Rathi VK, Bhattacharyya N. Contemporary incremental healthcare costs for allergic rhinitis in the United States. Laryngoscope. 2021;https://doi.org/10.1002/lary.29846
- 1175. Roland LT, Wise SK, Wang H, Zhang P, Mehta C, Levy JM. The cost of rhinitis in the United States: a national insurance claims analysis. Int Forum Allergy Rhinol. 2021;11(5):946–948. https://doi.org/10.1002/alr.22748
- 1176. Meltzer EO, Bukstein DA. The economic impact of allergic rhinitis and current guidelines for treatment. Ann Allergy Asthma Immunol. 2011;106(2 suppl):S12–S16. https://doi.org/10.1016/j.anai.2010.10.014
- 1177. Law AW, Reed SD, Sundy JS, Schulman KA. Direct costs of allergic rhinitis in the United States: estimates from the 1996 Medical Expenditure Panel Survey. J Allergy Clin Immunol. 2003;111(2):296–300. https://doi.org/10.1067/mai.2003.68
- 1178. Reed SD, Lee TA, McCrory DC. The economic burden of allergic rhinitis: a critical evaluation of the literature. Pharmacoeconomics. 2004;22(6):345–361. https://doi.org/10.2165/00019053‐200422060‐00002
- 1179. Avdeeva KS, Reitsma S, Fokkens WJ. Direct and indirect costs of allergic and non‐allergic rhinitis in the Netherlands. Allergy. 2020;75(11):2993–2996. https://doi.org/10.1111/all.14457
- 1180. Bousquet J, Schroder‐Bernhardi D, Bachert C, et al. Heterogeneity of the pharmacologic treatment of allergic rhinitis in Europe based on MIDAS and OTCims platforms. Clin Exp Allergy. 2021;51(8):1033–1045. https://doi.org/10.1111/cea.13884
- 1181. Smith P, Price D, Harvey R, et al. Medication‐related costs of rhinitis in Australia: a NostraData cross‐sectional study of pharmacy purchases. J Asthma Allergy. 2017;10:153–161. https://doi.org/10.2147/JAA.S128431
- 1182. Bousquet J, Schunemann HJ, Togias A, et al. Next‐generation Allergic Rhinitis and Its Impact on Asthma (ARIA) guidelines for allergic rhinitis based on Grading of Recommendations Assessment, Development and Evaluation (GRADE) and real‐world evidence. J Allergy Clin Immunol. 2020;145(1):70–80.e3. https://doi.org/10.1016/j.jaci.2019.06.049
- 1183. Price D, Scadding G, Ryan D, et al. The hidden burden of adult allergic rhinitis: UK healthcare resource utilisation survey. Clin Transl Allergy. 2015;5:39. https://doi.org/10.1186/s13601‐015‐0083‐6
- 1184. Belhassen M, Demoly P, Bloch‐Morot E, et al. Costs of perennial allergic rhinitis and allergic asthma increase with severity and poor disease control. Allergy. 2017;72(6):948–958. https://doi.org/10.1111/all.13098
- 1185. Celik G, Mungan D, Abadoglu O, Pinar NM, Misirligil Z. Direct cost assessments in subjects with seasonal allergic rhinitis living in Ankara, Turkey. Allergy Asthma Proc. 2004;25(2):107–113.
- 1186. Yoo KH, Ahn HR, Park JK, et al. Burden of respiratory disease in Korea: an observational study on allergic rhinitis, asthma, COPD, and rhinosinusitis. Allergy Asthma Immunol Res. 2016;8(6):527–534. https://doi.org/10.4168/aair.2016.8.6.527
- 1187. Kim SY, Yoon SJ, Jo MW, Kim EJ, Kim HJ, Oh IH. Economic burden of allergic rhinitis in Korea. Am J Rhinol Allergy. 2010;24(5):e110–e113. https://doi.org/10.2500/ajra.2010.24.3513
- 1188. Ghoshal AG, Ravindran GD, Gangwal P, et al. The burden of segregated respiratory diseases in India and the quality of care in these patients: results from the Asia‐Pacific Burden of Respiratory Diseases study. Lung India. 2016;33(6):611–619. https://doi.org/10.4103/0970‐2113.192878
- 1189. Nathan RA. The burden of allergic rhinitis. Allergy Asthma Proc. 2007;28(1):3–9. https://doi.org/10.2500/aap.2007.28.2934
- 1190. Crystal‐Peters J, Crown WH, Goetzel RZ, Schutt DC. The cost of productivity losses associated with allergic rhinitis. Am J Manag Care. 2000;6(3):373–378.
- 1191. Fineman SM. The burden of allergic rhinitis: beyond dollars and cents. Ann Allergy Asthma Immunol. 2002;88(4 suppl 1):2–7. https://doi.org/10.1016/s1081‐1206(10)62022‐4
- 1192. Colas C, Brosa M, Anton E, et al. Estimate of the total costs of allergic rhinitis in specialized care based on real‐world data: the FERIN Study. Allergy. 2017;72(6):959–966. https://doi.org/10.1111/all.13099
- 1193. Canonica GW, Klimek L, Acaster S, et al. Burden of allergic rhinitis and impact of MP‐AzeFlu from the patient perspective: pan European patient survey. Curr Med Res Opin. 2021;37(7):1259–1272. https://doi.org/10.1080/03007995.2021.1911973
- 1194. Vandenplas O, Vinnikov D, Blanc PD, et al. Impact of rhinitis on work productivity: a systematic review. J Allergy Clin Immunol Pract. 2018;6(4):1274–1286.e9. https://doi.org/10.1016/j.jaip.2017.09.002
- 1195. Lamb CE, Ratner PH, Johnson CE, et al. Economic impact of workplace productivity losses due to allergic rhinitis compared with select medical conditions in the United States from an employer perspective. Curr Med Res Opin. 2006;22(6):1203–1210. https://doi.org/10.1185/030079906X112552
- 1196. Hellgren J, Cervin A, Nordling S, Bergman A, Cardell LO. Allergic rhinitis and the common cold–high cost to society. Allergy. 2010;65(6):776–783. https://doi.org/10.1111/j.1398‐9995.2009.02269.x
- 1197. Roger A, Arcala Campillo E, Torres MC, et al. Reduced work/academic performance and quality of life in patients with allergic rhinitis and impact of allergen immunotherapy. Allergy Asthma Clin Immunol. 2016;12:40. https://doi.org/10.1186/s13223‐016‐0146‐9
- 1198. Jauregui I, Mullol J, Davila I, et al. Allergic rhinitis and school performance. J Investig Allergol Clin Immunol. 2009;19(suppl 1):32–39.
- 1199. Schoenwetter WF, Dupclay Jr L, Appajosyula S, Botteman MF, Pashos CL. Economic impact and quality‐of‐life burden of allergic rhinitis. Curr Med Res Opin. 2004;20(3):305–317. https://doi.org/10.1185/030079903125003053
- 1200. Mir E, Panjabi C, Shah A. Impact of allergic rhinitis in school going children. Asia Pac Allergy. 2012;2(2):93–100. https://doi.org/10.5415/apallergy.2012.2.2.93
- 1201. Trikojat K, Buske‐Kirschbaum A, Plessow F, Schmitt J, Fischer R. Memory and multitasking performance during acute allergic inflammation in seasonal allergic rhinitis. Clin Exp Allergy. 2017;47(4):479–487. https://doi.org/10.1111/cea.12893
- 1202. Wang J, Xiao D, Chen H, Hu J. Cumulative evidence for association of rhinitis and depression. Allergy Asthma Clin Immunol. 2021;17(1):111. https://doi.org/10.1186/s13223‐021‐00615‐5
- 1203. Klimek L, Bachert C, Pfaar O, et al. ARIA guideline 2019: treatment of allergic rhinitis in the German health system. Allergol Select. 2019;3(1):22–50. https://doi.org/10.5414/ALX02120E
- 1204. Cingi C, Bayar Muluk N, Scadding GK. Will every child have allergic rhinitis soon? Int J Pediatr Otorhinolaryngol. 2019;118:53–58. https://doi.org/10.1016/j.ijporl.2018.12.019
- 1205. Small P, Frenkiel A, Becker A, Boisvert P, Bouchard J. The Canadian Rhinitis Working Group: rhinitis – a practical and comprehensive approach to assessment and therapt. J Otolaryngol. 2007;36(1):S5–S27.
- 1206. Scadding GK, Kariyawasam HH, Scadding G, et al. BSACI guideline for the diagnosis and management of allergic and non‐allergic rhinitis (Revised 2017; First edition 2007). Clin Exp Allergy. 2017;47(7):856–889. https://doi.org/10.1111/cea.12953
- 1207. Schatz M. A survey of the burden of allergic rhinitis in the USA. Allergy. 2007;62(suppl 85):9–16. https://doi.org/10.1111/j.1398‐9995.2007.01548.x
- 1208. Bousquet J, Devillier P, Anto JM, et al. Daily allergic multimorbidity in rhinitis using mobile technology: a novel concept of the MASK study. Allergy. 2018;73(8):1622–1631. https://doi.org/10.1111/all.13448
- 1209. Ng ML, Warlow RS, Chrishanthan N, Ellis C, Walls R. Preliminary criteria for the definition of allergic rhinitis: a systematic evaluation of clinical parameters in a disease cohort (I). Clin Exp Allergy. 2000;30(9):1314–1331. https://doi.org/10.1046/j.1365‐2222.2000.00853.x
- 1210. Scadding GK, Hellings PW, Bachert C, et al. Allergic respiratory disease care in the COVID‐19 era: A EUFOREA statement. World Allergy Organ J. 2020;13(5):100124. https://doi.org/10.1016/j.waojou.2020.100124
- 1211. Costa DJ, Amouyal M, Lambert P, et al. How representative are clinical study patients with allergic rhinitis in primary care? J Allergy Clin Immunol. 2011;127(4):920–926.e1. https://doi.org/10.1016/j.jaci.2010.10.058
- 1212. Raza SN, Yousuf K, Small P, Frenkiel S. Diagnosing allergic rhinitis: effectiveness of the physical examination in comparison to conventional skin testing. J Otolaryngol Head Neck Surg. 2011;40(5):407–412.
- 1213. Shaker MS, Oppenheimer J, Grayson M, et al. COVID‐19: pandemic contingency planning for the allergy and immunology clinic. J Allergy Clin Immunol Pract. 2020;8(5):1477–1488.e5. https://doi.org/10.1016/j.jaip.2020.03.012
- 1214. Ziade GK, Karami RA, Fakhri GB, et al. Reliability assessment of the endoscopic examination in patients with allergic rhinitis. Allergy Rhinol (Providence). 2016;7(3):135–138. https://doi.org/10.2500/ar.2016.7.0176
- 1215. Jareoncharsri P, Thitadilok V, Bunnag C, Ungkanont K, Voraprayoon S, Tansuriyawong P. Nasal endoscopic findings in patients with perennial allergic rhinitis. Asian Pac J Allergy Immunol. 1999;17(4):261–267.
- 1216. Eren E, Aktas A, Arslanoglu S, et al. Diagnosis of allergic rhinitis: inter‐rater reliability and predictive value of nasal endoscopic examination: a prospective observational study. Clin Otolaryngol. 2013;38(6):481–486. https://doi.org/10.1111/coa.12171
- 1217. Ameli F, Tosca MA, Licari A, Gallo F, Ciprandi G. Can an otorhinolaryngological visit induce the suspect of allergic rhinitis in children? Eur Ann Allergy Clin Immunol. 2019;51(6):273–282. https://doi.org/10.23822/EurAnnACI.1764‐1489.105
- 1218. Ameli F, Brocchetti F, Tosca MA, Signori A, Ciprandi G. Nasal endoscopy in children with suspected allergic rhinitis. Laryngoscope. 2011;121(10):2055–2059. https://doi.org/10.1002/lary.22156
- 1219. White LJ, Rotella MR, DelGaudio JM. Polypoid changes of the middle turbinate as an indicator of atopic disease. Int Forum Allergy Rhinol. 2014;4(5):376–380. https://doi.org/10.1002/alr.21290
- 1220. Hamizan AW, Christensen JM, Ebenzer J, et al. Middle turbinate edema as a diagnostic marker of inhalant allergy. Int Forum Allergy Rhinol. 2017;7(1):37–42. https://doi.org/10.1002/alr.21835
- 1221. Brunner JP, Jawad BA, McCoul ED. Polypoid change of the middle turbinate and paranasal sinus polyposis are distinct entities. Otolaryngol Head Neck Surg. 2017;157(3):519–523. https://doi.org/10.1177/0194599817711887
- 1222. DelGaudio JM, Loftus PA, Hamizan AW, Harvey RJ, Wise SK. Central compartment atopic disease. Am J Rhinol Allergy. 2017;31(4):228–234. https://doi.org/10.2500/ajra.2017.31.4443
- 1223. DelGaudio JM, Levy JM, Wise SK. Central compartment involvement in aspirin‐exacerbated respiratory disease: the role of allergy and previous sinus surgery. Int Forum Allergy Rhinol. 2019;9(9):1017–1022. https://doi.org/10.1002/alr.22367
- 1224. Hamizan AW, Loftus PA, Alvarado R, et al. Allergic phenotype of chronic rhinosinusitis based on radiologic pattern of disease. Laryngoscope. 2018;128(9):2015–2021. https://doi.org/10.1002/lary.27180
- 1225. Marcus S, Schertzer J, Roland LT, Wise SK, Levy JM, DelGaudio JM. Central compartment atopic disease: prevalence of allergy and asthma compared with other subtypes of chronic rhinosinusitis with nasal polyps. Int Forum Allergy Rhinol. 2020;10(2):183–189. https://doi.org/10.1002/alr.22454
- 1226. Roland LT, Marcus S, Schertzer JS, Wise SK, Levy JM, DelGaudio JM. Computed tomography findings can help identify different chronic rhinosinusitis with nasal polyp phenotypes. Am J Rhinol Allergy. 2020;34(5):679–685. https://doi.org/10.1177/1945892420923926
- 1227. Lee K, Kim TH, Lee SH, Kang CH, Je BK, Oh S. Predictive value of radiologic central compartment atopic disease for identifying allergy and asthma in pediatric patients. Ear Nose Throat J. 2021:145561321997546. https://doi.org/10.1177/0145561321997546
- 1228. Abdullah B, Vengathajalam S, Md Daud MK, Wan Mohammad Z, Hamizan A, Husain S. The clinical and radiological characterizations of the allergic phenotype of chronic rhinosinusitis with nasal polyps. J Asthma Allergy. 2020;13:523–531. https://doi.org/10.2147/JAA.S275536
- 1229. Kaymakci M, Erel F, Bulbul E, Yazici H, Acar M, Yanik B. Maxillary sinus aeration in allergic rhinitis. J Craniofac Surg. 2015;26(4):e288–e290. https://doi.org/10.1097/SCS.0000000000001558
- 1230. Hizli O, Kayabasi S, Ozkan D. Is Nasal Septal Body Size Associated With Inferior Turbinate Hypertrophy and Allergic Rhinitis? J Craniofac Surg. 2020;31(3):778–781. https://doi.org/10.1097/SCS.0000000000006107
- 1231. Pearce MS, Salotti JA, Little MP, et al. Radiation exposure from CT scans in childhood and subsequent risk of leukaemia and brain tumours: a retrospective cohort study. Lancet. 2012;380(9840):499–505. https://doi.org/10.1016/S0140‐6736(12)60815‐0
- 1232. Meulepas JM, Ronckers CM, Smets A, et al. Radiation exposure from pediatric CT scans and subsequent cancer risk in the Netherlands. J Natl Cancer Inst. 2019;111(3):256–263. https://doi.org/10.1093/jnci/djy104
- 1233. Mathews JD, Forsythe AV, Brady Z, et al. Cancer risk in 680,000 people exposed to computed tomography scans in childhood or adolescence: data linkage study of 11 million Australians. BMJ. 2013;346:f2360. https://doi.org/10.1136/bmj.f2360
- 1234. Sharhan SSA, Lee EJ, Hwang CS, et al. Radiological comparison of inferior turbinate hypertrophy between allergic and non‐allergic rhinitis: does allergy really augment turbinate hypertrophy? Eur Arch Otorhinolaryngol. 2018;275(4):923–929. https://doi.org/10.1007/s00405‐018‐4893‐8
- 1235. Brown HM, Su S, Thantrey N. Prick testing for allergens standardized by using a precision needle. Clin Allergy. 1981;11(1):95–98. https://doi.org/10.1111/j.1365‐2222.1981.tb01571.x
- 1236. Ates A, Kinikli G, Turgay M, Aydogan N, Duman M. The results of skin prick testing in patients with allergic rhinitis: a comparison between a multiple lancet device and a single lancet. Asian Pac J Allergy Immunol. 2004;22(2‐3):109–114.
- 1237. Phagoo SB, Wilson NM, Silverman M. Skin prick testing using allergen‐coated lancets: a comparison between a multiple lancet device and a single lancet applied with varying pressures. Clin Exp Allergy. 1991;21(5):589–593. https://doi.org/10.1111/j.1365‐2222.1991.tb00851.x
- 1238. Rhodius R, Wickens K, Cheng S, Crane J. A comparison of two skin test methodologies and allergens from two different manufacturers. Ann Allergy Asthma Immunol. 2002;88(4):374–379. https://doi.org/10.1016/S1081‐1206(10)62367‐8
- 1239. Anon JB. Introduction to in vivo allergy testing. Otolaryngol Head Neck Surg. 1993;109(3 pt 2):593–600.
- 1240. Kim BJ, Mun SK. Objective measurements using the skin prick test in allergic rhinitis. Arch Otolaryngol Head Neck Surg. 2010;136(11):1104–1106. https://doi.org/10.1001/archoto.2010.185
- 1241. Piette V, Bourret E, Bousquet J, Demoly P. Prick tests to aeroallergens: is it possible simply to wipe the device between tests? Allergy. 2002;57(10):940–942. https://doi.org/10.1034/j.1398‐9995.2002.23536.x
- 1242. Sander I, Fleischer C, Meurer U, Bruning T, Raulf‐Heimsoth M. Allergen content of grass pollen preparations for skin prick testing and sublingual immunotherapy. Allergy. 2009;64(10):1486–1492. https://doi.org/10.1111/j.1398‐9995.2009.02040.x
- 1243. Curin M, Reininger R, Swoboda I, Focke M, Valenta R, Spitzauer S. Skin prick test extracts for dog allergy diagnosis show considerable variations regarding the content of major and minor dog allergens. Int Arch Allergy Immunol. 2011;154(3):258–263. https://doi.org/10.1159/000321113
- 1244. Bousquet J, Heinzerling L, Bachert C, et al. Practical guide to skin prick tests in allergy to aeroallergens. Allergy. 2012;67(1):18–24. https://doi.org/10.1111/j.1398‐9995.2011.02728.x
- 1245. Canonica GW, Ansotegui IJ, Pawankar R, et al. A WAO ‐ ARIA ‐ GA(2)LEN consensus document on molecular‐based allergy diagnostics. World Allergy Organ J. 2013;6(1):17. https://doi.org/10.1186/1939‐4551‐6‐17
- 1246. Bernstein IL, Li JT, Bernstein DI, et al. Allergy diagnostic testing: an updated practice parameter. Ann Allergy Asthma Immunol. 2008;100(3 suppl 3):S1–S148. https://doi.org/10.1016/s1081‐1206(10)60305‐5
- 1247. Oppenheimer J, Nelson HS. Skin testing: a survey of allergists. Ann Allergy Asthma Immunol. 2006;96(1):19–23. https://doi.org/10.1016/S1081‐1206(10)61034‐4
- 1248. Chafen JJ, Newberry SJ, Riedl MA, et al. Diagnosing and managing common food allergies: a systematic review. JAMA. 2010;303(18):1848–1856. https://doi.org/10.1001/jama.2010.582
- 1249. Tschopp JM, Sistek D, Schindler C, et al. Current allergic asthma and rhinitis: diagnostic efficiency of three commonly used atopic markers (IgE, skin prick tests, and Phadiatop). Results from 8329 randomized adults from the SAPALDIA Study. Swiss Study on Air Pollution and Lung Diseases in Adults. Allergy. 1998;53(6):608–613. https://doi.org/10.1111/j.1398‐9995.1998.tb03937.x
- 1250. Corren J, Shapiro G, Reimann J, et al. Allergen skin tests and free IgE levels during reduction and cessation of omalizumab therapy. J Allergy Clin Immunol. 2008;121(2):506–511. https://doi.org/10.1016/j.jaci.2007.11.026
- 1251. Ansotegui IJ, Melioli G, Canonica GW, et al. IgE allergy diagnostics and other relevant tests in allergy, a World Allergy Organization position paper. World Allergy Organ J. 2020;13(2):100080. https://doi.org/10.1016/j.waojou.2019.100080
- 1252. Nevis IF, Binkley K, Kabali C. Diagnostic accuracy of skin‐prick testing for allergic rhinitis: a systematic review and meta‐analysis. Allergy Asthma Clin Immunol. 2016;12:20. https://doi.org/10.1186/s13223‐016‐0126‐0
- 1253. Gungor A, Houser SM, Aquino BF, et al. A comparison of skin endpoint titration and skin‐prick testing in the diagnosis of allergic rhinitis. Ear Nose Throat J. 2004;83(1):54–60.
- 1254. Krouse JH, Shah AG, Kerswill K. Skin testing in predicting response to nasal provocation with alternaria. Laryngoscope. 2004;114(8):1389–1393. https://doi.org/10.1097/00005537‐200408000‐00013
- 1255. Krouse JH, Sadrazodi K, Kerswill K. Sensitivity and specificity of prick and intradermal testing in predicting response to nasal provocation with timothy grass antigen. Otolaryngol Head Neck Surg. 2004;131(3):215–219. https://doi.org/10.1016/j.otohns.2004.03.024
- 1256. Zarei M, Remer CF, Kaplan MS, et al. Optimal skin prick wheal size for diagnosis of cat allergy. Ann Allergy Asthma Immunol. 2004;92(6):604–610. https://doi.org/10.1016/S1081‐1206(10)61425‐1
- 1257. Pumhirun P, Jane‐Trakoonroj S, Wasuwat P. Comparison of in vitro assay for specific IgE and skin prick test with intradermal test in patients with allergic rhinitis. Asian Pac J Allergy Immunol. 2000;18(3):157–160.
- 1258. Heinzerling L, Mari A, Bergmann KC, et al. The skin prick test – European standards. Clin Transl Allergy. 2013;3(1):3. https://doi.org/10.1186/2045‐7022‐3‐3
- 1259. Escudero AI, Sanchez‐Guerrero IM, Mora AM, et al. Cost‐effectiveness of various methods of diagnosing hypersensitivity to Alternaria. Allergol Immunopathol (Madr). 1993;21(4):153–157.
- 1260. Trevino RJ, Veling MC. The importance of quantifying skin reactivity in treating allergic rhinitis with immunotherapy. Ear Nose Throat J. 2000;79(5):362–364, 366.
- 1261. Peltier J, Ryan MW. Comparison of intradermal dilutional testing, skin prick testing, and modified quantitative testing for common allergens. Otolaryngol Head Neck Surg. 2007;137(2):246–249. https://doi.org/10.1016/j.otohns.2007.05.002
- 1262. Calabria CW, Hagan L. The role of intradermal skin testing in inhalant allergy. Ann Allergy Asthma Immunol. 2008;101(4):337–347, quiz 347, 418. https://doi.org/10.1016/S1081‐1206(10)60307‐9
- 1263. Niemeijer NR, Fluks AF, de Monchy JG. Optimization of skin testing. II. Evaluation of concentration and cutoff values, as compared with RAST and clinical history, in a multicenter study. Allergy. 1993;48(7):498–503. https://doi.org/10.1111/j.1398‐9995.1993.tb01105.x
- 1264. Health Quality Ontario . Skin testing for allergic rhinitis: a health technology assessment. Ont Health Technol Assess Ser. 2016;16(10):1–45.
- 1265. Larrabee YC, Reisacher W. Intradermal testing after negative skin prick testing for patients with high suspicion of allergy. Int Forum Allergy Rhinol. 2015;5(6):547–550. https://doi.org/10.1002/alr.21512
- 1266. Nelson HS, Oppenheimer J, Buchmeier A, Kordash TR, Freshwater LL. An assessment of the role of intradermal skin testing in the diagnosis of clinically relevant allergy to timothy grass. J Allergy Clin Immunol. 1996;97(6):1193–1201. https://doi.org/10.1016/s0091‐6749(96)70184‐7
- 1267. Schwindt CD, Hutcheson PS, Leu SY, Dykewicz MS. Role of intradermal skin tests in the evaluation of clinically relevant respiratory allergy assessed using patient history and nasal challenges. Ann Allergy Asthma Immunol. 2005;94(6):627–633. https://doi.org/10.1016/S1081‐1206(10)61319‐1
- 1268. Lockey RF, Benedict LM, Turkeltaub PC, Bukantz SC. Fatalities from immunotherapy (IT) and skin testing (ST). J Allergy Clin Immunol. 1987;79(4):660–677. https://doi.org/10.1016/s0091‐6749(87)80164‐1
- 1269. Sharma HP, Wood RA, Bravo AR, Matsui EC. A comparison of skin prick tests, intradermal skin tests, and specific IgE in the diagnosis of mouse allergy. J Allergy Clin Immunol. 2008;121(4):933–939. https://doi.org/10.1016/j.jaci.2008.01.023
- 1270. Simons JP, Rubinstein EN, Kogut VJ, Melfi PJ, Ferguson BJ. Comparison of Multi‐Test II skin prick testing to intradermal dilutional testing. Otolaryngol Head Neck Surg. 2004;130(5):536–544. https://doi.org/10.1016/j.otohns.2004.02.005
- 1271. Niemeijer NR, Goedewaagen B, Kauffman HF, de Monchy JG. Optimization of skin testing. I. Choosing allergen concentrations and cutoff values by factorial design. Allergy. 1993;48(7):491–497. https://doi.org/10.1111/j.1398‐9995.1993.tb01104.x
- 1272. Hurst DS, McDaniel AB. Clinical relevance and advantages of intradermal test results in 371 patients with allergic rhinitis, asthma and/or otitis media with effusion. Cells. 2021;10(11):3224. https://doi.org/10.3390/cells10113224
- 1273. Erel F, Sarioglu N, Kose M, et al. Intradermal skin testing in allergic rhinitis and asthma with negative skin prick tests. Iran J Allergy Asthma Immunol. 2017;16(3):193–197.
- 1274. Peltier J, Ryan MW. Comparison of intradermal dilutional testing with the Multi‐Test II applicator in testing for mold allergy. Otolaryngol Head Neck Surg. 2006;134(2):240–244. https://doi.org/10.1016/j.otohns.2005.10.051
- 1275. Seshul M, Pillsbury H 3rd, Eby T. Use of intradermal dilutional testing and skin prick testing: clinical relevance and cost efficiency. Laryngoscope. 2006;116(9):1530–1538. https://doi.org/10.1097/01.mlg.0000234916.43285.f8
- 1276. Purohit A, Laffer S, Metz‐Favre C, et al. Poor association between allergen‐specific serum immunoglobulin E levels, skin sensitivity and basophil degranulation: a study with recombinant birch pollen allergen Bet v 1 and an immunoglobulin E detection system measuring immunoglobulin E capable of binding to Fc epsilon RI. Clin Exp Allergy. 2005;35(2):186–192. https://doi.org/10.1111/j.1365‐2222.2005.02156.x
- 1277. Brown WG, Halonen MJ, Kaltenborn WT, Barbee RA. The relationship of respiratory allergy, skin test reactivity, and serum IgE in a community population sample. J Allergy Clin Immunol. 1979;63(5):328–335. https://doi.org/10.1016/0091‐6749(79)90127‐1
- 1278. Reddy PM, Nagaya H, Pascual HC, et al. Reappraisal of intracutaneous tests in the diagnosis of reaginic allergy. J Allergy Clin Immunol. 1978;61(1):36–41. https://doi.org/10.1016/0091‐6749(78)90471‐2
- 1279. Fornadley JA. Skin testing for inhalant allergy. Int Forum Allergy Rhinol. 2014;4(Suppl 2):S41–S45. https://doi.org/10.1002/alr.21393
- 1280. Krouse JH, Krouse HJ. Modulation of immune mediators with MQT‐based immunotherapy. Otolaryngol Head Neck Surg. 2006;134(5):746–750. https://doi.org/10.1016/j.otohns.2006.01.007
- 1281. Tantilipikorn P, Danpornprasert P, Ngaotepprutaram P, Assanasen P, Bunnag C, Thinkhamrop B. The correlation between intradermal testing and serum specific IgE to house dust mite in negative skin prick test allergic rhinitis adult patients. Asian Pac J Allergy Immunol. 2015;33(4):308–311. https://doi.org/10.12932/AP0579.33.4.2015
- 1282. Lewis AF, Franzese C, Stringer SP. Diagnostic evaluation of inhalant allergies: a cost‐effectiveness analysis. Am J Rhinol. 2008;22(3):246–252. https://doi.org/10.2500/ajr.2008.22.3163
- 1283. Long WF, Taylor RJ, Wagner CJ, Leavengood DC, Nelson HS. Skin test suppression by antihistamines and the development of subsensitivity. J Allergy Clin Immunol. 1985;76(1):113–117. https://doi.org/10.1016/0091‐6749(85)90813‐9
- 1284. Phillips MJ, Meyrick Thomas RH, Moodley I, Davies RJ. A comparison of the in vivo effects of ketotifen, clemastine, chlorpheniramine and sodium cromoglycate on histamine and allergen induced weals in human skin. Br J Clin Pharmacol. 1983;15(3):277–286. https://doi.org/10.1111/j.1365‐2125.1983.tb01500.x
- 1285. Simons FE, Simons KJ. Clinical pharmacology of new histamine H1 receptor antagonists. Clin Pharmacokinet. 1999;36(5):329–352. https://doi.org/10.2165/00003088‐199936050‐00003
- 1286. Cook TJ, MacQueen DM, Wittig HJ, Thornby JI, Lantos RL, Virtue CM. Degree and duration of skin test suppression and side effects with antihistamines. A double blind controlled study with five antihistamines. J Allergy Clin Immunol. 1973;51(2):71–77. https://doi.org/10.1016/s0091‐6749(73)80002‐8
- 1287. Almind M, Dirksen A, Nielsen NH, Svendsen UG. Duration of the inhibitory activity on histamine‐induced skin weals of sedative and non‐sedative antihistamines. Allergy. 1988;43(8):593–596. https://doi.org/10.1111/j.1398‐9995.1988.tb00932.x
- 1288. Pearlman DS, Grossman J, Meltzer EO. Histamine skin test reactivity following single and multiple doses of azelastine nasal spray in patients with seasonal allergic rhinitis. Ann Allergy Asthma Immunol. 2003;91(3):258–262. https://doi.org/10.1016/S1081‐1206(10)63527‐2
- 1289. Miller J, Nelson HS. Suppression of immediate skin tests by ranitidine. J Allergy Clin Immunol. 1989;84(6 pt 1):895–899. https://doi.org/10.1016/0091‐6749(89)90386‐2
- 1290. Kupczyk M, Kuprys I, Bochenska‐Marciniak M, Gorski P, Kuna P. Ranitidine (150 mg daily) inhibits wheal, flare, and itching reactions in skin‐prick tests. Allergy Asthma Proc. 2007;28(6):711–715. https://doi.org/10.2500/aap.2007.28.3064
- 1291. Harvey RP, Schocket AL. The effect of H1 and H2 blockade on cutaneous histamine response in man. J Allergy Clin Immunol. 1990;65(2):136–139.
- 1292. Rao KS, Menon PK, Hilman BC, Sebastian CS, Bairnsfather L. Duration of the suppressive effect of tricyclic antidepressants on histamine‐induced wheal‐and‐flare reactions in human skin. J Allergy Clin Immunol. 1988;82(5 pt 1):752–757. https://doi.org/10.1016/0091‐6749(88)90075‐9
- 1293. Isik SR, Celikel S, Karakaya G, Ulug B, Kalyoncu AF. The effects of antidepressants on the results of skin prick tests used in the diagnosis of allergic diseases. Int Arch Allergy Immunol. 2011;154(1):63–68. https://doi.org/10.1159/000319210
- 1294. Noga O, Hanf G, Kunkel G. Immunological and clinical changes in allergic asthmatics following treatment with omalizumab. Int Arch Allergy Immunol. 2003;131(1):46–52. https://doi.org/10.1159/000070434
- 1295. Hill 3rd SL, Krouse JH. The effects of montelukast on intradermal wheal and flare. Otolaryngol Head Neck Surg. 2003;129(3):199–203. https://doi.org/10.1016/S0194‐5998(03)00607‐7
- 1296. Simons FE, Johnston L, Gu X, Simons KJ. Suppression of the early and late cutaneous allergic responses using fexofenadine and montelukast. Ann Allergy Asthma Immunol. 2001;86(1):44–50. https://doi.org/10.1016/S1081‐1206(10)62354‐X
- 1297. Cuhadaroglu C, Erelel M, Kiyan E, Ece T, Erkan F. Role of Zafirlukast on skin prick test. Allergol Immunopathol (Madr). 2001;29(2):66–68. https://doi.org/10.1016/s0301‐0546(01)79020‐9
- 1298. Des Roches A, Paradis L, Bougeard YH, Godard P, Bousquet J, Chanez P. Long‐term oral corticosteroid therapy does not alter the results of immediate‐type allergy skin prick tests. J Allergy Clin Immunol. 1996;98(3):522–527. https://doi.org/10.1016/s0091‐6749(96)70085‐4
- 1299. Slott RI, Zweiman B. A controlled study of the effect of corticosteroids on immediate skin test reactivity. J Allergy Clin Immunol. 1974;54(4):229–234. https://doi.org/10.1016/0091‐6749(74)90065‐7
- 1300. Olson R, Karpink MH, Shelanski S, Atkins PC, Zweiman B. Skin reactivity to codeine and histamine during prolonged corticosteroid therapy. J Allergy Clin Immunol. 1990;86(2):153–159. https://doi.org/10.1016/s0091‐6749(05)80060‐0
- 1301. Geng B, Thakor A, Clayton E, Finkas L, Riedl MA. Factors associated with negative histamine control for penicillin allergy skin testing in the inpatient setting. Ann Allergy Asthma Immunol. 2015;115(1):33–38. https://doi.org/10.1016/j.anai.2015.04.012
- 1302. Narasimha SK, Srinivas CR, Mathew AC. Effect of topical corticosteroid application frequency on histamine‐induced wheals. Int J Dermatol. 2005;44(5):425–427. https://doi.org/10.1111/j.1365‐4632.2005.02482.x
- 1303. Andersson M, Pipkorn U. Inhibition of the dermal immediate allergic reaction through prolonged treatment with topical glucocorticosteroids. J Allergy Clin Immunol. 1987;79(2):345–349. https://doi.org/10.1016/0091‐6749(87)90153‐9
- 1304. Pipkorn U, Hammarlund A, Enerback L. Prolonged treatment with topical glucocorticoids results in an inhibition of the allergen‐induced weal‐and‐flare response and a reduction in skin mast cell numbers and histamine content. Clin Exp Allergy. 1989;19(1):19–25. https://doi.org/10.1111/j.1365‐2222.1989.tb02338.x
- 1305. Gradman J, Wolthers OD. Suppressive effects of topical mometasone furoate and tacrolimus on skin prick testing in children. Pediatr Dermatol. 2008;25(2):269–270. https://doi.org/10.1111/j.1525‐1470.2008.00651.x
- 1306. Shah KM, Rank MA, Dave SA, Oslie CL, Butterfield JH. Predicting which medication classes interfere with allergy skin testing. Allergy Asthma Proc. 2010;31(6):477–482. https://doi.org/10.2500/aap.2010.31.3382
- 1307. Duenas‐Laita A, Ruiz‐Munoz P, Armentia A, Pinacho F, Martin‐Armentia B. Successful treatment of chronic drug‐resistant urticaria with alprazolam. J Allergy Clin Immunol. 2009;123(2):504–505. https://doi.org/10.1016/j.jaci.2008.12.005
- 1308. Spergel JM, Nurse N, Taylor P, ParneixSpake A. Effect of topical pimecrolimus on epicutaneous skin testing. J Allergy Clin Immunol. 2004;114(3):695–697. https://doi.org/10.1016/j.jaci.2004.05.067
- 1309. More DR, Napoli DC, Hagan LL. Herbal supplements and skin testing: the lack of effect of commonly used herbal supplements on histamine skin prick testing. Allergy. 2003;58(6):492–494. https://doi.org/10.1034/j.1398‐9995.2003.00140.x
- 1310. Simons FE, Simons KJ. Peripheral H1‐blockade effect of fexofenadine. Ann Allergy Asthma Immunol. 1997;79(6):530–532. https://doi.org/10.1016/S1081‐1206(10)63061‐X
- 1311. Komarow HD, Arceo S, Young M, Nelson C, Metcalfe DD. Dissociation between history and challenge in patients with physical urticaria. J Allergy Clin Immunol Pract. 2014;2(6):786–790. https://doi.org/10.1016/j.jaip.2014.07.008
- 1312. Ando M, Shima M. Serum interleukins 12 and 18 and immunoglobulin E concentrations and allergic symptoms in Japanese schoolchildren. J Investig Allergol Clin Immunol. 2007;17(1):14–19.
- 1313. Marinho S, Simpson A, Soderstrom L, Woodcock A, Ahlstedt S, Custovic A. Quantification of atopy and the probability of rhinitis in preschool children: a population‐based birth cohort study. Allergy. 2007;62(12):1379–1386. https://doi.org/10.1111/j.1398‐9995.2007.01502.x
- 1314. Salo PM, Calatroni A, Gergen PJ, et al. Allergy‐related outcomes in relation to serum IgE: results from the National Health and Nutrition Examination Survey 2005‐2006. J Allergy Clin Immunol. 2011;127(5):1226–1235.e7. https://doi.org/10.1016/j.jaci.2010.12.1106
- 1315. Jacobs TS, Forno E, Brehm JM, et al. Underdiagnosis of allergic rhinitis in underserved children. J Allergy Clin Immunol. 2014;134(3):737–739.e6. https://doi.org/10.1016/j.jaci.2014.03.028
- 1316. Tu YL, Chang SW, Tsai HJ, et al. Total serum IgE in a population‐based study of Asian children in Taiwan: reference value and significance in the diagnosis of allergy. PLoS One. 2013;8(11):e80996. https://doi.org/10.1371/journal.pone.0080996
- 1317. Park SC, Kim JH, Lee KH, Hong SC, Lee HS, Kang JW. Association of serum eosinophilia and total immunoglobulin E concentration with the risk of allergic symptoms and allergic sensitization, respectively: a 2‐year follow‐up study. Int J Pediatr Otorhinolaryngol. 2016;86:167–171. https://doi.org/10.1016/j.ijporl.2016.05.005
- 1318. Kalpaklioglu AF, Kavut AB. Allergic and nonallergic rhinitis: can we find the differences/similarities between the two pictures? J Asthma. 2009;46(5):481–485. https://doi.org/10.1080/02770900902849897
- 1319. Jung YG, Kim KH, Kim HY, Dhong HJ, Chung SK. Predictive capabilities of serum eosinophil cationic protein, percentage of eosinophils and total immunoglobulin E in allergic rhinitis without bronchial asthma. J Int Med Res. 2011;39(6):2209–2216. https://doi.org/10.1177/147323001103900617
- 1320. Li Y, Wu R, Tian Y, Bao T, Tian Z. The correlation of serum eosinophil cationic protein level with eosinophil count, and total IgE level in Korean adult allergic rhinitis patients. Asian Pac J Allergy Immunol. 2016;34(1):33–37. https://doi.org/10.12932/AP0746
- 1321. Sharma M, Khaitan T, Raman S, Jain R, Kabiraj A. Determination of Serum IgE and Eosinophils as a Diagnostic Indicator in Allergic Rhinitis. Indian J Otolaryngol Head Neck Surg. 2019;71(suppl 3):1957–1961. https://doi.org/10.1007/s12070‐018‐1383‐7
- 1322. Qamar S, Awan N, Cheema KM, Raza N, Ashraf S, Rehman A. Comparative analysis of nasal smear eosinophilia and serum IgE levels for the diagnosis of allergic rhinitis. J Coll Physicians Surg Pak. 2020;30(12):1297–1300. https://doi.org/10.29271/jcpsp.2020.12.1297
- 1323. Karli R, Balbaloglu E, Uzun L, Cinar F, Ugur MB. Correlation of symptoms with total IgE and specific IgE levels in patients presenting with allergic rhinitis. Ther Adv Respir Dis. 2013;7(2):75–79. https://doi.org/10.1177/1753465812468500
- 1324. Chung D, Park KT, Yarlagadda B, Davis EM, Platt M. The significance of serum total immunoglobulin E for in vitro diagnosis of allergic rhinitis. Int Forum Allergy Rhinol. 2014;4(1):56–60. https://doi.org/10.1002/alr.21240
- 1325. Satwani H, Rehman A, Ashraf S, Hassan A. Is serum total IgE levels a good predictor of allergies in children? J Pak Med Assoc. 2009;59(10):698–702.
- 1326. Demirjian M, Rumbyrt JS, Gowda VC, Klaustermeyer WB. Serum IgE and eosinophil count in allergic rhinitis–analysis using a modified Bayes' theorem. Allergol Immunopathol (Madr). 2012;40(5):281–287. https://doi.org/10.1016/j.aller.2011.05.016
- 1327. Shamji MH, Kappen JH, Akdis M, et al. Biomarkers for monitoring clinical efficacy of allergen immunotherapy for allergic rhinoconjunctivitis and allergic asthma: an EAACI Position Paper. Allergy. 2017;72(8):1156–1173. https://doi.org/10.1111/all.13138
- 1328. Goodman RE, Chapman MD, Slater JE. The allergen: sources, extracts, and molecules for diagnosis of allergic disease. J Allergy Clin Immunol Pract. 2020;8(8):2506–2514. https://doi.org/10.1016/j.jaip.2020.06.043
- 1329. Hamilton RG. Clinical laboratory assessment of immediate‐type hypersensitivity. J Allergy Clin Immunol. 2010;125(2 suppl 2):S284–S296. https://doi.org/10.1016/j.jaci.2009.09.055
- 1330. Steering Committee Authors , Review Panel Members . A WAO ‐ ARIA ‐ GA(2)LEN consensus document on molecular‐based allergy diagnosis (PAMD@): update 2020. World Allergy Organ J. 2020;13(2):100091. https://doi.org/10.1016/j.waojou.2019.100091
- 1331. Westwood M, Ramaekers B, Lang S, et al. ImmunoCAP(R) ISAC and Microtest for multiplex allergen testing in people with difficult to manage allergic disease: a systematic review and cost analysis. Health Technol Assess. 2016;20(67):1–178. https://doi.org/10.3310/hta20670
- 1332. Cox L. Overview of serological‐specific IgE antibody testing in children. Curr Allergy Asthma Rep. 2011;11(6):447–453. https://doi.org/10.1007/s11882‐011‐0226‐3
- 1333. Emanuel IA. In vitro testing for allergy diagnosis. Otolaryngol Clin North Am. 2003;36(5):879–893. https://doi.org/10.1016/s0030‐6665(03)00051‐3
- 1334. Incorvaia C, Al‐Ahmad M, Ansotegui IJ, et al. Personalized medicine for allergy treatment: allergen immunotherapy still a unique and unmatched model. Allergy. 2021;76(4):1041–1052. https://doi.org/10.1111/all.14575
- 1335. Corsico AG, De Amici M, Ronzoni V, et al. Allergen‐specific immunoglobulin E and allergic rhinitis severity. Allergy Rhinol (Providence). 2017;8(1):1–4. https://doi.org/10.2500/ar.2017.8.0187
- 1336. Ciprandi G, De Amici M, Giunta V, Marseglia GL. Comparison of serum specific IgE and skin prick test in polysensitized patients. Int J Immunopathol Pharmacol. 2010;23(4):1293–1295. https://doi.org/10.1177/039463201002300438
- 1337. Chen ST, Sun HL, Lu KH, Lue KH, Chou MC. Correlation of immunoglobulin E, eosinophil cationic protein, and eosinophil count with the severity of childhood perennial allergic rhinitis. J Microbiol Immunol Infect. 2006;39(3):212–218.
- 1338. Ciprandi G, Comite P, Ferrero F, Fontana V, Bruzzone M, Mussap M. Serum allergen‐specific IgE, allergic rhinitis severity, and age. Rhinology. 2016;54(3):231–238. https://doi.org/10.4193/Rhino15.300
- 1339. Ciprandi G, Comite P, Ferrero F, et al. Birch allergy and oral allergy syndrome: the practical relevance of serum immunoglobulin E to Bet v 1. Allergy Asthma Proc. 2016;37(1):43–49. https://doi.org/10.2500/aap.2016.37.3914
- 1340. Howarth P, Malling HJ, Molimard M, Devillier P. Analysis of allergen immunotherapy studies shows increased clinical efficacy in highly symptomatic patients. Allergy. 2012;67(3):321–327. https://doi.org/10.1111/j.1398‐9995.2011.02759.x
- 1341. Kowalski ML, Ansotegui I, Aberer W, et al. Risk and safety requirements for diagnostic and therapeutic procedures in allergology: World Allergy Organization Statement. World Allergy Organ J. 2016;9(1):33. https://doi.org/10.1186/s40413‐016‐0122‐3
- 1342. Brown CE, Jones CJ, Stuttaford L, Robertson A, Rashid RS, Smith HE. A qualitative study of the allergy testing experiences, views and preferences of adult patients. Clin Transl Allergy. 2016;6(1):34. https://doi.org/10.1186/s13601‐016‐0125‐8
- 1343. Williams PB, Barnes JH, Szeinbach SL, Sullivan TJ. Analytic precision and accuracy of commercial immunoassays for specific IgE: establishing a standard. J Allergy Clin Immunol. 2000;105(6 pt 1):1221–1230. https://doi.org/10.1067/mai.2000.105219
- 1344. Wood RA, Segall N, Ahlstedt S, Williams PB. Accuracy of IgE antibody laboratory results. Ann Allergy Asthma Immunol. 2007;99(1):34–41. https://doi.org/10.1016/S1081‐1206(10)60618‐7
- 1345. Wang J, Godbold JH, Sampson HA. Correlation of serum allergy (IgE) tests performed by different assay systems. J Allergy Clin Immunol. 2008;121(5):1219–1224. https://doi.org/10.1016/j.jaci.2007.12.1150
- 1346. Ownby DR, Bailey J. Comparison of MAST with radioallergosorbent and skin tests for diagnosis of allergy in children. Am J Dis Child. 1986;140(1):45–48. https://doi.org/10.1001/archpedi.1986.02140150047031
- 1347. Ferguson AC, Murray AB. Predictive value of skin prick tests and radioallergosorbent tests for clinical allergy to dogs and cats. CMAJ. 1986;134(12):1365–1368.
- 1348. Wide L, Bennich H, Johansson SG. Diagnosis of allergy by an in‐vitro test for allergen antibodies. Lancet. 1967;2(7526):1105–1107. https://doi.org/10.1016/s0140‐6736(67)90615‐0
- 1349. Tian M, Zhou Y, Zhang W, Cui Y. Der p 1 and Der p 2 specific immunoglobulin E measurement for diagnosis of Dermatophagoides pteronyssinus allergy: a systematic review and meta‐analysis. Allergy Asthma Proc. 2017;38(5):333–342. https://doi.org/10.2500/aap.2017.38.4073
- 1350. Knight V, Wolf ML, Trikha A, Curran‐Everett D, Hiserote M, Harbeck RJ. A comparison of specific IgE and skin prick test results to common environmental allergens using the HYTEC 288. J Immunol Methods. 2018;462:9–12. https://doi.org/10.1016/j.jim.2018.07.005
- 1351. van Hage M, Schmid‐Grendelmeier P, Skevaki C, et al. Performance evaluation of ImmunoCAP(R) ISAC 112: a multi‐site study. Clin Chem Lab Med. 2017;55(4):571–577. https://doi.org/10.1515/cclm‐2016‐0586
- 1352. Chinoy B, Yee E, Bahna SL. Skin testing versus radioallergosorbent testing for indoor allergens. Clin Mol Allergy. 2005;3(1):4. https://doi.org/10.1186/1476‐7961‐3‐4
- 1353. Bignardi D, Comite P, Mori I, et al. Allergen‐specific IgE: comparison between skin prick test and serum assay in real life. Allergol Select. 2019;3(1):9–14. https://doi.org/10.5414/ALX01891E
- 1354. KleinJan A, Godthelp T, van Toornenenbergen AW, Fokkens WJ. Allergen binding to specific IgE in the nasal mucosa of allergic patients. J Allergy Clin Immunol. 1997;99(4):515–521. https://doi.org/10.1016/s0091‐6749(97)70079‐4
- 1355. Naclerio RM, Creticos PS, Norman PS, Lichtenstein LM. Mediator release after nasal airway challenge with allergen. Am Rev Respir Dis. 1986;134(5):1102. https://doi.org/10.1164/arrd.1986.134.5.1102
- 1356. Ahn JY, Hong SJ, Choi BS. Clinical evaluation of techniques for measuring nasal‐specific immunoglobulin E in pediatric patients. J Korean Med Sci. 2017;32(12):2005–2008. https://doi.org/10.3346/jkms.2017.32.12.2005
- 1357. Campo P, Del Carmen Plaza‐Seron M, Eguiluz‐Gracia I, et al. Direct intranasal application of the solid phase of ImmunoCAP(R) increases nasal specific immunoglobulin E detection in local allergic rhinitis patients. Int Forum Allergy Rhinol. 2018;8(1):15–19. https://doi.org/10.1002/alr.22039
- 1358. Ota Y, Ikemiyagi Y, Sato T, et al. Measuring local immunoglobulin E in the inferior turbinate nasal mucosa in patients with allergic rhinitis. Allergol Int. 2016;65(4):396–399. https://doi.org/10.1016/j.alit.2016.03.009
- 1359. Hamizan A, Alvarado R, Rimmer J, et al. Nasal mucosal brushing as a diagnostic method for allergic rhinitis. Allergy Asthma Proc. 2019;40(3):167–172. https://doi.org/10.2500/aap.2019.40.4209
- 1360. Reisacher WR. Mucosal brush biopsy testing of the inferior turbinate to detect local, antigen‐specific immunoglobulin E. Int Forum Allergy Rhinol. 2012;2(1):69–74. https://doi.org/10.1002/alr.20103
- 1361. Hamizan AW, Rimmer J, Alvarado R, et al. Turbinate‐specific IgE in normal and rhinitic patients. Am J Rhinol Allergy. 2019;33(2):178–183. https://doi.org/10.1177/1945892418825224
- 1362. Saricilar EC, Hamizan A, Alvarado R, et al. Optimizing protein harvest from nasal brushings for determining local allergy responses. Am J Rhinol Allergy. 2018;32(4):244–251. https://doi.org/10.1177/1945892418777668
- 1363. Fuiano N, Fusilli S, Incorvaia C. A role for measurement of nasal IgE antibodies in diagnosis of Alternaria‐induced rhinitis in children. Allergol Immunopathol (Madr). 2012;40(2):71–74. https://doi.org/10.1016/j.aller.2011.03.010
- 1364. Krajewska‐Wojtys A, Jarzab J, Gawlik R, Bozek A. Local allergic rhinitis to pollens is underdiagnosed in young patients. Am J Rhinol Allergy. 2016;30(6):198–201. https://doi.org/10.2500/ajra.2016.30.4369
- 1365. Gelardi M, Guglielmi AV, Iannuzzi L, et al. Local allergic rhinitis: entopy or spontaneous response? World Allergy Organ J. 2016;9(1):39. https://doi.org/10.1186/s40413‐016‐0126‐z
- 1366. Santamaria L, Calle A, Tejada‐Giraldo Biol M, Calvo V, Sanchez J, Cardona R. Nasal specific IgE to Der p is not an acceptable screening test to predict the outcome of the nasal challenge test in patients with non‐allergic rhinitis. World Allergy Organ J. 2020;13(9):100461. https://doi.org/10.1016/j.waojou.2020.100461
- 1367. Kim JH, Yoon MG, Seo DH, et al. Detection of allergen specific antibodies from nasal secretion of allergic rhinitis patients. Allergy Asthma Immunol Res. 2016;8(4):329–337. https://doi.org/10.4168/aair.2016.8.4.329
- 1368. Hamizan AW, Rimmer J, Husain S, et al. Local specific immunoglobulin E among patients with nonallergic rhinitis: a systematic review. Rhinology. 2019;57(1):10–20. https://doi.org/10.4193/Rhin18.074
- 1369. Eguiluz‐Gracia I, Ariza A, Testera‐Montes A, Rondon C, Campo P. Allergen Immunotherapy for local respiratory allergy. Curr Allergy Asthma Rep. 2020;20(7):23. https://doi.org/10.1007/s11882‐020‐00920‐w
- 1370. Schiavi L, Brindisi G, De Castro G, et al. Nasal reactivity evaluation in children with allergic rhinitis receiving grass pollen sublingual immunotherapy. Allergy Asthma Proc. 2020;41(5):357–362. https://doi.org/10.2500/aap.2020.41.200063
- 1371. Lee KS, Yu J, Shim D, et al. Local immune responses in children and adults with allergic and nonallergic rhinitis. PLoS One. 2016;11(6):e0156979. https://doi.org/10.1371/journal.pone.0156979
- 1372. Sakaida H, Masuda S, Takeuchi K. Measurement of Japanese cedar pollen‐specific IgE in nasal secretions. Allergol Int. 2014;63(3):467–473. https://doi.org/10.2332/allergolint.13‐OA‐0668
- 1373. Powe DG, Groot Kormelink T, Sisson M, et al. Evidence for the involvement of free light chain immunoglobulins in allergic and nonallergic rhinitis. J Allergy Clin Immunol. 2010;125(1):139–145.e1‐3. https://doi.org/10.1016/j.jaci.2009.07.025
- 1374. Ahn CN, Wise SK, Lathers DM, Mulligan RM, Harvey RJ, Schlosser RJ. Local production of antigen‐specific IgE in different anatomic subsites of allergic fungal rhinosinusitis patients. Otolaryngol Head Neck Surg. 2009;141(1):97–103. https://doi.org/10.1016/j.otohns.2009.03.002
- 1375. Castelli S, Arasi S, Tripodi S, et al. IgE antibody repertoire in nasal secretions of children and adults with seasonal allergic rhinitis: a molecular analysis. Pediatr Allergy Immunol. 2020;31(3):273–280. https://doi.org/10.1111/pai.13148
- 1376. Sensi LG, Piacentini GL, Nobile E, et al. Changes in nasal specific IgE to mites after periods of allergen exposure‐avoidance: a comparison with serum levels. Clin Exp Allergy. 1994;24(4):377–382. https://doi.org/10.1111/j.1365‐2222.1994.tb00250.x
- 1377. Nelson HS, Lahr J, Buchmeier A, McCormick D. Evaluation of devices for skin prick testing. J Allergy Clin Immunol. 1998;101(2 pt 1):153–156. https://doi.org/10.1016/S0091‐6749(98)70409‐9
- 1378. Andersen HH, Lundgaard AC, Petersen AS, et al. The Lancet weight determines wheal diameter in response to skin prick testing with histamine. PLoS One. 2016;11(5):e0156211. https://doi.org/10.1371/journal.pone.0156211
- 1379. Carr WW, Martin B, Howard RS, et al. Comparison of test devices for skin prick testing. J Allergy Clin Immunol. 2005;116(2):341–346. https://doi.org/10.1016/j.jaci.2005.03.035
- 1380. Seibert SM, King TS, Kline D, Mende C, Craig T. Reliability of skin test results when read at different time points. Allergy Asthma Proc. 2011;32(3):203–205. https://doi.org/10.2500/aap.2011.32.3436
- 1381. van der Veen MJ, Mulder M, Witteman AM, et al. False‐positive skin prick test responses to commercially available dog dander extracts caused by contamination with house dust mite (Dermatophagoides pteronyssinus) allergens. J Allergy Clin Immunol. 1996;98(6 pt 1):1028–1034. https://doi.org/10.1016/s0091‐6749(96)80187‐4
- 1382. McCann WA, Ownby DR. The reproducibility of the allergy skin test scoring and interpretation by board‐certified/board‐eligible allergists. Ann Allergy Asthma Immunol. 2002;89(4):368–371. https://doi.org/10.1016/S1081‐1206(10)62037‐6
- 1383. Choi IS, Koh YI, Koh JS, Lee MG. Sensitivity of the skin prick test and specificity of the serum‐specific IgE test for airway responsiveness to house dust mites in asthma. J Asthma. 2005;42(3):197–202.
- 1384. Jung YG, Cho HJ, Park GY, et al. Comparison of the skin‐prick test and Phadia ImmunoCAP as tools to diagnose house‐dust mite allergy. Am J Rhinol Allergy. 2010;24(3):226–229. https://doi.org/10.2500/ajra.2010.24.3459
- 1385. Pastorello EA, Incorvaia C, Ortolani C, et al. Studies on the relationship between the level of specific IgE antibodies and the clinical expression of allergy: I. Definition of levels distinguishing patients with symptomatic from patients with asymptomatic allergy to common aeroallergens. J Allergy Clin Immunol. 1995;96(5 pt 1):580–587. https://doi.org/10.1016/s0091‐6749(95)70255‐5
- 1386. Haxel BR, Huppertz T, Boessert P, Bast F, Fruth K. Correlation of skin test results and specific immunoglobulin E blood levels with nasal provocation testing for house‐dust mite allergies. Am J Rhinol Allergy. 2016;30(1):60–64. https://doi.org/10.2500/ajra.2016.30.4262
- 1387. Gendo K, Larson EB. Evidence‐based diagnostic strategies for evaluating suspected allergic rhinitis. Ann Intern Med. 2004;140(4):278–289. https://doi.org/10.7326/0003‐4819‐140‐4‐200402170‐00010
- 1388. de Vos G, Nazari R, Ferastraoaru D, et al. Discordance between aeroallergen specific serum IgE and skin testing in children younger than 4 years. Ann Allergy Asthma Immunol. 2013;110(6):438–443. https://doi.org/10.1016/j.anai.2013.03.006
- 1389. Hoffmann HJ, Santos AF, Mayorga C, et al. The clinical utility of basophil activation testing in diagnosis and monitoring of allergic disease. Allergy. 2015;70(11):1393–1405. https://doi.org/10.1111/all.12698
- 1390. Gonzalez‐Munoz M, Villota J, Moneo I. Analysis of basophil activation by flow cytometry in pediatric house dust mite allergy. Pediatr Allergy Immunol. 2008;19(4):342–347. https://doi.org/10.1111/j.1399‐3038.2007.00656.x
- 1391. Ozdemir SK, Guloglu D, Sin BA, Elhan AH, Ikinciogullari A, Misirligil Z. Reliability of basophil activation test using CD203c expression in diagnosis of pollen allergy. Am J Rhinol Allergy. 2011;25(6):e225–e231. https://doi.org/10.2500/ajra.2011.25.3723
- 1392. Ogulur I, Kiykim A, Baris S, Ozen A, Yuce EG, Karakoc‐Aydiner E. Basophil activation test for inhalant allergens in pediatric patients with allergic rhinitis. Int J Pediatr Otorhinolaryngol. 2017;97:197–201. https://doi.org/10.1016/j.ijporl.2017.04.006
- 1393. Zidarn M, Robic M, Krivec A, et al. Clinical and immunological differences between asymptomatic HDM‐sensitized and HDM‐allergic rhinitis patients. Clin Exp Allergy. 2019;49(6):808–818. https://doi.org/10.1111/cea.13361
- 1394. Mahmood F, Hetland G, Nentwich I, Mirlashari MR, Ghiasvand R, Nissen‐Meyer LSH. Agaricus blazei‐based mushroom extract supplementation to birch allergic blood donors: a randomized clinical trial. Nutrients. 2019;11(10):2339. https://doi.org/10.3390/nu11102339
- 1395. Saporta M, Kamei S, Persi L, Bousquet J, Arnoux B. Basophil activation during pollen season in patients monosensitized to grass pollens. Allergy. 2001;56(5):442–445. https://doi.org/10.1034/j.1398‐9995.2001.056005442.x
- 1396. Qiao Y, Chen J. Investigating the inflammatory cascade effect of basophil activation in children with allergic rhinitis or asthma, via the IgE‐FcepsilonRI‐NF‐kappaB signaling pathway. Adv Clin Exp Med. 2021;30(7):673–679. https://doi.org/10.17219/acem/135756
- 1397. Nagao M, Hiraguchi Y, Hosoki K, et al. Allergen‐induced basophil CD203c expression as a biomarker for rush immunotherapy in patients with Japanese cedar pollinosis. Int Arch Allergy Immunol. 2008;146(suppl 1):47–53. https://doi.org/10.1159/000126061
- 1398. Van Overtvelt L, Baron‐Bodo V, Horiot S, et al. Changes in basophil activation during grass‐pollen sublingual immunotherapy do not correlate with clinical efficacy. Allergy. 2011;66(12):1530–1537. https://doi.org/10.1111/j.1398‐9995.2011.02696.x
- 1399. Swamy RS, Reshamwala N, Hunter T, et al. Epigenetic modifications and improved regulatory T‐cell function in subjects undergoing dual sublingual immunotherapy. J Allergy Clin Immunol. 2012;130(1):215–224.e7. https://doi.org/10.1016/j.jaci.2012.04.021
- 1400. Caruso M, Cibella F, Emma R, et al. Basophil biomarkers as useful predictors for sublingual immunotherapy in allergic rhinitis. Int Immunopharmacol. 2018;60:50–58. https://doi.org/10.1016/j.intimp.2018.04.034
- 1401. Kepil Ozdemir S, Sin BA, Guloglu D, Ikinciogullari A, Gencturk Z, Misirligil Z. Short‐term preseasonal immunotherapy: is early clinical efficacy related to the basophil response? Int Arch Allergy Immunol. 2014;164(3):237–245. https://doi.org/10.1159/000365628
- 1402. Zidarn M, Kosnik M, Silar M, Bajrovic N, Korosec P. Sustained effect of grass pollen subcutaneous immunotherapy on suppression of allergen‐specific basophil response; a real‐life, nonrandomized controlled study. Allergy. 2015;70(5):547–555. https://doi.org/10.1111/all.12581
- 1403. Schmid JM, Wurtzen PA, Siddhuraj P, et al. Basophil sensitivity reflects long‐term clinical outcome of subcutaneous immunotherapy in grass pollen‐allergic patients. Allergy. 2021;76(5):1528–1538. https://doi.org/10.1111/all.14264
- 1404. Kim SH, Kim SH, Chung SJ, et al. Changes in basophil activation during immunotherapy with house dust mite and mugwort in patients with allergic rhinitis. Asia Pac Allergy. 2018;8(1):e6. https://doi.org/10.5415/apallergy.2018.8.e6
- 1405. Feng M, Zeng X, Su Q, et al. Allergen Immunotherapy‐induced immunoglobulin G4 reduces basophil activation in house dust mite‐allergic asthma patients. Front Cell Dev Biol. 2020;8:30. https://doi.org/10.3389/fcell.2020.00030
- 1406. Soyyigit S, Guloglu D, Ikinciogullari A, et al. Immunologic alterations and efficacy of subcutaneous immunotherapy with Dermatophagoides pteronyssinus in monosensitized and polysensitized patients. Ann Allergy Asthma Immunol. 2016;116(3):244–251.e2. https://doi.org/10.1016/j.anai.2016.01.002
- 1407. Aasbjerg K, Backer V, Lund G, et al. Immunological comparison of allergen immunotherapy tablet treatment and subcutaneous immunotherapy against grass allergy. Clin Exp Allergy. 2014;44(3):417–428. https://doi.org/10.1111/cea.12241
- 1408. Ma S, Qiao Y. [Changes of basophil activation before and after treatment in children with allergic rhinitis and its clinical significance]. Revue Francaise D'Allergologie. 2021;61(6):393–397.
- 1409. Matricardi PM, Kleine‐Tebbe J, Hoffmann HJ, et al. EAACI molecular allergology user's guide. Pediatr Allergy Immunol. 2016;27(suppl 23):1–250. https://doi.org/10.1111/pai.12563
- 1410. Sastre‐Ibanez M, Sastre J. Molecular allergy diagnosis for the clinical characterization of asthma. Expert Rev Mol Diagn. 2015;15(6):789–799. https://doi.org/10.1586/14737159.2015.1036745
- 1411. Barber D, Diaz‐Perales A, Escribese MM, et al. Molecular allergology and its impact in specific allergy diagnosis and therapy. Allergy. 2021;76(12):3642–3658. https://doi.org/10.1111/all.14969
- 1412. Sastre J, Sastre‐Ibanez M. Molecular diagnosis and immunotherapy. Curr Opin Allergy Clin Immunol. 2016;16(6):565–570. https://doi.org/10.1097/ACI.0000000000000318
- 1413. Sastre J. Molecular diagnosis and immunotherapy. Curr Opin Allergy Clin Immunol. 2013;13(6):646–650. https://doi.org/10.1097/ACI.0b013e328364f4c6
- 1414. Saltabayeva U, Garib V, Morenko M, et al. Greater real‐life diagnostic efficacy of allergen molecule‐based diagnosis for prescription of immunotherapy in an area with multiple pollen exposure. Int Arch Allergy Immunol. 2017;173(2):93–98. https://doi.org/10.1159/000477442
- 1415. Scala E, Abeni D, Pomponi D, et al. Ole e 1, Ole e 7, and Ole e 9: identifying distinct clinical subsets of olive tree‐allergic patients. J Allergy Clin Immunol. 2016;137(2):629–631.e3. https://doi.org/10.1016/j.jaci.2015.07.009
- 1416. Sastre J, Rodriguez F, Campo P, Laffond E, Marin A, Alonso MD. Adverse reactions to immunotherapy are associated with different patterns of sensitization to grass allergens. Allergy. 2015;70(5):598–600. https://doi.org/10.1111/all.12575
- 1417. Posa D, Perna S, Resch Y, et al. Evolution and predictive value of IgE responses toward a comprehensive panel of house dust mite allergens during the first 2 decades of life. J Allergy Clin Immunol. 2017;139(2):541–549.e8. https://doi.org/10.1016/j.jaci.2016.08.014
- 1418. Bronnert M, Mancini J, Birnbaum J, et al. Component‐resolved diagnosis with commercially available D. pteronyssinus Der p 1, Der p 2 and Der p 10: relevant markers for house dust mite allergy. Clin Exp Allergy. 2012;42(9):1406–1415. https://doi.org/10.1111/j.1365‐2222.2012.04035.x
- 1419. Celi G, Brusca I, Scala E, et al. House dust mite allergy in Italy‐Diagnostic and clinical relevance of Der p 23 (and of minor allergens): a real‐life, multicenter study. Allergy. 2019;74(9):1787–1789. https://doi.org/10.1111/all.13776
- 1420. Barber D, Arias J, Boquete M, et al. Analysis of mite allergic patients in a diverse territory by improved diagnostic tools. Clin Exp Allergy. 2012;42(7):1129–1138. https://doi.org/10.1111/j.1365‐2222.2012.03993.x
- 1421. Carvalho Kdos A, de Melo‐Neto OP, Magalhaes FB, et al. Blomia tropicalis Blo t 5 and Blo t 21 recombinant allergens might confer higher specificity to serodiagnostic assays than whole mite extract. BMC Immunol. 2013;14:11. https://doi.org/10.1186/1471‐2172‐14‐11
- 1422. Ayuso R, Reese G, Leong‐Kee S, Plante M, Lehrer SB. Molecular basis of arthropod cross‐reactivity: IgE‐binding cross‐reactive epitopes of shrimp, house dust mite and cockroach tropomyosins. Int Arch Allergy Immunol. 2002;129(1):38–48. https://doi.org/10.1159/000065172
- 1423. Gamez C, Sanchez‐Garcia S, Ibanez MD, et al. Tropomyosin IgE‐positive results are a good predictor of shrimp allergy. Allergy. 2011;66(10):1375–1383. https://doi.org/10.1111/j.1398‐9995.2011.02663.x
- 1424. Rodriguez‐Dominguez A, Berings M, Rohrbach A, et al. Molecular profiling of allergen‐specific antibody responses may enhance success of specific immunotherapy. J Allergy Clin Immunol. 2020;146(5):1097–1108. https://doi.org/10.1016/j.jaci.2020.03.029
- 1425. Saarelainen S, Taivainen A, Rytkonen‐Nissinen M, et al. Assessment of recombinant dog allergens Can f 1 and Can f 2 for the diagnosis of dog allergy. Clin Exp Allergy. 2004;34(10):1576–1582. https://doi.org/10.1111/j.1365‐2222.2004.02071.x
- 1426. Mattsson L, Lundgren T, Everberg H, Larsson H, Lidholm J. Prostatic kallikrein: a new major dog allergen. J Allergy Clin Immunol. 2009;123(2):362–368. https://doi.org/10.1016/j.jaci.2008.11.021
- 1427. Uriarte SA, Sastre J. Clinical relevance of molecular diagnosis in pet allergy. Allergy. 2016;71(7):1066–1068. https://doi.org/10.1111/all.12917
- 1428. Schoos AM, Nwaru BI, Borres MP. Component‐resolved diagnostics in pet allergy: current perspectives and future directions. J Allergy Clin Immunol. 2021;147(4):1164–1173. https://doi.org/10.1016/j.jaci.2020.12.640
- 1429. Wintersand A, Asplund K, Binnmyr J, et al. Allergens in dog extracts: implication for diagnosis and treatment. Allergy. 2019;74(8):1472–1479. https://doi.org/10.1111/all.13785
- 1430. Eder K, Becker S, San Nicolo M, Berghaus A, Groger M. Usefulness of component resolved analysis of cat allergy in routine clinical practice. Allergy Asthma Clin Immunol. 2016;12:58. https://doi.org/10.1186/s13223‐016‐0163‐8
- 1431. Smith W, Butler AJ, Hazell LA, et al. Fel d 4, a cat lipocalin allergen. Clin Exp Allergy. 2004;34(11):1732–1738. https://doi.org/10.1111/j.1365‐2222.2004.02090.x
- 1432. Saarelainen S, Rytkonen‐Nissinen M, Rouvinen J, et al. Animal‐derived lipocalin allergens exhibit immunoglobulin E cross‐reactivity. Clin Exp Allergy. 2008;38(2):374–381. https://doi.org/10.1111/j.1365‐2222.2007.02895.x
- 1433. Arruda LK, Vailes LD, Ferriani VP, Santos AB, Pomes A, Chapman MD. Cockroach allergens and asthma. J Allergy Clin Immunol. 2001;107(3):419–428. https://doi.org/10.1067/mai.2001.112854
- 1434. Postigo I, Gutierrez‐Rodriguez A, Fernandez J, Guisantes JA, Sunen E, Martinez J. Diagnostic value of Alt a 1, fungal enolase and manganese‐dependent superoxide dismutase in the component‐resolved diagnosis of allergy to Pleosporaceae. Clin Exp Allergy. 2011;41(3):443–451. https://doi.org/10.1111/j.1365‐2222.2010.03671.x
- 1435. Kespohl S, Raulf M. Mould allergens: where do we stand with molecular allergy diagnostics?: Part 13 of the series Molecular Allergology. Allergo J Int. 2014;23(4):120–125. https://doi.org/10.1007/s40629‐014‐0014‐4
- 1436. Barber D, Moreno C, Ledesma A, et al. Degree of olive pollen exposure and sensitization patterns. Clinical implications. J Investig Allergol Clin Immunol. 2007;17(suppl 1):11–16.
- 1437. Sastre J. Molecular diagnosis in allergy. Clin Exp Allergy. 2010;40(10):1442–1460. https://doi.org/10.1111/j.1365‐2222.2010.03585.x
- 1438. Martinez‐Canavate Burgos A, Torres‐Borrego J, Molina Teran AB, et al. Molecular sensitization patterns and influence of molecular diagnosis in immunotherapy prescription in children sensitized to both grass and olive pollen. Pediatr Allergy Immunol. 2018;29(4):369–374. https://doi.org/10.1111/pai.12866
- 1439. Moreno C, Justicia JL, Quiralte J, et al. Olive, grass or both? Molecular diagnosis for the allergen immunotherapy selection in polysensitized pollinic patients. Allergy. 2014;69(10):1357–1363. https://doi.org/10.1111/all.12474
- 1440. Stringari G, Tripodi S, Caffarelli C, et al. The effect of component‐resolved diagnosis on specific immunotherapy prescription in children with hay fever. J Allergy Clin Immunol. 2014;134(1):75–81. https://doi.org/10.1016/j.jaci.2014.01.042
- 1441. Letran A, Espinazo M, Moreno F. Measurement of IgE to pollen allergen components is helpful in selecting patients for immunotherapy. Ann Allergy Asthma Immunol. 2013;111(4):295–297. https://doi.org/10.1016/j.anai.2013.07.005
- 1442. Nolte M, Barber D, Maloney J, et al. Timothy specific IgE levels are associated with efficacy and safety of timothy grass sublingual immunotherapy tablet. Ann Allergy Asthma Immunol. 2015;115(6):509–515.e2. https://doi.org/10.1016/j.anai.2015.09.018
- 1443. Rodinkova VV, Yuriev SD, Kryvopustova MV, Mokin VB, Kryzhanovskyi YM, Kurchenko AI. Molecular profile sensitization to house dust mites as an important aspect for predicting the efficiency of allergen immunotherapy. Front Immunol. 2022;13:848616. https://doi.org/10.3389/fimmu.2022.848616
- 1444. Arroabarren E, Echechipia S, Galbete A, Lizaso MT, Olaguibel JM, Tabar AI. Association between component‐resolved diagnosis of house dust mite allergy and efficacy and safety of specific immunotherapy. J Investig Allergol Clin Immunol. 2019;29(2):164–167. https://doi.org/10.18176/jiaci.0359
- 1445. Chen KW, Zieglmayer P, Zieglmayer R, et al. Selection of house dust mite‐allergic patients by molecular diagnosis may enhance success of specific immunotherapy. J Allergy Clin Immunol. 2019;143(3):1248–1252.e12. https://doi.org/10.1016/j.jaci.2018.10.048
- 1446. di Coste A, Occasi F, De Castro G, et al. Predictivity of clinical efficacy of sublingual immunotherapy (SLIT) based on sensitisation pattern to molecular allergens in children with allergic rhinoconjunctivitis. Allergol Immunopathol (Madr). 2017;45(5):452–456. https://doi.org/10.1016/j.aller.2017.01.001
- 1447. Darsow U, Brockow K, Pfab F, et al. Allergens. Heterogeneity of molecular sensitization profiles in grass pollen allergy–implications for immunotherapy? Clin Exp Allergy. 2014;44(5):778–786. https://doi.org/10.1111/cea.12303
- 1448. Sastre J, Landivar ME, Ruiz‐Garcia M, Andregnette‐Rosigno MV, Mahillo I. How molecular diagnosis can change allergen‐specific immunotherapy prescription in a complex pollen area. Allergy. 2012;67(5):709–711. https://doi.org/10.1111/j.1398‐9995.2012.02808.x
- 1449. Tripodi S, Frediani T, Lucarelli S, et al. Molecular profiles of IgE to Phleum pratense in children with grass pollen allergy: implications for specific immunotherapy. J Allergy Clin Immunol. 2012;129(3):834–839.e8. https://doi.org/10.1016/j.jaci.2011.10.045
- 1450. Duffort O, Palomares O, Lombardero M, et al. Variability of Ole e 9 allergen in olive pollen extracts: relevance of minor allergens in immunotherapy treatments. Int Arch Allergy Immunol. 2006;140(2):131–138. https://doi.org/10.1159/000092532
- 1451. Pfaar O, Calderon MA, Andrews CP, et al. Allergen exposure chambers: harmonizing current concepts and projecting the needs for the future – an EAACI Position Paper. Allergy. 2017;72(7):1035–1042. https://doi.org/10.1111/all.13133
- 1452. Werfel T, Heratizadeh A, Niebuhr M, et al. Exacerbation of atopic dermatitis on grass pollen exposure in an environmental challenge chamber. J Allergy Clin Immunol. 2015;136(1):96–103.e9. https://doi.org/10.1016/j.jaci.2015.04.015
- 1453. Badorrek P, Dick M, Emmert L, et al. Pollen starch granules in bronchial inflammation. Ann Allergy Asthma Immunol. 2012;109(3):208–214.e6. https://doi.org/10.1016/j.anai.2012.06.019
- 1454. Ahuja SK, Manoharan MS, Harper NL, et al. Preservation of epithelial cell barrier function and muted inflammation in resistance to allergic rhinoconjunctivitis from house dust mite challenge. J Allergy Clin Immunol. 2017;139(3):844–854. https://doi.org/10.1016/j.jaci.2016.08.019
- 1455. Smith AM, Ramirez RM, Harper N, et al. Large‐scale provocation studies identify maladaptive responses to ubiquitous aeroallergens as a correlate of severe allergic rhinoconjunctivitis and asthma. Allergy. 2022;77(6):1797–1814. https://doi.org/10.1111/all.15124
- 1456. Smith AM, Harper N, Meunier JA, et al. Repetitive aeroallergen challenges elucidate maladaptive epithelial and inflammatory traits that underpin allergic airway diseases. J Allergy Clin Immunol. 2021;148(2):533–549. https://doi.org/10.1016/j.jaci.2021.01.008
- 1457. Ellis AK, Steacy LM, Hobsbawn B, Conway CE, Walker TJ. Clinical validation of controlled grass pollen challenge in the Environmental Exposure Unit (EEU). Allergy Asthma Clin Immunol. 2015;11(1):5. https://doi.org/10.1186/s13223‐015‐0071‐3
- 1458. Ellis AK, Soliman M, Steacy LM, Adams DE, Hobsbawn B, Walker TJ. Clinical validation of controlled exposure to birch pollen in the Environmental Exposure Unit (EEU). Allergy Asthma Clin Immunol. 2016;12:53. https://doi.org/10.1186/s13223‐016‐0156‐7
- 1459. Enomoto T, Ide T, Ogino S. Construction of an environmental exposure unit and investigation of the effects of cetirizine hydrochloride on symptoms of cedar pollinosis in Japan. J Investig Allergol Clin Immunol. 2007;17(3):173–81.
- 1460. Hashiguchi K, Tang H, Fujita T, et al. Validation study of the OHIO Chamber in patients with Japanese cedar pollinosis. Int Arch Allergy Immunol. 2009;149(2):141–149. https://doi.org/10.1159/000189197
- 1461. Jacobs RL, Ramirez DA, Andrews CP. Validation of the biogenics research chamber for Juniperus ashei (mountain cedar) pollen. Ann Allergy Asthma Immunol. 2011;107(2):133–138. https://doi.org/10.1016/j.anai.2011.04.009
- 1462. Krug N, Hohlfeld JM, Larbig M, et al. Validation of an environmental exposure unit for controlled human inhalation studies with grass pollen in patients with seasonal allergic rhinitis. Clin Exp Allergy. 2003;33(12):1667–1674. https://doi.org/10.1111/j.1365‐2222.2003.01810.x
- 1463. Lueer K, Biller H, Casper A, et al. Safety, efficacy and repeatability of a novel house dust mite allergen challenge technique in the Fraunhofer allergen challenge chamber. Allergy. 2016;71(12):1693–1700. https://doi.org/10.1111/all.12947
- 1464. Ronborg SM, Mosbech H, Poulsen LK. Exposure chamber for allergen challenge. A placebo‐controlled, double‐blind trial in house‐dust‐mite asthma. Allergy. 1997;52(8):821–828. https://doi.org/10.1111/j.1398‐9995.1997.tb02153.x
- 1465. Zuberbier T, Abelson MB, Akdis CA, et al. Validation of the Global Allergy and Asthma European Network (GA(2)LEN) chamber for trials in allergy: innovation of a mobile allergen exposure chamber. J Allergy Clin Immunol. 2017;139(4):1158–1166. https://doi.org/10.1016/j.jaci.2016.08.025
- 1466. Hohlfeld JM, Holland‐Letz T, Larbig M, et al. Diagnostic value of outcome measures following allergen exposure in an environmental challenge chamber compared with natural conditions. Clin Exp Allergy. 2010;40(7):998–1006. https://doi.org/10.1111/j.1365‐2222.2010.03498.x
- 1467. Boelke G, Berger U, Bergmann KC, et al. Peak nasal inspiratory flow as outcome for provocation studies in allergen exposure chambers: a GA(2)LEN study. Clin Transl Allergy. 2017;7:33. https://doi.org/10.1186/s13601‐017‐0169‐4
- 1468. Gherasim A, Fauquert JL, Domis N, Siomboing X, de Blay F. Birch allergen challenges in allergic conjunctivitis using standard conjunctival allergen challenge and environmental exposure chamber. Clin Transl Allergy. 2021;11(6):e12053. https://doi.org/10.1002/clt2.12053
- 1469. Jacobs RL, Ramirez DA, Rather CG, et al. Redness response phenotypes of allergic conjunctivitis in an allergen challenge chamber. Ann Allergy Asthma Immunol. 2017;118(1):86–93.e2. https://doi.org/10.1016/j.anai.2016.10.023
- 1470. Badorrek P, Muller M, Koch W, Hohlfeld JM, Krug N. Specificity and reproducibility of nasal biomarkers in patients with allergic rhinitis after allergen challenge chamber exposure. Ann Allergy Asthma Immunol. 2017;118(3):290–297. https://doi.org/10.1016/j.anai.2017.01.018
- 1471. North ML, Jones MJ, MacIsaac JL, et al. Blood and nasal epigenetics correlate with allergic rhinitis symptom development in the environmental exposure unit. Allergy. 2018;73(1):196–205. https://doi.org/10.1111/all.13263
- 1472. Krug N, Gupta A, Badorrek P, et al. Efficacy of the oral chemoattractant receptor homologous molecule on TH2 cells antagonist BI 671800 in patients with seasonal allergic rhinitis. J Allergy Clin Immunol. 2014;133(2):414–419. https://doi.org/10.1016/j.jaci.2013.10.013
- 1473. Horak FF, Jager S, Nirnberger G, et al. Dose‐related control of allergic rhinitis symptoms by a H1‐receptor antagonist. Finding the proper doses [correction of dosis] of dimethindene maleate in patients with allergic rhinitis. Int Arch Allergy Immunol. 1994;103(3):298–302. https://doi.org/10.1159/000236643
- 1474. Horak F, Jager S, Nirnberger G, et al. Pharmacodynamic dose finding of dimetindene in a sustained release formulation. Arzneimittelforschung. 1993;43(11):1193–1195.
- 1475. Day JH, Briscoe MP, Ratz JD, Ellis AK, Yao R, Danzig M. Onset of action of loratadine/montelukast in seasonal allergic rhinitis subjects exposed to ragweed pollen in the Environmental Exposure Unit. Allergy Asthma Proc. 2009;30(3):270–276. https://doi.org/10.2500/aap.2009.30.3234
- 1476. Horak F, Zieglmayer P, Zieglmayer R, Lemell P. Onset of action of loratadine/montelukast in seasonal allergic rhinitis patients exposed to grass pollen. Arzneimittelforschung. 2010;60(5):249–255. https://doi.org/10.1055/s‐0031‐1296281
- 1477. Berkowitz RB, Woodworth GG, Lutz C, et al. Onset of action, efficacy, and safety of fexofenadine 60 mg/pseudoephedrine 120 mg versus placebo in the Atlanta allergen exposure unit. Ann Allergy Asthma Immunol. 2002;89(1):38–45. https://doi.org/10.1016/S1081‐1206(10)61909‐6
- 1478. Day JH, Briscoe MP, Rafeiro E, Ratz JD. Comparative clinical efficacy, onset and duration of action of levocetirizine and desloratadine for symptoms of seasonal allergic rhinitis in subjects evaluated in the Environmental Exposure Unit (EEU). Int J Clin Pract. 2004;58(2):109–118. https://doi.org/10.1111/j.1368‐5031.2004.0117.x
- 1479. Horak F, Zieglmayer UP, Zieglmayer R, et al. Azelastine nasal spray and desloratadine tablets in pollen‐induced seasonal allergic rhinitis: a pharmacodynamic study of onset of action and efficacy. Curr Med Res Opin. 2006;22(1):151–157. https://doi.org/10.1185/030079906X80305
- 1480. Bousquet J, Meltzer EO, Couroux P, et al. Onset of action of the fixed combination intranasal azelastine‐fluticasone propionate in an allergen exposure chamber. J Allergy Clin Immunol Pract. 2018;6(5):1726–1732.e6. https://doi.org/10.1016/j.jaip.2018.01.031
- 1481. Tenn MW, Steacy LM, Ng CC, Ellis AK. Onset of action for loratadine tablets for the symptomatic control of seasonal allergic rhinitis in adults challenged with ragweed pollen in the Environmental Exposure Unit: a post hoc analysis of total symptom score. Allergy Asthma Clin Immunol. 2018;14:5. https://doi.org/10.1186/s13223‐017‐0227‐4
- 1482. Day JH, Briscoe MP, Rafeiro E, Hewlett D Jr., Chapman D, Kramer B. Randomized double‐blind comparison of cetirizine and fexofenadine after pollen challenge in the Environmental Exposure Unit: duration of effect in subjects with seasonal allergic rhinitis. Allergy Asthma Proc. 2004;25(1):59–68.
- 1483. Murdoch RD, Bareille P, Ignar D, et al. Once‐daily dosing of levocabastine has comparable efficacy to twice‐daily dosing in the treatment of allergic rhinitis assessed in an allergen challenge chamber. Int J Clin Pharmacol Ther. 2015;53(10):811–818. https://doi.org/10.5414/CP202389
- 1484. Horak F, Zieglmayer PU, Zieglmayer R, Kavina A, Lemell P. Levocetirizine has a longer duration of action on improving total nasal symptoms score than fexofenadine after single administration. Br J Clin Pharmacol. 2005;60(1):24–31. https://doi.org/10.1111/j.1365‐2125.2005.02377.x
- 1485. Krug N, Hohlfeld JM, Geldmacher H, et al. Effect of loteprednol etabonate nasal spray suspension on seasonal allergic rhinitis assessed by allergen challenge in an environmental exposure unit. Allergy. 2005;60(3):354–359. https://doi.org/10.1111/j.1398‐9995.2005.00703.x
- 1486. Salapatek AM, Patel P, Gopalan G, Varghese ST. Mometasone furoate nasal spray provides early, continuing relief of nasal congestion and improves nasal patency in allergic patients. Am J Rhinol Allergy. 2010;24(6):433–438. https://doi.org/10.2500/ajra.2010.24.3548
- 1487. Zieglmayer P, Zieglmayer R, Bareille P, Rousell V, Salmon E, Horak F. Fluticasone furoate versus placebo in symptoms of grass‐pollen allergic rhinitis induced by exposure in the Vienna Challenge Chamber. Curr Med Res Opin. 2008;24(6):1833–1840. https://doi.org/10.1185/03007990802155792
- 1488. Ng CC, Romaikin D, Steacy LM, et al. Comparative nasal airflow with loratadine‐pseudoephedrine and fluticasone nasal spray for allergic rhinitis. Ann Allergy Asthma Immunol. 2021;127(3):342–348.e2. https://doi.org/10.1016/j.anai.2021.05.001
- 1489. Zieglmayer P, Schmutz R, Lemell P, et al. Fast effectiveness of a solubilized low‐dose budesonide nasal spray in allergic rhinitis. Clin Exp Allergy. 2020;50(9):1065–1077. https://doi.org/10.1111/cea.13691
- 1490. Badorrek P, Dick M, Schauerte A, et al. A combination of cetirizine and pseudoephedrine has therapeutic benefits when compared to single drug treatment in allergic rhinitis. Int J Clin Pharmacol Ther. 2009;47(2):71–77. https://doi.org/10.5414/cpp47071
- 1491. Barchuk WT, Salapatek AM, Ge T, D'Angelo P, Liu X. A proof‐of‐concept study of the effect of a novel H3‐receptor antagonist in allergen‐induced nasal congestion. J Allergy Clin Immunol. 2013;132(4):838‐846.e1‐6. https://doi.org/10.1016/j.jaci.2013.05.001
- 1492. Horak F, Toth J, Marks B, et al. Efficacy and safety relative to placebo of an oral formulation of cetirizine and sustained‐release pseudoephedrine in the management of nasal congestion. Allergy. 1998;53(9):849–856. https://doi.org/10.1111/j.1398‐9995.1998.tb03990.x
- 1493. Yonekura S, Okamoto Y, Yamamoto H, et al. Randomized double‐blind study of prophylactic treatment with an antihistamine for seasonal allergic rhinitis. Int Arch Allergy Immunol. 2013;162(1):71–78. https://doi.org/10.1159/000350926
- 1494. Jordakieva G, Kundi M, Lemell P, et al. Cetirizine inhibits gender‐specific blood cell dynamics upon allergen contact in allergic rhinitis. Clin Immunol. 2020;215:108422. https://doi.org/10.1016/j.clim.2020.108422
- 1495. Yonekura S, Okamoto Y, Sakurai D, et al. Efficacy of Desloratadine and levocetirizine in patients with cedar pollen‐induced allergic rhinitis: a randomized, double‐blind study. Int Arch Allergy Immunol. 2019;180(4):274–283. https://doi.org/10.1159/000503065
- 1496. Hashiguchi K, Wakabayashi KI, Togawa M, Saito A, Okubo K. Therapeutic effect of bilastine in Japanese cedar pollinosis using an artificial exposure chamber (OHIO Chamber). Allergol Int. 2017;66(1):123–131. https://doi.org/10.1016/j.alit.2016.06.009
- 1497. Bareille P, Murdoch RD, Denyer J, et al. The effects of a TRPV1 antagonist, SB‐705498, in the treatment of seasonal allergic rhinitis. Int J Clin Pharmacol Ther. 2013;51(7):576–584. https://doi.org/10.5414/CP201890
- 1498. Corren J, Wood RA, Patel D, et al. Effects of omalizumab on changes in pulmonary function induced by controlled cat room challenge. J Allergy Clin Immunol. 2011;127(2):398–405. https://doi.org/10.1016/j.jaci.2010.09.043
- 1499. Horak F. VTX‐1463, a novel TLR8 agonist for the treatment of allergic rhinitis. Expert Opin Investig Drugs. 2011;20(7):981–986. https://doi.org/10.1517/13543784.2011.583237
- 1500. Horak F, Zieglmayer P, Zieglmayer R, et al. The CRTH2 antagonist OC000459 reduces nasal and ocular symptoms in allergic subjects exposed to grass pollen, a randomised, placebo‐controlled, double‐blind trial. Allergy. 2012;67(12):1572–1579. https://doi.org/10.1111/all.12042
- 1501. Gomes PJ, Abelson MB, Stein L, Viirre E, Villafranca JE, Lasser EC. Iodixanol nasal solution reduces allergic rhinoconjunctivitis signs and symptoms in Allergen BioCube((R)): a randomized clinical trial. J Asthma Allergy. 2019;12:71–81. https://doi.org/10.2147/JAA.S150251
- 1502. Struss N, Badorrek P, Mattern C, Mattern U, Hohlfeld JM. The effect of a thixotropic nasal gel on nasal symptoms and inflammatory biomarkers in seasonal allergic rhinitis. Int Arch Allergy Immunol. 2020;181(5):385–394. https://doi.org/10.1159/000506129
- 1503. Salapatek AM, Werkhauser N, Ismail B, Mosges R, Raskopf E, Bilstein A. Effects of ectoine containing nasal spray and eye drops on symptoms of seasonal allergic rhinoconjunctivitis. Clin Transl Allergy. 2021;11(1):e12006. https://doi.org/10.1002/clt2.12006
- 1504. Xiao JZ, Kondo S, Yanagisawa N, et al. Clinical efficacy of probiotic Bifidobacterium longum for the treatment of symptoms of Japanese cedar pollen allergy in subjects evaluated in an environmental exposure unit. Allergol Int. 2007;56(1):67–75. https://doi.org/10.2332/allergolint.O‐06‐455
- 1505. Bergmann KC, Krause L, Hiller J, et al. First evaluation of a symbiotic food supplement in an allergen exposure chamber in birch pollen allergic patients. World Allergy Organ J. 2021;14(1):100494. https://doi.org/10.1016/j.waojou.2020.100494
- 1506. Ellis AK, Frankish CW, Armstrong K, et al. Persistence of the clinical effect of grass allergen peptide immunotherapy after the second and third grass pollen seasons. J Allergy Clin Immunol. 2020;145(2):610–618.e9. https://doi.org/10.1016/j.jaci.2019.09.010
- 1507. Wagenmann M, Worm M, Akboga Y, Karjalainen M, Hohlfeld JM. Randomized immunotherapy trial in dual‐allergic patients using "active allergen placebo" as control. Allergy. 2019;74(8):1480–1489. https://doi.org/10.1111/all.13842
- 1508. Couroux P, Ipsen H, Stage BS, et al. A birch sublingual allergy immunotherapy tablet reduces rhinoconjunctivitis symptoms when exposed to birch and oak and induces IgG4 to allergens from all trees in the birch homologous group. Allergy. 2019;74(2):361–369. https://doi.org/10.1111/all.13606
- 1509. Ellis AK, Tenn MW, Steacy LM, et al. Lack of effect of Timothy grass pollen sublingual immunotherapy tablet on birch pollen‐induced allergic rhinoconjunctivitis in an environmental exposure unit. Ann Allergy Asthma Immunol. 2018;120(5):495–503. e2. https://doi.org/10.1016/j.anai.2018.02.003
- 1510. Pfaar O, Hohlfeld JM, Al‐Kadah B, et al. Dose‐response relationship of a new Timothy grass pollen allergoid in comparison with a 6‐grass pollen allergoid. Clin Exp Allergy. 2017;47(11):1445–1455. https://doi.org/10.1111/cea.12977
- 1511. Ellis AK, Frankish CW, O'Hehir RE, et al. Treatment with grass allergen peptides improves symptoms of grass pollen‐induced allergic rhinoconjunctivitis. J Allergy Clin Immunol. 2017;140(2):486–496. https://doi.org/10.1016/j.jaci.2016.11.043
- 1512. Zieglmayer P, Nolte H, Nelson HS, et al. Long‐term effects of a house dust mite sublingual immunotherapy tablet in an environmental exposure chamber trial. Ann Allergy Asthma Immunol. 2016;117(6):690–696.e1. https://doi.org/10.1016/j.anai.2016.10.015
- 1513. Horak F, Zieglmayer P, Zieglmayer R, et al. Early onset of action of a 5‐grass‐pollen 300‐IR sublingual immunotherapy tablet evaluated in an allergen challenge chamber. J Allergy Clin Immunol. 2009;124(3):471–477, 477.e1. https://doi.org/10.1016/j.jaci.2009.06.006
- 1514. Meyer W, Narkus A, Salapatek AM, Hafner D. Double‐blind, placebo‐controlled, dose‐ranging study of new recombinant hypoallergenic Bet v 1 in an environmental exposure chamber. Allergy. 2013;68(6):724–731. https://doi.org/10.1111/all.12148
- 1515. Nolte H, Maloney J, Nelson HS, et al. Onset and dose‐related efficacy of house dust mite sublingual immunotherapy tablets in an environmental exposure chamber. J Allergy Clin Immunol. 2015;135(6):1494–1501.e6. https://doi.org/10.1016/j.jaci.2014.12.1911
- 1516. Patel D, Couroux P, Hickey P, et al. Fel d 1‐derived peptide antigen desensitization shows a persistent treatment effect 1 year after the start of dosing: a randomized, placebo‐controlled study. J Allergy Clin Immunol. 2013;131(1):103‐109.e1‐7. https://doi.org/10.1016/j.jaci.2012.07.028
- 1517. Patel P, Holdich T, Fischer von Weikersthal‐Drachenberg KJ, Huber B. Efficacy of a short course of specific immunotherapy in patients with allergic rhinoconjunctivitis to ragweed pollen. J Allergy Clin Immunol. 2014;133(1):121‐129.e1‐2. https://doi.org/10.1016/j.jaci.2013.05.032
- 1518. Gherasim A, de Blay F. Does air filtration work for cat allergen exposure? Curr Allergy Asthma Rep. 2020;20(6):18. https://doi.org/10.1007/s11882‐020‐00912‐w
- 1519. Gherasim A, Jacob A, Schoettel F, Domis N, de Blay F. Efficacy of air cleaners in asthmatics allergic to cat in ALYATEC((R)) environmental exposure chamber. Clin Exp Allergy. 2020;50(2):160–169. https://doi.org/10.1111/cea.13511
- 1520. Rogol AD, Tkachenko N, Badorrek P, Hohlfeld JM, Bryson N. Phase 1 pharmacokinetics and phase 3 efficacy of testosterone nasal gel in subjects with seasonal allergies. Can Urol Assoc J. 2018;12(7):E349–E356. https://doi.org/10.5489/cuaj.4898
- 1521. Khayath N, Doyen V, Gherasim A, et al. Validation of Strasbourg environmental exposure chamber (EEC) ALYATEC((R)) in mite allergic subjects with asthma. J Asthma. 2020;57(2):140–148. https://doi.org/10.1080/02770903.2018.1563902
- 1522. Zieglmayer P, Lemell P, Chen KW, et al. Clinical validation of a house dust mite environmental challenge chamber model. J Allergy Clin Immunol. 2017;140(1):266–268.e5. https://doi.org/10.1016/j.jaci.2016.12.986
- 1523. Koriyama M, Okamoto Y, Suzuki T, et al. Characteristics of Japanese cypress pollen‐induced allergic rhinitis by environmental challenge chamber. Allergol Int. 2022;71(1):144–146. https://doi.org/10.1016/j.alit.2021.08.013
- 1524. Gomes PJ, Lane KJ, Angjeli E, Stein L, Abelson MB. Technical and clinical validation of an environmental exposure unit for ragweed. J Asthma Allergy. 2016;9:215–221. https://doi.org/10.2147/JAA.S123547
- 1525. Pfaar O, Zieglmayer P. Allergen exposure chambers: implementation in clinical trials in allergen immunotherapy. Clin Transl Allergy. 2020;10:33. https://doi.org/10.1186/s13601‐020‐00336‐9
- 1526. Pfaar O, Agache I, de Blay F, et al. Perspectives in allergen immunotherapy: 2019 and beyond. Allergy. 2019;74(suppl 108):3–25. https://doi.org/10.1111/all.14077
- 1527. Pfaar O, Alvaro M, Cardona V, Hamelmann E, Mosges R, Kleine‐Tebbe J. Clinical trials in allergen immunotherapy: current concepts and future needs. Allergy. 2018;73(9):1775–1783. https://doi.org/10.1111/all.13429
- 1528. Pfaar O, Bergmann KC, Bonini S, et al. Technical standards in allergen exposure chambers worldwide – an EAACI Task Force Report. Allergy. 2021;76(12):3589–3612. https://doi.org/10.1111/all.14957
- 1529. Ellis AK, DeVeaux M, Steacy L, et al. Environmental exposure unit simulates natural seasonal birch pollen exposures while maximizing change in allergic symptoms. Ann Allergy Asthma Immunol. 2021;127(4):488–495.e5. https://doi.org/10.1016/j.anai.2021.06.015
- 1530. Agache I, Bilo M, Braunstahl GJ, et al. In vivo diagnosis of allergic diseases–allergen provocation tests. Allergy. 2015;70(4):355–365. https://doi.org/10.1111/all.12586
- 1531. Riechelmann H, Epple B, Gropper G. Comparison of conjunctival and nasal provocation test in allergic rhinitis to house dust mite. Int Arch Allergy Immunol. 2003;130(1):51–59. https://doi.org/10.1159/000068369
- 1532. Dordal MT, Lluch‐Bernal M, Sanchez MC, et al. Allergen‐specific nasal provocation testing: review by the rhinoconjunctivitis committee of the Spanish Society of Allergy and Clinical Immunology. J Investig Allergol Clin Immunol. 2011;21(1):1–12, quiz follow 12.
- 1533. Malm L, Gerth van Wijk R, Bachert C. Guidelines for nasal provocations with aspects on nasal patency, airflow, and airflow resistance. International Committee on Objective Assessment of the Nasal Airways, International Rhinologic Society. Rhinology. 2000;38(1):1–6.
- 1534. Gosepath J, Amedee RG, Mann WJ. Nasal provocation testing as an international standard for evaluation of allergic and nonallergic rhinitis. Laryngoscope. 2005;115(3):512–516. https://doi.org/10.1097/01.MLG.0000149682.56426.6B
- 1535. Auge J, Vent J, Agache I, et al. EAACI Position paper on the standardization of nasal allergen challenges. Allergy. 2018;73(8):1597–1608. https://doi.org/10.1111/all.13416
- 1536. Casset A, Khayath N, de Blay F. How in vitro assays contribute to allergy diagnosis. Curr Allergy Asthma Rep. 2016;16(11):82. https://doi.org/10.1007/s11882‐016‐0659‐9
- 1537. Santos AF, Alpan O, Hoffmann HJ. Basophil activation test: mechanisms and considerations for use in clinical trials and clinical practice. Allergy. 2021;76(8):2420–2432. https://doi.org/10.1111/all.14747
- 1538. Larson D, Patel P, Salapatek AM, et al. Nasal allergen challenge and environmental exposure chamber challenge: a randomized trial comparing clinical and biological responses to cat allergen. J Allergy Clin Immunol. 2020;145(6):1585–1597. https://doi.org/10.1016/j.jaci.2020.02.024
- 1539. Wanjun W, Qiurong H, Yanqing X, Mo X, Nili W, Jing L. Responsiveness of nasal provocation testing‐but not skin test and specific immunoglobulin E blood level‐correlates with severity of allergic rhinitis in dermatophagoides species‐sensitized patients. Am J Rhinol Allergy. 2018;32(4):236–243. https://doi.org/10.1177/1945892418779435
- 1540. Joo SH, Hyun KJ, Kim YH. Korean modification of the nasal provocation test with house dust mite antigen following the EAACI guidelines. Clin Exp Otorhinolaryngol. 2021;14(4):382–389. https://doi.org/10.21053/ceo.2020.00563
- 1541. Xiao H, Jia Q, Zhang H, Zhang L, Liu G, Meng J. The importance of nasal provocation testing in the diagnosis of dermatophagoides pteronyssinus‐induced allergic rhinitis. Am J Rhinol Allergy. 2022;36(2):191–197. https://doi.org/10.1177/19458924211037913
- 1542. EAACI Task Force on Occupational Rhinitis , Moscato G, Vandenplas O, et al. Occupational rhinitis. Allergy. 2008;63(8):969–980. https://doi.org/10.1111/j.1398‐9995.2008.01801.x
- 1543. Rondon C, Campo P, Herrera R, et al. Nasal allergen provocation test with multiple aeroallergens detects polysensitization in local allergic rhinitis. J Allergy Clin Immunol. 2011;128(6):1192–1197. https://doi.org/10.1016/j.jaci.2011.06.012
- 1544. Tantilipikorn P, Siriboonkoom P, Sookrung N, et al. Prevalence of local allergic rhinitis to Dermatophagoides pteronyssinus in chronic rhinitis with negative skin prick test. Asian Pac J Allergy Immunol. 2021;39(2):111–116. https://doi.org/10.12932/AP‐170918‐0408
- 1545. Moller C, Bjorksten B, Nilsson G, Dreborg S. The precision of the conjunctival provocation test. Allergy. 1984;39(1):37–41. https://doi.org/10.1111/j.1398‐9995.1984.tb01931.x
- 1546. Bertel F, Mortemousque B, Sicard H, André C. Test de provocation conjonctival au Dermatophagoïdes pteronyssinus dans le diagnostic des conjonctivites allergiques aux acariens domestiques [Conjunctival provocation test with Dermatophagoides pteronyssinus in the diagnosis of allergic conjunctivitis from house mites]. J Fr Ophtalmol. 2001;24(6):581–589.
- 1547. Fauquert JL, Jedrzejczak‐Czechowicz M, Rondon C, et al. Conjunctival allergen provocation test : guidelines for daily practice. Allergy. 2017;72(1):43–54. https://doi.org/10.1111/all.12986
- 1548. Schoos AM, Chawes BL, Bloch J, et al. Children monosensitized to Can f 5 show different reactions to male and female dog allergen extract provocation: a randomized controlled trial. J Allergy Clin Immunol Pract. 2020;8(5):1592–1597.e2. https://doi.org/10.1016/j.jaip.2019.12.012
- 1549. Gelis S, Rueda M, Pascal M, et al., Usefulness of the Nasal Allergen Provocation Test in the Diagnosis of Shellfish Allergy. J Investig Allergol Clin Immunol. 2022;32(6):460–470. doi: https://doi.org/10.18176/jiaci.0736
- 1550. Krzych‐Falta E, Furmanczyk K, Samolinski B. Specificity and sensitivity assessment of selected nasal provocation testing techniques. Postepy Dermatol Alergol. 2016;33(6):464–468. https://doi.org/10.5114/pdia.2016.61339
- 1551. de Blay F, Doyen V, Lutz C, et al. A new, faster, and safe nasal provocation test method for diagnosing mite allergic rhinitis. Ann Allergy Asthma Immunol. 2015;115(5):385–390.e1. https://doi.org/10.1016/j.anai.2015.07.014
- 1552. Agarwal G, Hernandez D, Citardi MJ, Fakhri S, Luong A. End‐organ testing for allergic rhinitis with fungi is poorly correlated with fungal sensitivity. Otolaryngol Head Neck Surg. 2013;148(3):391–395. https://doi.org/10.1177/0194599812474224
- 1553. Gelardi M, Iannuzzi L, Quaranta N, Landi M, Passalacqua G. NASAL cytology: practical aspects and clinical relevance. Clin Exp Allergy. 2016;46(6):785–792. https://doi.org/10.1111/cea.12730
- 1554. Waecker NJ Jr., Shope TR, Weber PA, Buck ML, Domingo RC, Hooper DG. The Rhino‐Probe nasal curette for detecting respiratory syncytial virus in children. Pediatr Infect Dis J. 1993;12(4):326–329. https://doi.org/10.1097/00006454‐199304000‐00012
- 1555. Gelardi M, Passalacqua G, Fiorella ML, Quaranta N. Assessment of biofilm by nasal cytology in different forms of rhinitis and its functional correlations. Eur Ann Allergy Clin Immunol. 2013;45(1):25–29.
- 1556. Canakcioglu S, Tahamiler R, Saritzali G, et al. Evaluation of nasal cytology in subjects with chronic rhinitis: a 7‐year study. Am J Otolaryngol. 2009;30(5):312–317. https://doi.org/10.1016/j.amjoto.2008.06.015
- 1557. Di Lorenzo G, Pacor ML, Amodio E, et al. Differences and similarities between allergic and nonallergic rhinitis in a large sample of adult patients with rhinitis symptoms. Int Arch Allergy Immunol. 2011;155(3):263–270. https://doi.org/10.1159/000320050
- 1558. Gelardi M, Ciprandi G, Incorvaia C, et al. Allergic rhinitis phenotypes based on mono‐allergy or poly‐allergy. Inflamm Res. 2015;64(6):373–375. https://doi.org/10.1007/s00011‐015‐0826‐9
- 1559. Gelardi M, Incorvaia C, Passalacqua G, Quaranta N, Frati F. The classification of allergic rhinitis and its cytological correlate. Allergy. 2011;66(12):1624–1625. https://doi.org/10.1111/j.1398‐9995.2011.02741.x
- 1560. Gelardi M, Peroni DG, Incorvaia C, et al. Seasonal changes in nasal cytology in mite‐allergic patients. J Inflamm Res. 2014;7:39–44. https://doi.org/10.2147/JIR.S54581
- 1561. Ciofalo A, Cavaliere C, Incorvaia C, et al. Diagnostic performance of nasal cytology. Eur Arch Otorhinolaryngol. 2022;279(5):2451–2455. https://doi.org/10.1007/s00405‐021‐07044‐5
- 1562. Cavaliere C, Masieri S, Greco A, Lambiase A, Segatto M. Nasal expression of the vascular endothelial growth factor and its receptors is reduced by mepolizumab in chronic rhinosinusitis with nasal polyposis. Ann Allergy Asthma Immunol. 2021;126(4):442–443. https://doi.org/10.1016/j.anai.2021.01.010
- 1563. Chen Y, Yang M, Deng J, Wang K, Shi J, Sun Y. Elevated levels of activated and pathogenic eosinophils characterize moderate‐severe house dust mite allergic rhinitis. J Immunol Res. 2020;2020:8085615. https://doi.org/10.1155/2020/8085615
- 1564. Spector SL, English G, Jones L. Clinical and nasal biopsy response to treatment of perennial rhinitis. J Allergy Clin Immunol. 1980;66(2):129–137. https://doi.org/10.1016/0091‐6749(80)90060‐3
- 1565. Lim MC, Taylor RM, Naclerio RM. The histology of allergic rhinitis and its comparison to cellular changes in nasal lavage. Am J Respir Crit Care Med. 1995;151(1):136–144. https://doi.org/10.1164/ajrccm.151.1.7812543
- 1566. Howarth PH, Persson CG, Meltzer EO, Jacobson MR, Durham SR, Silkoff PE. Objective monitoring of nasal airway inflammation in rhinitis. J Allergy Clin Immunol. 2005;115(3 suppl 1):S414–S441. https://doi.org/10.1016/j.jaci.2004.12.1134
- 1567. De Corso E, Seccia V, Ottaviano G, et al. Clinical Evidence of Type 2 inflammation in non‐allergic rhinitis with eosinophilia syndrome: a systematic review. Curr Allergy Asthma Rep. 2022;22(4):29–42. https://doi.org/10.1007/s11882‐022‐01027‐0
- 1568. Phothijindakul N, Chusakul S, Aeumjaturapat S, et al. Nasal cytology as a diagnostic tool for local allergic rhinitis. Am J Rhinol Allergy. 2019;33(5):540–544. https://doi.org/10.1177/1945892419850926
- 1569. Gelardi M. "Overlapped" rhinitis: a real trap for rhinoallergologists. Eur Ann Allergy Clin Immunol. 2014;46(6):234–236.
- 1570. McHugh T, Levin M, Snidvongs K, Banglawala SM, Sommer DD. Comorbidities associated with eosinophilic chronic rhinosinusitis: a systematic review and meta‐analysis. Clin Otolaryngol. 2020;45(4):574–583. https://doi.org/10.1111/coa.13536
- 1571. Sivam A, Jeswani S, Reder L, et al. Olfactory cleft inflammation is present in seasonal allergic rhinitis and is reduced with intranasal steroids. Am J Rhinol Allergy. 2010;24(4):286–290. https://doi.org/10.2500/ajra.2010.24.3478
- 1572. Uller L, Emanuelsson CA, Andersson M, Erjefalt JS, Greiff L, Persson CG. Early phase resolution of mucosal eosinophilic inflammation in allergic rhinitis. Respir Res. 2010;11:54. https://doi.org/10.1186/1465‐9921‐11‐54
- 1573. Asai K, Foley SC, Sumi Y, et al. Amb a 1‐immunostimulatory oligodeoxynucleotide conjugate immunotherapy increases CD4+CD25+ T cells in the nasal mucosa of subjects with allergic rhinitis. Allergol Int. 2008;57(4):377–381. https://doi.org/10.2332/allergolint.O‐07‐528
- 1574. Rak S, Heinrich C, Scheynius A. Comparison of nasal immunohistology in patients with seasonal rhinoconjunctivitis treated with topical steroids or specific allergen immunotherapy. Allergy. 2005;60(5):643–649. https://doi.org/10.1111/j.1398‐9995.2005.00763.x
- 1575. Plewako H, Arvidsson M, Petruson K, et al. The effect of omalizumab on nasal allergic inflammation. J Allergy Clin Immunol. 2002;110(1):68–71. https://doi.org/10.1067/mai.2002.125488
- 1576. Pullerits T, Linden A, Malmhall C, Lotvall J. Effect of seasonal allergen exposure on mucosal IL‐16 and CD4+ cells in patients with allergic rhinitis. Allergy. 2001;56(9):871–877.
- 1577. Wilson DR, Nouri‐Aria KT, Walker SM, et al. Grass pollen immunotherapy: symptomatic improvement correlates with reductions in eosinophils and IL‐5 mRNA expression in the nasal mucosa during the pollen season. J Allergy Clin Immunol. 2001;107(6):971–976. https://doi.org/10.1067/mai.2001.115483
- 1578. Radulovic S, Jacobson MR, Durham SR, Nouri‐Aria KT. Grass pollen immunotherapy induces Foxp3‐expressing CD4+ CD25+ cells in the nasal mucosa. J Allergy Clin Immunol. 2008;121(6):1467–1472, 1472.e1. https://doi.org/10.1016/j.jaci.2008.03.013
- 1579. Till SJ, Jacobson MR, O'Brien F, et al. Recruitment of CD1a+ Langerhans cells to the nasal mucosa in seasonal allergic rhinitis and effects of topical corticosteroid therapy. Allergy. 2001;56(2):126–131. https://doi.org/10.1034/j.1398‐9995.2001.056002126.x
- 1580. Vogt K, Bachmann‐Harildstad G, Lintermann A, Nechyporenko A, Peters F, Wernecke KD. The new agreement of the international RIGA consensus conference on nasal airway function tests. Rhinology. 2018;56(2):133–143. https://doi.org/10.4193/Rhin17.084
- 1581. Vogt K, Wernecke KD, Behrbohm H, Gubisch W, Argale M. Four‐phase rhinomanometry: a multicentric retrospective analysis of 36,563 clinical measurements. Eur Arch Otorhinolaryngol. 2016;273(5):1185–1198. https://doi.org/10.1007/s00405‐015‐3723‐5
- 1582. Rimmer J, Hellings P, Lund VJ, et al. European position paper on diagnostic tools in rhinology. Rhinology. 2019;57(suppl S28):1–41. https://doi.org/10.4193/Rhin19.410
- 1583. Clement PA. Committee report on standardization of rhinomanometry. Rhinology. 1984;22(3):151–155.
- 1584. Ohki M, Naito K, Cole P. Dimensions and resistances of the human nose: racial differences. Laryngoscope. 1991;101(3):276–278. https://doi.org/10.1288/00005537‐199103000‐00009
- 1585. Jones AS, Lancer JM, Stevens JC, Beckingham E. Nasal resistance to airflow (its measurement, reproducibility and normal parameters). J Laryngol Otol. 1987;101(8):800–808. https://doi.org/10.1017/s0022215100102762
- 1586. Cole P. Stability of nasal airflow resistance. Clin Otolaryngol Allied Sci. 1989;14(2):177–1782. https://doi.org/10.1111/j.1365‐2273.1989.tb00357.x
- 1587. Shelton DM, Eiser NM. Evaluation of active anterior and posterior rhinomanometry in normal subjects. Clin Otolaryngol Allied Sci. 1992;17(2):178–182. https://doi.org/10.1111/j.1365‐2273.1992.tb01068.x
- 1588. Chen IC, Lin YT, Hsu JH, Liu YC, Wu JR, Dai ZK. Nasal airflow measured by rhinomanometry correlates with FeNO in children with asthma. PLoS One. 2016;11(10):e0165440. https://doi.org/10.1371/journal.pone.0165440
- 1589. Merkle J, Kohlhas L, Zadoyan G, Mosges R, Hellmich M. Rhinomanometric reference intervals for normal total nasal airflow resistance. Rhinology. 2014;52(4):292–299. https://doi.org/10.4193/Rhino13.220
- 1590. Suzina AH, Hamzah M, Samsudin AR. Active anterior rhinomanometry analysis in normal adult Malays. J Laryngol Otol. 2003;117(8):605–608. https://doi.org/10.1258/002221503768199924
- 1591. Vogt K, Jalowayski AA, Althaus W, et al. 4‐Phase‐Rhinomanometry (4PR)–basics and practice 2010. Rhinol Suppl. 2010;21:1–50.
- 1592. Andre RF, Vuyk HD, Ahmed A, Graamans K, Nolst Trenite GJ. Correlation between subjective and objective evaluation of the nasal airway. A systematic review of the highest level of evidence. Clin Otolaryngol. 2009;34(6):518–525. https://doi.org/10.1111/j.1749‐4486.2009.02042.x
- 1593. Mohan S, Fuller JC, Ford SF, Lindsay RW. Diagnostic and therapeutic management of nasal airway obstruction: advances in diagnosis and treatment. JAMA Facial Plast Surg. 2018;20(5):409–418. https://doi.org/10.1001/jamafacial.2018.0279
- 1594. Tarhan E, Coskun M, Cakmak O, Celik H, Cankurtaran M. Acoustic rhinometry in humans: accuracy of nasal passage area estimates, and ability to quantify paranasal sinus volume and ostium size. J Appl Physiol (1985). 2005;99(2):616–623. https://doi.org/10.1152/japplphysiol.00106.2005
- 1595. Timperley D, Srubisky A, Stow N, Marcells GN, Harvey RJ. Minimal clinically important differences in nasal peak inspiratory flow. Rhinology. 2011;49(1):37–40. https://doi.org/10.4193/Rhino10.097
- 1596. Chin D, Marcells G, Malek J, et al. Nasal peak inspiratory flow (NPIF) as a diagnostic tool for differentiating decongestable from structural nasal obstruction. Rhinology. 2014;52(2):116–121. https://doi.org/10.4193/Rhino13.126
- 1597. Kirtsreesakul V, Leelapong J, Ruttanaphol S. Correlation between peak nasal flow reversibility and mucociliary clearance in allergic rhinitis. Laryngoscope. 2020;130(6):1372–1376. https://doi.org/10.1002/lary.28226
- 1598. Mo S, Gupta SS, Stroud A, et al. Nasal peak inspiratory flow in healthy and obstructed patients: systematic review and meta‐analysis. Laryngoscope. 2021;131(2):260–267. https://doi.org/10.1002/lary.28682
- 1599. Krzych‐Falta E, Samolinski BK. Objectification of the nasal patency assessment techniques used in nasal allergen provocation testing. Postepy Dermatol Alergol. 2020;37(5):635–640. https://doi.org/10.5114/ada.2019.81404
- 1600. Ottaviano G, Ermolao A, Nardello E, et al. Breathing parameters associated to two different external nasal dilator strips in endurance athletes. Auris Nasus Larynx. 2017;44(6):713–718. https://doi.org/10.1016/j.anl.2017.01.006
- 1601. Kirtsreesakul V, Leelapong J, Ruttanaphol S. Nasal peak inspiratory and expiratory flow measurements for assessing nasal obstruction in allergic rhinitis. Am J Rhinol Allergy. 2014;28(2):126–130. https://doi.org/10.2500/ajra.2014.28.4008
- 1602. Wong DK, Saim L, Idrus RB, Saim A. Evaluating the incidence and severity of rhinitis using an peak nasal inspiratory flow meter and the SNOT‐22 questionnaire. J Med Assoc Thai. 2021;104(5):701–708.
- 1603. Canakcioglu S, Tahamiler R, Saritzali G, Isildak H, Alimoglu Y. Nasal patency by rhinomanometry in patients with sensation of nasal obstruction. Am J Rhinol Allergy. 2009;23(3):300–302. https://doi.org/10.2500/ajra.2009.23.3312
- 1604. Passali D, Mezzedimi C, Passali GC, Nuti D, Bellussi L. The role of rhinomanometry, acoustic rhinometry, and mucociliary transport time in the assessment of nasal patency. Ear Nose Throat J. 2000;79(5):397–400.
- 1605. Garcia GJM, Hariri BM, Patel RG, Rhee JS. The relationship between nasal resistance to airflow and the airspace minimal cross‐sectional area. J Biomech. 2016;49(9):1670–1678. https://doi.org/10.1016/j.jbiomech.2016.03.051
- 1606. Aksoy C, Elsurer C, Artac H, Bozkurt MK. Evaluation of olfactory function in children with seasonal allergic rhinitis and its correlation with acoustic rhinometry. Int J Pediatr Otorhinolaryngol. 2018;113:188–191. https://doi.org/10.1016/j.ijporl.2018.07.051
- 1607. Barnes ML, Lipworth BJ. Removing nasal valve obstruction in peak nasal inspiratory flow measurement. Ann Allergy Asthma Immunol. 2007;99(1):59–60. https://doi.org/10.1016/S1081‐1206(10)60622‐9
- 1608. Burrow A, Eccles R, Jones AS. The effects of camphor, eucalyptus and menthol vapour on nasal resistance to airflow and nasal sensation. Acta Otolaryngol. 1983;96(1‐2):157–161. https://doi.org/10.3109/00016488309132886
- 1609. Eccles R, Jones AS. The effect of menthol on nasal resistance to air flow. J Laryngol Otol. 1983;97(8):705–709. https://doi.org/10.1017/s002221510009486x
- 1610. Jones AS, Lancer JM, Shone G, Stevens JC. The effect of lignocaine on nasal resistance and nasal sensation of airflow. Acta Otolaryngol. 1986;101(3‐4):328–330. https://doi.org/10.3109/00016488609132846
- 1611. Eccles R, Griffiths DH, Newton CG, Tolley NS. The effects of menthol isomers on nasal sensation of airflow. Clin Otolaryngol Allied Sci. 1988;13(1):25–29. https://doi.org/10.1111/j.1365‐2273.1988.tb00277.x
- 1612. Naito K, Ohoka E, Kato R, Kondo Y, Iwata S. The effect of L‐menthol stimulation of the major palatine nerve on nasal patency. Auris Nasus Larynx. 1991;18(3):221–226. https://doi.org/10.1016/s0385‐8146(12)80260‐4
- 1613. Naito K, Komori M, Kondo Y, Takeuchi M, Iwata S. The effect of L‐menthol stimulation of the major palatine nerve on subjective and objective nasal patency. Auris Nasus Larynx. 1997;24(2):159–162. https://doi.org/10.1016/S0385‐8146(96)00005‐3
- 1614. Jones AS, Crosher R, Wight RG, Lancer JM, Beckingham E. The effect of local anaesthesia of the nasal vestibule on nasal sensation of airflow and nasal resistance. Clin Otolaryngol Allied Sci. 1987;12(6):461–464. https://doi.org/10.1111/j.1365‐2273.1987.tb00233.x
- 1615. Barnes ML, White PS, Gardiner Q. Re: correlation between subjective and objective evaluation of the nasal airway. Clin Otolaryngol. 2010;35(2):152–153; author reply 153. https://doi.org/10.1111/j.1749‐4486.2010.02110.x
- 1616. van Spronsen E, Ingels KJ, Jansen AH, Graamans K, Fokkens WJ. Evidence‐based recommendations regarding the differential diagnosis and assessment of nasal congestion: using the new GRADE system. Allergy. 2008;63(7):820–833. https://doi.org/10.1111/j.1398‐9995.2008.01729.x
- 1617. Ta NH, Gao J, Philpott C. A systematic review to examine the relationship between objective and patient‐reported outcome measures in sinonasal disorders: recommendations for use in research and clinical practice. Int Forum Allergy Rhinol. 2021;11(5):910–923. https://doi.org/10.1002/alr.22744
- 1618. Vogt K, Hasse W, Jalowayski AA. New resistance patterns in rhinomanometry: clinical evaluation of 5000 measurements. presented at: European Rhinologic Society; 2002.
- 1619. Iyer A, Athavale A. Nasal airway resistance and latent lower airway involvement in allergic rhinitis. J Assoc Physicians India. 2020;68(3):43–47.
- 1620. Pantin CT, Southworth T, Wetzel K, Singh D. Reproducibility of nasal allergen challenge responses in adults with allergic rhinitis. Clin Pharmacol. 2019;11:67–76. https://doi.org/10.2147/CPAA.S184404
- 1621. Wong EH, Eccles R. Comparison of classic and 4‐phase rhinomanometry methods, is there any difference? Rhinology. 2014;52(4):360–365. https://doi.org/10.4193/Rhino13.187
- 1622. Brindisi G, De Vittori V, De Nola R, et al. The role of nasal nitric oxide and anterior active rhinomanometry in the diagnosis of allergic rhinitis and asthma: a message for pediatric clinical practice. J Asthma Allergy. 2021;14:265–274. https://doi.org/10.2147/JAA.S275692
- 1623. Wandalsen GF, Mendes AI, Matsumoto F, Sole D. Acoustic rhinometry in nasal provocation tests in children and adolescents. J Investig Allergol Clin Immunol. 2016;26(3):156–160. https://doi.org/10.18176/jiaci.0036
- 1624. Malizia V, Ferrante G, Cilluffo G, Fasola S, Montalbano L, La Grutta S. Rhinomanometry: point of care test (POCT) for allergic rhinitis in children? Allergol Immunopathol (Madr). 2021;49(5):28–31. https://doi.org/10.15586/aei.v49i5.429
- 1625. Valero A, Navarro AM, Del Cuvillo A, et al. Position paper on nasal obstruction: evaluation and treatment. J Investig Allergol Clin Immunol. 2018;28(2):67–90. https://doi.org/10.18176/jiaci.0232
- 1626. Takeno S, Okabayashi Y, Kohno T, Yumii K, Hirakawa K. The role of nasal fractional exhaled nitric oxide as an objective parameter independent of nasal airflow resistance in the diagnosis of allergic rhinitis. Auris Nasus Larynx. 2017;44(4):435–441. https://doi.org/10.1016/j.anl.2016.09.007
- 1627. Demirbas D, Cingi C, Cakli H, Kaya E. Use of rhinomanometry in common rhinologic disorders. Expert Rev Med Devices. 2011;8(6):769–777. https://doi.org/10.1586/erd.11.45
- 1628. Eguiluz‐Gracia I, Testera‐Montes A, Salas M, et al. Comparison of diagnostic accuracy of acoustic rhinometry and symptoms score for nasal allergen challenge monitoring. Allergy. 2021;76(1):371–375. https://doi.org/10.1111/all.14499
- 1629. Isaac A, Major M, Witmans M, et al. Correlations between acoustic rhinometry, subjective symptoms, and endoscopic findings in symptomatic children with nasal obstruction. JAMA Otolaryngol Head Neck Surg. 2015;141(6):550–555. https://doi.org/10.1001/jamaoto.2015.0468
- 1630. Wandalsen GF, Mendes AI, Sole D. Correlation between nasal resistance and different acoustic rhinometry parameters in children and adolescents with and without allergic rhinitis. Braz J Otorhinolaryngol. 2012;78(6):81–86. https://doi.org/10.5935/1808‐8694.20120038
- 1631. Ozturk F, Turktas I, Asal K, Ileri F, Munevver Pinar N. Effect of intranasal triamcinolone acetonide on bronchial hyper‐responsiveness in children with seasonal allergic rhinitis and comparison of perceptional nasal obstruction with acoustic rhinometric assessment. Int J Pediatr Otorhinolaryngol. 2004;68(8):1007–1015. https://doi.org/10.1016/j.ijporl.2004.03.006
- 1632. Sikorska‐Szaflik H, Sozanska B. Peak nasal inspiratory flow in children with allergic rhinitis. Is it related to the quality of life? Allergol Immunopathol (Madr). 2020;48(2):187–193. https://doi.org/10.1016/j.aller.2019.08.002
- 1633. Neighbour H, Soliman M, Steacy LM, et al. The Allergic Rhinitis Clinical Investigator Collaborative (AR‐CIC): verification of nasal allergen challenge procedures in a study utilizing an investigational immunotherapy for cat allergy. Clin Transl Allergy. 2018;8:15. https://doi.org/10.1186/s13601‐018‐0198‐7
- 1634. Gupta N, Goel N, Kumar R. Correlation of exhaled nitric oxide, nasal nitric oxide and atopic status: a cross‐sectional study in bronchial asthma and allergic rhinitis. Lung India. 2014;31(4):342–347. https://doi.org/10.4103/0970‐2113.142107
- 1635. Kimberly B, Nejadnik B, Giraud GD, Holden WE. Nasal contribution to exhaled nitric oxide at rest and during breathholding in humans. Am J Respir Crit Care Med. 1996;153(2):829–836. https://doi.org/10.1164/ajrccm.153.2.8564139
- 1636. Lundberg JO, Farkas‐Szallasi T, Weitzberg E, et al. High nitric oxide production in human paranasal sinuses. Nat Med. 1995;1(4):370–373. https://doi.org/10.1038/nm0495‐370
- 1637. Chatkin JM, Qian W, McClean PA, Zamel N, Haight J, Silkoff P. Nitric oxide accumulation in the nonventilated nasal cavity. Arch Otolaryngol Head Neck Surg. 1999;125(6):682–685. https://doi.org/10.1001/archotol.125.6.682
- 1638. American Thoracic S, European Respiratory S. ATS/ERS recommendations for standardized procedures for the online and offline measurement of exhaled lower respiratory nitric oxide and nasal nitric oxide, 2005. Am J Respir Crit Care Med. 2005;171(8):912–930. https://doi.org/10.1164/rccm.200406‐710ST
- 1639. Malmberg LP, Petays T, Haahtela T, et al. Exhaled nitric oxide in healthy nonatopic school‐age children: determinants and height‐adjusted reference values. Pediatr Pulmonol. 2006;41(7):635–642. https://doi.org/10.1002/ppul.20417
- 1640. Franklin PJ, Taplin R, Stick SM. A community study of exhaled nitric oxide in healthy children. Am J Respir Crit Care Med. 1999;159(1):69–73. https://doi.org/10.1164/ajrccm.159.1.9804134
- 1641. Franklin PJ, Turner SW, Le Souef PN, Stick SM. Exhaled nitric oxide and asthma: complex interactions between atopy, airway responsiveness, and symptoms in a community population of children. Thorax. 2003;58(12):1048–1052. https://doi.org/10.1136/thorax.58.12.1048
- 1642. Khatri SB, Iaccarino JM, Barochia A, et al. Use of fractional exhaled nitric oxide to guide the treatment of asthma: an official american thoracic society clinical practice guideline. Am J Respir Crit Care Med. 2021;204(10):e97–e109. https://doi.org/10.1164/rccm.202109‐2093ST
- 1643. van Asch CJ, Balemans WA, Rovers MM, Schilder AG, van der Ent CK. Atopic disease and exhaled nitric oxide in an unselected population of young adults. Ann Allergy Asthma Immunol. 2008;100(1):59–65. https://doi.org/10.1016/S1081‐1206(10)60406‐1
- 1644. Dweik RA, Sorkness RL, Wenzel S, et al. Use of exhaled nitric oxide measurement to identify a reactive, at‐risk phenotype among patients with asthma. Am J Respir Crit Care Med. 2010;181(10):1033–1041. https://doi.org/10.1164/rccm.200905‐0695OC
- 1645. Moore WC, Meyers DA, Wenzel SE, et al. Identification of asthma phenotypes using cluster analysis in the Severe Asthma Research Program. Am J Respir Crit Care Med. 2010;181(4):315–323. https://doi.org/10.1164/rccm.200906‐0896OC
- 1646. Maniscalco M, Calabrese C, D'Amato M, et al. Association between exhaled nitric oxide and nasal polyposis in severe asthma. Respir Med. 2019;152:20–24. https://doi.org/10.1016/j.rmed.2019.04.017
- 1647. Lipworth B, Kuo CR, Chan R. 2020 Updated Asthma Guidelines: clinical utility of fractional exhaled nitric oxide (Feno) in asthma management. J Allergy Clin Immunol. 2020;146(6):1281–1282. https://doi.org/10.1016/j.jaci.2020.03.006
- 1648. Bencova A, Rozborilova E, Antosova M. Bidirectional link between upper and lower airways in patients with allergic rhinitis. Eur J Med Res. 2009;(14 suppl 4):18–20. https://doi.org/10.1186/2047‐783x‐14‐s4‐18
- 1649. Hervas D, Rodriguez R, Garde J. Role of aeroallergen nasal challenge in asthmatic children. Allergol Immunopathol (Madr). 2011;39(1):17–22. https://doi.org/10.1016/j.aller.2010.03.003
- 1650. Jang YY, Ahn JY. Evaluation of fractional exhaled nitric oxide in pediatric asthma and allergic rhinitis. Children (Basel). 2020;8(1):3. https://doi.org/10.3390/children8010003
- 1651. Choi BS, Kim KW, Lee YJ, et al. Exhaled nitric oxide is associated with allergic inflammation in children. J Korean Med Sci. 2011;26(10):1265–1269. https://doi.org/10.3346/jkms.2011.26.10.1265
- 1652. Lundberg JO, Nordvall SL, Weitzberg E, Kollberg H, Alving K. Exhaled nitric oxide in paediatric asthma and cystic fibrosis. Arch Dis Child. 1996;75(4):323–326. https://doi.org/10.1136/adc.75.4.323
- 1653. Byrnes CA, Dinarevic S, Shinebourne EA, Barnes PJ, Bush A. Exhaled nitric oxide measurements in normal and asthmatic children. Pediatr Pulmonol. 1997;24(5):312–318. https://doi.org/10.1002/(sici)1099‐0496(199711)24:5<312::aid‐ppul2>3.0.co;2‐k
- 1654. Baraldi E, Azzolin NM, Carra S, Dario C, Marchesini L, Zacchello F. Effect of topical steroids on nasal nitric oxide production in children with perennial allergic rhinitis: a pilot study. Respir Med. 1998;92(3):558–561. https://doi.org/10.1016/s0954‐6111(98)90308‐0
- 1655. Dweik RA, Boggs PB, Erzurum SC, et al. An official ATS clinical practice guideline: interpretation of exhaled nitric oxide levels (FENO) for clinical applications. Am J Respir Crit Care Med. 2011;184(5):602–615. https://doi.org/10.1164/rccm.9120‐11ST
- 1656. Shapiro AJ, Dell SD, Gaston B, et al. Nasal nitric oxide measurement in primary ciliary dyskinesia. A technical paper on standardized testing protocols. Ann Am Thorac Soc. 2020;17(2):e1–e12. https://doi.org/10.1513/AnnalsATS.201904‐347OT
- 1657. Lee KJ, Cho SH, Lee SH, et al. Nasal and exhaled nitric oxide in allergic rhinitis. Clin Exp Otorhinolaryngol. 2012;5(4):228–33. https://doi.org/10.3342/ceo.2012.5.4.228
- 1658. Dotsch J, Demirakca S, Terbrack HG, Huls G, Rascher W, Kuhl PG. Airway nitric oxide in asthmatic children and patients with cystic fibrosis. Eur Respir J. 1996;9(12):2537–2540. https://doi.org/10.1183/09031936.96.09122537
- 1659. Martin U, Bryden K, Devoy M, Howarth P. Increased levels of exhaled nitric oxide during nasal and oral breathing in subjects with seasonal rhinitis. J Allergy Clin Immunol. 1996;97(3):768–772. https://doi.org/10.1016/s0091‐6749(96)80154‐0
- 1660. Kalpaklioglu AF, Baccioglu A, Yalim SA. Can nasal nitric oxide be a biomarker to differentiate allergic and non‐allergic rhinitis? Egypt J Otolaryngol. 2021;37(1):91.
- 1661. Ambrosino P, Parrella P, Formisano R, et al. Clinical application of nasal nitric oxide measurement in allergic rhinitis: a systematic review and meta‐analysis. Ann Allergy Asthma Immunol. 2020;125(4):447–459.e5. https://doi.org/10.1016/j.anai.2020.07.003
- 1662. Wang B, Wu Z, Wang F, Yin Z, Shi L, Liu Y. Nasal nitric oxide testing for allergic rhinitis patients: Systematic review and meta‐analysis. Immun Inflamm Dis. 2021;9(3):635–648. https://doi.org/10.1002/iid3.439
- 1663. Maniscalco M, Sofia M, Carratu L, Higenbottam T. Effect of nitric oxide inhibition on nasal airway resistance after nasal allergen challenge in allergic rhinitis. Eur J Clin Invest. 2001;31(5):462–466. https://doi.org/10.1046/j.1365‐2362.2001.00825.x
- 1664. Moody A, Fergusson W, Wells A, Bartley J, Kolbe J. Nasal levels of nitric oxide as an outcome variable in allergic upper respiratory tract disease: influence of atopy and hayfever on nNO. Am J Rhinol. 2006;20(5):425–429. https://doi.org/10.2500/ajr.2006.20.2921
- 1665. Henriksen AH, Sue‐Chu M, Holmen TL, Langhammer A, Bjermer L. Exhaled and nasal NO levels in allergic rhinitis: relation to sensitization, pollen season and bronchial hyperresponsiveness. Eur Respir J. 1999;13(2):301–306. https://doi.org/10.1034/j.1399‐3003.1999.13b14.x
- 1666. Phillips PS, Sacks R, Marcells GN, Cohen NA, Harvey RJ. Nasal nitric oxide and sinonasal disease: a systematic review of published evidence. Otolaryngol Head Neck Surg. 2011;144(2):159–169. https://doi.org/10.1177/0194599810392667
- 1667. Hervas D, Milan JM, Garde J. Differences in exhaled nitric oxide in atopic children. Allergol Immunopathol (Madr). 2008;36(6):331–335. https://doi.org/10.1016/s0301‐0546(08)75865‐8
- 1668. Mucci T, Govindaraj S, Tversky J. Allergic rhinitis. Mt Sinai J Med. 2011;78(5):634–644. https://doi.org/10.1002/msj.20287
- 1669. Tversky J, McGlashan D. Short wave infrared (SWIR) camera as a novel approach to allergy skin testing. J Allergy Clin Immunol. 2017;139(2):AB156.
- 1670. Deshpande PR, Rajan S, Sudeepthi BL, Abdul Nazir CP. Patient‐reported outcomes: a new era in clinical research. Perspect Clin Res. 2011;2(4):137–144. https://doi.org/10.4103/2229‐3485.86879
- 1671. Scadding GW, Calderon MA, Shamji MH, et al. Effect of 2 years of treatment with sublingual grass pollen immunotherapy on nasal response to allergen challenge at 3 years among patients with moderate to severe seasonal allergic rhinitis: the GRASS randomized clinical trial. JAMA. 2017;317(6):615–625. https://doi.org/10.1001/jama.2016.21040
- 1672. Zieglmayer P, Focke‐Tejkl M, Schmutz R, et al. Mechanisms, safety and efficacy of a B cell epitope‐based vaccine for immunotherapy of grass pollen allergy. EBioMedicine. 2016;11:43–57. https://doi.org/10.1016/j.ebiom.2016.08.022
- 1673. Mosbech H, Canonica GW, Backer V, et al. SQ house dust mite sublingually administered immunotherapy tablet (ALK) improves allergic rhinitis in patients with house dust mite allergic asthma and rhinitis symptoms. Ann Allergy Asthma Immunol. 2015;114(2):134–140. https://doi.org/10.1016/j.anai.2014.11.015
- 1674. Casale TB. Anti‐immunoglobulin E (omalizumab) therapy in seasonal allergic rhinitis. Am J Respir Crit Care Med. 2001;164(8 pt 2):S18–S21. https://doi.org/10.1164/ajrccm.164.supplement_1.2103023
- 1675. Calderon MA, Bernstein DI, Blaiss M, Andersen JS, Nolte H. A comparative analysis of symptom and medication scoring methods used in clinical trials of sublingual immunotherapy for seasonal allergic rhinitis. Clin Exp Allergy. 2014;44(10):1228–1239. https://doi.org/10.1111/cea.12331
- 1676. Devillier P, Bousquet PJ, Grassin‐Delyle S, et al. Comparison of outcome measures in allergic rhinitis in children, adolescents and adults. Pediatr Allergy Immunol. 2016;27(4):375–381. https://doi.org/10.1111/pai.12561
- 1677. Bedard A, Basagana X, Anto JM, et al. Mobile technology offers novel insights into the control and treatment of allergic rhinitis: The MASK study. J Allergy Clin Immunol. 2019;144(1):135–143.e6. https://doi.org/10.1016/j.jaci.2019.01.053
- 1678. Glattacker M, Boeker M, Anger R, et al. Evaluation of a Mobile Phone App for patients with pollen‐related allergic rhinitis: prospective longitudinal field study. JMIR Mhealth Uhealth. 2020;8(4):e15514. https://doi.org/10.2196/15514
- 1679. Bousquet J, Bewick M, Arnavielhe S, et al. Work productivity in rhinitis using cell phones: The MASK pilot study. Allergy. 2017;72(10):1475–1484. https://doi.org/10.1111/all.13177
- 1680. Sousa‐Pinto B, Eklund P, Pfaar O, et al. Validity, reliability, and responsiveness of daily monitoring visual analog scales in MASK‐air(R). Clin Transl Allergy. 2021;11(7):e12062. https://doi.org/10.1002/clt2.12062
- 1681. Zhou AH, Patel VR, Baredes S, Eloy JA, Hsueh WD. Mobile applications for allergic rhinitis. Ann Otol Rhinol Laryngol. 2018;127(11):836–840. https://doi.org/10.1177/0003489418798385
- 1682. Jacome C, Pereira R, Almeida R, et al. Validation of App and Phone Versions of the Control of Allergic Rhinitis and Asthma Test (CARAT). J Investig Allergol Clin Immunol. 2021;31(3):270–273. https://doi.org/10.18176/jiaci.0640
- 1683. Hafner D, Reich K, Matricardi PM, Meyer H, Kettner J, Narkus A. Prospective validation of 'Allergy‐Control‐SCORE(TM)': a novel symptom‐medication score for clinical trials. Allergy. 2011;66(5):629–636. https://doi.org/10.1111/j.1398‐9995.2010.02531.x
- 1684. Demoly P, Jankowski R, Chassany O, Bessah Y, Allaert FA. Validation of a self‐questionnaire for assessing the control of allergic rhinitis. Clin Exp Allergy. 2011;41(6):860–868. https://doi.org/10.1111/j.1365‐2222.2011.03734.x
- 1685. Demoly P, Calderon MA, Casale T, et al. Assessment of disease control in allergic rhinitis. Clin Transl Allergy. 2013;3(1):7. https://doi.org/10.1186/2045‐7022‐3‐7
- 1686. Meltzer EO, Schatz M, Nathan R, Garris C, Stanford RH, Kosinski M. Reliability, validity, and responsiveness of the Rhinitis Control Assessment Test in patients with rhinitis. J Allergy Clin Immunol. 2013;131(2):379–386. https://doi.org/10.1016/j.jaci.2012.10.022
- 1687. Spector SL, Nicklas RA, Chapman JA, et al. Symptom severity assessment of allergic rhinitis: part 1. Ann Allergy Asthma Immunol. 2003;91(2):105–114. https://doi.org/10.1016/s1081‐1206(10)62160‐6
- 1688. Annesi‐Maesano I, Didier A, Klossek M, Chanal I, Moreau D, Bousquet J. The score for allergic rhinitis (SFAR): a simple and valid assessment method in population studies. Allergy. 2002;57(2):107–114. https://doi.org/10.1034/j.1398‐9995.2002.1o3170.x
- 1689. Bousquet PJ, Combescure C, Neukirch F, et al. Visual analog scales can assess the severity of rhinitis graded according to ARIA guidelines. Allergy. 2007;62(4):367–372. https://doi.org/10.1111/j.1398‐9995.2006.01276.x
- 1690. Devillier P, Chassany O, Vicaut E, et al. The minimally important difference in the Rhinoconjunctivitis Total Symptom Score in grass‐pollen‐induced allergic rhinoconjunctivitis. Allergy. 2014;69(12):1689–1695. https://doi.org/10.1111/all.12518
- 1691. Fonseca JA, Nogueira‐Silva L, Morais‐Almeida M, et al. Validation of a questionnaire (CARAT10) to assess rhinitis and asthma in patients with asthma. Allergy. 2010;65(8):1042–1048. https://doi.org/10.1111/j.1398‐9995.2009.02310.x
- 1692. Baiardini I, Pasquali M, Giardini A, et al. Rhinasthma: a new specific QoL questionnaire for patients with rhinitis and asthma. Allergy. 2003;58(4):289–294. https://doi.org/10.1034/j.1398‐9995.2003.00079.x
- 1693. Tosca MA, Del Barba P, Licari A, Ciprandi G, Asthma and Rhinitis Control Study Group . The measurement of asthma and allergic rhinitis control in children and adolescents. Children (Basel). 2020;7(5):43. https://doi.org/10.3390/children7050043
- 1694. Demoly P, Emminger W, Rehm D, Backer V, Tommerup L, Kleine‐Tebbe J. Effective treatment of house dust mite‐induced allergic rhinitis with 2 doses of the SQ HDM SLIT‐tablet: results from a randomized, double‐blind, placebo‐controlled phase III trial. J Allergy Clin Immunol. 2016;137(2):444–451.e8. https://doi.org/10.1016/j.jaci.2015.06.036
- 1695. Galimberti M, Passalacqua G, Incorvaia C, et al. Catching allergy by a simple questionnaire. World Allergy Organ J. 2015;8(1):16. https://doi.org/10.1186/s40413‐015‐0067‐y
- 1696. Klimek L, Bachert C, Lukat KF, Pfaar O, Meyer H, Narkus A. Allergy immunotherapy with a hypoallergenic recombinant birch pollen allergen rBet v 1‐FV in a randomized controlled trial. Clin Transl Allergy. 2015;5:28. https://doi.org/10.1186/s13601‐015‐0071‐x
- 1697. Benninger MS, Senior BA. The development of the Rhinosinusitis Disability Index. Arch Otolaryngol Head Neck Surg. 1997;123(11):1175–1179. https://doi.org/10.1001/archotol.1997.01900110025004
- 1698. Benninger MS, Benninger RM. The impact of allergic rhinitis on sexual activity, sleep, and fatigue. Allergy Asthma Proc. 2009;30(4):358–365. https://doi.org/10.2500/aap.2009.30.3244
- 1699. Kupczyk M, Baiardini I, Molinengo G, et al. Cross‐cultural adaptation and validation of the RhinAsthma Patient Perspective (RAPP) in the Polish population. Postepy Dermatol Alergol. 2020;37(1):97–102. https://doi.org/10.5114/ada.2020.93387
- 1700. Werner CU, Koch L, Linde K, et al. Prospective observational study validating the German version of the Control of Allergic Rhinitis and Asthma Test (CARAT10). NPJ Prim Care Respir Med. 2018;28(1):45. https://doi.org/10.1038/s41533‐018‐0112‐8
- 1701. Emons JA, Flokstra BM, de Jong C, et al. Use of the Control of Allergic Rhinitis and Asthma Test (CARATkids) in children and adolescents: validation in Dutch. Pediatr Allergy Immunol. 2017;28(2):185–190. https://doi.org/10.1111/pai.12678
- 1702. Nurmatov U, van Schayck CP, Hurwitz B, Sheikh A. House dust mite avoidance measures for perennial allergic rhinitis: an updated Cochrane systematic review. Allergy. 2012;67(2):158–165. https://doi.org/10.1111/j.1398‐9995.2011.02752.x
- 1703. International Consensus Report on the diagnosis and management of rhinitis. International Rhinitis Management Working Group. Allergy. 1994;49(19 suppl):1–34.
- 1704. Mackay IS, Durham SR. ABC of allergies. Perennial rhinitis. BMJ. 1998;316(7135):917–920. https://doi.org/10.1136/bmj.316.7135.917
- 1705. Woodcock A, Custovic A. ABC of allergies. Avoiding exposure to indoor allergens. BMJ. 1998;316(7137):1075–1078. https://doi.org/10.1136/bmj.316.7137.1075
- 1706. Krouse HJ. Environmental controls and avoidance measures. Int Forum Allergy Rhinol. 2014;4(suppl 2):S32‐S34. https://doi.org/10.1002/alr.21383
- 1707. Ghazala L, Schmid F, Helbling A, Pichler WJ, Pichler CE. Efficacy of house dust mite and allergen impermeable encasisgs in patients with house dust mite allergy [German]. Allergologie. 2004;27:26–34.
- 1708. Kniest FM, Wolfs BJ, Vos H, et al. Mechanisms and patient compliance of dust‐mite avoidance regimens in dwellings of mite‐allergic rhinitic patients. Clin Exp Allergy. 1992;22(7):681–689. https://doi.org/10.1111/j.1365‐2222.1992.tb00191.x
- 1709. Moon JS, Choi SO. Environmental controls in reducing house dust mites and nasal symptoms in patients with allergic rhinitis. Yonsei Med J. 1999;40(3):238–243. https://doi.org/10.3349/ymj.1999.40.3.238
- 1710. Reisman RE, Mauriello PM, Davis GB, Georgitis JW, DeMasi JM. A double‐blind study of the effectiveness of a high‐efficiency particulate air (HEPA) filter in the treatment of patients with perennial allergic rhinitis and asthma. J Allergy Clin Immunol. 1990;85(6):1050–1057. https://doi.org/10.1016/0091‐6749(90)90050‐e
- 1711. Terreehorst I, Hak E, Oosting AJ, et al. Evaluation of impermeable covers for bedding in patients with allergic rhinitis. N Engl J Med. 2003;349(3):237–246. https://doi.org/10.1056/NEJMoa023171
- 1712. Berings M, Jult A, Vermeulen H, et al. Probiotics‐impregnated bedding covers for house dust mite allergic rhinitis: a pilot randomized clinical trial. Clin Exp Allergy. 2017;47(8):1092–1096. https://doi.org/10.1111/cea.12937
- 1713. Jeon YH, Lee YJ, Sohn MH, Lee HR. Effects of vacuuming mattresses on allergic rhinitis symptoms in children. Allergy Asthma Immunol Res. 2019;11(5):655–663. https://doi.org/10.4168/aair.2019.11.5.655
- 1714. Antonicelli L, Bilo MB, Pucci S, Schou C, Bonifazi F. Efficacy of an air‐cleaning device equipped with a high efficiency particulate air filter in house dust mite respiratory allergy. Allergy. 1991;46(8):594–600. https://doi.org/10.1111/j.1398‐9995.1991.tb00629.x
- 1715. Geller‐Bernstein C, Pibourdin JM, Dornelas A, Fondarai J. Efficacy of the acaricide: acardust for the prevention of asthma and rhinitis due to dust mite allergy, in children. Allerg Immunol (Paris). 1995;27(5):147–154.
- 1716. Chen M, Wu Y, Yuan S, et al. Allergic rhinitis improvement in asthmatic children after using acaricidal bait: a randomized, double‐blind, cross‐placebo study. Front Pediatr. 2021;9:709139. https://doi.org/10.3389/fped.2021.709139
- 1717. Sheikh A, Hurwitz B, Nurmatov U, van Schayck CP. House dust mite avoidance measures for perennial allergic rhinitis. Cochrane Database Syst Rev. 2010;4.
- 1718. Stillerman A, Nachtsheim C, Li W, Albrecht M, Waldman J. Efficacy of a novel air filtration pillow for avoidance of perennial allergens in symptomatic adults. Ann Allergy Asthma Immunol. 2010;104(5):440–449. https://doi.org/10.1016/j.anai.2010.03.006
- 1719. Brehler R, Kniest F. Encasing study in mite‐allergic patients: one‐year, double‐blind placebo and environment‐controlled investigation. Allergy Clin Immunol Inter. 2006;18:15–19.
- 1720. Le Cann P, Paulus H, Glorennec P, Le Bot B, Frain S, Gangneux JP. Home environmental interventions for the prevention or control of allergic and respiratory diseases: what really works. J Allergy Clin Immunol Pract. 2017;5(1):66–79. https://doi.org/10.1016/j.jaip.2016.07.011
- 1721. Rosenstreich DL, Eggleston P, Kattan M, et al. The role of cockroach allergy and exposure to cockroach allergen in causing morbidity among inner‐city children with asthma. N Engl J Med. 1997;336(19):1356–1363. https://doi.org/10.1056/NEJM199705083361904
- 1722. Chew GL. Assessment of environmental cockroach allergen exposure. Curr Allergy Asthma Rep. 2012;12(5):456–464. https://doi.org/10.1007/s11882‐012‐0287‐y
- 1723. Sever ML, Arbes SJ Jr., Gore JC, et al. Cockroach allergen reduction by cockroach control alone in low‐income urban homes: a randomized control trial. J Allergy Clin Immunol. 2007;120(4):849–855. https://doi.org/10.1016/j.jaci.2007.07.003
- 1724. McConnell R, Milam J, Richardson J, et al. Educational intervention to control cockroach allergen exposure in the homes of hispanic children in Los Angeles: results of the La Casa study. Clin Exp Allergy. 2005;35(4):426–433. https://doi.org/10.1111/j.1365‐2222.2005.02196.x
- 1725. Arbes SJ Jr., Sever M, Mehta J, et al. Abatement of cockroach allergens (Bla g 1 and Bla g 2) in low‐income, urban housing: month 12 continuation results. J Allergy Clin Immunol. 2004;113(1):109–114. https://doi.org/10.1016/j.jaci.2003.10.042
- 1726. McConnell R, Jones C, Milam J, et al. Cockroach counts and house dust allergen concentrations after professional cockroach control and cleaning. Ann Allergy Asthma Immunol. 2003;91(6):546–552. https://doi.org/10.1016/S1081‐1206(10)61532‐3
- 1727. Wood RA, Eggleston PA, Rand C, Nixon WJ, Kanchanaraksa S. Cockroach allergen abatement with extermination and sodium hypochlorite cleaning in inner‐city homes. Ann Allergy Asthma Immunol. 2001;87(1):60–64. https://doi.org/10.1016/S1081‐1206(10)62324‐1
- 1728. Gergen PJ, Mortimer KM, Eggleston PA, et al. Results of the National Cooperative Inner‐City Asthma Study (NCICAS) environmental intervention to reduce cockroach allergen exposure in inner‐city homes. J Allergy Clin Immunol. 1999;103(3 pt 1):501–506. https://doi.org/10.1016/s0091‐6749(99)70477‐x
- 1729. Eggleston PA, Wood RA, Rand C, Nixon WJ, Chen PH, Lukk P. Removal of cockroach allergen from inner‐city homes. J Allergy Clin Immunol. 1999;104(4 pt 1):842–846. https://doi.org/10.1016/s0091‐6749(99)70296‐4
- 1730. Williams LW, Reinfried P, Brenner RJ. Cockroach extermination does not rapidly reduce allergen in settled dust. J Allergy Clin Immunol. 1999;104(3 pt 1):702–703. https://doi.org/10.1016/s0091‐6749(99)70346‐5
- 1731. Wang C, Eiden AL, Cooper R, Zha C, Wang D, Hamilton RG. Abatement of cockroach allergens by effective cockroach management in apartments. J Allergy Clin Immunol Pract. 2020;8(10):3608–3609. https://doi.org/10.1016/j.jaip.2020.06.040
- 1732. Eggleston PA, Butz A, Rand C, et al. Home environmental intervention in inner‐city asthma: a randomized controlled clinical trial. Ann Allergy Asthma Immunol. 2005;95(6):518–524. https://doi.org/10.1016/S1081‐1206(10)61012‐5
- 1733. Morgan WJ, Crain EF, Gruchalla RS, et al. Results of a home‐based environmental intervention among urban children with asthma. N Engl J Med. 2004;351(11):1068–1080. https://doi.org/10.1056/NEJMoa032097
- 1734. Sever ML, Salo PM, Haynes AK, Zeldin DC. Inner‐city environments and mitigation of cockroach allergen. Am J Prev Med. 2011;41(2 suppl 1):S55–S56. https://doi.org/10.1016/j.amepre.2011.05.007
- 1735. Sanchez J, Diez S, Cardona R. Pet avoidance in allergy cases: is it possible to implement it? Biomedica. 2015;35(3):357–362. https://doi.org/10.7705/biomedica.v35i3.2634
- 1736. Custovic A, Wijk RG. The effectiveness of measures to change the indoor environment in the treatment of allergic rhinitis and asthma: ARIA update (in collaboration with GA(2)LEN). Allergy. 2005;60(9):1112–1115. https://doi.org/10.1111/j.1398‐9995.2005.00934.x
- 1737. Portnoy J, Kennedy K, Sublett J, et al. Environmental assessment and exposure control: a practice parameter–furry animals. Ann Allergy Asthma Immunol. 2012;108(4):223. e1‐15. https://doi.org/10.1016/j.anai.2012.02.015
- 1738. Arshad SH. Environmental control for secondary prevention of asthma. Clin Exp Allergy. 2010;40(1):2–4. https://doi.org/10.1111/j.1365‐2222.2009.03407.x
- 1739. National Heart Lung and Blood Institute . National Asthma Education and Prevention Program. Expert Panel Report 3 (EPR‐3): Guidelines for the diagnosis and management of asthma. US Department of Health and Human Services, 2007.
- 1740. Matsui EC, Perzanowski M, Peng RD, et al. Effect of an integrated pest management intervention on asthma symptoms among mouse‐sensitized children and adolescents with asthma: A randomized clinical trial. JAMA. 2017;317(10):1027–1036. https://doi.org/10.1001/jama.2016.21048
- 1741. Wood RA, Johnson EF, Van Natta ML, Chen PH, Eggleston PA. A placebo‐controlled trial of a HEPA air cleaner in the treatment of cat allergy. Am J Respir Crit Care Med. 1998;158(1):115–120. https://doi.org/10.1164/ajrccm.158.1.9712110
- 1742. Avner DB, Perzanowski MS, Platts‐Mills TA, Woodfolk JA. Evaluation of different techniques for washing cats: quantitation of allergen removed from the cat and the effect on airborne Fel d 1. J Allergy Clin Immunol. 1997;100(3):307–312. https://doi.org/10.1016/s0091‐6749(97)70242‐2
- 1743. Hodson T, Custovic A, Simpson A, Chapman M, Woodcock A, Green R. Washing the dog reduces dog allergen levels, but the dog needs to be washed twice a week. J Allergy Clin Immunol. 1999;103(4):581–585. https://doi.org/10.1016/s0091‐6749(99)70227‐7
- 1744. Matsui EC, Simons E, Rand C, et al. Airborne mouse allergen in the homes of inner‐city children with asthma. J Allergy Clin Immunol. 2005;115(2):358–363. https://doi.org/10.1016/j.jaci.2004.11.007
- 1745. Grant T, Phipatanakul W, Perzanowski M, et al. Reduction in mouse allergen exposure is associated with greater lung function growth. J Allergy Clin Immunol. 2020;145(2):646–653.e1. https://doi.org/10.1016/j.jaci.2019.08.043
- 1746. Pongracic JA, Visness CM, Gruchalla RS, Evans R 3rd, Mitchell HE. Effect of mouse allergen and rodent environmental intervention on asthma in inner‐city children. Ann Allergy Asthma Immunol. 2008;101(1):35–41. https://doi.org/10.1016/S1081‐1206(10)60832‐0
- 1747. Phipatanakul W, Cronin B, Wood RA, et al. Effect of environmental intervention on mouse allergen levels in homes of inner‐city Boston children with asthma. Ann Allergy Asthma Immunol. 2004;92(4):420–425. https://doi.org/10.1016/S1081‐1206(10)61777‐2
- 1748. DiMango E, Serebrisky D, Narula S, et al. Individualized household allergen intervention lowers allergen level but not asthma medication use: a randomized controlled trial. J Allergy Clin Immunol Pract. 2016;4(4):671–679.e4. https://doi.org/10.1016/j.jaip.2016.01.016
- 1749. Jacobs TS, Forno E, Brehm JM, et al. Mouse allergen exposure and decreased risk of allergic rhinitis in school‐aged children. Ann Allergy Asthma Immunol. 2014;113(6):614–618.e2. https://doi.org/10.1016/j.anai.2014.09.007
- 1750. Sakaguchi M, Inouye S, Miyazawa H, Kamimura H, Kimura M, Yamazaki S. Evaluation of dust respirators for elimination of mouse aeroallergens. Lab Anim Sci. 1989;39(1):63–66.
- 1751. Bertelsen RJ, Carlsen KC, Granum B, et al. Do allergic families avoid keeping furry pets? Indoor Air. 2010;20(3):187–195. https://doi.org/10.1111/j.1600‐0668.2009.00640.x
- 1752. Curtin‐Brosnan J, Saams J, Breysse P, Diette G, Bradley H, Matsui E. Relationship between cat and mouse allergen levels in the homes of inner city children with asthma. J Allergy Clin Immunol. 2009;123:64.
- 1753. Dykewicz MS, Wallace DV, Baroody F, et al. Treatment of seasonal allergic rhinitis: an evidence‐based focused 2017 guideline update. Ann Allergy Asthma Immunol. 2017;119(6):489–511.e41. https://doi.org/10.1016/j.anai.2017.08.012
- 1754. Reisacher WR. Allergy treatment: environmental control strategies. Otolaryngol Clin North Am. 2011;44(3):711–725, x. https://doi.org/10.1016/j.otc.2011.03.019
- 1755. Ferguson BJ. Environmental controls of allergies. Otolaryngol Clin North Am. 2008;41(2):411–417, viii‐ix. https://doi.org/10.1016/j.otc.2007.11.006
- 1756. Bergmann KC, Berger M, Klimek L, et al. Nonpharmacological measures to prevent allergic symptoms in pollen allergy: a critical review. Allergol Select. 2021;5:349–360. https://doi.org/10.5414/ALX02294E
- 1757. Li L, Zhang L, Mo JH, et al. Efficacy of indoor air purification in the treatment of Artemisia pollen‐allergic rhinitis: a randomised, double‐blind, clinical controlled trial. Clin Otolaryngol. 2020;45(3):394–401. https://doi.org/10.1111/coa.13514
- 1758. Green BJ, Levetin E, Horner WE, Codina R, Barnes CS, Filley WV. Landscape plant selection criteria for the allergic patient. J Allergy Clin Immunol Pract. 2018;6(6):1869–1876. https://doi.org/10.1016/j.jaip.2018.05.020
- 1759. van Cauwenberge P, Bachert C, Passalacqua G, et al. Consensus statement on the treatment of allergic rhinitis. European Academy of Allergology and Clinical Immunology. Allergy. 2000;55(2):116–134. https://doi.org/10.1034/j.1398‐9995.2000.00526.x
- 1760. Comert S, Karakaya G, Kalyoncu AF. Wraparound eyeglasses improve symptoms and quality of life in patients with seasonal allergic rhinoconjunctivitis. Int Forum Allergy Rhinol. 2016;6(7):722–730. https://doi.org/10.1002/alr.21737
- 1761. Kenney P, Hilberg O, Laursen AC, Peel RG, Sigsgaard T. Preventive effect of nasal filters on allergic rhinitis: a randomized, double‐blind, placebo‐controlled crossover park study. J Allergy Clin Immunol. 2015;136(6):1566–1572.e5. https://doi.org/10.1016/j.jaci.2015.05.015
- 1762. Chen X, Deng C, Mi J, et al. Barrier protection measures for the management of allergic rhinitis: a systematic review and meta‐analysis. Am J Rhinol Allergy. 2020;34(4):564–572. https://doi.org/10.1177/1945892420912370
- 1763. Chen QY, Li L, Zhang L, et al. Efficacy of indoor air purification in treating Artemisia (mugwort) pollen allergic rhinitis: study protocol for a randomised controlled trial. BMC Public Health. 2018;18(1):841. https://doi.org/10.1186/s12889‐018‐5678‐0
- 1764. Gautrin D, Desrosiers M, Castano R. Occupational rhinitis. Curr Opin Allergy Clin Immunol. 2006;6(2):77–84. https://doi.org/10.1097/01.all.0000216848.87699.38
- 1765. Hox V, Steelant B, Fokkens W, Nemery B, Hellings PW. Occupational upper airway disease: how work affects the nose. Allergy. 2014;69(3):282–291. https://doi.org/10.1111/all.12347
- 1766. Castano R, Trudeau C, Castellanos L, Malo JL. Prospective outcome assessment of occupational rhinitis after removal from exposure. J Occup Environ Med. 2013;55(5):579–585. https://doi.org/10.1097/JOM.0b013e318289ee17
- 1767. Airaksinen LK, Luukkonen RA, Lindstrom I, Lauerma AI, Toskala EM. Long‐term exposure and health‐related quality of life among patients with occupational rhinitis. J Occup Environ Med. 2009;51(11):1288–1297. https://doi.org/10.1097/JOM.0b013e3181b9b242
- 1768. Vandenplas O, Jamart J, Delwiche JP, Evrard G, Larbanois A. Occupational asthma caused by natural rubber latex: outcome according to cessation or reduction of exposure. J Allergy Clin Immunol. 2002;109(1):125–130. https://doi.org/10.1067/mai.2002.120760
- 1769. Merget R, Schulte A, Gebler A, et al. Outcome of occupational asthma due to platinum salts after transferral to low‐exposure areas. Int Arch Occup Environ Health. 1999;72(1):33–39. https://doi.org/10.1007/s004200050331
- 1770. Taivainen AI, Tukiainen HO, Terho EO, Husman KR. Powered dust respirator helmets in the prevention of occupational asthma among farmers. Scand J Work Environ Health. 1998;24(6):503–507. https://doi.org/10.5271/sjweh.375
- 1771. Golightly LK, Greos LS. Second‐generation antihistamines: actions and efficacy in the management of allergic disorders. Drugs. 2005;65(3):341–384. https://doi.org/10.2165/00003495‐200565030‐00004
- 1772. Lieberman P. The basics of histamine biology. Ann Allergy Asthma Immunol. 2011;106(2 suppl):S2–S5. https://doi.org/10.1016/j.anai.2010.08.005
- 1773. Fein MN, Fischer DA, O'Keefe AW, Sussman GL. CSACI position statement: newer generation H1‐antihistamines are safer than first‐generation H1‐antihistamines and should be the first‐line antihistamines for the treatment of allergic rhinitis and urticaria. Allergy Asthma Clin Immunol. 2019;15:61. https://doi.org/10.1186/s13223‐019‐0375‐9
- 1774. Sanchez‐Borges M, Ansotegui IJ. Second generation antihistamines: an update. Curr Opin Allergy Clin Immunol. 2019;19(4):358–364. https://doi.org/10.1097/ACI.0000000000000556
- 1775. Bousquet J, Bindslev‐Jensen C, Canonica GW, et al. The ARIA/EAACI criteria for antihistamines: an assessment of the efficacy, safety and pharmacology of desloratadine. Allergy. 2004;59(suppl 77):4–16. https://doi.org/10.1111/j.1398‐9995.2004.00577.x
- 1776. Kardas G, Panek M, Kuna P, Cieszynski J, Kardas P. Primary non‐adherence to antihistamines‐conclusions from e‐prescription pilot data in Poland. Front Pharmacol. 2020;11:783. https://doi.org/10.3389/fphar.2020.00783
- 1777. Blaiss MS. Cost‐effectiveness of H1‐antihistamines. Clin Allergy Immunol. 2002;17:319–336.
- 1778. Miligkos M, Dakoutrou M, Statha E, et al. Newer‐generation antihistamines and the risk of adverse events in children: a systematic review. Pediatr Allergy Immunol. 2021;32(7):1533–1558. https://doi.org/10.1111/pai.13522
- 1779. Sastre J. Ebastine in the treatment of allergic rhinitis and urticaria: 30 years of clinical studies and real‐world experience. J Investig Allergol Clin Immunol. 2020;30(3):156–168. https://doi.org/10.18176/jiaci.0401
- 1780. Mullol J, Bousquet J, Bachert C, et al. Update on rupatadine in the management of allergic disorders. Allergy. 2015;70(suppl 100):1–24. https://doi.org/10.1111/all.12531
- 1781. Ridolo E, Montagni M, Bonzano L, Incorvaia C, Canonica GW. Bilastine: new insight into antihistamine treatment. Clin Mol Allergy. 2015;13(1):1. https://doi.org/10.1186/s12948‐015‐0008‐x
- 1782. Compalati E, Canonica GW. Efficacy and safety of rupatadine for allergic rhino‐conjunctivitis: a systematic review of randomized, double‐blind, placebo‐controlled studies with meta‐analysis. Curr Med Res Opin. 2013;29(11):1539–1551. https://doi.org/10.1185/03007995.2013.822855
- 1783. Mosges R, Konig V, Koberlein J. The effectiveness of modern antihistamines for treatment of allergic rhinitis – an IPD meta‐analysis of 140,853 patients. Allergol Int. 2013;62(2):215–222. https://doi.org/10.2332/allergolint.12‐OA‐0486
- 1784. Compalati E, Baena‐Cagnani R, Penagos M, et al. Systematic review on the efficacy of fexofenadine in seasonal allergic rhinitis: a meta‐analysis of randomized, double‐blind, placebo‐controlled clinical trials. Int Arch Allergy Immunol. 2011;156(1):1–15. https://doi.org/10.1159/000321896
- 1785. Ferrer M. Pharmacokinetic evaluation of levocetirizine. Expert Opin Drug Metab Toxicol. 2011;7(8):1035–1047. https://doi.org/10.1517/17425255.2011.590131
- 1786. Mosges R, Konig V, Koberlein J. The effectiveness of levocetirizine in comparison with loratadine in treatment of allergic rhinitis – a meta‐analysis. Allergol Int. 2011;60(4):541–546. https://doi.org/10.2332/allergolint.10‐OA‐0300
- 1787. Bachert C. A review of the efficacy of desloratadine, fexofenadine, and levocetirizine in the treatment of nasal congestion in patients with allergic rhinitis. Clin Ther. 2009;31(5):921–944. https://doi.org/10.1016/j.clinthera.2009.05.017
- 1788. Katiyar S, Prakash S. Pharmacological profile, efficacy and safety of rupatadine in allergic rhinitis. Prim Care Respir J. 2009;18(2):57–68. https://doi.org/10.3132/pcrj.2008.00043
- 1789. Bachert C, van Cauwenberge P. Desloratadine treatment for intermittent and persistent allergic rhinitis: a review. Clin Ther. 2007;29(9):1795–1802. https://doi.org/10.1016/j.clinthera.2007.09.009
- 1790. Canonica GW, Tarantini F, Compalati E, Penagos M. Efficacy of desloratadine in the treatment of allergic rhinitis: a meta‐analysis of randomized, double‐blind, controlled trials. Allergy. 2007;62(4):359–366. https://doi.org/10.1111/j.1398‐9995.2006.01277.x
- 1791. Patou J, De Smedt H, van Cauwenberge P, Bachert C. Pathophysiology of nasal obstruction and meta‐analysis of early and late effects of levocetirizine. Clin Exp Allergy. 2006;36(8):972–981. https://doi.org/10.1111/j.1365‐2222.2006.02544.x
- 1792. Hore I, Georgalas C, Scadding G. Oral antihistamines for the symptom of nasal obstruction in persistent allergic rhinitis – a systematic review of randomized controlled trials. Clin Exp Allergy. 2005;35(2):207–212. https://doi.org/10.1111/j.1365‐2222.2005.02159.x
- 1793. Passalacqua G, Canonica GW. A review of the evidence from comparative studies of levocetirizine and desloratadine for the symptoms of allergic rhinitis. Clin Ther. 2005;27(7):979–992. https://doi.org/10.1016/j.clinthera.2005.07.011
- 1794. Greisner WA 3rd. Onset of action for the relief of allergic rhinitis symptoms with second‐generation antihistamines. Allergy Asthma Proc. 2004;25(2):81–83.
- 1795. Limon L, Kockler DR. Desloratadine: a nonsedating antihistamine. Ann Pharmacother. 2003;37(2):237–246, quiz 313‐6. https://doi.org/10.1177/106002800303700216
- 1796. Scadding GK. Optimal management of allergic rhinitis. Arch Dis Child. 2015;100(6):576–582. https://doi.org/10.1136/archdischild‐2014‐306300
- 1797. Penston J, Wormsley KG. Adverse reactions and interactions with H2‐receptor antagonists. Med Toxicol. 1986;1(3):192–216. https://doi.org/10.1007/BF03259837
- 1798. Wood‐Baker R, Lau L, Howarth PH. Histamine and the nasal vasculature: the influence of H1 and H2‐histamine receptor antagonism. Clin Otolaryngol Allied Sci. 1996;21(4):348–352. https://doi.org/10.1111/j.1365‐2273.1996.tb01085.x
- 1799. Taylor‐Clark T, Sodha R, Warner B, Foreman J. Histamine receptors that influence blockage of the normal human nasal airway. Br J Pharmacol. 2005;144(6):867–874. https://doi.org/10.1038/sj.bjp.0706118
- 1800. Wang D, Clement P, Smitz J. Effect of H1 and H2 antagonists on nasal symptoms and mediator release in atopic patients after nasal allergen challenge during the pollen season. Acta Otolaryngol. 1996;116(1):91–96. https://doi.org/10.3109/00016489609137720
- 1801. Havas TE, Cole P, Parker L, Oprysk D, Ayiomamitis A. The effects of combined H1 and H2 histamine antagonists on alterations in nasal airflow resistance induced by topical histamine provocation. J Allergy Clin Immunol. 1986;78(5 pt 1):856–860. https://doi.org/10.1016/0091‐6749(86)90230‐7
- 1802. Juliusson S, Bende M. Effect of systemically administered H1‐ and H2‐receptor antagonists on nasal blood flow as measured with laser Doppler flowmetry in a provoked allergic reaction. Rhinology. 1996;34(1):24–27.
- 1803. Brooks CD, Butler D, Metzler C. Effect of H2 blockade in the challenged allergic nose. J Allergy Clin Immunol. 1982;70(5):373–376. https://doi.org/10.1016/0091‐6749(82)90027‐6
- 1804. Carpenter GB, Bunker‐Soler AL, Nelson HS. Evaluation of combined H1‐ and H2‐receptor blocking agents in the treatment of seasonal allergic rhinitis. J Allergy Clin Immunol. 1983;71(4):412–417. https://doi.org/10.1016/0091‐6749(83)90071‐4
- 1805. Carr WW, Ratner P, Munzel U, et al. Comparison of intranasal azelastine to intranasal fluticasone propionate for symptom control in moderate‐to‐severe seasonal allergic rhinitis. Allergy Asthma Proc. 2012;33(6):450–458. https://doi.org/10.2500/aap.2012.33.3626
- 1806. Kalpaklioglu AF, Kavut AB. Comparison of azelastine versus triamcinolone nasal spray in allergic and nonallergic rhinitis. Am J Rhinol Allergy. 2010;24(1):29–33. https://doi.org/10.2500/ajra.2010.24.3423
- 1807. Kaliner MA, Storms W, Tilles S, et al. Comparison of olopatadine 0.6% nasal spray versus fluticasone propionate 50 microg in the treatment of seasonal allergic rhinitis. Allergy Asthma Proc. 2009;30(3):255–262. https://doi.org/10.2500/aap.2009.30.3232
- 1808. Patel P, D'Andrea C, Sacks HJ. Onset of action of azelastine nasal spray compared with mometasone nasal spray and placebo in subjects with seasonal allergic rhinitis evaluated in an environmental exposure chamber. Am J Rhinol. 2007;21(4):499–503. https://doi.org/10.2500/ajr.2007.21.3058
- 1809. Patel D, Garadi R, Brubaker M, et al. Onset and duration of action of nasal sprays in seasonal allergic rhinitis patients: olopatadine hydrochloride versus mometasone furoate monohydrate. Allergy Asthma Proc. 2007;28(5):592–599. https://doi.org/10.2500/aap2007.28.3033
- 1810. Berger W, Hampel Jr F, Bernstein J, Shah S, Sacks H, Meltzer EO. Impact of azelastine nasal spray on symptoms and quality of life compared with cetirizine oral tablets in patients with seasonal allergic rhinitis. Ann Allergy Asthma Immunol. 2006;97(3):375–381. https://doi.org/10.1016/S1081‐1206(10)60804‐6
- 1811. Corren J, Storms W, Bernstein J, et al. Effectiveness of azelastine nasal spray compared with oral cetirizine in patients with seasonal allergic rhinitis. Clin Ther. 2005;27(5):543–553. https://doi.org/10.1016/j.clinthera.2005.04.012
- 1812. LaForce CF, Corren J, Wheeler WJ, Berger WE, Rhinitis Study Group . Efficacy of azelastine nasal spray in seasonal allergic rhinitis patients who remain symptomatic after treatment with fexofenadine. Ann Allergy Asthma Immunol. 2004;93(2):154–159. https://doi.org/10.1016/S1081‐1206(10)61468‐8
- 1813. Berger WE, White MV, Rhinitis Study Group . Efficacy of azelastine nasal spray in patients with an unsatisfactory response to loratadine. Ann Allergy Asthma Immunol. 2003;91(2):205–211. https://doi.org/10.1016/S1081‐1206(10)62179‐5
- 1814. Berlin JM, Golden SJ, Teets S, Lehman EB, Lucas T, Craig TJ. Efficacy of a steroid nasal spray compared with an antihistamine nasal spray in the treatment of perennial allergic rhinitis. J Am Osteopath Assoc. 2000;100(7 suppl):S8–S13.
- 1815. Berger WE, Fineman SM, Lieberman P, Miles RM. Double‐blind trials of azelastine nasal spray monotherapy versus combination therapy with loratadine tablets and beclomethasone nasal spray in patients with seasonal allergic rhinitis. Rhinitis Study Groups. Ann Allergy Asthma Immunol. 1999;82(6):535–541. https://doi.org/10.1016/s1081‐1206(10)63161‐4
- 1816. Stern MA, Wade AG, Ridout SM, Cambell LM. Nasal budesonide offers superior symptom relief in perennial allergic rhinitis in comparison to nasal azelastine. Ann Allergy Asthma Immunol. 1998;81(4):354–358. https://doi.org/10.1016/s1081‐1206(10)63128‐6
- 1817. Jarvis D, Newson R, Lotvall J, et al. Asthma in adults and its association with chronic rhinosinusitis: the GA2LEN survey in Europe. Allergy. 2012;67(1):91–98. https://doi.org/10.1111/j.1398‐9995.2011.02709.x
- 1818. LaForce C, Dockhorn RJ, Prenner BM, et al. Safety and efficacy of azelastine nasal spray (Astelin NS) for seasonal allergic rhinitis: a 4‐week comparative multicenter trial. Ann Allergy Asthma Immunol. 1996;76(2):181–188. https://doi.org/10.1016/S1081‐1206(10)63420‐5
- 1819. Charpin D, Godard P, Garay RP, Baehre M, Herman D, Michel FB. A multicenter clinical study of the efficacy and tolerability of azelastine nasal spray in the treatment of seasonal allergic rhinitis: a comparison with oral cetirizine. Eur Arch Otorhinolaryngol. 1995;252(8):455–458. https://doi.org/10.1007/BF02114749
- 1820. Pelucchi A, Chiapparino A, Mastropasqua B, Marazzini L, Hernandez A, Foresi A. Effect of intranasal azelastine and beclomethasone dipropionate on nasal symptoms, nasal cytology, and bronchial responsiveness to methacholine in allergic rhinitis in response to grass pollens. J Allergy Clin Immunol. 1995;95(2):515–523. https://doi.org/10.1016/s0091‐6749(95)70313‐6
- 1821. Gastpar H, Nolte D, Aurich R, et al. Comparative efficacy of azelastine nasal spray and terfenadine in seasonal and perennial rhinitis. Allergy. 1994;49(3):152–158. https://doi.org/10.1111/j.1398‐9995.1994.tb00818.x
- 1822. Meltzer EO, Weiler JM, Dockhorn RJ, Widlitz MD, Freitag JJ. Azelastine nasal spray in the management of seasonal allergic rhinitis. Ann Allergy. 1994;72(4):354–359.
- 1823. Passali D, Piragine F. A comparison of azelastine nasal spray and cetirizine tablets in the treatment of allergic rhinitis. J Int Med Res. 1994;22(1):17–23. https://doi.org/10.1177/030006059402200102
- 1824. Davies RJ, Lund VJ, Harten‐Ash VJ. The effect of intranasal azelastine and beclomethasone on the symptoms and signs of nasal allergy in patients with perennial allergic rhinitis. Rhinology. 1993;31(4):159–164.
- 1825. Dorow P, Aurich R, Petzold U. Efficacy and tolerability of azelastine nasal spray in patients with allergic rhinitis compared to placebo and budesonide. Arzneimittelforschung. 1993;43(8):909–912.
- 1826. Gambardella R. A comparison of the efficacy of azelastine nasal spray and loratidine tablets in the treatment of seasonal allergic rhinitis. J Int Med Res. 1993;21(5):268–275. https://doi.org/10.1177/030006059302100505
- 1827. Gastpar H, Aurich R, Petzold U, et al. Intranasal treatment of perennial allergic rhinitis. Comparison of azelastine nasal spray and budesonide nasal aerosol. Arzneimittelforschung. 1993;43(4):475–479.
- 1828. Howland WC, Amar NJ, Wheeler W, Sacks H. Efficacy and safety of azelastine 0.15% nasal spray administered once daily in patients with allergy to Texas mountain cedar pollen. Int Forum Allergy Rhinol. 2011;1(4):275–279. https://doi.org/10.1002/alr.20065
- 1829. Meltzer EO, Blaiss M, Fairchild CJ. Comprehensive report of olopatadine 0.6% nasal spray as treatment for children with seasonal allergic rhinitis. Allergy Asthma Proc. 2011;32(3):213–220. https://doi.org/10.2500/aap.2011.32.3448
- 1830. Berger WE, Ratner PH, Casale TB, Meltzer EO, Wall GM. Safety and efficacy of olopatadine hydrochloride nasal spray 0.6% in pediatric subjects with allergic rhinitis. Allergy Asthma Proc. 2009;30(6):612–623. https://doi.org/10.2500/aap.2009.30.3298
- 1831. Bernstein JA, Prenner B, Ferguson BJ, Portnoy J, Wheeler WJ, Sacks HJ. Double‐blind, placebo‐controlled trial of reformulated azelastine nasal spray in patients with seasonal allergic rhinitis. Am J Rhinol Allergy. 2009;23(5):512–517. https://doi.org/10.2500/ajra.2009.23.3396
- 1832. Shah S, Berger W, Lumry W, La Force C, Wheeler W, Sacks H. Efficacy and safety of azelastine 0.15% nasal spray and azelastine 0.10% nasal spray in patients with seasonal allergic rhinitis. Allergy Asthma Proc. 2009;30(6):628–633. https://doi.org/10.2500/aap.2009.30.3296
- 1833. Shah SR, Nayak A, Ratner P, Roland P, Michael Wall G. Effects of olopatadine hydrochloride nasal spray 0.6% in the treatment of seasonal allergic rhinitis: a phase III, multicenter, randomized, double‐blind, active‐ and placebo‐controlled study in adolescents and adults. Clin Ther. 2009;31(1):99–107. https://doi.org/10.1016/j.clinthera.2009.01.016
- 1834. van Bavel J, Howland WC, Amar NJ, Wheeler W, Sacks H. Efficacy and safety of azelastine 0.15% nasal spray administered once daily in subjects with seasonal allergic rhinitis. Allergy Asthma Proc. 2009;30(5):512–518. https://doi.org/10.2500/aap.2009.30.3284
- 1835. Pipkorn P, Costantini C, Reynolds C, et al. The effects of the nasal antihistamines olopatadine and azelastine in nasal allergen provocation. Ann Allergy Asthma Immunol. 2008;101(1):82–89. https://doi.org/10.1016/S1081‐1206(10)60839‐3
- 1836. Lumry W, Prenner B, Corren J, Wheeler W. Efficacy and safety of azelastine nasal spray at a dose of 1 spray per nostril twice daily. Ann Allergy Asthma Immunol. 2007;99(3):267–272. https://doi.org/10.1016/S1081‐1206(10)60663‐1
- 1837. Hampel Jr FC, Ratner PH, Amar NJ, et al. Improved quality of life among seasonal allergic rhinitis patients treated with olopatadine HCl nasal spray 0.4% and olopatadine HCl nasal spray 0.6% compared with vehicle placebo. Allergy Asthma Proc. 2006;27(3):202–207. https://doi.org/10.2500/aap.2006.27.2862
- 1838. Meltzer EO, Hampel FC, Ratner PH, et al. Safety and efficacy of olopatadine hydrochloride nasal spray for the treatment of seasonal allergic rhinitis. Ann Allergy Asthma Immunol. 2005;95(6):600–606. https://doi.org/10.1016/S1081‐1206(10)61025‐3
- 1839. Ratner PH, Hampel FC, Amar NJ, et al. Safety and efficacy of olopatadine hydrochloride nasal spray for the treatment of seasonal allergic rhinitis to mountain cedar. Ann Allergy Asthma Immunol. 2005;95(5):474–479. https://doi.org/10.1016/S1081‐1206(10)61174‐X
- 1840. Saengpanich S, Assanasen P, deTineo M, Haney L, Naclerio RM, Baroody FM. Effects of intranasal azelastine on the response to nasal allergen challenge. Laryngoscope. 2002;112(1):47–52. https://doi.org/10.1097/00005537‐200201000‐00009
- 1841. Golden S, Teets SJ, Lehman EB, et al. Effect of topical nasal azelastine on the symptoms of rhinitis, sleep, and daytime somnolence in perennial allergic rhinitis. Ann Allergy Asthma Immunol. 2000;85(1):53–57. https://doi.org/10.1016/S1081‐1206(10)62434‐9
- 1842. Herman D, Garay R, Le Gal M. A randomized double‐blind placebo controlled study of azelastine nasal spray in children with perennial rhinitis. Int J Pediatr Otorhinolaryngol. 1997;39(1):1–8. https://doi.org/10.1016/S0165‐5876(96)01457‐7
- 1843. Newson‐Smith G, Powell M, Baehre M, Garnham SP, MacMahon MT. A placebo controlled study comparing the efficacy of intranasal azelastine and beclomethasone in the treatment of seasonal allergic rhinitis. Eur Arch Otorhinolaryngol. 1997;254(5):236–241. https://doi.org/10.1007/BF00874095
- 1844. Weiler JM, Meltzer EO. Azelastine nasal spray as adjunctive therapy to azelastine tablets in the management of seasonal allergic rhinitis. Ann Allergy Asthma Immunol. 1997;79(4):327–332. https://doi.org/10.1016/S1081‐1206(10)63023‐2
- 1845. Ratner PH, Findlay SR, Hampel Jr F, van Bavel J, Widlitz MD, Freitag JJ. A double‐blind, controlled trial to assess the safety and efficacy of azelastine nasal spray in seasonal allergic rhinitis. J Allergy Clin Immunol. 1994;94(5):818–825. https://doi.org/10.1016/0091‐6749(94)90148‐1
- 1846. Han D, Chen L, Cheng L, et al. A multicenter randomized double‐blind 2‐week comparison study of azelastine nasal spray 0.1% versus levocabastine nasal spray 0.05% in patients with moderate‐to‐severe allergic rhinitis. ORL J Otorhinolaryngol Relat Spec. 2011;73(5):260–265. https://doi.org/10.1159/000330269
- 1847. Meltzer EO, Garadi R, Laforce C, et al. Comparative study of sensory attributes of two antihistamine nasal sprays: olopatadine 0.6% and azelastine 0.1%. Allergy Asthma Proc. 2008;29(6):659–668. https://doi.org/10.2500/aap.2008.29.3181
- 1848. Falser N, Wober W, Rahlfs VW, Baehre M. Comparative efficacy and safety of azelastine and levocabastine nasal sprays in patients with seasonal allergic rhinitis. Arzneimittelforschung. 2001;51(5):387–393. https://doi.org/10.1055/s‐0031‐1300052
- 1849. Pipkorn U, Proud D, Lichtenstein LM, et al. Effect of short‐term systemic glucocorticoid treatment on human nasal mediator release after antigen challenge. J Clin Invest. 1987;80(4):957–961. https://doi.org/10.1172/JCI113188
- 1850. Bascom R, Pipkorn U, Lichtenstein LM, Naclerio RM. The influx of inflammatory cells into nasal washings during the late response to antigen challenge. Effect of systemic steroid pretreatment. Am Rev Respir Dis. 1988;138(2):406–412. https://doi.org/10.1164/ajrccm/138.2.406
- 1851. Bascom R, Pipkorn U, Proud D, et al. Major basic protein and eosinophil‐derived neurotoxin concentrations in nasal‐lavage fluid after antigen challenge: effect of systemic corticosteroids and relationship to eosinophil influx. J Allergy Clin Immunol. 1989;84(3):338–346. https://doi.org/10.1016/0091‐6749(89)90418‐1
- 1852. Schwartz E, Levin L, Leibowitz H, et al. Oral cortisone therapy in ragweed hay fever. J Allergy. 1952;23(1):32–38. https://doi.org/10.1016/0021‐8707(52)90071‐3
- 1853. Schiller IW, Lowell FC. Oral cortisone in the treatment of hay fever. J Allergy. 1953;24(4):297–301. https://doi.org/10.1016/0021‐8707(53)90172‐5
- 1854. Schwartz E. Oral hydrocortisone therapy in bronchial asthma and bay fever. J Allergy. 1954;25(2):112–119. https://doi.org/10.1016/0021‐8707(54)90149‐5
- 1855. Brooks CD, Karl KJ, Francom SF. Oral methylprednisolone acetate (Medrol Tablets) for seasonal rhinitis: examination of dose and symptom response. J Clin Pharmacol. 1993;33(9):816–822. https://doi.org/10.1002/j.1552‐4604.1993.tb01957.x
- 1856. Snyman JR, Potter PC, Groenewald M, Levin J, Claricort Study Group . Effect of betamethasone‐loratadine combination therapy on severe exacerbations of allergic rhinitis: a randomised, controlled trial. Clin Drug Investig. 2004;24(5):265–274. https://doi.org/10.2165/00044011‐200424050‐00003
- 1857. Kwaselow A, McLean J, Busse W, et al. A comparison of intranasal and oral flunisolide in the therapy of allergic rhinitis. Evidence for a topical effect. Allergy. 1985;40(5):363–367. https://doi.org/10.1111/j.1398‐9995.1985.tb00248.x
- 1858. Karaki M, Akiyama K, Mori N. Efficacy of intranasal steroid spray (mometasone furoate) on treatment of patients with seasonal allergic rhinitis: comparison with oral corticosteroids. Auris Nasus Larynx. 2013;40(3):277–281. https://doi.org/10.1016/j.anl.2012.09.004
- 1859. Bascom R, Wachs M, Naclerio RM, Pipkorn U, Galli SJ, Lichtenstein LM. Basophil influx occurs after nasal antigen challenge: effects of topical corticosteroid pretreatment. J Allergy Clin Immunol. 1988;81(3):580–589.
- 1860. Erin EM, Leaker BR, Zacharasiewicz AS, et al. Single dose topical corticosteroid inhibits IL‐5 and IL‐13 in nasal lavage following grass pollen challenge. Allergy. 2005;60(12):1524–1529. https://doi.org/10.1111/j.1398‐9995.2005.00928.x
- 1861. Meltzer EO, Jalowayski AA, Orgel HA, Harris AG. Subjective and objective assessments in patients with seasonal allergic rhinitis: effects of therapy with mometasone furoate nasal spray. J Allergy Clin Immunol. 1998;102(1):39–49. https://doi.org/10.1016/s0091‐6749(98)70053‐3
- 1862. Baroody FM, Cruz AA, Lichtenstein LM, Kagey‐Sobotka A, Proud D, Naclerio RM. Intranasal beclomethasone inhibits antigen‐induced nasal hyperresponsiveness to histamine. J Allergy Clin Immunol. 1992;90(3 pt 1):373–376. https://doi.org/10.1016/s0091‐6749(05)80017‐x
- 1863. Meyer P, Andersson M, Persson CG, Greiff L. Steroid‐sensitive indices of airway inflammation in children with seasonal allergic rhinitis. Pediatr Allergy Immunol. 2003;14(1):60–65. https://doi.org/10.1034/j.1399‐3038.2003.02102.x
- 1864. Penagos M, Compalati E, Tarantini F, Baena‐Cagnani CE, Passalacqua G, Canonica GW. Efficacy of mometasone furoate nasal spray in the treatment of allergic rhinitis. Meta‐analysis of randomized, double‐blind, placebo‐controlled, clinical trials. Allergy. 2008;63(10):1280–1291. https://doi.org/10.1111/j.1398‐9995.2008.01808.x
- 1865. Rodrigo GJ, Neffen H. Efficacy of fluticasone furoate nasal spray vs. placebo for the treatment of ocular and nasal symptoms of allergic rhinitis: a systematic review. Clin Exp Allergy. 2011;41(2):160–170. https://doi.org/10.1111/j.1365‐2222.2010.03654.x
- 1866. Urdaneta E, Tunceli K, Gates D. Effect of mometasone furoate nasal spray on moderate‐to‐severe nasal congestion in seasonal allergic rhinitis: a responder analysis. Allergy Asthma Proc. 2019;40(3):173–179. https://doi.org/10.2500/aap.2019.40.4214
- 1867. Herman H. Once‐daily administration of intranasal corticosteroids for allergic rhinitis: a comparative review of efficacy, safety, patient preference, and cost. Am J Rhinol. 2007;21(1):70–79. https://doi.org/10.2500/ajr.2007.21.2896
- 1868. Rachelefsky G, Farrar JR. A control model to evaluate pharmacotherapy for allergic rhinitis in children. JAMA Pediatr. 2013;167(4):380–386. https://doi.org/10.1001/jamapediatrics.2013.623
- 1869. Craig TJ, Mende C, Hughes K, Kakumanu S, Lehman EB, Chinchilli V. The effect of topical nasal fluticasone on objective sleep testing and the symptoms of rhinitis, sleep, and daytime somnolence in perennial allergic rhinitis. Allergy Asthma Proc. 2003;24(1):53–58.
- 1870. Meltzer EO, Munafo DA, Chung W, Gopalan G, Varghese ST. Intranasal mometasone furoate therapy for allergic rhinitis symptoms and rhinitis‐disturbed sleep. Ann Allergy Asthma Immunol. 2010;105(1):65–74. https://doi.org/10.1016/j.anai.2010.04.020
- 1871. Day JH, Briscoe MP, Rafeiro E, Ellis AK, Pettersson E, Akerlund A. Onset of action of intranasal budesonide (Rhinocort aqua) in seasonal allergic rhinitis studied in a controlled exposure model. J Allergy Clin Immunol. 2000;105(3):489–494. https://doi.org/10.1067/mai.2000.104550
- 1872. Fokkens WJ, Cserhati E, dos Santos JM, et al. Budesonide aqueous nasal spray is an effective treatment in children with perennial allergic rhinitis, with an onset of action within 12 hours. Ann Allergy Asthma Immunol. 2002;89(3):279–284. https://doi.org/10.1016/s1081‐1206(10)61955‐2
- 1873. Kaiser HB, Naclerio RM, Given J, Toler TN, Ellsworth A, Philpot EE. Fluticasone furoate nasal spray: a single treatment option for the symptoms of seasonal allergic rhinitis. J Allergy Clin Immunol. 2007;119(6):1430–1437. https://doi.org/10.1016/j.jaci.2007.02.022
- 1874. Day J, Carrillo T. Comparison of the efficacy of budesonide and fluticasone propionate aqueous nasal spray for once daily treatment of perennial allergic rhinitis. J Allergy Clin Immunol. 1998;102(6 pt 1):902–908. https://doi.org/10.1016/s0091‐6749(98)70326‐4
- 1875. Juniper EF, Guyatt GH, O'Byrne PM, Viveiros M. Aqueous beclomethasone diproprionate nasal spray: regular versus "as required" use in the treatment of seasonal allergic rhinitis. J Allergy Clin Immunol. 1990;86(3 pt 1):380–386. https://doi.org/10.1016/s0091‐6749(05)80101‐0
- 1876. Juniper EF, Guyatt GH, Archer B, Ferrie PJ. Aqueous beclomethasone dipropionate in the treatment of ragweed pollen‐induced rhinitis: further exploration of "as needed" use. J Allergy Clin Immunol. 1993;92(1 pt 1):66–72. https://doi.org/10.1016/0091‐6749(93)90039‐i
- 1877. Jen A, Baroody F, de Tineo M, Haney L, Blair C, Naclerio R. As‐needed use of fluticasone propionate nasal spray reduces symptoms of seasonal allergic rhinitis. J Allergy Clin Immunol. 2000;105(4):732–738. https://doi.org/10.1067/mai.2000.105225
- 1878. Dykewicz MS, Kaiser HB, Nathan RA, et al. Fluticasone propionate aqueous nasal spray improves nasal symptoms of seasonal allergic rhinitis when used as needed (prn). Ann Allergy Asthma Immunol. 2003;91(1):44–48. https://doi.org/10.1016/S1081‐1206(10)62057‐1
- 1879. Thongngarm T, Wongsa C, Phinyo P, Assanasen P, Tantilipikorn P, Sompornrattanaphan M. As‐needed versus regular use of fluticasone furoate nasal spray in patients with moderate to severe, persistent, perennial allergic rhinitis: a randomized controlled trial. J Allergy Clin Immunol Pract. 2021;9(3):1365–1373.e6. https://doi.org/10.1016/j.jaip.2020.09.057
- 1880. DeWester J, Philpot EE, Westlund RE, Cook CK, Rickard KA. The efficacy of intranasal fluticasone propionate in the relief of ocular symptoms associated with seasonal allergic rhinitis. Allergy Asthma Proc. 2003;24(5):331–337.
- 1881. Bielory L, Chun Y, Bielory BP, Canonica GW. Impact of mometasone furoate nasal spray on individual ocular symptoms of allergic rhinitis: a meta‐analysis. Allergy. 2011;66(5):686–693. https://doi.org/10.1111/j.1398‐9995.2010.02543.x
- 1882. Ratner P, Van Bavel J, Mohar D, et al. Efficacy of daily intranasal fluticasone propionate on ocular symptoms associated with seasonal allergic rhinitis. Ann Allergy Asthma Immunol. 2015;114(2):141–147. https://doi.org/10.1016/j.anai.2014.11.012
- 1883. Bielory L, Gross GN, Letierce A, Melas‐Melt L, Lucio L. Ocular symptoms improvement from intranasal triamcinolone compared with placebo and intranasal fluticasone propionate: a meta‐analysis. Ann Allergy Asthma Immunol. 2020;124(6):616–621.e3. https://doi.org/10.1016/j.anai.2020.01.012
- 1884. Baroody FM, Shenaq D, DeTineo M, Wang J, Naclerio RM. Fluticasone furoate nasal spray reduces the nasal‐ocular reflex: a mechanism for the efficacy of topical steroids in controlling allergic eye symptoms. J Allergy Clin Immunol. 2009;123(6):1342–1348. https://doi.org/10.1016/j.jaci.2009.03.015
- 1885. Keith PK, Scadding GK. Are intranasal corticosteroids all equally consistent in managing ocular symptoms of seasonal allergic rhinitis? Curr Med Res Opin. 2009;25(8):2021–2041. https://doi.org/10.1185/03007990903094106
- 1886. Taramarcaz P, Gibson PG. Intranasal corticosteroids for asthma control in people with coexisting asthma and rhinitis. Cochrane Database Syst Rev. 2003;(4):CD003570. https://doi.org/10.1002/14651858.CD003570
- 1887. Lohia S, Schlosser RJ, Soler ZM. Impact of intranasal corticosteroids on asthma outcomes in allergic rhinitis: a meta‐analysis. Allergy. 2013;68(5):569–579. https://doi.org/10.1111/all.12124
- 1888. Yu CL, Huang WT, Wang CM. Treatment of allergic rhinitis reduces acute asthma exacerbation risk among asthmatic children aged 2‐18 years. J Microbiol Immunol Infect. 2019;52(6):991–999. https://doi.org/10.1016/j.jmii.2018.10.003
- 1889. Khattiyawittayakun L, Seresirikachorn K, Chitsuthipakorn W, Kanjanawasee D, Snidvongs K. Effects of double‐dose intranasal corticosteroid for allergic rhinitis: a systematic review and meta‐analysis. Int Forum Allergy Rhinol. 2019;9(1):72–78. https://doi.org/10.1002/alr.22204
- 1890. Weiner JM, Abramson MJ, Puy RM. Intranasal corticosteroids versus oral H1 receptor antagonists in allergic rhinitis: systematic review of randomised controlled trials. BMJ. 1998;317(7173):1624–1629. https://doi.org/10.1136/bmj.317.7173.1624
- 1891. Yanez A, Rodrigo GJ. Intranasal corticosteroids versus topical H1 receptor antagonists for the treatment of allergic rhinitis: a systematic review with meta‐analysis. Ann Allergy Asthma Immunol. 2002;89(5):479–484. https://doi.org/10.1016/S1081‐1206(10)62085‐6
- 1892. Benninger M, Farrar JR, Blaiss M, et al. Evaluating approved medications to treat allergic rhinitis in the United States: an evidence‐based review of efficacy for nasal symptoms by class. Ann Allergy Asthma Immunol. 2010;104(1):13–29. https://doi.org/10.1016/j.anai.2009.11.020
- 1893. Wilson AM, O'Byrne PM, Parameswaran K. Leukotriene receptor antagonists for allergic rhinitis: a systematic review and meta‐analysis. Am J Med. 2004;116(5):338–344. https://doi.org/10.1016/j.amjmed.2003.10.030
- 1894. Bhattachan S, Neupane Y, Pradhan B, Thapa N. Comparison of outcomes between mometasone furoate intranasal spray and oral montelukast in patients with allergic rhinitis. J Nepal Health Res Counc. 2020;18(2):268–270. https://doi.org/10.33314/jnhrc.v18i2.2509
- 1895. Meltzer EO. Formulation considerations of intranasal corticosteroids for the treatment of allergic rhinitis. Ann Allergy Asthma Immunol. 2007;98(1):12–21. https://doi.org/10.1016/S1081‐1206(10)60854‐X
- 1896. May JR, Dolen WK. Evaluation of intranasal corticosteroid sensory attributes and patient preference for fluticasone furoate for the treatment of allergic rhinitis. Clin Ther. 2019;41(8):1589–1596. https://doi.org/10.1016/j.clinthera.2019.05.017
- 1897. van Bavel JH, Ratner PH, Amar NJ, et al. Efficacy and safety of once‐daily treatment with beclomethasone dipropionate nasal aerosol in subjects with seasonal allergic rhinitis. Allergy Asthma Proc. 2012;33(5):386–396. https://doi.org/10.2500/aap.2012.33.3593
- 1898. Meltzer EO, Jacobs RL, LaForce CF, Kelley CL, Dunbar SA, Tantry SK. Safety and efficacy of once‐daily treatment with beclomethasone dipropionate nasal aerosol in subjects with perennial allergic rhinitis. Allergy Asthma Proc. 2012;33(3):249–257. https://doi.org/10.2500/aap.2012.33.3571
- 1899. Ratner PH, Andrews C, Martin B, et al. A study of the efficacy and safety of ciclesonide hydrofluoroalkane nasal aerosol in patients with seasonal allergic rhinitis from mountain cedar pollen. Allergy Asthma Proc. 2012;33(1):27–35. https://doi.org/10.2500/aap.2012.33.3490
- 1900. LaForce C, van Bavel J, Meltzer EO, Wingertzahn MA. Efficacy and safety of ciclesonide hydrofluoroalkane nasal aerosol once daily for the treatment of seasonal allergic rhinitis. Ann Allergy Asthma Immunol. 2009;103(2):166–173. https://doi.org/10.1016/S1081‐1206(10)60171‐8
- 1901. Yang Q, Wang F, Li B, et al. The efficacy and safety of ciclesonide for the treatment of perennial allergic rhinitis: a systematic review and meta‐analysis. Braz J Otorhinolaryngol. 2019;85(3):371–378. https://doi.org/10.1016/j.bjorl.2018.10.008
- 1902. Maspero JF, Rosenblut A, Finn Jr A, Lim J, Wu W, Philpot E. Safety and efficacy of fluticasone furoate in pediatric patients with perennial allergic rhinitis. Otolaryngol Head Neck Surg. 2008;138(1):30–37. https://doi.org/10.1016/j.otohns.2007.10.023
- 1903. Meltzer EO, Tripathy I, Maspero JF, Wu W, Philpot E. Safety and tolerability of fluticasone furoate nasal spray once daily in paediatric patients aged 6‐11 years with allergic rhinitis: subanalysis of three randomized, double‐blind, placebo‐controlled, multicentre studies. Clin Drug Investig. 2009;29(2):79–86. https://doi.org/10.2165/0044011‐200929020‐00002
- 1904. Rosenblut A, Bardin PG, Muller B, et al. Long‐term safety of fluticasone furoate nasal spray in adults and adolescents with perennial allergic rhinitis. Allergy. 2007;62(9):1071–1077. https://doi.org/10.1111/j.1398‐9995.2007.01521.x
- 1905. Ratner PH, Meltzer EO, Teper A. Mometasone furoate nasal spray is safe and effective for 1‐year treatment of children with perennial allergic rhinitis. Int J Pediatr Otorhinolaryngol. 2009;73(5):651–657. https://doi.org/10.1016/j.ijporl.2008.12.025
- 1906. Verkerk MM, Bhatia D, Rimmer J, Earls P, Sacks R, Harvey RJ. Intranasal steroids and the myth of mucosal atrophy: a systematic review of original histological assessments. Am J Rhinol Allergy. 2015;29(1):3–18. https://doi.org/10.2500/ajra.2015.29.4111
- 1907. van As A, Bronsky EA, Dockhorn RJ, et al. Once daily fluticasone propionate is as effective for perennial allergic rhinitis as twice daily beclomethasone diproprionate. J Allergy Clin Immunol. 1993;91(6):1146–1154. https://doi.org/10.1016/0091‐6749(93)90317‐9
- 1908. Brannan MD, Herron JM, Reidenberg P, Affrime MB. Lack of hypothalamic‐pituitary‐adrenal axis suppression with once‐daily or twice‐daily beclomethasone dipropionate aqueous nasal spray administered to patients with allergic rhinitis. Clin Ther. 1995;17(4):637–647. https://doi.org/10.1016/0149‐2918(95)80040‐9
- 1909. Vargas R, Dockhorn RJ, Findlay SR, Korenblat PE, Field EA, Kral KM. Effect of fluticasone propionate aqueous nasal spray versus oral prednisone on the hypothalamic‐pituitary‐adrenal axis. J Allergy Clin Immunol. 1998;102(2):191–197. https://doi.org/10.1016/s0091‐6749(98)70085‐5
- 1910. Howland 3rd WC, Dockhorn R, Gillman S, et al. A comparison of effects of triamcinolone acetonide aqueous nasal spray, oral prednisone, and placebo on adrenocortical function in male patients with allergic rhinitis. J Allergy Clin Immunol. 1996;98(1):32–38. https://doi.org/10.1016/s0091‐6749(96)70223‐3
- 1911. Nayak AS, Ellis MH, Gross GN, et al. The effects of triamcinolone acetonide aqueous nasal spray on adrenocortical function in children with allergic rhinitis. J Allergy Clin Immunol. 1998;101(2 pt 1):157–162. https://doi.org/10.1016/S0091‐6749(98)70379‐3
- 1912. Galant SP, Melamed IR, Nayak AS, et al. Lack of effect of fluticasone propionate aqueous nasal spray on the hypothalamic‐pituitary‐adrenal axis in 2‐ and 3‐year‐old patients. Pediatrics. 2003;112(1 pt 1):96–100. https://doi.org/10.1542/peds.112.1.96
- 1913. Kim K, Weiswasser M, Nave R, et al. Safety of once‐daily ciclesonide nasal spray in cildren 2 to 5 years of age with perennial allergic rhinitis. Ped Asthma Allergy Immunol. 2007;20:229–242.
- 1914. Chervinsky P, Kunjibettu S, Miller DL, et al. Long‐term safety and efficacy of intranasal ciclesonide in adult and adolescent patients with perennial allergic rhinitis. Ann Allergy Asthma Immunol. 2007;99(1):69–76. https://doi.org/10.1016/S1081‐1206(10)60624‐2
- 1915. Patel D, Ratner P, Clements D, Wu W, Faris M, Philpot E. Lack of effect on adult and adolescent hypothalamic‐pituitary‐adrenal axis function with use of fluticasone furoate nasal spray. Ann Allergy Asthma Immunol. 2008;100(5):490–496. https://doi.org/10.1016/S1081‐1206(10)60476‐0
- 1916. Weinstein S, Qaqundah P, Georges G, Nayak A. Efficacy and safety of triamcinolone acetonide aqueous nasal spray in children aged 2 to 5 years with perennial allergic rhinitis: a randomized, double‐blind, placebo‐controlled study with an open‐label extension. Ann Allergy Asthma Immunol. 2009;102(4):339–347. https://doi.org/10.1016/S1081‐1206(10)60340‐7
- 1917. Tripathy I, Levy A, Ratner P, Clements D, Wu W, Philpot E. HPA axis safety of fluticasone furoate nasal spray once daily in children with perennial allergic rhinitis. Pediatr Allergy Immunol. 2009;20(3):287–294. https://doi.org/10.1111/j.1399‐3038.2008.00775.x
- 1918. Hampel Jr FC, Nayak NA, Segall N, Small CJ, Li J, Tantry SK. No hypothalamic‐pituitary‐adrenal function effect with beclomethasone dipropionate nasal aerosol, based on 24‐hour serum cortisol in pediatric allergic rhinitis. Ann Allergy Asthma Immunol. 2015;115(2):137–142. https://doi.org/10.1016/j.anai.2015.05.019
- 1919. Sampieri G, Namavarian A, Lee JJW, Hamour AF, Lee JM. Hypothalamic‐pituitary‐adrenal axis suppression and intranasal corticosteroid use: a systematic review and meta‐analysis. Int Forum Allergy Rhinol. 2022;12(1):11–27. https://doi.org/10.1002/alr.22863
- 1920. Liu A, Manche EE. Bilateral posterior subcapsular cataracts associated with long‐term intranasal steroid use. J Cataract Refract Surg. 2011;37(8):1555–1558. https://doi.org/10.1016/j.jcrs.2011.05.020
- 1921. Ahmadi N, Snidvongs K, Kalish L, et al. Intranasal corticosteroids do not affect intraocular pressure or lens opacity: a systematic review of controlled trials. Rhinology. 2015;53(4):290–302. https://doi.org/10.4193/Rhino15.020
- 1922. Valenzuela CV, Liu JC, Vila PM, Simon L, Doering M, Lieu JEC. Intranasal corticosteroids do not lead to ocular changes: a systematic review and meta‐analysis. Laryngoscope. 2019;129(1):6–12. https://doi.org/10.1002/lary.27209
- 1923. Mener DJ, Shargorodsky J, Varadhan R, Lin SY. Topical intranasal corticosteroids and growth velocity in children: a meta‐analysis. Int Forum Allergy Rhinol. 2015;5(2):95–103. https://doi.org/10.1002/alr.21430
- 1924. Periasamy N, Pujary K, Bhandarkar AM, Bhandarkar ND, Ramaswamy B. Budesonide vs saline nasal irrigation in allergic rhinitis: a randomized placebo‐controlled trial. Otolaryngol Head Neck Surg. 2020;162(6):979–984. https://doi.org/10.1177/0194599820919363
- 1925. Brown K, Lane J, Silva MP, DeTineo M, Naclerio RM, Baroody FM. A pilot study of the effects of intranasal budesonide delivered by NasoNeb(R) on patients with perennial allergic rhinitis. Int Forum Allergy Rhinol. 2014;4(1):43–48. https://doi.org/10.1002/alr.21239
- 1926. Profita M, Riccobono L, Bonanno A, et al. Effect of nebulized beclomethasone on airway inflammation and clinical status of children with allergic asthma and rhinitis: a randomized, double‐blind, placebo‐controlled study. Int Arch Allergy Immunol. 2013;161(1):53–64. https://doi.org/10.1159/000343137
- 1927. Hehar SS, Mason JD, Stephen AB, et al. Twenty‐four hour ambulatory nasal pH monitoring. Clin Otolaryngol Allied Sci. 1999;24(1):24–25. https://doi.org/10.1046/j.1365‐2273.1999.00190.x
- 1928. Camargos P, Ibiapina C, Lasmar L, Cruz AA. Obtaining concomitant control of allergic rhinitis and asthma with a nasally inhaled corticosteroid. Allergy. 2007;62(3):310–316. https://doi.org/10.1111/j.1398‐9995.2007.01241.x
- 1929. Shaikh WA. Exhaling a budesonide inhaler through the nose results in a significant reduction in dose requirement of budesonide nasal spray in patients having asthma with rhinitis. J Investig Allergol Clin Immunol. 1999;9(1):45–49.
- 1930. Daman Willems CE, Dinwiddie R, Grant DB, Rivers RP, Zahir M. Temporary inhibition of growth and adrenal suppression associated with the use of steroid nose drops. Eur J Pediatr. 1994;153(9):632–634. https://doi.org/10.1007/BF02190681
- 1931. Kimmerle R, Rolla AR. Iatrogenic Cushing's syndrome due to dexamethasone nasal drops. Am J Med. 1985;79(4):535–537. https://doi.org/10.1016/0002‐9343(85)90046‐4
- 1932. Daley‐Yates PT, Baker RC. Systemic bioavailability of fluticasone propionate administered as nasal drops and aqueous nasal spray formulations. Br J Clin Pharmacol. 2001;51(1):103–105. https://doi.org/10.1046/j.1365‐2125.2001.01325.x
- 1933. Brown EB, Seidemen T, Siegelaub AB, Popovits C. Depo‐methylprednisolone in the treatment of ragweed hay fever. Ann Allergy. 1960;18:1321–1330.
- 1934. Borum P, Gronborg H, Mygind N. Seasonal allergic rhinitis and depot injection of a corticosteroid. Evaluation of the efficacy of medication early and late in the season based on detailed symptom recording. Allergy. 1987;42(1):26–32. https://doi.org/10.1111/j.1398‐9995.1987.tb02183.x
- 1935. Axelsson A, Lindholm B. The effect of triamcinolone acetonide on allergic and vasomotor rhinitis. Acta Otolaryngol. 1972;73(1):64–67. https://doi.org/10.3109/00016487209138195
- 1936. Laursen LC, Faurschou P, Munch EP. Intramuscular betamethasone dipropionate vs. topical beclomethasone dipropionate and placebo in hay fever. Allergy. 1988;43(6):420–424. https://doi.org/10.1111/j.1398‐9995.1988.tb00912.x
- 1937. Kronholm A. Injectable depot corticosteroid therapy in hay fever. J Int Med Res. 1979;7(4):314–317. https://doi.org/10.1177/030006057900700410
- 1938. Ohlander BO, Hansson RE, Karlsson KE. A comparison of three injectable corticosteroids for the treatment of patients with seasonal hay fever. J Int Med Res. 1980;8(1):63–69. https://doi.org/10.1177/030006058000800111
- 1939. Hermance WE, Gerardi A, Popovits CJ, Brown EB. Dexamethasone acetate suspension in the treatment of allergic rhinitis. Ann Allergy. 1969;27(12):617–621.
- 1940. Chervinsky P. Treatment of seasonal allergic rhinitis with long‐acting steroid injections. A comparison of four preparations. Ann Allergy. 1968;26(4):190–193.
- 1941. Laursen LC, Faurschou P, Pals H, Svendsen UG, Weeke B. Intramuscular betamethasone dipropionate vs. oral prednisolone in hay fever patients. Allergy. 1987;42(3):168–172. https://doi.org/10.1111/j.1398‐9995.1987.tb02194.x
- 1942. Pichler WJ, Klint T, Blaser M, et al. Clinical comparison of systemic methylprednisolone acetate versus topical budesonide in patients with seasonal allergic rhinitis. Allergy. 1988;43(2):87–92. https://doi.org/10.1111/j.1398‐9995.1988.tb00399.x
- 1943. Bayoumy AB, van Schie F, Stegeman I, Blijleven EB, van der Veen EL, de Ru JA. Intramuscular corticosteroid injections in seasonal allergic rhinitis: a systematic review. Laryngoscope Investig Otolaryngol. 2021;6(5):911–923. https://doi.org/10.1002/lio2.645
- 1944. Mygind N, Laursen LC, Dahl M. Systemic corticosteroid treatment for seasonal allergic rhinitis: a common but poorly documented therapy. Allergy. 2000;55(1):11–15. https://doi.org/10.1034/j.1398‐9995.2000.00108.x
- 1945. Aasbjerg K, Torp‐Pedersen C, Vaag A, Backer V. Treating allergic rhinitis with depot‐steroid injections increase risk of osteoporosis and diabetes. Respir Med. 2013;107(12):1852–1858. https://doi.org/10.1016/j.rmed.2013.09.007
- 1946. Wall JW, Shure N. Intranasal cortisone; preliminary study. AMA Arch Otolaryngol. 1952;56(2):172–176.
- 1947. Sidi E, Tardif R. Traitement des rhinites allergiques accompagnées d'eczéma par des injections d'acétate d'hydrocortisone au niveau de la muqueuse nasale [Treatment of allergic rhinitis accompanied by eczema with hydrocortisone acetate injected into nasal mucous membrane]. Sem Hop. 1955;31(33):1922–1923.
- 1948. Simmons MW. Intranasal injection of corticosteroids. Calif Med. 1960;92:155–158.
- 1949. Baker Jr DC, Strauss RB. Intranasal injections of long acting corticosteroids. Ann Otol Rhinol Laryngol. 1962;71:525–531. https://doi.org/10.1177/000348946207100224
- 1950. Mabry RL. Intraturbinal steroid injection: indications, results, and complications. South Med J. 1978;71(7):789–791, 794. https://doi.org/10.1097/00007611‐197807000‐00015
- 1951. Yang TY, Jung YG, Kim YH, Jang TY. A comparison of the effects of botulinum toxin A and steroid injection on nasal allergy. Otolaryngol Head Neck Surg. 2008;139(3):367–371. https://doi.org/10.1016/j.otohns.2008.06.031
- 1952. Rowe RJ, Dusler TW, Kinkella AM. Visual changes and triamcinolone. J Am Med Assoc. 1967;201:333.
- 1953. Byers B. Blindness secondary to steroid injections into the nasal turbinates. Arch Ophthalmol. 1979;97(1):79–80. https://doi.org/10.1001/archopht.1979.01020010019004
- 1954. Martin PA, Church CA, Petti Jr GH, Hedayi R. Visual loss after intraturbinate steroid injection. Otolaryngol Head Neck Surg. 2003;128(2):280–281. https://doi.org/10.1067/mhn.2003.81
- 1955. Nagasato D, Ikeda N, Masuda A, Kashimoto R, Ikeda T. Progression of glaucomatous optic neuropathy associated with chorioretinal microvascular embolism after intranasal injection of a corticosteroid suspension. Indian J Ophthalmol. 2020;68(8):1686–1687. https://doi.org/10.4103/ijo.IJO_2332_19
- 1956. Moss WJ, Kjos KB, Karnezis TT, Lebovits MJ. Intranasal steroid injections and blindness: our personal experience and a review of the past 60 years. Laryngoscope. 2015;125(4):796–800. https://doi.org/10.1002/lary.25000
- 1957. Eccles R. Substitution of phenylephrine for pseudoephedrine as a nasal decongeststant. An illogical way to control methamphetamine abuse. Br J Clin Pharmacol. 2007;63(1):10–14. https://doi.org/10.1111/j.1365‐2125.2006.02833.x
- 1958. Salerno SM, Jackson JL, Berbano EP. Effect of oral pseudoephedrine on blood pressure and heart rate: a meta‐analysis. Arch Intern Med. 2005;165(15):1686–1694. https://doi.org/10.1001/archinte.165.15.1686
- 1959. Bronsky E, Boggs P, Findlay S, et al. Comparative efficacy and safety of a once‐daily loratadine‐pseudoephedrine combination versus its components alone and placebo in the management of seasonal allergic rhinitis. J Allergy Clin Immunol. 1995;96(2):139–147. https://doi.org/10.1016/s0091‐6749(95)70001‐3
- 1960. Grosclaude M, Mees K, Pinelli ME, Lucas M, Van de Venne H. Cetirizine and pseudoephedrine retard, given alone or in combination, in patients with seasonal allergic rhinitis. Rhinology. 1997;35(2):67–73.
- 1961. Dockhorn RJ, Williams BO, Sanders RL. Efficacy of acrivastine with pseudoephedrine in treatment of allergic rhinitis due to ragweed. Ann Allergy Asthma Immunol. 1996;76(2):204–208. https://doi.org/10.1016/S1081‐1206(10)63423‐0
- 1962. Grubbe RE, Lumry WR, Anolik R. Efficacy and safety of desloratadine/pseudoephedrine combination vs its components in seasonal allergic rhinitis. J Investig Allergol Clin Immunol. 2009;19(2):117–124.
- 1963. Bertrand B, Jamart J, Marchal JL, Arendt C. Cetirizine and pseudoephedrine retard alone and in combination in the treatment of perennial allergic rhinitis: a double‐blind multicentre study. Rhinology. 1996;34(2):91–96.
- 1964. Sussman GL, Mason J, Compton D, Stewart J, Ricard N. The efficacy and safety of fexofenadine HCl and pseudoephedrine, alone and in combination, in seasonal allergic rhinitis. J Allergy Clin Immunol. 1999;104(1):100–106. https://doi.org/10.1016/s0091‐6749(99)70120‐x
- 1965. Mucha SM, deTineo M, Naclerio RM, Baroody FM. Comparison of montelukast and pseudoephedrine in the treatment of allergic rhinitis. Arch Otolaryngol Head Neck Surg. 2006;132(2):164–172. https://doi.org/10.1001/archotol.132.2.164
- 1966. Henauer S, Seppey M, Huguenot C, Pecoud A. Effects of terfenadine and pseudoephedrine, alone and in combination in a nasal provocation test and in perennial rhinitis. Eur J Clin Pharmacol. 1991;41(4):321–324. https://doi.org/10.1007/BF00314960
- 1967. Empey DW, Frosolono MF, Hughes DT, Perkins JG. Comparison of pseudoephedrine and triprolidine, alone and in combination in preventing nasal congestion in subjects with allergic rhinitis using nasal histamine challenge. Br J Clin Pharmacol. 1984;18(1):86–89. https://doi.org/10.1111/j.1365‐2125.1984.tb05026.x
- 1968. Howarth PH, Harrison K, Smith S. The influence of terfenadine and pseudo‐ephedrine alone and in combination on allergen‐induced rhinitis. Int Arch Allergy Immunol. 1993;101(3):318–321. https://doi.org/10.1159/000236470
- 1969. Meltzer EO, Ratner PH, McGraw T. Oral phenylephrine HCl for nasal congestion in seasonal allergic rhinitis: a randomized, open‐label, placebo‐controlled study. J Allergy Clin Immunol Pract. 2015;3(5):702–708. https://doi.org/10.1016/j.jaip.2015.05.007
- 1970. Pleskow W, Grubbe R, Weiss S, Lutsky B. Efficacy and safety of an extended‐release formulation of desloratadine and pseudoephedrine vs the individual components in the treatment of seasonal allergic rhinitis. Ann Allergy Asthma Immunol. 2005;94(3):348–354. https://doi.org/10.1016/S1081‐1206(10)60986‐6
- 1971. Svensson C, Pipkorn U, Alkner U, Baumgarten CR, Persson CG. Topical vasoconstrictor (oxymetazoline) does not affect histamine‐induced mucosal exudation of plasma in human nasal airways. Clin Exp Allergy. 1992;22(3):411–416. https://doi.org/10.1111/j.1365‐2222.1992.tb03103.x
- 1972. Bickford L, Shakib S, Taverner D. The nasal airways response in normal subjects to oxymetazoline spray: randomized double‐blind placebo‐controlled trial. Br J Clin Pharmacol. 1999;48(1):53–56. https://doi.org/10.1046/j.1365‐2125.1999.00972.x
- 1973. Gomez‐Hervas J, Garcia‐Valdecasas Bernal J, Fernandez‐Prada M, Palomeque‐Vera JM, Garcia‐Ramos A, Fernandez‐Castanys BF. Effects of oxymetazoline on nasal flow and maximum aerobic exercise performance in patients with inferior turbinate hypertrophy. Laryngoscope. 2015;125(6):1301–1306. https://doi.org/10.1002/lary.25107
- 1974. Taverner D, Bickford L, Shakib S, Tonkin A. Evaluation of the dose‐response relationship for intra‐nasal oxymetazoline hydrochloride in normal adults. Eur J Clin Pharmacol. 1999;55(7):509–513. https://doi.org/10.1007/s002280050665
- 1975. Barnes ML, Biallosterski BT, Gray RD, Fardon TC, Lipworth BJ. Decongestant effects of nasal xylometazoline and mometasone furoate in persistent allergic rhinitis. Rhinology. 2005;43(4):291–295.
- 1976. Pritchard S, Glover M, Guthrie G, et al. Effectiveness of 0.05% oxymetazoline (Vicks Sinex Micromist(R)) nasal spray in the treatment of objective nasal congestion demonstrated to 12 h post‐administration by magnetic resonance imaging. Pulm Pharmacol Ther. 2014;27(1):121–126. https://doi.org/10.1016/j.pupt.2013.08.002
- 1977. Druce HM, Ramsey DL, Karnati S, Carr AN. Topical nasal decongestant oxymetazoline (0.05%) provides relief of nasal symptoms for 12 hours. Rhinology. 2018;56(4):343–350. https://doi.org/10.4193/Rhin17.150
- 1978. Watanabe H, Foo TH, Djazaeri B, Duncombe P, Mackay IS, Durham SR. Oxymetazoline nasal spray three times daily for four weeks in normal subjects is not associated with rebound congestion or tachyphylaxis. Rhinology. 2003;41(3):167–174.
- 1979. Graf P, Hallen H. Effect on the nasal mucosa of long‐term treatment with oxymetazoline, benzalkonium chloride, and placebo nasal sprays. Laryngoscope. 1996;106(5 pt 1):605–609. https://doi.org/10.1097/00005537‐199605000‐00016
- 1980. Petruson B. Treatment with xylometazoline (Otrivin) nosedrops over a six‐week period. Rhinology. 1981;19(3):167–172.
- 1981. Song XH, Zhang L, Han DM, Wang KJ, Wang H, Zhang W. [Effects of oxymetazoline hydrochloride on ex vivo human nasal cilia movement measured with high‐speed digital microscopy]. Zhonghua Er Bi Yan Hou Tou Jing Wai Ke Za Zhi. 2008;43(4):268–271.
- 1982. Meltzer EO, Wallace D, Friedman HS, Navaratnam P, Scott EP, Nolte H. Meta‐analyses of the efficacy of pharmacotherapies and sublingual allergy immunotherapy tablets for allergic rhinitis in adults and children. Rhinology. 2021;59(5):422–432. https://doi.org/10.4193/Rhin21.054
- 1983. Krishnamoorthy M, Mohd Noor N, Mat Lazim N, Abdullah B. Efficacy of montelukast in allergic rhinitis treatment: a systematic review and meta‐analysis. Drugs. 2020;80(17):1831–1851. https://doi.org/10.1007/s40265‐020‐01406‐9
- 1984. Okubo K, Hashiguchi K, Takeda T, et al. A randomized controlled phase II clinical trial comparing ONO‐4053, a novel DP1 antagonist, with a leukotriene receptor antagonist pranlukast in patients with seasonal allergic rhinitis. Allergy. 2017;72(10):1565–1575. https://doi.org/10.1111/all.13174
- 1985. Wei C. The efficacy and safety of H1‐antihistamine versus Montelukast for allergic rhinitis: a systematic review and meta‐analysis. Biomed Pharmacother. 2016;83:989–997. https://doi.org/10.1016/j.biopha.2016.08.003
- 1986. Durham SR, Creticos PS, Nelson HS, et al. Treatment effect of sublingual immunotherapy tablets and pharmacotherapies for seasonal and perennial allergic rhinitis: pooled analyses. J Allergy Clin Immunol. 2016;138(4):1081–1088.e4. https://doi.org/10.1016/j.jaci.2016.04.061
- 1987. Hashiguchi K, Okubo K, Inoue Y, et al. Evaluation of montelukast for the treatment of children with japanese cedar pollinosis using an artificial exposure chamber (OHIO Chamber). Allergy Rhinol (Providence). 2018;9:2152656718783599. https://doi.org/10.1177/2152656718783599
- 1988. Yoshihara S, Kikuchi Y, Saitou M, et al. Efficacy of a leukotriene receptor antagonist for pediatric cedar pollen allergy complicated by asthma. Exp Ther Med. 2017;14(4):3233–3238. https://doi.org/10.3892/etm.2017.4893
- 1989. Chen H, Lou H, Wang Y, Cao F, Zhang L, Wang C. Comparison of the efficacy and mechanisms of intranasal budesonide, montelukast, and their combination in treatment of patients with seasonal allergic rhinitis. Int Forum Allergy Rhinol. 2018;8(11):1242–1252. https://doi.org/10.1002/alr.22197
- 1990. Jindal A, Suriyan S, Sagadevan S, et al. Comparison of oral montelukast and intranasal fluticasone in patients with asthma and allergic rhinitis. J Clin Diagn Res. 2016;10(8):OC06–OC10. https://doi.org/10.7860/JCDR/2016/20741.8268
- 1991. Dalgic A, Dinc ME, Ulusoy S, Dizdar D, Is A, Topak M. Comparison of the effects of nasal steroids and montelukast on olfactory functions in patients with allergic rhinitis. Eur Ann Otorhinolaryngol Head Neck Dis. 2017;134(4):213–216. https://doi.org/10.1016/j.anorl.2016.05.012
- 1992. Feng Y, Meng YP, Dong YY, Qiu CY, Cheng L. Management of allergic rhinitis with leukotriene receptor antagonists versus selective H1‐antihistamines: a meta‐analysis of current evidence. Allergy Asthma Clin Immunol. 2021;17(1):62. https://doi.org/10.1186/s13223‐021‐00564‐z
- 1993. Xiao J, Wu WX, Ye YY, Lin WJ, Wang L. A network meta‐analysis of randomized controlled trials focusing on different allergic rhinitis medications. Am J Ther. 2016;23(6):e1568–e1578. https://doi.org/10.1097/MJT.0000000000000242
- 1994. Xu Y, Zhang J, Wang J. The efficacy and safety of selective H1‐antihistamine versus leukotriene receptor antagonist for seasonal allergic rhinitis: a meta‐analysis. PLoS One. 2014;9(11):e112815. https://doi.org/10.1371/journal.pone.0112815
- 1995. Devillier P, Dreyfus JF, Demoly P, Calderon MA. A meta‐analysis of sublingual allergen immunotherapy and pharmacotherapy in pollen‐induced seasonal allergic rhinoconjunctivitis. BMC Med. 2014;12:71. https://doi.org/10.1186/1741‐7015‐12‐71
- 1996. Li YJ, Zong M, Ding LF, Rui XQ, Ma BY, Qin LP. Efficacy of Chinese medicine acupoint application combined with montelukast on children with perennial allergic rhinitis: a randomized controlled trial. Chin J Integr Med. 2020;26(11):845–852. https://doi.org/10.1007/s11655‐020‐3099‐2
- 1997. U.S. Food and Drug Administration . FDA requires Boxed Warning about serious mental health side effects for asthma and allergy drug montelukast (Singulair); advises restricting use for allergic rhinitis. 2020. Accessed January 15, 2022. https://www.fda.gov/drugs/fda‐requires‐boxed‐warning‐about‐serious‐mental‐health‐side‐effects‐asthma‐and‐allergy‐drug
- 1998. American Academy of Family Physicians . Clinical practice guidelines: allergic rhinitis. Updated July 6, 2020. Accessed November 1, 2021. https://www.aafp.org/family‐physician/patient‐care/clinical‐recommendations/all‐clinical‐recommendations/allergic‐rhinitis.html
- 1999. Goodman MJ, Jhaveri M, Saverno K, Meyer K, Nightengale B. Cost‐effectiveness of second‐generation antihistamines and montelukast in relieving allergic rhinitis nasal symptoms. Am Health Drug Benefits. 2008;1(8):26–34.
- 2000. Grainger J, Drake‐Lee A. Montelukast in allergic rhinitis: a systematic review and meta‐analysis. Clin Otolaryngol. 2006;31(5):360–367. https://doi.org/10.1111/j.1749‐4486.2006.01276.x
- 2001. Rodrigo GJ, Yanez A. The role of antileukotriene therapy in seasonal allergic rhinitis: a systematic review of randomized trials. Ann Allergy Asthma Immunol. 2006;96(6):779–786. https://doi.org/10.1016/S1081‐1206(10)61339‐7
- 2002. Gonyeau MJ, Partisan AM. A clinical review of montelukast in the treatment of seasonal allergic rhinitis. Formulary. 2003;38:368–378.
- 2003. Endo S, Gotoh M, Okubo K, Hashiguchi K, Suzuki H, Masuyama K. Trial of pranlukast inhibitory effect for cedar exposure using an OHIO chamber. J Drug Assess. 2012;1(1):48–54. https://doi.org/10.3109/21556660.2012.703630
- 2004. Wakabayashi K, Hashiguchi K, Kanzaki S, et al. Pranlukast dry syrup inhibits symptoms of Japanese cedar pollinosis in children using OHIO Chamber. Allergy Asthma Proc. 2012;33(1):102–109. https://doi.org/10.2500/aap.2012.33.3517
- 2005. Day JH, Briscoe MP, Ratz JD. Efficacy of levocetirizine compared with montelukast in subjects with ragweed‐induced seasonal allergic rhinitis in the Environmental Exposure Unit. Allergy Asthma Proc. 2008;29(3):304–312. https://doi.org/10.2500/aap.2008.29.3109
- 2006. Jiang RS. Efficacy of a leukotriene receptor antagonist in the treatment of perennial allergic rhinitis. J Otolaryngol. 2006;35(2):117–121. https://doi.org/10.2310/7070.2005.5007
- 2007. Patel P, Philip G, Yang W, et al. Randomized, double‐blind, placebo‐controlled study of montelukast for treating perennial allergic rhinitis. Ann Allergy Asthma Immunol. 2005;95(6):551–557. https://doi.org/10.1016/S1081‐1206(10)61018‐6
- 2008. Chervinsky P, Philip G, Malice MP, et al. Montelukast for treating fall allergic rhinitis: effect of pollen exposure in 3 studies. Ann Allergy Asthma Immunol. 2004;92(3):367–373. https://doi.org/10.1016/S1081‐1206(10)61576‐1
- 2009. Philip G, Nayak AS, Berger WE, et al. The effect of montelukast on rhinitis symptoms in patients with asthma and seasonal allergic rhinitis. Curr Med Res Opin. 2004;20(10):1549–1558. https://doi.org/10.1185/030079904x3348
- 2010. Ratner PH, Howland 3rd WC, Arastu R, et al. Fluticasone propionate aqueous nasal spray provided significantly greater improvement in daytime and nighttime nasal symptoms of seasonal allergic rhinitis compared with montelukast. Ann Allergy Asthma Immunol. 2003;90(5):536–542. https://doi.org/10.1016/S1081‐1206(10)61847‐9
- 2011. van Adelsberg J, Philip G, Pedinoff AJ, et al. Montelukast improves symptoms of seasonal allergic rhinitis over a 4‐week treatment period. Allergy. 2003;58(12):1268–1276. https://doi.org/10.1046/j.1398‐9995.2003.00261.x
- 2012. van Adelsberg J, Philip G, LaForce CF, et al. Randomized controlled trial evaluating the clinical benefit of montelukast for treating spring seasonal allergic rhinitis. Ann Allergy Asthma Immunol. 2003;90(2):214–222. https://doi.org/10.1016/S1081‐1206(10)62144‐8
- 2013. Philip G, Malmstrom K, Hampel FC, et al. Montelukast for treating seasonal allergic rhinitis: a randomized, double‐blind, placebo‐controlled trial performed in the spring. Clin Exp Allergy. 2002;32(7):1020–1028. https://doi.org/10.1046/j.1365‐2222.2002.01422.x
- 2014. Pullerits T, Praks L, Skoogh BE, Ani R, Lotvall J. Randomized placebo‐controlled study comparing a leukotriene receptor antagonist and a nasal glucocorticoid in seasonal allergic rhinitis. Am J Respir Crit Care Med. 1999;159(6):1814–1818. https://doi.org/10.1164/ajrccm.159.6.9810016
- 2015. Altounyan RE. Review of clinical activity and mode of action of sodium cromoglycate. Clin Allergy. 1980;(10 suppl):481–489. https://doi.org/10.1111/j.1365‐2222.1980.tb02162.x
- 2016. Kay AB, Walsh GM, Moqbel R, et al. Disodium cromoglycate inhibits activation of human inflammatory cells in vitro. J Allergy Clin Immunol. 1987;80(1):1–8. https://doi.org/10.1016/s0091‐6749(87)80183‐5
- 2017. Taylor G, Shivalkar PR. Disodium cromoglycate: laboratory studies and clinical trial in allergic rhinitis. Clin Allergy. 1971;1(2):189–198. https://doi.org/10.1111/j.1365‐2222.1971.tb03018.x
- 2018. Pelikan Z. The diagnostic approach to immediate hypersensitivity in patients with allergic rhinitis; a comparison of nasal challenges and serum rast. Ann Allergy. 1983;51(3):395–400.
- 2019. Kolly M, Pecoud A. Comparison of levocabastine, a new selective H1‐receptor antagonist, and disodium cromoglycate, in a nasal provocation test with allergen. Br J Clin Pharmacol. 1986;22(4):389–394. https://doi.org/10.1111/j.1365‐2125.1986.tb02907.x
- 2020. Davies HJ. Exposure of hay fever subjects to an indoor environmental grass pollen challenge system. Clin Allergy. 1985;15(5):419–427. https://doi.org/10.1111/j.1365‐2222.1985.tb02291.x
- 2021. Ibanez MD, Laso MT, Martinez‐San Irineo M, Alonso E. Anaphylaxis to disodium cromoglycate. Ann Allergy Asthma Immunol. 1996;77(3):185–186. https://doi.org/10.1016/s1081‐1206(10)63252‐8
- 2022. Wass U, Plaschke P, Bjorkander J, Belin L. Assay of specific IgE antibodies to disodium cromoglycate in serum from a patient with an immediate hypersensitivity reaction. J Allergy Clin Immunol. 1988;81(4):750–757. https://doi.org/10.1016/0091‐6749(88)91049‐4
- 2023. Schatz M, Zeiger RS, Harden K, Hoffman CC, Chilingar L, Petitti D. The safety of asthma and allergy medications during pregnancy. J Allergy Clin Immunol. 1997;100(3):301–306. https://doi.org/10.1016/s0091‐6749(97)70241‐0
- 2024. Mazzotta P, Loebstein R, Koren G. Treating allergic rhinitis in pregnancy. Safety considerations. Drug Saf. 1999;20(4):361–375. https://doi.org/10.2165/00002018‐199920040‐00005
- 2025. Meltzer EO, NasalCrom Study Group . Efficacy and patient satisfaction with cromolyn sodium nasal solution in the treatment of seasonal allergic rhinitis: a placebo‐controlled study. Clin Ther. 2002;24(6):942–952. https://doi.org/10.1016/s0149‐2918(02)80009‐1
- 2026. Chandra RK, Heresi G, Woodford G. Double‐blind controlled crossover trial of 4% intranasal sodium cromoglycate solution in patients with seasonal allergic rhinitis. Ann Allergy. 1982;49(3):131–134.
- 2027. Handelman NI, Friday GA, Schwartz HJ, et al. Cromolyn sodium nasal solution in the prophylactic treatment of pollen‐induced seasonal allergic rhinitis. J Allergy Clin Immunol. 1977;59(3):237–242. https://doi.org/10.1016/0091‐6749(77)90156‐7
- 2028. Nizami RM, Baboo MT. Efficacy double‐blind, crossover study of sodium cromoglycate in patients with seasonal allergic rhinitis. Ann Allergy. 1977;38(1):42–45.
- 2029. Knight A, Underdown BJ, Demanuele F, Hargreave FE. Disodium cromoglycate in ragweed‐allergic rhinitis. J Allergy Clin Immunol. 1976;58(2):278–283. https://doi.org/10.1016/0091‐6749(76)90132‐9
- 2030. Lejeune M, Lefebvre PP, Delvenne P, El‐Shazly AE. Nasal sodium cromoglycate (Lomusol) modulates the early phase reaction of mild to moderate persistent allergic rhinitis in patients mono‐sensitized to house dust mite: a preliminary study. Int Immunopharmacol. 2015;26(1):272–276. https://doi.org/10.1016/j.intimp.2015.02.004
- 2031. Tandon MK, Strahan EG. Double‐blind crossover trial comparing beclomethasone dipropionate and sodium cromoglycate in perennial allergic rhinitis. Clin Allergy. 1980;10(4):459–462. https://doi.org/10.1111/j.1365‐2222.1980.tb02129.x
- 2032. McDowell MK, Spitz E. Treatment of chronic perennial allergic rhinitis: a double‐blind trial of cromolyn sodium. Ann Allergy. 1977;39(3):169–174.
- 2033. Warland A, Kapstad B. The effect of disodium cromoglycate in perennial allergic rhinitis. A controlled clinical study. Acta Allergol. 1977;32(3):195–199. https://doi.org/10.1111/j.1398‐9995.1977.tb01350.x
- 2034. Cohan RH, Bloom FL, Rhoades RB, Wittig HJ, Haugh LD. Treatment of perennial allergic rhinitis with cromolyn sodium. Double‐blind study on 34 adult patients. J Allergy Clin Immunol. 1976;58(1 pt. 2):121–128. https://doi.org/10.1016/0091‐6749(76)90147‐0
- 2035. Orgel HA, Meltzer EO, Kemp JP, Ostrom NK, Welch MJ. Comparison of intranasal cromolyn sodium, 4%, and oral terfenadine for allergic rhinitis: symptoms, nasal cytology, nasal ciliary clearance, and rhinomanometry. Ann Allergy. 1991;66(3):237–244.
- 2036. Schata M, Jorde W, Richarz‐Barthauer U. Levocabastine nasal spray better than sodium cromoglycate and placebo in the topical treatment of seasonal allergic rhinitis. J Allergy Clin Immunol. 1991;87(4):873–878. https://doi.org/10.1016/0091‐6749(91)90136‐c
- 2037. Lange B, Lukat KF, Rettig K, Holtappels G, Bachert C. Efficacy, cost‐effectiveness, and tolerability of mometasone furoate, levocabastine, and disodium cromoglycate nasal sprays in the treatment of seasonal allergic rhinitis. Ann Allergy Asthma Immunol. 2005;95(3):272–282. https://doi.org/10.1016/S1081‐1206(10)61225‐2
- 2038. Fisher WG. Comparison of budesonide and disodium cromoglycate for the treatment of seasonal allergic rhinitis in children. Ann Allergy. 1994;73(6):515–520.
- 2039. Bousquet J, Chanal I, Alquie MC, et al. Prevention of pollen rhinitis symptoms: comparison of fluticasone propionate aqueous nasal spray and disodium cromoglycate aqueous nasal spray. A multicenter, double‐blind, double‐dummy, parallel‐group study. Allergy. 1993;48(5):327–333. https://doi.org/10.1111/j.1398‐9995.1993.tb02401.x
- 2040. Welsh PW, Stricker WE, Chu CP, et al. Efficacy of beclomethasone nasal solution, flunisolide, and cromolyn in relieving symptoms of ragweed allergy. Mayo Clin Proc. 1987;62(2):125–134. https://doi.org/10.1016/s0025‐6196(12)61882‐5
- 2041. Bjerrum P, Illum P. Treatment of seasonal allergic rhinitis with budesonide and disodium cromoglycate. A double‐blind clinical comparison between budesonide and disodium cromoglycate. Allergy. 1985;40(1):65–69. https://doi.org/10.1111/j.1398‐9995.1985.tb04156.x
- 2042. Morrow‐Brown H, Jackson FA, Pover GM. A comparison of beclomethasone dipropionate aqueous nasal spray and sodium cromoglycate nasal spray in the management of seasonal allergic rhinitis. Allergol Immunopathol (Madr). 1984;12(5):355–361.
- 2043. Brown HM, Engler C, English JR. A comparative trial of flunisolide and sodium cromoglycate nasal sprays in the treatment of seasonal allergic rhinitis. Clin Allergy. 1981;11(2):169–173. https://doi.org/10.1111/j.1365‐2222.1981.tb01581.x
- 2044. Wilson JA, Walker SR. A clinical study of the prophylactic use of betamethasone valerate and sodium cromoglycate in the treatment of seasonal allergic rhinitis. J Laryngol Otol. 1976;90(2):201–206. https://doi.org/10.1017/s0022215100081962
- 2045. Frankland AW, Walker SR. A comparison of intranasal betamethasone valerate and sodium cromoglycate in seasonal allergic rhinitis. Clin Allergy. 1975;5(3):295–300. https://doi.org/10.1111/j.1365‐2222.1975.tb01866.x
- 2046. Pitsios C, Papadopoulos D, Kompoti E, et al. Efficacy and safety of mometasone furoate vs nedocromil sodium as prophylactic treatment for moderate/severe seasonal allergic rhinitis. Ann Allergy Asthma Immunol. 2006;96(5):673–678. https://doi.org/10.1016/S1081‐1206(10)61064‐2
- 2047. Schuller DE, Selcow JE, Joos TH, et al. A multicenter trial of nedocromil sodium, 1% nasal solution, compared with cromolyn sodium and placebo in ragweed seasonal allergic rhinitis. J Allergy Clin Immunol. 1990;86(4 pt 1):554–561. https://doi.org/10.1016/s0091‐6749(05)80212‐x
- 2048. Craig S, Rubinstein E, Reisman RE, Arbesman CE. Treatment of ragweed hay fever with intranasally administered disodium cromoglycate. Clin Allergy. 1977;7(6):569–576. https://doi.org/10.1111/j.1365‐2222.1977.tb01487.x
- 2049. Posey WC, Nelson HS. Controlled trials with four per cent cromolyn spray in seasonal allergic rhinitis. Clin Allergy. 1977;7(5):485–496. https://doi.org/10.1111/j.1365‐2222.1977.tb01479.x
- 2050. Becker B, Borum S, Nielsen K, Mygind N, Borum P. A time‐dose study of the effect of topical ipratropium bromide on methacholine‐induced rhinorrhoea in patients with perennial non‐allergic rhinitis. Clin Otolaryngol Allied Sci. 1997;22(2):132–134. https://doi.org/10.1046/j.1365‐2273.1997.00875.x
- 2051. Sanwikarja S, Schmitz PI, Dieges PH. The effect of locally applied ipratropium aerosol on the nasal methacholine challenge in patients with allergic and non‐allergic rhinitis. Ann Allergy. 1986;56(2):162–166.
- 2052. Ostberg B, Winther B, Mygind N. Cold air‐induced rhinorrhea and high‐dose ipratropium. Arch Otolaryngol Head Neck Surg. 1987;113(2):160–162. https://doi.org/10.1001/archotol.1987.01860020052011
- 2053. Kirkegaard J, Mygind N, Molgaard F, et al. Ordinary and high‐dose ipratropium in perennial nonallergic rhinitis. J Allergy Clin Immunol. 1987;79(4):585–590. https://doi.org/10.1016/s0091‐6749(87)80153‐7
- 2054. Kirkegaard J, Mygind N, Molgaard F, et al. Ipratropium treatment of rhinorrhea in perennial nonallergic rhinitis. A Nordic multicenter study. Acta Otolaryngol Suppl. 1988;449:93–95. https://doi.org/10.3109/00016488809106386
- 2055. Bonadonna P, Senna G, Zanon P, et al. Cold‐induced rhinitis in skiers–clinical aspects and treatment with ipratropium bromide nasal spray: a randomized controlled trial. Am J Rhinol. 2001;15(5):297–301.
- 2056. Kaiser HB, Findlay SR, Georgitis JW, et al. The anticholinergic agent, ipratropium bromide, is useful in the treatment of rhinorrhea associated with perennial allergic rhinitis. Allergy Asthma Proc. 1998;19(1):23–29. https://doi.org/10.2500/108854198778557962
- 2057. Kaiser HB, Findlay SR, Georgitis JW, et al. Long‐term treatment of perennial allergic rhinitis with ipratropium bromide nasal spray 0.06%. J Allergy Clin Immunol. 1995;95(5 pt 2):1128–1132. https://doi.org/10.1016/s0091‐6749(95)70217‐2
- 2058. Bronsky EA, Druce H, Findlay SR, et al. A clinical trial of ipratropium bromide nasal spray in patients with perennial nonallergic rhinitis. J Allergy Clin Immunol. 1995;95(5 pt 2):1117–1122. https://doi.org/10.1016/s0091‐6749(95)70215‐6
- 2059. Kim KT, Kerwin E, Landwehr L, et al. Use of 0.06% ipratropium bromide nasal spray in children aged 2 to 5 years with rhinorrhea due to a common cold or allergies. Ann Allergy Asthma Immunol. 2005;94(1):73–79. https://doi.org/10.1016/s1081‐1206(10)61289‐6
- 2060. Ensing K, de Zeeuw RA, Nossent GD, Koeter GH, Cornelissen PJ. Pharmacokinetics of ipratropium bromide after single dose inhalation and oral and intravenous administration. Eur J Clin Pharmacol. 1989;36(2):189–194. https://doi.org/10.1007/BF00609193
- 2061. Meltzer EO, Orgel HA, Biondi R, et al. Ipratropium nasal spray in children with perennial rhinitis. Ann Allergy Asthma Immunol. 1997;78(5):485–491. https://doi.org/10.1016/S1081‐1206(10)63236‐X
- 2062. Milgrom H, Biondi R, Georgitis JW, et al. Comparison of ipratropium bromide 0.03% with beclomethasone dipropionate in the treatment of perennial rhinitis in children. Ann Allergy Asthma Immunol. 1999;83(2):105–111. https://doi.org/10.1016/S1081‐1206(10)62620‐8
- 2063. Dockhorn R, Aaronson D, Bronsky E, et al. Ipratropium bromide nasal spray 0.03% and beclomethasone nasal spray alone and in combination for the treatment of rhinorrhea in perennial rhinitis. Ann Allergy Asthma Immunol. 1999;82(4):349–359. https://doi.org/10.1016/S1081‐1206(10)63284‐X
- 2064. Finn Jr AF, Aaronson D, Korenblat P, et al. Ipratropium bromide nasal spray 0.03% provides additional relief from rhinorrhea when combined with terfenadine in perennial rhinitis patients; a randomized, double‐blind, active‐controlled trial. Am J Rhinol. 1998;12(6):441–449. https://doi.org/10.2500/105065898780707919
- 2065. Gorski P, Pazdrak K, Ruta U. Effect of ipratropium on nasal reactivity to histamine and eosinophil influx in perennial allergic rhinitis. Eur J Clin Pharmacol. 1993;44(6):545–547. https://doi.org/10.1007/BF02440856
- 2066. Meltzer EO, Orgel HA, Bronsky EA, et al. Ipratropium bromide aqueous nasal spray for patients with perennial allergic rhinitis: a study of its effect on their symptoms, quality of life, and nasal cytology. J Allergy Clin Immunol. 1992;90(2):242–249. https://doi.org/10.1016/0091‐6749(92)90078‐g
- 2067. Schultz Larsen F, Mygind N, Larsen FS. Ipratropium treatment for rhinorrhoea in patients with perennial rhinitis. An open follow‐up study of efficacy and safety. Clin Otolaryngol Allied Sci. 1983;8(4):267–272. https://doi.org/10.1111/j.1365‐2273.1983.tb01440.x
- 2068. Borum P, Mygind N, Schultz Larsen F. Intranasal ipratropium: a new treatment for perennial rhinitis. Clin Otolaryngol Allied Sci. 1979;4(6):407–411. https://doi.org/10.1111/j.1365‐2273.1979.tb01773.x
- 2069. Cox L. Biologics and allergy immunotherapy in the treatment of allergic diseases. Immunol Allergy Clin North Am. 2020;40(4):687–700. https://doi.org/10.1016/j.iac.2020.06.008
- 2070. Eschenbacher W, Straesser M, Knoeddler A, Li RC, Borish L. Biologics for the treatment of allergic rhinitis, chronic rhinosinusitis, and nasal polyposis. Immunol Allergy Clin North Am. 2020;40(4):539–547. https://doi.org/10.1016/j.iac.2020.06.001
- 2071. Licari A, Marseglia G, Castagnoli R, Marseglia A, Ciprandi G. The discovery and development of omalizumab for the treatment of asthma. Expert Opin Drug Discov. 2015;10(9):1033–1042. https://doi.org/10.1517/17460441.2015.1048220
- 2072. Tsabouri S, Tseretopoulou X, Priftis K, Ntzani EE. Omalizumab for the treatment of inadequately controlled allergic rhinitis: a systematic review and meta‐analysis of randomized clinical trials. J Allergy Clin Immunol Pract. 2014;2(3):332–340.e1. https://doi.org/10.1016/j.jaip.2014.02.001
- 2073. Yu C, Wang K, Cui X, et al. Clinical efficacy and safety of omalizumab in the treatment of allergic rhinitis: a systematic review and meta‐analysis of randomized clinical trials. Am J Rhinol Allergy. 2020;34(2):196–208. https://doi.org/10.1177/1945892419884774
- 2074. Casale TB, Bernstein IL, Busse WW, et al. Use of an anti‐IgE humanized monoclonal antibody in ragweed‐induced allergic rhinitis. J Allergy Clin Immunol. 1997;100(1):110–121. https://doi.org/10.1016/s0091‐6749(97)70202‐1
- 2075. Adelroth E, Rak S, Haahtela T, et al. Recombinant humanized mAb‐E25, an anti‐IgE mAb, in birch pollen‐induced seasonal allergic rhinitis. J Allergy Clin Immunol. 2000;106(2):253–259. https://doi.org/10.1067/mai.2000.108310
- 2076. Casale TB, Condemi J, LaForce C, et al. Effect of omalizumab on symptoms of seasonal allergic rhinitis: a randomized controlled trial. JAMA. 2001;286(23):2956–2967. https://doi.org/10.1001/jama.286.23.2956
- 2077. Chervinsky P, Casale T, Townley R, et al. Omalizumab, an anti‐IgE antibody, in the treatment of adults and adolescents with perennial allergic rhinitis. Ann Allergy Asthma Immunol. 2003;91(2):160–167. https://doi.org/10.1016/S1081‐1206(10)62171‐0
- 2078. Okubo K, Ogino S, Nagakura T, Ishikawa T. Omalizumab is effective and safe in the treatment of Japanese cedar pollen‐induced seasonal allergic rhinitis. Allergol Int. 2006;55(4):379–386. https://doi.org/10.2332/allergolint.55.379
- 2079. Corren J, Diaz‐Sanchez D, Saxon A, et al. Effects of omalizumab, a humanized monoclonal anti‐IgE antibody, on nasal reactivity to allergen and local IgE synthesis. Ann Allergy Asthma Immunol. 2004;93(3):243–248. https://doi.org/10.1016/S1081‐1206(10)61495‐0
- 2080. Bez C, Schubert R, Kopp M, et al. Effect of anti‐immunoglobulin E on nasal inflammation in patients with seasonal allergic rhinoconjunctivitis. Clin Exp Allergy. 2004;34(7):1079–1085. https://doi.org/10.1111/j.1365‐2222.2004.01998.x
- 2081. Nagakura T, Ogino S, Okubo K, Sato N, Takahashi M, Ishikawa T. Omalizumab is more effective than suplatast tosilate in the treatment of Japanese cedar pollen‐induced seasonal allergic rhinitis. Clin Exp Allergy. 2008;38(2):329–337. https://doi.org/10.1111/j.1365‐2222.2007.02894.x
- 2082. Davydov L. Omalizumab (Xolair) for treatment of asthma. Am Fam Physician. 2005;71(2):341–342.
- 2083. Bachert C, Hellings PW, Mullol J, et al. Dupilumab improves health‐related quality of life in patients with chronic rhinosinusitis with nasal polyposis. Allergy. 2020;75(1):148–157. https://doi.org/10.1111/all.13984
- 2084. Weinstein SF, Katial R, Jayawardena S, et al. Efficacy and safety of dupilumab in perennial allergic rhinitis and comorbid asthma. J Allergy Clin Immunol. 2018;142(1):171–177.e1. https://doi.org/10.1016/j.jaci.2017.11.051
- 2085. Busse WW, Maspero JF, Lu Y, et al. Efficacy of dupilumab on clinical outcomes in patients with asthma and perennial allergic rhinitis. Ann Allergy Asthma Immunol. 2020;125(5):565–576.e1. https://doi.org/10.1016/j.anai.2020.05.026
- 2086. Corren J, Saini SS, Gagnon R, et al. Short‐term subcutaneous allergy immunotherapy and dupilumab are well tolerated in allergic rhinitis: a randomized trial. J Asthma Allergy. 2021;14:1045–1063. https://doi.org/10.2147/JAA.S318892
- 2087. Casale TB, Busse WW, Kline JN, et al. Omalizumab pretreatment decreases acute reactions after rush immunotherapy for ragweed‐induced seasonal allergic rhinitis. J Allergy Clin Immunol. 2006;117(1):134–140. https://doi.org/10.1016/j.jaci.2005.09.036
- 2088. Kuehr J, Brauburger J, Zielen S, et al. Efficacy of combination treatment with anti‐IgE plus specific immunotherapy in polysensitized children and adolescents with seasonal allergic rhinitis. J Allergy Clin Immunol. 2002;109(2):274–280. https://doi.org/10.1067/mai.2002.121949
- 2089. Cordray S, Harjo JB, Miner L. Comparison of intranasal hypertonic dead sea saline spray and intranasal aqueous triamcinolone spray in seasonal allergic rhinitis. Ear Nose Throat J. 2005;84(7):426–430.
- 2090. Rogkakou A, Guerra L, Massacane P, et al. Effects on symptoms and quality of life of hypertonic saline nasal spray added to antihistamine in persistent allergic rhinitis – a randomized controlled study. Eur Ann Allergy Clin Immunol. 2005;37(9):353–36.
- 2091. Ural A, Oktemer TK, Kizil Y, Ileri F, Uslu S. Impact of isotonic and hypertonic saline solutions on mucociliary activity in various nasal pathologies: clinical study. J Laryngol Otol. 2009;123(5):517–521. https://doi.org/10.1017/S0022215108003964
- 2092. Garavello W, Somigliana E, Acaia B, Gaini L, Pignataro L, Gaini RM. Nasal lavage in pregnant women with seasonal allergic rhinitis: a randomized study. Int Arch Allergy Immunol. 2010;151(2):137–141. https://doi.org/10.1159/000236003
- 2093. Chusakul S, Warathanasin S, Suksangpanya N, et al. Comparison of buffered and nonbuffered nasal saline irrigations in treating allergic rhinitis. Laryngoscope. 2013;123(1):53–56. https://doi.org/10.1002/lary.23617
- 2094. Di Berardino F, Zanetti D, D'Amato G. Nasal rinsing with an atomized spray improves mucociliary clearance and clinical symptoms during peak grass pollen season. Am J Rhinol Allergy. 2017;31(1):40–43. https://doi.org/10.2500/ajra.2016.30.4383
- 2095. Sansila K, Eiamprapai P, Sawangjit R. Effects of self‐prepared hypertonic nasal saline irrigation in allergic rhinitis: a randomized controlled trial. Asian Pac J Allergy Immunol. 2020;38(3):200–207. https://doi.org/10.12932/AP‐090618‐0331
- 2096. Lin L, Chen Z, Cao Y, Sun G. Normal saline solution nasal‐pharyngeal irrigation improves chronic cough associated with allergic rhinitis. Am J Rhinol Allergy. 2017;31(2):96–104. https://doi.org/10.2500/ajra.2017.31.4418
- 2097. Yata K, Srivanitchapoom C. The comparison of nasal irrigation outcome between 3% NaCl and 0.9% NaCl in adults majority with intermittent allergic rhinitis: a randomized double‐blind study. Asian Pac J Allergy Immunol. 2021;39(1):9–14. https://doi.org/10.12932/AP‐140520‐0844
- 2098. Li CL, Lin HC, Lin CY, Hsu TF. Effectiveness of hypertonic saline nasal irrigation for alleviating allergic rhinitis in children: a systematic review and meta‐analysis. J Clin Med. 2019;8(1):64. https://doi.org/10.3390/jcm8010064
- 2099. Li H, Sha Q, Zuo K, et al. Nasal saline irrigation facilitates control of allergic rhinitis by topical steroid in children. ORL J Otorhinolaryngol Relat Spec. 2009;71(1):50–55. https://doi.org/10.1159/000178165
- 2100. Garavello W, Romagnoli M, Sordo L, Gaini RM, Di Berardino C, Angrisano A. Hypersaline nasal irrigation in children with symptomatic seasonal allergic rhinitis: a randomized study. Pediatr Allergy Immunol. 2003;14(2):140–143. https://doi.org/10.1034/j.1399‐3038.2003.00021.x
- 2101. Garavello W, Di Berardino F, Romagnoli M, Sambataro G, Gaini RM. Nasal rinsing with hypertonic solution: an adjunctive treatment for pediatric seasonal allergic rhinoconjunctivitis. Int Arch Allergy Immunol. 2005;137(4):310–314. https://doi.org/10.1159/000086462
- 2102. Marchisio P, Varricchio A, Baggi E, et al. Hypertonic saline is more effective than normal saline in seasonal allergic rhinitis in children. Int J Immunopathol Pharmacol. 2012;25(3):721–730. https://doi.org/10.1177/039463201202500318
- 2103. Satdhabudha A, Poachanukoon O. Efficacy of buffered hypertonic saline nasal irrigation in children with symptomatic allergic rhinitis: a randomized double‐blind study. Int J Pediatr Otorhinolaryngol. 2012;76(4):583–588. https://doi.org/10.1016/j.ijporl.2012.01.022
- 2104. Chen JR, Jin L, Li XY. The effectiveness of nasal saline irrigation (seawater) in treatment of allergic rhinitis in children. Int J Pediatr Otorhinolaryngol. 2014;78(7):1115–1118. https://doi.org/10.1016/j.ijporl.2014.04.026
- 2105. Malizia V, Fasola S, Ferrante G, et al. Efficacy of buffered hypertonic saline nasal irrigation for nasal symptoms in children with seasonal allergic rhinitis: a randomized controlled trial. Int Arch Allergy Immunol. 2017;174(2):97–103. https://doi.org/10.1159/000481093
- 2106. Jung M, Lee JY, Ryu G, et al. Beneficial effect of nasal saline irrigation in children with allergic rhinitis and asthma: a randomized clinical trial. Asian Pac J Allergy Immunol. 2020;38(4):251–257. https://doi.org/10.12932/AP‐070918‐0403
- 2107. Hermelingmeier KE, Weber RK, Hellmich M, Heubach CP, Mosges R. Nasal irrigation as an adjunctive treatment in allergic rhinitis: a systematic review and meta‐analysis. Am J Rhinol Allergy. 2012;26(5):e119–e125. https://doi.org/10.2500/ajra.2012.26.3787
- 2108. Head K, Snidvongs K, Glew S, et al. Saline irrigation for allergic rhinitis. Cochrane Database Syst Rev. 2018;6:CD012597. https://doi.org/10.1002/14651858.CD012597.pub2
- 2109. Wang Y, Jin L, Liu SX, Fan K, Qin ML, Yu SQ. Role of nasal saline irrigation in the treatment of allergic rhinitis in children and adults: a systematic analysis. Allergol Immunopathol (Madr). 2020;48(4):360–367. https://doi.org/10.1016/j.aller.2020.01.002
- 2110. Ozdemir O. Various effects of different probiotic strains in allergic disorders: an update from laboratory and clinical data. Clin Exp Immunol. 2010;160(3):295–304. https://doi.org/10.1111/j.1365‐2249.2010.04109.x
- 2111. Guvenc IA, Muluk NB, Mutlu FS, et al. Do probiotics have a role in the treatment of allergic rhinitis? A comprehensive systematic review and meta‐analysis. Am J Rhinol Allergy. 2016;30(5):157–175. https://doi.org/10.2500/ajra.2016.30.4354
- 2112. Zajac AE, Adams AS, Turner JH. A systematic review and meta‐analysis of probiotics for the treatment of allergic rhinitis. Int Forum Allergy Rhinol. 2015;5(6):524–532. https://doi.org/10.1002/alr.21492
- 2113. Du X, Wang L, Wu S, et al. Efficacy of probiotic supplementary therapy for asthma, allergic rhinitis, and wheeze: a meta‐analysis of randomized controlled trials. Allergy Asthma Proc. 2019;40(4):250–260. https://doi.org/10.2500/aap.2019.40.4227
- 2114. Zuccotti G, Meneghin F, Aceti A, et al. Probiotics for prevention of atopic diseases in infants: systematic review and meta‐analysis. Allergy. 2015;70(11):1356–1371. https://doi.org/10.1111/all.12700
- 2115. Anania C, Di Marino VP, Olivero F, et al. Treatment with a probiotic mixture containing Bifidobacterium animalis Subsp. Lactis BB12 and Enterococcus faecium L3 for the prevention of allergic rhinitis symptoms in children: a randomized controlled trial. Nutrients. 2021;13(4):1315. https://doi.org/10.3390/nu13041315
- 2116. Jalali MM, Soleimani R, Alavi Foumani A, Ganjeh Khosravi H. Add‐on probiotics in patients with persistent allergic rhinitis: a randomized crossover clinical trial. Laryngoscope. 2019;129(8):1744–1750. https://doi.org/10.1002/lary.27858
- 2117. Sumadiono S, Satria CD, Mardhiah N, Susanti GI. Immunotherapy and probiotic treatment for allergic rhinitis in children. Paediatr Indones. 2018;58(6):280–285.
- 2118. Dennis‐Wall JC, Culpepper T, Nieves Jr C, et al. Probiotics (Lactobacillus gasseri KS‐13, Bifidobacterium bifidum G9‐1, and Bifidobacterium longum MM‐2) improve rhinoconjunctivitis‐specific quality of life in individuals with seasonal allergies: a double‐blind, placebo‐controlled, randomized trial. Am J Clin Nutr. 2017;105(3):758–767. https://doi.org/10.3945/ajcn.116.140012
- 2119. Miraglia Del Giudice M, Indolfi C, Capasso M, Maiello N, Decimo F, Ciprandi G. Bifidobacterium mixture (B longum BB536, B infantis M‐63, B breve M‐16V) treatment in children with seasonal allergic rhinitis and intermittent asthma. Ital J Pediatr. 2017;43(1):25. https://doi.org/10.1186/s13052‐017‐0340‐5
- 2120. Beard S. Rhinitis. Prim Care. 2014;41(1):33–46. https://doi.org/10.1016/j.pop.2013.10.005
- 2121. North ML, Walker TJ, Steacy LM, et al. Add‐on histamine receptor‐3 antagonist for allergic rhinitis: a double blind randomized crossover trial using the environmental exposure unit. Allergy Asthma Clin Immunol. 2014;10(1):33. https://doi.org/10.1186/1710‐1492‐10‐33
- 2122. Nathan RA, Finn Jr AF, LaForce C, et al. Comparison of cetirizine‐pseudoephedrine and placebo in patients with seasonal allergic rhinitis and concomitant mild‐to‐moderate asthma: randomized, double‐blind study. Ann Allergy Asthma Immunol. 2006;97(3):389–396. https://doi.org/10.1016/S1081‐1206(10)60806‐X
- 2123. Chervinsky P, Nayak A, Rooklin A, Danzig M. Efficacy and safety of desloratadine/pseudoephedrine tablet, 2.5/120 mg two times a day, versus individual components in the treatment of patients with seasonal allergic rhinitis. Allergy Asthma Proc. 2005;26(5):391–396.
- 2124. Meltzer EO, Casale TB, Gold MS, et al. Efficacy and safety of clemastine‐pseudoephedrine‐acetaminophen versus pseudoephedrine‐acetaminophen in the treatment of seasonal allergic rhinitis in a 1‐day, placebo‐controlled park study. Ann Allergy Asthma Immunol. 2003;90(1):79–86. https://doi.org/10.1016/S1081‐1206(10)63618‐6
- 2125. McFadden EA, Gungor A, Ng B, Mamikoglu B, Moinuddin R, Corey J. Loratadine/pseudoephedrine for nasal symptoms in seasonal allergic rhinitis: a double‐blind, placebo‐controlled study. Ear Nose Throat J. 2000;79(4):254, 257‐8, 260 passim.
- 2126. Serra HA, Alves O, Rizzo LF, Devoto FM, Ascierto H. Loratadine‐pseudoephedrine in children with allergic rhinitis, a controlled double‐blind trial. Br J Clin Pharmacol. 1998;45(2):147–150. https://doi.org/10.1046/j.1365‐2125.1998.00657.x
- 2127. Corren J, Harris AG, Aaronson D, et al. Efficacy and safety of loratadine plus pseudoephedrine in patients with seasonal allergic rhinitis and mild asthma. J Allergy Clin Immunol. 1997;100(6 pt 1):781–788. https://doi.org/10.1016/s0091‐6749(97)70274‐4
- 2128. Williams BO, Hull H, McSorley P, Frosolono MF, Sanders RL. Efficacy of acrivastine plus pseudoephedrine for symptomatic relief of seasonal allergic rhinitis due to mountain cedar. Ann Allergy Asthma Immunol. 1996;76(5):432–438. https://doi.org/10.1016/S1081‐1206(10)63460‐6
- 2129. Grossman J, Bronsky EA, Lanier BQ, et al. Loratadine‐pseudoephedrine combination versus placebo in patients with seasonal allergic rhinitis. Ann Allergy. 1989;63(4):317–321.
- 2130. Storms WW, Bodman SF, Nathan RA, et al. SCH 434: a new antihistamine/decongestant for seasonal allergic rhinitis. J Allergy Clin Immunol. 1989;83(6):1083–1090. https://doi.org/10.1016/0091‐6749(89)90450‐8
- 2131. Chen YA, Chang KP, Lin YS, Hao SP. A randomized, double‐blind, parallel‐group study to compare the efficacy and safety of a once‐daily loratadine‐pseudoephedrine combination with that of a twice‐daily loratadine‐pseudoephedrine combination in the treatment of allergic rhinitis. Eur Arch Otorhinolaryngol. 2007;264(9):1019–1025. https://doi.org/10.1007/s00405‐007‐0316‐y
- 2132. Chiang YC, Shyur SD, Chen TL, et al. A randomized controlled trial of cetirizine plus pseudoephedrine versus loratadine plus pseudoephedrine for perennial allergic rhinitis. Asian Pac J Allergy Immunol. 2006;24(2‐3):97–103.
- 2133. Moinuddin R, deTineo M, Maleckar B, Naclerio RM, Baroody FM. Comparison of the combinations of fexofenadine‐pseudoephedrine and loratadine‐montelukast in the treatment of seasonal allergic rhinitis. Ann Allergy Asthma Immunol. 2004;92(1):73–79. https://doi.org/10.1016/S1081‐1206(10)61713‐9
- 2134. Simola M, Boss I, Holopainen E, et al. Astemizole in combination with pseudoephedrine in the treatment of seasonal allergic rhinitis. Rhinology. 1996;34(1):21–23.
- 2135. Prevost M, Turenne Y, Moote DW, et al. Comparative study of SCH 434 and CTM‐D in the treatment of seasonal allergic rhinitis. Clin Ther. 1994;16(1):50–56.
- 2136. Segal AT, Falliers CJ, Grant JA, et al. Safety and efficacy of terfenadine/pseudoephedrine versus clemastine/phenylpropanolamine in the treatment of seasonal allergic rhinitis. Ann Allergy. 1993;70(5):389–394.
- 2137. Zieglmayer UP, Horak F, Toth J, Marks B, Berger UE, Burtin B. Efficacy and safety of an oral formulation of cetirizine and prolonged‐release pseudoephedrine versus budesonide nasal spray in the management of nasal congestion in allergic rhinitis. Treat Respir Med. 2005;4(4):283–287. https://doi.org/10.2165/00151829‐200504040‐00006
- 2138. Negrini AC, Troise C, Voltolini S, Horak F, Bachert C, Janssens M. Oral antihistamine/decongestant treatment compared with intranasal corticosteroids in seasonal allergic rhinitis. Clin Exp Allergy. 1995;25(1):60–65. https://doi.org/10.1111/j.1365‐2222.1995.tb01003.x
- 2139. Stubner UP, Toth J, Marks B, Berger UE, Burtin B, Horak F. Efficacy and safety of an oral formulation of cetirizine and prolonged‐release pseudoephedrine versus xylometazoline nasal spray in nasal congestion. Arzneimittelforschung. 2001;51(11):904–910. https://doi.org/10.1055/s‐0031‐1300135
- 2140. Kaiser HB, Banov CH, Berkowitz RR, et al. Comparative efficacy and safety of once‐daily versus twice‐daily loratadine‐pseudoephedrine combinations versus placebo in seasonal allergic rhinitis. Am J Ther. 1998;5(4):245–251. https://doi.org/10.1097/00045391‐199807000‐00007
- 2141. Pinar E, Eryigit O, Oncel S, Calli C, Yilmaz O, Yuksel H. Efficacy of nasal corticosteroids alone or combined with antihistamines or montelukast in treatment of allergic rhinitis. Auris Nasus Larynx. 2008;35(1):61–66. https://doi.org/10.1016/j.anl.2007.06.004
- 2142. Anolik R, Mometasone Furoate Nasal Spray With Loratadine Study Group . Clinical benefits of combination treatment with mometasone furoate nasal spray and loratadine vs monotherapy with mometasone furoate in the treatment of seasonal allergic rhinitis. Ann Allergy Asthma Immunol. 2008;100(3):264–271. https://doi.org/10.1016/S1081‐1206(10)60452‐8
- 2143. Barnes ML, Ward JH, Fardon TC, Lipworth BJ. Effects of levocetirizine as add‐on therapy to fluticasone in seasonal allergic rhinitis. Clin Exp Allergy. 2006;36(5):676–684. https://doi.org/10.1111/j.1365‐2222.2006.02478.x
- 2144. Di Lorenzo G, Pacor ML, Pellitteri ME, et al. Randomized placebo‐controlled trial comparing fluticasone aqueous nasal spray in mono‐therapy, fluticasone plus cetirizine, fluticasone plus montelukast and cetirizine plus montelukast for seasonal allergic rhinitis. Clin Exp Allergy. 2004;34(2):259–267. https://doi.org/10.1111/j.1365‐2222.2004.01877.x
- 2145. Ratner PH, van Bavel JH, Martin BG, et al. A comparison of the efficacy of fluticasone propionate aqueous nasal spray and loratadine, alone and in combination, for the treatment of seasonal allergic rhinitis. J Fam Pract. 1998;47(2):118–125.
- 2146. Seresirikachorn K, Chitsuthipakorn W, Kanjanawasee D, Khattiyawittayakun L, Snidvongs K. Effects of H1 antihistamine addition to intranasal corticosteroid for allergic rhinitis: a systematic review and meta‐analysis. Int Forum Allergy Rhinol. 2018;8(10):1083–1092. https://doi.org/10.1002/alr.22166
- 2147. Wang R, Zhang C. [Clinical evaluation of Montelukast plus Budesonide nasal spray and Desloratadine citrate disodium in treating moderate and severe persistent allergic rhinitis]. Lin Chung Er Bi Yan Hou Tou Jing Wai Ke Za Zhi. 2015;29(23):2041–2043.
- 2148. Modgill V, Badyal DK, Verghese A. Efficacy and safety of montelukast add‐on therapy in allergic rhinitis. Methods Find Exp Clin Pharmacol. 2010;32(9):669–674. https://doi.org/10.1358/mf.2010.32.9.1533686
- 2149. Benitez HH, Arvizu VM, Gutiérrez DJ, et al., Budesonida nasal más zafirlukast vs budesonida nasal más loratadina‐ pseudoefedrina en el control de los síntomas de la rinitis y el asma [Nasal budesonide plus zafirlukast vs nasal budesonide plus loratadine‐pseudoephedrine for controlling the symptoms of rhinitis and asthma]. Rev Alerg Mex. 2005;52(2):90–95.
- 2150. Lanier BQ, Abelson MB, Berger WE, et al. Comparison of the efficacy of combined fluticasone propionate and olopatadine versus combined fluticasone propionate and fexofenadine for the treatment of allergic rhinoconjunctivitis induced by conjunctival allergen challenge. Clin Ther. 2002;24(7):1161–1174. https://doi.org/10.1016/s0149‐2918(02)80027‐3
- 2151. Wilson A, Dempsey OJ, Sims EJ, Coutie WJ, Paterson MC, Lipworth BJ. Evaluation of treatment response in patients with seasonal allergic rhinitis using domiciliary nasal peak inspiratory flow. Clin Exp Allergy. 2000;30(6):833–838. https://doi.org/10.1046/j.1365‐2222.2000.00749.x
- 2152. Juniper EF, Kline PA, Hargreave FE, Dolovich J. Comparison of beclomethasone dipropionate aqueous nasal spray, astemizole, and the combination in the prophylactic treatment of ragweed pollen‐induced rhinoconjunctivitis. J Allergy Clin Immunol. 1989;83(3):627–633. https://doi.org/10.1016/0091‐6749(89)90075‐4
- 2153. Kim MK, Lee SY, Park HS, et al. A randomized, multicenter, double‐blind, phase III study to evaluate the efficacy on allergic rhinitis and safety of a combination therapy of Montelukast and Levocetirizine in patients with asthma and allergic rhinitis. Clin Ther. 2018;40(7):1096–1107. e1. https://doi.org/10.1016/j.clinthera.2018.04.021
- 2154. Liu G, Zhou X, Chen J, Liu F. Oral Antihistamines alone vs in combination with leukotriene receptor antagonists for allergic rhinitis: a meta‐analysis. Otolaryngol Head Neck Surg. 2018;158(3):450–458. https://doi.org/10.1177/0194599817752624
- 2155. Mahatme MS, Dakhale GN, Tadke K, Hiware SK, Dudhgaonkar SD, Wankhede S. Comparison of efficacy, safety, and cost‐effectiveness of montelukast‐levocetirizine and montelukast‐fexofenadine in patients of allergic rhinitis: a randomized, double‐blind clinical trial. Indian J Pharmacol. 2016;48(6):649–653. https://doi.org/10.4103/0253‐7613.194854
- 2156. Lu S, Malice MP, Dass SB, Reiss TF. Clinical studies of combination montelukast and loratadine in patients with seasonal allergic rhinitis. J Asthma. 2009;46(9):878–883. https://doi.org/10.3109/02770900903104540
- 2157. Saengpanich S, deTineo M, Naclerio RM, Baroody FM. Fluticasone nasal spray and the combination of loratadine and montelukast in seasonal allergic rhinitis. Arch Otolaryngol Head Neck Surg. 2003;129(5):557–562. https://doi.org/10.1001/archotol.129.5.557
- 2158. Cingi C, Gunhan K, Gage‐White L, Unlu H. Efficacy of leukotriene antagonists as concomitant therapy in allergic rhinitis. Laryngoscope. 2010;120(9):1718–1723. https://doi.org/10.1002/lary.20941
- 2159. Li AM, Abdullah VJ, Tsen CS, et al. Leukotriene receptor antagonist in the treatment of childhood allergic rhinitis – a randomized placebo‐controlled study. Pediatr Pulmonol. 2009;44(11):1085–1092. https://doi.org/10.1002/ppul.21102
- 2160. Ciebiada M, Barylski M, Gorska Ciebiada M. Nasal eosinophilia and serum soluble intercellular adhesion molecule 1 in patients with allergic rhinitis treated with montelukast alone or in combination with desloratadine or levocetirizine. Am J Rhinol Allergy. 2013;27(2):e58–e62. https://doi.org/10.2500/ajra.2013.27.3881
- 2161. Yamamoto H, Yamada T, Sakashita M, et al. Efficacy of prophylactic treatment with montelukast and montelukast plus add‐on loratadine for seasonal allergic rhinitis. Allergy Asthma Proc. 2012;33(2):e17–e22. https://doi.org/10.2500/aap.2012.33.3514
- 2162. Watanasomsiri A, Poachanukoon O, Vichyanond P. Efficacy of montelukast and loratadine as treatment for allergic rhinitis in children. Asian Pac J Allergy Immunol. 2008;26(2‐3):89–95.
- 2163. Nayak AS, Philip G, Lu S, Malice MP, Reiss TF, Montelukast Fall Rhinitis Investigator Group . Efficacy and tolerability of montelukast alone or in combination with loratadine in seasonal allergic rhinitis: a multicenter, randomized, double‐blind, placebo‐controlled trial performed in the fall. Ann Allergy Asthma Immunol. 2002;88(6):592–600. https://doi.org/10.1016/S1081‐1206(10)61891‐1
- 2164. Meltzer EO, Malmstrom K, Lu S, et al. Concomitant montelukast and loratadine as treatment for seasonal allergic rhinitis: a randomized, placebo‐controlled clinical trial. J Allergy Clin Immunol. 2000;105(5):917–922. https://doi.org/10.1067/mai.2000.106040
- 2165. Andrews CP, Mohar D, Salhi Y, Tantry SK. Efficacy and safety of twice‐daily and once‐daily olopatadine‐mometasone combination nasal spray for seasonal allergic rhinitis. Ann Allergy Asthma Immunol. 2020;124(2):171–178.e2. https://doi.org/10.1016/j.anai.2019.11.007
- 2166. Hampel FC, Pedinoff AJ, Jacobs RL, Caracta CF, Tantry SK. Olopatadine‐mometasone combination nasal spray: evaluation of efficacy and safety in patients with seasonal allergic rhinitis. Allergy Asthma Proc. 2019;40(4):261–272. https://doi.org/10.2500/aap.2019.40.4223
- 2167. Gross GN, Berman G, Amar NJ, Caracta CF, Tantry SK. Efficacy and safety of olopatadine‐mometasone combination nasal spray for the treatment of seasonal allergic rhinitis. Ann Allergy Asthma Immunol. 2019;122(6):630–638.e3. https://doi.org/10.1016/j.anai.2019.03.017
- 2168. Patel P, Salapatek AM, Tantry SK. Effect of olopatadine‐mometasone combination nasal spray on seasonal allergic rhinitis symptoms in an environmental exposure chamber study. Ann Allergy Asthma Immunol. 2019;122(2):160–166.e1. https://doi.org/10.1016/j.anai.2018.10.011
- 2169. Kortekaas Krohn I, Callebaut I, Alpizar YA, et al. MP29‐02 reduces nasal hyperreactivity and nasal mediators in patients with house dust mite‐allergic rhinitis. Allergy. 2018;73(5):1084–1093. https://doi.org/10.1111/all.13349
- 2170. Berger W, Meltzer EO, Amar N, et al. Efficacy of MP‐AzeFlu in children with seasonal allergic rhinitis: importance of paediatric symptom assessment. Pediatr Allergy Immunol. 2016;27(2):126–133. https://doi.org/10.1111/pai.12540
- 2171. Meltzer E, Ratner P, Bachert C, et al. Clinically relevant effect of a new intranasal therapy (MP29‐02) in allergic rhinitis assessed by responder analysis. Int Arch Allergy Immunol. 2013;161(4):369–377. https://doi.org/10.1159/000351404
- 2172. Price D, Shah S, Bhatia S, et al. A new therapy (MP29‐02) is effective for the long‐term treatment of chronic rhinitis. J Investig Allergol Clin Immunol. 2013;23(7):495–503.
- 2173. Carr W, Bernstein J, Lieberman P, et al. A novel intranasal therapy of azelastine with fluticasone for the treatment of allergic rhinitis. J Allergy Clin Immunol. 2012;129(5):1282–1289.e10. https://doi.org/10.1016/j.jaci.2012.01.077
- 2174. Meltzer EO, LaForce C, Ratner P, Price D, Ginsberg D, Carr W. MP29‐02 (a novel intranasal formulation of azelastine hydrochloride and fluticasone propionate) in the treatment of seasonal allergic rhinitis: a randomized, double‐blind, placebo‐controlled trial of efficacy and safety. Allergy Asthma Proc. 2012;33(4):324–332. https://doi.org/10.2500/aap.2012.33.3587
- 2175. Salapatek AM, Lee J, Patel D, et al. Solubilized nasal steroid (CDX‐947) when combined in the same solution nasal spray with an antihistamine (CDX‐313) provides improved, fast‐acting symptom relief in patients with allergic rhinitis. Allergy Asthma Proc. 2011;32(3):221–229. https://doi.org/10.2500/aap.2011.32.3444
- 2176. Hampel FC, Ratner PH, Van Bavel J, et al. Double‐blind, placebo‐controlled study of azelastine and fluticasone in a single nasal spray delivery device. Ann Allergy Asthma Immunol. 2010;105(2):168–173. https://doi.org/10.1016/j.anai.2010.06.008
- 2177. LaForce CF, Carr W, Tilles SA, et al. Evaluation of olopatadine hydrochloride nasal spray,.6%, used in combination with an intranasal corticosteroid in seasonal allergic rhinitis. Allergy Asthma Proc. 2010;31(2):132–140. https://doi.org/10.2500/aap.2010.31.3326
- 2178. Ratner PH, Hampel F, Van Bavel J, et al. Combination therapy with azelastine hydrochloride nasal spray and fluticasone propionate nasal spray in the treatment of patients with seasonal allergic rhinitis. Ann Allergy Asthma Immunol. 2008;100(1):74–81. https://doi.org/10.1016/S1081‐1206(10)60408‐5
- 2179. Ilyina NI, Edin AS, Astafieva NG, et al. Efficacy of a novel intranasal formulation of azelastine hydrochloride and fluticasone propionate, delivered in a single spray, for the treatment of seasonal allergic rhinitis: results from Russia. Int Arch Allergy Immunol. 2019;178(3):255–263. https://doi.org/10.1159/000494507
- 2180. Berger W, Bousquet J, Fox AT, et al. MP‐AzeFlu is more effective than fluticasone propionate for the treatment of allergic rhinitis in children. Allergy. 2016;71(8):1219–1222. https://doi.org/10.1111/all.12903
- 2181. Klimek L, Bachert C, Stjarne P, et al. MP‐AzeFlu provides rapid and effective allergic rhinitis control in real life: A pan‐European study. Allergy Asthma Proc. 2016;37(5):376–386. https://doi.org/10.2500/aap.2016.37.3979
- 2182. Klimek L, Bachert C, Mosges R, et al. Effectiveness of MP29‐02 for the treatment of allergic rhinitis in real‐life: results from a noninterventional study. Allergy Asthma Proc. 2015;36(1):40–47. https://doi.org/10.2500/aap.2015.36.3823
- 2183. Klimek L, Poletti SC, Sperl A, et al. Olfaction in patients with allergic rhinitis: an indicator of successful MP‐AzeFlu therapy. Int Forum Allergy Rhinol. 2017;7(3):287–292. https://doi.org/10.1002/alr.21877
- 2184. Debbaneh PM, Bareiss AK, Wise SK, McCoul ED. Intranasal azelastine and fluticasone as combination therapy for allergic rhinitis: systematic review and meta‐analysis. Otolaryngol Head Neck Surg. 2019;161(3):412–418. https://doi.org/10.1177/0194599819841883
- 2185. Du K, Qing H, Zheng M, Wang X, Zhang L. Intranasal antihistamine is superior to oral H1 antihistamine as an add‐on therapy to intranasal corticosteroid for treating allergic rhinitis. Ann Allergy Asthma Immunol. 2020;125(5):589–596.e3. https://doi.org/10.1016/j.anai.2020.06.038
- 2186. Seresirikachorn K, Mullol J, Limitlaohaphan K, Asvapoositkul V, Snidvongs K. Leukotriene receptor antagonist addition to intranasal steroid: systematic review and meta‐analysis. Rhinology. 2021;59(1):2–9. https://doi.org/10.4193/Rhin20.126
- 2187. Chen H, Zhang L, Lou H, Wang Y, Cao F, Wang C. A randomized trial of comparing a combination of montelukast and budesonide with budesonide in allergic rhinitis. Laryngoscope. 2021;131(4):E1054–E1061. https://doi.org/10.1002/lary.28433
- 2188. Goh BS, Ismail MI, Husain S. Quality of life assessment in patients with moderate to severe allergic rhinitis treated with montelukast and/or intranasal steroids: a randomised, double‐blind, placebo‐controlled study. J Laryngol Otol. 2014;128(3):242–248. https://doi.org/10.1017/S002221511400036X
- 2189. Esteitie R, deTineo M, Naclerio RM, Baroody FM. Effect of the addition of montelukast to fluticasone propionate for the treatment of perennial allergic rhinitis. Ann Allergy Asthma Immunol. 2010;105(2):155–161. https://doi.org/10.1016/j.anai.2010.05.017
- 2190. Florinescu‐Gheorghe N‐A, Popescu F, Alexandru DO. Treatment evaluation with mometasone furoate, alone or in combination with desloratadine/montelukast, in moderate severe allergic rhinitis. Acta Med Marisienis. 2014;60:106–108.
- 2191. Haenisch B, Walstab J, Herberhold S, et al. Alpha‐adrenoceptor agonistic activity of oxymetazoline and xylometazoline. Fundam Clin Pharmacol. 2010;24(6):729–739. https://doi.org/10.1111/j.1472‐8206.2009.00805.x
- 2192. Kirtsreesakul V, Khanuengkitkong T, Ruttanaphol S. Does oxymetazoline increase the efficacy of nasal steroids in treating nasal polyposis? Am J Rhinol Allergy. 2016;30(3):195–200. https://doi.org/10.2500/ajra.2016.30.4294
- 2193. Matreja PS, Gupta V, Kaur J, Singh S. Efficacy of fluticasona nd oxymetazoline as the treatment for allergic rhinitis. J Clin Diagnostic Res. 2012;6:4.
- 2194. Meltzer EO, Bernstein DI, Prenner BM, Berger WE, Shekar T, Teper AA. Mometasone furoate nasal spray plus oxymetazoline nasal spray: short‐term efficacy and safety in seasonal allergic rhinitis. Am J Rhinol Allergy. 2013;27(2):102–108. https://doi.org/10.2500/ajra.2013.27.3864
- 2195. Rael EL, Ramey J, Lockey RF. Oxymetazoline hydrochloride combined with mometasone nasal spray for persistent nasal congestion (pilot study). World Allergy Organ J. 2011;4(3):65–67. https://doi.org/10.1097/WOX.0b013e31820f8fae
- 2196. Thongngarm T, Assanasen P, Pradubpongsa P, Tantilipikorn P. The effectiveness of oxymetazoline plus intranasal steroid in the treatment of chronic rhinitis: a randomised controlled trial. Asian Pac J Allergy Immunol. 2016;34(1):30–37. https://doi.org/10.12932/AP0649.34.1.2016
- 2197. Baroody FM, Brown D, Gavanescu L, DeTineo M, Naclerio RM. Oxymetazoline adds to the effectiveness of fluticasone furoate in the treatment of perennial allergic rhinitis. J Allergy Clin Immunol. 2011;127(4):927–934. https://doi.org/10.1016/j.jaci.2011.01.037
- 2198. Khattiyawittayakun L, Seresirikachorn K, Chitsuthipakorn W, Kanjanawasee D, Snidvongs K. Effects of decongestant addition to intranasal corticosteroid for chronic rhinitis: a systematic review and meta‐analysis. Int Forum Allergy Rhinol. 2018;8(12):1445–1453. https://doi.org/10.1002/alr.22193
- 2199. Kilic G. Treatment of allergic rhinitis in children. Antiinflamm Antiallergy Agents Med Chem. 2008;7:38–44.
- 2200. Scadding GK. Allergic rhinitis in children. Paediatrics Child Health. 2008;18:323–328.
- 2201. Yap L, Pothula VB, Warner J, Akhtar S, Yates E. The root and development of otorhinolaryngology in traditional Chinese medicine. Eur Arch Otorhinolaryngol. 2009;266(9):1353–1359. https://doi.org/10.1007/s00405‐009‐1041‐5
- 2202. Kaptchuk TJ. Acupuncture: theory, efficacy, and practice. Ann Intern Med. 2002;136(5):374–383. https://doi.org/10.7326/0003‐4819‐136‐5‐200203050‐00010
- 2203. Yang XY, Shi GX, Li QQ, Zhang ZH, Xu Q, Liu CZ. Characterization of deqi sensation and acupuncture effect. Evid Based Complement Alternat Med. 2013;2013:319734. https://doi.org/10.1155/2013/319734
- 2204. Petti FB, Liguori A, Ippoliti F. Study on cytokines IL‐2, IL‐6, IL‐10 in patients of chronic allergic rhinitis treated with acupuncture. J Tradit Chin Med. 2002;22(2):104–111.
- 2205. Feng S, Han M, Fan Y, et al. Acupuncture for the treatment of allergic rhinitis: a systematic review and meta‐analysis. Am J Rhinol Allergy. 2015;29(1):57–62. https://doi.org/10.2500/ajra.2015.29.4116
- 2206. Roberts J, Huissoon A, Dretzke J, Wang D, Hyde C. A systematic review of the clinical effectiveness of acupuncture for allergic rhinitis. BMC Complement Altern Med. 2008;8:13. https://doi.org/10.1186/1472‐6882‐8‐13
- 2207. Lee MS, Pittler MH, Shin BC, Kim JI, Ernst E. Acupuncture for allergic rhinitis: a systematic review. Ann Allergy Asthma Immunol. 2009;102(4):269–279; quiz 279‐81, 307. https://doi.org/10.1016/S1081‐1206(10)60330‐4
- 2208. Brinkhaus B, Ortiz M, Witt CM, et al. Acupuncture in patients with seasonal allergic rhinitis: a randomized trial. Ann Intern Med. 2013;158(4):225–234. https://doi.org/10.7326/0003‐4819‐158‐4‐201302190‐00002
- 2209. Wu AW, Gettelfinger JD, Ting JY, Mort C, Higgins TS. Alternative therapies for sinusitis and rhinitis: a systematic review utilizing a modified Delphi method. Int Forum Allergy Rhinol. 2020;10(4):496–504. https://doi.org/10.1002/alr.22488
- 2210. Yin Z, Geng G, Xu G, Zhao L, Liang F. Acupuncture methods for allergic rhinitis: a systematic review and bayesian meta‐analysis of randomized controlled trials. Chin Med. 2020;15:109. https://doi.org/10.1186/s13020‐020‐00389‐9
- 2211. Taw MB, Reddy WD, Omole FS, Seidman MD. Acupuncture and allergic rhinitis. Curr Opin Otolaryngol Head Neck Surg. 2015;23(3):216–220. https://doi.org/10.1097/MOO.0000000000000161
- 2212. Zhang CS, Yang AW, Zhang AL, et al. Ear‐acupressure for allergic rhinitis: a systematic review. Clin Otolaryngol. 2010;35(1):6–12. https://doi.org/10.1111/j.1749‐4486.2009.02067.x
- 2213. Li XR, Zhang QX, Liu M, et al. Catgut implantation at acupoints for allergic rhinitis: a systematic review. Chin J Integr Med. 2014;20(3):235–240. https://doi.org/10.1007/s11655‐014‐1748‐z
- 2214. Zhou F, Yan LJ, Yang GY, Liu JP. Acupoint herbal patching for allergic rhinitis: a systematic review and meta‐analysis of randomised controlled trials. Clin Otolaryngol. 2015;40(6):551–568. https://doi.org/10.1111/coa.12410
- 2215. Fu Q, Zhang L, Liu Y, et al. Effectiveness of acupuncturing at the sphenopalatine ganglion acupoint alone for treatment of allergic rhinitis: a systematic review and meta‐analysis. Evid Based Complement Alternat Med. 2019;2019:6478102. https://doi.org/10.1155/2019/6478102
- 2216. Li X. The mechanism analysis of treating nasal disease by sphenopalatine ganglion (acupoint “ZhiBi 3”) stimulation with acupuncture needle and an introduction to the relevant needling method. J Clin Otorhinolaryngol Head Neck Surg. 2011;5:193.
- 2217. Li XW, Tian ZP. A preliminary summary of the treatment on rhinitis puncturing sphenopalatine ganglion. Beijing J Chinese Med. 1990;9:36–38.
- 2218. Zhang J, Zhang Y, Huang X, et al. Different acupuncture therapies for allergic rhinitis: overview of systematic reviews and network meta‐analysis. Evid Based Complement Alternat Med. 2020;2020:8363027. https://doi.org/10.1155/2020/8363027
- 2219. Wang LL. [Characteristic of moxibustion and its warming‐dredging effect]. Zhongguo Zhen Jiu. 2011;31(10):865–868.
- 2220. Yuan T, Xiong J, Yang J, et al. The effectiveness and safety of thunder fire moxibustion for treating allergic rhinitis: a PRISMA compliant systematic review and meta‐analysis. Evid Based Complement Alternat Med. 2020;2020:6760436. https://doi.org/10.1155/2020/6760436
- 2221. Kacaniova M, Pavlicova S, Hascik P, et al. Microbial communities in bees, pollen and honey from Slovakia. Acta Microbiol Immunol Hung. 2009;56(3):285–295. https://doi.org/10.1556/AMicr.56.2009.3.7
- 2222. Duddukuri GR, Kumar PS, Kumar VB, Athota RR. Immunosuppressive effect of honey on the induction of allergen‐specific humoral antibody response in mice. Int Arch Allergy Immunol. 1997;114(4):385–388. https://doi.org/10.1159/000237699
- 2223. Ishikawa Y, Tokura T, Nakano N, et al. Inhibitory effect of honeybee‐collected pollen on mast cell degranulation in vivo and in vitro. J Med Food. 2008;11(1):14–20. https://doi.org/10.1089/jmf.2006.163
- 2224. Ishikawa Y, Tokura T, Ushio H, et al. Lipid‐soluble components of honeybee‐collected pollen exert antiallergic effect by inhibiting IgE‐mediated mast cell activation in vivo. Phytother Res. 2009;23(11):1581–1586. https://doi.org/10.1002/ptr.2824
- 2225. Subrahmanyam M. A prospective randomised clinical and histological study of superficial burn wound healing with honey and silver sulfadiazine. Burns. 1998;24(2):157–161. https://doi.org/10.1016/s0305‐4179(97)00113‐7
- 2226. Al‐Waili NS, Boni NS. Natural honey lowers plasma prostaglandin concentrations in normal individuals. J Med Food. 2003;6(2):129–133. https://doi.org/10.1089/109662003322233530
- 2227. Asha'ari ZA, Ahmad MZ, Jihan WS, Che CM, Leman I. Ingestion of honey improves the symptoms of allergic rhinitis: evidence from a randomized placebo‐controlled trial in the East coast of Peninsular Malaysia. Ann Saudi Med. 2013;33(5):469–475. https://doi.org/10.5144/0256‐4947.2013.469
- 2228. Saarinen K, Jantunen J, Haahtela T. Birch pollen honey for birch pollen allergy – a randomized controlled pilot study. Int Arch Allergy Immunol. 2011;155(2):160–166. https://doi.org/10.1159/000319821
- 2229. Rajan TV, Tennen H, Lindquist RL, Cohen L, Clive J. Effect of ingestion of honey on symptoms of rhinoconjunctivitis. Ann Allergy Asthma Immunol. 2002;88(2):198–203. https://doi.org/10.1016/S1081‐1206(10)61996‐5
- 2230. Bogdanov S, Jurendic T, Sieber R, Gallmann P. Honey for nutrition and health: a review. J Am Coll Nutr. 2008;27(6):677–689. https://doi.org/10.1080/07315724.2008.10719745
- 2231. Passalacqua G, Bousquet PJ, Carlsen KH, et al. ARIA update: I – systematic review of complementary and alternative medicine for rhinitis and asthma. J Allergy Clin Immunol. 2006;117(5):1054–1062. https://doi.org/10.1016/j.jaci.2005.12.1308
- 2232. Enomoto T, Nagasako‐Akazome Y, Kanda T, Ikeda M, Dake Y. Clinical effects of apple polyphenols on persistent allergic rhinitis: a randomized double‐blind placebo‐controlled parallel arm study. J Investig Allergol Clin Immunol. 2006;16(5):283–289.
- 2233. Matkovic Z, Zivkovic V, Korica M, Plavec D, Pecanic S, Tudoric N. Efficacy and safety of Astragalus membranaceus in the treatment of patients with seasonal allergic rhinitis. Phytother Res. 2010;24(2):175–181. https://doi.org/10.1002/ptr.2877
- 2234. D'Souza P, Amit A, Saxena VS, Bagchi D, Bagchi M, Stohs SJ. Antioxidant properties of Aller‐7, a novel polyherbal formulation for allergic rhinitis. Drugs Exp Clin Res. 2004;30(3):99–109.
- 2235. Pratibha N, Saxena VS, Amit A, D'Souza P, Bagchi M, Bagchi D. Anti‐inflammatory activities of Aller‐7, a novel polyherbal formulation for allergic rhinitis. Int J Tissue React. 2004;26(1‐2):43–51.
- 2236. Amit A, Saxena VS, Pratibha N, et al. Mast cell stabilization, lipoxygenase inhibition, hyaluronidase inhibition, antihistaminic and antispasmodic activities of Aller‐7, a novel botanical formulation for allergic rhinitis. Drugs Exp Clin Res. 2003;29(3):107–115.
- 2237. Guo R, Pittler MH, Ernst E. Herbal medicines for the treatment of allergic rhinitis: a systematic review. Ann Allergy Asthma Immunol. 2007;99(6):483–495. https://doi.org/10.1016/S1081‐1206(10)60375‐4
- 2238. Suzuki M, Yoshino K, Maeda‐Yamamoto M, Miyase T, Sano M. Inhibitory effects of tea catechins and O‐methylated derivatives of (‐)‐epigallocatechin‐3‐O‐gallate on mouse type IV allergy. J Agric Food Chem. 2000;48(11):5649–5653. https://doi.org/10.1021/jf000313d
- 2239. Maeda‐Yamamoto M, Inagaki N, Kitaura J, et al. O‐methylated catechins from tea leaves inhibit multiple protein kinases in mast cells. J Immunol. 2004;172(7):4486–4492. https://doi.org/10.4049/jimmunol.172.7.4486
- 2240. Masuda S, Maeda‐Yamamoto M, Usui S, Fujisawa T. 'Benifuuki' green tea containing o‐methylated catechin reduces symptoms of Japanese cedar pollinosis: a randomized, double‐blind, placebo‐controlled trial. Allergol Int. 2014;63(2):211–217. https://doi.org/10.2332/allergolint.13‐OA‐0620
- 2241. Hu G, Walls RS, Bass D, et al. The Chinese herbal formulation biminne in management of perennial allergic rhinitis: a randomized, double‐blind, placebo‐controlled, 12‐week clinical trial. Ann Allergy Asthma Immunol. 2002;88(5):478–487. https://doi.org/10.1016/s1081‐1206(10)62386‐1
- 2242. Shimoda H, Tanaka J, Yamada E, Morikawa T, Kasajima N, Yoshikawa M. Anti type I allergic property of Japanese butterbur extract and its mast cell degranulation inhibitory ingredients. J Agric Food Chem. 2006;54(8):2915–2920. https://doi.org/10.1021/jf052994o
- 2243. Russell LC, Burchiel KJ. Neurophysiological effects of capsaicin. Brain Res. 1984;320(2‐3):165–176. https://doi.org/10.1016/0165‐0173(84)90005‐5
- 2244. Philip G, Baroody FM, Proud D, Naclerio RM, Togias AG. The human nasal response to capsaicin. J Allergy Clin Immunol. 1994;94(6 pt 1):1035–1045. https://doi.org/10.1016/0091‐6749(94)90122‐8
- 2245. Cheng J, Yang XN, Liu X, Zhang SP. Capsaicin for allergic rhinitis in adults. Cochrane Database Syst Rev. 2006;(2):CD004460. https://doi.org/10.1002/14651858.CD004460.pub2
- 2246. Fujiwara T, Nishida N, Nota J, et al. Efficacy of chlorophyll c2 for seasonal allergic rhinitis: single‐center double‐blind randomized control trial. Eur Arch Otorhinolaryngol. 2016;273(12):4289–4294. https://doi.org/10.1007/s00405‐016‐4133‐z
- 2247. Corren J, Lemay M, Lin Y, Rozga L, Randolph RK. Clinical and biochemical effects of a combination botanical product (ClearGuard) for allergy: a pilot randomized double‐blind placebo‐controlled trial. Nutr J. 2008;7:20. https://doi.org/10.1186/1475‐2891‐7‐20
- 2248. Turpeinen AM, Ylonen N, von Willebrand E, Basu S, Aro A. Immunological and metabolic effects of cis‐9, trans‐11‐conjugated linoleic acid in subjects with birch pollen allergy. Br J Nutr. 2008;100(1):112–119. https://doi.org/10.1017/S0007114507886326
- 2249. Bernstein DI, Bernstein CK, Deng C, et al. Evaluation of the clinical efficacy and safety of grapeseed extract in the treatment of fall seasonal allergic rhinitis: a pilot study. Ann Allergy Asthma Immunol. 2002;88(3):272–278. https://doi.org/10.1016/S1081‐1206(10)62008‐X
- 2250. Hirano T, Kawai M, Arimitsu J, et al. Preventative effect of a flavonoid, enzymatically modified isoquercitrin on ocular symptoms of Japanese cedar pollinosis. Allergol Int. 2009;58(3):373–382. https://doi.org/10.2332/allergolint.08‐OA‐0070
- 2251. Kawai M, Hirano T, Arimitsu J, et al. Effect of enzymatically modified isoquercitrin, a flavonoid, on symptoms of Japanese cedar pollinosis: a randomized double‐blind placebo‐controlled trial. Int Arch Allergy Immunol. 2009;149(4):359–368. https://doi.org/10.1159/000205582
- 2252. Yamprasert R, Chanvimalueng W, Mukkasombut N, Itharat A. Ginger extract versus Loratadine in the treatment of allergic rhinitis: a randomized controlled trial. BMC Complement Med Ther. 2020;20(1):119. https://doi.org/10.1186/s12906‐020‐2875‐z
- 2253. Hewlings S, Kalman DS. Evaluating the impacts of methylsulfonylmethane on allergic rhinitis after a standard allergen challenge: randomized double‐blind exploratory study. JMIR Res Protoc. 2018;7(11):e11139. https://doi.org/10.2196/11139
- 2254. Chakravarty N. Inhibition of histamine release from mast cells by nigellone. Ann Allergy. 1993;70(3):237–242.
- 2255. El Gazzar M, El Mezayen R, Marecki JC, Nicolls MR, Canastar A, Dreskin SC. Anti‐inflammatory effect of thymoquinone in a mouse model of allergic lung inflammation. Int Immunopharmacol. 2006;6(7):1135–1142. https://doi.org/10.1016/j.intimp.2006.02.004
- 2256. Kalus U, Pruss A, Bystron J, et al. Effect of Nigella sativa (black seed) on subjective feeling in patients with allergic diseases. Phytother Res. 2003;17(10):1209–1214. https://doi.org/10.1002/ptr.1356
- 2257. Nikakhlagh S, Rahim F, Aryani FH, Syahpoush A, Brougerdnya MG, Saki N. Herbal treatment of allergic rhinitis: the use of Nigella sativa. Am J Otolaryngol. 2011;32(5):402–407. https://doi.org/10.1016/j.amjoto.2010.07.019
- 2258. Alsamarai AM, Abdulsatar M, Ahmed Alobaidi AH. Evaluation of topical black seed oil in the treatment of allergic rhinitis. Antiinflamm Antiallergy Agents Med Chem. 2014;13(1):75–82. https://doi.org/10.2174/18715230113129990014
- 2259. Rotondo S, Rajtar G, Manarini S, et al. Effect of trans‐resveratrol, a natural polyphenolic compound, on human polymorphonuclear leukocyte function. Br J Pharmacol. 1998;123(8):1691–1699. https://doi.org/10.1038/sj.bjp.0701784
- 2260. Varilek GW, Yang F, Lee EY, et al. Green tea polyphenol extract attenuates inflammation in interleukin‐2‐deficient mice, a model of autoimmunity. J Nutr. 2001;131(7):2034–2039. https://doi.org/10.1093/jn/131.7.2034
- 2261. Yang F, de Villiers WJ, McClain CJ, Varilek GW. Green tea polyphenols block endotoxin‐induced tumor necrosis factor‐production and lethality in a murine model. J Nutr. 1998;128(12):2334–2340. https://doi.org/10.1093/jn/128.12.2334
- 2262. Makino T, Furuta Y, Wakushima H, Fujii H, Saito K, Kano Y. Anti‐allergic effect of Perilla frutescens and its active constituents. Phytother Res. 2003;17(3):240–243. https://doi.org/10.1002/ptr.1115
- 2263. Takano H, Osakabe N, Sanbongi C, et al. Extract of Perilla frutescens enriched for rosmarinic acid, a polyphenolic phytochemical, inhibits seasonal allergic rhinoconjunctivitis in humans. Exp Biol Med (Maywood). 2004;229(3):247–254. https://doi.org/10.1177/153537020422900305
- 2264. Wassenberg J, Nutten S, Audran R, et al. Effect of Lactobacillus paracasei ST11 on a nasal provocation test with grass pollen in allergic rhinitis. Clin Exp Allergy. 2011;41(4):565–573. https://doi.org/10.1111/j.1365‐2222.2011.03695.x
- 2265. Perrin Y, Nutten S, Audran R, et al. Comparison of two oral probiotic preparations in a randomized crossover trial highlights a potentially beneficial effect of Lactobacillus paracasei NCC2461 in patients with allergic rhinitis. Clin Transl Allergy. 2014;4(1):1. https://doi.org/10.1186/2045‐7022‐4‐1
- 2266. Lenon GB, Xue CC, Story DF, Thien FC, McPhee S, Li CG. Inhibition of release of inflammatory mediators in primary and cultured cells by a Chinese herbal medicine formula for allergic rhinitis. Chin Med. 2007;2:2. https://doi.org/10.1186/1749‐8546‐2‐2
- 2267. Lenon GB, Li CG, Xue CC, Thien FC, Story DF. Inhibition of release of vasoactive and inflammatory mediators in airway and vascular tissues and macrophages by a chinese herbal medicine formula for allergic rhinitis. Evid Based Complement Alternat Med. 2007;4(2):209–217. https://doi.org/10.1093/ecam/nel083
- 2268. Xue CC, Thien FC, Zhang JJ, Da Costa C, Li CG. Treatment for seasonal allergic rhinitis by Chinese herbal medicine: a randomized placebo controlled trial. Altern Ther Health Med. 2003;9(5):80–87.
- 2269. Mao TK, Van de Water J, Gershwin ME. Effects of a Spirulina‐based dietary supplement on cytokine production from allergic rhinitis patients. J Med Food. 2005;8(1):27–30. https://doi.org/10.1089/jmf.2005.8.27
- 2270. Karkos PD, Leong SC, Karkos CD, Sivaji N, Assimakopoulos DA. Spirulina in clinical practice: evidence‐based human applications. Evid Based Complement Alternat Med. 2011;2011:531053. https://doi.org/10.1093/ecam/nen058
- 2271. Cingi C, Conk‐Dalay M, Cakli H, Bal C. The effects of spirulina on allergic rhinitis. Eur Arch Otorhinolaryngol. 2008;265(10):1219–1223. https://doi.org/10.1007/s00405‐008‐0642‐8
- 2272. Ishikura Y, Suwa Y, Okada T. Anti‐allergic effects of Rubus suavissimus extract. Japanese J Inflamm. 1995;15:167–173.
- 2273. Yonekura S, Okamoto Y, Yamasaki K, et al. A randomized, double‐blind, placebo‐controlled study of ten‐cha (Rubus suavissimus) on house dust mite allergic rhinitis. Auris Nasus Larynx. 2011;38(5):600–607. https://doi.org/10.1016/j.anl.2010.11.017
- 2274. Das AK, Mizuguchi H, Kodama M, et al. Sho‐seiryu‐to suppresses histamine signaling at the transcriptional level in TDI‐sensitized nasal allergy model rats. Allergol Int. 2009;58(1):81–88. https://doi.org/10.2332/allergolint.O‐07‐526
- 2275. Baba S. Double‐blind clinical trial of Sho‐seiryu‐to (TJ‐19) for perennial allergic rhinitis. Pract Otol. 1995;88:389–405.
- 2276. Badar VA, Thawani VR, Wakode PT, et al. Efficacy of Tinospora cordifolia in allergic rhinitis. J Ethnopharmacol. 2005;96(3):445–449. https://doi.org/10.1016/j.jep.2004.09.034
- 2277. Yoshimura M, Enomoto T, Dake Y, et al. An evaluation of the clinical efficacy of tomato extract for perennial allergic rhinitis. Allergol Int. 2007;56(3):225–230. https://doi.org/10.2332/allergolint.O‐06‐443
- 2278. Roschek Jr B, Fink RC, McMichael M, Alberte RS. Nettle extract (Urtica dioica) affects key receptors and enzymes associated with allergic rhinitis. Phytother Res. 2009;23(7):920–926. https://doi.org/10.1002/ptr.2763
- 2279. Mittman P. Randomized, double‐blind study of freeze‐dried Urtica dioica in the treatment of allergic rhinitis. Planta Med. 1990;56(1):44–47. https://doi.org/10.1055/s‐2006‐960881
- 2280. Podoshin L, Gertner R, Fradis M. Treatment of perennial allergic rhinitis with ascorbic acid solution. Ear Nose Throat J. 1991;70(1):54–55.
- 2281. Shahar E, Hassoun G, Pollack S. Effect of vitamin E supplementation on the regular treatment of seasonal allergic rhinitis. Ann Allergy Asthma Immunol. 2004;92(6):654–658. https://doi.org/10.1016/S1081‐1206(10)61432‐9
- 2282. Montano Velazquez BB, Jauregui‐Renaud K, Banuelos Arias Adel C, et al. Vitamin E effects on nasal symptoms and serum specific IgE levels in patients with perennial allergic rhinitis. Ann Allergy Asthma Immunol. 2006;96(1):45–50. https://doi.org/10.1016/s1081‐1206(10)61039‐3
- 2283. Cheng L, Chen J, Fu Q, et al. Chinese Society of Allergy Guidelines for diagnosis and treatment of allergic rhinitis. Allergy Asthma Immunol Res. 2018;10(4):300–353. https://doi.org/10.4168/aair.2018.10.4.300
- 2284. Chen S, Guo SN, Marmori F, et al. Clinical Practice Guideline for allergic rhinitis treatment with acupuncture. Chin J Integr Med. 2021;27(2):83–90. https://doi.org/10.1007/s11655‐020‐3161‐0
- 2285. Fjermedal O, Saunte C, Pedersen S. Septoplasty and/or submucous resection? 5 years nasal septum operations. J Laryngol Otol. 1988;102(9):796–798. https://doi.org/10.1017/s0022215100106486
- 2286. Karatzanis AD, Fragiadakis G, Moshandrea J, Zenk J, Iro H, Velegrakis GA. Septoplasty outcome in patients with and without allergic rhinitis. Rhinology. 2009;47(4):444–449. https://doi.org/10.4193/Rhin08.126
- 2287. Mondina M, Marro M, Maurice S, Stoll D, de Gabory L. Assessment of nasal septoplasty using NOSE and RhinoQoL questionnaires. Eur Arch Otorhinolaryngol. 2012;269(10):2189–2195. https://doi.org/10.1007/s00405‐011‐1916‐0
- 2288. Stoksted P, Gutierrez C. The nasal passage following rhinoplastic surgery. J Laryngol Otol. 1983;97(1):49–54. https://doi.org/10.1017/s0022215100093798
- 2289. Stewart MG, Smith TL, Weaver EM, et al. Outcomes after nasal septoplasty: results from the Nasal Obstruction Septoplasty Effectiveness (NOSE) study. Otolaryngol Head Neck Surg. 2004;130(3):283–290. https://doi.org/10.1016/j.otohns.2003.12.004
- 2290. Bugten V, Nilsen AH, Thorstensen WM, Moxness MH, Amundsen MF, Nordgard S. Quality of life and symptoms before and after nasal septoplasty compared with healthy individuals. BMC Ear Nose Throat Disord. 2016;16:13. https://doi.org/10.1186/s12901‐016‐0031‐7
- 2291. Manteghi A, Din H, Bundogji N, Leuin SC. Pediatric septoplasty and functional septorhinoplasty: a quality of life outcome study. Int J Pediatr Otorhinolaryngol. 2018;111:16–20. https://doi.org/10.1016/j.ijporl.2018.05.016
- 2292. Sokoya M, Gonzalez JR, Winkler AA. Effect of allergic rhinitis on nasal obstruction outcomes after functional open septorhinoplasty. Am J Otolaryngol. 2018;39(3):303–306. https://doi.org/10.1016/j.amjoto.2018.03.014
- 2293. Kokubo LCP, Carvalho TBO, Fornazieri MA, Gomes EMC, Alves CMF, Sampaio ALL. Effects of septorhinoplasty on smell perception. Eur Arch Otorhinolaryngol. 2019;276(4):1247–1250. https://doi.org/10.1007/s00405‐019‐05356‐1
- 2294. Gerecci D, Casanueva FJ, Mace JC, et al. Nasal obstruction symptom evaluation (NOSE) score outcomes after septorhinoplasty. Laryngoscope. 2019;129(4):841–846. https://doi.org/10.1002/lary.27578
- 2295. Kim SD, Jung DW, Lee JW, Park JH, Mun SJ, Cho KS. Relationship between allergic rhinitis and nasal surgery success in patients with obstructive sleep apnea. Am J Otolaryngol. 2021;42(6):103079. https://doi.org/10.1016/j.amjoto.2021.103079
- 2296. Ghosh SK, Dutta M, Haldar D. Role of bilateral inferior turbinoplasty as an adjunct to septoplasty in improving nasal obstruction and subjective performance in patients with deviated nasal septum associated with allergic rhinitis: an interventional, prospective study. Ear Nose Throat J. 2021:1455613211015440. https://doi.org/10.1177/01455613211015440
- 2297. Topal O, Celik SB, Erbek S, Erbek SS. Risk of nasal septal perforation following septoplasty in patients with allergic rhinitis. Eur Arch Otorhinolaryngol. 2011;268(2):231–233. https://doi.org/10.1007/s00405‐010‐1323‐y
- 2298. Eren E, Balci MK, Islek A. Analysis of patient‐ and procedure‐related risk factors for nasal septal perforations following septoplasty. Eur Arch Otorhinolaryngol. 2022;279(3):1357–1361. https://doi.org/10.1007/s00405‐021‐06887‐2
- 2299. Kim YH, Kim BJ, Bang KH, Hwang Y, Jang TY. Septoplasty improves life quality related to allergy in patients with septal deviation and allergic rhinitis. Otolaryngol Head Neck Surg. 2011;145(6):910–914. https://doi.org/10.1177/0194599811424119
- 2300. Chhabra N, Houser SM. The surgical management of allergic rhinitis. Otolaryngol Clin North Am. 2011;44(3):779–795, xi. https://doi.org/10.1016/j.otc.2011.03.007
- 2301. Chhabra N, Houser SM. Surgery for allergic rhinitis. Int Forum Allergy Rhinol. 2014;4(suppl 2):S79–S83. https://doi.org/10.1002/alr.21387
- 2302. Aksoy F, Yildirim YS, Veyseller B, Ozturan O, Demirhan H. Midterm outcomes of outfracture of the inferior turbinate. Otolaryngol Head Neck Surg. 2010;143(4):579–584. https://doi.org/10.1016/j.otohns.2010.06.915
- 2303. Mori S, Fujieda S, Igarashi M, Fan GK, Saito H. Submucous turbinectomy decreases not only nasal stiffness but also sneezing and rhinorrhea in patients with perennial allergic rhinitis. Clin Exp Allergy. 1999;29(11):1542–1548. https://doi.org/10.1046/j.1365‐2222.1999.00645.x
- 2304. Kaymakci M, Gur OE, Ozdem C. Nasal obstruction: comparison of radiofrequency with lateral displacement of the inferior turbinate and radiofrequency alone. J Pak Med Assoc. 2014;64(1):33–37.
- 2305. Assanasen P, Banhiran W, Tantilipikorn P, Pinkaew B. Combined radiofrequency volumetric tissue reduction and lateral outfracture of hypertrophic inferior turbinate in the treatment of chronic rhinitis: short‐term and long‐term outcome. Int Forum Allergy Rhinol. 2014;4(4):339–344. https://doi.org/10.1002/alr.21278
- 2306. Li KK, Powell NB, Riley RW, Troell RJ, Guilleminault C. Radiofrequency volumetric tissue reduction for treatment of turbinate hypertrophy: a pilot study. Otolaryngol Head Neck Surg. 1998;119(6):569–573. https://doi.org/10.1016/S0194‐5998(98)70013‐0
- 2307. Passali D, Lauriello M, Anselmi M, Bellussi L. Treatment of hypertrophy of the inferior turbinate: long‐term results in 382 patients randomly assigned to therapy. Ann Otol Rhinol Laryngol. 1999;108(6):569–575. https://doi.org/10.1177/000348949910800608
- 2308. Zagólski O. Skuteczność mukotomii przy uzyciu pesety bipolarnej w leczeniu spływania do gardła u chorych z przewlekłym niezytem nosa–doniesienie wstepne [Effectiveness of bipolar coagulation in treatment of post‐nasal drip in patients with chronic rhinitis–preliminary report]. Przegl Lek. 2007;64(1):9–11.
- 2309. Tani T, Seno S, Hanamitsu M, Shimizu T. [Clinical effectiveness of coblation‐assisted inferior turbinoplasty]. Arerugi. 2008;57(8):1053–1060.
- 2310. Liu CM, Tan CD, Lee FP, Lin KN, Huang HM. Microdebrider‐assisted versus radiofrequency‐assisted inferior turbinoplasty. Laryngoscope. 2009;119(2):414–418. https://doi.org/10.1002/lary.20088
- 2311. Hytonen ML, Back LJ, Malmivaara AV, Roine RP. Radiofrequency thermal ablation for patients with nasal symptoms: a systematic review of effectiveness and complications. Eur Arch Otorhinolaryngol. 2009;266(8):1257–1266. https://doi.org/10.1007/s00405‐009‐0914‐y
- 2312. Iwasaki A, Tokano H, Kamiyama R, Suzuki Y, Kitamura K. A 24‐month‐follow‐up study of argon plasma coagulation of the inferior turbinate in patients with perennial nasal allergy. J Med Dent Sci. 2010;57(1):11–15.
- 2313. Zagolski O. Factors affecting outcome of inferior turbinate mucotomy in treatment of postnasal drip syndrome. Am J Rhinol Allergy. 2010;24(6):459–463. https://doi.org/10.2500/ajra.2010.24.3524
- 2314. Lin HC, Lin PW, Friedman M, et al. Long‐term results of radiofrequency turbinoplasty for allergic rhinitis refractory to medical therapy. Arch Otolaryngol Head Neck Surg. 2010;136(9):892–895. https://doi.org/10.1001/archoto.2010.135
- 2315. Simeon R, Soufflet B, Souchal Delacour I. Coblation turbinate reduction in childhood allergic rhinitis. Eur Ann Otorhinolaryngol Head Neck Dis. 2010;127(2):77–82. https://doi.org/10.1016/j.anorl.2010.04.004
- 2316. Gunhan K, Unlu H, Yuceturk AV, Songu M. Intranasal steroids or radiofrequency turbinoplasty in persistent allergic rhinitis: effects on quality of life and objective parameters. Eur Arch Otorhinolaryngol. 2011;268(6):845–850. https://doi.org/10.1007/s00405‐010‐1462‐1
- 2317. Di Rienzo Businco L, Di Rienzo Businco A, Ventura L, Laurino S, Lauriello M. Turbinoplasty with quantic molecular resonance in the treatment of persistent moderate‐severe allergic rhinitis: Comparative analysis of efficacy. Am J Rhinol Allergy. 2014;28(2):164–168. https://doi.org/10.2500/ajra.2014.28.3990
- 2318. Garzaro M, Pezzoli M, Pecorari G, Landolfo V, Defilippi S, Giordano C. Radiofrequency inferior turbinate reduction: an evaluation of olfactory and respiratory function. Otolaryngol Head Neck Surg. 2010;143(3):348–352. https://doi.org/10.1016/j.otohns.2010.06.908
- 2319. Banhiran W, Tantilipikorn P, Metheetrairut C, Assanasen P, Bunnag C. Quality of life in patients with chronic rhinitis after radiofrequency inferior turbinate reduction. J Med Assoc Thai. 2010;93(8):950–960.
- 2320. Deenadayal DS, Kumar MN, Sudhakshin P, Hameed S. Radiofrequency reduction of inferior turbinates in allergic and non allergic rhinitis. Indian J Otolaryngol Head Neck Surg. 2014;66(suppl 1):231–236. https://doi.org/10.1007/s12070‐011‐0445‐x
- 2321. Ungkhara G, Purpermpulsiri P. Acoustic rhinometry evaluation in allergic rhinitis patients before and after turbinate radiofrequency ablation. J Med Assoc Thai. 2011;94(2):200–204.
- 2322. Parida PK, Santhosh K, Ganesan S, Surianarayanan G, Saxena SK. The efficacy of radiofrequency volumetric tissue reduction of hypertrophied inferior turbinate in allergic rhinitis. Indian J Med Sci. 2011;65(7):269–77.
- 2323. Lavinsky‐Wolff M, Camargo Jr HL, Barone CR, et al. Effect of turbinate surgery in rhinoseptoplasty on quality‐of‐life and acoustic rhinometry outcomes: a randomized clinical trial. Laryngoscope. 2013;123(1):82–89. https://doi.org/10.1002/lary.23628
- 2324. Garzaro M, Pezzoli M, Landolfo V, Defilippi S, Giordano C, Pecorari G. Radiofrequency inferior turbinate reduction: long‐term olfactory and functional outcomes. Otolaryngol Head Neck Surg. 2012;146(1):146–150. https://doi.org/10.1177/0194599811423008
- 2325. Incandela C, Calamusa G, Massenti MF, Incandela S, Speciale R, Amodio E. Long‐term efficacy of radiofrequency treatment of turbinate hypertrophy: a patient based point of view. Indian J Otolaryngol Head Neck Surg. 2013;65(suppl 2):226–230. https://doi.org/10.1007/s12070‐011‐0337‐0
- 2326. Fradis M, Malatskey S, Magamsa I, Golz A. Effect of submucosal diathermy in chronic nasal obstruction due to turbinate enlargement. Am J Otolaryngol. 2002;23(6):332–336. https://doi.org/10.1053/ajot.2002.126857
- 2327. Kojima Y, Tsuzuki K, Takebayashi H, Oka H, Sakagami M. Therapeutic evaluation of outpatient submucosal inferior turbinate surgery for patients with severe allergic rhinitis. Allergol Int. 2013;62(4):479–485. https://doi.org/10.2332/allergolint.12‐OA‐0533
- 2328. Bitar MA, Kanaan AA, Sinno S. Efficacy and safety of inferior turbinates coblation in children. J Laryngol Otol. 2014;128(suppl 2):S48–S54. https://doi.org/10.1017/S0022215114000206
- 2329. Akdag M, Dasdag S, Ozkurt FE, et al. Long‐term effect of radiofrequency turbinoplasty in nasal obstruction. Biotechnol Biotechnol Equip. 2014;28(2):285–294. https://doi.org/10.1080/13102818.2014.909083
- 2330. Assanasen P, Choochurn P, Banhiran W, Bunnag C. Radiofrequency inferior turbinate reduction improves smell ability of patients with chronic rhinitis and inferior turbinate hypertrophy. Allergy Rhinol (Providence). 2014;5(1):12–16. https://doi.org/10.2500/ar.2014.5.0077
- 2331. Acevedo JL, Camacho M, Brietzke SE. Radiofrequency Ablation turbinoplasty versus microdebrider‐assisted turbinoplasty: a systematic review and meta‐analysis. Otolaryngol Head Neck Surg. 2015;153(6):951–956. https://doi.org/10.1177/0194599815607211
- 2332. Arganbright JM, Jensen EL, Mattingly J, Gao D, Chan KH. Utility of inferior turbinoplasty for the treatment of nasal obstruction in children: a 10‐year review. JAMA Otolaryngol Head Neck Surg. 2015;141(10):901–904. https://doi.org/10.1001/jamaoto.2015.1560
- 2333. Shah AN, Brewster D, Mitzen K, Mullin D. Radiofrequency coblation versus intramural bipolar cautery for the treatment of inferior turbinate hypertrophy. Ann Otol Rhinol Laryngol. 2015;124(9):691–697. https://doi.org/10.1177/0003489415578709
- 2334. Banhiran W, Assanasen P, Tantilipikorn P, Nujchanart N, Voraprayoon S, Bunnag C. A randomized study of temperature‐controlled versus bipolar radiofrequency for inferior turbinate reduction. Eur Arch Otorhinolaryngol. 2015;272(10):2877–2884. https://doi.org/10.1007/s00405‐014‐3410‐y
- 2335. Sinno S, Mehta K, Lee ZH, Kidwai S, Saadeh PB, Lee MR. Inferior turbinate hypertrophy in rhinoplasty: systematic review of surgical techniques. Plast Reconstr Surg. 2016;138(3):419e–429e. https://doi.org/10.1097/PRS.0000000000002433
- 2336. Ishida H, Yoshida T, Hasegawa T, Mohri M, Amatsu M. Submucous electrocautery following submucous resection of turbinate bone – a rationale of surgical treatment for allergic rhinitis. Auris Nasus Larynx. 2003;30(2):147–152. https://doi.org/10.1016/s0385‐8146(03)00010‐5
- 2337. De Corso E, Bastanza G, Di Donfrancesco V, et al., Radiofrequency volumetric inferior turbinate reduction: long‐term clinical results. Acta Otorhinolaryngol Ital. 2016;36(3):199–205. doi: https://doi.org/10.14639/0392‐100X‐964
- 2338. Lukka VK, Jacob TM, Jeyaseelan V, Rupa V. Do turbinate reduction procedures restore epithelial integrity in patients with turbinate hypertrophy secondary to allergic rhinitis? A histopathological study. Eur Arch Otorhinolaryngol. 2018;275(6):1457–1467. https://doi.org/10.1007/s00405‐018‐4955‐y
- 2339. Turk B, Korkut AY, Kaya KS, et al. Results of radiofrequency ablation of inferior turbinate hypertrophy in patients with allergic and non‐allergic rhinitis. Sisli Etfal Hastan Tip Bul. 2018;52(4):296–301. https://doi.org/10.14744/SEMB.2018.77992
- 2340. Zhong B, Li LK, Deng D, et al. Effect of high‐intensity focused ultrasound versus plasma radiofrequency ablation on recurrent allergic rhinitis. Med Sci Monit. 2019;25:6775–6781. https://doi.org/10.12659/MSM.916228
- 2341. Kang T, Sung CM, Yang HC. Radiofrequency ablation of turbinates after septoplasty has no effect on allergic rhinitis symptoms other than nasal obstruction. Int Forum Allergy Rhinol. 2019;9(11):1257–1262. https://doi.org/10.1002/alr.22420
- 2342. Mun IK, Yoo SH, Mo JH. Long‐term outcome of concurrent coblator turbinoplasty with adenotonsillectomy in children with allergic rhinitis. Acta Otolaryngol. 2021;141(3):286–292. https://doi.org/10.1080/00016489.2020.1846782
- 2343. Pecorari G, Riva G, Bartoli C, et al. Nasal cytology in radiofrequency turbinate volume reduction. ORL J Otorhinolaryngol Relat Spec. 2021;83(4):252–257. https://doi.org/10.1159/000513629
- 2344. Whelan RL, Shaffer AD, Stapleton AL. Efficacy of inferior turbinate reduction in pediatric patients: a prospective analysis. Int Forum Allergy Rhinol. 2021;11(12):1654–1662. https://doi.org/10.1002/alr.22849
- 2345. Ogino‐Nishimura E, Okamura HO, Takiguchi Y. Argon plasma coagulation for intractable nasal obstruction occurring in patients with allergic rhinitis. Fukushima J Med Sci. 2003;49(1):15–22. https://doi.org/10.5387/fms.49.15
- 2346. Lin HC, Lin PW, Su CY, Chang HW. Radiofrequency for the treatment of allergic rhinitis refractory to medical therapy. Laryngoscope. 2003;113(4):673–678. https://doi.org/10.1097/00005537‐200304000‐00017
- 2347. Tokano H, Maehara H, Nakamura H, Makino N, Iwasaki A, Kitamura K. Short‐term effect of argon plasma coagulation of the inferior turbinate in patients with perennial nasal allergy. Auris Nasus Larynx. 2005;32(2):145–50. https://doi.org/10.1016/j.anl.2005.01.002
- 2348. Fang CX, Zhen SS. [Nasal endoscopy combined with multiple radiofrequency for perennial allergic rhinitis]. Di Yi Jun Yi Da Xue Xue Bao. 2005;25(7):876–877.
- 2349. Ding H, Liu J, Wang T, Xia G, Liu W. [Combination application of radiofrequency ablation in nasal operation]. Lin Chuang Er Bi Yan Hou Ke Za Zhi. 2005;19(20):918–919. 922.
- 2350. Quine SM, Aitken PM, Eccles R. Effect of submucosal diathermy to the inferior turbinates on unilateral and total nasal airflow in patients with rhinitis. Acta Otolaryngol. 1999;119(8):911–915. https://doi.org/10.1080/00016489950180270
- 2351. Sroka R, Janda P, Killian T, Vaz F, Betz CS, Leunig A. Comparison of long term results after Ho:YAG and diode laser treatment of hyperplastic inferior nasal turbinates. Lasers Surg Med. 2007;39(4):324–331. https://doi.org/10.1002/lsm.20479
- 2352. Chusakul S, Choktaweekarn T, Snidvongs K, Phannaso C, Aeumjaturapat S. Effect of the KTP laser in inferior turbinate surgery on eosinophil influx in allergic rhinitis. Otolaryngol Head Neck Surg. 2011;144(2):237–240. https://doi.org/10.1177/0194599810390448
- 2353. Caffier PP, Scherer H, Neumann K, Luck S, Enzmann H, Haisch A. Diode laser treatment in therapy‐resistant allergic rhinitis: impact on nasal obstruction and associated symptoms. Lasers Med Sci. 2011;26(1):57–67. https://doi.org/10.1007/s10103‐010‐0813‐x
- 2354. Gupta P, Kc T, Regmi D. Diode laser turbinate reduction in allergic rhinitis: a cross‐sectional study. JNMA J Nepal Med Assoc. 2018;56(214):949–952.
- 2355. Sasaki K, Ohshiro T, Sakio R, et al. Efficacy of seasonal allergic rhinitis using an 810 nm diode laser system. Laser Ther. 2019;28(1):11–18. https://doi.org/10.5978/islsm.28_19‐OR‐01
- 2356. Tanigawa T, Yashiki T, Hayashi K, Sato T. Carbon dioxide laser vaporization for turbinate: optimal conditions and indications. Auris Nasus Larynx. 2000;27(2):137–140. https://doi.org/10.1016/s0385‐8146(99)00061‐9
- 2357. Kunachak S, Kulapaditharom B, Prakunhungsit S. Minimally invasive KTP laser treatment of perennial allergic rhinitis: a preliminary report. J Otolaryngol. 2000;29(3):139–143.
- 2358. Janda P, Sroka R, Betz CS, Grevers G, Leunig A. [Ho:YAG and diode laser treatment of hyperplastic inferior nasal turbinates]. Die Laserkonchotomie mit Ho:YAG‐ und Dioden‐Laser zur Behandlung von hyperplastischen Nasenmuscheln. Laryngorhinootologie. 2002;81(7):484–490. https://doi.org/10.1055/s‐2002‐33288
- 2359. Janda P, Sroka R, Tauber S, Baumgartner R, Grevers G, Leunig A. Diode laser treatment of hyperplastic inferior nasal turbinates. Lasers Surg Med. 2000;27(2):129–39. https://doi.org/10.1002/1096‐9101(2000)27:2<129::aid‐lsm4>3.0.co;2‐r
- 2360. Takeno S, Osada R, Ishino T, Yajin K. Laser surgery of the inferior turbinate for allergic rhinitis with seasonal exacerbation: an acoustic rhinometry study. Ann Otol Rhinol Laryngol. 2003;112(5):455–460. https://doi.org/10.1177/000348940311200513
- 2361. Imamura S, Honda H. Carbon dioxide laser vaporization of the inferior turbinate for allergic rhinitis: short‐term results. Ann Otol Rhinol Laryngol. 2003;112(12):1043–1049. https://doi.org/10.1177/000348940311201209
- 2362. Sandhu AS, Temple RH, Timms MS. Partial laser turbinectomy: two year outcomes in patients with allergic and non‐allergic rhinitis. Rhinology. 2004;42(2):81–84.
- 2363. Takeno S, Osada R, Furukido K, Yajin K. Analysis of local cytokine gene expression in patients with allergic rhinitis treated with CO2 laser surgery. Laryngoscope. 2000;110(11):1968–1974. https://doi.org/10.1097/00005537‐200011000‐00038
- 2364. Lee JY. Efficacy of intra‐ and extraturbinal microdebrider turbinoplasty in perennial allergic rhinitis. Laryngoscope. 2013;123(12):2945–9. https://doi.org/10.1002/lary.24215
- 2365. Parthasarathi K, Christensen JM, Alvarado R, Barham HP, Sacks R, Harvey RJ. Airflow and symptom outcomes between allergic and non‐allergic rhinitis patients from turbinoplasty. Rhinology. 2017;55(4):332–338. https://doi.org/10.4193/Rhin16.210
- 2366. Ikeda K, Oshima T, Suzuki M, Suzuki H, Shimomura A. Functional inferior turbinosurgery (FITS) for the treatment of resistant chronic rhinitis. Acta Otolaryngol. 2006;126(7):739–745. https://doi.org/10.1080/00016480500472853
- 2367. Huang TW, Cheng PW. Changes in nasal resistance and quality of life after endoscopic microdebrider‐assisted inferior turbinoplasty in patients with perennial allergic rhinitis. Arch Otolaryngol Head Neck Surg. 2006;132(9):990–993. https://doi.org/10.1001/archotol.132.9.990
- 2368. Wu CC, Lee SY, Hsu CJ, Yeh TH. Patients with positive allergen test have less favorable outcome after endoscopic microdebrider‐assisted inferior turbinoplasty. Am J Rhinol. 2008;22(1):20–23. https://doi.org/10.2500/ajr.2008.22.3116
- 2369. Chen YL, Tan CT, Huang HM. Long‐term efficacy of microdebrider‐assisted inferior turbinoplasty with lateralization for hypertrophic inferior turbinates in patients with perennial allergic rhinitis. Laryngoscope. 2008;118(7):1270–1274. https://doi.org/10.1097/MLG.0b013e31816d728e
- 2370. Neri G, Mastronardi V, Traini T, D'Orazio F, Pugliese M, Cazzato F. Respecting nasal mucosa during turbinate surgery: end of the dogma? Rhinology. 2013;51(4):368–375. https://doi.org/10.4193/Rhino12.124
- 2371. de Moura BH, Migliavacca RO, Lima RK, et al. Partial inferior turbinectomy in rhinoseptoplasty has no effect in quality‐of‐life outcomes: a randomized clinical trial. Laryngoscope. 2018;128(1):57–63. https://doi.org/10.1002/lary.26831
- 2372. Suzuki M, Yokota M, Ozaki S, Murakami S. The effects of resection of the peripheral branches of the posterior nasal nerves in the inferior turbinate, with special focus on olfactory dysfunction. J Laryngol Otol. 2019;133(12):1046–1049. https://doi.org/10.1017/S0022215119002238
- 2373. Mori S, Fujieda S, Yamada T, Kimura Y, Takahashi N, Saito H. Long‐term effect of submucous turbinectomy in patients with perennial allergic rhinitis. Laryngoscope. 2002;112(5):865–869. https://doi.org/10.1097/00005537‐200205000‐00016
- 2374. Ogawa T, Takeno S, Ishino T, Hirakawa K. Submucous turbinectomy combined with posterior nasal neurectomy in the management of severe allergic rhinitis: clinical outcomes and local cytokine changes. Auris Nasus Larynx. 2007;34(3):319–326. https://doi.org/10.1016/j.anl.2007.01.008
- 2375. Tan G, Ma Y, Li H, Li W, Wang J. Long‐term results of bilateral endoscopic vidian neurectomy in the management of moderate to severe persistent allergic rhinitis. Arch Otolaryngol Head Neck Surg. 2012;138(5):492–497. https://doi.org/10.1001/archoto.2012.284
- 2376. Hamerschmidt R, Hamerschmidt R, Moreira AT, Tenorio SB, Timi JR. Comparison of turbinoplasty surgery efficacy in patients with and without allergic rhinitis. Braz J Otorhinolaryngol. 2016;82(2):131–139. https://doi.org/10.1016/j.bjorl.2015.10.010
- 2377. Piromchai P, Pornumnouy W, Saeseow P, Chainansamit S. The minimum effective dose of abobotulinum toxin A injection for allergic rhinitis: a dose‐escalation randomized controlled trial. Laryngoscope Investig Otolaryngol. 2021;6(1):6–12. https://doi.org/10.1002/lio2.499
- 2378. Abtahi SM, Hashemi SM, Abtahi SH, Bastani B. Septal injection in comparison with inferior turbinates injection of botulinum toxin A in patients with allergic rhinitis. J Res Med Sci. 2013;18(5):400–404.
- 2379. Unal M, Sevim S, Dogu O, Vayisoglu Y, Kanik A. Effect of botulinum toxin type A on nasal symptoms in patients with allergic rhinitis: a double‐blind, placebo‐controlled clinical trial. Acta Otolaryngol. 2003;123(9):1060–1063. https://doi.org/10.1080/00016480310000755
- 2380. Wei H, Zhang Y, Shi L, et al. Higher dosage of HIFU treatment may lead to higher and longer efficacy for moderate to severe perennial allergic rhinitis. Int J Med Sci. 2013;10(13):1914–1920. https://doi.org/10.7150/ijms.7117
- 2381. Wei H, Shi L, Zhang J, et al. High‐intensity focused ultrasound leads to histopathologic changes of the inferior turbinate mucosa with allergic inflammation. Ultrasound Med Biol. 2014;40(10):2425–2430. https://doi.org/10.1016/j.ultrasmedbio.2014.05.016
- 2382. Ibrahim N, Tyler MA, Borchard NA, Rathor A, Nayak JV. Nasal vestibular body treatment for recalcitrant nasal obstruction. Int Forum Allergy Rhinol. 2020;10(3):388–394. https://doi.org/10.1002/alr.22463
- 2383. Kim SJ, Kim HT, Park YH, Kim JY, Bae JH. Coblation nasal septal swell body reduction for treatment of nasal obstruction: a preliminary report. Eur Arch Otorhinolaryngol. 2016;273(9):2575–2578. https://doi.org/10.1007/s00405‐016‐3946‐0
- 2384. Karpishchenko S, Ulupov M, Gindryuk A , Kaplun D. Using thermal effect of 970 nm diode laser to reduce nasal swell body. Am J Otolaryngol. 2021;42(6):103165. https://doi.org/10.1016/j.amjoto.2021.103165
- 2385. Catalano P, Ashmead MG, Carlson D. Radiofrequency ablation of septal swell body. Ann Otolaryngol Rhinol. 2015;2(11):1069.
- 2386. Yu MS, Kim JY, Kim BH, Kang SH, Lim DJ. Feasibility of septal body volume reduction for patients with nasal obstruction. Laryngoscope. 2015;125(7):1523–1528. https://doi.org/10.1002/lary.25154
- 2387. Lai WS, Cheng SY, Lin YY, et al. Clinical assessment of diode laser‐assisted endoscopic intrasphenoidal vidian neurectomy in the treatment of refractory rhinitis. Lasers Med Sci. 2017;32(9):2097–2104. https://doi.org/10.1007/s10103‐017‐2330‐7
- 2388. Su WF, Liu SC, Chiu FS, Lee CH. Antegrade transsphenoidal vidian neurectomy: short‐term surgical outcome analysis. Am J Rhinol Allergy. 2011;25(6):e217–e220. https://doi.org/10.2500/ajra.2011.25.3704
- 2389. Ai J, Xie Z, Qing X, et al. Clinical effect of endoscopic vidian neurectomy on bronchial asthma outcomes in patients with coexisting refractory allergic rhinitis and asthma. Am J Rhinol Allergy. 2018;32(3):139–146. https://doi.org/10.1177/1945892418764964
- 2390. Shen L, Wang J, Kang X, et al. Clinical efficacy and possible mechanism of endoscopic vidian neurectomy for house dust mite‐sensitive allergic rhinitis. ORL J Otorhinolaryngol Relat Spec. 2021;83(2):75–84. https://doi.org/10.1159/000511711
- 2391. Maimaitiaili G, Kahaer K, Tang L, Zhang J. The effect of vidian neurectomy on pulmonary function in patients with allergic rhinitis and chronic rhinosinusitis with nasal polyps. Am J Med Sci. 2020;360(2):137–145. https://doi.org/10.1016/j.amjms.2020.04.024
- 2392. Qi Y, Liu J, Peng S, Hou S, Zhang M, Wang Z. Efficacy of selective vidian neurectomy for allergic rhinitis combined with chronic rhinosinusitis. ORL J Otorhinolaryngol Relat Spec. 2021;83(5):327–334. https://doi.org/10.1159/000512083
- 2393. Konno A. Historical, pathophysiological, and therapeutic aspects of vidian neurectomy. Curr Allergy Asthma Rep. 2010;10(2):105–112. https://doi.org/10.1007/s11882‐010‐0093‐3
- 2394. Bleier BS, Schlosser RJ. Endoscopic anatomy of the postganglionic pterygopalatine innervation of the posterolateral nasal mucosa. Int Forum Allergy Rhinol. 2011;1(2):113–117. https://doi.org/10.1002/alr.20011
- 2395. Wang EW, Gardner PA, Fraser S, Stefko ST, Fernandez‐Miranda JC, Snyderman CH. Reduced tearing with stable quality of life after vidian neurectomy: a prospective controlled trial. Laryngoscope. 2021;131(7):1487–1491. https://doi.org/10.1002/lary.29287
- 2396. Makihara S, Okano M, Miyamoto S, et al. Underwater posterior nasal neurectomy compared to resection of peripheral branches of posterior nerve in severe allergic rhinitis. Acta Otolaryngol. 2021;141(8):780–785. https://doi.org/10.1080/00016489.2021.1925151
- 2397. Hua H, Wang G, Zhao Y, Wang D, Qiu Z, Fang P. The long‐term outcomes of posterior nasal neurectomy with or without pharyngeal neurectomy in patients with allergic rhinitis: a randomized controlled trial. Braz J Otorhinolaryngol. 2022;88(Suppl 1):S147–S155. https://doi.org/10.1016/j.bjorl.2021.05.006
- 2398. Wang L, Chen M, Xu M. Effect of posterior nasal neurectomy on the suppression of allergic rhinitis. Am J Otolaryngol. 2020;41(3):102410. https://doi.org/10.1016/j.amjoto.2020.102410
- 2399. Li S, Cheng J, Yang J, et al. Efficacy of posterior nasal neurectomy for allergic rhinitis combined with chronic rhinosinusitis with nasal polyps. Acta Otolaryngol. 2019;139(10):890–894. https://doi.org/10.1080/00016489.2019.1654132
- 2400. Takahara D, Takeno S, Hamamoto T, Ishino T, Hirakawa K. Management of intractable nasal hyperreactivity by selective resection of posterior nasal nerve branches. Int J Otolaryngol. 2017;2017:1907862. https://doi.org/10.1155/2017/1907862
- 2401. Ogi K, Manabe Y, Mori S, et al. Long‐term effect of combined submucous turbinectomy and posterior nasal neurectomy in patients with allergic rhinitis. SN Comp Clin Med. 2019;1:540–546.
- 2402. Albu S, Trombitas V, Nagy A. Endoscopic microdebrider‐assisted inferior turbinoplasty with and without posterior nasal neurectomy. Auris Nasus Larynx. 2014;41(3):273–277. https://doi.org/10.1016/j.anl.2013.10.018
- 2403. Terao A, Meshitsuka K, Suzaki H, Fukuda S. Cryosurgery on postganglionic fibers (posterior nasal branches) of the pterygopalatine ganglion for vasomotor rhinitis. Acta Otolaryngol. 1983;96(1‐2):139–148. https://doi.org/10.3109/00016488309132884
- 2404. M Yen D, B Conley D, O'Malley EM, Byerly TA, Johnson J. Multiple site cryoablation treatment of the posterior nasal nerve for treatment of chronic rhinitis: an observational feasibility study. Allergy Rhinol (Providence). 2020;11:2152656720946996. https://doi.org/10.1177/2152656720946996
- 2405. Del Signore AG, Greene JB, Russell JL, Yen DM, O'Malley EM, Schlosser RJ. Cryotherapy for treatment of chronic rhinitis: 3‐month outcomes of a randomized, sham‐controlled trial. Int Forum Allergy Rhinol. 2022;12(1):51–61. https://doi.org/10.1002/alr.22868
- 2406. Ow RA, O'Malley EM, Han JK, Lam KK, Yen DM. Cryosurgical ablation for treatment of rhinitis: two‐year results of a prospective multicenter study. Laryngoscope. 2021;131(9):1952–1957. https://doi.org/10.1002/lary.29453
- 2407. Gerka Stuyt JA, Luk L, Keschner D, Garg R. Evaluation of in‐office cryoablation of posterior nasal nerves for the treatment of rhinitis. Allergy Rhinol (Providence). 2021;12:2152656720988565. https://doi.org/10.1177/2152656720988565
- 2408. Chang MT, Song S, Hwang PH. Cryosurgical ablation for treatment of rhinitis: a prospective multicenter study. Laryngoscope. 2020;130(8):1877–1884. https://doi.org/10.1002/lary.28301
- 2409. Yoo F, Kuan EC, Batra PS, Chan CK, Tajudeen BA, Craig JR. Predictors of rhinorrhea response after posterior nasal nerve cryoablation for chronic rhinitis. Int Forum Allergy Rhinol. 2020;10(7):913–919. https://doi.org/10.1002/alr.22574
- 2410. Ehmer D, McDuffie CM, Scurry Jr WC, et al. Temperature‐controlled radiofrequency neurolysis for the treatment of rhinitis. Am J Rhinol Allergy. 2022;36(1):149–156. https://doi.org/10.1177/19458924211033400
- 2411. Stolovitzky JP, Ow RA, Silvers SL, Bikhazi NB, Johnson CD, Takashima M. Effect of radiofrequency neurolysis on the symptoms of chronic rhinitis: a randomized controlled trial. OTO Open. 2021;5(3):2473974X211041124. https://doi.org/10.1177/2473974X211041124
- 2412. Krespi YP, Wilson KA, Kizhner V. Laser ablation of posterior nasal nerves for rhinitis. Am J Otolaryngol. 2020;41(3):102396. https://doi.org/10.1016/j.amjoto.2020.102396
- 2413. Singh AK, Kasle DA, Torabi SJ, Manes RP. Adverse events associated with ClariFix posterior nasal nerve cryoablation: a MAUDE database analysis. Otolaryngol Head Neck Surg. 2021;165(4):597–601. https://doi.org/10.1177/0194599820986581
- 2414. Jose J, Coatesworth AP. Inferior turbinate surgery for nasal obstruction in allergic rhinitis after failed medical treatment. Cochrane Database Syst Rev. 2010;(12):CD005235. https://doi.org/10.1002/14651858.CD005235.pub2
- 2415. Langille M, El‐Hakim H. Pediatric inferior turbinoplasty with or without adenoidectomy: preliminary report on improvement of quality of life, symptom control, and safety. J Otolaryngol Head Neck Surg. 2011;40(5):420–426.
- 2416. Di Rienzo Businco L, Di Rienzo Businco A, Lauriello M. Comparative study on the effectiveness of Coblation‐assisted turbinoplasty in allergic rhinitis. Rhinology. 2010;48(2):174–178. https://doi.org/10.4193/Rhin09.149
- 2417. Kobayashi T, Hyodo M, Nakamura K, Komobuchi H, Honda N. Resection of peripheral branches of the posterior nasal nerve compared to conventional posterior neurectomy in severe allergic rhinitis. Auris Nasus Larynx. 2012;39(6):593–596. https://doi.org/10.1016/j.anl.2011.11.006
- 2418. Roberts G, Pfaar O, Akdis CA, et al. EAACI Guidelines on allergen immunotherapy: allergic rhinoconjunctivitis. Allergy. 2018;73(4):765–798. https://doi.org/10.1111/all.13317
- 2419. Cox L, Nelson H, Lockey R, et al. Allergen immunotherapy: a practice parameter third update. J Allergy Clin Immunol. 2011;127(1 suppl):S1–S55. https://doi.org/10.1016/j.jaci.2010.09.034
- 2420. Matricardi PM, Kuna P, Panetta V, Wahn U, Narkus A. Subcutaneous immunotherapy and pharmacotherapy in seasonal allergic rhinitis: a comparison based on meta‐analyses. J Allergy Clin Immunol. 2011;128(4):791–799.e6. https://doi.org/10.1016/j.jaci.2011.03.049
- 2421. Radulovic S, Calderon MA, Wilson D, Durham S. Sublingual immunotherapy for allergic rhinitis. Cochrane Database Syst Rev. 2010;(12):CD002893. https://doi.org/10.1002/14651858.CD002893.pub2
- 2422. Tie K, Miller C, Zanation AM, Ebert Jr CS. Subcutaneous versus sublingual immunotherapy for adults with allergic rhinitis: a systematic review with meta‐analyses. Laryngoscope. 2022;132(3):499–508. https://doi.org/10.1002/lary.29586
- 2423. Durham SR, Walker SM, Varga EM, et al. Long‐term clinical efficacy of grass‐pollen immunotherapy. N Engl J Med. 1999;341(7):468–475. https://doi.org/10.1056/NEJM199908123410702
- 2424. Ebner C, Kraft D, Ebner H. Booster immunotherapy (BIT). Allergy. 1994;49(1):38–42. https://doi.org/10.1111/j.1398‐9995.1994.tb00771.x
- 2425. Arroabarren E, Tabar AI, Echechipia S, Cambra K, Garcia BE, Alvarez‐Puebla MJ. Optimal duration of allergen immunotherapy in children with dust mite respiratory allergy. Pediatr Allergy Immunol. 2015;26(1):34–41. https://doi.org/10.1111/pai.12296
- 2426. Kristiansen M, Dhami S, Netuveli G, et al. Allergen immunotherapy for the prevention of allergy: a systematic review and meta‐analysis. Pediatr Allergy Immunol. 2017;28(1):18–29. https://doi.org/10.1111/pai.12661
- 2427. Schmitt J, Schwarz K, Stadler E, Wustenberg EG. Allergy immunotherapy for allergic rhinitis effectively prevents asthma: results from a large retrospective cohort study. J Allergy Clin Immunol. 2015;136(6):1511–1516. https://doi.org/10.1016/j.jaci.2015.07.038
- 2428. Jacobsen L, Niggemann B, Dreborg S, et al. Specific immunotherapy has long‐term preventive effect of seasonal and perennial asthma: 10‐year follow‐up on the PAT study. Allergy. 2007;62(8):943–948. https://doi.org/10.1111/j.1398‐9995.2007.01451.x
- 2429. Valovirta E, Petersen TH, Piotrowska T, et al. Results from the 5‐year SQ grass sublingual immunotherapy tablet asthma prevention (GAP) trial in children with grass pollen allergy. J Allergy Clin Immunol. 2018;141(2):529–538.e13. https://doi.org/10.1016/j.jaci.2017.06.014
- 2430. Fiocchi A, Pajno G, La Grutta S, et al. Safety of sublingual‐swallow immunotherapy in children aged 3 to 7 years. Ann Allergy Asthma Immunol. 2005;95(3):254–258. https://doi.org/10.1016/S1081‐1206(10)61222‐7
- 2431. Agostinis F, Tellarini L, Canonica GW, Falagiani P, Passalacqua G. Safety of sublingual immunotherapy with a monomeric allergoid in very young children. Allergy. 2005;60(1):133. https://doi.org/10.1111/j.1398‐9995.2004.00616.x
- 2432. Darsow U. Allergen‐specific immunotherapy for atopic eczema: updated. Curr Opin Allergy Clin Immunol. 2012;12(6):665–669. https://doi.org/10.1097/ACI.0b013e3283588cf4
- 2433. Passalacqua G, Canonica GW. Allergen immunotherapy: history and future developments. Immunol Allergy Clin North Am. 2016;36(1):1–12. https://doi.org/10.1016/j.iac.2015.08.001
- 2434. Greenhawt M, Oppenheimer J, Nelson M, et al. Sublingual immunotherapy: a focused allergen immunotherapy practice parameter update. Ann Allergy Asthma Immunol. 2017;118(3):276–282.e2. https://doi.org/10.1016/j.anai.2016.12.009
- 2435. Akdis M, Akdis CA. Mechanisms of allergen‐specific immunotherapy: multiple suppressor factors at work in immune tolerance to allergens. J Allergy Clin Immunol. 2014;133(3):621–631. https://doi.org/10.1016/j.jaci.2013.12.1088
- 2436. Nelson HS, Makatsori M, Calderon MA. Subcutaneous immunotherapy and sublingual immunotherapy: comparative efficacy, current and potential indications, and warnings – United States Versus Europe. Immunol Allergy Clin North Am. 2016;36(1):13–24. https://doi.org/10.1016/j.iac.2015.08.005
- 2437. Lawrence MG, Steinke JW, Borish L. Basic science for the clinician: mechanisms of sublingual and subcutaneous immunotherapy. Ann Allergy Asthma Immunol. 2016;117(2):138–142. https://doi.org/10.1016/j.anai.2016.06.027
- 2438. Dhami S, Nurmatov U, Arasi S, et al. Allergen immunotherapy for allergic rhinoconjunctivitis: a systematic review and meta‐analysis. Allergy. 2017;72(11):1597–1631. https://doi.org/10.1111/all.13201
- 2439. Nurmatov U, Dhami S, Arasi S, et al. Allergen immunotherapy for allergic rhinoconjunctivitis: a systematic overview of systematic reviews. Clin Transl Allergy. 2017;7:24. https://doi.org/10.1186/s13601‐017‐0159‐6
- 2440. Lin SY, Erekosima N, Suarez‐Cuervo C, et al. Agency for Healthcare Research and Quality Comparative Effectiveness Reviews. Allergen‐Specific Immunotherapy for the Treatment of Allergic Rhinoconjunctivitis and/or Asthma: Comparative Effectiveness Review. 2013.
- 2441. Epstein TG, Murphy‐Berendts K, Liss GM, Bernstein DI. Risk factors for fatal and nonfatal reactions to immunotherapy (2008‐2018): postinjection monitoring and severe asthma. Ann Allergy Asthma Immunol. 2021;127(1):64–69.e1. https://doi.org/10.1016/j.anai.2021.03.011
- 2442. Bernstein DI, Wanner M, Borish L, Liss GM, Immunotherapy Committee, American Academy of Allergy, Asthma and Immunology . Twelve‐year survey of fatal reactions to allergen injections and skin testing: 1990‐2001. J Allergy Clin Immunol. 2004;113(6):1129–1136. https://doi.org/10.1016/j.jaci.2004.02.006
- 2443. Epstein TG, Liss GM, Murphy‐Berendts K, Bernstein DI. AAAAI and ACAAI surveillance study of subcutaneous immunotherapy, Year 3: what practices modify the risk of systemic reactions? Ann Allergy Asthma Immunol. 2013;110(4):274–278, 278.e1. https://doi.org/10.1016/j.anai.2013.01.015
- 2444. Lee S, Hess EP, Nestler DM, et al. Antihypertensive medication use is associated with increased organ system involvement and hospitalization in emergency department patients with anaphylaxis. J Allergy Clin Immunol. 2013;131(4):1103–1108. https://doi.org/10.1016/j.jaci.2013.01.011
- 2445. Muller UR, Haeberli G. Use of beta‐blockers during immunotherapy for Hymenoptera venom allergy. J Allergy Clin Immunol. 2005;115(3):606–610. https://doi.org/10.1016/j.jaci.2004.11.012
- 2446. Pitsios C, Tsoumani M, Bilo MB, et al. Contraindications to immunotherapy: a global approach. Clin Transl Allergy. 2019;9:45. https://doi.org/10.1186/s13601‐019‐0285‐4
- 2447. Wohrl S, Kinaciyan T, Jalili A, Stingl G, Moritz KB. Malignancy and specific allergen immunotherapy: the results of a case series. Int Arch Allergy Immunol. 2011;156(3):313–319. https://doi.org/10.1159/000323519
- 2448. Linneberg A, Jacobsen RK, Jespersen L, Abildstrom SZ. Association of subcutaneous allergen‐specific immunotherapy with incidence of autoimmune disease, ischemic heart disease, and mortality. J Allergy Clin Immunol. 2012;129(2):413–419. https://doi.org/10.1016/j.jaci.2011.09.007
- 2449. Bozek A, Kolodziejczyk K, Bednarski P. The relationship between autoimmunity and specific immunotherapy for allergic diseases. Hum Vaccin Immunother. 2015;11(12):2764–2768. https://doi.org/10.1080/21645515.2015.1087627
- 2450. Shaikh WA. A retrospective study on the safety of immunotherapy in pregnancy. Clin Exp Allergy. 1993;23(10):857–860. https://doi.org/10.1111/j.1365‐2222.1993.tb00264.x
- 2451. Shaikh WA, Shaikh SW. A prospective study on the safety of sublingual immunotherapy in pregnancy. Allergy. 2012;67(6):741–743. https://doi.org/10.1111/j.1398‐9995.2012.02815.x
- 2452. Odactra House Dust Mite . (Dermatophagoides farinae and Dermatophagoides pteronyssinus) allergen extract tablet for sublingual use. Accessed October 8, 2021. fda.gov/media/103380/download
- 2453. ORALAIR . (Sweet Vernal, Orchard, Perennial Rye, Timothy, and Kentucky Blue Grass Mixed Pollens Allergen Extract) tablet for sublingual use. Accessed July 3, 2022. https://www.fda.gov/media/87935/download
- 2454. Grastek Timothy grass pollen extract tablet for sublingual use. Accessed July 3, 2022. https://www.fda.gov/media/88510/download
- 2455. Ragwitek short ragweed pollen extract tablet for sublingual use. Accessed July 3, 2022. https://www.fda.gov/media/88712/download
- 2456. Osguthorpe JD. The evolution of understanding inhalant allergy. Otolaryngol Clin North Am. 2011;44(3):519–535, vii. https://doi.org/10.1016/j.otc.2011.03.008
- 2457. Noon L. Prophylactic inoculation against hayfever. Lancet. 1911;1:1572–1573.
- 2458. Mason WW, Ward WA. Standardized extracts. Otolaryngol Clin North Am. 1992;25(1):101–17.
- 2459. Zimmer J, Vieths S, Kaul S. Standardization and regulation of allergen products in the European Union. Curr Allergy Asthma Rep. 2016;16(3):21. https://doi.org/10.1007/s11882‐016‐0599‐4
- 2460. Carnes J, Iraola V, Gallego M, Leonor JR. Control process for manufacturing and standardization of allergenic molecules. Curr Allergy Asthma Rep. 2015;15(7):37. https://doi.org/10.1007/s11882‐015‐0541‐1
- 2461. Zimmer J, Bridgewater J, Ferreira F, van Ree R, Rabin RL, Vieths S. The history, present and future of allergen standardization in the United States and Europe. Front Immunol. 2021;12:725831. https://doi.org/10.3389/fimmu.2021.725831
- 2462. Park KH, Son M, Choi SY, et al. In vitro evaluation of allergen potencies of commercial house dust mite sublingual immunotherapy reagents. Allergy Asthma Immunol Res. 2015;7(2):124–129. https://doi.org/10.4168/aair.2015.7.2.124
- 2463. Thomsen GF, Schlunssen V, Skadhauge LR, et al. Are allergen batch differences and the use of double skin prick test important? BMC Pulm Med. 2015;15:33. https://doi.org/10.1186/s12890‐015‐0021‐3
- 2464. Slater JE. Standardized allergen extracts in the United States. Clin Allergy Immunol. 2004;18:421–432.
- 2465. Jutel M, Agache I, Bonini S, et al. International consensus on allergen immunotherapy II: mechanisms, standardization, and pharmacoeconomics. J Allergy Clin Immunol. 2016;137(2):358–368. https://doi.org/10.1016/j.jaci.2015.12.1300
- 2466. Zakzuk J, Kilimajer J, Lockey R. World Allergy Organization Allergen Standardization and Characterization. Updated January 2019. Accessed June 3, 2022. https://www.worldallergy.org/education‐and‐programs/education/allergic‐disease‐resource‐center/professionals/allergen‐standardization‐and‐characterization
- 2467. van Ree R, Chapman MD, Ferreira F, et al. The CREATE project: development of certified reference materials for allergenic products and validation of methods for their quantification. Allergy. 2008;63(3):310–326. https://doi.org/10.1111/j.1398‐9995.2007.01612.x
- 2468. Petrovsky N. Comparative Safety of vaccine adjuvants: a summary of current evidence and future needs. Drug Saf. 2015;38(11):1059–1074. https://doi.org/10.1007/s40264‐015‐0350‐4
- 2469. Gunawardana NC, Durham SR. New approaches to allergen immunotherapy. Ann Allergy Asthma Immunol. 2018;121(3):293–305. https://doi.org/10.1016/j.anai.2018.07.014
- 2470. Leuthard DS, Duda A, Freiberger SN, et al. Microcrystalline tyrosine and aluminum as adjuvants in allergen‐specific immunotherapy protect from IgE‐mediated reactivity in mouse models and act independently of inflammasome and TLR signaling. J Immunol. 2018;200(9):3151–3159. https://doi.org/10.4049/jimmunol.1800035
- 2471. Zubeldia JM, Ferrer M, Davila I, Justicia JL. adjuvants in allergen‐specific immunotherapy: modulating and enhancing the immune response. J Investig Allergol Clin Immunol. 2019;29(2):103–111. https://doi.org/10.18176/jiaci.0349
- 2472. Johnson L, Duschl A, Himly M. Nanotechnology‐based vaccines for allergen‐specific immunotherapy: potentials and challenges of conventional and novel adjuvants under research. Vaccines (Basel). 2020;8(2):237. https://doi.org/10.3390/vaccines8020237
- 2473. Kirtland ME, Tsitoura DC, Durham SR, Shamji MH. Toll‐like receptor agonists as adjuvants for allergen immunotherapy. Front Immunol. 2020;11:599083. https://doi.org/10.3389/fimmu.2020.599083
- 2474. Feng Z, Yi X, Hajavi J. New and old adjuvants in allergen‐specific immunotherapy: with a focus on nanoparticles. J Cell Physiol. 2021;236(2):863–876. https://doi.org/10.1002/jcp.29941
- 2475. Norman PS, Lichtenstein LM. Comparisons of alum‐precipitated and unprecipitated aqueous ragweed pollen extracts in the treatment of hay fever. J Allergy Clin Immunol. 1978;61(6):384–389. https://doi.org/10.1016/0091‐6749(78)90118‐5
- 2476. Jensen‐Jarolim E, Bachmann MF, Bonini S, et al. State‐of‐the‐art in marketed adjuvants and formulations in allergen immunotherapy: a position paper of the European Academy of Allergy and Clinical Immunology (EAACI). Allergy. 2020;75(4):746–760. https://doi.org/10.1111/all.14134
- 2477. Corrigan CJ, Kettner J, Doemer C, Cromwell O, Narkus A, Study G. Efficacy and safety of preseasonal‐specific immunotherapy with an aluminium‐adsorbed six‐grass pollen allergoid. Allergy. 2005;60(6):801–807. https://doi.org/10.1111/j.1398‐9995.2005.00790.x
- 2478. Drachenberg KJ, Wheeler AW, Stuebner P, Horak F. A well‐tolerated grass pollen‐specific allergy vaccine containing a novel adjuvant, monophosphoryl lipid A, reduces allergic symptoms after only four preseasonal injections. Allergy. 2001;56(6):498–505. https://doi.org/10.1034/j.1398‐9995.2001.056006498.x
- 2479. Tulic MK, Fiset PO, Christodoulopoulos P, et al. Amb a 1‐immunostimulatory oligodeoxynucleotide conjugate immunotherapy decreases the nasal inflammatory response. J Allergy Clin Immunol. 2004;113(2):235–241. https://doi.org/10.1016/j.jaci.2003.11.001
- 2480. Torii Y, Ito T, Amakawa R, et al. Imidazoquinoline acts as immune adjuvant for functional alteration of thymic stromal lymphopoietin‐mediated allergic T cell response. J Immunol. 2008;181(8):5340–5349. https://doi.org/10.4049/jimmunol.181.8.5340
- 2481. Tversky JR, Bieneman AP, Chichester KL, Hamilton RG, Schroeder JT. Subcutaneous allergen immunotherapy restores human dendritic cell innate immune function. Clin Exp Allergy. 2010;40(1):94–102. https://doi.org/10.1111/j.1365‐2222.2009.03388.x
- 2482. Tversky JR, Le TV, Bieneman AP, Chichester KL, Hamilton RG, Schroeder JT. Human blood dendritic cells from allergic subjects have impaired capacity to produce interferon‐alpha via Toll‐like receptor 9. Clin Exp Allergy. 2008;38(5):781–788. https://doi.org/10.1111/j.1365‐2222.2008.02954.x
- 2483. Creticos PS, Schroeder JT, Hamilton RG, et al. Immunotherapy with a ragweed‐toll‐like receptor 9 agonist vaccine for allergic rhinitis. N Engl J Med. 2006;355(14):1445–1455. https://doi.org/10.1056/NEJMoa052916
- 2484. Busse W, Korenblat P, Nayak A, et al. Phase 2/3 study of the novel vaccine Amb a 1 immunostimulatory oligodeoxyriboneucleotide conjugate AIC in ragweed allergic adults. J Allergy Clin Immunol. 2006;117:S88–S89.
- 2485. Leonard C, Montamat G, Davril C, et al. Comprehensive mapping of immune tolerance yields a regulatory TNF receptor 2 signature in a murine model of successful Fel d 1‐specific immunotherapy using high‐dose CpG adjuvant. Allergy. 2021;76(7):2153–2165. https://doi.org/10.1111/all.14716
- 2486. Senti G, Johansen P, Haug S, et al. Use of A‐type CpG oligodeoxynucleotides as an adjuvant in allergen‐specific immunotherapy in humans: a phase I/IIa clinical trial. Clin Exp Allergy. 2009;39(4):562–570. https://doi.org/10.1111/j.1365‐2222.2008.03191.x
- 2487. Rosewich M, Lee D, Zielen S. Pollinex Quattro: an innovative four injections immunotherapy in allergic rhinitis. Hum Vaccin Immunother. 2013;9(7):1523–1531. https://doi.org/10.4161/hv.24631
- 2488. DuBuske LM, Frew AJ, Horak F, et al. Ultrashort‐specific immunotherapy successfully treats seasonal allergic rhinoconjunctivitis to grass pollen. Allergy Asthma Proc. 2011;32(6):466. https://doi.org/10.2500/108854111798840203
- 2489. Feynman R. Plenty of room at the bottom. 1959. Lecture to the American Physical Society.
- 2490. Marty JJ, Oppenheim RC, Speiser P. Nanoparticles–a new colloidal drug delivery system. Pharm Acta Helv. 1978;53(1):17–23.
- 2491. Toh ZQ, Anzela A, Tang ML, Licciardi PV. Probiotic therapy as a novel approach for allergic disease. Front Pharmacol. 2012;3:171. https://doi.org/10.3389/fphar.2012.00171
- 2492. Ho HE, Bunyavanich S. Microbial adjuncts for food allergen immunotherapy. Curr Allergy Asthma Rep. 2019;19(5):25. https://doi.org/10.1007/s11882‐019‐0859‐1
- 2493. Liu PT, Stenger S, Li H, et al. Toll‐like receptor triggering of a vitamin D‐mediated human antimicrobial response. Science. 2006;311(5768):1770–1773. https://doi.org/10.1126/science.1123933
- 2494. Hesse L, van Ieperen N, Petersen AH, Elberink J, van Oosterhout AJM, Nawijn MC. High dose vitamin D3 empowers effects of subcutaneous immunotherapy in a grass pollen‐driven mouse model of asthma. Sci Rep. 2020;10(1):20876. https://doi.org/10.1038/s41598‐020‐77947‐6
- 2495. Heine G, Francuzik W, Doelle‐Bierke S, et al. Immunomodulation of high‐dose vitamin D supplementation during allergen‐specific immunotherapy. Allergy. 2021;76(3):930–933. https://doi.org/10.1111/all.14541
- 2496. Grundstrom J, Neimert‐Andersson T, Kemi C, et al. Covalent coupling of vitamin D3 to the major cat allergen Fel d 1 improves the effects of allergen‐specific immunotherapy in a mouse model for cat allergy. Int Arch Allergy Immunol. 2012;157(2):136–146. https://doi.org/10.1159/000327546
- 2497. Liu C, Yang N, Song Y, et al. Ganoderic acid C1 isolated from the anti‐asthma formula, ASHMI suppresses TNF‐alpha production by mouse macrophages and peripheral blood mononuclear cells from asthma patients. Int Immunopharmacol. 2015;27(2):224–231. https://doi.org/10.1016/j.intimp.2015.05.018
- 2498. Wen MC, Wei CH, Hu ZQ, et al. Efficacy and tolerability of anti‐asthma herbal medicine intervention in adult patients with moderate‐severe allergic asthma. J Allergy Clin Immunol. 2005;116(3):517–524. https://doi.org/10.1016/j.jaci.2005.05.029
- 2499. Mahler V, Esch RE, Kleine‐Tebbe J, et al. Understanding differences in allergen immunotherapy products and practices in North America and Europe. J Allergy Clin Immunol. 2019;143(3):813–828. https://doi.org/10.1016/j.jaci.2019.01.024
- 2500. Valenta R, Niespodziana K, Focke‐Tejkl M, et al. Recombinant allergens: what does the future hold? J Allergy Clin Immunol. 2011;127(4):860–864. https://doi.org/10.1016/j.jaci.2011.02.016
- 2501. Zhernov Y, Curin M, Khaitov M, Karaulov A, Valenta R. Recombinant allergens for immunotherapy: state of the art. Curr Opin Allergy Clin Immunol. 2019;19(4):402–414. https://doi.org/10.1097/ACI.0000000000000536
- 2502. Jutel M, Jaeger L, Suck R, Meyer H, Fiebig H, Cromwell O. Allergen‐specific immunotherapy with recombinant grass pollen allergens. J Allergy Clin Immunol. 2005;116(3):608–613. https://doi.org/10.1016/j.jaci.2005.06.004
- 2503. Klimek L, Schendzielorz P, Pinol R, Pfaar O. Specific subcutaneous immunotherapy with recombinant grass pollen allergens: first randomized dose‐ranging safety study. Clin Exp Allergy. 2012;42(6):936–945. https://doi.org/10.1111/j.1365‐2222.2012.03971.x
- 2504. Pauli G, Larsen TH, Rak S, et al. Efficacy of recombinant birch pollen vaccine for the treatment of birch‐allergic rhinoconjunctivitis. J Allergy Clin Immunol. 2008;122(5):951–960. https://doi.org/10.1016/j.jaci.2008.09.017
- 2505. Nony E, Bouley J, Le Mignon M, et al. Development and evaluation of a sublingual tablet based on recombinant Bet v 1 in birch pollen‐allergic patients. Allergy. 2015;70(7):795–804. https://doi.org/10.1111/all.12622
- 2506. Litwin A, Pesce AJ, Fischer T, Michael M, Michael JG. Regulation of the human immune response to ragweed pollen by immunotherapy. A controlled trial comparing the effect of immunosuppressive peptic fragments of short ragweed with standard treatment. Clin Exp Allergy. 1991;21(4):457–465. https://doi.org/10.1111/j.1365‐2222.1991.tb01686.x
- 2507. Purohit A, Niederberger V, Kronqvist M, et al. Clinical effects of immunotherapy with genetically modified recombinant birch pollen Bet v 1 derivatives. Clin Exp Allergy. 2008;38(9):1514–1525. https://doi.org/10.1111/j.1365‐2222.2008.03042.x
- 2508. Couroux P, Patel D, Armstrong K, Larche M, Hafner RP. Fel d 1‐derived synthetic peptide immuno‐regulatory epitopes show a long‐term treatment effect in cat allergic subjects. Clin Exp Allergy. 2015;45(5):974–981. https://doi.org/10.1111/cea.12488
- 2509. Maguire P, Nicodemus C, Robinson D, Aaronson D, Umetsu DT. The safety and efficacy of ALLERVAX CAT in cat allergic patients. Clin Immunol. 1999;93(3):222–231. https://doi.org/10.1006/clim.1999.4795
- 2510. Norman PS, Ohman Jr JL, Long AA, et al. Treatment of cat allergy with T‐cell reactive peptides. Am J Respir Crit Care Med. 1996;154(6 pt 1):1623–1628. https://doi.org/10.1164/ajrccm.154.6.8970345
- 2511. Spertini F, Perrin Y, Audran R, et al. Safety and immunogenicity of immunotherapy with Bet v 1‐derived contiguous overlapping peptides. J Allergy Clin Immunol. 2014;134(1):239–240.e13. https://doi.org/10.1016/j.jaci.2014.04.001
- 2512. Spertini F, DellaCorte G, Kettner A, et al. Efficacy of 2 months of allergen‐specific immunotherapy with Bet v 1‐derived contiguous overlapping peptides in patients with allergic rhinoconjunctivitis: Results of a phase IIb study. J Allergy Clin Immunol. 2016;138(1):162–168. https://doi.org/10.1016/j.jaci.2016.02.044
- 2513. Kettner A, DellaCorte G, de Blay F, et al. Benefit of Bet v 1 contiguous overlapping peptide immunotherapy persists during first follow‐up season. J Allergy Clin Immunol. 2018;142(2):678–680.e7. https://doi.org/10.1016/j.jaci.2018.01.052
- 2514. Jutel M, Kosowska A, Smolinska S. Allergen immunotherapy: past, present, and future. Allergy Asthma Immunol Res. 2016;8(3):191–197. https://doi.org/10.4168/aair.2016.8.3.191
- 2515. Valenta R, Campana R, Focke‐Tejkl M, Niederberger V. Vaccine development for allergen‐specific immunotherapy based on recombinant allergens and synthetic allergen peptides: Lessons from the past and novel mechanisms of action for the future. J Allergy Clin Immunol. 2016;137(2):351–357. https://doi.org/10.1016/j.jaci.2015.12.1299
- 2516. Norman PS, Lichtenstein LM, Marsh DG. Studies on allergoids from naturally occurring allergens. IV. Efficacy and safety of long‐term allergoid treatment of ragweed hay fever. J Allergy Clin Immunol. 1981;68(6):460–470. https://doi.org/10.1016/0091‐6749(81)90200‐1
- 2517. Grammer LC, Zeiss CR, Suszko IM, Shaughnessy MA, Patterson R. A double‐blind, placebo‐controlled trial of polymerized whole ragweed for immunotherapy of ragweed allergy. J Allergy Clin Immunol. 1982;69(6):494–499. https://doi.org/10.1016/0091‐6749(82)90173‐7
- 2518. Bousquet J, Hejjaoui A, Soussana M, Michel FB. Double‐blind, placebo‐controlled immunotherapy with mixed grass‐pollen allergoids. IV. Comparison of the safety and efficacy of two dosages of a high‐molecular‐weight allergoid. J Allergy Clin Immunol. 1990;85(2):490–497. https://doi.org/10.1016/0091‐6749(90)90160‐6
- 2519. Bousquet J, Maasch HJ, Hejjaoui A, et al. Double‐blind, placebo‐controlled immunotherapy with mixed grass‐pollen allergoids. III. Efficacy and safety of unfractionated and high‐molecular‐weight preparations in rhinoconjunctivitis and asthma. J Allergy Clin Immunol. 1989;84(4 pt 1):546–556. https://doi.org/10.1016/0091‐6749(89)90369‐2
- 2520. Riechelmann H, Schmutzhard J, van der Werf JF, Distler A, Kleinjans HA. Efficacy and safety of a glutaraldehyde‐modified house dust mite extract in allergic rhinitis. Am J Rhinol Allergy. 2010;24(5):e104–e109. https://doi.org/10.2500/ajra.2010.24.3508
- 2521. Passalacqua G, Albano M, Fregonese L, et al. Randomised controlled trial of local allergoid immunotherapy on allergic inflammation in mite‐induced rhinoconjunctivitis. Lancet. 1998;351(9103):629–632. https://doi.org/10.1016/S0140‐6736(97)07055‐4
- 2522. Rauber MM, Wu HK, Adams B, et al. Birch pollen allergen‐specific immunotherapy with glutaraldehyde‐modified allergoid induces IL‐10 secretion and protective antibody responses. Allergy. 2019;74(8):1575–1579. https://doi.org/10.1111/all.13774
- 2523. Ramesh M, Karagic M. New modalities of allergen immunotherapy. Hum Vaccin Immunother. 2018;14(12):2848–2863. https://doi.org/10.1080/21645515.2018.1502126
- 2524. Pohlit H, Bellinghausen I, Frey H, Saloga J. Recent advances in the use of nanoparticles for allergen‐specific immunotherapy. Allergy. 2017;72(10):1461–1474. https://doi.org/10.1111/all.13199
- 2525. Basomba A, Tabar AI, de Rojas DH, et al. Allergen vaccination with a liposome‐encapsulated extract of Dermatophagoides pteronyssinus: a randomized, double‐blind, placebo‐controlled trial in asthmatic patients. J Allergy Clin Immunol. 2002;109(6):943–948. https://doi.org/10.1067/mai.2002.124465
- 2526. TePas EC, Hoyte EG, McIntire JJ, Umetsu DT. Clinical efficacy of microencapsulated timothy grass pollen extract in grass‐allergic individuals. Ann Allergy Asthma Immunol. 2004;92(1):25–31. https://doi.org/10.1016/S1081‐1206(10)61706‐1
- 2527. Frankland AW, Augustin R. Prophylaxis of summer hay‐fever and asthma: a controlled trial comparing crude grass‐pollen extracts with the isolated main protein component. Lancet. 1954;266(6821):1055–1057. https://doi.org/10.1016/s0140‐6736(54)91620‐7
- 2528. Penagos M, Eifan AO, Durham SR, Scadding GW. Duration of allergen immunotherapy for long‐term efficacy in allergic rhinoconjunctivitis. Curr Treat Options Allergy. 2018;5(3):275–290. https://doi.org/10.1007/s40521‐018‐0176‐2
- 2529. Bachert C, Larche M, Bonini S, et al. Allergen immunotherapy on the way to product‐based evaluation – a WAO statement. World Allergy Organ J. 2015;8(1):29. https://doi.org/10.1186/s40413‐015‐0078‐8
- 2530. McEldowney SJ, Bush RK. Pollen immunotherapy: selection,prevention, and future directions. Curr Allergy Asthma Rep. 2006;6(5):420–426. https://doi.org/10.1007/s11882‐996‐0016‐5
- 2531. Coop CA. Immunotherapy for mold allergy. Clin Rev Allergy Immunol. 2014;47(3):289–298. https://doi.org/10.1007/s12016‐013‐8389‐4
- 2532. Nelson HS. Immunotherapy for house‐dust mite allergy. Allergy Asthma Proc. 2018;39(4):264–272. https://doi.org/10.2500/aap.2018.39.4145
- 2533. Dhami S, Agarwal A. Does evidence support the use of cat allergen immunotherapy? Curr Opin Allergy Clin Immunol. 2018;18(4):350–355. https://doi.org/10.1097/ACI.0000000000000457
- 2534. Larenas‐Linnemann D. Allergen immunotherapy: an update on protocols of administration. Curr Opin Allergy Clin Immunol. 2015;15(6):556–567. https://doi.org/10.1097/ACI.0000000000000220
- 2535. Epstein TG, Liss GM, Murphy‐Berendts K, Bernstein DI. AAAAI/ACAAI surveillance study of subcutaneous immunotherapy, years 2008‐2012: an update on fatal and nonfatal systemic allergic reactions. J Allergy Clin Immunol Pract. 2014;2(2):161–167. https://doi.org/10.1016/j.jaip.2014.01.004
- 2536. Epstein TG, Liss GM, Berendts KM, Bernstein DI. AAAAI/ACAAI Subcutaneous Immunotherapy Surveillance Study (2013‐2017): fatalities, infections, delayed reactions, and use of epinephrine autoinjectors. J Allergy Clin Immunol Pract. 2019;7(6):1996–2003.e1. https://doi.org/10.1016/j.jaip.2019.01.058
- 2537. DaVeiga SP, Liu X, Caruso K, Golubski S, Xu M, Lang DM. Systemic reactions associated with subcutaneous allergen immunotherapy: timing and risk assessment. Ann Allergy Asthma Immunol. 2011;106(6):533–537.e2. https://doi.org/10.1016/j.anai.2011.02.007
- 2538. Hankin CS, Cox L, Lang D, et al. Allergy immunotherapy among Medicaid‐enrolled children with allergic rhinitis: patterns of care, resource use, and costs. J Allergy Clin Immunol. 2008;121(1):227–232. https://doi.org/10.1016/j.jaci.2007.10.026
- 2539. Hankin CS, Cox L, Lang D, et al. Allergen immunotherapy and health care cost benefits for children with allergic rhinitis: a large‐scale, retrospective, matched cohort study. Ann Allergy Asthma Immunol. 2010;104(1):79–85. https://doi.org/10.1016/j.anai.2009.11.010
- 2540. Sun D, Cafone J, Shaker M, Greenhawt M. The cost‐effectiveness of requiring universal vs contextual self‐injectable epinephrine autoinjector for allergen immunotherapy. Ann Allergy Asthma Immunol. 2019;123(6):582–589. https://doi.org/10.1016/j.anai.2019.09.009
- 2541. Eng PA, Borer‐Reinhold M, Heijnen IA, Gnehm HP. Twelve‐year follow‐up after discontinuation of preseasonal grass pollen immunotherapy in childhood. Allergy. 2006;61(2):198–201. https://doi.org/10.1111/j.1398‐9995.2006.01011.x
- 2542. Halken S, Larenas‐Linnemann D, Roberts G, et al. EAACI guidelines on allergen immunotherapy: prevention of allergy. Pediatr Allergy Immunol. 2017;28(8):728–745. https://doi.org/10.1111/pai.12807
- 2543. Des Roches A, Paradis L, Menardo JL, Bouges S, Daures JP, Bousquet J. Immunotherapy with a standardized Dermatophagoides pteronyssinus extract. VI. Specific immunotherapy prevents the onset of new sensitizations in children. J Allergy Clin Immunol. 1997;99(4):450–453. https://doi.org/10.1016/s0091‐6749(97)70069‐1
- 2544. Pajno GB, Barberio G, De Luca F, Morabito L, Parmiani S. Prevention of new sensitizations in asthmatic children monosensitized to house dust mite by specific immunotherapy. A six‐year follow‐up study. Clin Exp Allergy. 2001;31(9):1392–1397. https://doi.org/10.1046/j.1365‐2222.2001.01161.x
- 2545. Worm M, Rak S, Samolinski B, et al. Efficacy and safety of birch pollen allergoid subcutaneous immunotherapy: a 2‐year double‐blind, placebo‐controlled, randomized trial plus 1‐year open‐label extension. Clin Exp Allergy. 2019;49(4):516–525. https://doi.org/10.1111/cea.13331
- 2546. Xian M, Feng M, Dong Y, Wei N, Su Q, Li J. Changes in CD4+CD25+FoxP3+ regulatory T cells and serum cytokines in sublingual and subcutaneous immunotherapy in allergic rhinitis with or without asthma. Int Arch Allergy Immunol. 2020;181(1):71–80. https://doi.org/10.1159/000503143
- 2547. Wang ZX, Shi H. Single‐allergen sublingual immunotherapy versus multi‐allergen subcutaneous immunotherapy for children with allergic rhinitis. J Huazhong Univ Sci Technolog Med Sci. 2017;37(3):407–411. https://doi.org/10.1007/s11596‐017‐1748‐2
- 2548. Bozek A, Kolodziejczyk K, Krajewska‐Wojtys A, Jarzab J. Pre‐seasonal, subcutaneous immunotherapy: a double‐blinded, placebo‐controlled study in elderly patients with an allergy to grass. Ann Allergy Asthma Immunol. 2016;116(2):156–161. https://doi.org/10.1016/j.anai.2015.12.013
- 2549. Bozek A, Kolodziejczyk K, Kozlowska R, Canonica GW. Evidence of the efficacy and safety of house dust mite subcutaneous immunotherapy in elderly allergic rhinitis patients: a randomized, double‐blind placebo‐controlled trial. Clin Transl Allergy. 2017;7:43. https://doi.org/10.1186/s13601‐017‐0180‐9
- 2550. Kim JY, Jang MJ, Kim DY, Park SW, Han DH. Efficacy of subcutaneous and sublingual immunotherapy for house dust mite allergy: a network meta‐analysis‐based comparison. J Allergy Clin Immunol Pract. 2021;9(12):4450–4458.e6. https://doi.org/10.1016/j.jaip.2021.08.018
- 2551. Jutel M, Agache I, Bonini S, et al. International consensus on allergy immunotherapy. J Allergy Clin Immunol. 2015;136(3):556–568. https://doi.org/10.1016/j.jaci.2015.04.047
- 2552. Shamji MH, Larson D, Eifan A, et al. Differential induction of allergen‐specific IgA responses following timothy grass subcutaneous and sublingual immunotherapy. J Allergy Clin Immunol. 2021;148(4):1061–1071.e11. https://doi.org/10.1016/j.jaci.2021.03.030
- 2553. Rondon C, Campo P, Salas M, et al. Efficacy and safety of D. pteronyssinus immunotherapy in local allergic rhinitis: a double‐blind placebo‐controlled clinical trial. Allergy. 2016;71(7):1057–1061. https://doi.org/10.1111/all.12889
- 2554. Kleine‐Tebbe J, Walmar M, Bitsch‐Jensen K, et al. Negative clinical results from a randomised, double‐blind, placebo‐controlled trial evaluating the efficacy of two doses of immunologically enhanced, grass subcutaneous immunotherapy despite dose‐dependent immunological response. Clin Drug Investig. 2014;34(8):577–586. https://doi.org/10.1007/s40261‐014‐0216‐z
- 2555. Klimek L, Uhlig J, Mosges R, Rettig K, Pfaar O. A high polymerized grass pollen extract is efficacious and safe in a randomized double‐blind, placebo‐controlled study using a novel up‐dosing cluster‐protocol. Allergy. 2014;69(12):1629–1638. https://doi.org/10.1111/all.12513
- 2556. Tworek D, Bochenska‐Marciniak M, Kuprys‐Lipinska I, Kupczyk M, Kuna P. Perennial is more effective than preseasonal subcutaneous immunotherapy in the treatment of seasonal allergic rhinoconjunctivitis. Am J Rhinol Allergy. 2013;27(4):304–308. https://doi.org/10.2500/ajra.2013.27.3935
- 2557. James LK, Shamji MH, Walker SM, et al. Long‐term tolerance after allergen immunotherapy is accompanied by selective persistence of blocking antibodies. J Allergy Clin Immunol. 2011;127(2):509‐516.e1‐5. https://doi.org/10.1016/j.jaci.2010.12.1080
- 2558. Kuna P, Kaczmarek J, Kupczyk M. Efficacy and safety of immunotherapy for allergies to Alternaria alternata in children. J Allergy Clin Immunol. 2011;127(2):502‐508.e1‐6. https://doi.org/10.1016/j.jaci.2010.11.036
- 2559. Pfaar O, Robinson DS, Sager A, Emuzyte R. Immunotherapy with depigmented‐polymerized mixed tree pollen extract: a clinical trial and responder analysis. Allergy. 2010;65(12):1614–1621. https://doi.org/10.1111/j.1398‐9995.2010.02413.x
- 2560. Tabar AI, Lizaso MT, Garcia BE, et al. Double‐blind, placebo‐controlled study of Alternaria alternata immunotherapy: clinical efficacy and safety. Pediatr Allergy Immunol. 2008;19(1):67–75. https://doi.org/10.1111/j.1399‐3038.2007.00589.x
- 2561. Charpin D, Gouitaa M, Dron‐Gonzalvez M, et al. Immunotherapy with an aluminum hydroxide‐adsorbed Juniperus ashei foreign pollen extract in seasonal indigenous cypress pollen rhinoconjunctivitis. A double‐blind, placebo‐controlled study. Int Arch Allergy Immunol. 2007;143(2):83–91. https://doi.org/10.1159/000098656
- 2562. Powell RJ, Frew AJ, Corrigan CJ, Durham SR. Effect of grass pollen immunotherapy with Alutard SQ on quality of life in seasonal allergic rhinoconjunctivitis. Allergy. 2007;62(11):1335–1338. https://doi.org/10.1111/j.1398‐9995.2007.01455.x
- 2563. Alvarez‐Cuesta E, Aragoneses‐Gilsanz E, Martin‐Garcia C, Berges‐Gimeno P, Gonzalez‐Mancebo E, Cuesta‐Herranz J. Immunotherapy with depigmented glutaraldehyde‐polymerized extracts: changes in quality of life. Clin Exp Allergy. 2005;35(5):572–578. https://doi.org/10.1111/j.1365‐2222.2005.02245.x
- 2564. Dokic D, Schnitker J, Narkus A, Cromwell O, Frank E. Clinical effects of specific immunotherapy: a two‐year double‐blind, placebo‐controlled study with a one year follow‐up. Prilozi. 2005;26(2):113–129.
- 2565. Ferrer M, Burches E, Pelaez A, et al. Double‐blind, placebo‐controlled study of immunotherapy with Parietaria judaica: clinical efficacy and tolerance. J Investig Allergol Clin Immunol. 2005;15(4):283–292.
- 2566. Tabar AI, Echechipia S, Garcia BE, et al. Double‐blind comparative study of cluster and conventional immunotherapy schedules with Dermatophagoides pteronyssinus. J Allergy Clin Immunol. 2005;116(1):109–118. https://doi.org/10.1016/j.jaci.2005.05.005
- 2567. Crimi N, Li Gotti F, Mangano G, et al. A randomized, controlled study of specific immunotherapy in monosensitized subjects with seasonal rhinitis: effect on bronchial hyperresponsiveness, sputum inflammatory markers and development of asthma symptoms. Ann Ital Med Int. 2004;19(2):98–108.
- 2568. Mirone C, Albert F, Tosi A, et al. Efficacy and safety of subcutaneous immunotherapy with a biologically standardized extract of Ambrosia artemisiifolia pollen: a double‐blind, placebo‐controlled study. Clin Exp Allergy. 2004;34(9):1408–1414. https://doi.org/10.1111/j.1365‐2222.2004.02056.x
- 2569. Varney VA, Tabbah K, Mavroleon G, Frew AJ. Usefulness of specific immunotherapy in patients with severe perennial allergic rhinitis induced by house dust mite: a double‐blind, randomized, placebo‐controlled trial. Clin Exp Allergy. 2003;33(8):1076–1082. https://doi.org/10.1046/j.1365‐2222.2003.01735.x
- 2570. Arvidsson MB, Lowhagen O, Rak S. Effect of 2‐year placebo‐controlled immunotherapy on airway symptoms and medication in patients with birch pollen allergy. J Allergy Clin Immunol. 2002;109(5):777–783. https://doi.org/10.1067/mai.2002.123868
- 2571. Bodtger U, Poulsen LK, Jacobi HH, Malling HJ. The safety and efficacy of subcutaneous birch pollen immunotherapy – a one‐year, randomised, double‐blind, placebo‐controlled study. Allergy. 2002;57(4):297–305. https://doi.org/10.1034/j.1398‐9995.2002.1o3532.x
- 2572. Drachenberg K, Heinzkill M, Urban E. Short‐term immunotherapy with tree pollen allergoids and the adjuvant monophosphoryl lipid‐A – results from a multicentre, placebo‐controlled, randomised, double‐blind study. [Kurzzeit‐Immuntherapie mit Baumpollen – Allergoiden und dem Adjuvans Monophosphoryl Lipid‐A: Ergebnisse einer randomisierten, doppelblinden, plazebokontrollierten Multicenterstudie]. Allergologie. 2002;25:466–474.
- 2573. Leynadier F, Banoun L, Dollois B, et al. Immunotherapy with a calcium phosphate‐adsorbed five‐grass‐pollen extract in seasonal rhinoconjunctivitis: a double‐blind, placebo‐controlled study. Clin Exp Allergy. 2001;31(7):988–996. https://doi.org/10.1046/j.1365‐2222.2001.01145.x
- 2574. Walker SM, Pajno GB, Lima MT, Wilson DR, Durham SR. Grass pollen immunotherapy for seasonal rhinitis and asthma: a randomized, controlled trial. J Allergy Clin Immunol. 2001;107(1):87–93. https://doi.org/10.1067/mai.2001.112027
- 2575. Balda BR, Wolf H, Baumgarten C, et al. Tree‐pollen allergy is efficiently treated by short‐term immunotherapy (STI) with seven preseasonal injections of molecular standardized allergens. Allergy. 1998;53(8):740–748. https://doi.org/10.1111/j.1398‐9995.1998.tb03969.x
- 2576. Zenner HP, Baumgarten C, Rasp G, et al. Short‐term immunotherapy: a prospective, randomized, double‐blind, placebo‐controlled multicenter study of molecular standardized grass and rye allergens in patients with grass pollen‐induced allergic rhinitis. J Allergy Clin Immunol. 1997;100(1):23–29. https://doi.org/10.1016/s0091‐6749(97)70190‐8
- 2577. Olsen OT, Frolund L, Heinig J, Jacobsen L, Svendsen UG. A double‐blind, randomized study investigating the efficacy and specificity of immunotherapy with Artemisia vulgaris or Phleum pratense/betula verrucosa. Allergol Immunopathol (Madr). 1995;23(2):73–78.
- 2578. Ortolani C, Pastorello EA, Incorvaia C, et al. A double‐blind, placebo‐controlled study of immunotherapy with an alginate‐conjugated extract of Parietaria judaica in patients with Parietaria hay fever. Allergy. 1994;49(1):13–21. https://doi.org/10.1111/j.1398‐9995.1994.tb00767.x
- 2579. Pastorello EA, Pravettoni V, Incorvaia C, et al. Clinical and immunological effects of immunotherapy with alum‐absorbed grass allergoid in grass‐pollen‐induced hay fever. Allergy. 1992;47(4 pt 1):281–290. https://doi.org/10.1111/j.1398‐9995.1992.tb02054.x
- 2580. Varney VA, Gaga M, Frew AJ, Aber VR, Kay AB, Durham SR. Usefulness of immunotherapy in patients with severe summer hay fever uncontrolled by antiallergic drugs. BMJ. 1991;302(6771):265–269. https://doi.org/10.1136/bmj.302.6771.265
- 2581. Grammer LC, Shaughnessy MA, Suszko IM, Shaughnessy JJ, Patterson R. A double‐blind histamine placebo‐controlled trial of polymerized whole grass for immunotherapy of grass allergy. J Allergy Clin Immunol. 1983;72(5 pt 1):448–453. https://doi.org/10.1016/0091‐6749(83)90580‐8
- 2582. Weyer A, Donat N, L'Heritier C, et al. Grass pollen hyposensitization versus placebo therapy. I. Clinical effectiveness and methodological aspects of a pre‐seasonal course of desensitization with a four‐grass pollen extract. Allergy. 1981;36(5):309–317. https://doi.org/10.1111/j.1398‐9995.1981.tb01582.x
- 2583. Moreno V, Alvarino M, Rodriguez F, et al. Randomized dose‐response study of subcutaneous immunotherapy with a Dermatophagoides pteronyssinus extract in patients with respiratory allergy. Immunotherapy. 2016;8(3):265–77. https://doi.org/10.2217/imt.15.124
- 2584. Pfaar O, Urry Z, Robinson DS, et al. A randomized placebo‐controlled trial of rush preseasonal depigmented polymerized grass pollen immunotherapy. Allergy. 2012;67(2):272–279. https://doi.org/10.1111/j.1398‐9995.2011.02736.x
- 2585. DuBuske LM, Frew AJ, Horak F, et al. Ultrashort‐specific immunotherapy successfully treats seasonal allergic rhinoconjunctivitis to grass pollen. Allergy Asthma Proc. 2011;32(3):239–247. https://doi.org/10.2500/aap.2011.32.3453
- 2586. Ceuppens JL, Bullens D, Kleinjans H, van der Werf J, Group PBES. Immunotherapy with a modified birch pollen extract in allergic rhinoconjunctivitis: clinical and immunological effects. Clin Exp Allergy. 2009;39(12):1903–1909. https://doi.org/10.1111/j.1365‐2222.2009.03379.x
- 2587. Chakraborty P, Roy I, Chatterjee S, Chanda S, Gupta‐Bharracharya S. Phoenix sylvestris Roxb pollen allergy: a 2‐year randomized controlled trial and follow‐up study of immunotherapy in patients with seasonal allergy in an agricultural area of West Bengal, India. J Investig Allergol Clin Immunol. 2006;16(6):377–384.
- 2588. Frew AJ, Powell RJ, Corrigan CJ, Durham SR, Group UKIS. Efficacy and safety of specific immunotherapy with SQ allergen extract in treatment‐resistant seasonal allergic rhinoconjunctivitis. J Allergy Clin Immunol. 2006;117(2):319–325. https://doi.org/10.1016/j.jaci.2005.11.014
- 2589. Rak S, Heinrich C, Jacobsen L, Scheynius A, Venge P. A double‐blinded, comparative study of the effects of short preseason specific immunotherapy and topical steroids in patients with allergic rhinoconjunctivitis and asthma. J Allergy Clin Immunol. 2001;108(6):921–928. https://doi.org/10.1067/mai.2001.119743
- 2590. Ariano R, Kroon AM, Augeri G, Canonica GW, Passalacqua G. Long‐term treatment with allergoid immunotherapy with Parietaria. Clinical and immunologic effects in a randomized, controlled trial. Allergy. 1999;54(4):313–319. https://doi.org/10.1034/j.1398‐9995.1999.00900.x
- 2591. Tari MG, Mancino M, Ghezzi E, Frank E, Cromwell O. Immunotherapy with an alum‐adsorbed Parietaria‐pollen allergoid: a 2‐year, double‐blind, placebo‐controlled study. Allergy. 1997;52(1):65–74. https://doi.org/10.1111/j.1398‐9995.1997.tb02547.x
- 2592. Dolz I, Martinez‐Cocera C, Bartolome JM, Cimarra M. A double‐blind, placebo‐controlled study of immunotherapy with grass‐pollen extract Alutard SQ during a 3‐year period with initial rush immunotherapy. Allergy. 1996;51(7):489–500. https://doi.org/10.1111/j.1398‐9995.1996.tb04655.x
- 2593. Brunet C, Bedard PM, Lavoie A, Jobin M, Hebert J. Allergic rhinitis to ragweed pollen. I. Reassessment of the effects of immunotherapy on cellular and humoral responses. J Allergy Clin Immunol. 1992;89(1 pt 1):76–86. https://doi.org/10.1016/s0091‐6749(05)80043‐0
- 2594. Bousquet J, Becker WM, Hejjaoui A, et al. Differences in clinical and immunologic reactivity of patients allergic to grass pollens and to multiple‐pollen species. II. Efficacy of a double‐blind, placebo‐controlled, specific immunotherapy with standardized extracts. J Allergy Clin Immunol. 1991;88(1):43–53. https://doi.org/10.1016/0091‐6749(91)90299‐4
- 2595. Iliopoulos O, Proud D, Adkinson Jr NF, et al. Effects of immunotherapy on the early, late, and rechallenge nasal reaction to provocation with allergen: changes in inflammatory mediators and cells. J Allergy Clin Immunol. 1991;87(4):855–866. https://doi.org/10.1016/0091‐6749(91)90134‐a
- 2596. Fell P, Brostoff J. A single dose desensitization for summer hay fever. Results of a double blind study‐1988. Eur J Clin Pharmacol. 1990;38(1):77–79. https://doi.org/10.1007/BF00314808
- 2597. Horst M, Hejjaoui A, Horst V, Michel FB, Bousquet J. Double‐blind, placebo‐controlled rush immunotherapy with a standardized Alternaria extract. J Allergy Clin Immunol. 1990;85(2):460–472. https://doi.org/10.1016/0091‐6749(90)90156‐x
- 2598. Juniper EF, Kline PA, Ramsdale EH, Hargreave FE. Comparison of the efficacy and side effects of aqueous steroid nasal spray (budesonide) and allergen‐injection therapy (Pollinex‐R) in the treatment of seasonal allergic rhinoconjunctivitis. J Allergy Clin Immunol. 1990;85(3):606–611. https://doi.org/10.1016/0091‐6749(90)90100‐i
- 2599. Ewan PW, Alexander MM, Snape C, Ind PW, Agrell B, Dreborg S. Effective hyposensitization in allergic rhinitis using a potent partially purified extract of house dust mite. Clin Allergy. 1988;18(5):501–508. https://doi.org/10.1111/j.1365‐2222.1988.tb02900.x
- 2600. Bousquet J, Hejjaoui A, Skassa‐Brociek W, et al. Double‐blind, placebo‐controlled immunotherapy with mixed grass‐pollen allergoids. I. Rush immunotherapy with allergoids and standardized orchard grass‐pollen extract. J Allergy Clin Immunol. 1987;80(4):591–598. https://doi.org/10.1016/0091‐6749(87)90013‐3
- 2601. Grammer LC, Shaughnessy MA, Bernhard MI, et al. The safety and activity of polymerized ragweed: a double‐blind, placebo‐controlled trial in 81 patients with ragweed rhinitis. J Allergy Clin Immunol. 1987;80(2):177–183. https://doi.org/10.1016/0091‐6749(87)90127‐8
- 2602. Grammer LC, Shaughnessy MA, Suszko IM, Shaughnessy JJ, Patterson R. Persistence of efficacy after a brief course of polymerized ragweed allergen: a controlled study. J Allergy Clin Immunol. 1984;73(4):484–489. https://doi.org/10.1016/0091‐6749(84)90359‐2
- 2603. Metzger WJ, Dorminey HC, Richerson HB, Weiler JM, Donnelly A, Moran D. Clinical and immunologic evaluation of glutaraldehyde‐modified tyrosine‐adsorbed short ragweed extract: a double‐blind, placebo‐controlled trial. J Allergy Clin Immunol. 1981;68(6):442–448. https://doi.org/10.1016/0091‐6749(81)90198‐6
- 2604. Cox L. Accelerated immunotherapy schedules: review of efficacy and safety. Ann Allergy Asthma Immunol. 2006;97(2):126–137; quiz 137‐140, 202. https://doi.org/10.1016/S1081‐1206(10)60003‐8
- 2605. More DR, Hagan LL. Factors affecting compliance with allergen immunotherapy at a military medical center. Ann Allergy Asthma Immunol. 2002;88(4):391–394. https://doi.org/10.1016/S1081‐1206(10)62370‐8
- 2606. Pfaar O, Biedermann T, Klimek L, Sager A, Robinson DS. Depigmented‐polymerized mixed grass/birch pollen extract immunotherapy is effective in polysensitized patients. Allergy. 2013;68(10):1306–1313. https://doi.org/10.1111/all.12219
- 2607. Klunker S, Saggar LR, Seyfert‐Margolis V, et al. Combination treatment with omalizumab and rush immunotherapy for ragweed‐induced allergic rhinitis: Inhibition of IgE‐facilitated allergen binding. J Allergy Clin Immunol. 2007;120(3):688–695. https://doi.org/10.1016/j.jaci.2007.05.034
- 2608. Morais‐Almeida M, Arede C, Sampaio G, Borrego LM. Ultrarush schedule of subcutaneous immunotherapy with modified allergen extracts is safe in paediatric age. Asia Pac Allergy. 2016;6(1):35–42. https://doi.org/10.5415/apallergy.2016.6.1.35
- 2609. Akmanlar N, Altintas DU, Guneser KS, Yilmaz M, Bingol G. Comparison of conventional and rush immunotherapy with der PI in childhood respiratory allergy. Allergol Immunopathol (Madr). 2000;28(4):213–218.
- 2610. Lilja G, Sundin B, Graff‐Lonnevig V, et al. Immunotherapy with cat‐ and dog‐dander extracts. IV. Effects of 2 years of treatment. J Allergy Clin Immunol. 1989;83(1):37–44. https://doi.org/10.1016/0091‐6749(89)90475‐2
- 2611. Bousquet J, Hejjaoui A, Dhivert H, Clauzel AM, Michel FB. Immunotherapy with a standardized Dermatophagoides pteronyssinus extract. Systemic reactions during the rush protocol in patients suffering from asthma. J Allergy Clin Immunol. 1989;83(4):797–802. https://doi.org/10.1016/0091‐6749(89)90017‐1
- 2612. Winslow AW, Turbyville JC, Sublett JW, Sublett JL, Pollard SJ. Comparison of systemic reactions in rush, cluster, and standard‐build aeroallergen immunotherapy. Ann Allergy Asthma Immunol. 2016;117(5):542–545. https://doi.org/10.1016/j.anai.2016.09.005
- 2613. Casanovas M, Martin R, Jimenez C, Caballero R, Fernandez‐Caldas E. Safety of an ultra‐rush immunotherapy build‐up schedule with therapeutic vaccines containing depigmented and polymerized allergen extracts. Int Arch Allergy Immunol. 2006;139(2):153–158. https://doi.org/10.1159/000090392
- 2614. Cook KA, Kelso JM, White AA. Increased risk of systemic reactions extends beyond completion of rush immunotherapy. J Allergy Clin Immunol Pract. 2017;5(6):1773–1775. https://doi.org/10.1016/j.jaip.2017.04.015
- 2615. Portnoy J, Bagstad K, Kanarek H, Pacheco F, Hall B, Barnes C. Premedication reduces the incidence of systemic reactions during inhalant rush immunotherapy with mixtures of allergenic extracts. Ann Allergy. 1994;73(5):409–418.
- 2616. Hejjaoui A, Dhivert H, Michel FB, Bousquet J. Immunotherapy with a standardized Dermatophagoides pteronyssinus extract. IV. Systemic reactions according to the immunotherapy schedule. J Allergy Clin Immunol. 1990;85(2):473–479. https://doi.org/10.1016/0091‐6749(90)90157‐y
- 2617. Feng S, Xu Y, Ma R, Sun Y, Luo X, Li H. Cluster subcutaneous allergen specific immunotherapy for the treatment of allergic rhinitis: a systematic review and meta‐analysis. PLoS One. 2014;9(1):e86529. https://doi.org/10.1371/journal.pone.0086529
- 2618. Jiang Z, Xiao H, Zhang H, Liu S, Meng J. Comparison of adverse events between cluster and conventional immunotherapy for allergic rhinitis patients with or without asthma: A systematic review and meta‐analysis. Am J Otolaryngol. 2019;40(6):102269. https://doi.org/10.1016/j.amjoto.2019.07.013
- 2619. Fan Q, Liu X, Gao J, Huang S, Ni L. Comparative analysis of cluster versus conventional immunotherapy in patients with allergic rhinitis. Exp Ther Med. 2017;13(2):717–722. https://doi.org/10.3892/etm.2017.4032
- 2620. Nanda A, O'Connor M, Anand M, et al. Dose dependence and time course of the immunologic response to administration of standardized cat allergen extract. J Allergy Clin Immunol. 2004;114(6):1339–1344. https://doi.org/10.1016/j.jaci.2004.08.049
- 2621. Subiza J, Feliu A, Subiza JL, Uhlig J, Fernandez‐Caldas E. Cluster immunotherapy with a glutaraldehyde‐modified mixture of grasses results in an improvement in specific nasal provocation tests in less than 2.5 months of treatment. Clin Exp Allergy. 2008;38(6):987–994. https://doi.org/10.1111/j.1365‐2222.2008.02995.x
- 2622. Yu J, Zhong N, Luo Q, et al. Early efficacy analysis of cluster and conventional immunotherapy in patients with allergic rhinitis. Ear Nose Throat J. 2021;100(5):378–385. https://doi.org/10.1177/0145561319863370
- 2623. Zhang L, Wang C, Han D, Wang X, Zhao Y, Liu J. Comparative study of cluster and conventional immunotherapy schedules with dermatophagoides pteronyssinus in the treatment of persistent allergic rhinitis. Int Arch Allergy Immunol. 2009;148(2):161–169. https://doi.org/10.1159/000155747
- 2624. Wang CS, Zhang W, Wang XD, et al. [Comparative study on cluster and conventional immunotherapy with Dermatophagoides pteronyssinus in patients with allergic rhinitis]. Zhonghua Er Bi Yan Hou Tou Jing Wai Ke Za Zhi. 2011;46(12):981–985.
- 2625. Cook KA, Ford CM, Leyvas EA, Waalen J, White AA. Half of systemic reactions to allergen immunotherapy are delayed, majority require treatment with epinephrine. J Allergy Clin Immunol Pract. 2017;5(5):1415–1417. https://doi.org/10.1016/j.jaip.2017.03.025
- 2626. Nielsen L, Johnsen CR, Mosbech H, Poulsen LK, Malling HJ. Antihistamine premedication in specific cluster immunotherapy: a double‐blind, placebo‐controlled study. J Allergy Clin Immunol. 1996;97(6):1207–1213. https://doi.org/10.1016/s0091‐6749(96)70186‐0
- 2627. Larenas Linnemann DE. One hundred years of immunotherapy: review of the first landmark studies. Allergy Asthma Proc. 2012;33(2):122–128. https://doi.org/10.2500/aap.2012.33.3515
- 2628. Scadding GK, Brostoff J. Low dose sublingual therapy in patients with allergic rhinitis due to house dust mite. Clin Allergy. 1986;16(5):483–491. https://doi.org/10.1111/j.1365‐2222.1986.tb01983.x
- 2629. Durham SR, Yang WH, Pedersen MR, Johansen N, Rak S. Sublingual immunotherapy with once‐daily grass allergen tablets: a randomized controlled trial in seasonal allergic rhinoconjunctivitis. J Allergy Clin Immunol. 2006;117(4):802–809. https://doi.org/10.1016/j.jaci.2005.12.1358
- 2630. Didier A, Malling HJ, Worm M, et al. Optimal dose, efficacy, and safety of once‐daily sublingual immunotherapy with a 5‐grass pollen tablet for seasonal allergic rhinitis. J Allergy Clin Immunol. 2007;120(6):1338–1345. https://doi.org/10.1016/j.jaci.2007.07.046
- 2631. Calderon MA. Meta‐analyses of specific immunotherapy trials. Drugs Today (Barc). 2008;44(suppl B):31–34.
- 2632. Cohen J. Statistical Power Analysis for the Behavioral Scienes. 2nd ed. Lawrence Erlbaum Associates; 1988.
- 2633. de Bot CM, Moed H, Berger MY, Roder E, van Wijk RG, van der Wouden JC. Sublingual immunotherapy in children with allergic rhinitis: quality of systematic reviews. Pediatr Allergy Immunol. 2011;22(6):548–558. https://doi.org/10.1111/j.1399‐3038.2011.01165.x
- 2634. Kim JM, Lin SY, Suarez‐Cuervo C, et al. Allergen‐specific immunotherapy for pediatric asthma and rhinoconjunctivitis: a systematic review. Pediatrics. 2013;131(6):1155–1167. https://doi.org/10.1542/peds.2013‐0343
- 2635. Larenas‐Linnemann D, Blaiss M, Van Bever HP, Compalati E, Baena‐Cagnani CE. Pediatric sublingual immunotherapy efficacy: evidence analysis, 2009‐2012. Ann Allergy Asthma Immunol. 2013;110(6):402–415.e9. https://doi.org/10.1016/j.anai.2013.02.017
- 2636. Chen L, Lei L, Cai Y, Li T. Specific sublingual immunotherapy in children with perennial rhinitis: a systemic review and meta‐analysis. Int Forum Allergy Rhinol. 2020;10(11):1226–1235. https://doi.org/10.1002/alr.22589
- 2637. Feng B, Wu J, Chen B, et al. Efficacy and safety of sublingual immunotherapy for allergic rhinitis in pediatric patients: a meta‐analysis of randomized controlled trials. Am J Rhinol Allergy. 2017;31(1):27–35. https://doi.org/10.2500/ajra.2017.31.4382
- 2638. Di Bona D, Plaia A, Leto‐Barone MS, La Piana S, Di Lorenzo G. Efficacy of subcutaneous and sublingual immunotherapy with grass allergens for seasonal allergic rhinitis: a meta‐analysis‐based comparison. J Allergy Clin Immunol. 2012;130(5):1097–1107.e2. https://doi.org/10.1016/j.jaci.2012.08.012
- 2639. Nelson H, Cartier S, Allen‐Ramey F, Lawton S, Calderon MA. Network meta‐analysis shows commercialized subcutaneous and sublingual grass products have comparable efficacy. J Allergy Clin Immunol Pract. 2015;3(2):256–266.e3. https://doi.org/10.1016/j.jaip.2014.09.018
- 2640. Dranitsaris G, Ellis AK. Sublingual or subcutaneous immunotherapy for seasonal allergic rhinitis: an indirect analysis of efficacy, safety and cost. J Eval Clin Pract. 2014;20(3):225–238. https://doi.org/10.1111/jep.12112
- 2641. Chelladurai Y, Suarez‐Cuervo C, Erekosima N, et al. Effectiveness of subcutaneous versus sublingual immunotherapy for the treatment of allergic rhinoconjunctivitis and asthma: a systematic review. J Allergy Clin Immunol Pract. 2013;1(4):361–369. https://doi.org/10.1016/j.jaip.2013.04.005
- 2642. Aasbjerg K, Dalhoff KP, Backer V. Adverse events during immunotherapy against grass pollen‐induced allergic rhinitis – differences between subcutaneous and sublingual treatment. Basic Clin Pharmacol Toxicol. 2015;117(2):73–84. https://doi.org/10.1111/bcpt.12416
- 2643. Calderon MA, Casale TB, Nelson HS, Demoly P. An evidence‐based analysis of house dust mite allergen immunotherapy: a call for more rigorous clinical studies. J Allergy Clin Immunol. 2013;132(6):1322–1336. https://doi.org/10.1016/j.jaci.2013.09.004
- 2644. Ji DX, Tan JR, Yu HW. [Efficacy, safety and compliance of immunotherapy in the treatment of allergic rhinitis: a Meta‐analysis]. Zhonghua Er Bi Yan Hou Tou Jing Wai Ke Za Zhi. 2019;54(12):894–901. https://doi.org/10.3760/cma.j.issn.1673‐0860.2019.12.003
- 2645. Di Bona D, Plaia A, Leto‐Barone MS, La Piana S, Macchia L, Di Lorenzo G. Efficacy of allergen immunotherapy in reducing the likelihood of developing new allergen sensitizations: a systematic review. Allergy. 2017;72(5):691–704. https://doi.org/10.1111/all.13104
- 2646. Di Bona D, Plaia A, Leto‐Barone MS, La Piana S, Di Lorenzo G. Efficacy of grass pollen allergen sublingual immunotherapy tablets for seasonal allergic rhinoconjunctivitis: a systematic review and meta‐analysis. JAMA Intern Med. 2015;175(8):1301–1309. https://doi.org/10.1001/jamainternmed.2015.2840
- 2647. de Groot H, Bijl A. Anaphylactic reaction after the first dose of sublingual immunotherapy with grass pollen tablet. Allergy. 2009;64(6):963–964. https://doi.org/10.1111/j.1398‐9995.2009.01998.x
- 2648. Cochard MM, Eigenmann PA. Sublingual immunotherapy is not always a safe alternative to subcutaneous immunotherapy. J Allergy Clin Immunol. 2009;124(2):378–379. https://doi.org/10.1016/j.jaci.2009.04.040
- 2649. Janssens NS, van Ouwerkerk L, Gerth van Wijk R, Karim F. Acute systemic reactions to sublingual immunotherapy for house dust mite. Allergy. 2020;75(11):2962–2963. https://doi.org/10.1111/all.14417
- 2650. Creticos PS, Bernstein DI, Casale TB, Lockey RF, Maloney J, Nolte H. Coseasonal initiation of allergen immunotherapy: a systematic review. J Allergy Clin Immunol Pract. 2016;4(6):1194–1204.e4. https://doi.org/10.1016/j.jaip.2016.05.014
- 2651. Maloney J, Durham S, Skoner D, et al. Safety of sublingual immunotherapy Timothy grass tablet in subjects with allergic rhinitis with or without conjunctivitis and history of asthma. Allergy. 2015;70(3):302–309. https://doi.org/10.1111/all.12560
- 2652. Makatsori M, Scadding GW, Lombardo C, et al. Dropouts in sublingual allergen immunotherapy trials – a systematic review. Allergy. 2014;69(5):571–580. https://doi.org/10.1111/all.12385
- 2653. Cafone J, Capucilli P, Hill DA, Spergel JM. Eosinophilic esophagitis during sublingual and oral allergen immunotherapy. Curr Opin Allergy Clin Immunol. 2019;19(4):350–357. https://doi.org/10.1097/ACI.0000000000000537
- 2654. Oykhman P, Kim HL, Ellis AK. Allergen immunotherapy in pregnancy. Allergy Asthma Clin Immunol. 2015;11:31. https://doi.org/10.1186/s13223‐015‐0096‐7
- 2655. Larenas‐Linnemann D. How does the efficacy and safety of Oralair((R)) compare to other products on the market? Ther Clin Risk Manag. 2016;12:831–850. https://doi.org/10.2147/TCRM.S70363
- 2656. Larenas‐Linnemann D. Direct comparison of efficacy of sublingual immunotherapy tablets for rhinoconjunctivitis. Ann Allergy Asthma Immunol. 2016;116(4):274–286. https://doi.org/10.1016/j.anai.2016.02.008
- 2657. Meadows A, Kaambwa B, Novielli N, et al. A systematic review and economic evaluation of subcutaneous and sublingual allergen immunotherapy in adults and children with seasonal allergic rhinitis. Health Technol Assess. 2013;17(27):1–322, vi, xi‐xiv, https://doi.org/10.3310/hta17270
- 2658. Asaria M, Dhami S, van Ree R, et al. Health economic analysis of allergen immunotherapy for the management of allergic rhinitis, asthma, food allergy and venom allergy: a systematic overview. Allergy. 2018;73(2):269–283. https://doi.org/10.1111/all.13254
- 2659. Yonekura S, Gotoh M, Kaneko S, Maekawa Y, Okubo K, Okamoto Y. Disease‐modifying effect of Japanese cedar pollen sublingual immunotherapy tablets. J Allergy Clin Immunol Pract. 2021;9(11):4103–4116.e14. https://doi.org/10.1016/j.jaip.2021.06.060
- 2660. Nolte H, Waserman S, Ellis AK, Biedermann T, Wurtzen PA. Treatment effect of the tree pollen SLIT‐tablet on allergic rhinoconjunctivitis during oak pollen season. J Allergy Clin Immunol Pract. 2021;9(5):1871–1878. https://doi.org/10.1016/j.jaip.2021.01.035
- 2661. Kim JY, Hwang D, Jang M, Rhee CS, Han DH. Clinical effectiveness of house dust mite immunotherapy in mono‐ versus poly‐sensitised patients with allergic rhinitis: a systematic review and meta‐analysis. Rhinology. 2021;59(4):352–359. https://doi.org/10.4193/Rhin20.588
- 2662. Boldovjakova D, Cordoni S, Fraser CJ, et al. Sublingual immunotherapy vs placebo in the management of grass pollen‐induced allergic rhinitis in adults: a systematic review and meta‐analysis. Clin Otolaryngol. 2021;46(1):52–59. https://doi.org/10.1111/coa.13651
- 2663. Blanco C, Bazire R, Argiz L, Hernandez‐Pena J. Sublingual allergen immunotherapy for respiratory allergy: a systematic review. Drugs Context. 2018;7:212552. https://doi.org/10.7573/dic.212552
- 2664. Radulovic S, Wilson D, Calderon M, Durham S. Systematic reviews of sublingual immunotherapy (SLIT). Allergy. 2011;66(6):740–752. https://doi.org/10.1111/j.1398‐9995.2011.02583.x
- 2665. Di Bona D, Plaia A, Scafidi V, Leto‐Barone MS, Di Lorenzo G. Efficacy of sublingual immunotherapy with grass allergens for seasonal allergic rhinitis: a systematic review and meta‐analysis. J Allergy Clin Immunol. 2010;126(3):558–566. https://doi.org/10.1016/j.jaci.2010.06.013
- 2666. Lin SY, Erekosima N, Kim JM, et al. Sublingual immunotherapy for the treatment of allergic rhinoconjunctivitis and asthma: a systematic review. JAMA. 2013;309(12):1278–1288. https://doi.org/10.1001/jama.2013.2049
- 2667. Ortiz AS, McMains KC, Laury AM. Single vs multiallergen sublingual immunotherapy in the polysensitized patient: a pilot study. Int Forum Allergy Rhinol. 2018;8(4):490–494. https://doi.org/10.1002/alr.22071
- 2668. Li P, Li Q, Huang Z, Chen W, Lu Y, Tian M. Efficacy and safety of house dust mite sublingual immunotherapy in monosensitized and polysensitized children with respiratory allergic diseases. Int Forum Allergy Rhinol. 2014;4(10):796–801. https://doi.org/10.1002/alr.21397
- 2669. Amar SM, Harbeck RJ, Sills M, Silveira LJ, O'Brien H, Nelson HS. Response to sublingual immunotherapy with grass pollen extract: monotherapy versus combination in a multiallergen extract. J Allergy Clin Immunol. 2009;124(1):150‐156.e1‐5. https://doi.org/10.1016/j.jaci.2009.04.037
- 2670. Moreno‐Ancillo A, Moreno C, Ojeda P, et al. Efficacy and quality of life with once‐daily sublingual immunotherapy with grasses plus olive pollen extract without updosing. J Investig Allergol Clin Immunol. 2007;17(6):399–405.
- 2671. Lee JE, Choi YS, Kim MS, et al. Efficacy of sublingual immunotherapy with house dust mite extract in polyallergen sensitized patients with allergic rhinitis. Ann Allergy Asthma Immunol. 2011;107(1):79–84. https://doi.org/10.1016/j.anai.2011.03.012
- 2672. Li Y, Yu SY, Tang R, Zhao ZT, Sun JL. Sublingual immunotherapy tablets relieve symptoms in adults with allergic rhinitis: a meta‐analysis of randomized clinical trials. Chin Med J (Engl). 2018;131(21):2583–2588. https://doi.org/10.4103/0366‐6999.244108
- 2673. Roder E, Berger MY, de Groot H, van Wijk RG. Immunotherapy in children and adolescents with allergic rhinoconjunctivitis: a systematic review. Pediatr Allergy Immunol. 2008;19(3):197–207. https://doi.org/10.1111/j.1399‐3038.2007.00648.x
- 2674. Dretzke J, Meadows A, Novielli N, Huissoon A, Fry‐Smith A, Meads C. Subcutaneous and sublingual immunotherapy for seasonal allergic rhinitis: a systematic review and indirect comparison. J Allergy Clin Immunol. 2013;131(5):1361–1366. https://doi.org/10.1016/j.jaci.2013.02.013
- 2675. Durham SR, Emminger W, Kapp A, et al. SQ‐standardized sublingual grass immunotherapy: confirmation of disease modification 2 years after 3 years of treatment in a randomized trial. J Allergy Clin Immunol. 2012;129(3):717–725.e5. https://doi.org/10.1016/j.jaci.2011.12.973
- 2676. Didier A, Malling HJ, Worm M, Horak F, Sussman GL. Prolonged efficacy of the 300IR 5‐grass pollen tablet up to 2 years after treatment cessation, as measured by a recommended daily combined score. Clin Transl Allergy. 2015;5:12. https://doi.org/10.1186/s13601‐015‐0057‐8
- 2677. Valovirta E, Jacobsen L, Ljorring C, Koivikko A, Savolainen J. Clinical efficacy and safety of sublingual immunotherapy with tree pollen extract in children. Allergy. 2006;61(10):1177–1183. https://doi.org/10.1111/j.1398‐9995.2006.01190.x
- 2678. Nolte H, Hebert J, Berman G, et al. Randomized controlled trial of ragweed allergy immunotherapy tablet efficacy and safety in North American adults. Ann Allergy Asthma Immunol. 2013;110(6):450–456.e4. https://doi.org/10.1016/j.anai.2013.03.013
- 2679. Creticos PS, Maloney J, Bernstein DI, et al. Randomized controlled trial of a ragweed allergy immunotherapy tablet in North American and European adults. J Allergy Clin Immunol. 2013;131(5):1342–1349.e6. https://doi.org/10.1016/j.jaci.2013.03.019
- 2680. Skoner D, Gentile D, Bush R, Fasano MB, McLaughlin A, Esch RE. Sublingual immunotherapy in patients with allergic rhinoconjunctivitis caused by ragweed pollen. J Allergy Clin Immunol. 2010;125(3):660–666, 666.e1‐666.e4. https://doi.org/10.1016/j.jaci.2009.12.931
- 2681. Bergmann KC, Demoly P, Worm M, et al. Efficacy and safety of sublingual tablets of house dust mite allergen extracts in adults with allergic rhinitis. J Allergy Clin Immunol. 2014;133(6):1608–1614.e6. https://doi.org/10.1016/j.jaci.2013.11.012
- 2682. Cortellini G, Spadolini I, Patella V, et al. Sublingual immunotherapy for Alternaria‐induced allergic rhinitis: a randomized placebo‐controlled trial. Ann Allergy Asthma Immunol. 2010;105(5):382–386. https://doi.org/10.1016/j.anai.2010.08.007
- 2683. Horak F, Jaeger S, Worm M, Melac M, Didier A. Implementation of pre‐seasonal sublingual immunotherapy with a five‐grass pollen tablet during optimal dosage assessment. Clin Exp Allergy. 2009;39(3):394–400. https://doi.org/10.1111/j.1365‐2222.2008.03153.x
- 2684. Malling HJ, Montagut A, Melac M, et al. Efficacy and safety of 5‐grass pollen sublingual immunotherapy tablets in patients with different clinical profiles of allergic rhinoconjunctivitis. Clin Exp Allergy. 2009;39(3):387–393. https://doi.org/10.1111/j.1365‐2222.2008.03152.x
- 2685. Gotoh M, Okubo K, Yuta A, et al. Safety profile and immunological response of dual sublingual immunotherapy with house dust mite tablet and Japanese cedar pollen tablet. Allergol Int. 2020;69(1):104–110. https://doi.org/10.1016/j.alit.2019.07.007
- 2686. Leatherman BD, Khalid A, Lee S, et al. Dosing of sublingual immunotherapy for allergic rhinitis: evidence‐based review with recommendations. Int Forum Allergy Rhinol. 2015;5(9):773–783. https://doi.org/10.1002/alr.21561
- 2687. Jin JJ, Li JT, Klimek L, Pfaar O. Sublingual immunotherapy dosing regimens: what is ideal? J Allergy Clin Immunol Pract. 2017;5(1):1–10. https://doi.org/10.1016/j.jaip.2016.09.027
- 2688. Marogna M, Spadolini I, Massolo A, et al. Effects of sublingual immunotherapy for multiple or single allergens in polysensitized patients. Ann Allergy Asthma Immunol. 2007;98(3):274–280. https://doi.org/10.1016/S1081‐1206(10)60718‐1
- 2689. Pfaar O, Nell MJ, Boot JD, et al. A randomized, 5‐arm dose finding study with a mite allergoid SCIT in allergic rhinoconjunctivitis patients. Allergy. 2016;71(7):967–976. https://doi.org/10.1111/all.12860
- 2690. Bozek A, Ignasiak B, Filipowska B, Jarzab J. House dust mite sublingual immunotherapy: a double‐blind, placebo‐controlled study in elderly patients with allergic rhinitis. Clin Exp Allergy. 2013;43(2):242–248. https://doi.org/10.1111/cea.12039
- 2691. Didier A, Melac M, Montagut A, Lheritier‐Barrand M, Tabar A, Worm M. Agreement of efficacy assessments for five‐grass pollen sublingual tablet immunotherapy. Allergy. 2009;64(1):166–171. https://doi.org/10.1111/j.1398‐9995.2008.01767.x
- 2692. Saporta D. Efficacy of sublingual immunotherapy versus subcutaneous injection immunotherapy in allergic patients. J Environ Public Health. 2012;2012:492405. https://doi.org/10.1155/2012/492405
- 2693. Tsabouri S, Mavroudi A, Feketea G, Guibas GV. Subcutaneous and sublingual immunotherapy in allergic asthma in children. Front Pediatr. 2017;5:82. https://doi.org/10.3389/fped.2017.00082
- 2694. Dhami S, Kakourou A, Asamoah F, et al. Allergen immunotherapy for allergic asthma: A systematic review and meta‐analysis. Allergy. 2017;72(12):1825–1848. https://doi.org/10.1111/all.13208
- 2695. Rice JL, Diette GB, Suarez‐Cuervo C, et al. Allergen‐specific immunotherapy in the treatment of pediatric asthma: a systematic review. Pediatrics. 2018;141(5):e20173833. https://doi.org/10.1542/peds.2017‐3833
- 2696. Epstein TG, Calabria C, Cox LS, Dreborg S. Current Evidence on safety and practical considerations for administration of sublingual allergen immunotherapy (SLIT) in the United States. J Allergy Clin Immunol Pract. 2017;5(1):34–40.e2. https://doi.org/10.1016/j.jaip.2016.09.017
- 2697. Seiberling K, Hiebert J, Nyirady J, Lin S, Chang D. Cost of allergy immunotherapy: sublingual vs subcutaneous administration. Int Forum Allergy Rhinol. 2012;2(6):460–464. https://doi.org/10.1002/alr.21061
- 2698. Reinhold T, Bruggenjurgen B. Cost‐effectiveness of grass pollen SCIT compared with SLIT and symptomatic treatment. Allergo J Int. 2017;26(1):7–15. https://doi.org/10.1007/s40629‐016‐0002‐y
- 2699. Drazdauskaite G, Layhadi JA, Shamji MH. Mechanisms of allergen immunotherapy in allergic rhinitis. Curr Allergy Asthma Rep. 2020;21(1):2. https://doi.org/10.1007/s11882‐020‐00977‐7
- 2700. Pfaar O, Bachert C, Bufe A, et al. Guideline on allergen‐specific immunotherapy in IgE‐mediated allergic diseases: S2k Guideline of the German Society for Allergology and Clinical Immunology (DGAKI), the Society for Pediatric Allergy and Environmental Medicine (GPA), the Medical Association of German Allergologists (AeDA), the Austrian Society for Allergy and Immunology (OGAI), the Swiss Society for Allergy and Immunology (SGAI), the German Society of Dermatology (DDG), the German Society of Oto‐ Rhino‐Laryngology, Head and Neck Surgery (DGHNO‐KHC), the German Society of Pediatrics and Adolescent Medicine (DGKJ), the Society for Pediatric Pneumology (GPP), the German Respiratory Society (DGP), the German Association of ENT Surgeons (BV‐HNO), the Professional Federation of Paediatricians and Youth Doctors (BVKJ), the Federal Association of Pulmonologists (BDP) and the German Dermatologists Association (BVDD). Allergo J Int. 2014;23(8):282–319. https://doi.org/10.1007/s40629‐014‐0032‐2
- 2701. Ott H, Sieber J, Brehler R, et al. Efficacy of grass pollen sublingual immunotherapy for three consecutive seasons and after cessation of treatment: the ECRIT study. Allergy. 2009;64(9):1394–1401. https://doi.org/10.1111/j.1398‐9995.2009.02194.x
- 2702. Naclerio RM, Proud D, Moylan B, et al. A double‐blind study of the discontinuation of ragweed immunotherapy. J Allergy Clin Immunol. 1997;100(3):293–300. https://doi.org/10.1016/s0091‐6749(97)70240‐9
- 2703. Cox LS, Hankin C, Lockey R. Allergy immunotherapy adherence and delivery route: location does not matter. J Allergy Clin Immunol Pract. 2014;2(2):156–160. https://doi.org/10.1016/j.jaip.2014.01.010
- 2704. Vita D, Caminiti L, Ruggeri P, Pajno GB. Sublingual immunotherapy: adherence based on timing and monitoring control visits. Allergy. 2010;65(5):668–669. https://doi.org/10.1111/j.1398‐9995.2009.02223.x
- 2705. Hura N, Song S, Kamil RJ, Pierre G, Lin SY. Predictors of completion of sublingual immunotherapy. Laryngoscope. 2021;131(7):E2111–E2115. https://doi.org/10.1002/lary.29272
- 2706. Chaaban MR, Mansi A, Tripple JW, Wise SK. SCIT versus SLIT: which one do you recommend, doc? Am J Med Sci. 2019;357(5):442–447. https://doi.org/10.1016/j.amjms.2019.02.004
- 2707. Lin CH, Alandijani S, Lockey RF. Subcutaneous versus sublingual immunotherapy. Expert Rev Clin Immunol. 2016;12(8):801–803. https://doi.org/10.1080/1744666X.2016.1196137
- 2708. Borg M, Lokke A, Hilberg O. Compliance in subcutaneous and sublingual allergen immunotherapy: a nationwide study. Respir Med. 2020;170:106039. https://doi.org/10.1016/j.rmed.2020.106039
- 2709. Gotoh M, Kaminuma O. Sublingual immunotherapy: how sublingual allergen administration heals allergic diseases; current perspective about the mode of action. Pathogens. 2021;10(2):147. https://doi.org/10.3390/pathogens10020147
- 2710. Allergenics . FDA. Accessed October 29, 2021. https://www.fda.gov/vaccines‐blood‐biologics/allergenics
- 2711. Creticos PS. Sublingual immunotherapy for allergic rhinoconjunctivitis and asthma. Accessed October 26, 2021. https://www.uptodate.com/contents/sublingual‐immunotherapy‐for‐allergic‐rhinoconjunctivitis‐and‐asthma
- 2712. Nolte H, Casale TB, Lockey RF, et al. Epinephrine use in clinical trials of sublingual immunotherapy tablets. J Allergy Clin Immunol Pract. 2017;5(1):84–89.e3. https://doi.org/10.1016/j.jaip.2016.08.017
- 2713. Clark S, Wei W, Rudders SA, Camargo Jr CA. Risk factors for severe anaphylaxis in patients receiving anaphylaxis treatment in US emergency departments and hospitals. J Allergy Clin Immunol. 2014;134(5):1125–1130. https://doi.org/10.1016/j.jaci.2014.05.018
- 2714. Des Roches A, Paradis L, Knani J, et al. Immunotherapy with a standardized Dermatophagoides pteronyssinus extract. V. Duration of the efficacy of immunotherapy after its cessation. Allergy. 1996;51(6):430–433. https://doi.org/10.1111/j.1398‐9995.1996.tb04643.x
- 2715. Bousquet J, Maasch H, Martinot B, Hejjaoui A, Wahl R, Michel FB. Double‐blind, placebo‐controlled immunotherapy with mixed grass‐pollen allergoids. II. Comparison between parameters assessing the efficacy of immunotherapy. J Allergy Clin Immunol. 1988;82(3 pt 1):439–446. https://doi.org/10.1016/0091‐6749(88)90017‐6
- 2716. Pajno GB, Vita D, Caminiti L, et al. Children's compliance with allergen immunotherapy according to administration routes. J Allergy Clin Immunol. 2005;116(6):1380–1381. https://doi.org/10.1016/j.jaci.2005.07.034
- 2717. Kiel MA, Roder E, Gerth van Wijk R, Al MJ, Hop WC, Rutten‐van Molken MP. Real‐life compliance and persistence among users of subcutaneous and sublingual allergen immunotherapy. J Allergy Clin Immunol. 2013;132(2):353–360.e2 https://doi.org/10.1016/j.jaci.2013.03.013
- 2718. Sieber J, De Geest S, Shah‐Hosseini K, Mosges R. Medication persistence with long‐term, specific grass pollen immunotherapy measured by prescription renewal rates. Curr Med Res Opin. 2011;27(4):855–861. https://doi.org/10.1185/03007995.2011.559538
- 2719. Senna G, Ridolo E, Calderon M, Lombardi C, Canonica GW, Passalacqua G. Evidence of adherence to allergen‐specific immunotherapy. Curr Opin Allergy Clin Immunol. 2009;9(6):544–548. https://doi.org/10.1097/ACI.0b013e328332b8df
- 2720. Leader BA, Rotella M, Stillman L, DelGaudio JM, Patel ZM, Wise SK. Immunotherapy compliance: comparison of subcutaneous versus sublingual immunotherapy. Int Forum Allergy Rhinol. 2016;6(5):460–464. https://doi.org/10.1002/alr.21699
- 2721. Creticos PS. Subcutaneous immunotherapy (SCIT) for allergic disease: indications and efficacy. Accessed October 26, 2021. https://www.uptodate.com/contents/subcutaneous‐immunotherapy‐scit‐for‐allergic‐disease‐indications‐and‐efficacy
- 2722. Nelson H. Preparation of allergen extracts fro therapeutic use. Accessed October 26, 2021. https://www.uptodate.com/contents/scit‐preparation‐of‐allergen‐extracts‐for‐therapeutic‐use
- 2723. Egan M, Atkins D. What is the relationship between eosinophilic esophagitis (EoE) and aeroallergens? Implications for allergen immunotherapy. Curr Allergy Asthma Rep. 2018;18(8):43. https://doi.org/10.1007/s11882‐018‐0798‐2
- 2724. Bos JD, Meinardi MM. The 500 Dalton rule for the skin penetration of chemical compounds and drugs. Exp Dermatol. 2000;9(3):165–169. https://doi.org/10.1034/j.1600‐0625.2000.009003165.x
- 2725. Senti G, Kundig TM. Novel delivery routes for allergy immunotherapy: intralymphatic, epicutaneous, and intradermal. Immunol Allergy Clin North Am. 2016;36(1):25–37. https://doi.org/10.1016/j.iac.2015.08.006
- 2726. Wang Y, Kong Y, Wu MX. Innovative systems to deliver allergen powder for epicutaneous immunotherapy. Front Immunol. 2021;12:647954. https://doi.org/10.3389/fimmu.2021.647954
- 2727. Esposito S, Isidori C, Pacitto A, et al. Epicutaneous immunotherapy in rhino‐conjunctivitis and food allergies: a review of the literature. J Transl Med. 2018;16(1):329. https://doi.org/10.1186/s12967‐018‐1701‐6
- 2728. Senti G, Graf N, Haug S, et al. Epicutaneous allergen administration as a novel method of allergen‐specific immunotherapy. J Allergy Clin Immunol. 2009;124(5):997–1002. https://doi.org/10.1016/j.jaci.2009.07.019
- 2729. Agostinis F, Forti S, Di Berardino F. Grass transcutaneous immunotherapy in children with seasonal rhinoconjunctivitis. Allergy. 2010;65(3):410–411. https://doi.org/10.1111/j.1398‐9995.2009.02189.x
- 2730. Senti G, von Moos S, Tay F, et al. Epicutaneous allergen‐specific immunotherapy ameliorates grass pollen‐induced rhinoconjunctivitis: a double‐blind, placebo‐controlled dose escalation study. J Allergy Clin Immunol. 2012;129(1):128–135. https://doi.org/10.1016/j.jaci.2011.08.036
- 2731. Senti G, von Moos S, Tay F, Graf N, Johansen P, Kundig TM. Determinants of efficacy and safety in epicutaneous allergen immunotherapy: summary of three clinical trials. Allergy. 2015;70(6):707–710. https://doi.org/10.1111/all.12600
- 2732. Xiong L, Lin J, Luo Y, Chen W, Dai J. The efficacy and safety of epicutaneous immunotherapy for allergic diseases: a systematic review and meta‐analysis. Int Arch Allergy Immunol. 2020;181(3):170–182. https://doi.org/10.1159/000504366
- 2733. Senti G, Freiburghaus AU, Larenas‐Linnemann D, et al. Intralymphatic immunotherapy: update and unmet needs. Int Arch Allergy Immunol. 2019;178(2):141–149. https://doi.org/10.1159/000493647
- 2734. Hoang MP, Seresirikachorn K, Chitsuthipakorn W, Snidvongs K. Intralymphatic immunotherapy for allergic rhinoconjunctivitis: a systematic review and meta‐analysis. Rhinology. 2021;59(3):236–244. https://doi.org/10.4193/Rhin20.572
- 2735. Aini NR, Mohd Noor N, Md Daud MK, Wise SK, Abdullah B. Efficacy and safety of intralymphatic immunotherapy in allergic rhinitis: a systematic review and meta‐analysis. Clin Transl Allergy. 2021;11(6):e12055. https://doi.org/10.1002/clt2.12055
- 2736. Hylander T, Latif L, Petersson‐Westin U, Cardell LO. Intralymphatic allergen‐specific immunotherapy: an effective and safe alternative treatment route for pollen‐induced allergic rhinitis. J Allergy Clin Immunol. 2013;131(2):412–420. https://doi.org/10.1016/j.jaci.2012.10.056
- 2737. Senti G, Prinz Vavricka BM, Erdmann I, et al. Intralymphatic allergen administration renders specific immunotherapy faster and safer: a randomized controlled trial. Proc Natl Acad Sci U S A. 2008;105(46):17908–17912. https://doi.org/10.1073/pnas.0803725105
- 2738. Senti G, Crameri R, Kuster D, et al. Intralymphatic immunotherapy for cat allergy induces tolerance after only 3 injections. J Allergy Clin Immunol. 2012;129(5):1290–1296. https://doi.org/10.1016/j.jaci.2012.02.026
- 2739. Witten M, Malling HJ, Blom L, Poulsen BC, Poulsen LK. Is intralymphatic immunotherapy ready for clinical use in patients with grass pollen allergy? J Allergy Clin Immunol. 2013;132(5):1248–1252.e5. https://doi.org/10.1016/j.jaci.2013.07.033
- 2740. Patterson AM, Bonny AE, Shiels 2nd WE, Erwin EA. Three‐injection intralymphatic immunotherapy in adolescents and young adults with grass pollen rhinoconjunctivitis. Ann Allergy Asthma Immunol. 2016;116(2):168–170. https://doi.org/10.1016/j.anai.2015.11.010
- 2741. Hylander T, Larsson O, Petersson‐Westin U, et al. Intralymphatic immunotherapy of pollen‐induced rhinoconjunctivitis: a double‐blind placebo‐controlled trial. Respir Res. 2016;17:10. https://doi.org/10.1186/s12931‐016‐0324‐9
- 2742. Hellkvist L, Hjalmarsson E, Kumlien Georen S, et al. Intralymphatic immunotherapy with 2 concomitant allergens, birch and grass: a randomized, double‐blind, placebo‐controlled trial. J Allergy Clin Immunol. 2018;142(4):1338–1341.e9. https://doi.org/10.1016/j.jaci.2018.05.030
- 2743. Konradsen JR, Grundstrom J, Hellkvist L, et al. Intralymphatic immunotherapy in pollen‐allergic young adults with rhinoconjunctivitis and mild asthma: a randomized trial. J Allergy Clin Immunol. 2020;145(3):1005–1007.e7. https://doi.org/10.1016/j.jaci.2019.11.017
- 2744. Skaarup SH, Schmid JM, Skjold T, Graumann O, Hoffmann HJ. Intralymphatic immunotherapy improves grass pollen allergic rhinoconjunctivitis: a 3‐year randomized placebo‐controlled trial. J Allergy Clin Immunol. 2021;147(3):1011–1019. https://doi.org/10.1016/j.jaci.2020.07.002
- 2745. Thompson CP, Silvers S, Shapiro MA. Intralymphatic immunotherapy for mountain cedar pollinosis: a randomized, double‐blind, placebo‐controlled trial. Ann Allergy Asthma Immunol. 2020;125(3):311–318.e2. https://doi.org/10.1016/j.anai.2020.04.030
- 2746. Terada T, Omura S, Kikuoka Y, et al. Sustained effects of intralymphatic pollen‐specific immunotherapy on Japanese cedar pollinosis. Rhinology. 2020;58(3):241–247. https://doi.org/10.4193/Rhin19.301
- 2747. Wang K, Zheng R, Chen Y, et al. Clinical efficacy and safety of cervical intralymphatic immunotherapy for house dust mite allergic rhinitis: a pilot study. Am J Otolaryngol. 2019;40(6):102280. https://doi.org/10.1016/j.amjoto.2019.102280
- 2748. Lee SP, Choi SJ, Joe E, et al. A pilot study of intralymphatic immunotherapy for house dust mite, cat, and dog allergies. Allergy Asthma Immunol Res. 2017;9(3):272–277. https://doi.org/10.4168/aair.2017.9.3.272
- 2749. Schmid JM, Nezam H, Madsen HH, Schmitz A, Hoffmann HJ. Intralymphatic immunotherapy induces allergen specific plasmablasts and increases tolerance to skin prick testing in a pilot study. Clin Transl Allergy. 2016;6:19. https://doi.org/10.1186/s13601‐016‐0107‐x
- 2750. Taudorf E, Laursen LC, Lanner A, et al. Oral immunotherapy in birch pollen hay fever. J Allergy Clin Immunol. 1987;80(2):153–161. https://doi.org/10.1016/0091‐6749(87)90124‐2
- 2751. Oppenheimer J, Areson JG, Nelson HS. Safety and efficacy of oral immunotherapy with standardized cat extract. J Allergy Clin Immunol. 1994;93(1 pt 1):61–67. https://doi.org/10.1016/0091‐6749(94)90233‐x
- 2752. Van Deusen MA, Angelini BL, Cordoro KM, Seiler BA, Wood L, Skoner DP. Efficacy and safety of oral immunotherapy with short ragweed extract. Ann Allergy Asthma Immunol. 1997;78(6):573–580. https://doi.org/10.1016/S1081‐1206(10)63218‐8
- 2753. Vickery BP, Vereda A, Casale TB, et al. AR101 oral immunotherapy for peanut allergy. N Engl J Med. 2018;379(21):1991–2001. https://doi.org/10.1056/NEJMoa1812856
- 2754. Allam JP, Stojanovski G, Friedrichs N, et al. Distribution of Langerhans cells and mast cells within the human oral mucosa: new application sites of allergens in sublingual immunotherapy? Allergy. 2008;63(6):720–7. https://doi.org/10.1111/j.1398‐9995.2007.01611.x
- 2755. Canonica GW, Cox L, Pawankar R, et al. Sublingual immunotherapy: World Allergy Organization position paper 2013 update. World Allergy Organ J. 2014;7(1):6. https://doi.org/10.1186/1939‐4551‐7‐6
- 2756. Reisacher WR, Suurna MV, Rochlin K, Bremberg MG, Tropper G. Oral mucosal immunotherapy for allergic rhinitis: a pilot study. Allergy Rhinol (Providence). 2016;7(1):21–28. https://doi.org/10.2500/ar.2016.7.0150
- 2757. Passalacqua G, Albano M, Ruffoni S, et al. Nasal immunotherapy to Parietaria: evidence of reduction of local allergic inflammation. Am J Respir Crit Care Med. 1995;152(2):461–466. https://doi.org/10.1164/ajrccm.152.2.7633693
- 2758. Andri L, Senna G, Betteli C, Givanni S, Andri G, Falagiani P. Local nasal immunotherapy for Dermatophagoides‐induced rhinitis: efficacy of a powder extract. J Allergy Clin Immunol. 1993;91(5):987–996. https://doi.org/10.1016/0091‐6749(93)90211‐w
- 2759. Tari MG, Mancino M, Monti G. Immunotherapy by inhalation of allergen in powder in house dust allergic asthma – a double‐blind study. J Investig Allergol Clin Immunol. 1992;2(2):59–67.
- 2760. Saco T, Ugalde IC, Cardet JC, Casale TB. Strategies for choosing a biologic for your patient with allergy or asthma. Ann Allergy Asthma Immunol. 2021;127(6):627–637. https://doi.org/10.1016/j.anai.2021.09.009
- 2761. Kamin W, Kopp MV, Erdnuess F, Schauer U, Zielen S, Wahn U. Safety of anti‐IgE treatment with omalizumab in children with seasonal allergic rhinitis undergoing specific immunotherapy simultaneously. Pediatr Allergy Immunol. 2010;21(1 pt 2):e160–e165. https://doi.org/10.1111/j.1399‐3038.2009.00900.x
- 2762. Rolinck‐Werninghaus C, Hamelmann E, Keil T, et al. The co‐seasonal application of anti‐IgE after preseasonal specific immunotherapy decreases ocular and nasal symptom scores and rescue medication use in grass pollen allergic children. Allergy. 2004;59(9):973–979. https://doi.org/10.1111/j.1398‐9995.2004.00552.x
- 2763. Lin H, Boesel KM, Griffith DT, et al. Omalizumab rapidly decreases nasal allergic response and FcepsilonRI on basophils. J Allergy Clin Immunol. 2004;113(2):297–302. https://doi.org/10.1016/j.jaci.2003.11.044
- 2764. Prussin C, Griffith DT, Boesel KM, Lin H, Foster B, Casale TB. Omalizumab treatment downregulates dendritic cell FcepsilonRI expression. J Allergy Clin Immunol. 2003;112(6):1147–1154. https://doi.org/10.1016/j.jaci.2003.10.003
- 2765. Kopp MV, Hamelmann E, Zielen S, et al. Combination of omalizumab and specific immunotherapy is superior to immunotherapy in patients with seasonal allergic rhinoconjunctivitis and co‐morbid seasonal allergic asthma. Clin Exp Allergy. 2009;39(2):271–279. https://doi.org/10.1111/j.1365‐2222.2008.03121.x
- 2766. Kopp MV, Hamelmann E, Bendiks M, et al. Transient impact of omalizumab in pollen allergic patients undergoing specific immunotherapy. Pediatr Allergy Immunol. 2013;24(5):427–433. https://doi.org/10.1111/pai.12098
- 2767. Massanari M, Nelson H, Casale T, et al. Effect of pretreatment with omalizumab on the tolerability of specific immunotherapy in allergic asthma. J Allergy Clin Immunol. 2010;125(2):383–389. https://doi.org/10.1016/j.jaci.2009.11.022
- 2768. Slater JE. Preparation and standardization of allergen extracts. In: Adkinson NF, Burks AW, Busse WW, Holgate ST, Lemanske RFJ, eds. Allergy Principlses and Practice. 8 ed. Mosbe; 2014:470–481.
- 2769. Calderon MA, Cox L, Casale TB, Moingeon P, Demoly P. Multiple‐allergen and single‐allergen immunotherapy strategies in polysensitized patients: looking at the published evidence. J Allergy Clin Immunol. 2012;129(4):929–934. https://doi.org/10.1016/j.jaci.2011.11.019
- 2770. Zuberbier T, Bachert C, Bousquet PJ, et al. GA(2) LEN/EAACI pocket guide for allergen‐specific immunotherapy for allergic rhinitis and asthma. Allergy. 2010;65(12):1525–1530. https://doi.org/10.1111/j.1398‐9995.2010.02474.x
- 2771. Demoly P, Passalacqua G, Pfaar O, Sastre J, Wahn U. Management of the polyallergic patient with allergy immunotherapy: a practice‐based approach. Allergy Asthma Clin Immunol. 2016;12:2. https://doi.org/10.1186/s13223‐015‐0109‐6
- 2772. Wahn U, Calderon MA, Demoly P. Real‐life clinical practice and management of polysensitized patients with respiratory allergies: a large, global survey of clinicians prescribing allergen immunotherapy. Expert Rev Clin Immunol. 2017;13(3):283–289. https://doi.org/10.1080/1744666X.2017.1277142
- 2773. Franklin W, Lowell FC. Comparison of two dosages of ragweed extract in the treatment of pollenosis. JAMA. 1967;201(12):915–917.
- 2774. Lowell FC, Franklin W. A double‐blind study of the effectiveness and specificity of injecton therapy in ragweed hay fever. N Engl J Med. 1965;273(13):675–679. https://doi.org/10.1056/NEJM196509232731302
- 2775. Johnstone DE, Dutton A. The value of hyposensitization therapy for bronchial asthma in children – a 14‐year study. Pediatrics. 1968;42(5):793–802.
- 2776. Reid MJ, Moss RB, Hsu YP, Kwasnicki JM, Commerford TM, Nelson BL. Seasonal asthma in northern California: allergic causes and efficacy of immunotherapy. J Allergy Clin Immunol. 1986;78(4 pt 1):590–600. https://doi.org/10.1016/0091‐6749(86)90076‐x
- 2777. El‐Qutob D, Raducan I, Mencia G. A preliminary study to investigate effectiveness of a mixed extract of Dermatophagoides sp. house dust mites and Alternaria sp. mold. Eur Ann Allergy Clin Immunol. 2021;53(5):234–239. https://doi.org/10.23822/EurAnnACI.1764‐1489.185
- 2778. Nevot‐Falco S, Mancebo EG, Martorell A, et al. Safety and effectiveness of a single multiallergen subcutaneous immunotherapy in polyallergic patients. Int Arch Allergy Immunol. 2021;182(12):1226–1230. https://doi.org/10.1159/000517473
- 2779. Nelson HS. Multiallergen immunotherapy for allergic rhinitis and asthma. J Allergy Clin Immunol. 2009;123(4):763–769. https://doi.org/10.1016/j.jaci.2008.12.013
- 2780. Blume SW, Yeomans K, Allen‐Ramey F, et al. Administration and burden of subcutaneous immunotherapy for allergic rhinitis in U.S. and Canadian Clinical Practice. J Manag Care Spec Pharm. 2015;21(11):982–990. https://doi.org/10.18553/jmcp.2015.21.11.982
- 2781. Kim JY, Han DH, Won TB, et al. Immunologic modification in mono‐ and poly‐sensitized patients after sublingual immunotherapy. Laryngoscope. 2019;129(5):E170–E177. https://doi.org/10.1002/lary.27721
- 2782. Song Y, Long J, Wang T, Xie J, Wang M, Tan G. Long‐term efficacy of standardised specific subcutaneous immunotherapy in children with persistent allergic rhinitis due to multiple allergens including house dust mites. J Laryngol Otol. 2018;132(3):230–235. https://doi.org/10.1017/S0022215117002547
- 2783. Search of: allergy immunotherapy | Recruiting . Not yet recruiting, Active, not recruiting Studies | Allergic Rhinitis ‐ List Results. Accessed November 8, 2021. https://clinicaltrials.gov/ct2/results?term=allergy+immunotherapy&cond=Allergic+Rhinitis&Search=Apply&recrs=b&recrs=a&recrs=d&age_v=&gndr=&type=&rslt=
- 2784. Nelson HS. Subcutaneous injection immunotherapy for optimal effectiveness. Immunol Allergy Clin North Am. 2011;31(2):211–226, viii. https://doi.org/10.1016/j.iac.2011.02.010
- 2785. Esch RE. Allergen immunotherapy: what can and cannot be mixed? J Allergy Clin Immunol. 2008;122(3):659–660. https://doi.org/10.1016/j.jaci.2008.07.018
- 2786. Grier TJ, LeFevre DM, Duncan EA, Esch RE, Coyne TC. Allergen stabilities and compatibilities in mixtures of high‐protease fungal and insect extracts. Ann Allergy Asthma Immunol. 2012;108(6):439–447. https://doi.org/10.1016/j.anai.2012.04.012
- 2787. Bozek A, Kolodziejczyk K, Warkocka‐Szoltysek B, Jarzab J. Grass pollen sublingual immunotherapy: a double‐blind, placebo‐controlled study in elderly patients with seasonal allergic rhinitis. Am J Rhinol Allergy. 2014;28(5):423–427. https://doi.org/10.2500/ajra.2014.28.4091
- 2788. Roberts G, Hurley C, Turcanu V, Lack G. Grass pollen immunotherapy as an effective therapy for childhood seasonal allergic asthma. J Allergy Clin Immunol. 2006;117(2):263–268. https://doi.org/10.1016/j.jaci.2005.09.054
- 2789. Cools M, Van Bever HP, Weyler JJ, Stevens WJ. Long‐term effects of specific immunotherapy, administered during childhood, in asthmatic patients allergic to either house‐dust mite or to both house‐dust mite and grass pollen. Allergy. 2000;55(1):69–73. https://doi.org/10.1034/j.1398‐9995.2000.00191.x
- 2790. Stelmach I, Sobocinska A, Majak P, Smejda K, Jerzynska J, Stelmach W. Comparison of the long‐term efficacy of 3‐ and 5‐year house dust mite allergen immunotherapy. Ann Allergy Asthma Immunol. 2012;109(4):274–278. https://doi.org/10.1016/j.anai.2012.07.015
- 2791. Pfaar O, Sager A, Robinson DS. Safety and effect on reported symptoms of depigmented polymerized allergen immunotherapy: a retrospective study of 2927 paediatric patients. Pediatr Allergy Immunol. 2015;26(3):280–286. https://doi.org/10.1111/pai.12347
- 2792. Wahn U, Bachert C, Heinrich J, Richter H, Zielen S. Real‐world benefits of allergen immunotherapy for birch pollen‐associated allergic rhinitis and asthma. Allergy. 2019;74(3):594–604. https://doi.org/10.1111/all.13598
- 2793. Zielen S, Kardos P, Madonini E. Steroid‐sparing effects with allergen‐specific immunotherapy in children with asthma: a randomized controlled trial. J Allergy Clin Immunol. 2010;126(5):942–949. https://doi.org/10.1016/j.jaci.2010.06.002
- 2794. Moller C, Dreborg S, Ferdousi HA, et al. Pollen immunotherapy reduces the development of asthma in children with seasonal rhinoconjunctivitis (the PAT‐study). J Allergy Clin Immunol. 2002;109(2):251–256. https://doi.org/10.1067/mai.2002.121317
- 2795. Niggemann B, Jacobsen L, Dreborg S, et al. Five‐year follow‐up on the PAT study: specific immunotherapy and long‐term prevention of asthma in children. Allergy. 2006;61(7):855–859. https://doi.org/10.1111/j.1398‐9995.2006.01068.x
- 2796. Devillier P, Molimard M, Ansolabehere X, et al. Immunotherapy with grass pollen tablets reduces medication dispensing for allergic rhinitis and asthma: a retrospective database study in France. Allergy. 2019;74(7):1317–1326. https://doi.org/10.1111/all.13705
- 2797. Lim CE, Sison CP, Ponda P. Comparison of pediatric and adult systemic reactions to subcutaneous immunotherapy. J Allergy Clin Immunol Pract. 2017;5(5):1241–1247.e2. https://doi.org/10.1016/j.jaip.2017.01.014
- 2798. Liu JL, Ning WX, Li SX, et al. The safety profile of subcutaneous allergen immunotherapy in children with asthma in Hangzhou, East China. Allergol Immunopathol (Madr). 2017;45(6):541–548. https://doi.org/10.1016/j.aller.2017.04.002
- 2799. Bousquet PJ, Castelli C, Daures JP, et al. Assessment of allergen sensitization in a general population‐based survey (European Community Respiratory Health Survey I). Ann Epidemiol. 2010;20(11):797–803. https://doi.org/10.1016/j.annepidem.2010.05.012
- 2800. Arbes Jr SJ, Gergen PJ, Elliott L, Zeldin DC. Prevalences of positive skin test responses to 10 common allergens in the US population: results from the third National Health and Nutrition Examination Survey. J Allergy Clin Immunol. 2005;116(2):377–383. https://doi.org/10.1016/j.jaci.2005.05.017
- 2801. Ballardini N, Bergstrom A, Wahlgren CF, et al. IgE antibodies in relation to prevalence and multimorbidity of eczema, asthma, and rhinitis from birth to adolescence. Allergy. 2016;71(3):342–349. https://doi.org/10.1111/all.12798
- 2802. Hauser M, Roulias A, Ferreira F, Egger M. Panallergens and their impact on the allergic patient. Allergy Asthma Clin Immunol. 2010;6(1):1. https://doi.org/10.1186/1710‐1492‐6‐1
- 2803. Aalberse RC, Aalberse JA. Molecular allergen‐specific IgE assays as a complement to allergen extract‐based sensitization assessment. J Allergy Clin Immunol Pract. 2015;3(6):863–869; quiz 870. https://doi.org/10.1016/j.jaip.2015.09.013
- 2804. Senna G, Lombardi C, Canonica GW, Passalacqua G. How adherent to sublingual immunotherapy prescriptions are patients? The manufacturers' viewpoint. J Allergy Clin Immunol. 2010;126(3):668–669. https://doi.org/10.1016/j.jaci.2010.06.045
- 2805. Kiotseridis H, Arvidsson P, Backer V, Braendholt V, Tunsater A. Adherence and quality of life in adults and children during 3‐years of SLIT treatment with Grazax‐a real life study. NPJ Prim Care Respir Med. 2018;28(1):4. https://doi.org/10.1038/s41533‐018‐0072‐z
- 2806. Chen H, Chen Y, Lin B, et al. Efficacy and adherence of sublingual immunotherapy in patients aged 60 to 75 years old with house dust mite‐induced allergic rhinitis. Am J Otolaryngol. 2020;41(4):102538. https://doi.org/10.1016/j.amjoto.2020.102538
- 2807. Vogelberg C, Bruggenjurgen B, Richter H, Jutel M. Real‐world adherence and evidence of subcutaneous and sublingual immunotherapy in grass and tree pollen‐induced allergic rhinitis and asthma. Patient Prefer Adherence. 2020;14:817–827. https://doi.org/10.2147/PPA.S242957
- 2808. Lemberg ML, Berk T, Shah‐Hosseini K, Kasche EM, Mosges R. Sublingual versus subcutaneous immunotherapy: patient adherence at a large German allergy center. Patient Prefer Adherence. 2017;11:63–70. https://doi.org/10.2147/PPA.S122948
- 2809. Stone B, Rance K, Waddell D, Aagren M, Hammerby E, Tkacz JP. Real‐world mapping of allergy immunotherapy in the United States: the argument for improving adherence. Allergy Asthma Proc. 2021;42(1):55–64. https://doi.org/10.2500/aap.2021.42.200114
- 2810. Liu W, Zeng Q, He C, et al. Compliance, efficacy, and safety of subcutaneous and sublingual immunotherapy in children with allergic rhinitis. Pediatr Allergy Immunol. 2021;32(1):86–91. https://doi.org/10.1111/pai.13332
- 2811. Hsu NM, Reisacher WR. A comparison of attrition rates in patients undergoing sublingual immunotherapy vs subcutaneous immunotherapy. Int Forum Allergy Rhinol. 2012;2(4):280–284. https://doi.org/10.1002/alr.21037
- 2812. Allam JP, Andreasen JN, Mette J, Serup‐Hansen N, Wustenberg EG. Comparison of allergy immunotherapy medication persistence with a sublingual immunotherapy tablet versus subcutaneous immunotherapy in Germany. J Allergy Clin Immunol. 2018;141(5):1898–1901.e5. https://doi.org/10.1016/j.jaci.2017.12.999
- 2813. Sorri M, Hartikainen‐Sorri AL, Karja J. Rhinitis during pregnancy. Rhinology. 1980;18(2):83–86.
- 2814. Incuado GA. Diagnosis and treatment of allergic rhinitis and sinusitis during pregnancy and lactation. Clin Rev Allergy Immunol. 2004;27(2):159–177.
- 2815. Murphy VE, Clifton VL, Gibson PG. Asthma exacerbations during pregnancy: incidence and association with adverse pregnancy outcomes. Thorax. 2006;61(2):169–176. https://doi.org/10.1136/thx.2005.049718
- 2816. Metzger WJ, Turner E, Patterson R. The safety of immunotherapy during pregnancy. J Allergy Clin Immunol. 1978;61(4):268–272. https://doi.org/10.1016/0091‐6749(78)90202‐6
- 2817. Dowdee A, Ossege J. Assessment of childhood allergy for the primary care practitioner. J Am Acad Nurse Pract. 2007;19(2):53–62. https://doi.org/10.1111/j.1745‐7599.2006.00195.x
- 2818. Berger WE. Allergic rhinitis in children: diagnosis and management strategies. Paediatr Drugs. 2004;6(4):233–250. https://doi.org/10.2165/00148581‐200406040‐00003
- 2819. Doulaptsi M, Aoi N, Kawauchi H, Milioni A, Karatzanis A, Prokopakis E. Differentiating rhinitis in the paediatric population by giving focus on medical history and clinical examination. Med Sci (Basel). 2019;7(3):38. https://doi.org/10.3390/medsci7030038
- 2820. Tharpe CA, Kemp SF. Pediatric allergic rhinitis. Immunol Allergy Clin North Am. 2015;35(1):185–198. https://doi.org/10.1016/j.iac.2014.09.003
- 2821. Sanford T. Allergic rhinitis in children. Mo Med. 2008;105(3):230–234.
- 2822. Roberts G, Xatzipsalti M, Borrego LM, et al. Paediatric rhinitis: position paper of the European Academy of Allergy and Clinical Immunology. Allergy. 2013;68(9):1102–1116. https://doi.org/10.1111/all.12235
- 2823. Izquierdo‐Dominguez A, Valero AL, Mullol J. Comparative analysis of allergic rhinitis in children and adults. Curr Allergy Asthma Rep. 2013;13(2):142–151. https://doi.org/10.1007/s11882‐012‐0331‐y
- 2824. Reshma A, Baranwal AK. Child with allergies or allergic reactions. Indian J Pediatr. 2018;85(1):60–65. https://doi.org/10.1007/s12098‐017‐2436‐8
- 2825. Lee VS, Lin SY. Allergy and the pediatric otolaryngologist. Otolaryngol Clin North Am. 2019;52(5):863–873. https://doi.org/10.1016/j.otc.2019.05.005
- 2826. Mims JW, Veling MC. Inhalant allergies in children. Otolaryngol Clin North Am. 2011;44(3):797–814, xi. https://doi.org/10.1016/j.otc.2011.03.013
- 2827. Kwon C, Lee HY, Kim MG, Boo SH, Yeo SG. Allergic diseases in children with otitis media with effusion. Int J Pediatr Otorhinolaryngol. 2013;77(2):158–161. https://doi.org/10.1016/j.ijporl.2012.09.039
- 2828. Roditi RE, Veling M, Shin JJ. Age: an effect modifier of the association between allergic rhinitis and Otitis media with effusion. Laryngoscope. 2016;126(7):1687–1692. https://doi.org/10.1002/lary.25682
- 2829. Olusesi AD, Undie NB, Amodu JE. Allergy history as a predictor of early onset adenoids/adenotonsillar hypertrophy among Nigerian children. Int J Pediatr Otorhinolaryngol. 2013;77(6):1032–1035. https://doi.org/10.1016/j.ijporl.2013.04.004
- 2830. Evcimik MF, Dogru M, Cirik AA, Nepesov MI. Adenoid hypertrophy in children with allergic disease and influential factors. Int J Pediatr Otorhinolaryngol. 2015;79(5):694–697. https://doi.org/10.1016/j.ijporl.2015.02.017
- 2831. Huang SW, Giannoni C. The risk of adenoid hypertrophy in children with allergic rhinitis. Ann Allergy Asthma Immunol. 2001;87(4):350–355. https://doi.org/10.1016/S1081‐1206(10)62251‐X
- 2832. La Mantia I, Andaloro C. Demographics and clinical features predictive of allergic versus non‐allergic rhinitis in children aged 6‐18 years: a single‐center experience of 1535 patients. Int J Pediatr Otorhinolaryngol. 2017;98:103–109. https://doi.org/10.1016/j.ijporl.2017.04.044
- 2833. Brown T. Diagnosis and management of allergic rhinitis in children. Pediatr Ann. 2019;48(12):e485–e488. https://doi.org/10.3928/19382359‐20191111‐01
- 2834. Eigenmann PA, Atanaskovic‐Markovic M, O'B Hourihane J, et al. Testing children for allergies: why, how, who and when: an updated statement of the European Academy of Allergy and Clinical Immunology (EAACI) Section on Pediatrics and the EAACI‐Clemens von Pirquet Foundation. Pediatr Allergy Immunol. 2013;24(2):195–209. https://doi.org/10.1111/pai.12066
- 2835. Host A, Andrae S, Charkin S, et al. Allergy testing in children: why, who, when and how? Allergy. 2003;58(7):559–569. https://doi.org/10.1034/j.1398‐9995.2003.00238.x
- 2836. Chawes BLK, Kreiner‐Moller E, Bisgaard H. Objective assessments of allergic and nonallergic rhinitis in young children. Allergy. 2009;64(10):1547–1553. https://doi.org/10.1111/j.1398‐9995.2009.02085.x
- 2837. Miller RE, Paradise JL, Friday GA, Fireman P, Voith D. The nasal smear for eosinophils. Its value in children with seasonal allergic rhinitis. Am J Dis Child. 1982;136(11):1009–1011. https://doi.org/10.1001/archpedi.1982.03970470053015
- 2838. Mierzejewska A, Jung A, Kalicki B. Nasal cytology as a marker of atopy in children. Dis Markers. 2017;2017:4159251. https://doi.org/10.1155/2017/4159251
- 2839. Jirapongsananuruk O, Vichyanond P. Nasal cytology in the diagnosis of allergic rhinitis in children. Ann Allergy Asthma Immunol. 1998;80(2):165–170. https://doi.org/10.1016/S1081‐1206(10)62950‐X
- 2840. Kaliner MA, Berger WE, Ratner PH, Siegel CJ. The efficacy of intranasal antihistamines in the treatment of allergic rhinitis. Ann Allergy Asthma Immunol. 2011;106(2 suppl):S6–S11. https://doi.org/10.1016/j.anai.2010.08.010
- 2841. Dibildox J. Safety and efficacy of mometasone furoate aqueous nasal spray in children with allergic rhinitis: results of recent clinical trials. J Allergy Clin Immunol. 2001;108(1 suppl):S54–S58. https://doi.org/10.1067/mai.2001.115567
- 2842. Roberge RJ, Hirani KH, Rowland 3rd PL, Berkeley R, Krenzelok EP. Dextromethorphan‐ and pseudoephedrine‐induced agitated psychosis and ataxia: case report. J Emerg Med. 1999;17(2):285–288. https://doi.org/10.1016/s0736‐4679(98)00193‐0
- 2843. Sauder KL, Brady Jr WJ, Hennes H. Visual hallucinations in a toddler: accidental ingestion of a sympathomimetic over‐the‐counter nasal decongestant. Am J Emerg Med. 1997;15(5):521–526. https://doi.org/10.1016/s0735‐6757(97)90200‐x
- 2844. Halken S, Lau S, Valovirta E. New visions in specific immunotherapy in children: an iPAC summary and future trends. Pediatr Allergy Immunol. 2008;19(suppl 19):60–70. https://doi.org/10.1111/j.1399‐3038.2008.00768.x
- 2845. Marogna M, Tomassetti D, Bernasconi A, et al. Preventive effects of sublingual immunotherapy in childhood: an open randomized controlled study. Ann Allergy Asthma Immunol. 2008;101(2):206–11. https://doi.org/10.1016/s1081‐1206(10)60211‐6
- 2846. Bousquet J, Pfaar O, Agache I, et al. ARIA‐EAACI care pathways for allergen immunotherapy in respiratory allergy. Clin Transl Allergy. 2021;11(4):e12014. https://doi.org/10.1002/clt2.12014
- 2847. Pitsios C, Demoly P, Bilo MB, et al. Clinical contraindications to allergen immunotherapy: an EAACI position paper. Allergy. 2015;70(8):897–909. https://doi.org/10.1111/all.12638
- 2848. Wenzel SE. Asthma phenotypes: the evolution from clinical to molecular approaches. Nat Med. 2012;18(5):716–725. https://doi.org/10.1038/nm.2678
- 2849. von Mutius E, Drazen JM. A patient with asthma seeks medical advice in 1828, 1928, and 2012. N Engl J Med. 2012;366(9):827–834. https://doi.org/10.1056/NEJMra1102783
- 2850. Global strategy for asthma management and prevention, 2021. Accessed November 6, 2021. http://www.ginasthma.org
- 2851. Kavanagh J, Jackson DJ, Kent BD. Over‐ and under‐diagnosis in asthma. Breathe (Sheff). 2019;15(1):e20–e27. https://doi.org/10.1183/20734735.0362‐2018
- 2852. Pavord ID, Beasley R, Agusti A, et al. After asthma: redefining airways diseases. Lancet. 2018;391(10118):350–400. https://doi.org/10.1016/S0140‐6736(17)30879‐6
- 2853. Chung KF, Wenzel SE, Brozek JL, et al. International ERS/ATS guidelines on definition, evaluation and treatment of severe asthma. Eur Respir J. 2014;43(2):343–373. https://doi.org/10.1183/09031936.00202013
- 2854. Pedersen CJ, Uddin MJ, Saha SK, Darmstadt GL. Prevalence of atopic dermatitis, asthma and rhinitis from infancy through adulthood in rural Bangladesh: a population‐based, cross‐sectional survey. BMJ Open. 2020;10(11):e042380. https://doi.org/10.1136/bmjopen‐2020‐042380
- 2855. Tohidinik HR, Mallah N, Takkouche B. History of allergic rhinitis and risk of asthma; a systematic review and meta‐analysis. World Allergy Organ J. 2019;12(10):100069. https://doi.org/10.1016/j.waojou.2019.100069
- 2856. Machluf Y, Farkash R, Rotkopf R, Fink D, Chaiter Y. Asthma phenotypes and associated comorbidities in a large cohort of adolescents in Israel. J Asthma. 2020;57(7):722–735. https://doi.org/10.1080/02770903.2019.1604743
- 2857. Heck S, Al‐Shobash S, Rapp D, et al. High probability of comorbidities in bronchial asthma in Germany. NPJ Prim Care Respir Med. 2017;27(1):28. https://doi.org/10.1038/s41533‐017‐0026‐x
- 2858. Pols DHJ, Bohnen AM, Nielen MMJ, Korevaar JC, Bindels PJE. Risks for comorbidity in children with atopic disorders: an observational study in Dutch general practices. BMJ Open. 2017;7(11):e018091. https://doi.org/10.1136/bmjopen‐2017‐018091
- 2859. Magnan A, Meunier JP, Saugnac C, Gasteau J, Neukirch F. Frequency and impact of allergic rhinitis in asthma patients in everyday general medical practice: a French observational cross‐sectional study. Allergy. 2008;63(3):292–298. https://doi.org/10.1111/j.1398‐9995.2007.01584.x
- 2860. de Groot EP, Nijkamp A, Duiverman EJ, Brand PL. Allergic rhinitis is associated with poor asthma control in children with asthma. Thorax. 2012;67(7):582–587. https://doi.org/10.1136/thoraxjnl‐2011‐201168
- 2861. Tay TR, Radhakrishna N, Hore‐Lacy F, et al. Comorbidities in difficult asthma are independent risk factors for frequent exacerbations, poor control and diminished quality of life. Respirology. 2016;21(8):1384–1390. https://doi.org/10.1111/resp.12838
- 2862. Deliu M, Belgrave D, Simpson A, Murray CS, Kerry G, Custovic A. Impact of rhinitis on asthma severity in school‐age children. Allergy. 2014;69(11):1515–1521. https://doi.org/10.1111/all.12467
- 2863. Holgate ST, Wenzel S, Postma DS, Weiss ST, Renz H, Sly PD. Asthma. Nat Rev Dis Primers. 2015;1:15025. https://doi.org/10.1038/nrdp.2015.25
- 2864. Togias A, Gergen PJ, Hu JW, et al. Rhinitis in children and adolescents with asthma: ubiquitous, difficult to control, and associated with asthma outcomes. J Allergy Clin Immunol. 2019;143(3):1003–1011.e10. https://doi.org/10.1016/j.jaci.2018.07.041
- 2865. Shaaban R, Zureik M, Soussan D, et al. Rhinitis and onset of asthma: a longitudinal population‐based study. Lancet. 2008;372(9643):1049–1057. https://doi.org/10.1016/S0140‐6736(08)61446‐4
- 2866. Fahy JV. Type 2 inflammation in asthma–present in most, absent in many. Nat Rev Immunol. 2015;15(1):57–65. https://doi.org/10.1038/nri3786
- 2867. Kuruvilla ME, Lee FE, Lee GB. Understanding asthma phenotypes, endotypes, and mechanisms of disease. Clin Rev Allergy Immunol. 2019;56(2):219–233. https://doi.org/10.1007/s12016‐018‐8712‐1
- 2868. Todd S, Walsted ES, Grillo L, Livingston R, Menzies‐Gow A, Hull JH. Novel assessment tool to detect breathing pattern disorder in patients with refractory asthma. Respirology. 2018;23(3):284–290. https://doi.org/10.1111/resp.13173
- 2869. Barker N, Thevasagayam R, Ugonna K, Kirkby J. Pediatric dysfunctional breathing: proposed components, mechanisms, diagnosis, and management. Front Pediatr. 2020;8:379. https://doi.org/10.3389/fped.2020.00379
- 2870. Seumois G, Zapardiel‐Gonzalo J, White B, et al. Transcriptional profiling of Th2 cells identifies pathogenic features associated with asthma. J Immunol. 2016;197(2):655–664. https://doi.org/10.4049/jimmunol.1600397
- 2871. Samitas K, Carter A, Kariyawasam HH, Xanthou G. Upper and lower airway remodelling mechanisms in asthma, allergic rhinitis and chronic rhinosinusitis: the one airway concept revisited. Allergy. 2018;73(5):993–1002. https://doi.org/10.1111/all.13373
- 2872. Ullmann N, Mirra V, Di Marco A, et al. Asthma: differential diagnosis and comorbidities. Front Pediatr. 2018;6:276. https://doi.org/10.3389/fped.2018.00276
- 2873. Shen Y, Zeng JH, Hong SL, Kang HY. Prevalence of allergic rhinitis comorbidity with asthma and asthma with allergic rhinitis in China: a meta‐analysis. Asian Pac J Allergy Immunol. 2019;37(4):220–225. https://doi.org/10.12932/AP‐120417‐0072
- 2874. Kou W, Li X, Yao H, Wei P. Meta‐analysis of the comorbidity rate of allergic rhinitis and asthma in Chinese children. Int J Pediatr Otorhinolaryngol. 2018;107:131–134. https://doi.org/10.1016/j.ijporl.2018.02.001
- 2875. Carr TF, Stern DA, Halonen M, Wright AL, Martinez FD. Non‐atopic rhinitis at age 6 is associated with subsequent development of asthma. Clin Exp Allergy. 2019;49(1):35–43. https://doi.org/10.1111/cea.13276
- 2876. Tosca MA, Duse M, Marseglia G, Ciprandi G, “ControL'Asma” Study Group . The practical clinical relevance of rhinitis classification in children with asthma: outcomes of the "ControL'Asma" study. Ann Allergy Asthma Immunol. 2019;123(5):516–519. https://doi.org/10.1016/j.anai.2019.08.003
- 2877. Kisiel MA, Zhou X, Sundh J, et al. Data‐driven questionnaire‐based cluster analysis of asthma in Swedish adults. NPJ Prim Care Respir Med. 2020;30(1):14. https://doi.org/10.1038/s41533‐020‐0168‐0
- 2878. Heffler E, Blasi F, Latorre M, et al. The severe asthma network in italy: findings and perspectives. J Allergy Clin Immunol Pract. 2019;7(5):1462–1468. https://doi.org/10.1016/j.jaip.2018.10.016
- 2879. Huang K, Yang T, Xu J, et al. Prevalence, risk factors, and management of asthma in China: a national cross‐sectional study. Lancet. 2019;394(10196):407–418. https://doi.org/10.1016/S0140‐6736(19)31147‐X
- 2880. Ji H, Hu Y, Zhang T, et al. Allergic comorbidity of asthma or wheezing, allergic rhinitis, and eczema: result from 333 029 allergic children in Shanghai, China. Am J Rhinol Allergy. 2020;34(2):189–195. https://doi.org/10.1177/1945892419883238
- 2881. Sonia T, Meriem M, Yacine O, et al. Prevalence of asthma and rhinitis in a Tunisian population. Clin Respir J. 2018;12(2):608–615. https://doi.org/10.1111/crj.12570
- 2882. Ziyab AH. Prevalence and risk factors of asthma, rhinitis, and eczema and their multimorbidity among young adults in Kuwait: a cross‐sectional study. Biomed Res Int. 2017;2017:2184193. https://doi.org/10.1155/2017/2184193
- 2883. Wright AL, Holberg CJ, Martinez FD, Halonen M, Morgan W, Taussig LM. Epidemiology of physician‐diagnosed allergic rhinitis in childhood. Pediatrics. 1994;94(6 pt 1):895–901.
- 2884. Settipane RJ, Settipane GA. IgE and the allergy‐asthma connection in the 23‐year follow‐up of Brown University students. Allergy Asthma Proc. 2000;21(4):221–225. https://doi.org/10.2500/108854100778248890
- 2885. Guerra S, Sherrill DL, Baldacci S, et al. Rhinitis is an independent risk factor for developing cough apart from colds among adults. Allergy. 2005;60(3):343–349. https://doi.org/10.1111/j.1398‐9995.2005.00717.x
- 2886. Toren K, Olin AC, Hellgren J, Hermansson BA. Rhinitis increase the risk for adult‐onset asthma–a Swedish population‐based case‐control study (MAP‐study). Respir Med. 2002;96(8):635–641. https://doi.org/10.1053/rmed.2002.1319
- 2887. Shaaban R, Zureik M, Soussan D, et al. Allergic rhinitis and onset of bronchial hyperresponsiveness: a population‐based study. Am J Respir Crit Care Med. 2007;176(7):659–666. https://doi.org/10.1164/rccm.200703‐427OC
- 2888. Rochat MK, Illi S, Ege MJ, et al. Allergic rhinitis as a predictor for wheezing onset in school‐aged children. J Allergy Clin Immunol. 2010;126(6):1170–1175.e2. https://doi.org/10.1016/j.jaci.2010.09.008
- 2889. Hamouda S, Karila C, Connault T, Scheinmann P, de Blic J. Allergic rhinitis in children with asthma: a questionnaire‐based study. Clin Exp Allergy. 2008;38(5):761–766. https://doi.org/10.1111/j.1365‐2222.2008.02953.x
- 2890. Antonicelli L, Micucci C, Voltolini S, et al. Allergic rhinitis and asthma comorbidity: ARIA classification of rhinitis does not correlate with the prevalence of asthma. Clin Exp Allergy. 2007;37(6):954–960. https://doi.org/10.1111/j.1365‐2222.2007.02729.x
- 2891. Jung S, Lee SY, Yoon J, et al. Risk factors and comorbidities associated with the allergic rhinitis phenotype in children according to the ARIA classification. Allergy Asthma Immunol Res. 2020;12(1):72–85. https://doi.org/10.4168/aair.2020.12.1.72
- 2892. Sio YY, Pang SL, Say YH, et al. Sensitization to airborne fungal allergens associates with asthma and allergic rhinitis presentation and severity in the Singaporean/Malaysian population. Mycopathologia. 2021;186(5):583–588. https://doi.org/10.1007/s11046‐021‐00532‐6
- 2893. Arshad SH, Tariq SM, Matthews S, Hakim E. Sensitization to common allergens and its association with allergic disorders at age 4 years: a whole population birth cohort study. Pediatrics. 2001;108(2):E33. https://doi.org/10.1542/peds.108.2.e33
- 2894. Bonay M, Neukirch C, Grandsaigne M, et al. Changes in airway inflammation following nasal allergic challenge in patients with seasonal rhinitis. Allergy. 2006;61(1):111–118. https://doi.org/10.1111/j.1398‐9995.2006.00967.x
- 2895. Panganiban RP, Wang Y, Howrylak J, et al. Circulating microRNAs as biomarkers in patients with allergic rhinitis and asthma. J Allergy Clin Immunol. 2016;137(5):1423–1432. https://doi.org/10.1016/j.jaci.2016.01.029
- 2896. Deng Q, Lu C, Yu Y, Li Y, Sundell J, Norback D. Early life exposure to traffic‐related air pollution and allergic rhinitis in preschool children. Respir Med. 2016;121:67–73. https://doi.org/10.1016/j.rmed.2016.10.016
- 2897. Wang J, Zhang Y, Li B, et al. Asthma and allergic rhinitis among young parents in China in relation to outdoor air pollution, climate and home environment. Sci Total Environ. 2021;751:141734. https://doi.org/10.1016/j.scitotenv.2020.141734
- 2898. Nordeide Kuiper I, Svanes C, Markevych I, et al. Lifelong exposure to air pollution and greenness in relation to asthma, rhinitis and lung function in adulthood. Environ Int. 2021;146:106219. https://doi.org/10.1016/j.envint.2020.106219
- 2899. Polosa R, Knoke JD, Russo C, et al. Cigarette smoking is associated with a greater risk of incident asthma in allergic rhinitis. J Allergy Clin Immunol. 2008;121(6):1428–1434. https://doi.org/10.1016/j.jaci.2008.02.041
- 2900. Ma T, Chen Y, Pang Y, et al. Prevalence and risk factors of allergic rhinitis and asthma in the southern edge of the plateau grassland region of northern China: a cross‐sectional study. World Allergy Organ J. 2021;14(7):100537. https://doi.org/10.1016/j.waojou.2021.100537
- 2901. Ibanez MD, Valero AL, Montoro J, et al. Analysis of comorbidities and therapeutic approach for allergic rhinitis in a pediatric population in Spain. Pediatr Allergy Immunol. 2013;24(7):678–684. https://doi.org/10.1111/pai.12126
- 2902. Burgess JA, Walters EH, Byrnes GB, et al. Childhood allergic rhinitis predicts asthma incidence and persistence to middle age: a longitudinal study. J Allergy Clin Immunol. 2007;120(4):863–869. https://doi.org/10.1016/j.jaci.2007.07.020
- 2903. Bodtger U, Poulsen LK, Linneberg A. Rhinitis symptoms and IgE sensitization as risk factors for development of later allergic rhinitis in adults. Allergy. 2006;61(6):712–716. https://doi.org/10.1111/j.1398‐9995.2006.01140.x
- 2904. Porsbjerg C, von Linstow ML, Ulrik CS, Nepper‐Christensen S, Backer V. Risk factors for onset of asthma: a 12‐year prospective follow‐up study. Chest. 2006;129(2):309–316. https://doi.org/10.1378/chest.129.2.309
- 2905. Plaschke PP, Janson C, Norrman E, Bjornsson E, Ellbjar S, Jarvholm B. Onset and remission of allergic rhinitis and asthma and the relationship with atopic sensitization and smoking. Am J Respir Crit Care Med. 2000;162(3 pt 1):920–924. https://doi.org/10.1164/ajrccm.162.3.9912030
- 2906. Corren J. Allergic rhinitis and asthma: how important is the link? J Allergy Clin Immunol. 1997;99(2):S781–S786. https://doi.org/10.1016/s0091‐6749(97)70127‐1
- 2907. Corren J. The impact of allergic rhinitis on bronchial asthma. J Allergy Clin Immunol. 1998;101(2 pt 2):S352–S356. https://doi.org/10.1016/s0091‐6749(98)70218‐0
- 2908. Jeffery PK, Haahtela T. Allergic rhinitis and asthma: inflammation in a one‐airway condition. BMC Pulm Med. 2006;6(suppl 1):S5. https://doi.org/10.1186/1471‐2466‐6‐S1‐S5
- 2909. Bhimrao SK, Wilson SJ, Howarth PH. Airway inflammation in atopic patients: a comparison of the upper and lower airways. Otolaryngol Head Neck Surg. 2011;145(3):396–400. https://doi.org/10.1177/0194599811410531
- 2910. Eriksson J, Bjerg A, Lotvall J, et al. Rhinitis phenotypes correlate with different symptom presentation and risk factor patterns of asthma. Respir Med. 2011;105(11):1611–1621. https://doi.org/10.1016/j.rmed.2011.06.004
- 2911. Kersten ET, van Leeuwen JC, Brand PL, et al. Effect of an intranasal corticosteroid on exercise induced bronchoconstriction in asthmatic children. Pediatr Pulmonol. 2012;47(1):27–35. https://doi.org/10.1002/ppul.21511
- 2912. Reed CE, Marcoux JP, Welsh PW. Effects of topical nasal treatment on asthma symptoms. J Allergy Clin Immunol. 1988;81(5 pt 2):1042–1047. https://doi.org/10.1016/0091‐6749(88)90177‐7
- 2913. Corren J, Adinoff AD, Buchmeier AD, Irvin CG. Nasal beclomethasone prevents the seasonal increase in bronchial responsiveness in patients with allergic rhinitis and asthma. J Allergy Clin Immunol. 1992;90(2):250–256. https://doi.org/10.1016/0091‐6749(92)90079‐h
- 2914. De Jong HJI, Voorham J, Scadding GK, et al. Evaluating the real‐life effect of MP‐AzeFlu on asthma outcomes in patients with allergic rhinitis and asthma in UK primary care. World Allergy Organ J. 2020;13(12):100490. https://doi.org/10.1016/j.waojou.2020.100490
- 2915. Chyrek‐Borowska S, Siergiejko Z, Michalska I. The effects of a new generation of H1 antihistamines (cetirizine and loratadine) on histamine release and the bronchial response to histamine in atopic patients. J Investig Allergol Clin Immunol. 1995;5(2):103–107.
- 2916. Wasserfallen JB, Leuenberger P, Pecoud A. Effect of cetirizine, a new H1 antihistamine, on the early and late allergic reactions in a bronchial provocation test with allergen. J Allergy Clin Immunol. 1993;91(6):1189–1197. https://doi.org/10.1016/0091‐6749(93)90322‐7
- 2917. Nishimura M, Koga T, Kamimura T, et al. Comparison of leukotriene receptor antagonists and anti‐histamines as an add‐on therapy in patients with asthma complicated by allergic rhinitis. Kurume Med J. 2011;58(1):9–14. https://doi.org/10.2739/kurumemedj.58.9
- 2918. Grembiale RD, Camporota L, Naty S, Tranfa CM, Djukanovic R, Marsico SA. Effects of specific immunotherapy in allergic rhinitic individuals with bronchial hyperresponsiveness. Am J Respir Crit Care Med. 2000;162(6):2048–2052. https://doi.org/10.1164/ajrccm.162.6.9909087
- 2919. Rak S, Lowhagen O, Venge P. The effect of immunotherapy on bronchial hyperresponsiveness and eosinophil cationic protein in pollen‐allergic patients. J Allergy Clin Immunol. 1988;82(3 pt 1):470–480. https://doi.org/10.1016/0091‐6749(88)90021‐8
- 2920. Novembre E, Galli E, Landi F, et al. Coseasonal sublingual immunotherapy reduces the development of asthma in children with allergic rhinoconjunctivitis. J Allergy Clin Immunol. 2004;114(4):851–857. https://doi.org/10.1016/j.jaci.2004.07.012
- 2921. Suissa S, Ernst P. Bias in observational study of the effectiveness of nasal corticosteroids in asthma. J Allergy Clin Immunol. 2005;115(4):714–719. https://doi.org/10.1016/j.jaci.2004.12.1118
- 2922. D'Amato G, Ortega OPM, Annesi‐Maesano I, D'Amato M. Prevention of allergic asthma with allergen avoidance measures and the role of exposome. Curr Allergy Asthma Rep. 2020;20(3):8. https://doi.org/10.1007/s11882‐020‐0901‐3
- 2923. Gotzsche PC, Johansen HK. House dust mite control measures for asthma. Cochrane Database Syst Rev. 2008;(2):CD001187. https://doi.org/10.1002/14651858.CD001187.pub3
- 2924. Terreehorst I, Duivenvoorden HJ, Tempels‐Pavlica Z, et al. The effect of encasings on quality of life in adult house dust mite allergic patients with rhinitis, asthma and/or atopic dermatitis. Allergy. 2005;60(7):888–93. https://doi.org/10.1111/j.1398‐9995.2004.00677.x
- 2925. Pasquali M, Baiardini I, Rogkakou A, et al. Levocetirizine in persistent allergic rhinitis and asthma: effects on symptoms, quality of life and inflammatory parameters. Clin Exp Allergy. 2006;36(9):1161–1167. https://doi.org/10.1111/j.1365‐2222.2006.02548.x
- 2926. Baena‐Cagnani CE, Berger WE, DuBuske LM, et al. Comparative effects of desloratadine versus montelukast on asthma symptoms and use of beta 2‐agonists in patients with seasonal allergic rhinitis and asthma. Int Arch Allergy Immunol. 2003;130(4):307–313. https://doi.org/10.1159/000070218
- 2927. Berger WE, Schenkel EJ, Mansfield LE, Desloratadine Study Group . Safety and efficacy of desloratadine 5 mg in asthma patients with seasonal allergic rhinitis and nasal congestion. Ann Allergy Asthma Immunol. 2002;89(5):485–491. https://doi.org/10.1016/S1081‐1206(10)62086‐8
- 2928. Grant JA, Nicodemus CF, Findlay SR, et al. Cetirizine in patients with seasonal rhinitis and concomitant asthma: prospective, randomized, placebo‐controlled trial. J Allergy Clin Immunol. 1995;95(5 pt 1):923–932. https://doi.org/10.1016/s0091‐6749(95)70090‐0
- 2929. Aubier M, Neukirch C, Peiffer C, Melac M. Effect of cetirizine on bronchial hyperresponsiveness in patients with seasonal allergic rhinitis and asthma. Allergy. 2001;56(1):35–42. https://doi.org/10.1034/j.1398‐9995.2001.00629.x
- 2930. Aaronson DW. Evaluation of cetirizine in patients with allergic rhinitis and perennial asthma. Ann Allergy Asthma Immunol. 1996;76(5):440–446. https://doi.org/10.1016/S1081‐1206(10)63461‐8
- 2931. Simons FE. Is antihistamine (H1‐receptor antagonist) therapy useful in clinical asthma? Clin Exp Allergy. 1999;29(suppl 3):98–104. https://doi.org/10.1046/j.1365‐2222.1999.0290s3098.x
- 2932. Bousquet J, Emonot A, Germouty J, et al. Double‐blind multicenter study of cetirizine in grass‐pollen‐induced asthma. Ann Allergy. 1990;65(6):504–508.
- 2933. Van Ganse E, Kaufman L, Derde MP, Yernault JC, Delaunois L, Vincken W. Effects of antihistamines in adult asthma: a meta‐analysis of clinical trials. Eur Respir J. 1997;10(10):2216–2224. https://doi.org/10.1183/09031936.97.10102216
- 2934. Allergic factors associated with the development of asthma and the influence of cetirizine in a double‐blind, randomised, placebo‐controlled trial: first results of ETAC. Early Treatment of the Atopic Child. Pediatr Allergy Immunol. 1998;9(3):116–124.
- 2935. Bhargava S, Prakash A, Rehan HS, Gupta LK. Effect of systemic corticosteroids on serum apoptotic markers and quality of life in patients with asthma. Allergy Asthma Proc. 2015;36(4):275–282. https://doi.org/10.2500/aap.2015.36.3834
- 2936. Sood V, Rogers L, Khurana S. Managing corticosteroid‐related comorbidities in severe asthma. Chest. 2021;160(5):1614–1623. https://doi.org/10.1016/j.chest.2021.05.021
- 2937. Henriksen JM, Wenzel A. Effect of an intranasally administered corticosteroid (budesonide) on nasal obstruction, mouth breathing, and asthma. Am Rev Respir Dis. 1984;130(6):1014–1048. https://doi.org/10.1164/arrd.1984.130.6.1014
- 2938. Dahl R, Nielsen LP, Kips J, et al. Intranasal and inhaled fluticasone propionate for pollen‐induced rhinitis and asthma. Allergy. 2005;60(7):875–881. https://doi.org/10.1111/j.1398‐9995.2005.00819.x
- 2939. Nathan RA, Yancey SW, Waitkus‐Edwards K, et al. Fluticasone propionate nasal spray is superior to montelukast for allergic rhinitis while neither affects overall asthma control. Chest. 2005;128(4):1910–1920. https://doi.org/10.1378/chest.128.4.1910
- 2940. Stelmach R, do Patrocinio TNM, Ribeiro M, Cukier A. Effect of treating allergic rhinitis with corticosteroids in patients with mild‐to‐moderate persistent asthma. Chest. 2005;128(5):3140–3147. https://doi.org/10.1378/chest.128.5.3140
- 2941. Thio BJ, Slingerland GL, Fredriks AM, et al. Influence of intranasal steroids during the grass pollen season on bronchial responsiveness in children and young adults with asthma and hay fever. Thorax. 2000;55(10):826–832. https://doi.org/10.1136/thorax.55.10.826
- 2942. Baiardini I, Villa E, Rogkakou A, et al. Effects of mometasone furoate on the quality of life: a randomized placebo‐controlled trial in persistent allergic rhinitis and intermittent asthma using the Rhinasthma questionnaire. Clin Exp Allergy. 2011;41(3):417–423. https://doi.org/10.1111/j.1365‐2222.2010.03660.x
- 2943. Nair A, Vaidyanathan S, Clearie K, Williamson P, Meldrum K, Lipworth BJ. Steroid sparing effects of intranasal corticosteroids in asthma and allergic rhinitis. Allergy. 2010;65(3):359–367. https://doi.org/10.1111/j.1398‐9995.2009.02187.x
- 2944. Agondi RC, Machado ML, Kalil J, Giavina‐Bianchi P. Intranasal corticosteroid administration reduces nonspecific bronchial hyperresponsiveness and improves asthma symptoms. J Asthma. 2008;45(9):754–757. https://doi.org/10.1080/02770900802249149
- 2945. Pedroletti C, Lundahl J, Alving K, Hedlin G. Effect of nasal steroid treatment on airway inflammation determined by exhaled nitric oxide in allergic schoolchildren with perennial rhinitis and asthma. Pediatr Allergy Immunol. 2008;19(3):219–226. https://doi.org/10.1111/j.1399‐3038.2007.00613.x
- 2946. Watson WT, Becker AB, Simons FE. Treatment of allergic rhinitis with intranasal corticosteroids in patients with mild asthma: effect on lower airway responsiveness. J Allergy Clin Immunol. 1993;91(1 pt 1):97–101. https://doi.org/10.1016/0091‐6749(93)90301‐u
- 2947. Gani F, Pozzi E, Crivellaro MA, et al. The role of patient training in the management of seasonal rhinitis and asthma: clinical implications. Allergy. 2001;56(1):65–68. https://doi.org/10.1034/j.1398‐9995.2001.00794.x
- 2948. Meltzer EO. Role for cysteinyl leukotriene receptor antagonist therapy in asthma and their potential role in allergic rhinitis based on the concept of "one linked airway disease". Ann Allergy Asthma Immunol. 2000;84(2):176–185; quiz 185‐7. https://doi.org/10.1016/S1081‐1206(10)62750‐0
- 2949. Egan M, Bunyavanich S. Allergic rhinitis: the "Ghost Diagnosis" in patients with asthma. Asthma Res Pract. 2015;1:8. https://doi.org/10.1186/s40733‐015‐0008‐0
- 2950. Nowak D. Management of asthma with anti‐immunoglobulin E: a review of clinical trials of omalizumab. Respir Med. 2006;100(11):1907–1917. https://doi.org/10.1016/j.rmed.2005.10.004
- 2951. Bousquet J, Cabrera P, Berkman N, et al. The effect of treatment with omalizumab, an anti‐IgE antibody, on asthma exacerbations and emergency medical visits in patients with severe persistent asthma. Allergy. 2005;60(3):302–308. https://doi.org/10.1111/j.1398‐9995.2004.00770.x
- 2952. D'Amato G, Salzillo A, Piccolo A, D'Amato M, Liccardi G. A review of anti‐IgE monoclonal antibody (omalizumab) as add on therapy for severe allergic (IgE‐mediated) asthma. Ther Clin Risk Manag. 2007;3(4):613–619.
- 2953. Normansell R, Walker S, Milan SJ, Walters EH, Nair P. Omalizumab for asthma in adults and children. Cochrane Database Syst Rev. 2014;(1):CD003559. https://doi.org/10.1002/14651858.CD003559.pub4
- 2954. Humbert M, Boulet LP, Niven RM, Panahloo Z, Blogg M, Ayre G. Omalizumab therapy: patients who achieve greatest benefit for their asthma experience greatest benefit for rhinitis. Allergy. 2009;64(1):81–84. https://doi.org/10.1111/j.1398‐9995.2008.01846.x
- 2955. Vignola AM, Humbert M, Bousquet J, et al. Efficacy and tolerability of anti‐immunoglobulin E therapy with omalizumab in patients with concomitant allergic asthma and persistent allergic rhinitis: SOLAR. Allergy. 2004;59(7):709–717. https://doi.org/10.1111/j.1398‐9995.2004.00550.x
- 2956. Erekosima N, Suarez‐Cuervo C, Ramanathan M, et al. Effectiveness of subcutaneous immunotherapy for allergic rhinoconjunctivitis and asthma: a systematic review. Laryngoscope. 2014;124(3):616–627. https://doi.org/10.1002/lary.24295
- 2957. Sidenius K, Arvidsson P, Indbryn R, Emanuelsson CA. A real‐life one‐year non‐interventional study assessing safety, tolerability, and treatment outcome of the SQ HDM SLIT‐Tablet (Acarizax((R))) in house dust mite allergic rhinitis with or without asthma. Pulm Ther. 2021;7(1):221–236. https://doi.org/10.1007/s41030‐021‐00150‐z
- 2958. Inal A, Altintas DU, Yilmaz M, Karakoc GB, Kendirli SG, Sertdemir Y. Prevention of new sensitizations by specific immunotherapy in children with rhinitis and/or asthma monosensitized to house dust mite. J Investig Allergol Clin Immunol. 2007;17(2):85–91.
- 2959. Purello‐D'Ambrosio F, Gangemi S, Merendino RA, et al. Prevention of new sensitizations in monosensitized subjects submitted to specific immunotherapy or not. A retrospective study. Clin Exp Allergy. 2001;31(8):1295–1302. https://doi.org/10.1046/j.1365‐2222.2001.01027.x
- 2960. Di Lorenzo G, Leto‐Barone MS, La Piana S, Plaia A, Di Bona D. The effect of allergen immunotherapy in the onset of new sensitizations: a meta‐analysis. Int Forum Allergy Rhinol. 2017;7(7):660–669. https://doi.org/10.1002/alr.21946
- 2961. Fortescue R, Kew KM, Leung MST. Sublingual immunotherapy for asthma. Cochrane Database Syst Rev. 2020;9:CD011293. https://doi.org/10.1002/14651858.CD011293.pub3
- 2962. Global Initiative for Asthma . Global Strategy for Asthma Management and Prevention, 2019. Accessed July 4, 2022. http://www.ginasthma.org
- 2963. 2020 Focused Update to the Asthma Management Guidelines: Clinician's Guide. Accessed July 4, 2022. https://www.nhlbi.nih.gov/sites/default/files/publications/AsthmaCliniciansGuideDesign‐508.pdf
- 2964. Katial RK, Oppenheimer JJ, Ostrom NK, et al. Adding montelukast to fluticasone propionate/salmeterol for control of asthma and seasonal allergic rhinitis. Allergy Asthma Proc. 2010;31(1):68–75. https://doi.org/10.2500/aap.2010.31.3306
- 2965. Price DB, Swern A, Tozzi CA, Philip G, Polos P. Effect of montelukast on lung function in asthma patients with allergic rhinitis: analysis from the COMPACT trial. Allergy. 2006;61(6):737–742. https://doi.org/10.1111/j.1398‐9995.2006.01007.x
- 2966. Marcus S, Roland LT, DelGaudio JM, Wise SK. The relationship between allergy and chronic rhinosinusitis. Laryngoscope Investig Otolaryngol. 2019;4(1):13–17. https://doi.org/10.1002/lio2.236
- 2967. Wilson KF, McMains KC, Orlandi RR. The association between allergy and chronic rhinosinusitis with and without nasal polyps: an evidence‐based review with recommendations. Int Forum Allergy Rhinol. 2014;4(2):93–103. https://doi.org/10.1002/alr.21258
- 2968. Baroody FM, Mucha SM, Detineo M, Naclerio RM. Nasal challenge with allergen leads to maxillary sinus inflammation. J Allergy Clin Immunol. 2008;121(5):1126–1132.e7. https://doi.org/10.1016/j.jaci.2008.02.010
- 2969. Tan BK, Zirkle W, Chandra RK, et al. Atopic profile of patients failing medical therapy for chronic rhinosinusitis. Int Forum Allergy Rhinol. 2011;1(2):88–94. https://doi.org/10.1002/alr.20025
- 2970. Pearlman AN, Chandra RK, Chang D, et al. Relationships between severity of chronic rhinosinusitis and nasal polyposis, asthma, and atopy. Am J Rhinol Allergy. 2009;23(2):145–148. https://doi.org/10.2500/ajra.2009.23.3284
- 2971. Gelincik A, Buyukozturk S, Aslan I, et al. Allergic vs nonallergic rhinitis: which is more predisposing to chronic rhinosinusitis? Ann Allergy Asthma Immunol. 2008;101(1):18–22. https://doi.org/10.1016/S1081‐1206(10)60829‐0
- 2972. Kirtsreesakul V, Ruttanaphol S. The relationship between allergy and rhinosinusitis. Rhinology. 2008;46(3):204–208.
- 2973. Robinson S, Douglas R, Wormald PJ. The relationship between atopy and chronic rhinosinusitis. Am J Rhinol. 2006;20(6):625–628. https://doi.org/10.2500/ajr.2006.20.2907
- 2974. Alho OP, Karttunen R, Karttunen TJ. Nasal mucosa in natural colds: effects of allergic rhinitis and susceptibility to recurrent sinusitis. Clin Exp Immunol. 2004;137(2):366–372. https://doi.org/10.1111/j.1365‐2249.2004.02530.x
- 2975. Van Zele T, Gevaert P, Watelet JB, et al. Staphylococcus aureus colonization and IgE antibody formation to enterotoxins is increased in nasal polyposis. J Allergy Clin Immunol. 2004;114(4):981–983. https://doi.org/10.1016/j.jaci.2004.07.013
- 2976. Berrettini S, Carabelli A, Sellari‐Franceschini S, et al. Perennial allergic rhinitis and chronic sinusitis: correlation with rhinologic risk factors. Allergy. 1999;54(3):242–248. https://doi.org/10.1034/j.1398‐9995.1999.00813.x
- 2977. Al‐Qudah M. Food sensitization in medically resistant chronic rhinosinusitis with or without nasal polyposis. Int Arch Allergy Immunol. 2016;169(1):40–44. https://doi.org/10.1159/000443737
- 2978. Li QC, Cheng KJ, Wang F, Zhou SH. Role of atopy in chronic rhinosinusitis with nasal polyps: does an atopic condition affect the severity and recurrence of disease? J Laryngol Otol. 2016;130(7):640–644. https://doi.org/10.1017/S0022215116008112
- 2979. Houser SM, Keen KJ. The role of allergy and smoking in chronic rhinosinusitis and polyposis. Laryngoscope. 2008;118(9):1521–1527. https://doi.org/10.1097/MLG.0b013e31817d01b8
- 2980. Kirtsreesakul V. Role of allergy in the therapeutic response of nasal polyps. Asian Pac J Allergy Immunol. 2002;20(3):141–146.
- 2981. Gorgulu O, Ozdemir S, Canbolat EP, Sayar C, Olgun MK, Akbas Y. Analysis of the roles of smoking and allergy in nasal polyposis. Ann Otol Rhinol Laryngol. 2012;121(9):615–619. https://doi.org/10.1177/000348941212100909
- 2982. Lill C, Loader B, Seemann R, et al. Milk allergy is frequent in patients with chronic sinusitis and nasal polyposis. Am J Rhinol Allergy. 2011;25(6):e221–e224. https://doi.org/10.2500/ajra.2011.25.3686
- 2983. Munoz del Castillo F, Jurado‐Ramos A, Fernandez‐Conde BL, et al. Allergenic profile of nasal polyposis. J Investig Allergol Clin Immunol. 2009;19(2):110–116.
- 2984. Bonfils P, Malinvaud D. Influence of allergy in patients with nasal polyposis after endoscopic sinus surgery. Acta Otolaryngol. 2008;128(2):186–192. https://doi.org/10.1080/00016480701387165
- 2985. Erbek SS, Erbek S, Topal O, Cakmak O. The role of allergy in the severity of nasal polyposis. Am J Rhinol. 2007;21(6):686–690. https://doi.org/10.2500/ajr.2007.21.3062
- 2986. Bonfils P, Avan P, Malinvaud D. Influence of allergy on the symptoms and treatment of nasal polyposis. Acta Otolaryngol. 2006;126(8):839–844. https://doi.org/10.1080/00016480500504226
- 2987. Collins MM, Loughran S, Davidson P, Wilson JA. Nasal polyposis: prevalence of positive food and inhalant skin tests. Otolaryngol Head Neck Surg. 2006;135(5):680–683. https://doi.org/10.1016/j.otohns.2006.07.005
- 2988. Asero R, Bottazzi G. Nasal polyposis: a study of its association with airborne allergen hypersensitivity. Ann Allergy Asthma Immunol. 2001;86(3):283–285. https://doi.org/10.1016/S1081‐1206(10)63299‐1
- 2989. Voegels RL, Santoro P, Butugan O, Formigoni LG. Nasal polyposis and allergy: is there a correlation? Am J Rhinol. 2001;15(1):9–14. https://doi.org/10.2500/105065801781329365
- 2990. Asero R, Bottazzi G. Hypersensitivity to molds in patients with nasal polyposis: a clinical study. J Allergy Clin Immunol. 2000;105(1 pt 1):186–188. https://doi.org/10.1016/s0091‐6749(00)90198‐2
- 2991. Pang YT, Eskici O, Wilson JA. Nasal polyposis: role of subclinical delayed food hypersensitivity. Otolaryngol Head Neck Surg. 2000;122(2):298–301. https://doi.org/10.1016/S0194‐5998(00)70259‐2
- 2992. Pumhirun P, Limitlaohapanth C, Wasuwat P. Role of allergy in nasal polyps of Thai patients. Asian Pac J Allergy Immunol. 1999;17(1):13–15.
- 2993. Keith PK, Conway M, Evans S, et al. Nasal polyps: effects of seasonal allergen exposure. J Allergy Clin Immunol. 1994;93(3):567–574. https://doi.org/10.1016/s0091‐6749(94)70068‐0
- 2994. Ence BK, Gourley DS, Jorgensen NL, et al. Allergic fungal sinusitis. Am J Rhinol. 1990;4:169–178.
- 2995. Schubert MS. Allergic fungal sinusitis. Otolaryngol Clin North Am. 2004;37(2):301–326. https://doi.org/10.1016/S0030‐6665(03)00152‐X
- 2996. Bent 3rd JP, Kuhn FA. Diagnosis of allergic fungal sinusitis. Otolaryngol Head Neck Surg. 1994;111(5):580–588. https://doi.org/10.1177/019459989411100508
- 2997. Saravanan K, Panda NK, Chakrabarti A, Das A, Bapuraj RJ. Allergic fungal rhinosinusitis: an attempt to resolve the diagnostic dilemma. Arch Otolaryngol Head Neck Surg. 2006;132(2):173–178. https://doi.org/10.1001/archotol.132.2.173
- 2998. Hutcheson PS, Schubert MS, Slavin RG. Distinctions between allergic fungal rhinosinusitis and chronic rhinosinusitis. Am J Rhinol Allergy. 2010;24(6):405–408. https://doi.org/10.2500/ajra.2010.24.3533
- 2999. Manning SC, Mabry RL, Schaefer SD, Close LG. Evidence of IgE‐mediated hypersensitivity in allergic fungal sinusitis. Laryngoscope. 1993;103(7):717–721. https://doi.org/10.1288/00005537‐199307000‐00002
- 3000. Stewart AE, Hunsaker DH. Fungus‐specific IgG and IgE in allergic fungal rhinosinusitis. Otolaryngol Head Neck Surg. 2002;127(4):324–332. https://doi.org/10.1067/mhn.2002.126801
- 3001. Ryan MW, Marple BF. Allergic fungal rhinosinusitis: diagnosis and management. Curr Opin Otolaryngol Head Neck Surg. 2007;15(1):18–22. https://doi.org/10.1097/MOO.0b013e328013dbd9
- 3002. Collins M, Nair S, Smith W, Kette F, Gillis D, Wormald PJ. Role of local immunoglobulin E production in the pathophysiology of noninvasive fungal sinusitis. Laryngoscope. 2004;114(7):1242–1246. https://doi.org/10.1097/00005537‐200407000‐00019
- 3003. Wise SK, Ahn CN, Lathers DM, Mulligan RM, Schlosser RJ. Antigen‐specific IgE in sinus mucosa of allergic fungal rhinosinusitis patients. Am J Rhinol. 2008;22(5):451–456. https://doi.org/10.2500/ajr.2008.22.3227
- 3004. Chang YT, Fang SY. Tissue‐specific immunoglobulin E in maxillary sinus mucosa of allergic fungal sinusitis. Rhinology. 2008;46(3):226–230.
- 3005. Kuhn FA, Swain Jr R. Allergic fungal sinusitis: diagnosis and treatment. Curr Opin Otolaryngol Head Neck Surg. 2003;11(1):1–5. https://doi.org/10.1097/00020840‐200302000‐00001
- 3006. Mabry RL, Marple BF, Folker RJ, Mabry CS. Immunotherapy for allergic fungal sinusitis: three years' experience. Otolaryngol Head Neck Surg. 1998;119(6):648–651. https://doi.org/10.1016/S0194‐5998(98)70027‐0
- 3007. Folker RJ, Marple BF, Mabry RL, Mabry CS. Treatment of allergic fungal sinusitis: a comparison trial of postoperative immunotherapy with specific fungal antigens. Laryngoscope. 1998;108(11 pt 1):1623–1627. https://doi.org/10.1097/00005537‐199811000‐00007
- 3008. Gan EC, Thamboo A, Rudmik L, Hwang PH, Ferguson BJ, Javer AR. Medical management of allergic fungal rhinosinusitis following endoscopic sinus surgery: an evidence‐based review and recommendations. Int Forum Allergy Rhinol. 2014;4(9):702–715. https://doi.org/10.1002/alr.21352
- 3009. Doellman MS, Dion GR, Weitzel EK, Reyes EG. Immunotherapy in allergic fungal sinusitis: the controversy continues. A recent review of literature. Allergy Rhinol (Providence). 2013;4(1):e32–e35. https://doi.org/10.2500/ar.2013.4.0045
- 3010. Pant H, Kette FE, Smith WB, Wormald PJ, Macardle PJ. Fungal‐specific humoral response in eosinophilic mucus chronic rhinosinusitis. Laryngoscope. 2005;115(4):601–606. https://doi.org/10.1097/01.mlg.0000161341.00258.54
- 3011. Clark DW, Wenaas A, Luong A, Citardi MJ, Fakhri S. Staphylococcus aureus prevalence in allergic fungal rhinosinusitis vs other subsets of chronic rhinosinusitis with nasal polyps. Int Forum Allergy Rhinol. 2013;3(2):89–93. https://doi.org/10.1002/alr.21090
- 3012. Ponikau JU, Sherris DA, Kern EB, et al. The diagnosis and incidence of allergic fungal sinusitis. Mayo Clin Proc. 1999;74(9):877–884. https://doi.org/10.4065/74.9.877
- 3013. Cody 2nd DT, Neel 3rd HB, Ferreiro JA, Roberts GD. Allergic fungal sinusitis: the Mayo Clinic experience. Laryngoscope. 1994;104(9):1074–1079. https://doi.org/10.1288/00005537‐199409000‐00005
- 3014. Dykewicz MS, Rodrigues JM, Slavin RG. Allergic fungal rhinosinusitis. J Allergy Clin Immunol. 2018;142(2):341–351. https://doi.org/10.1016/j.jaci.2018.06.023
- 3015. Tyler MA, Luong AU. Current understanding of allergic fungal rhinosinusitis. World J Otorhinolaryngol Head Neck Surg. 2018;4(3):179–185. https://doi.org/10.1016/j.wjorl.2018.08.003
- 3016. Lin YT, Lin CF, Liao CK, Chiang BL, Yeh TH. Clinical characteristics and cytokine profiles of central‐compartment‐type chronic rhinosinusitis. Int Forum Allergy Rhinol. 2021;11(7):1064–1073. https://doi.org/10.1002/alr.22759
- 3017. Makary CA, Falco J, Sussman S, et al. Disease involvement in the central compartment in eosinophilic chronic rhinosinusitis. Int Forum Allergy Rhinol. 2021;11(10):1417–1423. https://doi.org/10.1002/alr.22803
- 3018. Schertzer JS, Levy JM, Wise SK, Magliocca KR, DelGaudio JM. Is respiratory epithelial adenomatoid hamartoma related to central compartment atopic disease? Am J Rhinol Allergy. 2020;34(5):610–617. https://doi.org/10.1177/1945892420914212
- 3019. Laidlaw TM, Boyce JA. Pathogenesis of aspirin‐exacerbated respiratory disease and reactions. Immunol Allergy Clin North Am. 2013;33(2):195–210. https://doi.org/10.1016/j.iac.2012.11.006
- 3020. Samter M, Beers Jr RF. Intolerance to aspirin. Clinical studies and consideration of its pathogenesis. Ann Intern Med. 1968;68(5):975–983. https://doi.org/10.7326/0003‐4819‐68‐5‐975
- 3021. Bochenek G, Kuschill‐Dziurda J, Szafraniec K, Plutecka H, Szczeklik A, Nizankowska‐Mogilnicka E. Certain subphenotypes of aspirin‐exacerbated respiratory disease distinguished by latent class analysis. J Allergy Clin Immunol. 2014;133(1):98‐103.e1‐6. https://doi.org/10.1016/j.jaci.2013.07.004
- 3022. Berges‐Gimeno MP, Simon RA, Stevenson DD. The natural history and clinical characteristics of aspirin‐exacerbated respiratory disease. Ann Allergy Asthma Immunol. 2002;89(5):474–478. https://doi.org/10.1016/S1081‐1206(10)62084‐4
- 3023. Jakiela B, Soja J, Sladek K, et al. Heterogeneity of lower airway inflammation in patients with NSAID‐exacerbated respiratory disease. J Allergy Clin Immunol. 2021;147(4):1269–1280. https://doi.org/10.1016/j.jaci.2020.08.007
- 3024. Dona I, Barrionuevo E, Salas M, et al. NSAIDs‐hypersensitivity often induces a blended reaction pattern involving multiple organs. Sci Rep. 2018;8(1):16710. https://doi.org/10.1038/s41598‐018‐34668‐1
- 3025. Bochenek G, Nizankowska E, Szczeklik A. The atopy trait in hypersensitivity to nonsteroidal anti‐inflammatory drugs. Allergy. 1996;51(1):16–23. https://doi.org/10.1111/j.1398‐9995.1996.tb04544.x
- 3026. Stevens WW, Peters AT, Hirsch AG, et al. Clinical characteristics of patients with chronic rhinosinusitis with nasal polyps, asthma, and aspirin‐exacerbated respiratory disease. J Allergy Clin Immunol Pract. 2017;5(4):1061–1070.e3. https://doi.org/10.1016/j.jaip.2016.12.027
- 3027. Brown HJ, Tajudeen BA, Kuhar HN, Gattuso P, Batra PS, Mahdavinia M. Defining the allergic endotype of chronic rhinosinusitis by structured histopathology and clinical variables. J Allergy Clin Immunol Pract. 2021;9(10):3797–3804. https://doi.org/10.1016/j.jaip.2021.06.013
- 3028. Buchheit KM, Dwyer DF, Ordovas‐Montanes J, et al. IL‐5 Ralpha marks nasal polyp IgG4‐ and IgE‐expressing cells in aspirin‐exacerbated respiratory disease. J Allergy Clin Immunol. 2020;145(6):1574–1584. https://doi.org/10.1016/j.jaci.2020.02.035
- 3029. Ta V, White AA. Survey‐defined patient experiences with aspirin‐exacerbated respiratory disease. J Allergy Clin Immunol Pract. 2015;3(5):711–718. https://doi.org/10.1016/j.jaip.2015.03.001
- 3030. Haque R, White AA, Jackson DJ, Hopkins C. Clinical evaluation and diagnosis of aspirin‐exacerbated respiratory disease. J Allergy Clin Immunol. 2021;148(2):283–291. https://doi.org/10.1016/j.jaci.2021.06.018
- 3031. Leonardi A, Castegnaro A, Valerio AL, Lazzarini D. Epidemiology of allergic conjunctivitis: clinical appearance and treatment patterns in a population‐based study. Curr Opin Allergy Clin Immunol. 2015;15(5):482–488. https://doi.org/10.1097/ACI.0000000000000204
- 3032. Miyazaki D, Fukagawa K, Okamoto S, et al. Epidemiological aspects of allergic conjunctivitis. Allergol Int. 2020;69(4):487–495. https://doi.org/10.1016/j.alit.2020.06.004
- 3033. Singh K, Axelrod S, Bielory L. The epidemiology of ocular and nasal allergy in the United States, 1988‐1994. J Allergy Clin Immunol. 2010;126(4):778–783.e6. https://doi.org/10.1016/j.jaci.2010.06.050
- 3034. Navarro A, Colas C, Anton E, et al. Epidemiology of allergic rhinitis in allergy consultations in Spain: Alergologica‐2005. J Investig Allergol Clin Immunol. 2009;19(suppl 2):7–13.
- 3035. Kosrirukvongs P, Visitsunthorn N, Vichyanond P, Bunnag C. Allergic conjunctivitis. Asian Pac J Allergy Immunol. 2001;19(4):237–244.
- 3036. Kim DH, Park YS, Jang HJ, Kim JH, Lim DH. Prevalence and allergen of allergic rhinitis in Korean children. Am J Rhinol Allergy. 2016;30(3):72–78. https://doi.org/10.2500/ajra.2013.27.4317
- 3037. Han DH, Ahn JC, Mun SJ, Park SK, Oh SY, Rhee CS. Novel risk factors for allergic rhinitis in korean elementary school children: ARCO‐kids Phase II in a Community. Allergy Asthma Immunol Res. 2015;7(3):234–240. https://doi.org/10.4168/aair.2015.7.3.234
- 3038. Cibella F, Ferrante G, Cuttitta G, et al. The burden of rhinitis and rhinoconjunctivitis in adolescents. Allergy Asthma Immunol Res. 2015;7(1):44–50. https://doi.org/10.4168/aair.2015.7.1.44
- 3039. Gradman J, Wolthers OD. Allergic conjunctivitis in children with asthma, rhinitis and eczema in a secondary outpatient clinic. Pediatr Allergy Immunol. 2006;17(7):524–526. https://doi.org/10.1111/j.1399‐3038.2006.00429.x
- 3040. Williams DC, Edney G, Maiden B, Smith PK. Recognition of allergic conjunctivitis in patients with allergic rhinitis. World Allergy Organ J. 2013;6(1):4. https://doi.org/10.1186/1939‐4551‐6‐4
- 3041. Wuthrich B, Brignoli R, Canevascini M, Gerber M. Epidemiological survey in hay fever patients: symptom prevalence and severity and influence on patient management. Schweiz Med Wochenschr. 1998;128(5):139–143.
- 3042. Sánchez‐Hernández MC, Dordal MT, Navarro AM, et al., Severity and duration of allergic conjunctivitis: are they associated with severity and duration of allergic rhinitis and asthma? Eur Ann Allergy Clin Immunol. 2022;54(6):277–283. doi: https://doi.org/10.23822/EurAnnACI.1764‐1489.231
- 3043. Zhang SY, Li J, Liu R, et al. Association of allergic conjunctivitis with health‐related quality of life in children and their parents. JAMA Ophthalmol. 2021;139(8):830–837. https://doi.org/10.1001/jamaophthalmol.2021.1708
- 3044. Bielory L, Katelaris CH, Lightman S, Naclerio RM. Treating the ocular component of allergic rhinoconjunctivitis and related eye disorders. MedGenMed. 2007;9(3):35.
- 3045. Bielory L, Skoner DP, Blaiss MS, et al. Ocular and nasal allergy symptom burden in America: the Allergies, Immunotherapy, and RhinoconjunctivitiS (AIRS) surveys. Allergy Asthma Proc. 2014;35(3):211–218. https://doi.org/10.2500/aap.2014.35.3750
- 3046. Frolund L, Durham SR, Calderon M, et al. Sustained effect of SQ‐standardized grass allergy immunotherapy tablet on rhinoconjunctivitis quality of life. Allergy. 2010;65(6):753–757. https://doi.org/10.1111/j.1398‐9995.2009.02238.x
- 3047. Sayed KM, Kamel AG, Ali AH. One‐year evaluation of clinical and immunological efficacy and safety of sublingual versus subcutaneous allergen immunotherapy in allergic conjunctivitis. Graefes Arch Clin Exp Ophthalmol. 2019;257(9):1989–1996. https://doi.org/10.1007/s00417‐019‐04389‐w
- 3048. Alexandropoulos T, Haidich AB, Pilalas D, Dardavessis T, Daniilidis M, Arvanitidou M. Characteristics of patients with allergic rhinitis in an outpatient clinic: a retrospective study. Allergol Immunopathol (Madr). 2013;41(3):194–200. https://doi.org/10.1016/j.aller.2011.12.008
- 3049. Almaliotis D, Michailopoulos P, Gioulekas D, et al. Allergic conjunctivitis and the most common allergens in Northern Greece. World Allergy Organ J. 2013;6(1):12. https://doi.org/10.1186/1939‐4551‐6‐12
- 3050. Langan SM, Irvine AD, Weidinger S. Atopic dermatitis. Lancet. 2020;396(10247):345–360. https://doi.org/10.1016/S0140‐6736(20)31286‐1
- 3051. Laughter MR, Maymone MBC, Mashayekhi S, et al. The global burden of atopic dermatitis: lessons from the Global Burden of Disease Study 1990‐2017. Br J Dermatol. 2021;184(2):304–309. https://doi.org/10.1111/bjd.19580
- 3052. Gabryszewski SJ, Chang X, Dudley JW, et al. Unsupervised modeling and genome‐wide association identify novel features of allergic march trajectories. J Allergy Clin Immunol. 2021;147(2):677–685.e10. https://doi.org/10.1016/j.jaci.2020.06.026
- 3053. Hill DA, Grundmeier RW, Ramos M, Spergel JM. Eosinophilic esophagitis is a late manifestation of the allergic march. J Allergy Clin Immunol Pract. 2018;6(5):1528–1533. https://doi.org/10.1016/j.jaip.2018.05.010
- 3054. Tan R, Cvetkovski B, Kritikos V, et al. Identifying the hidden burden of allergic rhinitis (AR) in community pharmacy: a global phenomenon. Asthma Res Pract. 2017;3:8. https://doi.org/10.1186/s40733‐017‐0036‐z
- 3055. González‐Mendoza T, Bedolla‐Barajas M, Bedolla‐Pulido TR, et al., La prevalencia de rinitis alérgica y dermatitis atópica en adolescentes tardíos difiere de acuerdo con el sexo [The prevalence of allergic rhinitis and atopic dermatitis in late adolescents differs according to their gender]. Rev Alerg Mex. 2019;66(2):147–153. doi: https://doi.org/10.29262/ram.v66i2.521
- 3056. Bekic S, Martinek V, Talapko J, Majnaric L, Vasilj Mihaljevic M, Skrlec I. Atopic dermatitis and comorbidity. Healthcare (Basel). 2020;8(2):70. https://doi.org/10.3390/healthcare8020070
- 3057. Huang YH, Huang LH, Kuo CF, Yu KH. Familial aggregation of atopic dermatitis and co‐aggregation of allergic diseases in affected families in Taiwan. J Dermatol Sci. 2020;100(1):15–22. https://doi.org/10.1016/j.jdermsci.2020.07.007
- 3058. Schoos AM, Chawes BL, Bønnelykke K, Stokholm J, Rasmussen MA, Bisgaard H. Increasing severity of early‐onset atopic dermatitis, but not late‐onset, associates with development of aeroallergen sensitization and allergic rhinitis in childhood. Allergy. 2022;77(4):1254–62.
- 3059. Wang LC, Chiang BL. Early‐onset‐early‐resolving atopic dermatitis does not increase the risk of development of allergic diseases at 3 years old. J Formos Med Assoc. 2020;119(12):1854–1861. https://doi.org/10.1016/j.jfma.2020.02.014
- 3060. Dharma C, Lefebvre DL, Tran MM, et al. Patterns of allergic sensitization and atopic dermatitis from 1 to 3 years: effects on allergic diseases. Clin Exp Allergy. 2018;48(1):48–59. https://doi.org/10.1111/cea.13063
- 3061. Huang Y, Zhang Y, Zhang L. Prevalence of allergic and nonallergic rhinitis in a rural area of northern China based on sensitization to specific aeroallergens. Allergy Asthma Clin Immunol. 2018;14:77. https://doi.org/10.1186/s13223‐018‐0299‐9
- 3062. Biagini JM, Kroner JW, Baatyrbek Kyzy A, et al., Longitudinal atopic dermatitis endotypes: an atopic march paradigm that includes Black children. J Allergy Clin Immunol. 2022;149(5):1702–1710.e4. https://doi.org/10.1016/j.jaci.2021.09.036
- 3063. Jeong JW, Lim KH, Lee WH, Won JY, Kwon JW. Heterogeneity of adult rhinitis for multimorbidity and age at onset among non‐sensitized rhinitis and mono‐/poly‐sensitized rhinitis: a retrospective cross‐sectional study. Int Arch Allergy Immunol. 2020;181(7):512–521. https://doi.org/10.1159/000507444
- 3064. Raciborski F, Bousquet J, Bousqet J, et al. Dissociating polysensitization and multimorbidity in children and adults from a Polish general population cohort. Clin Transl Allergy. 2019;9:4. https://doi.org/10.1186/s13601‐019‐0246‐y
- 3065. Moreno‐Lopez S, Perez‐Herrera LC, Penaranda D, Hernandez DC, Garcia E, Penaranda A. Prevalence and associated factors of allergic diseases in school children and adolescents aged 6‐7 and 13‐14 years from two rural areas in Colombia. Allergol Immunopathol (Madr). 2021;49(3):153–161. https://doi.org/10.15586/aei.v49i3.183
- 3066. Williams H, Robertson C, Stewart A, et al. Worldwide variations in the prevalence of symptoms of atopic eczema in the International Study of Asthma and Allergies in Childhood. J Allergy Clin Immunol. 1999;103(1 pt 1):125–138. https://doi.org/10.1016/s0091‐6749(99)70536‐1
- 3067. Kim JP, Chao LX, Simpson EL, Silverberg JI. Persistence of atopic dermatitis (AD): a systematic review and meta‐analysis. J Am Acad Dermatol. 2016;75(4):681–687.e11. https://doi.org/10.1016/j.jaad.2016.05.028
- 3068. Rhodes HL, Thomas P, Sporik R, Holgate ST, Cogswell JJ. A birth cohort study of subjects at risk of atopy: twenty‐two‐year follow‐up of wheeze and atopic status. Am J Respir Crit Care Med. 2002;165(2):176–180. https://doi.org/10.1164/ajrccm.165.2.2104032
- 3069. Gustafsson D, Sjoberg O, Foucard T. Development of allergies and asthma in infants and young children with atopic dermatitis – a prospective follow‐up to 7 years of age. Allergy. 2000;55(3):240–245. https://doi.org/10.1034/j.1398‐9995.2000.00391.x
- 3070. Schneider L, Hanifin J, Boguniewicz M, et al. Study of the atopic march: development of atopic comorbidities. Pediatr Dermatol. 2016;33(4):388–398. https://doi.org/10.1111/pde.12867
- 3071. Mortz CG, Andersen KE, Dellgren C, Barington T, Bindslev‐Jensen C. Atopic dermatitis from adolescence to adulthood in the TOACS cohort: prevalence, persistence and comorbidities. Allergy. 2015;70(7):836–845. https://doi.org/10.1111/all.12619
- 3072. Sybilski AJ, Raciborski F, Lipiec A, et al. Atopic dermatitis is a serious health problem in Poland. Epidemiology studies based on the ECAP study. Postepy Dermatol Alergol. 2015;32(1):1–10. https://doi.org/10.5114/pdia.2014.40935
- 3073. Bozek A, Jarzab J. Epidemiology of IgE‐dependent allergic diseases in elderly patients in Poland. Am J Rhinol Allergy. 2013;27(5):e140–e145. https://doi.org/10.2500/ajra.2013.27.3920
- 3074. Lowe AJ, Hosking CS, Bennett CM, et al. Skin prick test can identify eczematous infants at risk of asthma and allergic rhinitis. Clin Exp Allergy. 2007;37(11):1624–1631. https://doi.org/10.1111/j.1365‐2222.2007.02822.x
- 3075. Karaman O, Turgut CS, Uzuner N, et al. The determination of asthma, rhinitis, eczema, and atopy prevalence in 9‐ to 11‐year‐old children in the city of Izmir. Allergy Asthma Proc. 2006;27(4):319–324. https://doi.org/10.2500/aap.2006.27.2877
- 3076. Yemaneberhan H, Flohr C, Lewis SA, et al. Prevalence and associated factors of atopic dermatitis symptoms in rural and urban Ethiopia. Clin Exp Allergy. 2004;34(5):779–785. https://doi.org/10.1111/j.1365‐2222.2004.1946.x
- 3077. Min YG, Choi BY, Kwon SK, et al. Multicenter study on the prevalence of perennial allergic rhinitis and allergy‐associated disorders. J Korean Med Sci. 2001;16(6):697–701. https://doi.org/10.3346/jkms.2001.16.6.697
- 3078. Leung R, Ho P. Asthma, allergy, and atopy in three south‐east Asian populations. Thorax. 1994;49(12):1205–1210. https://doi.org/10.1136/thx.49.12.1205
- 3079. Batlles Garrido J, Torres‐Borrego J, Bonillo Perales A, et al. Prevalence and factors linked to atopic eczema in 10‐ and 11‐year‐old schoolchildren. Isaac 2 in Almeria, Spain. Allergol Immunopathol (Madr). 2010;38(4):174–180. https://doi.org/10.1016/j.aller.2009.10.008
- 3080. Peroni DG, Piacentini GL, Bodini A, Rigotti E, Pigozzi R, Boner AL. Prevalence and risk factors for atopic dermatitis in preschool children. Br J Dermatol. 2008;158(3):539–543. https://doi.org/10.1111/j.1365‐2133.2007.08344.x
- 3081. Kidon MI, Chiang WC, Liew WK, et al. Sensitization to dust mites in children with allergic rhinitis in Singapore: does it matter if you scratch while you sneeze? Clin Exp Allergy. 2005;35(4):434–440. https://doi.org/10.1111/j.1365‐2222.2005.02208.x
- 3082. Kusel MM, Holt PG, de Klerk N, Sly PD. Support for 2 variants of eczema. J Allergy Clin Immunol. 2005;116(5):1067–1072. https://doi.org/10.1016/j.jaci.2005.06.038
- 3083. Peroni DG, Piacentini GL, Alfonsi L, et al. Rhinitis in pre‐school children: prevalence, association with allergic diseases and risk factors. Clin Exp Allergy. 2003;33(10):1349–1354. https://doi.org/10.1046/j.1365‐2222.2003.01766.x
- 3084. Ozdemir N, Ucgun I, Metintas S, Kolsuz M, Metintas M. The prevalence of asthma and allergy among university freshmen in Eskisehir, Turkey. Respir Med. 2000;94(6):536–541. https://doi.org/10.1053/rmed.1999.0728
- 3085. Garcia‐Gonzalez JJ, Vega‐Chicote JM, Rico P, et al. Prevalence of atopy in students from Malaga, Spain. Ann Allergy Asthma Immunol. 1998;80(3):237–244. https://doi.org/10.1016/s1081‐1206(10)62964‐x
- 3086. Cosme‐Blanco W, Arroyo‐Flores E, Ale H. Food allergies. Pediatr Rev. 2020;41(8):403–415. https://doi.org/10.1542/pir.2019‐0037
- 3087. Katelaris CH. Food allergy and oral allergy or pollen‐food syndrome. Curr Opin Allergy Clin Immunol. 2010;10(3):246–251. https://doi.org/10.1097/ACI.0b013e32833973fb
- 3088. Ebner C, Birkner T, Valenta R, et al. Common epitopes of birch pollen and apples–studies by western and northern blot. J Allergy Clin Immunol. 1991;88(4):588–594. https://doi.org/10.1016/0091‐6749(91)90152‐e
- 3089. American Academy of Asthma Allergy & Immunology: Oral allergy syndrome – pollens and cross‐reacting foods. Accessed March 19, 2022. https://www.aaaai.org/Aaaai/media/Media‐Library‐PDFs/Tools%20for%20the%20Public/Conditions%20Library/Library%20‐%20Allergies/OAS‐table_revised.pdf
- 3090. Carlson G, Coop C. Pollen food allergy syndrome (PFAS): a review of current available literature. Ann Allergy Asthma Immunol. 2019;123(4):359–365. https://doi.org/10.1016/j.anai.2019.07.022
- 3091. Dondi A, Tripodi S, Panetta V, et al. Pollen‐induced allergic rhinitis in 1360 Italian children: comorbidities and determinants of severity. Pediatr Allergy Immunol. 2013;24(8):742–751. https://doi.org/10.1111/pai.12136
- 3092. Sicherer SH, Warren CM, Dant C, Gupta RS, Nadeau KC. Food allergy from infancy through adulthood. J Allergy Clin Immunol Pract. 2020;8(6):1854–1864. https://doi.org/10.1016/j.jaip.2020.02.010
- 3093. Ortolani C, Pastorello EA, Farioli L, et al. IgE‐mediated allergy from vegetable allergens. Ann Allergy. 1993;71(5):470–476.
- 3094. Lieberman P, Nicklas RA, Randolph C, et al. Anaphylaxis – a practice parameter update 2015. Ann Allergy Asthma Immunol. 2015;115(5):341–384. https://doi.org/10.1016/j.anai.2015.07.019
- 3095. Skamstrup Hansen K, Vestergaard H, Stahl Skov P, et al. Double‐blind, placebo‐controlled food challenge with apple. Allergy. 2001;56(2):109–117. https://doi.org/10.1034/j.1398‐9995.2001.056002109.x
- 3096. Rosen JP, Selcow JE, Mendelson LM, Grodofsky MP, Factor JM, Sampson HA. Skin testing with natural foods in patients suspected of having food allergies: is it a necessity? J Allergy Clin Immunol. 1994;93(6):1068–1070. https://doi.org/10.1016/s0091‐6749(94)70056‐7
- 3097. de Jong NW, Terlouw S, van Boven FE, et al. Birch pollen related pear allergy: a single‐blind oral challenge TRIAL with 2 pear cultivars. Nutrients. 2021;13(4):1355. https://doi.org/10.3390/nu13041355
- 3098. Lee SC, Kim SR, Park KH, Lee JH, Park JW. Clinical features and culprit food allergens of Korean adult food allergy patients: a cross‐sectional single‐institute study. Allergy Asthma Immunol Res. 2019;11(5):723–735. https://doi.org/10.4168/aair.2019.11.5.723
- 3099. Fuhrmann V, Huang HJ, Akarsu A, et al. From allergen molecules to molecular immunotherapy of nut allergy: a hard nut to crack. Front Immunol. 2021;12:742732. https://doi.org/10.3389/fimmu.2021.742732
- 3100. Thompson JC, Kroker GF. The role of component‐resolved testing in food allergy and oral allergy syndrome. Ann Allergy Asthma Immunol. 2010;104(6):543; author reply 543‐4. https://doi.org/10.1016/j.anai.2010.03.011
- 3101. Nicolaou N, Poorafshar M, Murray C, et al. Allergy or tolerance in children sensitized to peanut: prevalence and differentiation using component‐resolved diagnostics. J Allergy Clin Immunol. 2010;125(1):191‐197.e1‐13. https://doi.org/10.1016/j.jaci.2009.10.008
- 3102. Bolhaar ST, Tiemessen MM, Zuidmeer L, et al. Efficacy of birch‐pollen immunotherapy on cross‐reactive food allergy confirmed by skin tests and double‐blind food challenges. Clin Exp Allergy. 2004;34(5):761–769. https://doi.org/10.1111/j.1365‐2222.2004.1939.x
- 3103. Inuo C, Kondo Y, Tanaka K, et al. Japanese cedar pollen‐based subcutaneous immunotherapy decreases tomato fruit‐specific basophil activation. Int Arch Allergy Immunol. 2015;167(2):137–145. https://doi.org/10.1159/000437325
- 3104. Asero R. Effects of birch pollen‐specific immunotherapy on apple allergy in birch pollen‐hypersensitive patients. Clin Exp Allergy. 1998;28(11):1368–1373. https://doi.org/10.1046/j.1365‐2222.1998.00399.x
- 3105. Mauro M, Russello M, Incorvaia C, et al. Birch‐apple syndrome treated with birch pollen immunotherapy. Int Arch Allergy Immunol. 2011;156(4):416–422. https://doi.org/10.1159/000323909
- 3106. Krouse JH, Chadwick SJ, Gordon BR, Derebery J. Allergy and Immunology: An Otolaryngic Approach. Lippincott Williams & Wilkins; 2002.
- 3107. Cudowska B, Pawlowicz M, Lebensztejn DM. Pollen‐related food allergy in children with seasonal allergic rhinitis. Postepy Dermatol Alergol. 2021;38(2):96–101. https://doi.org/10.5114/ada.2021.104284
- 3108. Thong BY, Arulanandam S, Tan SC, et al. Shellfish/crustacean oral allergy syndrome among national service pre‐enlistees in Singapore. Asia Pac Allergy. 2018;8(2):e18. https://doi.org/10.5415/apallergy.2018.8.e18
- 3109. Diaz‐Cabrera NM, Sanchez‐Borges MA, Ledford DK. Atopy: a collection of comorbid conditions. J Allergy Clin Immunol Pract. 2021;9(11):3862–3866. https://doi.org/10.1016/j.jaip.2021.09.002
- 3110. Matsumoto M, Takenaka M, Aoyagi K, et al. Factors associated with the development of oral allergy syndrome: a retrospective questionnaire survey of Japanese university students. Allergol Int. 2021;70(4):458–462. https://doi.org/10.1016/j.alit.2021.02.003
- 3111. Ota M, Nishida Y, Yagi H, et al. Regional differences in the prevalence of oral allergy syndrome among Japanese children: a questionnaire‐based survey. Asian Pacific Journal of Allergy and Immunology. 2020. doi: https://doi.org/10.12932/ap‐130120‐0739
- 3112. Anvari S, Miller J, Yeh CY, Davis CM. IgE‐mediated food allergy. Clin Rev Allergy Immunol. 2019;57(2):244–260. https://doi.org/10.1007/s12016‐018‐8710‐3
- 3113. Tong X, Tong H, Gao L, et al. A multicenter study of prevalence and risk factors for allergic rhinitis in primary school children in 5 cities of Hubei Province, China. Int Arch Allergy Immunol. 2022;183(1):34–44. https://doi.org/10.1159/000517948
- 3114. Celakovska J, Bukac J. Analysis of food allergy in atopic dermatitis patients – association with concomitant allergic diseases. Indian J Dermatol. 2014;59(5):445–450. https://doi.org/10.4103/0019‐5154.139867
- 3115. Bilaver LA, Kanaley MK, Fierstein JL, Gupta RS. Prevalence and correlates of food allergy among medicaid‐enrolled United States children. Acad Pediatr. 2021;21(1):84–92. https://doi.org/10.1016/j.acap.2020.03.005
- 3116. Wang HT, Warren CM, Gupta RS, Davis CM. Prevalence and characteristics of shellfish allergy in the pediatric population of the United States. J Allergy Clin Immunol Pract. 2020;8(4):1359–1370.e2. https://doi.org/10.1016/j.jaip.2019.12.027
- 3117. Ruffner MA, Wang KY, Dudley JW, et al. Elevated atopic comorbidity in patients with food protein‐induced enterocolitis. J Allergy Clin Immunol Pract. 2020;8(3):1039–1046. https://doi.org/10.1016/j.jaip.2019.10.047
- 3118. Bedolla‐Pulido TR, Bedolla‐Barajas M, Morales‐Romero J, et al. Self‐reported hypersensitivity and allergy to foods amongst Mexican adolescents: prevalence and associated factors. Allergol Immunopathol (Madr). 2019;47(3):246–253. https://doi.org/10.1016/j.aller.2018.09.004
- 3119. Hill DA, Grundmeier RW, Ram G, Spergel JM. The epidemiologic characteristics of healthcare provider‐diagnosed eczema, asthma, allergic rhinitis, and food allergy in children: a retrospective cohort study. BMC Pediatr. 2016;16:133. https://doi.org/10.1186/s12887‐016‐0673‐z
- 3120. Huang Y, Wang C, Zhang Y, Zhang L. Developing nomograms for identifying allergic rhinitis among chronic rhinitis: a real‐world study. World Allergy Organ J. 2021;14(4):100534. https://doi.org/10.1016/j.waojou.2021.100534
- 3121. Walter G, Kalicinsky C. Adult‐onset IgE‐mediated food allergy at a Winnipeg allergy clinic: a case series. Allergy Asthma Clin Immunol. 2020;16:85. https://doi.org/10.1186/s13223‐020‐00483‐5
- 3122. Lyons SA, Knulst AC, Burney PGJ, et al. Predicting food allergy: the value of patient history reinforced. Allergy. 2021;76(5):1454–1462. https://doi.org/10.1111/all.14583
- 3123. Blaiss MS, Meadows JA, Yu S, et al. Economic burden of peanut allergy in pediatric patients with evidence of reactions to peanuts in the United States. J Manag Care Spec Pharm. 2021;27(4):516–527. https://doi.org/10.18553/jmcp.2021.20389
- 3124. Blumchen K, DunnGalvin A, Timmermans F, et al. APPEAL‐1: a pan‐European survey of patient/caregiver perceptions of peanut allergy management. Allergy. 2020;75(11):2920–2935. https://doi.org/10.1111/all.14414
- 3125. Scott LA, Jones BI, Berni TR, Berni ER, De Vries J, Currie CJ. Evaluation of the epidemiology of peanut allergy in the United Kingdom. Expert Rev Clin Immunol. 2019;15(12):1333–1339. https://doi.org/10.1080/1744666X.2020.1693264
- 3126. Bedolla‐Barajas M, Bedolla‐Pulido TR, Macriz‐Romero N, Morales‐Romero J, Robles‐Figueroa M. Prevalence of peanut, tree nut, sesame, and seafood allergy in Mexican adults. Rev Invest Clin. 2015;67(6):379–386.
- 3127. Taylor‐Black S, Wang J. The prevalence and characteristics of food allergy in urban minority children. Ann Allergy Asthma Immunol. 2012;109(6):431–437. https://doi.org/10.1016/j.anai.2012.09.012
- 3128. Diez S, Puerta L, Martinez D, Munoz M, Hernandez K, Sanchez J. Clinical relevance of shrimp sensitization in patients with allergic rhinitis: anti‐Der p 10 IgE as predictor. Int Arch Allergy Immunol. 2021;182(10):971–979. https://doi.org/10.1159/000516005
- 3129. Du Toit G, Roberts G, Sayre PH, et al. Identifying infants at high risk of peanut allergy: the Learning Early About Peanut Allergy (LEAP) screening study. J Allergy Clin Immunol. 2013;131(1):135‐143.e1‐12. https://doi.org/10.1016/j.jaci.2012.09.015
- 3130. Fleischer DM, Sicherer S, Greenhawt M, et al. Consensus communication on early peanut introduction and the prevention of peanut allergy in high‐risk infants. Pediatrics. 2015;136(3):600–604. https://doi.org/10.1542/peds.2015‐2394
- 3131. Logan K, Du Toit G, Giovannini M, Turcanu V, Lack G. Pediatric allergic diseases, food allergy, and oral tolerance. Annu Rev Cell Dev Biol. 2020;36:511–528. https://doi.org/10.1146/annurev‐cellbio‐100818‐125346
- 3132. Webber CM, England RW. Oral allergy syndrome: a clinical, diagnostic, and therapeutic challenge. Ann Allergy Asthma Immunol. 2010;104(2):101–108; quiz 109‐10, 117. https://doi.org/10.1016/j.anai.2009.11.007
- 3133. American College of Allergy, Asthma, & Immunology . Food allergy: a practice parameter. Ann Allergy Asthma Immunol. 2006;96(3 suppl 2):S1–S68.
- 3134. Lam HY, Tergaonkar V, Ahn KS. Mechanisms of allergen‐specific immunotherapy for allergic rhinitis and food allergies. Biosci Rep. 2020;40(4):BSR20200256. https://doi.org/10.1042/BSR20200256
- 3135. Schoos AM, Bullens D, Chawes BL, et al. Immunological outcomes of allergen‐specific immunotherapy in food allergy. Front Immunol. 2020;11:568598. https://doi.org/10.3389/fimmu.2020.568598
- 3136. Tordesillas L, Berin MC, Sampson HA. Immunology of food allergy. Immunity. 2017;47(1):32–50. https://doi.org/10.1016/j.immuni.2017.07.004
- 3137. Marseglia GL, Poddighe D, Caimmi D, et al. Role of adenoids and adenoiditis in children with allergy and otitis media. Curr Allergy Asthma Rep. 2009;9(6):460–464. https://doi.org/10.1007/s11882‐009‐0068‐4
- 3138. Cassano P, Gelardi M, Cassano M, Fiorella ML, Fiorella R. Adenoid tissue rhinopharyngeal obstruction grading based on fiberendoscopic findings: a novel approach to therapeutic management. Int J Pediatr Otorhinolaryngol. 2003;67(12):1303–1309. https://doi.org/10.1016/j.ijporl.2003.07.018
- 3139. Dogru M, Evcimik MF, Calim OF. Does adenoid hypertrophy affect disease severity in children with allergic rhinitis? Eur Arch Otorhinolaryngol. 2017;274(1):209–213. https://doi.org/10.1007/s00405‐016‐4196‐x
- 3140. Modrzynski M, Zawisza E. The influence of birch pollination on the adenoid size in children with intermittent allergic rhinitis. Int J Pediatr Otorhinolaryngol. 2007;71(7):1017–1023. https://doi.org/10.1016/j.ijporl.2007.02.018
- 3141. Atan Sahin O, Kececioglu N, Serdar M, Ozpinar A. The association of residential mold exposure and adenotonsillar hypertrophy in children living in damp environments. Int J Pediatr Otorhinolaryngol. 2016;88:233–238. https://doi.org/10.1016/j.ijporl.2016.07.018
- 3142. Karaca CT, Toros SZ, Noseri H, et al. Role of allergy in children with adenotonsillar hypertrophy. J Craniofac Surg. 2012;23(6):e611–e613. https://doi.org/10.1097/SCS.0b013e31826cf562
- 3143. Ameli F, Brocchetti F, Tosca MA, Signori A, Ciprandi G. Adenoidal hypertrophy and allergic rhinitis: is there an inverse relationship? Am J Rhinol Allergy. 2013;27(1):e5–e10. https://doi.org/10.2500/ajra.2013.27.3854
- 3144. Sadeghi‐Shabestari M, Jabbari Moghaddam Y, Ghaharri H. Is there any correlation between allergy and adenotonsillar tissue hypertrophy? Int J Pediatr Otorhinolaryngol. 2011;75(4):589–591. https://doi.org/10.1016/j.ijporl.2011.01.026
- 3145. Eren E, Arslanoglu S, Erdem SB, et al. Chicken or the egg: the dilemma of allergic rhinitis versus adenoid hypertrophy. Rhinology. 2015;53(2):154–159. https://doi.org/10.4193/Rhino14.013
- 3146. Karabulut B, Sahin‐Onder S, Erkmen B, Cetemen A, Gergin O. Predictive fiberoptic endoscopic findings of upper airway in children with allergic rhinitis. Int J Pediatr Otorhinolaryngol. 2019;124:143–146. https://doi.org/10.1016/j.ijporl.2019.06.004
- 3147. Ni K, Zhao L, Wu J, Chen W, HongyaYang, Li X. Th17/Treg balance in children with obstructive sleep apnea syndrome and the relationship with allergic rhinitis. Int J Pediatr Otorhinolaryngol. 2015;79(9):1448–1454. https://doi.org/10.1016/j.ijporl.2015.06.026
- 3148. Masieri S, Trabattoni D, Incorvaia C, et al. A role for Waldeyer's ring in immunological response to allergens. Curr Med Res Opin. 2014;30(2):203–205. https://doi.org/10.1185/03007995.2013.855185
- 3149. Zhu F, Sun K, Yu L, Sun S, Wan Y, Shi L. Tissue cytokine adenoid experssion in hypertrophic adenoid gland in children with allergic rhinitis. J Coll Physicians Surg Pak. 2021;31(8):903–909. https://doi.org/10.29271/jcpsp.2021.08.903
- 3150. Cho KS, Kim SH, Hong SL, et al. Local atopy in childhood adenotonsillar hypertrophy. Am J Rhinol Allergy. 2018;32(3):160–166. https://doi.org/10.1177/1945892418765003
- 3151. Shin SY, Choi SJ, Hur GY, et al. Local production of total IgE and specific antibodies to the house dust mite in adenoid tissue. Pediatr Allergy Immunol. 2009;20(2):134–141. https://doi.org/10.1111/j.1399‐3038.2008.00756.x
- 3152. Shin SY, Ye YM, Eun YG, Kim SW, Cho JS, Park HS. Local IgE‐mediated hypersensitivity to Alternaria in pediatric adenoid tissue. Int J Pediatr Otorhinolaryngol. 2012;76(10):1423–1428. https://doi.org/10.1016/j.ijporl.2012.06.015
- 3153. Scadding G. Non‐surgical treatment of adenoidal hypertrophy: the role of treating IgE‐mediated inflammation. Pediatr Allergy Immunol. 2010;21(8):1095–1106. https://doi.org/10.1111/j.1399‐3038.2010.01012.x
- 3154. Zhang L, Mendoza‐Sassi RA, Cesar JA, Chadha NK. Intranasal corticosteroids for nasal airway obstruction in children with moderate to severe adenoidal hypertrophy. Cochrane Database Syst Rev. 2008;(3):CD006286. https://doi.org/10.1002/14651858.CD006286.pub2
- 3155. Chohan A, Lal A, Chohan K, Chakravarti A, Gomber S. Systematic review and meta‐analysis of randomized controlled trials on the role of mometasone in adenoid hypertrophy in children. Int J Pediatr Otorhinolaryngol. 2015;79(10):1599–1608. https://doi.org/10.1016/j.ijporl.2015.07.009
- 3156. Warman M, Granot E, Halperin D. Improvement in allergic and nonallergic rhinitis: a secondary benefit of adenoidectomy in children. Ear Nose Throat J. 2015;94(6):220;222;, 224‐7. https://doi.org/10.1177/014556131509400607
- 3157. Patel A, Brook CD, Levi JR. Factors associated with refractory nasal congestion following adenoidectomy. Ann Otol Rhinol Laryngol. 2021;130(2):148–152. https://doi.org/10.1177/0003489420940349
- 3158. De Corso E, Galli J, Di Cesare T, et al. A systematic review of the clinical evidence and biomarkers linking allergy to adeno‐tonsillar disease. Int J Pediatr Otorhinolaryngol. 2021;147:110799. https://doi.org/10.1016/j.ijporl.2021.110799
- 3159. Pagella F, De Amici M, Pusateri A, et al. Adenoids and clinical symptoms: epidemiology of a cohort of 795 pediatric patients. Int J Pediatr Otorhinolaryngol. 2015;79(12):2137–2141. https://doi.org/10.1016/j.ijporl.2015.09.035
- 3160. Schilder AG, Bhutta MF, Butler CC, et al. Eustachian tube dysfunction: consensus statement on definition, types, clinical presentation and diagnosis. Clin Otolaryngol. 2015;40(5):407–411. https://doi.org/10.1111/coa.12475
- 3161. Yang B, Brook CD. The role of allergy in otologic disease. Otolaryngol Clin North Am. 2017;50(6):1091–1101. https://doi.org/10.1016/j.otc.2017.08.005
- 3162. Fireman P. Otitis media and eustachian tube dysfunction: connection to allergic rhinitis. J Allergy Clin Immunol. 1997;99(2):S787–S797. https://doi.org/10.1016/s0091‐6749(97)70130‐1
- 3163. Doyle WJ, Boehm S, Skoner DP. Physiologic responses to intranasal dose‐response challenges with histamine, methacholine, bradykinin, and prostaglandin in adult volunteers with and without nasal allergy. J Allergy Clin Immunol. 1990;86(6 pt 1):924–935. https://doi.org/10.1016/s0091‐6749(05)80156‐3
- 3164. Skoner DP, Doyle WJ, Boehm S, Fireman P. Priming of the nose and eustachian tube during nasal pollen exposure. Am J Rhinol Allergy. 1989;3(2):53–57.
- 3165. Friedman RA, Doyle WJ, Casselbrant ML, Bluestone C, Fireman P. Immunologic‐mediated eustachian tube obstruction: a double‐blind crossover study. J Allergy Clin Immunol. 1983;71(5):442–447. https://doi.org/10.1016/0091‐6749(83)90459‐1
- 3166. Skoner DP, Doyle WJ, Chamovitz AH, Fireman P. Eustachian tube obstruction after intranasal challenge with house dust mite. Arch Otolaryngol Head Neck Surg. 1986;112(8):840–842. https://doi.org/10.1001/archotol.1986.03780080040008
- 3167. Skoner DP, Doyle WJ, Fireman P. Eustachian tube obstruction (ETO) after histamine nasal provocation – a double‐blind dose‐response study. J Allergy Clin Immunol. 1987;79(1):27–31. https://doi.org/10.1016/s0091‐6749(87)80012‐x
- 3168. O'Connor RD, Ort H, Leong AB, Cook DA, Street D, Hamburger RN. Tympanometric changes following nasal antigen challenge in children with allergic rhinitis. Ann Allergy. 1984;53(6):468–471.
- 3169. Downs BW, Butehorn HF, Prazma J, Rose AS, Stamat JC, Pillsbury 3rd HC. Action of histamine on eustachian tube function. Otolaryngol Head Neck Surg. 2001;124(4):414–420. https://doi.org/10.1067/mhn.2001.113943
- 3170. Ebert Jr CS, Pollock HW, Dubin MG, et al. Effect of intranasal histamine challenge on Eustachian tube function. Int J Pediatr Otorhinolaryngol. 2002;63(3):189–198. https://doi.org/10.1016/s0165‐5876(02)00007‐1
- 3171. Hardy SM, Heavner SB, White DR, McQueen CT, Prazma J, Pillsbury HC. Late‐phase allergy and eustachian tube dysfunction. Otolaryngol Head Neck Surg. 2001;125(4):339–345. https://doi.org/10.1067/mhn.2001.119140
- 3172. Osur SL, Volovitz B, Dickson S, Enck DC, Bernstein JM. Eustachian tube dysfunction in children with ragweed hayfever during natural pollen exposure. Allergy Proc. 1989;10(2):133–139. https://doi.org/10.2500/108854189778961071
- 3173. Knight LC, Eccles R, Morris S. Seasonal allergic rhinitis and its effects on eustachian tube function and middle ear pressure. Clin Otolaryngol Allied Sci. 1992;17(4):308–312. https://doi.org/10.1111/j.1365‐2273.1992.tb01002.x
- 3174. Juszczak H, Aubin‐Pouliot A, Sharon JD, Loftus PA. Sinonasal risk factors for eustachian tube dysfunction: cross‐sectional findings from NHANES 2011‐2012. Int Forum Allergy Rhinol. 2019;9(5):466–472. https://doi.org/10.1002/alr.22275
- 3175. Lazo‐Saenz JG, Galvan‐Aguilera AA, Martinez‐Ordaz VA, Velasco‐Rodriguez VM, Nieves‐Renteria A, Rincon‐Castaneda C. Eustachian tube dysfunction in allergic rhinitis. Otolaryngol Head Neck Surg. 2005;132(4):626–629. https://doi.org/10.1016/j.otohns.2005.01.029
- 3176. Gluth MB, McDonald DR, Weaver AL, Bauch CD, Beatty CW, Orvidas LJ. Management of eustachian tube dysfunction with nasal steroid spray: a prospective, randomized, placebo‐controlled trial. Arch Otolaryngol Head Neck Surg. 2011;137(5):449–455. https://doi.org/10.1001/archoto.2011.56
- 3177. Tucci DL, McCoul ED, Rosenfeld RM, et al. Clinical consensus statement: balloon dilation of the eustachian tube. Otolaryngol Head Neck Surg. 2019;161(1):6–17. https://doi.org/10.1177/0194599819848423
- 3178. Pollock HW, Ebert CS, Dubin MG, White DR, Prazma J, Pillsbury 3rd HC. The role of soluble interleukin‐4 receptor and interleukin‐5 antibody in preventing late‐phase allergy‐induced eustachian tube dysfunction. Otolaryngol Head Neck Surg. 2002;127(3):169–176. https://doi.org/10.1067/mhn.2002.126901
- 3179. Derebery MJ, Berliner KI. Allergic eustachian tube dysfunction: diagnosis and treatment. Am J Otol. 1997;18(2):160–165.
- 3180. Palmu A, Puhakka H, Rahko T, Takala AK. Diagnostic value of tympanometry in infants in clinical practice. Int J Pediatr Otorhinolaryngol. 1999;49(3):207–213. https://doi.org/10.1016/s0165‐5876(99)00207‐4
- 3181. Rosenfeld RM, Shin JJ, Schwartz SR, et al. Clinical practice guideline: otitis media with effusion (update). Otolaryngol Head Neck Surg. 2016;154(1 suppl):S1–S41. https://doi.org/10.1177/0194599815623467
- 3182. Caffarelli C, Savini E, Giordano S, Gianlupi G, Cavagni G. Atopy in children with otitis media with effusion. Clin Exp Allergy. 1998;28(5):591–596. https://doi.org/10.1046/j.1365‐2222.1998.00284.x
- 3183. Yeo SG, Park DC, Eun YG, Cha CI. The role of allergic rhinitis in the development of otitis media with effusion: effect on eustachian tube function. Am J Otolaryngol. 2007;28(3):148–152. https://doi.org/10.1016/j.amjoto.2006.07.011
- 3184. Borge P. Atopy and secretory otitis media. Immunological studies and responses to topical corticosteroid therapy. J Laryngol Otol. 1983;97(2):117–129. https://doi.org/10.1017/s0022215100093890
- 3185. Tomonaga K, Kurono Y, Mogi G. The role of nasal allergy in otitis media with effusion. A clinical study. Acta Otolaryngol Suppl. 1988;458:41–47. https://doi.org/10.3109/00016488809125100
- 3186. Corey JP, Adham RE, Abbass AH, Seligman I. The role of IgE‐mediated hypersensitivity in otitis media with effusion. Am J Otolaryngol. 1994;15(2):138–144. https://doi.org/10.1016/0196‐0709(94)90063‐9
- 3187. Chantzi FM, Kafetzis DA, Bairamis T, et al. IgE sensitization, respiratory allergy symptoms, and heritability independently increase the risk of otitis media with effusion. Allergy. 2006;61(3):332–336. https://doi.org/10.1111/j.1398‐9995.2006.00971.x
- 3188. Gultekin E, Develioglu ON, Yener M, Ozdemir I, Kulekci M. Prevalence and risk factors for persistent otitis media with effusion in primary school children in Istanbul, Turkey. Auris Nasus Larynx. 2010;37(2):145–149. https://doi.org/10.1016/j.anl.2009.05.002
- 3189. Sharifian MR, Mahmoudi M, Pourmomenarabi B, Keramati MR. Correlation between allergic rhinitis and otitis media with effusion. Iran J Otorhinolaryngol. 2019;31(105):209–215.
- 3190. Songu M, Islek A, Imre A, et al. Risk factors for otitis media with effusion in children with adenoid hypertrophy. Acta Otorhinolaryngol Ital. 2020;40(2):133–137. https://doi.org/10.14639/0392‐100X‐2456
- 3191. McMahan JT, Calenoff E, Croft DJ, Barenholtz L, Weber LD. Chronic otitis media with effusion and allergy: modified RAST analysis of 119 cases. Otolaryngol Head Neck Surg. 1981;89(3 pt 1):427–431. https://doi.org/10.1177/019459988108900315
- 3192. Hurst DS. Allergy management of refractory serous otitis media. Otolaryngol Head Neck Surg. 1990;102(6):664–669. https://doi.org/10.1177/019459989010200607
- 3193. Hurst DS. Association of otitis media with effusion and allergy as demonstrated by intradermal skin testing and eosinophil cationic protein levels in both middle ear effusions and mucosal biopsies. Laryngoscope. 1996;106(9 pt 1):1128–1137. https://doi.org/10.1097/00005537‐199609000‐00017
- 3194. Alles R, Parikh A, Hawk L, Darby Y, Romero JN, Scadding G. The prevalence of atopic disorders in children with chronic otitis media with effusion. Pediatr Allergy Immunol. 2001;12(2):102–106. https://doi.org/10.1046/j.0905‐6157.2000.00008.x
- 3195. Hurst DS. Efficacy of allergy immunotherapy as a treatment for patients with chronic otitis media with effusion. Int J Pediatr Otorhinolaryngol. 2008;72(8):1215–1223. https://doi.org/10.1016/j.ijporl.2008.04.013
- 3196. Norhafizah S, Salina H, Goh BS. Prevalence of allergic rhinitis in children with otitis media with effusion. Eur Ann Allergy Clin Immunol. 2020;52(3):121–130. https://doi.org/10.23822/EurAnnACI.1764‐1489.119
- 3197. Kreiner‐Moller E, Chawes BL, Caye‐Thomasen P, Bonnelykke K, Bisgaard H. Allergic rhinitis is associated with otitis media with effusion: a birth cohort study. Clin Exp Allergy. 2012;42(11):1615–1620. https://doi.org/10.1111/j.1365‐2222.2012.04038.x
- 3198. Cheng X, Sheng H, Ma R, et al. Allergic rhinitis and allergy are risk factors for otitis media with effusion: a meta‐analysis. Allergol Immunopathol (Madr). 2017;45(1):25–32. https://doi.org/10.1016/j.aller.2016.03.004
- 3199. Byeon H. The association between allergic rhinitis and otitis media: a national representative sample of in South Korean children. Sci Rep. 2019;9(1):1610. https://doi.org/10.1038/s41598‐018‐38369‐7
- 3200. Torretta S, Pignataro L, Carioli D, et al. Phenotype profiling and allergy in otitis‐prone children. Front Pediatr. 2018;6:383. https://doi.org/10.3389/fped.2018.00383
- 3201. Bluestone CD. Current concepts in eustachian tube function as related to otitis media. Auris Nasus Larynx. 1985;12(suppl 1):S1–S4. https://doi.org/10.1016/s0385‐8146(85)80083‐3
- 3202. Bluestone CD. Eustachian tube function: physiology, pathophysiology, and role of allergy in pathogenesis of otitis media. J Allergy Clin Immunol. 1983;72(3):242–251. https://doi.org/10.1016/0091‐6749(83)90027‐1
- 3203. Bernstein JM, Ellis E, Li P. The role of IgE‐mediated hypersensitivity in otitis media with effusion. Otolaryngol Head Neck Surg. 1981;89(5):874–878. https://doi.org/10.1177/019459988108900534
- 3204. Bernstein JM, Lee J, Conboy K, Ellis E, Li P. The role of IgE‐mediated hypersensitivity in recurrent otitis media with effusion. Am J Otol. 1983;5(1):66–69.
- 3205. Bernstein JM, Lee J, Conboy K, Ellis E, Li P. Further observations on the role of IgE‐mediated hypersensitivity in recurrent otitis media with effusion. Otolaryngol Head Neck Surg. 1985;93(5):611–615. https://doi.org/10.1177/019459988509300508
- 3206. Hurst DS, Weekley M, Ramanarayanan MP. Evidence of possible localized specific immunoglobulin E production in middle ear fluid as demonstrated by ELISA testing. Otolaryngol Head Neck Surg. 1999;121(3):224–230. https://doi.org/10.1016/S0194‐5998(99)70176‐2
- 3207. Hurst DS, Venge P. Evidence of eosinophil, neutrophil, and mast‐cell mediators in the effusion of OME patients with and without atopy. Allergy. 2000;55(5):435–441. https://doi.org/10.1034/j.1398‐9995.2000.00289.x
- 3208. Sobol SE, Taha R, Schloss MD, et al. T(H)2 cytokine expression in atopic children with otitis media with effusion. J Allergy Clin Immunol. 2002;110(1):125–130. https://doi.org/10.1067/mai.2002.125697
- 3209. Tewfik TL, Mazer B. The links between allergy and otitis media with effusion. Curr Opin Otolaryngol Head Neck Surg. 2006;14(3):187–190. https://doi.org/10.1097/01.moo.0000193190.24849.f0
- 3210. Wright ED, Hurst D, Miotto D, Giguere C, Hamid Q. Increased expression of major basic protein (MBP) and interleukin‐5(IL‐5) in middle ear biopsy specimens from atopic patients with persistent otitis media with effusion. Otolaryngol Head Neck Surg. 2000;123(5):533–538. https://doi.org/10.1067/mhn.2000.109472
- 3211. Jang CH, Kim YH. Characterization of cytokines present in pediatric otitis media with effusion: comparison of allergy positive and negative. Int J Pediatr Otorhinolaryngol. 2002;66(1):37–40. https://doi.org/10.1016/s0165‐5876(02)00185‐4
- 3212. Jang CH, Kim YH. Demonstration of RANTES and eosinophilic cataionic protein in otitis media with effusion with allergy. Int J Pediatr Otorhinolaryngol. 2003;67(5):531–533. https://doi.org/10.1016/s0165‐5876(03)00015‐6
- 3213. Nguyen LH, Manoukian JJ, Tewfik TL, et al. Evidence of allergic inflammation in the middle ear and nasopharynx in atopic children with otitis media with effusion. J Otolaryngol. 2004;33(6):345–351. https://doi.org/10.2310/7070.2004.03073
- 3214. Lildholdt T, Kortholm B. Beclomethasone nasal spray in the treatment of middle‐ear effusion – a double‐blind study. Int J Pediatr Otorhinolaryngol. 1982;4(2):133–137. https://doi.org/10.1016/0165‐5876(82)90088‐x
- 3215. Williamson I, Benge S, Barton S, et al. Topical intranasal corticosteroids in 4‐11 year old children with persistent bilateral otitis media with effusion in primary care: double blind randomised placebo controlled trial. BMJ. 2009;339:b4984. https://doi.org/10.1136/bmj.b4984
- 3216. Griffin G, Flynn CA. Antihistamines and/or decongestants for otitis media with effusion (OME) in children. Cochrane Database Syst Rev. 2011;(9):CD003423. https://doi.org/10.1002/14651858.CD003423.pub3
- 3217. Simpson SA, Lewis R, van der Voort J, Butler CC. Oral or topical nasal steroids for hearing loss associated with otitis media with effusion in children. Cochrane Database Syst Rev. 2011;(5):CD001935. https://doi.org/10.1002/14651858.CD001935.pub3
- 3218. Schoem SR, Willard A, Combs JT. A prospective, randomized, placebo‐controlled, double‐blind study of montelukast's effect on persistent middle ear effusion. Ear Nose Throat J. 2010;89(9):434–437.
- 3219. Ertugay CK, Cingi C, Yaz A, et al. Effect of combination of montelukast and levocetirizine on otitis media with effusion: a prospective, placebo‐controlled trial. Acta Otolaryngol. 2013;133(12):1266–1272. https://doi.org/10.3109/00016489.2013.824113
- 3220. Sajjadi H, Paparella MM. Meniere's disease. Lancet. 2008;372(9636):406–414. https://doi.org/10.1016/S0140‐6736(08)61161‐7
- 3221. Derebery MJ. Allergic and immunologic aspects of Meniere's disease. Otolaryngol Head Neck Surg. 1996;114(3):360–365. https://doi.org/10.1016/s0194‐5998(96)70204‐8
- 3222. Derebery MJ, Berliner KI. Prevalence of allergy in Meniere's disease. Otolaryngol Head Neck Surg. 2000;123(1 pt 1):69–75. https://doi.org/10.1067/mhn.2000.105715
- 3223. Tyrrell JS, Whinney DJ, Ukoumunne OC, Fleming LE, Osborne NJ. Prevalence, associated factors, and comorbid conditions for Meniere's disease. Ear Hear. 2014;35(4):e162–e169. https://doi.org/10.1097/AUD.0000000000000041
- 3224. Sen P, Georgalas C, Papesch M. Co‐morbidity of migraine and Meniere's disease – is allergy the link? J Laryngol Otol. 2005;119(6):455–460. https://doi.org/10.1258/0022215054273133
- 3225. Keles E, Godekmerdan A, Kalidag T, et al. Meniere's disease and allergy: allergens and cytokines. J Laryngol Otol. 2004;118(9):688–693. https://doi.org/10.1258/0022215042244822
- 3226. Roomiani M, Dehghani Firouzabadi F, Delbandi AA, et al. Evaluation of serum immunoreactivity to common indigenous iranian inhalation and food allergens in patients with Meniere's disease. Immunol Invest. 2021:1–10. https://doi.org/10.1080/08820139.2020.1869252
- 3227. Ma Y, Sun Q, Zhang K, Bai L, Du L. High level of IgE in acute low‐tone sensorineural hearing loss: a predictor for recurrence and Meniere disease transformation. Am J Otolaryngol. 2021;42(2):102856. https://doi.org/10.1016/j.amjoto.2020.102856
- 3228. Hsu L, Zhu XN, Zhao YS. Immunoglobulin E and circulating immune complexes in endolymphatic hydrops. Ann Otol Rhinol Laryngol. 1990;99(7 pt 1):535–538. https://doi.org/10.1177/000348949009900707
- 3229. Viscomi GJ, Bojrab DI. Use of electrocochleography to monitor antigenic challenge in Meniere's disease. Otolaryngol Head Neck Surg. 1992;107(6 pt 1):733–737. https://doi.org/10.1177/019459988910700604.1
- 3230. Gibbs SR, Mabry RL, Roland PS, Shoup AG, Mabry CS. Electrocochleographic changes after intranasal allergen challenge: a possible diagnostic tool in patients with Meniere's disease. Otolaryngol Head Neck Surg. 1999;121(3):283–284. https://doi.org/10.1016/S0194‐5998(99)70186‐5
- 3231. Derebery MJ, Valenzuela S. Meniere's syndrome and allergy. Otolaryngol Clin North Am. 1992;25(1):213–224.
- 3232. Derebery MJ. Allergic management of Meniere's disease: an outcome study. Otolaryngol Head Neck Surg. 2000;122(2):174–182. https://doi.org/10.1016/S0194‐5998(00)70235‐X
- 3233. NCT04815187 – Repurposed use of allergic rhinitis and allergic asthma drug to reduce vertigo and hearing loss in Meniere's disease. Accessed March 6, 2021. https://clinicaltrials.gov/show/NCT04815187
- 3234. Singh S, Nagarkar AN, Bansal S, Vir D, Gupta AK. Audiological manifestations of allergic rhinitis. J Laryngol Otol. 2011;125(9):906–910. https://doi.org/10.1017/S0022215111001137
- 3235. Irwin RS, Baumann MH, Bolser DC, et al. Diagnosis and management of cough executive summary: ACCP evidence‐based clinical practice guidelines. Chest. 2006;129(1 suppl):1S–23S. https://doi.org/10.1378/chest.129.1_suppl.1S
- 3236. Passali D, de Benedetto F, de Benedetto M, et al. Rhino‐bronchial syndrome. The SIO‐AIMAR (Italian Society of Otorhinolaryngology, Head Neck Surgery‐Interdisciplinary Scientific Association for the Study of the Respiratory Diseases) survey. Acta Otorhinolaryngol Ital. 2011;31(1):27–34.
- 3237. Krzych‐Falta E, Piekarska B, Sybilski A, Wojas O, Samolinski B. The safety of nasal allergen challenge test assessed in lower airways. Iran J Allergy Asthma Immunol. 2015;14(6):581–588.
- 3238. Chakir J, Laviolette M, Turcotte H, Boutet M, Boulet LP. Cytokine expression in the lower airways of nonasthmatic subjects with allergic rhinitis: influence of natural allergen exposure. J Allergy Clin Immunol. 2000;106(5):904–910. https://doi.org/10.1067/mai.2000.110100
- 3239. Chakir J, Laviolette M, Boutet M, Laliberte R, Dube J, Boulet LP. Lower airways remodeling in nonasthmatic subjects with allergic rhinitis. Lab Invest. 1996;75(5):735–44.
- 3240. Buday T, Gavliakova S, Mokry J, Medvedova I, Kavalcikova‐Bogdanova N, Plevkova J. The guinea pig sensitized by house dust mite: a model of experimental cough studies. Adv Exp Med Biol. 2016;905:87–95. https://doi.org/10.1007/5584_2016_217
- 3241. Lin HC, Cho SH, Ghoshal AG, et al. Respiratory diseases and the impact of cough in Taiwan: results from the APBORD observational study. Medicine (Baltimore). 2016;95(27):e3854. https://doi.org/10.1097/MD.0000000000003854
- 3242. Cho SH, Lin HC, Ghoshal AG, et al. Respiratory disease in the Asia‐Pacific region: cough as a key symptom. Allergy Asthma Proc. 2016;37(2):131–140. https://doi.org/10.2500/aap.2016.37.3925
- 3243. He S, Li YJ, Chen J. Clinical features of allergic rhinitis in children of Shanghai, China. Genet Mol Res. 2016;15(2). https://doi.org/10.4238/gmr.15028118
- 3244. Dicpinigiatis P, Birring S, McGarvey L, Schelfhout J, Tzontcheva A, Muccino D. Comorbid conditions and medical history among patients with refractory or unexplained chronic cough in two phase 3 clinical trials (COUGH‐1 and COUGH‐2). J Allergy Clin Immunol. 2021;147(2):AB61.
- 3245. Kim JH, Kim SA, Ku JY, Cho WK, Shin CH. Comparison of allergens and symptoms in patients with allergic rhinitis between 1990s and 2010s. Allergy Asthma Clin Immunol. 2020;16:58. https://doi.org/10.1186/s13223‐020‐00455‐9
- 3246. Tang W, Zhou J, Miao L, Shi G. Clinical features in patients of cough variant asthma with normal and high level of exhaled fractional nitric oxide. Clin Respir J. 2018;12(2):595–600. https://doi.org/10.1111/crj.12568
- 3247. Nakajima T, Nagano T, Nishimura Y. Retrospective study of the effects of post‐nasal drip symptoms on cough duration. In Vivo. 2021;35(3):1799–1803. https://doi.org/10.21873/invivo.12440
- 3248. Chen LC, Zeng GS, Wu LL, et al. Diagnostic value of FeNO and MMEF for predicting cough variant asthma in chronic cough patients with or without allergic rhinitis. J Asthma. 2021;58(3):326–333. https://doi.org/10.1080/02770903.2019.1694035
- 3249. Liu X, Wang X, Yao X, Wang Y, Sun Y, Zhang L. Value of exhaled nitric oxide and FEF25‐75 in identifying factors associated with chronic cough in allergic rhinitis. Allergy Asthma Immunol Res. 2019;11(6):830–845. https://doi.org/10.4168/aair.2019.11.6.830
- 3250. Deot N, Barr J, Mankowski N, Brunner J, McCoul ED. Effect of intranasal corticosteroids on secondary sinonasal symptoms: a systematic review of randomized trials. Am J Rhinol Allergy. 2019;33(5):601–607. https://doi.org/10.1177/1945892419844397
- 3251. Reidy PM, Dworkin JP, Krouse JH. Laryngeal effects of antigen stimulation challenge with perennial allergen Dermatophagoides pteronyssinus. Otolaryngol Head Neck Surg. 2003;128(4):455–462. https://doi.org/10.1016/s0194‐5998(03)00003‐2
- 3252. Lee K, Young Kang C, Lee H, Choi IH, Lee SH, Kim TH. Association of sinonasal factors with chronic laryngitis in Korean adults. JAMA Otolaryngol Head Neck Surg. 2019;145(10):919–925. https://doi.org/10.1001/jamaoto.2019.2134
- 3253. Wang YT, Chang GH, Yang YH, et al. Allergic rhinitis and laryngeal pathology: real‐world evidence. Healthcare (Basel). 2021;9(1):36. https://doi.org/10.3390/healthcare9010036
- 3254. Millqvist E, Bende M, Brynnel M, Johansson I, Kappel S, Ohlsson AC. Voice change in seasonal allergic rhinitis. J Voice. 2008;22(4):512–515. https://doi.org/10.1016/j.jvoice.2006.12.003
- 3255. Koc EA, Koc B, Erbek S. Comparison of acoustic and stroboscopic findings and voice handicap index between allergic rhinitis patients and controls. Balkan Med J. 2014;31(4):340–344. https://doi.org/10.5152/balkanmedj.2014.14511
- 3256. Krouse JH, Dworkin JP, Carron MA, Stachler RJ. Baseline laryngeal effects among individuals with dust mite allergy. Otolaryngol Head Neck Surg. 2008;139(1):149–151. https://doi.org/10.1016/j.otohns.2008.04.001
- 3257. Randhawa PS, Nouraei S, Mansuri S, Rubin JS. Allergic laryngitis as a cause of dysphonia: a preliminary report. Logoped Phoniatr Vocol. 2010;35(4):169–174. https://doi.org/10.3109/14015431003599012
- 3258. Ohlsson AC, Drevsater A, Brynnel M, Johansson I. Allergic rhinitis and voice change. Logoped Phoniatr Vocol. 2016;41(4):143–148. https://doi.org/10.3109/14015439.2015.1049288
- 3259. Velickovic V, Simovic S, Zovanovic S, Stojanovic J, Koravovic M, Mihailovic N. The factors asociated with allergic rhinitis in dysphonic professional voice users. Mediconski Casopis. 2017;51(3):73–78.
- 3260. Hamdan AL, Sibai A, Youssef M, Deeb R, Zaitoun F. The use of a screening questionnaire to determine the incidence of allergic rhinitis in singers with dysphonia. Arch Otolaryngol Head Neck Surg. 2006;132(5):547–549. https://doi.org/10.1001/archotol.132.5.547
- 3261. Turley R, Cohen SM, Becker A, Ebert Jr CS. Role of rhinitis in laryngitis: another dimension of the unified airway. Ann Otol Rhinol Laryngol. 2011;120(8):505–510. https://doi.org/10.1177/000348941112000803
- 3262. Simberg S, Sala E, Tuomainen J, Ronnemaa AM. Vocal symptoms and allergy – a pilot study. J Voice. 2009;23(1):136–139. https://doi.org/10.1016/j.jvoice.2007.03.010
- 3263. Randhawa PS, Mansuri S, Rubin JS. Is dysphonia due to allergic laryngitis being misdiagnosed as laryngopharyngeal reflux? Logoped Phoniatr Vocol. 2010;35(1):1–5. https://doi.org/10.1080/14015430903002262
- 3264. Alharethy S, Baqays A, Mesallam TA, et al. Correlation between allergic rhinitis and laryngopharyngeal reflux. Biomed Res Int. 2018;2018:2951928. https://doi.org/10.1155/2018/2951928
- 3265. Roth DF, Ferguson BJ. Vocal allergy: recent advances in understanding the role of allergy in dysphonia. Curr Opin Otolaryngol Head Neck Surg. 2010;18(3):176–181. https://doi.org/10.1097/MOO.0b013e32833952af
- 3266. Eren E, Arslanoglu S, Aktas A, et al. Factors confusing the diagnosis of laryngopharyngeal reflux: the role of allergic rhinitis and inter‐rater variability of laryngeal findings. Eur Arch Otorhinolaryngol. 2014;271(4):743–747. https://doi.org/10.1007/s00405‐013‐2682‐y
- 3267. Jackson‐Menaldi CA, Dzul AI, Holland RW. Allergies and vocal fold edema: a preliminary report. J Voice. 1999;13(1):113–122. https://doi.org/10.1016/s0892‐1997(99)80065‐4
- 3268. Belafsky PC, Peake J, Smiley‐Jewell SM, Verma SP, Dworkin‐Valenti J, Pinkerton KE. Soot and house dust mite allergen cause eosinophilic laryngitis in an animal model. Laryngoscope. 2016;126(1):108–112. https://doi.org/10.1002/lary.25467
- 3269. Mouadeb DA, Belafsky PC, Birchall M, Hood C, Konia T, Pinkerton KE. The effects of allergens and tobacco smoke on the laryngeal mucosa of guinea pigs. Otolaryngol Head Neck Surg. 2009;140(4):493–497. https://doi.org/10.1016/j.otohns.2008.12.034
- 3270. Dworkin JP, Reidy PM, Stachler RJ, Krouse JH. Effects of sequential Dermatophagoides pteronyssinus antigen stimulation on anatomy and physiology of the larynx. Ear Nose Throat J. 2009;88(2):793–799.
- 3271. Roth DF, Abbott KV, Carroll TL, Ferguson BJ. Evidence for primary laryngeal inhalant allergy: a randomized, double‐blinded crossover study. Int Forum Allergy Rhinol. 2013;3(1):10–18. https://doi.org/10.1002/alr.21051
- 3272. Suzuki T, Okamoto Y, Yonekura S, Okuma Y, Sakurai T, Sakurai D. Characteristics of laryngeal symptoms induced in patients with allergic rhinitis in an environmental challenge chamber. Ann Allergy Asthma Immunol. 2016;116(6):491–496. https://doi.org/10.1016/j.anai.2016.03.011
- 3273. Brook CD, Platt MP, Reese S, Noordzij JP. Utility of allergy testing in patients with chronic laryngopharyngeal symptoms: is it allergic laryngitis? Otolaryngol Head Neck Surg. 2016;154(1):41–45. https://doi.org/10.1177/0194599815607850
- 3274. Brook C, Noordzij JP, Russell K, Aliphas A, Platt M. Predictive findings of allergic disease in fiberoptic nasolaryngoscopy. Laryngoscope. 2015;125(2):286–290. https://doi.org/10.1002/lary.24880
- 3275. Leigh LY, Spergel JM. An in‐depth characterization of a large cohort of adult patients with eosinophilic esophagitis. Ann Allergy Asthma Immunol. 2019;122(1):65–72.e1. https://doi.org/10.1016/j.anai.2018.09.452
- 3276. Furuta GT, Liacouras CA, Collins MH, et al. Eosinophilic esophagitis in children and adults: a systematic review and consensus recommendations for diagnosis and treatment. Gastroenterology. 2007;133(4):1342–1363. https://doi.org/10.1053/j.gastro.2007.08.017
- 3277. Benninger MS, Strohl M, Holy CE, Hanick AL, Bryson PC. Prevalence of atopic disease in patients with eosinophilic esophagitis. Int Forum Allergy Rhinol. 2017;7(8):757–762. https://doi.org/10.1002/alr.21968
- 3278. Azzano P, Villard Truc F, Collardeau‐Frachon S, Lachaux A. Children with eosinophilic esophagitis in real life: 10 years' experience with a focus on allergic management. Allergol Immunopathol (Madr). 2020;48(3):244–250. https://doi.org/10.1016/j.aller.2019.07.013
- 3279. Ancellin M, Ricolfi‐Waligova L, Clerc‐Urmes I, et al. Management of eosinophilic esophagitis in children according to atopic status: a retrospective cohort in northeast of France. Arch Pediatr. 2020;27(3):122–127. https://doi.org/10.1016/j.arcped.2020.02.001
- 3280. Mohammad AA, Wu SZ, Ibrahim O, et al. Prevalence of atopic comorbidities in eosinophilic esophagitis: a case‐control study of 449 patients. J Am Acad Dermatol. 2017;76(3):559–560. https://doi.org/10.1016/j.jaad.2016.08.068
- 3281. Alves Marcelino JL, Cardoso de Aguiar R, Cabral Duarte F, Celia Costa A, Pereira‐Barbosa MA. Pediatric eosinophilic esophagitis in Portugal. Eur Ann Allergy Clin Immunol. 2017;49(2):66–74.
- 3282. Olson AA, Evans MD, Johansson MW, et al. Role of food and aeroallergen sensitization in eosinophilic esophagitis in adults. Ann Allergy Asthma Immunol. 2016;117(4):387–393.e2. https://doi.org/10.1016/j.anai.2016.08.008
- 3283. Vernon N, Shah S, Lehman E, Ghaffari G. Comparison of atopic features between children and adults with eosinophilic esophagitis. Allergy Asthma Proc. 2014;35(5):409–414. https://doi.org/10.2500/aap.2014.35.3768
- 3284. Chadha SN, Wang L, Correa H, Moulton D, Hummell DS. Pediatric eosinophilic esophagitis: the Vanderbilt experience. Ann Allergy Asthma Immunol. 2014;113(4):445–451. https://doi.org/10.1016/j.anai.2014.07.020
- 3285. Castro Jimenez A, Gomez Torrijos E, Garcia Rodriguez R, et al. Demographic, clinical and allergological characteristics of Eosinophilic Esophagitis in a Spanish central region. Allergol Immunopathol (Madr). 2014;42(5):407–414. https://doi.org/10.1016/j.aller.2013.04.004
- 3286. Spergel JM, Brown‐Whitehorn TF, Beausoleil JL, et al. 14 years of eosinophilic esophagitis: clinical features and prognosis. J Pediatr Gastroenterol Nutr. 2009;48(1):30–36. https://doi.org/10.1097/MPG.0b013e3181788282
- 3287. Roy‐Ghanta S, Larosa DF, Katzka DA. Atopic characteristics of adult patients with eosinophilic esophagitis. Clin Gastroenterol Hepatol. 2008;6(5):531–535. https://doi.org/10.1016/j.cgh.2007.12.045
- 3288. Assa'ad AH, Putnam PE, Collins MH, et al. Pediatric patients with eosinophilic esophagitis: an 8‐year follow‐up. J Allergy Clin Immunol. 2007;119(3):731–738. https://doi.org/10.1016/j.jaci.2006.10.044
- 3289. Plaza‐Martin AM, Jimenez‐Feijoo R, Andaluz C, et al. Polysensitization to aeroallergens and food in eosinophilic esophagitis in a pediatric population. Allergol Immunopathol (Madr). 2007;35(1):35–37. https://doi.org/10.1016/s0301‐0546(07)70227‐6
- 3290. Sugnanam KK, Collins JT, Smith PK, et al. Dichotomy of food and inhalant allergen sensitization in eosinophilic esophagitis. Allergy. 2007;62(11):1257–1260. https://doi.org/10.1111/j.1398‐9995.2007.01454.x
- 3291. Remedios M, Campbell C, Jones DM, Kerlin P. Eosinophilic esophagitis in adults: clinical, endoscopic, histologic findings, and response to treatment with fluticasone propionate. Gastrointest Endosc. 2006;63(1):3–12. https://doi.org/10.1016/j.gie.2005.07.049
- 3292. Guajardo JR, Plotnick LM, Fende JM, Collins MH, Putnam PE, Rothenberg ME. Eosinophil‐associated gastrointestinal disorders: a world‐wide‐web based registry. J Pediatr. 2002;141(4):576–581. https://doi.org/10.1067/mpd.2002.127663
- 3293. Gonzalez‐Cervera J, Arias A, Redondo‐Gonzalez O, Cano‐Mollinedo MM, Terreehorst I, Lucendo AJ. Association between atopic manifestations and eosinophilic esophagitis: a systematic review and meta‐analysis. Ann Allergy Asthma Immunol. 2017;118(5):582–590.e2. https://doi.org/10.1016/j.anai.2017.02.006
- 3294. Imamura K, Haruma K, Matsumoto H, et al. Clinical and endoscopic characteristics of eosinophilic esophagitis in Japan: a case‐control study. Asia Pac Allergy. 2020;10(2):e16. https://doi.org/10.5415/apallergy.2020.10.e16
- 3295. Armentia A, Martin‐Armentia S, Alvarez‐Nogal R, Armentia BM, Gayoso MJ, Fernandez‐Gonzalez D. Germination of pollen grains in the oesophagus of individuals with eosinophilic oesophagitis. Clin Exp Allergy. 2019;49(4):471–473. https://doi.org/10.1111/cea.13312
- 3296. Reed CC, Iglesia EGA, Commins SP, Dellon ES. Seasonal exacerbation of eosinophilic esophagitis histologic activity in adults and children implicates role of aeroallergens. Ann Allergy Asthma Immunol. 2019;122(3):296–301. https://doi.org/10.1016/j.anai.2018.12.013
- 3297. Fahey L, Robinson G, Weinberger K, Giambrone AE, Solomon AB. Correlation between aeroallergen levels and new diagnosis of eosinophilic esophagitis in New York City. J Pediatr Gastroenterol Nutr. 2017;64(1):22–25. https://doi.org/10.1097/MPG.0000000000001245
- 3298. Ram G, Lee J, Ott M, et al. Seasonal exacerbation of esophageal eosinophilia in children with eosinophilic esophagitis and allergic rhinitis. Ann Allergy Asthma Immunol. 2015;115(3):224–228.e1. https://doi.org/10.1016/j.anai.2015.07.004
- 3299. Moawad FJ, Veerappan GR, Lake JM, et al. Correlation between eosinophilic oesophagitis and aeroallergens. Aliment Pharmacol Ther. 2010;31(4):509–515. https://doi.org/10.1111/j.1365‐2036.2009.04199.x
- 3300. Almansa C, Krishna M, Buchner AM, et al. Seasonal distribution in newly diagnosed cases of eosinophilic esophagitis in adults. Am J Gastroenterol. 2009;104(4):828–833. https://doi.org/10.1038/ajg.2008.169
- 3301. Wang FY, Gupta SK, Fitzgerald JF. Is there a seasonal variation in the incidence or intensity of allergic eosinophilic esophagitis in newly diagnosed children? J Clin Gastroenterol. 2007;41(5):451–453. https://doi.org/10.1097/01.mcg.0000248019.16139.67
- 3302. Fogg MI, Ruchelli E, Spergel JM. Pollen and eosinophilic esophagitis. J Allergy Clin Immunol. 2003;112(4):796–797. https://doi.org/10.1016/s0091‐6749(03)01715‐9
- 3303. Armentia A, Martin‐Armentia S, Martin‐Armentia B, et al. Is eosinophilic esophagitis an equivalent of pollen allergic asthma? Analysis of biopsies and therapy guided by component resolved diagnosis. Allergol Immunopathol (Madr). 2018;46(2):181–189. https://doi.org/10.1016/j.aller.2017.11.001
- 3304. Iglesia EGA, Commins SP, Dellon ES. Complete remission of eosinophilic esophagitis with multi‐aeroallergen subcutaneous immunotherapy: a case report. J Allergy Clin Immunol Pract. 2021;9(6):2517–2519.e2. https://doi.org/10.1016/j.jaip.2021.01.045
- 3305. Ramirez RM, Jacobs RL. Eosinophilic esophagitis treated with immunotherapy to dust mites. J Allergy Clin Immunol. 2013;132(2):503–504. https://doi.org/10.1016/j.jaci.2013.04.053
- 3306. Lucendo AJ, Arias A, Redondo‐Gonzalez O, Gonzalez‐Cervera J. Seasonal distribution of initial diagnosis and clinical recrudescence of eosinophilic esophagitis: a systematic review and meta‐analysis. Allergy. 2015;70(12):1640–1650. https://doi.org/10.1111/all.12767
- 3307. Elias MK, Kopacova J, Arora AS, et al. The diagnosis of esophageal eosinophilia is not increased in the summer months. Dysphagia. 2015;30(1):67–73. https://doi.org/10.1007/s00455‐014‐9574‐1
- 3308. Frederickson NW, Bayman L, Valestin J, et al. Lack of seasonal variation in the incidence of eosinophilic oesophagitis in adolescent and adult non‐PPI‐responsive oesophageal eosinophilia midwestern US populations. United European Gastroenterol J. 2014;2(2):69–76. https://doi.org/10.1177/2050640614525152
- 3309. Meltzer EO, Blaiss MS, Derebery MJ, et al. Burden of allergic rhinitis: results from the Pediatric Allergies in America survey. J Allergy Clin Immunol. 2009;124(3 suppl):S43–S70. https://doi.org/10.1016/j.jaci.2009.05.013
- 3310. Meltzer EO, Nathan R, Derebery J, et al. Sleep, quality of life, and productivity impact of nasal symptoms in the United States: findings from the Burden of Rhinitis in America survey. Allergy Asthma Proc. 2009;30(3):244–254. https://doi.org/10.2500/aap.2009.30.3230
- 3311. Young T, Finn L, Palta M. Chronic nasal congestion at night is a risk factor for snoring in a population‐based cohort study. Arch Intern Med. 2001;161(12):1514–1519. https://doi.org/10.1001/archinte.161.12.1514
- 3312. Staevska MT, Mandajieva MA, Dimitrov VD. Rhinitis and sleep apnea. Curr Allergy Asthma Rep. 2004;4(3):193–199. https://doi.org/10.1007/s11882‐004‐0026‐0
- 3313. Morris LG, Burschtin O, Lebowitz RA, Jacobs JB, Lee KC. Nasal obstruction and sleep‐disordered breathing: a study using acoustic rhinometry. Am J Rhinol. 2005;19(1):33–39.
- 3314. Storms WW. Pharmacologic approaches to daytime and nighttime symptoms of allergic rhinitis. J Allergy Clin Immunol. 2004;114(5 suppl):S146–S153. https://doi.org/10.1016/j.jaci.2004.08.045
- 3315. Shedden A. Impact of nasal congestion on quality of life and work productivity in allergic rhinitis: findings from a large online survey. Treat Respir Med. 2005;4(6):439–446. https://doi.org/10.2165/00151829‐200504060‐00007
- 3316. Reinberg A, Gervais P, Levi F, Smolensky M, Del Cerro L, Ugolini C. Circadian and circannual rhythms of allergic rhinitis: an epidemiologic study involving chronobiologic methods. J Allergy Clin Immunol. 1988;81(1):51–62. https://doi.org/10.1016/0091‐6749(88)90220‐5
- 3317. Tobaldini E, Costantino G, Solbiati M, et al. Sleep, sleep deprivation, autonomic nervous system and cardiovascular diseases. Neurosci Biobehav Rev. 2017;74(pt B):321–329. https://doi.org/10.1016/j.neubiorev.2016.07.004
- 3318. Ferguson BJ. Influences of allergic rhinitis on sleep. Otolaryngol Head Neck Surg. 2004;130(5):617–629. https://doi.org/10.1016/j.otohns.2004.02.001
- 3319. Tashiro M, Mochizuki H, Iwabuchi K, et al. Roles of histamine in regulation of arousal and cognition: functional neuroimaging of histamine H1 receptors in human brain. Life Sci. 2002;72(4‐5):409–414. https://doi.org/10.1016/s0024‐3205(02)02276‐2
- 3320. Sri Kantha S, Matsumura H, Kubo E, et al. Effects of prostaglandin D2, lipoxins and leukotrienes on sleep and brain temperature of rats. Prostaglandins Leukot Essent Fatty Acids. 1994;51(2):87–93. https://doi.org/10.1016/0952‐3278(94)90083‐3
- 3321. Pasha S, Kumar S, Chatterjee AB, Krishnaswamy G. An obstructive sleep apnea primer: what the practicing allergist needs to know. Ann Allergy Asthma Immunol. 2017;118(3):259–268. https://doi.org/10.1016/j.anai.2016.07.033
- 3322. Chirakalwasan N, Ruxrungtham K. The linkage of allergic rhinitis and obstructive sleep apnea. Asian Pac J Allergy Immunol. 2014;32(4):276–286.
- 3323. Mullington JM, Hinze‐Selch D, Pollmacher T. Mediators of inflammation and their interaction with sleep: relevance for chronic fatigue syndrome and related conditions. Ann N Y Acad Sci. 2001;933:201–210. https://doi.org/10.1111/j.1749‐6632.2001.tb05825.x
- 3324. Tan SN, Abdullah B. The association between obstructive aleep apnea and allergic rhinitis: current literature review. Curr Respir Med Rev. 2021;17(1):13–19.
- 3325. Tankere F, Maisonobe T, Naccache L, et al. Further evidence for a central reorganisation of synaptic connectivity in patients with hypoglossal‐facial anastomosis in man. Brain Res. 2000;864(1):87–94. https://doi.org/10.1016/s0006‐8993(00)02177‐6
- 3326. Horner RL, Innes JA, Murphy K, Guz A. Evidence for reflex upper airway dilator muscle activation by sudden negative airway pressure in man. J Physiol. 1991;436:15–29. https://doi.org/10.1113/jphysiol.1991.sp018536
- 3327. White DP, Edwards JK, Shea SA. Local reflex mechanisms: influence on basal genioglossal muscle activation in normal subjects. Sleep. 1998;21(7):719–728. https://doi.org/10.1093/sleep/21.7.719
- 3328. Lo YL, Jordan AS, Malhotra A, et al. Influence of wakefulness on pharyngeal airway muscle activity. Thorax. 2007;62(9):799–805. https://doi.org/10.1136/thx.2006.072488
- 3329. Baraniuk JN, Merck SJ. Nasal reflexes: implications for exercise, breathing, and sex. Curr Allergy Asthma Rep. 2008;8(2):147–153. https://doi.org/10.1007/s11882‐008‐0025‐7
- 3330. Basner RC, Simon PM, Schwartzstein RM, Weinberger SE, Weiss JW. Breathing route influences upper airway muscle activity in awake normal adults. J Appl Physiol (1985). 1989;66(4):1766–1771. https://doi.org/10.1152/jappl.1989.66.4.1766
- 3331. Shintaro C, Park CS. Establishing a patent nasal passage in obstructive sleep apnea. Sleep Med Clin. 2019;14(1):41–50. https://doi.org/10.1016/j.jsmc.2018.10.005
- 3332. Peppard PE, Young T, Palta M, Skatrud J. Prospective study of the association between sleep‐disordered breathing and hypertension. N Engl J Med. 2000;342(19):1378–1384. https://doi.org/10.1056/NEJM200005113421901
- 3333. Kuniyoshi FH, Garcia‐Touchard A, Gami AS, et al. Day‐night variation of acute myocardial infarction in obstructive sleep apnea. J Am Coll Cardiol. 2008;52(5):343–346. https://doi.org/10.1016/j.jacc.2008.04.027
- 3334. Arzt M, Young T, Finn L, Skatrud JB, Bradley TD. Association of sleep‐disordered breathing and the occurrence of stroke. Am J Respir Crit Care Med. 2005;172(11):1447–1451. https://doi.org/10.1164/rccm.200505‐702OC
- 3335. Kanagala R, Murali NS, Friedman PA, et al. Obstructive sleep apnea and the recurrence of atrial fibrillation. Circulation. 2003;107(20):2589–2594. https://doi.org/10.1161/01.CIR.0000068337.25994.21
- 3336. Wang H, Parker JD, Newton GE, et al. Influence of obstructive sleep apnea on mortality in patients with heart failure. J Am Coll Cardiol. 2007;49(15):1625–1631. https://doi.org/10.1016/j.jacc.2006.12.046
- 3337. Marcus CL, Brooks LJ, Draper KA, et al. Diagnosis and management of childhood obstructive sleep apnea syndrome. Pediatrics. 2012;130(3):576–584. https://doi.org/10.1542/peds.2012‐1671
- 3338. Ali NJ, Pitson D, Stradling JR. Natural history of snoring and related behaviour problems between the ages of 4 and 7 years. Arch Dis Child. 1994;71(1):74–76. https://doi.org/10.1136/adc.71.1.74
- 3339. Isaiah A, Ernst T, Cloak CC, Clark DB, Chang L. Association between habitual snoring and cognitive performance among a large sample of preadolescent children. JAMA Otolaryngol Head Neck Surg. 2021;147(5):426–433. https://doi.org/10.1001/jamaoto.2020.5712
- 3340. Jan JE, Reiter RJ, Bax MC, Ribary U, Freeman RD, Wasdell MB. Long‐term sleep disturbances in children: a cause of neuronal loss. Eur J Paediatr Neurol. 2010;14(5):380–390. https://doi.org/10.1016/j.ejpn.2010.05.001
- 3341. Morris LG, Burschtin O, Setlur J, et al. REM‐associated nasal obstruction: a study with acoustic rhinometry during sleep. Otolaryngol Head Neck Surg. 2008;139(5):619–623. https://doi.org/10.1016/j.otohns.2008.08.017
- 3342. Huseni S, Gutierrez MJ, Rodriguez‐Martinez CE, et al. The link between rhinitis and rapid‐eye‐movement sleep breathing disturbances in children with obstructive sleep apnea. Am J Rhinol Allergy. 2014;28(1):56–61. https://doi.org/10.2500/ajra.2014.28.3994
- 3343. Kimura A, Chiba S, Capasso R, et al. Phase of nasal cycle during sleep tends to be associated with sleep stage. Laryngoscope. 2013;123(8):2050–2055. https://doi.org/10.1002/lary.23986
- 3344. Skirko JR, James KT, Shusterman DJ, Weaver EM. Association of allergic rhinitis with change in nasal congestion in new continuous positive airway pressure users. JAMA Otolaryngol Head Neck Surg. 2020;146(6):523–529. https://doi.org/10.1001/jamaoto.2020.0261
- 3345. Inoue A, Chiba S, Matsuura K, Osafune H, Capasso R, Wada K. Nasal function and CPAP compliance. Auris Nasus Larynx. 2019;46(4):548–558. https://doi.org/10.1016/j.anl.2018.11.006
- 3346. Iwata N, Nakata S, Inada H, Kimura A, Hirata M, Yasuma F. Clinical indication of nasal surgery for the CPAP intolerance in obstructive sleep apnea with nasal obstruction. Auris Nasus Larynx. 2020;47(6):1018–1022. https://doi.org/10.1016/j.anl.2020.06.005
- 3347. Camacho M, Riaz M, Capasso R, et al. The effect of nasal surgery on continuous positive airway pressure device use and therapeutic treatment pressures: a systematic review and meta‐analysis. Sleep. 2015;38(2):279–286. https://doi.org/10.5665/sleep.4414
- 3348. Nakata S, Noda A, Yagi H, et al. Nasal resistance for determinant factor of nasal surgery in CPAP failure patients with obstructive sleep apnea syndrome. Rhinology. 2005;43(4):296–299.
- 3349. Poirier J, George C, Rotenberg B. The effect of nasal surgery on nasal continuous positive airway pressure compliance. Laryngoscope. 2014;124(1):317–319. https://doi.org/10.1002/lary.24131
- 3350. Awad MI, Kacker A. Nasal obstruction considerations in sleep apnea. Otolaryngol Clin North Am. 2018;51(5):1003–1009. https://doi.org/10.1016/j.otc.2018.05.012
- 3351. Craig TJ, Sherkat A, Safaee S. Congestion and sleep impairment in allergic rhinitis. Curr Allergy Asthma Rep. 2010;10(2):113–121. https://doi.org/10.1007/s11882‐010‐0091‐5
- 3352. Jalalia MM, Soleimanib R, Jalali SM, Mohisafata B. Evaluation of the effects of allergic rhinitis treatment on sexual functioning, sleep, and fatigue. Revue Francaise d‐Allergologie. 2020;60(2):55–60.
- 3353. Kiely JL, Nolan P, McNicholas WT. Intranasal corticosteroid therapy for obstructive sleep apnoea in patients with co‐existing rhinitis. Thorax. 2004;59(1):50–55.
- 3354. Jacobi H, Rehm D, Nolte H, Andersen KF, Demoly P. Effect of house dust mite SLIT‐tablet treatment on quality of sleep in allergic rhinitis patients. J Allergy Clin Immunol. 2019;143:AB286.
- 3355. Mann RD, Pearce GL, Dunn N, Shakir S. Sedation with "non‐sedating" antihistamines: four prescription‐event monitoring studies in general practice. BMJ. 2000;320(7243):1184–1186. https://doi.org/10.1136/bmj.320.7243.1184
- 3356. Hindmarch I, Shamsi Z. Antihistamines: models to assess sedative properties, assessment of sedation, safety and other side‐effects. Clin Exp Allergy. 1999;29(suppl 3):133–142. https://doi.org/10.1046/j.1365‐2222.1999.0290s3133.x
- 3357. Chen ST, Lu KH, Sun HL, Chang WT, Lue KH, Chou MC. Randomized placebo‐controlled trial comparing montelukast and cetirizine for treating perennial allergic rhinitis in children aged 2‐6 yr. Pediatr Allergy Immunol. 2006;17(1):49–54. https://doi.org/10.1111/j.1399‐3038.2005.00351.x
- 3358. Clarenbach CF, Kohler M, Senn O, Thurnheer R, Bloch KE. Does nasal decongestion improve obstructive sleep apnea? J Sleep Res. 2008;17(4):444–449. https://doi.org/10.1111/j.1365‐2869.2008.00667.x
- 3359. Na HG, Sung CM, Yang HC. Effect of continuous positive airway pressure on symptoms of allergic rhinitis in patients with obstructive sleep apnea. World Allergy Organization J. 2020;13(8):100271.
- 3360. Chuang C, Tsai M, Tsai Y, Kuo C, Hsu C, Hung J. Increased risk of sleep apnea in patients of allergic rhinitis: a nationwide population‐based study. presented at: American Thoracic Society; 2019; Dallas, TX.
- 3361. Wongvilairat S, Assanasen P, Banhiran W, Tantilipikorn P, Bunnag C. The prevalence of high risk of obstructive sleep apnea in patients with allergic rhinitis. Asian Pacific Journal of Allergy and Immunology. 2022;40(3):205–209. doi: https://doi.org/10.12932/ap‐141218‐0458
- 3362. WHO Director‐General's opening remarks at the media briefing on COVID‐19 – 11 March 2020. Accessed November 13, 2021. https://www.who.int/director‐general/speeches/detail/who‐director‐general‐s‐opening‐remarks‐at‐the‐media‐briefing‐on‐covid‐19—11‐march‐2020
- 3363. Mustafa SS, Shaker MS, Munblit D, Greenhawt M. Paediatric allergy practice in the era of coronavirus disease 2019. Curr Opin Allergy Clin Immunol. 2021;21(2):159–165. https://doi.org/10.1097/ACI.0000000000000727
- 3364. Izquierdo‐Dominguez A, Rojas‐Lechuga MJ, Alobid I. Management of allergic diseases during COVID‐19 outbreak. Curr Allergy Asthma Rep. 2021;21(2):8. https://doi.org/10.1007/s11882‐021‐00989‐x
- 3365. Searing DA, Dutmer CM, Fleischer DM, et al. A phased approach to resuming suspended allergy/immunology clinical services. J Allergy Clin Immunol Pract. 2020;8(7):2125–2134. https://doi.org/10.1016/j.jaip.2020.05.012
- 3366. Pfaar O, Klimek L, Jutel M, et al. COVID‐19 pandemic: practical considerations on the organization of an allergy clinic – an EAACI/ARIA Position Paper. Allergy. 2021;76(3):648–676. https://doi.org/10.1111/all.14453
- 3367. Ozturk AB, Baccioglu A, Soyer O, Civelek E, Sekerel BE, Bavbek S. Change in allergy practice during the COVID‐19 pandemic. Int Arch Allergy Immunol. 2021;182(1):49–52. https://doi.org/10.1159/000512079
- 3368. Winders T, DuBuske L, Bukstein DA, Meltzer EO, Wallace D, Rance K. Shifts in allergy practice in a COVID‐19 world: implications of pre‐COVID‐19 national health care provider and patient surveys of treatments for nasal allergies. Allergy Asthma Proc. 2021;42(4):301–309. https://doi.org/10.2500/aap.2021.42.210035
- 3369. Tsao LR, Villanueva SA, Pines DA, et al. Impact of rapid transition to telemedicine‐based delivery on allergy/immunology care during COVID‐19. J Allergy Clin Immunol Pract. 2021;9(7):2672–2679.e2. https://doi.org/10.1016/j.jaip.2021.04.018
- 3370. Ren J, Pang W, Luo Y, et al. Impact of allergic rhinitis and asthma on COVID‐19 infection, hospitalization, and mortality. J Allergy Clin Immunol Pract. 2022;10(1):124–133. https://doi.org/10.1016/j.jaip.2021.10.049
- 3371. Beken B, Ozturk GK, Aygun FD, Aydogmus C, Akar HH. Asthma and allergic diseases are not risk factors for hospitalization in children with coronavirus disease 2019. Ann Allergy Asthma Immunol. 2021;126(5):569–575. https://doi.org/10.1016/j.anai.2021.01.018
- 3372. Yao Y, Wang H, Liu Z. Expression of ACE2 in airways: Implication for COVID‐19 risk and disease management in patients with chronic inflammatory respiratory diseases. Clin Exp Allergy. 2020;50(12):1313–1324. https://doi.org/10.1111/cea.13746
- 3373. Keswani A, Dhana K, Rosenthal JA, Moore D, Mahdavinia M. Atopy is predictive of a decreased need for hospitalization for coronavirus disease 2019. Ann Allergy Asthma Immunol. 2020;125(4):479–481. https://doi.org/10.1016/j.anai.2020.07.012
- 3374. Du H, Dong X, Zhang JJ, et al. Clinical characteristics of 182 pediatric COVID‐19 patients with different severities and allergic status. Allergy. 2021;76(2):510–532. https://doi.org/10.1111/all.14452
- 3375. Darabi A, Dehghanfard M, Jozan S, et al. Investigating the association between allergic diseases and COVID‐19 in 400 Iranian patients. Allergol Immunopathol (Madr). 2021;49(5):9–15. https://doi.org/10.15586/aei.v49i5.105
- 3376. Ming W, Zuo J, Han J, Chen J. The impact of comorbid allergic airway disease on the severity and mortality of COVID‐19: a systematic review and meta‐analysis. Eur Arch Otorhinolaryngol. 2022;279(4):1675–1690. https://doi.org/10.1007/s00405‐021‐07072‐1
- 3377. Guvey A. How does allergic rhinitis impact the severity of COVID‐19?: a case‐control study. Eur Arch Otorhinolaryngol. 2021;278(11):4367–4371. https://doi.org/10.1007/s00405‐021‐06836‐z
- 3378. Hagemann J, Onorato GL, Jutel M, et al. Differentiation of COVID‐19 signs and symptoms from allergic rhinitis and common cold: an ARIA‐EAACI‐GA(2) LEN consensus. Allergy. 2021;76(8):2354–2366. https://doi.org/10.1111/all.14815
- 3379. Bruno C, Locatello LG, Cilona M, et al. Seasonal allergic rhinitis symptoms in relation to COVID‐19. Allergy Rhinol (Providence). 2020;11:2152656720968804. https://doi.org/10.1177/2152656720968804
- 3380. Ferreli F, Gaino F, Russo E, et al. Clinical presentation at the onset of COVID‐19 and allergic rhinoconjunctivitis. J Allergy Clin Immunol Pract. 2020;8(10):3587–3589. https://doi.org/10.1016/j.jaip.2020.08.009
- 3381. Jin L, Fan K, Tan S, Liu S, Wang Y, Yu S. Analysis of the characteristics of outpatient and emergency diseases in the department of otolaryngology during the "COVID‐19" pandemic. Sci Prog. 2021;104(3):368504211036319. https://doi.org/10.1177/00368504211036319
- 3382. Yoon D, Kim KE, Lee JE, Kim M, Kim JH. Impact of the coronavirus disease 2019 (COVID‐19) pandemic on medical use of military hospitals in Korea. J Korean Med Sci. 2021;36(28):e204. https://doi.org/10.3346/jkms.2021.36.e204
- 3383. Choi HG, Kong IG. Asthma, allergic rhinitis, and atopic dermatitis incidence in Korean adolescents before and after COVID‐19. J Clin Med. 2021;10(15):3446. https://doi.org/10.3390/jcm10153446
- 3384. Dayal AK, Sinha V. Trend of allergic rhinitis post COVID‐19 pandemic: a retrospective observational study. Indian J Otolaryngol Head Neck Surg. 2020:1–3. https://doi.org/10.1007/s12070‐020‐02223‐y
- 3385. Gelardi M, Trecca E, Fortunato F, et al. COVID‐19 lockdown and seasonal allergic rhinitis: our experience in 40 patients. Acta Biomed. 2021;92(2):e2021215. https://doi.org/10.23750/abm.v92i2.10953
- 3386. Sozener ZC, Ozturk BO, Aydin O, et al. Coincidence of pollen season and coronavirus disease 2019 pandemic: less time outdoors – lesser allergy symptoms in 2020. Asia Pac Allergy. 2021;11(2):e16. https://doi.org/10.5415/apallergy.2021.11.e16
- 3387. Zhang Y, Lan F, Zhang L. Advances and highlights in allergic rhinitis. Allergy. 2021;76(11):3383–3389. https://doi.org/10.1111/all.15044
- 3388. Yucel E, Suleyman A, Hizli Demirkale Z, Guler N, Tamay ZU, Ozdemir C. ‘Stay at home’: is it good or not for house dust mite sensitized children with respiratory allergies? Pediatr Allergy Immunol. 2021;32(5):963–970. https://doi.org/10.1111/pai.13477
- 3389. Gallo O, Bruno C, Orlando P, Locatello LG. The impact of lockdown on allergic rhinitis: what is good and what is bad? Laryngoscope Investig Otolaryngol. 2020;5(5):807–808. https://doi.org/10.1002/lio2.459
- 3390. Dror AA, Eisenbach N, Marshak T, et al. Reduction of allergic rhinitis symptoms with face mask usage during the COVID‐19 pandemic. J Allergy Clin Immunol Pract. 2020;8(10):3590–3593. https://doi.org/10.1016/j.jaip.2020.08.035
- 3391. Mengi E, Kara CO, Alpturk U, Topuz B. The effect of face mask usage on the allergic rhinitis symptoms in patients with pollen allergy during the covid‐19 pandemic. Am J Otolaryngol. 2022;43(1):103206. https://doi.org/10.1016/j.amjoto.2021.103206
- 3392. Patella V, Delfino G, Florio G, et al. Management of the patient with allergic and immunological disorders in the pandemic COVID‐19 era. Clin Mol Allergy. 2020;18:18. https://doi.org/10.1186/s12948‐020‐00134‐5
- 3393. Wang Y, Shi C, Yang Y, et al. Anxiety and depression in allergic rhinitis patients during COVID‐19 pandemic in Wuhan, China. Asian Pac J Allergy Immunol. 2021. https://doi.org/10.12932/AP‐140820‐0941
- 3394. Gonzalez‐Diaz SN, Martin B, Villarreal‐Gonzalez RV, et al. Psychological impact of the COVID‐19 pandemic on patients with allergic diseases. World Allergy Organ J. 2021;14(3):100510. https://doi.org/10.1016/j.waojou.2021.100510
- 3395. Radulesco T, Verillaud B, Bequignon E, et al. COVID‐19 and rhinology, from the consultation room to the operating theatre. Eur Ann Otorhinolaryngol Head Neck Dis. 2020;137(4):309–314. https://doi.org/10.1016/j.anorl.2020.04.013
- 3396. De Luca P, Scarpa A, Ralli M, et al. Nasal, pharyngeal and laryngeal endoscopy procedures during COVID‐19 pandemic: available recommendations from national and international societies. Eur Arch Otorhinolaryngol. 2020;277(7):2151–2153. https://doi.org/10.1007/s00405‐020‐06028‐1
- 3397. Klimek L, Jutel M, Bousquet J, et al. Management of patients with chronic rhinosinusitis during the COVID‐19 pandemic – an EAACI position paper. Allergy. 2021;76(3):677–688. https://doi.org/10.1111/all.14629
- 3398. Zhang Y, Zhang L. Management practice of allergic rhinitis in china during the COVID‐19 pandemic. Allergy Asthma Immunol Res. 2020;12(4):738–742. https://doi.org/10.4168/aair.2020.12.4.738
- 3399. McCarty EB, Soldatova L, Brant JA, Newman JG. Innovations in otorhinolaryngology in the age of COVID‐19: a systematic literature review. World J Otorhinolaryngol Head Neck Surg. 2022;8(3):224–238. https://doi.org/10.1016/j.wjorl.2021.01.001
- 3400. Lee JH, Lee Y, Lee SY, et al. Management of allergic patients during the COVID‐19 pandemic in Asia. Allergy Asthma Immunol Res. 2020;12(5):783–791. https://doi.org/10.4168/aair.2020.12.5.783
- 3401. CDC – COVID‐19 and Your Health – Centers for Disease Control and Prevention. Accessed November 13, 2021. https://www.cdc.gov/coronavirus/2019‐ncov/prevent‐getting‐sick/prevention.html
- 3402. Cianferoni A, Votto M. COVID‐19 and allergy: how to take care of allergic patients during a pandemic? Pediatr Allergy Immunol. 2020;31(suppl 26):96–101. https://doi.org/10.1111/pai.13367
- 3403. Klimek L, Pfaar O, Worm M, et al. Allergen immunotherapy in the current COVID‐19 pandemic: a position paper of AeDA, ARIA, EAACI, DGAKI and GPA: position paper of the German ARIA Group(A) in cooperation with the Austrian ARIA Group(B), the Swiss ARIA Group(C), German Society for Applied Allergology (AEDA)(D), German Society for Allergology and Clinical Immunology (DGAKI)(E), Society for Pediatric Allergology (GPA)(F) in cooperation with AG Clinical Immunology, Allergology and Environmental Medicine of the DGHNO‐KHC(G) and the European Academy of Allergy and Clinical Immunology (EAACI)(H). Allergol Select. 2020;4:44–52. https://doi.org/10.5414/ALX02147E
- 3404. Matos S, Sharma A, Crosby D. Objective assessment of aerosolization during transnasal endoscopy: a systematic review. Otolaryngol Head Neck Surg. 2021:1945998211050632. https://doi.org/10.1177/01945998211050632
- 3405. Thamboo A, Lea J, Sommer DD, et al. Clinical evidence based review and recommendations of aerosol generating medical procedures in otolaryngology – head and neck surgery during the COVID‐19 pandemic. J Otolaryngol Head Neck Surg. 2020;49(1):28. https://doi.org/10.1186/s40463‐020‐00425‐6
- 3406. Workman AD, Welling DB, Carter BS, et al. Endonasal instrumentation and aerosolization risk in the era of COVID‐19: simulation, literature review, and proposed mitigation strategies. Int Forum Allergy Rhinol. 2020;10(7):798–805. https://doi.org/10.1002/alr.22577
- 3407. Sharma D, Rubel KE, Ye MJ, et al. Cadaveric simulation of endoscopic endonasal procedures: analysis of droplet splatter patterns during the COVID‐19 pandemic. Otolaryngol Head Neck Surg. 2020;163(1):145–150. https://doi.org/10.1177/0194599820929274
- 3408. Workman AD, Jafari A, Welling DB, et al. Airborne aerosol generation during endonasal procedures in the era of COVID‐19: risks and recommendations. Otolaryngol Head Neck Surg. 2020;163(3):465–470. https://doi.org/10.1177/0194599820931805
- 3409. Sharma D, Campiti VJ, Ye MJ, et al. Aerosol generation during routine rhinologic surgeries and in‐office procedures. Laryngoscope Investig Otolaryngol. 2021;6(1):49–57. https://doi.org/10.1002/lio2.520
- 3410. Murr AT, Lenze NR, Gelpi MW, et al. Quantification of aerosol concentrations during endonasal instrumentation in the clinic setting. Laryngoscope. 2021;131(5):E1415–E1421. https://doi.org/10.1002/lary.29122
- 3411. Tan VYJ, Zhang EZY, Daniel D, et al. Respiratory droplet generation and dispersal during nasoendoscopy and upper respiratory swab testing. Head Neck. 2020;42(10):2779–2781. https://doi.org/10.1002/hed.26347
- 3412. Di Maio P, Traverso D, Iocca O, De Virgilio A, Spriano G, Giudice M. Endoscopic nasopharyngoscopy and ENT specialist safety in the COVID 19 era: the back endoscopy approach to the patient. Eur Arch Otorhinolaryngol. 2020;277(9):2647–2648. https://doi.org/10.1007/s00405‐020‐06093‐6
- 3413. Olaguibel JM, Alobid I, Alvarez Puebla M, et al. Functional examination of the upper and lower airways in asthma and respiratory allergic diseases: considerations in the post‐SARS‐CoV‐2 era. J Investig Allergol Clin Immunol. 2021;31(1):17–35. https://doi.org/10.18176/jiaci.0625
- 3414. Suzaki I, Kobayashi H. Coronavirus disease 2019 and nasal conditions: a review of current evidence. In Vivo. 2021;35(3):1409–1417. https://doi.org/10.21873/invivo.12393
- 3415. Liu DT, Phillips KM, Speth MM, Besser G, Mueller CA, Sedaghat AR. Portable HEPA purifiers to eliminate airborne SARS‐CoV‐2: a systematic review. Otolaryngol Head Neck Surg. 2022;166(4):615–622. https://doi.org/10.1177/01945998211022636
- 3416. Christopherson DA, Yao WC, Lu M, Vijayakumar R, Sedaghat AR. High‐efficiency particulate air filters in the era of COVID‐19: function and efficacy. Otolaryngol Head Neck Surg. 2020;163(6):1153–1155. https://doi.org/10.1177/0194599820941838
- 3417. Bousquet J, Akdis CA, Jutel M, et al. Intranasal corticosteroids in allergic rhinitis in COVID‐19 infected patients: an ARIA‐EAACI statement. Allergy. 2020;75(10):2440–2444. https://doi.org/10.1111/all.14302
- 3418. Klimek L, Pfaar O, Hamelmann E, et al. COVID‐19 vaccination and allergen immunotherapy (AIT) – a position paper of the German Society for Applied Allergology (AeDA) and the German Society for Allergology and Clinical Immunology (DGAKI). Allergol Select. 2021;5:251–259. https://doi.org/10.5414/ALX02245E
- 3419. Nittner‐Marszalska M, Rosiek‐Biegus M, Kopec A, et al. Pfizer‐BioNTech COVID‐19 vaccine tolerance in allergic versus non‐allergic individuals. Vaccines (Basel). 2021;9(6):553. https://doi.org/10.3390/vaccines9060553
- 3420. Ding M, Dong X, Sun YL, et al. Recent advances and developments in COVID‐19 in the context of allergic diseases. Clin Transl Allergy. 2021;11(7):e12065. https://doi.org/10.1002/clt2.12065
- 3421. Gani F, Cottini M, Landi M, et al., Allergic rhinitis and COVID‐19: friends or foes? Eur Ann Allergy Clin Immunol. 2022;54(2):53–59. doi: https://doi.org/10.23822/EurAnnACI.1764‐1489.234
- 3422. Adir Y, Humbert M, Saliba W. COVID‐19 risk and outcomes in adult asthmatic patients treated with biologics or systemic corticosteroids: nationwide real‐world evidence. J Allergy Clin Immunol. 2021;148(2):361–367.e13. https://doi.org/10.1016/j.jaci.2021.06.006
- 3423. Strauss R, Jawhari N, Attaway AH, et al. Intranasal corticosteroids are associated with better outcomes in coronavirus disease 2019. J Allergy Clin Immunol Pract. 2021;9(11):3934–3940.e9. https://doi.org/10.1016/j.jaip.2021.08.007
- 3424. Bozek A, Winterstein J. Montelukast's ability to fight COVID‐19 infection. J Asthma. 2021;58(10):1348–1349. https://doi.org/10.1080/02770903.2020.1786112
- 3425. Larenas‐Linnemann DE, Ortega‐Martell JA, Blandon‐Vijil MV, et al. Coronavirus disease 2019, allergic diseases, and allergen immunotherapy: possible favorable mechanisms of interaction. Allergy Asthma Proc. 2021;42(3):187–197. https://doi.org/10.2500/aap.2021.42.210013
- 3426. Pfaar O, Agache I, Bonini M, et al. COVID‐19 pandemic and allergen immunotherapy‐an EAACI survey. Allergy. 2021;76(11):3504–3516. https://doi.org/10.1111/all.14793
- 3427. Koca Kalkan I, Ates H, Aksu K, et al. Real‐life adherence to subcutaneous immunotherapy: what has changed in the era of the COVID‐19 pandemic. World Allergy Organ J. 2021;14(7):100558. https://doi.org/10.1016/j.waojou.2021.100558
- 3428. Aytekin ES, Soyer O, Sekerel BE, Sahiner UM. Subcutaneous allergen immunotherapy in children: real life compliance and effect of COVID‐19 pandemic on compliance. Int Arch Allergy Immunol. 2021;182(7):631–636. https://doi.org/10.1159/000514587
- 3429. Yegit OO, Demir S, Unal D, et al. Adherence to subcutaneous immunotherapy with aeroallergens in real‐life practice during the COVID‐19 pandemic. Allergy. 2022;77(1):197–206. https://doi.org/10.1111/all.14876
- 3430. Shaker MS, Mosnaim G, Oppenheimer J, Stukus D, Abrams EM, Greenhawt M. Health and economic outcomes of home maintenance allergen immunotherapy in select patients with high health literacy during the COVID‐19 pandemic: a cost‐effectiveness analysis during exceptional times. J Allergy Clin Immunol Pract. 2020;8(7):2310–2321.e4. https://doi.org/10.1016/j.jaip.2020.05.007
- 3431. Busse WW, Morgan WJ, Gergen PJ, et al. Randomized trial of omalizumab (anti‐IgE) for asthma in inner‐city children. N Engl J Med. 2011;364(11):1005–1015. https://doi.org/10.1056/NEJMoa1009705
- 3432. Teach SJ, Gill MA, Togias A, et al. Preseasonal treatment with either omalizumab or an inhaled corticosteroid boost to prevent fall asthma exacerbations. J Allergy Clin Immunol. 2015;136(6):1476–1485. https://doi.org/10.1016/j.jaci.2015.09.008
- 3433. Esquivel A, Busse WW, Calatroni A, et al. Effects of omalizumab on rhinovirus infections, illnesses, and exacerbations of asthma. Am J Respir Crit Care Med. 2017;196(8):985–992. https://doi.org/10.1164/rccm.201701‐0120OC
- 3434. Hammad H, Lambrecht BN. The basic immunology of asthma. Cell. 2021;184(6):1469–1485. https://doi.org/10.1016/j.cell.2021.02.016
- 3435. Denlinger LC, Phillips BR, Ramratnam S, et al. Inflammatory and comorbid features of patients with severe asthma and frequent exacerbations. Am J Respir Crit Care Med. 2017;195(3):302–313. https://doi.org/10.1164/rccm.201602‐0419OC
- 3436. Schroeder JT, Bieneman AP, Xiao H, et al. TLR9‐ and FcepsilonRI‐mediated responses oppose one another in plasmacytoid dendritic cells by down‐regulating receptor expression. J Immunol. 2005;175(9):5724–5731. https://doi.org/10.4049/jimmunol.175.9.5724
- 3437. Liang C, Yang Z, Zou Q, Zhou M, Liu H, Fan J. Construction of an irreversible allergic rhinitis‐induced olfactory loss mouse model. Biochem Biophys Res Commun. 2019;513(3):635–641. https://doi.org/10.1016/j.bbrc.2019.03.110
- 3438. Gupta N, Harit A, Taneja HC, Kumar R, Tripathi AK. Olfaction and its correlates in allergic rhinitis: a case control study. Indian J Otolaryngol Head Neck Surg. 2019;71(suppl 3):1782–1786. https://doi.org/10.1007/s12070‐017‐1149‐7
- 3439. Bahri R, Custovic A, Korosec P, et al. Mast cell activation test in the diagnosis of allergic disease and anaphylaxis. J Allergy Clin Immunol. 2018;142(2):485–496, e16. https://doi.org/10.1016/j.jaci.2018.01.043
- 3440. Bahri R, Bulfone‐Paus S. Mast cell activation test (MAT). Methods Mol Biol. 2020;2163:227–238. https://doi.org/10.1007/978‐1‐0716‐0696‐4_19