This is the first large prospective study to investigate whether the use of heated tobacco products (HTPs) is associated with an increased risk of hypertension.
We found that both exclusive HTP use and dual use of HTPs and cigarettes are associated with an elevated risk of hypertension.
These findings suggest that HTPs may not be a safer alternative to conventional cigarettes regarding hypertension risk.
Introduction
Cigarette smoking is a major risk factor for various diseases, including cancer, heart disease and other chronic diseases. Globally, the prevalence of smoking has declined over the past decades. Since Philip Morris International (PMI) launched its IQOS line of heated tobacco products (HTPs) and electronic cigarettes in 2014, HTPs have been marketed in over 70 countries., PMI is now introducing more affordable versions to expand their reach, potentially targeting vulnerable populations like adolescents and those in low and middle-income countries., Japan has emerged as one of the largest markets for HTPs, where nearly half of the current tobacco product users report using HTPs exclusively or concurrently with other tobacco products. HTPs heat tobacco within a specific temperature range, generating aerosols containing harmful or potentially harmful compounds similar to those in cigarette smoke, albeit at lower concentrations. Epidemiological evidence regarding the health risks associated with using these products is scarce.
Hypertension is the leading cause of premature cardiovascular death, affecting over one billion people globally. Cigarette smoke contains toxicants such as nicotine and heavy metals, which have been shown to increase blood pressure in animal experiments. Although conventional cigarette smoking causes a temporary rise in blood pressure, evidence of the association between cigarette smoking and hypertension is conflicting; some cohort studies have reported a positive association, whereas others have not., Notably, HTP aerosols contain comparable levels of nicotine, and HTP use has been found to induce endothelial dysfunction, raising concerns about the potential adverse effects of HTPs on hypertension. Clinical trials have demonstrated that HTP use can lead to temporary and acute increases in blood pressure., However, the long-term association between HTP use and the risk of hypertension remains unknown. Additionally, the dual use of cigarettes and HTPs further complicates the potential relationship between tobacco use and hypertension.
To address these knowledge gaps, this cohort study aimed to investigate the association between cigarette smoking, HTP use, dual use of cigarettes and HTPs and the risk of hypertension among the Japanese working population, with annual data collection on smoking status during the follow-up period.
Methods
Study setting
The Japan Epidemiology Collaboration on Occupational Health (J-ECOH) Study is an ongoing study involving workers from more than ten companies across various industries, including electric machinery, steel, chemical, automobile, plastics manufacturing and healthcare. We invited companies headquartered in the Kanto and Tokai regions of Japan, via an occupational physician network (convenience sampling). Data were collected annually from each participating company through company-organized health checkups, which included anthropometric measurements, physical exams, laboratory tests and self-administered questionnaires on medical history and lifestyle. Further details have been reported elsewhere.
The present sub-study used data from a large J-ECOH Study participating company, an electrical machinery manufacturer, which has been collecting annual data on new tobacco products, including HTPs, since 2018. The company, with ∼50 000 workers in 2018, implemented measures against passive smoking in indoor workplaces by either maintaining a completely smoke-free environment or providing spatially separated smoking areas, depending on the location.
Analytic cohort
We created an analytic cohort of health checkup examinees during the 2018 fiscal year (baseline) and followed them until May 2022, based on annual checkups. Of 49 731 examinees ≥20 years, we excluded individuals with hypertension (receiving antihypertensive treatment or blood pressure ≥140/90 mmHg) (n = 9093), those with missing information on either baseline hypertension status (n = 451) or smoking status (n = 6892), and individuals with a history of cancer or cardiovascular disease (n = 629) at baseline. Of the remaining 32 666 participants, we further excluded those who did not attend any subsequent health checkups (n = 2480) or who attended but did not undergo blood pressure measurement (n = 34), resulting in a final sample size of 30 152 participants. Workers included in the final sample were younger and had lower rates of diabetes and dyslipidaemia, as well as lower blood pressure levels compared with those excluded. Only minor differences were observed in terms of body mass index (BMI), tobacco product use and other health behaviours (Supplementary Table S1, available as Supplementary data at IJE online).
Exposure
Tobacco product use was ascertained using a self-administered questionnaire as part of a health checkup, with smoking-related questions detailed in Supplementary Table S2, available as Supplementary data at IJE online. Examinees were queried about their current use of any tobacco products, including conventional cigarettes, e-cigarettes and HTPs. The response options were ‘Never’, ‘Quit’, and ‘Yes, current use’, with ‘current use’ including both daily and non-daily use. Those who reported current use of tobacco products were further asked about the types of tobacco products they used, with responses of ‘Conventional cigarettes only’, ‘E-cigarettes/HTPs only’, or ‘Both (cigarettes and e-cigarettes/HTPs)’ In Japan, e-cigarettes without nicotine are not regulated or classified as tobacco products by law. Nicotine-containing e-cigarettes are regulated as medicinal products and cannot be legally sold, though they can be imported for personal use. It has been reported that only about 1% of people in Japan use e-cigarettes,, and our previous study showed that over 95% of new tobacco product users reported using HTPs. Since the questionnaire did not distinguish between HTPs and e-cigarettes, we assumed them to be HTPs. Based on the participants’ responses, the individuals were categorized into five groups: never smokers, former smokers, exclusive cigarette smokers, exclusive HTP users and dual users of cigarettes and HTPs.
The questionnaire also included a question about the average number of cigarettes/HTPs used per day. For dual users, this quantity represents a combined total of both products. To investigate the association between the intensity of cigarette/HTP use and the risk of hypertension, exclusive cigarette smokers, exclusive HTP users and dual users were further categorized into two groups based on their daily consumption: 1–10 and ≥11 cigarettes/HTPs.
Outcome
The annual health checkup was conducted at the company’s healthcare centre, local clinics or onsite in meeting rooms, depending on the worksite location. Blood pressure was measured using an automatic blood pressure monitor. Prior to the measurement, participants were instructed to rest in a chair for ∼5 min. For individuals with an initial systolic blood pressure measurement ≥140 mmHg and/or diastolic blood pressure ≥90 mmHg, blood pressure was measured twice, 2–3 min apart and the lower measurement was used. The onset of hypertension was determined using annual health checkup data collected from baseline until the 2021 fiscal year. Hypertension was defined as meeting at least one of the following criteria: a systolic blood pressure level of ≥140 mmHg, a diastolic blood pressure level of ≥90 mmHg or receiving antihypertensive treatment.
Covariates
Informed by previous research and guided by a Directed Acyclic Graph (Supplementary Figure S1, available as Supplementary data at IJE online), the following covariates were considered: age, sex, job position (as an indicator of socioeconomic status), sleep duration, leisure-time physical activity, alcohol consumption, high-sodium food consumption, BMI, dyslipidaemia, diabetes, and baseline systolic blood pressure. During our analysis, we observed high correlations (r ≈ 0.60) among dietary factors, including high-sodium food, vegetable, fruit and dairy consumption (dietary-related questions detailed in Supplementary Table S2, available as Supplementary data at IJE online). To avoid multicollinearity, we exclusively included high-sodium food consumption in our model due to its causal effect on hypertension.
Body weight and height were measured using a scale with the participants wearing light clothes and no shoes. BMI was calculated as weight in kilograms divided by height squared in meters. Job position (high rank or others), sleep duration (<5, 5 to <6, 6 to <7, ≥7 h/day), alcohol consumption [non-drinkers, drinkers consuming <1, 1 to <2 or ≥2 go/day; go is a traditional Japanese unit of volume and one go (180 mL) of sake contains ∼23 g of ethanol] and leisure-time physical activity (≥150 min/week or <150 min/week) were ascertained via a self-administered questionnaire. Additionally, participants were asked about the frequency of high-sodium food consumption, ranging from ‘rarely’ to ‘≥3 times per day’. We transformed this into a continuous measure (times per week) by assigning numeric values to each category, from 0 for ‘rarely’ to 21 times per week for ‘3 times per day’.
Dyslipidaemia was defined as a triglyceride level ≥150 mg/dL, low-density lipoprotein cholesterol level ≥140 mg/dL, high-density lipoprotein cholesterol level <40 mg/dL or receiving medical treatment for dyslipidaemia. Diabetes was defined as a fasting blood glucose ≥126 mg/dL, a HbA1c level ≥6.5% or receiving medical treatment for diabetes.
Statistical analysis
The baseline characteristics of the study participants were described as means for continuous variables and percentages for categorical variables. Chi-squared tests for categorical variables and analysis of variance for continuous variables were used to examine differences in baseline characteristics among different tobacco use groups. Time to event or time to censoring was calculated as the time between the baseline health checkup and the earliest of the following dates: (i) the estimated onset date of hypertension, determined as the midpoint between the checkup when hypertension was first detected and the previous checkup, (ii) the date of the checkup when a participant first reported a change in smoking status or (iii) the date of the last health checkup attended. We censored participants whose smoking status changed from baseline, excluding subsequent exposures such as switching from a former smoker at baseline to a current smoker during follow-up, due to their short duration in the study.
The Cox proportional hazards regression was used to estimate hazard ratios (HRs) and 95% confidence intervals (CIs) for the association between HTP use and hypertension. We assessed the proportional hazards assumption using Schoenfeld residuals and found no violations. In the first model, age, sex and job position were adjusted. In the second model, we further adjusted for health behaviours such as sleep duration, leisure-time physical activity, alcohol consumption and high-sodium food consumption. In the third model, we expanded the adjustments to include BMI, dyslipidaemia, diabetes and baseline systolic blood pressure. We performed multiple imputations by fully conditional specification to address missing data on covariates at baseline, with missingness ranging from 0% to 18%, and smoking status during follow-up visits (Supplementary Figure S2, available as Supplementary data at IJE online). This involved generating 15 imputed datasets, analysing each dataset with the respective model and combining the results using Rubin’s rule to obtain a final point estimate and standard error.
In the sensitivity analysis, we used the last observation carried forward method to impute missing smoking data at follow-ups, assessing the robustness of the main results. All the statistical analyses were performed using SAS version 9.4 (SAS Institute, Cary, NC, USA).
Results
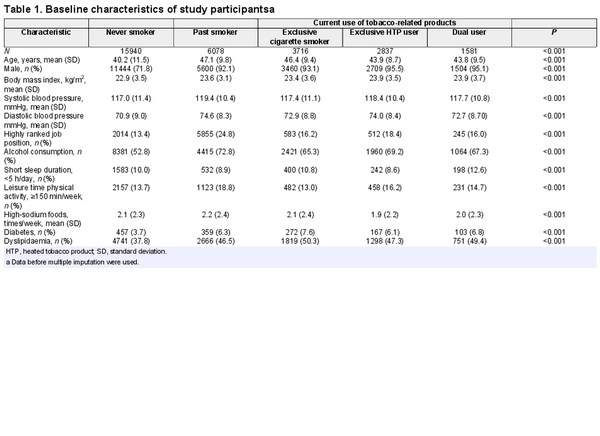
Of 30 152 participants included in the analysis, 82.0% were men and the mean (SD) age at baseline was 42.9 (11.0) years. The prevalence of exclusive cigarette smoking, exclusive HTP use and combined use was 12.3%, 9.4% and 5.2%, respectively. Table 1 presents the baseline characteristics of the study population according to tobacco product use. Notably, participants who exclusively used HTPs were younger than those who exclusively smoked cigarettes. Compared with never smokers, tobacco product users were older, more likely to be men, and had a higher prevalence of diabetes and dyslipidaemia. Mean blood pressure levels were similar across the groups.
As shown in Supplementary Figure S2, available as Supplementary data at IJE online, over 80% of participants attended all the subsequent health checkups between 2019 and 2021. The mean follow-up time for participants was 2.6 years (range: 0.1–4.0 years). A total of 3656 new-onset cases of hypertension were identified during 78 381 person-years of follow-up.
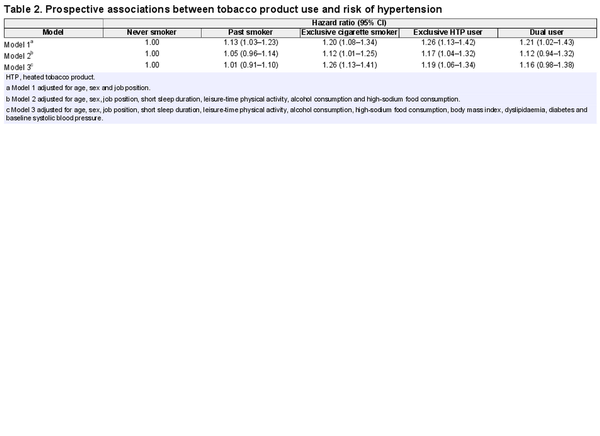
Table 2 shows adjusted HRs for hypertension according to tobacco product use. In the model adjusted for age, sex and job position (model 1), exclusive HTP users had a higher risk of hypertension (HR 1.26, 95% CI 1.13–1.42, P <0.001), followed by exclusive cigarette smokers (HR 1.20, 95% CI 1.08–1.34, P <0.001) and dual users (HR 1.21, 95% CI 1.02–1.43, P =0.03), compared with never smokers. This association persisted for exclusive HTP users (HR 1.17, 95% CI 1.04–1.32, P =0.008) after further adjusting for health behaviours (model 2), though somewhat attenuated. Even after adjusting for baseline BMI, diabetes, dyslipidaemia and systolic blood pressure (model 3), exclusive HTP users (HR 1.19, 95% CI 1.06–1.34, P =0.003) continued to have a higher risk of hypertension. An increased risk was also consistently observed among cigarette smokers in model 2 and model 3. Dual users (HR 1.16, 95% CI 0.98–1.38, P =0.09) had a marginally higher risk, likely due to the relatively small sample size.
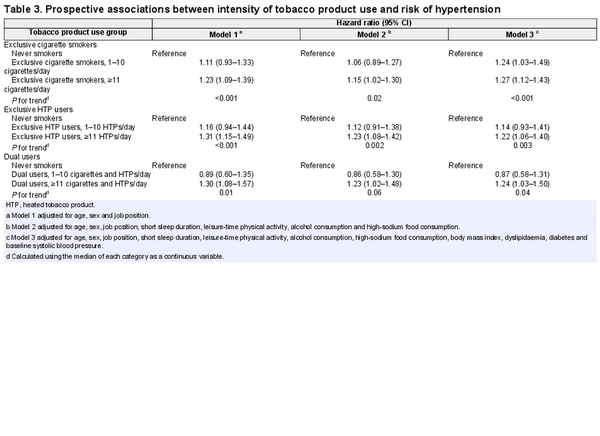
Table 3 shows that higher intensity of tobacco product use is associated with an increased risk of hypertension among exclusive HTP users, exclusive cigarette smokers and dual users. Among exclusive HTP users, the adjusted HRs (95% CIs) in model 3 were 1.14 (0.93–1.41) for people who used 1–10 HTPs per day and 1.22 (1.06–1.40) for those who used ≥11 HTPs per day (P for trend = 0.003), compared with never smokers. Among exclusive cigarette smokers, the adjusted HRs were 1.24 (1.03–1.49) for 1–10 cigarettes per day and 1.27 (1.12–1.43) for ≥11 cigarettes per day (P for trend < 0.001). For dual users, the adjusted HRs were 0.87 (0.58–1.31) for 1–10 cigarettes/HTPs per day and 1.24 (1.03–1.50) for ≥11 cigarettes/HTPs per day (P for trend = 0.04).
Sensitivity analyses found that the primary results did not change when using the last observation carried forward method to handle missing smoking data. The results are provided in Supplementary Tables S3 and S4, available as Supplementary data at IJE online.
Discussion
This large prospective cohort study found that the use of HTPs, whether alone or in combination with cigarettes, was associated with an increased risk of hypertension in a dose-response manner. To the best of our knowledge, this is the first study to provide evidence of an association between HTP use and the risk of hypertension.
Our finding of a higher risk of hypertension among exclusive cigarette smokers aligns with existing cohort studies. For example, a USA-based cohort study (n = 17 539; mean age = 39.0 years) reported an increased risk of self-reported hypertension with exclusive cigarette smoking (adjusted HR = 1.21, 95% CI 1.06–1.38). However, some studies have shown mixed results, with some reporting no significant association (n = 4549; age = 45–74 years) and others noting differing effects by sex.,, Despite these discrepancies, our study supports a positive association between cigarette smoking and hypertension risk, after adjusting for potential confounding factors.
Although a common belief among cigarette smokers is that HTPs are less harmful than conventional cigarettes, our study revealed a notable connection between exclusive HTP use and an elevated risk of hypertension, which is in line with the results of clinical trials investigating the acute effect of HTP use on blood pressure., More importantly, we found a comparable increased risk of hypertension among exclusive HTP users and exclusive cigarette smokers, indicating that HTPs may pose a health risk comparable to traditional cigarettes in relation to hypertension. The market for HTPs has grown significantly in recent years, and these products are now available in over 70 countries., Our study provides important and timely evidence that HTP use may increase the risk of hypertension.
In our study, dual users of HTPs and cigarettes exhibited an increased risk of hypertension, particularly among those who consumed 11 or more cigarettes and/or HTPs per day. Notably, the daily consumption of tobacco sticks among dual users (16.8 ± 8.5) was similar to that of exclusive cigarette smokers (15.5 ± 6.4). This suggests that while dual users may smoke fewer conventional cigarettes, their overall tobacco consumption does not decrease. This finding aligns partially with a cross-sectional study from Hong Kong, which reported lower cigarette consumption but higher total tobacco consumption among dual users. Thus, dual use does not reduce total tobacco intake and may contribute to the increased risk of hypertension observed in this group.
Our findings suggest that HTPs may not be a safer alternative to conventional cigarettes regarding hypertension risk, which accounts for approximately half of cardiovascular disease events worldwide. These findings align with the recent US FDA report, which concluded that PMI’s current data do not demonstrate that IQOS use will significantly decrease the risk of tobacco-induced diseases for individuals or reduce harm to the population. The current study provides evidence that policymakers should regulate HTPs as stringently as conventional cigarettes to mitigate the potential health effects of these tobacco products.
Several potential mechanisms exist through which the use of tobacco products contributes to hypertension. One such mechanism involves the toxicants found in tobacco products, such as nicotine and heavy metals, which have been linked to elevated blood pressure.,,,, Specifically, nicotine inhalation has been found to affect pulmonary and systemic blood pressure in mice by promoting the activation of mitogen-activated protein kinase pathways and the renin–angiotensin–aldosterone system. The use of tobacco products causes various physiological changes, including sympathetic activation, oxidative stress, endothelial dysfunction,, vascular injury, and increased arterial stiffness,,, all of which can contribute to hypertension.
Strengths and limitations
Our study has several strengths, including a well-powered comparison of never, past and current smokers with annual smoking status data collection for censoring when smoking behaviour changed and comprehensive covariate adjustment. However, this study has several limitations. First, smoking habits were self-reported and not biologically verified (e.g. exhaled carbon monoxide or salivary cotinine). The resulting misclassification of smoking status might have led to an inaccurate assessment of the association. Second, in blood pressure measurements, random errors introduce the possibility of non-differential misclassification of hypertension, often resulting in a bias toward the null hypothesis. Third, residual and unmeasured confounding factors, such as second-hand exposure to cigarettes and HTPs and prior use of tobacco products before the baseline survey, may have influenced our results. Fourth, this study did not differentiate between daily and non-daily users, acknowledging that these groups may have different levels of health risk associated with their usage patterns. In addition, the questionnaire did not distinguish between HTPs and e-cigarettes, so we assumed them to be HTPs. As a result, e-cigarette users could be misclassified as HTP users, potentially underestimating the association between HTP use and hypertension. Finally, as our study was conducted within a single large company, the generalizability of our findings to populations with different backgrounds or from other industries may be limited.
Conclusions
In this cohort study, cigarette smoking and HTP use were associated with an increased risk of hypertension. This suggests that HTPs should not be considered reduced-harm alternatives to traditional cigarettes for the prevention of hypertension.
Ethics approval
Before data collection, we informed workers through posters about the J-ECOH Study and the use of their health data, owned by the company, for research purposes after anonymization. Workers could opt-out by notifying their occupational physicians, in compliance with Japanese Ethical Guidelines for observational studies. The study protocol including consent procedure was approved by the Ethics Committee of the National Center for Global Health and Medicine, Japan (NCGM-G-001140).
References
- 1. Dai X, Gil GF, Reitsma MB et al Health effects associated with smoking: a Burden of Proof study. Nat Med2022;28:2045–55.
- 2. Dai X, Gakidou E, Lopez AD. Evolution of the global smoking epidemic over the past half century: strengthening the evidence base for policy action. Tob Control2022;31:129–37.
- 3. Sun T, Anandan A, Lim CCW et al Global prevalence of heated tobacco product use, 2015-22: a systematic review and meta-analysis. Addiction2023;118:1430–44.
- 4. Monzon J, Mus S, Davila G, Barnoya J, Kim M, Thrasher J. Lil Solid: a more affordable option for heated tobacco products in Guatemala. Tob Control2023;tc-2023-057954.
- 5. PMI Launches a More Affordable Heated Tobacco Product, Broadening Access to Better Alternatives to Cigarettes. https://www.pmi.com/our-transformation/pmi-launches-a-more-affordable-heated-tobacco-product-broadening-access-to-better-alternatives-to-cigarettes (14 May 2024, date last accessed).
- 6. Odani S, Tabuchi T. Prevalence and denial of current tobacco product use: combustible and heated tobacco products, Japan, 2022. Prev Med Rep2022;30:102031.
- 7. Upadhyay S, Rahman M, Johanson G, Palmberg L, Ganguly K. Heated tobacco products: insights into composition and toxicity. Toxics2023;11:667.
- 8. NCD Risk Factor Collaboration (NCD-RisC). Worldwide trends in hypertension prevalence and progress in treatment and control from 1990 to 2019: a pooled analysis of 1201 population-representative studies with 104 million participants. Lancet2021;398:957–80.
- 9. Oakes JM, Xu J, Morris TM et al Effects of chronic nicotine inhalation on systemic and pulmonary blood pressure and right ventricular remodeling in mice. Hypertension2020;75:1305–14.
- 10. Fioresi M, Simões MR, Furieri LB et al Chronic lead exposure increases blood pressure and myocardial contractility in rats. PLoS One2014;9:e96900.
- 11. Sanchez-Soria P, Broka D, Monks SL, Camenisch TD. Chronic low-level arsenite exposure through drinking water increases blood pressure and promotes concentric left ventricular hypertrophy in female mice. Toxicol Pathol2012;40:504–12.
- 12. Omvik P. How smoking affects blood pressure. Blood Press1996;5:71–77.
- 13. Bowman TS, Gaziano JM, Buring JE, Sesso HD. A prospective study of cigarette smoking and risk of incident hypertension in women. J Am Coll Cardiol2007;50:2085–92.
- 14. Halperin RO, Gaziano JM, Sesso HD. Smoking and the risk of incident hypertension in middle-aged and older men. Am J Hypertens2008;21:148–52.
- 15. Kaplan RC, Baldoni PL, Strizich GM et al Current smoking raises risk of incident hypertension: Hispanic Community Health Study-Study of Latinos. Am J Hypertens2021;34:190–97.
- 16. Cook S, Hirschtick JL, Barnes G et al Time-varying association between cigarette and ENDS use on incident hypertension among US adults: a prospective longitudinal study. BMJ Open2023;13:e062297.
- 17. Dochi M, Sakata K, Oishi M, Tanaka K, Kobayashi E, Suwazono Y. Smoking as an independent risk factor for hypertension: a 14-year longitudinal study in male Japanese workers. Tohoku J Exp Med2009;217:37–43.
- 18. Wang W, Lee ET, Fabsitz RR et al A longitudinal study of hypertension risk factors and their relation to cardiovascular disease: the Strong Heart Study. Hypertension2006;47:403–409.
- 19. Shi H, Leventhal AM, Wen Q, Ossip DJ, Li D. Sex differences in the association of E-cigarette and cigarette use and dual use with self-reported hypertension incidence in US adults. Nicotine Tob Res2023;25:478–85.
- 20. Klein J, Diaba-Nuhoho P, Giebe S, Brunssen C, Morawietz H. Regulation of endothelial function by cigarette smoke and next-generation tobacco and nicotine products. Pflugers Arch2023;475:835–44.
- 21. Ioakeimidis N, Emmanouil E, Terentes-Printzios D et al Acute effect of heat-not-burn versus standard cigarette smoking on arterial stiffness and wave reflections in young smokers. Eur J Prev Cardiol2021;28:e9–11.
- 22. Franzen KF, Belkin S, Goldmann T et al The impact of heated tobacco products on arterial stiffness. Vasc Med2020;25:572–74.
- 23. Hu H, Sasaki N, Ogasawara T et al; Japan Epidemiology Collaboration on Occupational Health Study Group. Smoking, smoking cessation, and the risk of hearing loss: Japan Epidemiology Collaboration on Occupational Health Study. Nicotine Tob Res2019;21:481–88.
- 24. Lau YK, Okawa S, Meza R, Katanoda K, Tabuchi T. Nicotine dependence of cigarette and heated tobacco users in Japan, 2019: a cross-sectional analysis of the JASTIS Study. Tob Control2022;31:e50–56.
- 25. Nakama C, Tabuchi T. Use of heated tobacco products by people with chronic diseases: the 2019 JASTIS study. PLoS One2021;16:e0260154.
- 26. Hu H, Nakagawa T, Honda T et al Heated tobacco products and circulating high-density lipoprotein cholesterol concentrations. Sci Rep2022;12:17385.
- 27. Umemura S, Arima H, Arima S et al The Japanese Society of Hypertension Guidelines for the Management of Hypertension (JSH 2019). Hypertens Res2019;42:1235–481.
- 28. Leng B, Jin Y, Li G, Chen L, Jin N. Socioeconomic status and hypertension: a meta-analysis. J Hypertens2015;33:221–29.
- 29. Wang Y, Mei H, Jiang YR et al Relationship between duration of sleep and hypertension in adults: a meta-analysis. J Clin Sleep Med2015;11:1047–56.
- 30. Huai P, Xun H, Reilly KH, Wang Y, Ma W, Xi B. Physical activity and risk of hypertension: a meta-analysis of prospective cohort studies. Hypertension2013;62:1021–26.
- 31. Roerecke M, Tobe SW, Kaczorowski J et al Sex-specific associations between alcohol consumption and incidence of hypertension: a systematic review and meta-analysis of cohort studies. J Am Heart Assoc2018;7:e008202.
- 32. Schwingshackl L, Schwedhelm C, Hoffmann G et al Food groups and risk of hypertension: a systematic review and dose-response meta-analysis of prospective studies. Adv Nutr2017;8:793–803.
- 33. Landsberg L, Aronne LJ, Beilin LJ et al Obesity-related hypertension: pathogenesis, cardiovascular risk, and treatment: a position paper of The Obesity Society and the American Society of Hypertension. J Clin Hypertens (Greenwich)2013;15:14–33.
- 34. Otsuka T, Takada H, Nishiyama Y et al Dyslipidemia and the risk of developing hypertension in a working-age male population. J Am Heart Assoc2016;5:e003053.
- 35. Sun D, Zhou T, Heianza Y et al Type 2 diabetes and hypertension. Circ Res2019;124:930–37.
- 36. Radi S, Lang T, Lauwers-Cancès V et al; IHPAF Group. One-year hypertension incidence and its predictors in a working population: the IHPAF study. J Hum Hypertens2004;18:487–94.
- 37. Sutanto E, Miller C, Smith DM et al Concurrent daily and non-daily use of heated tobacco products with combustible cigarettes: findings from the 2018 ITC Japan Survey. Int J Environ Res Public Health2020;17:2098.
- 38. Zhang X, Sun Y, Cheung YTD et al Cigarettes, heated tobacco products and dual use: exhaled carbon monoxide, saliva cotinine and total tobacco consumed by Hong Kong tobacco users. Tob Control2023;33:457–63.
- 39. Fuchs FD, Whelton PK. High blood pressure and cardiovascular disease. Hypertension2020;75:285–92.
- 40. U.S. Food and Drug Administration. Scientific Review of Modified Risk Tobacco Product Application (MRTPA) Under Section 911(d) of the FD&C Act-Technical Project Lead. Maryland: U.S. Food & Drug Administration. https://www.fda.gov/media/139796/download (12 June 2024, date last accessed).
- 41. Uguna CN, Snape CE. Should IQOS emissions be considered as smoke and harmful to health? A review of the chemical evidence. ACS Omega2022;7:22111–24.
- 42. Dimitriadis K, Narkiewicz K, Leontsinis I et al Acute effects of electronic and tobacco cigarette smoking on sympathetic nerve activity and blood pressure in humans. Int J Environ Res Public Health2022;19:3237.
- 43. Isik B, Ceylan A, Isik R. Oxidative stress in smokers and non-smokers. Inhal Toxicol2007;19:767–69.
- 44. Messner B, Bernhard D. Smoking and cardiovascular disease: mechanisms of endothelial dysfunction and early atherogenesis. Arterioscler Thromb Vasc Biol2014;34:509–15.
- 45. Mahmud A, Feely J. Effect of smoking on arterial stiffness and pulse pressure amplification. Hypertension2003;41:183–87.