Introduction
Atlantic salmon Salmo salar is a highly valued anadromous fish species that is exploited in commercial, subsistence and recreational fisheries (). After hatching in early summer, the juveniles typically spend 1–5 years in the rivers before migrating (the smolt stage) to sea, where the bulk of the growth occurs. After 1–4 years at sea, adults return to natal rivers to spawn in the autumn. Atlantic salmon are structured into more than 2000 genetically distinct populations distributed in watercourses flowing towards the North Atlantic Ocean (). The total abundance of Atlantic salmon has declined during the last three decades, both in terms of number of populations and reduced productivity in freshwater and the marine environment (; ; ; ). A number of anthropogenic factors may have contributed to this decline (), including hydropower regulation, migration barriers, habitat alterations or destruction, diseases, pollution, overexploitation, introduced parasites, climate change, genetic introgression through escaped farmed salmon spawning in the rivers and increased parasite loads linked to Atlantic salmon farming.
We could find no comprehensive and updated overview of impact factors and their relative importance on an international level, or in any of the major countries that support Atlantic salmon populations. provided information regarding declines and extirpation of Atlantic salmon on a global scale, but emphasized the need for more comprehensive studies, and evaluated causes of decline in North American populations. The lack of updated information on the relative importance of impact factors may hamper both international and national efforts to protect this species.
Norway currently has more than 400 watercourses with Atlantic salmon (Figure 1), and holds ∼25% of the world’s healthy populations (). Consequently, Norwegian authorities have taken a particular responsibility to protect the species and its populations (). Protection of Atlantic salmon populations requires managing various anthropogenic threats including sustainable exploitation (). In 2009, the Norwegian Environment Agency appointed the Norwegian Scientific Advisory Committee for Atlantic Salmon Management (hereafter termed Atlantic Salmon Committee), consisting of 12 scientists collectively covering all major areas relevant for providing scientific advice on wild Atlantic salmon management covered in this article. One of the responsibilities of the Atlantic Salmon Committee is to identify and rank the threat levels of the various anthropogenic impacts on Atlantic salmon populations, as a foundation for prioritizing mitigation measures. Here, we present a classification system developed by the Atlantic Salmon Committee, which ranks the different anthropogenic factors affecting the Norwegian Atlantic salmon populations. We use the system to identify the major threats to the populations, and to determine if the threats are stabilized or developing. We aimed to develop a system that can also be used as a template for ranking anthropogenic impact factors in other countries in support of international conservation efforts of Atlantic salmon.
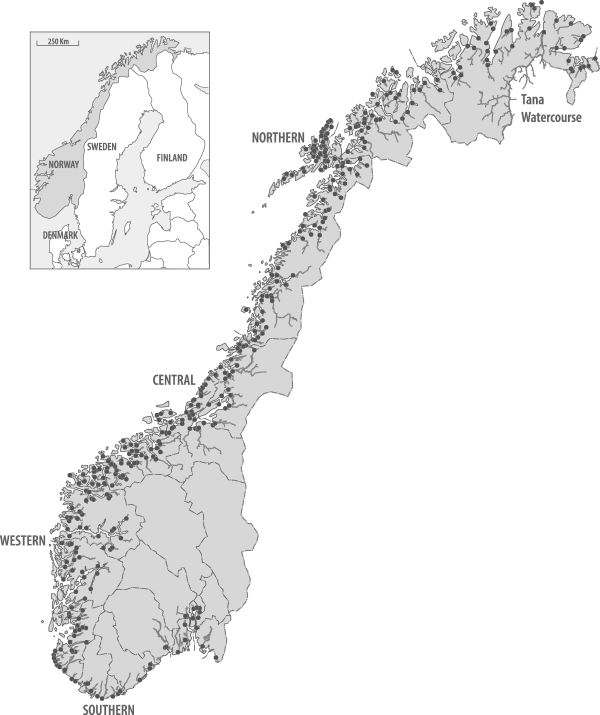
Figure 1
Map of Norway with location of the 400 Atlantic salmon watercourses, indicated by the location of the river outlets (dots) and the river area available to salmon. In addition there are 31 other watercourses with Atlantic salmon, but the available nursery area in these are likely too small to support viable populations in isolation. The major regions of Norway and the large Tana watercourse are also indicated.
The classification system
A semi-quantitative 2D classification system was developed to rank different anthropogenic impacts to Norwegian Atlantic salmon populations. The first dimension, the effect axis, describes the effect of each impact factor on the populations, and ranges from factors that cause loss in adult returns, to factors that threaten population viability and genetic integrity. The second dimension, the development axis, describes the likelihood for further reductions in population size or loss of additional populations in the future. Combined, these axes form a 2D continuous classification system in which the impact factors can be categorized into four major groups (Figure 2a):
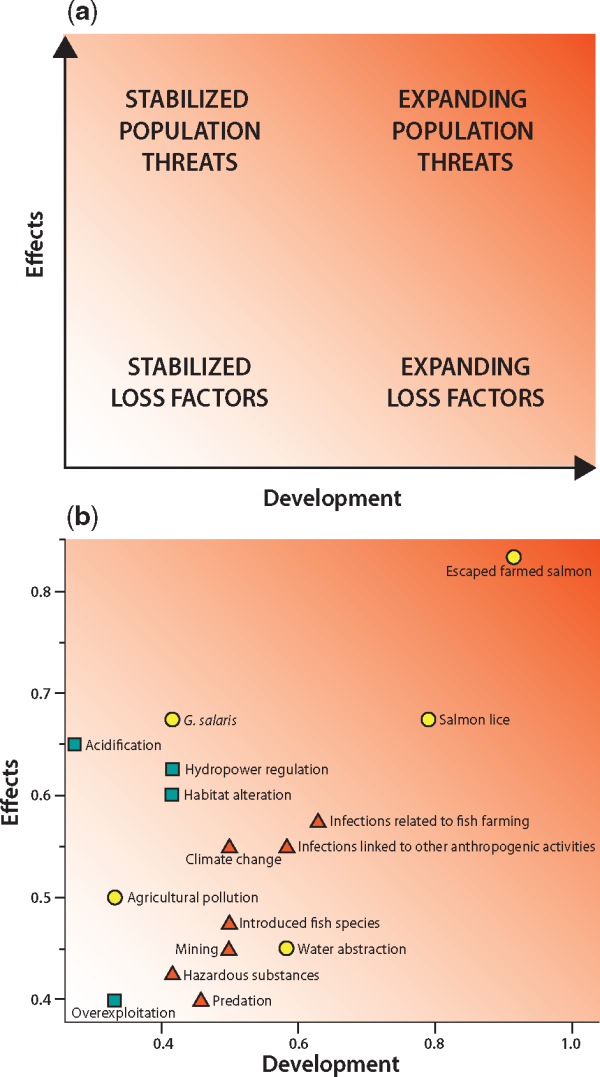
Figure 2
(a) The classification system developed to rank different anthropogenic impacts to Norwegian Atlantic salmon populations along the effect and development axes. The four major impact categories are indicated, but the system is continuous. Background coloring indicate severity of impacts, with dark as the most severe. (b) Location within the classification system of the 16 impact factors considered in 2015. For illustration, the knowledge on each impact factor and the uncertainty of future development is indicated by the color of the markers. Green squares = Extensive knowledge and small uncertainty (sum of scores 2–3), yellow circles = moderate knowledge and moderate uncertainty (sum of scores 4), and red triangles = poor knowledge and high uncertainty (sum of scores 5–6).
Expanding population threats—factors affecting populations to the extent that populations may be critically endangered or lost in nature and that have a high likelihood of causing even further reductions. Current mitigation measures are unable to hinder expansion of negative impacts in the future.
Stabilized population threats—factors that have contributed to populations becoming critically endangered or lost in nature, but that have a low likelihood of causing further reductions than they do already today. Mitigation measures taken are able to hinder expansion of negative impacts in the future.
Expanding loss factors—factors that cause loss in number of returning adults, and that have a high likelihood of causing further loss, but not to the extent that populations become threatened. Mitigation measures taken are unable to hinder expansion of negative impacts in the future.
Stabilized loss factors—factors that cause loss in number of returning adults, but not to the extent that populations become threatened, and that have a low likelihood of causing further loss. Mitigation measures taken are able to hinder expansion of negative impacts in the future.
Mechanisms and definition of terms
We define anthropogenic losses as reduction in the number of returning spawners due to the impact factors in freshwater or at sea caused by human activities. Anthropogenic factors can reduce Atlantic salmon populations through several mechanisms acting on different life stages. In freshwater, the carrying capacity may be reduced by decreased area or quality of the rearing habitat, ultimately causing reduced smolt production (). Several factors may increase juvenile mortality, and the effects of juvenile mortality on smolt production depend on when the mortality occurs relative to the strength and timing of density dependent population regulation (; ). In the absence of marine fisheries, mortality of post-smolts at sea will proportionally reduce the number of returning adults to natal rivers (Atlantic salmon show precise homing to their natal rivers, ; ), because there appears to be no density regulation at sea ().
We also consider the genetic integrity of populations in the classification system. Due to the extent of genetic structuring of Atlantic salmon, and evidence for genetically based adaptations among populations, conservation and management at the population level are recommended to protect diversity (; ). The genetic integrity of a population may be threatened in several ways (e.g. interspecific hybridization, outbreeding due to immigration, inbreeding, and genetic drift due to reduced population size). The major impact on Norwegian Atlantic salmon populations is introgression () from escaped farmed Atlantic salmon (, ; ).
We define a population as critically endangered or lost in nature when the number of returning adults is on average reduced by >75% over one generation (set to 5 years), or when significant introgression of farmed Atlantic salmon is documented (, ; ) and >10% (; ). We use the term “lost in nature”, because several Norwegian populations are conserved in the National Gene Banks (i.e. live fish kept in designated hatcheries and cryopreserved sperm; ).
Anthropogenic factors considered
Fifteen anthropogenic impact factors were considered in the ranking (see below and Table 1). Reduced growth and survival at sea seem to contribute to declines in Atlantic salmon over large parts of the distribution range (). Feeding conditions at sea was not included in the ranking, because we lack evidence to support that this should be regarded as an anthropogenic impact factor in Atlantic salmon (; ; ). However, correlations between Atlantic salmon productivity and ocean climate indexes are documented (e.g. ; , ; ; ; ), and this was considered in the ranking of climate change.
Characteristics considered for each impact factor and scoring
Several characteristics were used to describe each impact factor along the two axes (Table 1). For each factor, the characteristics were scored from one to four based on quantitative or qualitative criteria (Table 1), and thereafter scores were added up and expressed as a proportion of the potential maximum score. In a few cases, half points were given.
For the effect axis, five characteristics were considered for each impact factor. The number of affected populations was used as a measure of the extent of each factor. The geographical distribution was also considered, because impact factors operating on a regional rather than local level may promote loss of regional ecotypes or genotypes. The criteria ranged from effects on few dispersed populations to effects on a national level (affecting populations in at least 14 of the 16 counties with Atlantic salmon populations in Norway; Figure 1). The typical effect on affected populations was assessed in terms of reduction in number of adult returns, based on knowledge from published scientific papers and technical reports. The criteria ranged from small reductions (<10%) in adult returns to very large reductions (>75%). It can be difficult to characterize a “typical” effect, but classification into one of four relatively wide groups should be robust. The number of critically endangered or lost populations in nature was quantified based on the Norwegian Environment Agency database (), where lost populations (no longer self-reproducing) are listed. Critically endangered populations in terms of genetic integrity was added to these based on published studies (; , ; ). The criteria ranged from no affected populations to more than 20 affected populations. Finally, implemented mitigation measures that have reduced loss, or the likelihood of further populations becoming critically endangered or lost, were considered. Some of the impact factors have been active for many years and several mitigation measures have been implemented, such as the eradication programme against the introduced parasite Gyrodactylus salaris and large-scale liming in acidified watercourses (see below). Here, we assessed the documented effects of the measures, based on published papers, Norwegian reports and databases. The classification ranged from extensive measures with good effects to few or no measures or measures with no net effect in terms of reducing the impact. The extent of knowledge of each of the impact factors was classified as extensive, moderate or poor, based on assessment of published scientific papers and technical reports.
For the development axis, three characteristics were considered for each impact factor. The potential for effective measures to be implemented was based on a projection of the present situation and mitigation measures, primarily based on expert judgement of public information such as action plans, governmental White Papers and regulations and guidelines from the relevant management bodies, and general knowledge of primary experts within the Atlantic salmon committee. Classification ranged from few or no effective measures being planned to preparation of extensive and effective mitigation measures. Next, the likelihood of further losses in the number of returning adults and the likelihood of additional populations becoming critically endangered or lost were ranked from low to very high, based on expert judgement of public documents from the government departments responsible for each impact factor. The uncertainty of the projected development of each of the impact factors was classified as small, moderate, or high (scores 1–3).
Procedure for factor scoring
One or more primary experts from the Atlantic Salmon Committee presented initial scoring for their assigned impact factor at a committee meeting. The presentation included relevant scientific articles, Norwegian reports and other public documents. Scoring was extensively discussed within the committee, before arriving at the final scoring. The assessment of the primary experts was emphasized in the discussions. Each member could present alternative assessments and scores in the annual report if they did not agree in the scoring made by the committee majority, but this never happened because consensus was always reached.
Evaluation of the different impact factors
Hydropower regulation
Hydropower regulation includes hydropower facilities such as dams and power stations, but also altered water discharges and temperatures during the year due to water detraction or storage of water in reservoirs. The effects on Atlantic salmon vary substantially among rivers (; ). This impact factor ranked high along the effect axis because it affects nearly 20% of the populations, and 19 populations may have been lost due to hydropower development (). The effect in terms of reduced adult returns was classified as being between moderate and strong, based on published studies from Norway and elsewhere (; ; , ; ; ; ; ; ). Hydropower developments in Norway started in 1882, peaked during 1945–1961, and many of the extensive regulations were completed by 1980 (). Since then, few hydropower stations have been built in Atlantic salmon rivers. In two White Papers, the government designated 29 fjords distributed along the entire coastline as “national Atlantic salmon fjords” and 52 rivers draining into these fjords as “national Atlantic salmon rivers”, to protect the wild Atlantic salmon (, 2006–2007). These rivers, representing 72% of the conservation limits (CLs) for Atlantic salmon in Norway, were given protection against further hydropower development, water abstractions and flood control measures. There has been a general decrease in the proportion of hydropower development schemes that have been granted concessions or permissions after 2003, and this was particularly evident in the national Atlantic salmon rivers ().
Implementation of the European Water Framework Directive (WFD, ) is also expected to cause improvements in the management of regulated rivers. Moreover, in a joint report from the Norwegian Environment Agency and the Norwegian Water Resources and Energy Directorate, 35% of the regulated watercourses with anadromous salmonids were given top priority in a national plan for revision of hydropower concessions (). Such revisions will likely result in improved conditions for Atlantic salmon due to inclusion of minimum flow requirements and habitat restoration (). The regulatory framework (national Atlantic salmon rivers, WFD and revisions) and implemented mitigation measures resulted in low scores along the development axis. Increased hydropeaking caused by rapid increase or decrease in water discharge in accordance with fluctuations in the power demand (), cumulative effects of small hydropower projects in tributaries () and the increasing demand for renewable energy to reduce carbon emissions (e.g. the EU Renewable Energy Directive, ) add uncertainty to future development. Moreover, a recent White Paper signals that the Norwegian Government in some cases may allow for new hydropower developments in protected rivers, particularly in relation to flood protection ().
Water abstraction
Water abstraction in salmon rivers includes the use of water for fish hatcheries, industry and irrigation. Abstraction for irrigation purposes is limited due to a climate with high annual precipitation, and industrial water abstraction from rivers is not common in Norway. As a result, few rivers are severely impacted, and impacted rivers are often small and dominated by brown trout (Salmo trutta L.). A few populations have been lost, at least partly due to water abstraction for hatcheries, but the typical effects were regarded moderate. The low number of affected populations caused water abstraction to score low along the effect axis. Increased awareness through recent impact studies () and implementation of the WFD reduce future risk of populations being critically reduced by water abstraction. Therefore, water abstraction scored medium along the development axis. However, increased smolt production for aquaculture may increase the demand for further water abstraction () and adds uncertainty to future development.
Habitat alterations
Man-made structures and activities such as channelization, dredging, and building of levees and impoundments such as weirs lead to changes in river morphology and hydrology, which often lead to reduced habitat quality for fish, or even lost habitat (). Such habitat alterations are the results of flood protection or land reclamation and are common in Norwegian rivers (>200 rivers affected). However, extensive alterations are rare in Norway and typical effects were described as small. In summary, physical habitat alterations scored relatively high along the effect axis. However, on the development axis, the score was low, reflecting the river habitat protection offered by the Watercourse and Groundwater Act (2000) and implementation of the WFD, as well as several recent measures and plans to improve salmon habitats (; ). In western Norway, several recent major flooding events have resulted in new flood protection plans, including channelization, dredging and levees. These measures may challenge the protection of Atlantic salmon habitat.
Acidification
Deposition of long-range transported air pollutants (sulphur and nitric acids; acid rain) in areas of bedrock with low neutralizing capacity results in lowered pH and release of potentially toxic aluminium (Al) to surface waters (). This combined effect (acidification) has affected salmon populations in large areas in southern Norway (; ). Increased fish mortality may be related to both high concentrations of H+ ions (reduced pH) and inorganic monomeric Al (; ). Interaction of Al with the ion exchange and oxygen transport systems across the gills of juveniles and smolts accounts for the major part of the mortality during these life stages. Sub-lethal effects in freshwater may be lethal when the fish enter seawater, causing increased post-smolt mortality (; ). Large reductions in salmon catches due to acidification were already recorded around 1900 (). Fish mortality and population losses increased with the increase in sulphur emissions during 1960–1980. By 1990, Atlantic salmon populations were lost in 25 rivers and greatly reduced in at least eight more rivers (). Consequently, acidification scored high along the effect axis (typical effects described as very large and many populations have been lost), but the regional distribution and successful measures (see below) prevented top scores. From about 1990, significant reductions in acid deposition has resulted in increased surface water pH and reduced concentrations of Al in southern Norway (). This chemical recovery has not been sufficient to create acceptable water quality for Atlantic salmon.
A national liming programme was started in 1983 (), and 22 salmon rivers are limed by continuous addition of limestone powder. New salmon populations have established in affected rivers by re-stocking and straying of salmon from other rivers (). The national monitoring programme has documented only minor reductions in acid deposition since 2010, and further reductions will probably be negligible. Consequently, no significant change in this threat is expected in coming years. Moreover, as long as the liming programme continues (), no further losses of salmon populations are expected. Recently, increased funding has allowed optimization of the liming programme in some rivers and expansion of the programme by one or two rivers from 2016 to 2017. The likelihood of further losses was thus classified as low.
Gyrodactylus salaris
The ectoparasite G. salaris was accidently introduced to Norway during the 1970s, with devastating effects on infected Atlantic salmon populations (). Juvenile densities were typically reduced by 90% or more, and adult returns plummeted in affected watersheds (). In total, 50 Norwegian Atlantic salmon populations distributed in eight fjord systems have been infected. The parasite has limited survival in saline water (), but within each fjord system, G. salaris can disperse to new populations by infested fish moving within brackish water (). However, dispersal to new fjord systems across steep salinity gradients requires other transmission pathways (e.g. transport of fish or water). No other impact factor has caused more populations to become critically endangered or lost in Norway, and consequently it scored high along the effect axis. Various eradication programmes have been in operation, mainly using the toxin rotenone to kill all the fish in the impacted watersheds, because the parasite do not survive without its long-term hosts. Moreover, artificial barriers to reduce the area for chemical treatment and recently Al sulphate treatment (; ) have been used. Eradication efforts increased substantially after revisions of the National Action Plan against G. salaris in 2008 and formulation of the current plan in 2014 (). Consequently, by the end of 2015, G. salaris was present in 7 populations, 22 populations were declared free from the parasite, and 21 populations were in the process of being declared free of the parasite (requiring a minimum of five years without parasite observations). The action plan contains specific plans for removing the parasite from four of the remaining populations, whereas an expert group appointed by the Norwegian Environment Agency is currently exploring treatment options for the last three populations. The Atlantic salmon populations have been re-established from the National Gene Bank for Atlantic salmon () after eradication of the parasite. Thus, measures taken to reduce the effect of the parasite have been extensive and largely successful. Although the parasite has recently re-emerged in one population and been found in some small river systems within infected fjord systems, no dispersal to new fjord areas have been documented since 1996. The probability of further losses is thus moderate, the action plan outlines extensive future measures to reduce this threat, and consequently it scored low along the development axis.
Salmon lice
The salmon louse (Lepeophtheirus salmonis) is an ectoparasite of salmonids in the sea. Historically, salmon lice were observed in moderate numbers on wild salmonids, but because also farmed Atlantic salmon act as hosts, open net cage farming has increased the production of salmon lice in many coastal areas (; ). Since the late 1980s, salmon lice epizootics have been reported in wild salmonids in Norway, Scotland, Ireland, and Canada (, ; ; ; ). In farm-intensive areas, lice levels on wild salmonids are typically higher, and more variable than in farm-free areas (e.g. , ; ; ). The individual physiological and pathological effects of salmon lice on salmonids have been thoroughly described (), and population level effects have been well documented for Atlantic salmon. Studies in Norway, Scotland, and Ireland, based on comparison between chemically protected post-smolt and untreated controls, have shown average additional marine mortalities by salmon lice of 0.6–39% across locations and years (; ; ; ; ). Salmon lice may also increase age at maturity (). Hence, the effects in terms of reductions in the number of returning adults may be substantial, and typical effects in affected populations were classified as large (>25%). Salmon lice may reduce marine survival of wild Atlantic salmon in farmed areas along the Norwegian coast, especially in the southwestern and middle parts, but also in parts of northern Norway (; ). Thus, the number of affected populations is high, and the geographical distribution was classified as regional to national. Large effects on many populations gave high scores along the effect axis. The efforts to reduce salmon lice infestation pressures on Atlantic salmon are substantial (), and during the last 30 years, infestation pressures have varied with the size of the aquaculture industry and the implemented measures. However, the large growth of the industry has repeatedly nullified the effect of the measures (). Risk assessments of the environmental impacts of salmon farming (, ) have shown a general increase in infection pressure of salmon lice and its geographical distribution from 2010 to 2015. Moreover, resistance among salmon lice to the major drugs used to control lice levels in the farms is a major problem (). Warming of coastal waters () may extend the problem to the northernmost part of Norway. Consequently, the probability of further losses in number of returning Atlantic salmon was classified as high. High infection pressures over years may also reduce the number of returning adults to the extent that populations become critically endangered, and this probability was classified as moderate to high. A White Paper from the Ministry of Trade, Industry and Fisheries outlines further growth of the farming industry but also new instruments and management principles to ensure sustainable growth of salmon farming ().
Infections related to fish farming
Wild fish are the original source of pathogens causing diseases in farmed fish, but farming provides conditions for proliferation and spread among farmed fish and back to wild fish (). Moreover, farming practices have introduced new pathogens to naïve populations (). The use of fish from Scotland and Denmark in aquaculture operations in Norway provided a vector for the transmission of the bacterium Aeromonas salmonicida subsp. salmonicida, causing furunculosis in wild Atlantic salmon populations (). In 2013, outbreaks of one or more viral diseases were registered in 38% of the c. 600 active farming localities, and a large number of farms had outbreaks of bacterial and parasitic diseases (). Hence, the potential infection pressure from farmed to wild Atlantic salmon is large. The knowledge on pathogen transfer from farmed to wild Atlantic salmon is poor (except regarding salmon louse), partly due to the challenges of documenting the transfer and disease outbreaks in wild fish (). However, using Piscine orthoreovirus (PRV) as a model, presented a strong case for virus transmission from farmed to wild salmonids in Norway. Moreover, escaped Atlantic salmon infected with both salmon alphavirus and PRV have been found to ascend a river close to the likely source farms (). There is no studies documenting large effects in wild Atlantic salmon populations by infections originating from farmed salmon, and the typical effect was classified as small. However, this is an area where knowledge is largely lacking. Given the number of infective organisms (i.e. virus, bacteria, fungi, and parasites), and their effects in farmed fish, there is potential for strong effects in wild populations. Even though the typical effect was classified as small, increased knowledge through future studies may change this classification. The number of populations potentially affected is high, and the measures are likely unable to reduce the likelihood of further losses.
Infections linked to other anthropogenic activities than fish farming
For several infective organisms, disease outbreaks in Atlantic salmon can be related to changes in environmental conditions caused by other anthropogenic activities than fish farming. An outbreak of proliferative kidney disease (PKD) was linked to reduced flow and increased temperatures due to hydropower regulation, and the juvenile mortality was substantial (). The parasite Tetracapsuloides bryosalmonae causing PKD has been found in several other Norwegian rivers (). Furunculosis outbreaks may also occur under similar environmental conditions, with massive deaths of adult fish (). Due to the lack of a comprehensive national monitoring programme, the knowledge on this impact factor is poor and assessment was mainly based on expert judgement. The impact was ranked as moderate along both axes. Wide geographical distribution, the lack of effective measures, predicted increases in summer temperatures, and reductions in summer flow due to climate change () are important for the classification.
Escaped farmed Atlantic salmon
River scale experiments in Imsa and the Guddalselva in Norway and Burrishoole in Ireland have documented how escaped farmed Atlantic salmon, their offspring and hybrid offspring from mating with wild Atlantic salmon can affect wild populations negatively (; ; ). Introgression of farmed Atlantic salmon into wild populations may result in lower adult returns due to reduced smolt production () and reduced sea survival (). The effects from introgression of farmed Atlantic salmon on a given population may vary, because the effects depends on extent of local adaptation to environmental factors, which may differ among populations (). Loss of genetic integrity of wild populations due to introgression from farmed Atlantic salmon is a fundamental threat. Farmed Atlantic salmon have been through strong domestication selection and differ from wild Atlantic salmon in a number of genetically based traits (e.g. growth rate: ; , ; , , stress tolerance: ; behaviour: ), potentially leading to maladaptive trait values for life in nature (). Moreover, reduced genetic variability is documented in farmed Atlantic salmon strains, both due to the selection in the breeding programmes and the number of parents used (, , but see ). Repeated introgression of farmed Atlantic salmon into wild populations may over time cause the local Atlantic salmon populations to be replaced by less adapted and less genetically variable hybrid populations. showed genetic changes in microsatellite DNA in 6 of 21 studied populations (28%) that could be linked to introgression of farmed Atlantic salmon. In further studies using single nucleotide polymorphism (SNP) markers in 20 of the same populations, documented significant changes in five populations, and found that their present genetic profile was closer to a mixed farmed sample than historical samples from the population. The proportion of genes from farmed Atlantic salmon in the wild populations varied from 2 to 47%. recently documented that 51 of 109 Norwegian Atlantic salmon populations showed significant genetic introgression from farmed salmon (using diagnostic SNP marker developed by ). The mean introgression level in all rivers was 6.4%, and 27 populations (25%) had introgression levels above 10% (maximum 42%). Thus, we classified the number of critically endangered or lost populations due to this impact factor at the maximum level (>20 populations). found that introgression had occurred in all regions of Norway, but was highest in the regions with most farming. They also found a relationship between introgression levels and average proportion of escaped farmed salmon found in the rivers during monitoring (), although parts of the variation remain unexplained. The genetic studies and monitoring of farmed escapees in the rivers clearly show that escaped farmed Atlantic salmon is a national threat affecting a large proportion of the populations. The typical effect of introgression by farmed salmon in terms of reduced adult returns was classified as moderate.
The efforts to reduce the number of farmed Atlantic salmon escapes have been considerable, and the official number of reported escapees decreased from a peak level at more than 900 000 individuals in 2006 to generally below 300 000 thereafter (). Similarly, the incidence of farmed Atlantic salmon in samples from wild spawning populations has decreased from an average of 20–35% across monitored populations before 1998, to a level between 9 and 18% after 2003. However, since both the ecological effects (competition from farmed and hybrid offspring) and introgression are cumulative across generations (; , ), the measures taken are regarded as unable to reduce the likelihood of further losses.
Introduced fish species
Norway has 32 native self-sustaining freshwater fish species and at least 11 non-native self-sustaining species (; ). There is limited information on how introduced non-native and translocated native species affect Atlantic salmon, but they may potentially affect juvenile survival through competition for space and food. Some of the species may have significant impacts if they establish large populations.
Oncorhynchus-species use similar freshwater habitats as Atlantic salmon (). It is uncertain if rainbow trout (O. mykiss) has established viable populations in Norwegian rivers, even if some indications exist (). Rainbow trout is produced in net pen aquaculture, of which some escape into the wild (reported average at c. 80 000, range: 200–315 000, during 2001–2015). Potential spawning of rainbow trout in spring may lead to excavation of newly hatched Atlantic salmon larvae. Competition between rainbow trout and Atlantic salmon parr may also be expected. Pink salmon (Oncorhynchus gorbuscha) have been stocked in in Russian rivers (), leading to dispersal to Norwegian rivers, particularly in the north, where a few populations appear to have been established (; ; County Governor of Finnmark pers. Comm.). Pink salmon often utilize spawning habitats close to the estuary, and juveniles migrate to the sea soon after hatching (). Thus, competition with Atlantic salmon juveniles will be minor. The ecological effects of the establishment of rainbow trout and pink salmon on Atlantic salmon are basically unknown ().
Other introduced species, such as gudgeon (Gobio gobio) (), or translocated species such as pike (Esox lucius) and bullhead (Cottus gobio), may interact with Atlantic salmon through predation () or competition (; ; ).
The effects of these introductions are poorly studied and therefore uncertain. Because the few studies that exist on introduced or translocated species in Norway indicate small effects (; ), this factor was ranked relatively low along the effect axis. There is uncertainty to what extent introduced or translocated species will further expand their ranges, particularly given the observed climate change. The extensive aquaculture industry will continue to produce rainbow trout that escape from net pens, leading to a large propagule pressure (). The likelihood of further losses was considered as moderate.
Agricultural pollution
Many Norwegian Atlantic salmon rivers run through valleys with agricultural activity, while large parts of their catchments are located in sparsely populated low productive areas. Only 3–4% of the area of Norway is farmland (). Runoff of phosphorous (P) from agriculture may thus stimulate productivity in the generally nutrient-poor Norwegian salmon rivers (; ). Cultivation of new areas and runoff from exposed farmland (e.g. newly ploughed) may reduce Atlantic salmon habitat quality due to erosion and transport of fine particulate matters to the rivers (). Silage effluents and high stocking rate of grazing animals may result in high input of easily degradable organic matter and oxygen depletion in streams (), but salmonids in large rivers are probably less affected due to sufficient dilution and oxygen supply. Runoff of pesticides from treated areas is considered under “hazardous substances”.
Agricultural pollution potentially affects a number of Atlantic salmon populations and is a national impact factor. The National Action Plan against Agricultural Pollution for 1985–1988 developed mitigation strategies consisting of a set of legislative, regulatory, and economic instruments as well as information campaigns (). Long-term monitoring in small streams in agricultural areas has showed variable temporal trends in nutrient losses (; ), but a recent evaluation () concluded that implemented measures have positively influenced river water quality. The typical effect in salmon rivers was classified as small. Moderate nutrient loads stimulate productivity and high loads are rare due to relatively small agricultural areas in the catchments of most salmon rivers. No Atlantic salmon population has been classified as critically endangered or lost due to agricultural pollution (). Consequently, the rank along the effect axis is relatively low. Implementation of WFD management plans will likely initiate further countermeasures. This impact factor thus scored low also along the development axis. Increased runoff of nutrients and soil due to climate change () adds uncertainty to future development.
Hazardous substances
Atlantic salmon watercourses may receive heavy metals, pesticides, organic micropollutants and radionuclides from local (of natural and anthropogenic origin) and distant (long-range transported) sources (). Norway is a rural country with a relatively small onshore industrial activity. Major industrial facilities are typically situated in the lowermost parts of rivers and at estuaries where dilution may be adequate to avoid harmful effects. Also, industry effluents are regulated by discharge permits from the Norwegian Environment Agency.
The effects on fish vary from sub-lethal effects to long-term reductions in survival () depending on the substance and exposure. Some pollutants (so-called hormone mimics) may influence the development of sex and gonads, with potentially strong effects on fish reproduction (e.g. ). The typical effects were considered moderate, but knowledge on effects on Atlantic salmon populations are limited. Two Atlantic salmon populations have been lost at least partly due to industrial pollution, but new populations have been established in both rivers.
The EU Commission has listed several of the most relevant substances as prioritized harmful substances in the WFD due to their potential toxic effects and has set limits for their concentrations in freshwaters and the sea. The aim is to phase out the use of these compounds. Thus, this impact factor scored relatively low along the development axis.
Mining
Mining for metals and minerals, together with quarries for production of different crushed bedrock products may affect surface water quality. All these activities produce particles that may be transported to rivers as suspended solids. The effects are poorly documented, but may be both direct (e.g. mechanical damage of gills) and indirect (e.g. clogging of spawning sites). Leaching of heavy metals (e.g. copper) from waste rock dumps and from flooded mines has probably the greatest potential for effects on salmon populations. Smolt is the most sensitive life stage, and smolts may be affected at very low concentrations of heavy metals (). However, concentrations of heavy metals are generally below critical levels in Norwegian salmon rivers.
Potential effects depend on which minerals are mined, runoff treatment, and the downstream dilution. In Norway, harmful runoff from the mining industry has been related to abandoned mines, especially those based on blasting of sulphidic, metal-containing minerals. Sulphides are oxidized on exposure to air, producing sulphuric acid, potentially causing elevated concentrations of harmful Al and heavy metals such as copper, nickel, and zink. Sea deposits of waste rock, which are planned in Norway, may also influence Atlantic salmon negatively (), but studies from marine systems are lacking. Effects of mining on Atlantic salmon probably varies largely among sites, but was classified as small.
Overexploitation
After the establishment of CLs (in numbers of eggs or mass of females) based on stock-recruitment relationships (; ), overexploitation can be defined as reduction of the spawning population below the CL due to exploitation. Overexploitation is a dynamic impact factor, often with relatively rapid population responses to reduction in exploitation. Exploitation of Atlantic salmon was likely low until effective gears was developed during the 1800s (). In Norway, the exploitation level peaked during the 1970 and 1980s due to the large marine driftnet fishery (), but decreased after closure of this fishery in 1989 (), and has further decreased with recent reductions in other costal fisheries (). The Atlantic Salmon Committee estimates annually both attainment of the CLs () and overexploitation (as a percentage of the CL) for 160–180 of the largest Atlantic salmon populations in Norway (representing over 90% of the annual total river catches). Management targeted at reaching CLs seccessfully reduced exploitation both in freshwater and along the coast, and improved attainment of the CLs (). By 2015, average overexploitation of assessed populations (weighted by the CLs, as a measure of population size) was 14%, and average attainment of CLs was 87%. This result was largely impacted by overexploitation in the Tana watercourse, where overexploitation is considerable, and management according to CL has not been fully implemented because management is regulated by a bilateral agreement between Finland and Norway. The Tana watercourse affects the national result due its large size (if all CLs were reached, size of the salmon populations in this watercourse alone would amount to one-fifth of the total size of all assessed populations). Omitting the Tana population complex, average overexploitation was 7.7%, and was found in 52 of the 190 assessed populations. Thus, overexploitation scored low along both axes, largely due to the recent management measures that have reduced exploitation.
Predation
Atlantic salmon are vulnerable to predatory birds, mammals, and fish (). This is generally not considered an anthropogenic factor, but is included to the extent that predation is influenced by human activities. Human activities may increase the number of predators () and the vulnerability of salmon to predation. Overexploitation or habitat changes may reduce salmon abundance to the point that salmon become disproportionately vulnerable to predators (). Human activities may also increase the exposure to predation. Dams and reservoirs may for instance slow down Atlantic salmon migration, create favourable habitats for predators, and concentrate predators and prey (e.g. ; ; ). River regulations may also cause loss of ice cover, resulting in increased exposure to ectothermic predators ().
There is little data available to assess how and to what extent predation affect salmon populations, but there is little doubt that predation influences behaviour, recruitment, and population dynamics of salmon (). In Norway, there are likely few Atlantic salmon populations impacted by predation influenced by human activity. Removal of ice cover after hydropower regulation in northern rivers is likely the main challenge. The challenge with smolt predation in hydropower reservoirs occurs in some rivers, but is generally rare in Norway. Based on available information and expert judgements, predation due to human activity was ranked low along both axes.
Climate change
Recent and projected climate changes represent major demographic and adaptive challenges to Atlantic salmon (). Recent reviews conclude that populations have been and will be affected by climate change, both in freshwater, during seaward migration (, ; ) and in the marine environment (; ; , ; ; ). Negative effects are particularly likely to occur in the southern distribution rage of Atlantic salmon (), where physiological tolerance thresholds may be exceeded and summer droughts become more frequent (; ). An individual based mechanistic population model () was used to predict climate change effects on Atlantic salmon from three climatic regions of Norway () based on predicted local stream temperatures and discharges obtained from downscaled global climate models. According to these models, increased summer temperatures under future climate regimes increased Atlantic salmon production in western and northern Norway, whereas reduced summer wetted area caused lower predicted smolt production in southern Norway. Climate change may thus have both positive and negative effects on the Atlantic salmon freshwater production. The model included the whole life cycle, but did not consider effects in the marine environment. Several studies show correlations between growth or survival and ocean temperature or climate indices (; ), and a relationship between growth and survival has been established (). However, the mechanistic relationships are unclear, and correlations may be due to direct physiological effects of temperature, or due to indirect changes in prey abundance or quality (, ). The thermal scaling of Atlantic salmon growth at sea is poorly described in . Moreover, Atlantic salmon is a generalist and opportunistic predator at sea (). Given the wide oceanic distribution and limited knowledge of migration routes of different populations (), it is difficult to establish links between changes in prey abundance, growth, and survival. However, the general and continued decline in Atlantic salmon marine survival during the last decades (e.g. ; ; ; ), suggest observed changes in ocean climate () may be causative.
Considering both the freshwater and marine environments, the typical effect of climate change on Norwegian Atlantic salmon was classified as small, but the number of populations affected, the geographical distribution (regional) and the lack of effective measures caused moderate scores along both axes. However, current knowledge can be classified as poor and the uncertainty of future development is high.
The overall ranking of the threats to Norwegian Atlantic salmon
Four major groups of impact factors were identified according to the overall analysis of scores for each impact factor (Table 1, Figure 2). Escaped farmed Atlantic salmon and salmon lice were identified as expanding population threats with high scores along both axes. Escaped farmed Atlantic salmon had the highest scores along both axes, and was identified as the largest threat. The second group, G. salaris, acid rain, hydropower regulation and physical habitat alterations were also identified as population threats with high scores along the effect axis, but were classified as stabilized, mainly because effective mitigation measures have been implemented and there are plans for further mitigation measures in the near future.
The third group, positioned in the middle of the diagram (Figure 2), consisted of other infections related to fish farming, infections linked to other anthropogenic activities than fish farming, and climate change. The knowledge on the effects of these impact factors is generally poor and the uncertainty for the projected development particularly high.
The forth group, positioned towards the lower left corner of the diagram, represents stabilized loss factors. Among these, agricultural pollution ranked highest along the effect axis but low along the development axis. Water abstraction ranked highest in terms of risk of further losses. Overexploitation was ranked low both on the effect axis and development axis because of major reductions in fisheries due to implementation of management according to CLs (). Introduced fish species, mining, hazardous substances and predation were the other factors in this group.
Discussion
Escaped farmed Atlantic salmon and salmon lice were the two anthropogenic impact factors identified as expanding threats to Atlantic salmon populations in Norway, which affect wild salmon populations to the extent that they may be critically endangered or lost, and which have a large likelihood of causing even further reductions and losses in the future. The main reason for the heavy impact by these factors is the size (1.3 million tons farmed salmon produced in 2015) and expected growth in the production of farmed salmonids (). In 2015, there were 382 million farmed Atlantic salmon at nearly 600 farm localities along the coast (). In comparison, the number of wild adult Atlantic salmon returning to Norway the same year was estimated at 522 000 individuals (). Hence, the abundance of farmed Atlantic salmon was 732 times the abundance of wild Atlantic salmon. Indeed, the number of farmed Atlantic salmon in a single location typically exceeds the total abundance of adult wild Atlantic salmon in Norway. Although the proportion of farmed salmon that escape is low, between 0.04 and 0.16% in 2015, and their survival to adulthood is low (), the sheer numbers of farmed fish result in high risk of genetic changes in many populations (). Similarly, while the permitted level of salmon lice per fish in farms is strictly regulated, the large number of farmed fish results in a worst case daily release of more than 1 billion salmon lice larvae (). Consequently, monitoring indicates moderate or high risk for lice-related mortality in wild Atlantic salmon smolts at several locations along the coast ().
Salmon lice may only threaten population viability under strong infection pressures over several years. Average parasite induced mortality has been estimated in the range of 0.6–39.0% (across locations and years) in experiments based on protecting groups of smolts chemically against salmon lice (; ; ; ; ; ). However, in combination with other impacts, salmon lice may affect Atlantic salmon populations to the extent that they become critically endangered or lost (e.g. , ).
Escaped farmed Atlantic salmon is a direct threat to the genetic integrity of the wild populations, will likely reduce the number of returning adults over time (; ) and was classified as the most serious threat to the wild populations. Documentation of genetic introgression of farmed Atlantic salmon into a large number of the wild populations (, ; ) represents compelling evidence that escaped farmed Atlantic salmon threaten the genetic integrity of Atlantic salmon populations in Norway. The introgression risk may be higher when wild populations have been reduced by other impacts, due to density dependent spawning success of farmed fish (; ), but genetic introgression by farmed Atlantic salmon was also documented in salmon rivers with abundant wild populations ().
The introduced parasite G. salaris, acidification, hydropower regulation and habitat alterations were identified as stabilized population threats, which have contributed to Atlantic salmon populations becoming critically endangered or lost, but which have a low probability of causing further future loss. G. salaris has devastating effects on Atlantic salmon populations of Norway, but the eradication programme has successfully reduced the number of infected populations during recent decades. Similarly, the liming programme has successfully mitigated acidification in southern and southwestern parts of Norway and allowed reestablishment and population growth in 21 Norwegian Atlantic salmon rivers (). Moreover, international treaties have been signed by most European countries, and acid deposition over Norway has been reduced (). Although hydropower regulation continues to hamper Atlantic salmon production in as many as 54 regulated salmon rivers (), and has contributed to the loss of 19 populations, the progression of the industry, and national and international legislations, point towards improved conditions for Atlantic salmon in regulated rivers rather than further loss of production and populations. Similarly, further habitat alterations with large negative effects on Atlantic salmon populations are unlikely under the WFD. Moreover, habitat restoration measures seem to be expanding (; ).
The knowledge on all the six population threats discussed above was classified as good or moderate, and the uncertainty of future development as low or moderate. In contrast, knowledge on many of the remaining impact factors was classified as poor and uncertainty of future development as high. Particularly important is the lack of knowledge on effects of infections related to salmonid farming and other anthropogenic activities, which scored relatively high along both axes, and the uncertain effects of climate change. The combination of ranking and knowledge of the different impact factors may provide a tool for prioritizing applied research.
The impact factors were considered separately, whereas in most cases, several factors impact Atlantic salmon populations simultaneously. The interactive effects of two or more impact factors may be non-linear, unpredictable, and consequently difficult to study. An example may illustrate the complexity. , ) showed that Atlantic salmon smolts exposed to freshwater acidification were subsequently more vulnerable to salmon lice than control groups. However, vulnerability to salmon lice was reduced when the fish were given a sufficient recovery period between an acidification episode and the exposure to salmon lice. Thus, the relative timing and intensity of both acidification and the risk of salmon lice infestation may produce large variation among years in interactive effects of these two impact factors. Climate change is a factor that can affect all stages of the Atlantic salmon life cycle, and may thus interact with several other anthropogenic factors. Examination of nuanced interaction effects among impact factors was beyond the scope of this study.
The classification system developed is semi-quantitative and partly dependent on expert judgments. Although a more quantitate system would be preferable, the sheer number of Norwegian Atlantic salmon populations considered (400 watercourses) and the amount of data needed for quantitative assessment makes this unrealistic. On the other hand, classification into four wide classes for each impact factor and characteristics is likely robust to the lack of precise data from each population or watercourse. Classification systems are now widespread in conservation biology, including classification of threats to biodiversity at different levels (e.g. ; ), as well as in legislative frameworks for nature conservation (e.g. WFD). These are important tools to understand what threatens biodiversity, where risks occur and how fast threats are changing in type and intensity (). Expert judgments are also a broadly accepted approach to threats and environmental risk assessments (), although care should be taken to account for bias and ensure objectivity (). Carefully selected expert panels, analogous to the Atlantic salmon Committee, are typically used for expert judgements. The Atlantic Salmon Committee consists of 12 scientists collectively covering all major areas relevant for providing scientific advice on wild Atlantic salmon management, including expert knowledge on the majority of the anthropogenic threats. The members come from seven different research institutes and universities in Norway involved in salmonid research, but they do not attend as representatives for their institutions to reduce the risk of biased assessments. Moreover, the annual classification since 2010 is transparent through publication of the scoring for each impact factor in Norwegian reports, including detailed explanations for the scores, and the classification is open for discussion and critique, both from other experts, stakeholders, and the public.
Details of this classification system were developed for Norwegian conditions but can easily be adapted to other countries or for international assessments. The main feature of the system is that it combines the effect of each impact factor (until present) with projections for future development. This should make it particularly valuable for prioritizing research and conservation measures.
Acknowledgements
We acknowledge the contribution of previous members of the Atlantic Salmon Committee, Kjetil Hindar, and Frode Kroglund, who participated in the development of the classification system.
Funding
The work of the Atlantic salmon Committee is funded by the Norwegian Environment Agency.
References
- Aarestrup K., Jepsen N., Rasmussen G., Økland F. 1999. Movements of two strains of radio tagged Atlantic salmon, Salmo salar L., smolts through a reservoir. Fisheries Management and Ecology, 6: 97–107.
- Anon. 2001–2002. Om opprettelse av nasjonale laksevassdrag og laksefjorder (in Norwegian). St.prp. nr. 79. White Paper, Ministry of Environment.
- Anon. 2006–2007. Om vern av villaksen og ferdigstilling av nasjonale laksevassdrag og laksefjorder (in Norwegian). St.prp. nr. 32. White Paper, Ministry of Environment.
- Anon. 2014–2015. Forutsigbar og miljømessig bærekraftig vekst i norsk lakse- og ørretoppdrett (in Norwegian). St.prp. nr. 16. White Paper, Ministry of Trade, Industry and Fisheries.
- Anon. 2015–2016. Kraft til endring - Energipolitikken mot 2030 (in Norwegian). St.prp. nr. 25. White Paper, Ministry of Petroleum and Energy.
- Anon. 2016. The status for Norwegian Atlantic salmon populations in 2016 (in Norwegian). Report from the Norwegian Scientific Advisory Committee for Atlantic Salmon Management, 9: 1–191.
- Allendorf F. W., Leary R. F., Spruell P., Wenburg J. K. 2001. The problems with hybrids: setting conservation guidelines. Trends in Ecology and Evolution, 16: 613–622.
- Bates B. C., Kundzewicz Z. W., Wu S., Palutikof J. P. 2008. Climate change and water, Technical paper of the intergovernmental panel on climate change. Geneva, IPCC Secretary.
- Bechmann M., Deelstra J., Stålnacke P., Eggestad H. O., Øygarden L., Pengerud A. 2008. Monitoring catchment scale agricultural pollution in Norway: policy instruments, implementation of mitigation methods and trends in nutrient and sediment losses. Environmental Science and Policy, 11: 102–114.
- Benejam L., Saura-Mas S., Bardina M., Sola C., Munne A., Garcia-Berhou E. 2016. Ecological impacts of small hydropower plants on headwater stream fish: from individual to community effects. Ecology of Freshwater Fish, 25: 295–306.
- Bergan M. A. 2014. Challenges in anadromous watercourses in Søndre Fosen Water Area: Fish communities, historical information and hydromorphological alterations according to WFD in Frøya and Sunde, Sør-Trøndelag County (in Norwegian with English summary). NINA Report, 1077: 1–96.
- Bergan P. I., Gausen D., Hansen L. P. 1991. Attempts to reduce the impacts of reared Atlantic salmon on wild in Norway. Aquaculture, 98: 319–324.
- Beaugrand G., Reid P. C. 2003. Long-term changes in phytoplankton, zooplankton and salmon related to climate. Global Change Biology, 9: 801–817.
- Beaugrand G., Reid P. C. 2012. Relationships between North Atlantic salmon, plankton, and hydroclimatic change in the Northeast Atlantic. ICES Journal of Marine Science, 69: 1549–1562.
- Bilotta G. S., Burnside N. G., Gray J. C., Orr H. G. 2016. The effects of run-of-river hydroelectric power schemes on fish community composition in temperate streams and rivers. Plos One, 11: e0154271.
- Birkel C., Soulsby C., Ali G., Tetzlaff D. 2014. Assessing the cumulative impacts of hydropower regulation on the flow characteristics of a large Atlantic salmon river system. River Research and Application, 30: 456–475.
- Bjerknes V. 1977. Evidence of natural production of pink salmon fry Oncorhynchus gorbuscha in Finnmark North Norway. Astarte, 10: 5–8.
- Bjerknes V., Vaag A. B. 1980. Migration and capture of pink salmon, Oncorhynchus gorbuscha Walbaum in Finnmark, North Norway. Journal of Fish Biology, 16: 291–297.
- Blackwell B. F., Juanes F. 1998. Predation on Atlantic salmon smolts by striped bass after dam passage. North American Journal of Fisheries Management, 18: 936–939.
- Bornø G., Lie Linaker M. (eds.) 2015. The Fish Health Report 2014 (in Norwegian Norwegian). Veterinary Institute, Harstad. 47 pp.
- Boylan P., Adams C. E. 2006. The influence of broad scale climatic phenomena on long term trends in Atlantic salmon population size: an example from the River Foyle, Ireland. Journal of Fish Biology, 68: 276–283.
- Brander K. M. 2007. The role of growth changes in the decline and recovery of North Atlantic cod stocks since 1970. ICES Journal of Marine Science, 64: 211–217.
- Cairns D.K. (Ed.). 2001. An evaluation of possible causes of the decline in pre-fishery abundance of North American Atlantic salmon. Canadian Technical Report of Fisheries and Aquatic Sciences, No. 2358. 67 pp.
- Chaput G. 2012. Overview of the status of Atlantic salmon (Salmo salar) in the North Atlantic and trends in marine mortality. ICES Journal of Marine Science, 69: 1538–1548.
- Clair T. A., Hindar A. 2005. Liming for the mitigation of acid rain effects in freshwaters: a review of recent results. Environmental Review, 13: 91–128.
- Colautti R. I., Grigorovich I. A., MacIsaac H. J. 2006. Propagule pressure: a null model for biological invasions. Biological Invasions, 8: 1023–1037.
- Dadswell M. J., Spares A. D., Reader J. M., Stokesbury M. J. W. 2010. The North Atlantic subpolar gyre and the marine migration of Atlantic salmon Salmo salar: the ‘Merry-Go-Round’ hypothesis. Journal of Fish Biology, 77: 435–467.
- Deelstra J., Øygården L., Blankenberg A. G. B., Eggestad H. O. 2011. Climate change and runoff from agricultural catchments in Norway. International Journal of Climate Change Strategies and Management, 3: 345–360.
- Dickson R. R., Turrell W. R. 2000. The NAO: the dominant atmospheric process affecting oceanic variability in home, middle and distant waters of European Atlantic salmon. InThe Ocean Life of Atlantic Salmon—Environmental and Biological Factors influencing Survival, pp. 92–11. Ed. by Mills D.. Fishing News Books, Oxford.
- Driscoll C. T., Baker J. P., Bisogni J. J., Schofield C. L. 1980. Effect of aluminum speciation on fish in dilute acidified waters. Nature, 284: 161–164.
- Einum S., Fleming I. A. 1997. Genetic divergence and interactions in the wild among native, farmed and hybrid Atlantic salmon. Journal of Fish Biology, 50: 634–651.
- Einum S., Nislow K. H. 2011. Variation in population size through time and space: Theory and recent empirical advances from Atlantic salmon. InAtlantic Salmon Ecology, pp. 277–298. Ed. by Aas Ø., Einum S., Klemetsen A., Skurdal J.. Wiley-Blackwell, Oxford, UK.
- Eken M., Borgstrøm R. 1994. Gudgeon – a new fish species in Norway (in Norwegian). Fauna (Oslo), 47: 120–123.
- EU. 2000. Directive 2000/60/EC establishing a framework for community action in the field of water policy. Official Journal of the European Communities L, 327: 1–73.
- EU. 2009. Directive 2009/28/EC of the European parliament and of the Council of 23 April 2009 on the promotion of the use of energy from renewable sources and amending and subsequently repealing Directives 2001/77/EC and 2003/30/EC. Official Journal of the European Communities L, 140: 1–47.
- Ferguson A., Fleming I. A., Hindar K., Skaala Ø., McGinnity P., Gross T. F., Prodöhl P. 2007. Farm escapes. InThe Atlantic salmon. Genetics, conservation and management, pp. 357–398. Ed. by Verspoor E.. Blackwell Publishing, Oxford, UK.
- Finstad B., Bjørn P. A. 2011. Present status and implications of salmon lice on wild salmonids in Norwegian coastal zones. InSalmon Lice: An Integrated Approach to Understanding Parasite Abundance and Distribution, pp. 281–305. Ed. by Jones S., Beamish R.. Wiley-Blackwell, Oxford, UK.
- Finstad B., Bjørn P. A., Todd C. D., Whoriskey F., Gargan P. G., Forde G., 2011. The effect of sea lice on Atlantic salmon and other salmonid species. InAtlantic Salmon Ecology, pp. 253–276. Ed. by Aas Ø., Einum S., Klemetsen A., Skurdal J.. Wiley-Blackwell, Oxford, UK.
- Finstad B., Kroglund F., Bjørn P. A., Nilsen R., Pettersen K., Rosseland B. O., Teien H.-C., 2012. Salmon lice-induced mortality of Atlantic salmon postsmolts experiencing episodic acidification and recovery in freshwater. Aquaculture, 362–363: 193–199.
- Finstad B., Kroglund F., Strand R., Stefansson S. O., Bjørn P. A., Rosseland B. O., Nilsen T. O., 2007. Salmon lice or suboptimal water quality - reasons for reduced postsmolt survival?. Aquaculture, 273: 374–383.
- Fiske P., Lund R., Hansen L. P 2006. Relationships between the frequency of farmed Atlantic salmon, Salmo salar L., in wild salmon populations and fish farming activity in Norway, 1989-2004. ICES Journal of Marine Science, 63: 1182–1189.
- Fjeldstad H.-P., Barlaup B. T., Stickler M., Gabrielsen S. E., Alfredsen K. 2012. Removal of weirs and the influence of physical habitat for salmonids in a Norwegian river. River Research and Applications, 28: 753–763.
- Fleming I. A., Lamberg A., Jonsson B. 1997. Effects of early experience on the reproductive performance of Atlantic salmon. Behavioral Ecology, 8: 470–480.
- Fleming I. A., Hindar K., Mjølnerød I. B., Jonsson B., Balstad T., Lamberg A. 2000. Lifetime success and interactions of farm salmon invading a native population. Proceedings of the Royal Society of London Series B, 267: 1517–1523.
- Forseth T., Harby A. (eds.) 2014. Handbook for environmental design in regulated salmon rivers. NINA Special Report, 53: 1–90.
- Forseth T., Letcher B. H., Johansen M. 2011 The behavioural flexibility of salmon growth. InAtlantic Salmon Ecology, pp. 145–161. Ed. by Aas Ø., Einum S., Klemetsen A., Skurdal J.. Wiley-Blackwell, Oxford, UK.
- Forseth T., Fiske P., Gjøsæter H., Hindar K. 2013. Reference point based management of Norwegian Atlantic salmon populations. Environmental Conservation, 40: 356–366.
- Foy R. H., Kirk M. 1995. Agriculture and Water Quality: A Regional Study. Water and Environment Journal, 9: 247–256.
- Fraser D. J., Minto C., Calvert A. M., Eddington J. D., Hutchings J. A. 2010a. Potential for domesticated-wild interbreeding to induce maladaptive phenology across multiple populations of wild Atlantic salmon (Salmo salar). Canadian Journal of Fisheries and Aquatic Sciences, 67: 1768–1775.
- Fraser D. J., Houde A. L. S., Debes P. V., O’Reilly P., Eddington J. D., Hutchings J. A. 2010b. Consequences of farmed-wild hybridization across divergent wild populations and multiple traits in salmon. Ecological Applications, 20: 935–953.
- Friedland K. D. 1998. Ocean climate influences on critical Atlantic salmon (Salmo salar) life history events. Canadian Journal of Fisheries and Aquatic Sciences, 55(Suppl. 1): 119–130.
- Friedland K. D., Hansen L. P., Dunkley D. A. 1998. Marine temperatures experienced by postsmolts and the survival of Atlantic salmon, Salmo salar L., in the North Sea area. Fisheries Oceanography, 7: 22–34.
- Friedland K. D., Hansen L. P., Dunkley D. A., MacLean J. C. 2000. Linkage between ocean climate, post-smolt growth, and survival of Atlantic salmon (Salmo salar L.) in the North Sea area. ICES Journal of Marine Science, 57: 419–429.
- Friedland K. D., Reddin D. G., McMenemy J. R., Drinkwater K. F. 2003. Multidecadal trends in North American Atlantic salmon (Salmo salar) stocks and climate relevant to juvenile survival. Canadian Journal of Fisheries and Aquatic Sciences, 60: 563–583.
- Friedland K. D., MacLean J. C., Hansen L. P., Peyronnet A. J., Karlsson L., Reddin D. G., Ó Maoiléidigh N., McCarthy J. L. 2009. The recruitment of Atlantic salmon in Europe. ICES Journal of Marine Science, 66: 289–204.
- Gabler H. M., Amundsen P. A. 1999. Resource partitioning between Siberian sculpin (Cottus poecilopus Heckel) and Atlantic salmon parr (Salmo salar L.) in a sub-Arctic river, northern Norway. Ecology of Freshwater Fish, 8: 201–208.
- Garcia de Leaniz C., Fleming I. A., Einum S., Verspoor E., Jordan W. C., Consuegra S., Aubin-Horth N., Lajus D., Letcher B. H., Youngson A. F., Webb J. H., 2007. A critical review of adaptive genetic variation in Atlantic Atlantic salmon: implication for conservation. Biological Reviews, 88: 173–211.
- Gargan P. G., Forde G., Hazon N., Russell D. J. F., Todd C. D. 2012. Evidence for sea lice induced marine mortality of Atlantic salmon (Salmo salar) in western Ireland from experimental releases of ranched smolts treated with emamectin benzoate. Canadian Journal of Fisheries and Aquatic Sciences, 69: 343–353.
- Garseth A. H., Ekrem T., Biering E. 2013. Phylogenetic evidence of long distance dispersal and transmission of piscine reovirus (PRV) between farmed and wild Atlantic salmon. PLOS One, 8: e82202.
- Gensemer R. W., Playle R. C. 1999. The bioavailability and toxicity of aluminium in aquatic environments. Critical Reviews in Environmental Science and Technology, 29: 315–450.
- Glover K. A., Bergh O., Rudra H., Skaala Ø. 2006. Juvenile growth and susceptibility to Aeromonas salmonicida subsp salmonicida in Atlantic salmon (Salmo salar L.) of farmed, hybrid and wild parentage. Aquaculture, 254: 72–81.
- Glover K. A., Ottera H., Olsen R. E., Slinde E., Taranger G. L., Skaala Ø. 2009. A comparison of farmed, wild and hybrid Atlantic salmon (Salmo salar L.) reared under farming conditions. Aquaculture, 286: 203–210.
- Glover K. A., Pertoldi C., Besnier F., Wennevik V., Kent M., Skaala Ø. 2013. Atlantic salmon populations invaded by farmed escapees: quantifying genetic introgression with a Bayesian approach and SNPs. BMC Genetics, 14: 74.
- Glover K. A., Quintela M., Wennevik V., Besnier F., Sørvik A. G. E., Skaala Ø. 2012. Three decades of farmed escapees in the wild: a spatio-temporal analysis of Atlantic Atlantic salmon population genetic structure throughout Norway. PLOS One, 7: e43129.
- Gordeeva N. V., Salmenkova E. A. 2011. Experimental microevolution: transplantation of pink salmon into the European North. Evolutionary Ecology, 25: 657–679.
- Grøntvedt R. N., Jansen P. A., Horsberg T. A., Helgesen K., Tarpai A. 2016. The surveillance program for resistance to chemotherapeutants in L. salmonis in Norway 2015 (in Norwegian). Surveillance program for terrestrial and aquatic animals in Norway. Annual report 2015. Norwegian Veterinary Institute.
- Hansen L. P., Fiske P., Holm M., Jensen A. J., Sægrov H. 2008. Population status for Atlantic salmon in Norway, prognoses for 2008 (in Norwegian). Report from the working group. Investigation for DN, 2008-5: 1–66 pp.
- Harby A., Noack M. 2013. Rapid flow fluctuations and impact on fish and the aquatic ecosystem. InEcohydraulics: An Integrated Appraoch, pp. 323–335. Ed. by Maddock I., Harby A., Kemp P., Wood P.. Wiley Blackwell, West Sussex, UK.
- Hauer C., Pulg U., Gabrielsen S. E., Barlaup B. T. 2015. Application of step-backwater modelling for salmonid spawning habitat restoration in Western Norway. Ecohydrology, 7: 1239–1261.
- Hedger R. D., Sundt-Hansen L. E., Forseth T., Diserud O. H., Ugedal O., Finstad A. G. 2012. Modelling the complete life-cycle of Atlantic salmon (Salmo salar L.) using a spatially explicit individual-based approach. Ecological Modelling, 248: 119–129.
- Hedger R. D., Sundt-Hansen L. E., Forseth T., Ugedal O., Diserud O. H., Kvambekk ÅS., Finstad A. G. 2013. Predicting climate change effects on subarctic–Arctic populations of Atlantic salmon (Salmo salar). Canadian Journal of Fisheries and Aquatic Sciences, 70: 159–168.
- Helland I. P., Uglem I., Jansen P. A., Diserud O. H., Bjørn P. A., Finstad B. 2015. Statistical and ecological challenges of monitoring parasitic sea lice infestations in wild salmonid fish stocks. Aquaculture Environmental Interaction, 7: 267–280.
- Hesthagen T., Hansen L. P. 1991. Estimates of the annual loss of Atlantic salmon, Salmo salar L., in Norway due to acidification. Aquaculture and Fisheries Management, 22: 85–91.
- Hesthagen T., Sandlund O. T. 2007. Non-native freshwater fishes in Norway: history, consequences and perspectives. Journal of Fish Biology, 71: 173–183.
- Hesthagen T., Larsen B. M., Fiske P. 2011. Liming restores Atlantic salmon (Salmo salar) populations in acidified Norwegian rivers. Canadian Journal of Fisheries and Aquatic Sciences, 68: 224–231.
- Heuch P. A., Mo T. A. 2001. A model of salmon louse production in Norway: effects of increasing salmon production and public management measures. Diseases of Aquatic Organisms, 45: 145–152.
- Heuch P. A., Revie C. W., Gettinby G. 2003. A comparison of epidemiological patterns of salmon lice, Lepeophtheirus salmonis, infections on farmed Atlantic salmon, Salmo salar L., in Norway and Scotland. Journal of Fish Diseases, 26: 539–551.
- Heuch P. A., Bjørn P. A., Finstad B., Holst J. C., Asplin L., Nilsen F. 2005. A review of the Norwegian ‘National Action Plan Against Salmon Lice on Salmonids’: The effect on wild salmonids. Aquaculture, 246: 79–92.
- Hindar K., Hutchings J. A., Diserud O. H., Fiske P. 2011. Stock, recruitment and exploitation. InAtlantic Salmon Ecology, pp. 299–332. Ed. by Aas Ø., Einum S., Klemetsen A., Skurdal J.. Wiley-Blackwell, Oxford, UK.
- Hoegh-Guldberg O., Bruno J. F. 2010. The impact of climate change on the world’s marine ecosystems. Science, 328: 1523–1528.
- Huitfeldt-Kaas H. 1918. The Distribution and Immigration of freshwater Fishes in Norway, with a Supplement on Nobel Creyfish (in Norwegian). Centraltrykkeriet, Kristiania, Norway.
- Hvidsten N. A., Diserud O. H., Jensen A. J., Jensås J. G., Johnsen B. O., Ugedal O. 2015. Water discharge affects Atlantic salmon Salmo salar smolt production: a 27 year study in the River Orkla, Norway. Journal of Fish Biology, 86: 92–104.
- ICES. 2016. Report of the Working Group on North Atlantic Salmon (WGNAS). 30th March–8th April 2016. Copenhagen, Denmark. ICES CM 2016/ACOM:10: 1-321 pp.
- Jackson D., Cotter D., Newell J., McEvoy S., O’Donohoe P., Kane F., McDermott T., 2013. Impact of Lepeophtheirus salmonis infestations on migrating Atlantic salmon, Salmo salar L., smolts at eight locations in Ireland with an analysis of lice-induced marine mortality. Journal of Fish Diseases, 36: 273–281.
- Jensen A. J., Zubchenko A. V., Heggberget T. G., Hvidsten N. A., Johnsen B. O., Kuzmin O., Loenko A. A., 1999. Cessation of the Norwegian drift net fishery: changes observed in Norwegian and Russian populations of Atlantic salmon. ICES Journal of Marine Science, 56: 84–95.
- Jepsen N., Aarestrup K., Økland F., Rasmussen G. 1998. Survival of radio-tagged Atlantic salmon (Salmo salar L.) and trout (Salmo trutta L.) smolts passing a reservoir during seaward migration. Hydrobiologia, 372: 347–353.
- Johansen L. H., Jensen I., Mikkelsen H., Bjørn P. A., Jansen P. A., Bergh Ø. 2011. Disease interaction and pathogens exchange between wild and farmed fish populations with special reference to Norway. Aquaculture, 315: 167–186.
- Johnsen B. O., Jensen A. J. 1991. The Gyrodactylus story in Norway. Aquaculture, 98: 289–302.
- Johnsen B. O., Jensen A. J. 2005. The spread of furunculosis in salmonids in Norwegian rivers. Journal of Fish Biology, 45: 47–55.
- Johnsen B. O., Arnekleiv J. V., Asplin L., Barlaup B. T., Næsje T. F., Rosseland B. O., Saltveit S. J. 2010. Effect of hydropower developments on wild salmon (in Norwegian). Kunnskapsserien for Laks Og Vannmiljø, 3: 1–116.
- Johnsen B. O., Arnekleiv J. V., Asplin L., Barlaup B. T., Næsje T. F., Rosseland B. O., Saltveit S. J., 2011. Hydropower developments - ecological effects. InAtlantic Salmon Ecology, pp. 351–386. Ed. by Aas Ø., Einum S., Klemetsen A., Skurdal J.. Wiley-Blackwell, Oxford, UK.
- Jonsson B., Jonsson N. 2004. Factors affecting marine production of Atlantic salmon (Salmo salar). Canadian Journal of Fisheries and Aquatic Sciences, 61: 2369–2383.
- Jonsson B., Jonsson N. 2009. A review of the likely effects of climate change on anadromous Atlantic salmon Salmo salar and brown trout Salmo trutta, with particular reference to water temperature and flow. Journal of Fish Biology, 75: 2381–2447.
- Jonsson B., Jonsson N. 2011. Climatic effects on Atlantic salmon and brown trout. InEcology of Atlantic Salmon and Brown Trout: Habitat as Template for Life Histories, pp. 473–515. Ed. by B. Jonsson and N. Jonsson. Fish & Fisheries Series, 33. Springer Science+Business Media B.V.
- Jonsson B., Jonsson N., Hansen L. P. 2003. Atlantic salmon straying from the River Imsa. Journal of Fish Biology, 62: 641–657.
- Jonsson B., Jonsson N., Ugedal O. 2011. Production of juvenile salmonids in small Norwegian streams is affected by agricultural land use. Freshwater Biology, 56: 2529–2543.
- Jonsson N., Jonsson B., Hansen L. P. 1998. The relative role of density-dependent and density-independent survival in the life cycle of Atlantic salmon Salmo salar. Journal of Animal Ecology, 67: 751–762.
- Joppa L. N., O’Connor B., Visconti P., Smith C., Geldmann J., Hoffmann J. E., Watson M., 2016. Filling in biodiversity threat gaps. Science, 352: 416–418.
- Jørgensen L., Amundsen P.-A., Gabler H.-M., Halvorsen M., Erkinaro J., Niemelä E. 1999. Spatial distribution of Atlantic salmon parr (Salmo salar L.) and bullhead (Cottus gobio L.) in lotic and lentic habitats of a diversified watercourse in northern Fennoscandia. Fisheries Research, 41: 201–211.
- Karlsson S., Diserud O. H., Fiske P., Hindar K. 2016. Widespread genetic introgression of escaped farmed Atlantic salmon in wild salmon populations. ICES Journal of Marine Research, 73: 2488–2498.
- Karlsson S., Diserud O. H., Moen T., Hindar K. 2014. A standardized method for quantifying unidirectional introgression. Ecology and Evolution, 4: 3256–3263.
- Kekalainen J., Niva T., Huuskonen H. 2008. Pike predation on hatchery-reared Atlantic salmon smolts in a northern Baltic river. Ecology of Freshwater Fish, 17: 100–109.
- Kemp P. (ed.) 2010. Salmonid Fisheries. Freshwater Habitat Management. Wiley-Blackwell, Oxford, UK. 328 pp.
- Kittelsen A., Rosten T., Ulgenes Y., Selvik J. R., Alne H. 2006. Available freshwater resources for future production of smolts of salmon and trout (in Norwegian). Utredning fra Akvaforsk, SINTEF & NIVA, Report 2006.
- Krkošek M., Revie C., Gargan P., Skilbrei O. T., Finstad B., Todd C. D. 2013. Impact of parasites on salmon recruitment in the Northeast Atlantic Ocean. Proceedings of the Royal Society B, 280: 20122359.
- Kroglund F., Finstad B., Stefansson S. O., Nilsen T. O., Kristensen T., Rosseland B. O., Teien H. C., Salbu B. 2007. Exposure to moderate acid water and aluminum reduces Atlantic salmon post-smolt survival. Aquaculture, 273: 360–373.
- Lura H. 1995. Domesticated female Atlantic salmon in the wild: spawning success and contribution to local populations. Dr Scient. thesis, University of Bergen, Norway.
- Madhun A. S., Karlsbakk E., Isachsen C. H., Omdal L. M., Eide Sørvik A. G., Skaala Ø., Barlaup B. T., 2015. Potential disease interaction reinforced: double-virus-infected escaped farmed Atlantic salmon, Salmo salar L., recaptured in a nearby river. Journal of Fish Diseases, 38: 209–219.
- McCarthy J. L., Friedland K. D., Hansen L. P. 2008. Monthly indices of the post-smolt growth of Atlantic salmon from the Drammen River, Norway. Journal of Fish Biology, 72: 1572–1588.
- McGinnity P., Jennings E., DeEyto E., Allott N., Samuelsson P., Rogan G., Whelan K., 2003. Impact of naturally spawning captive-bred Atlantic salmon on wild populations: depressed recruitment and increased risk of climate-mediated extinction. Proceedings of the Royal Society B-Biological Sciences, 1673: 3601–3610.
- Milner N. J., Elliott J. M., Armstrong J. D., Gardiner R., Welton J. S., Ladle M. 2003. The natural control of Atlantic salmon and trout populations in streams. Fisheries Research, 62: 111–125.
- Mo T. A., Jørgensen M. 2017. A survey of the distribution of the PKD-parasite Tetracapsuloides bryosalmona (Cnidaria: Myxosoa: Malacosporea) in salmonids in Norwegian rivers – additional information gleaned from formerly collected fish. Journal of Fish Diseases, 40: 621–627.
- Moore A., Waring C. P. 2001. The effects of a synthetic pesticide on some aspects of reproduction in Atlantic salmon (Salmo salar L.). Aquatic Toxicology, 52: 1–12.
- Murchie K. J., Hair K. P. E., Pullen C. E., Redpath T. D., Stephens H. R., Cooke S. J. 2008. Fish response to modified flow regime in regulated rivers: Research methods, effects and opportunities. River Research and Applications, 24: 197–217.
- Norris A. T., Bradley D. G., Cunningham E. P. 1999. Microsatellite genetic variation between and within farmed and wild Atlantic salmon (Salmo salar) populations. Aquaculture, 180: 247–264.
- Norwegian Environment Agency. 2014. Handlingsplan mot lakseparasitten Gyrodatylus salaris for perioden 2014-2016 (in Norwegian). M-288 2014: 1–88 pp.
- Norwegian Environment Agency. 2016a. Action plan for liming of watercourses in Norway 2016-2021 (in Norwegian). Report M-488. 24 pp.
- Otero J., Jensen A. J., L’Abee-Lund J. H., Stenseth N. C., Storvik G. O., Vøllestad L. A. 2011. Quantifying the ocean, freshwater and human effects on year-to year variation of one-sea-winter Atlantic salmon angled in multiple Norwegian rivers. PLOS One, 6: e24005.
- Otero J., L’Abée-Lund J. H., Castro-Santos T., Leonardsson K., Storvik G. O., Jonsson B., Dempson B., 2014. Basin-scale phenology and effects of climate variability on global timing of initial seaward migration of Atlantic salmon (Salmo salar). Global Change Biology, 20: 61–75.
- Parrish D. L., Behnke R. J., Gephard S. R., McCormick S. D., Reeves G. H. 1998. Why aren’t there more Atlantic salmon (Salmo salar)?Canadian Journal of Fisheries and Aquatic Sciences, 55: 281–287.
- Peeler E. J., Oidtmann B. C., Midtlyng P. J., Miossec L., Gozlan R. E. 2011. Non-native aquatic animals introductions have driven disease emergence in Europe. Biological Invasions, 13: 1291–1303.
- Pettersen R. A., Vøllestad L. A., Flodmark L. E. W., Poléo A. B. S. 2006. Effects of aqueous aluminium on four fish ectoparasites. Science of the Total Environment, 369: 129–138.
- Quinn T. P. 2005. The Behavior and Ecology of Pacific Salmon and Trout. University of Washington Press, Seattle, WA, USA.
- Ramirez-Llodra E., Trannum H. C., Evenset A., Levin L. A., Andersson M., Finne T. E., Hilario A., 2015. Submarine and deep-sea mine tailing placements: A review of current practices, environmental issues, natural analogs and knowledge gaps in Norway and internationally. Marine Pollution Bulletin, 97: 13–35.
- Reist J. D., Wrona F. J., Prowse T. D., Power M., Dempson J. B., Beamish R. J., King J. R., 2006. General effects of climate change on Arctic fishes and fish populations. Ambio, 35: 370–380.
- Rengmark A. H., Slettan A., Skaala Ø., Lie Ø., Lingaas F. 2006. Genetic variability in wild and farmed Atlantic salmon (Salmo salar) strains estimated by SNP and microsatellites. Aquaculture, 253: 229–237.
- Revie C., Dill L., Finstad B., Todd C. D. 2009. Sea Lice Working Group Report. NINA Special Report, 39: 1–117 pp. Norwegian Institute for Nature Research, Trondheim.
- Rikardsen A. H., Dempson J. B. 2011. Dietary life-support: The marine feeding of Atlantic salmon. InAtlantic Salmon Ecology, pp. 115–144. Ed. by Aas Ø., Einum S., Klemetsen A., Skurdal J.. Wiley-Blackwell, Oxford, UK.
- Rosseland B. O., Kroglund F. 2011. Ecological consequences of pollution: lessons from acidification and pesticide. InAtlantic Salmon Ecology, pp. 387–408. Ed. by Aas Ø., Einum S., Klemetsen A., Skurdal J.. Wiley-Blackwell, Oxford, UK.
- Rosseland B. O., Staurnes M. 1994. Physiological mechanisms for toxic effects and resistance to acidic water: an ecophysiological and ecotoxicological approach. InAcidification of Freshwater Systems: Implications for the Future, pp. 227–246. Ed. by Steinberg C.E.W., Wright R.F.. John Wiley & Sons Ltd, West Sussex, UK.
- Ryman N., Utter F., Laikre L. 1995. Protection of intraspecific biodiversity of exploited fishes. Reviews in Fish Biology and Fisheries, 5: 417–446.
- Sægrov H., Hindar K., Urdal K. 1996. Natural reproduction of anadromous rainbow trout in Norway. Journal of Fish Biology, 48: 292–294.
- Salafsky N., Salzer D., Statteersfield A. J., Hilton-Taylor C., Neugarten R., Butchart S. H. M., Collen B., 2008. A standard lexicon for biodiversity conservation: unified classifications of threats and actions. Conservation Biology, 22: 897–911.
- Schneider C., Laizé C. L. R., Acreman M. C., Flörke M. 2013. How will climate change modify river flow regimes in Europe?. Hydrology and Earth System Sciences, 17: 325–339.
- Serra-Llinares R. M., Bjørn P. A., Finstad B., Nilsen R., Asplin L. 2016. Nearby farms are a source of lice for wild salmonids: a reply to Jansen et al. 2016. Aquaculture Environment Interactions, 8: 351–356.
- Serra-Llinares R., Bjørn P. A., Finstad B., Nilsen R., Harbitz A., Berg M., Asplin L. 2014. Salmon lice infection on wild salmonids in marine protected areas: an evaluation of the Norwegian “National Salmon Fjords”. Aquaculture Environment Interactions, 5: 1–16.
- Shearer W. M. 1992. The Atlantic Salmon: Natural History, Exploitation and Future Management. Oxford, Fishing News Books.
- Skaala Ø., Wennevik V., Glover K. A. 2006. Evidence of temporal genetic change in wild Atlantic Atlantic salmon, Salmo salar L., populations affected by farm escapees. ICES Journal of Marine Science, 63: 1224–1233.
- Skaala Ø., Høyheim B., Glover K., Dahle G. 2004. Microsatellite analysis in domesticated and wild Atlantic salmon (Salmo salar L.): allelic diversity and identification of individuals. Aquaculture, 240: 131–143.
- Skaala Ø., Glover K. A., Barlaup B. T., Svåsand T., Besnier F., Hansen M. M., Borgstrøm R. 2012. Performance of farmed, hybrid and wild Atlantic salmon (Salmo salar) families in a natural river environment. Canadian Journal of Fisheries and Aquatic Sciences, 69: 1994–2006.
- Skarbøvik E., Stålnacke P., Kaste Ø., Austnes K. 2014. Trends in nutrients and metals in Norwegian rivers and point sources 1990-2009. Hydrological Research, 45: 441–454.
- Skilbrei O. T., Heino M., Svåsand T. 2015. Using simulated escape events to assess the annual numbers and destinies of farned escaped farmed Atlantic salmon of different life stages from farm sites in Norway. ICES Journal of Marine Science, 72: 670–685.
- Skilbrei O. T., Finstad B., Urdal K., Bakke G., Kroglund F., Strand R. 2013. Impact of early salmon louse (Lepeophtheirus salmonis) infestation, and differences in survival and marine growth of sea-ranched Atlantic salmon (Salmo salar) smolts 1997-2009. Journal of Fish Diseases, 36: 249–260.
- Skjelkvåle B. L., Evans C., Larssen T., Hindar A., Raddum G. G. 2003. Recovery from acidification in European surface waters: a view to the future. Ambio, 32: 170–175.
- Solberg M. F., Skaala O., Nilsen F., Glover K. A. 2013a. Does domestication cause changes in growth reaction norms? A study of farmed, wild and hybrid Atlantic salmon families exposed to environmental stress. PLOS One, 8: e54469.
- Solberg M. F., Zhang Z., Nilsen F., Glover K. A. 2013b. Growth reaction norms of domesticated, wild and hybrid Atlantic salmon families in response to differing social and physical environments. BMC Evolutionary Biology, 13: 234.
- Soleng A., Bakke T. A. 1997. Salinity tolerance of Gyrodactylus salaris (Platyhelminthes, Monogenea): laboratory studies. Canadian Journal of Fisheries and Aquatic Sciences, 54: 1837–1845.
- Soleng A., Bakke T. A., Hansen L. P. 1998. Potential for dispersal of Gyrodactylus salaris (Platyhelminthes, Monogenea) by sea-running stages of the Atlantic salmon (Salmo salar): field and laboratory studies. Canadian Journal of Fisheries and Aquatic Sciences, 55: 507–514.
- Soleng A., Poléo A. B. S., Alstad N. E. W., Bakke T. A. 1999. Aqueous aluminium eliminates Gyrodactylus salaris (Platyhelminthes, Monogenea) infections in Atlantic salmon. Parasitology, 119: 19–25.
- Sørensen J., Brodtkorb E., Haug I., Fjellanger J. 2013. Hydropower concessions that may be revise by 2022. National overview and priorities (in Norwegian). The Norwegian Water Resources and Energy Directorate Report, 49: 1–311.
- Stabell O. B. 1984. Homing and olfaction in Atlantic salmonids: a critical review with special reference to the Atlantic salmon. Biological Reviews, 59: 333–388.
- Stålnacke P., Aakerøy P. A., Blicher-Mathiesen G., Iital A., Jansons V., Koskiaho J., Kyllmar K., 2014. Temporal trends in nitrogen concentrations and losses from agricultural catchments in the Nordic and Baltic countries. Agriculture Ecosystems and Environment, 198: 94–103.
- Sterud E., Forseth T., Ugedal O., Poppe T. T., Jørgensen A., Bruheim T., Fjeldstad H. P., 2007. Severe mortality in wild Atlantic salmon Salmo salar due to proliferative kidney disease (PKD) caused by Tetracapsuloides bryosalmonae (Myxozoa). Diseases of Aquatic Organisms, 77: 191–198.
- Stich D. S., Bailey M. M., Holbrook C. M., Kinnison M. T., Zydlewski J. D. 2015. Catchment-wide survival of wild- and hatchery-reared Atlantic salmon smolts in a changing system. Canadian Journal of Fisheries and Aquatic Sciences, 72: 1352–1365.
- Sutherland W. J. 2006. Predicting the ecological consequences of environmental change: a review of the methods. Journal of Applied Ecology, 43: 599–616.
- Sutherland W. J., Burgman M. A. 2015. Use experts wisely. Nature, 526: 317–318.
- Svåsand T., Karlsen Ø., Kvamme B. O., Stien L. H., Taranger G. L., Boxaspen K. K. (eds.) 2016. Risk assessment of environmental impacts of Norwegian Atlantic salmon farming 2016, (in Norwegian). Fisken og havet, særnummer. 2-2016: 1–189 pp. Institute of Marine Research, Bergen.
- Tammi J., Appelberg M., Beier U., Hesthagen T., Lappalainen A., Rask M. 2003. Fish status survey of Nordic lakes: Effects of acidification, eutrophication and stocking activity on present fish species composition. Ambio, 32: 98–105.
- Taranger G. L., Karlsen Ø., Bannister R. J., Glover K. A., Husa V., Karlsbakk E., Kvamme B. O., 2015. Risk assessment of the environmental impact of Norwegian Atlantic Atlantic salmon farming. ICES Journal of Marine Science, 72: 997–1021.
- Thodesen J., Grisdale-Helland B., Helland S. J., Gjerde B. 1999. Feed intake, growth and feed utilization of offspring from wild and selected Atlantic salmon (Salmo salar). Aquaculture, 180: 237–246.
- Thorstad E. B., Todd C. D., Uglem I., Bjørn P. A., Gargan P. G., Vollset K. W., Halttunen E., 2015. Effects of Atlantic salmon lice on wild sea trout - a literature review. Aquaculture Environment Interactions, 7: 91–113.
- Thorstad E. B., Uglem I., Finstad B., Kroglund F., Einarsdottir I. E., Kristensen T., Diserud O., 2013. Reduced marine survival of hatchery Atlantic salmon post-smolts exposed to aluminium and moderate acidification in freshwater. Estuarine, Coastal and Shelf Science, 124: 34–43.
- Todd C. D., Friedland K. D., MacLean J. C., Hazon N., Jensen A. J. 2011. Getting into hot water? Atlantic salmon responses to climate change in freshwater and marine environments. InAtlantic Salmon Ecology, pp. 409–444. Ed. by Aas Ø., Einum S., Klemetsen A., Skurdal J.. Wiley-Blackwell, Oxford, UK.
- Torrissen O., Jones S., Asche F., Guttormsen A., Skilbrei O. T., Nilsen F., Horsberg T. E., 2013. Salmon lice - impact on wild salmonids and salmon aquaculture. Journal of Fish Diseases, 36: 171–194.
- Ugedal O., Næsje T. F., Thorstad E. B., Forseth T., Saksgård L. M., Heggberget T. G. 2008. Twenty years of hydropower regulation in the River Alta: long-term changes in abundance of juvenile and adult Atlantic salmon. Hydrobiologia, 609: 9–23.
- Valdimarsson S., Metcalfe N. 1998. Shelter selection in juvenile Atlantic salmon, or why do salmon seek shelter in winter?. Journal of Fish Biology, 52: 42–49.
- Verspoor E., Strandmeyer L., Nielsen L. 2007. The Atlantic salmon. Genetics, Conservation and Management. Blackwell Publishing Ltd., Oxford.
- Vøllestad L. A., Skurdal J., L'Abée-Lund J. H. 2014. Evaluation of a new management scheme for Norwegian Atlantic salmon Salmo salar. Fisheries Management and Ecology, 21: 133–139.
- Vollset K. W., Barlaup B. T., Skoglund H., Normann E. S., Skilbrei O. T. 2014. Salmon lice increase the age of returning Atlantic salmon. Biology Letters, 10: 20130896.
- Vollset K. W., Krontveit R. I., Jansen P., Finstad B., Barlaup B. T., Skilbrei O. T., Krkošek M., 2016. Impacts of parasites on marine survival of Atlantic salmon: a meta-analysis. Fish and Fisheries, 17: 714–730.
- Ward D. M., Hvidsten N. A. 2011. Predation: Compensation and context dependence. InAtlantic Salmon Ecology, pp. 199–220. Ed. by Aas Ø., Einum S., Klemetsen A., Skurdal J.. Wiley-Blackwell, Oxford, UK.
- Ward D. M., Nislow K. H., Folt C. L. 2008. Predators reverse the direction of density dependence for juvenile salmon mortality. Oecologia, 156: 515–522.
- Water Resources Act, 2000. https://lovdata.no/dokument/NL/lov/2000-11-24-82 (last accessed 1 December 2016).