Myasthenia gravis (MG) is an auto-immune disorder affecting the neuro-muscular junctions of skeletal muscles; 90% of MG patients present initially as ocular myasthenia.[] There is no established gold standard test for the diagnosis of ocular myasthenia.[] Ocular myasthenia can mimic cranial nerve palsy, inter-nuclear ophthalmoplegia, or thyroid eye disease.[]
Edrophonium test is often used for confirmation and has a specificity of 97% but may be complicated by bradycardia and bronchiolar constriction,[] Single-fiber electromyogram (SFEMG) is sensitive and specific for ocular myasthenia but is not widely available.[] Acetylcholine receptor (AChR) antibody testing and repetitive nerve stimulation (RNS) tests are highly specific but less sensitive in ocular myasthenia (approximately 50%) compared with generalized MG (85%–90%).[]
Our clinical experience suggests that easily available clinical bedside tests—forced eyelid closure test (FECT) and ice pack test (IPT)—have more diagnostic accuracy in cases of ocular myasthenia compared to the laboratory tests, namely RNS and AChR antibodies. We also noted an association between AChR antibody positivity and progression from ocular myasthenia to generalized disease. To test these hypotheses, we attempted the following:
Compare the diagnostic accuracy of clinical tests (FECT and IPT) and laboratory tests (RNS and AChR antibody test) in patients with suspected ocular myasthenia
Assess the clinical utility of AChR antibody test in predicting disease progression
Methods
A retrospective cohort design was used. This is a single-center study at a neuro-ophthalmology clinic in a tertiary eye hospital. Following approval by the institutional review board, medical records were searched to identify patients diagnosed with ocular myasthenia from January 2016 to July 2017. Follow up period was 36 months from the time of diagnosis till July 2020. All patients included in this study have given written informed consent to utilize their de-identified data for research purposes. Our study adhered to the tenets of the Declaration of Helsinki.
Data abstracted for each patient included age, gender, ocular symptoms (ptosis and/or diplopia), duration of follow-up, and progression time to generalized MG (if this occurred).
All patients with suspected ocular myasthenia referred to neuro-ophthalmology clinic underwent the following clinical evaluation:
Best-corrected visual acuity
Anterior and posterior segment examination using torchlight, slit-lamp biomicroscope, and + 90D fundoscopy lens
9 gaze extraocular movement assessment
Hess and diplopia chart
Complete neurological exam
Fatigability test: Fatigability phenomenon was tested using forced eyelid closure test (FECT). To perform FECT, the patient was asked to squeeze his or her eyelids shut for 5–10 s and then open quickly and fixate in the primary position. The excessive upwards overshoot of eyelids movement indicated a positive FECT.
Ice pack test: The ice pack was held in place against the eyelids for 2 min. The evaluation was performed by recording the upper MRD with a millimeter ruler, immediately before and after applying the ice pack. The test was considered positive if there was an improvement of more than 2 mm of MRD or symptomatic relief in diplopia.
Intraocular pressure using noncontact tonometer
Blood pressure- manual sphygmomanometer
Following a probable clinical diagnosis of ocular myasthenia based on the above tests, patients were referred for the following tests:
A)
RNS: The facial nerve supplying orbicularis oculi was electrically stimulated 6–10 times at 3 or 5 Hz, and the compound muscle action potential (CMAP) was recorded with surface electrodes. Decremental response in CMAP greater than 10% was considered to be indicative of ocular myasthenia.
B)
AchR antibodies - serum testing for the AChR binding antibody (muscle AChR complexed with 125I-labeled-a-bungarotoxin; Lister Laboratories and Diagnostics), with values > 0.02 nmol/L considered a positive result
C)
CT chest to rule out thymoma
In addition, thyroid function test and blood glucose were also done. Neuro-imaging was not routinely done in all patients unless indicated. Single-fiber electromyogram (SFEMG) and MUSK antibody test were not done in our patients due to nonavailability of these tests.
The diagnosis of ocular myasthenia was made when at least one of the standard diagnostic tests for myasthenia gravis was positive in the absence of other causes. These included:
Presence of AChR antibodies
Decrement >10% of the third to fifth compound muscle action potential with respect to the first after RNS
Positive intramuscular neostigmine test (done when other tests are inconclusive)
Unequivocal response to oral steroid and/or acetylcholinesterase inhibitors for a period of at least 3 months
The results of FECT and IPT were not used for establishing the final diagnosis.
Patients received treatment under the combined care of a neurologist and a neuro-ophthalmologist. Following confirmation of diagnosis, patients were started on pyridostigmine, an acetylcholinesterase inhibitor (AChEI) as first-line therapy. Patients not responding to pyridostigmine were switched to low dose alternate days oral corticosteroids (prednisone 20 mg/day) with or without continued pyridostigmine. Patients who could not tolerate long-term steroids were initiated on immune suppressants including azathioprine or mycophenolate mofetil. All patients were followed up for a period of 36 months to monitor progression to generalized myasthenia. Of the 68 patients, 46 patients required steroids in addition to AChEI for adequate symptomatic relief.
Sample size
Given the absence of validated methods for a priori sample size estimate in the context of retrospective studies, we used past years’ estimates of population size and population proportion to arrive at a sample size.
Population proportion: 4.4%
Population size: 2500
Sample size needed to have a CI of 95% and margin of error 5%: 63 or more.
Statistical analysis
Continuous variables are presented as mean ± standard deviation (SD), and categorical variables as absolute and relative frequency (%). Fisher’s exact test was used to evaluate the univariate association between the results of test combination and other variables. Results are presented as sensitivity, specificity, positive predictive value (PPV), and negative predictive value (NPV) with respective 95% confidence intervals (CI). ROC analyses were used to assess the diagnostic accuracy of FECT, IPT, and RNS. DeLong test was used to compare the area under the curve (AUC) of the different tests and their combination with the reference test. Bonferroni correction was applied when appropriate. Multiple logistic regression was used to measure the association between all measured clinical variables and antibody result. Progression to generalized MG was compared by age and sex as well as antibody status with Kaplan–Meier estimates and log-rank tests. Statistical analysis was performed using statistical package Stata SE, 14.2.
Results
Population
During the study period, 112 patients with suspected ocular myasthenia were referred from the general ophthalmology outpatient services to our neuro-ophthalmology clinic. Of these, 68 patients were included in the study. Following were excluded:
Thirteen patients had other diagnoses (vasculopathic cranial nerve palsy: 5, chronic progressive external ophthalmoplegia: 1, Internuclear ophthalmoplegia: 3, cavernous sinus Tolossa Hunt syndrome: 1, thalamic lacunar infarct: 1, gaze palsy: 2).
Nineteen patients did not complete the 36-months follow-up.
Forced eyelid closure test was not documented in six patients.
Nine patients had not undergone antibody testing.
Three patients were in the pediatric age group. Patients less than 18 years were not included in the study.
Of the 68 patients included, 43 (63.2%) were males and 25 (36.8%) females. The mean (SD) age at the time of diagnosis was 48.96 years. Presenting symptoms included ptosis in 10 (14.7%), diplopia in 24 (35.3%), and ptosis and diplopia in 34 (50%) [Table 1].
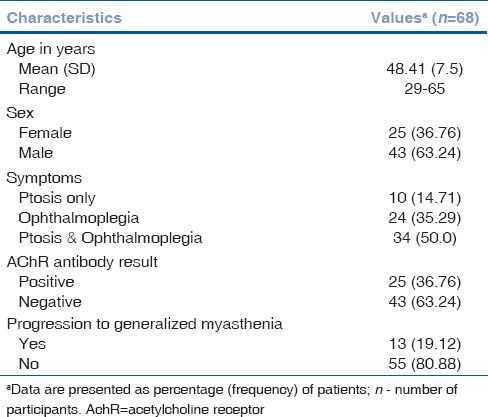
Table 1
Patient characteristics
Diagnostic yield of clinical and laboratory tests
The sensitivity, specificity, and positive predictive value of various tests are given in Table 2.
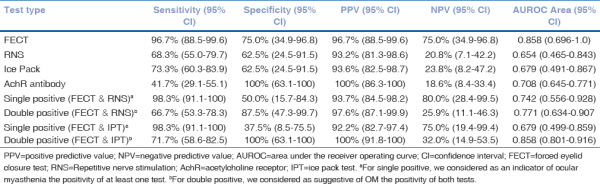
Table 2
Accuracy of FECT, IPT, RNS, and AchR antibody test in patients with ocular myasthenia
FECT was positive in 64 (94.1%) patients. IPT was positive in 49 (72.1%) patients. IPT’s positivity was more frequent in patients with isolated ptosis compared to patients with diplopia and ptosis (97.3% vs. 83.6%, P = 0.04) and in those with severe ptosis compared to patients with moderate (100% vs. 84.2%, P = 0.002) or mild (100% vs. 58.8%, P < 0.0001) ptosis. Patients with isolated diplopia showed no response to IPT. RNS showed decremental response in 44 (64.7%) patients. AChR antibody was positive in 25 (36.8%) patients. Sixty patients responded to the initial AChEI and the remaining eight responded to steroid therapy.
The majority of cases had at least one diagnostic criteria before receiving therapy; however, in up to 11.7% of cases, the diagnosis was based only on the response to steroid therapy and the absence of other identifiable causes.
FECT and IPT in patients with double-negative RNS and AChR-abs
As the FECT and IPT can be especially useful in those patients with suspected ocular myasthenia who have negative RNS and AChR-abs, we evaluated the accuracy of FECT, IPT, and combined FECT and IPT (at least one positive) in these patients [n = 15; Table 3]. In this population of double negative RNS and AChR antibody, the sensitivity and specificity of FECT was 100% (95% CI: 69.2–100) and 80% (95% CI: 28.4–99.5), respectively; Sensitivity and specificity of IPT was 70% (95% CI: 34.8–93.3) and 40% (95% CI: 5.3–85.3), respectively.
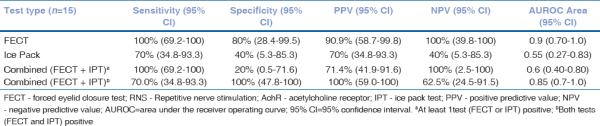
Table 3
FECT and IPT in patients with double negative RNS and AchR antibodies
Specificity of combined FECT and IPT (at least one positive) was 20% (95% CI: 0.5–71.6); However, specificity of combined FECT and IPT (both positive) was 100% (95% CI: 63.1–100).
Utility of AChR antibody in ocular myasthenia
Acetylcholine receptor antibody test results were positive in 25 (36.8%) patients. Thirteen (19.1%) patients out of the 68 patients developed generalized MG, out of which AChR antibody was positive in 11 (16.2%) patients at the time of diagnosis. All patients included were followed up for 36 months.
Patients who developed generalized symptoms were more likely to have a positive AChR antibody test result (OR: 25.07; 95% CI: 4.08–154.06; P = 0.001). Presentation with diplopia, ptosis, or both did not significantly predict antibody status. Age at diagnosis [OR: 1.03, 95% CI: 0.95–1.12, P = 0.455), gender (OR: 0.37, 95% CI: 0.09–1.46, P = 0.155), and combined variable looking at the interaction of age and sex did not significantly predict positive antibody status [Table 4]. None of our patients included in our study had symptoms of generalized myasthenia at presentation. Kaplan–Meier curves for progression to generalized MG based on antibody status are illustrated in Fig. 1. The log-rank test was significant for antibody status (P = 0.001) but not for age (P = 0.455) or sex (P = 0.155).
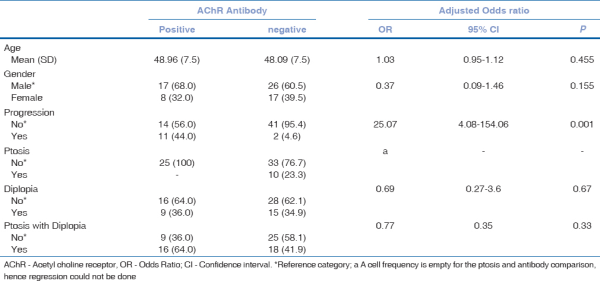
Table 4
Multiple logistic regression predicting acetylcholine antibody positivity
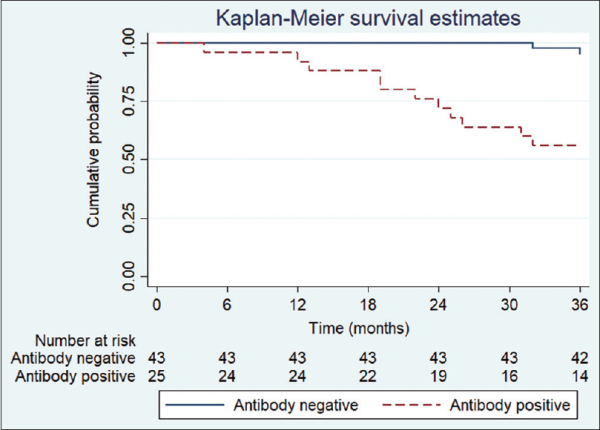
Figure 1
Proportion of patients by antibody status with ocular myasthenia that did not progress to generalized myasthenia (n = 68). Antibody status was significant by the log-rank test (P < 0.001)
Table 5 shows the distribution of patients according to FECT and treatment response. The presence of double positivity was significantly associated with the presence of AchR antibodies.
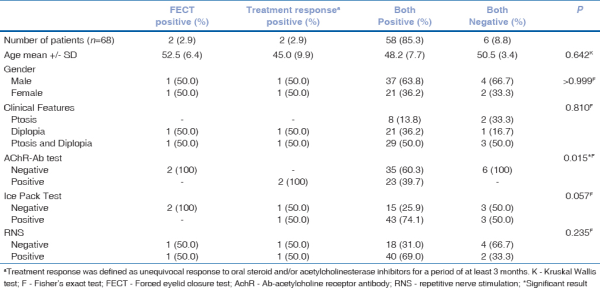
Table 5
Distribution of ocular myasthenia cases according to forced eyelid closure test and treatment response
Discussion
Around 50%–85% of patients with ocular myasthenia progress to generalized myasthenia; 90% of these patients progress within 2 years of onset of the disease.[] Currently available diagnostic tests, including RNS, SFEMG, and AChR-Abs, have higher diagnostic accuracy in cases of generalized myasthenia but show varying levels of sensitivity in ocular myasthenia.
In our clinical experience, we find the clinical bedside noninvasive tests, including forced eyelid closure test and ice pack test, to be of more diagnostic value in identifying ocular myasthenia. To test this hypothesis, we conducted this study. We also wanted to assess the clinical utility of AChR antibody testing in predicting disease progression.
Forced eyelid closure test (FECT) was first described by Don C. Bienfang, MD, a neuro-ophthalmologist at Harvard Medical School, in 1982. This is a lesser-known test compared to Cogan’s lid twitch and ice pack test for clinical screening of ocular myasthenia.
Validity of FECT has been studied by Apinyawasisuk et al.[] in 2018 and was proven to be a sensitive and specific tool in screening ocular myasthenia. In our study, we found that FECT has the highest diagnostic yield compared to IPT, RNS, and AChR antibody test. Table 2 compares the sensitivity, specificity, and positive and negative predictive values of the clinical and laboratory tests used in this study. Treatment response was used as the reference standard to compare these tests [Fig. 2] as there is no established gold standard test for diagnosis of ocular myasthenia. Treatment response was defined as unequivocal response to oral steroid and/or acetyl cholinesterase inhibitors for a period of at least 3 months.
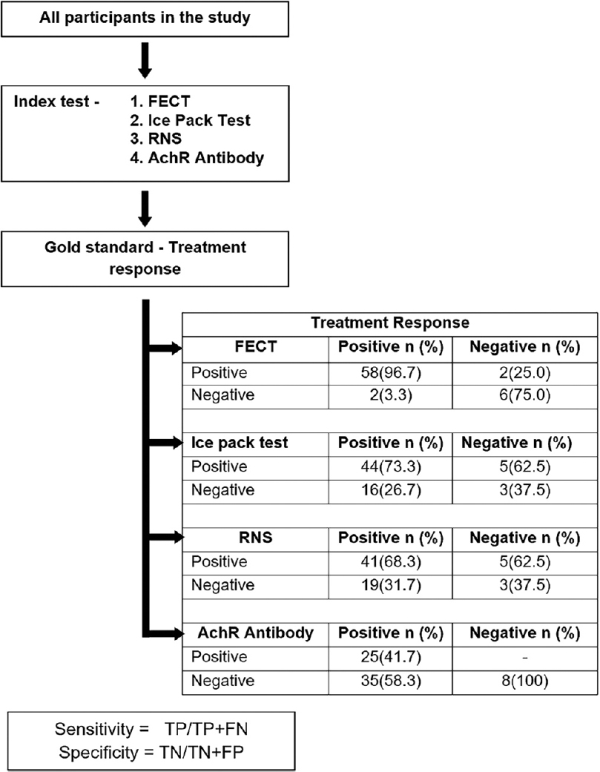
Figure 2
Comparison of the sensitivity, specificity, and positive and negative predictive values of the clinical and laboratory tests used in this study. Treatment response was used as the reference standard to compare these tests. Treatment response was defined as unequivocal response to oral steroid and/or acetylcholinesterase inhibitors for a period of at least 3 months. FECT = forced eyelid closure test; RNS = repetitive nerve stimulation; AchR = acetylcholine receptor
Sensitivity of FECT was 96.7% (95% CI: 88.5–99.6) with a specificity of 75% (95% CI: 34.9–96.8) and a positive predictive value of 96.7% (95% CI: 88.5–99.6). Combined FECT and IPT (at least one positive) had the highest sensitivity of 98.3% (95% CI: 91.1–100) with a positive predictive value of 92.2% (95% CI: 82.7–97.4). However, the sensitivity of combined FECT and IPT (both positive) was 71.7% (95% CI: 58.6–82.5). However, the specificity when both FECT and IPT were positive was 100% (95% CI: 63.1–100) with a positive predictive value of 100% (95% CI: 91.8–100).
False-negative rate of FECT was low in our study. Out of the 68 patients, four patients had false-negative FECT. At the time this false-negative FECT was performed, two patients had already received steroids from elsewhere for diplopia and one patient had dermatochalasis. False-positive FECT was noted in two patients whose final diagnoses were decompensated phoria and Lambert–Eaton myasthenia syndrome, respectively.
The sensitivity and the specificity of IPT in our cohort were 73.3% (95% CI: 60.3–83.9) and 62.5% (95% CI: 24.5–91.5), respectively. A study by Giannoccaro et al.[] reported that IPT and SFEMG have a similar diagnostic accuracy in patients with ocular myasthenia presenting with ptosis. Though IPT is shown to be equally accurate to SFEMG, it can be demonstrated only in patients presenting with ptosis. IPT cannot be used to screen patients presenting with isolated diplopia.
Sensitivity of RNS was 68.3% (95% CI: 55.0–79.7) with a positive predictive value of 93.2% (95% CI: 81.3–98.6). No statistical significance was found between the ROC curve of FECT, IPT, and RNS compared to the reference standard (treatment response).
A subset of 15 patients in our study was double negative for RNS and AChR-Ab.
The FECT and IPT still showed good diagnostic accuracy in these patients with normal RNS and negative AChR antibodies. FECT was positive in all 15 patients in this subset. Sensitivity of IPT was 70% (95% CI: 34.8–93.3). The positive predictive value of combined FECT and IPT (double-positive) was 100% (95% CI: 59.0–100). The negative predictive value of combined FECT and IPT (at least one positive) was 100% (95% CI: 2.5–100), indicating that if both these tests are negative, the diagnosis of ocular myasthenia is highly unlikely. Therefore, either the negativity or the positivity of both tests significantly changed the post-test probability of the diagnosis even in patients with double negative RNS and AChR antibodies.
We would like to emphasize that FECT formerly known as Bienfang test is a simple, quick, noninvasive test and should be used as a valuable screening tool for ocular myasthenia. It is especially valuable in patients of suspected ocular myasthenia with normal RNS and negative antibodies. Its advantage over IPT is that it can also be demonstrated in patients without ptosis. It has a higher sensitivity compared to RNS and AChR-abs in cases of ocular myasthenia and is demonstrable even in early stages of the disease.
Previous estimates of the rate of antibody positivity in patients with ocular myasthenia vary from 40% to 70%.[] Our result of 41% (95% CI: 29.1–55.1) sensitivity falls at the lower limit range. A possible explanation for our lower rate of AChR antibody sensitivity lies in the association between antibody levels and the duration of MG symptoms. Vincent and Newsom-Davis suggested that the high affinity of antibodies for end-plate AChRs at the neuromuscular junction could explain why some patients with OMG have negative serum test results early in the course of the disease but later seroconvert.[] It is not until an individual reaches a certain level of receptor-binding saturation that antibodies begin to freely circulate in the peripheral blood. Because many of our patients were seen in the early stages of the disease, it is possible that they had lower levels of circulating antibodies for detection.
Although the sensitivity of AChR-Abs was found to be low in our study, a significant effect of antibody positivity on the risk of progression to generalized MG was evident in our cohort based on the Kaplan–Meier estimate [Fig. 1] and log-rank tests. Kupersmith et al.[] and Peeler et al.[] also reported a significant association between positive AChR antibody test results in ocular myasthenia and the risk of progression to generalized MG.
In our study of 68 patients, 13 progressed to generalized myasthenia within the follow-up period of 36 months. Among the 13 patients, progression was noted as early as month 4 since the time of initial diagnosis in only one patient. The remaining patients showed progression between months 12–36 from the time of initial diagnosis. Rate of progression in our cohort was 19% at the end of 3 years follow-up as opposed to the existing literature that states that 50%–80% of ocular myasthenia will progress to generalized myasthenia among which 90% will progress within 2 years. The reason for lower rate of progression might be because of early initiation of corticosteroids in our patients.
Currently, there is limited evidence favoring the use of immune modulators in treating ocular myasthenia. Various studies on the effect of prednisone on the progression of ocular to generalized myasthenia have stated that the early use of steroids decreases the progression in these patients.[]
In our cohort of 68, 13 patients progressed to generalized myasthenia despite early AChEI/corticosteroid therapy. Out of these 13, 11 had AChR-Abs positivity at the time of diagnosis. We believe that this finding adds clinical value to antibody testing and will improve its utility as a prognostic tool. Although the sensitivity of antibody testing in ocular myasthenia is variable, it has a significant predictive value in disease progression, suggesting that patients with ocular myasthenia and positive AChR-abs require closer follow-up with consideration for early immunosuppressant therapy.
Conclusion
There are various limitations in our study. SF-EMG results could not be analyzed due to its nonavailability. The positivity and negativity of FECT were determined by a single neuro-ophthalmologist. Inter-observer reliability needs to be addressed in future studies. There is no generally accepted gold standard test for ocular myasthenia. Using an imperfect reference standard produces reference standard bias. To evaluate diagnostic tests with no existing reference standard, applying the concept of clinical test validation can provide a significant methodological advantage over the traditional test accuracy paradigm. In our study, we used treatment response as the reference standard to arrive at the diagnostic accuracy of FECT, IPT, RNS, and AChR-antibody test.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
Acknowledgements
The authors would like to thank Mr. Lakshmanan, statistician, Aravind eye care, for his support.
References
1
Grob D, Arsura EL, Brunner NG, Namba T The course of myasthenia gravis and therapies affecting outcome Ann N Y Acad Sci 1987 505 472 992
Kusner LL, Puwanant A, Kaminski HJ Ocular myasthenia: Diagnosis, treatment, and pathogenesis Neurologist 2006 12 231 93
Antonio-Santos AA, Eggenberger ER Medical treatment options for ocular myasthenia gravis Curr Opin Ophthalmol 2008 19 468 784
Kerty E, Elsais A, Argov Z, Evoli A, Gilhus NE EFNS/ENS guidelines for the treatment of ocular myasthenia Eur J Neurol 2014 21 687 935
Foorozan R, Bhatti MT, Falardeau J, Gordon LK, Lee MS, Subramanian PS Basic and Clinical Science Course Volume 5: Neuro-Ophthalmology 2013 San Francisco American Academy of Ophthalmology6
Padua L, Stalberg E, LoMonaco M, Evoli A, Batocchi A, Tonali P SFEMG in ocular myasthenia gravis diagnosis Clin Neurophysiol 2000 111 1203 77
Vincent A, Newsom-Davis J Acetylcholine receptor antibody as a diagnostic test for myasthenia gravis: Results in 153 validated cases and 2967 diagnostic assays J Neurol Neurosurg Psychiatry 1985 48 1246 528
Costa J, Evangelista T, Conceição I, de Carvalho M Repetitive nerve stimulation in myasthenia gravis –Relative sensitivity of different muscles Clin Neurophysiol 2004 115 2776 829
Grob D, Brunner N, Namba T, Pagala M Lifetime course of mysasthenia gravis Muscle Nerve 2008 37 141 910
Bever CT Jr, Aquino AV, Penn AS, Lovelace RE, Rowland LP Prognosis of ocular myasthenia Ann Neurol 1983 14 516 911
Apinyawasisuk S, Zhou X, Tian JJ, Garcia GA, Karanjia R, Sadun AA Validity of forced eyelid closure test: A novel clinical screening test for ocular myasthenia gravis J Neuroophthalmol 2017 37 253 712
Giannoccaro MP, Paolucci M, Zenesini C, Di Stasi V, Donadio V, Avoni P, et al. Comparison of ice-pack test and single fiber EMG diagnostic accuracy in patients referred for myasthenic ptosis Neurology 2020 95 e1800 613
Mayo Medical Laboratories, Mayo Clinic Test ID: ARBI: Acetylcholine receptor (muscle AChR) binding antibody, serum Available from: http://www.mayomedicallaboratories.com/test-catalog/Clinical+and+Interpretive/8338 Last accessed on 2014 Sep 2114
Vincent A, Newsom-Davis J Acetylcholine receptor antibody characteristics in myasthenia gravis, I: Patients with generalized myasthenia or disease restricted to ocular muscles Clin Exp Immunol 1982 49 257 6515
Kupersmith MJ, Latkany R, Homel P Development of generalized disease at 2 years in patients with ocular myasthenia gravis Arch Neurol 2003 60 243 816
Peeler CE, De Lott LB, Nagia L, Lemos J, Eggenberger ER, Cornblath WT Clinical utility of acetylcholine receptor antibody testing in ocular myasthenia gravis JAMA Neurol 2015 72 1170 417
Monsul NT, Patwa HS, Knorr AM, Lesser RL, Goldstein JM The effect of prednisone on the progression from ocular to generalized myasthenia gravis J Neurol Sci 2004 217 131 318
Bhanushali MJ, Wuu J, Benatar M Treatment of ocular symptoms in myasthenia gravis Neurology 2008 71 1335 41
