INTRODUCTION
Sperm defects are a major cause of male infertility. Epidemiological data indicate that there has been a notable decrease in sperm count over recent years.[] The consequences of sperm defects are profound, encompassing reduced fertility, impaired embryonic development, and an increased risk of genetic diseases, psychological stress, and overall health issues in males.[] Sperm formation and maturation are the results of a lengthy and complex process known as spermatogenesis. During spermatogenesis, sperm are susceptible to various internal and external stimuli, leading to adverse outcomes primarily manifested as reduced sperm motility and concentration, which are characteristics of oligospermia and asthenozoospermia, respectively.[] Reduced sperm count and decreased sperm motility often clinically co-occur as oligoasthenozoospermia (OAZS). Idiopathic OAZS is more common, as there is often no clear specific cause or pathophysiological mechanism to explain its occurrence. Currently, the major treatment for idiopathic OAZS is drug therapy, but its efficacy is variable and uncertain. Therefore, there is an urgent need to develop novel therapeutic drugs and uncover the mechanisms underlying their efficacy in treating OAZS.
An increasing number of studies have indicated that Chinese medicine holds great promise for treating male infertility.[] Yi-jing decoction (YJD) is a traditional Chinese medicine prescription formulated by Professor Jia Jinming of the Guang’anmen Hospital China Academy of Chinese Medical Sciences. This formulation helps treat OAZS by tonifying the kidneys and increasing blood circulation, according to Chinese medicine theory. Our previous clinical research validated YJD’s efficacy in improving sperm motility, density, and morphology.[] However, the specific action mechanism is unclear. YJD contains 12 Chinese medicinal materials, including Schisandra chinensis, Epimedium, Angelica sinensis, safflower, Semen cuscutae, prepared rehmannia root, mulberry, Rhizoma atractylodis, Cistanche deserticola, chive seed, Polygonatum sibiricum red, and Polygonatum odoratum. Some of those herb extracts appear to have the potential to protect against oxidative stress (OS) injury. The extracts of Schisandra chinensis, such as phenolic molecules and schisandrin, have been shown to protect mammalian sperm from oxidative damage and preserve their functional activity.[] Icaridin, a bioactive compound of Epimedium, can also protect human sperm cells from OS-induced damage.[,] Therefore, YJD shows promise as a predominant drug for treating OAZS, with its exact mechanism potentially related to the inhibition of OS.
It is well known that one of the major causes of reduced sperm quality is OS.[] Approximately 30%-80% of males with infertility have been shown to have elevated levels of reactive oxygen species (ROS) in their seminal fluid.[] This finding underscores the potential role of OS in male infertility. Adequate ROS levels are critical for maintaining sperm quality because they facilitate a variety of sperm tasks, such as triggering acrosomal responses, regulating flagellar motility, and mediating sperm-egg interactions. OS is formed when ROS production exceeds their scavenging capacity, and it can decrease sperm quality by controlling mitochondrial damage and apoptosis pathways.[,] This injury can occur throughout the spermatogenesis process. Research has demonstrated that OS can induce Leydig cell apoptosis and decrease testosterone production by disrupting the mitochondrial membrane structure and reducing membrane sites.[] It can also trigger Sertoli cell apoptosis, impacting the growth environment of spermatogenic cells and impeding spermatogenesis.
In the mammalian testis, mitogen-activated protein kinase (MAPK) is considered an important determinant of sperm development, and OS can regulate cellular activities through the activation of the MAPK signaling pathway.[,] p38MAPK, a crucial branch of the MAPK family, plays a significant role in mediating OS and activating the mitochondrial apoptosis pathway. Previous studies have shown that p38MAPK is involved not only in the regulation of sperm motility and the transcriptional repression of the steroidogenic acute regulatory (StAR) gene to regulate steroidogenesis but also in the apoptosis of Sertoli cells.[] Therefore, this study aims to explore the mechanism by which YJD improves OAZS through the OS-p38MAPK-mitochondrial apoptosis pathway using animal and cell experiments.
According to the aforementioned analysis, it is hypothesized that YJD alleviates OAZS by suppressing the mitochondrial apoptosis triggered by OS. This study elucidates the involvement of p38MAPK as a mediator in this pathway through related literature, which is subsequently confirmed through both in vivo and in vitro experiments.
METHODS
Herb preparation
The 12 herbs constituting YJD were provided and prepared by the Department of Pharmacy at Guang’anmen Hospital at the China Academy of Chinese Medical Sciences as aqueous decoctions at concentrations of 0.525 g/mL, 1.05 g/mL, and 2.1 g/mL. These three concentrations were used as the standards for the low-, medium-, and high-dose groups in later stages of the study.
Animals and treatment
Seventy-five male Bagg’s albino (BALB)/C mice (8 weeks old, 18-20 g) were obtained from Spero (Beijing) Biotechnology Co., Ltd. (Beijing, China; license No: SCXK [Jing] -2019-0010). The mice were maintained at 22 ± 1ºC with 60% relative humidity under a 12-h light/dark cycle and had free access to water and a standard laboratory rodent diet. After a 1-week adaptation period, the mice were randomly assigned to five groups (n = 15 per group): control, model (cyclophosphamide [CTX]), low-dose YJD, medium-dose YJD, and high-dose YJD. OS-related OAZS was induced in the model and YJD groups via intraperitoneal injections of CTX at 60 mg/kg for five consecutive days,[] while the control group received physiological saline (NS) at 120 mg/kg. From the 6th day, the three dose groups were given YJD oral gavage at a dose of 10 mL/kg/day at low, medium, and high drug concentrations for 5 consecutive weeks. These doses were approximately 0.5, 1.0, and 2.0 times, respectively, the daily clinical equivalent dose for a 60-kg person. On the second day following the end of treatment, blood was collected via eye enucleation between 7 a.m. and 9 a.m. The samples were centrifuged to separate the serum, which was then stored at -80°C. The bilateral testes and epididymides were quickly excised and weighed after removing the blood vessels, ligaments, and adipose tissue. The right epididymis was used to assess sperm motility and count, and the left epididymis and both testes were stored at -80°C for subsequent Western blotting and enzyme-linked immuno sorbent assay (ELISA) analysis. All protocols in this study were approved by the Institutional Animal Care and Use Committee (IACUC) of Guang’an Men Hospital, China Academy of Chinese Medical Sciences (IACUC Issue No. IACUC-GAMH-2024-009), in compliance with the Guide for the Care and Use of Laboratory Animals published by the US National Institutes of Health (NIH publication No. 85-23, revised 1996).
Assessment of sperm count and motility
The epididymal tail was placed in a culture dish containing Dulbecco’s Modified Eagle Medium (DMEM) and longitudinally dissected. After thorough washing with DMEM, the epididymal tissue was removed, and the supernatant containing sperm cells was incubated at 37ºC for 20-30 min.[] Subsequently, the sperm suspension was transferred into a white blood cell counting chamber, and 200 sperm cells were counted across 5-10 random fields. Three types of sperm were categorized—immotile, forward-moving (either slowly or swiftly), and nonforward-moving—with only the motile sperm considered viable. The percentage of motile sperm was calculated for each sample to determine the motility rate.
Biochemical analyses
In this study, various methods were employed to investigate the effects of OS on the morphology and function of mouse testicular tissue and germ cells. Paraffin sections of mouse testicular tissue were prepared and stained with hematoxylin and eosin (HE), and the morphology of the seminiferous tubules was observed using optical microscopy. Mitochondrial membrane potential (MMP) in testicular tissue cells was measured using the 5,5ʹ,6,6ʹ-Tetrachloro-1,1ʹ,3,3ʹ-tetraethyl-imidacarbocyanine iodide (JC-1) staining method. The ultrastructure of the seminiferous tubules and mitochondrial morphology was examined using transmission electron microscopy (TEM). Serum levels of follicle-stimulating hormone (FSH), luteinizing hormone (LH), free testosterone (FT), and total testosterone (TT) were quantified using specific ELISA kits (FANKEW). Additionally, OS markers, including ROS, superoxide dismutase (SOD), malondialdehyde (MDA), and glutathione peroxidase (GSH-Px), were assessed in testicular and epididymal tissues using specific ELISA kits. The optical density of the samples was measured at 450 nm within 15 min of the reaction using an ELISA reader.
Preparation of the YJD-containing serum
Eight adult male Wistar rats (weighing 250-280 g) were randomly assigned to either the YJD or control groups. Rats in the YJD group were administered YJD via oral gavage at a dose of 10 mL/kg body weight twice daily for 7 days. Rats in the control group received an equivalent volume of saline. After 7 days, blood samples were collected and left undisturbed at room temperature for 2 h before centrifugation for 20 min to obtain serum. The serum was then heat inactivated at 56°C for 30 min, filtered through a 0.22 µm membrane, and stored at -80°C.
Cell culture and treatment
The murine TM3 Leydig cell line was obtained from the Beijing Union Medical College Cell Resource Center. Primary Sertoli cells were isolated from mouse testicular tissues through physical disintegration and enzymatic digestion, and their purity was assessed using flow cytometry following staining with anti-FasL antibody (Abcam, ab134401). Both the TM3 Leydig cells and Sertoli cells were cultured in DMEM supplemented with 10% fetal bovine serum (FBS) at 37ºC in a 5% CO2 and 95% air environment.
The OS model of TM3 and Sertoli cells was induced using 100 µmol/L acrolein (ACR). To confirm the important role of p38MAPK in YJD inhibiting the OS-mediated mitochondrial apoptosis pathway, we used siRNA interference to knock down p38MAPK in the TM3 and Sertoli cells for experimental validation. The specific strategy was as follows: Target cells were inoculated in 24-well plates and cultured overnight at 37ºC. Ten pmol siRNA was mixed with 2 µL siRNA-mate, and after standing for 10 min, the mixture was added to the wells with fresh culture medium, achieving a final siRNA concentration of 16.7 nmol/L. The transfection efficiency was detected using reverse transcription-polymerase chain reaction (RT-PCR) 24-48 h post-transfection, and the siRNA sequence with the highest transfection efficiency for p38MAPK was selected for subsequent tests.
For experimental purposes, cells were divided into six groups: control (untreated), ACR, ACR + empty vector (NC), ACR + p38 siRNA, ACR + NC + 10% YJD serum, and ACR + p38 siRNA + 10% YJD serum. The cells in the last two groups were cultured in 10% YJD serum for 12 h.
Western blotting
Western blot analysis was conducted using proteins extracted from testicular tissues, TM3 Leydig cells, and Sertoli cells, following standard protocols. Primary antibodies specific to p38MAPK, cytochrome c (Cyt-C), P450scc, StAR, androgen receptor (AR), androgen-binding protein (ABP; diluted 1: 1000), BCL-2-associated X protein (BAX), B-cell lymphoma-2 (Bcl-2), and caspase 3 (diluted 1: 2000) were utilized. Positive bands were visualized using an enhanced chemiluminescence reagent, and band densities were quantified using ImageJ software.
Statistical analysis
Statistical analyses were performed using SPSS 22.0 software, and GraphPad Prism was used for graph plotting. Data are presented as mean ± standard deviation (SD). Dunnett’s t-test and one-way analysis of variance (ANOVA) were employed to evaluate significant differences between groups, with P < 0.05 considered statistically significant.
RESULTS
YJD enhances reproductive parameters in the mouse model of OAZS
CTX administration significantly decreased testicular and epididymal indices in mice compared to the healthy controls [Figure 1A]. However, YJD treatment did not significantly affect reproductive organ indices. In addition, CTX significantly reduced sperm motility and density, both of which were dose-dependently restored by YJD within a specific range [Figure 1B].
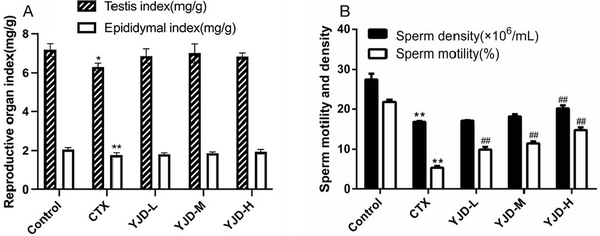
Figure 1.
YJD’s impact on reproductive parameters in mice with CTX-induced OAZS. (A) Reproductive organ index in the indicated groups. (B) Sperm quality and density in the indicated groups. Compared to the control group, *P < 0.05, **P < 0.01; compared to the CTX group, ##P < 0.01. YJD, Yi-jing decoction; CTX, cyclophosphamide; OAZS, oligoasthenozoospermia.
CTX administration elevated the serum levels of FSH and LH while reducing the TT and FT levels. However, YJD treatment restored all hormone levels in a dose-dependent manner [Figure 2].
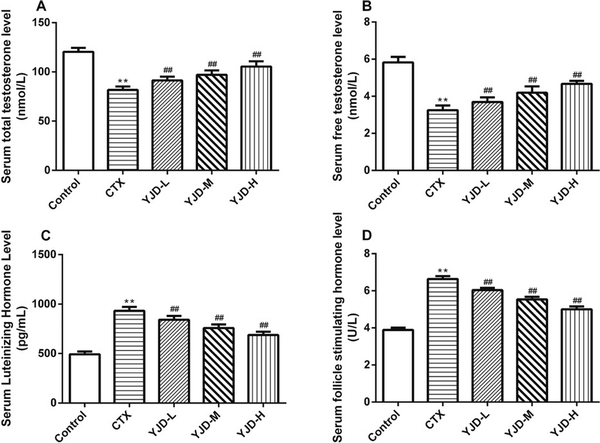
Figure 2.
Serum levels of (A) TT, (B) FT, (C) LH, and (D) FSH in the indicated groups. Compared to the control group, *P < 0.05, **P < 0.01; compared to the CTX group, ##P < 0.01. CTX, cyclophosphamide.
YJD decreases OS in the testes and epididymis of mice
Compared to the control group, CTX significantly increased the ROS and MDA levels in the testis and epididymis (P < 0.01), while the GSH-Px and SOD levels were significantly decreased (P < 0.01). Following administration of YJD across the three dose groups, the ROS and MDA levels in testicular tissue decreased significantly (P < 0.01), while the GSH-Px and SOD levels increased significantly (P < 0.01). These changes exhibited a dose-dependent trend but did not reach the normal levels observed in the control group. In epididymal tissue, the SOD levels significantly increased in all YJD dose groups compared to the model group (P < 0.01) and also showed a dose-dependent pattern. The MDA levels decreased (P < 0.05) and GSH-Px levels increased (P < 0.01) only in the medium-and high-dose groups, while the ROS levels decreased (P < 0.01) only in the high-dose group [Figure 3].
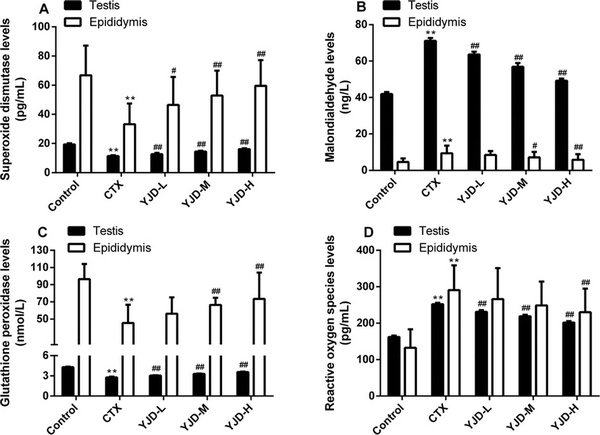
Figure 3.
YJD decreased OS in mice with CTX-induced OAZS. (A) SOD (ng/L). (B) MDA (ng/L). (C) GSH-Px (nmol/L). (D) ROS (ng/L). Compared to the control group, **P < 0.01; compared to the CTX group, #P < 0.05, ##P < 0.01. YJD, Yi-jing decoction; OS, oxidative stress; CTX, cyclophosphamide; OAZS, oligoasthenozoospermia; SOD, superoxide dismutase; MDA, malondialdehyde; GSH-Px, glutathione peroxidase; ROS, reactive oxygen species.
YJD ameliorates mitochondrial dysfunction and morphological damage in mouse testicular tissue induced by OS
As demonstrated in Figure 4A, the MMP levels in mouse testicular tissue cells were significantly reduced in the model group compared to the control group (P < 0.01). However, the MMP levels in all dose groups of YJD were significantly elevated relative to the model group (P < 0.01), with a dose-dependent increase observed. Figure 4B illustrates that the seminiferous epithelium and interstitium in the control group were normal, with orderly spermatogenic cells and visible mature sperm. In contrast, the model group exhibited disordered seminiferous epithelium, decreased spermatogenic cells, and an absence of sperm. The low-dose YJD group showed no significant improvement, while the medium- and high-dose YJD groups had partially restored morphology, approaching that of the control group. Ultrastructural analysis, as shown in Figure 4C, revealed mitochondrial swelling, cristae disappearance, vacuolization, increased lipid droplets, and lipofuscin deposition in the model group. In comparison, the low- and medium-dose YJD groups showed minimal differences, while the high-dose YJD group displayed near-normal mitochondrial morphology and structure. These findings underscore the protective effects of YJD against OS-induced damage in mouse testicular tissue.
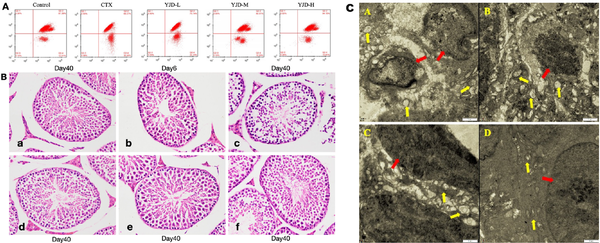
Figure 4.
Effects of YJD on testicular tissue and ultrastructure in mice. (A) MMP levels in testicular tissues across groups. (B) Light microscopy images of mouse testicular seminiferous tubules and interstitium (HE staining, ×400), a: Control; b: CTX-Day 6; c: CTX-Day 40; d: YJD-L; e: YJD-M; and f: YJD-H. (C) Mouse seminiferous tubule ultrastructure (TEM, ×11, 500): A: CTX; B: YJD-L; C: YJD-M; and D: YJD-H. Annotations: red arrow: cell nucleus; yellow arrow: mitochondria. MMP, Mitochondrial membrane potential; CTX, cyclophosphamide; YJD, Yi-jing decoction.
YJD alleviates apoptosis in mouse testicular cells and promotes testosterone synthesis and utilization
In the testicular tissues of the OAZS model, there was a significant increase in the relative expression levels of p38MAPK, BAX, Cyt-C, and caspase-3 proteins, as well as an elevated BAX/Bcl-2 ratio, accompanied by a decrease in the Bcl-2 protein levels [Figure 5A, B]. Additionally, CTX markedly suppressed the expression of StAR and cholesterol side-chain cleavage (P450scc) proteins [Figure 5C], which are essential enzymes in testosterone synthesis within the inner mitochondrial membrane. Correspondingly, the AR and ABP were downregulated in the model group [Figure 5D]. YJD treatment resulted in a dose-dependent restoration of these markers, with more pronounced effects observed at higher YJD concentrations. These findings indicate that YJD may enhance sperm quality by stimulating testosterone production in Leydig cells and optimizing testosterone utilization in Sertoli cells, achieved through the inhibition of the p38MAPK mitochondrial apoptosis pathway.
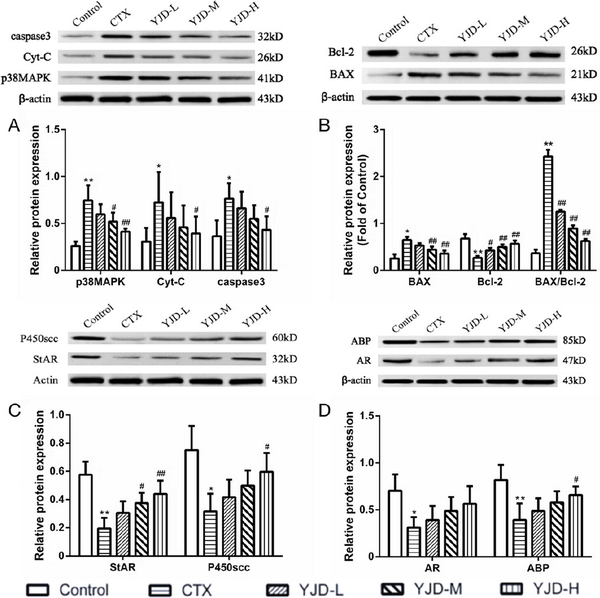
Figure 5.
YJD modulated the p38MAPK-mitochondrial apoptosis pathway and the pathways of testosterone synthesis and utilization in CTX-induced OAZS mice. Compared to the control group, *P < 0.05, **P < 0.01; compared to the CTX group, #P < 0.05, ##P < 0.01. YJD, Yi-jing decoction; CTX, cyclophosphamide; OAZS, oligoasthenozoospermia; MAPK, mitogen-activated protein kinase.
YJD inhibits OS by inhibiting the p38MAPK-mitochondrial apoptosis pathway in TM3 and Sertoli cells
As shown in Figure 6, ACR-induced OS significantly increased the relative expression levels of p38MAPK, Cyt-C, and caspase-3 (P < 0.01) as well as the BAX/Bcl-2 ratio (P < 0.01) in both cell types. Knockdown of the p38MAPK gene in TM3 and Sertoli cells resulted in a significant decrease in p38MAPK, Cyt-C, and caspase-3 expression (P < 0.05). However, the BAX/Bcl-2 ratio remained unchanged in TM3 cells after p38MAPK knockdown (P > 0.05) and increased in Sertoli cells (P < 0.01).
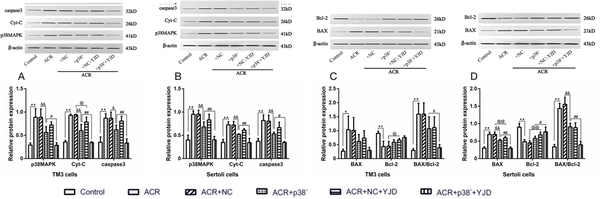
Figure 6.
YJD inhibited the p38MAPK mitochondrial apoptosis pathway in the TM3 and Sertoli cells in vitro. The relative expression levels of p38MAPK, Cyt-C, caspase-3, BAX, and Bcl-2 are shown in the indicated groups. Compared to the control group, *P < 0.05, **P < 0.01; compared to the ACR + p38- model group, @P < 0.05, @@P < 0.01; compared to the ACR + NC model group, &&P < 0.01; compared to the ACR + NC + YJD model group, #P < 0.05, ##P < 0.01. YJD, Yi-jing decoction; MAPK, mitogen-activated protein kinase; BAX, BCL-2-associated X protein; Bcl-2, B-cell lymphoma-2; ACR, acrolein; NC, empty vector.
Following 12 h incubation in YJD-containing serum, a significant reduction in the relative expression levels of p38MAPK, Cyt-C, and caspase-3 was observed in both TM3 and Sertoli cells (P < 0.05). However, the BAX/Bcl-2 ratio was decreased (P < 0.05). These findings collectively demonstrate that YJD attenuates ACR-induced OS and suppresses apoptosis in TM3 and Sertoli cells by modulating the mitochondrial p38MAPK pathway.
YJD regulates testosterone synthesis and utilization by inhibiting the p38MAPK-mitochondrial apoptosis pathway in TM3 and Sertoli cells
In contrast to the in vivo results, in vitro exposure to ACR significantly increased the expression levels of StAR and P450scc proteins in TM3 cells (P < 0.01) compared to untreated control cells [Figure 7A]. Interestingly, silencing p38MAPK resulted in a decrease in the StAR and P450scc protein levels. Following 12 h of culture in YJD-containing serum, the P450scc protein levels were significantly reduced (P < 0.01), while StAR expression remained unaffected (P > 0.05).
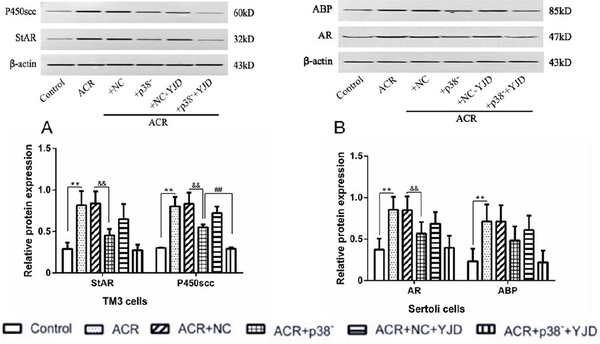
Figure 7.
YJD modulates testosterone synthesis and utilization. (A) Expression levels of StAR and P450scc in cultured TM3 cells. (B) Expression levels of ABP and AR in cultured Sertoli cells. Compared to the control group, **P < 0.01; compared to the ACR + NC model group, &&P < 0.01; compared to the ACR + NC + YJD model group, ##P < 0.01. StAR, steroidogenic acute regulatory; ABP, arterial blood pressure; AR, androgen receptor; ACR, acrolein; NC, empty vector; YJD, Yi-jing decoction.
In Sertoli cells, ACR-induced OS significantly increased the relative expression levels of AR and ABP proteins (P < 0.01; Figure 7B). Knockdown of p38MAPK led to a significant downregulation of AR protein (P < 0.01), while ABP expression remained unchanged (P > 0.05). Additionally, YJD treatment did not significantly alter the expression levels of AR and ABP proteins in Sertoli cells (P > 0.05).
In summary, these findings suggest that YJD may selectively regulate StAR and P450scc expression in TM3 cells while having a limited impact on AR and ABP expression in Sertoli cells. The discrepancies between the in vivo and in vitro findings underscore the importance of considering the cellular context when interpreting the experimental results.
DISCUSSION
OAZS develops from prolonged and chronic hypoxic conditions in the reproductive microenvironment, leading to increased levels of ROS and OS.[] ROS directly damages deoxyribonucleic acid (DNA), proteins, and lipids and indirectly triggers apoptosis through the activation of stress-sensitive intracellular signaling pathways, such as p38MAPK.[,] Our study demonstrates that YJD protects sperm motility and quality, reduces OS-induced sperm cell death, and enhances testosterone synthesis and utilization. These effects are likely mediated by inhibiting the p38MAPK-mitochondrial apoptosis pathway.
The pathogenesis of OAZS is complex, involving cell apoptosis, ROS/OS, inflammation, and hormone metabolism. The MAPK signaling pathway, activated by ROS, regulates sperm generation, apoptosis, and maturation.[,] p38MAPK, a key component of this pathway, is expressed in spermatozoa, influencing motility, and in testicular supporting cells, seminiferous tubules, and interstitial cells. Increased levels of p38MAPK and its phosphorylated forms have been observed in patients with OAZS,[] suggesting its involvement in sperm generation and motility.
Apoptosis, a crucial self-regulatory mechanism, eliminates undesirable or abnormal cells and plays an essential role in development, homeostasis, and pathogen clearance.[] Mitochondrial apoptosis, or the intrinsic apoptotic pathway, is central to programmed cell death.[] Members of the Bcl-2 protein family regulate mitochondrial permeability transition pores and control the release of Cyt-C from mitochondria in response to apoptotic signals. Cyt-C then binds to apoptotic protease activating factor-1, activating caspase-9 and initiating the downstream caspase-3-mediated apoptotic cascade.[] The release of Cyt-C is a hallmark event in mitochondrial apoptosis.
In our study, elevated levels of p38MAPK, BAX, Cyt-C, and caspase-3 proteins were observed in testicular tissues from the CTX-induced OAZS model, while the Bcl-2 levels were significantly reduced, resulting in a higher BAX/Bcl-2 ratio. YJD treatment restored these apoptosis-related protein levels in a dose-dependent manner, indicating its potential to prevent apoptosis by inhibiting the mitochondrial ROS/p38MAPK pathway. Additionally, MMP measurements showed a significant decrease in the model group, with YJD treatment improving the MMP levels, further supporting its protective role against mitochondrial dysfunction.
Histological examination with HE staining revealed normal seminiferous tubule morphology and well-ordered spermatogenic cells in the control group. In contrast, the model group exhibited disordered seminiferous epithelium, reduced spermatogenic cells, and an absence of sperm. Low-dose YJD showed no significant changes, while medium and high doses partially restored morphology to levels similar to those of the control group. Ultrastructural analysis using TEM revealed severe mitochondrial damage in the model group, including swelling, cristae disappearance, and vacuolization. The high-dose YJD group showed near-normal mitochondrial morphology, reinforcing YJD’s role in mitigating OS-induced damage.
Spermatogenesis and sperm maturation depend heavily on testosterone synthesis, secretion, and utilization.[] Leydig cells responsible for testosterone production rely on mitochondrial function.[] Key enzymes such as StAR and P450scc are crucial for this process, and mitochondrial dysfunction impairs testosterone synthesis.[] Our findings suggest that YJD protects against OS damage by blocking the ROS/p38MAPK mitochondrial pathway and inhibiting apoptosis in Leydig and Sertoli cells. Knocking down p38MAPK in TM3 and Sertoli cells under hypoxic conditions affected the expression of AR, P450scc, StAR, BAX/Bcl-2, Cyt-C, and caspase-3 but not ABP. YJD treatment downregulated p38MAPK, BAX/Bcl-2, Cyt-C, and caspase-3, while StAR and AR levels remained unaffected. YJD also augmented AR protein expression and increased testosterone utilization, promoting spermatogenesis, enhancing sperm density and vitality, and alleviating OAZS.
CONCLUSION
In conclusion, YJD improves spermatogenic dysfunction induced by OS through multiple mechanisms, including modulation of the p38MAPK-mitochondrial apoptosis pathway, maintaining MMP, and enhancing tissue morphology and mitochondrial ultrastructure. These findings highlight YJD’s multifaceted role in improving reproductive health and offer a comprehensive approach to managing OS-induced spermatogenic dysfunction.
Financial support and sponsorship
This study was supported by the National Natural Science Foundation of China (No. 81173444 and 81673987) and the High-Level Key Discipline Construction Project of Traditional Chinese Medicine from the National Administration of Traditional Chinese Medicine (zyyzdxk-2023238).
Author contributions
Cao DZ: Conceptualization, Writing—Original draft. Min X: Validation, Resources. Su L: Methodology, Data curation. Luo CL: Project administration. Cheng HY: Methodology. Zhang SZ: Methodology. Jia JM: Writing—Review and Editing. Jiao YZ: Writing—Review and Editing, Funding acquisition. All authors have read and approved the final version of the manuscript.
Ethics approval and consent to participate
All protocols in this study were approved by the Institutional Animal Care and Use Committee (IACUC) of Guang’an Men Hospital, China Academy of Chinese Medical Sciences (IACUC Issue No. IACUC-GAMH-2024-009), in compliance with the Guide for the Care and Use of Laboratory Animals published by the US National Institutes of Health (NIH publication No. 85-23, revised 1996).
Conflict of interest
The authors declare that they have no competing interests.
Data availability statement
The data presented in this study are available on request from the corresponding author.
How to cite: Cao DZ, Min X, Su L, et al. Yi-Jing decoction ameliorates oligoasthenozoospermia by Inhibiting the oxidative stress-p38 mitogen-activated protein kinase-mediated mitochondrial apoptosis pathway in leydig and sertoli cells. Integr Med Nephrol Androl. 2025;12:e24-00058. doi: 10.1097/IMNA-D-24-00058
REFERENCES
1.
Levine H, Jørgensen N, Martino-Andrade A, et al. Temporal trends in sperm count: a systematic review and meta-regression analysis of samples collected globally in the 20th and 21st centuries. Hum Reprod Update. 2023;29(2):157-176.2.
Leslie SW, Soon-Sutton TL, Khan MAB. Male Infertility. In: StatPearls. Treasure Island (FL): StatPearls Publishing. 2024.3.
Vahedi Raad M, Firouzabadi AM, Tofighi Niaki M, Henkel R, Fesahat F. The impact of mitochondrial impairments on sperm function and male fertility: a systematic review. Reprod Biol Endocrinol. 2024;22(1):83.4.
Zhou SH, Deng YF, Weng ZW, Weng HW, Liu ZD. Traditional Chinese Medicine as a Remedy for Male Infertility: A Review. World J Mens Health. 2019;37(2):175-185.5.
Luo SB, Jia JM, Ma WG, Dong JC, Jiao YZ. [Yijing Fang for the treatment of 207 cases of oligoasthenozoospermia]. Shaanxi J Tradit Chin Med. 2010;31(4):410-413.6.
Duracka M, Lukac N, Kacaniova M, et al. Antibiotics versus natural biomolecules: the case of in vitro induced bacteriospermia by Enterococcus faecalis in rabbit Semen. Molecules. 2019;24(23):4329.7.
Karna KK, Choi BR, Kim MJ, Kim HK, Park JK. The effect of Schisandra chinensis baillon on cross-talk between oxidative stress, endoplasmic reticulum stress, and mitochondrial signaling pathway in testes of varicocele-induced SD rat. Int J Mol Sci. 2019;20(22):5785.8.
Tvrdá E, Michalko J, Árvay J, et al. Characterization of the Omija (Schisandra chinensis) extract and its effects on the bovine sperm vitality and oxidative profile during in vitro storage. Evid Based Complement Alternat Med. 2020;2020:7123780.9.
Ma XY, Zhu KL, Yao ZL, et al. Icariin alleviates the injury of Sertoli cell junction function by upregulating PKR pathway via ERα/c-fos signaling in aged mice. J Ethnopharmacol. 2024;335:118673.10.
Zhao HY, Zhao TT, Yang JH, et al. Epimedium protects against dyszoospermia in mice with Pex3 knockout by exerting antioxidant effects and regulating the expression level of P16. Cell Death Dis. 2022;13(1):69.11.
Castleton PE, Deluao JC, Sharkey DJ, McPherson NO. Measuring Reactive Oxygen Species in Semen for Male Preconception Care: A Scientist Perspective. Antioxidants (Basel). 2022;11(2):264.12.
Pavuluri H, Bakhtiary Z, Panner Selvam MK, Hellstrom WJG. Oxidative stress-associated male infertility: current diagnostic and therapeutic approaches. Medicina (Kaunas). 2024;60(6):1008.13.
Moustakli E, Zikopoulos A, Skentou C, et al. Impact of Reductive Stress on Human Infertility: Underlying Mechanisms and Perspectives. Int J Mol Sci. 2024;25(21):11802.14.
Aitken RJ, Drevet JR, Moazamian A, Gharagozloo P. Male Infertility and Oxidative Stress: A Focus on the Underlying Mechanisms. Antioxidants (Basel). 2022;11(2):306.15.
Ni FD, Hao SL, Yang WX. Multiple signaling pathways in Sertoli cells: recent findings in spermatogenesis. Cell Death Dis. 2019;10(8):541.16.
Kumar L, Solanki S, Jain A, et al. MAPKs signaling is obligatory for male reproductive function in a development-specific manner. Front Reprod Health. 2024;6:1330161.17.
Li N, Dong XY, Fu S, et al. C-type natriuretic peptide (CNP) could improve sperm motility and reproductive function of asthenozoospermia. Int J Mol Sci. 2022;23(18):10370.18.
Fusco R, Salinaro AT, Siracusa R, et al. Hidrox® counteracts cyclophosphamide-induced male infertility through NRF2 pathways in a mouse model. Antioxidants (Basel). 2021;10(5):778.19.
Takeshima T, Usui K, Mori K, et al. Oxidative stress and male infertility. Reprod Med Biol. 2020;20(1):41-52.20.
Salem MA, Ismail RS, Zaki HF, Arafa HMM, El-Khatib ASN. L-carnitine extenuates endocrine disruption, inflammatory burst and oxidative stress in carbendazim-challenged male rats via upregulation of testicular StAR and FABP9, and downregulation of P38-MAPK pathways. Toxicology. 2021;457:152808.21.
Xie XM, Deng T, Duan JF, Xie J, Yuan JL, Chen MQ. Exposure to polystyrene microplastics causes reproductive toxicity through oxidative stress and activation of the p38 MAPK signaling pathway. Ecotoxicol Environ Saf. 2020;190:110133.22.
Almog T, Lazar S, Reiss N, et al. Identification of extracellular signal-regulated kinase 1/2 and p38 MAPK as regulators of human sperm motility and acrosome reaction and as predictors of poor spermatozoan quality. J Biol Chem. 2008;283(21):14479-14489.23.
Lin X, Zhu LJ, Gao XY, et al. Ameliorative effect of betulinic acid against Zearalenone exposure triggers testicular dysfunction and oxidative stress in mice via p38/ERK MAPK inhibition and Nrf2-mediated antioxidant defense activation. Ecotoxicol Environ Saf. 2022;238:113561.24.
Nano M, Montell DJ. Apoptotic signaling: Beyond cell death. Semin Cell Dev Biol. 2024;156:22-34.25.
Costa J, Braga PC, Rebelo I, Oliveira PF, Alves MG. Mitochondria Quality Control and Male Fertility. Biology (Basel). 2023;12(6):82726.
Budzinska M, Kamieniczna M, Wojnar L, et al. The role of the intrinsic pathway of apoptosis in human ejaculated sperm damage under a state of scrotal heat stress. J Assist Reprod Genet. 2024;41(1):99-108.27.
Naamneh Elzenaty R, du Toit T, Flück CE. Basics of androgen synthesis and action. Best Pract Res Clin Endocrinol Metab. 2022;36(4):101665.28.
Adamczewska D, Słowikowska-Hilczer J, Walczak-Jędrzejowska R. The Fate of Leydig Cells in Men with Spermatogenic Failure. Life (Basel). 2022;12(4):570.29.
Park YJ, Pang MG. Mitochondrial functionality in male fertility: from spermatogenesis to fertilization. Antioxidants (Basel). 2021;10(1):98.
