Introduction
Achieving and maintaining durable suppression of HIV replication require sufficient adherence to antiretroviral (ARV) therapy. Historically, near-perfect (≥95%) adherence was considered necessary for oral ARV treatment success, based primarily on data from older studies of early ARVs, unboosted PIs and mono or dual therapy. Since then, ARVs with improved potency, pharmacokinetics (PK) and safety profiles have become available, and combination therapy, including co-formulated regimens, has become the standard of care. Subsequently, the adherence threshold sufficient for viral suppression has become lower than previously thought and is likely ARV dependent. For example, two recent studies found that adherence to integrase strand transfer inhibitor (INSTI)-based regimens as low as 69% and 75% (based on prescription dispensation) achieved viral suppression in 90% of the populations studied.,
The INSTI-based single-tablet regimen bictegravir/emtricitabine/tenofovir alafenamide (B/F/TAF) is a guideline-recommended regimen for adults, adolescents and children weighing ≥14 kg, with demonstrated safety, efficacy and a high barrier to resistance., The Phase 3 clinical development programme for B/F/TAF included five double-blind, placebo-controlled studies in people with HIV-1 (treatment-naïve or virologically suppressed) who were randomized to B/F/TAF or dolutegravir plus 2 NRTIs (DTG + 2 NRTIs). Through 48 weeks of double-blinded treatment, high levels of virologic suppression were observed for both treatment groups in each study, and B/F/TAF had non-inferior efficacy and safety compared with DTG + 2 NRTIs. In the treatment-naïve studies, blinded treatment continued beyond Week 48, and high levels of virologic suppression were maintained at Weeks 96 and 144.,
Although overall virologic suppression was high in these studies, it was unclear how varying adherence levels affected viral suppression for B/F/TAF versus DTG + 2 NRTIs. To this end, we pooled data from these five studies, totalling more than 2600 participants, and retrospectively analysed the effect of varying adherence on virologic outcomes for B/F/TAF and DTG + 2 NRTIs.
Patients and methods
Ethics
All studies were undertaken in accordance with the Declaration of Helsinki and approved by central or site-specific review boards or ethics committees (approval numbers on file). All participants provided written informed consent.
Clinical trial design and participants
Details of the designs and primary endpoints for studies GS-US-380-1489, GS-US-380-1490, GS-US-380-4458, GS-US-380-1844 and GS-US-380-4030 (NCT02607930, NCT02607956, NCT03547908, NCT02603120 and NCT03110380, respectively) have been previously reported. GS-US-380-1489, GS-US-380-1490 and GS-US-380-4458 enrolled participants with HIV-1 who were treatment naïve. GS-US-380-1844 and GS-US-380-4030 enrolled participants with HIV-1 who were virologically suppressed on a stable ARV regimen consisting of dolutegravir and abacavir/lamivudine (DTG/ABC/3TC; GS-US-380-1844) or dolutegravir and emtricitabine/tenofovir alafenamide (DTG + F/TAF) or emtricitabine/tenofovir disoproxil fumarate (DTG + F/TDF; GS-US-380-4030). All participants were randomized 1:1 to receive B/F/TAF or one of the following DTG + 2 NRTI regimens: DTG/ABC/3TC (GS-US-380-1489 and GS-US-380-1844), DTG + F/TAF (GS-US-380-1490 and GS-US-380-4030) or DTG + F/TDF (GS-US-380-4458). In these double-blind studies, participants received active treatment plus placebo-to-match; therefore, all participants had two (GS-US-380-1489 and GS-US-380-1490) or three (GS-US-380-1490, GS-US-380-4030 and GS-US-380-4458) daily pills. Participants with at least one post-baseline HIV-1 RNA measurement and evaluable adherence data were included in this analysis.
Assessment of adherence and virologic outcomes
Plasma HIV-1 RNA concentrations were measured at Day 1 (baseline); Weeks 4, 8 and 12; and then every 12 weeks thereafter. Study regimen adherence (active treatment or placebo) was calculated by pill counts from returned pill bottles as a percentage using the following equation:
where the number of pills taken was imputed from the number of pills dispensed minus the number of pills returned at each dispensing period. Cumulative adherence represented overall adherence from baseline through a specified timepoint. At least one returned pill bottle was required for assessment of adherence. Only active study drug adherence is reported.
HIV-1 RNA and adherence were assessed through the blinded phase with timepoints at Week 48 for all five studies; at Week 96 for GS-US-380-1489, GS-US-380-1490 and GS-US-380-4458; and at Week 144 for GS-US-380-1489 and GS-US-380-1490. Virologic outcomes at each timepoint were determined by the last available on-treatment HIV-1 RNA value based on last observation carried forward imputation, by FDA-defined Snapshot algorithm and by on-treatment missing equals excluded (M = E) imputation. Virologic suppression was defined as HIV-1 RNA <50 copies/mL and viraemia as HIV-1 RNA ≥50 copies/mL. Adherence through each timepoint was categorized as high (≥95%), intermediate (≥85% to <95%) or low (<85%).
Resistance analyses
Baseline resistance was assessed by plasma HIV-1 RNA genotyping of integrase (IN) and/or protease (PR)/reverse transcriptase (RT) at screening for participants in the treatment-naïve studies and by proviral HIV-1 RNA genotyping of PR/RT/IN at Day 1 in the virologically suppressed switch studies. Resistance data from any prestudy genotypic testing were also collected if available.
Participants with HIV-1 RNA ≥ 200 copies/mL at their last visit or confirmed virologic failure (two consecutive visits with HIV-1 RNA ≥50 copies/mL or >1 log10 increase in HIV-1 RNA from nadir), without resuppression of HIV-1 RNA to <50 copies/mL while on study drugs, had plasma samples analysed for the development of HIV-1 drug resistance.
Statistical methods
Baseline demographics and clinical characteristics were summarized according to treatment group and adherence category. For continuous baseline data, differences between adherence categories were assessed by the two-sided Van Elteren test, stratified by participant population type (treatment-naïve versus virologically suppressed). The Cochran–Mantel–Haenszel test, adjusted by participant population type, was used to assess differences in categorical baseline data between high and intermediate versus low adherence, as well as differences in the percentages of participants with virological outcomes (HIV-1 RNA < 50 or ≥50 copies/mL) between high or intermediate versus low adherence categories within treatment groups or between treatment groups. The 95% CIs of differences in virologic outcomes were calculated based on an unconditional exact method using two inverted one-sided tests.
Results
Study population and adherence through Weeks 48, 96 and 144
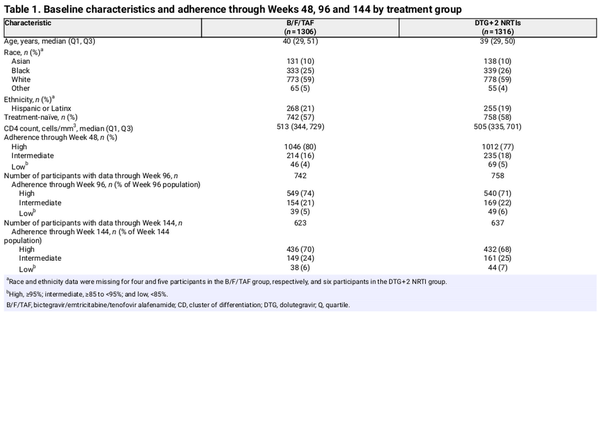
Overall, 2645 participants were randomized and treated, of whom 2622 with evaluable adherence and post-baseline HIV-1 RNA data were included in the analysis through Week 48 (B/F/TAF group, n = 1306; DTG + 2 NRTI group, n = 1316); 17 participants without returned pill bottles and a further 6 participants with no post-baseline HIV-1 RNA assessments were excluded. At screening, 1500/2622 (57%) of participants were treatment naïve (B/F/TAF: n = 742; DTG + 2 NRTIs: n = 758) and 1122/2622 (43%) were virologically suppressed (B/F/TAF: n = 564; DTG + 2 NRTIs: n = 558). Participant demographics and baseline characteristics were similar between the two groups [Table 1; Table S1 (available as Supplementary data at JAC Online)]. During blinded treatment, participants in DTG + 2 NRTI group (n = 1316) received DTG + F/TAF [600 (46%)], DTG/ABC/3TC [595 (45%)] or DTG + F/TDF [121 (9%)].
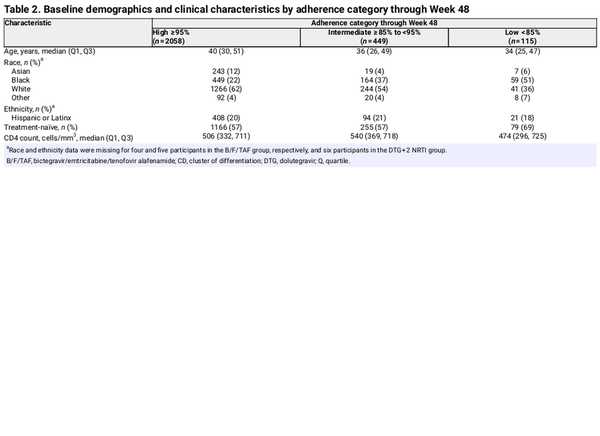
Through 48 weeks, 2058 (78%) participants had high (≥95%) adherence, 449 (17%) had intermediate (≥85% to <95%) adherence and 115 (4%) had low (<85%) adherence (Table 2), with similar distribution between the B/F/TAF and DTG + 2 NRTI treatment groups. Participants with low adherence were younger, more often Black and more often treatment-naïve compared with participants with high and intermediate adherence (P ≤ 0.02 for these comparisons; Table S2). Among participants with low adherence, median adherence through Week 48 was 78% for the B/F/TAF group and 80% for the DTG + 2 NRTI group.
After 48 weeks, all 1500 participants in the treatment-naïve trials continued blinded treatment. The proportions of participants in each adherence category through Week 96 for the B/F/TAF and DTG + 2 NRTI groups, respectively, were high: 549/742 (74%) and 540/758 (71%); intermediate: 154/724 (21%) and 169/758 (22%); and low: 39/742 (5%) and 49/758 (6%). Adherence was assessed again for participants in GS-US-380-1489 and GS-US-380-1490 (n = 1260) who continued blinded treatment through 144 weeks (B/F/TAF, n = 623; DTG + 2 NRTIs, n = 637). The proportions of participants in each adherence category through Week 144 for the B/F/TAF and DTG + 2 NRTI groups, respectively, were high: 436/623 (70%) and 432/637 (68%); intermediate: 149/623 (24%) and 161/637 (25%); and low: 38/623 (6%) and 44/637 (7%). Median adherence through Weeks 96 and 144 in the low adherence category was 80% for both the B/F/TAF and DTG + 2 NRTI groups.
Effect of adherence on virologic outcomes at last visit through 48, 96 and 144 weeks of blinded treatment
Overall virologic suppression at the last visit was high in both treatment groups during blinded treatments (Figure S1). Virologic outcomes were then stratified by adherence and compared between adherence categories within each treatment group. Through Week 48, virologic suppression was similarly high in all adherence categories in the B/F/TAF group; 44/46 (96%) of participants with low adherence had HIV-1 RNA <50 copies/mL at last visit compared with 210/214 (98%) with intermediate adherence and 1033/1046 (99%) with high adherence (P > 0.05 for all comparisons; Figure 1a). In the DTG + 2 NRTI group, however, virologic suppression through Week 48 was significantly lower for participants with low adherence [62/69 (90%)] compared with participants with intermediate adherence [231/235 (98%); difference 8.4%, 95% CI 1.6–18.3; P = 0.002] or with high adherence [999/1012 (99%); difference 8.9%, 95% CI 3.1–19.3; P < 0.0001; Table S3]. This same pattern was observed after 96 and 144 weeks of blinded treatment: virologic suppression was similar for participants in all adherence categories in the B/F/TAF group, but suppression was significantly lower (P ≤ 0.02) for participants with low adherence compared with participants with intermediate or high adherence in the DTG + 2 NRTI group (Figure 1b and c).
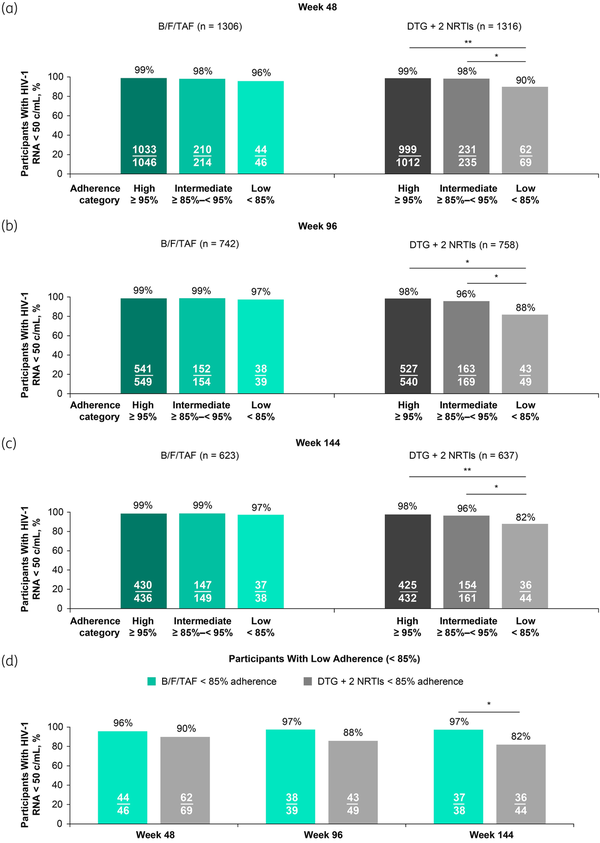
Figure 1
Virologic suppression (HIV-1 RNA < 50 copies/mL) at last visit through Weeks 48, 96 and 144 by treatment group and adherence category. Proportions of participants with virologic suppression were compared within or between treatment groups, with P-values determined by Cochran–Mantel–Haenszel test, adjusted by participant population type. At Week 48, studies GS-US-380-1489, GS-US-380-1490, GS-US-380-4458, GS-US-380-1844 and GS-US-380-4030 were included; DTG + 2 NRTI regimens were DTG/ABC/3TC (n = 595), DTG + F/TAF (n = 600) or DTG + F/TDF (n = 121) (a, d). At Week 96, studies GS-US-380-1489, GS-US-380-1490 and GS-US-380-4458 were included; DTG + 2 NRTI regimens were DTG/ABC/3TC (n = 314), DTG + F/TAF (n = 323) or DTG + F/TDF (n = 121) (b, d). At Week 144, studies GS-US-380-1489 and GS-US-380-1490 were included; DTG + 2 NRTI regimens were DTG/ABC/3TC (n = 314) or DTG + F/TAF (n = 323) (c, d). 3TC, lamivudine; ABC, abacavir; B/F/TAF, bictegravir/emtricitabine/tenofovir alafenamide; DTG, dolutegravir; F, emtricitabine; TAF, tenofovir alafenamide; TDF, tenofovir disoproxil fumarate. *P < 0.05, **P < 0.0001. This figure appears in colour in the online version of JAC and in black and white in the print version of JAC.
Next, differences in virologic outcomes for the B/F/TAF versus DTG + 2 NRTI groups were investigated for participants with low adherence. Through Weeks 48 and 96, virologic suppression was numerically higher for the B/F/TAF group compared with the DTG + 2 NRTI group [difference (95% CI) of 5.8% (−6.2 to 16.4) and 9.7% (−2.6 to 22.5), respectively; P > 0.05; Figure 1d]. Through Week 144, virologic suppression was statistically higher for participants with low adherence in the B/F/TAF group compared with participants with low adherence in the DTG + 2 NRTI group [37/38 (97%) versus 36/44 (82%), respectively; difference 15.6%, 95% CI 2.0–30.6; P = 0.03].
These analyses were repeated using data from only the three trials where DTG + F/TAF or DTG + F/TDF were the comparator regimens, with comparable results to the pooled analysis (Figure S2). In the B/F/TAF group, virologic suppression was similarly high across adherence categories at Weeks 96 and 144, though at Week 48, suppression was statistically lower for participants with low adherence versus high adherence for one of the two statistical tests used (P = 0.03). Participants receiving DTG + F/TAF or DTG + F/TDF with low adherence had significantly lower virologic suppression compared with participants with intermediate adherence (Weeks 48 and 144; P ≤ 0.01) or high adherence (Weeks 48, 96 and 144; P ≤ 0.002). No differences in virologic outcomes for B/F/TAF versus DTG + 2 NRTIs were observed in this subgroup analysis.
Virologic outcomes at Weeks 48, 96 and 144 by Snapshot algorithm and missing = excluded approach
Virologic outcomes for all 2622 participants were also assessed using the Snapshot algorithm, where a third possible outcome of ‘no virologic data’ was assigned for participants who did not have HIV-1 RNA data at Weeks 48, 96 or 144 (Figure 2a–c). Overall, participants with low adherence experienced higher rates of early study discontinuation and therefore had higher frequencies of missing data compared with participants with intermediate or high adherence. Within each treatment group, participants with low adherence had the lowest levels of virologic suppression and highest levels of viraemia and missing data compared with participants with intermediate and high adherence at all timepoints. Among participants with low adherence, greater proportions in the B/F/TAF group had no data available compared with the DTG + 2 NRTI group (28%, 41% and 47% for B/F/TAF versus 13%, 22% and 34% for DTG + 2 NRTIs, at Weeks 48, 96 and 144, respectively), while greater proportions in the DTG + 2 NRTI group had HIV-1 RNA ≥ 50 copies/mL compared with the B/F/TAF group (4, 3 and 3% for B/F/TAF versus 10, 12 and 16% for DTG + 2 NRTIs at Weeks 48, 96 and 144). At Week 144, there was a statistically lower incidence of viraemia among participants with low adherence in the B/F/TAF group versus the DTG + 2 NRTI group (−13.3% difference, 95% CI −28.3 to −0.4; P = 0.04; Figure 2d). Within the DTG + 2 NRTI group, viraemia was significantly higher for participants with low adherence compared with intermediate or high adherence at all timepoints (P ≤ 0.02), while levels of viraemia were similarly low in the B/F/TAF group for all adherence categories.
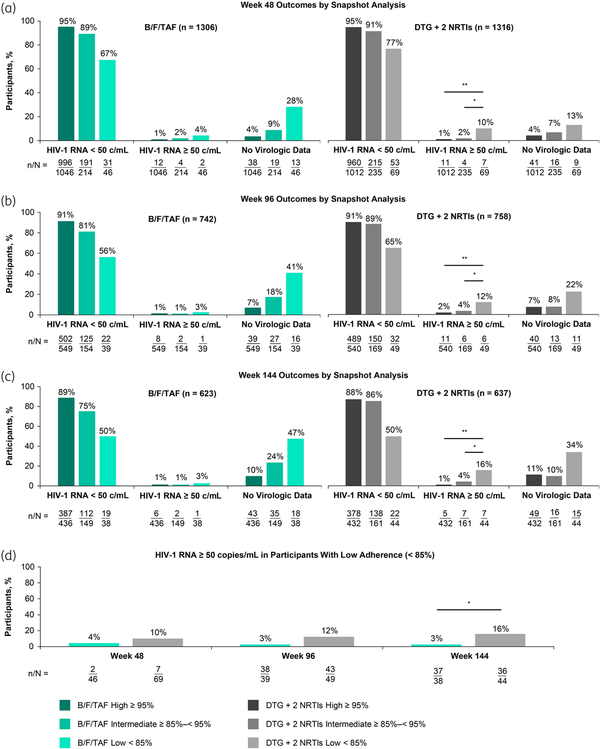
Figure 2
Virologic outcomes at Weeks 48, 96 and 144 (HIV-1 RNA cut-off at 50 copies/mL defined by Snapshot algorithm) by treatment group and adherence category. At Week 48, studies GS-US-380-1489, GS-US-380-1490, GS-US-380-4458, GS-US-380-1844 and GS-US-380-4030 were included; DTG + 2 NRTI regimens were DTG/ABC/3TC (n = 595), DTG + F/TAF (n = 600) or DTG + F/TDF (n = 121); DTG + 2 NRTI differences (95% CI) in HIV-1 RNA ≥50 copies/mL were −9.1% (−19.4% to −3.2%) for high versus low and −8.4% (−18.3% to −1.6%) for intermediate versus low adherence (a, d). At Week 96, studies GS-US-380-1489, GS-US-380-1490 and GS-US-380-4458 were included; DTG + 2 NRTI regimens were DTG/ABC/3TC (n = 314), DTG + F/TAF (n = 323) or DTG + F/TDF (n = 121); DTG + 2 NRTI differences (95% CI) in HIV-1 RNA ≥50 copies/mL were −10.2% (−23.5% to −2.3% for high versus low and −8.7% (−21.5% to −0.1%) for intermediate versus low adherence (b, d). At Week 144, studies GS-US-380-1489 and GS-US-380-1490 were included; DTG + 2 NRTI regimens were DTG/ABC/3TC (n = 314) or DTG + F/TAF (n = 323); DTG + 2 NRTI differences (95% CI) in HIV-1 RNA ≥50 copies/mL were −14.8% (−29.4% to −5.9%) for high versus low and −11.6% (−26.0% to −1.4%) for intermediate versus low adherence (c, d). Proportions of participants with low adherence who had HIV-1 RNA ≥ 50 copies/mL were compared within or between treatment groups, with P-values determined by Cochran–Mantel–Haenszel test, adjusted by participant population type (d). 3TC, lamivudine; ABC, abacavir; B/F/TAF, bictegravir/emtricitabine/tenofovir alafenamide; DTG, dolutegravir; F, emtricitabine; TAF, tenofovir alafenamide; TDF, tenofovir disoproxil fumarate. *P < 0.05, **P < 0.0001. This figure appears in colour in the online version of JAC and in black and white in the print version of JAC.
Virologic outcome on treatment was also analysed by M = E imputation (Figure 3; Table S4). In the B/F/TAF group, virologic suppression was similarly high (97−100%) among participants regardless of adherence level at all three timepoints. In the DTG + 2 NRTI group, virologic suppression was significantly lower for participants with low adherence compared with high adherence at Weeks 48 (difference of 6.5%, 95% CI 1.6–17.5; P < 0.0001) and 144 (difference of 4.1%, 95% CI −0.3 to 24.1; P = 0.007).
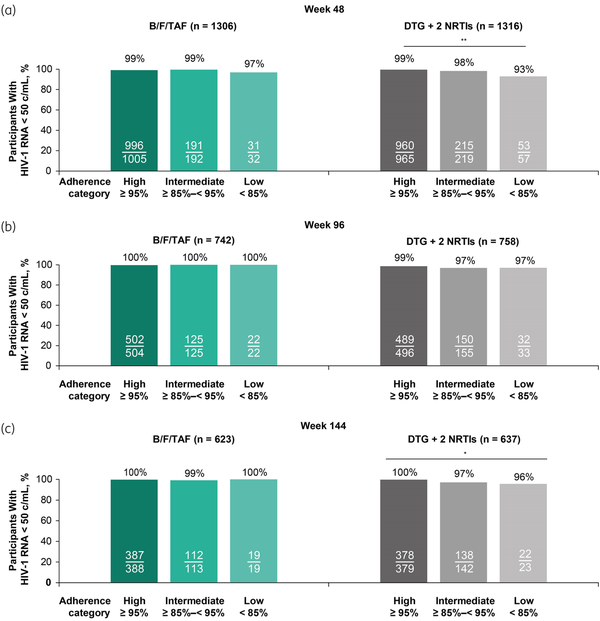
Figure 3
Virologic suppression (HIV-1 RNA < 50 copies/mL) at Weeks 48, 96 and 144 on treatment by missing = excluded imputation by treatment group and adherence category. All treated participants who had non-missing HIV-1 RNA data at Weeks 48 (a), 96 (b) and 144 (c) and evaluable adherence data were eligible. Proportions of participants with virologic suppression at were compared within treatment groups, with P-values determined using Cochran–Mantel–Haenszel test, adjusted by participant population type. 3TC, lamivudine; ABC, abacavir; B/F/TAF, bictegravir/emtricitabine/tenofovir alafenamide; DTG, dolutegravir; F, emtricitabine; TAF, tenofovir alafenamide; TDF, tenofovir disoproxil fumarate. *P < 0.05. **P<0.0001. This figure appears in colour in the online version of JAC and in black and white in the print version of JAC.
Baseline resistance and outcomes through Week 48
Baseline genotypic data were available for 2513/2622 (96%) of participants in the Week 48 analysis, including 109/115 (95%) with low adherence, 416/449 (93%) with intermediate adherence and 1968/2058 (96%) with high adherence. The M184V/I resistance substitution in RT was detected at baseline in eight participants with low adherence (B/F/TAF group, n = 5; DTG + 2 NRTI group, n = 3), with one participant in each treatment group also having five thymidine analogue mutations (TAMs). M184V/I was also detected at baseline in 19 participants with intermediate adherence (B/F/TAF group, n = 12; DTG + 2 NRTI group, n = 7). Three of these participants with baseline M184V/I in the B/F/TAF group also had three TAMs, one of whom also had the primary INSTI resistance substitution Y143H. Additionally, M184V/I was detected at baseline in 72 participants with high adherence (B/F/TAF group, n = 41; DTG + 2 NRTI group, n = 31), 32 of whom also had one or more TAMs (B/F/TAF group, n = 17; DTG + 2 NRTI group, n = 15) and 1 in the B/F/TAF group who also had the primary INSTI resistance substitution Q148H at baseline. All 99 of these participants with baseline resistance substitutions were from virologically suppressed switch studies, and all had HIV-1 RNA <50 copies/mL through Week 48.
Post-baseline resistance analyses
Overall, there were 68 participants with HIV-1 RNA ≥ 50 copies/mL at last visit through Weeks 48, 96 and/or 144 (B/F/TAF group: n = 23; DTG + 2 NRTI group: n = 45). Of these participants, 39 met criteria for post-baseline resistance analyses through Week 144 (B/F/TAF group, n = 15; DTG + 2 NRTI group, n = 24) and no participants in either group had treatment-emergent resistance. At Week 168 (last visit on blinded treatment), two participants receiving DTG/ABC/3TC had HIV-1 RNA ≥ 200 copies/mL with treatment-emergent M184V detected. One of these participants had 93% adherence through Week 144 and 2660 copies/mL HIV-1 RNA at Week 168. The other participant had 86% adherence through Week 144 and 279 copies/mL HIV-1 RNA at Week 168. Both participants switched to open-label B/F/TAF and subsequently resuppressed to HIV-1 RNA <50 copies/mL. No treatment-emergent resistance was observed in the B/F/TAF group at any time throughout the studies.
Discussion
In this large dataset pooled from all double-blind placebo-controlled Phase 3 studies conducted for B/F/TAF, we observed differential effects of adherence on virologic outcomes for B/F/TAF versus DTG + 2 NRTIs. Although B/F/TAF demonstrated similarly high efficacy regardless of adherence level, DTG + 2 NRTIs had reduced efficacy in participants with low adherence compared with intermediate and high adherence. Furthermore, two participants receiving DTG/ABC/3TC with intermediate adherence developed M184V, whereas there was no treatment-emergent resistance to B/F/TAF.
The word ‘forgiveness’ is sometimes used to describe the ability of an ARV regimen to achieve and maintain viral suppression despite imperfect medication adherence. For regimens with low forgiveness, suboptimal adherence can lead to a loss of virologic suppression, which over time increases the risk of negative outcomes such as drug resistance, disease progression and viral transmission. Although no specific quantifiable measure of forgiveness has been established, it is generally accepted that ARV regimens have differing forgiveness levels—driven primarily by the characteristics of the third agent—with newer INSTI-based regimens demonstrating very high forgiveness.,
Here, the forgiveness of INSTI-based three-drug regimens was directly compared for bictegravir versus dolutegravir, and results are consistent with previous studies evaluating each INSTI individually. Post hoc analyses of the GEMINI-1 and GEMINI-2 clinical trials showed that among participants receiving dolutegravir plus lamivudine or DTG + F/TDF, those with <90% adherence by pill count had significantly lower virologic suppression at last visit through Week 144 compared with participants with ≥90% adherence, with similar reductions in efficacy for both the two- and three-drug regimens. Differences in forgiveness between co-formulated dolutegravir/lamivudine and B/F/TAF have also been observed in observational cohorts with adherence analysed by pharmacy refills calculated as the proportion of days covered: adherence < 80% was unable to maintain viral suppression in any of the people with HIV receiving dolutegravir/lamivudine through a median of 117 weeks of follow-up, whereas adherence as low as 70% maintained viral suppression in those receiving B/F/TAF through a median of 84 weeks of follow-up., In studies of intermittent ARV treatment, B/F/TAF maintained viral suppression in 98% of participants through Week 96 when administered 4 or 5 days/week, but dolutegravir-based two-drug therapy administered 4 days/week had inferior efficacy compared with daily dosing though Week 48., Thus, our findings add to the body of clinical data demonstrating high forgiveness for B/F/TAF and lower forgiveness for dolutegravir-based regimens.
The reasons for the observed difference in forgiveness between B/F/TAF and DTG + 2 NRTIs may be attributed to the individual properties of the drugs in each regimen. Bictegravir and dolutegravir both have nanomolar potency in vitro, high barriers to resistance and similar plasma PK half-lives (17 h for bictegravir and 14 h for dolutegravir)., However, in a biochemical binding assay, bictegravir stayed bound to IN-DNA complexes for a significantly longer duration than dolutegravir, with a dissociation half-life of 163 h versus 96 h for dolutegravir. Thus, bictegravir may be able to retain antiviral activity for longer than dolutegravir during lapses of adherence. Additionally, the long plasma PK half-lives of emtricitabine and tenofovir (relative to abacavir and lamivudine) and the in vitro synergy observed between the components of B/F/TAF also likely contribute to the high forgiveness of B/F/TAF.,,
Other factors that could contribute to differences in adherence and outcomes were also considered, such as number of pills in a regimen. Previously, B/F/TAF, a single-tablet regimen, was associated with higher adherence than dolutegravir-based regimens containing multiple tablets. Although multi-tablet DTG + 2 NRTIs were used here, our studies were placebo controlled, so all participants received multiple daily pills, matched in number for each study, eliminating this potential bias. The inclusion of abacavir-containing regimens could also have implications for adherence due to possible side effects and pill size., We observed low adherence more frequently in participants receiving DTG/ABC/3TC versus DTG + F/TAF or DTG + F/TDF (data on file). However, after removing the studies where DTG/ABC/3TC was the comparator, we still observed reduced efficacy for low adherence with DTG + F/TAF or DTG + F/TDF, suggesting this finding was not driven by abacavir. Finally, since our definition of low adherence was broad (0% to <85%), differences in low adherence between treatment groups could have potentially skewed outcomes. However, the level of low adherence was similar between treatment groups, with median adherence levels among participants with low adherence ranging from 78% to 80% at all timepoints.
Our study has some limitations. Adherence is difficult to measure: pill count is based on returned pill bottles and the assumption that unreturned pills were taken. Also, the use of last observation carried forward imputation for determining virologic outcomes is limited by the uncertainty of imputing missing values, the assumption that outcomes do not change over time after the last non-missing value, the potential introduction of bias and the possible artificial increase in precision., Because participants with lower adherence had higher rates of study discontinuation, Snapshot and M = E analysis methods were unable to assign virologic outcomes to several of these participants. Given the small numbers of participants with low adherence, it was important to characterize outcomes for all participants and not exclude the very participants we aimed to describe. Whether or not study drugs could achieve viral suppression is still informative, even if treatment duration was not as long as the original study design. Furthermore, the results from this analysis were consistent with Snapshot- and M = E-derived virologic outcomes.
The high forgiveness of B/F/TAF has implications for long-term treatment success for people with HIV. Even though adherence was very high in these pooled trials, outside of clinical trials, adherence is often imperfect., Consequently, the use of ARV regimens with high forgiveness may be a critical HIV treatment strategy to increase the proportion of people with HIV with sustained virologic suppression. Additionally, other factors besides adherence may reduce drug levels in individuals with HIV. For example, two recent studies have shown that in pregnant women with HIV and in people with HIV receiving rifampicin treatment for TB, exposures to B/F/TAF were reduced but high efficacy was maintained, further demonstrating the forgiveness of B/F/TAF beyond adherence.
To conclude, in our analysis, B/F/TAF had higher efficacy than DTG + 2 NRTIs in achieving and maintaining virologic suppression in participants with suboptimal adherence (<85%), demonstrating the higher forgiveness of B/F/TAF despite similarly low levels of adherence.
Acknowledgements
We thank all participants and their families, participating sites, investigators and all staff involved in the study. We thank Hal Martin for his contributions to the study, Hailin Huang for his assistance with the statistical analyses, and Ernesto Scerpella for his helpful discussions. Medical writing support was provided by Christina Holleywood, PhD, at Aspire Scientific Limited (Bollington, UK), and funded by Gilead Sciences, Inc. (Foster City, CA, USA).
References
- 1. Bangsberg DR, Hecht FM, Charlebois ED, et al Adherence to protease inhibitors, HIV-1 viral load, and development of drug resistance in an indigent population. AIDS 2000; 14: 357–66.
- 2. de Olalla PG, Knobel H, Carmona A, et al Impact of adherence and highly active antiretroviral therapy on survival in HIV-infected patients. J Acquir Immune Defic Syndr 2002; 30: 105–10.
- 3. Paterson DL, Swindells S, Mohr J, et al Adherence to protease inhibitor therapy and outcomes in patients with HIV infection. Ann Intern Med 2000; 133: 21–30.
- 4. Rodriguez-Rosado R, Jimenez-Nacher I, Soriano V, et al Virological failure and adherence to antiretroviral therapy in HIV-infected patients. AIDS 1998; 12: 1112–3.
- 5. EACS. EACS guidelines version 12.0, October 2023. https://www.eacsociety.org/media/guidelines-12.0.pdf
- 6. Bezabhe WM, Chalmers L, Bereznicki LR, et al Adherence to antiretroviral therapy and virologic failure: a meta-analysis. Medicine (Baltimore) 2016; 95: e3361.
- 7. Gordon LL, Gharibian D, Chong K, et al Comparison of HIV virologic failure rates between patients with variable adherence to three antiretroviral regimen types. AIDS Patient Care STDS 2015; 29: 384–8.
- 8. Rosenblum M, Deeks SG, van der Laan M, et al The risk of virologic failure decreases with duration of HIV suppression, at greater than 50% adherence to antiretroviral therapy. PLoS One 2009; 4: e7196.
- 9. Shuter J, Sarlo JA, Kanmaz TJ, et al HIV-infected patients receiving lopinavir/ritonavir-based antiretroviral therapy achieve high rates of virologic suppression despite adherence rates less than 95%. J Acquir Immune Defic Syndr 2007; 45: 4–8.
- 10. Bangsberg DR. Less than 95% adherence to nonnucleoside reverse-transcriptase inhibitor therapy can lead to viral suppression. Clin Infect Dis 2006; 43: 939–41.
- 11. Maggiolo F, Ravasio L, Ripamonti D, et al Similar adherence rates favor different virologic outcomes for patients treated with nonnucleoside analogues or protease inhibitors. Clin Infect Dis 2005; 40: 158–63.
- 12. Viswanathan S, Justice AC, Alexander GC, et al Adherence and HIV RNA suppression in the current era of highly active antiretroviral therapy. J Acquir Immune Defic Syndr 2015; 69: 493–8.
- 13. Byrd KK, Hou JG, Hazen R, et al Antiretroviral adherence level necessary for HIV viral suppression using real-world data. J Acquir Immune Defic Syndr 2019; 82: 245–51.
- 14. Stover S, Milloy MJ, Grant C, et al Estimating the minimum antiretroviral adherence required for plasma HIV-1 RNA viral load suppression among people living with HIV who use unregulated drugs. AIDS 2022; 36: 1233–43.
- 15. Gandhi RT, Bedimo R, Hoy JF, et al Antiretroviral drugs for treatment and prevention of HIV infection in adults: 2022 recommendations of the International Antiviral Society-USA panel. JAMA 2023; 329: 63–84.
- 16. Gilead Sciences. BIKTARVY: summary of product characteristics. https://www.ema.europa.eu/en/documents/product-information/biktarvy-epar-product-information_en.pdf
- 17. Gilead Sciences. BIKTARVY: prescribing information. https://www.gilead.com/∼/media/files/pdfs/medicines/hiv/biktarvy/biktarvy_pi.pdf
- 18. Panel on Antiretroviral Guidelines for Adults and Adolescents. Guidelines for the use of antiretroviral agents in adults and adolescents with HIV. Department of Health and Human Services. https://clinicalinfo.hiv.gov/en/guidelines/adult-and-adolescent-arv
- 19. Avihingsanon A, Lu H, Leong CL, et al Bictegravir, emtricitabine, and tenofovir alafenamide versus dolutegravir, emtricitabine, and tenofovir disoproxil fumarate for initial treatment of HIV-1 and hepatitis B coinfection (ALLIANCE): a double-blind, multicentre, randomised controlled, Phase 3 non-inferiority trial. Lancet HIV 2023; 10: e640–52.
- 20. Gallant J, Lazzarin A, Mills A, et al Bictegravir, emtricitabine, and tenofovir alafenamide versus dolutegravir, abacavir, and lamivudine for initial treatment of HIV-1 infection (GS-US-380-1489): a double-blind, multicentre, Phase 3, randomised controlled non-inferiority trial. Lancet 2017; 390: 2063–72.
- 21. Molina JM, Ward D, Brar I, et al Switching to fixed-dose bictegravir, emtricitabine, and tenofovir alafenamide from dolutegravir plus abacavir and lamivudine in virologically suppressed adults with HIV-1: 48 week results of a randomised, double-blind, multicentre, active-controlled, Phase 3, non-inferiority trial. Lancet HIV 2018; 5: e357–65.
- 22. Sax PE, Pozniak A, Montes ML, et al Coformulated bictegravir, emtricitabine, and tenofovir alafenamide versus dolutegravir with emtricitabine and tenofovir alafenamide, for initial treatment of HIV-1 infection (GS-US-380-1490): a randomised, double-blind, multicentre, Phase 3, non-inferiority trial. Lancet 2017; 390: 2073–82.
- 23. Sax PE, Rockstroh JK, Luetkemeyer AF, et al Switching to bictegravir, emtricitabine, and tenofovir alafenamide in virologically suppressed adults with human immunodeficiency virus. Clin Infect Dis 2021; 73: e485–93.
- 24. Orkin C, DeJesus E, Sax PE, et al Fixed-dose combination bictegravir, emtricitabine, and tenofovir alafenamide versus dolutegravir-containing regimens for initial treatment of HIV-1 infection: Week 144 results from two randomised, double-blind, multicentre, Phase 3, non-inferiority trials. Lancet HIV 2020; 7: e389–400.
- 25. Stellbrink HJ, Arribas JR, Stephens JL, et al Co-formulated bictegravir, emtricitabine, and tenofovir alafenamide versus dolutegravir with emtricitabine and tenofovir alafenamide for initial treatment of HIV-1 infection: Week 96 results from a randomised, double-blind, multicentre, Phase 3, non-inferiority trial. Lancet HIV 2019; 6: e364–72.
- 26. Wohl DA, Yazdanpanah Y, Baumgarten A, et al Bictegravir combined with emtricitabine and tenofovir alafenamide versus dolutegravir, abacavir, and lamivudine for initial treatment of HIV-1 infection: Week 96 results from a randomised, double-blind, multicentre, Phase 3, non-inferiority trial. Lancet HIV 2019; 6: e355–63.
- 27. Chan IS, Zhang Z. Test-based exact confidence intervals for the difference of two binomial proportions. Biometrics 1999; 55: 1202–9.
- 28. Orkin C, Antinori A, Rockstroh JK, et al Switch to bictegravir/emtricitabine/tenofovir alafenamide from dolutegravir-based therapy. AIDS 2024; 38: 983–91.
- 29. Shuter J. Forgiveness of non-adherence to HIV-1 antiretroviral therapy. J Antimicrob Chemother 2008; 61: 769–73.
- 30. Martin M, Del Cacho E, Codina C, et al Relationship between adherence level, type of the antiretroviral regimen, and plasma HIV type 1 RNA viral load: a prospective cohort study. AIDS Res Hum Retroviruses 2008; 24: 1263–8.
- 31. Mannheimer S, Friedland G, Matts J, et al The consistency of adherence to antiretroviral therapy predicts biologic outcomes for human immunodeficiency virus-infected persons in clinical trials. Clin Infect Dis 2002; 34: 1115–21.
- 32. Bangsberg DR, Perry S, Charlebois ED, et al Non-adherence to highly active antiretroviral therapy predicts progression to AIDS. AIDS 2001; 15: 1181–3.
- 33. Harrigan PR, Hogg RS, Dong WW, et al Predictors of HIV drug-resistance mutations in a large antiretroviral-naive cohort initiating triple antiretroviral therapy. J Infect Dis 2005; 191: 339–47.
- 34. Lingappa JR, Hughes JP, Wang RS, et al Estimating the impact of plasma HIV-1 RNA reductions on heterosexual HIV-1 transmission risk. PLoS One 2010; 5: e12598.
- 35. Cheng Y, Nickman NA, Jamjian C, et al Predicting poor adherence to antiretroviral therapy among treatment-naive veterans infected with human immunodeficiency virus. Medicine (Baltimore) 2018; 97: e9495.
- 36. Fernvik E, Sierra Madero J, Espinosa N, et al Impact of treatment adherence on efficacy of dolutegravir + lamivudine and dolutegravir + tenofovir disoproxil fumarate/emtricitabine: pooled Week 144 analysis of the GEMINI-1 and GEMINI-2 clinical studies. J Acquir Immune Defic Syndr 2023; 94: e9–12.
- 37. Maggiolo F, Valenti D, Teocchi R, et al Real world data on forgiveness to uncomplete adherence to bictegravir/emtricitabine/tenofovir alafenamide. J Int Assoc Provid AIDS Care 2022; 21: 23259582221140208.
- 38. Maggiolo F, Valenti D, Teocchi R, et al Adherence to and forgiveness of 3TC/DTG in a real-world cohort. J Int Assoc Provid AIDS Care 2022; 21: 23259582221101815.
- 39. Landman R. ANRS 177 DUETTO randomized, open-label and multicentric trial evaluating the non-inferiority of maintenance antiretroviral dual therapy taken 4 consecutive days per week versus dual therapy 7/7 days per week [poster eP.A.106]. European AIDS Clinical Society (EACS) 2023. Warsaw, Poland, 2023.
- 40. Sellem B, Abdi B, Le M, et al Intermittent bictegravir/emtricitabine/tenofovir alafenamide treatment maintains high level of viral suppression in virally suppressed people living with HIV. J Pers Med 2023; 13: 583.
- 41. Johns BA, Kawasuji T, Taishi T, et al The discovery of S/GSK1349572: a once daily next generation integrase inhibitor with a superior resistance profile [abstract 55]. Conference on Retroviruses and Opportunistic Infections (CROI) 2010. San Francisco, CA, 2010.
- 42. Tsiang M, Jones GS, Goldsmith J, et al Antiviral activity of bictegravir (GS-9883), a novel potent HIV-1 integrase strand transfer inhibitor with an improved resistance profile. Antimicrob Agents Chemother 2016; 60: 7086–97.
- 43. ViiV Healthcare. TIVICAY: prescribing information. https://gskpro.com/content/dam/global/hcpportal/en_US/Prescribing_Information/Tivicay/pdf/TIVICAY-PI-PIL-IFU.PDF#page=1
- 44. White KL, Osman N, Cuadra-Foy E, et al Long dissociation of bictegravir from HIV-1 integrase-DNA complexes. Antimicrob Agents Chemother 2023; 65: e02406-20.
- 45. ViiV Healthcare. EPZICOM: prescribing information. https://www.accessdata.fda.gov/drugsatfda_docs/label/2017/021652s022lbl.pdf
- 46. Sax PE, Eron JJ, Frick A, et al Patterns of adherence in bictegravir- and dolutegravir-based regimens. Top Antivir Med 2020; 28: 483.
- 47. ViiV Healthcare. ZIAGEN: prescribing information. https://www.accessdata.fda.gov/drugsatfda_docs/label/2020/020977s035,020978s038lbl.pdf
- 48. ViiV Healthcare. TRIUMEQ: prescribing information. https://www.accessdata.fda.gov/drugsatfda_docs/label/2014/205551s000lbl.pdf
- 49. Little RJ, D'Agostino R, Cohen ML, et al The prevention and treatment of missing data in clinical trials. N Engl J Med 2012; 367: 1355–60.
- 50. Newgard CD, Lewis RJ. Missing data: how to best account for what is not known. JAMA 2015; 314: 940–1.
- 51. Benson C, Wang X, Dunn KJ, et al Antiretroviral adherence, drug resistance, and the impact of social determinants of health in HIV-1 patients in the US. AIDS Behav 2020; 24: 3562–73.
- 52. McComsey GA, Lingohr-Smith M, Rogers R, et al Real-world adherence to antiretroviral therapy among HIV-1 patients across the United States. Adv Ther 2021; 38: 4961–74.
- 53. Zhang H, Hindman JT, Lin L, et al A study of the pharmacokinetics, safety, and efficacy of bictegravir/emtricitabine/tenofovir alafenamide in virologically suppressed pregnant women with HIV. AIDS 2024; 38: F1–9.
- 54. Naidoo A, Naidoo K, Letsoalo MP, et al Efficacy, safety, and PK of BIC/FTC/TAF in adults with HIV and tuberculosis on rifampicin at Week 24 [oral 211]. Conference on Retroviruses and Opportunistic Infections (CROI) 2024. Denver, CO, 2024.