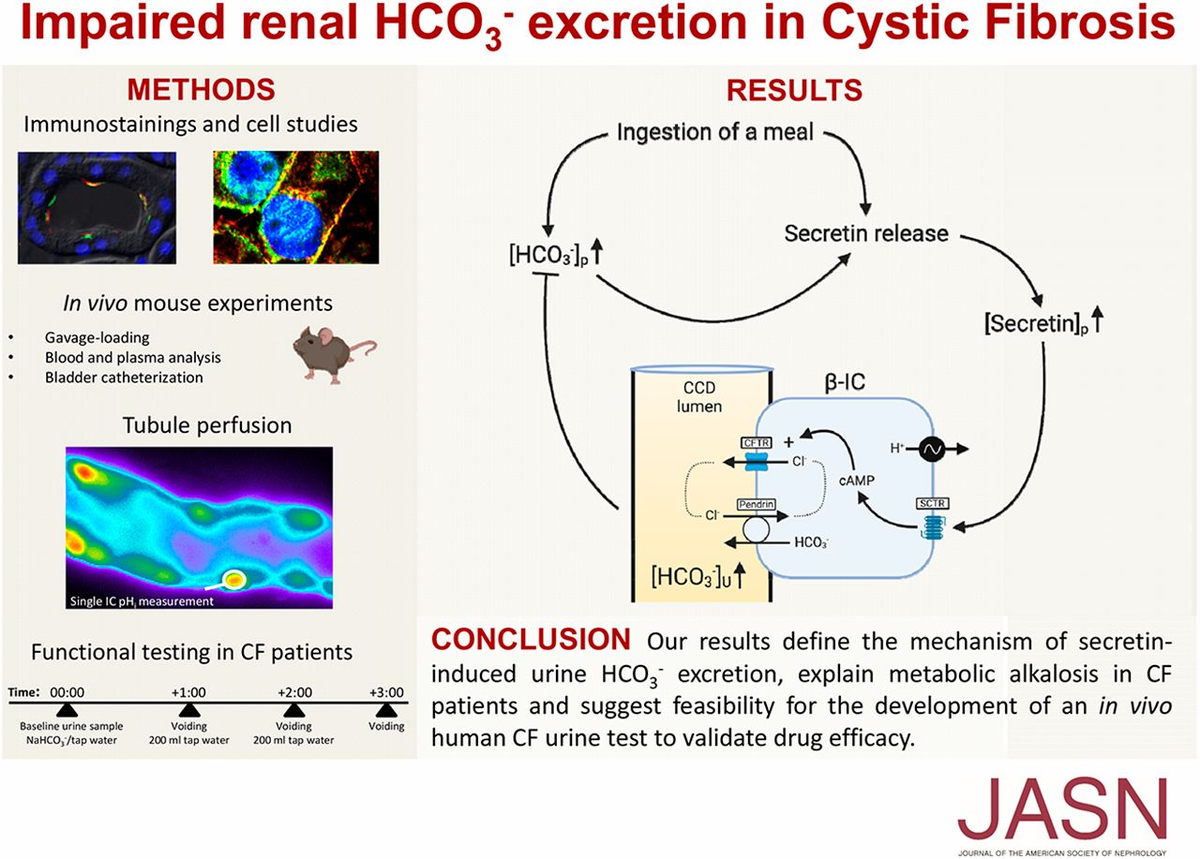
Corresponding Article
This Month's Highlights
Journal of the American Society of Nephrology 31(8):p i-i, August 2020. | DOI: 10.1681/ASN.2020060907
Corresponding Article
No Zoom Required: Meeting at the β-Intercalated Cells
- Lin, Wie-Yin
- Muallem, Shmuel
Journal of the American Society of Nephrology 31(8):p 1655-1657, August 2020. | DOI: 10.1681/ASN.2020060844