Graves disease (GD) is a common autoimmune thyroid disease driven by thyrotropin receptor antibodies (TRAbs). GD leads to reduced quality of life and is associated with increased cardiovascular morbidity and mortality (). The initial treatment aims to restore euthyroidism with antithyroid drugs (ATDs) or by inducing hypothyroidism with radioiodine (RAI) or thyroidectomy. ATD is first-line treatment, but has the drawback of about 50% recurrence with smoking, large goiter, and level of TRAbs as predictors of relapse (). The mechanism of remission in GD is unknown, and biomarkers predicting long-term outcome other than TRAbs are missing. Most studies have follow-up times of 2 to 5 years (), except a recent Swedish study with 6 to 10 years follow-up in which preserved thyroid function was found in 40% (). Longer observations do not exist.
Thyroid eye disease (TED), which affects up to 40% of GD patients (), considerably affects visual function, work ability, self-confidence, and quality of life (). The long-term risks of developing TED or progress of TED have been less studied. In addition, patients with GD have increased risk of developing other autoimmune diseases (, ), but the long-term risk and how it affects GD prognosis is unknown.
To address these knowledge gaps, we reinvestigated patients included in a randomized trial on ATD treatment 25 years ago ().
Materials and Methods
Patients and Design
In 1997 to 1999, a total of 218 patients with GD (age range, 16-75 years) were included in a prospective randomized clinical trial comparing block-replace or titration regimens for 12 months. For a subsequent 12 months, half of the patients in each group were randomly assigned to continued thyroxine treatment alone or to stop all medication. All were followed for 2 years after discontinuation of ATDs (). Blood samples were collected at inclusion and during follow-up and stored at −80 °C. In 2021 we invited all 195 alive patients to participate in the present study, and 155 (79%) agreed to participate. Thirty-six patients that either refused or did not respond were excluded. These patients did not differ regarding sex, age at disease onset, or relapse rate from those who agreed to participate. After ethics approval, deceased patients (n = 23) and those we were unable to reach (n = 4) were included in the analyses, leaving a total of 182 participants (Fig. 1). Plasma samples from 82 patients collected at baseline, 3 months, and 12 months were randomly selected for biomarkers studies. Clinical data on these patients are given in the supplemental materials (Supplementary Table S1 and S2 ()). Eighty-two age- and sex-matched blood donors were used as controls.
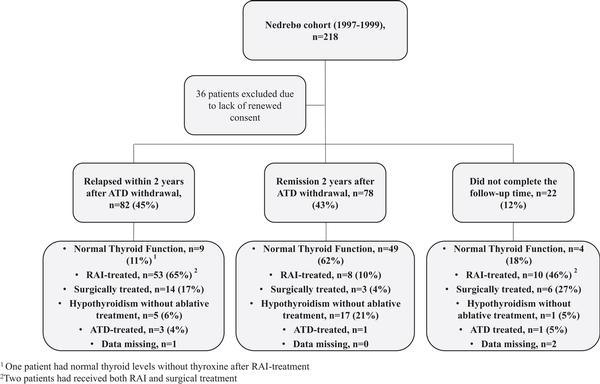
Figure 1
Flowchart of the study population. Showing outcome in the original study 2 years after discontinuation of antithyroid drugs (ATD) therapy and received treatment as of 2022. Twenty-two patients did not complete the treatment or follow-up time in the original study due to pregnancy, side effects of ATD, development of spontaneous hypothyroidism, change in residence, wish for ablative treatment, or high disease activity with indication for extended treatment duration with ATD or ablative treatment during the study period.
Clinical Data and Questionnaires
Study and hospital records were reviewed with respect to relapse of GD and treatment modalities, from inclusion in the original study until December 31, 2022, or death. Thyroid hormone levels were assessed to capture all cases of hyperthyroidism and hypothyroidism, and results were compared with self-reported information. If inconsistencies were identified, the information was validated against medical records, defined as the gold standard. ATD and levothyroxine usage was validated through each patient's Norwegian Summary Care Record, containing information on dispensed medications. Data were incomplete on 3 patients that had moved abroad after the original trial. A total of 136 patients completed the Thyroid-Specific Quality of Life Questionnaire (ThyPRO), which maps physical and mental symptoms, impaired function, and well-being. The Norwegian version is validated and has demonstrated good cross-cultural validity (see supplementary materials) (, ). ThyPRO scores from the Danish general population were used as controls ().
Definitions
Persisting or relapsing disease activity was defined as either patients that needed extended ATD treatment beyond 2 years or ablative treatment due to uncontrolled disease in the original study or had documented relapse after the initial study. A treatment period with ATD was defined as 12 months or longer. TED was defined according to current clinical guidelines (). Autoimmune hypothyroidism (Hashimoto disease) was defined as subclinical or overt hypothyroidism after GD remission with thyroid peroxidase autoantibodies (TPOAbs). Vitamin B12 deficiency was assumed to be present in patients who were given B12 supplementation.
Biochemical Data, Autoantibodies, and Biomarkers of Inflammation
Thyroid function tests and laboratory data from the original study were retrieved. Autoantibodies to intrinsic factor, parietal cells, and tissue transglutaminase were reanalyzed in original biobank samples (see supplementary materials) (). A total of 231 plasma samples from 82 GD patients and 82 controls were analyzed by multiplex proximity extension assay (Olink Bioscience). Olink’s inflammatory panel includes 92 proteins related to inflammatory diseases and related biological processes (Supplementary Table S3) (). Each of the 92 oligonucleotide antibody pairs contains unique DNA sequences allowing hybridization only to each other. Subsequent proximity extension creates 96 unique DNA reported sequences, which are amplified by real-time polymerase chain reaction (Olink). The data are normalized for intraplate and interplate variation, and a fixed correction factor is used.
Statistics
We report categorical data as absolute numbers and percentages and continuous data as medians and range (minimum-maximum). For comparison between proportions, Pearson chi-square test was used. For comparison between groups, unpaired t test was used for normally distributed data and the Mann-Whitney U test for nonnormally distributed data. For biomarkers analyses the Benjamini-Hochberg procedure for adjustment of P value was used to correct for possible false discovery rate due to multiple comparisons when analyzing differences between GD patients and healthy controls. To avoid excluding important observations, P values were not adjusted when differences between GD patients were analyzed. A 2-tailed P value less than .05 was considered statistically significant. For biomarkers with significant differences, logistic regression was used to explore their ability to predict relapse. The models were adjusted for TRAb level and smoking status. To display longitudinal patterns of biomarkers, repeated-measures analysis of variance simultaneous component analysis (RM-ASCA+) was used to summarize linear mixed-model results (). The analysis on quality of life was evaluated using multiple linear regression. All tests were adjusted for age and sex, and corrected for multiple comparison using the Benjamini-Hochberg procedure. Further details on statistics are given in the supplementary material ().
Ethics
The study was approved by the local ethics committee (REK No. 204586). All alive participants gave renewed informed consent.
Results
Clinical Characteristics
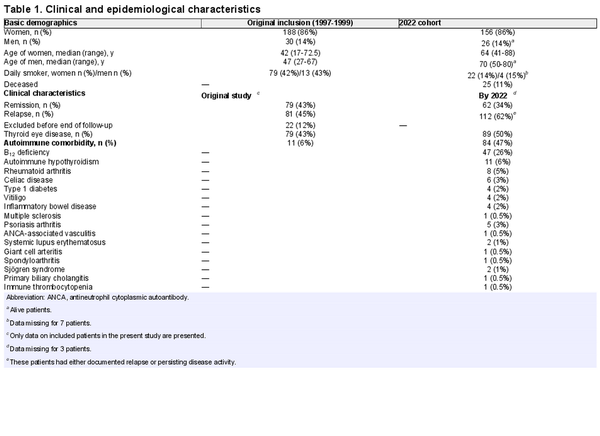
In the original study, 188 women (mean age, 41.5 years [17-73 years]) and 30 men (mean age, 48.5 years [27-67 years]) were included. A total of 189 patients completed 12 months of ATD treatment, 187 of whom completed the follow-up after therapy. Two years after discontinuation of therapy, 48% had relapsed.
In the present study, 156 women and 26 men were included (Table 1 and Fig. 1). Twenty-five of these patients had died by March 2023 at a median age of 74 years (range, 32-90 years). The most common cause of death was malignancy, occurring in 12 (48%).
Long-term Graves Disease Outcome, Treatment, and Relapse Rates
Thirty-five percent had only the GD episode that led to inclusion in the original trial, 62% had persisting or relapsing disease activity, while 3% were either lost to follow-up or received early ablative treatment due to side effects of ATD. Normal thyroid function without need for ATDs or levothyroxine replacement was achieved in 34% after more than 20 years of observation. Among those who relapsed within 2 years, 11% had normal thyroid function, compared to 62% among those who went into remission (see Fig. 1). Normal thyroid function was equally common in men (33%) and women (37%; odds ratio [OR] 1.17; CI, 0.47-2.92; P = .73). Age 40 years or younger and smoking at time of first episode of GD predicted persisting or relapsing disease (78% vs 53%; OR 3.22; CI, 1.64-6.34; P = .001% and 73% vs 55%; OR 2.21; CI, 1.18-4.16; P = .01, respectively) and gave lower likelihood of maintaining normal thyroid function at the end of follow-up (26% vs 41%; OR 0.52; CI, 0.27-0.99; P = .045% and 26% vs 43%; OR 0.46; CI, 0.24-0.86; P = .01, respectively). Sixteen patients (9%) had documented relapses beyond 10 years after primary diagnosis.
By December 2022, 71 (40%) had been treated with RAI and 23 (13%) had undergone subtotal or total thyroidectomy. Median time from initial presentation to ablative treatment was 2.5 years (range, 0.5-17 years). RAI treatment was given with the intention to render the patient hypothyroid, occurring in 99% of RAI-treated patients, including 5 patients (7%) who received more than one ablative dose and 1 (1%) patient who was thyroidectomized due to relapse. The most common indication for RAI treatment was one or more documented GD relapses (93%). The remaining patients were treated during their initial GD episode due to side effects of ATDs (4%), uncontrolled disease (1%), or wish for ablative therapy (1%). Deceased patients had more often received RAI treatment compared to those who were still alive by the end of follow-up (60% vs 36%; OR 2.63; CI, 1.11-6.23; P = .03). Out of 90 patients without ablative treatments, 23 (26%) used levothyroxine, of whom 21 (91%) had TPOAbs at inclusion in the original study. The indications to start treatment were subclinical or overt hypothyroidism with positive TPOAbs (n = 12), hypothyroidism due to blocking TRAbs (n = 2), neck radiation (n = 1), immunotherapy for cancer (n = 1), and unknown etiology (n = 7).
Inflammatory Markers in Graves Disease and Relation to Disease Course
Out of 92 biomarkers, 45 were significantly increased in GD, whereas 16 were decreased (Fig. 2A and Supplementary Table S4) (). PCA plotting revealed that GD patients and controls clustered separately at baseline, and that 12 months of treatment moved total inflammatory load incompletely toward the controls (Fig. 2B). There was no overall difference in level or trend of inflammatory markers between patients receiving block and replace vs a titration regimen (Fig. 2D).
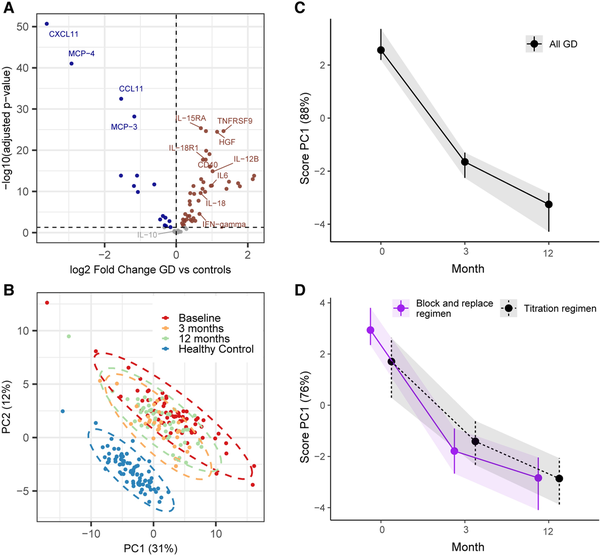
Figure 2
A, Volcano plot comparing healthy individuals and patients with Graves disease (GD) at baseline. Sixty-one out of 92 biomarkers show statistically significant difference between the 2 groups. B, Principal component analysis (PCA) scores with all included samples, showing healthy controls in light blue and samples from GD patients at baseline (dark blue), 3 months (red), and 12 months (green). The separate effect of all the normalized protein expression (NPX) biomarkers are compressed into single variables called principal components (PCs). The loading plot for the group analysis is shown in the supplementary material (Supplementary Fig. S2). C and D, Longitudinal changes in inflammation during treatment in all patients and between treatment groups. Score plots showing the development of inflammation profiles in all patients C, over time and D, grouped by antithyroid drug regimen from multivariate repeated-measures analysis of variance simultaneous component analyses. The separate effects over time of all the NPX biomarkers are compressed into PCs. PC1 explains most of the variation in biomarker development over time (88%). A high PC1 score (on the y-axis) indicates higher concentrations of biomarkers with positive loadings and lower concentrations of biomarkers with negative loadings. Vertical bars represent error bars from bootstrapping.
At baseline, interleukin 6 (IL-6) (P = .049) and tumor necrosis factor receptor superfamily member 9 (TNFRSF9) (P = .02) were significantly higher in 34 patients who relapsed within 2 years, compared with 31 patients who went into remission. TNFRSF9, but not IL-6, showed significant correlations with TRAb level (Supplementary Fig. S1) (). After adjusting for TRAbs and smoking status, IL-6 predicted relapse (OR 1 99; CI, 1.08-4.29; P = .04), while the ability of TNFRSF9 to predict relapse was not statistically significant (OR 2.36; CI, 0.96-6.50; P = .07). CD40 was lower (P = .04) at the end of treatment in 9 patients who had normal thyroid function at the end of follow-up compared to 18 patients who had hypothyroidism secondary to GD (Fig. 3). The level of CD40 did not correlate with TRAbs (see Supplementary Fig. S1) (). In receiver operating characteristic analysis, CD40 had an area under the curve of 0.77 to predict long-term outcome, but in logistic regression analysis, CD40 did not significantly predict outcome (OR 0.13; CI, 0.01-1.42; P = .13).
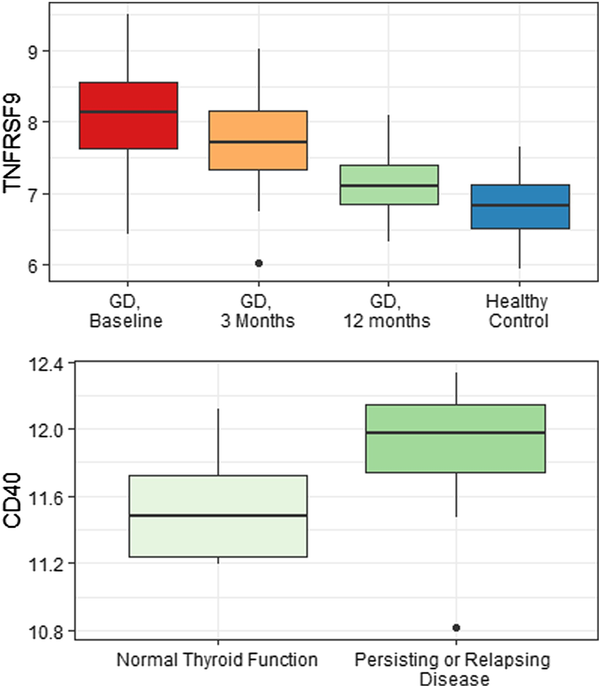
Figure 3
Box plot for value of TNFRSF9 at baseline, after 3 months of antithyroid drug (ATD) treatment and at end of therapy (12 months) compared with healthy controls (top), and CD40 at the end of treatment (12 months) in patients (n = 9) that had preserved thyroid function compared to patients that had persisting or relapsing disease that led to ablative treatment/hypothyroidism before the end of follow-up (n = 18). One patient with missing data and one patient with hypothyroidism of unknown cause were excluded from the analysis. The results are reported as relative quantification between samples by a unit named normalized protein expression (NPX). As NPX values are in log2 scale, a difference in one NPX equals a doubling in protein concentration.
Thyroid Eye Disease
During their first episode of GD, 79 patients (43%) had TED, including those with mild and transient eye disease. Only one patient (2%) experienced new worsening of TED in conjunction with GD relapse. After more than 20 years another 10 patients had developed TED, giving a total prevalence of 50%. Among the latter, 7 (70%) had de novo or flare-up of TED post RAI. Ten percent (18/179) required surgery or anti-inflammatory treatment. Twelve (67%) of these patients had TED in their first episode of GD, while 6 (33%) developed TED later in their disease course. Of the treatment-requiring patients, 7 of 18 (39%) could have been related to RAI therapy. De novo or flare-up of TED was seen in 11 of 72 (15%) RAI-treated patients, 3 (27%) of whom had received prednisolone prophylaxis (20-40 mg, gradually tapered). Seven (64%) of these patients did not have any eye symptoms or findings prior to RAI treatment. Six (55%) were current or former smokers. Excluding those with post-RAI TED, patients with TED had higher risk of relapsing or persisting disease compared with those without TED (73% vs 54%; OR 2.26; CI, 1.19-4.29; P = .01), but not reduced likelihood of having normal thyroid function at the end of follow-up (33% vs 39%; OR 0.75; CI, 0.40-1.40; P = .37).
Comorbidities
At time of inclusion in the original study, 11 of 182 (6%) patients had autoimmune comorbidity increasing to 85 of 179 (47%) at the end of follow-up (see Table 1). Forty-seven (26%) were supplemented for B12 deficiency, and in 45% of these, low or borderline levels of cobalamin were detected. For the remaining, biochemical data were not obtainable. Only 28% of those with antibodies to parietal cells at time of the original study developed B12 deficiency (Supplementary Table S5) (). After excluding patients with isolated B12 deficiency (n = 18) and hypothyroidism (n = 5) that could not be verified, 62 of 179 (35%) had at least one verified autoimmune disease. The most frequent autoimmune diseases other than B12 deficiency was autoimmune hypothyroidism (6%), and rheumatoid arthritis (RA) (5%) (see Table 1). Rates of relapsing or persisting disease were equal between patients with and without autoimmune comorbidity (P = .29). At the end of follow-up, malignancy had occurred in 17% and 22% were diagnosed with osteoporosis. Atrial fibrillation was observed in 9% of the patients, while 3% had heart failure and 5% had experienced stroke. Adding hypertension and coronary artery disease, 37% of the patients had developed one or more cardiovascular diseases during their disease course.
Quality of Life and Working Ability
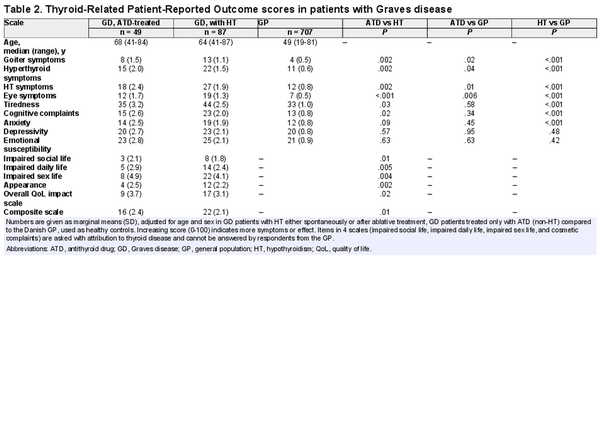
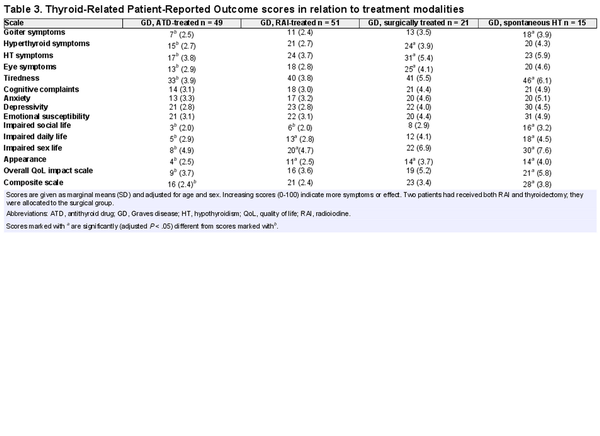
Of 136 patients, 24 (18%) replied that they had been on long-term sick leave or received permanent disability insurance due to thyroid disease. ThyPRO scale scores for patients with GD and a general reference population are summarized in Fig. 4. GD patients with hypothyroidism either spontaneously or after ablative treatment had higher symptom burden on most scales, compared to the general population and ATD-treated GD patients (Table 2). Patients treated only with ATDs had more comparable symptom scales with the general population, but higher scores on physical symptom scales (see Table 2). Table 3 shows ThyPRO scores in relation to treatment modality. There were no significant differences between RAI- and surgically treated patients. Patients diagnosed with TED did not have reduced quality of life compared with patients without (P = .92).
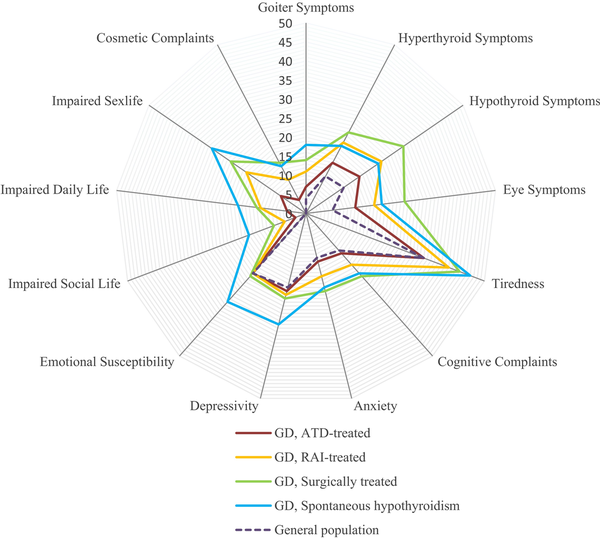
Figure 4
Mean Thyroid-Related Patient-Reported Outcome (ThyPRO) questionnaire scale scores in Graves disease (GD) patients treated with radioiodine (RAI) or surgery, compared to GD patients with spontaneous hypothyroidism, antithyroid drug (ATD)-treated GD patients, and the general population. Scale score ranges from 0 to 100, with higher score indicating more symptoms or effect. Items in 4 scales (impaired social life, impaired daily life, impaired sex life, and cosmetic complaints) are asked with attribution to thyroid disease and cannot be answered by respondents from the general population.
Discussion
Scrutiny of GD patients with the longest follow-up time ever reported confirms recent observations that only a third end up with normal thyroid function (). We extend this observation by showing that up to 50% developed autoimmune comorbidities and 10% treatment-requiring TED. Smoking, TED, and age below 40 years at onset were confirmed as risk factors associated with relapsing disease. GD patients displayed a broad systemic inflammatory response that improved during treatment. Especially IL-6, but also TNFRSF9 at baseline and CD40 at the end of therapy, emerge as potential new biomarkers. Development of hypothyroidism was associated with reduced quality of life compared to the general population and ATD-treated GD patients, indicating that long-term ATD therapy is a beneficial treatment option.
Intriguingly, only two-thirds of those in remission 2 years after discontinuation of therapy had preserved thyroid function after 25 years. Furthermore, 9% had relapse beyond 10 years after primary diagnosis, which indicates that late recurrences are common. Our findings support lifelong annual thyroid function tests for patients in remission to detect relapse or hypothyroidism (, ). Sjölin et al () reported that of the initial ATD-treated patients in remission, 23% needed thyroxine replacement after 6 to 10 years, but the indication to start treatment was uncertain. We found that 26% of patients who did not receive ablative treatment were supplemented with thyroxin at the end of follow-up; 91% of these were TPOAb positive at inclusion in the original trial. Our data indicate that coexistence of GD and Hashimoto disease is common. Thus, the risk of hypothyroidism after GD remission is substantial and patients should be informed and followed with this in mind.
The National Institute for Health and Care Excellence (NICE) guidelines recommend RAI as first-line treatment for adults with GD, unless ATDs are likely to achieve remission (). On the contrary, the European and American Thyroid Association guidelines both recommend continued low-dose ATD treatment to those who prefer this approach (, ). None of the guidelines provide specific advice on patient selection or how to determine treatment duration if long-term treatment is selected. Several risk factors for relapse prediction after ATD discontinuation have been proposed (, ), but with limited follow-up time and small predictive power for the individual patient. We found that age below 40 at time of diagnosis predicts poor outcome, supporting some studies (), but contradicting others (). Furthermore, we also show that smoking and TED predicted recurrence (, ), emphasizing that smoke cessation should be part of the treatment plan. Taken together, our data support that long-term ATD treatment now is a more common approach. Patients with young age, smoking, or TED at onset emerge as candidates for long-term treatment as a first-line option.
The original study is one of few published randomized ATD treatment trials and proved that block-and-replace treatment did not lead to higher remission rates (). If ATD had immunomodulatory properties, one would expect dose dependency. Still, ATDs’ effect on thyroid autoimmunity is debated, especially since long-term treatment gives higher remission rates (). We demonstrate for the first time profound inflammatory changes in GD, with the greatest reductions during the first 3 months, regardless of ATD regimen, which supports that remission is linked to restoration of the euthyroid state.
Biomarkers other than TRAbs to predict GD disease course are lacking. TNFRSF9 is a member of the tumor necrosis factor receptor family, expressed on activated immune cells. Interaction with antigen-presenting cells leads to secretion of proinflammatory cytokines including IL-6, while secretion of IL-10 is inhibited (). Our data support previous findings of high levels of IL-6 in untreated GD, with reduction during treatment (). Here we extend these observations by demonstrating that IL-6 levels at baseline predicted outcome. Taken together, TNFSF9 and IL-6 are more upregulated in patients with a severe phenotype () and could serve as new predictive biomarkers in the shorter term.
CD40 plays a central role in GD pathogenesis by promoting B-cell activation and TRAb production. Elevated levels of CD40 in serum have been reported to correlate with TRAb levels and thyroid volume (). For the first time, we demonstrate lower levels in patients who had preserved thyroid function at the end of follow-up. Interestingly, the monoclonal antibody iscalimab, which inhibits the interaction between CD154 and CD40, has shown to be clinically effective in a subgroup of patients (). Our data suggest that CD40 could become a potential biomarker for predicting favorable outcome in the longer term, and iscalimab is a promising treatment option.
A recent meta-analysis concluded that the prevalence of TED in patients with GD is 40% (), including papers discussing early onset or mild TED, which is in alignment with our findings. Tanda et al () found that two-thirds had no ocular involvement and only 6% had moderate to severe TED. Moreover, the risk of TED progression was low after 18 months. By extending the follow-up time to 20 years or longer, we demonstrate that patients without TED at baseline had low risk of TED development and patients with mild TED low risk of progression, except for those who received RAI. These findings align with a recent paper by Azizi et al () showing that progression and development of TED in the long term was more associated with RAI than ATD. Finally, we confirm that TED increases risk of relapse, suggesting careful follow-up and longer treatment duration with ATD in these patients ().
Autoimmune comorbidity was common, occurring in up to 47%, including B12 deficiency and autoimmune hypothyroidism, compared to 10% to 17% in previous reports that excluded autoimmune hypothyroidism (, ). Notably, we found that 26% were supplemented for B12 deficiency. The Norwegian Prescription Database reports that 61 of 1000 (6.1%) individuals in the same age group were prescribed B12 supplementation in 2020. Although we cannot validate if all patients had autoimmune gastritis, our data indicate that B12 deficiency is highly overrepresented in GD. Furthermore, screening with antibodies to parietal cells or intrinsic factor at onset did not predict who later developed B12 deficiency. RA is also frequent, 2 to 3 times higher than reported earlier (, ). This could be related to the high age of our patients, and that Norway is among countries with the highest prevalence of RA (). Autoimmune comorbidity did not increase relapse risk.
We confirm reduced quality of life in GD patients compared to the general population more than 20 years after primary diagnosis, but could not confirm worse thyroid-related outcome in RAI-treated patients (). Reduced quality of life, even many years after treatment, is described in patients with TED (, ). Surprisingly, having TED did not affect quality of life in our cohort. Older age in the patients compared to the controls could be another influencing factor, but we corrected for this in our analysis. ATD-treated patients had preserved quality of life on more scales, indicating that reduced quality of life is mainly driven by hypothyroidism. Clinicians should take this into account when treatment options are discussed, as long-term treatment with ATD emerges as a beneficial treatment option ().
There are some limitations to our study. We lack information on clinical activity scores in our TED patients. Furthermore, the storage of plasma samples for more than 20 years could affect their quality. However, large differences between controls and patients suggest that this is a minor problem. By analyzing 92 different biomarkers, there is an increased risk of chance findings and our sample sizes are relatively small. Smoking could be a confounder since it is reasonable to believe that very few blood donors smoked. Finally, at the time of the original study, long-term ATD treatment or giving a second course in case of relapse were less common. Higher use of these alternatives could have resulted in lower prevalence of hypothyroidism in our cohort.
In conclusion, we confirm that only one-third of GD patients obtain normal thyroid function. Many develop hypothyroidism and permanently reduced quality of life, either spontaneously or secondary to ablative treatment, arguing for longer ATD treatment as a beneficial treatment option. A higher risk of autoimmune comorbidity than previously described was identified, with a remarkably high risk of B12 deficiency, indicating that screening is indicated. IL-6 and TNFRSF9 at baseline and CD40 at the end of therapy emerge as a potential new biomarker for disease course to guide treatment decisions. Current clinical guidelines should consider recommending long-term ATD treatment as a standard approach in patients with young age, smoking, or TED at onset.
Abbreviations
ATD: antithyroid drug
GD: Graves disease
IL-6: interleukin 6
OR: odds ratio
RA: rheumatoid arthritis
RAI: radioiodine
RM-ASCA+: repeated-measures analysis of variance simultaneous component analysis
TED: thyroid eye disease
ThyPRO: Thyroid-Related Patient-Reported Outcome questionnaire
TNFSRF9: tumor necrosis factor receptor superfamily member 9
TPOAbs: thyroid peroxidase autoantibodies
TRAbs: thyrotropin receptor antibodies
References
- 1. Lillevang-Johansen M, Abrahamsen B, Jørgensen HL, Brix TH, Hegedüs L. Excess mortality in treated and untreated hyperthyroidism is related to cumulative periods of low Serum TSH. J Clin Endocrinol Metab. 2017;102(7):2301–2309.
- 2. Okosieme OE, Taylor PN, Evans C, et al Primary therapy of Graves’ disease and cardiovascular morbidity and mortality: a linked-record cohort study. Lancet Diabetes Endocrinol. 2019;7(4):278–287.
- 3. Törring O, Watt T, Sjölin G, et al Impaired quality of life after radioiodine therapy compared to antithyroid drugs or surgical treatment for graves’ hyperthyroidism: A long-term follow-up with the thyroid-related patient-reported outcome questionnaire and 36-item short form health Status survey. Thyroid. 2019;29(3):322–331.
- 4. Nedrebo BG, Holm PI, Uhlving S, et al Predictors of outcome and comparison of different drug regimens for the prevention of relapse in patients with graves’ disease. Eur J Endocrinol. 2002;147(5):583–589.
- 5. Abraham P, Avenell A, McGeoch SC, Clark LF, Bevan JS. Antithyroid drug regimen for treating graves’ hyperthyroidism. Cochrane Database Syst Rev. 2010;2010(1):Cd003420.
- 6. Sundaresh V, Brito JP, Thapa P, Bahn RS, Stan MN. Comparative effectiveness of treatment choices for graves’ hyperthyroidism: A historical cohort study. Thyroid. 2017;27(4):497–505.
- 7. Sjölin G, Holmberg M, Törring O, et al The long-term outcome of treatment for graves’ hyperthyroidism. Thyroid. 2019;29(11):1545–1557.
- 8. Chin YH, Ng CH, Lee MH, et al Prevalence of thyroid eye disease in graves’ disease: A meta-analysis and systematic review. Clin Endocrinol (Oxf). 2020;93(4):363–374.
- 9. Abraham-Nordling M, Wallin G, Träisk F, et al Thyroid-associated ophthalmopathy; quality of life follow-up of patients randomized to treatment with antithyroid drugs or radioiodine. Eur J Endocrinol. 2010;163(4):651–657.
- 10. Boelaert K, Newby PR, Simmonds MJ, et al Prevalence and relative risk of other autoimmune diseases in subjects with autoimmune thyroid disease. Am J Med. 2010;123(2):183.e1–9.
- 11. Ferrari SM, Fallahi P, Ruffilli I, et al The association of other autoimmune diseases in patients with Graves’ disease (with or without ophthalmopathy): review of the literature and report of a large series. Autoimmun Rev. 2019;18(3):287–292.
- 12. Meling Stokland AE, Austdal M, Nedrebø BG. Data from: Outcomes of Patients with Graves’ disease 25 Years after Initiating Anti-Thyroid Drug Therapy Zenodo. Updated Deposited August 22. 2023. https://doi.org/10.5281/zenodo.8274039
- 13. Cramon P, Bonnema SJ, Bjorner JB, et al Quality of life in patients with benign nontoxic goiter: impact of disease and treatment response, and comparison with the general population. Thyroid. 2015;25(3):284–291.
- 14. Bartalena L, Baldeschi L, Boboridis K, et al The 2016 European Thyroid Association/European Group on Graves’ Orbitopathy Guidelines for the Management of Graves’ Orbitopathy. Eur Thyroid J. 2016;5(1):9–26.
- 15. Jarmund AH, Madssen TS, Giskeødegård GF. ALASCA: an R package for longitudinal and cross-sectional analysis of multivariate data by ASCA-based methods. Front Mol Biosci. 2022;9:962431.
- 16. Ross DS, Burch HB, Cooper DS, et al 2016 American thyroid association guidelines for diagnosis and management of hyperthyroidism and other causes of thyrotoxicosis. Thyroid. 2016;26(10):1343–1421.
- 17. Kahaly GJ, Bartalena L, Hegedus L, Leenhardt L, Poppe K, Pearce SH. 2018 European thyroid association guideline for the management of graves’ hyperthyroidism. Eur Thyroid J. 2018;7(4):167–186.
- 18. National Institute for Health and Care Excellence: Guidelines. Thyroid disease: assessment and management. National Institute for Health and Care Excellence (NICE). Copyright © NICE 2019.; 2019.
- 19. Struja T, Fehlberg H, Kutz A, et al Can we predict relapse in graves’ disease? Results from a systematic review and meta-analysis. Eur J Endocrinol. 2017;176(1):87–97.
- 20. Shi H, Sheng R, Hu Y, et al Risk factors for the relapse of graves’ disease treated with antithyroid drugs: A systematic review and meta-analysis. Clin Ther. 2020;42(4):662–675.e4.
- 21. Vos XG, Endert E, Zwinderman AH, Tijssen JG, Wiersinga WM. Predicting the risk of recurrence before the start of antithyroid drug therapy in patients with graves’ hyperthyroidism. J Clin Endocrinol Metab. 2016;101(4):1381–1389.
- 22. Wiersinga WM. Smoking and thyroid. Clin Endocrinol (Oxf). 2013;79(2):145–151.
- 23. Azizi F, Amouzegar A, Tohidi M, et al Increased remission rates after long-term methimazole therapy in patients with graves’ disease: results of a randomized clinical trial. Thyroid. Sep 2019;29(9):1192–1200.
- 24. Dharmadhikari B, Wu M, Abdullah NS, et al CD137 And CD137L signals are main drivers of type 1, cell-mediated immune responses. Oncoimmunology. 2016;5(4):e1113367.
- 25. Pedro AB, Romaldini JH, Takei K. Changes of serum cytokines in hyperthyroid Graves’ disease patients at diagnosis and during methimazole treatment. Neuroimmunomodulation. 2011;18(1):45–51.
- 26. Ueland HO, Ueland G, Løvås K, et al Novel inflammatory biomarkers in thyroid eye disease. Eur J Endocrinol. 2022;187(2):293–300.
- 27. Yamamoto K, Itoh M, Okamura T, et al Relative levels of the inflammatory cytokine TNFα and the soluble CD40 ligand profile in serum correlate with the thyrotoxic activity of graves’ disease. Thyroid. 2012;22(5):516–521.
- 28. Kahaly GJ, Stan MN, Frommer L, et al A novel anti-CD40 monoclonal antibody, iscalimab, for control of graves hyperthyroidism-A proof-of-concept trial. J Clin Endocrinol Metab. 2020;105(3):dgz013.
- 29. Tanda ML, Piantanida E, Liparulo L, et al Prevalence and natural history of graves’ orbitopathy in a large series of patients with newly diagnosed graves’ hyperthyroidism seen at a single center. J Clin Endocrinol Metab. 2013;98(4):1443–1449.
- 30. Azizi F, Abdi H, Mehran L, Perros P, Masoumi S, Amouzegar A. Long-term follow-up of Graves’ orbitopathy after treatment with short-term or long-term methimazole or radioactive iodine. Endocr Pract. 2023;29(4):240–246.
- 31. Kiadaliri AA, Kristensen LE, Englund M. Burden of rheumatoid arthritis in the Nordic region, 1990-2015: a comparative analysis using the Global Burden of Disease Study 2015. Scand J Rheumatol. 2018;47(2):1–101.
- 32. Terwee C, Wakelkamp I, Tan S, Dekker F, Prummel MF, Wiersinga W. Long-term effects of Graves’ ophthalmopathy on health-related quality of life. Eur J Endocrinol. 2002;146(6):751–757.