INTRODUCTION
Patients with stage I human epidermal growth factor receptor 2 (HER2)–positive breast cancer have recurrence rates ranging between 5% and 30%, supporting the administration of adjuvant systemic therapy. The APT trial found that 12 weeks of adjuvant paclitaxel, combined with 1 year of trastuzumab (TH regimen), was associated with a 10-year invasive disease-free survival (iDFS) of 91.3% for patients with small node-negative HER2-positive tumors, leading to the recommendation by international guidelines and worldwide adoption of this regimen.
CONTEXT
Key Objective
To determine long-term outcomes of patients with stage I human epidermal growth factor receptor 2 (HER2)–positive breast cancer treated with adjuvant trastuzumab emtansine (T-DM1), and to investigate biomarkers able to identify patients with a higher risk of recurrence despite adjuvant treatment.
Knowledge Generated
We report outstanding long-term outcomes with adjuvant T-DM1 for stage I HER2-positive tumors, with a 5-year invasive disease-free survival of 97.0%. In addition, we report multiomic correlative analyses conducted on the study samples and describe the results of HER2DX testing, which identified a subset of patients with significantly higher risk of recurrence in ATEMPT.
Relevance (J.W. Friedberg)
One year of adjuvant T-DM1 is a treatment option for patients with stage I HER2 positive breast cancer who are not candidates of chemotherapy, resulting in durable responses.*
*Relevance section written by JCO Editor-in-Chief Jonathan W. Friedberg, MD.
Although TH is less toxic than trastuzumab combined with poly-chemotherapy regimens, it is still associated with adverse events that can affect quality of life including fatigue, neutropenia, peripheral neuropathy, and alopecia. Thus, optimizing adjuvant therapy remains an area of active interest in this setting. Trastuzumab emtansine (T-DM1) is currently approved for the adjuvant treatment of patients with HER2-positive breast cancer with residual invasive disease after neoadjuvant treatment or with metastatic disease, and in the metastatic setting, it has shown similar efficacy with lower toxicity compared with trastuzumab plus taxane treatment.,
The randomized phase II ATEMPT trial was designed to evaluate the activity of 1 year of adjuvant T-DM1 and to compare the rate of clinically relevant toxicities (CRT) with that of TH in patients with stage I centrally confirmed HER2-positive breast cancer. In the initial analysis, T-DM1 produced a 3-year iDFS of 97.8% (95% CI, 96.3 to 99.3), which rejected the null hypothesis for efficacy (P < .001). The CRT rate was 46% for patients on T-DM1 and 47% for patients on TH (P = .83). In this final preplanned analysis of the trial, we report the 5-year survival outcomes for patients enrolled in ATEMPT and conduct a multidimensional assessment of HER2 heterogeneity, evaluated through machine learning (ML) algorithms, single-cell fluorescence in situ hybridization (FISH), spatial proteomics, and the HER2DX genomic assay. Finally, we investigate genomic correlates of thrombocytopenia or bleeding with T-DM1 through germline whole-genome sequencing (WGS).
METHODS
Study Design
ATEMPT was a multicenter, randomized, investigator-initiated phase II trial. Details of the trial design and patient population have been previously reported. Briefly, eligible patients included those with pathologic stage I (N0 or N1mi) HER2-positive invasive breast cancer (HER2-positive by central testing according to the ASCO/CAP 2013 guidelines). Patients were required to have left ventricular ejection fraction ≥50%, have Eastern Cooperative Oncology Group performance status ≤1, be within 90 days of most recent breast surgery, and have no history of previous invasive breast cancer.
Patients were randomly assigned after surgery in a 3:1 ratio to receive T-DM1 (3.6 mg/kg once every 21 days for 17 cycles) or TH (once weekly paclitaxel 80 mg/m2 with concurrent once weekly trastuzumab at a loading dose of 4 mg/kg followed by 2 mg/kg for 12 weeks, followed by 6 mg/kg trastuzumab once every 21 days for 13 cycles). Adjuvant radiation therapy and endocrine therapy could be initiated after 12 weeks of T-DM1 or after the conclusion of paclitaxel therapy. Patients were followed for recurrence after the completion of study treatment. Those who discontinued treatment for unacceptable toxicity remained on study and were monitored for recurrence and resolution of toxicity. Patients were monitored for CRTs, defined as grade ≥3 nonhematologic toxicity, grade ≥2 neurotoxicity, grade ≥4 hematologic toxicity, febrile neutropenia, any serious adverse event, and any adverse event requiring dose delay or treatment discontinuation.
Outcomes
The coprimary end points, previously reported, were to compare the incidence of CRT in patients treated with T-DM1 versus TH and to evaluate iDFS in patients treated with T-DM1. Patients were monitored for iDFS, defined as invasive local or regional recurrence, contralateral invasive breast cancer, distant recurrence, or death from any cause. Recurrence-free interval (RFI) was a secondary end point and was defined as invasive local or regional recurrences, distant recurrences, and any death from breast cancer. Correlative analyses were conducted on the study samples, all of which were post hoc except the analysis of genomic predictors of thrombocytopenia, which was prespecified in the trial.
Statistical Analysis
The coprimary end points were evaluated separately, and the family-wise Type I error rate for the study was controlled at 10% using a Bonferroni correction. On the basis of historical data,,, a 3-year iDFS failure rate of 5% or less with T-DM1 was considered successful, whereas a 3-year iDFS failure rate of 9% was deemed to be unacceptable. With 375 patients in the T-DM1 arm, the study had 95% power to reject the null hypothesis for efficacy, with overall type I error controlled at 5% (one-sided).
HER2DX Testing
The standardized HER2DX genomic test was performed at Hospital Clinic of Barcelona (Spain) on 225 primary tumor samples from patients enrolled in ATEMPT and was successful for 187 samples with enough invasive component (T-DM1 arm: 147, TH arm: 40). The pre-established HER2DX risk score cutoff was used to create HER2DX risk groups (low [0-50] and high [50-100]). The association between HER2DX risk groups and survival outcomes was assessed among all patients treated in ATEMPT. Genomic analyses were performed blinded from clinical outcome data. HER2DX results were further compared among patients with and without HER2 genetic heterogeneity detected at single-cell FISH.
Machine Learning Analysis of HER2 Expression
PathAI's ML-based HER2 algorithms were deployed on whole slide images of the HER2-stained slides from the ATEMPT clinical trial. We calculated the proportion of invasive cancer cells with each stain intensity and the circumferential completeness on the basis of the ASCO/CAP 2018 guidelines. The distribution of each stain intensity proportion feature across cases was compared for multiple data set splits of interest using the Mann-Whitney U test. The following data splits were tested for significant differences in the distribution across patients: (1) RFI event versus no RFI event (RFI event n = 10, no RFI event n = 432), (2) estrogen receptor (ER)–positive versus ER-negative (ER-positive n = 318, ER-negative n = 124), and (3) progesterone receptor (PR)–positive versus PR-negative (PR-positive n = 239, PR-negative n = 203). The heterogeneity in stain intensity was quantified by calculating the entropy of the distribution across stain intensities across one slide and compared between the three data set splits listed above using the Mann-Whitney U test. All reported P values were corrected using the Benjamini-Hochberg method for false discovery rate (FDR) correction.
HER2 Genetic Heterogeneity
Central pathology evaluation of HER2 heterogeneity was performed at the European Institute of Oncology (Milan, Italy) on 13 baseline samples from patients experiencing RFI events (cases) and 39 controls matched on study arm, tumor size, and hormone receptor status. The study included 24 patients from the T-DM1 arm and 28 patients in the TH arm. HER2 gene status was assessed by FISH using the HER2 IQFISH pharmDx assay. FISH-stained slides were evaluated using a Zeiss Axio Imager Z2 microscope, equipped with Metafer 4 Metacyte software for automating microscopy. Each slide was evaluated visually and independently by two trained readers blinded to outcomes, counting at least 60 invasive tumor cells, and subsequently using the Metafer software counting an average of 300 invasive tumor cells. HER2 heterogeneity was defined as the existence of the population of tumor cells with HER2 amplification (ie, HER2/chromosome enumeration probe [CEP17] ratio >2.0 or a gene copy number [GCN] >6), representing more than 5% but < 50% of invasive tumor cells, following recommendations from dedicated guidelines.,
NanoString GeoMX Digital Spatial Profiling
FFPE tumor samples from 13 patients who experienced iDFS events and 21 matched controls were analyzed using the NanoString GeoMX Digital Spatial Profiler. Detailed methods are provided in the Data Supplement (online only).
WGS and Thrombocytopenia Associations
Germline WGS was conducted among 110 patients treated with T-DM1 in ATEMPT to identify genomic predictors of thrombocytopenia or bleeding with T-DM1. Detailed methods are provided in the Data Supplement.
Compliance With Ethical Standards
The study was conducted in accordance with the International Conference on Harmonization Good Clinical Practice Standards and the Declaration of Helsinki. Institutional review board approval was obtained from the Dana-Farber/Harvard Cancer Center and all participating sites. Safety and efficacy monitoring was conducted on a semiannual basis by a data safety monitoring board. The investigators obtained informed consent from each participant or each participant's guardian.
RESULTS
Among 512 patients recruited between May 2013 and December 2016, 384 and 128 were randomly assigned to the T-DM1 arm and to the TH arm, respectively. Of these, 383 patients on the T-DM1 arm and 114 patients on the TH arm received protocol treatment and were included in the analysis (Fig 1). Baseline patient and tumor characteristics were reported previously and did not differ significantly between study arms (Table 1). Approximately half of the patients had tumors larger than 1 cm, 373 (75%) had hormone receptor-positive disease, and 4% had micrometastatic nodal involvement, with all the remaining patients having node-negative disease.
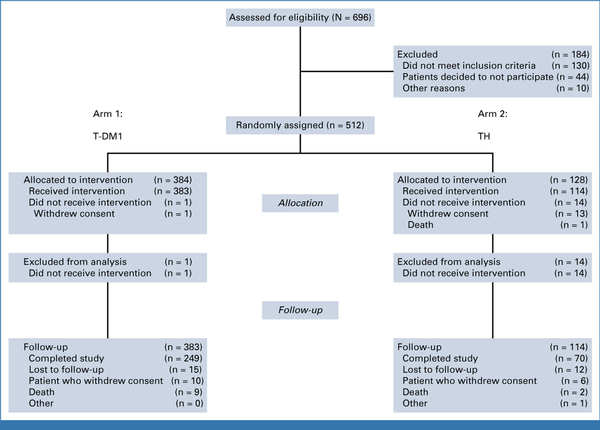
FIG 1.
CONSORT diagram. T-DM1, trastuzumab emtansine; TH, paclitaxel plus trastuzumab.
TABLE 1.
Patient and Tumor Characteristics
| Characteristic | Arm 1: T-DM1 | Arm 2: TH | ||||||
|---|---|---|---|---|---|---|---|---|
| ITT (n = 383) | No HER2DX (n = 236) | HER2DX (n = 147) | P | ITT (n = 114) | No HER2DX (n = 74) | HER2DX (n = 40) | P | |
| Age, years | ||||||||
| Median (range) | 56 (32-85) | 55 (33-79) | 57 (32-85) | .14 | 55.5 (23-82) | 56 (29-82) | 53 (23-82) | .64 |
| Sex, No. (%) | ||||||||
| Female | 378 (99) | 232 (98) | 146 (99) | .65 | 113 (99) | 74 (100) | 39 (98) | .35 |
| Male | 5 (1) | 4 (2) | 1 (1) | 1 (1) | 0 (0) | 1 (2) | ||
| Race, No. (%) | ||||||||
| White | 327 (85) | 198 (84) | 129 (88) | .82 | 93 (82) | 61 (82) | 32 (80) | .29 |
| Black/African American | 21 (5) | 14 (6) | 7 (5) | 7 (6) | 6 (8) | 1 (2) | ||
| Asian | 22 (6) | 14 (6) | 8 (5) | 4 (4) | 3 (4) | 1 (2) | ||
| More than one race | 2 (1) | 2 (1) | 0 (0) | 1 (1) | 0 (0) | 1 (2) | ||
| Others | 11 (3) | 8 (3) | 3 (2) | 9 (8) | 4 (5) | 5 (12) | ||
| Ethnicity, No. (%) | ||||||||
| Hispanic or Latino | 11 (3) | 8 (3) | 3 (2) | .60 | 1 (1) | 1 (1) | 0 (0) | .86 |
| Non-Hispanic | 352 (92) | 214 (91) | 138 (94) | 97 (85) | 62 (84) | 35 (88) | ||
| Unknown | 20 (5) | 14 (6) | 6 (4) | 16 (14) | 11 (15) | 5 (12) | ||
| Tumor size, cm, No. (%) | ||||||||
| ≤0.1 | 4 (1) | 4 (2) | 0 (0) | .03 | 7 (6) | 7 (9) | 0 (0) | .05 |
| >0.1-0.5 | 57 (15) | 40 (17) | 17 (12) | 13 (11) | 11 (15) | 2 (5) | ||
| >0.5-1.0 | 127 (33) | 80 (34) | 47 (32) | 40 (35) | 26 (35) | 14 (35) | ||
| >1.0-1.5 | 124 (32) | 64 (27) | 60 (41) | 29 (25) | 18 (24) | 11 (28) | ||
| >1.5-2.0 | 71 (19) | 48 (20) | 23 (16) | 25 (22) | 12 (16) | 13 (32) | ||
| Nodal (micrometastasis), No. (%) | ||||||||
| Yes | 18 (5) | 13 (6) | 5 (3) | .46 | 1 (1) | 1 (1) | 0 (0) | 1 |
| No | 365 (95) | 223 (94) | 142 (97) | 113 (99) | 73 (99) | 40 (100) | ||
| Histologic grade, No. (%) | ||||||||
| Well diff | 11 (3) | 6 (3) | 5 (3) | .96 | 4 (4) | 2 (3) | 2 (5) | .20 |
| Moderately diff | 148 (39) | 92 (39) | 56 (38) | 46 (40) | 34 (46) | 12 (30) | ||
| Poorly diff | 219 (57) | 135 (57) | 84 (57) | 62 (54) | 36 (49) | 26 (65) | ||
| Unknown | 5 (1) | 3 (1) | 2 (1) | 2 (2) | 2 (3) | 0 (0) | ||
| HER2 by IHC (central testing), No. (%) | ||||||||
| (0, 1+) | 5 (1) | 5 (3) | 0 (0) | <.001 | 1 (1) | 1 (1) | 0 (0) | <.001 |
| (2+) | 92 (24) | 86 (36) | 6 (4) | 25 (22) | 25 (34) | 0 (0) | ||
| (3+) | 277 (72) | 137 (58) | 140 (95) | 87 (76) | 47 (64) | 40 (100) | ||
| Not done/unknown | 9 (2) | 8 (2) | 1 (1) | 1 (1) | 1 (1) | 0 (0) | ||
| Hormone receptor status, No. (%) | ||||||||
| Positive | 289 (75) | 186 (79) | 103 (70) | .07 | 84 (74) | 52 (70) | 32 (80) | .37 |
| Negative | 94 (25) | 50 (21) | 44 (30) | 30 (26) | 22 (30) | 8 (20) | ||
After a median follow-up of 5.8 years, 11 iDFS events were observed in the T-DM1 arm, consistent with a 5-year iDFS of 97.0% (95% CI, 95.2 to 98.7; Fig 2A). The 5-year RFI was 98.3% (Fig 2B, 95% CI, 97.0 to 99.7), the 5-year overall survival (OS) was 97.8% (Fig 2C, 95% CI, 96.3 to 99.3), and the 5-year breast cancer-specific survival (BCSS) was 99.4% (Fig 2D, 95% CI, 98.6 to 100). Among the 11 iDFS events observed with T-DM1, only three were distant recurrences, with the remaining events being two ipsilateral recurrences (one of which was HER2-negative), three new contralateral primary breast cancers (all HER2-negative), and three non–breast cancer-related deaths (Data Supplement, Table S1).
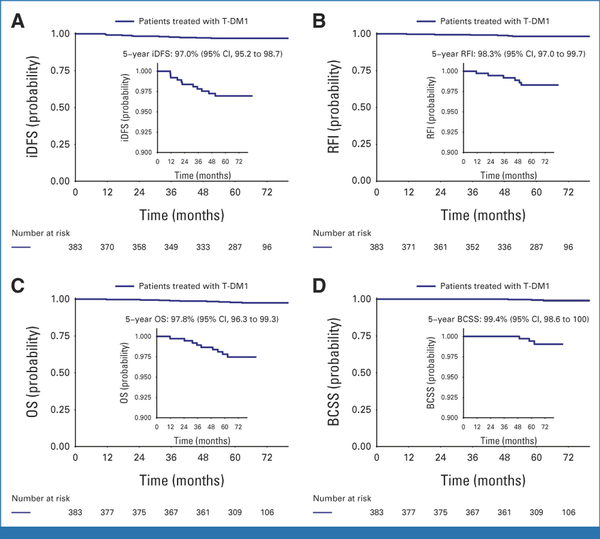
FIG 2.
Survival outcomes in the T-DM1 arm in terms of (A) iDFS, (B) RFI, (C) OS, and (D) BCSS. Outstanding long-term outcomes were observed among patients with stage I HER2-positive breast cancer receiving adjuvant T-DM1, with a 5-year iDFS of 97.0%, and only three distant recurrences were observed among 383 patients treated in the study arm. BCSS, breast cancer–specific survival; HER2, human epidermal growth factor receptor 2; iDFS, invasive disease-free survival; RFI, recurrence-free interval; T-DM1, trastuzumab emtansine.
Although the study was not powered to evaluate the efficacy of TH, a total of nine iDFS events were observed in the TH arm, consistent with a 5-year iDFS of 91.1% (Data Supplement, Fig S1A, 95% CI, 85.7 to 96.8). The 5-year RFI was 93.2% (Data Supplement, Fig S1B, 95% CI, 88.5 to 98.2), the 5-year OS was 97.9% (Data Supplement, Fig S1C, 95% CI, 95.2 to 100), and the 5-year BCSS was 99.0% (Data Supplement, Fig S1D, 95% CI, 97.2 to 100; Data Supplement, Table S1). Among the nine iDFS events observed with TH, only two were distant recurrences (Data Supplement, Table S1). Patient and tumor characteristics for the patients experiencing iDFS events are shown in the Data Supplement (Table S2).
Post hoc analyses were conducted to evaluate outcomes with T-DM1 among different clinically relevant groups of patients. Comparable survival outcomes were observed with T-DM1 irrespective of the tumor size and hormone receptor status. The 5-year iDFS was 98.7% (95% CI, 97.0 to 100) for patients having tumors <1 cm and 95.6% (95% CI, 92.9 to 98.5) for tumors ≥1 cm (Fig 3A). Similarly, the 5-year iDFS was 96.8% (95% CI, 94.7 to 98.7) for patients with hormone receptor-positive tumors and 97.6% (95% CI, 94.4 to 100) for patients with hormone receptor-negative tumors (Fig 3B). Similar survival outcomes were also observed for patients receiving T-DM1 with centrally determined HER2 immunohistochemistry (IHC) 3+ disease (n = 277 [72.3%], 5-year iDFS 96.9%, 95% CI, 94.9 to 99.1) and IHC ≤2+ disease (n = 97 [25.3%], 5-year iDFS 96.7%, 95% CI, 93.1 to 100; Fig 3C), with 10 patients not having central IHC testing. Analogous results were observed for RFI (Fig 3D).
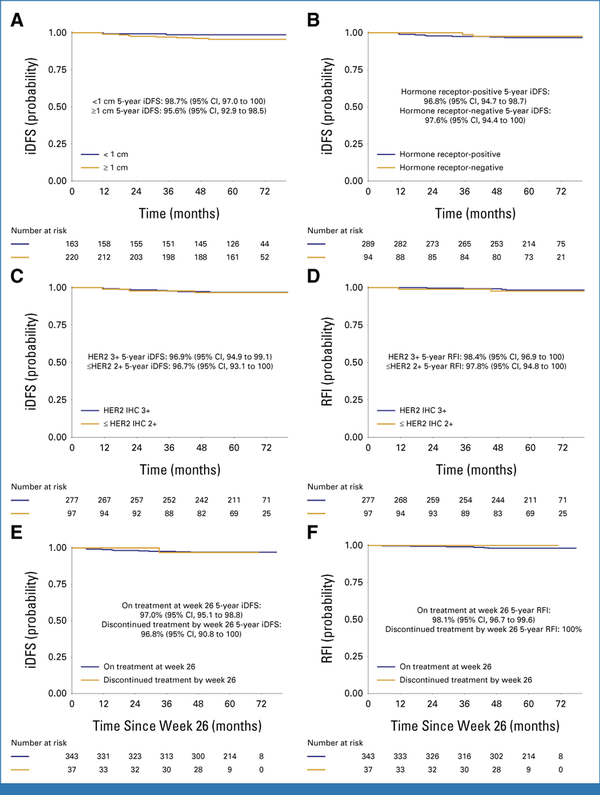
FIG 3.
Survival outcomes in the T-DM1 arm by tumor size ((A) iDFS), hormone receptor status ((B) iDFS), HER2 IHC score ((C) iDFS, (D) RFI), and exposure of T-DM1 for more or <26 weeks ((E) iDFS, (F) RFI). Comparable survival outcomes were observed among patients with stage I HER2-positive breast cancer receiving adjuvant T-DM1 irrespective of the baseline tumor size, hormone receptor status, HER2 IHC score, and exposure to more or less than 26 weeks of T-DM1. HER2, human epidermal growth factor receptor 2; iDFS, invasive disease-free survival; RFI, recurrence-free interval.
Among patients receiving T-DM1 for <6 months (n = 37, 9.7%), the 5-year iDFS was 96.8% (95% CI, 90.8 to 100) and the 5-year RFI was 100% (Fig 3E), whereas among patients receiving ≥6 months of T-DM1 (n = 343, 90.3%), the 5-year iDFS was 97.0% (95% CI, 95.1 to 98.8) and the 5-year RFI was 98.1% (95% CI, 96.7 to 99.6; Fig 3F).
HER2DX Analysis
The standardized HER2DX genomic test was obtained for 187 (37.6%) patients enrolled in ATEMPT (147 receiving T-DM1, 40 receiving TH). Baseline characteristics of the patients included in this study were comparable with those of the overall study cohort (Table 1, Data Supplement, Table S3). The 5-year iDFS and 5-year RFI in the cohort of patients with available HER2DX testing were 95.4% (95% CI, 92.3 to 98.6) and 97.0% (95% CI, 94.5 to 99.6), respectively. The proportion of patients with high expression of luminal, proliferation, and immunoglobulin G (IGG) signatures was 34.8%, 57.8%, and 48.1%, respectively, and 133 patients (71.1%) had high HER2 expression (Fig 4A, Data Supplement, Table S4). The proportion of patients with HER2DX low-risk disease was 93.6% (n = 175), whereas 6.4% (n = 12) had HER2DX high-risk disease (Fig 4B). Patients with HER2DX low-risk disease were found to have significantly more favorable RFI compared with patients having HER2DX high-risk disease (5-year 98.1% v 81.8%, hazard ratio [HR], 0.10 [95% CI, 0.02 to 0.57], P = .01) and better iDFS (5-year 96.3% v 81.8%, HR, 0.20 [95% CI, 0.04 to 0.98], P = .047; Figs 4C and 4D, Data Supplement, Table S5). A total of 26 patients (6.8%) had both HER2DX information and single-cell FISH results available to evaluate the HER2 heterogeneity (25 HER2 nonheterogeneous and one HER2 heterogeneous). The patient classified as HER2 heterogeneous showed the lowest level in HER2DX ERBB2 score, HER2DX HER2 amplicon signature, and HER2DX IGG score (Data Supplement, Fig S2B).
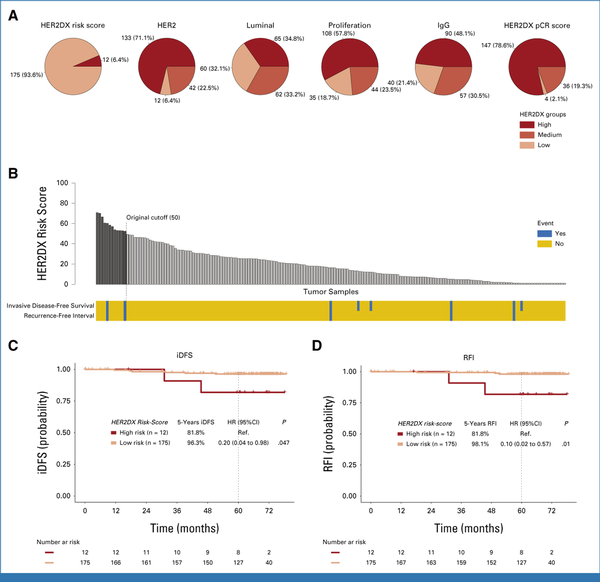
FIG 4.
The HER2DX risk score predicts the risk of recurrence in ATEMPT. (A) Distribution of the HER2DX risk score, pCR score, and main biologic features captured by HER2DX, including the expression of HER2 mRNA, the luminal signature, the proliferation signature, and the IgG signature. (B) HER2DX risk score ranking and association with iDFS events and RFI events. Five-year (C) iDFS and (D) 5-year RFI, stratified by HER2DX risk group. Compared with patients having HER2DX high-risk disease (n = 12, 6.4%), those with HER2DX low-risk disease (n = 175, 93.6%) had significantly better 5-year RFI (98.1% v 81.8%, HR, 0.10 [95% CI, 0.02 to 0.57], P = .01) and 5-year iDFS (96.3% v 81.8%, HR, 0.20 [95% CI, 0.04 to 0.98], P = .047). HER2, human epidermal growth factor receptor 2; HR, hazard ratio; iDFS, invasive disease-free survival; IgG, immunoglobulin G; pCR, pathologic complete response; ref., reference; RFI, recurrence-free interval.
Machine Learning Analysis of HER2 Heterogeneity
A wide range of variabilities in HER2 staining intensity was observed at a cancer cell level within and across the tumors (Fig 5A), with notable variability in both study arms (Fig 5B). Hormone receptor-positive tumors were found to be associated with a higher proportion of tumor cells expressing weak-to-moderate and incomplete membrane staining (ER: P = .002; PR: P < .001), whereas hormone receptor-negative tumors were associated with a higher proportion of tumor cells with intense and complete circumferential staining for HER2 (ER: P < .001; PR: P < .001, Figs 5C and 5D, Data Supplement, Tables S6 and S7). However, none of the HER2 stain intensity proportion features were significantly associated with the risk of recurrence among patients enrolled in ATEMPT (Fig 5E, Data Supplement, Table S8) although this analysis had limited power. The entropy of HER2 stain intensity distributions across each slide were evaluated as a measure of HER2 heterogeneity. Examples of tumors exhibiting high entropy (entropy = 1.55, ie, high HER2 heterogeneity) and low entropy (entropy = 0.39, ie, low HER2 heterogeneity) are shown in Figure 5F. Numerically higher entropy in HER2 staining distribution was observed among ER-positive versus ER-negative cases and PR-positive versus PR-negative cases although neither effect was significant (ER: U = 21,972, P = .062; PR: U = 26,866, P = .051; Figs 5G and 5H). No significant difference in entropy distribution was seen between patients experiencing or not experiencing recurrence, either in the overall study cohort (U = 2,649, P = .221; Fig 5I) or when evaluating each arm separately (T-DM1 arm: U = 1,209, P = .086; TH arm: U = 247, P = .982; Fig 5L).
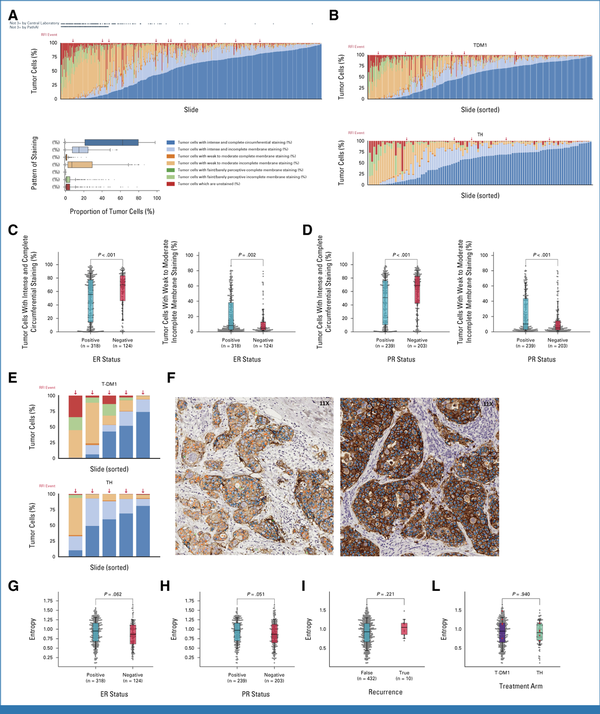
FIG 5.
Machine learning–assisted analysis of HER2 expression patterns. (A and B) Machine learning–derived HER2 stain intensity variability in the (A) overall population and (B) by study arm. (C and D) Percentage of tumor cells exhibiting intense and complete HER2 staining or weak and incomplete HER2 staining depending on (C) ER expression and (D) PR expression. (E) Distribution of tumor cells with different HER2 staining intensities among patients experiencing RFI events in each study arm. (F) Examples of tumors exhibiting high entropy (left panel, entropy = 1.55, ie, high HER2 heterogeneity) and low entropy (right panel, entropy = 0.39, ie, low HER2 heterogeneity). The colors of the dots in each cell represent different staining intensities and completeness, as defined in Figure 2A. Entropy distribution (ie, level of HER2 heterogeneity) according to (G) the ER expression and (H) PR expression of the tumor, (I) according to the recurrence status overall, and (J) depending on the study arm. ER, estrogen receptor; HER2, human epidermal growth factor receptor 2; PR, progesterone receptor; RFI, recurrence-free interval; T-DM1, trastuzumab emtansine; TH, paclitaxel plus trastuzumab.
Single-Cell FISH Analysis of HER2 Heterogeneity
A case-control study was conducted to characterize HER2 heterogeneity via single-cell FISH on primary tumors of 52 patients with evaluable tissue enrolled in ATEMPT, including 13 patients experiencing RFI events (seven ipsilateral recurrences, five distant recurrences, one breast cancer–related death; cases) and 39 matched controls. Fifty-one of the 52 samples showed HER2 gene amplification according to the ASCO/CAP guidelines. Visual and Metafer-assisted evaluations were highly concordant (Kendall's Tau: 0.93 for both HER2/CEP17 ratio and GCN) with mean ratio values of 5.44 (min 1.66, max 9.93) and 5.37 (1.64-9.79), respectively, and mean GCNs of 11.11 and 10.82. Intratumoral genetic heterogeneity was encountered in 4 of the 51 amplified cases (Figs 6A-6D); HER2 IHC was 2+ or 3+ in all but 8 of the 51 amplified cases (seven were 1+, one nonevaluable). No significant association was observed between HER2 genetic heterogeneity and recurrence (7.7% of patients had HER2 genetic heterogeneity among both those with and without recurrence, P = 1; Fig 6A). Among patients receiving T-DM1, 16.7% of those who experienced recurrence and 5.6% without recurrence had HER2 genetic heterogeneity (P = .45; Fig 6B). In a cross-analysis of heterogeneity assessed via single-cell FISH and ML-assisted HER2 evaluation, tumors with HER2 genetic heterogeneity were also found to harbor higher entropy of the IHC HER2 stain intensity distribution than tumors without genetic heterogeneity (U = 149, P = .024; Data Supplement, Fig S2A).
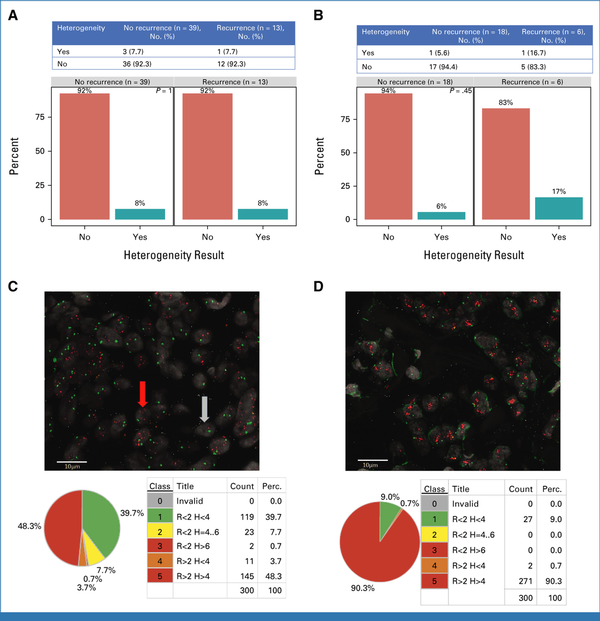
FIG 6.
Characterization of HER2 genetic heterogeneity through single-cell FISH. (A and B) Rates of recurrence by the presence or absence of HER2 genetic heterogeneity in (A) the overall case-control study population and (B) only among patients receiving adjuvant T-DM1. (C) Example of HER2 heterogenous cases with dissection in populations of cells (in terms of absolute numbers and percentage) having different HER2 ratios and copy numbers. (D) Example of HER2 nonheterogenous case with dissection in populations of cells (in terms of absolute numbers and percentage) having different HER2 ratios and copy numbers. H, copy number; FISH, fluorescence in situ hybridization; HER2, human epidermal growth factor receptor 2; Perc., percentage; R, ratio; T-DM1, trastuzumab emtansine.
Spatial Proteomic Analysis
To investigate biomarkers of treatment resistance among the patients enrolled in ATEMPT, spatial proteomic analyses were conducted on tumor samples of 13 patients experiencing iDFS events (cases) in the trial and 21 matched controls. Differential expression analyses between primary tumors of cases and controls revealed three upregulated (NF1, CTLA4, CD20) and five downregulated proteins (Cleaved Caspase 9, CD25, ICOS, GITR, GZMB) in the cases, with P < .05 and FDR <0.05 (Data Supplement, Figs S3 and S4). Expanded results are provided in the Data Supplement.
Thrombocytopenia Analysis
A total of 54 single nucleotide polymorphisms (SNPs) and 103 SNPs were found to be associated with the occurrence of thrombocytopenia/bleeding and with thrombocytopenia, respectively, with P values <10−4 (Data Supplement, Fig S5, Table S9). None of the variants, however, had a P value that reached the threshold for genome-wide significance. Expanded results are provided in the Data Supplement.
DISCUSSION
This final analysis of ATEMPT confirms the outstanding efficacy of T-DM1 at preventing recurrences of stage I HER2-positive breast cancer, with a 5-year RFI of 98.3%. In terms of tolerability, a similar CRT rate was observed with T-DM1 and TH in the study although patient-reported outcomes favored T-DM1, with less alopecia and higher work productivity compared to TH. Overall, on the basis of the excellent long-term outcomes and favorable tolerability,,, 1 year of adjuvant T-DM1 represents an alternative treatment option to TH for patients with stage 1 HER2-positive breast cancer. Importantly, to further improve treatment tolerability, a new randomized phase II trial (ATEMPT 2.0, ClinicalTrials.gov identifier: NCT04893109) is testing a shorter course of adjuvant T-DM1 (six cycles) for stage I HER2-positive breast cancer. In addition, the phase II ADEPT trial (ClinicalTrials.gov identifier: NCT04569747) is testing the chemotherapy-free regimen of endocrine treatment plus subcutaneous trastuzumab plus pertuzumab for stage I, hormone receptor-positive/HER2-positive breast cancer.
Despite favorable long-term outcomes observed with de-escalated regimens, a small number of recurrences were still observed in APT and ATEMPT, irrespective of the tumor size, HER2 IHC score, and hormone receptor status of the disease. Given that standard clinicopathologic features alone were not able to identify higher-risk tumors, we conducted multiomic analyses to evaluate innovative prognostic and predictive biomarkers in this setting. The presence of HER2 heterogeneity has been previously associated with the lack of response to T-DM1, and therefore, we focused on this feature, evaluated through comprehensive genomic and proteomic analyses and through novel ML-based algorithms. Single-cell FISH analysis confirmed that HER2 heterogenous tumors account for a minority of HER2-positive tumors, representing approximately 8% in ATEMPT. However, this feature had no significant impact on survival outcomes in ATEMPT. These results highlight the complex relationship between response to neoadjuvant treatment and survival in HER2-positive tumors, given that certain features (eg, luminal genes expression) can be concomitantly associated with not only decreased likelihood of response at surgery but also better long-term survival. Even when assessed through ML-based algorithms and using spatial proteomic analyses, HER2 heterogeneity did not show a clear relationship with survival although the small number of recurrences provided limited power for these analyses.
Of note, a comprehensive assessment of the tumor biology with the HER2DX tool showed that a pre-established risk score threshold was able to discriminate a small proportion of patients (6.4%) with significantly higher risk of recurrence in ATEMPT. This finding further reinforces what was previously observed in the APT trial, where a similar percentage of patients (4.9%) were found to have HER2DX high-risk disease, which was associated with increased risk of recurrence. The results from both trials underscore the promise of HER2DX in tailoring treatments for patients with early-stage HER2-positive tumors, warranting prospective validation.
In addition to looking at HER2 heterogeneity and HER2DX, we used spatial proteomic analyses to identify biomarkers associated with a higher risk of recurrence. NF1 and CTLA4 were found to be overexpressed in primary tumors of patients experiencing ipsilateral or contralateral events, whereas Cleaved Caspase 9, CD25, ICOS, GITR, and GZMB were found to be upregulated in matched controls. NF1 alterations have been previously found to be enriched in advanced breast cancers compared with matched primary tumors and potentially associated with increased sensitivity to T-DM1., CTLA4 is an immune checkpoint molecule that negatively regulates T-cell activation, often upregulated by tumors as a strategy to achieve immune evasion. Conversely, most proteins found to be upregulated in controls are associated either with the activation of the apoptotic pathway (Cleaved Caspase 9) or with immune activation (CD25, ICOS, GITR, and GZMB), suggesting that an active antitumor immune response, inducing a higher degree of apoptosis among cancer cells, may signal a better prognosis.
No germline genomic variants predictive of thrombocytopenia and bleeding with T-DM1 could be identified in our study, possibly because of little statistical power or the complex, multifactorial mechanism of T-DM1–induced thrombocytopenia.
In conclusion, the administration of 1 year of adjuvant T-DM1 led to outstanding long-term outcomes among patients with stage I HER2-positive breast cancer, with only three distant recurrences observed after more than a 5-year follow-up. The HER2DX score was found to be significantly associated with the risk of recurrence in ATEMPT.
PRIOR PRESENTATION
Presented at the San Antonio Breast Cancer Symposium, San Antonio, TX, December 9, 2022.
CLINICAL TRIAL INFORMATION
NCT01853748
DATA SHARING STATEMENT
Deidentified individual-level patient data that support the findings of this study are available from the corresponding author on reasonable request. A detailed proposal for how the data will be used is required, and we will assess applications on a case-by-case basis. All proposals should be submitted to the corresponding author. Whole-genome sequencing analysis will be made public through depositing in dbGAP.
AUTHOR CONTRIBUTIONS
Conception and design: Paolo Tarantino, Kathryn J. Ruddy, Michelle DeMeo, Eric P. Winer, Ian E. Krop, Sara M. Tolaney
Financial support: Sara M. Tolaney
Administrative support: Therese Mulvey
Provision of study materials or patients: Chau Dang, Steven J. Isakoff, Vicente Valero, Meredith Faggen, Therese Mulvey, Ron Bose, Douglas Weckstein, Hope S. Rugo, Bhuvaneswari Ramaswamy, Lowell Hart, Vijayakrishna K. Gadi, Michael Constantine, P. Kelly Marcom, Kathy Albain, Patricia DeFusco, Nadine Tung, Blair Ardman, Rita Nanda, Rachel C. Jankowitz, Paula R. Pohlmann, Catherine Van Poznak, Andres Forero-Torres, Minetta C. Liu, Harold J. Burstein, Ann H. Partridge, Ian E. Krop
Collection and assembly of data: Paolo Tarantino, Nabihah Tayob, Chau Dang, Denise A. Yardley, Steven J. Isakoff, Vicente Valero, Meredith Faggen, Therese Mulvey, Douglas Weckstein, Antonio C. Wolff, Dan Zuckerman, Lowell Hart, Vijayakrishna K. Gadi, Michael Constantine, Kit Cheng, Audrey Merrill Garrett, P. Kelly Marcom, Patricia DeFusco, Nadine Tung, Blair Ardman, Rita Nanda, Mothaffar Rimawi, Vandana Abramson, Paula R. Pohlmann, Catherine Van Poznak, Andres Forero-Torres, Minetta C. Liu, Michelle DeMeo, Ann H. Partridge, Patrizia Dell'Orto, Leila Russo, Aleix Prat, Sara M. Tolaney
Data analysis and interpretation: Paolo Tarantino, Nabihah Tayob, Guillermo Villacampa, Chau Dang, Denise A. Yardley, Steven J. Isakoff, Vicente Valero, Therese Mulvey, Ron Bose, Douglas Weckstein, Antonio C. Wolff, Katherine Reeder-Hayes, Hope S. Rugo, Bhuvaneswari Ramaswamy, Lowell Hart, Vijayakrishna K. Gadi, P. Kelly Marcom, Kathy Albain, Nadine Tung, Rachel C. Jankowitz, Mothaffar Rimawi, Paula R. Pohlmann, Catherine Van Poznak, Andres Forero-Torres, Adrienne G. Waks, Michelle DeMeo, Harold J. Burstein, Ann H. Partridge, Patrizia Dell'Orto, Emma Krause, Daniel J. Newhouse, Busem Binboğa Kurt, Elizabeth A. Mittendorf, Bryan Schneider, Aleix Prat, Ian E. Krop, Sara M. Tolaney
Manuscript writing: All authors
Final approval of manuscript: All authors
Accountable for all aspects of the work: All authors
AUTHORS' DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
Adjuvant Trastuzumab Emtansine Versus Paclitaxel Plus Trastuzumab for Stage I Human Epidermal Growth Factor Receptor 2–Positive Breast Cancer: 5-Year Results and Correlative Analyses From ATEMPT
The following represents disclosure information provided by authors of this manuscript. All relationships are considered compensated unless otherwise noted. Relationships are self-held unless noted. I = Immediate Family Member, Inst = My Institution. Relationships may not relate to the subject matter of this manuscript. For more information about ASCO's conflict of interest policy, please refer to www.asco.org/rwc or ascopubs.org/jco/authors/author-center.
Open Payments is a public database containing information reported by companies about payments made to US-licensed physicians (Open Payments).
Paolo Tarantino
Consulting or Advisory Role: Daiichi Sankyo/Astra Zeneca, Lilly, Gilead Sciences, Genentech/Roche, Novartis
Research Funding: AstraZeneca
Guillermo Villacampa
Honoraria: Reveal Genomics
Consulting or Advisory Role: AstraZeneca, GlaxoSmithKline
Speakers' Bureau: MSD Oncology, Pierre Fabre, Pfizer
Chau Dang
Consulting or Advisory Role: Daichii Sankyo, Pfizer, Gilead Sciences, Seagen, Novartis, ROCHE/Genentech, Race Oncology
Research Funding: Genentech/Roche (Inst)
Travel, Accommodations, Expenses: ROCHE/Genentech
Denise A. Yardley
Consulting or Advisory Role: Novartis (Inst), Immunomedics (Inst), AstraZeneca (Inst), Gilead Sciences (Inst), Daiichi Sankyo (Inst), IntegraConnect (Inst), Stemline Therapeutics (Inst)
Research Funding: Genentech/Roche (Inst), Novartis (Inst), MedImmune (Inst), Lilly (Inst), Medivation (Inst), Macrogenics (Inst), Abbvie (Inst), Merck (Inst), Amgen (Inst), Biomarin (Inst), Biothera (Inst), Dana Farber Cancer Hospital (Inst), Incyte (Inst), Innocrin Pharma (Inst), Odonate Therapeutics (Inst), Polyphor (Inst), Ambrx (Inst), G1 Therapeutics (Inst), Merrimack (Inst), AstraZeneca (Inst), Eisai (Inst), Gilead Sciences (Inst), Stemline Therapeutics (Inst), US Oncology Network (Inst), University of Texas Southwestern Medical Center—Simmons Cancer Center (Inst)
Steven J. Isakoff
Employment: Merus
Stock and Other Ownership Interests: Merus NV
Research Funding: Genentech (Inst), Abbvie (Inst), OncoPep (Inst), Merck (Inst), AstraZeneca/MedImmune (Inst)
Open Payments Link:https://openpaymentsdata.cms.gov/physician/110822/
Vicente Valero
Honoraria: Genentech/Roche, Novartis, AstraZeneca
Consulting or Advisory Role: Genentech/Roche, Novartis, AstraZeneca
Travel, Accommodations, Expenses: Genentech/Roche
Therese Mulvey
Honoraria: Cardinal Halth, Targeted Oncoloy
Ron Bose
Consulting or Advisory Role: Genentech
Research Funding: Puma Biotechnology (Inst)
Antonio C. Wolff
Research Funding: Genentech (Inst), Merck Sharp & Dohme (Inst), Array BioPharma (Inst)
Patents, Royalties, Other Intellectual Property: Antonio Wolff has been named as the inventor on one or more issued patents or pending patent applications relating to methylation in breast cancer, has assigned his rights to JHU, and participates in a royalty sharing agreement with JHU
Katherine Reeder-Hayes
Research Funding: Pfizer (Inst), Pfizer (Inst), Pfizer Global Medical Foundation (Inst)
Hope S. Rugo
Consulting or Advisory Role: Napo Pharmaceuticals, Puma Biotechnology, Mylan, Eisai, Daiichi Sankyo
Research Funding: OBI Pharma (Inst), Pfizer (Inst), Novartis (Inst), Lilly (Inst), Merck (Inst), Daiichi Sankyo (Inst), AstraZeneca (Inst), Gilead Sciences (Inst), Astellas Pharma (Inst), Taiho Oncology (Inst), Veru (Inst), GlaxoSmithKline (Inst), Hoffmann-La Roche AG/Genentech, Inc (Inst), Stemline Therapeutics (Inst)
Open Payments Link:https://openpaymentsdata.cms.gov/physician/183398
Bhuvaneswari Ramaswamy
Research Funding: Pfizer (Inst)
Dan Zuckerman
Leadership: OncoHealth Medical Group, PA
Consulting or Advisory Role: Oncology Analytics
Open Payments Link:https://openpaymentsdata.cms.gov/physician/791052
Lowell Hart
Honoraria: Novartis, G1 Therapeutics, Circulogene Theranostics
Consulting or Advisory Role: Genentech/Roche, Amgen, G1 Therapeutics, Merck, Seagen
Speakers' Bureau: Novartis, Circulogene Theranostics
Research Funding: Novartis (Inst), Genentech/Roche (Inst), Bristol Myers Squibb (Inst), G1 Therapeutics (Inst), Seagen (Inst)
Vijayakrishna K. Gadi
Stock and Other Ownership Interests: Sengine precision medicine, Novilla, 3rdEyeBio, Phoenix Molecular Designs, New Equilibrium Biosciences, Tahoma Therapeutics, Emerging Markets Cancer Ignition Fund
Consulting or Advisory Role: Puma Biotechnology, Hologic, Seagen/Pfizer, Stemline Therapeutics, Gilead Sciences, AstraZeneca
Speakers' Bureau: Seagen, Hologic, Puma Biotechnology, Stemline Therapeutics
Research Funding: Agendia (Inst), Tizona Therapeutics, Inc, Illumina
Travel, Accommodations, Expenses: Seagen, Genentech/Roche, Puma Biotechnology
Open Payments Link:https://openpaymentsdata.cms.gov/physician/2511
Kit Cheng
Honoraria: GlaxoSmithKline, bioTheranostics
P. Kelly Marcom
Employment: Veracyte
Stock and Other Ownership Interests: Veracyte
Open Payments Link:https://openpaymentsdata.cms.gov/physician/237508
Kathy Albain
Honoraria: Encore Medical Education
Research Funding: Seagen (Inst), Quantum Leap Healthcare Collaborative (Inst)
Other Relationship: Seagen
Patricia DeFusco
Employment: Hartford HealthCare, Hartford HealthCare
Leadership: Infinte Strength
Honoraria: Onc Live
Nadine Tung
Consulting or Advisory Role: AstraZeneca, GlaxoSmithKline
Research Funding: AstraZeneca (Inst)
Rita Nanda
Consulting or Advisory Role: Merck, Seagen, AstraZeneca, Gilead Sciences, GE Healthcare, Sanofi, Daiichi Sankyo/Astra Zeneca, Exact Sciences, Guardant Health, Moderna Therapeutics, Novartis, Stemline Therapeutics
Research Funding: Corcept Therapeutics (Inst), Celgene (Inst), Merck (Inst), Seagen (Inst), Genentech/Roche (Inst), Odonate Therapeutics (Inst), Pfizer (Inst), AstraZeneca (Inst), Immunomedics (Inst), OncoSec (Inst), Arvinas (Inst), Taiho Oncology (Inst), OBI Pharma (Inst), Sun Pharma (Inst), Relay Therapeutics (Inst)
Other Relationship: G1 Therapeutics
Rachel C. Jankowitz
Honoraria: Eisai
Consulting or Advisory Role: Merck
Research Funding: Biotheranostics
Mothaffar Rimawi
Consulting or Advisory Role: Macrogenics, Novartis, AstraZeneca, Pfizer, Sermonix Pharmaceuticals
Vandana Abramson
Consulting or Advisory Role: Daiichi Sankyo, AstraZeneca, Guardant Health
Research Funding: Bayer, Guardant Health
Paula R. Pohlmann
Leadership: Immunonet BioSciences
Stock and Other Ownership Interests: Immunonet BioSciences
Honoraria: Dava Oncology, OncLive/MJH Life Sciences, Frontiers Media, SABCS
Consulting or Advisory Role: Personalized Cancer Therapy, OncoPlex Diagnostics, Immunonet BioSciences, Pfizer, HERON, Puma Biotechnology, Sirtex Medical, Caris Life Sciences, Juniper Pharmaceuticals, Bolt Biotherapeutics, Abbvie, Pfizer
Speakers' Bureau: Genentech/Roche
Research Funding: Genentech/Roche (Inst), Genentech/Roche (Inst), Genentech/Roche (Inst), Genentech/Roche (Inst), Fabre-Kramer (Inst), Advanced Cancer Therapeutics (Inst), Caris Centers of Excellence (Inst), Caris Centers of Excellence (Inst), Pfizer (Inst), Pieris Pharmaceuticals (Inst), Cascadian Therapeutics (Inst), Pfizer (Inst), Bolt Biotherapeutics (Inst), Byondis (Inst), SEAGEN (Inst), SEAGEN (Inst), SEAGEN (Inst), Orum Therapeutics (Inst), Carisma Therapeutics (Inst)
Patents, Royalties, Other Intellectual Property: US Patent no. 8,486,413, US Patent no. 8,501,417, US Patent no. 9,023,362, US Patent no. 9,745,377, Patent application
Uncompensated Relationships: Seagen, Pfizer, Jazz Pharmaceuticals
Catherine Van Poznak
Research Funding: Bayer (Inst)
Patents, Royalties, Other Intellectual Property: UpToDate
Andres Forero-Torres
Employment: Seagen
Stock and Other Ownership Interests: Seagen
Minetta C. Liu
Employment: Natera
Stock and Other Ownership Interests: Natera
Research Funding: Eisai (Inst), Seagen (Inst), Novartis (Inst), Roche/Genentech (Inst), GRAIL (Inst), Merck (Inst), Tesaro (Inst), Menarini Silicon Biosystems (Inst), Genomic Health (Inst), Exact Sciences (Inst)
Travel, Accommodations, Expenses: GRAIL, Merck, Menarini Silicon Biosystems, Pfizer, Genomic Health, AstraZeneca (Inst), Natera
Kathryn J. Ruddy
Research Funding: Medtronic
Patents, Royalties, Other Intellectual Property: Spouse and Mayo Clinic have filed patents related to the application of artificial intelligence to the electrocardiogram for diagnosis and risk stratification
Adrienne G. Waks
Consulting or Advisory Role: AstraZeneca
Research Funding: Genentech (Inst), MacroGenics (Inst), Merck (Inst), Gilead Sciences (Inst)
Harold J. Burstein
This author is a Consultant Editor for Journal of Clinical Oncology. Journal policy recused the author from having any role in the peer review of this manuscript.
Ann H. Partridge
Research Funding: Novartis
Patents, Royalties, Other Intellectual Property: I receive small royalty payments for coauthoring the breast cancer survivorship section of UpToDate
Open Payments Link:https://openpaymentsdata.cms.gov/physician/835197
Patrizia Dell'Orto
Honoraria: MSD Oncology, Pfizer
Consulting or Advisory Role: Roche/Genentech
Speakers' Bureau: Roche/Genentech
Research Funding: Roche/Genentech
Travel, Accommodations, Expenses: Roche
Emma Krause
Employment: PathAI
Stock and Other Ownership Interests: PathAI
Travel, Accommodations, Expenses: PathAI
Daniel J. Newhouse
Other Relationship: NanoString Technologies
Elizabeth A. Mittendorf
Honoraria: Physicans' Education Resource
Consulting or Advisory Role: BioNTech SE, Merck, AstraZeneca, Moderna Therapeutics, Alight Sciences
Research Funding: Roche/Genentech (Inst), Gilead Sciences (Inst)
Travel, Accommodations, Expenses: Merck Sharpe and Dohme
Uncompensated Relationships: Bristol Myers Squibb, Roche/Genentech
Open Payments Link:https://openpaymentsdata.cms.gov/physician/899522
Bryan Schneider
Research Funding: Genentech/Roche, Pfizer, Foundation Medicine
Aleix Prat
Employment: Reveal Genomics
Leadership: Reveal Genomics
Stock and Other Ownership Interests: Reveal Genomics
Honoraria: Pfizer, Novartis, Roche, Daiichi Sankyo, AstraZeneca
Consulting or Advisory Role: Roche, Novartis, Pfizer, Oncolytics, Daiichi Sankyo, AstraZeneca
Research Funding: Roche (Inst), Novartis (Inst), Daiichi Sankyo, AstraZeneca
Patents, Royalties, Other Intellectual Property: PCT/EP2016/080056: HER2 as a Predictor of Response to Dual HER2 Blockade in the Absence of Cytotoxic Therapy, HER2DX filing, Methods for Breast Cancer Treatment and Prediction of Therapeutic Response (US 63/023785), TNBC-DX
Travel, Accommodations, Expenses: Daiichi Sankyo
Other Relationship: Oncolytics, Peptomyc
Eric P. Winer
Research Funding: Genentech (Inst)
Ian E. Krop
Employment: PureTech
Leadership: PureTech
Stock and Other Ownership Interests: PureTech
Honoraria: AstraZeneca, Daiichi Sankyo
Consulting or Advisory Role: Genentech/Roche, Seagen, Daiichi Sankyo, Merck, AstraZeneca, Novartis
Research Funding: Genentech (Inst), Pfizer (Inst), Macrogenics (Inst)
Patents, Royalties, Other Intellectual Property: Anti-murine CD19 monoclonal antibody licensed to PharMingen
Sara M. Tolaney
Consulting or Advisory Role: Novartis, Pfizer, Merck, Lilly, AstraZeneca, Genentech, Eisai, Sanofi, Bristol Myers Squibb, Seagen, CytomX Therapeutics, Daiichi Sankyo, Immunomedics/Gilead, BeyondSpring Pharmaceuticals, OncXerna Therapeutics, Zymeworks, Zentalis, Blueprint Medicines, Reveal Genomics, ARC Therapeutics, Umoja Biopharma, Menarini Group, AADi, Artios Biopharmaceuticals, Incyte, Zetagen, Bayer, Infinity Pharmaceuticals, Jazz Pharmaceuticals, Natera, Tango Therapeutics, Systimmune, eFFECTOR Therapeutics, Hengrui Pharmaceutical (USA), Sumitovant Biopharma
Research Funding: Genentech/Roche (Inst), Merck (Inst), Exelixis (Inst), Pfizer (Inst), Lilly (Inst), Novartis (Inst), Bristol Myers Squibb (Inst), Eisai (Inst), AstraZeneca (Inst), NanoString Technologies (Inst), Seagen (Inst), OncoPep (Inst), Gilead Sciences (Inst)
Travel, Accommodations, Expenses: Lilly, Sanofi, Gilead Sciences
No other potential conflicts of interest were reported.
Paolo Tarantino
Consulting or Advisory Role: Daiichi Sankyo/Astra Zeneca, Lilly, Gilead Sciences, Genentech/Roche, Novartis
Research Funding: AstraZeneca
Guillermo Villacampa
Honoraria: Reveal Genomics
Consulting or Advisory Role: AstraZeneca, GlaxoSmithKline
Speakers' Bureau: MSD Oncology, Pierre Fabre, Pfizer
Chau Dang
Consulting or Advisory Role: Daichii Sankyo, Pfizer, Gilead Sciences, Seagen, Novartis, ROCHE/Genentech, Race Oncology
Research Funding: Genentech/Roche (Inst)
Travel, Accommodations, Expenses: ROCHE/Genentech
Denise A. Yardley
Consulting or Advisory Role: Novartis (Inst), Immunomedics (Inst), AstraZeneca (Inst), Gilead Sciences (Inst), Daiichi Sankyo (Inst), IntegraConnect (Inst), Stemline Therapeutics (Inst)
Research Funding: Genentech/Roche (Inst), Novartis (Inst), MedImmune (Inst), Lilly (Inst), Medivation (Inst), Macrogenics (Inst), Abbvie (Inst), Merck (Inst), Amgen (Inst), Biomarin (Inst), Biothera (Inst), Dana Farber Cancer Hospital (Inst), Incyte (Inst), Innocrin Pharma (Inst), Odonate Therapeutics (Inst), Polyphor (Inst), Ambrx (Inst), G1 Therapeutics (Inst), Merrimack (Inst), AstraZeneca (Inst), Eisai (Inst), Gilead Sciences (Inst), Stemline Therapeutics (Inst), US Oncology Network (Inst), University of Texas Southwestern Medical Center—Simmons Cancer Center (Inst)
Steven J. Isakoff
Employment: Merus
Stock and Other Ownership Interests: Merus NV
Research Funding: Genentech (Inst), Abbvie (Inst), OncoPep (Inst), Merck (Inst), AstraZeneca/MedImmune (Inst)
Open Payments Link:https://openpaymentsdata.cms.gov/physician/110822/
Vicente Valero
Honoraria: Genentech/Roche, Novartis, AstraZeneca
Consulting or Advisory Role: Genentech/Roche, Novartis, AstraZeneca
Travel, Accommodations, Expenses: Genentech/Roche
Therese Mulvey
Honoraria: Cardinal Halth, Targeted Oncoloy
Ron Bose
Consulting or Advisory Role: Genentech
Research Funding: Puma Biotechnology (Inst)
Antonio C. Wolff
Research Funding: Genentech (Inst), Merck Sharp & Dohme (Inst), Array BioPharma (Inst)
Patents, Royalties, Other Intellectual Property: Antonio Wolff has been named as the inventor on one or more issued patents or pending patent applications relating to methylation in breast cancer, has assigned his rights to JHU, and participates in a royalty sharing agreement with JHU
Katherine Reeder-Hayes
Research Funding: Pfizer (Inst), Pfizer (Inst), Pfizer Global Medical Foundation (Inst)
Hope S. Rugo
Consulting or Advisory Role: Napo Pharmaceuticals, Puma Biotechnology, Mylan, Eisai, Daiichi Sankyo
Research Funding: OBI Pharma (Inst), Pfizer (Inst), Novartis (Inst), Lilly (Inst), Merck (Inst), Daiichi Sankyo (Inst), AstraZeneca (Inst), Gilead Sciences (Inst), Astellas Pharma (Inst), Taiho Oncology (Inst), Veru (Inst), GlaxoSmithKline (Inst), Hoffmann-La Roche AG/Genentech, Inc (Inst), Stemline Therapeutics (Inst)
Open Payments Link:https://openpaymentsdata.cms.gov/physician/183398
Bhuvaneswari Ramaswamy
Research Funding: Pfizer (Inst)
Dan Zuckerman
Leadership: OncoHealth Medical Group, PA
Consulting or Advisory Role: Oncology Analytics
Open Payments Link:https://openpaymentsdata.cms.gov/physician/791052
Lowell Hart
Honoraria: Novartis, G1 Therapeutics, Circulogene Theranostics
Consulting or Advisory Role: Genentech/Roche, Amgen, G1 Therapeutics, Merck, Seagen
Speakers' Bureau: Novartis, Circulogene Theranostics
Research Funding: Novartis (Inst), Genentech/Roche (Inst), Bristol Myers Squibb (Inst), G1 Therapeutics (Inst), Seagen (Inst)
Vijayakrishna K. Gadi
Stock and Other Ownership Interests: Sengine precision medicine, Novilla, 3rdEyeBio, Phoenix Molecular Designs, New Equilibrium Biosciences, Tahoma Therapeutics, Emerging Markets Cancer Ignition Fund
Consulting or Advisory Role: Puma Biotechnology, Hologic, Seagen/Pfizer, Stemline Therapeutics, Gilead Sciences, AstraZeneca
Speakers' Bureau: Seagen, Hologic, Puma Biotechnology, Stemline Therapeutics
Research Funding: Agendia (Inst), Tizona Therapeutics, Inc, Illumina
Travel, Accommodations, Expenses: Seagen, Genentech/Roche, Puma Biotechnology
Open Payments Link:https://openpaymentsdata.cms.gov/physician/2511
Kit Cheng
Honoraria: GlaxoSmithKline, bioTheranostics
P. Kelly Marcom
Employment: Veracyte
Stock and Other Ownership Interests: Veracyte
Open Payments Link:https://openpaymentsdata.cms.gov/physician/237508
Kathy Albain
Honoraria: Encore Medical Education
Research Funding: Seagen (Inst), Quantum Leap Healthcare Collaborative (Inst)
Other Relationship: Seagen
Patricia DeFusco
Employment: Hartford HealthCare, Hartford HealthCare
Leadership: Infinte Strength
Honoraria: Onc Live
Nadine Tung
Consulting or Advisory Role: AstraZeneca, GlaxoSmithKline
Research Funding: AstraZeneca (Inst)
Rita Nanda
Consulting or Advisory Role: Merck, Seagen, AstraZeneca, Gilead Sciences, GE Healthcare, Sanofi, Daiichi Sankyo/Astra Zeneca, Exact Sciences, Guardant Health, Moderna Therapeutics, Novartis, Stemline Therapeutics
Research Funding: Corcept Therapeutics (Inst), Celgene (Inst), Merck (Inst), Seagen (Inst), Genentech/Roche (Inst), Odonate Therapeutics (Inst), Pfizer (Inst), AstraZeneca (Inst), Immunomedics (Inst), OncoSec (Inst), Arvinas (Inst), Taiho Oncology (Inst), OBI Pharma (Inst), Sun Pharma (Inst), Relay Therapeutics (Inst)
Other Relationship: G1 Therapeutics
Rachel C. Jankowitz
Honoraria: Eisai
Consulting or Advisory Role: Merck
Research Funding: Biotheranostics
Mothaffar Rimawi
Consulting or Advisory Role: Macrogenics, Novartis, AstraZeneca, Pfizer, Sermonix Pharmaceuticals
Vandana Abramson
Consulting or Advisory Role: Daiichi Sankyo, AstraZeneca, Guardant Health
Research Funding: Bayer, Guardant Health
Paula R. Pohlmann
Leadership: Immunonet BioSciences
Stock and Other Ownership Interests: Immunonet BioSciences
Honoraria: Dava Oncology, OncLive/MJH Life Sciences, Frontiers Media, SABCS
Consulting or Advisory Role: Personalized Cancer Therapy, OncoPlex Diagnostics, Immunonet BioSciences, Pfizer, HERON, Puma Biotechnology, Sirtex Medical, Caris Life Sciences, Juniper Pharmaceuticals, Bolt Biotherapeutics, Abbvie, Pfizer
Speakers' Bureau: Genentech/Roche
Research Funding: Genentech/Roche (Inst), Genentech/Roche (Inst), Genentech/Roche (Inst), Genentech/Roche (Inst), Fabre-Kramer (Inst), Advanced Cancer Therapeutics (Inst), Caris Centers of Excellence (Inst), Caris Centers of Excellence (Inst), Pfizer (Inst), Pieris Pharmaceuticals (Inst), Cascadian Therapeutics (Inst), Pfizer (Inst), Bolt Biotherapeutics (Inst), Byondis (Inst), SEAGEN (Inst), SEAGEN (Inst), SEAGEN (Inst), Orum Therapeutics (Inst), Carisma Therapeutics (Inst)
Patents, Royalties, Other Intellectual Property: US Patent no. 8,486,413, US Patent no. 8,501,417, US Patent no. 9,023,362, US Patent no. 9,745,377, Patent application
Uncompensated Relationships: Seagen, Pfizer, Jazz Pharmaceuticals
Catherine Van Poznak
Research Funding: Bayer (Inst)
Patents, Royalties, Other Intellectual Property: UpToDate
Andres Forero-Torres
Employment: Seagen
Stock and Other Ownership Interests: Seagen
Minetta C. Liu
Employment: Natera
Stock and Other Ownership Interests: Natera
Research Funding: Eisai (Inst), Seagen (Inst), Novartis (Inst), Roche/Genentech (Inst), GRAIL (Inst), Merck (Inst), Tesaro (Inst), Menarini Silicon Biosystems (Inst), Genomic Health (Inst), Exact Sciences (Inst)
Travel, Accommodations, Expenses: GRAIL, Merck, Menarini Silicon Biosystems, Pfizer, Genomic Health, AstraZeneca (Inst), Natera
Kathryn J. Ruddy
Research Funding: Medtronic
Patents, Royalties, Other Intellectual Property: Spouse and Mayo Clinic have filed patents related to the application of artificial intelligence to the electrocardiogram for diagnosis and risk stratification
Adrienne G. Waks
Consulting or Advisory Role: AstraZeneca
Research Funding: Genentech (Inst), MacroGenics (Inst), Merck (Inst), Gilead Sciences (Inst)
Harold J. Burstein
This author is a Consultant Editor for Journal of Clinical Oncology. Journal policy recused the author from having any role in the peer review of this manuscript.
Ann H. Partridge
Research Funding: Novartis
Patents, Royalties, Other Intellectual Property: I receive small royalty payments for coauthoring the breast cancer survivorship section of UpToDate
Open Payments Link:https://openpaymentsdata.cms.gov/physician/835197
Patrizia Dell'Orto
Honoraria: MSD Oncology, Pfizer
Consulting or Advisory Role: Roche/Genentech
Speakers' Bureau: Roche/Genentech
Research Funding: Roche/Genentech
Travel, Accommodations, Expenses: Roche
Emma Krause
Employment: PathAI
Stock and Other Ownership Interests: PathAI
Travel, Accommodations, Expenses: PathAI
Daniel J. Newhouse
Other Relationship: NanoString Technologies
Elizabeth A. Mittendorf
Honoraria: Physicans' Education Resource
Consulting or Advisory Role: BioNTech SE, Merck, AstraZeneca, Moderna Therapeutics, Alight Sciences
Research Funding: Roche/Genentech (Inst), Gilead Sciences (Inst)
Travel, Accommodations, Expenses: Merck Sharpe and Dohme
Uncompensated Relationships: Bristol Myers Squibb, Roche/Genentech
Open Payments Link:https://openpaymentsdata.cms.gov/physician/899522
Bryan Schneider
Research Funding: Genentech/Roche, Pfizer, Foundation Medicine
Aleix Prat
Employment: Reveal Genomics
Leadership: Reveal Genomics
Stock and Other Ownership Interests: Reveal Genomics
Honoraria: Pfizer, Novartis, Roche, Daiichi Sankyo, AstraZeneca
Consulting or Advisory Role: Roche, Novartis, Pfizer, Oncolytics, Daiichi Sankyo, AstraZeneca
Research Funding: Roche (Inst), Novartis (Inst), Daiichi Sankyo, AstraZeneca
Patents, Royalties, Other Intellectual Property: PCT/EP2016/080056: HER2 as a Predictor of Response to Dual HER2 Blockade in the Absence of Cytotoxic Therapy, HER2DX filing, Methods for Breast Cancer Treatment and Prediction of Therapeutic Response (US 63/023785), TNBC-DX
Travel, Accommodations, Expenses: Daiichi Sankyo
Other Relationship: Oncolytics, Peptomyc
Eric P. Winer
Research Funding: Genentech (Inst)
Ian E. Krop
Employment: PureTech
Leadership: PureTech
Stock and Other Ownership Interests: PureTech
Honoraria: AstraZeneca, Daiichi Sankyo
Consulting or Advisory Role: Genentech/Roche, Seagen, Daiichi Sankyo, Merck, AstraZeneca, Novartis
Research Funding: Genentech (Inst), Pfizer (Inst), Macrogenics (Inst)
Patents, Royalties, Other Intellectual Property: Anti-murine CD19 monoclonal antibody licensed to PharMingen
Sara M. Tolaney
Consulting or Advisory Role: Novartis, Pfizer, Merck, Lilly, AstraZeneca, Genentech, Eisai, Sanofi, Bristol Myers Squibb, Seagen, CytomX Therapeutics, Daiichi Sankyo, Immunomedics/Gilead, BeyondSpring Pharmaceuticals, OncXerna Therapeutics, Zymeworks, Zentalis, Blueprint Medicines, Reveal Genomics, ARC Therapeutics, Umoja Biopharma, Menarini Group, AADi, Artios Biopharmaceuticals, Incyte, Zetagen, Bayer, Infinity Pharmaceuticals, Jazz Pharmaceuticals, Natera, Tango Therapeutics, Systimmune, eFFECTOR Therapeutics, Hengrui Pharmaceutical (USA), Sumitovant Biopharma
Research Funding: Genentech/Roche (Inst), Merck (Inst), Exelixis (Inst), Pfizer (Inst), Lilly (Inst), Novartis (Inst), Bristol Myers Squibb (Inst), Eisai (Inst), AstraZeneca (Inst), NanoString Technologies (Inst), Seagen (Inst), OncoPep (Inst), Gilead Sciences (Inst)
Travel, Accommodations, Expenses: Lilly, Sanofi, Gilead Sciences
No other potential conflicts of interest were reported.
APPENDIX 1. CONSORTIUM OF THE TBCRC TRANSLATIONAL INVESTIGATORS
Romualdo Barroso-Sousa, MD, PhD
DASA Oncology, Hospital Brasília, Brazil; Dasa Institute for Education and Research (IEPD), Brasília, Brazil
Giuseppe Curigliano, MD, PhD
Department of Oncology and Hemato-Oncology, University of Milan, Milan, Italy; IEO European Institute of Oncology, IRCCS, Milan, Italy
Molly DiLullo, BS
Breast Oncology Program, Dana-Farber Brigham Cancer Center, Boston, MA
Winnie Hui, PhD
NanoString Technologies, Inc, Boston, MA
Christian Kirkup, MS
PathAI, Boston, MA
Giuseppe Viale, MD
Department of Oncology and Hemato-Oncology, University of Milan, Milan, Italy; IEO European Institute of Oncology, IRCCS, Milan, Italy
Yue Zheng, MSc
Division of Data Science, Dana-Farber Cancer Institute, Boston, MA
ACKNOWLEDGMENT
We would like to thank Tim Erick for medical writing support and Kaitlyn T. Bifolck for editing and submission assistance; both are full-time employees of Dana-Farber Cancer Institute. The trial was supported by Genentech and the Gloria Spivak Faculty Advancement Fund (S.M.T.). We are grateful for the funding support to the Translational Breast Cancer Research Consortium (TBCRC), from The Breast Cancer Research Foundation and Susan G. Komen for funding support for translational analyses from MLSC Women's Health Collaboration. The funders had no role in the study design; in the collection, analysis, and interpretation of data; in the writing of the report; and in the decision to submit the paper for publication.
TBCRC Translational Investigators members listed in the Appendix 1 (online only).
REFERENCES
1.
Vaz-Luis I, Ottesen RA, Hughes ME, et al.: Outcomes by tumor subtype and treatment pattern in women with small, node-negative breast cancer: A multi-institutional study. J Clin Oncol 32:2142-2150, 20142.
Gonzalez-Angulo AM, Litton JK, Broglio KR, et al.: High risk of recurrence for patients with breast cancer who have human epidermal growth factor receptor 2-positive, node-negative tumors 1 cm or smaller. J Clin Oncol 27:5700-5706, 20093.
Curigliano G, Viale G, Bagnardi V, et al.: Clinical relevance of HER2 overexpression/amplification in patients with small tumor size and node-negative breast cancer. J Clin Oncol 27:5693-5699, 20094.
Tolaney SM, Tarantino P, Graham N, et al.: Adjuvant paclitaxel and trastuzumab for node-negative, HER2-positive breast cancer: Final 10-year analysis of the open-label, single-arm, phase 2 APT trial. Lancet Oncol 24:273-285, 20235.
Cardoso F, Kyriakides S, Ohno S, et al.: Early breast cancer: ESMO clinical practice guidelines for diagnosis, treatment and follow-up. Ann Oncol 30:1194-1220, 20196.
National Comprehensive Cancer Network: NCCN Clinical Practice Guidelines in Oncology (NCCN Guidelines): Breast Cancer V.4.2023. 2023. https://www.nccn.org7.
Burstein HJ, Curigliano G, Thürlimann B, et al.: Customizing local and systemic therapies for women with early breast cancer: The St. Gallen International Consensus Guidelines for treatment of early breast cancer 2021. Ann Oncol 32:1216-1235, 20218.
Timmins HC, Li T, Trinh T, et al.: Weekly paclitaxel-induced neurotoxicity in breast cancer: Outcomes and dose response. Oncologist 26:366-374, 20219.
von Minckwitz G, Huang CS, Mano MS, et al.: Trastuzumab emtansine for residual invasive HER2-positive breast cancer. N Engl J Med 380:617-628, 201910.
Verma S, Miles D, Gianni L, et al.: Trastuzumab emtansine for HER2-positive advanced breast cancer. N Engl J Med 367:1783-1791, 201211.
Krop IE, Kim SB, Gonzalez-Martin A, et al.: Trastuzumab emtansine versus treatment of physician's choice for pretreated HER2-positive advanced breast cancer (TH3RESA): A randomised, open-label, phase 3 trial. Lancet Oncol 15:689-699, 201412.
Krop IE, Kim SB, Martin AG, et al.: Trastuzumab emtansine versus treatment of physician's choice in patients with previously treated HER2-positive metastatic breast cancer (TH3RESA): Final overall survival results from a randomised open-label phase 3 trial. Lancet Oncol 18:743-754, 201713.
Perez EA, Barrios C, Eiermann W, et al.: Trastuzumab emtansine with or without pertuzumab versus trastuzumab plus taxane for human epidermal growth factor receptor 2-positive, advanced breast cancer: Primary results from the phase III MARIANNE study. J Clin Oncol 35:141-148, 201714.
Hurvitz SA, Dirix L, Kocsis J, et al.: Phase II randomized study of trastuzumab emtansine versus trastuzumab plus docetaxel in patients with human epidermal growth factor receptor 2-positive metastatic breast cancer. J Clin Oncol 31:1157-1163, 201315.
Tolaney SM, Tayob N, Dang C, et al.: Adjuvant trastuzumab emtansine versus paclitaxel in combination with trastuzumab for stage I HER2-positive breast cancer (ATEMPT): A randomized clinical trial. J Clin Oncol 39:2375-2385, 202116.
Wolff AC, Hammond MEH, Hicks DG, et al.: Recommendations for human epidermal growth factor receptor 2 testing in breast cancer: American Society of Clinical Oncology/College of American Pathologists clinical practice guideline update. J Clin Oncol 31:3997-4013, 201317.
Chia S, Norris B, Speers C, et al.: Human epidermal growth factor receptor 2 overexpression as a prognostic factor in a large tissue microarray series of node-negative breast cancers. J Clin Oncol 26:5697-5704, 200818.
Prat A, Guarneri V, Pascual T, et al.: Development and validation of the new HER2DX assay for predicting pathological response and survival outcome in early-stage HER2-positive breast cancer. EBioMedicine 75:103801, 202219.
Shannon CE: A mathematical theory of communication. Bell Syst Tech J 27:379-423, 623-656, 194820.
Vance GH, Barry TS, Bloom KJ, et al.: Genetic heterogeneity in HER2 testing in breast cancer: Panel summary and guidelines. Arch Pathol Lab Med 133:611-612, 200921.
Bartlett JMS, Starczynski J, Atkey N, et al.: HER2 testing in the UK: Recommendations for breast and gastric in-situ hybridisation methods. J Clin Pathol 64:649-653, 201122.
Sella T, Zheng Y, Tayob N, et al.: Treatment discontinuation, patient-reported toxicities and quality-of-life by age following trastuzumab emtansine or paclitaxel/trastuzumab (ATEMPT). NPJ Breast Cancer 8:127, 202223.
Partridge A, Zheng Y, Rosenberg S, et al.: Abstract PD10-02: Patient reported outcomes from the adjuvant trastuzumab emtansine (T-DM1) vs. paclitaxel + trastuzumab (TH) (ATEMPT) trial (TBCRC 033). Cancer Res 80, 2020 (suppl 4; abstr PD10-02)24.
Tarantino P, Villacampa G, Graham N, et al.: 6P—Combined analysis of the HER2DX genomic tool in adjuvant APT and ATEMPT trials. ESMO Open 8:101230, 202325.
Filho OM, Viale G, Stein S, et al.: Impact of HER2 heterogeneity on treatment response of early-stage HER2-positive breast cancer: Phase II neoadjuvant clinical trial of T-DM1 combined with pertuzumab. Cancer Discov 11:2474-2487, 202126.
Pearson A, Proszek P, Pascual J, et al.: Inactivating NF1 mutations are enriched in advanced breast cancer and contribute to endocrine therapy resistance. Clin Cancer Res 26:608-622, 202027.
Sokol ES, Feng YX, Jin DX, et al.: Loss of function of NF1 is a mechanism of acquired resistance to endocrine therapy in lobular breast cancer. Ann Oncol 30:115-123, 201928.
Aftimos P, Oliveira M, Irrthum A, et al.: Genomic and transcriptomic analyses of breast cancer primaries and matched metastases in AURORA, the Breast International Group (BIG) molecular screening initiative. Cancer Discov 11:2796-2811, 202129.
Duso BA, Dorronzoro EG, Tini G, et al.: Abstract 1164: Somatic NF1 loss in breast cancer leads to centrosome amplification, aneuploidy and increased sensitivity to T-DM1. Cancer Res 82:1164, 2022 (suppl 12)30.
Duso BA, Messuti E, Tini G, et al.: A novel, RAS-independent role for NF1 in microtubular dynamics and damage repair dictates sensitivity to T-DM1 in HER2-positive breast cancer. bioRxiv 2023.12.06.56957231.
Leach DR, Krummel MF, Allison JP: Enhancement of antitumor immunity by CTLA-4 blockade. Science 271:1734-1736, 199632.
Kern R, Panis C: CTLA-4 expression and its clinical significance in breast cancer. Arch Immunol Ther Exp (Warsz) 69:16, 202133.
Wolff AC, Hammond MEH, Allison KH, et al: Human epidermal growth factor receptor 2 testing in breast cancer: American Society of Clinical Oncology/College of American Pathologists clinical practice guideline focused update. J Clin Oncol 36:2105-2122, 2018
