1 Introduction
Acne scars, particularly atrophic scars, are a common dermatological concern, often resulting in significant textural irregularities and psychosocial distress []. Jacob's classification divides atrophic acne scars into ice pick, boxcar, and rolling scars, each requiring targeted approaches for optimal correction []. Among resurfacing modalities, fractional carbon dioxide (CO2) laser remains the gold standard for dermal remodeling. It effectively treats all three types of atrophic acne scars by inducing collagen remodeling, neocollagenesis, and fibroblast activation, with studies demonstrating its superiority over other resurfacing modalities [].
A major limitation of ablative CO2 laser resurfacing, particularly in darker skin types (Fitzpatrick III–VI), is the risk of post‐inflammatory hyperpigmentation (PIH) []. This occurs due to prolonged melanocyte stimulation and increased epidermal cytokine activity from inflammatory damage to basal keratinocytes, leading to dyschromia and potentially compromising treatment outcomes []. Up to 92% of patients with Fitzpatrick skin type IV and higher develop PIH following ablative CO2 laser treatment []. Given this high incidence, PIH represents the rate‐limiting factor in the use of CO2 laser for atrophic acne scars, particularly in higher Fitzpatrick skin types (FST).
To mitigate PIH risk, various approaches have been explored. Non‐ablative fractional lasers (NAFL) and radiofrequency microneedling (RFM) have gained popularity due to their lower PIH risk [, ]. However, these modalities do not match the efficacy of CO2 laser in dermal remodeling []. Within ablative laser resurfacing, fractional CO2 lasers offer advantages over fully ablative CO2 lasers, as fractional technology minimizes epidermal disruption while still inducing deep collagen remodeling []. Additional modifications to laser density, lasing patterns, pulse duration, and epidermal cooling have been employed to reduce heat accumulation and lower the risk of PIH [].
Once established, PIH can persist for several years, significantly impacting patient satisfaction and requiring prolonged intervention []. A wide range of topical, systemic, and procedural treatments exist for PIH, but prevention remains the primary objective (Table 1). Strategies for PIH prevention in CO2 laser resurfacing include topical tyrosinase inhibitors, corticosteroids, short‐pulsed lasers, photoprotection, and platelet‐rich plasma (PRP) [, , , , , , , ]. The use of post‐procedure agents such as topical fusidic acid has also been explored in clinical studies []. However, an optimal solution balancing effective scar remodeling with minimal PIH risk remains an unmet clinical need.
Here, we explore a novel peel for use in both pre‐ and post‐treatment to assess its efficacy in reducing PIH following CO2 laser resurfacing, particularly in patients with darker FST.
2 Materials and Methods
2.1 Study Design
This was a prospective, single‐center, randomized, controlled, evaluator‐blinded study conducted in accordance with the Declaration of Helsinki. Informed consent was obtained from all participants prior to study enrollment. The study was supervised by a Fellow of the Australasian College of Dermatologists (FACD) and conducted by a team comprising a cosmetic physician and dermatology nurses.
2.2 Study Objective
The primary objective was to compare the incidence of PIH in the treatment group receiving a MelanoPro Peel System (Dermalogica Pro, Carson, CA, USA) versus the control group receiving no peel.
2.3 Patient Recruitment and Randomization (Figure 1)
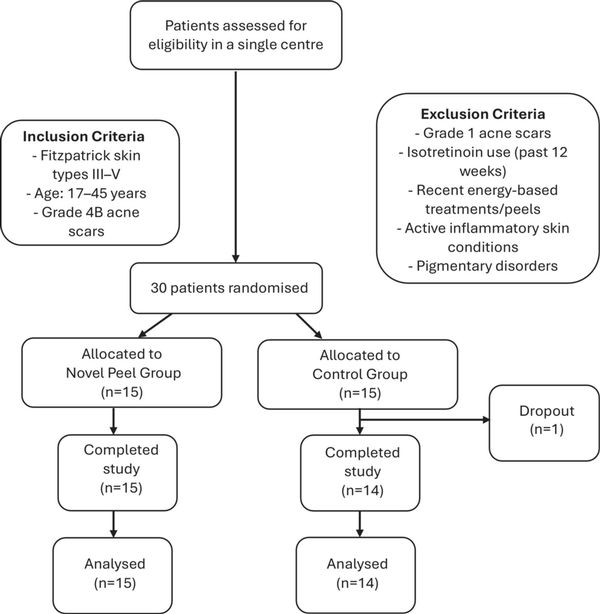
FIGURE 1
Flow chart: patient recruitment, randomization and analysis.
A total of 30 patients were recruited and randomized into two groups:
Treatment group (n = 15): Received the MelanoPro Peel System
Control group (n = 14): Received no peel. One patient dropped out, resulting in a final sample size of 14 patients in the control group.
2.4 Inclusion Criteria
FST III–V
Age range: 17–45 years
Grade 4B acne scars (as per the Goodman and Baron scale)
2.5 Exclusion Criteria
Patients with Grade 1 acne scars (macular erythematous scars or PIH)
Use of isotretinoin within 12 weeks prior to study enrollment
History of energy‐based device treatments or chemical peels within 12 weeks prior to the study
Presence of inflammatory skin conditions, including active acne, which could confound the Post‐Inflammatory Hyperpigmentation Area and Severity Index (PIHASI) scores
Patients with pigmentary disorders (melasma, Hori's nevus, or acquired pigmentation disorders), as ablative lasers may exacerbate pigmentation and affect study outcomes
2.6 Pre‐Treatment Protocol
Patients were instructed to discontinue all topical creams 4 weeks prior to the study, including Vitamin A analogues, bleaching agents, and exfoliants.
The only permitted topical product during this period was sunscreen, which patients were required to use consistently.
The study group, which was randomized to the peeling protocol, followed a two‐week peel regimen beginning 15 days before laser resurfacing, with one rest day (no application) prior to the procedure.
2.7 Treatment Protocol
All patients had topical 23% lidocaine and 7% tetracaine in an anhydrous base applied to the target areas 60 min prior to the procedure. The topical anesthetic cream was removed before ablative CO2 therapy. Patients underwent treatment with the fractional eCO2 Plus laser system (Lutronic Corporation, Billerica, MA, USA), with all procedures performed by a single dermatologist to ensure consistency. Each cosmetic area was initially treated with a single pass, followed by a second pass after all areas had been treated.
2.8 Laser Parameters
Fluences of 110–120 millijoules
Density of coverage: 5% per pass, for a total of 15% coverage over 3 passes
Spot size: 120 μm (standardized)
The study area was restricted to the right and left cheeks for standardization in PIHASI assessments. Though some patients received treatment on additional areas (e.g., temples, forehead, nose), these were excluded from the analysis. No epidermal cooling was applied in any cases.
2.9 Post‐Treatment Care
Patients in the peel group were restarted on the peel 7 days post‐resurfacing once complete re‐epithelialization was confirmed. They were advised to continue for 4 weeks.
2.9.1 Both Groups Received Standardized Post‐Laser Care, Which Included
Cetaphil Gentle Skin Cleanser (Galderma, Fort Worth, TX, USA) and white soft paraffin, applied three times daily for 4 days, then twice daily for 3 days.
Sunscreen (physical‐based SPF 50+), applied twice daily upon re‐epithelialization, regardless of sun exposure. The amount was standardized to two finger lengths per application.
Study group subjects were instructed to use only the novel peel, with no additional topical preparations.
2.9.2 Medications
Doxycycline 50 mg once daily for 7 days was prescribed for all participants.
Two subjects with a history of cold sores received valacyclovir 500 mg twice daily.
2.9.3 Adverse Events
Two cases of mild to moderate skin irritation, characterized by persistent desquamation, pruritus, and mild discomfort, were observed. One case occurred at Weeks 2–3, and the other at Weeks 3–4. Symptoms resolved following temporary cessation of the peel for 4 days, after which treatment was gradually reintroduced from day five.
No cases of prolonged erythema, persistent irritation, or allergic reactions were reported.
2.10 Skin Evaluation
All subjects underwent clinical evaluation at Weeks 3 and 6 post‐treatment.
2.10.1 Blinded Assessment
The primary dermatologist was blinded to treatment allocation and assessed outcomes based on standardized photography using the Clinical Imaging System (Sydney, Australia) [].
2.10.2 Photography Standardization
Comparative photographs were taken under controlled conditions to ensure consistent lighting, angle, and exposure. The imaging system was used to maintain fixed distances, lighting, exposure settings, and camera angles, ensuring standardization of photography. Pre‐treatment and 6‐week post‐treatment photographs were captured (Figures 2, 3, 4, 5, 6, 7, 8, 9).
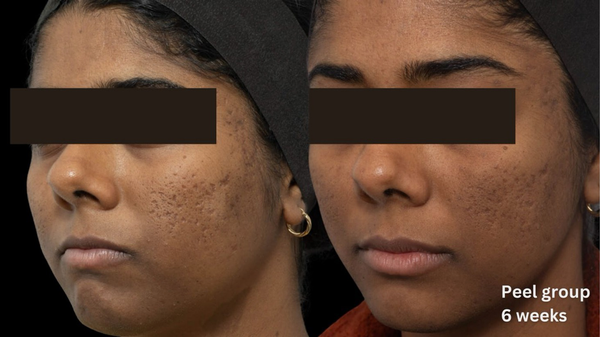
FIGURE 2
Skin evaluation at 6 weeks for peel group.
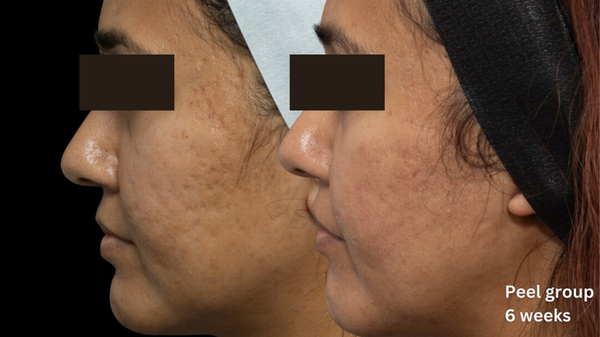
FIGURE 3
Skin evaluation at 6 weeks for peel group.
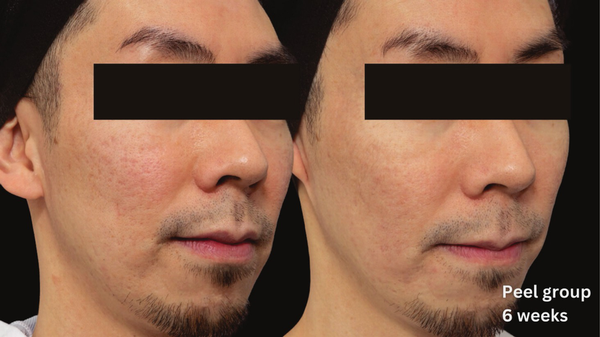
FIGURE 4
Skin evaluation at 6 weeks for peel group.
2.10.3 Outcome Assessments
2.10.3.1 Primary Outcome
The primary outcome was the comparison of PIH between the two groups, objectively evaluated at Week 6. The study was terminated at Week 6 post‐procedure, as PIH severity was maximal at Week 4 [].
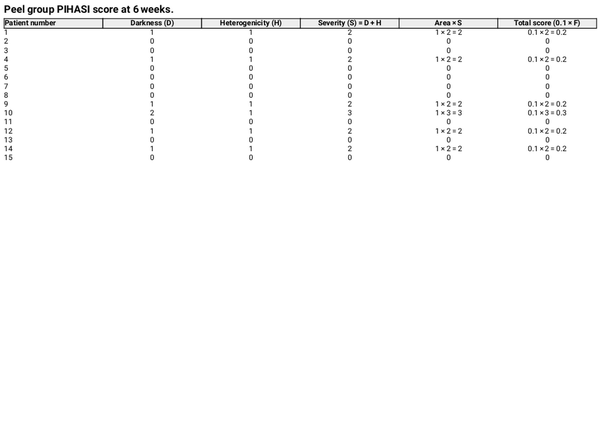
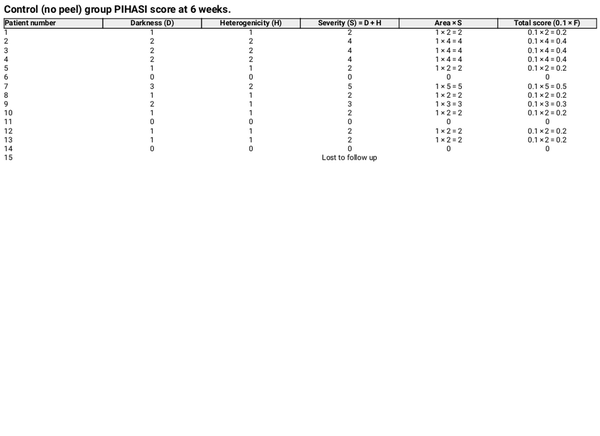
PIH is defined as a perceivable increase in pigmentation relative to the surrounding skin. The severity was assessed using the PIHASI score []. The PIHASI score was used due to its high interrater reliability, correlation with quantitative hyperpigmentation measures (melanin and Individual Typology Angle values), and concordance with established metrics like the IGA. It provides a broader numerical range, improves the assessment of relative hyperpigmentation, and enhances the specificity of treatment response measurement in inflammatory conditions [].
PIHASI evaluation included:
Darkness score (0–4)
Heterogeneity score (0–4)
Area percentage (0%–100%)
Severity was determined by summing the darkness and heterogeneity scores. The total score was limited to the face, calculated as 0.1 × F, where F is the product of area and severity.
2.10.3.2 Secondary Outcomes
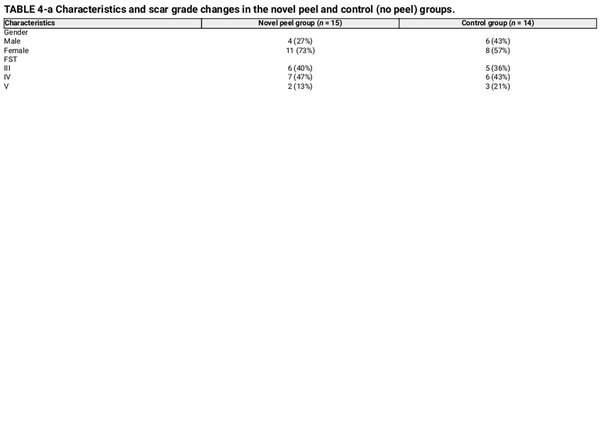
Healing time (re‐epithelization) and changes in acne scar grading were analyzed using the Goodman and Baron scale. At baseline, patients had grade 4 scars, which were visible at conversational distances and could not be stretched or concealed by normal facial hair growth.
2.11 Statistical Analysis
All statistical analyzes were performed using Stata version 18.0.
The primary outcome, PIHASI score at Week 6, was compared between the peel and no‐peel groups using the Mann–Whitney U test. Rank sums were used to assess differences between groups, and statistical significance was set at p < 0.05.
For secondary outcomes, healing time (re‐epithelialization) and changes in acne scar grading were assessed using mean ± standard deviation (SD) for each group. Differences between groups were analyzed using an independent t‐test (p < 0.05).
3 Results
3.1 Primary Outcome
At 6 weeks post‐treatment, the median PIHASI score in the peel‐treated group was 0.0 (IQR: 0.0–0.2), compared to 0.2 (IQR: 0.2–0.4) in the control group, indicating lower post‐laser pigmentation severity. The difference was statistically significant (p = 0.015), supporting the peel's efficacy in reducing PIH risk (Tables 2 and 3).
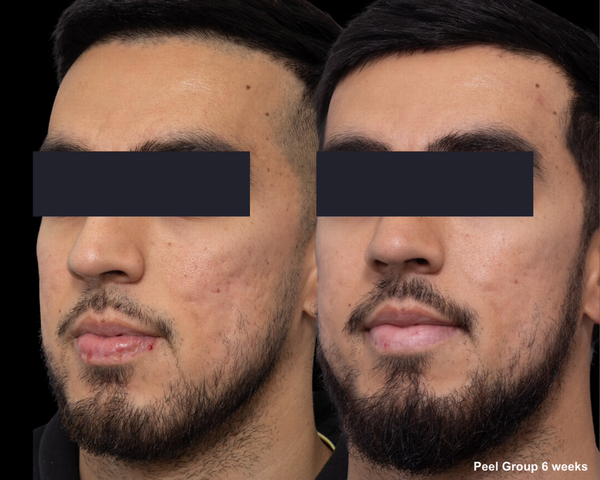
FIGURE 5
Skin evaluation at 6 weeks for peel group.
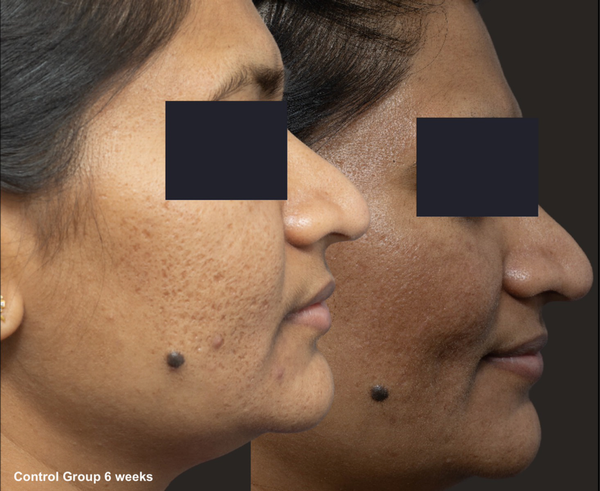
FIGURE 6
Skin evaluation at 6 weeks for no peel group.
3.2 Secondary Outcomes
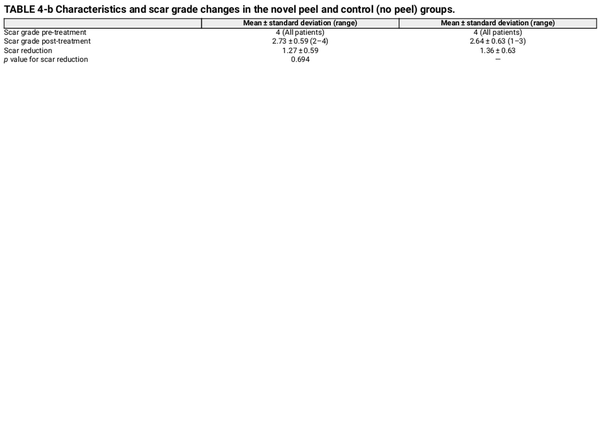
Re‐epithelialization, defined as the absence of crusting, was similar between groups, occurring at day 6.4 in the peel group and day 6.2 in the control group. Acne scar grading using the Goodman and Baron scores demonstrated no significant difference in both groups (p = 0.694), with a mean reduction of 1.27 ± 0.59 in the peel group and 1.36 ± 0.63 in the control group (Table 4).
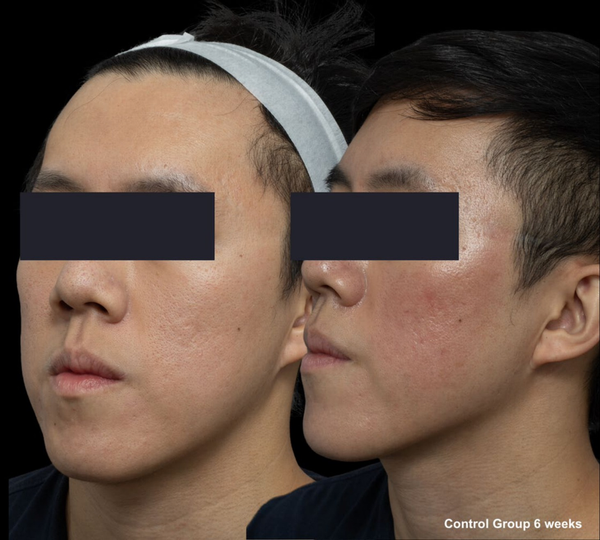
FIGURE 7
Skin evaluation at 6 weeks for no peel group.
These findings suggest that pre‐ and post‐treatment chemical peeling with the MelanoPro Peel System effectively reduces PIH risk following fractional CO2 laser resurfacing, particularly in Fitzpatrick III–V patients, who historically face high pigmentation complications. Notably, the 0.0 median PIHASI score in the peel group indicates an absence of visible pigmentation in most treated individuals, highlighting its potential as a standard adjunctive therapy for PIH prevention.
4 Discussion
4.1 Optimizing CO2 Laser Treatment for Acne Scar Remodeling and PIH Prevention
CO2 laser resurfacing remains the gold standard for acne scar remodeling, demonstrating efficacy across various scar types in FST I–V.
While not contraindicated in darker skin types (FST IV–VI), its use is challenging due to the high incidence of PIH, reported as high as 100% in some cases []. The combination of increased melanocyte reactivity in darker skin and the thermal load of CO2 lasers often leads to excessive pigment stimulation and post‐treatment hyperpigmentation.
4.2 Prevention and Treatment Strategies for PIH
Given the high risk of PIH, strategic prevention and early intervention are crucial. Various pre‐, intra‐, and post‐procedural strategies have been explored to mitigate pigmentary changes, with strict photoprotection for 2–4 weeks before and after treatment being a long‐standing recommendation. Laser settings are also adjusted to minimize thermal damage, reducing PIH risk [].
Among topical agents, tyrosinase inhibitors remain the mainstay of PIH prevention, with hydroquinone being the most effective. However, its high irritation potential, safety concerns, and regulatory restrictions limit its universal use []. Over the past decade, novel formulations combining multiple tyrosinase inhibitors and exfoliants have emerged as alternatives [, ].
4.3 The Role of Chemical Peeling in PIH Prevention
Chemical peeling, or chemo‐exfoliation, is a cost‐effective and widely used technique for treating pigmentation disorders. By regulating epidermal thickness, promoting even melanin distribution, and stimulating dermal collagen production, chemical peels improve skin tone and texture. Their efficacy in melasma, acne, PIH, and scarring has been well established, making them a safe and accessible option for pigmentation management [].
Most studies have focused on post‐treatment PIH management. Our approach, however, emphasizes PIH prevention by integrating a novel peel both as a priming agent before fractional CO2 laser resurfacing and post‐treatment intervention to minimize PIH risk. Proper priming is particularly essential in atrophic pigmented acne scars in darker skin types, where high‐fluence laser settings are limited due to PIH risk. Pre‐laser priming enhances skin tolerance, reduces wound healing time, and improves patient compliance []. Incorporating antioxidants and anti‐inflammatory cosmeceuticals helps protect against UV damage and prevent further pigmentation [].
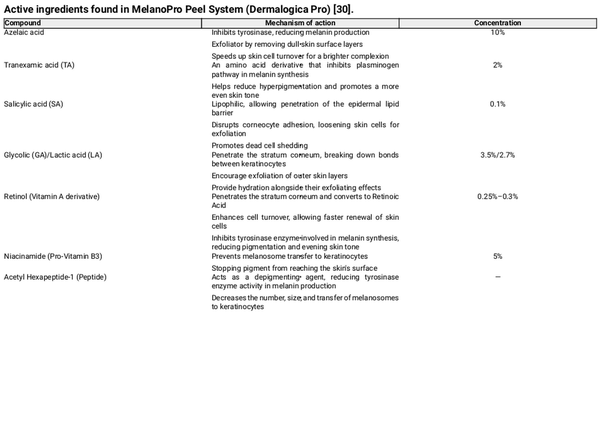
The MelanoPro Peel System is a novel formulation consisting of azelaic acid, tranexamic acid, salicylic acid, glycolic and lactic acids, retinol, niacinamide, and proprietary peptides (Table 5).
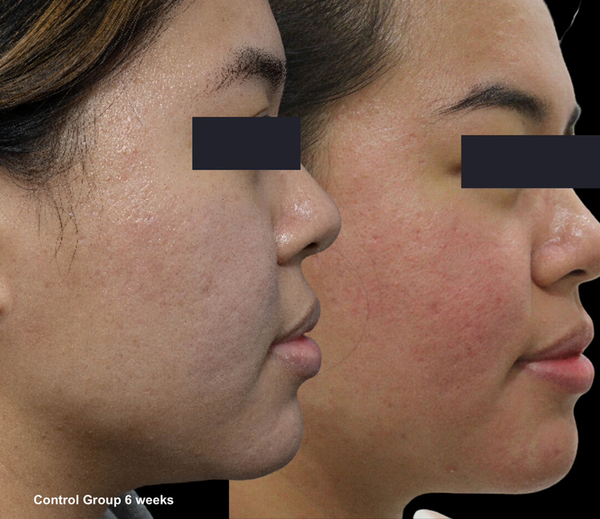
FIGURE 8
Skin evaluation at 6 weeks for no peel group.
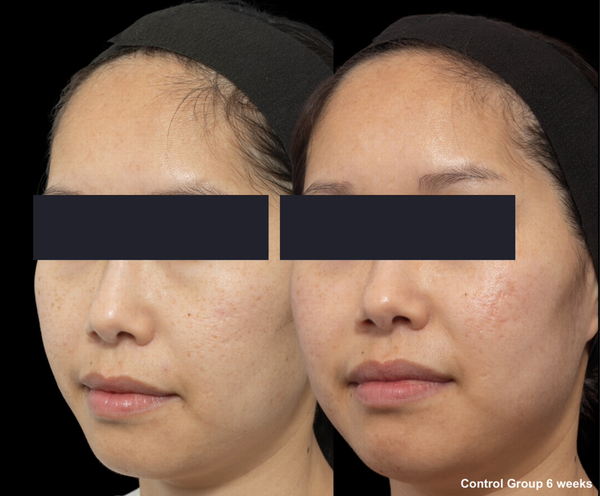
FIGURE 9
Skin evaluation at 6 weeks for no peel group.
4.4 Azelaic Acid and Its Role in PIH Treatment
Azelaic acid is one of the key ingredients in the MelanoPro Peel System at a concentration of 10%. It was initially formulated as a 20% cream for acne and later as a 15% gel for rosacea. It is now used for PIH due to its ability to inhibit tyrosinase, a key enzyme in melanin production []. Additionally, it selectively targets hyperactive melanocytes by inhibiting DNA synthesis and mitochondrial enzymes, leading to cytotoxic effects on abnormal pigment‐producing cells while sparing normal skin. Studies show that a 20% azelaic acid cream significantly reduces pigmentation, and when combined with 15%–20% glycolic acid, its efficacy in treating facial hyperpigmentation, including melasma and PIH, is comparable to 4% hydroquinone [, ].
4.5 Role of Tranexamic Acid (TA) in Hyperpigmentation Prevention
TA has been shown to effectively modulate melanogenesis by reducing melanin content and tyrosinase activity in melanocytes. It also downregulates key melanogenic proteins such as tyrosinase‐related protein 1 (TRP‐1) and TRP‐2, leading to a reduction in pigment production [].
Following laser treatments, TA helps mitigate inflammation‐induced melanogenesis by activating the ERK signaling pathway and suppressing melanocyte‐inducing transcription factor (MITF) expression, which plays a crucial role in melanin synthesis [].
TA has also demonstrated efficacy in melasma management, where it inhibits UV‐induced plasmin activity, thereby reducing arachidonic acid and prostaglandin levels, which are known to stimulate melanocytes and increase pigmentation [].
While studies indicate that high‐dose oral TA (1500 mg daily) post‐Q‐switched laser does not significantly prevent PIH, evidence suggests that oral administration may facilitate PIH clearance within 6 weeks. This has led to recommendations for further research into pre‐treatment administration to enhance outcomes [].
4.6 Other Active Ingredients in the MelanoPro Peel System
Salicylic acid (SA) is a beta hydroxy acid (BHA) with chemo‐exfoliant properties. It has been widely studied for its effectiveness in treating PIH, particularly in higher FST, both as a standalone chemical peel (30% SA) and in combination with hydroquinone 4% [, ]. Although hydroquinone is often used alongside SA, its long‐term inclusion in treatment regimens remains underexplored, and concerns persist regarding its safety profile and potential adverse effects.
Joshi et al. evaluated serial SA peels (20%–30%) in PIH patients (Fitzpatrick IV–VI), comparing treated and untreated facial halves. While mild clinical improvement was observed, the findings were not statistically significant [].
Ahn et al. reported a whitening effect with 30% SA peels in acne‐induced PIH, noting significant reductions in erythema, oiliness, dryness, and scaliness. This effect is particularly relevant when selecting chemical peels for darker skin tones [].
Glycolic acid (GA) and lactic acid (LA) are alpha hydroxy acids (AHAs) that suppress melanin formation by directly inhibiting tyrosinase activity, an effect that is independent of their acidic properties. Their role in treating pigmentary lesions extends beyond enhancing epidermal turnover, as they also actively inhibit melanin production within melanocytes. This dual mechanism makes GA and LA valuable agents for addressing hyperpigmentation and improving skin tone [].
Grover et al. reported that glycolic acid peels resulted in a significant improvement in PIH after 16 weeks []. Similarly, Burns et al. found that individuals with darker skin tones experienced faster and more pronounced improvement with glycolic acid peels compared to the control group []. This effect is attributed to the exfoliation of pigmented epidermal cells, followed by re‐epithelialization with cells containing less melanin.
Retinol, a derivative of vitamin A, plays a role in PIH treatment by inhibiting tyrosinase transcription and enhancing epidermal turnover, aiding in melanin removal and dispersion. It can be used alone or in combination with other depigmenting agents. Available in concentrations ranging from 0.01% to 0.1%, its use requires caution, particularly in darker skin types, as higher concentrations of tretinoin may cause irritant dermatitis, potentially worsening PIH []. The MelanoPro Peel System contains retinol at a concentration of 0.25%–0.3%, which may explain the transient irritant dermatitis observed in two cases within our study. However, symptoms were mild and self‐limiting.
Niacinamide, a form of Vitamin B3, has been studied for its role in treating pigmentary disorders, primarily by reducing melanosome transfer rather than directly inhibiting tyrosinase activity or melanocyte proliferation. Hakozaki et al. demonstrated that niacinamide decreases melanosome transfer by 35%–68%, likely by disrupting the signaling interaction between keratinocytes and melanocytes. Clinical studies have shown that formulations containing 2%–5% niacinamide effectively lighten melasma and UV‐induced hyperpigmentation []. However, further research is needed to establish its safety and efficacy in the management of PIH.
Acetyl hexapeptide‐1 is a peptide known for its depigmenting effects, as it decreases melanin production and melanosome transfer to keratinocytes []. While extensively used in anti‐aging skincare products for its ability to reduce static and dynamic wrinkles, there is limited published data on its role in pigmentation disorders []. This suggests that its application in improving hyperpigmentation may represent a novel and emerging use that warrants further investigation.
5 Conclusion
Although the study sample was small, these findings demonstrate that pre‐ and post‐treatment of facial skin in FST III–V with a novel, home‐based peeling system can significantly reduce the incidence of PIH following fractional CO2 laser resurfacing. The novel peel is an effective method for minimizing PIH, with high patient satisfaction, low adverse outcomes, and no requirement for hydroquinone prescriptions. In addition to reduced pigmentation, patients reported improvements in overall skin quality, including enhanced texture and reduced roughness. Its non‐prescriptive ingredient list provides a safer and more accessible alternative for patients seeking pigmentation control post‐laser resurfacing.
Author Contributions
Dr. Xiaozhun Hang and Dr. Davin Lim contributed equally as co‐first authors. Dr. Davin Lim conceptualized the study, led data collection, and drafted the manuscript. Dr. Xiaozhun Hang contributed to statistical analysis, data interpretation, and manuscript revisions. Both authors reviewed and approved the final manuscript.
Ethics Statement
This study was conducted in accordance with the Declaration of Helsinki. Written informed consent was obtained from all participants before enrolment. Participant confidentiality was maintained, and all data were anonymized before analysis.
Conflicts of Interest
The authors declare no conflicts of interest.
Acknowledgments
This study was partially funded by Dermalogica. The sponsor had no role in study design, data collection, analysis, or manuscript preparation.
References
- 1. J. Tan, S. Beissert, F. Cook‐Bolden, et al., “Evaluation of Psychological Well‐Being and Social Impact of Atrophic Acne Scarring: A Multinational, Mixed‐Methods Study,” JAAD International 6 (2022): 43–50.
- 2. C. I. Jacob, J. S. Dover, and M. S. Kaminer, “Acne Scarring: A Classification System and Review of Treatment Options,” Journal of the American Academy of Dermatology 45, no. 1 (2001): 109–117.
- 3. J. Chwalek and D. J. Goldberg, “Ablative Skin Resurfacing,” Current Problems in Dermatology 42 (2011): 40–47.
- 4. J. Preissig, K. Hamilton, and R. Markus, “Current Laser Resurfacing Technologies: A Review That Delves Beneath the Surface,” Seminars in Plastic Surgery 26, no. 3 (2012): 109–116.
- 5. N. Cheyasak, W. Manuskiatti, P. Maneeprasopchoke, and R. Wanitphakdeedecha, “Topical Corticosteroids Minimise the Risk of Postinflammatory Hyper‐Pigmentation After Ablative Fractional CO2 Laser Resurfacing in Asians,” Acta Dermato‐Venereologica 95, no. 2 (2015): 201–205.
- 6. H. H. Chan, D. Manstein, C. S. Yu, S. Shek, T. Kono, and W. I. Wei, “The Prevalence and Risk Factors of Post‐Inflammatory Hyperpigmentation After Fractional Resurfacing in Asians,” Lasers in Surgery and Medicine 39, no. 5 (2007): 381–385.
- 7. S. F. Weiner, “Radiofrequency Microneedling: Overview of Technology, Advantages, Differences in Devices, Studies, and Indications,” Facial Plastic Surgery Clinics of North America 27, no. 3 (2019): 291–303.
- 8. A. N. Kauvar, “Fractional Nonablative Laser Resurfacing: Is There a Skin Tightening Effect?,” Dermatologic Surgery 40, no. Suppl 12 (2014): S157–S163.
- 9. H. J. You, D. W. Kim, E. S. Yoon, and S. H. Park, “Comparison of Four Different Lasers for Acne Scars: Resurfacing and Fractional Lasers,” Journal of Plastic, Reconstructive & Aesthetic Surgery 69, no. 4 (2016): e87–e95.
- 10. J. H. M. Prohaska, Laser Complications (StatPearls Publishing, 2023), https://www.ncbi.nlm.nih.gov/books/NBK532248/.
- 11. S. Sriprachya‐anunt, N. L. Marchell, R. E. Fitzpatrick, M. P. Goldman, and E. F. Rostan, “Facial Resurfacing in Patients With Fitzpatrick Skin Type IV,” Lasers in Surgery and Medicine 30, no. 2 (2002): 86–92.
- 12. R. Wanitphakdeedecha, R. Phuardchantuk, and W. Manuskiatti, “The Use of Sunscreen Starting on the First Day After Ablative Fractional Skin Resurfacing,” Journal of the European Academy of Dermatology and Venereology 28, no. 11 (2014): 1522–1528.
- 13. S. Z. Arsiwala and S. R. Desai, “Fractional Carbon Dioxide Laser: Optimizing Treatment Outcomes for Pigmented Atrophic Acne Scars in Skin of Color,” Journal of Cutaneous and Aesthetic Surgery 12, no. 2 (2019): 85–94.
- 14. T. B. West and T. S. Alster, “Effect of Pretreatment on the Incidence of Hyperpigmentation Following Cutaneous CO2 Laser Resurfacing,” Dermatologic Surgery 25, no. 1 (1999): 15–17.
- 15. S. Lueangarun, A. Srituravanit, and T. Tempark, “Efficacy and Safety of Moisturizer Containing 5% Panthenol, Madecassoside, and Copper‐Zinc‐Manganese Versus 0.02% Triamcinolone Acetonide Cream in Decreasing Adverse Reaction and Downtime After Ablative Fractional Carbon Dioxide Laser Resurfacing: A Split‐Face, Double‐Blinded, Randomized, Controlled Trial,” Journal of Cosmetic Dermatology 18, no. 6 (2019): 1751–1757.
- 16. M. Wei, L. Li, X. F. Zhang, M. Li, B. Wang, and Y. Yan, “Fusidic Acid Cream Comparatively Minimizes Signs of Inflammation and Postinflammatory Hyperpigmentation After Ablative Fractional CO(2) Laser Resurfacing in Chinese Patients: A Randomized Controlled Trial,” Journal of Cosmetic Dermatology 20, no. 6 (2021): 1692–1699.
- 17. S. Rutnin, D. Pruettivorawongse, K. Thadanipon, and V. Vachiramon, “A Prospective Randomized Controlled Study of Oral Tranexamic Acid for the Prevention of Postinflammatory Hyperpigmentation After Q‐Switched 532‐Nm Nd:YAG Laser for Solar Lentigines,” Lasers in Surgery and Medicine 51, no. 10 (2019): 850–858.
- 18. S. Desai, E. Ayres, H. Bak, et al., “Effect of a Tranexamic Acid, Kojic Acid, and Niacinamide Containing Serum on Facial Dyschromia: A Clinical Evaluation,” Journal of Drugs in Dermatology 18, no. 5 (2019): 454–459.
- 19. S. E. Gad, Y. M. E. Neinaa, O. K. Rizk, and N. E. R. Ghaly, “Efficacy of Platelet‐Poor Plasma Gel in Combination With Fractional CO(2) Laser in Striae Distensae: A Clinical, Histological, and Immunohistochemical Study,” Journal of Cosmetic Dermatology 20, no. 10 (2021): 3236–3244.
- 20. J. A. Jung, B. J. Kim, M. S. Kim, et al., “Protective Effect of Botulinum Toxin Against Ultraviolet‐Induced Skin Pigmentation,” Plastic and Reconstructive Surgery 144, no. 2 (2019): 347–356.
- 21. T. K. Noh, B. Y. Chung, S. Y. Kim, et al., “Novel Anti‐Melanogenesis Properties of Polydeoxyribonucleotide, a Popular Wound Healing Booster,” International Journal of Molecular Sciences 17, no. 9 (2016): 1448.
- 22. B. Cho, J. Lee, Y. Won, et al., “Skin Brightening Efficacy of Exosomes Derived From Human Adipose Tissue‐Derived Stem/Stromal Cells: A Prospective, Split‐Face, Randomized Placebo‐Controlled Study,” Cosmetics 7 (2020): 90.
- 23. Imaging C , “Clinical Imaging System,” 2025, https://www.clinicalimaging.com.au/home.
- 24. M. Lazar, S. Shih, S. Rajanala, H. De La Garza, K. Kennedy, and N. Vashi, PIHASI, PIDASI, and IGA Scales, 1st ed. (Mendeley Data, 2024).
- 25. M. Lazar, S. Shih, S. Rajanala, H. De La Garza, K. F. Kennedy, and N. A. Vashi, “PIDASI and PIHASI: Scoring Tools for Assessment of Postinflammatory Hyperpigmentation,” Journal of the American Academy of Dermatology 91, no. 1 (2024): 142–144.
- 26. A. Bin Dakhil, A. Shadid, and S. Altalhab, “Post‐Inflammatory Hyperpigmentation After Carbon Dioxide Laser: Review of Prevention and Risk Factors,” Dermatology Reports 15, no. 4 (2023): 9703.
- 27. C. Schwartz, A. Jan, and P. M. Zito, Hydroquinone (StatPearls Publishing, 2023), https://www.ncbi.nlm.nih.gov/books/NBK539693/.
- 28. K. Mar, B. Khalid, M. Maazi, R. Ahmed, O. J. E. Wang, and T. Khosravi‐Hafshejani, “Treatment of Post‐Inflammatory Hyperpigmentation in Skin of Colour: A Systematic Review,” Journal of Cutaneous Medicine and Surgery 28, no. 5 (2024): 473–480.
- 29. S. Dayal, P. Sahu, B. Sangal, and S. Sharma, “Role of Chemical Peels in Postinflammatory Hyperpigmentation: A Comprehensive Review,” Pigment International 6, no. 2 (2019): 59.
- 30. Pro D. MelanoPro Peel System [PDF File] (Dermalogica Pro, 2024).
- 31. R. M. Halder and G. M. Richards, “Topical Agents Used in the Management of Hyperpigmentation,” Skin Therapy Letter 9, no. 6 (2004): 1–3.
- 32. N. J. Lowe, D. Rizk, P. Grimes, M. Billips, and S. Pincus, “Azelaic Acid 20% Cream in the Treatment of Facial Hyperpigmentation in Darker‐Skinned Patients,” Clinical Therapeutics 20, no. 5 (1998): 945–959.
- 33. L. S. Kakita and N. J. Lowe, “Azelaic Acid and Glycolic Acid Combination Therapy for Facial Hyperpigmentation in Darker‐Skinned Patients: A Clinical Comparison With Hydroquinone,” Clinical Therapeutics 20, no. 5 (1998): 960–970.
- 34. M. S. Kim, S. H. Bang, J. H. Kim, H. J. Shin, J. H. Choi, and S. E. Chang, “Tranexamic Acid Diminishes Laser‐Induced Melanogenesis,” Annals of Dermatology 27, no. 3 (2015): 250–256.
- 35. K. M. Kim and H. W. Lim, “The Uses of Tranexamic Acid in Dermatology: A Review,” International Journal of Dermatology 62, no. 5 (2023): 589–598.
- 36. P. E. Grimes, “The Safety and Efficacy of Salicylic Acid Chemical Peels in Darker Racial‐Ethnic Groups,” Dermatologic Surgery 25, no. 1 (1999): 18–22.
- 37. A. U. Bari, Z. Iqbal, and S. B. Rahman, “Tolerance and Safety of Superficial Chemical Peeling With Salicylic Acid in Various Facial Dermatoses,” Indian Journal of Dermatology, Venereology and Leprology 71, no. 2 (2005): 87–90.
- 38. S. S. Joshi, S. L. Boone, M. Alam, et al., “Effectiveness, Safety, and Effect on Quality of Life of Topical Salicylic Acid Peels for Treatment of Postinflammatory Hyperpigmentation in Dark Skin,” Dermatologic Surgery 35, no. 4 (2009): 638–644.
- 39. H. H. Ahn and I.‐H. Kim, “Whitening Effect of Salicylic Acid Peels in Asian Patients,” Dermatologic Surgery 32 (2006): 372–375.
- 40. A. Usuki, A. Ohashi, H. Sato, Y. Ochiai, M. Ichihashi, and Y. Funasaka, “The Inhibitory Effect of Glycolic Acid and Lactic Acid on Melanin Synthesis in Melanoma Cells,” Experimental Dermatology 12, no. Suppl 2 (2003): 43–50.
- 41. C. Grover and B. S. Reddu, “The Therapeutic Value of Glycolic Acid Peels in Dermatology,” Indian Journal of Dermatology, Venereology and Leprology 69, no. 2 (2003): 148–150.
- 42. R. L. Burns, P. L. Prevost‐Blank, M. A. Lawry, T. B. Lawry, D. T. Faria, and D. P. Fivenson, “Glycolic Acid Peels for Postinflammatory Hyperpigmentation in Black Patients. A Comparative Study,” Dermatologic Surgery 23, no. 3 (1997): 171–174.
- 43. V. D. Callender, S. St Surin‐Lord, E. C. Davis, and M. Maclin, “Postinflammatory Hyperpigmentation: Etiologic and Therapeutic Considerations,” American Journal of Clinical Dermatology 12, no. 2 (2011): 87–99.
- 44. T. Hakozaki, L. Minwalla, J. Zhuang, et al., “The Effect of Niacinamide on Reducing Cutaneous Pigmentation and Suppression of Melanosome Transfer,” British Journal of Dermatology 147, no. 1 (2002): 20–31.
- 45. Y. Ye, Y. Li, C. Xu, and X. Wei, “Improvement of Mild Photoaged Facial Skin in Middle‐Aged Chinese Females by a Supramolecular Retinol Plus Acetyl Hexapeptide‐1 Containing Essence,” Skin Health and Disease 3, no. 4 (2023): e239.