INTRODUCTION AND BACKGROUND
Over the past two decades, oncology has witnessed major advancements in the understanding, diagnosis, and treatment of cancer, which has led to an era of precision medicine and the proliferation of new therapies that can be tailored to patients. As one of the three pillars of cancer therapy, radiation oncology has undergone profound transformation, driven by advancements in technology, evolving treatment paradigms, and a growing understanding of the intricate interplay between cancer biology, systemic therapy, and radiation. Radiation therapy remains implicated in the treatment of more than 50% of all patients with cancer at least once during the course of their disease, with an increase in the use of reirradiation. Moreover, curative treatment strategies for many malignancies continue to include radiation therapy, further underscoring its importance in interdisciplinary cancer management.
CONTEXT
Key Objective
How has clinical trial activity in radiation oncology evolved over the past two decades, and what are the implications for global cancer research?
Knowledge Generated
A comprehensive analysis of 4,253 radiation oncology trials from ClinicalTrials.gov demonstrates a sustained increase in trial activity, with notable growth in research on digestive, CNS, and head and neck cancers. Emerging areas, such as oligometastasis, represent new focuses, while hematology has seen declining activity. Despite these advances, only 6% of radiation oncology trials received industry funding, highlighting persistent funding challenges.
Relevance
This study highlights the dynamic evolution of radiation oncology research, emphasizing the need for increased industry engagement, regional equity in trial distribution and research priorities, as well as continued exploration of innovative technologies and multimodal therapies. These efforts are critical to translating promising findings into clinical practice and improving patient outcomes globally.
This modern era has also witnessed an unprecedented surge in oncology clinical trials, which serve as a linchpin for translating scientific discoveries into tangible improvements in patient care. Clinical trials in radiation oncology test new treatments, technologies, and strategies to help researchers assess their safety, efficacy, and side effects. Through clinical trials, valuable data that inform evidence-based decision making are generated. Ultimately, clinical trials not only shape the current standard of oncologic care, but also provide a basis for developing more effective treatments in the future.
An important milestone in tracking this scientific progress, formalizing reporting, and enhancing transparency in clinical trial research was the launch of the ClinicalTrials.gov website in February 2000. Initially, the database primarily included information on federally and privately funded clinical trials conducted in the United States. However, over the years, its scope has expanded globally, and now includes trials from all over the world. In 2003, the International Committee of Medical Journal Editors (ICMJE) made it necessary for researchers to register their clinical trials in a public database, with ClinicalTrials.gov being the most widely used platform. Throughout the years, numerous updates and enhancements have been made to ClinicalTrials.gov to improve its usability and scope.
Leveraging ClinicalTrials.gov, several analyses have been conducted recently. In 2017, Trone et al found reporting limitations in phase III radiation therapy trials. Liu et al compared radiation therapy with oncology trials and found limited funding for the former. Aggarwal et al, using a bibliographic approach, analyzed radiation oncology trials in 25 leading research countries, described the diverse trial landscape, and highlighted the need for more investment. Odedina et al also highlighted the need for more clinical trials in Africa in particular. Park et al expanded on the analysis by Trone et al, suggesting diversified funding and more aggressive implementation of hypofractionated treatments. In 2021, Wells et al compared anticancer therapy trial activity from 2014 to 2017 in high-income countries versus low- and middle-income countries, concluding that most trials come from high-income countries and are not aligned with global cancer burden. In a similar effort, Dodkins et al looked at radiation oncology randomized trials from 2014 to 2017, and stressed the need for greater investment in trial infrastructure, especially in low- and middle-income countries. Most recently, Kim et al found evidence for challenges in data transparency and harmonization in neuro-oncology trials. All these analyses show that there is much to learn from clinical trial data.
Until today, no comprehensive analysis of the clinical trial landscape in radiation oncology from the past two decades has been conducted. The objectives of this analysis hence encompass an examination of the characteristics of all conducted trials over the past two decades and of all currently ongoing trials by synthesizing ClinicalTrial.gov data, identifying clinical trial patterns, and critically evaluating the identified trends. In looking at research priorities, understanding geographical dominance, assessing trial type, and evaluating funding sources, this analysis aspires to provide clinicians, researchers, and policymakers with a robust understanding of the current state of the clinical trial landscape in radiation oncology. This endeavor will help to highlight potential research domains and to chart the scientific trajectory for this discipline in the future.
MATERIALS AND METHODS
Database
The clinical trial database ClinicalTrials.gov, provided by the US National Institutes of Health (NIH), served as the sole data source for this analysis. Available variables included the National Clinical Trial (NCT) number, study title, study uniform resource locator (URL), study status, brief study summary, study results, condition/disease, intervention/treatment, primary and secondary outcome measures, sponsor, collaborators, sex, age, phases, enrollment status, funder type, study type, study design, start date, completion dates, postponement dates, and study location. The website allows for an advanced filter search and free download of trial data into Microsoft Excel.
Initial Screening and Study Selection Process
For this study, the ClinicalTrials.gov database was searched. Key word filters were “cancer” for condition/disease “radiation therapy” and for intervention/treatment. The search was limited to trials that started between January 1, 2003, and December 31, 2023. Selection was further restricted to interventional trials only, with treatment as the primary purpose. Trials that lacked one or more essential variables, such as sex, age, phases, funder type, and study location, were excluded from the analysis. To enhance the quality of the acquired database, further filtering steps were applied with the aim of removing false-positive trials that were not related to radiation oncology, with the aim of identifying any references to radiation therapy. This methodology was based on the definition of intervention/treatment, as provided by ClinicalTrials.gov for, namely, a process or action that is the focus of a clinical study. The interventions included drugs, medical devices, procedures, vaccines, and other products that were either investigational or already available. Therefore, the inclusion of radiotherapy as an intervention in the trial necessitates its explicit mention within this section. Trials that did not contain any references to radiotherapy in the intervention/treatment category were excluded from the data set. The list of radiotherapy-related terminology used in this filtering was designed through an iterative process with manual screening through intervention or treatment to ensure that the provided list was consistent.
Database Refining Process
The database content was subsequently vetted for consistency and was prepared for statistical analysis. Funding source was classified into FED, network, National Institutes of Health (NIH), industry, others, and other gov. on the basis of the variable funder type. Trial status was split into ongoing (if study status was not yet recruiting, recruiting, enrolling by invitation, active not recruiting, or suspended), stopped early (if study status was terminated or withdrawn), or completed (if study status was completed), and unknown. For each trial, the geographical location was determined by extracting information from the study location variable. Each country was assigned one of the six geographical locations, namely, Africa, Asia, Europe, North America, South America, and Oceania. The primary tumor site was extracted from the trial title and condition/disease parameters. Subsequently, tumor location was categorized into the following groups: breast, CNS, digestive, genitourinary, gynecology system, head and neck, hematology system, oligometastatic, thoracic, and others. The others category was reserved for trials that either did not clearly fit any of the other nine groups or included patients with two or more of the other nine primary tumor entities. Binning is based on bin-specific lists of words, morphemes, and word segments that represent various anatomic locations and primary tumor entities.
First, bins were allocated on the basis of the conditions/disease section as per the description provided on ClinicalTrials.gov, where the condition/disease section contained the disease, disorder, syndrome, illness, or injury being studied. If it was impossible to define any bin solely on the information provided in this section, the trial title was used for allocation purposes. If allocation to two or more bins was possible on the basis of the condition/disease information, manual screening was performed for all trials. Trials with ambiguous conditions/diseases and titles were subjected to additional manual inspection to assign them to one of the tumor categories. To identify trials concerning oligometastatic disease (OMD), which might include one or more primary cancer entities, all trials centered around OMD were filtered out and allocated to the oligometastatic rather than the respective primary cancer category. In the last step, filtering and categorization were independently performed by two radiation oncologists.
Statistical Analysis
Descriptive summary statistics were computed for all the variables under investigation. Trial data were stratified into two distinct periods: the first spanning from 2003 to 2012, and the second from 2013 to 2023. Associations between various variables across these two decades were assessed using the chi-squared or Fisher's exact tests. Statistical significance was defined as a P value of <.05. Data from ClinialTrials.gov were initially obtained and stored in the spreadsheet program Microsoft Excel, and data cleaning and analysis were subsequently carried out using the Python 3.11.0 programming language. All graphs and figures were generated using Python libraries Matplotlib 3.8.0 and GeoPandas 0.14.1. Statistical analyses were performed using Python library SciPy 1.11.2.
Ethical Approval and Data Reporting
Formal ethical approval was unnecessary as the study used publicly available data from an open-access database without personal information. To ensure transparency and reproducibility, all raw data, algorithms, commands, and methodologies have been included in the manuscript, supporting the scientific integrity and reproducibility of the results.
RESULTS
As of February 8, 2024, the ClinicalTrials.gov website compiled data from 482,151 registered studies. Filtering for cancer and radiation therapy trials conducted in the past two decades yielded an initial data set of 12,614 (2.6%) trials. After removing trials with insufficient data reporting, 5,795 (1.2%) trials remained. Subsequently, excluding false-positive trials yielded a remainder of 4,253 (0.9%) clinical trials, which were included in the analysis of this study. The flow diagram in Figure 1 shows a graphical depiction of the inclusion and exclusion criteria.
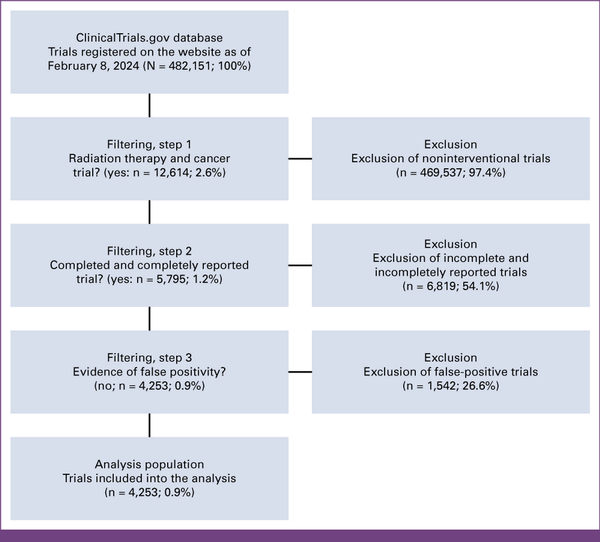
FIG 1
CONSORT diagram.
Over the past two decades, 4,253 radiation oncology trials have been registered at ClinicalTrials.gov. Clinical trial activity has steadily increased: 134 trials in 2003 (n = 4,253/n = 134; 3.2%) and 118 in 2004 (n = 4,253/n = 118; 2.8%), averaging 176 annually from 2005 to 2013 (n = 4,253/n = 176; 4.1%). In 2016, 234 trials were listed (n = 4,253/n = 234; 5.5%), rising to 244 in 2023 (n = 4,253/n = 244; 5.7%). The peak was in 2018, with 274 trials (n = 4,253/n = 274; 6.4%; Fig 2).
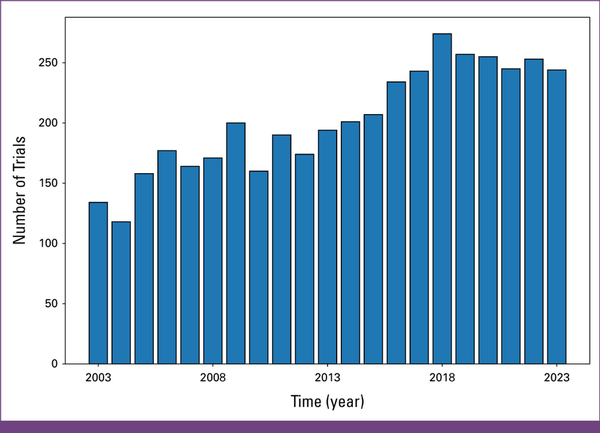
FIG 2
Number of trials per year.
Research activity is not uniformly distributed across organ systems. The digestive system emerged in most trials, that is, 972 (n = 4,253/n = 972; 22.9%). CNS and head and neck cancers were each represented in 577 (n = 4,253/n = 577; 13.6%) and 564 (n = 4,253/n = 564; 13.3%) trials, respectively. By contrast, the gynecologic system and breast cancers had the least number of trials, with 190 (n = 4,253/n = 190; 4.5%) and 236 (n = 4,253/n = 236; 5.5%) clinical trials, respectively. OMD was the subject of investigation in 95 (n = 4,253/n = 92; 2.2%) trials, underscoring the growing importance of better understanding and addressing this specific metastatic disease state (Appendix Fig A1A). The rise in trials focusing on OMD from one decade to the next was statistically significant, with an increase from 0.2% to 3.5% (P < .01). Hematology trials experienced a decrease in trial activity, with 175 (4.1%) trials reported for the period from 2013 to 2023 compared with 238 (5.6%) in the previous decade, representing a statistically significant drop from 13% to 7% (P < .01; Appendix Fig A2; Fig 3A).
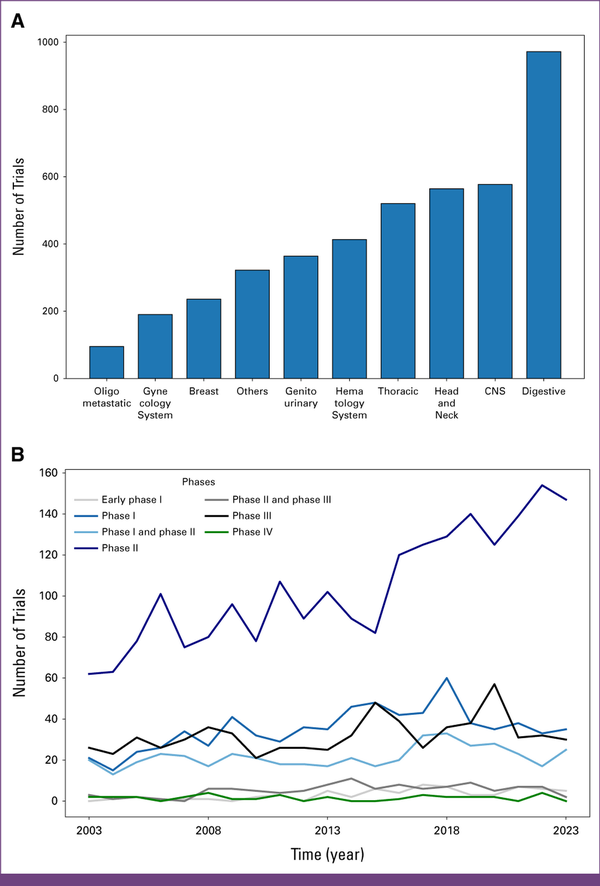
FIG 3
(A) Number of trials per year per tumor location. (B) Trends over time by study type.
Phase II studies emerged as the prevailing category throughout the years, with 2,181 (51.3%) registered trials falling into this category. From 2005 to 2014, an annual publication range of 75 (1.7%) to 107 (2.5%) phase II trials was observed, underlining a consistent focus on this particular clinical trial phase (first decade: 50.4%, second decade: 51.9%, P = .36). Phase I and phase III studies exhibited comparable levels of activity over the years, with numbers fluctuating between 15 (0.4%) and 60 (1.4%) clinical trials per year, totaling at 738 (17.4%) and 672 (15.8%) over the past 20 years, respectively. However, it is noteworthy to highlight a substantial deviation observed in the latter decade, characterized by a marked increase in early phase I studies, escalating from 0.6% to 2% (P < .01; Table 1). Phase IV trials manifested as the least published category, amounting to 33 (0.4%) of all registered trials, and maintained an average of approximately two (<0.1%) clinical trials per year (Fig 3B). This general distribution pattern of trial phases can also be observed in the OMD category (Appendix Fig A1B). Fewer trials terminated prematurely during the latter decade, accounting for only 10% of trials compared with the 22% observed in the first decade.
TABLE 1
Comparative Statistical Analysis Regarding Clinical Trial Patterns Across the Past Two Decades
| Parameter | Condition | 2003-2012, No. (%) | 2013-2023, No. (%) | P Value: Condition | P Value: Parameter |
|---|---|---|---|---|---|
| Study status | <.01 | ||||
| Ongoing | 129 (7.84) | 1,536 (58.92) | <.01 | ||
| Unknown | 229 (13.91) | 366 (14.04) | .94 | ||
| Completed | 926 (56.26) | 430 (16.49) | <.01 | ||
| Stopped early | 362 (21.99) | 275 (10.55) | <.01 | ||
| Conditions | <.01 | ||||
| Digestive | 351 (21.32) | 621 (23.82) | .06 | ||
| Others | 106 (6.44) | 216 (8.29) | .03 | ||
| CNS | 250 (15.19) | 327 (12.54) | .02 | ||
| Thoracic | 207 (12.58) | 313 (12.01) | .61 | ||
| Genitourinary | 131 (7.96) | 233 (8.94) | .29 | ||
| Breast | 96 (5.83) | 140 (5.37) | .57 | ||
| Oligometastatic | 4 (0.24) | 91 (3.49) | <.01 | ||
| Gynecology system | 86 (5.22) | 104 (3.99) | .07 | ||
| Head and neck | 195 (11.85) | 369 (14.15) | .03 | ||
| Hematology system | 220 (13.37) | 193 (7.4) | <.01 | ||
| Sex | .02 | ||||
| All | 1,372 (83.35) | 2,219 (85.12) | .13 | ||
| Male | 109 (6.62) | 189 (7.25) | .47 | ||
| Female | 165 (10.02) | 199 (7.63) | <.01 | ||
| Age | <.01 | ||||
| Adult, older adult | 1,343 (81.59) | 2,370 (90.91) | <.01 | ||
| Child, adult, older adult | 132 (8.02) | 98 (3.76) | <.01 | ||
| Child, adult | 117 (7.11) | 87 (3.34) | <.01 | ||
| Adult | 24 (1.46) | 16 (0.61) | <.01 | ||
| Older adult | 16 (0.97) | 31 (1.19) | .61 | ||
| Child | 14 (0.85) | 5 (0.19) | <.01 | ||
| Phases | <.01 | ||||
| II and III | 33 (2.0) | 76 (2.92) | .08 | ||
| II | 829 (50.36) | 1,352 (51.86) | .36 | ||
| I | 285 (17.31) | 453 (17.38) | .99 | ||
| III | 278 (16.89) | 394 (15.11) | .13 | ||
| I and II | 194 (11.79) | 260 (9.97) | .07 | ||
| IV | 17 (1.03) | 16 (0.61) | .18 | ||
| Early phase I | 10 (0.61) | 56 (2.15) | <.01 | ||
| Funder type | .07 | ||||
| Other | 1,461 (88.76) | 2,346 (89.99) | .22 | ||
| NIH | 84 (5.1) | 95 (3.64) | .03 | ||
| Industry | 101 (6.14) | 166 (6.37) | .81 | ||
| Continents | <.01 | ||||
| Africa | 16 (0.97) | 24 (0.92) | 1.0 | ||
| Asia | 238 (14.46) | 834 (31.99) | <.01 | ||
| Europe | 435 (26.43) | 468 (17.95) | <.01 | ||
| Oceania | 67 (4.07) | 86 (3.3) | .22 | ||
| South America | 27 (1.64) | 51 (1.96) | .53 | ||
| North America | 1,077 (65.43) | 1,400 (53.7) | <.01 |
North America contributed the majority of the studies (n = 2,477, 58.2%). The second most trials came from Asia (n = 1,072, 25.2%), while Europe maintained a stable contribution (n = 903, 21.2%). Africa contributed the least number of clinical studies (n = 40, 0.9%; Figs 4A and 4B).
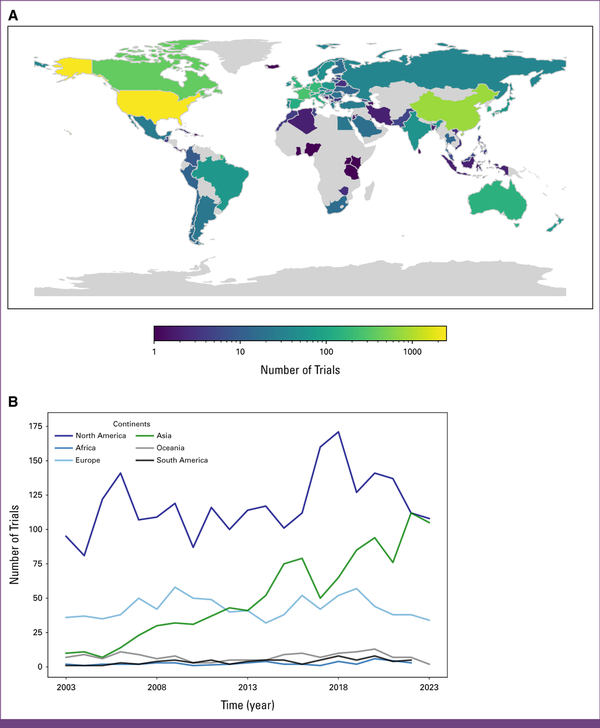
FIG 4
(A) Number of trials per country. (B) Number of trials per year per continent.
Industry funding remained stable at approximately 6% over the two decades (2003-2012: 6.1%; 2013-2023: 6.4%). Although other funding sources largely remained unchanged, the network funding category experienced a significant decrease (2003-2012: 10.2%; 2013-2023: 2.5%; P-value: <0.01), whereas the other category saw a significant increase over this period (2003-2012: 76.4%; 2013-2023: 85.7%; P value: <.01). For further details, see Appendix Figures A3A and A3B.
DISCUSSION
The sustained increase in the number of trials registered each year on ClinicalTrials.gov indicates growing interest in radiation oncology research. This trend is expected to continue. However, this does not provide a relative measure compared with other oncological disciplines, such as medical or surgical oncology. There is some evidence that radiation oncology trial activities are outnumbered and outfinanced by medical oncology. Medical oncology benefits significantly from pharmaceutical industry backing, with a focus on drug development, including immunotherapies and targeted treatments, which generates substantial funding and numerous trials. Surgical oncology, while less trial-heavy, emphasizes technical refinements and often collaborates with radiation oncology in multimodal treatment studies. A comparative analysis of oncologic research is required to holistically address this question.
One disease group that stands out, as it opposes this trend, is the hematologic system, where a decline in radiation oncology trial activity was observed. This decline coincides with advancements in systemic therapy for various hematologic diseases. Hodgkin and non-Hodgkin lymphomas have shifted the treatment paradigm away from primary radiation-based approaches.
The growing number of trials focusing on OMD, with an equitable distribution in terms of both publication and leadership between the United States and Europe, represents an important finding of our analysis. This finding reflects increasing recognition of the clinical relevance of OMD and the potential for targeted interventions in this specific patient population. The balanced leadership and publication contributions from the two regions highlight the importance of different research groups within the discipline attributed to the OMD state.
Although phase II trials are crucial for assessing treatment efficacy and generating hypotheses for further investigation, the preponderance of such studies prompts reflection on the lack of phase I trials and the translational gap between phase II and subsequent phase III trials. The abundance of phase II trials may suggest a need for improved strategies to investigate more drug-radiotherapy combinations in preclinical and phase I clinical trial settings and to facilitate the translation of promising therapies into larger, more definitive phase III trials. The prevalence of phase II trials also necessitates a critical evaluation of their role in informing clinical practice and shaping treatment guidelines, emphasizing the importance of well-designed and adequately powered trials in advancing the field.
North America has contributed to the majority of studies over the past two decades, yet other continents have steadily caught up in terms of research output. The remarkable surge in contributions from Asia is particularly noteworthy, signifying a significant leap in the region's involvement in clinical studies. Europe, while maintaining a stable presence, has demonstrated consistent growth in research endeavors. However, Africa remains relatively distant in terms of the number of clinical studies conducted, which in turn is also a reflection of lower patient volume, less cancer care resources, and research capacity. This evolving global distribution underscores the dynamic nature of scientific exploration and the expanding role of diverse regions in advancing medical knowledge.
Industry funding of approximately 6% over the past two decades has been notably low. Unlike medical oncology, which is heavily driven by pharmaceutical companies, radiation oncology relies on capital-intensive technologies, such as linear accelerators and proton therapy systems, which often have limited scalability and fewer repeat customers compared with drugs. Regulatory hurdles, high initial costs, and the lack of robust financial incentives for innovation further deter industry engagement. However, limited private-sector involvement arguably has the advantage of ensuring that most funding comes from publicly funded research institutions, which may reduce the potential biases associated with profit-driven motives. However, the downside is significant, as the private sector plays a crucial role in driving innovation, developing cutting-edge software programs, and advancing hardware technology. Increased industry funding can accelerate technological advancements and foster the development of novel treatment modalities in radiation oncology, ultimately improving patient care.
To our knowledge, the current analysis constitutes the first comprehensive overview of the clinical trial landscape in radiation oncology over the past two decades. One limitation of this analysis was that only trials registered on the ClinicalTrials.gov website were included. Another shortcoming was that only a limited number of parameters per trial were available via the database used, and that the quality and transparency of reported data remains imperfect. One implication for clinical routine and future research activity concerns the generalizability of clinical trial findings and recommendations to a global patient population, particularly considering variations in patient demographics, health care infrastructure, and socioeconomic factors, given the dominant contribution from the United States and Europe collectively shaping the majority of the studies investigated.
In conclusion, our examination of the radiation oncology clinical trial landscape over the past two decades has brought to light a concentration of studies in the United States and Europe, the prevalence of phase II trials, and an increasing emphasis on OMD trials. On the basis of these findings, the global research community should foster collaboration, address regional disparities, and strategically advance therapies from early-phase trials to impactful clinical applications. The field should also work toward attracting more industry funding for its trials.
AUTHOR CONTRIBUTIONS
Conception and design: Sebastian M. Christ, Gabriel Kobeissi
Collection and assembly of data: Sebastian M. Christ, Maksym Fritsak, Gabriel Kobeissi
Data analysis and interpretation: All authors
Manuscript writing: All authors
Final approval of manuscript: All authors
Accountable for all aspects of the work: All authors
AUTHORS' DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
The following represents disclosure information provided by authors of this manuscript. All relationships are considered compensated unless otherwise noted. Relationships are self-held unless noted. I = Immediate Family Member, Inst = My Institution. Relationships may not relate to the subject matter of this manuscript. For more information about ASCO's conflict of interest policy, please refer to www.asco.org/rwc or ascopubs.org/go/authors/author-center.
Open Payments is a public database containing information reported by companies about payments made to US-licensed physicians (Open Payments).
Rifaquat Rahman
Consulting or Advisory Role: Beijing Saint Lucia Consulting, Servier, NH TherAguix, Telix Pharmaceuticals
Research Funding: Bristol Myers Squibb (Inst), Puma Biotechnology (Inst), Lilly (Inst)
Uncompensated Relationships: Medicenna
Matthias Guckenberger
Consulting or Advisory Role: Varian Medical Systems, AstraZeneca
Research Funding: Varian Medical Systems (Inst)
Uncompensated Relationships: ESTRO
No other potential conflicts of interest were reported.
Rifaquat Rahman
Consulting or Advisory Role: Beijing Saint Lucia Consulting, Servier, NH TherAguix, Telix Pharmaceuticals
Research Funding: Bristol Myers Squibb (Inst), Puma Biotechnology (Inst), Lilly (Inst)
Uncompensated Relationships: Medicenna
Matthias Guckenberger
Consulting or Advisory Role: Varian Medical Systems, AstraZeneca
Research Funding: Varian Medical Systems (Inst)
Uncompensated Relationships: ESTRO
No other potential conflicts of interest were reported.
APPENDIX
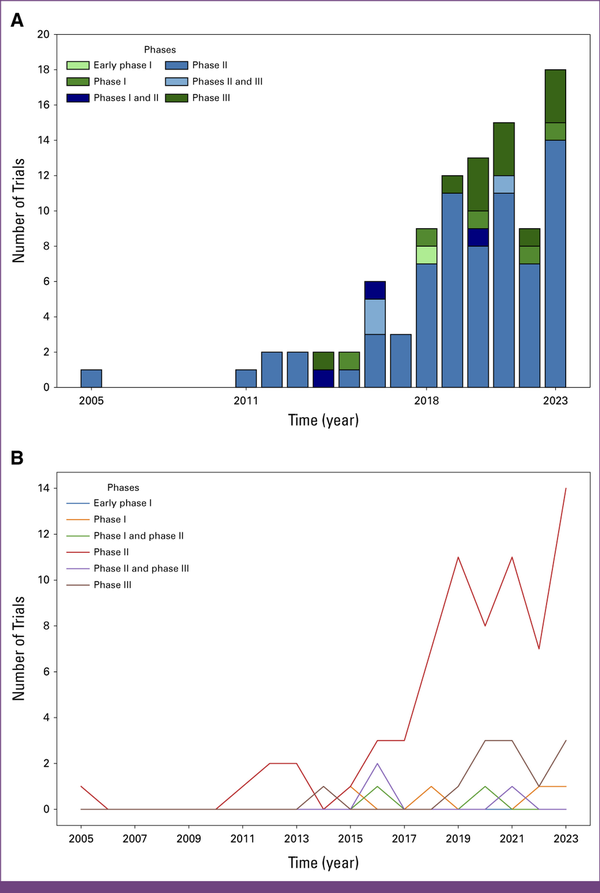
FIG A1
(A) Number of oligometastatic trials per year. (B) OMD trends over time by study phase. OMD, oligometastatic disease. Source: ClinicalTrials.gov data.
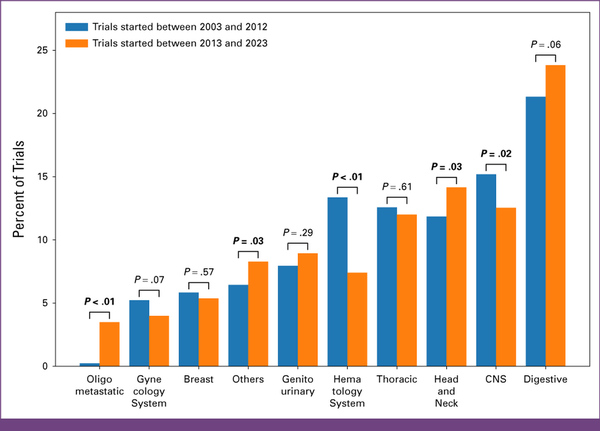
FIG A2
Percent of trials per tumor location per decade. Source: ClinicalTrials.gov data.
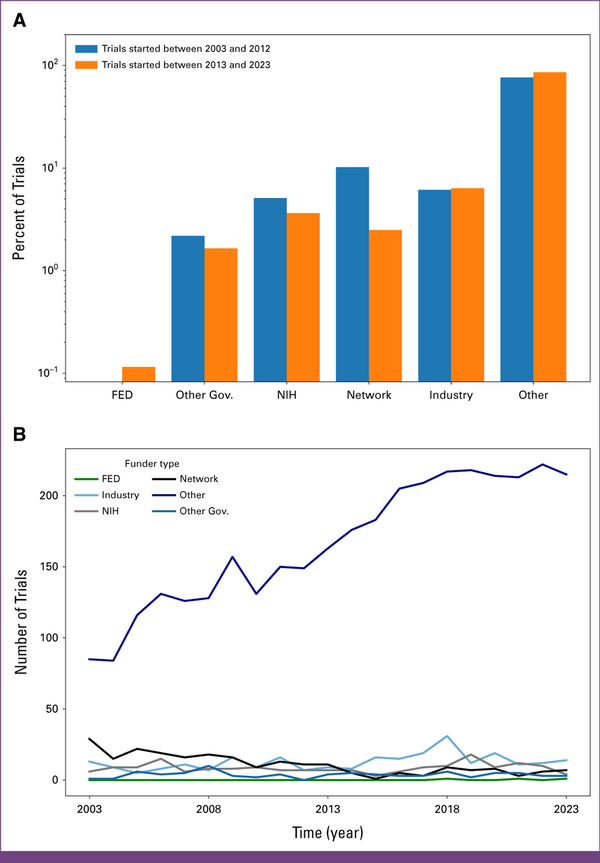
FIG A3
(A) Percent of trials per funder type per decade. (B) Trends over time by funder type. The FED category includes studies funded by US federal agencies other than the NIH. This includes a range of agencies such as The Department of Defense, The Department of Veterans Affairs, The Centers for Disease Control and Prevention, or The Food and Drug Administration. The other gov. category includes studies funded by government agencies outside the United States or by state and local government agencies within the United States. These studies often focus on public health initiatives, policy research, and regional health concerns. NIH is the US government’s primary agency for biomedical and public health research. The NIH comprises various institutes and centers focusing on different areas of health and disease. The network category refers to studies supported by research networks. These networks can be collaborations of multiple institutions, research groups, or consortia that pool resources and expertise to conduct large-scale, multicenter studies. Examples include clinical trial networks or consortia formed to tackle specific diseases or health issues. Finally, the other category encompasses funding from sources that do not fit into the other specified categories. It might include universities, nonprofit organizations, private foundations, and other nongovernmental organizations. FED, federal government; NIH, National Institutes of Health. Source: ClinicalTrials.gov data.
REFERENCES
1.
Chandra RA, Keane FK, Voncken FEM, et al.: Contemporary radiotherapy: Present and future. Lancet 398:171-184, 20212.
Barton MB, Jacob S, Shafiq J, et al.: Estimating the demand for radiotherapy from the evidence: A review of changes from 2003 to 2012. Radiother Oncol 112:140-144, 20143.
Christ SM, Ahmadsei M, Wilke L, et al.: Long-term cancer survivors treated with multiple courses of repeat radiation therapy. Radiat Oncol 16:1-8, 20214.
Valentini V, Boldrini L, Mariani S, et al.: Role of radiation oncology in modern multidisciplinary cancer treatment. Mol Oncol 14:1431-1441, 20205.
Trone JC, Espenel S, Rehailia-Blanchard A, et al.: Navigating the highlights of phase III trials: A watchful eye on evidence-based radiotherapy. Ann Oncol 28:2691-2697, 20176.
Liu X, Zhang Y, Tang LL, et al.: Characteristics of radiotherapy trials compared with other oncological clinical trials in the past 10 years. JAMA Oncol 4:1073-1079, 20187.
Aggarwal A, Lewison G, Rodin D, et al.: Radiation therapy research: A global analysis 2001-2015. Int J Radiat Oncol Biol Phys 101:767-778, 20188.
Odedina FT, Shamley D, Okoye I, et al.: Landscape of oncology clinical trials in Africa. JCO Glob Oncol 10.1200/JGO.19.001899.
Park S, Rim CH, Yoon WS: Where is clinical research for radiotherapy going? Cross-sectional comparison of past and contemporary phase III clinical trials. Radiat Oncol 15:1-7, 202010.
Wells JC, Sharma S, Del Paggio JC, et al.: An analysis of contemporary oncology randomized clinical trials from low/middle-income vs high-income countries. JAMA Oncol 7:379-385, 202111.
Dodkins J, Hopman WM, Wells JC, et al.: Is clinical research serving the needs of the global cancer burden? An analysis of contemporary global radiation therapy randomized controlled trials. Int J Radiat Oncol Biol Phys 113:500-508, 202212.
Kim Y, Armstrong TS, Gilbert MR, et al.: A critical analysis of neuro-oncology clinical trials. Neuro Oncol 25:1658-1671, 202313.
Specht L: Evolution, and current and future role of radiotherapy in the treatment of haematological malignancies. Lancet Haematol 11:e476-e479, 2024
