CONTEXT
Key Objective
Did the benefits of making cancer clinical trials easier to conduct, adopted in response to the COVID-19 pandemic in early 2020, outweigh the potential harms to data quality?
Knowledge Generated
Based on a meta-analysis of 67 trials comprising N = 12,000 US-based participants conducted by 10 sponsors, large declines in trial enrollment, major protocol deviations, dropouts, and severe toxicity were found during the acute phase of the COVID-19 pandemic in 2020. However, these metrics all returned to prepandemic levels by 2021-2022, suggesting limited or no adverse impact of trial flexibilities on data quality over the longer term.
Relevance (P.L. Kunz)
The COVID-19 pandemic disrupted the conduct of clinical trials and led to temporary measures to allow more flexibility, including remote consent, remote symptom monitoring, and distribution of oral anticancer agents directly to patients. A large meta-analysis showed that there were no negative effects of these changes on quality metrics. These findings suggest that we should routinely consider decentralized clinical trial principles.*
Plain Language Summary (M. Lewis)
The COVID-19 pandemic forced researchers to be more flexible with how clinical trials were conducted, including telehealth consents and visits and delivery of oral anti-cancer treatments. A research study showed that that these changes did not reduce the data quality from these trials. This suggests that we could consider adopting permanent changes to clinical trial conduct that are more patient centric.†
*Relevance section written by JCO Oncology Advances Editor-in-Chief Pamela L. Kunz, MD, FASCO.
†Plain Language Summary written by JCO Oncology Advances Associate Editor Mark Lewis, MD.
INTRODUCTION
The onset of the COVID-19 pandemic in early 2020 disrupted the conduct of cancer clinical trials, with steep drops in enrollment to existing trials and reductions in the activation of new trials. Given the challenges of recruiting patients to clinical trials during a pandemic, major federal agencies shared guidance to allow more flexibility in trial processes, supporting the continuity of clinical trials while ensuring patient safety and data integrity., Trial sponsors rapidly adopted these measures, including strategies such as remote patient consent to participate, remote symptom monitoring, and distribution of oral anticancer agents directly to trial patients. Enrollment in trials subsequently rebounded, especially for trials examining new cancer treatments.
The strategies adopted during the pandemic to facilitate access to trials, protocol treatment, monitoring, and follow-up have been advanced and studied previously., However, before the pandemic, their administration had not been widely adopted, largely out of concern about their impact on data quality. The COVID-19 public health emergency forced a rapid and systematic adoption of decentralized trial conduct procedures. A key question for researchers and policymakers is whether the benefits of adopting these strategies have outweighed the potential detriments to data quality.
To address this, in 2022, ASCO and the Friends of Cancer Research initiated an effort to systematically evaluate the impact of the COVID-19 experience for sponsors of oncology clinical trials. This report is based on original data obtained from 10 industry and federal sponsors of cancer clinical trials. To our knowledge, it represents a first-of-its-kind evaluation of the nature of key data quality indicators from trials during the COVID-19 pandemic.
METHODS
Sponsor and Trial Eligibility
Study participation was open to pharmaceutical companies and National Cancer Institute (NCI) Network groups that sponsored at least one anticancer treatment trial before the onset of the COVID-19 Public Health Emergency (PHE; January 2017-December 2019) and at least one anticancer treatment trial during the PHE (January 2020-December 2022). Eligible studies included phase I-III interventional anticancer treatment trials of any modality (eg, systemic therapy, surgery, etc) open in the United States from January 2017 to December 2022.
For global trials, we requested that sponsors provide data from US patients only. To limit the risk of identification of patients and sponsors, data were aggregated. The WCG Institutional Review Board approved this study.
Conceptual Framework
The COVID-19 outbreak had both direct and indirect effects on the conduct of cancer clinical trials. Direct effects included reduced patient willingness to participate in clinical trials and decreased institutional capacity and staffing. Indirect effects resulted from the declaration of a PHE and the accompanying mitigation strategies, such as shutdowns. Throughout this article, we generally refer to the impact of the COVID-19 pandemic itself (ie, the underlying causal mechanism of adverse consequences for trial conduct), even if, in some instances, the PHE was the more proximate cause.
Dependent Variables—Data Quality Indicators
Four data quality indicators served as the dependent variables (ie, the outcomes).
1. Enrollments were identified as the number of patients enrolled in a clinical trial. Enrollment data were chosen to reflect patients' access to clinical trials and willingness to participate in clinical trials throughout the pandemic.
2. Major protocol deviations were defined as any noncompliance with an IRB-approved protocol that presented a potential risk to participants or affected the integrity of study data. Protocol deviations were interpreted by sponsors to represent adherence to stated treatment, procedures, and data collection processes defined prospectively within trial protocols; thus, we interpreted an increase in the frequency of protocol deviations as a decrease in data integrity.
3. A patient dropout was defined as a patient withdrawal from protocol therapy early (ie, before achieving the primary end point as defined in the protocol) for any reason, excluding death. Common categories of dropout include withdrawal of consent, lost to follow-up, patient noncompliance, adverse events, or progressive disease. Patient dropouts reduce the overall power of trial designs, affect the integrity of trial data, and suggest the challenges that individual patients may face in adhering to study or protocol therapy.
4. Severe adverse events (grades 3-5), coded according to the Common Terminology Criteria for Adverse Events based on the initial onset, are significant treatment-related complications often requiring hospitalization. Only severe adverse events determined to be possibly, probably, or definitely related to treatment were considered. An increase in severe toxicity rates from prepandemic to during the pandemic may indicate compromised patient safety resulting from pandemic-related disruptions.
We hypothesized that enrollments declined and that major protocol deviations, patient dropouts, and severe adverse events increased beginning with the acute phase (ie, initial wave) of the pandemic.
Independent Variables
Prespecified COVID-19 pandemic–related landmark periods served as the key independent variables. The 3-year pre–COVID-19 pandemic period (pre-COVID) was defined as January 1, 2017-February 29, 2020, and was applied to enable the determination of a stable baseline period.
The initial pandemic wave was defined as beginning on March 1, 2020, commensurate with the first death because of COVID-19 observed in the United States and the announcement of a PHE., The initial wave was 2 months, ending on April 30, 2020, when the US COVID-19 mortality rate approximately peaked.
The initial recovery period was defined as May 1, 2020-December 31, 2020. The secondary recovery period was defined as January 1, 2021-December 31, 2022. Two recovery periods were used to determine whether recovery from pandemic effects occurred in stages.
Demographic variables included dichotomized age (<65 years v 65 or older), reflecting that those 65 years or older have access to social and medical support programs (eg, Social Security and Medicare); sex (female v male); race (Black v other race); and ethnicity (Hispanic v other ethnicity). Race and ethnicity were included given extensive research illustrating racial and ethnic barriers (especially for Black and Hispanic individuals) in access to care, including to COVID-19–related care.
Study phase was characterized as early (I and II)- versus late (III)-phase trials. Trials with combined strategies (ie, a phase II-III trial) were coded according to the highest phase.
Statistical Methods
Patients were considered at risk of an event if they had ≥30 days of follow-up within the prescribed landmark periods. Furthermore, to enable consistency in the amount of time at risk for the study outcomes across a broad set of trials with different follow-up periods, the follow-up period was specified to end at the completion of protocol therapy or 1 year after initial enrollment, whichever came first. In this context, the validity of the analysis is predicated on the idea that patients are uniformly at risk of a given event at any time within 1 year after trial enrollment. An individual patient's follow-up time could have spanned multiple study-specified periods. Within a given period, a patient who experienced an event was coded 1; otherwise, they were coded 0 (including unknowns). Thus, in aggregate, the trial- and period-level unit of analysis was a proportion, ranging from 0 to 1.
Trial enrollments were indexed from the date of initial registration to a trial. To account for a heterogeneous mix of trials with different enrollment goals over time, enrollment totals within each trial and period level were standardized on a 0-100 scale as the proportion of maximum study-level monthly enrollment across periods.
As the units of analysis were interval-level continuous proportions bounded on a 0-1 scale, we used multilevel beta-regression analyses. A Smithson transformation was used to accommodate 0 and 1 values. Multivariable analyses were conducted, adjusted for trial phase (early v late) with study and sponsor as random effects. For evaluations by time period, pre-COVID was considered the baseline (ie, reference) period, and indicator variables were used to compare outcomes between the initial wave, the initial recovery period, and the secondary recovery period and the pre-COVID baseline period. Only studies with both pre-COVID and follow-up data were included.
Interaction analyses by age, sex, race, and ethnicity were conducted to assess whether patterns of outcomes differed over time by these factors.
All P values were two-sided. For marginal comparisons, alpha = .05 was considered statistically significant, with no adjustment for multiple comparisons. For interaction tests by sociodemographic variables, we highlighted instances with alpha <.10 given more limited power for interaction analyses and for hypothesis generation.,
RESULTS
Among 41 eligible sponsors invited to participate, 10 (nine industry and one NCI) contributed data on 88 trials, among which 67 trials (76.1%) included sufficient data to analyze one or more of the specified outcomes, including enrollment (67, 100% of analyzable trials), protocol deviations (60, 89.6%), dropouts (61, 91.0%), and adverse events (61, 91.0%; Fig 1). The majority of evaluable trials (42, 62.9%) were sponsored by industry, with 25 (37.1%) sponsored by the NCI through its National Clinical Trials Network program. Fourteen trials (20.9%) were late-stage trials, and 53 (79.1%) were early-stage trials.
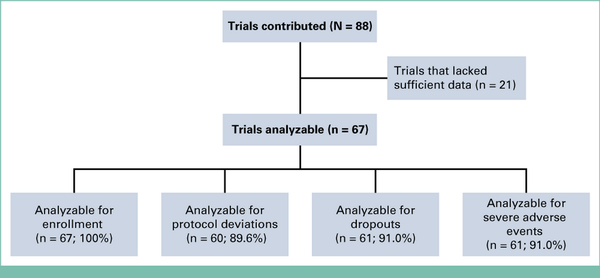
FIG 1.
Flow diagram.
Overall, the 67 analyzable trials represented N = 12,000 patients. The majority of patients were younger than 65 years (58.1%), and 42.8% was female (Table 1). Black and Hispanic representation was 10.6% and 8.5%, respectively. The most common cancers among patients were prostate (12.4%), breast (12.0%), bladder (11.3%), myeloma (11.2%), lymphoma (9.9%), and lung (6.5%).
TABLE 1.
Patient Characteristics (N = 12,000)
| Characteristic | No. (%) |
|---|---|
| Age, years | |
| <65 | 6,973 (58.1) |
| ≥65 | 5,027 (41.9) |
| Sex | |
| Female | 5,136 (42.8) |
| Male | 6,864 (57.2) |
| Race | |
| Black | 1,223 (10.6) |
| Other | 10,303 (89.4) |
| Unknown | 474 |
| Ethnicity | |
| Hispanic | 991 (8.5) |
| Other | 10,600 (91.5) |
| Unknown | 409 |
| Cancer type | |
| Biliary | 495 (4.1) |
| Bladder | 1,354 (11.3) |
| Breast | 1,440 (12.0) |
| Cervical | 169 (1.4) |
| Leukemia | 177 (1.5) |
| Lung | 779 (6.5) |
| Lymphoma | 1,187 (9.9) |
| Melanoma | 538 (4.5) |
| Myeloma | 1,339 (11.2) |
| Prostate | 1,484 (12.4) |
| Renal | 235 (2.0) |
| NOS | 2404 (20.0) |
| Other | 399 (3.3) |
Quality Metrics Over Time
There were large reductions in reported major protocol deviations, dropouts, and severe or worse toxicity in the initial wave compared with the pre-COVID period that rebounded to near-baseline proportions by the secondary recovery period. For instance, in the pre-COVID period, the estimated proportion of patients with a reported major protocol deviation was 15.7% (Table 2). By contrast, the proportion in the initial wave was 8.2%, representing a 58% reduction in the odds (odds ratio [OR], 0.42 [95% CI, 0.30 to 0.61], P < .001). The average estimated major protocol deviations increased to 12.5% in the initial recovery period, a 32% reduction compared with the pre-COVID period (OR, 0.68 [95% CI, 0.49 to 0.95], P = .02). By the secondary recovery period, the estimated major protocol deviations were 14.9%, a reduction of 24% in the odds that was not statistically significantly different from the baseline period (OR, 0.76 [95% CI, 0.53 to 1.07], P = .12). Similar large reductions in enrollments and in reported dropouts and severe or worse toxicity were observed during the initial wave, which also rebounded to near baseline proportions by the second recovery period (Fig 2).
TABLE 2.
Results for Quality Metrics Over Time
| End Point | Pre-COVID (January 2017-February 2020) | Initial Wave (March-April 2020) | Initial Recovery (May-December 2020) | Secondary Recovery (January 2021-December 2022) | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| % | OR (95% CI) | % | OR (95% CI) | P | % | OR (95% CI) | P | % | OR (95% CI) | P | |
| Mean monthly enrollment | 64.5 | 1.00 (reference) | 47.9 | 0.51 (0.30 to 0.86) | .01 | 65.8 | 1.06 (0.62 to 1.83) | .66 | 65.0 | 1.01 (0.56 to 1.81) | .97 |
| Major protocol deviations | 15.7 | 1.00 (reference) | 8.7 | 0.42 (0.30 to 0.61) | .001 | 12.5 | 0.68 (0.49 to 0.95) | .02 | 14.9 | 0.76 (0.53 to 1.07) | .12 |
| Dropouts | 37.6 | 1.00 (reference) | 8.5 | 0.10 (0.06 to 0.14) | .001 | 24.4 | 0.43 (0.31 to 0.58) | .001 | 30.7 | 0.74 (0.54 to 1.02) | .07 |
| Severe or worse toxicity | 35.7 | 1.00 (reference) | 18.6 | 0.36 (0.26 to 0.49) | .001 | 29.3 | 0.68 (0.51 to 0.91) | .001 | 32.7 | 0.86 (0.63 to 1.17) | .34 |
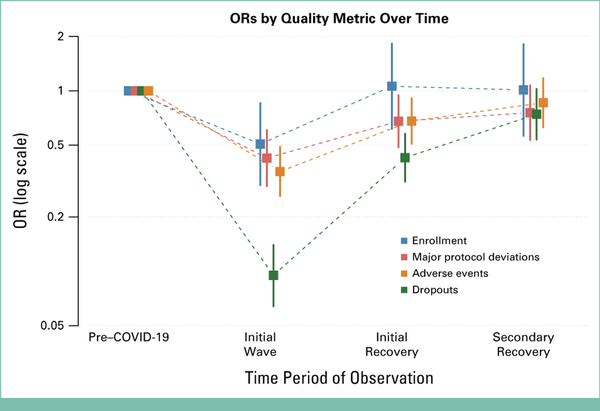
FIG 2.
ORs by quality metrics over time. OR, odds ratio.
Quality Metrics Over Time by Demographic Variables
Patterns of quality metrics over time by demographic factors reflected the overall aggregate patterns with a few exceptions (Fig 3). Black patients were less likely to enroll in trials than other patients during the initial wave. In addition, during the initial wave, a larger drop in reported protocol deviations was observed for patients 65 years or older (P = .06) and Black patients (P = .04). Finally, dropouts rebounded to pre-COVID levels during the initial recovery period for Black patients, but not for other patients (P = .007).
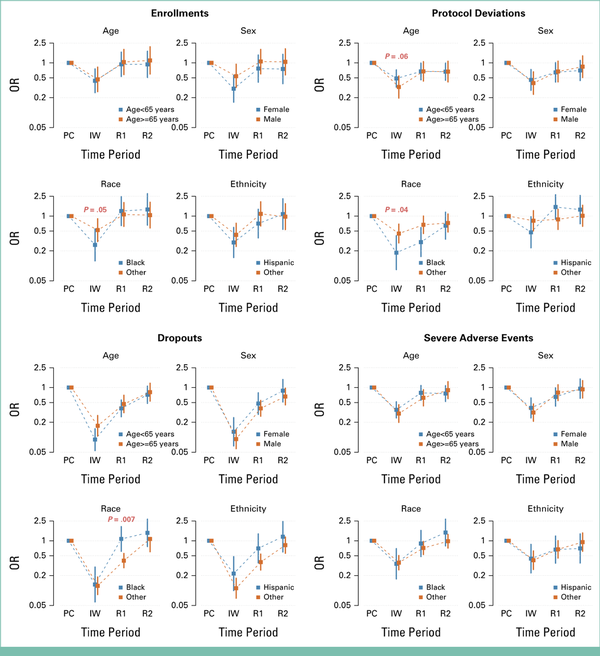
FIG 3.
ORs by quality metrics by demographic variables. P values indicate whether patterns of outcomes differed between specified groups (based on interaction analyses) at the given time period. P values <.10 are highlighted in red. IW, initial wave; OR, odds ratio; PC, Pre-COVID; R1, recovery period 1; R2, recovery period 2.
By the secondary recovery period, patterns of quality metrics were not statistically different from the pre-COVID baseline period for all demographic subgroups of patients except protocol deviations among patients younger than 65 years (OR, 0.67 [95% CI, 0.45 to 0.99], P = .04) and dropouts among male patients (OR, 0.66 [95% CI, 0.45 to 0.97], P = .03; Fig 3; Data Supplement, Table S1).
DISCUSSION
In this meta-analysis of multisponsor data, we found large declines in both enrollments to cancer clinical trials and reported major protocol deviations, dropouts, and severe toxicity for patients enrolled in trials during the initial wave of the COVID-19 pandemic. All metrics rebounded to approximately pre-COVID (ie, baseline) levels by the 2021-2022 period. During the initial wave of the pandemic, declines in enrollment were more pronounced among Black patients compared with all other racial groups combined. In addition, we observed a greater reduction in reported major protocol deviations for both Black patients and patients 65 years or older. Among virtually all demographic subgroups of patients, levels of the specified measures returned to baseline levels by the end of the study period.
The findings of reduced enrollment during the pandemic confirm previous observations of steep reductions in accrual during the initial wave of the pandemic.,, Data for federally sponsored trials suggested a 50% average reduction in enrollment early in the pandemic., Industry trials demonstrated reduced trial enrollments of about 30%., Enrollment declines were due to reductions in enrollment to active trials and reductions in the activation of new trials.
Previous evaluations suggested that protocol deviations, dropouts, and severe toxicity would be higher during the acute phase of the pandemic. A survey conducted by our team revealed that 90% of study sponsors reported a moderate or substantial rise in protocol deviations. However, this finding was based on sponsor perceptions rather than quantitative assessments. Bakouny et al showed an increase in protocol deviations during the initial wave although the evaluation was based on only 80 patients and the observed deviations were almost entirely minor (95%) and predominantly attributable to the COVID-19 virus.
Our data on 12,000 patients demonstrate a decline in the reported protocol deviations, dropouts, and severe toxicity during the initial pandemic wave. Several factors might have contributed to these declines. Guidance issued during the pandemic introduced modifications to trial conduct, potentially leading to fewer events classified as protocol deviations (ie, missed visits). This finding aligns with our previous qualitative analysis, which suggested that trial flexibility measures had an impact on the overall occurrence of protocol deviations. Logistical strategies to ease trial conduct, such as direct shipment of study drug to patients' homes, might have also reduced protocol deviations. In addition, survey results suggest that investigators and care teams adopted strategies to limit or avoid immunocompromising regimens early in the pandemic, which could have influenced the reporting of severe toxicities. However, it is unlikely that actual toxicity from cancer treatment dropped so precipitously immediately after the COVID-19 outbreak. The observed decrements in quality indicators may also reflect limitations in reporting and/or patient follow-up early in the pandemic. For example, a survey found that 60% of investigators reported that the COVID-19 pandemic had a moderate or high impact on patient visits and cancer centers experienced personnel shortages because of COVID-19., These factors together could have contributed to decreased data collection and reporting during the initial wave.
In May 2021, the CDC announced new guidance, stating that fully vaccinated individuals no longer needed to mask or practice social distancing. In addition, by 2022, health care utilization in the United States had largely rebounded from the pandemic's beginning. In this 2021-2022 timeframe, after the conclusion of the acute phase of the pandemic, we found that patterns of quality metrics had largely returned to pre-COVID (ie, baseline) levels, but importantly, did not exceed pre-COVID levels. This pattern emerged although trial mitigation processes and flexibilities had been widely adopted throughout the pandemic, including measures such as remote distribution of oral therapies, remote monitoring, and remote consent.,
The trial mitigation measures recommended early in the pandemic by federal agencies align with decentralized clinical trial principles., Although decentralized clinical trial elements have been considered over decades, the COVID-19 pandemic enabled their rapid adoption, creating the scenario for a natural experiment in which the impact on trial conduct could be feasibly evaluated. Throughout the pandemic, decentralized clinical trial elements were implemented to ensure continuity of research while minimizing risks to safety and data integrity. Despite these procedural modifications, quality metrics remained consistent with baseline levels, demonstrating that using DCT elements did not compromise trial quality. This finding suggests that decentralized clinical trial elements can be safely adopted as permanent fixtures in the conduct of cancer clinical trials without substantial reductions in data quality that could compromise study reporting or interpretation.
The permanent adoption of these new sets of trial procedures would represent a paradigm shift in the conduct of cancer clinical trials, with the potential to improve access to trials for all patients and thereby to conduct trials more rapidly in more diverse sets of patients. The US Food and Drug Administration recently provided guidance on the conduct of decentralized trials, with a focus on digital health technologies. Research and action statements by ASCO, a call to action by the American Cancer Society, and working group statements by the NCI have also highlighted the importance of further advancing decentralized clinical trial elements. The adoption of strategies that are patient-focused and reduce the burden of trial participation is a necessary adjunct to other measures aimed at improving access to clinical trials for vulnerable patient populations. Indeed, our own findings illustrated the disproportionate impact of the pandemic on some patient groups, with larger declines in enrollment among Black patients and larger reductions in protocol deviations reported for both Black patients and older patients. These differences likely reflect known differences in access to care by race and age during the pandemic, including access to clinical research studies.,,
This study has limitations. Although the study represented 12,000 patients, each random effect was at the study level, of which only 67 trials were available, which likely limited power to detect differences between patient subgroups. We did not adjust for multiple comparisons given the observational nature of this study. However, the strength of the observed differences during the initial wave was sufficient to be statistically significant under any multiplicity adjustment. Furthermore, our evaluation was completed at the end of 2022. Data beyond 2022 would have revealed the more enduring impact of the recovery in the presence of continuous adoption of modernized trial processes, especially as they are related to dropout rates and retention. In addition, data on other aspects of patients' backgrounds—such as rurality or additional categories of race—were not available for evaluation. Finally, data on the stage of disease were not available, and evaluations within disease type were limited by the number of studies for a given cancer.
In this comprehensive, multisponsor evaluation of quality metrics for cancer clinical trials during the COVID-19 pandemic, we found large declines in enrollment and in the occurrence of major protocol deviations, dropouts, and severe toxicity during the initial wave that rebounded to pre-COVID levels by the end of the study period. These findings highlight the temporary disruption to trial conduct during the acute phase of the COVID-19 pandemic. They also indicate that pandemic-related procedural flexibility did not lead to increased major protocol deviations, dropouts, or severe toxicity over the long term. As such, sponsors and regulators should consider broader adoption of trial flexibilities moving forward.
SUPPORT
NIH/NCI grant awards U10CA180888, U10CA180819 (J.M.U.).
AUTHOR CONTRIBUTIONS
Conception and design: Joseph M. Unger, Hillary S. Andrews, Laura A. Levit, Brittany A. McKelvey, Mark Stewart, Beverly Canin, Keith Flaherty, Suanna Bruinooge, Elizabeth Garrett-Mayer, Caroline Schenkel
Administrative support: Laura A. Levit, Adedayo Onitilo, Caroline Schenkel
Provision of study materials or patients: Laura A. Levit, Adedayo Onitilo
Collection and assembly of data: Joseph M. Unger, Hillary S. Andrews, Laura A. Levit, Denise Kimball, Caroline Schenkel
Data analysis and interpretation: Joseph M. Unger, Laura A. Levit, Mark Stewart, Keith Flaherty, Denise Kimball, Therica Miller, Adedayo Onitilo, Suanna Bruinooge, Elizabeth Garrett-Mayer
Manuscript writing: All authors
Final approval of manuscript: All authors
Accountable for all aspects of the work: All authors
AUTHORS' DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
The following represents disclosure information provided by authors of this manuscript. All relationships are considered compensated unless otherwise noted. Relationships are self-held unless noted. I = Immediate Family Member, Inst = My Institution. Relationships may not relate to the subject matter of this manuscript. For more information about ASCO's conflict of interest policy, please refer to https://ascopubs.org/authors.
Joseph M. Unger
Consulting or Advisory Role: Loxo/Lilly
Keith Flaherty
Leadership: Strata Oncology, Kinnate Biopharma, Scorpion Therapeutics, Clovis Oncology, Khora Therapeutics
Stock and Other Ownership Interests: Clovis Oncology, Loxo, X4 Pharma, Strata Oncology, PIC Therapeutics, Apricity Health, Oncoceutics, FOGPharma, Tvardi Therapeutics, Kinnate Biopharma, Scorpion Therapeutics, ALX Oncology, xCures, Monopteros Therapeutics, Vibliome Therapeutics, Transcode Therapeutics, Soley Therapeutics, Nextech Invest, Alterome Therapeutics, PreDICTA, Flinr Therapeutics, IntrECate, Tasca Therapeutics
Consulting or Advisory Role: Novartis, Tvardi Therapeutics, Takeda, Karkinos Healthcare
Denise Kimball
Employment: Johnson and Johnson
Leadership: Johnson and Johnson
Stock and Other Ownership Interests: Johnson and Johnson Innovative Medicine
Therica Miller
Employment: Mount Sinai Health System
Consulting or Advisory Role: Triomics, J&J, Flatiron Health, AOC Oncology
Research Funding: J&J
Travel, Accommodations, Expenses: American Society of Clinical Oncology
Other Relationship: University of Southern California, AOC Oncology, Alliance Foundation Trials, Children's Hospital Los Angeles, George Washington University, Alphasights
No other potential conflicts of interest were reported.
Joseph M. Unger
Consulting or Advisory Role: Loxo/Lilly
Keith Flaherty
Leadership: Strata Oncology, Kinnate Biopharma, Scorpion Therapeutics, Clovis Oncology, Khora Therapeutics
Stock and Other Ownership Interests: Clovis Oncology, Loxo, X4 Pharma, Strata Oncology, PIC Therapeutics, Apricity Health, Oncoceutics, FOGPharma, Tvardi Therapeutics, Kinnate Biopharma, Scorpion Therapeutics, ALX Oncology, xCures, Monopteros Therapeutics, Vibliome Therapeutics, Transcode Therapeutics, Soley Therapeutics, Nextech Invest, Alterome Therapeutics, PreDICTA, Flinr Therapeutics, IntrECate, Tasca Therapeutics
Consulting or Advisory Role: Novartis, Tvardi Therapeutics, Takeda, Karkinos Healthcare
Denise Kimball
Employment: Johnson and Johnson
Leadership: Johnson and Johnson
Stock and Other Ownership Interests: Johnson and Johnson Innovative Medicine
Therica Miller
Employment: Mount Sinai Health System
Consulting or Advisory Role: Triomics, J&J, Flatiron Health, AOC Oncology
Research Funding: J&J
Travel, Accommodations, Expenses: American Society of Clinical Oncology
Other Relationship: University of Southern California, AOC Oncology, Alliance Foundation Trials, Children's Hospital Los Angeles, George Washington University, Alphasights
No other potential conflicts of interest were reported.
ACKNOWLEDGMENT
We thank the following sponsors for contributing data to the meta-analysis: Amgen; Bayer; Eisai; EMD Serono/Merck KGaA; Genentech/Roche; Janssen/Johnson & Johnson; Merck & Co, Inc; Rahway, NJ, USA; Mirati Therapeutics (a Bristol Myers Squibb company); SWOG Cancer Research Network; and Takeda.
We also thank the following individuals for their contributions to the project: Margaret E McCann, DVM, PhD, Global Regulatory Affairs and Clinical Safety, Merck & Co, Inc, Rahway, NJ; Xiao Peng, PhD, Biostatistics and Research Decisions Sciences, Merck & Co, Inc, Rahway, NJ; Mathias Lebreton, Programming Lead, Johnson and Johnson Innovative Medicine; and Patricia Mader, MS, Kimberly Barnholt, PhD, Wei Yu, PhD, Yong Wang, MS, Bann-mo Day, PhD, Evidence Generation, US Medical Affairs, Genentech.
REFERENCES
1.
Unger JM, Blanke CD, LeBlanc M, et al.: Association of the Coronavirus disease 2019 (COVID-19) outbreak with enrollment in cancer clinical trials. JAMA Netw Open 3:e2010651, 20202.
Unger JM, Xiao H: The COVID-19 pandemic and new clinical trial activations. Trials 22:260, 20213.
Bakouny Z, Labaki C, Bhalla S, et al.: Oncology clinical trial disruption during the COVID-19 pandemic: A COVID-19 and cancer outcomes study. Ann Oncol 33:836-844, 20224.
National Cancer Institute: Coronavirus guidance, 2022. https://ctep.cancer.gov/investigatorResources/corona_virus_guidance.htm5.
US Food and Drug Administration: FDA guidance on conduct of clinical trials of medical products during COVID-19 pandemic, 2020. https://www.fda.gov/regulatory-information/search-fdaguidance-documents/fda-guidance-conduct-clinical-trials-medical-products-during-covid-19-pandemic6.
Unger JM, Stires H, Levit LA, et al.: Sponsor perspectives on the impact of the COVID-19 pandemic on interventional cancer clinical trial protocols and data quality. JCO Oncol Pract 19:907-916, 20237.
Unger JM, Xiao H, LeBlanc M, et al.: Cancer clinical trial participation at the 1-year anniversary of the outbreak of the COVID-19 pandemic. JAMA Netw Open 4:e2118433, 20218.
Khozin S, Coravos A: Decentralized trials in the age of real-world evidence and inclusivity in clinical investigations. Clin Pharmacol Ther 106:25-27, 20199.
Sommer C, Zuccolin D, Arnera V, et al.: Building clinical trials around patients: Evaluation and comparison of decentralized and conventional site models in patients with low back pain. Contemp Clin Trials Commun 11:120-126, 201810.
Friends of Cancer Research: Imact of the COVID-19 Pandemic Mitigation Strategies on Cancer Clinical Trials. Preliminary Findings of a Friends of Cancer Research-American Society of Clinical Oncology Study. Friends of Cancer Research Annual Meeting, 2022. https://friendsofcancerresearch.org/wp-content/uploads/Impact_COVID-19_Pandemic_Strategies_Cancer_Clinical_Trials.pdf11.
Fleury ME, Farner AM, Unger JM: Association of the COVID-19 outbreak with patient willingness to enroll in cancer clinical trials. JAMA Oncol 7:131-132, 202112.
Prindiville SA, Sarosy GA, Loose D, et al.: Patterns of enrollment in cancer treatment trials during the COVID-19 pandemic at National Cancer Institute-designated cancer centers. Cancer J 28:111-117, 202213.
Upadhaya S, Yu JX, Oliva C, et al.: Impact of COVID-19 on oncology clinical trials. Nat Rev Drug Discov 19:376-377, 202014.
US Food and Drug Administration: Protocol Deviations for Clinical Investigations of Drugs, Biological Products, and Devices: Guidance for Industry, 2024. https://www.fda.gov/media/184745/download15.
National Cancer Institute. Division of Cancer Treatment & Diagnosis: Common Terminology Criteria for Adverse Events (CTCAE). https://ctep.cancer.gov/protocoldevelopment/electronic_applications/ctc.htm16.
Centers for Disease Control and Prevention: CDC, Washington state report first COVID-19 death, 2020. https://www.cdc.gov/media/releases/2020/s0229-COVID-19-first-death.html17.
Cucinotta D, Vanelli M: WHO declares COVID-19 a pandemic. Acta Biomed 91:157-160, 202018.
Ahmad FB, Cisewski JA, Miniño A, et al.: Provisional mortality data—United States, 2020. MMWR Morb Mortal Wkly Rep 70:519-522, 202119.
Xiao H, Vaidya R, Liu F, et al.: Sex, racial, and ethnic representation in COVID-19 clinical trials: A systematic Review and meta-analysis. JAMA Intern Med 183:50-60, 202320.
Smithson M, Verkuilen J: A better lemon squeezer? Maximum-Likelihood regression with beta-distributed dependent variables. Psychol Methods 11:54-71, 200621.
Cascio WF, Zedeck S: Open a new window in rational research planning: Adjust alpha to maximize statistical power. Personnel Psychol 36:517-526, 198322.
Mathieu JE, Aguinis H, Culpepper SA, et al.: Understanding and estimating the power to detect cross-level interaction effects in multilevel modeling. J Appl Psychol 97:951-966, 201223.
Dizon DS, Szczepanek CM, Petrylak DP, et al.: National impact of the COVID-19 pandemic on clinical trial staff attrition: Results of the SWOG Cancer Research Network Survey of Oncology Research Professionals. J Clin Oncol 40, 2022 (suppl 16; abstr 11049)24.
Huggins A, Husaini M, Wang F, et al.: Care disruption during COVID-19: A national survey of hospital leaders. J Gen Intern Med 38:1232-1238, 202325.
Sun G, Dizon DS, Szczepanek CM, et al.: Crisis of the clinical trials staff attrition after the COVID-19 pandemic. JCO Oncol Pract 19:533-535, 202326.
Centers for Disease Control and Prevention: Interim Public Health Recommendations for Fully Vaccinated People, 2021. https://stacks.cdc.gov/view/cdc/10617727.
Peterson-KFF Health System Tracker: How has healthcare utilization changed since the pandemic? 2023. https://www.healthsystemtracker.org/chart-collection/how-has-healthcare-utilization-changed-since-the-pandemic/28.
Daly B, Brawley OW, Gospodarowicz MK, et al.: Remote monitoring and data collection for decentralized clinical trials. JAMA Netw Open 7:e246228, 202429.
US Department of Health and Human Services, Food and Drug Administration: Conducting Clinical Trials With Decentralized Elements: Guidance for Industry, Investigators, and Other Interested Parties. https://www.fda.gov/media/167696/download30.
Harvey RD, Miller TM, Hurley PA, et al.: A call to action to advance patient-focused and decentralized clinical trials. Cancer 130:1193-1203, 202431.
Thota R, Hurley PA, Miller TM, et al.: Improving access to patient-focused, decentralized clinical trials requires streamlined regulatory requirements: An ASCO research statement. J Clin Oncol 42:3986-3995, 202432.
American Society of Clinical Oncology: ASCO Welcomes FDA Efforts to Expand Access to Clinical Trials, Provides Agency with Additional Recommendations. ASCO, 2023. https://society.asco.org/news-initiatives/policy-news-analysis/asco-welcomes-fda-efforts-expand-access-clinical-trials33.
National Cancer Institute Clinical Trials and Translational Research Advisory Committee (CTAC) Strategic Planning Working Group: Working Group Report, 2020. https://deainfo.nci.nih.gov/advisory/ctac/1120/SPWGreport.pdf34.
Doroshow JH: Adapting NCI’s Clinical Trials System to a Changed Clinical Research Environment. National Cancer Advisory Board, 2023. https://deainfo.nci.nih.gov/advisory/ncab/0223/Doroshow.pdf35.
US Food and Drug Administration: Diversity Action Plans to Improve Enrollment of Participants from Underrepresented Populations in Clinical Studies. Draft Guidance for Industry, 2024. https://www.fda.gov/media/179593/download36.
Aburto JM, Tilstra AM, Floridi G, et al.: Significant impacts of the COVID-19 pandemic on race/ethnic differences in US mortality. Proc Natl Acad Sci USA 119:e2205813119, 202237.
Eberly LA, Kallan MJ, Julien HM, et al.: Patient Characteristics associated with telemedicine access for primary and specialty ambulatory care during the COVID-19 pandemic. JAMA Netw Open 3:e2031640, 2020
