CONTEXT
Key Objective
Can a structured medication reconciliation televisit (MRT) improve medication list accuracy and clinical trial readiness in early-phase oncology studies?
Knowledge Generated
A remote MRT model was implemented across 82 phase I trials and 525 patients. Among a prospectively evaluated subset, MRTs led to identification of unreported medications, allergy clarifications, and discontinuations of agents that could interfere with trial eligibility, including cannabis and herbal supplements.
Relevance (P.L. Kunz)
Integrating expert-led Medication Reconciliation Televisits (MRTs) into early trial screening improves protocol adherence and enhances patient safety. This approach is scalable across cancer centers using existing clinical trial infrastructure and team members.*
Plain Language Summary (M. Lewis)
For patients on cancer clinical trials, its critical to identify the most accurate list of all medications, called medication reconciliation. In this study, expert-led MRTs clarified allergies, increased accuracy of baseline records, and lowered the risk of unknown prohibited medications. Using this model has the potential to improve efficiency and patient safety.†
*Relevance section written by JCO Oncology Advances Editor-in-Chief Pamela L. Kunz, MD.
†Plain Language Summary written by JCO Oncology Advances Associate Editor Mark Lewis, MD.
INTRODUCTION
Accurate medication reconciliation is essential in early-phase oncology clinical trials, where drug-drug interactions (DDIs), eligibility criteria, and patient safety are closely scrutinized. Patients with cancer frequently manage complex medication regimens, including prescription (RX), over-the-counter (OTC), herbal, and cannabis products, often leading to polypharmacy. This can increase the risk of additive toxicities, potentially affect the efficacy of investigational agents, and lead to protocol deviations when these agents are introduced.
Despite institutional efforts to maintain up-to-date medication records, electronic medical records (EMRs) are frequently incomplete or outdated, particularly in research settings. These discrepancies can lead to delays in trial enrollment, which may result in ineligibility, missed safety signals, and increased workload for research staff. In addition, patients can find it distressing to be repeatedly asked about medications they report they are not taking, but staff members have been reluctant to discontinue.
To address these challenges, our institution developed and implemented a pharmacist-led medication reconciliation televisit (MRT) model. This intervention was designed to optimize baseline medication list accuracy before trial registration, streamline patient screening, and reduce the in-clinic burden for both patients and research teams. In this study, we describe the structure of this telemedicine model and evaluate its implementation and outcomes across multiple phase I oncology trials.
METHODS
Study Design and Setting
This quality improvement initiative was conducted at the Mass General Cancer Center, a founding member of the Havard-wide and National Cancer Institute–recognized Dana-Farber/Harvard Cancer Center Comprehensive Cancer Center. The pharmacist-led MRT program was implemented to support screening for phase I oncology clinical trials by improving baseline medication list accuracy and reducing the in-clinic burden on patients and research teams.
Patient Population
Patients were eligible for the MRT process if they
were screening for a phase I clinical trial,
were of age 18 years and older,
were English speaking or supported by interpreter services, and
provided informed consent to participate in the study.
Telemedicine Model for MRT
The MRT workflow is illustrated in Figure 1 (created using BioRender.com) and is subsequently outlined step by step for reproducibility.
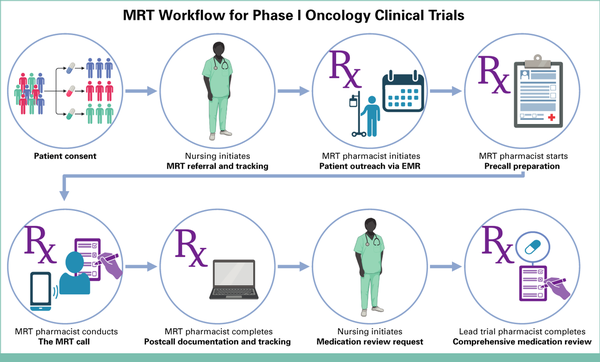
FIG 1.
Visual illustration of the pharmacist-led MRT workflow. EMR, electronic medical record; MRT, medication reconciliation televisit. Created in BioRender. Oneill S. (2025) https://BioRender.com/tmj2efv.
Patient Consent
First, the patient provided informed consent to participate in a phase I oncology clinical trial.
MRT Referral and Tracking
After obtaining trial consent but before the screening visit and registration, nursing staff entered referral information for MRTs into a secure, centralized internal tracking system to trigger pharmacist-led MRT initiation. Data fields included patient name, medical record number, name of referring staff, internal study number, date of MRT request, and the patient's screening appointment date. The system supports real-time, multiuser access, allowing both nurses and pharmacists to view and update records collaboratively.
Patient Outreach through Electronic Medical Records
Oncology clinical trial pharmacists responsible for MRTs review the tracker daily and initiate outreach to referred patients using a standardized message sent through the electronic medical record (EMR) patient portal (Appendix Fig A1). The message explains the purpose of the MRT; proposes a date and time for the call; and encourages patients to have all current medications readily available, including prescription, over-the-counter, herbal, and cannabis products. Caregivers involved in medication management were also encouraged to participate in the MRT. Patients could respond with their preferred times or alternate contact numbers. The pharmacist updates the internal tracker with the date of patient outreach, staff performing the outreach, as well as the planned date and time of call for nursing visibility.
Precall Preparation
MRT pharmacists review the patient's current EMR medication list (Appendix Fig A2), allergy history, and available dispense records before the call to prepare for a structured interview. Refill histories from outside pharmacies may reveal adherence challenges, such as difficulty accessing the pharmacy or affording copayments. At this stage, pharmacists conducting the MRT did not assess for protocol-specific eligibility, drug-drug interactions, or prohibited medications.
The MRT Call
The calls were conducted using a structured interview format. As part of the MRT, patients are asked to confirm all documented medication allergies and to report whether they have ever experienced a bad reaction to any medication, with particular attention given to prior reactions to cancer therapies. The medication list was then reviewed according to the patient's preference—either led by the patient or facilitated by a pharmacist. The details covered include the name, dose, frequency, indication, and approximate start date for each medication, including those used as needed or for symptom management. Pharmacists ensure conversations address all prescription, OTC, herbal, and cannabis-related products and review all possible administration routes. At the end of the call, pharmacists summarized the changes to the medication list, recapping any medication comments that will be documented. For example, the exact stop dates of the most recent cancer treatment and if any medications were started recently. Pharmacists conclude the call by offering the patient the opportunity to share any trial-related questions or concerns with the study team. The average call lasted 15-20 minutes. Caregivers were included when appropriate to support accurate medication reporting and often provided valuable insight into the challenges patients experienced.
Postcall Documentation and Tracking
Pharmacists document key outcomes in both the internal tracker and the EMR. The internal tracker was updated to confirm that the MRT is completed, the total time spent, and the staff completing the MRT. In the EMR, a standardized progress note was entered under the encounter type Medication Reconciliation Telephone Call (Appendix Fig A3). This note includes confirmation of the interview, sources used, allergy updates, individual medication details, and a closing statement that the best possible medication history had been completed. Duplicate or outdated medications were discontinued as appropriate.
In parallel, pharmacists enter deidentified MRT data into a secure, pharmacist-only database used for internal quality improvement. Fields included patient characteristics including age, sex, whether an interpreter was needed, primary cancer type, comorbidities, and internal study number. Fields also included MRT statistics including total number of medications on record and medication categories after MRT, turnaround time, and total time spent on MRT. Starting in April 2024, pharmacists started documenting additional data to better measure the clinical value of the MRT: whether EMR prompted medication reconciliation from outside sources; whether allergies were modified; total number of medications added, changed, or discontinued, duration of call; and total time spent including documentation. These data were used for retrospective analysis of MRT implementation and outcomes.
Medication Review Request
After the centralized internal tracking system was updated by the pharmacist to indicate the MRT and documentation was completed, the research nurse separately contacted the oncology clinical trial pharmacist designated as the lead for that study through email to initiate a medication review request.
Comprehensive Medication Review
The designated lead oncology clinical trial pharmacist for that trial then conducts a comprehensive, protocol-specific medication review on the basis of the MRT note to identify any drug-drug interactions with the study medications, protocol-prohibited medications, or safety concerns relevant to trial eligibility. Potential additive toxicities are identified, and when appropriate, alternative agents or suggestions for close monitoring were recommended. Recommendations were communicated to the study team for appropriate follow-up.
Pharmacist Training for MRT
Pharmacists conducting MRT calls are experienced oncology clinical trial pharmacists, who were previously fully trained in all aspects of clinical trial operations, including investigational drug handling and protocol review. Given the complexity of oncology trials and the advanced disease state of many patients being considered for phase I studies, sensitivity to the clinical and emotional context is an essential component of the MRT model.
Training for MRT implementation is guided by a detailed standard operating procedure, which outlines expectations for patient communication, verification of patient identity, documentation practices, key discussion points, and collaboration with the clinical research team.
As part of this process, pharmacists shadow multiple MRT calls before conducting independent visits. Only those who have completed all training components and demonstrate competency in supervised visits are authorized to perform MRT calls on their own.
Data Collection and Analysis
Descriptive statistics were used to summarize patient demographics, medication list characteristics, and outcomes of the MRT intervention. Categorical variables are reported as counts and percentages, and continuous variables are summarized using medians with ranges. Where applicable, proportions were calculated to describe the frequency of medication additions, discontinuations, allergy modifications, and documentation of OTC or nonprescription substances (eg, cannabis, herbal supplements) identified during the MRT.
All data were documented in real time, immediately after completion of each MRT. Beginning in April 2024, the clinical team implemented an expanded documentation framework to more systematically evaluate the clinical impact of the MRT. This included prospective tracking of specific interventions such as the number of medications added, changed, or discontinued, as well as instances of allergy clarification or modification. These enhancements were introduced to better capture the clinical relevance of the pharmacist-led MRT and to support continuous quality improvement.
A subset of 235 MRTs conducted between April 2024 and January 2025 was analyzed for these expanded outcomes, as this period reflects consistent use of the revised documentation process. No formal hypothesis testing or inferential statistical analyses were performed, as the objective of this quality improvement initiative was to describe and characterize the implementation and clinical utility of the MRT model in a real-world, prospective setting.
All analyses were performed using Microsoft Excel and institutional data review platforms. Clinical trial enrollment status and time from MRT to enrollment were determined using an internal research operations tracker that included MRN, study number, and MRT call date. Enrollment confirmation was verified through the institution's clinical trial registration portal (Cancer Center Protocol Office system). No access to patient medical records or identifiable health data beyond existing research infrastructure tools was required for this analysis.
Ethical Considerations and Regulatory Oversight
This initiative did not meet the criteria for human subject research as defined by Mass General Brigham Institutional Review Board (MGB IRB) policies and Health and Human Services regulations set forth in 45 CFR 46. Per the MGB IRB, this activity did not constitute human subjects research because it was not intended to be generalized knowledge, and the intent of this project was to improve the quality of clinical care by having clinical trial pharmacists conduct the medication reconciliation for patients in screening for oncology clinical trials. This quality improvement process assessed the feasibility and value of clinical trial pharmacists completing medication reconciliation and was exempt from formal consent requirements. No identifiable data were used in the analysis. All data used to determine trial enrollment status were obtained from institutional research support systems that track study registration and MRT scheduling.
RESULTS
Between December 2022 and January 2025, a total of 525 pharmacist-led MRTs were completed across 82 phase I oncology clinical trials. The implementation time line is shown in Appendix Figure A4, illustrating the distribution of completed MRTs over time and the progressive integration of the model into clinical workflows.
The median patient age was 61 years (range, 21-88 years), and 41% of patients were male. Table 1 presents the MRT statistics captured for all patients after the implementation of the MRT telemedicine model.
TABLE 1.
Patient Demographics, Medication List Characteristics, and Clinical Trial Enrollment Outcomes Associated With MRTs, Based on All MRTs Conducted Between December 2022 and January 2025 (n = 525)
| MRT Characteristics and Outcomes | Value |
|---|---|
| Patient characteristics | |
| Age, years, median (range) | 61 (21-88) |
| Male, No. (%) | 214 (41) |
| Interpreter needed, No. (%) | 25 (5) |
| Medication list characteristics | |
| Total No. of medications on record, median (range) | 12 (2-41) |
| Medication category characteristics after MRT, No. (%) | |
| Prescription (RX) | 519 (99) |
| OTC nonherbal | 503 (96) |
| OTC herbal | 100 (19) |
| Antacid/H2 blocker/PPI | 239 (46) |
| Cannabis | 117 (22) |
| MRT outcomes | |
| Turnaround time, days, median (range) | 2 (0-13) |
| Total time spent, minutes, median (range) | 45 (5-330) |
| Sum of total time spent on MRTs, hours | 418 |
| Clinical trial enrollment outcomes | |
| Total unique oncology clinical trials, No. | 82 |
| Patients enrolled in trial after MRT in total, No. (%) | 410 (78) |
| Time from MRT to enrollment, days, median (range) | 14 (0-63) |
Patients reported a median of 12 medications, and the median total time spent per MRT—including preparation, the call itself, and documentation—was 45 minutes (range 5-330 minutes). Twelve distinct primary cancer types were represented among these 525 MRTs, visualized in Figure 2 (created using BioRender.com). The most common disease categories included gastrointestinal (32%), breast (20%), and head and neck (11%) cancers. As expected, many of these patients with cancer had comorbidities that often required concomitant use of chronic medications, as illustrated in Figure 3.
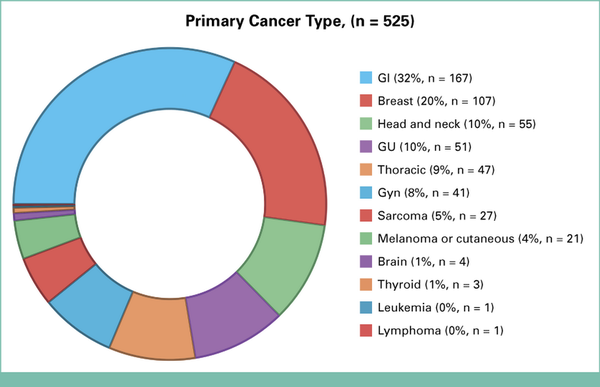
FIG 2.
Donut chart depicting primary cancer types of MRT patients. GU, genitourinary; Gyn, gynecologic; MRT, medication reconciliation televisit.
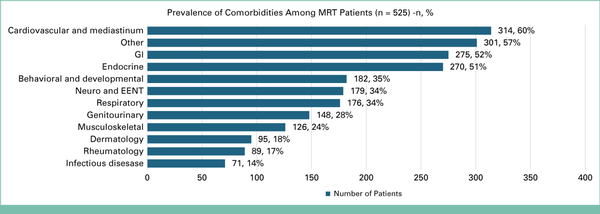
FIG 3.
Horizontal bar chart illustrating the prevalence of comorbidities among MRT patients. EENT, eyes, ears, nose, throat; MRT, medication reconciliation televisit.
Enhanced documentation in the later subset reveals data presented in Table 2. Among this subset of MRTs performed in April 2024 or later (n = 235), the median duration of the phone call alone was 18 minutes.
TABLE 2.
Clinical Interventions and Additional MRT Outcomes, Limited to a Subset of MRTs Conducted Between April 2024 and January 2025 (n = 235)
| Clinical Interventions and Additional MRT Outcomes | Value |
|---|---|
| Medications from outside sources need reconciliation, No. (%) | 151 (64) |
| Allergies modified, No. (%) | 75 (32) |
| Total No. of medications added, median (range) | 3 (0-37) |
| Total No. of medications changed, median (range) | 2 (0-13) |
| Total No. of medications discontinued, median (range) | 3 (0-18) |
| Duration of call, minutes, median (range) | 18 (5-65) |
Across these 235 MRT visits, a median of three medications were discontinued (range, 0-18). Importantly, this reflects a thoughtful and clinically guided process rather than a broad reduction effort. Medications were only discontinued when deemed potentially inappropriate or unnecessary in the context of the patient's current treatment plan or investigational regimen.
Symptom-directed therapies, such as pain management and antiemetics, are generally not discontinued, even when not currently in use, given their relevance in managing disease-related and treatment-related side effects. Instead, medication reconciliation emphasized clarifying intent, dose, and appropriateness, ensuring accurate documentation while preserving patient access to essential supportive care.
This distinction is critical, as merely documenting medications as not taking without deliberate evaluation may overlook risks or opportunities for optimization. Pharmacist judgment was central, and inappropriate discontinuation can have broad implications. This highlights the importance of discipline-specific training and context-aware review in complex oncology settings.
Table 3 summarizes the impact of MRTs on identifying previously undocumented medications with potential for DDIs in MRTs conducted in April 2024 and later (n = 235). Notably, documentation of cannabis products increased from 5% before MRT to 28% after MRT (+500%), highlighting the role of pharmacist outreach in uncovering nonprescription medication use.
TABLE 3.
Comparison of Medication Categories Documented Before and After MRT, Limited to a Subset of MRTs Conducted Between April 2024 and January 2025 (n = 235)
| Medication Category | Before MRT, No. (%) | After MRT, No. (%) | Absolute Change | % Increase |
|---|---|---|---|---|
| Prescription (RX) | 208 (98) | 232 (99) | +24 | +12 |
| OTC nonherbal | 179 (84) | 224 (95) | +45 | +25 |
| OTC herbal | 26 (12) | 46 (20) | +20 | +77 |
| Antacid/H2 blocker/PPI | 81 (38) | 107 (46) | +26 | +32 |
| Cannabis | 11 (5) | 66 (28) | +55 | +500 |
DISCUSSION
This quality improvement project demonstrates the feasibility, scalability, and clinical relevance of a telemedicine-based MRT model for patients undergoing screening in phase I oncology clinical trials. Implemented collaboratively by the clinical trials team as a quality improvement initiative across 82 studies, the MRT program improved the accuracy of baseline medication documentation, supported protocol compliance, and reduced clinic burden for both patients and research staff. While pharmacists played a key role in the development and implementation of this intervention at our site, the approach is designed to be adaptable across institutions and disciplines, supporting the broader application of expert televisit review as a standard component of trial readiness workflows.
The MRT model provided a structured opportunity for patients to report comprehensive medication use—including prescription, over-the-counter, herbal, and cannabis products—which are frequently under-reported in routine care. The notable increase in documentation of cannabis use (from 5% to 28%) and herbal supplements (from 12% to 20%) highlights the value of pharmacist outreach in identifying medications with potential for drug-drug interactions or trial ineligibility.
The model's remote format supported greater flexibility in scheduling and patient preparedness, with many patients referencing medications in real time from home. MRTs also allowed caregivers to participate, enhancing the accuracy of reported information. Importantly, the ability for pharmacists to conduct these calls remotely allowed them to structure their workflow efficiently, with ample time for chart review, call preparation, and documentation without the interruptions common in busy clinical or infusion settings. The median call duration was 18 minutes, and the total pharmacist time investment—including documentation—was 45 minutes per patient, supporting the model's efficiency and potential for wider implementation.
Importantly, the MRT was designed as a standardized history-taking process and did not include real-time protocol-specific review. That review occurred separately, initiated by research nurses and completed by the disease-specific lead pharmacist for each trial. This intentional separation ensured that medication reconciliation could be scaled across trials, while preserving the nuanced clinical assessment required for eligibility determination.
This model also introduced an early, nonclinical touchpoint between patients and pharmacists, which may enhance patient comfort and trust in the clinical trial process. Patients appreciated the opportunity to raise concerns in advance, allowing these to be addressed more efficiently at the next in-person visit setting that is often busy and, at times, overwhelming because of the number of providers involved.
In addition to supporting patient safety and trial efficiency, the MRT process received consistently positive feedback from advanced practice providers and nurses, who previously performed these medication reviews during time-constrained screening visits. Moreover, oncology clinical trial pharmacists completing the subsequent protocol-specific medication reviews reported increased confidence in the EMR medication lists. They expressed appreciation for the accuracy and completeness of the MRT documentation—including the inclusion of full product names, dosing, and ingredient-level details—which streamlined their reviews and reduced time spent evaluating medications the patient was no longer taking.
While this study reflects implementation at a single academic center, its consistency across multiple disease groups, trials, and documentation platforms supports its reproducibility. Future work may explore downstream outcomes that connect the MRT to the subsequent protocol-specific medication review process. In particular, medications identified during the MRT that were ultimately found to be prohibited or required modification before study enrollment. Drug-drug interaction questions often emerge after investigational treatment initiation, and the addition of new medications can complicate the clinical picture when unexpected side effects arise. To support longitudinal care, promote adherence, and enhance patient satisfaction, we propose introducing a follow-up call after investigational oral oncolytic treatment initiation.
The MRT model is a scalable, patient-centered intervention that improves the accuracy of medication histories during early-phase oncology clinical trial screening. By standardizing pharmacist outreach, supporting real-time patient engagement, and separating general medication history from protocol-specific eligibility review, this model enhances trial efficiency, patient safety, and multidisciplinary collaboration.
The flexibility of remote MRT scheduling benefits both patients and pharmacists by allowing for more thoughtful preparation and streamlined documentation. Positive feedback from advanced practice providers, nurses, and oncology clinical trial pharmacists underscores the model's operational value. While pharmacists played a central role in developing and piloting the model at our institution, MRTs are designed to be adapted by qualified team members across sites to support broader trial readiness. Future work should focus on linking MRT-identified medications to protocol eligibility outcomes and further evaluate the impact of this intervention on enrollment time lines.
This study was conducted at a single academic cancer center, which may limit generalizability to other institutions with different workflows, staffing structures, or EMR capabilities. While the MRT model was consistently applied across 82 trials, the impact of MRTs on clinical trial outcomes such as medication list changes relevant to eligibility, protocol deviations, or adverse events was not assessed. In addition, the separation of MRT documentation and protocol-specific medication review, while intentional, prevented real-time linkage of prohibited medications to enrollment decisions. These areas represent opportunities for future prospective evaluation.
AUTHOR CONTRIBUTIONS
Conception and design: Sarah O'Neill, Shannon Lerro, Elke Backman
Administrative support: Shannon Lerro, Elke Backman
Provision of study materials or patients: Elke Backman
Collection and assembly of data: All authors
Data analysis and interpretation: Sarah O'Neill, Shannon Lerro, Nitasha Sanil, Elke Backman
Manuscript writing: All authors
Final approval of manuscript: All authors
Accountable for all aspects of the work: All authors
AUTHORS' DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
The following represents disclosure information provided by authors of this manuscript. All relationships are considered compensated unless otherwise noted. Relationships are self-held unless noted. I = Immediate Family Member, Inst = My Institution. Relationships may not relate to the subject matter of this manuscript. For more information about ASCO's conflict of interest policy, please refer to https://ascopubs.org/authors.
Nitasha Sanil
Honoraria: American Society of Health-System Pharmacists, Massachusettes Society of Healthsystem Pharmacists (MSHP)
Uncompensated Relationships: Kelonia Therapeutics
No other potential conflicts of interest were reported.
Nitasha Sanil
Honoraria: American Society of Health-System Pharmacists, Massachusettes Society of Healthsystem Pharmacists (MSHP)
Uncompensated Relationships: Kelonia Therapeutics
No other potential conflicts of interest were reported.
APPENDIX
FIG A1.
Example template of a standardized research message sent by a pharmacist through the EMR patient portal directly to patients to schedule an MRT. EMR, electronic medical record; MRT, medication reconciliation televisit.
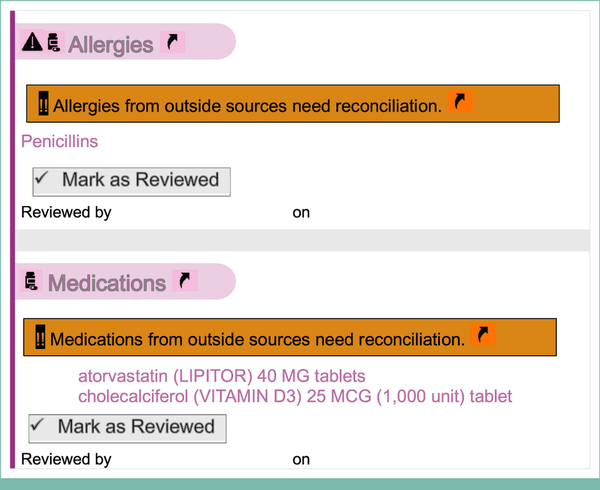
FIG A2.
Deidentified example of a patient allergy and medication list before an MRT. See Appendix Figure A3 for post-MRT documentation for the same patient. MRT, medication reconciliation televisit.
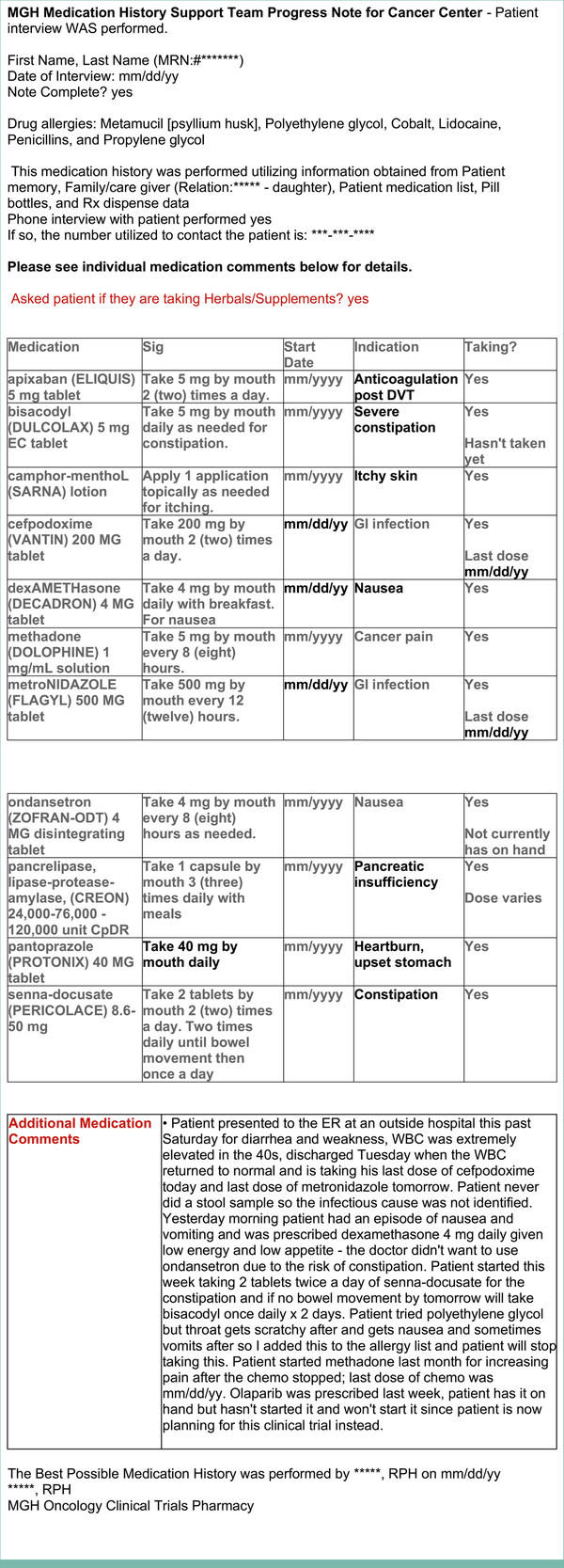
FIG A3.
Deidentified example of a standardized progress note documented by the pharmacist under the encounter type Medication Reconciliation Telephone Call after the MRT for the same patient whose allergies and medications before the MRT are illustrated in Appendix Figure A2. MRT, medication reconciliation televisit.
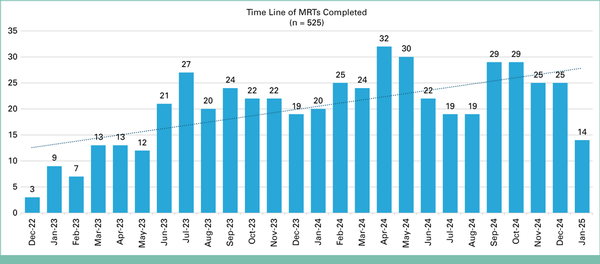
FIG A4.
Vertical bar chart illustrating the time line and volume of completed MRTs monthly from December 2022 to January 2025. MRT, medication reconciliation televisit.
ACKNOWLEDGMENT
We would like to thank the phase I research nurses and advanced practice providers at the Mass General Cancer Center's Henri and Belinda Termeer Center for Targeted Therapies for their collaboration on this quality improvement project. Their partnership was instrumental in developing an efficient internal tracking system and ensuring high-quality patient care throughout the implementation of the medication reconciliation televisit (MRT) process. We are deeply grateful to clinical trial pharmacists Lalit Joshi and Cecelia Madden for their dedicated efforts in completing the comprehensive medication reviews for phase I clinical trial patients following the MRT visits. We are also thankful to the MGH Department of Pharmacy for the ongoing support.
REFERENCES
1.
Malifarge A, Rousselot P, Chatelut E, et al.: Impact of medication reconciliation in oncology early phase studies: A drug–drug interaction retrospective study. JCO Oncol Pract 19:e536-e545, 20232.
Elbeddini A, To A, Tayefehchamani Y, et al.: Importance of medication reconciliation in cancer patients. J Pharm Policy Pract 14:98, 20213.
Wolff JL, Darer JD, Larsen KL: Family caregivers and medication management: Experiences and implications. J Am Board Fam Med 27:347-355, 20144.
Fu JB, Mohile SG, Culakova E, et al.: Association of patient comorbidities with cancer treatment and outcomes. Oncologist 18:1315-1320, 20135.
Mathes T, Antoine SL, Pieper D, et al.: Adherence influencing factors in patients taking oral anticancer agents: A systematic review. Cancer Epidemiol 38:214-226, 20146.
Powis M, Dara C, Macedo A, et al.: Implementation of medication reconciliation in outpatient cancer care. BMJ Open Qual 12:e002211, 20237.
Vega TG-C, Sierra-Sánchez JF, Martínez-Bautista MJ, et al.: Medication reconciliation in oncological patients: A randomized clinical trial. J Manag Care Spec Pharm 22:734-740, 20168.
Bell CM, Brener SS, Gunraj N, et al.: Association of primary care with readmission in cancer patients. BMC Fam Pract 18:30, 20179.
Signorelli J, Tran T, Sirek ME, et al.: Development of oral oncolytic nonadherence estimator (ORACLE): A pretreatment nonadherence risk assessment for oral oncolytics. J Oncol Pharm Pract 30:1307-1316, 2024
