Introduction
Leaf senescence is a highly regulated natural process during the life of a leaf, which involves degradation of various macromolecules, such as chlorophyll (Chl) and proteins (; ). Premature leaf senescence leads to demolished photosynthesis, retarded plant growth and productivity (). Chl degradation is the common hallmark of leaf senescence. Chl degradation involves multiple biochemical reactions catalyzed by at least six Chl catabolic enzymes (CCEs) in the chloroplast, namely pheophytin pheophorbide hydrolyase (PPH), Non-Yellow Coloring1 (NYC1), NYC1-like (NOL), Chl a reductase (HCAR), pheide a oxidase (PAO), and red Chl catabolite reductase (RCCR) (; ; ). PPH, also named NYC3 in rice (Oryza sativa) or CRN1 in Arabidopsis, is one of the key CCEs, which catalyzes conversion of pheophytin a to pheophorbide a by cleaving off the phytol group (). The PPH transcript level is positively correlated with the extent of leaf senescence during the natural leaf maturation process () or is induced by stresses, such as dark or shade (; ). However, the regulatory mechanisms of PPH expression associated with natural or stress-induced leaf senescence are not well understood.
Plant hormones, such as abscisic acid (ABA), ethylene, and cytokinin (CK), are known to play roles in regulating both natural leaf senescence and that induced by stress (; ; ; ; ; ). Recently, a number of independent studies suggested a possible link between Chl catabolism and ABA, ethylene, and CK signaling pathways (; ; ; ; ; ; ). ABA at high concentrations may accelerate leaf senescence and Chl degradation (). and found that exogenous spraying of ethylene also induced the Chl degradation associated with higher transcript levels of Chl catabolism genes in both pear and broccoli. In the model plant Arabidopsis, ethylene-insensitive mutants (etr1, ein2/ore2, and ein3) had a slower rate of Chl degradation during leaf senescence (; ; ; ). Furthermore, EIN3, together with ORE1/NAC2, transcription factors in the ethylene signaling pathway, promotes Chl degradation by enhancing the promoter activity of Chl degradation genes (such as NYE1 or SGR, NYC1, and PAO) (). In contrast to ABA and ethylene, CK is known to be negatively correlated with the level of leaf senescence (). The correlation between a decreased endogenous CK level in leaves and the onset and progression of senescence has been documented (). The increase of endogenous CK content through overexpression of the CK synthesis gene, isopentenyl-transferases (IPT), could significantly extend the lifespan of leaves in various plant species (; ; ). Despite the importance of the involvement of ABA, ethylene, and CK in leaf senescence, it is unclear whether ABA-, ethylene-, or CK- mediated leaf senescence is related to the regulation of PPH genes for Chl breakdown during leaf senescence.
Leaf senescence is a major problem for perennial grasses which are harvested for their green foliage as forage or maintained for a uniform green canopy as is the case for turfgrasses. Knowledge of mechanisms controlling leaf senescence is particularly important for developing stay-green grasses and improving forage or turf quality. As discussed earlier, PPH is a key gene involved in Chl degradation, but how this gene is regulated during leaf senescence in plants, particularly in perennial grass species, is still unclear. Manipulating the expression of PPH through genetic modification or mutagenesis could lead to a stay-green phenotype in plants (; ). In this study, we hypothesized that PPH cloned from a perennial grass species could possess the conserved functionality in Chl degradation, and PPH-mediated leaf senescence could be regulated by ABA, ethylene, or CK. The objectives of this study were (i) to determine the functional roles of PPH in leaf senescence in a perennial grass species (Lolium perenne L.) widely used as forage and turfgrass; (ii) to identify the regulatory roles of ABA, ethylene, and CK in PPH expression; and (iii) to predict the potential transcriptional factors activating or suppressing PPH expression conserved across diverse plant species.
Materials and methods
Plant materials and growth conditions
Perennial ryegrass (cv. ‘Pinnacle’) plants were used for cloning of the PPH gene and evaluation of PPH expression in relation to leaf senescence. Plants were grown in plastic pots (20cm in diameter and 25cm in height) filled with the mixture of soil and peat (3:1 v/v) and maintained in growth chambers (Environmental Growth Chamber, Chagrin Falls, OH, USA) set at 25/20 °C (day/night), 70% relative humidity, and 12h photoperiod with photosynthetically active radiation (PAR) of 750 µmol photons m–2 s–1.
The function of the PPH gene from perennial ryegrass in leaf senescence was confirmed using transient transformation and mutant complementation assay in two model plant species, wild tobacco (Nicotiana benthamiana) and Arabidopsis. Wild tobacco plants were cultured under the same conditions as for perennial ryegrass.
Arabidopsis thaliana wild-type (Col-0) and T-DNA insertion lines (at5g13800-1, SALK_000095) were grown in 10cm2 pots with peat soil, and were maintained in growth chambers (Environmental Growth Chamber) set at 22 °C with a 16h photoperiod and a PAR of 350 µmol photons m–2 s–1. The T-DNA line was obtained from the Arabidopsis Biological Resource Center (ABRC) (). All plants were well watered, and fertilized weekly with half-strength Hoagland’s nutrient solution ().
Evaluation of leaf senescence in perennial ryegrass induced by darkness and affected by ABA, ethephon, AVG, and CK
Leaf senescence affected by ethephon, aminoethoxyvinylglycine (AVG), ABA, and CK and induced by darkness was evaluated in perennial ryegrass. For dark-induced leaf senescence, the fully expanded leaves (~12 d after leaf emergence) were excised from perennial ryegrass plants, and were kept in between paper towels moistened with 3mM MES buffer (pH 5.8) at 25 °C for 8 d in the dark. For ethephon, AVG, ABA, and CK treatment, the protocol reported by was used. The fully expanded leaves (~12 d after leaf emergence) were excised from perennial ryegrass plants, and incubated in 3mM MES buffer (pH 5.8) supplemented with water (control), 200 µM ethephon, 25 µM AVG, 50 µM ABA, or 25 µM 6-benzylaminopurine (6-BA) at 25 °C.
Several physiological parameters commonly used as indicators of leaf senescence were evaluated, namely leaf Chl content, photochemical efficiency, and membrane stability (; ; ). Chl was extracted by soaking leaves in dimethylsulfoxide (DMSO) for 48h and the absorbance of extracts was measured at 663nm and 645nm using a spectrophotometer (Spectronic in Instruments, Rochester, NY, USA) (). Leaf photochemical efficiency was expressed as the ratio of the variable fluorescence (Fv) to the maximal fluorescence (Fm) (Fv/Fm) (). Chl fluorescence was determined using a fluorescence meter (Dynamax, Houston, TX, USA) after leaves were dark adapted for 30min. Leaf membrane stability was estimated by electrolyte leakage (EL) from leaves (). For EL measurement, 0.2g of leaves were collected, washed three times, and then immersed in 30ml of deionized water. Initial conductivity (Ci) was measured with a conductivity meter (YSI Model 32, Yellow Spring, OH, USA) after shaking overnight. Leaves were then boiled in an autoclave for 20min for measurement of the conductance (Cmax). The leaf relative EL was calculated as 100×Ci/Cmax.
Isolation and sequence analysis of PPH from perennial ryegrass (LpPPH)
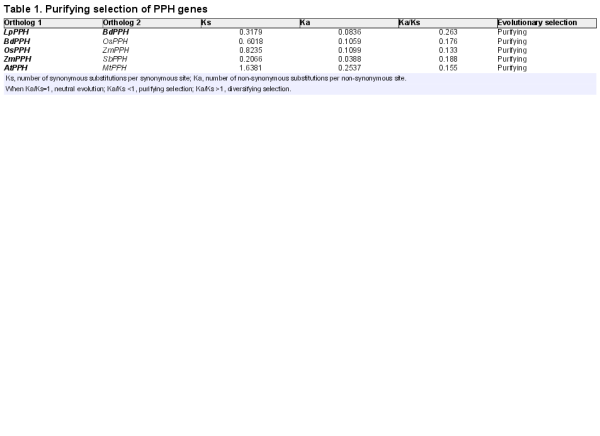
Total RNA was extracted from senescent leaf samples of perennial ryegrass. A pair of primers (LpPPH-F and LpPPH-R; Supplementary Table S1 available at JXB online) was designed for cloning the conserved nucleotide sequence of LpPPH according to the alignment of the PPH genes in rice and brachypodium. Two gene-specific primers (LpPPH-3' RACE and LpPPH-5' RACE; Supplementary Table S1) were designed to amplify the ends of the PPH gene. The cDNA used for random amplification of cDNA ends (RACE)-PCR was synthesized with a SMARTer® RACE 5'/3' Kit (Clontech Laboratories, Mountain View, CA, USA) following the manufacturer’s instructions. PCRs were performed in a 50 µl reaction volume using Q5 High-Fidelity 2× Master Mix (New England Biolabs, Ipswich, MA, USA). The DNA fragments obtained from the PCRs were sequenced and aligned to obtain the full-length open reading frame (ORF) of PPH. The ratio between non-synonymous and synonymous nucleotide substitutions (Ka/Ks) was calculated using DNAsp5 software (http://www.ub.edu/dnasp/) () for selected pairs of homologous genes. The cis-element analysis of selected PPH promoters (–2000bp from the transcription initiation site) was performed using the PLACE website (http://www.dna.affrc.go.jp/PLACE/) ().
Plasmid construction
The ORFs of LpPPH and AtPPH were amplified with gene-specific primers (LpPPH-CDSF, LpPPH-CDSR, AtPPH-CDSF, and AtPPH-CDSR), listed in Supplementary Table S1 at JXB online, using Q5 High-Fidelity 2× Master Mix (New England Biolabs), cloned into pENTR/D, their sequences were confirmed, and then they were moved into the destination vector pEarleyGate103 () for overexpression analysis or into a modified Gateway-compatible p2GWF7.0 vector () for subcellular localization analysis.
Subcellular localization of LpPPH
Arabidopsis mesophyll protoplasts were isolated from mature leaves of 6-week-old plants following the procedure described in . The 3'-green fluorescent protein (GFP)-tagged LpPPH was transformed into protoplasts using 20% polyethylene glycol, then incubated in the dark at 22 °C for 36h, and examined under a Zeiss LSM 780 laser scanning confocal microscope (Carl Zeiss SAS, Jena, Germany). GFP fluorescence images were viewed at an excitation wavelength of 488nm, and the emission signal was recovered between 495nm and 530nm according to published procedures (). Chl autofluorescence was viewed between 643nm and 730nm.
Transient overexpression of LpPPH in wild tobacco
Mature leaves of 4- to 6-week-old wild-type tobacco plants were used for the experiment applying the method described previously (). Briefly, Agrobacterium strain AGL1 harboring pEarleyGate103 with or without the target gene was injected into a tobacco leaf at the concentration of OD600=0.6. After injection, the tobacco leaves were excised and incubated on wet filter paper at 25 °C in the dark for up to 6 d. This experiment was repeated three times, each time with at least three biological replicates.
Arabidopsis mutant complementation assay
The homozygosity of the pph mutant of Arabidopsis was confirmed with three sets of primers, given in Supplementary Table S1 at JXB online. The standard floral dip method was applied for transformation (). Putative transgenic lines were selected on Murashige and Skoog (MS) medium with 20mg l–1 glufosinate, and were further verified by PCR. For the Arabidopsis senescence assay, detached leaves (numbers 4–6) of 28-day-old plants were excised and incubated on wet filter paper at 22 °C in total darkness for up to 6 d.
Analysis of gene expression with real-time RT-PCR
Total RNAs were extracted using an RNA cleaning kit (Qiagen, Valencia, CA, USA) following the manufacturer’s instructions. After DNA digestion with TURB DNA-free™ (Life Technologies, Grand Island, NY, USA), first-strand cDNA was synthesized using a High Capacity cDNA Reverse Transcription kit (Applied Biosystems, Grand Island, NY, USA). For semi-quantitative RT-PCR, the reaction was performed in a 20 μl reaction volume using PCR super mix (Life Technologies) for 26 cycles. Primers used are listed in Supplementary Table S1 at JXB online, with ACTIN2 as the reference gene.
For qRT-PCR, the reaction was performed in a 20 μl reaction volume with power SYBR® Green PCR Master mix (Applied Biosystems) using Roche Light Cycler® 480 II Real-Time PCR Systems. All reactions were performed with two technical and three biological replicates. Primers used for qRT-PCR are listed in Supplementary Table S1 . eIHF4A and TEF1 were used as reference genes ().
Statistical analysis
Data in this study were statistically analyzed using one-way ANOVA, and the means were compared by the LSD and Duncan test at a significance level of 0.05 by using SPSS (version 12, SPSS Inc., Chicago, IL, USA). The data are expressed as means ± SE.
Results
RACE-PCR cloning and phylogenetic analysis of LpPPH
According to the nucleotide sequence alignment between OsPPH and BdPPH, a pair of primers was designed to amplify a conserved sequence fragment of PPH from the cDNA derived from senescent leaves of ryegrass. The full-length coding sequnce (CDS) of LpPPH (GenBank accession no. KT345726), obtained using 5'- and 3'-RACE-PCR, encodes a deduced protein of 488 amino acids, with a pI of 6.55 (ExPASy server, http://web.expasy.org/computepi/) and the conserved domain of α/β hydrolases (InterPro IPR029058; PLN02824) () (Fig. 1).
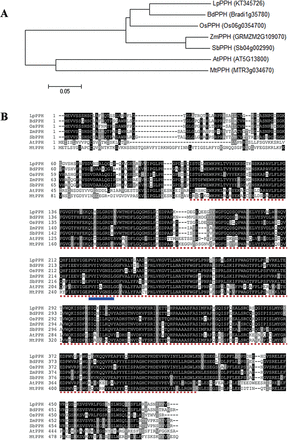
Fig. 1
Phylogenetic analysis and multiple sequence alignment of seven PPHs. (A) Phylogenetic analysis: the Neighbor–Joining phylogenetic tree was constructed using MEGA6 with the sum of branch length=1.16285194. In addition to LpPPH, its orthologous proteins in Arabidopsis (AtPPH), rice (OsPPH), brachypodium (BdPPH), maize (ZmPPH), sorghum (SbPPH), and Medicago truncatula (MtPPH) were used for the analysis. (B) Multiple sequence alignment: note that the region under dotted lines is the conserved domain of α/β hydrolase-fold family proteins, and the sequence under solid lines (VYIV/AGNSL) is the PPH motif (). (This figure is available in colour at JXB online.)
A phylogenetic tree of LpPPH and six PPH orthologs of different plant species was constructed (Fig. 1A), in which the phylogenetic relationship between the PPHs was consistent with that of the plant species, indicating that the cloned LpPPH should be the functional pheophytinase-encoding gene. The cloned LpPPH shared 87.9, 82.0, and 62.1% nucleotide sequence identity with BdPPH, OsPPH, and AtPPH, respectively. Based on the phylogenetic relationship of the PPH orthologs, the synonymous and non-synonymous nucleotide substitution rates were further compared between the closely related pairs, demonstrating that all PPH genes were under purifying selection (Ka/Ks <1) (Table 1).
LpPPH localized in chloroplasts
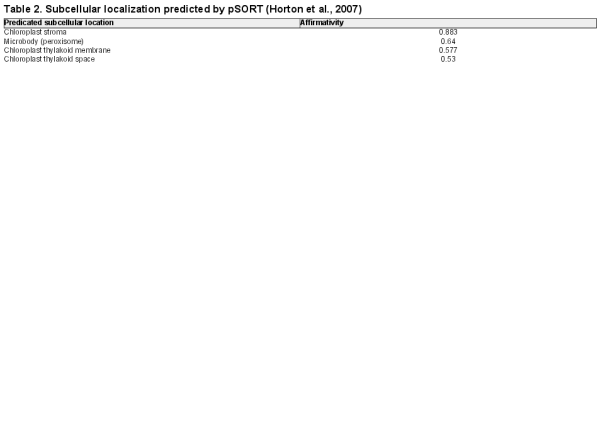
LpPPH protein was predicted to have a 72 amino acid chloroplast transit peptide at the N-terminus without a significant transmembrane domain using the ChloroP1.1 (), pSORT (), and TMHMM servers, respectively (the prediction result of pSORT is presented in Table 2). Transient overexpression of LpPPH fused with a GFP tag at the C-terminus in Arabidopsis mesophyll protoplasts confirmed that LpPPH was localized in chloroplasts (Fig. 2). Because LpPPH was predicted to contain no transmembrane domain, it may be mainly localized in the chloroplast stroma.
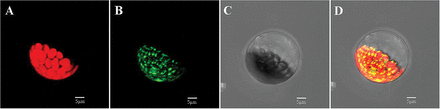
Fig. 2
Subcellular localization of LpPPH. (A) Chlorophyll autofluorescence; (B) green fluorescence from LpPPH–GFP; (C) bright field; (D) merge of green and autoflorescence.
Overexpression of LpPPH induced leaf senescence in wild tobacco and complemented the Arabidopsis pph mutant phenotype
In order to confirm the function of LpPPH in regulating leaf senescence, the transient overexpression of this gene in wild tobacco and a complementation assay of the Arabidopsis pph mutant were performed using both LpPPH and AtPPH driven under control of the Cauliflower mosaic virus (CaMV) 35S promoter. The transient overexpression of 35S:LpPPH and 35S:AtPPH both accelerated leaf senescence and resulted in a significantly lower Chl content and Chl a/Chl b ratio than those of the controls (Fig. 3).
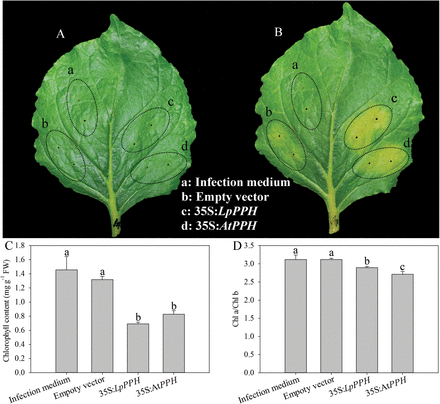
Fig. 3
Transient overexpression of LpPPH in wild tobacco (N. benthamiana). Agrobacterium strain AGL1 harboring either empty vector or LpPPH/AtPPH genes was injected into mature leaves of wild tobacco. (A) 0 day after infection; (B) 6 d after infection; (C) Chl content 6 d after infection; (D) Chl a/Chl b ratio 6 d after infection. Data are means ±SE (n=4 in C and D).
For pph mutant complementation assay, three T2 homologous transgenic lines of each gene ( 35S:LpPPH/pph-1, -3, and -12, and 35S:AtPPH/pph-2, -8, and -15) were compared together with the wild type and pph controls (Fig. 4). After 6 d of dark treatment, the detached leaves of the Arabidopsis pph mutant showed the stay-green phenotype, while Col-0 and all transgenic lines exhibited severe leaf Chl degradation (Fig. 4). Moreover, the expression levels of LpPPH and AtPPH were negatively correlated with Chl content in leaves of each transgenic line (Fig. 4C, D). In addition, LpPPH also rescued the stay-green phenotype of the seedpods of the Arabidopsis pph mutant (Fig. 5). Taken together, these results demonstrated that LpPPH complemented the phenotype of the Arabidopsis pph null mutant and shared the same functionality with AtPPH.
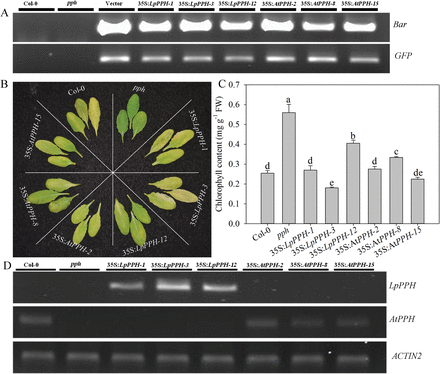
Fig. 4
LpPPH complements the leaf’s stay-green phenotype of the Arabidopsis pph mutant. (A) PCR confirmation of the presence of Bar and GFP genes in transgenic lines. (B) 35S:LpPPH as well as 35S:AtPPH complemented the phenotype of the pph mutant. The photograph was taken 6 d after dark-induced senescence on detached leaves. (C) Chl content of the detached leaves of each line 6 d after dark-induced senescence. (D) RT-PCR analysis of the expression levels of LpPPH or AtPPH in the tested Arabidopsis lines using detached leaves 6 d after dark-induced senescence. Data are means ±SE (n=4 in C and D).
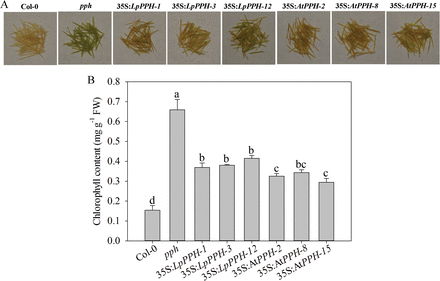
Fig. 5
LpPPH under the control of the CaMV 35S promoter partially restored the seedpod’s stay-green phenotype of the Arabidopsis pph mutant. (A) Phenotype of the wild type, pph mutant, and transgenic lines. (B) Chl content of the tested lines. Data are means ±SE (n=4 in C).
Increasing transcript levels of LpPPH in senescent leaves
Our previous test showed that the leaf of the tested ryegrass variety reached its full length and highest Chl content at 12 d after leaf emergence, and Chl content declined thereafter (data not shown), indicating that those leaves reached full maturation at 12 d after emergence. Therefore, in this study, changes in Chl content and LpPPH transcript level were examined during leaf senescence using the 12-day-old mature leaves (referred to as mature leaf) as the starting material (Fig. 6A). The transcript level of LpPPH did not significantly change during the early phase of leaf senescence (Chl declined by 32% from 3.4mg g–1 FW to 2.3mg g–1 FW), but significantly increased to the highest level when Chl decreased by 82% (from 3.4mg g–1 FW to 0.6mg g–1 FW) (Fig. 6B). The transcript level of LpPPH was also analyzed in different plant organs. The expression level of LpPPH was barely detectable in roots, and was relatively low in stems or leaf sheaths (Fig. 7C). The LpPPH gene had a ~30- and 80-fold higher expression level in leaves at early and late senescence stages (32 d and 36 d after leaf emergence), respectively, than in stems or in leaves at the expanding stage (6 d after leaf emergence) (Fig. 6C).
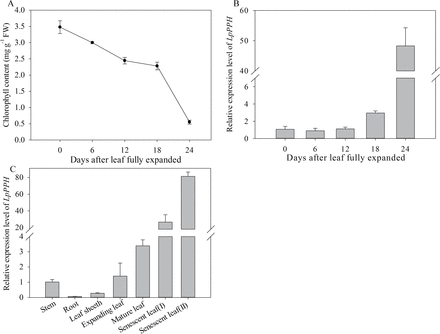
Fig. 6
Expression levels of LpPPH in leaves during senescence and in different organs. (A) Chl content changes in fully expanded leaves (note that perennial ryegrass leaves were fully expanded 12 d after leaf emergence). (B) Changes in relative expression of LpPPH in fully expanded leaves. (C) Relative expression of LpPPH in different organs. Data are means ±SE (n=4).
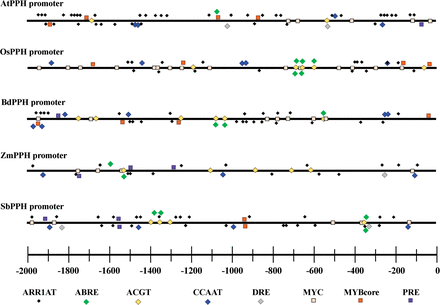
Fig. 7
List of conserved cis-elements involved in CK and ABA pathways and in response to abiotic stresses in five PPH promoters.
LpPPH is transcriptionally induced by ABA and ethephon, and suppressed by cytokinin and AVG
The analysis of promoters of PPH genes from five sequenced plant species demonstrated that all these PPH genes had multiple cis-elements involved in ABA or CK regulatory pathways. For example, ABRE (MACGYGB) and ACGT (G/AACGTC/T) are the core DNA-binding sequences for ABI3 and ABF4, respectively, and both transcription factors are in the ABA regulatory pathway. ARR1AT (NGATT) is the core sequence recognized and bound by Arabidopsis response regulators (ARRs), the transcription factors in the two-component CK signaling pathway (Fig. 7). In addition, a number of abiotic and biotic stress-responsive cis-elements were also found in all five PPH promoters, including a CCAAT box (heat shock element, CCAAT), a DRE (dehydration response element, RCCGAC), MYBCore (AtMYB1/2 DNA-binding site in response to water stress, CNGTTR), MYCATERD1 (MYC recognition sequence necessary for expression of erd1, and which can be bound by certain stress-inducible NAC proteins, CATGTG), and PRE (proline- and hypoosmolarity-responsive element, ACTCAT) (Fig. 7; Supplementary Table S2 at JXB online).
The test with exogenous treatment of leaves with ABA, ethephon, AVG, or 6-BA demonstrated that 6-BA and AVG significantly delayed leaf senescence, as manifested by increased Chl content and plasma membrane stability (or increased EL), and Fv/Fm; in contrast, ABA and ethephon treatment significantly accelerated leaf senescence, with leaves showing more a severe decline in Chl and Fv/Fm but increased EL (Fig. 8). Consistent with the pattern of physiological changes during leaf senescence, 6-BA and AVG significantly suppressed the expression of LpPPH, but ABA and ethephon significantly induced its expression (Fig. 8).
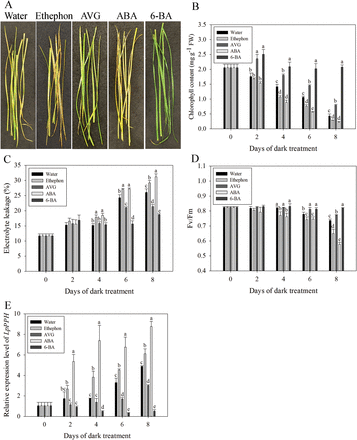
Fig. 8
Effects of ABA, ethephon, AVG, and 6-BA on leaf senescence and on the relative expression of LpPPH. (A) Effects of ABA, ethephon, AVG, and 6-BA on detached leaves after 8 d in the dark. (B–E) Chl content (B), electrolyte leakage (C), Fv/Fm (D), and relative expression of LpPPH changes after hormone treatment. Data are means ±SE (n=4 in A, B, and D, n=12 in C). (This figure is available in colour at JXB online.)
Discussion
A number of genes involved in the Chl degradation pathway were cloned from different plant species in recent years (review by ). The PPH genes have been cloned from different plant species, such as Arabidopsis (; ), rice (), and bamboo (Bambusa emeiensis) (), but not previously reported in perennial grass species. In this study, the PPH gene from perennial ryegrass was cloned and its function was confirmed. All PPHs have the conserved PPH motif (VYIA/VGNSL) () in which the serine residue carries out the nucleophilic attack of the substrate for the hydrolytic enzymatic activity (), although LpPPH only shared 87.9, 82.0, and 62.1% nucleotide sequence identities with BdPPH, OsPPH, and AtPPH, respectively. Transient expression of LpPPH fused to a GFP tag demonstrated that LpPPH was localized in the chloroplast, and the lack of a transmembrane domain in LpPPH further suggested that this protein may be mainly localized in the chloroplast stroma, which is consistent with the finding for AtPPH (). The synonymous and non-synonymous nucleotide substitution ratio (Ka/Ks) of <1.0 between the closely related PPH pairs demonstrated that PPH genes were under purifying selection (). Homologous characterization suggested that LpPPH from perennial ryegrass could share the conserved roles of PPH with other plant species in dephytylating Mg-free Chl during Chl catabolism.
The functionality of LpPPH was further confirmed experimentally with transient overexpression in wild tobacco and pph complementation assay in Arabidopsis. Overexpression of LpPPH resulted in leaf chlorosis in wild tobacco, while LpPPH rescued the stay-green phenotype in Arabidopsis pph null mutants. Furthermore, qPCR analysis demonstrated that the expression level of LpPPH was positively related to the greater extent of natural or dark-induced leaf senescence in perennial ryegrass, as manifested by the ~30- and 80-fold higher expression level in leaves at the early and late senescence stage in association with the decline in Chl content and increases in cell membrane stability. The association of increasing transcript levels of OsPPH with leaf senescence has also been reported in rice (). Our results strongly demonstrated that LpPPH served a function in Chl catabolism in perennial ryegrass, which could be manipulated to generate the stay-green trait that is desirable for perennial grass.
Further tests of LpPPH expression and physiological changes during leaf senescence in response to ethephon, AVG, ABA, and CK treatments showed that ethephon and ABA accelerated leaf senescence, and AVG and CK suppressed leaf senescence, which corresponded to up-regulated LpPPH and down-regulated LpPPH, respectively. This result supported that PPH is transcriptionally activated during leaf senescence and is regulated by sensing hormonal cues. The computational analysis of the cis-element components of five PPH genes showed that the PPH promoters shared conserved core nucleotide sequences potentially recognized by transcription factors in the ABA and CK signaling pathways, including ABRE, ACGT, and ARR1AT. ABRE and ACGT are the downstream core nucleotide sequences recognized by ABI3 and ABF4, respectively (; ), and both transcription factors are in the ABA regulatory pathway. The ACGT cis-element was also found in the promoter of another Chl degradation gene, AtNYC1, and was shown to be the DNA sequence targeted by ABF4 (). ARR1AT is the DNA recognition site for ARRs (; ), which are transcription factors in the CK signaling pathway. These results suggested that PPH genes might be transcriptionally regulated by the ABA, ethylene, and CK pathway transcription factors. The identities of these transcription factors and their exact nucleotide binding will be experimentally screened and tested in the future. From the combined data of gene expression, phenotypic changes, and the analysis of cis-elements, we hypothesized that CK suppressed the expression of PPH, probably through the ARR transcription factors by recognizing some of the ARR1AT cis-elements, and thereby circumvented the progress of Chl degradation, whereas ABA induced the expression of PPH, probably either by directly recognition of the ABRE or ACGT cis-elements by ABI3 or ABF4 transcription factors in the ABA pathway or by indirectly recognizing the other cis-elements through downstream abiotic stress-inducible transcription factors (e.g. MYBs or NACs). The transcription factors involved in ethylene-induced leaf senescence and ethylene-activated PPH expression are as yet unknown. The recent report by showed that most Chl catabolism genes but not PPH can be directly targeted by ORE1 in the ethylene signaling pathway. However, the fact that the promoter of the LpPPH gene lacked cis-elements in this study demonstrated that ethylene might not be directly involved in regulating the LpPPH gene through signaling and transcriptional activation. A number of cis-elements involved in abiotic stress were also found in the five PPH promoters in addition to ABA- or CK-responsive elements, including CCAAT box, DRE, MYBcore, MYCATERD1, and PRE. It is of interest that overexpressing DRE-binding proteins (DREBs) led to delayed instead of accelerated Chl breakdown under stressed growth conditions (; ). When and how the stress-inducible expression of transcription factors leads to accelerated or delayed Chl breakdown is still not well understood. found that a NAC protein, OsNAP, can directly bind to the promoter of PPH (NYC3). Yet the DNA-binding site of OsNAP has not yet been identified. The cis-element analysis suggested that the MYCATERD1 sequence that could be recognized by some stress-inducible NAC domain transcription factors () should be a candidate DNA-binding site recognized by OsNAP. The presence of these conserved cis-elements provides us with a strong clue for identifying important transcription factors that may directly regulate Chl breakdown and for understanding the mechanism of abiotic stress-induced Chl degradation. It remains intriguing to discover the integral regulatory network of Chl catabolism upon the sensing of external and internal cues; for example, senescence-related hormones. Also the mechanisms of and binding specificities for ethylene-, ABA,- or CK-responsive transcription factors that may activate or deactivate PPH expression deserves further investigation.
Conclusions
The LpPPH gene cloned from perennial ryegrass was confirmed to have a function in Chl phytol cleavage hydrolase, which could accelerate leaf senescence, depending on its expression levels, as demonstrated through the transient expression and mutant complementation assays, as well as qPCR analysis. LpPPH-mediated leaf senescence could be regulated by ethylene, ABA, and CK. Potential transcriptional factors in the ABA and CK signaling pathways were proposed as candidate upstream regulators of PPH that will be further confirmed by experimental approaches. The LpPPH gene could be used as a candidate gene by mutagenesis or gene editing techniques to create stay-green perennial grasses.
Supplementary data
Supplementary data are available at JXB online.
Table S1. Primers used in this study
Table S2. Prediction of PPH promoters and cis-elements.
Acknowledgements
We thank the Chinese Scholarship Council for providing a stipend during JZ’s study at Rutgers University. This study was supported by grant BK20140693 from the Natural Science Foundation of Jiangsu Province, China, by grant KYZ201552 from the Fundamental Research Funds for the Central Universities, and by grant 31572455 from the National Science Foundation of China.
References
- Alonso JM, Stepanova AN, Leisse TJ, Kim CJ, Chen H, Shinn P, Stevenson DK, Zimmerman J, Barajas P, Cheuk R. 2003. Genome-wide insertional mutagenesis of Arabidopsis thaliana. Science 301, 653–657.
- Büchert AM, Civello PM, Martínez GA. 2011. Chlorophyllase versus pheophytinase as candidates for chlorophyll dephytilation during senescence of broccoli. Journal of Plant Physiology 168, 337–343.
- Barnes J, Balaguer L, Manrique E, Elvira S, Davison A. 1992. A reappraisal of the use of DMSO for the extraction and determination of chlorophylls a and b in lichens and higher plants. Environmental and Experimental Botany 32, 85–100.
- Barry CS. 2009. The stay-green revolution: recent progress in deciphering the mechanisms of chlorophyll degradation in higher plants. Plant Science 176, 325–333.
- Bouaziz D, Pirrello J, Charfeddine M, Hammami A, Jbir R, Dhieb A, Bouzayen M, Gargouri-Bouzid R. 2013. Overexpression of StDREB1 transcription factor increases tolerance to salt in transgenic potato plants. Molecular Biotechnology 54, 803–817.
- Breeze E, Harrison E, McHattie S, Hughes L, Hickman R, Hill C, Kiddle S, Kim Y-s, Penfold CA, Jenkins D. 2011. High-resolution temporal profiling of transcripts during Arabidopsis leaf senescence reveals a distinct chronology of processes and regulation. The Plant Cell 23, 873–894.
- Büchert AM, Civello PM, Martínez GA. 2011. Chlorophyllase versus pheophytinase as candidates for chlorophyll dephytilation during senescence of broccoli. Journal of Plant Physiology 4, 337–343.
- Cheng Y, Guan J. 2014. Involvement of pheophytinase in ethylene-mediated chlorophyll degradation in the peel of harvested ‘Yali’ pear. Journal of Plant Growth Regulation 33, 364–372.
- Christ B, Hörtensteiner S. 2014. Mechanism and significance of chlorophyll breakdown. Journal of Plant Growth Regulation 33, 4–20.
- Clough SJ, Bent AF. 1998. Floral dip: a simplified method for Agrobacterium-mediated transformation of Arabidopsis thaliana. The Plant Journal 16, 735–743.
- Delmas F, Sankaranarayanan S, Deb S, Widdup E, Bournonville C, Bollier N, Northey JG, McCourt P, Samuel MA. 2013. ABI3 controls embryo degreening through Mendel’s I locus. Proceedings of the National Academy of Sciences, USA 110, E3888–E3894.
- Dodd IC, Beveridge CA. 2006. Xylem-borne cytokinins: still in search of a role? Journal of Experimental Botany 57, 1–4.
- Dodson G, Wlodawer A. 1998. Catalytic triads and their relatives. Trends in Biochemical Sciences 23, 347–352.
- Earley KW, Haag JR, Pontes O, Opper K, Juehne T, Song K, Pikaard CS. 2006. Gateway-compatible vectors for plant functional genomics and proteomics. The Plant Journal 45, 616–629.
- Emanuelsson O, Nielsen H, Von Heijne G. 1999. ChloroP, a neural network-based method for predicting chloroplast transit peptides and their cleavage sites. Protein Science 8, 978–984.
- Endler A, Meyer S, Schelbert S, Schneider T, Weschke W, Peters SW, Keller F, Baginsky S, Martinoia E, Schmidt UG. 2006. Identification of a vacuolar sucrose transporter in barley and Arabidopsis mesophyll cells by a tonoplast proteomic approach. Plant Physiology 141, 196–207.
- Fukao T, Yeung E, Bailey-Serres J. 2012. The submergence tolerance gene SUB1A delays leaf senescence under prolonged darkness through hormonal regulation in rice. Plant Physiology 160, 1795–1807.
- Gan S, Amasino RM. 1995. Inhibition of leaf senescence by autoregulated production of cytokinin. Science 270, 1986–1988.
- Grbić V, Bleecker AB. 1995. Ethylene regulates the timing of leaf senescence in Arabidopsis. The Plant Journal 8, 595–602.
- Guo Y, Gan S-S. 2014. Translational researches on leaf senescence for enhancing plant productivity and quality. Journal of Experimental Botany 65, 3901–3913.
- Hörtensteiner S. 2006. Chlorophyll degradation during senescence. Annual Review of Plant Biology . 57, 55–77.
- Hörtensteiner S, Kräutler B. 2011. Chlorophyll breakdown in higher plants. Biochimica et Biophysica Acta 1807, 977–988.
- Higo K, Ugawa Y, Iwamoto M, Korenaga T. 1999. Plant cis-acting regulatory DNA elements (PLACE) database: 1999. Nucleic Acids Research 27, 297–300.
- Hoagland DR, Arnon DI. 1950. The water-culture method for growing plants without soil. California Agricultural Experiment Station Circular 347, 32.
- Horton P, Park K-J, Obayashi T, Fujita N, Harada H, Adams-Collier C, Nakai K. 2007. WoLF PSORT: protein localization predictor. Nucleic Acids Research 35, W585–W587.
- Huang L, Yan H, Jiang X, Yin G, Zhang X, Qi X, Zhang Y, Yan Y, Ma X, Peng Y. 2014. Identification of candidate reference genes in perennial ryegrass for quantitative RT-PCR under various abiotic stress conditions. PLoS One 9, e93724.
- Karimi M, De Meyer B, Hilson P. 2005. Modular cloning in plant cells. Trends in Plant Science 10, 103–105.
- Kim HJ, Hong SH, Kim YW, Lee IH, Jun JH, Phee B-K, Rupak T, Jeong H, Lee Y, Hong BS. 2014. Gene regulatory cascade of senescence-associated NAC transcription factors activated by ETHYLENE-INSENSITIVE2-mediated leaf senescence signalling in Arabidopsis. Journal of Experimental Botany 65, 4023–4036.
- Kim J, Chang C, Tucker ML. 2015. To grow old: regulatory role of ethylene and jasmonic acid in senescence. Frontiers in Plant Science 6, 20.
- Koyama T. 2014. The roles of ethylene and transcription factors in the regulation of onset of leaf senescence. Frontiers in Plant Science 5, 6501.
- Li Z, Peng J, Wen X, Guo H. 2013. Ethylene-insensitive3 is a senescence-associated gene that accelerates age-dependent leaf senescence by directly repressing miR164 transcription in Arabidopsis. The Plant Cell 25, 3311–3328.
- Li Y, Hagen G, Guilfoyle TJ. 1992. Altered morphology in transgenic tobacco plants that overproduce cytokinins in specific tissues and organs. Developmental Biology 153, 386–395.
- Liang C, Wang Y, Zhu Y, et al. 2014. OsNAP connects abscisic acid and leaf senescence by fine-tuning abscisic acid biosynthesis and directly targeting senescence-associated genes in rice. Proceedings of the National Academy of Sciences, USA 111, 10013–10018.
- Librado P, Rozas J. 2009. DnaSP v5: a software for comprehensive analysis of DNA polymorphism data. Bioinformatics 25, 1451–1452.
- Lim PO, Kim HJ, Gil Nam H. 2007. Leaf senescence. Annual Review of Plant Biology 58, 115–136.
- Liu F, Guo F-Q. 2013. Nitric oxide deficiency accelerates chlorophyll breakdown and stability loss of thylakoid membranes during dark-induced leaf senescence in Arabidopsis. PLoS One 8, e56345.
- Merewitz EB, Gianfagna T, Huang B. 2010. Effects of SAG12-ipt and HSP18.2-ipt expression on cytokinin production, root growth, and leaf senescence in creeping bentgrass exposed to drought stress. Journal of the American Society for Horticultural Science 135, 230–239.
- Morita R, Sato Y, Masuda Y, Nishimura M, Kusaba M. 2009. Defect in non-yellow coloring 3, an alph/beta hydrolase-fold family protein, causes a stay-green phenotype during leaf senescence in rice. The Plant Journal 59, 940–952.
- Morran S, Eini O, Pyvovarenko T, Parent B, Singh R, Ismagul A, Eliby S, Shirley N, Langridge P, Lopato S. 2011. Improvement of stress tolerance of wheat and barley by modulation of expression of DREB/CBF factors. Plant Biotechnology Journal 9, 230–249.
- Murray M, Cape J, Fowler D. 1989. Quantification of frost damage in plant tissues by rates of electrolyte leakage. New Phytologist 113, 307–311.
- Nakajima S, Ito H, Tanaka R, Tanaka A. 2012. Chlorophyll b reductase plays an essential role in maturation and storability of Arabidopsis seeds. Plant Physiology 160, 261–273.
- Nakashima K, Yamaguchi-Shinozaki K. 2006. Regulons involved in osmotic stress-responsive and cold stress-responsive gene expression in plants. Physiologia Plantarum 126, 62–71.
- Oh SA, Park JH, Lee GI, Paek KH, Park SK, Nam HG. 1997. Identification of three genetic loci controlling leaf senescence in Arabidopsis thaliana. The Plant Journal 12, 527–535.
- Oxborough K, Baker NR. 1997. Resolving chlorophyll a fluorescence images of photosynthetic efficiency into photochemical and non-photochemical components—calculation of qP and Fv'/Fm' without measuring Fo'. Photosynthesis Research 54, 135–142.
- Qiu K, Li Z, Yang Z, Chen J, Wu S, Zhu X, Gao S, Gao J, Ren G, Kuai B. 2015. EIN3 and ORE1 accelerate degreening during ethylene-mediated leaf senescence by directly activating chlorophyll catabolic genes in Arabidopsis. PLoS Genetics 11, e1005399.
- Ren G, Zhou Q, Wu S, Zhang Y, Zhang L, Huang J, Sun Z, Kuai B. 2010. Reverse genetic identification of CRN1 and its distinctive role in chlorophyll degradation in Arabidopsis. Journal of Integrative Plant Biology 52, 496–504.
- Ross EJH. 2004. Activation of the Oryza sativa non-symbiotic haemoglobin-2 promoter by the cytokinin-regulated transcription factor, ARR1. Journal of Experimental Botany 55, 1721–1731.
- Sakai H, Aoyama T, Oka A. 2000. Arabidopsis ARR1 and ARR2 response regulators operate as transcriptional activators. The Plant Journal 24, 703–711.
- Sakai H, Hua J, Chen QG, Chang C, Medrano LJ, Bleecker AB, Meyerowitz EM. 1998. ETR2 is an ETR1-like gene involved in ethylene signaling in Arabidopsis. Proceedings of the National Academy of Sciences, USA 95, 5812–5817.
- Sato Y, Morita R, Katsuma S, Nishimura M, Tanaka A, Kusaba M. 2009. Two short-chain dehydrogenase/reductases, NON-YELLOW COLORING 1 and NYC1-LIKE, are required for chlorophyll b and light-harvesting complex II degradation during senescence in rice. The Plant Journal 57, 120–131.
- Schelbert S, Aubry S, Burla B, Agne B, Kessler F, Krupinska K, Hörtensteiner S. 2009. Pheophytin pheophorbide hydrolase (pheophytinase) is involved in chlorophyll breakdown during leaf senescence in Arabidopsis. The Plant Cell 21, 767–785.
- Simpson SD, Nakashima K, Narusaka Y, Seki M, Shinozaki K, Yamaguchi-Shinozaki K. 2003. Two different novel cis-acting elements of erd1, a clpA homologous Arabidopsis gene function in induction by dehydration stress and dark-induced senescence. The Plant Journal 33, 259–270.
- Tao G-Q, Letham D, Palni L, Summons R. 1983. Cytokinin biochemistry in relation to leaf senescence I. The metabolism of 6-benzylaminopurine and zeatin in oat leaf segments. Journal of Plant Growth Regulation 2, 89–102.
- Thomas H, Stoddart JL. 1980. Leaf senescence. Annual Review of Plant Physiology 31, 83–111.
- Tian F, Gong J, Zhang J, Zhang M, Wang G, Li A, Wang W. 2013. Enhanced stability of thylakoid membrane proteins and antioxidant competence contribute to drought stress resistance in the tasg1 wheat stay-green mutant. Journal of Experimental Botany 64, 1509–1520.
- Tran L-SP, Nakashima K, Sakuma Y, Simpson SD, Fujita Y, Maruyama K, Fujita M, Seki M, Shinozaki K, Yamaguchi-Shinozaki K. 2004. Isolation and functional analysis of Arabidopsis stress-inducible NAC transcription factors that bind to a drought-responsive cis-element in the early responsive to dehydration stress 1 promoter. The Plant Cell 16, 2481–2498.
- Wei Q, Cao H, Li Z, Kuai B, Ding Y. 2013. Identification of an AtCRN1-like chloroplast protein BeCRN1 and its distinctive role in chlorophyll breakdown during leaf senescence in bamboo (Bambusa emeiensis ‘Viridiflavus’). Plant Cell, Tissue and Organ Culture 114, 1–10.
- Wei Q, Guo Y, Kuai B. 2011. Isolation and characterization of a chlorophyll degradation regulatory gene from tall fescue. Plant Cell Reports 30, 1201–1207.
- Yamatani H, Sato Y, Masuda Y, Kato Y, Morita R, Fukunaga K, Nagamura Y, Nishimura M, Sakamoto W, Tanaka A. 2013. NYC4, the rice ortholog of Arabidopsis THF1, is involved in the degradation of chlorophyll–protein complexes during leaf senescence. The Plant Journal 74, 652–662.
- Yang J, Zhang J, Wang Z, Zhu Q, Liu L. 2002. Abscisic acid and cytokinins in the root exudates and leaves and their relationship to senescence and remobilization of carbon reserves in rice subjected to water stress during grain filling. Planta 215, 645–652.
- Yang K-Y, Liu Y, Zhang S. 2001. Activation of a mitogen-activated protein kinase pathway is involved in disease resistance in tobacco. Proceedings of the National Academy of Sciences, USA 98, 741–746.
- Zwack PJ, Rashotte AM. 2013. Cytokinin inhibition of leaf senescence. Plant Signaling and Behavior 8, e24737.