Respiratory syncytial virus (RSV) is a leading cause of respiratory tract infections and hospitalizations in children <5 years of age []. In recent years, there has been an increasing recognition of the burden RSV places on the health of older and immunocompromised adults [, ]. Although age and certain preexisting medical conditions are established risk factors, less focus has been placed on understanding the impact of socioeconomic status (SES) on severe RSV disease risk. SES has a well-documented role in influencing incidence and mortality rates for other respiratory diseases, including coronavirus disease 2019 (COVID-19) [, ]. These social inequities exist within the context of Canada's universal healthcare system, where individuals with lower SES experience worse health outcomes compared to their higher-SES counterparts [, ].
Although limited work has assessed RSV burden from this social determinants lens, it is evident that social inequities exist []. Populations experiencing higher levels of marginalization (in particular, Inuit infants living in Nunavut, who experience some of the highest rates of RSV hospitalization in the world) [] and with lower neighborhood-level income are disproportionately impacted by RSV [, , , ]. Furthermore, adherence to palivizumab, an effective (and expensive) monoclonal antibody given prophylactically to medically high-risk infants on a monthly basis, has previously been shown to be particularly low among infants of lower SES, including those with less-educated mothers [], foreign-born/minority parents, and limited physical access to administration sites []. Recently approved 1-dose prophylaxis and vaccination options for infants and older adults offer promise for improving equitable access and prevention of RSV across the life course [].
To date, work exploring the association between SES and severe RSV disease has primarily focused on a limited number of neighborhood-level SES indicators and on pediatric populations outside a Canadian context []. Although it is important to understand the influence of one's lived environment on health, use of area-based measures are subject to ecological fallacies and may not accurately reflect individual-level SES [, ]. Moreover, given that SES is a multifaceted concept, extending research beyond traditional SES measures and into other, largely unexplored health-influencing factors, such as housing quality [, , ], is essential; this is particularly important for RSV considering the role that housing may play in influencing respiratory disease severity, transmission risk, and access to adequate materials and preventive resources [, , ].
These gaps in existing research examining social inequities in RSV burden must be addressed to help inform future guidelines, program evaluations, and equitable allocation strategies for emerging RSV immunizations. Our study aimed to overcome these limitations by using data from a large population-based cohort study in Canada. This data linkage includes information on individual-level SES indicators not commonly available in health administrative data. Specifically, the goal of this work was to describe the preimmunization-era burden of RSV-related hospitalizations among noninstitutionalized Canadians (excluding Québec) ≥6 months of age, by largely unexplored individual-level SES indicators and age.
METHODS
Study Design, Cohort, and Measures
We conducted a longitudinal descriptive study in Canada to explore rates of International Classification of Diseases, 10th Revision, Canada (ICD-10-CA)–coded RSV-related hospitalizations across 3 respiratory seasons (defined as 1 September to 31 August), 2016–2017 to 2018–2019 (pre–RSV immunization and COVID-19), using data from the 2016 Canadian Census Health and Environment Cohort (CanCHEC) [].
The CanCHEC probabilistically linked datasets uniquely capture both individual-level census-based sociodemographic information, as well as health administrative data including hospitalization and death records from the Discharge Abstract Database (DAD) [] and the Canadian Vital Statistics–Death database [], respectively.
Specifically, the 2016 CanCHEC is a population-based cohort comprised of approximately 8.4 million Canadians who completed the 2016 long-form census questionnaire (25% of Canadian households) []. In brief, the census questionnaire is designed to provide a portrait of the demographic, social, and economic characteristics of households in Canada, and targeted the noninstitutionalized population that was living in Canada on census day (10 May 2016). The 2016 CanCHEC further excluded those who could not be successfully linked to the Derived Record Depository, a database containing personal identifiers necessary for linkage []. Recruitment and data processing are described in detail elsewhere [].
Given that the CanCHEC is a closed longitudinal cohort (those born after census day are not included) and our index date (1 September 2016) is 4 months after census day, infants are largely undercaptured. The study population included cohort members aged <105 years who were passively followed up using the DAD; as of the index date, person-time contributed from 6 months of age onward was included. The DAD does not record hospitalizations in Québec []; therefore, individuals residing in Québec at time of census were excluded.
Outcomes
Our primary outcome was RSV-related hospitalization as identified in the DAD. An individual was considered to have an RSV-related hospitalization based on a validated algorithm (presence of 1 of 4 RSV-related ICD-10-CA codes) shown to have moderate to high sensitivity and high specificity in both children and adults []. Postadmission diagnoses of RSV were excluded to attempt to limit cases to community-acquired infections. Hospitalization entries within 1 day of a previous discharge date for the same individual were assumed to reflect a single hospital care episode (eg, transfers between healthcare facilities) and were thus merged []. Given the potential for reinfection over time, we allowed for an individual to have 1 hospitalization per season. Among those with an RSV-related hospitalization, secondary outcomes including intensive care unit (ICU) admission, mechanical ventilation use, and in-hospital death were also captured.
Exposures of Interest
Our main exposures captured aspects of individual-, family-, and household-level SES (based on household and family identifiers), including income quintile based on economic family after-tax income (adjusted to account for regional differences and size of family), employment status (for those aged ≥15 years), highest level of education attained either individually or in one's census family (age and family-status dependent), household ownership status, type of dwelling, dwelling condition (reflecting degree of repairs required), residence suitability (reflecting crowding based on household composition), and household affordability. Age was captured at time of hospitalization for cases and as of index date for those without an RSV-related hospitalization. Detailed definitions of all variables are provided in Supplementary Table 1.
Statistical Analysis
Cohort characteristics and secondary outcomes were described using summary statistics (ie, frequencies or medians and interquartile ranges). Missing data were minimal but present for a subset of variables involving children <15 years of age whose parent information could not be obtained (Supplementary Table 1); all individuals remained in the cohort and rates were not presented for missing categories. Crude and age-stratified (6–59 months, 5–17 years, 18–64 years, 65–79 years, and ≥80 years) RSV-related hospitalization rates, with corresponding 95% confidence intervals (CIs), were calculated using Poisson regression with an offset term for the logarithm of subgroup-specific person-time. Rate ratios (RRs) and rate differences (RDs) were also estimated. Counts were randomly rounded to base 10 (Bernoulli distribution); rates were produced using unrounded values.
Each person contributed person-time at risk and events from 6 months of age until the earliest of 105 years of age, death, or 31 August 2019 (end of follow-up). To account for potential immunity from recent (same season) infections, individuals were further censored at the time of their first RSV-related hospitalization of the respiratory season with follow-up continuing at the start of the following season. Age-specific person-time was calculated to account for aging between age groups during follow-up.
All analyses were conducted in SAS software. RDs were calculated using the SAS Macro NLMeans (version 1.4). Analyses were performed using de-identified data at the Toronto Canadian Research Data Centre Network. Ethics approval was obtained from the University of Toronto's Health Sciences Research Ethics Board.
RESULTS
Cohort Characteristics
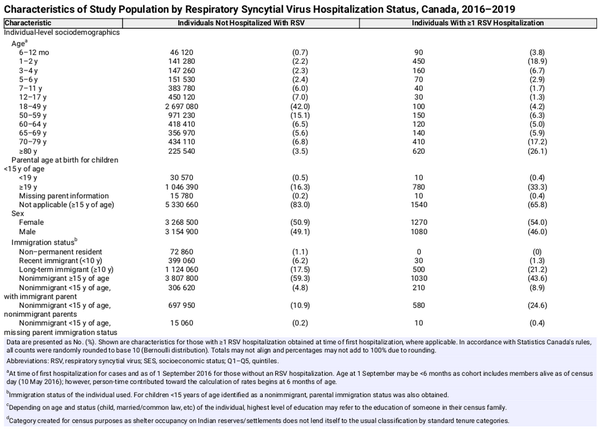
Of the 8.4 million Canadians in the 2016 CanCHEC, approximately 6.4 million individuals remained in our cohort after applying exclusion criteria (the majority reflecting the approximately 2 million excluded Québec residents) (Supplementary Figure 1). Approximately half of our cohort was female and resided in Ontario; just over three-quarters were Canadian citizens by birth. The majority of hospitalizations occurred among individuals aged ≥70 years, followed by those 6 months to 2 years of age (Table 1). Throughout our 3-year study period, individuals contributed a median of 2.995 years to follow-up.
Overall RSV Burden
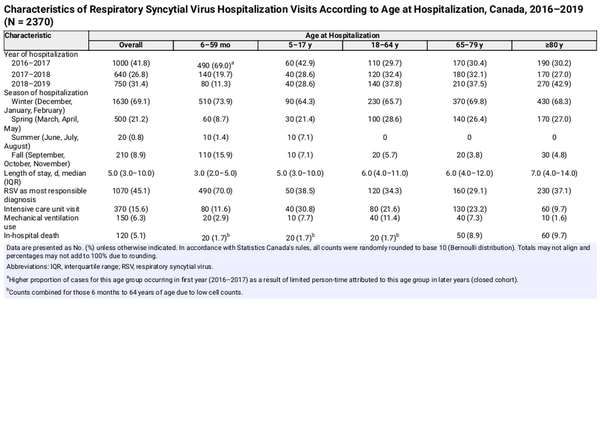
There were a total of 2370 RSV-related hospitalizations identified among 2350 individuals (maximum of 3 hospitalizations per individual), of which 15.6% resulted in an ICU visit, 6.3% required mechanical ventilation support, and 5.1% died in-hospital (Table 2). The overall rate of RSV-related hospitalizations was 12.4 (95% CI, 12.0–13.0) per 100 000 person-years. Rates were highest among children 6 months to 5 years of age (104.1 [95% CI, 96.7–112.1]) followed by adults ≥80 years (83.5 [95% CI, 77.2–90.3]), and lowest overall among 18- to 64-year-olds (2.9 [95% CI, 2.6–3.3]); this trend remained true across most indicators of SES (Supplementary Table 2).
RSV Burden by Individual-/Family-Level SES
Overall, there was a gradient effect present such that lower rates of RSV were observed with increasing SES, with respect to both family income and education attained. These inequities among the lowest-SES individuals (compared to the highest) were observed among all age groups except those aged ≥80 years (where rates remained exceptionally high regardless of income or education status); absolute differences were largest among <5-year-olds (income: RD, 33.4 [95% CI, 7.4–59.4]; education: RD, 104.3 [95% CI, 68.2–140.4]), whereas some of the greatest relative differences were observed among remaining age groups (income for 5- to 17-year-olds: RR, 4.6 [95% CI, 2.1–10.0]; education for 18- to 64-year-olds: RR, 4.5 [95% CI, 3.2–6.2]). Being unemployed did not significantly increase rates of RSV-related hospitalizations, but not being in the labor force did (RR, 11.5 [95% CI, 9.9–13.3]; RD, 22.9 [95% CI, 21.5–24.3] compared to employed individuals) (Figure 1; Supplementary Tables 3 and 4).
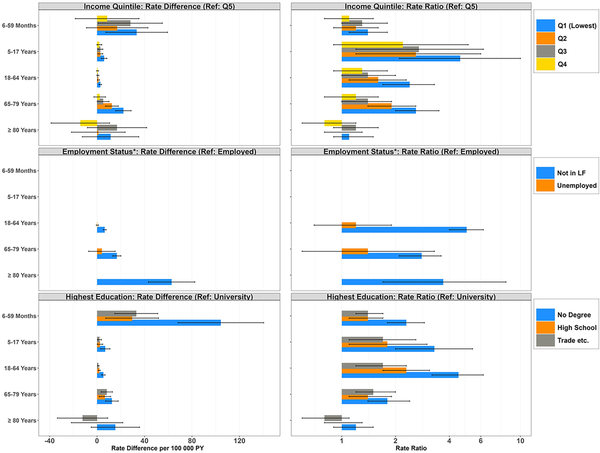
Figure 1
Age-stratified rate differences per 100 000 person-years and rate ratios (95% confidence interval [CI]) of respiratory syncytial virus hospitalizations according to income quintile of economic family, employment status, and highest level of education attained, Canada, 2016–2019. *Results not displayed for missing/not applicable categories (based on zero hospitalizations occurring in at least 1 comparator category). Rates are based on unrounded frequencies. Age groups based on age at time of hospitalization for numerator, and person-years contributed to age group for denominator. All rate estimates with 95% CIs estimated using Poisson regression with offset term for the log of person-time. Abbreviations: LF, labor force; PY, person-years; Q1–Q5, quintiles; Ref, reference; Trade etc., postsecondary nonuniversity/trade school.
RSV Burden by Household-Level SES
With respect to housing characteristics, the largest overall inequities were observed on the basis of household ownership and dwelling type. Compared to homeowners, individuals who resided on reserves/settlements or rented their home experienced a 1.6- and 1.3-fold increase in RSV-related hospitalizations, respectively (RR, 1.6 [95% CI, 1.3–1.9]; RR, 1.3 [95% CI, 1.2–1.5]); the magnitude of these differences also varied by age, with some of the largest absolute differences among renters aged ≥65 years and children aged <5 years on reserves (compared to homeowner counterparts). Further, individuals living in an apartment or in a mobile/movable home experienced higher rates compared to those in a single detached home (RR, 1.6 [95% CI, 1.5–1.8] and 1.6 [95% CI, 1.2–2.1]; RD, 6.8 [95% CI, 5.2–8.4] and 6.1 [95% CI, 1.1–11.1], respectively), a trend observed among adult age groups only (Figure 2; Supplementary Tables 3 and 4).
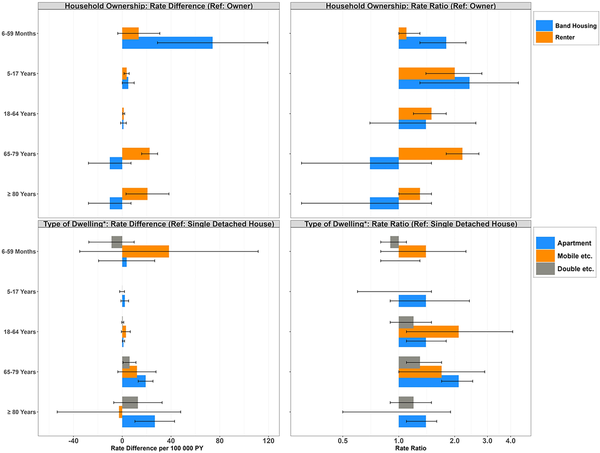
Figure 2
Age-stratified rate differences per 100 000 person-years and rate ratios (95% confidence interval [CI]) of respiratory syncytial virus hospitalizations according to household ownership status and type of dwelling, Canada, 2016–2019. *Results not displayed for missing/not applicable categories (based on zero hospitalizations occurring in at least 1 comparator category). In accordance with Statistics Canada's rules, rates based on frequencies <5 were not disclosed, and estimates were collapsed where appropriate. Rates are based on unrounded frequencies. Age groups based on age at time of hospitalization for numerator, and person-years contributed to age group for denominator. All rate estimates with 95% CIs estimated using Poisson regression with offset term for the log of person-time. Abbreviations: Double etc., double, row or terrace, duplex; Mobile etc., mobile/movable home; PY, person-years; Ref, reference.
In contrast, unsuitable living conditions demonstrated a significant increase in hospitalization rates among child age groups only (6–59 months: RR, 1.6 [95% CI, 1.3–1.9]; 5–17 years: RR, 1.6 [95% CI, 1.1–2.4]), with an absolute difference of 56.6 cases per 100 000 person-years for children aged <5 years living in unsuitable versus suitable homes. While individuals living in unaffordable homes experienced higher rates compared to those in affordable homes overall, these inequities were most apparent among adults 18–79 years old (18- to 64-year-olds: RR, 1.5 [95% CI, 1.2–1.9]; 65- to 79-year-olds: RR, 1.7 [95% CI, 1.4–2.0]), with the greatest absolute difference in 65- to 79-year-olds (RD, 13.5 [95% CI, 7.7–19.3]). Last, individuals living in homes requiring major repairs had higher RSV-related hospitalization rates relative to those in homes only requiring regular maintenance (RR, 1.2 [95% CI, 1.1–1.4]); the largest absolute differences were observed among those aged <5 years (RD, 34.9 [95% CI, 5.4–64.4]) and ≥80 years (RD, 39.4 [95% CI, 2.4–76.3]) (Figure 3; Supplementary Tables 3 and 4).
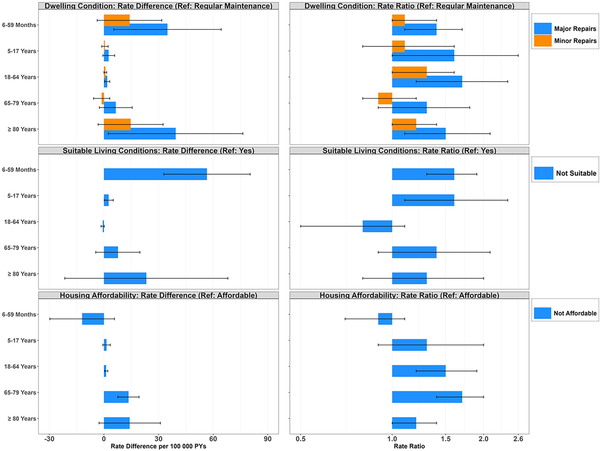
Figure 3
Age-stratified rate differences per 100 000 person-years and rate ratios (95% confidence interval [CI]) of respiratory syncytial virus hospitalizations according to dwelling condition, suitable living conditions, and housing affordability, Canada, 2016–2019. Rates are based on unrounded frequencies. Age groups based on age at time of hospitalization for numerator, and person-years contributed to age group for denominator. All rate estimates with 95% CIs estimated using Poisson regression with offset term for the log of person-time. Abbreviations: PY, person-years; Ref, reference.
DISCUSSION
This study reported rates of RSV-related hospitalizations among a cohort of noninstitutionalized Canadians (excluding Québec) 6 months of age and older from 2016 to 2019, across a range of largely unexplored individual-level SES indicators. We observed higher rates among lower-SES individuals as indicated through income, education, housing stability, and quality of one's dwelling. Importantly, inequities in burden and the relative importance of each SES indicator varied greatly by age. These findings indicate numerous pathways in which SES may influence RSV disease severity among Canadian populations.
Overall, we observed higher rates of RSV-related hospitalizations among individuals with lower compared to higher SES. Markedly, we observed an inverse gradient pattern between increasing family income and decreasing rates, a pattern well established for myriad other infectious and chronic diseases [, , ], including RSV [, , , ]. We further observed a trend of increasing rates with decreasing education across the majority of the lifespan; similar patterns have previously been identified among other respiratory diseases, with a recent population-based study in Ontario reporting an overall adjusted association between neighborhoods with the highest proportion of residents with less than a high school education and COVID-19 incidence []. In contrast, rates did not vary greatly by employment status, likely due to increased transmission of communicable diseases in many workplaces []. Higher rates were, however, noted among adults not in the labor force, potentially reflecting an individual's inability to participate in paid work due to disability, caring for people at risk, or other experiences that may increase one's likelihood of developing severe disease.
Our study further examined RSV hospitalization rates by housing characteristics. Given that the influence of income and, relatedly, education and employment status, on one's health is largely explained through their impact on access to adequate resources [], exploring housing stability and quality provides a direct perspective pertaining to the role that SES may play in disease burden. We identified lower rates of RSV among those with more favorable housing qualities. Particularly, we report heightened rates among individuals living in apartments, movable/mobile dwellings, crowded homes, unaffordable homes, and homes requiring major repairs, and in those living on reserves/settlements. These factors may influence various health-promoting determinants including access to healthcare and resources (eg, housing materials, vaccination) [, ], exposure to environmental factors known to exacerbate respiratory symptoms (eg, mold, air pollution) [, , ], poor ventilation and unsanitary conditions (eg, poor plumbing, water supply) [, , , ], and increased social contacts (eg, crowded homes/shared spaces) [, , ]. Individuals living on Indigenous reserves are at a higher risk of inadequate housing [] and face additional challenges related to various social determinants of health that may further contribute to RSV hospitalization. Similarly, higher rates of viral transmission (and respiratory disease in general) are more prevalent for Indigenous people living on reserves []. This is a reflection of historic and ongoing systemic biases and structural racism experienced by Indigenous people []. The solutions to these inequities are complex and beyond the scope of this article. Additional work is needed in partnership and under the leadership of Indigenous communities and organizations to better understand and address these unique risk factors for severe RSV disease among Indigenous people. This could include culturally appropriate public health interventions that support the health rights of Indigenous peoples both on- and off-reserve. This further underscores the difficulty in disentangling unique impacts of SES indicators, which often overlap with each other and with other factors including ethnicity [, , ]. As such, to appropriately target these inequities (eg, through targeting public health interventions), it is essential to consider all aspects of the social determinants of health in tandem.
Limited research in high-income countries has reported on the influence of individual-level housing characteristics on RSV burden, particularly across the life course. While differences in area-based measures may preclude us from comparing estimates directly, several neighborhood-level studies have identified similar trends as observed in the current study; future efforts will be required to assess comparability between such measures. Housing-related inequities were particularly described in Ontario (Canada), with age-standardized RSV-related hospitalization rates being 1.5 times higher in neighborhoods experiencing the greatest levels of household/dwelling marginalization compared to the least []. Similarly, a study in the United States (US) reported higher risk of RSV hospitalization among those in the most socially vulnerable areas compared to those in the least socially vulnerable areas based on indexes representing household characteristics, housing type, and transportation []. A study conducted by Beamer et al further found increased odds of being in an RSV cluster among children in areas in the US with greater air pollution and poorer housing conditions []. Similar to our findings among children, studies from the US, Canada, and Europe have also detected crowding as an indicator of increased risk [, ]. These trends have similarly been reported for other respiratory viruses [, , ]. For example, neighborhood-level measures of unaffordable housing and certain apartment living have also been shown to be associated with higher COVID-19 risk and mortality []. Our work contributes to this existing body of evidence pointing to influential downstream repercussions of SES, and helps emphasize the importance of improving housing quality, including finding long-term solutions to crowding and affordability [].
Our study further highlights the intertwined relationship that age has with SES risk factors. Despite evidence of lower-SES individuals having higher rates of RSV-related hospitalization across the age continuum, the majority of hospitalizations occurred among those aged <5 years and ≥80 years, leading to larger observable differences on the absolute scale for many indicators. Distinctively, despite estimates being sensitive to lower numerator counts, the relative burden of many indicators proved to be greatest in those 5–79 years of age, pointing to the influence that SES may have in severe RSV disease among other age groups not commonly identified as high risk. Crucially, however, different indicators of SES appeared more influential among certain age groups compared to others. For example, type of residence only significantly influenced risk among adults, potentially reflecting implications from a lifetime exposure to such conditions. Further, income and education did not appear to be significant risk factors in those ≥80 years of age, despite being important among remaining age groups. This aligns with prior research that has shown income to be a potentially less accurate indicator of SES in later life; older individuals may live on fixed, lower incomes in retirement but experience other differences in wealth []. Many indicators among this age group (≥80 years), however, did not result in significant differences in risk, with rates remaining exceptionally high regardless of SES; older age (a potential reflection of aging-related risk factors including accumulating comorbidities) may outweigh the influence of SES in shaping burden.
This study has many strengths. Foremost was our ability to capture high-quality, multidimensional SES data on a national cohort of individuals spanning multiple RSV seasons. Rather than relying solely on administrative health data, which often lack comprehensive individual-level SES information, CanCHEC allowed us to capture the breadth of sociodemographic data required to accurately measure health inequities at the individual level. In comparison to previous studies that have largely presented limited/composite indicators of SES [], this work provides novel insights into factors that may be potentially amenable or targeted through public health efforts, such as improved access to vaccines. As no single measure can capture the multifaceted nature of SES, operationalizing SES based on factors beyond income, and at different levels (ie, individual, household), offers critical insights into the potential mechanism that may drive social inequities in RSV risks. Last, as SES-related inequities in RSV burden are poorly characterized across life stages, our age-stratified results provide a strong baseline for future work, and highlight the role age may play in modifying the relationship between different aspects of SES and severe RSV disease.
This study also presents several limitations. Most notably, our cohort excludes young infants (<6 months) and institutionalized individuals (including long-term care residents) [], 2 groups for whom rates of RSV are often greatest [, ]; these exclusions may underestimate the true burden and inequities present in RSV disease among the full Canadian population. Our findings may not be generalizable to populations outside of our study population, including those in Québec (who did not contribute outcome data). Other limitations include the potential for exposure misclassification given that most covariates were self-reported. Individuals may be more inclined to report better SES than in reality (due to social desirability), thus potentially resulting in conservative estimates of inequities; however, income, a measure particularly vulnerable to bias [], was not self-reported in the 2016 census, helping to mitigate such concerns. Covariates were also captured at time of census, failing to account for the varying and accumulating effect that SES can have throughout one's life; however, as this study only takes place across 3 years, changes are likely minimal. Underestimation of the burden of RSV in hospitals is also plausible given that laboratory confirmation of RSV is known to be infrequently sought, or delayed, particularly among adults [, ]. Relatedly, there is the potential for outcome misclassification as we did not have access to laboratory data to differentiate community-acquired from nosocomial cases. Future work should aim to explore causal relationships by considering the role of comorbidities and other social determinants in shaping RSV burden.
This study highlights that, in addition to clinical risk factors, it is essential to understand the role that the social determinants of health play in perpetuating the risk of and inequities in severe RSV disease across the life course. The variety of novel indicators of SES examined in this study provides unique perspectives into these different pathways, including the role that housing may have in shaping RSV disease severity and transmission—an inequity potentially amenable through future policy efforts. Notably, this study also provides a quantification of age-specific inequities in RSV burden, which may offer direct insights for informing the targeted delivery and future evaluations of emerging RSV immunization programs.
References
- 1. Shi T, McAllister DA, O’Brien KL, et al Global, regional, and national disease burden estimates of acute lower respiratory infections due to respiratory syncytial virus in young children in 2015: a systematic review and modelling study. Lancet2017; 390:946–58.
- 2. Cong B, Dighero I, Zhang T, Chung A, Nair H, Li Y. Understanding the age spectrum of respiratory syncytial virus associated hospitalisation and mortality burden based on statistical modelling methods: a systematic analysis. BMC Med2023; 21:224.
- 3. Hamilton MA, Liu Y, Calzavara A, et al Predictors of all-cause mortality among patients hospitalized with influenza, respiratory syncytial virus, or SARS-CoV-2. Influenza Other Respir Viruses2022; 16:1072–81.
- 4. van Ingen T, Brown KA, Buchan SA, et al Neighbourhood-level socio-demographic characteristics and risk of COVID-19 incidence and mortality in Ontario, Canada: a population-based study. PLoS One2022; 17:e0276507.
- 5. Ahmad K, Erqou S, Shah N, et al Association of poor housing conditions with COVID-19 incidence and mortality across US counties. PLoS One2020; 15:e0241327.
- 6. Godley J, Tang KL. Income, education, and hospitalization in Canada: results from linked census and administrative data. Discov Soc Sci Health2022; 2:article 19.
- 7. Gupta N, Crouse DL, Balram A. Individual and community-level income and the risk of diabetes rehospitalization among women and men: a Canadian population-based cohort study. BMC Public Health2020; 20:article 60.
- 8. Zheng Z, Warren JL, Shapiro ED, Pitzer VE, Weinberger DM. Estimated incidence of respiratory hospitalizations attributable to RSV infections across age and socioeconomic groups. Pneumonia (Nathan)2022; 14:6.
- 9. Colosia AD, Masaquel A, Hall CB, Barrett AM, Mahadevia PJ, Yogev R. Residential crowding and severe respiratory syncytial virus disease among infants and young children: a systematic literature review. BMC Infect Dis2012; 12:95.
- 10. Holmen JE, Kim L, Cikesh B, et al Relationship between neighborhood census-tract level socioeconomic status and respiratory syncytial virus–associated hospitalizations in U.S. adults, 2015–2017. BMC Infect Dis2021; 21:293.
- 11. Beamer PI, Lothrop N, Lu Z, et al Spatial clusters of child lower respiratory illnesses associated with community-level risk factors. Pediatr Pulmonol2016; 51:633–42.
- 12. Franklin JA, Anderson EJ, Wu X, Ambrose CS, Simões EAF. Insurance status and the risk of severe respiratory syncytial virus disease in United States preterm infants born at 32–35 weeks gestational age. Open Forum Infect Dis2016; 3:ofw163.
- 13. Fitzpatrick T, McNally JD, Stukel TA, et al Family and child risk factors for early-life RSV illness. Pediatrics2021; 147:e2020029090.
- 14. Chen KYA, van Ingen T, Smith BT, et al Neighbourhood-level burden of social risk factors on respiratory syncytial virus hospitalization in Ontario, Canada, 2016–2019. Open Forum Infect Dis2024; 11:ofae384.
- 15. Buchan SA, Chung H, To T, et al Estimating the incidence of first RSV hospitalization in children born in Ontario, Canada. J Pediatric Infect Dis Soc2023; 12:421–30.
- 16. Radhakrishnan D, Ouedraogo A, Shariff SZ, McNally JD, Benchimol EI, Clemens KK. The association between climate, geography and respiratory syncytial virus hospitalizations among children in Ontario, Canada: a population-based study. BMC Infect Dis2020; 20:157.
- 17. Thomas CM, Raman R, Schaffner W, et al Respiratory syncytial virus hospitalizations associated with social vulnerability by census tract: an opportunity for intervention? Open Forum Infect Dis 2024; 11:ofae184.
- 18. Banerji A, Greenberg D, White LF, et al Risk factors and viruses associated with hospitalization due to lower respiratory tract infections in Canadian Inuit children: a case-control study. Pediatr Infect Dis J2009; 28:697–701.
- 19. Chan P, Li A, Paes B, Abraha H, Mitchell I, Lanctôt KLCARESS Investigators. Adherence to palivizumab for respiratory syncytial virus prevention in the Canadian registry of palivizumab. Pediatr Infect Dis J2015; 34:e290–7.
- 20. Frogel MP, Stewart DL, Hoopes M, Fernandes AW, Mahadevia PJ. A systematic review of compliance with palivizumab administration for RSV immunoprophylaxis. J Manag Care Pharm2010; 16:46–58.
- 21. Rzymski P, Gwenzi W. Respiratory syncytial virus immunoprophylaxis: novel opportunities and a call for equity. J Med Virol2024; 96:e29453.
- 22. Simoes EAF. Environmental and demographic risk factors for respiratory syncytial virus lower respiratory tract disease. J Pediatr2003; 143:S118–26.
- 23. Buajitti E, Chiodo S, Rosella LC. Agreement between area- and individual-level income measures in a population-based cohort: implications for population health research. SSM Popul Health2020; 10:100553.
- 24. Pichora E, Polsky JY, Catley C, Perumal N, Jin J, Allin S. Comparing individual and area-based income measures: impact on analysis of inequality in smoking, obesity, and diabetes rates in Canadians 2003–2013. Can J Public Health2018; 109:410–8.
- 25. Swope CB, Hernández D. Housing as a determinant of health equity: a conceptual model. Soc Sci Med2019; 243:112571.
- 26. Kantz ME, Enah C, Abdallah LM. The relationship between health and housing in low-income older adults: a secondary analysis of survey data. Public Health Nurs2023; 40:931–9.
- 27. Gan WQ, Sanderson WT, Browning SR, Mannino DM. Different types of housing and respiratory health outcomes. Prev Med Rep2017; 7:124–9.
- 28. Anwar N, Kirychuk S, Karunanayake CP, et al Associations between housing factors and respiratory symptoms in two Saskatchewan First Nations communities. Int J Environ Res Public Health2021; 18:3744.
- 29. Turcotte DA, Woskie S, Gore R, Chaves E, Adejumo KL. Asthma, COPD, and home environments: interventions with older adults. Ann Allergy Asthma Immunol2019; 122:486–91.
- 30. Wimalasena NN, Chang-Richards A, Wang KI, Dirks KN. Housing risk factors associated with respiratory disease: a systematic review. Int J Environ Res Public Health2021; 18:2815.
- 31. Statistics Canada. People living in apartments and larger households were at higher risk of dying from COVID–19 during the first wave of the pandemic. 2021. https://www150.statcan.gc.ca/n1/pub/45-28-0001/2021001/article/00004-eng.htm. Accessed 6 June 2024.
- 32. Islam M, Sultana ZZ, Iqbal A, Ali M, Hossain A. Effect of in-house crowding on childhood hospital admissions for acute respiratory infection: a matched case–control study in Bangladesh. Int J Infect Dis2021; 105:639–45.
- 33. Madewell ZJ, Yang Y, Longini IM Jr, Halloran ME, Dean NE. Household transmission of SARS-CoV-2: a systematic review and meta-analysis. JAMA Netw Open2020; 3:e2031756.
- 34. Statistics Canada. Canadian Census Health and Environment Cohorts (CanCHECs). 2024. https://www.statcan.gc.ca/en/microdata/data-centres/data/canchec. Accessed 6 June 2024.
- 35. Canadian Institute for Health Information. Discharge Abstract Database (DAD) metadata. 2024. https://www.cihi.ca/en/discharge-abstract-database-dad-metadata. Accessed 6 June 2024.
- 36. Statistics Canada. Statistics Canada, Canadian Vital Statistics—Death database (CVSD). 2024. https://www23.statcan.gc.ca/imdb/p2SV.pl?Function=getSurvey&SDDS=3233. Accessed 6 June 2024.
- 37. Tjepkema M, Christidis T, Bushnik T, Pinault L. Cohort profile: the Canadian Census Health and Environment Cohorts (CanCHECs). Health Rep2019; 30:18–26.
- 38. Statistics Canada. Guide to the census of population, 2016. 2017. https://www12.statcan.gc.ca/census-recensement/2016/ref/98-304/index-eng.cfm. Accessed 6 June 2024.
- 39. Hamilton MA, Calzavara A, Emerson SD, et al Validating International Classification of Disease 10th Revision algorithms for identifying influenza and respiratory syncytial virus hospitalizations. PLoS One2021; 16:e0244746.
- 40. Ng E, Quinlan J, Giovinazzo G, et al All-cause acute care hospitalization rates of immigrants and the Canadian-born population: a linkage study. Health Rep2021; 32:3–13.
- 41. Su CP, de Perio MA, Cummings KJ, McCague AB, Luckhaupt SE, Sweeney MH. Case investigations of infectious diseases occurring in workplaces, United States, 2006–2015. Emerg Infect Dis2019; 25:397–405.
- 42. Galobardes B, Shaw M, Lawlor DA, Lynch JW, Smith GD. Indicators of socioeconomic position (part 1). J Epidemiol Community Health2006; 60:7–12.
- 43. Thomas TK, Ritter T, Bruden D, et al Impact of providing in-home water service on the rates of infectious diseases: results from four communities in western Alaska. J Water Health2016; 14:132–41.
- 44. Lyeo JS, Wong MD, Clyke N, et al Ten questions concerning First Nations on-reserve housing in Canada. Build Environ2024; 257:111544.
- 45. Government of Canada. Delivering on Truth and Reconciliation Commission calls to action. 2024. https://www.rcaanc-cirnac.gc.ca/eng/1524494530110/1557511412801. Accessed 10 November 2024.
- 46. Spiers GF, Liddle JE, Stow D, et al Measuring older people's socioeconomic position: a scoping review of studies of self-rated health, health service and social care use. J Epidemiol Community Health2022; 76:572–9.
- 47. Narejos Pérez S, Ramón Torrell JM, Põder A, et al Respiratory syncytial virus disease burden in community-dwelling and long-term care facility older adults in Europe and the United States: a prospective study. Open Forum Infect Dis2023; 10:ofad111.
- 48. Prati A. Hedonic recall bias. Why you should not ask people how much they earn. J Econ Behav Organ2017; 143:78–97.
- 49. Lee N, Walsh EE, Sander I, et al Delayed diagnosis of respiratory syncytial virus infections in hospitalized adults: individual patient data, record review analysis and physician survey in the United States. J Infect Dis2019; 220:969–79.