Introduction
The prevalence of obesity and type 2 diabetes mellitus (T2DM) has been rapidly rising. Approximately 462 million people worldwide are affected by T2DM, among which >1 million die each year (). Compared to healthy individuals, those with either obesity or T2DM show disruptions to their transcriptomic and metabolomic profiles. These are mainly characterized by alterations in the glucose, lipid, and/or amino acid metabolism (; ). Lifestyle management of patients is a crucial factor in preventing and managing obesity and T2DM, and exercise intervention is a central component in all obesity and T2DM prevention programs. Regular physical activity helps not only prevent the onset of T2DM, but can also improve T2DM-related variables such as body mass index, glycemic control and variability, insulin sensitivity, lipid profile, oxidative stress/antioxidative capacity, and/or chronic inflammation (; ; ). Despite such well-recognized benefits of exercise on metabolic homeostasis, the pleotropic effects of biomarkers and molecular transducers regulated by exercise remain poorly understood.
The skeletal muscle, one of the predominant sites of glucose disposal, plays a critical role in glycemic and metabolic homeostasis (). Physical activity provides benefits partly through extensive metabolic and molecular remodeling of the skeletal muscle in response to exercise (). For example, the skeletal muscle uniquely responds to exercise with increased sensitivity to subsequent insulin stimulation (). Exercise training also alters the DNA methylation of specific genes and pathways within the skeletal muscle in people with varying degrees of insulin sensitivity (). The skeletal muscle adapts to exercise through a variety of pathways, including muscle contraction/ATP biosynthesis coupling, and energy utilization upon activation of mechano- and other metabolic sensors (; ; ). Although considerable effort has been made to reveal the comprehensive changes stimulated by exercise (; ; ), many regulators of the skeletal muscle remain undiscovered.
At present, the development of metabolic diseases such as obesity and T2DM are linked with the disruption of multiple interconnected ‘omic’ layers (such as those of the transcriptome, epigenome, and metabolome). These omic investigations help unravel the integrative physiology underlying such diseases. Some studies have identified the function of metabolic networks in the development of insulin resistance (; ), and others have elucidated the global transcriptional response of human muscle to exercise (). However, though often useful and insightful, most previous studies simply focused on single omic data of either genome, transcriptome, metabolome, or proteome, and thus were not able to comprehensively elucidate the integrative physiology of the complex diseases. To break through the limitation of single omic data, a multi-omic effort to discover the potential cross-talk between the transcriptome and metabolome on exercise-regulated pathways is urgently required.
Overall, the principal aim of this study was to assess and analyze transcriptional and metabolic networks regulated by exercise in high-fat diet (HFD)-induced obese mice. To this end, we established two models of exercise intervention in obese mice to provide mechanistic insight into the beneficial effects of exercise intervention on systemic energy homeostasis.
Results
Treadmill running mitigates HFD-induced systemic glucose homeostasis dysregulation
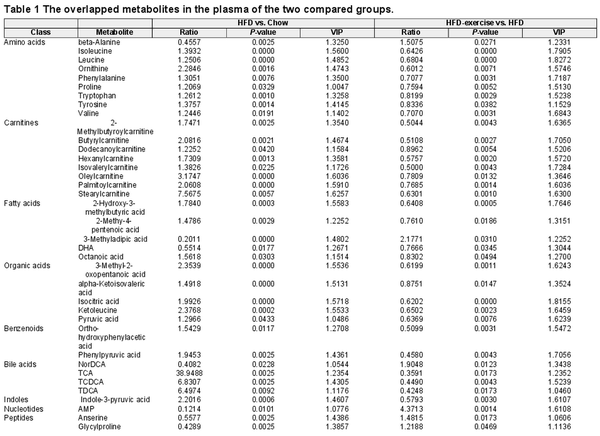
As shown in Figure 1, a murine prediabetic model was developed using HFD feeding. Exercise intervention (treadmill running) was conducted after 16-week HFD feeding (HFD-exercise group). The mice continuously fed a normal chow diet (Chow group) or HFD (HFD group) without exercise intervention were set up as control groups (Figure 1A). After another 8 weeks with or without exercise intervention, metabolic measurements including fasting glucose, glucose tolerance test (GTT), and insulin tolerance test (ITT), integrated multi-omics analysis (including metabolomics analysis in the plasma and skeletal muscle), and RNA sequencing (RNA-seq) analysis were performed for all groups (Figure 1A). As expected, HFD-fed mice showed sustained body weight gains compared to the Chow group, while exercise intervention significantly lessened the extent of the weight gain for the HFD-exercise group (Figure 1B). Exercise intervention also led to a reduction in the accumulation of fat mass and a reversal of lean mass loss induced by HFD (Figure 1C). Consistent with previous reports (), HFD led to hyperglycemia, hyperinsulinemia, and impairments in both glucose tolerance and insulin sensitivity compared to the Chow group (Figure 1D–G). Exercise intervention was able to mitigate HFD-induced systemic glucose homeostasis dysregulation and improve blood glucose control. Compared to the HFD group, the HFD-exercise group showed decreased fasting glucose and plasma insulin levels, as well as improved glucose tolerance and insulin sensitivity (Figure 1D–G). Further investigation showed that the insulin sensitivity of local muscle, liver, and adipose tissue was increased after exercise intervention (Supplementary Figure S1). These results demonstrated that exercise intervention at the prediabetic stage can partially restore glucose homeostasis for diabetes remission in mice.
Figure 1
Exercise intervention mitigates HFD-induced systemic glucose homeostasis dysregulation. (A) Schematic representation of the experimental procedures. (B) Dynamic body weight changes in mice with indicated treatments (n = 6 mice per group). ###P < 0.001, HFD vs. Chow; *P < 0.05, HFD-exercise vs. HFD; two-way ANOVA. (C) Body composition of mice in the indicated groups (n = 15 mice for the Chow group, n = 8 mice for for the HFD group, n = 11 mice for the HFD-exercise group). ###P < 0.001, HFD vs. Chow; *P < 0.05, HFD-exercise vs. HFD; one-way ANOVA followed by Tukey's multiple comparisons test. (D) Overnight fasting blood glucose levels at 6 weeks post exercise intervention (n = 6 mice per group). ###P < 0.001, HFD vs. Chow; ***P < 0.001, HFD-exercise vs. HFD; one-way ANOVA followed by Tukey's multiple comparisons test. (E) Overnight fasting blood insulin levels at 8 weeks post exercise intervention (n = 6 mice for the Chow group, n = 6 mice for the HFD group, n = 7 mice for the HFD-exercise group). ###P < 0.001, HFD vs. Chow; ***P < 0.001, HFD-exercise vs. HFD; one-way ANOVA followed by Tukey's multiple comparisons test. (F) GTT at 8 weeks post exercise intervention (n = 6 mice per group) (left). ##P < 0.01, ###P < 0.001, HFD vs. Chow; ***P < 0.001, HFD-exercise vs. HFD; two-way ANOVA followed by Tukey's multiple comparisons test. AOC quantification of GTT results (right). ###P < 0.001, HFD vs. Chow; **P < 0.01, HFD-exercise vs. HFD; one-way ANOVA followed by Tukey's multiple comparisons test. (G) ITT at 7 weeks post exercise intervention (n = 5 mice for the Chow group, n = 5 mice for for the HFD group, n = 6 mice for the HFD-exercise group) (left). #P < 0.05, ##P < 0.01, HFD vs. Chow; *P < 0.05, HFD-exercise vs. HFD; two-way ANOVA followed by Tukey's multiple comparisons test. AOC quantification of ITT results (right). #P < 0.05, HFD vs. Chow; *P < 0.05, HFD-exercise vs. HFD; one-way ANOVA followed by Tukey's multiple comparisons test.
Exercise training is linked to the altered plasma metabolism in HFD mice
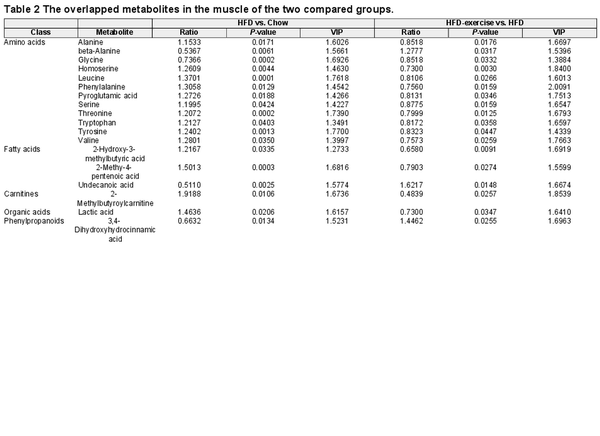
To globally evaluate exercise-induced circulating metabolites, targeted metabolomics was performed with blood plasma samples from HFD-exercise mice following long-term exercise of treadmill running and the control groups (Figure 2A). Notably, principal component analysis (PCA) of the metabonomics data showed a clear separation among the three groups. The pattern of plasma metabolites of the Chow group more resembled to that of the HFD-exercise group rather than the HFD group, suggesting that the HFD-altered plasma metabolites might have been partly reversed after exercise intervention (Figure 2B). Among all 198 detectable plasma metabolites from each group, amino acids (21.21%), fatty acids (20.20%), and organic acids (15.15%) were identified to have top-ranked proportions (Figure 2C). Volcano plots of an OPLS-DA model together with univariate statistics displayed variable contribution (variable importance in the projection, VIP), variable reliability (correlation coefficients, Corr.Coeffs), fold change (FC), and P-value for each metabolite (Figure 2D and E; Supplementary Figure S2). We then analyzed all the annotated metabolites in the plasma and found 93 differential metabolites between the HFD and Chow groups and 59 differential metabolites between the HFD-exercise and HFD groups (VIP > 1, P < 0.05). Among these differential metabolites, 38 overlapped (Figure 2F and Table 1), in which amino acids, carnitines, and fatty acids composed 23.7%, 21.4%, and 13.2%, respectively (Figure 2G). Further investigation demonstrated that 37 of the overlapped metabolites were induced by HFD and reversed by exercise training, leaving only one metabolite not showing such reversal (Figure 2H and I). The Kyoto Encyclopedia of Genes and Genomes (KEGG) pathway enrichment analysis revealed that these 37 reversed metabolites could be mapped onto 27 metabolic pathways. By removing the pathways with an impact value of 0, 15 metabolic pathways were established, in which 10 belonged to the amino acid metabolism and 3 were metabolically related to glucose (Figure 2J). These results suggested that exercise intervention can reverse some of the overnutrition-induced changes in plasma metabolite levels.
Figure 2
Treadmill running reverses HFD-induced changes in plasma metabolites. (A) Metabolomic data of plasma samples from the treadmill running experiment. (B) Scatter plot of PCA for metabolites of mice in the indicated groups. (C) Metabolite composition of plasma samples from indicated groups. SCFA, short-chain fatty acids. (D) Volcano plot of OPLS-DA model for each metabolite in HFD vs. Chow of plasma samples. The metabolites with log10(P-value) > 4 or |log2FC| ≥ 5 were labelled. (E) Volcano plot of OPLS-DA model for each metabolite in HFD-exercise vs. HFD of plasma samples. The metabolites with log10(P-value) > 3 or |log2FC| ≥ 2 were labelled. (F) Venn plot showing the overlap of differential metabolites shown in D and E. (G) Composition of overlapped metabolites (green shaded in F) in the Venn plot. (H) Scatter plot demonstrating that the changes of overlapped metabolites in the plasma were induced by HFD and mitigated by exercise. H v C, HFD vs. Chow; H-E v H, HFD-exercise vs. HFD. (I) Relative content heatmap for overlapped metabolites of mice in the indicated groups (n = 7 mice for the Chow group, n = 5 mice for the HFD group, n = 6 mice for the HFD-exercise group). Scale bar shows Z-score. (J) Bubble plot of significantly altered pathways (P < 0.05) reversed by exercise intervention. The horizontal coordinate is the extent to which the pathway is affected. The number of differential metabolites in the pathway is represented by graphs of different sizes. The P-values calculated by the enrichment analysis are described in terms of their color intensity.
Exercise training reverses metabolomic changes in HFD-fed mice
Targeted metabolomics analysis of quadriceps (Quad) was also performed to reveal changes of metabolites in the skeletal muscle (Figure 3A). Consistently, the metabolic profile of the Chow group shared more similarities with that of the HFD-exercise group rather than the HFD group (Figure 3B). A total of 211 metabolites from the skeletal muscle were identified, made up of amino acids (22.27%), fatty acids (21.80%), and organic acids (13.74%) (Figure 3C). According to VIP > 1 and P-value < 0.05, 57 and 29 differential metabolites were identified between the HFD and Chow groups and between the HFD-exercise and HFD groups, respectively (Figure 3D and E). Further analysis found that 18 of these metabolites overlapped (Figure 3F and Table 2), which mainly belonged to the classes of amino acids (66.67%) and fatty acids (16.67%) (Figure 3G). In addition, 17 of the overlapped metabolites showed reversibility of the overnutrition-induced changes after exercise intervention, among which 14 were increased by HFD feeding and relieved by exercise training (Figure 3H and I). The skeletal muscle metabolite profiles of HFD-exercise mice were similar to that of Chow mice in the heatmap (Figure 3I). KEGG pathway enrichment analysis showed that these differential metabolites were enriched in various metabolic pathways, especially those related to the amino acid metabolism (Figure 3J).
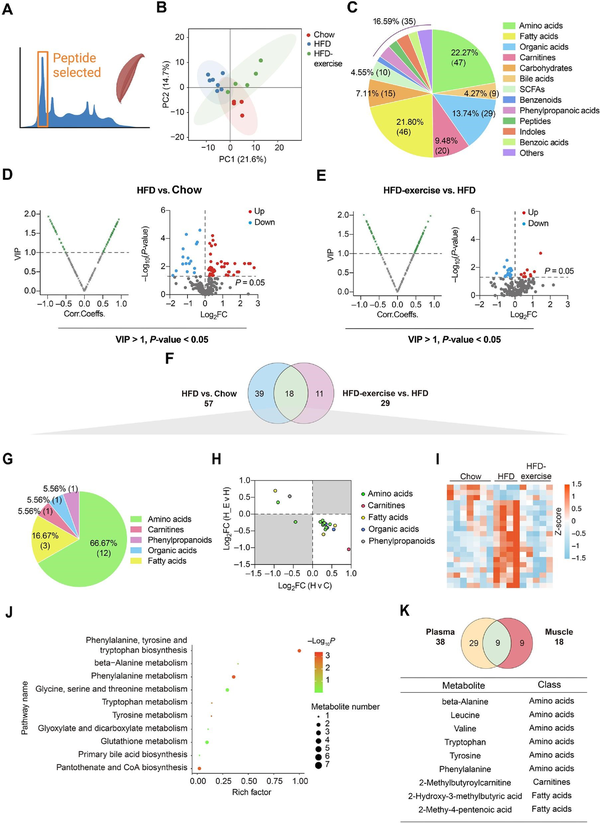
Figure 3
Treadmill running reverses HFD-induced changes of muscle metabolites. (A) Metabolomics of muscle samples from the treadmill running experiment. (B) Scatter plot of PCA for metabolites of mice in the indicated groups. (C) Metabolite composition of skeletal muscle samples from indicated groups. (D and E) Volcano plot of OPLS-DA model and univariate statistics for each metabolite in HFD vs. Chow (D) and HFD-exercise vs. HFD (E) of muscle tissues. (F) Venn plot showing the overlap of differential metabolites shown in D and E. (G) Composition of overlapped metabolites (green shaded in F) in the Venn plot. (H) Scatter plot demonstrating that the changes of overlapped metabolites in the skeletal muscle were induced by HFD and mitigated by exercise. H v C, HFD vs. Chow; H-E v H, HFD-exercise vs. HFD. (I) Relative content heatmap for overlapped metabolites of mice in the indicated groups (n = 7 mice for the Chow group, n = 4 mice for the HFD group, n = 5 mice for the HFD-exercise group). Scale bar shows Z-score. (J) Bubble plot of significantly altered pathways (P < 0.05) reversed by exercise intervention. The horizontal coordinate is the extent to which the pathway is affected, and the number of differential metabolites in the pathway is represented by graphs of different sizes. The P-values calculated by the enrichment analysis are described in terms of their color intensity. (K) Venn plot showing the overlap of exercise reversed metabolites in plasma and skeletal muscle samples. Table showing the overlapped metabolites (green shaded in the Venn plot) of plasma and skeletal muscle samples.
When comparing the differential metabolites in the plasma (38 metabolites) and muscle (18 metabolites) samples, the same variation trend was observed in nine metabolites, beta-alanine, leucine, valine, tryptophan, tyrosine, phenylalanine, 2-methylbutyroylcarnitine, 2-hydroxy-3-methylbutyric acid, and 2-methy-4-pentenoic acid (Figure 3K). These results suggested that exercise intervention has a great influence on the muscle and plasma metabolism in obese mice, primarily through altering the amino acid metabolism.
Exercise training reverses the skeletal muscle transcriptome in HFD-fed mice
To investigate whether the metabolic benefits coincide with the transcriptional signature, RNA-seq analysis was performed to examine the gene expression profiles in Quad muscles from the Chow, HFD, and HFD-exercise groups. Clear separation was found among the three groups via PCA (Figure 4A). Then, the RNA-seq data of genes related to the catabolic pathways were analyzed. Unexpectedly, the results showed that the expression levels of these related genes were not changed significantly in the HFD-exercise group compared to the HFD group, suggesting that exercise intervention regulated the amino acid changes in the muscle not through altering the catabolic pathway of the muscle itself (Figure 4B). Differential transcript analysis showed that a total of 1709 genes in Quad muscles expressed differentially after HFD feeding, while 772 genes expressed differentially after exercise intervention (Figure 4C). HFD feeding significantly upregulated the expression of 1296 genes in the skeletal muscle, and 386 of them were then significantly downregulated in response to exercise training. These 386 genes were classified into Set I. Among the 413 genes downregulated by HFD feeding, 79 genes were subsequently upregulated by exercise intervention and were classified into Set II (Figure 4C and D). Gene Ontology (GO) genetic pathway analysis on the 465 differential transcripts indicated that genes in Set I were mainly involved in the inflammation-related pathways, while genes in Set II were enriched in the areas of muscle development and function (Figure 4E). These findings suggested that exercise training may be effective in reversing HFD-induced loss of muscle tissues by regulating muscle contraction and development and reversing HFD-induced immune system disorders.
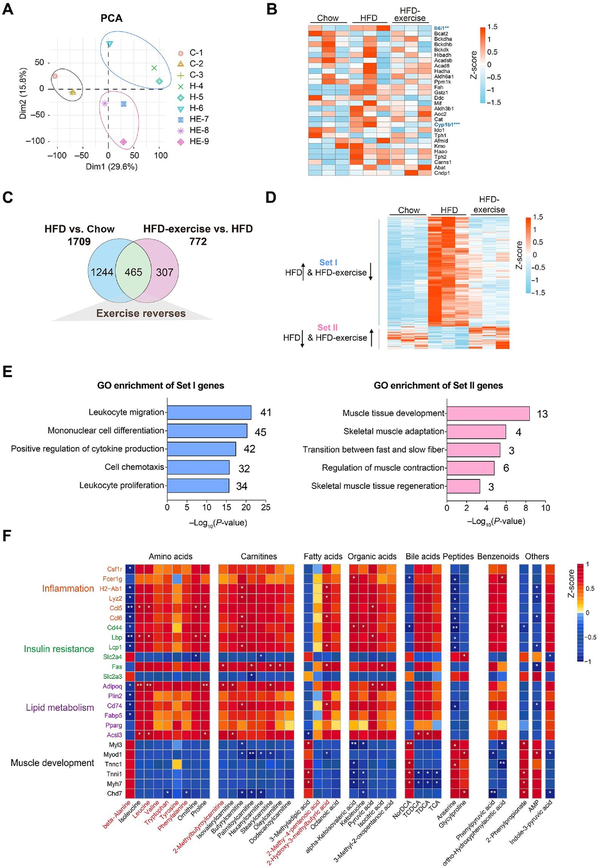
Figure 4
Exercise training reverses the skeletal muscle transcriptome in HFD-fed mice. (A) Scatter plot of PCA for genes of mice in the indicated groups. (B) Heatmap of metabolite-related genes for mice in the indicated groups (n = 3 mice per group). Scale bar shows Z-score. **P < 0.01 and ***P < 0.001. (C) Venn plot showing the number of genes with expression induced by HFD and/or reversed by HFD-exercise (|log2FC| > 0.3, P < 0.05). (D) Heatmap of reversed genes for mice in the indicated groups (n = 3 mice per group). Scale bar shows Z-score. Set I: genes with expression increased in the HFD group and decreased in the HFD-exercise group; Set II: genes with expression decreased in the HFD group and increased in the HFD-exercise group. (E) GO analysis of genes in Set I and Set II. (F) Heatmap of the Spearman's correlation coefficients between changes in reversed genes and metabolite alterations caused by exercise intervention. *P < 0.05 and **P < 0.01.
Integrated analysis reveals that exercise training is associated with insulin resistance pathways
According to the generally accepted associations of typical inflammation, insulin resistance, lipid metabolism, and muscle development pathways with the differential metabolisms, we discovered seven main metabolic categories that showed links to the expression of genes related to these pathways (Figure 4F). Most of amino acids, carnitines, fatty acids, organic acids, bile acids, and benzenoids were positively associated with inflammation, insulin resistance, and lipid metabolism pathways, whereas negatively associated with muscle morphogenesis pathways. The heatmap (Figure 4F) showed that exercise-induced upregulation of metabolites positively correlated with the induction of several inflammation-related genes (|r| > 0.7, P < 0.05) and fatty acid biosynthetic genes (|r| > 0.7, P < 0.05). To investigate whether macrophages were altered by exercise training, we analyzed the gene expression of M1-like and M2-like macrophage markers in the quadriceps from the Chow, HFD, and HFD-exercise groups, and found that almost all the measured genes, except Arg1, were upregulated in the HFD group and then downregulated after exercise intervention (Supplementary Figure S3).
Exercise-induced reductions in muscle amino acids, carnitines, fatty acids, and organic acids correlated with the upregulation of a similar set of genes involved in insulin resistance as well as the genes involved in muscle development (Figure 4F). Among these metabolites, beta-alanine, isoleucine, leucine, valine, tryptophan, tyrosine, 2-methylbutyroylcarnitine, isovalerylcarnitine, 2-methy-4-pentenoic acid, and 2-hydroxy-3-methylbutyric acid in plasma samples were regulated in the same trends as in muscle samples. In addition, isoleucine and leucine are not only essential amino acids but also belong to branched-chain amino acids (BCAAs) essential for the attainment of muscle mass and strength. This result demonstrated a lower BCAA level in the skeletal muscle of the HFD-exercise group compared with the HFD group. The transcriptomic data of the skeletal muscle showed that muscle contraction and development pathways were upregulated in HFD-exercise mice compared with HFD mice (Figure 4D and E), suggesting that exercise intervention might reverse HFD-induced elevation of BCAA levels by utilizing these amino acids as the substrate for protein synthesis and muscle growth. To further clarify the relationship between metabolites and the transcriptome in the muscle, we analyzed the expression of genes that take part in the catabolic process of metabolites in the muscle (Supplementary Figure S4). The results showed that, consistent with the alteration of the metabolites, the expression of the related genes (Il4i1, Cyp1b1, Tph2, Haao, Fah, and Gstz1) was upregulated by HFD feeding and then downregulated after exercise intervention. These results further suggested that exercise-induced alterations in metabolites contribute to the improvement of glucose metabolism in mice after exercise intervention.
Wheel running intervention also mitigates HFD-induced systemic glucose homeostasis dysregulation
In addition to the treadmill exercise, another form of exercise, wheel running, was also applied to examine the effect of exercise intervention on whole-body glucose metabolism and metabolites. We established a wheel running exercise mouse model, in which mice from the HFD-exercise group had free access to the running wheel in the cage, while mice from the HFD group lacked such access. Metabolic measurements, including fasting glucose, GTT, and ITT, were performed at the end of the intervention, with concurrent measurement of the amino acid content of the plasma (Figure 5A). Differing from treadmill running, wheel running did not lead to a significant reduction in body weight gain (Figure 5B). However, decreased fasting glucose and insulin levels and improved glucose tolerance and insulin sensitivity after wheel running were observed, in consistent with that by treadmill running intervention (Figure 5C–F). These results demonstrated that wheel running intervention could also improve systemic glucose homeostasis in mice with overnutrition.
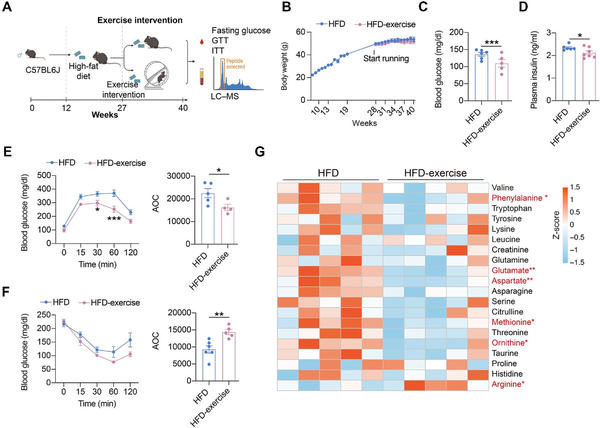
Figure 5
Wheel running can also alter glucose metabolism and metabolite levels. (A) Schematic representation of the experimental procedures. (B) Dynamic body weight changes in mice with indicated treatments (n = 11 mice for the HFD group, n = 9 mice for the HFD-exercise group). (C) Overnight fasting blood glucose levels at 7 weeks post exercise intervention (n = 6 mice for the HFD group, n = 5 mice for the HFD-exercise group). ***P < 0.001, two-tailed unpaired Student's t-test. (D) Overnight fasting plasma insulin levels at 7 weeks post exercise intervention (n = 6 mice for the HFD group, n = 7 mice for the HFD-exercise group). *P < 0.05, two-tailed unpaired Student's t-test. (E) GTT at 8 weeks post exercise intervention (n = 5 mice for the HFD group, n = 4 mice for the HFD-exercise group) (left). *P < 0.05, ***P < 0.001, two-way ANOVA followed by Sidak's multiple comparisons test. AOC quantification of GTT results (right). *P < 0.05, two-tailed unpaired Student's t-test. (F) ITT at 9 weeks post exercise intervention (n = 6 mice for the HFD group, n = 5 mice for the HFD-exercise group) (left). Two-way ANOVA followed by Sidak's multiple comparisons test. AOC quantification of ITT results (right). **P < 0.01, two-tailed unpaired Student's t-test. (G) Heatmap of relative abundance of amino acid metabolites in HFD and HFD-exercise mice (n = 5 mice per group). Scale bar shows Z-score. *P < 0.05, **P < 0.01, two-tailed unpaired Student's t-test.
Since the treadmill training model suggested that amino acid metabolism pathways play a key role in improving glucose metabolism in HFD mice, we then compared amino acid levels in the plasma of HFD and HFD-wheel running mice. The results showed a significant reduction in phenylalanine, glutamate, aspartate, methionine, and ornithine, as well as a decreasing trend in valine, tryptophan, tyrosine, lysine, leucine, creatinine, glutamine, asparagine, serine, citrulline, threonine, taurine, proline, and histidine, after wheel running intervention (Figure 5G). These results suggested that wheel running might also improve systemic glucose metabolism by altering the amino acid metabolism.
Discussion
Exercise training has gained increasing attention for its ability to counteract obesity and diabetes. In the present study, we used several unbiased approaches to assess the impact of exercise on the metabolomic and transcriptomic profiling of the plasma and skeletal muscle in HFD-fed mice. First, as previously recognized, we confirmed that exercise training indeed reversed HFD-induced obesity and impairments in glucose tolerance. We then went deeper to investigate several amino acids, such as beta-alanine, leucine, valine, and tryptophan, which may mediate the effects of exercise on obese mice. We further looked into the specific gene expression patterns linked with the initial phase of the adaptive response in the skeletal muscle via comparisons between exercise and non-exercise groups. We were finally able to provide a new conceptual framework by which the amino acid metabolism may contribute to exercise-mediated benefits upon the systemic glucose metabolism.
Given that a number of reports have demonstrated that exercise training can improve HFD-induced obesity and mitigate impaired glucose tolerance (; ), one possible mechanism is that such exercise improves these dysfunctions by remodeling the skeletal muscle (). If so, this remodeling response would likely involve the activation of intracellular signaling pathways and consequent genetic reprogramming, leading to alterations in muscle mass, contractile properties, and metabolic states (). Correspondingly, previous studies have reported that the movement of the muscle fibers enhances transmembrane glucose transport via the increase of glucose transporter 4, which leads to blood glucose reduction under physical exertion (; ; ; ). Other proposed mechanisms include stronger insulin binding to muscular insulin receptors, an increase in the number of muscular insulin receptors, increased activity of energy metabolism enzymes, and/or an increase in muscular capillary density (; ).
Exercise is known to enhance the catabolism of amino acids, including beta-alanine, leucine, valine, tryptophan, tyrosine, and phenylalanine, in both the plasma and skeletal muscle. Among these amino acids, both leucine and valine are BCAAs. Consistent with previous studies (), our study demonstrated lower BCAA levels in both the plasma and skeletal muscle of the HFD-exercise group compared with the HFD group. It is reported that BCAAs, and their metabolic by-products, strongly associate with insulin resistance (). Exercise accelerates BCAA biosynthesis and degradation, and a tight regulation of BCAA catabolism in the muscle is important for the homeostasis of the muscle energy metabolism and adaptation to exercise training (). The pathway leading to high levels of BCAAs in obesity is not completely understood but may involve chronic low-grade inflammation that prompts pro-inflammatory gene expression in the adipose tissue and determines further obesity impacts on metabolic health (; ). BCAAs have also been consistently identified as risk factors for cardiometabolic diseases due to their strong associations with insulin resistance () and with evidence accumulating for their causal effects (; ; ). Reduced BCAA concentrations achieved through lifestyle modifications over a prolonged period may therefore result in lower risks of T2DM (). However, discrepancies do appear in different studies related to this claim. For example, in one study, a bout of 12-week endurance and resistance-exercise training failed to result in any significant reduction in BCAAs levels, despite improvements in insulin sensitivity (). Previous studies have demonstrated that BCAA supplementation impaired the beneficial effect of exercise on glycolipid metabolism in obese mice (), and BCAA contributes to the development of obesity-associated insulin resistance in obese humans (). Meta-analysis showed that beta-alanine supplementation could increase muscle carnosine concentration and improve exercise capacity and performance (). found that tryptophan significantly improved glucose tolerance in both lean and diet-induced obese mice, but the extent of improvement was bigger in the obese mice with augmented glucose-stimulated insulin secretion enhancement (). Besides, a human study suggested that ingestion of L-phenylalanine, but not D-phenylalanine, increased insulin, glucagon, and GIP concentrations whereas reduced postprandial glucose levels without any obvious effects on appetite ().
Gene transcription is a key process controlling skeletal muscle phenotype and, consequently, metabolic health. Both pathological and physiological cues, as coupled with impaired and improved insulin sensitivity, respectively, link to the extensively remodeling of the skeletal muscle transcriptome (; ; ). In this study, exercise led to differential gene expression patterns in the skeletal muscle, including downregulation of immune-related gene categories, affecting leukocyte migration and mononuclear cell differentiation, and positive regulation of cytokine production, reflecting an anti-inflammatory effect. It is generally accepted that regular and moderate physical activity is inversely correlated with systemic low-grade inflammation, supporting the notion that exercise exerts a protective effect on patients with chronic diseases through its anti-inflammatory action (). Additionally, muscle cells are thought to have the capacity to produce several hundreds of secreted factors, which have been termed ‘myokines’ (; ). Myokines are believed to incorporate protective functions, including those with anti-inflammatory effects and/or specific effects, on the adipose tissue (; ). Therefore, the regulation of the expression of inflammatory genes may directly influence myokine secretion and therefore lower the muscle and global inflammatory status. This may partly explain the reduction in inflammatory markers such as C-reactive protein following long-term exercise (; ). In this study, we clearly observed the upregulated expression of genes related to the processes of muscle reconstruction and adaptation. During exercise, the skeletal muscle utilizes both muscle glycogen stores and circulating plasma glucose as fuel sources (). Muscle contractions, even at low intensity and low volume (), can activate both oxidative and non-oxidative glucose disposal and glucose uptake via insulin-dependent and insulin-independent mechanisms (), thus optimizing insulin actions on both glucose oxidation and storage.
In addition, we compared plasma amino acids between HFD and HFD-wheel running mice and found lower levels of valine, serine, phenylalanine, and tryptophan in the HFD-wheel running group. This result was broadly consistent with the treadmill experiment. We also demonstrated that, unlike the un-exercised HFD group, exercise resulted in a differential transcriptional response with GO terms associated with the regulation of cell immunity and skeletal muscle plasticity. Upon successfully implementing transcript–metabolite correlation analysis, we were able to further demonstrate the predominant effect of exercise on the amino acid metabolism, insulin resistance, fatty acid biosynthesis, and muscle morphogenesis.
In conclusion, in this study, metabolic and transcriptional profiling revealed that exercise training reverses HFD-induced changes in metabolism and transcription. Several amino acid metabolism pathways and muscle development pathways impacted by exercise training are implicated with important roles in the improvement of obesity and T2DM.
Materials and methods
Animal models
Wild-type mice on the C57BL/6 J background were obtained from GemPharmatech Co., Ltd (Nanjing, China). All animal studies were performed according to procedures approved by the University Committee on Use and Care of Animals at the Zhejiang University. Mice were housed in 12-h/12-h light/dark cycles at an ambient temperature of 23°C. All animal experiments used age-matched male mice.
To generate diet-induced obese mouse models, 12-week-old C57BL/6 J male mice were fed either a chow diet (10 kcal% fat, 70 kcal% carbohydrate, and 20 kcal% protein; Jiangsu Xietong Pharmaceutical Bio-engineering Co., Ltd, 1010088) or a HFD (60 kcal% fat, 20 kcal% carbohydrate, and 20 kcal% protein; Research Diets, D12492) for 16 weeks. For exercise intervention, mice fed with HFD for 16 weeks were subjected to treadmill running or wheel running for 8 weeks.
Metabolic measurements
Body fat and lean mass were measured using an NMR analyzer (Niumag, QMN06-090H). Fasting blood glucose levels were determined using a Bayer Contour blood glucometer by tail-snip blood sampling after overnight fasting (∼16 h).
GTT and ITT
GTT and ITT were performed as previously described (). For GTT, mice were fasted overnight (∼16 h) and intraperitoneally (i.p.) injected with glucose saline solution (1.2 g/kg body weight, glucose concentrations adjusted accordingly to obtain an equal injection volume for each mouse). Blood glucose levels were measured by tail-snip blood sampling pre-injection and 15, 30, 60, and 120 min post-injection. For ITT, mice were fasted for 4 h and i.p. injected with insulin saline solution (1 unit/kg body weight, insulin concentrations adjusted accordingly to equalize injection volume for each mouse). Blood glucose levels were measured by tail-snip blood sampling pre-injection and 15, 30, 60, and 120 min post-injection.
Metabolomic analysis
For treadmill running mice, plasma samples (50 μl) and muscle samples (50 mg) from the Chow, HFD, and HFD-exercise mice were subjected to identification and quantification using a Q300 Metabolite Array. Raw data files were analyzed by QuanMET (V2.0; Metabo-Profile) to identify and quantify metabolites.
For wheel running mice, plasma samples (50 μl) from HFD and HFD-exercise mice were subjected to identification and quantification using a Q300 Metabolite Array as previously described (; ).
RNA-seq and bioinformatics
For RNA-seq analysis, total muscle RNA samples were sent for library preparation and sequencing by the BGI group (Wuhan, China). In brief, mRNAs were enriched from total RNA and fragmented. These were then used for reverse transcription and second-strand cDNA synthesis. The cDNAs were tailed with adenine and ligated with adaptors for polymerase chain reaction (PCR) amplification and sequencing. PCR-amplified cDNA libraries were subjected to paired-end sequencing on a BGISEQ-500 system. Data were processed following the standard BGI mRNA analysis pipeline. Expression levels of mRNA were computed as fragments per kilobase of transcript per million mapped reads for statistical analysis, as performed using the Deseq2 (V.1.20.0) package (). Pathway grouping and enrichment studies, as well as GO analysis, were performed using clusterProfiler (V3.12.0). Pathway visualization was conducted using pathview (V1.26.0) (; ).
Statistical analysis
Statistical analyses were carried out using GraphPad Prism 8. Statistical differences were evaluated using two-tailed unpaired Student's t-test for comparisons between two groups and using analysis of variance (ANOVA) followed by appropriate post hoc analysis for comparisons of more than two groups. For ITT and GTT, two-way ANOVA with multiple comparisons test was used. The area of the curve (AOC) was calculated by subtracting the starting glucose value from the value at each time point () for each mouse, and statistical difference between two groups was evaluated using two-tailed unpaired Student's t-test. A P-value < 0.05 (*P < 0.05, **P < 0.01, ***P < 0.001, #P < 0.05, ##P < 0.01, and ###P < 0.001) was considered statistically significant. Statistical methods and corresponding P-values for data are included in the figure legends. No statistical method was used to predetermine the sample size. The experiments were not randomized, and the investigators were not blinded to allocation during the experiments and outcome assessment.
Acknowledgements
The authors thank the Meng Lab members for their helpful discussion and technical support to this study. We also thank the Core Facilities of Zhejiang University School of Medicine for technical support.
References
- Amin A., Frampton J., Liu Z., et al (2021). Differential effects of L- and D-phenylalanine on pancreatic and gastrointestinal hormone release in humans: a randomized crossover study. Diabetes Obes. Metab. 23, 147–157.
- Bassel-Duby R., Olson E.N. (2006). Signaling pathways in skeletal muscle remodeling. Annu. Rev. Biochem.75, 19–37.
- Bennetsen S.L., Feineis C.S., Legaard G.E., et al (2020). The impact of physical activity on glycemic variability assessed by continuous glucose monitoring in patients with type 2 diabetes mellitus: a systematic review. Front. Endocrinol.11, 486.
- Bergouignan A., Latouche C., Heywood S., et al (2016). Frequent interruptions of sedentary time modulates contraction- and insulin-stimulated glucose uptake pathways in muscle: ancillary analysis from randomized clinical trials. Sci. Rep.6, 32044.
- Bortoluzzi S., Scannapieco P., Cestaro A., et al (2006). Computational reconstruction of the human skeletal muscle secretome. Proteins62, 776–792.
- Camera D.M., Smiles W.J., Hawley J.A. (2016). Exercise-induced skeletal muscle signaling pathways and human athletic performance. Free Radic. Biol. Med.98, 131–143.
- Catoire M., Mensink M., Boekschoten M.V., et al (2012). Pronounced effects of acute endurance exercise on gene expression in resting and exercising human skeletal muscle. PLoS One7, e51066.
- Chen T., Ni Y., Ma X., et al (2016). Branched-chain and aromatic amino acid profiles and diabetes risk in Chinese populations. Sci. Rep.6, 20594.
- Cheng F., Dun Y., Cheng J., et al (2022). Exercise activates autophagy and regulates endoplasmic reticulum stress in muscle of high-fat diet mice to alleviate insulin resistance. Biochem. Biophys. Res. Commun.601, 45–51.
- Choi S., Liu X., Li P., et al (2005). Transcriptional profiling in mouse skeletal muscle following a single bout of voluntary running: evidence of increased cell proliferation. J. Appl. Physiol.99, 2406–2415.
- DeFronzo R.A., Tripathy D. (2009). Skeletal muscle insulin resistance is the primary defect in type 2 diabetes. Diabetes Care32, S157–S163.
- Duft R.G., Castro A., Bonfante I.L.P., et al (2020). Altered metabolomic profiling of overweight and obese adolescents after combined training is associated with reduced insulin resistance. Sci. Rep.10, 16880.
- Egan B., Zierath J.R. (2013). Exercise metabolism and the molecular regulation of skeletal muscle adaptation. Cell Metab.17, 162–184.
- Esefeld K., Kress S., Behrens M., et al (2021). Diabetes, sports and exercise. Exp. Clin. Endocrinol. Diabetes129, S52–S59.
- Fan W.W., Evans R.M. (2017). Exercise mimetics: impact on health and performance. Cell Metab.25, 242–247.
- Garcia L.A., Zapata-Bustos R., Day S.E., et al (2022). Can exercise training alter human skeletal muscle DNA methylation?Metabolites12, 222.
- Glynn E.L., Piner L.W., Huffman K.M., et al (2015). Impact of combined resistance and aerobic exercise training on branched-chain amino acid turnover, glycine metabolism and insulin sensitivity in overweight humans. Diabetologia58, 2324–2335.
- Gu H., Du J., Carnevale Neto F., et al (2015). Metabolomics method to comprehensively analyze amino acids in different domains. Analyst140, 2726–2734.
- Gunther S.H., Khoo C.M., Sim X., et al (2020). Diet, physical activity and adiposity as determinants of circulating amino acid levels in a multiethnic Asian population. Nutrients12, 2603.
- Howlett K.F., Andrikopoulos S., Proietto J., et al (2013). Exercise-induced muscle glucose uptake in mice with graded, muscle-specific GLUT-4 deletion. Physiol. Rep.1, e00065.
- Hussey S.E., McGee S.L., Garnham A., et al (2012). Exercise increases skeletal muscle GLUT4 gene expression in patients with type 2 diabetes. Diabetes Obes. Metab.14, 768–771.
- Ke C., Pan C.W., Zhang Y., et al (2019). Metabolomics facilitates the discovery of metabolic biomarkers and pathways for ischemic stroke: a systematic review. Metabolomics15, 152.
- Keller P., Vollaard N.B., Gustafsson T., et al (2011). A transcriptional map of the impact of endurance exercise training on skeletal muscle phenotype. J. Appl. Physiol.110, 46–59.
- Khan M.A.B., Hashim M.J., King J.K., et al (2020). Epidemiology of type 2 diabetes—global burden of disease and forecasted trends. J. Epidemiol. Glob. Health10, 107–111.
- Kujala U.M., Peltonen M., Laine M.K., et al (2016). Branched-chain amino acid levels are related with surrogates of disturbed lipid metabolism among older men. Front. Med.3, 57.
- Lang P., Hasselwander S., Li H., et al (2019). Effects of different diets used in diet-induced obesity models on insulin resistance and vascular dysfunction in C57BL/6 mice. Sci. Rep.9, 19556.
- Love M.I., Huber W., Anders S. (2014). Moderated estimation of fold change and dispersion for RNA-seq data with DESeq2. Genome Biol.15, 550.
- Luo W., Brouwer C. (2013). Pathview: an R/Bioconductor package for pathway-based data integration and visualization. Bioinformatics29, 1830–1831.
- Mahoney D.J., Parise G., Melov S., et al (2005). Analysis of global mRNA expression in human skeletal muscle during recovery from endurance exercise. FASEB J.19, 1498–1500.
- Mattusch F., Dufaux B., Heine O., et al (2000). Reduction of the plasma concentration of C-reactive protein following nine months of endurance training. Int. J. Sports Med.21, 21–24.
- Mazur-Biały A., Bilski J., Pocheć E., et al (2017). New insight into the direct anti-inflammatory activity of a myokine irisin against proinflammatory activation of adipocytes: implication for exercise in obesity. J. Physiol. Pharmacol.68, 243–251.
- Meng Z.-X., Gong J., Chen Z., et al (2017). Glucose sensing by skeletal myocytes couples nutrient signaling to systemic homeostasis. Mol. Cell66, 332–344.e4.
- Meuffels F.M., Isenmann E., Strube M., et al (2022). Exercise interventions combined with dietary supplements in type 2 diabetes mellitus patients—a systematic review of relevant health outcomes. Front. Nutr.9, 817724.
- Mu W.C., VanHoosier E., Elks C.M., et al (2018). Long-term effects of dietary protein and branched-chain amino acids on metabolism and inflammation in mice. Nutrients10, 918.
- Newgard C.B., An J., Bain J.R., et al (2009). A branched-chain amino acid-related metabolic signature that differentiates obese and lean humans and contributes to insulin resistance. Cell Metab.9, 311–326.
- Nogiec C., Burkart A., Dreyfuss J.M., et al (2015). Metabolic modeling of muscle metabolism identifies key reactions linked to insulin resistance phenotypes. Mol. Metab.4, 151–163.
- Pedersen B.K., Febbraio M.A. (2012). Muscles, exercise and obesity: skeletal muscle as a secretory organ. Nat. Rev. Endocrinol.8, 457–465.
- Pilegaard H., Ordway G.A., Saltin B., et al (2000). Transcriptional regulation of gene expression in human skeletal muscle during recovery from exercise. Am. J. Physiol. Endocrinol. Metab.279, E806–E814.
- Richter E.A., Hargreaves M. (2013). Exercise, GLUT4, and skeletal muscle glucose uptake. Physiol. Rev.93, 993–1017.
- Saunders B., Elliott-Sale K., Artioli G.G., et al (2017). Beta-alanine supplementation to improve exercise capacity and performance: a systematic review and meta-analysis. Br. J. Sports Med. 51, 658–669.
- Schnyder S., Handschin C. (2015). Skeletal muscle as an endocrine organ: pGC-1α, myokines and exercise. Bone80, 115–125.
- Scott L.J., Erdos M.R., Huyghe J.R., et al (2016). The genetic regulatory signature of type 2 diabetes in human skeletal muscle. Nat. Commun.7, 11764.
- Stanford K.I., Goodyear L.J. (2014). Exercise and type 2 diabetes: molecular mechanisms regulating glucose uptake in skeletal muscle. Adv. Physiol. Educ.38, 308–314.
- Stewart L.K., Flynn M.G., Campbell W.W., et al (2007). The influence of exercise training on inflammatory cytokines and C-reactive protein. Med. Sci. Sports Exercise.39, 1714–1719.
- Sylow L., Tokarz V.L., Richter E.A., et al (2021). The many actions of insulin in skeletal muscle, the paramount tissue determining glycemia. Cell Metab.33, 758–780.
- Thyfault J.P. (2008). Setting the stage: possible mechanisms by which acute contraction restores insulin sensitivity in muscle. Am. J. Physiol. Regul. Integr. Comp. Physiol.294, R1103–R1110.
- Thyfault J.P., Bergouignan A. (2020). Exercise and metabolic health: beyond skeletal muscle. Diabetologia63, 1464–1474.
- Ueda Y., Iwakura H., Bando M., et al (2018). Differential role of GPR142 in tryptophan-mediated enhancement of insulin secretion in obese and lean mice. PLoS One13, e0198762.
- Varemo L., Scheele C., Broholm C., et al (2015). Proteome- and transcriptome-driven reconstruction of the human myocyte metabolic network and its use for identification of markers for diabetes. Cell Rep.11, 921–933.
- Virtue S., Vidal-Puig A. (2021). GTTs and ITTs in mice: simple tests, complex answers. Nat. Metab.3, 883–886.
- Wang T.J., Larson M.G., Vasan R.S., et al (2011). Metabolite profiles and the risk of developing diabetes. Nat. Med.17, 448–453.
- Xu M., Kitaura Y., Ishikawa T., et al (2017). Endurance performance and energy metabolism during exercise in mice with a muscle-specific defect in the control of branched-chain amino acid catabolism. PLoS One12, e0180989.
- Yang Z., Huang G., Zhou P., et al (2022). Exercise ameliorates high-fat diet-induced insulin resistance accompanied by changes in protein levels of hepatic ATF3-related signaling in rats. Physiol. Behav.249, 113766.
- Yoon J.H., Yea K., Kim J., et al (2009). Comparative proteomic analysis of the insulin-induced L6 myotube secretome. Proteomics9, 51–60.
- Yoon M.S. (2016). The emerging role of branched-chain amino acids in insulin resistance and metabolism. Nutrients8, 405.
- Yu G., Wang L.-G., Han Y., et al (2012). Cluster profiler: an R package for comparing biological themes among gene clusters. OMICS16, 284–287.
- Zhang F., Zhao S., Yan W., et al (2016). Branched chain amino acids cause liver injury in obese/diabetic mice by promoting adipocyte lipolysis and inhibiting hepatic autophagy. EBioMedicine13, 157–167.
- Zhang H.S., Xiang L., Huo M.Y., et al (2022). Branched-chain amino acid supplementation impairs insulin sensitivity and promotes lipogenesis during exercise in diet-induced obese mice. Obesity30, 1205–1218.
- Zhang J., Wen X., Li Y., et al (2021). Diagnostic approach to thyroid cancer based on amino acid metabolomics in saliva by ultra-performance liquid chromatography with high resolution mass spectrometry. Talanta235, 122729.