Introduction
In mammals, the placenta ensures normal growth and development of the fetus and its dysfunction can cause spontaneous preterm births and abortions (). As the progenitor cells of the placenta, the trophectoderm (TE) can induce a primary decidual response and establish an efficient interaction between the conceptus and the uterus for implantation, thus ensuring a smooth pregnancy (; ). Primary trophoblast cells cannot be cultured long term in vitro, and thus it is difficult to investigate trophoblast expansion and differentiation in the primary trophoblast (). Trophoblast stem cells (TSCs) are derived from primary trophoblast cells and have the ability to differentiate in vitro to form all placental trophoblast cell types (). Therefore, TSCs are considered a good cell model for revealing molecular mechanisms of cell fate determination and lineage commitment during placental development, which provides a theoretical basis to resolve placental developmental dysfunction and ensure mammalian reproduction.
Kruppel-like factor 5 (Klf5), also known as Bteb2, contains three highly conserved and tandem zinc finger domains at its C-terminus () and recognizes CG-rich DNA-binding motifs. It has recently attracted attention due to its important roles in development (), differentiation (), and oncogenesis (; ). Klf5 is gradually expressed from the 2-cell stage to the blastocyst stage during early embryonic development and particularly shows stronger expression in TE than in the inner cell mass (ICM) (), emphasizing a more important role of Klf5 in maintaining trophoblast development. Klf5-knockout embryos die 6.5–8.5 days after fertilization due to defective extra-embryonic tissue development. However, reconstructed embryos that are aggregates of normal tetraploid embryos with Klf5-knockout embryonic stem cells (ESCs) have been shown to be capable of successful implantation (), suggesting that Klf5 preferentially affects trophoblast cell fate. Previous reports revealed that loss of Klf5 impaired TE specification, which was accompanied by the downregulation of trophoblast development-related genes, even with artificially supplemented fibroblast growth factor 4 (Fgf4), a cytokine used to activate the Mek/Erk signaling pathways and stimulate the expression of TE-specific genes (; ). However, the molecular mechanisms of Klf5 in regulating trophoblast cell development remain unclear.
Transcriptional network studies have identified that several transcription factors, including Tead4, Sox2, Esrrb, and Cdx2, play important regulatory roles in the development and self-renewal of TSCs at the transcriptional level (; ; ; ; ). Klf5 was reported to be enriched at promoter regions of TSC-specific genes and have a close correlation with Cdx2 (), indicating a possible role for Klf5 in directly activating TSC core transcription factors. A previous report demonstrated a regulatory mechanism involving Klf5 for self-renewal and homeostasis in intestinal stem cells by regulating histone modifications and chromatin accessibility of a distinct set of target genes (). However, how Klf5 regulates downstream genes for maintaining TSC pluripotency remains an open question.
In this study, we demonstrated that Klf5 served as a pivotal regulator involved in the transcriptional network of TSCs and played a critical role in TSC self-renewal and differentiation. By identifying Klf5 target TSC-specific genes and their chromatin accessibility patterns, we showed that Klf5 maintained an open chromatin structure of its target genes associated with TSC pluripotency. Moreover, Klf5-mediated occupancy of H3 lysine 27 acetylation (H3K27ac) at the promoter regions of TSC pluripotency genes is critical for TE development.
Results
Klf5 is critical for the maintenance of undifferentiated TSCs
To define the role of Klf5 in TSC differentiation, we performed a TSC differentiation assay with deprivation of Fgf4 () and collected samples from different time points. Quantitative polymerase chain reaction (qPCR) and western blotting results showed that both the Klf5 mRNA and protein levels were significantly decreased during TSC differentiation (Figure 1A; Supplementary Figure S1A). The expression pattern of Klf5 was similar to that of Cdx2 during TSC differentiation (Figure 1A and B). In addition, immunofluorescence staining results showed that Klf5 colocalized with Cdx2, Eomes, and Tead4 in undifferentiated cells (Supplementary Figure S1B), which suggested that high expression of Klf5 might be related to an undifferentiated TSC state. To gain insight into the possible roles of Klf5 in TSC pluripotency regulation, we employed a lentiviral knockdown system to target Klf5, whereas the scrambled shRNA was set as a control. Klf5 silencing resulted in the loss of tight TSC morphology (Figure 1C). In addition to shRNAs, siRNAs were selected to repeat the experiment to reduce off-target effects. In all cases, knockdown of Klf5 significantly reduced the mRNA levels of key TSC pluripotency genes (Figure 1D; Supplementary Figure S1C and D) and increased the mRNA levels of trophoblast developmental genes (Supplementary Figure S1E). Western blotting and immunostaining results showed that Klf5 silencing reduced the protein levels of TSC-specific genes (Figure 1E and F), suggesting that Klf5 plays a major role in self-renewal. To identify the functional domain of Klf5 in TSCs, an effective siRNA was designed to silence different domains of Klf5. Then, we constructed expression plasmids Klf5-ZF/DBD and Klf5-ΔZF/DBD. Klf5-ZF/DBD contains the zinc fingers and DNA-binding domain of Klf5, whereas Klf5-ΔZF/DBD lacks the zinc fingers and DNA-binding domain of Klf5 (Supplementary Figure S2A). Ectopic expression of Klf5-ZF/DBD, rather than Klf5-ΔZF/DBD, in Klf5-knockdown TSCs significantly rescued the expression levels of TSC-specific genes (Supplementary Figure S2B and C), suggesting that Klf5-ZF/DBD played a vital role in maintaining TSCs in an undifferentiated state. Hematoxylin–eosin (H&E) staining (Figure 1G) showed that Klf5-knockdown TSCs formed a smaller hemorrhagic lesion than wild-type TSCs, suggesting decreased invasion and differentiation of Klf5-knockdown TSCs in vivo. Furthermore, Klf5 knockdown reduced the proliferation rate of TSCs, toward that of TSCs under spontaneous differentiation (Figure 1H). Collectively, these results indicate that Klf5 acts as a critical regulator involved in TSC pluripotency maintenance.
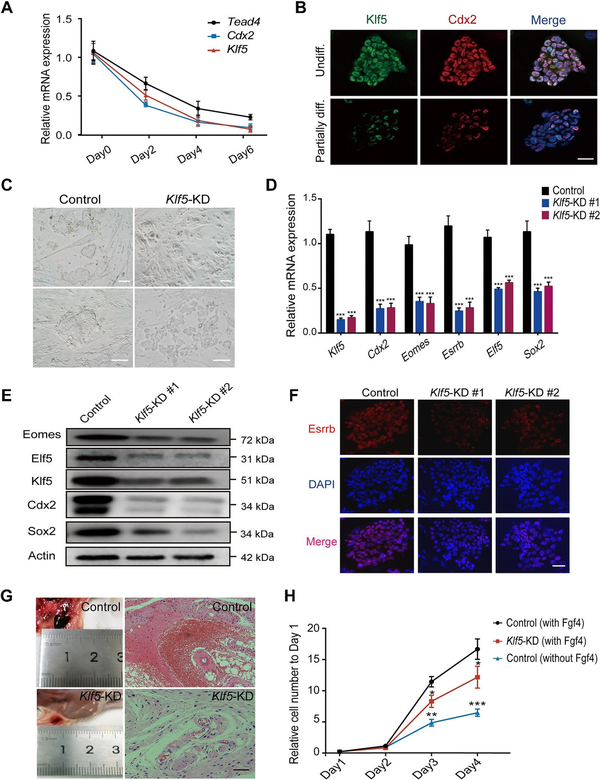
Figure 1
Klf5 is required for maintaining the stem cell state of TSCs. (A) The mRNA expression levels of Tead4, Cdx2, and Klf5 during TSC differentiation were determined by qPCR and normalized to that on Day 0. (B) Immunofluorescence staining of Klf5 and Cdx2 in TSCs during differentiation. Nuclei were stained with DAPI (blue). Top, an undifferentiated TSC colony (Undiff.). Bottom, a partially differentiated colony (Partially diff.). Scale bar, 50 μm. (C) Representative bright-field images of control and Klf5-knockdown TSCs. Scale bar, 100 μm. (D) qPCR analysis of TSC-specific genes in control and Klf5-knockdown TSCs. (E) Western blot analysis of TSC-specific proteins in control and Klf5-knockdown TSCs. (F) Immunofluorescence staining of Esrrb in control and Klf5-knockdown TSCs. Scale bar, 30 μm. (G) H&E staining of hemorrhagic lesions in immunodeficient nude mice 7 days after injection of control or Klf5-knockdown TSCs. Scale bar, 100 μm. (H) The proliferative capacity of TSCs cultured under different conditions. Cell numbers were counted at the indicated time points and normalized to that on Day 1. Data in A, D, and H are presented as mean ± SD (n = 3). *P < 0.05, **P < 0.01, and ***P < 0.001 by Student's t-test for comparison.
Klf5 directly targets and participates in the TSC core regulatory network
To understand how Klf5 regulates TSC pluripotency and maintains TSCs in an undifferentiated state, we carried out RNA sequencing (RNA-seq) in Klf5-knockdown and control TSCs. Analysis of differentially expressed genes (DEGs) revealed that 999 genes were downregulated (fold change <2; false discovery rate [FDR] <0.05), while only 164 genes were upregulated (fold change >2; FDR <0.05) after Klf5 knockdown (Figure 2A). Gene ontology (GO) analysis further showed that downregulated genes were enriched in cell proliferation and cell fate commitment, whereas upregulated genes were enriched in placental development (Figure 2B).
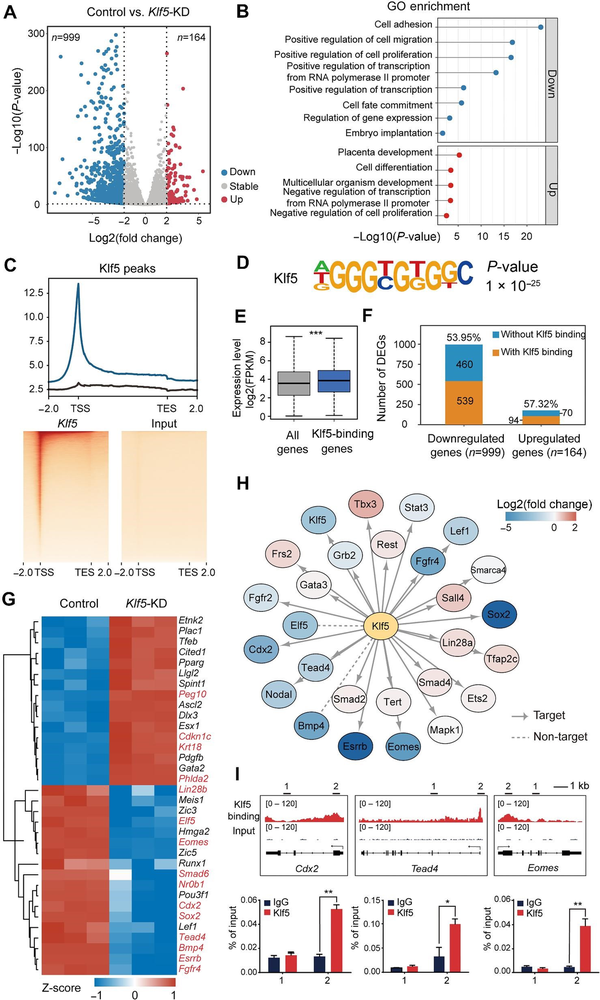
Figure 2
Klf5 acts as an activating transcription factor in the TSC core regulatory network. (A) Volcano map of DEGs after Klf5 knockdown. Upregulated genes are shown in red, and downregulated genes are shown in blue. (B) GO analysis of the upregulated (red bars) and downregulated (blue bars) genes after Klf5 knockdown. (C) Top, genomic distribution analysis for Klf5 ChIP-seq peaks. Bottom, heatmaps displaying the enrichment of Klf5-binding sites. Rabbit IgG was used as a control. TES, transcription termination site.(D) Motif analysis of Klf5 peaks in TSCs. (E) Expression levels of Klf5-binding genes in TSCs. (F) Percentages of Klf5-binding genes in DEGs upon Klf5 knockdown. (G) Heatmap showing the changes in the expression levels of Klf5-binding genes. (H) Protein–protein interaction network analysis between Klf5 and previously reported TSC-specific markers. (I) IGV and ChIP–qPCR analysis of the enrichment of Klf5 at the Cdx2, Tead4, and Eomes loci.
To further investigate how Klf5 regulates TSC pluripotency at the chromatin level, we performed chromatin immunoprecipitation sequencing (ChIP-seq) in undifferentiated TSCs with two biological replicates to identify genome-wide binding sites. A total of 27733 common peaks were identified in two biological replicates, and these peaks appeared in promoter regions, gene bodies, and intergenic regions, with 55.78% distributed in the promoter regions (Supplementary Figure S3A). Importantly, these Klf5-binding sites were particularly enriched within ± 1 kb of the transcription start site (TSS) (Figure 2C). Based on motif analysis, the main preference of Klf5-binding sites on the genome was 5′-AGGGTGTGGC-3′ (Figure 2D). We then analyzed Klf5 target genes, whose expression levels were significantly higher than the average expression levels of all genes (Figure 2E), and found that more than half of the downregulated genes upon Klf5 knockdown were directly regulated by Klf5 (Figure 2F). Interestingly, an interactome analysis of the top 100 DEGs demonstrated that the downregulated genes displayed closer interactions than the upregulated genes (Supplementary Figure S3C and D), suggesting that Klf5 target genes mainly functioned in repressing TSC differentiation. Consistently, the expression levels of TSC-specific genes, such as Tead4, Cdx2, Sox2, Eomes, and Elf5, was reduced in Klf5-knockdown TSCs (Figure 2G). In the same way, luciferase activity results showed that Klf5 functioned as an activating transcription factor directly regulating Tead4, Cdx2, and Eomes in undifferentiated TSCs (Supplementary Figure S4A and B). Furthermore, the protein–protein interaction analysis between Klf5 and previously reported TSC-specific markers () showed that Klf5 was closely related to Cdx2, Tead4, and Eomes (Figure 2H). The interaction of Klf5 with these key proteins was also confirmed by co-immunoprecipitation (co-IP) (Supplementary Figure S4C). Integrative Genomic Viewer (IGV) and ChIP–qPCR analyses revealed that Klf5 was located in the promoter regions of these genes (Figure 2I), which further confirmed that Klf5 directly targeted and activated TSC-specific marker genes. Collectively, our results indicate that Klf5, as an activating transcription factor, occupies proximal promoter regions of TSC-specific genes and participates in the TSC core regulatory network.
Klf5 maintains an open chromatin structure of its target TSC pluripotency genes
We next analyzed putative TSC pluripotency genes that were downregulated during TSC differentiation, based on published RNA-seq data (). Strikingly, >80% of putative TSC pluripotency genes were occupied by Klf5 at their promoter regions, and these 495 genes were defined as putative Klf5 target TSC pluripotency genes (Figure 3A). GO analysis showed that the putative target genes were enriched in TSC pluripotency regulation, such as multicellular organism development, sequence-specific DNA binding, chromatin accessibility, and cell differentiation (Figure 3B). Notably, these putative Klf5 target TSC pluripotency genes were highly expressed in TSCs, strongly suggesting that Klf5 maintained the undifferentiated TSC state upon activating the expression of these genes (Figure 3C). Therefore, these results indicate that Klf5 maintains undifferentiated TSCs by directly regulating a subset of putative target genes.
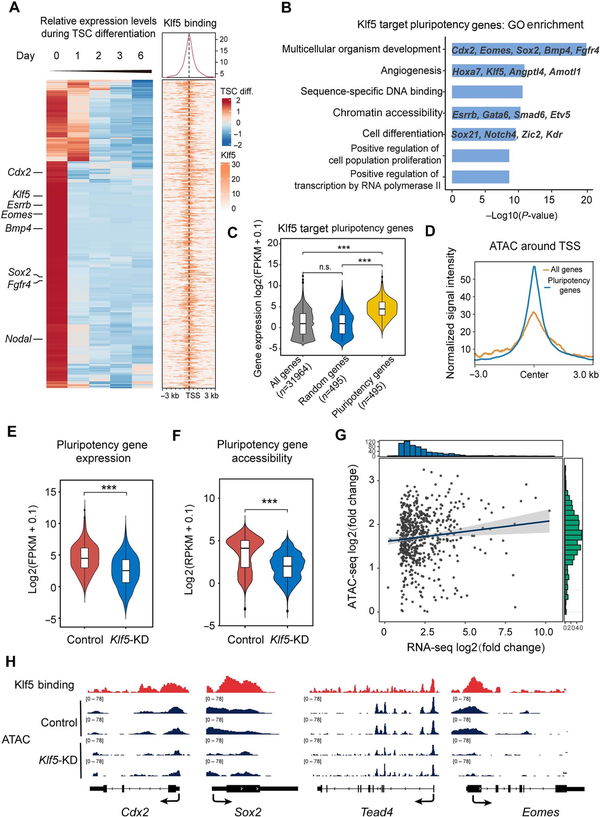
Figure 3
Klf5 maintains an open chromatin structure of its target genes. (A) Heatmaps displaying the enrichment of Klf5 at the indicated genes that are downregulated during TSC differentiation based on published RNA-seq data. (B) GO analysis of Klf5 target genes. (C) Box plot showing the expression levels of Klf5 target genes, all genes, and random genes. The number of genes analyzed is indicated at the bottom. (D) Average signal intensity of ATAC-seq reads around the TSS of Klf5 target genes. (E and F) Average gene expression (E) and genome accessibility (F) levels of Klf5 target genes in control and Klf5-knockdown TSCs. (G) Variance correlation of Klf5 target genes based on ATAC-seq and RNA-seq data. The horizontal coordinate represents log2(fold change) of gene expression. The vertical coordinate represents their differential accessibility. All analyzed genes were adjusted by multiple testing correction (P ≤ 0.05). (H) The IGV browser shows ATAC-seq of the promoter regions of Klf5 target genes (Cdx2, Sox2, Tead4, and Eomes) in control and Klf5-knockdown TSCs from two biologically independent experiments. P-values were calculated by Mann–Whitney–Wilcoxon two-sided tests in C, E, and F.
Chromatin accessibility is closely related to transcriptional output and thus plays a fundamental role in genome activation and cell differentiation (; ). Cell type-specific chromatin structures that facilitate gene expression are essential to the commitment of different lineages (; ). To determine whether Klf5 maintained an open chromatin structure of Klf5 target TSC pluripotency genes in undifferentiated TSCs, we carried out transposase accessible chromatin sequencing (ATAC-seq) in Klf5-knockdown and control TSCs. As expected, Klf5 target TSC pluripotency genes had an open chromatin structure in undifferentiated TSCs (Figure 3D). Importantly, both chromatin accessibility and transcription levels of Klf5 target TSC pluripotency genes were significantly reduced upon knockdown of Klf5 (Figure 3E and F). The changes in transcriptional output correlated strongly with the changes in promoter accessibility (Figure 3G), which further confirmed that decreased chromatin accessibility due to knockdown of Klf5 was responsible for cell differentiation. Moreover, we demonstrated that several Klf5 target TSC pluripotency genes showed loss of chromatin accessibility in the promoter regions, in line with reduced transcriptional levels compared with the control (Figure 3H). These results suggest that Klf5 regulates its target TSC pluripotency genes by maintaining their open chromatin structure.
Klf5 is required for H3K27 acetylation of its target TSC pluripotency genes
Then, we investigated how Klf5 maintains an open chromatin structure of these bound loci. As previously reported (), H3K27 acetylation neutralizes the positive charge of histone side chains to affect histone and DNA binding, and the regulation of gene expression mainly depends on chromatin stability. Accordantly, we observed globally decreased H3K27ac levels in Klf5-knockdown TSCs (Supplementary Figure 5A). Then, we carried out ChIP-seq using an H3K27ac-specific antibody to identify altered genomic loci between Klf5-knockdown and control TSCs. Notably, >69% of Klf5-binding regions overlapped with H3K27ac (Supplementary Figure 5B). After normalization of H3K27ac signals with the published HRT Atlas v1.0 database (), we found that H3K27ac signals only slightly decreased in Klf5-binding sites (Supplementary Figure 5C). Nevertheless, H3K27ac was closely related to high chromatin accessibility at the promoter regions of Klf5 target TSC pluripotency genes (Figure 4A–C), and knockdown of Klf5 reduced H3K27ac occupancy at the promoter regions of TSC pluripotency genes (Supplementary Figure 5D). These results suggest that H3K27ac plays a crucial role in regulating Klf5 target TSC pluripotency genes.
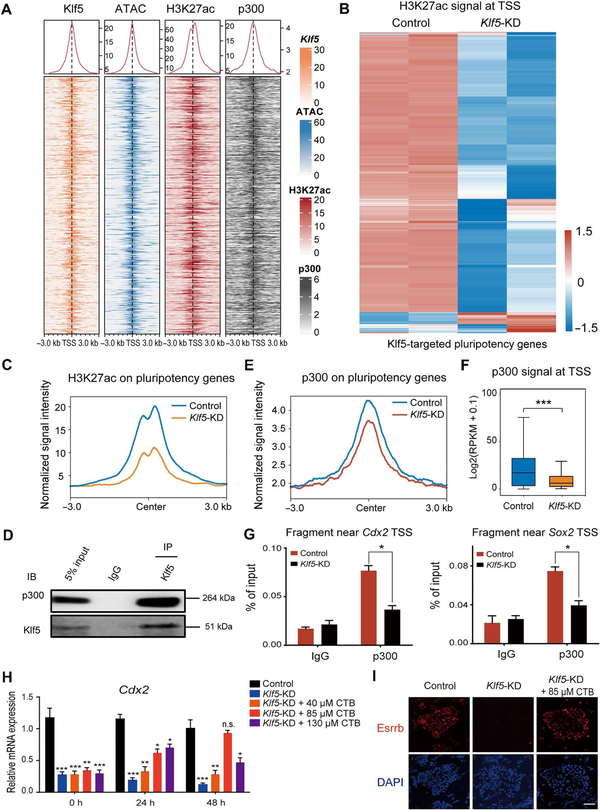
Figure 4
Klf5 maintains TSC-specific gene expression by recruiting p300, the acetylase of H3K27ac. (A) Heatmaps showing high chromatin accessibility and the enrichment of H3K27ac and p300 around the promoter regions of Klf5 target genes. (B) Heatmap demonstrating the change in H3K27ac signals at the TSS of Klf5 target genes in Klf5-knockdown TSCs. (C) Normalized signal intensity of H3K27ac around the promoter regions of Klf5 target genes in control and Klf5-knockdown TSCs. (D) Klf5 interactswith p300. Endogenous Klf5 was immunoprecipitated and analyzed with an antibody against p300. IgG and 5% input were used as controls. (E) Normalized signal intensity of p300 around the promoter regions of Klf5 target genes in control and Klf5-knockdown TSCs. (F) Box plot showing p300 signals at the TSS of Klf5 target genes in control and Klf5-knockdown TSCs. P-values were calculated by Mann–Whitney–Wilcoxon two-sided tests. (G) ChIP–qPCR showing p300 occupancy near the TSS of Cdx2 and Sox2. Data are presented as mean ± SD (n = 3). (H) qPCR analysis of Cdx2 in Klf5-knockdown TSCs treated with CTB at different concentrations for 0, 24, and 48 h. (I) Immunofluorescence staining of Esrrb in control and Klf5-knockdown TSCs treated with 85 μM CTB for 0 and 48 h. Scale bar, 30 μm. n.s., P > 0.05, not significant. *P < 0.05, **P < 0.01, and ***P < 0.001 by Student's t-test for comparison.
To determine whether Klf5 interacted with any H3K27 acetyltransferase complex, we performed unbiased protein identification co‐IP and mass spectroscopy using undifferentiated TSCs. We found that p300, a primary acetyltransferase that regulates histone acetylation (), interacted with Klf5 at the protein level (Figure 4D; Supplementary Table S2). Moreover, co-IP results showed that Klf5-ZF/DBD, rather than Klf5-ΔZF/DBD, interacted with p300 in undifferentiated TSCs (Supplementary Figure S6A). At the global chromatin level, p300 and H3K27ac mostly overlapped at the promoter regions of Klf5 target TSC pluripotency genes (Figure 4A). ChIP-seq for p300 in control and Klf5-knockdown TSCs showed that the occupancy of p300 at the Klf5-targeted regions decreased in Klf5-knockdown TSCs (Figure 4E and F), verifying that Klf5 recruited p300 to maintain deacetylation at these sites. Consistently, ChIP–qPCR and IGV analysis showed significantly decreased p300 signals at the promoter regions of Sox2, Cdx2, Fgfr4, and Esrrb (Figure 4G; Supplementary Figure 6B and C). Given that cholera toxin B (CTB) subunit treatment increased the mRNA and protein expression levels of p300 (; ), the expression levels of self-renewal markers (Cdx2, Eomes, and Esrrb) and trophoblast differentiation-associated genes (Krt18 and Gcm1) were rescued in Klf5-knockdown TSCs treated with 85 μM CTB for 48 h (Figure 4H and I; Supplementary Figure S6D). Moreover, ChIP–qPCR showed that the enrichment of H3K27ac at the promoter regions of well-characterized TSC-specific genes (Cdx2, Eomes, and Sox2) were rescued in Klf5-knockdown TSCs treated with 85 μM CTB for 48 h (Supplementary Figure S6E). Overall, these findings confirm that Klf5 can recruit p300 to the promoter regions of its target genes and maintain their open chromatin structure by H3K27ac.
Klf5-mediated acetylation of H3K27 is critical for TE development
To determine whether Klf5-mediated H3K27ac also affected pre-implantation development in vivo, we constructed a CRISPR/Cas9 system with sgRNAs to target the coding region of Klf5. After injection of sgRNA-Klf5 into the cytoplasm of zygotes, immunostaining results showed significantly reduced Klf5 expression levels in the injected embryos (Figure 5A). The Cdx2 protein level was also significantly reduced compared to the control (Figure 5B), which was in line with previous reports (; ). As expected, H3K27ac was downregulated and its acetylase p300 was not significantly changed in Klf5-knockout embryos (Figure 5C and D). Furthermore, Duolink® proximal ligation assay (PLA) staining revealed that Klf5 colocalized with p300 in E3.5 embryos (Figure 5E). To further investigate the impact of Klf5 deficiency on TSC derivation, wild-type and Klf5-knockout blastocysts were cultured in vitro to obtain TE-derived outgrowths. The results showed that the size of Klf5-knockout outgrowths was smaller than that of the wild-type controls (Figure 5F and G). Moreover, Klf5-knockout outgrowths impaired the expression of TE-specific genes (Figure 5H and I). Thus, Klf5 is critical for mouse TE development in vitro and affects the establishment of pluripotency.
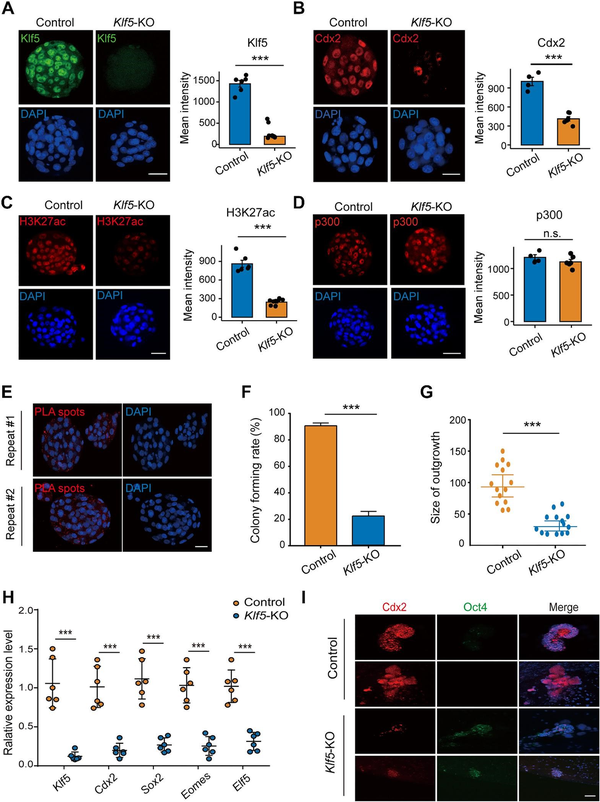
Figure 5
Klf5 is critical for mouse TE development and the establishment of TSCs. (A–D) Immunofluorescence staining of Klf5 (A), Cdx2 (B), H3K27ac (C), and p300 (D) in control and Klf5-knockout E3.5 embryos. The mean intensities of Klf5 (n = 6 embryos in each group), Cdx2 (n = 4 embryos in each group), H3K27ac (n = 6 embryos in each group), and p300 (n = 6 Klf5-KO embryos vs. n = 4 control embryos) were quantified. Scale bar, 30 μm. (E) Colocalization of Klf5 and Cdx2 in E3.5 embryos. PLA signals are shown in red and the nuclei in blue. Scale bar, 30 μm. (F and G) Colony forming rates and outgrowth sizes of control and Klf5-knockout E3.5 embryos in TSC maintenance medium. (H) qPCR analysis of TSC-specific gene expression levels in control and Klf5-knockout outgrowths (n = 4 outgrowths in each group). (I) Immunofluorescence staining of Cdx2 and Oct4 in control and Klf5-knockout outgrowths. Nuclei were stained with DAPI (blue). Scale bar, 20 μm. n.s., P > 0.05, not significant. ***P < 0.001 by Student's t-test for comparison.
Discussion
In the present study, we showed critical characteristics of Klf5 in TSC self-renewal and differentiation and unraveled a novel underlying mechanism. At the molecular level, Klf5 participates in TSC core regulation by occupying proximal promoter regions and maintaining an open chromatin structure of its target TSC pluripotency genes. In addition, histone modification of H3K27ac mediated by Klf5 was required for the maintenance of TSCs and proper mouse peri-implantation development. Together, our findings provide new evidence that Klf5 is essential for both the establishment and maintenance of TSC pluripotency, extending the understanding of the TSC core regulatory network.
Although Klf5, Tead4, Cdx2, and Sox2 are considered marker genes in multipotent TSCs (), the role of Klf5 in the TSC transcriptional circuitry has not been clearly defined. In this study, several lines of evidence suggested that Klf5 is a main activator for TSC pluripotency. First, our results (Figure 1A) were consistent with the previous finding that Klf5 was rapidly downregulated in diethylstilbestrol-induced TSC differentiation system (). Second, 86% of DEGs, including key TSC regulators such as Sox2, Eomes, and Cdx2, were downregulated upon Klf5 depletion (Figure 2A and G). Third, a significant proportion of downregulated genes were also bound by Klf5 at the promoter regions (Figure 2E and F). These results verified a functional role of Klf5 in the regulation of the core regulatory network. Interactome analysis indicated that Klf5 was a Cdx2-binding protein, which was also identified by mass spectrometry (). Consistently, our data demonstrated that Klf5 colocalized with Cdx2 in undifferentiated TSCs (Figure 1B; Supplementary Figure S1B). In addition, ESCs overexpressing Klf5 activated molecular markers of the TE (Cdx2 and Elf5) and gave rise to extra-embryonic lineages (). Our results further showed that Klf5 functioned upstream or in parallel with Cdx2, as 56% of the binding sites of Cdx2 overlapped with those of Klf5 (Supplementary Figure S3B). Hence, our findings provide new evidence that Klf5 may act as an activator to participate in the TSC core regulatory network. In contrast, the acetylated Klf5 downstream of TGFβ signaling acts as a transcriptional repressor for selected target genes in keratinocytes (). Taken together, Klf5 can act as a transcriptional activator or repressor depending on the cellular and genetic environment in which it resides.
Klf5 has been proposed to ensure bipotential cell fate through dual regulation of ICM and TE specification genes (). Importantly, Klf5 mediates different molecular mechanisms in ESCs and TSCs for maintaining self-renewal. Klf5 can link different signaling pathways or epigenetic modifications to different sets of transcription factors, resulting in cell type-specific responses to physiological processes. For example, Klf5 was reported as an upstream target of Nanog and Oct3/4 and further regulated ERK signaling (; ) in ESCs. In addition, acetylation of Klf5 was stimulated by TGF-β signaling, thereby enhancing Sox2 expression and facilitating the suppression of many differentiated genes to maintain undifferentiated ESCs (). However, Klf5 plays a more important role in the TE than in the ICM, because Klf5-null embryos showed obvious defects in forming a blastocoel cavity and could not be rescued by exogenous Fgf4 treatment or microinjection of wild-type ESCs into Klf5-null embryos (). Klf5 has cell-specific and context-specific properties in regulating target gene expression. In ESCs, Klf5 physically interacts with Oct4 and activates multiple pluripotency genes, including Nanog, Klf4, and Esrrb, to maintain pluripotency (; ). In contrast to ESCs, we identified 495 Klf5 target TSC-specific genes, including Cdx2, Tead4, and Eomes (Figure 3A and C). Thus, Klf5 mediates cell-specific mechanisms to maintain self-renewal by binding to and regulating different genes in different conditions. In addition to Sox2 (), Klf5 provides another clear example of a transcription factor with maximum flexibility to play various roles in different networks.
How does Klf5 manage to regulate a subset of genes in undifferentiated TSCs? Chromatin accessibility is closely related to transcription, and cell differentiation is often accompanied by chromatin remodeling (). Consistent with this notion, Klf5 deprivation in TSCs resulted in low accessibility of the TSS of Klf5 target TSC-specific genes (Figure 3D). Klf5 has been shown to establish and maintain chromatin accessibility in esophageal squamous cell carcinoma (). In our study, we showed that Klf5 maintained an open chromatin structure of its target TSC-specific genes to orchestrate the TSC core regulatory network (Figure 3G). Chromatin stability in eukaryotic transcription requires histone modifying enzymes, nucleosome remodeling complexes, and histone chaperones. Reduced levels of important cofactors of histone acetylation may be a common mechanism leading to evident changes in chromatin accessibility (). Recently, Klf5 was shown to increase acetylation of H3K27 to alter chromatin structure and activate cancer development-related genes through the recruitment of p300 (). In our study, disruption of Klf5 impaired the recruitment of p300 to its target loci, leading to a decrease in H3K27ac (Figure 4). In addition, given that p300 acts as a transcriptional co-activator of Klf5 (), we found that p300 protein levels were also downregulated after knockdown of Klf5. In contrast, p300-deficient TSCs maintained normal epithelial morphology, and Eomes expression level was unchanged whereas Cdx and Esrrb expression levels decreased by 25% in CBP/p300-knockdown cells (). In our data, Eomes, Esrrb, and Cdx2 were barely detectable at both the mRNA and protein levels in Klf5-knockdown cells (Figure 1D–G). H3K4me3 and H3K9ac are known to affect syncytization, a necessary prerequisite for adequate gestation and subsequent invasion, migration, and self-renewal of trophoblast cells (). Klf5 indirectly regulates H3K27me3 and H3K4me3 in the promoter regions of its target genes to regulate the G2/M cell cycle in gastric cancer (). Additionally, Klf5 was shown to interact with histone deacetylase 3 to inhibit the transcription of BECN1 (). Thus, the mechanism of Klf5 and additional histone modifications involved in maintaining the self-renewal process of TSCs needs further study. Moreover, we also found that 44.22% of Klf5-binding sites were located distally (Supplementary Figure S2A), suggesting potential distal regulation in TSCs. Further analysis of the specific regulatory mechanisms of Klf5 in 3D chromatin is warranted.
Taken together, our observations demonstrate that Klf5 recruits p300 to the promoter of individual TSC-specific gene and maintains its open chromatin structure by H3K27ac (Figure 6), which is essential for the maintenance of undifferentiated TSCs and trophoblast developmental capacity. Our findings shed light on how histone modifications orchestrate complicated transcriptional regulation and provide a theoretical basis for the treatment of pregnancy-related diseases or disorders.
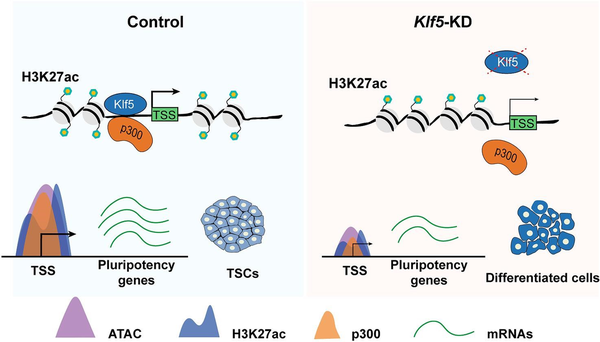
Figure 6
A working model showing the function of Klf5 in the maintenance of undifferentiated TSCs. Under undifferentiated TSC states, Klf5 recruits p300 to the promoter of individual pluripotency gene and maintains its open chromatin structure through H3K27ac. In Klf5-knockdown TSCs, reduced enrichment of p300 in the promoter region of specific pluripotency gene, followed by reduced enrichment of H3K27ac, suppresses chromatin accessibility and mRNA expression level of the pluripotency gene, ultimately leading to TSC differentiation.
Materials and methods
Mice and cell culture
Mice used for all experiments were housed in the animal facility of Huazhong Agricultural University, Wuhan, China, and all procedures were approved by the Animal Care and Use Committee of Huazhong Agriculture University (HZAUMO-2022-0052). Mice were maintained under specific pathogen-free conditions with a 12 h light–dark cycle (lights on at 6:00 AM and off at 6:00 PM) at a temperature of 23°C ± 2°C, with ad libitum access to food and water.
TSCs were maintained in proliferation medium containing 70% feeder condition medium, 30% TS medium (RPMI1640, 20% fetal bovine serum (FBS), 0.1 M 2-mercaptoethanol, 2 mM glutamine, 1 mM sodium pyruvate, and 100× Pen/Strep), 25 ng/ml Fgf4 (PeproTech), and 1 μg/ml heparin (Sigma) on mitomycin C-treated mouse embryonic fibroblasts (MEFs). For partially differentiated medium, Fgf4 was removed from TSC maintenance medium (). MEFs were cultured in DMEM supplemented with high glucose, 15% FBS, and 100× Pen/Strep. HEK293T cells used for lentivirus production were cultured in DMEM supplemented with high glucose, 10% FBS, and 100× Pen/Strep.
Klf5 knockdown by lentivirus and siRNA
For lentivirus production, we transfected pLKO.1-U6-shRNA-Puro, PSPAX2, and pMD2.G (3:2:1) into HEK293T cells with jetPRIME (Polyplus) transfection reagent. We replaced the medium with 10 ml of fresh medium in 10-cm plates 24 h after transfection. Lentivirus supernatant was collected at 48 and 72 h post-transfection, and the samples were pre-filtered with a 0.45 mm filter. The virus-containing medium was overlaid on 20% (w/w) lentivirus concentrate (ExCell bio) and centrifuged at 1500× g for 30 min at 4°C. For lentivirus transduction, TSCs were infected in proliferation medium containing 8 μg/ml polybrene (Merck Millipore) with an MOI = 1 (). After infection, targeted clones were selected for 3 days in puromycin (1 μg/ml). For Klf5 siRNA transfection, 100 μM siRNA was added to 200 μl of jetPRIME buffer and mixed. Then, 8 μl jetPRIME reagent was added and promptly mixed by vortexing and centrifuging. Afterward, the mixture was incubated at room temperature for 10 min. Subsequently, the transfection mixture in small drops was carefully added to the fresh TSC proliferation medium (). After 6 h, the transfection medium was removed and replaced with fresh TSC proliferation medium.
RNA extraction and qPCR
Total RNA was extracted from cells by TRIzol (Invitrogen, 15596-018), and 1 μg total RNA was reverse transcribed to obtain cDNA with the cDNA Synthesis Kit (Vazyme, R212-01) according to the manufacturer's protocol. Total RNA isolation from 20 embryos at E3.5 was performed by using the RNAprep Pure Micro Kit (TIANGEN, DP420). cDNA (1 μl) was used as a template for the 10 μl reaction, and reactions were performed with Universal SYBR qPCR Master Mix (Q321-02, Vazyme) run on a Bio-Rad CFX96. All gene relative expression levels were calculated using the ΔΔCt value and normalized to actin. The primers used are listed in Supplementary Table S1.
Immunofluorescence staining
For immunofluorescence, TSC colonies and blastocysts were fixed with 4% paraformaldehyde for 30 min at room temperature, permeabilized with 0.1% Triton X-100 for 15 min, and blocked with 3% bovine serum albumin for 1 h at room temperature.
TSCs and blastocysts were incubated overnight at 4°C with appropriate antibodies, including anti-Klf5 (1:500, Abcam, ab137676), anti-Cdx2 (1:500, Abcam, ab76541), anti-Esrrb (1:100, Santa Cruz, sc-376449), anti-p300 (1:100, Santa Cruz, sc-48343), and anti-H3K27ac (1:500, Abcam, ab4729). Cell nuclei were counterstained with DAPI. The secondary antibody was incubated with the samples after washing three times. Stained cells mounted on slides were observed by using a Zeiss confocal microscope (Zeiss LSM 800). ImageJ was used to analyze the mean fluorescence intensity of the acquired images.
For Duolink® PLA Fluorescence, E3.5 embryos were fixed with Duolink® Blocking Solution for 60 min at 37°C and then incubated overnight at 4°C with appropriate antibodies, including anti-Klf5 (1:100, Santa Cruz, SC-398470) and anti-p300 (1:500, Abcam, ab275378). Following the manufacturer's recommendations (Sigma, DUO92101), PLA signals were amplified after Duolink® PLA Probe incubation and ligation.
In vitro embryo culture and outgrowth formation
The fertilized eggs were collected from superovulated mice at the Institute of Cancer Research and cultured in G1 plus medium (Vitrolife, 10132) at 37°C and 5% CO2. To obtain Klf5-knockout embryos, we mixed Cas9 mRNA (100 ng/ml; Invitrogen, A29378) and sgRNAs (50 ng/ml) and injected them into the cytoplasm of fertilized eggs using a FemtoJet microinjector (Eppendorf). For outgrowth formation, wild-type and Klf5-knockout embryos were cultured in medium containing 70% feeder condition medium, 30% TS medium (RPMI1640, 20% FBS, 0.1 M 2-mercaptoethanol, 2 mM glutamine, 1 mM sodium pyruvate, and 100× Pen/Strep), 37.5 ng/ml Fgf4 (PeproTech), and 1.5 μg/ml heparin (Sigma) on mitomycin C-treated MEFs, and the medium was changed every 3 days until the 8th day.
Genetic modification and genotyping
Benchling (https://benchling.com) was used to design sgRNAs (Supplementary Table S1). Then, we used PCR amplification to add the T7 promoter to sgRNA. In vitro transcription employed the MEGAshortscript T7 kit (Life Technologies, AM1354) and was purified using the MEGAclear kit (Life Technologies, M1909). For genotyping, DNA was extracted according to the manufacturer's instructions (TIANGEN, DP304). PCR was performed for 34 cycles at 95°C for 30 sec, 62°C for 30 sec, and 72°C for 1 min, with a final extension at 72°C for 2 min. All performed PCR primers are listed in Supplementary Table S1.
Co-IP
IP and western blotting were performed as described previously (; ), except that IP was carried out with 4 μg of specific antibody. Antibodies used were anti-Klf5 (1:1000, Abcam, ab137676), anti-Cdx2 (1:1000, Abcam, ab76541), anti-Eomes (1:500, Santa Cruz, sc-293481), anti-p300 (1:500, Santa Cruz, sc-48343), anti-H3K27ac (1:1000, Abcam, ab4729), and anti-Elf5 (1:500, Santa Cruz, sc-166653).
ATAC-seq
ATAC-seq was performed as previously reported (). In brief, a total of 50000 cells were washed once with 50 μl of cold PBS and resuspended in 50 μl lysis buffer (10 mM Tris–HCl, pH 7.4, 10 mM NaCl, 3 mM MgCl2, and 0.1% IGEPAL CA-630). After centrifugation at 4°C, the suspension of nuclei was diluted to 5 ng/μl in elution buffer (10 mM Tris buffer, pH 8.0), followed by the addition of 50 μl transposition reaction mix from the DNA Library Prep Kit V2 for Illumina (Vazyme, TD501). The reaction mix was gently mixed and incubated at 55°C for 10 min. The ATAC-seq library was amplified in nine cycles. Libraries were purified using the Qiagen PCR Cleanup Kit according to the manufacturer's instructions. Library concentrations were measured by the KAPA Library Quantification Kit (KAPA Biosystems, KK4824) according to the manufacturer's instructions and checked by gel electrophoresis. Lastly, ATAC-seq libraries were sequenced on Illumina HiSeq X-Ten.
ChIP-seq and ChIP–qPCR
For ChIP-seq, TSCs were crosslinked with 1% formaldehyde for 10 min and quenched by 2 ml of 125 mM glycine for 5 min rotation at room temperature. Cells were washed 3 times with cold PBS. After centrifugation, cell pellets were lysed in buffer (50 mM HEPES–KOH, 140 mM NaCl, 1 mM EDTA, 10% glycerol, 0.5% NP-40, 0.25% Triton X-100, and 50 mM Tris–HCl, pH 8.0) for 10 min at 4°C. Chromatin was sonicated to 100–500 bp for subsequent experiments. The samples were pre-incubated with protein A/G Dynabeads (Life Technologies, 10015D), and the beads were then removed. Subsequently, 8 mg of Klf5 (Sigma, 09-822) or H3K27ac antibody was added, and the sample was rotated overnight at 4°C. Beads were washed four times with the washing buffer (10 mM Tris–HCl, pH 8.0, 140 mM NaCl, 1 mM EDTA, 1% glycerol, 0.5% Triton X-100, and 0.01% SDS), eluted, and reverse-crosslinked. Paired-end 125-bp sequencing was performed on Illumina HiSeq X-Ten. The resulting DNA was analyzed by qPCR, and the results were presented as the percentage of input using the indicated primers (Supplementary Table S1).
RNA-seq data analysis
Total RNA was extracted from TSCs by TRIzol and the RNA-seq library was generated using the RNA Library Prep Kit for Illumina® (NEB, E7530L) according to the manufacturer's recommendations. RNA-seq raw reads were trimmed to remove adapters and low-quality reads using TrimGalore (version 0.6.6) with the parameters ‘-q 25—phred33—stringency 3—length 36 -e 0.1’. These processed reads were then aligned to the mouse reference genome (Ensemble mm10) using STAR (version 2.7.3) with the default parameters. Mapped reads with high confidence were kept for further analysis using SAMtools (version 1.9). Expression levels for all Refseq genes were quantified to fragments per kilobase per million mapped reads (FPKM) using Stringtie (version 2.1.4) and FPKM values of replicates were averaged. To perform differential gene expression analysis, we first calculated the read counts of each RNA-seq sample using FeatureCounts (version 2.0.0). Then, we used DESeq2 in R to perform differential analysis. Genes with P ≤ 0.05 and fold change >2 or fold change <−2 were defined as DEGs. RNA-seq data of TSC time-course differentiation were downloaded from the Gene Expression Omnibus (accession number: GSE18507). Hierarchical clustering was performed to identify TSC pluripotency genes based on their expression patterns. Those gradually downregulated upon differentiation were identified as putative TSC-specific genes, including most known TSC-specific transcription factors (Elf5, Cdx2, Esrrb, Sox2, and Eomes).
ATAC-seq and ChIP-seq data analysis
ChIP-seq and ATAC-seq raw data were processed by TrimGalore (version 0.6.6) to remove adapters and low-quality reads using the following parameters ‘-q 25—phred33—stringency 3—length 36 -e 0.1’. The processed reads were then aligned to the mouse reference genome (Ensemble mm10) using Bowtie2 (version 2.4.4) with the parameters ‘—local—very-sensitive-local—no-mixed—no-discordant—phred33 -I 10 -X 700’. PCR duplicates were removed using Picard (version 2.23.9). ChIP-seq peaks were called with MACS2 (version 2.2.7.1) with the parameters ‘-f BAMPE—bdg—SPMR -q 0.05—call-summits—seed 11521—keep-dup all’. ATAC-seq peaks were called with the parameters ‘-f BAMPE -g 2472047704—shift -75—extsize 150—keep-dup all—bdg—cutoff-analysis’. Signal tracks for each sample were generated and normalized by calculating the reads per kilobase of transcript per million mapped reads (RPKM) using deepTools (version 3.5.0). Normalized signals were calculated for promoter regions (defined as ±1 kb around the TSS) of genes.
Statistical analysis
Statistical analyses were performed using R (www.r-project.org/) and GraphPad Prism software. Statistical significance was calculated with two-tailed unpaired Student's t-test (P < 0.05 was considered statistically significant). IGV (version 2.11.9) was performed to visualize all sequencing tracks. All experiments were repeated at least three times unless otherwise stated. Data are presented as mean ± standard deviation (SD).
Data availability
All datasets generated in this paper are openly available in NCBI Gene Expression Omnibus with accession code: GSE207053.
Acknowledgements
We thank Dr Heide Schatten (University of Missouri) for helping us to improve the English language.
References
- Abell A.N., Jordan N.V., Huang W., et al (2011). MAP3K4/CBP-regulated H2B acetylation controls epithelial-mesenchymal transition in trophoblast stem cells. Cell Stem Cell 8, 525–537.
- Adachi K., Nikaido I., Ohta H., et al (2013). Context-dependent wiring of Sox2 regulatory networks for self-renewal of embryonic and trophoblast stem cells. Mol. Cell 52, 380–392.
- Adamson S.L., Lu Y., Whiteley K.J., et al (2002). Interactions between trophoblast cells and the maternal and fetal circulation in the mouse placenta. Dev Biol. 250, 358–373.
- Azami T., Matsumoto K., Jeon H., et al (2018). Klf5 suppresses ERK signaling in mouse pluripotent stem cells. PLoS One 13, e0207321.
- Azami T., Waku T., Matsumoto K., et al (2017). Klf5 maintains the balance of primitive endoderm versus epiblast specification during mouse embryonic development by suppression of Fgf4. Development 144, 3706–3718.
- Benchetrit H., Herman S., van Wietmarschen N., et al (2015). Extensive nuclear reprogramming underlies lineage conversion into functional trophoblast stem-like cells. Cell Stem Cell 17, 543–556.
- Chen P.B., Zhu L.J., Hainer S.J., et al (2014). Unbiased chromatin accessibility profiling by RED-seq uncovers unique features of nucleosome variants in vivo. BMC Genomics 15, 1104.
- Creyghton M.P., Cheng A.W., Welstead G.G., et al (2010). Histone H3K27ac separates active from poised enhancers and predicts developmental state. Proc. Natl Acad. Sci. USA 107, 21931–21936.
- Cui T.T., Jiang L.Y., Li T.D., et al (2019). Derivation of mouse haploid trophoblast stem cells. Cell Rep. 26, 407–414.
- Dang D.T., Pevsner J., Yang V.W. (2000). The biology of the mammalian Kruppel-like family of transcription factors. Int. J. Biochem. Cell Biol. 32, 1103–1121.
- Dastjerdi M.N., Salahshoor M.R., Mardani M., et al (2013). The effect of CTB on P53 protein acetylation and consequence apoptosis on MCF-7 and MRC-5 cell lines. Adv. Biomed. Res. 2, 24.
- Devipriya B., Parameswari A.R., Rajalakshmi G., et al (2010). Exploring the binding affinities of p300 enzyme activators CTPB and CTB using docking method. Indian J. Biochem. Biophys. 47, 364–369.
- Diakiw S.M., D'Andrea R.J., Brown A.L. (2013). The double life of KLF5: opposing roles in regulation of gene-expression, cellular function, and transformation. IUBMB Life 65, 999–1011.
- Dixon J.R., Jung I., Selvaraj S., et al (2015). Chromatin architecture reorganization during stem cell differentiation. Nature 518, 331–336.
- Ema M., Mori D., Niwa H., et al (2008). Kruppel-like factor 5 is essential for blastocyst development and the normal self-renewal of mouse ESCs. Cell Stem Cell 3, 555–567.
- Erlebacher A., Price K.A., Glimcher L.H. (2004). Maintenance of mouse trophoblast stem cell proliferation by TGF-β/activin. Dev. Biol. 275, 158–169.
- Gao H.B., Gao R., Zhang L.F., et al (2019). Esrrb plays important roles in maintaining self-renewal of trophoblast stem cells (TSCs) and reprogramming somatic cells to induced TSCs. J. Mol. Cell Biol. 11, 463–473.
- He H.N., Wang J.L., Mou X.M., et al (2023). Selective autophagic degradation of ACLY (ATP citrate lyase) maintains citrate homeostasis and promotes oocyte maturation. Autophagy 19, 163–179.
- Hemberger M., Hanna C.W., Dean W. (2020). Mechanisms of early placental development in mouse and humans. Nat. Rev. Genet. 21, 27–43.
- Hou M., Han J., Li G., et al (2020). Multipotency of mouse trophoblast stem cells. Stem Cell Res. Ther. 11, 55.
- Hounkpe B.W., Chenou F., de Lima F., et al (2021). HRT Atlas v1.0 database: redefining human and mouse housekeeping genes and candidate reference transcripts by mining massive RNA-seq datasets. Nucleic Acids Res. 49, D947–D955.
- Ishiuchi T., Ohishi H., Sato T., et al (2019). Zfp281 shapes the transcriptome of trophoblast stem cells and is essential for placental development. Cell Rep. 27, 1742–1754.
- Jia J., Zhang H.B., Shi Q., et al (2019). KLF5 downregulation desensitizes castration-resistant prostate cancer cells to docetaxel by increasing BECN1 expression and inducing cell autophagy. Theranostics 9, 5464–5477.
- Jiang Y.Y., Jiang Y., Li C.Q., et al (2020). TP63, SOX2, and KLF5 establish a core regulatory circuitry that controls epigenetic and transcription patterns in esophageal squamous cell carcinoma cell lines. Gastroenterology 159, 1311–1327.
- Karahoda R., Kallol S., Groessl M., et al (2021). Revisiting steroidogenic pathways in the human placenta and primary human trophoblast cells. Int. J. Mol. Sci. 22, 1704.
- Kinisu M., Choi Y.J., Cattoglio C., et al (2021). Klf5 establishes bi-potential cell fate by dual regulation of ICM and TE specification genes. Cell Rep. 37, 109982.
- Knott J.G., Paul S. (2014). Transcriptional regulators of the trophoblast lineage in mammals with hemochorial placentation. Reproduction 148, R121–R136.
- Latos P.A., Goncalves A., Oxley D., et al (2015a). Fgf and Esrrb integrate epigenetic and transcriptional networks that regulate self-renewal of trophoblast stem cells. Nat. Commun. 6, 7776.
- Latos P.A., Sienerth A.R., Murray A., et al (2015b). Elf5-centered transcription factor hub controls trophoblast stem cell self-renewal and differentiation through stoichiometry-sensitive shifts in target gene networks. Genes Dev. 29, 2435–2448.
- Lee B.K., Jang Y.J., Kim M., et al (2019). Super-enhancer-guided mapping of regulatory networks controlling mouse trophoblast stem cells. Nat. Commun. 10, 4749.
- Lin S.C.J., Wani M.A., Whitsett J.A., et al (2010). Klf5 regulates lineage formation in the pre-implantation mouse embryo. Development 137, 3953–3963.
- Liu L.Q., Leng L.Z., Liu C.Y., et al (2019). An integrated chromatin accessibility and transcriptome landscape of human pre-implantation embryos. Nat. Commun. 10, 364.
- Liu Y.L., Guo B.Q., Aguilera-Jimenez E., et al (2020). Chromatin looping shapes KLF5-dependent transcriptional programs in human epithelial cancers. Cancer Res. 80, 5464–5477.
- Ma P., Pan Y., Yang F., et al (2020). KLF5-modulated lncRNA NEAT1 contributes to tumorigenesis by acting as a scaffold for BRG1 to silence GADD45A in gastric cancer. Mol. Ther. Nucleic Acids 22, 382–395.
- Matoba N., Mestan K.K., Collins J.W. (2021). Understanding Racial Disparities of Preterm Birth Through the Placenta. Clin. Ther. 43, 287–296.
- Meister S., Hahn L., Beyer S., et al (2021). Regulation of epigenetic modifications in the placenta during preeclampsia: PPARγ influences H3K4me3 and H3K9ac in extravillous trophoblast cells. Int. J. Mol. Sci. 22, 12469.
- Mieczkowski J., Cook A., Bowman S.K., et al (2016). MNase titration reveals differences between nucleosome occupancy and chromatin accessibility. Nat. Commun. 7, 11485.
- Miyamoto S., Suzuki T., Muto S., et al (2003). Positive and negative regulation of the cardiovascular transcription factor KLF5 by p300 and the oncogenic regulator SET through interaction and acetylation on the DNA-binding domain. Mol. Cell Biol. 23, 8528–8541.
- Ohinata Y., Tsukiyama T. (2014). Establishment of trophoblast stem cells under defined culture conditions in mice. PLoS One 9, e107308.
- Oldfield A.J., Yang P.Y., Conway A.E., et al (2014). Histone-fold domain protein NF-Y promotes chromatin accessibility for cell type-specific master transcription factors. Mol. Cell 55, 708–722.
- Parisi S., Cozzuto L., Tarantino C., et al (2010). Direct targets of Klf5 transcription factor contribute to the maintenance of mouse embryonic stem cell undifferentiated state. BMC Biol. 8, 128.
- Parisi S., Passaro F., Aloia L., et al (2008). Klf5 is involved in self-renewal of mouse embryonic stem cells. J. Cell Sci. 121, 2629–2634.
- Roberts R.M., Ezashi T., Sheridan M.A., et al (2018). Specification of trophoblast from embryonic stem cells exposed to BMP4. Biol. Reprod. 99, 212–224.
- Tessarz P., Kouzarides T. (2014). Histone core modifications regulating nucleosome structure and dynamics. Nat. Rev. Mol. Cell Biol. 15, 703–708.
- Wu D.Y., Li X.X., Sun Q.R., et al (2021). Defective chromatin architectures in embryonic stem cells derived from somatic cell nuclear transfer impair their differentiation potentials. Cell Death Dis. 12, 1085.
- Zhang B.T., Ci X.P., Tao R., et al (2020). Klf5 acetylation regulates luminal differentiation of basal progenitors in prostate development and regeneration. Nat. Commun. 11, 997.
- Zhang X.Y., Choi P.S., Francis J.M., et al (2018). Somatic superenhancer duplications and hotspot mutations lead to oncogenic activation of the KLF5 transcription factor. Cancer Discov. 8, 108–125.
- Zhao T., Liu C., Chen L.Y. (2015). Roles of Klf5 acetylation in the self-renewal and the differentiation of mouse embryonic stem cells. PLoS One 10, e0138168.
- Zhou J.L., He H.N., Zhang J.J., et al (2022). ATG7-mediated autophagy facilitates embryonic stem cell exit from naive pluripotency and marks commitment to differentiation. Autophagy 18, 2946–2968.
