INTRODUCTION
Alopecia, also known as hair loss, affects a sizable portion of the adult population worldwide. Androgenetic alopecia is the most popular genetic disorder, sometimes known as male or female pattern baldness. As a result of the progressive degeneration of hair follicles, escalating thinning phenomena are observed in specific regions of the scalp. Such places include the hairline, vertex, and crown.[]
Autologous platelet-rich plasma (PRP) injections have recently gained popularity in treating hair loss. PRP is taken from the blood of a patient and then processed to concentrate growth factor-rich platelets, which can theoretically help stimulate hair follicles, resulting in regrowth or improved hair thickness. The aim of this ongoing prospective clinical trial is to examine the effect and effectiveness of autologous PRP injections as a treatment for androgenetic male alopecia.[]
Mechanism of Action of PRP
PRP is derived from autologous blood—blood collected from a patient and centrifuged to separate out the plasma portion containing higher-than-baseline concentrations of platelets. Activated platelets release various growth factors, including:
Platelet-Derived Growth Factor (PDGF)
Transforming Growth Factor-β (TGF-β)
Vascular Endothelial Growth Factor (VEGF)
Insulin-Like Growth Factors (IGF)
These growth factors are important for cell growth, tissue rebuilding, and collagen synthesis. In the context of hair loss, they activate stem cells in the hair follicle to improve new growth and prolong the anagen (growth) phase of the hair cycle.[]
OBJECTIVE
This clinical trial is designed to assess the efficacy of using autologous PRP in ameliorating hair loss and improving hair regrowth among patients presenting with androgenetic alopecia.
METHODOLOGY
The study was conducted at the Department of Oral and Maxillofacial Surgery, Surendera Dental College, Sri Ganganagar, Rajasthan. Five patients with moderate to severe hair loss were included in the study. The institutional ethics committee provided ethical clearance, and informed consent was obtained from all participants.
Inclusion criteria
Males and females aged 20–65 years
Patients with moderate to severe androgenetic alopecia
Patients who were not previously treated with either topical or systemic treatments for hair loss in the past 6 months
Exclusion criteria
Patients with allergic history, immunosuppression, scalp affected by dermatological disorders, autoimmune diseases, or coagulopathies
Patients with cosmetic procedures such as stem cell therapy or visible scalp scars
Patients with hemostasis disorders or receiving treatment with anticoagulant or antiplatelet medications
PRP preparation and injection protocol
Sample collection
20 ml of blood was collected from the antecubital vein of each patient.
Preparation of PRP
The double-spin method was used to prepare PRP from the blood to generate increased numbers of platelets. The final PRP obtained was collected in two sterile syringes for injection.
Injections protocol
PRP was injected into the affected area using a 30-gauge needle after the application of local anesthesia on the scalp. Injections were given in three sessions over an interval of 4 weeks and included about 125 sites on the scalp.
Post treatment
Patients were instructed to avoid washing their hair, working out, sweating, sun exposure, and using different hair products for at least 3 days after treatment. Additionally, smoking, alcohol, and caffeine were off-limits for 2–3 days post-procedure.
Evaluation methods
Follow-ups occurred every 3 weeks, 6 weeks, and at quarterly intervals after the baseline assessment.
Hair pull test
A subjective method to evaluate the extent of hair shedding, repeated at each follow-up to track hair retention improvements.
Macroscopic photographs
Standardized photographs were taken during each visit to document changes in hair density and scalp coverage.
Patient satisfaction questionnaire (PSQ)
A self-assessment questionnaire based on the Mohebi Rate of Satisfaction Scale.
RESULTS
By the end of the treatment period, there was a marked decrease in hair shedding and a small increase in hair density.
Hair Pull Test:
At baseline, all patients showed positive results (i.e., >6 strands). By the end of the third session, no hair was obtained on the pull test [Table 1].
Macroscopic Photography:
Visual inspection revealed improved hair density, particularly in the vertex and frontal hairline regions.
Patient Satisfaction:
All patients reported improvements in hair quality, volume, and thickness. The average satisfaction score was 8.2/10 [Table 2].
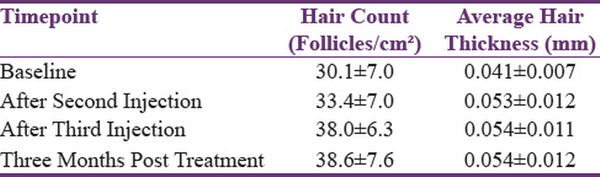
Table 1
Hair density changes in patients treated with PRP

Table 2
Patient satisfaction scores following PRP treatment
DISCUSSION
Our results align with several studies that have shown the effectiveness of PRP injections in treating androgenetic alopecia.[,] The higher concentration of growth factors like PDGF and VEGF plays a critical role in hair follicle regeneration and prolonging the anagen phase of the hair cycle. PRP treatments have been shown to increase hair density, thickness, and reduce hair loss.
PRP therapy is safe and involves little downtime. Minimal discomfort and swelling were experienced by a few patients, but no serious adverse events occurred. The results confirm PRP as a safe and effective alternative for treating androgenetic alopecia, with high patient satisfaction and minimal risks.[,]
CONCLUSION
This clinical trial demonstrates that autologous PRP injections are an effective treatment for androgenetic alopecia. Hair loss is reduced, hair density increases, and the overall quality of hair improves with this treatment. PRP is a promising option for non-surgical management of hair loss with few side effects and high patient satisfaction. More trials with larger sample sizes and long-term follow-up are needed to corroborate these findings and define ideal PRP treatment protocols.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
REFERENCES
1.
Bayat M, Yazdanpanah MJ, Hamidi Alamdari D, Banihashemi M, Salehi M. The effect of platelet-rich plasma injection in the treatment of androgenetic alopecia. J Cosmet Dermatol 2019;18:1624–8.2.
Elghblawi E. Platelet-rich plasma:The ultimate secret for youthful skin elixir and hair growth triggering. J Cosmet Dermatol 2018;17:423–30.3.
Khatu SS, More YE, Gokhale NR, Chavhan DC, Bendsure N. Platelet-rich plasma in androgenic alopecia:Myth or an effective tool. J Cutan Aesthet Surg 2014;7:107–10.4.
Butt G, Hussain I, Ahmed FJ, Choudhery MS. Efficacy of platelet-rich plasma in androgenetic alopecia patients. J Cosmet Dermatol 2019;18:996–1001.5.
Zhang J, Lin P, Lin H, Ma C, Hu Y, Wang Y, et al. Laser and light therapy combined with topical minoxidil for alopecia areata:A systematic review and meta-analysis of randomized controlled trials. Lasers Med Sci 2023;38:74.6.
Severi G, Sinclair R, Hopper JL, English DR, McCredie MRE, Boyle P, et al. Androgenetic alopecia in men aged 40–69 years:Prevalence and risk factors. Br J Dermatol 2003;149:1207–13.7.
Mysore V, Kumaresan M, Garg A, Dua A, Venkatram A, Dua K, et al. Hair transplant practice guidelines. J Cutan Aesthet Surg 2021;14:265–84.8.
Butt G, Hussain I, Ahmad FJ, Choudhery MS. Stromal vascular fraction-enriched platelet-rich plasma therapy reverses the effects of androgenetic alopecia. J Cosmet Dermatol 2020;19:1078–85.9.
Gkini MA, Kouskoukis AE, Tripsianis G, Rigopoulos D, Kouskoukis K. Study of platelet-rich plasma injections in the treatment of androgenetic alopecia through a one-year period. J Cutan Aesthet Surg 2014;7:213–9.10.
Verma K, Tegta GR, Verma G, Gupta M, Negi A, Sharma R. A study to compare the efficacy of platelet-rich plasma and minoxidil therapy for the treatment of androgenetic alopecia. Int J Trichol 2019;11:68–79.
