INTRODUCTION
Feeding a growing human population is a huge challenge owing to the extension of agricultural land exploitation, which results in serious global impacts such as land clearing and increased greenhouse gas emissions (). In recent years, researchers and plant breeders have applied evolutionary or ecological theories to improve crop yields while maintaining or increasing agricultural sustainability; this approach is called Darwinian agriculture (; ) or evolutionary agroecology (, ). This approach can provide an efficient and suitable solution to global crop risks through the group selection of crop genotypes with low individual fitness and high population yields (; ; ).
In close-planted crops such as wheat, genotypes with high competitiveness can allocate more photosynthetic products to vegetative organs to capture limited resources. This allows the plants to produce more seeds and hence are ‘naturally selected’. However, as their frequency increase, they start competing with each other. In such cases, there will no longer be any advantage to strong competitors simply because their neighbours are equally competitive. Because they pay a high cost of investment in resource-foraging structures (e.g. roots or shoots), they actually produce fewer seeds than the less competitive genotypes they displace.
first noted the ubiquity of the trade-off between individual competitiveness and group productivity, and then argued that high-yielding crop plants should be weak competitors, i.e. Donald’s ideotype. used another term, ‘growth redundancy’, to make it immediately transparent that ‘redundant’ growth in resource-foraging structures could be sacrificed to increase yields. also linked Donald’s ideotype or growth redundancy to the famous ‘tragedy of the commons’ (): every member of a crop could do better if they all agree to invest less in aggressive competition, but unilateral restraint will be exploited. Implicit acceptance of the trade-off between yield and individual competitiveness is the key to many past improvements (). For example, dwarf cereal varieties that contribute significantly to the green revolution () are less competitive for light than tall varieties (). Decreased tassel size, which lowers male fitness, has contributed significantly to increased maize yield since the 1960s in the USA (; ).
Theoretically, the costs of individual competition in crops arise not only from genetically fixed traits, such as growth redundancy in old varieties relative to modern varieties (), but also from plastic responses to competition among individuals within varieties (; ; ). When competing for a common pool of resources, crops ‘overproduce’ vegetative organs at the expense of reproductive growth (; ; ), which can also result in the tragedy of the commons (). Do crop genotypes with higher competitiveness and growth redundancy show greater expression of the plastic tragedy of the commons? However, direct empirical studies that test the sensitivity of crop varieties to the tragedy of the commons are rare.
The magnitude of the plastic tragedy of the commons caused by root competition in plants may decline in fertile soils because high soil fertility can decrease nutrient limitation for plant growth and alleviate below-ground competition between plants (; ; ). We expect crop varieties to possess different sensitivities to competition with neighbouring plants when they are artificially selected under different resource (water and fertilization) conditions (; ). However, little is known about how the magnitude of the plastic tragedy of the commons varies in relation to the resource availability.
Previous studies on the plastic tragedy of the commons have often involved treatments that provided a constant rooting space and amount of nutrients per plant (; ; ). As a result, plants that share rooting space with a neighbour potentially have access to twice as much volume as plants grown alone (; , ). Thus, the effects of neighbour detection may be confounded by the effects of rooting volume for each competitor (; ). Plant production increases in response to an increase in the volume to which a plant has access (; ). To solve this problem, used mesh partition to separate two plants in a pot. This mesh halved the volume but allowed competition, in which each plant had access to the same volume as the plants separated by a plastic partition.
In the current study, we employed the method of to set up mesh and plastic partitions and examined how wheat plants respond to the presence of an intra-variety neighbour. Plants were watered with low- and high-fertility conditions to determine the influence of soil fertility on their responses to competitors. Old Monkhead is a local landrace, and the modern 92-46 was released in 2000. Both are widely grown varieties in the semi-arid areas of Gansu Province; however, modern 92-46 has a smaller root system but higher grain yield than the old Monkhead (). This study hypothesized that (i) modern 92-46 has more resource allocation to reproductive components and less to non-reproductive components than old Monkhead, (ii) old Monkhead has greater expression of the plastic tragedy of the commons than modern 92-46 and (iii) the occurrence and magnitude of plastic tragedy of the commons depend on soil fertility.
MATERIALS AND METHODS
Materials
Spring wheat (Triticum aestivum) is the main grain crop in semi-arid areas of Northwest China. Monkhead, a local variety, was widely planted in the 1940s. Modern 92-46 was released in 2000 and has been widely planted since. Both varieties possess a similar phenology, but Monkhead is awnless and highly tillering; thus, it can be easily distinguished from 92-46 when harvested ().
Methods
We conducted greenhouse pot experiments in a randomized block design with three factors: root partition (plastic and mesh; Fig. 1), soil fertility (low and high) and variety (Monkhead and 92-46). All experimental treatments were replicated 20 times, and all pots were arranged into 20 blocks, with each including all treatment combinations.
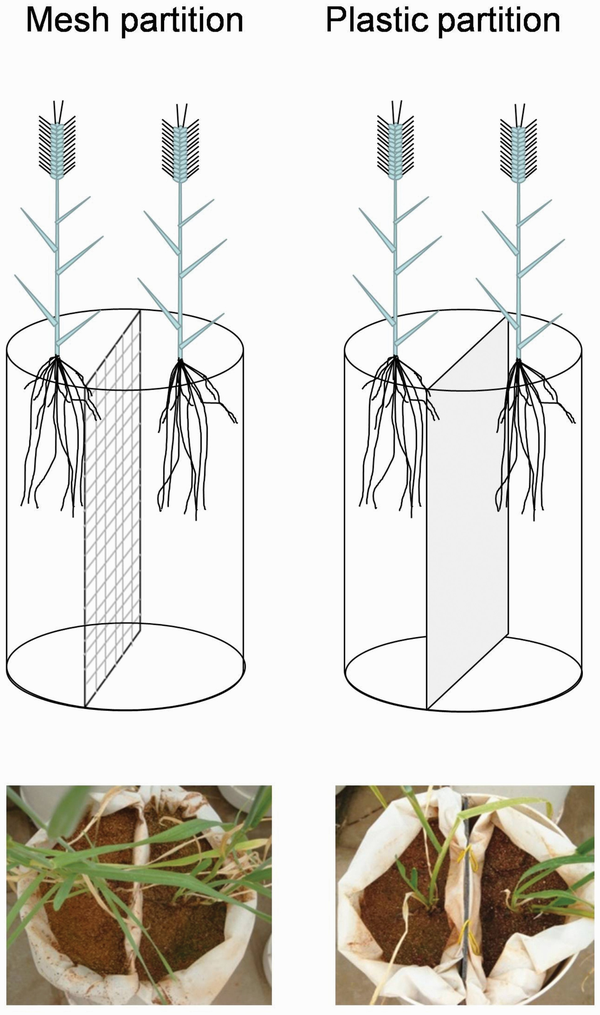
Figure 1
Schematic representation of mesh and plastic partition treatments.
To set up the partitions, we placed two nylon net bags (aperture size 20 µm) filled with vermiculite in one pot. Pots were constructed using PVC pipes (16 cm diameter and 48 cm length) with plastic composite panels as bottoms (holes were created for free draining), and each one was equally separated by a plastic composite panel. In the mesh partition, we removed the plastic composite panels to allow the movement of soil nutrients and root exudates from one side to the other while preventing direct contact between the roots of the two individuals. In the plastic partition, the edges between the pots and partitions were sealed with silicon (Fig. 1). In either plastic or mesh partitions, all individuals had access to half of the volume of the pot, which enabled us to eliminate the effect of soil volume that might confound the effects of neighbour detection (). Two weeks after transplanting, two soil fertility treatments were established based on the fertilization rate used in croplands in Gansu Province (). In the low-fertility treatments, pots were watered with 1.6 L of nutrient solution (0.2 g L−1 of Peter’s professional) every 14 days, and in the high-fertility treatments, 0.4 L of nutrient solution was added daily. During the entire experimental period, low- and high-fertility treatments were supplied with 0.248 g of N/P/K and 0.868 g of N/P/K in each pot. We did not separate the effects of soil nutrients and water because they are co-limited resources in the semi-arid croplands of the study site.
Seeds were vernalized at 4 °C for 1 day and germinated at 25 °C. Two seedlings were transplanted into pots, each in the centre of each half. Spike, stem + leaf (harvested together for each individual) and root samples were harvested at maturity from each individual plant. Roots were separated from the substrate and cleaned with tap water. All samples were dried at 65 °C to a constant weight and then weighed. Seeds of each plant peeled from the ears were counted and weighed.
Statistical analyses
We used a three-way ANOVA to test for the effects of partition treatments, wheat varieties and soil fertility on tiller number and plant biomass. Differences between treatments were determined by Student–Newman–Keuls multiple comparisons. ANOVA was conducted using SPSS statistical software (version 17.0; SPSS Inc., Chicago, IL, USA).
An allometric analysis was conducted to assess the resource allocation patterns. We compared static allometric relationships between treatments using standardized major axis (SMA) regression, in which whole-individual biomass was adopted as the independent variable and the biomass of components (i.e. root, stem + leaf and seed) was used as the dependent variable (; ). Given that biomass variables are interdependent, model type II regression was used to reduce the measurement errors associated with allometric data (; ). The slopes of the regression lines were compared using post hoc multiple comparisons. SMA analyses were conducted using standardized major axis tests and routines (SMATR) 2.0 (). Biomass data were log-transformed before analysis, when necessary, to improve the normality of residuals and homogeneity of variances.
RESULTS
Tiller number and plant biomass
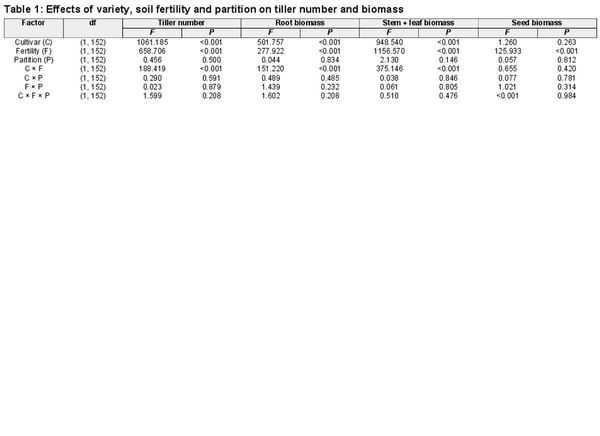
The tiller number was greater in the Monkhead than in the 92-46 variety. Application of nutrients increased tiller number in both varieties, but the degree of increase was higher in Monkhead (Table 1; Fig. 2a). Root and stem + leaf biomasses were greater in Monkhead over 92-46 (Table 1; Fig. 2b and c), but the seed biomass in Monkhead was similar to that in 92-46 (Table 1; Fig. 2d). In addition, fertilizer addition increased the plant biomass of all wheat components (Table 1; Fig. 2). However, in the root and stem + leaf, the degree of increase was higher in Monkhead than in 92-46 (Fig. 2b and c). For seed biomass, the interactions between variety and fertility were insignificant (Table 1; Fig. 2d). The partitions did not influence tiller number or biomass, and variety × partition interaction, fertility × partition interaction or variety × fertility × partition interaction were insignificant (Table 1).
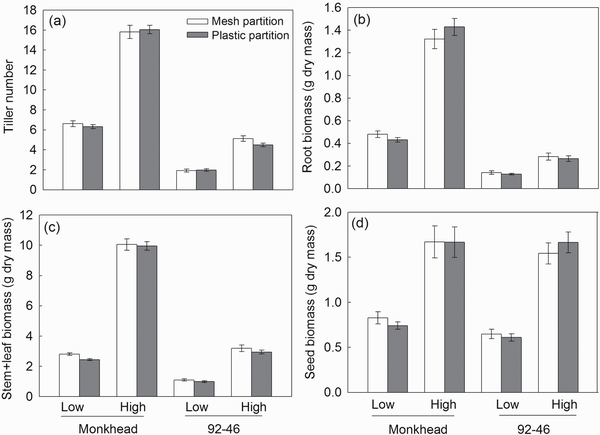
Figure 2
Effects of partition type, soil fertility and variety on tiller number (a), root biomass (b), stem + leaf biomass (c) and seed biomass (d). Abbreviations: High = high fertility, Low = low fertility. Mean ± SE are shown (n = 20).
Allometric relationships
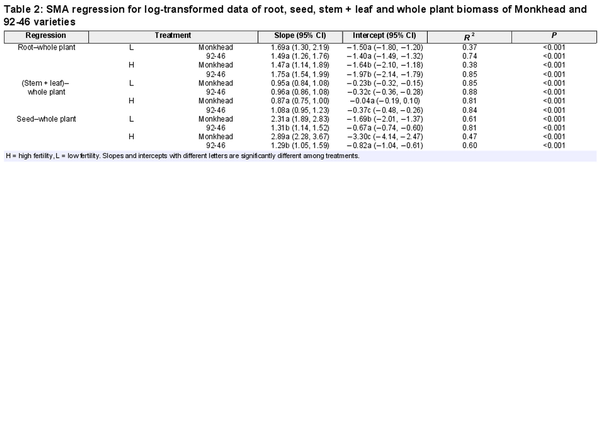
We found significant linear relationships between whole plant biomass (denoted as ‘total’ hereon) and reproductive or non-reproductive components across the treatments (Table 2). In the root–total relationships, both the slope and intercept were similar between Monkhead and 92-46 both fertility levels (Table 2; Fig. 3a and b). The use of fertilizer did not affect the slope but lowered the intercept for both varieties (Table 2; Fig. 3a and b). In the (stem + leaf)–total relationships, the slopes of the two varieties were similar, but the intercept for Monkhead was greater compared with that of 92-46 in fertility levels (Table 2; Fig. 3c and d). Fertilizer addition did not affect the slope for the two varieties or the intercept for 92-46, but it did increase the intercept for Monkhead (Table 2; Fig. 3c and d). In the seed–total relationships, the slope for Monkhead was greater than 92-46, and the intercept was low at both fertility levels (Table 2; Fig. 3e and f). Application of fertilizer did not affect the slopes for either variety or the intercept for 92-46, but it decreased the intercept for Monkhead (Table 2; Fig. 3e and f).
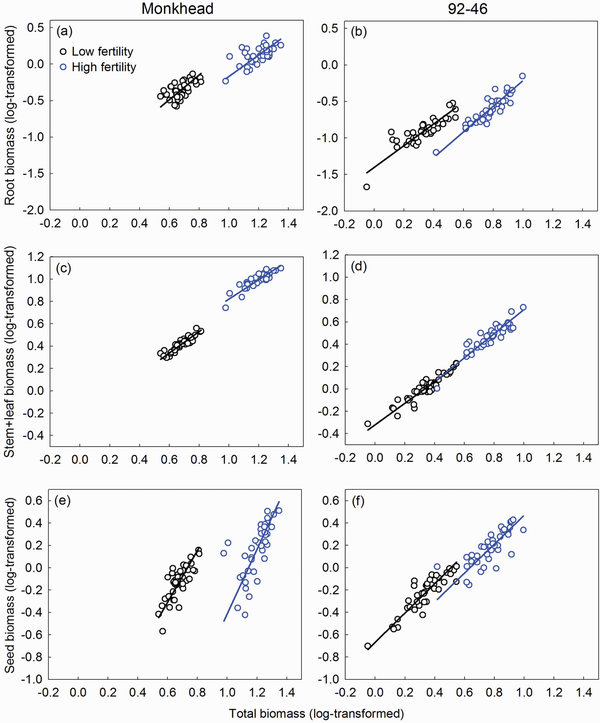
Figure 3
Regression between whole plant biomass and root biomass (a, b), stem + leaf biomass (c, d) and seed biomass (e, f) of Monkhead and 92-46. Regression lines are shown.
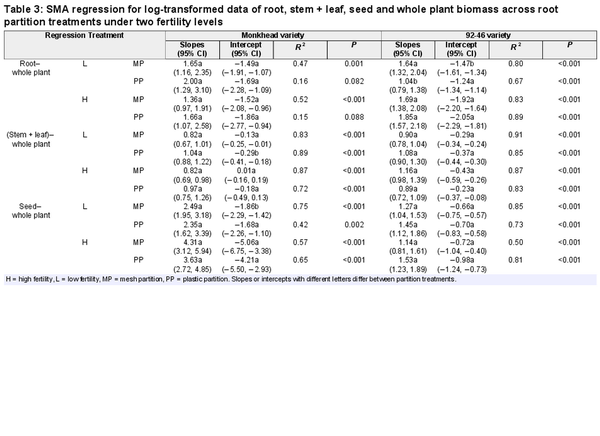
In 24 allometric comparisons (slopes and intercepts) between the mesh and plastic partitions, only four showed small differences in slopes or intercepts, all of which appeared only in the low-fertility treatments (Table 3; Supplementary Figs S1 and S2). Under the low-fertility treatments, the intercept in the seed–total biomass regression was slightly lower in the mesh partition than in the plastic partition for Monkhead. However, in the (stem + leaf)–total biomass regression, the intercept was higher (Table 3; Supplementary Fig. S1c and e). Under insufficient nutrient supply, the slope of the root–total biomass regression was high in the mesh partition, but the intercept was lower for the 92-46 variety (Table 3; Supplementary Fig. S1b).
DISCUSSION
Modern 92-46 allocates more resources to seeds at the expense of stem and leaves
Modern crop varieties possess high population yields, but low competitive capability and individual fitness (; ; ). Our first prediction was based on these findings and supported by the current results. Although old Monkhead had greater root biomass than modern 92-46 (Fig. 2), static allometric analysis suggested that Monkhead had similar root allocation as 92-46 regardless of fertility level (Table 2; Fig. 3a and b). Static allometric analysis also indicated that Monkhead allocated more biomass to stem + leaf than 92-46 (i.e. greater intercepts of the (stem + leaf)–total biomass regression; Table 2; Fig. 3c and d) and concurrently less to seeds (i.e. lower intercepts of the seed–total biomass regression; Table 2; Fig. 3e and f). The lowered seed allocation suggests that modern 92-46 consumes less resources to produce similar amounts of seeds, compared with old Monkhead. This reflects a more efficient conversion of vegetative to reproductive biomass (). The presence of stem + leaf redundancy in old Monkhead may reflect the greater tiller number in individual plants observed in this study (Fig. 2a). These data revealed a trade-off between above-ground non-reproductive and reproductive allocations, which results in a genetically fixed tragedy of the commons in old Monkhead and enhanced yield in 92-46. These data are consistent with the prediction of Darwinian agriculture that high-yield breeding in crops has inadvertently implemented group selection (; ) and selected ‘communal’ crop plants () with reduced growth redundancy (e.g. redundancy in stems and leaves in the present study) (). We suggest that further increase in yield can be obtained if breeders consciously implement this process. In fact, much effort in crop breeding has gone into using the harvest index (grain yield divided by biomass) as a selection criterion (), which is essentially a similar idea to the method of reducing growth redundancy.
However, one limitation of the present study is that our sample size was only one for each group of modern versus old cultivars. Any conclusions based on such a small sample size can only be tentative. Further work is required to test the generality of our findings using numerous new and old cultivars across different crop plants.
Similar to previous studies that claimed root redundancy in old grain varieties (; ; ; ), the current study also revealed a higher root biomass (Fig. 2b) and a higher root biomass-to-total biomass ratio (Supplementary Fig. S3) in old Monkhead compared with that in 92-46. However, approaches that use absolute root biomass or biomass ratios to examine resource allocation patterns have been criticized for confounding the effects of plant size, because plant growth is allometric (; ; ; ; ). In a study that conducted static allometric analysis, old wheat varieties allocated more biomass to roots and less to shoots compared with modern varieties; however, the old varieties reduced root allocation in the last 15 days (). It is likely that the magnitude of root redundancy in old varieties has been overestimated in previous studies. Consistent with the current study, a study on oats using static allometric analysis also found unchanged root allocation and a trade-off between stem + leaf and reproductive allocations (). Regardless of where the growth redundancy is (e.g. in roots), Darwinian agriculture/evolutionary agroecology can help breeders increase crop yield by focussing on traits that consider trade-offs between individual fitness and community performance (; ; , ; ) and not on traits that ignore trade-offs, such as photosynthetic efficiency, which has been optimized for thousands or even millions of years ().
A tragedy of the commons in wheat arises generally from genetically fixed traits rather than from plastic behaviour of individuals
The pot partitioning treatments allowed us to exclude the confounding effects of rooting space when testing plant responses to neighbours (). Our results were consistent with our second and third predictions: a plastic tragedy of the commons in response to an intra-variety neighbour existed only in treatments of old Monkhead and low fertility. Allocation to stems and leaves was slightly greater in the mesh partition than in the plastic partition (i.e. greater intercepts of the (stem + leaf)–total biomass regression in the mesh partition; Table 3; Supplementary Fig. S1c), and concurrently slightly lower allocation to seeds (i.e. lower intercepts of the seed–total biomass regression in the mesh partition; Table 3; Supplementary Fig. S1e). Stem + leaf biomass was also greater in the mesh partition (by 14.8%, Fig. 2c), and this consistency confirmed the occurrence of a plastic tragedy of the commons in old Monkhead at low-fertility levels. In the current study, tragedy may have little ecological significance owing to its small magnitude. also found a decreased tragedy of the commons in a modern variety relative to old Monkhead; however, they only used biomass as an index to test hypotheses and found significant root proliferation in response to competing neighbours.
A meta-analysis of 14 published papers showed that the plastic tragedy of the commons is phylogenetic. The ‘tragedy’ strategy clustered within Fabaceae but not in the Gramineae crops that were investigated, which included three Oryza sativa, two Zea mays and one Avena sativa () varieties. Consistent with wheat in the present study, all these Gramineae crop species exhibited genetically fixed growth redundancy (; ; , ). This consistency suggests that the tragedy of the commons in cereal crops generally arises from genetically fixed traits rather than from the plastic behaviour of individuals. Thus, for high-yield breeding of cereal crops, we speculate that focussing on reducing growth redundancy may be better than attempting to decrease or eliminate plastic proliferation in response to individual competition. Further research is required to confirm this hypothesis.
However, instead of observing a tragedy of the commons in response to a neighbour’s presence (; ; ), some studies have observed root segregation () and increased root allocation (; ). These seemingly inconsistencies between similar experiments can be explained by the effect of inter-plant distance on the expression of traits that result in a tragedy of the commons ().
In our experiments, pairs of wheat plants were physically isolated in both the mesh and plastic partitions, thus excluding the effect of physical space. The exchange of root exudates and nutrients was allowed in the mesh partition but not in the plastic partition. We excluded the size effect using SMA analysis in cases where it could be caused by nutrient exchange. Thus, the slight shift in resource allocation in response to the intra-variety neighbour in Monkhead at low fertility must have been caused by root exudates. This has been found to play a role in self–non-self and kin–strange recognition (; ; ; ). Our results suggest that wheat individuals may have recognized a neighbour through root exudates and transmitted the information above ground, which resulted in stem and leaf proliferation.
Modern 92-46 uses additional resources in a conservative way, whereas old Monkhead does it in an exploitative way
In the current study, all component biomass increased in wheat plants in response to fertilizer addition, but the degree differed in different components of Monkhead and 92-46 (Fig. 2), as manifested by their resource allocation patterns revealed by SMA analysis (Table 2; Fig. 3). Fertilizer application increased biomass allocation to the stems and leaves of Monkhead, but reduced biomass allocation to roots and seeds (Table 2; Fig. 3a, c and e). In contrast, for 92-46, fertilizer use did not alter biomass allocation to stems, leaves and seeds, but reduced biomass allocation to roots (Table 2; Fig. 3b, d and f). These data revealed a lowered response to fertilizer addition in modern 92-46, which demonstrates that old Monkhead adopts an exploitative way of resource use by allocating more to stems and leaves, whereas 92-46 takes a conservative approach, which tends to save resources for seed production. This shift in resource use strategy may result from crop breeding in cultivated systems with consistently high fertility. An alternative explanation is that this shift may be a natural result of high-yield breeding (mostly using the harvest index as a selection criterion), which unconsciously selects genotypes with reduced individual fitness (, ; ). Reduced individual fitness can be manifested as few tillers and numerous vertical stems (), a smaller root system (), few seminal roots (), reduced diameter of the metaxylem vessels of the seminal roots (), and in the present study, reduced allocation to non-reproductive growth in modern 92-46. Thus, from a resource use perspective, our results verify the breeding approach of selecting crop genotypes with low individual fitness (; ; ; ).
CONCLUSIONS
To increase yield, modern 92-46 decreased resource allocation to above-ground vegetative growth compared with old Monkhead, reflecting a trade-off between individual competitiveness and crop yield. The tragedy of the commons in wheat generally arises from genetically fixed traits in terms of growth redundancy in old Monkhead, rather than from the plastic behaviour of individuals as intra-variety neighbours compete. Modern 92-46 adopts a conservative strategy of resource use related to efficiency in seed production, whereas old Monkhead adopts an exploitative strategy related to high individual competitiveness. Based on a limited sample size, our results suggest that breeders can make further increases in yield by consciously selecting traits that reduce individual competitiveness, regardless of whether traditional breeding methods or modern molecular technologies are used. For breeding cereal crops, we speculate that focussing on decreasing genetically fixed growth redundancy may be better than attempting to reduce or eliminate plastic responses to individual competition. In conclusion, these data provide experimental support for the theory of Darwinian agriculture/evolutionary agroecology and explore a more specific and effective direction for breeding cereal crops in the future.
REFERENCES
- Bonser SP (2020) Size and phenology control plant reproduction and agricultural production. A commentary on: ‘How plant allometry influences bud phenology and fruit yield in two Vaccinium species’. Ann Bot126:vi–vii.
- Borlaug NE (2007) Sixty-two years of fighting hunger: personal recollections. Euphytica157:287–297.
- Cabal C, Martínez-García R, Aguilar A, et al. (2020) The exploitative segregation of plant roots. Science370:1197–1199.
- Chen BJW, During HJ, Anten NPR (2012) Detect thy neighbor: identity recognition at the root level in plants. Plant Sci195:157–167.
- Chen BJW, During HJ, Vermeulen PJ, et al. (2015) Corrections for rooting volume and plant size reveal negative effects of neighbour presence on root allocation in pea. Funct Ecol29:1383–1391.
- de Kroon H, Hendriks M, van Ruijven J, et al. (2012) Root responses to nutrients and soil biota: drivers of species coexistence and ecosystem productivity. J Ecol100:6–15.
- Denison RF (2012) Darwinian Agriculture: How Understanding Evolution Can Improve Agriculture. Princeton, NJ: Princeton University Press.
- Denison RF, Kiers ET, West SA (2003) Darwinian agriculture: when can humans find solutions beyond the reach of natural selection?Q Rev Biol78:145–168.
- Donald CM (1968) The breeding of crop ideotypes. Euphytica17:385–403.
- Donald CM (1981) Competitive plants, communal plants, and yield in wheat crops. In Evans LT, Peacock WJ (eds). Wheat Science—Today and Tomorrow. Cambridge, UK: Cambridge University Press, 223–247.
- Du YL, Xi Y, Cui T, et al. (2020) Yield components, reproductive allometry and the tradeoff between grain yield and yield stability in dryland spring wheat. Field Crops Res257:107930.
- Dudley SA, File AL (2007) Kin recognition in an annual plant. Biol Lett3:435–438.
- Duncan WG, Williams WA, Loomis RS (1967) Tassels and the productivity of maize. Crop Sci7:37–39.
- Duvick DN, Cassman KG (1999) Post–green revolution trends in yield potential of temperate maize in the north-central United States. Crop Sci39:1622–1630.
- Fang SQ, Clark RT, Zheng Y, et al. (2013) Genotypic recognition and spatial responses by rice roots. Proc Natl Acad Sci U S A110:2670–2675.
- Gersani M, Brown JS, O’Brien EE, et al. (2001) Tragedy of the commons as a result of root competition. J Ecol89:660–669.
- Hardin G (1968) The tragedy of the commons. Science162:1243–1248.
- Henry HAL, Aarssen LW (1999) The interpretation of stem diameter-height allometry in trees: biomechanical constraints, neighbour effects, or biased regressions?Ecol Lett2:89–97.
- Hess L, de Kroon H (2007) Effects of rooting volume and nutrient availability as an alternative explanation for root self/non-self discrimination. J Ecol95:241–251.
- Jennings PR, de Jesus J (1968) Studies on competition in rice. I. Competition in mixtures of varieties. Evolution22:119–124.
- Kong CH, Zhang SZ, Li YH, et al. (2018) Plant neighbor detection and allelochemical response are driven by root-secreted signaling chemicals. Nat Commun9:3867.
- Li L (2017) Genetic dissection of root morphology and drought-tolerant related physiological traits in wheat (Triticum aestivum L.). Ph.D. Thesis. Beijing: Chinese Academy of Agricultural Sciences.
- Li H, Zhang DY (1999) Morphological characteristics and growth redundancy of spring wheat root system in semi-arid regions. Chin J Appl Ecol10:26–30.
- Mahall BE, Callaway RM (1991) Root communication among desert shrubs. Proc Natl Acad Sci U S A88:874–876.
- Maina GG, Brown JS, Gersani M (2002) Intra-plant versus inter-plant root competition in beans: avoidance, resource matching or tragedy of the commons. Plant Ecol160:235–247.
- McConnaughay KDM, Bazzaz FA (1991) Is physical space a soil resource?Ecology72:94–103.
- Müller I, Schmid B, Weiner J (2000) The effect of nutrient availability on biomass allocation patterns in 27 species of herbaceous plants. Perspect Plant Ecol3:115–127.
- Niklas KJ (2006) Plant allometry, leaf nitrogen and phosphorus stoichiometry, and interspecific trends in annual growth rates. Ann Bot97:155–163.
- O’Brien EE, Gersani M, Brown JS (2005) Root proliferation and seed yield in response to spatial heterogeneity of below-ground competition. New Phytol168:401–412.
- Padilla FM, Mommer L, de Caluwe H, et al. (2013) Early root overproduction not triggered by nutrients decisive for competitive success belowground. PLoS One8:e55805.
- Passioura JB (1983) Roots and drought resistance. Agric Water Manage7:265–280.
- Preece C, Clamp NF, Warham G, et al. (2018) Cereal progenitors differ in stand harvest characteristics from related wild grasses. J Ecol106:1286–1297.
- Qin X, Niklas KJ, Qin L, et al. (2012) The effects of domestication on the scaling of below- vs. aboveground biomass in four selected wheat (Triticum; Poaceae) genotypes. Am J Bot99:1112–1117.
- Schenk HJ (2006) Root competition: beyond resource depletion. J Ecol94:725–739.
- Schenk HJ, Callaway RM, Mahall BE (1999) Spatial root segregation: are plants territorial?Adv Ecol Res28:145–180.
- Semchenko M, Hutchings MJ, John EA (2007) Challenging the tragedy of the commons in root competition: confounding effects of neighbour presence and substrate volume. J Ecol95:252–260.
- Semchenko M, Saar S, Lepik A (2017) Intraspecific genetic diversity modulates plant–soil feedback and nutrient cycling. New Phytol216:90–98.
- Semchenko M, Zobel K (2005) The effect of breeding on allometry and phenotypic plasticity in four varieties of oat (Avena sativa L.). Field Crops Res93:151–168.
- Siddique KHM, Belford RK, Tennant D (1990) Root: shoot ratios of old and modern, tall and semi-dwarf wheats in a Mediterranean environment. Plant Soil121:89–98.
- Smyčka J, Herben T (2017) Phylogenetic patterns of tragedy of commons in intraspecific root competition. Plant Soil417:87–97.
- Song L, Li FM, Fan XW, et al. (2009) Soil water availability and plant competition affect the yield of spring wheat. Eur J Agron31:51–60.
- Song L, Zhang DW, Li FM, et al. (2010) Drought stress: soil water availability alters the inter- and intra-cultivar competition of three spring wheat cultivars bred in different eras. J Agron Crop Sci196:323–335.
- Tilman D, Balzer C, Hill J, et al. (2011) Global food demand and the sustainable intensification of agriculture. Proc Natl Acad Sci U S A108:20260–20264.
- Warton DI, Wright IJ, Falster DS, et al. (2006) Bivariate line-fitting methods for allometry. Biol Rev81:259.
- Weiner J (2004) Allocation, plasticity and allometry in plants. Perspect Plant Ecol6:207–215.
- Weiner J (2019) Looking in the wrong direction for higher-yielding crop genotypes. Trends Plant Sci24:927–933.
- Weiner J, Andersen SB, Wille WKM, et al. (2010) Evolutionary agroecology: the potential for cooperative, high density, weed-suppressing cereals. Evol Appl3:473–479.
- Weiner J, Du YL, Zhang C, et al. (2017) Evolutionary agroecology: individual fitness and population yield in wheat (Triticum aestivum). Ecology98:2261–2266.
- Weiner J, Du YL, Zhao YM, et al. (2021) Allometry and yield stability of cereals. Front Plant Sci12:681490.
- Weiner J, Rosenmeier L, Massoni ES, et al. (2009) Is reproductive allocation in Senecio vulgaris plastic?Botany87:475–481.
- Yue K, Fornara DA, Li W, et al. (2021) Nitrogen addition affects plant biomass allocation but not allometric relationships among different organs across the globe.J Plant Ecol14:361–371.
- Zhai L, Xie R, Ma D, et al. (2015) Evaluation of individual competitiveness and the relationship between competitiveness and yield in maize. Crop Sci55:2307–2318.
- Zhai L, Xie R, Wang P, et al. (2016) Impact of recent breeding history on the competitiveness of Chinese maize hybrids. Field Crops Res191:75–82.
- Zhang DY, Sun GJ, Jiang XH (1999) Donald’s ideotype and growth redundancy: a game theoretical analysis. Field Crops Res61:179–187.
- Zhu YH, Weiner J, Li FM (2019a) Root proliferation in response to neighbouring roots in wheat (Triticum aestivum). Basic Appl Ecol39:10–14.
- Zhu YH, Weiner J, Yu MX, et al. (2019b) Evolutionary agroecology: trends in root architecture during wheat breeding. Evol Appl12:733–743.
- Zhu L, Zhang DY (2013) Donald’s ideotype and growth redundancy: a pot experimental test using an old and a modern spring wheat cultivar. PLoS One8:e70006.