INTRODUCTION
The relationship between biodiversity and ecosystem functioning has been a major focus of research in ecology. Previous studies have shown that increasing plant diversity has positive effects on ecosystem functioning, such as increasing primary productivity (; ), increasing ecosystem stability (; ), promoting nutrient cycling (; ; ) and improving resistance to diseases and pests (; ; ; ). In agriculture, increasing crop diversity, i.e. intercropping, aims to exploit this biodiversity effect to improve the efficiency of resource utilization and production (; ; ). However, due to the differences in phenology, plant type, grain size and harvest logistics, mixed planting of multiple crops often brings extra burden to farmers during sowing, maintenance and harvest. However, this would be well solved, if mixtures of varietal genotypes with similar growth and development can bring the same effects as species mixtures. In other words, genotype mixtures would lead to biodiversity benefits.
As early as the 1980s and 1990s, research has demonstrated that mixing several varieties of a crop could increase grain yield and reduce disease and pest pressure (; ). Like species diversity, genotype diversity can also affect the structure and functioning of ecosystems (). Some studies further verified the important role of genotype diversity in increasing plant biomass or productivity (; ; ; ; ; ) including crops (; ; ; ; ; ; ; ). However, the large variation in magnitude of genotype diversity effects questions whether genotype diversity can play a stable positive effect (; ; ). Besides, in crop systems, agronomists usually evaluate the success of crop mixture cultivation from agronomically relevant variables, such as biomass, yield and disease resistance, while ecologists assess the potential ecological factors and mechanisms underlying such effects. Here we aim to understand the ecological mechanisms supporting over-yielding and whether crop mixtures can be used to make agriculture more sustainable.
The sampling effect (SE) and complementarity effect (CE) are two components of the net genotype diversity effect (; , ; ). In other words, the net genotype diversity effect might be dominated by one of the two, or the combination of both effects. A previous study found that with the increase of genotypic richness of Oenothera biennis, the diversity of arthropods also increased and the additive (i.e. SE) and non-additive (i.e. CE) effects among genotypes jointly played a role (). In contrast, other studies found CEs play a greater role in aboveground net primary productivity than SEs (). Therefore, it remains unclear to what extent sampling and CEs contribute to genotype mixture effects on yield. Another way to study mechanisms underlying biodiversity effects is the quantification of plant–plant interaction intensities, which are supposed to be less negative with increasing complementarity among plants in a community (). Modern crop cultivars tend to represent weak competitors to reduce the mutual competition between neighboring plants, thus improving the population-level yield (; ). Furthermore, studies indicated that increasing genotype richness might further reduce negative plant–neighbor interactions through complementarity, and therefore further increase the population-level yield (; ; ). However, the ecological mechanisms of genotype diversity in crops have been little studied, and whether a mixture of multiple genotypes could reduce intraspecific competition intensity in wheat is still uncertain.
Recent studies have verified the importance of the environment on the effects of crop species diversity or genotype diversity on productivity (; ; ; ). However, the contrasting results limit our ability to resolve differences between studies. Some studies showed that genotype or species diversity often has more obvious effects under the condition of stress or limited resources (; ), but suggested the slopes of the biodiversity–productivity relationships increased with increasing levels of management intensity. Therefore, we need to understand how the over-yielding effects of genotype diversity vary under different environmental conditions.
Pot and field experiments are the two main methods in agricultural research along with their own advantages and disadvantages, such as pot experiments being easy to control and manage and field experiments being closer to the actual situation of crop production. Moreover, both pot and field experiments have often been used interchangeably to test hypotheses about biodiversity–ecosystem functioning relationships and their underlying mechanisms, but the results were not always completely consistent due to the objective, differential environmental conditions of pots compared with fields (; ). For example, researchers often found that plants growing in the glasshouse had higher specific leaf area or leaf biomass than in the field (; ; ). Thus, it is important to assess the genotype diversity effects in the situations of both pot and field experiments, and to judge how well pot experiments can represent field experiments.
In this study, we selected nine different genotypes of spring wheat (Triticum aestivum L.) and designed two planting patterns, a monoculture of one genotype and a mixture of nine genotypes, to simulate the possible effects of genotype richness. The experiment was conducted in both pot and field conditions for one growing season and we collected relevant trait data of spring wheat to verify our hypotheses: (i) genotypic diversity can promote productivity, such as biomass and yield; (ii) planting environment (i.e. pot and field) can affect character and strength of the diversity effect and (iii) CEs might contribute more to the net diversity effect than SEs.
MATERIALS AND METHODS
Experimental site and study species
We conducted the experiment in 2016 at the Dryland Agroecology Field Scientific Observation and Research Station, Yuzhong Campus, Lanzhou University, Gansu Province (35°56’ N, 104°09’ E, 1749 m a.s.l.). The station was a semi-arid dry farming area in the Loess Plateau, with a temperate continental climate. The annual average precipitation in the last 15 years is 289.6 ± 20.6 mm.
Nine spring wheat (T. aestivum L.) genotypes that are commonly planted in dry land and have similar growth periods (101 ± 5 days) were selected for pot and field experiments. The seeds were supplied by the Gansu Academy of Agricultural Sciences (GAAS), Dingxi Academy of Agricultural Sciences (DAAS) and the International Center for Agricultural Research in the Dry Areas (ICARDA) (Supplementary Table S1).
Experimental design
In this study, we selected two commonly used experimental methods to conduct an experiment: artificial pot conditions and real field conditions. The pot experiment was carried out from March to July 2016 in a rainout shelter near the field. The shelter was normally kept open but was closed manually when raining. We selected circular plastic pots (diameter 20 cm and height 26 cm) as the containers for planting. Soil in pots was taken from the top layer of the field and mixed with vermiculite at a uniform 1:3 ratio. We ensured each pot had the same soil weight (14.0 kg) and was watered with nutrient solution (1 g N, 0.27 g P and 0.35 g K per pot) before sowing. We conducted three planting treatments: monoculture, mixture and single plant (Fig. 1a–d). In mixture, one of the nine genotypes was used as the center plant, and the other eight genotypes were placed around it in random order and equal distance; while in monoculture, all the nine positions were of the same genotype. To ensure uniformity of mixture, each of the nine genotypes was used separately as a center plant. In addition, we had single plants in pots to observe the maximum potential of each genotype as a single plant without neighbors (Fig. 1d). Monocultures had three replications, mixtures and single treatments had four replicates, respectively. Therefore, there are a total of 99 pots in this pot experiment. All pots were distributed by using a completely randomized design. Pots were watered by the weighing method to 70% of the soil field capacity every day. Weeds were pulled by hand, and pests and diseases were controlled by spraying insecticide and fungicide once at each of the jointing stage, heading stage and grain filling stage, according to the local farmer’s management practices.
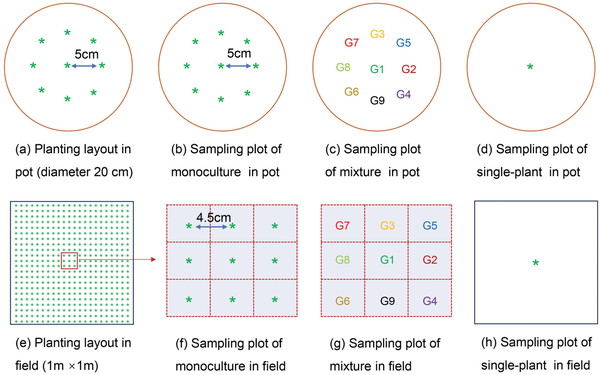
Figure 1
The schematic diagram of the experimental design in pots (a–d) and in the field (e–h).
The field experiment was conducted simultaneously with the pot experiment to test the realistic field production environment. Seeds were sown by hand at a depth of 3 cm with a planting density of 484 plants m−2 and the plot size was 1 m × 1 m. To ensure the same genotype layout as potted plants and to facilitate sampling of wheat individuals, we selected the central sampling plot as the sampling area (Fig. 1e–g). Genotypes outside the sampling plot were planted randomly, but they were not recorded but used for total aboveground biomass and yield data collection. The monocultures had three replicates, and to ensure the survival of central sampling plants in the mixture, we performed six replicates, and selected all the surviving five replicates for data analysis. In order to measure each genotype’s performance in the field, a single plant was sown in the center of the sample area (1 m × 1 m) with four replicates (Fig. 1h). Therefore, there are a total of 108 plots in this field experiment, 27 plots for monoculture, 45 plots for mixture and 36 plots for single plants. All plots in the field experiment were distributed using a completely randomized design. 4 mm rainfall sprinkler irrigation was carried out with micro-spray equipment to ensure seed germination. The fertilizer of N, P and K was 31.8, 8.6 and 11.1 g m−2, respectively, in the field according to the fertilization practices of local farmers. Weeds were pulled by hand, and pests and diseases were controlled by spraying insecticide and fungicide once at each of the jointing stage, heading stage and grain filling stage, following the local farmer’s management practices.
Data collection
In the field experiment, the daily precipitation data were obtained from the Lanzhou University Meteorological Station near the experimental site. In the pot experiment, the watering data of each pot were summarized every day. During the whole growth period, the rainfall in the field was about 118.9 mm and the average watering amount in pots was 327.9 mm.
The leaf area of each plant was measured manually at the time of flowering in every sampling plot. And after the spring wheat had matured, individual plants in pots and central sampling areas of plots in the field were harvested with scissors. All 9 plants per pot or plot were kept separately in sampling bags. The flag leaf height and plant height of the main stem and all tillers were measured and then all tillers were separated into stems, leaves, spikes and grain. All remaining spring wheat plants in the field plots (1 m × 1 m) were harvested together and dried to measure aboveground biomass and seed yield as a whole. The soil of every pot was sieved with a 0.5-mm mesh and roots were washed under flowing running water to collect the root biomass. All samples were dried at 75 °C to a constant weight and each individual plant was weighed on an electronic balance for stem weight, leaf weight, spike weight and seed weight of pots and field plots and root weight of pots.
Statistical analysis
We used linear mixed-effects models (LMMs) to test the effects of planting environment (pot or field), planting pattern (monoculture or mixture) and their interactions on plot-level variables (). These variables included aboveground biomass, grain yield, plant height, stem thickness, stem biomass, leaf biomass, spike biomass and grain number. In the analysis, planting environment and planting pattern were treated as fixed factors, composition (genotype_ID in monocultures and ‘MIX’ for all mixtures) was considered a random factor. Due to variations in the repetition of planting patterns, we incorporated a separate variance structure for the factor levels of planting patterns.
The additive partitioning method was used to calculate the net effect, CE and SE, respectively, for aboveground biomass and grain yield to evaluate variations in diversity effects (; ):
where ΔYield is the net effect, which is calculated as the difference between mixture and monoculture. N means the number of the genotypes, which is nine in this study. ΔRY represents the deviation from the anticipated relative yield of the genotypes within the mixture in the corresponding plot. This is determined by comparing the observed relative yield of the genotypes in the mixture to its yield in monoculture, expressed as the ratio. M denotes the yield of the genotypes when grown in monoculture. Yield refers to the aboveground biomass and the grain yield. is the expression for CE and is the expression for SE. A linear model was used to test differences of response between experimental treatments. The net effect, CE or SE were used as response variables.
A linear model was used to calculate the effect of neighbor plants on the grain yield and harvest index of the central plant. Planting environment (pot and field) and planting pattern (monoculture and mixture) were used as explanatory variables and the biomass of all eight neighboring plants was used as a covariable.
Relative interaction index (RII), an index representing the intensity of plant–plant interactions, was calculated Equation (2) ().
In Equation (2), Bc represents the biomass of a plant when grown in a community (monoculture and mixture), and Bs represents the biomass of a single plant grown without neighbors.
All analyses and graphical presentations were done in R software (4.2.3). The LMM was conducted using the ‘nlme’ package. The ‘emmeans’ package was used to test the comparison of all effects in the model. The standardized major axis regression was conducted using the ‘smatr’ package. Comparison analyses of RII between monoculture and mixture were conducted using the ‘rstatix’ package by Welch’s t-test. Package ‘ggplot2’ was used for graphing.
RESULTS
Effect of genotype diversity on spring wheat traits under different environments
Phenotypic characteristics at the sampling plot level were all significantly affected by the planting environment, with higher values observed in pots compared with those in the field (Fig. 2; Supplementary Table S2). The aboveground biomass, grain yield, stem weight, leaf weight, spike weight and grain number were affected by the interaction of planting environment and planting pattern: mixtures increased these variables under pot conditions but not under field conditions (Fig. 2; Supplementary Table S2).
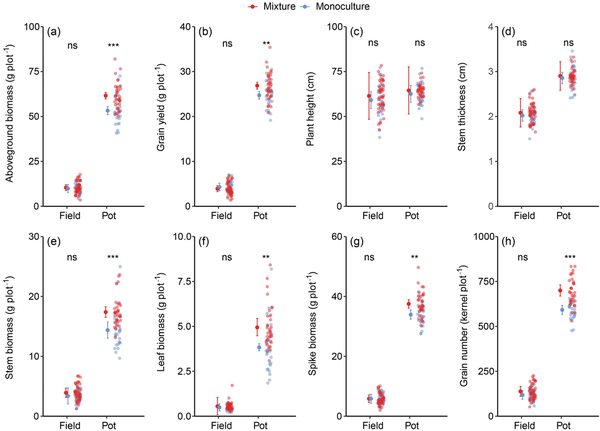
Figure 2
Effect of genotype diversity on plot-level performance of spring wheat under field and pot conditions. (a) Aboveground biomass, (b) grain yield, (c) plant height, (d) stem thickness, (e) stem weight, (f) leaf weight, (g) spike weight and (h) grain number. Apart from observed values, the mean and 95% CI are shown. n (monoculture in field) = 27, n (mixture in field) = 45, n (monoculture in pot) = 27 and n (mixture in pot) = 36. ** and *** indicate significant differences between monoculture and mixture at 0.01 and 0.001 levels, respectively, ns indicates no significant difference between the two.
Compared with monocultures, genotype mixtures significantly enhanced the aboveground biomass and grain yield by 14.5% and 8.2%, respectively, in pots (Fig. 2a and b). However, no significant effects of genotype diversity on aboveground biomass and seed yield were found in the field, neither at the sampling plot level (Fig. 2a and b) nor at the actual production level (Supplementary Fig. S1). Plant height and stem thickness in mixtures were slightly, but not significantly higher than in monocultures under the two conditions (Fig. 2c and d). The stem weight, leaf weight, spike weight and grain number showed the same characteristics, that was, they were significantly increased in mixtures compared with monocultures only under pot conditions (Fig. 2e–h).
SE, CE and net effect
The CE, rather than the SE, was more likely to influence the diversity effect; the values of CE were higher than those of SE both in pot and field conditions (Fig. 3). In the field, the CE of aboveground biomass was significantly positive (95% confidence interval [CI]: 0.19–2.73), while the SE was negative. But both CE and SE of grain yield were negative, which indicated that monocultures and mixtures did not produce differences in grain yield under field conditions. In the pot, the SEs of aboveground biomass and yield were negative, while the CEs were significantly positive (95% CIs: 9.88–12.86 for aboveground biomass and 2.68–5.68 for grain yield), and were higher than the SE, resulting in a positive net diversity effect (95% CIs: 7.23–10.83 for aboveground biomass and 0.65–4.25 for grain yield).
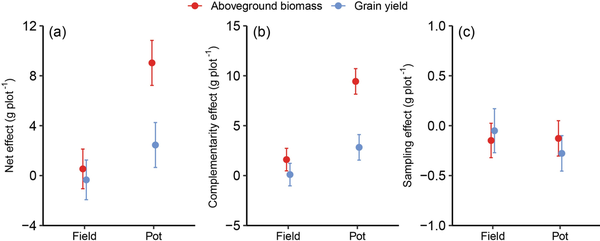
Figure 3
Diversity effects of spring wheat on aboveground biomass and grain yield. Data are mean and 95% CI for net effect (a), CE (b) and SE (c).
Plant–plant interactions in communities
We found that the effects of the neighbor plant’s biomass on the central plant’s grain yield and harvest index were significantly dependent on the planting pattern and planting environment (Fig. 4; Supplementary Table S3). In monocultures, the relationship between the neighboring biomass and grain yield of the central plant was negative (P = 0.30 and 0.37, respectively, in pot and field), and the relationship with the harvest index was significantly negative (P = 0.003 and 0.03 in pot and field, respectively). However, in mixtures, the relationship between the neighboring biomass and the central plant’s grain yield or harvest index changed to be a non-significant positive correlation. Furthermore, compared with monocultures, mixtures significantly changed these relationships in the pot environment, but not in the field environment (Supplementary Table S3). This indicated that mixtures reduced the negative impact of neighbors on the focal plant, especially in the pot environment. Additionally, the RII further demonstrated that mixtures significantly (P < 0.01, Welch’s t-test) reduced the intensity of plant–plant interactions compared with monocultures under pot conditions (Fig. 5).
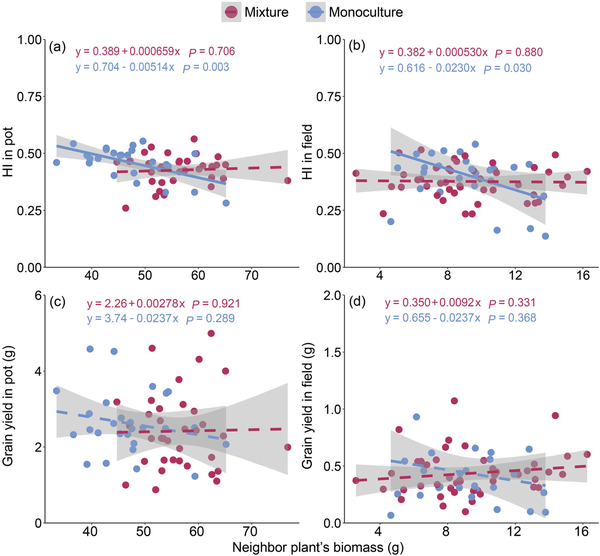
Figure 4
Relationships of the neighbor plant’s biomass with harvest index (HI) and grain yield of the central plant under pot (a and c) and field (b and d) conditions. Shaded regions indicate 95% CIs around the fitted lines. n (monoculture in field) = 27, n (mixture in field) = 45, n (monoculture in pot) = 27 and n (mixture in pot) = 36.
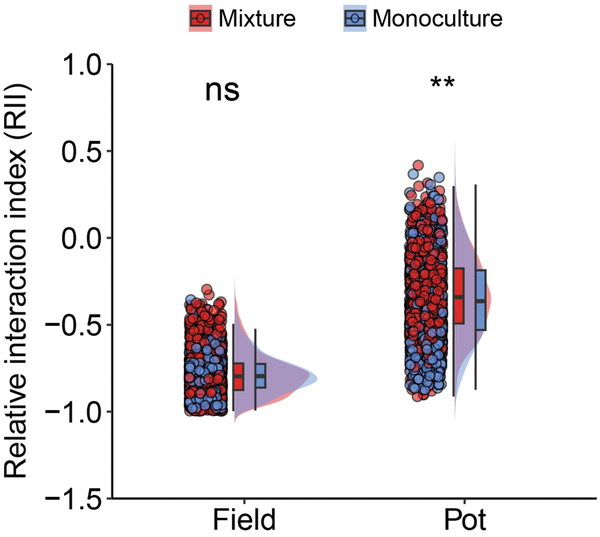
Figure 5
Effect of planting pattern on RII in pot and field conditions. n (monoculture in field) = 972, n (mixture in field) = 1620, n (monoculture in pot) = 972 and n (mixture in pot) = 1260. ** indicates significant differences between monoculture and mixture at 0.01 levels (Welch’s t-test), and ns indicates no significant difference between the two.
DISCUSSION
Previous studies on the impact of species diversity or genotypic diversity on productivity have shown that the environment plays a crucial role in the realization of diversity effects (; ; ; ). Our results are consistent with them, and we found that the positive effect of genotypic diversity was not expressed in the rainfed field environment but was observed in the artificial pot environment, which received more water supply. Although some studies indicated that with more effective use and sharing of resources, genotype diversity or species diversity often exhibit more positive effects under stressful conditions characterized by limited resources (; ; ), it is speculated that our opposite result can be explained by the varying effects of different planting environments on the ecological mechanisms underlying diversity effects.
From the perspective of diversity effect composition, we found that the SE exhibited a weak negative effect under both pot and field conditions (Fig. 3). This suggests that no specific spring wheat genotype dominated or suppressed others in mixtures, regardless of whether they were under field or pot conditions. However, the CE revealed distinct results between different environments, with no significant effect in the field but significant positive effects in the pot (Fig. 3). This suggests that the manifestation of the positive net effects of mixtures in pots is mainly driven by complementarities between genotypes. This is consistent with the findings of recent studies, which demonstrated that the yield increase of crop mixtures was primarily due to CEs (; ; ; ; ). Previous studies have demonstrated that the effectiveness of genotype diversity depends on the size of the potential genetic variation; the greater the difference in traits among individuals, the stronger the potential ecological CE (; ). Environmental conditions are important factors affecting the expression of this genetic variation (), and the full potential of genotypic differences is more easily expressed under relatively favorable conditions ().
In our study, the nine spring wheat genotypes, all of which are dryland wheat cultivars, have been adapted to the local water-scarce growing environment through a long-term drought domestication process. In the field environment, individual trait differences between genotypes were small. However, in the pot environment, greater water availability (2.8 times that of the field) promoted the expression of potential genetic variation, resulting in significant differences in individual traits among the spring wheat genotypes. We observed that the individual traits of spring wheat under pot conditions were much greater than under field conditions (Supplementary Figs S2 and S4) and showed higher ratios of coefficients of variance (Supplementary Fig. S3). This implies that mixed populations of spring wheat in pots exhibit greater ecological niche differentiation compared with those in fields, which is an important factor in promoting the emergence of CEs in mixtures.
Based on the ecological niche differentiation among spring wheat genotypes in pots, genotype mixtures can more fully utilize resources. For example, in pots we observed that the mixtures exhibited a higher specific leaf area and lower root biomass compared with the monocultures (Supplementary Fig. S5), showcasing the benefits of collective production in utilizing aboveground light and belowground resources. This is consistent with previous studies (; ), which have demonstrated that genotype mixtures can optimize canopy structure and enhance the efficiency of capturing light, thereby increasing grain yield. In addition, compared with monocultures, mixtures of spring wheat reduced intraspecific competition. The negative correlations in monocultures between neighbor biomass and the focal plant’s grain yield or harvest index turned to positive or unrelated in mixtures (Fig. 4) and the RII was increased (Fig. 5). These results are consistent with the results of and , who found that competition intensity is highest in monocultures, but decreased with higher genotype diversity, while the productivity also increased with genotypic richness. This reflects the important relationship between grain yield benefits driven by spring wheat genotype diversity and niche partitioning reducing plant interaction intensity and increasing complementarity.
Capitalizing on the positive effects of genotypic diversity to increase crop yields is currently a hot topic among ecologists and agronomists. By analyzing the underlying ecological mechanisms, mixtures designed using a trait-based approach prove to be more effective in generating positive effects (; ). It is interesting to note from this study that the influence of the planting environment on diversity effects is reflected in the realization of the unique genotypic potential. In the arid and semi-arid Loess Plateau region, where rainfed agriculture is prevalent, limited rainfall suppressed the genetic potential of the genotypes, leading to similar individual characteristics across all genotypes. This, in turn, limited the ecological niche differentiation within the mixtures, thereby restricting the emergence of effective positive complementarities. Therefore, we hypothesize that the over-yielding effect of genotype diversity may also be observed under water-stressed field conditions when appropriate water replenishment strategies are implemented. In the future, it will be necessary to investigate the positive effects of diversity by identifying varieties or management methods that are suitable for local conditions.
CONCLUSIONS
Our results showed that the positive diversity effect of genotype mixtures of spring wheat was only evident under pot conditions, not under field conditions. In pots, compared with the field rainfed environment, higher water availability promoted the expression of the genotypic potential of spring wheat genotypes, resulting in larger plant differences and ecological niche differentiation, and reduced competition between neighboring plants. This further led to a significantly positive CE and a net diversity effect on both biomass and grain yield. In the present study, the positive effects of genotype diversity occurred under the artificial pot condition but not under the real field conditions due to the different characteristics of the growing environment. This suggests that positive effects found under artificial (pot) conditions are not necessarily retained under more realistic field conditions.
REFERENCES
- Armas C, Ordiales R, Pugnaire FI (2004) Measuring plant interactions: a new comparative index. Ecology85:2682–2686. https://doi.org/10.1890/03-0650
- Barot S, Allard V, Cantarel A, et al. (2017) Designing mixtures of varieties for multifunctional agriculture with the help of ecology: a review. Agron Sustain Dev37:13. https://doi.org/10.1007/s13593-017-0418-x
- Borg J, Kiær LP, Lecarpentier C, et al. (2018) Unfolding the potential of wheat cultivar mixtures: a meta-analysis perspective and identification of knowledge gaps. Field Crops Res221:298–313. https://doi.org/10.1016/j.fcr.2017.09.006
- Bossdorf O, Shuja Z, Banta JA (2009) Genotype and maternal environment affect belowground interactions between Arabidopsis thaliana and its competitors. Oikos118:1541–1551. https://doi.org/10.1111/j.1600-0706.2009.17559.x
- Brooker RW, Hewison R, Mitchell C, et al. (2021) Does crop genetic diversity support positive biodiversity effects under experimental drought? Basic Appl Ecol56:431–445. https://doi.org/10.1016/j.baae.2021.05.001
- Cai T, Peng D, Wang R, et al. (2019) Can intercropping or mixed cropping of two genotypes enhance wheat lodging resistance? Field Crops Res239:10–18. https://doi.org/10.1016/j.fcr.2019.05.009
- Charmantier A, Garant D (2005) Environmental quality and evolutionary potential: lessons from wild populations. Proc Biol Sci272:1415–1425. https://doi.org/10.1098/rspb.2005.3117
- Chen J, Engbersen N, Stefan L, et al. (2021) Diversity increases yield but reduces harvest index in crop mixtures. Nat Plants7:893–898. https://doi.org/10.1038/s41477-021-00948-4
- Cong W-F, van Ruijven J, Mommer L, et al. (2014) Plant species richness promotes soil carbon and nitrogen stocks in grasslands without legumes. J Ecol102:1163–1170. https://doi.org/10.1111/1365-2745.12280
- Cook-Patton SC, McArt SH, Parachnowitsch AL, et al. (2011) A direct comparison of the consequences of plant genotypic and species diversity on communities and ecosystem function. Ecology92:915–923. https://doi.org/10.1890/10-0999.1
- Craven D, Eisenhauer N, Pearse WD, et al. (2018) Multiple facets of biodiversity drive the diversity-stability relationship. Nat Ecol Evol2:1579–1587. https://doi.org/10.1038/s41559-018-0647-7
- Crawford KM, Rudgers JA (2013) Genetic diversity within a dominant plant outweighs plant species diversity in structuring an arthropod community. Ecology94:1025–1035. https://doi.org/10.1890/12-1468.1
- Crawford KM, Whitney KD (2010) Population genetic diversity influences colonization success. Mol Ecol19:1253–1263. https://doi.org/10.1111/j.1365-294X.2010.04550.x
- Crutsinger GM, Collins MD, Fordyce JA, et al. (2006) Plant genotypic diversity predicts community structure and governs an ecosystem process. Science313:966–968. https://doi.org/10.1126/science.1128326
- Denison RF (2015) Evolutionary tradeoffs as opportunities to improve yield potential. Field Crops Res182:3–8. https://doi.org/10.1016/j.fcr.2015.04.004
- Denison RF, Kiers ET, West SA (2003) Darwinian agriculture: when can humans find solutions beyond the reach of natural selection? Q Rev Biol78:145–168. https://doi.org/10.1086/374951
- Drummond EB, Vellend M (2012) Genotypic diversity effects on the performance of Taraxacum officinale populations increase with time and environmental favorability. PLoS One7:e30314. https://doi.org/10.1371/journal.pone.0030314
- Engbersen N, Stefan L, Brooker RW, et al. (2022) Using plant traits to understand the contribution of biodiversity effects to annual crop community productivity. Ecol Appl32:e02479. https://doi.org/10.1002/eap.2479
- Fang Y, Xu B, Liu L, et al. (2014) Does a mixture of old and modern winter wheat cultivars increase yield and water use efficiency in water-limited environments? Field Crops Res156:12–21. https://doi.org/10.1016/j.fcr.2013.10.013
- Fridley JD, Grime JP, Bilton M (2007) Genetic identity of interspecific neighbours mediates plant responses to competition and environmental variation in a species-rich grassland. J Ecol95:908–915. https://doi.org/10.1111/j.1365-2745.2007.01256.x
- Gaba S, Lescourret F, Boudsocq S, et al. (2014) Multiple cropping systems as drivers for providing multiple ecosystem services: from concepts to design. Agron Sustain Dev35:607–623. https://doi.org/10.1007/s13593-014-0272-z
- Gallandt ER, Dofing SM, Reisenauer PE, et al. (2001) Diallel analysis of cultivar mixtures in winter wheat. Crop Sci41:792–796. https://doi.org/10.2135/cropsci2001.413792x
- Gardarin A, Celette F, Naudin C, et al. (2022) Intercropping with service crops provides multiple services in temperate arable systems: a review. Agron Sustain Dev42:39. https://doi.org/10.1007/s13593-022-00771-x
- Genung MA, Bailey JK, Schweitzer JA (2012) Welcome to the neighbourhood: interspecific genotype by genotype interactions in Solidago influence above- and belowground biomass and associated communities. Ecol Lett15:65–73. https://doi.org/10.1111/j.1461-0248.2011.01710.x
- Grange G, Finn JA, Brophy C (2021) Plant diversity enhanced yield and mitigated drought impacts in intensively managed grassland communities. J Appl Ecol58:1864–1875. https://doi.org/10.1111/1365-2664.13894
- Hahn PG, Cammarano JH (2023) Environmental context and herbivore traits mediate the strength of associational effects in a meta-analysis of crop diversity. J Appl Ecol60:875–885. https://doi.org/10.1111/1365-2664.14382
- Hautier Y, Tilman D, Isbell F, et al. (2015) Anthropogenic environmental changes affect ecosystem stability via biodiversity. Science348:336–340. https://doi.org/10.1126/science.aaa1788
- Hoffmann AA, Merilä J (1999) Heritable variation and evolution under favourable and unfavourable conditions. Trends Ecol Evol14:96–101. https://doi.org/10.1016/s0169-5347(99)01595-5
- Huang T, Döring TF, Zhao X, et al. (2024) Cultivar mixtures increase crop yields and temporal yield stability globally. A meta-analysis. Agron Sustain Dev44:28. https://doi.org/10.1007/s13593-024-00964-6
- Hughes AR, Hanley TC, Schenck FR, et al. (2016) Genetic diversity of seagrass seeds influences seedling morphology and biomass. Ecology97:3538–3546. https://doi.org/10.1002/ecy.1587
- Hughes AR, Inouye BD, Johnson MTJ, et al. (2008) Ecological consequences of genetic diversity. Ecol Lett11:609–623. https://doi.org/10.1111/j.1461-0248.2008.01179.x
- Hughes AR, Stachowicz JJ (2009) Ecological impacts of genotypic diversity in the clonal seagrass Zostera marina. Ecology90:1412–1419. https://doi.org/10.1890/07-2030.1
- Johnson MTJ, Lajeunesse MJ, Agrawal AA (2006) Additive and interactive effects of plant genotypic diversity on arthropod communities and plant fitness. Ecol Lett9:24–34. https://doi.org/10.1111/j.1461-0248.2005.00833.x
- Juskiw P, Helm J, Burnett P (2001) Three-component barley mixtures: ratio effects in replacement series. Can J Plant Sci81:651–656. https://doi.org/10.4141/p00-145
- Kathju S, Garg BK, Vyas SP, et al. (2003) Sustainable production of moth bean through genotype management under arid environments. J Arid Environ53:137–143. https://doi.org/10.1006/jare.2002.1026
- Kiær LP, Skovgaard IM, Østergård H (2009) Grain yield increase in cereal variety mixtures: a meta-analysis of field trials. Field Crops Res114:361–373. https://doi.org/10.1016/j.fcr.2009.09.006
- Kong X, Li L, Peng P, et al. (2023) Wheat cultivar mixtures increase grain yield under varied climate conditions. Basic Appl Ecol69:13–25. https://doi.org/10.1016/j.baae.2023.03.007
- Kong X, Zhao G (2023) Increasing yield through wheat cultivar mixture that optimizes functional traits within the canopy. Eur J Agron151:126977. https://doi.org/10.1016/j.eja.2023.126977
- Li C, Hoffland E, Kuyper TW, et al. (2020) Yield gain, complementarity and competitive dominance in intercropping in China: a meta-analysis of drivers of yield gain using additive partitioning. Eur J Agron113:125987. https://doi.org/10.1016/j.eja.2019.125987
- Li C, Li W, Luo Y, et al. (2023) Mixed cropping increases grain yield and lodging resistance by improving the canopy light environment of wheat populations. Eur J Agron147:126849. https://doi.org/10.1016/j.eja.2023.126849
- Lithourgidis A, Dordas C, Damalas CA, et al. (2011) Annual intercrops: an alternative pathway for sustainable agriculture. Aust J Crop Sci5:396–410.
- Lóopez-Castañeda C, Richards RA, Farquhar GD (1995) Variation in early vigor between wheat and barley. Crop Sci35:472–479. https://doi.org/10.2135/cropsci1995.0011183X003500020032x
- Loreau M, Hector A (2001) Partitioning selection and complementarity in biodiversity experiments. Nature412:72–76. https://doi.org/10.1038/35083573
- Mengistu N, Baenziger PS, Nelson LA, et al. (2010) Grain yield performance and stability of cultivar blends vs. component cultivars of hard winter wheat in Nebraska. Crop Sci50:617–623. https://doi.org/10.2135/cropsci2009.05.0280
- Mitchell CE, Tilman D, Groth JV (2002) Effects of grassland plant species diversity, abundance, and composition on foliar fungal disease. Ecology83:1713–1726. https://doi.org/10.2307/3071990
- Montazeaud G, Violle C, Roumet P, et al. (2020) Multifaceted functional diversity for multifaceted crop yield: towards ecological assembly rules for varietal mixtures. J Appl Ecol57:2285–2295. https://doi.org/10.1111/1365-2664.13735
- Parachnowitsch AL, Cook-Patton SC, McArt SH (2014) Neighbours matter: natural selection on plant size depends on the identity and diversity of the surrounding community. Evol Ecol28:1139–1153. https://doi.org/10.1007/s10682-014-9727-6
- Passioura JB (2006) The perils of pot experiments. Funct Plant Biol33:1075–1079. https://doi.org/10.1071/fp06223
- Peacock L, Hunter T, Turner H, et al. (2001) Does host genotype diversity affect the distribution of insect and disease damage in willow cropping systems? J Appl Ecol38:1070–1081. https://doi.org/10.1046/j.1365-2664.2001.00655.x
- Poorter H, Bühler J, van Dusschoten D, et al. (2012) Pot size matters: a meta-analysis of the effects of rooting volume on plant growth. Funct Plant Biol39:839–850. https://doi.org/10.1071/fp12049
- Poorter H, De Jong ROB (1999) A comparison of specific leaf area, chemical composition and leaf construction costs of field plants from 15 habitats differing in productivity. New Phytol143:163–176. https://doi.org/10.1046/j.1469-8137.1999.00428.x
- Rebetzke GJ, Botwright TL, Moore CS, et al. (2004) Genotypic variation in specific leaf area for genetic improvement of early vigour in wheat. Field Crops Res88:179–189. https://doi.org/10.1016/j.fcr.2004.01.007
- Reiss ER, Drinkwater LE (2018) Cultivar mixtures: a meta-analysis of the effect of intraspecific diversity on crop yield. Ecol Appl28:62–77. https://doi.org/10.1002/eap.1629
- Reusch TBH, Ehlers A, Hämmerli A, et al. (2005) Ecosystem recovery after climatic extremes enhanced by genotypic diversity. Proc Natl Acad Sci USA102:2826–2831. https://doi.org/10.1073/pnas.0500008102
- Schmid B, Baruffol M, Wang Z, et al. (2017) A guide to analyzing biodiversity experiments. J Plant Ecol10:91–110. https://doi.org/10.1093/jpe/rtw107
- Schöb C, Brooker RW, Zuppinger-Dingley D (2018) Evolution of facilitation requires diverse communities. Nat Ecol Evol2:1381–1385. https://doi.org/10.1038/s41559-018-0623-2
- Schöb C, Kerle S, Karley AJ, et al. (2015) Intraspecific genetic diversity and composition modify species-level diversity–productivity relationships. New Phytol205:720–730. https://doi.org/10.1111/nph.13043
- Smithson JB, Lenne JM (1996) Varietal mixtures: a viable strategy for sustainable productivity in subsistence agriculture. Ann Appl Biol128:127–158. https://doi.org/10.1111/j.1744-7348.1996.tb07096.x
- Steinbeiss S, Beßler H, Engels C, et al. (2008) Plant diversity positively affects short-term soil carbon storage in experimental grasslands. Glob Change Biol14:2937–2949. https://doi.org/10.1111/j.1365-2486.2008.01697.x
- Su Y, Yu R-P, Xu H-S, et al. (2023) Crop cultivar mixtures stabilize productivity, partly via facilitation, when conditions are less benign. Field Crops Res302:109046. https://doi.org/10.1016/j.fcr.2023.109046
- Tilman D, Reich PB, Knops J, et al. (2001) Diversity and productivity in a long-term grassland experiment. Science294:843–845. https://doi.org/10.1126/science.1060391
- Tomimatsu H, Nakano K, Yamamoto N, et al. (2014) Effects of genotypic diversity of Phragmites australis on primary productivity and water quality in an experimental wetland. Oecologia175:163–172. https://doi.org/10.1007/s00442-014-2896-8
- Weigelt A, Weisser WW, Buchmann N, et al. (2009) Biodiversity for multifunctional grasslands: equal productivity in high-diversity low-input and low-diversity high-input systems. Biogeosciences6:1695–1706. https://doi.org/10.5194/bg-6-1695-2009
- Wolfe MS (1985) The current status and prospects of multiline cultivars and variety mixtures for disease resistance. Annu Rev Phytopathol23:251–273. https://doi.org/10.1146/annurev.phyto.23.1.251
- Wuest SE, Peter R, Niklaus PA (2021) Ecological and evolutionary approaches to improving crop variety mixtures. Nat Ecol Evol5:1068–1077. https://doi.org/10.1038/s41559-021-01497-x
- Zhu Y, Chen H, Fan J, et al. (2000) Genetic diversity and disease control in rice. Nature406:718–722. https://doi.org/10.1038/35021046
- Zhu Z, Du H, Gao K, et al. (2023a) Plant species diversity enhances soil gross nitrogen transformations in a subtropical forest, Southwest China. J Appl Ecol60:1364–1375. https://doi.org/10.1111/1365-2664.14407
- Zhu S-G, Zhu H, Zhou R, et al. (2023b) Intercrop overyielding weakened by high inputs: global meta-analysis with experimental validation. Agric Ecosyst Environ342:108239. https://doi.org/10.1016/j.agee.2022.108239
