Introduction
Cardiovascular disease is a major health burden worldwide. It has long been established that lifestyle choices, including smoking, an unhealthy diet and physical inactivity, can increase the risk of cardiovascular disease (Lucaroni et al., ). We also know that the gene–environment interaction before birth can influence the cardiovascular risk in offspring. For example, evidence derived from human clinical studies and investigation in preclinical animal models shows that gestation complicated by adverse intrauterine conditions increases susceptibility to cardiovascular dysfunction in progeny via a process known as developmental programming (Crispi et al., ; Gluckman et al., ; Yzydorczyk et al., ). However, the mechanisms underlying developmental programming remain unclear, precluding intervention, particularly identifying therapy of human translational potential.
One of the most common adverse intrauterine conditions known to programme cardiovascular disease in offspring is gestational hypoxia leading to fetal growth restriction (FGR) (Ducsay et al., ; Galli et al., ; Giussani, ; Giussani & Davidge, ). Studies in animal models have established that underlying pathways stimulated by this condition include the genesis of oxidative stress in the developing fetal cardiovascular system (Giussani, ; Giussani & Davidge, ; Giussani et al., ; Herrera et al., ; Krause, Costello et al., ; Penaloza et al., ). Increased oxidative stress in the hypoxic fetus promotes endothelial dysfunction and increases cardiovascular complications in the offspring (Giussani, ; Giussani & Davidge, ; Giussani et al., ; Herrera et al., ; Krause, Costello et al., ; Penaloza et al., ). Therefore, numerous studies in preclinical animal models have reported that maternal treatment with antioxidants in hypoxic gestation can be cardioprotective in offspring (Botting et al., ; Brain et al., ; Giussani, ; Giussani & Davidge, ; Giussani et al., ; Thompson & Al‐Hasan, ). Interestingly, these studies have also revealed that maternal antioxidant treatment restores endothelial function in offspring of hypoxic/FGR gestation by enhancing nitric oxide (NO) independent components of vascular relaxation. However, the nature of this NO independent mechanism of rescued endothelial dysfunction in offspring of hypoxic/FGR gestation and its epigenetic regulation remain completely unknown.
A plausible NO independent candidate mechanism is the increased activity of the gasotransmitter hydrogen sulphide (H2S), which is also known to confer cardiovascular protection via several pathways (Kanagy et al., ; Saif et al., ). For example, H2S is cardioprotective through the opening of myocardial KATP channels in hypoxic and growth‐restricted chicken embryos (Hess et al., ). Furthermore, we and others have reported beneficial effects of the H2S‐precursor N‐acetylcysteine (NAC) against endothelial dysfunction in the hypoxic fetus in guinea‐pigs and rats (Hashimoto et al., ; Herrera et al., ; Patterson et al., ). In humans, maternal I.V. infusion with NAC in late gestation results in a greater NAC umbilical cord to maternal plasma ratio, suggesting rapid placental transfer and a slower rate of fetal NAC clearance, supporting its possible use in pregnancy to provide fetal antioxidant protection by maternal treatment, with potential direct fetal effects (Wiest et al., ). Consequently, NAC maternal therapy is currently approved by the US Food and Drug Administration for treating fetal acetaminophen intoxication at term (Crowell et al., ), with a growing number of clinical studies entertaining its use in other human pregnancy complications, such as chorioamnionitis, unexplained pregnancy loss and preterm labour (Amin et al., ; Conde‐Agudelo et al., ; Shahin et al., ). In addition to promoting H2S synthesis, NAC also has powerful antioxidant actions (Ezerina et al., ). It remains unclear how much of NAC‐induced cardiovascular protection in offspring of hypoxic/FGR gestation is a result of H2S.
Therefore, in the present study, we have investigated further the effects of NAC and H2S in protecting against cardiovascular dysfunction in hypoxic/FGR unborn offspring. We adopted a two‐pronged approach, combining cellular, molecular and functional studies in vessels from near‐term chicken embryos and from the human umbilical and placental circulations. The chicken embryo is the only established model system to isolate mechanisms acting directly on the embryonic vasculature independent of effects on maternal and/or placental physiology (Giussani, ; Skeffington et al., ). The study of human vessels clearly catalyses the translational potential of the biomedical research. Interestingly, the temporal profile of cardiovascular development between humans and chickens is similar, enhancing the added value of the species being compared (Giussani, ). The present study tested the hypothesis that the vascular expression and synthesis of H2S is enhanced and this acts to protect the embryonic and fetal vasculature in hypoxic/FGR progeny. Gene promoter DNA methylation patterns in differentially expressed transcripts were determined as possible underlying epigenetic markers of vascular programming.
Methods
Ethical approval
Studies in chicken embryos were performed under the UK Animals (Scientific Procedures) Act 1986 and were approved by the Ethical Review Board of the University of Cambridge (PPL, P0592D78B). Studies using human umbilical and placental vessels were performed after approval from the Ethics Committee of the Faculty of Medicine, Pontificia Universidad Católica de Chile (1 181 341), according to principles outlined in the Belmont Report for human studies. All experiments were designed and reported with reference to the ARRIVE guidelines (Kilkenny et al., ).
Human umbilical cord and chorionic artery samples
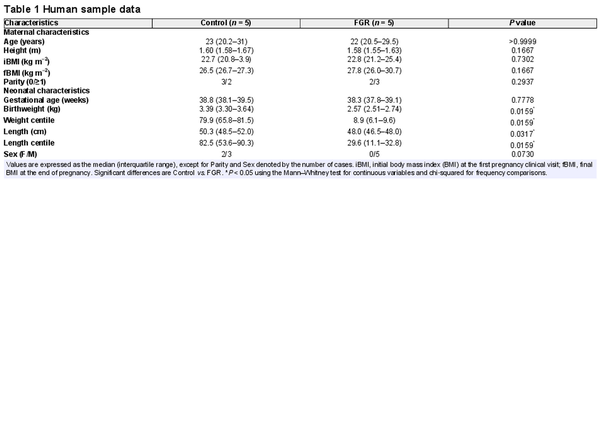
Umbilical cord and placenta samples were obtained from patients after written informed consent. Tissue was collected after delivery from singleton healthy term pregnancies (control group) and from pregnancies diagnosed with FGR, according to current clinical standard criteria (Lees et al., ). Normotensive pregnant women taking part in the study were non‐smokers who did not drink alcohol or take drugs during pregnancy or did not develop intrauterine infection or any other medical or obstetric complication. Gestational age was estimated by ultrasonography before week 12 of pregnancy. FGR was defined as fetal body weight below the tenth centile adjusted for gestational age and sex, together with a low abdominal circumference and/or abnormal Doppler recordings (Gordijn et al., ). Maternal and neonatal characteristics (Table 1) were recorded to confirm the prenatal diagnoses and the centiles were calculated using the web‐based app from Intergrowth‐21st (http://intergrowth21.ndog.ox.ac.uk/en/ManualEntry/Compute).
Chicken embryos
Fertilized Bovans Brown eggs (Gallus domesticus) were purchased from Medeggs (Henry Stewart & Co., Fakenham, UK), weighed and incubated under normoxic (21% O2) or hypoxic (14 ± 0.5% O2) conditions (12:12 h light/dark photocycle at 37.9°C and 45% relative humidity, with automatic rotation every hour; Mod‐75A incubator, equipped with electronic servo‐controlled humidity cool steam injection system HS‐Auto‐3.5L; Masalles, Barcelona, Spain) from day 1. The levels of oxygen, humidity and temperature inside the incubators were continuously monitored (DD103 DrDAQ Oxygen Sensor; Pico Technology, St Neots, UK) (Itani et al., , ; Teulings et al., ).
Chicken embryos incubating under normoxia, or chronic hypoxia were treated with NAC (33 μg kg–1 day–1; Sigma‐Aldrich, Dorset, UK) or vehicle (100 μL of saline) from day 13 to day 19 out of the 21 day incubation period, equivalent to 0.6–0.9 of human gestation. Treatment occurred daily via a 1 mm hole in the eggshell through the air cell and then topically onto the chorioallantoic membrane. The hole in the eggshell was covered with a small piece of tape at all other times. All of the treatment procedures were performed under sterile conditions. Treatment of hypoxic chicken embryos occurred inside a side chamber attached to the hypoxic incubator, which was maintained at the same level of oxygenation so that treatment occurred without losing the hypoxic exposure (Itani et al., , ; Teulings et al., ). The dose of NAC was derived from previous studies that investigated the protective effects of NAC during development on several species, including chicken embryos (Han et al., ; Hsieh et al., ). On day 19 out of the 21 day incubation period, chicken embryos were killed by cervical spinal transection. The embryo was removed and blotted. The body weight and body length (crown–rump length) were recorded. The brain was then dissected and weighed (Itani et al., , ; Teulings et al., ).
Isolation of human umbilical artery endothelial cells
Human umbilical artery endothelial cells (HUAEC) were isolated by collagenase digestion from umbilical cord from Control and FGR pregnancies using established techniques (Krause, Costello et al., ; Penaloza et al., ). Cells were cultured in medium 131 with Microvascular Growth Supplement (S00525; Invitrogen, Waltham, MA, USA) until the second passage and then starved (2% serum) overnight before DNA extraction, as previously described (Krause, Costello et al., ; Penaloza et al., ). In addition, HUAEC isolated from control pregnancies (n = 5) were exposed to normoxia (5% oxygen) or hypoxia (2% oxygen) for 48 h in a controlled chamber (Proox 110; Biospherix, Parish, NY, USA).
Quantitative PCR in the day 19 chicken embryo aorta and in HUAEC
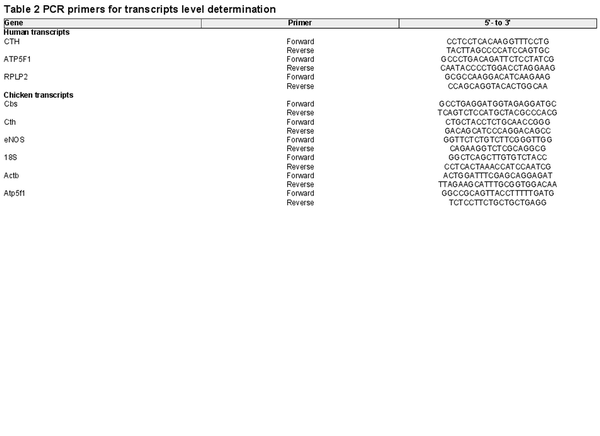
Total RNA was isolated using Trizol reagent (Invitrogen) and PCR was performed using established techniques, as described previously (Krause, Costello et al., ; Penaloza et al., ). Aliquots of 1 μg of total RNA were reverse transcribed using an IMPROM II RT kit (Promega, Madison, WI, USA). Three different RNAs, the ribosomal RNA 18S, and the messenger RNA for β‐actin and ATP5F1, were used as reference genes for mRNA quantification of the H2S‐related transcripts coding for the enzymes cystathionine β‐synthase (CBS) and cystathionine γ‐lyase (CTH), as well as for endothelial nitric oxide synthase (eNOS) by SYBR®‐green real‐time PCR. The same set was used as reference genes for human and chicken tissues following quantititive PCR guidelines (Kozera & Rapacz, ). The transcript‐specific primer sequences for humans and chickens are shown in Tables 2 and 3. Quantification was determined using the geometrical average of 2–ΔΔCT relative to the two or three different reference genes (Livak & Schmittgen, ).
In vitro H2S production in the day 19 chicken embryo aorta
The abdominal aorta was isolated from chicken embryos from normoxic and hypoxic incubations untreated and treated with NAC (n = 5 embryos in each group). Vessel segments were weighed, snap‐frozen in liquid nitrogen and stored at −80°C. In one cohort of frozen vessel segments, H2S synthesis was investigated. The H2S formation assay was based on a protocol described by Banerjee et al. () with minor modifications (Banerjee et al., ). Aortic tissue was homogenized on ice with a plastic pestle in 20 mM potassium phosphate‐bufferred saline (pH 7.4) with protease and phosphatase inhibitors, in a total volume of 300 μL. The homogenate was centrifuged (12,000 g for 20 min at 4°C), the supernatant recovered, and protein content quantified. Enzymatic activity was assayed in triplicate using 245 μL of reaction mixture containing lead acetate (0.4 mM), pyridoxal 5‐phosphate (1 mM) and crude protein extract (10 mg mL−1), using a 96‐well microplate pre‐incubated for 5 min at 37°C. The reaction was initiated by adding 5 μL of L‐cysteine to a final concentration of 10 mM, and lead sulphide formation was monitored by absorbance at 390 nm for 2 h. The specific activity was calculated using the molar extinction coefficient (5500 M−1 cm−1) for lead sulphide.
Vascular reactivity in the day 19 chicken embryo and human chorionic arteries
In chicken embryos incubated under normoxic or hypoxic conditions untreated and treated with NAC (n = 5–8 embryos in each group), a third‐order branch of the left femoral artery was isolated under a dissecting microscope (Stemi 2000; Zeiss, Oberkochen, Germany) (Itani et al., , ; Teulings et al., ). Similarly, third‐ to fourth‐order chorionic arteries were dissected from control (n = 5) and FGR (n = 5) human pregnancies under a dissecting microscope (Krause, Costello et al., ; Penaloza et al., ). For all vessels, connective tissue was removed, and two arterial segments per subject of 2 mm were threaded with two pieces of stainless‐steel wire (40 μm in diameter) before being mounted and secured in a four‐chamber microvascular myograph (Multi‐Wire Myograph System 610M; DMT, Aarhus, Denmark) (Itani et al., , ; Krause, Costello et al., ; Penaloza et al., ; Teulings et al., ). Each chamber was warmed gradually to 37°C and contained 5 mL of Kreb's buffer (NaCl: 118.5 mM, KCl: 4.75 mM, MgSO4.7H2O: 1.2 mM, KH2PO4: 1.2 mM, NaHCO3: 25.0 mM, CaCl2: 2.5 mM, glucose: 5.55 mM, gassed with 95% O2 and 5% CO2).
For chicken embryo arteries, dilator responses to cumulative doses of the H2S donor sodium hydrosulphide (NaHS) (10−6 to 10−2.5 M), the NO donor sodium nitroprusside (SNP) (10−9 to 10−4 M) and the endothelium‐dependent dilator methacholine (MetCh) (10−10 to 10−5 M) were assessed after pre‐constricting the vessel with a submaximal dose of phenylephrine (10−5 M). The partial contributions of NO‐dependent and NO independent mechanisms to the MetCh‐induced vasorelaxation were determined, as previously established (Itani et al., , ; Teulings et al., ). Briefly, a basal MetCh dose‐response curve was constructed in one segment of vessel from any one subject. In parallel, in another segment of vessel from the same subject, a MetCh dose–response curve was constructed after incubating the vessel with the NO synthase inhibitor ω‐nitro‐L‐arginine methyl ester hydrochloride (L‐NAME) (10−5 m for 10 min) (Itani et al., , ; Teulings et al., ). NO‐dependent and independent contributions were determined by calculating the difference in the areas above the curve using established techniques (Itani et al., , ; Teulings et al., ).
For human chorionic arteries, dilator responses to cumulative doses of the calcitonin gene‐related peptide (CGRP) (10−10 to 10−6 mol L–1) were assessed after pre‐constricting the vessel with a submaximal dose of KCl (31.2 mM). CGRP was used for the human vessels because previous studies have revealed consistent endothelium‐dependent dilator responses to this agonist in chorionic ateries (Herrera et al., ; Krause et al., ; Krause, Carrasco‐Wong et al., ). Vasodilator responses to CGRP in human chorionic arteries from FGR pregnancies were performed with and without incubation of the vessels for 20 min with NAC (10−5 M) (Sigma‐Aldrich).
Analysis of DNA methylation in the day 19 chicken embryo aorta
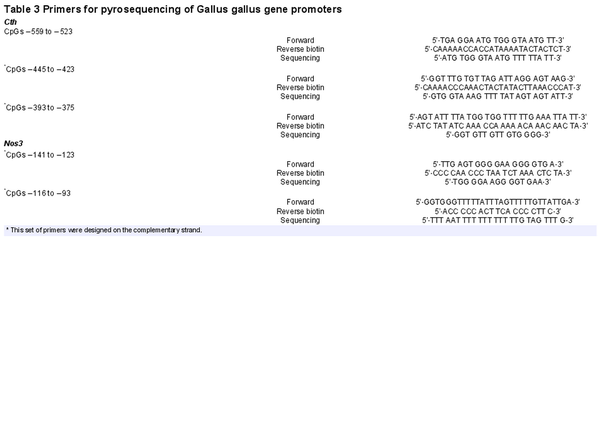
Another cohort of frozen abdominal aortas isolated from chicken embryos from normoxic and hypoxic incubations untreated and treated with NAC (n = 5 embryos in each group) was used for DNA methylation studies. The methylation status of the promoter region of the CTH and NOS3 genes Gallus gallus was determined using DNA bisulfite modification coupled with DNA sequencing, employing established techniques as previously described (Krause, Costello et al., ; Teichert et al., ). DNA was isolated from ∼10−6 EC using a DNeasy Blood & Tissue Kit (Qiagen, Hilden, Germany) and 500 ng of total DNA extracts were treated with sodium bisulfite using EpiTect Bisulfite Kit (Qiagen). Promoter regions of the CTH and NOS3 genes were amplified by PCR using specific primers for CTH and NOS3 (Table 3). Average core promoter and site‐specific CpG methylations were determined as a percentage using a PyroMark Q96 MD (Qiagen).
Transcription factors binding site prediction in the day 19 chicken embryo aorta
Prediction of binding sites for transcription factors in CTH and NOS3 G. gallus promoters was performed with the online software MatInspector (https://ngdc.cncb.ac.cn/biocode/tools/BT006439). Selected transcription factors were chosen considering a cut‐off of 0.900 for the matrix similarity index (Herrera et al., ; Krause, Costello et al., ). To avoid algorithm bias, predictions were further assessed using CIIDER (Gearing et al., ). The data generated were validated using the presence of conserved sequences for transcription factors that regulate eNOS expression in humans (Zhang et al., ) and mice (Teichert et al., ), as well as hypoxia‐related factors conserved in both human and chicken CTH gene promoters.
Data mining of HUAEC under chronic low and high oxygen conditions
To validate the study and identify differentially expressed genes related to cysteine and glutathione (GSH) metabolism in HUAEC under diverse oxygen conditions, we further performed a meta‐analysis of HUAEC data sets available on the Gene Expression Omnibus repository (https://www.ncbi.nlm.nih.gov/geo/), as previously described (Vega‐Tapia et al., ). These data sets include results from endothelial cells cultured for ∼25 days under standard (21%) and low (3%) oxygen conditions. To reduce heterogeneity, data sets assayed were selected from a unique platform (i.e. Affymetrix, HG‐U133 Plus 2, catalog number 900 466; Applied Biosystems; Thermo Fisher Scientific). We evaluated close to 47,000 transcripts, and analysed them using the software Transcriptome Analysis Console 4.0.2.15 (Applied Biosystems; Thermo Fisher Scientific) (Vega‐Tapia et al., ). Normalization was performed according to the standard workflow for this array, and analysis parameters considered the following cut‐off points: fold change < −1.2 and > 1.2, P < 0.01 and false discovery rate < 0.05. GEO codes for the data sets used are GSE29881, GSE43475, GSE47796 and GSE49958.
Statistical analysis
Values are expressed as the mean ± SEM unless otherwise stated. For the myography experiments, LabChart 6.0 was used for data acquisition and analysis (Labchart 6.0, Powerlab 8/30; AD Instruments, Sydney, NSW, Australia). Concentration–response curves were determined using an agonist‐response best‐fit line. For all data, comparisons were made using one or two‐way analysis of variance (ANOVA) with and without repeated measures, as appropriate. Relationships between variables were determined using the Pearson correlation. The Mann–Whitney test was used for continuous variables and chi‐squared was used for frequency comparisons. For all tests, P < 0.05 was considered statistically significant (Prism 7.0; GraphPad Software Inc., San Diego, CA, USA).
Results
Vascular CTH levels are regulated by hypoxia in chicken embryos and FGR in HUAEC
Relative to normoxia, exposure of HUAEC to hypoxia in vitro resulted in increased CTH levels after 48 h but not after 24 h (Fig. 1A). The effect of hypoxia on CTH expression and genes related to cysteine and H2S metabolism (CBS, GCLC and GSR) was also observed by data mining GEO transcriptome datasets from studies of HUAEC exposed to chronic (∼25 days) normoxia (21% O2) or low (3%) O2 levels (Fig. 2). CTH transcript levels in HUAEC isolated from control or FGR infants showed a negative correlation with birth weight (Fig. 1B). Comparable to results from FGR HUAEC, the expression of CTH was significantly greater in the aorta isolated from chicken embryos incubated under chronic hypoxia relative to normoxic incubation (Fig. 1C). CTH transcript levels in vessels isolated from control or hypoxic chicken embryos also showed a negative correlation with body weight measured at the end of incubation (Fig. 1D).
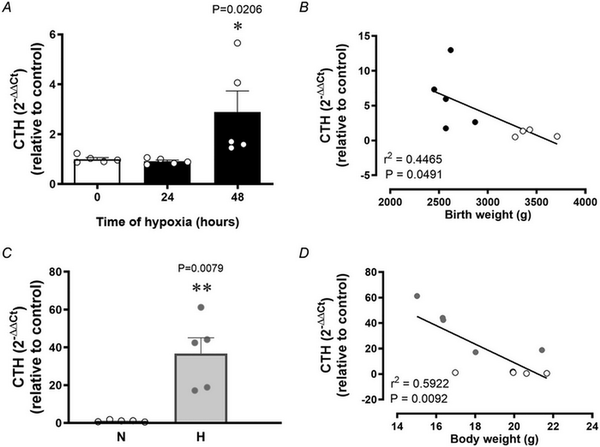
Figure 1
Vascular transcript levels of the H2S‐synthesizing gene CTH in human pregnancy and in chicken embryos
Top: CTH expression in control HUAEC (n = 5 pregnancies) exposed to hypoxia (2%) in vitro at 0, 24 and 48 h (A) and the correlation between HUAEC CTH transcript levels and birth weight in control (open circles) and FGR (solid circles) human infants (B). Bottom: CTH expression in abdominal aortas isolated from near‐term chicken embryos incubated under normoxic (N; n = 5 embryos) or hypoxic (H; n = 5 embryos) conditions (C) and the correlation between CTH transcript levels and body weight in normoxic (open circles) and hypoxic (grey circles) near‐term chicken embryos (D). Significant differences are vs. control HUAEC or normoxic chicken embryos *(P < 0.05) using one‐way ANOVA for (A) and (C), and the Pearson correlation for (B) and (D).
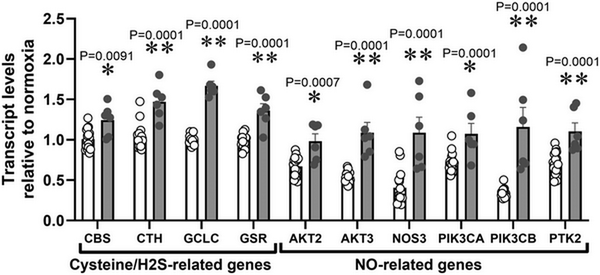
Figure 2
Data mining of GEO transcriptome datasets
Cysteine/H2S and NO‐related transcripts in HUAEC cultured under standard (21%, n = 15, open bars) or low (3%, n = 5, grey bars) oxygen levels. Significant differences are vs. N (*P < 0.05, **P < 0.01 and ***P < 0.001) using two‐way ANOVA + Benjamini–Hochberg FDR.
NAC promotes vascular H2S synthesis and H2S‐mediated vasodilatation in control and hypoxic chicken embryos
Relative to aortas isolated from normoxic chicken embryos, those isolated from hypoxic chicken embryos showed an increase in CTH transcript levels (Fig. 3A) and an increase in H2S synthesis (Fig. 3B,C) measured in vitro. Relative to aortas isolated from untreated normoxic chicken embryos, those isolated from NAC‐treated normoxic chicken embryos showed an increase in transcript levels of CTH (Fig. 3A) and in H2S synthesis (Fig. 3B,C). Relative to aortas isolated from untreated hypoxic chicken embryos, those isolated from NAC‐treated hypoxic chicken embryos showed a further increase in transcript levels of CTH (Fig. 3A) and in H2S synthesis (Fig. 3B,C). However, incubation of chicken embryos under normoxic or hypoxic conditions with or without treatment with NAC did not involve changes in aortic CBS expression (Fig. 4A). By contrast, aortic eNOS expression was increased in aortas isolated from hypoxic chicken embryos relative to normoxic chicken embryos, and this effect was prevented by NAC treatment (Fig. 4B).
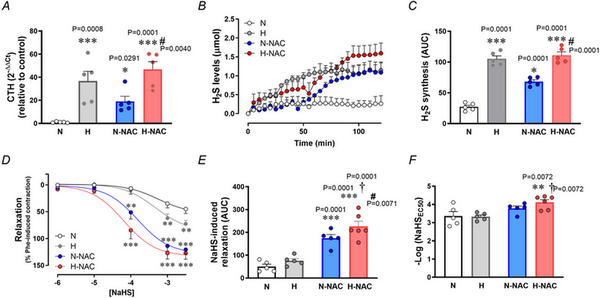
Figure 3
Aortic CTH levels and femoral artery hydrogen sulphide‐dependent relaxation in near‐term chicken embryos incubated under normoxic or hypoxic conditions with and without NAC treatment
Top: CTH transcripts levels (A), hydrogen sulphide formation (H2S) (B) and the cumulative H2S synthesis [area under the curve (AUC)] (C) in abdominal aortas isolated from near‐term chicken embryos incubated under normoxic (N; n = 5 embryos), hypoxic (H; n = 5 embryos), normoxic with NAC treatment (N‐NAC; n = 5 embryos) or hypoxic with NAC treatment (H‐NAC; n = 5 embryos) conditions. Bottom: relaxant responses to the H2S donor NaHS (D), the cumulative vasodilatation (AUC) (E) and potency (–logEC50) (F) in femoral arteries isolated from near‐term chicken embryos incubated under normoxic (N; n = 5 embryos), hypoxic (H; n = 5 embryos), normoxic with NAC treatment (N‐NAC; n = 5 embryos) or hypoxic with NAC treatment (H‐NAC; n = 5 embryos) conditions. Significant differences are vs. N (*P < 0.05, **P < 0.01, ***P < 0.001), H vs. H‐NAC (#P < 0.05) and N‐NAC vs. H‐NAC (†P < 0.05) using two‐way ANOVA + Benjamini–Hochberg FDR. [Colour figure can be viewed at wileyonlinelibrary.com]
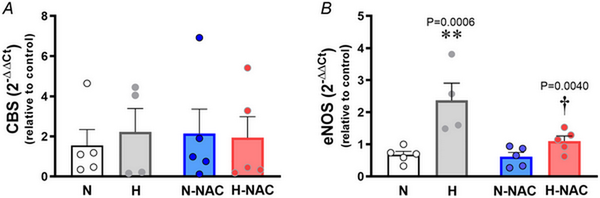
Figure 4
Aortic CBS and eNOS levels in near‐term chicken embryos incubated under normoxic or hypoxic conditions with and without NAC treatment
Values are dot plots over the mean ± SEM for mRNA levels of cystathionine‐β‐synthase (CBS) (A) and mRNA levels of endothelial nitric oxide synthase (eNOS) (B) in abdominal aortas isolated from chicken embryos. Groups are normoxic (N; n = 5), hypoxic (H; n = 5), normoxic treated with NAC (N‐NAC; n = 5) and hypoxic treated with NAC (H‐NAC; n = 3) chicken embryos. Significant differences are vs. N (**P < 0.01) and H vs. H‐NA (†P < 0.05) using two‐way ANOVA + Benjamini–Hochberg FDR. [Colour figure can be viewed at wileyonlinelibrary.com]
Relative to femoral arteries isolated from normoxic chicken embryos, those isolated from hypoxic chicken embryos showed enhanced H2S‐mediated femoral vasodilatation (Fig. 3D). Relative to femoral arteries isolated from untreated normoxic chicken embryos, those isolated from NAC‐treated normoxic chicken embryos showed a greater H2S‐mediated vasodilatation (Fig. 3D,E). Relative to femoral arteries isolated from untreated hypoxic chicken embryos, those isolated from NAC‐treated hypoxic chicken embryos showed the greatest H2S‐mediated vasodilatation (Fig. 3D–F).
NAC protects against endothelial dysfunction in human chorionic and chicken embryo arteries
Relative to chorionic plate arterial segments isolated from control pregnancies, those isolated from FGR pregnancies showed impaired endothelium‐dependent relaxation to CGRP (Fig. 5A–C). However, chorionic arterial segments isolated from FGR pregnancies incubated with NAC had improved relaxant responses to CGRP (Fig. 5A–C).
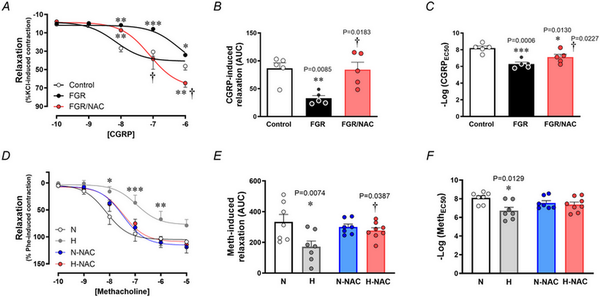
Figure 5
Endothelial‐dependent relaxation in chorionic arteries from human pregnancy and femoral arteries from chicken embryos with and without NAC treatment
Top: relaxant responses to the endothelium‐dependent vasodilator CGRP (A), the cumulative vasodilatation, expressed as area under the curve (AUC) (B) and the potency (–logEC50) (C) in chorionic arterial vessel segments from control (n = 5, open symbols) and FGR pregnancies with (n = 5, red symbols) and without (n = 5, black symbols) NAC pretreatment. Bottom: relaxant responses to the endothelium‐dependent vasodilator methacholine (D), the cumulative vasodilatation (AUC) (E) and the potency (–logEC50) (F) in femoral arteries isolated from chicken embryos incubated under normoxic (N; n = 5–7 embryos), hypoxic (H; n = 5–7 embryos), normoxic with NAC treatment (N‐NAC; n = 5–7 embryos) or hypoxic with NAC treatment (H‐NAC; n = 5–8 embryos) conditions. Significant differences are vs. N or Control (*P < 0.05, **P < 0.01, ***P < 0.001), FGR vs. FGR/NAC (†P < 0.05) or H vs. H‐NAC (†P < 0.05) using one‐ or two‐way ANOVA + Benjamini–Hochberg FDR. [Colour figure can be viewed at wileyonlinelibrary.com]
Similarly, relative to femoral arterial segments isolated from normoxic chicken embryos, those isolated from hypoxic chicken embryos showed impaired endothelium‐dependent relaxation to MetCh (Fig. 5D–F). However, femoral arterial segments isolated from hypoxic chicken embryos treated with NAC had similar relaxant responses to MetCh compared to normoxic chicken embryos (Fig. 5D–F). Femoral arterial segments isolated from normoxic chicken embryos treated with NAC had similar relaxant responses to MetCh compared to untreated normoxic chicken embryos (Fig. 5D–F).
To isolate NO‐dependent and NO independent mechanisms contributing to the effect of NAC in hypoxic chicken embryos, femoral relaxant responses to MetCh were determined before and after treatment with L‐NAME. The data show that the impaired femoral relaxant response to MetCh in hypoxic chicken embryos was the result of a reduced NO‐dependent mechanism (Fig. 6). Furthermore, the restoration of MetCh‐induced femoral dilatation in hypoxic chicken embryos treated with NAC was the result of an enhanced NO independent mechanism (Fig. 6). Femoral arteries from normoxic chicken embryos treated with NAC also showed enhanced NO independent vasodilatation (Fig. 6). Neither exposure to chronic hypoxia, nor treatment with NAC affected the femoral vasorelaxant responses to the NO donor SNP (Fig. 7).
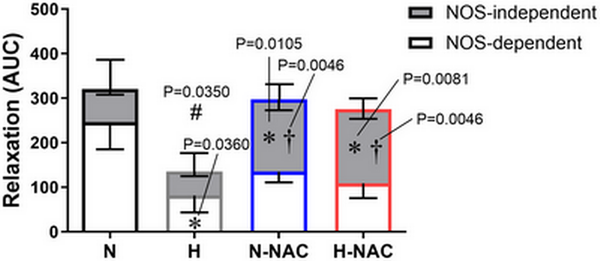
Figure 6
Contribution of NO‐dependent and NO independent mechanisms to relaxation in chicken embryo femoral arteries
The bar chart represents the mean ± SEM for the area under the curve (AUC) for the relaxation to methacholine in the absence and presence of the NOS‐inhibitor L‐NAME in femoral arteries isolated from chicken embryos incubated under normoxic (N; n = 5–7 embryos), hypoxic (H; n = 5–7 embryos), normoxic with NAC treatment (N‐NAC; n = 5–7 embryos) or hypoxic with NAC treatment (H‐NAC; n = 5–8 embryos) conditions. The overall histogram with positive SEM represents the overall relaxation. The grey compartmental histogram with negative SEM represents the AUC for NOS independent mechanisms. The white compartmental histogram with negative represents the AUC for NOS‐dependent mechanisms. Significant differences are vs. the corresponding component in N (*P < 0.05) vs. the corresponding component in H (†P < 0.05) and vs. the total AUC in N (#P < 0.05) using two‐way ANOVA + Benjamini–Hochberg FDR. [Colour figure can be viewed at wileyonlinelibrary.com]
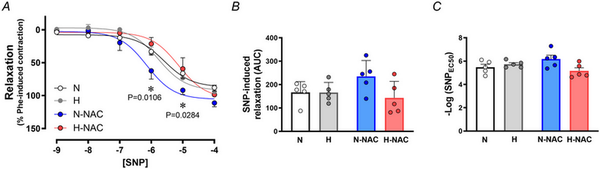
Figure 7
Smooth muscle‐dependent relaxation in femoral arteries from chicken embryos with and without NAC treatment
Values are the mean ± SEM and dot plots over histograms for relaxant responses to the NO donor SNP (A), cumulative vasodilatation [area under the curve (AUC)] (B) and potency (–logEC50) (C) in femoral arteries isolated from chicken embryos. Groups are normoxic (N; n = 5–7), hypoxic (H; n = 5–7), normoxic treated with NAC (N‐NAC; n = 5–7) and hypoxic treated with NAC (H‐NAC; n = 5–8) chicken embryos. Significant differences are vs. N (*P < 0.05) using two‐way repeated measures ANOVA + Benjamini–Hochberg FDR. [Colour figure can be viewed at wileyonlinelibrary.com]
NAC induces epigenetic effects on the CTH gene promoter in vessels from control and hypoxic chicken embryos
In silico analysis of the G. gallus CTH gene promoter showed a relatively high content of CpGs, and the presence of transcription factor binding sites related to basal gene expression (AP2 and Sp1) and three hypoxia response element (HRE) regions, located either in the coding (+) and non‐coding (–) DNA strand (Fig. 8A,B). Similar results were observed in human CTH gene promoter, supporting a potential regulation by hypoxia (Fig. 9). The pyrosequencing analysis focused on HRE regions of the CTH gene promoter showed lower levels of DNA methylation in CpGs located in the upstream HRE (−559 to −543), but increased DNA methylation in CpGs located in the downstream HRE (−401 to −384) in aorta from hypoxic embryos compared to those from normoxic embryos (Fig. 8C–E). Treatment with NAC was associated with CpG‐specific effects, inducing either restoration of lower levels of DNA methylation in CpGs located in the upstream HRE (−559 to −543) or an enhancement of the increased DNA methylation in CpGs located in the downstream HRE (−401 to −384) in aorta from hypoxic embryos without affecting those from normoxic embryos (Fig. 8C–E).
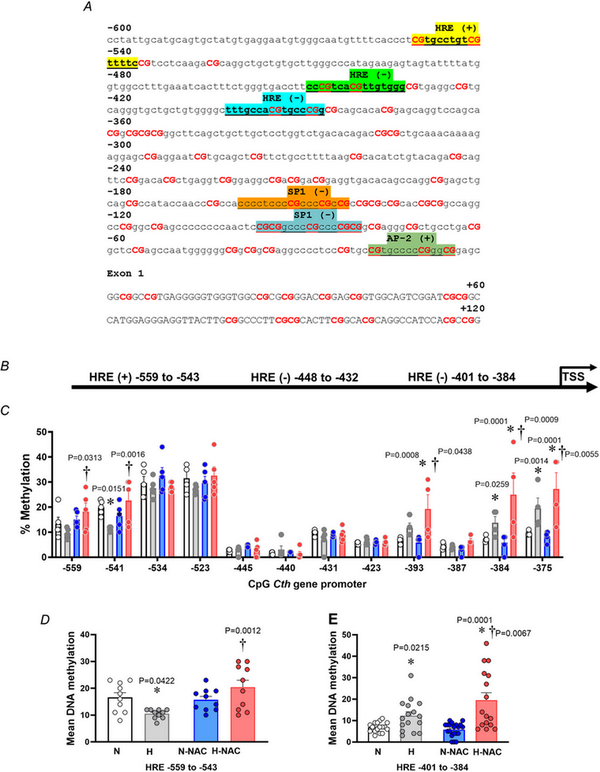
Figure 8
The vascular CTH promoter methylation profile in normoxic and hypoxic chicken embryos with and without NAC treatment
A, Gallus gallus CTH gene promoter (−600 bp from the transcription start site) and first exon sequence obtained from Ensembl genome browser. Binding sites are highlighted, and the related transcription factor is shown above. B, schematic diagram of the G. gallus CTH gene promoter predicted with MatInspector. C–E, abdominal aortas isolated from chicken embryos incubated under normoxic (N; n = 5 embryos), hypoxic (H; n = 5 embryos), normoxic with NAC treatment (N‐NAC; n = 5 embryos) or hypoxic with NAC treatment (H‐NAC; n = 5 embryos) conditions. C, dot plots over the mean ± SEM values for single‐CpG methylation levels across the CTH proximal promoter. D, mean ± SEM for DNA methylation levels in HRE sites located between −559 and −543. E, mean ± SEM for DNA methylation levels in HRE sites located between −401 and −384. Significant differences are vs. N (*P < 0.05) or H vs. H‐NA (†P < 0.05) using two‐way ANOVA + Benjamini–Hochberg FDR. [Colour figure can be viewed at wileyonlinelibrary.com]
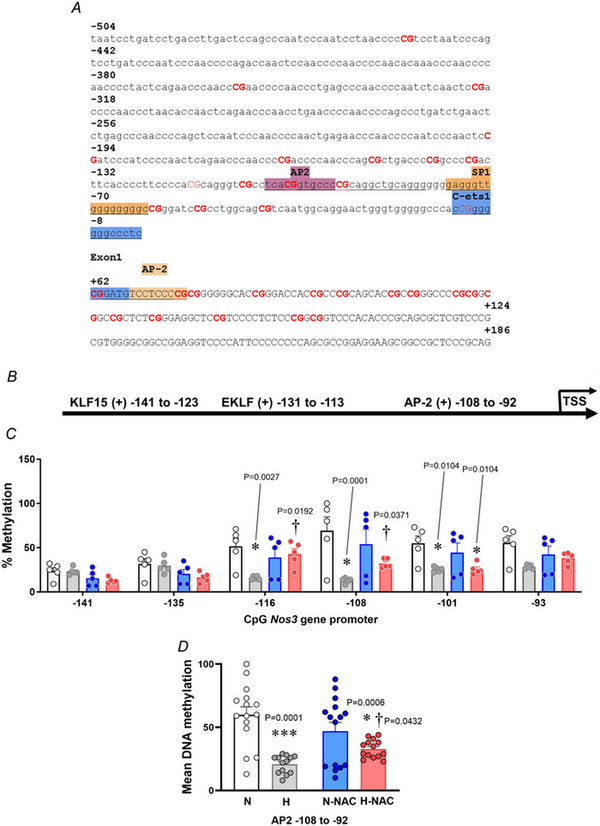
Figure 9
The vascular NOS3 promoter methylation profile in normoxic and hypoxic chicken embryos with and without NAC treatment
A, Gallus gallus NOS3 gene promoter and first exon sequence (−504 bp from the transcription start site), obtained from the Ensembl genome browser. The binding sites are highlighted, and the related transcription factor is shown above. B, schematic diagram of the G. gallus NOS3 gene promoter predicted with MatInspector. C, dot plots over the mean ± SEM for single‐CpG methylation levels across the NOS3 proximal promoter. D, mean DNA methylation levels in AP‐2 site located at −108 to −92 upstream for abdominal aortas isolated from chicken embryos. Groups are normoxic (N; n = 5), hypoxic (H; n = 5), normoxic treated with NAC (N‐NAC; n = 5) and hypoxic treated with NAC (H‐NAC; n = 5) chicken embryos. Significant differences are vs. N (*P < 0.05 and ***P < 0.001) or H vs. H‐NAC (†P < 0.05) using two‐way ANOVA + Benjamini–Hochberg FDR. [Colour figure can be viewed at wileyonlinelibrary.com]
NAC induces epigenetic effects on the NOS3 gene (eNOS) promoter in vessels from control and hypoxic chicken embryos
In silico analysis of the G. gallus NOS3 gene promoter showed the presence of AP2 and Sp1 transcription factor binding sites but no HRE regions, located either in the coding (+) and non‐coding (–) DNA strand (Fig. 9A,B). Pyrosequencing analysis showed lower levels of DNA methylation in CpGs in the aorta from hypoxic embryos compared to those from normoxic embryos (Fig. 9C). Treatment with NAC restored the lower levels of DNA methylation to control levels in the aorta from hypoxic embryos without affecting those from normoxic embryos (Fig. 9C). Consistent with these findings, relative to aortas from normoxic embryos, the mean DNA methylation of CpGs was lower in aortas from hypoxic embryos and this effect was reversed by NAC treatment (Fig. 9D). Conversely, treatment with NAC did not affect the Nos3 gene promoter methylation in aortas from normoxic chicken embryos.
Effects of hypoxia and NAC on growth in human pregnancy and in the chicken embryo
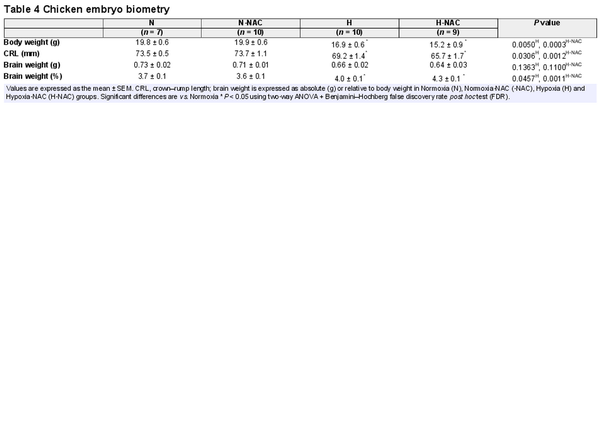
Relative to controls, infants from FGR pregnancies had significantly lower birth weights and values for body length (Table 1). Relative to controls, chicken embryos incubated under hypoxic conditions showed reduced body weight and an increase in brain weight when expressed as a percentage of body weight, indicating asymmetric growth restriction with evidence of brain sparing. Treatment of hypoxic chicken embryos with NAC did not prevent this effect (Table 4).
Discussion
The data obtained in the present study show that hypoxia increases the expression of the H2S gene CTH in the vasculature of the human fetus and the chicken embryos and that CTH expression in both species is negatively correlated with embryonic or fetal growth. Second, NAC increases the vascular expression of CTH and the production of H2S in normoxic and hypoxic chicken embryos. Third, treatment with NAC of chicken embryos during normoxic incubation enhances the relaxant responses in third‐order femoral arteries to the H2S donor NaHS and augments this effect in hypoxic chicken embryos. Fourth, treatment with NAC reverts impaired endothelial relaxation in third‐to‐fourth order chorionic arteries from FGR pregnancies and protects against endothelial dysfunction in third‐order femoral arteries from hypoxic chicken embryos. This NAC‐induced vascular protection in hypoxic chicken embryos is mediated by increasing NO independent mechanisms of the endothelial relaxation. Combined, the data support the hypothesis tested that the vascular expression and synthesis of H2S is enhanced and this acts to protect the vasculature in hypoxic/FGR unborn offspring.
Several studies in preclinical animal models support the beneficial effects of maternal antioxidant therapy in adverse gestation on the cardiovascular system of the offspring by improving endothelial‐dependent vasodilatation (Botting et al., ; Brain et al., ; Giussani, ; Giussani & Davidge, ; Itani et al., , ). Studies in ovine and rodent models and in human chorionic arteries support that chronic fetal hypoxia promotes oxidative stress in the placenta and the fetal cardiovascular system (Giussani, ; Giussani & Davidge, ; Herrera et al., ; Schneider et al., ). Chronic fetal hypoxia also increases placental vascular resistance (Giussani, ; Giussani & Davidge, ; Herrera et al., ; Schneider et al., ; Tong et al., ), leads to FGR and induces remodelling of the fetal cardiovascular system, thereby promoting an enhanced cardiovascular risk in the offspring in later life (Botting et al., ; Brain et al., ; Giussani, ; Giussani & Davidge, ; Krause et al., ). Based on this evidence, there has been accruing clinical interest in the use of antioxidants or agents that increase NO bioavailability, such as vitamins C and E, L‐arginine or sildenafil to treat the mother and thereby fetus in FGR gestation (Botting et al., ; Brain et al., ; Chen et al., ; Giussani, ; Giussani & Davidge, ; Itani et al., , ; Tenorio et al., ). However, human clinical studies have reported limited effectiveness of conventional antioxidant therapy in protecting the FGR offspring (Rumbold, Ota, Hori et al., ; Rumbold, Ota, Nagata et al., ). Exogenous treatment with antioxidants or agents that increase NO bioavailability may have too slow a reaction rate to protect against pro‐oxidant mechanisms (Aldini et al., ). Therefore, an alternative approach may be to enhance the synthesis of endogenous antioxidant mechanisms at their very site of physiological production.
One of the main endogenous antioxidant defences is the tonic synthesis of reduced GSH, which actively participates in the detection, signalling, and reduction of oxidative stress (Winterbourn & Hampton, , ). The ratio of reduced GSH to oxidized glutathione (GSSG) within cells is a robust measure of cellular oxidative stress (Lu, ; Pastore et al., ) where the increased GSSG‐to‐GSH ratio is indicative of greater oxidative stress. NAC is a derivative of the amino acid cysteine and a precursor for the endogenous production of GSH (Elbini Dhouib et al., ). Therefore, previous studies from our group and others have reported potent protective antioxidant effects of NAC on fetal and adult offspring (Hashimoto et al., ; Herrera et al., ; Krause et al., ; Patterson et al., ). However, the precise molecular mechanisms underlying the protection by NAC remain uncertain and, in addition to antioxidant effects (Aldini et al., ), they may include the generation of substrates for H2S synthesis (Ezerina et al., ). This is because the synthesis of H2S in vascular tissues is primarily catalysed by the activity of CTH, which in turn uses thiol‐containing substrates derived from cysteine or GSH metabolism (Kanagy et al., ). Upon endothelial stimulation, H2S has immediate effects as an endothelial‐derived vasorelaxant gas, with effects comparable to NO in vascular homeostasis (Yang & Wang, ; Yang et al., ).
Our results show that, when HUAECs isolated from healthy pregnancies are incubated under hypoxic conditions for 48 h, the transcript levels of the H2S‐synthesizing gene CTH are increased. Similarly, relative to controls, CTH expression is significantly greater in femoral arteries isolated from chicken embryos incubated under hypoxic conditions. In both cases, the expression of CTH is negatively correlated with size at term, such that the lightest babies or chicken embryos have the greatest expression of CTH. Therefore, these data show that the H2S gene CTH is regulated by hypoxia in the unborn offspring vasculature of both species and that it is related to embryonic or fetal growth restriction. Differences in the magnitude of CTH expression between species may be attributable to the duration of exposure to hypoxia. HUAECs were exposed to hypoxia in vitro for 48 h, whereas chicken embryos were incubated under hypoxic conditions throughout the incubation period. Additional data in the present study show that the expression of CTH, the formation of H2S and the dilator response to the H2S donor NaHS are all increased in arteries isolated from chicken embryos chronically treated with NAC during normoxic conditions, relative to untreated normoxic incubation, and the outcomes were elevated even further in NAC‐chronically treated hypoxic chicken embryos. Notably, data from eNOS knockout and transgenic mice show that H2S‐ and eNOS‐mediated vasorelaxant actions are reciprocally regulated, with enhanced H2S dilator effects under conditions of impaired NO synthesis (Ertuna et al., ). Therefore, the data in the present study show that chronic treatment with NAC enhances the synthesis and vasodilator function of H2S in normoxic chicken embryos and that these effects can be sensitized in hypoxic chicken embryos. Finally, in vitro acute treatment with NAC induced vasorelaxation in human chorionic plate arterial segments. We noted that, following washout, the vasodilatation returned immediately to basal levels. Therefore, this acute effect of NAC in human chorionic arteries probably represents the basal induction of CTH and H2S promoting vasorelaxation. Furthermore, acute treatment with NAC restored impaired endothelial function in human chorionic arterial segments isolated from FGR pregnancies, and in vivo treatment with NAC restored impaired endothelial function in femoral arteries isolated from hypoxic chicken embryos via NO independent pathways. Interestingly, recent data show that adverse alterations in H2S metabolism impairs the endothelial proteome affecting signalling pathways that mediate responses to flow (Bibli et al., ). Combined, the experiments reported in the present study suggest that NAC protects the vasculature in the human fetus and in the chicken embryo via the synthesis and actions of H2S, as well as via its antioxidant effects. Furthermore, the data are consistent with the concept that H2S underlies potent NO independent molecular mechanisms that protects endothelial function in hypoxic or FGR unborn offspring and that its endogenous synthesis at its very site of physiological production can be enhanced by acute and chronic NAC treatment.
Previous studies have reported that treatment with various antioxidants such as NAC, vitamin C, melatonin, allopurinol or MitoQ in ovine and rodent preclinical animal models of hypoxic gestation also protects against FGR (Botting et al., ; Brain et al., ; Giussani, ; Giussani et al., ; Herrera et al., ; Schneider et al., ). Conversely, neither treatment with NAC in the present study, nor with melatonin, pravastatin, sildenafil, or MitoQ in previous studies, protected against growth restriction in the hypoxic chicken embryo (Botting et al., ; Giussani, ; Giussani et al., ; Itani et al., , ; Itani et al., ). Therefore, past and present findings support a protective effect of antioxidants on fetal or embryonic growth at the level of the placenta, enhancing perfusion and fetal oxygen and nutrient delivery in mammals, and therefore not seen in avian species. Consistent with this idea, maternal treatment with antioxidants such as NAC, vitamin C, melatonin, allopurinol or MitoQ ameliorates placental oxidative stress and enhances placental and umbilical perfusion in mammalian species (Hansell et al., ; Herrera et al., ; Richter et al., ).
Additional data in the present study show that both hypoxia and NAC induced epigenetic effects on the CTH and NOS3 gene promoters. The data provide a novel characterization of the DNA methylation profile for these genes in chickens. Although DNA methylation is commonly referred to as a silencing process, increasing evidence supports that the effects of DNA methylation on transcriptional activity are context‐ and gene‐dependent (Angeloni & Bogdanovic, ; Jones, ). A previous study determined the effects of gene variation within the CTH promoter in mice models of essential hypertension, describing an important role for inflammatory mediators as regulatory factors of CTH transcriptional activity (Gupta et al., ). In the present study, by applying updated tools for transcription factor binding site prediction, potential regulatory elements responding to hypoxia were discovered in both the human and chicken CTH gene promoter. Pyrosequencing results confirmed that CTH gene promoter had a high density of CpG sites, which were differentially methylated in untreated‐ and NAC‐treated hypoxic embryos compared to the control. These data support that chronic exposure to hypoxia during fetal development imposes epigenetic marks at the level of the vasculature, which can be modified by therapeutic interventions. Although there was a reciprocal association between CTH DNA methylation at the higher HREs (−559 to −543) and gene expression in vessels from hypoxic embryos, there was no clear association between CTH DNA methylation at the lower HREs (−402 to −384) in vessels from hypoxic embryos or vessels from hypoxic embryos treated with NAC. Consistent with these data, other studies in fetal vessels, have reported that the DNA methylation of genes with CpG‐rich promoters did not show a correlation with transcriptional activity or gene expression (Angeloni & Bogdanovic, ; Jones, ; Krause, Costello et al., ). Conversely, consistent with findings reported in human (Krause, Costello et al., ), rat, mice and guinea‐pig (Herrera et al., ; Krause et al., ) pregnancy, the NOS3 promoter in chicken arteries had a low density of CpG sites, in which both mean and site‐specific DNA methylation were decreased in embryos incubated under hypoxic conditions and restored by NAC treatment, correlating with changes in eNOS transcript levels. Studies focused on eNOS regulation show that basal eNOS expression is tightly and inversely regulated by NOS3 DNA methylation (Krause, ; Krause, Costello et al., ), an effect that has been confirmed by transcriptomic and genome‐wide data (Arenas et al., ). Combined, therefore, the data suggest that embryonic or fetal hypoxia drives epigenetic changes in key vascular genes, with a remarkable effect on NOS3 shared across diverse mammalian and non‐mammalian species. Furthermore, early‐life interventions are effective in reverting the epigenetic effect on eNOS by increasing the contribution of alternative vasodilator pathways, such as those mediated by H2S. It is plausible that developmental hypoxia in the chicken embryo and human FGR pregnancy are associated with impaired NO‐dependent vasodilatation but enhanced H2S biology. The latter could be a compensatory response to offset the impaired mechanism and restore function, as is so often found in homeostasis. This is speculation. Silencing experiments to elucidate the role of H2S in developmental hypoxia and its contribution to the effects of NAC may be helpful in the future. In our studies using HUAEC, the sex split does represent a source of bias because all five FGR offspring were male. Future studies should incorporate greater numbers to determine possible differences imposed by the sex of the fetus.
In conclusion, the data reported in the present study show important effects of NAC and H2S that offer a mechanism for restoring impaired relaxant responses of the fetal vasculature in hypoxic offspring. Such effects may have therapeutic relevance against developmental origins of an increased risk of cardiovascular disease in the adult offspring, although this needs testing. This may be of human translational relevance because it could be applied to pregnancies complicated by FGR. However, clearly, further experiments are necessary for translation into safe and effective materno‐fetal therapies in human pregnancy.
Additional information
Competing interests
The authors declare that they have no competing interests.
Author contributions
D.A.G. and B.J.K. were responsible for conceptualization. B.J.K., A.A.P., T.A.C.G., E.P., F.V.‐T., S.G.F. and Y.N. were responsible for methodology. B.J.K., A.A.P., T.A.C.G., E.P., F.V.‐T., S.G.F., Y.N. and D.A.G. were responsible for data and statistical analysis. D.A.G. and B.J.K. were responsible for writing the original draft. D.A.G., Y.N. and B.J.K. were responsible for reviewing and editing. B.J.K., A.A.P., T.A.C.G., E.P., F.V.‐T., S.G.F., Y.N. and D.A.G. were responsible for visualization. D.A.G., Y.N., S.G.F. and B.J.K. were responsible for supervision. D.A.G. and B.J.K. were responsible for project administration. D.A.G. and B.J.K. were responsible for funding acquisition.
Funding
This study is supported by The British Heart Foundation (PG/10/99/28 656; DAG). Fondecyt Regular 1 220 421; Premio Santander a la Investigación Universitaria 2018; In Training Grant – Society for Reproductive Investigation.
Acknowledgements
We are grateful to the staff of the University of Cambridge Biological Services for helping with the maintenance of the animals. D.A.G. is a Fellow of the Lister Institute for Preventive Medicine and a Royal Society Wolfson Research Merit Award Holder.
References
- Aldini G., Altomare A., Baron G., Vistoli G., Carini M., Borsani L., & Sergio F. (2018). N‐Acetylcysteine as an antioxidant and disulphide breaking agent: The reasons why. Free Radical Research, 52(7), 751–762.
- Amin A. F., Shaaban O. M., & Bediawy M. A. (2008). N‐acetyl cysteine for treatment of recurrent unexplained pregnancy loss. Reproductive Biomedicine Online, 17(5), 722–726.
- Angeloni A., & Bogdanovic O. (2019). Enhancer DNA methylation: Implications for gene regulation. Essays in Biochemistry, 63(6), 707–715.
- Arenas G. A., Santander N., & Krause B. J. (2022). Transcriptional and epigenomic markers of the arterial‐venous and micro/macro‐vascular endothelial heterogeneity within the umbilical‐placental bed. International Journal of Molecular Sciences, 23(19), 11873.
- Banerjee R., Chiku T., Kabil O., Libiad M., Motl N., & Yadav P. K. (2015). Assay methods for H2S biogenesis and catabolism enzymes. Methods in Enzymology, 554, 189–200.
- Bibli S. I., Hu J., Looso M., Weigert A., Ratiu C., Wittig J., Drekolia M. K., Tombor L., Randriamboavonjy V., Leisegang M. S., Goymann P., Delgado Lagos F., Fisslthaler B., Zukunft S., Kyselova A., Justo A. F. O., Heidler J., Tsilimigras D., Brandes R. P., … Fleming I. (2021). Mapping the endothelial cell S‐sulfhydrome highlights the crucial role of integrin sulfhydration in vascular function. Circulation, 143(9), 935–948.
- Botting K. J., Skeffington K. L., Niu Y., Allison B. J., Brain K. L., Itani N., Beck C., Logan A., Murray A. J., Murphy M. P., & Giussani D. A. (2020). Translatable mitochondria‐targeted protection against programmed cardiovascular dysfunction. Science Advances, 6(34), eabb1929.
- Brain K. L., Allison B. J., Niu Y., Cross C. M., Itani N., Kane A. D., Herrera E. A., Skeffington K. L., Botting K. J., & Giussani D. A. (2019). Intervention against hypertension in the next generation programmed by developmental hypoxia. PLoS Biology, 17(1), e2006552.
- Chen J., Gong X., Chen P., Luo K., & Zhang X. (2016). Effect of L‐arginine and sildenafil citrate on intrauterine growth restriction fetuses: A meta‐analysis. BioMed Central Pregnancy Childbirth, 16(1), 225.
- Conde‐Agudelo A., Romero R., Jung E. J., & Garcia Sanchez A. J. (2020). Management of clinical chorioamnionitis: An evidence‐based approach. American Journal of Obstetrics and Gynecology, 223(6), 848–869.
- Crispi F., Miranda J., & Gratacos E. (2018). Long‐term cardiovascular consequences of fetal growth restriction: Biology, clinical implications, and opportunities for prevention of adult disease. American Journal of Obstetrics and Gynecology, 218(2S), S869–S879.
- Crowell C., Lyew R. V., Givens M., & Deering S. H. (2008). Caring for the mother, concentrating on the fetus: Intravenous N‐acetylcysteine in pregnancy. American Journal of Emergency Medicine, 26(6), 735.e1–735.e2.
- Ducsay C. A., Goyal R., Pearce W. J., Wilson S., Hu X.‐Q., & Zhang L. (2018). Gestational hypoxia and developmental plasticity. Physiological Reviews, 98(3), 1241–1334.
- Elbini Dhouib I., Jallouli M., Annabi A., Gharbi N., Elfazaa S., & Lasram M. M. (2016). A minireview on N‐acetylcysteine: An old drug with new approaches. Life Sciences, 151, 359–363.
- Ertuna E., Loot A. E., Fleming I., & Yetik‐Anacak G. (2017). The role of eNOS on the compensatory regulation of vascular tonus by H(2)S in mouse carotid arteries. Nitric Oxide, 69, 45–50.
- Ezeriņa D., Takano Y., Hanaoka K., Urano Y., & Dick T. P. (2018). N‐acetyl cysteine functions as a fast‐acting antioxidant by triggering intracellular H2S and sulfane sulfur production. Cell Chemical Biology, 25(4), 447–459.e4.
- Galli G. L. J., Lock M. C., Smith K. L. M., Giussani D. A., & Crossley D. A. 2nd (2023). Effects of developmental hypoxia on the vertebrate cardiovascular system. Physiology (Bethesda, Md.), 38(2), 0.
- Gearing L. J., Cumming H. E., Chapman R., Finkel A. M., Woodhouse I. B., Luu K., Gould J. A., Forster S. C., & Hertzog P. J. (2019). CiiiDER: A tool for predicting and analysing transcription factor binding sites. PLoS ONE, 14(9), e0215495.
- Giussani D. A. (2021). Breath of life: Heart disease link to developmental hypoxia. Circulation, 144(17), 1429–1443.
- Giussani D. A., Camm E. J., Niu Y., Richter H. G., Blanco C. E., Gottschalk R., Blake E. Z., Horder K. A., Thakor A. S., Hansell J. A., Kane A. D., Wooding F. B. P., Cross C. M., & Herrera E. A. (2012). Developmental programming of cardiovascular dysfunction by prenatal hypoxia and oxidative stress. PLoS ONE, 7(2), e31017.
- Giussani D. A., & Davidge S. T. (2013). Developmental programming of cardiovascular disease by prenatal hypoxia. The Journal of Developmental Origins of Health and Disease, 4(5), 328–337.
- Gluckman P. D., Hanson M. A., Cooper C., & Thornburg K. L. (2008). Effect of in utero and early‐life conditions on adult health and disease. New England Journal of Medicine, 359(1), 61–73.
- Gordijn S. J., Beune I. M., Thilaganathan B., Papageorghiou A., Baschat A. A., Baker P. N., Silver R. M., Wynia K., & Ganzevoort W. (2016). Consensus definition of fetal growth restriction: A Delphi procedure. Ultrasound in Obstetrics & Gynecology: The Official Journal of the International Society of Ultrasound in Obstetrics and Gynecology, 48(3), 333–339.
- Gupta V., Kapopara P. R., Khan A. A., Arige V., Subramanian L., Sonawane P. J., Sasi B. K., & Mahapatra N. R. (2017). Functional promoter polymorphisms direct the expression of cystathionine gamma‐lyase gene in mouse models of essential hypertension. Journal of Molecular and Cellular Cardiology, 102, 61–73.
- Han Z.‐J., Song G., Cui Y., Xia H.‐F., & Ma X. (2011). Oxidative stress is implicated in arsenic‐induced neural tube defects in chick embryos. International Journal of Developmental Neuroscience, 29(7), 673–680.
- Hansell J. A., Richter H. G., Camm E. J., Herrera E. A., Blanco C. E., Villamor E., Patey O. V., Lock M. C., Trafford A. W., Galli G. L. J., & Giussani D. A. (2022). Maternal melatonin: Effective intervention against developmental programming of cardiovascular dysfunction in adult offspring of complicated pregnancy. Journal of Pineal Research, 72(1), e12766.
- Hashimoto K., Pinkas G., Evans L., Liu H., Al‐Hasan Y., & Thompson L. P. (2012). Protective effect of N‐acetylcysteine on liver damage during chronic intrauterine hypoxia in fetal guinea pig. Reproductive Sciences, 19(9), 1001–1009.
- Herrera E. A., Cifuentes‐Zúñiga F., Figueroa E., Villanueva C., Hernández C., Alegría R., Arroyo‐Jousse V., Peñaloza E., Farías M., Uauy R., Casanello P., & Krause B. J. (2017a). N‐Acetylcysteine, a glutathione precursor, reverts vascular dysfunction and endothelial epigenetic programming in intrauterine growth restricted guinea pigs. The Journal of Physiology, 595(4), 1077–1092.
- Herrera E. A., Cifuentes‐Zúñiga F., Figueroa E., Villanueva C., Hernández C., Alegría R., Arroyo‐Jousse V., Peñaloza E., Farías M., Uauy R., Casanello P., & Krause B. J. (2017b). N‐Acetylcysteine, a glutathione precursor, reverts vascular dysfunction and endothelial epigenetic programming in intrauterine growth restricted guinea pigs. The Journal of Physiology, 595(4), 1077–1092.
- Herrera E. A., Krause B., Ebensperger G., Reyes R. V., Casanello P., Parra‐Cordero M., & Llanos A. J. (2014). The placental pursuit for an adequate oxidant balance between the mother and the fetus. Frontiers in Pharmacology, 5, 149.
- Hess R. M., Niu Y., Garrud T. A. C., Botting K. J., Ford S. G., & Giussani D. A. (2020). Embryonic cardioprotection by hydrogen sulphide: Studies of isolated cardiac function and ischaemia‐reperfusion injury in the chicken embryo. The Journal of Physiology, 598(19), 4197–4208.
- Hsieh C. L., Chen K. C., Ding C. Y., Tsai W. J., Wu J. F., & Peng C. C. (2013). Valproic acid substantially downregulated genes folr1, IGF2R, RGS2, COL6A3, EDNRB, KLF6, and pax‐3, N‐acetylcysteine alleviated most of the induced gene alterations in chicken embryo model. Romanian Journal of Morphology and Embryology, 54(4), 993–1004.
- Itani N., Salinas C. E., Villena M., Skeffington K. L., Beck C., Villamor E., Blanco C. E., & Giussani D. A. (2018). The highs and lows of programmed cardiovascular disease by developmental hypoxia: Studies in the chicken embryo. The Journal of Physiology, 596(15), 2991–3006.
- Itani N., Skeffington K. L., Beck C., Niu Y., Katzilieris‐Petras G., Smith N., & Giussani D. A. (2020). Protective effects of pravastatin on the embryonic cardiovascular system during hypoxic development. Federation of American Societies for Experimental Biology Journal, 34(12), 16504–16515.
- Itani N., Skeffington K. L., Beck C., Niu Y., & Giussani D. A. (2016). Melatonin rescues cardiovascular dysfunction during hypoxic development in the chick embryo. Journal of Pineal Research, 60(1), 16–26.
- Jones P. A. (2012). Functions of DNA methylation: Islands, start sites, gene bodies and beyond. Nature Reviews Genetics, 13(7), 484–492.
- Kanagy N. L., Szabo C., & Papapetropoulos A. (2017). Vascular biology of hydrogen sulfide. American Journal of Physiology‐Cell Physiology, 312(5), C537–C549.
- Kilkenny C., Browne W. J., Cuthill I. C., Emerson M., & Altman D. G. (2010). Improving bioscience research reporting: The ARRIVE guidelines for reporting animal research. PLoS Biology, 8(6), e1000412.
- Kozera B., & Rapacz M. (2013). Reference genes in real‐time PCR. Journal of Applied Genetics, 54(4), 391–406.
- Krause B. J. (2021). Novel insights for the role of nitric oxide in placental vascular function during and beyond pregnancy. Journal of Cellular Physiology.236(12), 7984–7999,
- Krause B. J., Carrasco‐Wong I., Caniuguir A., Carvajal J., Faras M., & Casanello P. (2013). Endothelial eNOS/arginase imbalance contributes to vascular dysfunction in IUGR umbilical and placental vessels. Placenta, 34(1), 20–28.
- Krause B. J., Costello P. M., Muñoz‐Urrutia E., Lillycrop K. A., Hanson M. A., & Casanello P. (2013). Role of DNA methyltransferase 1 on the altered eNOS expression in human umbilical endothelium from intrauterine growth restricted fetuses. Epigenetics : Official Journal of the DNA Methylation Society, 8(9), 944–952.
- Krause B. J., Peñaloza E., Candia A., Cañas D., Hernández C., Arenas G. A., Peralta‐Scholz M. J., Valenzuela R., García‐Herrera C., & Herrera E. A. (2019). Adult vascular dysfunction in foetal growth‐restricted guinea‐pigs is associated with a neonate‐adult switching in Nos3 DNA methylation. Acta Physiology (Oxford), 227(3), e13328.
- Krause B. J., Prieto C. P., Muñoz‐Urrutia E., San Martín S., Sobrevia L., & Casanello P. (2012). Role of arginase‐2 and eNOS in the differential vascular reactivity and hypoxia‐induced endothelial response in umbilical arteries and veins. Placenta, 33(5), 360–366.
- Lees C. C., Stampalija T., Baschat A. A., Da Silva Costa F., Ferrazzi E., Figueras F., Hecher K., Kingdom J., Poon L. C., Salomon L. J., & Unterscheider J. (2020). ISUOG Practice Guidelines: Diagnosis and management of small‐for‐gestational‐age fetus and fetal growth restriction. Ultrasound in Obstetrics & Gynecology: The Official Journal of the International Society of Ultrasound in Obstetrics and Gynecology, 56(2), 298–312.
- Livak K. J., & Schmittgen T. D. (2001). Analysis of relative gene expression data using real‐time quantitative PCR and the 2(‐Delta Delta C(T)) method. Methods (San Diego, California), 25(4), 402–408.
- Lu S. C. (2013). Glutathione synthesis. Biochimica Et Biophysica Acta, 1830(5), 3143–3153.
- Lucaroni F., Cicciarella Modica D., Macino M., Palombi L., Abbondanzieri A., Agosti G., Biondi G., Morciano L., & Vinci A. (2019). Can risk be predicted? An umbrella systematic review of current risk prediction models for cardiovascular diseases, diabetes and hypertension. British Medical Journal open, 9(12), e030234.
- Pastore A., Piemonte F., Locatelli M., Lo Russo A., Gaeta L. M., Tozzi G., & Federici G. (2001). Determination of blood total, reduced, and oxidized glutathione in pediatric subjects. Clinical Chemistry, 47(8), 1467–1469.
- Patterson A. J., Xiao D., Xiong F., Dixon B., & Zhang L. (2012). Hypoxia‐derived oxidative stress mediates epigenetic repression of PKCepsilon gene in foetal rat hearts. Cardiovascular Research, 93(2), 302–310.
- Peñaloza E., Soto‐Carrasco G., & Krause B. J. (2020). MiR‐21‐5p directly contributes to regulating eNOS expression in human artery endothelial cells under normoxia and hypoxia. Biochemical Pharmacology, 182, 114288.
- Richter H. G., Camm E. J., Modi B. N., Naeem F., Cross C. M., Cindrova‐Davies T., Spasic‐Boskovic O., Dunster C., Mudway I. S., Kelly F. J., Burton G. J., Poston L., & Giussani D. A. (2012). Ascorbate prevents placental oxidative stress and enhances birth weight in hypoxic pregnancy in rats. The Journal of Physiology, 590(6), 1377–1387.
- Rumbold A., Ota E., Hori H., Miyazaki C., & Crowther C. A. (2015). Vitamin E supplementation in pregnancy. Cochrane Database of Systematic Reviews (Online), CD004069.
- Rumbold A., Ota E., Nagata C., Shahrook S., & Crowther C. A. (2015). Vitamin C supplementation in pregnancy. Cochrane Database of Systematic Reviews (Online), 2015(9), CD004072.
- Saif J., Ahmad S., Rezai H., Litvinova K., Sparatore A., Alzahrani F. A., Wang K., & Ahmed A. (2021). Hydrogen sulfide releasing molecule MZe786 inhibits soluble Flt‐1 and prevents preeclampsia in a refined RUPP mouse model. Redox Biology, 38, 101814.
- Schneider D., Hernández C., Farías M., Uauy R., Krause B. J., & Casanello P. (2015). Oxidative stress as common trait of endothelial dysfunction in chorionic arteries from fetuses with IUGR and LGA. Placenta, 36(5), 552–558.
- Shahin A. Y., Hassanin I. M. A., Ismail A. M., Kruessel J. S., & Hirchenhain J. (2009). Effect of oral N‐acetyl cysteine on recurrent preterm labor following treatment for bacterial vaginosis. International Journal of Gynaecology and Obstetrics: The Official Organ of the International Federation of Gynaecology and Obstetrics, 104(1), 44–48.
- Skeffington K. L., Beck C., Itani N., & Giussani D. A. (2018). Isolating the direct effects of adverse developmental conditions on in vivo cardiovascular function at adulthood: the avian model. The Journal of Developmental Origins of Health and Disease, 9(4), 460–466.
- Teichert A.‐M., Karantzoulis‐Fegaras F., Wang Y., Mawji I. A., Bei X., Gnanapandithen K., & Marsden P. A. (1998). Characterization of the murine endothelial nitric oxide synthase promoter. Biochimica Et Biophysica Acta, 1443(3), 352–357.
- Tenório M. B., Ferreira R. C., Moura F. A., Bueno N. B., Goulart M. O. F., & Oliveira A. C. M. (2018). Oral antioxidant therapy for prevention and treatment of preeclampsia: Meta‐analysis of randomized controlled trials. Nutrition, Metabolism and Cardiovascular Diseases, 28(9), 865–876.
- Teulings N. E. W. D., Garrud T. A. C., Niu Y., Skeffington K. L., Beck C., Itani N., Conlon F. G., Botting K. J., Nicholas L. M., Ashmore T. J., Blackmore H. L., Tong W., Camm E. J., Derks J. B., Logan A., Murphy M. P., Ozanne S. E., & Giussani D. A. (2020). Isolating adverse effects of glucocorticoids on the embryonic cardiovascular system. Federation of American Societies for Experimental Biology Journal, 34(7), 9664–9677.
- Thompson L. P., & Al‐Hasan Y. (2012). Impact of oxidative stress in fetal programming. Journal of Pregnancy, 2012, 582748.
- Tong W., Allison B. J., Brain K. L., Patey O. V., Niu Y., Botting K. J., Ford S. G., Garrud T. A., Wooding P. F. B., Shaw C. J., Lyu Q., Zhang L., Ma J., Cindrova‐Davies T., Yung H. W., Burton G. J., & Giussani D. A. (2022). Chronic hypoxia in ovine pregnancy recapitulates physiological and molecular markers of preeclampsia in the mother, placenta, and offspring. Hypertension, 79(7), 1525–1535.
- Vega‐Tapia F., Peñaloza E., & Krause B. J. (2021). Specific arterio‐venous transcriptomic and ncRNA‐RNA interactions in human umbilical endothelial cells: A meta‐analysis. Iscience, 24(6), 102675.
- Wiest D. B., Chang E., Fanning D., Garner S., Cox T., & Jenkins D. D. (2014). Antenatal pharmacokinetics and placental transfer of N‐acetylcysteine in chorioamnionitis for fetal neuroprotection. The Journal of Pediatrics, 165(4), 672–677.e2.
- Winterbourn C. C., & Hampton M. B. (2008). Thiol chemistry and specificity in redox signaling. Free Radical Biology & Medicine, 45(5), 549–561.
- Winterbourn C. C., & Hampton M. B. (2015). Redox biology: Signaling via a peroxiredoxin sensor. Nature Chemical Biology, 11(1), 5–6.
- Yang G., An S. S., Ji Y., Zhang W., & Pei Y. (2015). Hydrogen sulfide signaling in oxidative stress and aging development. Oxidative Medicine and Cellular Longevity, 2015, 357824.
- Yang G., & Wang R. (2015). H2S and blood vessels: An overview. Handbook of Experimental Pharmacology, 230, 85–110.
- Yzydorczyk C., Armengaud J. B., Peyter A. C., Chehade H., Cachat F., Juvet C., Siddeek B., Simoncini S., Sabatier F., Dignat‐George F., Mitanchez D., & Simeoni U. (2017). Endothelial dysfunction in individuals born after fetal growth restriction: Cardiovascular and renal consequences and preventive approaches. The Journal of Developmental Origins of Health and Disease, 8(4), 448–464.
- Zhang R., Min W., & Sessa W. C. (1995). Functional analysis of the human endothelial nitric oxide synthase promoter. Sp1 and GATA factors are necessary for basal transcription in endothelial cells. Journal of Biological Chemistry, 270(25), 15320–15326.