Introduction
Lithium has recently been endorsed as the first-line maintenance treatment of bipolar affective disorder (BPAD; ) and treatment refractory depression (; ). Yet, its use is in decline in many countries (; ) due to the introduction of other mood stabilisers such as some anticonvulsants and second-generation antipsychotics (SGAs). Long-term lithium use is associated with an increased risk of loss of renal function (; ). To limit those risks, lithium levels are carefully monitored and kept as low as possible. Unfortunately, the risk of chronic kidney disease (CKD) cannot be eliminated by adherence to modern treatment principles (). When using lithium, we trade off a small morbidity and mortality risk of CKD against the morbidity and mortality risk of mental ill-health associated with lack of effective prophylaxis (; ).
Little is known about the incidence, clinical course and associated factors of acute lithium toxicity. suggest that lithium poisoning occurs frequently, ‘since it is used by individuals at high risk of taking an overdose’. A correlation between CKD and sudden lithium intoxication has been postulated (), but the relationship between both remains unclear. CKD may give rise to lithium intoxication, and lithium intoxication may increase the risk of CKD (; ).
The aims of this study were to determine the frequency of lithium intoxication, to evaluate associated factors, clinical course and treatment, and to clarify how great the risk is that toxic lithium levels cause acute or chronic renal failure. Such information can help to improve pharmacological treatment of patients suffering from severe affective disorders.
Methods and materials
We collected the data as part of a retrospective cohort study (LISIE) into side effects and effects of lithium treatment compared to other mood stabilisers for the maintenance treatment of BPAD. The Regional Ethics Review Board at Umeå University, Sweden, approved this study (DNR 2010-227-31M, DNR 2011-228-32M, DNR 2014-10-32M).
Participants
We identified all patients with BPAD in the Swedish county of Norrbotten who were at least 18 years of age and had been exposed to lithium. The study covered a 17-year period from 1997 to 2013. Lithium exposure was determined by at least one blood lithium concentration >0.2 mmol/L in the central laboratory database where all measurements were stored. We defined a lithium level of ≥1.2 mmol/L as our cut-off point for all intoxication, since this is the upper limit of recommended therapeutic levels. A lithium level of ≥1.5 mmol/L was set as the cut-off point for a risk of clinically significant intoxication (). We then determined how many patients had experienced such episodes.
To estimate the incidence of intoxication, we first calculated the episodes per patient treated over the entire 17-year observation period. Then, we estimated the incidence of lithium intoxication per treatment year based on lithium prescribing data from the Swedish National Board of Health and Welfare (Socialstyrelsen) from 2006 to 2013, to ensure that all patients having received lithium prescriptions were covered.
For all patients who had consented to participate in this study or whose records we were approved to access because the patients had deceased, we reviewed in detail the episodes with lithium levels of ≥1.5 mmol/L as documented in the electronic medical records. Both primary-care and secondary-care records were accessed. To control for selection bias, we compared key parameters that were available in anonymous form, including sex, age, maximum serum creatinine and maximum serum lithium concentration for consenting and non-consenting patients in accordance with the ethical approval granted.
Chart review and analysis
Regarding each episode of lithium intoxication eligible for review, we determined the mode of detection, presenting symptoms, somatic co-morbidities and co-medications, aetiology, treatment including need for dialysis, clinical outcome and renal function before and at least one month after lithium intoxication. Episodes with acute intoxication, defined as cases of supra-therapeutic lithium doses leading to toxic blood levels within 24 hours after ingestion, were classified as ‘acute’ in lithium naive patients and ‘acute on therapeutic’ in patients on lithium maintenance treatment. Cases in whom recent supra-therapeutic doses had been ruled out were defined as ‘chronic’. We used estimated glomerular filtration rate (eGFR) as a more reliable parameter of renal function than creatinine for baseline and one month after intoxication. eGFR was estimated with the CKD-EPI formula (). For acute kidney injury (AKI), we used creatinine, since eGFR would render false results. AKI was defined as a rise in creatinine ≥26.5 µmol/L/48 hours, according to the ). Baseline creatinine was defined as last creatinine measured before intoxication or occurrence of the relevant co-morbidity (e.g. infection). Co-morbidities and associated factors were classified according to the likelihood of causing AKI. Such were considered ‘probable’ if the clinical data showed patterns that would induce AKI in a lithium-naive patient. Episodes with co-morbidities essentially able to cause AKI but without enough clinical information were considered ‘possible’. All information was then entered into a database and anonymised before analysis.
We conducted a descriptive analysis, establishing the frequency of all variables in our database. Then, we compared moderate with severe intoxication, using a cut-off point of 2.5 mmol/L (). Group comparisons were made with a two-tailed t-test. When data of the same individual were compared over time, a paired t-test was used. Differences were considered statistically significant with a p-value of <0.05. We did not correct for multiple testing because our data were ‘observational’ but not ‘random’ in nature (). Statistical analysis was performed using IBM SPSS Statistics for Windows v23 (IBM Corp., Armonk, NY). We analysed categorical data using the chi-square test and Fisher’s exact test (GraphPad Prism; GraphPad Software, Inc., La Jolla, CA; ).
Figure 1
Identification of cases with lithium intoxication.
Results
Epidemiology
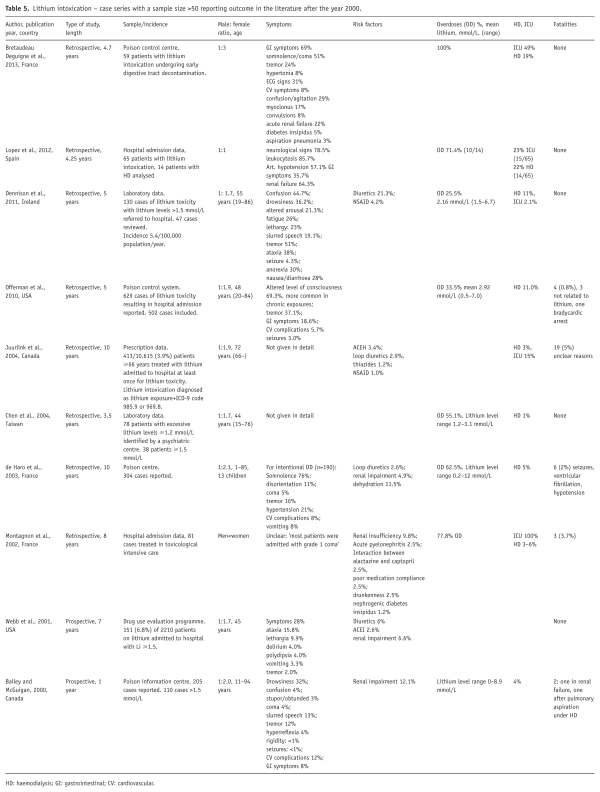
We identified 1340 patients who had been exposed to lithium over the 17-year period of observation. Of these, 228 had experi-enced at least one episode of lithium levels ≥1.2 mmol/L, and 96 (7.16%) had experienced at least one episode of lithium levels ≥1.5 mmol/L. The mean number of patients treated with lithium between 2006 and 2013 in the catchment area was 667 per year, corresponding to 2.67 patients/1000 inhabitants, according to national prescription data. These numbers were stable over the observation period. Our study covered an estimate of 11,339 patient-years on lithium. Under the assumption that the patients not consenting to have their charts reviewed had a similar distribution of episodes, the incidence of moderate to severe lithium intoxication amounted to 0.01 patients per year.
Case analysis
Of the eligible patients, 75% consented to the study. Including patients who had passed away, 1101 patients (82%) were analysed. Patients not consenting did not differ significantly in age, sex, maximal measured creatinine or maximum lithium level from the patients who were included.
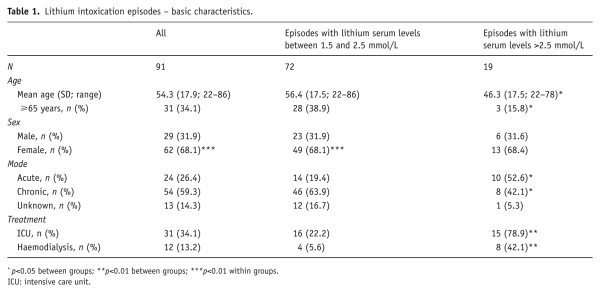
Of the 1101 patients who were analysed, 77 had experienced 91 episodes of lithium intoxication with lithium levels ≥1.5 mmol/L. Of these, 10 patients had two episodes and two patients had three episodes of intoxication, but there was no obvious pattern in these repeated episodes. Fourteen per cent had a diagnosis of paranoid schizophrenia, 7% schizoaffective disorder, 71% BPAD and 8% depression.
Twenty-nine episodes occurred during the first two years of lithium treatment. The proportion of cases of acute intoxication was significantly higher in this subgroup (p<0.01).
Seventy-two (79%) episodes concerned moderate plasma elevations in the range 1.50–2.5 mmol/L, and 19 (21%) episodes concerned severe plasma elevations >2.5 mmol/L. The highest lithium level detected was 9.26 mmol/L in the context of an intentional overdose. In all groups, women accounted for about two-thirds of intoxication episodes (p<0.01). The mean age for all these episodes was 54.3 years (SD=17.9 years, range 22–86 years). Patients with severe intoxication were significantly younger (p< 0.05; Table 1).
Episodes of intoxication were significantly more frequent in summer and autumn, with a p-value of ≤0.01 for the whole sample and a p-value of 0.025 for episodes not concerning intentional overdoses. There was no seasonal variation for episodes of unknown cause.
Symptoms of lithium intoxication
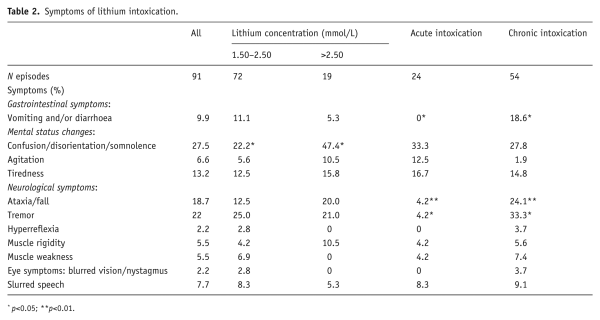
Symptoms of lithium intoxication were only recorded for a minority of patients. Most commonly, patients presented with altered level of consciousness (confusion, disorientation or somnolence). This was significantly more likely to occur in patients with severe intoxication (p<0.05). Less likely to occur in an acute overdose setting were tremor (p<0.05), vomiting and diarrhoea (p<0.05) and ataxia or falls (p<0.001) (see Table 2).
Mode of identification
Seventy-seven per cent of cases of intoxication were detected after unscheduled blood tests, and 22% were identified during routine monitoring conducted every four months. Twenty-four (26%) episodes were related to acute intoxication. Of these, 23 episodes occurred in patients with maintenance treatment (‘acute on therapeutic’) and one episode in a patient not previously treated with lithium. Fifty-four (59%) episodes related to chronic intoxication. For the remaining 13 episodes, there was insufficient data to attempt classification. Patients <65 years of age were significantly more likely to have taken an overdose than patients ≥65 years (odds ratio [OR]=6.7, confidence interval [CI] 1.8–24.4; p<0.01; Table 1).
Associated factors
Infections were associated with nine (9.9%) episodes of the cases of intoxication. Eleven (12.1%) cases of intoxication were associated with initiation of interacting drugs. Four (4.4%) of these episodes were linked to initiation of therapy with non-steroidal anti-inflammatory drugs (NSAID). Thiazide and loop-diuretics were associated with another three (3.3%) episodes. Blockade of the renin-angiotensin-aldosterone-system (RAAS) with ACE inhibitors, angiotensin receptor blockers or spironolactone accounted for a further seven (7.7%) episodes. Of the 1101 patients analysed, 760 had at least one prescription of NSAID, 247 patients RAAS-blocking drugs and 112 patients thiazides over the 17-year study period. Patients ≥65 years of age accounted for all of the RAAS-associated, none of the NSAID associated episodes and only one of the episodes related to infections.
Renal function
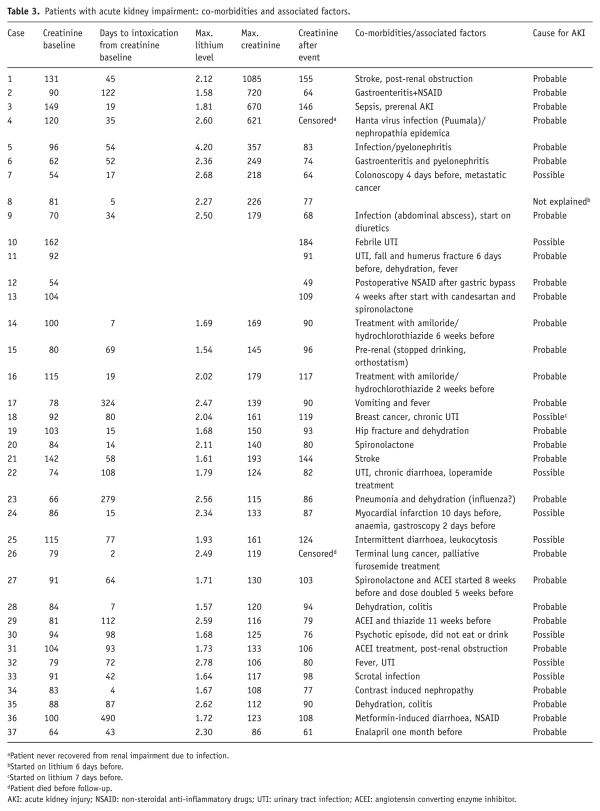
Mean eGFR at baseline was 80 mL/min/1.73 m2, corresponding to a mild decrease (category G2 according to the ). In 32 (34%) episodes, AKI was present. In another five patients, the rise of creatinine was borderline between 22 and 26 µmol/L. In 27/37 episodes, the decline of renal function was probably and in eight episodes possibly caused by co-morbidities or factors other than lithium. In two episodes, AKI occurred after the first week of lithium treatment (Table 3).
Patients ≥65 years of age were more likely to have had renal impairment prior to intoxication (p<0.01). No patient with acute intoxication had AKI.
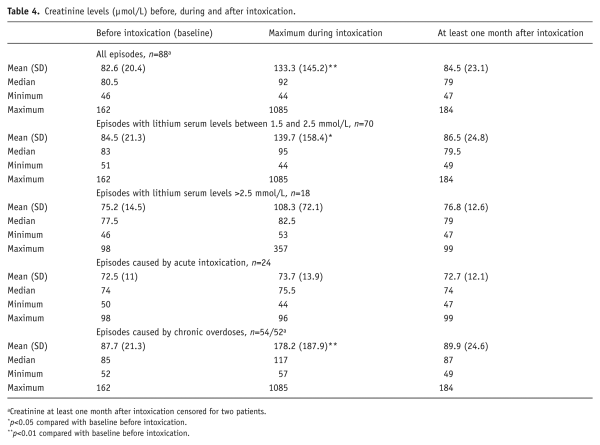
For 88 episodes, serum creatinine levels were analysed comparing baseline and maximum creatinine and creatinine at least one month after intoxication. For one episode, creatinine was not measured during intoxication. The other two episodes concerned a patient who died of lung cancer within one month of intoxication, and another patient who had nephropathia epidemica (Hanta virus infection) and did not recover from renal impairment.
Creatinine was significantly higher during intoxication (p<0.01). Kidney function did not differ before and after intoxication (Table 4). GFR at least one month after intoxication was 81.33 mL/min/1.73 m2. Eighteen (20%) patients had a lost >5 mL/min/1.73 m2 and six patients (7%) >10 mL/min/1.73 m2. The highest loss of GFR was 25 mL/min/1.73 m2. The median change in GFR was 0 mL/min for all episodes. A decline in GFR did not correlate with GFR at baseline, age ≥65 years, sex or maximum creatinine. However, it correlated inversely with maximal lithium level (R=−0.21, p<0.05).
Six patients had potassium levels >5.0 mmol/L, and two patients had levels >5.5 mmol/L.
Treatment and outcome
No fatalities occurred in connection with lithium intoxication. Detailed information on treatment was available for 77 episodes (Figure 2). Of these, 34.1% received intensive care. Patients with severe intoxication were significantly more likely to receive intensive care (p<0.01; Table 1). Most episodes were managed conservatively. Twelve episodes were treated with haemodialysis (HD; 15.6%). Nine patients received intermitted HD (IHD). Seven of these patients were dialysed between 2 and 5.5 hours; two received two treatment sessions. Two patients were treated with continuous venovenous HD (CVVHD) for 21 and 48 hours, respectively. One patient was started on IHD after an overdose (5.5 hours) and then continued on CVVHD. Despite CVVHD, the patient suffered from a rebound of lithium plasma levels and was switched back to IHD. HD did not induce adverse events.
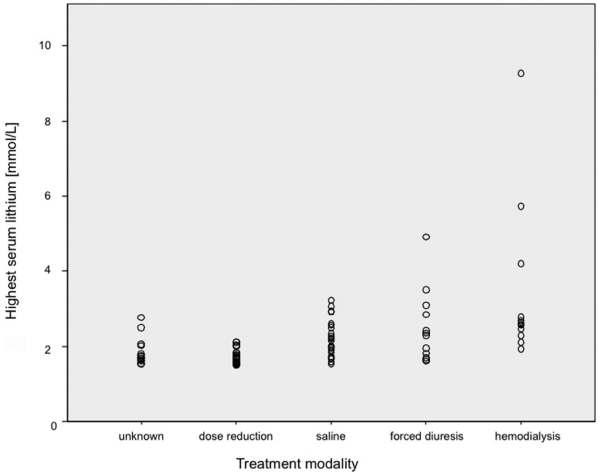
Figure 2
Treatment modality.
Twelve patients were treated with forced diuresis (e.g. concomitant infusion with sodium chloride 0.9% [saline] and furosemide). Three of these had moderate to severe renal impairment at presentation (creatinine 155–670 µmol/L). Three had nephrogenic diabetes insipidus (NDI), and for three, the NDI status was not known. Sodium at presentation was normal in 10 patients; one patient had low (130 mmol/L) and one patient high sodium (175 mmol/L). The patient with hyponatremia normalised under the treatment. The patient with hypernatremia decreased to 157 mmol/L during the first 48 hours under controlled infusion of dextrose 5%. Sodium follow-up was available in 9/10 of the patients with normal sodium. In these, sodium stayed normal during treatment, even in the presence of NDI. Twenty-three patients were treated with saline alone.
In the majority of cases (79.1%), long-term maintenance treatment with lithium was continued despite the intoxication episode.
Discussion
Concerns about the risk of acute and chronic intoxication and the need of regular monitoring with lithium treatment have led to the perception that other mood stabilisers such as some anticonvulsants and SGAs are safer than lithium and hence preferable. In this work, we examined the toxicity profile in 91 episodes observed over a 17-year period in our county.
The epidemiology of lithium intoxication
The incidence of lithium intoxication was 1/100 patient-years. Based on this incidence, only 1/100 patients per year treated with lithium can be expected to experience moderate to severe lithium intoxication ≥1.5 mmol/L. We reported an incidence of 7.2% patients with lithium intoxication over 17 years. This is in line with findings from a large population-based study from Canada in elderly patients treated with lithium (). As in other studies, we saw that women were nearly twice as likely to experience an episode of lithium intoxication (Table 4). However, our study was not sufficiently powered to examine potential sex differences arising from differences in body mass, kidney function and overdose propensity.
Symptoms of lithium toxicity
In many cases, symptoms of lithium intoxication were relatively bland or non-existent. Most symptoms related to mental status changes, which were more frequent in cases of severe intoxication. The statement from a recent expert consensus panel that ‘gastrointestinal symptoms tend to distinguish acute poisoning, where they are expected and prominent, from chronic toxicity, where they are almost invariably absent’ () could not be endorsed in our study. In our study, no patient having taken an overdose had gastrointestinal symptoms, and all cases with diarrhoea belonged to the group of chronic toxicity. As many patients present with unspecific or mild symptoms, it is important to have a low threshold to check lithium levels. It is important not to ascribe mental status changes to psychiatric symptoms automatically, since mental state changes are prominent in lithium intoxication. Equally, it is important not to discard falls in the elderly treated with lithium as age related and hence ‘normal’ and omit to check lithium levels. Ultimately, clinical symptoms may be poor indicators of actual lithium levels (; ). Conversely, it has even been suggested that intoxication can occur even despite normal lithium levels (; ). This could occur in the context of lithium-mediated serotonergic toxicity or in the context of acute overdoses, when levels are taken before lithium tablets have entered the bloodstream.
Aetiology of lithium intoxication
Drug interactions are an important cause of increased lithium levels. The risk of drug interactions does not seem limited to the elderly population. However, the type of drug interaction may depend on age. In our sample, all drug interactions with NSAID related to patients <65 years of age, and all drug interactions with RAAS-blockade to patients aged ≥65 years of age. Considering the number of prescriptions of potentially interacting drugs, lithium intoxication caused by these drugs is relatively uncommon. But this does not make co-prescription safe. Circumstances regarding the initiation of these interacting drugs, specifically the need for adaptation of lithium dosage, requires further exploration.
Renal function
Lithium is exclusively eliminated by the kidneys. Therefore, impaired renal function increases the risk for lithium retention and hence for lithium toxicity. This was confirmed by our study, since in the majority of cases, decline of renal function preceded lithium intoxication. Acute lithium exposure can lead to overt diabetes insipidus () and consequently to dehydration. This may partly explain a transient increase of serum creatinine during the acute intoxication period. Possibly, lithium-induced water loss and reduced fluid ingestion add up to pre-renal AKI. Patients suffering from NDI due to chronic lithium treatment must drink large volumes or receive large parenteral quantities of hypo-osmolar fluids to compensate for NDI associated water loss. As any other AKI, lithium-associated AKI can lead to CKD in individual patients. In a few patients in our study, kidney function was decreased after the episode; in others, it increased. It cannot be ruled out that creatinine-based estimated GFR might have been inaccurately high due to decreased muscle mass caused by inactivity and malnutrition in some patients. For the whole group, kidney function at least one month after intoxication did not differ from baseline. Baseline kidney function or maximum creatinine did not predict changes in GFR after one month. Our study was not designed to look at long-term changes of GFR. Hence, the question remains whether lithium intoxication can contribute to a long-term decline in renal function, even if the kidneys have recovered in the short term. Some studies have suggested that this may be so (; ). This would then suggest an increased renal vulnerability after lithium intoxication. In our study, higher lithium levels correlated with better kidney function. Lithium levels in acute overdoses were higher than those in chronic intoxication. Possibly, longer exposure to lower but still supra-therapeutic lithium levels is more toxic than short exposure to high levels (; ). Yet, the very nature of chronicity implies that such cases of intoxication may go undetected for a long time.
Treatment
Our study depicted real-life treatment. The type of treatment occurred in a hierarchy of invasiveness, from reducing or temporarily discontinuing lithium to saline rehydration to forced diuresis to HD. HD was performed in 13.2% of episodes with both intermittent HD and CVVHF. In the published cohorts, HD treatment was used in 1–11% of cases (Table 4). It remains unclear which patients to dialyze, and clinical practice remains variable (). Recent recommendations indicate a need for HD in patients with a lithium level >5 mmol/L or a level of 4 mmol/L in the presence of renal impairment as long as life-threatening symptoms are absent (). This is a much higher threshold than that used by the clinicians in our study. The lowest lithium level leading to treatment with HD in our study was 1.93 mmol/L; the highest level treated without HD treatment was 4.91 mmol/L. Both related to patients who had taken overdoses and presented with mild symptoms. Clearly, the decision of whether to instigate HD depends not only on the highest mean lithium level, but also on the associated symptoms, pharmacokinetic factors and even logistic considerations.
Interestingly, forced diuresis was used as frequently as HD, despite not being recommended in the guidelines. Yet, other studies in the field report a similar treatment approach. In a study by , ‘most’ patients received forced diuresis. In another cohort (), 12/22 patients received furosemide. The rationale for treating lithium toxicity with forced diuresis is based on furosemide increasing the lithium clearance by decreasing the reabsorption in the thick ascending limb of the Henle loop. If a patient were euvolemic and had preserved GFR, furosemide could theoretically increase endogenous clearance up to 20% (). The statement that forced diuresis is not effective and is potentially harmful (; ) seems based on two cases from (Hansen and Amdisen), where volume losses have been replaced by isotonic glucose. This leads to increased reabsorption in the proximal tubule. Another study investigating lithium clearances in an intoxication setting compared 12 patients under forced diuresis with a single reference patient not receiving diuretics and did not find any benefit (). As GFR varies greatly between patients, it is difficult to evaluate benefit of forced diuresis. In our study, no harm was associated with forced diuresis.
It is now widely accepted to address volume depletion and consecutive reabsorption of sodium and lithium by saline infusion (). Because of the risk of dehydration due to NDI in lithium-exposed patients, tight measurements of serum sodium and eventually treatment with dextrose are mandatory. This should allow patients to be hydrated generously with saline, even in the absence of overt hypovolemia.
Outcome in lithium intoxication
Lithium intoxication is potentially life-threatening. Large case series from poison-control centres (PCC) report neurological or cardiac causes (; ) or renal failure and aspiration pneumonia () for fatal outcomes. It is difficult to deduct mortality rates from PCC data due to selection bias and high variability in severity. Published case series investigating mortality on the base of hospital admission data or laboratory data (; ; ) did not report any deaths in 236 cases. had a much higher mortality in a cohort of elderly patients identified from prescription data, but it remains unclear how many fatalities were caused by lithium. Despite the life-threatening potential of lithium intoxication, only a minority will require treatment with HD, and surprisingly few result in death (Table 5).
Lithium may be potentially neurotoxic, but neurologic sequelae of lithium intoxication seem rare. A review of the literature between 1964 and 2004 revealed 90 patients with irreversible neurologic sequelae, also called syndrome of irreversible lithium-effectuated neurotoxicity (SILENT), presenting with persistent cerebellar dysfunction, extrapyramidal symptoms, brainstem dysfunction or ‘dementia with varying degrees of mental syndromes’. The authors suggested that this risk of neurotoxicity supports longer dialysis sessions as a potential preventive measure (). With regard to the risk of SILENT, it seems justified to have a low threshold for HD treatment. Current recommendations on when to start extra-corporal removal of lithium depend on expert consensus rather than on systematic evidence. Non-adherence to current recommendations has not led to adverse events ().
Strengths
All patients treated with lithium were registered in one single central laboratory database in our county. Together with national prescription data, this allowed the incidence of lithium intoxication to be estimated. The vast majority of patients consenting to access to their medical chart resulted in us being able to include 82% of patients. The electronic medical records for both primary and secondary care have been electronically available since 1997. Thus, we had a long period of observation, including all laboratory parameters and prescription data across services. To our knowledge, this is the first study reporting systematically on renal function before, during and after lithium intoxication.
Limitations
This study had some limitations. We limited our review to episodes with lithium levels of at least 1.5 mmol/L to avoid a bias towards mild and borderline intoxication. Also, we wished to avoid false positive results. Lithium levels to screen for toxicity tend to be taken at any time after the last lithium ingestion, in contrast to therapeutic plasma levels, which are taken as trough levels. Lithium intoxication may have been more frequent than shown but clinically not recognised. In such cases, the lithium levels may not have been taken.
The study depended on the quality of the information recorded in the medical records. Hence, we may have underestimated the prevalence of symptoms, since these were not always comprehensively recorded. This, coupled with a relatively small sample size, yielded insufficient power to explore further potential contributing factors to the renal outcome. Our relatively small sample size also made the detection of rare events such as deaths and SILENT unlikely.
In this study, we did not follow up on renal function longer than the next measurement of creatinine after at least one month; whether lithium intoxication leads to increased vulnerability and higher risk of risk of long-term decline of renal function was not addressed.
Conclusions
Severe to moderate cases of lithium intoxication are rare. If they occur, they can be managed safely in most cases. AKI occurs, but sustained loss of renal function is rare. In our study, in the majority of cases, renal impairment was most likely a cause rather than a consequence of lithium intoxication. Intensive care may be needed. Both intermittent HD and CVVHD can be used, but the clearance of CVVHD can be too low in cases where large amounts of lithium have been ingested. Both volume expansion with saline and forced diuresis have been used and are safe. As lithium can cause irreversible neurotoxic effects, it makes sense intuitively to remove lithium assertively, since HD has a low complication rate.
Ultimately, as lithium intoxication is rare and can be safely managed in most cases, physicians should not withhold lithium for fear of intoxication in patients who benefit from it. Yet, physicians should have a low threshold to screen for toxicity if changes in mental and somatic status occur. It is important to discuss such risks in the context of benefits with patients, who may have become concerned about the safety of lithium through reports on the Internet. In order to manage lithium treatment safely, it is important to educate patients about the risks of lithium toxicity and enable them to understand under which circumstances lithium levels can rise.
Declaration of Conflicting Interests The authors declared the following potential conflicts of interest with respect to the research, authorship, and/or publication of this article: Ursula Werneke received funding for educational activities (Masterclass Psychiatry Programme/EAPM 2016, Luleå, Sweden): Astra Zeneca, Janssen, Lilly, Lundbeck, Novartis, Servier, Otsuka and Shire. Michael Ott, Bernd Stegmayr and Ellinor Salander Renberg declare that there is no conflict of interest.
Funding The authors disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This work was supported by a grant of the Norrbotten County Research & Development Fund, Sweden and the Swedish Kidney Association (Njurförbundet Norrbotten).
References
- Adityanjee Munshi KR, Thampy A (2005) The syndrome of irreversible lithium-effectuated neurotoxicity. Clin Neuropharmacol 28: 38–49.
- Aiff H, Attman PO, Aurell M, et al. (2015) Effects of 10 to 30 years of lithium treatment on kidney function. J Psychopharmacol 29: 608–614.
- Azab AN, Shnaider A, Osher Y, et al. (2015) Lithium nephrotoxicity. Int J Bipolar Disord 3: 28.
- Bailey B, McGuigan M (2000) Lithium poisoning from a poison control center perspective. Ther Drug Monit 22: 650–655.
- Bocchetta A, Ardau R, Fanni T, et al. (2015) Renal function during long-term lithium treatment: a cross-sectional and longitudinal study. BMC Med 13: 12.
- Bretaudeau Deguigne M, Hamel JF, Boels D, et al. (2013) Lithium poisoning: the value of early digestive tract decontamination. Clin Toxicol (Phila) 51: 243–248.
- Chen KP, Shen WW, Lu ML (2004) Implication of serum concentration monitoring in patients with lithium intoxication. Psychiatry Clin Neurosci 58: 25–29.
- Cleare A, Pariante CM, Young AH, et al. (2015) Evidence-based guidelines for treating depressive disorders with antidepressants: a revision of the 2008 British Association for Psychopharmacology guidelines. J Psychopharmacol 29: 459–525.
- Close H, Reilly J, Mason JM, et al. (2014) Renal failure in lithium-treated bipolar disorder: a retrospective cohort study. PLoS One 9: e90169.
- Clos S, Rauchhaus P, Severn A, et al. (2015) Long-term effect of lithium maintenance therapy on estimated glomerular filtration rate in patients with affective disorders: a population-based cohort study. Lancet Psychiatry 2: 1075–1083.
- de Haro L, Roelandt J, Pommier P, et al. (2003) [Aetiologies of lithium overdose: 10-year experience of Marseille poison centre]. Ann Fr Anesth Reanim 22: 514–519.
- Decker BS, Goldfarb DS, Dargan PI, et al. (2015) Extracorporeal treatment for lithium poisoning: systematic review and recommendations from the EXTRIP workgroup. Clin J Am Soc Nephrol 10: 875–887.
- Dennison U, Clarkson M, O’Mullane J, et al. (2011) The incidence and clinical correlates of lithium toxicity: a retrospective review. Ir J Med Sci 180: 661–665.
- Edwards SJ, Hamilton V, Nherera L, et al. (2013) Lithium or an atypical antipsychotic drug in the management of treatment-resistant depression: a systematic review and economic evaluation. Health Technol Assess 17: 1–190.
- Erden A, Karagoz H, Basak M, et al. (2013) Lithium intoxication and nephrogenic diabetes insipidus: a case report and review of literature. Int J Gen Med 6: 535–539.
- Eyer F, Pfab R, Felgenhauer N, et al. (2006) Lithium poisoning: pharmacokinetics and clearance during different therapeutic measures. J Clin Psychopharmacol 26: 325–330.
- Hannedouche T, Natov S, Ikeni A, et al. (1990) Does lithium affect renal sodium handling and renal haemodynamics in normal man? Nephrol Dial Transplant 5: 1007–1012.
- Hansen HE, Amdisen A (1978) Lithium intoxication. (Report of 23 cases and review of 100 cases from the literature). Q J Med 47: 123–144.
- Juurlink DN, Mamdani MM, Kopp A, et al. (2004) Drug-induced lithium toxicity in the elderly: a population-based study. J Am Geriatr Soc 52: 794–798.
- Kehoe RF, Mander AJ (1992) Lithium treatment: prescribing and monitoring habits in hospital and general practice. BMJ 304: 552–554.
- Kidney Disease: Improving Global Outcomes (KDIGO) Acute Kidney Injury Work Group (2012) KDIGO Clinical Practice Guideline for Acute Kidney Injury 2011. Kidney Int Suppl 6: p. 8.
- Kidney Disease: Improving Global Outcomes (KDIGO) (2013) Clinical Practice Guideline for the Evaluation and Management of Chronic Kidney Disease 2012. Kidney Int Suppl 3: p. X.
- Lepkifker E, Sverdlik A, Iancu I, et al. (2004) Renal insufficiency in long-term lithium treatment. J Clin Psychiatry 65: 850–856.
- Levey AS, Stevens LA, Schmid CH, et al. (2009) A new equation to estimate glomerular filtration rate. Ann Intern Med 150: 604–612.
- Lopez JC, Perez X, Labad J, et al. (2012) Higher requirements of dialysis in severe lithium intoxication. Hemodial Int 16: 407–413.
- Montagnon F, Said S, Lepine JP (2002) Lithium: poisonings and suicide prevention. Eur Psychiatry 17: 92–95.
- Offerman SR, Alsop JA, Lee J, et al. (2010) Hospitalized lithium overdose cases reported to the California Poison Control System. Clin Toxicol (Phila) 48: 443–448.
- Oruch R, Elderbi MA, Khattab HA, et al. (2014) Lithium: a review of pharmacology, clinical uses, and toxicity. Eur J Pharmacol 740: 464–473.
- Peng J (2014) Case report on lithium intoxication with normal lithium levels. Shanghai Arch Psychiatry 26: 103–104.
- Price LH, Heninger GR (1994) Lithium in the treatment of mood disorders. N Engl J Med 331: 591–598.
- Roberts DM, Gosselin S (2014) Variability in the management of lithium poisoning. Semin Dial 27: 390–394.
- Rothman KJ (1990) No adjustments are needed for multiple comparisons. Epidemiology 1: 43–46.
- Scharman EJ (1997) Methods used to decrease lithium absorption or enhance elimination. J Toxicol Clin Toxicol 35: 601–608.
- Shorter E (2009) The history of lithium therapy. Bipolar Disord 11 Suppl 2: 4–9.
- Thompson JW, Johnson AC (2011) Acute lithium intoxication: properly directing an index of suspicion. South Med J 104: 371–372.
- Vermeire S, Vanbrabant P, Van Boxstael P, et al. (2010) Severity (and treatment) of chronic lithium poisoning: clinical signs or lab results as a criterion? Acta Clin Belg 65: 127–128.
- Waring WS, Laing WJ, Good AM, et al. (2007) Pattern of lithium exposure predicts poisoning severity: evaluation of referrals to a regional poisons unit. QJM 100: 271–276.
- Webb AL, Solomon DA, Ryan CE (2001) Lithium levels and toxicity among hospitalized patients. Psychiatr Serv 52: 229–231.
- Werneke U, Ott M (2014) Response to ‘The impact of modern treatment principles may have eliminated lithium-induced renal failure’ Aiff et al. 2014. J Psychopharmacol 28: 1189–1190.
- Werneke U, Ott M, Renberg ES, et al. (2012) A decision analysis of long-term lithium treatment and the risk of renal failure. Acta Psychiatr Scand 126: 186–197.
- Young AH, Hammond JM (2007) Lithium in mood disorders: increasing evidence base, declining use? Br J Psychiatry 191: 474–476.
- Zimmerman JL (2003) Poisonings and overdoses in the intensive care unit: general and specific management issues. Crit Care Med 31: 2794–2801.