Introduction
Reserpine is an alkaloid extracted from the root of the Rauwolfia serpentina plant, which became commercially available in Western medicine in 1952 after being used for centuries in Indian medicine for a variety of illnesses, including schizophrenia (). Reserpine was used as a first-line antihypertensive with clear efficacy (), including for individuals with refractory hypertension (). Currently, reserpine is considered a second-line treatment () but its use fell dramatically following reports of depression after treatment (; ).
The first reports of depression in humans, as a potential consequence of reserpine, emerged in the early 1950s. observed psychiatric complications including sadness, fatigue and suicidal ideation in five patients with hypertension treated with large doses of reserpine. These were not observed in patients taking other antihypertensives, and symptoms ceased after reserpine discontinuation. Other case series supported this, including in patients with a history of psychiatric illness (). Notably, at this time reserpine was also being used as an antipsychotic treatment and appeared effective in some individuals with refractory schizophrenia (), although it was not universally supported for this indication () and its popularity was short lived ().
This observation was also one of the foundations for the monoamine hypothesis of depression, suggesting deficiency of monoamines to be linked to depression. As reserpine depletes catecholamines so markedly (see below), and monoamine oxidase inhibitors were found to be beneficial at ameliorating depressive-like symptoms, improvements in depression were thought to be linked to catecholamine increases (). Partly as a result of the clinical observations of depression after reserpine, the monoamine hypothesis of depression has persisted in influencing conceptual thinking in behavioural pharmacology () through to the current day (; ).
Here, we briefly summarise the mechanism by which reserpine is thought to provoke depression, essentially through catecholamine depletion. Reserpine binds irreversibly to catecholamine storage vesicles, such as dopamine and norepinephrine, and blocks adrenergic neurotransmission by irreversibly inhibiting the vesicular monoamine transporter-2 (VMAT-2). This interference in the adrenergic neurotransmission pathway depletes catecholamine pumps, ultimately leading to inhibited uptake of neurotransmitters into pre-synaptic storage vesicles. This degradation of catecholamines from peripheral and central synapses occurs through intraneuronal monoamine oxidase in the cytoplasm ().
Nevertheless, there are reasons to question reserpine’s purported depressogenic effects. One argument is that the claims of reserpine-induced depression originated from observations made by physicians other than psychiatrists, and that when assessed by experienced psychiatrists, patients may rarely meet diagnostic criteria for depression (). An example is that akathisia is a side effect of reserpine (similar to other neuroleptics) and is notoriously challenging to diagnose, often misconstrued as affective episodes (; ); although a lack of association with depression has previously been reported (). A second argument – more related to a lack of high-quality research – is that pharmaceutical companies may have a conflict of interest in favour of declining reserpine use; the molecular structure of reserpine does not allow for chemical manipulations that can generate further patentable derivatives, so there is incentive for pharmaceutical companies to prioritise other more marketable compounds (). Third, it is argued that the ‘depressive syndromes’ observed under reserpine respond to stimulant treatment, indicating a physiological rather than psychopathological effect (). Finally, it is argued that psychiatrists’ views should be considered more closely as they have previously heralded its putative benefits both for affective and psychotic syndromes. These arguments do not deny tolerability or safety considerations, rather that reserpine may be like other antipsychotics that can cause tranquilisation and Parkinsonism symptoms when used for longer durations and at higher doses (an approximately 10-fold excess of recommended dose was used in some of the early case series) and can cause akathisia or dyskinesia during early treatment ().
This literature has been (non-systematically) reviewed previously: Considering 61 case reports (from 14 studies), a depression rate after reserpine was found from original articles to be 66%, which decreased to an approximately 10% when restricted to the reviewed group studies. The authors concluded that many of the depression cases were not necessarily caused by reserpine and that one reason why this has not been established in the literature is a reticence to disregard the monoamine hypothesis of depression ().
Objectives
Given long-standing and substantial clinical consequences which have arisen from the reserpine–depression link, it is surprising that a systematic review of this literature has not been published.
We therefore aimed to systematically review the literature to evaluate all available evidence related to the depression-related effects of reserpine. The primary aim of this review was to indicate the nature and extent of depressive episodes as consequences of reserpine treatment. Our secondary aims were to synthesise data pertaining to related symptoms (e.g. anxiety, suicide), overall tolerability and acceptability of reserpine treatment.
Methods
The review adheres to the preferred reporting items for systematic reviews and meta-analyses (PRISMA) guidelines (). The review protocol was pre-registered on the international prospective register of systematic reviews (registration CRD42021225227).
Search strategy
A systematic literature search was conducted of the electronic databases MEDLINE, Embase and PsycINFO (by RJ). The following search terms were used to identify publications from all dates up to 14 February 2021: (depress* OR MDD OR major depress* OR suicid* OR anxiety OR low mood) AND (reserpine OR serpasil). Reference lists from notable authors, review articles and articles eligible for inclusion were also hand searched for thorough data retrieval. ClinicalTrials.gov was also searched as part of the hand-searching process to identify unpublished trials. No language restrictions were implemented.
Study eligibility criteria
Inclusivity of evidence was maximised to consider all possible effects of reserpine on depression. There were no restrictions on types of study design eligible for inclusion. Only primary studies of at least 10 participants were included. Participants had to be human adults, but no other restrictions were placed on participants’ age, gender, diagnosis or treatment.
Studies had to have assessed the effect of reserpine, in any dose and duration, on symptoms of depression. There must have been an untreated versus treated comparison, wherein data included adult participants not treated with reserpine, compared with those treated with reserpine. This could be either between-subjects (between treated and untreated participants) and/or within-subjects (between pre- and post-treatment time points). There was no restriction regarding comparator treatments. The primary outcome was differences in depressive symptoms between reserpine-untreated and reserpine-treated conditions. Studies which had no outcome for depression were excluded.
Study selection and data extraction
Screening was conducted by two review authors (RJ and JC) independently assessing the search results against the pre-defined inclusion and exclusion criteria, blinded from one another’s selections. Any discrepancies between individual judgements were addressed by consensus through a third review author (RS or AHY). Upon agreement of included articles, data extraction was undertaken by two review authors (RJ and JC) independently, using a standardised extraction form. Data extracted pertained to study design characteristics, participant demographics and baseline characteristics (e.g. diagnoses), information regarding any between- or within-subjects characteristics, treatment characteristics (e.g. dose and duration of treatment), measures of outcome and relevant results. For the primary outcome, any assessment which captured depression was eligible, prioritising validated, clinician-rated measures of depression severity. Where this was not available, patient-rated validated depression measures, or non-standardised assessments of depression were considered. For secondary outcomes, data regarding extent of adherence to reserpine and any comparison interventions, such as trial dropout, non-completion, or other compliance data, were recorded. Additionally, extent of tolerability to reserpine was noted. This constituted data on side effects reported, highlighting those associated with mood or psychiatric symptoms, or discontinuation from the study for any reason. The effects of reserpine on other symptoms associated with depression, such as anxiety and suicide, or other individual symptoms of depression were also recorded.
Quality assessment
The methodological quality assessment was examined using a modified risk of bias (RoB) assessment from the risk of bias in non-randomised studies of interventions (ROBINS-I) tool (). Due to the heterogeneity of study designs in this review, tailoring multiple RoB tools (combining or excluding some ROBINS-I items) was considered appropriate (). This was modified to enable assessment of non-longitudinal studies as described below. Studies were assessed by two independent reviewers for the following nine domains: sequence generation and allocation concealment (for randomised studies), comparability of intervention groups at baseline, blinding (participant and intervention), equal treatment of groups, use of intention-to-treat analysis, appropriateness of outcomes measured, deviations from protocol and allegiance effect. Each study was subsequently allocated an overall RoB rating of high, moderate or low RoB.
Data analysis
Due to substantial methodological heterogeneity in study designs, populations studied and outcome measures employed, it was not statistically appropriate to conduct a meta-analysis in this review, as it may have obscured differences in effects or precluded a meaningful summary estimate of effect (). Therefore, a formal narrative synthesis on quantitative studies was conducted, in accordance with reporting guidelines of Synthesis Without Meta-analysis (), to strengthen the robustness of the narrative synthesis of results. Methodology and findings from the included articles are presented and analysed using a tabular method and narrative synthesis. To explore heterogeneity between studies, we considered potentially relevant subgroups or covarying factors comprised of study quality (RoB), diagnosis at baseline, duration and dose of reserpine. Additionally, based on data availability, we examined design (randomised, non-randomised, observational) and sample size of studies, and the type of depression measure (validated clinician rated, patient rated or non-validated) employed (). Where possible, pooled percentages for binary outcomes were calculated. Because studies varied greatly in how they measured and reported outcomes, their findings were synthesised using vote counting based on direction of effect (). Vote counting comprised comparing the number of effects showing a positive or negative association between reserpine and depression, with the number illustrating no association. The nature of the data did not allow us to assess the certainty of the synthesised findings. Secondary outcomes, namely tolerability, adherence and effects of reserpine on symptoms associated with depression were qualitatively explored.
Changes made since protocol registration
Due to the inconsistency of methodologies and reporting of effect sizes between the included studies, we altered the measures of effect. Rather than using a standardised effect size, we employed vote counting as this was deemed more appropriate for syntheses of this nature ().
Results
Systematic search
See Figure 1 for details of the search and study inclusion process. The systematic search yielded a total of 5037 records (3984 after removing duplicates). After an initial screen of eligibility based on article title and abstracts, 182 articles underwent a thorough full text review. Several articles were not accessible in a sufficiently detailed form to be considered (n = 52) or did not examine depression outcomes (n = 47); others were not primary studies (n = 20), did not administer reserpine (n = 13), did not have an untreated comparison (n = 6), did not include ≥10 participants (n = 5) or were not of human adults (n = 4). Thirty-five articles were included in the review.
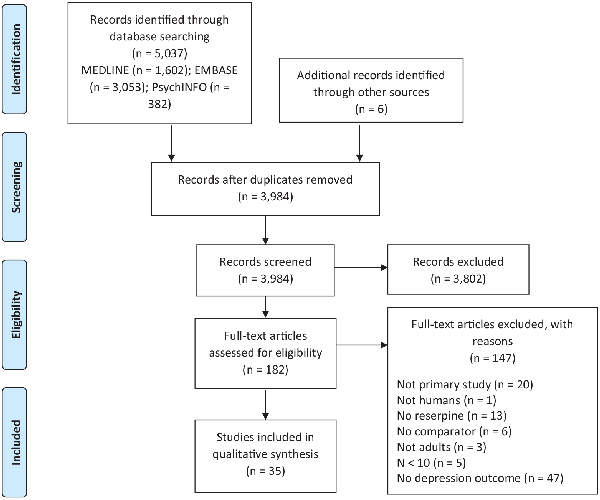
Figure 1
PRISMA flow chart.
Flow chart depicting the literature screening process according to the PRISMA.
Characteristics of included studies
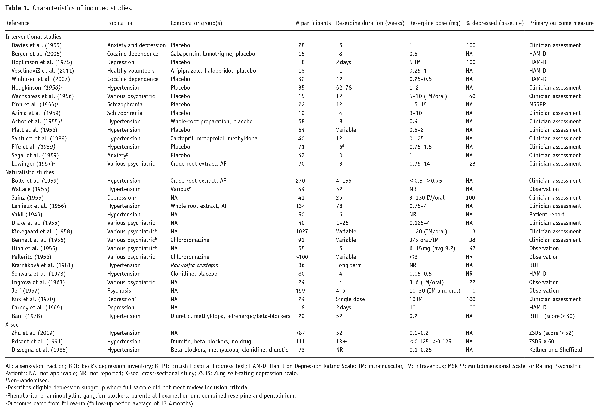
Of 35 included studies, 16 (46%) were conducted in Europe, 15 (43%) in North America, 2 (5%) in Asia, 1 (3%) in South America and 1 (3%) in Australia. Their study designs varied: 17 (49%) were naturalistic studies, 9 (26%) randomised controlled trials, 6 (17%) non-randomised interventional studies and 3 (8%) cross-sectional comparisons. The cross-sectional studies were comparing depression outcomes in a group of patients who had been treated with reserpine with a matched control group who had no treatment or who were treated with a comparator drug. The median sample size was 42 (interquartile range = 56). The duration of treatment with reserpine ranged from 2 days (; ) to 36 months (), with a median duration of 8 weeks (interquartile range = 22). Although most studies assessed reserpine alone (in addition to usual care), in one study, reserpine was augmented with imipramine, trimipramine and amitriptyline tricyclic antidepressants (), one combined reserpine with imipramine (), another with a ganglionic blocker (). In four studies, patients were also given psychological intervention, that is, cognitive behavioural therapy or group psychotherapy (; ; ; ). The dose of reserpine ranged from 0.05 mg () to 130 mg daily (), administered orally or injected intramuscularly or intravenously. Seven studies were not written in English; for these, data were extracted by one native speaker (see acknowledgements) in addition to one English speaker using internet translation (RJ).
Table 1 provides summary characteristics of included studies; additional characteristics can be found in Supplemental Table 1. Participants had a mean age of 48 years (s.d. = 11, range 26–70) and on average 55% were female. The population assessed varied between studies: 15 (43%) studied participants with hypertension, 9 (25%) included various psychiatric conditions, 4 (11%) had a depression population, 2 (6%) involved cocaine dependent participants, 2 (6%) had a schizophrenia population, 1 (3%) included anxiety, 1 (3%) used healthy volunteers and 1 (3%) included anxiety and depression.
Quality assessment
Supplemental Table 2 displays the RoB ratings across criteria and studies. The majority of studies were undertaken prior to the introduction of clear research conduct and reporting standards and were rated to have a high RoB, with only two rated as low RoB and three as moderate RoB. Each of the low or moderate RoB studies appeared to have higher quality controls than other studies in terms of being randomised, with a placebo condition and a clinician-rated validated depression outcome (although were not necessarily more recent or recruiting an adequate sample size). Notably, there was no evidence of pre-specified methods or outcomes in all but one study (). Only one study () was indicated to have a potential conflict of interest through being affiliated with an industrial sponsor.
Primary outcome
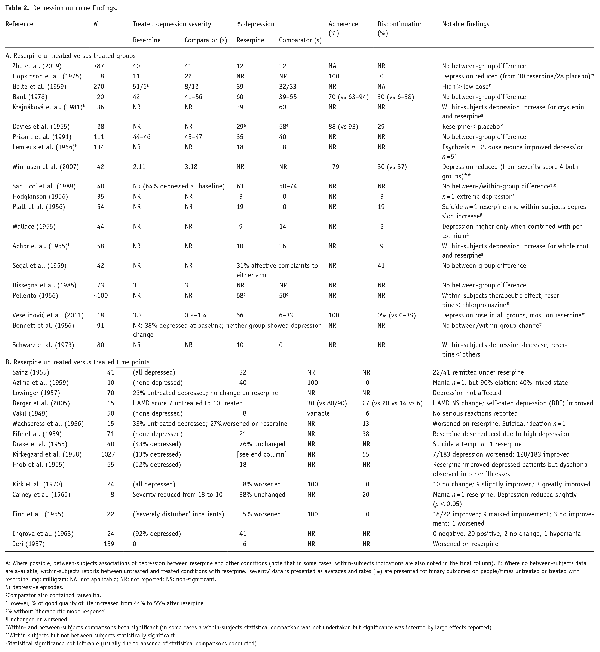
Depression rates after reserpine treatment ranged from 3% () to 76% () of participants across different study types. Table 2 displays these findings across studies. Notably, about half of the studies included a psychiatric population at baseline and non-psychiatric studies did not always exclude people with psychiatric symptoms prior to reserpine treatment.
Between-subjects effects
As presented in Table 2, of the 20 studies (n = 2071 participants) with a between-subjects comparison, numerically higher depression (severity scores or episode rates) was reported in reserpine-treated patients than in untreated patients in eight studies (n = 711 participants); no difference between treated and untreated (or other-treated) groups was reported in six studies (n = 1077) and lower depression in six studies (n = 283).
Where reserpine was associated with depression, this was reported as statistically significant in one study, compared with aripiprazole, haloperidol and placebo (). Rates were numerically higher (statistical tests not undertaken) in comparison to crude root extract and asteroxyon fraction (; ), placebo (), clonidine and placebo (). The difference was smaller in one study compared to chlorpromazine () and another to placebo () and in only one of these studies with a statistical comparison, non-significantly higher than diuretic, methyldopa, adrenergic blockers or beta-blockers ().
Clearer no-difference effects were in comparison with chlorpromazine (), placebo (), beta-blockers, methyldopa, clonidine, diuretic () and other-treated participants in a cross-sectional examination (). Additionally, one study reported no significant difference in reserpine monotherapy compared with a variety of other medications (although overall, depression was numerically lower than comparators), except for a higher rate of depression identified in participants treated with both reserpine and pentolinium than other comparators (); a final study identified (numerically) lower rates of depression in reserpine than alpha methyldopa but higher depression in reserpine than in either captopril or metoprolol ().
Finally, of the six studies finding less depression in reserpine-treated individuals, two were numerically lower (not statistically) than diuretic, beta-blockers, placebo () and another versus placebo (). Others, not tested statistically, were compared with whole-root extract and placebo () and crystepin (). However, both are also derived from R. serpentina. Finally, statistically lower depression was reported in two placebo-controlled studies (; ).
Despite these between-group effects, some of the studies where between-groups comparisons did not find reserpine to elicit higher depression did identify within-group worsening with reserpine (; ) and in others where more depression was reported in reserpine than other groups, within-subjects improvements even in reserpine groups were reported ().
Within-subjects effects
Twenty-seven studies provided evidence of changes in depression over time (n = 2320 participants). As above, most did not report statistical significance for within-subjects effects; therefore results are reported in terms of numerical direction. Eleven studies (n = 645) found some increase in depression under reserpine; 4 studies (n = 216) found no changes in depression and 12 studies (n = 1459) reported some reduction in depression following reserpine therapy. Most notably, one study found that 54% depressed individuals remitted under reserpine (); another study from the latter category did report an improvement in depressive illness for those with symptoms prior to treatment but dysphoria emerging in other patient groups (). One study recorded above as ‘no change’ reported a slight (non-significant) increase in clinician-rated depression severity scores, but a larger improvement in patient-rated scores after reserpine (). Two other ‘no change’ studies reported that despite a lack of overall improvement, reserpine had helped patients to engage with other concomitant antidepressant therapies and that it could be a useful adjunctive agent from this perspective (; ).
Relatedly, across within- and between-subjects findings, it is relevant that three of the studies finding no association and three reporting improvement were concomitantly treating patients with an antidepressant or psychotherapy (studies referenced above).
When pooling depression rates after reserpine across all available studies, the rate was 27% for participants with psychiatric illnesses at baseline (19 studies, n = 1821; rate not reported in an additional four studies) while the rate for non-psychiatric patients was 23% (16 studies, n = 1881; rate not reported in one study). What differed more between these participant groups was the rate of depression in placebo-treated participants; 10% across eight psychiatric population studies (n = 182) and 1% from six non-psychiatric studies (n = 316). If then calculating the percentage difference overall between participants in reserpine compared with control arms, an increase in depression of 17% is found in populations with mental illnesses (27% reserpine vs 10% placebo) and the increased rate of depression in populations without mental illnesses is 22% (23% reserpine vs 1% placebo). Speculatively, this could support the view that reserpine is less depressogenic in people with existing psychiatric illnesses.
Primary outcome effect modifiers
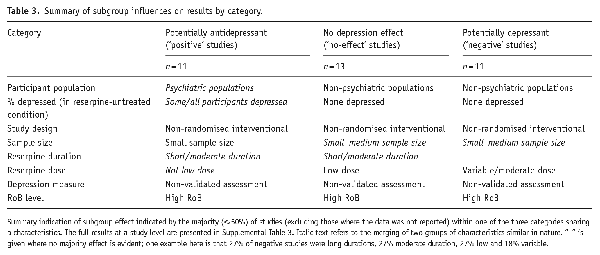
We considered potential effect modifiers by categorising all studies into three categories: Negative studies, where their findings overall suggested a significant or possible depressogenic effect of reserpine (11 studies); No-effect studies, where their findings did not demonstrate an effect (13 studies) and Positive studies, where a potential therapeutic effect on depression was suggested (11 studies). See Table 3 for a summary of the putative effect modifiers across each category, and Supplemental Table 3 contains a more detailed breakdown of all these findings.
Pre-existing mental illness: Positive studies were more likely to examine (not depression-specific) psychiatric populations, and none recruited non-psychiatric patients; non-psychiatric patient samples were more frequently examined in no-effect and negative studies. Some studies found that depressogenic effects of reserpine were less likely within depressed participants () and therefore the proportion of (reserpine-untreated) participants who were classified as depressed was considered as a separate potential effect modifier.
Baseline depression: 10/11 (91%) negative studies examined participants who were not depressed before starting treatment and this was also the case for no-effect studies. By contrast, 7/11 (64%) of positive studies included some/all participants with depression at baseline.
Depression outcome measure: Most studies employed a non-validated measure of depression; clinician-rated validated measures were slightly more frequent in the positive studies, and no-effect studies comprised the majority of patient-rated assessment.
Dose: Negative studies frequently used moderate and/or variable doses, whereas no-effect studies frequently used low doses, and positive studies were slightly less likely to use low doses. Notably, one negative and one no-effect study both reported higher rates of depression in participants taking a higher dose of reserpine (; ) and others have reported lowering doses of reserpine as a direct result of depressive symptoms emerging ().
Duration: No positive studies treated patients with reserpine for longer than 6 months (studies were equally distributed between short or moderate durations). Duration was more evenly distributed across categories for no-effect and negative studies (with long durations in 31% and 27% of studies, respectively).
Design: Naturalistic studies were well represented in all three categories, but no-effect studies were less likely to be randomised trials, and negative studies were slightly more likely to be non-randomised interventional designs.
Sample size: Positive studies were less likely to examine large samples (9%; 73% small samples). The other two groups were more evenly distributed between categories, although negative studies were more often either large or small than no-effect studies (more often medium).
Newer studies did not consistently assess larger samples than older studies, though one of the four studies published in this century was large and was classified as no effect. The other three newer studies were categorised as positive (two studies) or negative (one study). Two out of four newer studies comprised 2/5 of the low/moderate RoB studies (one was a positive study, the other was negative and both had small sample sizes).
Risk of bias: As most studies were categorised as having a high RoB, we report here only the classification of the five low/moderate RoB studies: 3/5 were positive and 2/5 were negative (all small samples).
Secondary outcomes
Adherence
These data are included in Table 2. Two-thirds of studies did not report adherence or discontinuation of treatment with reserpine or comparators. Across arms, the highest dropout rate was observed in a haloperidol comparator (39%; ()) in contrast with a reserpine discontinuation of 0%. There was a difference of >10% in participant discontinuation between arms in (17% antihypertensive drugs; 36% non-antihypertensive comparisons) and (27% reserpine; 7% lamotrigine; 7% placebo; 0% gabapentin). Ten studies reported adherence data, which was numerically lower in reserpine versus active and placebo controls in three studies (; ; ; ).
Tolerability and related symptoms
Seventeen studies reported an average proportion of the sample reporting adverse events. These were higher in reserpine than in some comparators (active/placebo) in six studies (; ; ; ; ; ), although two of these studies were higher in other rauwolfia formulations than in reserpine or placebo (; ). The most common side effect reported was sedation, although one manic onset was also reported in two studies (; ). One further study noted a psychotic reaction in two patients following reserpine ().
Increased anxiety was reported in six studies (see Supplemental Table 1) and suicide or suicide attempt or suicidal ideation reported in three studies (see Table 2). There were no suicidal trends reported in comparator arms. Individual symptoms of depression were reported as a side effect to reserpine treatment; fatigue was reported in 10 studies (; ; ; ; ; ; ; ; ; ). Concentration deficits were reported in ; insomnia and sleep disturbances were reported in , , and . Loss of motivation, interest or drive were reported in and . reported decreased appetite, while , and reported an increase in appetite. Reserpine was associated with weight gain in six studies (; ; ; ; ; ).
Discussion
This systematic review is, to our knowledge, the first to evaluate the effects of reserpine on depressive symptoms. Our findings highlight the limited evidence base, with few adequately powered controlled studies, high heterogeneity between studies and a high RoB.
The prevalence of depression following reserpine treatment ranged from 3% to 87% (although studies with higher depression symptoms post-reserpine also included depressed individuals in the untreated condition). Eleven studies reported depressive effects of reserpine, 13 reported an absence of effect and 11 reported potential benefits for depression symptoms with reserpine. Studies suggestive of a depressive effect were more likely to examine non-psychiatric patients at baseline, treat participants for longer and at lower doses compared with studies suggestive of an antidepressant effect. Despite indications that reserpine’s inhibition of VMAT function (explaining its widespread non-selective depletion of monoamines) could be dose and duration dependent (), its pharmacological effects have not been fully characterised. Thus, we are not able to attribute any potential impact of variation in interventional (e.g. dose/duration) factors’ influences on depression to biological effects.
Exploring heterogeneity
These conflicting findings reflect inconsistencies in the literature, from early case studies on reserpine () to more recent arguments challenging these concerns (). Because our systematic review was inclusive, the studies included would inevitably be highly variable in terms of methodology and quality. This was the case across a range of domains, including study design (from cross-sectional comparisons to naturalistic studies, randomised and non-randomised trials); RoB; date of publication; sample size; participant factors (most notably, psychiatric status prior to initiating reserpine); treatment factors (e.g. dose and duration) and outcome factors (e.g. measures used to assess depression). We examined these as subgroups to determine their relationship with study findings. Most of these subgroups did not show a definitive association with depression findings, although a therapeutic effect was observed only in studies recruiting psychiatric patients and none of these studies examined durations of reserpine treatment exceeding 6 months. Some studies treated participants particularly for a short duration, as little as 2–4 days in two studies that observed reductions in depression severity after reserpine (; ). Early work had suggested that at least 6 weeks were needed to observe the emergence of depression after reserpine ().
Almost two-thirds of included studies used a non-validated assessment of depression, usually clinician judgement or observation, and a further 14% used patient-report assessments. Both can generate false-positive cases, which would overestimate clinically significant depression switches after reserpine () and this is supported by non-systematic review findings. This leaves only seven studies evaluating depression using a potentially valid method (diagnostically), of which four had reported putative antidepressant effects.
Given the number of combinations of these factors alongside the small number of articles and patients studied, we cannot make conclusions regarding the circumstances under which reserpine causes or treats depressive symptoms. What we can conclude, though, is that the story of reserpine is not as straightforward as has been widely assumed; thus this work extends previous literature by highlighting that the broadly accepted notion that reserpine should not be used requires further investigation.
We did not assess all possible covarying factors, for example the age of participants: the prevalence of depression reported after reserpine treatment was approximate to the upper limit of point prevalence estimates for depression in the general population (using self-report to assess depression (), and rates of depression may be higher in older people ()). Many samples in our review were older hypertensive patients, and therefore rates of depression in these people may well be relatively large, though not necessarily larger than the general population of people in this age range.
Putative antidepressant effects of reserpine?
The 11 studies identifying potential therapeutic effects in our review align with a previous placebo-controlled investigation of reserpine for patients with treatment-resistant depression (n = 9; ineligible for this review) finding that reserpine produced rapid improvements when adjunctive to tricyclic antidepressants. However, treatment only lasted for a short period of 7 days (). Taken together, this supports the view that reserpine might be an antidepressant and underpins a potential use for reserpine in the treatment of depression when combined with an antidepressant or psychotherapy.
Some of the studies reporting reserpine’s benefits on depression administered concomitant antidepressant therapies, for example, tricyclic antidepressants or psychological input. Many found that reserpine improved the engagement or efficacy with other treatments, but of the 11 positive studies, two were delivering reserpine monotherapy (; ) and five treated reserpine only in addition to various (non-psychotropic) continuation treatments (; ; ; ; ).
Additionally, the study reporting the highest prevalence of post-reserpine depression (87%) was in a mixed psychiatric population of whom 38% were depressed before reserpine initiation (). This suggests reserpine can be depressogenic in those susceptible to the illness () and questions the idea that reserpine may be an antidepressant in those with pre-existing clinically significant symptoms, although this was one of the longer studies, treating patients for 6 months.
Conversely, the overall pooled percentages for depression across our included studies indicated a 3% difference in depression onset after reserpine treatment between psychiatric patients and non-psychiatric patients, whereas the risk for depression after placebo was 7% higher in psychiatric versus non-psychiatric participants. One possible explanation for this relates to a possible misdiagnosis (in some cases) of depression instead of akathisia () as akathisic reactions may emerge as an adverse effect more frequently in non-psychiatric populations () and therefore may explain the negative effects observed in samples with hypertension.
Depression-associated symptoms
Even if the psychiatric adverse effects are questionable, the consequences are significant. Previous research reported one suicide and three attempted suicides during 1 year’s treatment with reserpine in patients with schizophrenia () corroborating findings of three of our included studies (; ; ) and a previous theory that reserpine-induced depression is characterised by suicidal thoughts, albeit from a case study of six patients (). The quantity of evidence is not sufficient to disregard the possibility of this suicidality being attributable to natural courses of psychiatric illness. Relatedly, there remain uncertainties about risk of manic switch after reserpine (; ). The effect of reserpine on anxiety is questionable, as four of our studies found anxiogenic effects (some on patients with pre-existing anxiety) () while others reported anxiolytic effects potentially via sedation (; ).
Some articles reported increases in other individual symptoms of depression, for example reduction in concentration (), or anorexia (). These are non-specific symptoms though, and if documented in the absence of the core elements of depression (low mood, anhedonia), may inflate estimated depression rates ().
Relevance for mechanisms underlying antidepressant response and monoamines
Our findings pose a challenge to the original monoamine hypothesis of depression which has largely dominated the field, as reserpine acts by depleting catecholamines (), yet has been evidenced to improve depressive symptoms in some cases. The putative depressogenic effect of reserpine has been largely cited in support of the basic monoamine hypothesis (). Findings of some antidepressant properties of reserpine contradict this (), supplementing other literature that has identified no depressive effect from manipulating tryptophan (). This may also have implications for the reserpine animal model which is still used as a pharmacological challenge for putative antidepressants (), though it is acknowledged that animal models of depression are difficult to extrapolate to the complex behavioural phenotypes of depression. No studies included in this review examined biological markers before and after reserpine treatment. However, because almost half of included studies did report some depressogenic effects of reserpine, it is possible that a subset of people could be susceptible to reserpine-induced depression based on, for example, catecholamine activity. This is as yet an unsubstantiated speculation.
Strengths and limitations
Although the studies included in this review were highly heterogeneous (reflected in inconsistent findings between studies) and date back in many cases to the 1950s, we posit that this review was strengthened by its inclusive approach and adds to the extant literature as the first systematic examination of reserpine’s effects on depression. The small number and size of studies, their high RoB and variable designs limit conclusions that can be drawn and precluded a quantitative meta-analysis. We investigated a range of relevant potential effect modifiers to interpret our findings, although the quantity and heterogeneity (e.g. of population, dose, duration, methodology) limit interpretations that can be drawn. It is also worth acknowledging that some of the older studies had a low RoB.
Moreover, our RoB ratings are undertaken according to current research practices, one example being the pre-registration of protocols to ensure rigour and transparency of analysis and findings (). In this respect and others (e.g. many older studies did not conduct statistical analyses of depression emergence) the reviewed literature is outdated and new studies recruiting adequate sample sizes and employing bias minimisation strategies are required. Although we made maximum efforts to obtain all available data, there were articles that could not be accessed and authors we could not contact for more detailed data.
Conclusions
There has been long-standing controversy surrounding the notion that reserpine causes depression. This review has not uncovered conclusive evidence to elucidate the role reserpine has in inducing – or treating – depressive symptoms. However, it represents the first systematic consolidation of this literature, and we propose enhancing our present understanding of the effect of reserpine on depressive symptoms in humans. Given that the studies which have reported depressogenic effects of reserpine tended not to be randomised trials, administered reserpine for longer and at lower doses and were more likely to examine non-psychiatric patients, we call for rigorous controlled clinical studies to examine the outcomes of time-limited reserpine at a moderate dose with standardised mood stabilising or antidepressant therapies. Of equal importance, we urge a balanced and careful judgement regarding reserpine’s effects as being complex and multifaceted.
While the clinical studies of reserpine appear equivocal, the results of this review do cast doubt on simplistic notions underlying the initial monoamine hypothesis of depression. Wide individual variation in response of depression symptoms to this drug suggests a more nuanced approach is necessary when evaluating the effects of catecholamine depletion on depression syndromes.
We are extremely grateful to the following individuals who extracted information from articles not written in English: Alžběta Jamieson, Valeria de Angel, Irene Faiman and Julia Henke.
Declaration of conflicting interests The author(s) declared the following potential conflicts of interest with respect to the research, authorship, and/or publication of this article: In the last 3 years, RS declares an honorarium from Lundbeck. AHY declares honoraria for speaking from Astra Zeneca, Lundbeck, Eli Lilly, Sunovion; honoraria for consulting from Allergan, Livanova and Lundbeck, Sunovion, Janssen; and research grant support from Janssen. SJ has received honoraria for educational talks given for Lundbeck, Sunovian and Janssen, on antipsychotics. No other conflicts of interest are declared.
Funding The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This work is supported by the National Institute for Health Research (NIHR) Maudsley Biomedical Research Centre at South London and Maudsley NHS Foundation Trust and King’s College London. The views expressed are those of the authors and not necessarily those of the NIHR or the Department of Health and Social Care.
Rebecca Strawbridge
https://orcid.org/0000-0002-2984-1124
Sameer Jauhar
https://orcid.org/0000-0002-3878-3659
Supplemental material Supplemental material for this article is available online.
References
- Achor RW, Hanson NO, Gifford RW (1955) Hypertension treated with Rauwolfia serpentina (whole root) and with reserpine; controlled study disclosing occasional severe depression. JAMA 159: 841–845.
- Akagi H, Kumar TM (2002) Akathisia: Overlooked at a cost. Br Med J 324: 1506–1507.
- Al-Jundi A, Sakka S (2016) Protocol writing in clinical research. J Clin Diagn Res 10: ZE10–ZE13.
- Amsterdam JD, Berwish N (1987) Treatment of refractory depression with combination reserpine and tricyclic antidepressant therapy. J Clin Psychopharmacol 7: 238–242.
- Azima H, Azima FJ, Durost HB (1959) Psychoanalytic formulations of effects of reserpine on schizophrenic organization. AMA Arch Gen Psychiatry 1: 662–670.
- Bant WP (1978) Antihypertensive drugs and depression: A reappraisal. Psychol Med 8: 275–283.
- Baumeister AA, Hawkins MF, Uzelac SM (2003) The myth of reserpine-induced depression: Role in the historical development of the monoamine hypothesis. J History Neurosci 12: 207–220.
- Bennett AE, Ford FR, Turk RE (1956) Clinical investigation of chlorpromazine and reserpine in private psychiatric practice. Am J Psychiatry 112: 782–787.
- Berger SP, Winhusen TM, Somoza EC, et al. (2005) A medication screening trial evaluation of reserpine, gabapentin and lamotrigine pharmacotherapy of cocaine dependence. Addiction 100(Suppl 1): 58–67.
- Blasco-Serra A, Escrihuela-Vidal F, González-Soler EM, et al. (2015) Depressive-like symptoms in a reserpine-induced model of fibromyalgia in rats. Physiol Behav 151: 456–462.
- Bolte E, Marc-Aurele J, Brouillet J, et al. (1959) Mental depressive episodes during Rauwolfia therapy for arterial hypertension, with special reference to dosage. Can Med Assoc J 80: 291–293.
- Campbell M, McKenzie JE, Sowden A, et al. (2020) Synthesis without meta-analysis (SWiM) in systematic reviews: Reporting guideline. Br Med J 368: l6890.
- Carlsson A (2001) A half-century of neurotransmitter research: Impact on neurology and psychiatry. Nobel lecture. Biosci Rep 21: 691–710.
- Carney MW, Thakurdas H, Sebastian J (1969) Effects of imipramine and reserpine in depression. Psychopharmacologia 14: 349–350.
- Cheung M, Parmar M (2022) Reserpine. In: StatPearls. Treasure Island (FL): StatPearls Publishing. Available at: http://www.ncbi.nlm.nih.gov/books/NBK557767/ (accessed 1 March 2022).
- Davies DL, Shepherd M (1955) Reserpine in the treatment of anxious and depressed patients. Lancet (London, England) 269: 117–120.
- Deeks JJ, Higgins JPT, Altman DG (eds) (2022) Chapter 10: Analysing data and undertaking meta-analyses. In: Higgins JPT, Thomas J, Chandler J (eds) Cochrane Handbook for Systematic Reviews of Interventions version 6.3 (updated February 2022). Cochrane. Available at: http://www.training.cochrane.org/handbook (accessed 22 May 2022).
- Dissegna L, Ambrosio GB, Zamboni S, et al. (1985) [Psychological effects in the drug treatment of arterial hypertension. A study during a community preventive program]. Giornale Italiano Di Cardiologia 15: 571–575.
- Drake FR, Ebaugh FG (1955) The use of reserpine in office psychiatry: Preliminary report. Ann N Y Acad Sci 61: 198–205.
- El-Marasy SA, El Awdan SA, Hassan A, et al. (2021) Anti-depressant effect of cerebrolysin in reserpine-induced depression in rats: Behavioral, biochemical, molecular and immunohistochemical evidence. Chemico Biological Interactions 334: 109329.
- Farrah K, Young K, Tunis MC, et al. (2019) Risk of bias tools in systematic reviews of health interventions: An analysis of PROSPERO-registered protocols. Syst Rev 8: 280.
- Fife R, Paton JP, Whyte WG (1959) Treatment of hypertension in out patients; the effect of pentolinium. Scottish Med J 4(5): 242–248.
- Finn MH, Nadolski F, Guy W, et al. (1955) Clinical, psychological and myoneural changes in psychotic patients under ora serpasil medication. J Nerv Mental Dis 122: 458–462.
- Freis ED (1954) Mental depression in hypertensive patients treated for long periods with large doses of reserpine. New Engl J Med 251: 1006–1008.
- Furberg CD, Wright JT, Davis BR, et al. (2002) Major outcomes in high-risk hypertensive patients randomized to angiotensin-converting enzyme inhibitor or calcium channel blocker vs diuretic: The antihypertensive and lipid-lowering treatment to prevent heart attack trial (ALLHAT). J Am Med Assoc 288: 2981–2997.
- Halstead SM, Barnes TR, Speller JC (1994) Akathisia: Prevalence and associated dysphoria in an in-patient population with chronic schizophrenia. Br J Psychiatry 164: 177–183.
- Healy D, Farquhar G (1998) Immediate effects of droperidol. Human Psychopharmacol Clin Exp 13: 113–120.
- Healy D, Savage M (1998) Reserpine exhumed. Br J Psychiatry 172: 376–378.
- Hiob J, Hippius H (1955) [Clinical trials of Rauwolfia alkaloid, reserpine, in psychiatry]. Deutsche Medizinische Wochenschrift (1946) 80: 1497–1500.
- Hodgkinson R (1956) The effects of reserpine on hypertensive patients over a period of two years. Br Heart J 18: 523–528.
- Hopkinson G, Kenny F (1975) Treatment with reserpine of patients resistant to tricyclic antidepressants. A double-blind trial. Psychiatria Clinica 8: 109–114.
- Hull LD, Horita A (1964) Reserpine reversal response by iproniazid: A dose-dependent phenomenon. Nature 202: 604–605.
- Ingrova L, Bojanovsky J, Chloupkova K (1963) Treatment of endogenous depressions with intermittent doses of reserpine. Activitas Nervosa Superior 5: 183–184.
- Isharwal S, Gupta S (2006) Rustom Jal Vakil: His contributions to cardiology. Texas Heart Institute Journal 33: 161–170.
- Jeri R (1957) Experiencias con la reserpina en el tratamiento de algunas psicosis endógenas y sintomáticas. Rev Neuropsiquiatr 20: 342–358.
- Jorstad J (1956) Reserpine in psychoses. Nord Med 55: 794–798.
- Kim GE, Jo M-W, Shin Y-W (2020) Increased prevalence of depression in South Korea from 2002 to 2013. Sci Rep 10: 16979.
- Kirk L, Gram LF, Jenson PS (1970) Clinical experience with 10 mg reserpin injected i.m. in depressive disorders. Acta Psychiatrica Scandinavica 1: 55.
- Kirkegaard G, Lyager T, Nielsen J, et al. (1958) The serpasil treatment of 1,027 psychiatric patients. Acta Psychiatrica Scandinavica 33: 26–43.
- Krajnáková E, Mallineritsová E, Dobrotka G, et al. (1981) [Concerning psychological problems in the development of pharmacogenic depressions. Depressions during long-term treatment with hypotensive drugs (author’s transl)]. Bratislavske Lekarske Listy 75: 361–364.
- Lemieux G, Davignon A, Genest J (1956) Depressive states during Rauwolfia therapy for arterial hypertension; a report of 30 cases. Can Med Assoc J 74(7): 522–526.
- Leon AC, Portera L, Olfson M, et al. (1997) False positive results: A challenge for psychiatric screening in primary care. Am J Psychiatry 154: 1462–1464.
- Lim GY, Tam WW, Lu Y, et al. (2018) Prevalence of depression in the community from 30 countries between 1994 and 2014. Scientific reports 8 1–0.
- López-Muñoz F, Bhatara VS, Alamo C, et al. (2004) [Historical approach to reserpine discovery and its introduction in psychiatry]. Actas Espanolas De Psiquiatria 32: 387–395.
- Lowinger P (1957) Rauwolfia serpentina in the control of anxiety. Psychiatric Q 31: 445–453.
- Mahata M, Mahata SK, Parmer RJ, et al. Vesicular monoamine transport inhibitors: Novel action at calcium channels to prevent catecholamine secretion. Hypertension 1996; 28: 414–420.
- McKenzie JE, Brennan SE (2022) Chapter 12: Synthesizing and presenting findings using other methods. In: Higgins JPT, Thomas J, Chandler J, et al. (ed.) Cochrane Handbook for Systematic Reviews of Interventions version 6.3 (updated February 2022). Cochrane. Available at: http://www.training.cochrane.org/handbook (accessed 22 May 2022).
- Moulton CD, Strawbridge R, Tsapekos D, et al. (2021) The Maudsley 3-item Visual Analogue Scale (M3VAS): Validation of a scale measuring core symptoms of depression. J Affect Disorders 282: 280–283.
- Nick J (1955) [Melancholia in hypertensive patients caused by therapeutic use of Rauwolfia serpentina]. Bulletins Et Memoires De La Societe Medicale Des Hopitaux De Paris 71: 884–893.
- Nordman LO (1956) Reserpine-triggered depressive psychoses. Reserpinutlosta depressiva psykoser 53: 1641–1647.
- Page MJ, McKenzie JE, Bossuyt PM, et al. (2021) Updating guidance for reporting systematic reviews: Development of the PRISMA 2020 statement. J Clin Epidemiol 134: 103–112.
- Pellerito N (1956) [Action of chlorpromazine and reserpine on the anxiety of psychoneurotics]. Minerva Medica 47: 628–631.
- Peterfy G, Pinter EJ, Pattee CJ (1976) Psychosomatic aspects of catecholamine depletion: Comparative studies of metabolic, endocrine and affective changes. Psychoneuroendocrinology 1: 243–253.
- Platt R, Sears HT (1956) Reserpine in severe hypertension. Lancet 270: 401–403.
- Preskorn SH (2007) The evolution of antipsychotic drug therapy: Reserpine, chlorpromazine, and haloperidol. J Psychiatr Pract 13: 253–257.
- Prisant LM, Spruill WJ, Fincham JE, et al. (1991) Depression associated with antihypertensive drugs. J Family Pract 33: 481–485.
- Reeves BC, Deeks JJ, Higgins JPT, et al. (2022) Chapter 24: Including non-randomized studies on intervention effects. In: Higgins JPT, Thomas J, Chandler J, et al. (editors). Cochrane Handbook for Systematic Reviews of Interventions version 6.3. Cochrane. Available at: http://www.training.cochrane.org/handbook (accessed 22 May 2022).
- Ruhé HG, Mason NS, Schene AH (2007) Mood is indirectly related to serotonin, norepinephrine and dopamine levels in humans: A meta-analysis of monoamine depletion studies. Mol Psychiatry 12: 331–359.
- Sainz AA (1955) The use of reserpine in ambulatory and hospitalized geriatric psychotics. Ann N Y Acad Sci 61: 72–77.
- Santucci A, Puccetti F, Ficara C, et al. (1989) Angiotensin converting enzyme inhibition and quality of life: A randomized controlled trial. Curr Ther Res 46: 849–857.
- Schwarz D, Michel D, Strian F (1973) [Depressive effects during treatment with antihypertensive drugs (author’s transl)]. Arch Psychiatr Nervenkr 218: 41–50.
- Segal MM, Shapiro KL (1959) A clinical comparison study of the effects of reserpine and placebo on anxiety. AMA Arch Gen Psychiatry 81: 392–398.
- Shamon SD, Perez MI (2016) Blood pressure-lowering efficacy of reserpine for primary hypertension. Cochrane Database Syst Rev 12: CD007655.
- Shepherd M, Watt DC (1956) A controlled clinical study of chlorpromazine and reserpine in chronic schizophrenia. J Neurol Neurosurg Psychiatry 19: 232–235.
- Siddiqui M, Bhatt H, Judd EK, et al. (2020) Reserpine substantially lowers blood pressure in patients with refractory hypertension: A proof-of-concept study. Am J Hypertens 33: 741–747.
- Sterne JA, Hernán MA, Reeves BC, et al. (2016) ROBINS-I: A tool for assessing risk of bias in non-randomised studies of interventions. BMJ 355: i4919.
- Upthegrove R, Marwaha S, Birchwood M (2017) Depression and schizophrenia: Cause, consequence, or trans-diagnostic issue? Schizophr Bullet 43: 240–244.
- Vakil RJ (1949) A clinical trial of Rauwolfia serpentina in essential hypertension. Br Heart J 11: 350–355.
- Veselinović T, Schorn H, Vernaleken I, et al. (2011) Effects of antipsychotic treatment on psychopathology and motor symptoms. A placebo-controlled study in healthy volunteers. Psychopharmacology 218: 733–748.
- Wachspress M, Fink M, Blumberg A, et al. (1956) Evaluation of high dose reserpine therapy for the relief of anxiety. J Hillside Hosp 5: 67–77.
- Wallace DC (1955) Treatment of hypertension; hypotensive drugs and mental changes. Lancet (London, England) 269: 116–117.
- Winhusen T, Somoza E, Sarid-Segal O, et al. (2007) A double-blind, placebo-controlled trial of reserpine for the treatment of cocaine dependence. Drug Alcohol Depend 91: 205–212.
- Zhu G-H, Sun X-P, Li J, et al. (2019) No association between low-dose reserpine use and depression in older hypertensive patient: Result of a multicenter, cross-sectional study. J Geriatric Cardiol 16: 608–613.