Introduction
The 5-hydroxtryptamine (5-HT or serotonin) system has been widely implicated in the pathophysiology of mood and anxiety disorders, with a main treatment focus on the serotonin transporter (SERT), 5-HT1A, 5-HT1B, and 5-HT2A receptor subtypes (; ). More recently, this has extended to the 5-HT7 receptor, which was first identified in 1993 (; ; ). The 5-HT7 receptor is widely distributed across the brain, including the cortex, hippocampus, thalamus, and hypothalamus (; ; ; ) and has been implicated in a variety of brain functions such as mood, sleep, learning and memory, stress, seizures, and circadian rhythm regulation. Moreover, the 5-HT7 receptor may have a significant role in mediating cognition, especially in people with mood disorders (). Given these associations, the 5-HT7 receptor may be a promising treatment target for mood and anxiety disorders (; ; ).
Many second-generation antipsychotic drugs currently available show high affinity to the 5-HT7 receptor and two medications with 5-HT7 antagonist properties, vortioxetine and lurasidone, have been found to enhance cognitive functioning in people with major depressive disorder (MDD) and schizophrenia (; ). Furthermore, preclinical animal studies have indicated the potential use of 5-HT7 antagonists for the treatment of anxiety disorders ().
In this paper, we aimed to systematically review the relationship between 5-HT7 receptors and mood and anxiety disorders, and to further explore the pharmacology and therapeutic potential of medications that target the 5-HT7 receptor for the treatment of mood and anxiety disorders.
Methods
The systematic review study protocol was registered on the International Prospective Register for Systematic Review (PROSPERO) database (registration number CRD42019138174). All study procedures are documented and were reported following the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) reporting guidelines ().
Search methods
The systematic search was conducted using Medline, Cochrane Library, EMBASE, PsycINFO, the National Institute of Health website Clinicaltrials.gov, controlled-trials.com, and relevant grey literature) from 1993 inception to March 2021. The following search string was used:
[(5-HT7 OR serotonin receptor 7 OR 5-hydroxytryptamine 7) AND (depress* OR bipolar disorder OR anxiety disorder)] OR [(5-HT7 OR serotonin receptor 7 OR 5-hydroxytryptamine 7) AND (animals OR humans OR preclinical study OR clinical trial OR experimental medicine)] OR [(5-HT7 OR serotonin receptor 7 OR 5-hydroxytryptamine 7) AND (lurasidone OR vortioxetine)] OR (5-HT7 antagonists OR 5-HT7 agonists)
Reference lists of included articles were further searched for eligible studies. If papers were not written in English, we attempted to obtain a translated version.
Systematic searches of the preselected databases were carried out independently by two researchers (TYL and NG), using the predetermined search string. Results were compiled using Rayyan QCRI software (), and titles and abstracts were independently screened by both researchers. Any studies that appeared eligible, or if there was any uncertainty about eligibility, underwent a full-text review. Final inclusion lists were compared, and any disagreements were discussed until a consensus was reached. An additional (PRS) reviewer was consulted as needed.
Study selection
Study and participant type
Only full-text original research articles using an appropriate control group (sham or placebo) were included. Animal studies that used a mood or anxiety disorder model and a relevant genetic or pharmacological manipulation to the 5-HT7 system were included.
For human experimental medicine studies or clinical trials, randomized control trials (RCTs) using males and females over the age of 18 fulfilling International Classification of Diseases (ICD) or Diagnostic and Statistical Manual of Mental Disorders (DSM) criteria diagnosis for an MDD, major depressive episode (MDE), bipolar affective disorder (BD) or an anxiety disorder were included. All subtypes of MDD or MDE (mild, moderate, severe, with/without psychotic features) and bipolar disorder (rapid cycling, type I, type II and other) were included. Participants who only met criteria for dysthymia or cyclothymia were excluded.
Intervention type
Any studies that used an appropriate genetic or pharmacological manipulation to the 5-HT7 system were included in the present review. Pharmacological agents, in both preclinical and human studies, that have substantial selectivity for 5-HT7 receptors were included, such as selective agonists (AS-19, LP-44, LP-12, LP-211, E55888) (; ; , ; ) and antagonists (SB-238719, SB-269970, SB-656104, DR-4004, DR-4446, PZ-766, JNJ-18038683, asenapine, amisulpride, imipramine, and desipramine) (; ; ; ; ; ; ; ; ; ; ; ). Lurasidone and vortioxetine, antipsychotic and antidepressant medications, respectively, were also included due to their partial 5-HT7 receptor antagonism (; ). Studies that did not use pharmacological agents with reasonable 5-HT7 affinity were excluded.
Outcome measures
The primary outcome measure of interest was changes in mood and anxiety behaviors after pharmacological interventions with agents that bind to, or alter, 5-HT7 receptor function. In preclinical animal studies, these behavioral changes were evaluated by use of validated functional tests that may measure depressive or anxiety symptomatology (e.g. light dark transfer test, elevated plus-maze tests, forced swim tests, and tail suspension tests). Secondary outcome measures of interests were changes in sleep or cognition measures.
For human studies, changes from baseline to endpoint in mood status, assessed by change in mood-related symptoms measured by validated rating scales such as the Hamilton Depression Rating Scale (HAM-D) or Quick Inventory of Depressive Symptomology (QIDS-CR) was the primary outcome measure (; ). Any included data on change in sleep, cognition, or other experimental medicine markers (e.g. neuroimaging) were also collected.
Data collection and analysis
Data extraction was conducted after individual articles were assessed against inclusion criteria and discussed further where needed. All data extraction was completed independently by two authors (NG and TYL).
Quality measures
Study quality was measured by using the “Quality Assessment Tool for Quantitative Studies,” which was designed by Effective Public Health Practice Project (; ). Included studies were assessed in the following domains: selection bias, study design, confounders, blinding, data collection methods, withdrawal, and dropout. A global quality rating was scored independently, and any disagreements discussed by reviewers.
Results
Systematic search results
The initial search identified 8097 papers. After removal of duplicates, 4989 studies underwent initial title and abstract screening leaving 109 studies for full-text review. Review of full-text articles excluded 49 articles for the following reasons: article type (7 review or meta-analysis papers and 18 conference abstracts or posters), wrong disorder or outcome measure (20), data reported elsewhere (2), not a randomized control trials study (1), and article language (1). Two further studies were added from hand searching after the original search. In total, 64 studies were included (52 animal studies and 12 human studies), and details are outlined in Figure 1.
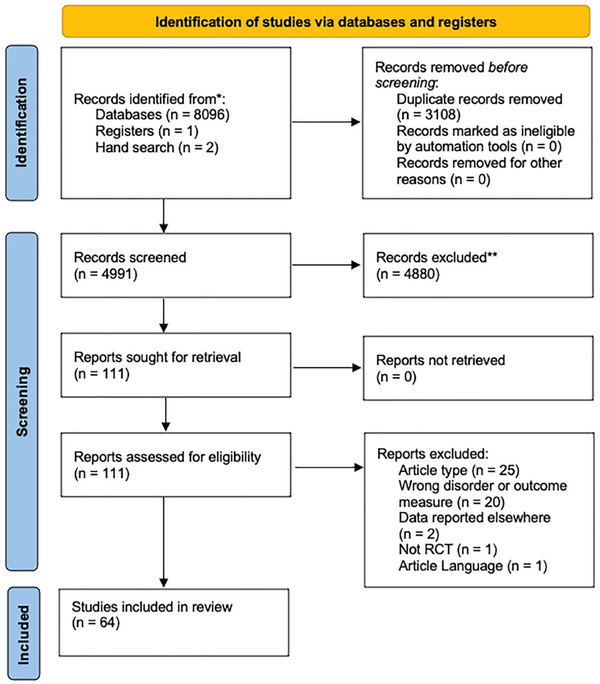
Figure 1
PRISMA diagram.
RCT: randomized controlled trial.
Preclinical study results
Study characteristics
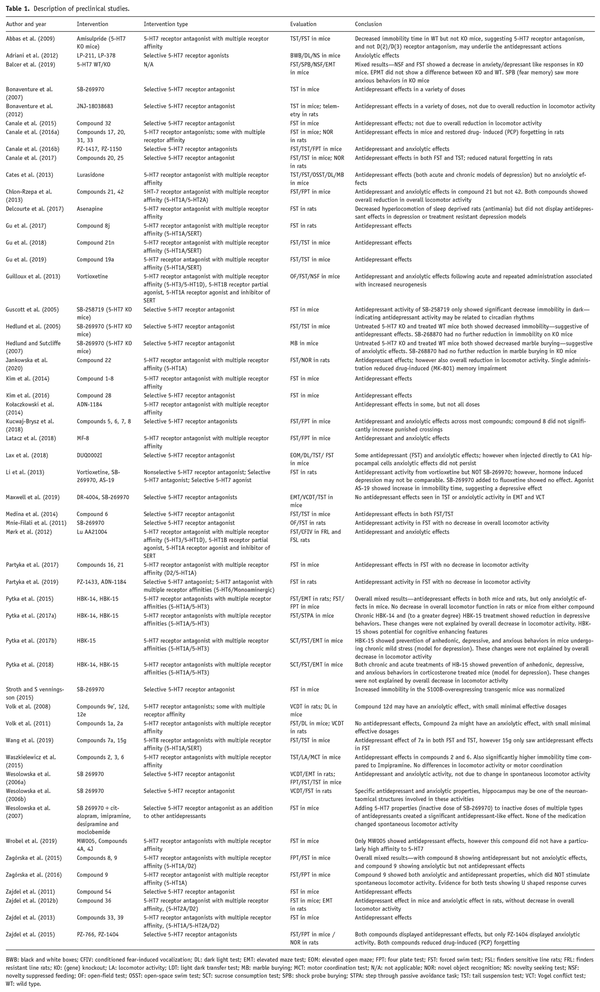
Fifty preclinical animal studies were included in the review. Most of the studies (48/52) examined 5-HT7 drug or knockout (KO) effects on preclinical models of depression, with a further 23 examining models of anxiety. Study details and summarized findings are listed in Table 1.
A comprehensive listing of all relevant study results, with corresponding mood-related behavioral paradigms and mood models, comparators, individual results and p-values can be found in Supplemental Information—Preclinical Behavioral Results.
While all included studies used compounds or genetic manipulations that reasonably targeted 5-HT7 receptors, some compounds had higher selectivity than others.
Thirty used nonspecific 5-HT7 antagonist agents, which also have moderate to high affinity to other receptors, including other 5-HT and dopaminergic receptors, while 25 studies used compounds selective to 5-HT7 receptors.
For the purpose of this review, selective compounds are considered those which had preferential binding defined as a 5-HT7 inhibition constant (Ki) at least fourfold less than all other receptors of interest. A complete list of compounds considered in this review, with their chemical (IUPAC) name and receptor binding profiles, can be found in Supplemental Information—Receptor Activity. Receptor bindings are described by inhibition constants (Ki in nanomoles) unless otherwise noted (e.g. IC50 or pKi values). The Psychoactive Drug Screening Program Ki database was used to reference all compounds (; ). If compounds were not listed in the database, this is noted in the Supplemental Information along with source data for receptor bindings.
Antidepressant effects
The forced swim test (FST) and the tail suspension test (TST) are often used to evaluate antidepressant agents in preclinical rodent studies, and these tests measure immobility time as a correlate of negative mood, or hopelessness (; ). Even though the FST and TST are not recognized as effective models of depression, they are still used for screening potential antidepressants in preclinical studies (; ). Within our systematic review, 43 studies used the FST, and 15 studies used the TST.
Twenty-five studies that used compounds nonspecific to the 5-HT7 receptor identified potential antidepressant effects depression such as immobility time, in both the FST or TST (; ; ; , , ; ; ; ; ; ; ; ; , ; , , , ; ; ; ; , ; ).
Furthermore, 19 studies using agents with selective 5-HT7 antagonist properties found significant improvements in potential markers of depression; for example, SB-269970 was found to decrease animals’ immobility time both in water (FST) and land (TST) (, ; , , , ; ; ; ; ; ; ; ; ; , , ; , ). An additional study found decreased immobility time in 5-HT7 gene KO mice, compared to wild type (WT) mice, in the FST (). Lastly, one study using a highly selective 5-HT7 agonist, AS-19, reported increased immobility (i.e. more depressed-like behavior) in the FST ().
Of note, the antipsychotic medication amisulpride and antidepressants with 5-HT7 antagonist properties, such as imipramine and desipramine, were found to have potential antidepressant effects in these tests (; ). Abbas and colleagues found that amisulpride, an antipsychotic that acts primarily as a dopaminergic receptor antagonist but also has potent 5-HT7 receptor antagonism (), showed potential antidepressant effects in both the TST and FST in WT mice. These effects were not seen in 5-HT7 KO mice, suggesting that activity at the 5-HT7 receptor may be specifically associated with potential antidepressant properties. Another study using citalopram, imipramine, desipramine, and moclobemide found that while low doses of these agents had no significant effect, antidepressant properties were evident once combined with SB-269970, a selective 5-HT7 receptor antagonist, suggesting that it was enhanced 5HT7 antagonism that produced potential antidepressant-like effects ().
Two studies found mixed results, each with a 5-HT7 antagonist compound showing potential antidepressant effects in the FST, but not TST (; ). A further three studies showed no significant antidepressant effects of their tested compounds in either the TST or FST (; ; ).
Anxiolytic effects
In addition to modulation of depressive symptoms, there is also evidence from preclinical animal studies that 5-HT7 receptor modulation may mediate changes in measures of anxiety. A total of 23 studies measured anxiety behaviors using a variety of paradigms.
Similarly to preclinical depression studies, 11 studies found potential anxiolytic effects in animal models associated with the use of 5-HT7 antagonist agents, which also have strong to moderate affinities at other serotonergic and dopaminergic receptors (; ; ; , , ; , ; , ; ). Eight studies found potential anxiolytic effects when investigating selective 5-HT7 receptor antagonists (; ; ; ; ; , ; ).
These studies used animal models of anxiety where anxiolytic effects were measured by a decrease in rodents’ motion or increase their searching behaviors in anxious environments (e.g. maintaining motionless to escape from electric shock in Four-Plate Test, increasing attempts to get food under electrical shocks in Vogel Conflict Drinking Test, or burying more marbles in their bedding in Marble Burying Test). The selective 5-HT7 antagonist, SB-269970, increased antianxiety behaviors in the Vogel Conflict Drinking Test and in the elevated plus-maze test (). A similar finding was identified in the shock threshold test and open-field test (). In addition, mice showed less anxious behavior in the marble burying test, which may measure obsessive-compulsive behaviors ().
Balcer and colleagues found mixed results, with 5-HT7 gene KO mice being significantly less anxious during a novelty suppressed feeding task, but not during an elevated maze plus task (). Two studies found no significant anxiolytic effects associated with any compounds with 5-HT7 antagonistic properties (; ). One study found that the 5-HT7 agonists, LP-211 and LP-378, improved potential anxiety-related behaviors in the light/dark test and increased exploration of black and white boxes ().
Other effects
In addition to antidepressant and anxiolytic effects, 5-HT7 antagonists have been investigated in animal models of mania, sleep, and cognition. One study found that hyperlocomotion in rodents induced by sleep deprivation, a behavioral model of mania, was reduced after treatment with asenapine (). Rodents treated with JNJ-18038683, a selective 5-HT7 antagonist, and 5-HT7 gene KO mice both displayed increased latency to REM sleep and decreased REM duration (; ). Additionally, the selective 5-HT7 antagonist SB-269970 increased REM latency, decreased REM sleep duration, and reversed increases in sleep fragmentation induced by citalopram ().
Several studies have also reported that compounds with 5-HT7 antagonist properties were associated potential pro-cognitive effects, such as reversing natural forgetting impairment in rats (; ) and reversing drug-induced memory impairments (; ; ).
Human study results
Study characteristics
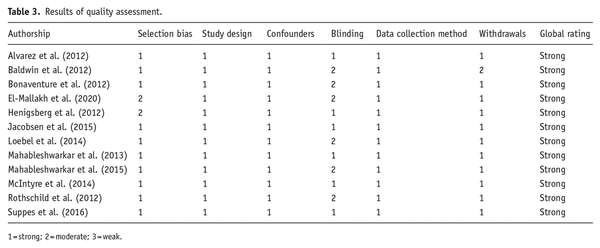
Twelve RCTs were included in the systematic review. Most of the studies (11) were between 6 and 8 weeks long (mean study duration 7.4 weeks); however, one study lasted for 32 weeks. Eight studies explored effects in participants with MDD, two in participants with bipolar disorder, and two in participants with generalized anxiety disorder (GAD). Only one study used a highly selective 5-HT7 antagonist (JNJ-18038683), while the others used medications that had higher affinity to other receptors, in addition to 5-HT7 receptor activity. All clinical trial details and findings are summarized in Table 2.
Major depressive disorder
Six studies investigated the effect of vortioxetine (LU AA21004), a medication with 5-HT1A, 5-HT1B, 5-HT3, and 5-HT7 receptors and 5-HT transporter affinities, on MDD symptoms. All studies administering vortioxetine at 20 mg found significant improvements in mood as measured by Montgomery–Åsberg Depression Rating Scale (MADRS) () between baseline and week 8 (; ). Three studies found significant differences in mood at a 10-mg dose (; ; ); however, two only found a trend toward significance at this dosage (; ). Lower doses of vortioxetine (5 mg or less) found no significant changes associated with treatment (; ; ). It is important to emphasize that vortioxetine is not a 5-HT7-specific agent, and vortioxetine also blocks 5-HT1D and 5HT-3 receptors and stimulates 5-HT1A and 5HT1B receptors.
Only one study examined change in depressive symptoms after 7 weeks of 20-mg JNJ-18038683, a highly selective 5-HT7 receptor antagonist, compared to placebo and escitalopram 20 mg (). Although JNJ-18038683 did not significantly decrease depression symptoms compared to placebo, escitalopram (20 mg) also did not significantly change mood scores in this study.
Anxiety disorders
Two studies examined the effects of medications with 5-HT7 antagonistic properties in participants with GAD. Rothschild and colleagues measured anxiety symptoms using the Hamilton Anxiety Scale (HAM-A) () before and after 8 weeks of treatment with 5 mg vortioxetine, but found no differences compared to placebo (). Baldwin and colleagues investigated the effect of either 5 or 10 mg of vortioxetine on time to relapse in participants with GAD. At the end of a 20-week open-label treatment period, participants who responded were then randomized to 24–56 weeks of a double-blind treatment of vortioxetine (n = 229) or placebo (n = 230). The study found a statistically significant effect of vortioxetine relative to the placebo in time to relapse ().
Bipolar disorders
Lurasidone and asenapine are both atypical antipsychotics often used to treat schizophrenia and bipolar disorders, and they both have moderate affinity to 5-HT7 receptors. Lurasidone is a dopaminergic D2 and D3 receptor, 5-HT2A, 5-HT7, and α2C-adrenergic receptor antagonist, and a partial 5-HT1A agonist (). In one study investigating bipolar depression, participants treated with lurasidone (at both 20–60 mg and 80–120 mg doses) experienced a significant improvement in MADRS scores after 6 weeks compared to placebo (). One study, with a sample size of nine participants, found significant improvements in both depressive and anxiety symptoms in those with bipolar depression after 8-week treatment with asenapine (). In participants with MDD with mixed features, who experienced least 2–3 manic or hypomanic episodes, depressive symptoms measured by MADRS and manic symptoms measured by the Young Mania Rating Scale (YMRS) improved after 6 weeks of treatment of 20–60 mg lurasidone (; ).
Other effects
As with rodent studies, human clinical trials have observed effects of 5-HT7 receptor modulation outside of mood symptoms. McIntyre and colleagues found that both 10- and 20-mg vortioxetine significantly improved participants’ composite cognition scores compared to placebo using a predefined efficacy analysis. Additionally, they also found significant improvements in most secondary objectives and subjective patient-reported cognitive measures (). Another study measuring cognitive impairment using the Cognitive and Physical Functioning Questionnaire (CPFQ) found while both 10- and 15-mg vortioxetine numerically improved scores, these improvements were not statistically significant versus placebo (; ).
Only one study in the systematic review examined changes in sleep in human participants. JNJ-18038683, a specific 5HT-7 antagonist, was found to prolong REM latency and reduced REM sleep duration in healthy participants, and enhanced REM sleep suppression induced by citalopram ().
Quality assessment
Overall, the clinical trials included in this systematic review were assessed to be between strong and moderate quality using the Quality Assessment Tool for Quantitative Studies, and the scores are listed in Table 3. All studies were considered to have a strong study design and strong blinding strategies. Some studies did not describe participant withdrawals and drop out, which is vital to a well described trial. Full quality assessment results for each study can be found in Table 3.
Discussion
Overall, the animal studies and human clinical trials included in this review provide preliminary evidence that 5-HT7 antagonists may potentially be useful for the treatment of mood and anxiety disorders, with 49 animal studies and 11 human studies reporting statistically significant changes in mood or behavior using 5-HT7-related pharmacological interventions. However, this review highlights that the evidence base is constrained by a lack of studies using specific 5-HT7 receptor agents, which do not influence dopaminergic or other 5-HT receptor subtypes. While many pharmacological interventions with activity at the 5-HT7 receptor have been found to induce significant changes in mood symptoms in clinical trials, these results are difficult to interpret due to the lack of 5-HT7 receptor specificity.
Preclinical studies report an improvement in depression-related (43 studies) and anxiety-related (21 studies) behaviors, further supporting that the notion that 5-HT7 receptor modulation may impact mood- and anxiety-related symptoms. For example, the selective 5-HT7 antagonist SB-269970 was associated with specific anxiolytic effects in the Vogel conflict drinking, elevated plus-maze, shock threshold, and open-field tests (), without influencing gross locomotion. Selective 5-HT7 antagonists have also been shown to induce possible antidepressant-like behaviors in preclinical animal models (, ; , , , ; ; ; ; ; ; ; ; ; , , ; , ) although the interpretation of these results is complicated by concerns over the validity of these tests measuring depressive symptoms and lack of validation of receptor binding profiles for many of the compounds used. The use of compounds with multiple receptor subtype affinity (such as MF-8, HBK-14, and HBK-15) was also associated with potential antidepressant-like and anxiolytic-like effects, although the lack of specificity of these compounds for the 5-HT7 receptor makes interpretation of these results difficult. For example, Wrobel and colleagues only found that MW005 may have antidepressant-related effects in animal models. However, this compound had lower 5-HT7 receptor selectivity compared to the newer compounds investigated in this study, which were not associated with antidepressant-related effects ().
In addition to 5-HT7 antagonism, 5-HT7 agonists were found to impact emotion in preclinical animal models. One study found that 5-HT7 agonist AS-19 exacerbated depressive symptoms in rodent models of progesterone withdrawal, which are believed to model hormonally induced mood disorders in women (). Conversely, Adriani and colleagues found that LP-211 and LP-378, two 5-HT7 agonists, were associated with increased disinhibition across a variety of tasks, including B/W boxes, dark/light, and novelty seeking tasks. The researchers observed pro-locomotor and pro-exploratory behaviors, with mice spending more time in the aversive light side and white boxes (). Further studies using 5-HT7 agonists are needed to clarify the mechanisms underlying these observations.
In human clinical trials, several studies found an improvement of symptoms in people with MDD after treatment with vortioxetine. All studies administering vortioxetine at 20 mg found significant improvements in mood (; ). Those using lower dosages had more mixed results, suggesting it may be only doses of vortioxetine greater than 10 mg are effective in symptom improvement. Lastly, two studies also found improvements in cognition with use of at least 10-mg vortioxetine in these participants with MDD (; ). As only these two studies measured changes in cognition, further research in needed.
In people with GAD, 5-mg vortioxetine was associated with no differences in anxiety symptoms (); however, vortioxetine at 5 and 10 mg did show a statistically significant effect of vortioxetine relative to the placebo in time to relapse in a longer 56-week study (). Taken together, these studies highlight the importance of dosage and treatment duration when considering efficacy in the treatment of affective symptoms.
In the only human trial using a selective 5-HT7 antagonist, Bonaventure and colleagues found that while there was no significant decrease in depressive symptoms compared to placebo, JNJ-18038683 prolonged REM latency and reduced REM sleep duration in healthy controls. It is important to note that in this study, neither escitalopram (20 mg), a commonly used antidepressant, nor JNJ-18038683 significantly decreased depressive symptomology as measured by the MADRS compared to placebo ().
The quality of the clinical trials included in this review was considered to be strong or moderate overall. However, one study had small sample size (n = 9) due to it being a subset of a larger study that was terminated early by the sponsor for non-safety-related issues (). Quality assessments were not carried out on preclinical rodent studies as the availability of standardized quality assessment for use in animal studies is lacking ().
Limitations and future directions
Despite the evidence for the potential role of the 5-HT7 receptor subtype in mood and anxiety disorders, this systematic review has several limitations.
While all the studies included in this review used pharmacological treatments with moderate to strong affinity to 5-HT7 receptor subtypes, many also have affinity to other receptor types including the SERT and 5-HT1A receptors, which are often targets for antidepressants and anxiolytics (; ). Only 21 of the original 52 preclinical studies measuring depressive symptomology used selective 5-HT7 pharmacological interventions. While the compounds described in this systematic review were defined as selective based on a preferential binding to 5-HT7 receptors compared to others, some of these compounds did have moderate affinities to other receptors with inhibition constants less than 100 nm. For example, while PZ-766 is a potent 5-HT7 antagonist, with a Ki value of 0.3 nm, there is also indication of activity at the D2 receptor (Ki = 52 nm) (). This may imply that changes in mood symptoms result in part from the modulation of SERT, 5-HT1A, or 5-HT2 receptor function. JNJ-18038683 was the only highly selective 5-HT7 antagonist used in human trials, highlighting that further research is required using more selective 5-HT7 compounds to better characterize the underlying pharmacology of the observed changes in mood and anxiety disorders.
Although antidepressant- and anxiolytic-related effects were widely examined, only one preclinical study and one clinical trial explored effects on mania-related measures using agents with wider therapeutic targets (; ). Sleep and cognition were examined as secondary outcome measures in nine animal studies and three human studies (; , ; , ; ; ; ). We would suggest that further research using more selective 5-HT7 compounds is necessary to fully understand the effects of 5-HT7 receptor modulation on mania, sleep, and cognition function ().
Finally, it is important to note that no unpublished data were identified to be included in this systematic review, which may indicate that negative findings could have been underreported.
Conclusions
This systematic review examined evidence for the 5-HT7 receptor as a therapeutic target for mood and anxiety disorders with 49 preclinical and 11 human studies demonstrating antidepressant- or anxiolytic-related effects using compounds that had at least moderate 5-HT7 receptor affinity. The 5-HT7 receptor may also be a potential target for the treatment of sleep disturbances or cognitive impairment associated with mood disorders, but further research is warranted. We would suggest that further studies investigate pharmacological agents with more distinct selectivity to the 5-HT7 receptor subtype, to ensure that effects are not related to their affinity to other receptors (such as 5-HT1A, D2, or SERT). Additionally, more research is needed to observe changes in cognition and sleep.
We would like to thank Dr. Anthony Vernon for his help and advice on interpretation of preclinical compound binding.
Author’s Note Natalie Gottlieb and Allan H Young are also affiliated to South London and Maudsley NHS Foundation Trust, Bethlem Royal Hospital, Monks Orchard Road, Beckenham, Kent, UK.
Declaration of conflicting interests The author(s) declared the following potential conflicts of interest with respect to the research, authorship, and/or publication of this article: AY declares honoraria for speaking from Astra Zeneca, Lundbeck, Eli Lilly, Sunovion; honoraria for consulting from Allergan, Livanova and Lundbeck, Sunovion, Janssen; and research grant support from Janssen. He is an editor for Journal of Psychopharmacology and Deputy Editor, BJPsych Open. PS reports personal fees and nonfinancial support from Frontiers in Psychiatry, personal fees from Allergan and a grant from H Lundbeck outside the submitted work. All authors report support from a grant from the Medical Research Council UK and nonfinancial support from Janssen Research and Development LLC during the conduct of the study. No other declarations of interest are reported.
Funding The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This study represents independent research partly funded a Medical Research Council UK grant (MR/R005885/1). This work was also supported by the Ministry of Education, Taiwan as a PhD scholarship to TYL. This paper also includes independent research part funded by the National Institute for Health Research (NIHR) Maudsley Biomedical Research Centre at South London and Maudsley NHS Foundation Trust and King’s College London. The funders were not involved in any aspects of this work’s planning, execution, article preparation or in the decision to submit the article for publication. The views expressed are those of the authors and not necessarily those of the funding Trusts, the NHS, the NIHR, or the Department of Health and Social Care.
Natalie Gottlieb
https://orcid.org/0000-0002-9220-1234
Supplemental material Supplemental material for this article is available online.
References
- Abbas AI, Hedlund PB, Huang X-P, et al (2009) Amisulpride is a potent 5-HT7 antagonist: Relevance for antidepressant actions in vivo. Psychopharmacology (Berl.) 205: 119–128. DOI:
- Adell A (2010) Lu-AA21004, a multimodal serotonergic agent, for the potential treatment of depression and anxiety. IDrugs 13: 900–910.
- Adriani W, Travaglini D, Lacivita E, et al (2012) Modulatory effects of two novel agonists for serotonin receptor 7 on emotion, motivation and circadian rhythm profiles in mice. Neuropharmacology 62: 833–842. DOI:
- Alvarez E, Perez V, Dragheim M, et al (2012) A double-blind, randomized, placebo-controlled, active reference study of Lu AA21004 in patients with major depressive disorder. Int J Neuropsychopharmacol 15: 589–600. DOI:
- Armijo-Olivo S, Stiles CR, Hagen NA, et al (2012) Assessment of study quality for systematic reviews: A comparison of the Cochrane Collaboration Risk of Bias Tool and the Effective Public Health Practice Project Quality Assessment Tool: Methodological research. J Eval Clin Pract 18: 12–18. DOI:
- Artigas F (2013) Serotonin receptors involved in antidepressant effects. Pharmacol Ther 137: 119–131. DOI:
- Azima H, Vispo RH (1958) Imipramine; a potent new anti-depressant compound. Am J Psychiatry 115: 245–246. DOI:
- Balcer OM, Seager MA, Gleason SD, et al (2019) Evaluation of 5-HT7 receptor antagonism for the treatment of anxiety, depression, and schizophrenia through the use of receptor-deficient mice. Behav Brain Res 360: 270–278. DOI:
- Baldwin DS, Loft H, Florea I (2012) Lu AA21004, a multimodal psychotropic agent, in the prevention of relapse in adult patients with generalized anxiety disorder. Int Clin Psychopharmacol 27: 197–207. DOI:
- Bard JA, Zgombick J, Adham N, et al (1993) Cloning of a novel human serotonin receptor (5-HT7) positively linked to adenylate cyclase. J Biol Chem 268: 23422–23426.
- Bawa R, Scarff JR (2015) Lurasidone: A new treatment option for bipolar depression—A review. Innov Clin Neurosci 12: 21–23.
- Bonaventure P, Dugovic C, Kramer M, et al (2012) Translational evaluation of JNJ-18038683, a 5-hydroxytryptamine type 7 receptor antagonist, on rapid eye movement sleep and in major depressive disorder. J Pharmacol Exp Ther 342: 429–440. DOI:
- Bonaventure P, Kelly L, Aluisio L, et al (2007) Selective blockade of 5-hydroxytryptamine (5-HT)7 receptors enhances 5-HT transmission, antidepressant-like behavior, and rapid eye movement sleep suppression induced by citalopram in rodents. J Pharmacol Exp Ther 321: 690–698. DOI:
- Bourin M, Fiocco AJ, Clenet F (2001) How valuable are animal models in defining antidepressant activity? Hum Psychopharmacol 16: 9–21. DOI:
- Brenchat A, Romero L, García M, et al (2009) 5-HT7 receptor activation inhibits mechanical hypersensitivity secondary to capsaicin sensitization in mice. Pain 141: 239–247. DOI:
- Brodie BB, Bickel MH, Sulser F (1961) Desmethylimipramine, a new type of antidepressant drug. Med Exp Int J Exp Med 5: 454–458. DOI:
- Can A, Dao DT, Terrillion CE, et al (2011) The tail suspension test. J Vis Exp 58: e3769. DOI:
- Canale V, Kurczab R, Partyka A, et al (2016a) Towards new 5-HT7 antagonists among arylsulfonamide derivatives of (aryloxy)ethyl-alkyl amines: Multiobjective based design, synthesis, and antidepressant and anxiolytic properties. Eur J Med Chem 108: 334–346. DOI:
- Canale V, Kurczab R, Partyka A, et al (2015) Towards novel 5-HT7 versus 5-HT1A receptor ligands among LCAPs with cyclic amino acid amide fragments: Design, synthesis, and antidepressant properties. Part II. Eur J Med Chem 92: 202–211. DOI:
- Canale V, Kurczab R, Partyka A, et al (2016b) N-Alkylated arylsulfonamides of (aryloxy)ethyl piperidines: 5-HT(7) receptor selectivity versus multireceptor profile. Bioorg Med Chem 24: 130–139. DOI:
- Canale V, Partyka A, Kurczab R, et al (2017) Novel 5-HT7R antagonists, arylsulfonamide derivatives of (aryloxy)propyl piperidines: Add-on effect to the antidepressant activity of SSRI and DRI, and pro-cognitive profile. Bioorg Med Chem 25: 2789–2799. DOI:
- Canese R, Zoratto F, Altabella L, et al (2015) Persistent modification of forebrain networks and metabolism in rats following adolescent exposure to a 5-HT7 receptor agonist. Psychopharmacology (Berl.) 232: 75–89. DOI:
- Cates LN, Roberts AJ, Huitron-Resendiz S, et al (2013) Effects of lurasidone in behavioral models of depression. Role of the 5-HT7 receptor subtype. Neuropharmacology 70: 211–217. DOI:
- Chlon-Rzepa G, Zmudzki P, Satala G, et al (2013) New 8-aminoalkyl derivatives of purine-2,6-dione with arylalkyl, allyl or propynyl substituents in position 7, their 5-HT1A, 5-HT2A, and 5-HT7 receptor affinity and pharmacological evaluation. Pharmacol Rep 65: 15–29. DOI:
- Delcourte S, Abrial E, Etievant A, et al (2017) Asenapine modulates mood-related behaviors and 5-HT1A/7 receptors-mediated neurotransmission. CNS Neurosci Ther 23: 518–525. DOI:
- El-Mallakh RS, Nuss S, Gao D, et al (2020) Asenapine in the treatment of bipolar depression. Psychopharmacol Bull 50: 8–18.
- Fakhoury M (2016) Revisiting the serotonin hypothesis: Implications for major depressive disorders. Mol Neurobiol 53: 2778–2786. DOI:
- Fava M, Iosifescu DV, Pedrelli P, et al (2009) Reliability and validity of the Massachusetts General Hospital cognitive and physical functioning questionnaire. Psychother Psychosom 78: 91–97. DOI:
- Forbes IT, Douglas S, Gribble AD, et al (2002) SB-656104-A: A novel 5-HT(7) receptor antagonist with improved in vivo properties. Bioorg Med Chem Lett 12: 3341–3344. DOI:
- Frånberg O, Wiker C, Marcus MM, et al (2008) Asenapine, a novel psychopharmacologic agent: Preclinical evidence for clinical effects in schizophrenia. Psychopharmacology (Berl.) 196: 417–429.
- Gasbarri A, Pompili A (2014) Serotonergic 5-HT7 receptors and cognition. Rev Neurosci 25: 311–23. DOI:
- Godínez-Chaparro B, Barragán-Iglesias P, Castañeda-Corral G, et al (2011) Role of peripheral 5-HT4, 5-HT6, and 5-HT7 receptors in development and maintenance of secondary mechanical allodynia and hyperalgesia. Pain 152: 687. DOI:
- Gu Z-S, Wang W-T, Qian H, et al (2019) Synthesis and antidepressant effect of novel aralkyl piperazine and piperidine derivatives targeting SSRI/5-HT1A/5-HT7. Bioorg Med Chem Lett 29: 126703. DOI:
- Gu Z-S, Xiao Y, Zhang Q-W, et al (2017) Synthesis and antidepressant activity of a series of arylalkanol and aralkyl piperazine derivatives targeting SSRI/5-HT1A/5-HT7. Bioorg Med Chem Lett 27: 5420–5423. DOI:
- Gu Z-S, Zhou A-N, Xiao Y, et al (2018) Synthesis and antidepressant-like activity of novel aralkyl piperazine derivatives targeting SSRI/5-HT1A/5-HT7. Eur J Med Chem 144: 701–715. DOI:
- Guilloux J-P, Mendez-David I, Pehrson A, et al (2013) Antidepressant and anxiolytic potential of the multimodal antidepressant vortioxetine (Lu AA21004) assessed by behavioural and neurogenesis outcomes in mice. Neuropharmacology 73: 147–59. DOI:
- Guscott M, Bristow LJ, Hadingham K, et al (2005) Genetic knockout and pharmacological blockade studies of the 5-HT7 receptor suggest therapeutic potential in depression. Neuropharmacology 48: 492–502.
- Hagan JJ, Price GW, Jeffrey P, et al (2000) Characterization of SB-269970-A, a selective 5-HT7 receptor antagonist. Br J Pharmacol 130: 539–548. DOI:
- Hamilton M (1960) A rating scale for depression. J Neurol Neurosurg Psychiatry 23: 56–62.
- Hamilton M (1959) The assessment of anxiety states by rating. Br J Med Psychol 32: 50–55. DOI:
- Harvey PD (2015) The clinical utility of lurasidone in schizophrenia: Patient considerations. Neuropsychiatr Dis Treat 11: 1103–1109. DOI:
- Hedlund PB, Huitron-Resendiz S, Henriksen SJ, et al (2005) 5-HT7 receptor inhibition and inactivation induce antidepressantlike behavior and sleep pattern. Biol Psychiatry 58: 831–837.
- Hedlund PB, Sutcliffe JG (2007) The 5-HT7 receptor influences stereotypic behavior in a model of obsessive-compulsive disorder. Neurosci Lett 414, 247–251.
- Helmuth L (2000) NETWATCH. Science 287, 543.
- Henigsberg N, Mahableshwarkar AR, Jacobsen P, et al (2012) A randomized, double-blind, placebo-controlled 8-week trial of the efficacy and tolerability of multiple doses of Lu AA21004 in adults with major depressive disorder. J Clin Psychiatry 73: 953–959. DOI:
- Horisawa T, Nishikawa H, Toma S, et al (2013) The role of 5-HT7 receptor antagonism in the amelioration of MK-801-induced learning and memory deficits by the novel atypical antipsychotic drug lurasidone. Behav Brain Res 244: 66–69. DOI:
- Jacobsen PL, Mahableshwarkar AR, Serenko M, et al (2015) A randomized, double-blind, placebo-controlled study of the efficacy and safety of vortioxetine 10 mg and 20 mg in adults with major depressive disorder. J Clin Psychiatry 76: 575–582. DOI:
- Jankowska A, Satała G, Kołaczkowski M, et al (2020) Novel anilide and benzylamide derivatives of arylpiperazinylalkanoic acids as 5-HT1A/5-HT7 receptor antagonists and phosphodiesterase 4/7 inhibitors with procognitive and antidepressant activity. Eur J Med Chem 201: 112437. DOI:
- Kikuchi C, Nagaso H, Hiranuma T, et al (1999) Tetrahydrobenzindoles: Selective antagonists of the 5-HT7 receptor. J Med Chem 42: 533–535.
- Kim Y, Tae J, Lee K, et al (2014) Novel N-biphenyl-2-ylmethyl 2-methoxyphenylpiperazinylalkanamides as 5-HT7R antagonists for the treatment of depression. Bioorg Med Chem 22: 4587–4596. DOI:
- Kim Y, Yeom M, Tae J, et al (2016) Novel N-acyl-carbazole derivatives as 5-HT7R antagonists. Eur J Med Chem 110: 302–310. DOI:
- Kołaczkowski M, Mierzejewski P, Bieńkowski P, et al (2014) ADN-1184 a monoaminergic ligand with 5-HT(6/7) receptor antagonist activity: Pharmacological profile and potential therapeutic utility. Br J Pharmacol 171: 973–984. DOI:
- Kucwaj-Brysz K, Kurczab R, Jastrzębska-Więsek M, et al (2018) Computer-aided insights into receptor-ligand interaction for novel 5-arylhydantoin derivatives as serotonin 5-HT7 receptor agents with antidepressant activity. Eur J Med Chem 147: 102–114. DOI:
- Latacz G, Lubelska A, Jastrzebska-Wiesek M, Partyka A, et al (2018) MF-8, a novel promising arylpiperazine-hydantoin based 5-HT7 receptor antagonist: In vitro drug-likeness studies and in vivo pharmacological evaluation. Bioorg Med Chem Lett 28: 878–883. DOI:
- Lax NC, Parker S-AJ, Hilton EJ, et al (2018) Cyanobacterial extract with serotonin receptor subtype 7 (5-HT7 R) affinity modulates depression and anxiety-like behavior in mice. Synap 72: e22059. DOI:
- Leopoldo M, Berardi F, Colabufo NA, et al (2004) Structure−affinity relationship study on N-(1,2,3,4-tetrahydronaphthalen-1-yl)-4-aryl-1-piperazinealkylamides, a new class of 5-hydroxytryptamine7 receptor agents. J Med Chem 47: 6616–6624. DOI:
- Leopoldo M, Lacivita E, De Giorgio P, et al (2008) Structural modifications of N-(1,2,3,4-tetrahydronaphthalen-1-yl)-4-aryl-1-piperazinehexanamides: influence on lipophilicity and 5-HT7 receptor activity. Part III. J Med Chem 51: 5813–5822. DOI:
- Li Y, Raaby KF, Sánchez C, et al (2013) Serotonergic receptor mechanisms underlying antidepressant-like action in the progesterone withdrawal model of hormonally induced depression in rats. Behav Brain Res 256: 520–528. DOI:
- Loebel A, Cucchiaro J, Silva R, et al (2014) Lurasidone monotherapy in the treatment of bipolar I depression: A randomized, double-blind, placebo-controlled study. Am J Psychiatry 171: 160–168. DOI:
- Lovenberg TW, Baron BM, De Lecea L, et al (1993) A novel adenylyl cyclase-activating serotonin receptor (5-HT7) implicated in the regulation of mammalian circadian rhythms. Neuron 11: 449–458. DOI:
- Mahableshwarkar AR, Jacobsen PL, Chen Y (2013) A randomized, double-blind trial of 2.5 mg and 5 mg vortioxetine (Lu AA21004) versus placebo for 8 weeks in adults with major depressive disorder. Curr Med Res Opin 29: 217–226. DOI:
- Mahableshwarkar AR, Jacobsen PL, Serenko M, et al (2015) A randomized, double-blind, placebo-controlled study of the efficacy and safety of 2 doses of vortioxetine in adults with major depressive disorder. J Clin Psychiatry 76: 583–591. DOI:
- Martin-Cora FJ, Pazos A (2004) Autoradiographic distribution of 5-HT7 receptors in the human brain using [3H]mesulergine: Comparison to other mammalian species. Br J Pharmacol 141: 92–104. DOI:
- Maxwell J, Gleason SD, Falcone J, et al (2019) Effects of 5-HT7 receptor antagonists on behaviors of mice that detect drugs used in the treatment of anxiety, depression, or schizophrenia. Behav Brain Res 359: 467–473. DOI:
- McIntyre RS, Lophaven S, Olsen CK (2014) A randomized, double-blind, placebo-controlled study of vortioxetine on cognitive function in depressed adults. Int J Neuropsychopharmacol 17: 1557–1567. DOI:
- McIntyre RS, Lophaven S, Olsen CK (2013) Randomized, double-blind, placebo-controlled study of the efficacy of vortioxetine on cognitive dysfunction in adult patients with major depressive disorder (MDD). Neuropsychopharmacology 38: S380–S381. DOI:
- Medina RA, Vazquez-Villa H, Gomez-Tamayo JC, et al (2014) The extracellular entrance provides selectivity to serotonin 5-HT7 receptor antagonists with antidepressant-like behavior in vivo. J Med Chem 57: 6879–84. DOI:
- Mnie-Filali O, Faure C, Lambas-Senas L, El Mansari M, et al (2011) Pharmacological blockade of 5-HT7 receptors as a putative fast acting antidepressant strategy. Neuropsychopharmacology 36: 1275–88. DOI:
- Montgomery SA, Asberg M (1979) A new depression scale designed to be sensitive to change. Br J Psychiatry J Ment Sci 134: 382–389. DOI:
- Mørk A, Pehrson A, Brennum LT, et al (2012) Pharmacological effects of Lu AA21004: A novel multimodal compound for the treatment of major depressive disorder. J Pharmacol Exp Ther 340: 666–675. DOI:
- Nautiyal KM, Hen R (2017) Serotonin receptors in depression: From A to B. F1000Research 6: 123. DOI:
- Ohno Y, Ishibashi T, Tokuda K, et al (1997) Behavioral characteristics of SM-13496, a novel atypical antipsychotic agent. Acta Neurobiol Exp (Warsz.) 57: 25.
- Ouzzani M, Hammady H, Fedorowicz Z, et al (2016) Rayyan—A web and mobile app for systematic reviews. Syst Rev 5: 210. DOI:
- Page MJ, McKenzie JE, Bossuyt PM, et al (2021) The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. BMJ 372: n71. DOI:
- Partyka A, Jastrzębska-Więsek M, Antkiewicz-Michaluk L, et al (2019) Novel antagonists of 5-HT6 and/or 5-HT7 receptors affect the brain monoamines metabolism and enhance the anti-immobility activity of different antidepressants in rats. Behav Brain Res 359: 9–16. DOI:
- Partyka A, Kurczab R, Canale V, et al (2017) The impact of the halogen bonding on D2 and 5-HT1A/5-HT7 receptor activity of azinesulfonamides of 4-[(2-ethyl)piperidinyl-1-yl]phenylpiperazines with antipsychotic and antidepressant properties. Bioorg Med Chem 25: 3638–3648. DOI:
- Puech A, Fleurot O, Rein W (1998). Amisulpride, an atypical antipsychotic, in the treatment of acute episodes of schizophrenia: A dose-ranging study versus haloperidol. Acta Psychiatr Scand 98: 65–72.
- Pytka K, Gawlik K, Pawlica-Gosiewska D, et al (2017a) HBK-14 and HBK-15 with antidepressant-like and/or memory-enhancing properties increase serotonin levels in the hippocampus after chronic treatment in mice. Metab Brain Dis 32: 547–556. DOI:
- Pytka K, Głuch-Lutwin M, Kotańska M, et al (2018) Single administration of HBK-15-a Triple 5-HT1A, 5-HT7, and 5-HT3 receptor antagonist-reverses depressive-like behaviors in mouse model of depression induced by corticosterone. Mol Neurobiol 55: 3931–3945. DOI:
- Pytka K, Gluch-Lutwin M, Kotanska M, et al (2017b) HBK-15 protects mice from stress-induced behavioral disturbances and changes in corticosterone, BDNF, and NGF levels. Behav Brain Res 333: 54–66. DOI:
- Pytka K, Partyka A, Jastrzebska-Wiesek M, et al (2015) Antidepressant- and anxiolytic-like effects of new dual 5-HT1A and 5-HT7 antagonists in animal models. PLoS One 10: e0142499. DOI:
- Roth BL, Lopez E, Patel S, et al (2000) The multiplicity of serotonin receptors: Uselessly diverse molecules or an embarrassment of riches? Neurosci 6: 252–262. DOI:
- Rothschild AJ, Mahableshwarkar AR, Jacobsen P, et al (2012) Vortioxetine (Lu AA21004) 5 mg in generalized anxiety disorder: Results of an 8-week randomized, double-blind, placebo-controlled clinical trial in the United States. Eur Neuropsychopharmacol 22: 858–866. DOI:
- Ruat M, Traiffort E, Leurs R, et al (1993) Molecular cloning, characterization, and localization of a high-affinity serotonin receptor (5-HT7) activating cAMP formation. Proc Natl Acad Sci U S A. 90: 8547–8551. DOI:
- Rush AJ, Trivedi MH, Ibrahim HM, et al (2003) The 16-Item quick inventory of depressive symptomatology (QIDS), clinician rating (QIDS-C), and self-report (QIDS-SR): A psychometric evaluation in patients with chronic major depression. Biol Psychiatry 54: 573–583. DOI:
- Sanin A, Brisander M, Rosqvist S, et al (2004) 5-aryl substituted (S)-2-(dimethylamino)-tetralins novel serotonin 5-HT7 ligands. In: Presented at the Proceedings of the 14th camerino-noordwijkerhout symposium: Ongoing progress in the receptor chemistry, Camerino, Italy, 7–11 September 2003, p. 24.
- Sewell F, Waterson I, Jones D, et al (2021) Preclinical screening for antidepressant activity—shifting focus away from the forced swim test to the use of translational biomarkers. Regul Toxicol Pharmacol 125: 105002. DOI:
- Stroth N, Svenningsson P (2015) S100B interacts with the serotonin 5-HT7 receptor to regulate a depressive-like behavior. Eur Neuropsychopharmacol 25: 2372–2380. DOI:
- Suppes T, Silva R, Cucchiaro J, et al (2016) Lurasidone for the treatment of major depressive disorder with mixed features: A randomized, double-blind, placebo-controlled study. Am J Psychiatry 173: 400–407. DOI:
- Thase ME, Denko T (2008) Pharmacotherapy of mood disorders. Ann Rev Clin Psychol 4: 53–91. DOI:
- Thomas BH, Ciliska D, Dobbins M, et al (2004) A process for systematically reviewing the literature: Providing the research evidence for public health nursing interventions. Worldviews Evid Based Nurs 1: 176–184. DOI:
- Thomas DR, Melotto S, Massagrande M, et al (2003) SB-656104-A, a novel selective 5-HT7 receptor antagonist, modulates REM sleep in rats. Br J Pharmacol 139: 705–714. DOI:
- Thomas DR, Middlemiss DN, Taylor SG, et al (1999) 5-CT stimulation of adenylyl cyclase activity in guinea-pig hippocampus: Evidence for involvement of 5-HT7 and 5-HT1A receptors. Br J Pharmacol 128: 158–164. DOI:
- To ZP, Bonhaus DW, Eglen RM, et al (1995) Characterization and distribution of putative 5-ht7 receptors in guinea-pig brain. Br J Pharmacol 115: 107–116. DOI:
- Volk B, Barkoczy J, Hegedus E, et al (2008) (Phenylpiperazinyl-butyl)oxindoles as selective 5-HT7 receptor antagonists. J Med Chem 51: 2522–2532. DOI:
- Volk B, Gacsalyi I, Pallagi K, et al (2011) Optimization of (arylpiperazinylbutyl)oxindoles exhibiting selective 5-HT7 receptor antagonist activity. J Med Chem 54: 6657–6669. DOI:
- Wang W-T, Qian H, Wu J-W, et al (2019) Synthesis and antidepressant-like activity of novel alkoxy-piperidine derivatives targeting SSRI/5-HT1A/5-HT7. Bioorg Med Chem Lett 29: 126769. DOI:
- Waszkielewicz AM, Pytka K, Rapacz A, et al (2015) Synthesis and evaluation of antidepressant-like activity of some 4-substituted 1-(2-methoxyphenyl)piperazine derivatives. Chem Biol Drug Des 85, 326–335. DOI:
- Wesolowska A, Nikiforuk A, Stachowicz K (2006a) Potential anxiolytic and antidepressant effects of the selective 5-HT7 receptor antagonist SB 269970 after intrahippocampal administration to rats. Eur J Pharmacol 553: 185–190.
- Wesolowska A, Nikiforuk A, Stachowicz K, et al (2006b) Effect of the selective 5-HT7 receptor antagonist SB 269970 in animal models of anxiety and depression. Neuropharmacology 51: 578–86.
- Wesolowska A, Tatarczynska E, Nikiforuk A, et al (2007) Enhancement of the anti-immobility action of antidepressants by a selective 5-HT7 receptor antagonist in the forced swimming test in mice. Eur J Pharmacol 555: 43–47.
- Wróbel MZ, Chodkowski A, Herold F, et al (2019) Synthesis and biological evaluation of new multi-target 3-(1H-indol-3-yl)pyrrolidine-2,5-dione derivatives with potential antidepressant effect. Eur J Med Chem 183: 111736. DOI:
- Yankelevitch-Yahav R, Franko M, Huly A, et al (2015) The forced swim test as a model of depressive-like behavior. J Vis Exp 97: 52587. DOI:
- Young RC, Biggs JT, Ziegler VE, et al (1978) A rating scale for mania: reliability, validity and sensitivity. Br J Psychiatry J Ment Sci 133: 429–435. DOI:
- Zagórska A, Bucki A, Kołaczkowski M, et al (2016) Synthesis and biological evaluation of 2-fluoro and 3-trifluoromethyl-phenyl-piperazinylalkyl derivatives of 1H-imidazo[2,1-f]purine-2,4(3H,8H)-dione as potential antidepressant agents. J Enzyme Inhib Med Chem 31: 10–24. DOI:
- Zagórska A, Kołaczkowski M, Bucki A, et al (2015) Structure-activity relationships and molecular studies of novel arylpiperazinylalkyl purine-2,4-diones and purine-2,4,8-triones with antidepressant and anxiolytic-like activity. Eur J Med Chem 97: 142–154. DOI:
- Zajdel P, Canale V, Partyka A, et al (2015) Arylsulfonamide derivatives of (aryloxy)ethylpiperidines as selective 5-HT7 receptor antagonists and their psychotropic properties. Med Chem Comm 6: 1272–1277. DOI:
- Zajdel P, Kurczab R, Grychowska K, et al (2012a) The multiobjective based design, synthesis and evaluation of the arylsulfonamide/amide derivatives of aryloxyethyl- and arylthioethyl- piperidines and pyrrolidines as a novel class of potent 5-HT7 receptor antagonists. Eur J Med Chem 56: 348–360. DOI:
- Zajdel P, Marciniec K, Maślankiewicz A, et al (2013) Antidepressant and antipsychotic activity of new quinoline- and isoquinoline-sulfonamide analogs of aripiprazole targeting serotonin 5-HT1A/5-HT2A/5-HT7 and dopamine D2/D3 receptors. Eur J Med Chem 60: 42–50. DOI:
- Zajdel P, Marciniec K, Maślankiewicz A, et al (2011) Arene- and quinoline-sulfonamides as novel 5-HT7 receptor ligands. Bioorg Med Chem 19: 6750–6759. DOI:
- Zajdel P, Marciniec K, Maslankiewicz A, et al (2012b) Quinoline- and isoquinoline-sulfonamide derivatives of LCAP as potent CNS multi-receptor-5-HT1A/5-HT2A/5-HT7 and D2/D3/D4-agents: The synthesis and pharmacological evaluation. Bioorg Med Chem 20: 1545–1556. DOI:
- Zeng X, Zhang Y, Kwong JSW, et al (2015) The methodological quality assessment tools for preclinical and clinical studies, systematic review and meta-analysis, and clinical practice guideline: A systematic review. J Evid Based Med 8: 2–10. DOI:
- Zhang M-R, Haradahira T, Maeda J, et al (2002) Synthesis and preliminary PET study of the 5-HT7 receptor antagonist [11C]DR4446. J Label Compd Radiopharm 45: 857–866. DOI: