The latest VisuMax 800 femtosecond laser (Carl Zeiss Meditec) has several advancements over its predecessor, the VisuMax 500 femtosecond laser, including a faster 2-MHz laser frequency, centration aid in the form of CentraLign, cyclotorsional aid in the form of OcuLign, separate laser and microscope arms, and heads-up docking achieved by lowering the laser arm instead of raising the surgical bed. A recent study published by our group showed the new features on the laser improved the overall workflow when performing bilateral SMILE.
In addition to improved workflow, early reports of small incision lenticule extraction (SMILE) with the VisuMax 800 have shown excellent visual and refractive outcomes. Reinstein et al and Saad et al published data sets retrospectively evaluating their early results while noting that the results are similar to their results after SMILE with the VisuMax 500 femtosecond laser. Yoo et al performed a retrospective analysis comparing cohort of patients who underwent SMILE with the VisuMax 800 to a matched cohort of patients who underwent SMILE with the VisuMax 500. Yoo et al found that, similar to what Reinstein et al and Saad et al predicted, the outcomes between groups were similar.
However, the studies did not fully evaluate the areas where there may be a difference between lasers given the upgrades available on the VisuMax 800. In addition, at the time of writing this article there have been no studies evaluating results beyond the standard visual and refractive outcomes. Therefore, in addition to evaluating standard visual and refractive outcomes, higher order aberrations (HOAs), and quality of vision metrics, this study also investigated patient satisfaction and surgical workflow differences following SMILE using the VisuMax 800 in one eye and VisuMax 500 in the contralateral eye of the same patient.
Patients and Methods
Patients enrolled in the study agreed to have one eye treated with the VisuMax 800 femtosecond laser and the contralateral eye treated with the VisuMax 500 femtosecond laser. Computer-generated randomization was used to determine which eye of the patient would receive treatment by each laser. Inclusion criteria were 21 to 40 years of age, myopia or myopic astigmatism with a spherical equivalent (SEQ) between −1.00 and −10.00 diopters (D), and manifest cylinder of 1.00 D or less, not mandating preoperative corneal marking. Exclusion criteria were eyes with corneal scars, keratoconus, pellucid marginal degeneration, pachymetry less than 480 microns, glaucoma, retinal disease, pregnancy, and medications such as immunosuppressants.
The study was approved by the institutional review board of Nethradhama Eye Hospital, Bangalore, India, and adhered to the tenets of the Declaration of Helsinki. Patients were appropriately counseled regarding the nature of the study, and both verbal and written consent was obtained from all participants prior to enrollment.
Preoperative Evaluation
All patients underwent a full preoperatively evaluation that included uncorrected distance visual acuity (UDVA) and corrected distance visual acuity (CDVA) using the Early Treatment of Diabetic Retinopathy Study chart at 4 m, manifest subjective refraction, slit-lamp examination, Pentacam Scheimpflug Tomography (Oculus Optikgeräte GmbH), ATLAS topography (Carl Zeiss Meditec) for x and y coordinates and angle kappa measurement, iTrace (Tracey Technologies) for aberrations, HD Analyzer (Visiometrics) for Objective Scatter Index (OSI), and modulation transfer function (MTF) and dilated fundus examination to rule out optic disc and retinal pathologies.
Operative Visit
Bilateral lenticule extraction was performed by two experienced surgeons (SG, SB) using a standardized technique. Eyes were treated as per the randomization protocol. Because the femtosecond lasers were installed in two separate operating rooms, approximately 6 m apart, the patient was shifted from the first operating room after the treatment of the first eye to the second operating room for completion of the bilateral procedure. In the VM800 group, docking and centration was achieved using the centration aid (CentraLign) based on the x,y pupil coordinates obtained from the ATLAS topographer. In the VM500 group, patient-controlled active fixation on visual axis was achieved. Lenticule extraction was accomplished using the Reinstein lenticule dissector. No interface wash was performed after the lenticule was removed.
Various timings were noted by an independent trained observer (DS), including:
Docking time (minutes): Time taken from preparation of the eye to activation of suction.
Suction time (seconds): Total time for which the eye was under suction (this includes the laser time).
Laser time (seconds): Time taken to complete the active laser process and formation of the refractive lenticule.
Lenticule dissection time (seconds): Time taken from insertion of the dissector into the cornea until extraction of the lenticule.
Total surgical time (minutes): Total surgical time including docking, suction, and lenticule dissection time.
Postoperative Assessment
Postoperative assessments were conducted on the same day and 1 day, 2 weeks, and 3 months after treatment. Postoperative medications included prednisolone (1% PredForte; Allergan) four times per day for 4 weeks in a tapering dosage, moxifloxacin (Milflox, 0.3%; Sun Pharmaceuticals Industries Ltd) eye drops four times a day for 3 days, and lubricant eye drops (Trehalube 0.5%; Entod Pharmaceuticals Ltd) four times a day for 45 days.
Immediately after treatment was completed, the surgeon was asked to grade the ease of lenticule dissection, with 1 being most difficult and 5 being effortless with no resistance. In addition, a questionnaire was presented to the patient while in the recovery room that included the following questions:
Grade the degree of pain you experienced during the procedure from 0 to 10 (0 being no pain, 10 being unbearable pain).
Grade the degree of foreign body sensation you experienced during the procedure from 0 to 10 (0 being none, 10 being unbearable).
Rate your overall experience with the procedure (0% to 100%), with 0% being unpleasant, 100% being extremely smooth and enjoyable.
Which machine would you prefer if you were to get the same procedure done a second time?
UDVA, CDVA, manifest refraction, OSI, contrast sensitivity (CSV-1000), and aberrometry were collected and analyzed. All preoperative and postoperative evaluations were performed by a single experienced optometrist and technician, who were masked to which laser was used to treat each eye.
Statistical Analysis
SPSS software for Windows version 17.0.0 (IBM Corporation) was used for statistical analysis. All values were expressed as mean ± standard deviation. Data were checked for normality. The paired t-test and independent t-test were used for intragroup and intergroup comparison between the means, respectively. A P value of .05 or less was considered statistically significant.
Results
A total of 60 eyes (30 eyes treated with the VisuMax 800 [VM800 group] and 30 eyes treated with the VisuMax 500 [VM500 group]) from 30 patients who underwent bilateral SMILE between February 2022 and August 2023 were included in the study.
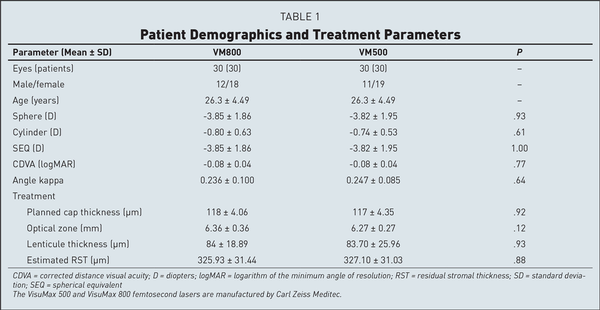
Both groups were comparable in terms of preoperative mean age, sphere, cylinder, SEQ, pachymetry, angle kappa, contrast sensitivity at all spatial frequencies, OSI, and MTF cut-off, as well as for treatment parameters including cap thickness, optical zone, residual bed thickness, and maximum and minimum lenticule thickness. Patient demographics and treatment parameters are listed in Table 1.
Surgical Workflow
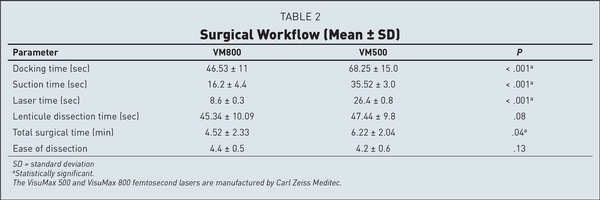
Table 2 shows the timings noted during the procedure for eyes in both groups. The mean docking time was 46.53 ± 11 seconds for the VM800 group and 68.25 ± 15 seconds for the VM500 group (P < .001). The mean lenticule creation time was 8.6 ± 0.3 seconds in the VM800 group compared to 26.4 ± 0.8 seconds in the VM500 group (P < .001). The mean overall surgical time was shorter at 4.52 ± 2.33 minutes in the VM800 group compared to 6.22 ± 2.04 minutes in the VM500 group (P < .001). There was no difference found between groups for lenticule dissection time. The mean score for ease of lenticule dissection was slightly better in the VM800 group (4.40 ± 0.5) compared to the VM500 group (4.20 ± 0.6), but the differences were not significant.
Subjective Questionnaire
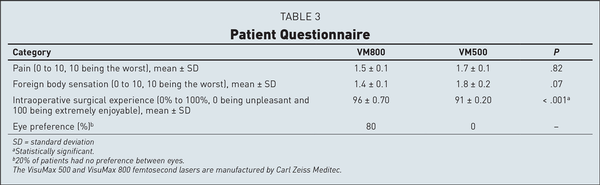
There were no significant differences observed for intraoperative pain and foreign body sensation. However, overall satisfaction with the intraoperative experience was rated significantly better in the VM800 group. Patients preferred the eye that had treatment with the VM800 80% of the time and had no preference 20% of the time. No patient reported a strong preference for the VM500 eye (Table 3).
Efficacy and Predictability
Figures 1–3 show the visual and refractive outcomes comparing the VM800 and VM500 groups. There were no statistically significant differences found for UDVA (Figure A) or CDVA between the VM800 and VM500 groups. At the 3-month postoperative visit, 97% of eyes in the VM800 group and 93% of eyes in the VM500 group had a UDVA of 20/20 or better. All (100%) eyes in the VM800 group were within ±0.50 D of intended SEQ correction and 97% of eyes in the VM500 group. Analysis of astigmatism correction showed that 97% of eyes in the VM800 group and 100% of eyes in the VM500 group had 0.50 D or less of residual refractive astigmatism. Both groups showed similar behavior of stability during the first 3 months after treatment.
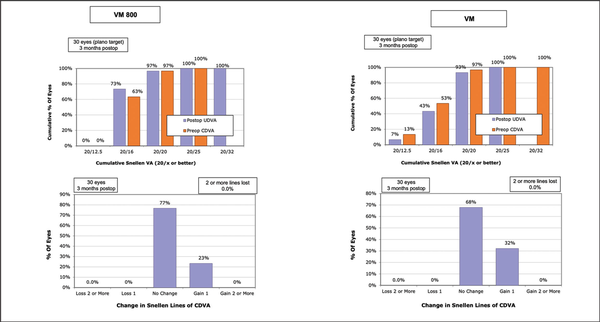
Figure 1
Standard graphs comparing the cumulative Snellen visual acuity (top row) and change in Snellen lines of corrected distance visual acuity (CDVA) (bottom row) for patients undergoing small incision lenticule extraction with the VisuMax 800 (VM800) and VisuMax 500 (VM) femtosecond lasers (Carl Zeiss Meditec). UDVA = uncorrected distance visual acuity
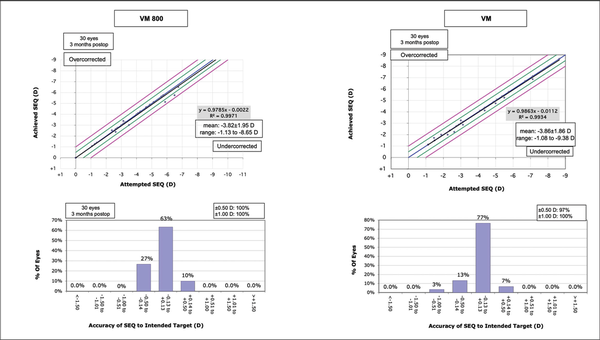
Figure 2
Standard graphs comparing the predicted versus achieved spherical equivalent (SEQ) (top row) and accuracy of the SEQ to the intended target (bottom row) for patients undergoing small incision lenticule extraction with the VisuMax 800 (VM800) and VisuMax 500 (VM) femtosecond lasers (Carl Zeiss Meditec). D = diopters
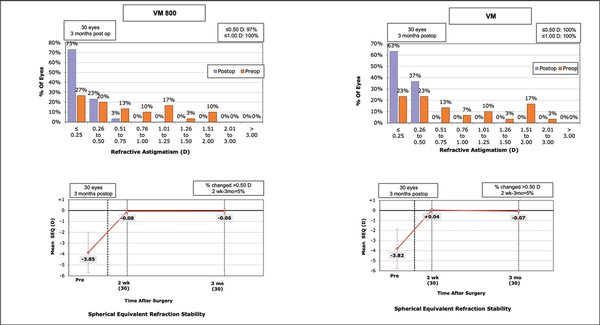
Figure 3
Standard graphs comparing the preoperative and postoperative refractive astigmatism (top row) and stability of the spherical equivalent (SEQ) (bottom row) for patients undergoing small incision lenticule extraction with the VisuMax 800 (VM800) and VisuMax 500 (VM) femtosecond lasers (Carl Zeiss Meditec). D = diopters
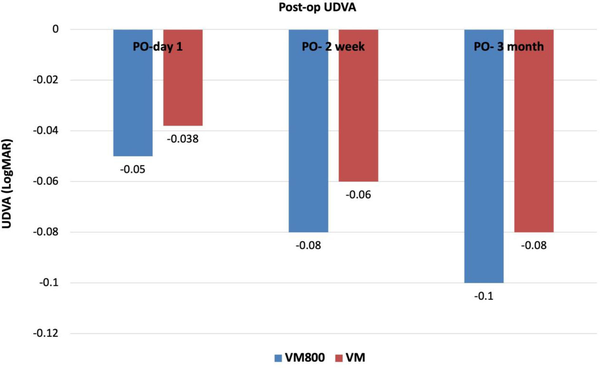
Figure A
Comparison of postoperative (PO) uncorrected distance visual acuity (UDVA) at 1 day, 2 weeks, and 3 months. The VisuMax 500 (VM) and VisuMax 800 (VM800) femtosecond lasers are manufactured by Carl Zeiss Meditec.
Quality of Vision
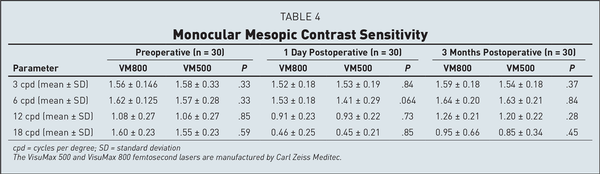
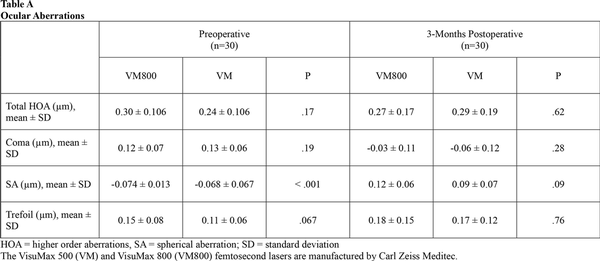
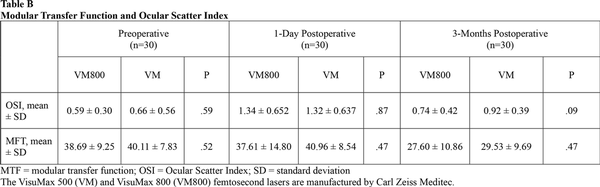
Table 4 shows the mean preoperative and postoperative values for contrast sensitivity. Both groups were similar at the 3-month visit. Eyes in both groups also showed similar results when compared to their preoperative levels at 3, 6, and 12 cycles per degree. At 18 cycles per degree, there was a slight decrease noted for both groups when compared to the preoperative levels. Table A show the preoperative and 3-month postoperative aberrations including total HOAs, coma, spherical aberration, and trefoil for both groups. Table B shows the MTF and OSI at the preoperative, 1-day, and 3-month postoperative visits. There were no significant differences found between groups for either of these categories. There was a slight increase in OSI and a slight decrease in MTF when comparing each group to its respective preoperative levels.
Safety and Complications
Postoperative CDVA was compared to preoperative CDVA for eyes in both groups. In the VM800 group, 23% of eyes gained one line of visual acuity, 77% of eyes remained the same, and no eyes lost any lines of CDVA. In the VM500 group, 32% of eyes gained one line of vision, 68% of eyes remained the same, and no eyes lost any lines of CDVA. There were no operative or postoperative complications such as excessive opaque bubble layer, black spots, incision or lenticule tears, suction loss, or inability to extract the lenticule, interface haze, or edema reported for any eyes in either group during this study.
Discussion
The results of this study showed excellent visual and refractive outcomes for eyes that underwent SMILE using the VisuMax 500 and VisuMax 800. The VM800 group showed a faster lenticule creation time and overall treatment time compared to the VM500 group and patients preferred the VM800 eye over the VM500 eye 80% of the time.
Visual and refractive outcomes with the second generation VisuMax 800 femtosecond laser have been recently published. Reinstein et al reported the first results in a retrospective analysis of consecutive eyes treated by SMILE with VisuMax 800. At 3 months, the postoperative SEQ was within ±0.50 D in 86% and ±1.00 D in 100% of eyes. UDVA was 20/20 or better in 91% of eyes and no eyes lost two or more lines of CDVA. There was a small but statistically significant increase in contrast sensitivity at 3, 6, 12, and 18 cycles per degree. The authors concluded that early outcomes data for SMILE with the second-generation laser demonstrated an effective and safe option and were equivalent to published first generation VisuMax 500 outcomes for the treatment myopia and astigmatism. Similarly, Saad et al reported high efficacy and safety indices of 0.93 and 1.00, respectively. In their study, 99% of eyes achieved a SEQ within ±1.00 D and 91% were within ±0.50 D of attempted correction at 3 months. Our results were consistent with the results of the above studies, with 97% eyes achieving 20/20 or better UDVA using the VM800, and no eye losing any lines of CDVA.
In addition, the mean postoperative UDVA, CDVA, SE, cylinder, OSI, HOA, and contrast sensitivity were comparable between the VM800 and VM500 groups, reinforcing their initial assumptions that there is little difference with regard to visual outcomes between groups. This is due to the fact that the optics and laser delivery design of the VisuMax 800 are identical to that of the VisuMax 500, and therefore one would expect similar clinical outcomes between the two devices.
In the current study, we clearly observed a significant advantage of the VisuMax 800 laser for docking time, suction time, and overall surgical time when compared to the VisuMax 500. Of these parameters, “docking time” deserves special attention here. This time may be divided into two parts: time taken after preparation of the eye until the contact lens applanates the eye and time taken to achieve satisfactory centration and activation of suction. While comparing the VisuMax 800 with VisuMax 500, certain fundamental differences exist between the two lasers. For the execution of time taken after preparation of the eye until the contact lens applanates the eye with the VisuMax 800, one needs to lower the laser arm to applanate the cornea, whereas with the VisuMax 500, the patient bed is raised to advance the patient's eye toward the contact glass. The movement of the laser arm toward the eye is faster in comparison to the movement of the bed. The time taken to achieve satisfactory centration is aided by a centration guide based on the x and y coordinates of the pupil in the VM800 group and therefore was faster than in the VM500 group where there is no software aid available, which may result in a variable, subjective, and time-consuming centration. Accurate treatment centration is crucial to avoid visual quality issues due to the induction of HOAs. This step may be especially challenging in eyes with large angle kappa, large and decentered pupils, high corneal astigmatism, and uncooperative patients, in the absence of a centration guide. The presence of the CentraLign feature makes it easier to perform docking with greater confidence, even in the challenging situations mentioned above. Finally, the suction and laser time is clearly determined by the speed of the laser. Therefore, the VisuMax 800, with a 2-MHz pulse repetition rate and average lenticule creation time of 8 to 9 seconds, has a definite advantage over the VisuMax 500, which has a 500-KHz frequency and average lenticule creation time of 25 to 27 seconds. The significantly reduced docking, suction, and laser times appear to have contributed to shortening the overall surgical time and patient eye preference in the current study.
One of the important factors determining the incidence of intraoperative suction loss is the duration of suction time. The significantly reduced suction and laser time with the VisuMax 800 is certainly expected to reduce the risk of inadvertent suction losses. No cases of suction loss were observed in either of the study groups during the current study. However, the reported incidence of suction loss in SMILE varies from 0.22% to 3.2%, and therefore it may be by chance that we did not observe suction loss in the relatively smaller sample size of 60 total eyes. In addition, this cohort was operated on by experienced SMILE surgeons. A difference in suction loss rates between lasers may be more apparent for novice SMILE surgeons early in their learning curve.
Optimization of laser settings such as spot and track spacings are crucial for determining the quality of the laser pattern, which in turn affect the ease of lenticule separation. In both groups, optimized energy settings and differential spot spacing patterns were used. The energy used in the VM800 group was 26 (130 nJ) versus 27 (135 nJ) in the VM500 group. The slightly better, yet comparable surgeon scores for ease of lenticule dissection in the VM800 group may be attributed to the faster scanning pattern in the VisuMax 800 resulting in less “cross talk” between the lenticule and cap interface resulting in less opaque bubble layer formation. Despite this, no significant differences in the 1-day or 3-month visual acuity or visual quality (OSI, MTF, and contrast sensitivity) were noticed.
This was the first study to our knowledge treating one eye of the patient with the VisuMax 800 and one eye with the VisuMax 500. Patients preferred the eye that had treatment with the VisuMax 800 80% of the time compared to the eyes that had treatment using the VisuMax 500. Patient satisfaction and intraoperative surgical experience was also better in the VM800 group. This was attributed to markedly reduced suction and laser time to create the refractive lenticule during which the patient cannot move, and less claustrophobia experienced with the VisuMax 800. Both factors significantly reduced the patient's anxiety during the procedure.
Conclusion
The new VisuMax 800 femtosecond laser with a 2-MHz frequency and a host of intuitive features appears to improve the surgical workflow and enhance patient experience. To our knowledge, this is the first contralateral eye study comparing the VisuMax 800 and VisuMax 500 femtosecond lasers. The results show a definite advantage in overall surgical times and patient experience when using the second-generation VisuMax 800 laser. Future studies with larger sample sizes are suggested to confirm these preliminary findings.
AUTHOR CONTRIBUTIONS
Study concept and design (SB, SG); data collection (DTS); analysis and interpretation of data (SB); writing the manuscript (SB); critical revision of the manuscript (SB, SG, DTS); statistical expertise (SB); administrative, technical, or material support (SG); supervision (SG)
References
- 1. Brar S, Ganesh S, Bhargav S. Comparison of intraoperative time taken for docking, lenticule dissection, and overall workflow for SMILE performed with the VisuMax 800 versus the VisuMax 500 femtosecond laser. J Refract Surg. 2023;39(9):648. PMID:
- 2. Reinstein DZ, Archer TJ, Potter JG, Gupta R, Wiltfang R. Refractive and visual outcomes of SMILE for compound myopic astigmatism with the VisuMax 800. J Refract Surg. 2023;39(5):294–301–. PMID:
- 3. Saad A, Klabe K, Kirca M, Kretz FAT, Auffarth G, Breyer DRH. Refractive outcomes of small lenticule extraction (SMILE) Pro® with a 2 MHz femtosecond laser. Int Ophthalmol. 2024;44(1):52. PMID:
- 4. Yoo TK, Kim D, Kim JS , , et al. Comparison of early visual outcomes after SMILE using VisuMax 800 and VisuMax 500 for myopia: a retrospective matched case-control study. Sci Rep. 2024;14(1):11989. PMID:
- 5. Kang DSY, Lee H, Reinstein DZ , , et al. Comparison of the distribution of lenticule decentration following SMILE by subjective patient fixation or triple marking centration. J Refract Surg. 2018;34(7):446–452–. PMID:
- 6. Shao T, Wang Y, Ng ALK , , et al. The effect of intraoperative angle kappa adjustment on higher-order aberrations before and after small incision lenticule extraction. Cornea. 2020;39(5):609–614–. PMID:
- 7. Lee H, Roberts CJ, Arba-Mosquera S, Kang DSY, Reinstein DZ, Kim TI. Relationship between decentration and induced corneal higher-order aberrations following small-incision lenticule extraction procedure. Invest Ophthalmol Vis Sci. 2018;59(6):2316–2324–. PMID:
- 8. Liu M, Wang J, Zhong W, Wang D, Zhou Y, Liu Q. Impact of suction loss during small incision lenticule extraction (SMILE). J Refract Surg. 2016;32(10):686–692–. PMID:
- 9. Hamed AM, Heikal MA, Soliman TT, Daifalla A, Said-Ahmed KE. SMILE intraoperative complications: incidence and management. Int J Ophthalmol. 2019;12(2):280–283–. PMID:
- 10. Qin B, Li M, Shen Y , , et al. Management of suction loss during SMILE in 12,057 eyes: incidence, outcomes, risk factors, and a novel method of same-day recutting of refractive lenticules. J Refract Surg. 2020;36(5):308–316–. PMID:
- 11. Wong CW, Chan C, Tan D, Mehta JS. Incidence and management of suction loss in refractive lenticule extraction. J Cataract Refract Surg. 2014;40(12):2002–2010–. PMID:
- 12. Reinstein DZ, Archer TJ, Vida RS, Carp GI. Suction stability management in small incision lenticule extraction: incidence and outcomes of suction loss in 4000 consecutive procedures. Acta Ophthalmol. 2020;98(1):e72–e80–. PMID:
- 13. Huang TZ, Shen L, Yu XN, Jin HY. Risk factors and incidence of suction loss during small incision lenticule extraction (SMILE) in 8493 eyes. BMC Ophthalmol. 2020;20(1):412. PMID:
- 14. Li L, Schallhorn JM, Ma J, Zhang L, Dou R, Wang Y. Risk factors for opaque bubble layer in small incision lenticule extraction (SMILE). J Refract Surg. 2017;33(11):759–764–. PMID:
- 15. Donate D, Thaëron R. Lower energy levels improve visual recovery in small incision lenticule extraction (SMILE). J Refract Surg. 2016;32(9):636–642–. PMID:
- 16. Brar S, Ganesh S, Gautam M, Meher S. Feasibility, safety, and outcomes with standard versus differential spot distance protocols in eyes undergoing SMILE for myopia and myopic astigmatism. J Refract Surg. 2021;37(5):294–302–. PMID: