We highlight a recent study published in Nature which explored the role of interleukin‐11 (IL‐11), a pro‐inflammatory cytokine, in aging. The study demonstrates how inhibiting IL‐11 signaling can positively impact both healthspan and lifespan in mice, suggesting a novel target for antiaging therapies with significant implications for enhancing human longevity and quality of life.
Aging is an inevitable process characterized by a gradual decline in physiological functions, increasing vulnerability to diseases as well as mortality. Therefore, understanding the molecular mechanisms that drive aging is crucial for developing interventions that can extend not only the lifespan of people, but also the healthspan defined as the period of life spent in good health. This study found that IL‐11 expression in mice increases with age in various tissues, including the liver, visceral gonadal white adipose tissue and skeletal muscle. This suggests that changes in IL‐11 may drive the decline in physical and metabolic health observed in older animals. To explore the influence of IL‐11, the researchers used genetically engineered mice lacking IL‐11 or its receptor. These models showed significant health benefits, including improved metabolic health, reduced inflammation and decreased cellular senescence compared to wild‐type controls. The absence of IL‐11 signaling also protected the mice from age‐related muscle decline, metabolic dysfunction and other age‐associated diseases. Importantly, the genetic deletion of IL‐11 extended the average lifespan by 24.9% (median lifespan increasing from 121 to 151 weeks) in both male and female mice.
In IL‐11‐deficient mice, some of the changes such as increased muscle strength were observed even in young mice. Therefore, the researchers administered an anti‐IL‐11 antibody to aged mice (75 weeks old) for 25 weeks. This resulted in significant improvements in metabolic function, such as enhanced glucose tolerance and insulin sensitivity. Additionally, the mice treated with the antibody exhibited better muscle strength and lower levels of aging biomarkers, including pro‐inflammatory cytokines, telomere length and tissue fibrosis. Similar to IL‐11 knockout, the administration of anti IL‐11 antibodies extended the median lifespan by 22.5% in male mice and 25% in female mice. Intriguingly, both IL‐11‐knockout mice and anti‐IL‐11‐treated mice showed fewer macroscopic tumors compared to wild type mice at autopsy, suggesting IL‐11 inhibition may have beneficial effects on cancer, a common cause of morbidity and mortality in old age. The study also evaluated the mechanisms by which IL‐11 influences aging. Inhibiting IL‐11 signaling reduced the activity of the ERK–AMPK–mTORC1 pathway, leading to reduced cellular senescence and inflammation. Additionally, IL‐11 increased the expression of senescence‐associated secretory phenotype factors, which exacerbate aging‐related conditions.
The study's findings have significant translational implications, particularly as IL‐11 inhibitors are currently tested in phase I clinical trials for fibrotic diseases (e.g., NCT05658107). These inhibitors could potentially be repurposed or further developed as antiaging therapies. This research also contributes to the broader understanding of aging biology by highlighting the critical role of chronic inflammation and cellular senescence. Chronic inflammation has been implicated in age related diseases such as cardiovascular disease and cancer. The anti‐IL‐11 antibody used in this study has been previously shown to demonstrate anti‐fibrotic effects in mouse models of pulmonary fibrosis and kidney injury. In idiopathic pulmonary fibrosis, a disease with poor prognosis often diagnosed in older adults, it has recently been recognized that half of patients have short telomere lengths, with some of these having germline mutations in telomere‐related genes. These genetic short telomere syndromes present with diverse features such as premature hair graying, pulmonary fibrosis and bone marrow failure, and it may be interesting to test if IL‐11 inhibition, shown in this study to reverse telomere shortening in mice, also provides therapeutic benefits in genetic disorders of premature aging.
We have previously reported that facial and fundus images can be used to predict the aging process using multi‐modal deep learning models. This model can predict biological age, which can be compared with chronological age to serve as a biomarker for the development and prognosis of chronic diseases. Other approaches to predict biological age using different proteomic markers exist. By combining the predictive power of the proteomic clock with biological age estimations and IL‐11 inhibition, a more comprehensive approach to delaying aging and preventing chronic diseases can be developed. Nevertheless, aging is an inherently heterogeneous process and no single aging process will be the same. Therefore, there is a critical need for reliable biomarkers that can more accurately predict biological age. Biomarkers such as telomere length, DNA methylation patterns (epigenetic clocks), and the approaches outlined above can be good indicators of biological age. Artificial intelligence (AI) approaches will no doubt play a big role in the elucidation of the intricate networks influenced by IL‐11 signaling, providing deeper insights into its role in aging (Figure 1).
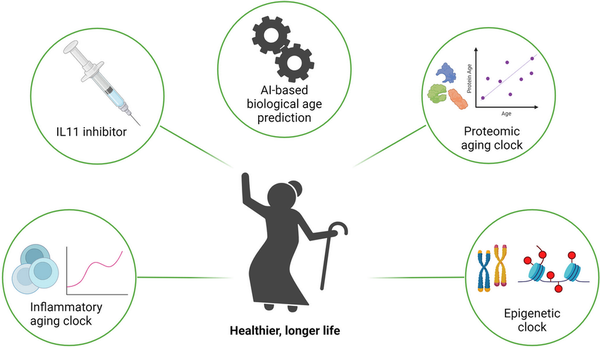
Figure 1
The potential of IL‐11 inhibitors as antiaging therapies, coupled with AI‐based biological age prediction and inflammatory, proteomic and epigenetic clocks offer promising avenues for promoting healthy aging and longevity.
While extending life may seem universally desirable, it must be balanced against the implications for population growth, resource distribution, and societal structures. Prolonging life without ensuring quality of life, particularly in terms of mental and physical health, could lead to extended periods of frailty rather than wellbeing. There are also ethical considerations related to the accessibility and equity of potential antiaging treatments. If therapies like IL‐11 inhibitors become available, it is crucial to ensure that they are accessible to all segments of society, not just the affluent. This raises broader questions about healthcare equity and global disparities in access to medical advancements. Nevertheless, this study provides compelling evidence that the inhibition of IL‐11 signaling represents a promising new approach to extending both healthspan and lifespan. As research continues to uncover the complex mechanisms underlying aging, targeting IL‐11 and similar pathways could become central to developing interventions that promote healthy aging and longevity.
AUTHOR CONTRIBUTIONS
L S C Lok: Methodology (equal); writing—original draft (equal); writing—review and editing (equal). O Monteiro: Methodology (equal); writing—original draft (equal); writing—review and editing (equal). D T Baptista‐Hon: Conceptualization (lead); methodology (equal); supervision (lead); writing—original draft (equal); writing—review and editing (equal). All authors have read and approved the final manuscript.
CONFLICT OF INTEREST STATEMENT
Author Daniel T. Baptista‐Hon is an editorial staff of MedComm‐Future Medicine. Author Daniel T. Baptista‐Hon was not involved in the journal's review of, or decisions related to, this manuscript. The other authors declared no conflict of interest.
ETHICS STATEMENT
Not applicable.
ACKNOWLEDGMENTS
Figure 1 was created in BioRender. Baptista‐hon, D. (2024) BioRender.com/i06d849. We would like to thank the Fundo para o Desenvolvimento das Ciências e da Tecnologia (FDCT 0109/2020/A3, FDCT 0106/2021/A and FDCT 0055/2022/A1).
REFERENCES
- 1. Widjaja AA, Lim WW, Viswanathan S, et al. Inhibition of IL‐11 signalling extends mammalian healthspan and lifespan. Nature. 2024;632:157–165.
- 2. Cook SA, Schafer S. Hiding in plain sight: interleukin‐11 emerges as a master regulator of fibrosis, tissue integrity, and stromal inflammation. Annu Rev Med. 2020;71:263–276.
- 3. Alder JK, Armanios M. Telomere‐mediated lung disease. Physiol Rev. 2022;102(4):1703–1720.
- 4. Wang J, Gao Y, Wang F, et al. Accurate estimation of biological age and its application in disease prediction using a multimodal image Transformer system. Proc Nat Acad Sci. 2024;121(3):e2308812120.
- 5. Li Z, Zhang W, Duan Y, et al. Progress in biological age research. Front Public Health. 2023;11:1074274.
