Background
The human papillomavirus (HPV) is responsible for causing 6 different types of anogenital and oropharyngeal cancers. Vaccination serves as an effective preventive measure against these cancers. In 2006, the Advisory Committee on Immunization Practices (ACIP) recommended routine HPV vaccination for adolescent girls aged 11 to 12 y, with catch-up vaccination available for females up to age 26 y. Later, in 2011, the routine recommendation was extended to include adolescent boys aged 11 to 12 y, with catch-up vaccination available for males until age 21 y or up to age 26 y for high-risk groups, such as men who have sex with men and immunocompromised individuals. More recently, the catch-up age of 26 y was harmonized for males and females. Despite effective vaccines being readily available, there has been a suboptimal adoption of the HPV vaccine among adolescents, leaving a significant number of adults susceptible to oncogenic HPV infections.–
In 2018, the US Food and Drug Administration granted approval for the 9-valent HPV vaccine for adults aged 27 to 45 y, subsequently leading to an updated recommendation from ACIP. In June 2019, ACIP made the decision to recommend the HPV vaccine as a shared clinical decision for individuals aged 27 to 45 y. This recommendation considers not only safety and efficacy but also considers factors such as vaccine effectiveness and cost-effectiveness. The current guideline for expanded HPV vaccination (i.e., adults aged 27–45 y) suggests that “shared clinical decision-making regarding HPV vaccination is recommended for some adults aged 27 through 45 y who are not adequately vaccinated.” Furthermore, the recommendation advises clinicians to consider discussing HPV vaccination with individuals who are most likely to benefit from it. However, there is a lack of clear guidance on which patients providers should approach for discussions regarding potential vaccination benefits.
Nonetheless, despite guidelines being available, there remains a pressing need for shared decision-making support in clinical practice. The expanded age authorization for the 9-valent HPV vaccine offers an opportunity to reach individuals who were previously considered too old or had exceeded the initial licensure age but who can still benefit from vaccination., While health care providers are aware of the mid-adult vaccination guidelines, there is a substantial gap in their understanding (e.g., which patients to discuss with, key points of the guidelines) for how to effectively translate and implement these recommendations., Hurley et al. recommended the use of a decision aid to support the shared clinical decision-making process and support physicians in the key talking points from ACIP recommendations. Moreover, the use of a decision aid that is patient facing can help reduce potential downstream inequities. The expanded age option is most likely to benefit populations who are already well-informed about HPV vaccination, aware of the changes to the 9-valent vaccine licensure, and empowered to advocate for their health care choices. Prior research has shown that 27- to 45-y-old US adults with higher educational attainment and engagement with health care settings were more likely to be aware of HPV and HPV vaccination. Moreover, to assist with decision making, adults in this age range described needing more information about HPV vaccine safety, effectiveness, personal benefit, provider recommendations, side effects, and risks. Thus, both patients and providers could benefit from decision support tools with this complicated guideline from ACIP. There are limited examples of decision tools for HPV vaccination. This is likely largely attributable to clear ACIP guidelines for HPV vaccination among adolescents and young adults rather than a shared clinical decision recommendation. As such, any creation of a decision-support tool for mid-adult HPV vaccination should rely on existing standards and frameworks related to decision-making science.
To support patients in shared clinical decision making, we have developed a decision tool– that incorporates best practices for shared decision making and international standards for decision tools. The purpose of this study was to determine the effect of the decision aid tool on HPV vaccination decision support outcomes among adults ages 27 to 45 y in the United States who had not been vaccinated. The primary outcome was decisional conflict, and secondary outcomes included intermediate variables related to decision making. Our primary hypothesis was that persons who received the decision tool intervention would have lower decisional conflict compared with those who received the control condition. Exploratory research questions included: What impact did the decision tool have on intermediate outcomes such as knowledge, behavioral expectancies, decisional self-efficacy, and perceived risk? We also described preferences for the implementation of the decision tool from the perspective of end-users.
Methods
The development of the patient decision aid tool was guided by the Ottawa Decision Support Framework, which assists in supporting decisions with multiple options. Moreover, the decision aid was constructed in collaboration with community and provider advisory boards. The community advisory board (CAB) consisted of individuals who were unvaccinated for HPV and within the mid-adult age group (27–45 y) targeted for this decision aid. Health care providers made up the practice advisory board (PAB), representing family medicine, internal medicine, and obstetrics/gynecology. The CAB and PAB members were identified through community partners and academic medical centers. The advisory groups met with the research team for 5 rounds to review study materials and provide feedback on the decision aid tool’s content, appearance, and flow. Additional feedback was gained during usability (i.e., content, flow, and visuals feedback; n = 10) and field testing (i.e., independent use of the tool with input on future implementation; n = 10) with members of the target population. The final phase of tool development included qualitative interviews with health care providers and stakeholders, specifically focusing on the implementation of the tool and its acceptability to medical providers (n = 24). During each stage of development, revisions were made incorporating feedback and further refining the decision aid before the study launch.
This study was approved by the North Texas Regional Institutional Review Board. Study participants were recruited through a national survey panel vendor, Centiment. The inclusion criteria were individuals within the mid-adult age range (27–45 y), unvaccinated for HPV, English speaking, and living in the United States. Individuals who were unsure of their HPV vaccination status were excluded from the study. Participants viewed a consent form prior to the screening survey and indicated consent by proceeding with the survey.
A Solomon, 4-group, pretest/posttest research design was used to test the main outcome. In this design, groups 1 and 3 received the intervention, while groups 2 and 4 did not. Groups 1 and 2 received a pretest, whereas groups 3 and 4 did not. This design protects against pretest sensitization in which participants may be “primed” to respond differently on the posttest because of items in the pretest. Screening questions were used to determine eligibility for participation. Individuals determined to be eligible were randomized using urn randomization within the Rivulent platform into 1 of 4 groups (Figure 1). Randomization was also balanced based on sex. Individuals determined to be ineligible during the screening questions were excluded from participation. The intervention group received the Web-based decision tool, HPV DECIDE, which was interactive and provided information on the mid-adult HPV vaccination guideline, the decision, pros and cons of the decisions, values clarification, information dissemination based on values, and a tailored action plan based on the decision. The control group viewed the Centers for Disease Control and Prevention’s Vaccine Information Sheet on HPV vaccination embedded within the survey.
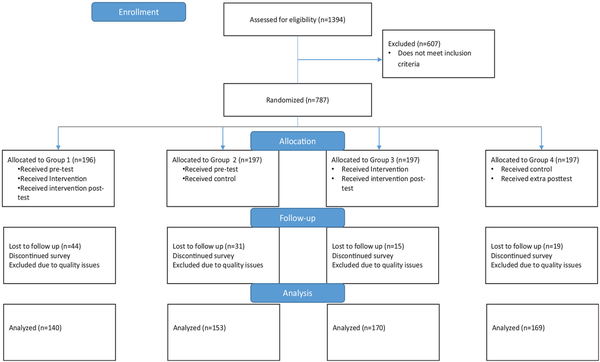
Figure 1
Consort diagram.
Sample
Participants were recruited between December 2023 and January 2024. There were 1,394 participants assessed for eligibility, and 787 individuals met inclusion criteria and were randomized into 1 of the 4 groups (Figure 1). Quotas were enabled within the survey to ensure equal distribution of males and females within each group. Participants who did not complete the survey or had quality issues in their responses were removed, leaving between 140 and 170 participants in each group used for analysis. The target sample size was estimated to detect a change in the decisional conflict scale. Anticipated effect size based on the Cochrane Database Systematic Review of decision aids on decisional conflict subscales ranged from 4.04 to 9.28. We calculated the variance of these effect sizes to be approximately 270 based on the average sample size (N∼ 300) across the studies reviewed. Based on these calculations, a sample size of 350 would enable us to detect effect sizes (i.e., mean differences in the decisional conflict subscales) as small as 3.5. Our analytic sample was 632.
Measures
Pre- and postsurveys were administered to participants in group 1 (subset of intervention recipients) and group 3 (subset of control recipients). Posttests were administered to all participants. Baseline measures were collected in the pretest survey. The primary outcome was decisional conflict. Secondary outcomes included vaccine decision, intentions, knowledge, expectations, decisional self-efficacy, and perceived risks.
Decisional conflict was measured using the 16-item Decisional Conflict Scale, with response options ranging from strongly agree (0) to strongly disagree (4); the scores ranged from 0 (no conflict) to 100 (extreme decisional conflict). The scale assesses an individual’s uncertainty in making decisions related to their health. Perceived uncertainty can be motivated by insufficient information about available options, lack of clarity on how choices align with personal values, and absence of support to inform decisions. We evaluated participants’ ability to make decisions about HPV vaccination based on personal values, social influence or pressure, availability of resources, and understanding of risks and benefits. The scale had excellent internal reliability, with the set of all Decisional Conflict posttest items having a Cronbach’s alpha of 0.96 and all subscales resulting in an alpha of 0.89 or 0.90.
Participants were expected to indicate their decision on getting the HPV vaccine. Response options included, “I have decided to get the HPV vaccine,”“I am unsure about getting the HPV vaccine,” and “I have decided not to get the HPV vaccine.” Intentions were assessed using a 4-item measure, with response options ranging from very unlikely to very likely. This covered participants’ intention within the next 6 mo to find out more information about the HPV vaccine, discuss the HPV vaccine with their health provider, and receive a dose of the HPV vaccine.
Knowledge about HPV was measured using the 13-item Mid-adult Human Papillomavirus Knowledge Scale. Three additional questions were asked to assess their knowledge on FDA’s approval of the vaccine for mid-adults, scientific evidence on HPV vaccination, and protection provided by HPV vaccination. Participants were expected to indicate either “true,”“false,” or “don’t know” for each statement. Knowledge was then computed as 1-point per correct response to a statement and 0 points for an incorrect or “don’t know” response. The Knowledge scale had good internal consistency (Cronbach’s alpha = 0.78).
Perceived expectation was assessed by asking the participant’s agreement on a 5-point Likert scale with the following statement: “The effectiveness of the HPV vaccine will vary from person to person.” This secondary outcome was selected due to the varied individual benefits of the HPV vaccination in the mid-adult age range.
An 11-item measure was adapted from the Decision Self-efficacy Scale to assess decisional self-efficacy among participants. The Decision Self-Efficacy Scale evaluates an individual’s self-confidence to make decisions on their own or with support from other sources. We measured participants’ level of confidence in getting information, asking questions, and making decisions about the HPV vaccine. Participants responded using a scale (0: not all confident to 4: very confident). Decisional Self-Efficacy items had excellent internal consistency (Cronbach’s alpha = 0.96).
Perceived risks of HPV infection were also assessed, including participants’ perceptions of getting an HPV-associated infection, genital/anal warts, or cancer in their lifetime. Response options ranged from very unlikely to very likely.
For the intervention groups, measures assessed decision-making outcomes based on their perceptions of the decision aid tool. These measures included rating of information (1 = poor to 4 = excellent), length of information (3 = too long to 1 = just right), amount of information (3 = too much information to 1 = just right), information balance (3 = slanted towards HPV vaccination to 1 = balanced), and trustworthiness (0 = not at all to 1 = completely). Furthermore, we assessed participants’ preferences for implementation of the decision tool. This included point of access, ideal time, ideal mode, acceptable provider to discuss the decision aid tool, and likelihoods of providing their contact information in the tool, printing a summary of the content, and taking a screenshot of the content.
Data Analysis
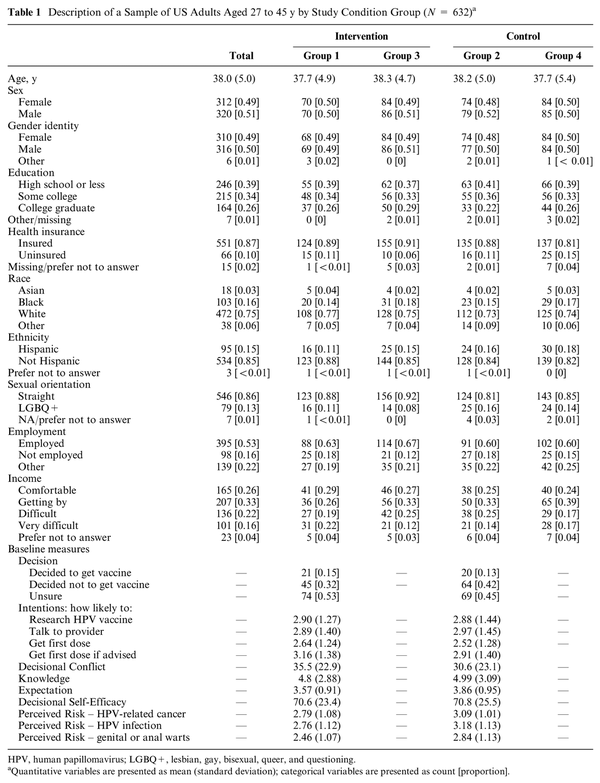
Descriptive statistics were calculated for demographic characteristics in the overall sample and stratified by experimental group. Pretest scores were computed for the groups that received the pretest. Total cell size and overall proportion were computed for categorical variables, and means and standard deviations were calculated for quantitative variables. Chi-square tests of independence and analysis of variance were conducted to determine any significant differences in demographic characteristics across experimental groups.
Data analysis was carried out in 2 steps for each outcome. The first step involved testing for pretest sensitization due to the Solomon experimental design. Individuals were assigned to 2 factors, with factor A being Treatment v. Control and factor B being Pretest v. No Pretest. Pretest sensitization is indicated if there is a significant interaction between factors A and B on the posttest measures., For continuous outcomes, these interaction terms were assessed via linear regression and, for categorical outcomes, proportional odds logistic regression for ordered categorical variables (Decision, Perceived Risks).
The second step gathered evidence of treatment effect across the 4 experimental groups in the case of no pretest sensitization. Braver and Braver described a meta-analytic procedure to achieve the largest statistical power in the Solomon design in which groups 1 and 2 are tested for treatment effects controlling for pretest scores while groups 3 and 4 are tested for treatment effects in a posttest-only analysis. These tests were carried out by linear models for continuous outcomes, multinomial regression for the Decision outcome, or proportional odds logistic regression for the Perceived Risk outcomes. The estimates were then meta-analyzed, ensuring that pretest information is controlled for when available and that all participants are included in the final inference. In our analyses, fixed-effects inverse-variance weighting was used to pool the linear or logistic coefficient estimates, calculate pooled confidence intervals (CIs) with the appropriate standard errors, and determine the statistical significance of a pooled test statistic. Logistic coefficients and CIs were exponentiated to reflect odds ratios (ORs). These effects are interpreted in the same manner as unstandardized regression coefficients or ORs from a single regression model.
Results
Group Descriptive Statistics
All descriptive statistics are presented in Table 1. There were no significant differences between the groups on any of the demographic characteristics, consistent with successful random assignment of the groups. Across all pretest measures, there were only 2 domains with significant differences between groups 1 and 2. The groups differed on the Expectation item, in which the control group scored 0.29 points higher on average regarding agreement that HPV vaccine effectiveness varies from person to person (t = −2.69, P = 0.008). In addition, the control group with pretest reported higher Perceived Risk than the intervention group with pretest across the 3 perceived risk items (HPV-related cancer item: P = 0.015; HPV infection item: P = 0.001; genital or anal warts item: P = 0.003). However, given that the family of pretest comparisons consists of 28 total variables tested, it is not surprising that some differences were detected by chance at the 0.05 level for each test, even if randomization was successful.
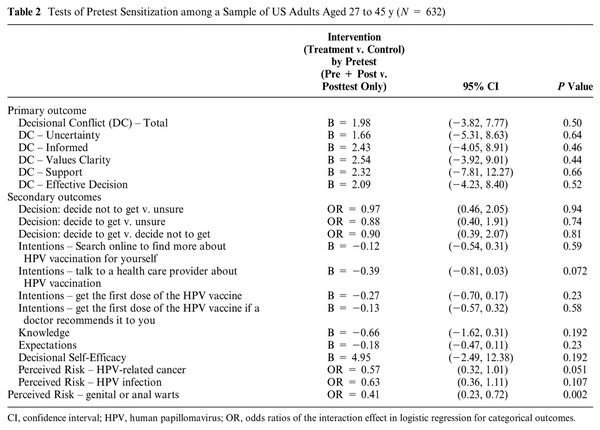
Pretest Sensitization
Table 2 presents all results for tests of pretest sensitization. Across all outcomes, only 1 variable showed evidence of pretest sensitization. The third question around perceived risk, which asked, “How likely are you to get anal or genital warts in your lifetime?” showed pretest sensitization. For all other outcomes, there was no evidence of pretest sensitization, as no interaction between factor A and factor B was significant. For quantitative outcome variables, we report the regression coefficient for the interaction term, the 95% CI, and its P value. For categorical outcomes, we report the interaction effect of OR, the 95% CI, and its P value. This result indicates that any effect of being in the treatment or control group on the posttest outcome is not modified by the administration of the pretest, except for risk perception of genital or anal warts.
Intervention Effectiveness
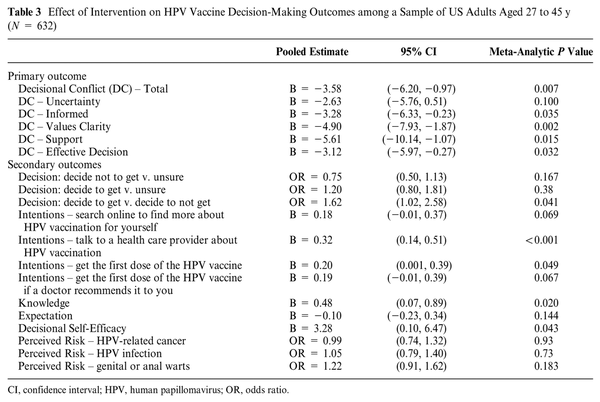
Evidence of an intervention effect was found for 10 of the 17 outcomes tested, and all meta-analyses of intervention effects are presented in Table 3. Of the primary outcomes, there was a significant intervention effect on the decisional conflict total score as well as all decisional conflict subscales except for uncertainty. The intervention group had lower decisional conflict scores by about 3.6 points, on average. The subscales with the largest reduction in decisional conflict due to intervention were the Support (5.6 points lower) and Values Clarity (4.9 points lower) subscales.
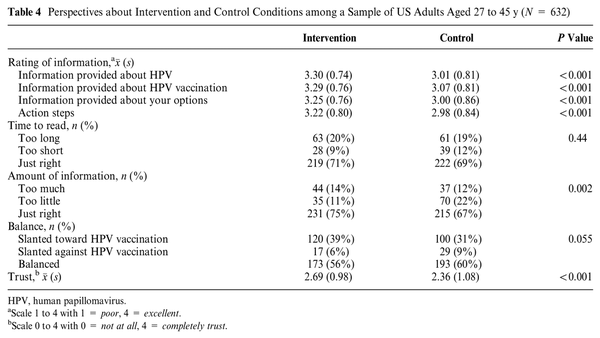
Regarding secondary outcomes, those receiving the intervention showed a significant increase in knowledge, greater intention to receive the vaccine and discuss the vaccine with their provider, and greater self-efficacy in their decision making about the HPV vaccine (see Table 3). The intervention was associated with higher odds of deciding to receive the vaccine compared with deciding not to receive the vaccine (OR = 1.62, 95% CI = 1.02, 2.56). The intervention was not associated with higher or lower odds of making a definitive decision on the vaccine compared with being unsure (decide not to get v. unsure: OR = 0.75, 95% CI = 0.50, 1.13; decide to get v. unsure: OR = 1.20, 95% CI = 0.80, 1.81). The intervention did not significantly alter the perceived risks of HPV infection, indicating that exposure to the decision tool did not inadvertently heighten fears around HPV infection and its consequences. Overall, the intervention was perceived more favorably than the control condition in terms of information shared, amount of information, and trustworthiness (Table 4).
Implementation Preferences
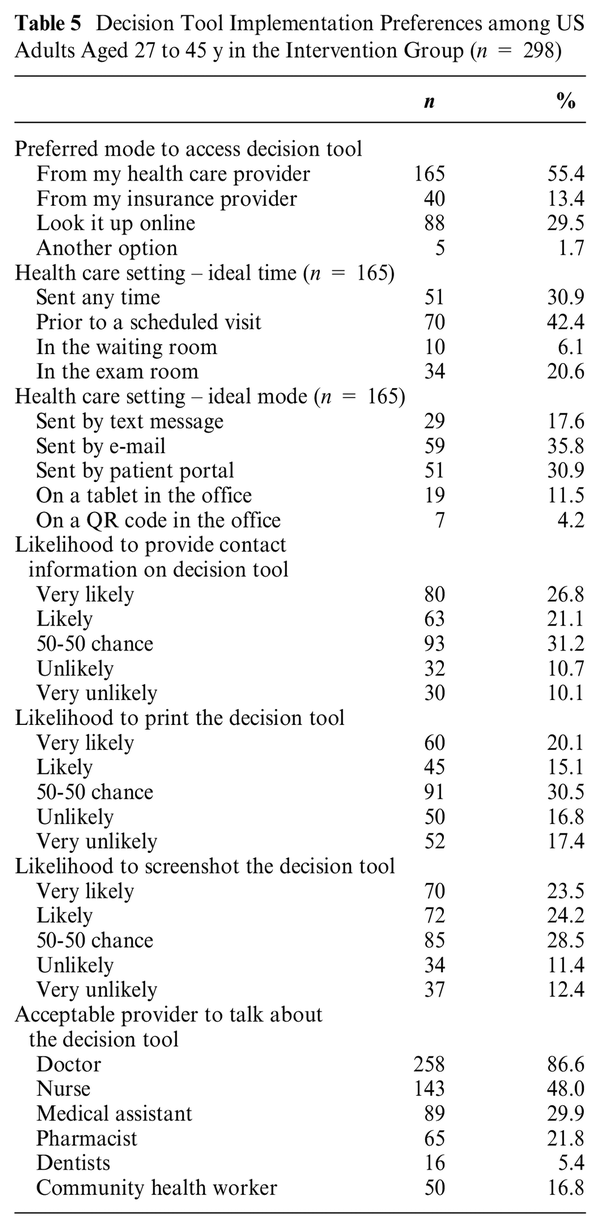
The intervention group received additional questions about their preferences for using this decision tool (Table 5). Most preferred to receive the decision tool from a health care provider (55.4%) or look it up online (29.5%). The most acceptable provider to receive this decision tool was a doctor (86.6%), followed by a nurse (48.0%). Among those preferring the health care provider, most wanted it prior to a scheduled visit (42.4%) or any time (30.9%). The ideal delivery mode from a health care provider was by e-mail (35.8%) or patient portal (30.9%). The final page of the tool included a summary of the decision, including inputs made by the user (e.g., values related to HPV vaccination). Participants were questioned about possible ways to share this personalized information with them. Almost half (48%) were likely to provide their contact information in the tool so results could be sent directly to them. Similarly, almost half (47%) would likely take a screenshot of the results screen. Fewer were likely to print the decision summary (35%).
Discussion
The HPV DECIDE tool was developed to assist with the decision-making process for HPV vaccination among mid-adults. After an iterative development process with mid-adult and health care provider input, the decision tool was tested in a randomized control trial. The goal was not to increase HPV vaccine intentions but rather to reduce decisional conflict and improve decisional outcomes. Overall, the HPV DECIDE tool was efficacious in reducing decisional conflict using the overall scale and subscales as well as improving knowledge and decision self-efficacy. Moreover, the decision tool was acceptable to the participants who also provided feedback on modes of delivery in a real-world setting.
As hypothesized, participants who received the decision tool compared with other control conditions had lower decisional conflict as reflected in the total scale score, in addition to the Information, Values, Support, and Effective Decisions subscores. These findings demonstrate that the decision tool helped to make the decision to get vaccinated explicit, provided useful information about the options, and helped to clarify personal values. The effect size (e.g., an average reduction of 3.58 on the total decimal conflict score) was consistent with similar tools informed by the Ottawa Decision Support Framework. Findings from secondary outcomes also reflect the utility of the tool in promoting shared clinical decision making with regard to mid-adult HPV vaccination. The use of the tool resulted in increased decisional self-efficacy and higher knowledge. Thus, participants were informed, engaged, and empowered to make a decision. The treatment group had a small increase in intentions to talk to a health care provider about HPV vaccination and, to a lesser degree, to initiate the HPV vaccine series. This is as expected as the tool was not designed to promote HPV vaccination. Similarly, the tool did not increase risk perceptions related to HPV infection. Overall, the outcomes support the concept of shared clinical decision making for HPV vaccination rather than promoting vaccine uptake and thus are appropriately aligned with the ACIP guideline.
Participants who received the HPV DECIDE tool reported that the time to read and length were largely acceptable (∼70% just right). The Web-based delivery of the tool allowed for the user to expand on additional information if desired (e.g., background on HPV) and present information aligned with value priorities. As such, this type of Web-based interface gives the user control of how much information to see and may also contribute to the acceptability, whereas a paper-based version would not have these features. The creation of a Web-based tool can also permit integration with electronic medical record systems and patient portals for ease of use. Moreover, participants had more favorable reactions to the information provided about HPV, HPV vaccination, options, and action steps compared with the vaccine information sheet control condition. Assessing user experiences is essential for determining the acceptability of a vaccination decision tool for future practice, and this decision tool likely benefited from several rounds of end-user input into the design of the tool. Future studies should examine the acceptability of the decision tool in a real-world setting when there are conflicting priorities and less dedicated time for use.
When analyzing the preferences of US adults for implementing the HPV decision support tool, as detailed in Table 5, participants predominantly (55.4%) preferred receiving the decision tool from a health care provider, with the most acceptable provider to talk about the decision tool being a doctor (86.6%), which emphasizes the trusted role of medical professionals and providers in facilitating health-related decisions. In addition, a significant proportion (29.5%) of respondents favored accessing the tool online, which aligns with the currently growing reliance on digital health solutions., As such, there may be two potential routes of dissemination of this decision tool to reach different audiences for mid-adults. First, disseminating the decision tool through different health systems and clinics will be appropriate for those mid-adults engaged in health care, particularly gynecological clinics, to reach women. Second, this tool can be disseminated through Web-based platforms in partnership with community and/or government public health organizations to reach persons who are not currently engaged in health care services. It cannot be a one-size-fits-all approach to dissemination.
Comparatively, this tool aligns well with other decision aid tools that underscore the importance of timely and relevant information delivery. Recent studies have shown that decision support tools are effectively integrated into clinical settings when their implementation occurs at critical points of care, such as prior to or during preventive health visits or consultations. Many participants reported that the ideal time to access the tool is prior to their scheduled visit (42.4%). Incorporating the decision tool prior to patient appointments is particularly effective as it allows patients ample time to review and digest the information, fostering a more informed health care experience. This strategy not only prepares patients to engage more actively in their care discussions but also enhances their understanding and satisfaction with the health care services provided. By strategically timing the introduction of decision tools to match these key interaction points within the health care system, institutions can maximize the benefits of these tools, supporting both clinical efficiency and patient outcomes.
Limitations
There were several limitations to this study that should be considered. First, we used a nonprobability sample, so the findings are not necessarily generalizable to the entire target population (i.e., unvaccinated adults aged 27 to 45 y in the United States). The use of an online panel likely introduces sampling biases, particularly around the comfort and acceptability of using Web-based tools for research purposes. Second, while we sought to strengthen internal validity through a randomized Solomon design, all outcomes were assessed with an immediate posttest; thus, we cannot determine how these effects were sustained over time. Future research should explore longer-term effects in a clinical and community environment.
Conclusions
The HPV DECIDE tool demonstrated efficacy in reducing decisional conflict and improving intermediate outcomes important in making informed decisions about mid-adult HPV vaccination. Therefore, this tool may be used to implement the ACIP shared clinical decision-making recommendation for mid-adult HPV vaccination. The tool is acceptable and rated highly by the priority population. Our findings suggest that the tool can be implemented to promote shared clinical decision making.
The authors declared the following potential conflicts of interest with respect to the research, authorship, and/or publication of this article: ELT has served as a consultant and speaker for Merck, Inc. for HPV vaccination work unrelated to this project. Unrelated to this project, GDZ has served as a consultant to Merck, Inc. for HPV vaccination work and has served on external advisory boards for Moderna and Pfizer. In addition, he has received investigator-initiated funding from Merck administered through Indiana University. The present study is funded from investigator-initiated funding from Merck and administered through the University of North Texas Health Science Center. The authors disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This project was supported by the Merck Investigator Studies Program administered through the University of North Texas Health Science Center. The funding agreement ensured the authors’ independence in designing the study, interpreting the data, writing, and publishing the report.
Ethical Considerations This study was approved by the North Texas Regional Institutional Review Board (IRB 2022-018). The study was registered with clinicaltrials.gov (NCT06435338).
Consent to Participate The North Texas Regional Institutional Review Board waived the requirement of documentation of consent as this would be the only identifier. Participants reviewed a written consent form and indicated consent by proceeding with the study.
Consent for Publication The North Texas Regional Institutional Review Board waived the requirement of documentation of consent as this would be the only identifier. Participants reviewed a written consent form and indicated consent by proceeding with the study.
Trial Registration The study was registered at NCT06435338.
Data Availability Deidentified data and data analytic code may be available upon reasonable request to the corresponding author.
Erika L. Thompson
https://orcid.org/0000-0002-7115-0001
References
- 1. Viens LJ, Henley SJ, Watson M, et al. Human papillomavirus–associated cancers—United States, 2008–2012. MMWR Morb Mortal Wkly Rep. 2016;65(26):661–6. Available from: https://www.jstor.org/stable/24858159 [Accessed 25 May, 2024].
- 2. Markowitz LE, Dunne EF, Saraiya M, et al. Human papillomavirus vaccination. MMWR Recomm Rep [Internet]. 2014;63(5):1–30. Available from: https://www.jstor.org/stable/24832595 [Accessed 25 May, 2024].
- 3. Meites E, Szilagyi PG, Chesson HW, Unger ER, Romero JR, Markowitz LE. Human papillomavirus vaccination for adults: updated recommendations of the Advisory Committee on Immunization Practices. MMWR Recomm Rep. 2019;68(32):698–702. DOI:
- 4. Guo Y, Bowling J. Human papillomavirus (HPV) vaccination initiation and completion among adult males in the United States. J Am Board Fam Med. 2020;33(4):592–9. DOI:
- 5. Yoo W, Koskan A, Scotch M, Pottinger H, Huh WK, Helitzer D. Patterns and disparities in human papillomavirus (HPV) vaccine uptake for young female adolescents among U.S. States: NIS-Teen (2008–2016). Cancer Epidemiol Biomarkers Prev. 2020;29(7):1458–67. DOI:
- 6. Newman PA, Logie CH, Lacombe-Duncan A, et al. Parents’ uptake of human papillomavirus vaccines for their children: a systematic review and meta-analysis of observational studies. BMJ Open. 2018;8(4):e019206. DOI:
- 7. US Food and Drug Administration. FDA approves expanded use of Gardasil 9 to include individuals 27 through 45 years old. FDA. Available from: https://www.fda.gov/news-events/press-announcements/fda-approves-expanded-use-gardasil-9-include-individuals-27-through-45-years-old [Accessed 28 May, 2024].
- 8. Wheeler CM, Skinner SR, Del Rosario-Raymundo MR, et al. Efficacy, safety, and immunogenicity of the human papillomavirus 16/18 AS04-adjuvanted vaccine in women older than 25 years: 7-year follow-up of the phase 3, double-blind, randomised controlled VIVIANE study. Lancet Infect Dis. 2016;16(10):1154–68. DOI:
- 9. Giuliano AR, Isaacs-Soriano K, Torres BN, et al. Immunogenicity and safety of Gardasil among mid-adult aged men (27–45 years)—the MAM study. Vaccine. 2015;33(42):5640–6. DOI:
- 10. Gidengil CA, Parker AM, Markowitz LE, et al. Health care provider knowledge around shared clinical decision-making regarding HPV vaccination of adults aged 27–45 years in the United States. Vaccine. 2023;41(16):2650–5. Available from: https://pubmed.ncbi.nlm.nih.gov/36990828/ [Accessed 25 May, 2024].
- 11. Hurley LP, O’Leary ST, Markowitz LE, et al. US primary care physicians’ viewpoints on HPV vaccination for adults 27 to 45 years. J Am Board Fam Med. 2021;34(1):162–70. Available from: https://pubmed.ncbi.nlm.nih.gov/33452094/ [Accessed 25 May, 2024].
- 12. Thompson EL, Wheldon CW, Rosen BL, Maness SB, Kasting ML, Massey PM. Awareness and knowledge of HPV and HPV vaccination among adults ages 27–45 years. Vaccine. 2020;38(15):3143–8. DOI:
- 13. Wheldon CW, Garg A, Galvin AM, Moore JD, Thompson EL. Decision support needs for shared clinical decision-making regarding HPV vaccination among adults 27–45 years of age. Patient Educ Couns. 2021;104(12):3079–85. Available from: https://pubmed.ncbi.nlm.nih.gov/33980398/ [Accessed 25 May, 2024].
- 14. Highet M, Jessiman-Perreault G, Hilton E, Law G, Allen-Scott L. Understanding the decision to immunize: insights into the information needs and priorities of people who have utilized an online human papillomavirus (HPV) vaccine decision aid tool. Can J Public Health. 2021;112(2):191–8. DOI:
- 15. Ottawa Hospital Research Institute. Decision aid summary. Ottawa (Canada): Ottawa Hospital Research Institute. Available from: https://decisionaid.ohri.ca/AZsumm.php?ID=2087 [Accessed 3 October, 2024].
- 16. Ottawa Hospital Research Institute. Ottawa decision support framework. Ottawa (Canada): Ottawa Hospital Research Institute. Available from: https://decisionaid.ohri.ca/odsf.html [Accessed 25 May, 2024].
- 17. Wheldon CW, Grace J, Zimet G, Daley EM, Akpan IN, Alkhatib SA, Thompson EL. Development and evaluation of a decision aid for HPV vaccination among adults aged 27–45 years old in the United States. Computers in Biology and Medicine. 2025 Feb 1;185:109557.
- 18. Centiment. Research services. Available from: https://www.centiment.co/research-services [Accessed 25 May, 2024].
- 19. Centers for Disease Control and Prevention. Vaccines & immunizations: HPV (Human Papillomavirus) VIS. US Department of Health & Human Services. Available from: https://www.cdc.gov/vaccines/hcp/vis/vis-statements/hpv.html [Accessed 3 October, 2024].
- 20. Ottawa Hospital Research Institute. User manuals for decisional conflict. Ottawa (Canada): Ottawa Hospital Research Institute. Available from: https://decisionaid.ohri.ca/docs/develop/User_Manuals/UM_decisional_conflict.pdf [Accessed 25 May, 2024].
- 21. Stacey D, Légaré F, Lewis K, et al. Decision aids for people facing health treatment or screening decisions. Cochrane Database Syst Rev. 2017;4(4):CD001431. DOI:
- 22. Thompson EL, Garg A, Galvin AM, Moore JD, Kasting ML, Wheldon CW. Correlates of HPV vaccination intentions among adults ages 27–45 years old in the U.S. J Community Health. 2021;46(5):893–2. Available from: https://pubmed.ncbi.nlm.nih.gov/33586085/ [Accessed 25 May, 2024].
- 23. Garg A, Wheldon CW, Galvin AM, Moore JD, Griner SB, Thompson EL. The development and psychometric evaluation of the mid-adult human papillomavirus vaccine knowledge scale in the United States. Sex Transm Dis. 2022;49(6):423–8. Available from: https://journals.lww.com/stdjournal/fulltext/2022/06000/the_development_and_psychometric_evaluation_of_the.5.aspx [Accessed 25 May, 2024].
- 24. O'Connor AM. User Manual - Decision Self-Efficacy Scale [document on the Internet]. Ottawa: Ottawa Hospital Research Institute; 1995 [modified 2002; cited 2024 12 16] 4p. Available from: https://decisionaid.ohri.ca/docs/develop/User_Manuals/UM_Decision_SelfEfficacy.pdf.
- 25. O'Connor AM, Cranney A. User Manual - Acceptability [document on the Internet]. Ottawa: Ottawa Hospital Research Institute; 1996 [modified 2002; cited 2024 12 16] 4p. Available from: https://decisionaid.ohri.ca/docs/develop/User_Manuals/UM_Acceptability.pdf.
- 26. Braver MW, Braver SL. Statistical treatment of the Solomon four-group design: a meta-analytic approach. Psychol Bull. 1988;104(1):150–4. DOI:
- 27. Campbell DT, Stanley JC, Gage NL. Experimental and Quasi-Experimental Designs for Research. Boston (MA): Houghton Mifflin and Company; 1963.
- 28. Borenstein M, Hedges LV, Higgins JP, Rothstein HR. A basic introduction to fixed-effect and random-effects models for meta-analysis. Res Synth Methods. 2010;1(2):97–11.
- 29. Hoefel L, Lewis KB, O’Connor A, Stacey D. 20th anniversary update of the Ottawa Decision Support Framework: part 2 subanalysis of a systematic review of patient decision AIDS. Med Decis Making. 2020;40(4):522–39. DOI:
- 30. Bruel S, Leclercq T, Ginzarly M, Botelho-Nevers E, Frappé P, Gagneux-Brunon A. Patient decision aid in vaccination: a systematic review of the literature. Expert Rev Vaccines. 2020;19(4):305–11. DOI:
- 31. Jolles MP, Richmond J, Thomas KC. Minority patient preferences, barriers, and facilitators for shared decision-making with health care providers in the USA: a systematic review. Patient Educ Couns. 2019;102(7):1251–62. Available from: https://www.sciencedirect.com/science/article/abs/pii/S0738399118306128 [Accessed 25 May, 2024].
- 32. Heponiemi T, Kaihlanen AM, Kouvonen A, Leemann L, Taipale S, Gluschkoff K. The role of age and digital competence on the use of online health and social care services: a cross-sectional population-based survey. Digital Health. 2022;8:205520762210744. DOI:
- 33. Graham TA, Ali S, Avdagovska M, Ballermann M. Effects of a web-based patient portal on patient satisfaction and missed appointment rates: survey study. J Med Internet Res. 2020;22(5):e17955. Available from: https://pubmed.ncbi.nlm.nih.gov/32427109/ [Accessed 25 May, 2024].
- 34. Chen Z, Liang N, Zhang H, et al. Harnessing the power of clinical decision support systems: challenges and opportunities. Open Heart. 2023;10(2):e002432. Available from: https://openheart.bmj.com/content/10/2/e002432 [Accessed 25 May, 2024].
- 35. Asmar MLE, Dharmayat KI, Vallejo-Vaz AJ, Irwin R, Mastellos N. Effect of computerised, knowledge-based, clinical decision support systems on patient-reported and clinical outcomes of patients with chronic disease managed in primary care settings: a systematic review. BMJ Open. 2021;11(12):e054659. Available from: https://bmjopen.bmj.com/content/11/12/e054659 [Accessed 25 May, 2024].