INTRODUCTION
Beginning in October 2001, the War on Terror in Iraq and Afghanistan is the longest war in U.S. history. As of 2019, more than 2.4 million active duty personnel who served in Post-9/11 combat operations became eligible for health care through the Department of Veterans Affairs (VA) (personal communication, Lauren Klepac).
Due to the frequent use of improvised explosive devices in Iraq and Afghanistan, a high proportion of combat casualties sustained polytrauma, with injuries to the extremities being predominant. As the case fatality rate of severe combat trauma declined due to advancements in combat casualty care and the implementation of a Joint Trauma System, there was also a rise in the rate of infectious complications. Although infections following deployment-related trauma typically develop in the immediate period (days to weeks) following the injury, they may also occur months to years following hospital discharge, resulting in rehospitalization, additional surgical procedures, and/or use of prolonged antibiotics. In particular, severe extremity trauma (e.g., traumatic amputations and open fractures) carries a high risk of both early onset and delayed infections. In one analysis, patients with severe lower extremity trauma who required a late leg amputation (>90 days post-injury) were more than three times more likely to have an infection and two times more likely to develop osteomyelitis prior to the amputation compared to patients with limb salvage. Among the patients with a late leg amputation, the proportion of osteomyelitis remained high in the years following injury (∼42% in Year 1, ∼30% in Year 2, and ∼10% in Year 3). Furthermore, nonunion fractures were also common in the first two years post-injury (∼30% in Year 1 and ∼25% in Year 2). For discussion of extremity wound infection research through the Infectious Disease Clinical Research Program (IDCRP), see Petfield et al. within this supplement.
Long-term physical and mental health may also be impacted by deployment-related trauma and the subsequent infectious complications. Among patients with upper extremity trauma (i.e., above elbow amputation, below elbow amputation, or severe injury), 41%–53% had a psychological diagnosis five years post-injury with post-traumatic stress disorder being the most frequent diagnosis (23%–36% of patients). Patients with late leg amputations were also more likely to have a mood or adjustment disorder during follow-up.
To fully understand the impact of deployment-related trauma on clinical outcomes, as well as physical, mental, and social health well-being, long-term follow-up with wounded warriors after their initial period of hospitalization is crucial. A difficulty in assessing long-term impacts of events occurring during military deployment is that health care data from two different government agencies and health care systems, the Department of Defense (DoD) and VA, must be linked at the individual level. Thus, collaborations between the VA and the DoD are essential to collect data needed for comprehensive analyses to improve the health of military personnel and veterans.
Since the War on Terror began, multiple collaborative projects or centers have been formed to address questions related to the long-term outcomes of battlefield trauma (Table I). In particular, the Extremity Trauma and Amputation Center of Excellence (EACE) was mandated by Congress in 2009 to enhance collaborative relationships and partnerships not only between the DoD and VA but also with academic institutions and appropriate public and private entities. Through the EACE, numerous studies comprehensively examining long-term outcomes (e.g., mental health, pain, osteoarthritis, and other health outcomes) following combat-related extremity amputations or serious extremity injuries have been funded (https://www.health.mil/About-MHS/OASDHA/HSPO/EACE). Additional collaborative centers and projects have focused on specific injury patterns, such as traumatic brain injury, ocular injuries, vascular injuries, and genitourinary trauma (Table I), providing valuable information to advance clinical care in these areas.
While collaborations between the DoD and VA offer many advantages (e.g., larger populations and increased follow-up), there are also challenges that may limit a successful partnership, including the need for additional agreements, training, and Institutional Review Board (IRB) approval. In the 2013 VA/DoD Collaboration Guidebook for Healthcare Research, the IDCRP DoD-VA multicenter observational Trauma Infectious Disease Outcomes Study (TIDOS) was used as an example of a successful research collaboration between the DoD and the VA St. Louis Health Care System. Herein, we provide a summary of the development, methodology, and status of the IDCRP DoD-VA TIDOS collaborative research efforts related to long-term clinical outcomes of battlefield trauma.
TIDOS DOD-VA COLLABORATIVE METHODS
In brief, TIDOS is a multicenter, observational study designed to evaluate the short- and long-term infectious outcomes among military personnel with deployment-related trauma., The protocol received approval in April 2009 from the centralized IRB at the Uniformed Services University of the Health Sciences (USU) and the protocol was initiated on June 1, 2009 (Fig. 1). Service members were eligible for inclusion in TIDOS if they were over 18 years of age, active duty personnel or DoD beneficiaries, and sustained a deployment-related injuring requiring medical evacuation to Landstuhl Regional Medical Center in Germany before being transferred to one of the participating military hospitals in the United States. The participating U.S. military hospitals were Walter Reed National Military Medical Center in the National Capital Region (Walter Reed Army Medical Center and National Naval Medical Center prior to September 2011) and Brooke Army Medical Center in San Antonio, TX. Prior to discharge from the U.S. military hospital, all patients eligible for TIDOS were given the opportunity to enroll in a longitudinal follow-up cohort by consenting to the collection of infection-related information from DoD medical records after they were discharged from the hospital (enrollment closed on January 31, 2015).
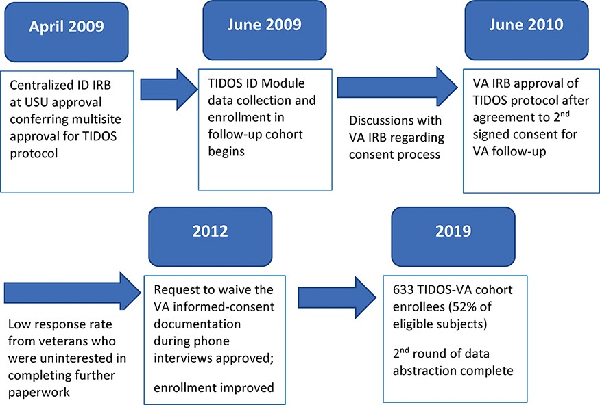
FIGURE 1
Timeline of events leading to approval of collaboration with the Department of Veterans Affairs (VA) for the Trauma Infectious Disease Outcomes Study (TIDOS) protocol. ID—infectious disease; IRB—Institutional Review Board; USU—Uniformed Services University of the Health Sciences.
Basic demographics and information on injury characteristics, trauma history, and early casualty care were collected through the DoD Trauma Registry for all patients admitted to Landstuhl Regional Medical Center during the study period (June 1, 2009–December 31, 2014). Infection-related data (e.g., syndromes, microbiology, and antibiotic use) from the initial hospitalization were obtained from all patients admitted to participating U.S. military hospitals through the TIDOS Infectious Disease Module designed to supplement the DoD Trauma Registry (see Tribble et al. within this supplement for discussion of the Infectious Disease Module)., Follow-up data for TIDOS cohort enrollees were collected from telephonic interviews at predefined intervals after discharge from the hospital and abstraction of DoD electronic medical records.,
At this point, telephone interview was the only follow-up mechanism after a subject left DoD health care, limiting the effective follow-up duration of the cohort. To allow more comprehensive follow-up for enrollees who left active duty status, a collaboration with VA investigators at the VA St. Louis Health Care System was established. Through this collaboration, TIDOS subjects enrolled in VA health care and consented to follow-up through the VA (referred to as the TIDOS-VA cohort) could then be followed longitudinally through VA health records, and relevant data could be abstracted and incorporated into TIDOS databases.
Although all IDCRP clinical sites had signed an agreement permitting institutional approval to be granted through a single review by the centralized USU IRB for IDCRP-related protocols, the TIDOS protocol needed to undergo additional scientific review by a VA IRB. Initially, it was proposed to allow the DoD TIDOS cohort enrollment to confer consent for data collection through the VA; however, the St. Louis VA IRB required a second, VA-specific informed consent to allow follow-up through the VA. Following an amendment to the TIDOS protocol to include a second consent process for veterans, the VA IRB approved the protocol in June 2010 (Fig. 1). A Data Use Agreement between the Veterans Health Administration, VA St. Louis Health Care System and USU was enacted.
Patients who had enrolled in the TIDOS follow-up cohort and received VA health care were identified by the VA investigative team through administrative databases. These subjects were contacted regarding enrollment in the TIDOS-VA cohort. The process of mailing documentation for the second informed consent, coordinating a follow-up telephone call, administering witnessed informed consent by telephone, and receiving back a completed enrollment packet signed by the subject and witness was piloted and found to be impractical due to a very low completion rate. As a result, the VA investigative team returned to the IRB and requested to waive the requirement for written informed-consent documentation, asking instead for a process of telephonic consent by an approved script, and not requiring a witness. This process was approved by the VA IRB in 2012. Following approval of the waiver, the rate of enrollment substantially increased.
Data abstraction from the VA medical records was done using CAPRI, the Compensation and Pension Records Interchange. The CAPRI is a tool that allows all VA clinical records from across all VA inpatient and outpatient facilities to be viewed through a single interface. It includes all narrative notes, labs, imaging, and pathology. In addition, narrative notes from each VA site can be searched by textword, allowing the standardization of searches of the medical record. Data abstractors at the St. Louis VA searched medical records of TIDOS-VA enrollees using standardized data abstraction protocols, and when infections related to prior combat injury were found, relevant data and covariates were abstracted into electronic abstraction forms in an encrypted interface, which incorporated the abstracted data directly into TIDOS databases housed within the DoD. The abstraction protocols used at the VA were designed to be as similar as possible to the DoD data abstraction protocols used by the DoD abstractors. This approach to data collection through CAPRI provided more detailed information than what could be collected through DoD follow-up using telephone interviews (Table II).
FINDINGS FROM TIDOS DOD-VA COLLABORATIVE STUDIES
TIDOS-VA Cohort Study
Among 1,336 TIDOS enrollees (excluding 23 withdrawals), 1,221 (91%) have received VA health care. All subjects who had received VA health care were contacted by telephone for enrollment in the TIDOS-VA cohort. Eighty-three declined enrollment, 481 were deferred or were unreachable, and 633 (52% of 1,221) enrolled in the TIDOS-VA cohort. In an analysis of data collected from the first 337 TIDOS-VA cohort enrollees, 127 (38%) enrollees developed a new trauma-related infection during the follow-up period. Overall, there were 239 unique trauma-related infections during follow-up with 170 (71%) infections identified through DoD sources and 69 (29%) infections identified through VA electronic medical records. Skin and soft-tissue infections (SSTIs) were predominant (68% of infections), followed by osteomyelitis (13%) and urinary tract infections (7%). The most common organisms associated with the SSTIs were Staphylococcus aureus (42% of organisms recovered), coagulase-negative staphylococci (9%), Pseudomonas aeruginosa (5%), and Streptococcus spp. (5%), while it was S. aureus (29% of organisms recovered), coagulase-negative staphylococci (19%), Escherichia coli (11%), and Enterobacter cloacae (7%) for osteomyelitis. Among the urinary tract infections, P. aeruginosa (21% of organisms recovered), E. coli (21%), and Klebsiella pneumoniae (14%) were most frequent. The time from hospital discharge to diagnosis of a new infection ranged from a median of 104 days for SSTIs to 54 days for osteomyelitis to 69 days for urinary tract infections (Fig. 2).
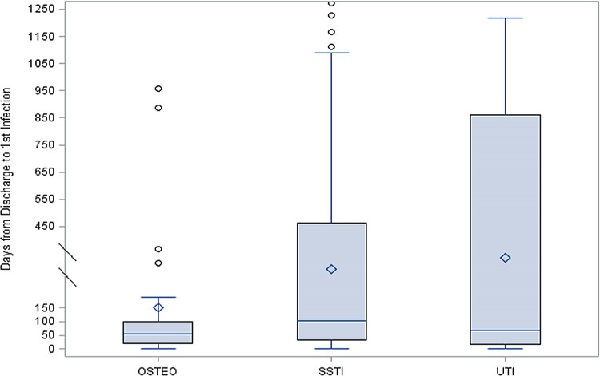
FIGURE 2
Time to new infection related to trauma injury following initial trauma hospital discharge. Figure is reprinted with permission of Oxford Academic Press
A reduced time to development of a new infection during follow-up was associated with severity of initial trauma based on injury severity score (ISS). Specifically, moderate injuries (ISS of 10–15) had a hazard ratio (HR) of 2.72 (95% confidence interval [CI]: 1.45–5.22), while severe injuries (ISS of 16–25) had a HR of 2.65 (95% CI: 1.45–4.84) and critical injuries (ISS ≥26) had a HR of 2.40 (95% CI: 1.24–4.62). Having at least one infection during the initial hospitalization was also an independent predictor of a shorter duration to the development of an infection during follow-up (HR: 1.81; 95% CI 1.16–2.82 for 1–2 inpatient infections and HR: 2.66; 95% CI: 1.50–4.73 for ≥3 inpatient infections). In Kaplan–Meier survival plots, ISS, volume of blood transfused within 24 hours of injury, duration of initial hospitalization, and number of infections during initial hospitalization were associated with risk of developing a new trauma-related infection during follow-up with the highest likelihood being within the first year after hospital discharge (Fig. 3). These findings confirm that incident infections related to trauma sustained while deployed may continue well past the initial hospitalization. Furthermore, as 29% of the infections were only identified through VA electronic medical records, it is clear that collaboration with the VA is critical to fully understanding the long-term impact of deployment-related trauma. Presently, data from the full population of 633 veterans are being assessed.
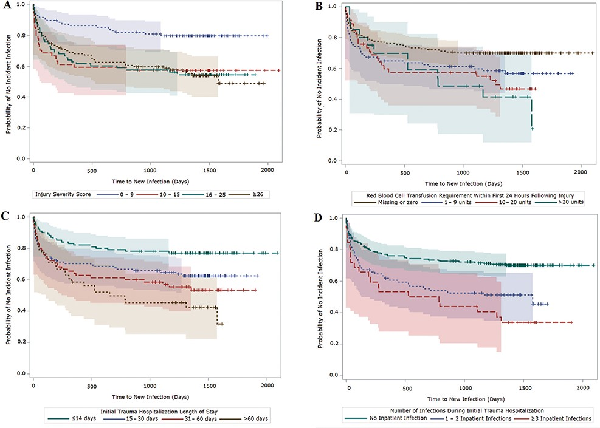
FIGURE 3
Kaplan–Meier survival plots (with 95% Hall–Wellner bands) of time to new infection following initial trauma hospital discharge. A, Plot stratified by injury severity score. Log-rank χ2, 16.8 (P < 0.001); Wilcoxon χ2, 16.2 (P = 0.001). B, Plot stratified by volume of blood transfusion within 24 hours of injury. Log-rank χ2, 11.2 (P = 0.011); Wilcoxon χ2, 7.4 (P = 0.060). C, Plot stratified by length of inpatient hospitalization. Log-rank χ2, 18.7 (P < 0.001); Wilcoxon χ2, 15.7 (P = 0.001). D, Plot stratified by number of inpatient infections. Log-rank χ2, 21.8 (P < 0.001); Wilcoxon χ2, 18.2 (P < 0.001). Figure is reprinted with permission of Oxford Academic Press.
Genitourinary Trauma and Urinary Complications
In another TIDOS-VA cohort analysis, 89 enrollees who had experienced genitourinary trauma were assessed for intermediate and long-term urological complications, including urinary tract infections. Twenty-one percent of enrollees had a urinary tract infection, of which approximately 25% were diagnosed during the initial hospitalization and 75% were identified during follow-up (28% through DoD sources and 48% through VA sources). Having a soft-tissue infection at the pelvis/hip (odds ratio [OR]: 28.11; 95% CI: 3.45–228.67), trauma involving the urinary tract (OR: 27.97; 95% CI: 2.04–383.19), and transtibial amputations (OR: 6.96; 95% CI: 1.22–39.73) were identified as independent risk factors for the development of urinary tract infections. Analyses to examine additional injury patterns (e.g., penetrating central nervous system injuries) are being developed.
DISCUSSION AND FUTURE DIRECTIONS
Information on the long-term infection burden following trauma is critical for improving the health of combat casualties. As more wounded warriors leave military service, partnerships between DoD and VA investigators are critical to conducting comprehensive analyses of the long-term impact of deployment-related trauma and identifying predictors of infectious complications. In addition to TIDOS, another IDCRP protocol is the Trauma-Associated Osteomyelitis study, which evaluates the characteristics of patients with open fractures to identify risk factors for development of osteomyelitis (incident infection and recurrence). Wounded warriors were eligible for inclusion in the study population if they sustained a combat-related open fracture of the tibia, femur, or upper extremity (humerus or radius/ulna) between March 2003 and December 2009. Through collaboration with the VA St. Louis Health Care System, there has been long-term follow-up (10–15 years) for 532 patients in the study who left military service and sought care through the VA. Analysis of the follow-up data is currently underway.
Information on the social, physical, and mental health well-being has also been collected from patients receiving VA health care, including diagnoses of depression and post-traumatic stress disorder, alcohol use, tobacco use, opioid use, relationship status, living situation, employment status, and quality of life. These data will be examined to determine the impact of infections on the long-term quality of life (and vice versa). Evaluation of health care utilization data of veterans with trauma-related is also planned.
These projects would not have been possible without close collaboration between DoD and VA personnel. Over the course of these efforts, we have learned many important lessons, which may be useful to others. First, the identification of ideal collaborators can be difficult, and is critically important to the success of similar projects. Ongoing networking between researchers in both agencies is crucial to establish relationships that will result in fruitful research. Second, careful attention must be paid to ensure that variable definitions are as similar as possible for DoD and VA data collection. Abstraction of data from one agency must mirror as closely as possible the abstraction at the other agency, despite differences in health record systems. Third, careful attention must be paid to establish mechanisms for the exchange of research funds between DoD and VA, as well as mechanisms for the secure transfer of data between agencies, both of which are achievable through collaboration with research offices and legal advisors. Data Use Agreements should be carefully written and vetted by the appropriate legal authorities at each agency. Finally, careful and thoughtful early discussion with the respective agency human subjects protection programs is critical to assure that subject protections are met while also avoiding unnecessary burden on subjects and regulatory redundancies that limit effective execution of joint DoD-VA research. Our experience with this inter-agency collaboration has been productive and enlightening, and we encourage others to explore opportunities in this area.
ACKNOWLEDGMENTS
We are indebted to the Infectious Disease Clinical Research Program Trauma Infectious Disease Outcomes Study (TIDOS) study team of nurses, investigators spanning multiple disciplines, clinical coordinators, microbiology technicians, data managers, clinical site managers, and administrative support personnel for their tireless hours to ensure the success of this project. Special thanks to Leigh Carson for her assistance in manuscript preparation.
REFERENCES
- 1.
- 2. Belmont PJ, Owens BD, Schoenfeld AJ: Musculoskeletal injuries in Iraq and Afghanistan: epidemiology and outcomes following a decade of war. J Am Acad Orthop Surg 2016; 24(6): 341–8.
- 3. Krueger CA, Wenke JC, Ficke JR: Ten years at war: comprehensive analysis of amputation trends. J Trauma Acute Care Surg 2012; 73(6 Suppl 5): S438–44.
- 4. Ficke JR, Eastridge BJ, Butler F, et al: Dismounted complex blast injury report of the Army Dismounted Complex Blast Injury Task Force. J Trauma Acute Care Surg 2012; 73(6 Suppl 5): S520–34.
- 5. Nessen SC, Gurney J, Rasmussen TE, et al: Unrealized potential of the US Military battlefield trauma system: DOW rate is higher in Iraq and Afghanistan than in Vietnam, but CFR and KIA rate are lower. J Trauma Acute Care Surg 2018; 85(1S): S4–12.
- 6. Tribble DR, Conger NG, Fraser S, et al: Infection-associated clinical outcomes in hospitalized medical evacuees after traumatic injury: Trauma Infectious Disease Outcome Study. J Trauma 2011; 71(Suppl 1): S33–42.
- 7. Murray CK, Hinkle MK, Yun HC: History of infections associated with combat-related injuries. J Trauma 2008; 64(Suppl 3): S221–31.
- 8. Blyth DM, Yun HC, Tribble DR, Murray CK: Lessons of war: combat-related injury infections during the Vietnam War and Operation Iraqi and Enduring Freedom. J Trauma Acute Care Surg 2015; 79(4 Suppl 2): S227–35. Proceedings of 2014 Military Health System Research Symposium.
- 9. Melcer T, Walker J, Franklin Sechriest V 2nd, et al: A retrospective comparison of five-year health outcomes following upper limb amputation and serious upper limb injury in the Iraq and Afghanistan conflicts. PM R 2019; 11(6): 577–89.
- 10. Melcer T, Walker J, Bhatnagar V, Richard E, Sechriest VF 2nd, Galarneau M: A comparison of four-year health outcomes following combat amputation and limb salvage. PLoS One 2017; 12(1): e0170569.
- 11. Yun HC, Branstetter JG, Murray CK: Osteomyelitis in military personnel wounded in Iraq and Afghanistan. J Trauma 2008; 64(Suppl 2): S163–8.
- 12. Casey K, Demers P, Deben S, Nelles ME, Weiss JS: Outcomes after long-term follow-up for combat-related extremity injuries in a multidisciplinary limb salvage clinic. Ann Vasc Surg 2015; 29(3): 496–501.
- 13. Petfield JL, Lewandowski LR, Stewart L, Murray CK, Tribble DR: IDCRP combat-related extremity wound infection research. Mil Med 2022; 187(Suppl 2): 25–33.
- 14.
- 15. Tribble DR, Murray CK, Lloyd BA, et al: After the battlefield: infectious complications among wounded warriors in the Trauma Infectious Disease Outcomes Study. Mil Med 2019; 184(Suppl 2): 18–25.
- 16. Tribble DR, Spott MA, Shackleford SA, Murray CK: Department of Defense Trauma Registry Infectious Disease Module impact on clinical practice. Mil Med 2022; 187(Suppl 2): 7–16.
- 17. Tribble DR, Krauss M, Murray CK, et al: Epidemiology of trauma-related infections among a combat casualty cohort after initial hospitalization: the Trauma Infectious Disease Outcomes Study. Surg Infect (Larchmt) 2018; 19(5): 494–503.
- 18. McDonald JR, Liang SY, Li P, et al: Infectious complications after deployment trauma: following wounded United States military personnel into Veterans Affairs care. Clin Infect Dis 2018; 67(8): 1205–12.
- 19. Liang SY, Jackson B, Kuhn J, et al: Urinary tract infections after combat-related genitourinary trauma. Surg Infect (Larchmt) 2019; 20(8): 611–8.
- 20. Tribble DR, Lewandowski LR, Potter BK, et al: Osteomyelitis risk factors related to combat trauma open tibia fractures: a case-control analysis. J Orthop Trauma 2018; 32(9): e344–53.
- 21. Ettenhofer ML, Remigio-Baker RA, Bailie JM, Cole WR, Gregory E: Best practices for progressive return to activity after concussion: lessons learned from a prospective study of U.S. military service members. Neurotrauma Rep 2020; 1(1): 137–45.
- 22. Flanagan G, Velez T, Gu W, Singman E: The relationship between severe visual acuity loss, traumatic brain injuries, and ocular injuries in American service members From 2001 to 2015. Mil Med 2020; 185(9-10): e1576–83.
- 23. Mahoney EJ, Silva MA, Reljic T, et al: Rehabilitation needs at 5 years post-traumatic brain injury: a VA TBI Model Systems Study. J Head Trauma Rehabil 2021; 36(3): 175–85.
- 24. Whyte J, Giacino JT, Heinemann AW, et al: Brain injury functional outcome measure (BI-FOM): a single instrument capturing the range of recovery in moderate-severe traumatic brain injury. Arch Phys Med Rehabil 2021; 102(1): 87–96.
- 25. Apostolova E, White HA, Morris PA, Eliason DA, Velez T: Open globe injury patient identification in warfare clinical notes. AMIA Annu Symp Proc 2017; 2017: 403–10.
- 26. Bhatnagar V, Richard E, Melcer T, Walker J, Galarneau M: Retrospective study of cardiovascular disease risk factors among a cohort of combat veterans with lower limb amputation. Vasc Health Risk Manag 2019; 15: 409–18.
- 27. Rhon DI, Perez KG, Eskridge SL: Risk of post-traumatic knee osteoarthritis after knee injury in military service members. Musculoskeletal Care 2019; 17(1): 113–9.
- 28. Dismuke-Greer CE, Gebregziabher M, Byers AL, et al: Comorbid TBI-depression costs in veterans: a chronic effect of neurotrauma consortium (CENC) study. Brain Inj 2019; 33(2): 198–204.
- 29. Eggleston B, Dismuke-Greer CE, Pogoda TK, et al: A prediction model of military combat and training exposures on VA service-connected disability: a CENC study. Brain Inj 2019; 33(13–14): 1602–14.
- 30. Guedes VA, Kenney K, Shahim P, et al: Exosomal neurofilament light: a prognostic biomarker for remote symptoms after mild traumatic brain injury? Neurology 2020; 94(23): e2412–23.
- 31. Rowland JA, Martindale SL, Spengler KM, Shura RD, Taber KH: Sequelae of blast events in Iraq and Afghanistan War veterans using the Salisbury Blast Interview: a CENC study. Brain Inj 2020; 34(5): 642–52.
- 32. Walker WC, Hirsch S, Carne W, et al: Chronic Effects of Neurotrauma Consortium (CENC) multicentre study interim analysis: differences between participants with positive versus negative mild TBI histories. Brain Inj 2018; 32(9): 1079–89.
- 33. Castillo RC, Carlini AR, Doukas WC, et al: Pain, depression, and PTSD following major extremity trauma among United States military serving in Iraq and Afghanistan: results from the METALS study. J Orthop Trauma 2021; 35(3): e96–e102.
- 34. Doukas WC, Hayda RA, Frisch HM, et al: The Military Extremity Trauma Amputation/Limb Salvage (METALS) study: outcomes of amputation versus limb salvage following major lower-extremity trauma. J Bone Joint Surg Am 2013; 95(2): 138–45.
- 35. Mitchell SL, Hayda R, Chen AT, et al: The Military Extremity Trauma Amputation/Limb Salvage (METALS) study: outcomes of amputation compared with limb salvage following major upper-extremity trauma. J Bone Joint Surg Am 2019; 101(16): 1470–8.
- 36. Janak JC, Orman JA, Soderdahl DW, Hudak SJ: Epidemiology of genitourinary injuries among male US service members deployed to Iraq and Afghanistan: early findings from the Trauma Outcomes and Urogenital Health (TOUGH) project. J Urol 2017; 197(2): 414–9.
- 37. Nnamani NS, Janak JC, Hudak SJ, et al: Genitourinary injuries and extremity amputation in Operations Enduring and Iraqi Freedom: early findings from the Trauma Outcomes and Urogenital Health (tough) project. J Trauma Acute Care Surg 2016; 81(5 Suppl 2): S95–9.
- 38. Nnamani NS, Pugh MJ, Amuan ME, et al: Outcomes of genitourinary injury in U.S. Iraq and Afghanistan War veterans receiving care from the Veterans Health Administration. Mil Med 2019; 184(3–4): e297–301.
- 39. Reed AM, Janak JC, Orman JA, Hudak SJ: Genitourinary injuries among female U.S. service members during Operation Iraqi Freedom and Operation Enduring Freedom: findings from the Trauma Outcomes and Urogenital Health (TOUGH) project. Mil Med 2018; 183(7–8): e304–9.
- 40. Shireman PK, Rasmussen TE, Jaramillo CA, Pugh MJ: VA Vascular Injury Study (VAVIS): VA-DoD extremity injury outcomes collaboration. BMC Surg 2015; 15(1): 13.


