INTRODUCTION
Emergence delirium (ED) is a condition characterized by psychomotor agitation, disorientation, confusion, and uncooperativeness that sometimes occurs in patients emerging from general anesthesia. Although most cases resolve quickly without sequelae, ED may result in physical injury to the patient or caregiver and contribute to future cognitive dysfunction.
Emergence delirium has a higher incidence in the pediatric population relative to the adult population; however, the incidence among adults can be surprisingly high, ranging between 2.1% and 22.2%.,, This wide incidence range among adults may be related to differing diagnostic measuring techniques as well as patient demographics. Many risk factors have been implicated, including age, gender, surgical case type and duration, anesthetic technique, medications, pain, and psychological comorbidities. Recently, combat exposure among U.S. military veterans has been identified as a risk factor for ED.,
Various medications have been investigated for the prevention or treatment of ED, including opioids, benzodiazepines, lidocaine, ketamine, tramadol, and dexmedetomidine. Dexmedetomidine is a specific α2-receptor agonist with unique properties advantageous in anesthesia practice because of its ability to produce anxiolysis, amnesia, analgesia, and sedation. Case studies reported that dexmedetomidine reduced ED in the perioperative period., Recent randomized-control trials reveal that dexmedetomidine reduces the incidence of ED in pediatric and elderly patients. Dexmedetomidine significantly reduces the duration of delerium in the elderly intensive care unit patients.
The purpose of this investigation was to determine if dexmedetomidine is an effective prophylactic treatment for ED among U.S. military combat veterans that present to surgery with high anxiety. The specific aims were (1) to utilize a standardized tool to quantify ED in a sample of combat veterans and (2) to compare intraoperative dexmedetomidine use to placebo in the reduction of ED incidence in the postoperative period. We hypothesized that combat veterans with high levels of anxiety treated with an intraoperative infusion of dexmedetomidine would experience a lower incidence of ED as compared to those who receive a placebo infusion.
METHODS
Design
This multi-center, prospective, observational, double-blind, randomized, placebo-controlled trial was funded by the Tri-Service Nursing Research Program Grant HU0001-14-TS05 (N14-PO3) and approved by the Institutional Review Boards at the Naval Medical Center San Diego, San Diego, CA, Womack Army Medical Center, Fort Bragg, NC, Walter Reed National Military Medical Center, Bethesda, MD, and the Uniformed Services University of the Health Sciences, Bethesda, MD. This manuscript adheres to the applicable CONSORT 2010 Statement and corresponding checklist for reporting randomized trials. The incidence of ED was measured using the Pediatric Anesthesia Emergence Delirium (PAED) Scale, an instrument validated for the evaluation of ED. The State-Trait Anxiety Inventory (STAI) was used to determine anxiety severity. Individuals deemed to have high anxiety (state ≥ 39) were selected to be in the experimental arm of the study to determine if intraoperative dexmedetomidine was an effective prophylactic treatment for ED when compared to a placebo.
Subjects were screened for possible inclusion during their preoperative anesthesia evaluation. Inclusion criteria included the following: English-speaking, U.S. military service member or veteran with a self-reported history of combat exposure defined as having fired a weapon and/or been fired upon in a combat theater of war since 2001, 18 years of age and older, and undergoing scheduled elective surgery requiring general anesthesia. Exclusion criteria included American Society of Anesthesiologists physical classification III or IV. We obtained signed written consent before the study enrollment, and all subjects received their surgical care in the main operating room (OR) at one of three military medical centers in the USA.
Measures
Demographic and clinical information was collected, and all participants were administered three questionnaires in a quiet, pre-anesthetic clinic setting: the Patient Health Questionnaire (PHQ-9) to assess depression, the PTSD Checklist—Military Version (PCL-M) to assess PTSD, and the STAI to assess both state and trait anxiety levels before surgery. The PHQ-9 is a validated instrument comprised of a nine-item questionnaire derived directly from the Diagnostic and Statistical Manual of Mental Disorders, 4th Edition (DSM-IV) to assess the signs and symptoms of major depression. Scores range from one to 27: 1-4 revealing minimal depression and 20-27 representing severe depression. Similarly, the PCL-M is a validated instrument designed to measure 17 symptoms of PTSD from the DSM-IV and has a long history of use among veterans with a history of physical and psychological injury. Possible scores range from 17 to 85. Although the PCL-M was not used in this study as a diagnostic tool, scores greater than 50 indicate a high likelihood of combat-related PTSD.
The STAI is a validated 40-question instrument widely used for the assessment of anxiety and is comprised of two parts: a state-anxiety score (may vary depending on the perioperative experience) and trait-anxiety (tendency to be stable). In each part, the score ranges from 20 (lowest anxiety possible) to 80 (highest anxiety possible). Previous research suggests that a STAI-State score ≥39 is associated with military individuals more likely to experience ED.
To assess ED, the PAED was used immediately following the emergence from general anesthesia in both the OR and the post-anesthesia care unit (PACU). Five behavioral profiles are subjectively scored to comprise the PAED Scale: makes eye contact with the caregiver, actions are purposeful, aware of his/her surroundings, degree of restlessness, and inconsolability. Each of the five behavioral descriptions are measured on a scale of 0 to 4, 0 = none and 4 = most. The scores are summed and represent the total PAED Scale score. The PAED Scale was content-validated for use in adults and has been reliably used to describe ED in the combat veteran population.
Protocol
After completion of all screening instruments, subjects with a STAI-State score <39 (n = 215) were placed in the observational arm of the study while subjects with a STAI-State score ≥39 were placed in the experimental arm (n = 153). Screening instruments were completed in the following order: PCL-M, PHQ-9, and STAI. A research pharmacist then randomly assigned patients according to a computer-generated table of randomization (STATA v13.0) to one of two groups: treatment group with a dexmedetomidine infusion (1 μg/kg bolus over 10 minutes at induction, followed by a 0.6 μg/kg/h infusion continued until approximately 15 minutes before the anticipated end of surgery) or a control group with placebo (normal saline infusion with similar volume). To ensure safe clinical practice, the anesthesia provider, who was not a member of the research team, was notified by the pharmacist as to the group assignment of each individual subject. The research pharmacist prepared and labeled dexmedetomidine and placebo infusions as “study drug.” The research pharmacist did not participate in data collection. The research subject, research ream, OR nurse, OR tech, operating surgeon, and PACU staff remained blinded. Otherwise, all participants received routine perioperative care. Decisions pertaining to monitoring and anesthesia were at the clinical discretion of the unblinded anesthesia provider.
Following emergence and extubation, all subjects were observed for signs of ED using the PAED Scale. The PAED Scale was utilized twice in the OR, once by a blinded study investigator and once by the OR nurse. Once the subject was transferred to the PACU and within the first 15 minutes, the PAED Scale was utilized twice, once by a blinded study investigator and once by the PACU nurse. Instrumentation was performed individually and not shared among others using the PAED Scale. All study investigators and nurses were trained in the use of the PAED Scale and blinded to the randomization group.
Data Analysis
Sample size determination was based on two previous reports that evaluated dexmedetomidine versus placebo in the treatment of ED using the PAED Scale., These reports agreed on an approximately 50% incidence of ED in the placebo group and 24% incidence in the dexmedetomidine group.,, A chi-square test was performed with a 5%, two-sided significance level having 80% power to detect a difference between dexmedetomidine and placebo groups resulting in a sample size of 53 per group in the experimental arm of the study. Accounting for 30% attrition, 152 subjects were required in the experimental arm of the study. Based on the prediction that 40% of combat veterans would present with high state-anxiety as revealed by a STAI-State score ≥39, the overall sample size was determined to be 380 subjects: 228 in the observational group and 152 in the randomized group.
Following completion of enrollment and data collection, the research pharmacist unmasked the random assignment log. All de-identified data were analyzed using R: A Language and Environment for Statistical Computing Version 3.3.2 (Vienna, Austria). Each subject’s final PAED Scale score was computed by selecting the larger of the average PAED Scale score from the OR and the average from the PACU (PAEDfinal = max{mean(PAEDOR), mean(PAEDPACU)}). Subjects without computable PAED Scale scores were omitted from descriptive and inferential analyses. Data from the experimental group were used for the analysis of variables as described below.
Descriptive statistics were used to analyze demographic and clinical data. The central tendency of each variable of interest was computed in terms of its median and interquartile interval (IQI) for ordinal variables and in terms of the proportion of observed events for dichotomous variables. Categorical data were compared using chi-square or two-tailed Fisher’s exact tests, as appropriate. Continuous numerical variables were compared with the two-sample bootstrapped Kolmogorov–Smirnov test. Although the Wilcoxon rank-sum test is often cited as the putative nonparametric analogue of the t-test, it is optimal only for continuous distributions and is inappropriate in the presence of ties, which is common when comparing discrete distributions like that of the PAED Scale score.P < .05 was accepted as statistically significant.
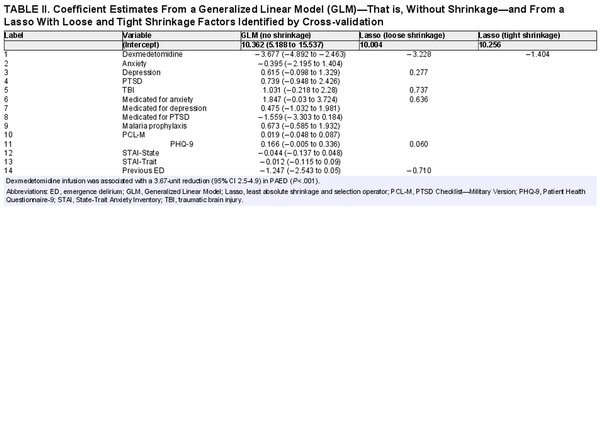
Two secondary inferential analyses were conducted: “variable importance” and “linear regression.” One ranked the importance of predictors of PAED score in terms of their contribution to the “gain” in accuracy of an ensemble machine learning model known as “eXtreme Gradient Boosting” (XGBoost), while the other measured the contribution of each predictor in terms of linear coefficient estimates by both classical linear regression and penalized linear regression using “least absolute shrinkage and selection operator”. Variable importance ranking was conducted with the XGBoost technique, which builds an ensemble of decision trees and then computes each variable’s overall contribution to the ensemble’s “gain” in accuracy., XGBoost hyperparameter tuning was conducted by bootstrap validation with an 80:20 split.
Linear regression was conducted within two frameworks: one was that of the classical linear model in which each variable features a coefficient estimate along with its 95% CI and P-value; the other was that of the penalized linear model in which the sum of the coefficients are constrained such that less important coefficients are shrunk to zero., For the penalized regression, the shrinkage parameter was optimized using cross-validation, from which two sets of penalized coefficients were computed: one in which the shrinkage parameter corresponded to the minimum cross-validated error (labeled “loose” in Table II), and a second in which the shrinkage parameter resulted in the error being 1 SD from the minimum cross-validated error (labeled “tight” in Table II).
RESULTS
Potential subjects were screened and recruited from pre-anesthesia clinics across three large military medical centers from October 2014 to December 2017. A total of 369 subjects were enrolled in the study: 215 in the observational group and 153 in the randomized group. Within the randomized group, 75 patients were assigned to the treatment group, 75 patients assigned to placebo, and 3 excluded. Subject recruitment ended secondary to reaching the minimum number of 53 subjects in each treatment group in the experimental arm. Thirty-two participants were excluded from data analyses. One subject did not receive a general anesthetic and the anesthesia provider for another subject did not follow the dexmedetomidine versus placebo protocol. Another 30 participants were excluded from data analyses based on one of three reasons: subject’s surgery was canceled and not rescheduled, subject did not arrive for surgery and never rescheduled, or subject had no computable PAED score (inability to attain one or more of the four PAED scores). Of the 32 excluded participants, 1 was excluded before completing the STAI, 3 were in the experimental group excluded before randomization, 8 were in the treatment group, 5 were in the control group, and 15 were in the non-randomized (observational) group.
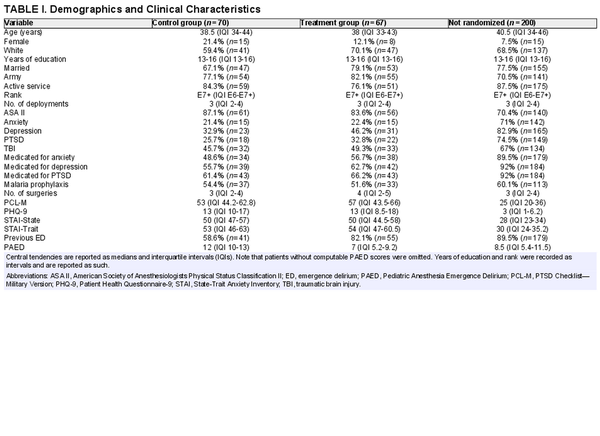
The demographic and clinical data are summarized in Table I. The study population represented a young, predominantly male cohort with comparable clinical and demographic characteristics. There were no statistically significant differences between age, gender, race, years of education, military rank, or number of combat deployments. Rates of anxiety, depression, and PTSD did not differ between the control and treatment groups (Table I). Self-reported medication rates for anxiety, depression, and PTSD did not differ between the control and treatment groups (Table I). Rates of anxiety, depression, and PTSD as well as self-reported medication rates for these conditions were higher in the non-randomized group (Table I). Surgical procedures were predominantly a mix of orthopedic (43.5%) and spine surgery (19.5%). The remainder of surgical procedures was as follows: general (15.4%); ear, nose, and throat (8.3%); gynecologic (3.8%); urologic (2.7%); and other (6.8%). All surgical procedures were evenly distributed among groups.
Among those subjects in the experimental arm, subjects randomized to receive the dexmedetomidine infusion were significantly less likely to experience ED (7, 5.2-9.2) than those randomized to receive the placebo infusion (median 12, IQI 10-13) (P < .0001) (Table I). The smoothed distributions of PAED stratified by group are shown in Figure 1.
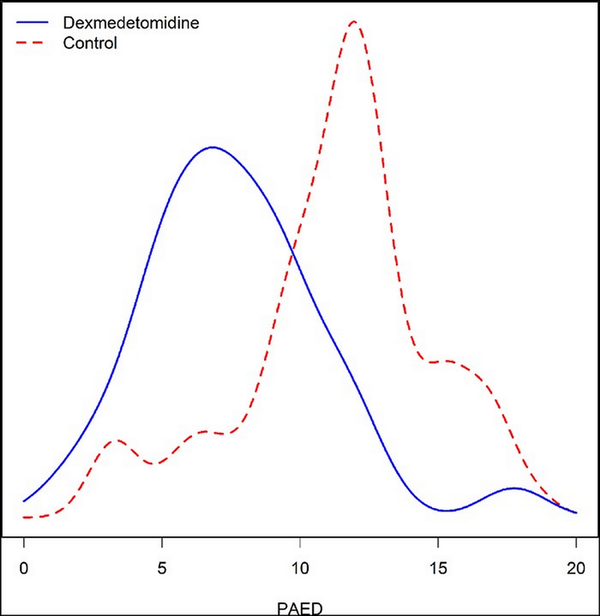
FIGURE 1
Smoothed distributions of PAED stratified by dexmedetomidine and control groups. PAED of patients randomized to dexmedetomidine (median 7, IQI 5.2-9.2) was significantly less (P < .001) than placebo group (median 12, IQI 10-13). IQI, interquartile interval; PAED, Pediatric Anesthesia Emergence Delirium.
XGBoost technique found dexmedetomidine infusion to be the most important predictor of PAED (35% relative importance), followed by PHQ-9 (14%), STAI-Trait (9%), and PCL-M (8%). The overall rankings are shown in Figure 2. Likewise, applying a linear model controlling for several variables, the directionality of the effect persisted upon shrinking the coefficients in a penalized linear model. Under these conditions, dexmedetomidine infusion was associated with a 3.7-unit reduction (95% CI 2.5-4.9) in PAED (P < .001) (Table II) compared to placebo.
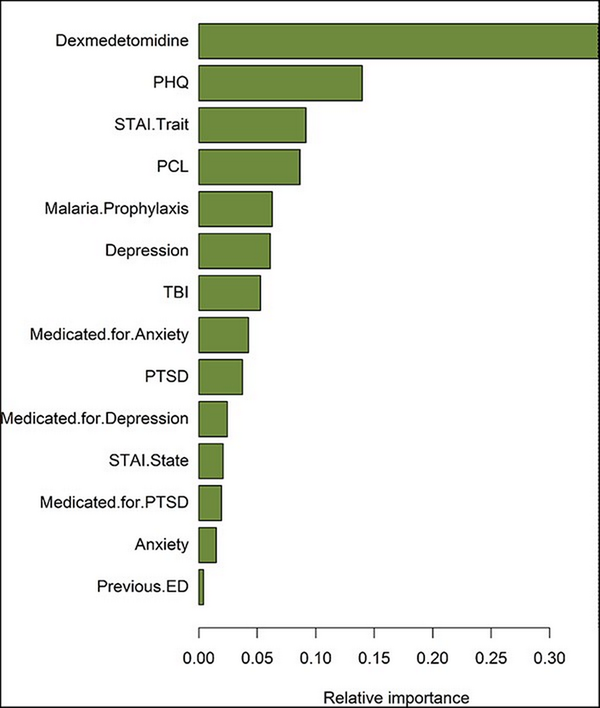
FIGURE 2
Variable contribution related to PAED among randomized patients. Variable importance was computed as gain from a tuned XGBoost model predicting PAED. Dexmedetomidine was the most important predictor of PAED (35% relative importance), followed by PHQ-9 (14%), STAI-Trait (9%), and PCL-M (8%). ED, emergence delerium; PAED, Pediatric Anesthesia Emergence Delirium; PCL-M, PTSD Checklist—Military Version; PHQ, Patient Health Questionnaire-9; STAI, State-Trait Anxiety Inventory; TBI, traumatic brain injury.
No patients or staff were injured due to ED, and there were no adverse events of symptomatic bradycardia or hypotension that required intervention.
DISCUSSION
The primary objective of this multi-center, double-blind, randomized study was to quantify ED and identify risk factors for ED in a high-risk adult population of combat veterans and secondarily to investigate the effectiveness of intraoperative dexmedetomidine in the prevention of ED. Emergence delirium was higher among combat veterans with high baseline anxiety compared to those without high baseline anxiety. The median postoperative PAED in combat veterans presenting for elective surgery with high baseline anxiety was 12, compared to 8.5 for combat veterans presenting for elective surgery without baseline anxiety. Risk factors contributing to this observed increased in ED in combat veterans undergoing elective surgery are elevated PHQ-9, STAI-Trait, and PCL-M. Furthermore, the study demonstrates that dexmedetomidine effectively reduced the incidence and severity of ED among combat veterans with high levels of state anxiety without prolonging PACU stays. To our knowledge, no other study has specifically examined the effect of intraoperative dexmedetomidine among adults with a known risk factor for ED such as combat exposure.
Interestingly, patients with low baseline anxiety (observational group) seemed more likely to self-report a diagnosis of PTSD and depression as well as taking medications for these conditions (Table I). As previously mentioned, PAED was less in this group. Conversely, combat veterans in the experimental group with high baseline anxiety seemed less likely to report a diagnosis of PTSD or depression and less likely to report taking medications for these conditions (Table I). This study was not designed or powered to evaluate the impact of behavioral health care on PAED, but these observations in our experimental and observational groups merit further investigation.
Previous studies have estimated the incidence among adult patients to range widely from 2.1 to 22.2, depending on study design and diagnostic criteria applied.,, Different scales of agitation exist and have been used in the assessment of ED including the Riker Agitation-Sedation Scale, Richmond Sedation-Agitation Scale, the Confusion Assessment Method, the New Sheffield Sedation Scale, the PAED Scale, Watcha Scale, as well as routine assessment and documentation of motor activity (thrashing and combative behavior).,, All formal scales with the exception of PAED have been developed for use in the intensive care unit. The PAED is the only scale specifically validated to measure ED. The PAED was selected because the scale has been validated in adults, specifically the adult military population and as such was selected for use in this study.
The etiology of ED is not well understood, and many risk factors have been implicated for adult patients, including various anesthetic agents such as benzodiazepines, opioids, sevoflourane, and propofol and type and duration of surgery (musculoskeletal, breast, and abdominal), but none have been consistently found to be associated with ED., Psychological comorbidities such as anxiety have long been associated with pediatric ED. A history of stressful medical encounters is hypothesized to increase a child’s anxiety before surgery, furthering postoperative anxiety and manifesting as ED. The child’s normal or pathological stress response, manifesting as preoperative anxiety, translates into an exaggerated stress response immediately following surgery. Among adults with ED, psychological comorbidities have been absent in literature. Studies that included adults have either failed to recognize psychological conditions as a risk factor for ED or specifically excluded patients with psychiatric conditions.
Although psychological morbidity is not unique to the military population, combat veterans carry some of the highest rates of anxiety, PTSD, and depression compared to any other sub-population. Indeed, the elevations of PAED in our study is consistent with recent studies evaluating high-risk adult populations. Studies of military combat veterans have revealed an elevated incidence of ED and identified psychological comorbidity as a significant risk factor., A study of 130 combat veterans revealed an overall incidence of ED to be 20%. Among those who reported a history of anxiety, depression, and/or PTSD, the incidence rose to 46%. Regression analysis of the data revealed anxiety, specifically state anxiety, to be associated with ED. Based on the data, a retrospective study was conducted on veterans at the VA Western New York Healthcare System to evaluate the relationship between PTSD and ED. Relying on the documentation in the patient record of “postoperative agitation” by PACU nurses and anesthesia providers, the study found PTSD to be an independent predictor of ED.
In addition to showing elevated PAED scores among combat veterans with high levels of anxiety, this study shows that intraoperative administration of dexmedetomidine reduced the incidence of ED in this high-risk population. In all analyses, dexmedetomidine infusion emerged as protective and the most important variable in the outcome of ED. In the sensitivity analysis using PAED as a categorical variable, dexmedetomidine infusion resulted in an OR = 0.093 (95% CI 0.042-0.20, P < .001), while in the logistic regression, dexmedetomidine was found to be the most important predictor of PAED (35%) and more than twice that of the next most important predictor, PHQ (Fig. 2). Using PAED as a continuous variable in a linear model, dexmedetomidine was associated with a 3.7-unit reduction (95% CI 2.5-4.9 in PAED, P < .0001).
The efficacy of dexmedetomidine as a prophylaxis in our study is consistent with the pediatric literature.,,, The most recent study, a 2018 double-blind randomized study of ED among children undergoing tonsillectomy, found patients who received a dexmedetomidine infusion similar to our patients (1 µg/kg bolus followed by 0.5 µg/kg/h infusion continuous intraoperative) exhibited significantly less ED at 20 and 30 minutes without prolonging extubation time. Another randomized controlled pediatric study comparing fentanyl and dexmedetomidine for the prophylactic treatment of ED showed that there is a significant decrease in the incidence of ED with the administration of intraoperative dexmedetomidine (45.9% versus 18%). Lastly, in a double-blind, randomized, prospective study, a nearly 3-fold reduction in the incidence of ED was found in children after sevoflurane-based general anesthesia when dexmedetomidine was compared to placebo. In all studies, no clinically meaningful adverse side effects or complications were reported.
In addition to reducing the risk of ED among adult patients, dexmedetomidine has been recently administered to treat ED in the PACU for adult patients for whom conventional therapy failed. Noteworthy is the fact that two of the three patients in the case series reportedly had comorbid psychiatric conditions. In another application, dexmedetomidine infusion improved the “quality of emergence” among adult patients as measured by postoperative cough, agitation, hypertension, tachycardia, and shivering.
Limitations of this study include lack of standardization in the surgical procedures and anesthetic technique, including opioid administration. Surgical procedure and duration introduce variability that may cause inconsistency in the data. In addition, we did not assess interrater reliability for PAED scoring. Our analysis focused on maximum PAED without regard for the time course of PAED throughout the perioperative stay. Moreover, although all data collection was completed by study investigators and nurses who were blinded to the randomized group, hemodynamic changes intrinsic to dexmedetomidine pharmacodynamics may have introduced bias. Finally, in our protocol, dexmedetomidine was administered via continuous infusion, which represented standard dosing at the time. Anesthetic practice has evolved to include bolus single dosing during emergence, which represents an opportunity for further research.
Our multi-center study of combat veterans found elevated ED in patients with elevated levels of anxiety. Further study of the relationship between psychological comorbid condition and ED and the efficacy of dexmedetomidine in reducing the risk of ED in the general adult population is warranted.
ACKNOWLEDGMENTS
None declared.
REFERENCES
- 1. Lepouse C, Lautner CA, Liu L, et al: Emergence delirium in adults in the post-anaesthesia care unit. Br J Anaesth2006; 96(6): 747–53.
- 2. Munk L, Andersen G, Moller AM: Post-anaesthetic emergence delirium in adults: incidence, predictors and consequences. Acta Anaesthesiol Scand2016; 60(8): 1059–66.
- 3. Radtke FM, Franck M, Hagemann L, et al: Risk factors for inadequate emergence after anesthesia: emergence delirium and hypoactive emergence. Minerva Anestesiol2010; 76(6): 394–403.
- 4. Yu D, Chai W, Sun X, Yao L: Emergence agitation in adults: risk factors in 2,000 patients. Can J Anesth2010; 57(9): 843–8.
- 5. Deiner S, Silverstein JH: Postoperative delirium and cognitive dysfunction. Br J Anaesth2009; 103(Suppl 1): i41–6.
- 6. McGuire JM: The incidence of and risk factors for emergence delirium in U.S. military combat veterans. J Perianesth Nurs2012; 27(4): 236–45.
- 7. Umholtz M, Cilnyk J, Wang CK, Porhomayon J, Pourafkari L, Nader ND: Postanesthesia emergence in patients with post-traumatic stress disorder. J Clin Anesth2016; 34: 3–10.
- 8. Wilson JT, Pokorny ME: Experiences of military CRNAs with service personnel who are emerging from general anesthesia. AANA J2012; 80(4): 260–5.
- 9. Read MD, Maani CV, Blackwell S: Dexmedetomidine as a rescue therapy for emergence delirium in adults: a case series. A A Case Rep2017; 9(1): 20–3.
- 10. Santos C, Fernandes C: Intraoperative use of dexmedetomidine in prevention of emergence agitation - a case report. Rev Port Cir Cardiotorac Vasc2018; 25(1-2): 65–7.
- 11. Su X, Meng ZT, Wu XH, et al: Dexmedetomidine for prevention of delerium in elderly patients after non-cardiac surgery: a randomised, double-blind, placebo-controlled trial. Lancet2016; 388(10054): 1893–902.
- 12. Chen F, Wang C, Lu Y, Huang M, Fu Z: Efficacy of different doses of dexmedetomidine as a rapid bolus for children: a double-blind, prospective, randomized study. BMC Anesthesiol2018; 18(1): 103.
- 13. Kim JA, Ahn HJ, Yang M, Lee SH, Jeong H, Seong BG: Intraoperative use of dexmedetomidine for the prevention of emergence agitation and postoperative delirium in thoracic surgery: a randomized-controlled trial. Can J Anesth2019; 66(4): 371–9.
- 14. Tsiotou AG, Malisiova A, Kouptsova E, Mavri M, Anagnostopoulou M, Kalliardou E: Dexmedetomidine for the reduction of emergence delirium in children undergoing tonsillectomy with propofol anesthesia: a double-blind, randomized study. Paediatr Anaesth2018; 28(7): 632–8.
- 15. Burry L, Hutton B, Williamson DR, et al: Pharmacological interventions for the treatment of delirium in critically adults. Cochrane Database Syst Rev2019; 9(9): CD011749.
- 16. Schulz KF, Altman DG, Moher D: CONSORT 2010 statement: updated guidelines for reporting parallel group randomised trials. BMJ2010; 340: c332.
- 17. Sikich N, Lerman J: Development and psychometric evaluation of the pediatric anesthesia emergence delirium scale. Anesthesiology2004; 100(5): 1138–45.
- 18. Kroenke K, Spitzer RL: The PHQ-9: a new depression and diagnostic severity measure. Psychiatr Ann2002; 32(9): 509–21.
- 19. Weathers FW: PTSD Checklist (PCL, civilian: PCL-C, military: PCL-M, specific: PCL-S). Available at http://www.ptsd.va.gov/professional/pages/assessments/ptsd-checklist.asp, 1993; accessed February 9, 2011.
- 20. Spielberger CD: State-Trait Anxiety Inventory for Adults Sampler Set: Manual, Test, Scoring Key. Mind Garden; 1983.
- 21. McGuire JM: Emergence Delirium in U. S. Military Combat Veterans. Hahn School of Nursing and Health Science, University of San Diego, Dissertation, San Diego, CA, 2011.
- 22. Kim SY, Kim JM, Lee JH, Song BM, Koo BN: Efficacy of intraoperative dexmedetomidine infusion on emergence agitation and quality of recovery after nasal surgery. Br J Anaesth2013; 111(2): 222–8.
- 23. Patel A, Davidson M, Tran MCJ, et al: Dexmedetomidine infusion for analgesia and prevention of emergence agitation in children with obstructive sleep apnea syndrome undergoing tonsillectomy and adenoidectomy. Anesth Analg2010; 111(4): 1004–10.
- 24. Sekhon JS: Multivariate and propensity score matching software with automated balance optimization: the matching package for R J Stat Softw. 2011; 42(7): 1–52.
- 25. Præstgaard JT: Permutation and bootstrap Kolmogorov-Smirnov tests for the equality of two distributions. Scand J Stat1995; 22(3): 305–22.
- 26. Chen T, Guestrin C: XGBoost: a scalable tree boosting system. In: Paper presented at Proceedings of the 22nd ACM SIGKDD International Conference on Knowledge Discovery and Data Mining. San Francisco, CA:2016.
- 27. Chen T, He T: XGBoost: eXtreme Gradient Boosting (version 0.6-4) [computer software]. 2017.
- 28. Friedman J, Hastie T, Tibshirani R: The Elements of Statistical Learning. Springer Series in Statisics; 2001.
- 29. Tibshirani R: Regression shrinkage and selection via the Lasso. J R Stat Soc Series1996; 58(1): 267–88.
- 30. Friedman J, Hastie T, Tibshirani R: Regularization paths for generalized linear models via coordinate descent. J Stat Softw2010; 33(1): 1–22.
- 31. Xará D, Silva A, Mendonca J, Abelha F: Inadequate emergence after anesthesia: emergence delirium and hypoactive emergence in the postanesthesia care unit. J Clin Anesth2013; 25(6): 439–46.
- 32. Kain ZN, Caldwell-Andrews A, Maranets I: Preoperative anxiety, emergence delirium and postoperative maladaptive behavioral changes: are they related?Anesth Analg2004; 99(6): 1648–54.
- 33. Lumley MA, Melamed BG, Abeles LA: Predicting children’s presurgical anxiety and subsequent behavior changes. J Pediatr Psychol1993; 18(4): 481–97.
- 34. Hoge CW, Castro CA, Messer SC, et al: Combat duty in Iraq and Afghanistan, mental health problems, and barriers to care. N Engl J Med2004; 351(1): 13–22.
- 35. Ali MA, Abdellatif AA: Prevention of sevoflurane related emergence agitation in children undergoing adenotonsillectomy: a comparison of dexmedetomidine and propofol. Saudi J Anaesth2013; 7(3): 296–300.
- 36. Meng Q, et al: Dexmedetomidine reduces emergence agitation after tonsillectomy in children by sevoflurane anesthesia: a case-control study. Int J Pediatr Otorhinolaryngol2012; 76(7): 1036–41.
- 37. Shukry M, et al: Does dexmedetomidine prevent emergence delirium in children after sevoflurane-based general anesthesia?Paediatr Anaesth2005; 15(12): 1098–104.
- 38. Aouad MT, et al: Dexmedetomidine for improved quality of emergence from general anesthesia: a dose-finding study. Anesth Analg2019; 129(6): 1504–11.