Therapy-resistant nephrotic syndrome in children is generally based on primary focal segmental glomerulosclerosis (pFSGS) and will lead to deterioration of kidney function. Kidney transplantation in such patients may result in a recurrence of FSGS (rFSGS), which has been reported in up to 50% of children []. Plasma circulating permeability factors (CPFs), the nature and origin of which still remain elusive, have been designated a pathological role in both pFSGS and rFSGS. Clinical evidence including posttransplant recurrence of the disease and response to extracorporeal therapies supports the presence of CPFs in patients. Moreover, in vitro experimental data with sera from rFSGS patients that induce glomerular permeability and podocyte injury highlights the pathophysiological role of CPFs in FSGS []. Furthermore, podocytopenia following podocyte injury is linked to elevated reactive oxygen species (ROS) levels that result in podocyte cell detachment and/or death []. Interestingly, higher levels of oxidative markers have been measured in children with active nephrotic syndrome [].
FSGS patients with a putative CPF often respond well to immunoadsorption (IA) or therapeutic plasma exchange (TPE). More importantly, preemptive IA or TPE has been shown to reduce the risk of rFSGS after transplantation []. Although TPE has a success rate of approximately 70% in pediatric patients, a semi-selective IA therapy removing immunoglobulins (Igs) but preserving other plasma proteins is increasingly used [, ]. The effectiveness of IA may suggest that the CPF could be a circulating Ig or lie in the Ig fraction, but this concept is not yet supported by in vitro experimental data. Here, we aimed to purify Ig fractions from the plasma of FSGS patients with a presumed CPF and investigate the effect of these fractions on human conditionally immortalized podocytes (hciPods) in vitro.
The study was conducted according to the recommendations of the appropriate version of the Helsinki Declaration and all subjects had given informed consent. TPE samples were collected from three FSGS patient with presumed CPF at active disease (Act 1, Act 2, Act 3). From one of these patients the remission plasma was also available (Rem) and included as control, as well as two non-kidney disease TPE patients (NR-Ctrl 1 and 2, both with neuromyelitis optica). Heparin plasma from seven healthy individuals was collected and pooled, and used as a healthy control plasma (hCtrl pool). Ig fractions were extracted from these seven samples using a protein A/G affinity chromatography column (Sigma). In addition to the column-bound Ig fractions (elution), the flow-through fractions of each sample were also collected. For in vitro assays, Ig fractions were concentrated to original plasma volume and the flow-through samples were corrected for the dilution factor. Sodium dodecyl sulfate polyacrylamide gel electrophoresis (SDS-PAGE) was performed to confirm that plasma Igs were isolated. Ig concentrations in the TPE and the flow-through fractions were determined by nephelometry. Oxidative stress in hciPods in response to Ig fractions and flow-throughs was assessed fluorometrically with a CM-H2DCFDA probe.
SDS-PAGE performed on the Ig fractions and flow-throughs showed two bands of 25 and 50 kDa only in the Ig fractions (Fig. 1A). The observed 25 and 50 kDa bands indicate the light chain and the glycosylated heavy chain of the IgG class of antibodies, respectively. Elevated ROS levels in hciPods was solely observed in response to the flow-through fractions of active FSGS patients and not in response to the Ig fractions (Fig. 1B). Flow-through fractions from FSGS patient at remission, non-kidney patients and healthy controls did not lead to oxidative stress in cultured podocytes. Furthermore, approximately 2% Igs was measured with nephelometry in the flow-through fractions obtained from three FSGS patients with presumed CPF at active disease when compared with the Ig fractions. Together, these data show that the putative CPFs in FSGS do not lie in Ig fractions, at least not in the three patients included in the current study.
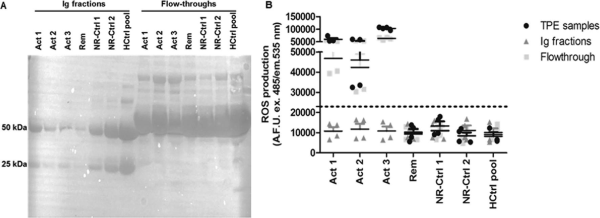
Figure 1
Ig fraction of FSGS patients with presumed CPF does not cause podocyte injury. (A) SDS-PAGE analysis of extracted Ig and flow-through fractions. (B) Significant ROS formation after 24 h was observed in response to flow-through fractions and TPE samples from three FSGS patients with presumed CPF.
Management of rFSGS in clinical practice remains a therapeutic challenge. Hence, currently there are no clear guidelines for the prevention of rFSGS after transplantation; treatment is mainly based on TPE or IA in combination with high dose of CD20-depleting antibody rituximab []. As no study has compared IA with TPE and case series have indicated a comparable efficacy, IA is increasingly used in various clinics to treat rFSGS. However, our results show that the putative CPF associated with FSGS does not seem to lie in Ig fractions of the three patients studied here. This suggests that IA solely may not be enough to efficiently remove CPFs. In a patient resistant to IA, it may be of benefit to use TPE to achieve remission of rFSGS. For the future, it may be beneficial to design randomized and prospective clinical trials to compare IA with TPE for the management of rFSGS.
ACKNOWLEDGEMENTS
We thank Prof. Moin Saleem for the conditionally immortalized human podocyte cell line.
REFERENCES
- 1. Trachtman R, Sran SS, Trachtman H. Recurrent focal segmental glomerulosclerosis after kidney transplantation. Pediatr Nephrol2015;30:1793–802. https://doi.org/10.1007/s00467-015-3062-1
- 2. Ha T-S. Circulating permeability factors in idiopathic nephrotic syndrome. Child Kidney Dis2019;23:7–21.
- 3. Mundel P, Shankland SJ. Podocyte biology and response to injury. J Am Soc Nephrol2002;13:3005–15. https://doi.org/10.1097/01.asn.0000039661.06947.fd
- 4. Reddy P, Sindgikar S, Shenoy R, et al Oxidative stress in childhood steroid sensitive nephrotic syndrome and its correlation with DNA damage. Int J Contemp Pediatrics2016;3:768–72. http://dx.doi.org/10.18203/2349-3291.ijcp20161853
- 5. Gohh RY, Yango AF, Morrissey PE, et al Preemptive plasmapheresis and recurrence of FSGS in high-risk renal transplant recipients. Am J Transplant2005;5:2907–12. https://doi.org/10.1111/j.1600-6143.2005.01112.x
- 6. Ponticelli C. Recurrence of focal segmental glomerular sclerosis (FSGS) after renal transplantation. Nephrol Dial Transplant2010;25:25–31. https://doi.org/10.1093/ndt/gfp538
- 7. Weber LT, Tönshoff B, Grenda R, et al Clinical practice recommendations for recurrence of focal and segmental glomerulosclerosis/steroid-resistant nephrotic syndrome. Pediatr Transplant2021;25:e13955. https://doi.org/10.1111/petr.13955
