Introduction
Gastric neuroendocrine neoplasms are a rare malignancy with relatively indolent biological behavior [, ]. In recent years, the World Health Organization (WHO) classified NENs of the stomach into three categories: gastric neuroendocrine tumor (G-NET), gastric neuroendocrine carcinoma (G-NEC), and gastric mixed adenoneuroendocrine carcinoma (G-MANEC) []. In 2006, the European Neuroendocrine Tumor Society (ENETS) published a consensus on tumor, node, metastasis (TNM) staging for G-NETs, which is also recommended for patients with G-NEC and G-MANEC []. However, previous studies have shown that the biological behavior of NETs was quite different from that of NECs, which were more invasive and had a worse prognosis [, ]. In 2016, the new (8th) edition of the AJCC staging system emphasized that the NENs staging system for gastroenteropancreatic (GEP)-NEN should only be applied to well-differentiated tumors (G1/G2 G-NET), while GEP-NEC and GEP-MANEC should be excluded and staged according to the TNM stages for gastric, intestinal, or pancreatic adenocarcinomas []. However, in recent years, some studies with a small sample size suggested that G-NECs have different clinical features from those of adenocarcinoma and that the prognosis of G-NECs was worse than that of gastric adenocarcinomas [, ]. To date, no large-scale studies have been performed to evaluate which staging system is better for G-NEC/MANEC. By using a multicenter, large-sample series, the goal of our study was to assess whether the ENETS classification system and the 8th AJCC staging system for GC are suitable for G-NEC/MANEC and to explore an optimal staging system, which was verified using a multicenter internal validation cohort and an external validation cohort from the SEER database.
Methods
Patients and Data Collection
The China Gastric Neuroendocrine Tumor Study Group (China-gNETSG) was established with a collaboration among 24 institutions. All patients with G-NEC/MANEC who underwent surgical resection with curative intent for a primary tumor between 2004 and 2018 were included. Patients who received neoadjuvant therapy, those with remnant GC, and those with incomplete data (including pathological stage and follow-up data) were excluded. Finally, 1,179 patients were included in the current study, and the number of cases from each center is shown in online supplementary Table 1 (see http://www.karger.com/doi/10.1159/000505924 for all online suppl. material). We used the data splitting method to randomly assign two-thirds of the patients to the training set (n = 786) and one-third to the validation set (n = 393; online suppl. Fig. 1a).
The external validation cohort was obtained from the SEER (1988–2015) database. Patients were retrieved based on the International Classification of Diseases for Oncology (3rd edition; ICD-O-3) site codes for tumors of the stomach: C16.0-C16.9. The following ICD-O-3 codes for histological type were included: large cell carcinoma (8012-8013), small cell carcinoma (8041-8044), carcinoid tumor (8240), mixed adenoneuroendocrine carcinoma (8244), adenocarcinoid tumor (8245), neuroendocrine carcinoid (8246), and atypical carcinoid tumor (8249) []. TNM staging was retrieved based on the following codes: derived AJCC stage group (7th edition; 2010+), derived AJCC stage group (6th edition; 2004+), derived SEER Summary Stage 1977 (2004+), collaborative stage (CS) tumor size 2004, CS extension 2004, CS lymph nodes 2004, CS metastases at DX 2004, extent of disease (EOD) 10-extent (1988–2003), EOD 10-nodes (1988–2003), and EOD 10-size (1988–2003). After excluding patients with unknown T and N categories, data from 471 patients from the SEER data set were analyzed in the present study (online suppl. Fig. 1b).
The survival duration was measured from the time of surgery to either the last date that survival information was collected or to the confirmed date of death. The median follow-up for the 2 data sets was 56 months (China-gNETSG) and 74.0 months (SEER). All staging data within the database were updated and coded to conform to the ENETS staging system and 8th AJCC staging manual for GC [, ].
Statistical Methods
Overall survival was analyzed using Kaplan-Meier (K-M) curves, and log-rank tests were used to evaluate the staging systems. A 3-step multivariate analysis was performed to investigate the validity of the 6th AJCC TNM staging manual for GC []. In the 1st step of the multivariate analysis, all the significant factors in the univariate analysis were included as well as the ENETS staging system, excluding the 8th and 6th AJCC systems for GC. In the 2nd step of the multivariate analysis, the 8th AJCC system was also included, but not the 6th AJCC system. In the 3rd step of the multivariate analysis, all three TNM systems were included. The relative discriminatory abilities of the different staging systems were assessed using the linear trend χ2 test, the Akaike Information Criterion (AIC), and the concordance index (C-index). A higher C-index indicates a better discriminatory ability [], smaller AIC values represent better prognostic stratification [], and a higher linear trend χ2 score shows better discriminatory ability and monotonicity. The prognostic abilities of the staging systems were also compared by generating time-dependent receiver operating characteristic (T-ROC) curves. T-ROC curve analysis is an extension of the ROC curve, which assesses the discriminatory power of continuous markers for time-dependent disease outcomes []. The “time-ROC” R packages (https://cran.r-project.org/web/packages/timeROC/index.html) were utilized to estimate T-ROC curves of each staging system (suppl. file 1). All statistical analyses were performed using SPSS statistical software (version 22.0, SPSS Inc., Chicago, IL, USA), STATA version 12.0 (StataCorp, College Station, TX, USA), and R software version 3.5.2 (http://www.r-project.org). The R packages “rms” was used for calculation of the C-index. Significant differences were assumed at p values <0.05 in a two-tailed test.
Results
Patient Characteristics
In total, 1,179 patients with pathologically confirmed G-NEC/MANEC from the China-gNETSG database were included in this study (online suppl. Table 2). This cohort of patients consisted of 925 male patients (78.5%) and 254 female patients (21.5%; the male-to-female ratio was 3.6:1) with a median age of 64.0 years. And 1,039 (88.1%) patients were with Ki-67 information. The proportion of patients with NEC was similar to that with MANEC (NEC-to-MANEC ratio, 1:1.2). K-M survival analysis showed that the prognosis of NEC was similar to that of MANEC (p = 0.101, online suppl. Fig. 2a). The multicentric databases were randomly divided into the training set (n = 786) and the China-gNETSG validation set (n = 393). No significant differences in clinicopathological characteristics were observed between the 2 sets (all p > 0.05, online suppl. Table 2). And the prognosis of the training set was similar to that of the China-gNETSG validation set (p = 0.716, online suppl. Fig. 2b).
Overall, 471 patients from the SEER database were included (online suppl. Table 3). The median age at diagnosis was 69.0 years, and men comprised 65.4% of the data set; moreover, 72.8% of the patients were white. Most of the tumors were located in the upper-third stomach (40.3%), and the main histological type was NEC (94.1%).
T Category and N Category of the ENETS and 8th AJCC Systems
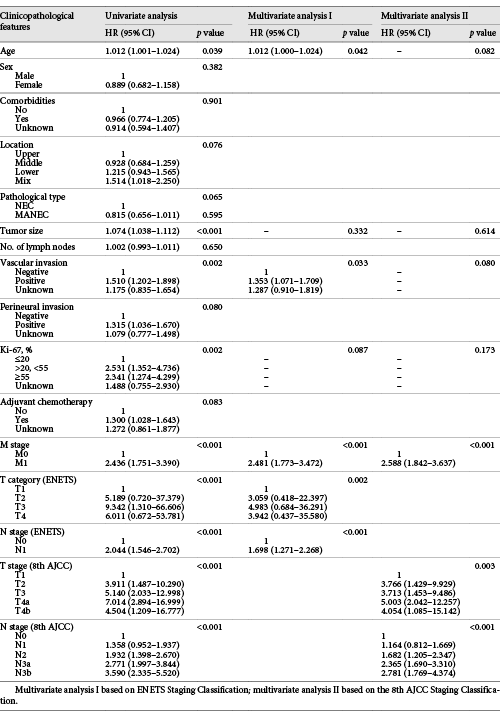
In the training set, univariate analyses of the variables showed that the ENETS T category and N category, and the 8th AJCC T category and N category were significant prognostic factors (all p < 0.05, Table 1). Multivariate analyses confirmed that the T category and N category of the ENETS and the 8th AJCC systems were all independent prognostic factors for G-NEC/MANEC (p < 0.05, Table 1).
The survival curve showed overlap in the ENETS system for T1 and T2 (p = 0.088, Fig. 1a) and in the 8th AJCC system for T2 and T3 (p = 0.322, Fig. 1c). For the N category, a significant difference in prognosis was observed between N0 and N1 in the ENETS system (p < 0.001, Fig. 1b). Although the heterogeneity of the 8th AJCC N category was better, there were no significant differences in survival between N0 and N1 as well as between N3a and N3b (both p > 0.05, Fig. 1d). Based on these survival analysis results, we obtained a new T classification in combination with T2 and T3 of the 8th AJCC system, which was consistent with the 6th AJCC T category for GC, and the survival curves were well separated (all p < 0.05, Fig. 1e). In addition, we recalculated the optimal cut-off values for the number of LN metastases using X-tile software: N1 = 1–6 metastatic LNs, N2 = 7–13 metastatic LNs, and N3 ≥14 metastatic LNs (online suppl. Fig. 3), which was also similar to the values for the 6th AJCC N category for GC. According to the 6th AJCC system, the survival curves were well separated within the N category (p < 0.05, Fig. 1f). These results suggest that the 6th AJCC staging system for GC may be more suitable than the other 2 systems for patients with G-NEC/MANEC.
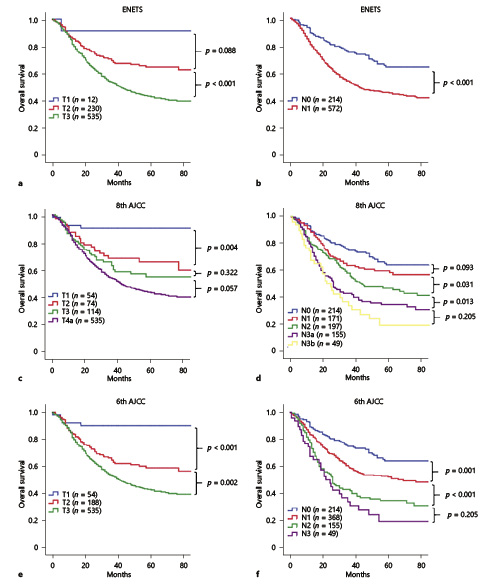
Fig. 1
Kaplan-Meier curves depict the overall survival of the training set according to the T category (a, c, e) and N category (b, d, f) of the ENETS (a, b) and the 8th (c, d) and 6th (e, f) editions of the TNM classification.
The ENETS, 8th Edition AJCC, and 6th Edition AJCC Staging Systems
K-M survival curves were used to compare the prognostic distribution of the ENETS, 8th AJCC, and 6th AJCC staging systems in the training set. For the 4-category stages (I, II, III, and IV), the survival curves were separated among the different stages of the three staging systems (all p < 0.05, Fig. 2a, c, e). However, in more detailed stages, some overlaps were noted for the ENETS classification of stage I and IIA disease and for stage IIB and IIIB diseases (both p > 0.05, Fig. 2b). There were only 3 patients with stage IIIA disease, and the distribution of each stage was unbalanced for the ENETS staging system. For the 8th AJCC classification, multiple survival curves of the substages overlapped, such as those for IB vs. IIA, IIB vs. IIIA, IIIB vs. IIIC, and IIIC vs. IV stages (all p > 0.05, Fig. 2d). However, for the 6th AJCC classification, significant differences were observed in survival for each stage (p < 0.05, Fig. 2f).
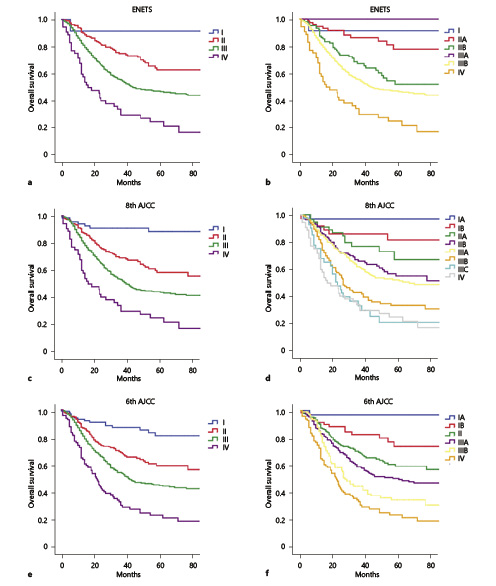
Fig. 2
Kaplan-Meier curves of different staging classifications for patients from the training set. a, b ENETS. c, d 8th AJCC. e, f 6th AJCC.
We further performed a 3-step multivariate analysis for prognosis. In the 1st step of the multivariate analysis, the ENETS system was confirmed to be an independent prognostic factor for G-NEC/MANEC (p < 0.05, Table 2). In the 2nd step, the 8th AJCC system was confirmed to be an independent prognostic factor (p < 0.05), whereas the ENETS system was no longer significant (p = 0.802). In the 3rd step, only the 6th AJCC system remained significant (p < 0.001), whereas the ENETS and the 8th AJCC system did not (both p > 0.05, Table 2).
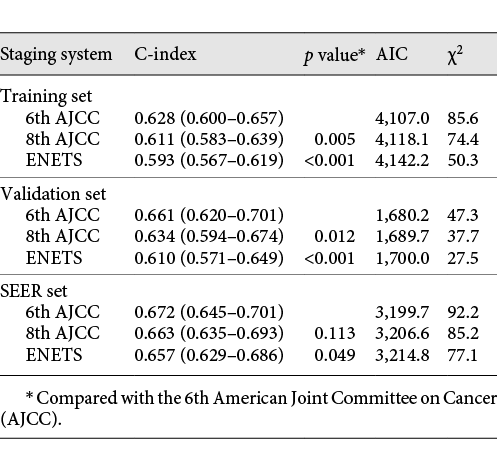
A comparison of the performance of the three staging systems is shown in Table 3. The C-index of the 6th AJCC system (0.628) was significantly better than that of the ENETS (0.593) and the 8th AJCC system (0.611; both p < 0.05, Table 3). In addition, the AIC of the 6th AJCC system was the smallest, and the χ2 value was the largest, confirming that the 6th AJCC system had better homogeneity and discriminatory ability. Furthermore, the T-ROC curve of the 6th AJCC system was continuously superior to that of the other 2 systems throughout the observation period (Fig. 3a).
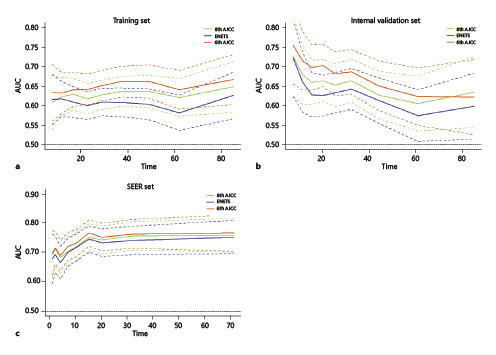
Fig. 3
Time-dependent AUC curves of ENETS and the 8th and 6th AJCC staging systems for the prediction of overall survival in the training set (a), internal validation set (b), and SEER set (c).
Internal and External Validation with China-gNETSG and SEER Database
In the China-gNETSG internal validation set, the survival curves were also separated among stages of the ENETS, 8th AJCC and 6th AJCC staging systems in 4-category stages (all p < 0.05, online suppl. Fig. 4a, c, e). However, in more detailed stages, the 6th AJCC system showed better discrimination in the survival curves than did the other 2 systems (online suppl. Fig. 4Bb, d, f). Similar results were found in the external validation cohorts by using the SEER database (online suppl. Fig. 5). In both internal and external validation cohorts, the 3rd step of the 3-step multivariate analysis showed only the 6th AJCC system was an independent prognostic factor (p < 0.001), whereas the ENETS and the 8th AJCC systems were no longer significant (both p > 0.05, Table 2; online suppl. Table 4). Additionally, in both validation cohorts, the C-index of the 6th AJCC system was significantly better than that of the ENETS and the 8th AJCC systems (all p < 0.05, Table 3). And the AIC of the 6th AJCC system was the smallest, and the χ2 value was the largest. Furthermore, the T-ROC curve of the 6th AJCC system was continuously superior to that of the other 2 systems throughout the observation period (Fig. 3b, c).
Stratified Validation according to G-NEC and G-MANEC
For the stratified validation, the China-gNETSG database was further divided into a G-NEC set (n = 645) and a G-MANEC set (n = 534) according to histological type. In both sets, the 3-step multivariate analysis confirmed that only the 6th AJCC system was an independent prognostic factor (p < 0.001), whereas the ENETS and 8th AJCC systems lost their significance (both p > 0.05, online suppl. Table 5). Further evaluation of performance showed that the C-index of the 6th AJCC system was significantly better than that of the other 2 systems in both histological types (online suppl. Table 6). Similarly, the T-ROC curve also showed that the 6th AJCC system was the best staging system (online suppl. Fig. 6a, b), which confirmed that the predictive efficacy of the 6th AJCC system was superior to that of the other 2 staging systems for both G-NEC and G-MANEC patients.
Discussion
High-grade NEC and MANEC are rare in the gastrointestinal (GI) tract and in other extrapulmonary primary tumor locations, and thus, published data on NEC/MANEC in the GI tract are scarce []. Survival is poor in patients with G-NEC/MANEC and ranges from 38 months for patients with localized disease to 5 months in patients with metastatic disease, according to the SEER population registry data []. To classify patients with G-NEC/MANEC, define prognosis, and determine the best treatment approaches, the 2 most common staging systems worldwide are the ENETS and AJCC staging systems. The 8th edition of the AJCC cancer staging manual recommends that G-NEC/G-MANEC be staged using the GC staging system and not the staging system for well-differentiated NETs of the stomach []. Until now, few large cohort studies have confirmed the prognostic value of these 2 staging systems for G-NEC/G-MANEC. Our current study first analyzed the prognostic value of the depth of tumor invasion (T category) and LN metastasis (N category) for G-NEC/MANEC based on our multicenter large-sample database. And found that the ENETS and 8th AJCC systems have some significant stage migration in evaluating the long-term prognosis of G-NEC and G-MANEC.
Cancer staging is not an exact science, and as new information becomes available about etiology and various methods of diagnosis and treatment, the classification and staging of cancer will change as will the accuracy [-]. A recent analysis of 6,792 small intestinal NETs in the SEER database found that the outcomes were similar between patients with T1 tumors and those with T2 tumors []. The 8th edition of the AJCC staging system for GC has been widely used in clinical practice, and according to this staging system, T2 in the 6th AJCC classification was subclassified into T2 (muscularis propria) and T3 (subserosa). Several studies have confirmed that these changes can better predict the prognosis of gastric cancer patients [, ]. However, in the current study, according to the T category of the 8th AJCC system, the survival between patients with T2 and T3 G-NEC/MANEC was not significantly different. When combined with the T2 and T3 patients, the T category was consistent with the 6th AJCC T category. Additionally, according to the survival analysis, the 6th AJCC T category showed the division well. This result can also explain the heterogeneity between G-NEC/MANEC and primary gastric adenocarcinoma.
LN metastasis is one of the most common metastasis pathways for GC and is also the most important prognostic factor. The current N classification for G-NEC/MANEC using the ENETS staging system only distinguishes between node-negative and node-positive disease and does not incorporate the number of positive metastatic LNs. Although the ENETS N category was an independent prognostic factor for G-NEC/MANEC, we also found that the prognosis of patients with different numbers of metastatic LNs showed significant heterogeneity according to the 8th AJCC system. Park et al. [] showed that lymphovascular invasion was more frequently identified in carcinomas with neuroendocrine differentiation, especially in patients with G-NEC and G-MANEC, which led to a poor prognosis. They also suggested the different characteristics of LN metastasis between G-NEC/MANEC and pure gastric adenocarcinoma. In our study, we also found some overlap in survival according to the 8th AJCC N category. In addition, we recalculated the optimal cut-off values of metastatic LN using the X-tile method and found that the cut-off numbers were basically consistent with the 6th AJCC N category. As a result, considering the simplicity and international generality of the staging system, we included the 6th AJCC system in our further study to determine which staging system could better predict the prognosis of G-NEC/MANEC.
Compared with the other 2 staging systems, the survival outcomes of each stage in the 6th AJCC system were significantly different with a better discriminatory ability (higher C-index and smaller AIC values) and monotonicity of gradients (higher linear trend χ2 score). However, before considering whether to use a clinical prediction model, it is essential to perform internal and external validation [-]. Because our multicentric databases contained information on patients from Eastern populations, whether the results are suitable for Western populations may affect the universality of an optimal staging system. Furthermore, in addition to the multicenter internal set, we also used the SEER database as an external validation set. The results also showed that for patients with G-NEC/MANEC, the predictive efficiency of the 6th AJCC staging system was significantly better than that of the other 2 staging systems and that this system demonstrated good universal applicability.
There were still several limitations to the current study. First, this study was limited by its retrospective nature, and the surgical procedure and pathological examination were not standardized across all the institutions, which may have led to variability in the reports. Second, although we included the SEER database, which contains mostly Western populations, as an external validation set, some missing information in the SEER database, such as Ki-67 index, may reduce the number of cases included and the integrity of the data in the validation set and, therefore, may have affected the validation. Third, in this study, we only included patients with resectable tumors; thus, the number of patients with tumors that had invaded adjacent organs (T4b) was limited (n = 9). Consequently, further investigation is needed to determine the accuracy of the staging system for predicting the prognosis of these patients. Nevertheless, using a large-scale multicenter database, our study first confirmed that the 6th AJCC staging system for GC is more suitable for predicting the prognosis and risk of patients with G-NEC and G-MANEC. This finding was also confirmed by the Western population-based validation cohort. Therefore, these results suggest that the 6th AJCC staging system for GC can be used in clinical practice for patients with G-NEC/MANEC and can provide an important reference value for improvements in the new G-NEC/MANEC staging system.
Acknowledgements
National Natural Science Foundation of China (No. 81871899); Scientific and technological innovation joint capital projects of Fujian Province (2016Y9031, 2017Y9011); Construction Project of Fujian Province Minimally Invasive Medical Center (No. [2017]171); The second batch of special support funds for Fujian Province innovation and entrepreneurship talents.
Statement of Ethics
All procedures followed were in accordance with the ethical standards of the responsible committee on human experimentation (institutional and national) and with the Helsinki Declaration of 1964 and later versions. Informed consent or substitute for it was obtained from all patients for being included in the study.
Conflict of Interest Statement
There are no conflicts of interest or financial ties to disclose from any author.
Author Contributions
Conception and design: J.X.L., Y.J.Z., Y.B.Z., H.K.H., and C.-M.H. Provision of study materials or patients: all authors. Collection and assembly of data: all authors. Data analysis and interpretation: J.X.L., Y.J.Z., Y.B.Z., H.K.H., Q.L.H., Y.T.T., B.B.Z., G.Z., X.T.Q., L.X.J., Z.L., Y.C.X., F.Q.X., W.H.F., Z.X., Y.X.L., S..LL., J.P.C., X.J.Z., Z.G.Z., H.L.L., Y.L., E.L., L.S.C., G.Q.J., J.-W.X., P.L., C.-H.Z., and C.-M.H. Manuscript writing: all authors. Final approval of manuscript: all authors. All authors are accountable for all aspects of the work.
References
- 1. Klöppel G, Perren A, Heitz PU. The gastroenteropancreatic neuroendocrine cell system and its tumors: the WHO classification. Ann N Y Acad Sci. 2004;1014(1):13–27.
- 2. Dasari A, Shen C, Halperin D, Zhao B, Zhou S, Xu Y, et al Trends in the Incidence, Prevalence, and Survival Outcomes in Patients With Neuroendocrine Tumors in the United States. JAMA Oncol. 2017;3(10):1335–42.
- 3. Rindi G, Arnold R, Bosman FT, et al Nomenclature and classification of neuroendocrine neoplasms of the digestive system. In: Bosman FT. WHO classification of tumours of the digestive system. 4th ed.Lyon, France: IARC Press; 2010. p. 64.
- 4. Rindi G, Klöppel G, Alhman H, Caplin M, Couvelard A, de Herder WW, et alall other Frascati Consensus Conference participantsEuropean Neuroendocrine Tumor Society (ENETS). TNM staging of foregut (neuro)endocrine tumors: a consensus proposal including a grading system. Virchows Arch. 2006;449(4):395–401.
- 5. Xie JW, Sun YQ, Feng CY, Zheng CH, Li P, Wang JB, et al Evaluation of clinicopathological factors related to the prognosis of gastric neuroendocrine carcinoma. Eur J Surg Oncol. 2016;42(10):1464–70.
- 6. Yao JC, Hassan M, Phan A, Dagohoy C, Leary C, Mares JE, et al One hundred years after “carcinoid”: epidemiology of and prognostic factors for neuroendocrine tumors in 35,825 cases in the United States. J Clin Oncol. 2008;26(18):3063–72.
- 7. Amin MB, Edge SB, Greene FL, et al AJCC Cancer Staging Manual. 8th ed.New York: Springer; 2016.
- 8. Kubota T, Ohyama S, Hiki N, Nunobe S, Yamamoto N, Yamaguchi T. Endocrine carcinoma of the stomach: clinicopathological analysis of 27 surgically treated cases in a single institute. Gastric Cancer. 2012;15(3):323–30.
- 9. Shia J, Tang LH, Weiser MR, Brenner B, Adsay NV, Stelow EB, et al Is nonsmall cell type high-grade neuroendocrine carcinoma of the tubular gastrointestinal tract a distinct disease entity? Am J Surg Pathol. 2008;32(5):719–31.
- 10. Dasari A, Mehta K, Byers LA, Sorbye H, Yao JC. Comparative study of lung and extrapulmonary poorly differentiated neuroendocrine carcinomas: A SEER database analysis of 162,983 cases. Cancer. 2018;124(4):807–15.
- 11. Sun Z, Wang ZN, Zhu Z, et al Evaluation of the seventh edition of American Joint Committee on Cancer TNM staging system for gastric cancer: results from a Chinese monoinstitutional study. Ann Surg Oncol 19:1918-27, 2012
- 12. Han DS, Suh YS, Kong SH, Lee HJ, Choi Y, Aikou S, et al Nomogram predicting long-term survival after d2 gastrectomy for gastric cancer. J Clin Oncol. 2012;30(31):3834–40.
- 13. Yoon HM, Ryu KW, Nam BH, Cho SJ, Park SR, Lee JY, et al Is the new seventh AJCC/UICC staging system appropriate for patients with gastric cancer? J Am Coll Surg. 2012;214(1):88–96.
- 14. Heagerty PJ, Lumley T, Pepe MS. Time-dependent ROC curves for censored survival data and a diagnostic marker. Biometrics. 2000;56(2):337–44.
- 15. Sorbye H, Strosberg J, Baudin E, Klimstra DS, Yao JC. Gastroenteropancreatic high-grade neuroendocrine carcinoma. Cancer. 2014;120(18):2814–23.
- 16. Chen ZH, Xing L, Min D, et al Comparison of a new prognostic system and five current staging systems in predicting the survival rate of patients with advanced hepatocellular carcinoma. J Clin Oncol. 2014;323_suppl:e193–193.
- 17. Lin JX, Lin JP, Li P, Xie JW, Wang JB, Lu J, et al Which staging system better predicts 10-year survival for gastric cancer? A study using an international multicenter database. Eur J Surg Oncol. 2018;44(8):1205–11.
- 18. Sano T, Coit DG, Kim HH, Roviello F, Kassab P, Wittekind C, et al Proposal of a new stage grouping of gastric cancer for TNM classification: International Gastric Cancer Association staging project. Gastric Cancer. 2017;20(2):217–25.
- 19. Kulke MH. Are neuroendocrine tumors going mainstream?J Clin Oncol. 2013;31(4):404–5.
- 20. Strosberg JR, Weber JM, Feldman M, Coppola D, Meredith K, Kvols LK. Prognostic validity of the American Joint Committee on Cancer staging classification for midgut neuroendocrine tumors. J Clin Oncol. 2013;31(4):420–5.
- 21. Kim MK, Warner RR, Roayaie S, Harpaz N, Ward SC, Itzkowitz S, et al Revised staging classification improves outcome prediction for small intestinal neuroendocrine tumors. J Clin Oncol. 2013;31(30):3776–81.
- 22. Warneke VS, Behrens HM, Hartmann JT, et al Cohort study based on the seventh edition of the TNM classification for gastric cancer: proposal of a new staging system. J Clin Oncol 29:2364-71, 2011
- 23. Zhang J, Niu Z, Zhou Y, Cao S. A comparison between the seventh and sixth editions of the American Joint Committee on Cancer/International Union Against classification of gastric cancer. Ann Surg 257:81-6, 2013
- 24. Park JY, Ryu MH, Park YS, Park HJ, Ryoo BY, Kim MG, et al Prognostic significance of neuroendocrine components in gastric carcinomas. Eur J Cancer. 2014;50(16):2802–9.
- 25. Collins GS, de Groot JA, Dutton S, Omar O, Shanyinde M, Tajar A, et al External validation of multivariable prediction models: a systematic review of methodological conduct and reporting. BMC Med Res Methodol. 2014;14(1):40.
- 26. Fang C, Wang W, Feng X, Sun J, Zhang Y, Zeng Y, et al Nomogram individually predicts the overall survival of patients with gastroenteropancreatic neuroendocrine neoplasms. Br J Cancer. 2017;117(10):1544–50.
- 27. Steyerberg EW, Harrell FE Jr. Prediction models need appropriate internal, internal-external, and external validation. J Clin Epidemiol. 2016;69:245–7.
J.X.L., Y.J.Z., Y.B.Z., H.K.H., and Q.L.H. contributed equally to this work and should be considered co-first authors.