Introduction
Acromegaly is a rare endocrine disease characterized by the overproduction of growth hormone (GH), and, consequently, of insulin-like growth factor 1 (IGF-1). The excess of these substances in the circulatory system results in enlargement of the face and extremities, overgrowth of organs, and the development of several comorbidities such as diabetes, sleep apnea, and osteoarthritis [, ]. Acromegaly and its related comorbidities significantly reduce the quality of life of patients [] and result in increased mortality [].
In most cases (≥95%), acromegaly is caused by a pituitary tumor. Therefore, the first treatment approach is surgery to debulk it [, ]. When a cure is not achieved with surgery, which happens in approximately 41% of the cases for macroadenomas and 19% for microadenomas [], drug treatment is recommended []. The drugs used in acromegaly treatment include the first-generation somatostatin analogues (SSAs) octreotide LAR and lanreotide autogel, the second-generation SSA pasireotide, and the GH receptor antagonist pegvisomant. Dopamine agonists such as cabergoline are also used in clinical practice, but their efficacy has never been assessed in clinical trials []. The first-generation SSAs are usually employed as a first-line strategy, and pasireotide or pegvisomant are only used if disease control is not achieved, usually because they are more expensive [, ].
Even though it is a rare disease, with prevalence estimated from 2.8 to 13.7 cases per 100,000 people and incidence from 0.2 to 1.1 cases per 100,000 people per year [], the burden of acromegaly must be acknowledged because of the high costs of its drug treatments, which must be taken lifelong to maintain disease control. A recent systematic review conducted by our research group showed that cost-effectiveness studies in acromegaly have been performed in recent years; however, they present several limitations and lack transparency []. Among these limitations are the inclusion of drug costs only, short time horizon, and the absence of quality of life outcomes. Considering these factors, the aim of this study was to present a transparent cost-utility analysis comparing first- and second-line drug treatment strategies used for acromegaly after an unsuccessful surgery, from a Brazilian public payer perspective. We also conducted a value of information (VOI) analysis to help elucidate where future research on acromegaly should be focused.
Methods
Target Population
The population under study consisted of patients with acromegaly not cured after a surgical procedure and, therefore, candidates for adjunctive treatment in order to achieve disease control. The study adopted a public payer perspective and was applied to the Brazilian population. The initial age of those in the cohort was 45 years, which is the mean age patients usually present at diagnosis and at treatment start [], and they were followed until death or 79 years of age, whichever came first. All patients entered the model with active disease.
Interventions Assessed
International guidelines, including the Endocrine Society and the Acromegaly Consensus Group, usually recommend after surgery failure the initial drug treatment with a first-generation SSA (octreotide long-acting release [LAR] or lanreotide autogel). If response is not achieved, second-line drug treatment can involve changing to pegvisomant or pasireotide or adding pegvisomant to the first-generation SSA. In cases of mild disease, the treatment with cabergoline is also suggested; however, this drug has never been evaluated in trials for acromegaly. In our analysis, we did not restrict the strategies to what is suggested in the guidelines and also explored other options (e.g., pegvisomant or pasireotide as fist-line drug treatments) in order to assess the cost-effectiveness of all treatments available with authorized use in acromegaly [, , ]. In Brazil, the strategies that are currently offered by the public health system are octreotide LAR, lanreotide autogel, and cabergoline. Pegvisomant and pasireotide are not reimbursed [].
The following strategies were compared: (a) octreotide LAR; (b) lanreotide autogel; (c) pasireotide LAR; (d) pegvisomant; (e) octreotide LAR and second-line pasireotide; (f) lanreotide autogel and second-line pasireotide; (g) octreotide LAR and second-line pegvisomant; (h) lanreotide autogel and second-line pegvisomant; (i) octreotide LAR and second-line pegvisomant in combination with octreotide LAR; (j) lanreotide autogel and second-line pegvisomant in combination with lanreotide autogel; (k) no treatment. In the base-case scenario, drugs were stopped if disease control was not achieved. Cabergoline and other dopamine agonists were not included in the analyses, as there is no randomized clinical trial evaluating their use in acromegaly.
Model Structure and Analyses
The model development, conduct, and reporting of this study followed SMDM-ISPOR recommendations, and cost-effectiveness analysis parameters were derived from Brazilian guidelines [-]. A Markov state-transition cohort model was constructed using TreeAge Pro 2016 (TreeAge Software Inc., Williamstown, MA, USA). The Markov model was chosen because acromegaly is a chronic disease with recurrent states. A scheme of the transition states can be seen in Figure 1.
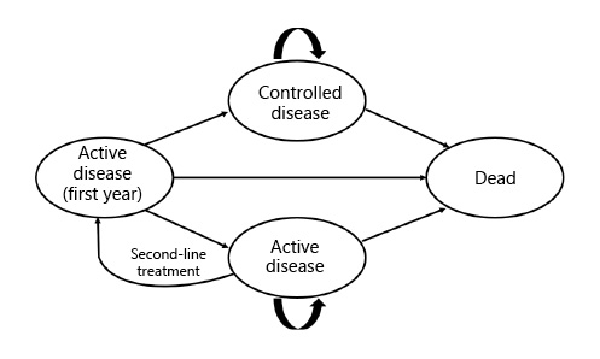
Fig. 1
State transition diagram for acromegaly pharmacological treatment.
The cost-utility of each strategy was estimated using direct costs (drug, drug administration, disease monitoring exams and consultations, hospitalization, day-hospital, drugs for comorbidities) and health utility values to obtain quality-adjusted life years (QALY).
The model was run probabilistically (Monte Carlo simulations) with 100,000 iterations. Within-cycle correction for state membership was used. A discount rate of 5% was applied for both costs and effectiveness. The probability of death per age was determined according to the Brazilian official life table, which estimates the risk of death until the age of 79 years []. Patients with active acromegaly have a standardized mortality ratio of approximately 2.4 when compared to the general population []. No cost-effectiveness threshold is defined in Brazil, but for the purpose of reporting results, we employed a cost-effectiveness threshold of three times the gross domestic product (GDP) per capita, equivalent to R$ 91,645/US$ 361,998 in 2016, the last official value available [].
The incremental cost-effectiveness ratio (ICER) was calculated in order to compare the strategies. A cost-effectiveness graph, cost-effectiveness acceptability curve, and Monte Carlo acceptability at cost threshold were constructed.
Base-Case Scenario
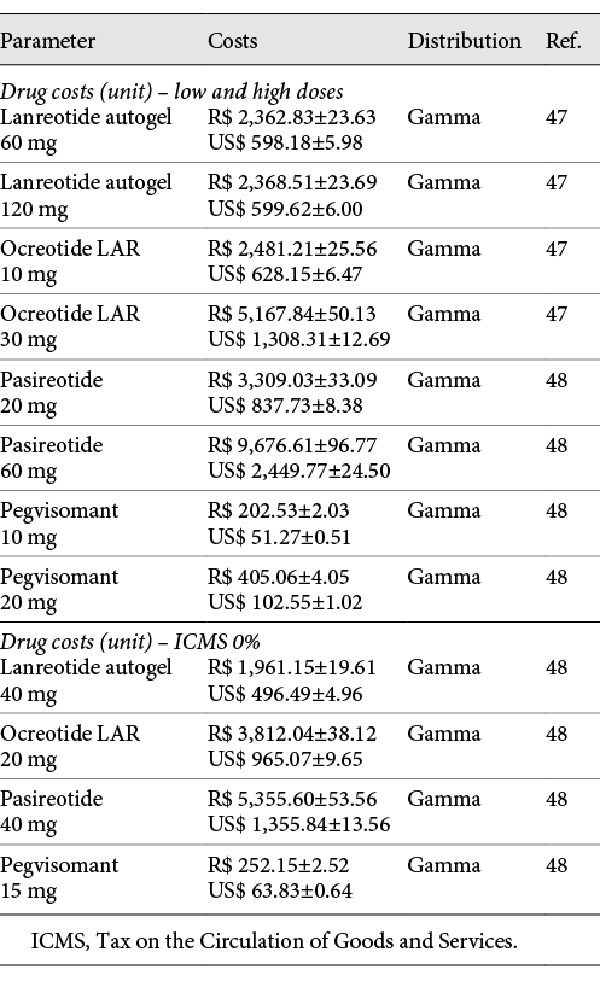
The base-case scenario considered that patients would stop receiving drug treatment if it was not effective. The drugs are presented in low, intermediate, and high doses. The dosage chosen for the base-case analysis was the intermediate one. A Tax on the Circulation of Goods and Services (referred in Brazilian Portuguese as ICMS) of 17% was levied on the drug costs retrieved from official price lists.
Model Assumptions
Some assumptions were considered for the model. We assumed that there would be no treatment discontinuation due to loss of efficacy. Therefore, after achieving the controlled state, patients could only remain in the controlled state for the following cycles or die. Discontinuation due to adverse events was considered only during the first year, as withdrawal for this reason is unlikely to occur after this period. The probability of discontinuation due to adverse events was considered equal for lanreotide and octreotide, as both drugs have the same mechanism of action [, ].
Costs
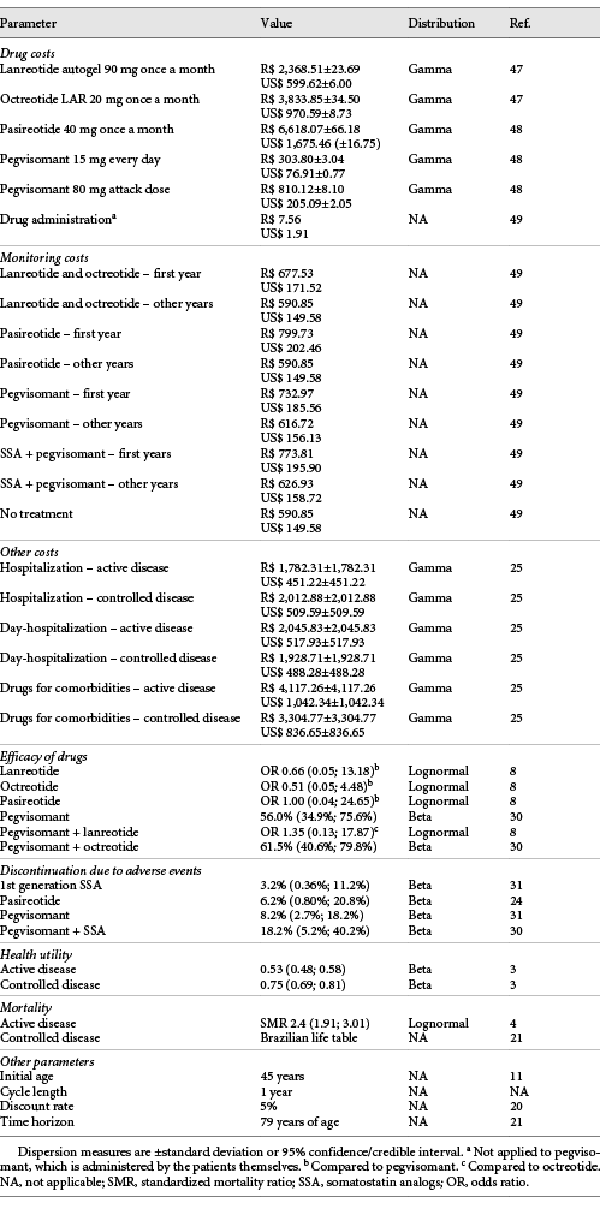
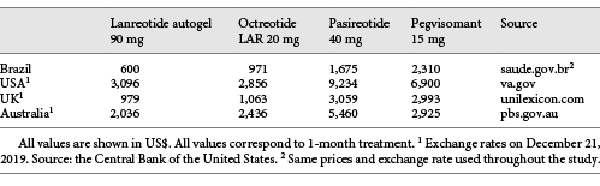
The costs of the drugs offered by the Brazilian public health system (octreotide LAR and lanreotide autogel) were obtained from the government database (Banco de Preços em Saúde –BPS) in order to get the actual prices charged on government purchases. The prices of drugs that are not offered by the public health system (pegvisomant and pasireotide) were retrieved from the Drug Market Regulation Chamber (known as CMED – Câmara de Regulação do Mercado de Medicamentos), considering the maximum selling price to the government. The fluctuation of the price of the drugs was based on octreotide real purchase data, as this was the only drug with several acquisitions. The standard deviation of the purchase costs was about 1% of the octreotide price. Therefore, the value of 1% was applied as standard deviation for the other drugs. All costs are shown in Table 1.
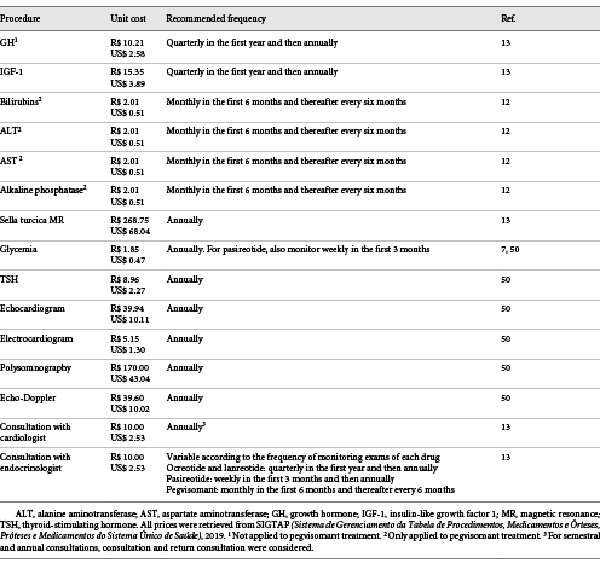
The costs of disease monitoring, including specialist consultation and exams, were obtained from the government database SIGTAP (Sistema de Gerenciamento da Tabela de Procedimentos, Medicamentos e Órteses, Próteses e Medicamentos do Sistema Único de Saúde). This database displays the costs reimbursed by the government for each procedure. Disease monitoring exams and frequency were selected according to national and international guidelines and were considered different between the first and the other years, because in the first year the patients must be more closely monitored for lack of efficacy and adverse events. These prices are not shown with a dispersion measure because they are standardized and fixed. The detailed costs of each procedure can be found in Table 2.
Other costs associated with acromegaly, such as drugs for comorbidities and costs of hospitalization and day-hospitalization were retrieved from a burden of disease study []. The costs were divided into those for patients with controlled disease and with uncontrolled disease. As the study was conducted in Italy some years ago, the costs were corrected for Italian monetary inflation using the consumer price index and converted to R$ according to the purchasing power parities [, ]. Since the primary study did not show a dispersion measure, we considered the value of the standard deviation to be the same as the mean []. All costs considered in the analyses are shown in Table 1. The costs throughout this article are shown in Brazilian Reais (R$) and in American Dollars (US$) for the year 2019. US$ 1.00 equals R$ 3.95 (average conversion rate in 2019) [].
Efficacy
The efficacy of drugs was retrieved from a systematic review [] and a clinical trial []. In both publications, treatment efficacy was defined as the achievement of IGF-1 normal levels for sex and age. Two utility values were considered, one for the state “controlled disease” and the other for the state “active disease,” based on the EQ-5D-3L questionnaire []. The probability of drug discontinuation due to adverse events was retrieved from clinical trials [, , ]. All parameters are shown in Table 1.
Scenario Analyses
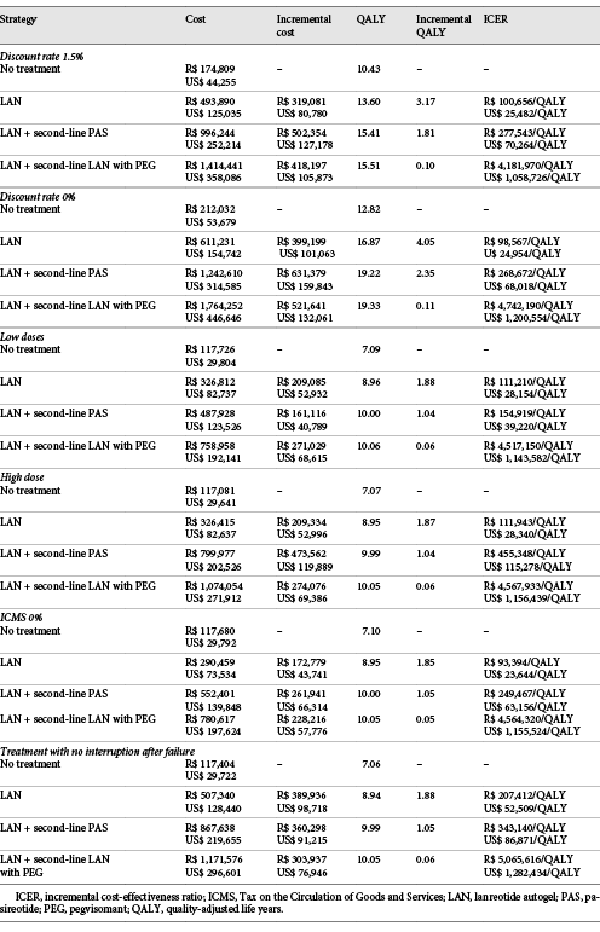
Alternative scenarios with 50,000 iterations each were conducted, considering low and high drug dosages, discount rates of 1.5 and 0%, an ICMS of 0%, and continuing the drug even if disease control was not achieved. The drug costs used in sensitivity analyses can be found in Table 3.
VOI Analysis
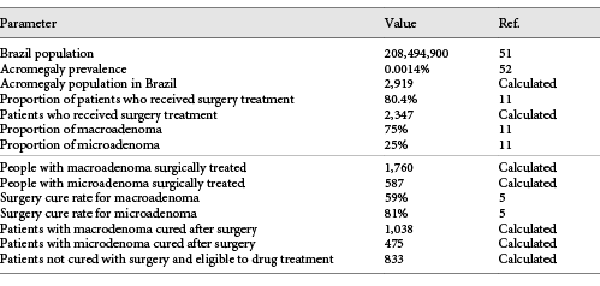
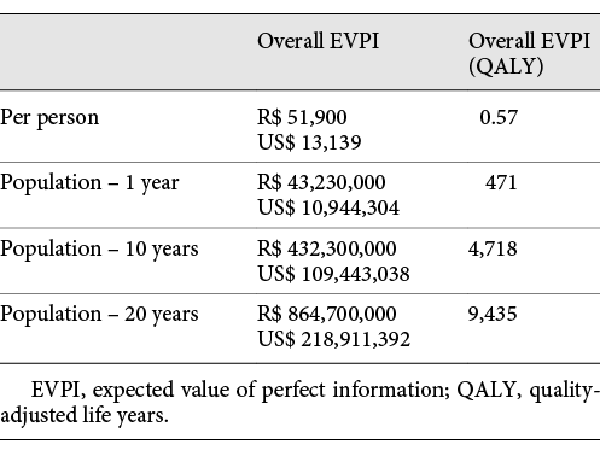
A VOI analysis was conducted using SAVI (Sheffield Accelerated Value of Information) v. 2.1.2. SAVI is an online application that calculates the expected value of perfect information (EVPI) and the expected value of partial perfect information (EVPPI) based on probabilistic analysis results through a nonparametric regression-based method []. Individual EVPI analysis estimates the opportunity loss of not choosing the ideal treatment option for each patient, which would only be possible with perfect information, and describes it in monetary values. Population EVPI considers the number of patients affected with the disease in the population and returns the maximum cost that should be spent with additional research to eliminate all the uncertainty []. After assessing the EVPI, an EVPPI analysis can be conducted to estimate the sensitivity of the decision to the uncertainty of each parameter individually, and therefore inform where future research should be focused []. For the population analyses, a total of 833 patients with acromegaly in Brazil was considered, based on disease prevalence, surgery failure, and Brazilian population. For more details, see Table 4.
Results
Base-Case Scenario
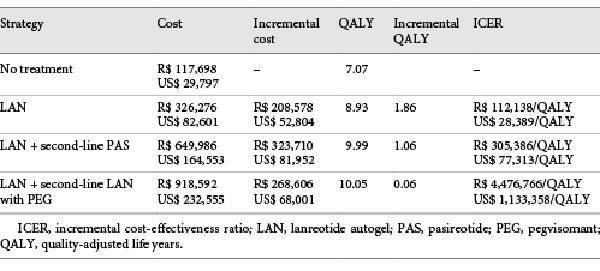
Eleven strategies were compared. The one with the lowest clinical effect was no treatment, with a QALY of 7.07, followed by octreotide LAR (8.74) and by lanreotide autogel (8.93). The strategies with the highest QALY values were lanreotide autogel + second-line lanreotide autogel with pegvisomant (10.05), and lanreotide autogel + second-line pasireotide (9.99). The less expensive strategy was no treatment, with a mean cost of R$ 117,698/US$ 29,797, followed by lanreotide autogel (R$ 326,276/US$ 82,601) and by octreotide LAR (R$ 423,707/US$ 107,267). The most expensive ones were octreotide + second-line octreotide with pegvisomant (R$ 1,120,628/US$ 283,703) and pegvisomant (R$ 1,023,381/US$ 259,084).
Of the 11 strategies compared, only four were not dominated. For these strategies, the ICERs were calculated and are shown in Table 5. Figure 2 depicts the cost-effectiveness graph. The strategies that are not connected by the line are dominated.
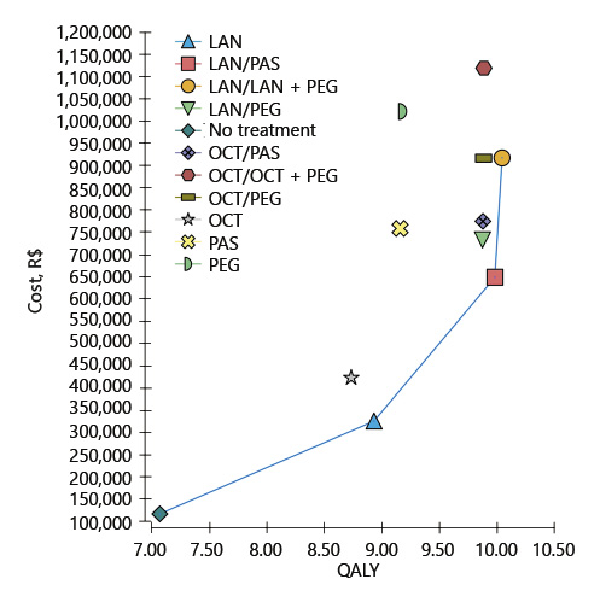
Fig. 2
Cost-effectiveness graph of the results of the base-case scenario. LAN, lanreotide autogel; OCT, octreotide LAR; PAS, pasireotide; PEG, pegvisomant; QALY, quality-adjusted life years. The bar separates the first- and second-line treatments and the plus sign represents drugs that are given in combination.
The only strategy that shows potential to be considered cost-effective is lanreotide autogel (ICER compared to no treatment of R$ 112,138/US$ 28,389 per QALY). The probability of it being the most cost-effective option compared to no treatment is shown in Figure 3 for different cost-effectiveness thresholds. When considering three times Brazil’s GDP per capita (R$ 91,645/US$ 23,201) as the cost threshold value [], lanreotide autogel was the most cost-effective strategy 38.19% of the time compared to no treatment.
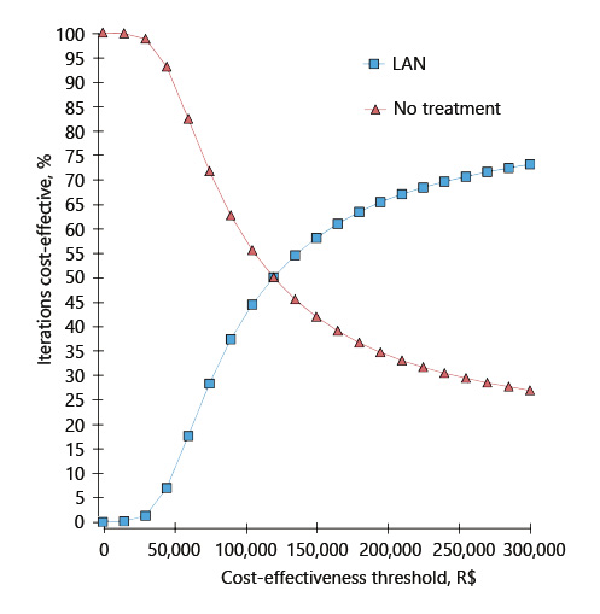
Fig. 3
Cost-effectiveness acceptability curve. LAN, lanreotide autogel.
Scenario Analyses
All scenario analyses showed the same cost-effectiveness rank as the base-case scenario (Table 6). When a discount rate of 1.5% is applied, the ICER of lanreotide autogel compared to no treatment drops from R$ 112,138/US$ 28,389 per QALY (base-case) to R$ 100,656/US$ 25,482 per QALY, and when the discount rate is equal to 0%, the value drops to R$ 98,567/US$ 24,954 per QALY. Varying the dosage of drugs had a low impact on the results (R$ 111,210/US$ 28,154 per QALY ICER of lanreotide compared to no treatment for low doses and R$ 111,943/US$ 28,340 per QALY for high doses). The lowest ICER was obtained when considering a 0% ICMS (R$ 93,394/US$ 23,644 per QALY ICER of lanreotide compared to no treatment), and the highest ICER was observed when considering that treatment would not be stopped even if efficacy was not achieved (R$ 207,412/US$ 52,509 per QALY ICER of lanreotide compared to no treatment). All scenarios showed higher ICERs than 3 times the Brazilian GDP.
VOI Analysis
The overall EVPI per person was estimated at R$ 51,900/US$ 13,139, which is equivalent to 0.57 QALYs per person. The overall population EVPI was estimated at R$ 43,230,000/US$ 10,944,304 per year. This is the expected cost of uncertainty surrounding the model and the maximum value that future research should cost to eliminate all relevant decision uncertainty. When considering other decision relevance horizons, the population EVPI is even higher (Table 7).
As can be seen in Figure 4, the individual parameters in which uncertainty affects the decision the most are, by far, the utility of active and controlled disease (R$ 30,466/US$ 7,713 and R$ 27,163/US$ 6,877 per person, respectively). This means that future research to reduce decision uncertainty should be focused on quality of life studies.
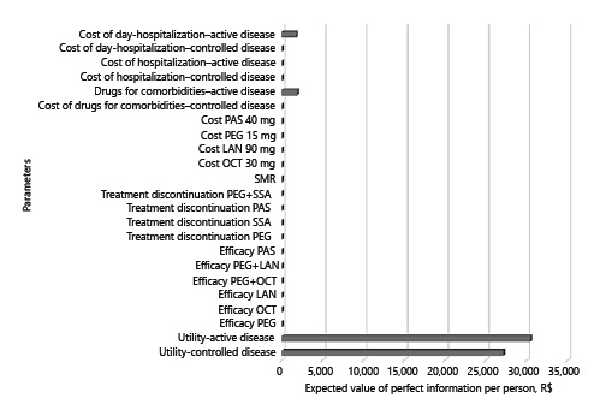
Fig. 4
Expected value of partial perfect information per person. LAN, lanreotide; OCT, octreotide; PAS, pasireotide; PEG, pegvisomant; SSA, somatostatin analogues; SMR, standardized mortality ratio.
Discussion
This is the first cost-utility study in acromegaly to simultaneously compare both first- and second-line drug treatments after an unsuccessful surgery, and the first one to present a VOI analysis [].
In Brazil, there is no defined cost-effectiveness threshold []. Therefore, we adopted the value of three times the GDP per capita just as a reference for the analyses; however, it should not be taken as an absolute value. Considering this threshold, none of the drug strategies was cost-effective, neither in the base-case analysis nor in the sensitivity scenario analyses. The only strategy that showed the potential to be considered cost-effective was first-line lanreotide autogel at this moment, with ICER estimated at R$ 112,138/US$ 28,389 per QALY compared to no treatment, a value not very far from the theoretical cost-effectiveness threshold (R$ 91,645/US$ 23,201). With this threshold, lanreotide autogel had a 38.19% chance of being cost-effective. According to a review of decisions by the National Institute of Health and Care Excellence (NICE), drugs that show a 40% certainty of being cost-effective based on probabilistic results are usually incorporated into the health system []. Considering that the analysis was performed for a different country, and that acromegaly is a rare disease (which can affect the value of the cost threshold []), lanreotide autogel should not be disregarded as a treatment option. The ICERs for all the other drugs were too elevated. Therefore, based on the results of our analyses, at this moment there is no cost-effective second-line drug treatment for acromegaly, and only lanreotide autogel showed the potential to be considered as a first-line drug option after surgery failure in Brazil.
Currently, the Brazilian health system offers both octreotide LAR and lanreotide autogel as options for acromegaly treatment, and no other drugs for acromegaly are reimbursed []. However, our analyses showed that octreotide is a dominated strategy. Therefore, we suggest reevaluating the offer of this drug by Brazilian public health system, and maybe focusing the reimbursement on lanreotide autogel. Octreotide and lanreotide are both first-generation SSAs, and both have the same mechanism of action []. Thus, if a patient does not achieve control with one of these drugs, changing to the other one is not recommended. One possible reason for the prescription of octreotide LAR as a first-line treatment for acromegaly patients, despite its higher cost, is that octreotide was the first SSA developed; thus, the physicians may be more familiar with its use [].
Previous cost-effectiveness analyses have been performed from the Brazilian public payer perspective [-]. However, they showed some limitations, such as considering a time horizon as short as 2 years, comparing octreotide LAR to a formulation of lanreotide that is no longer used (SR) [], and comparing a second-line drug to retreatment with a first-line drug without considering the option of no treatment [, ]. Furthermore, all three studies were sponsored by the drug producer. Only one study was a cost-utility analysis []; however, as it was published as a conference abstract, a deeper analysis could not be made.
In our study, the VOI analysis enabled the estimation of uncertainty. Through the EVPI analysis, it is possible to appraise how much money is lost if the decision is made in an environment of high uncertainty. Therefore, the population EVPI shows how much should be spent on future research to eliminate uncertainty. Our results demonstrated that a maximum amount per year of R$ 43,230,000/US$ 10,944,304 should be considered. Thus, it is safe to say that the performance of new clinical studies is mandatory to eliminate the uncertainty surrounding the parameters before definitive conclusions concerning the cost-effectiveness of the therapies are made.
The EVPPI helps to identify which parameters show uncertainty that can significantly affect the decision. Our EVPPI results showed that almost all the uncertainty involves health-related quality of life (utility) values. Therefore, future research should focus on determining utility values for patients with acromegaly. In our model, we used utility data from the study by Liu et al. [], which employed EQ-5D-3L to derive utility values from patients with more than, and fewer than four symptoms. Previous cost-utility studies [, ] used utility data from Rowles et al. []. However, in this study, a utility value was obtained for patients with acromegaly, irrespective of disease control, and this value was compared to the utility of the general population, derived from another nonrelated study []. Notwithstanding, the cited articles considered the former value as the one for patients with active disease, and the latter (general population) as the utility value for patients with controlled disease. This seems to be inappropriate, especially considering that acromegaly causes irreversible sequelae []. Therefore, considering the limitations of existing studies and the important role that uncertainty in utility plays in this decision analysis, future clinical research on acromegaly should focus on health-related quality of life.
Our study had some limitations. We utilized fixed medication doses throughout the treatment; however, in clinical practice, patients usually receive a titrated approach. We tried to overcome this issue by exploring other doses in the sensitivity analyses. We did not consider treatment discontinuation after the first year, neither due to loss of efficacy nor due to adverse events. We only considered these values for the first year, for which there is available data. We also did not consider the costs of adverse events, as they are usually mild and resolve with treatment discontinuation. Furthermore, in some cases, reoperation or radiotherapy can be considered as treatment option after drug failure and can potentially improve cost-effectiveness. Reoperation can be considered in patients that have uncontrolled disease despite drug treatment and that show a surgically approachable tumor. Radiotherapy is usually restricted to patients that still have active disease after surgery and drug treatment. However, radiotherapy can cause hypopituitarism, secondary tumors, and long-term neurocognitive impairment [, , ].
For an illustrative comparison, acromegaly drug costs in Brazil and other countries (USA, UK, and Australia) are shown in Table 8. All these countries have their own peculiarities concerning their health system. However, considering that drug costs are the main source of expenditure during acromegaly treatment, some conclusions can be speculated. In all countries, with the exception of the USA, lanreotide autogel is cheaper than octreotide LAR. Therefore, considering that the effects with lanreotide autogel are better than with octreotide LAR, the first dominates the latter. Hence, lanreotide autogel seems to be appropriate as the first drug treatment option after surgery failure for the majority of the countries. For the USA, a more detailed analysis should be made before any conclusion. In Brazil, pegvisomant is the most expensive drug. Nonetheless, in Australia, the UK, and the USA, pasireotide is the most expensive one. This suggests that, unlike the proposed for Brazil, pegvisomant can be a more cost-effective option to be associated with the first-generation SSA if the first-generation SSA does not control the disease, considering that the association with pegvisomant resulted in a slightly improved QALY compared to pasireotide (0.06). For deeper conclusions, it is recommended that the model is fully adapted to each country perspective. The structure of the model and the results of the VOI analysis can be applied to any country, as the data regarding drug efficacy and quality of life were retrieved from the international literature, and because the EVPPI analysis showed that almost all the uncertainty involves utility values.
Until better estimates of utility values for acromegaly are obtained, all cost-effectiveness analyses conducted in any country and from any perspective will carry considerable uncertainty that will compromise the conclusions of which drug treatment should be adopted for acromegaly after surgery failure.
Acknowledgements
We would like to thankCoordenação de Aperfeiçoamento de Pessoal de Nível Superior – Brasil (CAPES), Finance Code 001, and the Government of Canada through the Emerging Leaders in the Americas Program (ELAP) for the scholarships.
Statement of Ethics
Ethical approval was not required because this study only used public data from scientific articles or open databases.
Disclosure Statement
All authors declare that they have no conflict of interest.
Funding Sources
This study was not funded. Scholarships were provided by Coordenação de Aperfeiçoamento de Pessoal de Nível Superior (Brazil) and by the Emerging Leaders in the Americas Program (Canada).
Author Contributions
All authors contributed to the study concept and design and the acquisition, analysis, or interpretation of data, and the critical revision of the manuscript for important intellectual content. The first draft of the manuscript was written by L.P.L., and all authors commented on previous versions of the manuscript. All authors read and approved the final manuscript.
References
- 1. Melmed S. Acromegaly pathogenesis and treatment. J Clin Invest. 2009;119(11):3189–202. 0021-9738
- 2. Chanson P, Salenave S, Kamenicky P. Acromegaly. Handb Clin Neurol. 2014;124:197–219. 0072-9752
- 3. Liu S, Adelman DT, Xu Y, Sisco J, Begelman SM, Webb SM, et al Patient-centered assessment on disease burden, quality of life, and treatment satisfaction associated with acromegaly. J Investig Med. 2018;66(3):653–60. 1081-5589
- 4. Bolfi F, Neves AF, Boguszewski CL, Nunes-Nogueira VS. Mortality in acromegaly decreased in the last decade: a systematic review and meta-analysis. Eur J Endocrinol. 2018;179(1):59–71. 0804-4643
- 5. Buchfelder M, Schlaffer SM. The surgical treatment of acromegaly. Pituitary. 2017;20(1):76–83. 1386-341X
- 6. Vilar L, Vilar CF, Lyra R, Lyra R, Naves LA. Acromegaly: clinical features at diagnosis. Pituitary. 2017;20(1):22–32. 1386-341X
- 7. Melmed S, Bronstein MD, Chanson P, Klibanski A, Casanueva FF, Wass JA, et al A Consensus Statement on acromegaly therapeutic outcomes. Nat Rev Endocrinol. 2018;14(9):552–61. 1759-5029
- 8. Leonart LP, Ferreira VL, Tonin FS, Fernandez-Llimos F, Pontarolo R. Medical Treatments for Acromegaly: A Systematic Review and Network Meta-Analysis. Value Health. 2018;21(7):874–80. 1098-3015
- 9. Leonart LP, Borba HH, Ferreira VL, Riveros BS, Pontarolo R. Cost-effectiveness of acromegaly treatments: a systematic review. Pituitary. 2018;21(6):642–52. 1386-341X
- 10. Lavrentaki A, Paluzzi A, Wass JA, Karavitaki N. Epidemiology of acromegaly: review of population studies. Pituitary. 2017;20(1):4–9. 1386-341X
- 11. Maione L, Chanson P. National acromegaly registries. Best Pract Res Clin Endocrinol Metab. 2019;33(2):101264. 1521-690X
- 12. Katznelson L, Laws ER Jr, Melmed S, Molitch ME, Murad MH, Utz A, et alEndocrine Society. Acromegaly: an endocrine society clinical practice guideline. J Clin Endocrinol Metab. 2014;99(11):3933–51. 0021-972X
- 13. Brazil. Protocolo Clínico e Diretrizes Terapêuticas da Acromegalia - Ministry of Health. 2019.
- 14. Briggs AH, Weinstein MC, Fenwick EA, Karnon J, Sculpher MJ, Paltiel ADISPOR-SMDM Modeling Good Research Practices Task Force. Model parameter estimation and uncertainty analysis: a report of the ISPOR-SMDM Modeling Good Research Practices Task Force Working Group-6. Med Decis Making. 2012;32(5):722–32. 0272-989X
- 15. Caro JJ, Briggs AH, Siebert U, Kuntz KMISPOR-SMDM Modeling Good Research Practices Task Force. Modeling good research practices—overview: a report of the ISPOR-SMDM Modeling Good Research Practices Task Force-1. Med Decis Making. 2012;32(5):667–77. 0272-989X
- 16. Eddy DM, Hollingworth W, Caro JJ, Tsevat J, McDonald KM, Wong JBISPOR-SMDM Modeling Good Research Practices Task Force. Model transparency and validation: a report of the ISPOR-SMDM Modeling Good Research Practices Task Force-7. Med Decis Making. 2012;32(5):733–43. 0272-989X
- 17. Roberts M, Russell LB, Paltiel AD, Chambers M, McEwan P, Krahn MISPOR-SMDM Modeling Good Research Practices Task Force. Conceptualizing a model: a report of the ISPOR-SMDM Modeling Good Research Practices Task Force-2. Med Decis Making. 2012;32(5):678–89. 0272-989X
- 18. Siebert U, Alagoz O, Bayoumi AM, Jahn B, Owens DK, Cohen DJ, et alISPOR-SMDM Modeling Good Research Practices Task Force. State-transition modeling: a report of the ISPOR-SMDM Modeling Good Research Practices Task Force—3. Value Health. 2012;15(6):812–20. 1098-3015
- 19. Husereau D, Drummond M, Petrou S, Carswell C, Moher D, Greenberg D, et alCHEERS Task Force. Consolidated Health Economic Evaluation Reporting Standards (CHEERS) statement. Value Health. 2013;16(2):e1–5. 1098-3015
- 20. Brazil. Methodological guideline: economic evaluaton of health technologies - Ministry of Health. 2014.
- 21. IBGE. Brazilian life table.2017.
- 22. IBGE. 2016 Brazil gross domestic product per capita. 2019.
- 23. Ben-Shlomo A, Melmed S. Somatostatin agonists for treatment of acromegaly. Mol Cell Endocrinol. 2008;286(1-2):192–8. 0303-7207
- 24. Gadelha MR, Bronstein MD, Brue T, Coculescu M, Fleseriu M, Guitelman M, et alPasireotide C2402 Study Group. Pasireotide versus continued treatment with octreotide or lanreotide in patients with inadequately controlled acromegaly (PAOLA): a randomised, phase 3 trial. Lancet Diabetes Endocrinol. 2014;2(11):875–84. 2213-8587
- 25. Didoni G, Grottol S, Gasco V, Battistini M, Ferone D, Giusti M, et al Cost-of-illness study in acromegalic patients in Italy. J Endocrinol Invest. 2004;27(11):1034–9. 0391-4097
- 26. InflationTool. Value of 2003 Euro (Italy) today. 2019.
- 27. OECD. "Purchasing power parities (PPP)" (indicator). 2019.
- 28. Briggs A, Claxton K, Sculpher M. Decision modelling for hralth economic evaluation. Oxford University Press; 2011.
- 29. IPEA. Instituto de Pesquisa Econômica Aplicada - Taxa de câmbio comercial para venda. 2020.
- 30. Trainer PJ, Ezzat S, D’Souza GA, Layton G, Strasburger CJ. A randomized, controlled, multicentre trial comparing pegvisomant alone with combination therapy of pegvisomant and long-acting octreotide in patients with acromegaly. Clin Endocrinol (Oxf). 2009;71(4):549–57. 0300-0664
- 31. Ghigo E, Biller BM, Colao A, Kourides IA, Rajicic N, Hutson RK, et al Comparison of pegvisomant and long-acting octreotide in patients with acromegaly naïve to radiation and medical therapy. J Endocrinol Invest. 2009;32(11):924–33. 0391-4097
- 32. Strong M, Oakley JE, Brennan A. Estimating multiparameter partial expected value of perfect information from a probabilistic sensitivity analysis sample: a nonparametric regression approach. Med Decis Making. 2014;34(3):311–26. 0272-989X
- 33. Thorn J, Coast J, Andronis L. Interpretation of the Expected Value of Perfect Information and Research Recommendations: A Systematic Review and Empirical Investigation. Med Decis Making. 2016;36(3):285–95. 0272-989X
- 34. Soarez PC, Novaes HM. Cost-effectiveness thresholds and the Brazilian Unified National Health System. Cad Saude Publica. 2017;33(4):e00040717. 1678-4464
- 35. Adalsteinsson E, Toumi M. Benefits of probabilistic sensitivity analysis - a review of NICE decisions. J Mark Access Health Policy. 2013;1(1):1. 2001-6689
- 36. Ollendorf DA, Chapman RH, Pearson SD. Evaluating and Valuing Drugs for Rare Conditions: No Easy Answers. Value Health. 2018;21(5):547–52. 1098-3015
- 37. Gadelha MR, Wildemberg LE, Bronstein MD, Gatto F, Ferone D. Somatostatin receptor ligands in the treatment of acromegaly. Pituitary. 2017;20(1):100–8. 1386-341X
- 38. Pless J. The history of somatostatin analogs. J Endocrinol Invest. 2005;28(11 Suppl International):1-4.
- 39. Valentim J, Passos V, Mataveli F, Calabró A. Cost-effectiveness analysis of somatostatin analogues in the treatment of acromegaly in Brazil. Arq Bras Endocrinol Metabol. 2008;52(9):1452–60. 0004-2730
- 40. Fujii RK, Mould JF, Fernandes RA, Furlan F, Manfrin DF. Economic evaluation of pegvisomant for active acromegaly patients who failed available therapies in Brazil—public health care system perspective. Value Health. 2012;15(4):A105–105. 1098-3015
- 41. Souza CR, Ferreira CN, Ribeiro AP, Musolino NR. Economic evaluation of pegvisomanto for the treatment of patients with acromegaly with an inadequate response to a maximal dose of octreotide (alone or combined) in the context of the Unified Health System. Brazilian Journal of Health Economics.2014;6(2):8.
- 42. Moore DJ, Adi Y, Connock MJ, Bayliss S. Clinical effectiveness and cost-effectiveness of pegvisomant for the treatment of acromegaly: a systematic review and economic evaluation. BMC Endocr Disord. 2009;9(1):20. 1472-6823
- 43. Peral C, Cordido F, Gimeno-Ballester V, Mir N, Sanchez-Cenizo L, Rubio-Rodriguez D, et al Cost-effectiveness analysis of second-line pharmacological treatment of acromegaly in Spain. Expert Rev Pharmacoecon Outcomes Res. 2019;•••:1–10. 1473-7167
- 44. Rowles SV, Prieto L, Badia X, Shalet SM, Webb SM, Trainer PJ. Quality of life (QOL) in patients with acromegaly is severely impaired: use of a novel measure of QOL: acromegaly quality of life questionnaire. J Clin Endocrinol Metab. 2005;90(6):3337–41. 0021-972X
- 45. Burström K, Johannesson M, Diderichsen F. Swedish population health-related quality of life results using the EQ-5D. Qual Life Res. 2001;10(7):621–35. 0962-9343
- 46. Chanson P, Salenave S. Acromegaly. Orphanet J Rare Dis. 2008;3(1):17. 1750-1172
- 47. Banco de Preços em Saúde. Ministry of Health of Brazil. 2019.
- 48. Câmara de Regulação do Mercado de Medicamentos. Brazilian Health Regulatory Agency. 2019.
- 49. Sistema de Gerenciamento da Tabela de Procedimentos, Medicamentos e Órteses, Próteses e Medicamentos do Sistema Único de Saúde. Ministry of Health of Brazil. 2019.
- 50. Melmed S, Casanueva FF, Klibanski A, Klibanski A, Bronstein MD, Chanson P, et al. A consensus on the diagnosis and treatment of acromegaly complications. Pituitary. 2013 Sep;16(3):294–302.
- 51. IBGE. Population. 2018.
- 52. Day PF, Loto MC, Glerean M, Picasso MFR, Lovazzano S, Giunta DH. Incidence and prevalence of clinically relevant pituitary adenomas: retrospective cohort study in a Health Management Organization in Buenos Aires, Argentina. Arch Endocrinol Metab. 2016 Nov-Dec;60(6):554–561.