Introduction
Glucagon-like peptide-1 (GLP-1) is synthetized in 2 distinct cell populations. The endocrine L cells of the distal ileum and colon secrete this hormone to the circulation, while neuronal groups located primarily in the caudal nucleus tractus solitarii utilizes GLP-1 as a neurotransmitter [-]. These GLP-1 neurons have a wide projection field, innervating a large number of neuronal groups both in the forebrain and brain stem. The density of GLP-1-immunoreactive (GLP-1-IR) fibers is especially high in hypothalamic feeding-related nuclei. During starvation, plasma GLP-1 level is very low in the circulation, but after food intake, the secretion of GLP-1 is rapidly increased, especially after the ingestion of food rich in fat and carbohydrates [, ]. GLP-1 plays an important role in the regulation of glucose homeostasis, food intake, and body weight [, ].
Peripheral action of GLP-1 decreases the blood glucose level by slowing gastric emptying, glucose-dependently stimulating insulin secretion, and decreasing glucagon production []. High plasma concentration of GLP-1 also has a strong anorexigenic effect []. This anorexigenic effect of GLP-1 can also be observed when GLP-1 is directly injected to the central nervous system (CNS) [, ], indicating that the anorexigenic effect of GLP-1 is at least partly mediated by the CNS.
One CNS neuron group that plays a critical role in the regulation of food intake is the group of proopiomelanocortin (POMC) neurons in the arcuate (ARC) nucleus []. Ablation of these neurons results in increased body weight [], and lack of POMC or lack of the melanocortin 4 receptor, one of the receptors of the POMC-derived α-melanocyte-stimulating hormone, results in obesity both in rodents and humans []. These ARC POMC neurons play a critical role in the integration of peripheral adiposity and satiety signals like leptin, insulin, glucocorticoids, and circulating metabolites like amino acids, lipids, and glucose []. The POMC neurons also express GLP-1 receptor (GLP-1R) and are stimulated by GLP-1 agonists [, ]. Furthermore, the POMC neurons bind the GLP-1 agonist liraglutide after its peripheral administration [] in the rat. Other studies also indicated that GLP-1 directly stimulates the activity of POMC neurons []. As our recent ultrastructural studies [] demonstrated that GLP-1R is present not only on the perikarya but also in a high number of axon terminals in the ARC, we raised the possibility that in addition to its direct effect on the POMC neurons, GLP-1 may also influence the POMC neurons indirectly by modulating the activity of the GLP-1-sensitive presynaptic terminals. To test this hypothesis, we performed ultrastructural studies to determine the relationship of the POMC neurons and the GLP-1R-IR structures and examined the effect of GLP-1 signaling on the inputs of the POMC neurons.
Materials and Methods
Animals
Tg(POMC-Cre)16Lowl/J [] and Gt(ROSA)26Sor_CAG/tdTomato//C57Bl/6 J [] mice were crossed to generate double transgenic Tg(POMC-Cre)16Lowl/J//Gt(ROSA)26Sor_CAG/tdTomato//C57Bl/6 J mice. Adult male, double transgenic mice weighing 25–35 g were used for ultrastructural and electrophysiological investigations. Animals were housed under standard environmental conditions (lights on between 06.00 and 18.00 h, temperature 22 ± 1°C, and food and water were available ad libitum).
Tissue Preparation for Ultrastructural Studies
Mice were anesthetized with a mixture of ketamine and xylazine (ketamine 50 mg/kg body weight, xylazine 10 mg/kg body weight, intraperitoneally) and were perfused transcardially with 10 mL 0.1 M PBS (pH 7.4), followed by 50 mL 3% paraformaldehyde (PFA; Electron Microscopy Sciences, Fort Washington, PA, USA) and 1% acrolein (Sigma Aldrich) in 0.1 M phosphate buffer (PB, pH 7.4), and then followed by 10 mL 4% PFA in 0.1 M PB. The brains were rapidly removed and postfixed in 4% PFA in 0.1 M PB (pH 7.4) overnight at 4°C.
Double-Labeling Immunocytochemistry for GLP-1R and tdTomato
Serial, 25-µm-thick coronal sections were cut on a Leica VT 1000S vibratome (Leica Microsystems, Wetzlar, Germany) through the ARC. The sections were first pretreated in 1% sodium borohydride in 0.01 M PBS for 30 min, washed in 0.01 M PBS, and then treated with 0.5% H2O2 in 0.01 M PBS for 15 min. The sections were cryoprotected in 15% sucrose in 0.01 M PBS for 15 min at room temperature and in 30% sucrose in 0.01 M PBS overnight at 4°C, and then quickly frozen over liquid nitrogen and thawed 3 times to improve antibody penetration into the tissue. The nonspecific antibody binding was blocked by placing the sections in 2% normal horse serum diluted in PBS for 20 min.
The pretreated sections were placed in a mixture of sheep anti-tdTomato serum (1:80,000 raised in our laboratory []) and rabbit monoclonal antibody against GLP-1R (0.016 µg/mL, Clone 7F38; Novo Nordisk A/S []). After rinsing in 0.01 M PBS and 0.1% cold water fish gelatin/1% bovine serum albumin in PBS, the sections were incubated overnight in donkey anti-sheep IgG conjugated with 0.8 nm colloidal gold (Electron Microscopy Sciences, Fort Washington, PA, USA) diluted at 1:100 and biotinylated donkey anti-rabbit IgG diluted at 1:500 in 0.01 M PBS containing 0.1% cold water fish gelatin and 1% bovine serum albumin. After washing, the sections were fixed in 1.25% glutaraldehyde in 0.1 M PB for 10 min at room temperature. After further rinsing in 0.01 M PBS, the sections were washed in Aurion ECS buffer (Aurion) (1:10) diluted in distilled water for 2 × 10 min. The gold particles were silver intensified with the Aurion R-Gent SE-LM Kit after rinsing in 0.2 M sodium citrate, pH 7.5, followed by treatment in avidin-biotin complex (ABC Elite, PK-6100, 1:1,000; Vector Laboratories Ltd, Peterborough, UK). GLP-1R-immunoreactivity was detected with 0.05% DAB/0.15% Ni ammonium sulfate/0.005% H2O2 in 0.05 M Tris buffer, pH 7.6.
Embedding and Ultrastructural Examination of the Immunostained Sections
Sections were incubated in 1% osmium tetroxide (Electron Microscopy Sciences, Fort Washington, PA, USA), dissolved in 0.1 M PB for 60 min at room temperature, and then treated with 2% uranyl acetate (Electron Microscopy Sciences, Fort Washington, PA, USA) in 70% ethanol for 30 min. Following dehydration in an ascending series of ethanol and acetonitrile (Sigma Aldrich), the sections were flat embedded in Durcupan ACM epoxy resin (Fluka) on liquid release agent (Electron Microscopy Sciences, Fort Washington, PA, USA)-coated slides and polymerized at 56°C for 2 days. After polymerization, 60- to 70-nm-thick ultrathin sections were cut with the Leica UCT ultramicrotome (Leica Microsystems, Wetzlar, Germany). The ultrathin sections were mounted onto formvar-coated, single-slot grids, treated with lead citrate (Biomarker), and examined with a JEOL-100 C transmission electron microscope.
Slice Preparation for Electrophysiological Recordings
Mice were deeply anesthetized with isoflurane and subsequently decapitated. Brains were rapidly removed from the skull and placed into ice-cold cutting solution saturated with 95% O2 and 5% CO2 (carbogen gas). The cutting solution was prepared as follows (in mM): 140 NaCl, 3 KCl, 1.3 MgSO4, 1.4 NaH2PO4, 2.4 CaCl2, 11 glucose, and 5 HEPES (pH 7.4, 280–300 mOsm/L). Hypothalamic blocks were dissected from the mouse brains, and 200-μm-thick coronal slices were cut using a VT1200S vibratome (Leica). We collected and used slices for patch-clamp measurements exclusively from the bregma −1.7 to −2.5 mm containing the ARC. As precursors of POMC neurons are also precursors of some of the NPY neurons [], we targeted tdTomato-expressing neurons in the ARC of Tg(POMC-Cre)16Lowl/J//Gt(ROSA)26Sor_CAG/tdTomato//C57Bl/6 J mice exclusively from the lateral part of the nucleus where NPY neurons are not present. The slices were bisected along the third ventricle and transferred into a holding chamber containing artificial cerebrospinal fluid (ACSF) with the following composition (in mM): 140 NaCl, 3 KCl, 1.4 NaH2PO4, 2 MgCl2, 2.4 CaCl2, 26 NaHCO3, and 11 glucose, saturated with carbogen gas (pH 7.4; 280–300 mOsm/L) at room temperature for at least 1.5 h before the recording. Slices were transferred to a submersion-type recording chamber. During recording, the temperature was maintained at 32–33°C in the slice mini chamber with a temperature controller typ TC7 unit (Luigs & Neumann GmbH, Ratingen, Germany). The flow rate was set between 1.5 and 2 mL/min.
Whole-Cell Patch-Clamp Recording
POMC neurons were identified in the ARC based on the red fluorescence signal of tdTomato and were measured using the current-clamp configuration in whole-cell patch-clamp mode. The instruments used for data recording were as follows: a Multiclamp 700B patch-clamp amplifier, a Digidata-1440A data acquisition system, and pCLAMP 10.4 software (Axon Instruments-Molecular Devices Co.). Data were filtered at 3 kHz using the built-in Bessel filter of the amplifier and digitized at 20 kHz. The headstage of the amplifier was fitted to a Luigs & Neumann SM7 micromanipulator system. The cells were visualized by an Eclipse FN1 upright microscope (Nikon) equipped with infrared Nomarski differential interference contrast optics and a CCD camera (Zyla; ANDOR). The patch electrodes (OD = 1.5 mm, thin wall, Garner Co.) were pulled with a Flaming/Brown P-97 horizontal puller (Sutter Instrument Co.). The resistance of the patch electrodes was 3–5 MΩ.
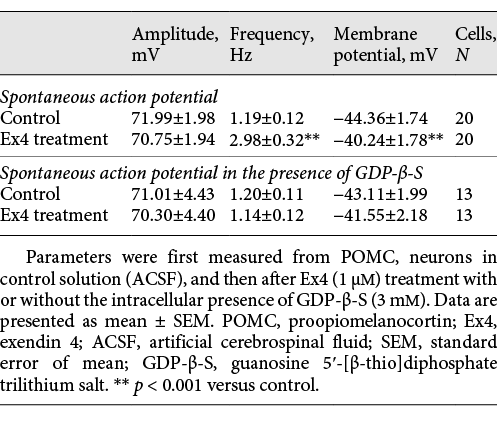
The intracellular and extracellular solutions used in the different experiments are summarized in Table 1. We used low-chloride intracellular pipette solution for electrophysiological recording of miniature excitatory postsynaptic currents (mEPSCs) or evoked EPSCs (eEPSCs) or when the mEPSCs and miniature inhibitory PSCs (mIPSCs) were recorded simultaneously. The low-chloride intracellular pipette solution contained (in mM) the following: 110 K-gluconate, 4 NaCl, 20 HEPES, 0.1 EGTA, 2 Mg-ATP, 0.3 Na-GTP, and 10 phosphocreatine salt (pH 7.25; 290–300 mOsm/L). High-chloride intracellular pipette solution was used for measuring mIPSCs or evoked IPSCs (eIPSCs). This solution contained (in mM) the following: 54 K-gluconate, 56 KCl, 4 NaCl, 20 HEPES, 0.1 EGTA, 2 Mg-ATP, 0.3 Na-GTP, and 10 phosphocreatine salt (pH 7.25; 290–300 mOsm/L). For voltage-clamp recordings, the holding current was set to −70 mV. In experiments when mEPSCs and mIPSCs were measured simultaneously, the holding potential was set to −40 mV. For the measurement of membrane potential changes and spontaneous firing rates, low-chloride intracellular solution and I = 0 configuration were used. To dissect pre- and postsynaptic effects of GLP-1, we blocked the GLP-1 signaling via inhibition of G-proteins in the POMC neurons by the addition of 3 mM G-protein blocker guanosine 5′-[β-thio]diphosphate trilithium salt (GDP-β-S) to the intracellular pipette solution []. When mPSCs were recorded, 660 mM tetrodotoxin (TTX; Tocris) was added to the ACSF.
In the case of the mEPSC or eEPSC recordings to block GABAA receptor-mediated IPSCs, picrotoxin (PTX: 100 µM; Sigma) was included in the ACSF. For recording mIPSCs or eIPSCs, the ACSF contained kynurenic acid (KYNA: 2 mM; Sigma).
To test the effect of GLP-1 signaling on the POMC neurons, a long-acting analog of GLP-1, exendin-4 (Ex4, 1 μM; Tocris), was pipetted into the recording chamber. The pipetting resulted in a short artifact on some of the recordings, but this part of recordings was excluded from the analysis.
For extracellular stimulation, a paired stimulus was delivered 50 milliseconds apart at 0.2 Hz, via theta electrode (World Precision Instruments) filled with ACSF and placed in the ARC within approximately 100 μm distance from the recorded POMC neuron. Thirty evoked stimuli were averaged and analyzed either for control or Ex4 (1 μM) treatment. The strength of the stimuli was set roughly to half of the maximal effective stimulus strength; this way we were able to detect both the decrease and increase in evoked currents after Ex4 treatment. During this extracellular stimulation paradigm, GDP-β-S (3 mM) was added to the intracellular solution to block the direct effect of Ex4 on the studied neuron. Unless otherwise stated, the chemicals for the intracellular and extracellular solutions were purchased from Sigma-Aldrich; inhibitors and antagonists were obtained from Tocris Bioscience.
Statistical Analysis of Electrophysiological Recordings
Event detection was performed using the Clampfit module of pClamp 10.4 software (Molecular Devices). All data for each experiment were normalized relative to baseline and described as mean ± standard error of mean. Measured parameters of the miniature potentials were as follows: peak amplitude (pA), event frequency (Hz), time to peak (ms), half-width (ms), rise time 10 90 (ms), and rise tau (ms). The criterion for separating measured neurons as responding to treatment was either a 15% increase or decrease compared to baseline frequency values (either spontaneous firing frequency or PSC frequency values). We did not separate responding cells based on changes in membrane potentials. Data were analyzed using the paired Student’s t test in the case of self-controlled experiments (before and after Ex4 treatment). An α-level of p < 0.05 was regarded as statistically significant in all statistical tests.
Results
Innervation of POMC-Positive Neurons by GLP-1R-IR Axons in the ARC
In agreement with the expression of GLP-1R on the POMC neurons described in the literature [], GLP-1R-immunoreactivity was detected on the surface of POMC perikarya (Fig. 1a). GLP-1R-immunoreactivity was however also observed in association with axons forming synapses on the perikarya of POMC neurons. GLP-1R-immunoreactivity was mostly attached to the outer cell membranes of the axons, but it was also detectable in a small proportion of synaptic vesicles. The GLP-1R-IR axons established both axosomatic (Fig. 1d) and axodendritic (Fig. 1b–c) synapses with the POMC containing structures. Both asymmetric (Fig. 1b) and symmetric type (Fig. 1c, d) GLP-1R-IR synapses were present on the POMC neurons. The presence of GLP-1R in the axons innervating the POMC neurons suggested that GLP-1 can regulate the inputs of POMC neurons at the level of presynaptic terminals.
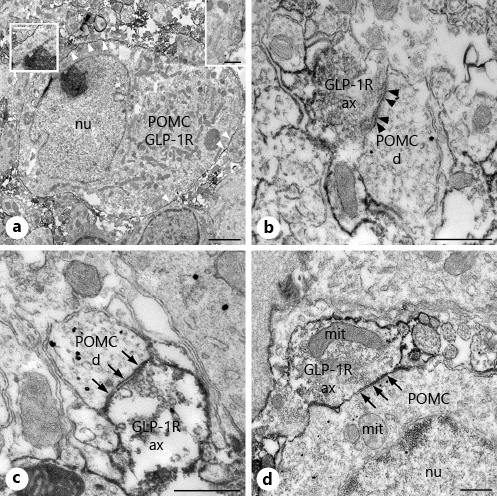
Fig. 1
Presence of POMC and GLP-1R-immunoreactivity in the ARC nucleus at the ultrastructural level. GLP-1R-immunoreactivity is associated with the membrane of POMC neurons and axons establishing synapses on POMC-IR dendrites and cell bodies in the ARC nucleus. Electron micrograph (a) illustrates the presence of both POMC (highly electron-dense gold-silver granules) and GLP-1R (electron-dense Ni-DAB precipitate) immunoreactivity in the same neuron. Note the association of GLP-1R-immunoreactivity with the cell membrane of POMC neurons (white arrowheads and insets). High magnification images illustrate asymmetric (b, black arrowheads)- and symmetric (c, arrows)-type synaptic associations between GLP-1R-IR axons and POMC-IR dendrites, while (d) demonstrates the presence of a symmetric-type axosomatic synapse (arrows) between a GLP-1R-IR axon and the perikaryon of a POMC neuron. POMC, proopiomelanocortin; GLP-1R-IR, glucagon-like peptide-1 receptor-immunoreactive; d, dendrite; ax, axon varicosity; mit, mitochondria; nu, nucleus; ARC, arcuate. Scale bar, 2 µm on a and 500 nm on b–d and on the insets.
Direct Regulation of POMC Neurons via GLP-1 Signaling in the ARC
Administration of Ex4 (1 μM) markedly increased the firing rate of all examined POMC neurons (249.86 ± 44.9%; p < 0.001, N = 20) (Fig. 2a) and depolarized their membrane potential (+4.12 ± 1.7 mV; p < 0.001, N = 20). To block G-protein signaling in the POMC neurons, the measured cells were intracellularly loaded with GDP-β-S (3 mM). The G-protein inhibition completely prevented the Ex4-induced increase in the firing rate (Fig. 2b) (p = 0.55; N = 13) and the effect of Ex4 on the membrane potential (p = 0.63). The GDP-β-S treatment itself did not significantly alter the basal membrane potential (membrane potential without GDP-β-S loading: −44.36 ± 1.74 mV vs. with loading: −43.11 ± 1.99; p = 0.70) or affect control firing frequency rate (1.19 ± 0.12 Hz without vs. 1.20 ± 0.11 Hz with GDP-β-S loading; p = 0.15). Data are summarized in Table 2.
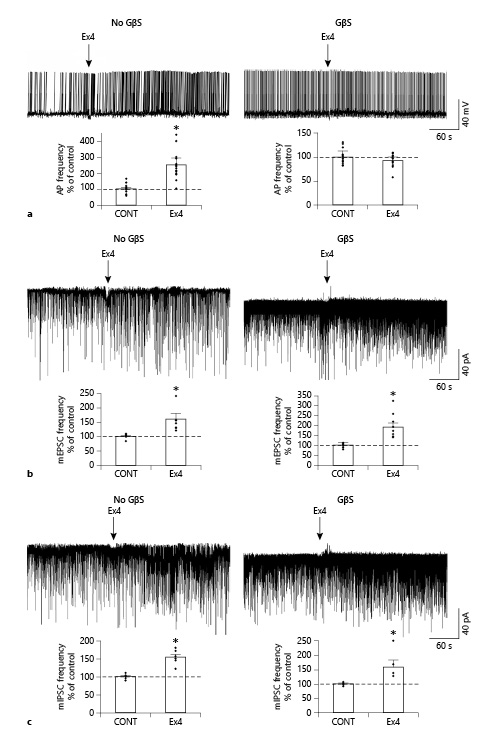
Fig. 2
GLP-1 analog Ex4 (1 μM) acts directly on the presynaptic input of POMC neurons. Representative traces (a) illustrate that Ex4 markedly increases the firing frequency of POMC neurons, but this effect is completely prevented by the intracellular administration of the G-protein blocker, GDP-β-S (GβS; 3 mM) indicating that the effect of Ex4 on the firing is exerted directly on the POMC neurons. GDP-β-S itself did not influence the firing frequency in the control period (1.19 ± 0.12 Hz without vs. 1.20 ± 0.11 Hz with GDP-β-S loading; p = 0.15). Bar graphs show the effects of Ex4 on the firing of POMC neurons in the absence (N = 20; p < 0.001) or the presence (N = 13; p = 0.55) of GDP-β-S. Arrows indicate the start of Ex4 treatment. Representative traces and bar graphs (b) illustrate the Ex4 treatment-induced increase in the frequency of the mEPSCs in the responsive POMC neurons in the absence (N = 7/15; p = 0.015) and presence (N = 8/15; p = 0.006) of GDP-β-S. Representative traces and bar graphs (c) illustrate the Ex4 treatment-induced increase in the frequency of the mIPSC in the responsive POMC neuron in the absence (N = 6/16; p = 0.012) and presence (N = 5/16; p = 0.038) of GDP-β-S. Intracellular administration of GDP-β-S did not influence the effect of Ex4 on the mPSCs indicating that this effect of GLP-1 signaling is exerted directly on the presynaptic inputs of POMC neurons. Neither GDP-β-S nor Ex4 altered the amplitude of mPSCs. Data were analyzed with paired Student’s t test. PSC, postsynaptic current; mPSC, miniature PSC; mEPSC, miniature excitatory PSC; mIPSC, miniature inhibitory PSC; POMC, proopiomelanocortin; GLP-1, glucagon-like peptide-1; Ex4, exendin 4; GDP-β-S, guanosine 5′-[β-thio]diphosphate trilithium salt. *p < 0.05.
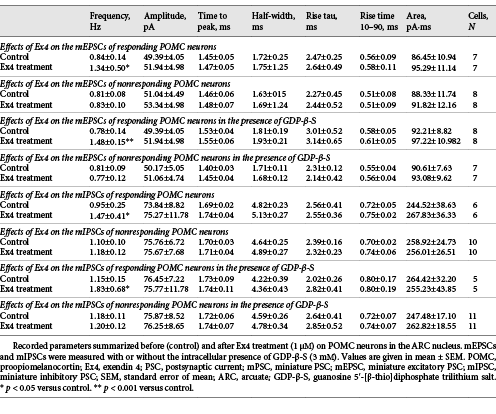
Effects of GLP-1 Analog Ex4 on the Presynaptic Inputs of POMC Neurons in the ARC
Ex4 (1 μM) increased the frequency of mEPSCs in 46.6% (N = 7) of the examined POMC neurons (N = 15). In these POMC neurons, the frequency of mEPSCs was 159.83 ± 20.82% of the control value after Ex4 treatment (p = 0.015). Other measured parameters did not show any significant changes. Inhibition of G-proteins by GDP-β-S in the studied POMC neurons prevented Ex4-induced depolarization, but did not affect the Ex4-induced increase in mEPSC frequency in the same cells (p = 0.99). Ex4 significantly stimulated the mEPSC-frequency of 8 POMC neurons (190.20 ± 21.6% of control value; p = 0.006) from the fifteen studied cells (responsive cells: 53%) (Fig. 2c). Our data indicate that GLP-1 signaling has a direct stimulatory effect on the excitatory presynaptic terminals of approximately half of the POMC neurons.
When mIPSCs were examined, Ex4 (1 μM) influenced these currents in one-third of the studied POMC neurons (31.6%, 6 from the 16 studied neurons). In these cells, the mIPSC frequency was 154.49 ± 8.37% of control values during the Ex4 treatment (p = 0.002). In the presence of GDP-β-S, we could also detect a similar increase in the frequency of mIPSCs (158.98 ± 23.77%; p = 0.038) in approximately one-third of studied neurons (31.25%, 5 from the 16 studied neurons) (Fig. 2d). The intracellular administration of the G-protein inhibitor did not influence the effect of Ex4 either on the frequency of mIPSCs of responsive POMC neurons (p = 0.97) or the number of responsive cells (Nno-GDP-β-S = 6; NGDP-β-S = 5). These data demonstrate that presynaptic GLP-1 signaling has a facilitatory effect on the inhibitory inputs of one-third of POMC neurons. Data are summarized in Table 3.
To understand whether the mEPSCs and mIPSCs are stimulated by Ex4 in the same or different POMC neuron subpopulations, the mEPSCs (inward currents) and mIPSCs (outward currents) were recorded in the same POMC neurons at −40 mV holding potential in the presence of TTX in the ACSF and GDP-β-S (3 mM) in the intracellular solution. To verify the experimental setting, PTX (100 μM) or KYNA (2 mM) or the combination of 2 was applied to the recording ACSF (Fig. 3a). PTX treatment caused complete disappearance of outward currents, treatment with KYNA blocked the inward currents, while the combination of PTX and KYNA caused a complete loss of both types of currents (Fig. 3a) validating the experimental setup. These series of experiments confirmed our results that Ex4 increased the mEPSC frequency (165.04 ± 15.68%; p < 0.001) in half (N = 10; 47.62%) of the recorded POMC neurons (N = 21) and increased the frequency of mIPSCs (186.38 ± 8.31% of control values; p < 0.001) in one-third of the measured POMC neurons (N = 8; 38.1%) (Fig. 3b, c).
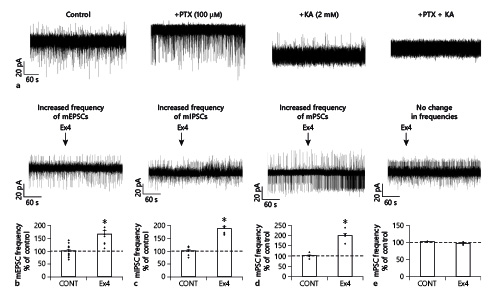
Fig. 3
Simultaneous effects of GLP-1 analog Ex4 on the frequency of mEPSCs and mIPSCs of the same POMC neurons of the ARC nucleus. Representative traces of control experiments in voltage-clamp mode (at −40 mV) (a) demonstrate the validity of the experimental setup for the simultaneous detection of mEPSCs and mIPSCs: without treatment, both mEPSCs (inward currents, downward) and mIPSCs (outward currents, upward) are readily visible; administration of PTX (100 μM) to the ACSF prevented the mIPSCs, while KYNA (2 mM) administration resulted in the absence of mEPSCs. Combined PTX and KYNA (100 μM and 2 mM, respectively) treatment caused the absence of both mIPSCs and mEPSCs. Representative traces and summary graphs illustrate (b–e) the effect of Ex4 on the frequency of mPSCs in different subgroups of POMC neurons: Ex4 stimulated only the frequency of mEPSCs (b), Ex4 stimulated only the frequency of mIPSCs (c), Ex4 influenced both types of mPSCs (d), Ex4 had no effect (e). All recordings (N = 21) were made in the presence of G-protein blocker GDP-β-S (3 mM) at 40 mV holding potential. POMC, proopiomelanocortin; GLP-1, glucagon-like peptide-1; Ex4, exendin 4; PSC, postsynaptic current; mPSC, miniature PSC; mEPSC, miniature excitatory PSC; mIPSC, miniature inhibitory PSC; ACSF, artificial cerebrospinal fluid; PTX, picrotoxin; KYNA, kynurenic acid; ARC, arcuate; GDP-β-S, guanosine 5′-[β-thio]diphosphate trilithium salt. *p < 0.05 (paired Student’s t test).
In one-quarter of the POMC neurons studied (N = 5; 23.81%), Ex4 treatment stimulated both the frequency of mEPSCs and mIPSCs, while in the second quarter of POMC neurons (N = 5; 23.81%) (Fig. 3b, c), Ex4 influenced only the frequency of mEPSCs. In 14.29% (N = 3) of the studied POMC neurons, Ex4 only increased the frequency of mIPSCs without influencing the mEPSCs. In 19.05% (N = 4) of POMC neurons, Ex4 did not affect the PSCs (Fig. 3b, c). Interestingly, with this experimental setup, we observed a few POMC neurons in which the Ex4 treatment decreased either the frequency of mEPSCs (70.36 ± 4.61%; p = 0.015; N = 3) or the frequency of mIPSCs (44.76%; N = 1).
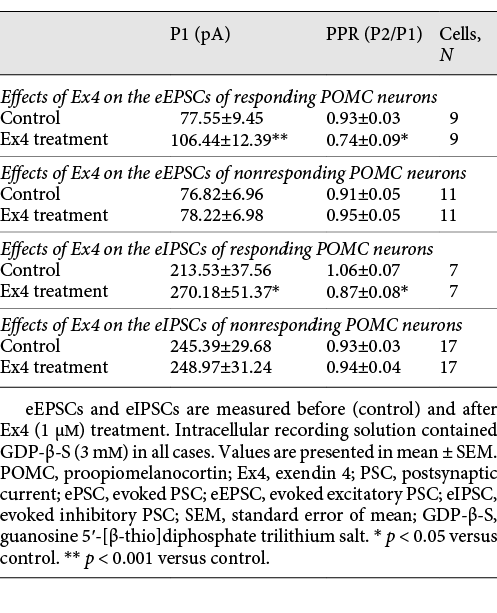
Effect of Ex4 Treatment on the Evoked Potentials of POMC Neurons
Evoked potentials of POMC neurons were also influenced by Ex4 (1 μM) treatment in the presence of GDP-β-S (3 mM). In the case of eEPSCs, we found that 45% of the examined POMC neurons (9 out of 20) showed significantly increased peak (P1) amplitude value of the first evoked potential (137.26 ± 4.54%; p < 0.001). Ex4 treatment significantly decreased the paired-pulse ratio (P2/P1) of these cells compared to the control values (79.51 ± 10.58%; p = 0.027). Ex4 treatment increased the first eIPSC amplitude (P1) (126.53 ± 4.44%; p = 0.006) in 29.17% of all examined POMC neurons (N = 24) and decreased significantly the paired-pulse ratio (P2/P1) (82.28 ± 6.66%; p < 0.022) of these cells. The amplitude of the second evoked peak (P2) did not change significantly in either case. Data are summarized in Table 4 and Figure 4.
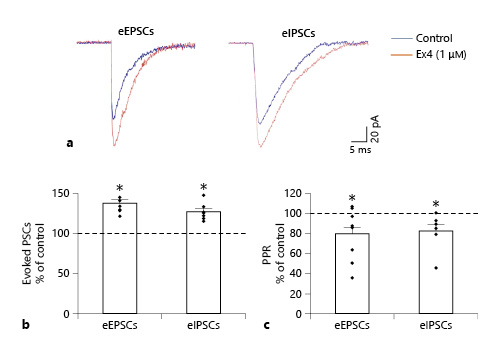
Fig. 4
Effects of GLP-1 analog Ex4 (1 μM) on the ePSCs of the POMC neurons in the ARC nucleus of mice. Superimposed, averaged traces (a) of the eEPSCs and eIPSCs of representative POMC neurons. Note the marked increase in the amplitude of the ePSCs after Ex4 treatment. Graph (b) shows that Ex4 treatment significantly increases the amplitude of the eEPSCs (NeEPSC = 9/20) and eIPSCs (NeIPSC = 7/24) of the responsive POMC neurons. Graph (c) illustrates that Ex4 treatment decreased the paired-pulse ratio (PPR) of both eEPSCs and eIPSCs significantly in the examined POMC neurons. All recordings were made in the presence of G-protein blocker GDP-β-S (3 mM). POMC, proopiomelanocortin; GLP-1, glucagon-like peptide-1; Ex4, exendin 4. PSC, postsynaptic current; eEPSC, evoked excitatory PSC; eIPSC, evoked inhibitory PSC; ARC, arcuate; GDP-β-S, guanosine 5′-[β-thio]diphosphate trilithium salt. *p < 0.05 (paired Student’s t test).
Discussion
Our results are in agreement with earlier findings demonstrating the presence of GLP-1R in the POMC neurons [, , ]. We observed that the GLP-1R-immunoreactivity covered the surface of POMC neurons in the ARC. This localization of the immunoreactivity indicates that the GLP-1R is functional on the POMC neurons. Furthermore, GLP-1R-immunoreactivity was also observed on the surface of axon varicosities forming synapses on POMC neurons. The GLP-1R-immunoreactivity covers almost the entire extrasynaptic surface of these varicosities, suggesting that GLP-1 originating from the periphery or extrasynaptic release regulates the function of presynaptic terminals of POMC neurons. GLP-1R-IR terminals formed both symmetric- and asymmetric-type synapses on the POMC neurons, indicating that GLP-1 may influence both excitatory and inhibitory inputs of POMC neurons, as the symmetric synapses are considered inhibitory, while the asymmetric synapses are considered excitatory synapses [].
In agreement with previous studies [, , ], we observed that GLP-1 signaling stimulates the POMC neurons. While Ex4 treatment markedly increased the firing of all studied POMC neurons and depolarized these cells in our experiments, there is a dispute in the literature about how large population of POMC neurons respond to GLP-1 signaling [, , ]. He et al. [] found that activation of GLP-1R influences only 37% of POMC neurons. In that study, the researchers examined POMC neurons from the retrochiasmatic area and in the most anterior part of the ARC []. In addition, they performed their measurements at room temperature that may highly influence the responsiveness of neurons []. In contrast, we studied POMC neurons from more caudal regions of the ARC at 32–33°C. Thus, both regional differences of POMC neurons and the different experimental temperatures may be behind these differences. Furthermore, He et al. [] counted a neuron as activated, if its membrane potential was depolarized, while we considered a neuron activated if the firing frequency was increased. Ronnekleiv et al. [] observed that 70% of POMC neurons were activated by a relatively low dose of Ex4. In contrast, the study by Secher et al. [], who used similar experimental setup as we in our experiments, observed that all POMC neurons responded to activation of GLP-1 signaling. They also observed by double-labeling in situ hybridization that virtually all POMC neurons express GLP-1R []. These data strongly support our finding that all studied POMC neurons increases its firing in response to Ex4 treatment.
As intracellular administration of the G-protein inhibitor GDP-β-S completely prevented the effect of Ex4 on the firing and membrane potential of POMC neurons, these effects are exerted directly on the studied POMC neurons. A similar conclusion was drawn by Secher et al. [] showing that the effect of GLP-1 was not prevented by simultaneous inhibition of GABAergic and glutamatergic signaling.
To understand the role of the presynaptic localization of GLP-1R in the regulation of the POMC neurons, the effect of Ex4 was studied on the mPSCs of these cells. As in earlier studies [, ], Ex4 markedly increased the frequency of mEPSCs in approximately half of the POMC neurons. The effect of Ex4 was also facilitatory on the mIPSCs of POMC neurons, but it influenced the mIPSCs only in one-third of these cells. The facilitatory effect of GLP-1 signaling on the frequency of mIPSCs was also observed by Secher et al. []. He et al. [] however did not observe the effect of GLP-1 signaling on the mIPSCs. We do not know the reason for these discrepancies. However, as discussed above, these authors used different experimental conditions on different subpopulations of POMC neurons. In addition, we observed the GLP1-induced facilitation in case of one-third of POMC neurons. As He et al. [] performed statistics on all examined POMC neurons together, the data of nonresponsive POMC neurons may have masked this effect.
While intracellular administration of G-protein inhibitor completely prevented the effect of Ex4 on the firing and membrane potential of all POMC neurons, the inhibition of G-protein signaling in the studied POMC cells did not influence the effect of Ex4 on the mPSCs. These data demonstrate that the effect of Ex4 on the presynaptic terminals of POMC neurons is exerted directly and excludes the possibility that this effect is mediated by retrograde signaling molecules synthesized by the POMC neurons. Furthermore, the presence of TTX in the extracellular solution also excludes the possibility that receptors on the perikarya of the presynaptic neurons are involved in the mediation of Ex4-induced facilitation of the presynaptic terminals as TTX completely blocks the spreading of action potentials [].
The effect of GLP-1R activation on both the excitatory and inhibitory inputs of POMC neurons is in agreement with our morphological finding demonstrating that GLP-1R-containing presynaptic terminals form both symmetric- and asymmetric-type synapses on the POMC neurons. Interestingly, He et al. [] did not observe GLP-1R activation-induced alteration of the mIPSC frequency of POMC neurons; we do not know the reason for this discrepancy; however, it is possible that they analyzed the data of all POMC neurons together, and the data of nonresponsive POMC neurons masked the frequency change of the responsive one-third of POMC neurons. Secher et al. [] however found an increased frequency of spontaneous IPSCs after liraglutide treatment, but they did not study whether the effect of treatment was exerted directly on the GABAergic terminals. The simultaneous regulation of excitatory and inhibitory inputs by peptide transmitters is though not surprising. For example, NPY inhibits both the excitatory and inhibitory inputs of the neurons in the PVN [].
Examination of the ePSCs of the POMC neurons further supported that GLP-1 signaling facilitates the inputs of POMC neurons. Ex4 significantly increased the peak amplitude of eEPSCs in almost half of the POMC neurons and the peak amplitude of eIPSCs in one-third of POMC neurons. The ratio of the POMC neurons with GLP-1-responsive inputs was very similar to experiments in which the mPSCs were studied. The Ex4 treatment significantly decreased the paired-pulse ratio both in the case of eEPSCs and eIPSCs, further demonstrating the direct effect of GLP-1 signaling on the inputs of POMC neurons.
While the effect of GLP-1 signaling on the excitatory input of POMC neurons is in agreement with its stimulatory effect on the POMC neurons, the role of the GLP-1R-induced facilitation of the inhibitory inputs of POMC neurons is not clear. This mechanism seems to work against the direct effect of GLP-1 signaling on the POMC neurons. To better understand the role of this regulatory mechanism, the source of the GLP-1R expressing input of the POMC neurons should be identified in future experiments.
Activation of GLP-1R stimulates the adenylyl cyclase-cAMP-PKA pathway, but it also increases PKC activity [, ]. Both of these signaling pathways were shown to play a role in presynaptic facilitation []. The adenylyl cyclase-cAMP-PKA-EPAC pathway was shown to increase the number of releasable synaptic vesicles and the release probability [], while PKC was shown to facilitate the activity of presynaptic terminals by increasing the Doc2α-Munc13-1 interaction []. Furthermore, the 2 pathways have additive effects on the synaptic activity []. Thus, it is likely that both the adenylyl cyclase-cAMP-PKA pathway and the stimulation of PKC activity are involved in the mediation of the GLP-1R-induced facilitation of presynaptic terminals.
The effect of Ex4 on the excitatory and inhibitory inputs of the POMC neurons raised the question whether the GLP-1 signaling facilitates the excitatory and inhibitory inputs of the same POMC neurons or the GLP-1R-containing excitatory and inhibitory inputs target separate POMC neuron populations. To answer this question, the excitatory and inhibitory mPSCs were detected simultaneously on the same POMC neurons at −40 mV holding potential. A similar experimental setup was earlier used by Melnick et al. [] to study the effect of NPY on the neurons of PVN. The inhibition of all inward currents by the glutamate receptor antagonist and all outward currents by the GABAA receptor antagonist demonstrated that this experimental setup can be used to study both types of mPSCs simultaneously in the same POMC neurons. Based on the response of their inputs to GLP-1 signaling, the POMC neurons can be divided into several subpopulations. Approximately one-quarter of POMC neurons receive both GLP-1R-containing excitatory and inhibitory inputs. A second quarter of POMC neurons have GLP-1R-containing excitatory inputs, but the inhibitory inputs of these POMC neurons are not GLP-1 responsive.
In contrast, a population of POMC neurons (14%) receive only inhibitory inputs that contain GLP-1R, while the input of approximately 20% of POMC neurons is not GLP-1 responsive. Interestingly, in this series of experiments, we observed few POMC neurons where Ex4 had an inhibitory effect on the inputs of the cell. However, due to the small number of these cells and the absence of any inhibitory effect in the other experiments of this work, we consider that rather the presynaptic facilitation is the typical presynaptic effect of the GLP-1 signaling.
Earlier publications also demonstrated the heterogeneity of the POMC neurons in the ARC. Based on their classical transmitter content, the POMC neurons are divided into 3 populations: 35% of POMC neurons are selectively GABAergic, 21% are selectively glutamatergic, while 38% of POMC neurons express both GABAergic and glutamatergic markers []. Single-cell analysis of the ARC POMC neurons also demonstrated that based on their gene expression profile, the POMC neurons can be clustered into 4 categories []. We do not know, however, whether the POMC neuron populations that differ in their GLP-1R-containing inputs overlap with the clusters of the single-cell sequencing.
Statement of Ethics
All experimental protocols were reviewed and approved by the Animal Welfare Committee at the Institute of Experimental Medicine and the National Animal Welfare Committee (PE/EA/2920-6/2016). All animal experiments were performed in accordance with relevant guidelines of Novo Nordisk.
Conflict of Interest Statement
C.P. and L.B.K. are shareholders of Novo Nordisk. C.F. has received funding from Novo Nordisk A/S. Z.P., A.Sz.-Sz., E.F., and Y.R. declare no competing interest.
Funding Sources
This work was supported by grants from the Hungarian Science Foundation (OTKA K124767), Hungarian National Brain Research Program (2017-1.2.1-NKP-2017-00002), EU H2020 THYRAGE No. 666869.
Author Contributions
Zoltán Péterfi performed the electrophysiological experiments, analyzed data, and were involved in manuscript preparation. Anett Szilvásy-Szabó performed ultrastructural studies, analyzed data, and were involved in manuscript preparation. Erzsébet Farkas and Yvette Ruska performed ultrastructural studies. Charles Pyke and Lotte Bjerre Knudsen edited the manuscript. Csaba Fekete planned the experiments, interpreted data, and was involved in manuscript writing.
References
- 1. Baggio LL, Drucker DJ. Biology of incretins: GLP-1 and GIP. Gastroenterology. 2007 May;132(6):2131–57.http://dx.doi.org/10.1053/j.gastro.2007.03.054.
- 2. Holst JJ. The physiology of glucagon-like peptide 1. Physiol Rev. 2007 Oct;87(4):1409–39.http://dx.doi.org/10.1152/physrev.00034.2006.
- 3. Llewellyn-Smith IJ, Reimann F, Gribble FM, Trapp S. Preproglucagon neurons project widely to autonomic control areas in the mouse brain. Neuroscience. 2011 Apr 28;180:111–21.http://dx.doi.org/10.1016/j.neuroscience.2011.02.023.
- 4. Nadkarni P, Chepurny OG, Holz GG. Regulation of glucose homeostasis by GLP-1. Prog Mol Biol Transl Sci. 2014;121:23–65.http://dx.doi.org/10.1016/B978-0-12-800101-1.00002-8.
- 5. NamKoong C, Kim MS, Jang BT, Lee YH, Cho YM, Choi HJ. Central administration of GLP-1 and GIP decreases feeding in mice. Biochem Biophys Res Commun. 2017 Aug 19;490(2):247–52.http://dx.doi.org/10.1016/j.bbrc.2017.06.031.
- 6. Drucker DJ. Biologic actions and therapeutic potential of the proglucagon-derived peptides. Nat Clin Pract Endocrinol Metab. 2005 Nov;1(1):22–31.http://dx.doi.org/10.1038/ncpendmet0017.
- 7. Yang Y, Moghadam AA, Cordner ZA, Liang NC, Moran TH. Long term exendin-4 treatment reduces food intake and body weight and alters expression of brain homeostatic and reward markers. Endocrinology. 2014 Sep;155(9):3473–83.http://dx.doi.org/10.1210/en.2014-1052.
- 8. Muller TD, Finan B, Bloom SR, D’Alessio D, Drucker DJ, Flatt PR, et al. Glucagon-like peptide 1 (GLP-1). Mol Metab. 2019 Dec;30:72–130.
- 9. Schwartz MW, Woods SC, Porte D Jr, Seeley RJ, Baskin DG. Central nervous system control of food intake. Nature. 2000 Apr 6;404(6778):661–71.http://dx.doi.org/10.1038/35007534.
- 10. Gropp E, Shanabrough M, Borok E, Xu AW, Janoschek R, Buch T, et al. Agouti-related peptide-expressing neurons are mandatory for feeding. Nat Neurosci. 2005 Oct;8(10):1289–91.http://dx.doi.org/10.1038/nn1548.
- 11. Secher A, Jelsing J, Baquero AF, Hecksher-Sørensen J, Cowley MA, Dalbøge LS, et al. The arcuate nucleus mediates GLP-1 receptor agonist liraglutide-dependent weight loss. J Clin Invest. 2014 Oct;124(10):4473–88.http://dx.doi.org/10.1172/JCI75276.
- 12. Knudsen LB, Secher A, Hecksher-Sørensen J, Pyke C. Long-acting glucagon-like peptide-1 receptor agonists have direct access to and effects on pro-opiomelanocortin/cocaine- and amphetamine-stimulated transcript neurons in the mouse hypothalamus. J Diabetes Investig. 2016 Apr;7(Suppl 1):56–63.http://dx.doi.org/10.1111/jdi.12463.
- 13. He Z, Gao Y, Lieu L, Afrin S, Cao J, Michael NJ, et al. Direct and indirect effects of liraglutide on hypothalamic POMC and NPY/AgRP neurons: implications for energy balance and glucose control. Mol Metab. 2019 Oct;28:120–34.http://dx.doi.org/10.1016/j.molmet.2019.07.008.
- 14.
- 15. McHugh TJ, Jones MW, Quinn JJ, Balthasar N, Coppari R, Elmquist JK, et al. Dentate gyrus NMDA receptors mediate rapid pattern separation in the hippocampal network. Science. 2007 Jul 6;317(5834):94–9.http://dx.doi.org/10.1126/science.1140263.
- 16. Madisen L, Zwingman TA, Sunkin SM, Oh SW, Zariwala HA, Gu H, et al. A robust and high-throughput Cre reporting and characterization system for the whole mouse brain. Nat Neurosci. 2010 Jan;13(1):133–40.http://dx.doi.org/10.1038/nn.2467.
- 17. Varga E, Farkas E, Zséli G, Kádár A, Venczel A, Kővári D, et al. Thyrotropin-releasing-hormone-synthesizing neurons of the hypothalamic paraventricular nucleus are inhibited by glycinergic inputs. Thyroid. 2019 Dec;29(12):1858–68.http://dx.doi.org/10.1089/thy.2019.0357.
- 18. Jensen CB, Pyke C, Rasch MG, Dahl AB, Knudsen LB, Secher A. Characterization of the glucagonlike peptide-1 receptor in male mouse brain using a novel antibody and in situ hybridization. Endocrinology. 2018 Feb 1;159(2):665–75.http://dx.doi.org/10.1210/en.2017-00812.
- 19. Padilla SL, Carmody JS, Zeltser LM. Pomc-expressing progenitors give rise to antagonistic neuronal populations in hypothalamic feeding circuits. Nat Med. 2010 Apr;16(4):403–5.http://dx.doi.org/10.1038/nm.2126.
- 20. McDermott CM, Schrader LA. Activation of κ opioid receptors increases intrinsic excitability of dentate gyrus granule cells. J Physiol. 2011 Jul 15;589(Pt 14):3517–32.http://dx.doi.org/10.1113/jphysiol.2011.211623.
- 21. Sandoval DA, Bagnol D, Woods SC, D’Alessio DA, Seeley RJ. Arcuate glucagon-like peptide 1 receptors regulate glucose homeostasis but not food intake. Diabetes. 2008 Aug;57(8):2046–54.http://dx.doi.org/10.2337/db07-1824.
- 22. Harris KM, Weinberg RJ. Ultrastructure of synapses in the mammalian brain. Cold Spring Harb Perspect Biol. 2012 May 1;4(5):a005587.http://dx.doi.org/10.1101/cshperspect.a005587.
- 23. Ronnekleiv OK, Fang Y, Zhang C, Nestor CC, Mao P, Kelly MJ. Research resource: gene profiling of G protein-coupled receptors in the arcuate nucleus of the female. Mol Endocrinol. 2014 Aug;28(8):1362–80.
- 24. Ruff RL. Effects of temperature on slow and fast inactivation of rat skeletal muscle Na(+) channels. Am J Physiol. 1999 Nov;277(5 Pt 1):C937–47.http://dx.doi.org/10.1152/ajpcell.1999.277.5.C937.
- 25. Evans MH. Tetrodotoxin, saxitoxin, and related substances: their applications in neurobiology. Int Rev Neurobiol. 1972;15:83–166.http://dx.doi.org/10.1016/s0074-7742(08)60329-3.
- 26. Peterfi Z, Farkas I, Denis RGP, Farkas E, Uchigashima M, Fuzesi T, et al. Endocannabinoid and nitric oxide systems of the hypothalamic paraventricular nucleus mediate effects of NPY on energy expenditure. Mol metab. 2018 Dec;18:120–33.
- 27. Doyle ME, Egan JM. Mechanisms of action of glucagon-like peptide 1 in the pancreas. Pharmacol Ther. 2007 Mar;113(3):546–93.http://dx.doi.org/10.1016/j.pharmthera.2006.11.007.
- 28. Shigeto M, Cha CY, Rorsman P, Kaku K. A role of PLC/PKC-dependent pathway in GLP-1-stimulated insulin secretion. J Mol Med. 2017 Apr;95(4):361–8.http://dx.doi.org/10.1007/s00109-017-1508-6.
- 29. Andrade-Talavera Y, Duque-Feria P, Sihra TS, Rodriguez-Moreno A. Pre-synaptic kainate receptor-mediated facilitation of glutamate release involves PKA and Ca(2+) -calmodulin at thalamocortical synapses. J Neurochem. 2013 Sep;126(5):565–78.http://dx.doi.org/10.1111/jnc.12310.
- 30. Hori T, Takai Y, Takahashi T. Presynaptic mechanism for phorbol ester-induced synaptic potentiation. J Neurosci. 1999 Sep 1;19(17):7262–7.http://dx.doi.org/10.1523/jneurosci.19-17-07262.1999.
- 31. Melnick I, Pronchuk N, Cowley MA, Grove KL, Colmers WF. Developmental switch in neuropeptide Y and melanocortin effects in the paraventricular nucleus of the hypothalamus. Neuron. 2007 Dec 20;56(6):1103–15.http://dx.doi.org/10.1016/j.neuron.2007.10.034.
- 32. Wittmann G, Hrabovszky E, Lechan RM, et al. Distinct glutamatergic and GABAergic subset of hypothalamic pro-opiomelanocortin neurons revealed by in situ hybridization in male rats and mice. J Comp Neurol. 2013 Oct;521(14):3287–3302. https://doi.org/10.1002/cne.23350.
- 33. Lam BYH, Cimino I, Polex-Wolf J, Nicole Kohnke S, Rimmington D, Iyemere V, et al. Heterogeneity of hypothalamic pro-opiomelanocortin-expressing neurons revealed by single-cell RNA sequencing. Mol Metab. 2017 May;6(5):383–92.http://dx.doi.org/10.1016/j.molmet.2017.02.007.
Zoltán Péterfia and Anett Szilvásy-Szabó contributed equally to the work.