Introduction
Growth hormone (GH) and insulin-like growth factor 1 (IGF-1) physiologically exert a beneficial role in skeletal health, enhancing bone formation and remodelling []. Based on these actions, acromegaly has long been neglected as a condition potentially associated with skeletal fragility [, ]. This paradigm, however, has been revisited in recent years, as an increasing body of evidence demonstrated that GH and IGF-1 excess may cause abnormalities in trabecular and cortical bone microstructure, with an overall increase in bone turnover and decrease in bone strength, and ultimately a higher risk of fractures [-].
Despite these advances in knowledge, however, the management of skeletal fragility in acromegaly remains challenging. Recent evidence suggests that standard bone-active therapies may be effective in preventing vertebral fractures in patients with active acromegaly []. However, guiding treatment choices based on the assessment of fracture risk according to standard algorithms might be inappropriate in acromegalic patients, as one of the most relevant “classical” determinants of skeletal fragility, i.e., the bone mineral density (BMD) does not clearly predict the occurrence of vertebral fractures, which in this setting may arise even in the presence of normal BMD values [-]. In fact, due to its peculiar pathophysiology, acromegalic osteopathy is not characterized by a significant impairment of BMD [, ]; in acromegalic patients, BMD values at the lumbar spine, femoral neck, and total hip are at least comparable or even higher than those of control subjects []. Therefore, alternative parameters of skeletal fragility should be sought, in place of BMD, for a better fracture risk assessment in acromegalic patients [].
In recent years, the trabecular bone score (TBS) has emerged as a novel index of bone micro-architectural health and skeletal fragility [, ]. TBS is a textural metric that captures the mean rate of gray-level variations in lumbar spine dual-energy X-ray absorptiometry (DXA) images [, ]; a high TBS value reflects a better trabecular bone microstructure, while a low TBS value indicates a trabecular microstructure impairment [, ].
Within the setting of acromegaly, some explorative studies noted that, contrary to BMD, TBS values might be lower in acromegalic patients with respect to control subjects [-]; in other papers, however, no significant differences in TBS between acromegalic patients and controls could be found [, ]. Other aspects of this topic are also matter of active debate, such as the role – within this context – of gonadal status [, , ] and acromegaly disease activity [, ]. Finally, and most importantly, the actual performance of TBS as a predictor of fracture risk in acromegalic patients still needs to be fully established and prospectively validated on large patient cohorts.
A previous meta-analysis [], which included articles published until September 2020, did not find any significant differences in TBS between acromegalic patients and controls; however, this result was limited by the low number of available studies; moreover, no data assessing the relationship between TBS and fragility fractures have been presented by the authors, as only one study evaluating this outcome was available at that time []. Within the last 2 years, additional research has been published in this regard, both about the difference in TBS between patients with and without acromegaly [, -] and about the comparison of TBS values between acromegalic patients with and without prevalent fragility fractures [].
The aim of this systematic review and meta-analysis was, thus, to evaluate all published data about TBS as an index of skeletal fragility in patients with acromegaly, in order to provide an up-to-date synthesis of the current knowledge in this regard and, more specifically, to quantitatively summarize the available evidence within the areas of uncertainty that we have previously outlined.
Methods
Search Strategy and Study Selection
This study was conducted according to the Preferred Reporting Items for Systematic Reviews and Meta-Analysis (PRISMA) guidelines []. The process of literature search and study selection was made by two independent reviewers (F.B. and M.B.); all disparities were resolved through consensus. The following electronic databases were queried until June 1, 2022: PubMed/Medline, EMBASE, Cochrane Library, OVID, and CINAHL. The search strategy was performed using a combination of relevant database-specific search terms to identify pertinent studies about the evaluation of TBS in acromegalic patients. The term “acromegaly” was combined with other relevant key words such as “TBS” and “trabecular bone score”. The full search strategy is presented in the online supplementary materials (Appendix 1; for all online suppl. material, see http://www.karger.com/doi/10.1159/000528199). No filters were applied for study design, language, and publication date. After duplicate removal, all studies found with the aforementioned search were evaluated for inclusion in the meta-analysis, first by title/abstract screening and then by full-text review. We excluded studies from our analysis according to the following exclusion criteria: (a) conference abstracts; (b) case reports or case series; (c) unavailability of any of the primary outcomes of interest, as defined in the following subsection.
Outcomes
The primary outcomes of interest were (i) the comparison of TBS values between acromegalic patients and non-acromegalic controls; (ii) the relationship, within acromegalic patients, between TBS values and fragility fractures. With respect to the first point, stratified data according to acromegaly disease activity and gonadal status were also collected, when provided. Moreover, in order to exclude possible biases arising from the specific criteria adopted for enrolment of the control group, the differences in BMD values between acromegalic patients and non-acromegalic controls were also assessed as an ancillary outcome, whenever available.
Data Extraction
Two authors (F.B. and M.B.) independently examined and extracted data from papers which met the inclusion criteria using pre-specified data extraction templates. For each eligible study, the following information was collected: (a) first author and publication year; (b) study design; (c) major selection criteria for each group; (d) matching criteria between acromegalic patients and controls; (e) number of subjects enrolled; (f) patients’ characteristics in terms of demographic data; (g) TBS and BMD values in acromegalic patients and controls, whenever available; (h) TBS values in acromegalic patients with and without prevalent fragility fractures, whenever available; (i) TBS values in acromegalic patients with and without incident fragility fractures, whenever available.
Risk of Bias Assessment
The risk of bias was independently assessed for each included study by two authors (F.B. and M.B.). The twenty components of the AXIS tool (Appraisal tool for Cross-Sectional Studies) [] were identified as the evaluation tool for any cross-sectional study that compared TBS values between acromegalic patients and controls and/or evaluated the association between TBS and the presence of prevalent fractures in acromegalic patients. The seven domains of the ROBINS-I tool (Risk Of Bias In Non-randomized Studies of Intervention) [] were identified as the evaluation tool for any longitudinal study that evaluated the association between TBS and the risk of incident fractures in acromegalic patients.
Statistical Analysis
Continuous variables and categorical variables were reported as numbers and percentages, respectively. Comparisons between groups were expressed as mean differences. A random-effect restricted maximum likelihood model was adopted for statistical pooling of data. The same approach was adopted for subgroup analyses. Higgins I2 statistics and the Cochran Q test were used to assess heterogeneity between studies. Publication bias was quantitatively assessed by Begg’s test. Statistical analysis was performed using STATA 17 (StataCorp, College Station, TX, USA).
Results
Search Results
A total of 218 records were identified in the initial literature search. Removal of duplicates led to an overall pool of 134 studies. An accurate title or abstract revision was sufficient to exclude 122 articles as not pertinent or not fulfilling our pre-specified inclusion or exclusion criteria. The remaining 12 studies were assessed in full-text for eligibility [-, -]; among these, 1 did not report any quantitative data on TBS [], while 3 others evaluated different outcomes from those of our interest [-]; the remaining 8 met all criteria for being included in the final analysis [-] (Fig. 1), encompassing 336 acromegalic patients and 490 non-acromegalic controls. All included studies [-] reported relevant data about the first outcome of interest (i.e., the comparison of TBS values between acromegalic patients and controls). Two studies [, ] also reported relevant data about the second outcome of interest (i.e., the relationship, within acromegalic patients, between TBS values and fragility fractures); both of them, in particular, evaluated the cross-sectional relationship between TBS and prevalent fragility fractures; on the other hand, the longitudinal relationship between baseline TBS values and incident fragility fractures at follow-up was not assessed in any study.
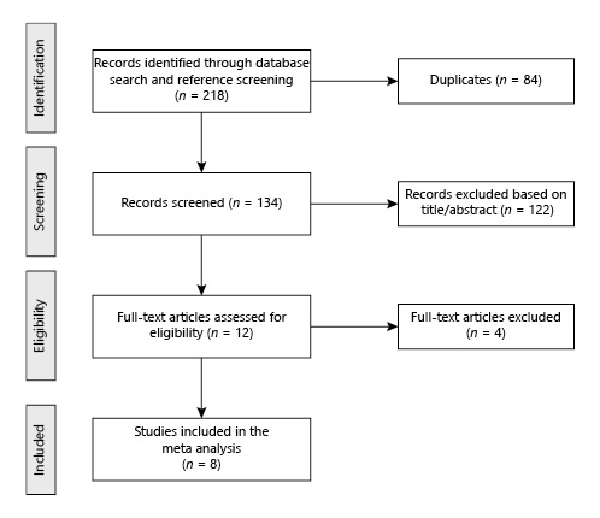
Fig. 1
Flowchart of study inclusion.
Characteristics of the Included Studies
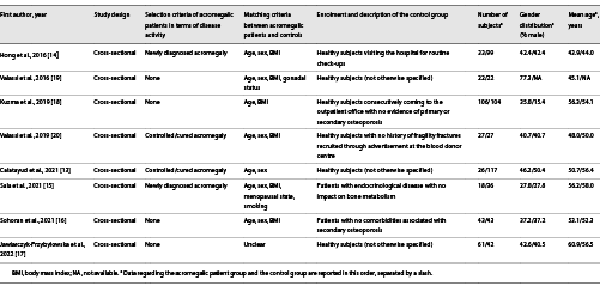
Table 1 summarizes the basic study characteristics. With respect to the outcomes of interest for this meta-analysis, all included studies had a cross-sectional design [-]. The acromegalic patient group was represented by subjects with newly diagnosed acromegaly in two studies [, ] and by subjects with controlled/cured acromegaly in two studies [, ], while the remaining four studies [-] did not apply any restriction in terms of disease activity. The matching criteria adopted for the selection of the control group included age in seven studies [-, -], sex in six studies [-, , ], and BMI in six studies [-, -]; gonadal status was considered as an adjunctive matching criterion in one study [], while menopausal state and smoking in another one [].
Comparison of TBS Values between Acromegalic Patients and Controls
As previously stated, eight studies [-] reported relevant data to compare TBS values between acromegalic patients and controls. Overall, TBS values were significantly lower in acromegalic patients compared to controls (−0.089, 95% CI: [−0.111, −0.067], p < 0.01), with no significant between-study heterogeneity (I2 = 35.78%, p = 0.17) (Fig. 2). These results were consistently confirmed also after stratifying the data according to the gonadal status of patients and controls, whenever possible (Fig. 3). At subgroup analysis, no differences emerged when subdividing the studies according to the selection criteria adopted by each in terms of acromegaly disease activity (p = 0.51) (Fig. 4). Among the 4 studies in which patients with any disease activity were enrolled [-], only two [, ] provided a head-to-head comparison of TBS values between patients with active and controlled/cured acromegaly, without highlighting any significant difference (p = 0.98) (online suppl. Fig. 1).
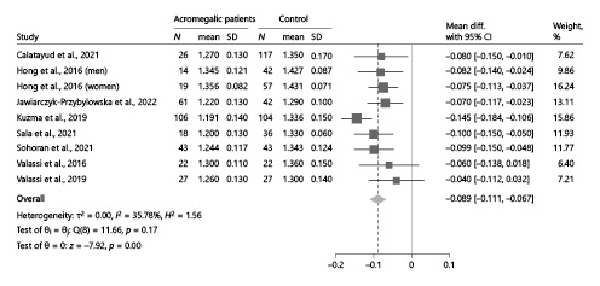
Fig. 2
Comparison of TBS values between acromegalic patients and controls. Data from the study by Hong et al. [] are reported separately by sex, as the authors did not provide the overall estimates of the outcomes in the study population as a whole. CI, confidence interval; N, number; SD, standard deviation; TBS, trabecular bone score.
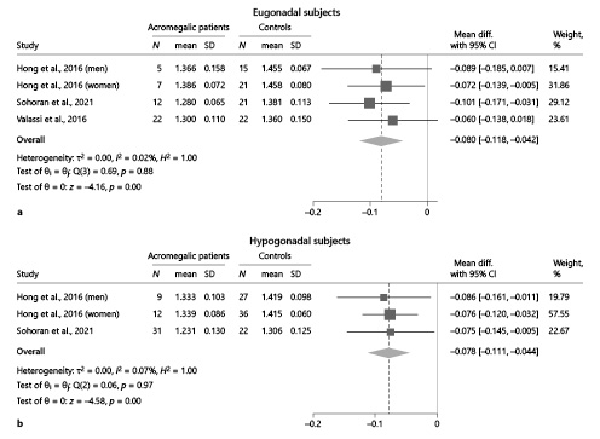
Fig. 3
Comparison of TBS values between acromegalic patients and controls, stratified according to gonadal status. a Comparison between eugonadal acromegalic patients and eugonadal controls. bComparison between hypogonadal acromegalic patients and hypogonadal controls. Data from the study by Hong et al. [] are reported separately by sex, as the authors did not provide the overall estimates of the outcomes in the study population as a whole. CI, confidence interval; N, number; SD, standard deviation; TBS, trabecular bone score.
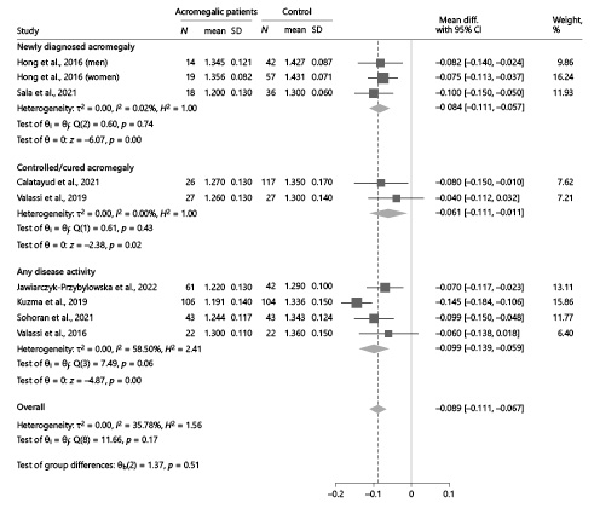
Fig. 4
Comparison of TBS values between acromegalic patients and controls, subdividing the analysis according to the selection criteria adopted by each study in terms of acromegaly disease activity. Data from the study by Hong et al. [] are reported separately by sex, as the authors did not provide the overall estimates of the outcomes in the study population as a whole. CI, confidence interval; N, number; SD, standard deviation; TBS, trabecular bone score.
As an ancillary analysis, in order to exclude possible biases arising from the specific criteria adopted for enrolment of the control group, data regarding the comparison between acromegalic patients and controls of BMD values at the lumbar spine, femoral neck, and total hip were also extracted from the included studies whenever available. The statistical pooling of the results did not highlight any significant difference in BMD values between acromegalic patients and controls, either at the lumbar spine (−0.019, 95% CI: [−0.046, 0.008], p = 0.16), femoral neck (0.012, 95% CI: [−0.013, 0.037], p = 0.35), or total hip (0.003, 95% CI: [−0.026, 0.031], p = 0.84) (online suppl. Fig. 2).
Relationship, within Acromegalic Patients, between TBS Values and Fracture Risk
Two studies [, ] reported relevant data to evaluate the relationship, within acromegalic patients, between TBS values and prevalent fragility fractures in a cross-sectional design. The presence of fractures was systematically assessed, in both studies, by performing lateral spine imaging (either with a densitometer [] or by conventional radiography []) in all enrolled patients. Overall, TBS values were significantly lower in acromegalic patients with vertebral fractures than in unfractured ones, with a pooled mean difference of −0.099 (95% CI: [−0.166, −0.032], p < 0.01) (Fig. 5).
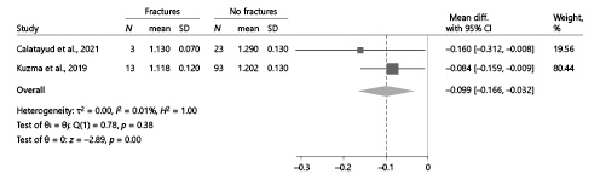
Fig. 5
Comparison of TBS values between acromegalic patients with and without vertebral fractures. CI, confidence interval; N, number; SD, standard deviation; TBS, trabecular bone score.
No studies, on the other hand, evaluated, in a longitudinal design, the relationship between TBS values at baseline and the occurrence of incident vertebral fractures at follow-up. One study [] reported prospective data about the relationship between incident fractures and TBS percentage change during follow-up, displaying a greater reduction over time of TBS values in patients with incident vertebral fractures compared to unfractured ones; however, data about the relationship between incident fractures and TBS values at baseline were not available, and therefore, the study did not fit our eligibility criteria for inclusion in the meta-analysis.
Quality Assessment and Publication Bias
The results of the quality assessment of the studies are reported in online supplementary Table 1. Altogether, the risk of bias appeared to be moderate-to-low in all studies. No significant publication bias was found at Begg’s test for the comparison of TBS values between acromegalic patients and controls (p = 0.25); the available data were insufficient to assess publication bias with respect to the other primary outcome.
Discussion
In this paper, we provided an updated systematic review and meta-analysis that specifically assessed the role of TBS as an index of bone structure quality and fracture risk in patients with acromegaly. Compared to a previous work [], which was based on articles published until September 2020 and could not retrieve any significant results, additional research has been published and could be included in our analysis. This allowed for greater statistical power in assessing the difference in TBS values between acromegalic patients and controls and for finer and more detailed sub-analyses; in addition, for the first time, the relationship between TBS values and prevalent fragility fractures could also be assessed.
In recent years, the correlation between TBS values and fracture risk has already been demonstrated in various settings of primary and secondary osteoporosis [, ]. In primary osteoporosis, TBS predicts both prevalent and incident fragility fractures []. In fact, in retrospective or cross-sectional studies, TBS has been shown to be consistently lower in subjects with an osteoporotic fracture than in those without [-]. A similar result holds true also when prospectively assessing the risk of incident fractures, as TBS was proven to be at least as good as BMD in predicting hip fractures, vertebral fractures, and all fractures [-]. In secondary osteoporosis, the role of TBS in assessing skeletal fragility and fracture risk seems to be even more prominent []. In fact, in these patients, the measurement of BMD often lacks sensitivity in predicting fracture risk []. For example, the increase in fracture risk associated with glucocorticoid exposure occurs before major bone loss can be measured by DXA []; this is also true for other causes of secondary osteoporosis, such as diabetes mellitus and asymptomatic primary hyperparathyroidism, in which bone mineralization is relatively well preserved [-], and the increase in fracture risk is not clearly governed by the expected relationship with BMD [-]. In these conditions, skeletal fragility is more related to subtler changes in bone micro-architecture [], which can be undetectable when simply evaluating BMD; nevertheless, they may be more clearly unveiled by TBS, which has been shown to outperform DXA for fracture risk prediction in many of these clinical contexts [-].
Within the setting of acromegaly, our meta-analysis highlighted the presence of lower TBS values in affected patients compared to unaffected controls. On the contrary, in the same studies, no overall differences in BMD values could be found between the two groups at any skeletal site; this latter outcome is in line with previous literature [, ], in which BMD was already shown to be either unaffected (at lumbar spine) or even slightly increased (at femoral neck and total hip) in acromegalic patients with respect to non-acromegalic controls. From a pathophysiological point of view, the finding of lower TBS values in acromegalic patients is coherent with the available data about the impairment in trabecular bone micro-architecture that is typical of acromegalic osteopathy: histomorphometric analyses, in fact, demonstrated a reduced trabecular bone volume, increased trabecular separation, and impaired trabecular biomechanical competence in acromegalic patients compared to controls [, ]; moreover, a reduced trabecular bone density and trabecular thickness were also displayed at high-resolution peripheral quantitative computed tomography (HR-pQCT), both at the distal radius and at the distal tibia [, ].
Gonadal status is known to be an important factor affecting bone strength by directly influencing osteoblast and osteoclast function; in women and men, in fact, oestradiol and testosterone, respectively, help in maintaining a correct balance between bone formation and bone resorption [, ]. Patients with acromegaly due to GH-secreting adenoma frequently present with pituitary hormone deficiencies, either due to compression of normal pituitary tissue by the adenoma or due to pituitary damage following surgery/radiotherapy, when performed []. Thus, when evaluating bone-related features in acromegalic patients, a possible interplay between GH dysregulation and gonadal status should always be considered. This point has been assessed by some of the studies included in this meta-analysis, though on relatively limited patient cohorts [, , ]. When pooling these data, notably, the impairment in TBS values in acromegalic patients was consistently confirmed both in eugonadal and hypogonadal patients. Thus, the detrimental role of GH hypersecretion on trabecular bone micro-architecture appeared to be evident irrespective of gonadal status.
Acromegaly disease activity has also been suggested as a possible determinant of bone fragility in acromegalic patients. Patients with active acromegaly have been shown to be at higher risk of vertebral fractures compared to those with a controlled/cured disease []. However, in a recent HR-pQCT study, Duan et al. [] suggested that the negative impact of acromegaly on bone microstructure may still not be fully reversible. With respect to TBS values, our meta-analysis could not highlight any significant difference in the outcome measure according to acromegaly disease activity. The strength of this result is certainly limited by the low number of available studies; nevertheless, it further supports the hypothesis that – in acromegalic patients – the changes in bone micro-architecture induced by persistent GH excess might not be easily reverted by disease cure or control, except possibly in the very long term.
With respect to fracture risk, our results highlighted an association between TBS values and prevalent vertebral fractures; in fact, when pooling the data from eligible studies, TBS values were significantly lower in acromegalic patients with vertebral fractures than in those without. This finding is in line with the available evidence from various other settings of primary and secondary osteoporosis [, ] and supports the use of TBS as a helpful index of skeletal fragility in acromegalic patients too. Of note, this is an even more relevant result in light of the fact that, as previously demonstrated, BMD is a poorly reliable measure of fracture risk in acromegalic disease [-]; nevertheless, it has to be noted that, to date, no longitudinal data are still available about the relationship between baseline TBS values and the occurrence of fragility fractures at follow-up, which should be the gold standard criterion for the definite establishment of TBS as a reliable predictor of fracture risk.
Our analysis had some limitations. Firstly, the strength of the conclusions was limited by the low number of available studies; this was particularly true for the analysis that examined the relationship, within acromegalic patients, between TBS values and fracture risk, as only two studies provided sufficient data for the assessment of this outcome. Secondly, the quality was limited by that of the included studies; nevertheless, according to the AXIS tool, the risk of bias was generally moderate-to-low, which reassured about the likely small impact of this issue on the final results. Thirdly, patients’ inclusion criteria were partly different between studies, and this could be responsible for a certain degree of heterogeneity in the considered outcomes; however, heterogeneity is a common limitation of all meta-analyses, and appropriate statistical methods – such as the use of a random-effect model – were adopted to account for it. Fourthly, the comparisons of TBS values between groups were all based on crude differences, as derived by univariate analyses; thus, the possible interplay with other predictors could not be evaluated. Fifthly, no studies evaluated, in a longitudinal design, the relationship between TBS values at baseline and the occurrence of incident vertebral fractures at follow-up; thus, prospective data about the utility of TBS as a predictor for future fractures in acromegalic patients are still lacking.
In conclusion, our meta-analysis supports the notion that acromegalic patients have an impaired bone quality, described by lower TBS values with respect to controls; conversely, BMD did not seem to be significantly affected, in line with previous results. Moreover, at cross-sectional evaluations, TBS values were lower in acromegalic patients with prevalent vertebral fractures than in those without. However, prospective data evaluating the capacity of TBS values to predict the future occurrence of incident fractures are still lacking. Overall, thus, our findings suggest that TBS could be valuable in the assessment and management of skeletal fragility in acromegalic patients, especially in light of the poor information provided in this setting by BMD. Nevertheless, larger prospective studies are needed for a more detailed evaluation of TBS as a predictor for future fractures.
Statement of Ethics
An ethics statement is not applicable because this study is based exclusively on published literature.
Conflict of Interest Statement
The authors have no conflicts of interest to declare.
Funding Sources
This research did not receive any specific funding.
Author Contributions
Fabio Bioletto and Marco Barale contributed to work conceptualization, data collection, data analysis, and manuscript writing. Nunzia Prencipe, Alessandro Maria Berton, and Mirko Parasiliti-Caprino contributed to data interpretation and manuscript writing. Valentina Gasco, Ezio Ghigo, Massimo Procopio, and Silvia Grottoli contributed to data interpretation and final draft supervision. All authors approved the manuscript in its final form.
Data Availability Statement
All data generated or analysed during this study are included in this article and its online supplementary materials. Further enquiries can be directed to the corresponding author.
References
- 1. Giustina A, Mazziotti G, Canalis E. Growth hormone, insulin-like growth factors, and the skeleton. Endocr Rev. 2008 Aug;29(5):535–59. https://doi.org/10.1210/er.2007-0036.
- 2. Vestergaard P, Jørgensen JOL, Hagen C, Hoeck HC, Laurberg P, Rejnmark L, et al. Fracture risk is increased in patients with GH deficiency or untreated prolactinomas: a case-control study. Clin Endocrinol. 2002;56(2):159–67. https://doi.org/10.1046/j.0300-0664.2001.01464.x.
- 3. Vestergaard P, Mosekilde L. Fracture risk is decreased in acromegaly: a potential beneficial effect of growth hormone. Osteoporos Int. 2004;15(2):155–9. https://doi.org/10.1007/s00198-003-1531-z.
- 4. Mazziotti G, Frara S, Giustina A. Pituitary diseases and bone. Endocr Rev. 2018;39(4):440–88. https://doi.org/10.1210/er.2018-00005.
- 5. Mazziotti G, Biagioli E, Maffezzoni F, Spinello M, Serra V, Maroldi R, et al. Bone turnover, bone mineral density, and fracture risk in acromegaly: a meta-analysis. J Clin Endocrinol Metab. 2015;100(2):384–94. https://doi.org/10.1210/jc.2014-2937.
- 6. Bonadonna S, Mazziotti G, Nuzzo M, Bianchi A, Fusco A, De Marinis L, et al. Increased prevalence of radiological spinal deformities in active acromegaly: a cross-sectional study in postmenopausal women. J Bone Miner Res. 2005;20(10):1837–44. https://doi.org/10.1359/JBMR.050603.
- 7. Mazziotti G, Bianchi A, Bonadonna S, Cimino V, Patelli I, Fusco A, et al. Prevalence of vertebral fractures in men with acromegaly. J Clin Endocrinol Metab. 2008;93(12):4649–55. https://doi.org/10.1210/jc.2008-0791.
- 8. Wassenaar MJ, Biermasz NR, Hamdy NA, Zillikens MC, van Meurs JB, Rivadeneira F, et al. High prevalence of vertebral fractures despite normal bone mineral density in patients with long-term controlled acromegaly. Eur J Endocrinol. 2011 Apr;164(4):475–83. https://doi.org/10.1530/EJE-10-1005.
- 9. Mazziotti G, Battista C, Maffezzoni F, Chiloiro S, Ferrante E, Prencipe N, et al. Treatment of acromegalic osteopathy in real-life clinical practice: the BAAC (bone active drugs in acromegaly) study. J Clin Endocrinol Metab. 2020 Sep;105(9):dgaa363–92. https://doi.org/10.1210/clinem/dgaa363.
- 10. Mazziotti G, Lania AGA, Canalis E. Management of endocrine disease: bone disorders associated with acromegaly-mechanisms and treatment. Eur J Endocrinol. 2019;181(2):R45–56. https://doi.org/10.1530/EJE-19-0184.
- 11. Silva BC, Leslie WD, Resch H, Lamy O, Lesnyak O, Binkley N, et al. Trabecular bone score: a noninvasive analytical method based upon the DXA image. J Bone Miner Res. 2014 Mar;29(3):518–30. https://doi.org/10.1002/jbmr.2176.
- 12. Ulivieri FM, Silva BC, Sardanelli F, Hans D, Bilezikian JP, Caudarella R. Utility of the trabecular bone score (TBS) in secondary osteoporosis. Endocrine. 2014;47(2):435–48. https://doi.org/10.1007/s12020-014-0280-4.
- 13. Calatayud M, Pérez-Olivares Martín L, Librizzi MS, Lora Pablos D, González Méndez V, Aramendi Ramos M, et al. Trabecular bone score and bone mineral density in patients with long-term controlled acromegaly. Clin Endocrinol. 2021;95(1):58–64. https://doi.org/10.1111/cen.14439.
- 14. Hong AR, Kim JH, Kim SW, Kim SY, Shin CS. Trabecular bone score as a skeletal fragility index in acromegaly patients. Osteoporos Int. 2016 Mar;27(3):1123–9. https://doi.org/10.1007/s00198-015-3344-2.
- 15. Sala E, Malchiodi E, Carosi G, Verrua E, Cairoli E, Ferrante E, et al. Spine bone texture assessed by trabecular bone score in active and controlled acromegaly: a prospective study. J Endocr Soc. 2021 Aug;5(8):bvab090. https://doi.org/10.1210/jendso/bvab090.
- 16. Sorohan MC, Dusceac R, Sorohan BM, Caragheorgheopol A, Poiana C. Trabecular bone score and bone mineral density in acromegalic osteopathy assessment: a cross-sectional study. Arch Osteoporos. 2021 Dec;16(1):134. https://doi.org/10.1007/s11657-021-00986-7.
- 17. Jawiarczyk-Przybyłowska A, Halupczok-Żyła J, Syrycka J, Zembska A, Kuliczkowska-Płaksej J, Bolanowski M. Trabecular bone score and osteoprotegerin as useful tools in the assessment of bone deterioration in acromegaly. Front Endocrinol. 2022 Apr;13:862845. https://doi.org/10.3389/fendo.2022.862845.
- 18. Kužma M, Vaňuga P, Ságova I, Pávai D, Jackuliak P, Killinger Z, et al. Non-invasive DXA-derived bone structure assessment of acromegaly patients: a cross-sectional study. Eur J Endocrinol. 2019 Mar;180(3):201–11. https://doi.org/10.1530/EJE-18-0881.
- 19. Valassi E, Crespo I, Malouf J, Vilades D, Leta R, Llauger J, et al. Epicardial fat is a negative predictor of spine volumetric bone mineral density and trabecular bone score in acromegaly. Endocrine. 2016 Sep;53(3):860–4. https://doi.org/10.1007/s12020-016-0945-2.
- 20. Valassi E, García-Giralt N, Malouf J, Crespo I, Llauger J, Díez-Pérez A, et al. Circulating miR-103a-3p and miR-660-5p are associated with bone parameters in patients with controlled acromegaly. Endocr Connect. 2019;8(1):39–49. https://doi.org/10.1530/EC-18-0482.
- 21. Ribeiro de Moura C, Campos Lopes S, Monteiro AM. Determinants of skeletal fragility in acromegaly: a systematic review and meta-analysis. Pituitary. 2022;25(6):780–94. https://doi.org/10.1007/s11102-022-01256-6.
- 22. Moher D, Liberati A, Tetzlaff J, Altman DG, Antes GPRISMA Group. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLoS Med. 2009 Jul;6(7):e1000097. https://doi.org/10.1371/journal.pmed.1000097.
- 23. Downes MJ, Brennan ML, Williams HC, Dean RS. Development of a critical appraisal tool to assess the quality of cross-sectional studies (AXIS). BMJ Open. 2016;6(12):e011458–7. https://doi.org/10.1136/bmjopen-2016-011458.
- 24. Sterne JA, Hernán MA, Reeves BC, Savović J, Berkman ND, Viswanathan M, et al. ROBINS-I: a tool for assessing risk of bias in non-randomised studies of interventions. BMJ. 2016;355:i4919. https://doi.org/10.1136/bmj.i4919.
- 25. Godang K, Olarescu NC, Bollerslev J, Heck A. Treatment of acromegaly increases BMD but reduces trabecular bone score: a longitudinal study. Eur J Endocrinol. 2016 Aug;175(2):155–64. https://doi.org/10.1530/EJE-16-0340.
- 26. Jawiarczyk-Przybyłowska A, Halupczok-Żyła J, Kolačkov K, Gojny Ł, Zembska A, Bolanowski M. Association of vitamin D receptor polymorphisms with activity of acromegaly, vitamin D status and risk of osteoporotic fractures in acromegaly patients. Front Endocrinol. 2019 Sep;10(SEP):643. https://doi.org/10.3389/fendo.2019.00643.
- 27. Kuzma M, Vanuga P, Sagova I, Pavai D, Jackuliak P, Killinger Z, et al. Vertebral fractures occur regardless of acromegaly activity and are best predicted by proximal femur cortical volumetric bone mineral density. J Endocr Soc. 2021;5(Suppl 1):A638. https://doi.org/10.1210/jendso/bvab048.1299.
- 28. Silva PPB, Pereira RMR, Takayama L, Borba CG, Duarte FH, Trarbach EB, et al. Impaired bone microarchitecture in premenopausal women with acromegaly: the possible role of wnt signaling. J Clin Endocrinol Metab. 2021;106(9):2690–706. https://doi.org/10.1210/clinem/dgab260.
- 29. Rabier B, Héraud A, Grand-Lenoir C, Winzenrieth R, Hans D. A multicentre, retrospective case-control study assessing the role of trabecular bone score (TBS) in menopausal Caucasian women with low areal bone mineral density (BMDa): analysing the odds of vertebral fracture. Bone. 2010;46(1):176–81. https://doi.org/10.1016/j.bone.2009.06.032.
- 30. Winzenrieth R, Dufour R, Pothuaud L, Hans D. A retrospective case-control study assessing the role of trabecular bone score in postmenopausal Caucasian women with osteopenia: analyzing the odds of vertebral fracture. Calcif Tissue Int. 2010 Feb;86(2):104–9. https://doi.org/10.1007/s00223-009-9322-y.
- 31. Del Rio LM, Winzenrieth R, Cormier C, Di Gregorio S. Is bone microarchitecture status of the lumbar spine assessed by TBS related to femoral neck fracture? A Spanish case-control study. Osteoporos Int. 2013 Mar;24(3):991–8. https://doi.org/10.1007/s00198-012-2008-8.
- 32. Leib E, Winzenrieth R, Aubry-Rozier B, Hans D. Vertebral microarchitecture and fragility fracture in men: a TBS study. Bone. 2014;62:51–5. https://doi.org/10.1016/j.bone.2013.12.015.
- 33. Krueger D, Fidler E, Libber J, Aubry-Rozier B, Hans D, Binkley N. Spine trabecular bone score subsequent to bone mineral density improves fracture discrimination in women. J Clin Densitom. 2014;17(1):60–5. https://doi.org/10.1016/j.jocd.2013.05.001.
- 34. Hans D, Goertzen AL, Krieg MA, Leslie WD. Bone microarchitecture assessed by TBS predicts osteoporotic fractures independent of bone density: the Manitoba study. J Bone Miner Res. 2011 Nov;26(11):2762–9. https://doi.org/10.1002/jbmr.499.
- 35. Iki M, Tamaki J, Kadowaki E, Sato Y, Dongmei N, Winzenrieth R, et al. Trabecular bone score (TBS) predicts vertebral fractures in Japanese women over 10 years independently of bone density and prevalent vertebral deformity: the Japanese Population-Based Osteoporosis (JPOS) cohort study. J Bone Miner Res. 2014 Feb;29(2):399–407. https://doi.org/10.1002/jbmr.2048.
- 36. Boutroy S, Hans D, Sornay-Rendu E, Vilayphiou N, Winzenrieth R, Chapurlat R. Trabecular bone score improves fracture risk prediction in non-osteoporotic women: the OFELY study. Osteoporos Int. 2013 Jan;24(1):77–85. https://doi.org/10.1007/s00198-012-2188-2.
- 37. Kanis JA, Johansson H, Oden A, Johnell O, De Laet C, Melton III LJ, et al. A meta-analysis of prior corticosteroid use and fracture risk. J Bone Miner Res. 2004;19(6):893–9. https://doi.org/10.1359/JBMR.040134.
- 38. Silverberg SJ, Shane E, de la Cruz L, Dempster DW, Feldman F, Seldin D, et al. Skeletal disease in primary hyperparathyroidism. J Bone Miner Res. 1989;4(3):283–91. https://doi.org/10.1002/jbmr.5650040302.
- 39. Strotmeyer ES, Cauley JA, Schwartz AV, Nevitt MC, Resnick HE, Bauer DC, et al. Nontraumatic fracture risk with diabetes mellitus and impaired fasting glucose in older white and black adults: the health, aging, and body composition study. Arch Intern Med. 2005 Jul;165(14):1612–7. https://doi.org/10.1001/archinte.165.14.1612.
- 40. Scillitani A, Mazziotti G, Di Somma C, Moretti S, Stigliano A, Pivonello R, et al. Treatment of skeletal impairment in patients with endogenous hypercortisolism: when and how?Osteoporos Int. 2014;25(2):441–6. https://doi.org/10.1007/s00198-013-2588-y.
- 41. Rubin MR. Type 2 diabetes and fractures: more information is needed. Endocrine. 2013;43(3):469–71. https://doi.org/10.1007/s12020-013-9901-6.
- 42. Chappard D, Legrand E, Basle MF, Fromont P, Racineux JL, Rebel A, et al. Altered trabecular architecture induced by corticosteroids: a bone histomorphometric study. J Bone Miner Res. 1996;11(5):676–85. https://doi.org/10.1002/jbmr.5650110516.
- 43. Paggiosi MA, Peel NFA, Eastell R. The impact of glucocorticoid therapy on trabecular bone score in older women. Osteoporos Int. 2015 Jun;26(6):1773–80. https://doi.org/10.1007/s00198-015-3078-1.
- 44. Romagnoli E, Cipriani C, Nofroni I, Castro C, Angelozzi M, Scarpiello A, et al. Trabecular Bone Score” (TBS): an indirect measure of bone micro-architecture in postmenopausal patients with primary hyperparathyroidism. Bone. 2013;53(1):154–9. https://doi.org/10.1016/j.bone.2012.11.041.
- 45. Eller-Vainicher C, Filopanti M, Palmieri S, Ulivieri FM, Morelli V, Zhukouskaya VV, et al. Bone quality, as measured by trabecular bone score, in patients with primary hyperparathyroidism. Eur J Endocrinol. 2013 Aug;169(2):155–62. https://doi.org/10.1530/EJE-13-0305.
- 46. Dalle Carbonare L, Micheletti V, Cosaro E, Valenti MT, Mottes M, Francia G, et al. Bone histomorphometry in acromegaly patients with fragility vertebral fractures. Pituitary. 2018 Feb;21(1):56–64. https://doi.org/10.1007/s11102-017-0847-1.
- 47. Ueland T, Ebbesen EN, Thomsen JS, Mosekilde L, Brixen K, Flyvbjerg A, et al. Decreased trabecular bone biomechanical competence, apparent density, IGF-II and IGFBP-5 content in acromegaly. Eur J Clin Invest. 2002;32(2):122–8. https://doi.org/10.1046/j.1365-2362.2002.00944.x.
- 48. Madeira M, Neto LV, De Paula Paranhos Neto F, Barbosa Lima IC, Carvalho de Mendonça LM, Gadelha MR. Acromegaly has a negative influence on trabecular bone, but not on cortical bone, as assessed by high-resolution peripheral quantitative computed tomography. J Clin Endocrinol Metab. 2013 Apr;98(4):1734–41. https://doi.org/10.1210/jc.2012-4073.
- 49. Silva PPB, Amlashi FG, Yu EW, Pulaski-Liebert KJ, Gerweck AV, Fazeli PK, et al. Bone microarchitecture and estimated bone strength in men with active acromegaly. Eur J Endocrinol. 2017 Nov;177(5):409–20. https://doi.org/10.1530/EJE-17-0468.
- 50. Seeman E, Delmas PD. Bone quality: the material and structural basis of bone strength and fragility. N Engl J Med. 2006;354(21):2250–61. https://doi.org/10.1056/NEJMra053077.
- 51. Fink HA, Ewing SK, Ensrud KE, Barrett-Connor E, Taylor BC, Cauley JA, et al. Association of testosterone and estradiol deficiency with osteoporosis and rapid bone loss in older men. J Clin Endocrinol Metab. 2006;91(10):3908–15. https://doi.org/10.1210/jc.2006-0173.
- 52. Katznelson L, Laws ER, Melmed S, Molitch ME, Murad MH, Utz A, et al. Acromegaly: an endocrine society clinical practice guideline. J Clin Endocrinol Metab. 2014 Nov;99(11):3933–51. https://doi.org/10.1210/jc.2014-2700.
- 53. Duan L, Yang S, Wang LJ, Zhang Y, Li R, Yang H, et al. The negative impacts of acromegaly on bone microstructure not fully reversible. Front Endocrinol. 2021;12:1–10. https://doi.org/10.3389/fendo.2021.738895.