Introduction
Symptoms arising from vestibular system dysfunction are common in people with multiple sclerosis (MS). Dizziness, that can include feelings of being off balance, lightheaded, or spinning movements of the person or the environment (vertigo) affects 37% to 59% of people with MS., Further, abnormalities in vestibular evoked ocular and spinal reflexes, that are important for the stabilization of gaze and balance, are seen in 71% of people with MS. Dysfunction to the peripheral or central vestibular pathways (vestibulopathy) causes perceptual deficits (eg, vertigo or dizziness and poor perception of vertical) and abnormalities in the control of eye movements and balance. Signs of vestibular dysfunction are associated with greater symptoms of fatigue and walking deficits MS.- Although MS affects the central nervous system (CNS), people with MS can present with peripheral symptoms if the lesion affects the vestibular nerve in isolation as it enters the CNS.,
Given the high prevalence of vestibular dysfunction in MS and its potential impact on function and quality of life targeted treatments to address these signs may be beneficial. Vestibular rehabilitation (VR) is the recommended treatment for persons with vestibulopathy. VR involves progressive exercises including eye, head, and body movements in sitting, standing, and walking. VR has been assessed in people with MS.- Meta-analyses and systematic reviews highlight that compared to no intervention, VR is more effective for improving balance and symptoms of dizziness in people with MS. However, no statistical differences have been observed when VR is compared to other exercise interventions., This may reflect the fact that a baseline vestibulopathy was not diagnosed using standardized neuro-otological tests.
In people with an isolated peripheral vestibulopathy not caused by MS, a customized, individualized VR program that targets patient-specific problems has been demonstrated to be more effective than a standard format, generic exercise program. However, in MS, given the multiple factors other than vestibulopathy that could affect balance and mobility, it may be that a booklet-based generic exercise program is as clinically effective as a customized approach. The primary aim of this trial was therefore to compare the clinical and cost effectiveness of a 12-week customized VR program (12 face-to-face sessions and a tailored home-based program) to a 12-week booklet-based home program with telephone support in ambulant people with MS and associated peripheral and/or central vestibulopathy. The hypothesis is that the customized VR program would be clinically more effective and cost effective compared to the booklet-based program with telephone support.
Methods
Trial Design
The study was a multi-centre parallel group, superiority randomized controlled trial (RCT) with blinded outcome assessment. It was conducted at 2 sites in England, UK. Ethics approval was granted by the South West-Cornwall & Plymouth Research Ethics Committee (18/SW/0145) in 2018. The trial was registered with the International Standard Randomized Controlled Trial registry, ISRCTN27374299. The study protocol has been published.
Participants
Participants were adults (>18 years) diagnosed with MS according to McDonald’s revised criteria. Inclusion criteria were (i) a score 1 to 6 on Patient Determined Disease Steps, (ii) reporting one of the following at least 4 times/month (questions 4, 6, and 10 of the vertigo symptom scale): feeling that things are spinning or moving around; a feeling of being light-headed, “swimmy” or giddy or feeling unsteady, and about to lose balance, (iii) willing and able to travel to and participate in 12 face-to-face sessions should they be allocated to the customized VR program (intervention) and to commit to undertaking their individualized home-based program, and (iv) willing and able to travel to local assessment centers for blinded outcomes assessment. People were excluded if they (i) had neurological conditions other than MS as determined from clinical notes, (ii) had relapsed or received steroid treatment within the last month, (iii) currently or recently (within past 6 months) participated in a VR program, (iv) had an orthopedic deficit which may impact on postural and gait testing or significant pain or weakness (>4/10 on a numerical rating scale) associated with osteo- or rheumatoid arthritis, (v) had dizziness solely explained by other causes (eg, postural hypotension), (vi) had a headache or migraine associated with a subjective report of either nausea or vomiting at least 4 times/month, and (viii) had been taking vestibular sedatives specifically for the treatment of vertigo for more than 4 weeks. In the latter case, with approval of their neurologist and/or GP, they could stop vestibular sedatives and be eligible after a 6 weeks wash out period following re-screening.
Recruitment and Screening
Identification and recruitment occurred through screening regional MS databases, via neurology and physiotherapy clinics, and advertising in local MS support group newsletters. Potential participants were screened by telephone for subjective symptoms of vertigo and dizziness and poor balance (questions 4, 6, and 10 of the Vertigo Symptom Scale short version). During the first face-to-face visit, individuals were screened for posterior canal Benign Paroxysmal Positional Vertigo (BPPV) using the Dix-Hallpike maneuver and horizontal canal BPPV using a roll test. Those with a positive BPPV test were excluded from the RCT and treated with the appropriate re-positioning maneuver.
People who screened negative for BPPV were screened for signs of peripheral and central vestibulopathy using a clinical and videonystagmography (VNG)-based neuro-otological assessment including rotary chair testing as outlined in the protocol publication. Individuals with no signs of vestibular pathology were excluded from the study. The remaining participants were classified according to the following criteria:
○ Peripheral Unilateral vestibular impairment-based on a past history of sudden vertigo resolving within days-to-weeks and/or i-VNG unidirectional spontaneous nystagmus (>4°/second) on gaze testing with response enhancement on removal of optic fixation ii right-left asymmetry of slow-phase velocity >15% on rotation testing (calculated with Jongkees’ formula and based on departmental normative data).
○ Peripheral Bilateral vestibular hypofunction. Based on a Vestibulo Ocular Reflex (VOR) mean slow component velocity (SCV) and/or time constant at <2 standard deviation (SD) of the mean for healthy controls (SCV = 32°/second, SD = 8°/second; time constant = 13.35 second, SD = 4.45 second) for clockwise/counter-clockwise step rotation testing
○ Central vestibular impairment will be based on smooth pursuit, saccades, Optokinetic Nystagmus (OKN), and VOR suppression abnormalities.
○ Combined—a combination of central and peripheral vestibulopathy
Randomization
Participants were randomly allocated to the intervention group (customized VR program) or control (booklet-based VR program) group (1:1 ratio), using an online web-based system (REDCap projectredcap.org). Allocation used a minimization procedure, incorporating a random element, with minimization factors (i) Diagnosis: Peripheral (unilateral or bilateral) versus central/combined vestibulopathy. (ii) Severity of dizziness: Dizziness Handicap Inventory (DHI) ≥59 or DHI<59. (iii) Fampridine: Prescribed or not prescribed. (iv) Region: South West England or London.
Participants were not blinded to group allocation as the intervention arm involved weekly one-to-one sessions with the Treating Therapist. The Treating Therapists were not blinded due to the nature of the programs. The Research Therapists undertaking outcome assessments were blinded to allocation. The initial baseline assessment was undertaken prior to randomization ensuring these assessments were blinded.
Procedures
Face-to-face interventions took place in a university-based therapy gym.
Booklet-Based VR Program (Control Group)
The control group undertook a 1-hour face-to-face individualized physiotherapy session during which they received a self-management “Balance Rehabilitation” booklet providing comprehensive advice on VR exercises. Participants were asked to practice the exercises unsupervised at home, twice daily each for 6 to 10 minutes, over 12 weeks and to fill out a daily diary sheet indicating practice duration and content. Telephone support from the Treating Therapist in the form of two 15-minute phone calls, 1 in week 1 and 1 in week 4, was scheduled with a focus on adherence, barriers to adherence, and discussion of any concerns and queries regarding the exercise program.
Customized VR Program (Intervention Group)
The customized VR group was scheduled to receive 12 individualized, 1 hour, supervised, face-to-face VR sessions over a 12-week period, typically on a weekly basis. Each participant practiced a selection from the following type of exercises (i) Eye, head, and postural exercises that provoked symptoms. (ii) Gaze stabilization exercises. (iii) Exercises to re-train postural alignment and movement strategies. (iv) Re-training sensory strategies including using optokinetic or moving visual scenes. (v) Learning to adapt postural strategies to changing contexts. (vi) Dual task training while walking. (vii) Postural orientation exercises. (vii) Neuromuscular (ankle–hip-stepping motor strategies) postural strategies.
The exercises were chosen by the Treating Therapist in collaboration with the participant following an assessment (see Marsden et al for more detail). Each participant was provided with an individualized home exercise program of 3 to 5 exercises to practice each for 1-minute, twice daily on days they did not have a session with the Therapist. This included video links to a demonstration of the exercise and progression rules. Progress in specific areas was objectively assessed at each supervised session, any concerns discussed, exercises not yet included in the home program practiced, and exercises modified to gradually increase task difficulty. Prior to the trial, Treating Therapists undertook a 2-day training session regarding the theory and practice of VR and trial methods. In summary, both interventions provided VR with similarities being an emphasis on head movements and gaze stabilization exercises.
Both groups completed daily diaries to record the duration of home exercise practice. In addition, adherence in the customized group was reported as number of face-to-face sessions attended. Exercise compliance for home-based exercises was defined as reporting, via the diary, at least 6 minutes of exercises a day, for at least 4 days a week, for at least 8 weeks of the 12-week treatment period. Session compliance was defined as attending ≥9 out of the planned 12 face-to-face sessions for the intervention group or both planned telephone follow-up calls for the control group.
Impact of Coronavirus Disease 2019 (COVID-19)
Some participants were recruited into the intervention group and, due to COVID-19 lockdown restrictions, had to be moved from face-to-face to on-line weekly interventions.
Data Collection and Outcomes
Participant characteristics (demographics, type of MS, medication, and co-morbidities) were collected at baseline alongside potential factors affecting balance and mobility23: isometric knee extensor strength, distal leg sensation, reaction time, and neck range of motion (see Marsden et al).
Outcomes were measured at baseline prior to randomization using scales whose validity and reliability in MS have been summarized previously. Baseline measurements and randomization (T0) occurred ~2 weeks prior to the onset of either intervention or control program. Outcomes collected post-intervention at 14 weeks (±2 weeks) post-randomization (T14) and at 26 weeks (±2 weeks) post-randomization (T26). During the COVID-19 lockdown period, primary and secondary outcomes at week 14 and week 26 to be assessed remotely. This precluded an assessment of items that required face-to-face contact.
The primary outcome measure was the impact of dizziness on daily function assessed using the DHI at 26 weeks. The DHI is a 25-item self-report validated questionnaire that assesses 3 domains: functional, emotional, and physical resulting in a 0 to 100 score with greater values indicating greater perceived disability. It was chosen based on its measurement properties and consultation with patient representatives.
Secondary outcome measures were the Vertigo Symptom Scale-Short Form (VSS),; Dynamic Gait Index (DGI),; Situational Characteristic Questionnaire; dynamic visual acuity,; Rod and Disc test,; Symbol Digit Modalities Test; self-report MS Walking Scale (MSWS-12 Version 2.0); MSWS-12Vs2.0, Activities-Specific Balance Confidence Scale (ABC); Fatigue Scale for Motor and Cognitive functions; and Hospital Anxiety and Depression Scale (HADS). Health-related quality of life was measured using the EuroQol 5-Dimension 5-Level questionnaire (EQ-5D-5L) and the Multiple Sclerosis Impact Scale (MSIS-29Vs2.0). A retrospective diary of falls over the past month prior to the baseline measure and a prospective daily falls diary over 12 weeks for assessment 2 (T14) and follow up (T26) were also collected. Falls were defined within the diary as “an unexpected event in which you come to rest on the ground, floor, or lower level.”
Participants were monitored for adverse events (AEs) as defined in the protocol via completion of AEs forms, during telephone or face-to-face contacts as part of the VR program phase and during follow-up assessments.
Statistical Analysis
The target sample size was calculated to detect a standard effect size of 0.94 in DHI, with 90% power at the 2-sided 5% significance level. Calculations were based on data from a MS vestibular waitlist control study as outlined in the protocol paper. To account for participant dropout of up to 10%, the aim was to recruit at least 70 participants (35 per allocated group).
The statistical analysis plan was prespecified prior to database lock. It included sensitivity analyses to explore the impact of recruitment during the COVID-19 pandemic. By categorizing participants into either “pre-COVID-19” or “during COVID-19,” with participants providing week 26 data after 23/03/20 classified as the latter.
The analysis of the primary outcome was undertaken in line with a modified Intention-to-Treat (ITT) principle amongst participants with complete data at baseline and 26 weeks. The per-protocol population included participants who had completed their 26 weeks assessment within the ±2 weeks window and the safety analysis included all randomized participants. The primary analysis used analysis of covariance (ANCOVA) comparing DHI at 26 weeks between the 2 allocated groups, and adjusting for baseline DHI scores, recruitment site, fampridine, and vestibular diagnosis. Further analysis of the primary outcome included a mixed-effects repeated measures model for DHI at 14 and 26 weeks using all available data, adjusting for baseline score and recruitment site, fampridine, vestibular diagnosis, and the interaction between time point and allocated group. Secondary outcomes were analyzed in a similar manner, using ANCOVA. The analysis was conducted using STATA version 17 (StataCorp. 2021).
Exploratory statistical analyses included an assessment of the impact of the following covariates on changes in DHI with treatment by adding an interaction term of the following variables and treatment allocation into the ANCOVA model (variables with small group numbers were explored visually) (i) diagnosis (central vs peripheral vs combined vestibulopathy), (ii) visual dependency as determined by the rod and disc test, (iii) psychological state as determined by the HADS, and (iv) associated symptoms (knee extensor strength, reaction time, leg sensation, and neck range of motion).
Health Economic Evaluation
A full cost-effectiveness analysis was undertaken, using individual patient-level data over 26 weeks, to estimate the cost per quality-adjusted-life-year (QALY) of customized versus booklet-based generic VR, from a primary perspective of the National Health Service, UK (NHS) and social care. QALY weights were derived from the EQ-5D-5L using the “cross-walk” to the EQ-5D-3L UK tariff, as recommended by NICE at the time of analysis.
Intervention resource requirements and costs were collected via case report forms and discussion with intervention providers. Self-reported resource use data were collected at baseline, 14 weeks, and 26 weeks using a bespoke resource use questionnaire informed by previous resource use instruments, core items for resource use measures, and input from patient experts. This included primary, secondary, and social care, and participant and carer-related resource use. Unit costs were obtained from nationally recognized sources and applied to the resource use data.
Sensitivity analyses explored (i) the cost-effectiveness of the intervention from a societal perspective, including participant out-of-pocket costs, informal care and time off work, and (ii) the impact on results of using the Multiple Sclerosis Impact Scale-8D (MSIS-8D) as a condition-specific alternative to the EQ-5D-5L. QALY weights for the MSIS-8D were estimated from participant responses to the MSIS-29 using a published algorithm.
Study Monitoring
The conduct of the trial was overseen by an independent Trial Steering Committee and Ethics Committee. The funder of the study had no role in study design, data collection, data analysis, data interpretation, or writing of the report.
Results
Recruitment
Recruitment ran from 01/19 to 04/21 and was stopped when the recruitment target was reached. Recruitment and retention is summarized in the CONSORT diagram (Figure 1). BPPV was diagnosed in 1 out of 73 people screened. This was successfully managed over 2 treatment sessions using the Epley maneuvers. Seventy participants were deemed eligible for the RCT after further screening and were assessed at baseline and randomized. There were 35 people allocated to each group, with 3 in the control group and 1 in the intervention group fully withdrawing from the trial. Twenty-nine participants (82.9%) in the control group and 32 (91.4%) participants in the intervention group undertook assessments at week 14 and week 26 participants (74.3%) in the control group and 32 (91.4%) participants in the intervention group at week 26. The primary outcome measure (DHI) was recorded at week 14 and week 26 in 59 and 58 people respectively (28 (80.0%) control, 31 (88.6%) intervention at 14 weeks, 28 (80.0%) control, 30 (85.7%) intervention at 26 weeks). The sensitivity analysis assessing the difference pre and during COVID-19 included 57 participants (26 control and 31 intervention). In total per-protocol assessment occurred in 41/70 participants (20 control and 21 intervention).
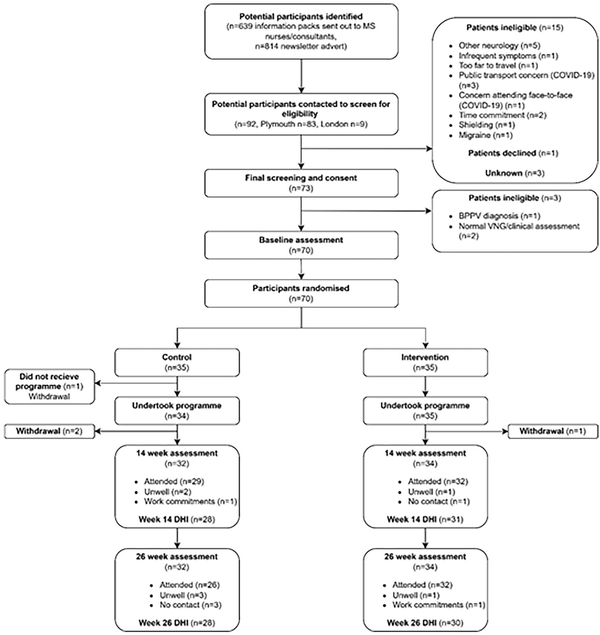
Figure 1
CONSORT diagram.
Baseline Demographics and Outcomes
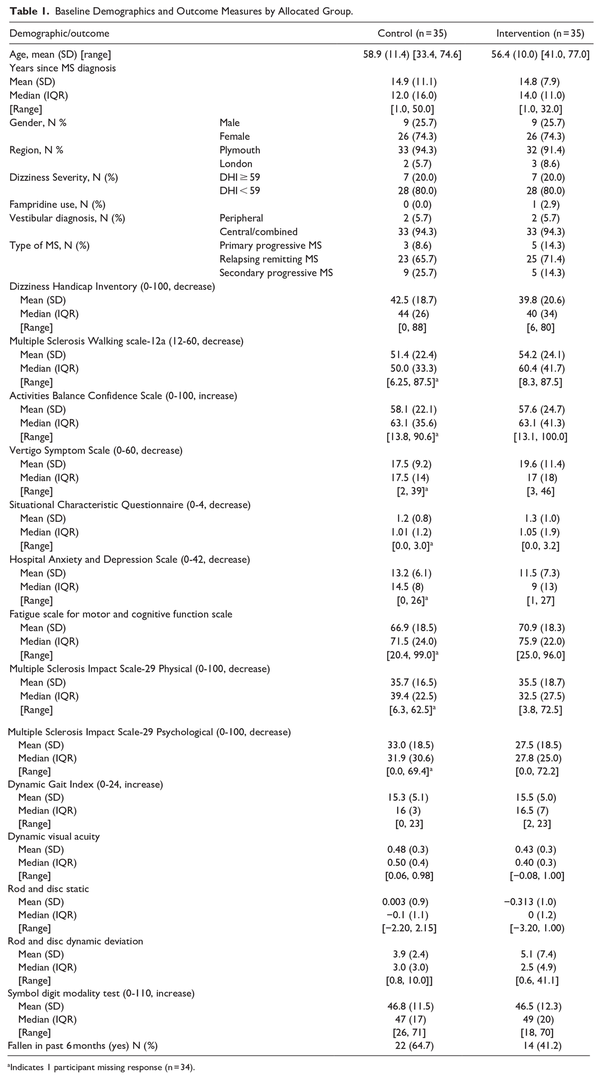
Baseline demographics are summarized in Table 1. Groups were similar in terms of their age, gender, MS type, severity on the DHI, fampridine use, and vestibular diagnosis (central or a combined central and peripheral presentation). The groups were similar in terms of the primary and secondary outcome measures at baseline (Table 1). The mean baseline DHI score was 42.5 (SD = 18.7) in the control group and 39.8 (20.6) in the intervention group; 7 people (20%) in each group were defined as having severe dizziness on the DHI (≥59).
Primary Outcome Measure
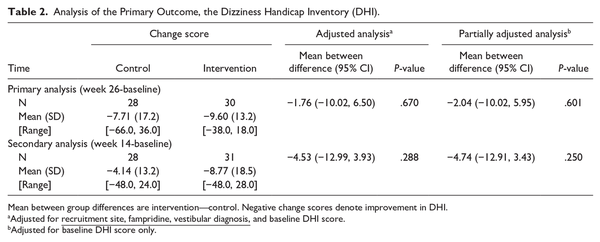
There was no evidence of a statistically significant difference between groups in terms of the primary outcome measure change score in DHI between week 26 (primary endpoint) and baseline. The primary analysis found a mean between-group difference of −1.76 (95% confidence interval [CI] −10.02, 6.50) at week 26 (P = .670) and −4.53 (−12.99, 3.93) at week 14 (P = .288), in favor of the intervention group (Table 2, Figure 1 in the Supplemental Material).
A pre-specified sensitivity analysis investigated differences in the DHI according to when the data was collected during the COVID-19 pandemic (Supplemental Material). This showed no between-group differences for those assessed per-protocol and those assessed prior to or during the pandemic (−1.73, 95% CI −19.98, 16.52, P = .845). A pre-specified per-protocol analysis included participants who had completed their 26-week assessment within the ±2 weeks window (41 participants) and found no between-group difference (−0.58, 95% CI −9.32, 8.16, P = 0.892). The mixed-effects repeated measures analysis found no between-group difference at both 14 and 26 weeks (−4.73, 95% CI = −12.89 to 3.43 and −1.90, 95% CI = −9.84 to 6.03, respectively) and there was no statistically significant interaction between time and allocation (interaction term = 2.83, 95% CI = −6.37 to 12.03).
The interactions between allocation and diagnosis and recruitment site were assessed visually due to small group sizes, however no indications of interactions were observed. There was no evidence of interactions between allocation and MS type (progressive vs relapsing-remitting) (interaction term = −16.5 95% CI = −34.1 to 1.2); psychological state (determined via the HADS) (interaction term = 0.1 95% CI = −1.3 to 1.4); visual dependence (determined via the maximal rod and disc deviation; interaction term = 0.7 95% CI = −2.1 to 3.6), or the associated symptoms of leg sensation (interaction term = −0.14 95% CI = −6.47 to 6.20), isometric leg strength (interaction term = −0.05 95% CI = −0.55 to 0.45), or reaction time (interaction term = −0.03 95% CI = −0.17 to 0.12) on the change in DHI from baseline (Table 3).
Secondary Outcome Measures
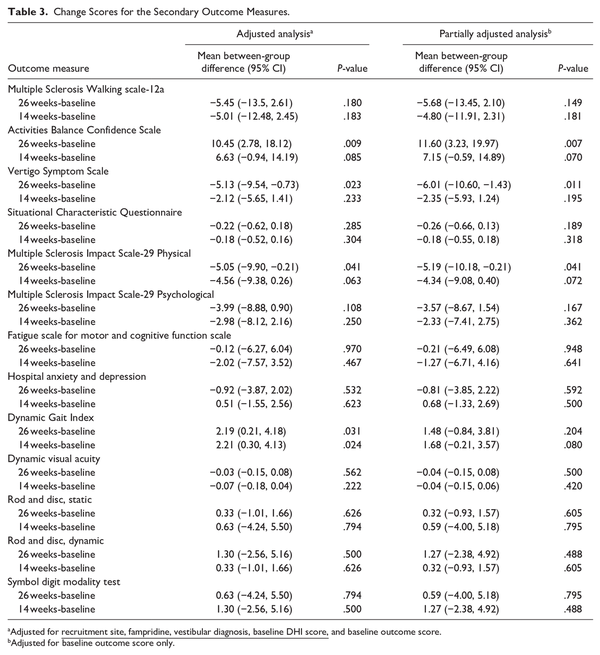
The secondary outcomes and the change in secondary outcome measures from baseline to week 14 and week 26 are summarized in Table 3. At week 14 and week 26 the DGI showed a significant difference in favor of the intervention group. The adjusted between mean group difference at week 14 was +2.21 (95% CI = 0.30, 4.13; P = .024) and at week 26 was +2.19 (95% CI = 0.21, 4.18, P = .031). Due to the impact of COVID-19 on collecting data face-to-face the DGI was collected in 49 people (74%) at week 14 and 37 people (56%) at week 26.
Significant improvements in favor of the intervention group at week 26 were seen for some patient-reported outcome measures. Here, as the data could be gathered remotely there was a more complete data set (>86% at week 26). There was a significant additional improvement in the VSS with a mean between-group difference of −5.13 (−9.54, −0.73; P = .023). There was a significant improvement in the ABC with a mean between-group difference of +10.45% (2.78, 18.12; P = .009). There was a significant improvement in the physical subscale of the MSIS-29 with a mean between-group difference of −5.05 (−9.90, −0.21; P = .041). There were no other between-group differences observed in the secondary outcomes (Table 3).
Compliance
Diaries were returned every month over the 3-month trial period up to week 14. In total 72.9% of participants returned all 3 diaries (62.9% control; 82.9% intervention). Compliance was 48.6% (n = 17) in the control group and 71.4% (n = 25) in the intervention group. Session compliance was 88.6% for the intervention and 65.7% for the control group.
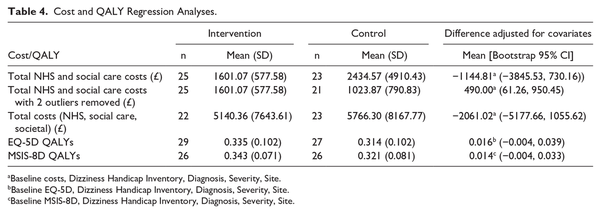
Health Economic Evaluation
The estimated mean cost per participant for delivery of the customized VR program was £1021, compared to £168 for the delivery of generic VR. The resources used to provide the intervention and their quantities are detailed in Supplemental Materials. Table 4 reports EQ-5D QALY weights at baseline and follow-up, and QALYs based on the EQ-5D. The customized VR group accrued more mean (95% CI) QALYs over the 26 weeks of follow-up than the generic VR group; 0.016 (−0.004, 0.039). NHS and social care resource use by participants across the 26-week follow-up period, and associated costs, are presented in Supplemental Materials. The estimated mean (95% CI) cost to the NHS and social care (including the cost of the VR programs) was −££1145 (−£3846, £730) less for the customized VR group than for the generic VR group (Table 4). The cost-effectiveness analysis conducted from the primary perspective of the NHS and social care, using the EQ-5D to estimate QALYs, showed the customized VR intervention to be dominant, that is it was less costly (−£1145) and more effective (0.016) than the generic VR approach.
The 2 planned sensitivity analyses also showed the customized VR intervention to be dominant over generic VR. Use of the MSIS-8D resulted in a similar mean (95% CI) adjusted difference of 0.014 (−0.004, 0.033) QALYs in favor of the customized VR group, compared to the results obtained from the EQ-5D. The estimated mean (95% CI) cost for broader societal resources was −££2061.02 (−££5178, £1056) less for the customized VR group than the generic group (Table 4).
A further sensitivity analysis was undertaken to explore the impact of specific cost outliers on the overall findings of the cost effectiveness analysis. Two participants had substantially greater costs to the NHS and social care during the follow-up period than all other individuals in the study (£12 925 and £21 569, whilst the mean cost was £2000). One participant stayed in hospital for 31 nights, and the other had personal care for 4 hours daily. Both individuals were in the comparator group. When these costs were excluded from the analysis, the mean NHS/social care costs shifted from −££1145 less in the customized VR group to £490 more in this group. Exclusion of these high costs resulted in the mean (95% CI) cost-per-QALY of the customized VR intervention being £30 147 (−££112617, £270 957). This had an associated probability that the intervention was cost-effective compared to generic VR of 0.34 at a willingness to pay threshold of £20 000 per QALY and 0.45 at a willingness to pay of £30 000 per QALY. With these 2 outliers excluded, customized VR was still dominant from a societal perspective (−££336 less cost and 0.016 more QALYs than generic VR).
Adverse Events
There were 6 reported Serious AEs in 5 people, all deemed unrelated to the trial. There were 120 AEs reported in 33 participants during the program delivery phase and 185 AEs reported in total by week 26. Of these, n = 2 (non-injurious falls) were considered to be related to the intervention. Non-injurious falls were additionally monitored via the diaries. There were 726 falls in 29 people over the 26-week trial period; the vast majority of which were reported by 3 people (2 control 41 [5.6%] and 390 [53.7%] falls) and 1 intervention (178 [24.5%) falls]). Thirty-eight injurious falls were reported, by 18 people (n = 9 control and n = 9 intervention). None of these were considered to be related to the intervention.
Discussion
This study found that in people with MS with a defined vestibulopathy a customized VR exercise group was more effective at improving measures of walking, balance confidence, and the perceived severity of dizziness and vertigo. The customized intervention was cost effective when both healthcare and societal costs were included. The study however did not find evidence of a statistically significant difference in the primary outcome of the DHI. Overall, the current cohort had baseline DHI scores of 41.5/100 similar to that in other studies of VR in people with MS., The change in DHI between baseline and week 26 was −7.71(17.2) in the control and −9.60 (13.2) in the intervention group. In comparison, a previous meta-analysis of the reduction in DHI score with VR compared to no intervention showed reductions of −17.43 (−29.99, −4.87). In patients with vestibular dysfunction the smallest detectable group difference for the DHI is 3.78 points and individual difference is 18 to 20 with a minimal important change of 11 points.,
The current study used an active control, that is, control participants also undertook a form of VR; which is not standard practice in people with MS. This suggests that the customized and booklet-based approaches have similar efficacy in improving function/participation and seem comparable to other exercise training. This may reflect that there are potentially multiple impairments in people with MS that may impact on function and participation. As a result it may be difficult for people to determine the contribution to function and participation caused by dizziness as requested in the DHI. It may be that greater improvements may be seen in the DHI if vestibulopathy is the sole or predominant condition.
There was, however, evidence of statistically significant between-group changes in some secondary outcomes, in favor of the intervention group. The VSS showed a significantly greater decrease (improvement) in the customized intervention group. This scale focuses more on the severity of vertigo and dizziness symptoms compared to the DHI that focuses on the resultant impact of dizziness. In the customized intervention group there was also a significantly greater reduction in the DGI at both week 14 and week 26. Further, there was a significantly greater improvement in both the ABC and the MSIS-29 physical component. This may reflect the greater emphasis on functional balance and walking training in the customized group compared to the generic group where exercises were mainly conducted in either sitting or standing depending on symptoms and their severity. Self-reported compliance was higher in the customized intervention group. This may reflect the goal-setting approach and support provided by face-to-face (or virtual during the pandemic) contact. Other secondary outcomes such as DVA and tests of vertical perception and visual dependence did not change with the intervention. This suggests that vestibular function may not improve with rehabilitation. Re-measuring vestibular function using VNG and rotary testing as occurred at screening could further explore this. Other factors such as fatigue also did not improve highlighting that although fatigue can be higher in people with MS and vestibuopathy- this is a complex multifactorial phenomenon.
The primary health economics analysis suggests that, taking an NHS and social care perspective, the customized VR intervention is less costly and more effective than the generic VR program. However, following the removal of 2 outliers it is unlikely that the customized VR intervention was cost-effective at the £30 000 cost-per-QALY threshold set by NICE for recommending interventions for funding on the NHS. Analysis from a broader societal perspective, incorporating participants own costs, informal care, and time off work, indicated that customized VR was less costly and more effective than generic VR. Further, all findings appear to hold when the MS-specific MSIS-8D is used in the health economics analyses in place of the generic EQ-5D. Adopting such a societal perspective seems key in capturing the often undocumented shift in economic load to people with MS and their family and friends when resources or services are not offered via the NHS/social care. The wide confidence intervals found, and the sensitivity of the health economic analysis to particular participants’ resource use, raises some uncertainty as to whether a similar pattern of findings would be replicated in larger samples of people with MS.
Study Limitations: The screening identified people with MS who had a defined vestibulopathy. However, we were unable to determine whether this was caused directly by MS or whether in some cases there could have been additional pathology (eg, a peripheral vestibular neuritis). The study was powered to show a change in the primary outcome, the DHI. It was not powered to show a change in the EQ-5D-5L, the measure used to calculate cost effectiveness. The majority of recruitment came from 1 study site meaning that biases caused by regional variations in intervention delivery could not be determined. As highlighted in the Methods section due to COVID-19 restrictions some alterations were made to the planned intervention and study design and some face to face measures were not taken. The intervention was conducted in a university setting within the UK meaning that it may not be generalizable to UK healthcare systems.
Overall, the results suggest that customized VR is beneficial in improving impairments if vestibulopathy is the predominant condition. However, to improve function and participation, more generic approaches incorporating VR may be required in people with MS with additional impairments.
Conclusion
Compared to a generic VR booklet-based home exercise plan, there was no additional benefit of customized VR intervention on the primary outcome the DHI. The secondary outcomes of the VSS; measures of balance confidence; walking; and the impact of physical symptoms in MS improved significantly more in the customized intervention group. The primary health economics analysis suggests that, taking an NHS and social care perspective, the customized VR intervention is less costly and more effective than the generic VR program but this was affected by high NHS/social costs in 2 control group participants. When broader health and social costs to people with MS, their carers, and society are also considered, the customized VR intervention is less costly and more effective than generic VR.
Author Contributions Jonathan Marsden: Conceptualization; Data curation; Funding acquisition; Investigation; Methodology; Project administration; Writing—original draft; and Writing—review & editing. Rachel Dennett: Investigation; Writing—original draft; and Writing—review & editing. Angela Gibbon: Investigation; Writing—original draft; and Writing—review & editing. Rachel Knight Lozano: Data curation; Investigation; Writing—original draft; and Writing—review & editing. Jennifer A. Freeman: Funding acquisition; Methodology; Writing—original draft; and Writing—review & editing. Doris-Eva Bamiou: Funding acquisition; Methodology; Writing—original draft; and Writing—review & editing. Chris Harris: Writing—original draft and Writing—review & editing. Annie Hawton: Formal analysis; Funding acquisition; Supervision; Writing—original draft; and Writing—review & editing. Elizabeth Goodwin: Formal analysis and Writing—review & editing. Siobhan Creanor: Conceptualization; Funding acquisition; Writing—original draft; and Writing—review & editing. Lexy Sorrell: Formal analysis; Writing—original draft; and Writing—review & editing. Joanne Hosking: Formal analysis; Funding acquisition; Writing—original draft; and Writing—review & editing. Marousa Pavlou: Conceptualization; Funding acquisition; Writing—original draft; and Writing—review & editing.
Declaration of Conflicting Interests The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This study is funded by the Multiple Sclerosis Society, United Kingdom (Ref 71). The article presents independent research. Under the University’s Rights Retention policy, authors grant the University a non-exclusive, irrevocable, worldwide, royalty-free license to make manuscripts of their scholarly articles publicly available under a Creative Commons Attribution (CC BY) license or similar license terms. SC is partially supported by the National Institute for Health Research Applied Research Collaboration South West Peninsula.
Jonathan Marsden
https://orcid.org/0000-0002-2037-4902
Rachel Dennett
https://orcid.org/0000-0003-0400-0502
Elizabeth Goodwin
https://orcid.org/0000-0003-1351-9170
Supplementary material for this article is available on the Neurorehabilitation & Neural Repair website along with the online version of this article.
References
- 1. Marrie RA, Cutter GR, Tyry T. Substantial burden of dizziness in multiple sclerosis. Mult Scler Relat Disord. 2013;2(1):21–28. doi:10.1016/j.msard.2012.08.004
- 2. Di Stadio A, Dipietro L, Ralli M, Greco A, Ricci G, Bernitsas E. The role of vestibular evoked myogenic potentials in multiple sclerosis-related vertigo. A systematic review of the literature. Mult Scler Relat Disord. 2019;28:159–164. doi:10.1016/j.msard.2018.12.031
- 3. Cochrane GD, Christy JB, Motl RW. Central vestibular functions correlate with fatigue and walking capacity in people with multiple sclerosis. Phys Ther. 2021;101(9):pzab168. doi:10.1093/ptj/pzab168
- 4. Cochrane GD, Christy JB, Sandroff BM, Motl RW. Cognitive and central vestibular functions correlate in people with multiple sclerosis. Neurorehabil Neural Repair. 2021;35(11):1030–1038. doi:10.1177/15459683211046268
- 5. Cochrane GD, Christy JB, Motl RW. Comprehensive clinical assessment of vestibular function in multiple sclerosis. J Neurol Phys Ther. 2021;45(3):228–234. doi:10.1097/npt.0000000000000358
- 6. Frohman EM, Kramer PD, Dewey RB, Kramer L, Frohman TC. Benign paroxysmal positioning vertigo in multiple sclerosis: diagnosis, pathophysiology and therapeutic techniques. Mult Scler. 2003;9(3):250–255.
- 7. Hall CD, Herdman SJ, Whitney SL, et al. Vestibular rehabilitation for peripheral vestibular hypofunction: an updated clinical practice guideline from the academy of neurologic physical therapy of the American Physical Therapy Association. J Neurol Phys Ther. 2022;46(2):118–177. doi:10.1097/npt.0000000000000382
- 8. Hebert JR, Corboy JR, Manago MM, Schenkman M. Effects of vestibular rehabilitation on multiple sclerosis-related fatigue and upright postural control: a randomized controlled trial. Phys Ther. 2011;91(8):1166–1183. doi:10.2522/ptj.20100399
- 9. Hebert JR, Corboy JR, Vollmer T, Forster JE, Schenkman M. Efficacy of balance and eye-movement exercises for persons with multiple sclerosis (BEEMS). Neurology. 2018;90(9):e797-e807. doi:10.1212/wnl.0000000000005013
- 10. Brichetto G, Piccardo E, Pedulla L, Battaglia MA, Tacchino A. Tailored balance exercises on people with multiple sclerosis: a pilot randomized, controlled study. Mult Scler. 2015;21(8):1055–1063. doi:10.1177/1352458514557985
- 11. Shady NAA, Shalaby NM, El Fayoumy NM, Youssef KH, Salem HMIA. Effect of vestibular rehabilitation on stability functions in patients with remitting relapse multiple sclerosis; A randomized controlled trial. J Adv Pharm Edu Res. 2018;8(4):39–44.
- 12. Afrasiabifar A, Karami F, Najafi Doulatabad S. Comparing the effect of Cawthorne-Cooksey and Frenkel exercises on balance in patients with multiple sclerosis: a randomized controlled trial. Clin Rehabil. 2018;32(1):57–65. doi:10.1177/0269215517714592
- 13. Tramontano M, Martino Cinnera A, Manzari L, et al. Vestibular rehabilitation has positive effects on balance, fatigue and activities of daily living in highly disabled multiple sclerosis people: a preliminary randomized controlled trial. Restor Neurol Neurosci. 2018;36(6):709–718. doi:10.3233/rnn-180850
- 14. Ozgen G, Karapolat H, Akkoc Y, Yuceyar N. Is customized vestibular rehabilitation effective in patients with multiple sclerosis? A randomized controlled trial. Eur J Phys Rehabil Med. 2016;52(4):466–478.
- 15. Karami F, Afrasiabifar A, Doulatabad NS. Comparing the effectiveness of vestibular rehabilitation and Frenkel exercise on fatigue reduction in patients with multiple sclerosis: a randomized controlled trial. Iran Red Crescent Med J. 2018;20:e68913.
- 16. Cattaneo D, Jonsdottir J, Regola A, Carabalona R. Stabilometric assessment of context dependent balance recovery in persons with multiple sclerosis: a randomized controlled study. J Neuroeng Rehabil. 2014;11:100. doi:10.1186/1743-0003-11-100
- 17. García-Muñoz C, Cortés-Vega MD, Heredia-Rizo AM, Martín-Valero R, García-Bernal MI, Casuso-Holgado MJ. Effectiveness of vestibular training for balance and dizziness rehabilitation in people with multiple sclerosis: a systematic review and meta-analysis. J Clin Med. 2020;9(2):590. doi:10.3390/jcm9020590
- 18. Synnott E, Baker K. The effectiveness of vestibular rehabilitation on balance related impairments among Multiple Sclerosis patients: a systematic review. J Mult Scler. 2020;7(1):1–8.
- 19. Whitney SL, Alghadir AH, Anwer S. Recent evidence about the effectiveness of vestibular rehabilitation. Curr Treat Options Neurol. 2016;18(3):13. doi:10.1007/s11940-016-0395-4
- 20. Marsden J, Pavlou M, Dennett R, et al. Vestibular rehabilitation in multiple sclerosis: study protocol for a randomised controlled trial and cost-effectiveness analysis comparing customised with booklet based vestibular rehabilitation for vestibulopathy and a 12 month observational cohort study of the symptom reduction and recurrence rate following treatment for benign paroxysmal positional vertigo. BMC Neurol. 2020;20(1):430. doi:10.1186/s12883-020-01983-y
- 21. Thompson AJ, Banwell BL, Barkhof F, et al. Diagnosis of multiple sclerosis: 2017 revisions of the McDonald criteria. Lancet Neurol. 2018;17(2):162–173. doi:10.1016/s1474-4422(17)30470-2
- 22. Learmonth YC, Motl RW, Sandroff BM, Pula JH, Cadavid D. Validation of patient determined disease steps (PDDS) scale scores in persons with multiple sclerosis. BMC Neurol. 2013;13:37. doi:10.1186/1471-2377-13-37
- 23. Lord SR, Menz HB, Tiedemann A. A physiological profile approach to falls risk assessment and prevention. Phys Ther. 2003;83(3):237–252.
- 24. Yardley L, Masson E, Verschuur C, Haacke N, Luxon L. Symptoms, anxiety and handicap in dizzy patients: development of the vertigo symptom scale. J Psychosom Res. 1992;36(8):731–741.
- 25. van Esch BF, Nobel-Hoff GE, van Benthem PP, van der Zaag-Loonen HJ, Bruintjes TD. Determining vestibular hypofunction: start with the video-head impulse test. Eur Arch Otorhino-laryngol. 2016;273(11):3733–3739. doi:10.1007/s00405-016-4055-9
- 26. Stell R, Bronstein AM, Marsden CD. Vestibulo-ocular abnormalities in spasmodic torticollis before and after botulinum toxin injections. J Neurol Neurosurg Psychiatry. 1989;52(1):57–62.
- 27. Yardley L. Balance Retraining; Exercises which speed recovery from dizziness and unsteadiness. 2012. Accessed 2020. http://www.menieres.org.uk/files/pdfs/balance-retraining-2012
- 28. Jacobson GP, Newman CW. The development of the Dizziness Handicap Inventory. Arch Otolaryngol Head Neck Surg. 1990;116(4):424–427. doi:10.1001/archotol.1990.01870040046011
- 29. Wilhelmsen K, Strand LI, Nordahl SH, Eide GE, Ljunggren AE. Psychometric properties of the Vertigo symptom scale: short form. BMC Ear Nose Throat Disord. 2008;8:2. doi:10.1186/1472-6815-8-2
- 30. Pavlou M, Davies RA, Bronstein AM. The assessment of increased sensitivity to visual stimuli in patients with chronic dizziness. J Vestib Res. 2006;16(4-5):223–231.
- 31. Dannenbaum E, Paquet N, Chilingaryan G, Fung J. Clinical evaluation of dynamic visual acuity in subjects with unilateral vestibular hypofunction. Otol Neurotol. 2009;30(3):368–372. doi:10.1097/MAO.0b013e31819bda35
- 32. Dannenbaum E, Paquet N, Hakim-Zadeh R, Feldman AG. Optimal parameters for the clinical test of dynamic visual acuity in patients with a unilateral vestibular deficit. J Otolaryngol. 2005;34(1):13–19.
- 33. Guerraz M, Yardley L, Bertholon P, et al. Visual vertigo: symptom assessment, spatial orientation and postural control. Brain. 2001;124(Pt 8):1646–1656.
- 34. Kaski D, Buttell J, Greenwood R. Targeted rehabilitation reduces visual dependency and improves balance in severe traumatic brain injury: a case study. Disabil Rehabil. 2018;40:856–858. doi:10.1080/09638288.2016.1276976
- 35. Sonder JM, Burggraaff J, Knol DL, Polman CH, Uitdehaag BM. Comparing long-term results of PASAT and SDMT scores in relation to neuropsychological testing in multiple sclerosis. Mult Scler. 2014;20(4):481–488. doi:10.1177/1352458513501570
- 36. Hobart JC, Riazi A, Lamping DL, Fitzpatrick R, Thompson AJ. Measuring the impact of MS on walking ability: the 12-Item MS Walking Scale (MSWS-12). Neurology. 2003;60(1):31–36.
- 37. Powell LE, Myers AM. The Activities-specific Balance Confidence (ABC) Scale. J Gerontol A Biol Sci Med Sci. 1995;50A(1):M28-M34.
- 38. Penner IK, Raselli C, Stocklin M, Opwis K, Kappos L, Calabrese P. The Fatigue Scale for Motor and Cognitive Functions (FSMC): validation of a new instrument to assess multiple sclerosis-related fatigue. Mult Scler. 2009;15(12):1509–1517. doi:10.1177/1352458509348519
- 39. Piker EG, Kaylie DM, Garrison D, Tucci DL. Hospital Anxiety and Depression Scale: factor structure, internal consistency and convergent validity in patients with dizziness. Audiol Neuro-otol. 2015;20(6):394–399. doi:10.1159/000438740
- 40. Herdman M, Gudex C, Lloyd A, et al. Development and preliminary testing of the new five-level version of EQ-5D (EQ-5D-5L). Qual Life Res. 2011;20(10):1727–1736. doi:10.1007/s11136-011-9903-x
- 41. Hobart JC, Lamping DL, Fitzpatrick R, Riazi A, Thompson AJ. The Multiple Sclerosis Impact Scale (MSIS-29): a new patient-based outcome measure. Brain. 2001;124:962–973.
- 42. van Hout B, Janssen MF, Feng YS, et al. Interim scoring for the EQ-5D-5L: mapping the EQ-5D-5L to EQ-5D-3L value sets. Value Health. 2012;15(5):708–715. doi:10.1016/j.jval.2012.02.008
- 43. Dolan P. Modeling valuations for EuroQol health states. Med Care. 1997;35(11):1095–1108. doi:10.1097/00005650-199711000-00002
- 44. National Institute for Care Research (NICE). Position statement on use of the EQ-5D-5L value set for England (updated October 2019). 2019.
- 45. Ridyard CH, Hughes DA. Development of a database of instruments for resource-use measurement: purpose, feasibility, and design. Value Health. 2012;15(5):650–655. doi:10.1016/j.jval.2012.03.004
- 46. Thorn JC, Brookes ST, Ridyard C, et al. Core items for a standardized resource use measure: expert Delphi Consensus Survey. Value Health. 2018;21(6):640–649. doi:10.1016/j.jval.2017.06.011
- 47. Curtis L, Burns A. Data from: Unit Costs of Health and Social Care 2020. Canterbury; 2020.
- 48. Goodwin E, Green C, Spencer A. Estimating a preference-based index for an eight-dimensional health state classification system for multiple sclerosis. Value Health. 2015;18(8):1025–1036. doi:10.1016/j.jval.2015.10.004
- 49. Cattaneo D, Regola A, Meotti M. Validity of six balance disorders scales in persons with multiple sclerosis. Disabil Rehabil. 2006;28(12):789–795.
- 50. Loyd BJ, Fangman A, Peterson DS, et al. Rehabilitation to improve gaze and postural stability in people with multiple sclerosis: study protocol for a prospective randomized clinical trial. BMC Neurol. 2019;19(1):119. doi:10.1186/s12883-019-1353-z
- 51. Tamber AL, Wilhelmsen KT, Strand LI. Measurement properties of the Dizziness Handicap Inventory by cross-sectional and longitudinal designs. Health Qual Life Outcomes. 2009;7:101. doi:10.1186/1477-7525-7-101
- 52. Loyd BJ, Fangman A, Peterson DS, et al. Rehabilitation to improve gaze and postural stability in people with multiple sclerosis: a randomized clinical trial. Neurorehabil Neural Repair. 2022;36(10-11):678–688. doi:10.1177/15459683221124126
- 53. Kalron A, Givon U, Frid L, Dolev M, Achiron A. Static posturography and falls according to pyramidal, sensory and cerebellar functional systems in people with multiple sclerosis. PLoS One. 2016;11(10):e0164467. doi:10.1371/journal.pone.0164467
- 54. Koppelaar-van Eijsden HM, Schermer TR, Bruintjes TD. Measurement properties of the dizziness handicap inventory: a systematic review. Otol Neurotol. 2022;43(3):e282-e297. doi:10.1097/mao.0000000000003448
- 55. Levack WM, Weatherall M, Hay-Smith JC, Dean SG, McPherson K, Siegert RJ. Goal setting and strategies to enhance goal pursuit in adult rehabilitation: summary of a Cochrane systematic review and meta-analysis. Eur J Phys Rehabil Med. 2016;52(3):400–416.
- 56. McCabe C, Claxton K, Culyer AJ. The NICE cost-effectiveness threshold: what it is and what that means. Pharmacoeconomics. 2008;26(9):733–744. doi:10.2165/00019053-200826090-00004
- 57. Goodwin E, Green C. A quality-adjusted life-year measure for multiple sclerosis: developing a patient-reported health state classification system for a multiple sclerosis-specific preference-based measure. Value Health. 2015;18(8):1016–1024. doi:10.1016/j.jval.2015.07.002