Key Points
A study on global trends in population-based survival from brain tumors in adults.
Five-year survival for 11 histology groups and, for glioblastoma, also 2-year and age-specific survival.
Wide variation in survival suggests inequalities in access to care.
Importance of the Study
We set out to provide a comprehensive examination of worldwide variation in population-based survival from brain tumors in adults, by histology. The analysis included more than 500,000 patients diagnosed with a primary brain tumor during 2000–2014 in 59 countries. A standardized protocol for data collection ensured that data were based on the same set of patient-related and tumor-related variables from all registries. We provided estimates of net survival 5 years after diagnosis for 11 histology groupings. For glioblastoma, we also estimated age-specific survival and survival at 2 years. We highlighted the remarkable gains in survival from glioblastoma since 2005, providing large-scale empirical evidence of the uptake of chemoradiation at population level. Survival improvements have been extensive, but some countries still lag behind. Our findings should enable clinicians involved in national and international tumor pathway boards to promote initiatives aimed at more extensive implementation of clinical guidelines. This should translate to more equitable access to care and continued progress in survival.
Tumors originating in the brain are rare. In England, the age-standardized (Europe) incidence rate for glioblastoma, the most common subtype in adults, was 5.0 per 100,000 in 2015.
Only a few treatment protocols of proven efficacy are available for brain tumors. After maximal safe resection, when feasible, patients may receive different combinations of radiotherapy and chemotherapy, depending on histology. For instance, the standard therapeutic regimen for glioblastoma is a course of radiotherapy given concomitantly with temozolomide and, subsequently, chemotherapy with temozolomide for 6 months.
Data from all patients registered by national or regional population-based cancer registries are the backbone of population-based cancer survival estimates. Survival for all patients in the population reflects the overall effectiveness of a health system in managing cancer. Worldwide disparities in survival can only be reliably explored through large studies that use a standard protocol for data collection, centralized procedures for data quality control, and the same statistical methods for all datasets.
The third cycle of the CONCORD program for global surveillance of cancer survival (CONCORD-3) obtained individual tumor records from 322 population-based cancer registries in 71 countries, for 37.5 million patients diagnosed during 2000–2014 with 1 of 18 common cancer types, including 742,145 adults diagnosed with a brain tumor. Age-standardized 5-year net survival for all brain tumors combined ranged from 14.7% in Thailand to 42.2% in Croatia for patients diagnosed during 2010–2014.
Access to high-quality health care is inequitable worldwide. Radiotherapy is critical to brain tumor management, but only 65% of middle-income countries have operational radiotherapy facilities., The neurosurgical workforce has been increasing in low-income and middle-income countries, but it is still largely suboptimal. Distribution of pathology services is uneven: in many countries, pathology services are simply not available and lack of neuropathology expertise is even more widespread.,
Survival for all brain tumors combined is likely to vary worldwide because of differences in access to health care, but given the clinical heterogeneity of the histological subtypes of brain tumors, it may also be affected by international differences in the distribution of histological subtypes.
Brain tumors comprise a heterogeneous group of neoplasms with distinct clinical behavior, and wide variation in survival by age. For example, in the United States, 5-year relative survival in young adults (15–39 years) diagnosed during 2001–2015 was 76% for diffuse astrocytoma, but only 26% for glioblastoma. For older adults (40 years or more), 5-year relative survival for these subtypes was 32% and 5%, respectively. The histology distribution of brain tumors varies widely between countries. In CONCORD-3, the proportion of glioblastomas diagnosed during 2000–2014 was less than 10% in China but more than 50% in Europe and North America. True geographical differences in incidence may occur for some brain tumor subtypes, but some of the observed variations may be attributable to international differences in the availability of neurosurgery and in the quality/completeness of neuropathological data, as much as to differences in cancer registration practice.
Treatment of brain tumors depends on histology, grade, and anatomic site, so clinically relevant survival analyses by histology are crucial for health systems that aim to monitor and improve cancer outcomes. So far, however, population-based survival estimates for brain tumors by histology have been limited to Europe and North America. Study designs vary widely, and hardly any studies have been conducted in low-income and middle-income countries. In addition, up-to-date international comparisons of survival trends by histology are not currently available., We aimed to address this gap by conducting a study of population-based survival trends with global coverage and up-to-date follow-up for vital status.
Patients and Methods
We analyzed all brain tumors included in the CONCORD-3 database. We considered adults (15–99 years) diagnosed with a primary malignant or nonmalignant tumor of the brain (topography code C71 in the International Classification of Diseases for Oncology, third edition, ICD-O-3) during 2000–2014. CONCORD-3 did not collect data for central nervous system tumors arising in the meninges (C70), the spinal cord, or the cranial nerves, including the optic chiasma (C72) or the pituitary gland (C75.1) or pineal gland (C75.3).
Data underwent stringent, 3-phase quality control, described elsewhere. In brief, diagnoses based solely on a death certificate or autopsy were excluded. Tumor records were not included if they contained errors in the date sequence or various unlikely combinations of age, site, and morphology, unless the records were confirmed as correct by the registry.
We categorized relevant ICD-O-3 codes into 11 histology groups, using the World Health Organisation Classification of Central Nervous System Tumours (4th edition) (Supplementary Table 1). The methodology and the principles for selecting the ICD-O-3 codes are explained elsewhere.
We estimated net survival up to 5 years after diagnosis, using the unbiased non-parametric Pohar Perme estimator. Net survival is the probability for cancer patients to survive their cancer, after controlling for competing risks of death (background mortality), which are higher in the elderly. Information on background mortality was obtained from life tables of all-cause mortality by single year of age, sex, and single calendar year in each country or territory. The data sources and methods for constructing these life tables have been described.
We estimated net survival by age (15–44, 45–54, 55–64, 65–74, and 75–99 years). We used the International Cancer Survival Standard (ICSS) weights (group 2, tumors with less variation in incidence by age) to produce age-standardized survival estimates for all ages combined. We did not estimate survival if fewer than 10 patients were available for analysis. If 10–49 patients were available for analysis in a given calendar period, we only estimated survival for all ages combined. If 50 or more patients were available, we attempted survival estimation for each age group. If a single age-specific estimate could not be obtained, we merged the data for adjacent age groups and assigned the combined estimate to both age groups before standardization for age. If 2 or more age-specific estimates could not be obtained, we present only the unstandardized estimate for all ages combined. We did not merge data between consecutive calendar periods.
We used the classical cohort approach for patients diagnosed during 2000–2004 and 2005–2009, because in most datasets, all the patients had been followed up for at least 5 years. We adopted the period approach for patients diagnosed during 2010–2014, because 5 years of follow-up were not available for all patients. This approach allowed estimation of 5-year net survival for patients diagnosed during 2010–2014, by combining the survival probabilities from the most recent follow-up data for patients diagnosed during 2010–2014 with the survival probabilities for patients diagnosed during the preceding 5 years who were still alive on January 1, 2010.,
We produced age-standardized 5-year survival estimates for each histology group, country, and calendar period. For glioblastoma, we also estimated 5-year survival by age, to examine the remarkable differences in tumor biology and outcome between age groups. For this purpose, we identified 3 age groups: 15–39, 40–70, and 71–99 years. The age boundaries for the 15–39 age group were chosen to match the methods used in previous studies. The age group 40–70 years was chosen to be in line with most treatment guidelines,, which recommend radiotherapy up to age 70 years. For patients diagnosed with glioblastoma, we also estimated 2-year survival by age, in light of the very poor prognosis for this subtype.
Survival estimates from a given cancer registry were deemed less reliable if 15% or more of patients were lost to follow-up or censored alive within 5 years of diagnosis, or the diagnosis was based only on a death certificate or autopsy, or were registered with incomplete dates. The pooled estimates for countries with more than 1 registry do not include data from registries for which the estimates were less reliable. Less reliable estimates are shown with a flag in figures and tables when they are the only available information from a given country or territory.
The CONCORD programme is approved by the United Kingdom’s statutory Health Research Authority (reference ECC 3-04(i)/2011; last update November 2, 2021), the National Health Service Research Ethics Service (11/LO/0331; January 12, 2022), and the London School of Hygiene & Tropical Medicine Ethics Committee (12171; November 21, 2021).
Results
For CONCORD-3, 286 cancer registries in 59 countries submitted 742,145 individual records for adults diagnosed with a primary brain tumor during 2000–2014. Of these, 671,085 (90.4%) passed the quality checks. The proportion of records with incomplete dates was low in most continents (0%–2%), but relatively high in Africa (26.2%). Patients registered only from a death certificate (DCO) accounted for 10.3% of records in Africa and 14.6% in Central and South America. Data quality indicators for all brain tumors combined are summarized in Supplementary Table 2. Countries are listed in alphabetical order within each continent in tables.
Records with morphology codes different from those selected for study (68,973, 10.3% of those potentially eligible for analysis) or from cancer registries in which the information was deemed less reliable for the purpose of survival analyses (45,887, 6.8%) were also excluded. The final study population comprised 556,237 adults (82.9% of the patients potentially eligible for analysis).
We focused on diffuse and anaplastic astrocytoma, glioblastoma, oligodendroglioma, unspecified astrocytoma, and unspecified glioma, and on countries for which reliable age-standardized survival estimates were available.
Age-standardized 5-year net survival for patients diagnosed with diffuse and anaplastic astrocytoma during 2010–2014 was in the range of 20%–29% in China, Israel, South Korea, Singapore, Taiwan, Turkey, and in 9 European countries (Denmark, Finland, Italy, Poland, Portugal, the Russian Federation, Slovakia, Slovenia, and Switzerland); in the range of 30%–39% in Canada, the United States, 9 European countries (Austria, Belgium, Czech Republic, Germany, the Netherlands, Norway, Spain, Sweden, and the United Kingdom), and in Australia and New Zealand (Supplementary Table 3A, Figure 1).
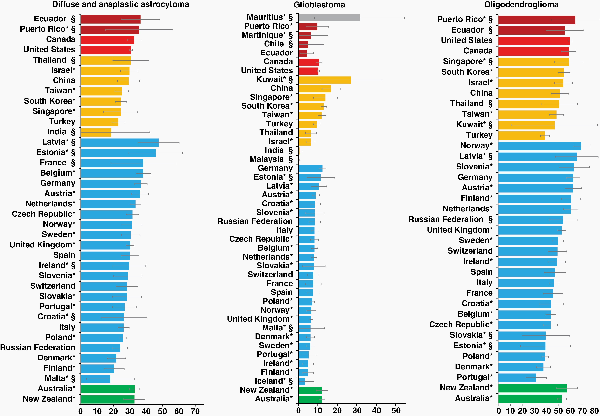
Fig. 1
Age-standardized 5-year net survival (%) with 95% confidence interval, by country: adults (15–99 years) diagnosed with diffuse and anaplastic astrocytoma, glioblastoma, or oligodendroglioma during 2010–2014. *Countries with 100% coverage of the national population. §Survival estimates are not age standardized. Continents are identified by different colors. In each panel, countries are ranked from highest to lowest, based on survival during 2010–2014.
Age-standardized 5-year survival from diffuse and anaplastic astrocytoma improved remarkably in Canada, most North European countries, Belgium, and the Netherlands (Western Europe), Spain (Southern Europe), Czech Republic (Eastern Europe), and in Australia. Survival was generally stable elsewhere. Overall, the largest improvements occurred between 2000–2004 and 2005–2009 (Figure 2).
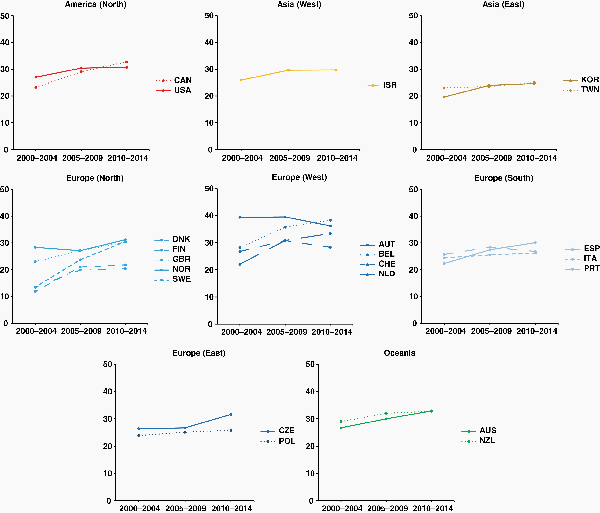
Fig. 2
Fifteen-year trends in age-standardized 5-year net survival (%) for adults (15–99 years) diagnosed with diffuse and anaplastic astrocytoma during 2000–2014, by continent (or continental region), and country. Countries are only included if age-standardized survival estimates were available for patients diagnosed during 2000–2004, 2005–2009, and 2010–2014. Continents (or continental regions) are identified by different colors. In each panel, countries are ranked from highest to lowest, based on survival during 2000–2004. X-axis: period of diagnosis; Y-axis: age-standardized 5-year net survival (%). International Organization for Standardization abbreviations for country names: Australia, AUS; Austria, AUT; Belgium, BEL; Canada, CAN; Czech Republic, CZE; Denmark, DNK; Finland, FIN; Israel, ISR; Italy, ITA; Netherlands, NLD; New Zealand, NZL; Norway, NOR; Poland, POL; Portugal, PRT; South Korea, KOR; Spain, ESP; Sweden, SWE; Switzerland, CHE; Taiwan, TWN; UK, GBR; USA, USA.
For patients diagnosed during 2010–2014, age-standardized 5-year survival for glioblastoma was generally poor, nowhere exceeding 17%. Survival was 4.4% in Ecuador; in the range 5%–9% in Puerto Rico, Israel, Thailand, Turkey, Singapore, and in 20 European countries, and between 10% and 15% in Canada, the United States, South Korea, Singapore, Taiwan, Germany, Latvia, Australia, and New Zealand. Five-year survival was 16.9% in China (Supplementary Table 3A, Figure 1).
Overall, age-standardized 5-year survival for glioblastoma improved over time, mainly between 2000–2004 and 2005–2009. Survival fluctuated or declined in Israel, Austria, Belgium, Croatia, Germany, Ireland, Latvia, and Norway, while it was substantially unchanged in Poland (Figure 3).
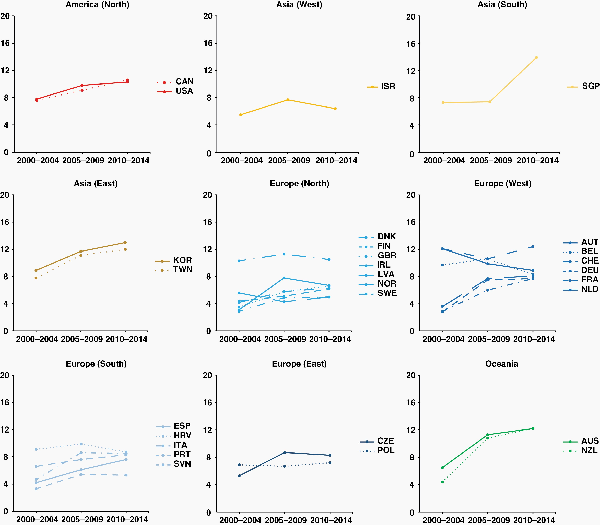
Fig. 3
Fifteen-year trends in age-standardized 5-year net survival (%) for adults (15–99 years) diagnosed with glioblastoma during 2000–2014, by continent (or continental region), and country. Countries are only included if age-standardized survival estimates were available for patients diagnosed during 2000–2004, 2005–2009, and 2010–2014. Continents (or continental regions) are identified by different colors. In each panel, countries are ranked from highest to lowest, based on survival during 2000–2004. X-axis: period of diagnosis; Y-axis: age-standardized 5-year net survival (%). International Organization for Standardization abbreviations for country names: Australia, AUS; Austria, AUT; Belgium, BEL; Canada, CAN; Croatia, HRV; Czech Republic, CZE; Denmark, DNK; Finland, FIN; France, FRA; Germany, DEU; Ireland, IRL; Israel, ISR; Italy, ITA; Latvia, LVA; Netherlands, NLD; New Zealand, NZL; Norway, NOR; Poland, POL; Portugal, PRT; Singapore, SGP; Slovenia, SVN; South Korea, KOR; Spain, ESP; Sweden, SWE; Switzerland, CHE; Taiwan, TWN; UK, GBR; USA, USA.
For glioblastoma, net survival at 2 years in young adults (15–39 years) varied between 30% and 70% worldwide. For patients diagnosed during 2010–2014, 2-year survival was in the range 31%–42% in Central and South American countries, 30%–62% in Asian countries, and 27%–72% in European countries. Two-year survival was around 48% in Canada, the United States, Australia, and New Zealand. For patients diagnosed aged 40–70 years during 2010–2014, two-year survival was 24% or lower in nearly all countries. Only in China, South Korea, Kuwait, Singapore, and Taiwan were outcomes more favorable (31%–42%). Among patients older than 70 years, 2-year net survival rarely exceeded 10% (Supplementary Table 4).
In young adults, 2-year survival for glioblastoma changed slightly during the 15-year period from 2000 to 2014. Steady, upward trends were observed in only 9e countries: Canada, South Korea, Denmark, Sweden, the United Kingdom, France, Germany, the Netherlands, and Slovenia. In a further 11 countries, early improvements in survival were offset by a decline during 2000–2014 (Supplementary Figure 1).
During 2000–2014, remarkable improvements in 2-year survival from glioblastoma for adults aged 40–70 years occurred in nearly all regions. The steepest increases were observed between 2000–2004 and 2005–2009, but trends were still upward for 2010–2014. The differential was slightly smaller in North America, and in Western and Southern European countries. In Israel, Singapore, Denmark, Sweden, Australia, and New Zealand, 2-year survival rose from around 10% to more than 20%. In Eastern Europe, however, 2-year survival was more stable during 2000–2014 and much lower than 20% (Supplementary Figure 2). Despite these improvements in short-term survival, however, survival at 5 years for patients aged 40–70 years improved only slightly throughout the 15 years from 2000 to 2014, remaining below 10% in almost all countries (Supplementary Table 5).
For patients diagnosed with an oligodendroglioma during 2010–2014, age-standardized 5-year survival varied widely, from less than 40% to 70%. Survival was less than 40% in Turkey, Denmark, Poland, and Portugal; it ranged between 40% and 49% in Taiwan and in 9 European countries (Belgium, Croatia, Czech Republic, France, Ireland, Italy, Spain, Sweden, and Switzerland). Survival varied between 50% and 59% in Canada, China, Israel, South Korea, the United Kingdom, Australia, and New Zealand. The highest values (60%–70%) were seen in Austria, Finland, Germany, the Netherlands, Norway, and Slovenia. Survival for oligodendroglioma was higher than for any category of astrocytoma in almost all countries where reliable estimates could be obtained (Supplementary Table 3A, Figure 1).
Steady improvements in 5-year survival for oligodendroglioma were observed in North America, Israel, South Korea, most North European countries, in France and the Netherlands (Western Europe), Croatia and Spain (Southern Europe), in East European countries, and in Australia. Survival wavered over time, or declined, in Taiwan, Finland, Austria, and New Zealand (Figure 4).
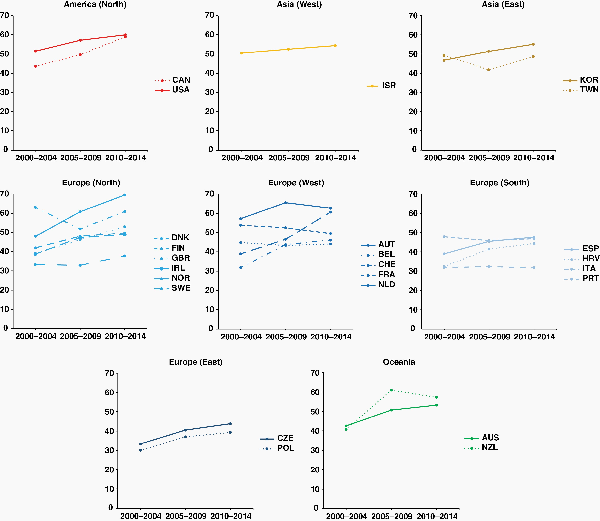
Fig. 4
Fifteen-year trends in age-standardized 5-year net survival (%) for adults (15–99 years) diagnosed with oligodendroglioma during 2000–2014, by continent (or continental region), and country. Countries are only included if age-standardized survival estimates were available for patients diagnosed during 2000–2004, 2005–2009, and 2010–2014. Continents (or continental regions) are identified by different colors. In each panel, countries are ranked from highest to lowest, based on survival during 2000–2004. X-axis: period of diagnosis; Y-axis: age-standardized 5-year net survival (%). International Organization for Standardization abbreviations for country names: Australia, AUS; Austria, AUT; Belgium, BEL; Canada, CAN; Croatia, HRV; Czech Republic, CZE; Denmark, DNK; Finland, FIN; France, FRA; Ireland, IRL; Israel, ISR; Italy, ITA; Netherlands, NLD; New Zealand, NZL; Norway, NOR; Poland, POL; Portugal, PRT; South Korea, KOR; Spain, ESP; Sweden, SWE; Switzerland, CHE; Taiwan, TWN; UK, GBR; USA, USA.
Global disparities in age-standardized 5-year net survival from astrocytoma without further specification of the histology (unspecified astrocytoma) were striking. Survival was 27.2% in Ecuador and around 40% in Canada and the United States. In Asia, it ranged between 13.1% (Thailand) and 45.5% (Turkey), while in Europe, it varied between 25.7% (Czech Republic) and 45.7% (Sweden). Five-year survival was 41.8% in Australia. Survival for unspecified astrocytoma was higher than for diffuse and anaplastic astrocytoma in many countries, with nonoverlapping CIs and absolute differences of at least 15% in South Korea, Taiwan, Turkey, Finland, and Sweden. By contrast, survival for unspecified astrocytoma was only slightly higher than for glioblastoma in a few countries. (Supplementary Table 3A).
Age-standardized 5-year survival for unspecified glioma in 2010–2014 was around 50% or less in all 20 countries where it could be reliably estimated. Survival ranged from below 20% in Croatia, Czech Republic, Poland, Russian Federation, and Spain to between 40% and 49% in the United States, South Korea, and Norway, and it was 53.6% in Belgium. Survival was higher than for diffuse and anaplastic astrocytoma in 9 of the 20 countries (Canada, the United States, Israel, South Korea, Taiwan, Turkey, Belgium, Norway, and Australia), but lower than for glioblastoma in the Russian Federation. Global disparities in survival for unspecified tumors were even wider than for unspecified astrocytoma, ranging from below 10% in Puerto Rico and Poland to between 65% and 69% in Belgium and Denmark and above 80% in Norway (Supplementary Table 3B).
Discussion
To our knowledge, this is the largest study to date of population-based survival from brain tumors by histology. Many of the countries represented here have never previously been included in international comparisons. We analyzed more than half a million individual patient records using a standard protocol for data collection, standardized data quality control procedures, and the same robust statistical methodology for all datasets, accounting for international differences in background mortality and in the age profile of cancer patients.
During 2010–2014, the global range in age-standardized 5-year survival within each histology group was remarkably wide: in the range 4%–17% for glioblastoma, 20%–38% for diffuse and anaplastic astrocytoma, and 32%–69% for oligodendroglioma. For patients diagnosed with glioblastoma, survival gains were most marked between 2000–2004 and 2005–2009; these improvements were more pronounced among adults aged 40–70 years than among adolescents and young adults (15–39 years).
Diffuse astrocytoma (WHO grade II) and anaplastic astrocytoma (WHO grade III) harbor a mutation in the isocitrate dehydrogenase 1 (IDH1) gene in approximately two-thirds of cases. This genetic hallmark has been found to be a more powerful predictor of outcome than the WHO grade,, and it was formally incorporated in the WHO Classification of Central Nervous System Tumours in 2016. Extent of resection, age, and performance status now drive the choice of subsequent treatment, rather than tumor histology. Moreover, interobserver variability in the pathological definition of WHO grade II or III astrocytic tumors is well established., Based on such evidence, we chose to pool tumors defined as diffuse astrocytoma or anaplastic astrocytoma. In a sensitivity analysis in which these 2 entities were kept separate, international variation in survival within the diffuse astrocytoma subgroup or the anaplastic astrocytoma subgroup was more pronounced than with the combined category (Supplementary Table 6). This finding suggests that nonuniform practices in the pathology definition of WHO grade II or III astrocytic tumors may amplify disparities in population-based survival for each of these tumor subtypes, hampering international comparisons.
In many countries, a steady decline in the proportion of brain tumors coded as diffuse astrocytoma and anaplastic astrocytoma during 2000–2014 was offset by increasing proportions of glioblastoma (data not shown). Refinements in brain tumor pathology may have led to a progressive reclassification of lower grade astrocytic tumors with a more aggressive phenotype as glioblastomas. Treatment options for diffuse and anaplastic astrocytoma are not yet widely agreed, and the definition of a standard treatment is still in progress, so reclassification of diffuse astrocytic tumors (WHO grade II or III) with features of glioblastoma, rather than changes in clinical practice, may be the reason for some of the observed improvements for diffuse and anaplastic astrocytoma during 2000–2014.
Glioblastoma is the most common brain tumor subtype in adults. The outcome is generally poor, and most tumors relapse or progress shortly after diagnosis and initial treatment. The current standard of care for glioblastoma was established in 2005, following the results of a large randomized clinical trial: 2-year survival for adults aged up to 70 years who received radiotherapy with concomitant chemotherapy was 26%, but only 10% for those treated with radiotherapy alone. In that trial, around 40% of the patients had received a complete surgical resection, one of the most important predictors of outcome for glioblastoma.
Here, we have aimed to assess whether the results of that trial for glioblastoma may have influenced clinical practice to the extent of improving survival at the population level. We assumed that most patients up to age 70 years were likely to have received chemoradiotherapy. That age boundary was used in the 2005 trial, and it has been implemented in clinical guidelines. We explored whether the survival benefit from treatment, barely visible at 5 years, was more pronounced in shorter term survival. We considered adolescents and young adults separately, because they may encounter barriers to optimal treatment due to the lack of age-appropriate psychosocial support services or centralized cancer care., In several countries, improvements in 2-year survival for adults aged 40–70 years were striking, with survival increasing markedly from 10% or less to values in the range 20%–30% over the period 2000–2014. However, trends were flatter in countries such as Poland and the Czech Republic, suggesting that constraints in the uptake of modern treatment protocols may still exist. In patients aged 15–39 years, outcomes were more favorable than in those aged 40–70 years, as expected in the light of the biological differences. In absolute terms, however, survival gains during 2000–2014 were much smaller than in patients aged 40–70 years, and limited to a few affluent countries (Canada, the Netherlands, South Korea). Clinical trials have not yet explored outcomes in adolescents and young adults, so it is still unclear whether the potential treatment benefit varies with age. However, the strikingly different trends in survival between patients aged 15–39 and those aged 40–70 years underline the age-related disparities in outcome, as well as the geographical disparities. Survival gains for glioblastoma were more marked for 2-year survival than 5-year survival, suggesting that improvements in early diagnosis or the quality of initial treatment may not have had a substantial effect on the longer term prognosis.
The CONCORD-3 protocol asked participating registries to submit treatment data, including the full date of the first course of chemotherapy or radiotherapy. For brain tumors, 23 of the 48 participating US cancer registries provided the full date of the first course of radiotherapy for at least 70% of the patients. The American Society for Radiation Oncology (ASTRO) and the American Society of Clinical Oncology (ASCO) recommend starting radiotherapy as soon as safely permissible. Patients enrolled in clinical trials usually start radiotherapy within 3–6 weeks after surgery,, but for patients not in trials, who are the great majority, the time between diagnosis and the initiation of treatment may be much longer if access to care is suboptimal. In the US CONCORD dataset, the proportion of patients receiving the first course of radiotherapy within 6 weeks of surgery or biopsy during 2000–2014 varied widely between US states, in the range 70%–86% (data not shown). More detailed data are needed to explore the impact on survival of delay in receiving treatment.
In previous work, we described the histology distribution of brain tumors worldwide. The proportion of brain tumors with nonspecific histology (ICD-O-3 codes 8000-8005) varied widely around the world. These neoplasms accounted for 64% of all brain tumor diagnoses in China and 41% in Denmark. The proportion of tumors with unspecified histology was higher than 30% in the data from all the participating regional registries in China (21 registries) and Italy (44 registries). In CONCORD-3, the proportion of brain tumors with a nonspecific histology code that were recorded as histologically verified was relatively high, ranging from 4.2% in Oceania to 65.2% in Africa. Strikingly, during 2010–2014, age-standardized 5-year net survival for brain tumors with nonspecific histology ranged from 7% in Puerto Rico to 82% in Norway. Very low survival may imply that the histology of these tumors could not be further specified because the patients were too unwell to undergo surgery or biopsy safely. It is difficult to draw firm conclusions, because the broad range of survival values suggests that barriers to the accurate reporting of the histology of a brain tumor may intervene at all levels between diagnosis and cancer registration. The exceptionally wide international variation in survival for tumors with unspecified histology and, to a lesser extent, for unspecified astrocytoma and unspecified glioma, suggests that there is also a wide international variation in the composition of these less specific diagnostic subgroups. In countries where the proportion of unspecified brain tumors is very high, age-standardized 5-year net survival for tumors in specific histology groups should be interpreted with caution.
Although we could not incorporate worldwide analyses of treatment, we have been able to provide robust population-based evidence that in some countries, the therapeutic standard that was established in a large trial in 2005 for glioblastoma, the most common subtype in adults, may still not have been implemented. Importantly, the wide disparities in brain tumor survival among adolescents and young adults warrant concerted efforts to provide equitable access to care for this vulnerable age group.
Adopting a multidisciplinary care (MDC) team approach can benefit both patients and health professionals. For patients with a brain tumor, the benefits of being managed by an MDC team include shorter intervals between diagnosis and treatment, greater likelihood of receiving care in accord with clinical guidelines, and higher survival. For health professionals, the benefits include improved patient care and outcomes through the development of an agreed treatment plan, streamlined treatment pathways, reduction in the duplication of services, and improved coordination of care.
Our findings may enable clinicians involved in national and international tumor pathway boards to promote initiatives aimed at more extensive implementation of clinical guidelines.
References
- 1. International Agency for Research on Cancer. Global Cancer Observatory. Cancer Today. Lyon: IARC (International Ahency for Research on Cancer); 2018. http://gco.iarc.fr/today/home
- 2. Davis FG, Smith TR, Gittleman HR, et al Glioblastoma incidence rate trends in Canada and the United States compared with England, 1995-2015. Neuro-Oncology.2020;22(2):301–302.
- 3. Stupp R, Mason WP, van den Bent MJ, et al Radiotherapy plus concomitant and adjuvant temozolomide for glioblastoma. N Eng J Med.2005;352(10):987–996.
- 4. Wick W, Hartmann C, Engel C, et al NOA-04 randomized phase III trial of sequential radiochemotherapy of anaplastic glioma with procarbazine, lomustine, and vincristine or temozolomide. J Clin Oncol.2009;27(35):5874–5880.
- 5. Buckner JC, Shaw EG, Pugh SL, et al Radiation plus procarbazine, CCNU, and vincristine in low-grade glioma. N Engl J Med.2016;374(14):1344–1355.
- 6. van den Bent MJ, Baumert B, Erridge SC, et al Interim results from the CATNON trial (EORTC study 26053-22054) of treatment with concurrent and adjuvant temozolomide for 1p/19q non-co-deleted anaplastic glioma: a phase 3, randomised, open-label intergroup study. Lancet.2017;390(10103):1645–1653.
- 7. Coleman MP. Cancer survival: global surveillance will stimulate health policy and improve equity. Lancet.2014;383(9916):564–573.
- 8. Allemani C, Matsuda T, Di Carlo V, et al Global surveillance of trends in cancer survival 2000-14 (CONCORD-3): analysis of individual records for 37,513,025 patients diagnosed with one of 18 cancers from 322 population-based registries in 71 countries. Lancet.2018;391(10125):1023–1075.
- 9. Atun R, Jaffray DA, Barton MB, et al Expanding global access to radiotherapy. Lancet Oncol.2015;16(10):1153–1186.
- 10. Abdel-Wahab M, Fidarova E, Polo A. Global access to radiotherapy in low- and middle-income countries. Clin Oncol.2017;29(2):99–104.
- 11. Kanmounye US, Lartigue JW, Sadler S, et al Emerging trends in the neurosurgical workforce of low- and middle-income countries: a cross-sectional study. World Neurosurg.2020;142:e420–e433.
- 12. Wilson ML, Fleming KA, Kuti MA, et al Access to pathology and laboratory medicine services: a crucial gap. Lancet.2018;391(10133):1927–1938.
- 13. Zerd F, Moore BE, Malango AE, et al Photomicrograph-based neuropathology consultation in Tanzania. Am J Clin Pathol.2020;154(5):656–670.
- 14. Ostrom QT, Cioffi G, Gittleman H, et al CBTRUS Statistical Report: primary brain and other central nervous system tumors diagnosed in the United States in 2012-2016. Neuro-Oncology.2019;21(suppl 5):v1–100.
- 15. Girardi F, Rous B, Stiller CA, et al The histology of brain tumours for 67,331 children and 671,085 adults diagnosed in 60 countries during 2000-2014: a global, population-based study (CONCORD-3). Neuro-Oncology.2021;23(10):1765–1776.
- 16. Leece R, Xu J, Ostrom QT, et al Global incidence of malignant brain and other central nervous system tumors by histology, 2003-2007. Neuro-Oncology.2017;19(11):1553–1564.
- 17. Girardi F, Allemani C, Coleman MP. Global trends in survival from astrocytic tumors in adolescents and young adults: a systematic review. JNCI Cancer Spectr.2020;4(5):pkaa049.
- 18. Visser O, Ardanaz E, Botta L, et al Survival of adults with primary malignant brain tumours in Europe; results of the EUROCARE-5 study. Eur J Cancer.2015;51(15):2231–2241.
- 19. Fuentes-Raspall R, Solans M, Roca-Barceló A, et al Descriptive epidemiology of primary malignant and non-malignant central nervous tumors in Spain: results from the Girona Cancer Registry (1994–2013). Cancer Epidemiol.2017;50(Pt A):1–8.
- 20. Fritz A, Percy C, Jack A, et al, eds. International Classification for Diseases in Oncology. 3rd ed., 1st rev. Geneva: WHO (World Health Organization); 2013. https://apps.who.int/iris/handle/10665/96612
- 21. Louis DN, Ohgaki H, Wiestler OD, et al The 2007 WHO classification of tumours of the central nervous system. Acta Neuropathol.2007 Aug;114(2):97–109.
- 22. Perme MP, Stare J, Estève J. On estimation in relative survival. Biometrics.2012;68(1):113–120.
- 23. Spika D, Bannon F, Bonaventure A, et al Life tables for global surveillance of cancer survival (the CONCORD programme): data sources and methods. BMC Cancer.2017;17(1):159.
- 24. Brenner H, Gefeller O. An alternative approach to monitoring cancer patient survival. Cancer.1996;78(9):2004–2010.
- 25. Brenner H, Spix C. Combining cohort and period methods for retrospective time trend analyses of long-term cancer patient survival rates. Br J Cancer.2003;89(7):1260–1265.
- 26. Trama A, Botta L, Foschi R, et al Survival of European adolescents and young adults diagnosed with cancer in 2000–07: population-based data from EUROCARE-5. Lancet Oncol.2016;17(7):896–906.
- 27. Ostrom QT, Gittleman H, Liao P, et al CBTRUS Statistical Report: primary brain and other central nervous system tumors diagnosed in the United States in 2010-2014. Neuro-Oncology. 2017; 19(suppl 5): v1–88.
- 28. Georgakis MK, Papathoma P, Ryzhov A, et al Malignant central nervous system tumors among adolescents and young adults (15-39 years old) in 14 Southern-Eastern European registries and the US surveillance, epidemiology, and end results program: mortality and survival patterns. Cancer.2017;123(22):4458–4471.
- 29. Stupp R, Brada M, van den Bent MJ, Tonn JC, Pentheroudakis G. High-grade glioma: ESMO clinical practice guidelines for diagnosis, treatment and follow-up. Ann Oncol.2014;25(Suppl 3):iii93–ii101.
- 30. Cabrera AR, Kirkpatrick JP, Fiveash JB, et al Radiation therapy for glioblastoma: executive summary of an American Society for Radiation Oncology evidence-based clinical practice guideline. Pract Radiat Oncol.2016;6(4):217–225.
- 31. Yan H, Parsons DW, Jin G, et al IDH1 and IDH2 mutations in gliomas. N Eng J Med.2009;360(8):765–773.
- 32. van den Bent MJ, Dubbink HJ, Marie Y, et al IDH1 and IDH2 mutations are prognostic but not predictive for outcome in anaplastic oligodendroglial tumors: a report of the European Organization for Research and Treatment of Cancer Brain Tumor Group. Clin Cancer Res.2010;16(5):1597–1604.
- 33. van den Bent MJ, Smits M, Kros JM, Chang SM. Diffuse infiltrating oligodendroglioma and astrocytoma. J Clin Oncol.2017;35(21):2394–2401.
- 34. Louis DN, Perry A, Reifenberger G, et al The 2016 World Health Organization classification of tumors of the central nervous system: a summary. Acta Neuropathol.2016;131(6):803–820.
- 35. Weller M, van den Bent M, Tonn JC, et al European Association for Neuro-Oncology (EANO) guideline on the diagnosis and treatment of adult astrocytic and oligodendroglial gliomas. Lancet Oncol.2017;18(6):e315–e329.
- 36. van den Bent MJ. Interobserver variation of the histopathological diagnosis in clinical trials on glioma: a clinician’s perspective. Acta Neuropathol.2010;120(3):297–304.
- 37. Schiff D, van den Bent M, Vogelbaum MA, et al Recent developments and future directions in adult lower-grade gliomas: Society for Neuro-Oncology (SNO) and European Association of Neuro-Oncology (EANO) consensus. Neuro-Oncology.2019;21(7):837–853.
- 38. Molinaro AM, Taylor JW, Wiencke JK, Wrensch MR. Genetic and molecular epidemiology of adult diffuse glioma. Nat Rev Neurol.2019;15(7):405–417.
- 39. Brat DJ, Aldape K, Colman H, et al cIMPACT-NOW update 3: recommended diagnostic criteria for “Diffuse astrocytic glioma, IDH-wildtype, with molecular features of glioblastoma, WHO grade IV”. Acta Neuropathol.2018;136(5):805–810.
- 40. Eckel-Passow JE, Lachance DH, Molinaro AM, et al Glioma groups based on 1p/19q, IDH, and TERT promoter mutations in tumors. N Eng J Med.2015;372(26):2499–2508.
- 41. Hartmann C, Hentschel B, Wick W, et al Patients with IDH1 wild type anaplastic astrocytomas exhibit worse prognosis than IDH1-mutated glioblastomas, and IDH1 mutation status accounts for the unfavorable prognostic effect of higher age: implications for classification of gliomas. Acta Neuropathol.2010;120(6):707–718.
- 42. Weller M, Weber RG, Willscher E, et al Molecular classification of diffuse cerebral WHO grade II/III gliomas using genome- and transcriptome-wide profiling improves stratification of prognostically distinct patient groups. Acta Neuropathol.2015;129(5):679–693.
- 43. Brown TJ, Brennan MC, Li M, et al Association of the extent of resection with survival in glioblastoma: a systematic review and meta-analysis. JAMA Oncol.2016;2(11):1460–1469.
- 44. Stark D, Bielack S, Brugieres L, et al Teenagers and young adults with cancer in Europe: from national programmes to a European integrated coordinated project. Eur J Cancer Care.2016;25(3):419–427.
- 45. Sender L, Zabokrtsky KB. Adolescent and young adult patients with cancer: a milieu of unique features. Nat Rev Clin Oncol.2015;12(8):465–480.
- 46. Fuentes-Raspall R, Puig-Vives M, Guerra-Prio S, Perez-Bueno F, Marcos-Gragera R. Population-based survival analyses of central nervous system tumors from 1994 to 2008. An up-dated study in the temozolomide-era. Cancer Epidemiol.2014;38(3):244–247.
- 47. Sulman EP, Ismaila N, Armstrong TS, et al Radiation therapy for glioblastoma: American Society of Clinical Oncology clinical practice guideline endorsement of the American Society for Radiation Oncology guideline. J Clin Oncol.2017;35(3):361–369.
- 48. Hegi ME, Diserens AC, Gorlia T, et al MGMT gene silencing and benefit from temozolomide in glioblastoma. N Eng J Med.2005;352(10):997–1003.
- 49. Herrlinger U, Tzaridis T, Mack F, et al Lomustine-temozolomide combination therapy versus standard temozolomide therapy in patients with newly diagnosed glioblastoma with methylated MGMT promoter (CeTeG/NOA-09): a randomised, open-label, phase 3 trial. Lancet.2019;393(10172):678–688.
