Key Points
A global study of trends in population-based survival from brain tumors in children.
Wide variation in survival suggests inequalities in access to care.
Survival estimates provide a baseline for evaluating the WHO Global Initiative for Childhood Cancer.
Importance of the Study
We conducted novel, up-to-date analyses of brain tumor survival in children, using CONCORD-3 data. The geographical coverage of CONCORD-3 was broader than any previous international comparison of cancer survival. A standardized protocol for data collection ensured that information was collected based on the same set of patient-related and tumor-related variables. For the first time in an international comparison of survival, we implemented a revised version of ICCC-3 to account for international differences in registration practice for low-grade tumors. We presented what are, to our knowledge, the first global survival estimates for low-grade tumors in children. Although low-grade glioma is 1 of the 6 index childhood cancers included by WHO in the Global Initiative for Childhood Cancer, global survival estimates for this histology group are not available. Our findings may be used as a benchmark to monitor improvements in survival from childhood brain tumors at a global level.
Tumors of the central nervous system (CNS) rank second after leukemia as a cause of cancer-related death in children. CNS tumors may originate in the brain, the meninges or the spinal cord, but the brain is by far the most common site. In children, age-standardized (world) incidence rates in 2018 ranged from an estimated 0.5 per 100,000 person-years in Africa to 2.3 in Europe and the Americas. Health care disparities, however, may lead to substantial under-diagnosis or under-registration. Up to 57% of childhood cancer diagnoses may be missed in Western Africa, compared with 3% in North America and Western Europe.
Health care facilities are unevenly distributed world-wide. In some countries, for instance, radiotherapy facilities are simply not available. Unmet need for treatment due to suboptimal access to the healthcare system translates to many years of life lost and extended periods of disability. Given that only 10% of children live in high-income countries, the social burden of childhood cancer in low-income and middle-income countries is disproportionately great in countries that are generally least well equipped to deal with that burden.,
Population-based survival is a key metric to evaluate the performance of the health care system in a given country in managing cancer. In 2015, the CONCORD programme began global surveillance of trends in cancer survival with data for patients diagnosed during the 15-year period 1995–2009. The third cycle of the programme (CONCORD-3), covering 71 countries, included individual data for more than 37 million patients diagnosed during 2000–2014 with one of 18 common cancer types, including childhood brain tumors. Global differences in age-standardized 5-year net survival for all childhood brain tumors combined were very wide, ranging between 29% in Brazil and 89% in Sweden.
Brain tumors represent a disparate group of subtypes, with more than 50 histological entities. Histology is an important determinant of outcome, so international comparisons in brain tumor survival can be more meaningful for health care planning if they account for histology. Survival estimates that take account of histology enable better interpretation of international differences in survival for all brain tumors combined, since these differences are confounded by the heterogeneity of clinical behavior and global variation in the distribution of histological types.
The third edition of the International Classification of Childhood Cancer (ICCC-3) has become established as the standard tool for categorizing childhood tumors by histology. ICCC-3 is a scheme with three progressively more granular tiers. However, the third tier does not contain distinct entities for astrocytoma, so it is not possible to analyze low-grade and high-grade astrocytic tumors separately using data classified with ICCC-3, and alternative approaches are seldom used. The third edition of the International Classification of Diseases for Oncology defines as non-malignant (ICD-O-3 behavior code 0 or 1) most of the low-grade brain tumors, including pilocytic astrocytoma, which alone comprises 70% of all childhood astrocytic tumors.,
This has important implications for international comparisons of brain tumor survival. Non-malignant tumors are not consistently recorded world-wide, due to differences in health regulations and cancer registration practice. Registration of non-malignant brain tumors is important because not only tumor behavior, but the anatomical site also has an effect on diagnosis, treatment choices and outcome. The World Health Organisation (WHO) grade of the tumor must therefore be incorporated in survival analyses for astrocytic tumors in children, because international comparisons of survival based on data coded to ICCC-3 are otherwise uninterpretable, owing to the very different proportions of low-grade and high-grade astrocytic tumors in cancer registry data.
To date, studies of survival from childhood brain tumors by histology have not been readily comparable because of differences in study design, especially as to the inclusion or exclusion of non-malignant brain tumors. Nearly all these studies have been conducted in high-income countries. No data are currently available for Africa, Central and South America, or most of Asia.
We set out to conduct a world-wide study of population-based survival from childhood brain tumors, using data collected with a central protocol, checked for quality using standardized rules and analyzed with the same robust statistical methods.
Patients and Methods
For CONCORD-3, individual tumor registrations for 71 526 children (0–14 years) diagnosed with a brain tumor (ICD-O-3 topography code C71), whether malignant or non-malignant, during 2000–2014 were provided by 261 cancer registries in 61 countries.
Each tumor record was subjected to rigorous quality checks for eligibility and definite or possible errors. Possible errors included implausible combinations of age, sex, site, and morphology. Each registry was invited to confirm or refute records with possible errors.
We defined 12 histology groups, each comprising a set of relevant ICD-O-3 codes. The methodology and the principles for selecting the ICD-O-3 codes are explained elsewhere. In brief, the histology groups were based on ICCC-3, but we devised more granular categories for astrocytic tumors by incorporating WHO grade, which forms part of the tumor subtype definition. The sixth digit of the ICD-O-3 code defines the grade of differentiation of a tumor (Rule G), as assigned by the pathologist or the tumor registrar. We used the sixth digit of the morphology code to reclassify tumors recorded as “astrocytoma NOS” to more specific astrocytic subtypes (Supplementary Table 1).
Net survival is the cumulative probability that cancer patients survive their cancer up to a given time since diagnosis (eg, 5 years), after accounting for competing risks of death (background mortality) and for informative censoring. Net survival can be directly estimated using the unbiased, nonparametric Pohar Perme estimator. Data on background mortality are derived from life tables of all-cause mortality specific for single year of age, sex, single calendar year, and race/ethnicity (where information was available) in the general population of each participating country or territory. We used the software package stns implemented in STATA (version 16).
Survival was not estimated if fewer than 10 patients were available for a given histology group, calendar period and country, or region. If 10–49 patients were available, we produced unstandardized estimates of survival for all ages combined. We attempted age standardization if 50 children or more were available. Standardization was obtained by applying equal weights to the age-specific survival estimates for children aged 0–4, 5–9, and 10–14 years., If a single age-specific estimate could not be computed, we pooled the records for two adjacent age groups and attributed the aggregated estimate to both age groups before age standardization. We did not combine data for consecutive calendar periods.
The cohort approach provides a survival estimate for a group of patients diagnosed in the same year and all followed up for the same amount of time, for example, for at least 5 years. We used the cohort approach for patients diagnosed during 2000–2004 and 2005–2009. For children diagnosed during 2010–2014, we adopted the period approach, since 5 years of follow-up were not available for most patients. This approach combines the most recent follow-up data for cancer patients diagnosed during a specified year and the follow-up data for patients diagnosed up to 5 years earlier and who were still alive at the start of the specified year of diagnosis. The survival prediction derived from this approach is conditional because it incorporates the survival probabilities matured over the preceding years when most of the individuals were diagnosed. Empirical evidence shows that period estimates provide a good approximation to the cohort estimates when they become available in due course.,
We produced 5-year survival estimates for tumors in each histology group, by country and calendar period. For selected tumor types, we also examined longer-term survival, up to 10 years from diagnosis.
We flagged survival estimates as less reliable if 15% or more of patients were lost to follow-up or censored within 5 years. We also considered estimates as less reliable if 15% or more of registrations were based solely on a death certificate or autopsy and excluded, because their survival time is unknown. Finally, survival estimates were flagged as less reliable if 15% or more of records were excluded from analysis because they contained one or more incomplete dates. Unreliable estimates were not included in pooled national survival estimates unless they were the only estimates available from that country, in which case the national estimate is flagged.
The CONCORD programme is approved by the UK’s statutory Health Research Authority (reference ECC 3-04(i)/2011; last update November 2, 2021), the National Health Service Research Ethics Service (11/LO/0331; January 12, 2022), and the London School of Hygiene & Tropical Medicine Ethics Committee (12171; November 21, 2021).
Results
The proportion of records with incomplete dates was less than 1% in North America, Asia, Europe, and Oceania, 2.4% in Central and South America, and 10.7% in Africa. Overall, children registered through a death certificate only (DCO) comprised 1.1% of all submissions. DCO proportions for Africa (6.8%) and Central and South America (5.9%) were higher than in other continents (2% or less). The proportion of brain tumors with histological confirmation was generally high, in the range 88%–98%. Brain tumors registered with a nonspecific histology (ICD-O-3 morphology code 8000–8005) only represented 3.2% of all brain tumor diagnoses in North America, but they accounted for 26.3% in Africa (Supplementary Table 2). Following quality checks, 67,776 records were retained for analysis (94.8% of those eligible for inclusion).
Of the 67,776 children potentially eligible for survival analyses, we excluded a further 6559 (9.7%) because the morphology code did not fall within one of the histology groups selected for this study. We also excluded 6310 (9.3%) records from 57 registries for which survival estimates were deemed less reliable. The analyzes included 54 907 tumor records (81.0% of eligible tumor records).
Comments in this section are focused on reliable, age-standardized survival estimates. When examining time trends, we only discuss countries for which reliable, age-standardized survival estimates were available for 2000–2004, 2005–2009, and 2010–2014. For each continent, countries are listed in alphabetical order.
For low-grade astrocytomas (WHO grade I and II; 26.6% of all brain tumors included), age-standardized 5-year net survival during 2010–2014 was in the range 80%–89% in Taiwan, Turkey, and Spain; 90%–94% in 8 of 20 European countries (Belarus, Belgium, France, Greece, Italy, the Netherlands, Slovakia, and Switzerland) and in Australia. Survival was highest (95%–100%) in Canada, the United States, Israel, Japan, two Eastern European countries (Czech Republic and Poland), Germany, 6 Northern European countries (Denmark, Finland, Ireland, Norway, Sweden, and the United Kingdom), and Portugal. (Supplementary Table 3A, Figure 1).
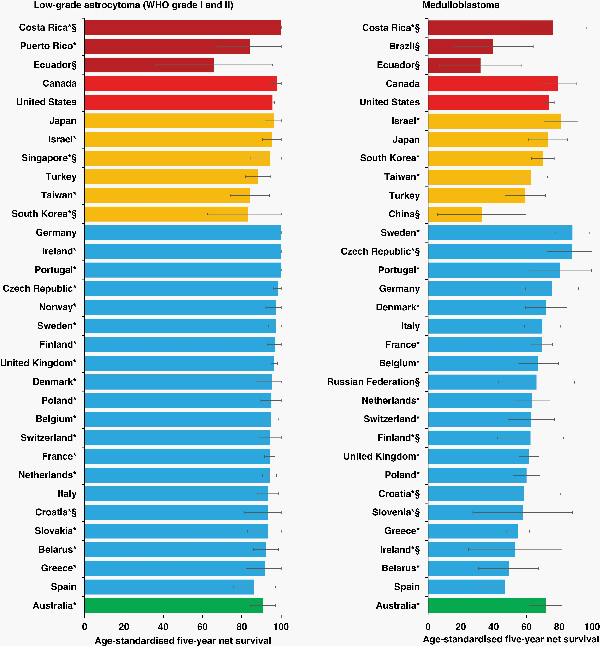
Fig. 1
Age-standardized 5-year net survival (%) with 95% confidence interval, by country: children (0–14 years) diagnosed with low-grade astrocytoma or medulloblastoma during 2010–2014. *Countries with 100% coverage of the national population. §Survival estimates not age-standardized. Continents are identified by different colors. In each panel, countries are ranked from highest to lowest, based on survival during 2010–2014.
For children diagnosed with a low-grade astrocytoma during the 15 years between 2000 and 2014, age-standardized 5-year net survival remained above 90%, largely unchanged, in North America, Israel, Northern Europe (Finland, Sweden, and the United Kingdom), Western Europe (France, the Netherlands, Italy, and Switzerland), and Italy. Survival in Spain, 92% during 2000–2004, subsided to values around 85% during 2005–2014. Marked improvements in survival occurred in Eastern Europe: survival rose from 78.1% to 92.4% in Belarus and from 86.5% to 95.1% in Poland. Survival in Australia, around 87% during 2000–2009, reached 90.9% during 2010–2014 (Supplementary Table 3A, Figure 2).
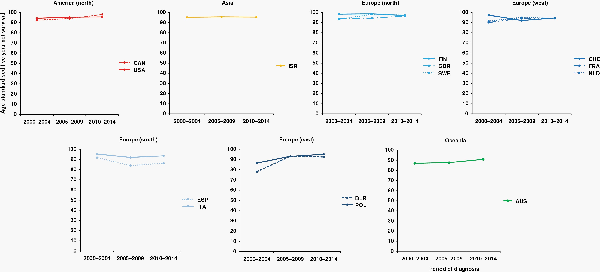
Fig. 2
15-year trends in age-standardized 5-year net survival (%) for children (0–14 years) diagnosed with WHO grade I and II astrocytoma during 2000-2014, continent (or continental region), and country. Countries are only included if age-standardized survival estimates were available for patients diagnosed during 2000–2004, 2005–2009, and 2010–2014. Continents (or continental regions) are identified by different colors. In each panel, countries are ranked from highest to lowest, based on survival during 2000–2004. X-axis: period of diagnosis; Y-axis: age-standardized 5-year net survival (%). International Organization for Standardization abbreviations for country names: AUS, Australia; BLR, Belarus; CAN, Canada; FIN, Finland; FRA, France; ISR, Israel; ITA, Italy; NLD, Netherlands; POL, Poland; ESP, Spain; SWE, Sweden; CHE, Switzerland; GBR, UK; USA, USA.
Outcomes for high-grade astrocytomas (WHO grade III and IV; 7.4% of all tumors included) were rather poor. Reliable, age-standardized estimates were only available for 8 countries. Five-year survival during 2010–2014 was 6.3% in France, 17.1% in the United Kingdom, in the range 20%–29% in the United States, South Korea, Taiwan, Italy, and Australia; and 31.2% in Poland (Supplementary Table 3A).
Of the 5 countries for which reliable, age-standardized survival estimates for high-grade astrocytoma were available throughout the study period, 4 (South Korea, Poland, the United Kingdom, and Australia) showed little consistent change in survival during 2000–2014, while 5-year survival in the United States declined steadily from 28.9% in 2000–2004 to 23.1% in 2010–2014 (Supplementary Table 3A, Figure 2).
Wide variation in survival was seen for medulloblastoma (15.7% of all brain tumors included), which is the most common embryonal CNS tumor. Age-standardized 5-year net survival for children diagnosed between 2010 and 2014 was less than 50% in Belarus and Spain; it ranged between 50% and 59% in Turkey and Greece; between 60% and 69% in Taiwan and 7 of 20 European countries (Belgium, France, Italy, the Netherlands, Poland, Switzerland, and the United Kingdom). Survival was in the range 70%–79% in Canada, the United States, Japan, South Korea, Denmark, Germany, and Australia. The highest survival was observed in Israel (81.0%), Portugal (80.6%), and Sweden (88.0%) (Supplementary Table 3A, Figure 1).
Fifteen-year trends in age-standardized 5-year net survival from medulloblastoma were only available for 9 countries. Survival was stable, or fluctuating slightly, in Poland (in the range 55%–60%), in France, Italy, and Australia (64%–72%), and in the United States (70%–75%). Survival from medulloblastoma rose from 60.5% to 70.0% in South Korea, from 56.4% to 62.7% in Taiwan, and from 51.8% to 63.3% in the Netherlands, while it fell from 68.8% to 61.5% in the United Kingdom (Supplementary Table 3A, Figure 3).
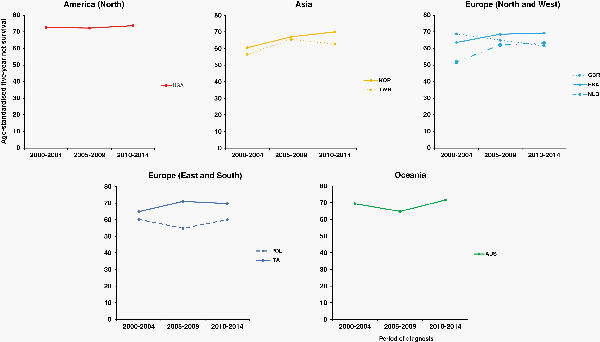
Fig. 3
15-year trends in age-standardized 5-year net survival (%) for children (0–14 years) diagnosed with medulloblastoma during 2000-2014, by continent (or continental region), and country. Countries are only included if age-standardized survival estimates were available for patients diagnosed during 2000–2004, 2005–2009, and 2010–2014. Continents (or continental regions) are identified by different colors. In each panel, countries are ranked from highest to lowest, based on survival during 2000–2004. X-axis: period of diagnosis; Y-axis: age-standardized 5-year net survival (%). International Organization for Standardization abbreviations for country names: AUS, Australia; FRA, France; ITA, Italy; NLD, Netherlands; KOR, South Korea; POL, Poland; TWN, Taiwan; GBR, UK; USA, USA.
The subgroup “other and unspecified embryonal tumors” (10.8% of children included in these analyses) consisted almost entirely of atypical/teratoid rhabdoid tumor and embryonal CNS tumor not otherwise specified (NOS) (formerly known as primitive neuroectodermal tumor). Age-standardized 5-year net survival (2010–2014) for children diagnosed with one of these tumors ranged between 30% and 39% in Canada and Israel; between 40% and 49% in Japan, Taiwan, Turkey, France, the Netherlands, and Australia, and between 50% and 59% in the United States, South Korea, Poland, Sweden, and the United Kingdom. Survival was 65.6% in Belgium, 83.5% in Italy, and 84.5% in Germany (Supplementary Table 3B).
Trends in age-standardized 5-year net survival for the “other and unspecified embryonal tumors” subgroup could be reliably estimated for 6 countries. In 4 of them (United States, South Korea, Poland, and the United Kingdom), survival remained within the range 48%–57% throughout. Survival increased between 2000–2004 and 2010–2014 from 40.7% to 48.6% in France and from 29.9% to 48.2% in Australia (Supplementary Table 3B).
Data for ependymoma were rather sparse (2685 records; 4.9% of all brain tumors included in the analyses). Age-standardized 5-year net survival for children diagnosed during 2010–2014 was 54.0% in Turkey, 59.7% in South Korea, 79.3% in Poland, and in the range 80%–90% in the United States, France, Italy, and the United Kingdom (Supplementary Table 3A).
Trends in age-standardized 5-year net survival for ependymoma could be reliably estimated for three countries (United States, France, and the United Kingdom). Survival increased in all three countries, from 65.1% to 75.8% for children diagnosed in 2000–2004 to 79.1%–82.3% in 2005–2009 and 81.3%–89.9% in 2010–2014 (Supplementary Table 3A).
Age-standardized 5-year net survival estimates for children diagnosed with neuronal and mixed neuronal–glial tumors during 2010–2014 were in the range 89%–100% for 10 countries (Canada, United States, Belgium, Finland, France, Italy, the Netherlands, Sweden, the United Kingdom, and Australia). For the other, less common histology groups, there were fewer countries with sufficient cases for age-standardized survival to be calculated. Survival of children with choroid plexus tumors during 2010–2014 was at least 90% in the United States, France, and the United Kingdom. Five-year survival for oligodendroglial tumors was 51.3% in France, and appreciably higher in the United Kingdom (70.4%), South Korea (73.4%), and the United States (83.0%), during 2010–2014. Age-standardized 5-year net survival for children diagnosed with neuroepithelial glial tumors of uncertain origin during 2010–2014 could only be estimated in the United States, where it was 67.1% (Supplementary Table 3A and B).
Age-standardized 5-year net survival for children diagnosed during 2010–2014 with glioma, otherwise unspecified (ICD-O-3 morphology code 9380/3) varied between 30% and 39% in Japan, France, the Netherlands, and Australia; between 40% and 49% in Canada, South Korea, Turkey, and the United Kingdom, and in the range 50%–61% in the United States, Israel, Belgium, and Italy (Supplementary Table 3B).
During 2010–2014, at least 10 children were diagnosed with a brain tumor labeled as unspecified (ICD-O-3 morphology codes 8000–8005) in 22 of 46 countries from which data were available. Variation in age-standardized 5-year net survival for these poorly specified neoplasms was remarkable: 35.8% in China, 58.5% in South Korea, 72.3% in Italy, 77.9% in the United Kingdom, and in the range 80%–89% in the United States, Japan, Turkey, Denmark, and Australia (Supplementary Table 3B).
We assessed survival at 10 years for children diagnosed with low-grade astrocytoma or medulloblastoma during 2000–2004 (Supplementary Table 5). For low-grade astrocytoma, age-standardized 10-year survival and 5-year survival differed by less than 3% in 12 of the 15 countries for which suitable data were available. The difference was slightly larger (3% or more) in Argentina, Belarus, and Australia. For medulloblastoma, the absolute difference between 5- and 10-year net survival was in the range 0%–4% in Argentina, the United States, South Korea, Italy, the Netherlands, and Australia, but in the range 6%–10% in Israel, Taiwan, France, Poland, and the United Kingdom.
Discussion
To our knowledge, this is the largest study on survival from childhood brain tumors to date. Individual records for over 50 000 children were provided to a standard protocol by 261 population-based cancer registries in 61 countries, prepared with the same rigorous quality checks, and analyzed with the same, robust statistical methodology.
Age-standardized 5-year net survival for low-grade astrocytoma (WHO grade I and II) was 90% or more during the whole period between 2000 and 2014 in most countries. World-wide variation in survival for medulloblastoma was much broader than for low-grade astrocytoma, with age-standardized 5-year net survival in the range 47%–86% during 2010–2014.
In most previous international comparisons of survival from childhood brain tumors, the broad definition “astrocytoma” has been adopted, in compliance with ICCC-3., Such survival estimates cannot be safely compared with those presented here, since we did not merge low-grade and high-grade astrocytic tumors.
More than two-thirds of low-grade brain tumors in children are pilocytic astrocytomas. ICD-O-3 classifies pilocytic astrocytoma as a non-malignant entity (ICD-O-3 behavior code 1). In the fourth cycle of the cancer registry based study on survival and care of cancer patients diagnosed in Europe during 1995–1999 (EUROCARE-4), 5-year observed survival for astrocytoma (broad group) was rather poor in Eastern Europe, around 64%, irrespective of inclusion of non-malignant tumors, and lower than in other European regions. These findings suggested under-registration of non-malignant brain tumors in Eastern Europe. In EUROCARE-5, covering the period 1999–2007, survival from childhood brain tumors was presented by tumor behavior (malignant or non-malignant), but this design does not account for histology. Alternatively, EUROCARE-5 provided survival estimates for the whole of Europe combined, by single ICD-O-3 morphology code, but this more granular approach cannot be readily implemented in large international comparisons of survival by histology.
Despite international recommendations, non-malignant tumors are still recorded inconsistently, not only in Europe, but world-wide. For instance, health regulations in New South Wales mandate registration of malignant tumors only, while Ecuador started recording non-malignant brain tumors only from 2010. In the CONCORD-3 data for children diagnosed with a brain tumor during 2000–2014, malignant tumors (ICD-O-3 behavior code 3) accounted for 80% of all tumor records in Australia (but 100% in New South Wales, which comprises 45% of the national population), and the totality of cases in South Korea, Taiwan, and New Zealand (data not shown). If these international differences in cancer registration practices are not properly considered, global disparities in survival for all astrocytic tumors may be wrongly interpreted. Survival in countries or regions that only include malignant brain tumors will be systematically lower than in countries where non-malignant tumors are also registered. We conducted a sensitivity analysis. Age-standardized 5-year net survival for all astrocytic tumors combined (ICCC-3 category) ranged from 43% to 88% world-wide during 2010–2014 (Supplementary Table 4). By contrast, survival was in the range 84%–100% for low-grade tumors and in the range 6%–30% for high-grade tumors. The remarkable difference in global disparities in survival when more granular categories are used confirms that international comparisons of survival for astrocytic tumors should take account of confounding by tumor grade. Where possible, estimates for low-grade and high-grade tumors should be reported separately.
Even where there is complete registration of non-malignant brain tumors, including pilocytic astrocytoma, variations in registration practice could affect reported survival estimates for low-grade astrocytoma. Pilocytic astrocytoma and the other specific types of WHO grade I astrocytoma all have specific morphology codes in ICD-O-3. Diffuse astrocytoma, however, which is the principal type of WHO grade II astrocytoma, shares the morphology code 9400/3 with astrocytoma NOS. Thus, it is only possible to identify most cases of WHO grade II astrocytoma in datasets from cancer registries that have routinely used and supplied the sixth digit of the morphology code (grade). In CONCORD-3, there were many datasets in which grade was never specified for cases with morphology code 9400/3, including seven where this code accounted for at least 30% of all astrocytomas (Argentina, Brazil, Colombia, Costa Rica, South Korea, Croatia, and Latvia), and others where it was very rarely specified. This would lead to a deficit of WHO grade II astrocytoma cases, with a poorer prognosis than WHO grade I, tending to overestimation of survival for low-grade astrocytoma.
In recent years, neuro-pathologists have increasingly classified diagnoses based on molecular characteristics, which has improved the identification of astrocytic tumors with more aggressive behavior. The relatively broad variation in survival for high-grade astrocytoma suggest that in some countries these strategies may have been implemented earlier than elsewhere. The decrease in survival over time for high-grade astrocytoma in the United States, with concurrent increases in survival for unspecified glioma and unspecified tumor, may also reflect improvements in diagnostic accuracy.
Comparisons of survival between countries could be affected by variations in diagnostic practice. There was formerly a marked tendency in France to regard some cases of WHO grade II and grade III astrocytoma as oligodendroglioma, as reflected in the unusually high proportion of childhood brain tumors classed as oligodendroglial tumors in CONCORD-3. Although in France this proportion fell from 7% to 8% in 2000–2009 to 4.4% in 2010–2014, it was still considerably higher than in most other countries and it seems likely that this would have resulted in underestimation of survival for high-grade astrocytoma. During 2000–2014, the proportion of embryonal tumors that were classed as medulloblastoma was lower in North America and Oceania than in Europe and Asia, and highest in Central and South America. While this could of course be due to real differences in incidence between populations, it seems likely that it was partly due to variation in the frequency with which, for example, atypical/teratoid rhabdoid tumor was identified as such rather than as medulloblastoma. Since atypical teratoid/rhabdoid tumor has a dismal prognosis, this could have led to underestimation of survival for medulloblastoma.
Five-year survival for medulloblastoma during 2010–2014 was in the range 70%–80% in several high-income countries. These values are in line with those from recent national studies assessing survival for children diagnosed during 2001–2009 in Germany (72%–80%) and the United States (also 72%–80%),, but higher than the survival levels seen during the 1990s. This may reflect recent advances in treatment protocols for children with medulloblastoma that revolve around two main pillars: reduction of the radiotherapy dose to minimize long-term neurological sequel, and treatment intensification only for high-risk patients. Other reasons for these survival gains may be the implementation of volumetric radiotherapy, better surgery with removal of larger tumor volumes and fewer complications, and more timely referral for postsurgical treatment., However, the wide global inequalities in survival strongly suggest that in some countries, children may still not have access to optimal treatment for medulloblastoma. Most childhood brain tumor subtypes have a favorable outcome, but timely surveillance for relapse and optimal follow-up care are both crucial. The comparisons of 5- and 10-year survival for low-grade astrocytoma and medulloblastoma should be interpreted with caution, because the changes are still small. However, it would seem that net survival tends to plateau after 5 years in some countries, suggesting low excess mortality among survivors to that point, whereas in other countries, brain tumor survivors may remain at higher long-term risk of death than children in the general population for more than 5 years.
Up to two-thirds of pilocytic astrocytomas originate in the cerebellum, while the most common supratentorial sites are the optic nerve and the optic chiasm. When pilocytic astrocytoma involves the optic pathways, it is also called “optic nerve glioma”. These tumors are often not biopsied, because of the high risk of visual loss, and the diagnosis is made through a combination of imaging and testing of the visual fields. These tumors may thus be incorrectly labeled in the cancer registry with the ICD-O-3 descriptor “glioma NOS” (ICD-O-3 morphology code 9380/3). CONCORD-3 only collected information for tumors originating in the brain. Nevertheless, we cannot exclude that, given the close anatomical proximity, inaccuracies at clinical record level may have led to some optic nerve gliomas being wrongly labeled, and submitted, with the ICD-O-3 topography code used for brain (C71), instead of the code for optic pathways (C72.3). We considered that some of the poorly specified gliomas might in fact have been pilocytic astrocytomas of the optic pathways. Five-year survival for unspecified glioma was much lower than for pilocytic astrocytoma, and for 87% of these records, the tumor grade was unspecified (sixth digit 9), so we could not confirm the non-malignant behavior.
In the CONCORD-3 childhood brain tumor dataset, the proportion of tumors of unspecified histology (ICD-O-3 morphology codes 8000–8005) varied widely, ranging from 5.0% in Europe to 21.7% in Africa during 2010–2014. In CONCORD-3, age-standardized 5-year net survival for all childhood brain tumors combined was only 29.9% in Brazil, but close to 80% in Denmark, Slovakia, and Sweden. After excluding children with tumors of unspecified histology, survival was still poor in Brazil (34.9%), but only 68.9% in Denmark, while it remained substantially unchanged in Slovakia and Sweden (data not shown). During 2010–2014, the proportion of tumors of unspecified histology was only 14.6% in Brazil, but 55.1% in Denmark. One may think that patients with tumors of unspecified histology may have been too unwell to undergo biopsy or surgery and, as a result, experience poor outcomes. However, 5-year survival during 2010–2014 for Danish children with tumors of unspecified histology was as high as 88.3%. This probably explains the impact of excluding these tumors on survival estimates for all brain tumors combined. Such discrepancies suggest that obstacles to accurate reporting of a brain tumor diagnosis may arise in both the hospital and the cancer registry. If the histology is known for only a subset of brain tumor patients, the interpretation of survival estimates requires great caution, whether for all childhood brain tumors combined or for specific tumor subtypes. These survival estimates may not be robustly comparable with estimates from countries where data on histology are more precise. Our findings should enable public health officials to prompt actions aimed at improving the reporting of brain tumors, such as audits at local and national level.
We excluded data from cancer registries that were considered less reliable, based on the criteria previously outlined. In a sensitivity analysis, we re-estimated survival by histology after inclusion of data from flagged cancer registries. Overall age-standardized survival estimates were slightly higher. This is not surprising, because one of the reasons for flagging was the high proportion (15% or more) of the patients lost to follow-up or censored before 5 years. The absolute increase, however, was unremarkable, with differences varying between 1.5% in the United States and 4.4% in Spain (data not shown). These findings suggest that the exclusion of less reliable records (9.3% of eligible submissions) did not explain the large differences in survival by country world-wide.
The Lancet Oncology Commission on Sustainable Care for Children with Cancer recently presented evidence for the implementation of cost-effective interventions to reduce the long-term clinical and economic burden of childhood cancer. The evidence included modeled estimates of survival for children diagnosed during 2015–2019, by histology, for over 200 countries and territories. Survival was modeled from CONCORD-3 estimates for 2000–2014. The Commission adopted ICCC-3 to classify childhood tumors, so it could not present any data for low-grade astrocytoma. This was also a major limitation of the Global Burden of Disease study, which cannot account for histology because it is based on topography descriptors from the International Classification of Diseases (ICD)., We have presented, for the first time to our knowledge, survival estimates for low-grade astrocytoma at a global level.
In the CONCORD-3 data for the United States, where registration of non-malignant tumors is statutory, astrocytomas accounted for 63.7% of all low-grade gliomas during 2000–2014. Low-grade glioma is 1 of the 6 index cancers included in the WHO Global Initiative for Childhood Cancer, which aims to improve 5-year net survival for these 6 childhood cancer types, world-wide, by 2030. The classifier “low-grade glioma” is very ill-defined, and it overlooks the complex histologic makeup of childhood brain tumors. ICCC-3 adopts more granular categories, but it does not consider tumor behavior. We overcame the limitations of ICCC-3 using a classification that preserves the ICCC-3 framework but incorporates tumor grade. Ultimately, our findings may form the basis for a revision of ICCC-3.
Our study has some limitations. The date of the first course of each major treatment modality was an optional variable in the CONCORD-3 data specification, and data on whether a patient underwent radiotherapy for a brain tumor was only provided by a few countries. In this context, granular treatment data may be used to assess adherence to treatment guidelines or abandonment of treatment. Data quality was suboptimal in some countries due to the low precision of histology data, or to the high proportion of patients whose duration of survival was not known. We decided to present, and flag, survival estimates based on these less reliable data as well, if they were the only data available for a given country or territory. We believe that countries should not be excluded from a study for reasons of lower or more questionable data quality, because their inclusion in large international comparisons is crucial to promote change.
Robust histology data can only become available if a common, statutory framework for data collection is in place. World-wide co-operation between international associations of pathologists and cancer registries will be essential to identify and remove obstacles to the accurate reporting of brain tumor diagnoses, and to promote transition to a more informative and up-to-date neuropathology lexicon. Moreover, the quality of cancer registration can only improve if specific funding to train tumor registrars and to strengthen health information systems in cancer registries is made available. High-quality data, and data from more low-income and middle-income countries, will enable robust global comparisons of survival. These will prove instrumental in monitoring progress toward the global targets for better control of childhood cancer set by the WHO.
References
- 1. Steliarova-Foucher E, Colombet M, Ries LAG, et al International incidence of childhood cancer, 2001-10: a population-based registry study. Lancet Oncol.2017; 18(6):719–731.
- 2. International Agency for Research on Cancer. Global Cancer Observatory; Cancer Today. Lyon: IARC (International Agency for Research on Cancer); 2018. Accessed on September 10, 2022. https://gco.iarc.fr/today/home
- 3. Ward ZJ, Yeh JM, Bhakta N, et al Global childhood cancer survival estimates and priority-setting: a simulation-based analysis. Lancet Oncol.2019; 20(7):972–983.
- 4. Atun R, Jaffray DA, Barton MB, et al Expanding global access to radiotherapy. Lancet Oncol.2015; 16(10):1153–1186.
- 5. Kanmounye US, Lartigue JW, Sadler S, et al Emerging trends in the neurosurgical workforce of low- and middle-income countries: a cross-sectional study. World Neurosurg.2020; 142:e420–e433.
- 6. Wilson ML, Fleming KA, Kuti MA, et al Access to pathology and laboratory medicine services: a crucial gap. Lancet.2018; 391(10133):1927–1938.
- 7. GBD 2017 Childhood Cancer Collaborators. The global burden of childhood and adolescent cancer in 2017: an analysis of the Global Burden of Disease Study 2017. Lancet Oncol.2019; 20(9):1211–1225.
- 8. Rodriguez-Galindo C, Friedrich P, Alcasabas P, et al Toward the cure of all children with cancer through collaborative efforts: pediatric oncology as a global challenge. J Clin Oncol.2015; 33(27):3065–3073.
- 9. Bhakta N, Force LM, Allemani C, et al Childhood cancer burden: a review of global estimates. Lancet Oncol.2019; 20(1):e42–e53.
- 10. Coleman MP. Cancer survival: global surveillance will stimulate health policy and improve equity. Lancet.2014; 383(9916):564–573.
- 11. Coleman MP, Allemani C. Cancer: the elephant in the room. Lancet.2015; 385(9973):1047–1048.
- 12. Organisation for Economic Co-operation and Development (OECD). Health at a Glance 2021: OECD Indicators. Paris: OECD (Organisationfor Economic Co-operation and Development); 2021. doi: 10.1787/ae3016b9-en
- 13. Allemani C, Weir HK, Carreira H, et al Global surveillance of cancer survival 1995-2009: analysis of individual data for 25,676,887 patients from 279 population-based registries in 67 countries (CONCORD-2). Lancet.2015; 385(9972):977–1010.
- 14. Allemani C, Matsuda T, Di Carlo V, et al Global surveillance of trends in cancer survival 2000-14 (CONCORD-3): analysis of individual records for 37,513,025 patients diagnosed with one of 18 cancers from 322 population-based registries in 71 countries. Lancet.2018; 391(10125):1023–1075.
- 15. Louis DN, Ohgaki H, Wiestler OD, Cavenee WK, eds. WHO Classification of Tumours of the Central Nervous System. 4th ed. Geneva: WHO (World Health Organization); 2007.
- 16. Girardi F, Rous B, Stiller CA, et al The histology of brain tumours for 67,331 children and 671,085 adults diagnosed in 60 countries during 2000-2014: a global, population-based study (CONCORD-3). Neuro Oncol.2021; 23(10):1765–1776.
- 17. Steliarova-Foucher E, Stiller C, Lacour B, Kaatsch P. International classification of childhood cancer, third edition. Cancer2005; 103(7):1457–1467.
- 18. Girardi F, Allemani C, Coleman MP. Worldwide trends in survival from common childhood brain tumors: a systematic review. J Glob Oncol2019; 5:1–25.
- 19. Fritz A, Percy C, Jack A, et al. eds. International Classification for Diseases in Oncology. 3rd ed., First Revision. Geneva: WHO (World Health Organization); 2013. Accessed on September 10, 2022. https://apps.who.int/iris/handle/10665/96612
- 20. Ostrom QT, Cioffi G, Gittleman H, et al CBTRUS Statistical Report: primary brain and other central nervous system tumors diagnosed in the United States in 2012-2016. Neuro Oncol.2019; 21(suppl 5):v1–100.
- 21. Allemani C, Harewood R, Johnson CJ, et al Population-based cancer survival in the United States: data, quality control, and statistical methods. Cancer.2017; 123(suppl 24):4982–4993.
- 22. Perme MP, Stare J, Estève J. On estimation in relative survival. Biometrics.2012; 68(1):113–120.
- 23. Spika D, Bannon F, Bonaventure A, et al Life tables for global surveillance of cancer survival (the CONCORD programme): data sources and methods. BMC Cancer.2017;17(1):159.
- 24. Clerc-Urmès I, Grzebyk M, Hédelin G. Net survival estimation with stns. Stata J.2014; 14:87–102.
- 25. Stiller CA, Bunch KJ. Trends in survival for childhood cancer in Britain diagnosed 1971-85. Br J Cancer.1990; 62(5):806–815.
- 26. Corazziari I, Quinn M, Capocaccia R. Standard cancer patient population for age standardising survival ratios. Eur J Cancer.2004; 40(15):2307–2316.
- 27. Brenner H, Gefeller O. An alternative approach to monitoring cancer patient survival. Cancer.1996; 78(9):2004–2010.
- 28. Brenner H, Spix C. Combining cohort and period methods for retrospective time trend analyses of long-term cancer patient survival rates. Br J Cancer.2003; 89(7):1260–1265.
- 29. Gatta G, Capocaccia R, Stiller C, et al Childhood cancer survival trends in Europe: a EUROCARE Working Group study. J Clin Oncol.2005; 23(16):3742–3751.
- 30. Gatta G, Zigon G, Capocaccia R, et al Survival of European children and young adults with cancer diagnosed 1995-2002. Eur J Cancer.2009; 45(6):992–1005.
- 31. Karalexi MA, Papathoma P, Thomopoulos TP, et al Childhood central nervous system tumour mortality and survival in Southern and Eastern Europe (1983-2014): gaps persist across 14 cancer registries. Eur J Cancer.2015; 51(17):2665–2677.
- 32. Trama A, Botta L, Foschi R, et al Survival of European adolescents and young adults diagnosed with cancer in 2000-07: population-based data from EUROCARE-5. Lancet Oncol.2016; 17(7):896–906.
- 33. Gatta G, Botta L, Rossi S, et al Childhood cancer survival in Europe 1999-2007: results of EUROCARE-5, a population-based study. Lancet Oncol.2014; 15(1):35–47.
- 34. Gatta G, Peris-Bonet R, Visser O, et al Geographical variability in survival of European children with central nervous system tumours. Eur J Cancer.2017; 82:137–148.
- 35. Bauchet L, Rigau V, Mathieu-Daudé H, et al Clinical epidemiology for childhood primary central nervous system tumors. J Neurooncol.2009; 92(1):87–98.
- 36. Ilveskoski I, Pihko H, Sankila R, et al Improving outcome of malignant brain tumours in very young children: a population-based study in Finland during 1975-93. Acta Paediatr.1997; 86(7):724–729.
- 37. Berger C, Trombert-Paviot B, Mitton N, et al Les cancers de l’enfant de la region Rhone-Alpes: incidence et survie 1987-1999. Arch Pediat.2006; 13(2):121–129.
- 38. Kaatsch P, Rickert CH, Kuhl J, Schuz J, Michaelis J. Population-based epidemiologic data on brain tumors in German children. Cancer.2001; 92(12):3155–3164.
- 39. Desandes E, Berger C, Tron I, et al Childhood cancer survival in France, 1990-1999. Eur J Cancer.2008; 44(2):205–215.
- 40. Dama E, Pastore G, Mosso ML, et al Time trends and prognostic factors for survival from childhood cancer: a report from the Childhood Cancer Registry of Piedmont (Italy). Eur J Pediat.2006; 165(4):240–249.
- 41. Alston RD, Newton R, Kelsey A, et al Childhood medulloblastoma in northwest England 1954 to 1997: incidence and survival. Dev Med Child Neurol.2003; 45(5):308–314.
- 42. Lannering B, Sandstrom PE, Holm S, et al Classification, incidence and survival analyses of children with CNS tumours diagnosed in Sweden 1984-2005. Acta Paediatr.2009; 98(10):1620–1627.
- 43. Fairley L, Picton SV, McNally RJQ, et al Incidence and survival of children and young people with central nervous system embryonal tumours in the North of England, 1990-2013. Eur J Cancer.2016; 61:36–43.
- 44. Ben Arush M, Rabinowicz R, Ramu N, Barchana M. Incidence and survival of first pediatric primary malignant central nervous system tumors in Israel, 1998-2007. Neuro Oncol. 2010; 12:ii46.
- 45. Smoll NR. Relative survival of childhood and adult medulloblastomas and primitive neuroectodermal tumors (PNETs). Cancer.2012; 118(5):1313–1222.
- 46. Schindler M, Belle FN, Grotzer MA, von der Weid NX, Kuehni CE. Childhood cancer survival in Switzerland (1976-2013): time-trends and predictors. Int J Cancer.2017; 140(1):62–74.
- 47. Khanna V, Achey RL, Ostrom QT, et al Incidence and survival trends for medulloblastomas in the United States from 2001 to 2013. J Neurooncol.2017; 135(3):433–441.
- 48. Tulla M, Berthold F, Graf N, et al Incidence, trends, and survival of children with embryonal tumors. Pediatrics.2015; 136(3):e623–e632.
- 49. Massimino M, Biassoni V, Gandola L, et al Childhood medulloblastoma. Crit Rev Oncol Hematol.2016; 105:35–51.
- 50. Padovani L, Horan G, Ajithkumar T. Radiotherapy advances in paediatric medulloblastoma treatment. Clin Oncol.2019; 31(3):171–181.
- 51. Bhakta N, Liu Q, Ness KK, et al The cumulative burden of surviving childhood cancer: an initial report from the St Jude Lifetime Cohort Study (SJLIFE). Lancet.2017; 390(10112):2569–2582.
- 52. Atun R, Bhakta N, Denburg A, et al Sustainable care for children with cancer: a Lancet Oncology Commission. Lancet Oncol.2020; 21(4):e185–e224.
- 53. Senate and House of Representatives of the United States of America . Public Law 107-260, 107th Congress, Benign Brain Tumor Cancer Registries Amendment Act. Washington, DC: The United States Congress; 2002. Accessed on September 10, 2022. https://www.congress.gov/107/plaws/publ260/PLAW-107publ260.pdf
- 54. World Health Organization. Global Initiative for Childhood Cancer. Geneva: WHO (World Health Organization); 2018. Accessed on September 10, 2022. https://www.who.int/cancer/childhood-cancer/en/
