Introduction
The menstrual cycle is suggested as a favorable model to investigate the effects of ovarian (estrogen and progesterone) hormones on cognition, emotion, and behavior []. The influence of ovarian hormones on both cortico-cortical and cortico-subcortical functional connectivity of the brain has been shown in various neuroimaging studies [-]. Findings exist that estrogen enhances the performance on cognitive tasks such as cognitive control [] mediated by frontal functions, selective attention [], and functional connectivity [-]. The effects of progesterone on the brain regions associated with negative emotion modulation have been shown; for instance, an increased reactivity in the amygdala was reported after acute progesterone administration []. Functional magnetic resonance imaging (fMRI) studies revealed that the changes in brain functions in the course of verbal [] and spatial [] tasks are associated with sex hormones. Besides, recent studies showed alterations in the resting-state functional connectivity (RS-FC) depending on the cycle phase and/or hormonal fluctuations [-].
Schizophrenia (SCZ) is described as a chronic disorder in an extensive symptom spectrum with abnormalities in perception, thinking, behavior, and presentation of negative symptoms []. Different influences of ovarian hormones on SCZ symptoms have been mentioned. The estrogen hypothesis of SCZ is based on the idea that in women, estrogen has a neuroprotective effect in clinical and cognitive symptoms of the illness []. In agreement with this, it was found that low estrogen levels correlated with more severe negative symptoms [] and reduced performance in cognitive functions [, ], increased number of hospitalizations [], and a required dose of antipsychotics (APs) [, ] in patients with SCZ. Also, the age of the onset of SCZ is younger in men (reviewed in []), while women, but not men, show a second incidence peak after the age of 50 years []. Moreover, the beneficial effect of estradiol supplementation on positive symptoms was reported in acutely ill women with SCZ []. Recently, it has been shown that the sensitivity of the dopamine receptors to estrogen and progesterone is increased during the luteal phase [], and this overactivity in dopamine pathways may cause the exacerbation of SCZ symptoms []. Additionally, menstrual dysfunction is widely observed as a result of hyperprolactinemia due to AP use in patients with SCZ (reviewed in []).
SCZ is also defined as a functional dysconnectivity or abnormal integration between distant brain regions []. Recently, besides the task-related fMRI method, another approach of examining the “functional connectivity” has been reported, which describes the operation of the integration and interrelations of multiple brain regions by fMRI at rest []. The activity of distributed cortical areas exhibits a pattern of low-frequency oscillations in the BOLD signal (<0.1 Hz) during rest [-]. The resting-state fMRI (RS-fMRI) technique is recommended as a powerful method providing a more comprehensive research opportunity to investigate the abnormalities in the intrinsic functional organization of the brain in patients with SCZ because it is not based on the ability to complete complex cognitive tasks []. Cognition-related frontoparietal networks (FPNs), which are also highly linked to sex differences [] in patients with SCZ, differentiate these patients from healthy individuals. The effects of the menstrual cycle on RS-FC have not been studied in SCZ. Also, a few task-related fMRI studies have been conducted [-]. These studies focused on examining the association between ovarian hormones and the disturbances of emotional processing in patients with SCZ. Mendrek et al. [], using a task-related fMRI method in female patients with SCZ (n = 21) and controls (n = 23) during different two phases of their menstrual cycle, have shown that atypical neural activation was associated with emotional processing depending on cycle phase and the affective valence of present stimuli in patients. Another task-related fMRI study [] explored the association between progesterone levels and brain activity while viewing emotionally positive, negative, and neutral images in patients with SCZ compared with controls. A positive correlation was found between progesterone levels and brain activity during the emotional processing in both healthy men and men with SCZ, but no significant relationship was detected in women []. On the contrary, a limited number of studies investigated the menstrual cycle effects on the RS-FC in healthy women, but they provided contradictory results. These studies reported alterations in the default mode network (DMN) connectivity depending on the cycle phase [-], increased functional connectivity of bilateral hippocampus during the late follicular phase [], and changes in the intrinsic connectivity of the right dorsolateral prefrontal cortex and bilateral sensory motor cortex associated with the progesterone levels []. However, some studies examining the cycle phase and/or hormonal effects on the functional connectivity of the resting-state networks (RSNs) in healthy women found that the connectivity of the RSNs was not altered across the menstrual cycle [, ].
The aims of the present study were to investigate the cycle-related hormonal and clinical changes in patients with SCZ (SCZs) compared with healthy controls (HCs) and to explore the possible menstrual cycle effects on the RS-FC alterations in the groups in two cycle phases with low and high hormone levels. We hypothesized the hormonal fluctuations across the menstrual cycle would differ in women with SCZ compared with controls. We also expected evidence regarding the alterations in the connectivity of the RSNs depending on ovarian hormone levels or/cycle phase of the groups. Finally, we assumed that the functional connectivity of the RSNs would differ between the 2 groups in each cycle phase.
Materials and Methods
Participants
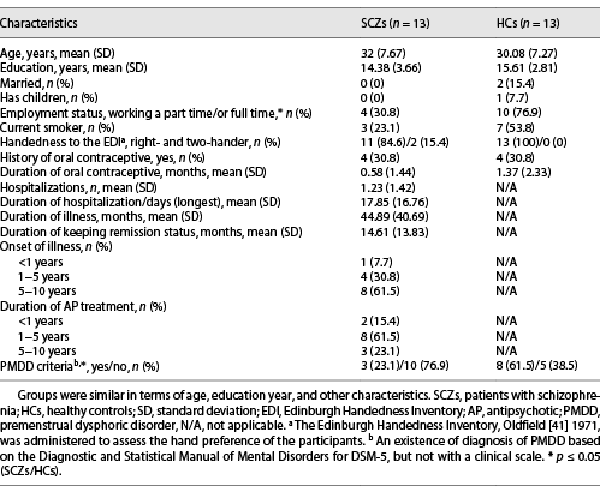
The study included 13 women with SCZ followed by the Psychotic Disorders Research Unit, Department of Psychiatry, Istanbul Faculty of Medicine, and 13 healthy women matched with the patients in terms of education year and age (HCs). Patients were diagnosed as SCZ by means of the Structured Clinical Interview for Diagnostic and Statistical Manual of Disorders (DSM-IV) axis I disorders, clinician version (SCID-CV) []. HC group, who had no current or previous history of psychiatric (except for the diagnosis of premenstrual dysphoric disorder [PMDD]) and neurologic disorders were chosen from a community sample after a face-to-face interview using the SCID-I/NP (nonpatient edition) []. The study was approved by the Ethics Committee of Istanbul University, Faculty of Medicine for Clinical Research Ethics, and all participants provided informed written consent for the study.
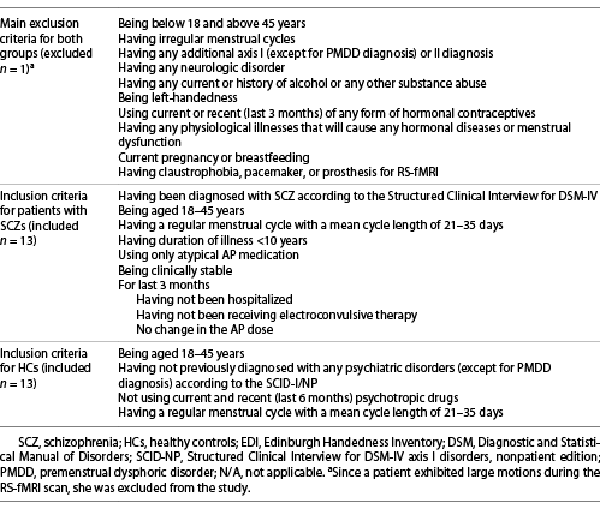
The exclusion and inclusion criteria of the participants are outlined in Table 1, while participants’ demographic and clinical features are given in Table 2. All patients were on atypical AP treatment, and the duration of illness was more than 1 year (Table 2). Most of our patients were treated with atypical APs (olanzapine, aripiprazole, and clozapine), which lacked significant effects on prolactin [] (review in []). Only 1 patient used risperidone (at a dose of 3 mg/day and its depot dose of 37.5 mg/2 weeks), which has been demonstrated to have a high prevalence of hyperprolactinemia among atypical APs []. However, all patients included in the study had a regular/normal menstrual cycle with a mean cycle length of 21–35 days []. Six patients were treated with clozapine at a mean dose of 137.5 mg/day, six were treated with aripiprazole at a mean dose of 22.5 mg/day, one was treated with olanzapine at a dose of 10 mg/day, and two were treated with aripiprazole depot dose of 400 mg/month. Three of these received a combination of aripiprazole and clozapine. The use of AP medication might affect daily brain dynamics, and hence the patients taking the AP dose in the morning were allowed to take their doses after RS-fMRI scanning.
Procedures
The RS-fMRI scanning, as well as hormonal and clinical assessments, was applied to each participant twice during two different cycle phases: early follicular (cycle days 2–6; low estrogen/progesterone) and mid-luteal (cycle days 20–22; high estrogen/progesterone), where “day 1” was the first day of menstrual bleeding. The cycle phases were selected in accordance with the previous literature [, ] and consultation to Departments of Endocrinology and Metabolic Diseases with Gynecology and Obstetrics of Istanbul Faculty of Medicine. A counting method was applied to estimate women’s cycle phases. This meant counting from the menstruation onset to 2–6 and 20−22 days to capture the early follicular and mid-luteal phase, respectively. A self-reported onset of menstruation was used as a starting point. The menstrual cycle phase was also confirmed by hormonal assays. The participants were randomized according to the cycle phase to control for a session effect. Approximately half of the participants in each group were tested first with their early follicular phase and another half with their mid-luteal phase.
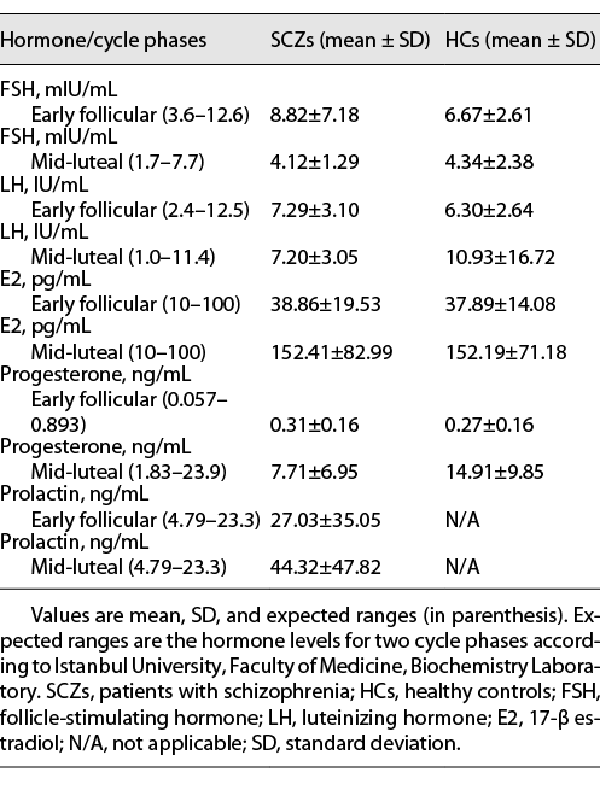
The clinical assessment of the patients with SCZ included the scales of Brief Psychiatric Rating Scale (BPRS) [], Clinical Global Impression (CGI) [], Calgary Depression Rating Scale for Schizophrenia (CDSS) [], and Global Assessment of Functioning (GAF) []. For the HCs, assessment interviews included the CDSS and the GAF. The CDSS is not only a standard assessment instrument for SCZ but also a scale that can be used for HCs []. Before the RS-fMRI scanning in each cycle phase, the fasting blood samples from all participants were taken to determine the serum hormone levels of follicle-stimulating hormone, luteinizing hormone, 17-beta estradiol, and progesterone. Furthermore, the serum prolactin level was measured in patients with SCZ for determining possible hyperprolactinemia. The blood samples were analyzed at the Clinical Biochemistry Laboratory of Istanbul Faculty of Medicine. The hormone concentrations in each cycle phase of groups are given in Table 3.
The participants completed two sessions of RS-fMRI. They were instructed to relax, stay awake, keep open-eyed, and look at a fixation cross. All RS-fMRI sessions were performed on a 3-Tesla Philips MRI scanner equipped with a 16-channel head array coil at the Department of Radiology, Yeditepe University Hospital. RS-fMRI data were acquired using a T2*-weighted gradient-echo echo-planar imaging sequence. The following parameters were used: 300 dynamics, acquisition matrix = 68 × 58, slice thickness = 3.5 mm, 30 axial slices, TR = 2,000 ms, TE = 30 ms, FOV = 230 × 230 mm, and flip angle = 77°. The total scanning time for RS-fMRI was 10 min.
Statistical Analysis of Hormone Assays and Clinical Assessment
Statistical analyses of the hormonal levels and the clinical variables were completed using the IBM SPSS 21.0 (SPSS Inc., Chicago IL, USA) software package. A Shapiro-Wilks test was used to determine whether the hormonal levels and the clinical variables were normally distributed (if all p > 0.05, the normal distribution was provided). The differences in hormone levels and clinical variables between the groups of SCZ and HC were analyzed using an independent-samples t test or a Mann-Whitney U test where applicable. The hormonal and clinical variables between the two cycle phases were compared using a paired-samples t test, or Wilcoxon rank-sum test where applicable within the groups. The associations between ovarian hormone levels and the scores of clinical assessment and their relations with the demographic (e.g., age for 2 groups) and clinical features (e.g., duration of illness for patients with SCZ) were assessed by Spearman’s correlation analysis in all groups for each cycle phase, separately. The threshold of statistical significance was set at two-tailed p ≤ 0.05. A clinical significance was estimated with Cohen’s d [] effect size (ES) using partial eta squared (η2) for the results based on statistical comparisons. The threshold was set at ES >1 for clinical significance, and all results were presented with p values.
Analysis of the RS-fMRI Data
Image preprocessing was performed using the Statistical Parametric Mapping Software SPM12 (http://www.fil.ion.ucl.ac.uk/spm) running on MATLAB 2014a (The MathWorks, Ins., Natick, MA, USA, http://www.mathworks.com). Preprocessing involved the realignment (none of the images had motion greater than 3 mm in the translational plane and 3 mm in the rotational plane), slice-timing correction, normalization to population template, and spatial smoothing (with a Gaussian kernel of 8-mm full width at half maximum) steps.
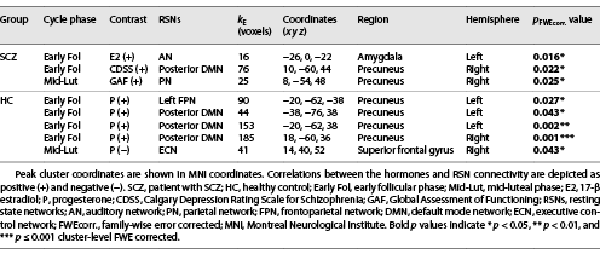
Group “independent component analysis (ICA)” was performed using GIFT fMRI toolbox version 3.0b (http://mialab.mrn.org/software/gift/) []. In the preprocessing step, the individual data were mean corrected by subtracting the image mean per time point. Thereafter, the data dimensionality was reduced for each participant using “principal component analysis.” The reduced data were used for the estimation of 27 independent components using the spatial group ICA based on the FastICA algorithm and repeated in ICASSO 20 times [, ]. The last step involved the back-reconstruction of individual spatial maps from the components estimated at the group level. Of the 27 components ICA produced, 12 RSNs (shown in Fig. 1) were identified in accordance with the previous literature [, , , -].
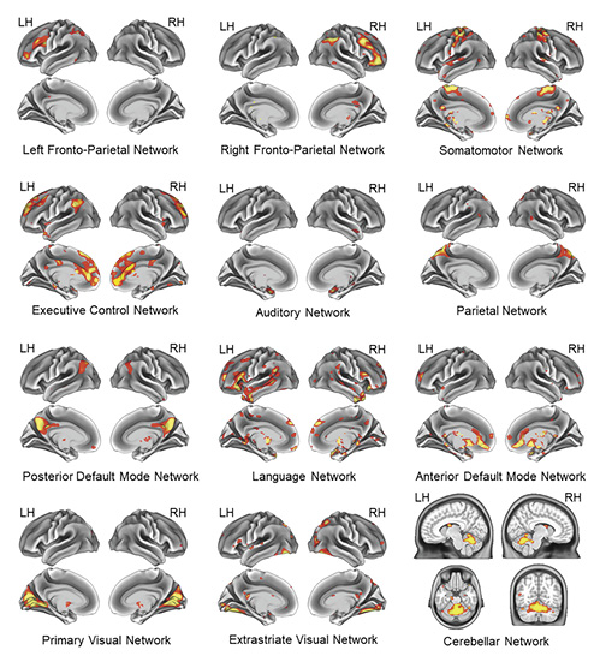
Fig. 1
Spatial maps of twelve RSNs included in the study, which were identified by group ICA on participants. RH, right hemisphere; LH, left hemisphere.
The statistical analysis was carried out with SPM12 using the subject-specific z-maps of the 12 RSNs studied. Explicit masks corresponding to each of the 12 components of interest were generated using one-sample t tests and used for filtering in further statistical analysis of the RSNs.
The paired-sample t tests were performed between the cycle phases in both groups to compare network connectivity for each RSN, and two contrasts were defined (i.e., early follicular > mid-luteal; early follicular < mid-luteal). Two-sample t tests were applied between groups in each cycle phase, and two contrasts were also defined (i.e., SCZs > HCs; SCZs < HCs). Multiple regression analyses were performed to investigate the correlations of the estradiol and progesterone levels with network connectivity in each cycle phase in both groups. In the analyses, all hormone levels other than the interest were imported as covariates in the design matrix. Therefore, the effect of each hormone was examined independent of the other hormone levels in the same cycle phase. Similarly, the associations between the severity of clinical symptoms and the RSN connectivity of the patients were investigated using the multiple regression analysis in each cycle phase separately. Two contrasts were specified to test for positive and negative correlations. The results of the t tests and multiple regression analyses were explored at a cluster-level significance threshold of the family-wise error (FWE, p < 0.05 corrected for multiple testing).
Sample Size Calculation
We executed a posteriori power analysis, which is also known as the “post hoc” power analysis using G*Power calculator (htttp://http://www.gpower.hhu.de/-version 3.1) []. Conducting the post hoc power analysis, we aimed to provide a required sample size to detect a significant difference in the statistical comparisons and find out the correlation of hormonal levels with the RS-FC, for future related studies. The values of ES (Cohen’s d) obtained from the current study were used to compute the required sample size providing a difference between the groups. The medium ES f2 of 0.15 [] was considered while exploring a significant correlation by multiple regression analysis. The threshold was set at two-tailed p < 0.05, with a power of 0.80 for all post hoc power analyses.
Results
Hormone Assays and the Clinical Assessment
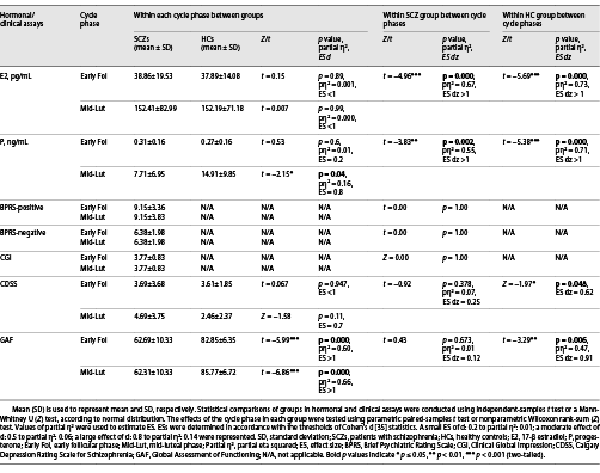
The groups’ means of all hormone levels were observed to be within approximately the expected normal physiologic range for each cycle phase assessed (Table 3). The differences in the levels of follicle-stimulating hormone (for the SCZs, Z = −2.97, p < 0.01, ES dz = 0.67; for the HCs, Z = −2.34, p < 0.05, ES dz = 0.68), estradiol (for the SCZs, t(12) = −4.96, p < 0.001; for the HCs, t(12) = −5.69, p < 0.001, for all, ES dz > 1), progesterone (for the SCZs, t(12) = −3.83, p < 0.01; for the HCs, t(12) = −5.38, p < 0.001, for all, ES dz > 1), and prolactin (for the SCZs, Z = −2.97, p < 0.01, ES dz = 0.57) between two cycle phases were statistically significant. For both the groups, luteinizing hormone levels were similar between the cycle phases (all p > 0.05). As expected, the means of estradiol and progesterone levels were significantly lower in the early follicular phase compared with the mid-luteal phase in both the groups. This finding also confirmed the cycle phases.
The groups differed significantly in the mean of mid-luteal phase progesterone levels (t(24) = −2.15, p < 0.05, partial η2 = 0.16, ES = 0.8). A large percentage of variance (16%) was explained by group factor for mid-luteal progesterone levels. The results of statistical comparisons between/within groups of the assays of hormone levels (for estradiol and progesterone) and clinical instruments are given in Table 4.
Hormonal and Clinical Correlations
The relationships between the hormonal, clinical, and demographic variables in the groups during two cycle phases were analyzed using Spearman’s rank correlation coefficients. In the SCZs during the early follicular phase, a significant positive correlation was found between age and the CDSS scores (rho = 0.58, p < 0.05). Also, the patients showed a strong positive correlation between the scores of CGI and the BPRS-positive symptom severity in this phase (rho = 0.72, p < 0.01). However, during their mid-luteal phase of the SCZs, age positively correlated with progesterone levels (rho = 0.72, p < 0.01). A strong negative correlation was observed between the mid-luteal estradiol levels and the sub-scores of the BPRS-negative symptom severity (rho = −0.61, p < 0.05). The BPRS-negative symptom severity was negatively associated with the GAF scores (rho = −0.59, p < 0.05), while the BPRS-positive symptom severity positively correlated with the CGI scores (rho = 0.74, p < 0.01) in this cycle phase with high hormone levels. Additionally, the correlations of ovarian hormone levels with the clinical features of patients, including duration of illness, number of hospitalization, duration of hospitalization (for longest), and keeping the remission status, showed a strong positive relationship between the duration of remission and mid-luteal progesterone levels (rho = 0.68, p = 0.01). No other significant correlations were found among demographic, hormonal, and clinical variables in the patients with SCZ for both cycle phases.
In the HCs during the early follicular phase, no significant correlation was noted between hormonal (estradiol and progesterone levels) and clinical variables (GAF and CDSS scores). Also, age was not correlated with any hormonal and clinical variables in this phase. However, the HCs showed significant correlations of age variable with the scores of GAF (rho = −0.58, p < 0.05) and CDSS (rho = 0.62, p < 0.05) during their mid-luteal phase. No other significant correlations were observed between hormonal and clinical variables of the HCs.
Cycle Phase-Related Alterations in the RS-FC
Comparing the functional connectivity of the brain regions represented by the 12 RSNs studied between the early follicular and mid-luteal phases, no significant differences (all p > 0.05, FWE corrected) were found either in patients with SCZ or in the HCs. Similarly, no difference was found in the functional connectivity of the 12 RSNs between the groups in each cycle phase (all p > 0.05, FWE corrected).
Correlations of Hormone Levels with the RS-FC
In each cycle phase, the correlations of the hormonal levels with the functional connectivity of the 12 RSNs studied within the groups were examined. Based on a significant positive correlation between age and mid-luteal progesterone levels in the patients with SCZ, the age was also entered as a covariate into the multiple regression model used to examine the correlation of hormonal levels with the RS-FC in patients during their mid-luteal phase.
Within the SCZs, our multiple regression analyses revealed that estradiol levels positively correlated with the connectivity of the auditory network (AN) in the left amygdala during the early follicular phase (p < 0.05, FWE corrected) (shown in Fig. 2; Table 5). No statistically significant clusters of ovarian hormone correlations with functional connectivity of other RSNs were studied in this phase. Also, estradiol and progesterone levels of the SCZs were not significantly correlated with the RSN connectivity in the mid-luteal phase in which age was controlled (all p > 0.05, FWE corrected).
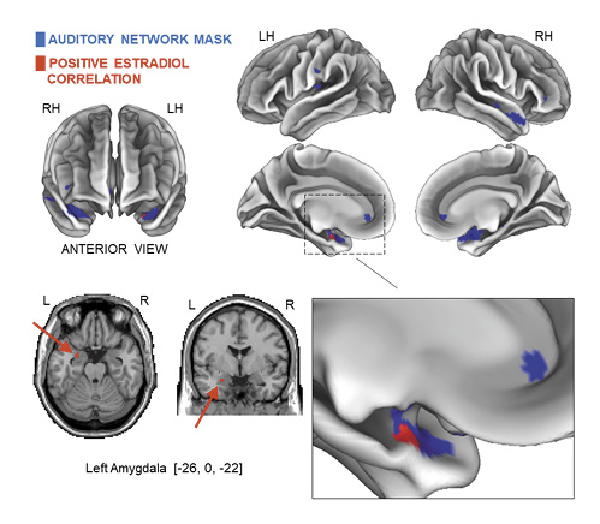
Fig. 2
Results of the hormonal correlations with the RS-FC in the patients with SCZ. Estradiol levels positively correlated with the functional connectivity of the AN in the left amygdala in the early follicular phase (p < 0.05, FWE corrected, peak difference at −26, 0, −22, kE = 16 voxels). Peak cluster coordinates are shown in the MNI152 T1 (2 mm). Note: The AN masks are represented in blue, and positive estradiol correlations are represented in red. R, right; L, left; RH, right hemisphere; LH, left hemisphere; MNI, Montreal Neurological Institute.
Within the HCs, in the early follicular phase, a positive correlation was observed between progesterone levels and the left FPN connectivity in the precuneus (p < 0.05, FWE corrected) (shown in Fig. 3a; Table 5). Additionally, the posterior DMN connectivity positively correlated with the progesterone levels for three clusters within the bilateral precuneus (p < 0.05; p < 0.01; p ≤ 0.001, FWE corrected) (shown in Fig. 3b; Table 5). However, in the mid-luteal phase, the progesterone levels correlated negatively with the connectivity of the executive control network (ECN) in the right superior frontal gyrus (p < 0.05, FWE corrected) (shown in Fig. 3c; Table 5). No statistically significant correlation was found between the levels of ovarian hormones with the functional connectivity of other RSNs studied (all p > 0.05, FWE corrected).
Fig. 3
Results of the hormonal correlations with the RS-FC in the HCs. Early follicular phase of HCs; the positive correlation between progesterone levels and the left FPN functional connectivity in the precuneus (p < 0.05, FWE corrected, peak difference at −20, −62, −38, kE = 90 voxels) (a); the positive correlations between the posterior DMN connectivity and progesterone levels for three clusters within the bilateral precuneus: two clusters in the left precuneus (p < 0.05, FWE corrected, peak difference at −38, −76, 38, kE = 44 voxels) CLUSTER #1 (b) and (p < 0.01, FWE corrected, peak difference at −20, −62, 38, kE = 153 voxels) CLUSTER #2 (b); one cluster in the right precuneus (p < 0.001, FWE corrected, peak difference at 18, –60, 36, kE = 185 voxels) CLUSTER #3 (b). Mid-luteal phase of the HCs; the negative correlation between progesterone levels and the ECN functional connectivity in the right superior frontal gyrus (p < 0.05, FWE corrected, peak difference at 14, 40, 52, kE = 41 voxels) (c). Peak cluster coordinates are shown in the MNI152 T1 (2 mm). Note: All related RSN masks are represented in blue, and negative and positive hormone correlations are represented in red. R, right; L, left; RH, right hemisphere; LH, left hemisphere; MNI, Montreal Neurological Institute.
Further, we examined whether the associations obtained between the levels of ovarian hormones and the RSN functionality in two cycle phases within each group differed between the patients with SCZ and HCs. Thus, the relationships between the interaction effect of group and ovarian hormone levels and the RSN connectivity for each cycle phase were estimated using the multiple regression analysis. The estimations of the interaction effect were only applied for the connectivity of four RSNs (AN, posterior DMN, left FPN, and ECN), which were found to be significantly associated with the estradiol or P levels in the groups during different cycle phases. The results showed no significant associations between the interaction effect of the group factor and ovarian hormone levels and the connectivity of the AN, posterior DMN, left FPN, and ECN for two cycle phases (all p > 0.05, FWE corrected).
Correlations of the Clinical Symptom Severity with RS-FC in Patients
For the patient group, the correlations between the severity of clinical symptoms and the functional connectivity of the 12 RSNs in each cycle phase were examined. Since age correlated with the CDSS scores in the early follicular phase and with the progesterone levels in the mid-luteal phase in the SCZs, age was included as a covariate in the multiple regression analyses for both cycle phases. The findings showed that the GAF scores of the SCZs were positively associated with the PN connectivity for one cluster in the right precuneus in their mid-luteal phase (p < 0.05, FWE corrected) (shown in Fig. 4a; Table 5) but not in the early follicular phase. However, in the early follicular phase, a positive correlation was found between CDSS scores and the posterior DMN connectivity for one cluster in the right precuneus (p < 0.05, FWE corrected) (shown in Fig. 4b; Table 5). No other statistically significant clusters of the correlations of age and clinical variables with the connectivity of RSNs studied in both cycle phases were detected.
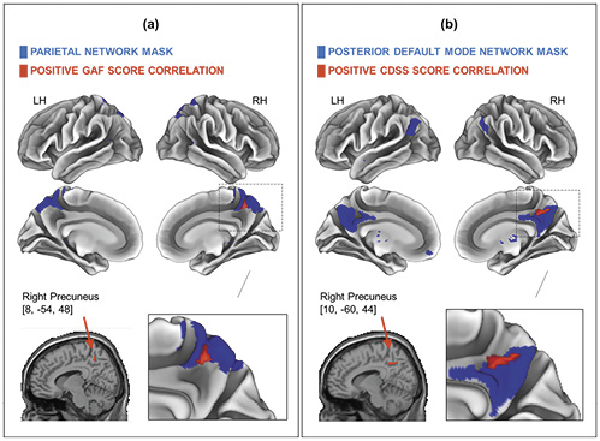
Fig. 4
Results of the correlations of clinical variables with the RS-FC in the patients with SCZ. The positive correlation of GAF scores with the PN connectivity in the right precuneus in the mid-luteal phase (p < 0.05, FWE corrected, peak difference at 8, –54, 48, kE = 25 voxels) (a); the positive correlation between CDSS scores and functional connectivity of the DMN in the right precuneus in the early follicular phase (p < 0.05, FWE corrected, peak difference at 10, –60, 44, kE = 76 voxels) (b). Peak cluster coordinates are shown in the MNI152 T1 (2 mm). Note: All related RSN masks are represented in blue; positive clinical correlations are represented in red. GAF, Global Assessment of Functioning; CDSS, Calgary Depression Rating Scale for Schizophrenia; RH, right hemisphere; LH, left hemisphere; MNI, Montreal Neurological Institute.
Sample Size Calculation
The mean of mid-luteal progesterone levels was 7.71 ± 6.95 in 13 patients and 14.91 ± 9.85 in 13 controls with a large ES of 0.8. A sample size of 24 is required in each group to detect a significant difference in mid-luteal progesterone levels among the 2 groups, considering a power of 0.80 and an alpha of 0.05. In controls, a sample size of 24 was required to find out a significant difference in the CDSS mean scores, using the current ES of 0.6. The sample size to detect a difference for the GAF scores among cycle phases was calculated as 12, using the current ES of 0.9. However, the required sample size for statistical significance in the comparisons of estradiol and progesterone levels between cycle phases within groups was found to be between 6 and 10. The required total sample size for each group to explore a significant correlation of hormonal levels with RS-FC by a linear multiple regression analysis with four hormone predictors was computed to be 85.
Discussion
We investigated hormonal and clinical changes and the RS-FC alterations depending on the menstrual cycle phase in women suffering from SCZ together with HCs. The present findings partially supported our hypothesis. We found that the mid-luteal progesterone levels of the patients were significantly lower than that of the controls. This difference was consistent with the results of the studies reporting the dysregulation of circulating progesterone levels in patients with SCZ, particularly in the mid-luteal phase of their menstrual cycle [, , -]. Also, the elevation in mid-luteal progesterone levels of the patients was associated with a longer duration of remission. In keeping with the findings of Ko et al. [], we found a negative correlation between the estradiol levels and the severity of negative symptoms of the patients in the mid-luteal phase. Our findings for HCs revealed that the healthy women had higher depressive symptoms and lower functionality in the early follicular phase compared with the mid-luteal phase. In the present study, the assessments of the severity of early follicular depression symptoms and functionality involved 10–12 days before menstruation, which was characterized as the premenstrual period in which the PMDD symptoms occurred []. Our findings obtained by the RS-fMRI method showed that the estradiol and progesterone levels depending on the cycle phase were associated with the intrinsic functional connectivity of the brain in both groups. Furthermore, in the patients, a higher level of functionality was related to the increased connectivity of the PN in the right precuneus in the cycle phase of high estradiol and peak progesterone levels. However, the severity of depressive symptoms of the patients was positively associated with the right precuneus connectivity of the DMN in the cycle phase of low hormone levels.
This study was novel in examining multiple effects of the menstrual cycle on the RS-FC of the brain in patients with SCZ. However, a few studies investigated the changes in task-related brain activations depending on the sex steroid hormones [, ] and menstrual cycle phase [] in patients with SCZ. The design of Mendrek et al. [] was the most similar to ours because the study was conducted in the follicular and luteal phases of the cycle in patients with SCZ. Mendrek et al. [] stated that healthy women rated the negative (but not positive and neutral) valanced images as more negative than patients in the luteal phase while they did not find a significant difference between the patient and control groups in terms of luteal phase progesterone levels. Consistently with their behavioral findings, a decrease was reported in the neural activations of the patients in the images charged negatively compared with the controls, only in the luteal phase, not in the follicular phase []. In keeping with these findings of Mendrek et al. [], it was reported that when progesterone levels were high, a greater tendency to perceive fearful and disgusted expressions was observed in healthy women []. Atypical emotional processing [] and difficulties in recognizing facial expressions, including negative emotions such as fear [], have been revealed in patients with SCZ. In line with all these previous findings and our current result, including that the patients had lower mid-luteal progesterone levels compared with the controls, it is thought that studies focusing on understanding the dysregulation in progesterone levels and its interaction with estradiol across the menstrual cycle in patients with SCZ are needed. Although several effects of estrogen have been widely demonstrated in patients with SCZ, the role of progesterone in the illness has still not been understood.
Correlations of the Hormone Levels with the RS-FC
Our results on the correlations of hormonal levels with the RS-FC in the patients with SCZ revealed that the estradiol levels positively correlated with the connectivity of the AN in the left amygdala in the cycle phase with low hormone levels. The finding might also be relevant to SCZ itself for various reasons. First, structural and functional abnormalities in the superior temporal gyrus, particularly on the left side, which are associated with the severity of psychotic symptoms such as auditory hallucinations and formal thought disorder, have been reported in patients with SCZ []. Second, decreased activation in relation to atypical emotional processing has been reported in the hippocampus, amygdala, and medial prefrontal, orbitofrontal, and cingulate cortex in patients with SCZ []. The amygdala is known to be involved in emotional stimulus detection and processing []. Consistently, deficits in recognizing facial expressions of negative emotions, especially fear, have been reported in patients []. Finally, the amygdala and limbic lobe are demonstrated to be the regions of the brain where estrogen [] and progesterone [] receptors are commonly located.
Our results on the correlations of hormonal levels with the RS-FC in the controls indicated that the progesterone levels were associated with the functional connectivity of the posterior DMN, left FPN, and ECN. The positive correlations of the progesterone levels with the connectivity of the posterior DMN and left FPN were found in the precuneus in the early follicular phase. The ECN connectivity negatively correlated with the progesterone levels in the right superior frontal gyrus in the mid-luteal phase. Consistent with our findings, the DMN connectivity has been related to estrogen and progesterone levels in healthy women [-, ]. However, three of the studies [-] have also reported changes in the DMN connectivity among the cycle phases.
Limitations and Strengths
The present study had several limitations. First, we conducted the study with a relatively small sample size, which might have been responsible for some of our nonsignificant findings due to a type-II error and might limit the generalizability of our results. Also, our patients were clinically stable during the study period. This might be a possible reason for the lack of difference in the RS-FC in patients between the cycle phases and in each cycle phase among the groups. In the studies on the menstrual cycle, comparing the cycle phases in which estradiol and progesterone levels were both low or high at the same time limited the interpretation of the findings and did not adequately eliminate the confounding effects. This limiting factor was also valid for our study. We did not exclude PMDD diagnosis from the study or did not evaluate PMDD and/or premenstrual syndrome symptoms using a clinical scale to determine its effectiveness and to control in statistical analyses. These might be considered as other restrictive factors of the study. Despite these limitations, our study also had some methodological strengths. First, the individuals in each group were examined in the two different cycle phases. Second, all the cycle-related hormone concentrations were analyzed, which increased the reliability by confirming the cycle phases. Third, all 12 RSNs, identified in the subject group, were included in the statistical analysis. Finally, the study included the HC group, and also, each participant created her own control using the repeated-measure design.
Conclusion
The patients showed lower levels of progesterone compared with the controls in their mid-luteal cycle phase. Also, our results pointed out that the changes in the severity of clinical symptoms or/features might be related to the cycle-dependent hormone levels in both groups. Our RS-fMRI findings indicated that the fluctuations in ovarian hormone levels across the cycle might be associated with the brain dynamics at rest in both groups. Additionally, in the patients, the functionality-level and depressive symptom severity depending on the cycle phase was found to correlate with the right precuneus connectivity of the PN and the posterior DMN. The RS-fMRI findings obtained from the controls were consistent with the previous findings indicating that the menstrual cycle effects might be associated with the intrinsic connectivity of the DMN and cognition-related networks in healthy women. However, it would be important to emphasize that our RS-fMRI findings must be interpreted with a larger sample size replication to understand multiple effects of the menstrual cycle-related changes on the RS-FC in patients with SCZ. Accordingly, the present study also contributed to determining the feasibility of a longitudinal RS-fMRI study design and a required sample size for future studies on exploring the cycle-dependent changes and/or effects in patients with SCZ. Finally, our study suggested that it could be important for clinicians to consider the changes related to the menstrual cycle in assessing symptom severity of patients with SCZ. Our next goal is to investigate the temporal correlations between the independent RSNs in different cycle phases based on the previous literature on functional disconnection in patients with SCZ.
Acknowledgments
The authors thank Filiz Er for collection of blood samples and are grateful to all patients and HCs who participated in this study.
Statement of Ethics
This study protocol was approved by the Ethics Committee of Istanbul University, Istanbul Faculty of Medicine for Clinical Research Ethics, Approval No. [1754], and conducted in accordance with the Declaration of Helsinki. All participants provided informed written consent for the study including fMRI scanning, blood-taking, and clinical interview.
Conflict of Interest Statement
The authors have no conflicts of interest to declare.
Funding Sources
AU received funding from the Istanbul University Scientific Research Projects Unit by the Project No. [59873].
Author Contributions
H.N. designed the study, collected the data, conducted the statistical data analyses, interpreted the data, and prepared the manuscript. A.H. helped with performing and interpreting the resting state fMRI data statistical analyses and preparation of the manuscript and made the resting state fMRI data visualization. Z.F. and A.S. managed and conducted the resting state fMRI scanning. A.U.. supervised the study at providing patients, conducting clinical interviews, preparation of the manuscript, and acquired the funding. All authors contributed to and have approved the final manuscript and provided intellectual input.
Data Availability Statement
The datasets used and/or analyzed in the present study are available from the corresponding author on reasonable request.
References
- 1. Sundstro[Combining Diaeresis]m-Poromaa I, Gingnell M. Menstrual cycle influence on cognitive function and emotion processing-from a reproductive perspective. Front Neurosci. 2014 Nov;8:380.
- 2. Ottowitz WE, Derro D, Dougherty DD, Lindquist MA, Fischman AJ, Hall JE. FDG-PET analysis of amygdalar-cortical network covariance during pre-versus post-menopausal estrogen levels: potential relevance to resting state networks, mood, and cognition. Neuro Endocrinol Lett. 2008 Aug;29(4):467–74.
- 3. Ottowitz WE, Siedlecki KL, Lindquist MA, Dougherty DD, Fischman AJ, Hall JE. Evaluation of prefrontal-hippocampal effective connectivity following 24 hours of estrogen infusion: an FDG-PET study. Psychoneuroendocrinology. 2008 Nov;33(10):1419–25. http://dx.doi.org/10.1016/j.psyneuen.2008.09.013.
- 4. Weis S, Hausmann M, Stoffers B, Vohn R, Kellermann T, Sturm W. Estradiol modulates functional brain organization during the menstrual cycle: an analysis of interhemispheric inhibition. J Neurosci. 2008 Dec;28(50):13401–10. http://dx.doi.org/10.1523/JNEUROSCI.4392-08.2008.
- 5. Hjelmervik H, Westerhausen R, Osnes B, Endresen CB, Hugdahl K, Hausmann M, et al. Language lateralization and cognitive control across the menstrual cycle assessed with a dichotic-listening paradigm. Psychoneuroendocrinology. 2012 Nov;37(11):1866–75.
- 6. Thimm M, Weis S, Hausmann M, Sturm W. Menstrual cycle effects on selective attention and its underlying cortical networks. Neuroscience. 2014 Jan;258:307–17. http://dx.doi.org/10.1016/j.neuroscience.2013.11.010.
- 7. van Wingen GA, van Broekhoven F, Verkes RJ, Petersson KM, Backstrom T, Buitelaar JK, et al. Progesterone selectively increases amygdala reactivity in women. Mol Psychiatry. 2008 Mar;13(3):325–33.
- 8. Weis S, Hausmann M, Stoffers B, Sturm W. Dynamic changes in functional cerebral connectivity of spatial cognition during the menstrual cycle. Hum Brain Mapp. 2011 Oct;32(10):1544–56. http://dx.doi.org/10.1002/hbm.21126.
- 9. Arélin K, Mueller K, Barth C, Rekkas PV, Kratzsch J, Burmann I, et al. Progesterone mediates brain functional connectivity changes during the menstrual cycle-a pilot resting state MRI study. Front Neurosci. 2015 Feb;9:44.
- 10. De Bondt T, Smeets D, Pullens P, Hecke WV, Jacquemyn Y, Parizel PM. Stability of resting state networks in the female brain during hormonal changes and their relation to premenstrual symptoms. Brain Res. 2015 Oct;1624:275–85.
- 11. Petersen N, Kilpatrick LA, Goharzad A, Cahill L. Oral contraceptive pill use and menstrual cycle phase are associated with altered resting state functional connectivity. NeuroImage. 2014 Apr;90:24–32. http://dx.doi.org/10.1016/j.neuroimage.2013.12.016.
- 12. Weis S, Hodgetts S, Hausmann M. Sex differences and menstrual cycle effects in cognitive and sensory resting state networks. Brain Cogn. 2019 Apr;131:66–73. http://dx.doi.org/10.1016/j.bandc.2017.09.003.
- 13. American Psychiatric Association (APA). Diagnostic and statistical manual of mental disorders: DSM-5, 5th Edition revised. 2013.
- 14. Bergemann N, Parzer P, Runnebaum B, Resch F, Mund C. Estrogen, menstrual cycle phases, and psychopathology in women suffering from schizophrenia. Psychol Med. 2007 Oct;37(10):1427–36. http://dx.doi.org/10.1017/S0033291707000578.
- 15. Ko YH, Joe SH, Cho W, Park JH, Lee JJ, Jung IK, et al. Estrogen, cognitive function and negative symptoms in female schizophrenia. Neuropsychobiology. 2006;53(4):169–75.
- 16. Hoff AL, Kremen WS, Wieneke MH, Lauriello J, Blankfeld HM, FaustmanWO, et al. Association of estrogen levels with neuropsychological performance in women with schizophrenia. Am J Psychiatry. 2001 Jul;158(7):1134–9.
- 17. Bergemann N, Parzer P, Nagl I, Salbach B, Runnebaum B, Mundt C, et al. Acute psychiatric admission and menstrual cycle phase in women with schizophrenia. Arch Womens Ment Health. 2002 Nov;5(3):119–26.
- 18. Gattaz WF, Vogel P, Riecher-Rössler A, Soddu G. Influence of the menstrual cycle phase on the therapeutic response in schizophrenia. Biol Psychiatry. 1994;36(2):137–9. http://dx.doi.org/10.1016/0006-3223(94)91195-9.
- 19. Seeman MV. Interaction of sex, age, and neuroleptic dose. Compr Psychiatry. 1983 Mar–Apr;24(2):125–8. http://dx.doi.org/10.1016/0010-440x(83)90100-1.
- 20. Leung MDDA, Chue MRC, Psych DP. Sex differences in schizophrenia, a review of the literature. Acta Psychiatr Scand Suppl. 2000;401:3–38. http://dx.doi.org/10.1111/j.0065-1591.2000.0ap25.x.
- 21. Riecher-Rössler A, Häfner H. Gender aspects in schizophrenia:bridging the border between social and biological psychiatry. Acta Psychiatr Scand Suppl. 2000;407:58–62.
- 22. Kulkarni J, Riedel A, de Castella AR, Fitzgerald PB, Rolfe TJ, Taffe J, et al. Estrogen- a potential treatment for schizophrenia. Schizophr Res. 2001 Mar;48(1):137–44.
- 23. Wieck A, Davies RA, Hirst AD, Brown N, Papadopoulos A, Marks MN, et al. Menstrual cycle effects on hypothalamic dopamine receptor function in women with a history of puerperal bipolar disorder. J Psychopharmacol. 2003 Jun;17(2):204–9.
- 24. Kendler KS, Schaffner KF. The dopamine hypothesis of schizophrenia: an historical and philosophical analysis. Philos Psychiatry Psychol. 2011 Mar;18(1):41–63. http://dx.doi.org/10.1353/ppp.2011.0005.
- 25. Wieck A, Haddad PM. Antipsychotic-induced hyperprolactinemia in women: pathophysiology, severity and consequences. Selective literature review. Br J Psychiatry. 2003 Mar;182:199–204.
- 26. Damaraju E, Allen EA, Belger A, Ford JM, McEwen S, Mathalon DH, et al. Dynamic functional connectivity analysis reveals transient states of dysconnectivity in schizophrenia. Neuroimage Clin. 2014 Jul;5:298–308.
- 27. Rogers BP, Morgan VL, Newton AT, Gore JC. Assessing functional connectivity in the human brain by fMRI. Magn Reson Imaging. 2007 Dec;25(10):1347–57. http://dx.doi.org/10.1016/j.mri.2007.03.007.
- 28. Biswal B, Yetkin FZ, Haughton VM, Hyde JS. Functional connectivity in the motor cortex of resting human brain using echo-planar MRI. Magn Reson Med. 1995 Oct;34(4):537–41. http://dx.doi.org/10.1002/mrm.1910340409.
- 29. Cordes D, Haughton VM, Arfanakis K, Carew JD, Turski PA, Moritz CH, et al. Frequencies contributing to functional connectivity in the cerebral cortex in “resting-state” data. AJNR Am J Neuroradiol. 2001 Aug;22(7):1326–33.
- 30. Fox MD, Raichle ME. Spontaneous fluctuations in brain activity observed with functional magnetic resonance imaging. Nat Rev Neurosci. 2007 Sep;8(9):700–11. http://dx.doi.org/10.1038/nrn2201.
- 31. Sheffield JM, Barch DM. Cognition and resting-state functional connectivity in schizophrenia. Neurosci Biobehav Rev. 2016 Feb;61:108–20. http://dx.doi.org/10.1016/j.neubiorev.2015.12.007.
- 32. Rotarska-Jagiela A, Van de Ven V, Oertel-Knochel V, Uhlhaas PJ, Vogeley K, Linden DE. Resting-state functional network correlates of psychotic symptoms in schizophrenia. Schizophr Res. 2010 Mar;117(1):21–30. http://dx.doi.org/10.1016/j.schres.2010.01.001.
- 33. Champagne J, Lakis N, Bourque J, Stip E, Lipp O, Mendrek A. Progesterone and cerebral function during emotion processing in men and women with schizophrenia. Schizophr Res Treatment. 2012;2012:917911. http://dx.doi.org/10.1155/2012/917901.
- 34. Mendrek A, Lakis N, Jiménez J. Associations of sex steroid hormones with cerebral activations during mental rotation in men and women with schizophrenia. Psychoneuroendocrinology. 2011 Oct;36(9):1422–6. http://dx.doi.org/10.1016/j.psyneuen.2011.03.016.
- 35. Mendrek A, Bourque J, Dubé A, Lakis N, Champagne J. Emotion processing in women with schizophrenia is menstrual cycle phase and affective valence dependent: an FMRI study. ISRN Psychiatry. 2012;2012:656274. http://dx.doi.org/10.5402/2012/656274.
- 36. Lisofsky N, Martensson J, Eckert A, Lindenberger U, Gallinat J, Ku[Combining Diaeresis]hn S. Hippocampal volume and functional connectivity changes during the female menstrual cycle. Neuroimage. 2015 Sep;118:154–62.
- 37. Hjelmervik H, Hausmann M, Osnes B, Westerhausen R, Specht K. Resting states are resting traits: an FMRI study of sex differences and menstrual cycle effects in resting state cognitive control networks. PLoS One. 2014 Jul;9(7):e103492. http://dx.doi.org/10.1371/journal.pone.0103492.
- 38. Syan SK, Minuzzi L, Costescu D, Smith M, Allega OR, Coote M, et al. Influence of endogenous estradiol, progesterone, allopregnanolone, and dehydroepiandrosterone sulfate on brain resting state functional connectivity across the menstrual cycle. Fertil Steril. 2017 May;107(5):1246–55.
- 39. First MB, Spitzer RL, Gibbon M, Williams JBW. Structured clinical interview for DSM-IV axis I disorders, clinician version (SCID-CV). New York: Biometrics Research, New York State Psychiatric Institute; 2002.
- 40. First MB, Spitzer RL, Gibbon M, Williams JBW. Structured clinical interview for DSM-IV axis I disorders, research version, non-patient edition. (SCID-I/NP). New York: Biometrics Research, New York State Psychiatric Institute; 2002.
- 41. Oldfield RC. The assessment and analysis of handedness: the Edinburgh inventory. Neuropsychologia. 1971 Mar;9(1):97–113. http://dx.doi.org/10.1016/0028-3932(71)90067-4.
- 42. Crismon ML, Buckley PF. Schizophrenia. In: DiPiro JT, Talbert RL, Yee GC, Matzke GR, Wells BG, Posey LM, editors. Pharmacotherapy a pathophysiologic approach. 6th ed.New York: McGraw-Hill Companies, Inc; 2005. p. 1209–33.
- 43. Bostwick JR, Guthrie SK, Ellingrod VL. Antipsychotic-induced hyperprolactinemia. Pharmacotherapy. 2009 Jan;29(1):64–73. http://dx.doi.org/10.1592/phco.29.1.64.
- 44. Kinon BJ, Gilmore JA, Liu H, Halbreich UM. Prevalence of hyperprolactinemia in schizophrenic patients treated with conventional antipsychotic medications or risperidone. Psychoneuroendocrinology. 2003 Apr;28:55–68. http://dx.doi.org/10.1016/s0306-4530(02)00127-0.
- 45. Lenton EA, Landgren BM, Sexton L, Harper R. Normal variation in the length of the follicular phase of the menstrual cycle: effect of chronological age. Br J Obstet Gynaecol. 1984 Jul;91(7):681–4. http://dx.doi.org/10.1111/j.1471-0528.1984.tb04830.x.
- 46. Rubin LH, Carter CS, Drogos LL, Pournajafi-Nazarloo H, Sweeney JA, Maki PM. Effects of sex, menstrual cycle phase, and endogenous hormones on cognition in schizophrenia. Schizophr Res. 2015 Aug;166(1–3):269–75. http://dx.doi.org/10.1016/j.schres.2015.04.039.
- 47. Lukoff D, Nuechterlein KH, Ventura J. Manual for the expanded brief psychiatric rating scale. Schizophr Bulletin. 1986;12:594–602.
- 48. Guy W. ECDEU assessment manual for psychopharmacology. Revised. US Department of Health, Education and Welfare Publication (ADM). Rockville, MD: National Institute of Mental Health; 1976. p. 76–338.
- 49. Addington D, Addington J, Maticka-Tyndale E, Joyce J. Reliability and validity of a depression rating scale for schizophrenics. Schizophr Res. 1992 Mar;6(3):201–8. http://dx.doi.org/10.1016/0920-9964(92)90003-n.
- 50. Moos RH, McCoy L, Moos BS. Global assessment of functioning (GAF) ratings: determinants and role as predictors of one-year treatment outcomes. J Clin Psychol. 2000 Apr;56(4):449–61. http://dx.doi.org/10.1002/(sici)1097-4679(200004)56:4<449::aid-jclp1>3.0.co;2-8.
- 51. Mu[Combining Diaeresis]ller MJ, Brening H, Gensch C, Klinga J, Kienzle B, Mu[Combining Diaeresis]ller KM. The calgary depression rating scale for schizophrenia in a healthy control group: psychometric properties and reference values. J Affect Disord. 2005 Sep;88(1):69–74.
- 52. Cohen J. Statistical power analysis for the behavioral sciences. 2nd ed.Hillsdale, NJ: L Erlbaum Associates; 1988.
- 53. Calhoun VD, Adalı T, Pearlson GD, Pekar JJ. A method for making group inferences from functional MRI data using independent component analysis. Hum Brain Mapp. 2001 Nov;14(3):140–51. http://dx.doi.org/10.1002/hbm.1048.
- 54. Correa N, Adalı T, Calhoun VD. Performance of blind source separation algorithms for fMRI analysis using a group ICA method. Magn Reson Imaging. 2007 Jun;25(5):684–94. http://dx.doi.org/10.1016/j.mri.2006.10.017.
- 55. Jafri MJ, Pearlson GD, Stevens M, Calhoun VD. A method for functional network connectivity among spatially independent resting-state components in schizophrenia. Neuroimage. 2008 Feb;39(4):1666–81. http://dx.doi.org/10.1016/j.neuroimage.2007.11.001.
- 56. Beckmann CF, DeLuca M, Devlin JT, Smith SM. Investigations into resting-state connectivity using independent component analysis. Philos Trans R Soc Lond B Biol Sci. 2005 May;360(1457):1001–13. http://dx.doi.org/10.1098/rstb.2005.1634.
- 57. Lee WH, Doucet GE, Leibu E, Frangou S. Resting-state network connectivity and metastability predict clinical symptoms in schizophrenia. Schizophr Res. 2018 Nov;201:208–16. http://dx.doi.org/10.1016/j.schres.2018.04.029.
- 58. Faul F, Erdfelder E, Buchner A, Lang AG. Statistical power analyses using G*Power 3.1: tests for correlation and regression analyses. Behav Res Methods. 2009 Nov;41(4):1149–60. http://dx.doi.org/10.3758/BRM.41.4.1149.
- 59. Cohen JA. Power primer. Psychol Bull. 1992;112(1):155–9. http://dx.doi.org/10.1037//0033-2909.112.1.155.
- 60. Bergemann N, Mundt C, Parzer P, Jannakos I, Nagl I, Salbach B, et al. Plasma concentrations of estradiol in women suffering from schizophrenia treated with conventional versus atypical antipsychotics. Schizophr Res. 2005 Mar;73(2–5):357–66.
- 61. Smith S, Wheeler MJ, Murray R, O’Keane V. The effects of antipsychotic-induced hyperprolactinaemia on the hypothalamic-pituitary-gonadal axis. J Clin Psychopharmacol. Apr 2002;22(2):109–14. http://dx.doi.org/10.1097/00004714-200204000-00002.
- 62. Thompson K, Sergejew A, Kulkarni J. Estrogen affects cognition in women with psychosis. Psychiatry Res. 2000 Jul;94(3):201–9. http://dx.doi.org/10.1016/s0165-1781(00)00161-x.
- 63. Conway CA, Jones BC, DeBruine LM, Welling LL, Law Smith MJ, Perrett DI, et al. Salience of emotional displays of danger and contagion in faces is enhanced when progesterone levels are raised. Horm Behav. 2007 Feb;51(2):202–6.
- 64. Gur RE, McGrath C, Chan RM, Schroeder L, Turner T, Turetsky BI. An fMRI study of facial emotion processing in patients with schizophrenia. Am J Psychiatry. 2002 Dec;159(12):1992–9. http://dx.doi.org/10.1176/appi.ajp.159.12.1992.
- 65. van’t Wout M, Van Dijke A, Aleman A, Kessels RPC, Pijpers W, Kahn RS. Fearful faces in schizophrenia: the relationship between patient characteristics and facial affect recognition. J Nerv Ment Dis. 2007 Sep;195(9):758–64.
- 66. Fusar-Poli P, Borgwardt S, Crescini A, Deste G, Kempton MJ, Lawrie S, et al. Neuroanatomy of vulnerability to psychosis: a voxel- based meta-analysis. Neurosci Biobehav Rev. 2011 Apr;35(5):1175–85.
- 67. Phillips ML, Drevets WC, Rauch SL, Lane R. Neurobiology of emotion perception I: the neural basis of normal emotion perception. Biol Psychiatry. 2003 Sep;54(5):504–14. http://dx.doi.org/10.1016/s0006-3223(03)00168-9.
- 68. Osterlund MK, Keller E, Hurd YL. The human forebrain has discrete estrogen receptor alpha messenger RNA expression: high levels in the amygdaloid complex. Neuroscience. 2000;95(2):333–42. http://dx.doi.org/10.1016/s0306-4522(99)00443-1.
- 69. Guerra-Araiza C, Cerbon MA, Morimoto S, Camacho-Arroyo I. Progesterone receptor isoforms expression pattern in the rat brain during the estrous cycle. Life Sci. 2000 Mar;66(18):1743–52. http://dx.doi.org/10.1016/s0024-3205(00)00497-5.