Introduction
Music is a ubiquitous component of human life, so that the “power” of music is perceived and experienced by human being, more or less consciously, in almost every moment of their life, since we constantly live immersed in sounds. Several studies investigated the potential of music and sounds on human cognition and social capabilities (e.g., []). In the last decades, the idea that music can be used for therapeutic purposes led to the birth of a new discipline: music therapy []. According to the psychiatrist and musician Rolando Omar Benenzon, music therapy is a psychotherapy technique in which sound, music, and musical instruments are used to foster a process of connection between the therapist and the patient or the patients’ group, with the aim of improving the quality of life and rehabilitating and recovering patients’ footing in society []. As a matter of fact, the discipline of music therapy has been developed since antiquity [].
William James defined our “susceptibility to music” []: music affects the human being relaxing, animating, comforting, exciting, and also representing a therapeutic potential in patients with different neurological conditions (e.g., [-]). Music has always been associated with the treatment of mental illness, given the potential of music in reducing anxiety, stress, tension, and pain (e.g., [-]). However, most studies only refer to self-reported information provided by the patients; therefore, potential associations between subjective and objective parameters – such as physiological values – remain to be deeply evaluated to show more reliable results and foster the use of music as a standardized method of intervention for anxiety and pain relief.
Over the years, various sound- and music-based treatments have been developed with different purposes (from relaxation to performance improvement, up to more complex therapeutic objectives). Examples of techniques are psychophony (sound is used as a direct factor of intervention), sound therapy (the diapason is used to stimulate certain points of the body), sound massage (an instrument is held close to various body areas to “massage” the body), and therapeutic listening (personal listening of classical music). Concerning the latter, a specific enhancement of cognitive skills has been described and it has been defined as the “Mozart effect” [, ].
The “Mozart Effect”
A study that appeared in 1993 described how listening to the Sonata for two pianos in W. A. Mozart’s D Major K 448 induced an improvement in cognitive learning []. Authors suggested that Mozart’s music helps organize neuronal circuits of the cerebral cortex (mainly the organization of firing pattern of the right hemisphere processes of spatiotemporal tasks performance) and strengthen the creative processes of the right hemisphere of the brain, predominantly associated with spatial abilities. Since then, the enhancement of cognitive performance induced by music exposition is known as the “Mozart effect.” This finding sparked a series of studies that investigated the cognitive effects of classical music more specifically. For a detailed description of these studies, see Figure 1 [, -]. All in all, the findings provide compelling evidence to previous experiments that the Mozart effect can be an artifact of arousal and mood [, , ].

Fig. 1
An overview of the main studies on the Mozart’s effect and its possible components.
Psychophysiology and Music
Generally, musical stimuli and sound stimuli can arouse organic and psychological manifestations of the dynamic functions of the human being []. Music cannot be separated from perceptual, symbolic, and personal processes []. Indeed, music can stimulate central emotions in the brain and release biochemical signals that change the physiological state [], as revealed, e.g., by heart rate (HR), blood pressure, and skin conductance. Several cardiac and neurological functions, as well as biochemical measurable effects, are triggered by different kinds of music []. For example, sedative and arousal music, compared to the silence condition, influenced the electrical function of the heart with a transitory effect on heartbeat and electrocardiographic signals []. Fried [] pointed out those factors affecting the relationship between the autonomic nervous reactions and music, such as age, sex, life-style, physical fitness, health, temporary states induced by alcohol, coffee, emotional factors, attitudes toward music and its role in one’s life, and attitude toward the musical selection. McClellan [] identified some features of pancultural music that seemed to be useful to calm and reduce tension, such as the rhythm of the music. Beyond heartbeat, HR variability (HRV) is related to neurocardiac function and is generated by heart-brain interactions and processes of the dynamic nonlinear autonomic nervous system (ANS) []. For details, see Figure 2 [-].

Fig. 2
A critical summary of current interpretations and possibilities offered by HRV metrics.
In addition to heartbeat system, some authors focused on breath as a response system and in the context of relaxation and stress techniques []. Indeed, some forms of music have been reliably shown to have a profound beneficial effect on breathing []. Abnormal respiratory rate (greater than 20 breaths per minute) indicates physical discomfort and can be an important predictor of serious clinical events [, ]. Koelsch and Jäncke [] summarized the effect of music on cardiorespiratory system, pointing out that music reduces HRV (in particular exciting music) and increases heart and respiratory rate (in particular exciting and/or pleasant music).
Purposes
The hypothesis that short-term musical experiences may have a direct causal influence on some cognitive functions is important for both practical and theoretical reasons []. The main effect of Mozart music has been argued to lie mainly with mood alteration due to music’s structure. However, focusing on the cognitive products of Mozart music has left a gap on the knowledge related to the process, i.e., the physiology during listening. The use of music in relaxation techniques is increasingly widespread since its use can induce relaxation and changes in the activity of the ANS, despite not much evidence of its psychophysiological effects have been collected so far. The multifaceted nature of emotional outcome produced by relaxing music is complex as it can provoke feelings that are usually, but not necessarily, positively toned and related to peacefulness and can also provoke feelings generally associated with an increase in arousal []. Within this framework, the concept of time, sound, and melody is fundamental to investigate whether and which sound elements can influence the psychological and physiological state in humans. Tempo can be the most significant feature affecting arousal []. Therefore, the main aim of this study was to identify a differential response to short-term exposure to Mozart music or to its Destructured – by sound and melody – components.
Beyond the most used Mozart’s Sonata K 448, other pieces of Mozart’s music have possible structural components affecting psychophysiology. To be used in educational or therapeutic setting, pieces for one piano – rather than two as in K 448 – can be better feasible for being played live by operators. Therefore, we used a Sonata for one piano with relaxing features to investigate the presence of changes both in subjective and objective parameters during listening. We hypothesized a positive mood effect of Mozart music and different relaxing effect of listening to Mozart’s music, as measured by both self-reported mood state and dynamics of heart rhythm, compared to the same piece but consisting only of beat, and to a control condition (silence).
Materials and Methods
Participants
This work is the first part of the “HRV & Breath Tune” project, aimed to identify a physiological signature of individual responses to different musical stimuli, to be linked with neuropsychological and behavioral variables. The whole sample consisted of 25 Western volunteers, mainly university students, aged 21–34 years, whose 68% (n = 17) were females and 32% (n = 8) were males. Exclusion criteria were therapy with any drugs capable to interfere with cardiorespiratory function, neurosis, psychosis, mood dysfunction, cardiovascular and respiratory disease. The 40% of participants reported none or poor musical competence, the 48% reported good competence, and the remaining 12% advanced competence (conservatory or other advanced musical courses attended). The study was conducted with a cross-over design.
Stimuli and Procedures
In three separate sessions, each participant listened to (i) the Mozart’s Piano Sonata in A major K 331 (Mozart), (ii) the same piece consisting only of beat (Destructured), and (iii) silence (Control). The whole “Sonata K 331” is composed of three movements, all of which are in the key of A major or minor: this type of composition is called “homotone.” The specific part of the piece used was constituted by the first movement “Go graceful” (6′48′ to 13′05′), characterized by the tempo “Andante,” that is part of the “slow tempo” group (56–108 bpm). The rhythm is in 6/8 and the piece can be defined as calm and with a lulling trend. The first movement of this Sonata is characterized by a theme repeated in 6 variations. The musical extract used referred to the III, IV, and part of the V variation. The third variation, in a minor key (A minor), is characterized by rhythmically regular notes that proceed by joint degree. In the fourth variation, the piece returns to the major key. In the V variation, unlike the III and IV, the theme is expanded; in fact, the tempo marking is “Adagio” (48–97 bpm). The music piece was chosen by a panel of pianists, due to the feasibility in therapeutic and leisure setting (it is a sonata for one piano) and for the rhythmic and melodic signature, adequate for the requirements of this project. The registered version of Daniel Barenboim was used.
The three conditions were carried out in three diverse days, carried out 5 to 10 days apart and in a random order. The psychological measurements were collected by a psychologist who administered the Italian version of the Depression Anxiety Stress Scales-21 (DASSs-21) that was administered before the first session, and the Italian Mood Scale (ITAMS) was administered immediately before and after each listening. A physiological measurement – cardiorespiratory assessment – was conducted during each listening session; both a psychologist and a physiologist set the physiological data acquisition.
The contextual variables were standardized and all the stimuli were administered to the volunteers lying in a supine position on a clinical bed. The experimenters ensured the experimental experience was always comfortable. The light was always kept at a low level and the temperature was maintained comfortable and stable across conditions. Volunteers stayed alone into the room. The room was not totally soundproofed, but in all the three conditions participants wore the same headphones (JVC HA-RX330) to be sensory isolated from the external world, thus avoiding possible biases in experimental conditions (to see an example of the procedural setting, see the image on online suppl. materials; for all online suppl. material, see http://www.karger.com/doi/10.1159/000525360). All the three listening conditions lasted about six and a half minutes.
Psychological Variables
The DASS-21 [] is a 21-item questionnaire consisting of three subscales measuring anxiety, depression, and stress; produces a total score showing the general distress, already validated in Italian language []. The final scores range from 0 to 21 for each subscale. Participants are required to select how well they agree with each statement for the past 7 days, using a 4-point Likert scale: 0 = not at all, 1 = somewhat, 2 = quite often, and 3 = always. The questionnaire was developed to account for a robust differentiation between depression and anxiety.
The ITAMS [] is the Italian version of the Brunel Mood Scale [] which consists of 24 descriptors for evaluating 6 dimensions of mood: anger, confusion, depression, fatigue, tension, and vigor. For each mood descriptor, participants are required to indicate how they feel on a 5-point Likert scale, from 0 = not at all to 4 = extremely. To the aim of this study, only the four sub-scales of anger, fatigue, vigor, and tension were considered.
Physiological Variables
From the ECG signal recording, the identification of R-R intervals (as the time elapsed between two successive peaks) allows the analysis of HRV metrics by the mean of time-domain, as standard deviation of normal R-R intervals (SDNN), and percentage of successive R-R intervals that differ by more than 50 ms (pNN50); frequency-domain, as absolute low-frequency (LF) and high-frequency (HF) power and their ratio (LF/HF); nonlinear parameters, as cardiac sympathetic index (CSI) and cardiac vagal index (CVI) from scatter plots, and Higuchi fractal dimension (HFD). Briefly, SDNN in short-term recording mostly reflects the parasympathetically mediated variations, pNN50 is associated with the activity of parasympathetic nervous system, HF is considered an index of parasympathetic regulation, while LF is modulated by both sympathetic and parasympathetic branches; nonlinear indices are used to capture fluctuations of heart rhythm across multiple time scales, accounting for a more adaptable and flexible system [, ].
The acquisition system consisted of a lightweight (≈50 g) chest strap developed by the National Research Council of Italy (CNR), based on a single-lead Shimmer2ECG sensor (Shimmer Sensing, Dublin, Ireland), with a sampling frequency of 500 Hz, in order to allow the estimation of the relevant HRV features according to the guidelines []. The sensor was attached to the volunteer’s body through a fitness-like chest strap (Polar Electro Oy, Kempele, Finland) avoiding discomfort for the participant.
Breaths per minute, breathing flow amplitude, and peripheral saturation (SpO2) were measured by a sleep apnea screening device (1-s intervals) attached to a finger [] and a nasal rubber probe (APN-100; Contec Medical Systems Co., China). The device measured SpO2 in a range of 70∼100% with a precision of 2% and breaths per minute in a range of 0∼40 rpm with a precision of 2 rpm.
Data Analysis
ECG signals were saved as .csv files and further processed in MATLAB (MathWorks, Inc., Natick, MA, USA) in a stepwise process: Kalman filter, IIR filter (order 29 and bandwidth 25–35 Hz), and parabolic interpolation. Then, the Pan-Tompkins method for the detection of QRS complex (the combination of three graphical deflections seen on an ECG) and the parametric autoregressive Yule-Walker model (order 9) for the calculation of power spectrum density were applied. The LF (0.04–0.15 Hz) and HF (0.15–0.40 Hz) power bands were estimated.
Statistical analyses were conducted with the software Jamovi version 1.2.5.0 (retrieved from https://www.jamovi.org). Assumption check consisted of Shapiro-Wilk’s test for normality of distribution, skewness and kurtosis calculation, Q-Q plots observation. A series of repeated measures analysis of variance (eventually with sphericity correction with the Greenhouse-Geisser method after Mauchly’s test), or the nonparametric analogous, i.e., Friedman’s test, was conducted to compare the physiological parameters by the three listening conditions. Considering the assumption check, psychological results were compared between phases with the paired sample Wilcoxon signed-rank test. For testing the association of psychological and physiological variables, first the percent change from pre- to poststimulus was computed as for the four variables resulted from ITAMS questionnaire; then, a series of nonparametric correlations were conducted separating for each of the three conditions; finally, an additional series of nonparametric partial correlations were conducted adjusting for the variable general distress as resulted from DASS-21 questionnaire; associations were considered if at least moderate (rho ≥ 0.4). Significance level was set for p < 0.05; greater values were possibly interpreted as tendencies on the basis of the effect sizes.
Results
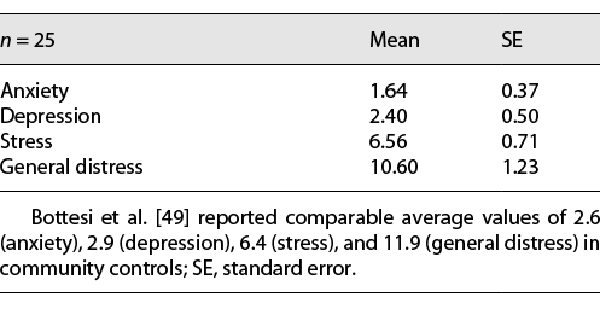
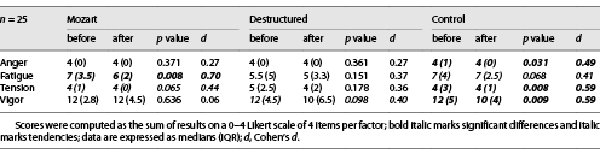
Participants were overall not affected by excessive values of general distress, anxiety, depression, and stress, as resulted from the DASS-21 data (see Table 1) compared to the data reported in previous studies []. As well, breathing rate [] and SpO2 were overall in a normal range. Among the four dimensions of the ITAMS questionnaire taken into account, anger, tension, and vigor were significantly reduced after Control condition, whereas no significance nor tendency was found after Destructured listening about the reduction of fatigue and tension, and a strong tendency emerged for the reduction of vigor. After Mozart listening, fatigue was significantly reduced (and a tendency emerged also for tension), whereas vigor was not affected (see Table 2).
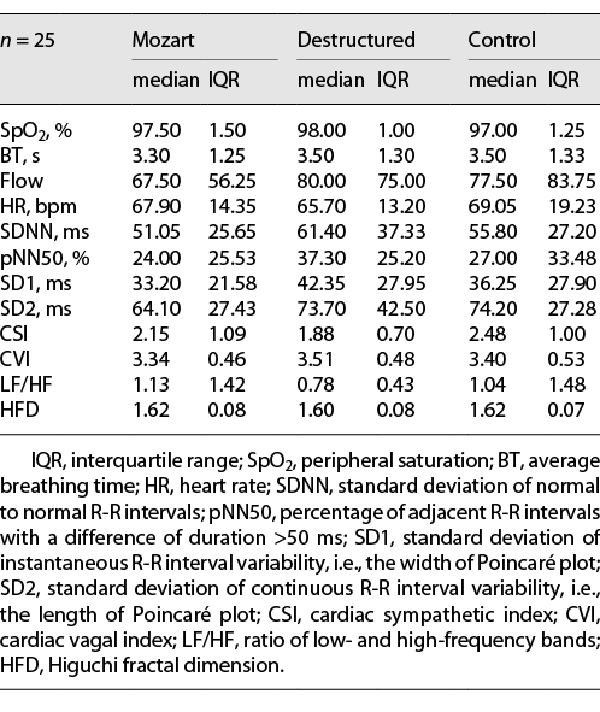
Comparing the measures acquired during each condition, no significant differences emerged for SpO2 (χ2(2) = 3.11, p = 0.212) and breathing flow (χ2(2) = 1.95, p = 0.378). Although almost statistically trivial, a difference emerged for breathing rate, which was higher during Mozart listening than in the other conditions (χ2(2) = 4.15, p = 0.126, η2p = 0.09). HR did not change by listening condition (F2, 34 = 0.66, p = 0.524), as well as the HRV metrics of SDNN (F2, 32 = 1.63, p = 0.218) and pNN50 (χ2(2) = 2.73, p = 0.255). Among nonlinear metrics derived from Poincaré plots, nonsignificant differences were found for both SD1 (F2, 34 = 2.56, p = 0.092, η2p = 0.13) and SD2 (F2, 34 = 2.16, p = 0.130, η2p = 0.11) with lower values during Mozart listening; instead, neither CSI (F2, 34 = 1.67, p = 0.204) nor CVI (F2, 34 = 1.11, p = 0.331) did change across conditions. LF/HF did not significantly change, (χ2(2) = 1.00, p = 0.607). The nonlinear parameter HFD, even though not significantly, was slightly lower during Destructured listening (F2, 34 = 2.54, p = 0.117, η2p = 0.13) (see Table 3).
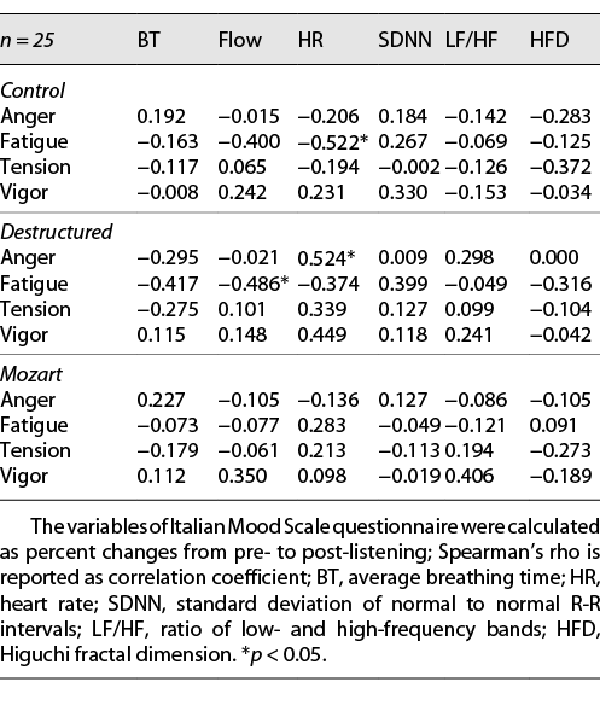
Partial correlations (see Table 4) revealed that in Control condition fatigue change was significantly associated with HR (rho = −0.552 and p = 0.021), and tendencies were found for fatigue change with breathing flow (rho = 0.400 and p = 0.059) and for tension change with HFD (rho = −0.372 and p = 0.073); controlling for general distress, the association of fatigue change with HR was mitigated (rho = −0.449 and p = 0.036), while the association of tension change with HFD was exacerbated (rho = −0.472 and p = 0.023). In Destructured condition, significant associations were found for anger change with HR (rho = 0.524 and p = 0.021) and for fatigue change with breathing flow (rho = −0.486 and p = 0.048) and tendencies for fatigue change with breathing rate (rho = −0.417 and p = 0.096) and for vigor with HR (rho = 0.449 and p = 0.054); after controlling for general distress, only trivial differences occurred. In Mozart condition, only a tendency in the association of vigor change with LF/HF was found (rho = 0.406 and p = 0.061); after controlling for general distress, only trivial differences occurred.
Discussion
The results of our study show that listening to Mozart did not empower the relaxation – neither subjective nor objective – in healthy young adults. In other words, Mozart’s Sonata in A major K 331 did not empower a contextual set which per se produces relaxation.
It was advocated to better define the influence of sound, noise, and music on psychological and physiological variables []. In our study, listening to the beat or silence reduced the vigor subscale, whereas listening to Mozart’s Sonata did not. We did not find any significant difference for SpO2, HR, SDNN, PNN50, CSI, CVI, LF/HF across the conditions but tendencies for SD1 and SD2 – with lower values listening to Mozart’s Sonata – and for HFD, with higher values during control. Breathing volume did not differ across conditions, and a tendency was found for breath frequency, with higher values when listening to Mozart. Almost surprisingly, we may affirm that Control was the most relaxing condition, and Mozart’s Sonata slightly supported arousal most of all. Orini and colleagues [] also reported silence to be the most relaxing condition assessing a panel of heart, breath, and HRV metrics to depict the changes while listening to pleasant or unpleasant music, compared to silence.
The musical components more conducive to relaxation (considered in terms of relaxing music for anxiety control and not relaxing music for any purpose), in descending order, can be listed as follows: tempo, melody, beat, harmony, rhythm, liking, complexity, key, scale, articulation, interval, melodic range, and familiarity []. Slow attacks, low rhythmic complexity, and low dynamic range define relaxing music []. The physiological relaxed state may be triggered by music with slow tempo, mellow timbre, and soft volume []. The most used piece for investigating the Mozart effect is Sonata K448, made up of three tempos, all in a major key: Allegro con spirito (D major), Andante (G major), and Allegro molto (D major). The first and third tempo are part of the “fast tempo” group (about 84–168 bpm), the second beat slows down for a while (Andante); the sensation that the piece arouses in the listener is of joy and cheer. The piece used for this study, extracted from Sonata K 331, has a moderately slow tempo and a lulling rhythmic and melodic trend and arouses calm; therefore, its characteristics, despite several musical variations, are more prone to a relaxing piece than K 448. Nonetheless, it produces a positive effect on mood, maintaining arousal compared to only beat or silence.
Considering the psychophysiological association, we argue that there was not a necessary entanglement between psychological effects and physiological dynamics of cardiovascular system. This is in line with other findings, such as those of Hernandez-Ruiz ad colleagues [], although they considered only the end of listening; they also argued that the profile of responders versus nonresponders may drive the results. The reasons for what physiological measures do not necessarily parallel psychological responses may lie on conscious interpretations of emotion, on the nondichotomous response of arousal or relaxation, and on the plethora of metrics of interest. The variations in mood variables in response to listening conditions are only partially associated with physiological features; those with higher HR encountered a higher reduction in fatigue, but only in Control condition, partially mitigated when controlling for general distress. This makes sense as fatigue change and general distress were negatively correlated (rho = −0.526, p = 0.008), i.e., the more stressed you are, the more you benefit of a relaxing condition. This was also revealed by the negative association of HFD with tension change, i.e., the reduction in tension was underlain by higher fluctuations of heart rhythm. In Destructured condition, the greater the reduction in fatigue, the more relaxed the breath, and the higher the HR, the lesser the reduction of anger and vigor. In Mozart condition, the higher the LF/HF ratio, the lesser the reduction of vigor. Therefore, each condition was underlain by meaningful, but unique, psychophysiological signatures. Id est, each condition was defined by a specific psychophysiological associative signature, possibly resulted from a different involvement of autonomic pathways.
As a limit of this study, it would have been helpful to contrast two or more different pieces of music balanced for emotionality on the basis of tempo and melody; the duration of the musical stimulus, lower than most of similar studies, limits the comparison of current findings with literature evidence. It would have been helpful to compare the psychophysiological response with personality, empathy, music preference, anthropometric variables, and basal status of ANS. For example, introverts can benefit from fast-tempo music for tasks requiring greater mental effort, while extroverts contrarily can predispose themselves to excessive risks []. Similar responder profile differences may lead to several psychophysiological outcomes when listening in relaxing conditions. Sensitivity to music features vary whether listeners are trained musicians []; although we assessed the level of musical competence, the percentage of volunteers with advanced competence (conservatory or other advanced musical courses attended) was too low for using this variable for statistics. Even though HRV complex metrics represent an intriguing hallmark of stress, disease, and aging, caveats should be highlighted about the use of HRV diverse metrics for feasible applications, as large misuse and overuse flaw such indexes.
Conclusions
Considering the potential of music in affecting neuropsychological and behavioral variables, we suggest that an individual approach to music should drive the music as medicine []. A very interesting example concerns the genetic regressive sounds, causing greater regressive effects in the human being than any other sound, more or less constant and above all independently of the pathology or individual characters. One of these sounds is the heartbeat []: infants exposed for 4 days to a heartbeat-like noise cried less and gained more weight than the control group; newborns in the womb undergo an auditory imprinting of the mother’s heartbeat probably causing cascading associative learning mechanisms lasting beyond early life []. More generally, this finding adds important evidence in the dynamic frame of the nature versus nurture preference for sounds and music (e.g., []). It would be interesting to furtherly investigate whether and how the psychophysiological effect induced by auditory stimulation could refer to its similarity to “biological musicality.” In this regard, sound identity (ISO) refers to the set of sounds that characterizes each human being []. As Benenzon argues, music therapy acts through our ISO, which is represented by sound, acoustic, and movement energies [, ]. The ISO approach may represent a music-based application of the personalized therapy model, which accounts for an ad personam modeling of dynamic processes and aims to increase both rapidity and effectiveness of psychopathological therapies []. The analysis of sonogram and spectrograms linked to individual features, as already proposed for psycholinguistic structures [], may unveil novel insights within this framework. All in all, further efforts are needed to extend the insights on the complex link between music and psychology, considering the effect of music on behavioral responses and personality traits [].
We have argued that listening to Mozart’s music may stimulate arousal even during relaxing conditions. Objective and subjective measures of relaxation represent a complex set of variables, not necessarily entangled. Finally, we advocated for further ad personam musical pieces, basing on physiological individual signature, to produce tailored musical psychotherapy.
Statement of Ethics
The research was conducted ethically in accordance with the World Medical Association Declaration of Helsinki and followed the ARRIVE guidelines. Ethics approval was not required in accordance with national guidelines as reported in the Italian Ministry of Health circular n. 6, 2002, and the Italian Legislative Decree n. 211/2003, which define interventional and noninterventional trials that are subjected to the ethical approval; this study does not involve patients, children, or animals, as well as drugs, genetic samples, or invasive techniques. Written informed consent to participate in the study was obtained from all the participants after a full explanation of the procedures.
Conflict of Interest Statement
The authors have no conflicts of interest to declare.
Funding Sources
This research was supported by the “Departments of Excellence 2018–2022” initiative of the Italian Ministry of Education, University and Research for the Department of Neuroscience, Imaging and Clinical Sciences (DNISC) of the University of Chieti-Pescara.
Author Contributions
Conceptualization: Margherita Di Cesare, Danilo Bondi, and Tiziana Pietrangelo; methodology: Margherita Di Cesare, Alessandro Tonacci, and Danilo Bondi; formal analysis: Alessandro Tonacci and Danilo Bondi; investigation: Margherita Di Cesare and Danilo Bondi; writing – original draft: Margherita Di Cesare, Alessandro Tonacci, Danilo Bondi, Giulia Prete, and Gianluca Malatesta; writing – review and editing: Margherita Di Cesare, Alessandro Tonacci, Danilo Bondi, Vittore Verratti, Giulia Prete, Gianluca Malatesta, and Tiziana Pietrangelo; funding acquisition: Tiziana Pietrangelo; resources: Alessandro Tonacci, Danilo Bondi, Vittore Verratti, and Tiziana Pietrangelo; and supervision: Vittore Verratti and Tiziana Pietrangelo.
Data Availability Statement
The data that support the findings of this study are not publicly available, as the key point of the procedure (the musical stimuli) is not owned by the authors, but are available, along with physiological time series and psychological results, from the corresponding author DB upon reasonable request.
References
- 1. Schulkin J, Raglan GB. The evolution of music and human social capability. Front Neurosci. 2014 Sep;8:292. http://dx.doi.org/10.3389/fnins.2014.00292.
- 2. Edwards J. The Oxford handbook of music therapy. Oxford University Press; 2016.
- 3. Benenzon RO. Music therapy manual. C.C. Thomas; 1981.
- 4. Horden P. Music as medicine: the history of music therapy since antiquity. Routledge; 2017.
- 5. Sacks O. Musicophilia: tales of music and the brain. Knopf; 2014.
- 6. Jung YH, Lee S, Kim WJ, Lee JH, Kim MJ, Han HJ. Effect of integrated cognitive intervention therapy in patients with mild to moderate Alzheimer’s disease. Dement Neurocogn Disord. 2020 Sep;19(3):86–95. http://dx.doi.org/10.12779/dnd.2020.19.3.86.
- 7. Moreno-Morales C, Calero R, Moreno-Morales P, Pintado C. Music therapy in the treatment of dementia: a systematic review and meta-analysis. Front Med. 2020;7:160. http://dx.doi.org/10.3389/fmed.2020.00160.
- 8. Štillová K, Kiska T, Koriťáková E, Strýček O, Mekyska J, Chrastina J, et al. Mozart effect in epilepsy: why is Mozart better than Haydn? Acoustic qualities-based analysis of stereoelectroencephalography. Eur J Neurol. 2021 Feb.
- 9. Dai WS, Huang ST, Xu N, Chen Q, Cao H. The effect of music therapy on pain, anxiety and depression in patients after coronary artery bypass grafting. J Cardiothorac Surg. 2020 May;15(1):81. http://dx.doi.org/10.1186/s13019-020-01141-y.
- 10. Low MY, Lacson C, Zhang F, Kesslick A, Bradt J. Vocal music therapy for chronic pain: a mixed methods feasibility study. J Altern Complement Med. 2020 Feb;26(2):113–22. http://dx.doi.org/10.1089/acm.2019.0249.
- 11. Najafi Ghezeljeh T, Mohades Ardebili F, Rafii F, Haghani H. The effects of patient-preferred music on anticipatory anxiety, post-procedural burn pain and relaxation level. Eur J Integr Med. 2017 Jan;9:141–7. http://dx.doi.org/10.1016/j.eujim.2016.12.004.
- 12. Nyashanu M, Ikhile D, Pfende F. Exploring the efficacy of music in palliative care: a scoping review. Palliat Support Care. 2021 Jun;19(3):355–60. http://dx.doi.org/10.1017/S1478951520001042.
- 13. Wulff V, Hepp P, Fehm T, Schaal NK. Music in obstetrics: an intervention option to reduce tension, pain and stress. Geburtshilfe Frauenheilkd. 2017 Sep;77(9):967–75. http://dx.doi.org/10.1055/s-0043-118414.
- 14. Ezzu A, Messaglia R. Introduzione alla musicoterapia. Storia, fondamenti, modelli, applicazioni cliniche, glossario. Musica Practica. 2006.
- 15. Rauscher FH, Shaw GL, Ky CN. Music and spatial task performance. Nature. 1993 Oct;365(6447):611. http://dx.doi.org/10.1038/365611a0.
- 16. Rauscher FH, Shaw GL, Ky KN. Listening to Mozart enhances spatial-temporal reasoning: towards a neurophysiological basis. Neurosci Lett. 1995 Feb;185(1):44–7. http://dx.doi.org/10.1016/0304-3940(94)11221-4.
- 17. Rideout BE, Dougherty S, Wernert L. Effect of music on spatial performance: a test of generality. Percept Mot Skills. 1998 Apr;86(2):512–4. http://dx.doi.org/10.2466/pms.1998.86.2.512.
- 18. Nantais KM, Schellenberg EG. The Mozart effect: an artifact of preference. Psychol Sci. 1999 Jul;10(4):370–3. http://dx.doi.org/10.1111/1467-9280.00170.
- 19. Pietschnig J, Voracek M, Formann AK. Mozart effect-Shmozart effect: a meta-analysis. Intelligence. 2010;38(3):314–23. http://dx.doi.org/10.1016/j.intell.2010.03.001.
- 20. Steele KM, Bass KE, Crook MD. The mystery of the Mozart effect: failure to replicate. Psychol Sci. 1999 Jul;10(4):366–9. http://dx.doi.org/10.1111/1467-9280.00169.
- 21. Lange-Küttner C, Rohloff S. Mozart Sharpens and Mahler degrades the word memory trace. Adv Res Psychol. 2020 May;1(1):13091.
- 22. Kenealy PM. Mood-state-dependent retrieval: the effects of induced mood on memory reconsidered. Q J Exp Psychol A. 1997;50(2):290–317. http://dx.doi.org/10.1080/713755711.
- 23. Spies K, Hesse F, Hummitzsch C. Mood and capacity in Baddeley’s model of human memory. Z Für Psychol Mit Z Für Angew Psychol. 1996;204(4):367–81.
- 24. Thompson WF, Schellenberg EG, Husain G. Arousal, mood, and the Mozart effect. Psychol Sci. 2001 May;12(3):248–51. http://dx.doi.org/10.1111/1467-9280.00345.
- 25. Chabris CF. Prelude or requiem for the “Mozart effect”?Nature. 1999 Aug;400(6747):826–8. http://dx.doi.org/10.1038/23608.
- 26. Schellenberg EG. Cognitive performance after listening to music: a review of the Mozart effect. Music, health, and wellbeing. New York, NY, USA: Oxford University Press; 2012. p. 324–38.
- 27. Taylor IA, Paperte F. Current theory and research in the effects of music on human behavior. J Aesthet Art Crit. 1958;17(2):251–8. http://dx.doi.org/10.1111/1540_6245.jaac17.2.0251.
- 28. Fancourt D, Ockelford A, Belai A. The psychoneuroimmunological effects of music: a systematic review and a new model. Brain Behav Immun. 2014 Feb;36:15–26. http://dx.doi.org/10.1016/j.bbi.2013.10.014.
- 29. Cervellin G, Lippi G. From music-beat to heart-beat: a journey in the complex interactions between music, brain and heart. Eur J Intern Med. 2011 Aug;22(4):371–4. http://dx.doi.org/10.1016/j.ejim.2011.02.019.
- 30. Dousty M, Daneshvar S, Haghjoo M. The effects of sedative music, arousal music, and silence on electrocardiography signals. J Electrocardiol. 2011 May;44(3):396–6. http://dx.doi.org/10.1016/j.jelectrocard.2011.01.005.
- 31. Fried R. Integrating music in breathing training and relaxation: I. Background, rationale, and relevant elements. Biofeedback Self Regul. 1990 Jun;15(2):161–9. http://dx.doi.org/10.1007/BF00999146.
- 32. McClellan R. The healing forces of music: history, theory, and practice. iUniverse; 2000.
- 33. Shaffer F, Ginsberg JP. An overview of heart rate variability metrics and norms. Front Public Health. 2017 Sep;5:258. http://dx.doi.org/10.3389/fpubh.2017.00258.
- 34. Sturmberg JP, Bennett JM, Picard M, Seely AJE. The trajectory of life. Decreasing physiological network complexity through changing fractal patterns. Front Physiol. 2015 Jun;6:169. http://dx.doi.org/10.3389/fphys.2015.00169.
- 35. West BJ. A mathematics for medicine: the network effect. Front Physiol. 2014 Dec;5:456. http://dx.doi.org/10.3389/fphys.2014.00456.
- 36. Mojtabavi H, Saghazadeh A, Valenti VE, Rezaei N. Can music influence cardiac autonomic system? A systematic review and narrative synthesis to evaluate its impact on heart rate variability. Complement Ther Clin Pract. 2020 May;39:101162. http://dx.doi.org/10.1016/j.ctcp.2020.101162.
- 37. Perez-Lloret S, Diez J, Domé MN, Delvenne AA, Braidot N, Cardinali DP, et al. Effects of different “relaxing” music styles on the autonomic nervous system. Noise Health. 2014 Oct;16(72):279–84. http://dx.doi.org/10.4103/1463-1741.140507.
- 38. Thomas BL, Claassen N, Becker P, Viljoen M. Validity of commonly used heart rate variability markers of autonomic nervous system function. Neuropsychobiology. 2019 Feb;78:14–26. http://dx.doi.org/10.1159/000495519.
- 39. Gomes RL, Vanderlei LCM, Garner DM, Vanderlei FM, Valenti VE. Higuchi fractal analysis of heart rate variability is sensitive during recovery from exercise in physically active men. Med Express. 2017;4(2). http://dx.doi.org/10.5935/medicalexpress.2017.02.03.
- 40. Dodo N, Hashimoto R. Autonomic nervous system activity during a speech task. Front Neurosci. 2019;13:406. http://dx.doi.org/10.3389/fnins.2019.00406.
- 41. Soni R, Muniyandi M. Breath rate variability: a novel measure to study the meditation effects. Int J Yoga. 2019;12(1):45–54. http://dx.doi.org/10.4103/ijoy.IJOY_27_17.
- 42. Chourpiliadis C, Bhardwaj A. Physiology, respiratory rate. In: StatPearls. Treasure Island, FL: StatPearls Publishing; 2020 [cited 2020 Apr 27]. Available from: http://www.ncbi.nlm.nih.gov/books/NBK537306/.
- 43. Cretikos MA, Bellomo R, Hillman K, Chen J, Finfer S, Flabouris A. Respiratory rate: the neglected vital sign. Med J Aust. 2008;188(11):657.
- 44. Koelsch S, Jäncke L. Music and the heart. Eur Heart J. 2015 Nov;36(44):3043–9. http://dx.doi.org/10.1093/eurheartj/ehv430.
- 45. Rauscher FH, Shaw GL. Key components of the Mozart effect. Percept Mot Skills. 1998 Jun;86(3 Pt 1):835–41. http://dx.doi.org/10.2466/pms.1998.86.3.835.
- 46. Elliott D, Polman R, McGregor R. Relaxing music for anxiety control. J Music Ther. 2011 Oct;48(3):264–88. http://dx.doi.org/10.1093/jmt/48.3.264.
- 47. Bernardi L, Porta C, Sleight P. Cardiovascular, cerebrovascular, and respiratory changes induced by different types of music in musicians and non-musicians: the importance of silence. Heart. 2006 Apr;92(4):445–52. http://dx.doi.org/10.1136/hrt.2005.064600.
- 48. Lovibond SH, Lovibond PF. Psychology foundation of Australia. Manual for the depression anxiety stress scales. Sydney, NSW: Psychology Foundation of Australia; 1995.
- 49. Bottesi G, Ghisi M, Altoè G, Conforti E, Melli G, Sica C. The Italian version of the depression anxiety stress scales-21: factor structure and psychometric properties on community and clinical samples. Compr Psychiatry. 2015 Jul;60:170–81. http://dx.doi.org/10.1016/j.comppsych.2015.04.005.
- 50. Quartiroli A, Terry PC, Fogarty GJ. Development and initial validation of the Italian Mood Scale (ITAMS) for use in sport and exercise contexts. Front Psychol. 2017 Sep;8:1483. http://dx.doi.org/10.3389/fpsyg.2017.01483.
- 51. Terry PC, Lane AM, Lane HJ, Keohane L. Development and validation of a mood measure for adolescents. J Sports Sci. 1999 Nov;17(11):861–72. http://dx.doi.org/10.1080/026404199365425.
- 52. Pham T, Lau ZJ, Chen SHA, Makowski D. Heart rate variability in psychology: a review of HRV indices and an analysis tutorial. Sensors. 2021 Jun;21(12):3998. http://dx.doi.org/10.3390/s21123998.
- 53. Malik M. Heart rate variability. Ann Noninvasive Electrocardiol. 1996;1(2):151–81.
- 54. World Health Oraganization. Pulse oximetry training manual. 2011. Available from: https://www.who.int/patientsafety/safesurgery/pulse_oximetry/who_ps_pulse_oxymetry_training_manual_en.pdf?ua=1.
- 55. Idrobo-Ávila EH, Loaiza-Correa H, van Noorden L, Muñoz-Bolaños FG, Vargas-Cañas R. Different types of sounds and their relationship with the electrocardiographic signals and the cardiovascular system – review. Front Physiol. 2018 May;9:525.
- 56. Orini M, Bailón R, Enk R, Koelsch S, Mainardi L, Laguna P. A method for continuously assessing the autonomic response to music-induced emotions through HRV analysis. Med Biol Eng Comput. 2010 May;48(5):423–33. http://dx.doi.org/10.1007/s11517-010-0592-3.
- 57. Wang WC. A study of the type and characteristics of relaxing music for college students. Proc Meet Acoust. 2014 May;21(1):035001.
- 58. Hernandez-Ruiz E, James B, Noll J, Chrysikou EG. What makes music relaxing? An investigation into musical elements. Psychol Music. 2020 May;48(3):327–43. http://dx.doi.org/10.1177/0305735618798027.
- 59. Karageorghis CI, Payre G, Howard E, Kuan W, Mouchlianitis LW, Reed N, et al. Influence of music on driver psychology and safety-relevant behaviours: a multi-study inductive content analysis. Theor Issues Ergon Sci. 2021 Nov;0(0):1–20. http://dx.doi.org/10.1080/1463922x.2021.2009933.
- 60. Salk L. Mothers’ heartbeat as an imprinting stimulus. Trans N Y Acad Sci. 1962 May;24:753–63. http://dx.doi.org/10.1111/j.2164-0947.1962.tb01441.x.
- 61. Prete G, Bondi D, Verratti V, Aloisi AM, Rai P, Tommasi L. Universality versus experience: a cross-cultural pilot study on the consonance effect in music at different altitudes. PeerJ. 2020 Jul;8:e9344. http://dx.doi.org/10.7717/peerj.9344.
- 62. Wagner G. The Benenzon model of music therapy. Nordic J Music Ther. 2007 Jan;16(2):146–7. http://dx.doi.org/10.1080/08098130709478184.
- 63. Wright AGC, Woods WC. Personalized models of psychopathology. Annu Rev Clin Psychol. 2020;16(1):49–74. http://dx.doi.org/10.1146/annurev-clinpsy-102419-125032.
- 64. Chaturvedi V, Kaur AB, Varshney V, Garg A, Chhabra GS, Kumar M. Music mood and human emotion recognition based on physiological signals: a systematic review. Multimed Syst. 2021 Apr.