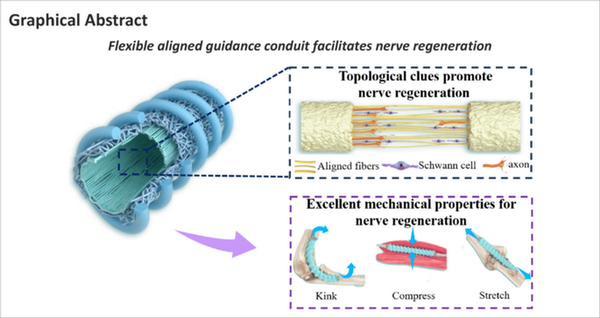
Introduction
The nervous system is a highly complex system that mediates and coordinates the host’s response to various environmental changes (Liebeskind et al., 2016). Peripheral nervous system injuries, whether caused by trauma or medical procedures, can result in pain and motor and sensory dysfunction (Lien et al., 2020; Park et al., 2020). For surgical treatment of short nerve gaps (< 5 mm), tension-free nerve anastomosis is typically sufficient, given the inherent regenerative capacity of the peripheral nervous system (Wieringa et al., 2018). Nevertheless, for longer nerve gaps, autografting using intercostal nerves or the superficial or deep peroneal nerves is the gold standard (Liu et al., 2022). However, autografting can be a “robbing Peter to pay Paul” situation, as it is associated with potential complications at the donor site such as sensory loss or pain, traumatic neuroma, and other problems (Ao, 2016; Liu et al., 2022). Artificial nerve guidance conduits (NGCs) are an alternative to autologous grafts which can overcome these limitations.
The ideal NGC for bridging damaged peripheral nerves should provide both structural and trophic support for axonal regrowth along the conduit and prevent the ingrowth of fibrous tissue (Sarker et al., 2018; Vijayavenkataraman, 2020; Yang et al., 2023; Zhao et al., 2023; Tang et al., 2024). Early NGC designs were simple hollow silicone shapes effective for short nerve gaps, which remained challenging to achieve functional regeneration (Carvalho et al., 2019). Advancements in tissue engineering and regenerative medicine have enabled the design and fabrication of NGCs with optimal mechanical, biological, and biochemical properties, which enhance nerve regeneration (Wieringa et al., 2018; Vijayavenkataraman, 2020). For instance, Chung et al. (2011) created a polycaprolactone (PCL) nerve conduit modified with nerve growth factor and tirofiban using photochemical techniques. In a 12 mm sciatic nerve defect replacement model, the mass of gastrocnemius muscle and the number of neurons in the PCL-nerve growth factor/tirofiban conduits were significantly higher than those in the pure PCL group. This finding suggests that peripheral nerve regeneration can be improved by loading bioactive factors (Chung et al., 2011). In addition, seeding cells onto the NGC promotes nerve regeneration. Zhou et al. (2020) developed such an NGC consisting of bone marrow stromal cells seeded onto a nanofiber PCL scaffold that significantly improved peripheral nerve regeneration over extended gaps of 15 mm. However, growth factor- and cell-based approaches have various drawbacks, such as short half-life, high cost, and limited cell survival time, which hinder their clinical translation.
Physical cues within NGCs such as topography, mechanical properties, and electrical properties regulate the proliferation, migration, and differentiation of nerve cells (Mohammadi et al., 2020). The primary benefit of incorporating physical cues into NGCs is their ability to regulate cellular behavior and the microenvironment in a controlled and sustained manner (Wei et al., 2023). Aligned fibers promote axonal and macrophage growth along the fibers (Yucel et al., 2010; Kim et al., 2016; Zhu et al., 2020). Quan et al. (2019a) used PCL and chitosan to create NGCs with aligned nanofiber arrangements and found that Schwann cells, PC12 cells, and dorsal root ganglion cells aggregated and promoted axonal elongation along the aligned nanofibers. Their in vivo evaluation demonstrated improved sciatic nerve function, increased gastrocnemius muscle mass, and distal nerve ultrastructural recovery (Quan et al., 2019a). These findings suggest that integrating aligned nanofibers enhances the efficacy of NGCs and their ability to effectively repair peripheral nerve defects.
Challenges in the clinical application of NGCs include lumen collapse caused by muscle extrusion (Koh et al., 2010; Sedaghati et al., 2011), suture pullout caused by movement, and inadequate flexibility to withstand deformation and kinks (Clements et al., 2016; Shahriari et al., 2017). Therefore, an ideal NGC should possess the following properties: 1) appropriate porosity (10–20 μm) to limit the growth of scar tissue while facilitating metabolite exchange; 2) provide conducive cues for nerve regeneration; 3) possess sufficient mechanical strength and flexibility to prevent collapse and avoid nerve compression as well as sufficient suture retention strength; 4) be non-immunogenic; 5) be degradable to avoid secondary removal; and 6) be readily available and suitable for sterilization (Duffy et al., 2019; Quan et al., 2019b; Zhang et al., 2020).
We have fabricated three types of NGC which resist mechanical kinking and compression and have conducive topographical features using electrospinning and melt-spinning techniques. In this study, we evaluate and compare their in vivo mechanical properties and ability to improve nerve regeneration and neuromuscular function recovery.
Methods
Animals
Thirty-two male Sprague–Dawley rats (weight, 220–250 g; age, 10–12 weeks; specific pathogen-free grade) were obtained from Beijing Vital River Laboratory Animal Technology Co., Ltd. (Beijing, China; license No. SCXK (Jing) 2021-0011). In line with previous NGC studies, male rats were selected to comprehensively assess the mechanical properties and topographically inductive capacity of NGCs for nerve regeneration, thereby eliminating any potential influence of sex differences. All rats were provided with unlimited access to food and water and housed in rooms maintained at a constant temperature (23–25°C) and humidity (45%–50%) with a 12-hour light/dark cycle. The animal experiments were approved by the Animal Ethics Committee of the Chinese PLA General Hospital (approval No. 2020-X16-26) on May 14, 2020 and were conducted in accordance with the National Institutes of Health Guide for the Care and Use of Laboratory Animals.
Fabrication of nerve guidance conduits
Three types of NGC were fabricated: NAH, NRH, and NR. The NAH consisted of aligned electrospun fibers in the lumen and helical melt-spun fibers on the outer surface. The NRH consisted of random electrospun fibers in the lumen and helical melt-spun fibers on the outer surface. The NR consisted of only random electrospun fibers. NGCs were fabricated using electrospinning and melt-spinning technology. First, a 15% (w/v) PCL solution was prepared by adding PCL pellets (Mn = 80,000; Sigma-Aldrich, St. Louis, MO, USA) into a chloroform and methanol mixture (5:1 v/v; Tianjin Chemical Reagent Company, Tianjin, China) which was stir-mixed on a magnetic stirrer for 12 hours to obtain a homogenous solution. The PCL solution was then placed in a 10 mL syringe with a stainless 21G needle. The voltage for electrospinning was set at 16 kV and the feeding rate was 4 mL/h. To obtain aligned fibers on NAH lumen, two separate stainless steel saw blades were placed 5 mm in front of the rotating stainless rod and connected to a negative high voltage power supply (2 kV). The distance between the needle and the receiving rod was 18 cm (Yucel et al., 2010; Kim et al., 2016; Zhu et al., 2020). To obtain random electrospun fibers on all three types, the negative charge was turned off and the rod continued to receive random fibers. Subsequently, melt-spinning was employed to fabricate helical fibers on the outer surface of the NAH and NRH conduits (but not the NR). Wall thickness was maintained similarly in all three types. NGCs were then dried in a vacuum for 48 hours to remove any residual solvent, immersed in 75% ethanol for 1 hour, and washed five times with physiological saline.
Characterization of nerve guidance conduits
NGC microstructure was observed using a scanning electron microscope (Phenom Pro, Phenom-World BV, Eindhoven, Netherlands) at an accelerating voltage of 15 kV after sputter coating with gold. Fiber diameter, wall thickness, and orientation of the electrospun fibers were measured using Image-Pro Plus software version 6.0 (Media Cybernetics, Rockville, MD, USA). Analysis was performed on at least three images per sample and five samples per group.
Mechanical properties and kink resistance testing
To mimic the in vivo environment, NGC samples were incubated in phosphate buffered saline at 37°C for 12 hours before mechanical testing. Compression and tensile tests were conducted as described in a previous report (Quan et al., 2019a) using a mechanical testing machine (BOSS 5100, Flemingham, MA, USA). For compression testing, the NGC was compressed by 1000 cycles and 50% strain radial compressive stiffness. For longitudinal tensile testing, two machine clamps were placed 1.5 cm apart on the NGC and the samples pulled longitudinally at a crosshead speed of 60 mm/min until rupture. Young’s modulus was calculated based on the slope of the stress-strain curve of the elastic region. The maximum strain and stress at rupture were also recorded. Additionally, suture retention strength was measured using an 8-0 suture, which was passed through the NGC 2 mm away from the edge, looped, and secured with three knots. To analyze kink resistance, a flexible steel wire was passed through the NGC and bent until a kink was observed in the NGC. The bending angle was defined as the angle of the bent wire.
Cell evaluation in vitro
PC12 cells (obtained from Beijing Xiehe Cell Resource Center, Beijing, China; RRID: CVCL_0481; identified by Procella (Wuhan, China)) were cultured in Dulbecco’s modified Eagle medium (Gibco Life Technologies, Grand Island, NY, USA) supplemented with 10% fetal calf serum (Gibco Life Technologies) and 1% penicillin/streptomycin solution (HyClone, Logan, UT, USA) in an incubator at 37°C with 5% CO2. The random and aligned electrospun fiber scaffolds were cut into circular pieces, sterilized using 75% ethanol, and exposed to UV radiation for 12 hours. Then, after the scaffolds were washed with phosphate buffered saline six times, PC12 cells were seeded onto them at a density of 1.0 × 104 cells/cm2. Cell proliferation of the seeded cells was evaluated at 1, 3, and 5 days using the Cell Counting Kit-8 assay (Beyotime Biotechnology, Beijing, China). The morphological evaluation of PC12 cells on the different scaffolds was conducted at 3 days. After fixation by 4% paraformaldehyde, phalloidin-Alexa Fluor 488 (Sigma-Aldrich, St. Louis, MO, USA) and 4′,6-diamino-2-phenylindole (DAPI, Sigma-Aldrich) staining was conducted to visualize cytoskeleton organization.
Fluorescence images were obtained using a laser scanning confocal microscope (Leica Microsystems, Heidelberg, Germany). To observe the morphology of the cells attached to the scaffold, the cells were fixed using a 2.5% glutaraldehyde solution after washing three times with phosphate buffered saline. Dehydration using graded ethanol was then performed and the samples examined using a scanning electron microscope. Three 2-day-old Sprague-Dawley rats (purchased from Beijing Vital River Laboratory Animal Technology Co., Ltd.) were anesthetized using inhalational isoflurane, sterilized with 75% alcohol, and decapitated. A dorsal root ganglion (DRG) was explanted and cultured on both aligned and random fibrous scaffolds using serum-free Dulbecco’s modified Eagle medium/F12 medium for 3 days. Immunofluorescence staining was then performed. Axonal length of DRG explants was measured using ImageJ 1.53e software (National Institutes of Health, Stapleton, NY, USA; Schneider et al., 2012). Average length of the 15 longest axons was used to evaluate neurite outgrowth.
In vivo implantation procedures
Thirty-two healthy adult male Sprague–Dawley rats were randomly divided into NAH, NRH, NR, and autograft (positive control) groups (8 rats each). The rats were anesthetized using intraperitoneal 1% sodium pentobarbital (40 mg/kg). The left sciatic nerve was then exposed and a 10 mm nerve defect created. The nerve defect was bridged using a group-appropriate NGC secured with 9-0 sutures or 10 mm autologous nerve graft.
Analysis of explanted samples
NGCs were harvested 4 weeks after implantation and then fixed in 4% paraformaldehyde for 24 hours. Four of the eight samples were longitudinally cut into 8 µm thick sections and four were transversely cut. Sample sections were used for hematoxylin-eosin (H&E) and immunofluorescence staining. H&E staining was performed using the H&E Stain Kit (Beyotime Biotechnology). Briefly, the sections were stained using the hematoxylin staining solution for 5 to 20 minutes and then introduced into the differentiation solution for 30 seconds. After washing with warm water (37°C), the sections were introduced in eosin solution for 30 to 120 seconds, washed and dehydrated, made transparent using xylene, and sealed with neutral gum. To evaluate nerve regeneration, sections were washed in phosphate buffered saline followed by immersion in 0.1% Triton-X100 for 10 minutes. After incubating in 0.5% bovine serum albumin for 30 minutes, the sections were incubated with rabbit anti-S100 beta (S100β, 1:100, Abcam, Cat# ab52642, RRID: AB_882426) and mouse anti-neurofilament 200 antibody (NF200, 1:200, Abcam, Cat# ab24574, RRID: AB_448151) at 4°C overnight. Then, the sections were incubated with secondary antibodies (1:500) including goat anti-mouse Alexa Fluor 488-conjugated IgG (1:500, Abcam, Cat# ab150077, RRID: AB_2630356) or goat anti-mouse Alexa Fluor 594-conjugated IgG (1:500, Abcam, Cat# ab150116, RRID:AB_2650601) for 2 hours at room temperature (20–25°C). Cell nuclei were stained with 4′,6-diamino-2-phenylindole and the samples were observed under a fluorescence microscope (DP70; Olympus, Tokyo, Japan). For histomorphometric evaluation, 2.5 μm thick series of regenerated nerve semithin transverse sections were obtained and observed using a transmission electron microscope (HT7700; Hitachi Ltd., Tokyo, Japan). Quantitative analysis of nerve fiber diameter and myelin thickness was conducted in five randomly selected fields in each group using ImageJ software.
Weight ratio and morphology of muscle
The gastrocnemius muscles of both lower limbs were excised and weighed to calculate the muscle weight ratio (ipsilateral/contralateral). Then, the muscle was fixed in a 4% paraformaldehyde fixative solution overnight and cut into 10 μm cross sections in paraffin-wax-embedded samples. After H&E staining was performed, the average area of gastrocnemius fibers in the sections was measured using Image-Pro Plus software.
Statistical analysis
Sample size was not predetermined using statistical methods; however, our sample size was similar to that reported in a previous study (Quan et al., 2019b). No animals were excluded from analysis. Differences among more than three groups were analyzed using one-way analysis of variance with Tukey’s post hoc test. Single comparisons between two independent data sets were performed using the unpaired t-test. Data are expressed as means ± standard error of the mean. P < 0.05 was considered significant. Analyses were performed using Prism software version 9.0.0 (GraphPad Software, Boston, MA, USA).
Results
Structural characteristics of nerve guidance conduits
Aligned electrospun fibers in the inner layer and helical melt-spun fibers on the outer surface were designed to promote nerve regeneration (Figure 1A) and resist kinking and compression (Figure 1B). The fabrication process was divided into three steps (Figure 1C). Thin, aligned electrospun fibers were first fabricated on the inner layer of the NGC by introducing an electric field parallel to the rotating steel rod. Then, the parallel electric field was removed and the random electrospun fibers collected to increase the wall thickness. Finally, the spiral melt-spun fibers were prepared on the outer surface. Total wall thickness of all three NGC types was controlled at 400 μm (Figure 1C). For NAH and NRH, the electrospun fiber thickness of the inner layer was controlled at 250 μm; wall thickness of the NR was controlled at 400 μm. Scanning electron microscope cross-sectional images showed that the melt-spun fibers of the outer layer were bonded to the electrospun fibers of the inner layer, indicating the stable structural anisotropy of the NGC. Images of the lumen surface (Figure 1D, fourth column) demonstrated that the electrospun fibers were aligned longitudinally in NAH and distributed randomly in NRH and NR. The fiber orientation distribution frequency indicated an optimal degree of orientation concentration in NAH (Figure 1E). The diameter of electrospun fibers in the NAH, NRH, and NR was 2.32 ± 0.27 μm, 1.86 ± 0.20 μm, and 2.30 ± 0.15 μm, respectively. The diameter of the outer spiral melt-spun fibers of NAH and NRH was 303.50 ± 1.93 μm and 302.09 ± 1.43 μm, respectively (Figure 1F).
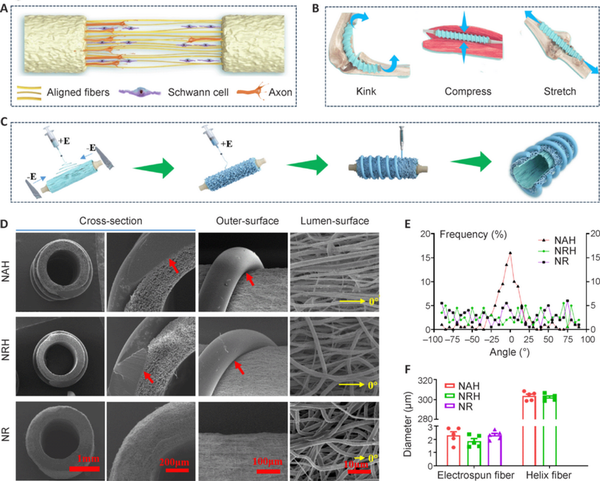
Figure 1
Structural characteristics of NGCs.
(A) Schematic diagram showing aligned electrospun fibers mediated nerve regeneration. (B) Schematic diagram displaying the role of the helix structure in resisting kinking and compression. (C) Schematic diagram of the NGC fabrication process. Panels A–C were created using CINEMA 4D (version R19) and Adobe Photoshop software (Version 9.0). (D) Scanning electron microscope images of an NGC. The first column is low-magnification and the second is high-magnification of a cross-section. The third column shows the outer surface and the fourth column shows the lumen surface. For NAH, aligned electrospun fibers are on the lumen surface, random electrospun fibers in the wall, and the helical melt-spun in the outer surface. For NRH, both the lumen surface and wall consist of random electrospun fibers and the outer surface consists of helical melt-spun fibers. The NR consists of random electrospun fibers without helical melt-spun fibers. The red arrows indicate the bonding sites between the inner electrospun fibers and the outer helical melt-spun fibers. The yellow arrows indicate the defined direction of 0°. (E) Statistics of fiber distribution frequency of the lumen surface. (F) Statistical analysis of fiber diameter. Fiber diameter was calculated based on scanning electron microscope images of the lumen surface (n = 5). Melt-spun fiber diameter was calculated based on images of the outer surface (n = 5). Data are expressed as means ± SEM and were analyzed by one-way analysis of variance with Tukey’s post hoc test. NAH: NGC with aligned electrospun fibers on the lumen and helix melt-spun fibers on the outer surface; NGC: nerve guide conduit; NR: NGC with random electrospun fibers only; NRH: NGC with random electrospun fibers on the lumen and helix melt-spun fibers on the outer surface.
Mechanical properties of nerve guidance conduits
To test the NGC anti-kinking properties, the conduit was passed through a flexible wire and then bent until kink formation (Quan et al., 2019b). Notably, both the NAH and NRH maintained their tubular shape even when bent into a loop (bend radius, approximately 0.5 cm) without any signs of kinking (Figure 2A). The bend angles of NAH and NRH were 116.20° ± 4.94° and 120.60° ± 4.82°, respectively. In contrast, the NR exhibited obvious kinks at a bending angle of 67.40° ± 3.14° (Figure 2B). The stress-strain curves of NAH and NRH were similar (Figure 2C), indicating that the inner thin electrospinning fibers did not significantly affect the mechanical properties of the electrospun fibers. However, the stress-strain curve of NR was distinct from that of NAH and NRH. The NAH and NRH stress decreased when the tensile strain exceeded 350% because the electrospun fiber layer in these two conduits was destroyed (Figure 2C). Notably, the Young’s modulus was lower in NAH and NRH than in NR, indicating that NAH and NRH were more flexible, probably because their layers of electrospun fibers were thinner (Figure 2D). The elongation at the break of NAH and NRH was significantly higher than that of NR (Figure 2E). Although NAH and NRH only broke when they were stretched to 2800% of strain, their electrospun fiber structure was destroyed at 350% of strain.
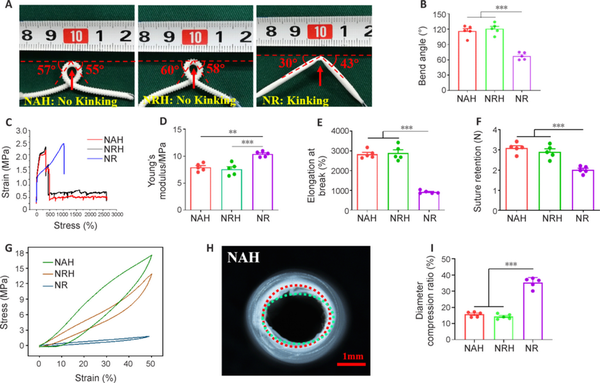
Figure 2
Mechanical properties of NGCs.
(A) Resistance to kinking. NAH and NRH maintained a tubular shape even when bent into a loop (bend radius, approximately 0.5 cm). Arrows indicate where the NGCs are most bent and likely to kink. The dashed lines indicate the horizontal. Bending angles of the loops are shown in degrees. (B) Quantitative bend angle analysis (n = 5). (C) Tensile stress-strain curves. (D, E) Statistical analysis of Young’s modulus (D), and elongation at break (E; n = 5). (F) Suture retention strength (n = 5). (G) Compression mechanics curve. (H) Luminal morphology of NAH after 1000 compression cycles. The red dashed line indicates the circular outline of the cross-section of the original NAH; the green dashed line indicates the outline after compression. (I) Luminal diameter loss rate after 1000 compressions (n = 5). Data are expressed as means ± SEM. **P < 0.01, ***P < 0.001 (one-way analysis of variance with Tukey’s post hoc test). NAH: NGC with aligned electrospun fibers on the lumen and helix melt-spun fibers on the outer surface; NGC: nerve guide conduit; NR: NGC with random electrospun fibers only; NRH: NGC with random electrospun fibers on the lumen and helix melt-spun fibers on the outer surface; ns: not significant.
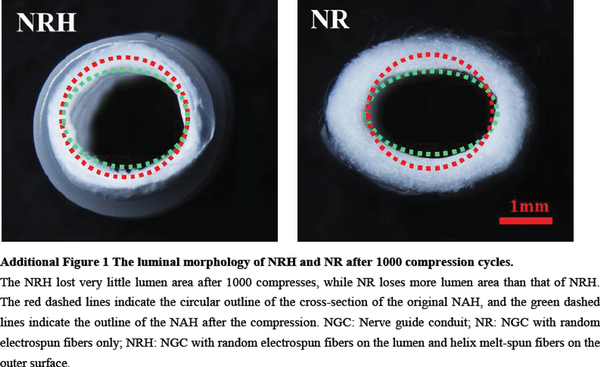
The suture retention strength was adequate to meet clinically acceptable requirements in all three NGC types (Huang et al., 2018) and was lowest in NR (Figure 2F). The high suture retention strength can be attributed to the strong adhesion between the conduits’ melt-spun fibers and electrospun fibers. The presence of helical melt-spun fibers on the outer surface also enhances resistance to compression. Compressive strength was higher in NAH and NRH than in NR (Figure 2G), indicating that melt-spun fibers enhance radial compressive strength. After 1000 cycles of compression, NAH and NRH retained their tubular shape (Figure 2H) while NR became deformed (Additional Figure 1). Lumen diameter loss was lower in NAH and NRH than in NR (Figure 2I). The mechanical properties of NAH, NRH, and NR are demonstrated in Additional Videos 1–3.
Effect of luminal topography on cell behavior
To investigate the effect of aligned and random fibers on nerve cell behavior, electrospun membranes with aligned and random electrospun fibers were seeded with PC12 cells and DRG cells, respectively. Phalloidin staining showed that PC12 cells aligned and extended along the direction of the aligned fibers; this did not occur along random fibers (Figure 3A). PC12 cell proliferation was significantly higher on the aligned fibrous membrane than the random fibrous membrane at 3 and 7 days (Figure 3B), indicating that aligned fibers promote nerve cell proliferation. Scanning electron microscope images confirmed the extension of PC12 cells on both aligned and random electrospun fibers (Figure 3C). DRG cells exhibited axons growing along the direction of the aligned fibers; however, axon growth was random on the random fibers (Figure 3D). Notably, DRG cell axon length was significantly longer on the aligned fibers than on the random fibers (Figure 3E). These results suggest that aligned fiber morphology on the NGC inner layer effectively guides neural and axon growth.
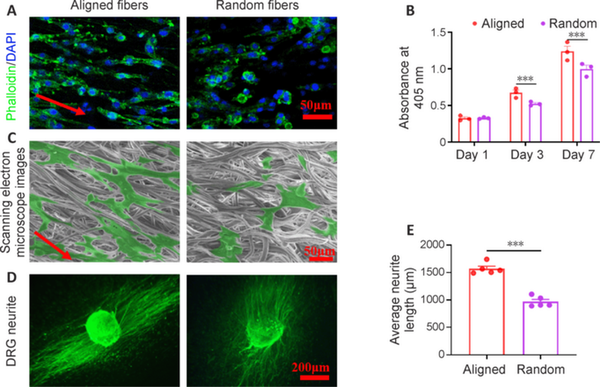
Figure 3
Effect of aligned fibers on guiding the growth of PC12 and DRG cells.
(A) The alignment of PC12 cells on the aligned and random fibers membranes was observed after staining with FITC-conjugated phalloidin and DAPI. The PC12 cells seeded on the aligned fibers showed a more obvious arrangement and stretching along the fibers than those seeded on random fibers. FITC-conjugated phalloidin, green; DAPI, blue. (B) PC12 cell proliferation was detected using CCK-8 (n = 3). From day 3 onwards, PC12 cell proliferation was higher on aligned fibers than on random fibers. (C) Scanning electron microscope images of PC12 cells seeded on aligned and random fiber membranes for 3 days. Those seeded on aligned fibers showed a more elongated shape. (D) FITC-conjugated NF200 staining images of DRG cells seeded on aligned and random fiber membranes for 3 days. The axons grew longitudinally in the same direction along aligned fibers but randomly along random fibers. FITC-conjugated NF200, green. (E) Quantitative analysis of DRG axon length on aligned and random fiber membranes at 3 days (n = 5). Axon length was higher on aligned fibers than random fibers. Data are expressed as means ± SEM. ***P < 0.001 (unpaired t-test). CCK-8: Cell Counting Kit-8; DAPI: 4′,6-diamino-2-phenylindole; DRG: Dorsal root ganglion; FITC: fluorescein isothiocyanate; NAH: NGC with aligned electrospun fibers on the lumen and helix melt-spun fibers on the outer surface; NGC: nerve guide conduit; NR: NGC with random electrospun fibers only; NRH: NGC with random electrospun fibers on the lumen and helix melt-spun fibers on the outer surface.
Resistance to kinking and compression in vivo
To evaluate NGC resistance to kinking and compression in vivo, each type was implanted into rat sciatic nerve defects. The NAH and NRH did not kink when subjected to a single pressure simulating surgically induced three-point bending. After removing the pressure, they returned to their tubular shape. Four weeks after implantation, the NAH and NRH maintained their tubular shape without any collapse. When subjected to unidirectional pressure, both NAH and NRH remained resistant to kinking and returned to their original shape after pressure removal; however, the NR still lacked the ability to resist kinking (Figure 4A). These results showed that NAH and NRH could maintain their tubular structure and exhibit the ability to resist pressure from muscles; in addition, they showed in vivo flexibility with joint bending. H&E staining confirmed that the NAH and NRH maintained their tubular shape 4 weeks after implantation (Figure 4B); however, the NR had already assumed an oval shape, probably because of pressure exerted by the surrounding muscle. Although the electrospun fiber layer is thinner in NAH and NRH than NR, no significant fibrotic scar tissue was observed in the NAH and NRH lumens. This suggests that NAH and NRH effectively prevent excessive infiltration of fibroblasts and formation of scar tissue. The in vivo anti-kinking and anti-compression properties of NAH and NRH, along with their ability to inhibit infiltration of fibroblasts and scar tissue, should promote nerve regeneration.
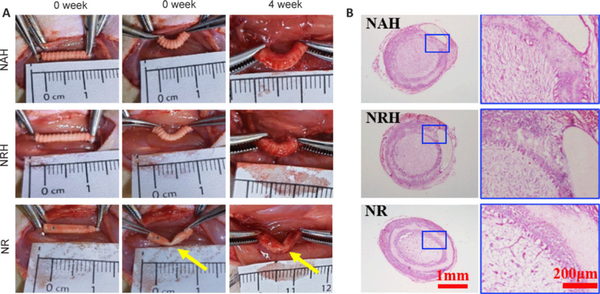
Figure 4
Evaluation of resistance to kinking and compression properties in vivo.
(A) Anti-kinking properties of NAH, NRH and NR were evaluated using the three-point bending test immediately and 4 weeks after surgery. NAH and NRH showed no kinking in the bent state during and after implantation, while NR showed obvious kinking. Yellow arrows indicate the kinking sites. (B) Lumen collapse was evaluated by H&E staining of cross-sections 4 weeks after implantation. No collapse was observed in NAH and NRH; NR showed collapse. H&E: Hematoxylin-eosin; NAH: NGC with aligned electrospun fibers on the lumen and helix melt-spun fibers on the outer surface; NGC: nerve guide conduit; NR: NGC with random electrospun fibers only; NRH: NGC with random electrospun fibers on the lumen and helix melt-spun fibers on the outer surface.
Ability to promote nerve regeneration
To evaluate the NGC’s efficacy in promoting nerve regeneration, Schwann cells and neurofilaments were examined in explanted NGC samples using S100 and NF200 antibodies, respectively. The results showed that S100 expression was significantly higher in NAH than in NRH and NR (Figure 5A). When co-staining with NF200 and S100 antibodies was performed on longitudinal sections, we observed that Schwann cells within the NAH migrated distally along the nerve defect; in addition, nerve filament regeneration extended up to half the length of the defect (Figure 5B). These findings occurred to lesser degrees in NRH and NR. Furthermore, transmission electron microscopy (TEM) was performed to examine axon regeneration at a finer structural level (Figure 5C). The TEM images revealed that while the myelin sheath in NAH exhibited variation, with some axons appearing unmyelinated or hypomyelinated, quantitative analysis of axon area showed that mean axon area was greater in NAH (0.48 ± 0.01 mm2) than in NRH (0.34 ± 0.02 mm2) and NR (0.30 ± 0.01 mm2) (Figure 5D). Myelin sheath thickness was also significantly greater in NAH (521.50 ± 27.19 μm) than NRH (419.25 ± 10.76 μm) and NR (385.75 ± 10.78 μm) 4 weeks after implantation (Figure 5E). However, nerve regeneration was lower in NAH than in autografts, as indicated by NF200 and S100 antibody co-staining as well as TEM. This may be because the evaluation was conducted at only 4 weeks. Although not significant, both axon area and myelin sheath thickness were higher in NRH than in NR, indicating that both the aligned fibers and the flexibility of NAH play important roles in promoting axon regeneration. These results suggest that NAH promotes nerve regeneration through its structural design and topological cues in the lumen.
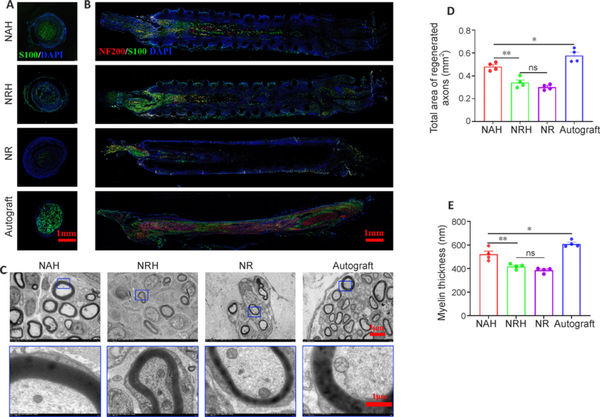
Figure 5
Analysis of nerve regeneration in NGC after 4 weeks of implantation.
(A) Representative S100 antibody staining images of cross-sections. The NAH showed better Schwann cells regeneration compared with that of NRH and NR, though still worse than that of autograft. S100, Alexa Fluor 488, green; DAPI, blue. (B) Representative co-staining images of longitudinal sections stained by S100 and NF200 antibodies. The NAH showed the best regeneration indicated by the Schwann cells and the nerve filaments compared with NRH and NR, but this was still not as good as that with the autograft. S100, Alexa Fluor 488, green; NF200, Alexa Fluor 594, red; DAPI, blue. Yellow arrows point to suture site. (C) The axon regeneration was observed by TEM. The NAH showed better axons regeneration in view of the myelin sheath, similar to that of autograft, while the NRH and NR group were worse. (D, E) quantitative analysis of axon area (D) based on the low-magnification TEM images and myelin thickness (E) based on high-magnification TEM images (n = 4). The NAH demonstrated higher axon area (D) and myelin thickness (E) compared with NRH and NR, but lower axon area and myelin thickness than autograft. Data are expressed as mean ± SEM. *P < 0.05, **P < 0.01 (one-way analysis of variance with Tukey’s post hoc test). DAPI: 4′,6-Diamino-2-phenylindole; NAH: NGC with aligned electrospun fibers on the lumen and helix melt-spun fibers on the outer surface; NF200: neurofilament 200; NGC: nerve guide conduit; NR: NGC with random electrospun fibers only; NRH: NGC with random electrospun fibers on the lumen and helix melt-spun fibers on the outer surface; ns: not significant; TEM: transmission electron microscope.
Neurological function
Rats in the NAH group had fewer foot ulcers and exhibited less toe self-harm than NRH and NR rats 4 weeks after implantation (Figure 6A). The NAH group displayed minimal ulceration and toe self-mutilation, whereas no significant ulcers or foot self-injury were observed in the autograft group, indicating that nerve recovery was superior in the NAH and autograft groups than the NRH and NR groups. Masson staining analysis showed that the NAH and autograft group rats had more red muscle fibers and less collagen than the NRH and NR rats (Figure 6B). Furthermore, the mass weight of the gastrocnemius muscle was higher in the NAH group than the NRH and NR groups (Figure 6C), as was the average area of muscle fibers (Figure 6D). Additionally, gastrocnemius muscle mass weight and muscle fiber area were higher in the autograft group than the NAH group. These findings suggest that NAH is more effective in guiding the repair of sciatic nerve function and regulating the activity of the gastrocnemius muscle than NRH and NR.
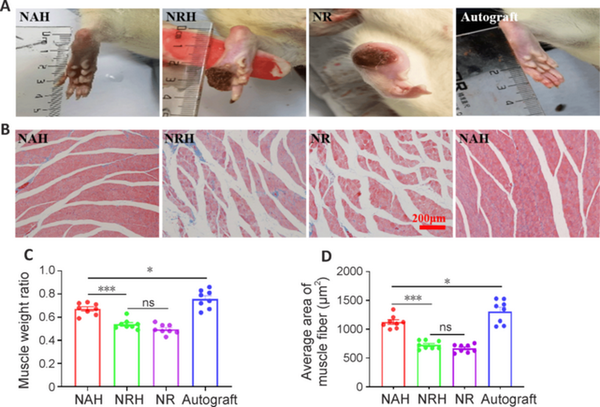
Figure 6
Analysis of toe survival and gastrocnemius muscle atrophy 4 weeks after implantation.
(A) Representative rat toe survival images. The NAH group had the fewest foot ulcers and least toe self-harm compared with that of NRH and NR groups. The NAH group displayed a slight degree of ulcer and toe self-harm, but it was less severe than that observed in the autograft group. No significant ulcers or foot self-injury were observed in the autograft group. The NR group had the most foot ulcers and exhibited the highest degree of toe self-harm. (B) Masson staining images of the gastrocnemius muscle. There were more muscle fibers and less collagen in the NAH and autograft groups than the NRH and NR groups. Red represents muscle fibers and blue represents collagen fibers. Quantitative analysis of (C) gastrocnemius muscle weight (n = 8) and (D) average area of gastrocnemius muscle fibers on Masson staining images (n = 8). Data are expressed as the means ± SEM. *P < 0.05, ***P < 0.001 (one-way analysis of variance with Tukey’s post hoc test). NAH: NGC with aligned electrospun fibers on the lumen and helix melt-spun fibers on the outer surface; NGC: nerve guide conduit; NR: NGC with random electrospun fibers only; NRH: NGC with random electrospun fibers on the lumen and helix melt-spun fibers on the outer surface; ns: not significant.
Discussion
The economic and social burdens of peripheral nerve injuries are immense, affecting millions of people worldwide (Yan et al., 2021). Although autologous transplantation remains the gold standard for repair, it is associated with various potential complications (Zhang et al., 2020; Kornfeld et al., 2021). The ultimate goal of tissue engineering is to improve clinical outcomes by supporting regeneration and repair of damaged tissue (Ashammakhi et al., 2022; Paternoster and Vranckx, 2022). NGC design has advanced from the initial hollow silicone tube to more complex designs that incorporate electrospinning (Frost et al., 2018), topological guidance (Yan et al., 2022), cell seeding (Thibodeau et al., 2022), or bioactive factor modifications (Li et al., 2022) to facilitate nerve regeneration and functional reconstruction. However, a significant gap remains between research and clinical practice owing to complex processing technology, product consistency control (Parker et al., 2021), and stringent regulatory requirements (Kasper et al., 2020). Therefore, it is of great practical significance to develop NGCs that can improve outcomes and enhance usability in clinical practice based on feedback from clinicians.
We have successfully developed an NGC, referred to as NAH in this study, which features an inner layer with aligned electrospun fibers and a tube wall with spiral melt-spun fibers. This design provides optimal topological guidance for nerve regeneration, resists compression, and provides flexibility. These features enable use in a challenging in vivo implantation environment. NAH is easily fabricated and readily available. Here, we have demonstrated its superior mechanical properties and ability to promote nerve regeneration in vivo. NAH holds promise for future clinical applications.
Nerve reconstruction in a cross-joint region is considered to be more challenging because the implantation of NGC near the joints is more prone to lumen collapse, suture pull-out and other adverse effects, which may be related to the poor anti-kinking and insufficient radial support properties of the NGC (Kaplan et al., 2015; Quan et al., 2019b; Sanchez Rezza et al., 2022; Liao et al., 2024). A commonly applied engineering solution to lumen compression is helical structure (Chiu et al., 2007; Zhang et al., 2022). Quan et al. (2019b) developed an NGC with helical groove structure that disperses stress concentration when bending. This design exhibited good flexibility and resistance to compression. We fabricated helical fibers on the outer surface of the NGC. NAH reached a bend radius of 0.5 cm and maintained a tubular shape after 1000 cycles of compression, which indicated good kinking resistance and structural stability. Fortunately, NAH and NRH were able to maintain good resistance to kinking after 4 weeks of in vivo implantation; however, NR lacked this ability. The explanted samples showed that the circular tube shape of the inner cavity of the NR group was inferior to that of the NAH and NRH groups. This may be due to insufficient mechanical support from the NGC and muscle compression at the site of nerve damage (Alarcón Apablaza et al., 2022). The excellent anti-kinking and anti-compression properties of NAH and NRH can avoid potential side effects caused by lumen collapse. It is worth noting that NAH and NRH showed a strong fusion between the helical melt-spinning fibers layer and the electrospinning fibers layer without any delamination. This may be due to the fusion and subsequent solidification of melt-spun PCL fibers on the outer surface of the electrospun fibers during fabrication. The robust integration of the different fiber layers in the NGC enhances its mechanical properties and structural stability, supporting its functional implementation after implantation. A narrowed lumen area within the NGC can hinder nerve regeneration (Carvalho et al., 2019). Four weeks after implantation, both the NAH and NRH maintained their structural integrity. This observation highlights that the NAH and NRH possess significant structural stability for bridging nerve defects. The addition of helical fibers on the outer surface of the NAH further enhances its resistance to kinking and its structural stability.
Adding physical cues that promote nerve regeneration to the NGCs while allowing them to maintain their tubular shape was a practical consideration in the design process. Aligned microfibers are widely reported to promote nerve regeneration via several possible mechanisms, including guidance and promotion of Schwann cell migration (Smith et al., 2022), effects on neural cell growth (Behtaj et al., 2022), polarization of macrophages to a pro-regenerative phenotype (Fang et al., 2023), and synergistic effects on Schwann cells, macrophages, and pan-neuronal cells (Dong et al., 2021). We also observed that the aligned microfibers of NAH promoted elongation and proliferation of PC12 cells along the microfiber direction. In the rat sciatic nerve defect repair model, NAH promoted nerve regeneration better than NRH. Regenerated axons in the NAH nearly reached the distal end of the nerve defect, indicating the promoting role of aligned fibers in the lumen. The ability of nerves to innervate muscles further supports nerve regeneration. However, both nerve regeneration and functional recovery in the NAH group rats were inferior to those in the autograft group, which may be due to the short 4-week implantation period. It is worth noting that although aligned fibers play a promoting role in guiding nerve cell growth, an excessive number of aligned fibers within the NGC causes insufficient radial support and uneven accumulation of fibers in the lumen (Yang et al., 2022). Therefore, balancing the aligned fibers’ roles of mechanical support and guidance remains an essential consideration. Since the aligned fibers guide nerve cells on the lumen surface, the fabrication process involves collecting these fibers in a short period of time to form a thin layer of aligned fibers. Then, the random electrospun fibers and helical melt-spun fibers were continued to be collected, which aim to provide structural and mechanical support.
The ideal tissue engineering scaffold is designed to encourage tissue regeneration (Amalakanti et al., 2024; Zhu et al., 2024). Scaffolds gradually degrade and are eventually absorbed after implantation, ultimately leading to complete tissue repair (Zhang and King, 2020). Although Schwann cells and axons had migrated almost to the distal end of the NAH 4 weeks after implantation in our study, the follow-up period was short. PCL degradation was not evaluated in this study because its time to degradation is 2 to 3 years (Lykins et al., 2022). Future studies should assess nerve regeneration and PCL degradation with the NAH conduit in long-term studies extending over several years. In addition, NAH should be evaluated to validate its mechanical support and the guiding effect of aligned fibers specifically at joint sites. We did not implant NGCs at joint sites because of constraints imposed by the rat model.
In conclusion, we have developed a novel NGC that features aligned microfibers in the lumen and helical melt-spun fibers on the outer surface, designated as NAH. The aligned microfibers on the lumen surface effectively promote nerve regeneration, while the helical melt-spun fibers provide exceptional resistance to kinking and compression. The dual-layer NGC is simple to manufacture and addresses practical challenges in clinical application, making it a promising candidate for clinical translation.
Author contributions:KW, PT and ZL supervised the overall study design. TL and QC designed and performed experimental and data analysis together with JZ, BL, YS, HW, LH, SZ, RZ, SW, and GL. TL, QC, and KW wrote and edited the manuscript. All authors discussed and interpreted results and approved the final version of the manuscript.
Conflicts of interest:The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Data availability statement:All data relevant to the study are included in the article or uploaded as Additional files.
Additional files:
Additional Figure 1:The luminal morphology of NRH and NR after 1000 compression cycles.
Additional Video 1:The compression of NAH.
Additional Video 1
Additional Video 2:The compression of NRH.
Additional Video 2
Additional Video 3:The compression of NR.
Additional Video 3
C-Editor: Zhao M; S-Editors: Yu J, Li CH; L-Editor: Song LP; T-Editor: Jia Y
References
- Alarcón Apablaza J, Lezcano MF, Godoy Sánchez K, Oporto GH, Dias FJ (2022) Optimal morphometric characteristics of a tubular polymeric scaffold to promote peripheral nerve regeneration: a scoping review. Polymers (Basel) 14:397.
- Amalakanti S, Mulpuri RP, Avula VCR (2024) Recent advances in biomaterial design for nerve guidance conduits: a narrative review. Adv Technol Neurosci DOI: 10.4103/ATN.ATN-D-23-00005.
- Ao Q (2016) Progress of nerve bridges in the treatment of peripheral nerve disruptions. J Neurorestoratol 4:107–113.
- Ashammakhi N, GhavamiNejad A, Tutar R, Fricker A, Roy I, Chatzistavrou X, Hoque Apu E, Nguyen KL, Ahsan T, Pountos I, Caterson EJ (2022) Highlights on advancing frontiers in tissue engineering. Tissue Eng Part B Rev 28:633–664.
- Behtaj S, Ekberg JAK, St John JA (2022) Advances in electrospun nerve guidance conduits for engineering neural regeneration. Pharmaceutics 14:219.
- Carvalho CR, Oliveira JM, Reis RL (2019) Modern trends for peripheral nerve repair and regeneration: beyond the hollow nerve guidance conduit. Front Bioeng Biotechnol 7:337.
- Chiu CH, Hwan CL, Tsai HS, Lee WP (2007) An experimental investigation into the mechanical behaviors of helical composite springs. Compos Struct 77:331–340.
- Chung TW, Yang MC, Tseng CC, Sheu SH, Wang SS, Huang YY, Chen SD (2011) Promoting regeneration of peripheral nerves in-vivo using new PCL-NGF/Tirofiban nerve conduits. Biomaterials 32:734–743.
- Clements BA, Bushman J, Murthy NS, Ezra M, Pastore CM, Kohn J (2016) Design of barrier coatings on kink-resistant peripheral nerve conduits. J Tissue Eng 7:2041731416629471.
- Dong X, Liu S, Yang Y, Gao S, Li W, Cao J, Wan Y, Huang Z, Fan G, Chen Q, Wang H, Zhu M, Kong D (2021) Aligned microfiber-induced macrophage polarization to guide schwann-cell-enabled peripheral nerve regeneration. Biomaterials 272:120767.
- Duffy P, McMahon S, Wang X, Keaveney S, O’Cearbhaill ED, Quintana I, Rodríguez FJ, Wang W (2019) Synthetic bioresorbable poly-α-hydroxyesters as peripheral nerve guidance conduits; a review of material properties, design strategies and their efficacy to date. Biomater Sci 7:4912–4943.
- Fang Y, Wang C, Liu Z, Ko J, Chen L, Zhang T, Xiong Z, Zhang L, Sun W (2023) 3D printed conductive multiscale nerve guidance conduit with hierarchical fibers for peripheral nerve regeneration. Adv Sci (Weinh) 10:e2205744.
- Frost HK, Andersson T, Johansson S, Englund-Johansson U, Ekström P, Dahlin LB, Johansson F (2018) Electrospun nerve guide conduits have the potential to bridge peripheral nerve injuries in vivo. Sci Rep 8:16716.
- Huang L, Zhu L, Shi X, Xia B, Liu Z, Zhu S, Yang Y, Ma T, Cheng P, Luo K, Huang J, Luo Z (2018) A compound scaffold with uniform longitudinally oriented guidance cues and a porous sheath promotes peripheral nerve regeneration in vivo. Acta Biomater 68:223–236.
- Kaplan HM, Mishra P, Kohn J (2015) The overwhelming use of rat models in nerve regeneration research may compromise designs of nerve guidance conduits for humans. J Mater Sci Mater Med 26:226.
- Kasper M, Deister C, Beck F, Schmidt CE (2020) Bench-to-bedside lessons learned: commercialization of an acellular nerve graft. Adv Healthc Mater 9:e2000174.
- Kim JI, Hwang TI, Aguilar LE, Park CH, Kim CS (2016) A controlled design of aligned and random nanofibers for 3D bi-functionalized nerve conduits fabricated via a novel electrospinning set-up. Sci Rep 6:23761.
- Koh HS, Yong T, Teo WE, Chan CK, Puhaindran ME, Tan TC, Lim A, Lim BH, Ramakrishna S (2010) In vivo study of novel nanofibrous intra-luminal guidance channels to promote nerve regeneration. J Neural Eng 7:046003.
- Kornfeld T, Nessler J, Helmer C, Hannemann R, Waldmann KH, Peck CT, Hoffmann P, Brandes G, Vogt PM, Radtke C (2021) Spider silk nerve graft promotes axonal regeneration on long distance nerve defect in a sheep model. Biomaterials 271:120692.
- Li C, Liu SY, Zhou LP, Min TT, Zhang M, Pi W, Wen YQ, Zhang PX (2022) Polydopamine-modified chitin conduits with sustained release of bioactive peptides enhance peripheral nerve regeneration in rats. Neural Regen Res 17:2544–2550.
- Liao M, Cui Q, Hu Y, Xing J, Wu D, Zheng S, Zhao Y, Yu Y, Sun J, Chai R (2024) Recent advances in the application of MXenes for neural tissue engineering and regeneration. Neural Regen Res 19:258–263.
- Liebeskind BJ, Hillis DM, Zakon HH, Hofmann HA (2016) Complex homology and the evolution of nervous systems. Trends Ecol Evol 31:127–135.
- Lien BV, Brown NJ, Ransom SC, Lehrich BM, Shahrestani S, Tafreshi AR, Ransom RC, Sahyouni R (2020) Enhancing peripheral nerve regeneration with neurotrophic factors and bioengineered scaffolds: a basic science and clinical perspective. J Peripher Nerv Syst 25:320–334.
- Liu K, Yan L, Li R, Song Z, Ding J, Liu B, Chen X (2022) 3D printed personalized nerve guide conduits for precision repair of peripheral nerve defects. Adv Sci (Weinh) 9:e2103875.
- Lykins WR, Bernards DA, Schlesinger EB, Wisniewski K, Desai TA (2022) Tuning polycaprolactone degradation for long acting implantables. Polymer 262:125473.
- Mohammadi M, Ramazani SaadatAbadi A, Mashayekhan S, Sanaei R (2020) Conductive multichannel PCL/gelatin conduit with tunable mechanical and structural properties for peripheral nerve regeneration. J Appl Polym Sci 137:49219.
- Park J, Jeon J, Kim B, Lee MS, Park S, Lim J, Yi J, Lee H, Yang HS, Lee JY (2020) Electrically conductive hydrogel nerve guidance conduits for peripheral nerve regeneration. Adv Funct Mater 30:2003759.
- Parker BJ, Rhodes DI, O’Brien CM, Rodda AE, Cameron NR (2021) Nerve guidance conduit development for primary treatment of peripheral nerve transection injuries: a commercial perspective. Acta Biomater 135:64–86.
- Paternoster JL, Vranckx JJ (2022) State of the art of clinical applications of tissue engineering in 2021. Tissue Eng Part B Rev 28:592–612.
- Quan Q, Meng HY, Chang B, Liu GB, Cheng XQ, Tang H, Wang Y, Peng J, Zhao Q, Lu SB (2019a) Aligned fibers enhance nerve guide conduits when bridging peripheral nerve defects focused on early repair stage. Neural Regen Res 14:903–912.
- Quan Q, Meng H, Chang B, Hong L, Li R, Liu G, Cheng X, Tang H, Liu P, Sun Y, Peng J, Zhao Q, Wang Y, Lu S (2019b) Novel 3-D helix-flexible nerve guide conduits repair nerve defects. Biomaterials 207:49–60.
- Sanchez Rezza A, Kulahci Y, Gorantla VS, Zor F, Drzeniek NM (2022) Implantable biomaterials for peripheral nerve regeneration-technology trends and translational tribulations. Front Bioeng Biotechnol 10:863969.
- Sarker MD, Naghieh S, McInnes AD, Schreyer DJ, Chen X (2018) Regeneration of peripheral nerves by nerve guidance conduits: Influence of design, biopolymers, cells, growth factors, and physical stimuli. Prog Neurobiol 171:125–150.
- Schneider CA, Rasband WS, Eliceiri KW (2012) NIH Image to ImageJ: 25 years of image analysis. Nat Methods 9:671–675.
- Sedaghati T, Yang SY, Mosahebi A, Alavijeh MS, Seifalian AM (2011) Nerve regeneration with aid of nanotechnology and cellular engineering. Biotechnol Appl Biochem 58:288–300.
- Shahriari D, Shibayama M, Lynam DA, Wolf KJ, Kubota G, Koffler JY, Tuszynski MH, Campana WM, Sakamoto JS (2017) Peripheral nerve growth within a hydrogel microchannel scaffold supported by a kink-resistant conduit. J Biomed Mater Res A 105:3392–3399.
- Smith CS, Orkwis JA, Bryan AE, Xu Z, Harris GM (2022) The impact of physical, biochemical, and electrical signaling on Schwann cell plasticity. Eur J Cell Biol 101:151277.
- Tang H, Li J, Wang H, Ren J, Ding H, Shang J, Wang M, Wei Z, Feng S (2024) Human umbilical cord mesenchymal stem cell-derived exosomes loaded into a composite conduit promote functional recovery after peripheral nerve injury in rats. Neural Regen Res 19:900–907.
- Thibodeau A, Galbraith T, Fauvel CM, Khuong HT, Berthod F (2022) Repair of peripheral nerve injuries using a prevascularized cell-based tissue-engineered nerve conduit. Biomaterials 280:121269.
- Vijayavenkataraman S (2020) Nerve guide conduits for peripheral nerve injury repair: a review on design, materials and fabrication methods. Acta Biomater 106:54–69.
- Wei Z, Jin F, Li T, Qian L, Zheng W, Wang T, Feng ZQ (2023) Physical cue-based strategies on peripheral nerve regeneration. Adv Funct Mater 33:2209658.
- Wieringa PA, Gonçalves de Pinho AR, Micera S, van Wezel RJA, Moroni L (2018) Biomimetic architectures for peripheral nerve repair: a review of biofabrication strategies. Adv Healthc Mater 7:e1701164.
- Yan Y, Yao R, Zhao J, Chen K, Duan L, Wang T, Zhang S, Guan J, Zheng Z, Wang X, Liu Z, Li Y, Li G (2022) Implantable nerve guidance conduits: Material combinations, multi-functional strategies and advanced engineering innovations. Bioact Mater 11:57–76.
- Yan Z, Chen C, Rosso G, Qian Y, Fan C (2021) Two-dimensional nanomaterials for peripheral nerve engineering: recent advances and potential mechanisms. Front Bioeng Biotechnol 9:746074.
- Yang J, Hsu CC, Cao TT, Ye H, Chen J, Li YQ (2023) A hyaluronic acid granular hydrogel nerve guidance conduit promotes regeneration and functional recovery of injured sciatic nerves in rats. Neural Regen Res 18:657–663.
- Yang X, Liu X, Xu F, Ji S, Sun Y, Song Z, Song J, Wu Y, Yin J (2022) Fabrication of microgroove poly(lactic-co-glycolic acid) nerve guide conduit using dry-jet wet spinning for rat laryngeal recurrent nerve regeneration. Mater Des 223:111151.
- Yucel D, Kose GT, Hasirci V (2010) Polyester based nerve guidance conduit design. Biomaterials 31:1596–1603.
- Zhang B, Han Q, Qin H, Zhang J, Niu S, Han Z, Ren L (2022) Bending resistance and anisotropy of basalt fibers laminate composite with bionic helical structure. J Bionic Eng 19:799–815.
- Zhang F, King MW (2020) Biodegradable polymers as the pivotal player in the design of tissue engineering scaffolds. Adv Healthc Mater 9:e1901358.
- Zhang X, Qu W, Li D, Shi K, Li R, Han Y, Jin E, Ding J, Chen X (2020) Functional polymer-based nerve guide conduits to promote peripheral nerve regeneration. Adv Mater Interfaces 7:2000225.
- Zhao YN, Wu P, Zhao ZY, Chen FX, Xiao A, Yue ZY, Han XW, Zheng Y, Chen Y (2023) Electrodeposition of chitosan/graphene oxide conduit to enhance peripheral nerve regeneration. Neural Regen Res 18:207–212.
- Zhou G, Chang W, Zhou X, Chen Y, Dai F, Anwar A, Yu X (2020) Nanofibrous nerve conduits with nerve growth factors and bone marrow stromal cells pre-cultured in bioreactors for peripheral nerve regeneration. ACS Appl Mater Interfaces 12:16168–16177.
- Zhu L, Jia S, Liu T, Yan L, Huang D, Wang Z, Chen S, Zhang Z, Zeng W, Zhang Y, Yang H, Hao D (2020) Aligned PCL fiber conduits immobilized with nerve growth factor gradients enhance and direct sciatic nerve regeneration. Adv Funct Mater 30:2002610.
- Zhu S, Liu X, Lu X, Liao Q, Luo H, Tian Y, Cheng X, Jiang Y, Liu G, Chen J (2024) Biomaterials and tissue engineering in traumatic brain injury: novel perspectives on promoting neural regeneration. Neural Regen Res 19:2157–2174.