Introduction
The consensus definition of diarrhoea, published by the World Health Organisation (WHO) in 1988 as stated by is the passage of three or more loose stools - one that takes the shape of a container, or watery stools in a 24-h period. According to WHO, diarrhoeal disease is the second leading cause of death among children under the age of 5 in the world (). The WHO’s recommendation for the treatment of diarrhoea is oral rehydration solution (ORS), consisting of a solution of clean water, sugar and salt along with a 10-14 day supplemental treatment course of dispersible 20 mg zinc tablets (). However, currently there is limited available information of the recommended ORS/zinc treatment especially in rural regions of some African countries (; ). In addition to poor access, a number of indigenous and culturally acceptable alternatives treatments based on fermented foods tend to be used in these regions ().
Fermented foods are consumed across the globe as traditional foods, within many societies and communities using fermented foods for the treatment of diarrhoea, especially in children (; ; ). This ancient food processing technique continues to be used both domestically and industrially as a food preservation method, prolonging shelf life of foods and beverages (; ; ). Fermentation additionally has the potential to enhance sensory properties of food product, which leads to some consumers to prefer (; ). To date, some research data support the potential of fermented foods in providing therapeutic benefits in the management or prevention of some gastrointestinal diseases and disorders (). Though not clearly understood, there are various hypotheses with respect to the possible ways in which fermented foods and beverages may be able to offer health benefits. Components of the fermented product matrix, including the fermentation organisms, fermentation products, including de novo organic compounds and exopolysaccharides produced during fermentation, which could collectively or individually result in the varied effects of fermented food products are reported in the literature ().
The most commonly found organisms in fermented foods and beverages are lactic acid bacteria (LAB) and yeast strains, which are generally regarded as safe (; ; ). These organisms are particularly common in dairy and cereal fermentation (; ). Some species of these organisms, particularly those of Lactobacilli and Saccharomyces have the potential to confer health benefits through mechanisms such as enhancement of gut immune response, competition with pathogenic organisms for biological niches in the gastrointestinal tract (GIT) or production of antibacterial substances (; ). Such organisms may be described as probiotic if they are able to survive the harsh environment of the upper GIT in order to reach the colon in significant numbers, where they may be able to enhance gut epithelial function (; ; ). However, a fermentation organism does not have to be probiotic in order to confer health benefits, as even dead cells may stimulate the production of helper T cells in the colon (; ; ). This was initially proposed by , who reported that dead LAB cells may enhance immune response in the GIT in an in-vivo model using rats. Upon reaching the gut, Lactobacilli along with other LAB, produce lactic acid which is metabolised by other organisms into butyrate (; ), a SCFA reported to have anti-inflammatory and anti-carcinogenic properties in the human GIT, whilst also boosting the GIT and systemic immune function ().
Diarrhoea occurs as a result alteration of the movement of water and ions in the colon where the absorption of water and electrolyte primarily takes place (; ). This can be induced by pathogenic infection or as a result of gut dysbiosis, for example, in antibiotic associated diarrhoea (; ). Extracellular polysaccharides (EPS) are carbohydrate polymers formed from simple or complex carbon substrates by the bacterial cells including fermentative organisms in foods and beverages (), as a protective surface in response to a hostile environment, enhancing colonization by the EPS producing organism (). EPS production influences the rheological and textural properties of fermented foods as seen in the viscosity of fermented milk. Some EPS produced by Lactobacilli organisms such as α-D-glucans, inulin-type fructans, oligosaccharides, (), may act as prebiotics in the human GIT (; ; ; ), selectively stimulating the growth of beneficial organisms in the colon (; ). Furthermore, enhanced production of SCFAs in the human gut has also been associated with EPS with the explanation that it serves as substrate for organisms, such as LAB and Bifidobacteria, which are involved in the production of SCFAs in the gut ().
In summary, fermented products may be useful in the prevention and management or treatment of diarrhoea by enhancing immune responses, potentially and/or selectively increasing the population of the beneficial organisms in the gut, inhibiting the growth of gut pathogenic organisms and enhancing gut homeostasis.
Although the potential of fermented products as alternative treatment for diarrhoeal disease has emerging supportive evidence in adults, there is a need for research to investigate its efficacy as an alternative treatment to ORS, especially in children. Randomised controlled trials (RCTs) and other clinical studies have examined the effect of fermented foods on diarrhoea amongst other gastrointestinal diseases or symptoms in adults (; ; ; ; ), but results have been inconsistent. To date, there is limited evidence and systematic analyses have investigated the efficacy of fermented foods in the prevention and treatment of diarrhoea jointly, using dairy products as the intervention (; ; ; ), although the potential mechanisms for both effects appear to be similar. This systematic review and meta-analysis aim to evaluate the available evidence with respect to the efficacy fermented foods and beverages in comparison with unfermented or heat treated products, including dairy and cereal products, focussing on the treatment of diarrhoea in infants.
Methods
The protocol for the systematic review and meta-analysis was registered with PROSPERO, registration number CRD42020201411. Cochrane Collaboration and Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) () guidelines for undertaking a systematic review and meta-analysis were followed.
Outcome measures
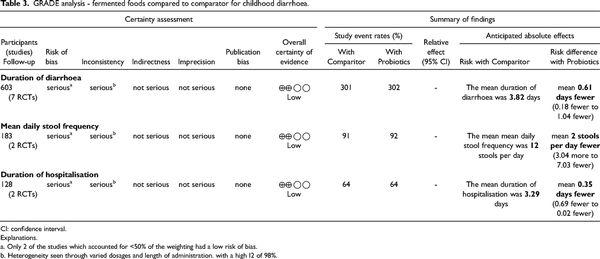
The primary outcome measure for this review was duration of diarrhoea. The secondary outcome measures were mean number of stools per day and duration of hospitalisation. A lack of data regarding these outcomes was not an exclusion criterion.
Electronic search
Electronic searches of the Cochrane Central Register of Controlled Trials (COCHRANE CENTRAL), OVID MEDLINE and PUBMED databases were undertaken in December 2020. There was no language restriction and publication date was left open till the date of search (December 2020) to avoid limited number of search output. Reference lists of relevant review articles, RCTs, systematic reviews and meta-analyses were hand search to identify any articles that the initial search may have missed. The search strategy included the use of validated filters in combination with topic-specific strategies. Search terms were included fermented food(s), diarrhoea/diarrhea, and gastrointestinal disease(s). The search strategy used both keywords and MeSH terms.
Inclusion and exclusion criteria
Randomised control trials (RCTs), clinical trials, controlled clinical trials or quasi-randomized trials were considered eligible for inclusion in this analysis. Studies must have included human subjects, with age no more than 5 years and with acute/chronic diarrhoea and must have had duration of diarrhoea as an outcome measure. Studies which included participants treated with antibiotics were excluded. Intervention had to be consumption of any fermented food product at any dosage scheme and duration of delivery with control being placebo, consumption of unfermented or ultra-heat treated or pasteurized fermented food product similar to the intervention or no intervention. Studies with no control, unspecified control and non-heat treated fermented controls were excluded.
Selection of studies
The title, abstract, and key words of every article identified during the search were initially screened. Articles that were irrelevant following an initial screening based on title, abstract and keywords were excluded. Full text of potentially relevant studies were then obtained for further screening. Consensus was reached by two reviewers (AO and NP) on the final chosen articles.
Data extraction
Information extracted from each article include: author; year of publication; baseline characteristics of the participants (age, setting, country of origin) sample size; type of intervention and comparator; dosage of intervention; duration of the intervention; definition of diarrhoea; outcome measures and results. Where comparable data were available, meta-analysis was undertaken using Review Manager (RevMan) (Version 5.3. Copenhagen: The Nordic Cochrane Centre, The Cochrane Collaboration, 2014). Mean difference between the experimental and control groups was selected to be the effect size of the continuous outcome with 95% confidence intervals. Duration of diarrhoea was identified as the only common outcome variable in the selected studies and therefore used in the meta-analysis. Where there were more than 1 intervention group, either similar experimental groups were combined to create a single pair-wise comparison or the more appropriate group was selected (). A conversion of result of outcome measure, shown as median and range to mean and standard deviation was achieved as recommended by (; ; ), in order to pool data together. It is however important to note that conversions such as this could result in overestimation of mean and standard deviation (), therefore, interpretation of pooled results of this meta-analysis would need to be made with caution.
Assessment of risk of bias in included trials
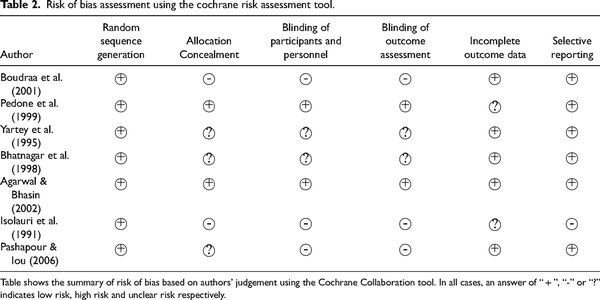
Risk of bias was assessed using the Cochrane Collaboration tool which includes the following criteria: adequacy of random sequence generation; allocation concealment; blinding of participants; personnel, and outcome assessors; incomplete outcome data and selective reporting. In all cases, an answer of “ + ”, “-” and “?” indicated low risk, high risk and unclear risk respectively. There was no formal assessment of publication bias using funnel plot due to small number (< 10) of selected articles ().
Results
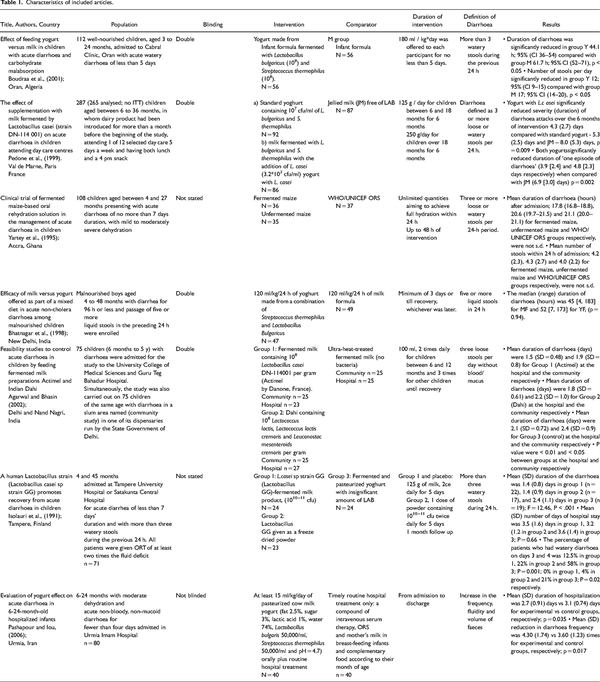
Seven RCTs (; ; ; ; ), were identified to have met the inclusion criteria. Figure 1 is the flow diagram showing the process of identification for the eligible trials and the characteristics of the excluded trials, with reasons for exclusion. Characteristics of these articles, assessment of risk of bias and Grading of Recommendations, Assessment, Development and Evaluations (GRADE#0 analysis can be found in Tables 1, 2 and 3 respectively. undertook these two studies independently and simultaneously in two different settings, the community and the hospital. Data from these settings were separately, hence eight studies were included for analysis. Only children aged between 3 months and 5 years old were recruited as participants in all selected studies. Two, of the 8 studies were not carried out with in-patients. These were studies by with children recuited from day care centres and , who recruited children from Nand Nagri for the community based study.
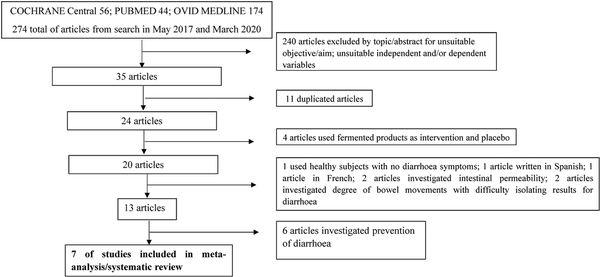
Figure 1
Shows the PRISMA flow chart for literature search.
and used WHO’s recommendations of oral rehydration solution (ORS), as control in their study. , , compared the effects of fermented milk against unfermented milk on the outcomes of diarhoea whilst studies by and used heat treated fermented milk as control. and administered ORS to all participants in both groups. However, whilst continued to administer the solution throughout the study, stopped the administration of ORS before patients were randomised into groups.
Duration of diarrhoea
This outcome was reported by six out for the seven RCT included in the current study using a total population of 603 children. The pooled results from the articles (Figure 2), showed that overall mean duration of diarrhoea was significantly less, in the experimental group in comparison with the control group, −0.61 days; 95% CI (−1.04, −0.18). Subgroup analysis separating studies which utilised a non-fermented control and ORS from those that used heat-treated fermented food as control found mean duration of diarrhoea was significantly lower in the group administered fermented foods in comparison with the heat-treated and ORS control group, −0.58 days; 95% CI (−0.97, −0.20) and −0.61 days; 95% CI (−0.19, −0.09) respectively, but not significantly different when compared with the non-fermented control −0.74 days; 95% CI (−1.52, 0.05).
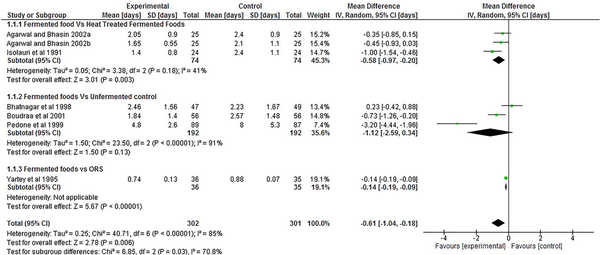
Figure 2
Forest plot showing the effect of intake of fermented food on duration of diarrhoea.
Secondary outcomes
Two RCTs each report mean daily number of stools and mean length of hospitalisation (days). Fermented foods did not significantly reduce mean daily stool frequency −2.00 95% CI (−7.03, 3.04), but significantly reduced the length of hospitalisation, −0.35 days 95% CI (−0.69, −0.02) in comparison to the control group (Figure 3).
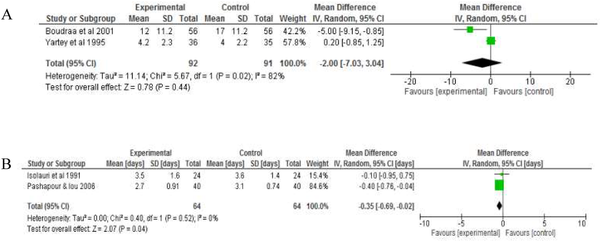
Figure 3
Forest plot showing the effect of intake of fermented food on A) mean daily stool frequency B) duration of hospitalisation. Data shows outcome of random effect models for both analysis analyses.
Discussion
This systematic review and meta-analyses of pooled data from six studies suggests that administering fermented foods during an episode of diarrhoea in infants under five years of age, may reduce the duration of the disease in comparison to the control groups. Fermented foods were shown to reduce the duration of diarrhoea compared to heat treated fermented food controls. This beneficial effect was observed when ORS was used as control, suggesting that fermented foods may have a potential role in the management of diarrhoeal disease. There was no clear effect on daily stool frequency, but duration of hospitalisation was reduced following administration of fermented foods.
The significant treatment effect observed in the mean duration of diarrhoea between the experimental group administered with fermented foods using the HT fermented food or ORS as control could an effect associated with a number of potentially active components within the fermented foods including the fermentation organisms and the products of fermentation process. The difference in the therapeutic effect observed in fermented foods versus heat treated fermented food is suggestive that the beneficial effects may be associated with the presence of viable fermentation organisms in the product. However, the plausible complementary effects of other fermentation by-products should not be ignored. Studies have investigated the effect of fermentation organisms in the fermented product, testing for probiotic effects of these organisms and using heated treated matching fermented product or products that do not contain the organism of interest as the comparator (; ; ). Species of LAB including Lb kefiri, Lb fermentum, Lb. acidophilus, Lb. rhamnosus, Lb. plantarum, Lb. casei, have been investigated in in vitro and in vivo studies and have been reported to have probiotic properties (; ; ; ; ; ). Nevertheless, LAB are able to also produce heat-stable bacteriocins as well as heat-labile antimicrobial proteins (), which may be effective in the restoration of gut health during an incident of diarrhoea.
According to the meta-analysis by , Lb rhamnosus GG (LGG) and Lb casei are LAB organisms which have most commonly been investigated for the management of gastrointestinal diseases including diarrhoea. The authors further reported in their study that these LAB had the potentials for preventive and therapeutic management of infectious diarrhoea but not for travellers’ diarrhoea in adults. There were variations in factors such as duration of administration, quantity administered varied and age of subject, across the studies included in the meta-analysis which may have caused the variations in the outcomes of both types of diarrhoea. Furthermore, the specificity of the health benefits that may be derived from organisms with probiotic properties may have contributed to inconsistencies that may have been observed between studies, as strains of the same species may not proffer the same health benefit to their host (; ; ; ). reported a more significant probiotic effect in combined species of Lactobacilli compared to studies that used only one species but higher doses of probiotic organisms did not significantly enhance the therapeutic effect of probiotics organisms against gastrointestinal diseases. They therefore suggested that dosage size of metabolites formed in fermented foods on the other hand may be crucial to the effectiveness of these foods in enhancing gastrointestinal health.
Majority of the studies found during the search, investigated the efficacy of fermented foods in the prevention rather than treatment of diarrhoea and other related gastrointestinal diseases (; ; ; ) with a combination of heat treated and unfermented controls. However, in vivo studies that have investigated the effect of cell free or heat treated fermented foods versus unfermented foods were unavailable. Such in vivo studies may be able to give insights on the effect of the combined components of the complex matrix of fermented food, excluding the fermentation organisms, on health. Though the results of this current study may indicate that the fermentation organisms may help improve gut health during an episode of diarrhoea, it also supports the possibility that the complex matrix of the fermented food, which includes the organisms, end-products and by-products of fermentation may be of greater significance, than the isolation of each component.
Furthermore, there is limited number of studies that have investigated treatment of diarrhoea using fermented foods other than dairy products in both adults and infants (; ; ; ). The potential of fermented cereals having similar outcomes as fermented dairy products should not be overlooked. Only one article, which was included in the current study. , also investigated that effect of a fermented cereal product in diarrhoea but was excluded from this study as it did not meet the inclusion criteria. Sub-analysis of fermented corn versus ORS in the current study suggests that fermented maize may be more effective in reducing the duration of diarrhoea than ORS, though the analysis was done with one article. This could imply that fermented corn may be a plausible effective alternative when ORS is not easily available especially in locations where corn is a staple meal. Furthermore, reported a significant difference, though marginal, in the reduction in the daily stool frequency with the group that was administered fermented milk having a decreased frequency compared to the group that was administered intravenous serum therapy and oral rehydration solution (ORS). This therefore buttresses the need for further research to be undertaken to investigate the efficacy of fermented food in the management of diarrhoea.
Limitation of meta-analysis
Due to limited number of studies that have investigated the effectiveness of fermented foods, in the treatment of diarrhoea, only seven articles were included in the current review due to the limited number of studies of fermented foods in infants. On the other hand, many more studies have investigated the effectiveness of fermentation organisms particularly those that have tested for probiotic potentials (; ; ; ) in the prevention or treatment of diarrhoea, especially in adults. Secondly, the disappearance of a significant outcome in the comparison between fermented foods versus unfermented foods in the sub-group analysis may be related to the variation in the types of controls that were used in the studies. But more importantly, the plausible overestimation of mean and standard deviation resulting from the conversion of the median and range values reported the included study by may have also notably contributed to the disappearance of this statistical significance. Thirdly, a significant degree of statistical heterogeneity was observed but not surprising as there were marked diversity in the ages of the participants, population size, location of study, duration and dosage of intervention between studies. Also, only 2 studies included in the review (; ) showed low risk of bias using the Cochrane assessment tool. The quality of trials included in a meta-analysis will have a great impact on the validity of the results (; ). declared support from DANONE and Foundation for Nutrition Research respectively for their studies.
Conclusion and future research
Fermented food products are widely available and consumed around the world. Probiotic potential of the organisms involved in the fermentation process have been documented. Health benefits of some of the end-products and by products of fermentation have also been suggested. Fermented foods are traditionally consumed in some African and Asian communities, not only for the nutritional benefits but also for possible therapeutic effects they are perceived to have in the treatment of gastrointestinal diseases such as diarrhoea. There is however limited number of studies to evidence this perception. The current review has attempted to do this using limited amount of data available on the effect of fermented food on infant diarrhoea. Results indicate that fermented foods may be helpful in the treatment of diarrhoea in infants up to the age of five, maybe to the same degree as, or better than as suggested by and , the ORS recommended by the WHO. Given the small number of trials and methodological limitations of the included studies, the evidence in this review should be viewed with caution. However, the efficacy of fermented foods has shown promise and warrants further investigation to inform health workers of the benefits gained in nutrition, health and well-being particularly amongst very young children. More good quality trials are required to investigate the complex matrix of fermented food products, other than dairy foods, in the management, particularly treatment of gastrointestinal diseases such as diarrhoea.
Abbreviations
WHO: World Health Organisation
GIT: gastrointestinal tract
LAB: lactic acid bacteria
ORS: oral rehydration solution
SCFA: Short Chain Fatty Acid
EPS: Extracellular polysaccharides
RCTs: Randomised controlled trials
PRISMA: Preferred Reporting Items for Systematic Reviews and Meta-Analyses
Ethical approval This analysis was exempt from ethics because there was no direct involvement of animal or human subjects.
Guarantor Not Applicable
Contributorship AO: Conceptualised the idea; Article is part of the PhD thesis; Undertook writing, data extraction and meta- analysis
Contributorship DM: Draft review and organisation
Contributorship YK: Draft review, organisation and re-write where necessary
Contributorship NP: PhD supervisor, data extraction, draft review
Availability of data and materials The data that support the findings of this study are available on request from the corresponding author.
Consent for publication All authors have approved the version of the manuscript submitted and agreed to publish this in Nutrition and Health. All authors also declare that this study is not published or submitted elsewhere for peer review.
Declaration of conflicting interests The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding The author(s) received no financial support for the research, authorship, and/or publication of this article.
ORCID iDs Adetokunbo Olayanju https://orcid.org/0000-0002-5021-2832
ORCID iDs Duane Mellor https://orcid.org/0000-0002-1369-3868
References
- Achi OK, Asamudo NU (2018) Cereal-based fermented foods of Africa as functional foods. In: Mérillon JM, Ramawat KG (eds) Bioactive Molecules in Food. Cham: Springer International Publishing, pp.1–32.
- Adams CA (2010) The probiotic paradox: Live and dead cells are biological response modifiers. Nutrition Research Reviews 23(1): 37–46.
- Agarwal K, Bhasin S (2002) Feasibility studies to control acute diarrhoea in children by feeding fermented milk preparations Actimel and Indian Dahi. European Journal of Clinical Nutrition 56(S4): S56–S59.
- Agostoni C, Goulet O, Kolacek S, et al. (2007) Fermented infant formulae without live bacteria. Journal of Pediatric Gastroenterology and Nutrition 44(3): 392–397.
- Ali AA (2010) Beneficial role of lactic acid Bacteria in food preservation and human health: A review. Research Journal of Microbiology 5(12): 1213–1221.
- Allen SJ, Martinez EG, Gregorio GV, et al. (2010) Probiotics for treating acute infectious diarrhoea. Cochrane Database Sysematict Review 11(11): CD003048.
- Anukam KC, Reid G (2009) African Traditional fermented foods and probiotics. Journal of Medical Foods 12(6): 1177–1184.
- Bao Y, Zhang Y, Zhang Y, et al. (2010) Screening of potential probiotic properties of Lactobacillus fermentum isolated from traditional dairy products. Food Control 21(5): 695–701.
- Beausoleil M, Fortier N, Guénette S, et al. (2007) Effect of a fermented milk combining Lactobacillus acidophilus Cl1285 and Lactobacillus casei in the prevention of antibiotic-associated diarrhea: A randomized, double-blind, placebo-controlled trial. Canadian Journal of Gastroenterology and Hepatology 21(11): 732–736.
- Beniwal RS, Arena VC, Thomas L, et al. (2003) A randomized trial of yogurt for prevention of antibiotic-associated diarrhea. Digestive Diease and Science 48(10): 2077–2082.
- Bhatnagar S, Singh KD, Sazawal S, et al. (1998) Efficacy of milk versus yogurt offered as part of a mixed diet in acute noncholera diarrhea among malnourished children. The Journal of Pediatrics 132(6): 999–1003.
- Boudraa G, Benbouabdellah M, Hachelaf W, et al. (2001) Effect of feeding yogurt versus milk in children with acute diarrhea and carbohydrate malabsorption. Journal of Pediatric Gastroenterology and Nutrition 33(3): 307–313.
- Campana R, van Hemert S, Baffone W (2017) Strain-specific probiotic properties of lactic acid bacteria and their interference with human intestinal pathogens invasion. Gut Pathogens 9(1): 12.
- Chelule P, Mokoena M, Gqaleni N (2010) Advantages of traditional lactic acid bacteria fermentation of food in Africa. In: Méndez-Vilas A (ed) Current Research, Technology and Education Topics in Applied Microbiology and Microbial Biotechnology. Badajoz, Spain: Formatex Research Center, pp.1160–1167.
- Das D, Baruah R, Goyal A (2014) A food additive with prebiotic properties of an α-d-glucan from Lactobacillus plantarum DM5. International Journal of Biological Macromolecules 69: 20–26.
- de Vrese M, Kristen H, Rautenberg P, et al. (2011) Probiotic lactobacilli and bifidobacteria in a fermented milk product with added fruit preparation reduce antibiotic associated diarrhea and Helicobacter pylori activity. Journal of Dairy Research 78(4): 396–403.
- Donot F, Fontana A, Baccou J, et al. (2012) Microbial exopolysaccharides: Main examples of synthesis, excretion, genetics and extraction. Carbohydrate Polymers 87(2): 951–962.
- Fox MJ, Ahuja KD, Robertson IK, et al. (2015) Can probiotic yogurt prevent diarrhoea in children on antibiotics? A double-blind, randomised, placebo-controlled study. British Medical Journal Open 5(1): e006474.
- Franz CM, Huch M, Mathara JM, et al. (2014) African Fermented foods and probiotics. International Journal of Food Microbiology 190: 84–96.
- Gibson GR, Probert HM, Van Loo J, et al. (2004) Dietary modulation of the human colonic microbiota: Updating the concept of prebiotics. Nutrition Research Reviews 17(2): 259–275.
- Gibson GR, Roberfroid MB (1995) Dietary modulation of the human colonic microbiota: Introducing the concept of prebiotics. The Journal of Nutrition 125(6): 1401–1412.
- Gomi A, Yamaji K, Watanabe O, et al. (2018) Bifidobacterium bifidum YIT 10347 fermented milk exerts beneficial effects on gastrointestinal discomfort and symptoms in healthy adults: A double-blind, randomized, placebo-controlled study. Journal of Dairy Science 101(6): 4830–4841.
- Gosálbez L, Ramón D (2015) Probiotics in transition: Novel strategies. Trends in Biotechnology 33(4): 195–196.
- Greco T, Zangrillo A, Biondi-Zoccai G, et al. (2013) Meta-analysis: Pitfalls and hints. Heart, Lung and Vessels 5(4): 219.
- Grosu-Tudor SS, Stancu MM, Pelinescu D, et al. (2014) Characterization of some bacteriocins produced by lactic acid bacteria isolated from fermented foods. World Journal of Microbiology and Biotechnology 30(9): 2459–2469.
- Higgins JPT, Thomas J, Chandler J, et al. (2011) Cochrane handbook for systematic reviews of interventions Version 6.2. Available at www.training.cochrane.org/handbook (accessed March 2021).
- Hill C, Guarner F, Reid G, et al. (2014) Expert consensus document: the international scientific association for probiotics and prebiotics consensus statement on the scope and appropriate use of the term probiotic. Nature Reviews Gastroenterology and Hepatology 11(8): 506–514.
- Hodges K, Gill R (2010) Infectious diarrhea: cellular and molecular mechanisms. Gut Microbes 1(1): 4–21.
- Hozo SP, Djulbegovic B, Hozo I (2005) Estimating the mean and variance from the median, range, and the size of a sample. BMC Medical Research Methodology 5(1): 13.
- Hutkins RW, Krumbeck JA, Bindels LB, et al. (2016) Prebiotics: Why definitions matter. Current Opinion in Biotechnology 37: .
- Isolauri E, Rautanen T, Juntunen M, et al. (1991) A human Lactobacillus strain (Lactobacillus casei sp strain GG) promotes recovery from acute diarrhea in children. Pediatrics 88(1): 90–97.
- Karovicova ZKJ (2007) Fermentation of cereals for specific purpose. Journal of Food and Nutrition Research 46(2): 51–57.
- Kiela PR, Ghishan FK (2016) Physiology of intestinal absorption and secretion. Best Practice and Research Clinical Gastroenterology 30(2): 145–159.
- Lei V, Friis H, Michaelsen KF (2006) Spontaneously fermented millet product as a natural probiotic treatment for diarrhoea in young children: An intervention study in northern Ghana. International Journal of Food Microbiology 110(3): 246–253.
- Levine GA, Walson JL, Atlas HE, et al. (2017) Defining pediatric diarrhea in low-resource settings. J Pediatric Infectious Disease Society 6(3): 289–293.
- Liu C, Lu J, Lu L, et al. (2010) Isolation, structural characterization and immunological activity of an exopolysaccharide produced by Bacillus licheniformis 8-37-0-1. Bioresource Technology 101(14): 5528–5533.
- Luo D, Wan X, Liu J, et al. (2018) Optimally estimating the sample mean from the sample size, median, mid-range, and/or mid-quartile range. Statistical Methods in Medical Research 27(6): 1785–1805.
- Marsh AJ, Hill C, Ross RP, et al. (2014) Fermented beverages with health-promoting potential: Past and future perspectives. Trends in Food Science and Technology 38(2): 113–124.
- Merenstein DJ, Foster J, D'Amico F (2009) A randomized clinical trial measuring the influence of kefir on antibiotic-associated diarrhea: The measuring the influence of Kefir (MILK) study. Archives of Pediatric and Adolescent Medicine 163(8): 750–754.
- Millette M, Luquet FM, Ruiz MT, et al. (2008) Characterization of probiotic properties of Lactobacillus strains. Dairy Science and Technology 88(6): 695–705.
- Moher D, Liberati A, Tetzlaff J, et al. (2009) Preferred reporting items for systematic reviews and meta-analyses: The PRISMA statement. PLoS Medicine 6(7): e1000097.
- Mokoena MP, Mutanda T, Olaniran AO (2016) Perspectives on the probiotic potential of lactic acid bacteria from African traditional fermented foods and beverages. Food and Nutrition Research 60: 1.
- Morrison DJ, Preston T (2016) Formation of short chain fatty acids by the gut microbiota and their impact on human metabolism. Gut Microbes 7(3): 189–200.
- Nicholson JK, Holmes E, Kinross J, et al. (2012) Host-gut microbiota metabolic interactions. Science (New York, N.Y.) 336(6086): 1262–1267.
- Njume C, Goduka NI (2012) Treatment of diarrhoea in rural African communities: An overview of measures to maximise the medicinal potentials of indigenous plants. Interantional Journal of Environmental Research and Public Health 9(11): 3911–3933.
- Nwodo UU, Green E, Okoh AI (2012) Bacterial exopolysaccharides: Functionality and prospects. International Journal of Molecular Sciences 13(11): 14002–14015.
- Nyanzi R, Jooste P (2012) Cereal-based functional foods. In: Rigobelo EC (ed) Probiotics. Rijeka: INTECH Open Access Publisher, pp.161–197.
- O'Callaghan A, van Sinderen D (2016) Bifidobacteria and their role as members of the human gut microbiota. Frontiers in Microbiology 7: 25.
- Park KY, Jeong JK (2015) Kimchi (Korean Fermented Vegetables) as a probiotic food. In: Watson RR, Preedy VR (eds) Probiotics, Prebiotics, and Synbiotics: Bioactive Foods in Health Promotion. London, UK: Academic Press, pp.391–404.
- Pashapour N, Iou SG (2006) Evaluation of yogurt effect on acute diarrhea in 6-24-month-old hospitalized infants. Turkish Journal of Pediatrics 48(2): 115–118.
- Patro-Golab B, Shamir R, Szajewska H (2015b) Yogurt for treating acute gastroenteritis in children: Systematic review and meta-analysis. Clinical Nutrition 34(5): 818–824.
- Patro-Golab B, Shamir R, Szajewska H (2015a) Yogurt for treating antibiotic-associated diarrhea: Systematic review and meta-analysis. Nutrition (Burbank, Los Angeles County, Calif.) 31(6): 796–800.
- Pedone C, Bernabeu A, Postaire E, et al. (1999) The effect of supplementation with milk fermented by Lactobacillus casei (strain DN-114 001) on acute diarrhoea in children attending day care centres. International Journal of Clinical Practice 53(3): 179–184.
- Pimentel RR (2003) Antibiotic-Associated Diarrhea. Available at: http://www.clevelandclinicmeded.com/diseasemanagement/gastro/antibioticdiarrhea/antibioticdiarrhea.htm (accessed 10th September 2019).
- Pisano MB, Viale S, Conti S, et al. (2014) Preliminary evaluation of probiotic properties of Lactobacillus strains isolated from Sardinian dairy products. Biomedical Research International 2014: .
- Pitkälä KH, Strandberg T, Finne-Soveri U, et al. (2007) Fermented cereal with specific bifidobacteria normalizes bowel movements in elderly nursing home residents. A randomized, controlled trial. The journal of nutrition. Health and Aging 11(4): 305.
- Ramos CL, Thorsen L, Schwan RF, et al. (2013) Strain-specific probiotics properties of Lactobacillus fermentum, Lactobacillus plantarum and Lactobacillus brevis isolates from Brazilian food products. Food Microbiology 36(1): 22–29.
- Rautiola E (2013) Short Chain Fatty Acid Production by Probiotic Organisms in the Gastrointestinal Tract. BSc (Hons) Thesis, Eastern Michigan University, USA.
- Ray M, Ghosh K, Singh S, et al. (2016) Folk to functional: An explorative overview of rice-based fermented foods and beverages in India. Journal of Ethnic Foods 3(1): 5–18.
- Ringel-Kulka T, Kotch JB, Jensen ET, et al. (2015) Randomized, double-blind, placebo-controlled study of synbiotic yogurt effect on the health of children. Journal of Pediatrics 166(6): 1475–1481.
- Ritchie ML, Romanuk TN (2012) A meta-analysis of probiotic efficacy for gastrointestinal diseases. PLoS One 7(4): e34938.
- Rivera-Espinoza Y, Gallardo-Navarro Y (2010) Non-dairy probiotic products. Food Microbiology 27(1): 1–11.
- Roberfroid M (2007) Prebiotics: the concept revisited. The Journal of Nutrition 137(3): 830S–837S.
- Salazar N, Gueimonde M, de los Reyes-Gavilán CG, et al. (2016) Exopolysaccharides produced by lactic acid bacteria and bifidobacteria as fermentable substrates by the intestinal microbiota. Critical Reviews in Food Science and Nutrition 56(9): 1440–1453.
- Sanlibaba P, Çakma GA (2016) Exopolysaccharides production by lactic acid Bacteria. Applied Microbiology Open Access 2(2): 5.
- Song D, Ibrahim S, Hayek S (2012) Recent application of probiotics in food and agricultural science. In: Rigobelo E (ed) Probiotics. London: Intechopen, pp.2–34.
- Sterne JA, Sutton AJ, Ioannidis JP, et al. (2011) Recommendations for examining and interpreting funnel plot asymmetry in meta-analyses of randomised controlled trials. British Medical Journal 343: d4002.
- Szajewska H, Skórka A, Pieścik-Lech M (2015) Fermented infant formulas without live bacteria: A systematic review. European Journal of Pediatrics 174(11): 1413–1420.
- Tamang JP, Watanabe K, Holzapfel WH (2016) Review: Diversity of microorganisms in global fermented foods and beverages. Frontiers in Microbiology 7: 377.
- Vinderola CG, Duarte J, Thangavel D, et al. (2005) Immunomodulating capacity of kefir. Journal of Dairy Research 72(2): 195–202.
- Wan X, Wang W, Liu J, et al. (2014) Estimating the sample mean and standard deviation from the sample size, median, range and/or interquartile range. BMC Medical Research Methodology 14(1): 35.
- Watson RR, Preedy VR (2015) Probiotics, Prebiotics, and Synbiotics. Amsterdam: Academic Press.
- Wenus C, Goll R, Loken EB, et al. (2008) Prevention of antibiotic-associated diarrhoea by a fermented probiotic milk drink. European Journal of Clinical Nutrition 62(2): 299–301.
- Whelan K (2013) Mechanisms and effectiveness of prebiotics in modifying the gastrointestinal microbiota for the management of digestive disorders. Proceedings of the Nutrition Society 72(3): 288–298.
- Widyastuti Y, Febrisiantosa A (2014) The role of lactic acid bacteria in milk fermentation. Food and Nutrition Sciences 5(4): 435–442.
- Wilson SE, Morris SS, Gilbert SS, et al. (2013) Scaling up access to oral rehydration solution for diarrhea: learning from historical experience in low- and high-performing countries. Journal of Global Health 3(1): 010404.
- World Health Organisation (2005) The treatment of diarrhoea: a manual for physicians and other senior health workers. Available at: http://apps.who.int/iris/bitstream/handle/10665/43209/9241593180.pdf?sequence = 1 (accesses 22 February 2019).
- Yartey J, Nkrumah F, Hori H, et al. (1995) Clinical trial of fermented maize-based oral rehydration solution in the management of acute diarrhoea in children. Annals of Tropical Paediatrics 15(1): 61–68.
- Zheng Y, Lu Y, Wang J, et al. (2013) Probiotic properties of Lactobacillus strains isolated from Tibetan kefir grains. PloS One 8(7): e69868.