Herpes zoster (HZ) or shingles, as its commonly known, results from reactivation of the varicella zoster virus (VZV), which lies dormant in the spinal and cranial sensory ganglia after primary infection in childhood []. Herpes zoster presents as a painful, erythematous, maculopapular rash in which lesions become fluid-filled before crusting over. Unique features that distinguish HZ from other dermatological rashes are unilateral presentation and restriction to a single dermatome []. Through various mechanisms, VZV is reactivated to cause HZ. Although treatment is available via antiviral therapy, there are many ophthalmic, vascular, visceral, and neurological complications of HZ [, ]. These complications lead to increased all-cause total healthcare cost and place financial burdens on patients []. The major complication associated with HZ is postherpetic neuralgia, pain persisting for over 90 days after shingles onset, that occurs within 20% of HZ patients [] with an estimated prevalence of 0.5–1 million [].
It is clear from the literature that millions of individuals are affected each year by shingles or HZ infection around the globe; in the United States, more than 1 million new cases of HZ are reported every year []. The incidence of HZ ranges from 3 to 5 per 100 000 in North America, Europe, and Asia, but more importantly, the incidence seems to be increasing with time, and it is unclear to what this may be related [, ]. Numerous studies have identified risk factors associated with reactivation of VZV, many of which are related to a decrease in T-cell immunity, such as aging and immunosuppression, but some are related to family history or stress []. We have previously conducted a meta-analysis that pooled data from randomized clinical trials and observational studies to determine the magnitude of risk with different immunosuppressive regimens in patients with rheumatoid arthritis (RA), systemic lupus erythematosus (SLE), and inflammatory bowel disease (IBD) []. This time, we have focused on pooling data from all studies evaluating the risk of developing HZ infection except those studies evaluating risk with taking immunosuppressive medications.
METHODS
This systematic review and meta-analysis was reported according to the MOOSE guidelines for the reporting of observational studies, in accordance with the PRISMA guidelines for conducting a meta-analysis [, ].
Data Sources and Search Strategy
We conducted a search of MEDLINE, EMBASE, Cochrane Central, Cochrane Systematic Reviews, Web of Science, and CAB Direct for articles reporting on HZ infection and associated risk from January 1, 1966 to January 31, 2019. Search terms as keywords, Mesh terms and subject headings included the following: zoster OR herpe* OR postherpe* OR shingle* AND risk OR immunosupp* OR stress OR trauma OR gender OR ethnicity OR race OR age OR diabetes OR asthma OR chronic obstructive pulmonary disease OR diabetes. After pooling the articles and deleting duplicates, a manual review of titles was conducted screening for relevant topics and keywords. Another final manual review of article abstract was conducted on shortlisted articles. The literature search was performed by an author (B.H.) and a university librarian, the article review was conducted by 2 authors (B.H. and N.V.), and uncertainty and revisions were resolved by consensus.
Inclusion and Exclusion Criteria
We included all English studies that evaluated the risk factors associated with HZ in the population. We excluded cases and case series reports and literature reviews. We also excluded all studies that used immunosuppressive medications, including biologics, disease-modifying antirheumatic drugs, and/or corticosteroids.
Data Extraction, Study Verification, and Quality Assessment
Data were extracted independently by 2 authors using a standardized abstraction form, with discrepancies being resolved through consensus. Data extracted from the studies included the author, date of the study, type of study, inclusion and exclusion criteria, risk factor, number of patients, confounders adjusted for, demographics, and study outcome data. Quality assessment of the studies was conducted by 2 authors independently using the Newcastle-Ottawa quality assessment scale [].
Statistical Analysis
Pooled risk ratios (RRs) with 95% confidence intervals (CI) were calculated for the risk of HZ associated with key risk factors, using natural logarithm of reported effect estimates, and their corresponding CI. Because these observational studies were conducted in different geographic locations, the true effect estimate will likely vary and represent a random sample of effect estimates; therefore, pooled estimates were obtained using a random-effects model.
We compared the risk of HZ in those with the following: (1) innate risk factors, such as race, sex, age, and family history; (2) immunosuppression (human immunodeficiency virus [HIV]/acquired immune deficiency syndrome or malignancy [AIDS]); (3) comorbidities including asthma, chronic obstructive pulmonary disease (COPD), cardiovascular diseases (CVDs), IBD, depression, diabetes, chronic renal disease, SLE; and (4) other studies (physical trauma, psychological stress, smoking).
We measured heterogeneity across studies using the I2 statistic, with higher values reflecting increasing heterogeneity []. Sources of heterogeneity were assessed by subgroup analysis and by meta-regression []. Subgroup analyses included disease subtypes, mean age, sex ratio, and study type. Using funnel plots, publication bias was assessed, and asymmetry was assessed by conducting the Egger test []. Statistical analyses were conducted in R version 3.3.2. All analyses were 2 sided with P < .05 defining statistical significance.
RESULTS
Search Results, Trial Characteristics, and Risk of Bias
The literature and manual references search identified 4417 studies (Figure 1). The majority of these studies were excluded based on the title and/or abstract screening and removal of duplicate records (N = 4192). Two hundred twenty-five studies were included for a full article review and 88 studies were included, corresponding to 68 cohort studies [], and 20 case-control studies []. Reasons for exclusion were mainly irrelevant topic, immunosuppressive therapy focus, study design, studies were evaluating herpes treatment and vaccine effect rather than risk factors, complications associated with HZ infection, or lack of quantitative data on the incidence of HZ associated with individual risk factors. One percent of the studies were published between 1965 and 1969, 5% were published from 1990 to 1999, 17% were published from 2000 to 2009, and 77% were published from 2010 to 2018.
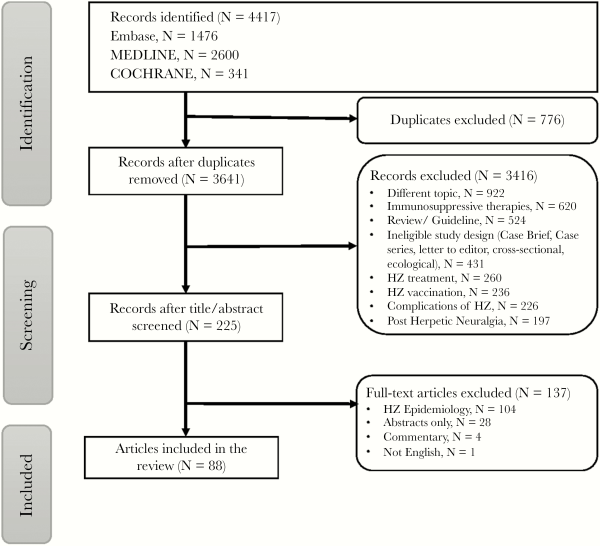
Figure 1
Screening of studies for inclusion into the study.
A summary of the baseline characteristics of patients included for analysis are presented in Supplementary eTable 1. A total study population of 198 751 846 was included, with 3 768 691 HZ cases reported in the included studies. The age ranged from 3 months to 104 years, the percentage of women in studies ranged from 0% to 100%, and the follow-up duration ranged from 1 to 62 years. Most studies were conducted in North America (Canada and United States; N = 35), followed by Asia (China, Iran, Israel, Japan, South Korea, and Taiwan; N = 31), and Europe (Belgium, Denmark, France, Germany, Italy, Spain, Netherlands, and United Kingdom; N = 20). Overall quality of the included studies ranged from fair to good, based on the Newcastle Ottawa scale assessment, ranging from 6 to 9 for cohort studies and 4 to 9 for case-control studies (Supplemental eTables 2 and 3).
Risk of Herpes Zoster
The estimates are presented in (Figure 2), and detailed forest plots for each outcome can be found in Supplemental eFigure 1–18). Within our categorization of innate characteristics, 56 studies evaluated gender and 39 studies looked at age as potential risk factors for development of HZ. A smaller number of studies evaluated race (N = 17) and family history of zoster (N = 9). Family history was strongly associated with an increased risk of HZ than controls (RR = 2.48, 95% CI, 1.70–3.60; I2 = 94.4%) (Figure 2). Both older age (RR = 1.65, 95% CI, 1.37–1.97; I2 = 100%) and gender (odds ratio [OR] = 1.19, 95% CI, 1.14–1.24; I2 = 99.4%) were associated with an increased HZ risk, but less so than family history. Of note, a lower risk of HZ was associated with black race (RR = 0.69, 95% CI, 0.56–0.85; I2 = 97.2%).
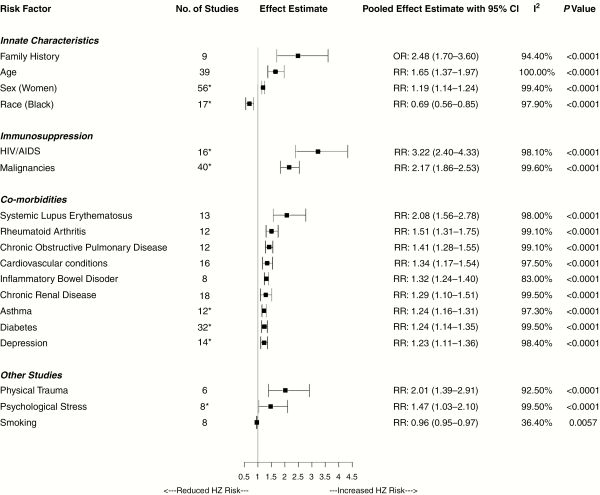
Figure 2
Pooled analysis of the risk of herpes zoster.
We found 40 studies that reported risk of HZ from malignancies and 18 studies on HIV/AIDS. Human immunodeficiency virus/AIDS was strongly associated with an increased risk of HZ compared with controls (RR = 3.22; 95% CI, 2.40–4.33; I2 = 98.1%). Malignancies, such as lymphoma and leukemia, were also strongly associated with risk of HZ (RR = 2.17; 95% CI, 1.86–2.53; I2 = 99.6%). Numerous studies evaluated risk of HZ with various concurrent illnesses including diabetes (N = 34), chronic renal disease (N = 18), CVD (N = 16), depression (N = 14), COPD (N = 13), SLE (N = 13), asthma (N = 12), RA (N = 12), and IBD (N = 8). Most of these comorbidities increased risk of HZ, RR ranging from 2.08 to 1.23, particularly SLE (RR = 2.08; 95% CI, 1.56–2.78; I2 = 98.00%) and RA (RR = 1.51; 95% CI, 1.31–1.75; I2 = 99.10%).
Other nonmedical factors evaluated in studies included psychological stress (N = 8), physical trauma (N = 6), and smoking (N = 8); HZ was strongly associated with physical trauma (RR = 2.01; 95% CI, 1.39–2.91; I2 = 92.5%), but the rest were not significant.
A considerable amount of heterogeneity was found through visual inspection of funnel plot assessment (data not shown), but after evaluation with Egger’s test only a handful of the risk factors (family history, SLE, psychological stress, and depression) were found to possess statistically significant heterogeneity. Most of the heterogeneity was found to be associated with study design, population, and geographic location.
Discussion
This is the most comprehensive systematic review assessing risk factors for HZ infection. Our meta-analysis indicates that immunosuppression through HIV/AIDS or malignancy places individuals at significant risk of reactivating the latent viral suppression. Family history of zoster, physical trauma, and older age also significantly increased risk. Although the risk is present with female gender, psychological stress, or presence of comorbidities, such as diabetes, RA, CVDs, renal disease, SLE, and IBD, it was slightly less than the former risk factors.
The elevated risk in the older patients is likely due to immunosenescence, in which the immune system progressively deteriorates as individuals age []. Varicella zoster-virus is normally kept dormant in dorsal root sensory ganglia via specific cell-mediated immunity (CMI) [, ]. Cell-mediated immunity naturally declines over time, but it is normally boosted by exogenous and endogenous methods, thereby limiting VZV’s ability to reactivate and cause HZ. Exogenous boosting occurs through repeated exposures to wild-type VZV causing subclinical infections that are immediately mediated by cellular processes []. Endogenously, subclinical reactivation of VZV stimulates CD4+ cells to release cytokines, including tumor necrosis factor (TNF)-alpha, interferon-gamma, and interleukin-2 (IL-2) [, ]. The latter agent enables T-helper cells to stimulate neutrophils and macrophages that phagocytize the zoster virus. In addition, IL-2 promotes CD8+ cells to release proteases, interferon-gamma, and lysins to destroy viral cells. Finally, CD4+ cells also play a role in memory B-cells stimulation and immunoglobulin G-mediated B-cell proliferation []. In elderly patients with reduced immune function, VZV- specific T-cell immunity (CD4, CD8, and memory T cells) is below the clinical threshold of maintaining virus latency, thus placing this population at elevated risk of developing HZ []. In our meta-analysis, most of the studies were conducted in the population aged 60 years and over (N = 36), with only 1 study reporting HZ risk in those aged 40 years and over [] and 2 studies reporting risk in individuals aged 50 years and above [, ]; the lack of data in these at-risk individuals meant we were unable to further characterize risk in these age groups.
It is well known that immunosuppressive conditions such as HIV/AIDS and malignancies [] result in decreased CMI that increases the risk of viral infections, such as zoster. In general, these individuals have low CD4+ and CD8+ cells and impaired lymphocyte proliferation. We were not able to do a separate analysis according to CD4 count because most studies reported HZ in HIV patients with >350 CD4 cell count and at the beginning of antiretroviral therapy. Immunosuppression also impacts recovery of CMI posttherapy, specifically towards IL-2 and CD4+, which play a significant role in limiting VZV reactivation to cause HZ []. Autoimmune diseases such as RA, IBD, and SLE also cause impaired CMI []. A study by Park et al [] found lower CD4+ cell counts (including TNF-alpha and interferon-gamma) in patients with SLE, which they stated may place individuals at higher risk of HZ. Furthermore, a study conducted by Nagasawa et al [] also discussed that SLE patients show altered immune function via tests for delayed hypersensitivity reactions, CD8+ activity, interferon production, and T-cell transformations. Elevated risk factors for both immunosuppressive conditions and autoimmune diseases were identified in our meta-analysis.
Only 1 other meta-analysis studied the impact of family history on developing HZ. Lai and Yew [] evaluated this relationship, as well as dose-response relationships and whether the number of relatives affected HZ rates. They included 5 case-control studies (N = 4169) and identified a statistically significant increase in the risk of family history with first-degree relatives (OR = 3.03; 95% CI, 1.86–4.94) []. One proposed genetic mechanism, discussed by Lai and Yew [], involves human leukocyte antigens (HLAs), specifically HLA-A, which is responsible for presenting peptides to CD8+ receptors to elicit an immune response. IE6862 is a VZV transcription factor protein and one of the main peptides responsible for eliciting CD8+ response; a study conducted by Meysman et al [] found that patients in Belgium who had lower HLA-A presentation ability of IE62 protein had a 60% greater risk of HZ.
Our meta-analysis found female gender places individuals at a slightly higher risk of HZ, but there is no definite explanation for this difference. However, a review conducted by Fleming et al [] proposed that gender biases during diagnosis may be a factor; another probable cause may be due to hormonal or biological differences between genders [].
Black race was seen to be protective against HZ in comparison to white individuals. There are few documented reasons for this occurrence. A possible explanation is due to differences in household composition; black individuals may have elevated exposure to varicella, which boosts CMI []. Other explanations may lie in genetic variations, racial differences in reporting disease or the frequency of medical interactions []. Finally, it may also be related to lower rates of healthcare access among black individuals, which may be due to mistrust or lack of a consistent healthcare source, and this may contribute to lower numbers of patients seen with HZ by a healthcare professional [, ].
Currently, there are 2 vaccines on the market, Zostavax (a live vaccine) and Shingrix (a recombinant zoster vaccine) []. Both vaccines increase cellular mediated immunity. Zostavax is no longer the recommended vaccination for HZ because vaccine effectiveness after 3 years is only slightly above 50% and is further diminished to ≤24% after 4 years []. In addition, Zostavax is less effective in older individuals and is contraindicated in immunosuppressive conditions (HIV/AIDs, malignancies), during immunosuppressive drug therapy and pregnancy []. Shingrix contains a recombinant VZV glycoprotein E and an adjuvant component that increase VZV-specific CMI and enhances specific humoral immunity, respectively [, ]. Shingrix is recommended to all adults ≥50 years old, in 2 separate intramuscular injections 2–6 months apart []. However, vaccination rates are still low and substantial efforts by healthcare professionals are required to increase uptake.
Our study was not without limitations. Most studies selected were observational (cohort or case-control studies) and, due to design, have a higher likelihood of bias. The potential for recall bias is possible in the studies that reported on family history and physical trauma []. Family history may have differential recall depending on various factors, such as the number of years since HZ events, variations in awareness of family history, or the strength of the relationships between families. Physical trauma can either impair patient’s ability to recall events or strengthen their memory of coinciding events. Finally, selection bias may also be present in studies that collected HZ cases from specialist clinics such as those from dermatologists []. However, bias was accounted for via risk of bias assessment, in which all studies scored low. Due to study design, studies were also at risk of confounding, although most studies minimized the risk by adjusting for some of the variables such as age, sex, or other comorbidities. It is likely that a patient with multiple risk factors is at higher risk for HZ compared with someone with single risk factor; however, because of the differences in study design and population, we were not able to determine cumulative risk in our study. Administrative data was the preferred method of data collection by a majority of the studies selected. This form of data can be miscoded, incorrect, or vary between practitioners []. Most studies accounted for the possible error through various methods such as the following: only including patients with first diagnosis of HZ, selecting databases that were validated for correctness and accuracy, or incorporating various sources to confirm diagnosis (International Classification of Diseases codes + antiviral prescriptions) [, , ]. Heterogenicity was high, which may be attributed to variations in study design, differences in outcome ascertainment (record linkage, hospital records, diagnosis by physician), characteristics of populations (age, gender, size), and countries in which studies were conducted.
Conclusions
In conclusion, HIV/AIDS, immunosuppression, family history, older age, trauma, females, and presence of comorbid conditions place individuals at an increased risk of HZ. With 2 vaccinations available on the market, physicians and other healthcare providers can target patient education based on their risk factors and improve the uptake of zoster vaccination.
Acknowledgments
Author contributions. F. M. conceived and designed the study; B. H. conducted literature research; K. P., B. H., and N. V. acquired the data; N. V. performed data analysis; F. M., N. V., B. H., and K. P. interpreted the data; F. M., K. P., and N. V. wrote the first draft of the article; F. M., N. V., K. P., and B. H. critically revised the article for important intellectual content; and F. M., N. V., K. P., and B. H. approved the version to be published.
Potential conflicts of interest. All authors: No reported conflicts of interest. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest.
References
- 1. Weller TH. Varicella and herpes zoster. Changing concepts of the natural history, control, and importance of a not-so-benign virus. N Engl J Med1983; 309:1434–40.
- 2. Cohen JI. Herpes zoster. N Engl J Med2013; 369:255–263.
- 3. Oxman MN. Clinical manifestations of herpes zoster. In: Arvin AM, Gershon AA, eds. Varicella-Zoster Virus: Virology and Clinical Management. Cambridge: Cambridge University Press; 2000: pp 246–75.
- 4. Somayaji R, Elliott JA, Sibbald RG. Dermatologic manifestations of herpes zoster. In: Watson CPN, Gershon AA, Oxman MN, eds. Herpes Zoster: Postherpetic Neuralgia and Other Complications: Focus on Treatment and Prevention. Cham: Springer International Publishing; 2017: pp 103–15.
- 5. Kovac M, Lal H, Cunningham AL, et al; ZOE-50/70 Study Group. Complications of herpes zoster in immunocompetent older adults: incidence in vaccine and placebo groups in two large phase 3 trials. Vaccine2018; 36:1537–41.
- 6. Rausch DA, Levin M, Meyers J, et al Cost of diagnosed herpes zoster complications in patients age ≥50 years: a U.S claims data analysis. Innovations in Aging2017; 1(Suppl_1):902.
- 7. Kost RG, Straus SE. Postherpetic neuralgia–pathogenesis, treatment, and prevention. N Engl J Med1996; 335:32–42.
- 8. Philip A, Thakur R. Post herpetic neuralgia. J Palliat Med2011; 14:765–73.
- 9. Oxman MN, Levin MJ, Johnson GR, et al; Shingles Prevention Study Group. A vaccine to prevent herpes zoster and postherpetic neuralgia in older adults. N Engl J Med2005; 352:2271–84.
- 10. Pinchinat S, Cebrián-Cuenca AM, Bricout H, Johnson RW. Similar herpes zoster incidence across Europe: results from a systematic literature review. BMC Infect Dis2013; 13:170.
- 11. Kawai K, Gebremeskel BG, Acosta CJ. Systematic review of incidence and complications of herpes zoster: towards a global perspective. BMJ Open2014; 4:e004833.
- 12. Thomas SL, Hall AJ. What does epidemiology tell us about risk factors for herpes zoster?Lancet Infect Dis2004; 4:26–33.
- 13. Marra F, Lo E, Kalashnikov V, Richardson K. Risk of herpes zoster in individuals on biologics, disease-modifying antirheumatic drugs, and/or corticosteroids for autoimmune diseases: a systematic review and meta-analysis. Open Forum Infect Dis2016; 3:ofw205.
- 14. Stroup DF, Berlin JA, Morton SC, et al Meta-analysis of observational studies in epidemiology: a proposal for reporting. Meta-analysis Of Observational Studies in Epidemiology (MOOSE) group. JAMA2000; 283:2008–12.
- 15. Moher D, Liberati A, Tetzlaff J, Altman DG; PRISMA Group. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. BMJ2009; 339:b2535.
- 16.
- 17. Higgins JP, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta-analyses. BMJ2003; 327:557–60.
- 18. Baker WL, White CM, Cappelleri JC, et al; Health Outcomes, Policy, and Economics (HOPE) Collaborative Group. Understanding heterogeneity in meta-analysis: the role of meta-regression. Int J Clin Pract2009; 63:1426–34.
- 19. Sterne JA, Egger M. Funnel plots for detecting bias in meta-analysis: guidelines on choice of axis. J Clin Epidemiol2001; 54:1046–55.
- 20. Antoniou T, Zheng H, Singh S, et al Statins and the risk of herpes zoster: a population-based cohort study. Clin Infect Dis2014; 58:350–6.
- 21. Ban J, Takao Y, Okuno Y, et al Association of cigarette smoking with a past history and incidence of herpes zoster in the general Japanese population: the SHEZ Study. Epidemiol Infect2017; 145:1270–5.
- 22. Blank LJ, Polydefkis MJ, Moore RD, Gebo KA. Herpes zoster among persons living with HIV in the current antiretroviral therapy era. J Acquir Immune Defic Syndr2012; 61:203–7.
- 23. Borkar DS, Tham VM, Esterberg E, et al Incidence of herpes zoster ophthalmicus: results from the Pacific Ocular Inflammation Study. Ophthalmology2013; 120:451–6.
- 24. Buchbinder SP, Katz MH, Hessol NA, et al Herpes zoster and human immunodeficiency virus infection. J Infect Dis1992; 166:1153–6.
- 25. Cebrián-Cuenca AM, Díez-Domingo J, Rodríguez MS, et al; ‘Herpes Zoster Research Group of the Valencian Community’. Epidemiology of herpes zoster infection among patients treated in primary care centres in the Valencian community (Spain). BMC Fam Pract2010; 11:33.
- 26. Chao DY, Chien YZ, Yeh YP, Hsu PS, Lian IB. The incidence of varicella and herpes zoster in Taiwan during a period of increasing varicella vaccine coverage, 2000–2008. Epidemiol Infect2012; 140:1131–40.
- 27. Chen HH, Chen YM, Chen TJ, et al Risk of herpes zoster in patients with systemic lupus erythematosus: a three-year follow-up study using a nationwide population-based cohort. Clinics 2011; 66:1177–82.
- 28. Chen HH, Lin CL, Yeh CJ, et al Statins can increase the risk of herpes zoster infection in Asia. Eur J Clin Microbiol Infect Dis2015; 34:1451–8.
- 29. Chen HH, Lin IC, Chen HJ, et al Association of herpes zoster and type 1 diabetes mellitus. PLoS One2016; 11:e0155175.
- 30. Chen D, Li H, Xie J, et al Herpes zoster in patients with systemic lupus erythematosus: clinical features, complications and risk factors. Exp Ther Med2017; 14:6222–8.
- 31. Côté-Daigneault J, Bessissow T, Nicolae MV, et al Herpes zoster incidence in inflammatory bowel disease patients: a population-based study. Inflamm Bowel Dis2019; 25:914–8.
- 32. Di Legami V, Gianino MM, Ciofi degli Atti M, et al; Zoster Study Group. Epidemiology and costs of herpes zoster: background data to estimate the impact of vaccination. Vaccine2007; 25:7598–604.
- 33. Esteban-Vasallo MD, Domínguez-Berjón MF, Gil-Prieto R, et al Sociodemographic characteristics and chronic medical conditions as risk factors for herpes zoster: a population-based study from primary care in Madrid (Spain). Hum Vaccin Immunother2014; 10:1650–60.
- 34. Fleming DM, Cross KW, Cobb WA, Chapman RS. Gender difference in the incidence of shingles. Epidemiol Infect2004; 132:1–5.
- 35. Gialloreti LE, Merito M, Pezzotti P, et al Epidemiology and economic burden of herpes zoster and post-herpetic neuralgia in Italy: a retrospective, population-based study. BMC Infect Dis2010; 10:230.
- 36. Glesby MJ, Hoover DR, Tan T, et al Herpes zoster in women with and at risk for HIV: data from the Women’s Interagency HIV Study. J Acquir Immune Defic Syndr2004; 37:1604–9.
- 37. Gonzalez Chiappe S, Sarazin M, Turbelin C, et al Herpes zoster: burden of disease in France. Vaccine2010; 28:7933–8.
- 38. Guignard AP, Greenberg M, Lu C, et al Risk of herpes zoster among diabetics: a matched cohort study in a US insurance claim database before introduction of vaccination, 1997-2006. Infection2014; 42:729–35.
- 39. Gupta G, Lautenbach E, Lewis JD. Incidence and risk factors for herpes zoster among patients with inflammatory bowel disease. Clin Gastroenterol Hepatol2006; 4:1483–90.
- 40. Hales CM, Harpaz R, Joesoef MR, Bialek SR. Examination of links between herpes zoster incidence and childhood varicella vaccination. Ann Intern Med2013; 159:739–45.
- 41. Harpaz R, Leung JW, Brown CJ, Zhou FJ. Psychological stress as a trigger for herpes zoster: might the conventional wisdom be wrong?Clin Infect Dis2015; 60:781–5.
- 42. Harpaz R, Dahl RM. Administrative data to explore the role of family history as a risk factor for herpes zoster. Mayo Clin Proc2018; 93:747–51.
- 43. Hata A, Kuniyoshi M, Ohkusa Y. Risk of herpes zoster in patients with underlying diseases: a retrospective hospital-based cohort study. Infection2011; 39:537–44.
- 44. Hodge WG, Seiff SR, Margolis TP. Ocular opportunistic infection incidences among patients who are HIV positive compared to patients who are HIV negative. Ophthalmology1998; 105:895–900.
- 45. Hope-Simpson RE. The nature of herpes zoster: a long-term study and a new hypothesis. Proc R Soc Med1965; 58:9–20.
- 46. Insinga RP, Itzler RF, Pellissier JM, et al The incidence of herpes zoster in a United States administrative database. J Gen Intern Med2005; 20:748–53.
- 47. Jih JS, Chen YJ, Lin MW, et al Epidemiological features and costs of herpes zoster in Taiwan: a national study 2000 to 2006. Acta Derm Venereol2009; 89:612–6.
- 48. Johnson BH, Palmer L, Gatwood J, et al Annual incidence rates of herpes zoster among an immunocompetent population in the United States. BMC Infect Dis2015; 15:502.
- 49. Kawai K, Yawn BP, Wollan P, Harpaz R. Increasing incidence of herpes zoster over a 60-year period from a population-based study. Clin Infect Dis2016; 63:221–6.
- 50. Kim MC, Yun SC, Lee SO, et al Statins increase the risk of herpes zoster: a propensity score-matched analysis. PLoS One2018; 13:e0198263.
- 51. Kuo CC, Lee CT, Lee IM, et al Risk of herpes zoster in patients treated with long-term hemodialysis: a matched cohort study. Am J Kidney Dis2012; 59:428–33.
- 52. Langan SM, Smeeth L, Margolis DJ, Thomas SL. Herpes zoster vaccine effectiveness against incident herpes zoster and post-herpetic neuralgia in an older US population: a cohort study. PLoS Med2013; 10:e1001420.
- 53. Li Y, An Z, Yin D, et al Disease burden due to herpes zoster among population aged ≥50 years old in china: a community based retrospective survey. PLoS One2016; 11:e0152660.
- 54. Liao CH, Chang CS, Muo CH, Kao CH. High prevalence of herpes zoster in patients with depression. J Clin Psychiatry2015; 76:e1099–104.
- 55. Lin SY, Liu JH, Lin CL, et al A comparison of herpes zoster incidence across the spectrum of chronic kidney disease, dialysis and transplantation. Am J Nephrol2012; 36:27–33.
- 56. Lin SY, Liu JH, Yeh HC, et al Association between herpes zoster and end stage renal disease entrance in chronic kidney disease patients: a population-based cohort study. Eur J Clin Microbiol Infect Dis2014; 33:1809–15.
- 57. Liu B, Heywood AE, Reekie J, et al Risk factors for herpes zoster in a large cohort of unvaccinated older adults: a prospective cohort study. Epidemiol Infect2015; 143:2871–81.
- 58. Long MD, Martin C, Sandler RS, Kappelman MD. Increased risk of herpes zoster among 108 604 patients with inflammatory bowel disease. Aliment Pharmacol Ther2013; 37:420–9.
- 59. Mirmirani P, Hessol NA, Maurer TA, et al Prevalence and predictors of skin disease in the Women’s Interagency HIV Study (WIHS). J Am Acad Dermatol2001; 44:785–8.
- 60. Morant-Talamante N, Diez-Domingo J, Martínez-Úbeda S, et al Herpes zoster surveillance using electronic databases in the Valencian Community (Spain). BMC Infect Dis2013; 13:463.
- 61. Morgan D, Mahe C, Malamba S, et al Herpes zoster and HIV-1 infection in a rural Ugandan cohort. AIDS2001; 15:223–9.
- 62. Mullooly JP, Riedlinger K, Chun C, Weinmann S, Houston H. Incidence of herpes zoster, 1997–2002. Epidemiol Infect2005; 133:245–53.
- 63. Muñoz-Quiles C, López-Lacort M, Ampudia-Blasco FJ, Díez-Domingo J. Risk and impact of herpes zoster on patients with diabetes: a population-based study, 2009–2014. Hum Vaccin Immunother2017; 13:2606–11.
- 64. Muñoz-Quiles C, López-Lacort M, Díez-Domingo J. Risk and impact of herpes zoster among COPD patients: a population-based study, 2009–2014. BMC Infect Dis2018; 18:203.
- 65. Ogunjimi B, Buntinx F, Bartholomeeusen S, et al Herpes zoster is associated with herpes simplex and other infections in under 60 year-olds. J Infect2015; 70:171–7.
- 66. Opstelten W, Van Essen GA, Schellevis F, et al Gender as an independent risk factor for herpes zoster: a population-based prospective study. Ann Epidemiol2006; 16:692–5.
- 67. Peng YH, Fang HY, Wu BR, et al Adult asthma is associated with an increased risk of herpes zoster: a population-based cohort study. J Asthma2017; 54:250–7.
- 68. Sato K, Adachi K, Nakamura H, et al Burden of herpes zoster and postherpetic neuralgia in Japanese adults 60 years of age or older: Results from an observational, prospective, physician practice-based cohort study. J Dermatol2017; 44:414–22.
- 69. Schmader K, George LK, Burchett BM, et al Racial differences in the occurrence of herpes zoster. J Infect Dis1995; 171:701–4.
- 70. Schmader K, George LK, Burchett BM, Pieper CF. Racial and psychosocial risk factors for herpes zoster in the elderly. J Infect Dis1998; 178(Suppl 1):S67–70.
- 71. Smitten AL, Choi HK, Hochberg MC, et al The risk of herpes zoster in patients with rheumatoid arthritis in the United States and the United Kingdom. Arthritis Rheum2007; 57:1431–8.
- 72. Suaya JA, Chen SY, Li Q, et al Incidence of herpes zoster and persistent post-zoster pain in adults with or without diabetes in the United States. Open Forum Infect Dis2014; 1:ofu049.
- 73. Tanuseputro P, Zagorski B, Chan KJ, Kwong JC. Population-based incidence of herpes zoster after introduction of a publicly funded varicella vaccination program. Vaccine2011; 29:8580–4.
- 74. Takao Y, Miyazaki Y, Okeda M, et al Incidences of herpes zoster and postherpetic neuralgia in Japanese adults aged 50 years and older from a community-based prospective cohort study: the SHEZ Study. J Epidemiol2015; 25:617–25.
- 75. Tsai SY, Yang TY, Lin CL, et al Increased risk of varicella zoster virus infection in inflammatory bowel disease in an Asian population: a nationwide population-based cohort study. Int J Clin Pract2015; 69:228–34.
- 76. Tseng HF, Smith N, Harpaz R, et al Herpes zoster vaccine in older adults and the risk of subsequent herpes zoster disease. JAMA2011; 305:160–6.
- 77. Tung YC, Tu HP, Tsai WC, et al Increased Incidence of herpes zoster and postherpetic neuralgia in adult patients following traumatic brain injury: a nationwide population-based study in Taiwan. PLoS One2015; 10:e0129043.
- 78. Ultsch B, Siedler A, Rieck T, et al Herpes zoster in Germany: quantifying the burden of disease. BMC Infect Dis2011; 11:173.
- 79. Veetil BM, Myasoedova E, Matteson EL, et al Incidence and time trends of herpes zoster in rheumatoid arthritis: a population-based cohort study. Arthritis Care Res2013; 65:854–61.
- 80. Weitzman D, Shavit O, Stein M, et al A population based study of the epidemiology of herpes zoster and its complications. J Infect2013; 67:463–9.
- 81. Wu MY, Hsu YH, Su CL, et al Risk of herpes zoster in CKD: a matched-cohort study based on administrative data. Am J Kidney Dis2012; 60:548–52.
- 82. Wu PH, Lin YT, Lin CY, et al A nationwide population-based cohort study to identify the correlation between heart failure and the subsequent risk of herpes zoster. BMC Infect Dis2015; 15:17.
- 83. Yang YW, Chen YH, Wang KH, et al Risk of herpes zoster among patients with chronic obstructive pulmonary disease: a population-based study. CMAJ2011; 183:E275–80.
- 84. Yang WS, Hu FC, Chen MK, et al High risk of herpes zoster among patients with advance acute kidney injury–a population-based study. Sci Rep2015; 5:13747.
- 85. Yawn BP, Saddier P, Wollan PC, et al A population-based study of the incidence and complication rates of herpes zoster before zoster vaccine introduction. Mayo Clin Proc2007; 82:1341–9.
- 86. Yawn BP, Wollan PC, St Sauver JL, Butterfield LC. Herpes zoster eye complications: rates and trends. Mayo Clin Proc2013; 88:562–70.
- 87. Yenikomshian MA, Guignard AP, Haguinet F, et al The epidemiology of herpes zoster and its complications in Medicare cancer patients. BMC Infect Dis2015; 15:106.
- 88. Ansar A, Farshchian M, Ghasemzadeh M, Sobhan MR. Association between family history and herpes zoster: a case-control study. J Res Health Sci2014; 14:111–4.
- 89. Chang K, Lee HS, Kim YJ, et al Increased risk of herpes zoster infection in patients with inflammatory bowel diseases in Korea. Clin Gastroenterol Hepatol2018; 16:1928–36.e2.
- 90. Chung SD, Tsai MC, Liu SP, et al Herpes zoster is associated with prior statin use: a population-based case-control study. PLoS One2014; 9:e111268.
- 91. Forbes HJ, Bhaskaran K, Thomas SL, et al Quantification of risk factors for herpes zoster: population based case-control study. BMJ2014; 348:g2911.
- 92. Hansson E, Forbes HJ, Langan SM, et al Herpes zoster risk after 21 specific cancers: population-based case-control study. Br J Cancer2017; 116:1643–51.
- 93. Heymann AD, Chodick G, Karpati T, et al Diabetes as a risk factor for herpes zoster infection: results of a population-based study in Israel. Infection2008; 36:226–30.
- 94. Hernandez PO, Javed S, Mendoza N, et al Family history and herpes zoster risk in the era of shingles vaccination. J Clin Virol2011; 52:344–8.
- 95. Hicks LD, Cook-Norris RH, Mendoza N, et al Family history as a risk factor for herpes zoster: a case-control study. Arch Dermatol2008; 144:603–8.
- 96. Joesoef RM, Harpaz R, Leung J, Bialek SR. Chronic medical conditions as risk factors for herpes zoster. Mayo Clin Proc2012; 87:961–7.
- 97. Ke CC, Lai HC, Lin CH, et al Increased risk of herpes zoster in diabetic patients comorbid with coronary artery disease and microvascular disorders: a population-based study in Taiwan. PLoS One2016; 11:e0146750.
- 98. Kwon HJ, Bang DW, Kim EN, et al Asthma as a risk factor for zoster in adults: a population-based case-control study. J Allergy Clin Immunol2016; 137:1406–12.
- 99. Lasserre A, Blaizeau F, Gorwood P, et al Herpes zoster: family history and psychological stress—case–control study. J Clin Virol2012; 55:153–7.
- 100. Marin M, Harpaz R, Zhang J, et al Risk factors for herpes zoster among adults. Open Forum Infect Dis2016; 3:ofw119.
- 101. Matthews A, Turkson M, Forbes H, Langan SM, Smeeth L, Bhaskaran K. Statin use and the risk of herpes zoster: a nested case–control study using primary care data from the U.K. clinical research practice datalink. Br J Dermatol2016; 175:1183–94.
- 102. Pope JE, Krizova A, Ouimet JM, et al Close association of herpes zoster reactivation and systemic lupus erythematosus (SLE) diagnosis: case-control study of patients with SLE or noninflammatory musculoskeletal disorders. J Rheumatol2004; 31:274–9.
- 103. Schmidt SA, Vestergaard M, Pedersen HS, et al Partner bereavement and risk of herpes zoster: results from two population-based case-control studies in Denmark and the United Kingdom. Clin Infect Dis2017; 64:572–9.
- 104. Schmidt SAJ, Langan SM, Pedersen HS, et al Mood disorders and risk of herpes zoster in 2 population-based case-control studies in Denmark and the United Kingdom. Am J Epidemiol2018; 187:1019–28.
- 105. Suo L, Lu L, Li J, et al A case control study on family history as a risk factor for herpes zoster and associated outcomes, Beijing, China. BMC Infect Dis2017; 17:334.
- 106. Tseng HF, Chi M, Hung P, et al Family history of zoster and risk of developing herpes zoster. Int J Infect Dis2018; 66:99–106.
- 107. Zhang JX, Joesoef RM, Bialek S, et al Association of physical trauma with risk of herpes zoster among Medicare beneficiaries in the United States. J Infect Dis2013; 207:1007–11.
- 108. Aw D, Silva AB, Palmer DB. Immunosenescence: emerging challenges for an ageing population. Immunology2007; 120:435–46.
- 109. Arvin AM. Varicella-zoster virus: pathogenesis, immunity, and clinical management in hematopoietic cell transplant recipients. Biol Blood Marrow Transplant2000; 6:219–30.
- 110. Oxman MN. Herpes zoster pathogenesis and cell-mediated immunity and immunosenescence. J Am Osteopath Assoc2009; 109:S13–7.
- 111. Gershon AA, Marin M, Seward JF. 62 - Varicella vaccines. In: Plotkin SA, Orenstein WA, Offit PA, Edwards KM, eds. Plotkin’s Vaccines. 7th ed. Philadelphia, PA: Elsevier; 2018: pp 1145–80.e17.
- 112. Park HB, Kim KC, Park JH, et al Association of reduced CD4 T cell responses specific to varicella zoster virus with high incidence of herpes zoster in patients with systemic lupus erythematosus. J Rheumatol2004; 31:2151–5.
- 113. Waller DG, Sampson AP. 38 - The immune response and immunosuppressant drugs. In: Waller DG, Sampson AP, eds. Medical Pharmacology and Therapeutics. 5th ed. Philadelphia, PA: Elsevier; 2018: pp 439–9.
- 114. Weinberg ED, Levin MJ. Current topics in microbiology and immunology. Volume 72. Microchem J1977; 22:590.
- 115. Chen SY, Suaya JA, Li Q, et al Incidence of herpes zoster in patients with altered immune function. Infection2014; 42:325–34.
- 116. Kang DH, Weaver MT, Park NJ, et al Significant impairment in immune recovery after cancer treatment. Nurs Res2009; 58:105–14.
- 117. Nagasawa K, Yamauchi Y, Tada Y, et al High incidence of herpes zoster in patients with systemic lupus erythematosus: an immunological analysis. Ann Rheum Dis1990; 49:630–3.
- 118. Lai YC, Yew YW. Risk of herpes zoster and family history: a meta-analysis of case-control studies. Indian J Dermatol2016; 61:157–62.
- 119. Meysman P, De Neuter N, Bartholomeus E, et al Increased herpes zoster risk associated with poor HLA-A immediate early 62 protein (IE62) affinity. Immunogenetics2018; 70:363–72.
- 120. Kawai K, Yawn BP. Risk factors for herpes zoster: a systematic review and meta-analysis. Mayo Clin Proc2017; 92:1806–21.
- 121. Schmader K, George LK, Burchett BM, et al Race and stress in the incidence of herpes zoster in older adults. J Am Geriatr Soc1998; 46:973–7.
- 122. Joon Lee T, Hayes S, Cummings DM, et al Herpes zoster knowledge, prevalence, and vaccination rate by race. J Am Board Fam Med2013; 26:45–51.
- 123. Hua C, Bardo AR, Brown JS. Mistrust in physicians does not explain black-white disparities in primary care and emergency department utilization: the importance of socialization during the Jim Crow era. J Natl Med Assoc2018; 110:540–6.
- 124. Mayberry RM, Mili F, Ofili E. Racial and ethnic differences in access to medical care. Med Care Res Rev2000; 57(Suppl 1):108–45.
- 125.
- 126. Dooling K, Guo A, Leung J, Belongia E, Harpaz R. Performance of zoster vaccine live (Zostavax): a systematic review of 12 years of experimental and observational evidence. Open Forum Infect Dis2017; 4(Suppl_1):S412–3.
- 127. Keating GM. Shingles (herpes zoster) vaccine (Zostavax(®)): a review in the prevention of herpes zoster and postherpetic neuralgia. BioDrugs2016; 30:243–54.
- 128. Sly JR, Harris AL. Recombinant zoster vaccine (Shingrix) to prevent herpes zoster. Nurs Womens Health2018; 22:417–22.
- 129.
- 130.
- 131. Szklo M, Nieto FJ. Epidemiology: Beyond the Basics. 3rd ed. Burlington, MA: Jones & Bartlett Learning; 2014.
- 132.
