CASE REPORT
A 33-year-old African American male who was virologically suppressed on antiretrovirals (ARVs) presented to the clinic complaining of significant weight gain. At the time of HIV diagnosis 8 years prior (November 2012), his initial HIV-1 RNA viral load was 8300 copies/mL with a baseline CD4 count of 590 cells/mm3. The patient had no other significant medical history except for mild tinea versicolor, treated with 2 short courses of fluconazole. One month after HIV diagnosis (December 2012), the patient was started on efavirenz/emtricitabine/tenofovir disoproxil fumarate (EFV/FTC/TDF; Atripla), and his HIV viral load was suppressed to <20 copies/mL within 4 weeks of treatment initiation. Approximately 3 years after starting EFV/FTC/TDF (March 2016), the patient was switched to elvitegravir/cobicistat/emtricitabine/tenofovir alafenamide (EVG/COBI/FTC/TAF; Genvoya) due to sleep disturbances and abdominal cramping with diarrhea, which were felt to be drug-related effects of efavirenz. He remained on EVG/COBI/FTC/TAF for the next 4 years (until April 2020), during which time he experienced dramatic weight gain in the absence of lifestyle changes.
The patient’s weight throughout the course of HIV treatment is presented in Figure 1. His weight at the time of EFV/FTC/TDF initiation was 66.7 kg (BMI 21.7 kg/m2), which he reported being his long-standing baseline. Following initiation of EFV/FTC/TDF, his weight dropped to a nadir of 60.3 kg 8 months later and remained relatively stable thereafter. At the time of switching to EVG/COBI/FTC/TAF, he weighed 62.6 kg. Over the next 4 years, his weight rose steeply to 85.4 kg (28% increase from baseline; BMI 27.8 kg/m2), at which time he was changed back to EFV/FTC/TDF per his request and the provider’s suggestion. Upon switching back, his weight decreased to 74.2 kg within 6 months. His weight continued to decline to 72.1 kg by 12 months. He reports intermittent vivid dreams but denies any gastrointestinal side effects since returning to EFV/FTC/TDF.
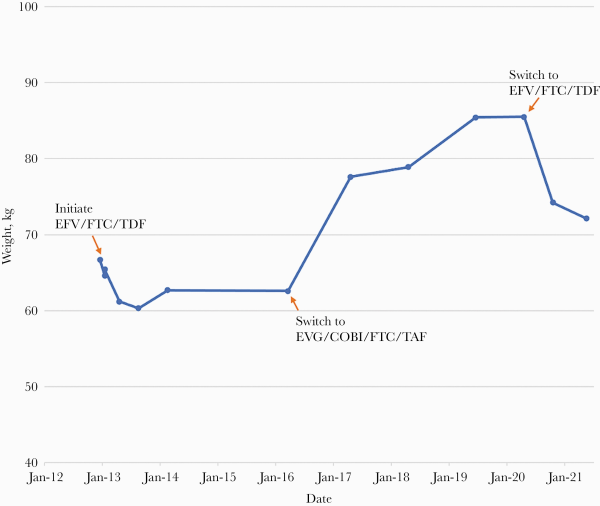
Figure 1
Patient weight annotated with antiretroviral regimens. Initiated EFV/FTC/TDF in December 2012. Switched to EVG/COBI/FTC/TAF in March 2016. Switched back to EFV/FTC/TDF in April 2020. Most recent weight May 2021. Abbreviations: COBI, cobicistat; EVG, elvitegravir; EFV, efavirenz; FTC, emtricitabine; TAF, tenofovir alafenamide; TDF, tenofovir disoproxil fumarate.
DISCUSSION
To our knowledge, this is the first reported case of significant weight reversal of antiretroviral therapy (ART) switch–induced weight gain upon switching back to the original regimen. Switching ART is associated with more weight gain than maintaining regimens, and the degree of weight gain may be influenced by both baseline and postswitch agents []. Therefore, our patient’s weight changes may be due to weight suppressive effects of EFV/FTC/TDF, weight gain effects of EVG/COBI/FTC/TAF, withdrawing weight suppressive/gain effects, or some combination.
Weight loss or suppressed weight gain associated with TDF use has been noted in several patient populations. In the iPrEx trial of TDF/FTC as pre-exposure prophylaxis, seronegative patients receiving TDF/FTC gained less weight, on average, than those receiving placebo and were significantly more likely to experience unintentional weight loss of ≥5% []. In the DISCOVER trial, which compared TDF with TAF in pre-exposure prophylaxis regimens, participants in the TDF arm lost weight in the first 24 weeks and returned to their baseline weight by week 48, similar to our patient’s initial weight loss and then return toward baseline []. Among people with HIV (PWH), patients in the GEMINI trials receiving dolutegravir (DTG)+TDF/FTC gained less weight than those receiving DTG+lamivudine (3TC; 3.7 kg vs 2.4 kg by week 144) []. This weight loss or weight gain suppression with ART may be an off-target effect, and perhaps not entirely healthy. In the IMPAACT 2010/VESTED trial of ART initiation in pregnant women, patients receiving EFV/FTC/TDF gained less weight than those receiving DTG/FTC/TAF or DTG/FTC/TDF, were furthest from the Institute of Medicine–recommended weekly weight gain in the second and third trimesters, and had more adverse pregnancy outcomes [].
Integrase strand transfer inhibitor (INSTI)–associated weight gain has been reported in a growing number of recent studies among both treatment-naïve and treatment-experienced patients []. An analysis of 8 randomized controlled clinical trials of ART-naïve PWH found that INSTI-containing regimens were associated with more weight gain than NNRTI- and PI-based ART regimens []. This appears to be a class effect, although bictegravir (BIC) and DTG were associated with more weight gain than cobicistat-boosted EVG (BIC, 4.24 kg; DTG, 4.07 kg; and EVG/COBI, 2.72 kg) over 96 weeks []. Significant weight gain among virologically suppressed PWH after switching from EFV/FTC/TDF to an INSTI-based regimen, similar to our patient, has also been reported []. Weight change with INSTI therapy appears to be heterogenous, however. While the average weight gain with INSTI use is moderate, some patients, such as our patient, experience substantial weight gain (>10% increase in bodyweight) [, ].
TAF has also been implicated as a potential cause of weight gain in several patient populations. In the DISCOVER trial, participants in the TAF arm had a mean body weight increase of 1.1 kg at week 48 compared with the initial weight loss and return to baseline in the TDF group []. Among virologically suppressed PWH, switching from TDF- to TAF-containing ART regimens, with or without changing other regimen components, is significantly associated with pronounced weight gain, which can lead to concerning metabolic complications including elevated serum lipid levels []. In the ADVANCE trial, the TAF arm was associated with treatment-emergent obesity, particularly among women []. TAF was significantly associated with substantial weight gain compared with all other NRTIs in trials among those initiating ART []. TAF appears to be associated with weight gain in HIV-negative, treatment-naïve, and virologically suppressed patients and may have contributed to the pronounced weight gain of our patient.
The mechanisms underlying specific susceptibility to ARV-associated weight changes are currently unknown. Patient- and HIV-specific factors may influence weight response, with substantial weight gain being more likely among female and Black patients as well as those with lower baseline BMI, lower CD4 cell counts, and higher HIV-1 RNA copies [, ]. This heterogeneous response may explain the lack of significant change in weight gain with INSTI therapy reported by some studies due to variations in demographics and HIV-specific factors among included participants [, ]. There is growing research regarding pharmacogenetics predicting weight gain or loss with specific ARVs. In a retrospective investigation, patients with CYP2B6 polymorphisms (slow metabolizers of EFV) gained more weight following a switch from an EFV- to INSTI-based regimen, potentially reflecting withdrawal of the inhibitory effect of higher EFV concentrations on weight gain []. Conversely, patients with CYP2B6 genotypes associated with extensive metabolism of EFV experienced similar weight gain with EFV-based ART as patients with an INSTI-based regimen []. The CYP2B6 alleles associated with impaired metabolism of EFV are more common among individuals of African ancestry, such as our patient, potentially contributing to observed differences in weight gain by race []. Further identification and understanding of patient- and HIV-specific factors associated with weight gain, particularly substantial weight gain, may influence treatment decisions.
CONCLUSIONS
We report a case of substantial weight gain (28% increase from baseline) in a virologically suppressed young African American male with HIV after switching his ART regimen from EFV/FTC/TDF to EVG/COBI/FTC/TAF. Remarkably, the patient quickly lost a significant portion of the weight gained within 6 months when switched back to his original regimen. Both INSTIs and TAF are associated with substantial weight gain, while TDF (especially with EFV) is associated with weight loss or weight gain suppression, with observed differences in susceptibility by patient- and HIV-specific factors. Understanding these factors may aid in predicting substantial weight gain to guide management for PWH.
Acknowledgments
Financial support. None.
Potential conflicts of interest. All authors: no reported conflicts of interest. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
Patient consent. Patient consent was obtained. This conforms with standards of the Duke Health Institutional Review Board for publishing case reports.
Author contributions. K.S.M. and M.S.M. contributed to clinical management. All authors coordinated the case report, collected clinical and laboratory data, discussed the results, and wrote and approved the manuscript.
References
- 1. Erlandson KM, Carter CC, Melbourne K, et al Weight change following antiretroviral therapy switch in people with viral suppression: pooled data from randomized clinical trials. Clin Infect Dis. 2021; ciab444.
- 2. Grant RM, Lama JR, Anderson PL, et al; iPrEx Study Team. Preexposure chemoprophylaxis for HIV prevention in men who have sex with men. N Engl J Med2010; 363:2587–99.
- 3. Mayer KH, Molina JM, Thompson MA, et al Emtricitabine and tenofovir alafenamide vs emtricitabine and tenofovir disoproxil fumarate for HIV pre-exposure prophylaxis (DISCOVER): primary results from a randomised, double-blind, multicentre, active-controlled, phase 3, non-inferiority trial. Lancet2020; 396:239–54.
- 4. Cahn P, Madero JS, Arribas JR, et al Durable efficacy of dolutegravir (DTG) plus lamivudine (3TC) in antiretroviral treatment–naive adults with HIV-1 infection—3-year results from the Gemini studies. J Acquir Immune Defic Syndr 2020; 83:310–8.
- 5. Lockman S, Brummel SS, Ziemba L, et al; IMPAACT 2010/VESTED Study Team and Investigators. Efficacy and safety of dolutegravir with emtricitabine and tenofovir alafenamide fumarate or tenofovir disoproxil fumarate, and efavirenz, emtricitabine, and tenofovir disoproxil fumarate HIV antiretroviral therapy regimens started in pregnancy (IMPAACT 2010/VESTED): a multicentre, open-label, randomised, controlled, phase 3 trial. Lancet2021; 397:1276–92.
- 6. Wu KS, Anderson C, Little SJ. Integrase strand transfer inhibitors play the main role in greater weight gain among men with acute and early HIV infection. Open Forum Infect Dis2021; 8:XXX–XX.
- 7. Bourgi K, Rebeiro PF, Turner M, et al Greater weight gain in treatment-naive persons starting dolutegravir-based antiretroviral therapy. Clin Infect Dis2020; 70:1267–74.
- 8. Eckard AR, McComsey GA. Weight gain and integrase inhibitors. Curr Opin Infect Dis2020; 33:10–9.
- 9. Bakal DR, Coelho LE, Luz PM, et al Obesity following ART initiation is common and influenced by both traditional and HIV-/ART-specific risk factors. J Antimicrob Chemother2018; 73:2177–85.
- 10. Sax PE, Erlandson KM, Lake JE, et al Weight gain following initiation of antiretroviral therapy: risk factors in randomized comparative clinical trials. Clin Infect Dis2020; 71:1379–89.
- 11. Norwood J, Turner M, Bofill C, et al Brief report: weight gain in persons with HIV switched from efavirenz-based to integrase strand transfer inhibitor-based regimens. J Acquir Immune Defic Syndr2017; 76:527–31.
- 12. Verboeket SO, Boyd A, Wit FW, et al; AGEhIV Cohort Study Group. Generally rare but occasionally severe weight gain after switching to an integrase inhibitor in virally suppressed AGEhIV cohort participants. PLoS One2021; 16:e0251205.
- 13. Schafer JJ, Sassa KN, O’Connor JR, Shimada A, Keith SW, DeSimone JA. Changes in body mass index and atherosclerotic disease risk score after switching from tenofovir disoproxil fumarate to tenofovir alafenamide. Open Forum Infect Dis2019; 6:ofz414.
- 14. Mallon PW, Brunet L, Hsu RK, et al Weight gain before and after switch from TDF to TAF in a U.S. cohort study. J Int AIDS Soc2021; 24:e25702.
- 15. Surial B, Mugglin C, Calmy A, et al; Swiss HIV Cohort Study. Weight and metabolic changes after switching from tenofovir disoproxil fumarate to tenofovir alafenamide in people living with HIV: a cohort study. Ann Intern Med2021; 174:758–67.
- 16. Venter WDF, Moorhouse M, Sokhela S, et al Dolutegravir plus two different prodrugs of tenofovir to treat HIV. N Engl J Med2019; 381:803–15.
- 17. Bedimo R, Adams-Huet B, Taylor BS, Lake J, Luque A. 538. Integrase inhibitor-based HAART is associated with greater BMI gains in Blacks, Hispanics, and women. Open Forum Infect Dis2018; 5(Suppl 1):S199.
- 18. Burns JE, Stirrup OT, Dunn D, et al No overall change in the rate of weight gain after switching to an integrase-inhibitor in virologically suppressed adults with HIV. AIDS2020; 34:109–14.
- 19. Tiraboschi J, Navarro-Alcaraz A, Giralt D, et al Changes in body fat distribution in antiretroviral-naive HIV-positive individuals initiating current ART regimens. J Clin Endocrinol Metab2019; 104:900–5.
- 20. Leonard MA, Cindi Z, Bradford Y, et al Efavirenz pharmacogenetics and weight gain following switch to integrase inhibitor–containing regimens. Clin Infect Dis. 2020; ciaa1219.
- 21. Griesel R, Maartens G, Chirehwa M, et al CYP2B6 genotype and weight gain differences between dolutegravir and efavirenz. Clin Infect Dis2020; 70:670–8.
- 22. Holzinger ER, Grady B, Ritchie MD, et al Genome-wide association study of plasma efavirenz pharmacokinetics in AIDS clinical trials group protocols implicates several CYP2B6 variants. Pharmacogenet Genomics2012; 22:858–67.
