Tetanus is a highly fatal disease caused by exotoxins of Clostridium tetani, a ubiquitous, Gram-positive bacilli []. The clinical pattern of tetanus can be characterized as generalized, local, cephalic, or neonatal []. Clinically, symptoms are caused by binding of tetanospasmin to synapses in the presynaptic knob, blocking calcium ion-dependent glycine release, which is an inhibitory neurotransmitter []. This results in uninhibited neuronal excitation resulting in continuous, muscle contraction and autonomic dysfunction [].
Since 1948, tetanus vaccination has been provided through a combined vaccination with diphtheria (Corynebacterium diphtheriae) and pertussis (Bordetella pertussis) vaccines as part of routine childhood immunization programs []. In addition, adolescent girls and women of childbearing age receive at least 2 doses of tetanus toxoid (TT) vaccine to prevent maternal tetanus and impart passive immunity to the newborn []. These efforts have substantially reduced the incidence of tetanus cases among mothers and children by over 96%, with Uganda undergoing an elimination validation in 2011 []. Tetanus toxoid vaccination is also recommended for prevention of nonneonatal tetanus among individuals suffering penetrating injuries and safe male circumcision volunteers [, ]. However, tetanus remains a health challenge and is associated with 34 700 deaths globally, disproportionately higher in the low-income countries where vaccination coverage is still low and unsafe delivery practices are still prevalent []. By 2016, Uganda had one of the highest nonneonatal tetanus incidence rates in the world (12.7/100 000) [], but this has since declined to 0.86/100 000 []. However, tetanus is still associated with high mortality rates [, ]. There were an estimated 0.65 per 100 000 deaths due to tetanus in Uganda in 2019 [].
Management of tetanus in adults often requires treatment in the intensive care unit (ICU), which is often not available or costly in most low- and middle-income countries [], resulting in preventable premature mortality. In fact, South Asia and sub-Saharan Africa contribute 45% and 44% of the global tetanus deaths, respectively []. Woldeamanuel et al [] noted that tetanus-associated mortality is often a result of suboptimal care, and Bae et al [] reported that the unit cost for treatment of an adult with tetanus is over US $18 000. In addition to antispasmodic therapy, the Uganda clinical guidelines recommend antitetanus immunoglobulin for individuals with a diagnosis of tetanus: this is often not readily available, resulting in preventable mortality [, , ]. Efforts to increase access to tetanus vaccination and appropriate therapy among adult populations have been limited by evidence to support resource allocation. This study therefore aimed to document trends in adult tetanus admissions, deaths, mortality rates, and associated factors on medical wards at a national referral hospital in Uganda.
METHODS
This was a retrospective cohort study. We obtained patient data from the Rainer Arnolds Senior House Officers Training support (RASHOTs) database [] for patients admitted to medical inpatient wards at Mulago and Kiruddu national referral hospitals (NRHs) from 2011 to 2020.
Study Population and Study Setting
We included all patients ≥12 years of age admitted to Mulago (2011–2014) and Kiruddu NRHs (2014–2020) with a diagnosis of tetanus. We excluded duplicate records and records with incomplete data (Figure 1). The RASHOTs database was the central repository for adult medical inpatient data for the study. In 2014, the Government of Uganda undertook a major renovation of Mulago NRH that necessitated migration of medical inpatient services to Kiruddu Hospital, which had been newly upgraded []. Mulago NRH is located in Kawempe Division, whereas Kiruddu NRH is located in Makindye division of the capital city.
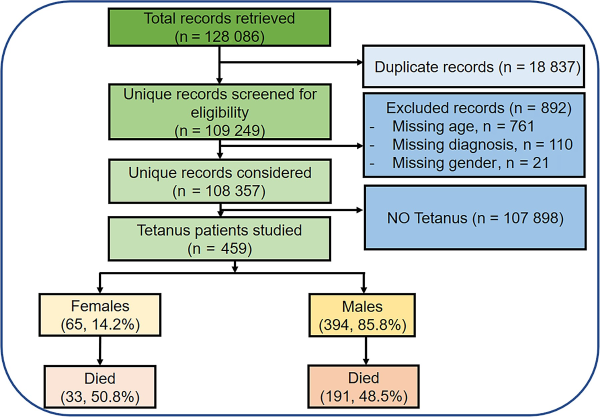
Figure 1
Study flow diagram.
Data Collection
We retrieved data on patient gender, age at admission, district of origin, human immunodeficiency virus (HIV) status, length of hospital stay, and treatment outcome for all individuals admitted with a diagnosis of tetanus; we downloaded these as spreadsheets, which we cleaned, and we conducted preliminary analyses using Microsoft Excel. Diagnoses were made by specialist physicians on inpatient wards using clinical algorithms involving patient clinical history, physical examination, laboratory tests, and radiological imaging, where applicable and available.
Data Analysis
We summarized patient characteristics as frequencies and determined temporal trends using the Mann-Kendall test. We calculated mortality rate as a percentage of deaths divided by number of admissions and compared average length of hospital stay between males and females using the Mann-Whitney U test. We performed Kaplan-Meier survival curves and the log-rank test for gender differences in in-patient survival. We used χ2 statistics for bivariate analysis to assess for association of gender with mortality. We generated tetanus burden maps using ArcGIS to demonstrate districts of origin. We carried out analyses and generated graphs using STATA/BE v.17.0 (StataCorp LLC). For all analyses, a P < .05 was considered statistically significant.
RESULTS
Baseline Characteristics
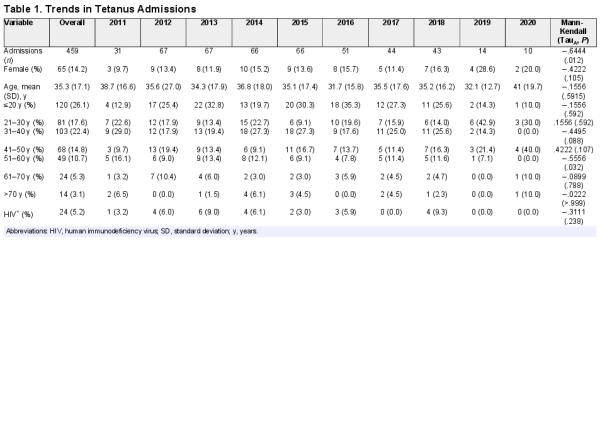
Data from 459 cases of tetanus were reviewed and analyzed. The majority of these were male (85.8%), aged 20 years or less (26.1%), and were HIV negative (94.8%). The median age of study participants was 35 years (range, 12–85; interquartile range, 20–47) (Table 1).
Trend in Tetanus Admissions
The total number of tetanus cases recorded declined over the decade (TauB = −.6593, P = .0116) (Table 1). Geospatial analysis revealed that 90% of the cases were from the central region of the country (Figure 2).
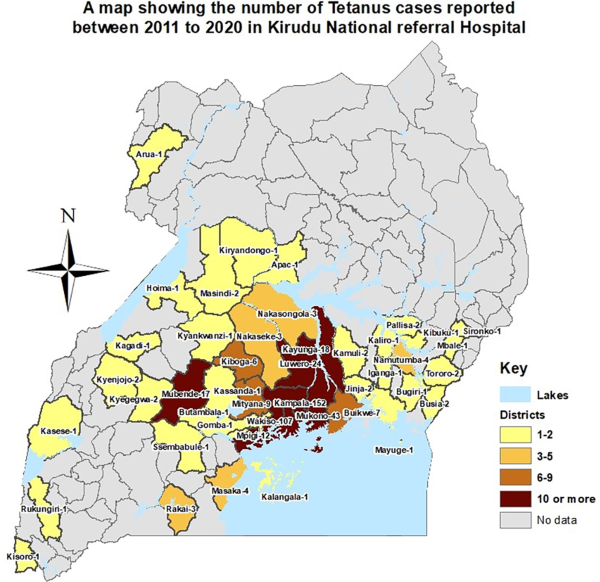
Figure 2
Map of districts of origin of individuals admitted with tetanus 2011–2020.
Average length of hospital stays was computed for 458 study participants, because data on length of stay was unavailable for one participant. Overall, average length of hospital stay was 8.1 days (standard deviation [SD], 7.5; range, 1–46). It was longest in 2017 (10.9; SD, 8.7; range, 1–37) and shortest in 2020 (3.4; SD, 2.2; range, 1–7). Whereas average length of stay was longer among males than females (8.3, SD = 7.6, range = 1–46 vs 7.0, SD = 7.2, range = 1–39), this was not statistically significant (P = .1693). The Kaplan-Meier survival curve demonstrated that females died earlier than males, although this was not statistically significant (P = .4513). Median survival time among males was 11 days and 7 days for females (Figure 3). Average length of hospital stay was lower among those who died compared to those who were discharged (3.8, SD = 3.8, range = 1–22 vs 12.6, SD = 7.8, range = 1–46; P = .001).
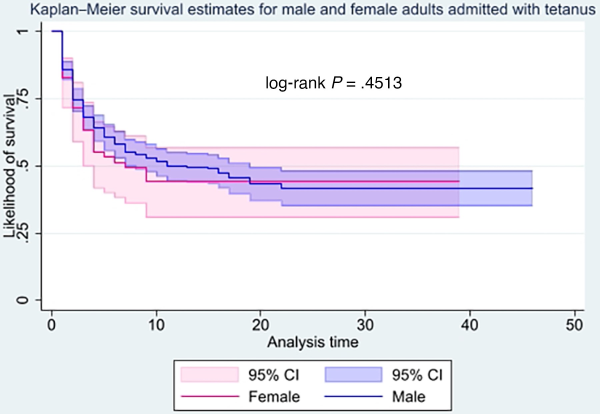
Figure 3
Kaplan-Meier inpatient survivor curves comparing adult males and females with tetanus. CI, confidence interval.
Trends in Tetanus Mortality Rate
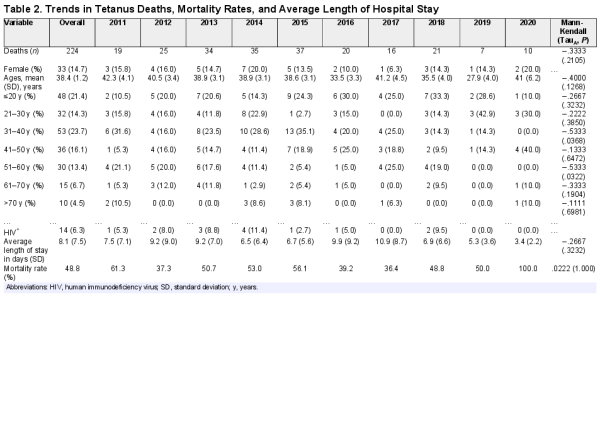
A total of 224 deaths were recorded over the period, representing a mortality rate of 48.8% (Table 2, Figure 4). Males and those aged 31–40 years accounted for the majority of the deaths (85.4% and 23.7%, respectively). Females had a higher mortality rate (50.8%, 33 of 65 vs 48.5%, 191 of 394), but there was no association between gender and mortality (Pearson χ2 = 1.7105, P = .425), nor HIV status (Fisher’s exact test = .658) The mortality rate tended to increase, without statistical significance (TauA = 0.0222, P > .999).
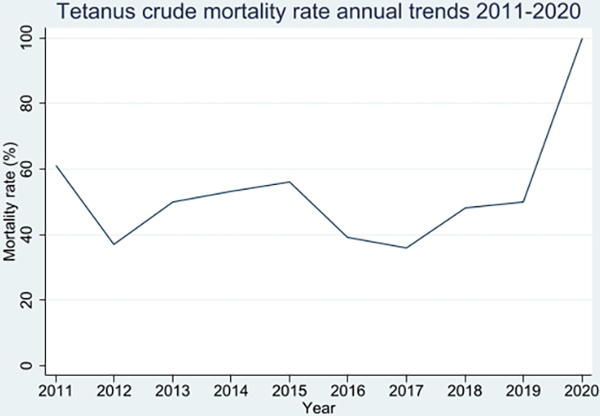
Figure 4
Trends in tetanus mortality rates.
The total time at risk of death in the study was 3721 person days. The incidence rate of mortality was 5.99 per 100 person days, highest in 2020 (29.4 per 100 person days), and among those aged 70 years and above (14.3 per 100 person days).
Availability of Data and Materials
All data used for this study are available in the content of the manuscript and the Supplementary Materials, and supporting information is in the Supplementary File, Tetanus dataset (.xls).
Ethics Approval and Consent to Participate
This study was approved under the machine learning project by the Makerere University School of Medicine Research and Ethics committee (Number: 2020/098) and the Uganda National Council for Science and Technology (Number: HS745ES). The study used secondary data and the need for informed consent was waived. Study participants were identified using patient identification numbers and records were anonymized.
DISCUSSION
We conducted a retrospective cohort study using inpatient hospital records at national referral hospitals to determine trends in tetanus admissions, deaths, and mortality rates over a period of 10 years (2011–2020). In this study, we observed a substantial decline in the number of patients hospitalized with tetanus. Tetanus predominantly affected males and very young adults in their youth. The majority of those admitted were from Uganda’s central region. Tetanus was associated with a high mortality rate (48.8%) that tended to increase over the years, registering 100% mortality in 2020. Tetanus mortality rate was higher among females than among males, although males contributed the highest number of admissions and deaths. Median survival was shorter among females than among males. However, the average length of hospital stay was short, which could be attributed to early fatality.
We reported that more than 80% of individuals admitted to adult medical wards with tetanus were male, similar to what was reported by Olum et al [] from Northern Uganda at 86.2% (94 of 109) and higher than the 76.4% reported by Derbie et al [] from North West Ethiopia. Similarly, at a district hospital in Eastern Uganda, Nanteza [] et al noted that all tetanus patients were male. Our findings and those of other studies underline the disproportionate burden of tetanus among adult males. This gender difference in admissions could be explained by routine TT vaccination among females of reproductive age in Uganda, which confers additional protection against tetanus on top of the diphtheria, tetanus, pertussis (DPT) vaccination in infancy []. In a study conducted across 5 regions of Uganda, Makumbi et al [] found that among 620 males who presented for safe male circumcision, 57.1% had insufficient tetanus immunity, determined from TT immunoglobulin antibody assays similar to a study among Chinese military personnel, where low antitetanus titers were frequent and yet a single booster shot was protective for more than 15 years []. This further stresses previous assertions that tetanus vaccination in infancy does not confer lifelong protection and hence the need for decennial booster vaccination among vulnerable males []. As such, the World Health Organization has recommended incorporation of tetanus vaccination for boys and men into national immunization programs []. In Uganda, however, this is currently only reserved for men undergoing safe male circumcision and penetrating trauma survivors [].
Furthermore, since males contributed more than three quarters of the tetanus admissions, they also contributed a similar proportion to the total number of tetanus deaths. The Global Burden of Disease Study estimated that there were more male than female tetanus deaths per 100 000 population (0.71 and 0.59, respectively) []. These estimates were lower than those for the neighboring countries of Tanzania (0.99 and 0.75), Burundi (1.94 and 1.49), South Sudan (2.73 and 2.14), and Kenya (4.66 and 4.69) but higher than for Rwanda (0.58 and 0.52) and the Democratic Republic of Congo (0.62 and 0.53) [].
Our study demonstrates clustering of cases around the urban areas, which may suggest increased occupational exposure to C tetani spores in these areas. This is not unexpected considering that the study sites, Mulago and Kiruddu NRHs, are located within Uganda’s capital city Kampala and are therefore accessible service centers for the city population. Noteworthy, however, from a national study by Nguna et al [], Kampala capital city was found to have the highest number of tetanus cases in the country. This probably suggests increased vulnerability to tetanus among urban dwellers and should be explored further. Additional risk factors among males for tetanus are associated with (1) reduced antitetanus antibody titers such as diabetes mellitus and HIV and (2) sociodemographic characteristics such as age, education level, employment, and marital status [, ].
Our study revealed that a number of women are also diagnosed with tetanus. This probably highlights missed opportunities for tetanus vaccination, and it calls for efforts to ensure universal access to tetanus vaccination. In a study by Islam et al [], TT vaccination among women correlated with living conditions, whereas another study in East Africa found that less educated women, those who traveled long distances to health facilities, and those who had less access to health information from electronic media were more likely to miss tetanus vaccination [].
Similar to what Oshinaike et al [] reported from Nigeria, our study also demonstrates a decline in tetanus admissions with nearly unchanged high mortality rates. High mortality rates (over 30%) were also reported by Tosun et al [] (32.5%), in northwest Nigeria (48.4%) [], Sudan (48.4%) [], and Afghanistan (50%) []. Whereas Van Hao et al [] reported a much lower mortality rate (2.8%) in Southern Vietnam where at least 50% of tetanus patients were mechanically ventilated, Olum et al [] reported a 57.8% mortality rate from Northern Uganda among tetanus patients admitted to the ICU, and Hasnain et al [] reported a 28.6% mortality rate from Bangladesh with only pharmacological without mechanical ventilation interventions among tetanus patients. The high rate of mortality reported by Olum et al [] and Nanteza et al [] could be because patients in their studies did not have access to human antitetanus immunoglobulin, and the extent to which this explained the high mortality in these studies was not analyzed. In the present study, we did not have data on the interventions that patients received.
Our study revealed a high proportion of tetanus deaths occurring among those aged 31–40 years. This is in contrast to what Khan et al [] reported from Bangladesh, where deaths mostly occurred among older individuals above 60 years of age. We recommend further studies to assess risk factors for mortality in our setting.
In Van Hao et al’s [] study, the average length of hospital stay was 15 days, much longer than what is reported in our study (8.1 days). This shorter length of hospital stay in our study is probably explained by high early premature mortality, demonstrated by a short average length of hospital stay among those who died (3.8 days). This could be due to inadequate care for those admitted with severe disease or late presentation []. A quality-of-care audit can be used to determine whether all tetanus patients received the standard care; and community sensitization on early recognition of tetanus symptoms may be implemented to address these.
Our study had some strengths and limitations. First, our data are limited to adults admitted to the national referral hospitals of Mulago and Kiruddu, excluding neonates, children under 5 years, pregnant and breastfeeding mothers, and cases treated at regional and lower level health facilities. This underestimates the national burden of tetanus. In addition, mortality rates could be overestimated due to referral bias if critically ill patients were preferentially referred to these facilities. Second, our study relied on routinely collected inpatient data extracted from a patient database. It therefore excludes important variables that would further explain the determinants of mortality in these patients such as quality of care provided, incubation period, entry wound, and severity of symptoms at admission. However, the strength of our study is in the large sample size, pooling participants over a 10-year period that allowed for description of trends in annual admissions and deaths from an inpatient setting.
CONCLUSIONS
Although the number of adults admitted with tetanus declined over a 10-year period, associated mortality remained high. The majority of admissions and deaths occurred among males. Quality-of-care audits are recommended to inform investments in improved inpatient care. We recommend further studies to describe risk factors for tetanus and tetanus mortality in our setting. Current tetanus vaccination efforts should be sustained and further refined to target vulnerable women and men.
Acknowledgments
We acknowledge support from the Rainer Arnolds Senior House Officers Training support (RASHOTs) staff (Namakula Cissy, Nagganja Cate, and Akullu Vicky) who played a pivotal role in retrieval and cleaning of the study dataset.
Author contributions. A. K. conceived and designed the study, collected and cleaned data, conducted data analysis, and contributed to writing the initial and final manuscript. N. E. O. collected and cleaned data, conducted data analysis, and contributed to writing the initial and final manuscript. F. B. contributed to the study design, data analysis, and writing the initial and final manuscript. A. M. N. contributed to writing the initial and final manuscript. J. B. B. contributed to data analysis and writing the initial and final manuscript. R. K. contributed to funding acquisition and writing the initial and final manuscript. M. K. contributed to fundings acquisition and writing the initial and final manuscript. I. A.-B. contributed to study conceptualization, design, data analysis, and writing the initial and final manuscript.
Financial support. Funding for this study was obtained by I. A.-B. through Makerere University Research and Innovations fund (https://rif.mak.ac.ug/) from the Government of Uganda . R. K. obtained funding for the RASHOTS database through the Uganda Foundation (https://ugandafoundation.com/).
References
- 1. Yen LM, Thwaites CL. Tetanus. Lancet2019; 393:1657–68.
- 2. Megighian A, Pirazzini M, Fabris F, Rossetto O, Montecucco C. Tetanus and tetanus neurotoxin: from peripheral uptake to central nervous tissue targets. J Neurochem2021; 158:1244–53.
- 3. Klein NP. Licensed pertussis vaccines in the United States. History and current state. Hum Vaccin Immunother2014; 10:2684–90.
- 4. Maertens K, Orije MRP, van Damme P, Leuridan E. Vaccination during pregnancy: current and possible future recommendations. Eur J Pediatr2020; 179:235–42.
- 5. World Health Organization. Validation of elimination: maternal and neonatal tetanus—Uganda, 2011. Wkly Epidemiol Rec2011; 86:565–75.
- 6. Ministry of Health. Uganda Clinical Guidelines 2016: National Guidelines for Management of Common Conditions. Kampala: Ministry of Health Uganda; 2016.
- 7. Nanteza B, Galukande M, Aceng J, et al The burden of tetanus in Uganda. Springerplus2016; 5:705.
- 8.
- 9. Nguna J, Kwesiga B, Ario AR. Distribution of tetanus in Uganda between 2012 and 2016. Uganda National Institute of Public Health Bulletin. Kampala: Uganda National Institute of Public Health; 2021.
- 10.
- 11. Olum S, Eyul J, Lukwiya DO, Scolding N. Tetanus in a rural low-income intensive care unit setting. Brain Commun2021; 3:fcab013.
- 12.
- 13. Rodrigo C, Fernando D, Rajapakse S. Pharmacological management of tetanus: an evidence-based review. Crit Care2014; 18:217.
- 14. Kyu HH, Mumford JE, Stanaway JD, et al Mortality from tetanus between 1990 and 2015: findings from the Global Burden of Disease Study 2015. BMC Public Health2017; 17:179.
- 15. Woldeamanuel YW, Andemeskel AT, Kyei K, Woldeamanuel MW, Woldeamanuel W. Case fatality of adult tetanus in Africa: systematic review and meta-analysis. J Neurol Sci2016; 368:292–9.
- 16. Bae S, Go M, Kim Y, et al Clinical outcomes and healthcare costs of inpatients with tetanus in Korea, 2011–2019. BMC Infect Dis2021; 21:247.
- 17. Kalyesubula R, Mutyaba I, Rabin T, et al Trends of admissions and case fatality rates among medical in-patients at a tertiary hospital in Uganda; a four-year retrospective study. PLoS One2019; 14:e0216060.
- 18.
- 19. Derbie A, Amdu A, Alamneh A, et al Clinical profile of tetanus patients attended at Felege Hiwot Referral Hospital, northwest Ethiopia: a retrospective cross sectional study. Springerplus2016; 5:892.
- 20. Makumbi F, Byabagambi J, Muwanika R, et al Prevalence of protective tetanus antibodies and immunological response following tetanus toxoid vaccination among men seeking medical circumcision services in Uganda. PLoS One2018; 13:e0209167.
- 21. Tong L, Jia Q, Li B, et al Investigation of the baseline tetanus antibody level and its persistence in a military unit. Vaccine2021; 39:4328–34.
- 22. Trucchi C, Zoppi G. Decennial diphtheria-tetanus adult boosters: are they really necessary?J Prev Med Hyg2015; 56:E44–8.
- 23. Dalal S, Samuelson J, Reed J, Yakubu A, Ncube B, Baggaley R. Tetanus disease and deaths in men reveal need for vaccination. Bull World Health Organ2016; 94:613–21.
- 24. Kutlu R, Dogan M, Çapa AR, Baykan M. Evaluation of tetanus immunoglobulin G levels according to age and sociodemographic characteristics: a community-based study. Konuralp Tıp Dergisi2021; 13:488–96.
- 25. Tavassol Z H, Sajjadpour Z, Hasani-Ranjbar S, Pejman Sani M, Aghaei Meybodi H, Larijani B. Do patients with diabetic foot ulcer need booster dose of tetanus vaccine?J Diabetes Metab Disord2022; 21:1023–7.
- 26. Islam UN, Sen KK, Bari W. Living standard and access to tetanus toxoid immunization among women in Bangladesh. BMC Public Health2022; 22:1037.
- 27. Belay AT, Fenta SM, Agegn SB, Muluneh MW. Prevalence and risk factors associated with rural women’s protected against tetanus in east Africa: evidence from demographic and health surveys of ten east African countries. PLoS One2022; 17:e0265906.
- 28. Oshinaike OO, Ojelabi OO, Ogbera AO, Ojo OO, Ajose FA, Okubadejo NU. Improving case fatality rate of adult tetanus in urban Nigeria: focus on better facilities of care. Trop Doct2012; 42:208–10.
- 29. Tosun S, Batirel A, Oluk AI, et al Tetanus in adults: results of the multicenter ID-IRI study. Eur J Clin Microbiol Infect Dis2017; 36:1455–62.
- 30. Aliyu A, Dahiru T, Obiako R, Amadu L, Biliaminu L, Akase E. Pattern and outcome of tetanus in a tertiary health facility in northwest Nigeria. Ethiop Med J2017; 19:1.
- 31. Dafallah MA, Ragab EA. Experience with tetanus in a tertiary care hospital in Sudan: a retrospective review. Emerg Med Int2021; 2021:4818312.
- 32. Qaderi S, Qaderi F, Tarki FE, et al Generalized, non-neonatial tetanus is a highly fatal disease in Afghanistan: a case series study. Int J Infect Dis2021; 103:568–72.
- 33. Van Hao N, Yen LM, Davies-Foote R, et al The management of tetanus in adults in an intensive care unit in southern Vietnam. Wellcome Open Res2021; 6:107.
- 34. Hasnain MG Managing severe tetanus without ventilation support in a resource-limited setting in Bangladesh. Int J Infect Dis2018; 73:164–5.
- 35. Khan MAS, Hasan MJ, Rashid MU, et al Factors associated with in-hospital mortality of adult tetanus patients–a multicenter study from Bangladesh. PLoS Negl Trop Dis2022; 16:e0010235.